Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory
____________________
1This document was prepared by the AEGL Development Team composed of Dana Glass (Oak Ridge National Laboratory), Lisa Ingerman (SRC, Inc.), Chemical Manager George Cushmac (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances), and Ernest V. Falke (U.S. Environmental Protection Agency). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold concentrations for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Perchloryl fluoride is a colorless, stable gas. It is used as a fluorinating agent, an oxidant in rocket fuels, and a gaseous dielectric for transformers. It is prepared by electrolysis of a saturated solution of sodium perchlorate in anhydrous hydrofluoric acid. Perchloryl fluoride is a strong oxidizer, and is strongly irritating to the eyes, mucous membranes, and lungs. Its systemic effects include induction of methemoglobinemia.
No human data were available for developing AEGL values, and only two relevant reports of studies in animals were found. Greene et al. (1960) performed several experiments in dogs, rats, mice, and guinea pigs. In acute studies with dogs, animals were treated with perchloryl fluoride at 224-622 ppm for 4 h, and hemoglobin and methemoglobin concentrations were evaluated. In studies with rats and mice, only 4-h LC50 (lethal concentration, 50% lethality) values were reported. Repeat-exposure studies in dogs, rats, mice, and guinea pigs also were performed. In the second report, mortality values were presented for rats at several time points, but details of the exposures to perchloryl fluoride were not included (Dost et al. 1974). No information relevant to time-scaling AEGL values for perchloryl fluoride was found.
The AEGL-1 values were derived from a study in which dogs and rats were exposed to perchloryl fluoride at 24 ppm for 6 h/day, 5 days/week for 26 weeks. All animals survived and no irritation or clinical signs of toxicity were
observed. The only long-term effect was increased fluoride deposition in the bone and urine. Therefore, 24 ppm was considered a no-effect level for an 8-h exposure, and was selected as the point of departure. That value was divided by a total uncertainty factor of 30 (3 for interspecies differences and 10 for intraspecies variability). An interspecies uncertainty factor of 3 was selected because lethality values for dogs, rats, and mice differed by less than a factor of 3. An intraspecies uncertainty factor of 10 was considered appropriate because infants are considerably more susceptible to methemoglobinemia than healthy adults. In the absence of time-scaling information, the 6-h value was scaled using the equation Cn × t = k, using the default values of n = 3 and n = 1 to extrapolate to shorter or longer exposure durations, respectively. Because of the uncertainty associated with scaling a 6-h exposure to 10 min, the 10-min AEGL value was set equal to the 30-min AEGL value.
No acute studies were available that addressed relevant AEGL-2 effects. In the absence of appropriate chemical-specific data, AEGL 2 values were set at one-third of the AEGL-3 values (NRC 2001). This approach is supported by the apparent steep-concentration response curve for perchloryl fluoride. Two of two dogs exposed to perchloryl fluoride at 425 ppm survived a 4-h exposure, but one of two dogs was found moribund after a 4-h exposure at a slightly higher concentration of 451 ppm (Green et al. 1960).
AEGL-3 values were based on moderate cyanosis and hyperpnea observed in dogs exposed to perchloryl fluoride at 224 ppm for 4-h. No dogs died at the next highest concentration of 451 ppm, but that concentration is greater than the rat 4-h LC50 of 385 ppm in the same study. A total uncertainty factor of 30 was applied (3 for interspecies differences and 10 for intraspecies variability). An interspecies uncertainty factor of 3 was selected because lethality values among dogs, rats, and mice differed by less than a factor of 3, and lethal values for the rat were considered in selecting the point of departure. An intraspecies uncertainty factor of 10 was considered appropriate because infants are considerably more susceptible to methemoglobinemia than healthy adults. In the absence of time-scaling information, the 4-h value was scaled to the shorter- and longer-exposure durations using the same approach as that for the AEGL-1 values. Because of uncertainty in time scaling from a 4-h exposure to 10 min, the 10-min value was set equal to the 30-min AEGL value.
AEGL values for perchloryl fluoride are presented in the Table 5-1.
1. INTRODUCTION
Perchloryl fluoride is a colorless gas with a characteristic sweet odor. Chemically, it is the acyl fluoride of perchloric acid, and is prepared by electrolysis of a saturated solution of sodium perchlorate in anhydrous hydrofluoric acid. It is a very stable compound. Perchloryl fluoride is used as a fluorinating agent, an oxidant in rocket fuels, and a gaseous dielectric for transformers (Mendiratta et al. 2005). It is a strong oxidizer, and acts as a strong irritant of the
eyes, mucous membranes, and lungs. Absorption of the chemical results in methemoglobinemia. Dermal contact with the liquid form of perchloryl fluoride can produce burns.
Production data were not found for perchloryl fluoride. Perchloryl fluoride does not burn and is not flammable, but it can support combustion. Chemical and physical properties are provided in Table 5-2.
TABLE 5-1 Summary of AEGL Values for Perchloryl Fluoride
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| AEGL-1 (nondisabling) | 1.8 ppm (7.6 mg/m3) | 1.8 ppm (7.6 mg/m3) | 1.5 ppm (6.3 mg/m3) | 0.92 ppm (3.9 mg/m3) | 0.60 ppm (2.5 mg/m3) | No effects in dog or rats after 26-wk exposure (Greene et al. 1960) |
| AEGL-2 (disabling) | 5.0 ppm (21 mg/m3) | 5.0 ppm (21 mg/m3) | 4.0 ppm (17 mg/m3) | 2.5 ppm (11 mg/m3) | 1.2 ppm (5.0 mg/m3) | One-third of the AEGL-3 values |
| AEGL-3 (lethal) | 15 ppm (63 mg/m3) | 15 ppm (63 mg/m3) | 12 ppm (50 mg/m3) | 7.5 ppm (32 mg/m3) | 3.7 ppm (16 mg/m3) | Highest concentration causing no deaths in mice, rats, and dogs after 4 h (Greene et al. 1960) |
TABLE 5-2 Chemical and Physical Properties of Perchloryl Fluoride
| Parameter | Value | References |
| Synonyms | Trioxychlorofluoride, chlorine oxyfluoride, chlorine fluoride oxide | ACGIH 2008; HSDB 2008 |
| CAS registry no. | 7616-94-6 | HSDB 2008 |
| Chemical formula | Cl-F-O3 | HSDB 2008 |
| Molecular weight | 102.45 | HSDB 2008 |
| Physical state | Colorless gas | HSDB 2008 |
| Melting point | -146°C | HSDB 2008 |
| Boiling point | -46.8°C | HSDB 2008 |
| Density | HSDB 2008 | |
| Vapor | 0.64 (air = 1) | |
| Liquid | 1.4 at 20°C (water = 1) | |
| Solubility in water | Miscible with water | HSDB 2008 |
| Vapor pressure | 8,943.9 mm Hg at 25°C | HSDB 2008 |
| Flammability limits | Not applicable, substance will not burn but can support combustion; strong oxidizer | HSDB 2008 |
| Conversion factors | 1 ppm = 4.2 mg/m3 1 mg/m3 = 0.24 ppm | ACGIH 1991 |
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
No human data were available on the acute lethality of perchloryl fluoride.
