8
Seafood Surveillance and Control Programs
ABSTRACT
This chapter considers the questions of how well existing governance efforts address and move to mitigate the risks in consuming seafood that have been identified thus far in this report. The chapter describes and evaluates efforts by federal and state regulatory authorities and private industry to minimize the level of seafood risk.
At the federal level, seafood safety falls primarily under the authority of the Food and Drug Administration (FDA). The FDA serves as the lead agency in setting and enforcing regulatory limits for seafood products. However, other federal agencies also play an important role. The Environmental Protection Agency is responsible for setting or recommending pesticide limits in seafood, and the National Marine Fisheries Service operates the Voluntary Seafood Inspection Program. The Centers for Disease Control is responsible for the collection and evaluation of data characterizing the source of seafood-borne illness.
Individual states play a dominant role in the control of seafood-borne risk. State public health, environmental protection, and resource management agencies have developed programs designed to mitigate that risk. The actions of state governments are fundamental to seafood safety because of the important differences in consumption and contaminant levels across regions of the country.
Furthermore, the international community, as well as individual foreign states, have developed practices and protocols impacting the regulation of seafood safety in the United States. The impacts of all these governance efforts are described and evaluated in this chapter.
INTRODUCTION
The purpose of this chapter is twofold. An initial effort is made to describe and characterize existing programs under the authority of federal, state, and local governments; other public bodies; and private organizations designed to ensure the safety of seafood consumed by the U.S. public. Based on an analysis of those programs, this report provides an overall evaluation of their effectiveness. Such a
broad-based evaluation is necessary to ensure a reasonable understanding of all governance and management programs currently in place, as well as some measure of protection for the U.S. seafood consuming public. This report recognizes that the question of seafood safety is being addressed by a network of governmental and nongovernmental efforts. The committee's evaluation attempts to incorporate as comprehensive an approach as possible in order to develop a realistic characterization of seafood safety.
The organization of this evaluation is designed to reflect as reasonably as possible this complex programmatic effort. The effort addresses both those programs carried out by federal administrative agencies and the responsibilities taken on by various state or local governments and the seafood industry. Further, in recognition of the international and interdependent nature of seafood commerce, efforts related to seafood safety carried out by other countries and by international economic and scientific organizations are also characterized.
RESPONSIBILITIES AND PROGRAMS OF THE FEDERAL GOVERNMENT
A number of federal agencies are involved in regulation of seafood (Martin, 1990). The primary federal agency with responsibility for the assurance of seafood safety is the U.S. Department of Health and Human Services' Food and Drug Administration (FDA). The agency houses a wide range of programs devoted to the research and management of seafood product safety. The FDA derives its authority over such programs primarily through two statutes: (1) the Federal Food, Drug and Cosmetic Act (FFDCA: 21 U.S.C. 301 et seq.), and (2) the Public Health Service Act (PHSA: 42 U.S.C. 262, 294 et seq.). Under the FFDCA, the FDA is assigned responsibility to ensure that seafood shipped or received in interstate commerce is "safe, wholesome, and not misbranded or deceptively packaged" (FDA, 1988d). Under the PHSA, FDA is empowered to control the spread of communicable disease from one state, territory, or possession to another. To carry out these statutory mandates, FDA has developed a series of regulatory and research programs described below.
Regulatory authority for seafood safety is partially shared, within the present federal system, with two other regulatory actors. The Environmental Protection Agency (EPA) is most fundamentally involved in setting and recommending regulatory guidelines for pesticides. The EPA also provides assistance to FDA in identifying the range of residual chemical contaminants that pose a human health risk and are most likely to accumulate in seafood. The National Marine Fisheries Service of the Department of Commerce conducts the Voluntary Seafood Inspection Program. The role and responsibilities of both these agencies are detailed further below. Other federal regulators are also responsible for seafood promotion and quality. However, although programs in the Departments of Agriculture, Interior, and Defense may enhance seafood safety, such efforts should be viewed as ancillary to the larger federal efforts described below.
Standards and Guidelines
The FDA is granted primary authority to set and enforce allowable levels of contaminants and pathogenic microorganisms in seafood, and has developed a number of regulatory guidelines designed to reduce public health risk. Section 402(a) of the FFDCA provides the FDA with its most broad-based power in controlling contaminants in seafood. Under this provision, FDA may control the production and trade of any "adulterated" seafood product. Under the FFDCA [Sec. 402(a)(1)] a food is deemed adulterated if "it bears or contains any poisonous or deleterious substance which may render it injurious to health. …"
The FDA has historically used three related strategies to determine whether or not a seafood product should be deemed adulterated. If significant and reliable toxicological data are available, the agency will set a formal "tolerance" that identifies a limit above which the food is deemed to be injurious (FFDCA, Sec. 406). A formal tolerance identifies the amount of a given substance or organism that must be present for an enforcement action or seizure to be initiated. If a product contaminant exceeds a tolerance level, FDA may automatically remove that product from interstate commerce. However, when toxicological data are scanty or conflicting, when additional data are being developed, or when other conditions are changing rapidly, the promulgation of formal tolerance may be deemed inappropriate. In such instances the agency can promulgate "action levels" [the authority to set such levels is defined in FDCA, Sections 306, 402(a) and 406] which, according to the agency, are designed to provide prosecutorial guidance. Action levels are not binding on the agency or industry, and FDA can recommend prosecution regardless of whether the action level is exceeded. If FDA recommends prosecution, then it must establish in court that the product is injurious to health.
Although the primary authority for the designation of formal tolerances and action levels resides with FDA, the agency shares authority with EPA regarding the regulatory limits for pesticides. With the creation of EPA, and by way of the Presidential Reorganization Order No. 3 of 1970 (DOC, 1970), primary responsibility for the regulation of pesticides in food was transferred to EPA. Under present agreements, EPA holds sole responsibility for setting formal tolerances in seafood that are then enforced by FDA. For pesticide action levels, EPA submits a recommendation to FDA for enforcement.
However, lacking a formal tolerance or action level, FDA may still act on an adulterated product by providing sufficient evidence that the product constitutes a problem for public health. These three approaches are detailed below.
Tolerances
According to FDA policy and a general interpretation of the U.S. Administrative Procedures Act (APA: 5 U.S.C. 551 et seq.), the setting of a formal tolerance requires (1) significant and reliable scientific evidence of the public health impacts of the specified tolerance level, and (2) a formal application of the notice-and-comment procedures enumerated in the APA. To date, FDA has specified only one formal tolerance designed specifically to mitigate human health impacts in seafood, that is, the 2.0-parts-per-million (ppm) tolerance for total polychlorinated biphenyls (PCBs). The
FDA has developed other tolerances for food, but none that are directly related to seafood safety.
Action Levels
Although FDA has used the vehicle of formal tolerances, setting regulatory guidelines for food, including seafood, has most often proceeded through the use of action levels. Unlike the establishment of tolerances, action levels do not require FDA to proceed through formal notice-and-comment rule making. Thus, setting seafood regulatory guidelines most often occurs via action levels.
However, action levels have been the focus of significant controversy in recent years. Until recently, FDA had developed a set of enforcement practices wherein tolerances and action levels were enforced with equivalent rigor. In fact, there was little difference in the certitude with which the agency would characterize and respond to an adulterated product. Indeed, the equivalence of these two types of regulatory limits was articulated by FDA. For example, FDA published a regulation [21 CFR 109.4 (1986)] that stated
[A]n action level for an added poisonous or deleterious substance … may be established to define the level of contamination at which food will be deemed adulterated. An action level may prohibit any detectable amount of substance in food.
Thus, in practice, there was no functional difference between an action level and a tolerance. However, in 1987 the legal status of FDA action levels was refined and clarified by the U.S. Court of Appeals for the District of Columbia Circuit. A suit was brought against FDA by a consortium of consumer groups and private organizations, known collectively as the Community Nutrition Institute (CNI), that challenged FDA regulation of aflatoxin in corn [Community Nutrition Institute vs. Young: 818 F. 2d 943 (D.C. Cir. 1987)]. FDA had set an action level for corn at 20 parts per billion (ppb). In addition, the agency allowed aflatoxin-contaminated and noncontaminated corn to be mixed, provided the mixture did not exceed the 20-ppb action level. The court did recognize that action levels have the benefit of apprising the regulated community of the agency's intention, as well as informing the exercise of discretion by agents and officers in the field; however, the court held [818 F. 2d 949 (D.C. Cir. 1987)] that
[o]ur limited holding is that the current action levels are treated as substantive rules by FDA and, as such, can only be permitted if notice-and-comment procedures are employed. If it so chooses, FDA could proceed by action levels that are purely policy statements. But in order to do so, FDA must avoid giving action levels the kind of substantive significance that it now so plainly attaches to them.
Because of the Community Nutrition Institute decision, FDA reevaluated its action level policy. The FDA has also published a new regulation that allows substantive rules, called regulatory limits, to be established by formal notice-and-comment rule making [55 Fed. Reg. 20,782 (May 21, 1990)].
The regulatory limit will establish the level of an unavoidable added poisonous or deleterious substance that renders a food adulterated within the meaning of the FFDCA. A regulatory limit will be established when (1) the substance cannot be
avoided by current good manufacturing practices (GMPs); (2) there is no tolerance established for the substance in the particular food; and (3) there is insufficient information by which a tolerance may be established for the substance, or technology changes that may affect the appropriateness of a tolerance appear reasonably possible [55 Fed. Reg. 20,782 (May 21, 1990)].
Although FDA has established a formal mechanism for creating regulatory limits for unavoidable deleterious or poisonous contaminants, the agency also recognizes that it will maintain the action levels. However, FDA has stressed that action levels are not binding on the agency or industry [55 Fed. Reg. 20,782 (May 21, 1990)]. As such, FDA has the discretion to recommend court enforcement regardless of whether the product is within the action level. Likewise, FDA can forgo recommending court action when a product exceeds an action level.
Although action levels are no longer binding on the FDA, they are valuable because they provide significant guidance and focus to field personnel who direct monitoring and inspection programs that contribute to the identification of adulterated seafood products. Specific action levels have been developed for several contaminants in seafoods and seafood products. The list of action levels for microbial and natural toxin contaminants includes Escherichia coli in fresh and frozen crabmeat, parasites in finfish, and paralytic shellfish poison in clams, mussels, and oysters. A complete list of current action levels for microbial and natural toxin contaminants, and of the measurement methods used to determine them, is presented as Table 8-1.
Additionally, FDA has published a set of action levels for residual chemical contaminants, including pesticides. As noted earlier, the list of 12 chlorinated pesticides represents a cooperative effort between FDA and EPA. Although EPA retains the right to generate formal tolerances for pesticides, its strategy has been to communicate pesticide limits in terms of action levels. According to EPA personnel, this approach allows the agency to continue to monitor pesticide levels in the environment and to modify the action level based on monitoring results. Although a broad-based reevaluation of current pesticide action levels is underway by EPA, they provide guidance for acceptable levels in seafood products. The complete list of FDA action levels for residual chemical contaminants is presented in Table 8-2.
Seafood Deemed Injurious to Public Health
The FDA need not avail itself of either a formal tolerance or an action level to make a determination of adulteration. It may monitor for any contaminant that might produce a product injurious to public health.
The most effective statement of existing FDA strategy addressing the development of regulatory guidelines was that recently presented by the agency's Acting Commissioner, during a hearing before Congress on February 7, 1990:
In the absence of a national standard, FDA brings individual enforcement actions to establish that the amount of the contaminant present in the food may render it injurious to health. This broad legal standard in the Federal Food, Drug, and Cosmetic Act applies across the board to all foods under FDA's jurisdiction and its application is the norm in food adulteration cases. Whether a national standard does or does not exist, the Agency can still take action against potentially unsafe products. This is an
TABLE 8-1 Food and Drug Administration Compliance Policy Guides (CPG) Relating to Seafood Safety
|
Microbiological Contamination |
|
Title: Crabmeat-Fresh and Frozen-Adulteration with Filth, Involving the Presence of the Organism Escherichia coli. |
|
Action Level: E. coli of at least 3.6 per gram (g). Calculation by most probable number (MPN) methodology. |
|
CPG 7108.02. Effective October 1, 1982. |
|
Title: Langostinos-Frozen, Cooked-Adulteration by Bacteriological Contamination. |
|
Action Level: (1) Coliform density greater than 20/g (MPN) in 20% of samples; (2) E. coli density greater than 3.6/g (MPN) in 20% of the subsamples; or, (3) coagulasepositive staphylococci density greater than 3.6/g in 20% of the subsamples or, aerobic plate count (at 35°C) greater than 100,000/g as a geometric average of all the subsamples. |
|
CPG 7108.09. Effective October 1, 1980. |
|
Title: Raw Breaded Shrimp-Microbiological Defect Action Levels |
|
Action Level: Actionable if one or more of the following conditions are met: |
|
1. Aerobic Plate Counts (35°C)-The mean log of 16 units of finished product breaded shrimp collected prior to freezing is greater than 5.00 (i.e., geometric mean greater than 100,000/g) and exceeds the mean log of 16 units of stock shrimp by more than twice the standard error of their difference (2 SED). 2. E. coli-The mean log of 16 units of finished product breaded shrimp collected prior to freezing is greater than 0.56 (i.e., geometric mean greater than 3.6/g) and exceeds the mean log of 16 units of stock shrimp by more than twice the standard error of their differences. |
|
3. Staphylococcus aureus-The mean log of 16 units of finished product breaded shrimp collected prior to freezing is greater than 2.00 (i.e., geometric mean greater than 100/g) and exceeds the mean log of 16 units of stock shrimp by more than twice the standard error of their difference (2 SED). |
|
CPG 7108.25. Effective August 1, 1983. |
|
Title: Fish-Fresh and Frozen, as Listed-Adulteration by Parasites |
|
Action Level: For tullibees, ciscoes, inconnus, chubs, and whitefish: 50 cysts per 100 pounds (lbs.) provided that 20% of the fish examined are infested. For bluefish and other freshwater herring: fish averaging 1 lb or less, 60 cysts per 100 fish, provided that 20% of the fish examined are infested. For fish averaging over 1 lb, 60 cysts per 100 lbs of fish, provided that 20% of the fish examined are infested. For rosefish (redfish and ocean perch), 3% of the fillets examined contain one or more Copepoda accompanied by pus pockets. |
|
CPG 7108.06. Effective October 1, 1980 |
|
Natural Toxins |
|
Title: Clams, Mussels, Oysters, Fresh or Canned-Paralytic Shellfish Poison (PSP) |
|
Action Level: PSP at 80 micrograms (µg) per 100 g of meat. |
|
CPG 7108.20. Effective October 1, 1980 |
|
Title: Decomposition and Histamine in Canned Albacore, Skipjack, and Yellowfin Tuna |
|
Action Level: Histamine content at 50 milligrams (mg) per 100 g of meat. |
|
CPG 7108.25. Effective July 1, 1981 |
|
SOURCE: FDA (1985). |
TABLE 8-2 FDA Action Levels for Chemical Contaminants
|
Substance |
Action Level (ppm) |
Type of Food |
|
Methylmercury |
1.0 |
Fish, shellfish, crustaceans, and other aquatics |
|
PCBs |
2.0 |
Fish and shellfish |
|
Aldrin |
0.3 |
Fish and shellfish |
|
Chlordane |
0.3 |
Fish |
|
Dieldrin |
0.3 |
Fish and shellfish |
|
DDT, DDE, and TDEa |
5.0 |
Fish |
|
Endrin |
0.3 |
Fish and shellfish |
|
Heptachlor and heptachlor epoxide |
0.3 |
Fish and shellfish |
|
Kepone |
0.3 |
Fish and shellfish |
|
|
0.4 |
Crabmeat |
|
Mirex |
0.1 |
Fish |
|
Toxaphene |
5.0 |
Fish |
|
a DDT = dichlorodiphenyltrichloroethane; DDE = dichlorodiphenyldichloroethane; TDE (DDD) = diphenylethanedichlorophenylethane. SOURCE: FDA (1987). |
||
important point. Standards are not an essential prerequisite to Agency action. We regularly use an internal health hazard evaluation process to determine whether a contaminant in a particular sample would be likely to render that food injurious to public health (Benson, 1990).
Indeed, it is by way of these broad-based responsibilities to control deleterious substances in food that the agency controls microbiological pathogens in seafood. In certain instances the mere measurable presence of a pathogen will signify an adulterated product. The agency treats Shigella dysenteriae, Vibrio cholerae O-1, Salmonella, and Listeria , among others, in this fashion. The effective regulatory limit for these pathogens is equivalent to the ability of the measurement methodology to detect them. For others, the mere measurable presence of a pathogen does not constitute an automatic hazard, but significant populations would. For this class of pathogens the agency has developed a reactive strategy that allows for a broad-ranging and flexible approach to determine adulteration. Indeed, a majority of FDA enforcement actions for microbial pathogens are determined on the basis of this less formal, flexible response (John Kvenberg, FDA, personal communication, 1990).
However, this flexible system is presently under review by the National Advisory Committee on Microbiological Criteria for Food (NACMCF). The NACMCF was established and jointly funded by the Departments of Defense, Agriculture, Commerce, and Health and Human Services to provide advice on the further development of microbiological action levels in food. The committee recently proposed new criteria for cooked, ready-to-eat shrimp and cooked, ready-to-eat crabmeat.
In general, the present system for setting regulatory limits could be both
adequate and appropriate if such guidelines were effectively implemented. However, several critical points must be raised. First, there are areas in which guidelines are either inappropriate or nonexistent. For example, as noted in the section of this report addressing the question of chemical contaminants in seafood, the appropriateness of the present 2.0-ppm tolerance for PCBs, and the lack of specific regulatory limits for certain other chemical contaminants such as polycyclic aromatic hydrocarbons (PAHs), cadmium, lead, and dioxin, are questionable. Further, as noted elsewhere in this report, there remains a persistent concern about certain natural toxins for which the FDA has yet to determine a discernible regulatory strategy. Specifically, the committee suggests that the agency more fundamentally address the question of regulatory guidelines for domoic acid and ciguatera.
Product concepts are emerging, both in processing and in retail settings, that may pose safety questions as yet unaddressed by specific FDA guidelines (e.g., sous vide, modified atmosphere packaging, custom smoking techniques, and further processing in the retail setting). Finally, it should be noted that FDA has not communicated these regulatory limits effectively to intended parties. The regulatory limits thus far determined by the agency are communicated by way of FDA Compliance Policy Guides (FDA, 1985), notices in the Federal Register, and even in various FDA memoranda. It can be difficult to locate these regulations because FDA publishes in such a wide variety of sources. A more concerted effort to publish in a generally available, single-source, regularly updated volume would provide for more effective communication of these limits.
National Shellfish Sanitation Program
One of the primary seafood safety responsibilities of the FDA is its role as federal representative on the Interstate Shellfish Sanitation Conference (ISSC)–the organization that implements the National Shellfish Sanitation Program. The NSSP is a cooperative program in which FDA, state agencies, and private industry work to control the quality and safety of oysters, clams, and mussels sold in interstate commerce (FDA, 1989a,b). Within this program the agency is required to (1) set certain product guidelines and standards, (2) evaluate state compliance with those rules, and (3) certify continued state participation in the ISSC. The principal objective of the NSSP is to "provide a mechanism for certifying that shellfish shipped in interstate commerce meet agreed upon, specific sanitation and quality criteria" (FDA, 1989b).
The sanitary quality of shellfish is based on both growing water and wholesale market strategies. The most significant contribution of the program has been the creation of classification and monitoring strategies designed to ensure that shellfish are taken from harvesting waters significantly free of microbial contaminants. For a state to continue as a certified member of the program, it is required to survey all growing waters within its jurisdiction and classify those waters as to their acceptability for harvesting shellfish. Waters that have not been surveyed and classified must be closed. The microbiological requirements in this program are discussed in Chapter 3. The state must then test the sample within 24 hours of the shipment entering the state. If two successive samples from the same shipper are found to be in violation of both GMPs and microbiological criteria, "the shellfish regulatory authority at the source should be requested to supply information to the receiving jurisdiction concerning the
source of the product and the shipper's status may be subject to rejection by the receiving state shellfish regulatory authority. Acceptance of future shipments should depend upon satisfactory reports by shellfish regulatory authorities at the point of origin" (FDA, 1989b). These regulations have been the focus of some controversy. The move away from the independent use of microbiological criteria is viewed by some with concern. Others remain convinced that the use of fecal coliform as a broad-based indicator organism is inappropriate. Additionally, there remains a measure of concern because the NSSP is a voluntary program in which both the shipper and the receiver retain a significant amount of discretion in using the safety and quality information generated by program participation.
One contribution of the NSSP has been the characterization of a list of alert levels for several trace metal contaminants. These are listed in Table 8-3 (Ratcliffe and Wilt, 1971).
TABLE 8-3 NSSP Alert Levels for Trace Metals (ppm wet weight)
|
Metal |
Oysters |
Hard-Shell Clams |
Soft-Shell Clams |
||
|
Cadmium |
3.5 |
0.5 |
0.5 |
||
|
Lead |
2.0 |
4.0 |
5.0 |
||
|
Chromium |
2.0 |
1.0 |
5.0 |
||
|
Zinc |
2,000.0 |
65.0 |
30.0 |
||
|
Copper |
175.0 |
10.0 |
25.0 |
||
|
SOURCE: Ratcliffe and Wilt (1971). |
|||||
It is worth noting that these alert levels for metals were not formulated on the basis of toxicity assessment, but rather are based on a 20-year-old survey of average concentrations of metals in U.S. coastal waters (Isaac and Delany, 1975). The alert levels do not constitute a formal regulatory limit and require further, more rigorous analysis. Indeed, these levels were never formally accepted by the NSSP, but are being used by certain states to control trace-metal levels in shellfish (Robert Wetherell, FDA, personal communication, 1990).
Inspection and Enforcement
The setting of limits by an agency, however, is only an initial step in regulating contaminants in seafood. The agency must then determine the current levels in seafoods and the proportion of seafood products that exceeds the regulatory limit. To characterize the success of federal efforts in reasonably protecting the U.S. seafood consumer, the degree to which such regulations are being implemented by way of monitoring, inspection, and enforcement must also be characterized. At the federal level, several programmatic efforts are currently in place to inspect and survey seafood for public health-related contaminants.
U.S. Environmental Protection Agency
Because seafood is most typically harvested from the natural environment, considerations of monitoring in support of seafood safety should necessarily include EPA programs to monitor general water quality. The agency carries out several programs as part of its responsibilities under the Federal Water Pollution Control Act (hereafter referred to as the Clean Water Act) (CWA: 33 U.S.C. 1,251 et seq.) and the Marine Protection, Research and Sanctuaries Act (MPRSA: 33 U.S.C. 1,401 et seq.). Under the CWA, the agency is mandated to regulate all discharges into navigable waters of the United States (Sec. 520), including the territorial sea [Sec. 106(a)]. The MPRSA regulates the transportation and dumping of waste into federal waters seaward of the territorial sea (and dredged material within the territorial sea) (Sec. 502). The EPA has the authority, particularly under the CWA, to regulate a broad suite of environmental contaminants. Under Section 304(a)(4) the CWA identifies a list of "conventional pollutants," including oil and fecal coliform bacteria. The chemical toxins examined in the present study are addressed in Section 307(a)(1). The agency currently lists and regulates a total of 126 "priority pollutants" (40 CFR 122, App. D), which include both organic chemicals and metals. The identification of critical contaminants characterizes the initial effort of EPA both to monitor environmental quality in coastal areas and to assess the toxicity of chemical contaminants in seafood.
The importance of these strategies resides in the fact that a critical control point for ensuring the safety of the U.S. seafood product is the quality of the environment in which the fish are harvested. For the most part, subsequent safety assurance strategies (apart from cooking) are designed to ensure that contaminant levels are not further elevated. Therefore, any effective control strategy should begin with efforts to reduce the probability that contaminated seafood enters the processing and retail system. Further, by focusing on the quality of harvesting environments, one fundamentally achieves an opportunity to restrict products at the point of least added value.
However, current environmental monitoring programs do not focus directly on the question of seafood safety. Rather, they are designed to assess the general health of our marine and aquatic environments. Efforts to use such data directly for consideration of seafood safety suffer because most of the available environmental data (1) do not focus on seafood harvesting areas, (2) lack a common methodological approach, and (3) do not focus on the edible portion of seafood in order to determine public health hazards, as opposed to environmental health aspects. This last point is raised because most evaluations of contaminants in fish are done not on edible tissue but rather on the whole fish or on specific internal organs.
U.S. Food and Drug Administration
The Food and Drug Administration serves as the agency with primary authority for seafood product inspection. Seafood inspection within the FDA falls generally into four major categories: (1) general plant and product inspection, (2) import control inspection, (3) routine and periodic surveys for residual chemicals, and (4) compliance inspection for the National Shellfish Sanitation Program.
Any evaluation of such a monitoring and inspection program should address three related questions. First, are the inspections carried out with sufficient frequency to ensure compliance with regulatory guidelines? Second, is the sampling plan used by the program sufficient to develop a reliable estimate of the results? Third, is the program sufficiently broad to ensure that the regulatory guidelines provide a sufficient measure of protection to the public? This third point is a rather important one: that is, are contaminants being found in seafood for which the agency has not developed a sufficient regulatory response and for which there is evidence of public health concern? An effort is made here to address each of these questions in the characterization of federal monitoring, inspection, and enforcement programs.
As part of its general responsibilities under the FFDCA, FDA carries out a general inspection program involving periodic visits of its inspectors to processing facilities that prepare products destined for interstate commerce. The frequency and type of inspection are determined by the nature of plant operation, the volume of product produced, the record of previous compliance with regulations, the existence of consumer complaints, or other evidence of a problem (ICF, 1986). These inspections involve the entire suite of issues for which FDA has statutory authority. That is, they address overall plant sanitation and economic fraud (among other things), in addition to issues relating directly to seafood safety. Given the mandate of this study, the committee's effort is directed solely at programs developed to mitigate problems related to seafood safety.
Inspections directed at domestic products and processing are oriented primarily toward the maintenance of plant sanitation by way of enforcing good manufacturing practices. Although there is an acknowledged relationship between compliance with GMPs and seafood safety, the most direct impact of these inspections is on product quality. During the past several years, sanitation inspection has constituted a majority of the FDA's inspection effort. However, product evaluations for potential seafood contaminants also play a role. Data characterizing the agency's efforts to inspect for biological and chemical contaminants in both domestic and import programs are contained in Table 8-4.
In this program, biological hazards are limited almost exclusively to microbiological contaminants and parasites. Paralytic shellfish poison (PSP) and other natural toxins are more fundamentally monitored in other programs (i.e., the NSSP or state programs). Table 8-4 highlights two obvious issues: (1) that there has been a slight increase in the number of products evaluated in recent years, and (2) that most of the program effort is directed at imported products. The results of these evaluations are contained in Table 8-5. It should be noted here that individual states also carry out additional plant inspections that provide a substantial complementary contribution to this federal inspection effort.
Those data taken alone suggest that a significant proportion of both domestic and imported products are in violation of FDA regulations. However, they are derived from samples of a small proportion of seafoods that were selected as potentially troublesome lots because of previous experience or other information. It is questionable and probably unlikely that random sampling would yield such high violation rates. However, the precise relationship between directed and random inspections cannot be effectively calculated and therefore a general violation rate cannot be known.
TABLE 8-4 General FDA Seafood Inspection Program Product Evaluations
|
|
1987 |
1988 |
1989 |
|
Domestic samples analyzed Biological hazards |
797 |
1,137 |
1,109 |
|
Chemical contaminants |
1,007 |
715 |
541 |
|
Imported samples analyzed Biological hazards |
4,147 |
4,428 |
4,939 |
|
Chemical contaminants |
881 |
1,204 |
1,063 |
|
Annual totals |
6,832 |
7,484 |
7,652 |
|
SOURCE: FDA (1990). |
|||
TABLE 8-5 General FDA Seafood Inspection Program Results, 1989
|
Inspection |
Biological Hazards |
Chemical Contaminants |
|
Domestic samples analyzed |
1,109 |
541 |
|
Adverse |
231 (21%) |
20 (4%) |
|
Regulatory action not taken |
134 (12%) |
12 (2%) |
|
In compliance |
744 (67%) |
509 (94%) |
|
Import samples analyzed |
4,939 |
1,063 |
|
Adverse |
1,864 (38%) |
510 (48%) |
|
Regulatory action not taken |
194 (4%) |
12 (1%) |
|
In compliance |
2,881 (58%) |
541 (51%) |
|
SOURCE: FDA (1990). |
||
Import control program
The FFDCA requires all imported products to meet the same criteria for wholesomeness and safety imposed on U.S. products destined for interstate commerce. As shown in Table 8-4, a majority of the FDA inspection effort is, in fact, directed at imported products, partly because imports constitute a majority of the seafood products consumed in the United States. However, the predominance of imports in inspection is more fundamentally a result of FDA policy to direct resources toward areas suspected of safety problems. As part of its import program, and based on the results of past evaluations, the FDA has developed a strategy of automatic detention and evaluation of certain products with a history of violations. A program of automatic detention is currently in place for swordfish (evaluated for mercury/methylmercury content), mahimahi (evaluated for scombrotoxin), and raw in-shell or peeled frozen shrimp (evaluated for filth and the presence of Salmonella or Listeria) (FDA, 1986-1987). These products are detained at the point of entry and held until the importer can provide assurance, typically by using private laboratory testing, that the product is safe. The FDA reserves the right to retest private laboratory results. In 1989, 3,150 imported lots with a value of approximately $223 million were detained. This constituted about 4% of the total value of seafood imports ($5.6 billion for the year). It should be noted that neither private nor state laboratory evaluations are included in the total analyses for imported products described in Table 8-5.
