Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
____________________
1This document was prepared by the AEGL Development Team composed of Cheryl B. Bast (Oak Ridge National Laboratory), Heather Carlson-Lynch (SRC, Inc.), Chemical Manager Roberta Grant (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances), and Ernest V. Falke (U.S. Environmental Protection Agency). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold concentrations for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Bromoacetone is a colorless liquid with a pungent odor. It is described as a dermal, ocular, and respiratory irritant. Bromoacetone was first used as a chemical weapon during World War I, and may currently be used in organic synthesis, although production data were not found. Bromoacetone is prepared by treating acetone with bromine and sodium chlorate. It occurs naturally in the essential oil of a seaweed species that grows in the ocean around the Hawaiian Islands (HSDB 2011).
AEGL-1 values for bromoacetone were based on a concentration of 0.1 ppm that caused ocular irritation in humans (Dow Chemical 1968). An intraspecies uncertainty factor of 3 was applied because contact irritation is a portal-of-entry effect and is not expected to vary widely between individuals. An interspecies uncertainty factor of 1 was applied because the study was conducted in humans. Time scaling was not performed, because the critical effect (ocular irritation) is a function of direct contact with the bromoacetone vapor and is unlikely to increase with duration of exposure (NRC 2001). However, because of the lack of human data on exposure to bromoacetone longer than a few seconds and because the point of departure was a nominal concentration, a modifying factor of 3 was applied.
When rat irritation data were used to derive AEGL-2 values for bromoacetone, it yielded values essentially identical to the AEGL-3 values calculated from lethality data. Thus, although the concentration-response relationship for bromoacetone is not particularly steep, the AEGL-3 values were divided by 3 to calculate AEGL-2 values.
AEGL-3 values were based on lethality studies in rats exposed to bromoacetone at concentrations of 1-131 ppm and for durations of 6-120 min (Dow Chemical 1968). The threshold for lethality at each AEGL-3 exposure duration was calculated using probit analysis, based on the dose-response program of ten Berge (2006) (see Appendix B). The threshold for lethality was set at LC01 (lethal concentration, 1% lethality). The LC01 was chosen over the BMCL05 (benchmark concentration, 95% lower confidence limit with 5% response) because values derived using the BMCL05 were less consistent with human data (2.5 ppm for 10 min, 0.94 ppm for 30 min, 0.44 ppm for 1 h, 0.089 ppm for 4 h, and 0.039 ppm for 8 h; and only ocular irritation was found in humans exposed at 0.1 and 1.0 ppm). A time-scaling value of 1.3 (C1.3 × t = k) was derived from the data. Interspecies and intraspecies uncertainty factors of 3 each were applied (total of 10), because bromoacetone is an irritant (causes lacrimation, nasal discharge, gasping, wheezing, and labored breathing in rats and ocular irritation in humans), and clinical signs are likely caused by a direct chemical effect on the tissues. This type of portal-of-entry effect is not likely to vary greatly between species or among individuals.
The AEGL values for bromoacetone are summarized in Table 2-1.
1. INTRODUCTION
Bromoacetone is a colorless liquid that rapidly turns violet, even in the absence of air. It has a pungent odor. It was first used as a chemical weapon during World War I, and was referred to as BA by the British and B-stoff (white cross) by the Germans. It might currently be used in organic synthesis, although production data were not found. It is prepared by treating acetone with bromine and sodium chlorate. Bromoacetone occurs naturally in the essential oil of a seaweed species that grows in the ocean around the Hawaiian Islands (HSDB 2011). Chemical and physical data for bromoacetone is provided in Table 2-2.
TABLE 2-1 AEGL Values for Bromoacetone
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| AEGL-1 (nondisabling) | 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) | Ocular irritation in humans (Dow Chemical 1968) |
| AEGL-2 (disabling) | 1.4 ppm (7.8 mg/m3) | 0.57 ppm (3.2 mg/m3) | 0.33 ppm (1.8 mg/m3) | 0.11 ppm (0.62 mg/m3) | 0.063 ppm (0.35 mg/m3) | One-third AEGL-3 values |
| AEGL-3 (lethality) | 4.1 ppm (23 mg/m3) | 1.7 ppm (9.5 mg/m3) | 0.98 ppm (5.5 mg/m3) | 0.32 ppm (1.8 mg/m3) | 0.19 ppm (1.1 mg/m3) | Threshold for lethality (LC01) in rats (Dow Chemical 1968) |
TABLE 2-2 Chemical and Physical Data for Bromoacetone
| Parameter | Value | Reference |
| Synonyms | Acetonyl bromide; acetylmethyl bromide; bromomethyl methyl ketone; 1-bromo-2-propanone; bromo-2-propanone; monobromoacetone | HSDB 2011 |
| CAS registry no. | 598-31-2 | HSDB 2011 |
| Chemical formula | C3H5BrO | HSDB 2011 |
| Molecular weight | 136.98 | HSDB 2011 |
| Physical state | Colorless liquid | HSDB 2011 |
| Melting point | -36.5°C | HSDB 2011 |
| Boiling point | 138°C | HSDB 2011 |
| Specific gravity | 1.634 (air = 1) at 23°C | HSDB 2011 |
| Relative vapor density | 4.75 (air = 1) | HSDB 2011 |
| Solubility in water | “Slightly” soluble; soluble in alcohol, acetone, and ether | HSDB 2011 |
| Vapor pressure | 9 mm Hg at 20°C | HSDB 2011 |
| Flash point | 51.1°C | IPCS 2005 |
| Conversion factors in air | 1 ppm = 5.6 mg/m3 1 mg/m3 = 0.18 ppm |
HSDB 2011 |
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
Human lethality data on bromoacetone were not located.
