Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
____________________
1This document was prepared by the AEGL Development Team composed of Robert Young (Oak Ridge National Laboratory), Julie Klotzbach (SRC, Inc.), Chemical Manager Paul Tobin (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances), and Ernest V. Falke (U.S. Environmental Protection Agency). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold concentrations for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Hexafluoroacetone (HFA) is a colorless gas with a musty odor used in the synthesis of various polymers, medicines, agriculture chemicals, and as an intermediate in various organic syntheses. HFA is highly reactive, reacting vigorously with water and resulting in a series of hydrates (sesquihydrate, monohydrate, and dihydrate) and ultimately producing a stable trihydrate.
There are no inhalation exposure-response data on humans exposed to HFA and no information regarding an odor threshold.
Information on lethality was available from studies in rats and dogs, and evidence of testicular degeneration was found in rats after acute inhalation exposure to HFA. A 30-min LC50 (lethal concentration, 50% lethality) of 900 ppm and a 3-h LC50 of 275 ppm were reported for rats. Other studies reported no lethality after a single 30-min exposure to HFA at 3,600 ppm or after a single 4-h exposure at 200 ppm (300 ppm for HFA nonahydrate). Effects, including lethality, appeared to be mediated systemically and often occurred during postexposure periods. The most prevalent nonlethal responses were lacrimation and salivation during exposure and developmental effects in the offspring of dams exposed to HFA for several days during gestation. Exposure of male rats to HFA resulted in testicular degeneration after repeated exposures at 12 ppm or a single 4-h exposure at 200 ppm.
The mode of action for HFA-induced toxicity is uncertain. The effects of HFA appeared to be systemically mediated with pulmonary damage in rats occurring only at concentrations exceeding minimal lethality levels. Results of
available toxicity studies are indicative of contact irritation (lacrimation and signs of nasal irritation), as well as systemic effects (testicular atrophy, central nervous system depression and neuromuscular dysfunction, weight loss, and renal dysfunction).
Neither qualitative nor quantitative data were available for development of AEGL-1 values for HFA, so no values were established.
Few studies on HFA relevant to AEGL-2 effects were available. Several studies reported reproductive toxicity in male rats after acute inhalation exposure to HFA, and developmental toxicity after female rats were exposed during gestation. Testicular atrophy observed in male rats appeared to be reversible after exposure was stopped. Developmental toxicity was selected as the critical effect for developing AEGL-2 values because those effects occurred at concentrations lower than those linked with testicular effects. Specifically, exposure of pregnant rats to HFA at 1 ppm for 6 h/day on gestation days 7-16 resulted in a slight decrease in mean fetal weight. In the absence of notable maternal toxicity, these findings suggest that the fetus is more sensitive to HFA exposure. A concentration of 1 ppm was selected as the point of departure for calculating AEGL-2 values, under the assumption that a single 6-h exposure during gestation could be responsible for the observed effects. A total uncertainty factor of 30 was applied. A factor of 10 was use to account for uncertainties associated with extrapolating animal data to human exposure conditions. An uncertainty factor of 3 was used for intraspecies variability because HFA does not appear to undergo significant metabolism and because the fetus is considered a sensitive target. Further adjustment was considered unnecessary because of the assumption that the observed effects were the result of a single 6-h exposure during the 10-day gestational exposure period. Time scaling from the 6-h experimental duration to AEGL-specific durations was performed using the equation Cn × t = k; n = 1 was empirically determined from available data (ten Berge et al. 1986). Because of the uncertainty associated with extrapolating a 6-h point of departure to a 10-min exposure duration, the 10-min AEGL-2 value was set equivalent to the 30-min value (NRC 2001).
Studies in rats by E. I. du Pont de Nemours & Co. provided the most comprehensive data from which to develop AEGL-3 values. Two reports (E. I. du Pont de Nemours & Co. 1962a,b) showed that 4-h exposure of rats to HFA at 200 ppm (300 ppm for the nonahydrate) was without lethality and that mortality increased to 50% at 300 ppm (50-75% at 400 ppm for the HFA nonahydrate). The concentration of 200 ppm was selected as the point of departure for AEGL-3 development. An uncertainty factor of 3 was applied to account for uncertainties associated with extrapolating animal data to human exposure conditions. A factor of 3 was used for intraspecies variability because HFA does not appear to undergo significant metabolism. Further adjustment in calculating the AEGL-3 values did not appear justified because the values would similar to or below concentrations shown to be nonlethal in 13-week rat and dog studies (E. I. du Pont de Nemours & Co. 1971). Time scaling from the 4-h experimental duration to AEGL-specific durations was performed using the equation Cn × t = k; n = 1
was empirically determined from available data (ten Berge et al. 1986). Because of uncertainties associated with extrapolating a 4-h point of departure to a 10-min exposure duration, the 10-min AEGL-3 was set equivalent to the 30-min value (NRC 2001).
AEGL values for HFA are presented in Table 4-1.
1. INTRODUCTION
Hexafluoroacetone (HFA) is a colorless gas with a musty odor, and is used in the synthesis of various polymers, medicines, agriculture chemicals, and as an intermediate in various organic syntheses (HSDB 2009; NIOSH 2011). HFA is highly reactive, reacting vigorously with water resulting in a series of hydrates (sesquihydrate, monohydrate, and dihydrate) and ultimately producing a stable trihydrate (Kennedy 1970). Chemical and physical data for HFA are presented in Table 4-2.
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
No data were available on lethality in humans after inhalation exposure to HFA.
TABLE 4-1 Summary of AEGL Values for Hexafluoroacetone
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| AEGL-1 (nondisabling) | NRa | NRa | NRa | NRa | NRa | |
| AEGL-2 (disabling) | 0.40 ppm (2.7 mg/m3) | 0.40 ppm (2.7 mg/m3) | 0.20 ppm (1.4 mg/m3) | 0.050 ppm (0.34 mg/m3) | 0.025 ppm (0.17 mg/m3) | NOAEL for developmental effects in rats (E. I. du Pont de Nemours & Co. 1989) |
| AEGL-3 (lethality) | 160 ppm (1,100 mg/m3) | 160 ppm (1,100 mg/m3) | 80 ppm (540 mg/m3) | 20 ppm (140 mg/m3) | 10 ppm (68 mg/m3) | Lethality threshold estimated from rat LC50 data (E. I. du Pont de Nemours & Co. 1962a,b) |
Abbreviations: LC50, lethal concentration, 50% lethality; NOAEL, no observed adverse effect level; NR, not recommended.
aAbsence of AEGL-1 values does not imply that exposures below AEGL-2 are without effect.