The FDA also regularly releases import alert notices to communicate potential hazards. However, one difficulty with this strategy has been that the identification of specific import lots is often lost during subsequent product processing, which may reduce the impact of such alerts.
In addition to the general import strategy described above, FDA has recently developed a limited number of programs designed to focus and guide its efforts. These include the following:
-
Processed Seafood Program — This program is designed to offer a comparative evaluation for microbiological contamination of domestic and imported processed seafood. Seafoods included in the survey were crabmeat, shrimp, surimi,1 crawfish, smoked salmon, and lobster meat. The program evaluated a total of 369 domestic samples and 270 imported samples. Results indicated an adverse finding in 14.4% of the domestic samples and 11.1% of the imported products. In almost all instances the contaminant was identified as Listeria monocytogenes (FDA, 1988a; Matches et al., 1986).
-
Imported and Domestic Shrimp Program — This program was designed to compare problems associated with imported and domestic frozen raw shrimp. A total of 183 samples of imported products from 34 countries and 30 domestic samples were taken for evaluation. Results suggested that approximately 7% of the imports were clearly in violation, whereas only one domestic sample was (John Kvenberg, FDA, personal communication, 1989).
-
Imported Molluscan Shellfish Assignment — This assignment was designed to analyze and audit samples of shellfish for use by the FDA in refining its general inspection program and for use by states in their inspection and enforcement practices. On completion, the program will have evaluated 240 imported samples (FDA, 1989c).
These and other programs are used to periodically update and refine FDA inspection practices and to further direct the general inspection program so as to maximize the effectiveness of the limited resources dedicated to seafood inspection.
Routine and periodic surveys for residual chemicals
As noted in recent congressional testimony by FDA Acting Commissioner James Benson, the agency carries out — in addition to the general inspection program — an ongoing surveillance program for residual chemicals in seafood (Benson, 1990). The program is located in the Division of Contaminants Chemistry (DCC) of the FDA Center for Food Safety and Applied Nutrition. According to FDA personnel, agency field officers are required to collect a specified minimum number of seafood samples harvested or processed in that region. The general sampling protocol for this program has been articulated in the following way (Lombardo and Yess, 1989): Sampling for chemical contaminants is based on a variety of factors, including inspectional findings, historical problem areas, new toxicological concerns, changes in growing/harvesting techniques, new sources of food, and review of state/local shellfish control programs.
During the past three years, DCC has evaluated 2,214 seafood products for approximately 60 pesticides and industrial chemicals. This suite of residual chemicals represents, according to FDA, approximately 75% of the chemicals deemed by EPA to have the highest tendency to bioaccumulate in finfish and shellfish (George Hoskin, FDA, personal communication, 1990). The results of these evaluations have not been published, nor has this committee carried out independent analysis of the data. Therefore, the reliability of these analyses cannot be verified independently.
The DCC also carries out periodic evaluations of trace-metal contamination in shellfish. The last of these surveys was done during fiscal year 1985-1986. Approximately 300 samples were taken from 20 coastal states and evaluated for 18 trace metals (FDA, 1988c). The DCC also carries out additional evaluations of lead and cadmium in fresh shellfish and other seafood products; of mercury/methylmercury in swordfish, shark, and tuna; and of dioxins in fish. These data have led FDA to assert that its current regulatory effort to control exposure to chemical contaminants is sufficient. The agency argues that the current set of action levels, designed to guide the general inspection program, reflect current concerns over residual contaminants. This policy was recently expressed in the following terms (Benson, 1990):
Occasionally, there are good candidates for the establishment of national standards. … Generally speaking, a candidate for a binding standard is one that would warrant a national policy based on what is known about its toxicity, the amounts being found, and geographic factors such as how widespread or localized the problem may be. Various metals of potential concern for seafood, such as lead, cadmium and arsenic,
have not shown up in the sample surveys that we have taken in amounts that would warrant a national standard. Similarly, pesticides newer than the 13 for which action levels were originally set tend to break down quickly and thus are not generally found in samples. We continue to conduct surveys, however, to update our knowledge.
The committee's assessment of these programs is contained in Chapter 5.
National Marine Fisheries Service
The Inspection Services Division of the National Marine Fisheries Service (NMFS) conducts a voluntary, fee-for-service National Seafood Inspection Program of seafood destined for domestic consumption and for export. The program's regulatory authority derives primarily from the Agricultural Marketing Act of 1946, which allows for the creation of a voluntary inspection and certification program for products in interstate commerce. The Presidential Reorganization Plan No. 3 of 1970 (DOC, 1970) transferred the voluntary inspection program to the U.S. Department of Commerce (DOC), in which NMFS resides. The service is constituted by three general programmatic elements: programs designed to develop product standards and specifications; the voluntary fee-for-service inspection program; and a training and industry/consumer liaison effort.
A primary effort within the inspection program is to inspect and certify seafood processing plants and to issue a "Packed Under Federal Inspection" (PUFI) mark or a U.S. Grade mark to seafood products. Efforts in standard setting and product specification include the "development of product grade standards, Federal purchase specifications, cooperation and compliance with FDA regulations and policies, and the participation in the activities of international organizations (e.g., Codex Alimentarius Commission) as they relate to the development and implementation of international standards and codes of practice" (DOC, 1989). These product specifications are designed primarily to ensure product quality, as opposed to product safety, but do address safety issues to the degree to which they consider FDA seafood safety policies and to which assurances of product quality also provide a means to increase product safety.
The inspection service carries out a wide range of inspection activities including vessel and plant sanitation inspection and seafood product evaluation (which includes a limited number of laboratory evaluations, typically fewer than 100 per year, for biological and chemical contaminants). These inspections are carried out by DOC inspectors, inspectors cross-licensed with the U.S. Department of Agriculture (USDA), and inspectors cross-licensed with DOC-trained state inspectors. Two general types of inspections are carried out under the program: type I in-plant inspections, and type II product lot inspections. In-plant inspections are primarily designed to ensure compliance with minimum sanitation practices and with product-grade standards. A product may be certified U.S. Grade A, B, or C; be certified by a PUFI designation; or be inspected and graded by means of a "no mark" certification. PUFI and "no mark" certifications are driven and limited by the contractual agreement signed between the DOC and the processor (NOAA, 1988a,b). Such agreements may include product-grade specifications (particularly for PUFI certification), but may also be limited to such issues as a contractual assurance that minimum counts (such as the number of shrimp in a box) are being met.
The DOC also conducts product lot inspections for both domestic and exported products. These inspections are generally not carried out for product safety purposes; rather, they are almost exclusively designed to ensure product quality and condition.
General participation in the Voluntary Seafood Inspection Program has increased in recent years. The total amount of reimbursable contract activity increased from $4.4 million in fiscal year 1988 to a projected level of $7.6 million for fiscal year 1990. However, the total number of pounds inspected under the program has decreased significantly over the past decade. For example, in fiscal year 1981 a total of 625 million pounds was inspected, whereas for fiscal year 1988 that total had dropped to 495 million pounds. The reasons for this drop are somewhat unclear. One reason is that the cost of the program to the processor has increased significantly and some participants have dropped out. However, there has also been an apparent shift in the kind of processor contracting with the program. In recent years, more firms processing fresh fish products have been brought into the program, and fewer firms processing large lots of breaded fish have continued in the program. One consequence of this shift has been that although the program has been growing in terms of total contract reimbursement and personnel (the Northeast Inspection Office witnessed nearly a 100% increase in the number of inspectors in the past year), the amount of product (as measured in pounds annually inspected) has decreased. The reasoning here is that for an equivalent effort, an inspector will certify fewer pounds of a fresh or fresh frozen product than in large lots of processed product.
It should be reiterated that this voluntary program is not directed primarily at seafood safety. Rather, the program is directed almost exclusively at plant sanitation and product quality and condition. However, it does impact on the safety of seafood in two nontrivial ways. First, there is a strong correlation between product quality and safety. Although there are many safety questions in which the quality of the product does not play a role (e.g., residual chemical contaminants, natural toxins such as ciguatera and PSP, and certain microbial pathogens), a number of safety issues can be mitigated by a sanitary processing environment and the application of GMPs. Second, the program does provide that a trained inspector be in the plant on a regular basis, and if the product being processed is suspected of being unsafe, the inspector can collect a sample for further analysis or alert the FDA of a potential safety problem.
Training and Educational Programs
The final set of activities to be considered here that are carried out by the federal government in support of seafood safety are related to training and education. In general, these programs and efforts are designed to educate relevant state officials and industry representatives about the risks from various seafood contaminants and about the development of strategies to mitigate those risks. These programs are addressed later in this chapter.
Public Health Monitoring
Some of the more relevant and fundamental governmental monitoring responsibilities are those relating to the compilation and evaluation of data
characterizing the number of people who become ill from eating contaminated seafood. The issue of public health and food has, in recent years, occupied a central place on the nation's political agenda. With this increased emphasis has come an attendant rise in the expectations for, and emphasis on, the role of government reporting of food-borne and–in the context of the present study–seafood-borne illness. The Centers for Disease Control (CDC) of the U.S. Public Health Service (PHS) is the lead agency providing technical support and direction for a range of programs, including the surveillance and investigation of food-borne diseases.
The CDC (1985b) collects and analyzes data on reportable diseases from states and territories. These data are distributed in the Morbidity and Mortality Weekly Report (MMWR) and published in an annual summary (CDC, 1985b). The legislative authority for collecting and disseminating morbidity and mortality statistics goes back to an 1878 act authorizing the collection of morbidity reports by the PHS for quarantine purposes against such pestilential diseases as cholera and yellow fever; however, it was not until 1925 that all states began to submit reports routinely. State and territorial health officers established the Conference of State Epidemiologists in 1950 to determine which diseases should be reported to the PHS by states and what procedures should be followed in submitting weekly reports and annual summaries. This group, currently known as the Conference of State and Territorial Epidemiologists (CSTE), continues to determine the procedures for nationwide morbidity and mortality reporting.
In addition to routine disease-specific reporting, CDC administers other surveillance systems and has conducted national food-borne disease surveillance since 1967. This system also is based primarily on reports from local and state health departments. Reports of each outbreak are submitted on a standard questionnaire, which covers the number of cases, persons hospitalized, and fatalities; clinical characterization of diseases; incubation period and duration of illness; results of epidemiologic investigation, including information on the vehicle incriminated by the epidemiological evidence; the place of preparation and consumption of the suspect food; the manner in which the incriminated food was marketed; factors, such as improper food handling, that are believed to have contributed to the outbreak; and pertinent laboratory data. All questionnaires received are reviewed by CDC staff; missing information is added if possible; and the preliminary diagnosis is reevaluated by using established guidelines. During 1973-1987, 3,699 food-borne outbreaks of disease with known vehicles were reported through the food-borne disease surveillance system. These outbreaks affected 164,695 persons; shellfish accounted for 5.8%, and finfish for 14.6%, of the reported outbreaks.
Food-borne cases and outbreaks are reported to CDC by all states; however, a legal requirement to report is not universal. As with epidemics of other communicable diseases, prompt telephone or electronic notification of food-borne disease outbreaks involving commercially available food products or potential interstate consumption is important for control purposes. However, the quality and completeness of routine surveillance data collected by local or county public health officials and forwarded to state or territorial health departments for transmission to CDC are not uniform and are inadequate for planning and evaluating food safety programs. More important, reporting to CDC does not identify sufficiently the species of fish or shellfish deemed to be the source of disease. Likewise, species verification relies on menu listings (which may not be accurate) or on inexperienced opinion.
In a poll of food-borne disease cases reported in 13 selected states and territories that was conducted by the committee, the general consensus was that reporting was "good" only for outbreaks of those diseases for which an etiologic agent could be identified and for which reporting by the laboratory was required by state regulation. This last point is by no means trivial. The data collected by CDC represent efforts by state health officers, whose resources are largely directed toward the investigation and reporting of illnesses that are officially reportable by that state. The two major sources of seafood-related illness, viruses and vibrios, are not consistently reported by states (Chorba et al., 1989). Indeed, vibriosis is a reportable illness in only 10 of the 56 U.S. jurisdictions (states and affiliated areas). The impact of this point was articulated by a senior public health official in Florida (Karl Klontz, Disease Control Epidemiology Section, Florida Department of Health and Rehabilitation Services, Tallahassee, personal communication, 1989) who suggested that "food-borne outbreaks in Florida are reported if the etiological agent is on the list of reportable diseases."
For those cases in which the disease was mild or had nonspecific symptoms, no specific diagnostic test was available, or a specific food vehicle was not readily apparent, especially when occurrence was mainly restricted to single cases or to small clusters, reporting tended to be very poor. All state health departments that were questioned reported that local, county, or state personnel attempted to identify and trace implicated food vehicles but acknowledged that the success rate was low.
Therefore, it is crucial to understand the limitations of the data gathered by the national food-borne disease surveillance system before attempting to analyze or interpret them. The number of outbreaks may bear little relationship to the total number of outbreak-associated and sporadic cases that occur. For example, Campylobacter infections are at least as common as Salmonella, but many more Salmonella outbreaks are reported. Individual cases of illness caused by seafoods are unlikely to be linked to the responsible seafood unless the illness is typically seafood-related, such as ciguatera or scombroid fish poisoning; the illness is severe enough to lead the victim to seek medical attention; and the doctor recognizes the disease and is one of the few who report faithfully to the local health department. Outbreaks may not be recognized because only a small number of people are ill or because people eating the food disperse after the meal and do not know that others became ill. Even when victims realize that an outbreak has occurred, only a small proportion of recognized outbreaks are investigated thoroughly enough to incriminate a food and determine the pathogen or toxin that caused the illness. A second reason for the inadequacy of reported outbreaks alone in defining the burden of food-borne disease on society is that most such disease, including that transmitted by seafood, occurs as sporadic cases rather than as part of recognized outbreaks. As already noted, the cases reported by CDC are outbreak-related cases rather than a characterization of all seafood-related cases. For example, persons with liver disease who eat raw oysters can get a devastating, frequently fatal infection with Vibrio vulnificus, but no outbreaks caused by this bacterium have ever been reported. Thus, data in addition to those from reported outbreaks are needed to assess the magnitude of the problem.
By using CDC food-borne disease outbreak and reportable disease surveillance data bases, various attempts have been made to estimate the total societal burden posed by food-borne disease. It has been estimated that more than 6 million cases of food-borne disease (excluding V. vulnificus and fish parasite infections, and scombroid,
ciguatera, or shellfish poisons) occur each year in the United States (Bennett et al., 1987). Todd (1989) estimated that 12.6 million cases of seafood-borne diseases annually are not considered in the report by Bennett et al. (1987). An attempt was made to rank various food vehicles by the risk they posed to public health, based on CDC data and published reports from small-scale community health surveys (Douglas Archer, FDA, personal communication, 1990). By those calculations, the consumption of raw molluscan shellfish represented a 1-2 log10 greater risk than cooked chicken, and consumption of cooked chicken represented a 1-2 log10 greater risk than cooked seafood. Clearly, existing data reporting the level and source of seafood-borne illness do not represent accurately either the level or the source of disease. Data currently available are too limited to lead to fully effective, scientifically valid, risk-based control programs, or even to valid comparisons of the hazards posed by different food vehicles. The CDC outbreak data indicate that illness due to seafood is a public health problem and provide information on the characteristics of these illnesses. However, they do not provide reliable information on the magnitude of the problem or its importance relative to that posed by other foods.
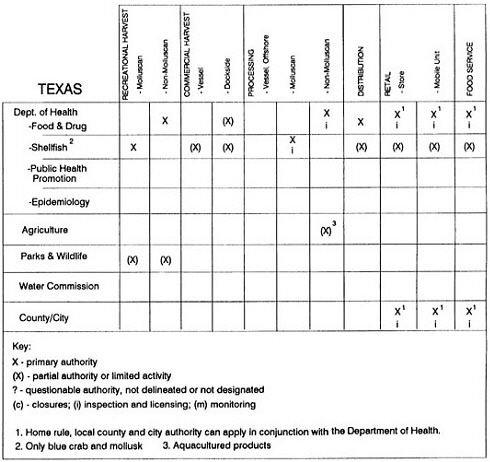
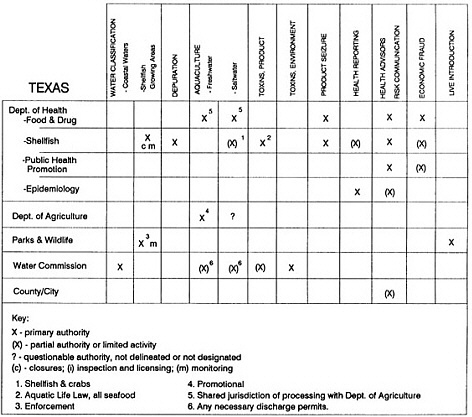
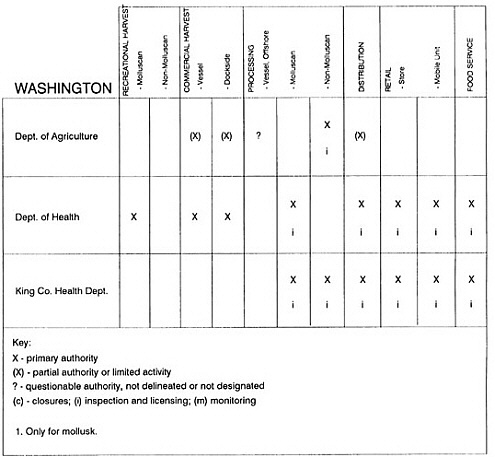
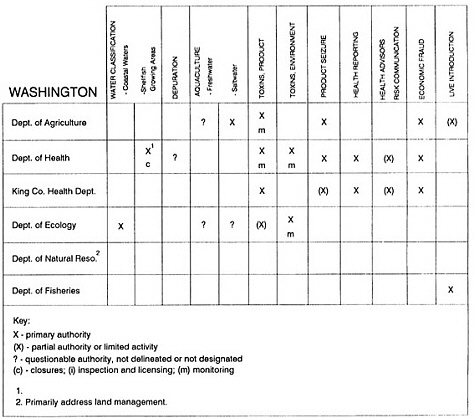
RESPONSIBILITIES AND PROGRAMS OF STATE AGENCIES
As noted earlier, the effort to enhance seafood safety in the United States consists of a set of highly integrated programs within the federal government, various state agencies, and private industry. In this section, the committee considers that suite of programs not focused exclusively within the federal environment.
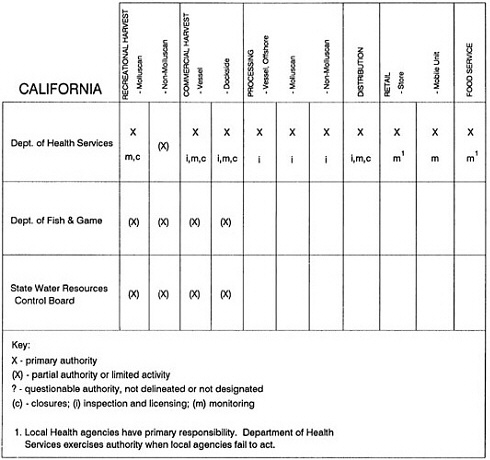
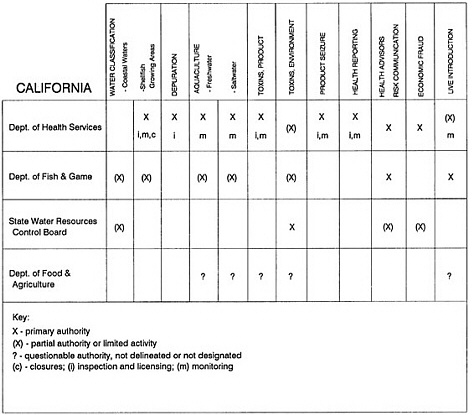
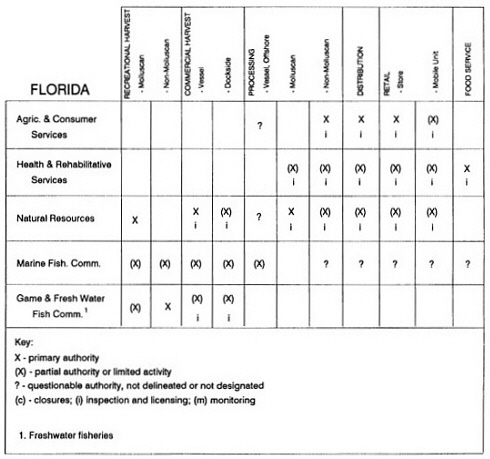
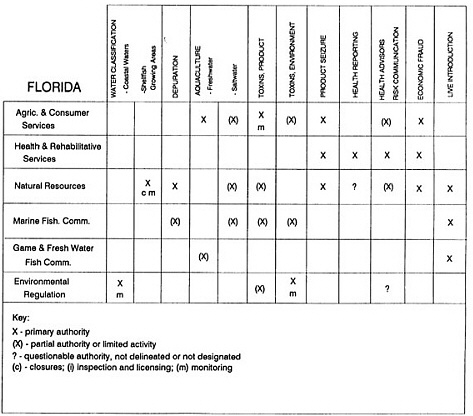
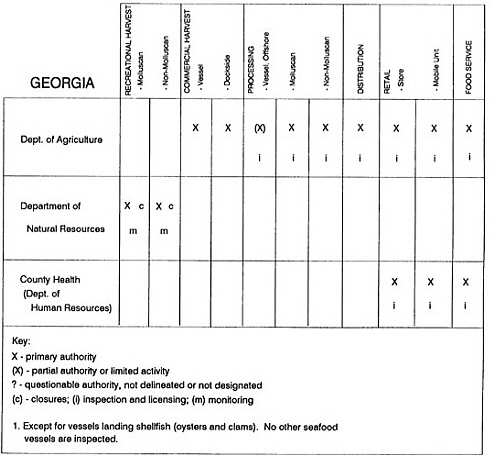
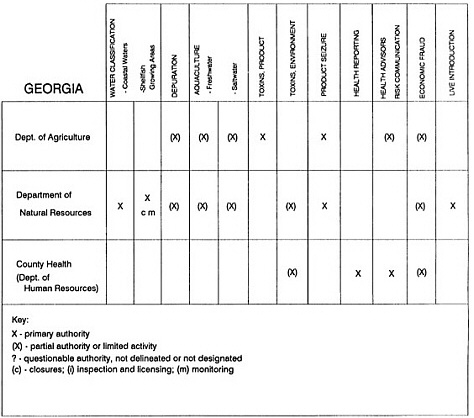
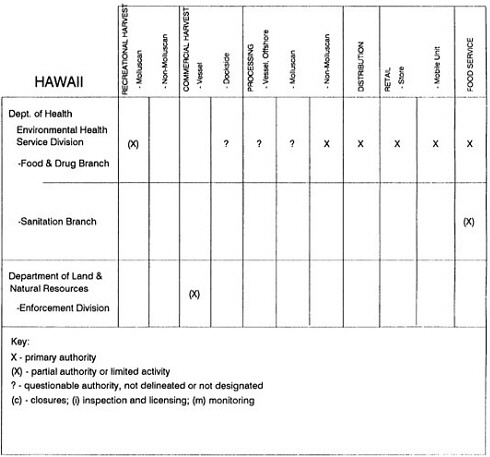
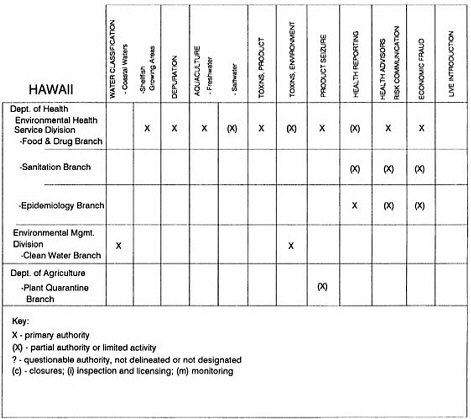
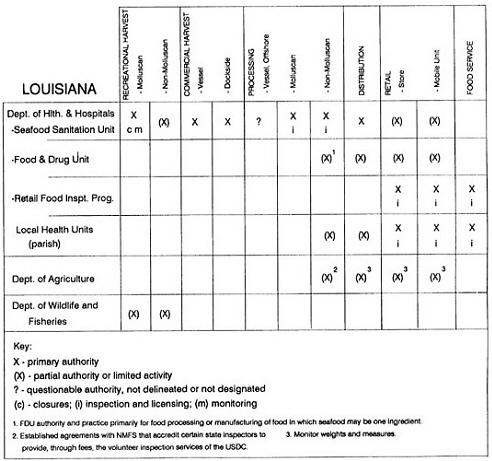
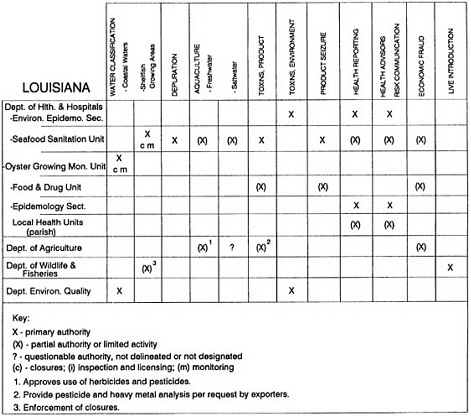
The information used to develop this characterization was compiled by way of discussions from group meetings and individual interviews with a variety of coastal state regulatory agencies and respective industries in an effort to better assess the "typical" state role in assuring seafood safety (see appendix to this chapter). The selection of states for review was directed by contractual obligations referencing locations, historical seafood commerce, and prior reported seafood-related illnesses. Garrett (1988) summarized annual CDC data indicating that 81% of all seafood-related illness in the United States is reported from only nine states or territories: California, Connecticut, Florida, Guam, Hawaii, New York, Puerto Rico, the Virgin Islands, and Washington State, and that the listed territories alone account for more than 49% of all seafood-borne illnesses reported annually.
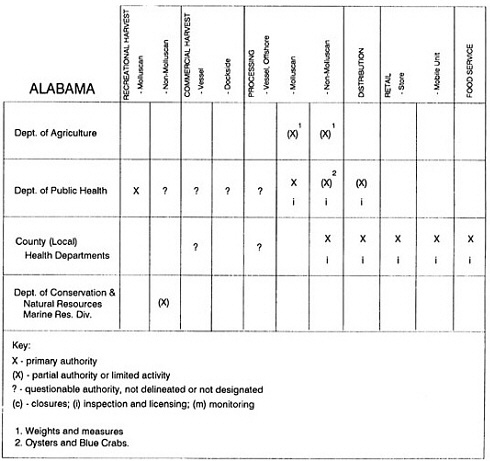
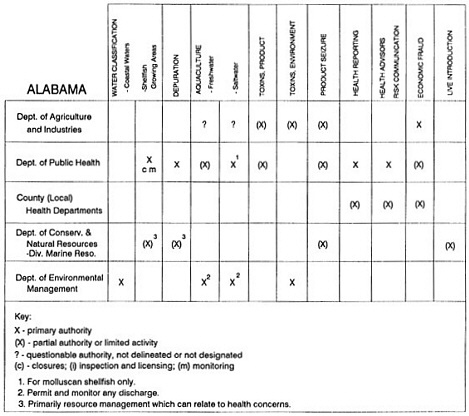
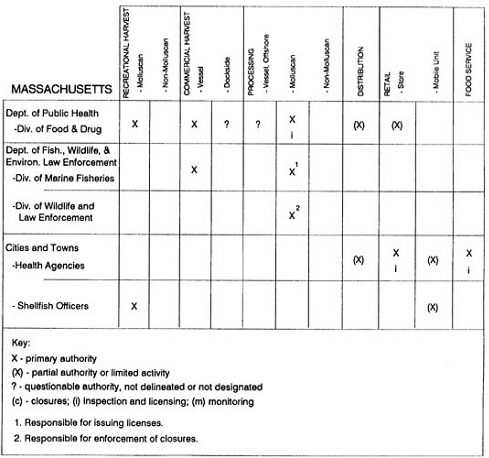
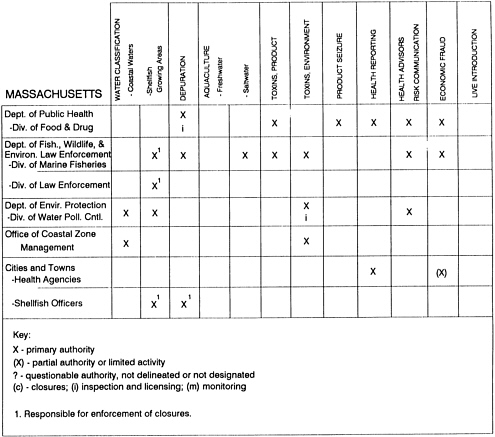
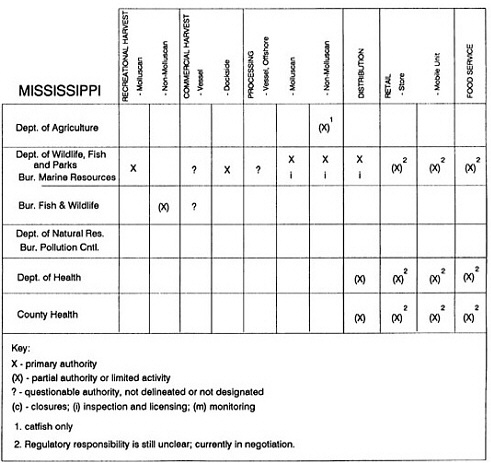
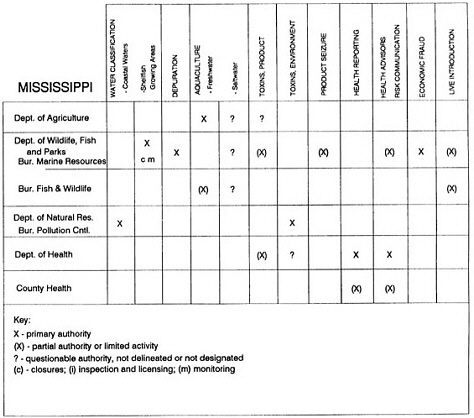
Group meetings in Hawaii, California, Washington, and Massachusetts were conducted by committee members and staff, and supplemented by results from a similar project conducted by the Southeastern Fisheries Association (Tallahassee, Florida) in Texas, Louisiana, Mississippi, Alabama, Florida, Georgia, South Carolina, North Carolina, and Puerto Rico. The meetings involved actual visits to the respective state or territory, and subsequent reviews for additional information. Information from other states and territories (Alaska, Wisconsin, Connecticut, New York, the U.S. Virgin Islands, and Guam) was obtained through individual interviews. The following observations are based on a collective assessment for all these states. Such condensed observations cannot represent all states; nor are they intended to represent, rate, or rank any individual state program. The committee's intention is to provide some initial indications on how state regulations and practices address seafood safety and how state regulations characterize the source and level of seafood risk.
