5
Occurrence of Chemical Contaminants in Seafood and Variability of Contaminant Levels
ABSTRACT
This chapter and the following one should be considered as a unit. Although the committee has not attempted a comprehensive quantitative assessment of the risks of chemical contaminants in seafood, this chapter performs the functions of the "hazard identification" portion of a chemical risk assessment–giving a broad overview of many different potential seafood contamination problems, as well as an extensive summary of available data for characterizing contaminant concentrations in aquatic organisms in the environment. Chapter 6 provides a discussion of the directions needed to improve quantitative risk assessment in this area [including a detailed treatment of the methods used to assess two specific hazards (polychlorinated biphenyls and methylmercury)] and, more broadly, covers the issues that are usually found under the headings "dose-response assessment," "exposure assessment," and "risk characterization," as well as some risk management considerations.
The inorganic contaminants with the greatest potential for toxicity appear to be antimony, arsenic, cadmium, lead, mercury, selenium, and sulfites (used in shrimp processing). Among organic compounds, polychlorinated biphenyls, dioxins, several chlorinated hydrocarbon insecticides, certain processing-related contaminants (nitrosamines and possibly products of chlorination), and contaminants related to aquaculture pose sufficient potential risks for consumers to be worthy of additional study.
In addition to providing a broad survey of data on chemical contamination of aquatic organisms and potential risks, this chapter undertakes an extensive set of analyses of the variability of concentrations of certain contaminants across geographic areas and the implications of this variability for control. In general, lognormal distributions appear to provide good descriptions of the pattern of variation of chemical contaminant concentrations among different geographic areas, and some contaminants (mostly organics) appear to be much more variable than others. The variability of contaminant concentrations among geographic areas is important because it indicates the potential for reduction of exposure through restrictions on the harvesting of aquatic organisms from specific sites. Based on analyses of data for inshore marine waters, for the most variable contaminants/sets of species, it would be possible theoretically to reduce the population dosage delivered by more than 50% by restricting harvesting/marketing from only the 5% most intensely contaminated sites. There is, therefore, considerable potential for management of the overall population dosage of contaminants by measures that would restrict harvesting in specific ways.
INTRODUCTION
There is no area of the committee's work that poses greater challenges to both the scientific tools for understanding likely health hazards and the social tools for managing risks, than the diverse collection of chemical residues that find their way into the human diet partly by way of seafood. Moreover, the confusion between technical questions and social control problems is connected. The understanding of toxicology and environmental health has made important strides since the multitiered structure of federal food protection law was erected (principally by legislation in 1906, 1938, and 1958). Older concepts, which shaped the legislative framework within which food protection agencies attempt to function, suggested sharp distinctions between "poisons" and other substances, or between "safe" and "unsafe" levels of exposure to important categories of environmental toxicants.1 These ideas are gradually giving way to a more quantitative (although generally still highly uncertain) conception of risks, based on more detailed information about the mechanisms by which different substances interact with intricate biological systems and the diversity of those systems in different individuals in the large and disparate human population. To the extent that increased understanding indicates that certain categories of risks cannot be eliminated entirely, the tools for social control of these risks will have to be adapted to manage toxicant exposures and risks in the light of explicitly formulated trade-offs between the costs of forgoing certain portions of our food resources and the costs of potential adverse effects.
The technical advances that have occurred in risk assessment in recent years have been applied most readily to issues of health protection by governmental institutions of relatively late vintage, operating under legislation adopted within the last 20 years – most notably, the different branches of the U.S. Environmental Protection Agency (EPA) and analogous state authorities. When the more modern techniques and assumptions for quantitatively assessing risks are applied to seafood contaminants, there are a number of areas of mismatch2 that give the appearance of inconsistency in the social and technical judgments on risks made by different agencies.
Both this chapter and the next deal with aspects of chemical residues in aquatic organisms. In this chapter the committee focuses on the tasks that are usually thought of as part of the hazard identification portion of a quantitative chemical health risk assessment. Chapter 6 deals broadly with issues in the assessment of dose-response relationships, estimation of exposures, and characterization of risks.
The committee has not, however, attempted a formal and comprehensive assessment of the risks of chemical residues in aquatic organisms. Aside from the fact that the available data on both contaminant3 levels and risks are inadequate for such a task at this time, the charge to the committee emphasized the review of the adequacy of current risk recognition, risk assessment, and risk management procedures in governmental agencies. In the next section of this chapter, the committee gives a broad overview of the types of toxic agents that are known or believed to be contaminants of seafood. Then, the various data bases available for characterizing the geographic and species distribution of chemical contaminants are reviewed, followed by the quantitative insights gleaned from these sources. The variability of contaminants by geography and species, which provides some of the most potentially important opportunities for reduction of exposures, is then considered. Finally, preliminary
conclusions and recommendations are offered, based on the material discussed in this chapter.
Chapter 6 articulates basic concepts underlying the mechanisms of action of toxic substances and quantitative ideas about dose-time-response relationships. Then, a focused examination is provided of available Food and Drug Administration (FDA) risk assessment and risk management analyses for two types of residues — one a set of organic carcinogens, polychlorinated biphenyls (PCBs), and the other an organometallic residue with reproductive and neurological effects (methylmercury). These two important examples are used to fulfill the committee's charge to critique the adequacy of current governmental procedures for assessing risks and the opportunities for risk reduction. Finally, the balance of Chapter 6 provides a more general overview of what can be said very approximately about the quantitative risks of other contaminants in seafood.
TOXIC AGENTS AND POTENTIAL TYPES OF HEALTH EFFECTS
Metals and Other Inorganics
The human and veterinary medical literature is replete with information regarding the toxicity of heavy metals. Based on this information, different metals can be classified as having major, modest, minor, or no potential for toxicity. Those with major potential for toxicity, in the committee's view, are antimony, arsenic, cadmium, chromium, lead, mercury, and nickel. Contaminants with a modest potential for toxicity include copper, iron, manganese, selenium, and zinc. Those of minor or no toxicity are aluminum, silver, strontium, thallium, and tin. This classification is based, among other parameters, on potency for producing effects and accessibility of the toxicant. Thus, such metals as nickel and chromium, known inhalant carcinogens, are among those of greatest toxicity, whereas selenium and tin are placed in the lesser categories. When considering the same metals as contaminants of an aquatic food source, however, their relative toxicities will certainly change. Criteria for identifying contaminants (hazard assessment, hazard analysis) of public health concern in the aquatic environment may vary but have been defined (PTI, 1987). These include (1) persistence, (2) bioconcentration potential, (3) toxicity to humans (or suspected toxicity), (4) sources of contaminants in the area of interest, and (5) high concentration in fish and shellfish from the area of interest.
By applying such criteria, both nickel (except for its carbonyl form) and chromium (at least in its hexavalent form), inhalant carcinogens and elicitors of dermal hypersensitivity, would be suspect as contaminants of public health concern (Haines and Nieboer, 1988). However, both are poorly absorbed from the gastrointestinal tract, and there is little evidence that this route of exposure results in systemic toxicity (Beliles, 1978; Nieboer and Jusys, 1988). Similarly, the use of trin-butyltin (TBT) to control marine fouling of vessels and aquaculture sea pens has been followed by the accumulation of butyl- and elemental tins in the muscles of fish and invertebrates (Short and Thrower, 1987a,b). Organic tin compounds tend to be more toxic than inorganic salts, and organic forms in particular may be of public health concern. Although little information exists about the toxicity of tin to man, there is sufficient information regarding dosage levels without observable effect to eliminate the
probability of tin poisoning from contaminated seafood (WHO, 1980). Conversely, selenium is well recognized as toxic by ingestion and, at existing levels in some seafoods, may be a source of risk (Fan et al., 1988). Antimony has been recognized as both an occupational and an iatrogenic toxicant (Anonymous, 1988a,b; Groth et al., 1986). Recent seafood residue studies, however, either have failed to sample for this metal or indicate concentrations above detectable levels in few contaminated sites (Lowe et al., 1985; NOAA, 1987). Such findings and reasoning, coupled with estimates of ingestion levels, suggest a preliminary list of heavy-metal contaminants found in the edible portions of aquatic animals that may be detrimental to human health. The metals identified in this hazard analysis include arsenic, cadmium, lead, mercury, and selenium. Some toxicity information related to antimony is given later, in the section where current dosage is compared to "acceptable daily intake" levels and other recommended standards.
Specific Trace Metals
Arsenic
Arsenic has a long history as a potent poison of humans and other animals. Previously used as a chemotherapeutic and homicidal drug, much information has been collected regarding its toxicity. It exists as the toxic trivalent form (arsenic trioxide, sodium arsenate, arsenic trichloride, etc.), as the less toxic pentavalent form (arsenic pentoxide, arsenic acid, lead arsenate, calcium arsenate, etc.), and as numerous organic forms (arsanilic acid, bimethyl arsenate, etc.). When ingested, inorganic arsenic may cause acute or chronic toxicity and is of primary concern as a carcinogen responsible for pulmonary carcinoma, hemangiosarcomas, and dermal basal cell and squamous cell carcinomas. Its toxicity is dependent on oxidation state and route of exposure. In its chronic manifestations, arsenic is responsible for gastroenteritis, nephritis, hepatomegaly, peripheral symmetrical neuropathy, and a number of lesions of the skin including plantar and palmar hyperkeratosis and generalized melanosis. Some of these lesions appear related to destruction of capillary endothelium, with consequent edema and even circulatory failure. At the molecular level the metal is known to uncouple phosphorylation; to react with sulfhydryl groups, thus upsetting cellular metabolism; to damage deoxyribonucleic acid (DNA) directly and to inhibit its repair (Buck, 1978). In addition, as sodium arsenate and arsenite it is teratogenic in lower animals (Earl and Vish, 1978). The metal, therefore, places at special risk pregnant and nursing mothers and their children.
However, the predominant form of arsenic that exists in the edible portions of aquatic animals is the organic form, either arsenobetaine or arsenocholine. These forms have been named "fish arsenic" and no toxic effects from their ingestion have been reported in animals [at doses of 10,000 milligrams (mg) per kilogram (kg)] or in humans. Furthermore, there is no evidence of mutagenicity by arsenobetaine (Penrose, 1975; Tam et al., 1982). Although arsenobetaine constitutes the bulk of arsenic in fish, available studies are inadequate to conclude that the amounts of more toxic inorganic forms of arsenic (or organic forms that can be metabolized to inorganic arsenic in humans) are negligible in all fish. It is known, however, that the trivalent form (inorganic) is toxic to man and that long-term effects include dermal hyperkeratosis,
dermal melanosis and carcinoma, hepatomegaly, peripheral neuropathy, and in cases of inhalation, pulmonary carcinoma (ATSDR, 1989a; Goyer, 1986).
Arsenic is used in the manufacture of pesticides, herbicides, and other agricultural products and is a by-product of mining and smelting operations (Buck, 1978).
Cadmium
Cadmium is unique among toxic metals because it is a relatively recent (50 years) contaminant of the aquatic environment. Its sources are solid waste dumping (pigment in paint) and cadmium-containing sewage sludge, the use of phosphatic fertilizers, electroplating and galvanizing manufacture, and mining (zinc, lead) wastewater (Sherlock, 1986; Sloan and Karcher, 1985). Cadmium is commonly found in its metallic form and as sulfides and sulfates. Invertebrates, both crustacea and bivalves, tend to accumulate metallic cadmium in large amounts by binding to various high-molecular-weight metallothioneine ligands. There is a differential affinity between crustacean muscle and hepatopancreas, the latter organ containing 10-20 times the concentration of the former. Because hepatopancreas may be considered a delicacy or marketed as "brown crab meat," the potential for ingesting large amounts of cadmium when eating lobsters or crabs is increased (McKenzie-Parnell et al., 1988; Sloan and Karcher, 1985).
Cadmium may damage cells by its activity in the plasmalemma where it reacts with phosphate groups of the lipid bilayer to alter permeability, in the nucleus where it is mutagenic, on lysosomal membranes, and as an inhibitor of mitochondrial activity. Its ability to stimulate metallothioneine production in aquatic animals, however, does much to decrease its toxicity (Viarengo, 1985).
Cadmium has been responsible for major human poisoning incidents as a contaminant of wastewater used for irrigation in Japan where the illness is known as itai-itai (ouch-ouch) disease. It is a chronic osteoporotic and osteomalacic condition that primarily affects multiparous females (Kobayashi, 1978). Although the highest accumulation of cadmium is found in bone, the liver and kidney also have a propensity for accumulating the metal, and the kidney is often seriously damaged in chronic occupational exposures (Lauwerys and De Wals, 1981). Clinically, patients suffer tubular dysfunction resulting in aminoaciduria, proteinuria, and glucosuria. Although the half-life of cadmium in kidneys of humans is uncertain, it may be as long as 30 years. Under such circumstances it has been conjectured that critical concentrations [kidney = 200 micrograms (µg) per gram (g) by age 50] could be used to establish maximum levels of daily exposure (Kjellström et al., 1977). What makes cadmium of dietary concern is that ordinary background dietary exposures were estimated to yield kidney concentrations of about one-quarter the hypothesized critical level. The segment of the population at greatest risk would appear to be older adults (ATSDR, 1989b).
Studies in maternal-fetal tissues have provided evidence for accumulation and transplacental transfer of metals. In one study, placental cadmium levels were one to two times those in maternal or cord blood. It was observed also that erythrocyte cadmium levels were roughly three to five times plasma cadmium levels, and that maternal erythrocyte cadmium levels were somewhat higher (27%) than those of the fetus.
Lead
Of all the heavy metals, lead probably has the longest history of environmental contamination and toxicity to humans (Green et al., 1978). For this reason, lead poisoning, or plumbism, has been intensely studied, and a large body of information is available for examination. Sources of lead found in the environment are multiple, and the metal is truly ubiquitous, being commonly found in food, water, and air. Evidence exists that lead in the environment has increased during the past 200 years, and it is not surprising that it can be found as a contaminant of aquatic animals (Shukla and Leland, 1973). Environmental lead is a product of storage battery, ammunition, solder, pigment, pipe, brass, and red lead manufacture. Tetraethyllead is a component of gasoline antiknock additives, although in recent years this use has been drastically reduced. There are at least five pools of lead in the body, two of which reside in the skeleton (90%) in cortical and trabecular bone. Lead in cortical bone is similar in half-life to cadmium (approximately 20 years). Other body compartments for lead include the kidney, lung, and central nervous system (Goyer, 1986). It is not surprising therefore that major lesions and clinical signs in humans suffering frank plumbism are referable to the blood (anemia), brain (convulsions, paralysis), and kidney (proteinuria).
The condition in humans is best known because of its chronic toxicity to young children who ingest lead-base paint chips or lead in soil, house dust from paint, industrial dust, and automotive emissions. Oral ingestion of inorganic lead is without doubt the primary port of entry into humans. Of the lead ingested, only 5-15% is absorbed in adults but considerably more in children (Goyer, 1986). Recent studies suggest that very low levels ingested by pregnant women may result in learning and behavioral disabilities in neonates and preschool children (Waternaux et al., 1989). Excretion is primarily via the bile and the gastrointestinal tract. Organic lead compounds such as tetraethyllead may be absorbed in large quantities through the skin, but as toxicants these forms of lead are primarily a problem in the petroleum industry. All forms of lead toxicity are less frequent in adults; any occurrence is usually acute and occupationally related (Green et al., 1978).
Lead's toxicological mode of action depends on its molecular configuration, inorganic lead being less toxic than and producing clinical signs different from tetraethyllead. Inorganic lead is an inhibitor of aminolevulinic acid dehydratase (ALAD) and heme synthetase, which leads to anemia (Hammond, 1978). The metal causes necrosis of neurons, myelin sheath degeneration, and especially, brain vascular damage with increased cerebrospinal fluid (CSF) pressure. These lead to encephalopathy and eventual mental retardation in children. Lead crosses the placental barrier, and there is a good correlation between maternal and fetal blood lead values (Van Gelder, 1978). Therefore, at primary risk from contaminated seafoods are the fetus and neonates.
Mercury
Mercury exists in elemental form, as monovalent (mercurous) or divalent (mercuric) salts, and methylated. The methylated form is the most toxic to humans (Harada, 1978). Methylmercury is formed in the environment from the divalent salts
by anaerobic bacteria. It is quite easily absorbed after ingestion and has a variable half-life of 60-120 days in man but is reported to have a half-life of up to 2 years in fish, where it is the predominant form (Al-Shahristani and Shihab, 1974; Stopford and Goldwater, 1975).
The metal is known to produce c-mitosis and chromosomal alterations resulting in cellular damage, with the kidney and brain as target organs. Neuronal damage and axonal demyelination result in the clinical signs and symptoms of paresthesia, incoordination, tremor, and epileptic seizures. The metal also binds strongly to sulfhydral groups (mercaptans), thereby inactivating certain enzymes (Hammond, 1978).
In its methylated form, mercury quite easily passes the placental barrier, placing the fetus at particular risk (Amin-Zaki et al., 1979). The relationship of clinical signs in humans to blood, hair, and urine mercury levels has been reviewed (Tollefson and Cordle, 1986). Children of symptom-free pregnant and nursing mothers with relatively low blood and hair levels may suffer from mental retardation.
Selenium
Selenium is an enigmatic metal because it functions both as an essential nutrient and, at slightly higher levels, as a poison. It is present in various enzymes, has been reported to possess anticarcinogenic effects in animals, is an antioxidant, and yet is a well-documented toxicant of domestic animals as well as a mutagen (Griffin, 1979; Schamberger, 1985; Schnell and Angle, 1983). As an animal toxicant it is a regional problem of the Southwest and Far West. Seleniferous (alkaline, oxidizing) soils give rise to high levels in selenium accumulator plants that are grazed by cattle, sheep, horses, and swine. Poisoned animals develop conditions known as "alkali disease" [subacute, <50 parts per million (ppm)] and "blind staggers" (acute, >100 ppm). Signs include anorexia, tooth and hair loss, watery diarrhea, lassitude, progressive paralysis, and eventual death (Harr and Muth, 1972).
Selenium levels in water from seleniferous areas are often quite high so it is not surprising that selenium has been found as a contaminant of fresh and marine aquatic animals. Its source however is not solely natural. Anthropogenic contamination occurs and is the product of fossil fuel combustion (fly ash) and of paint, alloy, photoelectric battery, and rectifier manufacture (Fishbein, 1983; Sorensen et al., 1984).
Selenium exists in a number of chemical forms, elemental selenium (Seo), selenide (Se2+), selenite (Se4+), and selenate (Se6+). These forms may bond with other metals or organic substances such as amino acids (Ewan, 1978). The selenates are most soluble and easily enter biological systems. In one study, approximately 15-30% of the selenium found in fish muscle was the selenate form (Cappon and Smith, 1981). The selenites and elemental selenium are relatively insoluble. This is not to say that selenite when ingested will not act as a toxicant, merely that its innate insolubility may affect its absorption and distribution within the body (Goyer, 1986). The biochemistry of selenium is poorly understood but has been reviewed recently (Reddy and Massaro, 1983).
The mode of action of selenium as a toxicant at the cellular and biochemical levels is uncertain. The metal appears to damage endothelium selectively, resulting in edema and hemorrhage in both humans and animals. It is also responsible for toxic hepatitis with eventual fibrosis (not constituting cirrhosis) in chronic exposures.
Selenosis in animals is reported to produce infertility and congenital malformations (Harr, 1978). Selenosis in man appears to be a relatively rare occurrence, most often due to acute occupational exposure or chronic exposure to contaminated water or food sources. There appears to be very little information regarding the effect on man of chronically high levels of selenium in the diet and its potential risk (Wilber, 1983). Recently, however, levels have been reached in fish that have prompted health alerts in California (Fan et al., 1988).
Organic Compounds
In this section, some of the potential contaminants of seafood that have come to the committee's attention, and about which there are at least some minimal data, are surveyed. These include the chlorinated hydrocarbon pesticides that came into widespread use in the United States and elsewhere immediately after World War II (Hansen et al., 1985). Among the chlorinated hydrocarbon pesticides detected in seafood were benzene hexachloride (BHC) or hexachlorobenzene (HCB), chlordane, dieldrin, dichlorodiphenyltrichloroethane (DDT), endrin, heptachlor, lindane, nonachlor, octachlor, and pentachlorophenol. In addition, industrial chemicals and by-products such as PCBs and dioxins are routinely detected in seafood. Less frequently detected pesticides included chlorpyrifos, dacthal (DCPA), diazinon, ethylene dibromide (EDB), malathion, mirex, omethoate, pentachloroaniline, tecnazene, and trifluralin (FDA, 1988; Gunderson, 1988). In quite specific circumstances, such as in farm ponds in heavily agricultural areas, other chemicals — even those that are not known to bioconcentrate, such as atrazine — can be found in fish (Kansas DHE, 1988). Some pesticides detected are specific to various regions. The carboxylic acid herbicide 2,4-(dichlorophenoxy)acetic acid (2,4-D) has been found in oysters from the northern Chesapeake Bay and Alaskan bivalves (NOAA, 1988). Fish from the Arroyo Colorado and adjacent lower Laguna Madre in Texas contained measurable concentrations of pesticides such as ethion, carbophenothion, ethyl parathion, and methyl parathion (NOAA, 1988). The organic compounds classified here have been reviewed by Murphy (1980).