TABLE 4-2 Chemical and Physical Data for Hexafluoroacetone
| Parameter | Value | Reference |
| Synonyms | Hexafluor-2-propane; 1,1,1,3,3,3-hexafluoro-2-propanone; HFA; perfluoroacetone | AIHA 1996; NIOSH 2011 |
| CAS registry no. | 684-16-2 (anhydrous gas) | NIOSH 2011 |
| Chemical formula | C3F6O | NIOSH 2011 |
| Structure | C(C(C(F)(F)F)=O)(F)(F)F | HSDB 2009 |
| Molecular weight | 166.0 | NIOSH 2011 |
| Physical state | Colorless gas | NIOSH 2011 |
| Melting point | -125.45°C | HSDB 2009 |
| Boiling point | -27°C | HSDB 2009 |
| Density/specific gravity | 1.33 g/mL at 25°C | HSDB 2009 |
| Relative vapor density | 5.76 | NIOSH 2011 |
| Solubility in water | Highly reactive | NIOSH 2011 |
| Vapor pressure | 5 mm Hg at 25°C | HSDB 2009 |
| Conversion factors in air | 1 mg/m3 = 0.15 ppm 1 ppm = 6.8 mg/m3 |
NIOSH 2011 |
2.2. Nonlethal Toxicity
No definitive data were available regarding nonlethal effects in humans following inhalation exposure to HFA. It is likely that inhaling HFA would be irritating but quantitative data are only available from an abstract reporting that exposure at 4 ppm was irritating to the upper respiratory tract (Kuznetsova 1972). No odor threshold values were available.
2.3. Developmental and Reproductive Effects
No human developmental or reproductive toxicity data on HFA were available.
2.4. Genotoxicity
No human genotoxicity data on HFA were available.
2.5. Carcinogenicity
No data on the carcinogenic potential of HFA in humans were found.
2.6. Summary
There is very little information on the effects of HFA in humans.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
3.1.1. Rats
In a preliminary study conducted by Haskell Laboratory (E. I. du Pont de Nemours & Co. 1962a), groups of four male rats (250-300 g; age and strain not specified) were exposed to HFA dihydrate at nominal concentrations of 200, 400, or 800 ppm for 4 h. Test atmospheres were generated by vaporization (50-60°C) in dried air of a known amount of the test article. Mortality ratios were 0/4, 3/4, and 4/4 for the 200-, 400-, and 800-ppm groups, respectively. At 800 ppm, two rats died within 2.5 h, one died on day 3, and one on day 5. At 400 ppm, three rats died on day 4. Clinical signs in rats exposed at 400 and 800 ppm included unconsciousness, prostration, hind-leg paralysis, diarrhea, and labored respiration. In some cases, apparent recovery occurred followed by death. Postmortem examination revealed exposure-dependent involvement of the brain, spinal cord, liver, kidney, and pancreas, as well as severe effects on stem cells and developing sperm. Specific responses in rats exposed at 200 ppm is uncertain, because no clinical signs were reported in study’s tabulated data but elsewhere in the report it was stated that rats in this group exhibited similar but less severe signs as those in the 400-ppm group (hind-leg dysfunction, diarrhea, and chromodacorryhea). Histopathologic examination, however, noted involvement (non-specified) of the gastrointestinal tract, spleen, and pituitary (hypoplasia) in all exposure groups with lesser severity and incidence in the 200-ppm group.
The acute lethal toxicity of HFA and HFA nonahydrate was evaluated using groups of four ChR-CD rats exposed for 4 h (E. I. du Pont de Nemours & Co. 1962b). Nominal HFA concentrations were 100, 200, 300, and 400 ppm and HFA nonahydrate concentrations were 300, 400, 500, and 1,000 ppm. There was a 14-day post-exposure observation period. Lethal concentrations were estimated to be 300 ppm for HFA and 400 ppm for HFA nonahydrate (see Table 4-3). Pathologic examination revealed marked concentration-dependent testicular damage (aspermatogenesis, interstitial damage).
Additional studies at Haskell Laboratory examined the lethal response of male ChR-CD rats after exposure to HFA for 15-30 min, and the course of testicular effects after exposure at 200 ppm for 4 h (E. I. du Pont de Nemours & Co. 1965). A control group was placed in the same exposure system but without HFA. In the lethality assessment, groups of four rats (235-327 g) were exposed to HFA at nominal concentrations of 1,200, 3,600, 4,800, or 6,000 ppm for 30 min or at 9,600 ppm for 15 min. Test atmospheres were generated as described in previous Haskell Laboratory studies. At all concentrations, rats exhibited lac-
rimation, salivation, nasal discharge, intermittent gasping, and inactivity during exposure; those that died were cyanotic and exhibited weakness of the extremities. Mortality results are summarized in Table 4-4. All rats lost weight during the post-exposure period. The most prevalent histopathologic finding in both surviving rats and rats that died was marked degeneration and necrosis of the germinal cells of the testes. Lung and thymus changes (no specifics provided) also were observed in all groups, including rats killed at 14 days post-exposure.
Borzelleca and Lester (1965) reported a 30-min LC50 (lethal concentration, 50% lethality) of 900 ppm and a 3-h LC50 of 275 ppm for male and females Wistar rats (150 g; 5/sex/group) exposed at a series of non-specified concentrations of HFA (99.99%) for 0.5, 3, or 6 h. Exposure atmospheres were prepared by mixing a stream of HFA with dry air. Concentrations in the 10-L exposure chamber were adjusted by a calibrated flow meter before mixing or by means of a motor-driven syringe (for low concentrations). Rats that survived the exposure were observed for 15 days. There were no sex-related differences observed, little or no lung damage, and no histopathologic findings in the heart, kidneys, or liver.
TABLE 4-3 Lethal Toxicity in Male Rats Exposed to Hexafluoroacetone and Hexafluoroacetone Nonahydrate for Four Hours
| Chemical | Concentration (ppm) | Mortality ratio | Details | |
| HFA | 100 | 0/4 | ||
| 200 | 0/4 | |||
| 300 | 2/4 | Deaths on post-exposure days 3 and 6 | ||
| 400 | 2/4 | Deaths on post-exposure days 5 and 7 | ||
| HFA | 300 | 0/4 | ||
| nonahydrate | 400 | 1/4 | Death on post-exposure day 5 | |
| 500 | 3/4 | Deaths on post-exposure days 4, 7, and 10 | ||
| 1,000 | 4/4 | Deaths within 17 h to post-exposure day 5 | ||
Source: E. I. du Pont de Nemours & Co. 1962b.
TABLE 4-4 Lethal Toxicity of Hexafluoroacetone in Male Rats After Acute
| Concentration (ppm) | Duration (min) | Mortality Ratio | Details | |
| 2,400 | 30 | 0/4 | ||
| 3,600 | 30 | 0/4 | ||
| 4,800 | 30 | 3/4 | Deaths at 4-days post-exposure | |
| 6,000 | 30 | 4/4 | Deaths at 1-4 days post-exposure | |
| 9,600 | 15 | 3/4 | Deaths at 1-2 days post exposure | |
Source: E. I. du Pont de Nemours & Co. 1965.
