9
The Committee's Dosimetric Model for Radon and Thoron Progeny
INTRODUCTION
The purpose of this chapter is to describe the underlying assumptions, the specific experimental data, and the formulation of the theoretical dosimetry model that the committee has used to carry out its task of comparing the dose per unit exposure received by underground miners with those received by subjects exposed to radon progeny at home. The modeling procedures that are reviewed and adapted here by the committee were developed as part of an overall review of models and experimental data for radon progeny dosimetry that was sponsored by the U.S. Department of Energy (James, 1990).
DOSIMETRIC ASSUMPTIONS AND MODEL
Both secretory and basal cells in the bronchial epithelium and, to a lesser extent, secretory cells in the bronchioles were identified in Chapter 8 as the principal targets for induction of bronchogenic cancer. The location of these cells within the ciliated epithelium is illustrated schematically in Figure 9-1, which shows a diagrammatic section through the wall of a bronchus. When radon progeny decay, the alpha particles that they emit lose all of their energy within very short distances in the fluid lining the bronchial or bronchiolar airway surfaces and in the epithelial tissue that contains the target cells. The range of the 6-MeV alpha particle from polonium-218 (218Po) in fluid or tissue is 48 µm, and that for the 7.7-MeV alpha particle of 214Po is 71 µm. The location of the target cells in relation to the points at which radon progeny decay (the source
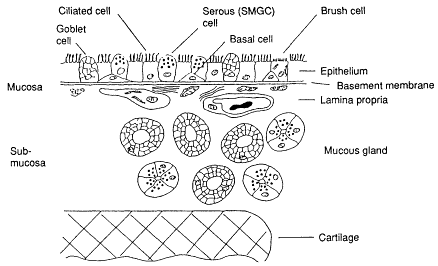
Figure 9-1 Diagrammatic section through the wall of a bronchus showing the types of cells found in the epithelium.
of alpha particles) is therefore a critical factor in determining dose. To evaluate the dose received by the respective populations of secretory or basal target cells from each decay of 218Po or 214Po, it is necessary to represent the positions of the source and targets by a geometrical model. The geometry of each bronchial or bronchiolar airway is approximated by a cylindrical tube (Figure 9-2). In the model, the inner surface of the tube is considered to be lined by a thin layer, or sheath, of fluid representing mucus. This inner sheath of mucus is separated from the underlying epithelium by a band of hair-like cilia, which are responsible for clearing the mucus in the direction of the trachea. The cilia are bathed in an aqueous fluid that forms a second, thin layer of shielding material. Both fluid layers have the protective effect of absorbing some of the energy from radon progeny alpha particles. The geometrical model of the airway wall is used to calculate the radiation dose at all points in the underlying epithelium where sensitive targets are found, and particularly the doses received by the nuclear DNA of sensitive cells from alpha-particle decays of the radon progeny source wherever this may be located. Figure 9-2 illustrates two possible locations of the source, within the sheath of mucous ''gel'' overlying the cilia and within the epithelium itself, if the progeny move into the epithelium.
In a dosimetric model, the distribution of target cell nuclei in the bronchial epithelium can be approximated by the idealized structure shown in Figure 9-3. A recent, detailed study has shown that the thickness of histologically normal human bronchial epithelium is typically about 58 µm in the larger bronchial airways and 50 µm in the most distal bronchi (Mercer et al., in
press). Mercer and colleagues found that secretory cell nuclei are distributed approximately uniformly between about 10 and 40 µm below the epithelial surface and that basal cell nuclei are located between about 35 and 50 µm below the surface, as illustrated schematically in Figure 9-3. The thickness of the mucous gel overlying the cilia is difficult to determine in histological preparations. Using the technique of fixation by vascular perfusion, Mercer et al. (1989)estimated that the gel is typically only 2-µm thick in the bronchi. It has been assumed previously that bronchial mucus is substantially thicker, for example, Harley and Pasternack (1982) and National Council on Radiation Protection and Measurements (NCRP, 1984) assume a value of 15 µm for the overall thickness of protective mucus (which, in their case, includes the 6-µm-thick fluid layer bathing the cilia). In view of the uncertainty in the actual value, the committee assumed for the purpose of modeling bronchial doses that the normal thickness of the mucous gel has an intermediate value of 5 µm (Figure 9-3). The thickness of mucus in smokers, and particularly in subjects with chronic bronchitis, may be substantially greater (Chapter 7).
The calculated variation of dose with depth below the surface of the normal bronchial epithelium for the decay of one alpha particle of 218Po or 214Po per cm2 of airway surface is shown in Figure 9-4. The calculation is based on the mathematical technique described by Harley (1971), except that it uses Armstrong and Chandler's (1973) theoretical stopping power of tissue as a function of alpha-particle energy (Nuclear Energy Agency Group of Experts [NEA], 1983). Both calculations take into account. the additional dose contributed by any alpha particles that cross the airway lumen from the opposite wall. An airway caliber of 5 mm in diameter is assumed to typify a bronchus. In adults, the actual airway caliber varies from about 1 cm in
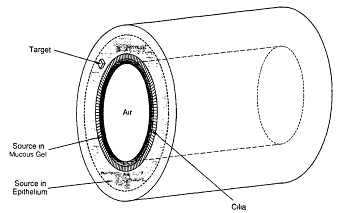
Figure 9-2 Cylindrical model of a bronchial or bronchiolar airway used to calculate doses received by target cell nuclei from alpha-particle decays of radon progeny located in mucus or in the epithelium.
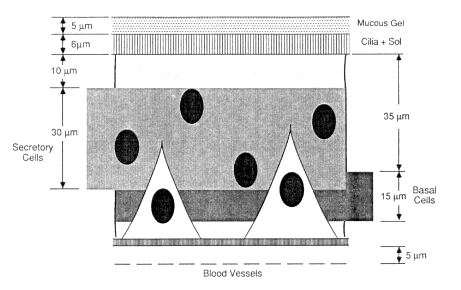
Figure 9-3 Model of the location of secretory and basal cell nuclei in bronchial epithelium and of the structure of the mucous layers at the epithelial surface.
the main bronchi (referred to as the first bronchial generation) to about 2 mm in the eighth generation, which the committee took to represent the last and smallest bronchi. Since the range of radon progeny alpha particles in air is on the order of 5 cm, however, it is found that the actual airway caliber has little effect on the dose received by epithelial target cells from a given number of alpha-particle decays per unit surface area.
Figure 9-4 shows two curves, labeled activity in mucus, for each of 218Po and 214Po. In each case, the lower curve represents activity retained in the 5-µm-thick mucous gel overlying the cilia (see Figure 9-3). The corresponding upper curves represent the higher doses calculated for activity mixed in the 6-µm-thick aqueous layer that bathes the cilia. This amount of variation in the location of the mucous source has a relatively minor impact on calculated doses. In contrast, however, the same number of alpha-particle decays from radon progeny located in the epithelium gives rise to a relatively constant dose throughout the tissue. Figure 9-4 also indicates the range of depths at which secretory or basal cell nuclei are assumed to occur. When the depth-dose curves are averaged over these ranges of target depths, it is found that the average dose received by secretory cell nuclei is relatively independent of the location of radon progeny alpha-particle decays, but the dose received by basal cell nuclei is significantly higher if radon progeny decay in the epithelium rather than in mucus. The degree to which radon progeny are taken up by the epithelium is an uncertain factor in the dosimetry model. Its overall impact on the calculated
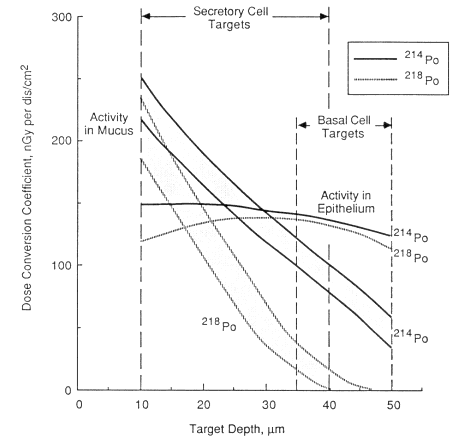
Figure 9-4 Variation of dose with depth below the surface of bronchial epithelium for alpha-particle decays of radon progeny in mucus or in the epithelium itself. The ranges of depth at which secretory or basal cell nuclei occur are shown for comparison.
conversion coefficient between exposure and critical dose is examined later in this chapter.
Figure 9-5 illustrates the model of target cell nuclei and mucus assumed by the committee to represent the epithelial lining of the bronchioles. These airways are devoid of basal cells. The sensitive targets are assumed to be the nuclei of secretory cells, which are located between 4 and 12 µm below the epithelial surface (Mercer et al., in press). Both the cilia (the mucous sol layer) and the overlying mucous gel are assumed to be thinner than those in the bronchi (4-µm high and 2-µm thick, respectively). The depth-dose curves calculated for secretory cell nuclei in the bronchiolar epithelium are shown in
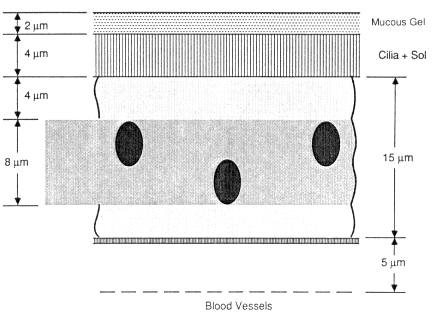
Figure 9-5 Model of the location of secretory cell nuclei in bronchiolar epithelium and of the structure of bronchiolar mucus.
Figure 9-6. In this case, it is found that the average dose received by target cell nuclei does not vary significantly with the location of the radon progeny alpha-particle decays, whether or not these occur in mucus (of the assumed normal thickness) or in the epithelium itself.
The values of target cell dose calculated respectively for bronchial and bronchiolar epithelia from the alpha decay of 218Po or 214Po per cm2 of airway wall are given in Table 9-1. It has been found that the epithelial thickness is the same in infants and children as that in the adult (Gehr, 1987). The tabulated dose conversion coefficients are therefore assumed to apply to all subjects.
The doses received by these various target cell populations for a given subject, under given conditions of exposure, are evaluated by first modeling the number of 218Po and 214Po alpha decays that occur in each airway generation, using the procedures described below. In order to apply the dose conversion coefficients given in Table 9-1, it is necessary to specify the surface areas of the respective airways in each subject. To do this, a model of the variation of airway caliber and length throughout the bronchial tree must be assumed. Several, more or less complete, models of bronchial and bronchiolar airway dimensions in the adult male have been used previously for radon progeny dosimetry (Weibel, 1963; Yeh and Schum, 1980; Phalen et al., 1985). The airway models of these investigators were derived by measuring casts of human lungs that were made
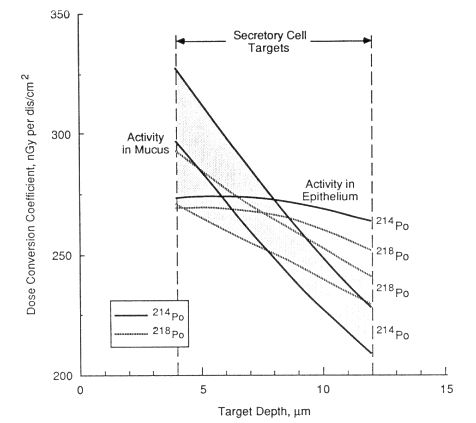
Figure 9-6 Variation of dose with depth below the epithelial surface in the bronchioles. This is shown over the range of depths at which secretory cell nuclei are assumed to occur.
at different degrees of inflation in each case. However, even when the reported airway dimensions are scaled to a common lung volume, the different models still exhibit significantly different dimensions (Yu and Diu, 1982). For the purpose of dosimetry, the committee assumes that a "representative" model for the adult male is obtained by averaging the airway dimensions reported in the three studies cited above, after these have been scaled to the normal functional residual capacity (FRC) of 3,000 ml (James, 1988).