Specific Organics
Polychlorinated biphenyls (PCBs)
Polychlorinated biphenyls include more than 200 different compounds ("congeners") that were used in various formulations as liquid insulators in electrical equipment, as encapsulating agents, in carbonless carbon paper, and in hydraulic fluids. The use of PCBs in "open" applications such as carbonless carbon paper was phased out in the early 1970s, and any new use for the remaining applications was stopped in the late 1970s with the passage of the Toxic Substances Control Act. The U.S. usage of approximately 500,000 tons of PCBs in 1930-1970 accounted for about half of the total world production. However, the unusually slow rate of environmental degradation of the more highly chlorinated PCBs in the environment and in higher organisms, and slow continued discharge of PCBs from old equipment and dump sites, have led to a
relatively slow rate of decline of PCB concentrations in fish from large freshwater bodies (e.g., the Great Lakes). The PCBs are a paradigmatic case for the phenomenon of bioconcentration. The more highly chlorinated congeners in particular tend to be both highly lipophilic and very slowly degraded by most organisms. Thus, PCBs that are passed "up" the food chain tend to become much more concentrated as predators are consumed by successively larger predators. In contrast, terrestrial animals that are used for human food are generally vegetarians (first-level consumers of the primary producing organisms).
The principal potential health concerns from PCB exposure include carcinogenesis (on the basis of extensive animal evidence and some suggestive findings in human epidemiological studies), changes in human birth weights, and some loss of neurological performance in the offspring of mothers with relatively high dietary exposures or body burdens (Bertazzi et al., 1987; Brown, 1987; Cordle et al., 1982; Fein et al., 1984; Gladen et al., 1988; J.L. Jacobson et al., 1989, 1990; S.W. Jacobson et al., 1985; Rogan et al., 1986; Sunahara et al., 1987; Taylor et al., 1989).
All carcinogens–in particular, the PCBs and dioxins–are not thought to act primarily by causing DNA mutations (Safe, 1989). This subject is discussed extensively in Chapter 6. Suffice it to say here that lack of knowledge of the precise mechanisms by which PCBs cause cancers makes quantitative assessment of their cancer risk more uncertain than is usual for other chemicals.
The PCB mixtures that are delivered to humans via seafood are likely to be systematically different from the original mixtures that were used in animal testing because of more rapid degradation of some (particularly less chlorinated) congeners in the environment and in aquatic organisms. The selection for relatively persistent congeners in aquatic organisms might tend to increase human risk relative to that expected from a naive extrapolation; other factors might have the opposite effect. In any event, the numbers of cases that could be expected seem large enough to warrant exploration of further options for risk reduction.
Dioxins
2,3,7,8-Tetrachlorodibenzo-p-dioxin (hereafter known as TCDD) is a contaminant of products made from trichlorophenol, including some chlorophenoxy herbicides. In humans, its effect has been linked to a severe dermatitis; fetal toxicity and numerous other effects have been observed in experimental animals at very low doses. In standard animal test systems, it is one of the most potent carcinogens known. Using its standard procedures for cancer potency estimation and certain consumption estimates, EPA estimated a lifetime cancer risk of approximately 1 in 100,000 from eating fish contaminated at the nominal detection level of 1 part per trillion (EPA, 1987). Using considerably different methodology for assessing the risk, FDA has advised that, for consumption patterns and species typical to the Great Lakes area, fish consumption should be limited if concentrations in the edible portion exceed 25 parts per trillion and should be banned if concentrations exceed 50 parts per trillion (Kociba et al., 1978).4 These profound differences in risk assessment indicate the tremendous uncertainty about the true potency of TCDD to cause human cancer.
Polycyclic aromatic hydrocarbons (PAHs)
Polycyclic aromatic hydrocarbons are common environmental contaminants found in petroleum, soot, or tar from incomplete combustion, lubricants, and domestic sewage. Many are well-established carcinogens and are highly toxic. Their pervasiveness in the environment assures widespread contamination of aquatic organisms. Because they are poorly metabolized by bivalves and are easily accessible to these animals, they may pose important potential hazards to humans.
Chlorinated hydrocarbon pesticides
Like PCBs, the broad group of relatively lipid-soluble, persistent chlorinated hydrocarbons was largely phased out of production in the United States during the 1970s because of concerns for carcinogenicity and ecological effects. Fortunately, few members of the group have proved to be as persistent as PCBs in the environment. Like PCBs, however, the precise mechanisms of action of many chlorinated hydrocarbons in causing cancer appear not to be by direct or indirect reactions with DNA; accordingly, quantitative assessments of risk for this group are more uncertain than usual.
DDT and metabolites: Both DDT and its metabolites [primarily dichlorodiphenyldichloroethane (DDE)] are persistent (slowly eliminated from organisms) lipophilic substances of uncertain health significance in humans, and are among the most widespread and frequently sampled of the chlorinated hydrocarbons. They are also persistent in ecosystems and bioaccumulate at higher levels of the food chain, resulting in toxicity to birds and aquatic organisms. The use of DDT was essentially banned in the United States in December 1972. Subacute effects of these chemicals at high doses in humans include central nervous system signs and, in rodents, liver toxicity and estrogenic effects. In addition, DDE has been observed to cause liver tumors in rodents.
Dieldrin: Dieldrin (an epoxide of aldrin) is a cyclodiene insecticide that, like DDT, affects the central nervous system, but is more toxic and has caused human fatalities. It too is lipophilic and may be released from fat stores long after exposure, to cause toxicity. It has led to increased liver tumors when fed at relatively low levels to rodents.
Chlordane: Chlordane is similar in molecular structure and mode of action to dieldrin, but is less toxic.
Heptachlor and heptachlor epoxide compounds: Heptachlor and heptachlor epoxide are also chlorinated cyclodienes, and the epoxide is known to be stored in human fat. They have toxicity similar to dieldrin.
Endosulfan: Endosulfan is a cyclodiene pesticide and a problem contaminant in estuaries near agricultural drainage areas due to its widespread use (NOAA, 1989).
Endrin: Endrin is similar in its toxic effects to other cyclodiene pesticides and is more acutely toxic than DDT.
Chlorinated benzenes and phenols: Lindane (γ-isomer of 1,2,3,4,5,6-hexachlorohexane), also known as benzene hexachloride (BHC), a mixture of α-, β-, and γ -isomers of 1,2,3,4,5,6-hexachlorocyclohexane depending on the manufacturer, is a neurotoxin but has also been found to cause aplastic anemia in humans.
Hexachlorobenzene has never been manufactured in the United States, but it is a ubiquitous fungicide and contaminant often found in other pesticides such as pentachloronitrobenzene (PCNB), which is used in the United States.
Pentachlorophenol: Also known as PCP and penta, pentachlorophenol is a wood preservative, slimicide, and metabolite of the fungicide hexachlorobenzene. Like other polychlorinated phenols, it is contaminated with carcinogenic dioxins (NOAA, 1988).
Mirex: Mirex is a pesticide used to control the fire ant in the southeastern United States. Like other organochlorine pesticides, it is lipophilic and has been reported to be a carcinogen on the basis of rodent studies. It may be a precursor of chlordecone (kepone), is persistent in the environment, and bioconcentrates in the food chain.
Kepone: Kepone has produced appreciable toxicity in exposed workers. It can cause neurological lesions, liver damage, and reproductive failure and is similar in its bioconcentration properties to mirex.
Toxaphene: Toxaphene is a very common domestically used insecticide of complex and often uncertain molecular structure. It is made by chlorinating a mixture of terpenes. It therefore may vary in toxicity from batch to batch depending on the proportion of its isomers. Fortunately, it is of relatively low persistence in the body. Carcinogenic activity is suspected.
Carboxylic herbicides: The herbicides DCPA, 2,4-D, and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) are chlorophenoxy compounds commonly used in agriculture, and by government agencies and utility companies to control woody plants in rights of way and along roadsides. Although 2,4,5-T has been found to have teratogenic activity, there has long been discussion about whether this is attributable to small amounts of dioxin contaminants. Aside from teratogenesis, these agents can affect animals by impairing neurotransmission, resulting in muscle weakness, ventricular fibrillation, and neuritis.
Atrazine: Atrazine is a herbicide commercially known as AAtrex. It is of low oral acute toxicity. However, its chronic effects are unknown. Structurally similar compounds have been shown to produce thyroid adenocarcinomas in rodents.
Contamination Problems in Aquaculture
Fish culture uses a variety of chemicals that represent potential threats to the health of the cultured animal, indigenous biota, and even the human consumer (Meyer and Schnick, 1989). A number of chemicals of potential toxicity to humans that are not registered for use in the United States are employed in other nations (Fox, 1990). These include furazolidone, nitrofurazone, carofur, chloramphenicol, and silvex–all of which are known or suspected carcinogens.
Chemicals employed in aquaculture include (1) drugs used to treat disease (chemotherapy), (2) those introduced through construction materials, (3) chemicals to treat parasites (formaldehyde), (4) hormones used to alter reproductive viability, sex, and growth rates, and (5) water quality treatments (copper compounds). Of these groups, those of greatest potential concern are the chemotherapeutic drugs. Chemicals used in construction and hormones are not considered in this section because they are relatively nontoxic or have been considered under other headings (organics, pesticides).
Disease is a limiting factor in the culture of aquatic animals. In recent years, culturists have dealt with this problem by developing rapid, immunological diagnostic tests, followed by treatment with drugs commonly used in veterinary medicine. Such chemotherapeutic drugs include, but are not limited to, the potentiated sulfonamides, antibiotics, and nitrofurans. Their widespread use, both nationally and especially internationally, may pose a threat to human health if residues persist in the edible portions of fish and shellfish. Both residue tolerance levels and withdrawal times (the period prior to slaughter during which no drug may be administered) have been determined and set by FDA for major drugs used by veterinarians in the treatment of terrestrial food producing animals. Hence, the problem of persistent residues has long been recognized by the medical profession, but only recently has it been investigated in cultured aquatic animals. The Center for Veterinary Medicine of FDA is aware of the seriousness of this problem and is actively attempting to deal with it, as evidenced by its recent requests for proposals in the April 9, 1990 issue of Business Commerce Weekly (Guarino et al., 1988; Mitchell, 1989). The proposals will study the pharmacokinetics and methods of monitoring some six drugs, including malachite green and chloramphenicol, presently used in fish and crustacean culture. In addition to this organization, active research is being conducted by the Fisheries Research Branch of the FDA and the Fisheries Research Center, La Crosse, Wisconsin of the U.S. Fish and Wildlife Service (Schnick, 1988). The latter organization has published a booklet entitled "A Guide to Approved Chemicals in Fish Production and Fishery Resource Management" (Schnick et al., 1989). Although there is much interest in the potential toxicity of chemotherapeutic drug residues in seafood, to date only a potentiated sulfonamide (Romet-30), oxytetracycline (Terramycin), and Formalin are approved for use in food producing aquatic animals.
The nature, use, and residues of the drugs applicable to aquaculture that are used to control disease in veterinary medicine have been reviewed (Bevill and Huber, 1977; Booth, 1977; Huber, 1977a-c; Michel, 1986).
Chemotherapeutic drugs used in aquaculture are sulfonamides, antibiotics, and drugs used in the chemotherapy of protozoal, mycotic, and helminthic infections.
In addition to these examples of deliberately used chemicals, fish raised in aquaculture are also susceptible to contamination via pesticides present in feed, agricultural runoff water, and sediments. The magnitude of human exposure to these sources has not yet been assessed and should be examined periodically in light of the growth and change in this sector of the seafood industry.
Sulfonamides
The sulfonamides are readily synthesized derivatives of sulfanilic acid. They are bacteriostatic drugs, and their efficacy may be increased by the addition of ormetoprim (Plakas et al., 1990). Commonly, sulfadimethoxine is potentiated with ormetoprim (Romet-30) and fed to cultured fish at the rate of 50 mg/kg body weight for 5 days. Similarly, sulfamethoxazole is potentiated with trimethoprim (Tribrissen) and fed at the rate of 1-4 mg/kg body weight for 10-14 days. The latter drug is not approved for use in aquaculture. The FDA tolerance level for both these drugs in cattle and chickens is 0.1 ppm (Booth, 1977).
The sulfonamides have been implicated in renal damage, urinary obstructions, and hematopoietic disorders. For that reason, FDA has set withdrawal times and residue tolerance levels to protect public health. The withdrawal period for catfish is 3 days and for trout 6 weeks (Schnick et al., 1989).
Antibiotics
The term antibiotic includes a large number of drugs inimical to the growth of microorganisms. Of greatest interest in aquaculture are oxytetracycline, ampicillin, chloramphenicol, and erythromycin.
Oxytetracycline (Terramycin) is a product of the mold Streptomyces rimosus. It is a broad-spectrum antibiotic used to treat a number of bacterial diseases in fish and crustaceans. It is the only antibiotic approved by the FDA for use in aquaculture. Contamination of the food supply with the drug is a public health concern because chronic exposures at low levels may lead to a higher incidence of antibiotic-resistant bacterial strains, poor growth of teeth, and the possibility of photosensitivity. The FDA tolerance level for oxytetracycline in meat is 0.25 ppm, whereas in fish it is 0.1 ppm with a withdrawal time of 21 days. Elimination time from muscle appears longer for salmonids than for catfish and is dependent on the water temperature (Plakas et al., 1988).
Ampicillin is a newer, semisynthetic penicillin that has activity against both gram-positive and gram-negative organisms. Although not approved for use in the United States and seldom used domestically, it is commonly used in Japan to control pasteurellosis in yellowtail culture (Hawke et al., 1987). Ampicillin, like its congeners, may result in severe hypersensitivity reactions in some people (Huber, 1977a). Because an initial exposure to some form of penicillin is necessary to produce an eventual drug allergy, ingestion of ampicillin as a residue in seafood is of public health interest. The FDA has set a tolerance level of 0.01 ppm and a preslaughter withdrawal time in cattle of 6 days. No information is available regarding residues or withdrawal time in fish or crustaceans.
Chloramphenicol is a product of the mold Streptomyces venezuelae and is a potent antibiotic that is effective against most bacteria, as well as rickettsia and the psittacosis-lymphogranuloma group of organisms. Development of resistant bacteria may follow its widespread and uncontrolled use. Chloramphenicol administered for long periods may cause blood dyscrasias such as aplastic anemia and has recently been incriminated as a carcinogen. It is not approved for use in the United States in food producing animals. The drug is used, however, with impunity to control bacterial diseases of shrimp, especially in Ecuador and in European fish culture (Manci, 1990; Meyer and Schnick, 1989). Because imported seafood is not checked for antibiotics, no information is available regarding its residue levels.
Erythromycin is a product of the mold Streptomyces erythreus and is primarily effective against gram-positive organisms. The drug is not approved for use in domestic fish culture but is used in Europe. The tolerance level in U.S. swine is 0.01 ppm, and the withdrawal time is 7 days. The drug is not considered an important cause of hypersensitivity in man and is relatively nontoxic.
Nitrofurans
Nitrofurans are synthetic compounds that are active against most gram-negative bacteria, some fungi, and some protozoal organisms. Because of their toxicity, their clinical use is limited and FDA has attempted unsuccessfully for over 15 years to ban their use in food producing animals and to have them withdrawn from the market (FDA, 1982). Side effects include bleeding, gastrointestinal upsets, and allergic reactions. Tolerance levels for the drug have been set at zero. In aquaculture, their use has been suggested for the treatment of Ichthyophthirius multifillis.
Conclusions
-
Certain drugs with potential toxicity to humans are used to control disease in cultured food producing aquatic animals.
-
There is a paucity of information regarding the withdrawal times, residue levels, and pharmacokinetics in the cultured aquatic animals receiving these drugs.
-
There is some reason for concern that large amounts of imported cultured seafood, which is routinely treated with drugs, are consumed by the American public, although the magnitude of ultimate human exposure from this source is as yet uncertain.
-
Cultured seafood imported into the United States is not presently inspected for drug residues.
Contaminants as a Result of Processing
Nitrosamines
Nitrosamines are formed in smoked fish products and in the human stomach as the result of the simultaneous presence of secondary amines and nitrite. Since the late 1960s, the use of nitrite in smoked fish has been authorized to avoid the repetition of botulism incidents in the early 1960s that resulted from the mishandling of vacuum packed smoked fish. [Earlier joint industry/FDA recommendations on the time and temperature cooking of smoked fish products were reportedly not complied with by Great Lakes producers, and the food additive petition was later granted after it was shown that nitrosamine formation in the fish itself did not exceed a specific level of sensitivity specified by FDA (Hattis, 1972).] The exact extent of the extra exposure to specific nitrosamines attributable to the use of nitrite as a food additive in smoked fish has apparently not been reappraised recently by using more sensitive analytical techniques and current risk assessment procedures.
Products of chlorination, bromination, and iodination
Chlorine and some other active halogen compounds are widely used as disinfectants in seafood processing, as well as in the treatment of drinking water and sewage effluents (Fukayama et al., 1986). This process is known to generate some
levels of halogenated amines, aromatics, and methanes (e.g., chloroform), and at least one test in chiller water used in chicken processing detected mutagenic activity when the chlorination level was raised to 250 ppm (Masri, 1986). The extent of contamination of seafood with products resulting from the use of chlorine and other halogen compounds does not appear to have been assessed, and there are no relevant appraisals of the associated risks in the available literature. Some relevant information may be contained in food additive petitions for disinfectants that have been submitted to FDA, but the committee has not obtained these documents.
Residues of ozonation
Ozone treatment is frequently used in foreign settings as a method of depuration of some shellfish (Fauvel et al., 1982). This technology has also been introduced into the United States for use in icing fish (Rice et al., 1982), washing seafoods, and cleansing saltwater for use in molluscan shellfish depuration. In recent years, FDA has raised questions about the safety of this process because of the likelihood of residual reaction by-products of the resulting oxidation.5 This concern was recently debated during the First International Molluscan Shellfish Depuration Conference, November 5-8, 1989 in Orlando, Florida (W.S. Otwell, University of Florida, Gainesville, personal communication, 1989) and later in letters from the FDA Compliance Branch (J.A. Baca, FDA, personal communication, June 22, 1990) explaining its position on the use of ozone in food manufacturing facilities. The primary concern is any ozone contact with seafood whereby the ozone could become a component of the food or affect the character of the food. The prevailing differences of opinion await more technical resolution in studies to monitor the consequences from direct and indirect (treating depuration water) applications. Cognizant of the current data, the FDA regulatory interpretations to restrict use will most likely prevail.
Sulfites
Sulfites have traditionally been used to prevent melanosis in crustaceans (Camber et al., 1956). Sanctioned procedures include a 1-minute dip in concentrations of up to 1.25% sodium bisulfite or metabisulfite. This has been shown to impart a residual sulfite level of less than 100 ppm on the edible portion of penaeid shrimp.6 Product treated in this way must be labeled to designate prior use and residuals in excess of 10 ppm. General concern for allergic-like reactions, particularly in some asthmatic people, has given rise to concern about the continued use and appropriate labeling of sulfites (Lecos, 1985). No equally effective alternative processes have yet been developed for crustaceans.
DATA ON THE DISTRIBUTION OF CHEMICAL CONTAMINATION
Introduction
Some modest concentrations of contaminants are ubiquitous in the clean (natural, pristine, nonenhanced, unimpacted) aquatic environment. A few metals, such as copper, selenium, iron, and zinc, are essential nutrients for fish and shellfish. Contamination occurs from both natural and anthropogenic sources, and can be said to exist when there is a statistically significant increase in geometric mean levels in comparable organisms, suitably adjusted for confounders.
Evidence for such higher detectable levels may be found in a number of studies conducted by university scientists and by state and federal agencies. Of major importance are ongoing studies by the National Oceanic and Atmospheric Administration (NOAA) and the U.S. Fish and Wildlife Service (USF&WS). The NOAA data are generated by the National Status and Trends Program, which examines fish and shellfish annually from more than 145 coastal sites in the United States (NOAA, 1987, 1989). Similarly, the USF&WS conducts the National Pesticides Monitoring Program, which examines fish from 115 freshwater sites in the 50 states (Lowe et al., 1985). In addition, the FDA conducts periodic inspections of domestic and imported seafoods (FDA, 1988). These federal programs are bolstered by extensive studies and reviews conducted on a regional basis by various researchers (Capuzzo et al., 1987; Clark et al., 1984; Landolt et al., 1985, 1987; Malins et al., 1980, 1982; Murphy, 1988a-c; Rohrer et al., 1982; St. Amant et al., 1983). Further, a careful literature review reveals a large number (100+) of publications on the subject of trace-metal contamination both in peer-reviewed journals and among state documents (Duling, 1988; Sloan et al., 1987).
This data base confirms that high levels of contaminants exist in various aquatic animals in some places. It has, however, a number of shortcomings for use in risk assessment. First, the more extensive studies have considered metal levels in the nonedible portions of finfish or in the whole fish. This prevents accurate determination of dosages. Second, reports vary in the data presentation (geometric versus arithmetic means), some failing to report sample size, mean values, or animal size, thus further preventing careful statistical analysis and risk assessment.