2.2. Nonlethal Toxicity
Six human volunteers (age and sex not specified) were self-exposed to bromoacetone at 0.1 ppm (nominal concentration) or 1.0 ppm (analytic concentration) to investigate acute irritative effects and odor (Dow Chemical 1968). Exposure duration was not report, but it appears to have been no more than a few seconds. The ocular irritation test was conducted by passing the prepared vapor sample from a Saran™ bag into modified chemical-worker goggles worn by the subject. The odor test was conducted by having the subjects sniff the gas from the exposure chamber or gas sampling bag. All six subjects reported considerable ocular irritation at 1.0 ppm, and two at 0.1 ppm. None of the subjects reported an objectionable odor at 1.0 ppm. No information on respiratory irritation or other respiratory effects was provided in the study report.
2.3. Case Reports
No case reports were found.
2.4. Developmental and Reproductive Effects
No information on the developmental or reproductive toxicity of bromoacetone in humans was available.
2.5. Genotoxicity
No information on the genotoxicity of bromoacetone in humans was available.
2.6. Carcinogenicity
No information on the carcinogenicity of bromoacetone in humans was available.
2.7. Summary
There is little human inhalation data on bromoacetone. Bromoacetone caused ocular irritation in two of six subjects at 0.1 ppm and in all six subjects at 1.0 ppm; however, no information on respiratory irritation or other respiratory effects was provided in the study report. No objectionable odor was reported at 1.0 ppm. No data on the developmental toxicity, reproductive toxicity, genotoxicity, or carcinogenicity of bromoacetone were available.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
3.1.1. Rats
Groups of five male rats (strain not specified) were exposed to bromoacetone at 17-131 ppm (analytic concentrations) for durations of 6-120 min, followed by a 14-day observation period (Dow Chemical 1968). Concentration-duration information is presented in Table 2-3. The tests were performed in a 160-L glass exposure chamber. Constant chamber airflow was maintained by means of a rotary air pump located at the exhaust side of the chamber; airflow was monitored with a rotameter. Bromoacetone was vaporized by aerosolizing a metered quantity of liquid with a positive pressure spray nozzle enclosed within a temperature-controlled round bottomed flask. Vapor laden air was then diluted with filtered room air, and passed through an aerosol trap before being drawn into the exposure chamber. Chamber concentrations were measured by gas chromatography.
During exposure, all animals showed severe ocular and nasal irritation, as evidenced by profuse lacrimation and salivation. Dyspnea followed these effects. After exposure ended, all animals exhibited mucoid nasal and oral secretions, respiratory wheezing, and severe dyspnea. Death occurred within hours and at up to 2 weeks, depending on exposure concentration and duration. Gross necropsies of animals dying during the observation period showed congested nasal passages and slight congestion and patchy hemorrhages in the lungs. The most notable observation was enormous distension of the stomach and intestines by gas, to the extent that pressure on the diaphragm may have interfered with respiratory function. No abnormalities were noted in the liver, kidneys, or other major organs. Necropsies of animals surviving the 14-day observation period showed only moderate focal pneumonia. Experimental parameters, clinical signs, and mortality incidence data are summarized in Table 2-3.
TABLE 2-3 Acute Inhalation Toxicity of Bromoacetone in Male Rats
| Concentration (ppm) | Duration (min) | Mortality | Day of Death | Effects During Exposure | Effects After Exposure |
| 17 | 12 | 0/5 | – | Lacrimation, nasal discharge, and labored breathing. | None. |
| 28 | 30 | 2/5 | 1 | Bloody nasal discharge and weight loss. | |
| 28 | 60 | 4/5 | 14 | Gasping, wheezing, bloody nasal discharge, and weight loss. | |
| 28 | 120 | 5/5 | 1 | Gasping, wheezing, and bloody nasal discharge. | |
| 48 | 60 | 5/5 | 12 | Same as above, except more rapid onset and more severe. | Wheezing and bloody nasal discharge. |
| 48 | 120 | 5/5 | 1 | Gasping, wheezing, and bloody nasal discharge. | |
| 51 | 6 | 0/5 | – | Weight loss | |
| 51 | 12 | 0/5 | – | None | |
| 51 | 30 | 3/5 | 2 | Wheezing, bloody nasal discharge, and weight loss. | |
| 131 | 10 | 5/5 | 11 | Same as above, except much faster onset and more severe. | Wheezing, bloody nasal discharge, and weight loss. |
| 131 | 30 | 5/5 | 3 | Gasping, wheezing, and bloody nasal discharge. | |
Source: Dow Chemical 1968.
3.1.2. Summary of Animal Lethality Data
Only one study on lethality from bromoacetone was available. That study demonstrated that bromoacetone is an irritant in rats, causing lacrimation, nasal discharge, gasping, wheezing, and labored breathing).
3.2. Nonlethal Toxicity
3.2.1. Rats
In a companion study to the acute lethality study described earlier, groups of four male rats (strain not specified) were exposed to bromoacetone at 0-18 ppm (analytic concentrations) for 15-87 min, followed by a 14-day observation period (Dow Chemical 1968). The experimental methodology was as described in Section 3.1.1, and concentration-duration information is presented in Table 2-4. The purpose of the study was to determine an irritation threshold in rats. Rats exposed to bromoacetone at 2 ppm showed definite signs of ocular irritation, and those exposed at 10 ppm had signs of respiratory tract irritation. Postexposure nasal discharge persisted only in rats exposed at 18 ppm. During the observation period, weight loss was reported in the 10 and 18 ppm groups; however, the magnitude of the weight loss was not reported. No treatment-related abnormalities were observed in any of the rats at necropsy. Experimental parameters, clinical signs, and mortality incidence data are summarized in Table 2-4.