2.2. Nonlethal Toxicity
No human data were available on the nonlethal toxicity of perchloryl fluoride. Anecdotal information indicates symptoms of upper respiratory irritation, headaches, and dizziness after exposure to perchloryl fluoride vapors (HSDB 2008).
Perchloryl fluoride has a characteristic sweet odor. Greene et al. (1960) used human volunteers (number and gender of participants not specified) to estimate the median detectable concentration of perchloryl fluoride gas. The gas, mixed with air from the room, was metered into an inhalation chamber using a Fair-Wells osmoscope (no further study details provided). At 41 ppm, 50% of the volunteers detected the odor and described it as sweet, musty, or similar to nitric acid.
2.3. Neurotoxicity
No human data were available on the neurotoxicity of perchloryl fluoride.
2.4. Developmental and Reproductive Toxicity
No data human were available on the developmental or reproductive toxicity of perchloryl fluoride.
2.5. Genotoxicity
No human data were available on the genotoxicity of perchloryl fluoride.
2.6. Carcinogenicity
No human data were available on the carcinogenicity of perchloryl fluoride.
2.7. Summary
Data on perchloryl fluoride exposure in humans either by occupational exposure or under experimental conditions were not available. Greene et al. (1960) conducted an odor-threshold clinical study with human volunteers and reported that 50% of participants could detect perchloryl fluoride at 41 ppm. However, details of this study were minimal.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
3.1.1. Dogs
Groups of two adult male beagle dogs were exposed to perchloryl fluoride at concentrations of 224, 425, or 451 ppm for 4 h (Greene et al. 1960). Two additional dogs were exposed at 622 ppm for 2.5 h (see Table 5-3). Perchloryl fluoride was metered through a calibrated flow meter and was diluted with air from the room before to entering the exposure chamber. Atmospheric concentrations of the chemical in the chamber were estimated from the weight of the compound. Blood was collected before and after exposure to determine hemoglobin and methemoglobin concentrations, and gross and histopathologic examinations were performed. Two of the eight dogs died; one exposed at 451 ppm for 4 h was moribund when removed from the chamber and one exposed at 622 ppm for 2.5 h was dead, but the time of death was not specified. The remaining dogs exposed at 451 or 622 ppm were injected with methylene blue (2 mg/kg) to counteract methemoglobinemia. All dogs experienced concentration-related cyanosis and hyperpnea. Dogs also exhibited convulsions and motor instability when exposed at 451 or 622 ppm. At 425 ppm, methemoglobin concentrations increased to 15.5% immediately after exposure, and decreased to 4.8% after an 18-h recovery period. Methemoglobin concentrations reached 28.7% and 70.9% in dogs exposed at 451 and 622 ppm, respectively, before methylene blue therapy. After methylene-blue therapy was initiated, methemoglobin concentrations were reduced to 3.8% and 0.0% in dogs exposed at 451 ppm and 622 ppm, respectively. No further study details were provided.
3.1.2. Rats
Greene et al. (1960) exposed rats to perchloryl fluoride in a manner similar to that described above for dogs. Groups of 10 adult male rats (derived Wistar CF-1 strain) were exposed to perchloryl fluoride at concentrations of 220-885 ppm for 4 h (individual concentrations not reported). The gas was metered through a calibrated flow meter and was diluted with air from the room before to entering the chamber. Atmospheric concentration of the chemical in the chamber was analyzed by using quantitative hydrolysis of perchloryl fluoride with 10% alcoholic potassium hydroxide in a series of bubblers. Rats that died had labored breathing, cyanosis, pronounced gasping, and convulsions. Most deaths occurred during exposure or within 2 days after exposure. Surviving rats were kept for 7 days postexposure. The 4-h LC50 for rats was 385 ppm (95% confidence limits of 367-404 ppm). Rats that died had moderate discoloration of the blood with accompanying discoloration of the viscera, especially the lungs. Microscopic examination showed marked congestion in the pulmonary vasculature
with some areas of hemorrhage in the alveoli. No raw data to confirm the results were included in the study report.
Dost et al. (1974) exposed male Sprague-Dawley rats to perchloryl fluoride at 5,000 ppm for 15 min, 2,000 ppm for 25 or 40 min, or 1,000 ppm for 60 min (number of rats not specified). Rats were placed in a 3.6-L chamber that accommodated two rats at a time. Exposure at 5,000 ppm for 15 min and 2,000 ppm for 40 min was lethal to all rats. All rats survived exposure at 2,000 ppm for 25 min and 1,000 ppm for 60 min. Methemoglobinemia was observed at all concentrations; at lethal concentrations, methemoglobin exceeded 60% of total hemoglobin. No further details were available.
3.1.3. Mice
In a mouse lethality study, Greene et al. (1960) exposed groups of 20 female Carworth Farms CF-1 strain mice to perchloryl fluoride at concentrations of 220 to 885 ppm for 4 h. The delivery system and concentration analysis were the same as those described for the rat above. Mice that died had labored breathing, cyanosis, pronounced gasping, and convulsions; most deaths occurred during or within 2 days postexposure. Surviving mice were kept for 14-days postexposure. The 4-h LC50 for mice was 630 ppm (95% confidence limits of 569 - 697 ppm). The mice that died had the same discoloration of the internal organs as observed in the rats, but to a lesser degree. No raw data to confirm the results were included in the study report.
3.1.4. Guinea Pigs
Kushneva (1999) reported an LC50 for perchloryl fluoride of 220 mg/m3 (52 ppm) in guinea pigs, but the exposure duration was not specified.
3.2. Nonlethal Toxicity
Greene et al. (1960) conducted several repeat-exposure studies of perchloryl fluoride, in which dogs, rats, mice, and guinea pigs were exposed in various scenarios ranging from 5-26 weeks in duration. Chamber concentrations were determined analytically with samples collected by quantitative hydrolysis of perchloryl fluoride with 10% alcoholic potassium hydroxide in a series of three bubblers. Sample collections were 0.125-0.150 L/min. In all of the studies, data were reported in graphic format without providing specific values.