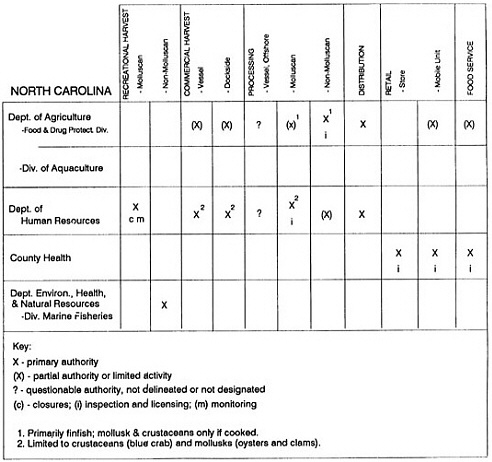
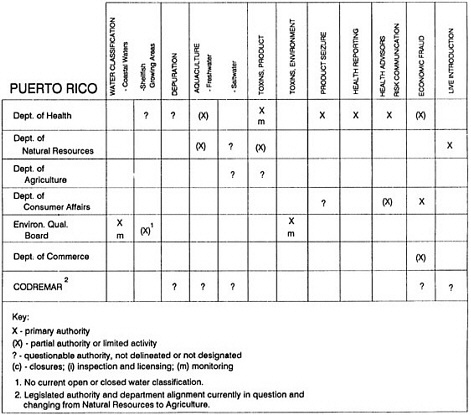
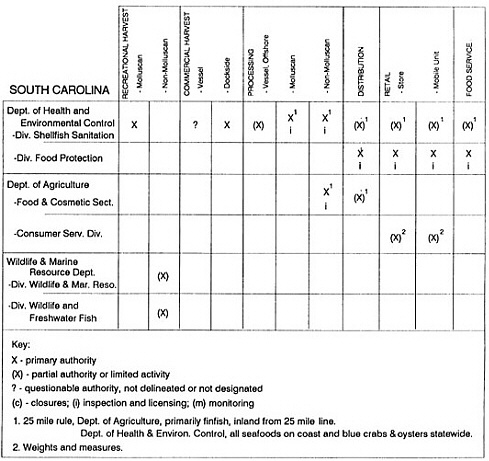
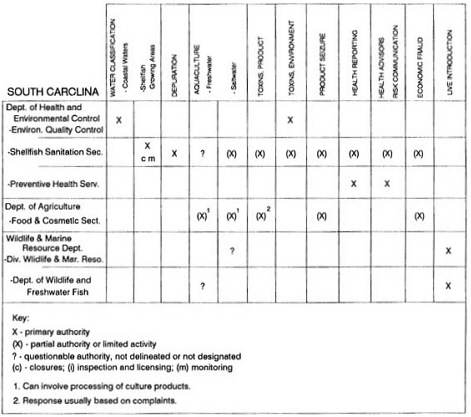
State regulatory authorities pertinent to seafood safety are typically structured and directed to address the prevalent concerns particular to their regional environments, production schemes, and product types. A review of current state regulatory jurisdictions and programs reveals that efforts are segmented with respect to environments (freshwater and saltwater sources), species (shellfish and finfish), product forms (raw and cooked), and stage of product handling (production, processing, retail, etc). In most instances the respective state regulatory organizations are justified by efficient use of resources and expertise, but this inherent diversity can confuse public and industry perception and may hamper intergovernmental cooperation.
In general, seafood safety assurance requires three areas of regulatory focus: (1) harvesting, (2) processing, and (3) distribution and marketing. The remainder of this chapter is organized around these themes. Each of these categories presents attributes unique to specific regions, states, and–in some instances–local sites and species. For example, the adverse health consequence from consumption of raw oysters harboring Vibrio vulnificus is primarily a problem in warmer waters typical of the coastal regions of the Gulf of Mexico. Similarly, ciguatera is more prevalent in tropical regions. The increasing popularity in fresh markets of certain pelagic fish species (e.g., mahimahi, mackerel, tuna) has led to concern about regional incidents of histamine poisoning. Although the less acute health concerns related to environmental contaminants may appear more ubiquitous in distribution, their occurrence and reasons for concern are usually site and species specific. The point to be made is that seafood diversity, in type and distribution, and the association of harvest environment warrant more "customized" state and local regulatory attention than currently practiced for many other foods.
Harvest
Seafood safety at harvest must consider the initial condition of the resource prior to capture and the immediate consequences of handling on the vessel during delivery to the dock. These considerations are similar for the harvest from natural resources or from a cultured stock in fresh-or saltwater environments.
Microbial Contaminants
State efforts in environmental monitoring to protect public health from microbial contaminants are primarily carried out under the auspices of the NSSP and addressed elsewhere in this chapter.
Although molluscan shellfish-borne illnesses remain the dominant, immediate regulatory concern for most states, many felt their molluscan shellfish monitoring programs were adequate and appreciated the guidance provided by NSSP. Of particular concern is law enforcement vulnerability to adequate field staffing to enforce closures for water quality and subsequent legal consequences. The geographic distribution of the resource, surface distinction of waters, and variable harvest time complicate field enforcement. Likewise, in some instances, consequences for illegal harvest have been compromised by court delays, limited penalties, and political influence.
Natural Toxins
State regulatory monitoring for natural toxins in seafoods is largely the responsibility of the departments of health and, in some instances, agriculture–in concert with the equivalent departments of natural resources or fisheries. The role of the latter is usually necessary due to their "on the water" access for resource management. Program activities are distinctly customized to toxins of regional concern. For example, red tides vary in occurrence, duration, toxicity, and public health significance, depending on the causative dinoflagellates. Species in coastal regions of New England and states along the Pacific Coast require closer monitoring for public health reasons than do species more typical of the Gulf of Mexico and south Atlantic regions. For all occurrences, public warnings are appropriate and effective, but the toxigenicity of the northern varieties can be more threatening to public health (see Chapter 4). Epidemiology and pertinent state interviews confirm that current state regulatory programs are adequately protecting public health from toxins associated with red tide. Recreational harvesting carried out by individuals unaware of PSP risks is the major reason for continued warnings. These programs should be continued and expanded through additional public education. Further consideration should be given to future federal assistance because red tides can originate in federal waters and involve adjacent states. As suggested elsewhere in this report, serious consideration should be given to the general development of marine recreational fishing licenses to serve as a vehicle to communicate risk information.
In contrast, ciguatera is a natural toxin of historical occurrence in regions of the United States that still defies state or federal efforts, beyond education, to curb the level of incidents. The lack of a reliable method for detection and the randomness of occurrence among fish samples limit prevention. Fortunately, occurrence is somewhat localized within certain states (Florida, Hawaii) and territories (Puerto Rico, the Virgin Islands, Guam). This situation could change with the expanding demand for fresh tropical fish and recent documented increases of fish imports from tropical regions (Adams and Lawlor, 1989). Likewise, increasing recreational fishing means more harvesting effort in likely ciguatoxic areas. Although extensive research continues to attempt to define and identify the toxins involved, adequate controls have yet to be implemented by the respective states. Given the many sources of suspect fish and the complexity in documenting more accurate occurrence data (from the fish habitat or as a consequence of consumption), state regulatory authorities alone should not be expected to resolve the ciguatera problem. Cooperative federal and state approaches should be considered to restrict the harvesting and marketing of particular species and of fish from particular areas. It should be noted, however, that in some instances, notably Hawaii, the state lacks the legal authority to close ciguatoxic areas to recreational fishing. In many cases the ability to restrict fishing is limited to scientific concerns relating to the health of the stock, not to the human health of seafood consumers.
Chemical Contaminants
State monitoring for potential chemical contaminants in the harvest environments is most often the responsibility of an equivalent department of environmental regulation. The principal objective in state waters is ensuring
environmental quality, which often does not include assessment of the safety of edible resources for consumption. Data on potential contaminants in edible portions of fish or shellfish are usually derived from studies directed by probable cause relative to constituents, species, or locations of concern. Edible product assessments are usually the responsibility of the departments of health or agriculture. Some routine product assessments are conducted for fish and shellfish in the environment, where there is probable cause of risk.
Most state authorities agree that additional edible product assessments should be conducted in a more continuous and expanded manner. Prior hesitancy in state programs was explained by a collection of reasons, presented here in no particular order:
-
Lack of sufficient funds for appropriate sampling, and the need for more analytical equipment and personnel
-
Lack of federal guidance in criteria, common risk assessments, and cooperative encouragement through shared expertise and funds
-
Insufficient evidence to rank environmental contaminants higher than other more prevalent food safety concerns
-
Fear of public misinterpretation and initiation of costly "witch-hunts"
-
A general, professional opinion that environmental contaminants pose no significant threat in most domestic sources of seafoods
States indicated plans for environmental and seafood product assessments in the future. The impact of these assessments could be greatly enhanced by a more coordinated and effective federal-state relationship. This kind of cooperation will be more readily accessible through a recently formed Federal-State Standing Committee on Residues in Fish. These intentions could lead to a more solid foundation for action within and among states if accompanied by agreement on analytical and risk assessment methodologies, more complete listings for constituents of concern, and compilation of and screening for a national data base.
Vessels and Recreational Fishing
If state waters yield safe products, the next harvest segment for regulatory surveillance is the vessel or fishing activity. This commercial activity receives the least amount of regulatory surveillance relative to product safety. States justify their regulatory posture with the assertion that little evidence exists to support, with the exception of molluscan shellfish, direct regulatory action for seafood product safety on commercial vessels. State officials and various segments of the commercial industry contend that fishing vessels can have a profound effect on product quality. This position recognizes that thermal abuse or cross-contamination from unsanitary conditions or chemical spills could result in products compromised by microbial contaminants, elevated histamines, or exposure to sanitizing agents, petrochemicals, or other chemicals. These potential safety issues, however, have not yet been fully recognized as justifying expanded vessel regulations. Thus, many state regulations do not specify the jurisdiction or responsible authority for inspection of fishing vessels. Where states do specify or assume this regulatory role, their efforts have been minimal and typically in response to a recurrent or highly suspicious activity (such as Maryland
regulation of shellfish vessels).
State regulatory agencies realize that steps to initiate more vessel seafood safety surveillance would be quite complicated and time-consuming due to the diversity of vessel types, variable harvest schedules, numerous dockside landings, and multiple products. A least-cost effort that also offers potential educational benefits, might involve initial orientation and certification for newly licensed vessels, annual certification linked to licensing, and a "terminal" inspection program based on unannounced inspections at dockside. These approaches require careful consideration to take into account vessels licensed in other states, variable handling methods per vessel and fishery, multipurpose fishing vessels, ownership of product at dockside, off-loading requirements, and additional harvest variables unique to different fisheries.
Offshore processing vessels present additional confusion for the state with such activity. In most instances, federal versus state jurisdiction would have to be resolved relative to the waters of harvest and the location when processing. Current federal efforts include processing vessels working in U.S. waters. States have not developed discrete regulatory responses to offshore processing in state waters, largely because of the limited amount of activity under their jurisdiction.
A dockside preharvest and postprocessing inspection of processing vessels to include access to records that reflect process conditions is the most plausible approach. In effect, the processing vessel would be subject to inspection similar to that used for shore-based facilities, but regulatory access during actual processing would be limited.
Because many states condone commercialization of a recreational catch, this type of vessel or shoreside harvest activity also requires regulatory consideration for product safety. In some state regulations, recreational and commercial fish harvests are distinguished by licensing, declared intent with permits, and harvest restrictions. These regulations are typically within the jurisdiction of the state agency protecting the aquatic resources and attempting to resolve recreational and commercial conflicts. Seafood product safety is rarely considered.
Whether commercial or recreational activities, all boat or shore-based seafood harvests should be made mindful of restricted areas and species, and should be properly equipped to preserve the catch. Again, certification with licensing and dockside surveillance could apply to recreational vessels, but additional requirements should be considered to ensure product safety in commercialization of the recreational harvest. State efforts to prevent seafood-borne illnesses associated with a recreational catch are typically limited to public warnings to restrict certain harvest sites, species, and consumption. Recreational closures are not common. Indeed, many states lack the legal competence to close recreational fisheries for reasons of public health. Many state-based institutions and agencies continue to issue information to encourage proper seafood handling and preparation. Public awareness and practice suggest that these state-based educational activities should continue and be more focused on prevalent seafood safety concerns.
Processing
Seafood processing is any postharvest handling of the catch or cultured product in preparation for distribution through retail or institutional settings. This activity is defined differently by fisheries and agencies of concern. It may begin with dockside
off-loading and repacking firms, progress through established commercial processing firms that alter the form and appearance of the domestically harvested or imported products, and continue into retail firms that are instituting more innovative processing programs at the store level. These variable levels of seafood processing, in combination with the diversity of seafoods, complicate state regulatory responsibilities.
Organization of state regulatory authority for seafood safety during processing is less uniform among states than that for harvesting, distribution, and marketing. State health departments are most often responsible for product safety in seafood processing. In some states this responsibility is shared with the department of agriculture, with jurisdictions delineated by species and product forms (e.g., Florida, North Carolina, Alabama, Wisconsin), or geographic setting (e.g., South Carolina). Likewise, in some states, processing authority for certain species is housed in the equivalent departments for natural resources or fisheries that justify their role through linking with resource access and "on the water" enforcement capability. Shellfish (oysters and clams) and blue crab processing are the usual candidates for such regulatory distinction in the southeastern United States. A state department of agriculture with sole authority for seafood safety during processing is the exception among states and, when present, usually includes a division of health. This array of state regulatory organizations for seafood safety in processing must be considered in decisions that mandate concomitant federal authority. The alignment of jurisdictions, programs, and regulatory philosophy is essential to fostering more cooperative, responsive, and uniform programs.
In general, most state regulatory officials believe that seafood processing does not present a major food safety problem and that, where potential problems exist, they are being addressed. Therefore, limited state-based resources are directed at inspection and control of commercial processing. The four most prevalent recorded seafood-borne illnesses – ciguatera, histamine poisoning, microbial infections from raw molluscan shellfish consumption, and cross-contamination with Vibrio parahaemolyticus (Garrett, 1988) – occur primarily from consequences outside the processing sector. Thus, state regulatory attention to seafood safety during processing has focused on the critical points of product procurement and entry that initiate processing. This preprocessing consideration complements state recommendations to expand environmental monitoring efforts that ensure product safety prior to processing. These recommendations are supported further by the fact that imported products destined for further processing constitute the major portion of seafood consumption in the United States. Likewise, to support postprocessing concerns, states emphasize the importance of increased education for proper product handling in retail institutions and in the home because seafood product safety can be significantly compromised in these settings.
State records for common seafood processing violations usually involve GMPs and adulteration or mislabeling that constitutes economic fraud. These quality problems can be resolved by existing state authorities. State access to permitted processing firms and point-of-sale transactions represents the first and most immediate regulatory influence. The effectiveness depends on frequency of inspection and more education to direct compliance. Most state process inspections are directed by prior firm performance, probable cause, and complaints. In many instances, regulatory state and industry representatives recommend additional, routine inspections but do not expect regulatory activity to serve as a quality control program.
Certain seafood processing quality violations could constitute potential product safety problems. For example, excessive and improper application of sulfiting agents to prevent crustacean melanosis could pose a health threat for consumers in asthmatic risk groups. Fish species substitution, intentionally or by error, could present a natural toxin not common to the intended fish product. Such adverse events are possible, yet in terms of food safety reports they are rarely if ever recorded, even in states more prone to these processing considerations.
Raw molluscan shellfish production and processing were considered the most dominant seafood safety concerns for most states. Prior state concern culminated in the development of the ISSC, which helped strengthen shellfish processing guidelines specified in the federal NSSP manuals (FDA, 1989b). In addition to basic GMPs, states enforce stringent tagging and labeling requirements that identify the source and time of harvest for live shellstock and shucked meats. The tagging requirement extends the processor's responsibility to the point of harvest and through all forms of marketing. Processors remain liable for 90 days posttagging. This product identification system represents one of the most tedious forms of inter- and intra-state seafood safety regulation. In addition, specified durations for in-plant processing and terminal sale-by-date requirements can be imposed for additional public protection. Combined with the commitment to monitor shellfish growing waters, state regulatory attention to ensure safe, raw molluscan shellfish often represents the most extensive regulatory effort per single seafood commodity. Continuing raw molluscan-related illnesses justify this commitment, along with the realization that monitoring for harvestable waters represents a stalwart effort to prevent coastal pollution in producer states.
In most instances the continued level of state regulatory attention for all seafood processing will require more manpower, additional support funds, and better analytical capability. States typically lack sufficient administrators and inspectors experienced with seafoods and with the subtleties of pertinent regulations. There is interagency confusion about certain regulatory interpretations and authority. A common industry complaint is lack of knowledge of the applicable regulations and authorities. Industry processors complain of the absence of a single, condensed, and instructive manual delineating the regulations and regulators. A single source booklet would undoubtedly improve both understanding of, and compliance with, state regulations on seafood. Interestingly, many processing firms have experienced and appreciate a more "instructive" form of state inspection and regulation than evident in federal activity. In some instances, state regulations are criticized by the respective authorities and the seafood industry for lacking specificity and the ability to adapt to innovative processing concepts. Dependence on federal guidance and difficulties in changing or amending statutes are common excuses for this lack of flexibility and adaptivity in state seafood safety regulations. In general, state regulations concerning seafood quality and safety – from harvest through processing, distribution, and retail – should be reviewed periodically for changes and supplements to take into account evolving practices and better industry guidance.
Distribution and Marketing
Seafood safety in distribution and marketing usually involves the regulatory responsibility of county and city health departments inspecting restaurants, retail outlets,
and institutions. Following guidelines from their respective state departments of health, and in some instances agriculture or natural resources and fisheries, these more localized authorities combine unannounced inspections with periodic training to ensure compliance with basic food safety practices. Additional guidance for food safety in retail has been provided through the national Food Retail Code Programs (AFDO/FDA, 1982) and a federal initiative to establish a Unicode that offers more uniform standards for sanitation in all retail practice (Anonymous, 1989a). These codes are basically GMPs and list critical points for control, including some specific concerns for seafoods.
Localized regulatory authority is more general, to account for the variety of food types and markets in cities and counties. Responsibilities can also include mobile retail units. The authorities are not commodity specific, rely on more specialized state-based seafood authority and training, and encourage additional and continuous training that addresses seafood safety and quality issues. This request is of particular concern in counties and cities that have a higher proportion of seafood marketing activity or experience a disruptive amount of annual inspector turnover.
The importance of localized regulation of seafood safety should be considered more in decisions to improve or expand state and federal regulatory programs. Combatting the high proportion of seafood-borne illness that occurs in restaurant settings and dealing with health concerns related to recreational fishing activities require more localized attention. Cross-contamination of ready-to-eat seafoods is a common consequence of poor handling and thermal abuse in restaurants, retail stores, or homes (Bryan, 1986). Sale of a potentially ciguatoxic or scombrotoxic fish can result from a local recreational activity. Similarly, prevention of uninformed recreational shellfish harvest from closed, contaminated waters requires local, accessible advice.
Although some county programs are extensive and may be more seafood specific, as exemplified by the King County Health Department in Washington, most county and city health authorities lack sufficient personnel and funds to afford more seafood-specific attention. Their impact on seafood safety must depend on public and institutional education available through cooperation with state authorities and academically based programs (e.g., Agricultural Cooperative Extension Services and Sea Grant Marine Advisory Services).
Additional State Considerations
Meetings and interviews with state groups revealed a number of common concerns that warrant additional consideration in evaluating state regulatory roles in ensuring seafood safety. These concerns vary by state but essentially involve every coastal state. Currently, these concerns are not considered major problems but are discussed as points for future consideration.
Aquaculture
Freshwater and marine-based culture of aquatic foods (cultured seafood) is increasing significantly in amount and diversity in domestic settings and imported
products (Redmayne, 1989). Domestic aquaculture is currently estimated at more than 790 million pounds, valued at approximately $700 million and expected to surpass 2 billion pounds by the year 2000 (Anonymous, 1989b). Initially, state regulatory agencies are faced with this evolving industry through decisions for land and water use relative to zoning, quality, and public use conflicts. Most of these decisions, viewed as constraints, involve production but public health questions will eventually arise. Anticipating this situation, most states have initiated discussions of jurisdictions and the necessary aquacultured food safety priorities.
Fortunately, aquaculture offers a more controlled form of seafood production that is less prone to common seafood-borne illnesses (Fong and Brooks, 1989; Otwell, 1989; Rodrick and Cheng, 1989; Ward, 1989), but its safety can be compromised by environmental contaminants and mishandling during processing or marketing. Basic food safety considerations suggest that cultured seafoods simply represent another protein-based aquatic food that can be addressed by the existing scheme of state regulations. What is contrary to logic is the jurisdictional confusion due to the production environment (natural versus man-made, freshwater versus saltwater), the distinction between product types (traditional harvesting versus cultured), and state and federal assistance programs established to promote aquaculture. All of these issue were evident in discussions with state authorities and respective industry representatives.
The existing situation may offer a favorable incentive for all states to reevaluate their current seafood regulatory programs. Careful deliberation and federal guidance are necessary to ensure uniformity and to avoid regulatory dichotomy that could dilute and further confuse the necessary authorities for food safety and quality. There are no aquatic food safety distinctions relative to chemical or microbial contaminants to justify separate aquacultured food safety regulatory authorities, and food safety concerns should not be compromised by efforts properly aligned to direct and promote production and market development.
Admittedly, federal and state regulatory attention for safety in aquacultured products lags behind culture development, but there has been little research to characterize food safety problems with such products. Concerns for chemical and microbial contaminants in the culture water, and the use of therapeutic drugs, which are being addressed by FDA efforts, should be expanded to include more state involvement.
Recreational Fisheries
Recreational fishing activities involve public seafood safety concerns that require state scrutiny relative to the harvest and the point of sale. The catch destined for personal use or commercialization must be taken from approved waters and properly handled prior to consumption. Safety for individual use is best ensured through public education and controlling resource access. Public protection through commercialization of the recreational catch presents more challenges. Current licensing of recreational activity as a commercial practice does not address seafood safety.
State authorities have difficulty in determining when a recreational harvest becomes a wholesale or retail item. Except for dockside sales to tourists, contemplated sales to licensed firms for further seafood marketing should constitute a commercial transaction. Certain states require some form of permit for declared harvest intent or
an equivalent form of "wholesale" dealers license to cover this event. These permits and licenses are usually intended to resolve recreational and commercial conflicts and to protect resources through harvest restrictions and information obtained for resource assessment. The buyer assumes responsibility for the safety of the catch.
For most recreational harvest a subsequent commercial event does not present a seafood safety problem, but states must prepare to address certain real and previously violated concerns. For example, the sale of recreationally caught fish harvested with a commercially licensed party boat from known or suspected ciguatoxic areas has resulted in ciguatera in Palm Beach County, Florida (PBCHD, 1988). Histamine poisoning is possible from the sale of certain scombroid-type fish that are popular recreational targets. Similar situations could arise from the sale of molluscan shellfish taken from local, unapproved waters. In each event, liability would rest with the initial buyer, but subsequent buyers, be they commercial or public, may not be aware of the source. If states continue to allow commercialization of recreational catch, they must consider some controls to license the event in a manner that identifies the product and the source. This situation may also involve federal authorities responsible for any harvest in federal or foreign waters.
Imports
Regulatory scrutiny for product safety of imported seafoods is the primary and initial responsibility of the FDA, but eventual domestic distribution can evoke additional state responsibility. Concerns for foreign product identification, country of origin declarations, certified sources, adulteration, and other seafood safety-related attributes become a state regulatory responsibility after the imports enter state commerce. By design, FDA attempts to alert states when specific products may pose problems, but the effectiveness of this system is compromised by limited federal surveillance of imports. Indeed, difficulties in identifying final product destination further complicate state-FDA communications. For example, as previously noted, the importation of shellfish from countries without formal NSSP memoranda of understanding2 is currently controlled by reference to state regulations requiring purchase and handling of shellfish from approved harvesting grounds. Clearly, state regulations can have little direct impact on the certification of foreign sources. These issues are generally appropriate to a rather broad set of seafood products.
Training and Education
Recommendations solicited during state group meetings and interviews were unanimous in calling for additional employee training with orientation to seafood safety and similar continuing education for the industry. Periodic programs are currently provided by the NSSP to ensure uniformity among state molluscan shellfish regulatory programs, and FDA workshops are occasionally offered for a variety of topics (e.g., retail sanitation, importing requirements, labeling issues). Some of these programs include seafood-related training for inspectors and industry. Likewise, the USDA conducts workshops to ensure better aquacultured products. The USDA has conducted extensive work in sensory evaluation of pond-cultured catfish (Johnsen, 1989). The
states acknowledge and appreciate these federal efforts, and suggest they be increased and made more convenient for regional and field staff.
Similar concerns are noted for the variety of state-based educational efforts provided through regulatory agencies or in the complementary extension and advisory programs offered by state universities and community colleges. In many programs, education for seafood safety has focused on critical points of concern for the vessel harvest through processing and retail. A few programs have included guidance in recordkeeping as an introduction to the Hazard Analysis Critical Control Point (HACCP) concept for product quality and safety. Despite these prior and ongoing efforts, most seafood industry representatives were not familiar with HACCP in a seafood processing or retail setting, yet their daily practice intuitively employs the critical point assessments without necessarily recording the daily events.
Innovative recommendations have suggested that more educational efforts should combine regulatory and seafood industry personnel in the same audience. Subsequent field education could be arranged to orient the inspector and the industry personnel to each of the respective settings. An industry-based internship for future inspectors could be instituted by trade associations to enhance regulators' appreciation of daily processing procedures. Standardized educational programs could be used to initiate a "certification" program with mandatory seafood training for plant employees, particularly managerial staff.
Despite the excellence of most programs, most seafood safety training efforts have not been well coordinated across regions (a group of states) or with respect to more national guidance for uniform content. Again, the unique attributes of a region or state require a certain seafood-specific orientation, but there needs to be greater consideration of uniformity, especially in the education of seafood inspectors and process managers.
CAUSE OF ILLNESS IN TARGETED STATES AND TERRITORIES
The situation regarding seafood-borne illness was examined specifically in a number of states identified by NMFS as having particular importance. In general, the examination confirmed conclusions of the broad national study that such illness is strongly geographic in incidence and that shellfish eaten raw are the principal cause of individual sickness in most coastal states. Data on outbreaks reported to CDC by the states under consideration for the period 1978-1987 are listed in Table 8-6 (CDC 1981a-c, 1983a,b, 1984, 1985a, 1989). Large differences are apparent in the relative importance of seafood-related illness among the states and territories. Hawaii, Puerto Rico, and the Virgin Islands report that seafood(s) are responsible for the majority of outbreaks of food-borne illness; this is due dominantly to seafood toxins. The other states report 15-33% (mean 26%). Cases per outbreak are relatively low so that the average for the nine states and territories represents only 7% of all food-borne illnesses. Nevertheless, Hawaii and New York report 39 and 23%, respectively, whereas Virgin Islands report 100% of cases. Discussions with state health department personnel and perusal of state food-borne disease reports have revealed discrepancies between CDC tabulations and in-state reported incidents of seafood-related illness. In part, these are a consequence of the limited range of diseases reported (e.g., Vibrio vulnificus was not reported) and the widespread occurrence of single-case incidents that
are not reported. Thus, in most cases, the incidence of seafood-related illness is underreported, but this is also true for other food-borne illnesses. State health officials provided estimates for actual incidence of seafood-borne disease ranging from 8 to 25% of all food-borne illness.