TABLE 2-4 Acute Irritation of Bromoacetone in Male Rats
| Concentration (ppm) | Duration (min) | Mortality | Effects During Exposure | Time to Response (sec) | Effects After Exposure |
| 0 | 15 | 0/4 | None | – | None |
| 1.0 | 15 | 0/4 | Mild blinking (reported as +/-) | 101 | None |
| 2.0 | 20 | 0/4 | Blinking | 98 | None |
| 6.3 | 74 | 0/4 | Blinking | 56 | None |
| 10.0 | 43 | 0/4 | Blinking, lacrimation, and sneezing | 68 | Body weight loss |
| 18.0 | 87 | Not reported | Blinking, lacrimation, sneezing, and dyspnea. | 25 | Nasal discharge and body weight loss |
Source: Dow Chemical 1968.
3.2.2. Summary of Nonlethal Toxicity in Animals
Only one study on nonlethal effects from acute inhalation of bromoacetone was available (Dow Chemical 1968). Bromoacetone was an irritant in rats, causing blinking, lacrimation, sneezing, and shortness of breath.
3.3. Developmental and Reproductive Effects
No developmental or reproductive data on bromoacetone were found.
3.4. Genotoxicity
No genotoxicity data on bromoacetone were found.
3.5. Carcinogenicity
No carcinogenicity data on bromoacetone were found.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
No metabolism data on bromoacetone were available.
4.2. Mechanism of Toxicity
The few inhalation studies available on bromoacetone suggest that bromoacetone is an irritant. Two of six human subjects reported ocular irritation at 0.1 ppm, and all six had irritation at 1 ppm. Rats exhibited lacrimation, nasal discharge, gasping, wheezing, and labored breathing after being exposed to bromoacetone (Dow Chemical 1968). HSDB (2011 also describes bromoacetone as a dermal, ocular, and respiratory irritant.
4.3. Structure-Activity Relationships
Bromoacetone is structurally similar to chloroacetone, which is also an irritant. However, bromoacetone appears to be a more potent irritant than chloroacetone. Humans exposed to chloroacetone first experience lacrimation at approximately 5 ppm (Sargent et al. 1986), whereas ocular irritation from bromoacetone has been reported at lower concentrations of 0.1 and 1.0 ppm (Dow Chemical 1968).
A 1-h LC50 of 316 ppm was reported for chloroacetone in male rats, and no mortality occurred in rats exposed at 132 ppm for 1 h (Arts and Zwart 1987).
However, in studies with bromoacetone, death occurred in four of five male rats exposed at 28 ppm and in all five rats exposed at 48 ppm for 1 h (Dow Chemical 1968).
4.4. Species Variability
Data are insufficient to determine species variability for bromoacetone. Bromoacetone is an irritant and clinical signs are likely caused by a direct chemical effect on tissues. This type of portal-of-entry effect is not likely to vary greatly between species.
4.5. Temporal Extrapolation
The concentration-time relationship for many irritant and systemically-acting vapors and gases can be described by the relationship Cn × t = k, where the exponent, n, ranges from 0.8 to 3.5 (ten Berge et al. 1986). The value of n was determined by analyzing the available lethality data on bromoacetone in rats using the DoseResp software of ten Berge (2006). On the basis of the concentration-specific data presented in Tables 2-3 and 2-4, the analysis produced an exponent value of 1.3, with confidence limits of 0.80 and 1.7. Details of the analysis are provided in Appendix B.
5. DATA ANALYSIS FOR AEGL-1
5.1. Human Data Relevant to AEGL-1
Immediate ocular irritation was observed in six of six humans exposed to bromoacetone at 1.0 ppm, and in two of six humans exposed at 0.1 ppm (Dow Chemical 1968).
5.2. Animal Data Relevant to AEGL-1
Slight blinking was observed in rats exposed to bromoacetone at 1.0 ppm for 15 min (Dow Chemical 1968).
5.3. Derivation of AEGL-1 Values
A concentration of 0.1 ppm was selected as the point of departure for deriving AEGL-1 values for bromoacetone. That concentration caused ocular irritation in two of six humans (Dow Chemical 1968). An intraspecies uncertainty factor of 3 was applied because contact irritation is a portal-of-entry effect and is not expected to vary widely between individuals. An interspecies uncertainty factor of 1 was applied because the study was conducted in humans. Time scal-
ing was performed, because ocular irritation is a function of direct contact with the bromoacetone vapor and is unlikely to increase with duration of exposure (NRC 2001). However, because of the lack of human data on exposures longer than a few seconds and because the point of departure is a nominal concentration, a modifying factor of 3 was applied. AEGL-1 values for bromoacetone are presented in Table 2-5, and their derivation presented in Appendix A.
6. DATA ANALYSIS FOR AEGL-2
6.1. Human Data Relevant to AEGL-2
No human data relevant to derivation of AEGL-2 values were found.
6.2. Animal Data Relevant to AEGL-2
Blinking, lacrimation, sneezing, and body weight loss were observed in rats exposed to bromoacetone at 10 ppm for 43 min (Dow Chemical 1968).
