Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory
____________________
1This document was prepared by the AEGL Development Team composed of Kowetha Davidson (Oak Ridge National Laboratory), Julie Klotzbach (SRC, Inc.), Chemical Managers Mark A. McClanahan and Susan Ripple (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances), and Ernest V. Falke (U.S. Environmental Protection Agency). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold concentrations for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Piperidine is a cyclic aliphatic amine (Eller et al. 2000). It is a clear, colorless, and flammable liquid that produces vapors that reach explosive concentrations at room temperature. Piperidine has a dissociation constant (pKb) of 2.88 and a pH of 12.6 (100 g/L, 20°C). Therefore, it is expected to be very corrosive. It has a strong pepper- or amine-like and pungent odor. Piperidine has many commercial uses, including use as a solvent, a curing agent for rubber and epoxy resins, an intermediate in organic synthesis, a food additive, and a constituent in the manufacturing of pharmaceuticals.
Daily exposure to piperidine is evidenced by its presence in the food supply and its excretion in human urine. It is a natural constituent in white and black pepper. Piperidine is formed naturally in the body from the degradation of lysine, cadaverine, and pipecolic acid. Exogenous piperidine is absorbed from the respiratory tract, gastrointestinal tract, and skin. It is found in most tissues of the body, including the brain, and is excreted as unchanged piperidine or its metabolites.
Studies in rats showed that nasal irritation and signs of ocular irritation occur at the lower concentrations of piperidine followed by corrosion around the nose and dyspnea at higher concentrations. Corneal damage, central nervous system (CNS) toxicity, and prostration occurred at the highest concentrations;
however, death occurred only at concentrations that caused dyspnea, CNS toxicity, and prostration. Therefore, the severity of effects from piperidine shows a clear continuum ranging from nasal irritation to death. Piperidine has no demonstrated carcinogenic activity, it is not genotoxic in Salmonella typhimurium, and it is not toxic to the developing rat fetus at the concentrations tested.
The database on piperidine in humans is very small. Inhalation exposure to piperidine may cause sore throat, coughing, labored breathing, and dizziness. The odor threshold is reported to be <2 ppm, and 2-5 ppm is reported to be tolerated by unacclimated individuals for only a brief time because of its pungent odor. The irritation threshold for humans was reported to be 26 ppm. At an odor threshold of 0.37 ppm, a level of distinct odor awareness would be 5.9 ppm (van Doorn et al. 2002).
AEGL-1 values were based on the no-effect level (20 ppm for 6 h) for nasal irritation in rats. Uncertainty factors of 3 for interspecies differences and 3 for intraspecies variability were applied. The rationale for selecting those factors included the following: (1) the effect observed at 50 ppm was mediated by direct contact of piperidine with the nasal epithelium without involvement of other regions of the respiratory tract; and (2) the cell composition of the nasal mucosa is similar between species and among individuals within the population, although the cell distribution and nasal morphology differ among species. In addition, the relationship between concentration vs. time for LC50 (lethal concentration, 50% lethality) values was similar in mice, guinea pigs, and rats; they did not vary by more than 30%. The linear correlation coefficient was -0.96. After applying a total uncertainty factor of 10, the resulting value of 5 ppm was time scaled based on the equation, Cn × t = k, where n = 1.5. The value of n was derived from a regression analysis of the LC50 values for the mouse, guinea pig, and rat.
AEGL-2 values were based on exposure of rats to piperidine at 200 ppm for 6 h, which caused nasal irritation without salivation or evidence of ocular irritation. The rationale for selecting uncertainty factors and the time-scaling procedure were the same as those described for the AEGL-1 values.
AEGL-3 values were based on the LC01 (lethal concentration, 1% lethality) calculated from a 4-h acute inhalation study in rats. The LC01 of 448 ppm is less than the lowest concentration that caused one death among 20 rats (5% lethality) and greater than the highest concentration that caused no deaths or clinical moribund signs. Therefore, the LC01 appeared to be a good estimate of the threshold for lethality. Uncertainty factors of 3 for interspecies differences and 3 for intraspecies variability were applied to the LC01. The rationale for selecting the uncertainty factors was the same as described for AEGL-1 values. In addition, larger factors for interspecies differences or intraspecies variability would have produced values for the 4-h and 8-h durations that were lower than the irritation threshold of 26 ppm. The time-scaling procedure was the same as described for AEGL-1 values.
AEGL values for piperidine are presented in Table 6-1.
TABLE 6-1 Summar of AEGL Values for Pi eridine
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| AEGL-1 (nondisabling) | 10 ppm (35 mg/m3) | 10 ppm (35 mg/m3) | 6.6 ppm (23 mg/m3) | 2.6 ppm (9 mg/m3) | 1.7 ppm (6 mg/m3) | No nasal irritation (BASF 1993) |
| AEGL-2 (disabling) | 50 ppm (175 mg/m3) | 50 ppm (175 mg/m3) | 33 ppm (116 mg/m3) | 13 ppm (46 mg/m3) | 8.3 ppm (29 mg/m3) | Nasal irritation (BASF 1990) |
| AEGL-3 (lethal) | 370 ppm (1,295 mg/m3) | 180 ppm (630 mg/m3) | 110 ppm (385 mg/m3) | 45 ppm (158 mg/m3) | 28 ppm (98 mg/m3) | Threshold for lethality (BASF 1980) |
1. INTRODUCTION
Piperidine is a cyclic aliphatic amine (Eller et al. 2000). It is flammable (Trochimowicz et al. 1994) and produces explosive vapors at room temperature (HSDB 2008). Piperidine is a clear, colorless liquid and has a strong pepper- or amine-like pungent odor (Lewis 1993; Trochimowicz et al. 1994). Piperidine is a very strong base with a dissociation constant (pKb) of 2.88 (Reed 1990); thus, it is a very corrosive agent. The vapor pressure indicates that exposure to piperidine could occur by the inhalation route under ambient conditions. Chemical and physical properties of piperidine are presented in Table 6-2.
Piperidine has many commercial uses. It is used as a solvent, a curing agent for rubber and epoxy resins, a catalyst in silicone esters, an intermediate in organic synthesis, and a wetting agent. It is used in the manufacture of pharmaceuticals (analgesics, anesthetics, and germicides) and as a food additive (Reed 1990; Trochimowicz et al. 1994; HSDB 2008). In 1983, the United States produced 2.75 × 108 g (~606,000 pounds) of piperidine (HSDB 2008).
Humans are exposed to piperidine on a daily basis, as evidenced by its wide presence in the food supply and, consequently, in human urine. As a food additive, piperidine is found at 2.5-3.33 ppm in nonalcoholic beverages, 4-5.67 ppm in candy, 9.69 ppm in baked goods, and 0.04-1.66 ppm in condiments, meats, and soups (HSDB 2008). Piperidine also occurs naturally in food products, including vegetables (Neurath et al. 1977). Pulverized white pepper contains as much as 1,322 ppm of piperidine, and black pepper up to 703 ppm (Lin et al. 1981). Baked ham contains 0.2 ppm of piperidine, milk 0.11 ppm, and dry coffee 1 ppm (Reed, 1990). Piperidine also is found in boiled beef (Golovnya et al. 1979). von Euler (1945) reported that humans excrete 7.6-8.5 mg of piperidine in a 24-h period; more recently, Tricker et al. (1992) reported excretion rates of 26.1-31.7 mg/day.
The toxicology database on piperidine consists of anecdotal human data and a small amount of animal data.
TABLE 6-2 Chemical and Physical Data on Piperidine
| Parameter | Value | Reference |
| Chemical name | Piperidine | |
| Synonyms | Azacyclohexane, cyclopentimine, hexahydropyridine, UN2401 | RTECS 2009 |
| CAS registry no. | 110-89-4 | RTECS 2009 |
| Chemical formula | C5H11N | Budavari et al. 1996 |
| Molecular weight | 85.15 | Budavari et al. 1996 |
| Physical state | Colorless liquid | Lewis 1993 |
| Boiling point | 106.3°C | Howard and Meylan 1997 |
| Freezing point | -13 to -7°C | Budavari et al. 1996 |
| Vapor density | 3.0 (air = 1) | Trochimowicz et al. 1994 |
| Specific gravity | 0.8622 at 20°C | Reed 1990 |
| Solubility | 1.6 × 106 mg/L of water at 20°C | Howard and Meylan 1997 |
| Vapor pressure | 32.1 mm Hg at 25°C 40 mm Hg at 29.2°C |
Howard and Meylan 1997 Trochimowicz et al. 1994 |
| Flash point | 16.11°C (61°F) | Trochimowicz et al. 1994 |
| Refractive index (no) | 1.4530 | Weast et al. 1985 |
| pH | 12.6 at 100 g/L at 20°C | BG Chemie 2000 |
| pKb | 2.88 | Reed 1990 |
| Log P | 0.84 | Howard and Meylan 1997 |
| Conversion factors | 1 ppm = 3.5 mg/m3 at 25°C, 1 atm 1 mg/m3 = 0.29 ppm | |
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
No data were found on the acute lethality of piperidine in humans.
2.2. Nonlethal Toxicity
2.2.1. Experimental Studies, Case Reports, and Anecdotal Data
Bazarova and Migoukina (1975) reported an irritation threshold for piperidine of 90 mg/m3 (26 ppm) in human volunteers. No additional details were provided.
Concentrations of 2-5 ppm (7.0-17.5 mg/m3) were measured in a semi-closed environment as piperidine was transferred from drums. The report stated that unacclimated individuals could tolerate the pungent odor for only a brief time, although irritation was not perceived (A.C. Nawakowski, Upjohn Company, unpublished material, 1980, as cited in Trochimowicz et al. 1994). No additional details were provided.
EPA (1985) reported that piperidine is a strong local irritant that can cause permanent injury after a short exposure to small amounts. DASE (1980) reported that inhalation exposure causes sore throat, coughing, labored breathing, and dizziness. No exposure concentrations were provided in either report.
2.2.2. Other Studies
No human studies were found on the neurotoxicity, developmental toxicity, reproductive toxicity, carcinogenicity, or genetic toxicity of piperidine.
