3
Toxicologic Issues
INTRODUCTION
Tissue monitoring for chemicals in the environment is best viewed as a component of a comprehensive environmental monitoring program. This chapter focuses on the relation of analytic measurements of chemicals in tissues to broad toxicologic issues. Tissue-monitoring data alone can alert those concerned with public health to the need to conduct studies on specific environmental chemicals. Three examples that illustrate the importance of tissue monitoring are monitoring of blood to determine the extent of lead toxicity in the United States, monitoring of fish and wildlife to determine concentrations of pesticides in tissues, and monitoring of acute tissue damage to identify conditions common to such damage.
Such examples show that tissue monitoring can be described as a component of an environmental-health and public-health program. Tissue monitoring can reveal some of the associations between the entry of a chemical into the environment and an adverse effect on human health.
RELATION BETWEEN ENVIRONMENTAL MONITORING AND TISSUE MONITORING
Knowing the concentrations of a chemical in exposure media is helpful in determining the potential risk associated with exposure to it, but such information must be supplemented by information on exposure itself. For example, assume that the concentration of a chemical in some medium of interest is 1 ppm. Obviously, the appropriate magnitude of concern will depend on whether consumption of the medium is, say 5 ml/day (about 1 teaspoon/day) or 2
L/day (about 2 qt/day). Estimates of exposure typically include a concentration component and a consumption component, or equivalent.
For many chemicals of interest, exposure is not limited to one medium. Pesticides can be sprayed directly on crops, but runoff water enters the water supply and spray can travel by air to enter the food supply remote from the point of application. Routes of exposure to chemicals with diverse commercial applications (e.g., lead) can include air, water, food, cosmetics, and drugs, both prescription and over-the-counter. Measurement of exposure through one of many media cannot always provide a full picture of human exposure. Tissue monitoring, however, yields a measurement of the amount of a chemical transferred into an organism, whatever the source and medium. To be transferred into tissues, the chemical must be absorbed, i.e., it must pass through various membranes, such as intestinal membranes, the alveolar membrane of the lung, the conjunctival membrane, and the skin.
The completeness of absorption of a substance from environmental media is referred to as bioavailability. Bioavailability differs according to route of exposure, chemical and physical properties of the substance, and physiologic and nutritional status of the organism. Furthermore, many chemicals that are rather benign in the form in which they exist in the environment might be converted to a more toxic form when they are absorbed.
Clearly, tissue monitoring can be of great value for associating adverse health effects with exposure to environmental chemicals, but several problems arise in attempts to understand the association. Most toxicologic concerns are related to chronic diseases that can take decades to develop (e.g., dementias, neurologic disorders, cancer, osteoporosis, and arthritis). The environmental conditions that may have been significant in the development of a disease and the mechanisms of most of those diseases are not completely understood (e.g., those diseases might be produced by infections or injury).
Given the complexity of chronic disease processes, it is sometimes difficult to associate the onset and development of a case of a disease with a specific chemical. The association of some chemicals with a disease process is well accepted (e.g., appearance of specific proteins in familial Alzheimer’s disease); where the association of others is not definite (e.g., DNA adducts). Exposure to some chemicals that do not definitely produce disease (or do not produce it through an understood mechanism) might nevertheless lead to the appearance of some effect. Such an effect is commonly referred to as a biologic marker.
RELEVANCE OF HUMAN TISSUE MONITORING TO RISK ASSESSMENT
The process of predicting the likelihood of disease in association with exposure to a foreign substance is referred to as risk assessment. The process is now generally considered to include the identification of a potential disease risk due to exposure to a hazard, determination of sources and magnitudes of exposure to the agent, estimation of the relationship between the potential risk or severity of disease and the dose of the agent, and the integration of this information into estimates of potential risk associated with various exposure conditions (NRC, 1984a). The quantitative estimation of potential human health risk associated with exposure to a chemical generally involves an assessment of biomedical data to determine the likelihood that the chemical can produce human disease and an assessment of the dose of the chemical. Most human risk assessment today is based on estimates of external exposure (in parts per million or mg/m3), which can be used to estimate dose (commonly expressed in mg of chemical inhaled, ingested, or absorbed per day per kg of body weight or per unit of surface area) (Anderson, 1982). A dose can be multiplied by the estimated potency of the chemical to estimate the risk per unit dose. A major limitation of that approach is that exposure data are generally imprecise and contribute considerably to uncertainty in the risk estimate. Tissue concentration, which can provide quantitative data on internal dose or biologically effective dose of a chemical or on the resulting biologic effects, can in theory introduce much greater precision into the risk-assessment process than the use of crude exposure data. Concentrations of environmental chemicals in human tissues can be used to assess the likelihood that disease will result from chemical exposures. The monitoring of human tissues for toxic substances is concerned primarily with measurement of exposure, although some information is relevant to biologic effects.
For some diseases and exposure conditions, threshold doses have traditionally been assumed—i.e., doses below which discernible effects are not elicited. For example, thresholds may be produced by reserve biologic capacity or by repair mechanisms that are fully effective at low doses. In such situations, the doses of concern are the threshold dose and larger doses. However, the occurrence of effects might not be a linear function of dose. For example, a threshold may vary from person to person. Carcinogens and some chemicals that augment existing disease processes are commonly assumed to carry some risk (perhaps very small) at even the smallest exposure (Peto, 1978). Even if a chemical is present below a threshold dose when acting in isolation, the combination of endogenous and exogenous factors already present may surpass a threshold dose.
A change or difference in the concentration of a toxic chemical in a particular tissue is generally assumed to reflect a difference in risk. For example, if tissue concentrations of DDT decrease, the potential risk of an adverse health outcome of DDT exposure is assumed to decrease. Tissue concentrations of a substance can provide an integrated assessment of the exposure to the substance by all routes, such as dermal exposure, inhalation, and ingestion.