6.3. Derivation of AEGL-2 Values
Although the concentration-response relationship for bromoacetone is not particularly steep, the AEGL-3 values were divided by 3 to calculate AEGL-2 values for bromoacetone. This approach was used instead of basing the values on rat data, because when rat irritation data were used to calculate AEGL-2 values, they were essentially identical to the AEGL-3 values based on lethality data. (AEGL-2 values derived with a point of departure of 10 ppm for 43 min, with interspecies and intraspecies uncertainty factors of 3 each, and with a time-scaling exponent of 1.3 [see Section 4.5] resulted in the following values: 3.2 ppm for 10 min, 1.3 ppm for 30 min, 0.77 ppm for 1 h, 0.26 ppm for 4 h, and 0.15 ppm for 8 h.)
AEGL-2 values for bromoacetone are presented in Table 2-6, and calculations are presented in Appendix A.
7. DATA ANALYSIS FOR AEGL-3
7.1. Human Data Relevant to AEGL-3
No human data relevant to derivation of AEGL-3 values were found.
TABLE 2-5 AEGL-1 Values for Bromoacetone
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) |
TABLE 2-6 AEGL-2 Values for Bromoacetone
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 1.4 ppm (7.8 mg/m3) | 0.57 ppm (3.2 mg/m3) | 0.33 ppm (1.8 mg/m3) | 0.11 ppm (0.62 mg/m3) | 0.063 ppm (0.35 mg/m3) |
7.2. Animal Data Relevant to AEGL-3
Animal lethality data were available for rats exposed to varying concentrations of bromoacetone for different time periods (Dow Chemical 1968). Exposure durations ranged from 6 to 120 min and concentrations ranged from 1.0 to 131 ppm. Mortality incidences ranged from 0 to 100%, depending on concentration-duration pairings. The experimental parameters were summarized earlier in Tables 2-3 and 2-4.
7.3. Derivation of AEGL-3 Values
Using the data from the studies by Dow Chemical (1968) presented in Tables 2-3 and 2-4, the lethality threshold at each AEGL-3 exposure duration was calculated using the probit analysis based on the dose-response program of ten Berge (2006) (see Appendix B). The lethality threshold was set at the LC01 (lethal concentration, 1% lethality). The LC01 was chosen over the BMCL05 (benchmark concentration, 95% lower confidence limit with 5% response) because values derived with the BMCL05 were less consistent with human data (2.5 ppm for 10 min, 0.94 ppm for 30 min, 0.44 ppm for 1 h, 0.089 ppm for 4 h, and 0.039 ppm for 8 h; and only ocular irritation was noted in humans at 0.1 and 1.0 ppm). A time-scaling value of 1.3 (C1.3 × t = k) was determined from the data. These calculated values were used as the basis for the AEGL-3 values.
Interspecies and intraspecies uncertainty factors of 3 each were applied (total of 10) and were considered sufficient because bromoacetone is an irritant, and clinical signs are likely caused by a direct chemical effect on the tissues. This type of portal-of-entry effect is not likely to vary greatly between species or among individuals.
The resulting AEGL-3 values are shown in Table 2-7, and their derivation summarized in Appendix A.
8. SUMMARY OF AEGLs
8.1. AEGL Values and Toxicity End Points
AEGL values for bromoacetone are summarized in Table 2-8. AEGL-1 values are based on ocular irritation in humans, and AEGL-3 values are based on a lethality threshold (LC01) in rats. AEGL-2 values were derived by dividing the AEGL-3 values by 3.
TABLE 2-7 AEGL-3 Values for Bromoacetone
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 4.1 ppm (23 mg/m3) | 1.7 ppm (9.5 mg/m3) | 0.98 ppm (5.5 mg/m3) | 0.32 ppm (1.8 mg/m3) | 0.19 ppm (1.1 mg/m3) |
TABLE 2-8 AEGL Values for Bromoacetone
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h |
| AEGL-1 (nondisabling) | 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) |
| AEGL-2 (disabling) | 1.4 ppm (7.8 mg/m3) | 0.57 ppm (3.2 mg/m3) | 0.33 ppm (1.8 mg/m3) | 0.11 ppm (0.62 mg/m3) | 0.063 ppm (0.35 mg/m3) |
| AEGL-3 (lethality) | 4.1 ppm (23 mg/m3) | 1.7 ppm (9.5 mg/m3) | 0.98 ppm (5.5 mg/m3) | 0.32 ppm (1.8 mg/m3) | 0.19 ppm (1.1 mg/m3) |
8.2. Comparisons with Other Standards and Guidelines
There are no other standards or guidelines for bromoacetone.
8.3. Data Adequacy and Research Needs
Additional acute animal studies in species other than rats would be helpful.
9. REFERENCES
Arts, J.H.E., and A. Zwart. 1987. Acute (One-h) Inhalation Toxicity Study of Chloroacetone in Rats. TNO Report No. V87.093/261236. Civo Institutes, Zeist, The Netherlands. EPA Document No. 88870000029. Microfiche No. OTS0513466.
Dow Chemical. 1968. Inhalation Exposure Toxicity of Bromoacetone and a Fumigant Mixture Containing Bromoacetone with Cover Letter Dated 041086. EPA Document No. 86860000027. Microfiche No. OTS0510179.
HSDB (Hazardous Substances Data Bank). 2011. Bromoacetone (CAS Reg. No. 598-31-2). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Sept. 10, 2012].
