6
Chemical Health Risk Assessment–Critique of Existing Practices and Suggestions for Improvement
ABSTRACT
This chapter and the previous one should be considered as a unit. A fourfold classification system of the mechanisms underlying adverse health effects is outlined below, which forms the basis for developing quantitative risk assessment approaches for both cancer and noncancer effects.
A detailed critique is then provided of existing Food and Drug Administration (FDA) risk assessments for polychlorinated biphenyls (PCBs) and methylmercury–representing the two most extensively documented examples of analyses underlying current regulatory levels for a carcinogen and a noncarcinogen in seafood, respectively. (In both cases, the committee finds considerable opportunity for improvement.)
The difficult issue of determining human intakes for a broad (though far from comprehensive) range of chemical contaminants in seafood is subsequently addressed. Estimates are made of national average daily intakes of various inorganic and organic contaminants via commercially marketed seafood, and for several organic carcinogens, upper-confidence-limit estimates of possible cancer risk are made. However, aside from the methylmercury example,1 in the absence of better information on the population distribution of the dosage of contaminants to the U.S. population, it is impossible to make even tentative quantitative estimates of potentially significant noncancer risks. Of additional serious concern are the appreciable quantities of seafood consumed following noncommercial sport and subsistence tribal fishing.
Finally, an overview of opportunities for research on different categories of potential health impacts is presented, and conclusions are drawn from both this and the previous chapter. The principal conclusions are the following:
-
From both natural and human sources, a small proportion of seafood is contaminated with appreciable concentrations of potentially hazardous organic and inorganic chemicals. Some of the risks that may be significant include reproductive effects from PCBs and methylmercury, and carcinogenesis from selected PCB congeners, dioxins, and some chlorinated hydrocarbon pesticides.
-
Consumption of some types of contaminated seafood poses enough risk that efforts toward evaluation, education, and control of that risk must be improved.
-
Present quantitative risk assessment procedures used by government agencies can and should be improved and extended to noncancer effects.
-
Current monitoring and surveillance programs provide an inadequate representation of the presence of contaminants in edible portions of domestic and imported seafood, resulting in serious difficulties in assessing both risks and specific opportunities for control.
-
Because of the unevenness of contamination among species and geographic areas, it is feasible to narrowly target control efforts and still achieve meaningful reductions in exposures.
-
The data base for evaluating the safety of certain chemicals that find their way into seafood via aquaculture and processing is too weak to support a conclusion that these products are being effectively controlled.
The principal recommendations of the committee are as follows:
-
Existing regulations to minimize chemical and biological contamination of the aquatic environment should be strengthened and enforced.
-
Existing FDA and state regulations should be strengthened and enforced to reduce the human consumption of aquatic organisms with relatively high contaminant levels (e.g., certain species from the Great Lakes with high levels of PCBs, swordfish and other species with high methylmercury levels).
-
Federal agencies should actively support further research to determine the actual risks from the consumption of contaminants associated with seafood and to develop specific approaches for decreasing these risks.
-
Increased environmental monitoring should be initiated at the state level, as part of an overall federal exposure management system.
-
States should continue to be responsible for site closures, and for issuing health and contamination advisories tailored to the specific consumption habits, reproductive or other special risks, and information sources of specific groups of consumers.
-
There should be an expanded program of public education on specific chemical contaminant hazards via governmental agencies and the health professions.
INTRODUCTION
Part of the committee's charge was to review and summarize "the current status of regulations, guidelines, and advisory statements issued by Federal and State public health authorities on environmental contaminants in seafood." Its review was to specifically address contaminants defined by Food and Drug Administration (FDA) regulations as "avoidable or unavoidable." Then, based on this, the committee was asked to "assess how well the current regulatory framework protects the public health."
The committee was also charged with the task of reviewing and summarizing, specifically, the health risk assessment procedures used by FDA, the Environmental Protection Agency (EPA), and other regulatory authorities for priority environmental pollutants, including toxic metals and synthetic organic chemicals. In addition, the committee was asked to "recommend future research directions, as appropriate."
To set the stage for an examination of how current risk assessment procedures can be improved, the basic concepts underlying the mechanisms of action of toxic substances are articulated in the following section, along with quantitative ideas about dose-time-response relationships. Then an extensive critique of agency risk assessments for PCBs and methylmercury is provided. Finally, the committee addresses issues of exposure assessment and risks from other substances, and opportunities for further research on potential chemical health hazards.
BROAD CATEGORIZATION OF MECHANISMS OF DIFFERENT ADVERSE EFFECTS AND IMPLICATIONS FOR DOSE-RESPONSE RELATIONSHIPS
The concern from which much of our regulatory history has resulted is the potential carcinogenic effect of some contaminants. To a certain extent, this concern is based on the mutagenic mechanisms of cancer. For carcinogens that act by primary genetic mechanisms, there are good theoretical reasons to believe that at the limit of low dosage, the risk will be a linear function of exposure (Ehrenberg et al., 1983; Hattis, 1990a). However, as more is learned about the mechanisms of some other types of toxic effects–particularly reproductive effects and chronic degenerative neurological conditions (NRC, 1989, 1990)–concern about potential low-dose effects of other types has tended to increase. It is therefore important to clarify what the expectations should be for dose-response relationships from first principles, given the full range of causal processes that can lead to impairment of health.
Table 6-1 shows a categorization system for biological damage mechanisms that can be helpful in guiding basic choices in risk assessment modeling (Hattis, 1982, 1986). The system is intended to distinguish between different ways of looking at the likely mechanisms of disease causation that are encouraged by different groups of scientific disciplines.2
The focus of the scheme in Table 6-1 is to sort adverse effects according to the kinds of events that are likely to be occurring at either (1) subclinical dosage levels (doses that do not produce unusual function) or (2) preclinical stages in the development of the pathological process (i.e., the time before an overt manifestation of a latent disease, such as cancer, occurs). Under these conditions, one first asks
-
Are the events occurring ordinarily fully reversible (or very nearly so), given a prolonged period with no further exposure to the hazard?
TABLE 6-1 Types of Health Hazards Requiring Fundamentally Different Risk Assessment Approaches
|
1. "Traditional" toxicity resulting from overwhelming body compensatory processes: below some threshold, in individuals who are not already beyond the limits of normal function without exposure, response is reversible. |
|
• Traditional acute toxicity–Toxic action is completely reversible or proceeds to long-term damage within about three days of exposure (paralytic shellfish poisoning, puffer fish poisoning; probably many teratogenic effects). • Traditional chronic toxicity—Toxic process typically proceeds to permanent damage over a period of several days to several months, due to either (1) reversible accumulation of a toxic agent (e.g., methylmercury, lead) or (2) accumulation of a slowly reversible toxic response (e.g., cholinesterase inhibition). |
|
2. Effects resulting from insidious processes that are irreversible or poorly reversible at low doses or early stages of causation. |
|
• Molecular biological (stochastic process) effects—Effects occur as a result of one or a small number of irreversible changes in information coded in DNA: mutagenesis, most carcinogenesis, and some teratogenesis. • Chronic cumulative effects–Effects occur as a result of a chronic accumulation of many small-scale damage events: emphysema, noise-induced hearing loss, atherosclerosis, and probably hypertension; possibly depletion of mature oocytes. |
If the answer to the first question is yes, then it will generally be appropriate to treat the condition within the framework of traditional toxicology.3 Some examples of such reversible changes are the following:
-
Buildup of a contaminant in blood or other tissues. It is rare for there to be a zero rate of excretion of any material. Given time and no further exposure, toxicant buildup should be reversible, although it can be quite prolonged. [Current estimates are that only about 9% of more persistent polychlorinated biphenyl (PCB) isomers may be metabolized or excreted per year in humans (Yakushiji et al., 1984).]
-
Most enzyme inhibition (generally, even irreversible inactivation of enzyme molecules can be "reversed" through the synthesis of replacement molecules)
-
Induction of short-term biological responses that act to maintain homeostasis (e.g., sweating in response to heat, tearing in response to eye irritation)
If the answer to the above question is no and events are likely to be occurring at subclinical exposure levels or preclinical stages that are not ordinarily reversible, the modeling of biological risks will have to be based on concepts that are fundamentally different from the homeostatic system/threshold paradigm. Examples of such irreversible or poorly reversible events include
-
changes in genetic information or the heritable pattern of gene expression after these are effectively "fixed" into a cell's genome expression by replication;
-
death of nonreplicating types of cells (adult neurons);
-
destruction of nonregenerating structures (alveolar septa); and
-
generation and buildup of incompletely repaired lesions (atherosclerotic plaques).
Appropriate modeling for conditions that are the result of irreversible or poorly reversible processes must be based fundamentally on the likely dose-response characteristics of the events that cause the basic irreversible changes. Once the primacy of such changes is established for a particular event, the analyst should then ask whether clinical manifestations are likely to be the direct result of only a few, or very many, individual irreversible damage events. If only a few events are believed to contribute directly to a particular clinical manifestation (e.g., a small number of heritable changes within a singe cell line leading to cancer), the effect can be considered a "molecular biological" disease. The risk assessment models used must follow from an understanding of the stochastic nature of the basic process. On the other hand, if thousands, millions, or billions of individual irreversible events directly contribute to a particular condition (e.g., very large numbers of individual neurons must die to cause the clinical manifestations of Alzheimer's or Parkinson's disease), the biological harm should be dealt with under the novel category of chronic cumulative conditions (see below).
Traditional Acute Toxicity
Three kinds of insights for acute toxicity risk assessment follow naturally from the homeostatic system paradigm of physiology and traditional toxicology:
-
There will be a series of toxic effects as different compensatory processes are overwhelmed and as impairment broadens from more-to less-sensitive cells and functions.
-
For each effect in each individual who is not already beyond the limits of normal functioning in the absence of exposure, there will be some subthreshold level of exposure that will be insufficient to produce the effect.
-
Individuals will differ in their thresholds.
A caveat to the general expectation of individual thresholds is that some tasks may so tax the capabilities of a system (perhaps, during fetal life, the struggle to mobilize metabolic resources to grow and differentiate as fast as possible so as to cope with the external world at birth) that any impairment of a key limiting functional parameter required for the task could compromise function to some degree. (This would also apply to reaction time for a driving task, for example.) Of particular relevance to the committee's task in this regard is the suggestion of some studies that dietary PCB exposure may be associated with either changes in birth weight (Fein et al., 1984; Sunahara et al., 1987; Taylor et al., 1989) or indices of neurological function in infants (Jacobson et al., 1985; Rogan et al., 1986).
The first job in assessing acute toxic effects is to define the series of acute responses to the disturbing influence in question. Ideally, the analysis should then attempt to determine (to whatever degree of precision is possible) the nature and magnitude of the dosage and the disturbance of physiological parameter(s) that are necessary to cause each type of acute toxic response, along with the frequency of each response in a diverse human population.4
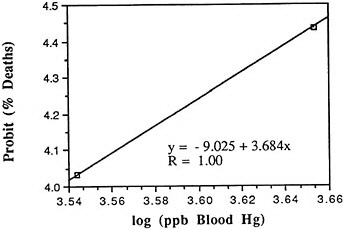
Such mechanism-based analysis is, however, not common in the field. Rather, the current state of the art in those rare cases where acute toxic effects are treated quantitatively is to use probit equations (Finney, 1971) of the general form
Prohibit of response = a + b ln (Cn T),
where a, b, and n are constants, C is external concentration, and T is exposure time; n represents the basic trade-off between intensity and duration of exposure, and b defines the breadth of an assumed lognormal distribution of threshold responses. Although some of the available animal data on irritant gases appear to be well summarized by equations of this form (Appelman et al., 1982), this is basically an empirical formula that does not incorporate quantitative representations of the various processes underlying toxicity. It is therefore difficult to decide what adaptations should be made in applying the empirical relationships to diverse subsets of humans.
Even more common, unfortunately, is the simple use of the no-effect level (NOEL)/"safety factor" analysis for arriving at acceptable daily intake (ADI) levels for chemical contaminants. Rather than estimate the numbers of people with specific degrees of particular effects, the general approach is to arrive at an ADI by a rule-of-thumb
procedure derived from observed NOELs [or, more recently, no-adverse effect levels (NOAEL), after often contentious discussion over what effects are considered "adverse"], or sometimes low effect levels (LOELs) in animal experiments or human studies. When projections are made from animal data, generally a 100-fold "uncertainty factor" is allowed between the NOEL and the ADI (Dourson and Starra, 1983). The 100-fold factor is often decomposed as 10-fold to account for possible differences in sensitivity between humans and the most sensitive species tested and 10-fold to account for possible interindividual differences in susceptibility among humans.
This approach has a few advantages:
-
It is "quick and dirty"—relatively straightforward to apply and does not require complicated model building or analysis.
-
Through thousands of applications in the past, it is not yet known to have led to catastrophic adverse effects in humans (using Ozonoff's working definition of a "catastrophe" as an effect so large that even an epidemiological study can detect it (D. Ozonoff, Boston University School of Public Health, personal communication, 1990).
On the other hand, for the long term, the simple uncertainty factor approach has a number of disadvantages:
-
No one knows how protective it really is, either in general or in specific cases. What fraction of the diverse human population can be expected to experience adverse effects when exposed at the level calculated to be "acceptable" under the formula? (In general, there may be some finite fraction of individuals who, because of disease or other reasons, are marginal for biological functions affected by the chemical and who may be pushed beyond a functional threshold for an adverse effect by a small finite dose of the chemical.)5
-
The procedure incorporates one specific social policy standard for setting "acceptable" levels without making clear where technical analysis leaves off and policy/value analysis begins.
-
Effects are generally scored as either present (operationally, statistically significant) or not present (not statistically significant) at a particular dose. There is usually no quantitative analysis of the effects of sample size or the dose-response relationship for the effect in question.
-
There is no defined or obvious way to incorporate newer types of relevant data on human interindividual differences in
-
rates of uptake/absorption for a constant environmental exposure ("exposure variability");
-
rates of activating or detoxifying metabolism and excretion, producing differences in the concentration x time of active metabolites per unit of absorbed dose at the site of toxic action ("pharmacokinetic variability"); and
-
differential risk of response ("response variability") for a given concentration x time of active metabolites at the site of toxic action.
In particular, the committee suspects that the inability of the uncertainty factor paradigm as usually formulated to incorporate newer types of relevant information into
a systematic procedure for updating assessments of health hazards has tended to discourage both the collection and the analysis of potentially important data. One example of this is information on human interindividual variability in parameters that could affect susceptibility.
Table 6-2 outlines some idealized components of a full quantitative analysis of a noncancer health effect that is mediated by what is called a "functional intermediate" parameter. Such a parameter is generally a continuous variable that has a strong causal influence on performance of an important biological function (although it will not generally be the sole determinant of performance). It should, in turn, be affected by the toxin/exposure under study, and it should be reasonably likely that effects on the final health condition of concern are primarily mediated through effects on this functional intermediate parameter. For example, a key functional intermediate for some reproductive effects of PCBs may well be changes in birth weights (Fein et al., 1984; Jacobson et al., 1985; Sunahara et al., 1987; Taylor et al., 1989). Similarly, blood or tissue concentrations of lead constitute a useful intermediate parameter for lead toxicity.
TABLE 6-2 Elements of a New Analysis for Noncancer Health Effects Mediated by a "Functional Intermediate" Parameter
|
1. Elucidate the quantitative relationships between internal dose/time of toxin exposure and changes in the functional intermediate parameter. |
|
2. Assess the preexisting "background" distribution of the functional intermediate parameter in the human population. |
|
3. Assess the relationship between the functional intermediate parameter and diminished physiological performance or adverse health effects. |
|
4. Assess the magnitude of parameter changes likely to result from specific exposures in humans (taking into account human interindividual variability in metabolism and other determinants of pharmacokinetics) and consequent changes in the incidence and severity of health effects. |
|
5. Do not attempt, from the biology alone, to determine "acceptable" levels of parameter change or exposure. (Let policymakers decide what changes in the incidence and severity of health effects are "acceptable" in the context of modes of exposure and in light of the feasibility of reducing or avoiding exposure.) |
The illustrative calculations in Table 6-3 (from Ballew and Hattis, 1989) show how modest changes in the population distribution of a key parameter such as birth weight can be reflected in serious changes in the outcome of infant mortality. It can be seen that birth weights are very strongly related to infant mortality and that the relationship is continuous. Although very low birth weight infants are at dramatically higher risk than infants in the normal weight range, even infants weighing about 3,000 grams (g) can be expected to have their risks increased somewhat by an agent that causes a marginal change in birth weight. As indicated in the table, because there are many more infants in the 2,500-3,500-g weight range, the expected population aggregate mortality increase is as large for these categories as the population aggregate mortality increase for infants in the very low birth weight range (500-1,500 g).
TABLE 6-3 Expected Infant Mortality Effects of a 1% (33.66-g) Reduction in Birth Weight
|
Weight Range (g) |
Fraction of Births |
Mortality Risk per 1,000 Births in Category |
Fraction of Births x Mortality Risks/1,000 |
|||
|
Original Population |
After 1% Weight Reduction |
Without Birth Weight Reduction |
After 1% Birth Weight Reduction |
Net Change |
||
|
White Infants |
||||||
|
Under 500 |
0.0006912 |
0.0007701 |
1,000 |
0.6912 |
0.7701 |
0.0789 |
|
500-999 |
0.002171 |
0.002335 |
673.31 |
1.4612 |
1.5720 |
0.1107 |
|
1,000-1,499 |
0.005249 |
0.005488 |
237.85 |
1.2485 |
1.3053 |
0.0568 |
|
1,500-1,999 |
0.009182 |
0.009575 |
76.86 |
0.7057 |
0.7360 |
0.0302 |
|
2,000-2,499 |
0.029192 |
0.032804 |
26.746 |
0.7808 |
0.8774 |
0.0966 |
|
2,500-2,999 |
0.15164 |
0.16568 |
8.3565 |
1.2672 |
1.3845 |
0.1174 |
|
3,000-3,499 |
0.36237 |
0.37081 |
4.2566 |
1.5424 |
1.5784 |
0.0359 |
|
3,500-3,999 |
0.31749 |
0.30337 |
3.0451 |
0.9668 |
0.9238 |
0.0430 |
|
4,000-4,499 |
0.10100 |
0.09027 |
3.0293 |
0.3060 |
0.2734 |
0.0325 |
|
4,500+ |
0.021021 |
0.01890 |
4.941 |
0.1039 |
0.0934 |
0.0105 |
|
Total |
1 |
1 |
|
9.0736 |
9.5142 |
0.4406 |
|
Black Infants |
||||||
|
Under 500 |
0.0026095 |
0.0028661 |
1,000 |
2.6095 |
2.8661 |
0.2566 |
|
500-999 |
0.006279 |
0.006666 |
645.90 |
4.0558 |
4.3058 |
0.2500 |
|
1,000-1,499 |
0.012709 |
0.013154 |
167.98 |
2.1348 |
2.2096 |
0.0748 |
|
1,500-1,999 |
0.020673 |
0.02165 |
57.72 |
1.1932 |
1.2495 |
0.0563 |
|
2,000-2,499 |
0.067052 |
0.074351 |
21.482 |
1.4404 |
1.5972 |
0.1568 |
|
2,500-2,999 |
0.24894 |
0.26444 |
9.832 |
2.4476 |
2.6000 |
0.1524 |
|
3,000-3,499 |
0.38248 |
0.37936 |
6.636 |
2.5381 |
2.5174 |
0.0207 |
|
3,500-3,999 |
0.20683 |
0.19100 |
5.581 |
1.1543 |
1.0660 |
0.0883 |
|
4,000-4,499 |
0.04418 |
0.04029 |
5.89 |
0.2602 |
0.2373 |
0.0229 |
|
4,500+ |
0.008253 |
0.006226 |
12.33 |
0.1018 |
0.0768 |
0.0250 |
|
Total |
1 |
1 |
|
17.9358 |
18.7257 |
0.7899 |
In principle, the use of such intermediate parameters can provide windows on the pathological processes that occur earlier in the development of toxicity, are more sensitive to the action of potential toxicants (compared with attempts to observe actual cases of illness), and are more accessible to direct comparative measurement in both animal models and humans. It is desirable, for these purposes, that the intermediate parameters chosen be as close as possible to the actual causal pathway leading to harm. However, even a parameter such as birth weight, which may not itself bear a direct causal relation to infant deaths, may be a close enough indicator of the actual causal processes to serve as a useful intermediate predictor.
Because there will generally be a series of steps in the sequence between toxin uptake and ultimate manifestation of adverse effects, the analyst may often have choices of which parameter(s) to use for assessing human risk. These choices will generally be based on the availability of measurement techniques and theory for observing or estimating the parameter(s) in question.
Traditional Chronic Toxicity
The basic principles that govern the analysis of acute toxic effects are by and large directly applicable to cases of chronic toxicity. Chronic toxic analyses tend to differ from analyses of acute toxic effects primarily in that considerable emphasis must be placed on the "slow step" of the process, which causes the effect to be chronic rather than acute. This slow step is generally either a long-term accumulation of a toxic agent that is poorly excreted under ordinary conditions (e.g., lead, mercury) or an accumulation of some slowly reversed residual effect (e.g., acrylamide).
With lead as an example, the following statements can be made:
-
Appreciable information is available on the pharmacokinetics of lead absorption, transport, storage, and excretion (Barry, 1975; Bernard, 1977; Campbell et al., 1984; Chamberlain, 1985; Marcus, 1985a-c; Rabinowitz et al., 1976); and much better information could be obtained with the aid of natural experiments such as strikes among lead-exposed workers, which can given information on the rate of decrease of blood lead levels after a reduction in exposure (Hattis, 1981).
-
Inhibition of heme synthesis enzymes at essentially all dose levels is well characterized (Haeger-Aronsen et al., 1974), and the inhibition of heme synthesis may be important in producing some of the neurotoxic effects of lead (Silbergeld et al., 1982), although the short- and long-term functional significance of different degrees of inhibition in different individuals is far from clear.
-
Effects on some measures of neurological function and kidney function are susceptible to study in reasonably straightforward ways. Effects on higher-order development of central nervous system functions are more difficult to determine because of an ignorance of basic mechanisms; however, some good studies have become available in recent years (Baker et al., 1983; Bellinger et al., 1987, 1990; HHS, 1988; Needleman et al., 1979, 1990; Waternaux et al., 1989). The impairment of very complex neurological functions by lead raises a significant issue in the application of the traditional toxicological paradigm to risk analysis. As indicated above, the usual assumption is that there is some functional reserve capacity in "normal" individuals that maintains "adequate" performance despite a "small" degree of perturbation of a biological parameter by a "low" dose of toxic material. However, if the function is already taxed to its limit in certain situations, even in the absence of exposure (perhaps for a first grader learning to read or for a developing fetus mobilizing all its available metabolic energy to grow and differentiate), and if the biological parameter being perturbed is limiting to the performance of that function, then any level of exposure may produce at least some reduction in performance.
Addressing the issues of the population distribution of different functional reserve capacities, and the relationship of functional reserve capacities to specific biochemical parameters, is essential to the future research needs of risk assessment for classical chronic toxic agents. Also in the area of neurotoxicology, Silbergeld (1982) has written of the potential of new radioimmunoassay and functional measurement techniques to help shift the focus of research away from traditional morphological criteria of neurological damage toward more sensitive and sophisticated measures of performance.
Molecular Biological (Stochastic Process) Diseases
In addition to most carcinogenesis, molecular biological diseases include mutagenesis and at least some teratogenesis. The subject of quantitative risk assessment for carcinogenic hazards has been discussed extensively elsewhere (American Industrial Health Council, 1987; Bishop, 1987; Crump et al., 1976, 1977; EPA, 1986a; Hattis, 1982; Hattis and Smith, 1986; Moolgavkar, 1986; Rai and Ryzin, 1981; Whittemore, 1980). However, it is worth briefly recapitulating some basic features of the carcinogenic process and their implications for cancer dose-response relationships.
Science is now much closer than it was a decade ago to understanding the fundamental mechanisms involved in carcinogenic transformation. For some time it has been clear that tumors arise as a result of a series of changes or rearrangements of information coded in DNA within single cells (Cleaver and Bootsma, 1975; Fialkow, 1977; Hattis, 1982; Knudson, 1973, 1977; McCann et al., 1975; Vogel and Motulsky, 1979). These changes are often induced by electrophilic metabolites of the parent compounds to which organisms are exposed (Miller and Miller, 1981). With the identification of "oncogenes," some detailed molecular characterization is being provided of the changes resulting in DNA (Fischinger and DeVita, 1984; Hoel, 1985; Modali and Yang, 1984; Yunis, 1983).
It has also been apparent for some time that further headway cannot be made in elucidating the shapes of carcinogenesis dose-response relationships at low dosages simply by increasing the numbers of animals studied in conventional bioassays. A variety of mathematical models with dramatically different consequences for low-dose risk can always be found that fit the observations about equally well (Maugh, 1978; Whittemore, 1983). Low-dose risk projections are, therefore, inevitably much more determined by the choice of model than by the available data (Guess et al., 1977; Whittemore, 1980), if what is meant by "data" is restricted to observations of the incidence of ultimate adverse effects in small groups of animals.
Because of sample size limitations, animal carcinogenesis bioassays must be done within a limited range of relatively high dose levels. Typically, the difference between the minimum detectable response and a response that effectively saturates the system or causes interference through overt toxicity is only one to two orders of magnitude (often even less). Over this high dose range near levels where the agent produces overt toxic effects, enzyme saturation and other forms of pharmacokinetic nonlinearities are most likely. If in dose-response modeling for risk assessment, the nonlinearities of pharmacokinetic origin are not separated from the nonlinearities that may arise from the multiple mutation mechanism that is central to carcinogenesis, our ordinary curve-fitting procedures will implicitly attribute the pharmacokinetic nonlinearities to the fundamental carcinogenic process (Hoel et al., 1983). The resulting errors are particularly serious if one wishes to produce the best point estimates of carcinogenic risk in addition to upper confidence limits.
Clearly, to make real progress in modeling carcinogenic risks, knowledge of the fundamental processes involved must be used to break open the black box between external exposure levels and ultimate production of tumors. The use of pharmacokinetic models and intermediate parameters ("markers") to characterize the dose-response characteristics of small segments of the causal pathway to carcinogenesis has considerable potential to improve dose-response modeling for the process as a
whole (Hattis, 1988). Such markers can include both those that may lie directly along the causal pathway, such as DNA adduct formation, and putative correlates, such as hemoglobin adduct formation, that can be good indicators of the concentration-time product of active intermediates in the systemic circulation.
One key fact must be recognized from the beginning about pharmacokinetic modeling, however. Whatever nonlinearities may be produced at high doses by the saturation of enzymes, the saturation of active transport processes, the depletion of cellular reactants for electrophilic agents, or changes in cell division rates to make up for cell killing due to overt toxicity, all of these nonlinearities must necessarily disappear as one approaches very low dose rates (Hattis, 1990a). The slope of the line relating ultimate DNA lesions in replicating cells to external dose may well be very different at low than at high doses, but it must be linear. The basic reason for this is that at low doses the rates of the transport and transformation processes that lead to DNA damage and repair depend directly on the number of collisions between molecules of an "input" chemical (or activated intermediate or DNA adduct) and a resident cellular reactant (or hole in a membrane or repair enzyme molecule). At low doses the number of resident cellular reactant molecules does not change appreciably as a function of the concentration of the input. Therefore, the number of relevant collisions and the rates of reactions and side reactions in the causal sequence at low dosage must be direct linear functions of the amounts of input chemical and its activated derivatives. Some finite fraction of the ultimate DNA lesions must escape repair before the next cell replication as long as the cells affected have a nonzero turnover rate, there are a finite number of repair enzyme molecules, and the repair molecules operate at a finite rate.
All carcinogens – in particular the PCBs and dioxins – are not thought to act primarily by causing DNA mutations (Safe, 1989). Table 6-4 lists a variety of other types of mechanisms whereby chemicals can affect carcinogenesis. There has been a tendency in some quarters to assume that if a chemical does not act via a primary genetic mechanism, one should revert to the traditional toxicological paradigm for analysis, including all of the old presumptions about thresholds and safety factors (Weisburger and Williams, 1983). As Rodericks (1989) has noted,
There is disagreement about how to estimate risks from carcinogens. In the United States, regulatory agencies generally estimate risks in the same way for both genotoxic and non-genotoxic carcinogens. Regulatory agencies adopt this position because they believe that full knowledge of the mechanism of action of non-genotoxic agents is needed before they can be assumed to exhibit thresholds. In several foreign countries, non-genotoxic carcinogens are generally assumed to have thresholds below which there is expected to be no risk. The disagreement is not confined to official government positions; some scientists prefer to treat non-genotoxic carcinogens as having thresholds, some do not.