In analysis of tissues for concentrations of chemicals, compositing of specimens (i.e., pooling of tissue specimens and analysis of the pooled sample) is a satisfactory technique if one needs only the average potential risk for the population represented by the composite. Compositing usually is not a good choice, except under the most extreme pressure. When n equaled-sized specimens are composited and found to have mean concentration ![]() , every one of the original specimens must have concentrations = n ·
, every one of the original specimens must have concentrations = n · ![]() . If the distribution of potential risk among individuals is of interest, as is often the case, individual specimens are required.
. If the distribution of potential risk among individuals is of interest, as is often the case, individual specimens are required.
Where no trends in average tissue concentration are noted for a population, then data over that period of time can be cumulated to improve estimates of potential risk. (Estimates might also be derived when there is a trend, but the statistical procedures are more complex). Establishment of baseline concentrations of chemicals in tissues can be useful in determining whether tissue concentrations, and hence potential risks for various populations, are abnormal.
Description of dose gives information on the intensity of exposure. Dose has been defined in many different ways, but fundamentally refers to an integrated assessment of environmental exposure (frequently termed “external dose”) or of the quantity of the chemical in the body (termed “internal dose”). Relevant information includes the amount (e.g., mg/kg body weight), duration, time pattern (e.g., continuous or intermittent), and route of exposure. For environmental chemicals, such information rarely is well established. Consequently, many studies of environmental chemicals use biologic monitoring of human tissues.
It is important to distinguish between the current use of human-tissue monitoring data and their potential use in risk assessment. Data on tissue concentrations of xenobiotics constitute measures of internal dose and so can help to identify a hazard (i.e., a qualitative risk) for further surveillance or followup studies. Tissue concentrations of environmental agents in different populations can be compared (e.g., urban vs. rural and children vs. adults). Tissue concentrations can also reveal exposure trends. However, those measures of internal dose generally are not themselves directly usable for predicting potential health effects. Estimates of potency of toxic agents are generally expressed as risk per unit of whole-body dose, so the supporting estimates of
exposure currently used to calculate absolute risk in risk assessment are based on external (usually environmental) concentrations. This may change as data and experience accumulate for the estimation of health effects from internal doses.
Mechanistic models relating tissue concentrations and environmental concentrations would require information on variations of contamination over time, routes of exposure, biologic factors (bioavailability and rates of uptake, distribution, metabolism, and elimination). Those kinds of information are rarely available, and estimates of exposure based on tissue concentrations typically use simplified models that incorporate limiting assumptions. The implication regarding the utility of tissue monitoring data is that their applications depend heavily on input from other fields, such as toxicology and exposure assessment, and that precise estimates of exposure based on tissue concentrations (or vice versa) are ordinarily beyond the reach of present models. For example, it is not possible to “back-calculate” the history of exposure from a single blood sample. Thus, the use of tissue monitoring data to estimate potential risks with the conventional approach is not always accurate. However, it is often possible to estimate past exposures, given limiting assumptions (i.e., a steady state) and groups of samples (in which some random effects can be averaged out). This approach is useful in comparing exposures and potential risks among population groups.
Viewed another way, tissue concentrations can sometimes be used as a direct measure of internal dose, and internal dose can sometimes replace estimated exposure as the dose variable in risk assessment. But, relating risk directly to internal dose requires more data. These relationships hold the promise of an exposure or dose measure that will yield a prediction of effect (i.e., potential risk) that is physiologically relevant. For reasons discussed above, the promise cannot yet be realized.
Biologic Markers
Sophisticated laboratory techniques developed during the last decade can now detect exposures to pollutants at very low concentrations, and can assess their behavior, fate, and effect at the cellular or molecular level. The methods have stimulated interest in the use of biologic markers in environmental research, particularly in the study of the somatic effects of exposure to environmental carcinogens and mutagens.
A biologic marker is an alteration that occurs at the biochemical, cellular, or molecular level on the continuum between exposure and disease and that can be measured with assays of body fluids, cells, or tissues. Biologic markers
are discussed in depth by Perera and Weinstein, 1982; Harris et al., 1987; Perera, 1987; Schulte, 1987; Hulka and Wilcosky, 1988; and NRC, 1989. For an exposure to result in an adverse health effect, it must generate a chain of events, each of which theoretically can be reflected in a biologic marker.
Conventionally, three broad categories of markers have been distinguished: markers of exposure or dose, markers of effect, and markers of susceptibility. A distinction can be drawn between a marker of exposure (which gives qualitative information as to whether an organism has been exposed) and a marker of dose (which provides a quantitative measure.) Markers of internal dose directly reflect the parent substance, its metabolites, or its derivatives in cells, tissues, or body fluids. Sensitive physicochemical and immunologic methods can detect and measure very low concentrations of foreign substances in the body. Exhaled air, blood, and urine are ordinarily used, but other body fluids—such as breast milk, semen, and adipose tissue—have also been used. Each biologic material has its own relevance to both exposure and health outcome, and the differences affect interpretation of results.