In a pilot study conducted by Haskell Laboratories (E. I. du Pont de Nemours & Co. 1988) to determine exposures for assessing the developmental toxicity of HFA, four of six female rats exposed at 60 ppm for 6 h per day for 2 days had to be killed in extremis. Four of six dams exposed at 30 ppm during gestation days 7-16 died, but the day of each death was not specified.
3.1.2. Dogs
The effect of HFA on anesthetized mongrel dogs was studied by Borzelleca and Lester (1965). Male and female dogs were anesthetized with sodium pentobarbitol (intravenous injection of 30 mg/kg) and exposed to HFA at concentrations of 5,000 or 10,000 ppm. HFA concentrations were generated similar to that described for rats (see Section 3.1.1), with HFA mixing with room air in a bag and the mixture being administered to the dogs via an endotracheal tube. Dogs inhaled through the mixing bag and exhaled into a hood. At 5,000 ppm, all three dogs survived a 30-min exposure and one of two survived a 45-min exposure. At 10,000 ppm, two of three dogs each survived a 30-min or 45-min exposure. Deaths occurred 1-3 days post-exposure. Postmortem exams revealed pulmonary hemorrhage and edema but no observable changes in the trachea, heart, spleen, liver, kidneys, gastrointestinal tract, or urinary bladder.
3.2. Nonlethal Toxicity
3.2.1. Rats
A single 4-h exposure of male rats to HFA at 100 or 200 ppm or to HFA nonahydrate at 300 ppm was not lethal (E. I. du Pont de Nemours & Co. 1962b). Lethality in this study was evaluated over a 14-day post-exposure period. This study also reported no lethality in rats repeatedly exposed (10 times) to HFA at 60 ppm for 4 h.
Exposure of groups of four ChR-CD male rats to HFA at 3,600 or 2,400 ppm for 30 min was not lethal (E. I. du Pont de Nemours & Co. 1965). Lethality was assessed up to a 14 days post-exposure. Another phase of this study examined the post-exposure course of testicular effects in rats following a 4-h exposure to HFA at 200 ppm (see Section 3.3). During exposure, rats exhibited deeper respiration than controls, lacrimation, salivation, and redness of the ears. Some rats exhibited chromodacryorrhea for 1-7 days post-exposure, and all treated rats experienced body weight loss for 1-3 days.
A 13-week inhalation exposure study, groups of 30 male and 30 female ChR-CD rats (245-327 g and 180-248 g, respectively) were exposed to HFA at 0, 0.1, 1.0, or 12 ppm for 6 h/day, 5 days/week (E. I. du Pont de Nemours & Co. 1971). Post-exposure assessments were conducted at 28 and 84 days. Test atmospheres were generated via metered distribution from supply cylinders and mixing with dry air and supply air (50% relative humidity). Air from each
chamber was sampled daily and analyzed for HFA by gas chromatography analysis of trifluoromethane generated by reacting the HFA-containing samples with sodium hydroxide. There were no observations at exposure durations consistent with AEGL durations. No gross, biochemical, hematologic, or histopathologic changes were found in rats exposed at 0.1 ppm, and the only treatment-related effects noted in the 1.0-ppm group was reversible kidney dysfunction. In the 12-ppm group, the most notable effects were testicular atrophy with interstitial edema and oligospermia at 30 days, and cessation of spermiogenesis, severe interstitial edema, and sloughing of germinal cells at 90 days. The investigators reported that these effects were reversible on the basis of 28- and 84-day postexposure observations.
3.2.2. Dogs
Groups of six male beagles (8.6-10 kg) were exposed to HFA at 0, 0.1, 1.0, or 12 ppm for 6 h/day, 5 days/week for 13 weeks, followed by postexposure assessment at 45 days (E. I. du Pont de Nemours & Co. 1971). Testes weight was decreased and pituitary and lung weights were increased, but the effects were reversible. Reversible testicular damage was observed in dogs at 12 ppm, but no testicular effects were observed at 0.1 or 1 ppm.
3.3. Developmental and Reproductive Effects
As previously noted in Section 3.1.1, no clinical signs were observed in a group of four rats exposed to HFA dehydrate at 200 ppm for 4 h (E. I. du Pont de Nemours & Co. 1962b). However, pathologic examination revealed effects on the gastrointestinal tract, spleen, pituitary gland, and spermatazoa.
Subsequent studies at Haskell Laboratory examined the course of testicular effects in rats exposed to HFA by inhalation (E. I. du Pont de Nemours & Co. 1965). Twelve rats were exposed to HFA at 200 ppm for 4 h. A control group was placed in the same exposure system but without HFA. Three rats were killed on post-exposure days 7, 14, 28, and 57 for histopathologic assessment. Rats in this phase of the study exhibited similar signs of exposure (lacrimation, salivation, and post-exposure weight loss) as did those exposed to nonlethal concentrations in the previous experiments assessing lethality. Both absolute and relative (to body weight) weights of the testes decreased in exposed animals compared with controls. Although some recovery from testicular degeneration was noted by day 57, some spermatogenic tubules still had no germinal cells.
An additional study conducted by E. I. du Pont de Nemours & Co. (1989) examined the developmental toxicity of HFA in groups of 24 Crl:CD7BR rats exposed by nose-only (0, 0.11, 1.0, or 6.9 ppm, mean chamber concentrations) for 6 h/day on gestation days 7-16. The exposure system was described in detail and affirmed uniform distribution of the test atmosphere. Exposure concentra-
tions in the test chamber were measured hourly and determined by HFA-hydrate formation and its analysis by gas chromatography. Nose-only exposure was used to minimize hydrate aerosol formation, dermal and oral absorption, and subsequent deposition onto the pelt of the animals. All female rats survived to scheduled sacrifice on gestation day 22, although a significant (p ≤ 0.05) decrease in body weight change relative to controls was found in the high-dose group on gestation days 17-22. However, absolute body weights adjusted to eliminate the products of conception (live and dead fetuses) were not significantly different from controls. Both absolute and relative (to body weight) liver weights were significantly greater (p ≤ 0.05) in rats of the 1- and 6.9-ppm groups. Reproductive effects included a significant (p ≤ 0.05) treatment-related decrease in total live fetuses and number of female live fetuses in the 6.9-ppm group. Fetal effects included significantly lower (p ≤ 0.05) fetal body weights in the 1- and 6.9-ppm groups, increased incidences of malformations, and external and skeletal developmental variations in the 6.9-ppm group. Major findings of this study are summarized in Table 4-5. The investigators concluded that HFA at 6.9 ppm resulted in significant increases in resorptions, malformations, developmental variations, and variations due to retarded development, and that exposure at 1 ppm resulted in increased incidences of skeletal developmental variations and decreases in fetal weights. Developmental effects at 1 and 6.9 ppm were considered by the investigators to be of greater severity than the severity of concurrent maternal responses.