National Status and Trends Program
The NOAA National Status and Trends (NS&T) program is an extensive federal program under the direction and management of the Ocean Assessments Division of NOAA (OAD) that monitors levels of toxicants annually (routine surveillance) in shellfish (Mussel Watch) and finfish (Benthic Surveillance) from approximately 150 coastal and estuarine sites in the continental United States, Alaska, and Hawaii (NOAA, 1989). Structured in a three-tiered design, NOAA states the objectives as:
-
to determine toxic contaminants as a basis for the identification of potential geographic differences (Mussel Watch, Tier 1);
-
to identify areas where environmental quality may be significantly compromised (Mussel Watch);
-
to determine significant temporal trends in toxic contaminant levels on a national basis (Mussel Watch); and
-
to evaluate and synthesize existing sources of information pertinent to the status of contaminants in selected areas (Historic Trend Assessment, Tier 2), and in Tier 3 (Verification) to augment the basic monitoring program as needed in areas indicated by Tier 1 results.
The NS&T has been in existence since 1984 and was an outgrowth of the previous Mussel Watch program. It produces an excellent data base from which scientists may evaluate levels of contamination and their spatial and temporal differences. Sites are numerous and evenly distributed geographically. Species of animals examined well represent the particular area monitored and, in the case of invertebrates, may be in the same genus nationally, thus decreasing physiological differences. Most important, the NOAA Quality Assurance Program used by NS&T establishes analytical protocols that ensure reliability of data.
It is unfortunate, from the point of view of human exposure assessment, that the NS&T program examines fish liver samples rather than edible portions. Extrapolation from liver to muscle contaminant levels is fraught with uncertainty, and the data are essentially useless for this purpose. In all fairness, however, it was and is not the objective of the NS&T Benthic Surveillance program to supply such information, and its design fulfills its objectives admirably.
Federal Survey of PCBs in Atlantic Coast Bluefish
A NOAA (1987) interpretative report is based on a sampling of hundreds of bluefish (Pomatomus saltaltrix) from New England to the Atlantic Coast of Florida that was undertaken as part of a 1984 congressional mandate resulting from the discovery of relatively high PCB contamination in bluefish from New Jersey and New York waters. The wide-ranging migratory nature of bluefish, which are found along the entire Atlantic Coast of the United States, led to speculation that this highly prized and abundant sport species could be of special concern from a public health perspective. In fact, bluefish constitute the principal recreational species in terms of landings (130-155 million pounds annually) along the Atlantic Coast.
National Contaminant Biomonitoring Program
The U.S. Fish and Wildlife Service National Contaminant Biomonitoring Program (NCBP) is a continuing survey in which freshwater fish are collected from 112 stations located throughout the United States (Lowe et al., 1985). Three composite samples of three to five fish are collected at half the stations in odd years and the other half in even years. During 1978-1981, 60 species were collected; however, a common species was collected at only 39 stations and no species were collected in common at 24 stations (Schmitt et al., 1983). Analyses are conducted on whole fish samples that are homogenized and lyophilized. Precision and accuracy of analysis are estimated by duplicate samples and by the use of reference materials from the National Bureau of Standards and FDA. Data generated by the NCBP may be used
to identify geographic areas of greatest concern and temporal variations. However, although the NCBP is an excellent, synoptic, national approach to the contamination of freshwater fish, it suffers from the same inadequacy as the NS&T program with regard to human exposure assessment because the samples examined are not just edible portions but include the whole animal. Furthermore, the large variation in species among collection points and between years adds to the difficulty. In this case, however, the difficulty in projection seems much less serious than in the case of fish liver.
Regional Reports
Regional reports provide other important data for examining industrial chemical and pesticide concentrations in certain species of aquatic organisms and for estimating the intake of sport and subsistence fishers. The committee relied on reports from Quincy Bay, Massachusetts; New York; southern California; and Puget Sound, Washington.
EVIDENCE FOR TRACE-METAL AND ORGANIC CONTAMINATION
This section provides an overview of both the level and the variability of contaminant concentrations, as inferred from the data bases reviewed above. The variability of contaminant concentrations among geographic areas is important because it indicates the potential for reduction of exposure through restrictions on the harvesting of aquatic organisms from specific sites. Therefore, wherever possible, the committee summarizes geographic variability data in the form of figures that show the data analyzed as lognormal distributions. In these figures, conformance to the assumption of lognormality is indicated by the correspondence of the points to a straight line. The lognormal standard deviations (slopes of the lines in these figures) allow an approximate calculation of the percentage of the aggregate fish or shellfish dose of the contaminant that could be avoided by restricting harvesting from various proportions of the sites in order of their mean concentrations. This analysis will be pursued further below.
Molluscan Shellfish
Trace Metals
National Status and Trends data for shellfish residues are summarized in Table 5-1.
Arsenic (As)
-
Mean levels: Descriptive statistics of all NS&T shellfish data are presented in Table 5-1 and Figure 5-1.7 The arithmetic grand mean arsenic levels of all bivalves
TABLE 5-1 Shellfish Contaminants (ppm wet weight)a
|
Contaminant |
Mean ± SD |
Percentiles |
Geographic Distribution and Sites Exceeding 95th Percentile |
Temporal Trends 1986-1988b (% change) |
||||
|
5th |
25th |
50th |
75th |
95th |
||||
|
PCB |
0.052 ± 0.102 |
0.003 |
0.008 |
0.017 |
0.047 |
0.200 |
All coastal sites. AB, H/R, NYSB, SD, BB, BH |
-13 |
|
DDT |
0.010 ± 0.02 |
0.001 |
0.002 |
0.004 |
0.011 |
0.032 |
All coastal sites. H/R, SPH, PV, AB, SFB, CBF, NYSB, BB |
+4; -6 |
|
PAH |
0.158 ± 0.354 |
0 |
0.009 |
0.049 |
0.157 |
0.509 |
All coastal sites. EB, H/R, SFB, BP, BH, LIS |
+8; -10 |
|
Arsenic |
1.390 ± 0.81 |
0.600 |
0.892 |
1.12 |
1.694 |
2.879 |
All coastal sites. CF, CK, CH, CRH, RB, SS, SRE |
+6; -8 |
|
Cadmium |
0.434 ± 0.275 |
0.106 |
0.247 |
0.376 |
0.552 |
0.925 |
All coastal sites. COP, DB, CB, H/R, BH/LIS, NBR |
+4; -17 |
|
Lead |
0.230 ± 0.366 |
0.02 |
0.05 |
0.09 |
0.289 |
0.733 |
All coastal sites. MD, AB, H/R, BH, LIS, NBR |
+5; -1 |
|
Mercury |
0.015 ± 0.01 |
0.004 |
0.008 |
0.012 |
0.020 |
0.036 |
All coastal sites. TB, H/R, BP, MB, BH, MRB, CH |
+11; -4 |
|
Selenium |
0.318 ± 0.122 |
0.15 |
0.244 |
0.304 |
0.360 |
0.523 |
All coastal sites. HH, BP, ABT, EST, UI, PC, COP |
+12; -2 |
|
NOTE: AB Anaheim Bay, Calif.; ABT Aransas Bay, Tex.; BB Buzzards Bay, Mass.; BH Boston Harbor, Mass.; BP Barbers Pt., Hawaii; CB Chesapeake Bay, Md.; CBF Choctawatchee, Fla.; CF Cape Fear, N.C.; CH Charlotte Harbor, Fla.; CK Cedar Key, Fla.; COP Copano Bay, Tex.; CRH Charleston Harbor, S.C.; DB Delaware Bay, Del.; EST Espiritu Santo, Tex.; EB Elliot Bay, Wash.; HH Honolulu Harbor, Hawaii; H/R Hudson/Raritan Bay, N.Y.; LIS Long Island Sound, N.Y.; MB Matagorda Bay, Tex.; MD Marina Del Ray, Calif.; MRB Moriches Bay, N.Y.; NBR Narragansett Bay, R.I.; NYSB New York State Bight of New Jersey; PC Point Concepcion, Calif.; PV Palos Verdes, Calif.; RB Rookery Bay, Fla.; SD San Diego Bay, Calif.; SFB San Francisco Bay, Calif.; SPH San Pedro Harbor, Calif.; SRE Savannah River Estuary, Ga.; SS Sapelo Sound, Fla.; TB Tampa Bay, Fla.; UI Unakit Inlet, Alaska. a 1987 NS&T data. b + = sites increased in number; - = sites decreased. SOURCE: NOAA (1989). |
||||||||
-
from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 2.763 ppm wet weight with a standard deviation of 0.9340 and a range of 1.920-5.131 ppm wet weight (using a dry weight/wet weight conversion factor of 0.12). Eight of the most contaminated sites (2.9604 to 5.1312 ppm wet weight) exceed the 95th percentile (2.8794).
-
Median levels: The median of the arithmetic grand means of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 2.559 ppm wet weight (range 1.920-5.1310). The 1986 Mussel Watch (145 sites) national grand median for arsenic is 1.120 ppm wet weight with a range of 0.2352-5.119 ppm (dry weight/wet weight conversion factor = 0.12).
-
Geographic distribution: Arsenic was present in both oysters and mussels from all sites examined by the 1986 Mussel Watch Survey. Of the 25 most contaminated sites, 10 were in the Southeast (North and South Carolina, Georgia, and Florida) 8 in California, 1 in the mid-Atlantic region (Chesapeake Bay), and none in the Northeast or Northwest. The eight sites exceeding the 95th percentile are Cape Fear, N.C.; Cedar Key, Fla.; Charlotte Harbor, Fla.; Charleston Harbor (CHFJ and CHSF), S.C.; Rookery Bay, Fla.; Sapelo Sound, Ga.; and Savannah River Estuary, Ga.
-
Temporal trends: The most recent NS&T data indicate increases in 6 and decreases in 8 of 177 sites studied. None of the most contaminated sites showed decreases.
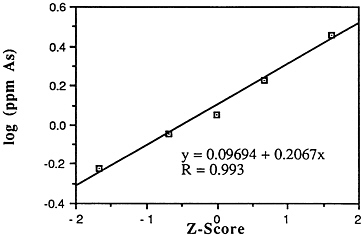
FIGURE 5-1 Lognormal distribution of wet weight concentrations of arsenic in bivalves (NS&T data set)
Cadmium (Cd)
-
Mean levels: Descriptive statistics of all NS&T shellfish data are presented in Table 5-1 and Figure 5-2. The arithmetic grand mean level for cadmium of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 0.9039 ppm wet weight with a standard deviation of 0.2621 and a range of 0.6276-1.560 ppm (using a dry weight/wet weight conversion factor of 0.12). Eight of the most
-
contaminated sites (0.9324-1.56 ppm wet weight) exceed the 95th percentile (0.9252).
-
Median levels: The median of the arithmetic grand means of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 0.8244 ppm wet weight (range 1.920-5.1310). The 1986 Mussel Watch (145 sites) national grand median for cadmium is 0.3756 ppm wet weight with a range of 0.0240-1.5600 ppm (dry weight/wet weight conversion factor = 0.12).
-
Geographic distribution: All sites examined by the NS&T program contained bivalves with tissue cadmium burdens. Of the 25 most contaminated sites, 10 were located in the Gulf of Mexico, 6 in the Chesapeake Bay area, 7 on the West Coast (6 California), and 2 in the Northeast. The eight most contaminated sites are Copano Bay, Tex.; Delaware Bay (DBAP), Del.; Chesapeake Bay, Md.; Delaware Bay (DBKI), Del.; Hudson/Raritan Estuary, N.Y.; Corpus Christi, Tex.; Mississippi Sound, Miss.; and Delaware Bay (DBBD), Del.
-
Temporal trends: The most recent NS&T data indicate increases in only 4 and decreases in 17 of 177 sites studied; 4 of the sites reported as having decreased levels are sites previously listed among the 10 most contaminated.
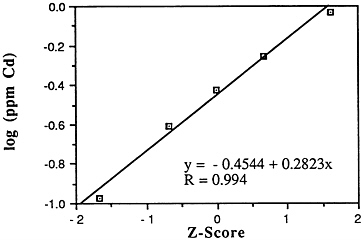
FIGURE 5-2 Lognormal distribution of wet weight concentrations of cadmium in bivalves (NS&T data set)
Lead (Pb)
-
Mean levels: Descriptive statistics of all NS&T shellfish data are presented in Table 5-1 and Figure 5-3. The arithmetic grand mean lead level of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 0.8203 ppm wet weight with a standard deviation of 0.5684 and a range of 0.3804-2.799 ppm (using a dry weight/wet weight conversion factor of 0.12). Eight of the most contaminated sites (0.7356-2.7996 ppm wet weight) exceed the 95th percentile (0.7326).
-
Median levels: The median of the arithmetic grand means of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 0.6240 ppm wet weight (range 0.3804-2.799). The 1986 Mussel Watch (145 sites) national grand median for lead is 0.0900 ppm wet weight with a range of 0.0108-2.7996 ppm (dry weight/wet weight conversion factor = 0.12).
-
Geographic distribution: All Mussel Watch sites reported bivalves containing lead. Of the 25 most contaminated sites, 15 were in the Northeast including the Hudson River/Raritan Estuary, Boston Harbor, Long Island Sound, New York Bight, and Narragansett Bay. Eight of the remaining sites–including the most contaminated (Marina Del Rey)–were in California. Those sites exceeding the 95th percentile are Marina Del Rey, Calif.; Anaheim Bay, Calif.; Hudson/Raritan Estuary, N.Y.; Boston Harbor (BHDB, BHBB, and BHDI), Mass.; Long Island Sound, N.Y.; and Narragansett Bay, R.I.
-
Temporal trends: The most recent NS&T data indicate increases in 5 sites and decreases in only 1 of the 177 studied (Barber's Point, Hawaii). None of the increases occurred in the previously reported most contaminated sites.
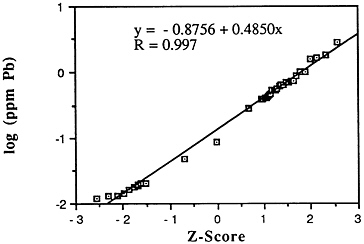
FIGURE 5-3 Lognormal distribution of wet weight concentrations of lead in bivalves (NS&T data set)
Mercury (Hg)
-
Mean levels: Descriptive statistics of all NS&T shellfish data are presented in Table 5-1 and Figure 5-4. The arithmetic grand mean mercury level of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 0.0351 ppm wet weight with a standard deviation of 0.0084 and a range of 0.0276-0.0576 ppm (using a dry weight/wet weight conversion factor of 0.12); 7 of the most contaminated sites (0.0372-0.0576 ppm wet weight) exceed the 95th percentile (0.0363).
-
Median levels: The median of the arithmetic grand means of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 0.0324 ppm wet weight (range 0.0276 to 0.0576). The 1986 Mussel Watch (145 sites) national grand median for mercury is 0.0120 ppm wet weight with a range of 0.0012-0.0576 ppm (dry weight/wet weight conversion factor = 0.12).
-
Geographic distribution: Mercury was found in bivalves from all coastal sites (145) examined by the Mussel Watch Survey. Distribution of the 25 most contaminated sites included all major geographic areas; 9 sites were in the Southeast, all in Florida; 7 sites in the Northeast, most in the New York/New Jersey area; 5 in California; 2 in the Pacific Northwest; and 2 in Hawaii. The seven sites exceeding the
-
95th percentile are Tampa Bay, Fla.; Hudson/Raritan Estuary, N.Y.; Barber's Point, Hawaii; Matagorda Bay, Tex; Boston Harbor, Mass; Moriches Bay, N.Y.; and Charlotte Harbor, Fla.
-
Temporal trends: The most recent NS&T data indicate increases in shellfish tissue mercury in 11 sites and decreases in only 4 sites of 177 studied. Of the ten most contaminated sites, one (Barber's Point, Hawaii) showed a decrease, whereas another (Hudson Raritan Estuary) had an increase. Increases occurred primarily in the northeastern and southern coastal areas.
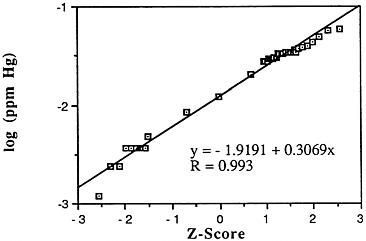
FIGURE 5-4 Lognormal distribution of wet weight concentrations of mercury in bivalves (NS&T data set)
Selenium (Se)
-
Mean levels: Descriptive statistics of all NS&T shellfish data are presented in Table 5-1 and Figure 5-5. The arithmetic grand mean selenium level of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 0.5145 ppm wet weight with a standard deviation of 0.1391 and a range of 0.3996-0.9800 ppm (using a dry weight/wet weight conversion factor of 0.12); 8 of the most contaminated sites (0.5364-0.9804 ppm) exceed the 95th percentile (0.5232).
-
Median levels: The median of the arithmetic grand means of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 0.4560 ppm wet weight (range 0.3996-0.9800). The 1986 Mussel Watch (145 sites) national grand median for selenium is 0.3036 ppm wet weight with a range of 0.1116-0.9800 ppm (dry weight/wet weight conversion factor = 0.12).
-
Geographic distribution: All 145 sites examined by the NS&T program revealed selenium in indigenous bivalves; 14 of the 25 most contaminated sites were in Texas (Arkansas Bay, Espiritu Santo, Copano Bay, Matagorda Bay, San Antonio Bay, Galveston Bay, Mesquite Bay) or California (Marina Del Rey, Pt. Concepcion, Pt. Delgada, Santa Catalina Island, Bodega Bay, La Jolla, Pt. Dume). Highest levels were found in oysters in Honolulu Harbor and Barber's Pt., Hawaii. Other sites included
-
Chesapeake Bay and Hudson River/Raritan estuary. Sites that exceed the 95th percentile are Honolulu Harbor, Hawaii; Barber's Point, Hawaii; Arkansas Bay, Tex.; Espiritu Santo, Tex.; Marina Del Rey, Calif.; Unakwit Inlet, Alaska; Point Concepcion, Calif.; and Copano Bay, Tex.
-
Temporal trends: The most recent NS&T data indicate shellfish tissue increases in 12 sites and decreases in only 2 sites (Commencement Bay, Wash., and Honolulu Harbor) of 177 sites studied. Those sites recording increases in selenium tissue content were primarily in the southern region, including four in Florida, five in Louisiana, and one each in Mississippi and Texas.
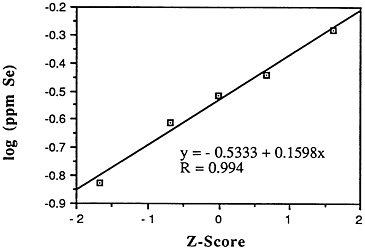
FIGURE 5-5 Lognormal distribution of wet weight concentrations of selenium in bivalves (NS&T data set)
Organics
Polychlorinated biphenyls
Polychlorinated biphenyls have been detected as contaminants in the marine environment for nearly five decades and in marine fish for nearly four decades (NOAA, 1988). Concentrations of total PCBs have ranged in fish muscle from below detection to 730 ppm wet weight in an American eel collected from New Bedford Harbor, Mass., in 1979 (NOAA, 1988). For bivalve molluscs, the most contaminated sites are located along the Northeast coast and in southern California harbors. The grand national median for PCBs calibrated against the commercial mixture Aroclor 12428 in the 1976 Mussel Watch Survey at 86 sites was 0.009 ppm wet weight with a range of 0.0008-2.09 ppm. Although not strictly comparable because of analytical, site, and species differences, preliminary calculations indicate that the grand national median of total PCBs in the 1986 Mussel Watch Survey at 144 sites was 0.017 ppm wet weight with a range of 0.0009-0.68 ppm (Table 5-1, Figure 5-6).
Descriptive statistics of all NS&T shellfish data are presented in Table 5-1. The arithmetic grand mean PCB levels of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 0.205 ppm wet weight with a standard deviation of 0.176 (range 0.0728-0.817 ppm) using a dry weight/wet weight conversion factor of 0.12.
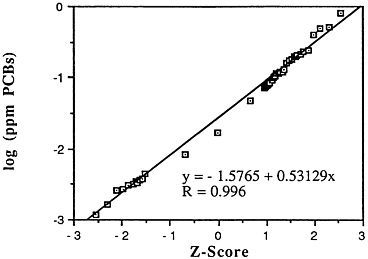
FIGURE 5-6 Lognormal distribution of wet weight concentrations of PCBs in bivalves (NS&T data set)
The median of the arithmetic grand means of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 0.1287 ppm wet weight (range 0.0728-0.8169). The national grand median for total polychlorinated biphenyls (tPCBs) in 145 sites was 0.0172 ppm wet weight with a range of 0.0011-0.8170 ppm (dry weight/wet weight conversion factor, 0.12). The most contaminated sites (exceeding the 95th percentile) were Buzzards Bay, Mass.; Hudson/Raritan Estuary (HRLB), N.Y.; New York Bight (NYSR), N.J.; Hudson/Raritan Estuary (HRUB), N.Y.; San Diego Bay, Calif.; Galveston Bay, Tex.; New York Bight (NYSH), N.J.; and Boston Harbor, Mass.