2.3. Summary
No human lethality data were found on piperidine. The irritation threshold for piperidine is 26 ppm (90 mg/m3). Inhalation exposure to piperidine causes sore throat, coughing, labored breathing, and dizziness. Piperidine at 2-5 ppm (7.0-17.5 mg/m3) is not irritating, but could be tolerated for only a brief time because of its pungent odor. These data indicate that the odor threshold for piperidine is less than 2 ppm (7.0 mg/m3).
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
BASF (1980) exposed groups of 10 male and 10 female Sprague-Dawley rats to piperidine (99%) at analytic concentrations of 2,190, 1,540, 1,190, 810, or 290 ppm (7,540, 5,300, 4,100, 2,800, or 1,000 mg/m3, respectively) for 4 h, and the rats were observed for 14 days. Rats were exposed (whole body) in a glass-steel chamber under dynamic conditions. Vapor was generated with an evaporation unit at 69°C and mixed with fresh air to obtain the desired concentration. Clinical signs, mortality, food consumption, body weights, and gross and microscopic findings were evaluated during or after exposure. Multiple clinical signs were observed at all concentrations. Prostration was observed only at 1,540 and 2,190 ppm. Corrosion around the nose, smoky-milky clouded cornea, crouched position, tremors, and clonic convulsions were observed at concentrations >1,190 ppm. Rubbing of the snout, dyspnea, and corrosion around the nose were observed at concentrations >810 ppm; nasal corrosion was observed in
only one male rat. A strong watery-reddish or reddish secretion from the eyes and nose, lid closure, and ragged fur were observed at all concentrations, and spasmodic respiration was observed only at 290 ppm. No clinical signs were observed more than 2 days after exposure at 290 ppm. Mortality data are summarized in Table 6-3.
A clear dose-response relationship was shown for male rats but not female rats. LC50 (lethal concentration, 50% lethality) values for piperidine in rats were 1,330, 1,420, 1,390 ppm (4,600, 4,900, and 4,800 mg/m3) for males, females, and both sexes combined, respectively. None of the rats exposed at 2,190 ppm survived until day 7. All male and female rats that did not survive until the end of the observation period died before day 7, except for one female rat exposed at 1,540 ppm and three female rats exposed at 1,190 ppm. Male and female rats exposed at 1,190 ppm and 1,540 ppm and females exposed at 810 ppm lost weight during the first week of observation, gained weight during the second week, and the males weighed 13-29% less than controls and the females weighed 14-19% less than controls at the end of the observation period. A postmortem evaluation was conducted, but no data were provided.
BASF (1981, as cited in BG Chemie 2000) reported that two of 12 and three of six Wistar rats died after exposure to an atmosphere of saturated piperidine vapor at 20°C (~45,000 ppm) for 3 or 10 min, respectively. No additional details were available.
Smyth et al. (1962) reported no deaths among six rats exposed to piperidine at 2,000 ppm (7,000 mg/m3) for 4 h. However, six of six rats died after exposure to piperidine at 4,000 ppm (14,000 mg/m3) for 4 h. The investigators also reported that inhalation of concentrated piperidine vapor for 15 min killed six of six rats; the exposure concentration was not reported.
Zayeva et al. (1968) reported a median lethal time (LT50) of 80 min for an unknown mammalian species exposed to an unknown concentration of piperidine by inhalation. Bazarova and Migoukina (1975) reported an LC50 of 1,885 ppm (6,500 mg/m3) for an unidentified mammalian species exposed for an unknown period of time. A 2-h LC50 in mice was reported to be 1,740 ppm (6,000 mg/m3) (BG Chemie 2000; AIHA 2001), and a 1-h LC50 in guinea pigs was 3,480 ppm (12,000 mg/m3) (AIHA 2001). Those lethality values were cited in a secondary source, and the primary sources could not be located to verify the values.
3.2. Nonlethal Toxicity
3.2.1. Rats
Groups of five male and five female Wistar rats were exposed to piperidine vapor (99.4% purity) at nominal concentrations of 0, 50, 100, and 200 ppm (175, 350, and 700 mg/m3, respectively) for 6 h/day for 5 days (BASF
TABLE 6-3 Lethalit Data for Pi eridine
| Concentration, ppm (mg/m3) | Mortality | |||
| Males | Females | Males and Females | ||
| 290 (1,000) | 0/10 | 0/10 | 0/20 | |
| 810 (2,800) | 0/10 | 1/10 | 1/20 | |
| 1,190 (4,100) | 3/10 | 7/10 | 10/20 | |
| 1,540 (5,300) | 6/10 | 1/10 | 7/20 | |
| 2,190 (7,540) | 10/10 | 10/10 | 20/20 | |
| LC50 [ppm (mg/m3)]a | 1,330 (4,600) | 1,420 (4,900) | 1,390 (4,800) | |
aCalculated using Number Cruncher Statistical System Survival Analysis, Version 5.5, published by Jerry L. Hintze, July 1991.
Source: BASF 1980.
1990). Analytic concentrations were 0, 49, 102, and 203 ppm (0, 170, 360, and 710 mg/m3), respectively. No animals died during the study. Clinical signs were observed during or immediately after exposure, and were concentration- and time-dependent. Nasal secretions and bloody encrustation on the edge of the nares were observed at all concentrations. “Stretched respiration posture,” lid closure, and salivation were observed at 200 ppm. Males exposed at 100 and 200 ppm had decreased body weights after the first days of exposure, but body weight and weight gain were not affected in females. No treatment-related changes in clinical pathology or post-mortem pathology were observed at any concentration. Because clinical signs were observed and recorded after each exposure, this study can be used for derivation of AEGL values.
In a 28-day study, two groups of five male and five female Wistar rats each were exposed to piperidine vapor (99.4% purity) at concentrations of 0, 5, 20, or 100 ppm (0, 18, 70, and 350 mg/m3, respectively) for 6 h/day, 5 days/week for 28 days (BASF 1993). The rats received 20 exposures. Additional groups of five male and five female rats exposed similarly at 0 or 100 ppm (0 and 350 mg/m3) were maintained for an additional 2 weeks without exposure to piperidine to evaluate recovery. The animals were exposed whole body under dynamic conditions in a glass-steel inhalation chamber. The atmosphere in the breathing zones of the animals was monitored approximately every 20 min using a total hydrocarbon analyzer equipped with a flame ionization detector. Rats were observed daily for clinical signs before, during, and after exposure, and body weights were measured at the beginning of the study and at 1-week intervals thereafter. Subgroups of five animals/sex/group were subjected to an extensive battery of neurofunctional tests before exposure and on days 2, 8, 14, and 28. Subgroups of five rats/sex/group were used for clinical pathology evaluations of blood and urine. Post-mortem evaluations consisted of gross examination, organ weight measurements, and microscopic examination of selected tissues.
Treatment-related clinical signs at 100 ppm consisted of a reddish crust (positive for blood) observed on the nasal edges of three male rats on day 2 of the study, all males from day 3 to the end of the study, two females on day 3, one female on day 4, almost all females starting on day 8, and all females by the end of the study. The reddish crust was indicative of upper respiratory tract irritation. Each subgroup of five male rats exposed at 100 ppm weighed 3.4% (n.s.) and 5.7% (n.s.) less than controls. Females exposed at 100 ppm did not show a trend toward decreased body weights. The only notable effects on the neurofunctional battery were increased hindlimb grip strength on day 8 in males exposed at 100 ppm and decreased response to the hot plate test on day 14 in males exposed at 5 and 100 ppm. Because these effects were transient or showed no dose-related trend, they are unlikely to be treatment related. No treatment-related effects were observed on ocular, hematologic, or clinical chemistry parameters, or on post-mortem findings. Treatment-related effects were not observed at 5 or 20 ppm (BASF 1993). This study is of marginal use for deriving AEGL values, because adverse effects were observed after the second exposure but not after the first exposure. BASF (1993) reported no nervous system effects; however, Bazarova and Migoukina (1975) reported an acute-exposure threshold of 5.8 ppm (20 mg/m3) for nervous system response in rats. No additional details were available in the translation.
Bazarova (1973) conducted a study in which groups of 20 rats (strain and sex not specified) were exposed to piperidine vapors at analytically measured concentrations of 0.002 ± = 0.0003 or 0.01 ± 0.001 mg/L (2 or 10 mg/m3 [0.6 or 3 ppm]) for 4 h/day, 5 days/week for 4 months followed by a 1-month recovery period. A group of 20 rats served as the control. Animals were exposed in a 700-L dynamic chamber (not otherwise described), and chamber atmospheres were measured eight times during each 4-h exposure. The investigators assessed body weight changes, blood vessel penetrability, erythrocyte parameters, liver and kidney function, testicular morphology, and neural activity.
Rats exposed at 10 mg/m3 weighed 14% less than the controls after 14 exposures and 16% less than controls at the end of the recovery period. Evidence of increased neural and muscular excitability was observed after exposure at 10 mg/m3 for 1.5 months or at 2 mg/m3 for 2.5 months. Respiration was decreased after exposure at 2 mg/m3 for 1.5 months and increased after exposure at 10 mg/m3 for 2.5 months. At both concentrations, blood vessels in the skin showed decreased penetrability (measured after application of xylol) during the early phase of the study, followed by increased penetrability during the late phase of the study that remained evident until the end of the recovery period. In addition, blood vessel stability was decreased throughout the study in the 10-mg/m3 group, as measured by increased petechia (submucosal hemorrhage). Decreases in erythrocyte count (80% of control) and hemoglobin concentration (89% of control) were observed in this group at the beginning of exposure and remained lower after 1.5 months, but were increased compared with controls at the end of the recovery period. Leukocyte count was decreased after 2.5 months (53% of control) due to a decrease in the lymphocyte count (47.9% of control) in rats
exposed at 10 mg/m3. Blood pressure was significantly decreased in rats exposed at 10 mg/m3 after 2.5 and 4 months. At the end of exposure, an effect on liver function was evidenced by a 47% decrease in urinary hippuric acid, and effects on kidney function were evidenced by a 46% decrease in urinary volume, increase in specific gravity of the urine, and 65% increase in urinary protein. Histopathologic findings in the 10 mg/m3 group included a decrease in the number of normal spermatogonia and degeneration of the seminiferous tubules in the testes, focal swelling of the interalveolar septa in the lungs, albuminous degeneration in the liver, hyalin droplet and albuminous degeneration in the kidney, stromal atrophy in the spleen, and necrosis and scaring in the cardiac muscle (Bazarova 1973). Descriptive details were lacking for an adequate evaluation of this study; some information about this study was also obtained from BG Chemie (2000).
