Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory
____________________
1This document was prepared by the AEGL Development Team composed of Cheryl Bast (Oak Ridge National Laboratory), Julie Klotzbach (SRC, Inc.), Chemical Manager Susan Ripple (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances), and Ernest V. Falke (U.S. Environmental Protection Agency). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold concentrations for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Chloroacetone is produced by the direct chlorination of acetone. It also has been manufactured by reacting chlorine with diketene followed by boiling with water. It is used in the manufacture of couplers for color photography, as a photosensitizer for polyester-vinyl polymerization, as a fungicide and bactericide, and as an intermediate in the production of perfumes, antioxidants, and pharmaceuticals (Sargent et al. 1986). Chloroacetone has a pungent, suffocating odor similar to hydrogen chloride. It is toxic by inhalation, ingestion, and dermal contact, and causes immediate lacrimation at low concentrations. Other effects from exposure to chloroacetone include contact burns of the skin and eyes, nausea, bronchospasm, delayed pulmonary edema, and death.
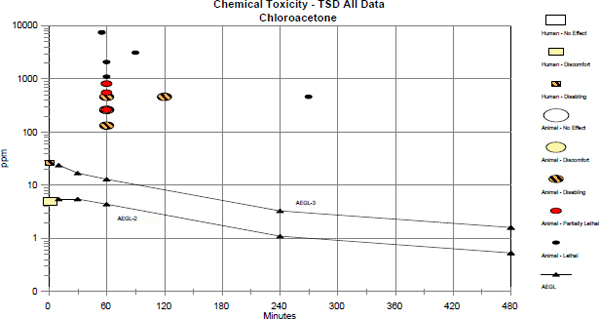
Data were insufficient for deriving AEGL-1 and AEGL-2 values for chloroacetone. The available data on acute toxicity suggest that chloroacetone has a steep dose-response relationship. Therefore, the AEGL-2 values were calculated by taking a three-fold reduction in the corresponding AEGL-3 values; those values are considered estimates of a threshold for irreversible effects.
A 1-h BMCL05 (benchmark concentration, 95% lower confidence limit with 5% response) of 131 ppm in the male rat was used as the basis of the AEGL-3 values (Arts and Zwart 1987). Interspecies and intraspecies uncertainty factors of 3 each were applied, because the preponderance of the data suggests that the effects of inhaled chloroacetone are likely caused by a direct chemical
effect on the tissues; this type of port-of-entry effect does not exhibit toxicokinetic variability and, thus, is not expected to vary greatly between species or among individuals. The interspecies uncertainty factor of 3 also is supported by data suggesting little species variability in lethality from oral and dermal exposure to chloroacetone (rat oral LD50 values: 100-141 mg/kg; mouse oral LD50 values: 127-141 mg/kg; rabbit dermal LD50 = 141 mg/kg), and the 1-h LC50 of 500 ppm for male and female rats (Arts and Zwart 1987) is approximately a dose of 114 mg/kg, which corresponds to the oral LD50 values (assuming 100% retention, 245 mL minute volume, and a rat body weight of 250 g). The intraspecies uncertainty factor of 3 also is considered sufficient because data from male rats, which are more sensitive than female rats, were used as the point-of-departure. Thus, the total uncertainty factor is 10. It has been shown that the concentration-exposure time relationship for many irritant and systemically acting vapors and gases may be described by the equation Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). Data were unavailable for an empirical derivation of n for chloroacetone, so default values were applied (NRC 2001). An n of 3 was applied to extrapolate to the 10- and 30-min AEGL durations, and an n of 1 was applied to extrapolate to the 4- and 8-h durations (NRC 2001). The calculated values are presented Table 3-1.
1. INTRODUCTION
Chloroacetone is a colorless to amber liquid at ambient temperature and pressure. It has a pungent, suffocating odor similar to hydrogen chloride (Sargent et al. 1986). It is toxic by inhalation, ingestion, and dermal contact, and causes immediate lacrimation at low concentrations. Other effects from exposure to chloroacetone include contact burns of the skin and eyes, nausea, bronchospasm, delayed pulmonary edema, and death (HSDB 2011).
TABLE 3-1 Summary of AEGL Values for Cloroacetone
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| AEGL-1 (nondisabling) | NRa | NRa | NRa | NRa | NRa | Insufficient data |
| AEGL-2 (disabling) | 8.0 ppm (30 mg/m3) | 5.5 ppm (21 mg/m3) | 4.4 ppm (17 mg/m3) | 1.1 ppm (4.2 mg/m3) | 0.53 ppm (2.0 mg/m3) | One-third of AEGL-3 values |
| AEGL-3 (lethal) | 24 ppm (91 mg/m3) | 17 ppm (65 mg/m3) | 13 ppm (49 mg/m3) | 3.3 ppm (13 mg/m3) | 1.6 ppm (6.1 mg/m3) | Estimated lethality threshold for male rats (BMD05) (Arts and Zwart 1987) |
aNot recommended. Absence of an AEGL-1 value does not imply that exposure below the AEGL-2 value is without adverse effects.
Chloroacetone is produced by the direct chlorination of acetone. It has also been manufactured by reacting chlorine with diketene followed by boiling with water (Sargent et al. 1986). In 1914, the French introduced chloroacetone as a war gas in hand and rifle grenades. It is now used in the manufacture of couplers for color photography, as a photosensitizer for polyester-vinyl polymerization, as a fungicide and bactericide, and as an intermediate in the production of perfumes, antioxidants, and pharmaceuticals (Sargent et al. 1986). Production is listed for only one manufacturer in the United States and four manufacturers worldwide (HSDB 2011). In 1977, U.S. production was reported to be at least 4.54 × 107 g, and U.S. imports were at least 4.54 × 105 g. In 1982, U.S. production was reported to be greater than 4.54 × 106 g (HSDB 2011).
The chemical structure of chloroacetone is depicted below, and the physicochemical properties of chloroacetone are presented in Table 3-2.