The model of airway dimensions is needed for two purposes: first, to calculate the fractions of inhaled radon progeny activity that are deposited in each airway generation throughout the bronchial tree (and also in the alveolated respiratory airways) and then to calculate the surface densities of alpha-particle decays throughout the bronchi and bronchioles, in order to evaluate target cell doses. Previous models of radon progeny dosimetry for adult females (who
TABLE 9-1 Calculated Mean Doses to Bronchial and Bronchiolar Target Cell Nuclei (in nGy) from 1 α-Decay of 218Po or 214Po per cm2 of Airway Surface
|
|
Mucous Gel |
Epithelium |
||
|
Location of Radon Progeny |
218Po |
214Po |
218Po |
214Po |
|
Target Cell Nuclei in Bronchi: |
||||
|
Secretory cells |
78 |
142 |
131 |
264 |
|
Basal cells |
3 |
68 |
126 |
132 |
|
Target Cell Nuclei in Bronchioles: |
||||
|
Secretory cells |
251 |
250 |
264 |
271 |
typically have lungs smaller than those of adult males) and children have assumed uniform scaling of airway dimensions with lung volume (Harley and Pasternack, 1982; Hofmann, 1982; NEA, 1983; NCRP, 1984) or, alternatively (James, 1988), have adopted the scaling of bronchial and bronchiolar dimensions with body height reported by Phalen et al. (1985) from measurements of airway casts that were taken from children and infants. However, the data of Phalen et al. surprisingly indicate that the small bronchioles in young children and infants are not substantially different in size from those in adults. These data yield unrealistically high values of the physiological dead space in young subjects. To give values of dead space that are comparable with physiological data (Table 9-2), the committee has adopted the scaling procedure introduced by Yu and Xu (1987) and also used by Egan et al. (1989). The committee assumes that all airway dimensions in the fully grown lungs of adults (male and female) are scaled according to the one-third power of the subject's FRC.
The factors reported by Phalen et al. (1985) to scale bronchial airway dimensions for young subjects in terms of their height are based on reasonably complete samplings of all airways. These data can therefore be used to evaluate the size of the trachea and bronchi in growing subjects relative to the standard values adopted for the adult male. However, in the absence of comparable data for the bronchioles, their dimensions must be inferred. It is reasonable to adopt Weibel's (1963) inference that, on average, the diameter and length of bronchioles decrease exponentially in successive generations, until they match the dimensions of the first respiratory bronchioles. The size of the latter is determined by considering how the lung grows from birth to maturity. Once the lung is fully developed, the respiratory airways are scaled replicas of those in the adult. Recent data indicate that this development process is complete by age 2, when the growing lung has a full complement of airways and most of
TABLE 9-2 Comparison of Tracheobronchiolar Dead Space Predicted Using the Committee's Model of Airway Dimensions Vis-à-Vis Physiological Data
|
|
Anatomical Dead Space (cm3) |
||||||
|
|
Physiological Data |
Model |
|||||
|
Subject Age (yr) |
Cook et al (1955) |
Hart et al. (1963) |
Wood et al. (1971) |
Mean VTOT |
VTOT |
VTB |
VET |
|
Newborn |
8.5 |
— |
— |
8.5 |
— |
— |
1.5 |
|
1/12 |
11 |
— |
— |
11 |
13 |
10.7 |
1.9 |
|
1 |
— |
— |
— |
— |
25 |
15.9 |
4.6 |
|
5 |
— |
51 |
— |
51 |
47 |
33 |
14 |
|
10 |
— |
88 |
81 |
85 |
85 |
59 |
26 |
|
Woman |
— |
126 |
127 |
127 |
118 |
78 |
40 |
|
Man |
— |
151 |
160 |
155 |
147 |
97 |
50 |
the final number of alveoli (Gehr, 1987; Zeltner et al., 1987). The respiratory airways are therefore scaled according to the growth in lung volume (FRC).
In order to estimate typical dimensions of respiratory airways in the developmental stage from birth to age 2 yr, it is reasonable to assume that the number of airways is virtually complete at birth, but that alveolization of these immature airways is not. The data of Zeltner et al. (1987) indicate that the number of alveoli at age 1 mo is about 20% of the typical adult value, and that at age 1 yr it is about 80%. Hansen et al. (1975) provided a complete description of the branching structure and dimensions of the respiratory acinus in an adult male. This model has been adopted by the committee to derive respiratory airway dimensions for the lungs of children and infants by scaling for lung volume and number of alveoli, respectively (Egan et al., private communication). The derived dimensions of respiratory bronchioles are then used to infer the size of the most distal generation of bronchioles in young subjects. Figures 9-7 and 9-8 show, respectively, the values of airway diameter and length that are given by the above scaling procedures for adult females, children aged 10 or 5 yrs, and infants aged 1 yr or 1 mo. The reference values for the adult male are also shown, for comparison. Table 9-3 gives the corresponding estimates of the total surface area of the airways in each generation of the bronchial and bronchiolar model for each subject. These values are used with the coefficients given in Table 9-1, to convert the calculated number of radon progeny alpha-particle decays in each airway generation into radiation doses absorbed by target cell nuclei.
CLEARANCE MODEL
The process of breathing continuously deposits radon progeny at all levels in the bronchial tree. The process of mucociliary clearance, which acts in all
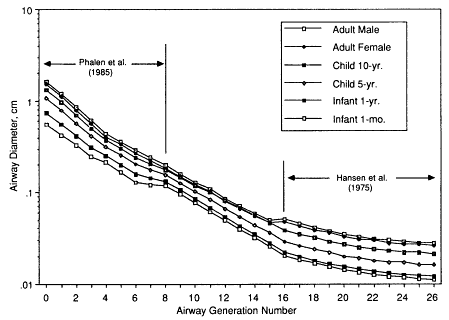
Figure 9-7 Scaling of airway diameter throughout the lung respiratory pathway assumed for the calculation of radon progeny deposition and dose in each generation for adults, children, and infants. The trachea is labeled generation 0. Airway generations 1-8 represent the bronchi. These are scaled with body height according to data from Phalen et al. (1985). Generations 9-15 represent the bronchioles. Generations 16-26 represent the respiratory airways whose dimensions are based on the model of Hansen et al. (1975).
ciliated airways, continually redistributes these progeny in a proximal direction (toward the trachea and larynx). This tends to concentrate the alpha particle decays on smaller areas of airway surface (as shown in Table 9-3), since the number of airways is halved each time subsidiary branches converge into a common parent. The combination of these processes with that of radioactive decay determines the number of alpha decays that occur in each airway generation, the surface density of the decays, and thus the dose received by target cells. This complex movement and decay of radon progeny within the bronchial tree is represented mathematically by a so-called clearance model.
Figure 9-9 shows the model used by the committee to calculate the number of radon progeny decays that occur in each airway generation. The model represents the average time taken by a theoretical, discrete packet of mucous gel to traverse a given airway generation by the parameter 1/λ2, where λi is a constant that represents the rate of mucous transport in the ith generation. If k represents each of the short-lived radon progeny in turn (218Po through 214Pb
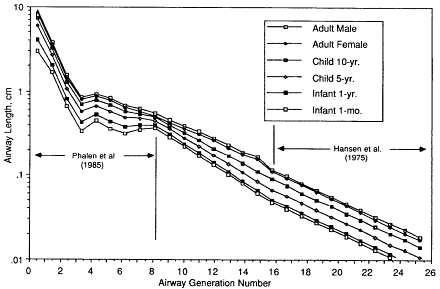
Figure 9-8 Scaling of airway length as described for Figure 9-7.
TABLE 9-3 Total Surface Area of the Airways in Each Generation of the Committee's Model of the Bronchial Tree for Adults, Children, and Infants
|
|
Surface Area (cm2) |
||||||
|
|
Adult |
Child |
Infant |
||||
|
Airway |
Gen. No. |
Male |
Female |
10 yr |
5 yr |
1 yr |
1 mo |
|
Trachea |
0 |
47 |
41 |
30 |
20 |
9.3 |
5.1 |
|
Bronchi |
1 |
29 |
25 |
19 |
13 |
7.1 |
4.4 |
|
|
2 |
16 |
14 |
11 |
7 |
4.1 |
2.6 |
|
|
3 |
13 |
11 |
9 |
6 |
3.2 |
2.0 |
|
|
4 |
20 |
17 |
14 |
10 |
6.5 |
4.6 |
|
|
5 |
29 |
25 |
20 |
14 |
8.5 |
5.7 |
|
|
6 |
39 |
33 |
27 |
19 |
11.7 |
8.0 |
|
|
7 |
58 |
50 |
43 |
33 |
22 |
17 |
|
|
8 |
85 |
74 |
68 |
55 |
41 |
34 |
|
Bronchioles |
9 |
113 |
98 |
90 |
70 |
51 |
42 |
|
|
10 |
154 |
134 |
120 |
89 |
63 |
52 |
|
|
11 |
227 |
196 |
161 |
113 |
77 |
64 |
|
|
12 |
298 |
258 |
212 |
142 |
95 |
80 |
|
|
13 |
402 |
348 |
284 |
181 |
116 |
99 |
|
|
14 |
545 |
476 |
378 |
231 |
145 |
122 |
|
|
15 |
824 |
721 |
508 |
297 |
179 |
152 |
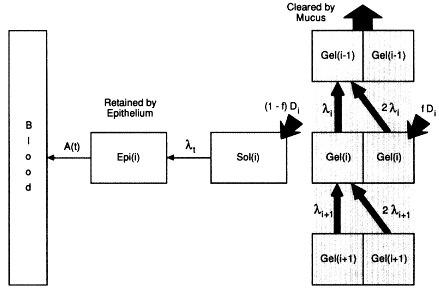
Figure 9-9 Model of mucous clearance through the ith airway generation. The model also has a pathway along which radon progeny may be transferred through the mucous sol layer to be retained temporarily in epithelial tissue, before radioactive decay or absorption into the blood.
through 214Bi), the number of atoms, nk, of the kth progeny that decays in generation i is given by the sum of three components
where
and Ak is the activity of the kth progeny that is cleared into generation i from generation i + 1, ![]() is the activity of the kth progeny that is produced in generation i by decay of its parent for k > 1, and Dk is the activity of the kth progeny that is deposited from inhaled and exhaled air.
is the activity of the kth progeny that is produced in generation i by decay of its parent for k > 1, and Dk is the activity of the kth progeny that is deposited from inhaled and exhaled air.
In these equations, the effective rate of mucociliary clearance, 2λi, is taken to be twice the value that applies to activity entering generation i from below, since, on average, progeny produced by decay of the parent and by direct deposition have only half the distance to travel (Jacobi and Eisfeld, 1980; NEA, 1983).