3.2.2. Rabbits
Bazarova (1973) exposed groups of six rabbits under the same conditions as rats to piperidine at 0.01 or 0.002 mg/L (10 and 2 mg/m3, respectively) for 4 h/day, 5 days/week for 4 months, followed by a 1-month recovery period. The only effect described for the rabbit was a 29% and 27% decrease in arterial blood pressure after exposure at 10 and 2 mg/m3, respectively, for 14 days, and an 8% increase in pressure after exposure at 10 mg/m3 for 4 months.
3.3. Developmental and Reproductive Toxicity
Hughes et al. (1990) reported on the developmental effects in rats exposed to piperidine vapor during organogenesis. Groups of 25 pregnant Crl:CD(SD) GR VAF/Plus strain rats were exposed whole body to piperidine at concentrations of 0, 5, 20, or 80 ppm (0, 18, 70, and 280 mg/m3, respectively) for 6 h/day on gestation days 6-15. Dams were observed daily for clinical signs, weighed on gestation days 2, 3, and 6 and at 2-day intervals until gestation day 20, and had food consumption measured at intervals between weighing days. Dams were killed on gestation day 20 and their ovaries and uteri were examined. All dams survived to the end of the study. No treatment-related effects were observed on any litter parameter examined, including litter size, post-implantation loss, mean litter weight, or mean fetal weight. In addition, the incidences of visceral and skeletal malformations were similar between the exposed and control groups. Therefore, no effects were observed in developing fetuses of female rats exposed to piperidine concentrations up to 80 ppm during organogenesis.
However, a number of maternal effects were observed. During each exposure to piperidine at 80 ppm, clinical signs in dams included a lack of response to noise (a knock on chamber door) and closed or half-closed eyes. Other signs observed during exposure at that concentration included licking the inside of the
mouth (frequency not reported), piloerection (frequency not reported), hunched posture during almost all exposures, and increased respiration, salivation, and rubbing the chin and paws on the cage during one or two exposures. After daily exposures at 80 ppm, some rats had red/brown staining on the fur, two had “snuffles,” and one showed sneezing and salivation. At 20 ppm, lack of response to a knock on the chamber door was noted on each exposure occasion, and closed or half-closed eyes and hunched posture were observed once during the study. No clinical signs were reported after daily exposure to piperidine at 20 ppm, and no clinical signs related to exposure were observed at 5 ppm. Body weights and weight gain at 80 ppm were reduced compared with controls during the exposure period, but showed signs of recovery after exposure ended and was similar to controls at the end of the study. Food consumption also was reduced at 80 ppm during the exposure period and remained reduced after exposure ended. No treatment-related effects were observed on body weights or food consumption in the 5- or 20-ppm groups and no treatment-related necropsy findings were observed at any exposure concentration. The lack of response to a knock on the chamber door was the only clinical sign observed daily in rats exposed at 20 ppm. It is doubtful that this nonspecific clinical signs is treatment related or toxicologically significant in the absence of any corroborating evidence of central nervous system toxicity. BASF (1993) observed no treatment-related effects in their battery of neurofunctional tests conducted in a 28-day study of rats exposed repeatedly to piperidine at concentrations up to 100 ppm. Nevertheless, this study can be used for AEGL derivation because of the maternal clinical signs observed at 80 ppm.
In a study by Timofievskaya and Silantyeva (1975), groups of 6-13 pregnant rats were exposed to piperidine vapor at concentrations of 0, 0.9, 4, or 30 ppm (0, 3, 15, or 100 mg/m3, respectively) throughout pregnancy or on gestation day 9 or at 0.9 or 30 ppm on gestation day 4. Two control groups were included in this study. Dams were killed on gestation day 21 for assessment of maternal and fetal parameters. The duration of each exposure was not reported, so it was assumed that animals were exposed continuously. No behavioral effects were noted in the dams, but body weight gain was lower in dams exposed at 4 and 30 ppm compared with controls. In rats exposed at 30 ppm on gestation day 4, significant decreases in the number of fetuses per dam (5.5 vs. 8.5 and 11.08 for the two control groups) and in the number of implantation sites (6.1 vs. 9.4 for control) were observed. Piperidine at 30 ppm on gestation day 9 or throughout pregnancy had no effect on these parameters. Fetal body weights were decreased in dams exposed at all concentrations throughout pregnancy (66-78% of control fetal weight). Fetal body weights were 76% of control weights after exposure at 0.9 ppm on gestation day 4 or 9; no significant reductions in fetal weights were observed after exposure at 30 ppm on gestation day 4 or 9 or at 4 ppm on gestation day 9. Decreases in fetal body weights appeared to be unrelated to exposure to piperidine. A concentration-response relationship was not observed for the single exposures. In addition, no corresponding changes were observed in other
fetal parameters consistent with pronounced decreases in fetal weights. Fetal length and placental size did not show concentration-response relationships. However, the placenta coefficient (not described) was significantly decreased at all concentrations throughout gestation, at 0.9 ppm on gestation day 4, and at 0.9 and 4 ppm on gestation day 9. Description of this study lacks adequate detail to be used for AEGL derivation. Some of the information for this study was obtained from BG Chemie (2000).
3.4. Carcinogenicity
No inhalation carcinogenicity studies on piperidine were found, but one oral study was available. Lijinsky and Taylor (1977) conducted a study in which groups of 15 male and 15 female Sprague-Dawley rats were administered piperidine at 0.09% (9,000 ppm) in drinking water with and without 0.2% sodium nitrite for 50 weeks. No treatment-related neoplasms developed during the lifetime of either test group.
3.5. Genotoxicity
Piperidine was negative in Salmonella typhimurium assays using strains TA98, TA100, and TA1537 with and without metabolic activation with S9 from phenobarbital-induced mouse liver; piperidine was tested at concentrations of 1.25-25 mM (Riebe et al. 1982). Green and Savage (1978) tested piperidine in the Ames and mouse host-mediated mutagenicity assays. Piperidine was negative in the Ames assay using S. typhimurium strains TA1531, TA1532, TA1964, and TA1530 with and without metabolic activation with mouse liver S9 and in the host-mediated assay using S. typhimurium strains TA1534, TA1950, TA1951, and TA1952. Riebe et al. (1982) also obtained negative results when they tested piperidine in the Escherichia coli pol A+/pol AB recombination assay. Nevertheless, piperidine was mutagenic in the mouse lymphoma assay without metabolic activation with rat liver S9, but negative with S9 activation (Wangenheim and Bolcsfoldi 1988). Garberg et al. (1988) reported that piperidine induced DNA strand breaks in mouse lymphoma cells with rat liver S9, but not alkaline unwinding with or without S9.
3.6. Summary
Inhalation toxicity data on piperidine are summarized in Table 6-4. The LT50 for mice exposed to an unknown preset absolute concentration of piperidine was 80 min (Zayeva et al. 1968). The 4-h LC50 for piperidine in rats ranged from 1,330-1,420 ppm (4,600-4,900 mg/m3) (BASF 1980). Smyth et al. (1962) reported, however, that no rats died after exposure at 2,000 ppm
TABLE 6-4 Summar of Acute Inhalation Toxicit Data of Pi eridine in Laborator Animals
| Species | Exposure Conditions | Effects | Reference | |
| Mouse | 2-h LC100 | LT50 = 80 min | Zayeva et al. 1968 | |
| 2 h | LC50 = 1,740 ppm | AIHA 2001 | ||
| Rat | 290-2,190 ppm (1,000-7,540 mg/m3) for 4 h | Respiratory irritation at all concentrations; deaths at >810 ppm (>2,800 mg/m3); LC50 = 1,330 ppm (4,600 mg/m3) (males); 1,420 ppm (4,900 mg/m3) (females); 1,390 ppm (4,800 mg/m3) (males and females) | BASF 1980 | |
| 2,000 ppm (7,000 mg/m3) for 4 h | 0/6 deaths | Smyth et al. 1962 | ||
| 4,000 ppm (14,000 mg/m3) for 4 h | 6/6 deaths | Smyth et al. 1962 | ||
| Concentrated vapor: | ||||
| 15 min | 6/6 deaths | Smyth et al. 1962 | ||
| 10 min | 3/6 | BASF 1981 | ||
| 3 min | 2/12 | BASF 1981 | ||
| NR | NR | LC50 = 1,885 ppm (6,500 mg/m3) | Bazarova and Migoukina 1975 | |
| Rats | 50, 100, 200 ppm (175, 360, 710 mg/m3), 6 h/d for 5 d | Upper respiratory tract irritation at all concentrations; closed eyes and salivation at 200 ppm. | BASF 1990 | |
| 5, 20, 100 ppm (20, 70, 350 mg/m3), 6 h/d for 28 d | Upper respiratory tract irritation at 100 ppm (350 mg/m3) on day 2. | BASF 1993 | ||
| 3 ppm (10 mg/m3), 4 h/d, 5 d/wk for 4 mos | No effects after a single exposure. After repeated exposure, body weight decreases; neural, muscular, cardiovascular, hematologic, and respiratory effects; and hepatic, renal, testicular, splenic, and cardiac muscle toxicity. | Bazarova 1973 | ||
| 0.6 ppm (2 mg/m3), 4 h/d, 5 d/wk for 4 mos | Cardiovascular, respiratory, neural, and muscular effects after multiple exposures. | Bazarova 1973 | ||
| Species | Exposure Conditions | Effects | Reference | |
| Rabbit | 0.6 or 3 ppm (2 or 10 mg/m3), 4 h/d, 5 d/wk for 4 mos | Decreased arterial blood pressure at both concentrations. | Bazarova 1973 | |
| Rat | 5.8 ppm (20 mg/m3) | Threshold for nervous system response. | Bazarova and Migoukina 1975 | |
| Rat, pregnant | 5, 20, 80 ppm (20, 70, 280 mg/m3), gestation days 6-15 | Dams: at 80 ppm, respiratory and ocular irritation, salivation during or after first exposure, hunched posture after most exposures; at 20 ppm, ocular irritation and hunched posture observed once Fetuses: no effects. | Hughes et al. 1990 | |
| Guinea pig | 1 h | LC50 = 3,480 ppm | AIHA 2001 | |
Abbreviations: LC100, lethal concentration, 100% lethality; LC50, lethal concentration, 50% lethality; LT50, median lethal time; NR, not reported.