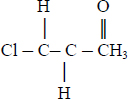
TABLE 3-2 Physical and Chemical Properties for Chloroacetone
| Parameter | Data | Reference |
| Common name | Chloroacetone | IPCS 2006 |
| Synonyms | 1-Chloro-2-propanone; chloropropanone; acetonyl chloride; monochloroacetone | IPCS 2006 |
| CAS registry no. | 78-95-5 | IPCS 2006 |
| Chemical formula | ClCH2COCH3 | IPCS 2006 |
| Molecular weight | 92.5 | IPCS 2006 |
| Physical state | Colorless liquid (turns dark on exposure to light) | IPCS 2006 |
| Melting point | -45°C | IPCS 2006 |
| Boiling point | 120°C | IPCS 2006 |
| Specific gravity | 1.123 (25°C) | HSDB 2011 |
| Relative Vapor density | 3.2 (air = 1) | IPCS 2006 |
| Solubility | Soluble in water; miscible with alcohol, ether, and chloroform | HSDB 2011 |
| Vapor pressure | 12.0 mm Hg (25°C) | HSDB 2011 |
| Flash point | 40°C (open cup) | OSHA 2012 |
| Octanol/water partition coefficient (log Pow) | 0.28 | IPCS 2006 |
| Conversion factors in air | 1 ppm = 3.8 mg/m3 | |
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
Chloroacetone at a concentration of 605 ppm was reported to be lethal to humans after 10 min (Prentiss 1937). No further details were available.
2.2. Nonlethal Toxicity
Prentiss (1937) reported that chloroacetone was extremely effective as a war gas unless a full-face gas mask was deployed quickly; a concentration of 26 ppm was reportedly intolerable after 1 min of exposure. No further details were provided.
Sargent et al. (1986) provided the only information on human exposure to chloroacetone. The authors reported that employee occupational health monitoring in 1981-1986 indicated that 25 employees reported to “Health Services” as a result of exposure to chloroacetone. Of these, eight had ocular irritation, seven had localized dermal irritation, one had contact dermatitis, and the remaining nine showed no clinical signs.
Sargent et al. (1986) also reported a case of direct exposure of one employee to hot chloroacetone as a result of a line break. The line break resulted in the release of chloroacetone vapors and hot liquid under pressure with combined inhalation and dermal exposure. The employee was hospitalized. Effects included immediate lacrimation and ocular irritation, upper-respiratory-tract irritation, and dermal irritation, producing slight erythema. Erythema subsided, but the exposed skin began to blister and the eyelids reddened and swelled and became painful to touch 8-h after exposure. After 24 h, the skin areas had completely blistered, were swollen, and were painful to touch, suggesting that some major dermal effects are delayed. All effects resolved within 7 days, and there was no evidence of pulmonary edema at the low concentration, despite the initial upper-respiratory-tract irritation. No additional information to quantify exposure for this worker was available from the investigators.
The Sargent et al. (1986) report included a summary table in which a chloroacetone concentration of 4.7 ppm was associated with lacrimation and burning sensation of the skin. However, the study authors did not provide information regarding the basis for that value (e.g., method of sampling or analysis, exposure duration, number of exposed individuals, number of affected individuals). An odor threshold for chloroacetone was not found.
2.3. Developmental and Reproductive Toxicity
Developmental and reproductive studies regarding acute human exposure to chloroacetone were not available.
2.4. Genotoxicity
Genotoxicity studies on acute human exposure to chloroacetone were not available.
2.5. Carcinogenicity
Carcinogenicity studies on human exposure to chloroacetone were not available.
2.6. Summary
There are few human studies of the toxicity of chloroacetone. The chemical is highly irritating and causes ocular, upper-respiratory tract, and dermal irritation. Immediate lacrimation has been reported at a concentration of approximately 5 ppm. Chloroacetone was reportedly intolerable at 26 ppm after 1 min, and lethal after 10 min of exposure at 605 ppm. No reports on developmental and reproductive toxicity, genotoxicity, or carcinogenicity of chloroacetone in humans were available.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
3.1.1. Rats
Groups of five male and five female SPF (Bor:WISW) rats were exposed to chloroacetone at 132, 263, 553, 816, 1,105, or 2,079 ppm (analytic concentrations) for 1 h, followed by a 14-day observation period (Arts and Zwart 1987). Animals were exposed in a horizontally placed glass tube that allowed observation of all animals during exposure. The volume of the exposure chamber was 0.015 m3, and air flow was 1.2 m3/h; relative humidity and temperature were measured at least once per hour. The test atmosphere was generated by delivering appropriate quantities of chloroacetone to an evaporator at the inlet port of the chamber, and the concentration of chloroacetone was determined by vapor phase infrared spectrometry and calibrated in a closed-loop system. Exposure concentration was calculated as the mean of recorded concentrations during the entire exposure period. Rats were observed during exposure and daily during the observation period for clinical signs. Body weight was recorded before exposure and on days 1, 2, 4, 7, and 14. Surviving rats were killed at the end of the observation period and subjected to gross necropsy. “Shortly” after the start of exposure, restlessness, rubbing of snouts, closed eyes, and humped posture were observed. Salivation, wet nares, and nasal discharge was observed within 3-5 min; these effects were noted “especially in those animals exposed to higher concentrations.” The skin of the extremities became
red during the second half of the exposure period in rats exposed at the higher concentrations. In the “highest concentration groups,” all rats showed labored respiration, accompanied by dyspnea and mouth breathing. Treatment-related mortality occurred shortly after exposure, usually within hours, or within 1-2 days after exposure. Mortality was greater in males than in females. The rats that died within the first two days of the observation period had pulmonary edema, accompanied by hydrothorax. The stomachs of these rats were filled with air, and some also had air in the cecum and intestine. Grey, discolored lungs was the only effect noted in animals necropsied at the end of the observation period. Animals surviving the study showed no treatment-related effect on body weight gain. One-hour LC50 values of 500 ppm (95% confidence interval [CI]: 421-579 ppm; males and females combined), 316 ppm (95% CI: 289-342 ppm; male rats), and 710 ppm (95% CI: 658-753 ppm; female rats) were calculated. One-hour BMC01 (benchmark concentration, 1% response) values of 170 ppm (males and females combined), 223 ppm (males), and 394 ppm (females) were calculated. One-hour BMCL05 (benchmark concentration, 95% lower confidence limit with 5% response) values of 144 ppm (males and females combined), 131 (males), and 258 ppm (females) were calculated. Mortality data are summarized in Table 3-3.
