Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
____________________
1This document was prepared by the AEGL Development Team composed of Claudia Troxel (Oak Ridge National Laboratory), Julie Klotzbach (SRC, Inc.), Chemical Manager George Rusch (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances), and Ernest V. Falke (U.S. Environmental Protection Agency). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold concentrations for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Boron trifluoride-dimethyl ether is one of several different complexes that can be formed with boron trifluoride. The complexes are generally formed for ease of handling boron trifluoride. The ether complexes consist of a 1:1 molar ratio of boron trifluoride and the dimethyl or diethyl ether and can dissociate under the proper temperature and pressure conditions. A single study was found that addressed the toxicity of boron trifluoride-dimethyl ether, but it reported only nominal concentrations. Because the complex can dissociate to form boron trifluoride, the AEGL values are based on this one chemical species.
Boron trifluoride is a colorless gas with an odor described both as pungent and suffocating and as pleasant. Although the gas is stable in dry air, it immediately forms a dense white mist or cloud when exposed to moist air. Boron trifluoride reacts with moisture (even at low concentrations) to form the dihydrate, BF3•2H2O. Boron trifluoride dihydrate is strongly corrosive to the eyes and skin of rabbits. Boron trifluoride is an excellent catalyst, and has fire retardant and antioxidant properties, nuclear applications, and insecticidal properties.
No definitive data were available on the toxicity of inhaled boron trifluoride in humans. One study reported that a worker could detect the odor of boron trifluoride at a concentration of 4.1 mg/m3 (1.5 ppm) (Torkelson et al. 1961). Acute toxicity studies with dogs, rats, mice, and guinea pigs were available, but exposure concentrations were generally expressed only in terms of nominal concentrations. Studies that measured exposure concentrations and compared them with nominal concentrations found that actual concentrations
ranged from 2.7-56% of nominal concentrations (Torkelson et al. 1961; Rusch et al. 1986; Bowden 2005). Studies identifying end points other than mortality were few. No data were available to evaluate the potential for boron trifluoride to cause developmental or reproductive toxicity or carcinogenicity in animals. Boron trifluoride was not mutagenic in several stains of Salmonella typhimurium.
AEGL-1 values are based on a no-effect level for irritation. A group of 10 rats exposed for 4 h to measured concentrations of boron trifluoride at 25 mg/m3 had no abnormal findings, whereas rats exposed to the next higher concentration of 74 mg/m3 had histopathologic changes in the larynx and tracheal bifurcation indicative of irritation (Bowden 2005). The concentration of 25 mg/m3 was selected as the point of departure for calculating AEGL-1 values. The irritant effects seen at 74 mg/m3 are more severe than the threshold effects for the AEGL-1values. A total uncertainty factor of 10 was applied. An interspecies uncertainty factor of 3 was applied because irritation is a direct contact effect and is not expected to vary greatly among species. An intraspecies uncertainty factor of 3 was applied because the mechanism of irritation is not expected to vary greatly in subpopulations. The same AEGL value was applied to all AEGL durations because the point of departure is a no-effect level for mild irritation.
Relevant data for deriving AEGL-2 values were not available. Therefore, the AEGL-3 values were divided by 3 to obtain reasonable estimates of the AEGL-2 values. Dividing AEGL-3 values by 3 is supported by the steep dose-response curve (Rusch et al. 1986).
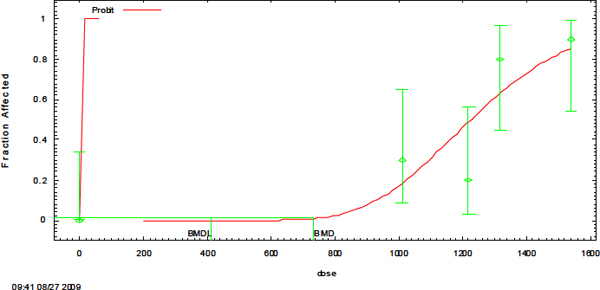
The derivation of AEGL-3 values was based on the threshold for lethality. Rusch et al. (1986) calculated a 4-h LC50 (lethal concentration 50% lethality) of 1,210 mg/m3 (exposures were to liquid aerosols of boron trifluoride dihydrate; concentrations reported are based on boron trifluoride). Using individual mortality data, a 4-h BMCL05 (benchmark concentration, 95% lower confidence limit with 5% response) was calculated by a log-probit analysis using EPA Benchmark Dose Software version 1.4.1c [2007] (EPA 2012). The resulting 4-h BMCL05 of 554 mg/m3 was used to derive the AEGL-3 values. An interspecies uncertainty factor of 3 was applied because boron trifluoride is a corrosive irritant, and the mechanism of action is not expected to vary greatly among species. An intraspecies uncertainty factor of 3 was chosen, because the mechanism of irritation is not expected to vary greatly among subpopulations. An intraspecies uncertainty factor of 3 is also supported by the steep dose-response curve for lethality (3/10 rats died at 1,010 mg/m3, while 9/10 rats died at 1,540 mg/m3), which indicates little variability in the response within a population. The Rusch et al. (1986) study is supported by the Kasparov and Kiriĭ (1972) study that reported a 4-h LC50 of 1,180 mg/m3 in rats. Time scaling was performed using the concentration-time relationship equation Cn × t = k, where C = concentration, t = time, k is a constant, and n generally ranges from 0.8 to 3.5 (ten Berge et al. 1986). An empirical value for n could not be determined because of inadequate data, so the default value of n = 1 was used for extrapolating from shorter to longer exposure periods and a value of n = 3 was used to extrapolate from
longer to shorter exposure periods. The 10-min value was set equal to the 30-min value because of the uncertainty associated with extrapolating data from a 4-h exposure duration to a 10-min AEGL value.
The AEGL values for boron trifluoride are presented in Table 1-1. Although the gas is stable in dry air, boron trifluoride reacts to form the dihydrate when exposed to even low levels of moisture in the air (NIOSH 1976; Hoffman, 1981). Therefore, all AEGL values are reported only in mg/m3.
1. INTRODUCTION
Boron trifluoride-dimethyl ether is one of several different complexes that can be formed with boron trifluoride. The complexes are generally formed for ease of handling of boron trifluoride (NIOSH 1976). Other complexes that can be formed include boron trifluoride with monoethylamine, water, phenol, phosphoric acid, piperidine, dimethyl aniline, methanol, or diethyl ether (NIOSH 1976). A summary table of acute toxicity studies with some of the boron trifluoride complexes can be found in Appendix E. The ether complexes consist of a 1:1 molar ratio of boron trifluoride and either the dimethyl or diethyl ether, and can dissociate under the proper temperature and pressure conditions. A single study was found that evaluated the toxicity of boron trifluoride-dimethyl ether, but it reported only nominal concentrations. Because the complex can dissociate to form boron trifluoride, the AEGL derivations are based on this one chemical species.
Boron trifluoride is a colorless gas with an odor that has been described both as pungent and suffocating (Budavari et al. 1996) and as a “rather pleasant acidic” odor (Torkelson et al. 1961). Chemical and physical data for boron trifluoride and boron trifluoride-dimethyl ether (when available) are listed in Table 1-2. Although the gas is stable in dry air, it immediately forms a dense white mist or cloud when exposed to moist air (NIOSH 1976). Hoffman (1981) reported that when exposed to moisture in the air, even at low levels, boron trifluoride reacts to form the dihydrate, BF3•2H2O. In water, boron trifluoride is believed to form the following products: fluoboric acid (HBF4), monohydroxyfluoboric acid (HBF3OH), dihydroxyfluoboric acid (HBF2(OH)2), trihydroxyfluroboric acid (HBF(OH)3), and boric acid (H3BO3) (NIOSH 1976). Boron trifluoride probably reacts slowly with water to form hydrogen fluoride. It has been suggested that if hydrogen fluoride is formed, it is almost immediately complexed with other species (NIOSH 1976). Dunn (1980) demonstrated that boron trifluoride dihydrate is strongly corrosive to the eyes and skin of rabbits. Topical administration of undiluted boron trifluoride dihydrate (0.1 mL) to the eye caused complete corneal opacity and necrosis of the conjunctivae, nictating membrane, and upper and lower eyelids. The corrosive action was not alleviated by irrigation of the eye with tap water. Topical application of undiluted boron trifluoride dihydrate (0.5 mL) to the skin under a semi-occlusive patch for 24 h resulted in total corrosion of the skin.
TABLE 1-1 Summar of AEGL Values for Boron Trifluoride
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) | |||
| AEGL-1 (nondisabling) | 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 | No-effect level for irritation at 25 mg/m3 for 4 h (Bowden 2005). | |||
| AEGL-2 (disabling) | 37 mg/m3 | 37 mg/m3 | 29 mg/m3 | 18 mg/m3 | 9.3 mg/m3 | One-third AEGL-3 values | |||
| AEGL-3 (lethal) | 110 mg/m3 | 110 mg/m3 | 88 mg/m3 | 55 mg/m3 | 28 mg/m3 | 4-h BMCL05 in rats of 554 mg/m3 (Rusch et al. 1986). | |||
TABLE 1-2 Chemical and Physical Data for Boron Trifluoride
| Parameter | Valuea | Reference |
| Synonyms | Trifluoroborane; boron trifluoride-dimethyl ether (1:1); boron trifluoride-dimethyl etherate; fluoride bority-diemthylether (1:1) | NIOSH 2011 Lewis 1996 |
| CAS registry no. | 7637-07-2 353-42-4 (BF3-dimethyl ether) |
|
| Chemical formula | BF3 C2H60•BF3 (BF3-dimethyl ether) |
|
| Molecular weight | 67.81 g 113.89 g (BF3-dimethyl ether) |
Budavari et al. 1996 Lewis 1996 |
| Physical state | Gas Liquid (BF3-dimethyl ether) | Budavari et al. 1996 |
| Color | Colorless | Budavari et al. 1996 |
| Melting point | -128.37°C -14°C (BF3-dimethyl ether) |
AIHA 1999 Lewis 1996 |
| Boiling point (760 mm Hg) | -100.4°C | Budavari et al. 1996 |
| Vapor density (air =1) | 3.077 g/L | Budavari et al. 1996 |
| Solubility in water | 332 g/100 g at 0°C | Budavari et al. 1996 |
| Vapor pressure | >1 Torr at 20°C | ACGIH 1991 |
| Conversion factors | 1 ppm = 2.76 mg/m3 1 mg/m3 = 0.36 ppm (v/v) 1 ppm = 4.65 mg/m3 (BF3-dimethyl ether) |
AIHA 1999 Calculated |
| aData are for boron trifluoride unless specified otherwise. | ||
Boron trifluoride is a strong Lewis acid and is, therefore, an excellent catalyst that is used in polymerizations, esterifications, and alkylations (NIOSH 1976). Its fire retardant and antioxidant properties are used by the magnesium
industry for protecting molten magnesium and its alloys from oxidation (NIOSH 1976; Budavari et al. 1996), and its nuclear applications include use in neutron detection instruments, boron-10 enrichment, and in the production of neutron-absorbing salts for molten-salt breeder reactors (NIOSH 1976). Boron trifluoride also has insecticidal properties (NIOSH 1976; Budavari et al. 1996).
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
No data on lethality in humans following acute inhalation exposure to boron trifluoride were found.
2.2. Nonlethal Toxicity
In the description of animal studies by Torkelson et al. (1961) discussed in Section 3 below, it was mentioned that a worker noted that boron trifluoride had a rather pleasant acidic odor a concentration of 4.1 mg/m3 (1.5 ppm).
Stewart and Waisberg (1988) reported the only documented case of boron trifluoride poisoning found in the literature. A part-time scrap merchant knocked a valve off a gas cylinder containing boron trifluoride in his garage. A choking white gas was released, quickly overcoming him, his infant son, and his pregnant wife. Upon admission to the hospital, hypoxemia with minimal acidosis was noted. The three patients were treated with ventilation and oxygen. The father and son recovered within 48 h, while the pregnant mother remained unconscious for 36 h and recovered slowly. Urinary concentrations of fluoride were not increased in the 24-h urine samples collected from the subject. The authors stated that all three patients recovered uneventfully. No other details, such as the pregnancy outcome, were provided.
2.3. Developmental and Reproductive Toxicity
No data on the developmental or reproductive toxicity of boron trifluoride in humans were found.
2.4. Genotoxicity
No data on the genotoxicity of boron trifluoride in humans were found.
2.5. Carcinogenicity
No data on the carcinogenicity of boron trifluoride in humans were found.