(7,000 mg/m3) for 4 h, but that six of six died after exposure at 4,000 ppm (14,000 mg/m3) for 4 h. The LC50 for an unidentified mammalian species exposed for an unknown period of time was reported as 1,885 ppm (6,500 mg/m3) (Bazarova and Migoukina 1975). A 2-h LC50 for the mouse was reported as 1,740 ppm (6,000 mg/m3) and a 1-h LC50 for the guinea pig was 3,480 ppm (12,000 mg/m3) (AIHA 2001).
Male and female rats exposed to piperidine at concentrations ranging from 50 to 200 ppm (175-710 mg/m3) for 6 h/day caused nasal irritation signs of ocular irritation and salivation also were observed after each daily exposure at 200 ppm (BASF 1990, 1993; Hughes et al. 1990). More severe nasal and ocular irritation were observed in rats exposed at 290 ppm (1,000 mg/m3) for 4 h followed by the first evidence of corrosion around the nose and dyspnea at 810 ppm (2,800 mg/m3). CNS toxicity, corneal damage, and more severe corrosion around the nose were observed at concentrations >1,190 ppm (>4,100 mg/m3) followed by prostration at 1,540 and 2,190 ppm (5,300 and 7,540 mg/m3) (BASF 1980). Clinical signs likely associated with death included prostration, CNS toxicity, and dyspnea, and the lowest concentration causing death in one rat was the lowest concentration that caused dyspnea. These data showed an exposure-related continuum that increased in severity from nasal irritation to death. Other studies were not reliable. Bazarova (1973) reported that repeated exposure of rats to piperidine at much lower concentrations of 0.6 and 3 ppm (2 or 10 mg/m3) for 4 months caused multiple effects, including neurotoxicity, cardiovascular toxicity, hematologic effects, hepatic effects, renal effects, and testicular effects at one or both concentrations. Insufficient details were available for adequately evaluating this study for AEGL derivation.
Two developmental toxicity studies were available. Hughes et al. (1990) found no effects on the fetuses of rat dams exposed to piperidine at concentrations up to 80 ppm (280 mg/m3) for 6 h/day during organogenesis. Timofievskaya and Silantyeva (1975) reported inconsistent results regarding the effect of piperidine on the fetuses of rat dams exposed at 0.9-30 ppm (3-100 mg/m3) throughout gestation or on gestation day 9 or at 0.9 or 30 ppm (3 or 100 mg/m3) on gestation day 4.
No inhalation studies were found on the carcinogenicity of piperidine. In an oral study, carcinogenic activity was not observed in rats administered piperidine at 0.09% in drinking water for 50 weeks (Lijinsky and Taylor 1977). Genotoxicity studies showed that piperidine was not mutagenic in S. typhimurium with or without metabolic activation in either the Ames or the host-mediated assay (Riebe et al. 1982). Piperidine also was negative in the E. coli recombination assay (Riebe et al. 1982), but was positive in the mouse lymphoma cell assay without metabolic activation (Wangenheim and Bolcsfoldi 1988). Piperidine induced DNA strand breaks in mouse lymphoma cells (Garberg et al. 1988).
4. SPECIAL CONSIDERATIONS
4.1. Metabolism, Disposition, and Kinetics
Piperidine is absorbed from the respiratory tract, digestive tract, and skin (Gehring 1983). Piperidine also is synthesized endogenously from lysine, cadaverine, and pipecolic acid. Pipecolic acid is a product of lysine degradation, and homogenates of brain tissue can convert pipecolic acid to piperidine. Radioactive piperidine is recovered from rat brain after intraperitoneal injection of radioactive pipecolic acid. Piperidine has been found in the muscles, liver, heart, kidneys, spleen, testes, small intestine, and lungs of rats and mice at concentrations ranging from 0.115-0.440 nmol/g of tissue. However, the highest endogenous concentrations are found in the brain, where concentrations reported for whole mouse and rat brain ranged from 0.016-3.28 nmol/g depending on the extraction and analysis procedures. The concentration of piperidine in whole human brain was reported to be 1.8 nmol/g. In other species, piperidine was found in the cerebellum of dogs at 3.9-5.18 nmol/g, in mice at 3.07-3.17 nmol/g, in cats at 0.8 nmol/g, and in rats at 0.047 nmol/g. Concentration in other brain regions varied considerably (Giacobini 1976). Perry et al. (1964) reported that piperidine is found in human cerebral spinal fluid at very low concentrations.
Piperidine and its metabolites are excreted in urine. Unchanged piperidine, 3-hydroxypiperidine, 4-hydroxypiperidine, and two unidentified metabolites were found in urine collected over 72 h after intraperitoneal injection of rats with [3H]piperidine (Okano et al. 1978).
von Euler (1945) used a colorimetric method to determine the amount piperidine in the urine of nonsmoking human subjects (eight male and four female medical students). Male subjects had an average urinary piperidine concentration of 0.49 mg/dL and females an average of 0.88 mg/dL in 24-h pooled specimens; the total amount excreted in 24 h was 8.5 mg for males and 7.6 mg for females.
4.2. Mechanism of Toxicity
Piperidine is a very strong alkaline agent with a pKb of 2.88 at 25°C; consequently, it is severely corrosive to skin producing severe third degree burns in a human after less than 3 min of contact (Linch 1965). Linch (1965) considered piperidine to be more corrosive than “strong primary irritants.” Because of its corrosive properties, piperidine is expected to cause irritation to the eyes and respiratory tract. Piperidine is found naturally in the brain and other tissues of vertebrates and invertebrates; it is a biogenic amine and acts as a neuromodulator (Giacobini 1976). Piperidine stimulates and blocks actions on ganglia, chemoreceptors, and neuromuscular junctions. It acts on chemoreceptors, which stimulates respiration; acts on sympathetic ganglia releasing catecholamines, which raises blood pressure; acts on parasympathetic ganglia, which stimulates
contraction of smooth muscle; and acts on end plates, which stimulates contraction of skeletal muscle (Kase and Miyata 1976). Piperidine interacts with cholinergic receptor sites of muscle end plates and with nicotinic receptors on sympathetic and parasympathetic ganglia to cause effects mimicking those of acetylcholine (Giacobini 1976). Piperidine also acts on the CNS where it mimics the nicotinic effects of acetylcholine on synaptic sites in the brain (Kase and Miyata 1976). Piperidine affects CNS responses related to emotional behavior, physiologic processes of sleep, and extrapyramidal motor function (ataxia, head turning, and nystagmus) (Giacobini 1976; Kase and Miyata 1976).
4.3. Structure-Activity Relationships
Pyrrolidine (CAS registry no. 123-75-1) is a five-membered alicyclic secondary amine that is structurally similar to piperidine (Trochimowicz et al. 1994). It produces CNS effects similar to those of piperidine (Giacobini 1976). The 2-h LC50 of pyrrolidine in mice is 1,300 mg/m3; inhalation exposure causes irritation, excitement, and convulsions. The oral LD50 (lethal dose, 50% lethality) is 300 mg/kg for rats, 450 mg/kg for mice, and 250 mg/kg for rabbits and guinea pigs. Intravenous administration of pyrrolidine causes increases in blood pressure and respiration in dogs and cats (Trochimowicz et al. 1994). Piperazine (CAS registry no. 110-85-0) is a six-membered alicyclic amine that is also structurally similar to piperidine (Trochimowicz et al. 1994). Piperazine produced signs of respiratory irritation in rats after exposure at 40 mg/L (4,000 mg/m3) for 2 h (DuPont 1968, as cited in Trochimowicz et al. 1994). Mice showed changes in motor activities and muscle contraction inhalation exposure at 5,400 mg/m3 for 2 h (Timofievskaya 1979, as cited in Trochimowicz et al. 1994). Oral LD50 values range from 2,050 to 3,000 mg/kg for rats and from 600 to 1,900 mg/kg for mice. Piperazine caused an initial fall in blood pressure and heart rate in rats, followed by a transient rise in both parameters (Trochimowicz et al. 1994).
4.4. Other Relevant Information
One case was reported in the literature concerning chemical burns associated with skin contact with piperidine. A worker was sprayed with piperidine when transferring the chemical under room temperature conditions. The worker suffered first degree burns on the face, left ear, and neck; second degree burns on the forearms and abdomen; and third-degree burns on the chest. Contact time with the chemical was less than 3 min (Linch 1965).
Smyth et al. (1962) reported an oral LD50 for piperidine of 520 mg/kg for the rat; values reported by Trochimowicz et al. (1994) ranged from 133 to 337 mg/kg. Oral administration of piperidine causes weakness, respiratory distress, and convulsions. van den Heuvel et al. (1990) reported LD50 values of 445 mg/kg for male and female rats combined; clinical signs included ptosis, respiratory effects, lethargy, ataxia, tremors, salivation, and lacrimation.
4.4.1. Species Variability
Little information was available to evaluate species variability in response to piperidine vapor. The LC50 is 3,480 ppm for a 1-h exposure to the guinea pig, 1,740 ppm for a 2-h exposure to the mouse, and 1,390 ppm for a 4-h exposure to the rat. The linear correlation for concentration vs. time for these three species is -0.96, indicating the response does not vary because of species.
4.4.2. Susceptible Populations
No data were available for determining human variability to piperidine after inhalation exposure.
