4—
Lakes
OVERVIEW
The fact that lakes occupy such a small fraction of the landscape belies their importance as environmental systems and resources for human use. They are major recreational attractions for Americans. Sport fishing, swimming, and boating are highly popular pastimes, and lake-front property has a high economic value. Large lakes and reservoirs are used as drinking water supplies; the Great Lakes alone serve as the domestic water supply for approximately 24 million Americans, and many more Americans rely on man-made reservoirs and smaller lakes for their source of drinking water. Lakes are used by humans for many commercial purposes, including fishing, transportation, irrigation, industrial water supplies, and receiving waters for wastewater effluents. Aside from their importance for human use, lakes have intrinsic ecological and environmental values. They moderate temperatures and affect the climate of the surrounding land. They store water, thereby helping to regulate stream flow; recharge ground water aquifers; and moderate droughts. They provide habitat to aquatic and semiaquatic plants and animals, which in turn provide food for many terrestrial animals; and they add to the diversity of the landscape.
The myriad ways in which humans use lakes, along with the numerous pollutant-generating activities of society, have stressed lake ecosystems in diverse ways, frequently causing impairment of lake quality for other human uses. Stresses to lakes arise from easily
identifiable point sources such as municipal and industrial wastewater, from nonpoint degradation, from urban and agricultural runoff within a lake's watershed, and from more insidious long-range atmospheric transport of contaminants. Major categories of stresses include excessive eutrophication from nutrient and organic matter loadings; siltation from inadequate erosion control in agricultural, construction, logging, and mining activities; introduction of exotic species; acidification from atmospheric sources and acid mine drainage; and contamination by toxic (or potentially toxic) metals such as mercury and organic compounds such as polychlorinated biphenyls (PCBs) and pesticides. In addition, physical changes at the land-lake interface (e.g., draining of riparian wetlands) and hydrologic manipulations (e.g., damming outlets to stabilize water levels) also have major impacts on the structure and functioning of lake ecosystems.
No lake in the United States is entirely free from such stresses, but the stresses are not always severe enough to impair lake ecosystems or their usefulness for human activities. Nonetheless, thousands of U.S. lakes (and reservoirs) covering several million acres of water surface have become degraded to the extent that some type of activity is necessary to make them more usable resources and ecosystems.
Lake restoration is a relatively recent activity. Historically, the term restoration has been applied broadly in lake management to an array of actions aimed at improving lake conditions for designated human uses (e.g., contact recreation, fishing, water supply). Return of a lake to its pristine condition has not been an explicit goal of most lake restoration projects, although these actions often improve some aspects of a lake's ecological attributes. As such, most so-called lake restoration projects are actually rehabilitation efforts (in the sense of the definitions in Chapter 1), and many are merely designed to manage (mitigate) undesirable consequences of human perturbations. For reasons of historical precedence, a broader definition of the term restoration is used in this chapter, but a distinction is made between methods that improve ecosystem structure and function (restoration in the broad sense) and methods that merely manage the symptoms of stress. Lake restoration began in the United States about 20 years ago, primarily in response to problems of nutrient overenrichment. A lake improvement program, the Clean Lakes Program was established in 1975 within the U.S. Environmental Protection Agency by Section 314 of the 1972 Federal Water Pollution Control Act Amendments (P.L. 92-500). Between 1975 and 1985, federal funds were provided for Clean Lakes projects on 313 lakes in 47 states and Puerto Rico; 87 percent of the Clean Lakes funds have been used for lake improvement projects (U.S. EPA, 1985). Matching state and/or local
funds typically are involved in these projects, and several states with large numbers of lakes have developed their own programs. As problems of lake acidification became more widely recognized during the past decade, restoration of acidified lakes by addition of limestone has become a relatively common practice in some northeastern states, as well as in Scandinavia.
For long-term restoration, it is essential to control the source of the problem. In the case of eutrophication, this means decreasing the loading of nutrients, particularly phosphorus, from various watershed sources. In some cases, this also means that loadings of silt and organic matter must be decreased. Control of external sources is sufficient to return some lakes to their former conditions, but in many cases the changes in the lake have been so dramatic—major shifts in biota, loss of habitat, physical changes in bottom sediments, and lake hydrology—that merely turning off the loadings is not sufficient to improve water quality and ecosystem structure, at least in a reasonable time frame. In-lake restoration techniques must be employed.
Numerous methods have been developed to restore lakes or improve their condition; this chapter describes more than 25 such methods. Available methods range widely in effectiveness, cost, frequency of use, and range of applicability. For example, methods that require addition of chemical agents to lake water are limited to small-and medium-sized lakes for economic reasons. Methods that use biological agents are potentially effective at low cost even in large systems because of low initial costs and the absence of labor and maintenance expenses. Many methods are applicable only to a single type of problem (e.g., liming to mitigate acidification). Others are potentially useful in restoring lakes degraded by a range of stresses; for example, dredging may be used for siltation, nutrient buildup, and toxic contaminant problems. Because eutrophication is the most widespread and longest-studied lake problem, more methods have been developed to restore eutrophic lakes than to address all other problems put together. Aside from removing contaminated sediments by dredging or covering them with uncontaminated sediment, few methods are available to restore lakes degraded by toxic substances.
Our ability to assess the effectiveness of past lake restoration projects and to compare the effectiveness of different restoration methods is severely limited by three factors. First, and perhaps most important, surveillance of lake conditions for an adequate period of time before and after a restoration attempt has been done on relatively few lakes. In some cases, sufficient surveillance probably was done, but rigorous analysis and interpretation of the data were not a part of the surveillance effort. All too often the data are not readily
available for others to assess. Second, lake restoration projects usually are considered to be operational activities rather than research and development projects, and as a result they are designed to produce the desired effect—a restored lake—by whatever combination of methods seems likely to succeed. It usually is not possible to determine which of several techniques used simultaneously on a lake actually produced the measured improvements, even if detailed monitoring is done. Third, the goals of restoration projects are not always clearly defined, and it is difficult to judge the degree of success when clear objectives have not been set.
The above comments notwithstanding, many successful lake "restorations" have been documented, starting perhaps with the widely publicized case of Lake Washington, a large, deep lake in Seattle that was becoming increasingly eutrophic from municipal sewage effluent and was restored in the mid-1960s by diverting the effluent from the lake. Success in this and other cases generally has been defined in terms of restoring an aquatic resource for some human activities rather than restoring an ecosystem to its original condition. It is often assumed that improvements that benefit human uses of lakes lead to an improvement in the lake's ecology. There is no basis to assume, however, that water quality enhancements such as improved water clarity actually restore lake ecosystems to their original (presettlement) conditions. Restoration failures are less widely publicized, of course, but several cases have been described in which a project produced fewer improvements than anticipated in lake quality (see Appendix A). Analysis of these failures is important because we can learn as much about the factors leading to successful restoration from such projects as we can from success stories.
Lake restoration projects typically focus on restoring only one part (the lake) of a connected stream-wetland-lake system within a watershed. When wetlands are considered at all in lake restoration projects, it is typically for diversion of nutrient-laden storm water runoff or sewage effluent into the wetland in an effort to obtain nutrient uptake by wetland vegetation. Such diversions may provide a temporary lowering of nutrient loadings to lakes, but wetland flushing during high flow periods may result in little net annual retention of nutrients by the wetlands. The impacts of diversion on wetland ecology generally are not taken into account in deciding whether to proceed with such projects.
Although many techniques are potentially available to restore degraded lakes, the science of lake restoration is inexact, and the outcome of applying a given technique to a particular lake is difficult to predict accurately. Lake restoration technology can be advanced by
ensuring that projects are monitored adequately so that the effects of various manipulations can be assessed properly. In this context, a lake restoration project should be considered as part of a long-term, ongoing management program rather than a one-time, permanent solution to a lake's problems.
INTRODUCTION-IMPORTANCE OF LAKES
Humans have always been attracted to lakes. Human settlement on lakeshores can be explained by practical reasons—lakes provide food and drinking water and a convenient means for personal transport and conveyance of goods—but can there be any doubt that even the Neolithic Swiss lake dwellers enjoyed their homes partly because of the beauty of their surroundings? Today, we prize small inland lakes especially for their recreational assets, including their visual appeal and the feeling of being close to nature that a "day at the lake" provides. Fishing, swimming, and boating are highly popular pastimes throughout the United States. Recreational fishing on inland lakes is estimated to generate more than $1.3 billion (1985 dollars) in economic activity annually in the state of Minnesota alone (Minnesota Department of Natural Resources, Office of Public Information, unpublished data, 1990), and comparable figures can be cited for many other states. In urban areas, lakefront homes are in high demand and command premium price tags; lakefront property in rural areas has a high commercial value for development of vacation homes. All too often, the attributes that give rise to a lake's recreational value—clear, high-quality water; scenic shorelines; prized game fish—are impaired by developments that were stimulated by the presence of these values.
There are about 100,000 lakes with areas greater than 40 hectares (1 ha = 2.47 acres) in the conterminous United States (Duda et al., 1987). Although natural lakes are found in most of the 50 states, they are especially common in several regions, owing to specific geological conditions: in the Upper Midwest, New England, New York, and Alaska, as a result of glacial activity; in Florida, where most lake basins are the result of chemical dissolution of underlying limestone; along major rivers like the Mississippi, where channel meandering has formed lake basins; and in mountainous areas of the Far West, where glaciers and volcanic activity have produced most of the lakes. In regions where natural lakes are rare or absent, artificial lakes (reservoirs) have been developed by damming rivers and streams to provide the benefits (e.g., recreation, water supply, water storage capacity) that natural lakes provide elsewhere.
Large lakes and reservoirs are used as public water supplies; the American Water Works Association (Achtermann, 1989) estimates that 68 percent of the water used for domestic purposes by the 600 largest utilities (>50,000 customers) comes from impounded surface waters (natural lakes and man-made reservoirs). For simplicity, in this chapter the term lake refers both to natural impoundments and to man-made reservoirs. The five Great Lakes alone supply domestic water to some 24 million Americans. Lakes provide many other economic benefits to society and are used for such diverse purposes as commercial fishing, transportation, irrigation, and dilution of wastewater effluents. Not all of these uses are compatible. The use of lakes as receptacles for wastewater obviously is likely to impair their usefulness as water supplies and recreational resources, but more subtle incompatibilities also exist. For example, the production of warmwater game fish is enhanced by increasing nutrient levels, at least up to a point, but swimmers prefer water to be as clear (hence, unproductive) as possible.
STRESSES ON LAKES
Classes of Stresses and Their Effects
Lake ecosystems are subject to stress from a wide range of human activities within their watersheds and along their shorelines and from the variety of ways that humans use them. These stresses often have caused significant impairment of lake quality. Six major classes of stresses have been important in degrading the quality of U.S. lakes in recent decades:
-
excessive inputs of nutrient and organic matter, leading to eutrophication;
-
hydrologic and physical changes such as water-level stabilization;
-
siltation from inadequate erosion control in agricultural and mining activities;
-
introduction of exotic species;
-
acidification from atmospheric sources and acid mine drainage; and
-
contamination by toxic (or potentially toxic) metals such as mercury and organic compounds such as PCBs and pesticides.
In addition, chemical stresses to lakes can be categorized according to source as (1) point sources (such as municipal wastewater), which generally are the easiest to identify and control; (2) nonpoint or diffuse
sources such as urban and agricultural runoff from a lake's watershed; and (3) long-range atmospheric transport of contaminants (the most difficult to measure and control). These stresses result in a variety of impacts on lake quality relative to human use and ecological integrity.
The specific impacts of stresses on lake ecosystems depend on the nature of the stress and the characteristics of the lake, but some responses are common to several categories of stress. For example, stress-impacted lakes tend to lose sensitive native species. Their replacement by stress-tolerant native or exotic species often does not fully compensate for the loss and leads to lower biodiversity and simplified food webs. Many types of stress result in loss of habitat; often this is the proximate cause of species losses. Many kinds of stress produce ''nuisance conditions," that is, proliferation of a native or exotic organism or deterioration in a physical-chemical property (such as water clarity) to the extent that beneficial uses of the lake are impaired. Finally, the development of toxic levels of contaminants in biota results not only from direct loading of toxic materials to lakes but also from indirect effects of other stresses (e.g., solubilization of aluminum as pH is decreased by acid deposition).
EUTROPHICATION
Of the six categories of stress, problems related to nutrient overenrichment and excessive plant production are probably the most common and have received public and scientific attention for the longest time. Concern about lake eutrophication from municipal wastewater extends back at least to the 1940s and the classic studies of Sawyer (1947) on the relationship between springtime concentrations of inorganic phosphorus and nitrogen and the occurrence of algal blooms in summer. By the 1960s, widespread concern existed about increasing eutrophication of the Great Lakes, and nutrient enrichment problems were recognized in numerous inland lakes. A large-scale research program funded primarily by federal agencies was undertaken on eutrophication in the 1960s and 1970s. This program led to improved understanding of the extent of the problem in U.S. lakes, delineated specific causes of the problem in some lakes, generated quantitative relationships between rates of nutrient loadings (especially of phosphorus) to lakes and water column responses in the lakes, and developed techniques to restore lakes degraded by eutrophication.
Eutrophication results in numerous ecological and water quality changes in lakes. The chain of events leading to use impairment is
roughly as follows. Increased input of nutrients, especially phosphorus, leads to an increased incidence of nuisance blooms of algae (especially blue-green algae), leading to a loss of water clarity, a buildup of organic and nutrient-rich sediments, loss of oxygen from the bottom waters of the lake (which in turn, accelerates nutrient recycling processes), and changes in the lake's food web structure. Secondary nutrient limitation by silica or nitrogen that results when phosphorus levels are elevated also leads to changes in the phytoplankton community and to the development of nuisance species of algae (e.g., blue-green forms). Proliferation of macrophytes is also associated with eutrophication, especially in shallow lakes, but these problems are not tied directly to excessive rates of nutrient loading (see "Exotic Species," below). Although increases in nutrient levels enhance fish production, the loss of habitat (e.g., by sediment buildup, deoxygenation, undesirable proliferation of macrophytes) and food sources (by food web simplification) causes a shift from more desirable game fish to less desirable species, especially in more extreme cases of eutrophication. Stocking of exotics and overfishing exacerbate this problem. From a human use perspective these changes create numerous problems, including the following: fouling of boats and structures (by algal growths), loss of aesthetic appeal, accessibility problems for swimmers and boaters (because of macrophyte proliferation), economic damage to resort and property owners, and increased costs and technical difficulties of treating water for drinking purposes (because of taste and odor problems and increased potential for trihalomethane production).
The causes of eutrophication resulting from human activity are reasonably well understood. Once an oligotrophic lake has been made eutrophic, processes develop that may delay recovery after nutrient loadings have been decreased. If the hypolimnion becomes anoxic, recycling of phosphorus from the sediments is enhanced, in effect increasing the efficiency of use of the phosphorus input. During the eutrophic phase many changes may occur that will not be automatically reversed by a reduction in nutrient supply, such as loss of desirable macrophyte, invertebrate, and fish species. Nutrient reduction is a necessary, but not always a sufficient, condition for reversal of eutrophy.
Point sources of nutrients are the primary cause of excessive loadings in some lakes, but nonpoint sources (urban and agricultural runoff) contribute most of the nutrient input to the majority of U.S. lakes. Based on a modeling exercise with loading data on phosphorus for 255 lakes in the eastern United States, Gakstatter et al. (1978) concluded that only 18 to 22 percent of the lakes would show a
measurable improvement in trophic conditions (which they assumed would require at least a 25 percent reduction in phosphorus inputs) if an effluent standard of 1 mg of phosphorus per liter were imposed on municipal wastewater treatment plants. Only 28 percent of the lakes would show measurable improvement if all their point sources of phosphorus were removed. Thus, most of the lakes (72 to 82 percent) in this analysis would require control of nonpoint sources of nutrients to achieve measurable improvements in trophic conditions.
HYDROLOGICAL AND RELATED PHYSICAL CHANGES
The watersheds of lakes in urban and agricultural areas clearly are no longer ecologically the same as they were in presettlement days, and such land use changes are a primary cause of the stresses described in this section. What is not so widely recognized is the fact that important physical properties of lakes themselves, such as water residence time, water level, and basin morphology, are often modified significantly in developed areas. In turn, these changes can have untoward effects on water quality and ecological conditions. The importance of morphology in determining a lake's basic level of productivity is a fundamental concept in limnology.
Diversion of stream flow into lakes to provide water for urban or agricultural uses outside the watershed has occurred in some western states; Mono Lake, California is probably the best known example. The resulting decline in water supply to the lake has caused long-term lowering of the lake level, an increase in the lake's salinity, and ecological damage to tributary streams and to the lake itself (NRC, 1987). A much more widespread practice nationwide is the stabilization of lake levels by regulating outflows with a control structure (dam) at the lake outlet. This practice minimizes flooding of shoreline developments during wet periods and prevents loss of access to the lake due to receding shorelines during dry periods. However, long-term water-level stabilization also leads to loss of ephemeral wetlands in nearshore areas, converting them either to permanently dry upland areas or to lake littoral area. Fluctuating water levels are thought to have a cleansing effect on littoral sediments (oxidizing organic deposits); accumulation of such deposits in nearshore areas of lakes with stabilized water levels contributes to poor water quality and loss of fish spawning areas.
Changes in water level also affect fish reproduction directly by regulating access to spawning areas in the littoral zone, streams, or surrounding wetlands. Consequently, coordination between agencies that regulate water level and agencies that manage fisheries can
have significant benefits. For example, the level of Lake Mendota, Wisconsin, had generally been lowered in winter to protect shoreline structures from ice damage. As a consequence, northern pike were prevented from spawning in the marshes around the lake. This problem was recognized in 1987. Beginning in 1988, the water level was raised about 15 cm during the spawning season (late March to early April). Numbers of spawning northern pike increased about two-fold in 1988 and about eightfold in 1989 (Johnson et al., 1992). There has been no increase in the incidence of ice damage to shoreline structures.
Water residence times of lakes in developed areas are affected by water-level stabilization, as well as by diversion of streams into or out of a lake's drainage basin (thus also affecting watershed size and loading rates of nutrients and pollutants). Lake Okeechobee, Florida, is an extreme case of human-induced changes in lake morphometry, watershed area, water level, and other hydrologic characteristics that resulted in a variety of water quality problems (see Kissimmee River case study, Appendix A).
SEDIMENTATION
Problems of excessive sediment loading occur in lakes with large drainage basins where agricultural practices result in excessive soil erosion. Such problems are common in the central and southeastern parts of the United States, where row crop farming and erosive soils coexist, but some large reservoirs in the arid West also suffer from excessive sediment buildup. Siltation problems are significant in urban lakes as well. In extreme cases, excessive sedimentation leads to significant loss of reservoir storage capacity, diminishing the usefulness of lakes for regulating water availability (i.e., supplying water during droughts and controlling floods). Excessive sediment buildup renders large areas of lakes unusable for recreational purposes, as well as for fish spawning and habitat. Because nutrients (especially phosphorus) tend to adsorb onto sediments and because suspended sediments prevent penetration of light, lakes with very high loadings of sediment may not have sufficient plant productivity to support a good sport fishery; Lake Chicot, Arkansas, is an example (Stefan et al., 1990).
EXOTIC SPECIES
Lakes are island habitats. Like islands, they are highly susceptible to invasion by exotic species that lead to extirpation of native species
(Magnuson, 1976). In some cases, invasions by exotic species have had severe environmental and economic consequences. The most notorious species invasions have widespread effects that reverberate throughout an ecosystem. The seemingly random nature and explosive development of biological invasions have fascinated ecologists for many years (Elton, 1958); the status of basic research on this topic was reviewed by Mooney and Drake (1986).