In the view of the committee, the quantitative implications of the many and diverse mechanisms listed in Table 6-4 must be worked out on a case by case basis. Even for specific types of mechanisms for which some data are available, as in the receptor binding studies that provide a framework for understanding the multiple effects of PCBs and dioxins, the implications for the shape of the dose-response relationship at
TABLE 6-4 "Indirect" Mechanisms of Carcinogenesis
|
Indirect processes that enhance the rate of "initiation" (the initial change or rearrangement of information in DNA that places a cell on a pathway to cancer): |
|
1. Changes in basic transport processes (e.g., low-fiber diets may prolong the residence time of feces in the gut, leading to greater exposure of the intestinal epithelium to reactive agents). |
|
2. Changes in metabolic processing (e.g., induction of enzymes such as some mixed function oxidases that convert chemicals to forms that can directly react with DNA). |
|
3. Changes in the effective amount of target tissue available for carcinogenesis (e.g., by simple hyperplasia). |
|
4. Changes in the efficiency of DNA repair [e.g., inhibition of DNA repair by some metals (Zakour et al., 1981) or enhancement of cell replication (leaving less time for repair before a DNA lesion can be fixed into the genome as a permanent mutation)]. |
|
Indirect processes that alter the frequency with which "initiated" cells progress through subsequent stages in the carcinogenic process: |
|
1. Induction of subsequent genetic changes: Many promoters appear to be capable of inducing the expression of Epstein-Barr virus antigens (Takada and Zur Hausen, 1984). Additionally, some "promoters" reportedly lead to the generation of active oxygen species that may damage DNA and lead to subsequent somatic mutations along the pathway to carcinogenesis without themselves being converted to compounds that react directly with DNA (Kinsella and Radman, 1978). |
|
2. Changes in the frequency with which initiated cells are effectively removed by terminal differentiation [e.g., effects of early and multiple pregnancies in reducing later breast cancer risk (Kampert et al., 1988; Layde et al., 1989; Moolgavkar et al., 1980)]. |
|
3. Release of initiated cells from growth control by neighboring cells |
|
• Mimicry of the action of a growth regulator or hormone by an introduced substance. [Phorbol esters alter the binding and phosphorylation of epidermal growth factor receptors (Friedman et al., 1984; McCaffrey et al., 1984). The very high-affinity binding of 2,3,7,8-tetrachlorodibenzodioxin (TCDD) to the AH receptor induces an increase in transcription of the genes that code for certain P-450 isozymes, among other effects (Whitlock, 1989). Genetic studies suggest that the AH receptor is necessary but not sufficient for the activity of halogenated aryl hydrocarbons as skin tumor promoters in hairless mice (Safe, 1989).] • Inhibition of the action of normal growth suppressing substances. [Phorbol esters inhibit the binding of somatostatin (Zeggari et al., 1985).] • Inhibition of the passage of growth inhibitors among cells by many chlorinated aromatic compounds (Tsushimoto et al., 1983). • Killing of neighboring cells responsible for repression of initiated cells. • Induction of cell replication among initiated cells, interfering with the ability of repressors to pass tight junctions or isolating some daughter cells from tight junctions. |
|
4. Changes in the rates of proliferation or survival (without terminal differentiation) of initiated cells relative to the proliferation and survival of normal cells (theoretical mechanism suggested by Moolgavkar and Knudson, 1981). |
|
Indirect processes that might alter the survival, growth and spread of tumors, or the progression of tumors to increased malignancy: |
|
1. Changes in hormonally mediated processes that might speed up the growth of specific cell types (e.g., estrogens and some breast cancers). |
|
2. Changes in the efficiency of immune surveillance in destroying incipient tumors at early stages. Some observations suggest that tumor promoters may alter the functioning of "natural killer" cells (Kabelitz, 1985). Immunosuppressive effects have also been observed for some promoters in vivo (Pasquinelli et al., 1985). |
|
3. Changes in local tissue conditions that favor colonization of new tissues by metastases (e.g., the establishment of tumor blood supplies). |
|
SOURCE: Adapted from Hattis and Strauss (1986). |
low doses will depend on some key facts that are not yet known. For receptor-binding mechanisms, the number of receptors that must be bound per relevant cell to cause or contribute to the carcinogenic transition must be known. If that number is large, and if only a few of these receptors are usually occupied by their normal substrate, then the dose-response relationship for PCB and dioxin effects might well be threshold-like, or at least highly nonlinear. On the other hand, if the occupation of only a single receptor site can lead to a relevant cellular transition, then as for a mutagenic mechanism, a linear dose-response relationship would be expected at low doses.
Generally, U.S. regulatory agencies adopt linear, no-threshold models and do not depart from them unless there is overwhelming scientific evidence to show that they are incorrect in specific cases. This is a prudent posture to take, until evidence of the details of specific nongenetic mechanisms is detailed enough for different low-dose assumptions to be indicated very clearly.
Chronic Cumulative Conditions
Chronic cumulative conditions include neurological conditions caused by the cumulative loss of neurons (e.g., Parkinsonism, Alzheimer's disease), emphysema and other chronic lung diseases, atherosclerosis, and hypertension. This new category is required because the underlying mechanism of these conditions – slow accumulation of many irreversible or poorly reversible damage steps – departs significantly from paradigmatic mechanisms that provide the basis for the three other, more traditional categories. These conditions are likely to be increasingly important as the population shifts to progressively older ages, and as continued progress is made in preventing and treating diseases that afflict younger people. For example, from 1969 to 1985, the percentage of nursing home residents with a primary diagnosis of a mental disorder rose from 11 to 22% (Hing, 1989, p. 9).
Theoretically, the approach that should be taken in developing risk assessment models for individual conditions within this new category is to
-
describe the fundamental mechanism(s) that causes individual damage events to accumulate (especially the quantitative significance of various contributory factors);
-
elucidate quantitatively the ways in which specific environmental agents enhance the production or prevent the repair of individual damage events; and
-
describe the relationships between the numbers, types, and physical distribution of individual damage events and the loss of biological function or clinical illness.
Unfortunately, no examples are known in which there has been successful quantitative modeling of any of these three types for any chronic cumulative condition. Often, the qualitative nature of individual damage events is not difficult to discern. For example, atherosclerotic lesions are thought to be produced by a sequence of events described by Ross and Glomset (1976). However, no one has yet successfully developed a predictive model that relates the frequency or severity of these events to the various internal and external causal factors that must be involved. This is clearly an area that requires basic biomedical research, as well as creative interaction between
mathematical modelers and experimentalists.
A couple of consequences of importance here, however, follow from the basic nature of the cumulative irreversible processes that define this category. First, it is important to recognize that the damage processes must be proceeding continually under quite usual everyday situations. This requires viewing with some suspicion the usual presumption of the traditional toxicological paradigm that the common adaptive responses to everyday insults are harmless. For diseases such as atherosclerosis, which proceed silently and chronically in ordinary individuals throughout their lives, homeostatic protective mechanisms must be failing in subtle ways rather frequently. It is likely that there are thresholds that give rise to the small-scale damage events of chronic cardiovascular disease processes [for example, perhaps the lining of the arterial wall in a particular region only suffers appreciable damage when systolic blood pressure temporarily goes above 180 millimeters (mm) of mercury (Hg)]. However, whatever thresholds exist must be low enough to produce a sufficient accumulation of net damage to account for the observation that atherosclerosis and long-term blood pressure increases with age occur in very large numbers of "normal" people.
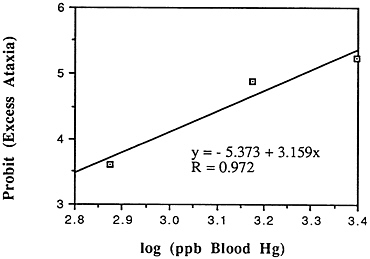
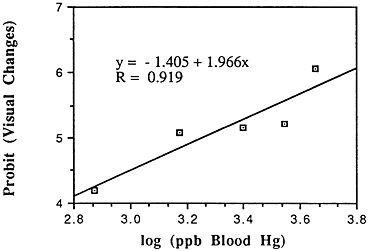
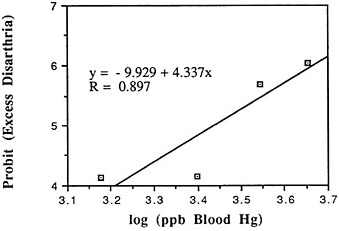
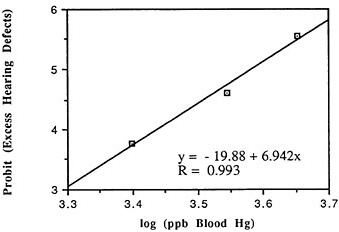
TABLE 6-5 Dose-Response Relationship of Parkinson's Disease Risk and Blood or Hair Mercury (Hg) Levels by Approximate Subject Tertiles
|
Hg Levela |
No. of Cases |
No. of Controls |
Adjusted Odds Ratiob |
95% Confidence Interval |
|
Blood (ng/mL) |
|
|
|
|
|
0.0-5.8 |
6 |
46 |
1.0 |
|
|
5.9-14.2 |
20 |
28 |
8.5 |
2.2-33.2 |
|
≥ 14.3 |
28 |
21 |
9.4 |
2.5-35.9 |
|
Hair (ppm) |
|
|
|
|
|
0.0-4.0 |
10 |
42 |
1.0 |
|
|
4.1-6.9 |
15 |
33 |
1.8 |
0.6-5.2 |
|
≥ 7.0 |
29 |
20 |
4.1 |
1.6-10.5 |
|
a ng = nanograms; mL = milliliters; ppm = parts per million. b Odds ratio after adjustment by conditional logistic regression for cigarette smoking, alcohol consumption, seafood intake, and ethnic medication. SOURCE: Ngim and Devathasan (1989). |
||||
Second, the basic concept of these diseases as accumulations of many small damage events implies that the number of damage events accumulated by different individuals will show a broad continuous distribution. Because of this, any agent that accelerates the production or prevents the repair of such damage events will shift all, or a very large part, of the exposed population in the direction of worse function. For example, noise exposure will cause people who would otherwise have excellent hearing to have less excellent hearing; people who might have fair hearing without noise can expect to be shifted toward poor hearing, and so forth.
Many years ago, Weiss and Spyker (1974) suggested that methylmercury might accelerate the loss of neurons in adult life and contribute to a "chronic cumulative" process that would fall within this category. Recently, an apparently very sound case control epidemiological study among people in Singapore found a strong association
between blood levels of mercury and the risk of Parkinson's disease (Ngim and Devathasan, 1989) (Table 6-5). Parkinson's disease occurs when a large portion of the neurons responsible for making dopamine in the substantia nigra area of the brain is lost. Thus, if the association with mercury exposure holds and proves causal in later studies, it would be a very important basis on which to quantitatively reevaluate the long-term risks of seafood mercury consumption.
CRITIQUE OF RISK ASSESSMENTS USED IN FORMULATING CONTAMINANT GUIDELINES/TOLERANCES FOR SPECIFIC CHEMICALS AND SUGGESTIONS FOR IMPROVEMENT
One of the charges to the committee was to review and critique previous agency efforts to assess the likely human risks of chemical contaminants of aquatic organisms. The committee was asked to suggest directions for the improvement of risk assessment approaches to better serve the needs of decision making on chemical risks, but not to attempt to develop its own set of final risk numbers. The committee has chosen to focus on PCBs and methylmercury as two paradigmatic cases, representing one carcinogen and one noncarcinogen, for which there is a relatively sufficient public record of FDA efforts to assess the risks and benefits of alternative control options.
The PCBs are of special interest in light of the results presented in this chapter (Table 6-30). By using conventional carcinogenic risk assessment techniques (although these have considerable difficulties in general and especially as applied to PCBs) and available data on likely average daily intakes in the United States, PCBs are shown to pose by far the largest potential carcinogenic risk of any environmental contaminants for which measurements exist. According to EPA's cancer potency factor, the aggregate U.S. lifetime risk could be as high as 6 × 10-5, corresponding to approximately 120 cancers per year. With FDA's lower estimate of the upper confidence limit (UCL) of the PCB cancer potency, the aggregate risk for the U.S. population would be considerably less, but still not insignificant, 2.7 × 10-6, implying a maximum toll from commercial seafood of about 5 cancers per year. These risks would be much greater for the subpopulation consuming relatively large amounts of sport or subsistence caught fish near the FDA tolerance level of 2 parts per million (ppm). For someone who consumes 20 kilograms (kg) per year of 20-ppm fish [which is probably not far from the actual consumption of some individuals in some areas of Lake Michigan (Humphrey, 1983a,b)], the upper-confidence-limit lifetime risk that would be expected by using the FDA cancer potency estimates is about 5 × 10-4; for the EPA cancer potency estimate, the lifetime upper-confidence-limit risk would be somewhat more than 1%. Perhaps in part because of the difference in cancer potency factors, there is also a great difference in the practical policies of the two agencies in regulating PCBs, with FDA generally taking a far less protective posture toward PCB exposure than EPA.6
Methylmercury is also of special interest because it shows the operation of the old no-effect level/safety factor paradigm in a practical case where, as it happens, an alternative type of quantitative assessment is also possible based on existing data. In this case, therefore, one can examine what levels of risk might implicitly be accepted by using FDA's rule-of-thumb procedure for dealing with noncancer effects.
In the following material, the committee will assess for both cases
-
the reasonableness of the calculations that went into FDA's decision making in terms of the information available at the time; and
-
the improvements that could and should be made by applying more modern analytical techniques to recently available or feasibly obtainable data on population exposures, the nature and mechanisms of potential adverse effects, and likely population dose-response relationships.
To briefly foreshadow the committee's conclusions, three statements can be made:
-
Both assessments (Cordle, 1983; Cordle et al., 1982; FDA, 1979; Tollefson and Cordle, 1986), even at the times they were done, suffered from ad hoc unsystematic approaches to the treatment of population heterogeneity in dosage and (for methylmercury) susceptibility to adverse effects. For mercury, the likely special susceptibility of developing fetuses was mentioned in discussion. However, a tenfold "safety factor" was applied to the lowest blood level reported to produce effects for adults (rather than to a no-effect level of intake, which would have been more consistent with established procedures) in the cited epidemiological studies, without numerical allowance for extra sensitivity of fetuses, without a quantitative dose-response treatment of the data then available for adults to gauge the potential adult risk at the blood level selected as the highest permissible for U.S. consumers, and without a quantitative treatment of the effects of pharmacokinetic differences among people that would tend to make blood levels and risks as a function of dietary intake more variable than blood levels themselves.
-
Procedures for the systematic quantitative analysis and communication of uncertainties are absent. Both analyses use the "method" of compounding a series of ad hoc allegedly "conservative" assumptions (such as the use of the consumption level of the 90th percentile consumer, the upper 99% confidence limit for the PCB animal potency calculation, but a body weight rather than a surface area scaling rule to translate animal to human dose). The difficulty in picking a single series of point estimates of uncertain parameter values is that after the first few such assumptions have been entered into an analysis, no person on earth can determine where one actually is on an overall distribution of the likelihoods of different outcomes. Monte Carlo simulation procedures are now readily available via personal computer-based software for the calculation of probability density functions of different outcomes, given uncertainties in multiple parameters affecting population risk. Such procedures have been effectively advanced in the context of risk assessment in a recent report by Finkel (1990).
-
Both assessments have been rendered substantially obsolete by the development of recent information related to PCB and to methylmercury risks, changes in the economic conditions assumed in the cost analyses, and possibly changes in residue levels and available options for population exposure reduction.
-
In the case of PCBs, understanding of mechanisms has advanced to the point where there are now serious proposals for a congener-specific assessment of relative activity (Clement Associates, 1989; Jones, 1988). In the view of the
-
-
committee, this congener-specific activity analysis must be coupled with a congener-specific treatment of pharmacokinetics, which now seems possible based in part on the human dietary exposure and serum concentration data being collected by Humphrey and coworkers (H. Humphrey, Michigan Department of Public Health, Lansing, personal communication, 1989). Both congener-specific activity estimates and pharmacokinetics should be used to evaluate not only existing animal carcinogenesis data, but also recently emerging epidemiological information from studies in workers.
-
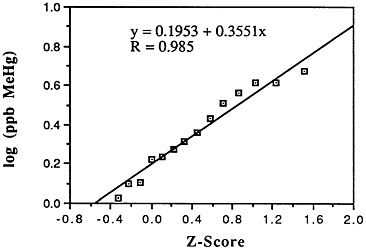
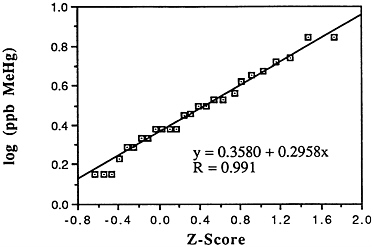
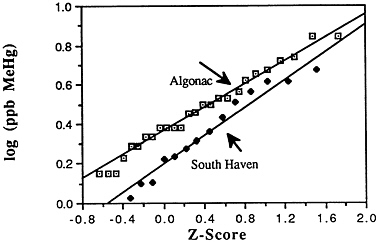
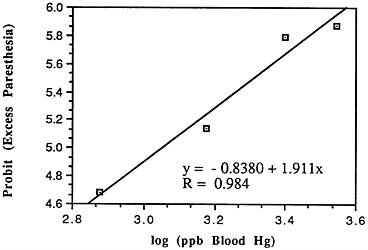
In the case of methylmercury, recent data allow preliminary evaluation of the population dose-response relationship for a number of long-term neurological effects from exposure during fetal development (Marsh et al., 1987). The analysis in the following section indicates that the apparent interindividual variability in susceptibility for fetal effects is much broader than that for adult effects. Consequently, although the tenfold safety factor, as applied, appears to offer a reasonable degree of protection for adult effects, projections based on an additional toxicological assumption of lognormal distribution of threshold for effects (logprobit projections) of the fetal dose-response data suggest the possibility of appreciable risk from methylmercury exposure, even at levels to which many people are exposed via the diet. Published projections applying a logistic model to the same data come to similar conclusions (Cox et al., 1989).
Polychlorinated Biphenyls (PCBs)
Background
The PCBs originally came to the attention of FDA through a series of contamination incidents in which PCB-containing oils leaked from electrical equipment directly into food substances, such as rice oil in Japan and Taiwan (the "Yusho" incidents), and by various routes found their way into U.S. livestock.7 The initial concerns were direct overt toxicity and reproductive effects, and acceptable daily intakes were defined as 100-fold and 10-fold below the no-effect levels in animals and humans, respectively. Table 6-6 is from Scheuplein (1988).
To this day, FDA analyses continue to reflect and even emphasize calculations within the NOEL/safety factor paradigm that was initially used to interpret animal and human data on the risk of overt chronic toxicity. Thus, Scheuplein (1988) compares FDA's 1 microgram (µg)/kg/day ADI to the observations of Humphrey (1983a) that average PCB intakes in a group of heavy eaters of fish8 from Lake Michigan were 1.75 µg/kg/day:
No symptoms or adverse health effects clearly related to PCB ingestion could be identified in the exposed group. This suggests that FDA's acceptable intake level of 1.0 µg/kg-day provides reasonable safety, although it is possible that effects too subtle for detection are occurring or that the latency periods for cancer are very long.
From work reported to EPA (Humphrey, 1983b) and other data reported later by Humphrey 91988), it appears that the cited work had only a very small health component (major emphasis was on defining exposures). The health component seems
to have been a simple medical history questionnaire administered to the participants, asking them about 18 specific conditions. Table 6-7 shows the percentage of fish eaters and the comparison group that reported various past medical complaints. This limited presentation of the data, without confidence limits or sample sizes, may not do justice to the underlying work. Clearly, however, this kind of study was not designed to be a very sensitive detector of carcinogenic risk or other types of effects (e.g., on fetal development) that are the concerns at low doses. Carcinogenic risks are traditionally studied in human populations by case control studies and detailed comparisons of the frequency of specific cancers or cancer deaths in defined cohorts, adjusted for age. Reproductive effects must clearly involve careful follow-up of offspring in groups where – through either dietary analysis or serum studies – it is possible to define prenatal exposures. The fact that the Humphrey (1983a) work is seriously cited as evidence of no effect – and that other work (e.g., Fein et al., 1984; Jacobson et al., 1985) that does suggest fetal effects from the same general Michigan fish eater population is not cited – is perhaps testimony to FDA's general lack of enthusiasm at the prospect of departing from the NOEL/safety factor paradigm.
TABLE 6-6 FDA Projection of Human Acceptable Levels for PCBs
|
Study |
No-Effect Level (µg/kg body weight/day)a |
Acceptable Daily Intake (µg/kg body weight/day)a |
Reference |
|
Subchronic (rats and dogs) |
250-300 |
2.5-3.0 |
FDA file data |
|
Yusho data (actual 50-day exposure) |
200 |
20 |
Karatsune and Fukuoka, Acta Med. Med. 23(117), 1971b |
|
Yusho data (assuming 1,000-day exposure) |
10 |
1 |
Fed. Reg. 38(129), July 6, 1973 |
|
a µg = micrograms. b This is the reference given by Scheuplein (1988). The Toxline data base lists a paper that seems to roughly correspond to Yamaguchi, A., T. Yoshimura, and M. Kuratsune. 1971. A survey on pregnant women having consumed rice oil contaminated with chlorobiphenyls and their babies. Fukuoka, Acta Med. 62(1):117-122 (1971). SOURCE: Scheuplein (1988). |
|||
TABLE 6-7 Percentage of Humphrey (1983b) Study Participants Reporting Histories of Various Medical Conditions
|
Condition |
Fish Eaters |
Comparison Group |
|
Diabetes |
4.6 |
5.9 |
|
Heart attack |
6.8 |
3.9 |
|
Hypertension |
21.1 |
18.4 |
|
Kidney problems |
2.2 |
1.2 |
|
Cancer |
5.5 |
4.7 |
|
Liver problems |
2.4 |
2.0 |
|
Other conditions |
11.5 |
14.3 |
|
SOURCE: Humphrey (1988). |
||
FDA Assessment of Costs and Risks for PCBs in Fish
The most recent detailed statements concerning FDA assessment of the carcinogenic risks of PCBs appear to be in two papers by Cordle (1983) and Cordle et al. (1982). These recapitulate and give more details of the analysis underlying the official risk assessment that appeared in the Federal Register (FDA, 1979) promulgating the 2-ppm tolerance (lower than the former 5-ppm guideline).
When the committee reviewed FDA's official analysis (FDA, 1979), it was surprised to find that FDA had actually calculated a series of aggregate estimates of economic costs incurred and cancer cases prevented by going to different standard levels (Table 6-8). This analysis appears to have been done in part to satisfy requirements for a balancing analysis of benefits and costs that became a government-wide requirement for regulatory action in the mid-1970s. For this purpose, however, the calculation of societal aggregate cancer risks from 90th percentile values of consumption for fish consumers is problematic because, of course, the 90th percentile overstates the average. Therefore, the committee has recalculated the balance struck by FDA in terms of the 50th percentile of fish eaters (Table 6-9). This probably results in a small error in the opposite direction, because of the likely skewed distribution of PCB consumption among fish consumers.9 The committee's aggregate national average estimate of current PCB exposure from fish (see Table 6-31) is slightly less than 0.01 µg/kg/day. This compares quite well with the national aggregate estimate implied by Table 6-9 for the situation after implementation of the 2-ppm tolerance (0.012 µg/kg/day), if there is essentially no exposure of any but the 15% of the population covered by the FDA fish eater analysis.
It should be stressed that FDA (1979) did not juxtapose the incremental costs and benefits of different possible tolerance reductions, as has been done in Tables 6-8 and 6-9. Had it done so, FDA might at least have been led to some explicit comment on the closeness (within twofold) of the incremental cost/benefit ratio of the regulatory step it declined to take (from 2 to 1 ppm) to the incremental cost/benefit ratio of the regulatory step it did take (from 5 to 2 ppm). If FDA really intended this result, then an unusually specific insight is afforded into the value it attached implicitly to the prevention of a unit of upper-confidence-limit cancer risk.
Instead, it appears that the FDA decision was most influenced by the concentration of the regulatory impact expected for commercial harvesting of freshwater fish. Whereas the 2-ppm tolerance was expected to cause the loss of a negligible portion of the commercial marine catch (less than 0.2%), the same regulation was expected to cause the loss of 14% of the freshwater catch. For the 1-ppm standard, the loss of freshwater fish was expected to increase to 35%. In its conclusion commenting on the choice of the 2-ppm level, FDA focuses on the absolute magnitude of the losses expected from the 2-ppm and 1-ppm alternatives:
… the agency estimates that under a tolerance of 1 ppm, approximately $16 million worth of the commercial fish catch would be violative and thus, presumably, removed from commerce. This is nearly triple the $5.7 million worth estimated to be violative under a 2 ppm tolerance. It is far more likely under a 1 ppm tolerance than under a 2 ppm tolerance that the more heavily contaminated species of freshwater fish would be violative in percentages high enough to put an end to their commercial exploitation and, possibly, force some segments of the freshwater fishing industry to cease operations completely. Thus, the actual loss of food resulting from the 1 ppm tolerance could
greatly exceed even the $16 million landed value (1974 dollars) estimated above. Second, for the average consumer, current exposure to PCBs in fish is at a tolerably low level, when considered in the light of the criteria of section 406 of the act [Food, Drug, and Cosmetic Act], without a 1 ppm tolerance. The average consumer eats a modest amount of fish from a variety of sources, both freshwater and marine, most of which yield fish with PCB levels below 1 ppm. Because their exposure is thus low to begin with, they are adequately protected by a 2 ppm tolerance, which ensures that they will not be exposed to the unusually high levels of PCBs found in some species of fish. The slight additional protection these average consumers might gain from a 1 ppm tolerance does not justify the significantly greater impact such a tolerance would have on the availability of food. On the other hand, atypical heavy consumers (e.g., the Great Lakes sport fisher who catches and consumes large quantities of the contaminated species) would likely not be adequately protected by even a 1 ppm tolerance because of the amount of fish they eat and because those fish are seldom affected by FDA tolerances (either because they are sport fish or are from intrastate commercial channels and, in either case, are outside FDA's jurisdiction).
TABLE 6-8 FDA Risk and Cost Calculations for PCBs
|
A. Incremental Health Benefits |
||||
|
PCB Fish Tolerance Level (ppm) |
Heavy Consumers' Projected Dose (µg/day) |
UCLa Lifetime Individual Risk for Heavy Consumers |
Aggregate UCL No. of New Cancers per Year for Heavy Consumers |
Incrementalb Savings of UCL Cancers per Year |
|
5 |
20.1 |
9.8 × 10-5 |
46.8 |
|
|
2 |
14.9 |
7.2 × 10-5 |
34.3 |
12.5 |
|
1 |
9.1 |
4.4 × 10-5 |
21 |
13.3 |
|
B. Incremental Costs and Cost/Benefit Ratios |
||||
|
PCB Fish Tolerance Level (ppm) |
Projected Cost in Lost Landings (1974 $/yr) |
Incrementalc Cost (1974 $/yr) |
Implied Incremental Cost/Benefit Ratio (1974 $/UCL cancer) |
|
|
5 |
600,000 |
|
|
|
|
2 |
5,700,000 |
5,100,000 |
410,000 |
|
|
1 |
16,700,000 |
10,300,000 |
770,000 |
|
|
a Upper confidence limit. The numbers in this column imply a low-dose cancer potency of about 0.34 cancer case per lifetime average mg/kg/day exposure. b Numbers in this column reflect the reduction in annual UCL-estimated cancers attributable to lowering the standard from 5 to 2 ppm, and from 2 to 1 ppm. c Numbers in this column reflect the increase in annual lost landings cost attributable to lowering the standard from 5 to 2 ppm, and from 2 to 1 ppm. NOTE: Aggregate projections were apparently made by assuming that all 33 million consumers of the species of interest had intakes at the 90th percentile. SOURCE: FDA (1979). |
||||
Table 6-8 also derives the FDA implied upper-confidence-limit cancer potency factor for PCBs of about 0.34 case/(mg/kg/day), compared to the current EPA estimate of 7.7 cases (EPA, 1989). The bulk of this difference stems from a traditional disagreement between EPA and FDA on how to perform interspecies extrapolation, with FDA favoring the translation of effective doses in mg/kg of body weight and EPA favoring the "surface area" extrapolation rule – mg/(kg body weight)2/3. Some additional portion of the difference derives from the fact that EPA has used more recent experiments with Aroclor 1260 for its risk calculations (Norback and Weltman, 1985), whereas FDA used data on the overall tumor risk from an experiment with Aroclor 1254, which produced the highest estimate of risk from studies then available (Cordle et al., 1982).