4.4.3. Concentration-Exposure Duration Relationship
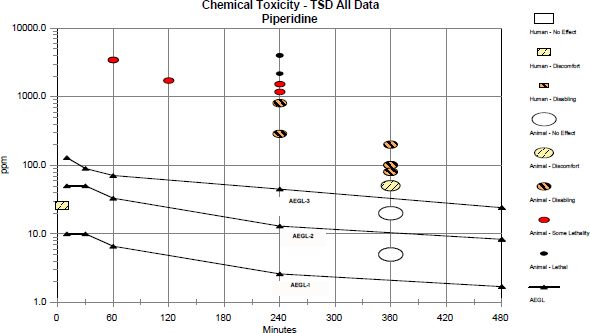
Data from a single species were not available for establishing a concentration-exposure duration relationship for piperidine. However, LC50 data were available for three different species that allowed derivation of the value for n, which is used in the equation Cn × t = k for extrapolating data to the pertinent AEGL time frames. The 1-h LC50 for the guinea pig is 3,480 ppm (AIHA 2001); the 2-h LC50 for the mouse is 1,740 ppm (BG Chemie 2000; AIHA 2001); and the 4-h LC50 for the rat is 1,390 ppm (BASF 1980). The correlation coefficient is -0.96, and the value of n derived from these data is 1.5. Figure 6-1 shows the concentration-exposure duration relationship.
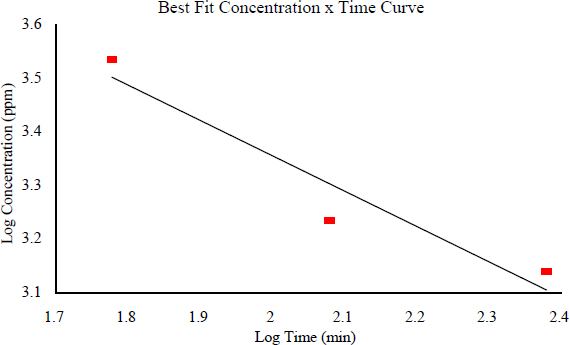
FIGURE 6-1 Concentration-exposure duration relationship for piperidine.
4.4.4. Concurrent Exposure Issues
There are no known concurrent exposure issues related to inhalation of piperidine.
5. DATA ANALYSIS FOR AEGL-1
5.1. Human Data Relevant to AEGL-1
Piperidine has an amine-like pungent (Trochimowicz et al. 1994) or pepper-like odor (Lewis 1993; Trochimowicz et al. 1994). At concentrations of 2-5 ppm, piperidine has an odor reported to be tolerated for only a brief time by unacclimated individuals. The odor threshold for piperidine was reported to be <2 ppm (A.C. Nawakowski, Upjohn Company, unpublished material, 1980, as cited in Trochimowicz et al. 1994) and 0.37 ppm by van Doorn et al. (2002). Bazarova and Migoukina (1975) reported that the irritation threshold for inhalation exposure to piperidine was 90 mg/m3 (26 ppm). These data are from secondary sources and are not be verified.
5.2. Animal Data Relevant to AEGL-1
Bazarova and Migoukina (1975) reported that the acute inhalation threshold for nervous system response to piperidine was 5.7 ppm for an unidentified species. In a repeat exposure study by Bazarova (1973), no effects were described in rats after the first exposure to piperidine at 0.6 or 3 ppm for 4 h. A concentration-related increase in the severity of nasal irritation (secretions and bloody encrustation) was observed during or after each 6-h exposure at 50-200 ppm for 5 days, and eye lid closure and salivation were observed at 200 ppm (BASF 1990). Nasal irritation also was observed after the second 6-h exposure to piperidine at 100 ppm in a 28-day inhalation study (BASF 1993). No effects were observed after exposure at 5 or 20 ppm. In a developmental toxicity study, Hughes et al. (1990) observed eye closure, increased respiration, hunched posture, piloerection, salivation, a lack of response to a knock on the chamber door after the first exposure at 80 ppm in pregnant rats exposed for 6 h/day during gestation days 6-15. At 20 ppm, dams did not respond to a knock on the chamber door. No clinical signs were observed at 5 ppm. An extensive battery of neurofunctional test showed no treatment-related CNS toxicity after repeated 6-h daily exposures at concentrations up to 100 ppm. Studies by BASF (1990, 1993) and Hughes et al. (1990) appeared to follow standard protocol for repeat exposure studies and were conducted under Good Laboratory Practice.
5.3. Derivation of AEGL-1 Values
Data on odor detection, threshold, or tolerance as well as the data on irritation threshold were obtained from secondary sources, and the primary sources
were not available for verification. Three studies showed nasal secretions, bloody or reddish encrustation or other evidence of upper respiratory tract irritation, ocular irritation, or general discomfort in rats exposed to piperidine at 50-200 ppm (Hughes et al. 1990; BASF 1990, 1993). The lowest concentration causing nasal irritation was 50 ppm for a 6-h exposure in rats (BASF 1990), and no nasal irritation was observed in rats exposed at 20 ppm for 6 h (BASF 1993). A lack of response to a knock was observed in pregnant rats exposed to piperidine at 20 ppm for 6 h, but the toxicologic significance of this observation is uncertain because a functional observation battery showed no treatment-related effects after repeated exposures at 100 ppm for 6 h. A no-effect level for nasal irritation of 20 ppm for 6 h was selected as the end point for deriving AEGL-1 values. An uncertainty factor of 3 for interspecies differences and a factor 3 for intraspecies variability were applied. The rationale for selecting these factors included the following: (1) the irritant effects are mediated by direct contact of piperidine (corrosive agent) with the nasal epithelium without involvement of other regions of the respiratory tract; and (2) the cell composition of the nasal mucosa is similar among species and individuals in the population, although the cell distribution and nasal morphology differ among species. An interspecies factor of 3 is also supported by an analysis of the LC50 data for three species exposed for three different time periods. The LC50 values are 3,480 ppm for a 1-h exposure to the guinea pig, 1,740 ppm for a 2-h exposure to the mouse, and 1,390 ppm for a 4-h exposure to the rat. The linear correlation coefficient for regression analysis of the LC50 values for the three species is –0.96, and the concentration and time relationships for three species do not vary by more than 30%, indicating the response is similar between the species. Therefore, these data support an interspecies factor of 3. After applying a total uncertainty factor of 10, the resulting value of 2 ppm was time scaled based on the equation Cn × t = k, where n = 1.5. The value of n was derived from a regression analysis of the LC50 values for the mouse, guinea pig, and rat (see section 4.4.3). The 30-min AEGL-1 value was applied to the 10-min exposure, because of the uncertainty in scaling 6-h duration to a 10-min exposure. AEGL-1 values for piperidine are presented in Table 6-5. All AEGL-1 values are below the irritation threshold of 26 ppm reported by Bazarova and Migoukina (1975).
6. DATA ANALYSIS FOR AEGL-2
6.1. Human Data Relevant to AEGL-2
No human data relevant to AEGL-2 end points were found. The irritation threshold for piperidine was reported to be 26 ppm (Bazarova and Migoukina 1975); however, no information was provided on how that value was derived. Secondary sources reported that piperidine can cause sore throat, signs
TABLE 6-5 AEGL-1 Values for Pi eridine
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 10 ppm (35 mg/m3) | 10 ppm (35 mg/m3) | 6.6 ppm (23 mg/m3) | 2.6 ppm (9 mg/m3) | 1.7 ppm (6 mg/m3) |
of respiratory tract irritation (coughing, labored breathing), and dizziness (probably CNS related), but exposure concentrations and durations were not specified.
6.2. Animal Data Relevant to AEGL-2
Smyth et al. (1962) observed no deaths among six rats exposed to piperidine at 2,000 ppm for 4 h, and BASF (1980) reported no deaths among 20 rats exposed at 290 ppm for 4 h. Nasal secretions and bloody encrustation were observed after exposure at 50-200 ppm for 6 h (BASF 1990). In addition, closed eyes (possibly indicative of ocular irritation) and salivation (probably from attempted mouth breathing) occurred in rats during each exposure to piperidine at 200 ppm. Severity of nasal and ocular irritation and general discomfort showed a concentration-related increase at piperidine concentrations of 50-200 ppm. Clinical signs observed at 287 ppm included ocular and nasal irritation, spasmodic respiration (probably from attempted mouth breathing because of the pungent odor), and ragged fur; none of these clinical signs were indicative of death. Thus, the clinical signs observed in rats exposed at 290 ppm for 4 h are similar but slightly more severe than those observed in rats exposed to 200 ppm for 4 h. In rats exposed at 10 mg/m3 (3 ppm) for 4 h/day for 4 months, Bazarova (1973) noted cardiovascular and hematologic effects at the beginning of exposure (not otherwise described), but did not state whether the effects were observed after the first exposure; the results have not been corroborated in another study. Studies by BASF (1980, 1990) appeared to have been conducted using the standard protocols for acute inhalation and repeat exposure studies.
No developmental toxicity was found in rat fetuses after dams were exposed to piperidine at 0.9, 4, or 30 ppm throughout gestation, on gestation day 4, or on gestation day 9 (Timofievskaya and Silantyeva 1975). Developmental toxicity also was not observed after exposure of pregnant rats at 5, 20, or 80 ppm for 6 h/day on gestation days 6-15 (Hughes et al. 1990).
6.3. Derivation of AEGL-2 Values
AEGL-2 values were derived from the study showing nasal irritation but no salivation or eye closure, possibly indicative of ocular irritation, in rats exposed to piperidine at 100 ppm for 6 h (BASF 1990). The uncertainty factors,
justification for the uncertainty factors, and time scaling were the same as those described for AEGL-1 values (see Section 5.3). AEGL-2 values for piperidine are presented in Table 6-6.
7. DATA ANALYSIS FOR AEGL-3
7.1. Human Data Relevant to AEGL-3
No human lethality data on piperidine were found.