It is clear, as noted elsewhere in this report (see Chapter 3), that special circumstances influence the number and type of seafood-borne illnesses reported. These include the source and condition of the seafood supply, the eating and food preparation practices of the local population, and actions by state or local authorities. Officials from coastal states in the Northeast including Massachusetts, New York, and Connecticut indicated that the seafood of major concern was raw molluscan shellfish and the causal agents were viruses. This reflects concerns over both domestically produced and imported molluscs, mostly clams, and the prevalence of local consumption of uncooked or very lightly cooked shellfish. One factor in this situation is the difficulty in controlling distribution of clams illegally harvested from areas closed because of contaminated growing waters. However, there is a question concerning the reliability of fecal indicator bacteria as an index of viral contamination because of the somewhat greater persistence of contaminant viruses. It is also known that depuration procedures used in some supplier countries are not fully effective in eliminating viruses
TABLE 8-6 CDC Food-borne Disease Outbreak Surveillance Data, 1978-1987a
from clams. There is evidence that both these deficiencies have been factors in shellfish-related incidents in the Northeast during the past 10 years. These states are also concerned with paralytic shellfish poisoning and scombroid fish poisoning. There is general satisfaction with present PSP control measures, but more rapid test methods are desired. The scombroid problem is not a major health hazard but is seen primarily as a result of poor industry practices, usually occurring outside state jurisdiction. Disease due to vibrios is not considered a major problem in these states, though sporadic illness due to V. parahaemolyticus does occur. Northeastern states are concerned about (industrial) chemical contamination of inshore waters and have closed
areas to fishing for this reason.
Michigan was the only inland state reviewed, and health personnel indicated that on the basis of recorded illness, hepatitis A virus (HAV) and scombroid poisoning were the major concerns for seafoods. However, it was emphasized that hepatitis A was most commonly transmitted by food handlers. Thus, it is not a specific seafood-associated disease. Michigan has effected closures and warnings in its freshwater lake and river fisheries because of chemical contaminants. The West Coast states California and Washington also indicated shellfish as the major public health concern, and in both cases vibrios and PSP were identified as significant problems. V. parahaemolyticus is a consistent cause of sporadic seafood-related illness in both states, and V. vulnificus has been encountered in California. Health officials in both states expressed concern over imports, particularly from Pacific Islands and Southeast Asia. This concern is related to the large number of recent immigrants from these areas and the associated increase in production and consumption of ethnic foods. This includes different species of marine animals and consumption of parts of the animal (e.g., intestines) normally discarded by more traditional U.S. consumers. There is also concern over scombroid fish poisoning which is relatively common, and ciguatera, which is still relatively rare but expected to increase due to importation of Pacific Island fish.
A third Pacific Coast state, Alaska, shares the PSP concern, but has a unique problem of botulism associated with consumption of fish and marine animals. The occurrence of botulism is an ethnic food problem of native Alaskans resulting from consumption of "fermented" products held under conditions that encourage the growth of Clostridium botulinum. Scombroid fish poisoning from imported fish also occurs in Alaska.
The island states and territories Hawaii, Puerto Rico, Virgin Islands, and Guam show disease patterns that are a consequence of both the major local fish supply and local eating customs. In all areas, seafood constitutes a major part of the diet and is the cause of significant food-borne disease dominated by ciguatera and, to a lesser extent, scombroid fish poisoning. The ciguatera problem is intractable. All health officials emphasized the need for development of rapid test(s) for ciguatera toxins. Without such tests, it is impossible to ensure the safety of fish consumers in these areas. At present, the only available procedures involve warnings issued by health authorities and, in Hawaii, some voluntary control of commercial distribution of dangerous species by the industry. Ciguatera is of particular importance in the islands because fish is a major source of protein for permanent inhabitants and because tourism is a major industry. Tourists in the Caribbean are increasingly at risk from ciguatera (Morris et al., 1982; F. Quevedo, Regional Advisor for Pan American Health Organization, Washington, D.C., personal communication, 1989), although this problem does not seem to be a significant cause of illness among visitors to Hawaii (M. Sugi, Epidemiology Branch, Department of Health, State of Hawaii, personal communication, 1990).
Guam presents a somewhat different set of circumstances. Apparently, seafoods are commonly eaten raw or lightly processed so that in addition to the typical ciguatera problem, there is a significant incidence of vibrio disease. This is illustrated in Table 8-7, which tabulates seafood-related disease incidents reported by the territorial epidemiologist Dr. Robert L. Haddock (Haddock, 1989). Guam has experienced small outbreaks of true cholera related to consumption of seafoods, but most cases have been caused by V. parahaemolyticus or V. cholerae non-O1. The large number of
single-case incidents are the principal reason that the data in Table 8-7 are at variance with the CDC report in Table 8-6. Although the high incidence of illness from Vibrio is certainly due in part to eating patterns in the territory, this cannot be the only factor. Consumption of raw seafood is also quite high in Hawaii. The territorial epidemiologist in Guam notes the importance of actually testing for vibrios in suspected seafood-related illness by the use of thiosulfate-citrate-bile salts-sucrose (TCBS) selective agar medium. The differences and similarities between patterns of seafood-related illness in the various island states and territories underscore both the commonality of problems in tropical areas and the unique features that distinguish one area from another. This supports the view that problems must be dealt with at the state and local levels.
Florida is a mainland state that abuts a tropical ocean; it is not surprising therefore that ciguatera is a concern of health officials in that state. The state is an importation point for fish from the Caribbean area, including potentially scombrotoxic fish, and is also subject to periodic red tides due to Gymnodinium breve that make local shellfish toxic. However, the major seafood-related health concern is vibrio disease related to raw shellfish consumption. High water temperatures encourage vibrio growth including that of V. vulnificus , and if oysters are not cooled quickly after harvest, vibrios will multiply and present a hazard to people eating shellfish raw. Vibrios (V. parahaemolyticus, cholerae non-O1, and vulnificus) seem to present the greatest hazard for consumers of raw molluscs harvested in states such as Florida and Louisiana, whereas viruses are more significant in the Northeast. Of course, cases due to vibrios also occur in the North, and viruses have caused mollusc-related illness in warmwater states. In West Coast states, shellfish problems appear to be PSP and vibrios, with lesser incidence of virus-related disease.
One conclusion that can be drawn from this fragmentary review of the status of seafood-related illness in selected states and territories is that the particular circumstances of each state requires that control systems be tailored–at least in emphasis–to local conditions. However, it is also obvious that states need help in developing procedures and methods of analysis that will be effective. They also lack the skilled personnel, technical facilities, and money to undertake this development themselves.
Allergies and Intolerances
This report does not directly address illnesses caused by seafood intolerance or allergies in the sense of reported occurrences and regulatory responses. Intolerance to eating certain types of seafoods is rare and more typically associated with certain individuals in risk categories predisposed by other health complications. Seafood allergies, distinguished as immunological reactions rather than the inability to digest, appear to be more prevalent, but they are difficult to diagnose and document. Specific allergens in seafood have thus far been only grossly characterized in few studies. In most seafoods of concern (e.g., certain crustaceans and "pink flesh" fish), very little has been done to identify the chemical offender (Kilara, 1982). In some cases a food
TABLE 8-7 Food-borne Illness in Guam
|
Year |
Fish and Shellfish |
All Foods and Etiologies |
||||||||||
|
Salmonella |
Vibrio cholerae |
Vibrio parahaemolyticus |
Ciguatera |
Unknown |
||||||||
|
Outbreaks |
Cases |
Outbreaks |
Cases |
Outbreaks |
Cases |
Outbreaks |
Cases |
Outbreaks |
Cases |
Outbreaks |
Cases |
|
|
1978 |
|
|
|
|
3 |
22 |
4 |
6 |
1 |
2 |
10 |
56 |
|
1979 |
|
|
|
|
2 |
14 |
6 |
10 |
|
|
12 |
50 |
|
1980 |
|
|
|
|
2 |
6 |
5 |
10 |
|
|
13 |
37 |
|
1981 |
|
|
1 |
4 |
|
|
3 |
3 |
|
|
10 |
38 |
|
1982 |
|
|
2 |
9 |
|
|
3 |
3 |
|
|
9 |
31 |
|
1983 |
|
|
|
|
1 |
4 |
11 |
21 |
2 |
2 |
22 |
42 |
|
1984 |
|
|
|
|
6 |
8 |
8 |
22 |
2 |
5 |
40 |
70 |
|
1985 |
|
|
3 |
3 |
15 |
22 |
13 |
28 |
9 |
16 |
65 |
107 |
|
1986 |
1 |
2 |
2 |
4 |
16 |
24 |
3 |
9 |
4 |
5 |
50 |
121 |
|
1987 |
1 |
5 |
|
|
9 |
15 |
4 |
5 |
1 |
1 |
23 |
57 |
|
Totals |
2 |
7 |
8 |
20 |
54 |
115 |
60 |
117 |
19 |
31 |
254 |
609 |
|
Totals |
Seafoods: Outbreaks |
143 (124)a |
Cases |
290 (259)a |
|
|
|
|
|
|
||
|
|
All Foods |
254 |
|
609 |
|
|
|
|
|
|
||
|
|
% Seafoodsb |
56% |
|
48% |
|
|
|
|
|
|
||
|
a Number in parentheses indicates total for confirmed etiologies. b Indicates outbreaks or cases as a percentage of total food-borne incidents. SOURCE: Haddock (1989). |
||||||||||||
additive (e.g., sulfiting agents on shrimp) or contaminants may cause the symptoms and confuse the diagnosis. In light of this level of information on cause and occurrence of this somewhat limited form of seafood-borne illness, regulatory response must depend on proper labeling to distinguish (1) species or types of seafood, (2) ingredients in formulated and fabricated seafoods (e.g., fish base surimi formed to resemble crab), and (3) ingredients used in preservation and processing (e.g., sulfites to retard shrimp melanosis). Future responses will require additional investigations of the biochemical and immunological characteristics of seafood allergens and their significance in producing illnesses.
STATE-FEDERAL REGULATORY LIAISON
Because seafood diversity poses region-specific concerns in monitoring coastal waters, in addressing species unique to local harvests and process settings, and in accessing point-of-sale transactions and recreational fishing, state regulations have played the more immediate and dominant role in surveillance of seafood safety and quality. However, federal cooperation and support is essential. All pertinent federal authorities are represented by an equivalent agency at the state level that, in most states, models and adopts regulations in accordance with its federal counterpart.
In most states the primary enforcing agency for seafood safety is the state department of health equivalent to the U.S. Food and Drug Administration (Hui, 1986). In some states this authority is shared across species or commercial settings by the departments of health, agriculture, and equivalent divisions within a department of natural resources. In a few states the department of agriculture maintains sole authority over all seafood, usually in a division or bureau of health. This diversity among states reflects the unique attributes of seafood and the challenge for statefederal liaisons.
The FDA exerts a significant effort to support state-based seafood safety surveillance through (1) maintaining inspection procedures and directives, (2) commissioning state officials to perform federal regulatory activities, (3) issuing contracts for routine and specific investigations, (4) advising actions and policy, and (5) administering training and education programs. In an effort to direct inspection activities, FDA maintains and, for retail programs, assists the Association of Food and Drug Officials (AFDO) and regional AFDO affiliates in preparing various retail sanitation codes (AFDO/FDA, 1982). "Unicode" is an ongoing FDA attempt to eventually combine the variety of food store codes into a single retail code (Anonymous, 1989a). Similar attempts at regulatory standardization are evident in FDA's manuals being developed for the HACCP approach in molluscan shellfish sanitation, smoked fish guidelines, course outlines on modified atmosphere packaging, and the comprehensive Fish List to designate the official nomenclature for all fish and, eventually, for molluscs and crustaceans (FDA, 1988a,b). Immediate FDA-state liaison is possible through electronic linkage now available in over 125 metropolitan centers. Periodic liaison is possible through federal contractual obligations for production or food processing and retail surveys. In 1988-1989, Colorado, Florida, Oregon, and Wisconsin participated in seafood-specific contractual surveys for microbial consequences in commerce.
Further, the Environmental Protection Agency plays an active and often aggressive liaison role. For example, a recently formed Fish Contaminants Advisory Committee combines FDA and EPA efforts to direct and encourage states to use more uniform methods of risk assessment and to advise on analytical methods and decisions concerning potential waterborne seafood contaminants. The EPA National Estuary Program can be viewed as a useful effort to work with states to determine sources and levels of contaminants in coastal waters that serve as both spawning and harvesting areas for important commercial species. Clearly, these programs are not specifically designed to deal solely with issues of seafood safety; however, the results of this sponsored research are essential to a comprehensive evaluation of the quality of the nation's harvesting environments.
The Interstate Shellfish Sanitation Conference represents the most comprehensive, routine state-federal liaison specifically established to address seafood safety concerns. This national organization ensures cooperation among state authorities, NSSP, and respective molluscan industries in sharing the responsibility to establish federal and state regulations for molluscan shellfish product safety.
Cooperative voluntary inspection services have been established between requesting states and the National Marine Fisheries Service. Under specific agreements, NMFS provides state inspector training and certification to perform DOC inspections on a per-fee basis paid by the industry user. States with varying degrees of prior participation include Alabama, Alaska, Colorado, Florida, Michigan, Minnesota, New Jersey, New York, Oregon, and Tennessee. The lack of DOC fish inspectors, particularly in certain areas, encourages continuation of these coinspectional agreements.
Efforts to promote domestic aquaculture have been enhanced by USDA's cooperative programs in regional training, research services, and extension services. The 1980 National Aquaculture Act designated USDA as the lead federal agency in promoting aquaculture. The USDA offers specific programs to improve marketing (Federal-State Marketing Program) and to resolve quality problems such as the occurrence of off-flavors in catfish (Johnsen, 1989). Likewise, USDA's Animal and Plant Health Inspection Service provides diagnostic assistance for identification and treatment of fish disease. These activities relate to cultured product quality and safety; however, FDA is still the primary food safety regulatory authority, and it maintains open cooperation with USDA and the states on all pertinent aquaculture issues.
Overall, the advantages and necessity of federal-state liaison for seafood safety could be enhanced with better and more routine communications focused on more seafood-specific issues as evidenced for molluscan shellfish. The ISSC, in design, exemplifies the mode of cooperation that can improve surveillance for seafood safety. The ISSC strength lies in equal state participation, direct industry involvement, and eventually national implementation through federal guidance. Similar efforts should be explored through established associations that offer the same essential participation and could be restructured to allow a more regulatory development forum. Candidate associations include AFDO and regional affiliates of the International Association of Milk, Food and Environmental Sanitarians (IAMFES). These groups of experts have not traditionally featured or excluded seafoods, and although they may not be amenable to new mandates, they certainly represent collective talent to initiate better seafood regulatory liaison between state and federal authorities. Recent seafood-specific concerns of the AFDO and its southern affiliate, the Association of Food and
Drug Officials of Southern States (AFDOSS), were voiced in a national letter dated September 9, 1989 of state regulatory position on seafood inspection mandates and a resolution calling for a federal position on new GMPs for smoked fish (AFDO, 1989). These actions reflect a concerned organization that can be channeled to better facilitate federal-state liaison for seafood safety.
STATE AND INDUSTRY INITIATIVES
Initiatives to foster better seafood quality and safety come in a variety of forms, both state and industry based, that are developed primarily to promote a particular product or seafoods in general. The focus is usually on product quality, and efforts are structured to direct industry compliance or to offer general operating guidelines and product specifications. Most of these efforts are based on voluntary participation.
The most structured industry initiative is the Canned Salmon Control Plan initiated in the early 1920s and formalized in 1936 (NFPA, 1989). Today the plan includes participation for nearly 99% of all domestic canned salmon, which in 1989 represented over 50% of domestic salmon consumption. Basically, the plan provides routine, uniform surveillance for particular product quality concerns, in addition to the paramount processing requirements to prevent botulism. This program is a voluntary cooperative agreement among participating packers of canned salmon, the National Food Processors Association (NFPA), and FDA; it is based in NFPA's northwest regional laboratory in Seattle. The plan incorporates all the appropriate federal regulations and clearly specifies all procedures from raw product procurement through each stage in processing. Records for operations and coding of the production lots exemplify the basic HACCP concept used for other low-acid, canned food industries. A firm's voluntary participation is confirmed by an authorized company signature that subjects the company to inspection, frequent product sampling, and detention or product destruction in the event of adverse findings. The plan requirements do not exempt products or packers from established legal action. The inspections can be conducted by NFPA or FDA staff. Every packer must designate a fully authorized person for communication in surveillance and other provisions of the plan. Production lots are specifically defined and subject to mandatory sampling per lot. Sample evaluations involve destructive laboratory analysis to judge the can seam, container integrity, and product wholesomeness. These routine lot samplings can be supplemented with random, unannounced checks. Participating firms are listed for buyer reference, including firms that may have been suspended pending any necessary corrective action.
A similar state-directed plan to ensure fish quality, and possibly safety, is the Maine Fresh Groundfish Quality Control Program initiated in 1980. This plan also requires signed agreements to participate. Currently, the program includes 12 fish processing firms that represent over 85% of Maine's fresh fillet production (Griffen, 1986). These firms have volunteered to be subject to periodic, usually weekly, unannounced inspections by trained inspectors from the Maine Department of Marine Resources. Inspections include lot assessments and rating of the facilities' operations. Program criteria for facilities and products are specified in Maine Department of Marine Resources statutes (Maine DMR, 1989). Continued compliance is denoted by a listing of participants and use of the program logo "Maine Certified Fresh Fish." The entire program is financed by the state and supplemented by additional state marketing
promotions that focus on the program and logo. Lack of compliance can result in a three-to six-month suspension and possibly permanent removal from the list of certified participants. The Maine program does not evaluate or monitor for safety concerns but could alert responsible authorities in the Maine Department of Agriculture in the event of any adverse finding or suspicion. Interagency agreements have recognized the program as an effective quality assessment program, such that the Maine Department of Agriculture recognizes and does not intend to duplicate inspection efforts. Most recently, the State of Maine resolved to specify that the Maine Department of Marine Resources would do all seafood inspections if federal authority of seafood inspections is shifted to the USDA. Continued efforts in the current program, and especially in consideration of additional species (e.g., cultured trout, mussels), could be threatened by its dependence on state funds and available manpower.
The Catfish Institute (TCI) in Belzoni, Mississippi, initiated the Mississippi Prime program in 1986. The intent was to promote product quality by all participants and to build consumer confidence in cultured catfish. Since 1988, all participating firms have been obligated to maintain satisfactory sanitary status as determined by NMFS voluntary inspections in accordance with 50 CFR Part 267 (United States Standards for Grade of North American Freshwater Catfish and Products Made Therefrom). This inspection service qualifies the firm for participation and establishes its listing on DOC's Sanitary Fish Establishment List. Subsequent weekly DOC lot inspections are contracted through TCI such that the confidential results are compiled and reported by TCI to ensure compliance and encourage improvements by individual companies. Program standards include sampling procedures for flavor evaluations and product conditions. Firms maintaining a satisfactory rating based on a standard DOC inspection score can use the Mississippi Prime seal. Failure to meet standards or make necessary corrections can lead to probations or suspensions that disallow use of the program seal. The seal designates quality commitment, backed by federal TCI inspection and promoted by extensive marketing efforts.
In 1986 the Southeastern Fisheries Association (SFA, 1990a) in Tallahassee, Florida introduced a seafood Product Quality Code program. The premise of the code was to improve seafood product quality and safety through buyer education. The intended audience was any wholesale or retail seafood buyer in the nation. The code was simply a loose-leaf binder of seafood products or species as produced and processed in the southeastern coastal states, from Texas through North Carolina. Each species represented a code entry developed by selected SFA members experienced with the specific product forms and processing requirements. Each product entry was drafted to explain product quality attributes, different product forms and types, packaging recommendations, and labeling requirements. The final draft was subject to a majority vote of SFA's standing Quality Control Committee. As of July 1990, SFA had completed 14 entries: raw (headless) shrimp, rock shrimp, breaded shrimp, spiny lobster, blue crab, stone crab, oysters, hard clams, calico scallops, mullet, tuna, catfish, grouper, and snapper, and had initiated 5 new entries: swordfish, Spanish mackerel, crawfish, shark, and king mackerel. This entire effort was made possible by federal grants provided through the Saltonstall-Kennedy Fisheries Funds administered by DOC. Initial acceptance and use has exceeded expectations, and the Food Marketing Institute (FMI) representing all major supermarket chains in North America has promised extended distribution.
In 1984, the National Blue Crab Industry Association (NBCIA), in affiliation with the National Fisheries Institute (NFI), introduced recommended standards for blue crab pasteurization (NBCIA, 1984). The manual, developed by experienced members in reviews and actual field tests with various federal and state authorities, was offered for regulatory adoption by the respective states. The standards specify requirements for all processing procedures from initial raw product quality through cooking, packaging, thermal treatment, labeling, and recordkeeping. States have referenced these standards in regulatory practice, and some authorities have recodified regulations to adopt portions of these industry recommendations.
Recently, some industry- and state-based marketing efforts have used reference to advancing seafood technology and inspections in an effort to combat negative publicity and to promote consumer confidence. The Virginia Marine Products Board advertises and distributes flyers about the "fingerprinting" system available to monitor potential chemical hazards in Virginia seafood. Similarly, many state trade associations promote the extensive regulatory efforts in monitoring water quality and processing requirements to ensure safe molluscan shellfish. Their shellfish message includes definitive cooking recommendations for consumers in certain potential health risk categories. Some supermarket advertising has adopted these same approaches to promote consumer confidence; use of the DOC voluntary inspection is a common boast.
Industry initiatives and concomitant government-based promotional efforts to encourage seafood product quality and safety are all well intended and can be helpful, but they must remain aware of possible legal requirements and risks. Greenberg's (1985) review of prior legislative and Federal Trade Commission attempts to stipulate requirements for establishing voluntary standards and certifications suggests that these efforts should be referenced. Nonprofit trade associations engaged in standard setting can be subject to antitrust laws for anticompetitive action. Greenberg (1985) recommends
In order to protect itself against potential liability in standard setting, associations should follow certain minimum procedural steps, including notification to interested parties, opportunity to participate in standard setting proceedings, complaint mechanisms, recordkeeping, and disclosure of intended scope, indicating any products or product attributes not covered by the standard that users of the standard would reasonably presume were covered … [and] any serious risks or limitations associated with the use of products that conform to the standard, when such risks or limitations would not be apparent to reasonable buyers. [See proposed 16 CFR 457.10, 43 Fed. Reg. 57,269 (Dec. 7, 1978).]
Furthermore, associations should adhere to certain minimum substantive requirements:
-
Standards should have logical and technical justification in light of their stated or implied policy goals.
-
Standards should not exclude products that are equivalent to those products that are included.
-
Standards should not exclude products if there is a less restrictive alternative (i.e., one that preserves or increases buyer options and the opportunity of sellers to compete).
-
Standards should not promote false assumptions that two or more conforming products are identical in performance or safety.
-
Standards should not lead to misplaced buyer confidence that results in economic loss or unforeseen or unreasonable risk.
Risks beyond establishing standards involve fewer legal concerns. Again, although helpful in intent, seafood product quality and safety declarations can result in confusion and false warnings. Statement accuracy and clarity, particularly from the buyer's perspective, are essential. A seal awarded for quality may not ensure product safety. An acknowledged quality processing firm conforming to programs issuing logos or seals cannot always control the handling consequences for its products in subsequent distribution. These concerns do not preclude the necessity and value of industry and marketing initiatives, but they do give reason for scrutiny of promotional assurances.
Health Advisories
Public health advisories, warnings, and related educational activities are commonly used by state agencies to better inform choices for seafood harvest and consumption. The majority of these advisories address abiotic contaminants that may pose problems in freshwater sources and microbial concerns related to the consumption of raw molluscan shellfish. Additional issues can involve particular species or products (i.e., improperly prepared items), problematic sources (i.e., suspect ciguatoxic region), and particular constituents (e.g., mercury in certain species or locations, or PCB contamination of striped bass in New York). These advisories are deemed appropriate in terms of responsibility for public health, yet their effectiveness has typically received no assessment.
The need for such warnings for raw molluscan shellfish has recently been reinforced by legal actions in defense of clients from particular health risk categories, who have experienced severe consequences from ingestion of Vibrio vulnificus on raw oysters. State concern for such liability prompted the Louisiana Department of Public Health to propose a requirement for labels and public displays to deter raw molluscan consumption by persons with liver, stomach, blood, or immune system disorders (J.C. Nitzkin, Director of Office of Public Health, Louisiana Department of Public Health, New Orleans, personal communication, 1990).
In most states, criteria and authority are established to clear and direct public health advisories, but the perceived urgency of some problems, the magnitude of local situations, and media pressure or entrapment can short-circuit the system. City- and county-based authorities have released warnings, intentionally and through media coverage, that have not been addressed through official channels. The results can be confusion, mistargeted messages, and questioned credibility. Similar mishaps can occur among the variety of state agencies sharing authority over aquatic resources.
Typically, official state authorization for public health warnings rests with a department of health or equivalent public health agency housing the state's public health officer. This authority relies on the advice of state-based technical expertise and federal guidance. In many instances involving seafood, the consistency and clarity of federal advice have been questioned, particularly relating to guidelines for levels of exposure, analytical procedures, risk assessments, and mode of public notification.
Because of the diversity among seafoods and their increasing association with environmental factors that come under state jurisdiction, a more formalized national seafood health advisory program should be considered for uniform assurance of public health and confidence.
SEAFOOD SAFETY IN THE INTERNATIONAL ENVIRONMENT
The active interest in seafood safety so robustly manifest in the United States is not limited to domestic markets. Indeed, the question of seafood safety is one of increasing prominence in the policy debates of several international organizations and countries. The potential importance of this international debate exists for one rather straightforward reason. That is, the commerce of seafood products is conducted in a strongly interdependent, international market. However, international regulation of seafood is both complex and, at present, rather inconsistent.
The international program with the most direct competence in seafood safety is the Joint Food Standards Program implemented by the Codex Alimentarius Commission, which is composed of member states and associate members of the Food and Agricultural Organization (FAO) and the World Health Organization (WHO), who have notified the commission of their wish to become members. As of late 1981, the commission was comprised of 121 members. The purpose of the commission (FAO, 1983) is
to protect the health of consumers and to ensure fair practices in the food trade; to promote coordination of all food standards work undertaken by international governmental and non-governmental organizations; to determine priorities and initiate and guide the preparation of draft standards through and with aid of appropriate organizations; to finalize standards and after acceptance by governments, publish them in a Codex Alimentarius either as regional or world-wide standards.
Upon promulgation of standards, each member state is provided a measure of latitude in accepting them. A member may (1) accept the standard fully, (2) accept it with specified deviations, or (3) target an acceptance to specific foods or food groups. Volume V of the Codex Alimentarius (FAO, 1983) sets the standards for 13 fish and fishery products. These are primarily standards of quality and identity criteria. However, a limited suite of microbiological and chemical limits has been agreed upon. Those addressing residual chemical contaminants are presented as Table 8-8 (with equivalent standards from the United States and Canada included for comparison). The United States has completed action on approximately one-third of the existing Codex standards. Food safety regulations by major importing countries have been tabulated and published by the Food and Agriculture Organization of the United Nations (FAO, 1989).
Perhaps a more important international influence on the U.S. seafood industry is the standards and inspection practices developed by the Canadian government (DFO, 1988). The Canadian seafood inspection program is based on an HACCP-type TABLE approach, and includes quality- and safety-oriented plant inspection, vessel and landing site inspection, and compliance with a broad list of contaminant action levels (which include the regulation of all agricultural chemicals and their derivatives). The list of Canadian regulatory limits appears in Table 8-8.
TABLE 8-8 Regulatory Limits for Toxic Contaminants in Seafood Extracted from United Nations, United States, and Canadian Regulations
|
Contaminant |
FAO/WHO (ppm)a |
FDA Action Level (ppm) |
Canada Health and Welfare Action Level (ppm) |
|
Arsenic |
- |
- |
- |
|
Cadmium |
3.6-4.4 |
- |
- |
|
Lead |
26.7 |
- |
0.5 |
|
Mercury |
2.7 |
1.0 |
0.5 |
|
Methylmercury |
1.8 |
- |
- |
|
Fluoride |
- |
- |
150 |
|
Dioxin |
- |
- |
20b |
|
DDT/metabolites |
1.9 |
5.0 |
5.0 |
|
Heptachlor/heptachlor epoxide |
1.9 |
0.3 |
0.1b |
|
Endrin |
- |
- |
0.1c |
|
Aldin/dieldrin |
- |
0.3 |
0.1c |
|
Chlordane |
- |
0.3 |
0.1c |
|
Mirex |
- |
0.1 |
0.1c |
|
PCBs |
- |
2.0 |
2.0 |
|
Toxaphene |
- |
5.0 |
0.1c |
|
All other agricultural chemicals |
- |
- |
0.1c |
|
a Based on conversion of FAO/WHO provisional tolerable weekly intake (PTWI) to U.S. population assuming average per capita seafood consumption to be 18.7 grams per 70 kilogram adult body weight per day. b Parts per trillion. c Characterized by way of "All Other Agricultural Chemicals" in Canadian regulations. SOURCE: DFO (1988); FAO (1989); FDA (1987). |
|||
The existing Canadian program derives from the Fish Inspection Act passed in 1970 and amended most recently in 1985 (DFO, 1985). The inspection system mandated by that legislation is designed to be comprehensive and includes all fish imported to Canada, or exported from one province to another or out of the country. Complementary legislation exists at the provincial level that effectively includes within the same regulatory structure all intraprovincial trade in seafood. The Department of Fisheries and Oceans (DFO) has primary authority to implement the program, although some authority is shared with Agriculture Canada (which has limited oversight and auditing competence), Consumer and Corporate Affairs, and Health and Welfare Canada (which sets regulatory limits for seafood contaminants). However, in recognizing the potential confusion for both industry and consumers in such a shared regulatory environment, memoranda have been signed by the agencies involved specifying a single federal contact for each regulated industry.