TABLE 9-4 Cuddihy and Yeh's (1988) Estimates of the Time Taken by Mucus to Traverse Each Airway Generation Throughout the Bronchial Tree
|
 |
The committee adopted values of λi derived recently by Cuddihy and Yeh (1988) from a review of the available human data. These investigators matched the time taken by volunteer subjects to clear various fractions of insoluble radioactive particles with theoretical estimates of the amount of activity deposited at various depths in the bronchial tree. The estimated times taken to traverse each airway generation, 1/λi, are given in Table 9-4. In the absence of contrary data, the committee assumed that these values also typify mucous clearance times in children and infants.
The clearance model shown in Figure 9-9 also enables the effect on doses to target cells of any transfer of deposited radon progeny from the surface fluid to the underlying epithelium to be evaluated (NEA, 1983). This is represented by partitioning the activity Di deposited in generation i into a fraction, fDi, that is retained and cleared by the mucous gel and a complementary fraction, (1-f)Di, that is transferred through the sol layer to be retained temporarily in the epithelium. It can be assumed that the rate of transfer through the sol layer is rapid in comparison with the rates at which the progeny decay. However, once the progeny have been taken up in tissue, their biological retention time is likely to be significant. A half-time of approximately 10 h has been observed for the absorption of 210Pb ions from human lung into the blood (Booker et al., 1969; Hursh and Mercer, 1970), and a similar value was measured in dogs (Bianco et al., 1974).
James et al. (1977) observed in rabbits that insoluble particles are distributed more proximally in the bronchial tree than are 212Pb ions introduced
simultaneously in a small volume of fluid. Greenhalgh et al. (1982) found that the fraction of 212Pb ions retained in the nasal airways of rats was always greater than that of insoluble particles. They reported that, on average, only 57% of 212Pb ions that were attached to insoluble particles on instillation into the noses of the rats were cleared by mucus over an observation period of about 1 h, whereas 75% of particles were cleared. Lead ions are clearly more mobile on the epithelial surface than insoluble particles are. Greenhalgh et al. (1982) interpreted their observed excess of retained ions as evidence in support of the uptake of radon progeny by bronchial epithelium in rats that was reported earlier by Kirichenko et al. (1970). On the basis of these limited experimental data, it is difficult to determine the fractions of deposited radon progeny that are taken up by the epithelium and the factors that influence uptake. However, the impact of this uncertain aspect of radon progeny behavior on doses to target cells is examined in Chapter 3.
DEPOSITION MODEL
The committee used the theoretical model of aerosol transport and deposition in the lung that was developed by Egan and Nixon (1985) and updated by Egan et al. (1989) to calculate the fractions of inhaled radon progeny that are deposited in each airway generation. This model applies the same mathematical approach as that used by Taulbee and Yu (1975).
The lungs of various subjects (adults, children, and infants) are represented by the regular system of branching airways that was discussed earlier in Chapter 5. In this model, each airway branch within one generation is taken to have identical dimensions, which are a characteristic of the subject. Each point within the lung is then characterized by its axial distance, x, from the origin of the trachea. The properties of the airway generation at that level in the lung are associated with each value of x, for example, the number of branches, the branch diameter and length, and the number of alveoli. Within these model airways, the variation of aerosol concentration, c(x,t), is governed by convection at velocity u(x,t) through the airways, diffusion along the airways [represented by an effective diffusivity D(x,t)], and deposition onto the various lung surfaces, which is characterized by the deposition rate per unit length of airway, L(x,t). As described by Egan and Nixon (1985), the combined effect of these processes is represented by the following equation:
In this formalism, AA(x, t), which is represented in Equation 9-4 by the simpler term AA, is the cross-sectional area for aerosol transport. This is summed over all airways at distance x from the entrance of the trachea. In the same manner, AT(x, t) is a cross-sectional area function that allows for the
alveolar volume associated with the respiratory airways, where (AT — AA) l is the additional alveolar volume associated with an airway generation of length, l. All of these variables are functions of time, t, and distance, x.
Equation 9-4 is solved numerically for c(x, t) over several breaths, until ventilation and deposition rates reach equilibrium. The breathing process is simulated by allowing the cross-sectional areas of the alveolated airways to expand and contract about their mean values. Thus, for both AA and AT,
where A(x) is the mean cross-sectional area, and f(t) is a function of time. The function f(t) is chosen to represent the subject's breathing pattern.
The flow fields that are produced in the lung during breathing are complicated, and they induce irreversible mixing between the tidal and reserve air within the lung. Characteristics of this mixing process have been investigated experimentally by Scherer et al. (1975). These investigators represented the dispersion of tidal flow that they measured in a hollow airway cast by an axial diffusion coefficient. The diffusion coefficient was found to be higher on inhalation than on exhalation. This process has the effect of "washing in" radon progeny that are attached to small ambient particles (the so-called accumulation aerosol), to increase their rate of deposition within the lung over successive breaths (Egan and Nixon, 1987). On the basis of the analysis of Scherer and colleagues, the committee's deposition model represents the effective diffusivity of particles within the lung [denoted earlier as D(x, t) in Equation 9-4] by:
(for inspiration)
(for expiration)
where DB is the Brownian diffusion coefficient (which depends on particle size), u(x, t) is the convective flow velocity, and d is the diameter of an individual airway through which the flow is being considered (Pack et al., 1977; Egan and Nixon, 1985).
The deposition term L(x,t), or L in Equation 9-5, represents the rate at which particles are removed from the airstream per unit length of airway because of the combined effects of impaction, gravitational settling, and Brownian diffusion. The contribution to L(x, t) made by inertial impaction is modeled directly from the experimental data obtained with hollow bronchial casts of human lung (Gurman et al., 1984). Figure 9-10 shows that the efficiencies, ηI with which particles are deposited by impaction in each bronchus of a particular airway generation, i, can be approximated by an expression of the form
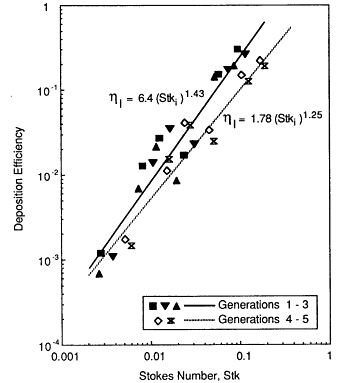
Figure 9-10 Efficiency of particle deposition by impaction measured by Gurman et al. (1984) in a hollow cast of a human trachea and five generations of bronchial airways. The symbols show the mean deposition efficiencies measured in each airway generation (1 through 5) as a function of stokes number. The stokes number was varied experimentally by changing both the particle size and the airflow rate through the cast.
where Stki is the stokes number of the flow in that airway generation, given by ![]() , where ρ is the particle density, dp is the particle diameter, μ is the fluid viscosity, and u2 and di are the mean flow velocity and airway diameter, respectively, in generation i. The values of the constants α and β are found to be significantly higher in generations 1 through 3 than they are thereafter (these fitted values are shown in Figure 9-10). The experimental data were obtained under a sinusoidally varying flow that approximates the variation of flow rate in vivo over a normal inhalation cycle (Gurman et al., 1980).
, where ρ is the particle density, dp is the particle diameter, μ is the fluid viscosity, and u2 and di are the mean flow velocity and airway diameter, respectively, in generation i. The values of the constants α and β are found to be significantly higher in generations 1 through 3 than they are thereafter (these fitted values are shown in Figure 9-10). The experimental data were obtained under a sinusoidally varying flow that approximates the variation of flow rate in vivo over a normal inhalation cycle (Gurman et al., 1980).
The bulk of deposition by gravitational sedimentation and Brownian diffusion occurs in the smaller airways, where the air velocity is low and the flow field is less complicated than that in the bronchi. It is therefore reasonable to apply theoretical treatments to represent these processes. Pich's (1972) theory
was found by Heyder and Gebhart (1977) to predict the observed deposition of particles by sedimentation in inclined circular tubes. The committee's deposition model (Egan et al., 1989) uses the theoretical expression derived by Ingham (1975) to evaluate deposition by Brownian diffusion.
The probability that an aerosol particle will be deposited on the wall of a given airway is determined by considering the interaction of the thermodynamic process of Brownian diffusion with the aerodynamic processes of gravitational sedimentation and impaction. The aerodynamic mechanisms can be treated as mutually exclusive. As discussed by Heyder et al. (1985), deposition (DE) in an aerosol filter can then be described by:
where ηI and ηG are the mean probabilities that a particle undergoing impaction or gravitational sedimentation is captured in the filter. However, in airways where aerodynamic deposition is predominantly due to sedimentation, this process is competing for the same particles as thermodynamic Brownian diffusion, so that the aerodynamic and thermodynamic processes cannot be considered to be independent of each other. Heyder et al. (1985) studied experimentally the interaction of diffusional and gravitational particle transport in various types of aerosol filters. They found empirically that the interaction can be described by:
Therefore, Equation 9-9, which has the same form as Equation 9-8, is appropriate to evaluate the effect of simultaneous deposition by thermodynamic (th) and aerodynamic (ae) processes.
An expression of the same form as Equation 9-7 is inappropriate, but this is commonly applied in aerosol deposition models. The proper treatment of thermodynamic and aerodynamic processes as competing processes (represented by Equation 9-9 gives significantly lower estimates of their combined efficiency.
There is some uncertainty in the use of a theoretical expression to evaluate the thermodynamic deposition efficiency in the complex flow fields that occur in the upper bronchial airways. Recent experimental studies of particle deposition by diffusion in hollow casts of bronchial generations 1 through 6 (taken from human lungs) have shown that Ingham's (1975) theory tends to underestimate deposition in these airways (Cohen, 1987; Cohen et al., 1990). The factor by which Ingham's expression is found to under-predict values measured by Cohen et al. (1990) is shown in Figure 9-11. In this figure the correction
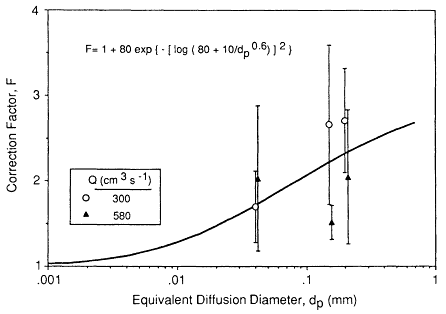
Figure 9-11 Factors by which the deposition efficiencies of sub-micron-sized particles measured by Cohen et al. (1990) for cyclic flow in hollow bronchial casts exceed the values calculated using the Ingham (1975) expression for diffusion in constant laminar flow. The symbols represent the values of the correction factor, F, averaged over airway generations 1 through 6, for each combination of mean tracheal flow rate and particle size. The error bars show ±1 standard deviation of individual values of F observed in different airway generations (there was no apparent correlation of F with airway generation number). The curve shows the variation of F with particle diffusion diameter, dp, that is derived from these data.
factors observed in airways sampled from the six generations included in the bronchial casts are averaged over all generations, since there was found to be no distinct variation with generation number. The error bars shown in the Figure 9-11 reflect a combination of variability in deposition efficiency between the sampled airways and measurement uncertainties. These studies were carded out under conditions of cyclic inspiratory flow, to simulate the in vitro variation of airflow, and at two values of the average inspiratory flow rate. The so-called correction factor, F, is found to be insensitive to the flow rate (denoted in Figure 9-11 by Q, in cm3/s). As also shown in Figure 9-11, data were obtained for particles of different sizes (of about 0.04, 0.15, and 0.2 µm in diameter. Despite this limited range of particle sizes, Cohen et al. (1990) observed a clear trend in their data which indicates that the enhancement of thermodynamic deposition efficiency decreases for smaller particles. Cohen et al., (1990) reported that the measured deposition in each airway can be approximated by an expression of the form
where ∆ = πLD/(4 Q), L is airway length, D is the particle diffusion coefficient, and Q is the flow rate through the airway. For particles in the size range of unattached radon progeny (of approximately 0.001 µm in diameter), it can be shown that Equation 9-10 converges to predict a slightly lower value of deposition efficiency than that predicted by the Ingham (1975) expression. The variation of the correction factor for thermodynamic deposition efficiency with particle size that is indicated by these experimental data is found empirically to be represented by:
This empirical function is compared in Figure 9-11 with the data of Cohen et al. (1990) from which it is derived.