Although a large body of data exists from state local programs, to date these have not been carefully analyzed. Gulf Coast sites appear undersampled. With present data it is difficult to determine if PCBs are increasing or decreasing nationally. Certain specific sites such as Whites Pt., Calif.; Escambia Bay, Fla.; Narragansett Bay, R.I.; and Chesapeake Bay, Md. have shown major decreases. However, other sites such as Boston Harbor and Beaufort, N.C. have shown no change or slight increases (Mearns et al., 1988). Most recent NS&T data (1986-1988) now including 177 sites indicate no increases in PCBs and decreases in 13 sites including 2 of the most contaminated (Boston Harbor and the Hudson River).
Polyaromatic hydrocarbons
Polyaromatic hydrocarbons contain some prominent carcinogens such as benzo[a]pyrene. In a recent survey of contaminants in hard-shell clams (Mercenaria mercenaria) in the vicinity of Alan Harbor, R.I. (the location of a military hazardous waste disposal site), PAHs were the only group of contaminants that appeared to show a gradient of increasing concentration in areas nearest the site (Hattis, 1989). Unfortunately, for purposes of risk assessment, benzo[a]pyrene, whose carcinogenic activity is relatively well characterized, constitutes only a minor fraction of the total PAHs found either in marine sediments or in shellfish (in the Alan Harbor data, benzo[a]pyrene averaged about 1% of the total PAHs measured—total PAHs in clams were about 0.8 ppm dry weight, about half of that elsewhere in Narragansett Bay). Innovative approaches to assessing the relative hazard of some other PAHs have been proposed (Rugen et al., 1989).
Very little good data exist nationwide on the extent of PAH contamination. This is of particular concern for animals lower on the food chain such as bivalves. Researchers purchased quahogs (Mercenaria mercenaria ) from 13 markets throughout Rhode Island and analyzed them for PAHs (Pruell et al., 1984). Levels observed varied widely between stores and also between repeat visits to the same store. These data indicate clearly that shellfish consumers have the potential to purchase quahogs with elevated levels of nonregulated, carcinogenic organic contaminants.
Descriptive statistics of all the NS&T shellfish data are presented in Table 5-1 and Figure 5-7. The arithmetic grand mean of PAH levels of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 0.6471 ppm wet weight with a standard deviation of 0.6458 (range 0.2400-2.760 ppm) using a dry weight/wet weight conversion factor of 0.12; 7 of the most contaminated sites (0.6360-2.7600 ppm wet weight) exceed the 95th percentile (0.5090).
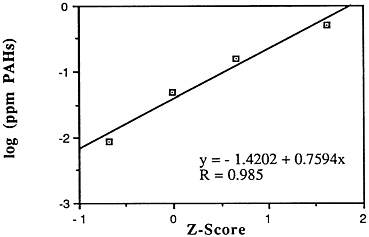
FIGURE 5-7 Lognormal distribution of wet weight concentrations of PAHs in bivalves (NS&T data set)
The median of the arithmetic grand means of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 0.396 ppm wet weight (range 0.240-2.76). The national median PAH for 145 sites is 0.0492 ppm wet weight with a range of 0.0000-2.79 ppm (dry weight/wet weight conversion factor, 0.12).
The geographic distribution is widespread, from Maine to Washington including Alaska and Hawaii. The seven most heavily contaminated sites ranked from highest to lowest are Elliot Bay, Wash.; Hudson/Raritan Estuary, N.Y.; St Andrew Bay, Fla.; Barber's Point, Hawaii; Boston Harbor (BHDB and BHHB), Mass.; and Long Island Sound, N.Y.
Most recent NS&T data (1986-1988) now including 177 sites indicate increases in PAHs (high- and low-molecular-weight data combined) in 8 sites and decreases in 10 sites, including only one of the most contaminated (Hudson Raritan Estuary).
Chlorinated hydrocarbon pesticides
DDT and metabolites: According to NOAA, the pesticide DDT and its metabolites are among the most widespread and frequently sampled chlorinated hydrocarbons. In contrast with PCBs, DDT concentrations in seafood have declined dramatically in the last 15 years, perhaps as much as 100-fold nationally (NOAA, 1988). Traces of DDT have been found in marine samples from every coastal state, at many offshore and deep-water sites, and from nearly every estuary. In fact, DDT and metabolites were found in 63% of the 8,095 oysters, clams, and mussels from 180 sites sampled during the National Pesticides Monitoring Program of NOAA (NPMP) (NOAA, 1988). Mean concentrations ranged from below detection to 1.4 ppm wet weight at Iona Point in southeast Florida. When the NPMP survey resampled all sites in 1977, total DDT concentrations had fallen below the 0.01 ppm wet weight detection limit everywhere except at the Point Mugu Lagoon site near Oxnard in southern California and at sites in upper Delaware Bay. A 1976-1978 Mussel Watch Survey analyzing for DDE and using more sensitive detection limits yielded many more positive detection results (ranging from 0.001 to 0.010 ppm wet weight) from sites where there were previously no detectable levels. Still, total DDT (tDDT) concentrations in bivalves have declined nearly an order of magnitude during the past two decades.
Descriptive statistics of all NS&T shellfish DDT data are presented in Table 5-1 and Figure 5-8. The arithmetic grand mean of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 0.0365 ppm wet weight with a standard deviation of 0.0353 (range 0.0570-0.1330 ppm) using a dry weight/wet weight conversion factor of 0.12. The eight most contaminated sites (0.0322-0.1330 ppm wet weight) exceed the 95th percentile (0.0321).
The median of the arithmetic grand means of all bivalves from the 25 most contaminated sites reported in the 1986 Mussel Watch Survey is 0.0229 ppm wet weight (range 0.0570-0.1330 ppm). The national grand median of tDDT for 145 sites was 0.0039 ppm wet weight with a range of 0.00002-0.133 ppm (dry weight/wet weight conversion factor, 0.12) (NOAA, 1987).
Contamination of coastal sites extends from Maine to Washington and includes Hawaii and Alaska. Of the 25 most contaminated sites, 9 are located in California (San Pedro Harbor, Palos Verdes, Anaheim Bay, San Francisco Bay, Imperial Beach),
5 in New York (Hudson/Raritan Estuary, Long Island Sound), 3 in New Jersey (New York Bight), and 1 site each in Texas, Virginia, and Massachusetts. Those exceeding the 95th percentile are Hudson/Raritan Estuary (HRLB), N.Y.; San Pedro Harbor, Palos Verdes, Anaheim Bay, and San Francisco Bay, Calif.; Choctawahatchee Bay, Fla.; New York Bight (NYSH), N.J.; and Buzzards Bay, Mass.
Since the estuarine mollusc survey of 1965-1972, the median tDDT level has decreased nationally nearly an order of magnitude from 0.024 to 0.003 ppm wet weight (Mearns et al., 1988). Most recent NS&T data (1986-1988) now including 177 sites indicate increases in DDT in 4 sites and decreases in 6 sites, including 3 of the most contaminated (Buzzards Bay, Chesapeake Bay, and HRLB).
Chlordane: According to NOAA, chlordane did not occur above the detection limit of 0.01 ppm wet weight in any of the more than 8,000 samples analyzed during the 1965-1972 or 1977 NPMP estuarine bivalve monitoring activities. However, chlordane compounds have frequently occurred in shellfish from other local and regional surveys, such as the California Mussel Watch. Chlordane was second only to DDT and PCBs in abundance in 1981-1982 samples of marine life from the Gulf of Alaska and the Bering Sea (NOAA, 1988).
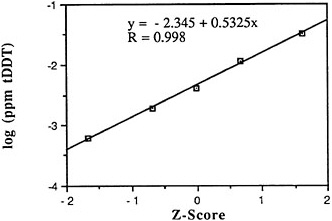
FIGURE 5-8 Lognormal distribution of wet weight concentrations of DDT and metabolites in bivalves (NS&T data set)
Heptachlor and heptachlor epoxide: Heptachlor and heptachlor epoxide did not occur above the 0.01-ppm wet weight detection limit in any of more than 8,000 shellfish samples analyzed between 1965 and 1977 during the NPMP.
Endosulfan: Endosulfan may be a problem contaminant in estuaries near agricultural drainage areas (NOAA, 1988). It should be noted that endosulfan is currently in widespread use as an agricultural pesticide and that data are clearly insufficient to judge current nationwide environmental contamination. The highest concentration of total endosulfan detected in marine shellfish is about 1.4 ppm wet weight in a 1983-1984 sample of bay mussels from Moss Landing, Monterey County, California (NOAA, 1988).
Chlorinated benzenes and phenols: Galveston Bay oysters contained pentachlorophenol (penta) concentrations ranging from 0.003 to 0.008 ppm wet weight. In
Puget Sound, clams from Eagle Harbor, site of a wood-treatment operation, had concentrations of 0.003-0.008 ppm wet weight. Contamination has been documented from native and transplanted mussels in the northern part of Humbolt Bay. Pentachlor – anisole – a metabolite of penta – was detected in 24% of the whole fish sampled in the 1980-1981 NPMP surveys of the interior United States. Sites that produced contaminated samples include the Raritan River (New Jersey), Cape Fear River (North Carolina), Penobscott River (Maine), Mississippi River (Louisiana), and Willamette River (Oregon). It is possible that a nationwide survey of PCP and related chlorophenols would identify additional freshwater and estuarine contamination.
Kepone: By the mid-1980s, concentrations of kepone in oysters in the Virginia James River ecosystems were generally below 0.1 ppm wet weight (NOAA, 1988). This contamination is geographically limited.
Carboxylic acid herbicides: According to NOAA, the herbicide 2,4-D was documented in northern Chesapeake Bay oysters in 1979 and 1981, and there has been one confirmed occurrence in Alaska (NOAA, 1988).
Finfish
Data on contaminant levels in finish are summarized in Table 5-2 which includes information on both the liver and the edible portions of fish (fillets), segregated according to data source.
Trace Metals
Arsenic
-
Mean levels: Descriptive statistics of all data (fillet, liver) are presented in Table 5-2. The arithmetic grand mean level in the marine edible portions of 10 2-pound fillet samples (one sample being a within-species duplicate) from 10+ species of finfish taken from the Atlantic Ocean, the Gulf of Mexico, and the Pacific Ocean was 2.36 ppm wet weight with a standard deviation of 2.02 ppm and a range of 0.3-6.6 ppm. The arithmetic grand mean level of arsenic in marine fish livers from the 10 most contaminated sites reported in the 1986 NOAA NS&T is 5.20 ppm wet weight with a standard deviation of 1.94 and a range of 2.99-8.17 ppm. The grand mean level of all sites (45) is 2.34 ppm wet weight with a standard deviation of 2.06 and a range of 0.150-8.16 ppm (dry weight/wet weight conversion factor of 0.25). The geometric grand (national) mean level of arsenic in freshwater whole fish samples from 60 species sampled at 112 locations was, for 1978-1979, 0.16 ppm wet weight with a range of 0.04-2.08 ppm. For 1980-1981 the national mean was 0.14 ppm wet weight with a range of 0.05-1.69 ppm.
-
Median levels: The arithmetic median of the grand means of arsenic in the edible portions of fish in the Zook et al. (1976) survey is 1.80 ppm wet weight. The arithmetic median of the grand means of arsenic in marine fish livers from the 10 most contaminated sites reported in the 1986 NOAA NS&T is 4.80 ppm wet weight.
TABLE 5-2 Finfish Contaminants (PPM Wet Weight)a
|
Contaminant |
Mean ± SD |
Percentile |
Geographic Distribution and Sites Exceeding 95th Percentile |
Temporal Trends 1986-1988 |
||
|
5th |
50th |
90th |
||||
|
PCB |
0.420 ± 1.4b |
0.02b |
0.10b |
All coastal sites. SD, EB, BH, SB |
Uncertain |
|
|
|
0.755 ± 1.0 |
|
|
|
|
|
|
DDT |
0.840 ± 1.78d |
0.01d |
0.69d |
All coastal sites. SP, SB, SM, SD |
Uncertain |
|
|
|
0.378 ± 0.88 |
0.005 |
0.06 |
0.81 |
|
|
|
Arsenic |
2.355 ± 2.0d |
0.14e |
1.8e |
All sites examined (45). CBO, LI, NBA, DP, NBR |
Uncertain |
|
|
|
2.335 ± 2.1 |
0.27 |
1.66 |
5.14 |
|
|
|
|
0.15f |
- |
- |
- |
|
|
|
Cadmium |
0.044 ± 0.01e |
0.001e |
0.05e |
All sites examined (45). SS, NRW, DP, CRO |
Freshwater decline 1972-79 |
|
|
|
0.518 ± 0.87 |
0.02 |
0.17 |
1.31 |
|
No change 1978-81 |
|
|
0.035c |
- |
- |
- |
|
|
|
Lead |
0.473 ± 0.11e |
0.014e |
0.45e |
All sites examined. CB, EB, CBW, BB, NBR |
Like cadmium |
|
|
|
0.133 ± 0.29 |
0.002 |
0.04 |
0.27 |
|
|
|
|
0.11f |
- |
- |
- |
|
|
|
Mercury |
0.230 ± 0.16e |
0.003e |
0.17e |
All sites examined. DP, SS, SD, OH |
Like cadmium |
|
|
|
0.158 ± 0.32 |
0.003 |
0.17 |
0.22 |
|
|
|
|
0.11f |
- |
- |
- |
|
|
|
Slenium |
3.22 ± 0.2 |
0.46 |
2.52 |
5.70 |
All sites examined. SS, CC, ABF, DP, MR |
Like cadmium |
|
|
0.46e |
- |
- |
- |
|
|
|
NOTE: ABF Appalachacola, Fla.; BB Buzzards Bay, Mass.; BH Boston Harbor, Mass.: CBO Coos Bay, Ore.; CBW Commencement Bay, Wash.; CC Corpus Christi, Tex.; CB Casco Bay, Maine; CRO Columbia River, Ore.; DP Dana Pt., Calif.; EB Elliot Bay, Wash.; LI Lutak Inlet, Alaska; MR Mississippi River, La.; NBR Narragansett Bay, R.I.; NRV Nisqually Reach, Wash.; NBA Nabhu Bay, Alaska; OH Oakland Harbor, Calif.; SB Seal Beach, Calif.; SD San Diego, Calif.; SMB Santa Monica Bay, Calif.; SP San Pedro Beach, Calif.; SS Southampton Shoal, Calif.; WLI West Long Island Sound, N.Y. a NS&T (1987), livers. b Fillets (Gadbois and Maney, 1983). c 95th percentile. d Fillets (Gossett et al., 1983; Stout, 1980). e Fillets (Zook et al., 1976). f Freshwater; geometric mean; whole body (Lowe et al., 1985). SOURCE: NOAA (1989). |
||||||
-
The 1986 NS&T grand median for fish livers from all sites (45) is 1.678 ppm wet weight (NOAA, 1987).
-
Georgraphic distribution: The five most contaminated sites (6.16-8.17 ppm wet weight) exceed the 90th percentile (5.14) and are Coos Bay, Ore.; Lutak Inlet and Nahbu Bay, Alaska; Dana Pt., Calif.; and Narragansett Bay, R.I. Freshwater sites reporting the highest individual contamination were in Lake Michigan at Saugatuck, Mich. and Sheboygan, Wisc.
-
Temporal trends: Information about trends in marine fish should be available upon publication of the new NS&T data. Arsenic concentrations for freshwater fish were about midway between those reported for earlier collection periods. No increase was noted in the 1978-1981 sampling period.
Cadmium
-
Mean levels: Descriptive statistics of all data (fillet, liver) are presented in Table 5-2. The arithmetic grand mean level in the marine edible portions of 10 2-pound fillet samples (one sample being a within-species duplicate) from 10+ species of finfish taken from the Atlantic Ocean, the Gulf of Mexico, and the Pacific Ocean was 0.0439 ppm wet weight with a standard deviation of 0.0136 ppm and a range of 0.02-0.0690 ppm. The grand mean level of cadmium in marine fish livers from the 10 most contaminated sites reported in the 1986 NS&T is 1.42 ppm wet weight with a standard deviation of 1.08 and a range of 0.530-3.94 ppm. The grand mean level of all sites (45) is 0.519 ppm wet weight with a standard deviation of 0.870 and a range of 0.0150-4.89 ppm (dry weight/wet weight conversion factor of 0.25). The geometric grand (national) mean level of cadmium in freshwater whole fish samples from 60 species sampled at 112 locations for 1978-1979 was 0.04 ppm wet weight with a range of 0.01-0.41 ppm, and for 1980-1981 was 0.03 ppm wet weight with a range of 0.01-0.35 ppm.
-
Median levels: The arithmetic median of the grand means of cadmium in the edible portions of fish in the Zook et al. (1976) survey is 0.0460 ppm wet weight. The arithmetic median of the grand means of cadmium in marine fish livers from the 10 most contaminated sites reported in the 1986 NOAA NS&T is 1.310 ppm wet weight. The 1986 NS&T grand median for fish livers from all sites (45) is 0.1750 ppm wet weight.
-
Geographic distribution: The five most contaminated sites (1.31-4.89 ppm wet weight) exceed the 90th percentile (1.3063) and are Southampton Shoal, Calif.; Nisqually Reach, Wash.; Dana Pt., Calif.; Columbia River, Ore.; and Dana Pt., Calif. Freshwater sites reporting the highest individual contamination were in the Columbia River Grand Coulee, Wash.; the Colorado River at Lake Powell, Ariz.; Verdigris River, Oologah, Okla.; and Kansas River at Bonner Springs, Kans.
-
Temporal trends: Information about trends in marine fish should be available upon publication of the new NS&T data. Cadmium concentrations for freshwater fish declined significantly from 1972 to 1979; however, no decline was noted between 1978 and 1981.
Lead
-
Mean levels: descriptive statistics of all data (fillet, liver) are presented in Table 5-2. The arithmetic grand mean level in the marine edible portions of 10 2-pound fillet samples (one sample being a within-species duplicate) from 10+ species of finfish taken from the Atlantic Ocean, the Gulf of Mexico, and the Pacific Ocean was 0.474 ppm wet weight with a standard deviation of 0.111 ppm and a range of 0.320-0.630 ppm. The grand mean level of lead in marine fish livers from the 10 most contaminated sites reported in the 1986 NOAA NS&T is 0.414 ppm wet weight with a standard deviation of 0.523 and a range of 0.140-1.85 ppm. The grand mean level of all sites (43) is 0.133 ppm wet weight with a standard deviation of 0.294 and a range of 0.0075-1.85 ppm (dry weight/wet weight conversion factor of 0.25). The geometric grand (national) mean level of lead in freshwater whole fish samples from 60 species sampled at 112 locations for 1978-1979 was 0.19 ppm wet weight with a range of 0.10-6.73 ppm, and for 1980-1981 was 0.17 ppm wet weight with a range of 0.10-1.94 ppm.
-
Median levels: The median of the grand means of lead in the edible portions of fish in the Zook et al. (1976) survey is 0.510 ppm wet weight. The arithmetic median of the grand means of lead in marine fish livers from the 10 most contaminated sites reported in the 1986 NS&T is 0.230 ppm wet weight. The 1986 NS&T grand median for fish livers from all sites (43) is 0.0450 ppm wet weight.
-
Geographic distribution: The five most contaminated sites (0.288-1.85 ppm wet weight) exceed the 90th percentile (0.271) and are Casco Bay, Maine; Elliot Bay and Commencement Bay, Wash.; West Long Island Sound, N.Y.; Buzzards Bay, Mass.; and Narragansett Bay, R.I. Freshwater sites reporting the highest individual contamination were Manoa Stream, Honolulu, Hawaii; the Connecticut River at Windsor Locks, Conn.; and the Hudson River at Poughkeepsie, N.Y.
-
Temporal trends: Information about trends in marine fish should be available upon publication of the new NS&T data. Lead concentrations for freshwater fish declined significantly from 1972 to 1979; however, no decline was noted between 1978 and 1981.
Mercury
-
Mean levels: Descriptive statistics of all data (fillet, liver) are presented in Table 5-2. The arithmetic grand mean level in the marine edible portion of 10 2-pound fillet samples (one sample being a within-species duplicate) from 10+ species of finfish taken from the Atlantic Ocean, the Gulf of Mexico, and the Pacific Ocean was 0.230 ppm wet weight with a standard deviation of 0.160 ppm and a range of 0.070-0.600 ppm. The grand mean level of mercury in marine fish livers from the 10 most contaminated sites reported in the 1986 NS&T is 0.372 ppm wet weight with a standard deviation of 0.432 and a range of 0.120-1.46 ppm. The grand mean level of all sites (43) is 0.158 ppm wet weight with a standard deviation of 0.319 and a range of 0.0100-1.55 ppm (dry weight/wet weight conversion factor of 0.25). The geometric grand (national) mean level of mercury in freshwater whole fish samples from 60 species sampled at 112 locations for 1978-1979 was 0.11 ppm wet weight with a range of 0.01 to 1.10 ppm, and for 1980-1981 was 0.11 ppm wet weight with a range of 0.01-0.77 ppm.