The decision to develop a multifaceted seafood safety program that includes programmatic efforts from harvesting through wholesale distribution was based on several assumptions about seafood commerce. First, a program limited to product sampling plans "has limitations and cannot by itself perform adequately to deliver the levels of assurance demanded by the public and buyers at a reasonable cost." Second, limiting a program to product or plant inspection detects problems only after value has been added to the product. Third, final product evaluation for any contaminant is
expensive and would demand higher inspection costs than measures designed to prevent contaminated products from entering the processing system or to prevent the processing system from introducing or elevating contaminants in the product. As recently noted by a senior administrator in DFO, "this type of system will prevent problems in the final product rather than inspect problems out of final product in an attempt to reduce the incidence of problems or unacceptable lots reaching the marketplace." The frequency of the various inspection activities mandated by the Canadian program is generally described in Table 8-9 and further detailed in Table 8-10.
The cost of the entire DFO inspection was approximately Canadian $35 million in 1988 which, distributed across the amount of total seafood production, provides an estimated cost per pound of approximately $0.01 (David Bevan, Canadian Department of Fisheries and Oceans, personal communication, 1989).
TABLE 8-9 Frequency Totals for Various Inspection Classes in Canada
|
Total site inspections |
37,500 |
|
Total field product inspections |
93,000 |
|
Total laboratory product inspections |
40,600 |
|
Total inspections for export certification |
18,900 |
|
SOURCE: Bevan (1989). |
|
An additional aspect of the Canadian inspection program worthy of note is that relating to imported products. The focus of the import inspection program is on importing plants or producers, rather than importing countries. The first time a producer attempts to import a product that product is inspected. If the product fails inspection, the producer is placed on the "Mandatory Inspection List" and required to pay for all subsequent inspections until the product passes four consecutive inspections, at which time the producer is removed from the list. If a producer is not listed for mandatory inspection, it is still subject to periodic inspection.
To date, the existence of the Canadian seafood inspection program has not had a particularly significant impact on U.S.-Canadian trade, but the situation may be in the process of changing. The recent Canada-U.S. Free Trade Agreement addresses the question of a consistent approach in regulations and standards to "protect human, animal and plant life and to facilitate commerce between the Parties." Section 708:1(a) calls for the harmonization of "technical regulatory requirements and inspection procedures, taking into account appropriate international standards, or, where harmonization is not feasible, to make equivalent their respective technical regulatory requirements and inspection procedures" (DEA, 1988).
According to the current Canadian interpretation of this provision, the Free Trade Agreement calls for an equivalence in U.S. and Canadian seafood inspection systems and regulatory guidelines. At present, the United States does not appear to meet the criteria of equivalence. The potential for impact on U.S. seafood exports is not limited to trade with Canada. Ongoing negotiations within the European Economic Community, directed at further uniting European economies in 1992, suggest that similar provisions relating to programmatic harmonization may be imposed on seafood imports within the next few years (CEC, 1987a,b). Indeed, the question of harmonization of national seafood safety programs is one that is taking an increasingly
TABLE 8-10 Canadian Seafood Inspections
|
Facility Inspection |
|
|
Approximate no. of fishing vessels |
39,800 |
|
Approximate no. of fishing vessel inspections/year |
13,200 |
|
Approximate no. of fish processing plants |
1,400 |
|
Approximate no. of fish processing plant inspections/year |
18,000 |
|
Approximate no. of unloading sites |
2,000 |
|
Approximate no. of unloading site inspections/year |
3,300 |
|
Approximate no. of transport vehicle inspections/year |
3,300 |
|
Product Inspection |
|
|
Field inspections/year |
|
|
Raw material |
13,200 |
|
Domestic final product |
65,200 |
|
Imported product |
14,600 |
|
Lab inspections/year |
|
|
Domestic product (chemical, bacteriological, and sensory analyses) |
25,900 |
|
Imported product |
14,700 |
|
Inspections/year for export certification |
18,900 |
|
SOURCE: Bevan (1989). |
|
prominent place in international trade and in the domestic policy debate to refine the U.S. program. However, as illustrated in Table 8-5, there is a lack of consistency in the generation and specification of regulatory guidelines. Indeed, a recent study carried out by the EPA revealed that differences in regulatory limits for individual contaminants vary as much as two orders of magnitude between countries. Clearly, present and future efforts to make regulatory practices equivalent must take these variances into account.
COMPARATIVE RISKS FOR CONSUMERS OF VARIOUS SEAFOOD PRODUCTS
A clearer view of the actual risks faced by seafood consumers can be obtained by looking at the fish and seafood products most widely and frequently consumed by Americans. Consumption and supply data (USDA, 1985a,b; NMFS, 1989, 1990) indicate that the most widely consumed item is shrimp, either produced domestically or imported from all over the world. Only the muscle of the tail of shrimp is eaten
and generally after cooking, but a considerable amount is eaten cold without further cooking after a cooking operation at the processing level. The most common problem with shrimp seems to be postprocessing contamination, but the reported incidents of disease are relatively few. This is probably due to the fact that shrimp are usually frozen quickly after cooking and sold in this form to the consumer. Imported shrimp receive particular scrutiny by FDA because of periodic problems with Salmonella contamination, and this may also reduce the level of risk to the consumer. There is no evidence of exceptionally high levels of contaminant chemicals in shrimp tail meat, although the data are scanty. In general, therefore, unless there is a failure of process control or contamination at the food service level, shrimp is a relatively safe diet item.
The second most commonly eaten seafood products are fillets of bottom-living white-fleshed species of fish such as cod, haddock, and pollock, formed into compressed blocks, frozen, and cut to produce fish sticks, fish portions, fish nuggets, and similar products. These may be breaded, battered, and precooked before refreezing and distribution. The disease record of these products is good, with incidents of sickness mostly being due (as with shrimp) to contamination at the food service level. The fish used to manufacture these products are generally harvested from deep offshore waters and, consequently, carry low levels of chemical contamination.
Most tuna is eaten as canned fish, and this is rarely a cause of food-borne illness unless mishandled during preparation for eating. However, unprocessed tuna has been implicated as a major cause of scombroid fish poisoning. Canned tuna is protected by an aggressive preprocess sampling and histamine analysis program by canners, who also exert pressure on catchers to chill the fish rapidly. Scombroid poisoning comes mainly from improperly handled fresh or frozen tuna, which is a minor component of tuna consumption in most parts of the United States. As a pelagic, high-seas fish, tuna generally has low levels of environmental contamination, but in a few ocean regions tuna may carry marginally high levels of mercury. This is controlled by analysis and rejection of such fish by both processors and regulators. Canned tuna must fall below the FDA action levels. For most consumers this is probably safe, but there are some questions in relation to sensitive groups such as babies and young children. Thus, in tuna products targeted for these groups, much lower levels of mercury should be maintained.
Marine and cultured freshwater fish purchased as fillets or whole fish in a fresh or frozen state probably are the next most abundantly consumed items. Microbiological risks from these products when eaten cooked seem to be minor, but potential hazards from toxins or environmental chemicals vary depending on species and origin. Fish associated with tropical reef communities present a risk of ciguatera, but these fish are rarely eaten outside of tropical islands and southern Florida. Certain other marine species besides tuna present a risk of scombroid poisoning when they are mishandled. The greatest risk would seem to come from mahimahi, with a lesser risk from bluefish and mackerel (see Chapter 4). Fortunately, this is a mild disease of short duration, and the hazard is not serious. Nevertheless, consumers should be aware of it. Bluefish from certain regions and variety of fresh fish species taken from "hotspot" regions, including the Great Lakes and adjacent river systems, other lakes and rivers, and specific inshore marine areas along both Pacific and Atlantic coastlines, may carry undesirable levels of chemical contaminants. Such species should be thoroughly screened and excluded from commercial channels if chemical contaminant levels are
higher than FDA/EPA limits. Consumers should be made aware of the risk of eating sport caught fish of this type. Naturally high mercury levels may occur in species of shark, swordfish, and halibut, but these are quite closely regulated (especially swordfish) by state agencies and FDA, so it is unlikely that the consumer will experience a dangerously high level in fish sold at retail. The proviso concerning the susceptibility of children and other sensitive groups (e.g., pregnant women) to lower levels should, however, be kept in mind.
Fish caught by subsistence and sports anglers represent a significant component of U.S. seafood consumption, approximately 4 live pounds (or 1.6 edible pounds) per person above the commercial consumption of 15.9 pounds per capita in 1989 (NMFS, 1990). Catfish, trout, salmon, and other cultured fish have a good record as far as food-borne disease is concerned. Because all are usually eaten after cooking, it is not surprising that microbiological hazards are limited. There is less information concerning chemical residues in cultured fish, although there is no evidence of acute concern. Because these fish are raised under controlled conditions it should be possible to produce animals with very low contamination levels, equivalent to other farmed animals. There is concern about agriculture and chemical runoff and drugs in feed as discussed in previous chapters. Risks from recreationally caught fish may be higher than from commercially available seafoods because temperature control after capture is generally poor. This is true, for example, of bluefish and tuna, where poor temperature control after capture might be expected to occur more frequently in a sport fishing context. Ciguatera incidence data from Hawaii support the view that sport fishers are at high risk from reef fish consumption. The hazard to freshwater anglers and others consuming their catch from the Great Lakes and several large river systems is well recognized and arises from the egregious chemical contamination of these waters by human activities. A similar hazard exists for some marine species taken in inshore marine fishing areas of California, Washington, New England, and the Chesapeake Bay. Local authorities have issued warnings in the form of specific advisories and listing of hazards in fish and game regulations made available to recreationists. The risks of delayed disease effects, including cancer from consumption of fish containing substances such as PCBs and dioxin, are sufficiently high that an increased effort to discourage anglers from consuming contaminated fish seems warranted.
Canned fish in general seems to be among the safest of seafood items. Canning is designed to destroy dangerous microorganisms and to protect the fish from future contamination. Most canning operations are large enough to support technical staff, and tests for both sterility and the presence of toxic substances are commonplace; moreover, because canned products are stable they are available for testing over a long period of time, whereas fresh and often even frozen products must be moved quickly through trade channels.
Clearly, the highest-risk category of seafood products for the general U.S. consumer is molluscan shellfish eaten raw. Traditionally, whole clams, mussels, and oysters are consumed raw, including the intestines and other viscera. This practice exposes the consumer to all possible sources of contamination, including any potentially pathogenic microorganisms or chemicals present in the gut contents. In a few cases where risk is recognized, the viscera may be removed before eating (this procedure is followed with the Pacific razor clam to reduce risks of PSP occurrence). The hazards of contaminating pathogenic bacteria from human sewage are greatly reduced for legally
harvested molluscs in commercial trade by the surveillance of water quality in growing areas under the NSSP, but are less consistent in terms of human enteric viruses and bacteria that are natural marine inhabitants. Unfortunately, the NSSP program is circumvented by infiltration of shellfish harvested illegally from closed areas. The integrity of the shellfish supply is further compromised by unregulated importation of shellfish from foreign sources. The sessile nature and filter feeding of molluscs make them likely sites for accumulation of waste chemicals, which constitute another source of potential hazard in unregulated systems. Thus, there is always some risk in consuming uncooked whole molluscan shellfish, and this practice–although of long standing–should perhaps be discouraged. Fortunately, the consumption of raw shellfish is confined to a minority of the fish-eating population, but among this group the risk of at least mild disease is quite high. Some reduction in risk may come from improved processing methods and better education of consumers.
Other risks associated with finish and shellfish consumption are more disparate in origin. Some are associated with specific eating practices such as the consumption of the tomalley of lobsters, which can expose the eater to excessively high levels of chemicals, or the consumption of whole scallops, which can expose the consumer to PSP. Others are related to unsafe home preparation practices, including marinating, pickling, or fermenting seafoods and even improper home canning of fish. Educational materials are necessary, including handbooks, public service spots, etc., to inform consumers of some of these dangers and alert them to proper practices. Public service ads or labels may also be useful.
Risks are unevenly distributed among fish consumers. There is little risk of acute illness being contracted by people eating most fish obtained from regular commercial channels when such products are eaten after cooking. The extent of the risk due to chemical contamination is uncertain because of lack of understanding of the effects of particular chemicals and poor information on the extent of contamination. Nevertheless, there is no evidence of an urgently critical situation as far as the general population is concerned. It should be apparent from the preceding discussion that people at greatest risk of seafood-borne illness are (1) consumers of raw molluscan shellfish, (2) sports anglers who eat their catch, (3) inhabitants of tropical islands, and (4) consumers of fresh/frozen mahimahi, tuna, and bluefish. Most people who become ill from seafood-borne disease suffer mild and transient symptoms, but certain groups of people including the elderly, immunosuppressed individuals, and those with underlying chronic disease conditions may suffer severe and even life-threatening illness. Young children and pregnant women are at risk from long-term effects of ingestion of certain chemical contaminants and should be protected from eating fish from contaminated areas.
CONCLUSIONS AND RECOMMENDATIONS
The preceding analysis suggests the following conclusory notes and general recommendations.
Microbial and Natural Toxin Contaminants
The process used to set federal regulatory guidelines for microbial and natural toxin contaminants is housed solely within the Food and Drug Administration. The process used by FDA to determine safe levels of these contaminants is largely reactive. Guidelines have been set primarily on an as-needed basis. However, as a group, if effectively implemented, these guidelines could provide an adequate and appropriate safeguard for the U.S. seafood consuming public.
However, the current system for these contaminant classes is not without its problems. A large number of questions remain fundamentally unresolved under present regulations and guidelines. For example, although FDA has research efforts in place, there has been no formal response by way of explicit regulatory guidelines for either ciguatoxin or domoic acid–areas of clear and acknowledged concern in domestic and imported products. Furthermore, additional product forms and emerging processing or handling concepts necessitate continued evaluation and supplementation (e.g., sous vide, modified atmosphere packaging, custom smoking techniques, and further processing in the retail setting). Without more fundamental assistance from the federal government, these responsibilities will be left to states, which may lack the required financial resources and technical expertise.
Although the FDA has, in the opinion of this committee, developed a set of adequate and appropriate regulatory limits for seafood deemed injurious to public health, the agency has not sufficiently communicated those limits to either the regulated industry or the consuming public. The various tolerances, action levels, and other regulatory guidelines used by the agency to carry out its statutory responsibilities can be fully discerned only by an extensive and rather circuitous search of FDA Compliance Policy Guides, announcements in the Federal Register , and copies of various FDA memoranda. A more concise, comprehensive, and generally available single source for all FDA guidelines relating to seafood safety should be developed and updated on a regular basis. This information should be disseminated to industry and integrated into state regulatory programs through more routine and uniform training and education.
Residual Chemical Contaminants
An assertion that current federal regulatory guidelines are sufficient to protect consumers from residual chemical risks is rather more difficult to support. At present, FDA enforcement activities focus primarily on those 15 residual chemical contaminants for which the FDA has developed action levels. That this list is sufficiently comprehensive to ensure an acceptable level of carcinogenic or chronic health risk must be questioned. The Food and Drug Administration contends that its present surveillance program supports an assertion that other contaminants do not pose a sufficient risk in seafood to warrant a more active regulatory response. However, a systematic review of FDA, EPA, National Oceanic and Atmospheric Administration (NOAA), and state-sponsored efforts to determine contaminant levels in fish and shellfish, carried out as part of this study, suggests a range of additional elements and compounds worthy of more fundamental review. These issues are addressed in detail in Chapter 5.
The committee also notes that the development or reassessment of contaminant guidelines should take into account critical regional differences in seafood harvesting and consumption patterns. A focus on the creation of national contaminant standards (as opposed to regionally based standards, for example) could lead to a situation in which a higher than anticipated seafood risk could be characterized for critical subpopulations (e.g., pregnant women, women of childbearing age, children, and recreational fishermen). Among other things, this leads to a recommendation that regional and high-risk group consumption data be collected so that more realistic seafood risk characterizations can be developed.
The development of regulatory guidelines for seafood safety is a responsibility shared by EPA and FDA (for pesticides), and by FDA and states (for shellfish). Although a rather impressive number of interagency boards, councils, and formal understandings exists, these arrangements have not been used sufficiently to reduce the problems that result from the lack of interagency cooperation. Of particular concern are the rather important differences between EPA and FDA in their respective risk assessment methodologies. The development of an interagency structure with a single focus on seafood safety could contribute significantly toward reducing these difficulties.
Monitoring and Inspection
In general, the present monitoring and inspection program carried out by all federal agencies lacks both the frequency and the direction sufficient to ensure effective implementation of the nation's regulatory limits for seafood safety. However, the need for a renewed and redesigned seafood inspection effort is now almost universally acknowledged. This committee is not in the position to evaluate prospectively the likely success of alternative inspection models currently under consideration by Congress and the administration. However, whatever inspection does emerge from this effort should address several discrete questions and concerns.
Future efforts must recognize the importance of understanding and quantifying the source and level of contaminants in seafood harvesting areas. Existing environmental monitoring efforts are not, for the most part, designed to be of direct use in evaluating seafood safety concerns. Among other things, they lack (1) sufficient geographic scope, (2) a common methodological approach, and (3) sufficient focus on the edible portion of seafood in order to determine public health risks, as opposed to environmental health impacts. This last point is of particular importance. Save for the monitoring of harvesting waters carried out as part of the National Shellfish Sanitation Program, data evaluating contaminant levels in fish and shellfish do not consistently focus on the analysis of edible tissue. More often the focus is on whole fish, liver, or gallbladder analysis which, by design, offers insufficient insights into contaminant levels in the marketable seafood product. In addition, any expanded seafood safety effort must focus on the level of hazard at the point of capture. One strategy to mitigate such risks is for agencies, both federal and state, to more explicitly develop regulations allowing for fishing closure based on questions of public health.
There is widespread application of HACCP-based systems for ensuring control of the safety of food products. Such systems are well designed to control the safety of processing operations in which human activities can either decrease or increase risk. However, control of the intrinsic safety of the raw material may not be easily achieved.
This is a particular problem for seafoods derived from wild stocks. It is clear from analyses presented in the preceding chapters that most of the reported incidents of human disease caused by seafood in the United States derive from species of fish and shellfish that have been contaminated in the ocean and are consumed in a nonprocessed form. The Critical Control Points (CCPs) in such cases are difficult to define. In the case of molluscs, which are the single largest cause of illness, the CCP is apparently prior to harvest and involves environmental (water) testing with subsequent closure of areas. However, the system is flawed by the unreliability of tests (viruses and vibrios are not detected) and the imperfect nature of distribution controls. A second example is ciguateric fish for which the CCP should be at or before harvest, but testing methods are lacking and the legal basis for fishery control is questionable. It is easy to draw a box at the beginning of a seafood process flow diagram that says "Harvest CCP," but this does not resolve the problem.
Statistical sampling concerns are certainly an important aspect of raw material safety control in such situations (see Chapter 7). To deal with these, more information is needed on the distribution of hazards among fish and shellfish stocks, particularly of chemicals and viruses, and additional research is required along the lines noted in previous chapters.
Recreational Fishing
Any future monitoring and inspection effort must take greater account of that part of seafood caught recreationally. This fraction of the total domestic catch imposes a significant and largely unregulated risk on the consumer. Strong consideration should be given to creating a marine recreational fishing license system that is linked to the distribution of information characterizing the level and scope of potential risk from eating recreationally caught seafood. Strong consideration should also be given to the recreational closure of harvesting areas deemed to pose a threat to human health.
Public Health Monitoring
A fundamental contribution to the reasoned development of regulatory responses to seafood safety questions is the development of accurate and reliable information on seafood-borne illness. The present CDC system is useful for identifying seafood-related hazards but is inadequate to quantify and fully characterize risks that would permit the development of risk-based intervention strategies. Rather, CDC should develop an active program, founded on community-based health surveys, to better determine the level and source of seafood-borne illness in the U.S. population.
Regulation of Imported Products
One of the important differences between the regulation of domestic and imported seafood products is that, for the most part, any assurance of the quality of foreign harvesting environments and postharvest handling practices is lacking. Therefore, to further enhance the safety of imported seafood, consideration should be given to the
development of agreements with foreign authorities or individual producers to ensure that imported products are produced in a manner consistent with and equivalent to domestic products. Such a system is currently in place, for example, for imported meat and poultry. The current practice of developing memoranda of understanding (MOUs) with other countries constitutes a reasonable, if initial, effort. However, given the proportion of imported seafood products, the committee suggests that the question of imported product regulation is in need of fundamental reconsideration.
The large portion of the U.S. seafood supply that is imported presents another serious problem for control. It has been proposed that HACCP-type control systems be required for imported seafoods. This would require either extranational inspections by U.S. personnel or MOUs with the exporting countries and some reliable cross-checking. Unfortunately, many seafood exporting countries probably lack the technical ability to meet this proposed requirement. Fish are often transshipped through several countries (and jurisdictions) before appearing in U.S. markets as finished products, and despite country-of-origin labeling requirements, it is difficult to identify the actual location in which animals were harvested. This implies that some type of lot testing will probably continue to be necessary for a significant proportion of imported products. The U.S. food safety authorities should make every effort, working with international agencies, to develop a system for effective identification of harvest areas for fish and shellfish in international commerce. There should also be some ongoing process to identify toxigenic areas, regions of high chemical contamination, and fecally polluted regions in parts of the world from which fish imported to the U.S. originate. Some aspects of these issues could conceivably be undertaken under the Codex Alimentarius process or through international programs concerned with water pollution.
Because of the large international trade in seafood and the very diversified nature of both the fish and the environments from which they come, it is essential that the United States consider hazard from a global viewpoint. There needs to be constant awareness of the health-related conditions in areas from which seafoods are imported, in terms of both environmental effects and food-borne disease. Such considerations, together with an improved data base on contaminants and rapid, effective test methods, could lead to more strongly directed sampling and testing with a high probability of success in protecting public health.
Seafood Health Advisories
The determination of seafood health advisories has been made almost exclusively by state regulatory agencies. The committee suggests that a more pronounced and consistently defined federal role in the risk characterizations leading to these advisories would be of significant benefit. A more consistent and focused effort in the determination and communication of public health risks from contaminated seafood should be developed. Without such an effort the public to whom these advisories are directed cannot make effective risk judgments. As already noted, strong consideration should be given to the development of a marine recreational licensing system that would serve, primarily, as a vehicle to communicate risks to recreational fishers.
Educational Programs
Although federal and state training and education do exist for both industry and regulatory authorities, these programs should be expanded, offered on a more routine basis, and structured for uniformity across industries and states. Furthermore, one issue in seafood education that has been consistently undervalued is the integration of food and seafood safety with continuing education for the medical and public health professions. Finally, and as noted elsewhere in this report, the value of a greatly expand public education effort focusing on proper seafood handling in the home should not be underestimated. Such programs should take advantage of existing educational networks available through cooperative extension services and other state institutions.
State Regulations and Programs
At present, states play a fundamental role in the current governance effort to regulate seafood safety in the United States. Given the diversity of our nation's harvesting waters, seafood species, and seafood processing practices, states should continue to play a prominent role in the development of seafood safety standards and inspection practices. Unlike most other food commodities with more uniform production and product forms, seafoods require more regional or local control and surveillance.
In general, the committee's evaluation of existing state regulatory programs for seafood safety suggests that states have developed, with some notable exceptions, a sufficient authority to respond reasonably to problems related to seafood safety. However, the implementation of that authority is extremely uneven. The importance of effective and well-funded seafood surveillance and inspection programs is underscored by the fact that the authority of the FDA is limited to seafood products sold in interstate commerce. For seafood both harvested and consumed within the boundaries of a single state the only controls for product safety are those imposed by the state.
State seafood regulatory programs are very dependent on federal guidance in statutes and advice, but federal rules do not always take into account the regional specificity of seafoods. The ISSC is an ongoing example of communication between federal authorities and state programs. The ISSC concept could be expanded to ensure more state involvement in federal policy in order to reflect regional considerations. Established professional organizations (e.g., AFDO) or respective state and federal officials should be better utilized in facilitating communication and decisions for more uniform and consistent surveillance.
State-based educational programs through cooperative extension services, Sea Grant College Programs, and community education offer experienced networks of expertise that have not been fully utilized to assist regulatory communication, industry compliance, and public perception and protection. These programs offer additional links to research advances in seafood safety and environmental/risk assessments. Together, these educational and research programs warrant further and direct funding considerations as a primary source of future expertise to address food safety in the United States.
Industry Initiatives
Although initiatives on the part of private industry do exist and do, in some instances, add measurably to the enhancement of seafood safety, these programs are, for the most part, designed as quality assurance and product promotion programs. However, the manner in which these programs are communicated to the consumer does not clearly distinguish between those specifically designed to enhance product safety and those with other intentions. Regulations should be developed to qualify and to affirm initiatives for seafood safety claims.
International Issues
One final recommendation relating to setting standards for seafood safety evolves out of a recognition of the importance of international trade in the seafood industry. Several countries have instituted extensive and formal regulatory standards designed to reduce health risks from seafood consumption. There is an emerging recognition within the U.S. government that the development or reevaluation of domestic regulatory guidelines must be cognizant of and take into account other such efforts. However, it should be noted that the regulatory guidelines developed by individual countries are not consistent. Indeed, for certain contaminants, regulatory limits may differ by two orders of magnitude. As more states require equivalency for domestic and imported products, it is increasingly apparent that the time has come for the international community to begin a process to minimize the existing differences in national regulatory guidelines and approaches.
NOTES
REFERENCES
Adams, C.M., and F.J. Lawlor, III. 1989. Trends in the importation of selected fresh and frozen seafood products into the southeastern United States. Florida Sea Grant Tech. Report No. 59. 70 pp.
AFDO (Association of Food and Drug Officials). 1989. A national letter on seafood inspection mandate. September 9, 1989. Association of Food and Drug Officials, York, Pennsylvania.
AFDO/FDA (Association of Food and Drug Officials/Food and Drug Administration). 1982. Retail Food Store Sanitation Code. Association of Food and Drug Officials, York, Pennsylvania, and
Food and Drug Administration, Washington, D.C. 74 pp.
Anonymous. 1989a. "The Unicode": A dynamic food safety system. Dairy, Food and Environ. Sanitation 9:459.
Anonymous. 1989b. Aquaculture: New markets for meals, fats and oils. J. Am. Oil Chemist. Soc. 66:1531-1546.
Bennett, J.V., S.C. Holmberg, M.F. Rogers, and S.L. Solomon. 1987. Infectious and parasitic diseases. Pp. 102-114 in R.W. Amler and J.B. Dull, eds. Closing the Gap: The Burden of Unnecessary Illness. Oxford University Press, New York.
Benson, J.S. 1990. Statement Before Subcommittee on Department Operations, Research, and Foreign Agriculture, Committee on Agriculture, United States House of Representatives, February 7.
Bevan, D. 1989. Effectiveness of Canadian fish inspection in protecting consumer health. Paper presented at the Workshop on Assessing and Controlling Health Hazards from Fishery Products, Food and Nutrition Board, Institute of Medicine, National Academy of Sciences Study Center, Woods Hole, Mass., July 26. 18 pp.
Bryan, F.L. 1986. Seafood-transmitted infections and intoxications in recent years. Pp. 319-337 in D.E. Kramer and J. Liston, eds. Seafood Quality Determination. Proceedings of an International Symposium Coordinated by the University of Alaska November 10-14, 1986. Elsevier Science Publishers, Amsterdam, The Netherlands.
CDC (Centers for Disease Control). 1981a. Salmonella Surveillance, Annual Summary, 1978. HHS Publ. No. (CDC)81-8219. Public Health Service, U.S. Department of Health and Human Services, Atlanta, Ga. 25 pp.
CDC (Centers for Disease Control). 1981b. Annual Summary of Foodborne Disease, 1978. HHS Publ. No. (CDC)81-8185. Public Health Service, U.S. Department of Health and Human Services, Atlanta, Ga. 53 pp.
CDC (Centers for Disease Control). 1981c. Annual Summary of Foodborne Disease, 1979. HHS Publ. No. (CDC) 81-8185. Public Health Service, U.S. Department of Health and Human Services, Atlanta, Ga. 40 pp.
CDC (Centers for Disease Control). 1983a. Food-Borne Disease Outbreaks, Annual Summary 1980. HHS Publ. No. (CDC) 83-8185. Public Health Service, U.S. Department of Health and Human Services, Atlanta, Ga. 32 pp.
CDC (Centers for Disease Control). 1983b. Food-Borne Disease Outbreaks, Annual Summary 1981. HHS Publ. No. (CDC) 83-8185. Public Health Service, U.S. Department of Health and Human Services, Atlanta, Ga. 41 pp.
CDC (Centers for Disease Control). 1984. Food-Borne Disease Outbreaks, Annual summary 1983: Reported morbidity and mortality in the United States. Morbid. Mortal. Weekly Rep. (annual suppl.) 32 pp.