2.6. Summary
No definitive data on the toxicity of boron trifluoride in humans were found. One paper reported that three individuals (an adult male, an infant, and a pregnant woman) survived exposure to an unknown but debilitating concentration of boron trifluoride. A worker exposed to approximately 4.1 mg/m3 (1.5 ppm) boron trifluoride reported the odor to be rather pleasant and acidic.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
3.1.1. Rats
Vernot et al. (1977) determined 1-h LC50 values for boron trifluoride in rats. Groups of five male and five female Sprague-Dawley rats were exposed at various concentrations of boron trifluoride (individual concentrations not specified; not clear whether values were measured or nominal concentrations) for 1 h in either a bell jar or large desiccator (not stated which). The 1-h LC50 values, calculated by probit analysis, were 1,100 mg/m3 (95% CI [confidence interval]: 883-1,289 mg/m3) (equivalent to 387 ppm (95% CI: 320-467 ppm) for male rats, and 1,000 mg/m3 (95% CI: 809-1,294 mg/m3) (equivalent to 371 ppm; 95% CI: 293-469 ppm) for female rats. No other experimental details were provided.
A series of experiments in rats investigated the acute toxicity of inhaled boron trifluoride (DuPont Company 1948); most of these tests are described below in Section 3.2.2. In one experiment, a group of six rats was exposed for 4 h to boron trifluoride at a nominal concentration of 3,900 mg/m3 (1,400 ppm). One rat died after 148 min of exposure, and another died within 24 h of exposure. Necropsy of the rat that died during the exposure revealed general cyanosis, acute inflammatory reaction of the larynx and upper trachea, and slightly edematous lungs with moderate congestion of alveolar walls. The other rat had consolidation of the upper part of all the lobes of the lung, pus in the bronchi, desquamated mucosa, and areas of emphysema. The four surviving rats were observed for 4 days, and were observed to only experience weight loss. Necropsy of the animals revealed areas of the lungs with thickened alveolar walls as a result of swelling of the lining cells.
Groups of 10 rats, 10 mice, and 10 guinea pigs were exposed to nominal concentrations of boron trifluoride (Stokinger and Spiegl 1953). Rats were exposed at 2,100 mg/m3 (750 ppm) for 5.5 h or 370 mg/m3 (135 ppm) for 10.9 h. The actual exposure concentrations may have been much less since only nominal concentrations were reported. Exposures were conducted in a dynamic exposure chamber measuring 28 ×12 × 6 inches, with plastic sides and a removable plastic top. Three screens were placed inside the chamber to separate the three
species that were exposed simultaneously. Gaseous boron trifluoride was diluted with nitrogen, and continuously metered by a flow meter through copper lines into one end of the chamber with removal at the opposite end of the chamber using a small, motor driven compressor (approximately 1 air change/min). Animals were observed for 14 days following the exposure. The chemical purity was unknown, and the age, sex, and strain of the animals were not specified. At 2,100 mg/m3 for 5.5 h, 1/10 rats died, whereas no rats died at 370 mg/m3 for 10.9 h. Animals surviving the exposure did not exhibit any weight loss during the postexposure observation period.
Kasparov and Kiriĭ (1972) reported a 4-h LC50 of 1,180 mg/m3 for 50 albino rats exposed to boron trifluoride. It was not stated if animals were exposed to nominal or measured concentrations. Necropsy of exposed animals revealed cyanosis of mucous membranes and hemorrhage of internal organs, including the lungs. Lung weights were increased, and pulmonary examination revealed edema, alveolar duct destruction, and vascular dilation. Hyperemia and edema were observed in the kidneys, spleen, and brain. The mucous membranes of the eyes showed evidence of irritation.
F344 rats were exposed by inhalation to boron trifluoride dihydrate for 4 h, 9 days, or 13 weeks (Rusch et al. 1986). The stable dihydrate of boron trifluoride was used to avoid the secondary reaction of dihydrate formation that would occur in the inhalation chamber because of the hygroscopic nature of boron trifluoride gas. A nebulizer was used to generate the aerosol, and the concentration was controlled by regulating the airflow of the compressed, breathing-grade air through the nebulizer. Exposure concentrations were measured hourly by trapping a known volume of test atmosphere, dissolving the aerosol in distilled water, and analyzing the sample using an ion-selective electrode technique. Particle size was measured hourly during the acute exposure, three times per week during the 9-day exposure, and twice per week during the subchronic exposure. The mean particle size ranged from 1.5-2.2 microns; more than 97% of the particles were smaller than 10 microns.
For the acute study, groups of five male and five female 9-week-old F344 rats were exposed for 4 h to liquid aerosols of boron trifluoride dihydrate at mean measured concentrations of 0, 1,010, 1,220, 1,320, or 1,540 mg/m3 (animals were exposed to boron trifluoride dihydrate, but the concentrations are based on boron trifluoride) (Hoffman 1981; Rusch et al. 1986). Nominal concentrations ranged from 4,100 mg/m3 for the low-concentration group to 6,200 mg/m3 for the high-concentration group. Loss of the chemical was attributed to losses associated with high aerosol generation. Rats were observed during the exposure and daily for 14 days postexposure. Body weight was recorded prior to exposure (day 0) and on days 1, 2, 4, 7, and 14 postexposure. Gross necropsy was conducted on all animals. Mortality was observed in all exposure groups (see Table 1-3). A 4-h LC50 of 1,210 mg/m3 (95% CI: 1,080-1,350 mg/m3) was calculated using the method of Litchfield and Wilcoxon (1949). Clinical signs observed during exposure included reduced activity, closed eyes, excessive
TABLE 1-3 Mortality in Rats Exposed to Boron Trifluoride Aerosol for 4 Hours
| Concentration (mg/m3) | Mortality (%) [day of death] | |
| 0 | 0/10 (0) | |
| 1,010 | 3/10 (30) [0, 3, 6] | |
| 1,220 | 2/10 (20) [0, 3] | |
| 1,320 | 8/10 (80) [1, 1, 2, 3, 3, 3, 4, 5] | |
| 1,540 | 9/10 (90) [0, 0, 0, 1, 2, 3, 4, 5, 5] | |
| Source: Rusch et al. 1986. | ||
lacrimation, and excessive oral and nasal discharge. The high-concentration group also exhibited gasping. Clinical signs of respiratory distress (dry rales, moist rales, gasping) and/or irritation (excessive oral and nasal discharge and lacrimation) were noted 4 hours after exposure in most of the exposed animals. Two rats (one each from the 1,320- and 1,540-mg/m3 groups) had corneal opacities when removed from the chamber and later died. Most clinical signs in surviving rats were no longer present by day 6. All rats lost weight following exposure, but the control rats lost less (4-11 g) than the exposed rats (19-56 g). All rats gained weight by day 14, indicating reversibility of toxicity. Necropsy of exposed animals revealed red discoloration of the lungs in all exposure groups. Discoloration of the thymus, kidney, and liver were reported in animals dying spontaneously in all groups, but it was unclear whether these changes were an effect of treatment. Slight increases in liver and lung weight were observed in exposed females (lung weight was 2-5% greater than controls; liver weight was 13-15% greater than controls).
In the subacute study, groups of five male and five female F344 rats were exposed to liquid aerosols of boron trifluoride dihydrate for 6 h/day, 5 days/week for 9 days, at mean measured concentrations of 0, 24, 66, or 180 mg/m3 (animals were exposed to boron trifluoride dihydrate, but concentrations given are based on boron trifluoride) (Hoffman and Rusch 1982b; Rusch et al. 1986). Nominal concentrations were 48, 117, and 390 mg/m3, respectively. The differences between nominal and measured concentrations were attributed to absorption by the chamber wall. Animals were observed twice daily, clinical signs were recorded twice a week (exposure days 2, 5, 9, and 11), and body weight was recorded weekly. At study termination, the brains, gonads, kidneys, liver, lungs, spleen, and thymus were weighed, and the lungs, trachea, turbinates, liver, kidneys, stomach, duodenum, testis, and epididymis were examined microscopically. All 10 rats from the high-concentration group died before the sixth exposure. Clinical signs after two days included mucoid and/or red nasal discharge, dry or moist rales, dried red material around nose or mouth, lacrimation, and yellow anal-genital staining, whereas three control females exhibited mucoid nasal discharge and one control male had mucoid nasal discharge and dry rales (see Table 1-4). Clinical signs did not appear to be related to concen-
tration at day 2. Clinical signs were increased in incidence and severity after 5 days of exposure. Mean body weight and body weight gain were statistically decreased in males of all exposure groups and in females of the mid- and high-concentration groups. Concentration-related increases in absolute and relative lung weights were observed in both sexes of the low- and mid-concentration groups (increased by 12% and 21%, respectively, compared with controls), while absolute and relative liver weights were decreased in the mid-concentration groups (approximately 79% of controls). The only treatment-related histopathologic finding was necrosis and pyknosis of the proximal tubular epithelium in the kidneys from animals exposed at 180 mg/m3.
TABLE 1-4 Clinical Signs in Rats Exposed to Boron Trifluoride for Nine Days
| Observation | 0 mg/m3 | 24 mg/m3 | 66 mg/m3 | 180 mg/m3 |
| Exposure day 2 | ||||
| Number examined | 10 | 10 | 10 | 10 |
| Number affected | 4 | 7 | 7 | 7 |
| No signs | 6 | 3 | 3 | 3 |
| Mucoid nasal discharge | 4 | 5 | 4 | 5 |
| Red nasal discharge, dried red material around nose or mouth | 0 | 2 | 2 | 1 |
| Dry rales | 1 | 2 | 2 | 1 |
| Moist rales | 0 | 1 | 0 | 2 |
| Lacrimation | 0 | 0 | 0 | 1 |
| Stained anal-genital area | 0 | 2 | 1 | 1 |
| Exposure day 5 | ||||
| Number examined | 10 | 10 | 10 | 10 |
| Number affected | 0 | 6 | 10 | 10 |
| No signs | 10 | 4 | 0 | 0 |
| Dead | 0 | 0 | 0 | 3 |
| Mucoid nasal discharge | 0 | 0 | 9 | 2 |
| Red nasal discharge, dried red material around nose or mouth | 0 | 6 | 6 | 4 |
| Dry rales | 0 | 0 | 3 | 0 |
| Moist rales | 0 | 2 | 7 | 1 |
| Lacrimation | 0 | 0 | 0 | 7 |
| Stained anal-genital area | 0 | 1 | 5 | 5 |
| Gasping, shallow or labored breathing | 0 | 0 | 0 | 3 |
| Poor condition | 0 | 0 | 0 | 8 |
Source: Adapted from Hoffman and Rusch 1982b; Rusch et al. 1986.
In the subchronic study, groups of 20 male and 20 female F344 rats were exposed to liquid aerosols of boron trifluoride dihydrate for 6 h/day, 5 days/week for 13 weeks, at mean measured concentrations of 0, 2.0, 6.0, or 17 mg/m3 (animals were exposed to boron trifluoride dihydrate, but the concentrations given are based on boron trifluoride) (Hoffman and Rusch 1982a; Rusch et al. 1986). Nominal concentrations were 6.4, 24, and 54 mg/m3, respectively. Differences between nominal and measured concentrations were again attributed to absorption by the chamber wall. Animals were observed twice daily, a detailed clinical assessment was performed on all animals weekly, and body weight was recorded weekly. Hematology and clinical chemistry analysis, urinalysis, and urinary ionic and total fluoride and serum total fluoride amounts were determined after 1 month of exposure (5 rats/sex/dose), during the final week of exposure (15 rats/sex/dose), or 2 weeks after the last exposure (retained group of 5 rats/sex/dose). In addition, urine ionic and total fluoride measurements were taken after 2 months of exposure, and bone fluoride analysis was conducted on all animals. All rats were subjected to gross necropsy, and the brain, lungs, heart, liver, spleen, kidneys, and gonads were weighed. Tissues from the control and high-concentration groups were examined for histopathologic changes, and sections of the kidneys, nasal turbinates, lungs, and liver were examined from all animals.
One male rat from the high-concentration group died during week 12 (Hoffman and Rusch 1982a; Rusch et al. 1986). Clinical signs in exposed animals included an increased incidence of dried red material around the nose, dried material around the mouth, excessive lacrimation, and dry rales, primarily in the high-concentration group. In the low-concentration groups, irritation in the form of excessive lacrimation was observed in 5 rats of the 2 mg/m3 group (1-2 times starting at week 10), and in 16 rats of the 6-mg/m3 group (1-2 times starting at week 2). No differences in body weight, ophthalmologic findings, hematology analysis, organ weight, or gross necropsy findings were observed in exposed animals compared with controls. Urinalysis revealed a concentration-related decrease in urinary calcium and concentration-related increase in urinary fluoride. Clinical chemistry analysis revealed concentration-related decreases in serum protein and globulin concentrations, and one male rat with elevated blood urea nitrogen (BUN) concentration. A concentration-related increase in fluoride concentration in the femurs of exposed animals was observed. The concentrations persisted during the recovery period, suggesting either slow release from the bone or irreversible binding. Histopathologic examination of the male rat in the high-concentration group that died had findings consistent with toxic renal tubular necrosis, and the rat with elevated BUN concentrations exhibited mild renal lesions that probably would not have affected its survival.