TABLE 4-5 Effects of Hexafluoroacetone in Rats Exposed During Gestation
| Effect | Control | 0.1 ppm | 1.0 ppm | 6.9 ppm |
| Maternal effects | ||||
| Liver weight (absolute) | 14.3 g | 14.7 g | 15.7 ga | 16.2 ga |
| Liver weight (relative) | 4.9 g | 4.8 g | 5.2 ga | 5.4 ga |
| Reproductive effects | ||||
| No. live fetuses | 300 | 270 | 339 | 277 |
| Live fetuses/litter | 14.3 | 13.5 | 14.1 | 11.5a |
| Total resoprtions/litter | 1.0 | 1.8 | 1.0 | 3.5a |
| Fetal effects | ||||
| Mean fetal weight | 5.30 | 5.21 | 4.94a | 4.11a |
| Malformationsb | 0 | 0 | 3 | 68a |
| Variationsb | ||||
| Developmental | 60 | 36 | 86 | 204 |
| Retarded development | 27 | 26 | 64 | 194 |
ap ≤ 0.05 relative to untreated controls.
bTotal number of fetuses affected; includes external, visceral, head, and skeletal malformations.
Source: E. I. du Pont de Nemours & Co. 1989.
Testicular atrophy was also reported for Crl:CD7BR rats exposed to HFA at 12 ppm for 6 h/day, 5 days/week for 30 days (Lee and Kennedy 1991). After 90 days of exposure, more severe atrophy was observed. No significant testicular effects were observed in rats exposed at 0.1 or 1.0 ppm, and some evidence of regeneration of atrophic testes was observed in the 12-ppm group at postexposure day 28, but normal spermatogenesis was only partially restored at postexposure day 84.
3.4. Genotoxicity
HFA sesquihydrate was not mutagenic in Salmonella typhimurium strains TA 1535, 1537, and 1538 with or without activation (S-9) at concentrations up to 7,500 µg/plate (E. I. du Pont de Nemours & Co. 1975).
3.5. Carcinogenicity
There were no data with which to evaluate the carcinogenic potential of HFA.
3.6. Summary
A 30-min LC50 of 900 ppm and a 3-h LC50 of 275 ppm have been reported for HFA in rats. Other studies reported no lethality after a single 30-min exposure to HFA at 3,600 ppm or a single 4-h exposure at 200 ppm. Effects, including lethality, appeared to be mediated systemically and often occurred during post-exposure periods. Mortality results of repeated exposures to HFA are equivocal; four of six rats died after being exposed to HFA at 60 ppm for 6 h/day for two days, but in another study it was reported that no lethality was observed when rat were exposed 10 times to HFA at 60 ppm for 4 h/day. At 30 ppm, it was reported that four of six pregnant rats died after being exposed to HFA for 10 days. The most prevalent nonlethal responses to HFA after inhalation exposure were lacrimation and salivation during exposure and developmental effects in rats when dams were exposed to HFA for several days during gestation. Exposure of male rats to HFA consistently resulted in testicular degeneration after multiple exposures at 12 ppm or a single 4-h exposure at 200 ppm.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
No information was available regarding the metabolism and disposition of HFA following inhalation exposure. Gillies and Rickard (1984) reported that [14C]HFA exhibited biphasic elimination from the blood after it was adminis-
tered subcutaneously in rats at 3 mg/kg. The half-life for the initial and elimination phases were 22.6 and 75.1 h, respectively. HFA was eliminated unchanged in the urine (81% at 120 h) and feces (9% at 120 h). Although toxicity data clearly indicate the testes as a target organ, there was no unusual accumulation or sequestration of [C14]HFA.
4.2. Mechanism of Toxicity
The mechanism(s) by which HFA exerts toxic effects is uncertain. No studies were available that specifically addressed the topic. Borzelleca and Lester (1965) noted that HFA effects appeared to be systemically mediated with pulmonary damage in rats occurring only at air concentrations exceeding minimal lethality levels. Studies described in Section 3 indicate contact irritation effects (lacrimation and signs of nasal irritation) and systemic effects (testicular atrophy, central nervous system depression, neuromuscular dysfunction, weight loss, and renal dysfunction).
Results of a study by Gillies and Lee (1983) suggested that HFA-induced alteration of lipid metabolism and the resulting inhibition of sterol synthesis was associated with testicular atrophy in rats.
4.3. Structure-Activity Relationships
No data were available with which to develop definitive structure-activity analyses.
5. DATA ANALYSIS FOR AEGL-1
5.1. Human Data Relevant to AEGL-1
No data were available regarding AEGL-1 type effects in humans.
5.2. Animal Data Relevant to AEGL-1
No exposure-response data in animals consistent with AEGL-1 effects were available.
5.3. Derivation of AEGL-1
Neither qualitative nor quantitative data were available regarding AEGL-1 type effects resulting from acute inhalation exposure to HFA. Therefore, no AEGL-1 values are recommended. Although some studies reported exposures that were without serious or lethal effects, no details were available about the severity of responses relevant to AEGL-1 effects.
6. DATA ANALYSIS FOR AEGL-2
6.1. Human Data Relevant to AEGL-2
No data were available on nonlethal adverse effects in humans resulting from inhalation exposure to HFA.
6.2. Animal Data Relevant to AEGL-2
Information regarding the adverse effects of HFA in animals after acute inhalation exposure was only available from studies conducted by E. I. du Pont de Nemours & Co. (see Sections 3.2 and 3.3). A single 4-h exposure of male rats to HFA at 200 ppm did not result in any effects consistent with AEGL-2 severity. However, follow-up evaluation for 57 days revealed decreased absolute and relative testicular weights compared with unexposed controls. Results of a developmental study in rats showed that nose-only exposure to HFA at 7 ppm for 6 h/day on gestation days 7-16 resulted in a significantly decreased mean fetal weight, decreased number of live fetuses per litter, and an increase in total resorptions per litter that, according to the investigators, was not a function of maternal effects (significantly decreased body weight [although not significant when adjusted for products of conception] and increased absolute and relative liver weight).
6.3. Derivation of AEGL-2
There is a paucity of information on HFA with which to develop AEGL-2 values. No human data were available. Several studies have reported developmental and reproductive toxicity of HFA after acute inhalation exposure of male rats (E. I. du Pont de Nemours & Co. 1965) and exposure of female rats during gestation (E. I. du Pont de Nemours & Co. 1989). Testicular atrophy observed in male rats tended to be reversible. Evidence of developmental toxicity in rats occurred at lower concentrations than did testicular effects and were selected as the critical effect for development of AEGL-2 values for HFA.