CDC (Centers for Disease Control). 1985a. Food-borne Disease Outbreaks, Annual Summary 1982. DHHS Publ. No. (CDC) 85-8185. Public Health Service, U.S. Department of Health and Human Services, Atlanta, Ga. 38 pp.
CDC (Centers for Disease Control). 1985b. Manual of Procedures for National Morbidity Reporting and Public Health Surveillance Archives. Public Health Service. U.S. Department of Health and Human Services, Atlanta, Ga.
CDC (Centers for Disease Control). 1989. Food-Borne Surveillance Data for All Pathogens in Fish/Shellfish for Years 1973-1987. Public Health Service, U.S. Department of Health and Human Services, Atlanta, Ga.
CEC (Commission of the European Communities). 1987a. Working document of the health conditions affecting the production and the placing on the market of live bivalve molluscs. Directorate-General for Agriculture. VI/206/87-EN Rev. 3. 37 pp.
CEC (Commission of the European Communities). 1987b. Working paper of health conditions affecting the production and the placing on the market of fishery products. Directorate-General for Agriculture. VI/2078/87-EN Rev. 4. 33 pp.
Chorba, T.L., R.L. Berkelman, S.K. Safford, N.P. Gibbs, and H.F. Hull. 1989. Mandatory reporting of infectious diseases by clinicians. J. Am. Med. Assoc. 262:3018-3026.
DEA (Canadian Department of External Affairs). 1988. The Canada-U.S. Free Trade Agreement, 2nd ed., November 17. Ottawa.
DFO (Canadian Department of Fisheries and Oceans). 1985. Fish Inspection Act. Amendment List, December 11 and 12. Ottawa.
DFO (Canadian Department of Fisheries and Oceans). 1988. Fish Inspection Regulations, Amendment
List, June 13. Ottawa.
DFO (Canadian Department of Fisheries and Oceans). 1989. Canadian Guidelines for Chemical Contaminants in Fish and Fish Products. Government of Canada, Ottawa.
DOC (Department of Commerce). 1970. Organization Order 25-5A, Sec. 4, October 9.
DOC (Department of Commerce). 1989. A Report to GAO: Review of Federal Food Safety and Quality Activities. DOC/NOAA/NMFS, October. 23 pp.
FAO (Food and Agricultural Organization). 1983. Codex Alimentarius Codex Standards for Fish and Fishery Products. Joint FAO/WHO Food Standards Programme, Vol. V, 1st ed. Food and Agricultural Organization of the United Nations, and World Health Organization, Geneva, Switzerland. 135 pp.
FAO (Food and Agriculture Organization). 1989. Food Safety Regulations Applied for Fish by Major Importing Countries. FAO Fisheries Circular No. 825. Food and Agricultural Organization of the United Nations and World Health Organization, Rome. 107pp.
FDA (Food and Drug Administration). 1975. Interagency Agreements and Memorandum of Understanding. Staff Manual Guide IV. 2820.1. GT No. 75-34, February 19. Food and Drug Administration, Washington, D.C., 5 pp.
FDA (Food and Drug Administration). 1985. Compliance Policy Guide, Chapter 8: Fish and Seafood. 7108.01-7108.25, 10/80 through 6/85, July 1. Food and Drug Administration, Washington, D.C. 41 pp.
FDA (Food and Drug Administration). 1986-1987. Import Seafood Detentions Information. Food and Drug Administrations, Washington, D.C. 51 pp.
FDA (Food and Drug Administration). 1987. Compliance Policy Guide, Chapter 41-Pesticides. 7141.01, April 1. Food and Drug Administration, Washington, D.C.
FDA (Food and Drug Administration). 1988a. Processed Seafoods Assignment (FY 89). March 31. Food and Drug Administration, Washington, D.C. 16 pp.
FDA (Food and Drug Administration). 1988b. Fish List: FDA Guide to Acceptable Market Names for Food Fish Sold in Interstate Commerce. Washington, D.C. 50 pp.
FDA (Food and Drug Administration). 1988c. Summary of FY '85/86 Field Program "Lead, cadmium and other elements in domestic shellfish" (Program 7304.004C). Pesticides and Chemical Contaminants Program. CESAN/DCC, April 19. Division of Contaminants Chemistry, Center for Food Safety and Applied Nutrition, Washington, D.C. 25 pp.
FDA (Food and Drug Administration). 1988d. Seafood Regulation: An Analysis of FDA Strategy. Report of the Seafood Task Force. Food and Drug Administration, Washington, D.C. 62 pp.
FDA (Food and Drug Administration). 1989a. NSSP Shellfish Sanitation Program. Manual of Operations. Part I: Sanitation of Shellfish Growing Areas.
FDA (Food and Drug Administration). 1989b. NSSP Shellfish Sanitation Program. Manual of Operations. Part II: Sanitation of the Harvesting, Processing and Distribution of Shellfish.
FDA (Food and Drug Administration). 1989c. Imported Molluscan Shellfish Assignment (FY 89)--Revised. January 17. Washington, D.C. 20 pp.
FDA (Food and Drug Administration). 1990. Summary Seafood Operational Accomplishments and Resource Expenditures, FY 1984-89, January. Program Evaluation Branch (HFC 42), Food and Drug Administration, Rockville, Md. 15 pp.
Federal Register. 1978. Federal Trade Commission (FTC). FTC proposes prohibition. Fed. Reg. 43(December 7):57269-57284.
Federal Register. 1990. Department of Health and Human Services. Food and Drug Administration. 21 CFR Parts 109 and 509. Action levels for added poisonous or deleterious substances in food. Fed. Reg. 55(May 21):20782-20787.
Fong, W.g., and G.M. Brooks. 1989. Regulation of chemicals for aquaculture use. Food Technol. 43:88-93.
Garrett, E.S., III. 1988. Microbiological standards, guidelines and specification and inspection of seafood products. Food Technol. 42:90-93, 103.
Greenberg, E.V.C. 1985. The debate over fish product quality and inspection. Paper presented at the Fourth Annual National Fishery Law Symposium, Seattle, Wash., October 11-12. 13 pp.
Griffen, N. 1986. Finding new markets for Maine fish. Seafood Leader Magazine 5:74-76.
Haddock, R.L. 1989. Letter report on food-borne disease incidence dated September 8, 1989 from Dr. Robert L. Haddock, Territorial Epidemiologist, Department of Public Health and Social Services, Government of Guam to Dr. Farid E. Ahmed, Project Director, Committee on
Evaluation of the Safety of Fishery Products, Institute of Medicine, National Academy of Sciences, Washington, D.C.
Hui, Y.H. 1986. United States Food Laws, Regulations and Standards, Vol. II. John Wiley & Sons, New York. 704 pp.
ICF. 1986. Preliminary Analysis of a National Seafood Inspection Program. Prepared for NMFS/NOAA/DOC by ICF, Inc., Washington D.C. December. 363 pp.
Isaac, R., and J. Delany. 1975. Toxic Element Survey: Final Report, Research and Demonstration Project 71-06. Commonwealth of Massachusetts, Department of Environmental Quality Engineering, Division of Water Pollution Control, November. 25 pp.
Johnsen, P.B. 1989. Factors influencing the flavor quality of farm-raised catfish. Food Technol. 43:94-97.
Kilara, A. 1982. Food allergies–Facts or fiction. Nutr. Letter 1:1-4.
Kvenberg, J. 1989. FDA Pathogen Surveillance Program. Paper presented at the Workshop on Assessing and Controlling Health Hazards from Fishery Products conducted by the Committee on Evaluation of Safety of Fishery Products, Food and Nutrition Board, Institute of Medicine, National Academy of Sciences Study Center, Woods Hole, Mass., July 26 . 25 pp.
Lombardo, P., and N.J. Yess. 1989. FDA program on chemical contaminants in seafood. Paper presented at the 1989 Interstate Seafood Conference, Ocean City, Md., October 17-18. 9 pp.
Maine DMR (Department of Marine Resource). 1989. The Best Is Fresh from Maine. Maine Department of Marine Resources, Augusta. 2 pp.
Martin, R. 1990. Regulations. Pp. 351-380 in R.E. Martin and G.J. Flick, eds., The Seafood Industry. VanNostrand and Reinhold, New York.
Matches, J.R., E. Raghubeer, I.A. Yoon, and R.E. Martin. 1986. Microbiology of Surimi-based products. Pp. 373-387 in D.E. Kramer and J. Liston, eds. Seafood Quality Determination. Elsevier Science Publishers, Amsterdam, The Netherlands.
Morris, J.G., Jr., P. Lewin, C.W. Smith, P.A. Blake, and R. Schneider. 1982. Ciguatera fish poisoning: Epidemiology of the disease on St. Thomas, U.S. Virgin Islands. Am. J. Trop. Med. Hyg. 31:574-578.
NBCIA (National Blue Crab Industry Association). 1984. National Crabmeat Industry Pasteurization Standards. National Fisheries Institute, Arlington, Va. 8 pp.
NFPA (National Food Processors Association). 1989. The 1989 Canned Salmon Control Plan and Container Integrity Program. A voluntary cooperative agreement between participating salmon packers, U.S. Food and Drug Administration, and the National Food Processors Association in Seattle, Wash. April. 20 pp.
NMFS (National Marine Fisheries Service). 1989. Fisheries of the United States, 1988. DOC/NOAA/NMFS, U.S. Government Printing Office, Washington, D.C. 116 pp.
NMFS (National Marine Fisheries Service). 1990. Fisheries of the United States, 1989. DOC/NOAA/NMFS, U.S. Government Printing Office, Washington, D.C. 111 pp.
NOAA (National Oceanic and Atmospheric Administration). 1988a. Fishery Products Inspection Manual. Part I: Inspection. Handbook No. 25. DOC/NOAA/NMFS, October.
NOAA (National Oceanic and Atmospheric Administration). 1988b. Fishery Products Inspection Manual. Part III: Certification. Handbook No. 25. DOC/NOAA/NMFS, October.
Otwell, W.S. 1989. Regulatory status of aquaculture products. Food Technol. 43:103-105.
PBCHD (Palm Beach County Health Department). 1988. Episode Care Notes. A comprehensive report prepared by Palm Beach County Health Department. Palm Beach, Fla., May 25.
Ratcliffe, S., and D.S. Wilt, eds. 1971. Proceedings Seventh National Shellfish Sanitation Workshop, Washington, D.C., October 20-22. 412 pp.
Redmayne, P.C. 1989. World aquaculture developments. Food Technol. 43:80-81.
Rodrick, G.E., and T.C. Cheng. 1989. Parasites: Occurrence and signficance in marine animals. Food Technol. 43:98-102.
SFA (Southeastern Fisheries Association). 1990a. Product Quality Code. Southeastern Fisheries Association, Tallahassee, Fla. 1107 pp.
SFA (Southeastern Fisheries Association). 1990b. Industry and Regulatory Interface to Address Concerns for Seafood Product Quality and Safety. Southeastern Fisheries Association, Tallahassee, Fla. June., 215 pp.
Todd, E.C.D. 1989. Preliminary estimates of costs of foodborne disease in the United States. J. Food Protect. 52:595-601.
USDA (United States Department of Agriculture). 1985a. Nationwide Food Consumption Survey .
Continuing Survey of Food Intakes by Individuals
. Men 19-50 Years, 1 Day. NFCS, CSFII Report No. 85-3. Nutrition Monitoring Division, Human Nutrition Information Service, United States Department of Agriculture, Hyattsville, Md. 94 pp.USDA (United States Department of Agriculture). 1985b. Nationwide Food Consumption Survey. Continuing Survey of Food Intakes by Individuals. Women 19-50 Years and Their Children 1-5 Years, 1 Day. NFCS, CSFII, Report No. 85-1. Nutrition Monitoring Division, Human Nutrition Information Service, United States Department of Agriculture, Hyattsville, Md. 102 pp.
Ward, D.R. 1989. Microbiology of aquacultured products. Food Technol. 43:82-87.
Appendix to Chapter 8
SUMMARIES FROM GROUP MEETINGS WITH INDUSTRY AND VARIOUS STATE REGULATORY PERSONNEL
This appendix material includes summaries from group meetings with industry representatives and various state regulatory authorities responsible for some related aspect of surveillance and monitoring to ensure seafood product safety and quality. The meetings in California, Hawaii, Massachusetts, and Washington were conducted by the staff/members of the Committee on Evaluation of the Safety of Fishery Products of the National Academy of Sciences' Institute of Medicine. Summaries from meetings in Alabama, Florida, Georgia, Louisiana, Mississippi, North Carolina, Puerto Rico, South Carolina, and Texas were referenced from a similar project being conducted by the Southeastern Fisheries Association (SFA), a trade association based in Tallahassee, Florida (SFA, 1990b). The SFA project was supported by Saltonstall-Kennedy funds, project number NA 88-WC-J-06065. The SFA project was separate from, yet similar to, the work conducted by the committee.
The basic approach for each group meeting was to assemble industry and respective state regulatory representatives to discuss the current state regulatory structure and practices in addressing seafood safety in production, processing, and marketing. The open discussion format was directed to explain activities from environmental monitoring and production (harvest, culture, or import); through product and processing inspection; through further preparation in retail outlets, restaurants, or other routes; to final consumption. The objective was to illustrate authorities and programs and to record experiences, concerns, and recommendations.
Table A Review of State Regulatory and Industry Responses (November 1, 1988) Concerning Seafood Quality and Safety
|
ALABAMA |
|
|
Industry Character (1988) |
|
|
1. |
Ranked 17th in total state production (22.3 million pounds) and 18th in value ($39.7 million). |
|
2. |
State production primarily from warm waters with significant portion of additional product coming from out-of-state sources. |
|
3. |
Most fishing vessels independently owned and operated. |
|
4. |
Diverse state production, principal species including shrimp, oysters, blue crabs, and assorted finfish from nearshore and deepwater fisheries. |
|
5. |
Diversity in levels and types of processing firms, to include smaller packing houses at dockside; blue crab, shrimp, and oyster processors; large shrimp canneries, and cultured catfish processors. Permitted processing firms totaled 236 in 1988. |
|
6. |
Consumption includes demand from some large metropolitan regions and some seasonal tourism. |
|
7. |
Aquaculture development primarily for catfish with annual production ranked third among state productions. |
|
8. |
Recreational fisheries–all species. Recreational harvest does enter commercial settings. |
|
9. |
Imports play significant role as supply for processors and product for consumers, particularly in metropolitan regions. |
|
Primary Product Safety Problems |
|
|
1. |
Records and industry practice reflect no major concerns for seafood safety in Alabama. Any potential problem areas are being addressed by current regulatory programs (i.e., molluscan shellfish harvest). The principal concern is consumption of raw molluscan shellfish. |
|
Primary Product Quality Problems |
|
|
1. |
Most common violations involve GMPs. |
|
2. |
Industry is concerned about the continuing and increasing use and misuse of chemicals in treatment to prolong shelf life and increase product yields without required labeling. |
|
3. |
Enforcement for country of origin labeling. |
|
Additional Problems |
|
|
1. |
Aquaculture may introduce new species to the environment that could influence existing stocks. |
|
2. |
Aquaculture may introduce cultured varieties that will be difficult to distinguish from traditionally harvested species. |
|
Regulatory Concerns for Seafood Industry |
|
|
1. |
In all levels of seafood industry there is some general confusion coupled with a lack of awareness of the current, pertinent seafood quality and safety regulations and regulatory authorities. |
|
Regulatory Concerns for Their Respective Programs |
|
|
1. |
Training for inspectors is lacking, particularly in relation to seafoods and in noncoastal counties. |
|
2. |
Program effectiveness and ability to build a better-informed program is compromised by employee turnover and limited career incentives. |
|
3. |
Seafood regulatory and educational efforts in Alabama must consider the significant portion of Vietnamese workers. |
|
Industry Concerns for Regulations |
|
|
1. |
Alabama has recently received a disproportionate amount of federal regulatory scrutiny through site- and species-specific sampling programs. |
|
2. |
Regulatory authorities need to initiate more active programs to enforce proper species identification and net weights. |
|
3. |
Regulations for net weights/content declarations require more clarification and increased awareness for industry compliance (i.e., water content in oysters). |
|
4. |
Regulatory surveillance is primarily complaint activated and oriented. |
|
5. |
County health inspections look for obviously ill workers, with little or no screening for less obvious illnesses. |
|
6. |
Regulations are not apparent or easily accessible for guidance or explanations in commercial practice. |
|
7. |
Regulatory surveillance, particularly federal based, should be more instructive and helpful to ensure compliance. Currently most technical guidance is provided through financially limited academic-based programs and advice (i.e., Alabama Sea Grant Program). |
|
8. |
Line of authority between state and counties is not clear relative to enforcing labeling, net weight/content declarations, country of origin, etc. |
|
9. |
Need more regulatory surveillance of seafood imports. |
|
10. |
A better understanding of regulations would encourage more compliance. |
|
Recommendations |
|
|
1. |
Consider a certification program for various levels of industry that is based on voluntary participation with acknowledged credits for completion. |
|
2. |
Publish a simple, usable version of all county, state, and federal seafood regulations for vessels, processors, and retailers. |
|
3. |
Consider a structured, periodic training program on seafood safety and regulations for all segments of the industry and regulatory agencies. |
|
4. |
Need more accessible means to note complaints in industry practice or regulatory activity without drawing regulatory attention to the complainer. |
|
Regulatory Initiatives |
|
|
None recorded. |
|
|
Industry Initiatives |
|
|
None recorded. |
|
|
Summary |
|
|
Production and processing of seafoods in Alabama are typical and modest in comparison with other coastal states around the Gulf of Mexico. Records and practice reflect a safe, reputable product, both from harvests and from well-established aquaculture. The main regulatory responsibility is in the Department of Public Health (DPH). Oyster and blue crab production and processing operations, primary in the coastal area, are monitored by a specific state program (DPH), whereas other seafoods and segments of the industry are monitored by the respective county health departments referencing DPH regulations. All agencies and industry representatives request more education and regulatory orientation. |
|
Table A Review of State Regulatory and Industry Responses (December 13, 1989) Concerning Seafood Quality and Safety
|
CALIFORNIA |
|
|
Industry Character (1989) |
|
|
1. |
Ranked 4th in production and value among all seafood producing states. |
|
2. |
Production includes a variety of fish [e.g., salmon, rockfishes, assorted ground fish and some tuna, traditional squid and dungeness crab landings, and limited molluscan shellfish (production limited by suitable and approved growing waters)]. |
|
3. |
Processing operations (approximately 85%) are concentrated around Los Angeles, including firms producing value added items (e.g., breading, smoking surimi). |
|
4. |
Company owned vessels are principally the larger seiners and trawlers, but most fishing vessels are independently owned. |
|
5. |
Recreational fisheries are increasing for mackerel, salmon, rockfishes, some tuna and other assorted ground species and a variety of freshwater fish. |
|
6. |
Aquaculture is established for oysters, trout, catfish, tilapia, sturgeon, some crayfish, and limited abalone. |
|
7. |
A substantial amount of seafood imports are consumed in California. |
|
Primary Product Safety Problems |
|
|
1. |
Raw molluscan shellfish consumption is a principal seafood safety concern relative to environmental contaminants. |
|
2. |
The occurrence of paralytic shellfish poison (PSP) requires a continuous monitoring commitment to manage commercial and recreational harvest for molluscan shellfish. |
|
3. |
Popularity of sushi (raw fish consumption) poses concern for parasitic infections. |
|
4. |
High influx and establishment of ethnic populous introduces concern for particular dietary habits, product selections, and methods of preparation that require specific surveillance. Likewise, health and seafood safety advisories must be multilingual. |
|
5. |
Significant recreational fishery poses increasing challenge to assure safety of fish relative to environmental contaminants per location and species. State resorts to strong dependence on contaminant and site specific fishing advisories. |
|
Regulatory Concerns |
|
|
1. |
Constant demand for environment and product monitoring and public advisement to assure product safety relative to contaminants is a growing multi-agency commitment that requires more interagency cooperation and better federal liaison relative to "guidelines" and "risk assessment" methods. |
|
2. |
State agencies need better exposure and consumption data to assist in state-based risk assessments relative to environmental contaminants. |
|
3. |
Better and more rapid analytical methods are needed for monitoring PSP and addressing "indicators" for approved shellfish growing waters. Likewise, there is an information gap for the survival and behavior of many aquatic microbial pathogens. |
|
4. |
State agencies are continually frustrated by perceived delays in federal communications to immediate and recurring seafood safety problems. For example, there is a lack in understanding or existence of federal "action levels" for many environmental contaminants. |
|
5. |
Some autonomy in certain local and county health and food safety related programs can hamper some interagency communications. |
|
6. |
Recreational harvest can enter commercial distribution. |
|
7. |
Agencies need to improve their data clearing and storage methods relative to seafood epidemiology, water quality, and seafood product safety assessments in order to better prioritize programs and to assess effectiveness of the programs. |
|
8. |
In comparisons with most other states, imports represent a higher proportion of the seafoods consumed in California, yet there are no data to judge the safety of imports versus domestic sources. |
|
Industry Concerns |
|
|
1. |
The seafood processors should not be blamed for perceived safety problems due to environmental consequences. |
|
2. |
There needs to be more coordination and consideration for consolidating of the multitude of agency efforts in assessing waters and seafood products relative to environmental contaminants. |
|
3. |
Industry is often confused by the diversity of agencies and pertinent regulations. There is no single source manual or guide to clarify responsibility and regulatory requirements. |
|
4. |
Many processing facilities are located on public lands, thus limiting incentive to invest in improvements requiring long-term returns. |
|
5. |
Fishing (safety) advisories for commercial or recreational sectors should be subject to a central, standard authority prior to "official" release. |
|
6. |
Regulatory inspectors and portions of the seafood industry need more specific, dedicated and continuous training in aquatic product quality and safety. In some instances, this concern is more evident in local settings. |
|
7. |
The HACCP vogue does not address the primary seafood quality and safety violations. The primary causes are a few less scrupulous firms or individuals that are not deterred due to lack of penalties and an ineffective enforcement |
|
|
of existing regulations. |
|
8. |
The NMFS volunteer inspection program, taunted by some retail advertising, is primarily promotional with a perceived "safety assurance" message, yet the program is quality oriented. |
|
9. |
The FDA regulatory response is often too complaint oriented. |
|
Regulatory and Industry Initiatives |
|
|
1. |
State has an ongoing effort, soon to be completed, for monitoring radioactivity in fish around Farallon Islands and adjacent locations. |
|
2. |
New California statute will focus more attention on "Recommended Public Health Levels" for contaminants, whereby enforcement can be based on a regulatory review and judgment that can be more inclusive and conservative than current federal guidelines or action levels. |
|
3. |
Trade associations are encouraging more compliance with commercial licensing and need for regulatory orientation for industry participants, particularly new entries. |
|
Recommendations |
|
|
1. |
Need to improve industry knowledge of pertinent regulations and regulators. |
|
2. |
Need to address more interagency cooperation relative to environmental monitoring pertinent to aquatic food safety, advisories, and industry liaison. |
|
3. |
Expand public education relative to seafood safety perception and advisories. |
|
Summary |
|
|
Regulatory structure and activity in California represents one of the nation's most responsive and current state-based programs to assure seafood product safety. Where problems occur they are being addressed or have been mandated for attention. The higher proportion of imports, recreational fishing, immigrants, various ethnic groups, and extensive coast pose challenging problems. |
|
|
The seafood processing industry is not considered a primary culprit in any seafood safety problems. The principal concerns are environmental contaminants which involve commercial and recreational sectors. |
|
|
California industry and regulatory commitments to seafood safety represent one of the most significant state efforts in the nation. |
|
Table A Review of State Regulatory and Industry Responses (March 29, 1988) Concerning Seafood Quality and Safety
|
FLORIDA |
|
|
Industry Character (1988) |
|
|
1. |
Ranked 8th in total production (182.2 million pounds) and 7th in dockside value ($169.6 million). |
|
2. |
Primarily warmwater, subtropical source of production with significant imports (shrimp and warmwater species) and advanced processing. |
|
3. |
Most fishing vessels independently owned and operated and few company vessels in shrimp fishery. |
|
4. |
Very diverse production through finfish and shellfish; principal species shrimp, spiny lobster, stone and blue crab, grouper and snapper, tuna, oysters, scallops and clams, etc. |
|
5. |
Largest number of licensed seafood processing firms per state. Over 570 firms licensed to process in 1988. |
|
6. |
Consumption includes significant portion of tourist and elderly. |
|
7. |
Aquaculture – initial developments with oysters, clams, catfish, tilapia, and alligators; established crawfish and soft crabs production. |
|
8. |
Major recreational fisheries – all species. Recreational harvest can commercial settings. |
|
9. |
Imports include an increasing influx of tropical, subtropical species to complement traditional species and shrimp destined for large shrimp processing firms. |
|
Primary Product Safety Problems |
|
|
1. |
Raw molluscan shellfish harvest, processing and consumption versus water quality "indicator" regulations relative to potential pathogens (e.g., Vibrio vulnificus). |
|
2. |
Occurrence of ciguatera primarily as a recreational event or from imported fish and fish taken in foreign waters. Public perception is thought to exceed actual incidence. |
|
3. |
Ensuring product safety for precooked, ready-to-eat items (e.g., cooked shrimp, pasteurized crabmeat). |
|
4. |
Lack of funds and personnel to better address issues in a preventive rather than a reactive role. |
|
5. |
Need to assess product quality and potential toxins of concern in the variety of freshwater sources. |
|
Primary Product Quality Problems |
|
|
1. |
General product spoilage for certain fish species. |
|
2. |
Net weight or content declarations for certain frozen crustaceans, fresh shucked oysters, or shellstock. |
|
3. |
Improper species identification and mislabeling. |
|
4. |
Improper storage conditions, (e.g., temperature, packaging). |
|
Additional Problems |
|
|
1. |
Ability to distinguish gamefish versus commercial fish, and aquacultured products versus naturally harvested products. |
|
2. |
Continuing concern for introduction of exotic and potentially disease-bearing species for commercial sales or aquaculture. |
|
3. |
Aquaculture development versus environmental regulations for water quality. |
|
Regulatory Concern for Seafood Industry |
|
|
1. |
Production definitions, (e.g., gallon or pint of oysters, bushel of oysters). |
|
2. |
Mislabeling of ingredients (i.e., use of sulfiting or phosphating agents). |
|
3. |
Monitoring product quality at the vessel level. |
|
4. |
Frozen substitution for product declared fresh (never frozen). |
|
Regulatory Concerns for Their Respective Programs |
|
|
1. |
No or limited seafood-specific training for agencies and inspectors, with exception of federally assisted training for regulation of molluscan shellstock harvest and processing. |
|
2. |
Low salaries; for some agencies there is no geographic adjustments for cost of living. |
|
3. |
Job pressure and diversity for Marine Patrol officers limits their time to address seafood harvest and quality within jurisdiction of Department of Natural Resources (DNR). |
|
4. |
Lack of job advancement. |
|
5. |
Excessive ratio of establishments and products to inspection staff and analytical capability. |
|
Industry Concern for Regulations |
|
|
1. |
Overlapping and sometimes inconsistent regulations pertinent to product quality and sanitation. |
|
2. |
Clarification of product seizure, or destruction authority, particularly for Florida Marine Patrol. |
|
3. |
Clarification of the food quality/safety regulatory role of DNR's Florida |
|
Marine Patrol and Marine Fisheries Commission. |
|
|
4. |
Clarification of vessel or processing equipment confiscation by respective authorities relative to product handling. |
|
5. |
Clarification of state authority and procedures for any product reclamation. |
|
6. |
Extensive product retention time and destruction during regulatory sampling, particularly by FDA with imports. |
|
7. |
Ensuring product quality and safety for recreational harvest sold, legally or illegally, directly to wholesalers or to food service establishments (e.g., potential ciguatoxic fish). |
|
8. |
Better and necessary full inspection and certification before licensing processing/wholesale participants. |
|
9. |
Better communication or regulatory changes and reemphasis versus too many and too diverse agency meetings, particularly by Marine Fisheries Commission to warrant beneficial participation. |
|
10. |
Difficulty in regulating seafood wholesale and retail mobile units. |
|
11. |
Better means to orient and identify "newcomers" to the industry less familiar with regional regulations and practices. |
|
Recommendations |
|
|
1. |
Expand education of regulatory staff with more specific attention to seafoods. |
|
2. |
Add seafood specialists to regulatory staff. |
|
3. |
Establish a "complaint" system that does not result in compliance repercussions. |
|
4. |
Explore utility of a Florida seafood quality "seal" program. |
|
5. |
Explore the development of an industry and regulator agent "certification" program to ensure basic, necessary education and compliance with food safety. |
|
Regulatory Initiatives |
|
|
1. |
DNR's oyster producer "certification" training program. |
|
2. |
Establishing species (frozen) library for Department of Agriculture reference in product identity. |
|
3. |
Expanding academic-regulatory interface to enhance education of regulatory staff and industry. |
|
4. |
Florida legislated "promotional campaign" to be administered by Agriculture and Consumer Services with potential use of Florida Seal of Quality. |
|
5. |
In-state construction of new, state-of-the-art linear accelerator with partial goal to advance technology in irradiation for food safety with nonradioactive source. |
|
6. |
Authorization of molluscan shellfish depuration (e.g., clams) with specific restrictions to ensure product safety. |
|
Industry Initiatives |
|
|
1. |
SFA Product Quality Control Code. |
|
2. |
SFA Seafood Import Surveillance project. |
|
Summary |
|
|
Florida is more prone to seafood product safety problems due to its geographic setting and particular products. The industry is very diverse in product forms and levels of processing, ranging from one-man harvest-sell operations to the world's largest processing firms for shrimp and scallops. The populace contains a significant portion of at risk consumers relative to age. |
|
|
Regulatory structures are equally diverse and are often perceived as redundant in jurisdiction. Despite limited funds and personnel, they have identified and are addressing all major seafood safety and quality issues. The primary safety issues are raw molluscan shellfish consumption and ciguatoxin. |
|
Table A Review of State Regulatory and Industry Responses (October 11, 1988) Concerning Seafood Quality and Safety
|
GEORGIA |
|
|
Industry Character (1988) |
|
|
1. |
Ranked 20th in total production (16.6 million pounds) and 19th in dockside value ($21.5 million). |
|
2. |
Includes temperate and warmwater sources of state production with significant portion of product coming from out of state. |
|
3. |
Most fishing vessels independently owned and operated, whereas few company vessels participate in the shrimp fishery. |
|
4. |
Diverse state production, principal species including shrimp, blue crabs, oysters, clams, whelks, and assorted nearshore finfishes. |
|
5. |
Processing firms range in size and sophistication from fully integrated shrimp breading firms to typical dockside packing houses or off-loading operations. |
|
6. |
Consumption includes demand from large metropolitan regions and some tourism. |
|
7. |
Aquaculture has yet to develop. Includes some progress with soft-shell blue crabs and attempts with crawfish. |
|
8. |
Recreational fisheries — all species. Recreational catch can enter commercial settings. |
|
9. |
Imports play significant role in meeting state demand, particularly in metropolitan regions and as a necessary source of shrimp for established processing firms. |
|
10. |
There are approximately 150 seafood wholesalers and 50 processors/packers in Georgia. |
|
Primary Product Safety Problems |
|
|
1. |
Seafood safety is not evidenced or considered a major concern in production, processing, or retailing in Georgia. |
|
2. |
In ranking problems relative to the amount of regulatory attention, raw molluscan shellfish harvest, shellfish processing and consumption, and blue crab processing are the primary concerns. Concerns for the oyster and clam harvests are controlled through a unique "closed fishery" leasing only concept. Likewise, the industry receives advisory assistance in processing through the University of Georgia Sea Grant Program. |
|
Primary Product Quality Problems |
|
|
1. |
