Although large fluctuations in atmospheric CO2 levels are common in Earth’s history, past increases in CO2 occurred over periods of hundreds of thousands to millions of years, and thus differ considerably from the very rapid present day increase related to human activities. The current rate of increase in the level of atmospheric carbon dioxide is unprecedented over at least the past 55 million years (Kump et al., 2009; Zeebe et al., 2009). The rate is far greater than occurred in even the most rapid events known from Earth history, and each of these past events were accompanied by important changes in ocean chemistry and mass extinctions of ocean or terrestrial life or both. Currently, atmospheric CO2 levels are approaching 395 ppm, a value 40% higher than the preindustrial period and greater than has occurred for at least 800,000 years. Approximately one-third of the CO2 added to the atmosphere since the beginning of the Industrial Revolution in the mid-18th century has been absorbed by the ocean, and recent estimates suggest that the ocean is continuing to absorb each year approximately one-quarter of global anthropogenic CO2 emissions (Sabine et al., 2004; Khatiwala et al., 2009; Le Quéré et al., 2009; Sabine and Tanhua 2010). By absorbing a substantial share of the CO2 released through such human activities as fossil fuel combustion, cement production and land-use change, the ocean plays a critical role in moderating human-induced climate change. However, this beneficial effect of CO2 uptake by the ocean is coupled with potentially damaging consequences due to changes in ocean carbonate chemistry and
BOX 1.1
Ocean Acidification
Definition and Drivers of Ocean Acidification: Acidity is measured using the pH scale, where pH is the negative of the base 10 logarithm of hydrogen ion activity. Note that ocean acidification does not necessarily mean that seawater will become acidic, i.e., attain a pH below 7. Rather, it refers to the increase in hydrogen ion activity and thus the lowering of pH from any point on the pH scale.
The expression “ocean acidification” has been defined in somewhat different ways in the literature. It is important to differentiate between pH reduction due to natural processes like volcanic activity and sea floor CO2 venting and acidification due to anthropogenic activities that result in rapid increases in atmospheric CO2 levels. The Committee adopts the definition of Field et al. (2011) that was developed at an IPCC workshop on ocean acidification: “Ocean acidification refers
SOURCE: Adapted from Kleypas, 2012 (unpublished presentation).
pH; processes collectively termed “ocean acidification” (see definitions in Box 1.1).1
To date, global warming has been the primary focus of public interest and scientific investigation concerning effects of CO2 emissions. Ocean
_________________________
1 Because the rate of increase is more rapid than in the past, sources and sinks of alkalinity are no longer in balance, and both ocean pH and CaCO3 saturation are changing in the ocean (Hönisch et al., 2012)
to a reduction in the pH of the ocean over an extended period, typically decades or longer, which is caused primarily by uptake of carbon dioxide from the atmosphere, but can also be caused by other chemical additions or subtractions from the ocean Anthropogenic ocean acidification refers to the component of pH reduction that is caused by human activity.”
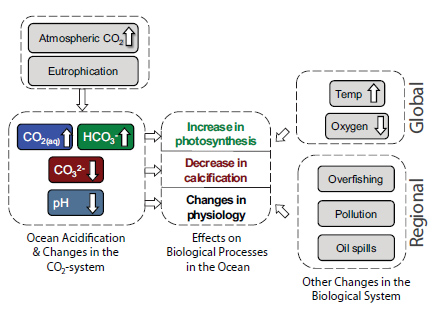
Ocean Acidification and Changes in the CO2 and Carbonate System: Atmospheric carbon dioxide is absorbed by the ocean, where it reacts with seawater to form carbonic acid, which then dissociates to form bicarbonate ions (HCO3-) and hydrogen ions (H+). The increase in hydrogen ion activity (decrease in pH) is buffered by the carbonate system: some of the added hydrogen ions react with carbonate ions (CO32-) to form more bicarbonate, which makes CO32- less abundant. If atmospheric carbon dioxide rises slowly, ocean pH and carbonate ion levels will remain relatively stable due to dissolution of existing calcium carbonate deposits in the ocean (1,000s+ of years), weathering of terrestrial rock (100,000s+ of years), and tectonic processes (millions of years). However, the current rapid rise in atmospheric CO2 is faster than the time required for natural processes to buffer changes in the ocean carbonate system and avoid large changes in pH or ocean carbonate levels. Increased nutrient input from runoff can result in larger than usual algal blooms (i.e., eutrophication) that produce organic matter, which contributes to increases in CO2 when respired.