7.2. Animal Data Relevant to AEGL-3
Zayeva et al. (1968) reported an LT50 of 80 min for piperidine, but an exposure concentration was not specified. Bazarova and Migoukina (1975) reported an LC50 of 1,885 ppm (6,500 mg/m3) for an unknown species exposed for an unknown duration. BASF (1981) reported that two of 12 rats exposed to a saturated piperidine atmosphere at 20 C died after 3 min and three of six rats died after 10 min. The 4-h LC50 for rats was 1,390 ppm (4,800 mg/m3); no rats died at 290 ppm (1,000 mg/m3) and all 20 rats exposed at 2,190 ppm (7,540 mg/m3) died (BASF 1980). No deaths occurred in six rats exposed to piperidine at 2,000 ppm (7,000 mg/m3) for 4 h, but all six rats exposed at 4,000 ppm (14,000 mg/m3) for 4 h died (Smyth et al. 1962). LC50 values of 1,740 ppm (6,000 mg/m3) were reported for a 2-h exposure to the mouse and 3,480 ppm (12,000 mg/m3) for a 1-h exposure to the guinea pig. The mouse and guinea pig values were cited from secondary sources; the primary sources could not be located.
7.3. Derivation of AEGL-3 Values
One acute inhalation study showing increased mortality with concentration was available for deriving AEGL-3 values for piperidine. Clinical signs at lethal doses included effects on the eyes, upper and lower respiratory tracts, and the CNS. Dyspnea, tremors, clonic convulsions, and prostration were the most severe signs that appeared to be associated with death. One death occurred among 20 rats exposed at 810 ppm, and dyspnea was the only clinical sign that was potentially related to the death. The 4-h LC50 for inhalation exposure to piperidine is 1,390 ppm. The lethality threshold (LC01) for 4 h was estimated by probit analysis to be 448 ppm (NCSS, Version 5.5). The LC01 benchmark dose (BMD01) estimated from the probit model using EPA’s Benchmark Dose Software, Version 1.3.2 (EPA 2003), was 415 ppm and the lower confidence limit (BMDL05) was 474 ppm. AEGL-3 values were derived from the LC01 of 448 ppm for a 4-h exposure. That value is below the lowest concentration (810 ppm) that caused one death among 20 rats (5% lethality) and above than the highest
TABLE 6-6 AEGL-2 Values for Piperidine
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 50 ppm (175 mg/m3) | 50 ppm (175 mg/m3) | 33 ppm (116 mg/m3) | 13 ppm (46 mg/m3) | 8.3 ppm (29 mg/m3) |
concentration (290 ppm) that caused no deaths or clinical signs indicative of death. Therefore, the LC01 appears to be a good estimate of the threshold for lethality. The uncertainty factors, justification for the uncertainty factors, and time scaling were the same as those described for AEGL-1 values (see Section 5.3). In addition, larger uncertainty factors of 10 for either interspecies differences or intraspecies variability would lower the AEGL-3 values for the 4- and 8-h durations below the irritation threshold of 26 ppm for piperidine (Bazarova and Migoukina 1975). AEGL-3 values for piperidine are summarized in Table 6-7.
8. SUMMARY OF PROPOSED AEGLs
8.1. AEGL Values and Acute Toxicity End Points
No human data were available for deriving any of the AEGL values. AEGL-1 values were derived on the basis of a no-effect level (20 ppm for 6 h) for nasal irritation in rats exposed to piperidine. Uncertainty factors of 3 for interspecies differences and 3 for intraspecies variability were applied, and time scaling was performed using the equation Cn × t = k, with n = 1.5. AEGL-2 values were derived on the basis of the highest concentration (100 ppm for 6 h) that caused nasal irritation but no salivation or eye closure. The basis of AEGL-3 was the LC01 derived from a 4-h acute lethality study in rats. The LC01 (448 ppm) was lower than the lowest concentration associated with death or clinical signs indicative of death (810 ppm) and greater than the highest concentration that caused no deaths or clinical signs indicative of death (290 ppm). Uncertainty factors and time-scaling methods for AEGL-2 and AEGL-3 values were the same as described for AEGL-1 values. In addition, the interspecies uncertainty factor for AEGL-3 is supported by an analysis of LC50 values for three species. AEGL values are summarized in Table 6-8.
8.2. Comparison with Other Standards and Guidelines
The only standards available for piperidine are the workplace environmental exposure level (WEEL) established by the American Industrial Hygiene Association (AIHA) and the United Kingdom Occupational Exposure Level (OEL). The WEEL for piperidine is 1 ppm (8-h time-weighted average), with a skin notation (AIHA 1996, 2001, 2007). AIHA based the WEEL on secondary
TABLE 6-7 AEGL-3 Values for Piperidine
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 370 ppm (1,295 mg/m3) | 180 ppm (630 mg/m3) | 110 ppm (385 mg/m3) | 45 ppm (158 mg/m3) | 28 ppm (98 mg/m3) |
TABLE 6-8 AEGL Values for Piperidine
| Classification | 10 min | 30 min | 1 h | 4h | 8h |
| AEGL-1 (nondisabling) | 10 ppm (35 mg/m3) | 10 ppm (35 mg/m3) | 6.6 ppm (23 mg/m3) | 2.6 ppm (9 mg/m3) | 1.7 ppm (6 mg/m3) |
| AEGL-2 (disabling) | 50 ppm (175 mg/m3) | 50 ppm (175 mg/m3) | 33 ppm (116 mg/m3) | 13 ppm (46 mg/m3) | 8.3 ppm (29 mg/m3) |
| AEGL-3 (lethal) | 370 ppm (1,295 mg/m3) | 180 ppm (630 mg/m3) | 110 ppm (385 mg/m3) | 45 ppm (158 mg/m3) | 28 ppm (98 mg/m3) |
sources and comparison to standards for 2-aminopyridine of 0.5 ppm (2 mg/m3) set by various organizations and an immediately dangerous to life and health value of 5 ppm (20 mg/m3). The source documentation for the WEEL (AIHA 2007) cites three dermal LD50 values: 275 mg/kg in rats (van den Heuval et al. 1990), 320 mg/kg in rabbits (Royal Society 1987), and 1,000 mg/kg in rabbits (D. Conine, Abbott Laboratories, personal conversation, 1994, as cited in AIHA 1996). The latter value was from an unpublished study. These dermal LD50 values are very high, and it is unlikely that such concentrations would occur during an accidental release of piperidine vapors. It appears that the data used to derive AEGL values were not available to the organizations that developed the standards and guidelines for piperidine.
8.3. Data Quality and Research Needs
The studies by Bazarova (1973), Bazarova and Migoukina (1975), Zayeva et al. (1968), and Timofievskaya and Silantyeva (1975) did not provide adequate experimental detail and explanation of results. Studies by BASF (1980, 1990, 1993) and Hughes et al. (1990) were well conducted and useful for deriving the AEGL values. Overall, the BASF and Hughes et al. studies showed an exposure-response continuum from 5 to 2,190 ppm; therefore, the database for piperidine provided sufficient data for deriving AEGLs. Nevertheless, the evaluation and conclusions regarding the acute inhalation toxicity of piperidine vapor and AEGL derivations could be strengthen with definitive data on the odor and irritation threshold in humans and with 1- and 8-h acute inhalation toxicity studies at concentrations in rats that would encompass lethal and nonlethal end points.
9. REFERENCES
AIHA (American Industrial Hygiene Association). 1996. Workplace Environmental Exposure Level Guide: Piperidine. American Industrial Hygiene Association, Fairfax, VA.
AIHA (American Industrial Hygiene Association). 2001. Workplace Environmental Exposure Level Guide: Piperidine (CAS Reg. No. 110-89-4). In 2001 WEELs Complete Set. American Industrial Hygiene Association, Fairfax, VA.
AIHA (American Industrial Hygiene Association). 2007. Piperidine (CAS Reg. No. 110-89-4). P. 8 in The AIHA 2007 Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guides Handbook. American Industrial Hygiene Association, Fairfax, VA.
BASF. 1980. Determination of the Acute Inhalation Toxicity LC50 of Piperidine as Vapor in Sprague-Dawley Rats after a 4-Hour Exposure [in German]. BASF Gewerbehygiene und Toxikologie. November 17, 1980.
BASF. 1981. Piperidin-akutes inhalationsrisiko. BASF Gewerbehygiene und Toxikologie (as cited in BG Chemie 2000).
BASF. 1990. Range-finding Study on the Inhalation Toxicity of Piperidine as Vapor in Rats: 5-Day Study [in German]. Project No. 3010523-89017. BASF Aktiengesellschaft, Ludwigshafen/Rhein, Germany. January 2, 1990.
BASF. 1993. Study on the Inhalation Toxicity of Piperidine as a Vapor in Rats: 28-Day Test Including an About 2-Week Post-Exposure Observation Period Including Neurotoxicological Examinations. Project No. 4610523-89065. BASF Aktiengesellschaft, Ludwigshafen/Rhein, Germany.
Bazarova, L.A. 1973. Evaluation of general toxic and specific effects of piperidine at chronic exposure [in Russian]. Toksikol. Nov. Prom. Khim. Veshchestv. 13:100-107.
Bazarova, L.A., and N.V. Migoukina. 1975. Comparative evaluation of the toxicity, hazards and mode of action of piperidine and morpholine [in Russian]. Toksikol. Nov. Khim. Veshchestv. 14:90-95.
BG Chemie. 2000. Piperidine (CAS Reg. No. 110-89-4). Toxicological Evaluations No.72 [in German]. Berufsgenossenschaft der Chemischen Industrie (Employment Accident Insurance Fund of the Chemical Industry), Heidelberg, Germany [online]. Available: http://www.bgrci.de/fileadmin/BGRCI/Downloads/DL_Praevention/Fachwissen/Gefahrstoffe/TOXIKOLOGISCHE_BEWERTUNGEN/Bewertungen/ToxBew072-L.pdf [accessed Nov. 1, 2012].
Budavari, S., M.J. O’Neil, A. Smith, P.E. Heckelman, and J.F. Kinneary, eds. 1996. P. 1285 in The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 12th Ed. Whitehouse Station, NJ: Merck.
DASE (Dutch Association of Safety Experts). 1980. Piperidine. P. 757 in Handling Chemicals Safely. Dutch Association of Safety Experts, Dutch Chemistry Industrial Association, and Dutch Safety Institute, The Hague.