3.1.2. Mice
Groups of 10 mice, 10 rats, and 10 guinea pigs were exposed to nominal concentrations of boron trifluoride (Stokinger and Spiegl 1953). Mice were ex-
posed at 2,100 mg/m3 (750 ppm) for 5.5 h or 370 mg/m3 (135 ppm) for 10.9 h. Actual exposure concentrations may have been less since only nominal concentrations were reported. Exposures were conducted in a dynamic exposure chamber measuring 28 × 12 × 6 inches, with plastic sides and a removable top. Three screens were placed inside the chamber to separate the different species that were exposed simultaneously. Gaseous boron trifluoride was diluted with nitrogen, and continuously metered by a flow meter through copper lines into one end of the chamber with removal at the opposite end of the chamber using a small, motor driven compressor (approximately 1 air change/min). Animals were observed for 14 days after the exposure. The chemical purity was unknown, and the age, sex, and strain of the animals used was not specified. One of 10 mice exposed at 2,100 mg/m3 for 5.5 h died, whereas no deaths occurred in mice exposed at 370 mg/m3 for 10.9 h. Mice that survived did not to exhibit any weight loss during the postexposure observation period.
Kasparov and Kiriĭ (1972) reported a 2-h LC50 of 3,460 mg/m3 for 70 albino mice exposed to boron trifluoride. It was not stated if animals were exposed to nominal or measured concentrations. Necropsy of exposed animals revealed cyanosis of mucous membranes and hemorrhage of internal organs, including the lungs. Lung weight was increased, and examination revealed edema, alveolar duct destruction, and vascular dilation. Hyperemia and edema were observed in the kidneys, spleen, and brain. The mucous membranes of the eyes showed evidence of irritation.
3.1.3. Guinea Pigs
A series of experiments was carried out in guinea pigs to investigate the acute toxicity of inhaled boron trifluoride (DuPont Company 1948). Most of the experimental details were not provided, including the chemical purity, sex and strain of animals, description of exposure chamber, and generation of test material aerosol. In the first experiment, two guinea pigs exposed to boron trifluoride at approximately 2,760 mg/m3 (1,000 ppm) died during the first 5 min of exposure. Necropsy revealed pale and distended lungs, and histopathologic examination found emphysema with partially detached bronchial epithelium. No edema was observed. In the next experiment, two guinea pigs were exposed for 3 h at a nominal concentration of 720 mg/m3 (260 ppm). Guinea pigs exhibited signs of respiratory distress within a few minutes of exposure, and had labored breathing throughout the first hour of exposure. One guinea pig died 90 min into the exposure, and necropsy revealed distended lungs and pulmonary edema. The other guinea pig survived the 3-h exposure and was sacrificed the next day. Examination of the lungs revealed thickening of the pleura, atelectasis of some portions of the lungs, and areas of emphysema. Pretreatment with pyribenzamine or adrenaline (20 mg/kg or 0.1 mg, respectively, injected subcutaneously 15 min before exposure to boron trifluoride) did not modify the response of guinea pigs when they were subsequently exposed to boron trifluoride at 2,760 mg/m3 (1,000 ppm) or 280-550 mg/m3 (100-200 ppm).
Groups of 10 rats, 10 mice, and 10 guinea pigs were exposed to nominal concentrations of boron trifluoride (Stokinger and Spiegl 1953). Guinea pigs were exposed at 2,100 mg/m3 (750 ppm) for 5.5 h, 970 mg/m3 (350 ppm) for 1.4 h, or 370 mg/m3 (135 ppm) for 10.9 h. Actual exposure concentrations may have been less since only nominal concentrations were reported. Exposures were conducted in a dynamic exposure chamber measuring 28 × 12 × 6 inches, with plastic sides and a removable top. Three screens were placed inside the chamber to separate the different species that were exposed simultaneously. Gaseous boron trifluoride was diluted with nitrogen, and continuously metered by a flow meter through copper lines into one end of the chamber with removal at the opposite end of the chamber using a small, motor driven compressor (approximately 1 air change/min). Animals were observed for 14 days after the exposure. The chemical purity was unknown, and the age, sex, and strain of the animals used were not specified. A summary of the mortality data is presented in Table 1-5. Surviving animals did not exhibit any weight loss during the postexposure observation period.
Kasparov and Kiriĭ (1972) reported a 4-h LC50 of 109 mg/m3 in 42 guinea pigs exposed to boron trifluoride. It was not stated if animals were exposed to nominal or measured concentrations. Necropsy of exposed animals revealed cyanosis of mucous membranes and hemorrhage of internal organs, including the lungs. Lung weight was increased, and examination revealed edema, alveolar duct destruction, and vascular dilation. Hyperemia and edema were observed in the kidneys, spleen, and brain. The mucous membranes of the eyes showed evidence of irritation.
Torkelson et al. (1961) conducted a number of repeated exposure studies in which guinea pigs were exposed to nominal concentrations of boron trifluoride at 8, 21, or 35 mg/m3 (3, 7.7, or 12.8 ppm) for 7 h/day, 5 days/week for various durations. Measured concentrations of the 8 and 21 mg/m3 exposures were 4 mg/m3 and 8-11 mg/m3, respectively. The exposure chamber used for the 21- and 35-mg/m3 exposures was a 160-L cubical chamber, whereas the 8-mg/m3 exposures occurred in a 3,700-L rectangular, vault-type chamber. The chemical purity was not determined. Dry boron trifluoride gas was metered into the exposure chamber with a stream of nitrogen to prevent loss of the chemical. To determine actual exposure concentrations, air samples were taken from the chamber by drawing chamber air through a fritted glass scrubber containing distilled water. Samples also were taken from several areas within the chambers to ensure reasonable distribution of the boron trifluoride. A carbamic acid technique was used to determine the concentration of total boron, and the equivalent concentration of boron trifluoride was calculated.
In the first experiment, 10 male guinea pigs were exposed to boron trifluoride at a nominal concentration of 35 mg/m3 for 7 h/day, 5 days/week, for up to 42-45 exposures in 62-65 days (Torkelson et al. 1961). The exposed animals had obvious difficulty breathing and appeared asthmatic. Death from respiratory irritation and asphyxia occurred in 7/10 guinea pigs starting after the nineteenth exposure. Lung weight was increased, and gross examination of the lungs
revealed pneumonitis primarily in the hilar regions. Microscopic examination of the lungs revealed vessels in the alveolar walls distended with red-blood cells, erythrocytes on the alveoli, phagocytic cells containing a yellow material, thickened alveolar walls separated from the vascular epithelium, and areas of collapse and emphysema. In a second experiment, 10 male guinea pigs were exposed to boron trifluoride at a nominal concentration of 21 mg/m3 (average measured concentration of 8-11 mg/m3) for 7 h/day, 5 days/week for up to 29 exposures. Four guinea pigs developed what appeared to be asthmatic attacks and died; one animal died during the second, fifth, sixth, and eleventh exposure. Six guinea pigs survived 28 exposures, but had difficulty breathing and exhibited signs discomfort. They were accidentally killed after exposure to high concentrations of boron trifluoride and nitrogen on the twenty-ninth exposure day. In a third experiment, a group of 10 male and 10 female guinea pigs were exposed to boron trifluoride at a nominal concentration of 8 mg/m3 (average measured concentration of 4 mg/m3) for 7 h/day, 5 days/week for 127-128 exposures. All animals were killed after the last exposure and subjected to gross necropsy. The heart, lungs, liver, kidneys, spleen, and testes were weighed; these organs plus the adrenal glands and pancreas were also examined microscopically. No exposure-related deaths were observed, and no changes in growth rate, appearance, organ weights, or gross findings were noted in exposed animals compared with unexposed controls. Exposed guinea pigs had a slightly higher incidence of pneumonitis than controls.
3.2. Nonlethal Toxicity
3.2.1. Dogs
Two dogs were exposed to boron trifluoride at a concentration estimated to be between 1,380-2,760 mg/m3 (500-1,000 ppm) for 30 min (one dog) or 2 h (one dog) (DuPont Company 1948). Most of the experimental details were not provided, including the chemical purity, sex and strain of animals, description of exposure chamber, generation of test material aerosol, and method used for determining the concentration. The dog exposed for 30 min gagged, wheezed, and spit up frothy mucous during the exposure, but recovered after the exposure ended. The dog exposed for 2 h exhibited similar clinical signs during the exposure. For 40 min after exposure ended, the dog breathed slowly with a prolonged expiratory phase, breath sounds were noisy and bronchial, and musical rales
TABLE 1-5 Mortality of Guinea Pigs Exposed to Boron Trifluoride
| Concentration | Duration | Mortality |
| 750 ppm (2,100 mg/m3) | 5.5 h | 10/10 (100%) |
| 350 ppm (970 mg/m3) | 1.4 h | 7/10 (70%) |
| 135 ppm (370 mg/m3) | 10.9 h | 1/10 (10%) |
| Source: Adapted from Stokinger and Spiegl 1953. | ||
were heard over all areas of the chest. Noisy breath sounds and moist rales were still present after 160 min. The dog coughed frequently and blood pressure and body temperature became elevated. By 24 h postexposure, respiration was slow but not labored, moist rales were heard on inspiration, and a cough was still present. By 48-h postexposure, the dog’s chest sounded clear; the dog was killed and necropsied. Findings included acute inflammation of the epiglottic and laryngeal tissues above the cords and slightly congested trachea and bronchi. Microscopic examination revealed marked edema of the larynx with surface necrosis, desquamation, and polymorphonuclear exudate; emphysema in the lung with areas of congestion and edema; mucopurulent exudate in the bronchi; and renal capsular spaces and convoluted tubules distended with albuminous fluid.
3.2.2. Rats
Groups of 10 male and 10 female Sprague-Dawley rats received whole body exposures to boron trifluoride hydrate vapor and aerosol (99.8% purity) at measured concentrations of 9, 25, or 74 mg/m3 (nominal concentrations of 319, 734, and 982 mg/m3, respectively) for 4 h (Bowden 2005). The droplet sizes at the three concentrations had mass median aerodynamic diameters of 0.7, 2.3, and 3.3 μm, respectively; 99, 89, and 79% of the droplets, respectively, were less than 7 μm. A control group was exposed to clean air. The control group and 25-mg/m3 group were exposed on February 17, 2004; the 74-mg/m3 group was exposed on April 21, 2004; and the 9-mg/m3 group was exposed on September 21, 2004. Animals were from the same source and generally of the same age. Five male and five female rats/group were killed 24-h postexposure (subgroup 1), while the remaining five male and five female rats/group were observed for 14-days postexposure before termination (subgroup 2). Clinical signs in all animals were recorded are various times, including at the end of chamber equilibration; 15 min, 30 min, and 1 h into the exposure; hourly intervals during the exposure; immediately after exposure ended; and 1- and 2-h postexposure. Clinical signs were also recorded twice daily during the observation period (subgroup 2). Body weight was recorded prior to exposure (day 0), daily during the observation period (subgroup 2), and at necropsy. A visual inspection of water consumption was conducted daily. All animals were subjected to gross necropsy, and the lung and kidney weights were recorded. The respiratory tract and kidneys were fixed and examined by light microscopy. No exposure-related effects were noted on body weight, water consumption, lung or kidney weights, or macroscopic findings. Clinical signs were limited to the 25-mg/m3 group, which exhibited no response to outside stimuli after 4 h of exposure, and one female in the 74-mg/m3 group, which had brown staining around the snout and jaw immediately after exposure that persisted to 1-h postexposure. The clinical signs in the 25-mg/m3 group were not considered an effect of treatment because they were not related to concentration, and there were no other supporting data that boron trifluoride is a neurotoxicant. Exposure-related histopathologic findings
were noted only in the 74-mg/m3 group (see Table 1-6). Examination of the larynx 24-h postexposure revealed ventral cartilage necrosis (minimal to slight) in 4/5 males and 4/5 females and anterior ventral hemorrhage (minimal) in 2/5 males. These changes were not observed in the control or other exposure groups. Animals from the 74-mg/m3 group also had an increase in the severity of ventral epithelial hyperplasia and ventral inflammatory cell infiltration in the larynx compared with control animals. Following a 2-week recovery period, ventral cartilage necrosis was still noted in one male and one female. Histopathologic findings in the trachea included an increased incidence cilia loss (minimal) at the point of tracheal bifurcation 24-h postexposure, particularly in the 74-mg/m3 females. Incidences were comparable to controls after 2-weeks postexposure.