TABLE 3-3 Mortality in Rats Exposed to Chloroacetone for One Hour
| Concentration (ppm) | Males | Females | Males and Females | |
| Observed | ||||
| 132 | 0/5 | 0/5 | 0/10 | |
| 263 | 1/5 | 0/5 | 1/10 | |
| 553 | 5/5 | 1/5 | 6/10 | |
| 816 | 5/5 | 3/5 | 8/10 | |
| 1,105 | 5/5 | 5/5 | 10/10 | |
| 2,079 | 5/5 | 5/5 | 10/10 | |
| Calculated | ||||
| 316 (289-342) | LC50 | — | — | |
| 500 (421-579) | — | — | LC50 | |
| 710 (658-753) | — | LC50 | — | |
| 170 | BMC01 | |||
| 223 | BMC01 | — | — | |
| 394 | — | BMC01 | — | |
| 131 | BMCL05 | — | — | |
| 144 | — | — | BMCL05 | |
| 258 | — | BMCL05 | — | |
Abbreviations: BMC01, benchmark concentration, 5% response; BMCL01, benchmark concentration, 95% lower confidence limit with 5% response; LC50, lethal concentration, 50% lethality.
Source: Arts and Zwart 1987.
Eastman Kodak (1992) conducted a series of experiments each using groups of three male rats (strain not specified). Rats exposed to chloroacetone at 462 ppm exhibited nasal irritation, gasping, and pink extremities within 1 h, rough coat after 2 h, and all were dead after 4.5 h. Another group exposed at 3,120 ppm exhibited nasal irritation, gasping, and pink extremities in 0.5 h, and all were dead after 1.5 h. Finally, groups of rats was sequentially exposed to chloroacetone at 52 and 105 ppm for 6 h at each concentration. At 52 ppm, pink extremities were noted in 2.25 h, but no rats died. Nasal irritation, gasping, and pink extremities were noted after 2.5 h at 105 ppm, and all rats died within 24 h of the initiation of exposure. The authors estimated a 6-h LC50 of 50-100 ppm. No other experimental details were provided.
Sargent et al. (1986) exposed a group of five male and five female Sprague-Dawley rats to chloroacetone at 7,522 ppm for up to 1 h. A vapor-laden stream of chloroacetone was produced by bubbling air through the test material at a flow rate of 4 L/min. Lacrimation and excessive salivation were observed immediately, and all rats died within 55 min. No other experimental details were provided.
In another experiment, Sargent et al. (1986) exposed groups of five male and five female Sprague-Dawley rats to chloroacetone at 95, 204, 254, 302, or 874 ppm (nominal concentrations) for 1 h, followed by a 14-day observation period. An LC50 of 262 ppm was calculated. No other experimental details were provided.
Groups of five male and five female Wistar rats were administered chloroacetone at 0, 50, 71, 100, 140, or 200 mg/kg by gavage in corn oil, followed by a 2-day and 14-day observation period (Sargent et al. 1986). Clinical signs observed in all treatment groups included ataxia, red nasal discharge, urinary and fecal staining of the abdomen, decreased activity, and piloerection. An oral LD50 of 100 mg/kg was determined with the 14-day observation period, and an oral LD50 of 113 mg/kg was determined with the 2-day observation period.
Eastman Kodak (1992) reported an oral LD50 of 141 mg/kg for male rats. Clinical signs included rough coat, diarrhea, ataxia, and prostration. No other experimental details were provided.
3.1.2. Mice
Groups of five female CF1S mice were administered chloroacetone at 0, 50, 71, 100, 140, or 200 mg/kg by gavage in corn oil, followed by a 14-day observation period (Sargent et al. 1986). Clinical signs included ataxia, lethargy, prostration, piloerection, and a generally unhealthy appearance. An oral LD50 of 127 mg/kg was determined.
Eastman Kodak (1992) reported an oral LD50 of 141 mg/kg for male mice. Clinical signs included rough coat, diarrhea, ataxia, and prostration. No other experimental details were provided.
3.1.3. Rabbits
In an acute dermal toxicity study, four New Zealand white rabbits were administered chloroacetone at 50, 100, 200, or 400 mg/kg and were observed for 14 days (Sargent et al. 1986). The test substance was applied to the clipped skin, covered with impervious plastic sheeting, and allowed to remain in contact with the skin for 24 h. The rabbits were fitted with collars to prevent ingestion of the chloroacetone. In the high-dose groups, signs of toxicity presented within 24 h and included ataxia, clear oral discharge, general unhealthy appearance, soft stools, and decreased activity. Moderate to severe erythema and edema were observed in all treatment groups, and eschar formation and necrosis were observed in all surviving animals during the second week of the study. An acute dermal LD50 of 141 mg/kg was calculated.
3.1.4. Guinea Pigs
Eastman Kodak (1992) reported that the dermal LD50 of chloroacetone in guinea pigs is “probably between 0.1 and 1.0 mL/kg.” No other information was provided.
3.2. Nonlethal Toxicity
No acute toxicity studies of nonlethal effect of chloroacetone in animals were found.
3.3. Repeated-Exposure Studies
Eastman Kodak (1992) conducted a series of experiments each using one rat (strain and sex not specified). The rats were repeatedly exposed to chloroacetone by inhalation for up to 11 exposures. No additional experimental details were reported. Data are summarized in Table 3-4.
Groups of five male rats (strain not specified) were administered chloroacetone at 0, 10, 50, or 100 mg/kg by gavage, 5 days/week for up to 13 days (Eastman Kodak 1992). One rat in the 100-mg/kg group died after three doses, and the other four were sacrificed on day four because of poor condition. Food intake and body weight gain were “severely depressed” at 100 mg/kg, and food intake was “moderately depressed” and weight gain “severely depressed” 50 mg/kg. At 10 mg/kg, food consumption and body weight gain were “moderately depressed.” Clinical signs in the 50-mg/kg group included salivation, slight hyperactivity, pale eyes, and dark urine. No clinical signs were noted in the 10-mg/kg group. Gross necropsy of high-dose animals revealed necrotizing gastritis, fluid in the thoracic and abdominal cavities, adhesions
TABLE 3-4 Effects of Chloroacetone on Rats Repeatedly Exposed to Chloroacetone
| Average Concentration (ppm) | No. of exposures | No. of rats | Observations | |
| 20 | 11 | 1 | Pink extremities, gasping, nasal irritation, rough hair, body weight loss, survived | |
| 22 | 9 | 1 | Pink extremities, gasping, nasal irritation, rough hair, body weight loss | |
| 25 | 11 | 1 | Pink extremities, gasping, nasal irritation, rough hair, body weight loss, survived | |
| 39 | 4 | 1 | Pink extremities, gasping, nasal irritation, rough hair, body weight loss, death | |
| 58 | 2 | 1 | Pink extremities, gasping, nasal irritation, death in 2 days | |
Source: Eastman Kodak 1992.