The committee applied Equation 9-11 to correct values of bronchial deposition predicted by the theoretical model of Egan et al. (1989) for the enhancement effects observed by Cohen and coworkers (1990). It was found that for particles larger than those studied experimentally and at the higher flow rates that occur in exercising subjects, the effect of enhanced thermodynamic deposition is diminished by increased impaction. Figure 9-12 shows how the resulting correction factor for the combined deposition in each bronchial airway calculated by the model of Egan et al. (1989) varies with particle size and flow rate. The committee used the net correction factors shown in Figure 9-12 to evaluate bronchial deposition as a function of radon progeny aerosol size. The effect of this correction for enhanced thermodynamic deposition on calculated doses from inhaled radon progeny is examined in Chapter 4.
Figure 9-13 shows the variation in deposition efficiency with particle size that is predicted for each airway generation in the model of the adult female lung over the size range of concern for deposition of radon progeny. Figure 9-13 relates the number of particles deposited in each airway to the number that enter the trachea on inhalation. Similar patterns of deposition have been calculated for adult males and young subjects. The effect of prefiltration of particles in the nose or mouth, which is important for very small micron-sized particles, is not included in this calculation. It is considered below.
Figure 9-13 shows that the deposition efficiency of particles with diameters of 0.001 µm is calculated to be uniformly high throughout the bronchi (generations 1 to 8). By the time the inspired air reaches the bronchioles (generations 9 to 15), the number of airborne, 0.001-µm particles available for deposition is low. Therefore, deposition is found to decrease rapidly in succeeding generations of the bronchiolar airways. This very small particle diameter of 0.001 µm is within the typical size range of "unattached" radon progeny (Chapter 2). If the particle size is increased to 0.02 µm, the deposition efficiency is found
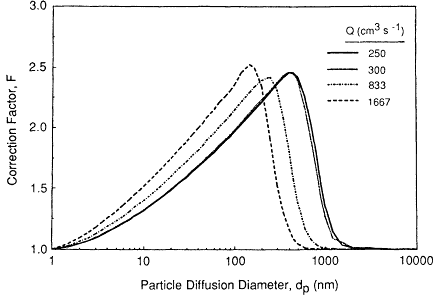
Figure 9-12 Factor to correct bronchial deposition calculated by Egan et al. (1989) for the thermodynamic deposition efficiencies measured by Cohen et al. (1990). The correction factor, F, is reduced for larger particles and higher flow rates because of competitive deposition by impaction.
to be about an order of magnitude lower in the bronchi and to reach a broad peak in the respiratory airways (generations 16 to 26). Particles of this size are typical of the radon progeny "growth" mode produced by human activities such as cooking or vacuum cleaning (Chapter 2). It is assumed that the size distributions of both the unattached and growth modes of radon progeny are not affected by the humid environment of the respiratory tract.
The bronchial deposition efficiencies of particles in the size range of attached radon progeny are found to be about two orders of magnitude lower than those of "unattached" progeny. This is shown below to lead to disproportionately large contributions to the dose from exposure to small fractions of radon progeny in the unattached state. The attached or so-called accumulation mode of the radon progeny aerosol has a median size in ambient air that ranges from about 0.15 to 0.25 µm diameter (Chapter 6). However, the carrier aerosol particles are considered to be partly hygroscopic and to grow in the respiratory tract to about double their ambient size (Chapter 6; Sinclair et al., 1974). Figure 9-13 compares the profiles of deposition throughout the lung that are calculated for particles that attain equilibrium sizes of 0.3 and 0.5 µm within the respiratory tract. It is seen that deposition is expected to be relatively independent of particle size over this limited range, at least in resting subjects.
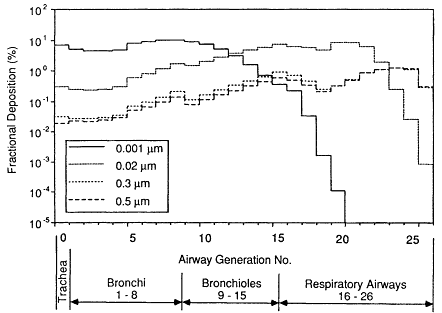
Figure 9-13 Fraction of particles entering the trachea that is calculated to deposit in each airway generation in the lung of an adult female subject. Histograms are shown for particles of 0.001, 0.02, 0.3 and 0.5 µm diameter within the respiratory tract. The calculation relates to a resting subject (ventilation rate assumed to be 0.39 m3/h).
Figure 9-14 illustrates how the deposition profiles of unattached and attached progeny are expected to be influenced by a subject's breathing rate. An increase in the breathing rate (in this case, from rest to light work) is found to decrease the efficiencies with which inhaled progeny are deposited in the bronchi. In the example considered here (the adult female), as the ventilation rate increases from 0.39 to 1.26 m3/h, the average deposition efficiencies of airway generations 1 through 8 are expected to decrease by 26% for 0.001-µm particles and by 50% for 0.3-µm particles. However, these reduced deposition efficiencies are more than offset by the increased amount of activity inhaled at the higher ventilation rate. The inhaled activity is increased by the factor 1.26/0.39 = 3.23. The net effects of variations in breathing rate and radon progeny aerosol size distributions on doses received by different subjects are examined below.
Another significant factor that must be accounted for in calculating the deposition profile of radon progeny within the respiratory tract is the typical variability or dispersion in size of the aerosol particles. Unattached radon progeny have a relatively narrow distribution of particle size, whereas the
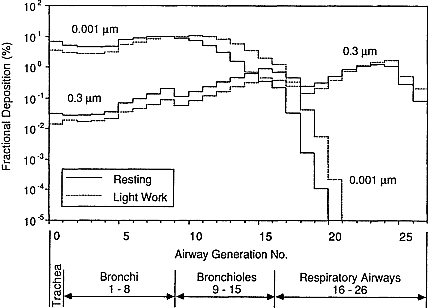
Figure 9-14 Effect of exercise on fraction of particles entering the trachea that is calculated to deposit in each airway generation. Histograms are shown for 0.001- and 0.3-µm-diameter particles, for an adult female at two levels of physical activity (resting, 0.39 m3/h, and light work, 1.26 m3/h).
activity-size distribution of progeny attached to ambient aerosol particles is typically broad (Chapter 2). Each component or mode of the radon progeny aerosol is assumed to be represented by a log normal distribution of activity with particle size, in which the geometric standard deviation (denoted by σg) is related to the activity median diameter (denoted by AMD) by
This function is illustrated in Figure 9-15.
NASAL AND ORAL FILTRATION
In evaluating dose to the lung from exposure to radon progeny, it has been customary to assume that the nose filters out 50 to 60% of the unattached progeny from the inhaled air (Jacobi and Eisfeld, 1980; Harley and Pasternack, 1982; NEA, 1983; James, 1984, 1988; NCRP, 1984). Likewise, it has generally been assumed for attached progeny that the filtration efficiency of the nose is either zero or, at most, a few percent. These assumptions were based on the experimental data obtained by George and Breslin (1969) in several
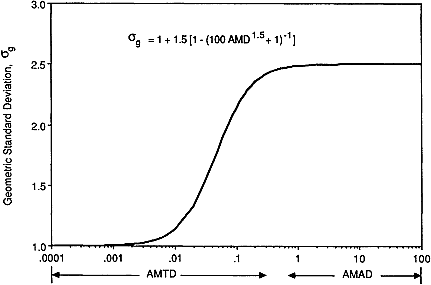
Figure 9-15 Variation of geometric standard deviation of particle size with activity median diameter of radon progeny aerosols that is assumed in calculations of deposition in the respiratory tract.
volunteer subjects. These investigators drew freshly formed 218Po in through each subject's nose and out through a mouthpiece at various flow rates. They measured the fractional penetration of the unattached 218Po activity through the nasal passages and oral cavity. Penetration of radon progeny attached to condensation nuclei was measured in the same way. Their experimental data are reproduced in Figure 9-16.
More recently, hollow casts of the human nasal and oral passages have been used to study the mechanisms of particle deposition, in particular, the influence of flow rate and particle diffusion coefficient on the deposition of particles in the size range of 0.2 to 0.005 µm in diameter (Cheng et al., 1988, 1990). For these particles, the deposition efficiency, E, of both the nasal and oral passages was found to be represented by an empirical expression of the form
where k is a constant, Q is the flow rate (in liters/rain), and D is the particle diffusion coefficient (in cm2/s). Using postmortem casts, Cheng et al. (1988, 1990) found similar deposition efficiencies for the nasal and the oral pathways through to the trachea and, thus, similar values of the constant k in Equation 9-13.
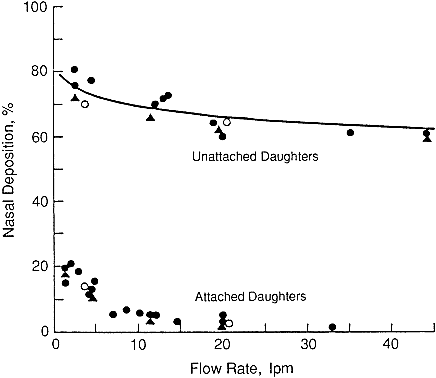
Figure 9-16 Nasal deposition of unattached and attached radon progeny measured by George and Breslin (1969) in several human subjects.
On extending this work to include two casts made with magnetic resonance imaging (MRI) data of the nasal and oral passages obtained in vivo and also to include smaller particles (unattached 212Pb with a measured diffusion coefficient of 0.02 ± 0.004 cm2/s, corresponding to a 0.0018-μm particle diameter), Cheng et al. (1989) found that it was necessary to modify the exponent of the particle diffusion coefficient in Equation 9-13 from 2/3 to 1/2 to fit the measured deposition efficiencies. Thus, their revised expression is:
The data of Cheng et al. (1989) for inspiration of particles through the nasal passages are shown in Figure 9-17, together with the fitted efficiency function given by k' = 13.2. Figure 9-16 also shows the deposition efficiencies measured by Strong and Swift (in press) in the same nasal casts. Strong and Swift used unattached 218Po for which the measured diffusion coefficient was found to vary between 0.05 cm2/s for freshly formed 218Po to 0.02 cm2/s after
aging. Figure 9-17 shows that the deposition efficiencies measured by Strong and Swift, represented by k' = 7.7, are consistently lower than those obtained by Cheng et al. (1989), which are represented by k' = 13.2. The difference in nasal deposition efficiencies measured by Cheng et al. (1989), although relatively small, is significant because it implies approximately a factor of two uncertainty in the fractions of inhaled unattached progeny that are able to penetrate the nose to be deposited in the bronchi. The impact of this uncertainty on the respective predictions of nasal penetration efficiency for unattached 218Po is illustrated in Figure 9-18. The data of George and Breslin (1969) are replotted in Figure 9-18 and compared with the penetration efficiencies predicted as a function of flow rate by Strong and Swift (1990) and Cheng et al. (1989) for an assumed value of the diffusion coefficient of unattached 218Po. Unfortunately, George and Breslin were not able to measure the diffusion coefficient of unattached 218Po at the time of their experiments. For this comparison, it is assumed that the most likely value was 0.035 cm2/s (E. O. Knutson, Environmental Measurements Laboratory, New York, personal communication, 1990). The diffusion coefficient implied by the data of Cheng et al. (1989) is 0.015 cm2/s, which is probably too low.