IPCS (International Programme on Chemical Safety). 2005. Bromoacetone (CAS Reg. No. 598-31-2). International Chemical Safety Card No. 1074. International Programme on Chemical Safety, Commission of the European Communities, Brussel, Belgium [online]. Available: http://www.inchem.org/documents/icsc/icsc/eics1074.htm [accessed Sept. 10, 2012].
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
Sargent, E.V., G.D. Kirk and M. Hite. 1986. Hazard evaluation of monochloroacetone. Am. Ind. Hyg. Assoc. J. 47(7):375-378.
ten Berge. W.F. 2006. Concentration-time Response in Acute Inhalation Toxicity, Online Excel Program. Santoxar, The Netherlands [online]. Available: http://home.wxs.nl/~wtberge/doseresp.html [accessed Sept. 10, 2012].
ten Berge, W.F., A. Zwart, and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Mater. 13(3):301-309.
APPENDIX A
DERIVATION OF AEGL VALUES FOR BROMOACETONE
Derivation of AEGL-1 Values
| Key study: | Dow Chemical. 1968. Inhalation Exposure Toxicity of Bromoacetone and a Fumigant Mixture Containing Bromoacetone with Cover Letter Dated 041086. EPA Document No. 86860000027. Microfiche No. OTS0510179. |
| Critical effect: | Ocular irritation in two of six humans at 0.1 ppm |
| Time scaling: | None applied. The critical effect (ocular irritation) is a function of direct contact with the bromoacetone vapors and unlikely to increase with duration of exposure (NRC 2001). |
| Uncertainty factors: | 1 for interspecies differences 3 for intraspecies variability; contact irritation is a portal-of-entry effect and is not expected to vary widely between individuals. Total uncertainty factor of 3 |
| Modifying factor: | 3 because of the lack of human data on exposure durations longer than a few seconds and because the point of departure is a nominal concentration. |
| Calculations: | |
| 10- and 30-min, 1-, 4-, and 8-h AEGL-1: | 0.1 ppm ÷ 3 ÷ 3 = 0.011 ppm |
Derivation of AEGL-2 Values for Bromoacetone
AEGL-2 values were derived by taking one-third of the respective AEGL-3 values, even though there were data relevant to AEGL-2 values and the
concentration-response relationship for bromoacetone is not particularly steep. However, when rat irritation data was used the point of departure, AEGL-2 values were essentially identical to AEGL-3 values calculated from lethality data (see below).
Calculations based on one-third of the AEGL-3 values:
| 10-min AEGL-2: | 4.1 ppm (10-min AEGL-3) ÷ 3 = 1.4 ppm |
| 30-min AEGL-2: | 1.7 ppm (30-min AEGL-3) ÷ 3 = 0.57 ppm |
| 1-h AEGL-2: | 0.98 ppm (1-h AEGL-3) ÷ 3 = 0.33 ppm |
| 4-h AEGL-2: | 0.32 ppm (4-h AEGL-3) ÷ 3 = 0.11 ppm |
| 8-h AEGL-2: | 0.19 ppm (8-h AEGL-3) ÷ 3 = 0.063 ppm |
| Calculations based on relevant animal data: | |
| Key study: | Dow Chemical. 1968. Inhalation Exposure Toxicity of Bromoacetone and a Fumigant Mixture Containing Bromoacetone with Cover Letter Dated 041086. EPA Document No. 86860000027. Microfiche No. OTS0510179. |
| Toxicity end point: | Irritation in rats at 10 ppm for 43 min (0.717 h) |
| Time scaling: | Cn × t = k where n = 1.3 C1.3 × t = k (10 ppm)1.3 × 0.717 h = k k = 14.3 ppm-h |
| Uncertainty factors | 3 for interspecies differences 3 for intraspecies variability: 3 Total uncertainty factor of 10 |
| 10-min AEGL-2: | C1.3 × 0.167 h = 14.3 ppm-h C1.3 = 85.6 ppm C = 30.6 ppm 30.6 ÷ 10 = 3.1 ppm |
| 30-min AEGL-2: | C1.3 × 0.5 h = 14.3 ppm-h C1.3 = 28.6 ppm C = 13.2 ppm 13.2 ÷ 10 = 1.3 ppm |
| 1-h AEGL-2: | C1.3 × 1 h = 14.3 ppm-h C1.3 = 14.3 ppm C = 7.7 ppm 7.7 ÷ 10 = 0.77 ppm |
| 4-h AEGL-2: | C1.3 × 4 h = 14.3 ppm-h C1.3 = 3.57 ppm C = 2.66 ppm 2.66 ÷ 10 = 0.27 ppm |
| 8-h AEGL-2: | C1.3 × 8 h = 14.3 ppm-h C1.3 = 1.79 ppm C = 1.56 ppm 1.56 ÷ 10 = 0.16 ppm |
Derivation of AEGL-3 Values for Bromoacetone
| Key study: | Dow Chemical. 1968. Inhalation Exposure Toxicity of Bromoacetone and a Fumigant Mixture Containing Bromoacetone with Cover Letter Dated 041086. EPA Document No. 86860000027. Microfiche No. OTS0510179. |
| Toxicity end point: | Threshold for lethality in rats (L01) calculated using probit-analysis dose-response program of ten Berge (2006). LC01 point estimates obtained for 10 and 30 min, and 1, 4, and 8 h. |