Need more regulatory scrutiny for product integrity [e.g., proper species identification, net weight/content declarations, particularly for out-of-state products in metropolitan regions (retail settings)]. |
|
Additional Problems |
|
|
1. |
Aquaculture products not yet an issue, but should evolve in compliance with Department of Agriculture regulations for product quality and safety, and with reference to species and water quality concerns of Department of Natural Resources. |
|
Regulatory Concerns for Seafood Industry |
|
|
1. |
Industry should request more specific training and involve a trade association. |
|
2. |
Industry should establish more quality control programs with more emphasis on sanitation. |
|
Regulatory Concern for Their Respective Programs |
|
|
1. |
Primary limitations to providing additional and specific regulatory seafood scrutiny from vessel through retail and food service include (in priority order): funds, personnel, equipment, and training. Predictions suggest these limitations will impose more restrictions in the near future. |
|
2. |
Revision and promulgation of additional regulations applicable to the seafood industry have not been adequate. |
|
3. |
Seafood quality and safety are not major concerns or problems in Georgia; thus, it is difficult to justify more regulations or regulatory scrutiny. |
|
4. |
Most (seafood) inspectors initially dependent on on-the-job training in industry settings. |
|
5. |
There are no specific state guidelines for molluscan depuration other than reference to ISSC guidance. |
|
6. |
Consider better regulatory scrutiny, routine and compliance driven, for seafood species identification, particularly in retail and food service sectors in metropolitan regions. |
|
7. |
State regulations reflect dependence on federal and industry guidance (i.e., shellfish regulations in reference to ISSC-NSSP directions, and blue crab processing regulations currently referencing recommendation from the National Blue Crab Processors Association). |
|
8. |
Consider mandatory education for other segments of the seafood industry as required for food service certification. Best to start on voluntary basis. |
|
9. |
Inspection authority and practice begin at the dock. There is no inspection of fishing vessels, with the exception of guidelines for oyster and clam harvest as provided through the ISSC (oysters). |
|
10. |
Evaluate all current regulations for any necessary change. Evaluations should include industry liaison. |
|
Industry Concern for Regulations |
|
|
1. |
Increase awareness and understanding of current state seafood regulations and regulators (i.e., who does what, who approves labels). More active involvement in state/federal regulatory revisions. |
|
2. |
New, better-structured liaison between seafood industry and respective regulatory agencies to consider new and amended regulations, education-certification programs, etc. |
|
3. |
More training and orientation for industry personnel at all levels. |
|
4. |
Department of Agriculture has only one seafood specialist with statewide orientation for seafood quality and safety from vessel through retail, but most inspections are under the supervision of 20 general food inspectors. |
|
5. |
Need more regulatory scrutiny of out-of-state seafoods, which constitute bulk of Georgia consumption. |
|
6. |
In certain settings, NMFS's voluntary inspection service is cooperatively oriented, rather than taking a more advisory approach as in other federal inspection programs. |
|
Recommendations |
|
|
1. |
Initiate seafood educational/training programs for industry and for Department of Agriculture and county health inspectors (Department of Human Resources). |
|
Regulatory Initiatives |
|
|
1. |
Recent interagency meetings to draft plans to address future aquaculture developments. |
|
Industry Initiatives |
|
|
1. |
Currently planning more in-service training in seafood quality and safety for regulatory agencies and industry as provided through Georgia Sea Grant Program and Marine Extension Service. |
|
Summary |
|
Seafood production in Georgia is comparatively modest, yet the processing sector is well established, including large shrimp firms and blue crab processors. The retail and food service sector, particularly in large metropolitan regions, assures a traditional and increasing demand for all seafoods. |
|
The current state regulatory scheme is well organized, with one dominant department adequately addressing most seafood safety concerns. Requests for more specific training and orientation have been noted both for the industry and for regulatory agencies. Academic programs and state agencies are experienced and poised to assist. Additional surveillance is deemed necessary for product in retail, food service, and transport from out of state. |
Table A Review of State Regulatory and Industry Responses (January 2, 1990) Concerning Seafood Quality and Safety
|
HAWAII |
|
|
Industry Character (1990) |
|
|
1. |
Ranked 17th (21.1 million pounds) in production and 19th ($39.7 million) in value. |
|
2. |
Exclusively warmwater fishery, with significant contribution from reef species. |
|
3. |
No commercial shellfishing at present. |
|
4. |
Seafood production primarily for fresh and frozen market, with little additional processing carried out within the state. |
|
5. |
Per capita annual consumption exceeds 22 pounds with a significant proportion of seafood (˜16%) consumed raw. Estimated that 94% of the residents of the island of Oahu consumed seafood during 1987. |
|
6. |
Expanding exports directed at exotic and high-quality markets. The state has developed a "Hawaii Seafood" marketing label to enhance export sales of Hawaiian seafood products. |
|
7. |
Recreational fishery important to overall consumption patterns and to the tourist industry. |
|
8. |
Imports of traditional Hawaiian seafood products (such as mahimahi) have begun to play an increasingly important role in the market. |
|
9. |
Aquaculture is an expanding industry with total industry value (production and aquaculture service sector) reaching $18.2 million in 1988. |
|
Primary Product Safety Problems |
|
|
1. |
State seafood safety problems are almost exclusively limited to three categories: (1) ciguatera poisoning, (2) scombroid poisoning, and (3) "hallucinogenic fish poisoning." |
|
2. |
Cases of ciguatera may number in the hundreds on an annual basis. State officials report that between 1983 and 1987 there were 417 individuals involved in 127 incidents of ciguatera/hallucinogenic fish poisoning. A more recent report places the number of cases at a higher level, with trend analysis suggesting a significant worsening of the problem during the 1980s. |
|
3. |
It has been suggested that as few as one-quarter of ciguatera cases are reported; 80% of the cases are attributable to recreational fishing. |
|
4. |
Ciguatera limited to fish caught within reef environments. Evidence that coastal development activities may serve as a catalyst for elevated levels of ciguatoxin in adjacent areas. |
|
5. |
Ongoing research has produced a simple and inexpensive stick test for ciguatera and other polyether toxins. The test is characterized as being 100% reliable for results indicating the absence of polyether toxins but may produce overly sensitive positive results. |
|
6. |
The number of reported cases of scombroid poisoning has increased in recent years. However, a majority of cases have been attributed to imported products. |
|
7. |
The relative lack of industrialization and enviable water quality in commercial harvesting areas suggest the lack of a significant residual chemical problem in locally harvested seafood. |
|
Issues in the Regulation of Seafood Safety |
|
|
1. |
At present, there is little formal regulatory response, beyond public education, to recreationally caught fish. Further, no state agency has direct responsibility for regulating offshore vessel processors. |
|
2. |
The state fisheries resource agency (Department of Land and Natural Resources, Division of Aquatic Resources) has no legal competency to regulate fisheries on the basis of human health impacts. |
|
3. |
Effective management of ciguatera will require a more fundamental understanding of the temporal and spatial relationship between the occurrence and density of Gambierdiscus toxicus (the dinoflagellate that produces the toxin) and levels of ciguatoxin in fish. |
|
Industry Initiatives |
|
|
1. |
Sponsorship by the Hawaii Seafood Promotion Committee of position papers related to seafood safety. Specifically, a recent report, "The Wholesomeness of Raw Tuna: The Case Against Freezing" prepared by Dr. John Kaneko in response to recent FDA recommendations that all fish destined for raw or rare consumption be previously frozen to eliminate the potential health risk from parasites. |
|
Recommendations |
|
|
1. |
FDA recommendation that all seafood destined for raw or rare consumption be frozen should be reconsidered and rejected. Risk from parasites is limited to a small number of species and regulations requiring freezing should be species specific. |
|
2. |
The state of Hawaii should develop a more proactive strategy to identify and regulate fishing grounds populated by ciguatoxic fish. Such a strategy should incorporate research that would allow for a more effective forecast of likely ciguatoxic areas. |
|
Summary |
|
|
Hawaii provides an unusual case in seafood safety. Good coastal water quality and the present lack of an indigenous shellfish industry mean the absence of a broad |
|
|
suite of seafood safety issues in evidence in much of the continental United States. However, the state does have a significant problem with ciguatera and, to a somewhat lesser degree, with scombroid poisoning, the preponderance of cases being attributable to either recreationally caught fish or imported products. |
|
Regulatory agencies are generally knowledgeable and well trained to deal with the state's significant seafood safety issues. However, there are important gaps in the state's jurisdiction. Particularly, there is no well-defined regulatory competence to manage fisheries resources on the basis of human health. |
Table A Review of State Regulatory and Industry Responses (May 24, 1988) Concerning Seafood Quality and Safety
|
LOUISIANA |
|
|
Industry Character (1988) |
|
|
1. |
Ranked 2nd in total state production (1,356.5 million pounds) and 2nd in value ($317.3 million). production includes a substantial portion of industrial fishery products (e.g., menhaden, fish meal) and valuable traditional edible products (e.g., shrimp, oysters, blue crabs, crawfish). |
|
2. |
State production centers about warm coastal and intercoastal waters supplemented by nutrient-rich flow of the Mississippi delta region. A significant portion of additional products comes from out-of-state sources. |
|
3. |
Other state-based processors dependent on Louisiana's production of certain species (i.e., oysters and shrimp). |
|
4. |
Most fishing vessels independently owned and operated. |
|
5. |
Diversity in levels and types of processing firms include smaller packing houses at dockside; blue crab, shrimp, and oyster processors; large shrimp canneries; and cultured catfish and crawfish processors. There were more than 300 licensed seafood processors in Louisiana in 1988. |
|
6. |
Consumption includes demand in some large metropolitan regions and some seasonal tourism. |
|
7. |
Aquaculture is well established for crawfish and catfish and developing for other food fish. |
|
8. |
Recreational fisheries – all species. Recreational catch can enter commercial settings. |
|
9. |
Imports play significant role as supply for processors and product for consumers, particularly in metropolitan regions. |
|
Primary Product Safety Problems |
|
|
1. |
Primary seafood product safety problems in Louisiana involved the necessary regulatory attention and industry compliance concerns for production and consumption of unprocessed, raw molluscs. |
|
Primary Product Quality Problems |
|
|
1. |
Principal product integrity concerns in commerce are net weight declarations and species identification. |
|
Additional Problems |
|
|
1. |
Producers and processors contend a limited number of commercial buyers will emphasize price over quality and occasionally adjust pricing to provide outlets for inferior product. |
|
2. |
The Louisiana Department of Health and Hospitals has the authority under the State Food, Drug and Cosmetic Law to inspect any vessel or vehicle carrying a commercial food product, but there are no routine vessel inspections with the exception of some molluscan shellfish harvest. |
|
3. |
Industry is often unaware or confused by Louisiana Department of Agriculture versus Louisiana Department of Health inspections for quality or sanitation, despite the fact that the latter agency is mandated by law to permit, inspect, and regulate seafood processing and distribution. |
|
4. |
Current regulatory distinction for cultured products (e.g., alligators, catfish, and crawfish) can be confusing. Inspections can depend on the processor's decision to receive per fee services from the Louisiana Department of Agriculture (through agreements with the USDC-NMFS volunteer Inspection Services), whereas the Louisiana Department of Health (in reference to FDA standards) still maintains concomitant authority which it may not exercise. In addition, the USDA role in assisting cultured product can add to the confusion. |
|
5. |
Any attempts to initiate a state-based "quality seal" program would depend on a realistic mode of standard enforcement that could compete for financing necessary for current regulatory programs in need of more support. |
|
6. |
Efforts to educate the public are difficult due to extreme product diversity. |
|
Regulatory Concerns for Seafood Industry |
|
|
1. |
Department of Agriculture is typically poised to address processing of a "live" harvest (e.g., red meat), yet seafoods are usually landed dead, thus initiating postmortem quality and safety concerns. |
|
2. |
It is difficult to define or distinguish all seafood processors relative to the diversity in levels of product procurement and handling. Distinction based on "altering the original product form" can eliminate firms that only purchase, repack, and distribute. |
|
Regulatory Concerns for Their Respective Programs |
|
|
1. |
Basically, state agencies need more manpower, support funds, and analytical capability to better address seafood quality and safety. |
|
2. |
There are no major concerns to warrant specific, routine vessel inspections for attributes that may compromise product safety. |
|
Industry Concerns forn Regulations |
|
|
1. |
Basically, seafood inspections in Louisiana need to be more equitable and consistent across all segments and sizes of industry. |
|
2. |
Knowledge of regulations and regulatory authority often depends on one or a few individuals' knowledge and possible opinion. |
|
3. |
Seafood processors are deemed the primary target for regulatory surveillance, although they are positioned between two minimally regulated commercial segments, production and retail, which can significantly influence product quality and safety. |
|
4. |
Seafood product quality and safety liabilities must be adjusted to include more responsibility setting. In general, more seafood regulatory surveillance is needed beyond production and processing. |
|
5. |
Federal inspections (i.e., FDA) are focused only on faults, with no offer to advise or direct compliance. |
|
6. |
State inspectors need more seafood-specific orientation and experience in their credentials and training. |
|
7. |
Need clarification and more enforcement for country-of-origin labeling. |
|
8. |
Methods and sources by which to register seafood quality and safety complaints from industrial settings are not obvious, encouraged, or convenient. |
|
9. |
Fishery regulations promulgated to protect stocks can influence product quality and processing modes [i.e., state restrictions that require all fish be landed with head and tail intact (for size verification)]. |
|
Recommendations |
|
|
1. |
Need better coordination and clarification of regulations, regulatory authorities, and safety criteria within and among respective agencies and industry. |
|
2. |
Need to review and improve the state's current seafood quality and safety regulations. Some specific concerns cited are • production and processing of pasteurized blue crab meat and soft-shell crabs; • oyster "sell-by" dating; • clarify regulations and authorities for alligator production and processing; and • address emerging industries (e.g., soft-shell crawfish). |
|
3. |
Need condensed, user friendly version of current seafood quality- and safety-related regulations and regulators. |
|
4. |
Education necessary for any seafood buyer group (i.e., retailers, consumers, institutions). |
|
5. |
Need to consider some forms of uniform, annual training for seafood inspectors and permitted industry participants, both general and species specific. |
|
6. |
Continuing and additional seafood education available through universities (e.g., Louisiana Sea Grant Programs) would be best assisted by initiatives and |
|
|
support from the respective state agencies and industry groups. |
|
7. |
Need more regulatory attention directed on a probable concern basis to address vessel product quality (e.g., cleanliness, proper ice, product abuses). |
|
8. |
Federal authorities need to improve efforts to interface with state regulatory authorities and respective industry participants with particular attention on assisting compliance. |
|
9. |
Need more product quality and safety assurance in state-based promotional programs to address errant publicity. |
|
Regulatory Initiatives |
|
|
None identified. |
|
|
Industry Initiatives |
|
|
None identified. |
|
|
Summary |
|
|
Louisiana represents a state with very abundant and valuable seafood production, which is essential to suit the traditional demands of consumers and utility of in-state and neighboring state processors. This production includes species of primary product safety concern (i.e., molluscan shellfish and certain fish species). Regulatory ability to continue surveillance that ensures product safety will depend on future supplements for manpower with financial and analytical support. Current regulatory jurisdictions can be confusing, particularly pertinent to processing of cultured products. |
|
|
Recommendations for improvements focus on general knowledge and application of existing regulations rather than any need for more regulations. |
|
Table A Review of State Regulatory and Industry Responses (July 25, 1989) Concerning Seafood Quality and Safety
|
MASSACHUSETTS |
|
|
Industry Character (1989) |
|
|
1. |
Ranked 15th in production and 14th in landed value (1987). |
|
2. |
Port of New Bedford ranked 1st in value landed by port (1987). |
|
3. |
Primarily coldwater source production, dominant offshore finfishery with shellfishing predominantly inshore (within the territorial sea). Sea scallops and surf clams also taken offshore. |
|
4. |
Diverse production with principal species including lobster, sea scallops, flounder (particularly yellowtail), cod, shrimp, bay scallop, soft- and hard-shell clams. |
|
5. |
Consumption is representative of principal species, with significant recreational fishery for lobster, cod, winter flounder, bay scallops and soft-shell clams, bluefish and striped bass. |
|
6. |
Seafood contributes significantly to state tourist industry. |
|
7. |
Aquaculture is an emerging, rather than an established, industry in the state. |
|
8. |
Imports constitute a significant portion of consumption in the state, with Canada playing a dominant role, particularly with lobster, flounder and cod (including haddock). |
|
Primary Product Safety Problems |
|
|
1. |
Significant organic chemical contamination of fisheries in Boston and New Bedford Harbors (principally PCBs). |
|
2. |
Widespread closures of shellfish beds throughout the state. Boston Harbor beds are all either closed or restricted to commercial harvesting and depuration. |
|
3. |
PSP closures are common but well controlled by regulatory closures. |
|
4. |
Mercury warnings have been issued for selected inland locations. |
|
Additional Problems |
|
|
1. |
The impact of shellfish depuration on public health (particularly concerning reduction of virus-associated disease) is not well understood. |
|
2. |
The Commonwealth has tested and embargoed a significant proportion of shellfish imported from other states (particularly Maryland). |
|
Regulatory Concerns |
|
|
1. |
The Commonwealth is presently experiencing a severe fiscal crisis. Funding needed for regulatory positions to perform seafood inspection and for harvesting water quality has proven difficult to secure. |
|
2. |
Public health reporting is focused at the level of cities and towns. Less than one-third of Massachusetts cities and towns have fulltime health officers. |
|
3. |
The regulation of shellfish harvest is controlled primarily at the level of cities and towns where level of training can be very uneven. |
|
4. |
Lack of more effective federal guidance for the establishment of regulatory limits is in evidence. Massachusetts waters are contaminated with a number of chemical contaminants not addressed by existing federal limits. |
|
5. |
Aquaculture is a growing industry that, at present, is not well regulated in any discreet fashion. |
|
6. |
The state has established vessel-based regulations, but these regulations have not been well communicated to the industry. |
|
7. |
Massachusetts does not enforce the FDA action level for mercury in marine fish. |
|
Industry Initiatives |
|
|
1. |
Certain seafood restaurants have established independent laboratories to determine levels of pathogens. |
|
Summary |
|
|
The Commonwealth of Massachusetts produces and imports a wide variety of fresh, fresh-frozen, and processed seafood products. Recreational harvesting for both fish and shellfish is common. Significant near-shore problems with chemical contaminants have led to a closure to the taking of all seafood within the Acushnet River estuary and to limited advisories in Quincy Bay. State fiscal problems have severely limited the ability of the Commonwealth to regulate seafood safety. |
|
Table A Review of State Regulatory and Industry Responses (February 9, 1989) Concerning Seafood Quality and Safety
|
MISSISSIPPI |
|
|
Industry Character (1988) |
|
|
1. |
Ranked 5th in total state production (336.4 million pounds) and 14th in value ($61.2 million). Production includes a significant portion of industrial, nonedible fish species. |
|
2. |
State production primarily from warm waters with significant portion of additional product coming from out-of-state sources. |
|
3. |
Most fishing vessels independently owned and operated. |
|
4. |
Diverse state production, principal species including shrimp, oysters, blue crabs, and assorted finish from nearshore and deepwater fisheries. |
|
5. |
Diversity in levels and types of processing firms to include smaller packing houses at dockside; blue crab, shrimp, and oyster processors; large shrimp canneries; and cultured catfish processors. |
|
6. |
Consumption includes some metropolitan regions and some seasonal tourism. |
|
7. |
Aquaculture is well established for catfish and being developed for other food fish. |
|
8. |
Recreational fisheries – all species. Recreational catch can enter commercial settings. |
|
9. |
Imports play significant role as supply for processors and product for consumers, particularly in metropolitan regions. |
|
Primary Product Safety Problems |
|
|
1. |
Attending representative felt that Mississippi agencies and experienced firms believe that state seafood production and processing pose no significant food safety problems, with exception of customary concerns for raw molluscan shellfish. |
|
Primary Product Quality Problems |
|
|
1. |
Most seafood product quality problems involve out-of-state products and retail settings. |
|
2. |
Most quality problems noted in processing firms deal with basic compliance with GMPs. |
|
Additional Problems |
|
|
1. |
Established aquaculture industry is well managed and yields safe, quality products. Pending aquaculture bill will address more regulatory responsibility. |
|
Regulatory Concerns for Seafood Industry |
|
|
1. |
There is general lack of understanding of the regulations, regulatory authorities, and applications. |
|
Regulatory Concerns for Their Respective Programs |
|
|
1. |
Identified needs for additional regulatory/inspection personnel and funds are difficult to achieve in light of limited product safety and quality problems. |
|
2. |
No specific regulations exist to direct inspection or certification of fishing vessels relative to sanitation with exception of molluscan harvest. |
|
3. |
State seafood and food regulatory authority and regulations are currently adjusting to the recent and ongoing programmatic changes in which the major portion of responsibility for seafoods was placed in the Bureau of Marine Resources. The bureau is currently trying to adapt to the new assignment. |
|
Industry Concerns for Regulations |
|
|
1. |
Many nonspecific or flexible regulations are left to regulatory interpretation which has caused confusion. |
|
2. |
It is good to recognize regional differences, but there is a need for more uniformity in regulations across counties and states. |
|
3. |
Regulatory distinction is not clear for authorities dealing with general seafoods versus oysters and crabs versus shrimp in the various industry levels from vessel through processing and retail across the state. |
|
4. |
There should be more permit consolidation for various seafood handling and processing operations. |
|
5. |
Need more equitable regulation of imports. |
|
6. |
General lack of understandable permitting process for processors versus variety of required permits. |
|
Recommendations |
|
|
1. |
Marketing programs need to incorporate more quality and safety in their promotional messages. |
|
2. |
Initiate more training for industry and inspectors. |
|
3. |
Develop a condensed, readable explanation of pertinent state regulations. |
|
Regulatory Initiatives |
|
|
1. |
Agencies in continuing effort to accommodate recent program changes in jurisdictions and authorities. |
|
Industry Initiatives |
|
|
None identified. |
|
|
Summary |
|
|
Mississippi has recently undergone major changes in regulatory responsibility for seafood quality and safety. The changes are to be more efficient, yet they are initially confusing. Most pertinent authority is now housed in the Bureau of Marine Resources, with primary emphasis on oysters. Authority of the bureau extends to distribution, whereas the Department of Health and county health programs assume responsibility for seafood safety in retail and food service. The well-established catfish processing industry has been positioned under surveillance by a separate department (Agriculture). |
|
|
Despite some transitional confusion in state regulatory authority, attending industry and regulatory personnel believe there are no major seafood safety or problems in Mississippi that are not or cannot be resolved by the current regulatory scheme. The efficiency and responsiveness of current programs are restricted only by limited personnel, meager funds, and no specific seafood training. |
|
Table A Review of State Regulatory and Industry Responses (August 22, 1988) Concerning Seafood Quality and Safety
|
NORTH CAROLINA |
|
|
Industry Character (1988) |
|
|
1. |
Ranked 7th in total state production (192 million pounds) and 11th in dockside value ($78 million). A significant portion of the annual landings includes industrial, nonedible fish (i.e., menhaden). |
|
2. |
State production includes temperate and some warmwater sources complemented by a significant portion of out-of-state products. |
|
3. |
Most fishing vessels independently owned and operated and some traditional harvest based on shoreline and shallow-water landing (seines). |
|
4. |
Diverse state production; principal edible species include shrimp, blue crab, and assorted nearshore finfish. |
|
5. |
Processing firms are diversified by size and species. There were more than 130 processing firms licensed to operate in North Carolina in 1988. The most advanced processing firms produce fresh and pasteurized crabmeat. |
|
6. |
Seafood consumption centers about some large metropolitan regions, plus seasonal tourism. |
|
7. |
Aquaculture development is established with production of freshwater trout and emerging efforts for catfish and striped bass. |
|
8. |
Recreational fisheries – all species. Recreational harvest can enter commercial channels. |
|
9. |
Imports play major role in meeting state demand, particularly in metropolitan regions. |
|
Primary Product Safety Problems |
|
|
1. |
Seafood safety is not considered a major problem in North Carolina, and where problems exist or recur the current regulatory agencies feel that they are addressing these issues in a cooperative and reactive manner. |
|
2. |
One of the most difficult seafood safety-related issues is classification and monitoring of molluscan shellfish growing waters. |
|
Primary Product Quality Problems |
|
|
1. |
The most common yet easily resolved seafood product quality problem is temperature abuse. |
|
2. |
Lack of some basic food quality control practice in some industry settings. |
|
3. |
Fishing vessels are not inspected routinely relative to food quality and sanitation, but there are no significant or persistent problems. As problems |
|
|
occur (e.g., proper use of sulfites, handling of scallops), current regulatory programs can adequately address these issues. |
|
Additional Problems |
|
|
1. |
North Carolina institutional buying specifications and guidelines for product quality and sanitation could be more aligned in support of its seafood industry's products and expectations. A state program for seafood marketing that emphasizes product quality and safety has been slow to develop. |
|
2. |
Product safety and quality regulations for aquaculture should mesh into existing regulatory programs rather than initiating new ones. |
|
Regulatory Concerns for Seafood Industry |
|
|
1. |
Trade associations can be more involved in regulatory/industry training. |
|
2. |
Industry, especially newcomers, need more regulatory orientation. |
|
Regulatory Concerns for Their Respective Programs |
|
|
1. |
Regulatory attention is often complaint directed or planned to address most-probable problems. |
|
2. |
State inspectors get very limited seafood orientation outside of on-the-job training. There is a need for more orientation to seafoods and the seafood industry. |
|
3. |
Inspection for retail settings is largely left up to local, county authorities, which may or may not have a specific ordinance to direct activity. Although there are no specific agreements, county (health) authorities often reference respective state regulations. More formal agreements and regulatory attention may be warranted. |
|
4. |
County authorities are confronted with a troublesome turnover rate (1 of 7 inspectors per year) that can compromise effectiveness and training commitments. |
|
5. |
Mobile units are difficult to monitor, but they have not presented a food safety problem. |
|
6. |
Regulation for product quality and safety in interstate commerce can be complicated by lack of uniformity across states for particular seafoods (e.g., boiled versus steamed crabmeat, raw oysters, calico and bay scallops, general grade enforcements). |
|
Industry Concern for Regulations |
|
|
1. |
Industry and regulatory authorities should coparticipate in more training and orientations to ensure continued product quality and safety. |
|
2. |
Regulatory authorities need new, improved techniques to better accommodate processing developments and changes. |
|
3. |
Retail inspections for seafood concerns appear more complaint directed than routine. |
|
4. |
Need more regulatory surveillance for imports. |
|
Recommendations |
|
|
1. |
Consider "certification" program for processors and inspectors to ensure some education from established academic/extension service programs. |
|
2. |
Consider industry "internship" programs as part of the training and orientation inspectors that will deal with seafoods. |
|
3. |
Start industry/regulatory-based awards program to recognize regulatory/inspector service. |
|
4. |
Produce a publication to consolidate and simplify the introduction to North Carolina's seafood quality and safety requirements and concerns. |
|
5. |
Improve media liaison and public advisories on seafood safety issues in North Carolina. |
|
6. |
Various state regulatory programs and industry associations should increase participation in professional organizations established to foster more interstate liaison (i.e., AFDO and AFDOSS). |
|
Regulatory Initiatives |
|
|
1. |
Agency liaison ongoing to consider more direction and structure for inspection of retail firms. |
|
2. |
Agency liaison ongoing to plan for aquaculture products in commerce. |
|
3. |
Agency liaison ongoing to consider seafood promotional activity with more product quality and safety emphasis. |
|
Industry Initiatives |
|
|
None identified. |
|
|
Summary |
|
North Carolina's edible seafood production is comparatively modest and predicted to be approaching annual maximum yield. There are no major biological indications for significant increases in state commercial harvests. Imports, out-of-state production, and some aquaculture represent the future supplies to meet North Carolina's growing product demands. |
|
North Carolina's seafood production and processing have not and are not predicted to pose any significant seafood safety problems that cannot be addressed by existing regulatory authorities and industry cooperation. Most safety and product quality issues are known and under control. The existing surveillance system provides sufficient routine monitoring for particular concerns (e.g., molluscan harvest and blue crab processing). Likewise, existing programs have the ability to respond to emerging problems and complaints. Program effectiveness can be improved through more and specific seafood training and orientation, with coparticipation by the industry. |
Table A Review of Regulatory and Industry Responses (November 29, 1989) Concerning Seafood Quality and Safety
|
PUERTO RICO |
|
|
Industry Character (1989) |
|
|
1. |
Local seafood production in Puerto Rico is modest and primarily artisanal. In 1987 the total recorded seafood landings just exceeded 2.0 million pounds, with a dockside value of $3.1 million. These figures do not account for tuna destined for major Puerto Rican-based tuna canning operations. |