Many thousands of acres of inland lakes suffer from problems of excessive macrophyte growths, and in most cases the problem plants are exotic (nonnative) species. Some of these plants were introduced to this country by the aquarium industry; others, such as water hyacinth (Eichhornia crassipes), were imported because they were regarded as visually attractive. The natural predators and pathogens that tend to keep the plant populations in check in their native lands usually are not present in this country. The resulting uncontrolled growth causes a variety of problems: clogging of irrigation canals, hydro-electric systems, and navigational waterways; flooding due to obstructed drainage systems; and impairment of boating and contact recreational activities (Barrett, 1989). Cases have been reported of swimmers becoming entangled in excessive growths of macrophytes and drowning. Dense beds of plants alter water chemistry and habitat structure, leading to changes in invertebrate and fish communities, and they are a major source of organic matter to the water column and sediments. Some exotic plants (e.g., purple loosestrife and water hyacinth) have low nutritive value to aquatic animals and provide a poor base for the food chain. Aquatic weed invasions contributing to major management problems include water hyacinth in 50 countries on five continents, kariba weed (Salvinia molesta) in tropical regions worldwide, hydrilla (Hydrilla verticillata) and Eurasian water milfoil (Myriophyllum spicatum) in North America, and Elodea canadensis in Europe (Hutchinson, 1975; Barrett, 1989).
Exotic species problems are by no means limited to plants. Benthic invertebrate invaders also have created problems. An example is the invasion of lakes throughout northern Wisconsin and Minnesota by the rusty crayfish, Orconectes rusticus (Lodge et al., 1985). This species displaces native species from their burrows, exposing them to predation. Rusty crayfish are voracious consumers of game fish eggs and obliterate macrophyte beds, essential habitat for recruitment of game fish (Lodge et al., 1985). Thus, the crayfish tend to eliminate their main predators, smallmouth bass. Ironically, the invasion originated with releases from anglers' bait buckets. Spread of the crayfish is now perpetuated by the development of commercial harvesting of the rusty crayfish (primarily for export to Scandinavia). Crayfishers
have transplanted rusty crayfish to new lakes to increase the harvestable resource.
Exotic fish have displaced native species, contributed to the collapse of fisheries, and even led to water quality problems (Magnuson, 1976; see Lake Michigan case study, Appendix A). The common carp, Cyprinus carpio, is not native to this country but was introduced to many northern lakes and rivers in the late 1870s by the U.S. Fish Commission in response to requests from European immigrants. Carp are widely eaten in European countries but are rarely consumed in this country and are not a sought-after game fish. Because carp are benthivorous (bottom feeders) and stir up bottom sediments, they accelerate nutrient recycling from sediments, destroy spawning areas for other fish, and cause turbidity problems in lakes and rivers.
The Great Lakes have a long and unfortunate history of invasions by exotic species. The sea lamprey (Petromyzon marinus), a large parasite of game fish, is a native of the Atlantic Ocean that made its way into Lake Erie through the Welland Canal in 1921. It gradually worked its way as far as Lake Superior, where it remains a significant cause of fish mortality (especially for lake trout). The lamprey has been controlled (but not eliminated) by applying a "lampricide," 3-trifluoromethyl-4 nitrophenol (TFM), to tributary streams where adult lamprey spawn. The TFM selectively kills young lamprey. The alewife, a small forage fish, was also introduced into the Great Lakes inadvertently, as a result of development of the St. Lawrence Seaway. The fish grew to great abundance in the 1960s, and episodes of massive mortality in alewife populations caused problems along urban beaches. The fish was controlled in the Great Lakes primarily by stocking the lakes with other exotic fish, coho, and Chinook salmon.
The latest in a series of exotic species to invade the Great Lakes, and potentially the most devastating, is the zebra mussel (Dreissena polymorpha). First found in Lake St. Clair in 1988, this rapidly spreading species was found throughout the western basin of Lake Erie in 1989 and as far as the Duluth-Superior harbor in western Lake Superior in 1990. The organism was most likely introduced to the Great Lakes by discharge of ballast water from oceangoing vessels. A native of Asia, the zebra mussel has been a problem in European waters for more than 100 years. It is already causing obstruction problems with water intake for power plants and municipal and industrial water treatment plants in Lake Erie. Because fouling organisms historically have not been a problem in inland waters of the United States and Canada, most facilities have not been designed to control or compensate for these problems, and the potential costs are enormous (Mackie et al., 1989). The zebra mussel has become abundant enough that it
may already have had an impact on the food web in Lake Erie. A filter feeder, it is thought to be responsible for an increase in water clarity in the lake during 1989 and 1990. Fishery scientists are concerned that the organism will divert enough primary and secondary production from pathways that support fish growth to affect the lake's economically important walleye fishery. No control techniques are currently available to address a problem of this magnitude. Although it is not yet found in U.S. waters outside the Great Lakes, the zebra mussel is expected to spread widely throughout the surface waters of the eastern United States over the next several years.
ACIDIFICATION
Acidification of poorly buffered lakes (and other surface waters) by acidic precipitation has been a major environmental issue in the United States and Canada (as well as parts of western Europe) for the past two decades. The ecological changes caused by acidification are fairly well understood (e.g., Schindler, 1988), but the severity of the problem is still controversial, despite more than a decade of extensive research. Acidification tends to simplify the biotic structure of lakes, as acid-sensitive species are lost and relatively fewer acid-tolerant species remain. However, ecological impacts generally are greater at the population level than at the community level, and effects on some integrative measures of ecosystem performance, such as total primary production and community respiration, have not been demonstrated conclusively, especially for mild levels of acidification.
In contrast, rates of decomposition of organic matter, especially leaves and other terrestrially produced materials, are slowed in acidic lakes (Perry et al., 1987; Brezonik et al., 1991 a), and certain pathways in the biogeochemical cycles of major elements such as nitrogen and sulfur may be altered or inhibited under acidic conditions (e.g., Rudd et al., 1988). Water column concentrations of several minor metals (manganese, iron, and especially aluminum) and trace metals (cadmium, lead, zinc, and mercury) are higher in acidic lakes because of increased solubility and decreased tendency to adsorb onto particles, and the free (uncomplexed) chemical forms of the metal ions trend to predominate in acidic waters. Other factors being equal this trend should increase metal bioaccumulation and toxicity to aquatic biota. Indeed, increased aluminum toxicity is thought to be a major factor in the loss of fish species in many acidic lakes, but the situation is less certain for other potentially toxic trace metals (Campbell and Stokes, 1985; Brezonik et al., 1991a). Increased competition for metal-binding sites on organisms by the higher H+ concentrations in acidic
waters may actually decrease biological uptake of trace metals and reduce their toxicity to aquatic biota.
From a perspective of water use, lake acidification has three major effects: loss of fish populations; increased water clarity, caused primarily by loss of colored organic matter (so-called humic material) from the water column; and increased abundance of acid-tolerant, filamentous algae (primarily Mougeotia), huge, unsightly masses of which may cover the bottom in littoral areas. Fish species differ widely in their sensitivity to acidity (Table 4.1). Smallmouth bass are much more sensitive than largemouth bass. Rainbow trout are impacted in the pH range 5.5 to 6.0; brook trout are much less sensitive. Perch survive and reproduce at pH 5, but survival of young-of-the-year perch is strongly affected at pH 4.7 (Brezonik et al., 1991b). Some Florida lakes with a pH as low as 4.5 have apparently healthy fish communities, although fish production is low because acidic lakes tend to be very oligotrophic. In general, fish production is much more closely related to a lake's nutritional status than to its pH. In a given species, adults are more tolerant than immature forms; lack of
TABLE 4.1 Approximate pH Range in Which Various Fish Species Suffer Reproductive Failure or Mortality
|
pH |
Species |
||||||
|
6.0 to 5.5 |
Smallmouth bass (Micropterus dolomieui) Walleye (Stizostedion vitreum) Rainbow trout (Oncorhynchus mykiss) Common shiner (Notropis comutus) Burbot (Lota lota) |
||||||
|
5.5 to 5.2 |
Lake trout (Salvelinus namaycush) Trout perch (Percopsis omiscomaycus) Fathead minnow (Pimephales promelas) |
||||||
|
5.2 to 4.7 |
Brook trout (Salvelinus fontinalis) Brown bullhead (Ictalurus nebulosus) White sucker (Catostomus commersoni) Largemouth bass (Micropterus salmoides) Rock bass (Ambloplites rupestris) |
||||||
|
4.7 to 4.5 |
Cisco (Coregonus artedii) Yellow perch (Perca flavescens) Lake chub (Couesius plumbeus) |
||||||
|
NOTE: Compiled by the committee from various sources. |
|||||||
spawning success and year-class recruitment failures occur before the condition of adults or their mortality is affected. Without question, potential damage to fishing has caused the greatest public concern about lake acidification, but the actual extent of losses has been very difficult to quantify. Several early studies purporting to show that acidification caused a significant loss of game fish (e.g., trout) in Adirondack lakes over the past 50 to 60 years were shown later to be flawed. For example, trout populations disappeared from some lakes because fishery management practices changed (i.e., stocking of young fish was stopped for unknown reasons). Nonetheless, the recently completed integrated assessment of the National Acid Precipitation Program (NAPAP, 1990a) concluded "with reasonable confidence" that acidification had resulted in a loss of one or more fish populations in about 16 percent of the Adirondack lakes.
On the one hand, the lakes most sensitive to acidification tend to be small and relatively unproductive (oligotrophic). On the other hand, these lakes tend to occur in relatively unspoiled forested areas and are valued for their pristine nature. It is difficult to compare the value of the total experience of catching a trout in such a lake (on a dollar-per-fish or dollar-per-pound basis) with that of catching a perch in a more highly developed lake. Moreover, small lakes do act as sensitive indicators of environmental damage and may be viewed as early warning indicators of environmental stress.
CONTAMINATION BY TOXIC SUBSTANCES
Lakes are sinks for many materials (i.e., inputs from their drainage basins exceed losses through outlet streams). Such materials tend to accumulate in certain compartments of lakes—ultimately in bottom sediments, but also (and more importantly) in biotic components. In several well-documented cases, toxic substances (metals or synthetic organic compounds) have accumulated to problem proportions in the food web of a lake (particularly in game fish) because of industrial accidents or inadequate disposal practices, but in other cases, the source of the toxic material is more diffuse—nonpoint source runoff or deposition from the atmosphere.
The list of metals that have been identified with use impairment in lakes is lengthy and includes silver, arsenic, cadmium, copper, mercury, manganese, lead, selenium, and zinc. Excessive levels of selenium in two North Carolina reservoirs resulted from discharges from coal-fired power plants (U.S. EPA, 1989) and caused drastic declines in fish populations and reproduction. Mining and mineral processing
activities caused accumulations of toxic metals in biota of Lake Coeur d'Alene, Idaho.
Long-range atmospheric transport from widespread sources is blamed for high body burdens of mercury (Hg) in the fish of many otherwise pristine lakes in forested regions of the Upper Midwest (Henning, 1989; Swain and Helwig, 1989). The problem in these states is more pronounced in low-alkalinity (acid-sensitive) lakes, but levels of bioaccumulation are not closely correlated with water pH. Mercury contamination of fish is at least indirectly related to acidic deposition in that fossil fuel burning by power plants contributes to both problems. The accumulation of mercury varies widely among different species of fish; biomagnification proceeds as mercury moves through the food web, and top carnivores such as walleye have the highest body burdens. Within a given species, body burdens increase with size (and age) of the fish.
Several states routinely issue consumption advisories related to mercury contamination of fish in lakes, and there is much concern about the economic impacts of these advisories on sport fishing in the affected regions. The nature of the advisories varies from state to state, and depending on the level of contamination, the advisories may recommend that a certain size range and species of fish not be eaten at all or that consumption be limited to one meal per week or per month. Problems caused by mercury in lakes are not limited to human consumption of contaminated fish; wildlife whose diet includes fish are also at risk. Body burdens of mercury in piscivorous loons in northern Minnesota are high enough to cause acute toxicity and may explain some incidents of loon mortality (Swain and Helwig, 1989).
Contamination problems involving organochlorine compounds such as pesticides and PCBs have been induced in lakes by all three types of sources for chemical stress (point sources, nonpoint watershed sources, and long-range atmospheric transport). High levels of PCBs in fish of the lower Great Lakes are attributed to general, widespread use of these chemicals from the 1920s to the 1970s, but localized cases of sediment contamination can usually be traced to one or a few specific industrial operations. For example, severe contamination of sediments in Waukegan harbor (Lake Michigan) occured as the result of disposal practices by one manufacturer. At the other extreme, high body burdens of PCBs are found in some large lake trout in Lake Superior (at levels sufficient to cause a consumption advisory), in spite of the fact that the lake has only minor point sources and nonpoint watershed sources of PCBs. Atmospheric transport (on scales of hundreds or even thousands of miles) is the
principal source of PCBs in Lake Superior (Eisenreich, 1987) and the major source of toxaphene for all the Great Lakes. A chlorinated insecticide, toxaphene was used principally on cotton fields in southern states to control the boll weevil until it was banned in the 1970s. Nonetheless, residues of toxaphene are commonly found in water and fish of the Great Lakes (Camanzo et al., 1987). Another illustration of the importance of long-range atmospheric transport is provided by Siskiwit Lake, on Isle Royale, more than 90 km from the nearest shore in western Lake Superior. The island is a wilderness area (and a national park), and Siskiwit Lake has no watershed sources of contamination (past or present). Nonetheless, elevated levels of polychlorinated dibenzodioxins (PCDDs) and PCBs are found in fish from the lake (Swain, 1978; Czuczwa et al., 1984, 1985), a fact that can be attributed only to atmospheric inputs.
Responses to Stresses-Status of U.S. Lakes
OVERVIEW
Several assessments of conditions in U.S. lakes have been made in the past two decades (Ketelle and Uttormark, 1971; Duda and Johnson, 1984; ASIWPCA, 1984, 1985; U.S. EPA, 1989, 1990b). All were based on responses to questionnaires to administrators of state water agencies. The earliest surveys focused on trophic conditions (eutrophication was considered the major lake problem in the 1960s and 1970s), but more recent surveys also considered other types of degradation. The responses are largely qualitative and vary widely among the states in accuracy and completeness. States use differing criteria for classifying lakes and defining problem conditions, and all have incomplete data. Some states reported on only a small fraction of their lakes in a given assessment. For example, Florida, which has about 7,700 lakes, assessed trophic conditions in only 91 lakes for EPA's 1988 water quality survey (U.S. Environmental Protection Agency, 1989, 1990). Only one Florida lake was listed as hypereutrophic and thirteen as eutrophic; this grossly understates the seriousness of eutrophication problems in that state. States that lack active lake programs did not participate in some of the surveys. Omitted from the data are thousands of private lakes and small water bodies. All U.S. Army Corps of Engineers projects, which include 783 reservoirs with a total of 27,000 km2(66.7 million acres; Kennedy and Gaugush, 1988), have also been excluded from the surveys.
The most recent survey (U.S. EPA, 1989, 1990), which was conducted in 1988, compiled data from 40 responding states or territories.
All 40 respondents provided some assessment of surface acreage of lakes supporting designated uses versus acreage of lakes with impaired or partially impaired uses, and threatened lakes (Table 4.2), but the percentage of total acreage that was assessed in a given state ranged from about 25 to 100 percent, and only 32 states specified the basis of their assessment decisions. Only 26 states provided information on the degree of impairment (minor, moderate, or major), 33 provided data on the nature (causes) of impairment (e.g., nutrients, siltation, and toxic substances), and 28 provided information on the sources of pollution (agriculture, storm sewers, municipal wastewater, and so on). The survey concluded that 26 percent of the assessed lake acreage suffered from some kind of use impairment (Table 4.2); of the 16.3 million acres assessed, almost 4.3 million acres were impaired (defined here as acreage not supporting or only partially supporting the designated uses). An additional 18 percent of the assessed acreage (2.9 million acres) was reported to be threatened. Four states (Florida, North Dakota, South Dakota, and Wisconsin) each had more than 600,000 acres of threatened or impaired waters (Table 4.2). Eight states had 120,000 acres of lakes with threatened or impaired waters, and an additional nine states had at least 160 km2 (40,000 acres) of lake waters in those categories. These 21 states accounted for most of the threatened or impaired waters in this survey.
The EPA's 1988 survey identified 12 causes of impairment and estimated the percentage of total use-impaired lake acreage affected by each of these, as well as by identified sources of pollution for 33 responding states (Table 4.3A,B). Because of the nature of the survey, the numbers reported in Tables 4.2 and 4.3A and B cannot be extrapolated to the total population of lakes in the country, and probably should not be used even to estimate total numbers or acreage of impaired or threatened lakes in a given state. It is clear even from these limited statistics, however, that many of the nation's lakes are degraded to the extent that their use is impaired and that a wide variety of problems and causes are responsible for this situation. Moreover, the data in Table 4.2 probably represent highly conservative estimates of the surface area of impounded water that could be improved by proper restoration and management measures.