The committee concluded from these data that the nasal penetration efficiency for unattached radon progeny is uncertain by about a factor of two, but that the data of George and Breslin (1969) still provide the best estimate. The impact of this uncertainty on estimates of lung dose in mine and home environments was examined elsewhere in this report.*
To evaluate lung dose in subject's who breathe habitually through their mouth, it has been customary to assume that the filtration efficiency of the oral passageway for unattached radon progeny is negligibly low (NCRP, 1984). However, the recent studies with hollow casts of these airways indicate that oral filtration is substantial. In Figure 9-19, the oral filtration efficiencies measured by Cheng et al. (1989) are compared with their values for the nasal passages. It is seen that oral filtration is approximately 75% of the values for nasal filtration. A similar ratio of oral:nasal efficiencies was observed by Strong and Swift (1990), although the absolute values were lower, as noted above. However,
|
* |
After the committee completed its work, further experimental studies of the penetration of unattached radon progeny through hollow casts of the human nasal passages, pharynx, and larynx were carded out by J. C. Strong and D. L. Swift (at the Biomedical Research Laboratory, AEA Technology, Harwell, England) and also by P. K. Hopke and D. L. Swift (at Clarkson University, Potsdam, New York). These researchers studied two casts that had been reconstructed from MRI scans of subjects in vivo. One of the casts, for an adult male, had previously been studied by Cheng et al. (1989). The second was taken from a 1.5-year-old infant. The results obtained for both casts were found to support Cheng et al.'s experimental observations. This emerging congruence of experimental data now indicates that George and Breslin's (1969) study of human subjects may not in fact provide the best estimate of nasal penetration efficiency. Further study of this possibility is recommended in the Summary and Recommendations. Tables 3-4 and 9-6 include data for both values of nasal deposition. |
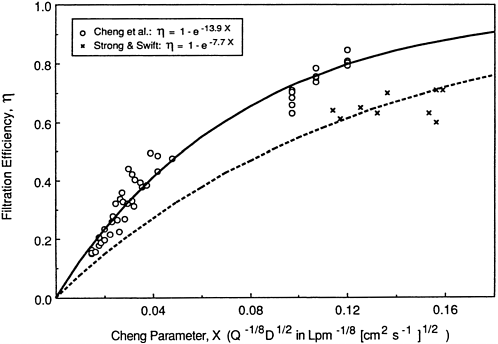
Figure 9-17 Filtration efficiencies for submicron particles measured in hollow casts of the human nasal passages. The data are plotted against Cheng et al.'s (1989) turbulent diffusion parameter, X. Cheng et al.'s data for particles in the size range 0.2 µm to 0.05 µm diameter (X ≤ 0.05), and also their data for 0.0018-µm-diameter particles of unattached 212Pb (0.10 ≤ X ≤ 0.12), are compared with Strong and Swift's (1990) data for unattached 218Po. The curves show the respective efficiency functions fitted to these data.
in view of the preliminary nature of these data and the lack of confirmatory evidence in vivo, the committee took a more conservative approach by assuming that the filtration efficiency of the oral passageway for unattached radon progeny is only 50% of the value for nasal filtration efficiency.
It has been shown that so-called mouth breathers normally inhale partly through the nose (Niinimaa et al., 1980, 1981). Therefore, for the purpose of evaluating dose to the lung from exposure to radon progeny, it is unrealistic to consider the example of a pure mouth breather. On the other hand, at a sufficiently high rate of ventilation in response to heavy work, referred to as the ''switch point,'' a normal nose breather will augment nasal flow by breathing partly through the mouth. The typical pattern of change between nasal and oral breathing found in adult subjects by Niinimaa et al. (1980, 1981) is shown in Figure 9-20. The proportion of the total airflow inhaled nasally by so-called mouth breathers is typically found to decrease from a major fraction at rest to a minor fraction for heavy work. Normal nose breathers were found to switch from 100% nasal breathing to partial mouth breathing at a total respiratory ventilation rate (VE) of about 2.1 m3/h. As discussed above, the committee
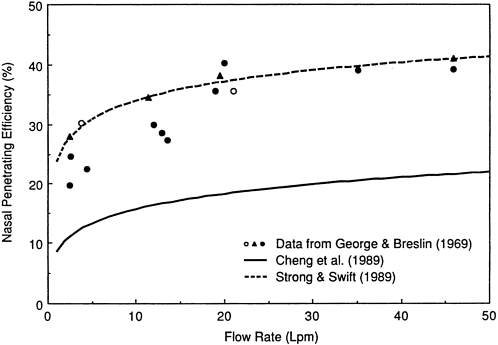
Figure 9-18 Comparison of nasal penetration efficiency of unattached 218Po measured in human subjects by George and Breslin (1969) with values predicted by Strong and Swift (1990) and Cheng et al. (1989) on the basis of an assumed particle diffusion coefficient.
assumed that the deposition efficiencies of the nose and mouth for radon progeny particles in the thermodynamic size range are given by Equation 9-14, with k' = 7.7 and k' = 3.9, respectively. This expression is assumed to apply for an adult male subject. In order to scale the nasal and oral deposition efficiencies for other subjects (adult females, children, and infants), the committee adopted the procedure described by Swift (1989). It is assumed for scaling purposes that the flow rate Q in Equation 9-14 can be replaced by the dimensionless Reynolds number Re (Cheng et al., 1988):
where L is a hydraulic diameter (in cm), V is the fluid velocity (in cm/s), and v is the kinematic fluid viscosity (in cm2/s). Substituting the volumetric flow rate Q for V in Equation 9-15 gives
Therefore, to scale nasal and oral deposition efficiency for body size, the factor to be applied to Q in Equation 9-14 is Lref/Ls, where Lref is a characteristic airway dimension for the reference adult male and Ls is the
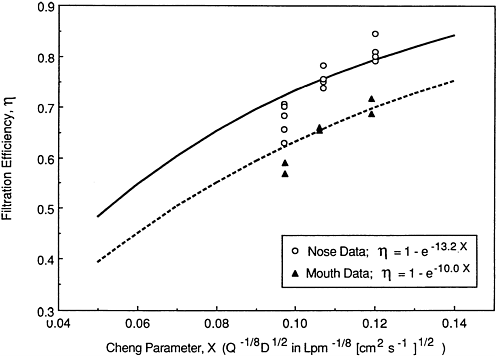
Figure 9-19 Comparative filtration efficiencies of the nasal and oral passages for unattached 212Pb measured by Cheng et al. (1989). The measurements were made in hollow casts constructed using in vivo MRI data.
corresponding dimension for the subject considered. Yu and Xu (1987) and Swift (1989) considered that the diameter of the trachea provides an adequate index dimension. The values of tracheal diameter assumed by the committee to represent adults, children, and infants were shown in Figure 9-7.
Finally, it is also necessary to consider the efficiencies of the nose and mouth for removing that part of the radon progeny aerosol spectrum that overlaps the aerodynamic size range. The experimental data on nasal deposition of particles in the size range from about 1 to 10 µm in aerodynamic diameter in human subjects are shown in Figure 9-21 (Stahlhofen et al., 1989). Rudolf et al. (1986) represented these data on the aerodynamic deposition efficiency of the nose, ηae (nose), by
The aerodynamic deposition efficiency of the oral cavity and oropharynx is found to be lower than that of the nose. The bulk of deposition in the oral passageway during mouth breathing is considered to occur in the larynx (Rudolf et al., 1986). Rudolf and colleagues represented the experimental data on oral deposition in adult male subjects by:
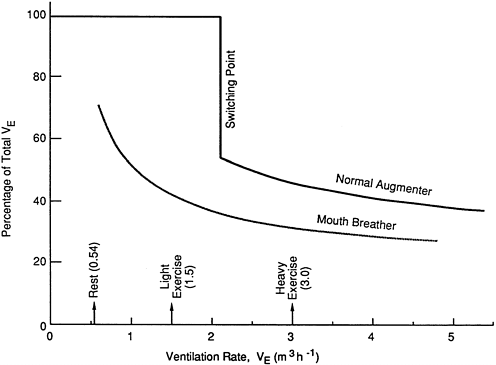
Figure 9-20 Percentage of total ventilatory airflow passing through the nasal route in "normal augmenters" and in "mouth breathers." The average ventilation rate, VE, at which "normal augmenters" switch from 100% nasal breathing to partial breathing through the mouth is shown at VE = 2.1 m3/h.
In order to scale these nasal and oral aerodynamic deposition efficiencies for body size, Equations 9-17 and 9-18 can be rewritten in terms of the stokes inertial parameter (Stk) (defined earlier in this chapter), where
where V is air velocity (in cm/s), h is dynamic air viscosity (in g cm-2 s-1), and L is characteristic airway dimension (in cm).
For a given aerodynamic particle size, the inertial parameter depends on the ratio V/L, and thus on the ratio Q/L3. The factor 1/L3 can therefore be applied to Q to scale inertial effects in the nasal or oral passages for airway dimensions. As assumed above to scale thermodynamic deposition efficiencies, the committee adopted the diameter of the trachea as the reference airway dimension (Yu and Xu, 1987; Swift, 1989).
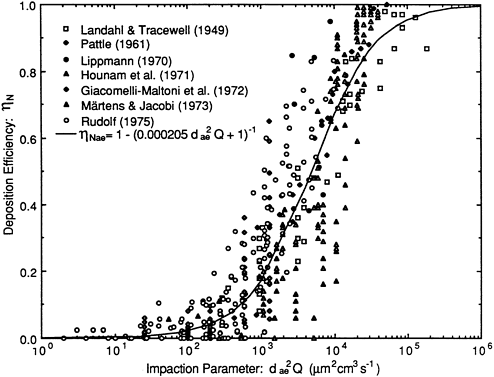
Figure 9-21 Data on deposition efficiency of the human nose for particles with aerodynamic diameter greater than 1 µm. The solid curve shows a hyperbolic approximation to the median values fitted as a function of the inertial parameter ![]() Q.
Q.
The expressions given above have been used to evaluate nasal and oral filtration of radon progeny aerosols inhaled by adults, children, and infants in all calculations of doses to the lung. The thermodynamic and aerodynamic filtration efficiencies are combined as described earlier (Heyder et al., 1985).