Effects on Biological Processes: The increase in CO2 and HCO3- availability has the potential to increase photosynthesis by some but not all photosynthesizers in the ocean. The decreased availability of CO32- at calcification sites makes it more difficult for many types of calcifying organisms, including some phytoplankton, corals and bivalves (clams and mussels) to build their calcareous shells or skeletons. Lastly, a decrease in pH may cause important physiological changes, many of which are associated with negative impacts such as increased energetic costs for regulating internal H+ concentrations.
Simultaneous Changes Impacting Biological Processes: Global increase in ocean temperature and decrease in dissolved oxygen are stressors for many marine organisms that will likely add to or amplify the impacts of ocean acidification, resulting in changes in the composition, abundance, and production of biological communities. In addition, regional human impacts—such as overfishing, eutrophication, pollution, or oil spills to name a few—also affect biological processes.
acidification is generally unknown to the public and has been the subject of substantially less scientific research than have the effects of CO2 on climate. However, the relative lack of attention given to ocean acidification belies its potential importance as a threat to marine organisms, ecosystems, and socioeconomic activities dependent on a healthy ocean (e.g., IPCC, 2011, Caldeira and Wickett, 2003; Gattuso and Hansson, 2011). A few key background data and the interactions illustrated in Box 1.1 help
to more clearly define the contributors to ocean acidification and put its consequences for marine life and human societies into perspective.
Since the beginning of the Industrial Revolution, the average pH of the upper ocean has decreased by about 0.1 pH unit, which corresponds to an approximately 30% rise in acidity (activity of hydrogen ions (H+)). Shallow ocean pH is projected to decrease by an additional 0.2-0.3 pH units by the end of this century, corresponding to a rise in acidity of 100-150% since the mid-18th century (IPCC, 2007 WGI; under the IS92a scenario). The rates of relevant chemical change in deep waters may not necessarily be that much slower than in surface waters because deeper waters naturally have higher concentrations of inorganic carbon, a lower buffer capacity, and are thus more susceptible to CO2 perturbations. This rate of acidification is faster than any rates inferred from the geological record for at least the past 55 million years (Zeebe and Ridgwell, 2011; Hönisch et al., 2012). Due to the mixing of ocean waters across depths, pH is decreasing—and will continue to decrease—in deeper regions of the marine water column as well as at shallow depths.
These changes in pH and carbonate chemistry are expected to have effects on marine organisms at all levels of biological organization, including the physiologies of individual organisms and the composition, productivity, and health of diverse marine ecosystems. Furthermore, the effects of ocean acidification may be compounded by stresses arising from other features of global change, notably rising temperatures and decreases in concentrations of dissolved oxygen. Currently, we are in the early stages of discovering what these diverse and interacting effects are and how they may affect marine ecosystems and the socioeconomic activities that depend on ocean-derived resources. However, even though the field of ocean acidification research is relatively new, it is growing rapidly and beginning to reveal the scope and magnitude of biological, ecological, and societal consequences projected to arise from future acidification. Early studies focused primarily on the many organisms that build shells and skeletons of calcium carbonate, such as reef-building corals and the small calcareous phytoplankton that lie at the base of the marine food web. Recent studies now illustrate that the biological impacts of ocean acidification go far beyond calcification processes and also include photosynthesis, respiration, nutrient acquisition, behavior, growth, reproduction, and survival per se (Gattuso et al., 2011).