between abdominal viscera, pale livers, and small seminal vesicles. In the 50-mg/kg group, one rat had adhesions between the stomach and diaphragm and thickening of the nonglandular mucosa of the stomach; another rat in this group had a raised firm red area on the visceral surface of the stomach. No gross abnormalities were noted in the 10-mg/kg group. Histopathologic observations in the 100-mg/kg rats included gastric necrosis, ulceration, and perforation. Necrosis of the liver, spleen, adrenal glands, and testes were considered secondary to severe gastric irritation and subsequent peritonitis. Moderate to severe cortical atrophy of the thymus was noted in all rats of the 100-mg/kg group. In the 50-mg/kg group, one rat had necrosis of the nonglandular stomach mucosa, one had necrosis of the glandular stomach mucosa, one had ulceration of the nonglandular stomach, and all had hyperkeratosis of the esophageal mucosa. Three rats in the 10-mg/kg group had minor hyperkeratosis of the gastric nonglandular stomach.
3.4. Developmental and Reproductive Toxicity
Developmental and reproductive toxicity studies of animal exposure to chloroacetone were not available.
3.5. Genotoxicity
Chloroacetone at concentrations of 100-2,000 nmole/plate did not induce mutation in Salmonella typhimurium strains TA1535 or TA100, with or without exogenous metabolic activation (Merrick et al. 1987). Negative results were also obtained in S. typhimurium strains TA1535, TA1537, TA98, TA100, and hisG46 at concentrations of 1,500-3,000 μg/plate, with and without metabolic activation (Sargent et al. 1986). Chloroacetone was negative in the SOS chromotest at
concentrations of 0.01-3,000 μg/mL without S9 activation and at 0.1-100 μg/mL with activation, and was also negative for clastogenicity in a newt micronucleus test at 0.1-0.4 μg/mL (Le Curieux et al. 1994). Chloroacetone was positive in an Ames-fluctuation test with S. typhimurium strain TA100 at concentrations of 1-30 μg/mL with metabolic activation, but was negative without activation (Le Curieux et al. 1994). Chloroacetone increased the frequency of sex-linked recessive lethals in Drosophila melanogaster exposed via inhalation (Lee et al. 1983).
3.6. Carcinogenicity
Robinson et al. (1989) gave 40 female SENCAR mice dermal treatments of chloroacetone at 0 or 50 mg/kg in 0.2 mL ethanol six times over a 2-week period or oral doses of chloroacetone at 0 or 50 mg/kg three times over a 2-week period (total dermal dose was 300 mg/kg; total oral dose was 150 mg/kg). Two-weeks after the final doses, 1.0 μg TPA (12-O-tetradecanoly-phorbol-13-acetate) in 0.2 mL acetone was applied to the skin three times per week for 20 weeks. No evidence of increased tumor incidence was found in animals treated with chloroacetone by either route compared with controls.
In another study, groups of 10 male and 10 female outbred stock albino mice were given dermal treatments of chloroacetone (0.2 mL of a 0.3% chloroacetone solution in acetone) twice a week for 12 weeks (Searle 1966). Mice were then given dermal treatments of croton oil (0.2 mL, 0.5% in acetone) twice a week for 20 weeks; surviving mice were killed after another 20 weeks without treatment. Controls were treated with acetone followed by croton oil. There was no treatment-related effect on mortality; however, a greater number of papillomas wasfound in treated mice than in controls during subsequent croton oil treatment. The overall tumor incidences appeared to be similar between treated and control groups (cumulative incidence was not reported, and statistical analysis was not performed), but the total number of tumors was higher in treated mice compared with controls, and males developed more tumors than females. Total numbers of tumors observed at 40 weeks were as follows: three for male controls, seven for female controls, 29 for chloroacetone-treated males, and 16 for chloroacetone-treated females. Both the incidences and the total number of tumors were lower at 40 weeks than at 30 weeks, suggesting that some of the tumors regressed; the authors reported that there were no malignant tumors in chloroacetone-treated mice.
3.7. Summary
Animal toxicity data are limited to acute lethality studies in rats, mice, and rabbits, and repeated-exposure studies in rats. The data suggest that male rats are approximately 2.3 times more sensitive than female rats to the effects of chloroacetone administered by inhalation. Oral lethality data suggest that mice
and rats have similar sensitivities. Oral and dermal LD50 values show little variability with regard to species and route of exposure. Clinical signs included restlessness, labored breathing, nasal irritation, salivation, lacrimation, dyspnea, and pulmonary edema at necropsy. No developmental or reproductive data were available. Genotoxicity results were equivocal; findings were negative for reverse mutation in five S. typhimurium strains, negative in SOS chromotest, and negative for clastogenicity, but were positive in an Ames fluctuation test and a sex-linked recessive lethal assay. Searle (1966) showed that chloroacetone increased the numbers of skin tumors, but not the skin tumor incidence, in male and female mice given dermal applications of chloroacetone twice per week for 12 weeks and subsequently administered croton oil. However, there was no increase in tumor incidence in female mice administered chloroacetone orally or dermally over two weeks, followed by TPA administration (Robinson et al. 1989). The inconsistent results might be due to differences in mouse strain, vehicle, dose, or length of exposure. In summary, the carcinogenic potential of chloroacetone cannot be evaluated with the available data.
Selected acute toxicity data are summarized in Table 3-5.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism
No in vivo metabolism information was found; however, in vitro data suggest that chloroacetone reacts directly with and depletes glutathione (GSH) (Merrick et al. 1987). The purpose of the in vitro study was to determine whether chloroacetone was directly cytotoxic as a result of chemical reactivity with sulfhydryl nucleophiles and to compare the toxicity of single and multiple chlorinated propanones. When equimolar (10 mM) concentrations of GSH and 1,3-dichloropropanone (1,3-DCP), 1,1-dichloropropopanone (1,1-DCP), or chloroacetone (monochloropropanone) were allowed to react in phosphate buffer, concentrations of GSH decreased within 1 min, reaching less than 15% of initial values within 20 min. The greatest decrease occurred with 1,3-DCP, followed by chloroacetone, and then 1,1-DCP. There was a concentration-related increase in aspartate transaminase (AST) enzyme leakage after 1 h, when primary hepatocyte cultures from male Sprague-Dawley rats were incubated with 1,3-DCP, 1,1-DCP, or chloroacetone. The greatest leakage occurred with 1,3-DCP, followed by chloroacetone, and then 1,1-DCP. AST release from the hepatocytes was associated with reduction of GSH, with relative order being the same.