Exposure of dams to HFA at 6.9 ppm for 6 h/day on gestation days 7-16 resulted in a significant decrease in the number of live fetuses per litter, total resorptions per litter, and mean fetal weight, and exposure at 1 ppm resulted in a slight decrease in mean fetal weight (E. I. du Pont de Nemours & Co. 1989). In the absence of notable maternal toxicity, these findings suggest that the fetus is uniquely sensitive to HFA exposure. The concentration of 1.0 ppm was selected as the point of departure for AEGL-2 development under the assumption that a single 6-h exposure during gestation could be responsible for the observed effects. A total uncertainty factor of 30 was applied. A factor of 10 was applied to account for uncertainties associated with extrapolating animal data to human exposure conditions. An uncertainty factor of 3 was used to account for intra-
species variability because HFA does not appear to undergo significant metabolism and because the fetus is considered a sensitive target. Further adjustment was considered unnecessary because of the assumption that the observed effects were the result of a single 6-h exposure during the 10-day gestational exposure period. For time scaling from the 6-h experimental duration to AEGL-specific durations, the equation Cn × t = k was applied. The value of n was empirically determined from the available data to be 1 (ten Berge et al. 1986).
AEGL-2 values for HFA are presented in Table 4-6, and their derivation is summarized in Appendix A. Because of the uncertainty associated with extrapolating a 6-h point of departure to a 10-min exposure duration, the 30-min AEGL-2 value was set equal to the 30-min value.
7. DATA ANALYSIS FOR AEGL-3
7.1. Human Data Relevant to AEGL-3
No data were available regarding lethality of inhaled HFA in humans.
7.2. Animal Data Relevant to AEGL-3
Lethality data on HFA were available from studies in rats and dogs, although the latter involved the use of anesthetized animals and was not definitive regarding a lethality threshold. Results of acute inhalation exposure studies in rats by E. I. du Pont de Nemours & Co. (1962a,b) indicated that 200 ppm was a 4-h nonlethal exposure, but that 300 ppm produced 50% mortality and 400 ppm produced 75% mortality. A study of HFA nonahydrate suggested slightly lower toxicity; 25% mortality occurred in rats after a 4-h exposure at 400 ppm. A subsequent study by E. I. du Pont de Nemours & Co. (1965) showed that higher concentrations were required for a lethal effect when exposure duration was decreased. Specifically, 30-min exposure at 2,400 or 3,600 ppm was not lethal to groups of four rats. However, exposure at 9,600 ppm for 15 min or at 4,800 ppm for 30 min resulted in 75% mortality. A 30-min and a 3-h LC50 of 900 and 275 ppm, respectively, were reported by Borzelleca and Lester (1965). Post-exposure observation periods in most of the studies revealed systemic involvement, and deaths often occurred after exposure ended. Two 6-h/day exposures at 60 ppm resulted in four of six female rats being killed in extremis (E. I. du Pont de Nemours & Co. 1988).
TABLE 4-6 AEGL-2 Values for Hexafluoroacetone
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 0.40 ppm (2.7 mg/m3) | 0.40 ppm (2.7 mg/m3) | 0.20 ppm (1.4 mg/m3) | 0.050 ppm (0.34 mg/m3) | 0.025 ppm (0.17 mg/m3) |
7.3. Derivation of AEGL-3
The E. I. du Pont de Nemours & Co. studies in rats provided the most comprehensive data from which to develop AEGL-3 values. Two reports (E. I. du Pont de Nemours & Co. 1962 a,b) showed that 4-h exposure of rats to HFA at 200 ppm (300 ppm for the nonahydrate) was without lethality and that mortality increased to 50% at 300 ppm and 50-75% at 400 ppm. A 4-h exposure at 200 ppm was selected as the point of departure for AEGL-3 development. A factor of 3 was applied to account for uncertainties associated with extrapolating animal data to human exposure conditions. A factor of 3 was applied to account for individual variability, because HFA does not appear to undergo significant metabolism. Additional uncertainty factors were not applied because AEGL-3 values would be similar to or below the concentrations shown to be nonlethal in 13-week rat and dog studies (E. I. du Pont de Nemours & Co. 1971). For time scaling from the 4-h experimental duration to AEGL-specific durations, the equation Cn × t = k was applied. The value of n was empirically determined from the available data to be 1 (ten Berge et al. 1986).
AEGL-3 values for HFA are presented in Table 4-7 and their derivation is summarized in Appendix A. Because of the uncertainty in extrapolating from a 4-h point of departure to a 10 min exposure duration, the 30-min AEGL-2 value was set equal to the 30-min value.
8. SUMMARY OF AEGLs
8.1. AEGL Values and Toxicity End Points
There were inadequate data for developing AEGL-1 values. AEGL-2 values were based on results of studies in rats conducted by E. I. du Pont de Nemours & Co. (1989) indicating that HFA was toxic to the fetus in the absence of significant maternal toxicity. Although the developmental study selected as the basis for AEGL-2 development used a multiple exposure protocol (gestation days 7-16), the point of departure was based on the assumption that the observed effects could have been the result of a single-day exposure. AEGL-3 values were developed using a no-effect level for lethality (200 ppm for 4 h) in rats as the point of departure. AEGL values for HFA are presented in Table 4-8. The relationship of the AEGL-2 and AEGL-3 values to available toxicity data are shown in a category plot in Appendix D.
TABLE 4-7 AEGL-3 Values for Hexafluoroacetone
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 160 ppm (1,100 mg/m3) | 160 ppm (1,100 mg/m3) | 80 ppm (540 mg/m3) | 20 ppm (140 mg/m3) | 10 ppm (68 mg/m3) |
8.2. Comparisons with Other Standards and Guidelines
Standards and guidance levels for workplace and community exposures in the United States are presented in Table 4-9. Additionally, the Health Council of the Netherlands Committee on Updating of Occupational Exposure Limits developed an occupational exposure limit of 0.05 mg/m3 (≈0.0075 ppm) for HFA and its hydrates on the basis of HFA-induced developmental toxicity (Health Council of the Netherlands 2001).
TABLE 4-8 AEGL Values for Hexafluoroacetone
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h |
| AEGL-1 (nondisabling) | NRa | NRa | NRa | NRa | NRa |
| AEGL-2 (disabling) | 0.40 ppm (2.7 mg/m3) | 0.40 ppm (2.7 mg/m3) | 0.20 ppm (1.4 mg/m3) | 0.050 ppm (0.34 mg/m3) | 0.025 ppm (0.17 mg/m3) |
| AEGL-3 (lethality) | 160 ppm (1,100 mg/m3) | 160 ppm (1,100 mg/m3) | 80 ppm (540 mg/m3) | 20 ppm (140 mg/m3) | 10 ppm (68 mg/m3) |
aAbsence of AEGL-1 values does not imply that exposures below the AEGL-2 values are without effect.
TABLE 4-9 Extant Standards and Guidelines for Hexafluoroacetone
| Guideline | Exposure Duration | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h | |
| AEGL-1 | NRa | NRa | NRa | NRa | NRa |
| AEGL-2 | 0.40 ppm | 0.40 ppm | 0.20 ppm | 0.050 ppm | 0.025 ppm |
| AEGL-3 | 160 ppm | 160 ppm | 80 ppm | 20 ppm | 10 ppm |
| ERPG-1 (AIHA)b | — | ||||
| ERPG-2 (AIHA) | 1 ppm | ||||
| ERPG-3 (AIHA) | 50 ppm | ||||
| TLV-TWA (ACGIH)c | 0.1 ppm | ||||
| REL-TWA (NIOSH)d | 0.1 ppm (skin) | ||||
| MAC (the Netherlands)e | 0.0075 ppm | ||||
aNR, not recommended; absence of AEGL-1 values does not imply that exposure below the AEGL-2 values is without adverse effects.
bERPG (emergency response planning guidelines, American Industrial Hygiene Association [AIHA] 1996).
ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to one h without experiencing other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. Not applicable for HFA.
ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to one h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protection action.
ERPG-3 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to one h without experiencing or developing life-threatening health effects.
cTLV-TWA (threshold limit value - time weighted average, American Conference of Governmental Industrial Hygienists [ACGIH 2003]) is the time-weighted average concentration for a normal 8-h workday and a 40-h workweek, to which nearly all workers may be repeatedly exposed, day after day, without adverse effect.
dREL-TWA (recommended exposure limits - time weighted average, National Institute for Occupational Safety and Health [NIOSH 2011]) is defined analogous to the ACGIH TLV-TWA. The skin notation indicates the potential for dermal absorption; skin exposure should be prevented as necessary.
eMAC (maximaal aanvaaarde concentratie [maximum accepted concentration], SDU Uitgevers [under the auspices of the Ministry of Social Affairs and Employment], The Hague, The Netherlands, MSZW 2004) is defined analogous to the ACGIH TLV-TWA.
8.3. Data Adequacy and Research Needs
Human data on HFA are lacking. The available animal data on HFA include cursory lethality studies which used relatively small numbers of animals. Studies were conducted primarily in rats, with a few in dogs, so little information was available on species variability. There was no definitive information on exposure-response relationships for clinical effects or on the mode action of HFA.
9. REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 2003. Threshold Limit Values and Biological Exposure Indices. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
AIHA (American Industrial Hygiene Association). 1996. The AIHA 1996 Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guidelines Handbook. Fairfax VA: AIHA Press.
Borzelleca, J.F., and D. Lester. 1965. Acute toxicity of some perhalogenated acetones. Toxicol. Appl. Pharmacol. 7(4):592-597.
E. I. du Pont de Nemours & Co. 1962a. Inhalation Toxicity of Hexafluoroacetone Dihydrate in Rats. Haskell Laboratory Report No. 47-62. Haskell Laboratory for Toxicology and Industrial Hygiene, E. I. du Pont de Nemours Co. June 27, 1962.
E. I. du Pont de Nemours & Co. 1962b. Inhalation Toxicity of Hexafluoroacetone Compound in Rats. Haskell Laboratory Report No. 46-62. Haskell Laboratory for Toxicology and Industrial Hygiene, E. I. du Pont de Nemours Co.
E. I. du Pont de Nemours & Co. 1965. Inhalation Studies on Hexafluoroacetone. Part II. A. The Lethality of Short (<1 hr.). B. The Persistence of Tissue Effects. Haskell
Laboratory Report No. 6-65. Haskell Laboratory for Toxicology and Industrial Hygiene, E. I. du Pont de Nemours & Co. January 25, 1965.
E. I. du Pont de Nemours & Co. 1971. Thirteen-Week Inhalation Exposure of Rats and Dogs to Hexafluoroacetone (HFA). Haskell Laboratory Report No. 4-71. Haskell Laboratory for Toxicology and Industrial Medicine, E. I. du Pont de Nemours & Co., Inc. January 7, 1971.
E. I. du Pont de Nemours & Co. 1975. In vitro Microbial Mutagenicity Studies of Hexafluoroacetone Sesquihydrate. Haskell Laboratory Report No. 340-75. Haskell Laboratory for Toxicology and Industrial Medicine, E. I. du Pont de Nemours & Co., Inc. June 19, 1975.
E. I. du Pont de Nemours & Co. 1988. Pilot Developmental Toxicity Study of Hexafluoroacetone in the Rat - Summary of Reproductive Outcome with Attached Letter and Receipt Dated April 13, 1988 and Cover Letter Dated 122988. Haskell Laboratory for Toxicology E. I. du Pont de Nemours & Co.
E. I. du Pont de Nemours & Co. 1989. Developmental Toxicity Study of Hexafluoroacetone (HFA) in the Rat with Cover Letter dated 042889. E. I. du Pont de Nemours & Co., Inc. Medical Research No. 8166-001. Du Pont HLR 776-88.
Gillies, P.J., and K.P. Lee. 1983. Effects of hexafluoroacetone on testicular morphology and lipid metabolism in the rat. Toxicol. Appl. Pharmacol. 68(2):188-197.
Gillies, P.J., and R.W. Rickard. 1984. Toxicokinetics of [14C]hexafluoroacetone in the rat. Toxicol. Appl. Pharmacol. 73(1):23-29.
Haber, F. 1924.On the history of the gas war. Pp. 76-92 in Five Lectures from the Year 1920-1923 [in German]. Berlin: Springer-Verlag.
Health Council of the Netherlands. 2001. Hexafluoroacetone; Health-Based Reassessment of Administrative Occupational Exposure Limits. Committee on Updating of Occupational Exposure Limits. Publication No. 2000/150SH/023. The Hague: Health Council of the Netherlands [online]. Available: http://www.gezondheidsraad.nl/sites/default/files/00@15023OSH.PDF [accessed Oct. 1, 2012].
HSDB (Hazardous Substances Data Bank). 2009. 1,1,1,3,3,3-hexafluoro-2-propanone (CASRN 684-16-2). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Oct. 1, 2012].
Kennedy, G.L., Jr. 1990. Toxicology of fluorine-containing monomers. Crit. Rev. Toxicol. 21(2):149-170.
Kuznetsova, E.E. 1972. Hygienic standardization of perfluoroacetone dihydrate in air of working zones [in Russian]. Nauch. Tr. Irkutsk. Med. Inst. 115:54-56 (as cited in Health Council of the Netherlands 2001).
Lee, K.P., and G.L. Kennedy, Jr. 1991. Testicular toxicity of rats exposed to hexafluoracetone (HFA) for 90 days. Toxicology 67(3):249-265.
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: Hexafluoraceton. Den Haag: SDU Uitgevers [online]. Available: http://www.lasrook.net/lasrookNL/maclijst2004.htm [accessed Oct. 1, 2012].
NIOSH (National Institute for Occupational Safety and Health). 2011. NIOSH Pocket Guide to Chemical Hazards: Hexafluoroacetone. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH [online]. Available: http://www.cdc.gov/niosh/npg/npgd0319.html [accessed Oct. 1, 2012].
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
Rinehart, W.E., and T. Hatch. 1964. Concentration-time product (CT) as an expression of dose in sublethal exposures to phosgene. Ind. Hyg. J. 25(6):545-553.
ten Berge, W.F., A. Zwart, and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Mater. 13(3):301-309.