|
2. |
There are more than 2,000 local, nearshore commercial fishers with an average annual production of only 3,000 pounds per fisherman. Most local, nearshore commercial fishing is from small-scale and individually owned vessels, whereas most of the larger, offshore vessels fishing tuna, swordfish, etc., are company owned or based outside Puerto Rico. |
|
3. |
Harvest from nearshore waters is diverse, including a typical variety of tropical and subtropical species. Principal species include snapper, grouper, grunt, tuna, mackerel, lobster, and conch. In 1987 the recorded landing for barracuda was 27.1 thousand pounds, and reported landings for oysters and clams totaled only 52 pounds. (These shellfish landings are obviously lower than actual due to limited reporting.) |
|
4. |
Puerto Rican and neighboring Caribbean coastal waters do represent some current sources for certain popular fish species (e.g., swordfish, tunas, shark) destined for U.S. fresh and frozen seafood markets. |
|
5. |
Local fisheries support popular recreational fishing. Recreational harvest can enter commercial settings. |
|
6. |
With the exception of modern, large-scale tuna canning operations there is very little seafood processing in Puerto Rico. Large-scale tuna canning operations shipped over 500 million pounds of canned tuna to the United States in 1987. |
|
7. |
The average annual Puerto Rican per capita seafood consumption is expected to exceed the average amount (~15 pounds per person) recorded for mainland U.S. consumers. The substantial influx of tourists expects seafood availability. |
|
8. |
Estimates suggest that nearly 90 to 95% of the seafood consumed in Puerto Rico is purchased from other regions. In 1986, seafood shipments from the United States alone exceeded 33.5 million pounds, excluding tuna. |
|
9. |
Seafoods shipped to Puerto Rico can include "true" imports originating in countries outside of the United States or as products merchandized through the mainland United States to Puerto Rico. True imports coming into Puerto Rico are subject to clearance through U.S. Customs and FDA, whereas seafoods originating in mainland U.S. ports can be reshipped to Puerto Rico without additional federal scrutiny. |
|
10. |
According to previously recorded CDC data, 49% of all seafood-related illnesses are reported from four states or territories: Hawaii, Puerto Rico, the Virgin Islands, and Guam. These tropical settings include unique species, ambient temperatures, and handling difficulties that contribute to seafood quality and safety problems. Previous public health reports (via CDC) suggest ciguatera is a primary seafood safety problem in Puerto Rico. |
|
11. |
Aquaculture is limited in Puerto Rico. Initial efforts are in progress for shrimp, tilapia, and some molluscan shellfish. |
|
Primary Product Safety Problems |
|
|
1. |
The primary seafood safety problems in Puerto Rico are typical for tropical settings (i.e., ciguatera, scombroid poisoning, and adverse microbial pathogens associated with consumption of raw molluscs). |
|
2. |
Industry practices in production (harvest and culture) and retail/restaurant handling represent the primary areas of seafood product safety concern for local authorities. There is little seafood processing, with the exception of tuna canning operations that pose no significant seafood safety problems. |
|
Primary Product Quality Problems |
|
|
1. |
On-board handling practices by local, nearshore fishermen are suspect for seafood quality problems. Their tendency to employ traditional methods and their inability to finance new vessels or gear contribute to product quality concerns. Despite the concerns, most fishermen can usually sell all they catch because local demand exceeds supply, and in some instances, local product scrutiny lacks knowledge in quality judgments. |
|
2. |
Seafood products from the mainland United States, less subject to federal scrutiny in Puerto Rico, are suspect for inferior quality. |
|
3. |
In some instances, retail and restaurant personnel lack sufficient training to prevent and identify seafood quality problems. |
|
Additional Problems |
|
|
1. |
Regulatory and industry representatives believe that Puerto Rico's ciguatoxic reputation is exaggerated and perpetuated without sound epidemiological confirmation. This general belief does not diminish regulatory concern for ciguatera but encourages efforts to address the problem with better epidemiology, education, and development of reasonable surveillance methods. Continuing exaggerated media coverage of ciguatera on the island results in large economic losses to the already weak fishing industry of Puerto Rico. |
|
2. |
Epidemiology in the Department of Health needs to improve efforts to confirm etiological agents contributing to the large number of annual cases of gastroenteritis and associated ailments. In 1988 there were over 67,000 |
|
|
reported cases of gastroenteritis without etiological confirmation. Efforts are seriously compromised by infrastructure in Puerto Rico and social practices/beliefs. This situation confuses any interpretation of seafood-borne illnesses. For example, local authorities are confused by conflicting reports citing incidences for ciguatera, whereas their program does not include ciguatera as a disease in weekly reports to CDC. In 1989, only three cases of ciguatera were reported. There is serious concern about the probable incidence of hepatitis as a consequence of raw mollusc consumption. |
|
3. |
CODREMAR (Corporation for the Development and Administration of Marine Resources) of Puerto Rico was established in 1979 basically as a commercial development program to assist fisheries and aquaculture. Legislated structure aligned programs with the Department of Natural Resources and issued authority ranging from assessing (records access) and licensing of harvest and cultured production, to quality control. The corporation was given authority to establish, by regulation, standards for establishments, and for sanitary and quality controls of the fish harvest and the processing of fish and fishing products. It could conduct inspections, suspend rights to process, and confiscate (seafood) products in any establishment in which it was determined that public health was jeopardized. This board authority, although directed to work in agreement with other established agencies for food quality and safety, was not generally known or understood by the respective segments of the seafood industry and pertinent agencies. In practice, CODREMAR has focused primarily on the development of fisheries and aquatic product cultivation. Recent legislative considerations indicate change may be implemented by June 1990 to reorient the program and realign efforts with the Department of Agriculture. |
|
4. |
Regulatory authority is shared, in part, by the Department of Consumer Affairs and the Department of Health. The Department of Health conducts inspections for public health reasons, can seize decomposed and potentially injurious foods, and can use court assistance for cease and desist action. The Department of Consumer Affairs has the authority to act against a business through issuing administrative fines and conducting hearings. These actions can be in support of the Department of Health's activities but are primarily concerned with protecting the consumer against fraud and deceit (species substitution, selling previously frozen fish as fresh fish, net weight violations, etc.). In effect, the Department of Health's complaints or actions against a seafood proprietor can be cause for the Department of Consumer Affairs to conduct an administrative hearing that could decide for cease and desist action. Distinction of authority is not always clear or understood, and can be confused by the multiple market levels unique to seafood commerce, particularly dockside sales and mobile units. |
|
5. |
Despite significant attempts to educate and warn the public and industry about ciguatoxic concerns there have been no efforts to assess the success, in terms of decreased illness of these programs. In a general public forum these efforts are thought to deter tourism and business. |
|
Regulatory Concerns for Seafood Industry |
|
|
1. |
Local industry from production through retail generally lacks understanding of the principal authorities and pertinent regulations that ensure seafood quality and safety. |
|
2. |
There is a continuing lack of knowledge and commitment among a substantial portion of local fishermen about proper onboard handling and seafood safety concerns. |
|
3. |
Recreational harvest, which may or may not be licensed as a commercial event, can enter local seafood markets through channels less subject to regulatory surveillance. |
|
4. |
Although local fishermen believe their knowledge of potential ciguatoxic areas provides avoidance of ciguatoxic fish, there has not been any routine confirmation by water or product sampling to "map" problematic regions for regulatory control. In 1979 the Department of Natural Resources and CODREMAR issued a notice that prohibits buying, selling, and marketing three species that can cause ciguatera: barracuda, amberjack, and black jack. This notice also says that if these species are found in the market, CODREMAR will embargo, seize, or destroy them. It is still displayed in some fishing villages and, in the past, was also used in restaurants. (Note: CODREMAR's program status is currently in question.) |
|
5. |
The major portion of seafood products consumed in Puerto Rico is shipped from the United States and not subject to routine "import" surveillance by FDA authorities based in Puerto Rico. Although there are no major documented safety concerns associated with these products, product quality has been questioned. Puerto Rico, like some other U.S. territories, has been noted as a potential "dumping" ground for inferior quality. Regulatory scrutiny for this concern relies on limited local authority currently stressed by concerns for local production. |
|
Regulatory Concerns for Their Respective Programs |
|
|
1. |
There are no specific regulations or directed regulatory authority to routinely address seafood handling at the vessel production level. |
|
2. |
The Department of Health and Department of Consumer Affairs should offer more guidance and training for all postharvest segments of the seafood industry, particularly restaurants and retailers. |
|
3. |
An interdepartmental review of current regulations and regulatory activity pertinent to seafoods is warranted, but promulgation of additional and more specific regulations and surveillance would be limited by the lack of program personnel and funds. |
|
4. |
Local molluscan shellfish production by harvest or cultivation is very limited, but the confirmed presence of certain microbial pathogens (e.g., viruses, Vibrio vulnificus), no specific monitoring and approval of harvestable waters, and associated illnesses from raw consumption warrant consideration for regulatory attention. Any regulatory initiative must realize the limited production relative to program operational costs and incorporate cost-effective |
|
|
use of public and professional education. |
|
5. |
Recent additions to the Department of Health's analytical capability for environmental contaminants should consider aquatic product assessments in terms of edible portions. |
|
6. |
Regulatory authority for aquaculture product quality and safety is not distinct and can be confused with programs aligned to promote development of cultured production. |
|
Industry Concern for Regulations |
|
|
1. |
Local merchants welcome additional regulatory surveillance for seafood quality and safety, which they currently feel is limited in activity and visibility. This concern results from a combination of actual regulatory limitations and general industry confusion about responsible regulatory activity. |
|
2. |
Local fishermen's concern for harvest quality and safety is compromised by limited to no available, reasonable financing to purchase new vessels and gear to allow better preservation and access to less problematic offshore resources. This economic situation compromises local efforts and incentives to resolve any quality and safety problems, while allowing resource access to more modernized non-Puerto Rican fishing competition. |
|
3. |
Regulatory surveillance and liaison with the tuna canning operations are believed adequate and appreciated. This position is based on the processors' intuitive HACCP approach common for canning operations. |
|
Recommendations |
|
|
1. |
Develop a financial support program that allows local fishermen to invest in vessels and gear that provide better preservation of the harvest and access to offshore resources. |
|
2. |
Additional, routine food quality, safety, and general sanitation training for seafood proprietors and respective regulatory authorities is strongly encouraged. A single, comprehensible publication that outlines the pertinent seafood regulations and the regulators should be the first educational objective. |
|
3. |
Aquacultural development, particularly in the event of molluscan shellfish aquaculture, should be made more mindful of water quality in terms of site selection and various operating parameters that may adversely contribute to product quality and safety. |
|
4. |
Research, education, and regulatory efforts must be continued to prevent seafood-borne illnesses in Puerto Rico, particularly ciguatera. Goals should include rapid, analytical methods for prevention, "targeted" education for avoidance, and considerations of regulatory positions on parameters to minimize occurrence (e.g., restrictions by species or harvest location). |
|
5. |
Local trade associations (e.g., "Meat Institute") should join forces with academic-based programs (e.g., University of Puerto Rico Sea Grant College Program) to design and support seafood quality and safety training efforts |
|
|
for all segments of the seafood industry and regulatory authorities. This effort could establish an industry-based seafood quality and safety advisory board to direct and attract necessary support and participation. |
|
6. |
An interagency committee should be established to better communicate the responsibilities, current and planned efforts, and industry liaison pertinent to seafood quality and safety. This committee should incorporate expertise from the industry and advisory/research programs in academic institutions. |
|
7. |
Because the major portion (˜95%) of seafoods consumed in Puerto Rico is imported from various countries and shipped from the mainland United States, these products warrant more equivalent surveillance to ensure seafood safety and quality. This recommendation is most applicable to seafood reshipped from the mainland United States and not subject to additional clearance through customs or FDA. |
|
Initiatives |
|
|
1. |
The Governor of Puerto Rico has established a Food and Nutrition Committee composed of many agency representatives to prepare an advisory paper on food nutrition, quality, and safety problems in Puerto Rico. This report is due by mid-1990 and plans to include problems related to seafoods as produced and consumed in Puerto Rico. |
|
2. |
Recent FDA regional programming has rearranged federal authority for Puerto Rico within the Atlanta-based jurisdiction. This arrangement is intended to direct more attention to seafood safety concerns in import surveillance and further assistance for local authorities, principally the Puerto Rico Department of Health, in addressing seafood safety of molluscan shellfish, reshipped products, and any specific issues through various levels of commerce. |
|
3. |
Besides preparing and distributing publications to consumers and fishermen, the University of Puerto Rico Sea Grant College Program also offers seminars and workshops directed to the entire distribution chain, including housewives, students, fishermen, fish inspectors from the Health Department, restaurants, and supermarkets. Besides stressing proper fish handling techniques, these workshops and seminars cover an array of subjects such as hazards posed by these products to the consumer [e.g., ciguatera, contaminants in the water (especially for shellfish), parasites, scombroid poisoning, naturally toxic fish, seafood nutritional value, preparation, display techniques]. |
|
Summary |
|
Puerto Rico is more prone to seafood product quality and safety problems due to its particular geographic setting, artisanal nearshore fisheries, and dependence on "imported" products. Local fishing activity lacks abundant nearshore seafood resources to justify financial support for vessel and gear improvements for better on-board preservation or offshore sourcing in less problematic waters. Seafood processing is limited to large tuna canning operations that maintain sufficient product quality and safety assurance programs. The per capita seafood consumption exceeds the average amount for the United States and includes tourist trade. |
|
Regulatory programs and authority are present and can be effective in surveillance for seafood safety and quality. Their efforts are limited by lack of enough personnel and operating funds to allow more specific attention to seafoods. Industry compliance and cooperation are hampered by a clear distinction between pertinent regulations and regulatory authorities. Additional regulatory liaison and surveillance, and more industry, regulatory, and public education are requested to ensure better seafood quality and safety. |
Table A Review of State Regulatory and Industry Responses (November 10, 1988) Concerning Seafood Quality and Safety
|
SOUTH CAROLINA |
|
|
Industry Character (1988) |
|
|
1. |
Ranked 21st in total production (16.2 million pounds) and 20th in dockside value ($21.1 million). |
|
2. |
Includes temperate and warmwater sources of state production with significant portion of product coming from out of state. |
|
3. |
Most fishing vessels independently owned and operated. |
|
4. |
Diverse state production; principal species include shrimp, blue crabs, oysters, and assorted nearshore finfish. |
|
5. |
Most processing firms are comparatively small with majority involved in operations for picking and pasteurizing blue crab meat. |
|
6. |
Consumption includes large metropolitan regions and seasonal tourism. |
|
7. |
Aquaculture limited and in infancy with catfish, hybrid striped bass, clams, and soft-shell crabs. |
|
8. |
Recreational fisheries – all species |
|
9. |
Imports play significant role in meeting state demand, particularly in metropolitan regions. |
|
Primary Product Safety Problems |
|
|
1. |
Regulatory agencies and state-based industry firms believe seafood production and processing in South Carolina do not pose any major seafood product safety concerns. |
|
2. |
Concerns for molluscan shellfish harvest and processing, and blue crab processing, are being adequately addressed with reference to established federal and state guidelines. |
|
Primary Product Quality Problems |
|
|
1. |
Product integrity in terms of proper species identification, net weight/content declarations, and use of ingredients are the principal concerns, particularly for the major portion of seafoods from out-of-state sources. |
|
Additional Problems |
|
|
1. |
Aquaculture could introduce additional species identification problems (e.g., cultured striped bass versus natural striped bass stocks). |
|
2. |
The "25-mile line" distinguishing regulatory authorities to inspect fish handling/processing operations offers economic advantages for operations, but can be confusing if not well coordinated. |
|
Regulatory Concerns for Seafood Industry |
|||
|
1. |
In general, industry has a poor understanding of the pertinent seafood regulations and regulators from vessel through retail. |
||
|
Regulatory Concerns for Their Respective Programs |
|||
|
1. |
Need more training and orientation for regulator inspectors and less dependence on on-the-job training. |
||
|
2. |
Employee turnover due to weak salaries and lack of career orientation hampers regulatory capability and limits justification of more significant, structured education. |
||
|
3. |
Level of current state production and predicted decreases cannot support justification for increased regulatory emphasis, particularly in reference to primary species and limited safety problems. |
||
|
Industry Concerns for Regulations |
|||
|
1. |
Want more active than reactive regulatory program, especially for product integrity (i.e., species identification and incoming product quality). |
||
|
2. |
Need more regulatory consolidation and explanation of the pertinent regulations and regulators for product quality and safety. |
||
|
3. |
Regulatory activity is primarily compliance oriented, particularly concerning product integrity (e.g., fresh versus frozen declaration, species identification, proper net weights). Would like to see more routine surveillance, particularly for products from out of state. |
||
|
4. |
Need more equitable inspection of imports and products from out of state. |
||
|
Recommendations |
|||
|
1. |
Plan periodic training on seafood quality and safety for all levels of industry and regulatory agencies. |
||
|
Regulatory Initiatives |
|||
|
None identified. |
|||
|
Industry Initiatives |
|
|
None identified. |
|
|
Summary |
|
|
South Carolina is not a major seafood producing and processing state, yet the seafood industry and products are a traditional and expected part of the state's economy and image. The major portion of seafood consumed comes from out-of-state sources. State production and processing of oysters and blue crabs are being adequately monitored to ensure consumer safety. Increasing product demand and operational requirements for the regulatory agencies could compromise future surveillance for product quality and safety. |
|
|
The industry and respective regulatory agencies are content with the current "25-mile line" distinguishing jurisdictions for inspection of fish processing, but this economic operational agreement could be compromised by steady-state and reduced budgets. |
|
Table A Review of State Regulatory and Industry Responses (March 6, 1990) Concerning Seafood Quality and Safety
|
TEXAS |
|
|
Industry Character (1990) |
|
|
1. |
In 1988, dockside seafood landings in Texas ranked 14th in production (96.0 million pounds) and 5th in value ($175.7 million). |
|
2. |
Primarily warmwater, nearshore fisheries from subtropical sources were supplemented with significant imports including foreign entries via ground transport from Mexico. |
|
3. |
Fish vessel ownership split between individuals and company-based operations. |
|
4. |
The high value for Texas seafood production is attributed to the shrimp harvest, which generates approximately 80% of the landed value. Blue crab and oyster production constitutes the next major portion of the industry, whereas finfish production is meager due to management decisions for recreational interest. |
|
5. |
Most significant segment of seafood processing involves blue crabs and oysters; larger shrimp harvest supports a traditional off-loading/packing practice destined for fresh and frozen markets or further processing outside Texas. |
|
6. |
Consumption includes significant portion of ethnic groups and some particular tastes (e.g., Texas consumes 40% of nation's cultured catfish production). |
|
7. |
Aquaculture is in a fledgling yet developing state, with primary focus on catfish and growing interest in tilapia and crawfish. Saltwater aquaculture is not evident to date. |
|
8. |
Major recreational finfish fisheries exist for near and inshore species. |
|
9. |
There are strong regulatory distinction and enforcement between recreational and commercial fishing activity. |
|
10. |
Imports continue to increase to suit regional demands. |
|
Primary Product Safety Problems |
|
|
1. |
Raw molluscan shellfish consumption constitutes most important concern for regulatory scrutiny among Texas-based seafood production and use, but it is not considered a major problem without effective controls. |
|
2. |
Stronger enforcement penalties have proved effective and necessary to curb illegal harvest or bootleg activity from unapproved growing areas. |
|
3. |
There is a growing concern about use of further processing in retail settings. |
|
Primary Product Quality Problems |
|
|
1. |
Assuring postharvest product quality and maintaining product quality in wholesale and retail settings (e.g., basic time temperature abuse). |
|
2. |
Recurrent problems include initial spoilage, excessive or undeclared use of sulfites, and misuse of phosphates and net control labeling. |
|
Additional Problems |
|
|
1. |
There is no specific legislated authority to monitor postharvest product quality with the exception of molluscan shellfish. |
|
2. |
Blue crab processing in Mexico has not evolved to a level of concern for more surveillance of ground imports (via Mexico), yet should activity increase the only regulatory recourse for Texas health authorities is reliance on limited federal screening upon entry. This situation places strong dependence on foreign government assurance that plants comply with adequate GMPs for potential ready-to-eat seafood items. |
|
3. |
More routine product assessments are warranted to ensure that regional and site-specific aquatic resources do not harbor potential contaminants. Despite interagency cooperation, authorities lack sufficient allocations to support activity under the "Aquatic Life Law" that directs field sampling for routine product analysis of potential chemical contaminants. The respective health authorities rely on data from agency programs more directed to assess the water quality and consequences on aquatic life. |
|
4. |
Authorities question if illegal harvest restrictions should be imposed to deter catching finfish from areas identified as potentially toxic (e.g., closures, advisories, bans), similar to the authority to halt harvest of shellfish from prohibited waters. |
|
Regulatory Concerns for Seafood Industry |
|
|
1. |
An ethnic related problem exists for bootleg crabmeat. |
|
2. |
Principal seafood surveillance programs focus primarily on ensuring seafood safety, with concomitant inference for quality, thus leaving seafood quality assurance an industry responsibility. |
|
Regulatory Concerns for Their Respective Programs |
|
|
1. |
Based on home rule, counties and cities establish their own surveillance for retail and restaurant operations. They rely on help from their state-level counterparts, which can be directly involved in the absences of local authorities. This system warrants additional attention to ensure more uniform and specific activity across the retail sector, particularly in reference to seafood products and handling. |
|
2. |
State health authorities do not have the mandate or support to conduct technical work (research) to assess new processing innovations (e.g., alternative crab |
|
|
washing procedures). Current regulatory programs must rely on developments and verification in industry or academic settings, or from the federal government. |
|
3. |
Current shellfish program, like all state programs, lacks ability to confidently trace molluscan shellfish back to the source of actual harvest, thus limiting resolution of any adverse consequence beyond the processor. |
|
4. |
Current surveillance programs for seafood safety need more manpower and funds to support additional and more uniform coverage from dock, through processing, to retail sectors. |
|
5. |
Although academic and federally based programs offer annual educational support for the agency staff, additional seafood-specific training is necessary particularly at county and city levels. Current programs depend on substantial amounts of on-the-job training. |
|
6. |
Federal inspection activity in Texas had decreased, leaving a larger burden for state health authorities. |
|
7. |
Coastal, inshore water quality assessments need to include more work with long-term trend assessments and "bioaccumulation." |
|
8. |
Initial lack of interagency coordination in legislation and program development for aquaculture has confused regulatory distinction in authority for surveillance of processing cultured products. |
|
Industry Concern for Regulations |
|
|
1. |
There is some confusion over the respective roles and rules of the divisions within the Texas Department of Health and other agencies relative to permitting and surveillance of traditional seafood processing. |
|
2. |
To avoid federal inspection, some seafood processors can cease operation, yet maintain their license to process, then resume processing once the probability of inspection is lower. |
|
3. |
Licensing and permitting requirements with Texas Departments of Health, Agriculture, Parks and Wildlife, and Texas Water Commission can be confusing and discouraging for aquaculture development. |
|
Recommendations |
|
|
1. |
Additional emphasis and support should be directed to increase and standardize seafood quality and safety surveillance in the retail and restaurant sectors as monitored by respective county and city authorities. |
|
2. |
Aquaculture development, particularly in processing sector, warrants more interagency coordination relative to pertinent product quality and safety assurances. |
|
3. |
Regulatory seafood inspectors for processing through retail need additional and more uniform training in seafood processing and handling. |
|
4. |
A condensed compilation of all related Texas regulations on seafood quality and seafood safety should be published for industry reference. |
|
5. |
Health authorities and seafood processors should consider a "certification" program based on minimal, standardized training for inspectors, processing |
|
|
personnel, and plant managers. |
|
6. |
Additional manpower is needed for adequate surveillance of seafood processors and wholesalers. |
|
Regulatory Initiatives |
|
|
None identified. |
|
|
Industry Initiatives |
|
|
None identified. |
|
|
Summary |
|
|
Texas seafood production and processing do not pose any major acute seafood safety concerns that are not being addressed by current regulatory programs. The processing sector is primarily limited to two species, blue crabs and oysters. Product quality compromised prior to processing or in wholesale retail/restaurant settings is the principal concern. Regulatory efforts at county and city level may warrant reassessment for the surveillance for seafoods in retail. The evolving aquaculture industry offers an opportunity for agencies to evaluate their roles and initiate interagency cooperation to better address cultured product quality and safety. Continuing concern for potential contaminants in edible, aquatic resources destined for commercial or recreational harvest will require sufficient funds to support established health programs and encourage additional interagency cooperation. |
|
Table A Review of State Regulatory and Industry Responses (May 24, 1989) Concerning Seafood Quality and Safety
|
WASHINGTON |
|
|
Industry Character (1989) |
|
|
1. |
Ranked 9th in production and 6th in landed value. |
|
2. |
Primarily coldwater source production and pass-through processing for Alaska and offshore vessels. |
|
3. |
Principal species – salmon, halibut, albacore, tanner and dungeness crab, shrimp, and oysters. |
|
4. |
Introduction of large-scale offshore processing. |
|
5. |
Significant portion of seafood utilization in King County. |
|
6. |
Aquaculture principal species – salmon and oysters. |
|
7. |
Recreational fisheries increasing. |
|
8. |
Imports constitute significant and increasing portion of commerce. |
|
Primary Product Safety Problems |
|
|
1. |
Molluscan shellfish safety – microbial consequences and PSP on the product and in the environment as encountered by both commercial and recreational interests. |
|
2. |
Monitoring for toxic contaminants in the environment with need to increase surveillance and interprogram coordination for prevention and assessments. |
|
Regulatory Concerns |
|
|
1. |
Use of live tanks (wet storage) and mixed molluscan species should be addressed. |
|
2. |
Need to improve epidemiology including commercial and recreational consequence. |
|
3. |
Need more equitable surveillance for imports versus domestic products, and products during and from offshore processing. |
|
4. |
Currently programs have experienced some poor coordination in identifying import problems, general food safety/quality concerns and species marketing and introduction. |
|
5. |
There are occasional conflicts with federal program as pertains to concomitant state responsibility (e.g., state versus FDA versus NMFS on foreign certification for wholesomeness). |
|
8. |
Public perception versus reality (education) for seafood safety. |
|
9. |
Lack of adequate funding, staff and analytical tools, with emphasis on rapid methods. |
|
Regulatory Initiatives |
|
|
1. |
Statewide recreational mollusc harvest regulation. |
|
2. |
Expand labeling to inform public. |
|
3. |
Initiate education programs for regulatory agencies, industry, and public. |
|
Industry Initiatives |
|
|
1. |
Canned Salmon Control Plan. |
|
2. |
Annual training/education in cooperation with academic based expertise (e.g., Sea Grant Programs). |
|
Recommendations |
|
|
1. |
Formalize and exercise HACCP concept in regulatory and commercial practice. |
|
2. |
Improve current regulations and analytical capability. |
|
3. |
Address microbiological standards for high-risk products (e.g., raw, cooked ready to eat). |
|
4. |
Address safety issues for molluscan shellfish in retail (e.g., use of live tanks, tagging, and pull dates). |
|
5. |
Expand education of • public (high-risk groups and special issues); • industry (consider "certification"); and • regulators (more and more frequent seafood training). |
|
Summary |
|
|
Washington State is not a problem area due primarily to unique features of environment, specific species involved, nature of processing (largely pass-through operations), and effective, responsible regulatory programs. |
|
|
Where problems exist they have been identified and are being addressed with current capability and recommendations. The primary seafood safety concerns are molluscan shellfish consumption and specific environmental contaminants. |
|
|
Washington State stands as an excellent example that regulation (inspection, surveillance) does exist and does work at the state level. |
|