4.2. Mechanism of Toxicity
No information regarding the mechanism of toxicity of chloroacetone was found. Symptoms of acute inhalation poisoning with chloroacetone suggest that
TABLE 3-5 Summary of Selected Acute Toxicity Data on Chloroacetone in Animals
| Species | Concentration or Dose | Exposure Duration | Effect | Reference |
| Inhalation | ||||
| Rat | 52 ppm | 6 h | Pink extremities after 2.25 h; no mortality (0/3) | Eastman Kodak 1992 |
| Rat | 50-100 ppm | 6 h | Estimated LC50 | Eastman Kodak 1992 |
| Rat | 105 ppm (same rats exposed to 52 ppm above) | 6 h | Nasal irritation, gasping, and pink extremities within 2.5 h; 100% mortality within 24 h | Eastman Kodak 1992 |
| Rat | 132 ppm | 1 h | No mortality (0/10); restlessness, rubbing of snouts, lacrimation, salivation, and humped posture | Arts and Zwart 1987 |
| Rat | 262 ppm | 1 h | LC50 (male and female combined) | Sargent et al. 1986 |
| Rat | 316 ppm | 1 h | LC50 (male) | Arts and Zwart 1987 |
| Rat | 462 ppm | 4.5 h | Nasal irritation, gasping, and pink extremities after 1 h; rough coat after 2 h; 100% mortality (3/3 males) at 4.5 h | Eastman Kodak 1992 |
| Rat | 500 ppm | 1 h | LC50 (male and female combined) | Arts and Zwart 1987 |
| Rat | 710 ppm | 1 h | LC50 (female) | Arts and Zwart 1987 |
| Rat | 1,105 ppm | 1 h | 100% mortality | Arts and Zwart 1987 |
| Rat | 3,120 ppm | 1.5 h | Nasal irritation, gasping, and pink extremities after 0.5 h; 100% mortality (3/3 males) at 1.5 h | Eastman Kodak 1992 |
| Rat | 7,522 ppm | 1 h | Immediate lacrimation and salivation; 100% mortality (10/10) | Sargent et al. 1986 |
| Oral | ||||
| Rat | 100 mg/kg | Single gavage | LD50 (male and female) | Sargent et al. 1986 |
| Rat | 141 mg/kg | Single gavage | LD50 (male) | Eastman Kodak, 1992 |
| Mouse | 127 mg/kg | Single gavage | LD50 (female) | Sargent et al. 1986 |
| Mouse | 141 mg/kg | Single gavage | LD50 (male) | Eastman Kodak, 1992 |
| Dermal | ||||
| Rabbit | 141 mg/kg | 24 h | LD50 | Sargent et al. 1986 |
it acts as an irritant, causing immediate lacrimation at low concentrations and contact burns to the skin and eyes, nausea, bronchospasm, delayed pulmonary edema, and death at higher concentrations (HSDB 2011). In a study of repeated oral exposure to chloroacetone (Eastman Kodak 1992), the primary effects were from irritation, and included gastric necrosis, ulceration, and perforation. However, sex differences in the response to inhaled chloroacetone have been observed (e.g., rat LC50 values in males are lower than in females). Such differences are not consistent with a mode of action of direct-acting irritation, which would be unlikely to vary significantly within or across species.
Some information from oral and dermal lethality studies suggests the possibility that chloroacetone might exert systemic effects. Ataxia and lethargy were noted in rats and mice exposed via gavage to chloroacetone (Sargent et al. 1986). Rabbits exposed topically to lethal concentrations of chloroacetone (under conditions designed to limit or prevent oral and inhalation exposure) exhibited clinical signs of toxicity, including ataxia, hypoactivity, clear oral discharge, and soft stools (Sargent et al. 1986). Whether these clinical signs are indicative of systemic absorption and toxicity of chloroacetone or occur as a consequence of profound irritation and injury at the site of exposure is uncertain. In summary, while there is suggestive evidence for systemic effects after oral and dermal exposure, the preponderance of the available information suggests that the primary effects of chloroacetone inhalation are due to direct-acting irritation.
4.3. Concurrent Exposure Issues
No information was found.
4.4. Structure-Activity Relationships
Structure-activity data were only available from the in vitro metabolism study described in Section 4.1 and genotoxicity data. Merrick et al. (1987) found that 1,3-dichloropropanone was highly mutagenic in S. typhimurium, 1,1-dichloropropanone was a weaker mutagen, and chloroacetone was not mutagenic with or without metabolic activation. Le Curieux et al. (1994) found that chloropropanones with chlorine substituents on both carbon positions (1,3-dichloropropanone and 1,1,3-trichloropropanone) were more genotoxic than chloropropanones with substituents on only one carbon position (1,1-dichloropropanone and 1,1,1-trichloropropanone), which were, in turn, more genotoxic than chloroacetone.
4.5. Species Differences
The few available studies suggest that mice and rats have similar sensitivities to orally administered chloroacetone with regard to lethality. Oral
and dermal LD50 values show little variability with regard to species and route of exposure (see Table 3-5). For example, oral LD50 values range from 100 to 141 mg/kg for rats and from 127 to 141 mg/kg in mice, and a dermal LD50 of 141 mg/kg was reported for rabbits. Furthermore, the 1-h LC50 of 500 ppm for male and female rats (Arts and Zwart 1987) gives an approximate dose of 114 mg/kg, which corresponds to the oral LD50 values (assuming 100% retention, 245 mL minute volume, and a rat body weight of 250 g).
4.6. Concentration-Exposure Duration Relationship
The concentration-time relationship for many irritant and systemically-acting vapors and gases may be described by the equation Cn × t = k, where the exponent, n, ranges from 0.8 to 3.5 (ten Berge et al. 1986). Data were inadequate for deriving an empirically-derived chemical-specific scaling exponent for chloroacetone. Available toxicity data for chloroacetone are limited to 1-h exposures (calculated LC50 values) and 6-h exposures (0/3 deaths at 52 ppm and 3/3 deaths at the 105 ppm). Thus, data from different exposure durations were not adequate for use in plotting and calculating a value for n. However, the available data suggest that exposure duration may alter the lethal concentration of chloroacetone; specifically, the 1-h LC50 in male and female rats (combined) was estimated to be in the range of 262-500 ppm (Sargent et al. 1986; Arts and Zwart 1987) whereas an estimate of the 6-h rat LC50 is 50-100 ppm (Eastman Kodak 1992). To obtain conservative and protective AEGL values in the absence of an empirically-derived chemical-specific scaling exponent, temporal scaling was be performed using the default values of n = 3 when extrapolating to shorter time points and n = 1 when extrapolating to longer time points.