3.2.1. Dogs
Groups of three beagles were exposed to perchloryl fluoride at 0 or 24 ppm for 6 h/day, 5 days/week for 26 weeks (Greene et al. 1960). Dogs were
either killed at the end of the exposure period or allowed a 6-week recovery period (number of dogs not specified). Fluoride concentrations were measured in the blood and urine throughout the study and in the bone (femur) at the end of the exposure period or at the end of the recovery. All dogs survived, and no clinical signs of toxicity were observed. Urinary fluoride concentrations increased 4-fold over 6 months, but were comparable those of the controls at the end of the exposure and remained normal during the recovery period. Bone fluoride concentrations in treated dogs were 46% greater than that of control dogs. However, the investigators stated that the amount of fluoride in the bone did not reach 4,000 ppm, the concentration thought to cause histopathologic changes. Spleen congestion containing iron-bearing pigments was found in treated dogs killed after the final exposure, but not in treated dogs after a 6-week recovery period. No effects were observed in the lungs of any dogs.
3.2.2. Rats
Groups of 20 adult male rats (derived Wistar CF-1 strain) were exposed to perchloryl fluoride at 0, 104 ppm, or 185 ppm for 6 h/day, 5 days/week for 5 weeks (104 ppm) or 7 weeks (185 ppm) (Greene et al. 1960). Groups of three rats were killed immediately and 18 h after the first and fourth exposure period of each week. Blood was taken by cardiac puncture and the following hematology parameters were measured: red-blood-cell count, white-blood-cell count (with differential), reticulocyte count, methemoglobin, hemoglobin, fragility, sedimentation rate, and hematocrit. Select tissues were prepared for histologic examination. Fluoride deposition in the femur and urinary and blood fluoride were determined.
Mortality was 90% (18/20) in the 185-ppm group after 35 days and 5% (1/20) in the 104-ppm group after 25 days. Cyanosis was observed at both concentrations, and dyspnea at the highest concentration. After 1 week, rats exposed at 185 ppm had a 23% increase in methemoglobin concentrations and a 25% decrease in total hemoglobin compared with controls. Methemoglobin concentrations returned to normal after an overnight recovery period. Methemoglobin and hemoglobin measurements in the treated rats were comparable to those of controls after the second week. At gross examination, rats exposed at 185 ppm had darkened organs and splenic weight was increased 4-5 times that of the controls. Histopathologic lesions included splenic, hepatic, and renal hemosiderosis and pulmonary lesions, including alveolar edema that developed into bronchopneumonia. Rats also exhibited stained incisors from fluorosis. At 104 ppm, similar blood and tissue changes were observed, but were less severe. The only raw data provided for this study were graphs showing the findings for the rats exposed at 104 ppm.
Groups of 10 adult male rats (derived Wistar CF-1 strain) were also exposed to perchloryl fluoride at 0 or 24 ppm for 6 h/day, 5 days/week for 26 weeks (Greene et al. 1960). All rats survived the study. Bone (femur) fluoride
concentrations were three times greater than those of the controls at the end of the study. Urinary fluoride concentrations were not reported. As with dogs, rats had splenic congestion that disappeared after a recovery period, and no effects were observed in the lungs.
3.2.3. Mice
Groups of 20 adult female mice (Carworth Farms CF-1 strain) were exposed to perchloryl fluoride at 0 or 185 ppm for 6 h/day, 5 days/week for 7 weeks (Greene et al. 1960). Animals were observed for toxic effects and killed at the end of the study. Mortality was 51% (20/39) in the mice after 35 days of exposure, with dyspnea and cyanosis observed.
3.2.4. Guinea Pigs
Groups of 10 adult male guinea pigs (strain not specified) were exposed to perchloryl fluoride at 0, 104, or 185 ppm for 6 h/day, 5 days/week for 5 weeks (104 ppm) or 7 weeks (185 ppm) (Greene et al. 1960). Mortality was 100% in guinea pigs exposed at 185 ppm for 3 days or at 104 ppm for 25 days. Dyspnea and cyanosis were observed in the animals exposed at 185 ppm. Only cyanosis was observed at 104 ppm.
Groups of 30 adult male guinea pigs (strain not specified) were exposed to perchloryl fluoride at 0 or 24 ppm for 6 h/day, 5 days/week for 26 weeks (Greene et al. 1960). Animals were monitored for toxic effects and 10 animals per group were killed periodically for blood and tissue samples. Mortality was 3% (1/30) in the controls and 47% (14/30) in the treated animals, but the times of deaths were not provided. Clinical signs of toxicity were not observed. Although total hemoglobin was consistently lower in treated guinea pigs, the differences were not statistically significant. Blood fluoride concentrations were slightly greater (data not provided) in the treated guinea pigs, but blood volumes obtained were small making the data unreliable. Urinary fluoride concentrations increased 5-fold over a 6-month period but were comparable to those of controls at the end of the exposure and remained normal during the postexposure period. Bone (femur) fluoride concentrations in treated guinea pigs increased to four times that of controls at the end of the study. During the recovery period, the amount of fluoride in the femur did not diminish. Marked changes in the lungs were observed in both control and treated guinea pigs. Localized damage suggesting a chronic condition was observed in approximately 80-85% of the guinea pigs in the control and treated groups, indicating a cause other than perchloryl fluoride. Bordetella bronchiseptica was isolated in some guinea pigs, making the results of this study questionable and unreliable for determining AEGL values. Treated guinea pigs also had congestion of the spleen and a corresponding increase in splenic weight, but these effects were reversed after exposure stopped.
3.3. Developmental and Reproductive Toxicity
No animal data were available on the developmental or reproductive toxicity of perchloryl fluoride.
3.4. Genotoxicity
No animal data were available on the genotoxicity of perchloryl fluoride.
3.5. Chronic Toxicity and Carcinogenicity
No animal data were available on the chronic toxicity or carcinogenicity of perchloryl fluoride.
3.6. Summary
Greene et al. (1960) conducted both acute- and repeat-exposure studies. Dogs exposed for 4 h to perchloryl fluoride at 224 or 425 ppm survived, whereas 4-h exposures at 451 and 622 ppm were partially lethal. The 4-h LC50 values for rats and mice were 385 and 630 ppm, respectively. In repeat-exposure studies, dogs and rats survived a 26-week exposure to perchloryl fluoride at 24 ppm for 6 h/day, 5 days/week. Evidence of contact irritation (dyspnea, labored breathing) and of systemic absorption leading to methemoglobinemia (cyanosis) and fluoride deposition were observed in all species. Dost et al. (1974) reported that perchloryl fluoride at 2,000 ppm for 25 min or at 1,000 ppm for 60 min was not lethal to rats. A summary of the available animal data on perchloryl fluoride is presented in Table 5-3.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
There were no studies available on the metabolism and disposition of perchloryl fluoride. In the longer-term repeat studies by Greene et al. (1960), rats, guinea pigs, and dogs had fluoride deposition in the bone (femur) and in the urine of dogs and guinea pigs, indicating that the molecule is broken down and fluoride is released. Enamel fluorosis was also observed in rats.
