5
Dosimetry and Dosimetric Models for Inhaled Radon and Progeny
Dosimetry is defined as the measurement of the amount, type, rate, and distribution of radiation emitted from a source of ionizing radiation and the calculation of both spatial and temporal patterns of energy deposition in any material of interest as a result of ionizing radiation. Instruments and techniques exist to measure fields of penetrating radiation such as X rays or gamma rays that are external to the body, and provide means for directly quantifying the amount of energy deposited per unit mass of material (air, tissue, water). These dose measurements can then be related to a person present in the radiation field and the radiation dose that he or she would receive. In the case of internally deposited radionuclides, however, direct measurement of the energy absorbed from the ionizing radiation emitted by the decaying radionuclide is rarely, if ever, possible. Therefore, one must rely on dosimetric models to obtain estimates of the spatial and temporal patterns of energy deposition in tissues and organs of the body. In the simplest case, when the radionuclide is uniformly distributed throughout the volume of a tissue of homogeneous composition and when the size of the tissue is large compared with the range of the particulate emissions of the radionuclide, then the dose rate within the tissue is also uniform and calculation of absorbed dose can proceed without complication. However, if nonuniformities in the spatial and temporal distributions of radionuclide are coupled with heterogeneous tissue composition, then calculation of absorbed radiation dose becomes complex and uncertain. Such is the case with the dosimetry of inhaled radon and radon progeny in the respiratory tract.
The objective of this chapter is to provide both background and a historical perspective of the development of dosimetric models for radon and radon
progeny and to provide a basis for more detailed descriptions of the scientific issues that relate to the different component parts of the present radon progeny dosimetric model. The physical dosimetry and microdosimetry of alpha particles in tissue are discussed in Appendix I of the BEIR IV report (NRC, 1988).
SOME PHYSICAL CHARACTERISTICS OF RADON AND RADON PROGENY
Three isotopes of radon occur naturally in the environment as a result of being part of the so-called uranium (238U), thorium (232Th), and actinium (235U) decay series. These are 222Rn, 220Rn, and 219Rn, respectively. Their importance as environmental sources of radioactivity stems from the fact that radon is a noble gas that can be carried in air or water streams far from its source of creation in soil and rock and can reach the indoor environment where people work and live. Radon's importance as an environmental source of radiation depends principally on the local concentrations of the parent radionuclide, the physical characteristics of the rocks and soil, and on its half-life. The last two factors determine the time available to migrate into air and water. The decay characteristics of the isotopes of the three decay series are summarized in Figures 5-1A through C. Of least importance is 219Rn, which, because of its 3.96 s half-life, has limited capacity for migration in the environment. Moreover, its progenitor, 235U, has a relatively low concentration in the environment. 220Rn, with a 55.6 s half-life, is more readily available to the environment, and in geographic regions that have shallow deposits of thorium-rich soils, it can be a major contributor to the potential radiation dose to people. However, the majority of the concern for risks from radon exposure is due to the environmental presence of the isotope 222Rn, which has the longest half-life of the radon isotopes, 3.824 days, and is ubiquitous because of the pervasive presence of its precursor radionuclides, 226Ra and 238U, in the earth's crust.
The apparent complexity of the 222Rn decay scheme in Figure 1-1 can be simplified by neglecting the decays ![]() and
and ![]() , both of which occur with very low probabilities
, both of which occur with very low probabilities ![]() , and by truncating the decay scheme at 210Pb, which has a 22-yr half-life and is of little consequence as a respiratory hazard. Only three alpha-emitting radionuclides of consequence. then, remain, 222Rn, 218Po, and 214Po. Table 5-1 provides additional physical data on 222Rn and its progeny, including the historical designations for the decay products. Note that because the very short half-life for 214Po (164 s), it is in virtual equilibrium with its parent 214Bi under any conditions. Thus, decay chain calculations need only be done for the chain
, and by truncating the decay scheme at 210Pb, which has a 22-yr half-life and is of little consequence as a respiratory hazard. Only three alpha-emitting radionuclides of consequence. then, remain, 222Rn, 218Po, and 214Po. Table 5-1 provides additional physical data on 222Rn and its progeny, including the historical designations for the decay products. Note that because the very short half-life for 214Po (164 s), it is in virtual equilibrium with its parent 214Bi under any conditions. Thus, decay chain calculations need only be done for the chain ![]() .
.
MORPHOMETRIC MODELS OF THE RESPIRATORY TRACT
A morphometric model of the respiratory tract is a fundamental component
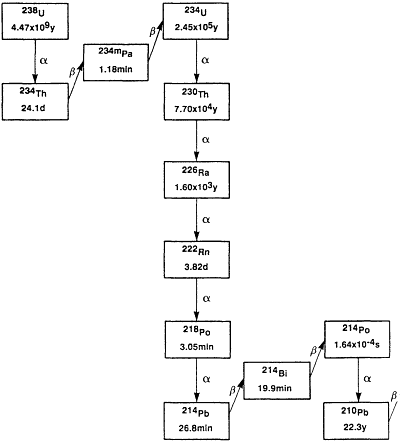
Figure 5-1A Uranium decay series.
of any dosimetric model intended for use in estimating alpha-radiation doses from radon and radon progeny, because of the heterogeneous distribution of deposited activity that occurs upon inhalation and the diverse structures, airway sizes, and geometries that are found at the different levels of the respiratory tract. Without an accurate anatomic description of the respiratory tract, one is limited in the ability to apply theoretical or empirical principles of airflow patterns and aerosol deposition at the level of resolution that is presumed to be necessary for properly modeling the radiation dose distribution from inhalation of radon and radon progeny, i.e., at the millimeter and submillimeter levels (see Chapter 9). Although morphometric models of the respiratory tract below the level of the larynx have existed for some time, there is currently no
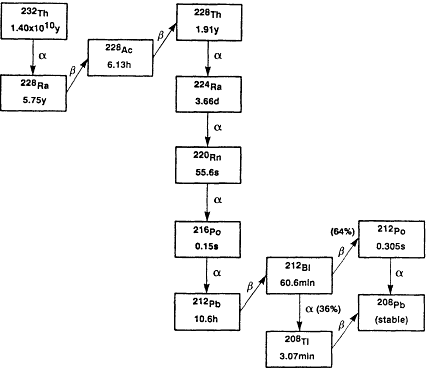
Figure 5-1B Thorium decay series.
single morphometric model considered to be fully adequate for describing the morphometry of the entire respiratory tract, which includes the nasal airways, oral cavity, nasopharynx and oropharynx, larynx, trachea, bronchi, bronchioles, and alveoli. Additionally, the roles of subject size and age on the dimensions of the various regions of the respiratory tract have only recently received attention by researchers.
HEAD AIRWAY MORPHOMETRY
For several reasons, morphometric models have not yet been developed for either the nasal or the oral airways. First nasal airways in humans, although much simpler geometrically than those in most other animal species, are complex anatomic structures that do not lend themselves to description in simple geometric terms, e.g., as tubes with circular or elliptical cross sections. Second, significant interindividual variabilities in the size and shape of nasal airways, particularly as a function of the size of the individual, have been recognized.
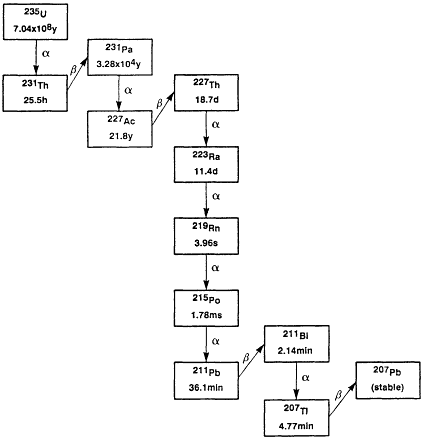
Figure 5-1C Actinium decay series.
Third, although the nasal airways have long been recognized as an important deposition site for large-sized aerosol particles ![]() , their importance in filtering out smaller particles (<0.1 µm) has only recently been appreciated (Cheng et al., 1988).
, their importance in filtering out smaller particles (<0.1 µm) has only recently been appreciated (Cheng et al., 1988).
The nasal airways consist of several distinct anatomic regions that differ in terms of their size, shape, physiological function, and types of epithelial cells lining the airways (Swift and Proctor, 1976). Air enters through elliptically shaped openings, the anterior nares, and passes into a conically shaped vestibular region that extends approximately 10 to 15 mm in the posterior direction. The cross-sectional area decreases posteriorly to a minimum value at the location of the nasal valve or ostium internum, which has been determined by Haight and Cole (1983) to be the location of maximum airway resistance. Proctor
TABLE 5-1 Some Physical Properties of 222Rn and Its Short-Lived Decay Products
|
|
No. of Atoms |
|||||
|
Element |
Historical Symbol |
Principal Radiation(s) |
Decay Energies (MeV) |
Half-Life |
Per µCi |
Per Bq |
|
226Ra |
Ra |
α |
4.8 |
1,620 yr |
2.7 × 1015 |
7.4 × 1010 |
|
222Rn |
Rn |
α |
5.5 |
3.82 day |
1.8 × 1010 |
4.8 × 105 |
|
218Po |
RaA |
α |
6.0 |
3.05 min |
9.77 × 106 |
2.6 × 102 |
|
214Pb |
RaB |
β,γ |
1.0 max |
26.8 min |
8.58 × 107 |
2.3 × 103 |
|
214Bi |
RaC |
β,γ |
3.3 max |
19.7 min |
6.31 × 107 |
1.7 × 103 |
|
214Po |
RaC |
α |
7.7 |
164 µs |
8.8 |
2.4 × 10-4 |
and Swift (1971) estimated that the average cross-sectional area at this point is probably between 20 and 40 mm2 per side. Posterior to the nasal valve are the nasal fossae, which contain the inferior, medial, and superior turbinates attached to the lateral walls. The left and right nasal airway cavities are separated by a thin cartilaginous nasal septum, whose thickness and shape can vary significantly among individuals, but it is typically 3- to 7-mm thick in the anterior cartilaginous part and 2- to 3-mm thick in the posterior bony part (International Commission on Radiological Protection [ICRP], 1974). Because of the intrusion of the turbinates, the air passages of the nasal fossae have ribbon-like cross sections that are complexly folded within the turbinate structures. Because of this complexity, there is a high surface area-to-volume ratio in this region of the airways (ICRP, 1974). At the posterior end of the turbinate region, about 50 mm in length, the nasal septum terminates, and the two nasal passages combine (nasal choanae) and join the pharynx, which then bends 70° downward to the remainder of the respiratory tract. The length of the nasal passage from nostril to oropharynx is about 130 mm (Swift, 1981).