APPENDIX A
DERIVATION OF AEGL VALUES FOR HEXAFLUOROACETONE
Derivation of AEGL-1 Values
No AEGL-1 values were recommended because of inadequate data. Absence of AEGL-1 values does not imply that exposure below the AEGL-2 values is without effect.
Derivation of AEGL-2 Values
| Key study: | E. I. du Pont de Nemours & Co. 1989. Developmental Toxicity Study of Hexafluoroacetone (HFA) in the Rat with Cover Letter Dated 042889. E. I. du Pont de Nemours & Co., Inc. Medical Research No. 8166-001. Du Pont HLR 776-88. Unpublished report. |
| Critical effect: | A significant (p ≤ 0.05) decrease in live fetuses per litter, total resorptions per litter, and mean fetal weight was observed in pregnant rats exposed to HFA at 6.9 ppm for 6 h/day on gestation days 7-16. At 1 ppm, only mean fetal body weight was decreased. Thus, 1 ppm was selected as the point of departure. It was assumed that the observed effects could be induced by a single 6-h exposure. |
| Time scaling: | Cn × t = k; n = 1 was determined empirically from available data (ten Berge et al. 1986). (1 ppm)1 × 6 h = 6 ppm-h |
| Uncertainty factors: | 10 for interspecies differences 3 for intraspecies variability, because HFA does not appear to undergo significant metabolism and because the fetus is considered a sensitive target. A larger factor was considered unnecessary because of the assumption that the effects reported in the key study were the result of |
| a single 6-h exposure during the 10-day gestational exposure period. Total uncertainty factor of 30 |
|
| Calculations: | |
| 10-min AEGL-2: | Set equivalent to the 30-min AEGL-2 value of 0.40 ppm, because of the uncertainty with extrapolating a 6-h point of departure to a 10-min exposure duration (NRC 2001). |
| 30-min AEGL-2: | C × 0.5 h = 6 ppm-h 12 ppm ÷ 30 = 0.40 ppm |
| 1-h AEGL-2: | C × 1 h = 6 ppm-h 6 ppm ÷ 30 = 0.20 ppm |
| 4-h AEGL-2: | C × 4 h = 6 ppm-h 1.5 ppm ÷ 30 = 0.050 ppm |
| 8-h AEGL-2: | C × 8 h = 6 ppm-h 0.75 ppm ÷ 30 = 0.025 ppm |
Derivation of AEGL-3 Values
| Key studies: | E. I. du Pont de Nemours & Co. 1962b. Inhalation Toxicity of Hexafluoroacetone Compound in Rats. Haskell Laboratory report No. 46-62. Haskell Laboratory for Toxicology and Industrial Hygiene, E. I. du Pont de Nemours & Co. Unpublished report. |
| E. I. du Pont de Nemours & Co. 1962a. Inhalation Toxicity of Hexafluoroacetone Dihydrate in Rats. Haskell Laboratory report No. 47-62. Haskell Laboratory for Toxicology and Industrial Hygiene, E. I. du Pont de Nemours & Co. June 27, 1962. Unpublished report. | |
| Critical effect: | No lethality in male rats exposed at 200 ppm for 4 h. |
| Time scaling: | Cn × t = k; n = 1 was determined empirically from available data (ten Berge et al. 1986). (200 ppm)1 × 4 h = 800 ppm-h |
| Uncertainty factors: | 3 for interspecies differences; no irreversible effects were observed in studies of rats and dogs exposed to HFA at 12 ppm for up to 13 weeks (6 h/day, 5 days/week). 3 for intraspecies variability; HFA does not appear to undergo significant metabolism and a larger adjustment would result in exposure concentrations below those shown to be nonlethal in multiple-exposure rat and dog studies (E. I. du Pont de Nemours & Co. 1971). Total uncertainty factor of 10 |
| Calculations: | |
| 10-min AEGL-3: | Set equivalent to the 30-min AEGL-3 value of 160 ppm, because of the uncertainty with extrapolating a 4-h point of departure to a 10-min exposure duration (NRC 2001). |
| 30-min AEGL-3: | C × 0.5 h = 800 ppm-h 1,600 ppm ÷ 10 = 160 ppm |
| 1-h AEGL-3: | C × 1 h = 800 ppm-h 800 ppm ÷ 10 = 80 ppm |
| 4-h AEGL-3: | C × 4 h = 800 ppm-h 200 ppm ÷ 10 = 20 ppm |
| 8-h AEGL-3: | C × 8 h = 800 ppm-h 100 ppm ÷ 10 = 10 ppm |
APPENDIX B
TIME SCALING CALCULATIONS
The relationship between dose and time for any given chemical is a function of the physical and chemical properties of the substance and its toxicologic and pharmacologic properties. Historically, the relationship according to Haber (1924), commonly called Haber’s Law (NRC 1993) or Haber’s Rule (C × t = k, where C = exposure concentration, t = exposure duration, and k = a constant) has been used to relate exposure concentration and duration to effect (Rinehart and Hatch 1964). According to this concept, exposure concentration and exposure duration may be reciprocally adjusted to maintain a cumulative exposure constant (k) and this cumulative exposure constant will always reflect a specific quantitative and qualitative response. This inverse relationship of concentration and time may be valid when the toxic response to a chemical is dependent equally on the concentration and the exposure duration.
75% Mortality Response in Rats (E. I. Du Pont de Nemours & Co. 1962a, 1965)
| Time | Concentration | Log | Log | Regression output | |
| Time | Concentration | ||||
| 15 | 9,600 | 1.1761 | 3.9823 | Intercept | 5.2508 |
| 30 | 4,800 | 1.4771 | 3.6812 | Slope | -1.0708 |
| 240 | 500 | 2.3802 | 2.6990 | R squared | 0.9997 |
| Correlation | -0.9999 | ||||
| Degrees of freedom | 1 | ||||
| Observations | 3 | ||||
However, an assessment by ten Berge et al. (1986) of LC50 data for certain chemicals revealed chemical-specific relationships between exposure concentration and exposure duration that were often exponential. The relationship can be expressed by the equation Cn × t = k, where n represents a chemical-specific, and even a toxic-end-point specific, exponent. The relationship described by this equation is basically the form of a linear regression analysis of the log-log transformation of a plot of C vs. t. ten Berge et al. (1986) examined the airborne concentration (C) and short-term exposure duration (t) relationship relative to death for approximately 20 chemicals and found that the empirically derived value of n ranged from 0.8 to 3.5 among this group of chemicals. Hence, the value of the exponent (n) in the equation Cn × t = k quantitatively defines the relationship between exposure concentration and exposure duration for a given chemical and for a specific health-effect end point. Haber’s Rule is the special case where n = 1. As the value of n increases, the plot of C vs. t yields a progressive decrease in the slope of the curve.
AEGL values for HFA were derived on the basis of 6-h (AEGL-2) and 4-h (AEGL-3) experimental durations. The equation Cn × t = k was applied. The value of n was empirically determined from available data to be 1 (ten Berge et al. 1986).