DuPont Company. 1968. Report HLR 158-68 (as cited in Trochimowicz et al. 1994).
Eller, K., E. Henkes, R. Rossbacher, and H. Höke. 2000. Amines, aliphatic. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH [online]. Available: http://onlinelibrary.wiley.com/doi/10.1002/14356007.a02_001/abstract;jsessionid=7E07931380494CD0DB64275764D4B544.d01t01 [accessed Nov. 1, 2012].
EPA (U.S. Environmental Protection Agency). 1985. Chemical Profile: Piperidine (CAS Reg. No. 110-89-4). U.S. Environmental Protection Agency, Washington, DC.
EPA (U.S. Environmental Protection Agency). 2003. EPA’s Benchmark Dose Software, Version 1.3.2. U.S. Environmental Protection Agency: Washington, DC.
Garberg, P., E.L. Akerblom, and G. Bolcsfoldi. 1988. Evaluation of a genotoxicity test measuring DNA-strand breaks in mouse lymphoma cells by alkaline unwinding and hydroxyapatite elution. Mutat. Res. 203(3):155-176.
Gehring, P.J. 1983. Pyridine, homologues and derivatives. Pp. 1810-1812 in Encyclopedia of Occupational Health and Safety, Vol. 2, 3rd Ed., L. Parmeggiani, ed. Geneva, Switzerland: International Labour Organization.
Giacobini, E. 1976. Piperidine: A new neuromodulator or a hypnogenic substance? Adv. Biochem. Pyschopharmacol. 15:17-56.
Golovnya, R.V., I.L. Zhuravleva, and Y.P. Kapustin. 1979. Gas chromatographic analysis of volatile nitrogen bases of boiled beef as possible precursors of N-nitrosamines. Chem. Senses 4(2):97-105.
Green, N.R., and J.R. Savage. 1978. Screening of safrole, eugenol, their ninhydrin positive metabolites and selected secondary amines for potential mutagenicity. Mutat. Res. 57(2):115-121.
Howard, P.H., and W.M. Meylan, eds. 1997. P. 203 in Handbook of Physical Properties of Organic Chemicals. Boca Raton, FL: CRC Press.
HSDB (Hazard Substance Data Bank). 2008. Piperidine (CAS Reg. No. 110-89-4). TOXNET, Specialized Information Services. U.S. National Library of Medicine: Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen? HSDB [accessed Nov. 1, 2012].
Hughes, E.W., B.A. Homan, D.M. John, T.J. Kenny, D.W. Coombs, and C.J. Hardy. 1990. A Study of the Effect of Piperidine on Pregnancy of the Rats. Report No. BGH 9/9097. PE18 6ES. Huntingdon Research Centre, Ltd., Huntingdon, England.
Kase, Y., and T. Miyata. 1976. Neurobiology of piperidine: Its relevance to CNS function. Adv. Biochem. Pyschopharmacol. 15:5-16.
Lewis, R.J., Sr. 1993. P. 919 in Hawley’s Condensed Chemical Dictionary, 12th Ed. New York: Van Nostrand Reinhold Co.
Lijinsky, W., and H.W. Taylor. 1977. Feeding tests in rats on mixtures of nitrite with secondary and tertiary amines of environmental importance. Food Cosmet. Toxicol. 15(4):269-274.
Lin, J.K., J.J. Hwa, and Y.J. Lee. 1981. Chemical toxicants in Chinese foods: 4. The contents and biological significance of piperidine in black pepper, white pepper, red pepper and other species. Nat. Sci. Counc. Mon. 9(7):557-566.
Linch, A.L. 1965. Piperidine - A hazardous chemical. Am. Ind. Hyg. Assoc. J. 26(1):95-96.
Neurath, G.B., M. Dünger, F.G. Pein, D. Ambrosius, and O. Schreiber. 1977. Primary and secondary amines in the human environment. Food Cosmet. Toxicol. 15(4):275-282.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
Okano, Y., T. Miyata, S.H. Hung, T. Motoya, M. Kataoka, K. Takahama, and Y. Kasé. 1978. Metabolites of piperidine in rat urine. Jpn. J. Pharmacol. 28(1):41-47.
Perry, T.L., S. Hansen, and L.C. Jenkins. 1964. Amine content of normal human cerebrospinal fluid. J. Neurochem. 11(1):49-53.
Reed, R.L. 1990. Piperidine. Pp. 251-258 in Ethel Browning’s Toxicity and Metabolism of Industrial Solvents, Vol. II: Nitrogen and Phosphorus Solvents, 2nd Ed., D.R. Buhler, and D.J. Reed, eds. New York: Elsevier.
Riebe, M., K. Westphal, and P. Fortnagel. 1982. Mutagenicity testing, in bacterial test systems, of some constituents of tobacco. Mutat. Res. 101(1):39-43.
Royal Society. 1987. Piperidine. Pp. 207-210 in Chemical Safety Data Sheets (as cited in AIHA 1996).
RTECS (Registry of Toxic Effects of Chemical Substances). 2009. Piperidine. RTECS No. TM3500000 National Institute for Occupational Safety and Health [online]. Available: http://www.cdc.gov/niosh-rtecs/tm3567e0.html [accessed Nov. 1, 2012].
Smyth, H.F., Jr., C.P. Carpenter, C.S. Weil, U.C. Pozzani, and J.A. Striegel. 1962. Range-finding toxicity data: List VI. Am. Ind. Hyg. Assoc. J. 23:95-107.
Timofievskaya, L.A. 1979. Comparative evaluation of the toxicity of piperazine and N-methyl piperazine in Russian]. Toksikol. Nov. Prom. Khim. Veshchestv. 15:116-123.
Timofievskaya, L.A., and I.V. Silantyeva. 1975. Study of the effect of piperidine on embryogenesis [in Russian]. Toksikol. Nov. Prom. Khim. Veshchestv. 14:40-46.
Trochimowicz, H.J., G.L. Kennedy, Jr., and N.D. Krivanek. 1994. Heterocyclic and miscellaneous nitrogen compounds. Pp. 3285-3521 in Patty’s Industrial Hygiene and Toxicology, Vol. IIB, 4th Ed., G.D. Clayton, and F.E. Clayton, eds. New York: John Wiley & Sons.
Tricker, A.R., B. Pfundstein, T. Kaelble, and R. Preussmann. 1992. Secondary amine precursor in human saliva, gastric juice, blood, urine and faeces. Carcinogenesis 13(4):563-568.
van den Heuvel, M.J., D.G. Clark, R.J. Fielder, P.P. Koundakjian, G.J. Oliver, D. Pelling, N.J. Tomlinson, and A.P. Walker. 1990. The international validation of a fixed-dose procedure as an alternative to the classical LD50 test. Food. Chem. Toxicol. 28(7):469-482.
van Doorn, R., M. Ruijten, and T. van Harreveld. 2002. Guidance for the Application of Odor in 44 Chemical Emergency Responses, Version 2.1. August 29, 2002. Presented at the NAC/AEGL Meeting, September 2002.
von Euler, U.S. 1945. The occurrence and determination of piperidine in human and animal urine. Acta Pharmacol. Toxicol. 1(1):29-49.
Wangenheim, J., and G. Bolcsfoldi. 1988. Mouse lymphoma L5178Y thymidine kinase locus assay of 50 compounds. Mutagenesis 3(3):193-205.
Weast, R.C., M.J. Astle, and W.H. Beyer, eds. 1985. CRC Handbook of Chemistry and Physics, 66th Ed. Boca Raton: CRC Press.
Zayeva, G.N., L.A. Timofievskaya, K.P. Stasenkova, and L.A. Bazarova. 1968. Use of time/effect plots in toxicological experiments [in Russian]. Toksikol. Nov. Prom. Khim. Veshchestv. 10:5-9.
APPENDIX A
DERIVATION OF AEGL VALUES FOR PIPERIDINE
Derivation of AEGL-1 Values
| Key study: | BASF. 1993. Study on the Inhalation Toxicity of Piperidine as a Vapor in Rats: 28-day Test. Project No. 4610523-89065. BASF Aktiengesellschaft, Ludwigshafen/Rhein, Germany. |
| Toxicity end point: | No-effect level for nasal irritation (20 ppm for 6 h). |
| Time scaling: | Cn × t = k; n = 1.5 (derived by regression analysis of LC50 data for rats, guinea pigs, and mice). |
| Uncertainty factors: |
3 for interspecies differences because the effects are mediated by direct contact with nasal epithelium, which has similar cell composition among species but different cell distribution and nasal morphology; linear correlation for the concentration vs. time relationship for LC50 values for three species is -0.96 and the concentration-time relationships are similar, not varying by more than 30%, indicating that the response was similar among the three species
3 for intraspecies variability because the nasal epithelium does not vary among individuals in the population. |
| Calculations: | C = 20 ppm ÷ 10 (total uncertainty factor) = 2 ppm Cn × t = k; C = 2 ppm, t = 360 minutes, n = 1.5 k = (2 ppm)1.5 × 360 min = 1,018.2338 ppm-min |
| 10-min AEGL-1: | Set equal to the 30-min AGEL-1 values |
| 30-min AEGL-1: | C = (1,018.2338 ppm-min ÷ 30 min)1/1.5 C = 10 ppm |
| 1-h AEGL-1: | C = (1,018.2338 ppm-min ÷ 60 min)1/1.5 C = 6.6 ppm |
| 4-h AEGL-1: | C = (1,018.2338 ppm-min ÷ 240 min)1/1.5 C = 2.6 ppm |
| 8-h AEGL-1: | C = (1,018.2338 ppm-min ÷ 480 min)1/1.5 C = 1.7 ppm |
Derivation of AEGL-2 Values
| Key study: | BASF. 1990. Range-finding Study on the Inhalation Toxicity of Piperidine as Vapor in Rats: 5-day Study. Project No. 3010523-89017, BASF Aktiengesellschaft, Ludwigshafen/Rhein, Germany. |
| Toxicity end point: | Nasal irritation without eye closure or salivation (100 ppm for 6 h). |
| Time scaling: | Cn × t = k; n = 1.5 (derived by regression analysis of LC50 data for rats, guinea pigs, and mice). |
| Uncertainty factors: |
3 for interspecies differences because the effects are mediated by direct contact with nasal mucosa, which has similar cell composition among species but different cell distribution and nasal morphology; the data indicate only small variations in LC50 values for three different species
3 for intraspecies variability because the nasal epithelium does not vary among individuals in the population. |
| Calculations: | C = 100 ppm ÷ 10 (total uncertainty factor) = 10 ppm Cn × t = k; C = 10 ppm, t = 360 min, n = 1.5 k = 11,384.1996 ppm-min |
| 10-min AEGL-2: | Set equal to the 30-min AGEL-1 values |
| 30-min AEGL-2: | C = (11,384.1996 ppm-min ÷ 30 min)1/1.5 C = 50 ppm |
| 1-h AEGL-2: | C = (11,384.1996 ppm-min ÷ 60 min)1/1.5 C = 33 ppm |
| 4-h AEGL-2: | C = (11,384.1996 ppm-min ÷ 240 min)1/1.5 C = 13 ppm |
| 8-h AEGL-2: | C = (11,384.1996 ppm-min ÷ 480 min)1/1.5 C = 8.3 ppm |
Derivation of AEGL-3 Values
| Key study: | BASF. 1980. Determination of the Acute Inhalation Toxicity LC50 of Piperidine as Vapor in Sprague-Dawley Rats After a 4-Hour Exposure. BASF Gewerbehygiene and Toxikologie. |
| Toxicity end point: | LC01 (lethality threshold) of 448 ppm calculated by probit analysis. |
| Time scaling: | Cn × t = k; n = 1.5 (derived by regression analysis of LC50 data for rats, guinea pigs, and mice). |
| Uncertainty factors: |
3 for interspecies differences because the data showed only small variations in LC50 values for three species.