Markers of Exposure or Dose
Monitoring for these markers is based on direct measurement of concentrations of the parent compound in cells, tissues or body fluids. Physical, chemical, and immunologic methods can now detect and quantify very low concentrations of xenobiotic substances in the body. Biologic markers of internal dose can be characterized according to their chemical-specificity/selectivity, with selective markers representing measures of pollutants or their metabolites detected in biologic media. Examples of internal dosimeters include blood levels of styrene, pesticides, and metals; exhaled volatile organic chemicals (VOCs); concentrations of polychlorinated biphenyls (PCBs), DDE (a metabolite of DDT), and TCDD (dioxin) in adipose tissue; and urinary mandelic acid resulting from styrene exposure. Various nonselective markers such as urinary excretion of thioethers and the mutagenicity of urine and other body fluids have also been assessed in humans, the latter fairly widely. Disadvantages of internal dosimeters are that, while the laboratory methods may be highly sensitive, in the absence of bioaccumulation, markers can reflect only recent exposure. This can be a limitation if exposure has been interrupted or if past exposures must be estimated. In addition, internal dosimeters do not reflect critical interactions with macromolecules in target cells (Lucier and Thompson, 1987). Biologic monitoring of dose may also be based on measurement of a metabolite of the environmental chemical or of a compound produced by cells as a result of interference of the environmental chemical in
the normal metabolic process. For example, biologic monitoring for the effects of lead exposure have frequently been based on measurement of the concentration of various intermediates of heme metabolism, which is disrupted by lead. Because estimates of the amount of the chemical present in the entire body are rarely possible, and may not be as useful, a particular tissue is typically chosen as an indicator of body burdens or stores of the chemical. The specific tissue chosen in biologic monitoring may be selected because this tissue accumulates the chemical (e.g., adipose tissue for some pesticides; bone for lead or strontium) or is affected by the chemical. Measurements can also be made on exhaled air, blood, and urine, and other body fluids such as breast milk or semen have sometimes been used. Each of those biologic media has a different relationship (e.g., temporal) to both past exposure and future health outcomes. Those differences can markedly affect interpretation of results.
Markers of Effect
Biologic monitoring for the effect of an environmental chemical may be based on a change at the tissue, cellular, or molecular level in response to exposure to the chemical. Such changes may be biologic indicators that do not impair function, but serve as indicators of exposure. Generally, the effect is the biologic response (i.e., the reaction or the response of the person) following exposure to the chemical. This reaction may vary with respect to the quality, strength, onset, and duration of exposure to the chemical. A large number of factors influence this reaction. Biologic monitoring that is based on the effect produced by the chemical frequently has far greater implications than does direct monitoring of tissues for the chemical of interest. In some cases, the biologic indicator may be useful as a surrogate for measurement of the chemical or physical agent per se. The biologic indicator may be of particular value if data are available to determine the relationship between external exposure, the biologic indicator, and the toxic effect(s) of the chemical (Mahaffey, 1987).
Effect monitoring for chemical exposures can be based on assessment of a variety of biochemical indicators, including enzyme activities that can be measured either in target organs or in body fluids such as blood and urine. Examples include the inhibition of pseudocholinesterase in plasma, elevation of glutaryltranspeptidase in serum or urine, increased excretion of urinary or fecal porphyrins, D-glutaryltranspeptidase in serum or urine, and DNA adducts in urine. Other effect indicators include changes in genetic material, as identified by chromosomal aberrations, effects on the immune system (e.g.,
surface markers and induction of lymphocyte subpopulations), and the endocrine system. Markers in this category also include DNA and hemoglobin adducts in peripheral blood and other cells and tissues (e.g., lung macrophages, buccal mucosa, bone marrow, placental tissue, lung tissue).
To estimate risk of disease from human tissue concentrations requires knowing the relationship between concentration and the risk or extent of the development of the disease process—that is, the association of internal dose and response. This information generally does not exist and often requires extensive biokinetic research. However, relative risks (e.g., male vs. female, urban vs. rural, current vs. past) can be estimated if the incidence of disease is proportional to internal dose as measured by tissue and/or blood levels. If risk is proportional to blood or tissue levels of a chemical, then relative risks can be estimated. Uncertainties exist in relating tissue concentrations to dose and large uncertainties exist in relating risk to dose (Allen et al., 1988). Uncertainties in interspecies extrapolation of risk estimates among rodents are up to a factor of 10 to 100 for carcinogens (Gaylor and Chen, 1986). Hence, smaller uncertainties in the estimates of tissue concentrations or dose may not negate their usefulness for risk assessment.
Markers of Biologically Effective Dose
Markers of biologically effective dose measure the amount of a pollutant or its metabolites that has interacted with cellular macromolecules at a target site or an established surrogate. Measures of biologically effective dose include DNA adducts and hemoglobin adducts in a range of cells and tissues, including peripheral blood, bone marrow, lung macrophages, buccal mucosa, placental tissue, and lung tissue. Many carcinogens are metabolically activated to electrophilic metabolites that covalently bind to DNA. Adducts on critical sites of DNA, if unrepaired, can cause gene mutation, which is a critical initial step in the multistage carcinogenic process. Several methods to detect DNA-chemical adducts in lymphocytes and target tissues are currently available, including radio- and enzyme-linked immunoassays utilizing polyclonal or monoclonal antibodies, 32P post-labeling, and synchronous fluorescence spectrophotometry (Santella, 1988).
For example, antibodies were used to detect the presence of polycyclic aromatic hydrocarbon-DNA (PAH-DNA) adducts in lung tissue and peripheral white blood cells (WBC) from lung cancer patients and controls. Antibody methods have also been used to analyze white blood cells and other tissues of individuals exposed to polycyclic aromatic hydrocarbons (PAHs) in cigarette smoke and in occupational settings (Shamsuddin et al., 1985; Haugen et al.,
1986; Harris et al., 1987; Perera et al., 1988b; Hemminki et al., 1990). Examples of antibodies available to assess formation of DNA adducts in humans include those to aflatoxin B1, alkylating agents, 4-aminobiphenyl, benzo[a]pyrene (BP) and PAHs, cisplatinum, and 8-methoxypsoralen. Immunoassays can detect adduct concentration as low as one adduct per 108 nucleotides.
The 32P post-labeling technique is even more sensitive, as it can measure one adduct per 109–1010 nucleotides (Randerath et al., 1988). It has been applied to the measurement of adducts formed by various alkylating and methylating agents, aromatic compounds (e.g., BP/PAH) and cigarette smoke constituents (Dunn and Stich, 1986; Everson et al., 1986; Phillips et al., 1988; Hemminki et al., 1990). The method produces images that are considered idiosyncratic “fingerprints” of exposure.