According to Duda and Johnson (1984), EPA Regions IV (Southeast), V (North Central), and VIII (Missouri Basin) have the highest fractions of impaired lakes (accounting for >80 percent of the impaired acreage described in their report). Duda et al. (1987) reported that 22 of the 32 major Tennessee Valley Authority (TVA) reservoirs have some form of use impairment, and 16 of the 21 non-TVA reservoirs in the region are impaired or threatened. Aside from the Great
TABLE 4.2 Designated Use Support in Lakes and Reservoirs
|
State |
Number of Lakes |
Total Acres of Lakes |
Total Acres Surveyed |
Acres Fully Supporting |
Acres Partially Supporting |
Acres Not Supporting |
|
AL |
43 |
504,336 |
491,566 |
405,486 |
0 |
86,080 |
|
CA |
4,955 |
1,417,540 |
1,076,891 |
568,739 |
95,505 |
412,647 |
|
CO |
4,069 |
265,982 |
124,973 |
123,300 |
1,673 |
0 |
|
CT |
6,000 |
82,900 |
21,701 |
9,312 |
12,389 |
0 |
|
DC |
8 |
377 |
136 |
0 |
0 |
136 |
|
FL |
7,712 |
2,085,120 |
947,200 |
309,760 |
536,320 |
101,120 |
|
GA |
175 |
417,730 |
417,730 |
412,357 |
5,347 |
26 |
|
IL |
2,940 |
247,188 |
183,572 |
22,931 |
100,591 |
60,050 |
|
IN |
560 |
104,540 |
104,540 |
104,361 |
63 |
116 |
|
IA |
282 |
81,400 |
80,249 |
26,801 |
52,058 |
1,390 |
|
KS |
232 |
175,189 |
173,911 |
116,655 |
48,141 |
9,115 |
|
KY |
92 |
228,385 |
214,483 |
179,335 |
31,471 |
3,677 |
|
LA |
101 |
713,719 |
517,476 |
376,335 |
141,141 |
0 |
|
ME |
5,779 |
994,560 |
994,560 |
958,080 |
36,480 |
0 |
|
MD |
59 |
17,448 |
17,448 |
14,838 |
2,603 |
7 |
|
MI |
35,000 |
840,960 |
424,021 |
304,185 |
62,834 |
57,002 |
|
MN |
12,034 |
3,411,200 |
1,435,554 |
1,198,709 |
67,622 |
169,223 |
|
MS |
— |
500,000 |
500,000 |
481,740 |
18,260 |
0 |
|
MO |
362 |
288,012 |
288,012 |
285,701 |
2,311 |
0 |
|
MT |
4,018 |
756,450 |
663,363 |
345,367 |
305,396 |
12,600 |
|
NB |
412 |
145,300 |
85,518 |
82,304 |
2,779 |
435 |
|
NH |
1,300 |
151,000 |
149,854 |
130,708 |
18,756 |
390 |
|
NM |
— |
126,500 |
119,666 |
72,358 |
47,308 |
0 |
|
NY |
7,500 |
750,000 |
750,000 |
454,668 |
267,343 |
27,989 |
|
NC |
1,500 |
305,367 |
305,367 |
293,470 |
2,075 |
9,822 |
|
ND |
216 |
625,503 |
619,333 |
571,208 |
48,125 |
0 |
|
OH |
2,500 |
117,323 |
90,771 |
30,936 |
50,988 |
8,847 |
|
OR |
6,095 |
610,808 |
504,928 |
374,303 |
58,918 |
71,707 |
|
PR |
38 |
11,146 |
11,146 |
3,801 |
4,240 |
3,105 |
|
RI |
113 |
16,520 |
16,089 |
14,688 |
787 |
614 |
|
SC |
1,418 |
525,000 |
410,407 |
409,242 |
840 |
325 |
|
SD |
789 |
1,598,285 |
662,532 |
567,812 |
17,984 |
76,736 |
|
TN |
117 |
538,657 |
538,657 |
452,009 |
50,830 |
35,818 |
|
TX |
5,700 |
1,410,240 |
1,410,240 |
1,225,629 |
0 |
184,611 |
|
VT |
719 |
229,146 |
227,121 |
177,915 |
37,713 |
11,493 |
|
VA |
248 |
161,562 |
161,089 |
147,352 |
13,737 |
0 |
|
WA |
808 |
613,582 |
156,518 |
122,834 |
33,104 |
580 |
|
WV |
94 |
19,171 |
19,171 |
0 |
17,441 |
1,730 |
|
WI |
14,998 |
971,000 |
971,000 |
249,000 |
478,000 |
244,000 |
|
WY |
2,629 |
427,219 |
427,219 |
396,815 |
30,404 |
0 |
|
Total |
131,615 |
22,486,365 |
16,314,012 |
12,021,044 |
2,701,577 |
1,591,391 |
|
SOURCE: Reprinted from U.S. Environmental Protection Agency, 1990. |
||||||
TABLE 4.3A Lake Acres Affected by Causes of Impairment
|
Total Impaired Watersa |
Nutrients |
Siltation |
Organic Enrichment |
Salinity |
Habitat Mod |
Pathogens |
Priority Organics |
Suspended Solids |
Metals |
Pesticides |
pH |
Flow Alteration |
|
2,658,839 |
1,297,044 |
676,664 |
671,923 |
380,831 |
301,354 |
228,246 |
217,258 |
200,239 |
197,803 |
141,136 |
136,723 |
86,737 |
|
|
48.8% |
25.4% |
25.3% |
14.3% |
11.3% |
8.6% |
8.2% |
7.5% |
7.4% |
5.3% |
5.1% |
3.3% |
|
a The sum of partially and nonsupporting lake acres. SOURCE: U.S. Environmental Protection Agency, 1990. |
||||||||||||
TABLE 4.3B Impaired Lake Acres Affected by Sources of Pollution
|
Total Impaired Watersa |
Agriculture |
Hydro Habitat Mod |
Storm Sewers / Runoff |
Land Disposal |
Municipal |
Industrial |
Resource Extraction |
Construction |
Silviculture |
Combined Sewers |
|
2,686,889 |
1,564,382 |
889,760 |
744,214 |
710,998 |
404,846 |
207,591 |
112,977 |
87,879 |
25,034 |
7,981 |
|
|
58.2% |
33.1% |
27.7% |
26.5% |
15.1% |
7.7% |
4.2% |
3.3% |
0.9% |
0.3% |
|
a The sum of partially and nonsupporting lake acres. SOURCE: U.S. Environmental Protection Agency, 1990. |
||||||||||
LAKES
Lakes, many lakes of national or regional significance are impaired (e.g., Lakes Apopka and Okeechobee, Florida; Ocean Lake, Wyoming; and Reelfoot Lake, Tennessee). Reelfoot Lake, a natural lake in the south-central United States, is a classic example. Silt and nutrients from agriculture and channelization of inflowing streams have increased sedimentation rates in the lake, and associated weed and algal growths have reduced its area from 208 km(2) (51,400 acres), to 52 km(2) (12,800 acres). The habitat of two endangered species of birds is threatened, and changes in the lake itself have affected the economy of the area (Duda and Johnson, 1984). Based on current rates of sedimentation, McIntyre and Naney (1990) predicted that the lake will become too shallow for recreational purposes in as little as 60 years (for the shallowest of its three basins). Changes in land management are needed to alter this situation.
The condition of the nation's lakes appears to be deteriorating. The 1984 ASIWPCA survey assessed changes from 1972 to 1982 and concluded that the acreage of lakes that had degraded was four times that of the acreage that had improved during the decade. Similarly, a 1983 survey of state lake administrators by the North American Lake Management Society (NALMS; Duda and Johnson, 1984) showed an alarming increase in problem lakes since the survey of Ketelle and Uttormark (1971). The NALMS survey was marred by the lack of lake programs in many states or the inability of some states to respond, but good documentation appears to be available in nine states located in six EPA regions. The number of problem lakes reported in these states increased by a factor of 20 between 1971 and 1983 (Duda and Johnson, 1984).
TROPHIC STATE
The National Eutrophication Survey (NES), conducted by EPA in 1973 to 1976, sampled several hundred lakes throughout the continental United States and constructed nutrient budgets on many of the lakes. Results of the survey showed that the great majority of surveyed lakes had eutrophic conditions and experienced some form of water quality degradation. Lakes were not selected for the NES based on a random sampling of U.S. lakes. The survey was designed to assess the severity of eutrophication in lakes with municipal sewage treatment plants in their drainage basin, and it would not be appropriate to extrapolate NES statistics to estimate the trophic status of the nation's lakes.
The surveys listed at the beginning of this section did attempt nationwide trophic state assessments. In the most recent (1988) assessment
by EPA, 39 states provided trophic classification on a total of 15,514 lakes (U.S. EPA, 1990b). About 30 percent of the surveyed lakes were classified as eutrophic or hypereutrophic, and 23 percent were mesotrophic. Trophic conditions were unknown in about 30 percent of the lakes included in the survey. In some cases, a lake is eutrophic simply as a result of natural circumstances (e.g., ecoregional characteristics), but nonpoint pollution from agricultural and urban run-off is the cause of use impairment from excess nutrients in most lakes.
The trophic status of the North American Great Lakes, including Great Bear Lake and Great Slave Lake, was summarized by Robertson and Scavia (1984). They concluded that Lakes Ontario and Erie are eutrophic and that Green Bay (Lake Michigan), Saginaw Bay (Lake Huron), and the Lake Erie western basin are highly eutrophic. The other lakes are mesotrophic or oligotrophic.
Canada has the largest acreage of lakes in the world, and a complete inventory, much less an assessment of their trophic states, is not available at this time. Most of them are thought to be oligotrophic, and in terms of raw numbers, the great majority of Canadian lakes lie in wilderness or undeveloped forests. Nonetheless, many lakes in agricultural areas of southern Canada have water quality problems resulting from excessive nutrients, and recreational developments have led to impaired water quality in some lakes located within driving distance of major urban areas such as Toronto. A small sample of 130 Canadian lakes found 16 of them to be eutrophic (Janus and Vollenweider, 1981).
Summary reports (e.g., Vollenweider and Kerekes, 1981; Forsberg, 1987) show that eutrophication problems are widespread throughout Europe. Reports of this nature do not exist for other continents, but accounts of extensive soil erosion and massive siltation of reservoirs everywhere, coupled with the absence of wastewater treatment in many areas (Brown and Wolf, 1984; Postel, 1985), suggest that water bodies worldwide are affected by excessive biological production and its consequences. Rapid in-filling of major impoundments in Third World nations is particularly troubling in view of their needs for irrigation water, potable supplies, and flood control. Deforestation and cultivation of marginal lands are causing soil losses at rates that will fill some impoundments in these countries in 5 to 20 years (Brown and Wolf, 1984).
ACIDIFICATION
The National Surface Water Survey (NSWS), a major survey of lakes and streams in acid-sensitive regions of the United States, was
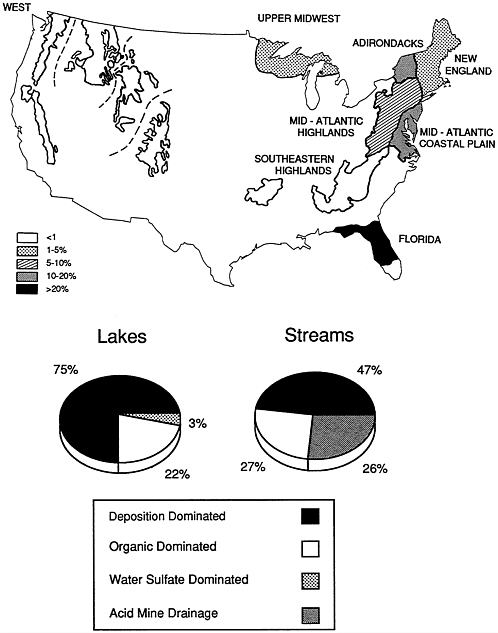
conducted by EPA in the mid-1980s. Because the survey design was based on stratified-random sampling procedures, the results can be extrapolated to the population of surface waters in acid-sensitive regions of the United States. According to the survey (NAPAP, 1990a,b), 4.2 percent of the NSWS lakes (representing about 1,180 lakes in the total population of lakes in the sampled regions) were acidic, defined as having an alkalinity less than 0 (pH<ca. 5.0-5.5). The acidic lakes are about equally divided among three regions: (1) the Northeast (primarily the Adirondacks), (2) the Upper Midwest (primarily northeastern Wisconsin and the Upper Peninsula of Michigan, and (3) interior Florida. Because the total number of lakes occurring in each region is different, the percentage of acidic lakes varies regionally (Figure 4.1), with Florida having the highest percentage (23) and the Upper Midwest having the lowest (3). About three-fourths of the acidic lakes were attributable to acidic deposition; most of the remainder were colored and were thought to be acidic from the presence of natural organic acids.
Although only a small percentage of lakes in the NSWS were found to be acidic, several cautions must be considered before the results are accepted as an accurate portrayal of the impact of acidic precipitation on U.S. lakes. First, the NSWS sampled lakes only one time — in the fall — and this is not the season during which the pH is lowest in lakes or the most critical season for biological impacts. Second, the NSWS did not sample small lakes (those <4 ha in area and >1 ha in the western lakes survey, where acidification is of less concern; no acidic lakes were found in western regions), and survey results indicate that the frequency of acidic conditions increases as lake size decreases. Third, the definition of acidic conditions used in the NSWS is arbitrary; ecological damage may occur at higher pH and alkalinity values than the NSWS used for its criteria. The criterion (acid-neutralizing capacity) was selected because it is considered to be a fairly unambiguous indicator of anthropogenic acidification, at least for lakes not affected by natural organic acids. Finally, the survey data indicate the status of lakes at a particular point in time and do not indicate the extent to which any lake has become more acidic as a result of acidic deposition.
The NSWS concluded that about 8 percent of the streams sampled were chronically acidic (acid-neutralizing capacity). On a length basis, approximately 7,900 km of streams were acidic; this represents about 4 percent of the total length of streams (211,000 km) in the NSWS regions. The acidic streams occurred mainly in the Mid-Atlantic Highlands and Mid-Atlantic Coastal Plain regions (Figure 4.1). The NSWS also concluded that 26,400 km (13 percent) of streams
had very low alkalinity values (&2264;50 µeq per liter). These streams are especially susceptible to episodic acidification (e.g., during spring snowmelt or large rain events), and this is considered to be a significant problem in the Mid-Atlantic Highlands. Many miles of streams in Appalachia are acidic from acid mine drainage; the NSWS concluded that about 60 percent of the acidic stream length in the Mid-Atlantic Highlands is from this source and about 40 percent is caused by atmospheric deposition. Acidic mine drainage results from oxidation of pyritic minerals exposed to the atmosphere during mining activity. Oxidation of the minerals produces sulfuric acid. Regions in which acid mine drainage is a problem have few natural lakes, and this source accounts for only a few acidic lakes nationwide.
TOXIC SUBSTANCES
The importance of toxic substances in lake degradation can be illustrated with data from the Great Lakes. The International Joint Commission has identified 42 areas of concern in the Great Lakes, and 41 of these have problems associated with toxic substances (Hartig and Thomas, 1988). As mentioned earlier, all Great Lakes have fish contaminated by PCBs and organochlorine pesticides. Toxic substances reported from the analysis of sediments include metals (arsenic, cadmium, chromium, copper, lead, mercury, manganese, and zinc), cyanide, grease and oil, and a variety of chlorinated organic compounds: polychlorinated biphenyls (PCBs), polynuclear aromatic hydrocarbons (PAHs), hexachlorobenzene (HCB), dioxins, phthalates, and dibenzofurans. A much longer list has been identified from the analysis of water samples.
No large-scale field surveys have been conducted to determine the status of U.S. lakes with regard to the problem of toxic chemicals, but EPA's 1988 water quality assessment (based on questionnaires) shows that such problems are widespread (U.S. EPA, 1990b). A total of 556,000 acres of lakes in 18 states was reported to be impaired by priority organic pollutants, metals, or pesticides; this represents almost 21 percent of the total impaired acreage for which the cause of impairment is known (Table 4.3A). Some inland lakes and many rivers have fish consumption advisories because of contamination by these compounds. Elevated chlordane levels have been found in fish from Kansas lakes; PCB contamination is a common problem in New York lakes and has also led to consumption advisories for some Minnesota and Wisconsin lakes. Reliable statistics are lacking on the
pervasiveness and seriousness of such problems because adequate surveys have not been done.
Accurate data are also lacking on the number of lakes degraded by toxic metals such as mercury, but several lines of evidence suggest the number could be very large. For example, 21 states currently issue fish consumption advisories because of mercury contamination problems. Almost 90 percent of the Minnesota lakes from which fish have been analyzed for mercury (233 out of 261 lakes) had at least one species with burdens high enough to issue a consumption advisory (>0.16 ug/g for a one-meal-per-week advisory), and 98 of the lakes (38 percent) had fish with mercury levels higher than 0.65 ug/g (the trigger level for a one-meal-per-month advisory) (D. Helwig, Minnesota Pollution Control Agency, personal communication, 1991). Most of these lakes are in undeveloped forested areas of northeastern Minnesota. The 1990 consumption advisory of the Wisconsin Department of Natural Resources includes 157 lakes and 11 rivers with mercury-contaminated fish, as well as parts of 11 rivers, Green Bay, Lake Michigan, and Lake Superior for PCBs. The cited lakes and rivers are found throughout the state. The number of lakes with consumption advisories increases as more lakes are sampled, and problems are not limited to low-alkalinity lakes or to the Midwest. Mercury-contaminated fish have been reported in several western and many East Coast states, including Florida and New York.
OTHER STRESSES
According to the 1988 EPA survey, almost 700,000 lake acres are impaired by siltation in the United States (Table 4.3A; U.S. EPA, 1990b). Given the incompleteness of the data on which this number is based, the actual area impacted by excess sediment is probably significantly greater. No national statistics are available on the extent of lakes impaired by exotic species. Nonetheless, it is common knowledge that problems with exotic macrophytes are pervasive, especially in southern states, and that many thousands of acres are affected. The Great Lakes all suffer from a variety of exotic species problems, and with the recent zebra mussel invasion, problems caused by exotic species appear to be getting worse. No national statistics are available on the extent of damage caused by physical and hydrologic changes to lakes; in many cases, these manipulations are not even recognized as a factor in lake degradation.
LAKE RESTORATION AND MANAGEMENT
Definitions
The definitions of restoration, rehabilitation, mitigation, and management discussed in Chapter 1 apply to lakes as well as to other aquatic systems, but as noted earlier in this chapter, limnologists have applied the term restoration rather broadly to actions designed to alleviate degraded conditions in lakes. There are some important differences between lakes and other surface waters relative to ease of restoration, and many of the methods used to restore lakes are not applicable to the restoration of wetlands and running waters. For example, rivers and streams degraded by chemical contaminants can be restored in many cases simply by eliminating the source of contamination and relying on their self-cleansing properties, but this approach seldom is sufficient for lakes, which tend to have long water and substance residence times and behave more as closed systems. In-lake manipulations are usually necessary (in addition to source controls) to restore lakes. Loss of habitat by physical alterations (channelization, installation of flow-regulating structures) is probably the most common reason that rivers need restoration, but most degraded lakes suffer from some sort of chemical contamination by excess nutrients, organic matter, toxic substances, or acidity. (Loss of littoral habitat (macrophyte beds) is a common condition in recreational lakes but often is not recognized as a problem. Instead, such losses are viewed by swimmers and boating enthusiasts as an ''improvement.") Differences in the source of degradation lead to differing approaches in restoring lakes versus restoring rivers or wetlands, as well as to different approaches to managing the three types of aquatic systems to prevent further degradation or minimize the impacts of stress.
RESTORATION USING THE CONCEPT OF ECOREGIONS
A major determinant of lake and reservoir productivity is the steady-state, long-term average concentration of nutrients, especially those that can be growth limiting, such as phosphorus, nitrogen, and silica. Increased nutrient and organic matter loading, usually from cultural sources such as wastewater treatment plants and runoff from urban or agricultural land, often leads to sharply increased nutrient concentrations in the water column and ultimately to algal blooms, dissolved
oxygen depletion, and other symptoms of cultural eutrophication. Elimination or significant reduction of these cultural sources of stress is essential if a lake or reservoir is to be restored to its previous condition.
The nutrient concentration attainable in a lake following significant reduction or elimination of cultural loading will depend on several factors, including basin morphometry, hydrologic conditions, land use, and the geographic region in which the lake is located. Lake morphometry plays a major role in determining the amount of "internal loading" of nutrients from the sediments to the water column. Shallow lakes, particularly those exposed to wind-induced mixing, are likely to have high internal loading rates. Water residence time also plays a role in determining lake water column nutrient concentration. As water residence time decreases, the concentration of nutrients approaches the concentration in incoming streams or rivers, and sedimentation of nutrients becomes less of a factor.
Morphometric features and hydrologic factors can vary widely from lake to lake even within a small region, but nonetheless the earth can be characterized as containing ecological regions (or "ecoregions") that have broad similarities of soil, relief, and dominant vegetation. Omernik (1987) divided the conterminous United States into 76 ecoregions, or areas of regional similarity in soil, land use, land surface form, and potential natural vegetation (Figure 4.2). The water quality of streams within an ecoregion would be expected to be more similar (in terms of nutrients, silt, organic matter, and major ions) than would the water quality of streams of different ecoregions (Hughes et al., 1986). It follows that trophic conditions of lakes in an ecoregions characterized by highly erodible, nutrient-rich soils would differ, even without any cultural nutrient loading, from those of lakes in an area of sandy soils and low relief, simply because of differences in loading from their drainage basins.
These expectations have been verified through studies of phosphorus concentrations, fish and invertebrates in streams of Arkansas, Kansas, Minnesota, Ohio, and Oregon, and lakes of Michigan, Minnesota, Ohio, and Wisconsin (Hawkes et al., 1986; Hughes and Larsen, 1988; Omernik et al., 1988; Wilson and Walker, 1989; Fulmer and Cooke, 1990; and others). For example, Larsen et al. (1988) described the patterns of water quality in streams of the five ecoregions that extend into Ohio. Strong differences were found between ecoregions with regard to nutrients and major ion variables, and with regard to the complexity and health of fish assemblages. Heiskary et al. (1987) and Wilson and Walker (1989) used the ecoregion concept to develop lake restoration priorities and strategies for Minnesota.