TABLE 1-6 Summary of Histopathologic Findings in Rats Exposed Boron Trifluoride for Four Hours
| Finding | Severity | Number of males Concentration (mg/m3) | Number of females Concentration (mg/m3) | ||||||
| 0 | 9 | 25 | 74 | 0 | 9 | 25 | 74 | ||
| Number examined | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Larynx (24-h postexposure) | |||||||||
| Cartilage necrosis | Total | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 4 |
| Minimal | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | |
| Slight | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | |
| Anterior ventral hemorrhage | Minimal | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Ventral epithelial hyperplasia | Total | 2 | 1 | 3 | 4 | 2 | 0 | 1 | 5 |
| Minimal | 1 | 1 | 3 | 1 | 2 | 0 | 1 | 3 | |
| Slight | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | |
| Ventral inflammatory-cell | Total | 3 | 3 | 3 | 5 | 2 | 1 | 4 | 5 |
| infiltration | Minimal | 2 | 2 | 2 | 2 | 2 | 1 | 4 | 2 |
| Slight | 1 | 1 | 1 | 3 | 0 | 0 | 0 | 3 | |
| Larynx (2-wk postexposure) | |||||||||
| Cartilage necrosis | Total | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Minimal | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Slight | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Anterior ventral hemorrhage | Minimal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ventral epithelial hyperplasia | Total | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 |
| Minimal | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Slight | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Ventral inflammatory-cell | Total | 1 | 2 | 2 | 1 | 1 | 1 | 0 | 1 |
| infiltration | Minimal | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Slight | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Tracheal bifurcation (24-h postexposure) | |||||||||
| Loss of cilia | Minimal | 0 | 3 | 2 | 2 | 1 | 3 | 2 | 5 |
| Tracheal bifurcation (2-wk postexposure) | |||||||||
| Loss of cilia | Minimal | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
Source: Bowden 2005.
A series of experiments was carried out on rats to investigate the acute toxicity of inhaled boron trifluoride (DuPont Company 1948). Most of the experimental details were not provided, including chemical purity, sex and strain of animals, description of exposure chamber, generation of test material vapor, and whether exposure concentrations were nominal or analytic. In the first experiment, two rats were exposed to boron trifluoride at approximately 2,760 mg/m3 (1,000 ppm) for 1 h. Both rats survived the exposure (no mention was made of clinical signs), and were killed and examined 24 h later. Abnormal lung findings included pulmonary congestion with swelling of the cells lining the alveoli. In the second experiment, two rats exposed for 3 h to a nominal concentration of 720 mg/m3 (260 ppm) showed no clinical signs during exposure and no gross or microscopic changes in the lungs or other organs when killed and examined the following day. In the third experiment, a group of six rats was exposed for 4 h to a nominal concentration of boron trifluoride at 3,900 mg/m3 (1,400 ppm). The results of this experiment are reported in Section 3.1.1 of this document because mortalities were reported.
Torkelson et al. (1961) conducted a number of repeated-exposure studies in which rats were exposed to boron trifluoride at nominal concentrations of 8, 21, or 35 mg/m3 for 7 h/day, 5 days/week for various durations. Average measured concentrations for the 8- and 21-mg/m3 exposures were 4 and 8-11 mg/m3, respectively. The exposure chamber used for the 21- and 35-mg/m3 exposures was a 160-L cubical chamber, whereas the 8-mg/m3 exposures occurred in a 3,700-L rectangular, vault-type chamber. The chemical purity was not determined. Dry boron trifluoride gas was metered into the exposure chamber with a stream of nitrogen to prevent loss of the chemical. To determine actual exposure concentrations, air samples were taken from the chamber during exposure by drawing chamber air through a fritted glass scrubber containing distilled water. Samples were also taken from several areas within the chambers to ensure reasonable distribution of the boron trifluoride. A carbamic acid technique was used to determine the concentration of total boron, and the equivalent concentration of boron trifluoride was calculated.
In the first experiment, 14 female rats were exposed to nominal concentrations of boron trifluoride of 35 mg/m3 for 7 h/day, 5 days/week for up to 60 exposures total (Torkelson et al. 1961). One rat died after 34 exposures, but the cause of death was not determined. Groups of four rats were killed for examination after 45 or 60 exposures. No changes in appearance, mortality, or organ weights were noted. Gross examination revealed changes in the lungs indicative of chemical irritation, with microscopic evaluation revealing pneumonitis. Five rats exposed for 60 exposures and allowed to recover for 1 month did not exhibit any changes in body weight or appearance. In a second experiment, five female rats were exposed at 21 mg/m3 for 7 h/day, 5 days/week for 33 exposures in 51 days. No changes in appearance or body weight were observed. Fluorosis was not observed in the teeth, but the fluoride content in the bones and teeth was increased. In a third experiment, groups of 12 male and 12 female rats were exposed to boron trifluoride at a nominal concentration of 8 mg/m3 for 7 h/day, 5
days/week for 127-128 times. All animals were killed after the last exposure and subjected to gross necropsy. The heart, lungs, liver, kidneys, spleen, and testes were weighed; these organs plus the adrenal glands and pancreas were also examined microscopically. The lower jaws were examined for fluorosis. All animals survived. No changes in growth rate, appearance, organ weights, or gross findings were observed in exposed animals compared with unexposed controls. Microscopic examination of the lungs revealed areas of pneumonitis, peribronchiole round cell infiltration, and areas of congestion of capillaries lining the alveolar walls. Fluorosis was not evident.
3.2.3. Rabbits
No changes in body weight, gross necropsy findings, organ weights (heart, lungs, liver, kidneys, spleen, and testes), or microscopic findings (adrenal glands and pancreas, in addition to the organs that were weighed) were observed in a group of three male and tree female rabbits exposed to boron trifluoride at a nominal concentration of 8 mg/m3 (average measured concentration of 4 mg/m3) for 7 h/day, 5 days/week for a total of 127-128 exposures (Torkelson et al. 1961).
3.3. Developmental and Reproductive Toxicity
No data were found regarding the potential for inhaled boron trifluoride to cause developmental or reproductive toxicity in laboratory animals.
3.4. Genotoxicity
Boron trifluoride at concentrations of 0.01-5 mg/plate was not mutagenic to Salmonella typhimurium strains TA-1535, TA-1537, TA-98, or TA-100 in the presence or absence of metabolic activation; however, it was toxic to all strains at the highest concentration (Wudl and Goode 1982).
3.5. Carcinogenicity
No data were found regarding the potential for boron trifluoride to cause cancer in laboratory animals.
3.6. Summary
A summary of lethal and nonlethal effects of boron trifluoride is presented in Tables 1-7 and 1-8. Unfortunately, exposure concentrations were not analyzed or clearly defined in most of the reports. Studies which actually measured the exposure concentrations and compared them with nominal concentrations found
TABLE 1-7 Summary of Acute Lethal Inhalation Data on Laboratory Animals
| Concentration | |||||||||
| mg/m3 | ppma | Duration | Effect | Reference | |||||
| Rat | |||||||||
| 1,100b | 387 | 1 h | LC50 in male rats | Vernot et al. 1977 | |||||
| 1,000b | 371 | 1 h | LC50 in female rats | ||||||
| 1,210 | 4 h | LC50 in male and female rats | Rusch et al. 1986 | ||||||
| 1,180b | 4 h | LC50 | Kasparov and Kiriĭ 1972 | ||||||
| 3,900c | 1,400 | 4 h | 2/6 died; one 148 min into exposure, the other within 24 h of exposure | DuPont Company 1948 | |||||
| 2,100c | 750 | 5.5 h | 1/10 died | Stokinger and Spiegl 1953 | |||||
| Mouse | |||||||||
| 3,460b | 2 h | LC50 | Kasparov and Kiriĭ 1972 | ||||||
| 2,100c | 750 | 5.5 h | 1/10 died | Stokinger and Spiegl 1953 | |||||
| Guinea pig | |||||||||
| 2,760c | 1,000 | 5 min | 2/2 died | DuPont Company 1948 | |||||
| 720c | 260 | 3 h | 1/2 died | ||||||
| 109b | 4 h | LC50 | Kasparov and Kiriĭ 1972 | ||||||
| 210c | 750 | 5.5 h | 10/10 died | Stokinger and Spiegl 1953 | |||||
| 970c c | 350 | 1.4 h | 7/10 died | ||||||
aConcentration provided in ppm only if the study authors reported concentrations in those units.
bNot known if nominal or measured concentrations.
cNominal concentration.
TABLE 1-8 Summary of Nonlethal Inhalation Data on Laboratory Animals
| Concentration | ||||
| mg/m3 | ppma | Durationb | Effect | Reference |
| Dog | ||||
| 1,380-2,760c | 500-1,000 | 30 min | 1 dog; gagged, wheezed, spit up frothy mucous during exposure; recovered after exposure. | DuPont Company 1948 |
| 2 h | 1 dog; similar clinical signs, necropsy 48-h postexposure revealed edema of larynx, emphysema in lungs, exudate in bronchi, renal capsular spaces, convoluted tubules distended with fluid. | |||
| Rat | ||||
| 2,760c | 1,000 | 1 h | 2/2 rats survived; pulmonary congestion. | DuPont Company 1948 |
| 720c | 260 | 3 h | No clinical signs, no gross or microscopic changes. | |
| 370c | 135 | 10.9 h | 10/10 rats survived. | Stokinger and Spiegl 1953 |
| 9 | 4 h | No effects. | Bowden 2005 | |
| 25 | No effects. | |||
| 74 | Histopathologic changes in larynx and tracheal bifurcation indicative of irritation. | |||
| 24 | 6 h/d, 5 d/wk for 9 d | 10/10 survived; clinical signs included oral and nasal discharge, lacrimation, dry and moist rales, gasping, poor condition; increased lung weight. | Rusch et al. 1986 | |
| 66 | 10/10 survived; same clinical signs; decreased body weights; increased lung weight. | |||
| 35c | 12.8c | 7 h/d, 5 d/wk up to 60 exposures | 14 rats; no changes in appearance or organ weights; gross/microscopic changes in lungs, pneumonitis. | Torkelson et al. 1961 |
| 8-11 | 3-4 | 7 h/d, 5 d/wk for 33 exposures | 5 rats; no changes in appearance or body weight. | |
| 4 | 1.5 | 7 h/d, 5 d/wk for 127-128 exposures | 12 males, 12 females; no changes in appearance, body or organ weights, or gross necropsy findings. | |
| Mouse | ||||
| 370c | 135 | 10.9 h | 10/10 survived. | Stokinger and Spiegl 1953 |
| Guinea pig | ||||
| 4 | 1.5 | 7 h/d, 5 d/wk for 127-128 exposures | 10 males, 10 females; no changes in appearance, body or organ weights, increased incidence of pneumonitis. | Torkelson et al. 1961 |
| Rabbit | ||||
| 4 | 1.5 | 7 h/d, 5 d/wk for 127-128 exposures | 3 males, 3 females; no changes in appearance, body or organ weights, or gross or microscopic findings. | Torkelson et al. 1961 |
aConcentration provided in ppm only if the study authors reported the exposure concentrations in those units.
bSome repeated-exposure studies are included in the table if the data were deemed relevant.
cNominal concentration.
that actual concentrations ranged from 2.7-56% of nominal concentrations (Torkelson et al. 1961; Rusch et al. 1986; Bowden 2005). This is, therefore, an important consideration when analyzing studies based on nominal exposure concentrations.
Several LC50 values were reported. Rusch et al. (1986) calculated a 4-h LC50 of 1,210 mg/m3 in male and female rats based on exposure to measured concentrations of boron trifluoride. Vernot et al. (1977) reported 1-h LC50 values of 1,100 and 1,000 mg/m3 for male and female rats, respectively (unknown whether the concentrations were nominal or measured). Kasparov and Kiriĭ (1972) reported a 2-h LC50 of 3,460 mg/m3 in mice, a 4-h LC50 of 1,180 mg/m3 in rats, and a 4-h LC50 of 109 mg/m3 in guinea pigs (unknown whether exposure concentrations were nominal or measured). Stokinger and Spiegl (1953) reported no mortality in mice and rats exposed at 370 mg/m3 (nominal) for 10.9 h, whereas 1/10 guinea pigs died under the same exposure conditions.
Acute toxicity studies identifying end points other than those of mortality were few. Exposure of dogs to boron trifluoride at 1,380-2,760 mg/m3 (nominal) for 30 min or 2 h resulted in gagging, wheezing, and frothy mucous production, with pulmonary and renal effects evident at microscopic examination in the dog exposed for 2 h (DuPont Company 1948). Pulmonary congestion was found in rats exposed to boron trifluoride at 2,760 mg/m3 (nominal) for 1 h, but no effects were found in rats exposed at 720 mg/m3 (nominal) for 3 h, or in rats and mice exposed at 370 mg/m3 (nominal) for 10.9 h (DuPont Company 1948; Stokinger and Spiegl 1953).