Limited morphometric information is available regarding the sizes of the different parts of the nasal airways. Additionally, most of the published measurements on nasal airway dimensions were obtained from cadaver studies. Postmortem measurements result in overestimation of the cross-sectional areas and airway volumes of the region containing the turbinates, because of the marked shrinkage of the nasal mucosa after death (Guilmette et al., 1989).
For calculational purposes, Landahl (1950) assumed the following schematic representation of the nasal airways: Each of the external nares had a cross-sectional area of 75 mm2, with hairs of 100 µm in diameter occupying one-half of the projected area. The second region was the region containing the nasal valve, 2 cm behind the opening of the nares, and was assumed to have a rectangular cross section 1.2-cm high and 0.25-cm wide and to bend at an angle of 30°. The third region was also assumed to have a cross-sectional profile of a rectangular tube 3-cm tall, 0.2-cm wide, and 1-cm long, with a bend of 20°. The fourth region was subdivided into two elements. The first was the narrow and tortuous upper passage of 1 mm in width; the second was the more direct lower passage of 2 mm in width. The total height for both elements was 40 mm, with a length of 50 mm, so that the total effective surface area for both nasal passages was 80 cm2. The appropriateness of the various simplifying assumptions used in this nasal airway model has not been evaluated to date.
Scott et al. (1978) also formulated a geometrical model for calculating aerosol particle deposition within the nasal airways. In this model, a symmetrical, bilateral model was divided into five contiguous parts that represented the anterior nares, the nasal valve, the expansion region just posterior to the nasal valve, the turbinate area, and the posterior bend. There are many similarities between this model and that of Landahl (1950). The major differences between them are the increased geometrical complexity of the turbinate areas, which
is accomplished by using coupled rectangular shapes that simulate the meatal folds, the graded cross-sectional area in the region of the nasal valve, and the presence of the posterior nasopharyngeal bend. Again, as with the Landahl model, the adequacy of this particular model in representing nasal deposition has not been evaluated experimentally. However, the model, together with the accompanying theory of particle deposition in the nasal airways, did show reasonable agreement with the data available from studies of aerosol deposition in human nasal airways (Scott et al., 1978).
Except for the dosimetry model of Bailey (1984), in which the nasal airways were assumed to be two sets of parallel disks, representing the anterior and posterior portions of the airway, no other morphometric models of the nasal airways have been presented. To date, no morphometric models of the oral airways have been used either experimentally or for theoretical calculations. The reasons for this lack are not clean It is clear, however, that oral breathing can be important, and even predominant, in cases of acute or chronic nasal airway obstruction and in cases of significant physical exertion, in which increased ventilation can only be accomplished by decreasing respiratory resistance, i.e., by compensatory oral breathing (see Chapter 7). Intuitively, it would appear to be difficult to construct a single morphometric model of the oral cavity that would be adequate for deposition and dosimetry purposes, as the cavity dimensions are likely to depend significantly on the size of the individual, as well as the position of the tongue and jaw. These latter positions depend on the ventilation rate requirements and the fractionation of breathing between the nasal and oral routes.
TRACHEOBRONCHIAL AIRWAY MORPHOMETRY
Anatomically, the tracheobronchial airways consist of a series of bifurcating tubes with circular or near-circular cross sections and ever-decreasing diameters that extend from the trachea at the proximal end to the terminal bronchioles in the distal region of the lung. The trachea divides into the left and right major bronchi, which in turn divide by dichotomous branching into smaller bronchi and bronchioles. In humans, there are about 18 to 20 branchings before the level of the respiratory bronchioles is reached. The bronchi have a ciliated epithelium covered by a layer of mucus, which is produced by the goblet cells and mucous glands. The bronchi also have irregularly shaped cartilage plates situated on the outside of the bronchial walls that, along with the smooth muscle layers, provide structural support to maintain airway patency during ventilation. The smallest conducting airways, the bronchioles, occur at the ends of the bronchi and have neither cartilage nor mucous glands. They do, however, have a ciliated epithelium and mucus-producing cells, although the ciliated regions and the mucus blanket are no longer continuous, as they tend to be in the larger bronchi.
Morphometric models of the conducting airways have been developed based on measurements made by using airway corrosion casts or in vivo bronchoscopic or radiographic measurements or at autopsy. These measurements have included airway diameters, segment lengths, and branching angles. From these, luminal surface areas and volumes can be calculated. The earliest models of the respiratory airways were made assuming symmetry of size and length for airways of a given generation (Findeisen, 1935; Landahl, 1950; Davies, 1961). These models have been summarized by Raabe (1982).
More recent morphometric models have been based on sets of data obtained primarily from measurements of conducting airway dimensions by using corrosion cast techniques (Weibel, 1963; Horsfield et al., 1971; Yeh and Schum, 1980; Phalen et al., 1985). Weibel (1963) used a plastic airway cast prepared by Liebow and measured the lengths, diameters, and branching angles completely for the first 5 airway generations and incompletely through 10 airway generations. Sampling frequencies decreased gradually from 91 to 95% for generations 6 to 18 to 21% for generation 10. The results confirmed earlier views that the conducting airway system has a characteristic irregular dichotomous branching.
For a given generation, there was a dispersion of both airway diameters and airway lengths, with a greater variability being seen in the lengths of the airway segments. The above measurements were then combined with data collected from the more peripheral regions of the lung by quantitative morphometry of histologically prepared sections. These measurements were used to construct two morphometric models of the human lung: the first (Weibel lung model A), which emphasized the regular features of the airways and their patterns, and the second (Weibel lung model B), which attempted to take into account the irregularities encountered in the measurements (Weibel, 1963). The Weibel morphometry models, particularly Weibel lung model A, have been incorporated into deposition and dosimetric models by many investigators.
Horsfield et al. (1971) described a morphometric model based on data obtained from measurement of a resin cast of a normal human bronchial tree (Horsfield and Cumming, 1968; Parker et al., 1971). Their measurements, which included airway diameters, segment lengths, and branching patterns, indicated that asymmetric branching was needed to produce a more realistic model of the airway structure. Thus, they developed two models that included asymmetric branching, unequal numbers of airways in different lung lobes, and variations in the airway segment lengths (Horsfield et al., 1971). Of note was their determination that the mean diameter of the smaller of a pair of daughter bronchi was similar to that of a larger bronchial branch occurring four generations distal to the original. Their model has been implemented mathematically by Yeates and Aspin (1978).
Yeh and Schum (1980) prepared a flexible silicone rubber airway cast of the lungs of a 60-yr-old male. Their measurements included diameters and lengths of each airway segment together with the respective branching angles
and angle of each airway with respect to gravity. Measurements were made of all airways to 2 or 3 mm in diameter, with randomized sampling of 20% of the smaller-diameter airways being made to the level of the terminal bronchioles. They then developed a typical path lung model in which n number of identical pathways (down to the level of the terminal bronchioles) was used to represent n number of actual or estimated pathways of a complicated tree structure such as those present in each lobe of a lung or the lung as a whole. The concept is similar to that of Weibel lung model A, except that it does not impose a symmetry requirement on the bronchial tree structure.
Phalen et al. (1985) developed a morphometric model of the tracheobronchial airways based on measurements obtained from 20 humans ranging in age from 11 days to 21 yr. Complete measurements of diameters, lengths, branching angles, and angles with respect to gravity were made for the first three generations; successive generations were sampled, and pathways to the level of the terminal bronchioles were marked and measured at random. Data were then analyzed with respect to the height of the subject, and linear regressions were performed on the data sets grouped by airway generation.
Although several studies have measured morphometric parameters of human conducting airways, no single study has made a complete set of measurements for the length of the tracheobronchial tree. This is understandable, considering that analysis of all airways for 15 generations of a symmetrical dichotomous tree would require more than 65,000 measurements. Thus, each investigator must make assumptions as to the structure of the tracheobronchial airways and how representative their sampling is with respect to the real morphology of the conducting airways. Additionally, very few human lung specimens have been analyzed, and their ages and sizes have differed significantly. Thus, a standard human conducting airway model is not available, nor has variability been adequately described by age, size, gender, or other anthropomorphic characteristic. However, James (1988) compared the generation-specific surface areas and volumes of airways from Weibel lung model A, the Yeh and Schum model, and the Phalen (University of California, Irvine [UCI]) model. The data for the Weibel and Yeh and Schum models were derived from Yu and Diu (1982), in which the respective models were scaled to an initial lung volume of 3,000 cm3. Figure 5-2 illustrates the relationships between the generation-specific volumes for the three models. Although there is relatively good agreement between the model values for the first four generations, there is a significant divergence of the volumes of the Yeh and Schum model for generations 5 to 12 from those of the other two models, with a maximum variance of a factor of 3 in generation 8.
It is not known whether the present models accurately reflect the morphology of the conducting airways of the human lung, nor is it known to what degree the variabilities in the morphometric models can affect dosimetry calculations. Since most of the models currently being used for deposition and dosime
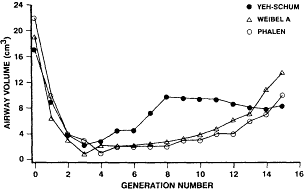
Figure 5-2 Calculated airway volumes of tracheobronchial airways using different models.
try calculations rely on simplifying assumptions about the generation-specific structure and size of the airways, it would be useful to evaluate the effect of introducing asymmetry assumptions into the morphometric models. These assumptions, which can now be characterized by using statistical distributions for the generation-specific variables of airway size, length, and branching angle, would undoubtedly add complexity to the models. However, with current computer technologies, it would not be difficult to perform the necessary calculations. Weibel, in lung model B, attempted to account for the irregular dichotomy of the lung by applying a binomial distribution to the occurrence of airways with 2-mm diameters with respect to generation number. The distance from the root of the trachea at which these 2-mm bronchi occurred was then described by a normal distribution with a mean of 24.5 cm. Asymmetries for the smaller airways (1.0-and 0.5-mm diameters) were then estimated based on the assumption of normal distributions analogous to that used for the 2-mm-diameter airways. Thus, a model accounting for airway diameter and length asymmetries was developed. To date, however, this model has not been applied to deposition or dosimetry calculations, although there is no reason why this could not be done.