Although seven ecoregions extend into Minnesota, 98 percent of the state's 12,500 lakes with surface areas greater than 10 ha occur in four of them. It is apparent from Table 4.4 and Figure 4.3 that lakes in the North Central Hardwood Forest (NCHF) and Northern Lakes and Forests (NLF) ecoregions differ substantially from lakes in the Western Corn Belt Plains (WCBP) and Northern Glaciated Plains (NGP) ecoregions. Lakes in the latter two ecoregions are unlikely to have water with few algal blooms, regardless of the amount of lake management activity. However, lakes with high algal biomass, low transparency, and severe dissolved oxygen depletion in the NCHF or NLF ecoregions are likely to have deviated significantly from their
TABLE 4.4 Summary of Land Use and Water Quality Data for Four Ecoregions in Minnesota
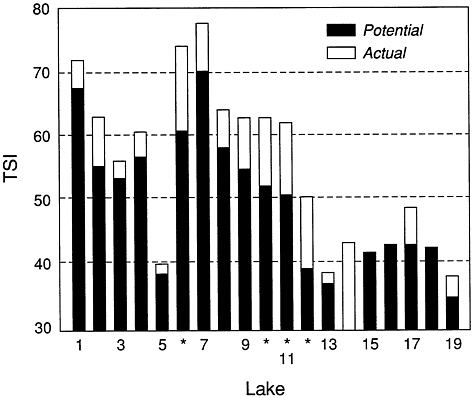
FIGURE 4.3 Actual and attainable trophic state (as indicated by Carlson trophic state index values) in 19 Ohio reservoirs (TSI<40 = oligotrophy; TSI>50 = Eutrophy).
attainable conditions, probably through cultural nutrient loading. Lake restoration is possible for a eutrophic lake in any of the ecoregions, but the attainable trophic state for lakes can vary significantly between adjacent ecoregions.
The ecoregion idea has been used to predict the restoration potential of Ohio reservoirs subjected to varying degrees of nutrient, silt, and organic matter loading (Fulmer and Cooke, 1990). These authors used the 25th percentile values of stream phosphorus concentrations in the least affected streams of the ecoregion for each reservoirs (from Larsen et al., 1988), along with hydrologic and morphometric data for the reservoirs and Canfield and Bachmann's (1981) loading model, to predict the steady-state phosphorus concentration attainable in the deep water zone of each reservoir. The 25th percentile concentration was chosen for purposes of illustration as a stream concentration that probably can be reached through technologically feasible changes in the watershed, such as advanced wastewater treatment, feedlot
runoff detention systems, and other land management practices. Other concentrations appropriate to a specific stream or ecoregion could be chosen. The predicted phosphorus concentrations in the reservoirs were compared with measured values, and the data were transformed into an index number, a Carlson trophic state index (TSI) value (see "Water Quality and Human Use Criteria," below) to describe the lake conditions expected for that concentration.
Four reservoirs were identified that have much higher phosphorus concentrations and trophic states than those predicted by the model (Figure 4.4). These eutrophic reservoirs have trophic conditions in the mesotrophic range (TSI of 40–50; i.e., they can be shifted from conditions of prolonged and severe algal blooms to conditions of higher transparency and fewer problems with nuisance algae). Additional studies are needed to ascertain causes of the deviations from attainable quality, but the four reservoirs represent the best opportunities among the 19 studied reservoirs for obtaining significant lake improvements.
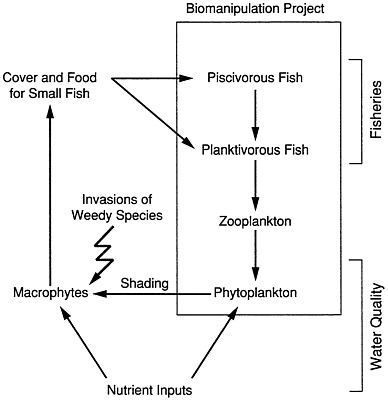
FIGURE 4.4 Linkages of algae, macrophytes, and fisheries in lakes.
The above approach to determining the best candidates for lake restoration differs significantly from the traditional one of simply selecting the lakes with the worst trophic states and then spending the available money in an attempt to restore them. The desired trophic state may not be attainable or may require large and continued expenditures of energy and money to be maintained. For example, lake 1 in figure 4.3 is hypereutrophic, and its water quality is among Ohio's poorest. It is located in an ecoregion with rich humic soils in which the principal land use is agricultural. Its attainable trophic state (Figure 4.3) does not differ significantly from its current state. Although management activities (e.g., aeration, weed harvesting, dredging) could improve the lake for recreation, continued loading will refill it with silt and maintain its current trophic state. If this lake were assigned a top priority for restoration without considering its attainable condition, scarce restoration funds could be wasted. Lakes 6, 10, 11, and 12 (Figure 4.3) have much better attainable quality, have deviated significantly from this condition, and are thus better candidates for restoration.
One of the values of the ecoregion concept in lake restoration and management is that it provides a rational basis for setting regional rather than national lake water quality standards. The approach can take into account regional factors related to attainable water quality and thus can be used to designate lakes for protection and to establish lake restoration goals appropriate for each ecoregion.
Stream water quality in some watersheds of an ecoregion, and ultimately lake trophic state, can be greatly improved through changes in land use (e.g., wetland restoration, improved agricultural practices) and through stream restoration itself. In these cases, the additional use of in-lake procedures, such as enhancement of biological controls on algal populations or application of chemicals to control sediment phosphorus release, may improve a lake beyond expectations based on original ecoregion characteristics. Although no lake in the Eastern Corn Belt Plains (ECBP) ecoregion of Indiana and Ohio will look like the oligotrophic lakes of the Northern Lakes and Forests ecoregion of Minnesota, Wisconsin, and Michigan, it is important to recognize that the various ecoregions were defined based on existing land use conditions and that intensive row crop farming is not the native condition of land in the ECBP. If farming became less intensive or less prevalent in the ECBP, or if best management practices became effective in reducing the export of soil and nutrients to streams in the region, land use would become a reduced factor in determining stream quality, and lakes could improve to some degree beyond the conditions currently defined as attainable.
There are few case histories of the uses of in-lake procedures on lakes that also have had wastewater inflows eliminated and major improvements in land use practices. It is not yet known whether and how far lakes can be restored beyond the attainable condition associated with their ecoregion.
Criteria for Successful Restoration and Measures of Success
Most lake restoration projects undertaken in the United States over the past 20 years have focused on improving the fitness of a degraded lake for human uses such as swimming, other water contact sports, fishing, or drinking water supply. Undoing ecological damage from past human perturbations and restoring the lake's ecosystem to its pristine state are not the primary goals of typical restoration efforts. Nonetheless, restoration proponents generally assume (at least implicitly) that improving a lake's usability for human activities will improve functions of the lake's ecosystem, and indeed there is merit in the assumption. To the extent that a project stops excessive (human-induced) inputs of nutrients, sediments, or acids; controls or eliminates nonnative species; or removes unnatural toxic substances, it will promote return of the aquatic ecosystem to a less-stressed (more "natural") condition. The structural and functional properties of ecosystems change over time, however, because of natural ecological succession, as well as long-term shifts in climate and hydrology. Consequently, it is unrealistic to think that we can restore a lake precisely to the ecological state it was in before a stress occurred or to the unknown (and unknowable) state to which it would have evolved in the absence of the stress.
ECOLOGICAL CRITERIA
Improving the ecology of a lake is a laudable goal, and the success of lake restoration projects should be measured, at least in part, on the basis of ecological criteria, not just on economic or human use criteria. Although ecologists may disagree on the detailed characteristics of a functioning ecosystem, some general principles can be stated. Restoration should promote a self-sustaining, stable system. The system should have the ability to resist stress and the resiliency to rebound from stresses once they have been removed. Production and respiration should be roughly in balance, and the food web should be complicated enough (involving many checks and balances and negative feedback loops) to minimize uncontrolled growth and wild
population swings by one or a few species. Often this is best achieved by restoring native species. Although ecologists no longer equate diversity directly with ecosystem stability, biotic diversity, often stated in terms of species or genetic diversity, is still an important measure of ecosystem quality.
Many so-called lake restoration projects really are only mitigation and management efforts to rid a lake, by whatever means, of some nuisance. Criteria for selection of the procedure are based primarily on cost and effectiveness relative to the specific target (e.g., elimination of a nuisance organism). Some procedures that rank highly based on those criteria fare poorly when evaluated more broadly in terms of total ecosystem restoration. For example, chemical herbicides are commonly used to control rampant macrophyte growths in littoral zones of lakes and, if applied properly, can achieve the goal of removing the nuisance, at least temporarily. However, the dead plant material may release a substantial load of nutrients to the water column, inducing excessive algal growth (substituting one problem for another), or the herbicide may adversely affect nontarget organisms. Most important, herbicide treatments and similar palliatives do not get at the underlying causes of a problem. In the example given, once the herbicide disappears, the macrophyte problem probably will return.
WATER QUALITY AND HUMAN USE CRITERIA
As stated above, the goal of most lake restoration projects is to remove a specific problem — a nuisance organism, excess chemical(s), or unwanted physical condition — and make the lake more desirable (based on human-centered criteria) and more usable for specific human purposes. The success of a restoration project is (and must be) evaluated according to the extent to which these human-oriented goals are met (e.g., Was the fishery restored? Was swimming improved?). Chemical measures of water quality (and associated numerical criteria for specific chemical species) may be used to measure the success of restoration when the problem has a simple cause (e.g., presence of a toxic chemical), but more typically the causes and symptoms of degradation are much more complicated. Quantitative measurements of improvements in recreational and aesthetic attributes are notoriously difficult to obtain, however, and therefore success commonly is measured in terms of quantifiable water quality characteristics such as Secchi disk transparency (a measure of water clarity) and concentration of chlorophyll a (a measure of algal biomass) that are loosely related to recreational and aesthetic conditions.
Quantitive evaluation of trophic state conditions has been aided by use of simple trophic state indices. The most widely used TSIs are those developed by Carlson (1977), based on Secchi disk transparency and on concentrations of total phosphate and chlorophyll a. These strongly intercorrelated parameters are, respectively, the best quantified physical, chemical, and biological measures of trophic conditions, and Carlson developed a simple index based on each parameter (Table 4.5). The approximate range of each index is 0 to 100, and values greater than about 50 denote eutrophic conditions. An increase of 10 units in an index represents a doubling of algal biomass. Carlson recommended that the indices be considered separately in evaluating trophic state, but others (e.g., Kratzer and Brezonik, 1981) recommended averaging the three values to obtain a single number integrating the components contributing to trophic state.
More complicated, multidimensional indices have been proposed to express the concepts of trophic state (Shannon and Brezonik, 1972; Uttormark and Wall, 1975) or water quality (Brown et al., 1972; Harkins, 1974; Walski and Parker, 1974) in a single number, but none of these indices has been used routinely in lake management and restoration programs. Dierberg et al. (1988b) used modified versions of Carlson's
TABLE 4.5 Values of Secchi Disk Transparency, Total Phosphorus Concentration, and Chloryphyll a Concentration Corresponding to Carlson Trophic State Index (TSI) Values
|
TSI |
Secchi Disk Transparency (m) |
Total Phosphorus Concentration (ug/liter) |
Chlorophyll a Concentration (ug/liter) |
|
0 |
64 |
1 |
0.04 |
|
10 |
32 |
2 |
0.12 |
|
20 |
16 |
4 |
0.34 |
|
30 |
8 |
8 |
0.94 |
|
40 |
4 |
16 |
2.6 |
|
50 |
2 |
32 |
6.4 |
|
60 |
1 |
65 |
20 |
|
70 |
0.5 |
130 |
56 |
|
80 |
0.25 |
260 |
154 |
|
90 |
0.12 |
519 |
427 |
|
100 |
0.06 |
1,032 |
1,183 |
|
SOURCE: Reprinted by permission from Carlson, 1977. Copyright © 1977 by the American Society of Limnology and Oceanography, Inc. |
|||
TSIs to evaluate water quality changes in Florida lakes that had undergone restoration.
The cost-effectiveness of a restoration effort must be measured in terms of the economic benefits obtained relative to expenses incurred. The difficulties in assigning a dollar amount to such elusive attributes as ecological health and wilderness values are well known, however, and cost-effectiveness as measured by conventional economic procedures should not be the sole measure of success. Restoration projects should also be evaluated in terms of ecological criteria (i.e., the extent to which a project improves lake ecosystems as measured by the criteria described above). In most cases, these criteria do not conflict with those related to human-centered goals, but success in attaining the latter goals does not necessarily translate to success relative to the former. As a minimum, lake restoration professionals should strive to ensure that human-centered goals are not achieved at the expense of ecological goals, for that would be shortsighted. Finally, because human and financial resources are limited, success should be measured in terms of the longevity of effects and the extent to which a restored lake is self-sustaining.
FEDERAL AND STATE PROGRAMS FOR LAKE RESTORATION AND MANAGEMENT
Federal Programs
The principal federal program dealing with restoration of degraded lakes is EPA's Clean Lakes Program (CLP), which was established by P.L. 92-500, the Federal Water Pollution Control Act Amendments of 1972. The CLP began in 1975 through a congressional appropriation pursuant to Section 314 of P.L. 92-500. The purpose of Section 314 was to develop a national program to clean up publicly owned fresh-water lakes. The CLP requires that all point sources of pollution be treated or have treatment planned under Sections 201 and 402 of the 1972 Clean Water Act before a grant is awarded for in-lake restoration activities (Duda et al., 1987).
From 1975 to 1978, $35 million in research and development grants was issued to identify restoration techniques and restore specific lakes. In 1980, a four-part program was established that included (1) a classification survey, wherein states were to identify and rank their lakes according to trophic state; (2) ''Phase I" projects, which were awarded for diagnosis and feasibility studies on lakes ranked by the states as having the greatest need (for restoration); (3) "Phase II" projects, in which funds were awarded to implement Phase I recommendations;
and (4) "Phase III" projects to assess the responses of restored lakes (U.S. EPA, 1985). Some Phase III funds are now being awarded. The Water Quality Act of 1987 (P.L. 100-4) reauthorized the CLP and mandated some new initiatives and requirements. For example, to remain eligible for CLP grant funds, each state is required to submit a biennial report that includes a revised lake classification list, an assessment of status and trends in lake water quality, and a restoration plan for degraded lakes. In addition, EPA was authorized to establish a Clean Lakes demonstration program to enhance understanding of the effectiveness of various lake restoration techniques, and the Water Quality Act directs that specific attention be paid to mitigation of acidified lakes.
A key feature of the CLP is its emphasis on assisting states in setting up their own programs. Federal funds have been limited to 70 percent of the cost of the classification studies and Phase I projects (up to a maximum of $100,000 in each category). The federal share of Phase II projects is 50 percent. States administer their own programs, and there is considerable emphasis on local involvement in raising the matching funds.
In fiscal years 1976 to 1980, more than $60 million was spent on classification, Phase I, and the initial funding of Phase II projects. In fiscal years 1981 to 1985, no funds for lake restoration and protection were requested in the President's budgets, but a total of $32.64 million was added to the EPA's budget by Congress for Phase II lake programs (Duda and Johnson, 1984; U.S. EPA, 1985).
The CLP has had some success in stimulating states to develop lake programs and in encouraging citizen involvement. During the period 1975 to 1985, 313 CLP studies and projects were funded. Four percent of the total funds ($93 million) were spent on classification, 9 percent on diagnosis and feasibility studies (Phase I), and 87 percent on restoration (Phase II). Projects were distributed among 47 states; only Alabama, West Virginia, and Hawaii did not participate. Through 1985, 67 Phase II projects had been completed, and 92 Phase II projects were in progress in 29 states (U.S. EPA, 1985). Half of the projects completed by 1985 were in four states: New York, Wisconsin, South Dakota, and Minnesota.
Many other federal programs are concerned, at least indirectly, with restoration of lakes. For example, EPA's Section 201 program deals with improvement of municipal wastewater treatment facilities in urban areas, and its Section 208 program (the numbers refer to sections of P.L. 92-500) is concerned with areawide planning for water quality management (with emphasis on nonpoint sources of pollution in storm water runoff). The U.S. Army Corps of Engineers has
responsibility for managing numerous impoundments and for authorizing permits for dredge and fill operations on surface waters. The U.S. Fish and Wildlife Service has interest and activities related to accumulation of toxic substances in fish and waterfowl. Nonetheless, EPA's Clean Lakes Program is the only federal program involved directly in restoring lakes nationwide, and it provides the primary federal support (technical, administrative, and financial) for state lake programs.
State Programs
Obtaining detailed information on state programs is beyond the scope of this study. Information on lake management and restoration activities compiled by the North American Lake Management Society from EPA's 1988 survey of state agencies (U.S. EPA, 1990b) does provide comparative data on state programs, however. Only 42 states reported on lake management activities in the broadest sense, and in most of the states only a few people were involved in the program. More than 30 of the states reported that the federal Clean Lakes Program was the major source of funding for proposed lake restoration projects.
Only a few states are involved in lake restoration activities beyond those associated with the CLP. Notable among these are Florida, Minnesota, South Dakota, Vermont, Washington, and Wisconsin. Data on the numbers of lakes restored through state-sponsored programs are not readily available. Some of the programs are too recent to have established a track record. For example, Florida's Surface Water Improvement and Management (SWIM) Program was initiated in 1987, and to date most of its funds have been devoted to feasibility and planning studies. Minnesota's Clean Water Partnership (also initiated in 1987) provides funding to local governments to improve water resources (lakes, wetlands, streams, ground water aquifers) that have been degraded by activities related to land use (nonpoint source pollution), as well as to protect aquatic systems threatened by such degradation. Operation of the program is somewhat similar to that of the federal CLP. To date the program has awarded funds to 30 projects and has allocated $2.6 million in state funds (which have been matched equally by local units of government).
The NALMS survey also showed that many states have citizen groups actively involved in lake management activities. Programs such as "Water Watch" in Kentucky, "Lake Watch" in Florida, "Volunteer Lake Assessment Program" in New Hampshire, and "Volunteer Lake Monitoring Program in Illinois" all use specially trained
volunteers to monitor environmental trends in lakes. These activities include measuring Secchi disk transparency, recording rainfall and lake levels, and collecting and storing water samples for analysis in a central laboratory. These self-help programs are designed primarily to assist lake associations in collecting information and interpreting data. Collection of long-term data sets is essential in determining the need for restoration activities and in evaluating these activities if they are undertaken.
Evaluation of Past Restoration Efforts: Need for Monitoring Data
Without question, state and federal programs affecting the quality of lakes have made major strides in three areas over the past 20 years: (1) eliminating or decreasing pollution sources to lakes (especially point sources of pollutants), (2) cleaning up pollution problems (e.g., removing specific pollutants and contaminated sediments from lakes), and (3) restoring or improving user-oriented qualities of some lakes.
A 1980 study by EPA evaluated economic benefits resulting from the CLP and concluded that the program was highly cost-effective (U.S. EPA, 1980). The analysis was based on 28 projects in 16 states that received a total of $15.35 million in federal grants and an approximately equal sum from state and local sources. Twelve categories of benefits were considered in the assessment, but many benefits could not be quantified in monetary terms. The 1980 value of the benefits that could be quantified was estimated to be $127.5 million (Table 4.6), which represents a return of $4.15 per total project dollar.