Repeated-exposure studies reporting measured exposure concentrations were included in the discussion of the toxicity of boron trifluoride to provide a complete description of the chemical’s toxicity at quantified levels of exposure. Studies by Torkelson et al. (1961) found no adverse effects in rats exposed at 8-11 mg/m3 for 7 h/day, 5 days/week for 33 exposures, or in rats and rabbits exposed at 4 mg/m3 for 7 h/day, 5 days/week for 127-128 exposures. Four guinea pigs exposed at 8-11 mg/m3 for 7 h/day, 5 days/week died by the eleventh exposure. A slightly higher incidence of pneumonitis was the only effect observed in guinea pigs exposed at 4 mg/m3 for 7 h/day, 5 days/week for 127-128 exposures. In a nine-day exposure study by Rusch et al. (1986), mortality from renal toxicity was observed in all rats of the high-concentration group (180 mg/m3), whereas all rats survived exposure at 24 or 66 mg/m3. Clinical signs were recorded on exposure days 2, 5, 9, and 11. Signs of irritation were observed in exposed groups, but were not related to concentration on exposure day 2. However, clinical signs worsened with increasing concentration and with continued exposure. A subchronic study was conducted by Rusch et al. (1986), in which groups of rats were exposed to boron trifluoride at 2, 6, or 17 mg/m3 for 6 h/day, 5 days/week for 13 weeks. One rat died in the high-concentration group at week 12, but all rats from the 2- and 6-mg/m3 group survived. Excessive lacrimation was observed in five rats of the 2-mg/m3 group (1-2 times starting at week 10), and in 16 rats of the 6-mg/m3 group in 16 (1-2 times starting at week 2).
No data were available to evaluate the potential for boron trifluoride to cause developmental or reproductive toxicity or carcinogenicity in animals. Boron trifluoride was not mutagenic in several strains of S. typhimurium.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
Female rats exposed to boron trifluoride aerosol for approximately 6 weeks had elevated fluoride content in the bones and teeth (Torkelson et al. 1961). A subchronic inhalation study in rats reported a concentration-related decrease in urinary calcium and concentration-related increase in urinary ionic fluoride and fluorine amounts, and clinical chemistry analysis revealed concentration-related decreases in serum protein and globulin levels, concentration-related increase in serum fluorine, and one male rat with elevated BUN levels (Rusch et al. 1986). The study authors propose that the depression of urinary calcium may have been related to increased calcium utilization as a consequence of the higher body amounts of fluoride. A comparison of urinary fluoride amounts to total urinary fluorine indicated that less than half of the boron trifluoride dissociated to free fluoride while the remainder appeared to have been excreted as undissociated boron trifluoride. Elimination of boron trifluoride (undissociated) increased to a greater degree with increasing exposure concentration compared to urinary ionic fluoride. A concentration-related increase in fluoride levels in the bones of exposed animals was also observed. The bone concentration of fluoride continued to increase even after the final exposure. It was assumed that the continued deposition in the bone occurred as a consequence of the serum fluoride source. This increase is consistent with a pattern of deposition of free fluoride from the blood.
4.2. Mechanism of Toxicity
Data specifically addressing the mechanism of inhaled boron trifluoride toxicity were not available. An examination of the toxicity data on inhaled boron trifluoride indicates that acute exposure to a lethal concentration results in effects related to the corrosive, irritant nature of this compound. Findings observed during necropsy of animals exposed once to a lethal concentration of boron trifluoride included:
• an acute inflammatory reaction of the larynx and upper trachea and slightly edematous lungs with moderate congestion of alveolar wall in one rat and consolidation of the upper part of the lobes of the lungs and pus in the bronchi in another rat (DuPont Company 1948);
• increased lung weight, pulmonary edema, and alveolar duct destruction in albino rats and guinea pigs (Kasparov and Kiriĭ 1972);
• distended lungs and pulmonary edema in one guinea pig and thickening of the pleura, atelectasis, and areas of emphysema in another guinea pig (DuPont Company 1948);
• marked edema of the larynx with surface necrosis, desquamation, and polymorphonuclear exudate, pulmonary emphysema with areas of congestion, and mucopurulent exudate in the bronchi of dogs (DuPont Company 1948); and
• renal capsular spaces and convoluted tubules distended with albuminous fluid in dogs (DuPont Company 1948).
The concentration-response for lethality resulting from an acute 4-h exposure is very steep; 3/10 rats died at 1,010 mg/m3, while 9/10 rats died at 1,540 mg/m3 (Rusch et al. 1986).
In contrast to the pulmonary effects observed from short inhalation exposures to high concentrations of boron trifluoride, repeated exposure at lower concentrations can result in fatal renal toxicity. When rats were exposed at 180 mg/m3 for 6 h/day, 5 days/week for 9 days, all 10 died by the sixth exposure from renal toxicity (renal necrosis and pyknosis) (Rusch et al. 1986). When exposed at 17 mg/m3 for 6 h/day for 13 weeks, one rat died during week 12 from renal toxicity (necrosis of the proximal tubule epithelium and tip of the renal papillae), while another exhibited renal lesions that were mild in severity (elevated BUN, hypertrophied tubule epithelial cells with karyomegaly, remnants of necrotic epithelial cells in the lumina of scattered tubules) and most likely would not have affected the survival of the animal (Rusch et al. 1986). The mechanism of action for the renal toxicity is not known, but appears to follow a steep concentration-response curve during a 9-day exposure, all rats exposed at 180 mg/m3 died, whereas no rats died or exhibited any signs of renal toxicity at 66 mg/m3.
4.3. Structure Activity Relationships
Boron trifluoride-dimethyl ether is one of several different complexes that can be formed with boron trifluoride. A single study was found that addressed the toxicity of boron trifluoride-dimethyl ether, but it reported only nominal concentrations. Because the complex can dissociate to form boron trifluoride, the AEGL values are based on this one chemical species alone.
4.4. Other Relevant Information
4.4.1. Species Variability
Guinea pigs were much more sensitive than other species to inhaled boron trifluoride. Guinea pigs are known to be much more sensitive to some respiratory irritants, sometimes demonstrating strong allergic anaphylactic lung responses. Sensitivity to histamine is a recognized phenomenon in guinea pigs,
and is one of the reasons it is a good model for pulmonary sensitivity (Karol 1992). Because of this sensitivity, the guinea pig data for boron trifluoride were not considered in the derivation of AEGL values.
4.4.2. Susceptible Subpopulations
Information on subpopulations susceptible to boron trifluoride was not available.
4.4.3. Concentration-Exposure Duration Relationship
The relationship between concentration of boron trifluoride and duration of exposure as related to lethality was examined by ten Berge et al. (1986) for approximately 20 irritant or systemically-acting vapors and gases. The authors subjected the individual animal data sets to probit analysis with exposure duration and exposure concentration as independent variables. An exponential function of Cn × t = k, where the value of n ranged from 0.8 to 3.5 for different chemicals, was found to be an accurate quantitative descriptor for the chemicals evaluated. Approximately 90% of the values of n range between 1 and 3. Consequently, these values were selected as the reasonable lower and upper bounds of n. A value of n = 1 is used when extrapolating from shorter to longer time periods, because the extrapolated values represent the most conservative approach in the absence of other data. Conversely, a value of n = 3 is used when extrapolating from longer to shorter time periods, because the extrapolated values are more conservative in the absence of other data.
4.4.4. Toxicity of Boron Trifluoride-Dimethyl Ether
The study by Stokinger and Spiegl (1953) is the only publication that addressed the toxicity of boron trifluoride-dimethyl ether. In a pilot study, groups of 2 or 3 guinea pigs, groups of 6 or 10 mice, and groups of 4 rats were exposed to various nominal concentrations of boron trifluoride-dimethyl ether for various durations and observed for 14 days for mortality. A summary of the mortality data is presented in Table 1-9. The authors reported that mortality occurred within 14 h in the rat and mouse, but after less than 4 h in the guinea pig.
In a repeated-exposure experiment, groups of dogs, rats, mice, guinea pigs, and rabbits were exposed to boron trifluoride at nominal concentrations of 74 or 138 mg/m3 (27 or 50 ppm) for 6 h/day, 6 days/week for a total of 30 exposure days. A summary of the mortality can be found in Table 1-10. The authors noted that a few deaths occurred after only 5 h at 138 mg/m3. Pathologic examination of the animals revealed mild pulmonary irritation and some degree of thyroid colloid depletion. No changes were observed in the liver, spleen, kidneys, intestines, lymph nodes, bone marrow, gonads, or adrenal glands. However, none of the dying animals were examined.
TABLE 1-9 Mortality in Laboratory Animals Exposed to Boron Trifluoride-Dimethyl Ether
| Concentration (nominal) | |||
| ppm | mg/m3 | Duration (h) | Mortality (%) |
| Rat | |||
| 4,580 | 21,000 | 6.5 | 4/4 (100) |
| 3,450 | 16,000 | 9.5 | 4/4 (100) |
| 2,580 | 12,000 | 3.5 | 2/4 (50) |
| 1,290 | 6,000 | 14 | 1/4 (25) |
| 850 | 4,000 | 14 | 3/4 (75) |
| 550 | 2,600 | 14 | 1/4 (25) |
| 485 | 2,300 | 14 | 1/4 (25) |
| 345 | 1,600 | 14 | 0/4 (0) |
| 265 | 1,200 | 14 | 0/4 (0) |
| Mouse | |||
| 880 | 4,100 | 5.6 | 3/6 (50) |
| 380 | 1,800 | 7 | 10/10 (100) |
| 225 | 1,000 | 14 | 3/10 (30) |
| 155 | 720 | 14 | 0/10 (0) |
| 100 | 470 | 14 | 0/10 (0) |
| Guinea pig | |||
| 3,135 | 15,000 | 0.8 | 2/2 (100) |
| 2,000 | 9,300 | 0.3 | 2/2 (100) |
| 1,105 | 5,100 | 0.3 | 2/2 (100) |
| 695 | 3,200 | 2.8 | 3/3 (100) |
| 570 | 2,700 | 0.9 | 2/2 (100) |
| 405 | 1,900 | 0.3 | 2/2 (100) |
| 225 | 1,000 | 0.8 | 2/2 (100) |
| 110 | 510 | 2.3 | 1/2 (50) |
| 50 | 230 | 3.8 | 1/2 (50) |
| 38 | 180 | 14 | 0/2 (0) |
Source: Stokinger and Spiegl 1953.
TABLE 1-10 Mortality in Laboratory Animals Exposed to Boron Trifluoride Dimeth l Ether for 30 Days
| Species | Mortality (%) | |
| 27 ppm (130 mg/m3) | 50 ppm (230 mg/m3) | |
| Dog | 0/5 (0) | 0/5 (0) |
| Rat | 6/100 (6) | 18/99 (18) |
| Mouse | 9/47 (19) | 89/89 (100) |
| Guinea pig | 2/30 (7) | 23/30 (77) |
| Rabbit | 0/12 (0) | 0/12 (0) |
| Cat | 0/6 (0) | 2/6 (33) |
Source: Stokinger and Spiegl 1953.
5. DATA ANALYSIS FOR AEGL-1
5.1. Summary of Human Data Relevant to AEGL-1
No human data relevant to deriving AEGL-1 values were available.
5.2. Summary of Animal Data Relevant to AEGL-1
Rats exposed for 4 h to a measured concentration of boron trifluoride at 25 mg/m3 had no abnormal findings, whereas rats exposed at the next higher concentration of 74 mg/m3 had histopathologic changes in the larynx and tracheal bifurcation indicative of irritation. The histopathologic changes in the larynx included minimal to slight ventral cartilage necrosis, minimal anterior ventral hemorrhage, and an increase in the severity of ventral epithelial hyperplasia and ventral inflammatory cell infiltration. Histopathologic changes in the trachea included an increased incidence of cilia loss (minimal) at the point of tracheal bifurcation. Following a two-week recovery period, only ventral cartilage necrosis was still noted (in one male and one female) (Bowden 2005).
5.3. Derivation of AEGL-1 Values
AEGL-1 values are based on a no-effect level for irritation in rats of 25 mg/m3. (Irritant effects observed at 74.4 mg/m3 were considered more severe than the threshold effects for AEGL-1 values.) A total uncertainty factor of 10 was applied. Because irritation is a direct contact effect, an interspecies uncertainty factor of 3 was applied because the mechanism of action is not expected to vary greatly among species (see NRC 2001, Section 2.5.3.2.3.), and an intraspecies uncertainty factor of 3 was applied because the mechanism of action is not expected to vary greatly in subpopulations (see NRC 2001; Section 2.5.3.4.4.). The 4-h value was set equal for all AEGL durations because the point of departure is a no-effect level for mild irritation.
AEGL-1 values are presented in Table 1-11. Although the gas is stable in dry air, boron trifluoride reacts to form the dihydrate upon exposure to even low levels of moisture in the air (NIOSH 1976; Hoffman 1981). Therefore, AEGL values are reported only in mg/m3.