3 for intraspecies variability because a factor of 10 produces unusually low values that are not supported by available data. |
| Calculations: | C = 448 ppm ÷ 10 (total uncertainty factor) = 44.8 ppm Cn × t = k; C = 44.8 ppm, t = 240 min, n = 1.5 k = 71,966.1488 ppm-min |
| 10-min AEGL-3: | C = (71,966.1488 ppm-min ÷ 10 min)1/1.5 C = 370 ppm |
| 30-min AEGL-3: | C = (71,966.1488 ppm-min ÷ 30 min)1/1.5 C = 180 ppm |
| 1-h AEGL-3: | C = (71,966.1488 ppm-min ÷ 60 min)1/1.5 C = 110 ppm |
| 4-h AEGL-3: | C = (71,966.1488 ppm-min ÷ 240 min)1/1.5 C = 45 ppm |
| 8-h AEGL-3: | C = (71,966.1488 ppm-min ÷ 480 min)1/1.5 C = 28 ppm |
APPENDIX B
ACUTE EXPOSURE GUIDELINES FOR PIPERIDINE
Derivation Summary for Piperidine
AEGL-1 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 10 ppm (35 mg/m3\) | 10 ppm (35 mg/m3) | 6.6 ppm (32 mg/m3) | 2.6 ppm (9 mg/m3) | 1.7 ppm (6 mg/m3) |
| Reference: BASF. 1993. Study on the Inhalation Toxicity of Piperidine as a Vapor in Rats: 28-day Test. Project No. 4610523-89065. BASF Aktiengesellschaft, Ludwigshafen/Rhein, Germany. | ||||
| Test species/Strain/Number: Rat, Wistar, five of each sex | ||||
| Exposure route/Concentration/Durations: Inhalation; 0, 20, and 100 ppm; 6 h/day for 28 days | ||||
| Effects: Nasal irritation (red crusts on nasal edge) at 100 ppm starting on day 2; no effect at 20 ppm. | ||||
| End point/Concentration/Rationale: No-effect level for nasal irritation at 100 ppm for 6 h | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 10 Interspecies: 3, because the effects are mediated by direct contact with nasal epithelium, which has similar cell composition among species, although cell distribution and nasal morphology differ; the linear correlation coefficient for the concentration vs. time relationship for LC50 values for three species is -0.96 and the concentration-time relationships are similar, not varying by more than 30%, indicating the response is similar among the three species Intraspecies: 3, because the nasal epithelium does not vary among individuals in the population |
||||
| Modifying factor: None | ||||
| Animal-to-human dosimetric adjustment: None | ||||
| Time scaling: Cn × t = k; n = 1.5 based on regression analysis of LC50 values for the rat exposed for 4 h, the mouse exposed for 2 h, and the guinea pig exposed for 1 h. | ||||
| Confidence and support of AEGL values: Time scaling was based on LC50 values of three different species. The key study was a 28-day study in which the animals were observed daily for clinical signs. The exposure concentration from which the AEGL values were derived was a no-effect level for nasal irritation in a well-conducted study; concentrations ≤50 ppm of piperidine vapor caused exposure-related effects on the upper respiratory tract and eyes. The AEGL values are below the reported irritation threshold of 26 ppm. | ||||
AEGL-2 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 50 ppm (175 mg/m3) | 50 ppm (175 mg/m3) | 33 ppm (116 mg/m3) | 13 ppm (46 mg/m3) | 8.3 ppm (29 mg/m3) |
| Reference: BASF. 1990. Range-finding Study on the Inhalation Toxicity of Piperidine as Vapor in Rats: 5-day Study. Project No. 3010523-89017, BASF Aktiengesellschaft, Ludwigshafen/Rhein, Germany. | ||||
| Test species/Strain/Number: Rat, Wistar, five of each sex | ||||
| Exposure route/Concentration/Durations: Inhalation; 0, 50, 100, and 200 ppm; 6 h/day for 5 days | ||||
| Effects: Nasal irritation at all concentrations (severity increased with concentration and time); “stretched respiration posture,” eye closure, and salivation at 200 ppm. | ||||
| End point/Concentration/Rationale: 100 ppm for 6 h was the highest concentration at which nasal irritation (reddish crusts on the nasal edge) was observed without eye closure or salivation. Severity of nasal irritation in the rat increased with increasing exposure concentration, but there was no involvement of other regions of the respiratory tract. | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 10 Interspecies: 3, because the effects are mediated by direct contact with nasal epithelium, which has similar cellular composition among species, although cell distribution and morphology differ; the linear correlation coefficient for the concentration vs. time relationship for LC50 values for three species is -0.96 and the concentration-time relationships are similar, not varying by more than 30%, indicating the response is similar among the three species. Intraspecies: 3, because the nasal epithelium does not vary among individuals in the population. |
||||
| Modifying factor: None | ||||
| Animal-to-human dosimetric adjustment: None | ||||
| Time scaling: Cn × t = k; n = 1.5 based on regression analysis of LC50 values for the rat exposed for 4 h, the mouse exposed for 2 h, and the guinea pig exposed for 1 h. | ||||
| Confidence and support of AEGL values: Time scaling was based on LC50 values of three different species. The key study used for deriving AEGL-2 values was conducted according to standard protocol. The resulting AEGL-2 values for 10-60 min are greater than the reported irritation threshold for humans. Nasal irritation was the most sensitive end point in rats. The concentration of piperidine at which nasal irritation occurred and from which AEGL-2 values were derived caused no respiratory effects that extended beyond the nasal region and did not cause eye closure or salivation. The experimental concentration did not cause CNS toxicity. Therefore, the AEGL-2 values are well within the limits that would protect against long-term or irreversible effects of piperidine vapor. | ||||
AEGL-3 VALUES FOR PIPERIDINE
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 370 ppm (1,295 mg/m3) | 180 ppm (630 mg/m3) | 110 ppm (385 mg/m3) | 45 ppm (158 mg/m3) | 28 ppm (98 mg/m3) |
| Reference: BASF. 1980. Determination of the Acute Inhalation Toxicity LC50 of Piperidine as Vapor in Sprague-Dawley Rats After a 4-h Exposure. BASF Gewerbehygiene und Toxikologie. | ||||
| Test species/Strain/Number: Rats, Sprague-Dawley, 10 of each sex | ||||
| Exposure route/Concentration/Durations: Inhalation; 290, 810, 1,190, 1,540, and 2,190 ppm; 4 h (single exposure) | ||||
| Effects: 290 ppm: No deaths; nasal and ocular irritation. 810 ppm: One of 20 rats died; nasal and ocular irritation, corrosion around the nose (1 rat), and dyspnea. 1,190 ppm: 10 of 20 rats died; nasal and ocular irritation, corneal damage, corrosion around the nose, dyspnea, and CNS toxicity. 1,540 ppm: seven of 20 rats died; prostration and same effects noted at 1,190 ppm 2,190 ppm: 20 of 20 rats died; effects same as at 1,540 ppm |
||||
| End point/Concentration/Rationale: Lethality threshold (LC01) for piperidine is 448 ppm. That concentration is lower than the lowest concentration (810 ppm) where one of 20 rats died and had signs of dyspnea, which could be associated with death, and is greater than the highest concentration (290 ppm) that caused no deaths or clinical moribund signs. | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 10 Interspecies: 3, because the linear correlation coefficient for the concentration vs. time relationship for LC50 values for three species is -0.96 and the concentrationtime relationships are similar, not varying by more than 30%, indicating the response is similar among the three species. Intraspecies: 3, because a factor of 10 would produce AEGL values for the 4- and 8-h durations that are lower than the irritation threshold. |
||||
| Modifying factor: None | ||||
| Animal-to-human dosimetric adjustment: None | ||||
| Time scaling: Cn × t = k; n = 1.5 based on regression analysis of LC50 values for the rat exposed for 4 h, the mouse exposed for 2 h, and the guinea pig exposed for 1 h. | ||||
| Confidence and support of AEGL values: Time scaling was based on LC50 values of three different species. The acute inhalation study was conducted according to standard protocol and showed a reasonable concentration-response relationship for lethality and a clear concentration-response relationship for severity of clinical signs. The LC01 was a good approximation of the lethality threshold; therefore, the AEGL-3 values should be within the limits that would protect humans from lethal exposure to piperidine vapor. | ||||