In the context of the carcinogenic risk assessment practices of its time, the FDA quantitative analysis does not greatly depart from established principles. On the theory (discussed above) that cancer is the result of a multiple series of somatic mutations, a hypothesis of low-dose linearity in carcinogenic response is not at all unreasonable, particularly if carcinogenic transitions caused by one agent can interact with some of the processes that cause the vast number of "background" cancers in the human population. An upper-confidence-limit risk derived from the multistage theory guarantees low-dose linearity in risk estimates and thus provides a convenient benchmark for risk even though a "mean-estimate" (which would be more helpful for cost/benefit comparisons of the type shown in Table 6-9) risk is not easily produced.
TABLE 6-9 Results of Revising the Aggregate Risk Calculations to Reflect and Assumption that Consumers Had Intakes at the 50th Percentilea
|
A. Incremental Health Benefits |
||||
|
PCB Fish Tolerance Level (ppm) |
Median Consumers' Projected Dose (µg/day) |
UCLb Lifetime Individual Risk for Heavy Consumers |
Aggregate UCL No. of New Cancers per Year for Heavy Consumers |
Incremental Savings of UCL Cancers per Year |
|
5 |
7.57 |
3.7 × 10-5 |
17.6 |
|
|
2 |
5.59 |
2.7 × 10-5 |
12.9 |
4.7 |
|
1 |
3.3 |
1.6 × 10-5 |
7.7 |
5.2 |
One can question the practice of summing up tumors from all sites in terms of the multistage theory, but this is not unusual in the context of risk assessment practices in the late 1970s and early 1980s. The choice of animal to human extrapolation formulas is open to considerable discussion in the field to this day, although in the specific case of PCBs, from some limited positive epidemiological studies in PCB-exposed workers,10 Allen et al. (1987) have estimated a TD25 (the lifetime dose estimated to produce cancers in 25% of exposed people) of 0.15 mg/kg/day (for 45-year, 240-day/year occupational exposures).11 This translates into the equivalent of a best-estimate cancer potency factor of 3.9 cases per lifetime mg/kg/day dosage–rather closer to the EPA upper confidence limit estimate of 7.7 than to the FDA estimate of 0.34 case per lifetime mg/kg/day.
Overall, FDA (1979) has been quite cautious in presenting the difficulties and uncertainties of carcinogenic risk analysis for PCBs from the data it had available:
… the utility of this risk assessment for evaluating actual risk to humans from exposure to PCBs is extremely limited. This is due both to difficulties inherent in making such extrapolations from animals to humans and, perhaps more importantly in this instance, to gaps and uncertainties in the data available for this particular risk assessment. For example, the toxicity studies on which the risk assessment is based used commercial preparations of PCBs, which are chemically different from the PCB residues found in fish and which contain small amounts of highly toxic impurities (e.g., dibenzofurans) not known to be present in fish residues. [It should be noted, however, that these contaminants are also not known not to be present in fish.] Also, in making the exposure estimates required for the risk assessment, it was necessary to use existing data on the numerical distribution of PCB levels in fish and rely on the assumption that the effect of a given tolerance level is to remove from commerce all fish containing PCBs exceeding the tolerances. It is possible that neither the assumption nor the data precisely reflect what actually occurs.
For these reasons and others … the risk assessment does not provide a basis for precise quantification of the amount of risk reduction accomplished by reducing the fish tolerance. Despite the limitations inherent in the risk assessment, however, the agency regards it as illustrative of the basic validity of the toxicological rationale for reducing the tolerance for PCBs in fish: Reduction of the tolerance will result in a significant reduction in risk among those who consume PCB-contaminated fish. FDA considers this risk reduction to be of significant public health value, even though it cannot be precisely quantified.
For this limited purpose, the analysis as presented may well have served an appropriate and useful function. However, there are now both an opportunity and a need to gather and analyze newer information, so that by the early to mid-1990s it will be possible to significantly improve on this 1979 effort.
Suggestions for Improved Analysis
First, a better regulatory impact analysis might evaluate a wider range of choices of regulatory alternatives–including not just different tolerance levels, but possible rules restricting the location of harvest, species, and size. Choices in these areas are likely to allow a more narrowly targeted regulatory action that will reduce human exposure to PCBs at less overall cost of forgone food resources. By being keyed to characteristics of fish that do not require expensive and time-consuming chemical analyses, such regulations might be much more efficiently implementable than tolerances based simply on chemical content.
Second, an improved analysis of carcinogenic risks should be based on a congener-specific assessment of (1) pharmacokinetics and (2) relative potency at the site(s) of action. The FDA analysis mentions the fact that the distribution of PCB congeners to which consumers are exposed is not the same as found in the commercial PCB preparations (e.g., Aroclor 1254) that were the subjects of carcinogenicity testing. The FDA specifically mentions the possibility of dibenzofuran contamination in the commercial mixtures used for carcinogenicity testing. Not mentioned, however, is the likely possibility that fish PCB residues may be relatively enriched in just those congeners that are more persistent in biological systems. More degradable congeners are almost by definition likely to be preferentially destroyed either in the fish themselves or in organisms that are lower on the food chain, whereas the original commercial mixtures would contain the full mix of more- and less-degradable congeners. Humphrey (1983b) noted a change over time in the distribution of PCB congeners found in human serum toward more highly chlorinated congeners, which tend to be more persistent in biological systems. Data now exist that would allow a detailed comparison of congener distributions in fish and humans with those in commercial mixtures (McFarland and Clarke, 1989).
At the same time, a long series of comparative studies by Safe (1989) and others has provided basic insights into the mechanisms of action of PCBs, dibenzofurans, and dioxins. It now appears likely that PCBs enhance carcinogenesis not by direct interaction with DNA, but through a receptor-binding mechanism (similar to dioxins) whose precise dose-response implications have not been elucidated. Receptor binding and related activities vary enormously among different congeners, with congeners that are planar in shape (and relatively highly chlorinated) appearing to have the greatest potency. Careful experiments relating tumor enhancement to the number, type, and persistence of specific PCB-receptor interactions in animal systems could yield important insights into the likely form of the dose-response relationships that should be applied to human risk assessment. There are now serious proposals for a congener-specific assessment of relative activity by using the existing data base (Clement Associates, 1989; Jones, 1988). This approach needs to be pursued and refined with additional laboratory and carcinogenesis studies.
A good opportunity may exist to reevaluate the accumulating evidence of human carcinogenic response in occupationally exposed populations with relation to serum PCB levels and to relate the human response per unit of delivered dose to the response per serum level likely to have been present in rodent bioassays. [There are two very limited but apparently positive epidemiological studies in groups of capacitor workers (Bertazzi et al., 1987; Brown, 1987) and at least one negative study at a plant in Massachusetts whose results have not yet been published in detail. In at least one case, there are measurements of serum PCB levels in the exposed group.] Harold Humphrey of the Michigan Department of Health reportedly has an extensive data set of PCB concentrations in blood in relation to dietary exposure levels. A series of congener-specific measurements is now in progress together with published measurements of metabolic rates of PCBs in humans (Buehler et al., 1988; Phillips et al., 1989; Yakushiji et al., 1984); this is likely to be useful in establishing metabolic rates of PCBs for comparison with animal models and measurements after the work by Matthews and Dedrick (1984) and Tanabe et al. (1981). Animal work, together with the newer metabolism information, appears capable of providing a basis for estimating serum PCB levels in the rats and mice that showed positive cancer
responses in chronic bioassays. Projections to human risk could then be made on the basis of a more appropriate measure of "delivered dose" (weighted for different congeners by receptor binding and pharmacokinetics) rather than gross dietary intake. There already is reasonably good information on the population distribution of overall plasma PCB levels in the United States (as can be seen by the correspondence of the points to the straight line in Figure 6-1, these data are reasonably well described as lognormal).12 Using such a distribution, if one had an estimate of cancer risk per unit of plasma PCB concentration, an inference could be made not only about the average risk to the population, but about the distribution of risk to various individuals that derives from differences in both dietary habits and PCB elimination rates. Plasma and whole body burden PCB levels could also be productively used as indicators of long-term dosage in epidemiological case control studies of cancer patients (versus other people) in communities near the Great Lakes with greater than usual numbers of people having high exposures to fish with appreciable levels of PCBs.
A better analysis should also include a quantitative assessment of the risks posed by prenatal exposure to PCBs, via such parameters as gestational period and birth weight, as well as subtle neurological measures of effect. The presence of at least modest effects on birth weight has recently been supported by an epidemiological study in workers (Taylor et al., 1989). Because birth weight is such an easy parameter to study (measurements are made routinely on nearly all newborns) and because it has a very strong relationship to infant mortality (Hogue et al., 1987), additional large population studies of birth weight and gestational period in relation to fish consumption and relevant serum PCB congener levels should be a high priority. Methodology exists to interpret birth weight changes in terms of other relevant endpoints such as infant mortality (Ballew and Hattis, 1989), but these techniques require validation with an actual case having large enough numbers of subjects to provide data on both the intermediate parameters (birth weight and gestational age) and the ultimate parameters of concern (infant mortality, impairment of neurological development, etc.).
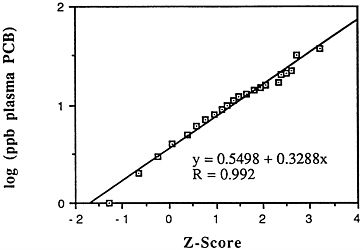
FIGURE 6-1 Plasma PCB levels in 738 Southern Californians screened prior to employment [data of Sahl et al. (1985)]
Finally, there is a need for improved and more systematic treatment of analytical uncertainties, with ultimate results in the form of cumulative probability density functions or, failing this, at least a statement of both best-estimate and upper-confidence-limit risks, under consistent and defensible theories.
Methylmercury
FDA Risk Assessment and Current Regulations
The published analysis of Tollefson and Cordle (1986) appears to be the most definitive public assessment by FDA personnel of the risks of methylmercury in fish. It is patterned after the NOEL/uncertainty factor analysis illustrated for PCBs in Table 6-6, but has distinctive elements that alter the usual risk acceptance posture somewhat.
The principal benchmark that Tollefson and Cordle (1986) use in defining an acceptable intake of methylmercury is the lowest blood methylmercury level that appeared to be associated with overt neurological symptoms of toxicity in a few studied mass poisoning incidents (two that occurred from fish taken from Minamata Bay and Niigata in Japan in the 1950s and 1960s, and one incident that arose from the consumption of contaminated seed grain in Iraq):
A Swedish expert group evaluated data on human methylmercury toxicity derived from cases which occurred in Minamata and Niigata. The Swedish group determined (by extrapolation) that the lowest blood mercury level associated with toxic effects was 220 ppb [parts per billion] and the lowest hair mercury level associated with toxic effects was 50 ppm (Berglund et al., 1971).
The Iraqi outbreak of methylmercury poisoning has been extensively studied by many investigators. The blood level of mercury at which symptoms of toxicity were first detected in the Iraqi episode was approximately 240 ppb [parts per billion] (Clarkson et al., 1976). This calculation was made on samples collected 65 days after the end of exposure, which is the approximate half-life of methylmercury in humans. Since the actual clearance times from the blood are not known, the level may lie between 240 and 480 ppb. These values are for adult exposures only.
Studies of people in Sweden who consumed relatively large amounts of fish allowed these blood levels to be related to dietary levels–at least on a population average basis (Tollefson and Cordle, 1986): "A linear relationship was found between daily ingested methylmercury and the level of methylmercury in blood, and the data indicated that a steady daily intake of approximately 300 µg Hg [mercury] as methylmercury for a 70-kg person would result in a blood concentration of roughly 200 ppb at steady state (Skerfving, 1974)."
This 300-µg/day dose rate, calculated to correspond to 200 ppb in blood for an average person, was interpreted by Tollefson and Cordle (1986) as
a threshold value at which symptoms of toxicity associated with methylmercury are first noticeable. … Dose-response relationships below this range of intake are not known. In addition, there is concern about the relative sensitivity of the developing fetus. The question of interaction of other chemical factors such as selenium on the toxicity of methylmercury has not been conclusively demonstrated at this time, but may be a factor to be considered when more information is developed. Because of these areas of uncertainty, a safety factor of ten has been used to provide a sufficient margin of safety.
Thus a maximum tolerable level would be 30 µg methylmercury daily in the diet, resulting in 20 ppb of methylmercury in blood and 5 ppm in hair.
The following limitations to this approach were recognized: (1) it was not known to what extent particular individuals are more or less sensitive to mercury than others; (2) the estimates were based on the "lowest level that caused an effect" rather than the normal procedure of using a "no-effect dose level"; (3) paresthesia is usually the first symptom of methylmercury toxicity noted but is not sufficient to diagnose poisoning because it can be caused by many other factors (Clarkson et al., 1983); (4) questions about dose-response relationships in human fetuses and newborn infants were unanswered; and (5) there is a possibility of subclinical effects arising from exposure to very low levels of methylmercury.
Tollefson and Cordle (1986) therefore realize that they are departing from the usual procedure for the NOEL/safety factor analysis and that, in particular, risks to fetuses may not be represented adequately in the available data. However, they make no additional numerical adjustment for these uncertainties, presuming implicitly that the usual 10-fold safety factor has enough of a margin built into it to avoid an unacceptable level of risk.
In addition to these enumerated factors, there is a subtle point that makes the calculation on the basis of the average diet/blood relationship less protective than the usual calculation based on a 10-fold reduction from the dietary dose that is directly observed to be without effects. The 10-fold factor is supposed to represent the protection needed to guard against interindividual variability in the complete pathway from dietary intake to the production of biological responses. Some portion of that human interindividual variability, however, occurs in characteristics that affect individual blood levels–especially individual half-lives for elimination of methylmercury from the body. For those with relatively slow elimination rates, toxicity would be expected to be associated with lower average dietary intakes than for those with average elimination rates. Therefore, by doing the dose extrapolation in terms of blood levels, and translating the corresponding average dietary levels, the authors are being less protective than would have been the case had the 10-fold factor been applied to the minimum dietary dosage associated with an observed effect (or no observed effect). Later in the paper, Tollefson and Cordle (1986) discuss interindividual differences in biological half-lives for methylmercury as follows:
Additional data developed on the biological half-life of methylmercury in humans, however, indicate a need to take into account the problem of variations among individuals. In the Iraqi episode, 90% of the individuals studied had a biological half-life of methylmercury between 60 and 70 days, but 10% showed values of 110 to 120 days (Al-Shahristani and Shihab, 1974). Individuals having a long biological half-life would accumulate much higher steady-state levels than those having short biological half-lives and would thus be at greater risk from the same level of methylmercury intake.
Tollefson and Cordle have unfortunately misread the population distribution of methylmercury half-lives presented in the cited paper. A figure in the paper is misleading in that the highest single observed biological half-life (189 days) has somehow been excluded, but there is no way that even the figure can be construed to indicate that 90% of the values are between 60 and 70 days. Had Tollefson and Cordle checked the detailed data presented in a table on the following page in the Al-Shahristani and Shihab 1974 paper, they would have seen the distribution shown in
TABLE 6-10 Biological Half-Lives for Individuals Reported by Al-Shahristani and Shihab (1974)
|
Half-Life (days) |
Rank Order |
Percentage Scorea [100 × (Rank -0.5)/N] |
|
37 |
1 |
1.04 |
|
38 |
2 |
3.13 |
|
43 |
3 |
5.21 |
|
43 |
4 |
7.29 |
|
44 |
5 |
9.38 |
|
45 |
6 |
11.46 |
|
45 |
7 |
13.54 |
|
50 |
8 |
15.63 |
|
51 |
9 |
17.71 |
|
51 |
10 |
19.79 |
|
51 |
11 |
21.88 |
|
52 |
12 |
23.96 |
|
54 |
13 |
26.04 |
|
57 |
14 |
28.13 |
|
57 |
15 |
30.21 |
|
58 |
16 |
32.29 |
|
58 |
17 |
34.38 |
|
62 |
18 |
36.46 |
|
62 |
19 |
38.54 |
|
62 |
20 |
40.63 |
|
65 |
21 |
42.71 |
|
66 |
22 |
44.79 |
|
66 |
23 |
46.88 |
|
66 |
24 |
48.96 |
|
67 |
25 |
51.04 |
|
69 |
26 |
53.13 |
|
69 |
27 |
55.21 |
|
71 |
28 |
57.29 |
|
73 |
29 |
59.38 |
|
75 |
30 |
61.46 |
|
75 |
31 |
63.54 |
|
75 |
32 |
65.63 |
|
76 |
33 |
67.71 |
|
78 |
34 |
69.79 |
|
78 |
35 |
71.88 |
|
79 |
36 |
73.96 |
|
83 |
37 |
76.04 |
|
85 |
38 |
78.13 |
|
87 |
39 |
80.21 |
|
89 |
40 |
82.29 |
|
91 |
41 |
84.38 |
|
93 |
42 |
86.46 |
|
117 |
43 |
88.54 |
|
118 |
44 |
90.63 |
|
119 |
45 |
92.71 |
|
120 |
46 |
94.79 |
|
120 |
47 |
96.88 |
|
189 |
48 |
98.96 |
|
a This is the percentage of an infinite sample that would be expected to be equal to or below the stated half-life. |
||
Table 6-10. It is apparent that overall there is a fivefold range of half-lives among the 48 people studied. Only 10 values (a little over 20%) fall between 60 and 70 days. As can be seen in Figure 6-2, the data are reasonably well described as a lognormal distribution. From the geometric standard deviation of that distribution (1.403), it would be expected that approximately 2% of the population would have half-lives more than twice the geometric mean of 68.3 days.
Tollefson and Cordle (1986) go on to support the results of their analysis by citing studies failing to find obvious methylmercury toxicity in heavy fish-eating population groups in Peru and American Samoa with average blood methylmercury concentrations in the range of 60-80 ppb (Marsh et al., 1974; Turner et al., 1980). However, without careful analysis of the distributions of thresholds implied by the earlier data, the sample sizes, and the degree of background symptoms in control groups in the negative studies cited, neither the reader nor the authors can judge the sensitivity of those studies to detect adverse effects and whatever quantitative implications there might be for the level of risk at lower doses.
FIGURE 6-2 Lognormal plot of the distribution of methylbercury biological half-lives (data of Al-Shahristani and Shihab, 1974)
Tollefson and Cordle's apparent reluctance to construct and analyze the implications of quantitative population distributions is also seen in their treatment of the expected dietary dosage of methylmercury to consumers. They had available the results of a fairly extensive study commissioned by the Tuna Research Foundation (TRF) of one-month diary reports of consumption of different types of fish by 7,662 families, including 25,165 people, and measurements of mercury levels in different species of fish. However, instead of using these data to describe the population distribution of monthly mercury intakes from fish as a whole and the possible effects of different tolerances, Tollefson and Cordle (1986) opted for an ad hoc treatment of the data for only a few species of interest:
Data from the TRF survey have indicated that the average consumption of all fish among the fish-eating population of the United States is 18.58 oz/month or approximately 18 g/day. Daily consumption of species containing relatively high levels
of methylmercury such as tuna, swordfish, or halibut would be considerably less. For example, the mean daily consumption of halibut is 7.2 g with two standard deviations increasing the total to 16.6 g. This would include some 97.5% of all consumers of halibut. The consumption of 16.6 g of halibut with 0.179 ppm mercury would provide a daily mercury intake of approximately 2.9 µg.
The mean daily consumption of swordfish is 6.53 g with a standard deviation of 2.5 g. If 11.53 g of swordfish with a mercury level of 1.5 ppm were consumed each day, and this would include over 95% of all swordfish eaters,13 the daily mercury intake would be 17.3 µg, still below the ADI of 30 μg.
At the highest level of swordfish consumption shown by the NPD [National Purchase Dairy Panel, Inc.] study, i.e. 511 g in a month or 17 g per day, with a mercury residue of 1.5 ppm the daily mercury intake would be 25.5 µg, still below the ADI of 30 µg. If in addition to the highest level of consumption of swordfish in the NMFS [National Marine Fisheries Service] study, i.e. 17 g per day with a mercury residue of 1.5 ppm, the same individual consumed the average daily amounts of tuna, halibut, and salmon at the present average residue levels of mercury for each of the three species, then the total daily intake of mercury from all four species would average 31.7 µg, only 1.7 µg above the ADI. Such consumption, which would realistically be on a periodic basis to derive daily exposure, seems very unlikely, particularly since the cost of swordfish, halibut, and salmon is prohibitively high.
Tollefson and Cordle are certainly correct in concluding that very few consumers will exceed the 30-µg/day level for prolonged periods. However, this type of analysis cannot give an estimate of how many might, or how many might fall short of the 30-µg/day level by various amounts. The failure to analyze the data more rigorously is all the more unfortunate because the FDA had available an example providing statistically sophisticated treatment of these same data (NMFS, 1978). 14
Tollefson and Cordle (1986) conclude with a somewhat unusual statement of the relevant risk issues:
The data currently available for evaluating quantitatively the association of neurological symptoms of toxicity with exposure to methylmercury are sparse and inconsistent. Additional studies are being carried out on the prenatal effects of methylmercury to determine that this lifestage continues to be protected by the 1.0 ppm regulatory level for mercury in fish. However, even with the above-outlined uncertainties concerning the results of exposure in Japan and Iraq, where exposures were considerably higher than anything experienced in other countries, U.S. fish consumption data do not indicate any cause for concern of methylmercury poisoning for the average American. The majority of fish consumers could easily double their intake and still remain below the mercury ADI. The current 1.0 ppm regulatory level for marine species provides more than adequate protection at the current average fish consumption level in the U.S. In addition, the enforced limit of 1.0 ppm mercury in marine fish provides a sufficient margin of safety for young children and for significant numbers of consumers exceeding the acceptable daily intake. (Emphasis added.)
The potential for a more quantitative analysis of methylmercury risks with the aid of available data on the distributions of blood methylmercury concentrations and dose-response data from the Iraqi poisoning incident is illustrated below. The aim is not to provide a definitive assessment of methylmercury risks, but to show an approach for improving the type of assessment now done by FDA.
Available Data on Blood Levels in the U.S. Population
Gathering and analyzing data on the actual distribution of blood mercury and methylmercury levels in the population allow one to bypass a great deal of the complexity of modeling individual dietary intakes over times long enough to determine biologically significant systemic exposures. Figure 6-3 shows a sample of mercury (Hg) blood levels from a fishing village in the United Kingdom (Haxton et al., 1979). Full data describing this distribution are not available; however, a plot of the median and extreme values given indicates that the distribution is well described as lognormal. The variability indicated by the log10 geometric standard deviation of 0.328 indicates that 95% of the population is spread out over a 20-fold range – between 4.5-fold above and 4.5-fold below the median blood level for the population. This variability includes both dietary and pharmacokinetic differences among individuals.
FIGURE 6-3 Lognormal plot of the Haxton et al. (1979) data on blood mercury concentrations (only the median and extreme values of the distribution were available for plotting)
Figures 6-4 – 6-6 show methylmercury (MeHg) blood level distribution data from two towns in Michigan, generously supplied to us by Harold Humphrey from unpublished data collected in the early 1970s. Algonac (population 3,684) was chosen because of its proximity to both Lake St. Clair and the St. Clair River – sites of a famous mercury contamination episode discovered by a Canadian graduate student in 1970. South Haven (population 6,471) was chosen as a control community with similar proximity to Lake Michigan. Figures 6-4 – 6-6 show the results from a portion of the study that consisted of randomly selected residents of each community. The distributions appear to be well described as lognormal (perfect lognormal distributions would appear as straight lines on this kind of plot), and the Algonac community distribution is translated distinctly upward from that of South Haven (medians at about 2.3 and 1.6 ppb, respectively). The degree of interindividual variability (the slope of the lines; the shallower the slope, the greater is the variability) is in each case similar to that inferred from the total mercury distribution observed by Haxton et al. (1979) (Figure 6-3).
Methylmercury dose, blood levels, and population risks
Recently, Marsh et al. (1987) published detailed information on the incidence of a variety of fetal mercury effects in relation to the maximal levels of mercury found in the hair of the mothers during gestation. (The observations come from an Iraqi mass poisoning incident that resulted from the distribution of methylmercury-treated Green Revolution seed grain.) In all, 81 mother-infant pairs were studied. Table 6-11 shows the numerical results, grouped in fourfold ranges of dosage.
Figures 6-7 through 6-11 show log10 probit dose-response relationships for these effects. It can be seen that the log10 probit slopes for the five different fetal mercury effects range from 0.66 to 1.5 (the log10 probit slope is the number of standard deviations of the population distribution of effect thresholds per 10-fold change in dose, smaller numbers indicated a broader lognormal distribution of thresholds). These results show a very large range of variability. A slope of 1 indicates that 95% of the individual thresholds for effect would be spread over a 10,000-fold range, from 100-fold lower to 100-fold higher than the dose that would cause an effect in a median individual. Observations of the interindividual variability of human pharmacokinetic parameters indicate considerably narrower ranges of interindividual variability than suggested in this case (average chemicals giving log10 probit slope values on the order of 8-10, although a few chemicals are in the range of 2-3) (Hattis et al., 1987a,b).
One possible criticism of the fitting procedure represented in Figures 6-7 – 6-11 is that individual points have not been weighted for their relative degree of statistical power. Moreover, these simple probit plots necessarily exclude points at which either none or all of the subjects showed the response. To refine the analysis, therefore, the classical maximum likelihood procedure of Finney (1971) was used to fit the data in Table 6-11. This method corrects both statistical deficiencies mentioned above, provides a means to test the overall fit of the logprobit model to the data, and also measures the uncertainties of the fitted parameters (Table 6-12).15
By comparing these results with the regression equations given in Figures 6-7 – 6-11, it can be seen that in general the probit slopes calculated by the Finney (1971) procedure, with its fine weighing of the points and maximum likelihood estimation methods, are somewhat higher (indicating slightly less interindividual variability) than the simpler procedure without weighing. Overall, however, there is reasonably close agreement, and in any case, the conclusion appears unchanged that the Marsh et al. (1987) data indicate quite a large amount of interindividual variability. The mean log10 probit slope for the five effects as determined by the Finney (1971) procedure is about 1.18.
Such a conclusion, however, depends on whether the biomarker of exposure used in this case – the maximum hair mercury found at any time during gestation – is the most appropriate direct causal predictor of response that can be developed. Other possibilities might well include the concentration of mercury at a specific sensitive time during gestation or a weighted sum of concentration x duration over a specific set of sensitive periods. Accurate assessment of the degree of interindividual variability in susceptibility in humans, and consequent low-dose risks, might well depend on quantitative measurement and modeling of the causal processes involved in this case and reanalysis of the data according to the most likely causally predictive summary measure of delivered dose.