-
Median levels: The arithmetic median of the grand means of mercury in the edible portions of fish in the Zook et al. (1976) survey is 0.180 ppm wet weight. The arithmetic median of the grand means of mercury in marine fish livers from the 10 most contaminated sites reported in the 1986 NS&T is 0.1700 ppm wet weight. The 1986 NS&T grand median for fish livers from all sites (43) is 0.070 ppm wet weight.
-
Geographic distribution: The five most contaminated sites (0.238-1.55 ppm wet weight) exceed the 90th percentile (0.215) and are Dana Pt. (bsb), Southampton Shoal, Dana Pt. (wc), San Diego Harbor, and Oakland Harbor, Calif. Freshwater sites reporting the highest individual contamination were the Columbia River, Cascades Locks, Wash.; the Red River of the North at Noyes, Minn.; the Colorado River at Imperial Reservoir, Calif.; the Truckee River at Fernley, Nev.; and the Merrimack River at Lowell, Mass.
-
Temporal trends: Information about trends in marine fish should be available upon publication of the new NS&T data. Mercury concentrations for freshwater fish declined significantly from 1972 to 1977; however, no decline was noted between 1978 and 1981.
Selenium
-
Mean levels: Descriptive statistics of all data (fillet, liver) are presented in Table 5-2. National data on the mean levels of selenium in the edible portions of marine fish were not available. The grand mean level of selenium in marine fish livers from the 10 most contaminated sites reported in the 1986 NS&T is 6.15 ppm wet weight with a standard deviation of 1.589 and a range of 4.79-9.05 ppm. The grand mean level of all sites (45) is 3.22 ppm wet weight with a standard deviation of 2.16 and a range of 0.292-9.05 ppm (dry weight/wet weight conversion factor of 0.25). The geometric grand (national) mean level of selenium in freshwater whole fish samples from 60 species sampled at 112 locations for 1978-1979 was 0.46 ppm wet weight with a range of 0.09-3.65 ppm, and for 1980-1981 was 0.47 ppm wet weight with a range of 0.09-2.47 ppm.
-
Geographic distribution: The five most contaminated sites (5.96-9.05 ppm wet weight) exceed the 90th percentile (5.70) and are Southampton Shoal, Calif.; Corpus Christi Bay, Tex.; Apalachicola Bay, Fla.; Dana Pt., Calif.; and Mississippi River Delta, La. Freshwater sites reporting the highest individual contamination were the Colorado River at Imperial Reservoir, Calif.; Lake Havasu, Arizona-California; Lake Powell, Ariz.; and Yuma, Arizona-California.
-
Median levels: The arithmetic median of the grand means of selenium in the edible portions of marine fish was not available. The arithmetic median of the grand means of selenium in marine fish livers from the 10 most contaminated sites reported in the 1986 NS&T is 5.28 ppm wet weight. The 1986 NS&T grand median for fish livers from all sites (45) is 2.57 ppm wet weight.
-
Temporal trends: Information about trends in marine fish should be available upon publication of the new NS&T data. Selenium concentrations for freshwater fish declined significantly from 1972 to 1979; however, no decline was noted between 1980 and 1981.
Organics
Polychlorinated biphenyls
Polychlorinated biphenyl contamination varies greatly from region to region and site to site within regions. In a 1979-1980 survey of fillets of a mix of pelagic and nearshore predatory fish from sites in 15 coastal and estuarine areas, 63 of 70 samples contained PCBs with concentrations as high as 22.0 ppm wet weight in Hudson River white perch (NOAA, 1988). Sites producing the highest mean concentrations were the New York Bight Apex (1.1 ppm wet weight) and East Bay, near Panama City, Florida (0.42 ppm wet weight). Sites producing fish with the lowest PCB concentrations were at Catalina Island, offshore of Los Angeles (less than 0.04 ppm wet weight), and Chandeleur Sound, east of New Orleans (0.05 ppm wet weight in 13 species). The lognormal distribution of contaminant concentrations in edible portions of fish is illustrated in Figure 5-9. It is evident from the relatively large slope (the log10 of the geometric standard deviation of the distribution) that the concentrations in fish may vary greatly from site to site. Finally, data from nationwide, large-scale sampling programs confirm the fact that PCBs occur in fish and shellfish from all estuaries sampled, including remote nonindustrialized sites in Alaska, the Virgin Islands, and Hawaii. Data also indicate that the highest concentrations have occurred in fish from urban embayments on the Pacific and East coasts and near Pensacola, Florida, with lower concentrations in fish from the southeastern and Gulf of Mexico estuaries. On the basis of available comparable data, there has been no dramatic national change, or at most a minor decline, in PCB contamination of fish and shellfish over the past 10-15 years (NOAA, 1988).
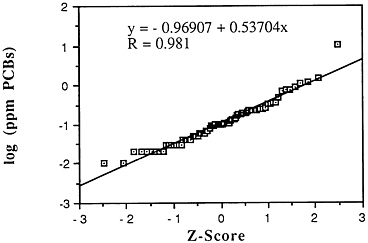
FIGURE 5-9 Lognormal distribution of wet weight concentrations of PCBs in edible portions of inshore marine fish from different locations
Descriptive statistics of all fish PCB data (fillet, liver) are presented in Table 5-2. The arithmetic grand mean PCB level in the edible portions of 188 samples (10 fillets per sample) from 32 species of finfish taken from 20 coastal sites was 0.412 ppm wet weight with a standard deviation of 0.655 ppm and a range of 0.03-2.9 ppm.
The arithmetic grand mean level of PCBs in marine fish livers from the 10 most contaminated sites reported in the 1986 NS&T is 1.90 ppm wet weight with a standard deviation of 0.90 and a range of 0.933-3.97 ppm wet weight. The grand mean level of all sites (46) is 0.755 ppm wet weight with a standard deviation of 1.03 and a range of 0.0063-4.93 ppm (dry weight/wet weight conversion factor of 0.25). The five most contaminated sites (1.99-4.93 ppm wet weight) are San Diego Harbor (bst), Calif.; Elliot Bay, Wash.; Boston Harbor, Mass.; San Diego Harbor (dt) and Seal Beach, Calif.
The arithmetic median of the grand means of the edible portions of fish in the Gadbois and Maney (1983) survey is 0.1100 ppm wet weight. The arithmetic median of the grand means of PCB in marine fish livers from the 10 most contaminated sites reported in the 1986 NS&T is 1.765 ppm wet weight. The 1986 NS&T grand median for fish livers from all sites (43) is 0.2874 ppm wet weight.
Dioxins
As part of its national dioxin strategy, EPA conducted a survey of the extent of TCDD contamination in the environment (EPA, 1987). One significant finding from this study was contamination of freshwater fish in areas of pulp and paper mills that utilize chlorine and chlorine compounds as part of that bleaching process. Dioxin contamination was discovered at approximately 85 sites throughout the country including Alaska (EPA, 1988a,b). Levels of TCDD found in whole fish were as high as 85 parts per trillion, whereas levels found in fillets ranged up to 41 parts per trillion. Two fish consumption advisories have been issued based on findings from the study. A high proportion (23 of 29) of Great Lakes fish sampling sites were found to have detectable levels of TCDD. Outside the Great Lakes, fish contamination was primarily found in major river systems, such as the Ohio and Mississippi Rivers, or in waterways with significant industry activity. Levels of TCDD in fish fillet samples may be a cause for concern at specific locations for certain consumption patterns; local exposure conditions should be evaluated to determine a level of concern for those areas. Fish and shellfish from estuarine and coastal waters were rarely contaminated with TCDD; three of the four contaminated sites were in areas heavily influenced by industrial discharge. Paper mills using chlorine bleaching are being investigated by EPA, the states, and the paper industry to determine possible sources of TCDD contamination within the mills. Because recent studies indicate that TCDD has a half-life of less than 1 year in fish, the implication is that dioxin contamination of fish is a current and continuing phenomenon.
Chlorinated hydrocarbon pesticides
DDT and metabolites: Although notable declines have occurred in DDT and metabolite concentrations in fish muscle, potentially significant concentrations of these materials continue to be found in fish from historic hot spots such as Whites Point, California. For example, the mean concentration of DDT in white croaker (a southern
California sport fish) caught from various areas in nearby Santa Monica Bay ranged from 0.22 to 0.69 ppm wet weight, but the mean was 0.6 ppm wet weight for white croaker caught from Whites Point, a site where DDT-laced waste sludge from a pesticide manufacturing plant was dumped (Gossett et al., 1982).
Descriptive statistics of all finfish DDT data (fillet, liver) are presented in Table 5-2 and Figure 5-10. The arithmetic grand mean level in the edible portions of 765 fillet samples from six species of finfish taken from the northwest Atlantic Ocean, the Gulf of Mexico, and Los Angeles Harbor was 0.841 ppm wet weight with a standard deviation of 1.78 ppm and a range of 0.008-7.6 ppm. These data come from a compilation of two studies: Stout (1980) and Gossett et al. (1983).
The grand mean level of DDT and all its metabolites in marine fish livers from the 10 most contaminated sites reported in the 1986 NS&T is 1.24 ppm wet weight with a standard deviation of 1.49 and a range of 0.2070-4.665 ppm. The grand mean level of all sites (48) is 0.3781 ppm wet weight with a standard deviation of 0.884 and a range of 0.0025-4.66 ppm (dry weight/wet weight conversion factor of 0.25). The five most contaminated sites (0.967-4.66 ppm wet weight), which exceed the 90th percentile (0.812 ppm), are San Pedro Beach, Seal Beach (wc), Seal Beach (ht), Santa Monica Beach, and San Diego Harbor, California.
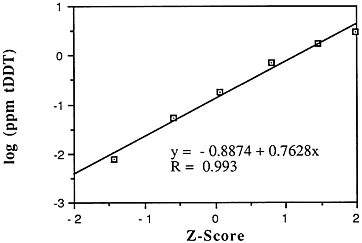
FIGURE 5-10 Lognormal distribution of wet weight concentrations of DDT and metabolites in edible portions of inshore marine fish from different locations
The arithmetic median of the grand means of tDDT in the edible portions of fish in the survey is 0.177 ppm wet weight. The arithmetic median of the grand means of tDDT in marine fish livers from the 10 most contaminated sites reported in the 1986 NS&T is 0.441 ppm wet weight. The 1986 NS&T grand median for fish livers from all sites (48) is 0.0566 ppm wet weight.
Dieldrin: According to NOAA, dieldrin has historically been the most frequently detected cyclodiene pesticide in coastal fish and shellfish (NOAA, 1988). More than 15,000 samples have been analyzed for dieldrin. Concentrations in muscle tissue ranged from below detection to a maximum of 1.56 ppm wet weight in a 1970 sample of milkfish from Ali Wai Canal, Honolulu, Hawaii. Besides milkfish, tarpon from Honolulu had levels ranging from 0.36 to 1.56 ppm wet weight or from one to five
times the FDA action limit. These results, however, are approximately 20 years old. Dieldrin was a common contaminant in the 1984 NS&T Benthic Surveillance fish liver survey, occurring in fish livers in some sites at concentrations above 0.001 ppm wet weight. The range was less than 0.001 to a high of 0.104 ppm wet weight in liver of winter flounder from a site in Salem Harbor, Massachusetts (NOAA, 1989). The next highest concentrations occurred in livers of starry flounder and white croaker from two collection sites in San Francisco Bay (0.09 ppm wet weight). Urbanization does not appear to be the overriding determinant of dieldrin contamination, because some of the fish with the lowest dieldrin concentrations were in urbanized embayments such as San Diego Harbor (less than 0.0003 ppm wet weight) and Elliott Bay, Puget Sound (less than 0.01 ppm wet weight). Intermediate concentrations occurred in fish from St. Johns River, Florida; the Mississippi River Delta; San Pablo Bay, California; and Boston Harbor, Massachusetts. In summary, dieldrin is a common contaminant nationwide – particularly of inland and estuarine fish. Definite declines in concentrations can be confirmed for inland sites and some marine sites. Analyses of FDA data appear to support the hypotheses that inland fish are considerably more contaminated with dieldrin than open water marine fish and even estuarine fish. Although virtually all dieldrin uses in agriculture were restricted more than 15 years ago, its residues remain in soils and in the food web. Dieldrin was the second most frequently documented pesticide in the 1980-1981 NPMP whole composites of inland freshwater fish national surveys, occurring at 75% of the sites nationwide. Mean concentrations in whole freshwater fish on a nationwide basis declined only slightly from 0.05 ppm wet weight in 1976-1977 to 0.04 ppm wet weight in 1980-1981, but maximum concentrations over this time decreased from 5.0 to 0.72 ppm wet weight, a trend due primarily to declines in Hawaiian stream fish. In some cases, particularly with much of the freshwater harvest including farm raised fish from areas of past agricultural use, regular consumption of dieldrin-contaminated fish could be reason for concern.
Chlordane and heptachlor compounds: According to NOAA, chlordane compounds have been contaminants of fish from several estuarines for many years and are among several pesticides that contaminated coastal fish of Hawaii as well as freshwater fish from throughout the U.S. mainland. Although concentrations do not appear to be increasing, neither are they decreasing dramatically. In some states such as Kansas and New York, chlordane contamination of freshwater finfish is pervasive, based on the committee's review of data bases for these states. The contamination problem stems in part from the widespread use of chlordane as a killer of termites through 1986 and its subsequent environmental distribution and persistence.
Two chlordane compounds, alpha-chlordane and trans-nonachlor, were measured in livers of fish from 48 NS&T site collections made by NOAA in 1984. With the use of detection limits lower than those used in earlier national surveys of the 1970s, at least one of these compounds was detected in 95% of the site collections. To obtain a better grasp of the extent of chlordane contamination of fish and possibly bivalves, a comprehensive sampling program should be undertaken in which detection limits are 5-10 times lower than those used for regulatory purposes, along with a compilation of all available, current local and state data. Chlordane is a probable human carcinogen, but there are significant gaps in our knowledge of whether it is a teratogen, reproductive toxin, or mutagen (EPA, 1986).
Heptachlor and heptachlor epoxide: According to NOAA, heptachlor and its metabolite heptachlor epoxide have been looked for in more than 12,000 samples, mainly from the NPMP (NOAA, 1988). In contrast with shellfish, total heptachlor occurred in 39% of inland fish samples at concentrations above 0.01 ppm wet weight in the 1980-1981 NPMP surveys. Presently, heptachlor does not appear to be a prominent contaminant of marine fish anywhere.
Endosulfan: According to NOAA, the highest confirmed concentration of endosulfan in marine fish was 0.05 ppm wet weight in liver of a 1983 sample of fringehead sculpin from Elkhorn Slough near the same Moss Landing site (NOAA, 1988). Besides the Elkhorn Slough samples, endosulfan was detected in fish from an Oregon estuary and in a Pacific Coast estuary in Mexico. Fish and invertebrates from sloughs and bays of coastal Monterey County, California, were notably contaminated with endosulfan according to surveys conducted in 1980-1981, and contamination of mussels from Elkhorn Slough continued through 1984. NOAA reported that concentrations in Elkhorn Slough mussels ranged up to 0.7 ppm wet weight. Freshwater fish from the nearby Salinas River contained concentrations as high as 1.2 ppm wet weight. In 1983, seven species of marine and estuarine fish contained total endosulfan concentrations ranging from 0.021 to 0.052 ppm wet weight. Several other California sites also produced mussels containing endosulfan: Trinidad Head, Bodega Head, four San Francisco Bay sites, Bolinas Lagoon, Pacific Grove, Newport Bay, Port Hueneme, and Santa Cruz. The last two sites, plus the Elkhorn Slough site, also experienced increasing endosulfan concentrations between 1979 and 1981, and continued contamination occurred into 1986. Because California sites with significant endosulfan contamination are in heavily agricultural regions, it is important to determine the extent of recreational harvesting in such areas and the present contamination levels of endosulfan.
Lindane and benzene hexachloride: In early surveys, lindane and gamma-BHC have been looked for in nearly 12,000 marine or estuarine fish and invertebrate samples, but were found above the detection limit of 0.01 ppm wet weight in only a few samples. However, with improved detection limits in the 1984 NS&T Benthic Surveillance project, lindane occurred in 47% of the fish liver site collections at concentrations above 0.001 ppm wet weight. The nationwide average was about 0.002 ppm wet weight and the highest mean concentration was 0.014 ppm wet weight in the livers of Atlantic croaker from a site in the Chesapeake Bay (NOAA, 1988). Lindane was detected in 44% of 64 California Mussel Watch samples in 1980-1981 through 1985-1986 surveys; most of these were from San Francisco Bay and the Los Angeles area, at concentrations slightly exceeding 0.001 ppm wet weight. However, in inland waters, lindane was documented in 16% of the 1980-1981 NPMP fish samples at concentrations exceeding 0.01 ppm wet weight. Highest concentrations were in freshwater fish from a stream in Honolulu, from Lake Mead (Colorado River), and from several Great Lakes sites. The low levels observed in fish livers during the 1984 NS&T Benthic Surveillance suggest that there is no significant nationwide lindane contamination today (NOAA, 1988).
Chlorinated benzenes and phenols: According to NOAA, the highest reported concentration of hexachlorobenzene (HCB) (the fully aromatic form of benzene with 6 chlorines) used as a fungicide was about 0.7 ppm wet weight in the liver of English sole collected in 1979 from the Hylebos Waterway in Commencement Bay, Washington (NOAA, 1988). Measurable, but lower, concentrations were also reported in fish and
shellfish from the New York Bight, Galveston Bay, the Upper Chesapeake Bay, and from Palos Verdes Peninsula and the Santa Monica Bay outfalls in California. However, HCB is probably a more significant inland contaminant. It was detected at concentrations above 0.01 ppm wet weight in 24% of the samples of whole fish from the 1980-1981 inland NPMP freshwater fish survey. The highest concentrations of 0.12-0.13 ppm wet weight occurred in whole fish from the Tombigbee River, Alabama and from the Mississippi River at a site in Louisiana. There is evidence that, nationwide, there has been a decline in HCB contamination since the 1976-1977 surveys.
Pentachlorophenol is a wood preservative, slimicide, metabolite of the fungicide HCB, and carrier of carcinogenic dioxins (NOAA, 1988). It has not been widely surveyed in marine and estuarine fish on a national scale. Concentrations have been found in blue crabs and brown shrimp from San Luis Pass, Texas.
Other chlorobenzenes that may be important include di- and trichlorinated benzenes as well as other monocyclic chlorinated aromatic hydrocarbons. In southern California, 1,2,4-trichlorobenzene was measured in flatfish livers near a sewage discharge site (NOAA, 1988). Monocyclic chlorinated aromatic hydrocarbons have been detected in striped bass from the San Francisco Bay-Delta region. Although none have been surveyed nationally, all are considered EPA priority pollutants. Thus, their inclusion in future national surveys is warranted.
Mirex: According to NOAA, mirex is an ant poison once thought to be a serious contaminant of Southeast U.S. estuarine organisms. Mirex was measured in inland fish NPMP surveys for the first time during the 1980-1981 surveys and occurred at 18% of the stations, mainly in the Great Lakes and the Southeast (NOAA, 1988). Because of its persistence and long term threats, fish advisories have been issued for mirex existence in Lake Ontario. Monitoring for mirex in fish from the Great Lakes, Georgia, and South Carolina waters is warranted.
Kepone: According to NOAA, since kepone was first discovered in the James River in 1973 as a result of illegal discharges from a pesticide manufacturing plant, it has since been found in thousands of samples of fish, crabs, and oysters (NOAA, 1988). By the mid-1980s, concentrations in fish and crabs were on the order of 0.2-0.8 ppm wet weight. Concentrations in some fish exceeded 7 ppm wet weight in the mid-1970s and levels above 1 ppm wet weight were common. Kepone has not been included as a target chemical in any post-1973 national or regional survey. State monitoring programs in Maryland and North Carolina included kepone, and its residues were detected at low concentration–0.01 ppm wet weight–in the flesh of some sport fish taken near inlets in North Carolina in 1976. It is not certain whether kepone contamination is more pervasive than in the lower James River area of Virginia. Kepone levels should continue to be monitored.
Toxaphene: According to NOAA, toxaphene is possibly an important regional contaminant. Toxaphene is a mix of chlorinated camphenes and has been measured in more than 12,000 samples but was consistently present above detection limits in only a few regions including southern Georgia and southern Laguna Madre, Texas (NOAA, 1988). Secondary occurrences have been reported for fish from the San Francisco Bay-Delta area; from East Bay, Los Angeles; and from Oso Bay, Texas. The highest concentration in muscle was 35.6 ppm wet weight in both a mullet and a goatfish from the Back River near Brunswick, Georgia.
Inland sampling indicates that the chemical was recorded above detection limits of 0.01 ppm wet weight in nearly 88% of the stations in one survey. Highest
concentrations were in whole fish from the Mississippi River, the Great Lakes, and the Cape Fear River in North Carolina. It is possible that toxaphene remained a significant contaminant at some estuarine sites into the 1980s and may even have increased locally or nationally. Toxaphene contamination appears to be limited to certain regions. Historically sampled sites in at least three states–Georgia, California, and Texas–should be resampled.