Soong et al. (1979) also used Weibel lung model A as a basis for constructing a tracheobronchial airway morphometry model that included variability in airway size. Initially, they estimated coefficients of variation for 24 generations of airways based on data obtained from available morphometric studies, and then applied the coefficients to the mean diameters and lengths for the different generations of airways taken from Weibel (1963). They later determined that the distribution of airway diameters and lengths could be characterized by either log-normal or gamma probability distributions. Yu et al. (1979) then applied
the above statistical morphometric model to human data on total and regional deposition of inhaled aerosols and stated that the model agreed well with the experimental data, suggesting that intersubject variability in deposition was due primarily to differences in airway dimensions.
Koblinger and Hofmann (1985) also developed a statistical morphometric model for calculating aerosol deposition. Their model, which was based on the extensive data set developed by Raabe et al. (1976), included the asymmetries measured in human airways in terms of airway diameters, lengths, and branching angles. They then used Monte Carlo methods to select pathways randomly, but the pathway selections were weighted by the measured statistical distributions for each of the morphometric parameters.
Nikiforov and Schlesinger (1985) made morphometric measurements of the upper airways of right lungs from eight young adult males ages 17 to 45 yr to estimate the range of interindividual variability that could be expected from a human population. Their mean morphometric values agreed reasonably well with those of the Weibel (1963) and Yeh and Schum (1980) models, particularly considering that their measurements involved only right lungs, whereas both lungs were used by Weibel (1963) and Yeh and Schum (1980). The greatest intersubject and intrasubject variability was found in branching angles, and the least variability was found in airway diameter, with airway length variability being moderate.
Significant effort has been expended over the last 30 yr in determining quantitatively the structure of the conducting airways in humans. It is clear that the structure can be described as asymmetric dichotomy. More recent studies have attempted to quantitate the bounds of variability that characterize the morphometric parameters in the lung airways. Some attempts have been made to incorporate statistical variability into morphometric parameters into deposition and dosimetric models. It is not clear, however, what level of accuracy in the morphometric model is needed for the specific case of constructing an adequate dosimetric model for exposure of the conducting airways to radon and radon progeny. In this context, other important facets of the dosimetric model, e.g., thickness of epithelium and mucus, deposition and clearance parameters, and identification of cells at risk, must also be considered.
RESPIRATORY AIRWAY MORPHOMETRY
The respiratory or gas-exchange region of the respiratory tract is generally considered to include the airway structures that occur beyond the terminal bronchioles. Each terminal bronchiole supplies air to a pulmonary lobule or acinus, which consists of about three generations of bronchioles that are partially alveolated (respiratory bronchioles), followed by three more generations of ducts that are fully alveolated (alveolar ducts) and that lead to the final generation of alveolar sacs (Weibel, 1963). The vast majority of the airway volume and
surface area occurs in this region of the lung. Morphometric analyses have indicated that there are about 3 × 108 alveoli in a human lung (Weibel, 1963). The total surface area of the respiratory airways is in the range of 70 to 100 m2 (Weibel, 1963; Thudbeck, 1967; Gehr et al., 1978). More recent morphometric studies done by electron microscopy have focused on the size and structure of the pulmonary acinus (Haefeli-Bleuer and Weibel, 1988; Ciurea and Gil, 1989). Based on their studies, Haefeli-Bleuer and Weibel have constructed an idealized model of the acinar airways that features a regularized dichotomy between transitional (respiratory) bronchiole and alveolar ducts. Their model also includes airway diameters and lengths, as well as total cross-sectional areas and volumes of the ducts per generation, and the total alveolar surface area.
Past radon dosimetric models have not emphasized radiation doses to the respiratory region of the lung, probably because of the distribution of lung cancers that have been observed in uranium and other metal miners, in whom most of the tumors have been said to originate in the upper bronchial airways (see Chapter 8). Although this may have been appropriate for this population, it is not clear whether differences in exposure aerosol characteristics, age and size of respiratory tracts, and health status may affect the distribution of alpha-radiation dose. It is clear from dose-response studies in several animal species that the cells in the parenchymal lung are capable of being irradiated as a result of exposure to aerosols containing alpha-emitting radionuclides and that such irradiation can lead to lung cancer (Sanders et al., 1977; Lambert et al., 1982; Lundgren et al., 1983; National Council on Radiation Protection and Measurements [NCRP], 1984; Dagle et al., 1989). Therefore, it may be prudent to include some form of morphometric model for the respiratory airway portion of the respiratory tract, if for no other purpose than to facilitate comparison of results from experimental animal studies to humans.
DEPOSITION MODELS OF THE RESPIRATORY TRACT
One of the important factors controlling the distribution of alpha-radiation dose to the different portions of the human respiratory tract is the deposition pattern of radon progeny-containing aerosols. Much research has been done to determine experimentally the deposition fractions for different sizes and types of aerosols in different experimental animal models, including humans. This large body of information has been summarized in numerous articles and chapters (e.g., Raabe et al., 1977; Brain and Valberg, 1979; Lippmann et al., 1980; Clarke and Pavia, 1984) and is considered in detail from the point of view of the special particle characteristics associated with radon progeny-containing aerosols later in this volume (see Chapter 7).
Deposition of different sizes of aerosols in the nasal and oral airways of humans has been measured experimentally both in vivo (Landahl and Black, 1947; Pattle, 1961; Lippmann, 1970; Hounam et al., 1971; Fry and Black, 1973;
Heyder and Rudolf, 1977; Bowes and Swift, 1989) and in physical replica casts (Cheng et al., 1988; Gradon and Yu, 1989; Phalen et al., 1989). Most of the early data obtained in humans consisted of deposition of aerosol particles whose sizes ranged from 0.5 to 10 µm in diameter (Landahl and Black, 1947; Pattle, 1961; Lippmann, 1970; Hounam et al., 1971; Fry and Black, 1973; Heyder and Rudolf, 1977; Bowes and Swift, 1989). Their results showed that the deposition fractions could be modeled by assuming that impaction mechanisms were dominant. Deposition fractions increased with increasing particle diameter and with increasing flow rate. Scott et al. (1978) developed a five-stage theoretical model of the nasal airways and calculated deposition fractions based on considerations of impaction and secondary flow generation posterior to the area of the nasal valve. Their calculations were in general agreement with published deposition data in humans. Their model considered the particle size range of 0.2 to 10 µm. Yu et al. (1981) used all of the available data on nasal and oral airway deposition of particles ranging from 0.3 to 23 µm and developed empirical fits of deposition fraction with the impaction parameter ρd2Q, where ρ is the particle density, d is the particle diameter, and Q is the flow rate. They also used the variabilities in the data sets that were probably due to intersubject and intrasubject variabilities to calculate variances for the calculated mean deposition fractions, assuming normal statistics. Although these data and models are relevant to many types of inhalation exposures, the particle size range for the studies described above does not include the sizes of most radon progeny-containing aerosols, which are significantly smaller.
Most of the data relative to the deposition of ultrafine aerosols in the nasal or oral airways have thus far been obtained by using physical replicas of the nasal airways (Cheng et al., 1988; Gradon and Yu, 1989). These data showed that deposition within the nasal airways increased with decreasing particle diameter and with decreasing flow rate, indicating that diffusion was the dominant deposition mechanism. In particular, both Cheng et al. (1988) and Gradon and Yu (1989) have stated that turbulent diffusion was the dominant mechanism, and each group has developed empirical descriptions of their research results. Because of the small sizes of most radon progeny-containing aerosols, these new results concerning ultrafine aerosol deposition need to be considered in any radon dosimetric model.
The experimental basis for modeling the deposition of aerosols in the conducting airways arises from analysis of deposition data from a large number of studies in which people have inhaled test aerosols under controlled experimental conditions. The tracheobronchial region can be simply defined from an anatomic viewpoint, i.e., airways beginning at the trachea and extending to the level of the terminal bronchioles. However, it is not straightforward to define what fraction of an aerosol has deposited in these conducting airways as a result of an inhalation exposure, mainly because of deposition of aerosols in the other parts of the respiratory tract. Using radioactively labeled aerosols,
several investigators have functionally defined tracheobronchial deposition as being equal to the amount of total aerosol deposited in the lung that has been cleared from the thorax in 24 h after a brief inhalation exposure (Albert et al., 1967; Lippmann et al., 1971; Camner and Philipson, 1978). Although it is reasonable to assume that essentially all of the aerosol that is cleared in this 24-h period is due to particles that were deposited on the ciliated airway surfaces, it cannot be easily demonstrated that all of the aerosol particles that had originally deposited on the conducting airways had been cleared by that time. It is, therefore, likely that tracheobronchial deposition has been underestimated by the 24-h clearance criterion; however, it is unlikely that the underestimate is of significant magnitude.
Considerable effort has been expended in developing deposition models for the conducting airways. Despite the morphological complexity of the airways and the known variability in airway size and geometry among different people, many investigators have used a simplified morphometry that consists of a series of bifurcating cylindrical tubes with varying degrees of symmetry (Findeisen, 1935; Landahl, 1950; Beekmans, 1965; Task Group on Lung Dynamics, 1966; Gerrity et al., 1979; Yeh and Schum, 1980; Egan and Nixon, 1985). Because of the preponderance of human deposition data available for particle sizes greater than 0.5 µm, most of the theoretical models have focused on the range of particle sizes in which inertial impaction and gravitational settling are the predominant mechanisms of deposition (D), i.e., for diameters ![]() . Many of these model predictions, compared to the available human deposition data, have been summarized in review articles (Mercer, 1975; Chan and Lippmann, 1980; Raabe, 1982; Stuart, 1984; Morrow and Yu, 1985). In general, for nasal breathing and for a particle size range of greater than 0.5 µm, less than 10% deposition of aerosol is found in the conducting airways, within wide limits of assumed airway sizes and breathing patterns. Oral breathing tends to result in increased deposition of large aerosol particles in the conducting airways by impaction; this is a result of increased penetration into the lung because of the absence of nasal filtration.