A third approach, synchronous fluorescence spectrophotometry, has recently been applied to human samples with a reported sensitivity of one BP adduct per 107 nucleotides (Vahakangas et al., 1985). High pressure liquid chromatography (HPLC) and fluorescence spectroscopy have been used to detect excised carcinogen-DNA adducts in urine (Autrup et al., 1983).
Assays that measure carcinogen-protein adducts, especially the binding of metabolites with hemoglobin, are in some cases a good surrogate for DNA-adduct measurements. Methods available for measuring these adducts include immunoassays, gas chromatography-mass spectrometry (GC-MS), ion-exchange amino acid analysis, and negative chemical ionization mass spectrometry (NCIMS). This last method has been successfully applied to the quantitation of hemoglobin adducts formed by ethylene oxide (Osterman-Golkar et al., 1984) and 4-aminobiphenyl-hemoglobin (4-ABP) (Bryant et al., 1987). Because of the 3-month lifespan of hemoglobin, these assays reflect relatively recent exposures, while DNA adducts can assess exposure integrated over a much longer period.
Markers of Early Biologic Effect
Whereas markers of biologically effective dose indicate an interaction with critical macromolecules that might potentially result in disease, these markers may also be repaired, or otherwise “lost.” In contrast, markers of early biologic effect indicate the occurrence of irreversible toxic interactions either at the target or an analogous site.
Markers of early biologic effect indicate an event resulting from a toxic interaction of a xenobiotic substance, at either the target or an analogous site, which is known or believed to be a step in the pathogenesis of disease or to be qualitatively or quantitatively correlated with the disease process. Like
markers of biologically effective dose, markers of early biological effect provide integrated “black box” measurements of the net result of all the biological processes that occur when the body is exposed to a particular pollutant or pollutants (Hoel et al., 1983). As understanding of the range and complexity of the mechanisms of action of chemicals expands, such concepts may require revision in response to more fundamental mechanistic information on how toxicity occurs at a subcellular level. These include pharmacokinetic events occurring on the cellular or systemic level such as absorption, metabolism, detoxification and elimination, as well as macromolecular processes such as binding, repair, and immune response. An irreversible effect can be due to direct attack by the chemical (e.g., genotoxic effect, allergic effect, cytotoxic effect), to an indirect reaction of an organ which is not directly attacked (e.g., central nervous system damage following carbon monoxide intoxication), or to inappropriate tissue repair (Mahaffey, 1987).
Cytogenetic techniques provide a direct, though nonspecific, method of assessing changes at the chromosomal level. These changes include alterations in chromosome number, structural changes such as breakage and rearrangement, and exchanges between reciprocal portions of a single chromosome (sister chromatid exchanges or SCE). Elevated frequencies of chromosomal aberrations and/or SCEs have been observed in persons exposed to ionizing radiation or to a variety of chemicals including vinyl chloride, styrene, ethylene oxide, and organophosphates (Evans, 1982; Vainio et al., 1984). Although SCEs are a biologic effect, the significance of this increase in relation to disease outcome in unclear. Micronuclei (MN)—fragments of nuclear material left in the cytoplasm following replication—are generally considered to indicate prior chromosomal aberrations.
Another approach to assessing genetic effects involves measurement of single-strand breaks in lymphocyte DNA (Walles et al., 1988). In addition, DNA hyperploidy measured in exfoliated bladder and lung cells has been shown to be a biologic marker of response to carcinogens (Hemstreet et al., 1988).
An important new marker is the activated oncogene and its protein products. During oncogenesis, a normal segment of DNA (termed a proto-oncogene) is activated to a form that causes cells to become malignant. Activation can occur through several mechanisms including gene mutation, chromosome breaks, and rearrangements. Activation of oncogenes or their protein products can be measured by complex immunoblotting techniques or by the polymerase chain reaction (PCR) technique. More studies are needed to understand the precise role of oncogenes and their protein products in tumorigenesis and, in particular, the nature of barriers to malignant transformation (Weinberg, 1989).
Markers of Susceptibility
Generally, the effect of interest is the biological response following exposure to the chemical. This response may vary with respect to the quality, strength, time of onset, and duration of exposure to the chemical. A large number of factors influence this reaction, including age, genetic makeup, gender, developmental stage, physical activity, and normal physiological states such as pregnancy and lactation. Susceptibility to the effects of a chemical results from differences in transfer of the toxic chemical from the external dose to the internal dose, the biokinetics of distribution among and within tissues, and the responsiveness of the target tissue. Not all tissues are equally responsive to the potential effects of exposure to chemical contaminants. For example, the severe neurological effects of exposure to methylmercury during gestation are highly dependent on the developmental stage of the nervous system when exposures occur.
Markers of susceptibility reflect inherent or acquired differences affecting an individual’s response to exposure. These differences can serve as effect modifiers and thereby increase or decrease risk at any point on the continuum between exposure and the emergence of symptomatic disease. Markers of susceptibility may indicate the presence of inherited genetic factors that affect the individual or a population of which he/she is a part. They may also reflect certain host factors, such as lifestyle, activity patterns, prior exposures to environmental toxicants, or nutritional status.
For example, nutritional status and individual variability affect the cytochrome P-450 system for metabolism of xenobiotics, and individual variability in cytochrome P-450 metabolism may explain differences in lung cancer risk, although results of various studies have been conflicting (Karki et al., 1987). Enzyme activity as measured by metabolism on indicator drugs such as debrisoquin and antipyrine may be useful in identifying genetic polymorphisms that modulate the effects of exposure (Conney, 1982; Harris et al., 1987). Nutrients influence the rate of cellular metabolism that may increase or decrease toxicity of an environmental chemical. For example, cytochrome P-450 concentration in liver microsomes can be lowered by protein deficient diets (Marshall and McLean, 1969). Concentrations of various cytochromes, including P-450, are decreased in the liver, kidney and adrenals of guinea pigs following ascorbic acid deprivation (Degkwitz et al., 1975).