6. DATA ANALYSIS FOR AEGL-2
6.1. Summary of Human Data Relevant to AEGL-2
No human data relevant to derivation of AEGL-2 values were available.
TABLE 1-11 AEGL-1 Values for Boron Trifluoride
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 |
6.2. Summary of Animal Data Relevant to AEGL-2
Acute toxicity data from animal studies meeting the definition of AEGL-2 end points were not available. Histopathologic findings in rats exposed for 4 h to boron trifluoride at 74 mg/mg3 were indicative of mild irritation (Bowden 2005), an effect less severe than that those defined by AEGL-2 values.
6.3. Derivation of AEGL-2 Values
In the absence of relevant data for deriving AEGL-2 values for boron trifluoride, the AEGL-3 values were divided by 3 to obtain a reasonable estimate. Dividing the AEGL-3 values by 3 is supported by the steep dose-response curve (Rusch et al. 1986).
AEGL-2 values are presented in Table 1-12. Although the gas is stable in dry air, boron trifluoride reacts to form the dihydrate upon exposure to even low levels of moisture in the air (NIOSH 1976; Hoffman 1981). Therefore, all AEGL values are reported only in mg/m3.
7. DATA ANALYSIS FOR AEGL-3
7.1. Summary of Human Data Relevant to AEGL-3
No human data relevant to derivation of AEGL-3 values were available.
7.2. Summary of Animal Data Relevant to AEGL-3
Rusch et al. (1986) calculated a 4-h LC50 of 1,210 mg/m3 for rats on the basis of measured concentrations of boron trifluoride dihydrate. A number of other studies also reported lethality end points: Vernot et al. (1977) reported a 1-h LC50 of 1,100 and 1,000 mg/m3 for male and female rats, respectively (unknown whether concentrations were nominal or measured); Kasparov and Kiriĭ (1972) reported a 2-h LC50 of 3,460 mg/m3 in mice, a 4-h LC50 of 1,180 mg/m3 in rats, and a 4-h LC50 of 109 mg/m3 in guinea pigs (unknown whether concentrations were nominal or measured); and Stokinger and Spiegl (1953) reported a no-effect level for death of 370 mg/m3 (nominal) for 10.9 h in mice and rats. Studies that measured exposure concentrations and compared them with nominal concentrations found that actual concentrations ranged from 2.7-56% of nominal (Torkelson et al. 1961; Rusch et al. 1986; Bowden 2005); therefore, this difference should be considered when basing AEGL values on a nominal concentration.
TABLE 1-12 AEGL-2 Values For Boron Trifluoride
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 37 mg/m3 | 37 mg/m3 | 29 mg/m3 | 18 mg/m3 | 9.3 mg/m3 |
7.3. Derivation of AEGL-3 Values
AEGL-3 values are based on the threshold for lethality found by Rusch et al. (1986). Using the individual mortality data from that study, a 4-h BMC01 (benchmark concentration with 1% response) of 736 mg/m3 and BMCL05 (benchmark concentration, 95% lower confidence limit with 5% response) of 554 mg/m3 were calculated by a log-probit analysis using EPA Benchmark Dose Software version 2.0 (2007) (see Appendix B). The 4-h BMCL05 of 554 mg/m3 was used to derive AEGL-3 values because it is the more conservative value. A total uncertainty factor of 10 was applied. An interspecies uncertainty factor of 3 was applied because boron trifluoride is a corrosive irritant and the mechanism of action is not expected to vary greatly among species. An intraspecies uncertainty factor of 3 was chosen because the mechanism of irritation is not expected to vary greatly among subpopulations. An intraspecies uncertainty factor of 3 is also supported by the steep dose-response curve for lethality (3/10 rats died at 1,010 mg/m3, while 9/10 rats died at 1,540 mg/m3), which indicates there is not much variability in the response within a population. The Rusch et al. (1986) study is supported by the Kasparov and Kiriĭ (1972) study that reported a 2-h LC50 of 1,180 mg/m3 in rats. Because the irritation occurring at the AEGL-3 level is severe irritation leading to death, the point of departure is not set equal across all AEGL time points. Instead, time-scaling was performed using the concentration-time relationship given by the equation Cn × t = k, where C = concentration, t = time, k is a constant, and n generally ranges from 0.8 to 3.5 (ten Berge et al. 1986). The value of n could not be empirically derived because of inadequate data. Therefore, the default value of n = 1 was used for extrapolating from shorter to longer exposure periods, and n = 3 was used to extrapolate from longer to shorter exposure periods. The 10-min value was set equal to the 30-min value because of the uncertainty in extrapolating from a 4-h exposure duration to a 10-min exposure duration.
AEGL-3 values for boron trifluoride are presented in Table 1-13. Although the gas is stable in dry air, boron trifluoride reacts to form the dihydrate upon exposure to even low levels of moisture in the air. Therefore, the values are reported only in mg/m3.
8. SUMMARY OF AEGL VALUES
8.1. AEGL Values and Toxicity End Points
AEGL values for boron trifluoride are summarized in Table 1-14. AEGL-1 values are based on a no-effect level for irritation. Because relevant data for deriving AEGL-2 values were not available, the AEGL-3 values were divided by 3 to provide a reasonable estimate of AEGL-2 values. AEGL-3 values are based on a BMCL05 derived measured concentrations in a rat mortality study. A limitation of the study was that mortality was observed at all exposure concen-
trations. AEGL values are reported in mg/m3 because boron trifluoride gas becomes an aerosol upon contact with moisture in the air.
A useful way to evaluate the AEGL values in context of existing empirical data is presented in Figure 1-1. For this plot, the toxic response was placed into severity categories. The severity categories fit into definitions of the AEGL health effects: no effects, discomfort, disabling, and lethal and partially lethal (an experimental concentration at which some of the animals died and some did not). The effects that place an experimental result into a particular category vary according to the spectrum of data available on a specific chemical and the effects from exposure to that chemical. The concentrations often span a several orders of magnitude, especially when human data exist. Therefore, the concentration is placed on a log scale. The graph in Figure 1-1 plots AEGL values for boron trifluoride along with the existing acute animal toxicity data in terms of the categories assigned to them (see data in Appendix C). This plot shows that the AEGL values are below exposure concentration in animals resulting in any effects, and should therefore be protective of human health.
8.2. Comparison with Other Standards and Guidelines
Standards and guidelines for short-term exposures to boron trifluoride are presented in Table 1-15. The 1-h AEGL-1, AEGL-2, and AEGL-3 are comparable to the Emergency Response Planning Guidelines—ERPG-1, ERPG-2, and ERPG-3, respectively (AIHA 1999, 2008). The 8-h AEGL-1 is comparable to the 8-h Recommended Exposure Limits - Time-Weighted Average (NIOSH 2011) and Maximaal Aanvaaarde Concentratie (SDU Uitgevers 2000). The 30-min AEGL-3 value is almost twice that of the Immediately Dangerous to Life and Health value (NIOSH 1994).
TABLE 1-13 AEGL-3 Values For Boron Trifluoride
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 110 mg/m3 | 110 mg/m3 | 88 mg/m3 | 55 mg/m3 | 28 mg/m3 |
TABLE 1-14 AEGL Values for Boron Trifluoride
| Exposure Duration | |||||
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h |
| AEGL-1 (nondisabling) | 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 |
| AEGL-2 (disabling) | 37 mg/m3 | 37 mg/m3 | 29 mg/m3 | 18 mg/m3 | 9.3 mg/m3 |
| AEGL-3 (lethal) | 110 mg/m3 | 110 mg/m3 | 88 mg/m3 | 55 mg/m3 | 28 mg/m3 |
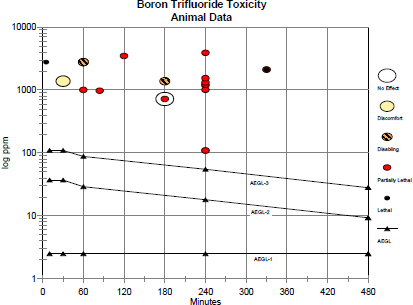
FIGURE 1-1 Category Plot of Animal Toxicity Data on Boron Trifluoride Compared with AEGL Values
TABLE 1-15 Extant Standards and Guidelines for Boron Trifluoride
| Exposure Duration | |||||
| Guideline | 10 min | 30 min | 1 h | 4 h | 8 h |
| AEGL-1 | 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 |
| AEGL-2 | 37 mg/m3 | 37 mg/m3 | 29 mg/m3 | 18 mg/m3 | 9.3 mg/m3 |
| AEGL-3 | 110 mg/m3 | 110 mg/m3 | 88 mg/m3 | 55 mg/m3 | 28 mg/m3 |
| ERPG-1 (AIHA)a | 2 mg/m3 | ||||
| ERPG-2 (AIHA) | 30 mg/m3 | ||||
| ERPG-3 (AIHA) | 100 mg/m3 | ||||
| IDLH (NIOSH)b | 25 ppm [69 mg/m3] | ||||
| TLV-STEL-Ceiling (ACGIH)c | 1 ppm [3 mg/m3] | 1 ppm [3 mg/m3] | 1 ppm [3 mg/m3] | 1 ppm [3 mg/m3] | 1 ppm [3 mg/m3] |
| PEL-Ceiling (OSHA)d | 1 ppm [3 mg/m3] | 1 ppm [3 mg/m3] | 1 ppm [3 mg/m3] | 1 ppm [3 mg/m3] | 1 ppm [3 mg/m3] |
| REL- Ceiling (NIOSH)e | 1 ppm [3 mg/m3] | 1 ppm [3 mg/m3] | 1 ppm [3 mg/m3] | 1 ppm [3 mg/m3] | 1 ppm [3 mg/m3] |
| MAC (The Netherlands)f | 1 ppm [3 mg/m3] | ||||
aERPG (emergency response planning guidelines) (American Industrial Hygiene Association [AIHA 1999, 2008])
ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing health effects more severe than mild transient adverse health effects or without perceiving a clearly defined objectionable odor. The ERPG-1 for boron trifluoride is based on the observation that while exposure to boron trifluoride at 6 mg/m3 for 6 h/day, 5 days/week for 3 months produced slight signs of irritation (excessive lacrimation in 1-3 rats of 40 during 6 of 15 observation periods), exposure at 2 mg/m3 for the same period did not. ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protective action. The ERPG-2 for boron trifluoride is based on the observations that all animals survived nine 6-h exposures at 66 mg/m3 and showed no histopathologic lesions associated with the exposure; however, a 6-h exposure at 6 mg/m3 and 17 mg/m3 for 13 weeks produced transient signs of irritation. Acute exposure of dogs at 500-1,000 ppm (1,380-2,760 mg/m3) resulted in marked irritation. Although these studies provided support for a level above 30 ppm, concern about impaired ability to escape because of the irritant effects of boron trifluoride suggested a lower value.
ERPG-3 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing life-threatening health effects. The ERPG-3 for boron trifluoride is based on animal studies. Two inhalation studies in rats determined a 4-h median lethal concentration of 1,200 mg/m3, while one study indicated that even a 1-h exposure of rats at approximately 1,000 mg/m3 could cause death. Furthermore, sensitivity to the respiratory irritant effects may be species dependent.
bIDLH (immediately dangerous to life or health) (National Institute for Occupational Safety and Health [NIOSH 1994]) represents the maximum concentration from which one could escape within 30 min without any escape-impairing symptoms, or any irreversible health effects. The IDLH for boron trifluoride is based on subchronic inhalation toxicity data in animals (Rusch et al. 1986).
cTLV-STEL-Ceiling (threshold limit value – short-term exposure limit - ceiling) (American Conference of Governmental Industrial Hygienists [ACGIH 2008]) is a 15-min TWA exposure that must not be exceeded during any part of the workday.
dPEL-Ceiling (permissible exposure limit – ceiling) (Occupational Health and Safety Administration [29CFR Part 1910.1000 [1996]) is a ceiling value that must not be exceeded during any part of the workday.
eREL-Ceiling (recommended exposure limit – ceiling) (National Institute for Occupational Safety and Health [NIOSH 2011) is a ceiling value that must not be exceeded during any part of the workday.
fMAC (maximaal aanvaaarde concentratie [maximal accepted concentration]) (SDU Uitgevers [under the auspices of the Ministry of Social Affairs and Employment], The Hague, The Netherlands, MSZW 2004) is defined analogous to the ACGIH TLV-TWA (a time-weighted average concentration for a normal 8-h workday and a 40-h workweek, to which nearly all workers may be repeatedly exposed, day after day, without adverse effect).
8.3. Data Quality and Research Needs
Inadequate data were available for deriving AEGL values for boron trifluoride-dimethyl ether. Therefore, the AEGLs were based on the dissociation
compounds boron trifluoride. No definitive toxicity data on boron trifluoride in humans was available, and animal studies generally relied on nominal exposure concentrations. Studies that measured the exposure concentrations and compared them with nominal concentrations found that actual concentrations ranged from 2.7-56% of nominal values. Therefore, nominal concentrations of boron trifluoride are unreliable.