Some discoveries of how acidification affects marine species have been unanticipated. For example, it was recently discovered that low pH impairs sensory and neurotransmitter systems of larval marine fish, which leads to maladaptive changes in their behavior and olfactory capabilities (Munday et al., 2009; Nilsson et al., 2012). It seems reasonable to
conclude that other unanticipated effects of acidification will be revealed as scientific research on this topic continues.
Whereas much remains to be learned about the scope and magnitude of the consequences of ocean acidification, existing data support a growing consensus in the research community that most documented responses to acidification reflect impairment of physiological capacity or performance. Certain physiological processes in some species may benefit from ocean acidification (e.g., enhancement of photosynthesis in sea grasses and some algae by increased levels of CO2 [Kroeker et al., 2010]). However, the beneficial effects on some species may directly lead to negative effects on other species in the same marine community (Kroeker et al., 2010).
Although our knowledge about the biological effects of ocean acidification is expanding quite rapidly, most of this research has either involved studies of single species under closely controlled laboratory conditions, or mesocosm-studies in which communities of organisms are confined under controlled conditions. Much remains to be learned about the effects of ocean acidification on natural ecosystems, but moving from laboratory and mesocosm experiments toward assessments and projections of the in situ, long-term responses of ecosystems presents not only scientific challenges, but logistical and financial ones as well. Simply extrapolating information on impacts from laboratory-derived species’ responses or short-term in situ observations (Gattuso and Riebesell, 2011) is hampered by the variability in the responses across species, and even within some single species. Lastly, as mentioned earlier, projecting long-term changes in marine ecosystems is complicated by the interactions of impacts due to ocean acidification with those resulting from alterations in water temperature and oxygen concentration or from other human activities (e.g., from agricultural run-off and extractive activities).
Socioeconomic impacts of ocean acidification are likely to be substantial, based, for example, on the dependence of humans on protein from marine species (approximately 6.5% of dietary protein in 2009) (FAO, 2012). However, projecting socioeconomic impacts of ocean acidification is currently challenging because of a dearth of research in this domain. Nonetheless, economically important natural resources may already be affected by ocean acidification resulting from upwelling events and, to a lesser extent, from increases in dissolved atmospheric CO2. For example, the Pacific Northwest Aquaculture industry, which is estimated to contribute approximately 270 million dollars per year and 3,200 jobs to local coastal communities, has recently experienced major failures in its oyster hatcheries due to effects of upwelling of low pH seawater on oyster larvae (Washington State Blue Ribbon Panel, 2012). In addition, complex ecological effects of low pH on coral reefs have been documented, notably
in studies of natural CO2 seeps where sharp pH gradients exist across an ecosystem (Fabricius et al., 2011). Effects of decreasing pH on coral reefs are likely to be amplified by influences of additional stressors such as increases in water temperature (Anthony et al., 2008) or run-off. Coral reefs are not only important in supporting healthy fisheries (Jones et al., 2004) but also support a vital tourist industry and can serve as important physical barriers to reduce the effects of storms on coastal communities.
In summary, the magnitude and rate of change in pH and the marine carbonate system and the likelihood that this change—in conjunction with climate change and other human impacts on the ocean—will have wide-ranging biological and socioeconomic effects argue for a comprehensive and integrated program to broaden our understanding of the scope of ocean acidification and its potential consequences for ocean ecosystems and society. The program’s purview needs to encompass such diverse activities as monitoring ongoing changes in carbonate chemistry and pH of seawater as well as associated changes in marine life; elucidating the fundamental physiological effects of acidification on diverse marine species, ranging from primary producers to animals higher in the trophic web; analyzing and predicting—with assistance from well-designed models—how ecosystems will change under acidification (and climate change); and predicting the socioeconomic consequences of acidification and how these impacts can most effectively be prevented or ameliorated. Only a broad and closely coordinated research and monitoring program supported by multiple federal agencies and that interacts effectively with relevant international programs will be able to deal with these complex and interacting facets of ocean acidification in a comprehensive and cost-effective manner.