Nutritional status may also influence the percent and rate of absorption of the toxicant, and nutrients can influence the quantity of the toxicant that reaches a critical target tissue by sequestering the toxic compound into body depots. In such depots as adipose tissue or bone mineral, the intensity of exposure to the compound in organs such as brain, liver, or kidney is relatively
less than would occur if the compound were more evenly deposited. On the other hand, exposure to target organs may be greatly improved and/or prolonged by storage in tissue depots, if these depots are mobilized.
Nutrients and toxicants may affect the same biological markers. For example, both lead toxicity and iron deficiency result in impairment of hematopoiesis, and increases in the concentration of erythrocyte protoporphyrin has been widely used as a marker for both iron deficiency and lead toxicity (Mahaffey and Annest, 1982). A potentially serious complication is that the effect of both conditions produces a greater than additive increase in impaired heme biosynthesis (Mahaffey and Annest, 1982).
Age of the subject can have a marked effect on a biologic marker. An important example is exposure during gestation. The toxicity of methylmercury and lead differ greatly when exposures occur during gestation and the first few years of life when contrasted to their effects among adults. A particular concentration of lead in bone must be considered relative to age. Adults store approximately 90 to 95% of their total body burden of lead in bone. Young children, by contrast, have only about 70% of their total body burden of lead in bone, with a far greater fraction of the total body burden present in brain and other tissues.
Markers of susceptibility may also indicate a pre-existing disease condition that could increase an individual’s risk. The presence of certain rare hereditary diseases (e.g., ataxia telangiectasia, Fanconi’s anemia, and Bloom’s syndrome) may indicate heightened susceptibility to potentially genotoxic ambient exposures (Carrano and Natarajan, 1988). Pre-existing conditions may either affect an individual’s metabolic status or establish sites within the DNA where initiating events such as point mutations or translocations are more likely to occur. Restriction enzyme DNA fragment-length analysis of genetic polymor-phism (RFLP) is a new approach using recombinant techniques to detect DNA conformations associated with genetic predisposition to cancers such as retinoblastoma (Francomano and Kazazian, 1986).
Summary
Although many biologic markers are in the validation stage, they have considerable potential in risk assessment and environmental epidemiology (reviewed in Perera, 1987; Hulka and Wilcosky, 1989). For example, as the above discussion has demonstrated, use of markers can improve exposure assessments and can provide timely identification of groups and individuals at potentially elevated risk of disease. Biologic markers can improve understanding of the mechanisms of disease causation and, in addition, serve as a bridge
between studies of experimental animals and humans experiencing the same exposure to chemicals. Thus, biologic markers can improve risk extrapolation between species and can provide a valuable tool for health risk assessments through human tissue monitoring.
Priorities for research, testing, and regulation are based on complex considerations that include estimates of risk attached to environmental contaminants—i.e., the likelihood that human disease will arise from exposure. If a precise relationship between risk of disease associated with chemicals and tissue concentrations of the chemicals can be established, the tissue concentrations corresponding to an allowable magnitude of risk can be estimated. The selection of tissues and chemicals for monitoring in the NHATS has so far been based on persistent chemicals, such as chlorinated hydrocarbons, that accumulate in adipose tissue. In a future national monitoring network, samples might also be analyzed for selected markers of biologically effective dose and effect to provide information on possible previous exposures.
CHOICE OF TISSUES TO MONITOR
Background
A monitoring program to survey xenobiotic chemicals in human tissues should provide an estimate of chemical concentrations in the tissues selected, identify the tissues and toxic effects relevant to each chemical, and identify potential risks. Those objectives are seldom achieved in the laboratory, and they are even less likely to be achieved in a survey of tissues from the general population. It is not feasible to study a broad range of tissues in a general population sample. One must instead try to identify tissues that most nearly account for the body burden of most of the chemicals of concern.
The concentration of any xenobiotic in tissues of humans or other animals depends on several factors. The most obvious and important are the magnitude of the exposure that led to the presence of the substance in tissue and the degree of persistence of the substance in tissue. Presence indicates some exposure, but that exposure might have occurred in the workplace, in the home, or in any of several other environments, and it might have occurred at any earlier time. Persistence is a property of both substance and the specific tissue. Most compounds to which humans are exposed, including environmental contaminants, are rapidly cleared through the conventional routes of metabolism and elimination. Some notable exceptions are 2,2,-bis(p-chlorophenyl)-1,1,1-trichloroethane (DDT), other halogenated insecticides, and certain industrial chemicals such as polychlorinated biphenyls (PCBs), which
are not readily cleared but accumulate in tissues in proportion to overall exposure. Those chemicals also accumulate and concentrate in the food chain and may reach toxic levels. This fact is of great concern to those responsible for the protection of the health of our population and has been a driving force behind the NHATS program.
When it was initiated, the NHATS was designed to survey concentrations of pesticides in tissues of the general population. The pesticides of greatest concern were halogenated hydrocarbons, primarily halogenated aromatic hydrocarbons. Those compounds are generally highly lipophilic and slowly metabolized—both properties that result in their accumulation in the environment and in the tissues of higher animals, including humans. The lipophilicity results in their moving from blood into tissues with a high fat content, particularly adipose tissue, where they concentrate with continued exposure. Therefore, the choice of adipose tissue to monitor for pesticides addressed the original purpose of the survey. However, the goals and objectives of the program have expanded greatly since its inception, and no tissue can be best for all purposes. Given the advances in analytic chemistry, the increased sensitivity of equipment, and the discontinuation of use of most halogenated aromatic pesticides and many halogenated aromatic industrial chemicals, including PCBs, it seems reasonable to reconsider which tissues to monitor.