. Many of these model predictions, compared to the available human deposition data, have been summarized in review articles (Mercer, 1975; Chan and Lippmann, 1980; Raabe, 1982; Stuart, 1984; Morrow and Yu, 1985). In general, for nasal breathing and for a particle size range of greater than 0.5 µm, less than 10% deposition of aerosol is found in the conducting airways, within wide limits of assumed airway sizes and breathing patterns. Oral breathing tends to result in increased deposition of large aerosol particles in the conducting airways by impaction; this is a result of increased penetration into the lung because of the absence of nasal filtration.
To supplement the data obtained in vivo in controlled inhalation studies in humans, several investigators have constructed physical models of the conducting airways (e.g., Martin and Jacobi, 1972; Ferron, 1977; Schlesinger et al., 1977; Chan and Lippmann, 1980; Martonen, 1983; Gurman et al., 1984). The models were constructed by either corrosion techniques by using human lung specimens or idealized bifurcating tube models built according to published morphometry models, usually Weibel A lung model (Weibel, 1963). The use of such models under conditions of controlled aerosol exposure provides opportunities for measuring regional deposition patterns within the models and comparing the qualitative and quantitative deposition patterns with the predictions of existing theoretical deposition models. These types of data cannot be obtained for the conducting airways in vivo by noninvasive methods.
Conducting airway models have been used experimentally both to determine deposition efficiencies for aerosols with different particle sizes as well as to evaluate fluid dynamic parameters such as flow patterns or velocity profiles within the model tubes. Olson et al. (1973) used replica casts to determine velocity profiles, dynamic and static pressures, and flow instabilities from the oral cavity to the segmental bronchi. Lee and Wang (1977) used a two-level bifurcation model based on Weibel morphometry to measure airflow characteristics and deposition velocities; they showed the importance of considering the effects of consecutive serial bifurcations on the prediction of local particle deposition patterns. Such localized deposition patterns were measured in replica hollow casts by Schlesinger et al. (1982) using a five-generation model. Their results with large respirable aerosols and results of other investigations (Ferron, 1977; Johnson et al., 1977; Chan and Lippmann, 1980; Martonen, 1983; Gurman et al., 1984) have been used to validate several theoretical deposition models that feature deposition by inertial impaction and gravitational sedimentation.
Although these previous studies, which were done with large aerosol particles, have been important in validating theoretical predictions of deposition, they are probably of lesser importance when considering the particle size distributions typically observed for radon progeny-containing aerosols, which tend to be significantly smaller than 1.0 µm. Very few experimental studies of the deposition of particles of ![]() in diameter in the human respiratory tract have been done (Dautrebande and Walkenhorst 1961; George and Breslin, 1969; Tu and Knutson, 1984), and only Tu and Knutson (1984) used monodisperse aerosols. The total respiratory tract deposition measurements of Tu and Knutson (1984) for hydrophobic particles were in general agreement with the mathematical predictions of Yeh and Schum (1980) and Yu et al. (1981), but were significantly less than those predicted by the Task Group on Lung Dynamics (1966). With respect to the deposition of ultrafine aerosols in the conducting airways, only cast studies provide data that can be compared with theoretical models (Chamberlain and Dyson, 1956; Martin and Jacobi, 1972; Cohen, 1987). In general, their results indicate that deposition efficiency for diffusion-controlled deposition is higher than that predicted by using assumptions of laminar flow within the airways (Ingham, 1976). This increased deposition is important to consider with respect to the deposition of radon progeny aerosols within the tracheobronchial region of the respiratory tract.
in diameter in the human respiratory tract have been done (Dautrebande and Walkenhorst 1961; George and Breslin, 1969; Tu and Knutson, 1984), and only Tu and Knutson (1984) used monodisperse aerosols. The total respiratory tract deposition measurements of Tu and Knutson (1984) for hydrophobic particles were in general agreement with the mathematical predictions of Yeh and Schum (1980) and Yu et al. (1981), but were significantly less than those predicted by the Task Group on Lung Dynamics (1966). With respect to the deposition of ultrafine aerosols in the conducting airways, only cast studies provide data that can be compared with theoretical models (Chamberlain and Dyson, 1956; Martin and Jacobi, 1972; Cohen, 1987). In general, their results indicate that deposition efficiency for diffusion-controlled deposition is higher than that predicted by using assumptions of laminar flow within the airways (Ingham, 1976). This increased deposition is important to consider with respect to the deposition of radon progeny aerosols within the tracheobronchial region of the respiratory tract.
Nonuniform enhanced deposition of aerosol particles within the regions of conducting airway bifurcations has been reported in studies in which cast replica models of the upper airways have been used (Martin and Jacobi, 1972; Gurman, 1983; Martonen and Lowe, 1983; Schlesinger et al., 1983; Cohen et al., 1988). Because of the short range of the alpha particles associated with radon progeny, such increased deposition, expressed in terms of particles per unit surface area, could result in radiation doses to these anatomical structures that exceed those calculated on the basis of an assumed uniform distribution of deposited
particles. For particles depositing primarily by impaction mechanisms, increased deposition bias toward bifurcations has been shown for increasing particle sizes and increasing flow rate (Schlesinger and Lippmann, 1972; Martonen and Lowe, 1983) for inhalation and for cyclic inspiratory airflow patterns (Gurman, 1983). For particle sizes ranging from 2 to 11 µm, the enhancement in deposition relative to that in the adjoining straight-tube sections of the airway models ranged from 20 to 360%. Using smaller particle sizes, Cohen et al. (1988) found an average of about 20% increase in deposition at bifurcations compared with airway lengths for 0.15-and 0.2-µm particles at low flow rates (<20 liters/min). However, the degree of enhancement varied significantly between the different generations of the airway cast model that they used. Additionally, at higher flow rates, no enhanced deposition was found. For 0.04-µm particles, there did not appear to be enhanced deposition at any of the flow rates measured (Cohen et al., 1988). These data agree with modeling predictions of Gradon and Orlicki (1990), who concluded that deposition enhancement in bifurcation regions was insignificant for particles with diameters of ![]() , in which diffusional mechanisms dominate. As diffusional mechanisms increasingly predominate with decreasing particle size, the effect of localized flow patterns becomes less important, leading to more and more uniform localized depositions (Gradon and Orlicki, 1990). Thus, although the data are very limited, it appears that enhanced deposition at airway bifurcations may not be a significant factor to be considered for the expected particle sizes to which radon progeny would be attached.
, in which diffusional mechanisms dominate. As diffusional mechanisms increasingly predominate with decreasing particle size, the effect of localized flow patterns becomes less important, leading to more and more uniform localized depositions (Gradon and Orlicki, 1990). Thus, although the data are very limited, it appears that enhanced deposition at airway bifurcations may not be a significant factor to be considered for the expected particle sizes to which radon progeny would be attached.
The experimental basis for the development of deposition models for the alveolar or nonciliated regions of the respiratory tract arises from the human deposition data of Lippmann and Albert (1969), Lippmann (1977), Foord et al. (1978), Camner and Philipson (1978), Emmett and Aitken (1982), Stahlhofen et al. (1980, 1983), and Heyder et al. (1986). These studies have focused on the inhalation of particles with sizes ![]() that deposit primarily by impaction and sedimentation mechanisms. However, the more recent studies of Heyder et al. (1986) and Schiller et al. (1988) have extended the particle size range down to 0.005 µm.
that deposit primarily by impaction and sedimentation mechanisms. However, the more recent studies of Heyder et al. (1986) and Schiller et al. (1988) have extended the particle size range down to 0.005 µm.
Many theoretical models of the deposition of aerosols in the alveolar regions of the lung have been reported (e.g., Findeisen, 1935; Landahl, 1950; Altshuler, 1959; Beekmans, 1965; Task Group on Lung Dynamics, 1966; Taulbee and Yu, 1975; Gerrity et al., 1979; Yeh and Schum, 1980; Rudolf et al., 1986; Egan and Nixon, 1985, 1988). The models differ in terms of their selection of morphometric model, gas dynamics model, particle transport model, and physiological model (Heyder and Rudolf, 1984). Although the number of theoretical models is large, basically they are all derived from three primary deposition models (Heyder and Rudolf, 1984), which differ in their basic mathematical formalisms. The Findeisen (1935) formalism depicts the human respiratory tract as a series of discrete morphometric compartments
through which air containing aerosol particles flows. The model is, therefore, spatially discrete; it is also temporally discrete in that it assumes constant airflow for the inspiratory and expiratory phases of the breathing cycle and includes a respiratory pause after inspiration. There have been over 20 secondary deposition models derived by using Findeisen's formalism, including that of the Task Group on Lung Dynamics (1966). The second formalism was developed by Altshuler (1959), who regarded the respiratory tract as a continuous filter bed. As such, this formalism is spatially continuous. However, it is temporally discrete in that it assumes constant airflow conditions for the inspiratory and expiratory parts of the breathing cycle. This formalism has only been used by Altshuler et al. (1967). The third formalism was developed by Taulbee and Yu (1975), who described the respiratory tract as a single channel with a variable cross-sectional area as a function of the depth of penetration, z, into the model. This morphometric ''trumpet'' model, which was originally proposed by Scherer et al. (1972), features a terminal volume component due to the alveoli that expand and contract uniformly with breathing. Scherer et al. (1972) also used an integral expression for the volumetric flow rate at the entrance of the trumpet model, so that the model is therefore both spatially and temporally continuous. Several investigations have used this model formalism in their deposition models (Yu, 1978; Yu and Diu, 1982; Egan and Nixon, 1985).