4.2. Mechanism of Toxicity
No studies were identified describing the mechanism of toxicity for perchloryl fluoride. Two mechanisms of action might be present. The oxidative properties of perchloryl fluoride might lead to direct-contact lung damage. Formation of methemoglobin in all species studied indicates that perchloryl fluoride
TABLE 5-3 Summary of Inhalation Data on Perchloryl Fluoride in Laboratory Animals
| Species | Concentration (ppm) | Exposure Duration | Effect | Reference |
| Acute Lethality Studies | ||||
| Doga | 224 | 4 h | Moderate cyanosis, hyperpnea. | Greene et al. 1960 |
| Dog | 425 | 4 h | Severe cyanosis, hyperpnea, emesis. | Greene et al. 1960 |
| Dog | 451 | 4 h | Severe cyanosis, hyperpnea, motor instability, convulsions; one dog moribund in chamber, other dog treated with methylene blue and survived. | Greene et al. 1960 |
| Dog | 622 | 2.5 h | Severe cyanosis, hyperpnea, salivation, motor instability; convulsions; one dog died, other dog treated with methylene blue and survived. | Greene et al. 1960 |
| Rat | 384 | 4 h | LC50 | Greene et al. 1960 |
| Rat | 5,000 | 15 min | 100% mortality | Dost et al. 1974 |
| Rat | 2,000 | 25 min 40 min | No mortality 100% mortality | Dost et al. 1974 |
| Rat | 1,000 | 60 min | No mortality | Dost et al. 1974 |
| Mouse | 630 | 4 h | LC50 | Greene et al. 1960 |
| Repeat-Exposure Studiesb | ||||
| Dog | 0, 24 | 6 h/d, 5 d/wk for 26 wk | All survived; no clinical signs; increased fluoride in femur after 6 mos | Greene et al. 1960 |
| Rat | 0, 104, 185 | 6 h/d, 5 d/wk for 5 wk (104 ppm) or 7 wk (185 ppm) | 104 ppm: 1/20 died (>25 exposure days), cyanosis, increased methemoglobin, decreased hemoglobin, histopathologic changes (liver, spleen, kidney) 185 ppm: 18/20 died (>35 exposure days), dyspnea, same effects as 104 ppm but more severe | Greene et al. 1960 |
| Species | Concentration (ppm) | Exposure Duration | Effect | Reference |
| Rat | 0, 24 | 6 h/d, 5 d/wk for 26 wk | All survived; no clinical signs; increased fluoride in femur after 6 mos | Greene et al. 1960 |
| Mouse | 0, 185 | 6 h/d, 5 d/wk for 7 wk | 20/39 died (after 35 exposure days); dyspnea and cyanosis | Greene et al. 1960 |
| Guinea pig | 0, 104, 185 | 6 h/d, 5 d/wk for 5 wk (104 ppm) or 7 wk (185 ppm) | 104 ppm: 10/10 died (>25 exposure days); cyanosis 185 ppm: 10/10 died (>3 exposure days); dyspnea and cyanosis | Greene et al. 1960 |
| Guinea pig | 0, 24 | 6 h/d, 5 d/wk for 26 wk | 1/30 control and 14/30 treated diedc, increased fluoride in bone, lung lesionsc | Greene et al. 1960 |
aTwo dogs per group.
bIn all repeat-exposure studies, there were no effects observed in the controls unless otherwise stated.
cThe high mortality and lung data are questionable because both the control and treated guinea pigs had Bordetella bronchiseptica infections.
or its metabolites oxidize the ferrous (Fe+2) iron in hemoglobin to the oxidized ferric form (Fe+3). Methemoglobin is unable to transport oxygen to the organs and tissues, resulting in cyanosis. Methemoglobin concentrations of more than 70% are considered the threshold for lethality (Kiese 1974; Seger 1992). Normal methemoglobin concentrations in humans are below 1%.
4.3. Structure-Activity Relationships
Greene et al. (1960) provided compared fluoride deposition in the femurs of rats exposed to perchloryl fluoride at 104 ppm and similar exposures to fluorine (F2) and hydrogen fluoride. The amount of fluoride deposited from perchloryl fluoride was about one-fourth that of fluorine and one-half that of hydrogen fluoride. A possible explanation for the lower absorption of perchloryl fluoride is that it has a more stable structure compared with the other fluorides.
4.4. Other Relevant Information
4.4.1. Species Variability
The available data suggest little interspecies variability. Greene et al. (1960) exposed rats, mice, guinea pigs, and dogs to similar concentrations of perchloryl fluoride and observed similar effects in all species. Effects included labored breathing, cyanosis, convulsions, and changes in hemoglobin and methemoglobin concentrations in blood. The 4-h concentration at which one of two dogs was moribund was 451 ppm, and the LC50s of 385 ppm for rats and 630 ppm for mice are within 3-fold of each other.
4.4.2. Susceptible Populations
As with other lung irritants, humans with compromised lung function would be more susceptible to the toxic effects of perchloryl fluoride. Young children and the elderly are naturally more susceptible to methemoglobinemia and could develop that condition more readily when exposed to perchloryl fluoride. Young children have increased hemoglobin concentrations, and the elderly may be on multiple oxidant medications that can lower their threshold for developing methemoglobinemia (Wilburn-Goo and Lloyd 1999). A rare genetic defect which causes a deficiency of nicotinamide adenine dinucleotide-cytochrome b5 reductase enzyme (NADH-cytochrome b5 reductase) has been documented. This enzyme cofactor reduces methemoglobin in normal erythrocytes. Fetal hemoglobin is more susceptible to oxidation than adult hemoglobin; this cofactor lacks full activity until infants are 4 months of age (Seger 1992). Humans can be carriers who show no clinical signs but have a lower threshold for developing acquired methemoglobinemia or, if autosomal recessive transmission occurs,
they exhibit cyanosis at birth and must be treated, as it can cause death within a few years (Da-Silva et al. 2003).
4.4.3. Concentration-Exposure Duration Relationship
The concentration-exposure duration relationship for many irritant and systemically-acting vapors and gases can be described by the relationship Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). Data were unavailable for an empirical derivation of n, so default values of n = 3 to extrapolate from longer-to-shorter durations and n = 1 for extrapolation from shorter-to-longer durations was used (NRC 2001).
5. DATA ANALYSIS FOR AEGL-1
5.1. Summary of Human Data Relevant to AEGL-1
No applicable human data were available for deriving AEGL-1 values for perchloryl fluoride.
5.2. Summary of Animal Data Relevant to AEGL-1
There were two studies of perchloryl fluoride in laboratory animals that addressed lethality (Greene et al. 1960; Dost et al. 1974). A longer-term study in which dogs and rats were exposed to perchloryl fluoride at 24 ppm for 6 h/day, 5 days/week for 26 weeks, reported no acute effects (Greene et al. 1960).