5. DATA ANALYSIS FOR AEGL-1
5.1. Human Data Relevant to AEGL-1
The study by Sargent et al. (1986) provides the only information on human experience with chloroacetone. A concentration of 4.7 ppm was associated with lacrimation and a burning sensation of the skin, but no further information (e.g., ambient or personal monitoring, method of analysis, exposure duration, number of individuals affected, number of exposed individuals) was provided to support this association. This information is not considered adequate for the purpose of deriving AEGL-1 values.
5.2. Animal Data Relevant to AEGL-1
No animal data consistent with the definition of AEGL-1 were available.
5.3. Derivation of AEGL-1
The available data on chloroacetone are insufficient, so AEGL-1 values are not recommended.
6. DATA ANALYSIS FOR AEGL-2
6.1. Human Data Relevant to AEGL-2
No adequate human data consistent with the definition of AEGL-2 were available. Immediate lacrimation and ocular and upper respiratory irritation were noted in a worker accidentally exposed to chloroacetone vapors and hot liquid for an undetermined duration at an unspecified concentration; the exposure included inhalation and dermal components (Sargent et al. 1986).
6.2. Animal Data Relevant to AEGL-2
Restlessness, rubbing of snouts, lacrimation, salivation, and humped posture were noted in male and female rats exposed to chloroacetone at 132 ppm for 1 h (Arts and Zwart 1987).
6.3. Derivation of AEGL-2
The only data consistent with the definition of AEGL-2 are the clinical signs observed in rats exposed to chloroacetone at 132-2,079 ppm for 1 h (Arts and Zwart 1987). Chloroacetone exhibited a steep dose-response relationship. In that study, 132 ppm was the only concentration causing no mortality and is greater than the concentration used as the point-of-departure for AEGL-3 values (BMCL05 of 131 ppm; see below). Due to the steep dose-response relationship and limitations in the available data, the AEGL-2 values for chloroacetone were determined by a taking 3-fold reduction in the AEGL-3 values (see below); this was considered an estimate of a threshold for irreversible effects (NRC 2001). AEGL-2 values for chloroacetone are presented in Table 3-6, and the calculations for these AEGL-2 values are presented in Appendix A.
7. DATA ANALYSIS FOR AEGL-3
7.1. Human Data Relevant to AEGL-3
No human data consistent with the definition of AEGL-3 were available.
7.2. Animal Data Relevant to AEGL-3
There are few animal studies with data consistent with the definition of AEGL-3. One-hour LC50 values of 500 ppm (95% CI: 421-579 ppm for male
and female rats combined), 316 ppm (95% CI: 289-342 ppm for male rats), and 710 ppm (95% CI: 658-753 ppm for female rats) were calculated. One-hour BMC01 values of 170 ppm (males and females combined), 223 ppm (males only), and 394 ppm (females only) were calculated. One-hour BMCL05 values of 144 ppm (males and females combined), 131 ppm (males only), and 258 ppm (females only) also were calculated (Arts and Zwart 1987).
7.3. Derivation of AEGL-3
The BMCL05 of 131 ppm (Arts and Zwart 1987) was be used as the basis for calculating AEGL-3 values for chloroacetone. Interspecies and intraspecies uncertainty factors of 3 each were applied. The mechanism of chloroacetone toxicity is uncertain; although direct irritation effects are observed after exposure through all routes, some information suggests the potential for systemic effects after dermal and oral exposure (see Section 4.2). However, the preponderance of the available information suggests that the primary effects of chloroacetone inhalation are due to direct-acting irritation; this type of port-of-entry effect does not exhibit toxicokinetic variability and thus is not expected to vary greatly between species or among individuals. The interspecies uncertainty factor of 3 is also supported by data suggesting little species variability with regard to lethality from oral and dermal exposure to chloroacetone (rat oral LD50 values: 100-141 mg/kg; mouse oral LD50 values: 127-141 mg/kg; rabbit dermal LD50 = 141 mg/kg). Furthermore, the 1-h LC50 of 500 ppm for male and female rats (Arts and Zwart 1987) is approximately a dose of 114 mg/kg, which corresponds to the oral LD50 values (assuming 100% retention, 245 mL minute volume, and a rat body weight of 250 g). The intraspecies uncertainty factor of 3 is also considered sufficient because data from the more sensitive males were used as the point-of-departure. Thus, the total adjustment was 10.
It has been shown that the concentration-time relationship for many irritant and systemically acting vapors and gases may be described by the equation Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). Data on chloroacetone were inadequate to derive an empirical value for n. The available information suggests that the lethal concentration is lower after longer exposure durations; the 1-h LC50 in male and female rats was 262-500 ppm (Sargent et al. 1986; Arts and Zwart 1987), while the 6-h LC50 is approximately 50-100 ppm (Eastman Kodak 1992). Therefore, default estimates of n were used to extrapolate from the 1-h point-of-departure to other time points. An n of 3 was applied to extrapolate to the 10- and 30-min time periods,
TABLE 3-6 AEGL-2 Values for Chloroacetone
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 8.0 ppm (30 mg/m3) | 5.5 ppm (21 mg/m3) | 4.4 ppm (17 mg/m3) | 1.1 ppm (4.2 mg/m3) | 0.53 ppm (2.0 mg/m3) |
and an n of 1 was applied to extrapolate to the 4- and 8-h time periods to provide AEGL values that would be protective of human health (NRC 2001). AEGL-3 values for chloroacetone are presented in Table 3-7, and the calculations for these AEGL-3 values are presented in Appendix A.
8. SUMMARY OF PROPOSED AEGLS
8.1. AEGL Values and Toxicity End Points
AEGL values for chloroacetone are summarized in Table 3-8. AEGL-1 values are not recommended because of insufficient data. AEGL-2 values were set at one-third the AEGL-3 values, and AEGL-3 values were based on a 1-h estimated threshold for lethality in male rats.