Comparison of the results of calculations using different deposition models with experimental data have been published (Yu and Diu, 1982; Heyder and Rudolf, 1984; Ferron et al., 1985). These comparisons have shown that the calculation of total deposition within the respiratory tract is relatively insensitive to the assumptions of the models and do not differ significantly from the original calculations of Findeisen (1935). The major differences between model predictions and experimental data are seen for regional depositions in the tracheobronchial and alveolar regions, where the models tend to overestimate the deposition in the tracheobronchial region and underestimate deposition in the alveolar region when the results are compared with the experimental results of Stahlhofen et al. (1980, 1983). It is difficult, however, to attempt to differentiate between model predictions strictly by comparison with the results from human experimental deposition studies. Those data arise from studies with substantially different experimental designs and with varying degrees of control imposed on the respiratory breathing patterns of the experimental subjects. Additionally, as pointed out earlier, it has not been possible to measure fractional deposition directly within the alveolar and conducting airway compartments of the lung. Such deposition values have been functionally defined based on observed clearance rates of particles from the thorax region.
CLEARANCE MODELS OF THE RESPIRATORY TRACT
Models of clearance of inhaled particles from the respiratory tract differ
significantly from those previously described for the deposition of particles. Clearance models are mostly empirical and reflect the lack of knowledge concerning mechanisms of clearance compared with knowledge of deposition models, which are based on a fluid dynamic understanding of the behavior of micrometer and submicrometer particles in moving fluids. As such, the models are based on the results of limited human studies.
Clearance of particles deposited in the nasopharyngeal region depends on the site of deposition. Studies of mucous and particle clearance have shown that the nasal airways can be divided into two anatomically distinct regions: (1) the anterior region, which includes the nostrils, vestibule, and nasal valve, and (2) the posterior nasal airways, which include the nasal turbinates and extend to the pharyngeal area. Because of the lack of mucociliary clearance owing to the absence of ciliated and secretory cells in the anterior region of the nose, clearance occurs via extrinsic means in this region and occurs generally toward the nostrils (Proctor et al. 1977). Particles deposited in this region may remain there for many hours.
Measurements of mucous clearance velocities and particle clearance for the ciliated region of the nasal airways have shown that clearance rates are large, but with substantial intersubject variability (Ewert, 1965; Proctor and Wagner, 1965; Bang et al., 1967; Quinlan et al., 1969; Andersen et al., 1971; Fry and Black, 1973; Proctor, 1973; Yergin et al., 1978). These results have been obtained by a combination of methods, including radiographic imaging of radiopaque particles, direct optical visualization of the streaming of dye particles, radiometric imaging of instilled radionuclide-containing particles, and subjective perception of a substance by taste (saccharine test). Proctor et al. (1977) have summarized the results of several studies and have determined that the average mucous clearance velocity for the ciliated portion of the nasal airways in normal adults without respiratory disease is 5.3 mm/min; however, the range of values extends from 0.5 to 24 mm/min. For a nasal passage of 10 cm in length, the average clearance time would be 19 min. The degree of variability in clearance rates should not be underestimated. Distributions of clearance rates are not normally distributed; i.e., there appears to be a bias toward slower clearance rates, giving the distribution a log-normal appearance, although statistical analysis of measured populations of normal people has not been done. In the study of Andersen et al. (1971), in which over 200 measurements were made in 58 subjects, 57% could be described as having constant unobstructed mucous flow rates, 25% as having heterogeneous flow rates in different regions of the nasal airways, and 18% as having uniformly slow clearances. However, they also pointed out that repeated measurements on the same individual often resulted in a shift of the classification. The reasons for the large variabilities in clearance rates in different individuals are not clear, although variability in mucous rheology and in ciliary beat frequency have been suggested (Bang et al., 1967).
Measurement of mucous clearance rates in the conducting airways below the larynx has been limited to measurements made in the trachea and major bronchi, but primarily the former. The techniques used to make these measurements have been similar to those used to measure nasal mucous clearance, i.e., roentgenographic localization of insufflated powder (Gamsu et al., 1973) or radiopaque Teflon disks emplaced bronchoscopically (Friedman et al., 1977; Goodman et al., 1978); direct observation of the movement of Teflon disks through a bronchoscope (Santa Cruz et al., 1974; Wood et al., 1975); or measurement of the clearance of radioactively labeled microscopic particles by using gamma imaging devices, the particles being emplaced either by bronchoscopy (Chopra et al., 1979) or by inhalation (Thomson and Short, 1969; Yeates et al., 1975; Foster et al., 1976; van Hengstum et al., 1989). The measured average values of tracheal mucous clearance velocities varied between studies that used normal nonsmoking adults: 10.1 ± 3.5 (young adult) and 5.8 ± 2.6 mm/min (Goodman et al., 1978), 15.5 ± 0.69 mm/min (Chopra et al., 1979), 21.5 ± 5.5 mm/min (Santa Cruz et al., 1974), 20.1 ± 1.4 mm/min (Wood et al., 1975), and 5.5 ± 0.4 mm/min (Foster et al., 1980). Yeates et al. (1982) described their measured tracheal mucous velocities in terms of a log-normal distribution with a geometric mean of 4.0 mm/min with a coefficient of variation of 48%. It is not clear whether the differences in the measured velocities among the various studies were due to differences in the subjects or in the different measurement methods used. In addition to the values for normal nonsmoking adults given above, other measurements have been made on smokers and on subjects with lung disease.
Foster et al. (1980) measured clearance velocities for ferric oxide particles deposited in the large bronchi by gamma camera imaging and found average velocities to be 2.4 ± 0.5 mm/min, compared with tracheal velocities of 5.5 mm/min. However, no studies have measured the mucous clearance rates for the different generations of the bronchial and bronchiolar airways. Since clearance of particles from the different levels of the tracheobronchial tree has been considered to be an important variable in assessing the radiation dose to the bronchial epithelium from radon progeny-containing aerosols, investigators have resorted to theoretical models to calculate the generation-by-generation mucous clearance rates for the conducting airways. Clearance velocities calculated by Harley and Pasternack (1972), Lee et al. (1979), Haque and Geary (1981), Yu et al. (1983), and Cuddihy and Yeh (1988) are summarized in Table 5-2. In general, the values differ within a range of a factor of three. The basis for the differences seen in these models is the selection of the appropriate tracheal mucous velocity, since with all of the model structures there is a mathematical scaling of generation-specific velocities to that of the trachea, which is the only experimentally measured value. The second basis for the differences lies in the assumptions that were made regarding the dynamics of mucous and lung water production and absorption. For example, Lee et al. (1979) assumed a
TABLE 5-2 Mucous Clearance Velocities Derived from Theoretical Models
|
|
Mucous Clearance Velocity (mm/min) from the Following Models |
||||
|
Weibel Airway Generation |
Harley and Pasternack |
Lee et al. |
Haque and Geary |
Yu et al. |
Cuddihy and Yeh |
|
0 |
15.0 |
5.5 |
10 |
13 |
5.5 |
|
1 |
8.0 |
4.1 |
4.9 |
8.98 |
3.5 |
|
2 |
2.5 |
3.0 |
4.9 |
3.45 |
2.0 |
|
3 |
2.5 |
2.2 |
4.9 |
1.52 |
1.1 |
|
4 |
0.9 |
1.4 |
0.4 |
0.94 |
0.9 |
|
5 |
0.9 |
0.88 |
0.4 |
0.53 |
0.9 |
|
6 |
0.9 |
0.55 |
0.4 |
0.28 |
0.7 |
|
7 |
0.9 |
0.34 |
0.09 |
0.15 |
0.6 |
|
8 |
0.25 |
0.21 |
0.09 |
0.14 |
0.4 |
|
9 |
0.25 |
0.13 |
0.09 |
0.14 |
0.3 |
|
10 |
0.01 |
0.074 |
|
0.15 |
0.2 |
|
11 |
0.01 |
0.044 |
|
0.14 |
0.1 |
|
12 |
0.01 |
0.025 |
|
0.13 |
0.05 |
|
13 |
0.01 |
0.015 |
|
0.12 |
0.02 |
|
14 |
0.01 |
0.0082 |
|
0.11 |
0.007 |
|
15 |
0.01 |
0.0046 |
|
0.04 |
0.001 |
|
SOURCES: Harley and Pasternack (1972), Lee et al. (1979), Haque and Geary (1981), Yu et al. (1983), and Cuddihy and Yeh (1988). |
|||||
uniformly thick mucous blanket that undergoes neither secretion nor absorption throughout the length of the conducting airways; thus, the generation-specific mucous velocities scale as the ratio of the total airway perimeters, i.e., vα = v0d0/2αdα, where vα is the velocity in airway generation a, da is the diameter of an airway of generation α, and v0 and d0 are the velocity of clearance and diameter of the trachea, respectively. On the other hand, Altshuler et al. (1964) assumed that mucus is produced throughout the length of the conducting airways and at different generation-specific rates. Their methodology was used by Harley and Pasternack (1972), albeit with a different morphometric model. Currently, there is no firm basis on which to select one theoretical clearance model because of the lack of biological understanding of the mechanisms of secretion and absorption of either lung water or mucus. Nor are the sites of production and absorption well known. It is interesting to note, however, that the elimination of clearance in the Harley and Pasternack (1972) model resulted in a 50% increase in alpha-radiation dose to the larger bronchi compared with that obtained by assuming normal clearance.
Clearance of particles from the nonciliated or parenchymal regions of the lung have generally been done by quantitating the long-term retention of inhaled gamma-emitting radioactive particles within the thorax region by using appropriate gamma-ray detectors. As described above, the clearance of particles during the first 24 h after a brief exposure have been attributed to clearance from the ciliated tracheobronchial airways. Likewise, decreases in radioactivity
beyond the first 24 h has been assigned to clearance of particles from the nonciliated regions of the lung. The use of insoluble particles in which the radioactive label has been firmly bound has facilitated the measurement of long-term clearance, as the confounding influence of clearance via translocation of the radiolabel to blood becomes minimized. Studies have been reported in which humans have inhaled 51Cr-labeled Teflon particles (Philipson et al., 1985), 198Au-Fe2O3 (Stahlhofen et al., 1986), or 85Sr-or 88Y-labeled fused aluminosilicate particles (Bailey et al., 1985). The half-lives for retention have varied somewhat, but all have been long. Philipson et al. (1985) measured a mean half-life of 1,200 days for 68% of the deposited particles; Bailey et al. (1985) estimated a mechanical clearance half-life of about 700 days beyond 200 days after inhalation. This latter value was corroborated by the modeling of Cuddihy and Yeh (1988). The Task Group on Lung Dynamics (1966) has assigned a clearance half-life of 500 days to "class Y" insoluble particles. Thus, it is clear that retention of particles that deposit in the respiratory region of the lung is very long, i.e., hundreds of days. When compared with the effective radiological half-life of radon progeny, ![]() , then retention of the progeny in the pulmonary region can be considered to be infinite.