However, our ability to assess the effectiveness of restoration projects funded by government programs in quantitative, scientific terms is greatly diminished by a paucity of data on lake quality before and after treatment. For example, in a review of 43 Florida lakes that had been sites of restoration projects, Dierberg et al. (1988a) found that only 7 lakes had sufficient data to permit an evaluation of water quality improvement. Baker and Swain (1989) found similarly dismal statistics in attempting to analyze lake restoration projects in Minnesota. Most restoration projects are required to include some pre-and posttreatment monitoring, but collection of data for an adequate period of time and at sufficient detail before and after restoration has been relatively uncommon. In some cases, sufficient monitoring may have been done, but rigorous analysis and interpretation of the results were not a part of the monitoring effort. All too often,
TABLE 4.6 Comparison of Economic Benefits and Project Costs (federal share only) for 28 Clean Lakes Projects
|
|
Economic Benefitsa |
||||||||||||
|
Lake |
Recreation |
Aesthetics |
Flood Control |
Economic Development |
Fish/ Wildlife |
Agriculture |
Property Value |
Public Health |
Water Supply |
Education R&D |
Miscellaneous |
Total Discounted Benefits (b)($) |
Grant Amount ($) |
|
Annabessacook |
+ |
+ |
$ |
+ |
$ |
|
+ |
|
|
|
|
23,246,100 |
497,906 |
|
Bomoseen |
$ |
+ |
|
|
|
|
|
|
|
+ |
+ |
1,830,500 |
74,640 |
|
Buckingham |
$ |
|
|
+ |
|
|
|
|
|
+ |
|
127,700 |
23,250 |
|
Charles |
$ |
$ |
|
|
|
|
|
|
|
+ |
|
2,286,600 |
387,163 |
|
Clear |
$ |
$ |
|
+ |
+ |
|
|
+ |
+ |
|
|
471,500 |
358,682 |
|
Cochrane |
$ |
+ |
|
|
$ |
|
+ |
|
|
+ |
|
52,500 |
9,906 |
|
Collins Park |
$ |
+ |
|
|
|
|
|
|
|
+ |
|
51,700 |
79,355 |
|
Ellis |
$ |
$ |
$ |
$ |
+ |
$ |
|
+ |
|
+ |
$ |
11,123,000 |
1,625,000 |
|
59th Sr. Pond |
$ |
+ |
+ |
|
|
|
|
+ |
|
|
|
4,837,000 |
498,035 |
|
Frank Holten |
$ |
$ |
|
+ |
|
|
|
+ |
|
+ |
+ |
1,862,300 |
927,000 |
|
Henry |
$ |
|
|
|
|
+ |
+ |
|
|
+ |
$ |
134,200 |
220,000 |
|
Jackson |
$ |
$ |
|
|
+ |
|
+ |
|
|
|
|
7,309,800 |
725,663 |
|
Lansing |
$ |
|
|
+ |
|
|
+ |
|
|
+ |
|
1,155,900 |
800,000 |
|
Liberty |
$ |
$ |
|
|
|
|
+ |
+ |
|
+ |
|
813,000 |
577,975 |
|
Lilly |
+ |
+ |
|
|
+ |
|
$ |
|
|
+ |
|
2,880,000 |
350,000 |
|
Little Pond |
|
+ |
|
|
|
|
|
|
$ |
+ |
|
212,200 |
9,946 |
|
Loch Raven |
$ |
$ |
|
|
|
|
|
|
$ |
+ |
|
11,944,100 |
150,900 |
|
Medical |
$ |
+ |
|
|
+ |
|
$ |
|
|
|
|
931,700 |
128,217 |
|
Mirror and Shadow |
$ |
|
|
|
|
|
+ |
+ |
|
+ |
|
312,700 |
215,000 |
|
Moses |
$ |
+ |
|
|
|
|
|
|
|
+ |
+ |
534,700 |
3,251,000 |
|
Nutting |
$ |
$ |
|
$ |
|
|
+ |
+ |
|
+ |
|
5,292,100 |
241,159 |
|
Penn |
$ |
$ |
|
+ |
+ |
|
|
|
|
+ |
$ |
186,000 |
87,900 |
|
Rivanna |
$ |
|
|
|
|
|
|
|
$ |
+ |
|
923,500 |
63,835 |
|
Steinmetz |
$ |
+ |
+ |
+ |
|
|
|
$ |
|
+ |
|
126,300 |
36,680 |
|
Temescal |
$ |
$ |
|
|
|
|
|
$ |
|
+ |
$ |
1,112,500 |
315,618 |
|
Tivoli |
$ |
+ |
+ |
+ |
|
|
|
+ |
|
+ |
|
240,100 |
202,645 |
|
Vancouver |
$ |
+ |
|
+ |
|
$ |
|
+ |
|
+ |
|
47,370,000 |
3,468,328 |
|
Washington Park |
$ |
$ |
|
|
|
|
|
|
|
+ |
|
120,800 |
23,250 |
|
Total |
|
|
|
|
|
|
|
|
|
|
|
127,488,500 |
15,349,053 |
|
a "$" in monetary terms; "+" in qualitative terms. b Total discounted benefits include "$" items only. Source: U.S. Environmental Protection Agency, 1980. |
|||||||||||||
monitoring data are simply filed away and are not accessible for others to evaluate.
Need for Coordination of Management Efforts
Water quality management and fisheries management have evolved as almost completely separate disciplines. Water quality experts and fisheries experts are trained in separate university departments, belong to different professional societies, attend different scientific meetings, and read different scientific journals. Wildlife and fisheries managers may be trained in the same college or department, but the disciplines remain segregated in many ways. Yet members of these distinct professions find themselves managing nuisance algal blooms, toxic chemicals, fish production, and waterfowl habitat on the same ecosystems. It is not surprising that management programs sometimes work at cross-purposes. For example, in Medical Lake, Washington, fish stocking caused a decline in herbivorous zooplankton, confounding efforts of water quality managers to control nuisance algal blooms (see Box 4.1).
|
Box 4.1 Medical Lake, Washington Medical Lake is a large (63 km 2 ),deep (maximum depth, 18 m; mean depth, 10 m), eutrophic lake in eastern Washington. Prior to 1964, the homes on its small watershed were serviced with septic tanks and cesspools. Dense blooms of blue-green algae continued after wastewater diversion because of high rates of internal nutrient loading from enriched sediments, and this led to frequent curtailments of swimming. More than half of the lake's volume was anoxic, and fish were rare or absent. Attempts by the Washington Department of Game to stock trout were unsuccessful because of high levels of ammonia and hydrogen sulfide (Bauman and Soltero, 1978; Soltero et al., 1981). In 1977, 936 m 3 of liquid aluminum sulfate were added to remove phosphorus from the water column and retard its recycling. After treatment, phosphorus concentrations and algal density were reduced; blue-green algae were largely replaced with less noxious species of green algae; water clarity increased; and large-bodied zooplankton, Daphnia pulex, became the primary regulators of phytoplankton density. The |
|
lake once again became attractive for various recreational and aesthetic uses (Mires et al., 1981; Soltero et al., 1981). The significant improvement in water quality encouraged the Washington Department of Game to attempt to establish a rainbow trout (Oncorhynchus mykiss) fishery in the lake to provide economic benefits to the area. Between 1978 and 1981, 45,330 trout were stocked. During this period, Knapp and Soltero (1983) determined that all age classes of trout in the lake fed almost exclusively on D. pulex, and this led to the near elimination of this algae grazer from the lake in 1981. Water quality again deteriorated; coupled with lack of food, this led to an extensive trout kill in autumn of 1981. Daphnia pulex again increased in the years after the trout kill (Scholz et al., 1985). The Medical Lake restoration project had the rare benefit of long-term monitoring. Not only did the project demonstrate the effectiveness of controlling phosphorus recycling by alum addition, it also showed that the switch in control of algal biomass from "bottom-up" resource limitation (by nutrients) to "top-down" control by grazers can be reversed easily by a poorly planned overstocking with game fish for short-term economic gain (Scholz et al., 1985). A moderate and balanced restocking of several fish species, along with establishment of a refuge for Daphnia, might have allowed continued high water quality and a resumption of multiple lake uses. Interestingly, the overstocking with trout may represent a brief (but significant) perturbation, and the lake may yet return to a grazer-controlled, more stable ecosystem. |
LAKE RESTORATION TECHNOLOGY
Overview
Lake restoration methods can be divided into two major categories: (1) watershed activities to eliminate stress loadings, and (2) in-lake activities to restore or improve the aquatic ecosystem itself. The former are necessary for the long-term success of restoration programs (except when the stress originates in the lake itself, e.g., lake stage regulation), but they are not restorative actions per se and moreover may not be sufficient. The latter are done to accelerate the return to earlier (more natural) conditions, to remove long-lived contaminants
or exotic species, or to reintroduce ecosystem components lost through the impact of stress. Numerous restoration techniques have been developed for lakes over recent decades (Table 4.7). Some methods are applicable to several types of stress: dredging is useful to remove sediments laden with nutrients or contaminated with toxic materials, or to deepen lakes prematurely filled in by excessive erosion. Others apply to only one type of stress: alum treatment is used to remove excess phosphorus from the water and prevent its recycling to the water from the sediments (by forming an aluminum hydroxide barrier at the sediment-water interface). Some methods (e.g., sediment removal) need be used only once to achieve long-term
TABLE 4.7 Restoration Techniques for Major Categories of Lake Degradation
|
|
Problem Category |
||||
|
Technique |
Eutrophication |
Siltation |
Acidification |
Exotic Species |
Toxic Contaminants |
|
Nutrient source reduction |
X |
X |
X |
|
X |
|
Diversion |
X |
X |
|
|
|
|
Land disposal |
X |
|
|
|
|
|
Product Modification |
X |
|
|
|
X |
|
Wastewater treatment |
X |
|
|
|
X |
|
Interception of nonpoint sources |
X |
X |
|
|
X |
|
Dilution |
X |
|
X |
|
X |
|
Flushing |
X |
|
X |
|
X |
|
In-lake methods |
X |
|
X |
X |
X |
|
Alum treatment |
X |
|
|
|
|
|
Sediment skimming |
X |
X |
|
|
X |
|
Sediment oxidation |
X |
|
|
|
|
|
Deep-water discharge |
X |
|
|
|
X |
|
Biomanipulation |
X |
|
|
X |
|
|
Artificial circulation |
X |
|
|
|
|
|
Biocides (algicides/herbicides/ piscicides) |
X |
|
|
X |
|
|
Biocontrol agents |
|
|
|
X |
|
|
Drawdown/sediment desiccation |
X |
X |
|
|
|
|
Bioharvesting |
X |
|
|
X |
|
|
Aeration |
X |
|
|
|
|
|
Dredging |
X |
X |
|
|
|
|
Liming |
|
|
X |
|
|
benefits (if the cause of the stress has been eliminated); others must be applied continuously (aeration) or repeatedly (liming of acidic lakes; herbicide treatment of macrophyte problems) to maintain the benefits of treatment.
Lake restoration and management techniques are listed in Table 4.7 according to the type of problems they seek to remedy, and the sections below briefly describe the most important of these techniques. More comprehensive reviews of the techniques are provided by Cooke et al. (1986) and Cooke and Kennedy(1989)
Problems caused by excess nutrients have received the most attention over the past two decades, and more techniques have been developed to address these problems than all other types of stresses combined. Excessive nutrient enrichment manifests itself in several distinct problems; algal blooms, macrophyte proliferation, oxygen depletion, and loss of sport fisheries are the most important.
Eutrophication
CONTROL OF ALGAL BLOOMS
Nutrient Source Reduction
High loading of nutrients to lakes produces algal blooms and other problems. In many cases, oxygen-demanding organic matter, silt, or toxic materials accompany the nutrient loadings. Reduction of nutrient loadings (and related inputs) can be accomplished (Table 4.8) by (1) diverting point sources of nutrients (e.g., municipal sewage effluents) or nutrient-laden streams out of the lakes watershed; (2) modifying products to contain lower amounts of nutrients (mainly phosphrous); (3) removing nutrients from wastewater in engineered treatment systems; (4) intercepting nutrients in pre-lake impoundments (storm water detention and retention ponds, natural or artificial wetlands); (5) decreasing nutrient runoff from agricultural lands by "best management practices"; and (6) instituting land use and management controls.
Diversion Wastewater effluent is rarely diverted out of watersheds because of the difficulty of finding an alternative disposal site, but a few well-known diversions have occured. The lower lakes of Madison, Wisconsin (Monona, Waubesa, and Kegonsa) have deteriorated drastically during the twentieth century because of sewage discharges (Sawyer, 1947; Lathrop, 1979). By 1958, most of the effluent have been diverted downstream from the lakes, and all effluent was
TABLE 4.8 Control Methods for External Sources of Nutrients
|
I. Stream or wastewater diversion |
|
II. Municipal wastewater treatment (tertiary treatment for N and P removal) |
|
III. Product modification (e.g., legislative ban of phospate in laundry detergents; slow release fertilizers) |
|
IV. Treatment of inflow streams |
|
A. Diversion |
|
1. Into wetlands |
|
2. Over upland vegetation |
|
B. In-stream methods |
|
1. Sedimentation basins to remove particulate N and P |
|
2. Channel aeration |
|
3. Chemical precipitation |
|
4. Biotic harvesting |
|
V. Land use practices |
|
A. Prospective zoning |
|
1. On-site storm water retention or detention regulations |
|
2. Setback and other shoreline restrictions on new construction |
|
3. Restrictions on shoreline vegetation removal |
|
4. Restrictive zoning in watershed to minimize development |
|
5. Minimization of impervious areas in developments |
|
6. Use of grassy swales instead of curb and gutter drainage |
|
B. Treatment of urban runoff (best management practices) |
|
1. Retention/detention basins |
|
2. Swirl concentrators |
|
3. First flush diversion (or low flow) to sanitary sewers |
|
4. Diversion of runoff into wetlands |
|
5. Street sweeping or vacuuming |
|
6. Public education (reduce litter accumulation, control lawn fertilizer losses) |
|
C. Treatment of agricultural runoff (best management practices) |
|
1. Runoff controls (change volume and peak flow) |
|
a. No or minimum tillage |
|
b. Winter cover crop |
|
c. Contour plowing and strip cropping |
|
d. Terraces |
|
e. Grassed outlets; vegetated borders on fields and along waterways |
|
f. Detention ponds |
|
2. Nutrient loss controls |
|
a. Timing and frequency of fertilizer applications |
|
b. Amount and type of fertilizer used |
|
c. Control in situ transformation of fertilizer to soluble forms |
|
d. Crop rotation with legumes |
|
e. Storage of manure during winter |
diverted from the Madison lakes by 1971. The diversion was partially successful. Severe blue-green algal problems were ameliorated, but the lakes remained eutrophic (Sonzogni et at., 1975). Diversion of sewage effluent from Seattle from nearby suburban areas out of Lake Washington in the mid-1960s restored the lake from a state of moderate eutrophy and declining water quality to a mesotrophic or an oligotrophic system with high water quality (see Box 4.2). Effluent from an advanced wastewater treatment plant at South Lake Tahoe, California, was diverted from Lake Tahoe into a man-made impoundment in the Nevada desert to provide added protection to that highly oligotrophic and pristine lake in the Sierra Nevadas (Goldman, 1988).
Land disposal of treated municipal wastewater (a form of nutrient diversion) is becoming common in warm climates; often the water is used for irrigation of agricultural lands (e.g., citrus groves in Florida) or golf courses. This practice is used as much for water conservation purposes (to decrease demands on ground water or surface supplies) as for protection or restoration of lakes or receiving streams.
Product Modification The most important example relative to reduction of nutrient loadings is the reduction or removal of phosphates from laundry detergents; other materials — carbonates, silicates, organic compounds are substituted to achieve the functions that phosphates provide. Laws banning phosphates or requiring lower levels of phosphates in laundry detergents are in effect in at least 10 states along the Great Lakes plus parts of several other states (Maki et al., 1984). Detergent phosphates account for about two-thirds of the phosphate in the municipal sewage of areas without detergent phosphate regulations. Phosphate levels in sewage from areas with laws regulating detergent phosphate levels are typically about 50 percent lower than levels in other areas. Bans are not instituted to reduce phosphorus loadings to a single lake — local ordinances are ineffective because consumers will obtain the products in nearby areas where they are not banned. This approach thus is used to lower phosphorus levels in surface waters on a statewide or regionwide basis. Although limitations on detergent phosphates usually are not sufficient to lower phosphorus loadings to lakes below the levels leading to eutrophication, they do lead to lower costs of removing phosphate from municipal sewage.
Removal of Phosphorus from Wastewater Removal of phosphorus from wastewater in municipal treatment plants, almost unheard of 20 years ago, is now a well-developed and widespread practice (see Box 4.3). It is required for wastewater entering the Great Lakes from all plants
|
Box 4.2 Lake Washington Lake Washington, an important recreational lake (area, 87 km2; maximum depth, 65 m), was first impacted by raw and treated sewage from Seattle early in this century. This first episode of pollution ended in the 1930s with the construction of a sewerage system that diverted the sewage effluent to Puget Sound. A second episode began in the 1940s when suburban growth spread along the lake north and south of Seattle. By 1963, effluent from 11 sewage treatment plants was being discharged to the lake or its tributaries and contributed 63 percent of the phosphorus load entering the lake. By 1955, accelerated nutrient enrichment had progressed to the point that the first bloom of Oscillatoria rubescens was observed. This blue-green alga is widely recognized as an indicator of excess nutrient enrichment. Deteriorating water quality from algal growths was reported in the press, and related issues were addressed by the scientific community (see Lehman, 1986; Edmondson, 1991). By 1957, public concern had resulted in state legislation to form a metropolitan governmental agency to address the problem of water supply and waste management. Establishment of this agency required a public referendum. The first proposal was defeated in March 1958, but a revised proposal passed in September 1958, and provided funds to construct facilities to divert sewage from the lake to Puget Sound. Diversion of effluent began in 1963, and by March 1967, 99 percent of the sewage effluent had been diverted from the lake. The lake responded rapidly to decreased nutrient loading, as limnologists had predicted. Noticeable differences occurred soon after diversion was complete. By the summer of 1971, Secchidisk transparency was greater than it had been in 1950. By 1975, large changes had occurred compared with conditions in 1963: Secchi transparency increased from 1.0 to 4.0 m, total phosphorus decreased from 70 to 16 µg per liter, and epilimnetic chlorophyll decreased from 35 to 4 μg per liter (Edmondson, 1979). Nitrogen was no longer limiting after diversion. It had become a limiting nutrient because of the large biomass of algae produced by increased phosphorus loads. Nuisance blooms of algae were no longer a threat to the lake. The much larger volume of Puget Sound enabled it to assimilate the treated effluent without suffering significant water quality impairment. |
|
The diversion project developed rapidly because of scientific analysis, legislative action, and public support. The project was effective because the scientific community, headed by W. T. Edmondson, convinced the public that water quality would become worse with no action and would be improved only by eliminating sewage inputs to the lake. Scientific projections for rapid improvement in water quality were borne out after diversion had taken place. Scientific information used to support the proposal for sewage diversion included data for nutrients, oxygen, and water transparency. Data from 1955 to 1959 were compared with those describing conditions in 1933 and the early 1950s. Scientific support for action also came from Edmondson's analysis of the problem in relation to earlier eutrophication problems of European lakes, which was simplified by Hasler's general review of cultural eutrophication (Hasler, 1947). In particular, Lake Zürich in Switzerland and other lakes had developed blooms of Oscillatoria rubescens as a result of pollution by domestic sewage. Diversion is more effective than tertiary treatment of sewage effluent for two reasons. Although tertiary treatment removes 90 to 95 percent of the phosphorus (P) from sewage, the effluent may still contain 0.5 to 1.0 mg of phosphorus per liter, which is 10-fold higher than the phosphorus content of Lake Washington when it was most enriched. In addition, diversion reduces inputs of other substances that might be secondary factors in promoting algal growth. Diversion thus completely removes phosphorus, the main contributor to eutrophication, as well as other substances that could promote algal growth but might not be removed completely in sewage treatment. |
with discharges exceeding 1 million gallons per day. Most plants remove phosphorus by chemical methods in ''tertiary" treatment units, installed after conventional "secondary" (biological) treatment units. Phosphorus removal is achieved by adding alum, calcium, or (more rarely) iron salts to the waste stream. The metal ions form hydrous precipitates, and phosphate is removed by coprecipitation and adsorption onto the surfaces of the precipitates (Hsu, 1975). Removal efficiencies far exceeding 90 percent are common. Effluent standards for phosphorus, where such exist, usually are 1 mg per liter (based on a conventional engineering goal of 90 percent removal efficiency
|
Box 4.3 Shagawa Lake, Minnesota In contrast to Lake Washington, Shagawa Lake, Minnesota, is an important recreational lake in which the effects of reduced phosphorus loading were not as great as predicted. Its main external source of phosphorus was the sewage treatment plant at Ely, Minnesota, a community of 5,000 residents. Ely was established around 1900 and attained its largest population (ca. 6,000) in the 1930s. The three-basin lake has a maximum depth of 13 m, mean depth of 5.6 m, mean volume of 5.2 x 10 (7) m (3), and surface area of 925 ha (Larsen et al., 1975). Shagawa Lake has had a long history of water quality deterioration as the result of cultural eutrophication. Wastewater from Ely initially flowed untreated into the lake, then received primary treatment in 1911 and secondary treatment in 1952. A tertiary treatment plant to remove phosphorus was constructed with EPA demonstration grant funds in 1973 and reduced the lake's external phosphorus loading by 80 to 85 percent (Larsen et al., 1979, 1981). The lake's average total phosphorus (P) concentration should have declined from 51 µg of phosphorus per liter (pretreatment) to about 12 µ g of phosphorus per liter in 1.5 years, according to a tank reactor model. Instead, only a 40 percent reduction occurred. By 1976, the average total phosphorus concentration had decreased from about 50 to 29 <CH:181> g per liter, and soluble reactive phosphorus decreased even more, from 21 to 4.5µg per liter. The only noticeable biological response was a small decline in the annual average chlorophyll concentration, caused by a decrease during May and June to less than half the pretreatment value of 15µg per liter. However, there was no trend in chlorophyll concentrations during the main recreational season (July to August), when the most severe blue-green algal blooms occurred (chlorophyll levels of 60 <CH:181> g per liter and sometimes up to 100µg per liter). The lake's water residence time is 8 to 9 months, and its phosphorus residence time was estimated to be less than 6 months (Larsen et al., 1975). A rapid response to nutrient reduction thus would be expected (based on loading model concepts). Problems with blue-green algal blooms continued in the lake during late summer throughout the 1980s. A large bloom in August of 1987 was thought to be responsible for the death of several dogs (apparently by ingestion of toxins excreted by the algae (B. Wilson, Minnesota Pollution Control Agency, personal communication, 1991). |
|
This demonstration project has been considered a failure by some because a rapid biological response to reduced phosphorus loading was not obtained, as predicted from simple models of phosphorus loading. These models failed to predict lake conditions because they do not account for internal loading of phosphorus, a process whose importance was not widely recognized prior to the Shagawa Lake project. Data collected after external loading was reduced showed that biological productivity is being maintained by release of phosphorus from recent sediments when deep-water oxygen depletion occurs (Larsen et al., 1981). Phosphorus transport from these deep waters to the surface was sufficient to maintain algal blooms. Restoration of the lake may take much longer than anticipated because of feedback from the sediment. Recovery could be accelerated by alum treatment, but the size of the lake makes this approach impractical. Evaluation of this project possibly should be delayed until it can be established how much internal loading will be reduced with time. Intuitively, one would expect that internal loads over the long term depend on external loads. Thus, with some unknown time lag, the rate of internal loading may decrease and water quality may improve. This appears to have occurred in Lake Sammamish, Washington, after wastewater was diverted from it (Welch et al., 1986). Continued research in Shagawa Lake to determine the long-term interplay between reduced external loading and internal loading would be helpful. Long-term data may be required to establish trends because of interannual variability in biological productivity caused by climatic factors (independent of nutrient loading). |
and a typical raw sewage concentration of 10 mg per liter), but well-run tertiary plants routinely produce effluents with 0.1 mg of phosphorus per liter or even less.