Carboxylic herbicides: No. 2,4-D and 2,4,5-T were detected among fish in the FDA monitoring program. There was sporadic detection of DCPA. Among the most notable cases of contamination with DCPA were fish from Arroyo Colorado and adjacent areas in southern Texas.
Atrazine: The fact that surveys do not often detect atrazine may be due either to a real lack of significant bioaccumulation or to the laboratory detection limits in use. Traces of atrazine at levels ranging from 0.2-0.3 mg/kg have been found in bluegill (Lepomis machrochirus ) in Kansas and, during periods of high use, may be found in other fish in farm ponds and nearby lakes or creeks (Kansas DHE, 1988).
Conclusions
Evidence exists that fish and shellfish from domestic freshwater and marine environments are contaminated with a number of inorganic and organic chemicals that are potentially toxic to humans. Although the contamination is widespread, it varies greatly with geographic location and species. Where adequate data are available, it appears that the distribution of contaminant levels is reasonably well described as lognormal in most cases.
POTENTIAL OPPORTUNITIES FOR REDUCING EXPOSURES
Without prejudging the need for additional controls on specific contaminant residues, it is reasonable to use the observations of contaminant variability derived in the previous sections to draw some preliminary inferences about the potential of different kinds of control measures to reduce seafood contaminant exposures in the United States. Three basic types of control measures can be considered:
-
The classical approach, now the primary control measure at the federal level, of setting maximum contaminant levels that are acceptable in seafood, analyzing a small fraction of the commercial seafood in interstate commerce, and, where excessive levels are found, seizing products with violative residues
-
Restrictions on harvesting/marketing based on relationships between contaminant levels and (1) species, (2) geographic area, and (3) size
-
Labeling and consumer information programs of various types, ranging from general advisories now issued by state health departments primarily to sport fishers, to possible programs to disclose the origin (or even average contaminant levels) for seafood sold in retail outlets.
The first type of option has obvious difficulties. Chemical analyses are both expensive and slow, relative to the usual pace of marketing fresh seafood products.
Aside from limited special programs, such as sampling for mercury compounds in imported swordfish, only a tiny fraction of product can be screened. Therefore, with the important exception of the use of analyses for detection of situations in which more intensive control efforts of other types may be indicated, direct chemical residue screening of seafood has little potential to achieve quantitative reductions in the delivery of contaminant residues to consumers.
The Food Drug and Cosmetic Act concept of "adulteration," evaluated on the basis of single items or, in practice, single-shipment lots of product, makes sense in protecting against an acute toxic hazard such as botulism or paralytic shellfish poisoning. In those cases an individual meal has a high probability of directly causing illness, and carefully excluding the tiny minority of dangerously contaminated items is an effective strategy for avoiding harm.
Many of the effects discussed in Chapter 6, however, are the products of long-term, even lifetime, levels of exposure. There simply is no neat dividing line between safety and hazard, defined on the basis of individual meals. The important risks may take the form of either a modestly increased long-term probability of cancer (in relation to the background of cancer from unrelated causes), a subtle shift in the distribution of birth weights and attained mental performance in offspring, or an increased long-term risk of a chronic cumulative condition such as Parkinson's disease. In structuring social control measures to reduce these types of risks it is important to have in mind the limitation of long-term average exposures—not simply to reduce the number of individual items that reach the market above some (arbitrarily defined) cutoff level.
The second set of options mentioned above—restrictions of various kinds on harvesting and marketing—has considerable potential to limit long-term average exposures and the exposures of selected groups for various kinds of toxic effects (e.g., women of childbearing age). This type of control measure has been utilized to some degree in closing selected areas to shellfish harvesting, particularly by state authorities, but it has not been comprehensively analyzed for the potential to reduce selected seafood-related chemical residue exposure on a national basis. Some of the existing, less-than-perfect data bases are used below to illustrate the process of analyzing the variability of residue levels by geography and size. This analysis seeks to assess the potential reduction in population doses achievable by 1-20% restrictions on harvesting/marketing. The basic concept is that the more variability there is in contaminant levels associated with geography or size, the greater is the potential for economically reducing population dosage by using those variables to control harvesting and marketing.
The advisability of expanded consumer information programs to inform people of the residual risks of different kinds of seafood products, especially people with unusual exposures (e.g., freshwater sport fishers and their families) and people of reproductive age, is discussed in Chapter 6.
Analysis of Potential Benefits from Geographic Restrictions on Harvesting/Marketing
In one of the least utilized, best structured, and most extensive data sets on inorganic contaminants complied during the 1970s (Hall et al., 1978), the authors write
The completion of this data report does not bring the Resource Survey to an end. No attempt has been made here, for example, to compare element levels in a particular species or to investigate possible relationships between element levels and fish location, size, or sex. The analytical values have been summarized by site only for each species and tissue, and very little of the history of the fish has been presented. … The complete collection of data is available for use in interpretive studies. It is also anticipated that the data will be made available to the public, in the future, through the National Oceanographic Data Center, Environmental Data Service, National Oceanic and Atmospheric Administration, Rockville, Md.
Unfortunately these hoped-for analyses of contaminant levels in relation to size, geography, and other fish characteristics readily ascertainable at harvest were never completed and published. The following illustrates what might have been done with such data.
Figures 5-1 through 5-10 are lognormal plots of the distribution of contaminant levels for geographic locations included within a few national data bases for specific contaminants in specific aquatic organisms. In general, the lognormal model appears to be reasonably accurate in describing these data, as judged by the correspondence of the points in those figures to the straight lines. In each plot there is a regression equation describing the straight line shown. The slope of those straight lines corresponds to the standard deviation of the log10 of the contaminant levels found at the different sites; the greater the slope (the number preceding the x), the more variability there is among geographic areas.
If, for purposes of this illustration, some simplifying assumptions are made (that the distributions are truly lognormal and that, in the absence of restrictions, all sites would make roughly equal contributions of specified seafood items to the U.S. food supply), then Table 5-3 shows how large a reduction could be made in the population aggregate dosage of specific contaminants (in specific sets of organisms) by restricting harvesting/marketing at specified percentages of the geographic sites covered in the underlying data.
The results in the top half of the table suggest that for most of the inorganics and contaminants in shellfish, the sole exception being lead, the site-to-site variability indicated in the NS&T data is small enough that even taking a relatively extreme measure (restricting harvesting from the worst 20% of locations) would be expected to reduce population aggregate dosage by less than 50%. The bottom half of the table suggests quite a different picture for organic contaminants. In each case, the indicated geographic variability is larger than the variability suggested for all five inorganics. For the most variable contaminants/sets of species, it would be theoretically possible to reduce the population dosage delivered by more than 50% through restricting harvesting/marketing from only 5% of the most intensely contaminated sites. For other cases, restrictions on slightly more than 10% of the sites would be required to achieve this goal.
TABLE 5-3 Illustrative Analysis of the Potential for Reducing the Aggregate Dosage of Specific Contaminants from Subsets of Seafood by Restricting Harvesting from the Sites with the Highest Contaminant Levelsa
|
Fraction of Sites Restricted (%) |
Inorganic Contaminantsb |
||||
|
|
Arsenic |
Cadmium |
Lead |
Mercury |
Selenium |
|
1 |
3.3 |
4.8 |
11.5 |
5.4 |
2.6 |
|
2 |
5.9 |
8.3 |
17.8 |
9.2 |
4.8 |
|
5 |
12.2 |
16.1 |
29.9 |
17.5 |
10.1 |
|
10 |
21.2 |
26.6 |
43.6 |
28.5 |
18.2 |
|
20 |
36.0 |
42.7 |
61.1 |
44.9 |
32.0 |
|
Site-to-site geometric standard deviation |
1.61 |
1.92 |
3.05 |
2.03 |
1.44 |
|
Ratio arithmetic/geometric mean |
1.11 |
1.23 |
1.86 |
1.28 |
1.06 |
Analysis of Potential for Reducing Population Exposure to Mercury from Swordfish and PCBs from Bluefish via Size Restrictions
Size is another potentially important control parameter associated with lipophilic contaminant concentrations that may prove useful in some cases. Data obtained by FDA and Canadian authorities in the early 1970s were reviewed on swordfish mercury concentrations in relation to fish size. An association between fish size and mercury concentration can be seen in Figure 5-11, although there is quite a bit of scatter in the results for individual fish. Unfortunately, when the fish are arranged in size classes to determine the cumulative population dose reduction that could be achieved by restricting harvesting/marketing of the biggest fish, the prospects for appreciably reducing population mercury dosage by modest size restrictions appear dim. As indicated in Table 5-4, the largest fish appear to have somewhat smaller average mercury concentrations than more moderate-sized ones, and in any event, reductions in average mercury concentrations are quite modest until the fish are very small, in which case they represent a trivial proportion of the aggregate weight of the catch.
This example is provided here for illustration of the method only. If analyses of these types were extended to other species and other contaminants, it is likely that size restrictions would be found to be feasible control measures in some cases. Based on data discussed in Chapter 6 (NOAA, 1987), one promising opportunity for size-based restrictions appears to be PCB concentrations in eastern bluefish. When data for the two sexes are averaged, small bluefish (less than 11.8 inches) average about 0.21 ppm PCB; medium (11.8-19.7 inches) average 0.42 ppm; and large average somewhat over 1.4 ppm – about seven times larger than the average concentrations in the small category. Ideally, such size-based restrictions could be structured with somewhat different cut points for different geographic areas, depending on local concentration/size data.
FIGURE 5-11 Mercury concentrations versus size of swordfish-log-log plot
TABLE 5-4 Potential Population Mercury Dose Reductions Achievable by Size Restrictions on Atlantic Swordfish (based on early 1970s data)
|
Weight Class (lb) |
Number of Fish |
Average Weight (lb) |
Average Hg Level (ppm) |
Cumulative Fish Weight (%) |
Cumulative Fish Hg Dose (%) |
|
Over 200 |
20 |
263.9 |
1.22 |
26.5 |
23.0 |
|
150-199 |
20 |
175.6 |
1.30 |
45.2 |
38.2 |
|
100-149 |
21 |
121.0 |
1.22 |
57.9 |
49.2 |
|
75-99 |
42 |
83.5 |
1.92 |
73.4 |
64.6 |
|
50-74 |
79 |
63.4 |
0.86 |
91.2 |
86.3 |
|
25-49 |
69 |
40.0 |
0.72 |
99.46 |
98.36 |
|
Under 25 |
23 |
16.4 |
0.32 |
100 |
100 |
NOTES
|
|||||||||||||||||||||||||||||||||||||||||||
Appendix to Chapter 5
PRESENT STATUS OF DOSE-RESPONSE DATA FOR TRACE METALS OF GREATEST POTENTIAL TOXICITY
Dose-response data used in risk assessment for trace-metal exposure are generated from animal studies and from occupational or accidental environmental exposure of humans. The uncertainty factor in the first instance (extrapolation) may be quite large, whereas data generated by occupational or accidental environmental exposures allow more accurate assessment. For four of the five metals identified as potential toxicants, arsenic, cadmium, lead, and mercury, an extensive body of literature exists documenting occupational and accidental exposure of humans. Although poisoning has been documented in humans from ingestion of selenium, far fewer dose-response data are available for accurate assessment. Dose-response data should allow the assessor to produce the following information:
-
Acceptable daily intake (ADI) or reference dose (RfD)
-
Toxic body (usually based on 70 kg) or organ burden
-
Steady daily intake for toxicity (acute/chronic)
This information can be calculated from other data obtained from controlled animal exposures or from inadvertently exposed humans. Needed for calculation are
-
human half-life of the metal (most often whole body);
-
blood LOAELs (low-observed-adverse-effect levels);
-
other tissue LOAELs (hair, nails, etc.);
-
absorption coefficient (percent absorption); and
-
age, sex, reproductive status, and interindividual variability of response.
The following are also desirable:
-
Pretoxic indicators (biomarkers)
-
Compartment kinetics (distribution within the organism)
Dose-response data used here come from three sources: the U.S. Environmental Protection Agency (EPA, 1988c) Integrated Risk Information System (IRIS); the U.S. Department of Health and Human Services (HHS) Agency for Toxic Substances and Disease Registry (ATSDR, 1989a,b); and specific papers in the world literature. These data are summarized in Table 5A-1.
TABLE 5A-1 Trace-Metal Dose-Response Data
|
Metal |
ADI (mg/day) |
Toxic Body Burden (mg) |
Steady Daily Intake for Toxicity (mg/day) |
Human Half-Lifea |
Blood LOAELb |
Gastrointestinal Absorption (%) |
Dependence of Toxicity on Age, Sex, Reproductive Status |
Long-Term Effects |
Biomarkers |
Tissue LOAEL (ppm) |
Relative Priority as a Seafood Hazard |
|
Arsenic |
Uncertain for seafood |
Uncertain for seafood |
Uncertain for seafood |
<20 h |
Uncertain for seafood |
Uncertain for Seafood |
Not reported for seafood |
Uncertain for seafood |
Uncertain for seafood |
Uncertain for seafood |
Very low |
|
Cadmium |
51-72 |
Not reported |
35 |
Three phases <200 d, +20 d, 10-30 yr |
Uncertain poor monitor |
5 |
>50 years old and multiparous female |
Nephropathy |
Urine retinol binding protein |
Kidney, 200-285 |
High |
|
Lead |
429 |
100-400 |
Uncertain |
Three phases 3-4 weeks, 5-30 yr |
25 ppb (5-15 ppb in child) |
10 |
Fetus and neonate |
Anemia and CNSc problems |
delta-Aminolevulinic acid (RBC)d |
Poor |
High |
|
Mercury |
0.23 |
25 |
0.003 (acute) |
70-110 d |
0.23 ppb adult (0.1 ppb fetus) |
95 |
Fetus, neonate, and pregnant female |
Retardation |
Porphyrinuria |
Hair, pregnant patients, 15-20 |
High |
|
Selenium |
Uncertain for seafood |
Not reported |
— |
Three phases 1 d, 8-20 d, 65-116 d |
Uncertain (0.179 ppt) |
40-80 as the selenite |
Not reported |
Uncertain |
Not reported |
Hair, 0.828 |
Uncertain |
|
a d = days; h = hours; yr = years. b ppb = parts per billion; ppt = parts per thousand. c CNS = central nervous system. d RBC = red blood corpuscles. |
|||||||||||
Arsenic
Although seafood is a major source of arsenic in the diet, its chemical form in seafood is organic—primarily arsenobetaine and arsenocholine. This so-called fish arsenic is much less toxic than inorganic forms of arsenic and is not generally considered a threat to human health (ATSDR, 1989a). The literature concerned with arsenic in the environment and its toxicology has been reviewed by the World Health Organization (WHO, 1981), Fowler (1983), and the ATSDR (1989a,b). Inorganic forms of arsenic are established carcinogens in humans (EPA, 1988c). To the degree that inorganic forms of arsenic are either present in seafood or produced as metabolites of the organic arsenic in seafood, there would be expected to be some carcinogenic risk.
Half-life: The half-life varies according to the compound studied. For organic arsenic in seafood (fish arsenic; arsenobetaine) the best estimate in humans is less than 20 hours. Humans eliminate 50-80% of the dose from seafood within 48 hours (Tam et al., 1982). In animal studies, arsenobetaine is also rapidly eliminated in the urine; the small portion retained is found in testes, cartilage, and muscle (Vahter et al., 1983). When inorganic arsenic was force-fed (intubation) to trout it was converted to organic arsenic in the gut, and more than 95% of the arsenic found in muscle after 12 hours was the organic form (Penrose, 1975).
For inorganic arsenic the trivalent arsenite has three recorded half-lives: 2 hours, 8 hours, and 8 days. For pentavalent arsenic, half-life phases were 2.1, 9.5, and 38 days.
Blood LOAEL: The blood LOAEL of arsenic is uncertain; levels are poor indicators of exposure. With organic arsenic, the turnover is so rapid that levels are of little value, but they do correspond to seafood in the diet.
Other Tissue LOAELs: Arsenobetaine does not accumulate in hair (Vahter et al., 1983), and inorganic arsenic hair and nail levels correlate poorly with intoxication. Furthermore, they tend to be contaminated by external factors. There is a large interindividual variability.
Percent Absorption: Both inorganic and organic forms of arsenic are similar. Absorption of inorganic form is approximately 90%; organic, reported from 70% to more than 90% (Tam et al., 1982).
Age, Sex, Reproductive Status, and Interindividual Variability: No response differences in age, sex, or reproductive status have been reported. Interindividual variability for the effects recorded may be large.
Pretoxic Indicators: Pretoxic indicators for arsenic are uncertain, with few biomonitoring options.
Long-term Effects: For inorganic arsenic in humans, the long-term effects include dermal hyperkeratosis, melanosis and carcinoma, hepatomegaly, peripheral neuropathy, and in cases of inhalation, pulmonary cancer. For the organic forms, no toxic effects have been reported in humans, and studies in animals indicate no toxic effects at an oral dose of 10,000 mg/kg. Further, there is no evidence of mutagenicity by arsenobetaine.
Kinetics: A two-compartment model is likely: initial buildup in, and clearance from, the liver, kidney, and lungs; and long-term retention in hair, skin, and skeletal system. Arsenobetaine is not biotransformed in vivo and is eliminated as such.
Acceptable Daily Intake (ADI): For inorganic arsenic trioxide the ADI is not agreed upon; it is estimated by the Food and Agriculture Organization (FAO) at 182 µg. None is reported for organic arsenic.
Toxic Body or Organ Burden: No information is available for fish arsenic.
Steady Daily Intake for Toxicity: Inorganic arsenic intake in children of 1.3-3.6 mg results in lesions in 33 days (subacute). Water levels of 0.9-3.4 mg per liter (L) result in skin lesions of adults. No information is available for fish arsenic.
Cadmium
Cadmium is both an occuptional and an environmental toxicant. Occupational toxicity is, for the most part, related to inhalation, whereas environmental outbreaks are related to ingestion via contaminated food or water. Cadmium toxicity in man and animals has been extensively reviewed (ATSDR, 1989b; Friberg et al., 1985; Nriagu, 1981). Acceptable daily intake levels for cadmium may be difficult to determine for that segment of the population who are smokers. The prevalence, among cadmium-exposed individuals, of beta-2-microglobulin excretion may be three times higher in smokers than nonsmokers (Ellis et al., 1979; Hansen et al., 1985; Kjellström et al., 1977).
Half-life: Three phases are suggested. For phases 1 (<20 days) and 2 (20+ days), the half-life is estimated at 170 days. Phase 3 is estimated at 10-30 years (Friberg et al., 1974).
Blood LOAEL: Blood levels of cadmium poorly reflect the body burden but do indicate recent exposure.
Tissue LOAEL: The critical level in kidney is 200-285 ppm (effects are nephropathy and proteinuria). The LOAEL normally reaches a peak of 40-50 µg/g at age 50. Hair levels may reflect long-term exposure (Whanger, 1979).
Percent Absorption: About 2.7-5.9% is absorbed, usually considered 5% for all forms; absorption is determined by feeding humans radiolabeled cadmium (Newton et al., 1984).
Age, Sex, Reproductive Status, and Interindividual Variability of Response: Toxic effects, especially renal damage, are closely related to age, with greatest prevalence among those over 50 years (Fukushima et al., 1974). Multiparous females appear more sensitive to skeletal lesions (Kobayashi, 1978). The extent of interindividual variability is uncertain.
Pretoxic Indicator: Urine retinol binding protein (RBP) is a biomarker.
Long-term Effects: Nephropathy with tubular dysfunction results in proteinuria. There are conflicting reports regarding the production of hypertension in women. There is a significant correlation among cadmium levels in food, urine cadmium, and tubular dysfunction (Nogawa et al., 1978).
Kinetics: An eight-compartment model is used; the highest levels are found in kidney and liver (Kjellström and Nordberg, 1985).
ADI: The ADI is 57-72 µg/day; for a 70-kg individual, it is 0.0008-0.00103 mg/kg.
Toxic Body or Organ Burden: The toxic burden for kidney is 200-285 ppm.
Steady Daily Intake for Toxicity: A daily intake of 0.35 mg (0.005 mg/kg/day) at 50 years
could lead to nephrosis (Friberg et al., 1974). The NOAEL is 0.2 mg/day (0.0029 mg/kg/day) (CEC, 1978).
Lead
Occupational and environmental poisoning with lead is well documented (NRC, 1972, 1980a). A plethora of dose-response information is available that has been used by risk assessors to calculate an acute ADI. Neurodevelopmental effects in children are presently being used to assess acceptable dosage and blood levels, although there is considerable skepticism that a true threshold exists. The latter is a difficult task because precise exposure doses associated with effects are not well known (Tsuchiya, 1980). Reports concerning dose-response are conflicting for similar effects, and a wide range of interindividual variability is apparent.
Half-life: The half-life is 5 years to decades for bones, depending on type and location. In blood, it is estimated at 21-28 days; in soft tissues, it is intermediate between these extremes. Lead accumulates in the skeletal system.
Blood Median Acceptable Toxicant Concentration (MATC): The Centers for Disease Control (CDC) puts the level for young children at 25 parts per billion (ppb) (but see long-term effects).