, then retention of the progeny in the pulmonary region can be considered to be infinite.
DOSIMETRY MODELS OF THE RESPIRATORY TRACT
Radon and radon progeny dosimetric models have evolved since the first efforts were made to develop themin the 1940s. During this radon period, a large number of models have been published. The impetus for the continued development of increasingly sophisticated models stems from the fact that the actual alpha-radiation doses to the presumed target tissue, the epithelial cells of the bronchial airways, cannot actually be measured, thus requiring dosimetric modeling, and because better data relating to the key components of the dosimetric models have become available, i.e., the physical characteristics of mine and home atmospheres, improved morphometric measurements of the respiratory tract, better deposition modeling of aerosols in the different regions of the respiratory tract, more detailed modeling of clearance phenomena, particularly in the tracheobronchial airways, and evolving views concerning the critical cells at risk to the development of alpha-radiation-induced lung cancer.
Several documents have summarized the radon dosimetric models that have developed over the years. NCRP (1984) lists 48 references through 1981 and remarks on the evolution in thinking and increasing sophistication of the models as well as the dose conversion factors in rad per working level month (WLM), and derived limits for exposure to both radon gas and radon progeny. The investigators noted a trend of decreasing values of the dose conversion factor toward 1 rad/WLM as the unattached fraction decreased and the activity becomes attached to intermediate-sized aerosols (tenths of a micrometer).
James (1988) has updated the listing of radon dosimetry models to include
the newer works of Hofmann (1982), Harley and Pasternack (1982), and the NEA Experts Report (1983), as well as the model of James (1988). In examining the dose conversion factors, James (1988) pointed out the importance of determining separately the dose conversion factors for attached and unattached radon progeny. This facilitates comparison of the different dosimetric models on a more equivalent basis. Thus, for the attached fraction, the dose conversion factors have converged somewhat since 1980, to a range of 0.2 to 1.3 rad/WLM; the variations were attributed mainly to the assumed aerosol particle size and the depth of the target cells. For unattached radon progeny, the dose conversion factors for most estimates were mostly in the range of 10 to 20 rad/WLM.
To date, there remains little consensus as to the atmospheric characteristics of radon progeny aerosols in mine and home environments (NCRP, 1984; ICRP, 1987; NRC, 1988; United Nations Scientific Committee on the Effects of Atomic Radiation, 1988). Nor is there agreement as to which choices in dosimetric model structure and parameter values are most appropriate. This difficulty arises because of a general lack of either relevant data (e.g. in upper airway morphometry, regional deposition, and mucous clearance) or understanding of the nature of the different processes that must be considered in calculating alpha-radiation doses to airway epithelial cells. As the knowledge base matures, construction of better models will be possible. Later sections of this report deal with updating knowledge of radon and radon progeny dosimetry as it applies to the different scenarios specific to exposure within a mine environment, for which epidemiological data are available, and in homes, for which human exposure-response data are being developed.
REFERENCES
Albert, R. E., M. Lippmann, J. Spiegelman, A. Liuzzi, and N. Nelson. 1967. The deposition and clearance of radioactive particles in the human lung. Arch. Environ. Health 14:10-15.
Altshuler, B. 1959. Calculation of regional deposition of aerosol in the respiratory tract. Bull. Math. Biophys. 21:257-270.
Altschuler, B., N. Nelson, and M. Kuschner. 1964. Estimation of lung tissue dose from the inhalation of radon and daughters. Health Phys. 10:1137-1161.
Altshuler, B., E. D. Palmes, and N. Nelson. 1967. Regional aerosol deposition in the human respiratory tract. Pp. 323-337 in Inhaled Particles and Vapors II, C. N. Davies, ed. Oxford: Pergamon Press.
Andersen, I., G. R. Lundquist, and D. F. Proctor. 1971. Human nasal mucosa . Arch. Environ. Health 23:408-420.
Bailey, M. R. 1984. Assessment of the dose to the nasopharyngeal region from inhaled radionuclides. Pp. 273-280 in Lung Modelling for Inhalation of Radioactive Materials, H. Smith, and G. Gerber, eds. Brussels: Commission of the European Communities.
Bailey, M. R., F. A. Fry, and A. C. James. 1985. Long-term retention of particles in the human respiratory tract. J. Aerosol Sci. 16(4):295-305.
Bang, B. G., A. L. Mukherjee, and F. B. Bang. 1967. Human nasal mucus flow rates. Johns Hopkins Med. J. 121:38-48.
Beekmans, J. M. 1965. Correction factor for size-selective sampling results, based on a new computed alveolar deposition curve. Ann. Occup. Hyg. 8:221-231.
Bowes, S. M., and D. L. Swift. 1989. Deposition of inhaled particles in the oral airway during oronasal breathing. Aerosol Sci. Tech. 11:157-167.
Brain, J. D., and P. A. Valberg. 1979. Deposition of aerosol in the respiratory tract. Am. Rev. Respir. Dis. 120:1325-1373.
Camner, P., and K. Philipson. 1978. Human alveolar deposition of 4 µm Teflon particles. Arch. Environ. Health 33:181-185.
Chamberlain, A. C., and E. D. Dyson. 1956. The dose to the trachea and bronchi from the decay products of radon and thoron. Br. J. Radiol. 29:317-325.
Chan, T. L., and M. Lippmann. 1980. Experimental measurements and empirical modelling of the regional deposition of inhaled particles in humans. Am. Ind. Hyg. Assoc. J. 41:399-409.
Cheng, Y. S., Y. Yamada, H. C. Yeh, and D. L. Swift. 1988. Diffusional deposition of ultrafine aerosols in a human nasal cast. J. Aerosol Sci. 19(6):741-751.
Chopra, S. K., G. V. Taplin, D. Elam, S. A. Carson, and D. Golde. 1979. Measurement of tracheal mucociliary transport velocity in humans—smokers versus non-smokers. Am. Rev. Respir. Dis. 119(Suppl.):205.
Ciurea, D., and J. Gil. 1989. Morphometric study of human alveolar ducts based on serial sections. J. Appl. Physiol. 67:2512-2521.
Clarke, S. W., and D. Pavia. 1984. Aerosols and the lung. Boston: Butterworths.
Cohen, B. S. 1987. Deposition of ultrafine particles in the human tracheobronchial tree: A determinant of the dose from radon daughters. Pp. 475-486 in Radon and Its Decay Products, P. H. Hopke, ed. Washington, D.C.: American Chemical Society.
Cohen, B. S., N. H. Harley, R. B. Schlesinger, and M. Lippmann. 1988. Nonuniform particle deposition on tracheobronchial airways: Implications for lung dosimetry. Ann. Occup. Hyg. 32:1045-1053.
Cuddihy, R. G., and H. C. Yeh. 1988. Respiratory tract clearance of particles and substances dissociated from particles. Pp. 169-193 in Inhalation Toxicology: The Design and Interpretation of Inhalation Studies and Their Use in Risk Assessment, D. Dungworth, G. Kimmerle, J. Lewkowski, R. McClellan, and W. Stober, eds. New York: Springer-Verlag.
Dagle, G. E., J. E Park., E. S. Gilbert, and R. E. Weller. 1989. Risk estimates for lung tumours from inhaled 239PuO2, and 238PuO2, and 239Pu(NO3)4 in beagle dogs . Radiat. Prot. Dosim. 26:173-176.
Dautrebande, L., and W. Walkenhorst. 1961. Deposition of NaCl microaerosols in the respiratory tract. Arch. Environ. Health 3:411-419.
Davies, C. N. 1961. A formalized anatomy of the human respiratory tract. Pp. 82-87 in Inhaled Particles and Vapors, C. N. Davies, ed. Oxford: Pergamon Press.
Egan, M. J., and W. Nixon. 1985. A model of aerosol deposition in the lung for use in inhalation dose assessments. Radiat. Prot. Dosim. 11(1):5-17.
Egan, M. J., and W. Nixon. 1988. A modelling study of regional deposition of inspired aerosols with reference to dosimetric assessments. Ann. Occup. Hyg. 32:909-918.
Emmett, P. C., and R. J. Aitken. 1982. Measurements of the total and regional deposition of inhaled particles in the human respiratory tract. J. Aerosol Sci. 13(6):549-560.
Ewert, G. 1965. On the mucus flow rate in the human nose. Acta Otolaryngol. 200 (Suppl.):7-62.
Ferron, G. A. 1977. Deposition of polydisperse aerosols in two glass models representing the upper human airways. J. Aerosol Sci. 8:409-427.
Ferron, G. A., S. Hornik, W. G. Kreyling, and B. Haider. 1985. Comparison of experimental and calculated data for the total and regional deposition in the human lung. J. Aerosol Sci. 16(2):133-143.
Findeisen, W. 1935. Über das Absetzen kleiner, in der Luft suspendierter Teilchen in der menschlichen Lunge bei der Atmung. Pflügert Arch Ges. Physiol. 236:367-379.
Foord, N., A. Black, and M. Walsh. 1978. Regional deposition of 2.5-7.5 µm diameter inhaled particles in healthy male non-smokers. J. Aerosol Sci. 9:343-357.
Foster, W. M., E. H. Bergofsky, D. E. Bohning, M. Lippmann, and R. E. Albert. 1976. Effect of adrenergic agents and their mode of action on mucociliary clearance in man. J. Appl. Physiol. 41(2):146-152.
Foster, W. M., E. Langenback, and E. H. Bergofsky. 1980. Measurement of tracheal and bronchial mucus velocities in man: Relation to lung clearance. J. Appl. Phys. Respir. Environ. Exercise Phys. 48(6):965-971.
Friedman, M., F. D. Stott, D. O. Poole, R. Dougherty, G. A. Chapman, H. Watson, M. A. Sackner. 1977. A new roentgenographic method for estimating mucous velocity in airways. Am. Rev. Respir. Dis. 115:67-72.