5.3. Derivation of AEGL-1
The 26-week study by Greene et al. (1960) was used to derive the AEGL-1 values. The point of departure was 24 ppm, the concentration at which dogs and rats exposed for 6 h/day, 5 days/week, exhibited no clinical signs or evidence of irritation. The only long-term effect observed was increased fluoride deposition in the bone and urine. Therefore, 24 ppm was considered a no-effect level for an 8-h exposure. To calculate the AEGL-1 values, an interspecies uncertainty factor of 3 was considered appropriate because lethality values for dogs, rats, and mice differed by less than a factor of 3. An intraspecies uncertainty factor of 10 was applied because infants are considerably more susceptible to methemoglobinemia than healthy adults. Time scaling was performed using the equation Cn × t = k, with n = 1 and n = 3 for longer- and shorter-exposure durations, respectively (NRC 2001). The 30-min AEGL-1 value was adopted as the 10-min value, because of the uncertainties associated with extrapolating a 6-h exposure to a 10-min AEGL value (NRC 2001). AEGL-1 values for perchloryl fluoride are presented in Table 5-4.
TABLE 5-4 AEGL-1 Values for Perchloryl Fluoride
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 1.8 ppm (7.6 mg/m3) | 1.8 ppm (7.6 mg/m3) | 1.5 ppm (6.3 mg/m3) | 0.92 ppm (3.9 mg/m3) | 0.60 ppm (2.5 mg/m3) |
6. DATA ANALYSIS FOR AEGL-2
6.1. Summary of Human Data Relevant to AEGL-2
No human data were available for deriving AEGL-2 values for perchloryl fluoride.
6.2. Summary of Animal Data Relevant to AEGL-2
No animal data were available that defined effects consistent with the definition of AEGL-2.
6.3. Derivation of AEGL-2
In the absence of appropriate chemical-specific data, AEGL 2 values for perchloryl fluoride were derived by reducing the AEGL-3 values by a third (NRC 2001). This approach is supported by the steep concentration-response curve for perchloryl fluoride. Two of two dogs exposed to perchloryl fluoride at 425 ppm survived a 4-h exposure, but one of two dogs was found moribund after a 4-h exposure at a slightly higher concentration of 451 ppm (Green et al. 1960). AEGL-2 values for perchloryl fluoride are presented in Table 5-5.
7. DATA ANALYSIS FOR AEGL-3
7.1. Summary of Human Data Relevant to AEGL-3
No human data were available for deriving AEGL-3 values for perchloryl fluoride.
7.2. Summary of Animal Data Relevant to AEGL-3
Greene et al. (1960) exposed dogs, rats, and mice to varying concentrations of perchloryl fluoride for 4-h. Dogs exhibited clinical signs (cyanosis and hyperpnea) at a concentration of 224 ppm, but no mortality occurred. Cyanosis worsened at 425 ppm and one dog exposed at 451 ppm was found moribund. LC50 values for rats and mice were 385 and 630 ppm, respectively. No mortality occurred in rats exposed for perchloryl fluoride at 2,000 ppm for 25 min or at 1,000 ppm for 60 min (Dost et al. 1974). Details of this latter study were not provided.
TABLE 5-5 AEGL-2 Values for Perchloryl Fluoride
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 5.0 ppm (21 mg/m3) | 5.0 ppm (21 mg/m3) | 4.0 ppm (17 mg/m3) | 2.5 ppm (11 mg/m3) | 1.2 ppm (5.0 mg/m3) |
7.3. Derivation of AEGL-3
The study of Greene et al. (1960), in which groups of two dogs were exposed at several concentrations of perchloryl fluoride for 4 h, was chosen as the key study. Dogs survived at concentrations of 224 and 451 ppm. The concentration of 224 ppm was chosen as the point of departure because it is lower than the LC50 value of 384 ppm for rats in the same study. The point of departure was divided by interspecies and intraspecies uncertainty factors of 3 and 10. An interspecies uncertainty factor of 3 was considered appropriate because lethality values for dogs, rats, and mice differed by less than a factor of 3, and lethal values for the rat were considered in selecting the point of departure. An intraspecies uncertainty factor of 10 was applied because infants are considerably more susceptible to methemoglobinemia than healthy adults. Time scaling was performed using the equation Cn × t = k, with n = 1 and n = 3 for longer- and shorter-exposure durations, respectively (NRC 2001). The 30-min AEGL-3 value was adopted as the 10-min value, because of the uncertainties associated with extrapolating a 4-h exposure to a 10-min AEGL value (NRC 2001). AEGL-3 values for perchloryl fluoride are provided in Table 5-6.
AEGL-3 values might appear low in light of the odor-threshold study in which human volunteers were exposed at several concentrations of perchloryl fluoride, including 41 ppm (Greene et al. 1960). However, the sparse database justifies the low concentrations.
8. SUMMARY OF AEGLS
8.1. AEGL Values and Toxicity End Points
Because of the lack of acute studies on perchloryl fluoride, a longer-term study at a concentration at which dogs and rats exhibited no acute effects was used for the derivation of AEGL-1 values. No studies that addressed effects consistent with the definition of an AEGL-2 were found. Because the concentration-response curve for lethality is steep, AEGL-2 values were derived by dividing the AEGL-3 values by 3. The point of departure for AEGL-3 values was a concentration of 224 ppm that caused no deaths in dogs exposed for 4 h, but was below the 4-h LC50 value of 384 ppm for rats. For AEGL-1 and AEGL-3, a total uncertainty factor of 30 (3 for interspecies differences and 10 for intraspecies variability) was applied. Time scaling was performed using the equation Cn × t = k, with n = 1 and n = 3 for longer- and shorter-exposure durations, respectively (NRC 2001). AEGL values for perchloryl fluoride are provided in Table 5-7.
TABLE 5-6 AEGL-3 Values for Perchloryl Fluoride
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 15 ppm (63 mg/m3) | 15 ppm (63 mg/m3) | 12 ppm (50 mg/m3) | 7.5 ppm (32 mg/m3) | 3.7 ppm (16 mg/m3) |
TABLE 5-7 Summary of AEGL Values for Perchloryl Fluoride
| Classification | Exposure Duration | ||||
| 10 min | 30 min | 1 h 4 h | 8 h | ||
| AEGL-1 (nondisabling) | 1.8 ppm (7.6 mg/m3) | 1.8 ppm (7.6 mg/m3) | 1.5 ppm 0.92 ppm (6.3 mg/m3) (3.9 mg/m3) | 0.60 ppm (2.5 mg/m3) | |
| AEGL-2 (disabling) | 5.0 ppm (21 mg/m3) | 5.0 ppm (21 mg/m3) | 4.0 ppm 2.5 ppm (17 mg/m3) (11 mg/m3) | 1.2 ppm (5.0 mg/m3) | |
| AEGL-3 (lethal) | 15 ppm (63 mg/m3) | 15 ppm (63 mg/m3) | 12 ppm 7.5 ppm (50 mg/m3) (32 mg/m3) | 3.7 ppm (16 mg/m3) | |
8.2. Comparison with Other Standards and Guidelines
The threshold limit value - time weighted average (TLV-TWA) for perchloryl fluoride established by the American Conference of Governmental Industrial Hygienists and the permissible exposure limit - time weighted average (PEL-TWA) of the Occupational Safety and Health Administration (OSHA) were both determined on the basis of the studies by Greene et al. (1960). For both guidelines, the recommended value of 3 ppm is approximately one-tenth of the 24-ppm concentration that caused enamel fluorosis and hematologic alterations in experimental animals after repeated exposures (26 weeks). The 8-h AEGL-2 and AEGL-3 values appear low in comparison with the TLV-TWA and PEL-TWA, but those standards are for healthy adults, whereas the AEGL values are protective of sensitive infant and elderly populations. The National Institute of Occupational Safety and Health established a value that is Immediately Dangerous to Life and Health of 100 ppm, which was based on the study by Greene et al. (1960) that found a 4-h LC50 for rats of 385 ppm. Standards and guidelines for perchloryl fluoride are provided in Table 5-8.