8.2. Comparison with Other Standards and Guidelines
Table 3-9 shows exposure criteria for chloroacetone that have been established. ACGIH (2012) recommended a threshold limit value (TLV) ceiling of 1.0 ppm for chloroacetone. The TLV-ceiling is a concentration that should not be exceeded during any part of the working exposure; as such, there is no parallel value among the AEGL exposure durations. The Dutch maximal accepted concentration (MAC) of 1 ppm is equivalent to an 8-h TLV. However, there is no published method for measuring occupational exposure to chloroacetone (OSHA 2012), and efforts to locate occupational monitoring data on chloroacetone were not successful. Thus, there is no information with which to determine whether workers have been exposed at concentrations at or approaching the 1 ppm limit without adverse effects.
TABLE 3-7 AEGL-3 Values for Chloroactone
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 24 ppm (91 mg/m3) | 17 ppm (65 mg/m3) | 13 ppm (49 mg/m3) | 3.3 ppm (13 mg/m3) | 1.6 ppm (6.1 mg/m3) |
TABLE 3-8 Summary of AEGL Values for Chloroacetone
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h |
| AEGL-1 (nondisabling) | NRa | NRa | NRa | NRa | NRa |
| AEGL-2 (disabling) | 8.0 ppm (30 mg/m3) | 5.5 ppm (21 mg/m3) | 4.4 ppm (17 mg/m3) | 1.1 ppm (4.2 mg/m3) | 0.53 ppm (2.0 mg/m3) |
| AEGL-3 (lethality) | 24 ppm (91 mg/m3) | 17 ppm (65 mg/m3) | 13 ppm (49 mg/m3) | 3.3 ppm (13 mg/m3) | 1.6 ppm (6.1 mg/m3) |
aNot recommended; absence of an AEGL-1 value does not imply that exposure below the AEGL-2 value is without adverse effects.
TABLE 3-9 Extant Standards and Guidelines for Chloroacetone
| Guideline | Exposure Duration | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h | |
| AEGL-1 | NR | NR | NR | NR | NR |
| AEGL-2 | 8.0 ppm | 5.5 ppm | 4.4 ppm | 1.1 ppm | 0.53 ppm |
| AEGL-3 | 24 ppm | 17 ppm | 13 ppm | 3.3 ppm | 1.6 ppm |
| TLV-Ceiling (ACGIH)a | 1 ppm | 1 ppm | 1 ppm | 1 ppm | 1 ppm |
| MAC (The Netherlands)b | 1 ppm | ||||
aTLV-Ceiling (threshold limit value - ceiling) (American Conference of Governmental Industrial Hygienists [ACGIH 2012]) is based on human exposure data (lacrimation and other irritation) reported by Sargent et al. (1986). The TLV-ceiling is a concentration that should not be exceeded during any part of the working exposure. Includes a skin notation.
bMAC (maximaal aanvaaarde concentratie [maximal accepted concentration]). SDU Uitgevers (under the auspices of the Ministry of Social Affairs and Employment), The Hague, The Netherlands, (MSZW 2004) is defined analogous to the ACGIH TLV-TWA (a time-weighted average concentration for a normal 8-h workday and a 40-h workweek, to which nearly all workers may be repeatedly exposed, day after day, without adverse effect).
8.3 Data Adequacy and Research Needs
The available human data have limitations because they are anecdotal and do not provide robust concentration or duration exposure parameters. Animal data also had limitations; however, oral exposure data corresponded well with inhalation data, showing similar effects at similar dose equivalents. Data were insufficient for derivation of AEGL-1 values.
9. REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 2012. Chloroacetone (CAS Reg. No.78-95-5). TLVs and BEIs Threshold Limit Values and Biological Exposure Indices. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
Arts, J.H.E., and A. Zwart. 1987. Acute (One-Hour) Inhalation Toxicity Study of Chloroacetone in Rats. TNO Report No. V87.093/261236. CIVO Institutes, Zeist, The Netherlands. EPA Document No. 88870000029. Microfiche No. OTS0513 466.
Eastman Kodak. 1992. Initial Submission: Basic Toxicity of Chloroacetone with Cover Letter Dated 090192. EPA Document No. 88920008853. Microfiche No. OTS057 0898.
HSDB (Hazardous Substances Data Bank). 2011. 1-Chloro-2-propanone (CAS Reg. No.78-95-5). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed May 25, 2012].
IPCS (International Programme on Chemical Safety). 2006. Chloroacetone. International Chemical Safety Cards (ICSCs). International Programme on Chemical Safety [online]. Available: http://www.inchem.org/documents/icsc/icsc/eics0760.htm [accessed May 25, 2012].
Le Curieux, F., D. Marzin, and F. Erb. 1994. Study of the genotoxic activity of five chlorinated propanones using the SOS chromotest, the Ames-fluctuation test and the newt micronucleus test. Mutat. Res. 341(1):1-15.
Lee, W.R., R. Abrahamson, R. Valencia, E.S. Von Halle, F.E. Wurgler, and S. Zimmering. 1983. The sex-linked recessive lethal test for mutagenesis in Drosophila melanogaster. Mutat. Res. 123(2):183-279.
Merrick, B.A., C.L. Smallwood, J.R. Meier, D.L. McKean, W.H. Kaylor, and L.W. Con-die. 1987. Chemical reactivity, cytotoxicity, and mutagenicity of chloropropanones. Toxicol. Appl. Pharmacol. 91(1):46-54.
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: Chlooraceton. Den Haag: SDU Uitgevers [online]. Available: http://www.lasrook.net/lasrookNL/maclijst2004.htm [accessed Sept. 11, 2012].
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
OSHA (Occupational Safety and Health Administration). 2012. Occupational Safety and Health Guideline for Chloroacetone. Occupational Safety and Health Administration, Washington, DC [online]. Available: http://www.osha.gov/SLTC/healthguidelines/chloroacetone/recognition.html [accessed May 25, 2012].
Prentiss, A.M. 1937. P. 121 in Chemicals in War: A Treatise on Chemical Warfare. New York: McGraw Hill.
Robinson, M., R.J. Bull, G.R. Olson, and J. Stober. 1989. Carcinogenic activity associated with halogenated acetones and acroleins in the mouse skin assay. Cancer Lett. 48(3):197-203.
Sargent, E.V., G.D. Kirk, and M. Hite. 1986. Hazard evaluation of monochloroacetone. Am. Ind. Hyg. Assoc. J. 47(7):375-378.