Biological techniques also have been developed that remove 80 to 90 percent of the phosphorus from wastewater (Shapiro et al., 1967; Marais et al., 1983). Conventional biological waste treatment plants have low phosphorus removal efficiencies (20 to 40 percent) because phosphorus levels in raw waste far exceed the stoichiometric needs
of the microorganisms (activated sludge) that consume the waste's organic matter. Efficient biological removal is obtained by manipulating operating variables such as aeration time, cell residence time, and organic loading rates and preventing the sludge from becoming anoxic, all of which promote "luxury" uptake and retention of phosphorus by the sludge. Biological techniques have the advantages of requiring no chemical doses and no additional treatment units and of costing less than chemical methods. However, chemical methods are easier to control and operate at higher removal efficiencies.
Interception of Nonpoint Sources of Nutrients Control or elimination of point sources of nutrients usually is insufficient to reverse eutrophication problems, and nonpoint sources must be managed as well. The costs of treating nonpoint sources by engineered systems are high, and diversion of inputs to other ecosystems may have high political and social costs, as well as untoward ecological and hydrological effects. This has led to the development of pre-lake interception systems as "low-technology" supplements to tertiary wastewater treatment and agricultural best management practices to decrease nonpoint sources of nutrient loading. Most interception systems function to remove other contaminants (loading silt, particulate organic matter, toxic metals) as well as nutrients. Three types of interception systems have been used: storm water detention and retention (siltation) basins, artificial or natural wetlands, and in-stream phosphorus precipitation.
Detention basins function by impounding storm water runoff (urban or agricultural) for a long enough time to allow settling of particulate materials. Retention ponds are designed to catch a certain amount of runoff (e.g., the first inch) and retain it until it infiltrates through the soil. Because much of the phosphorus (and other contaminants) in runoff water is associated with suspended particulates, detention and retention basins are effective as low-cost, low-maintenance treatment systems, and such ponds are required for new urban developments in many states. Design criteria and performance standards have been evaluated (Walker, 1987). Detention ponds eventually fill up with silt and organic matter, and must be reconstructed or dredged.
Prereservoir detention basins are a variation on the same idea. They are constructed on natural streams just upstream from their entry to a lake or reservoir. Such basins protect the reservoir from silt, phosphorus, and bacterial loadings, and are effective as long as they remain aerobic to prevent internal loading. Based on loading model calculations, Benndorf and Putz (1987) concluded that basins
with a 2-day retention time should achieve about 35 percent phosphorus removal, 5 days should yield about 40 percent removal, and 15 days should yield 50 to 60 percent removal. Actual data from the watershed of Jesenice Reservoir (Czechoslovakia) shows greater removal efficiency. A prereservoir detention basin with a water residence time of 5 days was found to retain 60 to 70 percent of the total phosphorus entering it (Fiala and Vasata, 1982). Removal efficiency information can be used along with a phosphorus budget of the downstream lake to determine the detention time (hence the basin size) required to decrease the phosphorus loading to a lake.
Man-made and engineered natural wetlands (see Box 4.4) have been successful, in some cases, in retaining materials suspended in water flowing through them. Wetlands are effective in retaining suspended solids, given adequate detention time, and most phosphorus removal is associated with this process. They are also highly effective in reducing stream loads of metals such as lead and zinc (Martin, 1988). Temperate wetland systems for this purpose generally emphasize
|
Box 4.4 Clear Lake, Minnesota Clear Lake, in Waseca, Minnesota, is an example of the use of an engineered wetland (Barten, 1987). This 257-ha, heavily used recreational lake became eutrophic from sanitary sewage and urban and agricultural runoff. In 1981, about 50 percent of the water load and 55 percent of the phosphorus load were diverted into a modified 21-ha marsh throughout the growing season. All cells of the marsh can be drained to harvest plants. About 40 percent of the lake's annual phosphorus load is retained in the marsh. Nonetheless, the average lake concentration of phosphorus fell only about 30 percent, apparently because excessive phosphorus loading over many years had led to high rates of hypolimnetic oxygen demand and high internal phosphorus recycling in the lake. A whole-lake fish reclamation project (rotenone treatment to remove rough fish) in the fall of 1986 caused only a small decrease in phosphorus concentrations but did reduce chlorophyll levels dramatically for about a year (because fish removal enhanced zooplankton grazing). Finally, hypolimnetic alum treatment in 1988 was successful in lowering the N-lake phosphorus levels and summertime chlorophyl concentrations. |
emergent plants such as cattails, whereas floating plants like water hyacinth are most effective in subtropical and tropical wetlands (Reddy and DeBush, 1987).
In contrast, diversion of streams or runoff into unmanaged natural wetlands appears to provide only limited long-term nutrient removal. Although such wetlands may assimilate nutrient inputs during the growing season, a large outflow of nutrients released from dead vegetation the following spring may offset the nutrients stored the previous growing season. In addition, large losses of nutrients from wetlands during high-flow, intensive rain events or through channelization tend to counterbalance the net storage of nutrients during longer periods of low or moderate flow rates (Richardson, 1988).
In a few cases, streams flowing into lakes have been treated by adding phosphorus-precipitating chemicals (iron, aluminum), but because volumes of water that need to be treated generally are large (compared with municipal wastewater), this usually is not a cost-effective approach. Iron is preferred for in-stream treatments because it has fewer toxicity problems than does aluminum, but binding of phosphorus to iron requires continuously aerobic conditions. Success in lowering phosphorus concentrations has been reported when relatively small flows can be treated. An example is the addition of ferric sulfate to water pumped into Foxcote Reservoir (England) to remove dissolved phosphorus. Although internal phosphorus loading in the reservoir has reduced the treatment's effectiveness, the length of time that Foxcote Reservoir cannot be used as a potable water supply during summer months has decreased (Young et al., 1988).
Wahnbach Reservoir, an important municipal water supply for Bonn, Germany, is protected from nutrient, silt, and organic matter loading from its main tributary by a prereservoir detention basin and phosphorus elimination plant (Bernhardt, 1980; Clasen, 1989). Water from the detention basin is treated with iron to remove phosphorus and then filtered through an ion exchanger and a series of activated carbon and sand filters. The plant removes 95 to 99 percent of phosphorus, coliform bacteria, algae, and turbidity; 77 percent of the water's biochemical oxygen demand; and 58 percent of the dissolved organic carbon. Grossly enriched river water is converted into nearly drinkable water before it enters the reservoir. Costs of this project have not been published.
Best Management Practices Numerous best management practices (BMPs) (Table 4.8) have been developed to decrease losses of soil,
nutrients, and other contaminants from agricultural lands and urban areas. (The interception methods described in the section immediately above are essentially one class of BMPs.) Effectiveness in preventing nutrient export, technical feasibility, social acceptability, and cost vary widely among the practices, and quantitative information on these aspects is lacking for many of them, which are still in the developmental stage. Some practices (e.g., restrictive zoning ordinances and setback requirements) are more suitable for new developments than for developed areas. Although BMPs seldom provide the complete solution in restoring degraded lakes, they are key elements in an evolving strategy that recognizes that lakes can be managed and protected effectively only in the context of the watershed in which they exist.
Dilution
Dilution is a procedure that can lower water column phosphorus (P) concentrations by adding water that is low in phosphorus. It will also increase washout of algal cells from a lake. In principle, addition of dilution water to a lake will increase its total phosphorus loading rate but decrease the mean inflow phosphorus concentration. The lake's flushing rate is also increased, and this tends to decrease phosphorus sedimentation. As a result, the water column phosphorus concentration will decrease, although increasing the amount of dilution water will not produce a proportionate reduction in water column phosphorus concentration. The best candidate lakes are those with high flushing rates and moderate problems with high phosphorus concentrations.
Moses Lake, Washington, is the best documented case history of dilution (Welch, 1981; Welch and Weiher, 1987). Columbia River water was diverted through the lake and then to agricultural areas for irrigation. Algal blooms were reduced by 50 percent and water clarity increased by 100 percent during the 9 years that dilution water entered the lake. Nonetheless, from an economic cost-benefit perspective, Moses Lake was one of the few unsuccessful projects noted by EPA (1980) in its analysis of benefits of the Clean Lakes Program (see Table 4.6). The project received about $3.25 million in federal funds (and about the same in local funds), but quantifiable benefits amounted to only $0.53 million.
Dilution does not appear to be associated with negative impacts on the lake, other than those associated with increased flow. Few case histories of its use exist, in part because of the general absence of sufficient supplies of nutrient-poor water to add to a lake's inflow.
In-Lake Methods to Reduce Phosphorus Concentrations and Cycling
Phosphorus Inactivation A significant reduction in nutrient loading to a eutrophic lake is a necessary but sometimes insufficient step in order to decrease water column phosphorus concentrations enough to reduce the amount of algae. Phosphorus release from lake sediments at high pH, or when dissolved oxygen in overlying water is low or zero, can be a major source of phosphorus to the water column. Under certain conditions, phosphorus released from lake sediments will be transported to the upper layers of a lake and stimulate an algal bloom. This process, in which sediments enriched in organic and inorganic matter from external loading and in-lake production cause dissolved oxygen consumption and phosphorus release, is known as internal loading. It can be great enough to delay or prevent a lake's recovery from nutrient diversion or interception (see Box 4.3).
Phosphorus inactivation reduces the rate of phosphorus release from lake sediments by the addition of aluminum salts (sodium aluminate, aluminum sulfate) to them (Cooke et al., 1986). Aluminum hydroxide is formed and appears as a visible floc that settles to the sediment and binds with phosphate ions to form a solid that is insoluble under low or zero dissolved oxygen. Phosphate ions diffusing from the sediment are trapped by the floc. The process has proved to be effective and long-lasting. Several Wisconsin lakes treated in the early 1970s exhibited improved conditions 10 years later (Garrison and Knauer, 1984; see Box 4.5). Treatment of shallow, well-mixed lakes can also be effective but appears not to have the longevity found with deep, thermally stratified lakes. A representative case history is Long Lake, Kitsap County Washington (Welch et al., 1988).
In contrast, Eau Galle Reservoir, a flood control impoundment in Wisconsin, illustrates the ineffectiveness of phosphorus inactivation when nutrient loading is not reduced significantly (Kennedy et al., 1987). The effects of treatment on the quality of this water body were overwhelmed in a few months by continued nutrient loading. Because reservoirs are difficult to protect from nutrient loading, this technique is not considered widely applicable to this type of water body (see Box 4.5).
Aluminum is a potentially toxic metal. At naturally occurring pH (6 to 8) in waters with carbonate alkalinity, nearly all aluminum is found as nontoxic aluminum hydroxide. If the pH falls much below 6, toxic forms of soluble aluminum will increase. Several observations of treated lakes with normal pH have failed to demonstrate any
|
BOX 4.5 West and East Twin Lakes, Ohio Some lakes have significant internal sources of nutrients from littoral wetland and macrophyte zones, and especially from bottom sediments. The presence of an anoxic hypolimnion greatly increases the rate of release of nutrients from sediments to the overlying water. Under certain circumstances (e.g., high mean depth, large area, exposure to winds), these nutrients are transported from the hypolimnion to the epilimnion and subsidize algal blooms. Diversion of cultural nutrient loading, although essential, may not be sufficient to return these lakes to their undisturbed condition; curtailment of internal loading may also be required. Such lakes may be more common than those in which water column nutrient concentrations are determined by external loading alone. Shagawa Lake, Minnesota, described earlier (see Box 4.3) is another example. West and East Twin Lakes are small (34 and 27 ha, respectively), thermally stratified lakes of glacial origin in a 335-ha forested, urbanized watershed (including lakes) in northeastern Ohio. Prior to 1973, domestic waste from about 360 homes was discharged to septic tanks and leach fields. Although most of these disposal systems were located in ideal soil, sloping lawns became saturated with effluents, and organic matter, nutrients, and bacteria were washed into the lakes with surface and shallow ground water flows. In 1969, high densities of algae and coliform bacteria caused the lakes to be closed to contact recreation. Between 1971 and 1973, all domestic wastewater was diverted out of the watershed of both lakes. Lake scientists predicted that internal release of phosphorus from anoxic hypolimnetic sediments, followed by vertical entrainment to the epilimnion, would delay recovery of the lakes. It was further predicted that application of aluminum sulfate (alum) to the hypolimnion would accelerate lake recovery by controlling phosphorus release from anoxic sediments (Cooke et al., 1982). External and internal phosphorus budgets were determined from 1971 to 1976, and changes in lake trophic state were monitored from 1969 to 1976 and at widely spaced intervals through 1989. A basis for the alum dose was determined by field and laboratory toxicity tests and by calculations of expected dissolved aluminum concentrations for various lake alkalinities. |
|
A pilot treatment of a small (3-ha) lake was carried out in 1974 as a test of dose and application guidelines. In 1975, West Twin's hypolimnion received 100 tons of liquid alum. East Twin, the downstream lake, served as a reference. Alum treatment sharply reduced phosphorus release from the anoxic hypolimnetic sediments. Prior to nutrient diversion, the lakes were classified as eutrophic, based on water transparency and on phosphorus and chlorophyll concentrations. In 1989, 14 years after treatment and 16 years after diversion, they were near the mesotrophicoligotrophic border, a state consistent with expectations in this ecoregion. In laboratory experiments under anaerobic conditions, phosphorus release rates from the treated West Twin sediments were still significantly lower than release rates from untreated East Twin sediments in 1989, showing that the alum treatment retained its effectiveness for 14 years (Cooke et al., 1986; Cooke and Martin, 1989). The key event to restoration of the two lakes was diversion of nutrient inflows. Alum treatment aided in the recovery of West Twin but would have had little long-term effect if loading had continued. Whereas hypolimnetic phosphorus concentrations in West Twin after treatment remained at less than a third of those in East Twin (and at about 20 percent of pretreatment concentrations through 1986), surface water phosphorus concentrations in the two lakes remained similar and declined over the years 1975 to 1989 in a nearly identical pattern. Several studies demonstrated that phosphorus release into the hypolimnion of West Twin was controlled by alum treatment. However, vertical entrainment appeared not to be as large a source of phosphorus to the epilimnion as was predicted or as has been calculated for deeper lakes with greater exposure to wind mixing (e.g., Lake Mendota, Shagawa Lake). The results demonstrate the importance of controlling the load of nutrients from the watershed and also underscore the importance of long-term monitoring of restoration projects. The initial results appeared to support the hypothesis that control of internal loading was the key to restoration, but longer-term data did not. Use of the reference lake was also an essential component in understanding the mechanism of recovery. Unfortunately, projects with this type of design are rare in lake restoration. |
deleterious effects to fish or invertebrate animals living in treated sediments over very long periods (years) of exposure. Phosphorus inactivation, mistakenly classified as an algicide treatment by some agencies, is considered to be safe and cost-effective when the aluminum sulfate dose is below that which will create low-pH conditions. The high water clarity that occurs after treatment can promote the invasion and/or spread of rooted macrophytes in shallow water. An alternative to aluminum salts for such purposes is calcium hydroxide (lime). This material has been used in one successful treatment (Prepas et al., 1990), but there are no data on treatment longevity.