9. REFERENCES
ACGIH (American Conference of Government and Industrial Hygienists). 1991. Boron Trifluoride (CAS Reg. No. 7637-07-2). Documentation of the Threshold Limit Values and Biological Exposure Indices, Sixth Ed. American Conference of Government and Industrial Hygienists, Cincinnati, OH.
ACGIH (American Conference of Government and Industrial Hygienists). 2008. Boron Trifluoride (CAS Reg. No. 7637-07-2). TLVs and BEIs: Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. American Conference of Government and Industrial Hygienists, Cincinnati, OH.
AIHA (American Industrial Hygiene Association). 1999. Boron trifluoride (CAS Reg. No. 7637-07-2). Pp. 1-7 in Emergency Response Planning Guidelines. American Industrial Hygiene Association, Fairfax VA.
AIHA (American Industrial Hygiene Association). 2008. Boron Trifluoride (CAS Reg. No. 7637-07-2). The AIHA 2008 Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guides Handbook. American Industrial Hygiene Association, Fairfax VA.
Bonnette, K.L. 2001. An Acute Oral Toxicity Study in Rats with Boron Trifluoride Di-ethyl Etherate (569-98A). SLI Study No. 3167.245. Conducted by Springborn Laboratories, Inc. (SLI); Sponsored by AlliedSignal Inc. April 30, 2001.
Bowden, A.M. 2005. Boron Trifluoride Dihydrate Acute (Four-Hour) Inhalation Irritation Threshold Study in Rats. Conducted by Huntingdon Life Sciences Ltd., Cambridgeshire, England; Sponsored by Honeywell International, Inc., Morristown, NJ.
Braun, W.G., and J.C. Killeen. 1975a. Rabbit Primary Dermal Irritation Compound Boron Trifluoride. TOX Computer Entry No. MA-40A-80-15. Allied Chem. Corporation. April 21, 1975.
Braun, W.G., and J.C. Killeen. 1975b. Acute Dermal Toxicity in Rabbits. Compound: Boron Trifluoride Di-n-butyl Ether Complex. TOX Computer Entry No. MA-40A-80-14. Allied Chem. Corporation. June 17, 1975.
Braun, W.G., and J.C. Killeen. 1975c. Rabbit Eye Irritation. Compound: Boron Trifluoride Di-n-butyl Ether Complex. TOX Computer Entry No. MA-40A-80-19. Allied Chem. Corporation. April 24, 1975.
Braun, W.G., and J.C. Killeen. 1975d. Acute Oral Toxicity in Rabbits. Compound: Boron Trifluoride Di-n-butyl Ether Complex. TOX Computer Entry No. MA-40A-80-13. Allied Chem. Corporation. May 6, 1975.
Braun, W.G., and J.C. Killeen. 1975e. Acute Oral Toxicity in Rats. Compound: Boron Trifluoride Di-n-butyl Ether Complex. TOX Computer Entry No. MA-40A-80-16. Allied Chem. Corporation. August 1, 1975.
Budavari, S., M.J. O'Neil, A. Smith, P.E. Heckelman, and J.F. Kinneary, eds. 1996. P. 206 in The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 12th Ed. Whitehouse, NJ: Merck.
Derelanko, M.J., and C.S. Gad. 1982. Acute Oral Toxicity Study of Boron Trifluoride Dihydrate. Report No. MA 40-80-5. Allied Signal Corporation, Morristown, NJ. June 7, 1982.
Dunn, B.J. 1980. Primary Eye and Dermal Irritation Studies of Boron Trifluoride Dihydrate. Report No. MA-40-80-1. Allied Corporation, Department of Toxicology, Morristown, NJ. October 24, 1980.
DuPont Company. 1948. Toxicity of Boron Trifluoride (BF3). Haskell Laboratory Report No. 13-48. E.I. DuPont de Nemours & Co., Newark, DE. April 15, 1948.
Eibert, J. 1969. Acute Oral Toxicity (LD50) Study in Rats; Acute Toxicity Test in Rabbits (MLD); Acute Dermal Toxicity (LD50) in Rabbits; Dermal Irritation in Rabbits; Eye Irritation in Rabbits; and Inhalation Toxicity in Rats. TOX Computer Entry No. MA-40A-80-18. Allied Chem. Corporation. July 2, 1969.
EPA (U.S. Environmental Protection Agency). 2012. Benchmark Dose Software. National Center for Environmental Assessment, Office of Research and Development: Washington, DC [online]. Available: http://www.epa.gov/ncea/bmds/about.html [accessed July 2, 2012].
Hoffman, G.M. 1981. An Acute Inhalation Toxicity Study of Boron Trifluoride Dihydrate in the Rat. Report No. MA-40-80-2. Allied Corporation, Department of Toxicology, Morristown, NJ. December 1, 1981.
Hoffman, G.M., and G.M. Rusch. 1982a. A Thirteen-Week Inhalation Toxicity Study of Boron Trifluoride Dihydrate in the Rat. Report No. MA-40-80-7. Allied Corporation, Department of Toxicology, Morristown, NJ. September 28, 1983.
Hoffman, G.M., and G.M. Rusch. 1982b. A Two-Week Inhalation Toxicity Study of Boron Trifluoride Dihydrate in the Rat. Report No. MA-40-80-3. Allied Corporation, Department of Toxicology, Morristown, NJ. September 1, 1981.
Karol, M.H. 1992. Design of animal models to probe the mechanisms of multiple chemical sensitivity. Pp. 65-76 in Multiple Chemical Sensitivities Addendum to Biologic Markers in Immunotoxicity. Washington, DC: National Academy Press.
Kasparov, A.A., and V.G. Kiriĭ. 1972. Toxicity of boron trifluoride [in Russian]. Farmakol. Toksikol. 35(3):369-372.
Lewis, R.J., Sr. 1996. P. 499 in Sax’s Dangerous Properties of Industrial Materials. New York: John Wiley & Sons.
Litchfield, J.T., and F. Wilcoxon. 1949. A simplified method of evaluating dose-effects experiment. J. Pharmacol. Exp. Ther. 96(2):99-113.
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: Boriumtrifloride. Den Haag: SDU Uitgevers [online]. Available: http://www.lasrook.net/lasrookNL/maclijst2004.htm [accessed July 2, 2012].
NIOSH (National Institute for Occupational Safety and Health). 1976. Criteria for a Recommended Standard. Occupational Exposure to Boron Trifluoride. DHEW [NIOSH] Pub. No. 77-122. U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health, Cincinnati, OH [online]. Available: http://www.cdc.gov/niosh/docs/1970/77-122.html [accessed July 2, 2012].
NIOSH (National Institute for Occupational Safety and Health). 1994. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs): Boron Triflouride. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health
[online]. Available: http://www.cdc.gov/niosh/idlh/7637072.html [accessed July 2, 2012].
NIOSH (National Institute for Occupational Safety and Health). 2011. NIOSH Pocket Guide to Chemical Hazards: Boron Triflouride. U.S. Department of Health and Human Services. Centers for Disease Control, National Institute for Occupational Safety and Health [online]. Available: http://www.cdc.gov/niosh/npg/npgd0062.html [accessed July 2, 2012].
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
Rusch, G.M., G.M. Hoffman, R.F. McConnell, and W.E. Rinehart. 1986. Inhalation toxicity studies with boron trifluoride. Toxicol. Appl. Pharmacol. 83(1):69-78.
Stewart, M.J., and R. Waisberg. 1988. Poisoning with boron trifluoride. S. Afr. Med. J. 88(12):1536-1537.
Stokinger, H.E., and C.J. Spiegl. 1953. Part A. Inhalation-toxicity studies of boron halide and certain fluorinated hydrocarbons. Pp. 2291-2328 in Pharmacology and Toxicology of Uranium Compounds, C. Voegtlin, and H.C. Hodge, eds. New York: McGraw-Hill Book.
ten Berge, W.F., A. Zwart, and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Mater. 13(3):301-309.
Torkelson, T.R., S.E. Sadek, and V.K. Rowe. 1961. The toxicity of boron trifluoride when inhaled by laboratory animals. Am. Ind. Hyg. Assoc. J. 22:263-270.
Vernot, E.H., J.D. MacEwen, C.C. Haun, and E.R. Kinkead. 1977. Acute toxicity and skin corrosion data for some organic and inorganic compounds and aqueous solutions. Toxicol. Appl. Pharmacol. 42(2):417-423.
Wudl, L.R., and S.M. Goode. 1982. Evaluation of Boron Trifluoride for Enzyme Mediated Mutagenicity in Salmonella typhimurium. Report No. MA-40-80-4. Allied Corporation, Department of Toxicology, Morristown, NJ. February 1, 1982.
APPENDIX A
DERIVATION OF AEGL VALUES FOR BORON TRIFLUORIDE
Derivation of AEGL-1 Values
| Key study: | Bowden, A.M. 2005. Boron Trifluoride Dihydrate Acute (Four-H) Inhalation Irritation Threshold Study in Rats. Conducted by Huntingdon Life Sciences Ltd.: Cambridgeshire, England; Sponsored by Honeywell International, Inc., Morristown, NJ. |
| Toxicity end points: | No-effect level for irritation of 25 mg/m3 for 4 h. |
| Time scaling | Values were set equal across all AEGL durations because the point of departure is a no-effect level for irritation. |
| Uncertainty factors: | 3 for interspecies variability 3 for intraspecies variability Combined uncertainty factor of 10 |
| Modifying factor: | Not applicable |
| 10-min AEGL-1: | Set equal to 4-h AEGL-1 value of 2.5 mg/m3 |
| 30-min AEGL-1: | Set equal to 4-h AEGL-1 value of 2.5 mg/m3 |
| 1-h AEGL-1: | Set equal to 4-h AEGL-1 value of 2.5 mg/m3 |
| 4-h AEGL-1: | 25 mg/m3 ÷ 10 = 2.5 mg/m3 |
| 8-h AEGL-1: | Set equal to 4-h AEGL-1 value of 2.5 mg/m3 |
Derivation of AEGL-2 Values
| Calculations: | Because there were no relevant data for deriving AEGL-2 values, AEGL-3 values were divided by 3 to estimate AEGL-2 values |
| 10-min AEGL-2: | 110 mg/m3 ÷ 3 = 37 mg/m3 |
| 30-min AEGL-2: | 110 mg/m3 ÷ 3 = 37 mg/m3 |
| 1-h AEGL-2: | 88 mg/m3 ÷ 3 = 29 mg/m3 |
| 4-h AEGL-2: | 55 mg/m3 ÷ 3 = 18 mg/m3 |
| 8-h AEGL-2: | 28 mg/m3 ÷ 3 = 9.3 mg/m3 |
Derivation of AEGL-3 Values
| Key study: | Rusch, G.M., G.M. Hoffman, R.F. McConnell, and W.E. Rinehart, W.E. 1986. Inhalation toxicity studies with boron trifluoride. Toxicol. Appl. Pharmacol. 83(1):69-78. |
| Toxicity end point: | 4-h BMCL05 in rats of 554 mg/m3 |
| Time scaling: | Cn × t = k (default of n = 3 for extrapolation from longer to shorter durations; default of n = 1 for extrapolation from shorter to longer durations) [(554 mg/m3 ÷ 10)]1 × 4 h = 221.6 mg/m3-h [(554 mg/m3) ÷ 10]3 × 4 h = 680,125.9 mg/m3-h |
| Uncertainty factors: | 3 for interspecies variability 3 for intraspecies variability Combined uncertainty factor of 10 |
| Modifying factor: | Not applicable |
| 10-min AEGL-3: | Set equal to the 30-min value of 110 mg/m3 because of uncertainty with extrapolating from a 4-h exposure to 10 min. |
| 30-min AEGL-3: | C3 × 0.5 h = 680,125.9 mg/m3-h C3 = 1,360,251.8 mg/m3 C = 110 mg/m3 |
| 1-h AEGL-3: | C3 × 1 h = 680,125.9 mg/m3 -h C3 = 680,125.9 mg/m3 C = 88 mg/m3 |
APPENDIX B
BENCHMARK DOSE CALCULATIONS FOR BORON TRIFLUORIDE
| Probit Model. (Version: 3.1; Date: 05/16/2008) Input Data File: C:\USEPA\BMDS2\Data\LogBF3Set.(d) Gnuplot Plotting File: C:\USEPA\BMDS2\Data\LogBF3Set.plt Thu Aug 27 09:29:46 2009 |
|
| BMDS Model Run | |
| The form of the probability function is: | |
| P[response] | = Background + (1-Background) * CumNorm(Intercept+Slope*Log[Dose]), |
| where CumNorm(.) is the cumulative normal distribution function | |
| Dependent variable = No._affected Independent variable = DOSE Slope parameter is restricted as slope > = 1 |
|
| Total number of observations = 5 Total number of records with missing values = 0 Maximum number of iterations = 250 Relative Function Convergence has been set = 1E-008 Parameter Convergence has been set = 1E-008 |
|
| User has chosen the log transformed model | |
| Default Initial (and Specified) Parameter Values Background = 0 Intercept = -33.8553 Slope = 4.76995 |
|
Asymptotic Correlation Matrix of Parameter Estimates
| Intercept | Slope | |
| Intercept | 1 | -1 |
| Slope | -1 | 1 |
| (***The model parameter(s) background has been estimated at a boundary point, or has been specified by the user, and do not appear in the correlation matrix). | ||
Parameter Estimates
| 95.0% Wald Confidence Interval | ||||
| Variable | Estimate | Standard Error | Lower Limit | Upper Limit |
| Background | 0 | NA | ||
| Intercept | -32.9607 | 10.9082 | -54.3404 | -11.581 |
| Slope | 4.64094 | 1.53091 | 1.64041 | 7.64146 |
NA: indicates that this parameter has hit a bound implied by some inequality constraint and thus has no standard error.