Fry, F. A., and A. Black. 1973. Regional deposition and clearance of particles in the human nose. Aerosol Sci. 4:113-124.
Gamsu, G., R. M. Weintraub, and J. A. Nadel. 1973. Clearance of tantalum from airways of different caliber in man evaluated by a roentgenographic method. Am. Rev. Respir. Dis. 107:214-224.
Gehr, P., M. Bachofen, and E. R. Weibel. 1978. The normal human lung: Ultrastructure and morphometric estimation of diffusion capacity. Respir Phys. 32:121-140.
George, A., and A. J. Breslin. 1969. Deposition of radon daughters in humans exposed to uranium mine atmospheres. Health Phys. 17:115-124.
Gerrity, T R., P. S. Lee, F. J. Hass, A. Marinelli, P. Werner, and R. V. Lourenco. 1979. Calculated deposition of inhaled particles in the airway generations of normal subjects. J. Appl. Physiol. 47:867-873.
Goodman, R. M., B. M. Yergin, J. F. Landa, M. H. Golinvaux, and M. A. Sackner. 1978. Relationship of smoking history and pulmonary function tests to tracheal mucous velocity in nonsmokers, young smokers, ex-smokers, and patients with chronic bronchitis. Am. Rev. Respir. Dis. 117:205-214.
Gradon, L., and D. Orlicki. 1990. Deposition of inhaled aerosol particles in a generation of the tracheobronchial tree. J. Aerosol Sci. 21:3-19.
Gradon, L., and C. P. Yu. 1989. Diffusional particle deposition in the human nose and mouth. Aerosol Sci. Tech. 11:213-220.
Guilmette, R. A., J. D. Wicks, and R. K. Wolff. 1989. Morphometry of human nasal airways in vivo using magnetic resonance imaging. J. Aerosol Med. 2:365-377.
Gurman, J. L. 1983. Particle deposition patterns in human upper bronchial airways undera simulated inspiratory flow. Ph.D. dissertation. Institute of Environmental Medicine, New York University, New York , N.Y.
Gurman, J. L., M. Lippmann, and R. B. Schlesinger. 1984. Particle deposition in replicate casts of the human upper tracheobronchial tree under constant and cyclic inspiratory flow. I. Experimental. Aerosol Sci. Tech. 3:245-252.
Haefeli-Bleuer, B., and E. R. Weibel. 1988. Morphometry of the human pulmonary acinus. Anatomic Rec. 220:401-414.
Haight, J. S., and P. Cole. 1983. The site and function of the nasal valve. Laryngoscope 93:49-55.
Haque, A. K. M. M., and M. J. Geary. 1981. The radiation dose to the respiratory system due to the daughter products of radon. Pp. 113-127 in Seventh Symposium on Microdosimetry, J. Booz, G. Ebert, and H. D. Hartfiel, eds. Oxford: Harwood Academic Publishers Ltd.
Harley, N. H., and B. S. Pasternack. 1972. Alpha absorption measurements applied to lung dose from radon daughters. Health Phys. 23:771-781.
Harley, N. H., and B. S. Pasternack. 1982. Environmental radon daughter alpha dose factors in a five-lobed human lung. Health Phys. 42:789-799.
Heyder, J., and G. Rudolf. 1977. Deposition of aerosol particles in the human nose. Pp. 107-126 in Inhaled Particles IV, Part 1, W. H. Walton and B. McGovern, eds. Oxford: Pergamon Press.
Heyder, J., and G. Rudolf. 1984. Mathematical models of particle deposition in the human respiratory tract. J. Aerosol Sci. 15:697-707.
Heyder, J., J. Gebhart, G. Rudolf, C. E Schiller, and W. Stahlhofen. 1986. Deposition of particles in the human respiratory tract in the size range 0.005-15 µm. Aerosol Sci. 17:811-825.
Hofmann, W. 1982. Cellular lung dosimetry for inhaled radon decay products as a base for radiation-induced lung cancer risk assessment. 1. Calculation of mean cellular doses. Radiat. Environ. Biophys. 20:95-112.
Horsfield, K., and G. Cumming. 1968. Morphology of the bronchial tree in man. J. Appl. Physiol. 24:373-383.
Horsfield, K., G. Dart, D. E. Olson, G. F. Filley, and G. Cumming. 1971. Models of the human bronchial tree. J. Appl. Physiol. 31:207-217.
Hounam, R. F., A. Black, and M. Walsh. 1971. The deposition of aerosol particles in the nasopharyngeal region of the human respiratory tract. Pp. 71-80 in Inhaled Particles III, Volume 1, W. H. Walton, ed. Old Woking, Surrey, England: The Gresham Press.
Ingham, D. B. 1976. Diffusion of aerosols from a stream flowing through a cylindrical tube. J. Aerosol Sci. 6:125-132.
International Commission on Radiological Protection (ICRP). 1974. Report of the Task Group on Reference Man, W. S. Snyder, M. J. Cook, E. S. Nasset, L. R. Karhausen, G. P. Howells, and I. H. Tipton, eds. Oxford: Pergamon Press.
International Commission on Radiological Protection (ICRP). 1987. Lung Cancer Risk from Indoor Exposure to Radon Daughters. Publ. No. 50. Oxford: Pergamon Press.
James, A. C. 1988. Lung dosimetry. Pp. 259-310 in Radon and Its Decay Products in Indoor Air , W. W. Nazaroff and A. V. Nero, eds. New York: John Wiley & Sons.
Johnson, J. R., K. D. Isles, and D. C. F. Muir. 1977. Inertial deposition of particles in human branching airways. Pp. 61-73 in Inhaled Particles IV, Part 1, W. H. Walton and B. McGovern, eds. Oxford: Pergamon Press.
Koblinger, L., and W. Hofmann. 1985. Analysis of human lung morphometric data for stochastic aerosol deposition calculations. Phys. Med. Biol. 30:541-556.
Lambert, B. E., M. L. Phipps, P. J. Lindop, A. Black, and S. R. Moores. 1982. Induction of lung tumours in mice following the inhalation of 239PuO2. Pp. 370-375 in Proceedings of the 3rd International Symposium on Radiation Protection—Adv. in Theory and Practice, Vol. 1. Inverness, Scotland: Society for Radiation Protection.
Landahl, H. D. 1950. On the removal of air-borne droplets by the human respiratory tract. II. The nasal passages. Bull. Math. Biophys. 12:161-169.
Landahl, H. D., and S. Black. 1947. Penetration of air-borne particulates through the human nose. J. Ind. Hyg. Toxicol. 29:269-277.
Lee, P. S., T. R. Gerrity, E J. Hass, and R. V. Lourenco. 1979. A model for tracheobronchial clearance of inhaled particles in man and a comparison with data. IEEE Trans. Biomed. Eng. BME-26:624-630.
Lee, W. C., and C. S. Wang. 1977. Particle deposition in systems of repeatedly bifurcating tubes. Pp. 49-59 in Inhaled Particles IV, Part 1, W. H. Walton and B. McGovern, eds. Oxford: Pergamon Press.
Lippmann, M. 1970. Deposition and clearance of inhaled particles in the human nose. Ann. Otol. Rhinol. Laryngol. 79:519-528.
Lippmann, M. 1977. Regional deposition of particles in the human respiratory tract. Pp. 213-232 in Reaction to Environmental Agents, D. H. K. Lee, H. L. Falk, S. D. Murphy, and S. R. Geiger, eds. Bethesda, Md.: American Physiological Society.
Lippmann, M., and R. E. Albert. 1969. The effect of particle size on the regional deposition of inhaled aerosols in the human respiratory tract. Am. Ind. Hyg. Assoc. J. May/June:257-275.
Lippmann, M., R. E. Albert, and H. T. Peterson. 1971. The regional deposition of inhaled aerosols in man. Pp. 105-122 in Inhaled Particles III, Volume 1, W. H. Walton, ed. Old Woking, Surrey: The Gresham Press.
Lippmann, M., D. B. Yeates, and R. E. Albert. 1980. Deposition retention and clearance of inhaled particles. Br. J. Ind. Med. 37:337.
Lundgren, D. L., F. F. Hahn, A. H. Rebar, and R. O. McClellan. 1983. Effects of the single or repeated inhalation exposure of Syrian hamsters to aerosols of 239PuO2. Int. J. Radiat. Biol. 43:1-18.
Martin, D., and W. Jacobi. 1972. Diffusion deposition of small-sized particles in the bronchial tree. Health Phys. 23:23-29.
Martonen, T. B. 1983. Measurement of particle dose distribution in a model of a human larynx and tracheobronchial tree. J. Aerosol Sci. 14:11-22.
Martonen, T. B., and J. Lowe. 1983. Assessment of aerosol deposition patterns in human respiratory tract casts. Pp. 151-164 in Aerosols in the Mining and Industrial Work Environments, Vol. 1, Fundamentals and Status, V. A. Marple and B. Y. H. Liu, eds. Ann Arbor, Mich.: Ann Arbor Science.
Mercer, T. T. 1975. The deposition model of the task group on lung dynamics: A comparison with recent experimental data. Health Phys. 29:673-680.
Morrow, P. E., and C. P. Yu. 1985. Models of aerosol behavior in airways. Pp. 150-191 in Aerosols in Medicine, Principles, Diagnosis and Therapy, M. F. Newhouse and M. B. Dolovich, eds. New York: Elsevier Science Publishers.
National Research Council (NRC). 1988. Health Risks of Radon and Other Internally Deposited Alpha Emmitters. BEIR IV. Committee on the Biological Effects of Ionizing Radiation. Washington, D.C.: National Academy Press.
National Council on Radiation Protection and Measurements (NCRP). 1984. Evaluation of Occupational and Environmental Exposures to Radon and Radon Daughters in the United States. Bethesda, Md.: National Council on Radiation Protection and Measurements.
Nikiforov, A. I., and R. B. Schlesinger. 1985. Morphometric variability of the human upper bronchial tree. Respir. Physiol. 59:289-299.
Nuclear Energy Agency Group Experts (NEA). 1983. Dosimetry Aspects of Exposure to Radon and Thoron Daughter Products. Paris: Organization for Economic Cooperation and Development.