Blood LOAEL: The LOAEL is not well known in humans and varies according to the effect measured. It is a poor indicator of an individual's dose-response but is of value in assessing population exposure. Some LOAELs for varying effects are delta-aminolevulinic acid dehydratase 10-20 µg/100 mL; changes in peripheral nerve conduction velocity 51-60 µg/100 mL; chronic encephalopathy (children) 50-60 µg/100 mL; acute encephalopathy 80 µg/100 mL. Recent measurements of developmental impairment show differences between groups in the normal range (5-15 g/100 mL) (see below).
Hair LOAEL: Hair is a poor estimator; there is no LOAEL.
Percent Absorption: The absorption is very variable, with the best estimate being 10%. Children may reach 50%. True mean percentage absorption remains uncertain (Rabinowitz et al., 1974).
Age, Sex, Reproductive Status, and Interindividual Variability of Response: Recent studies indicate that the brain of the fetus may be more sensitive to lead than that of the neonate; hence, CDC levels of 25 ppb in blood may not be acceptable (Waternaux et al., 1989).
Pretoxic Indicator: delta-Aminolevulinic acid dehydratase inhibition in erythrocytes correlates negatively with lead blood levels (EPA, 1979). Free erythrocyte protoporphyrin (FEP) increases with blood lead levels but varies in relation to sex.
Long-term Effects: Anemia due to inhibition of hemoglobin production and shortened life span of erythrocytes result. There is derangement of both the peripheral and the central nervous system (CNS), especially with regard to neurobehavioral problems. Slowed mental development in neonates has been measured by the Bailey Scales of Mental Development. Children with higher blood lead levels at 6 months (7.07 µg/100 mL, SD 1.18 µg/100 mL) have poorer scores at 18 months than those with lower blood levels (4.66 µg/100 mL, SD 0.50 µg/100 mL) (Waternaux et al., 1989). Irreversible
renal functional and morphological changes may occur (Tsuchiya, 1980).
Kinetics: There are essentially two compartments (three possible): blood and soft tissue (10% of burden) and skeletal system (90% of burden).
ADI: The ADI is estimated at 429 µg/day and the PTI at 6.1 µg/kg/day (FAO/WHO, 1972).
Toxic Body Burden: The body burden that would be toxic to a 70-kg individual is 100-400 mg.
Steady Daily Intake for Toxicity: The daily intake that would lead to toxicity is uncertain.
Mercury
As previously stated, the chemical form of the ingestible mercury in seafood is thought to be predominantly methylmercury. Both occupational and accidental environmental poisonings with the metal and its methyl or ethyl form have been extensively reported and reviewed in the literature. Observations of blood and other tissue LOAELs in affected individuals allow calculation of an acute ADI (Inskip and Piotrowski, 1985; Tollefson and Cordel, 1986).
Half-life: Whole body half-life values reveal an interindividual variability and differences with regard to reproductive status. The half-life is estimated at 70 days but is more than 110 days in some individuals and is 45 days for lactating females (Al-Shahristani and Shihab, 1974; Greenwood et al., 1978).
Blood LOAEL: In males and nonpregnant women the LOAEL in blood is 0.22-0.24 ppm; for pregnant women, 0.10 ppm; and due to fetal sensitivity, the maternal threshold is 0.05 ppm (Inskip and Piotrowski, 1985). A sound relationship exists between blood level and daily intake.
Hair LOAEL: The LOAEL in hair for adults is 25-50 ppm; for pregnant women, 37 ppm; maternal threshold, 15-20 ppm. The threshold may be as low as 10 ppm (10 µg/g; blood 0.03 ppm) in cases of extended periods of exposure (Inskip and Piotrowski, 1985). Hair appears to better reflect existing body mercury levels than urine or blood and is more resistant to sudden change. Hair levels also appear related to fish intake (Airey, 1983; Ohno et al., 1984). The hair/blood ratio is consistent and helpful in estimating exposure.
Percent Absorption: Gastrointestinal absorption is 95%.
Age, Sex, Reproductive Status, and Interindividual Variability of Response: There is little evidence for systematic differences in response due to age or sex of adults. Pregnant women may have a greater sensitivity; however, they deliver full-term, normal-weight children whose blood levels may be twice that of the mother. Such children develop CNS signs, including cerebral palsy, and delayed motor activity and speech. Lesions may increase in severity over long periods (Amin-Zaki et al., 1979). Effects of mild exposure are unknown.
Pretoxic Indicator: Porphyrinuria has been observed in early poisonings and is suggested as a possible indicator of exposure.
Long-term Effects: In children exposed prenatally to mercury, mental retardation can occur.
Kinetics: A single-compartment (possibly two-compartment) model is likely. There is
uncertainty as to how the methyl form complexes with tissue, but studies suggest binding to glutathione in human blood and rat brain. Of the total body burden, 3-7% is found in the brain (Naganuma et al., 1980).
ADI: The ADI is 0.0033 mg/kg body weight. Estimates vary, however; even at this level there is an 8% risk of effect.
Toxic Body Burden: The toxic body burden for a 70-kg individual is calculated to be 25 mg.
Steady Daily Intake for Toxicity: The acute daily intake level is 300 µg; the chronic level is uncertain.
Selenium
Selenium as an ingestible toxicant remains enigmatic because of its known protective and deleterious effects. As previously noted, the metal may exist in a number of forms. Elemental selenium is not water soluble. The reduced form (-2) is selenide. The dioxide (+4) in water forms selenous acid whose salts are selenites, whereas the trioxide (+6) in water forms selenic acid whose salts are selenates. These substances appear to vary in toxicity and distribution in the body. Among other missing dose-response data for selenium, knowledge concerning the chemical forms found in seafood is incomplete. This lack results in an inability to calculate no-observed-adverse-effect levels (NOAELs), LOAELs, and frank effect levels (FELs). Although some dose-response data are available for inhalation toxicity in humans, very few are available concerning oral exposure. Recent studies of humans chronically exposed to selenium in endemic areas of the United States revealed no adverse health effects (Fan et al., 1988). The literature on selenium and its toxicity has been reviewed by Hogberg and Alexander (1986).
Half-life: In humans, studies reveal three phases for selenite, which are 1 day, 8-20 days, and 65-116 days.
Blood LOAEL: For nail changes the LOAEL is 0.179 µg/mL. Depending on the definition of selenosis, other reports indicate no signs with blood levels of 0.44 µg/mL.
Blood FEL: The blood FEL is 1.3-7.5 µg/mL with a mean of 3.2 µg/mL.
Tissue LOAELs: For nail changes as measured by hair concentration, the LOAEL is 0.828 µg/mL. Depending on the definition of selenosis, some report no signs with hair levels of 3.7 µg/mL.
Hair FEL: The hair FEL is 4.1-100 µg/mL with a mean of 32.2 µg/mL.
Percent Absorption: Absorption is estimated in humans to be from 40 to 80% for selenite and 75 to 97% for selenomethionine (Bopp et al., 1982).
Age, Sex, Reproductive Status, and Interindividual Variability of Response: No information is available regarding the effect of human age, sex, reproductive status, or interindividual variability of response.
Pretoxic Indicator: The use of increased glutathione peroxidase activity as an indicator of exposure is apparently of no value (Valentine et al., 1988). No biomarkers have been identified.
Long-term Effects: The long-term effects are uncertain. Pathological nail changes, loss of hair, dermatitis, icterus, mottled teeth, and caries are some of the more obvious
ones. In a few instances, neurological upsets have been reported in adults. The target organ for chronic exposure in animals is the liver. This has not been documented in humans.
Kinetics: Initially, selenium is distributed to most organs, but the percentage of distribution may depend on the chemical form. Selenium in humans appears to bind to plasma lipoproteins, cross the placenta, and enter milk. Transient accumulation occurs in blood, muscle, liver, and kidney, with greater retention in the brain, thymus, and reproductive organs. Excretion in humans is primarily by urine and exhalation.
ADI: The ADI is uncertain. Yang et al. (1983) estimate 0.022 mg of organic selenium daily as a NOAEL for adult humans; the National Research Council (NRC, 1980b) gives the estimated safe and adequate daily dietary intake (ESADDI) for infants as 0.01-0.06 mg, for children 0.02-0.2 mg., and for adults 0.05-0.2 mg.
Toxic Body Burden: No information is available.
Steady Daily Intake for Toxicity: It has been estimated that 1 mg of selenium daily, as the selenite, would be toxic (Yang et al., 1983).
Conclusions
Thresholds of toxicity calculated for acute or short-term exposures, especially for mercury, may not reflect a threshold for chronic or long-term exposures. Recent assessment models have, therefore, included coefficients of cumulative toxicity (Inskip and Piotrowski, 1985; PTI, 1987). Such models, however, would be strengthened if data regarding chronic and cumulative toxicity could be generated. Models would be further strengthened by information regarding interindividual variability of response as a function of blood level. The existing dose-response data base with respect to human risk from seafoods contaminated with the trace metals arsenic, cadmium, lead, mercury, and selenium lacks sufficient information regarding the effects of chronic exposures, the sensitivity of certain subpopulations, and interindividual variability to make such an assessment. In the case of arsenic, although no sound human data exist, the primary form found in seafood is organic and appears to be of very low toxicity to animals. Therefore, identification of this trace metal as a potential hazard to humans may be premature.
REFERENCES
Airey, D. 1983. Total mercury concentrations in human hair from 13 countries in relation to fish consumptions and locations. Sci. Total Environ. 31:157-180.
Al-Shahristani, H., and K.M. Shihab. 1974. Variation of biological half-life of methylmercury in man. Arch. Environ. Health 28:342-344.
Amin-Zaki, L., M.A. Majeed, S.B. Elhassani, T.W. Clarkson, M.R. Greenwood, and R.A. Doherty. 1979. Prenatal methylmercury poisoning. Clinical observations over five years. Am. J. Dis. Child. 133:172-177.
Anonymous. 1988a. Health and environmental effects profile for antimony oxides. NTIS/PB88-175039. Government Reports Announcements and Index, Issue 12. 129 pp.
Anonymous. 1988b. Health effects assessment for antimony and compounds. NTIS/PB88-179445. Government Reports Announcements and Index, Issue 13. 46 pp.
ATSDR (Agency for Toxic Substances and Disease Registry). 1989a. Toxicological Profile for Arsenic. ATSDR/TP-88/02. Prepared by Life Systems, Inc. for ATSDR, U.S. Public Health Service in
collaboration with U.S. Environmental Protection Agency. 125 pp.
ATSDR (Agency for Toxic Substances and Disease Registry). 1989b. Toxicological Profile for Cadmium. ATSDR/TP-88/08. Prepared by Life Systems, Inc. for ATSDR, U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency. 107 pp.
Beliles, R.P. 1978. The lesser metals. Pp. 547-616 in F.W. Oehme, ed. Toxicity of Heavy Metals in the Environment, Part 2. Marcel Dekker, New York.
Bertazzi, P.A., L. Riboldi, A. Pesatori, L. Radice, and C. Zocchetti. 1987. Cancer mortality of capacitor manufacturing workers. Am. J. Indust. Med. 11:165-176.
Bevill, R.F., and W.G. Huber. 1977. Sulfonamides. Pp. 894-911 in L. Jones, N. Booth, and L. McDonald, eds. Veterinary Pharmacology and Therapeutics, 4th ed. Iowa State University Press, Ames.
Booth, N.H. 1977. Drug and chemical residues in the edible tissues of animals. Pp. 1299-1342 in L. Jones, N. Booth, and L. McDonald, eds. Veterinary Pharmacology and Therapeutics, 4th ed. Iowa State University Press, Ames.
Bopp, B.A., R.C. Sonders, and J.W. Kesterson. 1982. Metabolic fate of selected selenium compounds in laboratory animals and man. Drug Metab. Rev. 13:271-318.
Brown, D. P. 1987. Mortality of workers exposed to polychlorinated biphenyls: An update. Arch. Environ. Health 42:333-339.
Buck, W.B. 1978. Toxicity of inorganic and aliphatic organic arsenicals. Pp. 357-374 in F.W. Oehme, ed. Toxicity of the Heavy Metals in the Environment. Marcel Dekker, New York.
Camber, C.I., M.H. Vance, and J. Alexander. 1956. How to use sodium bisulfite to control "blackspot" on shrimp. Univ. Miami Special Bull. No. 12, 4 pp.
Cappon, C.J., and J.C. Smith. 1981. Mercury and selenium content and chemical form in fish muscle. Arch. Environ. Contam. Toxicol. 10:305-319.
Cappuzo, J., A. McElroy, and G. Wallace. 1987. Fish and shellfish contamination in New England waters: An evaluation and review of available data on the distribution of chemical contaminants. Report submitted to Coast Alliance, Washington, D.C. 85 pp.
CEC (Commission of the European Communities). 1978. Pp. 1-198 in Criteria (Dose/ Effect Relationships) for Cadmium. Pergamon Press. Oxford, England.
Clark, J.R., D. Devault, R.J. Bowden, and J.A. Weishaar. 1984. Contaminant analysis of fillets from Great Lakes coho salmon, 1980 . J. Great Lakes Res. 10:38-47.
Cordle, F., R. Locke, and J. Springer. 1982. Risk assessment in a federal regulatory agency: An assessment of risk associated with the human consumption of some species of fish contaminated with polychlorinated biphenyls (PCBs). Environ. Health Perspect. 45: 171-182.
Duling, L. 1988. Fish contaminant monitoring program 1988 annual report. Report MI/DNR/SWQ-88/090. Michigan Department of Natural Resources, Surface Water Quality Division, Lansing. 300 pp.
Earl, F.L., and T.J. Vish. 1978. Teratogenicity of heavy metals. Pp. 617-640 in F.W. Oehme, ed. Toxicity of Heavy Metals in the Environment, Part 2. Marcel Dekker, New York.
Ellis, K.J., D. Vartsky, I. Zanzi, S.H. Cohn, and S. Yasumura. 1979. Cadmium: In vivo measurement in smokers and nonsmokers. Science 205:323-324.
EPA (Environmental Protection Agency). 1979. Ambient Water Quality Criteria for Lead. Water Planning and Standards. EPA 440/5-80-057. U.S. Environmental Protection Agency, Washington, D.C. 161 pp.
EPA (Environmental Protection Agency). 1986. Pesticides Fact Sheet No. 109: Chlordane. EPA 540/FS 87/143. U.S. Environmental Protection Agency, Washington, D.C. 9 pp.
EPA (Environmental Protection Agency). 1987. The National Dioxin Study: Tiers 3,5,6, and 7. EPA 440/4-87-003. Office of Water Regulations and Standards, Monitoring and Data Support Division, U.S. Environmental Protection Agency, Washington, D.C. 208 pp.
EPA (Environmental Protection Agency). 1988a. Assessment of Dioxin Contamination of Water, Sediment and Fish in the Pigeon River System (a Synoptic Study) Report No. 001. U.S. Environmental Protection Agency, Region IV, Water Management Division, Atlanta, Georgia. 75 pp.
EPA (Environmental Protection Agency). 1988b. Dioxin Levels in Fish Near Pulp and Paper Mills. Interim report dated October 25. U.S. Environmental Protection Agency, Office of Water Regulations and Standards, Washington, D.C.
EPA (Environmental Protection Agency) 1988c. Integrated Risk Information System (IRIS). As, Cd, Pb, Hg and Se. DIALCOM, Inc., Washington, D.C.
Ewan, R.C. 1978. Toxicology and adverse effects of mineral imbalance with emphasis on selenium and other minerals. Pp. 445-490 in F.W. Oehme, ed. Toxicity of Heavy Metals in the Environment, Part 2. Marcel Dekker, New York.
Fan, A.M., S.A. Book, R.R. Neutra, and D.M. Epstein. 1988. Selenium and human health implications in California's San Joaquin Valley. J. Toxicol. Environ. Health 23:539-560.
Fauvel, Y., G. Pons, and J.P. Legeron 1982. Ozonation de l'eau de mer et épuration des coquillages. Science et Peche, Nantes 320:1-16.
FDA (Food and Drug Administration). 1982. Levels for Poisonous or Deleterious Substances in Human Food and Animals Feed. U.S. Food and Drug Administration, Washington, D.C. 13 pp.
FDA (Food and Drug Administration). 1988. Compliance Program Guidance Manual. FY86 Pesticides and Industrial Chemicals in Domestic Food. Program No. 7304-004. U.S. Department of Health and Human Services, Public Health Service, U.S. Food and Drug Administration, Bureau of Foods, Washington, D.C. 48 pp.
Fein, G.G., J.L. Jacobson, S.W. Jacobson, P.M. Schwartz, and J.K. Dowler. 1984. Prenatal exposure to polychlorinated biphenyls: Effects on birth size and gestational age. J. Pediatrics 105:315-320.
Fishbein, L. 1983. Environmental selenium and its significance. Fundam. Appl. Toxicol. 3:411-419.
Fowler, B.A. 1983. Pp. 1-281 in Biological and Environmental Effects of Arsenic, Vol. 6. Elsevier Science Publishers, Amsterdam, The Netherlands.
Fox, A. 1990. Fishery chemicals used in foreign countries. Intracenter memorandum, January 16. National Fisheries Research Center, Seattle, Wash.
Friberg, L., M. Piscator, G.F. Nordberg, and T. Kjellström. 1974. Cadmium in the Environment, 2nd ed. CRC Press, Cleveland, Ohio. Vol. I, 209 pp; Vol. II, 248 pp.
Friberg, L., C.-G. Elinder, T. Kjellström, and G.F. Nordberg. 1985. Cadmium and Health. A Toxicological and Epidemiological Appraisal. CRC Press, Boca Raton, Fla. 307 pp.
Fukayama, M.Y., H. Tan, W.B. Wheeler, and C.I. Wei. 1986. Reactions of aqueous chlorine and chlorine dioxide with model food compounds. Environ. Health Perspect. 69:267-274.
Fukushima, M. A. Ishizaki, K. Nogawa, M. Sakamoto, and E. Kobayashi. 1974. Epidemiological studies on renal failure of inhabitants in "itai-itai" disease endemic district (Part 1). Some urinary findings of inhabitants living in and around the endemic district of the Jinzu River basin. Jap. J. Pub. Health 21:65-73.
Gadbois, D.F., and R.S. Maney. 1983. Survey of polychlorinated biphenyls in selected finfish species from United States coastal waters. Fish. Bull. (USFWS) 81:389-396.
Gladen, B. C., W.J. Rogan, P. Hardy, J. Thullen, J. Tinglestad, and M. Tully. 1988. Development after exposure to polychlorinated biphenyls and dichlorodiphenyldichloroethene transplacentally and through human milk. J. Pediatr. 113:991-995.
Gossett, R.W., H.W. Puffer, R.H. Arthur, Jr., J. Alfafara, and D.R. Young. 1982. Pp. 29-37 in W. Bascon, ed. Levels of Trace Organic Compounds in Sportfish from Southern California. Coastal Water Research Project Biennial Report. Southern California Coastal Water Research Project 1981-1982, Long Beach, Calif.
Gossett, R.W., H.W. Puffer, R.H. Arthur Jr., and D.R. Young. 1983. DDT, PCB and Benzo(a)pyrene levels in white croaker (Genyonemus lineatus ) from southern California. Mar. Poll. Bull. 14:60-65.
Goyer, R.A. 1986. Toxic effects of metals. Pp. 582-635 in C. Klassen, M. Amdur, and J. Doull, eds. Casarett and Doull's Toxicology, 3rd ed. Macmillan, New York.
Green, V.A., G.W. Wise, and J.C. Callenbach. 1978. Lead poisoning. Pp. 123-141 in F.W. Oehme, ed. Toxicity of Heavy Metals in the Environment, Part 1. Marcel Dekker, New York.
Greenwood, M.R., T. W. Clarkson, R. A. Doherty, A. H. Gates, L. Amin-Zaki, S. Elhassani, and M. A. Majeed. 1978. Blood clearance half-times in lactating and non-lactating members of a population exposed to methylmercury. Environ. Res. 16:48-54.
Griffin, A.C. 1979. Role of selenium in the chemoprevention of cancer. Adv. Cancer Res. 29:419-422.
Groth, D.H., L.E. Stettler, J.R. Burg, W.M. Busey, G.C. Grant, and L. Wong. 1986. Carcinogenic effects of antimony trioxide and antimony ore concentrate in rats. J. Toxicol. Environ. Health 18:607-626.
Guarino, A.M., S.M. Plakas, R.W. Dickey, and M. Zeeman. 1988. Principles of drug absorption and recent studies of bioavailability in aquatic species. Vet. Human Toxicol. 30:41-44.
Gunderson, E. L. 1988. FDA Total Diet Study, April 1982-April 1984, Dietary intakes of pesticides,
selected elements and other chemicals. J. Assoc. Off. Anal. Chem. 71:1200-1209.
Haines, A.T., and E. Nieboer. 1988. Chromium hypersensitivity. Pp. 497-532 in J. Nrigau and E. Nieboer eds. Chromium in the Natural and Human Environment. John Wiley & Sons, New York.
Hall, R.A., E.G. Zook, and G.M. Meaburn. 1978. National Marine Fisheries Service Survey of Trace Elements in the Fishery Resource. NOAA Technical Report NMFS SSRF-721, National Technical Information Service No. PB 283 851, March. 313 pp.