| Time scaling: | Cn × t = k where n = 1.3 based on rat lethality data (see Appendix B for time-scaling calculations) |
| Uncertainty factors: | 3 for interspecies differences; considered sufficient because bromoacetone is an irritant (lacrimation, nasal discharge, gasping, wheezing, and labored breathing in rats and ocular irritation in humans) and |
|
clinical signs are likely caused by a direct chemical effect on the tissues. This type of portal-of-entry effect is not likely to vary greatly between species. 3 for intraspecies variability; considered sufficient because bromoacetone is an irritant and clinical signs are likely caused by a direct chemical effect on the tissues. This type of portal-of-entry effect is not likely to vary greatly among individuals. Total uncertainty factor of 10 |
Data for Calculations
| Concentration (ppm) | Exposure duration (min) | Mortality incidence |
| 28 | 120 | 5/5 |
| 28 | 60 | 4/5 |
| 28 | 30 | 2/5 |
| 17 | 12 | 0/5 |
| 48 | 120 | 5/5 |
| 48 | 60 | 5/5 |
| 51 | 30 | 3/5 |
| 51 | 12 | 0/5 |
| 51 | 6 | 0/5 |
| 131 | 30 | 5/5 |
| 131 | 10 | 5/5 |
| 1.0 | 15 | 0/4 |
| 2.0 | 20 | 0/4 |
| 6.3 | 74 | 0/4 |
| 10.0 | 43 | 0/4 |
ten Berge (2006) Program Output
| Exposure Duration | LC01 point estimate (ppm) |
| 10 min | 40.69 |
| 30 min | 16.97 |
| 1 h | 9.773 |
| 4 h | 3.241 |
| 8 h | 1.867 |
| n = 1.3 | |
APPENDIX B
Time-Scaling Calculations for Bromoacetone
An n of 1.3 was obtained after analysis of lethality data in rats (Dow Chemical 1968) using the software of ten Berge (2006). This exposure-time relationship for lethality was considered appropriate for AEGL-3 development but because bromoacetone-induced ocular irritation is the result of direct-contact irritation. No time scaling was used in the development of AEGL-1 values. AEGL-2 values were derived by taking one-third of the AEGL-3 values, so no time scaling was necessary.
| Used Probit Equation Y = B0 + B1*X1 + B2*X2 X1 = Concentration (ppm), ln-transformed X2 = Min, ln-transformed |
|
| Chi-square Degrees of freedom Probability Model |
= 4.50 = 12 = 9.73E-01 |
| Ln(Likelihood) | = -5.61 |
| B 0 = -1.3833E+01 B 1 = 2.9799E+00 B 2 = 2.3722E+00 |
Student t = -2.8141 Student t = 3.6728 Student t = 3.6582 |
| Variance B 0 Covariance B 0 Covariance B 0 Variance B 1 Covariance B 1 Variance B 2 |
0 = 2.4161E+01 1 = -3.8264E+00 2 = -2.9135E+00 1 = 6.5827E-01 2 = 4.0467E-01 2 = 4.2051E-01 |
| Estimation ratio between regression coefficients of ln(concentration) and ln(min) Point estimate = 1.256 Lower limit (95% CL) = 0.800 Upper limit (95% CL) = 1.713 |
|
| Estimation of concentration (ppm) at response of 1% Min = 10 Point estimate concentration (ppm) = 4.069E+01 for response of 1% Lower limit (95% CL) concentration (ppm) = 1.623E+01 for response of 1% Upper limit (95% CL) concentration (ppm) = 5.881E+01 for response of 1% |
|
Filename: Bromoacetone Dow Chemical Rat for Log Probit Model
Date: 08 September 2008 Time: 11:14:28
| Sequence Number | Concentration (ppm) | Min | Sex | Exposed | Responded |
| 1 | 28 | 120 | 1 | 5 | 5 |
| 2 | 28 | 60 | 1 | 5 | 4 |
| 3 | 28 | 30 | 1 | 5 | 2 |
| 4 | 17 | 12 | 1 | 5 | 0 |
| 5 | 48 | 120 | 1 | 5 | 5 |
| 6 | 48 | 60 | 1 | 5 | 5 |
| 7 | 51 | 30 | 1 | 5 | 3 |
| 8 | 51 | 12 | 1 | 5 | 0 |
| 9 | 51 | 6 | 1 | 5 | 0 |
| 10 | 131 | 30 | 1 | 5 | 5 |
| 11 | 131 | 10 | 1 | 5 | 5 |
| 12 | 10 | 43 | 1 | 4 | 0 |
| 13 | 6 | 74 | 1 | 4 | 0 |
| 14 | 2 | 20 | 1 | 4 | 0 |
| 15 | 1 | 15 | 1 | 4 | 0 |
| Observations 1 through 15 considered | |||||
| 1 | 28 | 120 | 5 | 5 | |
| 2 | 28 | 60 | 5 | 4 | |
| 3 | 28 | 30 | 5 | 2 | |
| 4 | 17 | 12 | 5 | 0 | |
| 5 | 48 | 120 | 5 | 5 | |
| 6 | 48 | 60 | 5 | 5 | |
| 7 | 51 | 30 | 5 | 3 | |
| 8 | 51 | 12 | 5 | 0 | |
| 9 | 51 | 6 | 5 | 0 | |
| 10 | 131 | 30 | 5 | 5 | |
| 11 | 131 | 10 | 5 | 5 | |
| 12 | 10 | 43 | 4 | 0 | |
| 13 | 6 | 74 | 4 | 0 | |
| 14 | 2 | 20 | 4 | 0 | |
| 15 | 1 | 15 | 4 | 0 | |
Estimation of concentration (ppm) at response of 1%
Min = 30
Point estimate concentration (ppm) = 1.697E+01 for response of 1%