Olson, D. E., M. F. Sudlow, K. Horsfield, and G. F. Filley. 1973. Convective patterns of flow during inspiration. Arch. Intern. Med. 131:51-57.
Parker, H., K. Horsfield, and G. Cumming. 1971. Morphology of distal airways in the human lung. J. Appl. Physiol. 31:386-391.
Pattle, R. E. 1961. The retention of gases and particles in the human nose. Pp. 302-309 in Inhaled Particles and Vapors I, C. N. Davies, ed. Oxford: Pergamon Press.
Phalen, R. F., M. J. Oldham, C. B. Beaucage, T. T. Crocker, and J. D. Mortensen. 1985. Postnatal enlargement of human tracheobronchial airways and implications for particle deposition. Anat. Rec. 212:363-380.
Phalen, R. F., M. J. Oldham, and W. J. Mautz. 1989. Aerosol deposition in the nose as a function of body size. Health Phys. 57:299-305.
Philipson, K., R. Falk, and P. Camner. 1985. Long-term lung clearance in humans studied with teflon particles labeled with chromium-51. Exp. Lung Res. 9:31-42.
Proctor, D. F. 1973. Clearance of inhaled particles from the human nose. Arch. Intern. Med. 131:132-139.
Proctor, D. F., and H. N. Wagner, Jr. 1965. Clearance of particles from the human nose. Arch. Environ. Health 11:377-381.
Proctor, D. F., and D. L. Swift. 1971. The nose—A defence against the atmospheric environment. Pp. 59-70 in Inhaled Particles III, Vol. 1, W. H. Walton, ed. Old Woking, Surrey: The Gresham Press.
Proctor, D. F., I. Andersen, and G. Lundquist. 1977. Nasal mucociliary function in humans. Pp. 427-452 in Respiratory Defense Mechanisms, Part I, J. D. Brain, D. F. Proctor, and L. M. Reid, eds. New York: Marcel Dekker.
Quinlan, M. F., S. D. Salman, D. L. Swift, H. N. Wagner, and D. F. Proctor . 1969. Measurement of mucociliary function in man. Am. Rev. Respir. Dis. 99:13-23.
Raabe, O. G. 1982. Deposition and clearance of inhaled aerosols. Pp. 28-68 in Mechanisms in Respiratory Toxicology, Vol. 1, H. Witschi and P. Nettesheim, eds. Boca Raton, Fla.: CRC Press.
Raabe, O. G., H. C. Yeh, G. M. Schum, and R. F. Phalen. 1976. Tracheobronchial geometry: human, dog, rat, hamster. Lovelace Foundation Report, U.S. Department of Energy Document No. LF-53. Springfield, Va.: NTIS.
Raabe, O. G., H. C. Yeh, G. J. Newton, R. F. Phalen, and D. J. Velasquez. 1977. Deposition of inhaled monodisperse aerosols in small rodents. P. 3 in Inhaled Particles IV, Part 1, W. H. Walton and B. McGovern, eds. Elmsford, N.Y.: Pergamon Press.
Rudolf, G., J. Gebhart, J. Heyder, C. F. Schiller, and W. Stahlhofen. 1986. An empirical formula describing aerosol deposition in man for any particle size. J. Aerosol Sci. 17:350-355.
Sanders, C. L., G. E. Dagle, W. C. Cannon, G. J. Powers, and D. M. Meier. 1977. Inhalation carcinogenesis of high fired 238puO2 in rats. Radiat. Res. 71:528-546.
Santa Cruz, R., J. Landa, J. Hirsch, and M. A. Sackner. 1974. Tracheal mucous velocity in normal man and patients with obstructive lung disease: Effects of terbutaline. Am. Rev. Respir. Dis. 109:458-463.
Scherer, P. W., L. H. Shendalman, and N. M. Greene. 1972. Simultaneous diffusion and convection in single breath lung washout. Bull. Math. Biophys. 34:393-412.
Schiller, C. F., G. J. Gebhart, G. Rudolf, and W. Stahlhofen. 1988. Deposition of monodisperse insoluble aerosol particles in the 0.005 to 0.2 µm size within the human respiratory tract. Ann. Occup. Hyg. 32:41-49.
Schlesinger, R. B., and M. Lippmann. 1972. Particle deposition in casts of the human upper tracheobronchial tree. Am. Ind. Hyg. Assoc. J. 33:237-251.
Schlesinger, R. B., D. E. Bohning, T. L. Chan, and M. Lippmann. 1977. Particle deposition in a hollow cast of the human tracheobronchial tree. J. Aerosol Sci. 8:429-445.
Schlesinger, R. B., J. L. Gurman, and M. Lippmann. 1982. Particle deposition within bronchial airways: Comparisons using constant and cyclic inspiratory flows. Ann. Occup. Hyg. 26:47-64.
Schlesinger, R. B., J. Concato, and M. Lippmann. 1983. Particle deposition during exhalation: A study in replicate casts of the human upper tracheobronchial tree. In Aerosols in the Mining and Industrial Work Environments, Vol. 1, Fundamentals and Status, V. A. Marple and B. Y. H. Liu, eds. Ann Arbor, Mich.: Ann Arbor Science.
Scott, W. R., D. B. Taulbee, and C. P. Yu. 1978. Theoretical study of nasal deposition. Bull. Math. Biol. 40:581-603.
Soong, T. T, P. Nicolaides, C. P. Yu, and S.C. Soong. 1979. A statistical description of the human tracheobronchial tree geometry. Respir. Physiol. 37:161-172.
Stahlhofen, W., J. Gebhart, and J. Heyder. 1980. Experimental determination of the regional deposition of aerosol particles in the human respiratory tract. Am. Ind. Hyg. Assoc. J. 41:385-398.
Stahlhofen, W., J. Gebhart, J. Heyder, and G. Scheuch. 1983. New regional deposition data of the human respiratory tract. J. Aerosol Sci. 14:186-188.
Stahlhofen, W., J. Gebhart, G. Rudolf, and G. Scheuch. 1986. Measurement of lung clearance with pulses of radioactively-labeled aerosols. J. Aerosol. Sci. 17:333-336.
Stuart, B. O. 1984. Deposition and clearance of inhaled particles. Environ. Health Perspect. 55:369-390.
Swift, D. L. 1981. Aerosol deposition and clearance in the human upper airways. Ann. Biomed. Eng. 9:593-604.
Swift, D. L., and D. F. Proctor. 1976. Access of air to the respiratory tract. Pp. 63-93 in Respiratory Defense Mechanisms, J. D. Brain, D. F. Proctor, and L. M. Reid, eds. New York: Marcel Dekker.
Task Group on Lung Dynamics. 1966. Deposition and retention models for internal dosimetry of the human respiratory tract. Health Phys. 12:173-207.
Taulbee, D. B., and C. P. Yu. 1975. A theory of aerosol deposition in the human respiratory tract. J. Appl. Physiol. 38:77-85.
Thomson, M. L., and M. D. Short. 1969. Mucociliary function in health, chronic obstructive airway disease, and asbestosis. J. Appl. Physiol. 26:535-539.
Thurlbeck, W. M. 1967. The internal surface area of nonemphysematous lungs. Am. Rev. Respir. Dis. 95:765-773.
Tu, K. W., and E. O. Knutson. 1984. Total deposition of ultrafine hydrophobic and hygroscopic aerosols in the human respiratory system. Aerosol Sci. Tech. 3:453-465.
United Nations Scientific Committee on the Effects of Atomic Radiation. 1988. Sources, Effects and Risks from Ionizing Radiation. 1988 Report to the General Assembly. New York: United Nations.
van Hengstum, M., J. Festen, W. Buijs, W. van den Broek, and F. Corstens. 1989. Variability of tracheobronchial clearance in healthy non-smoking subjects. Respiration 56:94-102.
Weibei, E. R., ed. 1963. Morphometry of the Human Lung. New York: Academic Press.
Wood, R. E., A. Wanner, J. Hirsch, and P.M. Farrell. 1975. Tracheal mucociliary transport in patients with cystic fibrosis and its stimulation by terbutaline. Am. Rev. Repir. Dis. 111:733-738.
Yeares, D. B., and N. Aspin. 1978. A mathematical description of the airways of the human lungs. Respir. Physiol. 32:91-104.
Yeates, D. B., N. Aspin, H. Levison, M. T. Jones, and A. C. Bryan. 1975. Mucociliary tracheal transport rates in man. J. Appl. Physiol. 39:487-495.
Yeates, D. B., T. R. Gerrity, and C. S. Garrard. 1982. Characteristics of tracheobronchial deposition and clearance in man. Ann. Occup. Hyg. 26:245-257.
Yeh, H. C., and G. M. Schum. 1980. Models of human lung airways and their application to inhaled particle deposition. Bull. Math. Biol. 42:461-480.
Yergin, B. M., K. Saketkhoo, E. D. Michaelson, S. M. Serafini, K. Kovitz, and M. A. Sackner. 1978. A roentgenographic method for measuring nasal mucous velocity. J. Appl. Physiol. Respir. Environ. Exercise Physiol. 44:964-968.
Yu, C. P. 1978. Exact analysis of aerosol deposition during steady state breathing. Powder Technol. 21:55-62.
Yu, C. P., and C. K. Diu. 1982. A comparative study of aerosol deposition in different lung models. J. Am. Ind. Hyg. Assoc. 43:54-65.
Yu, C. P., P. Nicolaides, and T. T. Soong. 1979. Effect of random airway sizes on aerosol deposition. J. Am. Ind. Hyg. Assoc. 40:999-1005.
Yu C. P., C. K. Diu, and T. T. Soong. 1981. Statistical analysis of aerosol deposition in nose and mouth. J. Am. Ind. Hyg. Assoc. 42:726-733.
Yu, C. P., J. P. Hu, G. Leikauf, D. Spektor, and M. Lippmann. 1983. Mucociliary transport and particle clearance in the human tracheobronchial tree. Pp. 177-184 in Aerosols in the Mining and Industrial Work Environments, Vol. 1, Fundamentals and Status, V. A. Marple and B. Y. H. Liu, eds. Ann Arbor, Mich.: Ann Arbor Science.






