8.3. Data Adequacy and Research
Human data on perchloryl fluoride are lacking. Animal data are also limited; although several species (dogs, rats, guinea pigs, and mice) were exposed by inhalation to perchloryl fluoride, most of the studies were conducted by the same laboratory and details were not reported.
TABLE 5-8 Extant Standards and Guidelines for Perchloryl Fluoride
| Guideline | Exposure Duration | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h | |
| AEGL-1 | 1.8 ppm (7.6 mg/m3) | 1.8 ppm (7.6 mg/m3) | 1.5 ppm (6.3 mg/m3) | 0.92 ppm (3.9 mg/m3) | 0.60 ppm (2.5 mg/m3) |
| AEGL-2 | 5.0 ppm (21 mg/m3) | 5.0 ppm (21 mg/m3) | 4.0 ppm (17 mg/m3) | 2.5 ppm (11 mg/m3) | 1.2 ppm (5.0 mg/m3) |
| AEGL-3 | 15 ppm (63 mg/m3) | 15 ppm (63 mg/m3) | 12 ppm (50 mg/m3) | 7.5 ppm (32 mg/m3) | 3.7 ppm (16 mg/m3) |
| IDLH (NIOSH)a | 100 ppm (420 mg/m3) | ||||
| TLV-TWA (ACGIH)b | 3 ppm (14 mg/m3) | ||||
| PEL-TWA (OSHA)c | 3 ppm (14 mg/m3) | ||||
| REL-TWA (NIOSH)d | 3 ppm (14 mg/m3) | ||||
| TLV-STEL (ACGIH)e | 6 ppm (25 mg/m3) (15 min) | ||||
| REL-STEL (NIOSH)f | 6 ppm (25 mg/m3) (15 min) | ||||
| MAC (MSZW)g | 3 ppm (14 mg/m3) | ||||
aIDLH (immediately dangerous for life or health, National Institute for Occupational Safety and Health) (NIOSH 1994) represents the maximum concentration from which one could escape within 30 min without any escape-impairing symptoms, or any irreversible health effects.
bTLV-TWA (threshold limit value-time weighted average, American Conference of Governmental Industrial Hygienists) (ACGIH 1991, 2008) is the time-weighted average concentration for a normal 8-h workday and a 40-h workweek, to which nearly all workers may be repeatedly exposed, day after day, without adverse effect.
cPEL-TWA (permissible exposure limit-time weighted average, Occupational Safety and Health Administration) (29 CFR Part 1910 [2005]) is defined analogous to the ACGIH TLV-TWA, but is for exposures of no more than 10 h/day, 40 h/week.
dREL-TWA (recommended exposure limit-time weighted average, National Institute for Occupational Safety and Health) (NIOSH 2010) is defined analogous to the ACGIH TLV-TWA.
eTLV-STEL (threshold limit value-short term exposure limit, American Conference of Governmental Industrial Hygienists) (ACGIH 2008) is defined as a 15-min TWA exposure which should not be exceeded at any time during the workday even if the 8-h TWA is within the TLV-TWA. Exposures above the TLV-TWA up to the STEL should not be longer than 15 min and should not occur more than four times per day. There should be at least 60 min between successive exposures in this range.
fREL-STEL (recommended exposure limit-short term exposure limit, National Institute for Occupational Safety and Health) (NIOSH 2010) is defined analogous to the ACGIH TLV-STEL.
gMAC (maximaal aanvaarde concentratie [maximal accepted concentration], Ministry of Social Affairs and Employment, The Hague, The Netherlands [MSZW 2004]) is defined analogous to the ACGIH TLV-TWA.
9. REFERENCES
ACGIH (American Conference of Government and Industrial Hygienists). 1991. Perchloryl Fluoride (CAS Reg. No. 7616-94-6). Documentation of the Threshold Limit Values and Biological Exposure Indices. American Conference of Government and Industrial Hygienists, Cincinnati, OH.
ACGIH (American Conference of Government and Industrial Hygienists). 2008. Perchloryl Fluoride (CAS Reg. No. 7616-94-6). P. 47 in Documentation of the Threshold Limit Values and Biological Exposure Indices. American Conference of Government and Industrial Hygienists, Cincinnati, OH.
Da-Silva, S., I.S. Sajan, and J. Underwood, III. 2003. Congenital methemoglobinemia: A rare cause of cyanosis in the newborn - a case report. Pediatrics 112(2):158-161.
Dost, F.N., D.J. Reed, V.N. Smith, and C.H. Wang. 1974. Toxic properties of chlorine trifluoride. Toxicol. Appl. Pharmacol. 27(3):527-536.
Greene, E.A., J.L. Colbourn, E. Donati, and M.H. Weeks. 1960. The Inhalation Toxicity of Perchloryl Fluoride. U.S. Army Chemical Research and Development Laboratories Technical Report CRDLR 3010. Army Chemical Center, Edgewood, MD.
HSDB (Hazardous Substances Databank). 2008. Trioxychlorofluoride (CAS Reg. No. 7616-94-6). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Oct. 31, 2008].
Kiese, M. 1974. Methemoglobinemia: A Comprehensive Treatise. Cleveland, OH: CRC Press.
Kushneva. 1999. Handbook of Toxicological and Hygienic Standards (PDK) of Potentially Hazardous Substances. Developed by the Institute of Biophysics and its (associated) branches.
Mendiratta, S.K., R.L. Dotson, and R.T. Brooker. 2005. Perchloric acid and perchlorates. In Kirk-Othmer Encyclopedia of Chemical Technology. New York: John Wiley and Sons.
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: Perchlorylfluoride Den Haag: SDU Uitgevers [online]. Available: http://www.lasrook.net/lasrookNL/maclijst2004.htm [accessed Aug. 28, 2012].