Publications by Bakir et al. (1973) and Clarkson et al. (1976) provide the basis
TABLE 6-11 Observations of Marsh et al. (1987) on the Incidence of Various Effects in Children Following in Utero Methylmercury Exposure
|
|
|
|
Effects |
|||||
|
Maximum Hair Hg During Gestation |
Late Walking (after 18 months) |
Late Talking (after 24 months) |
"Mental" Symptoms |
|||||
|
Range (ppm) |
Geometric Mean |
Number of Subjects |
Number of Cases |
Fraction Affected |
Number of Cases |
Fraction Affected |
Number of Cases |
Fraction Affected |
|
1-3 |
1.37 |
27 |
0 |
0.000 |
2 |
0.074 |
1 |
0.927 |
|
5-19 |
10.00 |
14 |
2 |
0.143 |
1 |
0.071 |
0 |
0 |
|
20-79 |
52.53 |
13 |
2 |
0.154 |
3 |
0.231 |
1 |
0.077 |
|
80-319 |
163.38 |
12 |
3 |
0.250 |
4 |
0.333 |
3 |
0.250 |
|
Over 319 |
436.60 |
15 |
12 |
0.800 |
11 |
0.733 |
4 |
0.267 |
|
|
|
|
Effects |
|||||
|
Maximum Hair Hg During Gestation |
Seizures |
Neurological Score over 3 |
Neurological Score over 4 |
|||||
|
Range (ppm) |
Geometric Mean |
Number of Subjects |
Number of Cases |
Fraction Affected |
Number of Cases |
Fraction Affected |
Number of Cases |
Fraction Affected |
|
1-3 |
1.37 |
27 |
0 |
0.000 |
3 |
0.111 |
0 |
0 |
|
5-19 |
10.00 |
14 |
0 |
0.000 |
1 |
0.071 |
1 |
0.071 |
|
20-79 |
52.53 |
13 |
1 |
0.077 |
4 |
0.308 |
2 |
0.154 |
|
80-319 |
163.38 |
12 |
2 |
0.167 |
3 |
0.250 |
2 |
0.167 |
|
Over 319 |
436.60 |
15 |
4 |
0.267 |
9 |
0.600 |
6 |
0.400 |
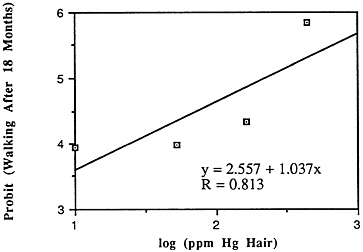
FIGURE 6-7 Logprobit dose-relationship for walking after 18 months in relation to maternal hair mercury (data of Marsh et al., 1987)
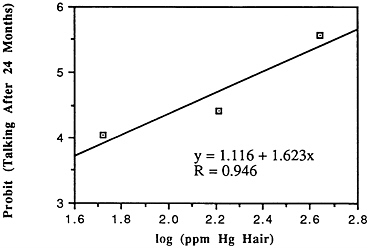
FIGURE 6-8 Logprobit dose-response relationship for talking after 24 months in relation to maternal hair mercury (data of Marsh et al., 1987)
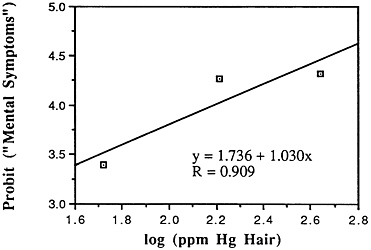
FIGURE 6-9 Logprobit dose-response relationship for "mental symptoms" in children in relation to maternal hair mercury (data of Marsh et al., 1987)
TABLE 6-12 Maximum Likelihood Fita of the Marsh et al. (1987) Fetal Effects Data by Using the Method of Finney (1971)
|
Effect |
Background Response (%b) |
Probit Slope |
Slope Standard Error |
Intercept |
ED50 (ppb blood) |
ED50 Geometric Standard Error |
Chi-Squared |
Degrees of Freedomc |
Pd |
|
Late walking |
0 |
1.21 |
0.30 |
2.19 |
205 |
1.49 |
6.093 |
3 |
0.11 |
|
Late talking |
7.3 |
1.76 |
0.71 |
0.81 |
244 |
1.38 |
0.689 |
1 |
0.41 |
|
Mental symptoms |
2.4 |
0.99 |
0.76 |
1.88 |
1,429 |
4.75 |
0.351 |
1 |
0.55 |
|
Seizures |
0 |
1.10 |
0.53 |
1.54 |
1,399 |
2.95 |
0.356 |
3 |
0.95 |
|
Neurological score >4 |
0 |
0.85 |
0.27 |
2.42 |
1,047 |
2.54 |
0.874 |
3 |
0.83 |
|
|
Average |
1.18 |
|
|
|
Sum |
8.363 |
11 |
0.68 |
|
a The equation fit is: probit of excess risk over background = intercept + (slope) × log10 (blood Hg in ppb). b Estimated from data in the lowest one to three dose groups. c The number of dose groups available for analysis, less 2 for the number of parameters estimated from the data (intercept and probit slope). d The probability that a deviation as large as that observed between the logprobit model and the data would have been expected by chance, even if the logprobit model were a perfect description of the underlying dose-response function. |
|||||||||
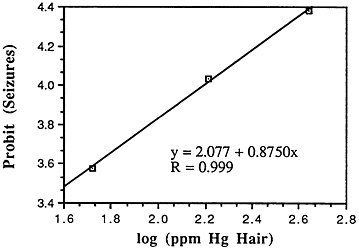
FIGURE 6-10 Logprobit dose-response relationship for seizures in children in relation to maternal hair mercury (data of Marsh et al., 1987)
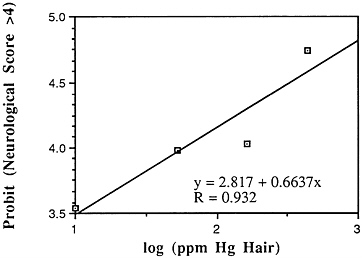
FIGURE 6-11 Logprobit dose-response relationship for adverse neurological scores in children in relation to maternal hair mercury (data of Marsh et al., 1987)
for similar treatment of the incidence of adult effects in relation to blood levels, at least as measured at a point approximately 65 days (on the order of one half-life) after the end of exposure. Table 6-13 summarizes the numerical results, and Figures 6-12–6-18 show the logprobit plots for the various effects (after subtraction of the apparent background incidence of the effects, as indicated by their observed frequencies in the lowest-dose groups). Figure 6-18 shows a plot of all the adult effects combined. The data can also
TABLE 6-13 Observations of the Incidence of Various Effects in Adults in the Iraqi Methylmercury Poisoning Incident
|
|
|
|
Observations of Various Adult Effects |
|||||
|
ppb Blood Hg Measured About 65 Days After Exposurea |
Paresthesias |
Ataxia |
"Visual Changes" |
|||||
|
Range |
Assumed Mean |
Number of Subjects |
Number of Casesb |
Fraction Affected |
Number of Cases |
Fraction Affected |
Number of Cases |
Fraction Affected |
|
0-100 |
50 |
21 |
2 |
0.095 |
1 |
0.05 |
0 |
0 |
|
101-500 |
350 |
19 |
1 |
0.05 |
0 |
0 |
0 |
0 |
|
501-1,000 |
750 |
19 |
8 |
0.42 |
2 |
0.11 |
4 |
0.21 |
|
1,001-2,000 |
1,500 |
17 |
10 |
0.6 |
8 |
0.47 |
9 |
0.53 |
|
2,001-3,000 |
2,500 |
25 |
20 |
0.79 |
15 |
0.60 |
14 |
0.56 |
|
3,001-4,000 |
3,500 |
17 |
14 |
0.82 |
17 |
1.00 |
10 |
0.58 |
|
4,001-5,000 |
4,500 |
7 |
7 |
1.00 |
7 |
1.00 |
6 |
0.83 |
|
|
|
|
Observations of Various Adult Effects |
|||||
|
ppb Blood Hg Measured About 65 Days After Exposurea |
Disarthria |
"Hearing Defects" |
Deaths |
|||||
|
Range |
Assumed Mean |
Number of Subjects |
Number of Casesb |
Fraction Affected |
Number of Cases |
Fraction Affected |
Number of Cases |
Fraction Affected |
|
0-100 |
50 |
21 |
1 |
0.05 |
0 |
0 |
0 |
0 |
|
101-500 |
350 |
19 |
1 |
0.05 |
0 |
0 |
0 |
0 |
|
501-1,000 |
750 |
19 |
1 |
0.05 |
1 |
0.05 |
0 |
0 |
|
1,001-2,000 |
1,500 |
17 |
4 |
0.24 |
0 |
0 |
0 |
0 |
|
2,001-3,000 |
2,500 |
25 |
6 |
0.25 |
3 |
0.125 |
0 |
0 |
|
3,001-4,000 |
3,500 |
17 |
13 |
0.75 |
6 |
0.36 |
3 |
0.17 |
|
4,001-5,000 |
4,500 |
7 |
6 |
0.85 |
5 |
0.66 |
2 |
0.28 |
|
a The numbers of cases shown here were inferred from percentages of affected subjects given in the paper and the numbers of subjects studied. b The numbers of cases shown here were inferred from percentages of affected subjects given in the paper and the number of subjects studied. NOTE: Because these measurements were done after the end of exposure, it is likely that they understate actual blood levels during exposure by about 2-fold. |
||||||||
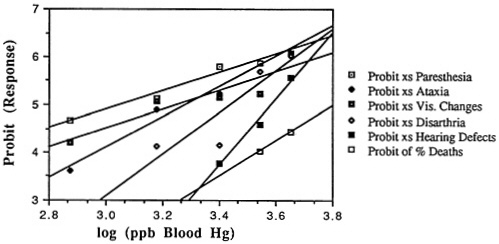
FIGURE 6-18 Logprobit dose-response relationships for all effects in adults–Iraqi data
be used to compare the probit slopes as determined by the Finney (1971) maximum likelihood fitting procedure (Table 6-15) with the simple linear regression procedure of Figures 6-7–6-11.
It can be seen that, as with fetal effects, the fuller analysis of data with the Finney (1971) procedure suggests somewhat larger probit slopes than the values determined by simple regression analysis of the points. It can also be seen that, in comparison with the fetal effects, the effects in adults tend to have larger probit slopes, indicating less interindividual variability and therefore less risk at low doses. Biologically, the implication is that the more complicated array of causal processes involved in producing fetal effects (allowing for variability in maternal mercury elimination, maternal/fetal transfer, and the time-sensitive processes of interference by methylmercury with development) tends to lead to more interindividual variability. From a social policy perspective, the implication is that the 10-fold safety factor rule, whatever its effectiveness in protecting exposed populations against the effects of interindividual variability in adults, appears likely to provide less protection for the developing organism.
Tables 6-12 and 6-14 show that the chi-squared statistical analysis indicates that the logprobit procedure provides an adequate overall fit for both the fetal and the adult data. Although this cannot be said to prove that the distribution of effect thresholds for methylmercury effects is in fact lognormal, it suggests that such an assumption is not grossly at variance with the available information. Moreover, it should be noted that logprobit analysis, as pioneered by Finney in earlier editions of his book, has been widely applied to toxicological data from animal experiments.
Tables 6-15 through 6-17 provide preliminary risk projections based on the lognormal threshold distribution model implicit in the Finney (1971) probit analysis and (for fetal effects) the same 4/1 translation of ppm hair levels into ppb blood levels used by Tollefson and Cordle (1986). Table 6-16 allows a preliminary appraisal of the difference in protection provided by the 10-fold safety factor in this case, when (as is not usual) the 10-fold factor is applied to blood levels. In part A of Tables 6-15 and 6-16, the committee has not corrected for the likely twofold underestimation of the adult blood levels associated with effects, which is related to the fact that the adult blood levels were measured 65 days after the end of exposure. The variants in part
TABLE 6-14 Maximum Likelihood Fitsa of the Iraqi Adult Methylmercury Effects Data by Using the Method of Finney (1971)
|
Effects |
Background Response (%b) |
Probit Slope |
Slope Standard Error |
Intercept |
ED50 (ppb blood) |
ED50 Geometric Standard Error |
Chi-Squared |
Degrees of Freedomc |
Pd |
|
Paresthesias |
7.5 |
2.17 |
0.63 |
-1.64 |
1,145 |
1.24 |
1.155 |
2 |
0.76 |
|
Ataxias |
2.5 |
3.92 |
0.76 |
-7.67 |
1,687 |
1.11 |
4.95 |
3 |
0.18 |
|
Visual changes |
0 |
2.19 |
0.43 |
-2.24 |
2,006 |
1.16 |
3.695 |
3 |
0.3 |
|
Disarthria |
5 |
4.67 |
1.36 |
-11.2 |
2,952 |
1.10 |
5.608 |
3 |
0.13 |
|
Hearing defects |
1.3 |
6.42 |
2.17 |
-18.05 |
3,877 |
1.10 |
0.209 |
1 |
0.65 |
|
Deaths |
0 |
7.58 |
3.19 |
-23.05 |
5,007 |
1.18 |
0.83 |
1 |
0.36 |
|
Sum for adult effects |
|
|
|
|
|
|
16.447 |
13 |
0.22 |
|
Sum for fetal and adult effects |
|
|
|
|
|
24.81 |
24 |
0.42 |
|
|
a The equation fit is: probit of excess risk over background = intercept + (slope) × log10 (blood Hg in ppb). b Estimated from data in the lowest one to three dose groups. c The number of dose groups available for analysis, less 2 for the number of parameters estimated from the data (intercept and probit slope). d The probability that a deviation as large as that observed between the logprobit model and the data would have been expected by chance, even if the logprobit model were a perfect description of the underlying dose-response function. |
|||||||||
B of the tables show the results after this adjustment (no adjustment is required for fetal effects data because measurements made sequentially along hair shafts reflect methylmercury concentrations in maternal blood during pregnancy). It can be seen in Tables 6-16A and B that although a 10-fold reduction in blood level from the presumed adult LOEL is expected to reduce the incidence of adult effects to quite low rates, the greater interindividual variability associated with fetal effects suggests the possibility that very significant fetal risks may remain. The incidence of fetal effects projected by these probit risk relationships is appreciable, even for the actual population distributions of blood methylmercury levels observed in the Michigan communities (Table 6-17). It can also be seen in Table 6-17 that the bulk of the population excess fetal risk is expected to be found in portions of the population that do not have unusually high blood methylmercury levels (≤ 10 ppb).
Projections of fetal risks here, of course, are greatly affected by the assumed lognormal form of the distribution of thresholds for both adult and fetal effects. Although lognormal distributions appear to be broadly compatible with the high-dose data (see earlier figures), this is not the only statistical form that the distribution of thresholds might take. Cox et al. (1989) have applied a logit model, which assumes a different distribution of thresholds, to predict possible low-dose risks for one of the fetal effects covered here (late walking), using the same data set (Cox and coworkers also made projections based on scores for neurological impairment, but using a different cutoff for defining effect than the committee, thus rendering the risk projections noncomparable). The risk predictions are compared in Table 6-18. In the dose range of interest, it appears that the two different assumptions about the form of the population distribution of thresholds yield very similar results.
As mentioned earlier, it would be more common for a 10-fold safety factor to be applied to the dietary dosage of a toxicant, rather than to blood levels. To represent this for methylmercury, one must add to the logprobit risk equations an estimate of the interindividual variability in internal blood levels as a function of dietary intake. In principle, two factors should be considered in accomplishing this: (1) interindividual variability in absorption, and (2) interindividual variability in overall elimination of mercury from the blood. Methylmercury is relatively efficiently absorbed from food, so the influence of the first factor may well be small. For the second factor, fortunately the data of Al-Shahristani and Shihab (1974) are available, based on measurements of the decline in mercury levels with distance along hair shafts, interpreted in terms of time with the aid of measurements of individual rates of hair growth (Figure 6-2). Tables 6-19A and B (analogous to Tables 6-16A and B) show the results of translating the ED50's into equivalent daily dietary doses [ED50 (diet) = ED50 (blood) × 300/200], including the extra variability represented by the geometric standard
TABLE 6-15A Logprobit Projections of Risks of Adult and Fetal Methylmercury Effects, Based on Observations from Iraq of Effects as a Function of Blood and Hair Levels
TABLE 6-15B Logprobit Projections of Risks of Adult and Fetal Methylmercury Effects, Based on Observations from Iraq of Effects as a Function of Blood and Hair Levels (After a Twofold Upward Adjustment of Observed Blood Levels Because of Measurement Delay)
TABLE 6-16A Projected Risks at the Presumed Adult Blood Methylmercury LOEL and at One-Tenth the Presumed Adult LOEL
TABLE 6-16B Projected Risks at the Presumed Adult Blood Methylmercury LOEL, and at One-Tenth the Presumed Adult LOEL (After a Twofold Upward Adjustment of Observed Blood Levels Because of Measurement Delay)
|
Effects |
Probit Slope |
Blood ED50 (ppb) |
SDs Below ED50 at Presumed Adult LOEL (200 ppb in blood) |
Risk (expected fraction affected) at Presumed Adult LOEL |
SDs Below ED50 at 1/10th Adult LOEL (20 ppb in blood) |
Risk (expected fraction affected) at 1/10th Adult LOEL |
|
Adult |
||||||
|
Paresthesias |
2.170 |
2,290 |
2.3 |
0.011 |
4.47 |
4.0 × 10-6 |
|
Ataxias |
3.926 |
3,370 |
4.82 |
7.3 × 10-7 |
8.74 |
Very small |
|
Visual changes |
2.192 |
4,010 |
2.85 |
0.0022 |
5.05 |
2.3 × 10-7 |
|
Disarthria |
4.67 |
5,900 |
6.87 |
Very small |
11.54 |
Very small |
|
Hearing defects |
6.423 |
7,750 |
10.20 |
Very small |
16.63 |
Very small |
|
Deaths |
7.582 |
10,020 |
12.89 |
Very small |
20.47 |
Very small |
|
Fetal |
||||||
|
Late walking |
1.214 |
819 |
0.74 |
0.229 |
1.96 |
0.0251 |
|
Late Talking |
1.756 |
978 |
1.21 |
0.113 |
2.97 |
0.0015 |
|
Mental symptoms |
0.988 |
5,720 |
1.44 |
0.075 |
2.43 |
0.0076 |
|
Seizures |
1.100 |
5,600 |
1.59 |
0.056 |
2.69 |
0.0035 |
|
Neurological score >4 |
0.854 |
4,190 |
1.13 |
0.130 |
1.98 |
0.0237 |
TABLE 6-17 Projected Effects for the Blood Methylmercury Distributions Observed in South Haven and Algonac, Michigan (Humphrey, 1974)
|
|
Population Risk for South Haven Distribution |
Population Risk for Algonac Distribution |
||||
|
Effects |
Fraction of People Expected to be Affected |
Population Risk (%) Due to Blood MeHg |
Fraction of People Expected to be Affected |
Population Risk(%) Due to Blood MeHg |
||
|
≤10 ppb |
≤20 ppb |
≤10 ppb |
≤20 ppb |
|||
|
Adult |
||||||
|
Paresthesias |
1.2 × 10-7 |
9 |
40 |
1.0 × 10-7 |
17 |
61 |
|
Ataxias |
Very smalla |
|
|
Very smalla |
|
|
|
Visual changes |
1.9 × 10-9 |
3 |
22 |
1.2 × 10-9 |
8 |
43 |
|
Disarthria |
Very smalla |
Very smalla |
|
|
|
|
|
Hearing defects |
Very smalla |
Very smalla |
|
|
|
|
|
Deaths |
Very smalla |
Very smalla |
|
|
|
|
|
Fetal |
||||||
|
Late walking |
3.7 × 10-3 |
88 |
98.2 |
5.3 × 10-3 |
90 |
99.0 |
|
Late talking |
1.6 × 10-5 |
48 |
82 |
2.1 × 10-5 |
58 |
91 |
|
Mental symptoms |
4.5 × 10-4 |
87 |
97.9 |
6.3 × 10-4 |
89 |
98.3 |
|
Seizures |
1.4 × 10-4 |
82 |
96.6 |
2.0 × 10-4 |
85 |
98.3 |
|
Neurological score >4 |
2.5 × 10-3 |
92.2 |
98.9 |
3.4 × 10-3 |
93.0 |
99.4 |
|
a Less than 10-10. SOURCE: Humphrey (1974). |
||||||
TABLE 6-18 Comparison of Logit Versus Probit Risk Projections
|
Maternal Hair Hg Level (ppm) |
Assumed Blood Hg (ppb) |
Cox et al. (1989) Logit Model Risk Estimates (% excess risk) |
Probit Model Risk Estimates (Table 6-16b) (% excess risk) |
|
1 |
4 |
0.52 |
0.25 |
|
5 |
20 |
2.5 |
2.5 |
|
50 |
200 |
19 |
23 |
deviation of the blood half-lives,16 and recalculating the risks expected at the presumed adult LOEL and 1/10th the presumed adult LOEL. The effect of this is to increase the estimate of the probit slope by a relatively greater amount for those effects that, in the absence of the adjustment, had relatively small amounts of interindividual variability (high probit slopes). The corresponding estimates of adult risks are increased, but generally not by a huge factor, for the adult effects expected to pose the numerically greatest risks.
Conclusions and Recommendations for Changes in Risk Assessment Practices
As indicated even by these two examples (PCBs and methylmercury), where FDA has devoted much more attention to two types of residues than to other potential chemical hazards of seafood consumption, the agency lags badly in the development of innovative methodology for assessing risks and evaluating the potential benefits of different options for control. This is all the more unfortunate because FDA has major technical resources available in both risk assessment and experimental toxicology (including reproductive toxicology) at the National Center for Toxicological Research in Arkansas. Neither the PCB nor the methylmercury analyses by FDA show evidence of input from this very capable group, which is under FDA's jurisdiction.
Carcinogenesis risk assessment procedures should be modified to give decision makers additional information about the uncertainties of the analysis, as well as both societal aggregate and individual risk estimates. In particular, to facilitate evaluation of the costs and benefits of measures to achieve quantitative reductions in exposure to carcinogenic inadvertent contaminants, procedures should be developed to supplement current "upper-confidence-limit" cancer potency estimates with estimates representing the central tendency of cancer risks, information on cancer risks from all available species, and comparative information on the pharmacokinetic and pharmacodynamic factors in different species.
The FDA is most conspicuously backward in the development of quantitative risk assessment approaches for noncarcinogens. The rule-of-thumb (ADI/NOEL/safety factor) procedure now universally in use has serious conceptual flaws, inappropriately mixes technical and social policy presumptions in analysis, and fails to encourage the development of better information on pharmacokinetics, human interindividual variability, and other topics that could allow better estimates to be made of human risk for noncancer effects.
TABLE 6-19A Projected Risks at the Presumed Adult Diet Methylmercury LOEL and at One-Tenth the Presumed Adult LOEL
TABLE 6-19B Projected Risks at the Presumed Adult Diet Methylmercury LOEL and at One-Tenth the Presumed Adult LOEL (After a Twofold Upward Adjustment of Observed Blood levels Because of Measurement Delay)
|
Effects |
Probit Slope |
Diet ED50 (µg/day) |
SDs Below ED50 at Presumed Adult LOEL (300 µg/day in diet) |
Risk (expected fraction affected) at Presumed Adult LOEL |
SDs Below ED50 at 1/10th Adult LOEL (30 µg/day in diet) |
Risk (expected fraction affected) at 1/10th Adult LOEL |
|
Adult |
||||||
|
Paresthesias |
2.067 |
3,430 |
2.19 |
0.014 |
4.26 |
1.0 × 10-5 |
|
Ataxias |
3.400 |
5,060 |
4.17 |
1.5 × 10-5 |
7.57 |
Very small |
|
Visual changes |
2.086 |
6,020 |
2.72 |
0.0033 |
4.80 |
7.8 × 10-7 |
|
Disarthria |
3.850 |
8,860 |
5.66 |
7.6 × 10-9 |
9.51 |
Very small |
|
Hearing defects |
4.670 |
11,600 |
7.42 |
Very small |
12.09 |
Very small |
|
Deaths |
5.063 |
15,000 |
8.61 |
Very small |
13.67 |
Very small |
|
Fetal |
||||||
|
Late walking |
1.195 |
1,229 |
0.73 |
0.232 |
1.93 |
0.0270 |
|
Late talking |
1.700 |
1,467 |
1.17 |
0.121 |
2.87 |
0.0020 |
|
Mental symptoms |
0.978 |
8,570 |
1.42 |
0.077 |
2.40 |
0.0082 |
|
Seizures |
1.086 |
8,390 |
1.57 |
0.058 |
2.66 |
0.0039 |
|
Neurological score >4 |
0.847 |
6,280 |
1.12 |
0.132 |
1.97 |
0.0246 |
ESTIMATING HUMAN INTAKE OF CONTAMINANTS FROM SEAFOOD AND SOME ASSOCIATED RISKS
In this section, the committee pursues a couple of different strategies for estimating exposures and, in some cases, risks for the array of other seafood hazards it was unable to investigate as deeply as PCBs and methylmercury. After available data bases for assessing exposures are reviewed briefly, national average intakes are estimated for various inorganic contaminants by using the FDA Total Diet Study and the Hall et al. (1978) data base, and making comparisons with regulatory levels for inorganics (similar comparisons are not presented for organic carcinogens, because the threshold assumptions implicit in the ADI methodology are not appropriate for the major effects of concern in these cases). After this, national average exposures for both organic and inorganic contaminants in commercial seafood are estimated tentatively from FDA surveillance data and, with the aid of EPA upper-confidence-limit estimates, the potential national cancer risk from organic carcinogenic contaminants is estimated. Finally, the section on exposure from sport, subsistence, and tribal fishing, and the section on the potential for reducing exposure through consumer information or labeling programs and fishing advisories, provide comments on the potential helpfulness of consumer and fisher advisories in reducing exposures.
Review of Data Bases Available for Estimating Exposures
FDA Total Diet Study
The FDA Total Diet Study provides a direct estimate of the dietary pesticide intake from a variety of foods including a limited number of seafood products (Gunderson, 1988). In the Total Diet Study, 234 different food items representing the diets of U.S. consumers are collected and analyzed four times each year throughout the United States. Each of four market basket samples is a composite of foods collected in three cities in a particular region (Gunderson, 1988). The foods are prepared table-ready and then analyzed for residues of industrial chemicals, pesticides, and metals such as cadmium and lead. The principal objective of the Total Diet Study is to develop dietary intake information on industrial chemicals and pesticides and to compare these intakes with acceptable daily intakes (ADIs). An ADI is the daily intake of a chemical which, if ingested over a lifetime, appears, according to FDA, to be without appreciable risk. The ADIs are established by scientific experts who have attended annual joint meetings of the United Nations Food and Agriculture Organization (FAO) and the World Health Organization (WHO) (Gunderson, 1988). The Total Diet Study could be a useful data base for determining the proportionate contributions of chemical and pesticide contamination of seafood and the contributions seafood makes to contamination, relative to other foods such as meat, poultry, dairy products, fruit, vegetables, grains, and oils. However, the FDA Total Diet Study included very few seafood samples among the 234 food items analyzed, and those items that were sampled – tuna, haddock, pollock, shrimp, and fish sticks – were among the products least likely to harbor significant chemical or pesticide contamination. Nonetheless, the pesticides detected in these products cast doubt on the ability of the
FDA regulatory monitoring program to detect all pesticides that may be in seafood products (Gunderson, 1988). For example, pesticides such as malathion, diazinon, penta, and chlorpropham detected in seafood products in the Total Diet Study were rarely, if ever, detected by the FDA regulatory monitoring program (Gunderson, 1988). Data from the Total Diet Study are presented later in this chapter.
FDA Pesticide Monitoring Files
The committee's use of FDA pesticide monitoring files is discussed at length in exposures from commercial seafood. Although there are appreciable concerns about the extent and representativeness of these data for specific species and areas of the country, overall they are the best source of information on organic contaminants in the commercial seafood market. However, information on inorganic contaminants in fish muscle is decidedly lacking, and the committee believes that FDA should consider bolstering its data base with measurements on edible portions of finfish for inorganic chemicals such as arsenic, cadmium, lead, and selenium.
The FDA seafood industrial pollutant and pesticide monitoring program has a tremendous potential to deliver important data about the contaminant levels in interstate seafood intended for human consumption. The committee acquired these files, numbering some 1,000 pages, for the years 1985-1988. They are rich in data, and fairly well organized and structured; yet they can also be quite superficial, with significant data gaps.
Although the full files for the most recent and the earliest years were not acquired by the committee, it is believed that complete files were acquired for 1986 and 1987. These files are among the most important sources of information about the concentration of industrial pollutants and pesticides in raw edible portions of finfish and shellfish intended to be sold in the nation's commercial markets. This monitoring effort stresses sampling of fish at the wholesale market level and is not generally useful for identifying the site of the catch and thus the exact source of the residues. However, the files are somewhat species specific. The program is designed primarily to satisfy FDA's legislative mandate of regulating shipments in interstate commerce. The fiscal year 1988 domestic monitoring program requires that a minimum of eight samples of locally produced fish or shellfish of commercial significance be collected per district as close to their origin as possible. Species selected are said to be those with the highest potential for residue contamination. In most cases, nonmigratory bottom feeders are the species of choice for pesticides and PCBs. Fresh fish are collected whenever possible. In addition to the formal domestic program requirements, several FDA district offices have ongoing cooperative programs in which samples from specific areas are submitted to a particular district laboratory for analysis.
The FDA monitoring program is divided into compliance and surveillance samples. The compliance samples are of food items the agency believes to be of special concern (i.e., to pose regulatory compliance problems). These include methylmercury in swordfish or the many freshwater fish that may contain excessive levels of pesticides and industrial chemicals such as PCBs and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). The surveillance samples are representative of normal interstate seafood items for which no compliance problems are expected.
However, it is important to note that data on 80-90% of the fish selected for
the committee's work were derived from FDA surveillance sampling. In only a few cases, such as tuna, swordfish, and shark, were samples listed under the compliance program. Thus, in the committee's estimation, the data should not be construed as being too heavily skewed toward the most elevated toxic concentrations. In calculations made from these data, residue levels below detection limits were considered to be zero. Because of this, in the committee's opinion, calculations based on FDA data are likely to underestimate the consumer's daily exposure to industrial chemicals, pesticides, and inorganic contaminants.
The FDA claims that the import monitoring component of this program stresses freshwater species, because strictly deep-sea species are not considered by FDA to be a significant source of pesticide residues. (However, the committee has noted that several deep-sea species such as swordfish and sablefish may indeed be sources of significant pesticide and inorganic contaminant residues.) The FDA does sample many imported saltwater species.
In fiscal year 1984, 453 samples were collected and analyzed (374 domestic and 79 imported samples), whereas in fiscal year 1985, 532 samples were analyzed (464 domestic and 68 imported). The fiscal year 1985-1986 reports appear to include a much larger number of domestic seafood samples; however these data include approximately 900 bluefish samples analyzed to fulfill the 1985-1986 congressionally mandated Atlantic Coast bluefish survey.