Definition of Exposure and Reference Breathing Rates
In the final part of this chapter, the results of combining all aspects of the panel's dosimetric model for radon and thoron progeny that have been described above are presented. This is done to evaluate the conversion coefficient between "exposure to potential α-energy" and the "dose" received by the tissues of the respiratory tract that are deemed sensitive to bronchogenic cancer. The exposure-dose conversion coefficient is examined as a function of the particular conditions of exposure and determined by the radon progeny aerosol size and characteristics of the exposed subject. Exposures to potential α-energy are expressed here in the familiar unit working level month (WLM), where one working level (WL) is any combination of short-lived radon (or thoron) progeny in 1 liter of air that will result in the emission of 1.3 × 105 MeV (2.08 × 10-8
J) of potential α-energy, and 1 WLM corresponds to an exposure for 1 working month (of 170 hours) to an airborne concentration of 1 WL. Thus, in terms of physical quantities (SI units), 1 WLM of exposure simply corresponds to 2.08 × 10-5 (J m-3) × 170 (h) = 3.5 × 10-3 J h m-3.
Contributions of Individual Radon Progeny to the WLM
The amounts (in Bq) of each individual radon progeny that is inhaled by a subject whose exposure to potential α-energy is 3.5 × 10-3 J h m-3 (1 WLM) are related to the degree of radioactive equilibrium between the progeny, and to the subject's breathing rate. If the breathing rate is denoted by B (in m3 h-1) and the activity-concentration ratios of 218Po, 214Pb, and 214Bi/214Po with respect to radon gas by FRaA, FRaB, and FRaC, the activities of each progeny inhaled are given by:
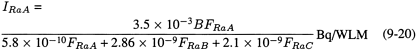
where 5.8 × 10-10 is the potential α-energy (in joule) associated with 1 Bq of the α-emitting progeny 218Po (RaA), 28.6 × 10-10 is the potential α-energy (J) associated with 1 Bq of the β-emitting progeny 214Pb (RaB), and 21.0 × 10-10 is the potential α-energy (J) associated with 1 Bq of the β-emitting progeny 214Bi (RaC).
The dosimetric model is solved for intake of a given mixture of activities. To represent the activity-intake from 1 WLM exposure to the "unattached" fraction of radon progeny, it is assumed (Reineking and Porstendörfer, 1990) that:
This mixture of 218Po and 214Bi activities is assumed below to calculate dose conversion coefficients for unit exposure to unattached radon progeny (1 WLM) in the size range 0.0006 µm (0.6 nm) to 0.01 µm (10 nm) activity median thermodynamic diameter (denoted by AMTD).
To represent the activity intake from unit exposure to the "attached" fraction of radon progeny, it is assumed that:

Exposure-dose conversion coefficients are derived below as functions of the attached radon progeny aerosol size over the continuous range of size that may extend from 0.01 µm (10 nm) to 1 µm (1000 nm) AMTD.
In practice, the activity-concentration ratios of 218Po, 214Pb, and 214Bi/214Po will vary with the particular environmental conditions of exposure. However, it can be shown that doses calculated in terms of unit exposure to potential α-energy are insensitive to the actual ratios of the progeny concentrations (Jacobi and Eisfeld, 1980; James, 1988) for both "unattached" and "attached" radon progeny.
Contributions of Individual Thoron Progeny to the WLM
In an analagous manner to Equations 9-20 through 9-22 for radon progeny, the activities of each of the thoron progeny that are inhaled per WLM exposure are given by:
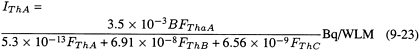
where 5.3 × 10-13 is the potential α-energy (in joule) associated with 1 Bq of the α-emitting progeny 216Po (ThA), 6.91 × 10-8 is the potential α-energy (J) associated with 1 Bq of the β-emitting progeny 212Pb (ThB), and 6.56 × 10-9 is the potential α-energy (J) associated with 1 Bq of the α-emitting progeny 212Bi (ThC).
Since the number of joule per Bq intake of 216Po is five orders of magnitude lower than the corresponding values for 212Pb and 212Bi, exposure to 216Po does not contribute significantly to calculated doses. Therefore, to represent the activity intake from 1 WLM exposure to the "unattached" fraction of thoron progeny, it is assumed that:

To represent the activity-intake from 1 WLM exposure to the "attached" fraction of thoron progeny, it is assumed that:
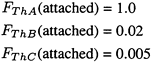
Reference Breathing Rates
To estimate the intakes of radon (or thoron) progeny activity by men, women, children, and infants for unit exposure to potential α-energy at various levels of physical exertion, the committee has assumed the breathing rates given in Table 9-5. These values were derived by Roy and Courtay (1990) from a review of Godfrey et al. (1971), Godfrey (1973), ICRP (1975), Taussig et al. (1977), Gaultier (1978), Cotes (1979), Gaultier et al. (1981), Scherrer (1981), Flandrois et al. (1982), Cooper and Weiler-Ravell (1984), and Zapletal (1987). The associated values of tidal volume (in cm3) and respiratory frequency (in min-1) for each subject are also given in Table 9-5 (Roy and Courtay, 1990), together with the fractions of the inspired airflow that are assumed to pass through the nose and the mouth in "normal" nose breathers and in subjects who habitually breath through the "mouth" (Niinimaa et al., 1980, 1981). These values have been substituted in the deposition and nasal/oral filtration models (described above) to calculate the amounts of radon (or thoron) progeny deposited in each airway generation, as a function of aerosol size and breathing rate, for each subject.
Exposure-Dose Conversion Coefficients
In this final section, the dependence of the radon progeny exposure-dose conversion coefficient on influencing factors such as aerosol size, breathing rate, the assumed clearance behavior of the progeny, the choice of target cell population for which dose is evaluated, the presence of airway disease, and the gender or age of the exposed subject are examined. In each case, the dose received by the nuclei of target cells is evaluated as a continuous function of the assumed size of radon progeny on entry into the respiratory tract. The calculations relate to the equilibrium size attained by inhaled radon progeny under physiological conditions, where the air is saturated with water vapor. As discussed above, the ambient aerosol particles to which radon progeny become attached are assumed to be unstable in saturated air and to grow rapidly in the nose or pharyngeal airways to double their ambient size.
The curves presented below give values of dose (as the ordinate) for unit exposure (1 WLM or 3.5 J h m-3) of each subject to the particular
TABLE 9-5 Summary of the Respiratory Data Assumed by the Panel to Calculate Exposure-Dose Conversion Coefficients for Various Subjects Exposed to Radon and Thoron Progeny
|
|
Subject |
||||||
|
Level of Exertion/ Respiratory Parameter |
Man |
Woman |
Child (10-yr) |
Child (5-yr) |
Infant (1-yr) |
Infant (1-mo) |
|
|
FRC |
(cm3) |
3,300 |
2,660 |
1,500 |
770 |
240 |
110 |
|
Sleep: F(normal) = 1.0, F(mouth) = 0.7 |
|||||||
|
VE |
(m3 h-1) |
0.45 |
0.32 |
0.31 |
0.24 |
0.153 |
0.079 |
|
f |
(min-1) |
12 |
12 |
17 |
21 |
34 |
40 |
|
Vt |
(cm3) |
625 |
450 |
305 |
190 |
75 |
33 |
|
Rest: F(normal) = 1.0, F(mouth) = 0.7 |
|||||||
|
VE |
(m3 h-1) |
0.54 |
0.39 |
0.38 |
0.32 |
0.22 |
— |
|
f |
(min-1) |
12 |
14 |
19 |
25 |
36 |
— |
|
Vt |
(cm3) |
750 |
460 |
330 |
210 |
100 |
— |
|
Light Exercise: F(normal 0) = 1.0, F(mouth) = 0.4 |
|||||||
|
VE |
(m3 h-1) |
1.5 |
1.26 |
1.11 |
0.57 |
0.35 |
0.12 |
|
f |
(min-1) |
20 |
21 |
32 |
39 |
46 |
50 |
|
Vt |
(cm3) |
1,250 |
1,000 |
580 |
245 |
125 |
39 |
|
Heavy Exercise: F(normal) = 0.47, F(mouth) = 0.3 |
|||||||
|
VE |
(m3 h-1) |
3 |
2.7 |
2.1 |
— |
— |
— |
|
f |
(min-1) |
26 |
28 |
45 |
— |
— |
— |
|
Vt |
|
(cm3) |
|
1,920 |
1,610 |
760 |
— |
radon progeny aerosol size shown on the abscissa. To enable quantitative comparison and application of the dose conversion coefficients evaluated for various conditions, these values are also presented in Table 9-6, which includes the two values of nasal deposition efficiency for the smallest particles based on findings of George and Breslin (1969) and Cheng et al. (1989).
Influence of Aerosol Size, Clearance Behavior, and Target Cells
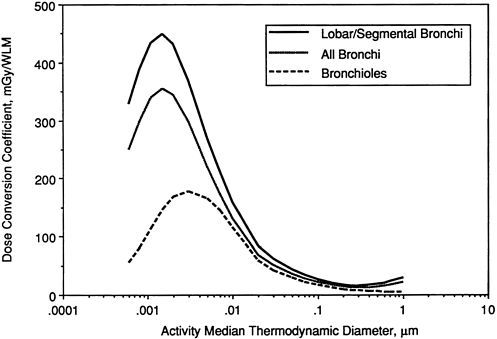
The calculated dependence on radon progeny aerosol size of doses received by the nuclei of various target cells in an adult male is examined in Figures 9-22 through 9-24. These figures relate to a man undergoing light exercise, who is assumed to breathe through his nose. The three curves shown in Figure 9-22 show doses calculated for secretory cell nuclei in different regions of the respiratory tract, on the assumption that radon progeny, once deposited, remain in mucus and are cleared continuously toward the throat. The highest values are obtained if the dose is averaged over secretory cell nuclei in just the lobar and segmental bronchi (generations 2 through 5 in the lung model). Intermediate values are obtained when doses are averaged for the larger population of
TABLE 9-6 Summary of Coefficients to Convert Exposure to Radon Progeny Potential Alpha-Energy into the Averagea Dose to Basal Cell Nuclei in the Bronchi of Different Subjects (Normal Nasal Breathers) as a Function of the Radon Progeny Aerosol Size and the Subject's Level of Physical Exertion
|
Subject |
Radon Progeny AMTD (µm) With Assumed Nasal Deposition |
Exposure-Dose Conversion Coefficient (mGy per WLM) for the Following Level of Physical Exertion |
|||
|
|
|
Sleep |
Rest |
Light Exercise |
Heavy Exercise |
|
Adult Male |
0.0011b |
48.9 |
59.6 |
153.0 |
321.2 |
|
|
0.0011c |
23.4 |
28.9 |
80.9 |
210.7 |
|
|
0.02 |
18.3 |
20.0 |
31.5 |
41.6 |
|
|
0.15 |
4.66 |
5.04 |
7.86 |
11.8 |
|
|
0.25 |
3.35 |
3.64 |
6.31 |
14.9 |
|
|
0.3 |
3.03 |
3.31 |
6.22 |
18.4 |
|
|
0.5 |
2.63 |
2.93 |
7.51 |
38.6 |
|
Adult Female |
0.0011b |
39.9 |
48.8 |
152.5 |
340.9 |
|
|
0.0011c |
18.6 |
23.1 |
80.0 |
223.2 |
|
|
0.02 |
19.1 |
21.4 |
36.2 |
49.2 |
|
|
0.15 |
4.66 |
5.17 |
8.62 |
13.4 |
|
|
0.25 |
3.29 |
3.64 |
6.75 |
17.1 |
|
|
0.3 |
2.95 |
3.27 |
6.59 |
21.1 |
|
|
0.5 |
2.48 |
2.77 |
7.77 |
44.4 |
|
Child age 10 yr |
0.0011b |
48.8 |
60.2 |
166.5 |
— |
|
|
0.0011c |
23.0 |
28.8 |
87.5 |
— |
|
|
0.02 |
22.3 |
24.9 |
40.1 |
— |
|
|
0.15 |
5.38 |
5.98 |
9.58 |
— |
|
|
0.25 |
3.81 |
4.25 |
7.61 |
— |
|
|
0.3 |
3.43 |
3.84 |
7.47 |
— |
|
|
0.5 |
2.89 |
3.30 |
8.80 |
— |
|
Child age 5 yr |
0.0011b |
55.7 |
74.9 |
129.4 |
— |
|
|
0.0011c |
26.1 |
36.0 |
65.6 |
— |
|
|
0.02 |
25.8 |
29.7 |
38.2 |
— |
|
|
0.15 |
6.04 |
6.98 |
8.94 |
— |
|
|
0.25 |
4.34 |
5.05 |
6.66 |
— |
|
|
0.3 |
3.93 |
4.61 |
6.26 |
— |
|
|
0.5 |
3.33 |
4.08 |
6.42 |
— |
|
Infant age 1 yr |
0.0011b |
63.8 |
94.3 |
148.6 |
— |
|
|
0.0011c |
29.6 |
45.2 |
74.3 |
— |
|
|
0.02 |
33.2 |
39.6 |
48.2 |
— |
|
|
0.15 |
7.77 |
9.06 |
10.9 |
— |
|
|
0.25 |
5.56 |
6.65 |
8.19 |
— |
|
|
0.3 |
5.02 |
6.11 |
7.69 |
— |
|
|
0.5 |
4.17 |
5.47 |
7.67 |
— |
|
Infant age 1 mo |
0.0011b |
50.1 |
— |
78.8 |
— |
|
|
0.0011c |
22.4 |
— |
36.7 |
— |
|
|
0.02 |
36.8 |
— |
45.9 |
— |
|
|
0.15 |
9.02 |
— |
10.9 |
— |
|
|
0.25 |
6.25 |
— |
7.35 |
— |
|
|
0.3 |
5.54 |
— |
6.47 |
— |
|
|
0.5 |
4.25 |
— |
5.01 |
— |
|
a Average value of the exposure-dose conversion coefficient calculated on the alternative assumptions that radon progeny are i) insoluble or ii) partially soluble in mucus. b Nasal deposition of unattached progeny according to George and Breslin (1969). c Nasal deposition of unattached progeny according to Cheng et al. (1989). |
|||||
secretory cells that occurs throughout the bronchi (generations 1 through 8 in the lung model). The reference doses are approximately twofold lower if they are averaged for secretory cells in the bronchioles (generations 9 through 15 in the lung model).