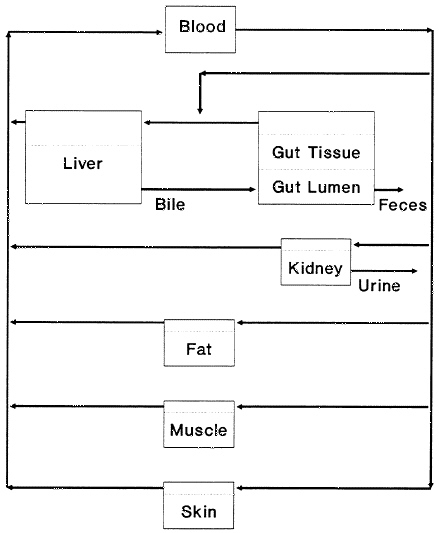
One critical element in the choice is tissue-to-blood ratios of chemicals of concern. Blood is the common intermediary among all tissues. As it transports nutrients, oxygen, and wastes to and from tissues, blood maintains intimate contact with each other tissue and thereby in effect keeps all tissues in contact with one another. That contact is critical to the maintenance of homeostasis by hormones and the other mechanisms that regulate biologic processes. Most xenobiotic chemicals in the body cross cell membranes by passive diffusion, so they readily partition, up to the tissue-to-blood ratios, from exposed tissues to blood and from blood to other tissues. The result of the various tissue-to-blood ratios is a dynamic equilibrium of xenobiotic concentrations between tissues, as is shown schematically for a pharmacokinetic model in Figure 3–1. At equilibrium, or steady state, the proportions of a chemical stored in the various tissue compartments depends on the affinities of the chemical for the tissues in question and the tissue volumes. Tissue-to-blood ratios for more polar compounds often are near unity (Table 3–1); the choice of a tissue to sample depends heavily on the properties of the chemical of interest, including lipophilicity, solubility, and ease of metabolic degradation. Tissues reach equilibrium at rates that depend in large part on the concentration of the chemical in blood, the rate of blood flow to the major tissue depots, and the tissue volumes. Final tissue-to-blood ratios reflect the affinities of the chemical for the individual tissues; the ratio for lipid-rich tissues is very high for highly lipophilic chemicals.
TABLE 3–1 Examples of Tissue-to-Blood Ratios for Various Classes of Chemicalsa
|
Chemical |
Adipose Tissue |
Liver |
Kidney |
Muscle |
References |
|
Halogenated aromatic compounds |
|||||
|
DDT |
184–792 |
2.4–4.4 |
2.7–3.9 |
— |
Wooley and Talens, 1971 |
|
DDE |
52–412 |
1.1–5.6 |
1.1–2.3 |
— |
Wooley and Talens, 1971 |
|
TCDD |
89–135 |
121–280 |
— |
— |
Birnbaum, 1986 |
|
4-Chlorobiphenyl |
30 |
1 |
— |
1 |
Lutz et al., 1977 |
|
4,4’-Dichlorobiphenyl |
70 |
3 |
— |
2 |
Lutz et al., 1977 |
|
2,4,5,2’,4’,5’-Hexachlorobiphenyl |
400 |
12 |
— |
4 |
Lutz et al., 1977 |
|
Halogenated aliphatic compounds |
|||||
|
1,2,3-Trichloropropane |
15.0 |
3.8 |
2.0 |
0.6 |
Volp et al., 1984 |
|
1,1,1-Trichloroethane |
16.0 |
2.7 |
3.2 |
1.0 |
Matthews, 1988 |
|
Bromodichloromethane |
1.0 |
14.3 |
6.5 |
0.6 |
Matthews et al., 1990 |
|
Aromatic amine compounds |
|||||
|
p-Nitroaniline |
7.0 |
0.9 |
0.4 |
0.8 |
Chopade and Matthews, 1984 |
|
Benzidine |
Lynn et al., 1984 |
||||
|
p-Phenylenediamine |
0.1 |
3.0 |
— |
0.7 |
Ioannou and Matthews, 1985 |
The passive diffusion of chemicals across cell membranes accounts for the fact that, if a chemical can be eliminated from any tissue, the constant equilibration between blood and all tissues will result in its elimination from the body. Conversely, if a chemical cannot be readily eliminated, a decrease in its concentration in one tissue will increase its concentrations in other tissues. That relationship accounts for the observation that rats exposed to DDT at nontoxic concentrations for 3 months died of DDT intoxication when placed on a restricted diet that reduced their total body fat and consequently increased concentrations (Kimbrough, 1982).
Blood
If blood-to-tissue ratios are known, determination of the blood concentration of a chemical can provide accurate estimates of other tissue concentrations. The characteristics that determine chemical partitions between tissues are the same in humans and animals, so blood-to-tissue ratios in rats and mice, such as those in Table 3–1, yield good approximations for humans. Furthermore, they are available for many compounds and so could be easily developed to support surveys of human exposure and tissue concentrations. Thus, assay of blood samples should permit accurate estimation of tissue burdens of many chemicals of interest.
Detection of a chemical in blood or another tissue does not, however, provide insight into the time or magnitude of exposure. Analysis of tissues for chemical content can confirm exposure or lack of exposure, but only serial samples from a single person or many samples from a population over a long period can yield an accurate estimate of exposure or persistence. Blood concentrations of many chemicals change greatly within hours or days of exposure. Some chemicals, such as benzidine and the glycol ether 2-butoxy-ethanol (Table 3–1), are no longer detectable in blood within a few hours of exposure and some (e.g., formaldehyde) disappear even faster; they are completely and rapidly cleared from other tissues as well, so no tissue has evidence of earlier exposure.