There are basic problems with FDA data. Among them are high detection limits for chemicals of concern, lack of monitoring for industrial pollutants such as PCBs and pesticides in interstate-trafficked bivalves, and lack of monitoring for inorganic contaminants such as arsenic, cadmium, and lead in edible portions of finfish.
National Marine Fisheries Service Survey
In 1978, the NMFS published a 300-page survey of trace elements in the fishery resource (Hall et al., 1978). This document reports trace element levels in tissues of 204 species of finfish, molluscs, and crustaceans from 198 sites around the coastal United States, including Alaska and Hawaii. Fifteen elements were determined: antimony (Sb), arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb), manganese (Mn), mercury (Hg), molybdenum (Mo), nickel (Ni), selenium (Se), silver (Ag), tin (Sn), vanadium (Va), and zinc (Zn). The species analyzed represented approximately 93% of the volume of the U.S. commercial and sport fish catch. It is an invaluable, well-conducted study.
Fisheries of the United States, 1987
The Fisheries of the United States, 1987 report (NMFS, 1988) details the amount of important commercial seafood species that were landed in 1987 by U.S. vessels and imported into the United States from other nations. The committee relied upon this report to estimate per capita consumption figures of important commercially caught seafood.
Exposures from Sport, Subsistence, and Tribal Fishing
Regional reports provided the principal available information for examining industrial chemical and pesticide concentrations in certain species of recreational/subsistence caught seafood and for estimating the intake of such species by anglers and their families. The committee relied on reports from Quincy Bay, Mass.; New York; southern California; and Puget Sound, Wash. These data, however, were not extensive enough to allow national estimates of exposure to be developed via this route.
Exposures from Commercial Seafood
Dietary Intake of Seafood of Various Species
Perhaps one of the most crucial tasks that the committee faced in determining the efficacy of present programs to protect the public health with regard to industrial chemicals and pesticides was determination of the daily dietary intake of specific commercial seafood species. There were several sources to which the committee had access to learn about the daily per capita intake of certain seafood species.
The NMFS Fisheries of the United States, 1987 report was the most important resource for estimating the intake of many individual commercial seafood species (NMFS, 1988). Unfortunately, trade associations, in general, have not conducted recent consumption surveys (Konz, 1988). The committee used several regional portraits to create consumption, daily exposure, and cancer risk scenarios for typical sport fishers.
Enough information was available for the committee to make regional and national projections of seafood consumption for both commercially sold seafood and sport harvested species. In the future, because each region has different consumption patterns with different pollution problems, a variety of data sources from specific areas will play an important role in aiding risk managers to protect particular consumers, especially regional sport fishing subgroups that may concentrate their intake on certain favored but contaminated species.
The nationwide daily per capita intake of domestic and imported seafood was estimated from landing information contained in Fisheries of the United States, 1987; landings data for each species were given in round (live) weight, that is, the weight of the catch as aquatic animals are taken from the water before any processing (NMFS, 1988). After subtracting exports of whole uneviscerated products and annual changes in inventories (when available), the edible quantity of each species was calculated with the aid of a conversion factor supplied by NMFS. Generally, these conversion figures ranged from 0.35 to 0.59% of the live round weight. Upon application of the conversion figures, exports were subtracted under the product categories of fillets, steaks, portions, and cured products (NMFS, 1988).
Shellfish landings for univalves and bivalve molluscs, such as clams, oysters, and scallops, are reported in weight of meat, excluding the shell (NMFS, 1988, p. 2). Conversion factors were, however, applied to crabs, shrimp, squid, and the miscellaneous category of other marine and freshwater shellfish.
After applying the appropriate conversion factors and subtracting exports and year-end inventory, the amount of product remaining was divided by 242 million, the
approximate U.S. population in 1987. This figure, which provided annual per capita consumption data, was then divided by 365 days to determine daily per capita consumption.
For imports, conversion factors were applied when whole or eviscerated fish were listed. Other imports were listed under fillets and steaks; canned products; balls, cakes, and puddings; and pickled or salted product; these categories, which dealt with edible portions, required no conversions. The amount imported – after the necessary conversions – was again divided by 242 million (the approximate U.S. population in 1987) and then by 365 days.
Unfortunately, the data provided for imports frequently neglected individual aquatic species. Thus, it was not possible to determine per capita consumption for certain aquatic species such as shark, swordfish, grouper, sea trout, and many others (NMFS, 1988). This is an important caveat in comparing differences between imported and U.S. seafood products, because import estimates probably underestimate the consumer's exposure to contaminants as a result of the highly generalized information provided.
The data on individual species landings, the conversion factors used, and estimates of the per capita consumption of individual categories of seafood are given in Tables 6-20 and 6-21. The resulting overall yearly per capita seafood consumption estimate, for U.S. and imported products, of about 18 pounds was a little high, but not grossly larger than the official estimate of 15.4 pounds, and well within range to offer the kind of reliability needed for a first-order appraisal of daily seafood intake patterns and exposures. (For proprietary reasons, some NMFS information that would have helped the committee further refine the per capita figures was withheld concerning year-end inventory figures underlying the data in Fisheries of the United States, 1987; obtaining a per capita number identical to the NMFS figure would be impossible without additional information). It can be seen that the leading contributors to U.S. per capita seafood consumption are cod, flounder, tuna, salmon, clams, shrimp, groundfish, and aquatic finfish species from two groupings of miscellaneous fish known as "other marine finfish" and "other freshwater finfish."
Inorganic Contaminant Exposures and Suggested Acceptable Intake
This section employs some of the best available data for estimating seafood-related exposure to inorganic contaminants and compares the indicated national average intakes via commercially marketed seafood with ADIs and related regulatory levels. This comparison provides a very broad indication of potential risk but is potentially misleading because the variability in exposures to different contaminants, and the variability of contaminant exposures from other nonseafood and noncommercial seafood sources, cannot be estimated from the available data. Therefore, the committee was unable to arrive at estimates of how many people are likely to exceed the stated ADI intake levels on either an acute or a chronic basis.
Mean levels and ranges of trace metals in the edible portions of certain fish and invertebrates may be obtained from combinations of the data base references previously cited in this chapter. Three agencies, the FDA, the Australian National Health and Medical Research Council (NHMRC), and the Canadian Health Protection Board (CHPB), have suggested allowable trace-metal levels for seafood and seafood
TABLE 6-20 U.S. Landings, Consumption/Live Weight Conversion Factors, and Annual Per Capita Consumption for Different Species
|
Species |
1987 U.S. Landings (103 lb) |
Conversion Factor (lb consumed/lb round wt) |
U.S. Average Annual Per Capita Consumption (lb)a |
|
Alewives |
20,541 |
|
|
|
Anchovies |
12,857 |
1 |
|
|
Bluefish |
15,226 |
0.43 |
0.027 |
|
Bonito |
11,420 |
0.43 |
0.020 |
|
Butterfish |
10,315 |
0.38 |
0.004 |
|
Cod |
229,711 |
0.5 |
0.305 |
|
Croaker |
11,044 |
0.5 |
0.023 |
|
Cusk |
3,064 |
0.5 |
0.006 |
|
Flounder |
199,711 |
0.45 |
0.373 |
|
Grouper |
9,494 |
0.5 |
0.002 |
|
Hake |
56,696 |
Not given |
0.117 |
|
Halibut |
76,107 |
0.45 |
0.116 |
|
Herring, sea |
0.59 |
|
|
|
Jack mackerel |
26,671 |
0.59 |
0.047 |
|
Lingcod |
7,027 |
0.5 |
0.015 |
|
Mackerel |
115,103 |
0.45 |
0.202 |
|
Mullet |
30,125 |
0.5 |
0.057 |
|
Ocean perch |
24,184 |
0.38 |
0.027 |
|
Pollock |
597,693 |
0.5 |
1.116 |
|
Rockfish |
117,881 |
0.5 |
0.244 |
|
Sablefish |
102,698 |
0.5 |
0.126 |
|
Salmon |
562,018 |
0.5 |
0.510 |
|
Scup or porgy |
14,295 |
0.38 |
0.022 |
|
Sea bass |
5,083 |
0.5 |
0.011 |
|
Sea trout |
21,871 |
0.5 |
0.045 |
|
Shark |
21,832 |
0.4 |
0.036 |
|
Snapper |
8,908 |
0.42 |
0.015 |
|
Striped bass |
431 |
0.5 |
0.001 |
|
Swordfish |
9,761 |
0.5 |
0.016 |
|
Tilefish |
7,950 |
0.5 |
0.016 |
|
Tuna |
593,444 |
0.45 |
1.104 |
|
Whiting |
34,673 |
0.45 |
0.044 |
|
Other marine finfish |
199,795 |
0.5 |
0.376 |
|
Other freshwater finfish |
117,880 |
0.5 |
0.244 |
|
Clams |
134,357 |
1.0 |
0.550 |
|
Crabs |
386,368 |
0.183 |
0.270 |
|
Lobsters |
51,313 |
0.22 |
0.044 |
|
Oysters |
39,807 |
1.0 |
0.164 |
|
Scallops |
40,773 |
1 |
0.165 |
|
Shrimp |
363,142 |
0.49 |
0.657 |
|
Squid |
82,049c |
0.6 |
0.151 |
|
Other shellfish |
89,702 |
0.4 |
0.116 |
|
Total |
4,655,826 |
|
7.362 |
|
a The numbers in this column are not in all cases equal to the product of the previous two columns because of adjustments for exports and year-to-year inventory changes. b Largely used for fish meal. c Largely used for bait. |
|||
TABLE 6-21 Volume of Imports, Consumption/Live Weight Conversion Factors, and Annual Per Capita Consumption for Different Species
|
Species |
1987 Imports (103 lb) |
Conversion Factor (lb consumed/lb landed wt) |
U.S. Average Annual Per Capita Consumption (lb) |
|
Whole or eviscerated |
|||
|
Cod, cusk, haddock, and flounder |
105,158 |
0.4875 |
0.206 |
|
Halibut |
9,295 |
0.45 |
0.017 |
|
Salmon |
41,902 |
0.5 |
0.087 |
|
Tuna |
787,133 |
0.45 |
1.464 |
|
Fillets and steaks |
|||
|
Flounder |
73,003 |
1 |
0.302 |
|
Groundfish |
315,418 |
1 |
1.303 |
|
Other |
232,564 |
1 |
0.961 |
|
Blocks and slabs |
403,577 |
1 |
1.668 |
|
Shrimp |
461,173 |
0.88a |
1.677 |
|
Crabmeat |
12,571 |
1 |
0.052 |
|
American lobster meat |
38,974 |
1 |
0.129 |
|
Spiny lobster meat |
41,949 |
1 |
0.139 |
|
Scallops (meats) |
39,934 |
1 |
0.165 |
|
Analogue products with shellfish |
30,539 |
1 |
0.126 |
|
Other fish and shellfish |
98,996 |
1 |
0.409 |
|
Canned and other products |
|||
|
Canned herring |
5,617 |
1 |
0.023 |
|
Sardines |
65,022 |
1 |
0.269 |
|
Tuna |
211,685 |
1 |
0.875 |
|
Analogue products without shellfish |
4,737 |
1 |
0.020 |
|
Other |
8,797 |
1 |
0.036 |
|
Abalone |
13,974 |
1 |
0.058 |
|
Crabmeat |
7,967 |
1 |
0.033 |
|
American lobsters |
637 |
1 |
0.003 |
|
Spiny lobsters |
136 |
1 |
0.001 |
|
Oysters |
32,668 |
1 |
0.135 |
|
Shrimp |
17,132 |
1 |
0.071 |
|
Other fish and shellfish |
57,579 |
1 |
0.238 |
|
Pickled or salted |
|||
|
Cod, haddock, hake, etc. |
31,893 |
1 |
0.132 |
|
Herring |
9,991 |
1 |
0.041 |
|
Other fish and shellfish |
16,108 |
1 |
0.067 |
|
Total |
3,176,129 |
|
10.707 |
|
a This is the result of a weighted calculation based on the importation of 310 million pounds of shrimp with the shell on, noting that consumed weight represents 80-85% of landed weight. The rest of the shrimp is assigned a conversion factor of 1. |
|||
products (Table 6-22). The FDA has set an acceptable concentration for mercury based on per capita consumption (FDA, 1982). The Australian NHMRC has recommended maximum concentrations in seafood for arsenic, cadmium, copper, lead, and zinc (Mackay et al., 1975). The Canadian government has set action levels based on the contaminant level of edible weight for arsenic, lead, and mercury (DFO, 1989). Alert levels for trace metals in molluscs have been set by the National Shellfish Sanitation Program (NSSP) of the Interstate Shellfish Sanitation Commission. However, these have no public health significance and are based on mean survey levels in U.S. coastal waters approximately two decades ago (see Table 8-3 and the accompanying discussion in Chapter 8 of this report).
The United Nations Food and Agricultural Organization in conjunction with the World Health Organization (FAO/WHO, 1972) has suggested provisional tolerable weekly intake limits (PTWIs) for cadmium, mercury, and lead. In addition, there are estimated safe and adequate daily dietary intake (ESADDI) levels for all foods set by the National Research Council (NRC) of the National Academy of Sciences (NRC, 1980). These values are listed in the first two columns of Table 6-23. In contrast to the values quoted in Table 6-22, these suggested levels apply to all foods, not just seafood.
Below are a number of comparisons of possible intake rates with these numbers:
-
The last column of Table 6-23 shows the committee's estimate of what the average daily intake of each element would be if a consumer were to eat 15 pounds of seafood per year at the weighted average concentration observed in a massive (more than 200,000 measurements) stratified survey of the U.S. marine fishery. In analyzing these data, the committee used the distribution of species-average concentrations in the NMFS survey, weighted by the percentage of the U.S. catch for each species intended for human consumption, according to the dietary patterns prevailing in 1970. (These detailed distributions are given in the discussion of individual elements following Table 6-23.) This analysis neglects freshwater species and imported seafood, but it is perhaps not an unreasonable set of provisional estimates of long-term average consumption. In comparing these numbers to the acceptable daily intake estimates and other standards, the reader should bear in mind three types of population variability that will cause some people to be at greater risk than average: (1) many people consume more seafood than the U.S. average intake of about 15 pounds per year; (2) many people may consume more of the species with higher average levels of a particular contaminant; and (3) in general, some levels of the toxicant in question can be expected to be contributed by other components of the diet (see fourth column in Table 6-23). The magnitude of these variabilities, in the context of the dynamics with which different kinds of damage may be produced by specific toxicants, will determine how many people may actually be at risk of significant harm from individual agents.
-
The fourth column of Table 6-23 gives FDA estimates of overall dietary exposure to specific elements from all foods, inferred from its market basket surveys (for the adolescent male consumer).
-
Finally, although most of the hazards from these inorganic elements are chronic in nature, one can get a feel for potential short-term dosage from shellfish by multiplying the ranges of contaminant levels found in different surveys by an assumed 250-g intake at a single sitting. These findings are discussed below in the separate
TABLE 6-22 Suggested Trace Metal Action Levels for Seafood and Seafood Products [µg/g (ppm)]
|
Metal |
FDA |
ANHMRCa |
CHPBb |
|
Arsenicc |
- |
1.53 |
3.5 |
|
Cadmium |
- |
2.0 |
- |
|
Cooper |
- |
30.0 |
- |
|
Lead |
- |
2.0 |
0.5 |
|
Mercury |
1.0 |
- |
0.5 |
|
Zinc |
- |
1,000.0 |
- |
|
a Australian National Health and Medical Research Council. b Canadian Health Protection Board. c As the trioxide. |
|||
TABLE 6-23 Acceptable Daily Intake Limits of Trace Metals as Suggested by FAO/WHO (PTDI) and NRC-NAS (ESADDI) Compared with Mean U.S. Daily Intake of Trace Metals Reported by FDA Total Diet Studies (µg/day)
|
Metal |
FAO/WHO PTDIa |
NRC-NAS ESADDI |
FDAb Mean Daily Intake |
Mean Daily Intake from 15-lb/yr U.S. Catchc |
|
Antimonyd |
|
|
15 |
|
|
Arsenic |
182e |
- |
45 |
82 (43.6)f |
|
Cadmium |
57-72 |
- |
15 |
2.0 |
|
Chromium |
- |
50-200 |
- |
3.7 |
|
Copper |
- |
2,000-3,000 |
- |
51 |
|
Lead |
429 |
- |
41 |
10i |
|
Mercuryg |
33-43 |
- |
3.9 |
2.1 |
|
Nickel |
|
|
|
5.6 |
|
Selenium |
- |
50-200 |
152h |
14 |
|
Zinc |
15,000 |
- |
17,700h |
|
|
a Calculated from the PTWI; 70-kg human. b Total Diet Studies for 25-30-year-old males (FDA market basket program); Gunderson et al. (1988) except as otherwise noted. c Calculated from data in Hall et al. (1978); for the breakdown of average levels for species, see Tables 6-10-6-18, which detail the distributions of species-average concentrations. d EPA RfD = 4 × 10-4 mg/kg/day = 28 µg/day. e Arsenic trioxide; not agreed upon but estimated. f Data in parentheses are FDA seafood measurements given in Gunderson et al. (1988). g Lowest value is methylmercury, highest total mercury. h From the older total diet data of Gartrell et al. (1985). i A study coauthor believes this result may be too high because of contamination. |
||||
-
subsections for each element. The data base used for this was Cappuzo et al. (1987) for the northeastern coastal region (review 1972-1983, 5 species); Landolt et al. (1985) for Puget Sound (review 1982-1985, 4 species, n = ?); Landolt et al. (1987) for Puget Sound (1985-1986, 11 species, n = 64); Murphy (1988a-c) for Chesapeake Bay (bluefish, n = 71; striper, n = 35). Chromium is mentioned only because oral intake of amounts as small as 50 µg of potassium dichromate may exacerbate existing chromium hypersensitivity, and reported levels in finfish and squid would fulfill that criterion (Haines and Nieboer, 1988) if they were in the hexavalent form (which is probably not likely).
Use of NMFS Survey of Trace-Element Data
Available data from the NMFS survey of trace elements (Hall et al., 1978) are now examined in relation to these advisory levels.
Antimony
The committee was surprised to find that the average U.S. consumption of antimony from seafood indicated by the Hall et al. (1978) data base (Table 6-24) is within a factor of two of EPA's reference dose (RfD) value intended to protect against systemic toxicity (EPA, 1986b). It is unclear what forms of antimony are present in seafood or whether those forms have toxicity similar to the forms that gave rise to the RfD. Antimony is below arsenic on the periodic table, and antimony dust appears to be carcinogenic for the lung when tested by inhalation (Groth et al., 1986). The committee is not aware of any long-term testing by oral routes. According to the abstract of an EPA document, increases in spontaneous abortions and premature births have been reported for pregnant female workers exposed to antimony (EPA, 1981). Further investigation of antimony's potential for hazard via seafood seems warranted.
Arsenic
As can be seen in Table 6-23, the committee's calculation of the average daily intake of arsenic from seafood from the data of Hall et al. (1978) (detailed in Table 6-25) exceeded somewhat the estimated total dietary intake of arsenic from the FDA's market basket survey program. Clearly, seafood is an important source of overall arsenic exposure in the diet, although as mentioned earlier, the organic forms of arsenic that appear to predominate in the seafood species studied in detail are considerably less hazardous than inorganic forms of arsenic.
Preliminary examination of muscle levels in fish from the areas of interest from other data bases indicates a range of 0.25-20.7 ppm in finfish and up to 15.9 ppm in squid. The estimate of intake in micrograms for a 250-g serving ranges from 62.5 to 5,157. In contrasting this with the estimated FAO PTDI of 182 µg (calculated as the trioxide to be 2 µg/kg/day for a 69.1-kg human), the highest possible acute daily value exceeds the PTDI by 4,975 µg, roughly a factor of 25. No ESADDI is given by the NRC.
TABLE 6-24 Distribution of Species-Average Antimony Concentrations, Weighted by the Percentage Contribution of Each Species to 1970 Human Consumption
|
Range (ppm) for Species Average Consumption |
Midpoint (ppm) of Range |
Percentage of U.S. Catch Intended for Human Consumption |
|
Finfish, muscle |
||
|
0.5-0.6 |
0.55 |
3.6 |
|
0.6-0.7 |
0.65 |
17.3 |
|
0.7-0.8 |
0.75 |
14.9 |
|
0.8-0.9 |
0.85 |
26 |
|
0.9-1.0 |
0.95 |
1.7 |
|
1.0-2.0 |
1.5 |
0.5 |
|
All finfish |
0.764 |
64 |
|
Molluscs, edible tissues |
||
|
<0.1 |
0.05 |
3.4 |
|
0.5-0.6 |
0.55 |
0.4 |
|
0.6-0.7 |
0.65 |
0.6 |
|
0.7-0.8 |
0.75 |
0.1 |
|
0.8-0.9 |
0.85 |
1.9 |
|
0.9-1.0 |
0.95 |
1.1 |
|
1.0-2.0 |
1.5 |
0 |
|
All, average |
0.469 |
7.5 |
|
Crustaceans, edible tissues |
||
|
0.2-0.3 |
0.25 |
0.1 |
|
0.8-0.9 |
0.85 |
8.7 |
|
0.9-1.0 |
0.95 |
11.7 |
|
1.0-2.0 |
1.50 |
7.8 |
|
All crustaceans |
1.07 |
28.3 |
|
Grand weighted average (ppm) |
0.83 |
99.8a |
|
Daily dose for 15 lb/yr (µ/day) |
15.4 |
|
|
a Total is not 100% because of rounding error and because some species were excluded from the study. SOURCE: Hall et al. (1978). |
||
TABLE 6-25 Distribution of Species-Average Arsenic Concentrations, Weighted by the Percentage Contribution of Each Species to 1970 Human Consumption
|
Range (ppm) for Species Average Consumption |
Midpoint (ppm) of Range |
Percentage of U.S. Catch Intended for Human Consumption |
|
Finfish, muscle |
||
|
0.6-0.7 |
0.65 |
0.2 |
|
1.0-2.0 |
1.5 |
2.6 |
|
2.0-3.0 |
2.5 |
35 |
|
3.0-4.0 |
3.5 |
13.8 |
|
4.0-5.0 |
4.5 |
3 |
|
5.0-6.0 |
5.5 |
2.8 |
|
6.0-7.0 |
6.5 |
0.3 |
|
7.0-8.0 |
7.5 |
5 |
|
8.0-9.0 |
8.5 |
0.1 |
|
9.0-10.0 |
9.5 |
0.2 |
|
10.0-20.0 |
15 |
0.7 |
|
20.0-30.0 |
25 |
0.2 |
|
All finfish |
3.54 |
63.9 |
|
Molluscs, edible tissues |
||
|
2.0-3.0 |
2.5 |
4 |
|
3.0-4.0 |
3.5 |
3.2 |
|
4.0-5.0 |
4.5 |
0.1 |
|
10.0-20.0 |
15 |
0.1 |
|
All, average |
3.13 |
7.4 |
|
Crustaceans, edible tissues |
||
|
3.0-4.0 |
3.5 |
1.8 |
|
4.0-5.0 |
4.5 |
14.2 |
|
5.0-6.0 |
5.5 |
2.5 |
|
6.0-7.0 |
6.5 |
5.8 |
|
9.0-10.0 |
9.5 |
2 |
|
10.0-20.0 |
15.0 |
0.5 |
|
20.0-30.0 |
25.0 |
1.5 |
|
All crustaceans |
6.56 |
28.3 |
|
Grand weighted average (ppm) |
4.37 |
99.6 |
|
Daily dose for 15 lb/yr (µg/day) |
81.5 |
|
|
SOURCE: Hall et al. (1978). |
||
TABLE 6-26 Distribution of Species-Average Cadmium Concentrations, Weighted by the Percentage Contribution of Each Species to 1970 Human Consumption
|
Range (ppm) for Species Average Consumption |
Midpoint (ppm) of Range |
Percentage of U.S. Catch Intended for Human Consumption |
|
Finfish, muscle |
||
|
<0.1 |
0.05 |
63.8 |
|
0.1-0.2 |
0.15 |
0.1 |
|
All finfish |
0.050 |
63.9 |
|
Molluscs, edible tissues |
||
|
0.1-0.2 |
0.15 |
3 |
|
0.2-0.3 |
0.25 |
1.2 |
|
0.7-0.8 |
0.75 |
0.6 |
|
0.9-1.0 |
0.95 |
2 |
|
1.0-2.0 |
1.5 |
0.6 |
|
2.0-3.0 |
2.5 |
0.1 |
|
All, average |
0.567 |
7.5 |
|
Crustaceans, edible tissues |
||
|
<0.1 |
0.05 |
9.8 |
|
0.1-0.2 |
0.15 |
17 |
|
0.2-0.3 |
0.25 |
1.5 |
|
0.3-0.4 |
0.35 |
0.1 |
|
All crustaceans |
0.12 |
28.4 |
|
Grand weighted average (ppm) |
0.11 |
99.8 |
|
Daily dose for 15 lb/yr (µg/day) |
2.0 |
|
|
SOURCE: Hall et al. (1978). |
||
Cadmium
The overall seafood-related consumption of cadmium estimated as 2 µg/day (Table 6-26) is a modest, but not completely insignificant (6%), portion of the overall dietary exposure estimated from the total diet program. Within seafood, cadmium is relatively highly concentrated in molluscan shellfish.
Muscle levels in fish and squid from the designated data base range from 0.0006 to 0.63 ppm. For a 250-g serving, intake would vary from 0.15 to 157.5 µg. The PTDI for cadmium is 57-72 µg, approximately one-half to one-third the highest calculated intake. No estimated safe daily dietary intake is suggested by the NRC.
TABLE 6-27 Distribution of Species-Average Chromium Concentrations, Weighted by the Percentage Contribution of Each Species to 1970 Human Consumption
|
Range (ppm) for Species Average Consumption |
Midpoint (ppm) of Range |
Percentage of U.S. Catch Intended for Human Consumption |
|
Finfish, muscle |
||
|
<0.1 |
0.05 |
0.3 |
|
0.1-0.2 |
0.15 |
51 |
|
0.2-0.3 |
0.250 |
12.5 |
|
All finish |
0.169 |
63.8 |
|
Molluscs, edible tissues |
||
|
0.1-0.2 |
0.15 |
0.6 |
|
0.2-0.3 |
0.25 |
0.7 |
|
0.3-0.4 |
0.35 |
6.1 |
|
All, average |
0.324 |
7.4 |
|
Crustaceans, edible tissues |
||
|
0.1-0.2 |
0.15 |
5.4 |
|
0.2-0.3 |
0.25 |
22.9 |
|
All crustaceans |
0.23 |
28.3 |
|
Grand weighted average (ppm) |
0.20 |
99.5 |
|
Daily dose for 15 lb/yr (µg/day) |
3.7 |
|
|
SOURCE: Hall et al. (1978). |
||
Chromium
The overall consumption of chromium from seafood indicated by the Hall et al. (1978) data (Table 6-27) appears well below the applicable ADI. Chromium levels in the muscles of finfish and squid for the areas of interest varied from 0.35 to 2.0 ppm. Calculated short-term intake levels are therefore 87.5-5,000 µg for a 250-g portion. The FAO has no suggested PTWI. There are no action levels reported for chromium in seafood. However, the ESADDI for chromium is 50-200 µg, which is far lower than the highest calculated intake (by a factor of 25). Again, whether a rare acute exposure of this magnitude is problematic is unclear.
Lead
If results from the Hall et al. (1978) data base (Table 6-28) are taken literally, seafood is contributing about one-eighth of the overall dietary exposure to lead for the general population. In the light of recent research indicating developmental toxicity even from levels of lead considered to be in the normal range, this would be worthy of further study. However, in this case, the committee has been cautioned by one of the coauthors of the Hall study (M. Meaburn) that the analyses were conducted under
TABLE 6-28 Distribution of Species-Average Lead Concentrations, Weighted by the Percentage Contribution of Each Species to 1970 Human Consumption
conditions that, in retrospect, did not rigorously enough exclude the possibility of postcollection contamination of samples, reagents, etc. Meaburn believes that the lead results would likely prove considerably overstated, if the analyses were redone with modern methods.
Muscle levels in finfish and squid from the designated data base range from 0.008 to 12 ppm. Calculated intake for a 250-g serving would be 2.0-3,000 µg. The higher range is certainly skewed by fish taken from Boston Harbor. A more realistic range would be from 0.008 to 2.3 ppm, resulting in an intake of 2.0-575 µg. The PTDI for lead is 429 µg. In the first instance, the ADI is exceeded by a factor of 6; in the second, by 1.3. No ESADDI is given. The normal estimated daily lead intake for children is less than 0.3 µg (Green et al., 1978).