Lower limit (95% CL) concentration (ppm) = 5.890E+00 for response of 1%
Upper limit (95% CL) concentration (ppm) = 2.405E+01 for response of 1%
Estimation of concentration (ppm) at response of 1%
Min = 60
Point estimate concentration (ppm) = 9.773E+00 for response of 1%
Lower limit (95% CL) concentration (ppm) = 2.829E+00 for response of 1%
Upper limit (95% CL) concentration (ppm) = 1.503E+01 for response of 1%
Estimation of concentration (ppm) at response of 1%
Min = 120
Point estimate concentration (ppm) = 5.628E+00 for response of 1%
Lower limit (95% CL) concentration (ppm) = 1.303E+00 for response of 1%
Upper limit (95% CL) concentration (ppm) = 9.794E+00 for response of 1%
Estimation of concentration (ppm) at response of 1%
Min = 240
Point estimate concentration (ppm) = 3.241E+00 for response of 1%
Lower limit (95% CL) concentration (ppm) = 5.857E-01 for response of 1%
Upper limit (95% CL) concentration (ppm) = 6.537E+00 for response of 1%
Estimation of concentration (ppm) at response of 1%
Min = 480
Point estimate concentration (ppm) = 1.867E+00 for response of 1%
Lower limit (95% CL) concentration (ppm) = 2.597E-01 for response of 1%
Upper limit (95% CL) concentration (ppm) = 4.424E+00 for response of 1%
APPENDIX C
ACUTE EXPOSURE GUIDELINE LEVESL FOR BROMOACETONE
Derivation Summary
AEGL-1 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) | 0.011 ppm (0.062 mg/m3) |
| Reference: Dow Chemical. 1968. Inhalation Exposure Toxicity of Bromoacetone and a Fumigant Mixture Containing Bromoacetone with Cover Letter Dated 041086. EPA Document No. 86860000027. Microfiche No. OTS0510179. | ||||
| Test Species/Strain/Number: Human subjects (age and sex not specified); six subjects | ||||
| Exposure route/Concentrations/Durations: Vapor exposure at 0.1 or 1 ppm; duration not reported, but appears to be seconds. | ||||
| Effects: Ocular irritation in two of six subjects at 0.1 ppm; considerable ocular irritation in all six subjects at 1 ppm. No objectionable odor reported. | ||||
| End point/Concentration/Rationale: Ocular irritation at 0.1 ppm in two of six subjects. | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 3 Interspecies: 1, because human data were used. Intraspecies: 3, because contact irritation is a portal-of-entry effect and is not expected to vary widely between individuals. |
||||
| Modifying factor: 3, because of the lack of human data on exposures longer than a few seconds and because the point of departure is a nominal concentration. | ||||
| Animal-to-human dosimetric adjustment: None | ||||
| Time scaling: none | ||||
| Data adequacy: Sparse data set; modifying factor necessary. | ||||
AEGL-2 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 1.4 ppm (7.8 mg/m3) | 0.57 ppm (3.2 mg/m3) | 0.33 ppm (1.8 mg/m3) | 0.11 ppm (0.62 mg/m3) | 0.063 ppm (0.35 mg/m3) |
| End point/Concentration/Rationale: Values were calculated as one-third the AEGL-3 values, because AEGL-2 values derived from relevant rat data were essentially identical to the AEGL-3 values calculated from lethality data. | ||||
| Data adequacy: Sparse data set for AEGL-2 effects. AEGL-2 values derived using clinical signs in rats exposed at 10 ppm for 43 min, with interspecies and intraspecies uncertainty factors of 3 each, and with a time-scaling exponent of 1.3 [see Section 4.5] would have resulted in the following values: 3.2 ppm for 10 min, 1.3 ppm for 30 min, 0.77 ppm for 1 h, 0.26 ppm for 4 h, and 0.15 ppm for 8 h. AEGL-2 values based on one-third of the AEGL-3 values are considered protective. | ||||
AEGL-3 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 4.1 ppm (23 mg/m3) | 1.7 ppm (9.5 mg/m3) | 0.98 ppm (5.5 mg/m3) | 0.32 ppm (1.8 mg/m3) | 0.19 ppm (1.1 mg/m3) |
| Reference: Dow Chemical, 1968. Inhalation Exposure Toxicity of Bromoacetone and a Fumigant Mixture Containing Bromoacetone with Cover Letter dated 041086. EPA Document No. 86860000027. Microfiche No. OTS0510179. | ||||
| Test species/Strain/Sex/Number: Rat, strain not specified, male, 4-5 per group | ||||
| Exposure route/Concentrations/Durations: Inhalation, 1.0-131 ppm for 6-120 min | ||||