NIOSH (National Institute for Occupational Safety and Health). 1994. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLH): Perchloryl fluoride. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH [online]. Available: http://www.cdc.gov/niosh/idlh/7616946.html [accessed Aug. 22, 2012].
NIOSH (National Institute for Occupational Safety and Health). 2010. NIOSH Pocket Guide to Chemical Hazards: Perchloryl fluoride. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute
for Occupational Safety and Health, Cincinnati, OH [online]. Available: http://www.cdc.gov/niosh/npg/default.html [accessed Aug. 22, 2012].
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standard Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
Seger, D.L. 1992. Methemoglobin-forming chemicals. Pp. 800-806 in Hazardous Materials Toxicology: Clinical Principles of Environmental Health, J.B. Sullivan, and G.R. Krieger, eds. Baltimore, MD: Williams & Wilkins.
ten Berge, W.F., A. Zwart, and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Mater. 13(3):301-309.
Wilburn-Goo, D., and L. Lloyd. 1999. When patients become cyanotic: Acquired methemoglobinemia. J. Am. Dent. Assoc. 130(6):826-831.
APPENDIX A
DERIVATION OF AEGL VALUES FOR PERCHLORYL FLUORIDE
Derivation of AEGL-1 Values
| Key study: | Greene, E.A., J.L. Colbourn, E. Donati, and M.H. Weeks. 1960. The Inhalation Toxicity of Perchloryl Fluoride. U.S. Army Chemical Research and Development Laboratories Technical Report CRDLR 3010. Army Chemical Center, Edgewood, MD. |
| Toxicity end point: | No treatment-related adverse effects. |
| Time scaling: |
Cn × t = k n = 3 for extrapolating to the 30-min and 1- and 4-h durations (24 ppm)3 × 6 h = 8.3 × 104 ppm-h n = 1 for extrapolating to the 8-h duration (24 ppm)1 × 6 h = 144 ppm-h |
| Uncertainty factors: | 3 for interspecies differences 10 for intraspecies variability |
| 10-min AEGL-1: | 1.8 ppm (set equal to the 30-min value because of the long exposure duration of the key study) |
| 30-min AEGL-1: | C3 × 0.5 h = 8.3 × 104 ppm-h C3 = 1.7 × 105 ppm C = 55.4 ppm 55.4 ppm ÷ 30 = 1.8 ppm |
| 1-h AEGL-1: | C3 × 1 h = 8.3 × 104 ppm-h C3 = 8.3 × 104 ppm C = 43.6 ppm 43.6 ppm ÷ 30 = 1.5 ppm |
| 4-h AEGL-1: | C3 × 4 h = 8.3 × 104 ppm-h C3 = 20,750 ppm C = 27.5 ppm 27.5 ppm ÷ 30 = 0.92 ppm |
| 8-h AEGL-1: | C1 × 8 h = 144 ppm-h C1 = 18 ppm 18 ppm ÷ 30 = 0.60 ppm |
Derivation of AEGL-2 Values
| Key study: | NRC (National Research Council). 2001. Standard Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press. |
| Toxicity end point: | For chemicals with a steep concentration-response curve, AEGL-2 values may be calculated by dividing AEGL-3 values by 3 (NRC 2001). |
| Time scaling: | See derivation of AEGL-3 |
| Uncertainty factors: | See derivation of AEGL-3 |
| 10-min AEGL-2: | 15 ppm ÷ 3 = 5.0 ppm |
| 30-min AEGL-2: | 15 ppm ÷ 3 = 5.0 ppm |
| 1-h AEGL-2: | 12 ppm ÷ 3 = 4.0 ppm |
| 4-h AEGL-2: | 7.5 ppm ÷ 3 = 2.5 ppm |
| 8-h AEGL-2: | 3.7 ppm ÷ 3 = 1.2 ppm |
Derivation of AEGL-3 Values
| Key study: | Greene, E.A., J.L. Colbourn, D. Donati, and M.H. Weeks. 1960. The Inhalation Toxicity of Perchloryl Fluoride. U.S. Army Chemical Research and Development Laboratories Technical Report CRDLR 3010. Army Chemical Center, Edgewood, MD. |
| Toxicity end point: | Highest 4-h concentration causing no mortality in dogs, but below the LC50 value for rats in the same study. |
| Scaling: | Cn × t = k n = 3 for extrapolating to the 30-min and 1-h durations (224 ppm)3 × 4 h = 4.5 × 107 ppm-h |
| n = 1 for extrapolating to the 8-h duration (224 ppm)1 × 4 h = 896 ppm-h |
|
| Uncertainty factors: | 3 for interspecies variability 10 for intraspecies variability |
| 10-min AEGL-3: | 15 ppm (set equal to the 30-min value because of the long exposure duration of the key study) |
| 30-min AEGL-3: | C3 × 0.5 h = 4.50 × 107 ppm-h C3 = 9.0 × 107 ppm C = 448 ppm 448 ppm ÷ 30 = 15 ppm |
| 1-h AEGL-3: | C3 × 1 h = 4.50 × 107 ppm-h C3 = 4.50 × 107 ppm C = 356 ppm 356 ppm ÷ 30 = 12 ppm |
| 4-h AEGL-3 | C = 224 ppm ÷ 30 C = 7.5 ppm |
| 8-h AEGL-3: | C1 × 8 h = 896 ppm-h C1 = 112 ppm 112 ppm ÷ 30 = 3.7 ppm |
APPENDIX B
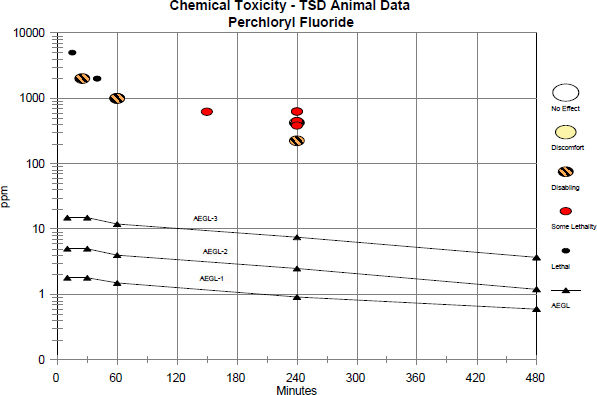
FIGURE B-1 Category plot of animal toxicity data on perchloryl fluoride compared with AEGL values.