A disadvantage of sampling blood is that it often contains lower concentrations of the most persistent xenobiotic chemicals than any other available tissue, so assay sensitivity is low. In contrast, although blood and adipose tissue can differ considerably in their concentrations of some highly lipophilic chemicals (Table 3–1), the difference is relatively minor for most other classes of chemicals. With improving analytic methods, this disadvantage is not as great as it was when the NHATS program was initiated, and it should become even less important as methods continue to improve. Another important issue
that must be addressed for blood collection is the safe and proper handling and storage of samples. The potential health consequences of careless or improper handling of blood can expose laboratory workers to viruses, such as hepatitis B and AIDS. Individuals involved in collection, analysis, and storage of blood must be given careful direction and supervision.
Numerous chemicals can be detected in the breath of humans (Wallace et al., 1986; Gordon et al., 1988). The chemicals so detected are most often volatile chemicals encountered in the home or workplace. Breath analysis is in effect a blood analysis, because there is a constant passive equilibration of chemicals between blood and air in the lungs, just as there is between blood and other tissues. The concentration of a chemical in exhaled air will be proportional to its volatility and its concentration in blood and inversely proportional to its solubility in blood. However, most chemicals are insufficiently volatile to permit detection in breath, and the ones that are sufficiently volatile are usually so depleted as to be undetectable within hours, or at most a day or so, of exposure. But breath analysis can yield estimates of relatively constant exposure, as sometimes occurs in the home or workplace.
An important advantage for blood collection, in contrast to collection of adipose samples from cadavers, is that samples to be obtained from living persons, so that interviews can be conducted with sample donors to obtain demographic and environmental information, which permits examination of causal relationships and risk factors. For example, interviews can yield data on the following:
-
Geographic location, including current residence and length of stay in previous residences. This information could be used for analysis by region, for urban-rural comparison, for separate analyses of persons living near heavy industry or chemical or petrochemical refineries, etc.
-
Demographic information, such as sex, age, race, and ethnicity. Separate analyses of such groups are of interest.
-
Information on occupation and industry, particularly whether employment is in a chemical plant or refinery, on a farm using chemical pesticides, etc.
-
Other environmental data, such as type of drinking water used (private well water or community system).
The number of samples in a single year might not be large enough to support detailed analysis of the relationship between the information obtained during interviews and contaminant concentrations in blood, but data from 2 years or more can be combined. With the larger number of samples, relation-
ships among tissue concentrations, demographic data, and occupational and environmental exposures can be studied with more precision.
In developing a monitoring system that relies on large samples of blood, multiple factors must be evaluated, including the need for special storage methods and containers. Many such issues may have been studied previously by other groups (e.g., the Red Cross); however, it is unlikely that all storage methods and containers have been evaluated to identify factors that might influence the measurements of environmentally important chemicals. Included in the questions that should be studied with great care are to what extent freezing/thawing will change concentrations of volatile contaminants; whether and how cells should be separated from plasma or serum to minimize analytical effects; and how samples should be stored before analysis, including temporal effects on volatile chemical concentrations.
Adipose Tissue
Adipose tissue contains the highest concentrations of some of the most persistent chemicals in the environment, including halogenated aromatic hydrocarbons. Those concentrations can be 100 or even 1,000 times greater than concentrations in blood or other lean tissues. Assay of adipose tissue can thus greatly enhance sensitivity. However, tissue-to-blood ratios of adipose tissue other tissues are much smaller for most chemicals (Table 3–1). The use of adipose tissue is also complicated by the difficulty of obtaining samples of fat. It is because of that difficulty that the NHATS program obtains approximately 80% of its samples from autopsies. Satisfactory samples can be obtained from autopsy, but reliance on this source limits both the representativeness and the numbers of samples available.
Whenever possible, samples of adipose tissue should be taken from persons who were known not to have died from long-term illnesses or wasting diseases (e.g., cancer or AIDS). Furthermore, samples should be taken from the same anatomic site in each individual from the sampled population, in order to minimize the inherent variability among sampled sites and individuals.
Lean Tissues
Of the lean tissues, liver and kidney usually contain the highest concentrations of organic chemicals. Muscle has the largest tissue volume and might contain a major portion of the body burden of a chemical, but concentrations in muscle are usually similar to those in blood (Table 3–1).
The kidneys are major excretory organs and can contain transient high concentrations of some chemicals. Other chemicals can be concentrated and retained by the kidneys as they recover water, minerals, and other essential substances from the glomerular filtrate.
The liver plays a dual role of xenobiotic degradation and excretion in bile. Most of the metabolism of foreign chemicals occurs in the liver, and some can be concentrated in the liver during metabolic degradation. Some chemicals, such as the tetrachlorodibenzo-p-dioxins (TCDDs), seem to have an affinity for the liver, even though they are not readily degraded (Birnbaum, 1986). Others, such as chlordecone (Kepone), are concentrated in the liver as a result of hepatic excretion in bile, reabsorption from the intestine, and return to the liver in enterohepatic recirculation (Bungay et al., 1979).
Lean tissues are primary depots for heavy metals (Comar and Bronner, 1964). Cadmium and mercury are concentrated in the kidneys, and cadmium in the liver. Lead is concentrated in the liver, but more concentrated in bone. Each lean tissue can be the preferred tissue to assay for one or more chemicals. Lean tissues are generally available for assay only from autopsies; however, once a collection system is established for collecting adipose tissues at autopsy, then other solid (lean) tissues, such as liver and kidney, can be collected without much additional expense or effort.
Biologic Fluids
Just as the equilibration of chemicals between blood and other tissues results in a dynamic equilibrium among all tissues, there is an equilibration between tissues and the fluids that they secrete or excrete. Almost all biologic fluids (urine, sweat, saliva, milk, etc.) contain chemicals at concentrations proportional to those in the tissues in which they originated. But most (milk is an exception) are rather polar media and contain low concentrations of the persistent lipophilic chemicals that have been of most concern.