Sediment Skimming Phosphorus release from lake sediments is greatest from the most recent phosphorus-rich surficial layers. Sediment skimming (see Box 4.6) involves the use of a hydraulic dredge to remove this layer. This procedure, although effective, is more costly than phosphorus inactivation. It does have a restorative effect without the addition of potentially toxic materials, especially when nutrient inflows have been reduced or eliminated. Once the equipment is set up for sediment skimming, it might be reasonable to proceed with a full-scale sediment removal to accomplish both lake deepening and control of internal loading (provided an adequate containment area for the sediment water slurry is available).
|
Box 4.6 Lake Trummen, Sweden Lake Trummen, Sweden, received domestic wastewater and flax mill discharges for many years, and algal blooms and fish winter kill were common. The loading was diverted from the lake, but no improvement occurred (because of internal nutrient loading). A sediment-skimming treatment removed the enriched surficial materials, and the phosphorus content of the remaining sediment was 10 percent of the material that had been removed. This was followed several years later by the removal of carp, which had become abundant, disturbed the sediments, and promoted phosphorus release. The lake improved greatly and remained in this improved state for at least 9 years. Continued removal of the carp was found to be essential to maintaining lake quality (Bjork, 1988). |
Sediment Oxidation
Phosphorus release from lake sediments can be controlled by accelerating the oxidation of sediment organic matter and providing a chemical environment that favors the binding of phosphorus by iron in the top 5 to 10 cm of lake sediment. Although the procedure is still in the development stage, it does not involve the addition of a potentially toxic element such as aluminum. Instead, calcium nitrate, Ca(NO3)2 , is injected into the sediments. The nitrate serves as an electron acceptor in the absence of oxygen, and decomposition of organic matter proceeds via denitrification (nitrate is reduced to N2, which evolves as a gas). At the same time iron sulfide, FeS, is oxidized, and phosphate ions are bound to the resulting ferric hydroxide. In some lakes, calcium hydroxide is added to bring the pH to the optimum for denitrification, and ferric chloride may be added if the lake is iron deficient (Ripl and Lindmark, 1978). Lake Lillesjon, Sweden, received a treatment with ferric chloride, lime, and calcium nitrate. The oxygen demand of the sediment decreased by 30 percent, and release of phosphorus from sediments to water was reduced to 10 to 20 percent of the pretreatment rate. 1 Some have suggested the use of nitrate-rich effluent from wastewater treatment plants to oxidize lake sediments, but field demonstration of this approach has not yet been conducted.
Deep-Water Discharge The impact of phosphorus release from lake sediments can be controlled by siphoning the nutrient-rich deep (hypolimnetic) water from a lake or discharging the hypolimnetic water of a reservoir through a deep gate in its dam. If release exceeds new external loading, the procedure should gradually deplete the sediments of phosphorus and could reduce the amount of nutrients entrained from deep to surface waters each summer. Summer and early autumn algal blooms should be reduced (Nurnberg, 1987). Continued high nutrient loading to a lake is likely to negate the effects of this technique.
Although deep-water discharge is not widely used, the few recorded attempts are encouraging. It appears that the greater the amount of phosphorus discharged in this way, the greater is the decrease in phosphorus concentration in the upper waters where algae grow. Moreover, the more years deep-water discharge operates, the
greater is the change in a lake's concentration of nutrients. However, there can be significant negative impacts of discharging nutrient-rich hypolimnetic waters to receiving streams. The dissolved oxygen content of such discharged waters may be near zero, and there will probably be high concentrations of soluble iron, manganese, hydrogen sulfide, ammonium, and phosphate. Treatment of the discharge would probably be required. Also, high discharge rates could induce a midsummer partial mixing of the water column, and this is likely to trigger an algal bloom when nutrient-rich bottom waters are mixed with surface waters.
Management of Symptoms
The techniques and procedures described above for control of algal blooms can be restorative because they produce a lasting decrease in nutrient concentrations. In some situations, however, it is not technically possible or economically practical to control external or internal nutrient loadings enough to prevent degraded water quality conditions. Several in-lake management tools are available to alleviate the symptoms of nutrient overenrichment and improve water quality for lake users. Some of these management tools (e.g., artificial circulation, use of algicides) require continuous or repeated applications (i.e., their benefits are short-lived), but others, such as biomanipulation, potentially can provide long-term benefits.
Biomanipulation Biomanipulation was broadly defined by Shapiro et al. (1975) to include a wide array of biological controls for water quality problems. They distinguished these from the many chemical and engineering approaches that exist for water quality improvement. More recently, a narrower definition, derived from the pioneering studies of Hrbacek et al. (1961), has been adopted by some limnologists: the manipulation of fish community structure to permit large herbivorous zooplankton grazers to flourish and to control nuisance algae (Shapiro, 1990b). This approach to biomanipulation is currently the object of substantial research programs in the United States, Canada, and several European nations (Gulati et al., 1990). Biomanipulation is not regarded as a substitute for reduction of nutrient loads. Important questions revolve around the capacity of biomanipulation to (1) reduce algal biomass where loads cannot be controlled and (2) augment or accelerate the effects of load reductions.
Results are still emerging, and it is unlikely that general principles concerning the efficacy of biomanipulation will be complete for several years (Gulati et al., 1990). However, certain patterns are clear.
Fish removal allows large, generalist grazers to become abundant and commonly reduces algal biomass and production by factors of 10 or more (Henrikson et al., 1980; Reinertsen et al., 1990). However, elimination of fish is neither practical nor desirable in many lakes. The alternative is to establish fish populations dominated by large, piscivorous fish. These predators reduce the biomass of smaller planktivorous fish, allow grazer biomass to increase, and reduce the biomass of algae. In whole-lake experiments, piscivore enhancements have improved water quality (Shapiro and Wright, 1984; Carpenter et al., 1987; Benndorf et al., 1988). Based on these experiments and case histories of fish kills, it appears that (1) the greatest improvements are possible in lakes dominated by planktivores prior to treatment, and (2) piscivore additions must achieve substantial (tenfold or greater) changes in planktivore biomass to influence water quality (Carpenter and Kitchell, 1988; Gulati et al., 1990).
Biomanipulation research is now expanding at two main interfaces. The first is the linkage between water quality and fisheries ecology. Management for large piscivores is a key element of biomanipulation (see Box 4.1). The high variability of fish stocks and the capacity of nutrient loads to destabilize lake food webs are key challenges that demand the best interactive efforts of fisheries ecology and limnology (Carpenter, 1988; Kitchell, 1991). The second interface is that between littoral zone ecology and the pelagic food web (Figure 4.4). In shallow lakes, fish removals that improved water clarity have been followed by expansion of submersed aquatic vegetation (Gulati et al., 1990). Once established, the submersed plants shelter fish that may eliminate grazers, causing declining water clarity and reduction in submersed vegetation. Abrupt transitions between alternate stable states of macrophyte and algal dominance may be triggered by nutrient mitigation, biomanipulation, or aquatic plant management. There is an obvious need for better understanding of the interactive effects of restoration and management of aquatic plants, fisheries, and phytoplankton.
Artificial Circulation Artificial circulation is a management technique whose goal is to achieve and maintain an isothermal and isochemical water column in a lake or reservoir that otherwise would exhibit stratification during summer. This is accomplished by injecting compressed air into a pipeline tethered at the lake's bottom in the deep zone. The last several meters of the pipe are perforated so that a vigorous bubble curtain is created, with enough energy to mix the water column rapidly. Even on the warmest days, a properly sized system will have a temperature vertical difference of less than 3ºC
(versus 20¹ C in typical stratified lakes). Pumps and whirling blades may also accomplish this goal (Cooke et al., 1986).
Expected improvements include (1) habitat expansion; (2) low concentrations of soluble iron and manganese, ammonium, hydrogen sulfide, and other reduced compounds associated with anoxic waters; (3) a reduction of algal biomass (in some cases); and (4) the elimination of surface thermal microstratification, a factor that favors the formation of blue-green algal scums.
Artificial circulation has been successful in improving potable water supplies (by eliminating iron, manganese, and hydrogen sulfide). Blue-green algae have declined in some cases, but not in others, and success in this respect seems to be depend on whether circulation reduces the pH of the surface water (Shapiro, 1973, 1984, 1990b; Shapiro et al., 1975). Even if artificial circulation does decrease the abundance of nuisance blue-green algae, it may not necessarily decrease the total amount of algae in the lake.
Most problems in the use of artificial circulation are associated with an underpowered compressor. A warm, uncirculated layer of water may develop on the lake's surface if the circulator cannot overcome the difference in water density created between surface and subsurface layers in hot weather. This would provide an ideal habitat for blue-green algae, which can regulate their depth with gas vacuoles and create surface scums. Another problem is the creation of turbid water if the bubble curtain disturbs flocculent sediments. This procedure provides little lasting benefit when it is shut off.
Algicides Algicides are chemicals that achieve control of nuisance algae through a toxic effect. The most common algicide is copper sulfate, to which blue-green algae are particularly sensitive. This is a purely symptomatic treatment; no lasting benefits are achieved, and a residue of copper is left in lake sediments. Copper sulfate treatments are effective only as long as the cupric ion (Cu 2+) concentration remains sufficiently high in the water, but concentrations usually fall rapidly (within hours or a few days) after treatment because copper adsorbs onto suspended particles and forms organic complexes and insoluble precipitates that settle to the lake's sediments (McKnight et al., 1983). Loss of copper is especially rapid in alkaline waters, and most lakes with algal bloom problems fall in this category.
Significant negative effects may occur as a result of copper sulfate treatment: dissolved oxygen depletion following decay of killed cells, sediment contamination, and toxicity to nontarget species, including fish and algae-grazing zooplankton. Repeated applications are required, making the cost-effectiveness poor. A summary of the chemistry,
effectiveness, dose, and negative effects of the use of copper sulfate is found in Cooke and Carlson (1989).
CONTROL OF AQUATIC MACROPHYTES
Overview
Macrophytes are natural and essential components of lake ecosystems. Small-bodied fish species and young fish of many species find food and shelter from predators in the littoral zone. Cultural eutrophication decreases water clarity and leads to the elimination of macrophytes that are important as food for waterfowl (e.g., Vallisneria americana). The simplified macrophyte community of eutrophic lakes usually is dominated by one or a few species, often exotics, that thrive in disturbed habitats. These species often concentrate their biomass near the water surface and thus are much more conspicuous to lake users than are native species that grow deeper in the water (Nichols et al., 1991).
To date, macrophyte restoration techniques have been limited to methods for killing nuisance plants. Most lake managers recognize that moderate macrophyte growth is essential for a healthy fishery and thus seek to control macrophytes rather than to eliminate them. Almost nothing is known about replacement of nuisance macrophytes by desirable species. Improved water clarity is probably essential for restoration of desirable macrophytes but may not be sufficient. Further steps such as sediment amendments and planting may be necessary. There is a need for research that moves beyond suppression of nuisance plants to the establishment of diverse macrophyte communities that provide essential habitat for waterfowl and fish (Nichols et al., 1991).
Biological Agents
Biological agents offer the prospect of long-term management of nuisance macrophytes at reasonable cost and minimal environmental impact, but the risks of escape and irruption of the biological control agent itself must be considered carefully (Magnuson, 1976). Many of the plants that cause nuisance problems are exotics, often imported for use in aquaria and inadvertently introduced to lakes. These plants have few pathogens or native animals that graze on them. Biological control research commonly involves the exploration for pathogens (bacteria, fungi, viruses) or predators (herbivorous insects) in the native habitat of the plants. These organisms are imported under controlled
conditions, and if found to be safe (i.e., to not attack nontarget organisms) and effective against the target plant, they are released at sites where the plant is a nuisance. Herbivorous fish, especially the white amur (or grass carp, Ctenopharyngodon idella Val.), that consume a variety of plant species also are common biological control agents. Sometimes biological agents are used with mechanical or chemical treatment for rapid relief while the bioagent develops to the density required to produce control.
Grass carp are permitted in 26 states. In contrast to phytophagous insects, which were brought into the United States under strict quarantine until their effectiveness and negative impacts could be evaluated, grass carp were introduced to some lakes with little or no prior testing. Grass carp have voracious appetites for certain plants and in warm waters may consume 50 to 60 percent of their body weight each day. Compared with mechanical and chemical procedures, grass carp are more cost-effective by a factor of 10. If properly stocked, they are not likely to produce negative environmental impacts (Cooke and Kennedy, 1989), and unlike the common carp, Cyprinus carpio, they are not likely to become a massive nuisance (Stanley et al., 1978). Nonetheless, grass carp are controversial for many reasons, and caution in their use is warranted. Not the least among these reasons is the fact that once grass carp are stocked in a lake, they are almost impossible to remove, and their effects on vegetation will remain for many years (Leslie et al., 1987). Their preferred diet does not include such nuisance plants as Eurasian water milfoil, water hyacinth, or alligator weed (Fowler and Robson, 1978). Preferred plants include hydrilla and native species such as elodea, and some pondweeds (Potamogeton). Their effectiveness is related to stocking rate, water temperature, length of growing season, size of fish, and types of plants to be controlled. If the stocking level is too low, then only palatable plants will be grazed, which actually may make problems with macrophytes worse (Leslie et al., 1987). Overstocking has resulted in eradication of submergent littoral vegetation and attendant loss in fish habitat, as well as increases in turbidity, algal blooms, and shoreline erosion (see Box 4.7).
In Florida, where their use is common, many lakes stocked with grass carp are very turbid (because of algal blooms), and shoreline erosion is so extensive, due to the absence of a ''damping" effect by submersed plants, that shoreline trees have fallen. Precautions must be taken to minimize fish movement to habitats where vegetation is desirable. Infertile hybrid grass carp were used in the 1970s and early 1980s to avoid potential problems of grass carp reproduction in open aquatic systems, but the hybrids have lower feeding rates than
|
BOX 4.7 LAKE BALDWIN, FLORIDA Lake Baldwin is an 80-ha eutrophic lake located on the Orlando Ridge in Orlando, Florida (Canfield et al., 1983). The lake was stocked with 34 grass carp (each >394mm length, 0.8 kg) per hectare of hydrilla during summer and fall of 1978. An earlier stocking of fingerling grass carp in 1974 failed to control hydrilla, presumably because of high predation pressure on the fingerlings. Shireman and Maceina (1981) reported that hydrilla was nearly eradicated 2 years after the second stocking. According to these authors, hydrilla control was evident when grass carp biomass reached 130 kg of fish per hectare of hydrilla beds. Phytoplankton chlorophyll increased from approximately 5 µg per liter before stocking to levels as high as 30 µg per liter after aquatic plants had been eradicated (Canfield et al., 1983). Secchi disk transparency readings decreased from 6 m during the height of hydrilla infestation to approximately 1.5 m after hydrilla had been eradicated by grass carp. Chlorophyll a and total alkalinity also increased in the lake after hydrilla had been controlled. The long-term effects of eliminating the lake's macrophytes on total fish biomass and species composition could not be determined from a relatively short period of study after treatment but could be significant (Canfield et al., 1983). |
the parental stock (Osborne, 1982; Shireman et al., 1983). This drawback has been overcome with the development of a sterile triploid form that has about the same feeding and growth characteristics as the fertile diploid form (Wiley and Wike, 1986).
Insect control of alligator weed and water hyacinth has been effective in Florida, Alabama, Mississippi, Texas, Louisiana, and Georgia. Alligator weed is controlled primarily by two insects: Agasicles hygrophila, commonly known as the alligator weed flea bettle, and Vogtia malloi pastrana, commonly known as the alligator weed stem borer. The species of insects involved include Sameodes ilbiguttalis (Warren) (Lepidoptera: Pyralidae) and Neochetina eichhornia Warner and N. bruchi Hustache (Coleoptera: Curculionidae). Techniques have been developed to concentrate the insects, allowing their reproduction, population growth, and subsequent spread through the lake. A characteristic
of the insects used in controlling these plants is that they can complete their life cycle only on the target species. The insects effective against alligator weed and water hyacinth are sensitive to cold weather, limiting their distribution to southern waters (Center et al., 1988), but the plants themselves are also limited to warm climates. Little is known of the effects of bird predation on these insects.
The development of effective plant pathogens has not been as rapid as that of herbivorous insects. Pathogenic fungi have many of the properties of an ideal biological control agent, including target specificity and low or zero pathogenicity to humans. Successful use of plant pathogens has been increased by combining their application with the use of a herbivorous insect and either chemical or mechanical control agents (Charudattan, 1986).
The use of biological controls, including manipulation of food webs to enhance grazing on algae or to reduce nutrient recycling, has been effective. This approach treating the symptoms of eutrophication or the invasion of exotic plant species has promise for providing lowcost improvements with long-term effectiveness, and it avoids the problems associated with chemical and mechanical technologies. More emphasis on research funding for this type of lake management is needed.
Water-Level Drawdown
Some aquatic plants are susceptible to exposure to dry, freezing conditions and can be controlled in temperate latitudes by lowering the lake level in November and refilling in early spring. Three to four weeks of continuous exposure to below-freezing air temperature will kill the roots and reproductive structure of some nuisance plants, including Eurasian water milfoil, coontail, and southern naiad. The procedure is most likely to be effective only in northern and some midwestern areas. Water removal also allows other lake restoration activities to occur, including fish management, sediment removal, and repair of dams and shoreline structures. Other benefits can occur in lakes with flocculent organic sediments through sediment consolidation and compaction. Drawdown in warm weather can also be effective for these purposes, but it may interfere with recreation, irrigation, and water supply.
Some aquatic plants are not affected by drawdown, including water hyacinth, elodea, hydrilla, and bushy pondweed (Najas flexilis). Negative effects include a failure to refill (if dry weather persists), the possible stimulation of algal blooms, and the potential for altering
or destroying wetlands. Cooke et al. (1986) summarized the use of this technique, including the responses of 74 aquatic plants to drawdown and desiccation.
Harvesting
Harvesting nuisance aquatic plants is a common lake management procedure, particularly in northern climates with short growing seasons and an absence of exotic plants with very high growth rates, such as water hyacinth and alligator weed (Cooke et al., 1986). Harvesting is not a restorative procedure, and its goal is to make a lake more usable for recreation. Harvesters are machines that combine a cutter bar and conveyor system with a large on-board storage area to receive the cut plants. The cutter bar is lowered to the sediment surface, or to a depth of 1.5 to 1.8 m, and the plants are cut, collected, stored on board, and then transported to a disposal site on land. Machines range in storage capacity from about 3 to 23 m3. At most, several hectares per day can be cut, which precludes use of this machinery to attempt plant eradication. In southern climates, particularly where exotic plant species have successfully invaded, plant densities are high and regrowth rates rapid, making harvesting largely impractical. In northern climates, harvesting once per season is generally adequate, and regrowth is reduced in the following season, especially if harvesting is done late in the growing season (Kimbel and Carpenter, 1981).
Harvesting has several positive features. No toxic materials are used, and the lake can be open for use during harvesting. Cut plants may have agronomic value as mulch and possibly as a supplement to livestock feed. In lakes with a high plant biomass and low external nutrient income, removal of organic matter and nutrients could have some restorative effect.
Harvesting constitutes habitat removal, and with this will come the removal or elimination of organisms living in this habitat. For example, harvesting may remove young-of-the-year fish, as well as larval insect forms associated with plants. Other negative effects include an increased likelihood of algal blooms and short-term increases in water column turbidity and nutrient concentrations (Cooke et al., 1986). Nicholson (1981) suggested that the replacement of native plant species with nuisance exotic species in Lake Chautauqua, New York, was caused by use of herbicides and harvesting. Fragmentation, dispersal, and rerooting of nuisance plants also may occur. Unless the harvester is operated to remove plant root crowns, plant regrowth can occur within weeks. Efforts are under way to
develop new machines, including diver-operated dredges and tilling machines to destroy roots.
Herbicides
Herbicides produce plant control through toxic actions. Most modern chemicals are effective and do not leave long-lived toxic residues or accumulate in food webs. In some cases, herbicide use is the only practical way to manage a plant-choked water body. Costs of harvesting and herbicide treatments are comparable in northern and midwestern areas, but herbicides are usually less costly than harvesting in southern areas. The herbicide fluridone has been shown to be highly effective against major nuisance plants such as hydrilla, and at the recommended dose it exhibits very low toxicity to nontarget organisms such as fish, benthic invertebrates, and birds (Hamelink et al., 1986), but high cost may deter use of this compound.