Hammond, P.B. 1978. Metabolism and metabolic action of lead and other heavy metals. Pp. 87-99 in F.W. Oehme, ed. Toxicity of Heavy Metals in the Environment, Part 1. Marcel Dekker, New York.
Hansen, J.C., H.C. Wulf, N. Kromann, and K. Alboge. 1985. Cadmium concentrations in blood samples from the East Greenlandic population. Dan. Med. Bull. 32:277-279.
Harada, M. 1978. Methyl mercury poisoning due to environmental contamination (Minamata disease). Pp. 261-302 in F.W. Oehme, ed. Toxicity of Heavy Metals in the Environment, Part 1. Marcel Dekker, New York.
Harr, J.R. 1978. Biological effects of selenium. Pp. 393-426 in F.W. Oehme, ed. Toxicity of Heavy Metals in the Environment, Part 2. Marcel Dekker, New York.
Harr, J.R., and O.H. Muth. 1972. Selenium poisoning in domestic animals and its relationship to man. Clin. Toxicol. 5:175-186.
Hattis, D. 1972. The FDA and nitrite—A case study of violations of the Food, Drug, and Cosmetic Act with respect to a particular food additive. Presented in hearings before the Select Committee on Nutrition and Food Needs of the United States Senate, September 21, pp. 1692-1720.
Hattis, D. 1987. A pharmacokinetic mechanism-based analysis of the carcinogenic risk of ethylene oxide. National Technical Information Service, No. NTIS/PB88-188784. MIT Center for Technology, Policy and Industrial Development, No CTPIC 87-1 , August. 176 pp.
Hattis, D. 1989. Letter report from Dr. Dale Hattis, MIT, to W. Munns, Environmental Research Laboratory, Narragansett, R.I., December 28. 4 pp.
Hawke, J.P., S.M. Plakas, R. Vernon Minton, R.M. McPhearson, T.G. Snider, and A.M. Guarino. 1987. Fish pasteurellosis of cultured striped bass (Morone saxatilis) in coastal Alabama. Aquaculture 65:193-204.
Hogberg, J., and J. Alexander. 1986. Selenium. Pp. 482-520 in L. Friberg, J. Parizek, and V. Vouk, ed. Handbook on the Toxicology of Metals, 2d end. Elsevier Science Publishers, Amsterdam, The Netherlands.
Huber, G. 1977a. Penicillins. Pp. 912-928 in L.M. Jones, N.H Booth, and L.E. McDonald, eds. Veterinary Pharmacology and Therapeutics, 4th ed. Iowa State University Press, Ames.
Huber, G. 1977b. Tetracyclines. Pp. 929-939 in L.M. Jones, N.H. Booth, and L.E. McDonald, eds. Veterinary Pharmacology and Therapeutics, 4th ed. Iowa State University Press, Ames.
Huber, G. 1977c. Streptomycin, chloramphenicol and other antibiotic agents. Pp. 940-971 in L.M. Jones, N.H. Booth, and L.E. McDonald, eds. Veterinary Pharmacology and Therapeutics, 4th ed. Iowa State University Press, Ames.
Inskip, M.J., and J.K. Piotrowski. 1985. Review of the health effects of methylmercury. J. Appl. Toxicol. 5:113-133.
Jacobson, J.L., H.E. Humphrey, S.W. Jacobson, S.L. Schantz, M.D. Mullin, and R. Welch. 1989. Determinants of polychlorinated biphenyls (PCBs), polybrominated biphenyls (PBBs), and dichlorodiphenyl trichloroethane (DDT) levels in the sera of young children. Am. J. Publ. Health 79:1401-1404.
Jacobson, J.L., S.W. Jacobson, and H.E. Humphrey. 1990. Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. J. Pediatr. 116:38-45.
Jacobson, S.W., G.G. Fein, J.L. Jacobson, P.M. Schwartz, and J.K. Dowler. 1985. The effect of intrauterine PCB exposure on visual recognition memory. Child Develop. 56:853-860.
Kansas DHE (Department of Health and Environment). 1988. A Survey of Pesticides in Tuttle Creek Lakes, Its Tributaries and the Upper Kansas River. Water Quality Assessment Section, Bureau of Water Protection, Kansas Department of Health and Environment, Topeka, Kansas. 29 pp.
Kjellström, T., and G.F. Nordberg. 1985. Kinetic model of cadmium metabolism. Pp. 179-197 in L. Friberg, C.G. Elinder, T. Kjellström, and G.F. Nordberg, eds. Cadmium and Health: A Toxicological and Epidemiological Appraisal, Vol. 1 Exposure, Dose and Metabolism. CRC Press, Boca Raton, Fla.
Kjellström, T., P.-E. Ervin, and B. Rahnster. 1977. Dose-response relationship of cadmium-induced tubular proteinuria. Environ. Res. 13:303-317.
Kobayashi, J. 1978. Pollution by cadmium and the itai-itai disease in Japan. Pp. 199-260 in F.W. Oheme, ed. Toxicity of Heavy Metals in the Environment, Part 1. Marcel Dekker, New York.
Kociba, R.J., D.G. Keyes, J.E. Beyer, R.M. Carreon, C.E. Wade, D.A. Dittenber, R.P. Kalmins, L.E. Frauson, C.N. Park, S.D. Barnard, R.A. Hummel, and C.G. Humiston. 1978. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol. Appl. Pharmacol. 46:279-303.
Landolt, M.L., F.R. Hafer, A. Nevissi, G. van Belle, K. Van Ness, and C. Rockwell. 1985. Potential toxicant exposures among consumers of recreationally caught fish from urban embayments of Puget Sound. NOAA Tech. Memo. NOS OMA 23. Rockville, Md. 104 pp.
Landolt, M.L., D. Kalman, A. Nevissi, G. van Belle, K. Van Ness, and F.R. Hafer. 1987. Potential toxicant exposures among consumers of recreationally caught fish from urban embayments of Puget Sound. NOAA Tech. Memo. NOS OMA 33. Rockville, Md. 111 pp.
Lauwerys, R., and Ph. De Wals. 1981. Environmental pollutions y cadmium and mortality from renal diseases. Lancet 1:383.
Lecos, C. 1985. Reacting to sulfites. FDA Consumer 19:22-25.
Lowe, T.P., T.W. May, W.G. Brumbaugh, and D.A. Kane. 1985. National contaminant biomonitoring program: Concentrations of seven elements in freshwater fish, 1978-1981. Arch. Environ. Contam. Toxicol. 14:363-388.
Malins, D.C., B.B. McCain, D.W. Brown, A.K. Sparks, and H.O. Hodgins. 1980. Chemical contaminants and biological abnormalities in central and southern Puget Sound. NOAA Tech. Memo. OMPA-2. PB 81/155/897. Boulder, Co. 312 pp.
Malins, D.C., B.B. McCain, D.W. Brown, A.K. Sparks, H.O. Hodgins, and S-L. Chan. 1982. Chemical contaminants and abnormalities in fish and invertebrates in Puget Sound. NOAA Tech. Memo. OMPA-19. PB 83/115/188. Springfield, Va. 189 pp.
Manci, W. 1990. Hope and caution highlight fish disease news. Catfish News/Aquaculture News 4:10.
Masri, M.S. 1986. Chlorinating poultry chiller water: The generation of mutagens and water reuse . Food Chem. Toxicol. 24:923-930.
McKenzie-Parnell, J.M., T.E. Kjellström, R.P. Sharma, and M.F. Robinson. 1988. Unusually high intake and fecal output of cadmium, and fecal output of other trace elements in New Zealand adults consuming dredge oysters. Environ. Res. 46:1-14.
Mearns, A.J., M.B. Matta, D. Simecek-Beatty, M.F. Buchman, G. Shigenaka, and W.A. Wert. 1988. PCB and chlorinated pesticide contamination in U.S. fish and shellfish: A historical assessment report. NOAA Tech. Memo. NOS OMA 39. Seattle, Wash. 140 pp.
Meyer, F.P., and R.A. Schnick. 1989. A review of chemicals used for the control of fish diseases. Aquat. Sci. 1:693-710.
Michel, C. 1986. Practical value, potential dangers and methods of using antibacterial drugs in fish. Rev. Sci. Tech. Off. Int. Epiz. 5:659-675.
Michigan CEHS (Center for Environmental Health Sciences). 1986. Background Document Concerning 1986 Fish Consumption Advisory for Dioxin Contaminated Fish. Prepared for the Michigan Environmental Review Board, Lansing, Mich. February. 13 pp.
Mitchell, G.A. 1989. The FDA role in aquaculture chemical and drug usage. Paper presented at Aquaculture Opportunities for Appalachia Meeting, November 13-15, 1989, Birmingham, Ala. 13 pp.
Murphy, D.L. 1988a. Basic water monitoring program. Fish tissue analysis, 1985. State of Maryland, Department of Environment, Division of Standards and Certification, Water Management Administration. Technical Report 59. Baltimore, Md. 38 pp.
Murphy, D.L. 1988b. Trace contaminants in Chesapeake Bay bluefish. Metals and organochlorine pesticides. State of Maryland, Department of the Environment, Division of Standards and Certification, Water Management Administration. Technical Report 73. Baltimore, Md. 21 pp.
Murphy, D.L. 1988c. Trace contaminants in striped bass from two Chesapeake Bay tributaries. Metals and organochlorine pesticides. State of Maryland, Department of the Environment, Division of Standards and Certification, Water Management Administration. Technical Planning and Evaluation Report 58. Baltimore, Md. 19 pp.
Murphy, S.D. 1980. Pesticides. Pp. 357-408 in J. Doull, C. Classen, and M. Amdur, eds. Casarett and Doull's Toxicology, 2nd ed. Macmillan, New York.
Naganuma, A., Y. Koyama, and N. Imura. 1980. Behaviour of methylmercury in mammalian
erythrocytes. Toxicol. Appl. Pharmacol. 54:405-410.
Newton, D., P. Johnson, A.E. Lally, R.J. Pentreath, and D.J. Swift. 1984. The uptake by man of cadmium ingested in crab meat. Human Toxicol. 3:23-28.
Nieboer, E., and A.A. Jusys. 1988. Biological chemistry of chromium. Pp. 21-80 in J. Nrigau and E. Nieboer, eds. Chromium in the Natural and Human Environments. John Wiley & Sons, New York.
NOAA (National Oceanic and Atmospheric Administration). 1987. A summary of selected data on chemical contaminants in tissues collected during 1984, 1985, and 1986. Progress Report. NS&T Program for Marine Environmental Quality. NOAA Technical Memorandum. NOS OMA 38. Rockville, Md. 104 pp.
NOAA (National Oceanic and Atmospheric Administration). 1988. PCB and chlorinated pesticide contamination in U.S. fish and shellfish: A historical assessment report. NOAA Technical Memorandum. NOS OMA 39. Seattle, Wash. 140 pp.
NOAA (National Oceanic and Atmospheric Administration). 1989. A summary of data on tissue contamination from the first three years (1986-1988) of the mussel watch project. Progress Report. NS&T Program for Marine Environmental Quality. NOAA Technical Memorandum. NOS OMA 49. Rockville, Md. 161 pp.
Nogawa, K., A. Ishizaki, and S. Kawano. 1978. Statistical observations of the dose-response relationships of cadmium based on epidemiological studies in the Kakehashi River basin. Environ. Res. 15:185-198.
NRC (National Research Council). 1972. Airborne Lead in Perspective. Committee on Biological Effects/Atmospheric Pollutants. National Academy Press, Washington, D.C. 333 pp.
NRC (National Research Council). 1980a. Lead in the Human Environment. Committee on Lead in Human Environment. National Academy Press, Washington, D.C. 525 pp.
NRC (National Research Council). 1980b. Recommended Dietary Allowances, 9th ed. Committee on Dietary Allowances, Food and Nutrition Board. National Academy Press, Washington, D.C. 185 pp.
Nriagu, J.O., ed. 1981. Cadmium in the Environment, Part II: Health Effects. John Wiley & Sons, New York. 908 pp.
Ohno, H., R. Doi, Y. Tani, and M. Harada. 1984. Mercury content of head hair from residents on the cost of Jakarta Bay. Bull. Env. Contamin. Toxicol. 33:382-385.
Penrose, W.R. 1975. Biosynthesis of organic arsenic compounds in the brown trout (Salmo trutta). J. Fish. Res. Bd. Canada 32:2385-2390.
Plakas, S.M., R.M. McPhearson, and A.M. Guarino. 1988. Disposition and bioavailability of 3H-tetracycline in the channel catfish (Ictalurus punctatus). Xenobiotica 18:83-93.
Plakas, S.M., R.W. Dickey, M.G. Barron, and A.M. Guarino. 1990. Tissue distribution and renal excretion of ormetoprim after intravascular and oral administration in the channel catfish (Ictalurus punctatus ). Can. J. Fish. Aquat. Sci. 47:1-6.
Pruell, R.J., E.J. Hoffman, and J.G. Quinn. 1984. Total hydrocarbons, polycyclic aromatic hydrocarbons and synthetic organic compounds in the hard shell clam, Mercenaria mercenaria, purchased at commercial seafood stores. Mar. Environ. Res. 11:163-181.
PTI. 1987. Guidance Manual for Assessing Human Health Risks from Chemically Contaminated Fish and Shellfish. Draft Report c737-01 submitted to Battelle New England Marine Research Laboratory, December 1987, by PTI Environmental Services, Inc., Bellevue, Wash. 64 pp.
Rabinowitz, M., G.W. Wetherill, and J.D. Kopple. 1974. Studies of human lead metabolism by use of stable isotope tracers. Environ. Health Perspect. 7:145-153.
Reddy, C.C., and E.J. Massaro. 1983. Biochemistry of selenium: A brief overview. Fundam. Appl. Toxicol. 3:431-436.
Rice, R.G., J.W. Farquhar, and L.J. Bollyky. 1982. Review of the applications of ozone for increasing storage times of perishable foods. Ozone: Science Engineer. 4:147-163.
Rogan, W.J., B.C. Gladen, J.D. McKinney, N. Carreras, P. Hardy, J. Thullen, J. Tinglestad, and M. Tully. 1986. Neonatal effects of transplacental exposure to PCBs and DDE. J. Pediatrics 109:335-341.
Rohrer, T.K., J.C. Forney, and J.H. Hartig. 1982. Organochlorine and heavy metal residues in standard fillets of coho and chinook salmon of the Great Lakes, 1980. J. Great Lakes Res. 8:623-634.
Rugen, P.J., C.D. Stern, and S.H. Lamm. 1989. Comparative carcinogenicity of the PAHs as a basis for acceptable exposure levels (AELs) in drinking water. Regul. Toxicol. Pharmacol. 9:273-283.
Safe, S. 1989. Polychlorinated biphenyls (PCBs): Mutagenicity and carcinogenicity . Mutat. Res. 220:31-47.
Schamberger, R.J. 1985. The genotoxicity of selenium. Mutation Res. 154:29-48.
Schmitt, C.J., M.A. Ribick, J.L. Ludke, and T.W. May. 1983. Organochlorine residues in freshwater fish, 1976-1979. National Pesticides Monitoring Program. Prepared by Columbia National Research Fisheries Laboratory, Columbia, Mo. 69 pp.
Schnell, R.C., and C.R. Angle. 1983. Selenium-Toxin or panacea? Fundam. Appl. Toxicol. 3:409-410.
Schnick, R.A. 1988. The impetus to register new therapeutants for aquaculture. Prog. Fish-Culturist 50:190-196.
Schnick, R.A., F.P. Meyer, and D.L. Gray. 1989. A guide to approved chemicals in fish production and fishery resource management. University of Arkansas, Cooperative Extension and U.S. Fish and Wildlife Service, Little Rock. Publication MP 241-5M-3-89RV. 27 pp.
Sherlock, J.C. 1986. Part III: Cadmium and human health. Experientia (supplement) 50:110-114.
Short, J.W., and F.P. Thrower. 1987a. Accumulations of butyltins in muscle tissue of chinook salmon reared in sea pens treated with tri-n-butyltin. Aquaculture 61:181-192.
Short, J.W., and F.P. Thrower. 1987b. Toxicity of tri-n-butyltin to chinook salmon, Oncorhynchus tshawytscha, adapted to seawater. Aquaculture 61:193-200.
Shukla, S.S., and H.V. Leland. 1973. Heavy metals: A review of lead. J. Water Poll. Control Fed. 45:1319-1331.
Sloan, R.J., and R. Karcher. 1985. On the origin of cadmium concentrations in Hudson River blue crab (Callinectes sapidus Rathbun). Northeastern Environ. Sci. 3:221-231.
Sloan, R.J., L.C. Skinner, E.G. Horn, and R. Karcher. 1987. An overview of mercury contamination in the fish of Onondaga Lake. New York State Department of Environmental Conservation, Division of Fish and Wildlife, Albany. Technical Report 87-1 (BEP). July. 44 pp.
Sorensen, E.M.B., P.M. Cumbie, T.L. Bauer, J.S. Bell, and C.W. Harlan. 1984. Histopathological, hematological, condition-factor, and organ weight changes associated with selenium accumulation in fish from Belews Lake, North Carolina. Arch. Environ. Contam. Toxicol. 13:153-162.
St. Amant, J.R., M.E. Pariso, and T.B. Sheffy. 1983. Final report on the toxic substances survey of the Lakes Michigan, Superior and tributary streams for the Wisconsin Coastal Management Program. Wisconsin Department of Natual Resources, Bureau of Water Resources Management, Water Quality Evaluation Section. Madison. 31 pp.
Stopford, W., and L.J. Goldwater. 1975. Methylmercury in the environment: A review of current understanding. Environ. Health Perspect. 12:115-118.
Stout, V.F. 1980. Organochlorine residues in fishes from the northwest Atlantic Ocean and the Gulf of Mexico. Fish. Bull. 78:51-58.
Sunahara, G.I., K.G. Nelson, T.K. Wong, and G.W. Lucier. 1987. Decreased human birth weights after in utero exposure to PCBs and PCDFs are associated with decreased placental EGF-stimulated receptor autophosphorylation capacity. Molec. Pharmacol. 32:572-578.
Tam. G.K.H., S.M. Charbonneau, F. Byrce, and E. Sandi. 1982. Excretion of a single oral dose of fish-arsenic in man. Bull. Environ. Contam. Toxicol. 28:669-673.
Taylor, P.R., J.M. Stelma, and C.E. Lawrence. 1989. The relation of polychlorinated biphenyls to birth weight and gestational age in the offspring of occupationally exposed mothers. Am. J. Epidem. 129:395-406.
Tollefson, L., and F. Cordle. 1986. Methylmercury in fish: Review of residue levels, fish consumption and regulatory action in the United States. Environ. Health Perspect. 68:203-208.
Tsuchiya, K. 1980. Lead. Pp. 451-484 in L. Friberg, G.F. Nordberg, and V.B. Vouk, eds. Handbook on the Toxicology of Metals, 2d ed. Elsevier Science Publishers, Amsterdam, The Netherlands.
Vahter, M., E. Marafante, and L. Denker. 1983. Metabolism of arsenobetaine in mice, rats and rabbits. Sci. Total Environ. 30:197-211.
Valentine, J.L., B. Faraji, and H.K. Kang. 1988. Human glutathione peroxidase activity in cases of high selenium exposures. Environ. Res. 45:16-27.
Van Gelder, G.A. 1978. Lead and the nervous system. Pp. 101-121 in F.W. Oehme, ed. Toxicity of Heavy Metals in the Environment, Part 1. Marcel Dekker, New York.
Viarengo, A. 1985. Biochemical effects of trace metals. Mar. Poll. Bull. 16:153-158.
Waternaux, C., N.M. Laird, and J.H. Ware. 1989. Methods for analysis of longitudinal data: Blood lead concentrations and cognitive development. J. Amer. Stat. Assoc. 84:33-41.
Whanger, P.D. 1979. Cadmium effects in rats on tissue iron, selenium, and blood pressure; Blood and hair cadmium in some Oregon residents. Environ. Health Perspect. 28:115-121.
WHO (World Health Organization). 1980. Tin and organotin compounds: A preliminary review.
Environmental Health Criteria 15. World Health Organization, Geneva, Switzerland. 109 pp.
WHO (World Health Organization). 1981. Pp. 1-174 in Environmental Health Criteria, Arsenic. World Health Organization, Geneva, Switzerland.
Wilber, C.G. 1983. Selenium: A potential environmental poison and a necessary food constitutent. Charles C. Thomas, Springfield, Ill. 126 pp.
Yang, G., S. Wang, R. Zhou, and S. Sun. 1983. Endemic selenium intoxication of humans in China. Am. J. Clin. Nutr. 37:872-881.
Zook, E.G., J.J. Powell, B.M. Hackley, J.A. Emerson, J.R. Brooker, and G.M. Knobl, Jr. 1976. National Marine Fisheries Service preliminary survey of selected seafoods for mercury, lead, cadmium, chromium and arsenic content. J. Agric. Food Chem. 24:47-53.





























