Milk contains lipid, as well as protein and water, and has been an important indicator of exposure in several incidents of environmental contamination with lipophilic compounds (Matthews, 1979). However, milk samples can be provided only by postpartum women, a highly limited and atypical segment of the population useful mostly for studies directly relevant to the fetus or nursing infant.
In studies of metabolism, urine is routinely assayed for parent substances and metabolites. Because it is polar, urine contains only low concentrations of lipid-soluble parent chemicals and has been used only sparingly to determine environmental exposures. Increased use of high-performance liquid
chromatography (HPLC) and characterization of major metabolites of chemicals of interest would increase the utility of urine as an assay medium.
Other biologic fluids are equally polar, but are used less than urine, because they are harder to collect, are present in smaller volumes, and generally offer no important advantages over urine. Sweat might have the greatest potential for development of a noninvasive assay for xenobiotic chemicals in humans, because it contains substantial amounts of skin oil, as well as water and minerals. If methods for standardization could be developed, sweat might be useful for assays for many xenobiotic chemicals.
Hair
Xenobiotics have been detected in hair, feathers, and nails of numerous species. Hair is the most easily obtained human growing tissue for monitoring and has received the most attention. Several toxic elements—including selenium, mercury, and arsenic—have an affinity for hair, probably as a result of their reaction with sulfur-containing amino acids, which are more highly concentrated in hair than in other tissues. Those elements are chemically bound and thus not easily removed by washing, so their presence in hair is proportional to the magnitude of exposure to them. Hair contains mercury at approximately 300 times the concentration found in blood and is considered a good indicator of the body burden of this element. But the probability of contamination from the external environment, such as selenium-containing shampoos, makes hair a less accurate indicator of exposure to other elements (EPA, 1976).
Organic compounds are generally not chemically bound to hair, but are more commonly found in oil produced by the body and associated with hair. That is particularly true of highly lipophilic substances, such as halogenated aromatic compounds. Halogenated insecticides and other halogenated organic compounds are easily detected in human hair by simple extraction and analysis with gas chromatography (Matthews et al., 1976). Because the quantities of oil and hair, the rate of hair growth, and personal hygiene all vary greatly among individuals, analysis of hair for organic compounds in hair oil can rarely be used to derive quantitative estimates of exposure; but given its ready availability and ease of assay, hair should not be overlooked as a qualitative indicator of human exposure to a wide variety of xenobiotics.
SUMMARY AND RECOMMENDATIONS
Given the central role of chemicals in modern society, people will be exposed to chemicals. It is prudent that the general population be monitored to document magnitudes of exposure and to determine the need for and effectiveness of regulations and other measures to limit risk. An essential part of monitoring exposure of the general population is a survey of chemical concentrations in human tissues. The original NHMP program was required to concentrate on chemicals in human adipose tissue. However, the objectives of the NHMP have broadened with time, the classes of chemicals currently surveyed or proposed for survey are much more varied than those originally targeted, and analytic instruments are much more sensitive than they were 20 years ago. Therefore, a survey of adipose tissue might no longer be the best way to obtain accurate estimates of concentrations of chemicals of current interest in the tissues of the general population.
After extensive discussion of the advantages and disadvantages of the major tissue groups and biologic fluids, the committee draws the following general conclusions regarding the choice of tissues on which to base a survey of chemical residues in human tissues.
-
Blood is the common intermediary among all tissues, and chemical concentrations in blood accurately reflect those in all other tissues. Blood also offers the advantages of availability by a widely accepted, relatively noninvasive technique and of being the most accessible tissue for assay. An assay of chemical concentrations in blood would permit sampling of a wider sector of the population, better comparison of exposed populations with national averages, repeat sampling of persons who have high tissue concentrations, and opportunities to follow chemical clearance with time. We recommend that any new program to assay chemical concentrations in tissues of the U.S. population be based primarily on analysis of blood.
-
Adipose tissue contains the highest concentrations of some of the most persistent chemicals to which humans are exposed, primarily halogenated hydrocarbons. Analysis of adipose tissue would provide the most sensitive assay of tissue concentrations of those chemicals and should be continued where feasible. Continued analysis of adipose tissue would also provide continuity with the present program, as well as confirmation that a survey based on blood also detects important tissue residues of persistent chemicals.
-
Analysis of lean tissues (although not specifically recommended by the committee) and fat taken at autopsy would yield data on chemical concentrations in specific tissues that could be used to calculate tissue-to-blood ratios for estimating tissue concentrations where only blood is available.
-
With additional development, analysis of hair, urine, and some other biologic tissues and fluids might provide noninvasive methods to estimate human tissue concentrations. However, for most organic compounds, those methods need additional refinement and supporting data before they can be used in general assays.
The question of which tissues to sample requires very careful consideration.
The present evidence leads the committee to conclude that the basis of a human tissue monitoring program should be the broad, random collection of samples of blood.
Implementation of this recommendation includes probability sampling; data needs cannot be satisfied by existing EPA plans regarding the proposed National Blood Network to sample blood donors.
Blood collection should be supplemented by the continued collection of adipose tissue, in part to maintain historical continuity while new long-term series of blood measures are established and in part be cause some important residues are most concentrated in fat.
As discussed elsewhere, fat samples are necessarily nonrandom and nonrepresentative, so careful study of the relation between blood concentrations and fat concentrations is needed to validate the latter.
Measurements of nonrandom adipose tissue (as in the present NHATS) will continue to be important for at least several years, although they might be replaced later with studies of the lipid fraction of blood.
While blood and adipose tissue are being collected, the program should devote some resources to study of the correlations between chemical measures of xenobiotics in these two tissues, so that the effects of nonrandomness in the adipose samples will be better un derstood and the continued contribution of the adipose samples can be property evaluated.
Whatever tissues are collected, samples should be accompanied by standardized information on demographics, illness (especially termi nal illness), and known occupational or other major exposures to chemicals.