TABLE 6-29 Distribution of Species-Average Mercury Concentrations, Weighted by the Percentage Contribution of Each Species to 1970 Human Consumption
|
Range (ppm) for Species Average Consumption |
Midpoint (ppm) of Range |
Percentage of U.S. Catch Intended for Human Consumption |
|
Finfish, muscle |
||
|
<0.1 |
0.05 |
30.7 |
|
0.1-0.2 |
0.15 |
26.1 |
|
0.2-0.3 |
0.25 |
3.9 |
|
0.3-0.4 |
0.35 |
2.2 |
|
0.4-0.5 |
0.45 |
0 |
|
0.5-0.6 |
0.55 |
0.7 |
|
0.6-0.7 |
0.65 |
0 |
|
0.7-0.8 |
0.75 |
0.1 |
|
All finfish (except swordfish)a |
0.120 |
63.7 |
|
Molluscs, edible tissues |
||
|
<0.1 |
0.05 |
7.5 |
|
Crustaceans, edible tissues |
||
|
<0.1 |
0.05 |
13.7 |
|
0.1-0.2 |
0.15 |
13.2 |
|
0.2-0.3 |
0.25 |
1.4 |
|
All crustacea |
0.107 |
28.3 |
|
Grand weighted average without swordfish (ppm) |
0.111 |
99.5 |
|
Swordfish |
0.95b |
0.107c |
|
Grand weighted average with swordfish (ppm) |
0.112 |
|
|
Daily dose for 15 lb/yr (µg/day) |
2.09 |
|
|
a According to one of the coauthors, for "policy reasons" swordfish was not included in the species sampled for the Hall et al. (1978) survey. b Average from FDA surveillance samples. c Based on total swordfish consumption of about 4 million lb/year (of about 9.7 million lb round weight landed). This represents an average of about 0.016 lb per person per year. [Landings data from NMFS (1988); consumed weight/round weight ratio taken from other NMFS data.] SOURCE: Hall et al. (1978). |
||
Mercury
The estimated population average consumption of mercury from aquatic animals of about 2.1 µg/day (Table 6-29) represents over half of the mercury estimated to be in the diet as a whole for the age and sex group (25-50-year-old males) with the highest daily mercury consumption in the FDA Total Diet Study. Aquatic organisms probably make a larger aggregate contribution to the methylated portion of total mercury (total mercury, of course, includes inorganic mercury, which has very different risks) (Gunderson, 1988). The 2.1-µg/day average is more than an order of magnitude below the official ADI of 33 µg/day (as methylmercury). As indicated earlier in this chapter, in one of its specific studies of FDA regulations the committee attempted to assess the degree of actual protection offered by this spread (and the degree of "safety" incorporated into the ADI itself) by analysis of data on the population distributions of internal dosage (as measured in blood or hair) and the interindividual variation in susceptibility to different effects in adults and developing fetuses.
Swordfish can routinely achieve even higher concentrations than the official 1-ppm guideline (which reportedly is not enforced in Massachusetts and perhaps elsewhere). The suggested PTDI for total mercury is 43 µg, which is exceeded by a factor of 6 in a serving containing 1 ppm. The FDA allowable level is 1.0 ppm and the Canadian action level is 0.5 ppm. The NRC has not suggested an estimated safe daily dietary intake level.
Selenium
The committee's overall average calculated selenium daily intake of slightly less than 14 µg/day (Table 6-30) represents somewhat less than 10% of the estimate of total dietary loading, and between 7 and 30% of NRC's ESADDI.
Selenium levels in teleosts (fish with bones) seldom exceed 1 ppm (Sorensen et al., 1984). The range of selenium levels from this data base was 0.0-0.49 ppm and came from determinations in Puget Sound animals only. Selenium is very much a regional, freshwater problem (most notably in a wildlife refuge that receives extensive irrigation tile drainage in California), and the chosen data base for this preliminary report does not reflect the apparent regional risk. Calculated intake levels are therefore somewhat lower than found in problem areas and range from 0.0 to 122.5 µg. This is less than the high range of the NRC ESADDI of 50-200 μg. When the data base includes site-specific studies, values for skeletal muscle may vary from 0.5 to 12.9 ppm (Cumbie and Van Horn, 1978; Sorensen et al., 1984). Calculated values of intake using this data base are 125-3,225 µg, the highest value exceeding the PTDI by a factor of 15. The Fish and Wildlife Service (F&WS) National Pesticide Monitoring Program (1980-1981) also reports higher values for selenium, which range from 0.09 to 2.47 ppm; however, these values are for the whole fish and not specifically for skeletal muscle. Thus, their use in assessing risk is devalued. The FAO/WHO Joint Committee has not suggested a PTWI for selenium. The toxic chronic dose of selenium for man is estimated as 2,400 to 3,000 µg/day (Wilber, 1983).
TABLE 6-30 Distribution of Species-Average Selenium Concentrations, Weighted by the Percentage Contribution of Each Species to 1970 Human Consumption
|
Range (ppm) for Species Average Consumption |
Midpoint (ppm) of Range |
Percentage of U.S. Catch Intended for Human Consumption |
|
Finfish, muscle |
||
|
0.1-0.2 |
0.15 |
0.5 |
|
0.3-0.4 |
0.35 |
2.3 |
|
0.4-0.5 |
0.45 |
21.2 |
|
0.5-0.6 |
0.55 |
8.3 |
|
0.6-0.7 |
0.65 |
4.9 |
|
0.7-0.8 |
0.75 |
2.1 |
|
0.8-0.9 |
0.85 |
5.4 |
|
0.9-1.0 |
0.95 |
1.5 |
|
1.0-2.0 |
1.50 |
17.7 |
|
All finfish |
0.819 |
63.9 |
|
Molluscs, edible tissues |
||
|
0.3-0.4 |
0.35 |
3.2 |
|
0.4-0.5 |
0.45 |
0.8 |
|
0.5-0.6 |
0.55 |
0.7 |
|
0.7-0.8 |
0.75 |
2.3 |
|
0.8-0.9 |
0.85 |
0.5 |
|
All, average |
0.54 |
7.5 |
|
Crustaceans, edible tissues |
||
|
0.2-0.3 |
0.25 |
0.4 |
|
0.3-0.4 |
0.35 |
5.1 |
|
0.4-0.5 |
0.45 |
2.1 |
|
0.5-0.6 |
0.55 |
1.1 |
|
0.6-0.7 |
0.65 |
11.9 |
|
0.7-0.8 |
0.75 |
6.2 |
|
0.8-0.9 |
0.85 |
1.4 |
|
1.0-2.0 |
1.50 |
0.1 |
|
All crustacea |
0.61 |
28.3 |
|
Grand weighted average (ppm) |
0.737 |
99.7 |
|
Daily dose for 15 lb/yr (µg/day) |
13.75 |
|
|
SOURCE: Hall et al. (1978). |
||
Estimates Of Cancer Risks From Organic Contaminants
Once the committee established per capita consumption patterns for a variety of seafood species as outlined earlier (Tables 6-20 and 6-21), average concentrations of contaminants calculated from the available FDA surveillance and compliance data were used to estimate the average amounts of industrial organic chemicals, pesticides, and selected inorganics delivered to U.S. consumers via commercially marketed seafood.
As discussed earlier, these FDA surveillance and compliance data have quite a few weaknesses. Improvements in monitoring can be made by the responsible agencies. Yet, despite these weaknesses, the committee believes that the data provide a realistic picture of national aggregate organic chemical exposure from commercially marketed seafood. These are the only data that enable even a preliminary estimate of national aggregate cancer risks from organic carcinogens.
Among the weaknesses in the FDA data are the following:
-
The detection limits used by FDA regulatory laboratories result in a generally gross underestimate of actual contaminant concentrations in seafood. For example, fish shown by other studies to be contaminated with low-level PCBs appeared to have no contamination in the FDA data set. This is true of California species such as bonito, mackerel, squid, and white croaker, which probably do have consistently low levels of PCBs according to studies performed by the Southern California Coastal Water Research Organization (Gossett et al., 1982). The committee believes that because of their frequency in the aquatic food chain and in some areas of the seafood supply, PCBs constitute an important contaminant even at levels below the present regulatory detection limits. Many contaminants probably go undetected by the FDA regulatory monitoring program because the laboratory detection limits used for enforcement of regulations have levels 5-10 times higher than the limits used in the Total Diet Study analyses (Gunderson, 1988, pp. 1200-1209).
-
There are significant problems of misidentifying some fish species. The FDA lists the Great Lakes chub under whitefish; yet according to the 1988 Fish List (FDA, 1988), chub and whitefish are not the same species. This has added a great deal of confusion to the FDA data base.
-
There is a decided lack of precise identification of species. In the FDA data base, there is only one classification of "mullet." In fact, however, 11 different species of mullet may be sold for food in interstate commerce. It would be helpful to know the precise species being sampled by FDA. In that way, it could be determined whether certain species of mullet have greater contamination than others. Another example is FDA's sampling of "croaker." There are more than 20 species of croaker that are sold for food fish according to the 1988 Fish List (FDA, 1988); yet it is unclear which croakers are actually being sampled and from which of the nation's coastlines. Because contaminant levels among different croaker species may vary dramatically, the committee believes that such information would prove extremely helpful in refining the understanding of which fish species, in particular, contribute the highest dietary exposure to toxic chemicals.
-
There is also a tremendous need for precise geographic information. The FDA should determine where each sample has been caught.
-
Because of the limited number of samples, FDA data are clearly inadequate and present absolutely no meaningful overall information on contamination of many species. Some aquatic organisms are sampled sporadically.
-
Many aquatic species are never analyzed for mercury concentrations.
-
Many finfish are never analyzed for inorganic contaminants, such as arsenic, cadmium, lead, and selenium, in edible portions.
-
Very few shellfish are analyzed for organic chemical contamination or for other contaminants of concern such as organotins and polycyclic aromatic hydrocarbons (PAHs) even though it is known that bivalves may well be contaminated with such chemicals.
-
It is uncertain for which pollutants FDA routinely tests, and at what laboratory detection limits. For example, the FDA Total Diet Study was able to detect pesticides such as chlorpropham, dacthal, diazinon and malathion on 45 occasions when analyzing 48 composite samples of seafood dishes such as cod and handdock fillets, canned tuna, and shrimp–pesticides that the FDA industrial chemical and pesticide monitoring program rarely, if ever, detected among these seafood items in some five years of sampling (Gunderson, 1988). This may occur because these pesticides are applied to the food product after harvest and during processing, because the analytical methods in use are not designed to detect these particular chemicals, or because the laboratory detection limits in the Total Diet Study have quantitation levels that are 5-10 times lower than the limits used in FDA enforcement of regulatory limits (Gunderson, 1988). In any event, FDA regulatory data appear to underestimate consumers' exposure to a variety of organic and inorganic contaminants including pesticides, metals, and PAHs.
The disparity in sampling detection limits between the Total Diet Study and regular FDA seafood monitoring programs raises serious doubts as to whether the FDA sampling program is accurately estimating the actual concentrations of industrial chemicals and pesticides in the seafood supply. Indeed, the FDA laboratories' relatively high detection levels–particularly for PCBs and dieldrin, which can occur in seafood at levels below the current detection limits–lead to so-called nondetected zero values when the PCB concentration might actually be several parts per billion. Because of the relatively high carcinogenic potency of dieldrin and PCBs, and their frequency in the seafood supply, findings of several parts per billion in widely consumed aquatic species might add appreciably to overall exposure and risk.
Nevertheless, FDA data can be used to provide a rough estimate of daily dietary exposure and at least a highly tentative indication of potential risk. Table 6-31 presents the committee's estimates of U.S. daily dietary exposure to selected organic and inorganic chemicals in terms of milligrams per kilogram of body weight per day, the form that is best suited to calculation of upper-confidence-limit cancer risks. These calculations assume a standard body weight of 70 kg. Table 6-31 also provides EPA upper-confidence-limit cancer potency estimates (where they exist) and the indicated upper-confidence-limit estimates of national aggregate lifetime cancer risk based on the committee's estimation of daily exposure to carcinogenic organic chemicals.17
TABLE 6-31 Dietary Exposures Estimated from Selected FDA Surveillance Data, 1984-1988
Using such calculations to determine the nationwide daily per capita intake of seafood species may well tend to obscure individual risks associated with industrial chemicals. Certain consumer subgroups probably favor certain seafood over others, whether their reasons be cultural, taste, health, or simply economic. Thus, any one individual or group of individuals may have a handful of favorite seafood items that are eaten repeatedly, which means that exposure is not spread evenly throughout the population but tends to be concentrated among certain subgroups. Based on landings data, a significant amount of freshwater fish, classified under the NMFS data as "other freshwater finfish," is being eaten; consumers of freshwater finfish probably are not spread out among the approximately 242 million citizens. Also, this category contains many of the most contaminated finfish.
It can be seen that the overall estimated cancer risk is dominated by the (highly uncertain) estimate for PCBs, dieldrin, DDT, and dioxin. Nevertheless, the overall risk as assessed here is clearly not negligible, if these cancer potency estimates are at all close to the mark, and there is no way to know at present that they are not.
As for metals, the set of exposure estimates in the second part of Table 6-31 can be usefully compared with estimates made from the much more extensive and statistically representative Hall et al. (1978) data set (for the U.S. catch) in Tables 6-23 through 6-30. In the case of mercury, the two data sets provide essentially identical estimates (2.5 and 2.2 µg/day). For arsenic and cadmium, the estimates from the FDA surveillance data are lower than the Hall et al. (1978) data set but are generally within twofold. This is probably because of the lack of monitoring data for these inorganic contaminants in the edible portions of finfish. For chromium, lead, and especially selenium, there are larger differences, with the FDA data set in all cases providing the lower estimates. It is quite possible that the paucity of coverage of some important species in the FDA data set, the probably higher detection limits, and the committee's practice of assigning zero contaminant values to both samples below detection limits and species that were not sampled for a particular contaminant, lead to a downward biasing of the exposure estimates from the FDA data. It would therefore not be surprising to find that some of the estimates of organic contaminants in Table 6-31 may have a similar downward bias.
Based on the FDA data set, the six U.S. commercially caught seafood products that appear to present the greatest daily per capita aggregate PCB exposures and their upper-confidence-limit cancer risks are other freshwater finfish (5.4 × 10-6), bluefish (6.3 × 10-7), mackerel (2.5 × 10-7), and sea trout, mullet, and scup or porgy (each 1.1 × 10-7). The reader should be cautioned that these data are not adequate to fully characterize PCB levels for individual species in a nationally representative way. Nevertheless, the result that probably does have some significance is that the bulk of the PCB risk is concentrated in a relatively obscure category of "other freshwater fish." This group includes fishery products from the Great Lakes and other inland waterways. To the extent that PCBs are considered to be a problem, the bulk of contamination comes from a minor, identifiable fraction of the overall seafood in commerce.
The U.S. commercially caught seafood species presenting the greatest daily per capita dieldrin exposures and upper-confidence-limit cancer risks to the general population are other freshwater fish (4.7 × 10-7), and mullet (1.0 × 10-8).
Comparison of Imported and Domestic Seafood
This analysis is also based on FDA monitoring data. Information on individual imported fish is statistically insignificant, but when taken as a whole the FDA monitoring program appears to suggest some relevant differences in terms of contamination of the domestic supply versus that of the imported supply. Imported seafood, overall, appears less contaminated than domestically landed seafood. The committee believes that this overall difference is largely the result of contamination of U.S. freshwater finfish products. If freshwater finfish were not part of the U.S. seafood supply, U.S. saltwater fish probably would not differ much from imported fish. This could be established with greater precision by a more directed and well-designed sampling effort. Such an effort would be valuable in protecting the consumer and more cost effective than a visual inspection program. In fact, the hypothesis that significant differences in contamination exist between species of fish, as well as between domestically landed and imported seafood, offers a rationale to help consumers reduce toxic exposures by accurately identifying species and the geographic origin of seafood products offered for sale. This could aid consumers in choosing the least contaminated products.
Overall, in only a few cases of contaminated finfish, did imports provide a greater exposure for the consumer than the domestic catch. The committee realizes, however, that this observation may be due to the fact that import consumption patterns were not narrowed down to individual species, that many imported species were not always identified, and that many were not analyzed.
Those cases in which imports had higher contaminant levels included chemicals such as lindane, tecnazene, cadmium, and mercury.
As for cadmium, canned tuna accounts for much of the consumer's exposure. Other, lesser contributors of cadmium include imported scallops. For mercury, the biggest contributors are shark, swordfish, and tuna. An estimation of the total mercury exposure from these imports could not be determined because NMFS import data do not detail specific consumption figures for imported shark and swordfish.
Among imports, salmon and herring accounted for primary sources of PCBs in the diet. Yet, only four samples of herring were taken by the FDA monitoring program to be sampled for organic contamination between 1983 and 1987. This lack of data for individual species is disconcerting, especially when there are definite warning signals indicating that more data gathering would be warranted. The PCB concentration in imported sea herring ranged from nondetectable to 0.36 ppm with three positive detections out of the four samples – a significant positive percentage and range. Furthermore, 11 different industrial pollutants and pesticides were found in these four imported sea herring samples, and although the levels were below FDA action guidelines, these four samples with 27 individual industrial chemicals and pesticide residues warrant further sampling.
Approximately 12 samples of imported salmon were analyzed for organic contaminants between 1986 and 1988. Two of the nine samples from Norway were positive for PCBs, tecnazene, and lindane. Because so little is known about noncancer long-term health consequences of PCBs and other toxic exposures, it seems prudent to reduce PCB exposure in any way possible, and with farm-raised products, the reduction of PCB and other organic chemical contamination should be possible.
Import data are virtually comprised entirely of finfish samples. Very few
samples of the imported seafood examined consisted of shellfish. There simply is not enough information available for the committee or anyone to determine the extent of contamination of imported shellfish.
Based on the data available, the biggest single potential risk to consumers in seafood is posed by exposure to PCBs. Yet, whereas the upper-confidence-limit risk per capita from PCBs alone (not counting DDT, dieldrin, and dioxin) is 5.8 × 10-5 for domestically landed finfish, the risk from imports appears significantly lower, slightly less than 3 × 10-6. For the next highest cancer risk chemical, dieldrin, the U.S. catch typically provides a risk to the average consumer of approximately 1 × 10-5. The risk per capita from dieldrin in imported fish also appears much smaller, less than 1 × 10-6. These findings should not be overgeneralized nor, based on present knowledge, can one assume that imported seafood is safer than domestically landed seafood.
Exposures from Sport, Subsistence, and Tribal Fishing
Noncommercial fishing is a significant source of overall fish for human consumption in the United States – estimated at 3-4 pounds per person on the average. It is even more significant as a vehicle for the delivery of bioaccumulating chemicals for the following reasons:
-
Noncommercial seafood harvesters often concentrate their fish/shellfish harvesting within specific areas, some of which may be heavily contaminated.
-
A minority of recreational and subsistence seafood harvesters engage in this activity very frequently and consume relatively large amounts of fish (Humphrey, 1983a,b).
-
Some of the most significant recreational/subsistence caught species also happen to be among those with the largest concentrations of contaminants (e.g., northeastern bluefish; southern California white croaker; Great Lakes trout, walleye, and salmon).
-
In some states (e.g., New York), relatively uncontaminated areas appear to be difficult to find (New York State DEC, 1987).
The primary mechanism by which state governments have alerted consumers about the potential risk of contaminated finfish is the fish consumption advisory (Zeitlin, 1989). In fact, among the 30 U.S. coastal and Great Lakes states, Hawaii, and Alaska (with the exception of Georgia, which did not respond to the survey), 2,094 advisories were issued for coastal marine water, estuaries, rivers, and inland waters. Overall, the vast majority of the advisories (87%) were issued for freshwater fish. Among the states indicated, Alaska, Alabama, New Hampshire, and Oregon each issued one advisory between 1984 and 1987. On the other hand, Minnesota issued 665. As Zeitlin (1989) notes
Consistently high numbers of advisories were issued by the Great Lakes states. Even if advisories are posted to warn anglers, some persons may not heed these warnings because they do not get sick immediately from the consumption of contaminated seafood. There is no way of knowing how effective they are.
The effectiveness of advisories in convincing the general public to voluntarily
alter their preparation and consumption habits is, for the most part, unknown. Very few epidemiological or social studies have been conducted. The inherent difficulty in changing pleasurable human behavior is not a novel concept and public perception of risk is often difficult to measure. Adherence to hazard warnings often depends on how much confidence the public has in the credibility of the agency issuing the warnings.
Thus, anglers may be constantly re-exposed to contaminants. Further, many of the nation's most contaminated areas are relatively accessible to the recreational seafood gatherer (e.g., estuarine regions and freshwater bodies). In addition, many "recreational" anglers may actually be subsistence anglers who fish as an important means of supplementing their diet, and they may share their catch with family members and friends.
According to Zeitlin, methods used to communicate potential risks include media announcements, printed brochures, posting notices in public places, and information in fishing license applications. Members of the medical community were contacted to disseminate information to patients in New York, Maine, Michigan, Minnesota, and Wisconsin. Indiana, New York, New Jersey, Michigan, Minnesota, Washington, and South Carolina have organized public outreach and education programs. Finally, contamination of the nation's recreational fishery appears to be pervasive, and although extensively documented as well as possible to date, the contamination is probably not very thoroughly documented – particularly for freshwater fishing.
Examination indicated that PCBs accounted for 43% of all advisories issued. Mercury accounted for 40%, followed by chlordane and dioxin at 8% and 2%, respectively. The remaining 7% was issued for chemicals such as dieldrin, kepone, DDT, PAHs, heptachlor epoxide, petroleum compounds, selenium, and chlorinated benzenes (Zeitlin, 1989).
Advisories issued according to families of freshwater fish were Percidae, including yellow perch and walleye, 21%; Salmonidae, 16% (trout, 11%; salmon, 5%); Centrachidae (sunfish and bass), 13%; Esocidae (pike), 12%; and Cyprinidae (carp), 11%. The remaining advisories were divided among Ictularidae (bullhead catfish), 10%; Catostomiae (suckers), 5%; Anguillidae (freshwater eels), 4%; striped bass and white perch, 4%; and other species, 4%. Based on regional case studies, recreational anglers probably eat far more seafood than nonrecreational seafood consumers. In the Puget Sound study, average daily consumption was 12.3 g (Landolt et al., 1987). The highest consumption rate for a smaller number of fishermen and women was estimated to be 95.1 g/day. These amounts are somewhat similar to findings for Los Angeles area recreational anglers.
Puget Sound Contaminated Recreational Fish Study
During 1986-1987 a broad-scale survey was sponsored by EPA Region X and supported by the Washington State Department of Ecology and the Department of Social and Health Services (DSHS) to characterize potential human health risks associated with chemical contaminants in Puget Sound seafood (Landolt et al., 1987). Research focused entirely on recreational harvesters who frequently collect fish, shellfish, or edible seaweed for use in personal consumption. Data were available for chemical contaminants in fish, shellfish, and macroalgae from 22 locations in Puget Sound. Fish, shellfish, and macroalgae evaluated in the risk assessments of chemically
contaminated seafood included pelagic species such as coho salmon, chinook salmon, Pacific hake, striped perch, rockfish, and sablefish; bottom-feeding species such as Pacific cod, walleye, pollock, and tomcod; bottom fish such as English sole, starry flounder, Pacific sanddab, rock sole, flathead sole, and buffalo sculpin; shellfish such as Dungeness crab and rock crab; bivalves including heart cockles, bent nose clams, sand clams, soft-shell clams, littleneck clams, butter clams, Manila clams, and horseneck clams; also examined were market squid, brown algae (kelp), green algae (also known as sea lettuce), and red algae (also known as nori). Thus, the study was both very thorough and very complete.
The chemicals of highest concern included carcinogens such as arsenic, PAHs including benz[a]anthracene, benzo[a]pyrene, benzo[b]fluoranthene, and chrysene; PCBs, alpha-hexachlorocyclohexane (HCH); and DDTs. Noncarcinogenic chemicals of concern included cadmium, lead, and mercury. Interestingly, FDA tolerances or action levels existed for only three of the eight chemicals of highest concern: mercury, PCBs, and DDTs. Overall, the average concentrations of these substances in the various species and categories of seafood were lower than the FDA tolerances or action levels for all locations in Puget Sound, with the notable exception of the concentration of PCBs (2.06 ppm) in English sole from Elliott Bay.
The average consumption rate for Puget Sound recreational anglers was 12.3 g daily. The highest consumption rate for a smaller number of anglers was estimated to be 95.1 g of fish per day from Puget Sound. In this study, upper-confidence-limit cancer risks for cumulative exposure to arsenic and the four organic chemicals of concern ranged from 2 × 10-4 for average anglers to 4 × 10-3 for those most highly exposed. The relatively high, assessed upper-confidence-limit cancer risk derived primarily from potential exposure to PCBs in fish from specific locations in Puget Sound such as Commencement Bay, Elliott Bay, Manchester and Sinclair Inlet. If the contribution of PCBs is excluded, the remaining risk is less than 10-5. No effort was made in this study to quantitatively evaluate the potential for noncancer risks.
It is also quite apparent from this study that recreational anglers have favorite spots and that this is important information for the distribution of risks among the community. As Landolt et al. (1987) note, "the potential cumulative health risks associated with the consumption of chemically contaminated seafood may exceed [one in 10,000 cancers in excess18] for certain locations in Puget Sound."
Regional Studies of Contaminants
Regional studies conducted in New York, Massachusetts, the District of Columbia, Alabama, Kansas, California, and Wisconsin indicate levels of inorganic and organic chemicals in recreationally important fish species (EPA, 1983, 1987; Kansas, 1988; New York State DEC, 1987; Rosen, 1989).
Using data generated by these regional studies, one can tentatively estimate upper-confidence-limit cancer risks that might be attributable to dioxin contamination of fish in selected areas. Given an average-size serving (based on recommendations of the Tolerance Assessment System in the EPA Office of Pesticide Programs) of approximately 114 g (0.25 pound) (PTI, 1987), if one were to eat a one-quarter pound serving of large-mouth bass with an average of 11.3 parts per trillion dioxin once a month for a lifetime, the upper-confidence-limit cancer risk implied by the EPA cancer potency factor would be 1 × 10-4. Therefore, cases may exist in which contamination
with dioxin could be a potentially serious hazard for recreational or subsistence anglers.19
As suggested earlier, another potential problem residue is methylmercury. Michigan's 1989 fish consumption advisory for anglers suggested that consumption of certain fish from inland lakes statewide be restricted because of mercury found in fish from Upper and Lower Peninsula lakes; approximately 60 of Michigan's inland lakes were tested since 1983, which indicated that about three out of four had at least some fish with mercury exceeding the Michigan Department of Public Health (MDPH) level of concern for state citizens (Michigan, 1988).20 Pregnant women, nursing mothers, women who intend to have children, and children age 15 or under were advised to eat no more than one meal per month of rock bass, crappie, and yellow perch over 9 inches in length. They were advised not to eat any large-mouth bass, small-mouth bass, walleye, northern pike, or muskie from inland lakes. Others were advised to eat no more than one meal per week of these fish.
Atlantic Coast Bluefish Contamination
Bluefish is the principal recreational species along the Atlantic Coast, with 130-155 million pounds landed annually (NOAA, 1987). Contaminant levels appear to correlate with geographic site of origin and size (fork length).
Small- and medium-sized bluefish tend to have, with few exceptions, lower PCB concentrations than large bluefish. For small and medium bluefish, no samples exceeded 2 ppm PCBs at any of the sampling sites, and no statistically significant differences were noted among the site percentages in these classes. Large bluefish did exceed 2 ppm PCBs.
The arithmetic mean concentration of PCBs in medium-size bluefish taken in January-February 1985 from North Carolina was 0.20 ppm in males and 0.53 in females, whereas large bluefish had concentrations of 1.94 ppm in males and 1.61 ppm in females. The arithmetic mean concentration of PCBs in small bluefish taken from New York Bight in May-June 1985 was 0.13 ppm in males and 0.20 ppm in females. The arithmetic mean concentration of PCBs in medium bluefish was 0.38 ppm in males and 0.37 ppm in females. The arithmetic mean concentration of PCBs in large bluefish was 1.00 ppm in males and 1.58 ppm in females.
In New England for May-June 1985, small bluefish averaged 0.20 ppm PCBs in males and 0.65 ppm in females. Medium bluefish averaged 0.39 ppm in males and 0.44 ppm in females. Large bluefish averaged 1.10 ppm in males and 0.99 ppm in females. In New England for October 1985, although no small bluefish were sampled, medium bluefish averaged 0.55 ppm PCBs in males and 0.50 ppm in females. Large bluefish averaged 1.35 ppm PCBs in males and 1.11 ppm in females.