TABLE B-1 Data Used in Category Plot of AEGL Values for Perchloryl Fluoride
| Source | Species | Concentration (ppm) | Duration (min) | Category |
| NAC/AEGL-1 | 1.8 | 10 | AEGL | |
| NAC/AEGL-1 | 1.8 | 30 | AEGL | |
| NAC/AEGL-1 | 1.5 | 60 | AEGL | |
| NAC/AEGL-1 | 0.92 | 240 | AEGL | |
| NAC/AEGL-1 | 0.60 | 480 | AEGL | |
| NAC/AEGL-2 | 5 | 10 | AEGL | |
| NAC/AEGL-2 | 5 | 30 | AEGL | |
| NAC/AEGL-2 | 4 | 60 | AEGL | |
| NAC/AEGL-2 | 2.5 | 240 | AEGL | |
| NAC/AEGL-2 | 1.2 | 480 | AEGL | |
| NAC/AEGL-3 | 15 | 10 | AEGL | |
| NAC/AEGL-3 | 15 | 30 | AEGL | |
| NAC/AEGL-3 | 12 | 60 | AEGL | |
| NAC/AEGL-3 | 7.5 | 240 | AEGL | |
| NAC/AEGL-3 | 3.7 | 480 | AEGL | |
| Greene et al. 1960 | Dog | 224 | 240 | 2 (moderate cyanosis, hyperpnea) |
| Dog | 425 | 240 | 2 (severe cyanosis, hyperpnea, emesis) | |
| Dog | 451 | 240 | SL (severe cyanosis, hyperpnea, motor instability, convulsions, death of one of two dogs) | |
| Dog | 622 | 150 | SL (severe cyanosis, hyperpnea, salivation, motor instability, convulsions; death of one of two dogs) | |
| Rat | 384 | 240 | SL (LC50) | |
| Mouse | 630 | 240 | SL (LC50) | |
| Dost et al. 1974 | Rat | 5,000 | 15 | 3 (100% mortality) |
| Rat | 2,000 | 25 | 2 (no mortality) | |
| Rat | 2,000 | 40 | 3 (100% mortality) | |
| Rat | 1,000 | 60 | 2 (no mortality) | |
For category: 0 = no effect; 1 = discomfort; 2 = disabling; SL = some lethality; and 3 = lethal.
APPENDIX C
ACUTE EXPOSURE GUIDELINE LEVELS FOR PERCHLORYL FLUORIDE
Derivation Summary
AEGL-1 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 1.8 ppm | 1.8 ppm | 1.5 ppm | 0.92 ppm | 0.60 ppm |
| Key reference: Greene, E.A., J.L. Colbourn, D. Donati, and M.H. Weeks. 1960. The Inhalation Toxicity of Perchloryl Fluoride. U.S. Army Chemical Research and Development Laboratories Technical Report CRDLR 3010. Army Chemical Center, Edgewood, MD. | ||||
| Test species/Strain/Number: Beagle dogs, 2 per group; Sprague-Dawley rats, 10 per group | ||||
| Exposure route/Concentrations/Durations: Inhalation, 24 ppm for 6 h/day, 5 days/week for 26 weeks | ||||
| Effects: 0 ppm: No effects observed 24 ppm: No clinical signs observed; some increases in fluoride deposition but only after long-term exposure. |
||||
| End point/Concentration/Rationale: No effect except for increased fluoride deposition in bones after 26 weeks | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 30 |
||||
| Interspecies: 3, because the 4-h concentration at which 1 of 2 dogs were moribund (451 ppm) and the LC50 concentrations for rats (385 ppm) and mice (630 ppm) are within 3-fold of each other, and all species developed similar symptoms (dyspnea and cyanosis). | ||||
| Intraspecies: 10, because perchloryl fluoride is systemically absorbed and the possible increased sensitivity of some humans, especially infants, for developing methemoglobinemia. | ||||
| Modifying factor: Not applied | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time scaling: Extrapolation to different exposure durations was performed using the equation Cn × t = k (ten Berge et al. 1986), where n = 3 for extrapolation to durations of 30-min, 1 h, and 4 h, and n = 1 for extrapolation to 8 h. The 30-min value was adopted as the 10-min value because extrapolating from 4 h to 10 min is not recommended (NRC 2001). | ||||
| Data adequacy: Adequate | ||||
AEGL-2 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 5.0 ppm | 5.0 ppm | 4.0 ppm | 2.5 ppm | 1.2 ppm |
| Key reference: NRC (National Research Council). 2001. Standard Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. National Research Council, National Academy Press: Washington, DC. | ||||
| Test species/Strain/Number: See derivation of AEGL-3 | ||||
| Exposure route/Concentrations/Durations: See derivation of AEGL-3 | ||||
| Effects: See derivation of AEGL-3 | ||||
| End point/Concentration/Rationale: In the absence of chemical-specific data, NRC (2001) recommends taking one-third of AEGL-3 values when there is evidence of a steep concentration-response curve. | ||||
| Uncertainty factors/Rationale: See derivation of AEGL-3 | ||||
| Modifying factor: See derivation of AEGL-3 | ||||
| Animal-to-human dosimetric adjustment: See derivation of AEGL-3 | ||||
| Time scaling: See derivation of AEGL-3 | ||||
| Data adequacy: Not adequate | ||||
AEGL-3 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 15 ppm | 15 ppm | 12 ppm | 7.5 ppm | 3.7 ppm |
| Key reference: Greene, E.A., J.L. Colbourn, D. Donati, and M.H. Weeks. 1960. The Inhalation Toxicity of Perchloryl Fluoride. U.S. Army Chemical Research and Development Laboratories Technical Report CRDLR 3010. Army Chemical Center, Edgewood, MD. | ||||
| Test species/Strain/Number: Male beagle dogs, 2 per group | ||||
| Exposure route/Concentrations/Durations: Inhalation, 224, 425, or 451 ppm for 4-h. | ||||
| Effects: Rat: LC50 = 385 ppm Mouse: LC50 = 630 ppm Dogs: 224 ppm, both dogs had cyanosis and hyperpnea; 425 ppm, both dogs had severe cyanosis and hyperpnea; 451 ppm, 1 of 2 dogs moribund |
||||
| End point/Concentration/Rationale: 224 ppm was highest nonlethal concentration in dogs with no projected mortality in rats and mice. | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 30 |
||||
| Interspecies: 3, because the 4-h concentration at which 1 of 2 dogs were moribund (451 ppm) and the LC50 concentrations for rats (385 ppm) and mice (630 ppm) are within 3-fold of each other, and all species developed similar symptoms (dyspnea and cyanosis). | ||||
| Intraspecies: 10, because perchloryl fluoride is systemically absorbed and the possible increased sensitivity of some humans, especially infants, for developing methemoglobinemia. | ||||
| Modifying factor: Not applied | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time scaling: Extrapolation to different exposure durations was performed using the equation Cn × t = k (ten Berge et al. 1986), where n = 3 for extrapolation to durations of 30-min, 1 h, and 4 h, and n = 1 for extrapolation to 8 h. The 30-min value was adopted as the 10-min value because extrapolating from 4 h to 10 min is not recommended (NRC 2001). | ||||
| Data adequacy: Adequate | ||||




