Congress recognized the potential seriousness of the ocean acidification issue several years ago and mandated that the issue receive sufficient study to enable the development of an effective research and monitoring program. In the Magnuson-Stevens Fishery Conservation and Management Reauthorization Act of 2006 (PL 109-479 sec 701), Congress asked the NRC to conduct a comprehensive study on ocean acidification. The resulting report (Ocean Acidification: A National Strategy to Meet the Challenges of a Changing Ocean [NRC, 2010]) summarized the latest scientific understanding of the issue and described the necessary elements of a national ocean acidification program (NRC, 2010).
While the NRC 2010 study leading to this report was under way, Congress in 2009 passed the Federal Ocean Acidification Research And Monitoring (FOARAM) Act (as part of PL 111-11). In October 2009, as man-
dated by the Act, the Subcommittee on Ocean Science and Technology (SOST) established the Interagency Working Group on Ocean Acidification (IWGOA), which includes representatives from the National Oceanic and Atmospheric Administration (NOAA), National Science Foundation (NSF), Bureau of Ocean Energy Management (BOEM), Department of State (DOS), Environmental Protection Agency (EPA), National Aeronautics and Space Administration (NASA), U.S. Fish and Wildlife Service (USFWS), U.S. Geological Survey (USGS), and the U.S. Navy. The group has been meeting regularly and has drafted the Strategic Plan for Federal Research and Monitoring of Ocean Acidification (from here on referred to as the Strategic Plan). As described in the FOARAM Act, the goals of the Strategic Plan are to “advance the understanding of ocean acidification and its physical, chemical, and biological impact;” and to “improve the ability to assess the socioeconomic impacts of ocean acidification; and provide information for the development of adaptation and mitigation strategies.” The Strategic Plan is to include five program elements: (1) monitoring of ocean chemistry and biological impacts associated with ocean acidification; (2) research to understand impacts on marine organisms and food webs and to track marine ecosystem responses; (3) modeling to predict changes in biogeochemical cycles and ecosystems; (4) technology development; and (5) assessment of socioeconomic impacts of ocean acidification and development of adaptation and mitigation strategies. The FOARAM Act also directs NOAA to request a review by the NRC of the Strategic Plan. Our report is the response to this charge (see committee’s task below).
The draft Strategic Plan was submitted to the Office of Science and Technology Policy (OSTP) for review in the summer of 2011 and was approved by OSTP in May 2012. The draft Strategic Plan was published for public comment in June of 2012. The creation of the Strategic Plan for Federal Research and Monitoring of Ocean Acidification, following the mandates of the FOARAM Act, represents an important next step forward in the development of a comprehensive, integrated, and cost-effective program for examining the diverse facets of ocean acidification.
As indicated in the previous section, our committee was asked to review the IWGOA Strategic Plan for Federal Research and Monitoring on Ocean Acidification based on the Program Elements described in the FOARAM Act of 2009 and the advice provided to the IWGOA through the 2010 NRC report, Ocean Acidification: A National Strategy to Meet the Challenges of a Changing Ocean. More specifically, the review is to consider the following elements: goals and objectives; metrics for evaluation; mechanisms for coordination, integration, and evaluation; means to transition
research and observational elements to operational status; coordination with existing and developing national and international programs; and community input and external review.
In the following chapters, our report analyzes the extent to which the seven Themes of the IWGOA Strategic Plan address the mandates of the FOARAM Act. During its review, the committee identified several common issues that arose across most, if not all, of the Themes of the Strategic Plan. These issues include the establishment of a National Ocean Acidification Program Office, the prioritization of research and monitoring efforts, and the development of metrics for evaluating the success of the different programs. These key issues are briefly discussed and summarized in Chapter 2, to provide a context for the more detailed and focused analysis that occurs in Chapter 3, where the committee reviews the seven individual Themes that comprise the core of the Strategic Plan.