When all sampling sites were combined, the differences continued to appear significant. Small male bluefish averaged 0.15 ppm PCBs, and small females averaged 0.27 ppm. Medium male bluefish and medium female bluefish each averaged 0.42 ppm. Large male bluefish averaged 1.40 ppm, and large female bluefish averaged 1.45 ppm PCBs.
The study divided bluefish into three size groupings: Small bluefish were less than or equal to 300 mm (11.8 inches). Medium-sized bluefish ranged from 301 to 500 mm (11.8-19.7 inches). Large bluefish were more than 500 mm (19.7 inches).
Mean PCBs were compared for each fork length class to assess possible differences due to sampling site. For small bluefish, the sample PCB means did not statistically differ among sites, whereas for medium-sized bluefish, the sample PCB means formed two statistically significant site groupings. The sample mean for New England in October was 0.94 ppm, which significantly exceeded the January-February mean for North Carolina, 0.71 ppm.
However, even small bluefish may not be perfectly safe for the recreational fisher to consume constantly. The FDA tolerance of 2 ppm in fish is frequently cited as a criterion for evaluating the significance of PCB residues. However, as shown earlier, the risks associated with this level of PCB contamination could be appreciable.
Southern California Sport Fishery
A 1978 southern California sport fishing survey carried out by the California Department of Fish and Game found that 75% of the catch was composed of 20 species and that one in three fish was a white croaker, a fish known to contain significant amounts of DDT and PCBs. Another report on Los Angeles metropolitan area recreational fishers during 1980 assessed the consumption rates of potentially hazardous marine fish and shellfish by local, nonprofessional anglers; identified subgroups with a significantly large consumption rate; and estimated the size of the population potentially exposed.
The median consumption rate was found to be 37 g/day, much higher than the average fish consumption for the U.S. population as a whole (estimated at about 18.7 g/day). At the 90th percentile the average consumption rate was 225 g/day. The results of this study also demonstrated that there exists a regular fishing population along the southern California shoreline: 14% of the subjects surveyed fished three to seven times per week, even at sites likely to be contaminated by waste discharge. Furthermore, fish caught by frequent as well as infrequent fishermen are generally shared and consumed among an estimated 342,000 family members.
The fish caught are dominated by a few species, including white croaker and Pacific bonito which have been found to accumulate both PCBs and DDT. At the median, the recreational fishers surveyed consumed 14.8 g of white croaker daily and 63.6 g of bonito daily. At the 90th percentile, those surveyed consumed 85.2 g of white croaker and 334 of bonito daily.
According to analyses performed by the Southern California Coastal Water Research Project and published in 1981-1982, white croaker sampled in Los Angeles harbor in the vicinity of Cabrillo pier, a popular fishing spot, averaged 1.7 ppm DDT and 0.18 ppm PCBs. Bonito sampled in the program averaged 0.184 ppm DDT and 0.029 ppm PCBs (Gossett et al., 1982).
By using the EPA upper-confidence-limit cancer potency factors for oral administration in conjunction with median consumption rates, the potential cancer risk for consumption of white croakers from Cabrillo pier would be approximately 4 × 10-4; the cancer risk for DDT would be 1.5 × 10-4. The total upper-confidence-limit cancer risk for average consumers of white croaker would be 5 × 10-4.
The results of this study suggest that certain subpopulations, characterized by age and ethnic group, may be at higher risk, including individuals over 65 years of age, Orientals, and Samoans.
In April 1985, warnings were issued by the California Department of Health Services that advised against eating white croakers and recommended reduced consumption of other fish to no more than once a week, particularly at the most contaminated sites in the Los Angeles Harbor area and near the Whites Point sewage outfall.
Posting signs is one means for advising people of some potential health threat from eating locally gathered seafood. It is not the whole answer, but it is an honest attempt to provide information and no doubt gives some fishers pause to consider whether to change their fishing locale.
Conclusions
-
Based on the committee's tentative analysis of FDA survey data, it appears that in the aggregate, freshwater fish tend to be more contaminated than open ocean saltwater fish. Past studies have reached a similar conclusion (GAO, 1988).
-
Many freshwater sport fish have levels of contamination with toxins such as chlordane, dieldrin, and PCBs that, based on current risk assessment numbers, may present an appreciable risk.
-
The health effects of concern go well beyond cancer and include reproductive effects and possibly other chronic conditions. For example, it is known that PCBs cross the placenta in women exposed to ordinary dietary levels (Fein et al., 1984). In one study of mothers who ate contaminated lake fish, PCB exposure, determined both in contaminated fish consumption and in cord serum PCB levels, predicted lower birth weight and smaller head circumference.
-
Serum monitoring of recreational anglers who frequent contaminated areas (and appropriate controls) should be conducted to ascertain the extent of recreational exposure in comparison to people who do not consume recreationally caught fish.
-
Although some problems, such as PCB contamination, result from historically poor public pollution control policy, continuing contamination with some pollutants is ongoing-most notably pesticide, PAH, and dioxin contamination of saltwater and freshwater fish-throughout the country. An active environmental stance aimed at pollution prevention (rather than mitigation after the damage has been done) should be implemented at the federal level to prevent future pollution disasters on the level of the PCB decimation of much of the nation's freshwater fishery.
-
One of the biggest weaknesses of present seafood monitoring programs is that only a certain group of chemicals, suspected to be unsafe, is monitored. However, the number of chemicals so classified is extremely small compared with the number of chemicals being added to the environment whose long-term effects are generally unknown (GAO, 1988). One researcher stated that he found 340 chemical compounds in the Chesapeake Bay, most of which have not been assessed for safety. As a result, he believes that oysters coming even from approved harvest areas are not necessarily safe to eat because of the long-term effects of accumulating toxic chemicals in humans.
-
Sampling programs are very limited-sampling too few species, too few contaminants, and too few sites (Zeitlin, 1989).
-
Exposure data, consumption estimates, and risk assessment models differ among state agencies and from state to state (Zeitlin, 1989).
-
Guidelines and criteria supplied by the federal government are rare and sometimes contradictory (Zeitlin, 1989).
-
Although different methods have been used to disseminate information concerning advisories, the most effective methods have not been identified through controlled testing (Zeitlin, 1989). Other questions relating to risk management must be considered in the development of fish consumption advisories based on quantitative health assessments. Many of these questions were discussed at a National Wildlife Federation workshop (NWF, 1989).
Consumer Information and Labeling Programs, and Fishing Advisories
Unfortunately, as noted in the discussion of sport fishing, there appears to have been relatively little systematic study of the efficacy of state advisory programs in reducing population exposures to chemical contaminants in seafood from areas known to have higher than usual levels. Consumer information programs do, nevertheless, have features that have attracted the strong support of some members of the committee.
Advisories and other information programs represent the least coercive type of governmental intervention in the marketplace. Such programs theoretically allow the diverse set of consumers to use their individual assessment of the importance of possible risks versus costs to choose appropriate risk control policies for themselves. Moreover, advisories represent a way governmental entities can take at least some action on problems that would be difficult to attack by more rigorous measures (e.g., sport fishers may tend to resist efforts to directly limit harvesting in some areas, and enforcement of those restrictions may involve more effort and expense than public authorities can exert in some cases). Some members of the committee have advocated that retail displays of seafood be accompanied by a score based on the assessed quantitative risk of the species being offered for sale and the geographic location from which it was taken.
On the other hand, placing the burden of choice with respect to risks on the individual consumer (or individual sport fisher) may require people to devote more time and effort than they consider reasonable, in light of competing needs to evaluate other health and economic choices. Even with additional public education, there is room for doubt about how accurate consumer perceptions would be in evaluating supermarket-delivered risk information. Public agencies have a clear advantage of scale in gathering information and evaluating risks that apply to their constituencies as a whole (or to significant subsections). Public health authorities can evaluate risks much more easily than individual consumers and are in a unique position of trust to take preventive action, in light of widely shared attitudes toward risk and risk control options. A measure of the disenchantment of many consumers with agencies who hold such trust is that public demands for direct consumer information programs (such as California's Proposition 65) seem to have expanded in recent years.
PROBABLE HEALTH RISKS FROM FISH AND SHELLFISH CONSUMPTION-RECOMMENDATIONS FOR RESEARCH
Classic Acute and Chronic Toxic Effects
There seems to be little potential for important classic acute toxic effects from the types and levels of chemical contaminants in U.S. seafood. Some classic chronic effects may be of significance, however, particularly the effects of cadmium on kidney function and of lead in impairing cognitive development in early childhood. Biomarkers of lead exposure (e.g., blood and bone lead levels) should be assessed in relation to the consumption of selected seafood items to assess the potential for control and the significance of the hazard in relation to other sources of exposure to these contaminants.
Reproductive Effects
Reproductive effects constitute a seriously understudied area, in which the information available gives appreciable cause for concern. The analysis in the mercury case study discussion above indicates that low-dose developmental risks from fetal exposures to methylmercury may be appreciable. Good case control studies using hair and other biomarkers of methylmercury exposure should be pursued. More generally, markers of modestly impaired status, such as the population distribution of birth weights, should be used to assess the potential effects of other contaminants in people with unusually high in utero exposures to PCBs and related contaminants. Alternative measures of PCB concentration need to be developed that will be better indices of the potential activity of different PCB congeners in producing both reproductive and other effects.
Carcinogenesis
The committee's analyses, using conventional approaches to carcinogenic risk assessment, indicate an appreciable potential of carcinogenic risk from some freshwater locations (e.g., Lake Michigan) and species (e.g., bluefish harvested in some locations in the eastern United States) and, to a lesser extent, from general commercial seafood (Table 6-31). Assessments of this risk will benefit from further research on the mechanisms whereby some PCB and dioxin congeners enhance carcinogenesis in animal systems, and from follow-up of human populations with relatively high exposure to both seafood and nonseafood sources of different PCB mixtures.
Chronic Cumulative Toxic Effects
Studies of chronic cumulative toxic effects are in their infancy. As mentioned earlier, a recent, apparently sound case control epidemiological study among people in Singapore has found a strong association between blood levels of mercury and risk of Parkinson's disease (Ngim and Devathasan, 1989). Similar studies, using both
biomarkers of exposure of selected seafood-borne toxicants and early biomarkers of progressive damage, may ultimately prove fruitful for prevention of this increasingly important area of health damage.
CONCLUSIONS AND RECOMMENDATIONS
Significance of the Risk
Seafood generally provides an important source of protein in the diet and has lower saturated fat content than most other high-protein foods. Because of this, seafood as a whole undoubtedly makes an important positive contribution to a healthy diet. Several different chemical contaminants of seafood have the potential to pose large enough hazards to public health to warrant additional societal efforts at control. However, they are not generally of such magnitude, in the aggregate, as to be comparable to the largest environmental health hazards characterized to date (e.g., indoor exposure to radon progeny). Some examples of the risks that may be significant include reproductive effects from PCBs and methylmercury; carcinogenesis from selected congeners of PCBs, dioxins, and dibenzofurans (all of which appear to act primarily by binding to a single type of receptor); and, based on a very recent and as yet unconfirmed epidemiological study from Singapore, parkinsonism in old age from very long-term mercury exposure. Several other metallic and pesticide residues also warrant attention.
Part of the reason some aquatic animals pose particular chemical contamination problems derives from their position in the food chain. Whereas the land animals used for human food are generally vegetarians, most of the aquatic animals that contribute to our diet are themselves predators of other animals-and, in some cases, predators of predators. Because of this, there is an opportunity for substances that are both poorly metabolized and poorly excreted by living organisms to become more concentrated through several successive sets of flesh consumers. Substances that tend to "bioconcentrate" in this way include chlorinated aromatic compounds such as PCBs, DDT, and related pesticides, and some metallic compounds such as methylmercury.
When these same chemical residues are consumed by humans, they also tend to persist in the body and to build up over prolonged periods. Some PCB congeners, cadmium, and lead have biological half-lives measured in several years or decades. Because of this, changes in risks from these substances will generally be manifest only long after changes have been made in exposures.
Potential for Control
Contaminant levels in aquatic animals are distributed very unevenly. The fact that some geographic areas (e.g., fresh versus salt water), some species, and some size classes of aquatic animals have much higher residue levels than others means that important quantitative reductions can be made in individual and societal aggregate health risks with measures that would restrict the overall commercial availability of fresh and marine seafood to only a modest degree. If the available data bases are improved, there is a potential for regulatory agencies to better target their efforts and
for interested consumers to modify individual risks by altering their consumption of specific species, and of fresh and marine seafood originating in specific areas. Such targeting should include efforts not only to close or reduce harvesting of high-risk species from high-risk areas, but also to reduce the input of contaminants to the local marine environment. A strong effort should be made to develop systems for containment of waste that do not involve atmospheric or aquatic dumping. Coordination of efforts to improve the health of aquatic ecosystems with efforts to improve the safe management of seafood resources will have benefits for both types of social objectives.
Performance of Current Federal Regulatory Authorities in Assessing and Managing Risks
The overall posture of relevant federal agencies, particularly FDA, appears to be almost totally reactive. Whether due to inadequate resources, priorities implicit in the relevant enabling legislation, or an ideological disinclination to raise issues that might run counter to the prevailing trend toward deregulation, it is the committee's overall judgment that there has been less effort than would be desirable to discover and quantify hazards, to evaluate options for the reduction of risks, and to implement prudent policies that protect both the health of consumers and the stability of commercial markets.
Data Gathering for Risk and Control Analysis
With the notable exception of a data base created by Hall et al. (1978) for inorganic elements and never completely analyzed, the data bases available for quantifying human exposure to seafood-borne toxicants, setting priorities for control measures, and appropriately advising consumers of risks are grossly inadequate. Even though 90% of FDA's analytical samples are classified as having been taken for "surveillance" rather than "compliance" purposes, the data do not adequately represent national seafood consumption. Several extensively consumed species have not been analyzed at all, and others have received minimal effort. There has been virtually no monitoring by FDA for PCBs or pesticides in bivalves.
Some of the available sampling data are difficult to interpret or entirely useless because of inaccuracies in classification of the species concerned and inadequate recording of the geographic areas of harvest. Relatively high detection limits have been tolerated for some analytical tests, probably because the agency has focused primarily on determining the incidence of residues over a certain level that would violate current standards, rather than quantifying the overall dose delivered to consumers.
Risk Assessment Practices
Carcinogenesis risk assessment procedures should be modified to give decision makers additional information about the uncertainties of analysis, as well as both aggregate and individual risk estimates. In particular, to facilitate evaluation of the
costs and benefits of measures to achieve quantitative reductions in exposure to inadvertent carcinogenic contaminants, procedures should be developed to supplement current upper-confidence-limit cancer potency estimates with estimates representing the central tendency of cancer risks, incorporating information on cancer risks from all available species, as well as comparative information on the pharmacokinetic and pharmacodynamic factors in different species.
The FDA is most conspicuously backward in the development of quantitative risk assessment approaches for noncarcinogens. The rule-of-thumb (ADI/NOEL/safety factor) procedure now universally in use has serious conceptual flaws, inappropriately mixes technical and social policy presumptions in analysis, and fails to encourage the development of additional information on pharmacokinetics, human interindividual variability, and other topics that could enable better estimates of human risk for noncancer effects.
Risk Management
The relevant federal enabling legislation is the product of a long and complex history in which different criteria and authorities have been layered on top of one another. It would be desirable to direct increased agency attention to problems involving inadvertent contaminants and natural toxins. To do this, Congress should consider a fundamental restructuring of food and fisheries management legislation. Designated federal authorities should be encouraged to evaluate the opportunities to achieve feasible reductions in risk by quantitatively analyzing the benefits and costs of control opportunities, ranging from the restriction of harvesting fish and shellfish in particular areas, through restricting the species harvested, to determining the size of fish that can be brought to market. In selected rare cases where contaminants are known to concentrate in particular organs of seafood species, such as the hepatopancreas ("tomalley") of lobsters and the gonads or roe of scallops, the relevant governmental authorities should promulgate organ-specific restrictions on marketing and consumer advisories. In light of the fact that most seafood commerce is interstate, the current restriction of FDA authority to items between states appears to be an unproductive complication. One federal agency should be given overall responsibility for managing the risk from chemical residues in seafood, to ensure at least the minimum performance of state programs, while allowing the states flexibility to implement more effective programs tailored to local hazards and consumption practices.
Despite repeated requests from the states for additional guidance on appropriate residue levels, FDA's output of contaminant standards is minuscule, although the committee understands that several informal shellfish guidance documents are expected to be completed in the summer of 1990; unfortunately these documents are not yet available (P. Lombardo, FDA, personal communication, 1991). Even the tolerances that have been promulgated were based – in at least two cases (PCBs and methylmercury) – on reasoning that was questionable at the time and has been rendered obsolete by more recent scientific information. Advances in understanding the likely mechanisms involved in PCB carcinogenesis, the relative potency of different PCB congeners, the findings of subtle noncancer risks at relatively low dose levels (via fetal exposure), and data on the uneven distribution of residue levels suggest
substantial unexploited opportunities to reduce risks. Information on the fetal and chronic neurological risks of methylmercury further suggests the need to reevaluate the current tolerance in that case.
In the development of advisories for reproductive effects, due weight must be given to the persistence of different toxicants in people. For methylmercury, with an elimination half-life averaging about 70 days, it may well be sufficient to direct advice to couples who intend to have children in the near future. For PCBs on the other hand, with half-lives measured in several years or even decades, reductions in intake only in the months prior to and during pregnancy can be expected to have little impact on effective body burdens and fetal exposure. In that case, cautionary advice may need to extend to the entire population of reproductive and prereproductive ages.
NOTES
|
8. |
This group was defined as those consuming more than 24 pounds per year (average consumption was 38.5 pounds). If they indeed received an average of 1.75 µg/kg/day, then the average concentration of PCBs in their fish would have been approximately 2.6 ppm; 70-kg average body weights are assumed. |
|
9. |
Unfortunately, a number of other difficulties with the calculation as presented are not so easily quantified. For example, on the cost side, FDA appears to have used landed weights of fish subject to seizure to estimate the economic losses expected from various policies. The assumption is thus that (1) every violative fish caught and presented to market is detected, and yet (2) fishermen make no changes in the locations they fish, species they fish for, and sizes of fish they bring in, in response to the assumed strict enforcement system. The shifting of fishing resources (people, equipment) to locations and species with fewer contamination problems would tend to reduce the long-term economic cost of the tolerance reduction. |
|
10. |
Not all such studies are positive, it should be noted, and the human evidence of PCB carcinogenicity is still not regarded as definitive. |
|
11. |
Note that the uncertainty bounds on this estimate extend to values that are higher and lower than the estimate by at least 10-fold; thus, the FDA potency factor is by no means ruled out by current epidemiological results. |
|
12. |
The Z-score in Figure 6-1 is simply the number of standard deviations above or below the midpoint of a standard normal or lognormal distribution, inferred from the rank of a specific individual value in a data set. To create this type of plot, measurements are first arranged in order and given ranks i (1 through N). Then, a "percentage score" is calculated for each ordered value as 100 x (i – 0.5)/N. (This is simply the percentage of an infinite sample that would be expected to be less than or equal to the observed value. It differs from the usual definition of a "percentile" in which the highest observation is assigned a score of 100.) Finally, from tables of probits in Finney (1971) or areas under a cumulative normal distribution, one calculates the number of standard deviations above or below the median of a normal distribution that would be expected to be associated with each "percentage score," if the distribution of values were in fact normal (Gaussian). In the regression line calculated from this type of plot, the intercept (Z = 0) represents the expected median, and the slope represents the standard deviation; R is regression coefficient. |
|
13. |
From the two preceding sentences, it can be inferred that Tollefson and Cordle (1986) are treating the distributions of consumption rates for individual species as normal (Gaussian), rather than lognormal, which may be more accurate. |
|
14. |
Unfortunately, although the NMFS (1978) report is statistically sophisticated it appears to be biologically naive, in that is seems to focus on the distribution of daily intake, rather than periods of a month or more to be as comparable as possible to the long biological half-life of methylmercury in humans. The report is unclear enough in its methodology and end product results that the committee is unable to effectively utilize its contents, but it provides at least an illustration of the kind of distributional treatment that, if based on appropriate periods of exposure, could be toxicologically informative. |
|
15. |
In other work (not shown) these data were fit to the lognormal risk model in Clement Associates ToxRisk2 statistical package (Crump et al., 1989). This model differs slightly from the classic probit model in that it is essentially a one-hit dose-response function, incorporating a lognormal distribution of susceptibilities. Despite |
|
|
this difference, the results obtained by this procedure were very similar to those shown for the classical Finney (1971) procedure. |
|
16. |
If the distributions of biological half-lives and thresholds for effect in terms of blood levels are in fact lognormal, if among individuals the two distributions are independent (uncorrelated), and if the two factors each act multiplicatively in affecting individuals' thresholds for effect in terms of long-term dietary dose, then the corresponding log10 variances can simply be added. Thus, the probit slope for dietary exposure = 1/{(1/blood prohibit slope)2 [log10 (half-life geometric standard deviation)2]}0.5 The log10 (half-life geometric standard deviation) calculated from the data in Figure 6-2 is 0.147016. |
|
17. |
The FDA does not publish a list of cancer potency estimates for these compounds using its own methodology, which differs from that of EPA (discussed earlier). As a standard practice, EPA accompanies the use of these cancer potency estimates with the following: "This level is an upper estimate and the actual risk may be as low as zero." Exactly how much, as a rule, these numbers are likely to overstate actual risks is the subject of much current controversy in the regulatory and toxicological communities. For a comparison of best estimates of cancer risk and EPA upper confidence limits in three cases with the aid of physiologically based pharmacokinetic analyses, see Hattis (1990b). |
|
18. |
This is in excess of the background cancer risk, which is about 1 in 5 for the U.S. population. |
|
19. |
For example, in the Alabama River, near Claiborne, Alabama, fillets sampled from large-mouth bass average 16.1 parts per trillion dioxin. If the consumption rate is 18 g daily, which is equivalent to about one-third of a pound per week, the cancer risk would be 6.7 × 10-4. |
|
20. |
Unlike the federal regulation, MDPH uses a concentration of 0.5 ppm of mercury in fish tissue as a trigger for issuance of fish consumption advisories. This level is based on a WHO recommendation that daily consumption of mercury not exceed 35 µg. This would result in a body burden approximately 10 times lower than that observed to cause effects in humans in mercury poisoning incidents in Japan and Iraq (Michigan, 1989). At the 0.5-ppm contamination level, a person could eat nearly a pound of fish per week without exceeding the WHO recommended maximum daily intake. Larger, older fish in many inland lakes throughout Michigan may have concentrations of mercury in the 0.5- to 1.5-ppm range. This discovery of mercury in fish from inland lakes is not limited to Michigan. Wisconsin, Minnesota, and Ontario have all experienced similar findings. The EPA and the upper Midwest states are currently evaluating whether factors such as acid rain may contribute to this problem. As for warning anglers about all sources of contamination, that task appears to be impossible. Michigan alone has approximately 10,000 inland lakes within its boundaries, and the state readily concedes that it will never be feasible for fish from all lakes to be tested for contaminants. |
REFERENCES
AIHC (American Industrial Health Council). 1987. A Discussion Memorandum of the Vinyl Chloride Decision: Improving the Science Base for Health Assessment. Submitted to EPA for consideration in its Carcinogen Risk Assessment Guidelines: Proposed Updating. September. AIHC, Washington, D.C. 19 pp.
Allen, B.C., A.M. Shipp, K.S. Crump, B. Killian, M.L. Hoge, and B.K. Tudor. 1987. Investigation of Cancer Risk Assessment Methods (four volumes). Report by Clement Associates, Inc. to the U.S. Environmental Protection Agency, EPA Report No. EPA/600/6-87/007a-d, September.
Al-Shahristani, H., and K. Shihab. 1974. Variation of biological half-life of methylmercury in man. Arch. Environ. Health 28:342-344.
Appelman, L.M., W.F. ten Berge, and P.G.J. Reuzel. 1982. Acute inhalation toxicity study of ammonia in rats with variable exposure periods, Am. Ind. Hyg. Assoc. J. 43:662-665.
Baker, E.L., R.G. Feldman, R.F. White, and J.P. Harley. 1983. The role of occupational lead exposure in the genesis of psychiatric and behavioral disturbances. Acta Psychiat. Scand. 67(Suppl. 303):38-48.
Bakir, F., S.F. Damluji, L. Amin-Zaki, M. Murtadha, A. Khalidi, N.Y. al-Rawi, S. Tikriti, H.I. Dahahir, T.W. Clarkson, J.C. Smith, and R.A. Doherty. 1973. Methylmercury poisoning in Iraq. Science 181:230-241.
Ballew, M., and D. Hattis. 1989. Reproductive Effects of Glycol Ethers in Females-A Quantitative Analysis. M.I.T. Center for Technology, Policy, and Industrial Development, CTPID 89-7, July.
Barry, P.S.I. 1975. A comparison of concentrations of lead in human tissues. Brit. J. Ind. Med. 32:119-139.
Bellinger, D., A. Leviton, C. Waternaux, H. Needleman, and M. Rabinowitz. 1987. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. N. Engl. J. Med. 316:1037-1043.
Bellinger, D., A. Leviton, and J. Sloman. 1990. Antecedents and correlates of improved cognitive performance in children exposed in utero to low levels of lead. Environ. Health Perspect. 89:5-11.
Berglund, F., M. Berlin, G. Birke, U. von Euler, L. Friberg, B. Holmstedt, E. Jonsson, C. Ramel, S. Skerfving, A. Swensson, and S. Tejning. 1971. Methylmercury in fish: A toxicological-epidemiologic evaluation of risks. Report from an expert group. Nord. Hyg. Tidskr. 4(Suppl.):19-290.
Bernard, S.R. 1977. Dosimetric data and metabolic model for lead. Health Physics 32:44-46.
Bertazzi, P.A., L. Riboldi, A. Pesatori, L. Radice, and C. Zocchetti. 1987. Cancer mortality of capacitor manufacturing workers. Am. J. Ind. Med. 11:165-76.
Bishop, J.M. 1987. The molecular genetics of cancer. Science 235:305-311.
Brown, D.P. 1987. Mortality of workers exposed to polychlorinated biphenyls: An update. Arch. Environ. Health 42:333-339.
Buchler, F., P. Schmid, and C. Schlatter. 1988. Kinetics of PCB elimination in man. Chemosphere 17:1717-1726.
Campbell, B.C., P.A. Meredith, M.R. Moore, and W.S. Watson. 1984. Kinetics of lead following intravenous administration in man. Toxicol. Lett. 21:231-235.
Cappuzo, J., A. McElroy, and G. Wallace. 1987. Fish and shellfish contamination in New England waters: An evaluation and review of available data on the distribution of chemical contaminants. Report submitted to Coast Alliance, Washington, D.C.
Chamberlain, A.C. 1985. Prediction of response of blood lead to airborne and dietary lead from volunteer experiments with lead isotopes. Proc. Roy. Soc. London B 224:149-182.
Clarkson, T.W., L. Amin-Zaki, and S.K. Al-Tikriti. 1976. An outbreak of methylmercury poisoning due to consumption of contaminated grain. Fed. Proc. 35:2395-2399.
Clarkson, T.W., B. Weiss, and C. Cox. 1983. Public health consequences of heavy metals in dump sites. Environ. Health Perspect. 48:113-127.
Cleaver, J.E., and D. Bootsma. 1975. Xeroderma pigmentosum: Biochemical and genetic characteristics. Ann. Rev. Genet. 9:19-38.
Clement Associates, Inc. 1989. Issues in Setting Drinking Water Standards for Polychlorinated Biphenyls-Alternative Approaches to Estimates of Risk for Polychlorinated Biphenyls. Clement Associates, Inc., Ruston, La.
Cordle, F. 1983. Use of epidemiology and clinical toxicology to determine human risk in regulating
polychlorinated biphenyls in the food supply
. Regul. Toxicol. Pharmacol. 3:252-274.Cordle, F., R. Locke, and J. Springer. 1982. Risk assessment in a federal regulatory agency: An assessment of risk associated with the human consumption of some species of fish contaminated with polychlorinated biphenyls (PCBs). Environ. Health Perspect. 45:171-182.
Cox, C., T.W. Clarkson, D.O. Marsh, L. Amin-Zaki, S. Tikriti, and G.G. Myers. 1989. Dose-response analysis of infants prenatally exposed to methylmercury: An application of a single compartment model to single-strand hair analysis. Environmental Research 49:318-332.
Crump, K., D. Hoel, and R. Peto. 1976. Fundamental carcinogenic processes and their implications for low dose risk assessment . Cancer Research 36:2973-2979.