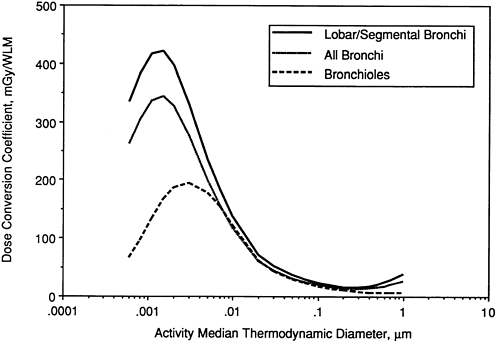
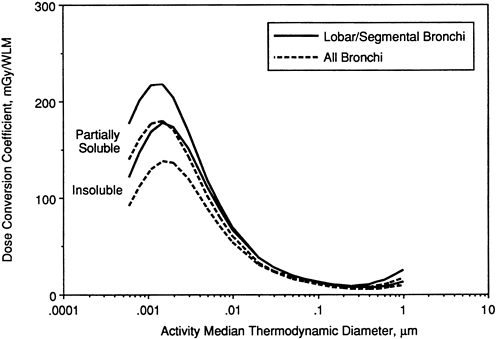
It is noted that, for targets in both "lobar/segmental bronchi" and "all bronchi," the dose per unit exposure is approximately 25-fold higher for unattached progeny (with AMTD ≈ 0.001 µm) than it is for attached progeny with equilibrium AMTD in the range 0.3 to 0.5 µm. This ratio is somewhat lower (at approximately 20-fold) for targets in the bronchioles. For ultrafine radon progeny aerosols (AMTD < 0.01 µm), the dose per unit exposure is strongly influenced by the assumed nasal filtration efficiency. Figure 9-23 shows the equivalent exposure-dose conversion coefficients that are calculated if the radon progeny are assumed to be partially (30%) taken up by epithelial tissue at the site of deposition. Comparison with Figure 9-22 shows that this degree of uncertainty in the clearance behavior of radon progeny has a small effect on doses calculated for secretory cell targets.
However, if basal cells are instead assumed to be the principal targets, significantly different values of the exposure-dose conversion coefficient are calculated (Figure 9-24). In this case, dose conversion coefficients are approximately twofold lower for ultrafine radon progeny aerosols than they are for secretory cells, and uncertainty in the clearance behavior of radon progeny has a greater impact.
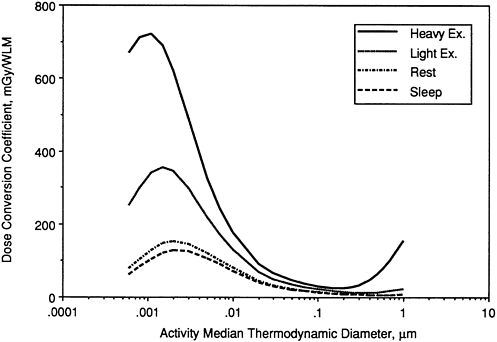
Influence of Exercise
Figure 9-25 shows exposure-dose conversion coefficients calculated for secretory cell nuclei throughout the bronchi for a man at various levels of physical exertion. It is seen that the calculated doses increase markedly with exercise for both unattached progeny and for large attached aerosols (with AMTD ≈ 0.5 µm) . These findings are significant for the evaluation of doses for exposure of underground miners.
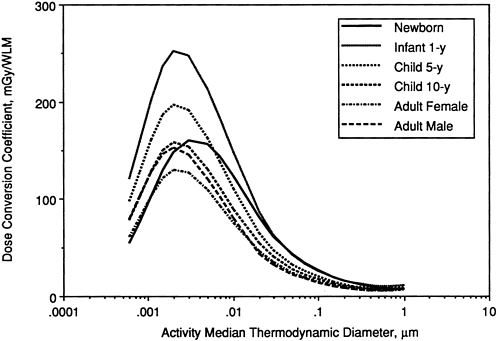
Influence of Age and Gender
Figure 9-26 shows exposure-dose conversion coefficients calculated for a man, a woman, and children and infants (of either sex) of different age. In this case, the subjects are taken to be resting, and the target cells are taken to be secretory cells throughout the bronchi. It is seen that dose conversion coefficients are calculated to be somewhat lower for a woman than for a man, whereas they are generally higher in children and in the 1-year-old infant.
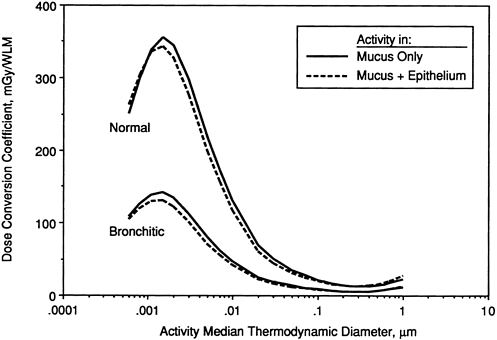
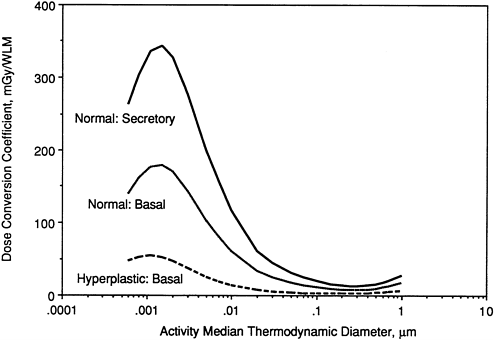
Influence of Airway Disease
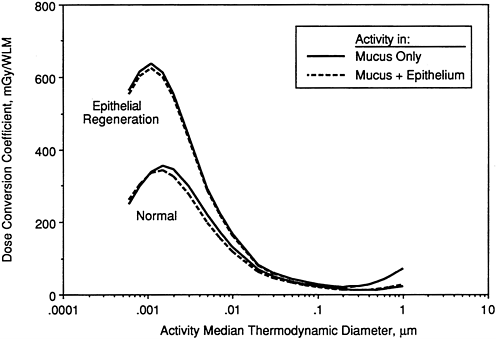
Figures 9-27 through 9-29 illustrate the effects of modeling various disease conditions on the calculated exposure-dose conversion coefficient. In the case of a man with bronchitis (Figure 9-27), the effect of thickened mucus is to reduce the calculated doses by about a factor two. Markedly lower doses are also calculated for target cells in areas of hyperplastic epithelium. Figure 9-28 compares doses calculated for basal cells in hyperplastic epithelium (where no secretory cells are present) with those calculated for both secretory and basal cell targets in normal epithelium. Finally, Figure 9-29 compares doses calculated for secretory cells in thinned epithelium that is undergoing regeneration with those in epithelium of normal thickness. In this case, target cells in the damaged epithelium are calculated to receive about twofold higher doses than those in normal epithelium.
REFERENCES
Armstrong, T. W., and K. C. Chandler. 1973. SPAR, a FORTRAN Program for Computing Stopping Powers and Ranges for Muons, Charged Pions, Protons and Heavy Ions. ORNL-4869. Oak Ridge, Tenn.: Oak Ridge National Laboratory .
Bianco, A., F. R. Gibb, and P. E. Morrow. 1974. Inhalation study of a submicron size lead-212 aerosol. Pp. 1214-1219 in Proceedings of the 3rd International Congress of the International Radiological Protection Association. CONF-730907. Washington, D.C.: U.S. Atomic Energy Commission.
Booker, D. V., A. C. Chamberlain, D. Newton, and A. N. B. Stott. 1969. Uptake of radioactive lead following inhalation and injection. Br. J. Radiol. 42:457-466.
Cheng, Y. S., Y. Yamada, H. C. Yeh, and D. L. Swift. 1988. Diffusional deposition of ultrafine aerosols in a human nasal cast. J. Aerosol Sci. 19:741-752.
Cheng, Y. S., D. L. Swift, Y. F. Su, and H. C. Yeh. 1989. Deposition of radon progeny in human head airways. Proceedings of the DOE Technical Exchange Meeting on Assessing Indoor Radon Health Risks, September 18-19, 1989, Grand Junction, Colo. Department of Energy CONF 8909190. Springfield, Va.: National Technical Information Service.
Cheng, Y. S., Y. Yamada, H. C. Yeh, and D. L. Swift. 1990. Deposition of ultrafine aerosols in a human oral cast. Aerosol Sci. Technol. 12:1075-1081.
Cohen, B. S. 1987. Deposition of ultrafine particles in the human tracheobronchial tree: A determinant of dose from radon daughters. Pp. 475-486 in Radon and Its Decay Products: Occurrence, Properties, and Health Effects, P. K. Hopke, ed. Washington, D.C.: American Chemical Society.