Searle, C.E. 1966. Tumor initiatory activity of some chloromononitrobenzenes and other compounds. Cancer Res. 26:12-17.
ten Berge, W.F., A. Zwart, and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Mater. 13(3):301-309.
APPENDIX A
DERIVATION OF AEGL VALUES FOR CHLOROACETONE
Derivation of AEGL-1 Values
Data are insufficient for derivation of AEGL-1 values for chloroacetone.
Derivation of AEGL-2 Values
| Key study: | Arts, J.H.E., and A. Zwart. 1987. Acute (one-hour) inhalation toxicity study of chloroacetone in rats. Civo Institutes, TNO. Report No. V87.093/261236. The Netherlands. EPA Document No. 88870000029. Microfiche No. OTS0513466. |
| Toxicity end point: | One-third of the AEGL-3 values |
| 10-min AEGL-2: | 24 ppm ÷ 3 = 8.0 ppm |
| 30-min AEGL-2: | 17 ÷ 3 = 5.5 ppm |
| 1-h AEGL-2: | 13 ÷ 3 = 4.4 ppm |
| 4-h AEGL-2: | 3.3 ÷ 3 = 1.1 ppm |
| 8-h AEGL-2: | 1.6 ÷ 3 = 0.53 ppm |
Derivation of AEGL-3 Values
| Key study: | Arts, J.H.E., and A. Zwart. 1987. Acute (one-hour) inhalation toxicity study of chloroacetone in rats. Civo Institutes, TNO. Report No. V87.093/261236. The Netherlands. EPA Document No. 88870000029. Microfiche No. OTS0513466. |
| Toxicity end point: | Male rat 1-hr BMCL05 = 131 ppm. |
| Scaling: | C3 × t = k (10-min, 30-min) (131 ppm)3 × 1 h = 2,248,091 ppm-h C1 × t = k (4-h, 8-h) (131 ppm)1 × 1 h = 131 ppm-h |
| Uncertainty factors: | 3 for interspecies differences 3 for intraspecies variability |
| 10-min AEGL-3: | C3 × 0.167 h = 2,248,091 ppm-h C3 = 13,461,623 ppm C = 238 ppm 238 ÷ 10 = 24 ppm |
| 30-min AEGL-3: | C3 × 0.5 h = 2,248,091 ppm-h C3 = 4,496,182 ppm C = 165 ppm 165 ÷ 10 = 17 ppm |
| 1-h AEGL-3: | C3 × 1 h = 2,248,091 ppm-h C3 = 2,248,091 ppm C = 131 ppm 131 ÷ 10 = 13 ppm |
| 4-h AEGL-3: | C1 × 4 h = 131 ppm-h C1 = 32.7 ppm C = 32.7 ppm 32.7 ÷ 10 = 3.3 ppm |
| 8-h AEGL-3: | C1 × 8 h = 131 ppm-h C1 = 16.4 ppm C = 16.4 ppm 16.4 ÷ 10 = 1.6 ppm |
APPENDIX B
ACUTE EXPOSURE GUIDELINE LEVELS FOR CHLOROACETONE
Derivation Summary
AEGL-1 VALUES
Data on chloroacetone were insufficient for derivation of AEGL-1 values. Absence of AEGL-1 values does not imply that exposure below the AEGL-2 values are without adverse effects.
AEGL-2 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 8.0 ppm | 5.5 ppm | 4.4 ppm | 1.1 ppm | 0.53 ppm |
| Data adequacy: No acute toxicity data relevant to deriving AEGL-2 values were available. Therefore, the AEGL-3 values were divided by 3. | ||||
AEGL-3 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 24 ppm | 17 ppm | 13 ppm | 3.3 ppm | 1.6 ppm |
| Key reference: Arts, J.H.E., and A. Zwart. 1987. Acute (one-hour) inhalation toxicity study of chloroacetone in rats. Civo Institutes, TNO Report No. V87.093/261236. The Netherlands. EPA Document No. 88870000029. Microfiche No. OTS0513466. | ||||
| Test species/Strain/Number: Rat; SPF (Bor:WISW); 5/sex/group | ||||
| Exposure route/Concentrations/Durations: Inhalation; 132, 263, 553, 816, 1,105, and 2,079 ppm for 1 h | ||||
| Effects: | ||||
| 132 ppm: | No mortality; clinical signs: restlessness, rubbing of snouts, lacrimation, salivation, and humped posture | |||
| 263 ppm: | Clinical signs; mortality: 1/5 males; 0/5 females | |||
| 553 ppm: | Clinical signs; mortality: 5/5 males; 1/5 females | |||
| 816 ppm: | Clinical signs; mortality: 5/5 males; 3/5 females | |||
| 1,105 ppm: | Clinical signs; mortality: 5/5 males; 5/5 females | |||
| 2,079 ppm: | Clinical signs; mortality: 5/5 males; 5/5 females | |||
| LC50: | 500 ppm for males and females; 316 ppm for males; 710 ppm for females | |||
| BMC01: | 170 ppm for males and females; 223 ppm for males; 394 ppm for females | |||
| BMLC05 | 144 ppm for males and females; 131 ppm for males; 258 ppm for females | |||
| End point/Concentration/Rationale: Threshold for death; BMCL05 for male rats of 131 ppm | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 10 Interspecies: 3, available information suggests that the primary effects of chloroacetone via inhalation are due to direct-acting irritation; this type of port-ofentry effect does not exhibit toxicokinetic variability and, thus, is not expected to vary greatly between species. Factor is also supported by data suggesting little species variability in lethality from oral and dermal exposure to chloroacetone (rat oral LD50 values: 100-141 mg/kg; mouse oral LD50 values: 127-141 mg/kg; rabbit dermal LD50 = 141 mg/kg), and the 1-h LC50 of 500 ppm for male and female rats is approximately a dose of 114 mg/kg, which corresponds to the oral LD50 values (assuming 100% retention, 245 mL minute volume, and a rat body weight of 250 g). Intraspecies: 3, available information suggests that the primary effects of chloroacetone via inhalation are due to direct-acting irritation; this type of port-ofentry effect does not exhibit toxicokinetic variability and, thus, is not expected to vary greatly among individuals. A factor of 3 is also considered sufficient because the point-of-departure was from more sensitive male rats. |
||||
| Modifying factor: Not applicable | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time scaling: Cn × t = k, where an n of 3 was applied to extrapolate to the 10- and 30-min durations and an n of 1 was applied to extrapolate to the 4- and 8-h durations (NRC 2001). | ||||