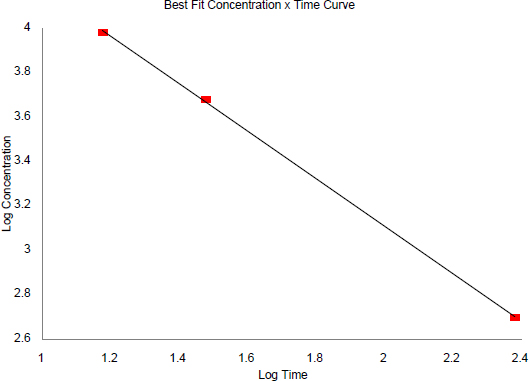
FIGURE B-1 Regression Plot of LC75 Values in Rats from Studies by E. I. Du Pont de Nemours & Co. (1962a, 1965).
APPENDIX C
ACUTE EXPOSURE GUIDELINE LEVELS FOR HEXAFLUOROACETONE
Derivation Summary
Inadequate data exist for deriving AEGL-1 values for HFA. Absence of AEGL-1 values does not indicate that exposure below AEGL-2 values is without effect.
AEGL-2 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 0.40 ppm | 0.40 ppm | 0.20 ppm | 0.050 ppm | 0.025 ppm |
| Reference: E. I. du Pont de Nemours & Co. 1989. Developmental Toxicity Study of Hexafluoroacetone (HFA) in the Rat with Cover Letter Dated 042889. E. I. du Pont de Nemours & Co., Inc. Medical Research No. 8166-001. Du Pont HLR 776-88. npublished report. | ||||
| Test species/Strain/Sex/Number: Rat, Crl:CD7BR, female, 24 | ||||
| Exposure route/Concentrations/Durations: Nose-only inhalation, HFA at 0, 0.11, 1.0 or 6.9 ppm (mean chamber concentrations) for 6 h/day on gestation days 7-16. | ||||
| Effects: Significant (p ≤ 0.05) decreases in live fetuses per litter, total resorptions per litter, and mean fetal weight were observed at 6.9 ppm. At 1 ppm, only mean fetal body weight was decreased; so, 1 ppm was selected as the point of departure. | ||||
| End point/Concentration/Rationale: It was assumed that the observed effects at 1 ppm could be induced by a single 6-h exposure. | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 30 Interspecies: 10 to account for extrapolating animal data to human exposure conditions. Intraspecies: 3 for intraspecies variability, because HFA does not appear to undergo significant metabolism and because the fetus is considered a sensitive target. A larger factor was considered unnecessary because of the assumption that the observed effects were the result of a single 6-h exposure during the 10-day gestational exposure period. |
||||
| Modifying factor: None | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time scaling: For time scaling from the 6-h experimental duration to AEGL-specific durations, the equation Cn × t = k was applied. The value of n was determined empirically from available data to be 1 (ten Berge et al. 1986). Because of the uncertainty in extrapolating a 6-h point of departure to a 10-min exposure duration, the 30-min AEGL-2 value was set equivalent to the 30-min value. | ||||
| Data adequacy: Data were considered adequate for AEGL-2 development. However, no human exposure data were available to compare with the AEGL values. | ||||
AEGL-3 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 160 ppm | 160 ppm | 80 ppm | 20 ppm | 10 ppm |
| Reference: E. I. du Pont de Nemours & Co. 1962b. Inhalation Toxicity of Hexafluoroacetone Compound in Rats. Haskell Laboratory report No. 46-62. Haskell Laboratory for Toxicology and Industrial Hygiene, E. I. du Pont de Nemours & Co. Unpublished report. E. I. du Pont de Nemours & Co. 1962a. Inhalation Toxicity of Hexafluoroacetone Dihydrate in Rats. Haskell Laboratory report No. 47-62. Haskell Laboratory for Toxicology and Industrial Hygiene, E. I. du Pont de Nemours & Co. June 27, 1962. Unpublished report. |
||||
| Test Species/Strain/Sex/Number: Rat, ChR-CD, male, 4 | ||||
| Exposure Route/Concentrations/Durations: Inhalation; HFA at 100, 200, 300, and 400 ppm (nominal) for 4 h; HFA nonahydrate at 300, 400, 500, and 1,000 ppm (nominal) for 4 h | ||||
| Effects: Lethality | ||||
| End point/Concentration/Rationale: No lethality observed with HFA at 200 ppm or with HFA nonahydrate at 300 ppm HFA. HFA at 300 ppm resulted in 50% mortality and HFA nonahydrate at 400 ppm resulted in 25% mortality. | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 10 Interspecies: 3 was applied to account for uncertainties associated with extrapolating animal data to human exposure conditions; no irreversible effects were observed in studies of rats and dogs exposed to HFA at 12 ppm for up to 13 weeks (6 h/day, 5 days/week). Intraspecies: 3 for intraspecies variability because HFA does not appear to undergo significant metabolism and because a larger factor would result in exposure concentrations below those shown to be nonlethal in multiple-exposure rat and dog studies (E. I. du Pont de Nemours & Co. 1971). |
||||
| Modifying factor: None | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time scaling: For the time scaling from the 4-h experimental duration to AEGLspecific durations, the equation Cn × t = k was applied, where n = 1 was determined empirically from available data (ten Berge et al. 1986). Because of the uncertainty in extrapolating a 4-h point of departure to a 10-min exposure duration, the 30-min AEGL-3 value was set equivalent to the 30-min value. | ||||
| Data adequacy: Lethality data were considered adequate for development of AEGL-3 values. | ||||
APPENDIX D
CATEGORY PLOT FOR HEXAFLUOROACETONE
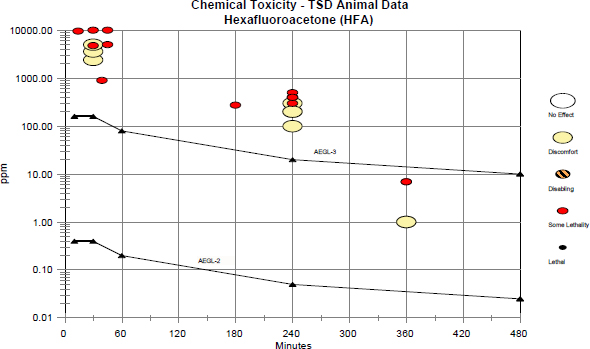
FIGURE D-1 Category plot of toxicity data and AEGL values for hexafluoroacetone. The 360-min data entries between the AEGL-2 and AEGL-3 values reflect multiple exposures during gestation (see Sections 3.3 and 6.3) and are not single 6-h exposures. Because of uncertainties in extrapolating from the experimental exposure durations to 10 min, the 30-min AEGL-2 and AEGL-3 values were set equivalent to the respective 30-min values. AEGL-1 values were not recommended because of insufficient data.



