Cohen, B. S., R. G. Sussman, and M. Lippmann. 1990. Ultrafine particle deposition in a human tracheobronchial cast. Aerosol Sci. Technol. 12:1082-1091.
Cook, C. D., R. B. Cherry, D. O'Brien, P. Karlberg, and C. A. Smith. 1955. Studies of respiratory physiology in the newborn infant. I. Observations on normal premature and full-term infants. J. Clin. Invest. 34:975-982.
Cooper, D. M., and D. Weiler-Ravell. 1984. Gas exchange response to exercise in children. Am. Rev. Respir. Dis. 129(Suppl.):S47-S48.
Cotes, J. E. 1979. Lung function assessment and application in medicine. Oxford: Blackwell Scientific Publications.
Cuddihy, R. G., and H. C. Yeh. 1988. Respiratory tract clearance of particles and substances dissociated from particles. Pp. 169-193 in Inhalation Toxicology: The Design and Interpretation of Inhalation Studies and Their Use in Risk Assessment, U. Mohr, ed. New York: Springer-Verlag.
Egan, M. J., and W. Nixon. 1985. A model of aerosol deposition in the lung for use in inhalation dose assessments. Radiat. Prot. Dosim. 11:5-17.
Egan, M. J., and W. Nixon. 1987. Mathematical modelling of fine particle deposition in the respiratory system. Pp. 34-40 in Deposition and Clearance of Aerosols in the Human Respiratory Tract, W. Hofmann, ed. Vienna: Facultas Universitätsverlag.
Egan, M. J., W. Nixon, N. I. Robinson, A. C. James, and R. F. Phalen. 1989. Inhaled aerosol transport and deposition calculations for the ICRP task group. J. Aerosol Sci. 20:1301-1304.
Flandrois, R., H. Grandmontagne, M. H. Mayet, R. Favier, et al. 1982. La consommation d'oxygene maximale chez le jeune français, sa variation avec l'âge et le sexe et l'entranement. J. Physiol. (Paris) 78:186-194.
Gaultier, C. 1978. Développement des volumes pulmonaires pendant les 36 premier mois de la vie chez l'homme. Doctoral thesis in human biology research. Paris: CHU St. Antoine.
Gaultier, C., L. Perret, N. Boule, A. Buvry, and F. Girard. 1981. Occlusion pressure and breathing pattern in healthy children. Respir. Physiol. 46:71-80.
Gehr, P. 1987. Inhalation pathways in relation to infants and children. Pp. 67-78 in Age-Related Factors in Radionuclide Metabolism and Dosimetry, G. B. Gerber, H. Métivier, and H. Smith, eds. Hingham, Maine: Kluwer Academic Publishers.
George, A. C., and A. J. Breslin. 1969. Deposition of radon daughters in humans exposed to uranium mine atmospheres. Health Phys. 17:115-124.
Godfrey, S. 1973. Exercise Testing in Children. London: Saunders.
Godfrey, S., C. T. M. Davies, E. Wozniak, and C. A. Barnes. 1971. Cardio-respiratory response to exercise in normal children. Clin. Sci. 40:419-431.
Greenhalgh, J. R., A. Birchall, A. C. James, H. Smith, and A. Hodgson. 1982. Differential retention of 212Pb ions and insoluble particles in nasal mucosa of the rat. Phys. Med. Biol. 27:837-851.
Gurman, J. L., R. B. Schlesinger, and M. Lippmann. 1980. A variable-opening mechanical larynx for use in aerosol deposition studies. Am. Ind. Hyg. Assoc. J. 41:678-680.
Gurman, J. L., M. Lippmann, and R. B. Schlesinger. 1984. Particle deposition in replicate casts of the human upper tracheobronchial tree under constant and cyclic inspiratory flow. I. Experimental. Aerosol Sci. Technol. 3:245-252.
Hansen, J. E., E. P. Ampaya, G. H. Bryant, and J. J. Navin. 1975. Branching pattern of airways and air spaces of a single human terminal bronchiole. J. Appl. Physiol. 38:983-986.
Harley, N.H. 1971. Spatial distribution of radon daughter and plutonium-239 alpha lung dose based on experimental energy absorption measurements. Ph.D. thesis. New York University, New York.
Harley, N.H., and B. S. Pastemack. 1982. Environmental radon daughter alpha dose factors in a five-lobed human lung. Health Phys. 42:789-799.
Hart, M. D., M. M. Orzalesi, and C. D. Cook. 1963. Relation between anatomic respiratory dead space and body size and lung volume. J. Appl. Physiol. 18:519-522.
Heyder, J., and J. Gebhart. 1977. Gravitational deposition of particles from laminar aerosol flow through inclined circular tubes. Aerosol Sci. 8:289-295.
Heyder, J., J. Gebhart, and G. Scheuch. 1985. Interaction of diffusional and gravitational particle transport in aerosols. Aerosol Sci. Technol. 4:315-326.
Hofmann, W. 1982. Dose calculations for the respiratory tract from inhaled natural radioactive nuclides as a function of age. II. Basal cell dose distributions and associated lung cancer risk. Health Phys. 43:31-44.
Hursh, J. B., and T. T. Mercer. 1970. Measurement of 212Pb loss rate from human lungs. J. Appl. Physiol. 28:268-274.
Ingham, D. B. 1975. Diffusion of aerosols from a stream flowing through a cylindrical tube. Aerosol Sci. 6:125-132.
International Commission on Radiological Protection (ICRP). 1975. Report of the Task Group on Reference Man. International Commission on Radiological Protection, Publication 23. Oxford: Pergamon.
Jacobi, W., and K. Eisfeld. 1980. Dose to tissues and effective dose equivalent by inhalation of radon-222, radon-220 and their short-lived daughters. GSF Report S-626. Munich-Neuherberg, West Germany: Gesellschaft für Strahlen und Umweltforschung.
James, A. C. 1984. Dosimetric approaches to risk assessment for indoor exposure to radon daughters. Radiat. Prot. Dosim. 7:353-366.
James, A. C. 1988. Lung dosimetry. Pp. 259-309 in Radon and Its Decay Products in Indoor Air, W. W. Nazaroff and A. V. Nero, eds. New York: Wiley Interscience.
James, A. C., J. R. Greenhalgh, and H. Smith. 1977. Clearance of lead-212 ions from rabbit bronchial epithelium to blood. Phys. Med. Biol. 22:932-948.
James, A. C. 1990. Experimental data and theoretical models for the dosimetry of human exposure to radon and thoron progeny. DOE/Technical Report Series (in preparation).
Kirichenko, V. N., D. G. Khachirov, S. A. Dubrovin, V. E. Klyuch, and A. V. Bykhovskii. 1970. Experimental study of the distribution of short-lived radon daughters in the respiratory tract. Gig. Sanit. 2:222-227.
Mercer, R. R., M. L. Russell, and J. D. Crapo. 1989. Airway cell and nuclear depth distribution in human and rat lungs. Health Phys., in press.
National Council on Radiation Protection and Measurements (NCRP). 1984. Evaluation of Occupational and Environmental Exposures to Radon and Radon Daughters in the United States. Report No. 78. Bethesda, Md.: National Council on Radiation Protection and Measurements.
Niinimaa, W., P. Cole, S. Mintz, and R. J. Shephard. 1980. The switching point from nasal to oronasal breathing. Respir. Physiol. 42:61-71.
Niinimaa, W., P. Cole, S. Mintz, and R. J. Shephard. 1981. Oronasal distribution of respiratory airflow. Respir. Physiol. 43:69-75.
Nuclear Energy Agency Group of Experts (NEA). 1983. Dosimetry Aspects of Exposure to Radon and Thoron Daughter Products. Paris: Organization for Economic Cooperation and Development (OECD).
Pack, A. I., M. B. Hooper, W. Nixon, and J. C. A. Taylor. 1977. A computational model of pulmonary gas transport incorporating effective diffusion. Respir. Physiol. 29:101-124.
Phalen, R. F., M. J. Oldham, C. B. Beaucage, T. T. Crocker, and J. D. Mortensen. 1985. Postnatal enlargement of human tracheobronchial airways. Anat. Rec. 212:368-380.
Pich, J. 1972. Theory of gravitational deposition of particles from laminar flow in channels. Aerosol Sci. 3:351-361.
Reineking, A., and J. Porstendörfer. 1990. Unattached fractions of short lived Rn decay products in indoor and outdoor environments: an improved single screen method and results. Health Phys. 58:715-728.
Roy, M., and C. Courtay. 1990. Daily activities and breathing parameters for use in respiratory tract dosimetry. Radiat. Prot. Dosim., in press.
Rudolf, G., J. Gebhart, J. Heyder, C. F. Schiller, and W. Stahlhofen. 1986. An empirical formula describing aerosol deposition in man for any particle size. J. Aerosol Sci. 17:350-355.
Scherrer, J. 1981. Précis de physiologie du yravail. In Notions Ergonomie. Masson: Paris.
Scherer, P. W., L. H. Shendalman, N. H. Greene, and A. Bouhuys. 1975. Measurement of axial diffusivities in a model of the bronchial airways. J. Appl. Physiol. 38:719-723.
Sinclair, D. R., R. J. Countess, and G. S. Hoopes. 1974. The effect of relative humidity on the size of atmospheric aerosol particles. Atmos. Environ. 8:1111-1117.
Stahlhofen, W., G. Rudolf, and A. C. James. 1989. Intercomparison of experimental regional aerosol deposition data. J. Aerosol Med. 2:285-308.
Strong, J. C., and D. L. Swift. 1990. Deposition of unattached radon daughters in models of human nasal airways. To be presented at the 29th Hanford Symposium on Health and the Environment Indoor Radon and Lung Cancer: Reality or Myth?, October 16-19, 1990, Richland, Wash.
Swift, D. L. 1989. Age-related scaling for aerosol and vapor deposition in the human respiratory tract. Health Phys. 57:293-297.
Taulbee, D. B., and C. P. Yu. 1975. A theory of aerosol deposition in the human respiratory tract. J. Appl. Physiol. 38:77-85.
Taussig, L. M., R. T. Harris, and M.D. Lebowitz. 1977. Lung function in infants and young children. Am. Rev. Respir. Dis. 116:233-239.
Weibel, E. R. 1963. Morphometry of the Human Lung. New York: Academic Press.
Wood, L. D. H., S. Pritchard, T. R. Weng, K. Kruger, A. C. Bryan, and H. Levison. 1971. Relationship between anatomic dead space and body size in health, asthma and cystic fibrosis. Am. Rev. Respir. Dis. 104:215-222.
Yeh, H. C., and G. M. Schum. 1980. Models of human lung airways and their application to particle deposition. Bull. Math. Biol. 42:461-480.
Yu, C. P., and C. K. Diu. 1982. A probabilistic model for intersubject deposition variability of inhaled particles. Aerosol Sci. Technol. 1:355-362.
Yu, C. P., and G. B. Xu. 1987. Predicted particle deposition of diesel particles in young humans. J. Aerosol Sci. 18:419-429.
Zapletal, A. 1987. Lung Function Testing in Children and Adoloescents, H. Herzog, ed. Basel: Karger.
Zeltner, T. B., J. H. Caduff, P. Gehr, J. Pfenninger, and P. H. Burri. 1987. The postnatal development and growth of the human lung. I. Morphometry. Respir. Physiol. 67:247-267.