Analysis of Deviance Table
| Model | Log (likelihood) | No. Parameters | Deviance Test | DF | P-value |
| Full model | -19.3675 | 5 | |||
| Fitted model | -22.3 | 2 | 5.86491 | 3 | 0.1184 |
| Reduced model | -34.2965 | 1 | 29.8579 | 4 | <0.0001 |
Goodness of Fit
| Scaled | |||||
| Dose | Estimated Probability | Expected | Observed | Size | Residual |
| 0 | 0 | 0 | 0 | 10 | 0 |
| 1,010 | 0.196 | 1.96 | 3 | 10 | 0.829 |
| 1,220 | 0.5082 | 5.082 | 2 | 10 | -1.95 |
| 1,320 | 0.6503 | 6.503 | 8 | 10 | 0.992 |
| 1,540 | 0.8647 | 8.647 | 9 | 10 | 0.326 |
Chi-square = 5.58; DF = 3 P-value = 0.1340
Benchmark Dose Computation
| Specified effect | = 0.01 | |
| Risk type | = Extra risk | |
| Confidence level | = 0.95 | |
| BMD | = 735.755 | |
| BMDL | = 408.953 |
Benchmark Dose Computation
| Specified effect | = 0.05 | |
| Risk type | = Extra risk | |
| Confidence level | = 0.95 | |
| BMD | = 852.132 | |
| BMDL | = 554.475 |
APPENDIX C
RAW DATA FOR BORON TRIFLUORIDE CATEGORY PLOT
TABLE C-1 Raw Data for Boron Trifluoride Category Plot
| Source | Species | Sex | No. Exposures | mg/m3 | Min | Category |
| NAC/AEGL-1 | 2.5 | 10 | AEGL | |||
| NAC/AEGL-1 | 2.5 | 30 | AEGL | |||
| NAC/AEGL-1 | 2.5 | 60 | AEGL | |||
| NAC/AEGL-1 | 2.5 | 240 | AEGL | |||
| NAC/AEGL-1 | 2.5 | 480 | AEGL | |||
| NAC/AEGL-2 | 37 | 10 | AEGL | |||
| NAC/AEGL-2 | 37 | 30 | AEGL | |||
| NAC/AEGL-2 | 29 | 60 | AEGL | |||
| NAC/AEGL-2 | 18 | 240 | AEGL | |||
| NAC/AEGL-2 | 9.3 | 480 | AEGL | |||
| NAC/AEGL-3 | 110 | 10 | AEGL | |||
| NAC/AEGL-3 | 110 | 30 | AEGL | |||
| NAC/AEGL-3 | 88 | 60 | AEGL | |||
| NAC/AEGL-3 | 55 | 240 | AEGL | |||
| NAC/AEGL-3 | 28 | 480 | AEGL | |||
| Vernot et al. 1977 | Rat | Male | 1,100 | 60 | PL | |
| Vernot et al. 1977 | Rat | Female | 1,000 | 60 | PL | |
| DuPont Company 1948 | Rat | 3,900 | 240 | PL | ||
| Stokinger and Spiegl 1953 | Rat | 2,100 | 330 | PL | ||
| Stokinger and Spiegl 1953 | Rat | 370 | 654 | 0 | ||
| Kasparov and Kiriĭ 1972 | Rat | 1,180 | 240 | PL | ||
| Rusch et al. 1986 | Rat | 1,010 | 240 | PL | ||
| Rat | 1,220 | 240 | PL | |||
| Rat | 1,320 | 240 | PL | |||
| Rat | 1,540 | 240 | PL | |||
| Stokinger and Spiegl 1953 | Mice | 2,100 | 330 | PL | ||
| Mice | 370 | 654 | 0 | |||
| Kasparov and Kiriĭ 1972 | Mice | 3,460 | 120 | PL | ||
| DuPont Company 1948 | Guinea pig | 2,760 | 5 | 3 | ||
| DuPont Company 1948 | Guinea pig | 720 | 180 | PL | ||
| Stokinger and Spiegl 1953 | Guinea pig | 2,100 | 330 | 3 | ||
| Stokinger and Spiegl 1953 | Guinea pig | 970 | 84 | PL | ||
| Stokinger and Spiegl 1953 | Guinea pig | 370 | 654 | PL | ||
| Kasparov and Kiriĭ 1972 | Guinea pig | 109 | 240 | PL | ||
| DuPont Company 1948 | Dog | 1,380 | 30 | 1 | ||
| DuPont Company 1948 | Dog | 1,380 | 180 | 2 | ||
| DuPont Company 1948 | Rat | 2,760 | 60 | 2 | ||
| DuPont Company 1948 | Rat | 720 | 180 | 0 | ||
| Bowden 2005 | Rat | 9 | 240 | 0 | ||
| Bowden 2005 | Rat | 25 | 240 | 0 | ||
| Bowden 2005 | Rat | 74 | 240 | 1 | ||
a0 = no effect; 1 = discomfort; 2 = disabling; PL = partially lethal; 3 = lethal.
APPENDIX D
ACUTE EXPOSURE GUIDELINE LEVELS FOR BORON TRIFLUORIDE
Derivation Summary
AEGL-1
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 | 2.5 mg/m3 |
| Key reference: Bowden, A.M. 2005. Boron Trifluoride Dihydrate Acute (Four-H) Inhalation Irritation Threshold Study in Rats. Conducted by Huntingdon Life Sciences Ltd., Cambridgeshire, England; Sponsored by Honeywell International, Inc., Morristown, NJ. | ||||
| Test species/Strain/Number: Rat, Sprague-Dawley, 10 male and 10 female per group | ||||
| Exposure route/Concentrations/Durations: Inhalation; 0, 9, 25, or 74 mg/m3 for 4 h | ||||
| Effects: 9 mg/m3: No effects 25 mg/m3: No effects 74 mg/m3: Histopathologic changes in the larynx and tracheal bifurcation indicative of irritation. |
||||
| End point/Concentration/Rationale: 25 mg/m3 is a no-effect level for irritation | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 10 Interspecies: 3, because the irritation is a direct contact effect, so mechanism of action is not expected to vary greatly among species (NRC 2001; Section 2.5.3.2.3.). Intraspecies: 3, because the mechanism of action is not expected to vary greatly in subpopulations (NRC 2001; Section 2.5.3.4.4.). |
||||
| Modifying factor: Not applicable | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time scaling: The 4-h AEGL value was applied to all AEGL durations because the point of departure is a no-effect level for mild irritation. | ||||
| Data adequacy: The Bowden (2005) study is the only acute study addressing nonlethal end points after exposure to a quantified concentration of boron trifluoride. Data inadequacies include data addressing acute, nonlethal exposures. | ||||
AEGL-2 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 37 mg/m3 | 37 mg/m3 | 29 mg/m3 | 18 mg/m3 | 9.3 mg/m3 |
| Data adequacy: No acute toxicity data relevant to deriving AEGL-2 values were available. Therefore, the AEGL-3 values wre divided by 3. | ||||
AEGL-3 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 110 mg/m3 | 110 mg/m3 | 88 mg/m3 | 55 mg/m3 | 28 mg/m3 |
| Key reference: Rusch, G.M., G.M. Hoffman, R.F. McConnell, and W.E. Rinehart. 1986. Inhalation toxicity studies with boron trifluoride. Toxicol. Appl. Pharmacol. 83(1):69-78. | ||||
| Test species/Strain/Number: Rat, F344, 5 male and 5 female per group | ||||
| Exposure route/Concentrations/Durations: Inhalation; 0, 1,010, 1,220, 1,320, or 1,540 mg/m3 for 4 h | ||||
| Effects: | ||||
| Concentration (mg/m3) | Mortality | |||
| 0 | 0/10 | |||
| 1,010 | 3/10 | |||
| 1,220 | 2/10 | |||
| 1,320 | 8/10 | |||
| 1,540 | 9/10 | |||
| LC50: 1,210 mg/m3 LC01: 736 mg/m3 BMCL05 = 554 mg/m3 |
||||
| End point/Concentration/Rationale: 4-h BMCL05 was chosen as point of departure for AEGL-3 to represent the threshold for lethality. | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 10 Intraspecies: 3, because boron trifluoride is a corrosive irritant and the mechanism of action is not expected to vary greatly among species. Intraspecies: 3, because the mechanism of irritation is not expected to vary greatly among subpopulations; an uncertainty factor of 3 is also supported by the steep doseresponse curve for lethality (3/10 rats died at 1,010 mg/m3, while 9/10 rats died at 1,540 mg/m3), which indicates there is not much variability in the response within a population. |
||||
| Modifying factor: Not applicable | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time scaling: Extrapolation to different exposure durations was performed using the equation Cn × t = k (ten Berge et al. 1986), where n = 3 for extrapolation to durations of 30-min and 1 h, and n = 1 for extrapolation to 8 h. The 30-min value was adopted as the 10-min value because of the uncertainty with extrapolating from a 4-h exposure to 10 min. | ||||
| Data adequacy: AEGL-3 values based on a calculated BMCL05 from a study with measured concentrations of boron trifluoride. A limitation of the study was that mortality was observed at all exposure concentrations. Another limiting factor is that other studies addressing mortality after acute exposure did not provide analytic exposure concentrations; therefore, there are no other studies using the same or other species to support the values. Nonetheless, a study by Kasparov and Kiri. (1972) provide some supporting evidence with a 4-h LC50 of 1,180 mg/m3 in rats. | ||||
APPENDIX E
SUMMARY OF ACUTE TOXICITY STUDIES WITH BORON TRIFLUORIDE COMPLEXES
TABLE E-1 Acute Toxicity Studies of Boron Trifluoride Complexes
| Test Substance | Study Design | Results | References | |
| Boron trifluoride dihydrate | Dermal irritation | 6 rabbits, 0.5 mL, 24 h, total corrosion | Dunn 1980 | |
| Eye irritation | 6 rabbits, 0.1 mL, corrosive | |||
| Oral LD50 | Male rats, LD50 = 464 mg/kg; Female rats, LD50 = 282-363 mg/kg | Derelanko and Gad 1982 | ||
| Boron trifluoride dinbutyl ether | Primary dermal irritation (PDI) | 6 rabbits, PDI = 5.5, corrosive | Braun and Killeen 1975a | |
| Acute dermal toxicity | Rabbits (2 males, 2 females/group); only 2 groups tested; LD50 = 1-2 g/kg | Braun and Killeen 1975b | ||
| Eye irritation | 6 rabbits, 0.1 mL, corrosive | Braun and Killeen 1975c,d | ||
| Oral LD50 | Rabbits (1 male and 1 female/dose), LD50 = 0.25 g/kg | |||
| Oral LD50 | Rats (5 males, 5 females/group), LD50 = 0.71 g/kg | Braun and Killeen 1975e | ||
| Boron trifluoride diethyl ether | Primary dermal irritation | 6 rabbits, 0.5 mL, corrosive | Eibert 1969 | |
| Dermal LD50 | Rabbits (6 males, 6 females/group, 2 groups), LD50 = 1-2 g/kg | |||
| Eye irritation | 6 rabbits, 1.1 mL, corrosive | |||
| Oral, minimal lethal dose | Rabbits (1 male, 1 female/group), 0.32-1.0 g/kg, lethality at 0.32 g/kg | |||
| Oral LD50 | Rats, 6/group, LD50 = 375 mg/kg | |||
| Inhalation screen, 1 h exposure | Rats (5 males, 5 females/group), 2.54 mg/L, 3 males and 1 female died, 1-h LC50 ≥2.54 mg/L | |||
| Boron trifluoride diethyl etherate | Oral | Rats (5 males, 5 females/group); male: LD50 >0.28 but <0.56 g/kg; females: 0.30 g/kg | Bonnette 2001 | |