Crump, K., H. Guess, and K. Deal. 1977. Confidence intervals and test of hypotheses concerning dose response relations inferred from animal carcinogenicity data. Biometrics 33:437-451.
Crump, K.S., R.B. Howe, and C. Van Landingham. 1989. Tox-Risk–Toxicology Risk Assessment Program. Clement Associates, Inc., Ruston, La.
Cumbie, P.M., and S.L. Van Horn. 1978. Selenium accumulation associated with fish mortality and reproductive failure. Proc. Am. Conf. S.E. Assoc. Fish Wildlife Agencies 32:612.
DFO (Canadian Department of Fisheries and Oceans). 1989. Canadian Guidelines for Chemical Contaminants in Fish and Fish Products. Government of Canada, Ottawa.
Dourson, M.L., and J.F. Stara. 1983. Regulatory history and experimental support of uncertainty (safety) factors. Reg. Toxicol. Pharmacol. 3:224-238.
Ehrenberg, L., E. Moustacchi, and S. Osterman-Golkar. 1983. International Commission for Protection Against Environmental Mutagens and Carcinogens. Dosimetry of genotoxic agents and dose-response relationships of their effects. Mutat. Res. 123:121-182.
EPA (Environmental Protection Agency). 1981. Chemical Hazard Information Profile Draft Report. Antimony Trioxide, CAS No. 1309-64-4. Office of Toxic Substances, U.S. Environmental Protection Agency, Washington, D.C. 41 pp.
EPA (Environmental Protection Agency). 1983. Lower Meramec River Water Quality Intensive Survey. EPA Region VII, Environmental Services Division, Environmental Monitoring and Compliance Branch, Environmental Evaluation Section.
EPA (Environmental Protection Agency). 1986a. Guidelines for carcinogen risk assessment, Parts II-VI. Federal Register 51(185):33992-34012.
EPA (Environmental Protection Agency). 1986b. Superfund Public Health Evaluation Manual EPA/540/1-86/060. Office of Emergency and Remedial Response, U.S. Environmental Protection Agency, Washington, D.C., October. 186 pp.
EPA (Environmental Protection Agency). 1987. The National Dioxin Study: Tiers 3, 5, 6, and 7. EPA 440/4-87-003. Office of Water Regulations and Standards, Monitoring and Data Support Division (WH-553), U.S. Environmental Protection Agency, Washington, D.C.
EPA (Environmental Protection Agency). 1989. Health Assessment Survey Tables-Third Quarter 1989. U.S. Environmental Protection Agency. Publication OERR 9200.6/303/(89-3), July.
FAO/WHO (Food and Agriculture Organization/World Health Organization). 1972. Evaluation of certain food additives and of the contaminants mercury, lead, and cadmium. Joint FAO/WHO Expert Committee on Food Additives. FAO Nutrition Meetings Report Series No. 51, Rome. 32 pp.
FDA (Food and Drug Administration). 1979. Polychlorinated biphenyls (PCB's); Reduction of tolerance. Federal Register 44(June 29):38330-38340.
FDA (Food and Drug Administration). 1982. Levels for poisonous or deleterious substances in human food and animals feed. Food and Drug Administration, Washington, D.C. 13 pp.
FDA (Food and Drug Administration). 1988. Fish List: Guide to Acceptable Market Names for Food Fish Sold in Interstate Commerce. U.S. Government Printing Office, Washington, D.C. 50 pp.
Fein, G.G., J.L. Jacobson, S.W. Jacobson, P.M. Schwartz, and J.K. Dowler. 1984. Prenatal exposure to polychlorinated biphenyls: Effects on birth size and gestational age. J. Pediatrics 105:315-320.
Fialkow, P.J. 1977. Clonal origin and stem cell evolution of human tumors. Pp. 439-453 in J.J. Mulvihill, R.W. Miller, and J.F. Fraumeni, Jr., eds. Genetics of Human Cancer. Raven Press, New York.
Finkel, A.M. 1990. Confronting Uncertainty in Risk Management–A Guide for Decision Makers. Resources for the Future, Washington, D.C. 68 pp.
Finney, D.J. 1971. Probit Analysis, 3rd ed. Cambridge University Press, Cambridge, England. 333 pp.
Fischinger, P.J., and V.T. DeVita, Jr. 1984. Governance of science at the National Cancer Institute: Perceptions and opportunities in oncogene research. Cancer Res. 44:4693-4696.
Friedman, B., A.R. Frackelton, Jr., A.H. Ross, J.M. Connors, H. Fujiki, T. Sugimura, and M.R. Rosner.
1984. Tumor promoters block tyrosine-specific phosphorylation of the epidermal growth factor receptor. Proc. Natl. Acad. Sci. 81:3034-3038.
GAO (General Accounting Office). 1988. Seafood Safety: Seriousness of Problems and Efforts to Protect Consumers, RCED-88-135. Report to the Chairman, Subcommittee on Commerce, Consumer and Monetary Affairs, Committee on Government Operations, House of Representatives. U.S. Government Printing Office, Washington, D.C.
Gartrell, M.J., J.C. Craun, D.S. Podrebarac, and E.L. Gunderson. 1985. Pesticides, selected elements, and other chemicals in adult total diet samples, October 1978-September 1979. J. Assoc. Off. Anal. Chem. 68:862-873.
Gossett, R., H. Puffer, R. Arthur, J. Alfafara, and D. Young. 1982. Levels of Trace Organic Compounds in Sportfish from Southern California. Coastal Water Research Project Biennial Report. Southern California Coastal Water Research Project, Long Beach, Calif.
Green, V.A., G.W. Wise, and J.C. Callenbach. 1978. Lead poisoning. Pp. 123-141 in F. Oehme, ed. Toxicity of Heavy Metals in the Environment, Part 1. Marcel Dekker, New York.
Groth, D.H., L.E. Stettler, J.R. Burg, W.M. Busey, G.C. Grant, and L. Wong. 1986. Carcinogenic effects of antimony trioxide and antimony ore concentrate in rats. J. Toxicol. Environ. Health 18:607-626.
Guess, H., K. Crump, and R. Peto. 1977. Uncertainty estimates from low-dose extrapolations of animal carcinogenicity data. Cancer Res. 37:3475-3483.
Gunderson, E.L. 1988. FDA Total Diet Study, April 1982-April 1984, Dietary intakes of pesticides, selected elements and other chemicals. J. Assoc. Off. Anal. Chem. 71:1200-1209.
Haeger-Aronsen, B., M. Abdulla, and B.I. Fristedt. 1974. Effect of lead on delta-aminolevulinic acid dehydratase activity in red blood cells. II. Regeneration of enzyme after cessation of lead exposure. Arch. Environ. Health 29:150-153.
Haines, A.T., and E. Nieboer. 1988. Chromium hypersensitivity. Pp. 497-532 in J. Nrigau and E. Nieboer, eds. Chromium in the Natural and Human Environment. John Wiley and Sons, New York.
Hall, R.A., E.G. Zook, and G.M. Meaburn. 1978. National Marine Fisheries Service Survey of Trace Elements in the Fishery Resource. NOAA Technical Report NMFS SSRF-721, National Technical Information Service No. PB 283 851, March. U.S. Government Printing Office, Washington, D.C.
Hattis, D. 1981. Dynamics of medical removal protection for lead–A reappraisal. Report to the National Institute for Occupational Safety and Health. M.I.T. Center for Policy Alternatives Report No. CPA-81-25. Cambridge, Mass. 47 pp.
Hattis, D. 1982. From presence to health impact: Models for relating presence to exposure to damage. Pp. 1-66 in N.A. Ashford and C.T. Hill, eds. Analyzing the Benefits of Health, Safety, and Environmental Regulations. M.I.T. Center for Policy Alternatives, Report No. CPA-82-16. Cambridge, Mass.
Hattis, D. 1986. The promise of molecular epidemiology for quantitative risk assessment. Risk Analysis 6:181-193.
Hattis, D. 1988. The use of biological markers in risk assessment. Statistical Science 3:358-366.
Hattis, D. 1990a. Pharmacokinetic principles for dose rate extrapolation of carcinogenic risk from genetically active agents. Risk Analysis 10:303-316.
Hattis, D. 1990b. Use of biological markers and pharmacokinetics in human health risk assessment. Environmental Health Perspectives (in press).
Hattis, D., and A. Smith. 1986. What's wrong with quantitative risk assessment? Pp. 57-79 in R. Almeder and J. Humber, eds. Biomedical Ethics Reviews 1986. The Humana Press, Clifton, N.J.
Hattis, D., and H. Strauss. 1986. Potential Indirect Mechanisms of Carcinogenesis, A Preliminary Taxonomy. National Technical Information Service No. NTIS/PB89-120513. Center for Technology, Policy, and Industrial Development, Report No. CTPID 86-3, Massachusetts Institute of Technology, Cambridge, Mass. 12 pp.
Hattis, D., L. Erdreich, and M. Ballew. 1987a. Human variability in susceptibility to toxic chemicals–A preliminary analysis of pharmacokinetic data from normal volunteers. Risk Anal. 7:415-426.
Hattis, D., S. Bird, and L. Erdreich. 1987b. Human Variability in Susceptibility to Anticholinesterase Agents. M.I.T. Center for Technology, Policy and Industrial Development, Report No. CTPID 87-4 . Cambridge, Mass.
Haxton, J., D.G. Lindsay, J.S. Hislop, L. Salmon, E.J. Dixon, W.H. Evans, J.R. Reid, C.J. Hewitt, and D.F. Jeffries. 1979. Duplicate diet study on fishing communities in the United Kingdom:
Mercury exposure in a "critical group". Environ. Res. 18:351-368.
HHS (Department of Health and Human Services), Agency for Toxic Substances and Disease Registry. 1988. The Nature and Extent of Lead Poisoning in Children in the United States: A Report to Congress. July. U.S. Government Printing Office, Washington, D.C.
Hing, E. 1989. Nursing Home Utilization by Current Residents: United States, 1985. National Center for Health Statistics, Vital Health Stat. 13(102), DHHS Publication No. (PHS) 89-1763, October. U.S. Government Printing Office, Washington, D.C.
Hoel, D.G. 1985. Epidemiology and the inference of cancer mechanisms. Natl. Cancer Inst. Monogr. 67:199-203.
Hoel, D.G., N.L. Kaplan, and M.W. Anderson. 1983. Implications of non-linear kinetics on risk estimation in carcinogenesis. Science 219:1032-1037.
Hogue, C.J.R, J.W. Buehler, M.A. Strauss, and J.C. Smith. 1987. Overview of the national infant mortality surveillance (NIMS) project–Design, methods, results. Public Hlth. Reports 102:126-138.
Humphrey, H.E.B. 1974. Mercury concentrations in humans and consumption of fish containing methylmercury. Mercury Project Progress Report. Michigan Department of Public Health, Lansing. 6 pp.
Humphrey, H. 1983a. Population studies of PCBs in Michigan residents. Pp. 299-310 in F.M. D'Itri and M.A. Kamrin, eds. PCBs: Human and Environmental Hazards. Butterworth, Boston, Mass.
Humphrey, H. 1983b. Evaluation of Humans Exposed to Water-Borne Chemicals in the Great Lakes. Final Report to the Environmental Protection Agency, Cooperative Agreement CR-807192. U.S. Environmental Protection Agency, Washington, D.C. 205 pp.
Humphrey, H.E.B. 1988. Chemical contaminants in the Great Lakes: The human health aspect. Pp. 153-165 in M.S. Evans, ed. Toxic Contaminants and Ecosystem Health: A Great Lakes Focus. John Wiley & Sons, New York.
Jacobson, S.W., G.G. Fein, J.L. Jacobson, P.M. Schwartz, and J.K. Dowler. 1985. The effect of intrauterine PCB exposure on visual recognition memory. Child Develop. 56:853-860.
Jones, K.C. 1988. Determination of polychlorinated biphenyls in human foodstuffs and tissues: Suggestions for a selective congener analytical approach. Science Total Environ. 68:141-159.
Kabelitz, D. 1985. Modulation of natural killing by tumor promoters. The regulatory influence of adherent cells varies with the type of target cell. Immunobiology 169:436-446.
Kampert, J.B., A.S. Whittemore, and R.S. Paffenbarger, Jr. 1988. Combined effect of childbearing, menstrual events, and body size on age-specific breast cancer risk, Am. J. Epidemiol. 128:962-979.
Kansas DHE (Department of Health and Environment). 1988. A Survey of Pesticides in Tuttle Creek Lakes, Its Tributaries and the Upper Kansas River. Water Quality Assessment Section, Bureau of Water Protection, Kansas Department of Health and Environment, Topeka.
Kinsella, A.R., and M. Radman. 1978. Tumor promoter induces sister chromatid exchanges. Proc. Natl. Acad. Sci. 75:6149-6153.
Konz, J. 1988. Fish intake study. A memo report dated September 19, 1988 from Jim Konz of Versar, Inc. to Jacqueline Moya of the Environmental Protection Agency. Versar, Inc., Springfield, Va.
Knudson, A.G. 1973. Mutation and human cancer. Adv. Cancer Res. 17:317-352.
Knudson, A.G. 1977. Genetics and etiology of human cancer. Adv. Hum. Genet. 8:1-66.
Landolt, M.L., F.R. Hafer, A. Nevissi, G. Van Belle, K. Van Ness, and C. Rockwell. 1985. Potential toxicant exposures among consumers of recreationally caught fish from urban embankments of Puget Sound. NOAA Tech. Memo, NOS OMA 23. Rockville, Md. 104 pp.
Landolt, M.L., D. Kalman, A. Nevissi, G. van Belle, K. Van Ness, and F.R. Hafer. 1987. Potential toxicant exposures among consumers of recreationally caught fish from urban embayments of Puget Sound. NOAA Tech. Memo, NOS OMA 33. Rockville, Md. 111 pp.
Layde, P.M., L.A. Webster, A.L. Baughman, P.A. Wingo, G.L. Rubin, and H.W. Ory. 1989. The independent associations of parity, age at first full term pregnancy, and duration of breastfeeding with the risk of breast cancer. Cancer and Steroid Hormone Study Group. J. Clin. Epidemiol. 42:963-973.
Mackay, N.J., R.J. Williams, J.L. Kacprzac, M.N. Kazacos, A.J. Collins, and E.H. Auty. 1975. Heavy metals in cultivated oysters (Crassostrea commercialis = Saccostrea cucullata) from the estuaries of New South Wales. Aust. J. Mar. Freshwater Res. 26:31-46.
Marcus, A.H. 1985a. Multicompartment kinetic models for lead. I. Bone diffusion models for long-term retention. Environ. Res. 36:441-458.
Marcus, A.H. 1985b. Multicompartment kinetic models for lead. II. Linear kinetics and variable absorption in humans without excessive lead exposures. Environ. Res. 36:459-472.
Marcus, A.H. 1985c. Multicompartment kinetic models for lead. III. Lead in blood plasma and erythrocytes. Environ. Res. 36:473-489.
Marsh, D.O., M.D. Turner, J.C. Smith, J.W. Choe, and T.W. Clarkson. 1974. Methylmercury in human populations eating large quantities of marine fish. I. Northern Peru. Pp. 235-239 in Proceedings of the 1st International Mercury Congress, May 6-10, 1974. Barcelona, Spain.
Marsh, D.O., T.W. Clarkson, C. Cox, G.J. Myers, L. Amin-Zaki, and S. Al-Tikriti. 1987. Fetal methylmercury poisoning. Relationship between concentration in single strands of maternal hair and child effects. Arch. Neurol. 44:1017-1022.
Matthews, H.B., and R.L. Dedrick. 1984. Pharmacokinetics of PCBs. Rev. Pharmacol. Toxicol. 24:85-103.
Maugh, T.H. 1978. Chemical carcinogens: How dangerous are low doses? Science 202:37.
McCaffrey, P.G., B. Friedman, and M.R. Rosner. 1984. Diacylglycerol modulates binding and phosphorylation of the epidermal growth factor receptor. J. Biol. Chem. 259:12502-12507.
McCann, J., E. Choi, E. Yamasaki, and B.N. Ames. 1975. Detection of carcinogens as mutagens in the salmonella/microsome test: Assay of 300 chemicals. Proc. Natl. Acad. Sci. 72:5135-5139.
McFarland, V.A., and J.U. Clarke. 1989. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: Considerations for a congener-specific analysis. Environ. Health Perspect. 81:225-239.
Michigan DPH (Department of Public Health). 1988. Fish Consumption Advisory Issued by State Health Department . Michigan Department of Public Health, Center for Environmental Health Sciences, December 14, 1988. Lansing. 8 pp.
Miller, E.C., and J.A. Miller. 1981. Mechanisms of chemical carcinogens. Cancer 47:1055-1064.
Modali, R., and S. S. Yang. 1986. Specificity of Aflatoxin B1 binding on human proto-oncogene nucleotide sequence. Pp. 147-158 in M. Sorsa and H. Norppa, eds. Monitoring of Occupational Genotoxicants. Proceedings of a Satellite Symposium to the Fourth International Conference on Environmental Mutagens, Helsinki, Finland, June 30-July 2, 1985. Alan R. Liss, New York.
Moolgavkar, S. 1986. Carcinogenesis modeling: From molecular biology to epidemiology, Ann. Rev. Public Hlth. 7:151-169.
Moolgavkar, S.H., and A.G. Knudson, Jr. 1981. Mutation and cancer: A model for human carcinogenesis. J. Natl. Cancer Inst. 66:1037-1052.
Moolgavkar, S.H., N.E. Day, and R.G. Stevens. 1980. Two-stage model for carcinogenesis: Epidemiology of breast cancer in females, J. Natl. Cancer Inst. 65:559-569.
Murphy, D.L. 1988a. Basic water monitoring program. Fish tissue analysis, 1985 . Water Management Admin. Tech. Report 59. State of Maryland, Department of the Environment, Baltimore. 38 pp.
Murphy, D.L. 1988b. Trace contaminants in Chesapeake Bay bluefish. Metals and organochlorine pesticides. Water Management Admin. Tech. Report 73. State of Maryland, Department of the Environment, Divisions of Standards and Certification, Baltimore. 21 pp.
Murphy, D.L. 1988c. Trace contaminants in striped bass from two Chesapeake Bay tributaries. Metals and organochlorine pesticides. Technical Report 58. State of Maryland, Department of the Environment, Divisions of Standards and Certification, Water Management Administration, Baltimore. 19 pp.
Needleman, H.L., C. Gunnoe, A. Leviton, R. Reed, H. Peresie, C. Maher, and P. Barrett. 1979. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N. Engl. J. Med. 300:689-695.
Needleman, H., A. Schell, D. Bellinger A. Leviton, and E. Allred. 1990. Long-term effect of childhood exposure to lead at low doses: An eleven-year follow-up report. N. Engl. J. Med. 322:83-88.
Ngim, C.N., and G. Devathasan. 1989. Epidemiologic study on the association between body burden mercury level and idiopathic Parkinson's disease. Neuroepidemiol. 8:128-141.
NMFS (National Marine Fisheries Service). 1978. On the Chance of U.S. Seafood Consumers Exceeding the Current Acceptable Daily Intake for Mercury: Recommendations, Regulatory Controls. Seafood Quality and Inspection Division, Office of Fisheries Development, National Marine Fisheries Service. 198 pp.
NMFS (National Marine Fisheries Service). 1988. Fisheries of the United States, 1987. Current Fisheries Statistics, No. 8700. U.S. Government Printing Office, Washington D.C. 115 pp.
NOAA (National Oceanic and Atmospheric Administration). 1987. Report on 1984-86 Federal Survey of PCBs in Atlantic Coast Bluefish. Interpretive Report. NOAA in cooperation with the Food and Drug Administration and Environmental Protection Agency. U.S. Government Printing Office,
Washington, D.C. March. 75 pp.
Norback, D.H., and R.H. Weltman. 1985. Polychlorinated biphenyl induction of hepatocellular carcinoma in the Sprague-Dawley rat. Environ. Health Perspect. 60:97-105.
NRC (National Research Council). 1980. Recommended Dietary Allowances, 9th ed. Committee on Dietary Allowances, Food and Nutrition Board. National Academy Press, Washington, D.C. 185 pp.
NRC (National Research Council). 1989. Biologic Markers in Reproductive Toxicology. Board on Environmental Studies and Toxicology. National Academy Press, Washington, D.C. 416 pp.
NRC (National Research Council). 1990. Managing Troubled Waters. Committee on a Systems Assessment of Marine Environmental Monitoring, Marine Board. National Academy Press, Washington, D.C. 125 pp.
NRC (National Research Council). 1991. Neurotoxicology and Models for Assessing Risk. Board on Environmental Studies and Epidemiology. National Academy Press, Washington, D.C. (in press).
NWF (National Wildlife Federation). 1989. Abbreviated summary of quantitative health assessments for PCB, dieldrin, DDT, and chlordane. Developed for the 13 April 1989 workshop on Managing the Health Risks of Consuming Contaminated Great Lakes Sport Fish. Great Lakes Natural Resource Center, National Wildlife Federation, Ann Arbor, Mich. 16 pp.
Pasquinelli, P., F. Bruschi, F. Saviozzi, and G. Malvaldi. 1985. Immunosuppressive effects and promotion of hepatic carcinogenesis by thiobenzamide. Bull. Soc. Ital. Biol. Sper. 61:61-66.
Phillips, D.L., A.B. Smith, V.W. Burse, G.K. Steele, L.L. Heedham, and W.H. Hannon. 1989. Half-life of polychlorinated biphenyls in occupationally exposed workers. Arch. Environ. Hlth. 44:351-354.
PTI. 1987. Guidance Manual for Assessing Human Health Risks from Chemically Contaminated Fish and Shellfish . Draft Report C737-2. Submitted to Batelle New England Marine Research Laboratory by TI Environmental Services, Inc., Bellevue, Wash., December. 64 pp.
Rabinowitz, M.B., G.W. Wetherill, and J.D. Kopple. 1976. Kinetic analysis of lead metabolism in healthy humans. J. Clin. Invest. 58:260-271.
Rai, K., and J. van Ryzin. 1981. A generalized multihit dose response model for low dose extrapolation. Biometrics 37:341-352.
Rodericks, J. 1989. Assessing and managing risks associated with the consumption of chemically contaminated seafoods. Paper presented at the Workshop on Assessing and Controlling Health Hazards from Fishery Products conducted by the Committee on Evaluation of the Safety of Fishery Products, Food and Nutrition Board, Institute of Medicine, National Academy of Sciences Study Center, Woods Hole, Mass., July 26. 49 pp.
Rogan, W.J., B.C. Gladen, J.D. McKinney, N. Carreras, P. Hardy, J. Thullen, J. Tinglestad, and M. Tully. 1986. Neonatal effects of transplacental exposure to PCBs and DDE. J. Pediatrics 109:335-341.
Ross, R., and J.A. Glomset. 1976. The pathogenesis of atherosclerosis (second of two parts). N. Engl. J. Med. 295:420-425.
Safe, S. 1989. Polychlorinated biphenyls (PCBs): Mutagenicity and carcinogenicity. Mutat. Res. 220:31-47.
Sahl, J.D., T.T. Crocker, R.J. Gordon, and E. J. Faeder. 1985. Polychlorinated biphenyl concentrations in the blood plasma of a selected sample of non-occupationally exposed southern California (U.S.A.) working adults. Sci. Total. Environ. 46:9-18.
Scheuplein, R.J. 1988. Risk assessment and risk management of environmental food contaminants by FDA. Pp. 109-122 in C.R. Cothern, M.A. Mehlman, and W.L. Marcus, eds. Advances in Modern Environmental Toxicology, Vol. 15: Risk Assessment and Risk Management of Industrial and Environmental Chemicals. Princeton Scientific Publishing Co., Princeton, N.J.
Silbergeld, E.K. 1982. Current status of neurotoxicology, basic and applied. Trends Neurosci. 5:291-294.
Silbergeld, E.K., R.E. Hruska, D. Bradley, J.M. Lamon, and B.C. Frykholm. 1982. Neurotoxic aspects of porphyrinopathies: Lead and succinylacetone. Environ. Res. 29:459-471.
Skerfving, S. 1974. Methylmercury exposure, mercury levels in blood and hair, and health status in Swedes consuming contaminated fish. Toxicology 2:3-23.
Sloan, R., E. O'Connell, and R. Diana. 1987. Toxic Substances in Fish and Wildlife-Analyses since May 1, 1982, Vol. 6. Technical Report 87-4 (BEP), September. New York State Department of Environmental Conservation, Division of Fish and Wildlife, Albany. 182 pp.
Sorensen, E.M.B., P.M. Cumbie, T.L. Bauer, J.S. Bell, and C.W. Harlan. 1984. Histopathological,
hematological, condition-factor, and organ weight changes associated with selenium accumulation in fish from Belews Lake, North Carolina
. Arch. Environ. Contam. Toxicol. 13:153-162.Sunahara, G.I., K.G. Nelson, T.K. Wong, and G.W. Lucier. 1987. Decreased human birth weights after in utero exposure to PCBs and PCDFs are associated with decreased placental EGF-stimulated receptor autophosphorylation capacity. Molecular Pharmacol. 32:572-578.
Takada, K., and H. Zur Hausen. 1984. Induction of Epstein-Barr virus antigens by tumor promoters for epidermal and nonepidermal tissues. Int. J. Cancer 33:491-496.
Tanabe, S., Y. Nakagawa, and R. Tatsukawa. 1981. Absorption efficiency and biological half-life of individual chlorobiphenyls in rats treated with Kanechlor products. Agricult. Biol. Chemist. 45:717-726.
Taylor, P.R., J.M. Stelma, and C.E. Lawrence. 1989. The relation of polychlorinated biphenyls to birth weight and gestational age in the offspring of occupationally exposed mothers. Am. J. Epidem. 129:395-406.
Tollefson, L., and F. Cordle. 1986. Methylmercury in fish: A review of residue levels, fish consumption and regulatory action in the United States. Environ. Hlth. Perspect. 68:203-208.
Tsushimoto, G., C.C. Chang, J.E. Trosko, and F. Matsumura. 1983. Cytotoxic, mutagenic, and cell-cell communication inhibitory properties of DDT, lindane, and chlordane on Chinese hamster cells in vitro. Arch. Environ. Contam. Toxicol. 12:721-729.
Turner, M.D., D.O. Marsh, J.C. Smith, J.B. Inglis, T.W. Clarkson, C.E. Rubio, J. Chiriboga, and C.C. Chiriboga. 1980. Methylmercury in populations eating large quantities of marine fish. Arch. Environ. Health. 35:367-378.
Vogel, F., and A.G. Motulsky. 1979. Pp. 326-329 in Human Genetics-Problems and Approaches. Springer-Verlag, New York.
Waternaux, C., N.M. Laird, and J.H. Ware. 1989. Methods for analysis of longitudinal data: Blood lead concentrations and cognitive development. J. Am. Stat. Assoc. 84:33-41.
Weisburger, J.H., and G.M. Williams. 1983. The distinct health risk analyses required for genotoxic carcinogens and promoting agents. Environ. Health Perspect. 50:233-245.
Weiss, B., and J.M. Spyker. 1974. Behavioral implications of prenatal and early postnatal exposure to chemical pollutants. Pediatrics 53:851-859.
Whitlock, J.P., Jr. 1989. The control of cytochrome P-450 gene expression by dioxin. Trends Pharmacol. Sci. 10:285-288.
Whittemore, A.S. 1980. Mathematical models of cancer and their use in risk assessment, J. Environ. Pathol. Toxicol. 3:353-362.
Whittemore, A.S. 1983. Facts and values in risk assessment for environmental toxicants. Risk Anal. 3:23-33.
Wilber, C.F. 1983. Selenium: A Potential Environmental Poison and a Necessary Food Constituent. Charles C. Thomas, Springfield, Ill. 126 pp.
Yakushiji, T., I. Watanabe, K. Kuwabara, R. Tanaka, T. Kashimoto, N. Kunita, and I. Hara. 1984. Rate of decrease and half-life of polychlorinated biphenyls (PCBs) in the blood of mothers and their children occupationally exposed to PCBs, Arch. Environ. Contam. Toxicol. 13:341-345.
Yunis, J.J. 1983. The chromosomal basis of human neoplasia. Science 221:227-236.
Zakour, R.A., T.A. Kunkel, and L.A. Loeb. 1981. Metal-induced infidelity of DNA synthesis. Environ. Health Perspect. 40:197-206.
Zeggari, M., C. Susini, N. Viguerie, J.P. Esteve, N. Vaysse, and A. Ribet. 1985. Tumor promoter inhibition of cellular binding of somatostatin. Biochem. Biophys. Res. Commun. 128:850-857.
Zeitlin, D. 1989. State-Issued Fish Consumption Advisories: A National Perspective. National Ocean Pollution Program Office, National Oceanic and Atmospheric Administration, Washington, D.C. November. 73 pp.