| Effects: Lethality | ||||
| Concentration (ppm) | Duration (min) | Mortality |
| 28 | 120 | 5/5 |
| 28 | 60 | 4/5 |
| 28 | 30 | 2/5 |
| 17 | 12 | 0/5 |
| 48 | 120 | 5/5 |
| 48 | 60 | 5/5 |
| 51 | 30 | 3/5 |
| 51 | 12 | 0/5 |
| 51 | 6 | 0/5 |
| 131 | 30 | 5/5 |
| 131 | 10 | 5/5 |
| 1 | 15 | 0/4 |
| 2 | 20 | 0/4 |
| 6.3 | 74 | 0/4 |
| 10 | 43 | 0/4 |
| End point/Concentration/Rationale: Threshold for lethality in rats (L01) calculated using probit-analysis dose-response program of ten Berge (2006). The LC01 was chosen over the BMCL05 because values derived with the BMCL05 were less consistent with human data (2.5 ppm for 10 min, 0.94 ppm for 30 min, 0.44 ppm for 1 h, 0.089 ppm for 4 h, and 0.039 ppm for 8 h; and only ocular irritation was noted in humans at 0.1 and 1.0 ppm). | ||
| Uncertainty factors/Rationale Total uncertainty factor: 10 Interspecies: 3, considered sufficient because bromoacetone is an irritant and clinical signs are likely caused by a direct chemical effect on the tissues. This type of portal-of-entry effect is not likely to vary greatly between species. Intraspecies: 3, considered sufficient because bromoacetone is an irritant and clinical signs are likely caused by a direct chemical effect on the tissues. This type of portal-of-entry effect is not likely to vary greatly among individuals. |
||
| Modifying factor: Not applicable | ||
| Animal-to-human dosimetric adjustment: Not applicable | ||
| Time scaling: Cn × t = k, where n = 1.3 as determined by analysis of rat lethality data using ten Berge (2006) software. | ||
| Data adequacy: Sparse data set. One well-conducted study in rats with effects relevant to AEGL-3 values. | ||
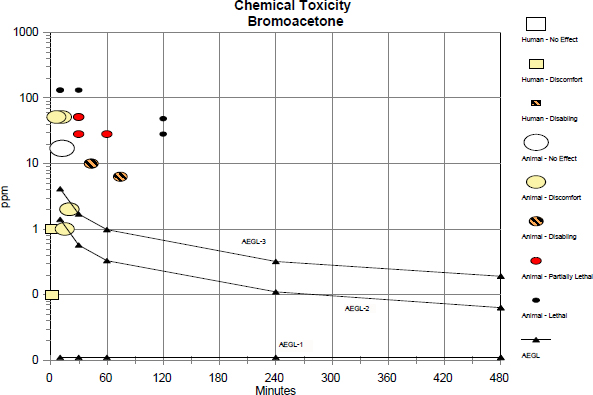
TABLE C-1 Data Used in the Category Graph
| Source | Species | Sex | No. of Exposures | ppm | Min | Category | Comments |
| AEGL-1 | 0.011 | 10 | AEGL | ||||
| AEGL-1 | 0.011 | 30 | AEGL | ||||
| AEGL-1 | 0.011 | 60 | AEGL | ||||
| AEGL-1 | 0.011 | 240 | AEGL | ||||
| AEGL-1 | 0.011 | 480 | AEGL | ||||
| AEGL-2 | 1.4 | 10 | AEGL | ||||
| AEGL-2 | 0.57 | 30 | AEGL | ||||
| AEGL-2 | 0.33 | 60 | AEGL | ||||
| AEGL-2 | 0.11 | 240 | AEGL | ||||
| AEGL-2 | 0.063 | 480 | AEGL | ||||
| AEGL-3 | 4.1 | 10 | AEGL | ||||
| AEGL-3 | 1.7 | 30 | AEGL | ||||
| AEGL-3 | 0.98 | 60 | AEGL | ||||
| AEGL-3 | 0.32 | 240 | AEGL | ||||
| AEGL-3 | 0.19 | 480 | AEGL | ||||
| Rat | Male | 1 | 28 | 120 | 3 | 5/5 mortality; lacrimation, gasping, wheezing, and nasal discharge. | |
| Rat | Male | 1 | 28 | 60 | PL | 4/5 mortality; lacrimation, gasping, wheezing, nasal discharge, and body weight loss. |
| Rat | Male | 1 | 28 | 30 | PL | 2/5 mortality; lacrimation, gasping, wheezing, nasal discharge, and body weight loss. |
| Rat | Male | 1 | 17 | 12 | 0 | No effects. |
| Rat | Male | 1 | 48 | 120 | 3 | 5/5 mortality; lacrimation, gasping, wheezing, and nasal discharge. |
| Rat | Male | 1 | 48 | 60 | 5 | 5/5 mortality; lacrimation, wheezing, and nasal discharge. |
| Rat | Male | 1 | 51 | 30 | PL | 3/5 mortality; lacrimation, wheezing, nasal discharge, and body weight loss. |
| Rat | Male | 1 | 51 | 12 | 1 | Lacrimation and nasal discharge. |
| Rat | Male | 1 | 51 | 6 | 1 | Lacrimation and nasal discharge. |
| Rat | Male | 1 | 131 | 30 | 3 | 5/5 mortality; lacrimation, gasping, wheezing, and nasal discharge. |
| Rat | Male | 1 | 131 | 10 | 3 | 5/5 mortality; lacrimation, gasping, wheezing, nasal discharge, and body weight loss. |
| Rat | Male | 1 | 1 | 15 | 1 | Mild blinking. |
| Source | Species | Sex | No. of Exposures | ppm | Min | Category | Comments |
| Rat | Male | 1 | 2 | 20 | 1 | Blinking. | |
| Rat | Male | 1 | 6.3 | 74 | 2 | Blinking, lacrimation, sneezing, and body weight loss. | |
| Rat | Male | 1 | 10 | 43 | 2 | Blinking, lacrimation, sneezing, dyspnea, and body weight loss. | |
| Human | 1 | 0.1 | 1 | 1 | 2/6 ocular irritation; estimated duration. | ||
| Human | 1 | 1 | 1 | 1 | 6/6 considerable ocular irritation; estimated duration. | ||
Categories: 0 = no effect; 1 = discomfort; 2 = disabling; PL = partially lethal; 3 = lethal.