Herbicides have the potential to produce water quality problems. If dead plants are left in the lake to decompose (a common practice), they consume oxygen and release nutrients. This can be avoided if a pelletized form of the herbicide is used before plant emergence. At least one herbicide (diquat) is toxic to some fish-food organisms. There is evidence that 2,4-dichlorophenoxyacetic acid is associated with the development of non-Hodgkin's lymphoma in applicators, and the photodegradation products of fluridone are embryotoxic (Hoar et al., 1986; Kennedy, 1986). (Nevertheless, fluridone is registered for use in potable water supply reservoirs.) Brooker and Edwards (1975) and Newbold (1975) reviewed the use of herbicides in aquatic ecosystems, and Cooke and Kennedy (1989) and Cooke and Carlson (1989) describe costs, effectiveness, and additional negative effects of aquatic herbicides.
LOW DISSOLVED OXYGEN
Hypolimnetic Aeration
The purpose of hypolimnetic aeration is to increase the dissolved oxygen content of the deep, stagnant, cold layer of a lake or reservoir (the hypolimnion) without destratification. Although several methods exist to do this, including direct injection of liquid oxygen (Prepas et al., 1990), the principal technology involves an airlift system called an aerator. This is a large double-sleeved cylinder, open at the bottom and vented from the closed top via a pipe to the atmosphere. The cylinder is placed in the hypolimnion, compressed air is injected
at the bottom of the inner cylinder, and the water is aerated as it rises in the cylinder. Gases such as carbon dioxide and methane are vented to the atmosphere via the pipe, and the aerated water returns down the outer cylinder and to the hypolimnion. The number of these units needed per lake depends on the hypolimnion volume and its oxygen demand (Pastorok et al., 1982).
Aerators are effective when properly sized and installed (McQueen and Lean, 1986). If aerators are operated continuously during the stratified period, a cold-water fishery can be restored, and the quality of raw potable water or deep-water discharge can be improved. Hypolimnetic aerators have not been shown to be effective in algal control, but there is evidence from ongoing work on Vadnais Lake, Minnesota, that an addition of ferric iron to the aerator can reduce internal phosphorus loading to the upper water column (D. Shuler, St. Paul Water Utility, personal communication, 1990).
Hypolimnetic aeration is not appropriate for every thermally stratified lake. In shallower systems, the temperature gradient through the metalimnion may not be steep, and the aerator could slowly destratify the lake and introduce low-oxygen, high-nutrient water to the lake's surface. An algal bloom would be likely. Hypolimnetic aeration is a management and not a restoration procedure. Dissolved oxygen consumption in deep-water and bottom sediments will again make this habitat anoxic if the aerator is shut off.
Artificial Circulation
A management technique described above—artificial circulation—will aerate an entire water column through the mixing energy imparted by a curtain of bubbles rising from a perforated pipe at the lake's bottom. Properly sized to maintain isothermal conditions, a circulator will eliminate low dissolved oxygen and problems associated with it, and may control nuisance blue-green algae. However, it will also eliminate the cold-water layer and thereby the possibility of a cold-water fishery or the use of cold water for a potable water supply.
Winter Aeration
Shallow, productive lakes in northern climates may experience oxygen depletion during the winter ice-cover period, especially during winters of high snowfall, which eliminates light penetration through the ice and prevents photosynthesis from continuing. Fish kills commonly result. Some otherwise productive northern lakes lack viable
sport fisheries because this problem occurs almost annually. Aerators are installed in many lakes in Minnesota and Wisconsin to avoid or reduce winter fish kills. Again, winter aeration is a management technique and does not solve the underlying cause-organic sediments with high oxygen demand. Wirth (1988) evaluated the effectiveness of winter aeration in 29 lakes and found good or satisfactory performance in 26 cases. The three cases judged marginal or failures involved improper operation of aeration systems or inadequate capacity for the size of the lake. Two problems remain to be solved regarding winter aeration. First, the efficiency of oxygen (O2 transfer needs to be improved to decrease energy consumption (the major cost involved in winter aeration). Second, aeration causes ice to weaken and open water patches to occur near aerators, a dangerous situation for persons using a lake for snowmobiling or ice fishing. Deaths from drowning have been recorded and are a serious concern on lakes with winter aerators, whose sites must be clearly marked as hazardous to lake users. A bubbleless aerator based on hollow fiber membranes has been proposed to solve these problems (Semmens et al., 1990).
Excess Sediment
Volume loss caused by excessive watershed and shoreline erosion and subsequent high sedimentation rates in a lake or reservoir is a common problem. High loading rates of inorganic sediments also reduce water clarity, possibly to the point of inhibiting primary production, which occurred in Lake Chicot, Arkansas, a riverine lake tributary to the Mississippi River (Stefan et al., 1990). Best management practices and land use controls to decrease soil erosion are the long-term solutions for such problems, but where significant volume losses have occured, sediment removal is the only practical method of restoring the original volume. Buildup of organic sediments from proliferating macrophyte growths also has caused significant volume losses in many shallow lakes. Hydraulic dredges are the usual means of removing excess sediment from lakes and reservoirs (see Box 4.8). These devices remove a mud-water slurry via a floating suction line and deposit the slurry in a containment area. Normally this area is on land; in some cases the recovered lake sediment can serve as a useful amendment to agricultural soils. In large lakes the dredged material may be deposited in a designated lake area. Dewatering occurs from the shore-based containment area, and the elutriate is often returned to the lake (sometimes after treatment). The solids remain in a properly designed containment area.
|
Box 4.8 SPRINGFIELD LAKE, ILLINOIS Springfield Lake, Illinois, is an example of successful dredging to restore an ensilted reservoir (Buckler et al., 1988). Over its 51-year history, this 1,635-ha potable water supply and recreation impoundment lost more than 13 percent of its storage capacity (9.5 x 106 m3) because of deposition of agricultural soils. This led to increased water treatment costs, loss of shoreline property values, algal blooms, weeds, turbidity, rough fish, and impaired recreation. A material balance study of silt and nutrients identified the major sources of loading, and land management practices were instituted in cooperation with city, county, state, and federal agencies at a cost of $1.6 million. Hydraulic dredging removed 2 x 10 m3 of sediment, which was pumped to adjacent farmland and reclaimed for agricultural uses. The water quality of the return flow from the disposal sites was within standards, and no negative effects on lake quality were noted during dredging operations. Dredging costs were $4.1 million. |
Several major problems are encountered in dredging projects. One is an inadequately designed containment area that allows turbid, nutrient-rich water to overflow and return to the lake. Normally, in-lake problems such as turbidity or nutrient release are minimal. A more common problem is that land management and shoreline protection steps are not taken to prevent a second episode of erosion and volume loss. Another possible problem is the occurrence of toxic materials in lake sediments, which then require special and expensive sediment disposal procedures. Finding adequate, inexpensive, and environmentally sound disposal sites is a serious problem even when sediments are not contaminated. A large literature exists on dredging, which is widely used to restore or maintain channel depths in rivers and harbors. Cooke et al. (1986) and Cooke and Kennedy (1989) have reviewed this technique in more detail.
Exotic Species
Extirpation of exotic species is far more difficult to accomplish than is their introduction (Magnuson, 1976). Successes are infrequent
and few generalizations can be derived from them. Control or management of the exotic species is usually the only practical alternative. Most attempts even to control exotic species in lakes fail. For success, control measures must be specific for the nuisance species and highly effective. The requisite combination of specificity and effectiveness is rarely found. The exotic sea lamprey (Petromyzon marinus) in the Great Lakes has been suppressed (but not eliminated) by the chemical 3-trifluoromethyl-4-nitrophenol (see Lake Michigan case study, Appendix A). In Australia and Papua New Guinea, infestations of the exotic kariba weed (Salvinia molesta) have been controlled (but not eliminated) by the herbivorous beetle Cyrtobagous salviniae (Barrett, 1989; also see discussion of control of aquatic macrophytes, above).
In small lakes and ponds, exotic or nuisance fish are sometimes removed by applying rotenone to kill all fish and then restocking with the desired species (Magnuson, 1976). The risks that accompany this drastic approach make it controversial among lake users, including anglers. The fish community after such treatment has fewer species than the system can support and thus is highly susceptible to invasion. The most likely invaders are undesirable species that lead to long-term degradation of the fishery (Magnuson, 1976). The result is a perpetual cycle of fish removal and restocking, rather than a restored, self-sustaining community.
In some cases, the invading species declines naturally in population after some years, eventually becoming a subdominant member of the community. Natural declines are known for the macrophytes Elodea canadensis in Europe (Hutchinson, 1975) and Myriophyllum spicatum in North America (Carpenter, 1980; painter and McCabe, 1988; Nichols et al. 1991). In the case of M. spicatum, management by dredging, drawdown, mechanical harvesting, and herbicides may actually prolong infestation (Smith and Barko, 1990). In general, the long-term community consequences of macrophyte control are poorly known.
In other cases, species invasions accompanied by extirpations of native species have permanently altered lake ecosystems. Depending on the outcome of efforts to establish reproducing populations of lake trout, Lake Michigan may be an example of a permanently altered ecosystem (see Lake Michigan case study, Appendix A).
Lake Victoria, East Africa, provides a spectacular and recent example of an ecosystem transformed by species introduction. Introduction of Nile perch (Lates nilotica) and Nile tilapia (Oreochromis niloticus), combined with heavy fishing pressure, has depleted native cichlid stocks, and the introduced species now are the mainstay of
the lake's fishery (Ogutu-Ohwayo, 1990). Although the fishery is economically successful, local processing techniques (frying and smoking) consume large amounts of wood, which is a scarce resource in the region (Bruton, 1990). The initial high productivity of the fishery was due in part to the high biomass of the native cichlid forage, but after depletion of the forage, growth rates and condition factors of the introduced predators declined (Ogutu-Ohwayo, 1990). The productivity of the fishery is likely to decline. Bruton (1990) is pessimistic about prospects for restoring the native fish community. Ogutu-Ohwayo (1990) noted that restoration of ancestral stocks would deprive an impoverished region of an important local industry and source of protein.
Introductions of exotic species sometimes lead to economic benefits (as occurred initially in Lake Victoria). However, economic benefits may be short-lived because of instabilities in the population density of the invader. In general, economic benefits deriving from the invasion must be balanced against the long-term costs of stabilizing the ecosystem. Lake Michigan (see case study, Appendix A) is an excellent example of an ecosystem with a profitable fishery for exotic species, sustained at the cost of perpetual management. Lakes dominated by exotic species tend to be more variable and less predictable than lakes that lack exotics. Unpredictability adds to the cost of management (Walters, 1986).
Acidification
The long-term solution to lake acidification, of course, is to decrease emissions of sulfur and nitrogen oxides to the atmosphere, because these are precursors of the sulfuric and nitric acids that cause acidic deposition. Control actions in the United States over the past 15 years already have had significant effects; emissions of SO2 and NOx peaked in the late 1970s and declined nationally by 10 to 20 percent from 1975 to 1985. Much higher percentage decreases were achieved in some states during the 1980s. The recently enacted Clean Air Act of 1990 (P.L. 101–549) mandates a further reduction in SO2 emissions of 10 million metric tons over the next 10 years (from current rates of nearly 21 million metric tons). These reductions will decrease the amount of acid deposition sufficiently to reverse the acidification process in some impacted lakes but will not be sufficient to restore all acid-sensitive systems (NAPAP, 1990b).
Liming is by far the most common in-lake restoration technique for acidified lakes, and a large amount of experience with this method has accumulated over the past decade. Liming is common in Scandinavia
(e.g., Sverdrup and Bjerle, 1983; Wright, 1985) and is used on a regional management scale in Sweden (Lessmark and Thornelof, 1986), but liming projects have also been undertaken in the United States, especially in the Adirondack region of New York (e.g., Porcella, 1989; Young et al., 1989), as well as in Canada (Molot et al., 1986) and Great Britain (Brown et al., 1988; Dalziel et al., 1988).
Most liming projects actually add calcium carbonate (calcite), the major constituent of limestone, rather than powdered lime (calcium oxide) or slurried lime (calcium hydroxide), directly to the lake by helicopter (in remote areas) or by boat (in lakes with road access). Calcite is preferred because it dissolves more slowly than calcium hydroxide and does not cause such extreme increases in pH. However, finely powdered calcite can produce short-term pH values of 8 to 9 (Fordham and Driscoll, 1989), which may cause stress to aquatic organisms acclimated to living in acidic environments. Dissolution of the calcite adds both calcium ions and carbonate alkalinity to the lake water. Calcite that does not dissolve immediately settles to the lake bottom and slowly dissolves at the sediment-water interface. Treatment of acidic lakes with calcium carbonate is not designed to convert them from soft-(low calcium and alkalinity) to hard-water systems (high calcium and alkalinity, pH > 7). Instead, only enough calcite is added to raise the pH to circumneutrality. Conversion of an acidic lake to a well-buffered hard-water lake would not constitute restoration, because acidic lakes inherently are soft-water systems with low buffering capacity.
Calcite treatments are often short-lived because many acidic lakes are in drainage systems with short water residence times (sometimes only several months, often a year or so). Continued input of acidic water from the drainage basin can reinduce acidic conditions in time periods equivalent to a few water residence times. Liming of watersheds (by applying calcium carbonate to the land portion of the watershed) is more costly, but this approach potentially can provide benefits for much longer periods. Watershed liming has been done in a few cases (Brocksen et al., 1988; Brown et al., 1988), but it is too early to judge the longevity of beneficial effects.
Limnological studies on acidic lakes restored by liming have shown that the aquatic ecosystem responds favorably in short periods of time: acid-sensitive species return, fish condition improves, and the symptoms of acidic systems decrease. Nonetheless, several detailed studies have indicated that recovery is incomplete and that not all of the species present before acidification return in a few years of liming.
An innovative approach to restoring acidic lakes currently under investigation in northern Wisconsin involves pumping ground water
into the lake (Garrison et al., 1992). Ground water is typically high in hardness and alkalinity. This is especially true of ground water in limestone aquifers, but even surficial (water table) aquifers in unconsolidated soil (e.g., glacial till) have higher hardness and alkalinity levels than do surface waters in acid-sensitive regions. Advantages of this approach involve low cost (no chemicals need be added to the lake) and ease of repeating the additions to maintain lake pH in the desired range. This method would not be useful in areas where ground water supplies are very limited (e.g., where granitic bedrock is near the surface).
Contaminants
Elimination or reduction of the input of contaminants (synthetic organic compounds and heavy metals) is necessary for remediation of contaminant effects. From a technical standpoint, input reduction is straightforward when point sources predominate. Where nonpoint inputs are substantial (see Lake Michigan case study, Appendix A), input reduction is much more difficult and costly.
Decontamination of lake ecosystems is most straightforward when contaminants are locally concentrated in sediments that can be removed by dredging. In other cases, contaminated sediments can be covered to retard recycling of contaminants to the overlying water. Where aquatic macrophytes concentrate metal contaminants, harvesting of the plants provides a means of biological decontamination of the system (Clark et al., 1981).
In many cases, contaminants are widely dispersed in lakes, and sediment or macrophyte removal is impractical. There is considerable interest in developing bioremediation techniques for dispersed organic pollutants. Bioremediation involves development of natural or mutant microbes that metabolize organic contaminants to nontoxic or less toxic compounds. These microbes can then be introduced to contaminated sites to degrade specific pollutants. Considerable research is under way concerning the use of altered microbes in ecosystems (Tiedje et al., 1989).
Remediation of chemical contaminants in lakes relates directly to management of fish and wildlife. Certain contaminants, such as halogenated hydrocarbons and methylmercury, are bioconcentrated, and they accumulate at increasingly higher concentrations in the tissues of organisms higher in the food chain. Consequently, piscivorous fish, birds, and mammals can develop in their tissues concentrations several orders of magnitude greater than those found in water (Thomann, 1989). Fish species differ widely in their tendency to
bioaccumulate contaminants because of differences in diet and growth rate. Therefore, contamination may determine which fish are exploited and the composition of the remaining stock. Fisheries management decisions also can affect the amounts of contaminants in fish at the top of the food chain by manipulating the composition of the fish stock (see Lake Michigan case study, Appendix A). Reproduction of piscivorous birds and mammals was severely affected by dichlorodiphenyltrichloroethane (DDT) in some ecosystems (NRC, 1986). Organochlorine contaminants remain a threat to populations of waterfowl raptorial birds, minks, and otters, and wildlife populations depend on the extent to which contaminants can be remediated.
INTEGRATED AQUATIC SYSTEMS
Lake restorations must be viewed in a watershed context. Abatement of eutrophication, siltation, and contaminant problems is far simpler, and generally more effective, when inputs can be controlled or reduced. This chapter has described many in-lake techniques that can ameliorate symptoms of eutrophication. Reduction of inputs enhances the long-term effectiveness of in-lake approaches.
Lake restoration has strong interactions with restoration of other watershed components. Restoration of influent streams affects the input of sediment, solutes (including nutrients and contaminants), and water to the lake. The surrounding wetlands affect water and solute fluxes and habitats for fish spawning. Conversely, lake restoration affects wetlands by influencing macrophyte distribution, water levels, and wave and ice impacts on littoral areas. Lake restorations and stream restorations interact through the life cycles of migratory fish.
From a technical standpoint, the watershed is the most logical scale at which to undertake restoration. However, institutional constraints, and occasional ecological surprises, can make watershed restoration more difficult than it appears. Institutional complexities are best illustrated by the Lake Michigan case study (Appendix A), in which the major participants include international commissions, two U.S. federal agencies, and water quality managers and fisheries managers from five states. The Lake Apopka case study (Appendix A) illustrates unexpected ecological consequences of watershed change. Draining, diking, and canal building left the lake vulnerable to the effects of a 1947 hurricane that unprooted and drastically reduced aquatic vegetation. Subsequent algal blooms left the water so turbid that macrophytes could not be reestablished. Fishery management contributed to water quality problems via deliberate, massive kills of
gizzard shad with rotenone. The dead fish were not removed, and this added more phosphorus to the lake water. Another case of miscommunication between water quality managers and fisheries managers occurred in the Medical Lake, Washington, restoration (see Box 4.1). Fish stocking caused a decline in herbivorous zooplankton, confounding the efforts of water quality managers to control nuisance algal blooms.
One consequence of fragmentation in the management of water quality, fisheries, and wildlife is missed opportunities to restore habitat. Aquatic plant management is an important example. Most macrophyte management is aimed at control or suppression of nuisance growths of exotic species. This is an important step, but habitat restoration requires reestablishment of native species important for fish and wildlife habitat. The biomanipulation concept and bioaccumulation of contaminants such as methylmercury and chlorinated hydrocarbons are further examples of links between management of water quality and fisheries.
In sum, restoration of lakes must extend beyond the shoreline to the watershed boundary. The watershed is the natural scale for many restorations. Restorations at this scale are more likely to be self-sustaining than piecemeal restorations. The major barriers to watershed restorations are institutional and educational. Political boundaries seldom correspond to watershed boundaries, and different agencies have responsibility for different ecological components of the watershed. Distinctly different scientific disciplines apply to different watershed components. Effective restoration requires collaboration among this diversity of scientists, economists, managers, and policymakers.
NEEDS IN LAKE RESTORATION
Needs in Federal Lakes Programs
The significance of lakes and reservoirs to the economy of the United States is apparent. Equally apparent are the deterioration of these resources over recent decades and the inadequacy of federal programs to restore lakes. At present, the collective federal water quality program emphasizes streams, rivers, and wetlands. The most recent report of the Council on Environmental Quality (CEQ, 1989) on environmental trends in the nation does not even mention lakes and reservoirs, except for the Great Lakes. Lakes were also neglected in a report by the National Research Council on the nation's water resources (NRC, 1982). The apparent assumption is that lakes will be
protected and will restore themselves if the water quality of streams and rivers is improved. This assumption does not recognize that many lakes and reservoirs have lost significant volume from siltation or that their excessive productivity and cycling of toxic materials through food webs are subsidized by contaminated sediments. Some lakes have had components of their biological communities replaced by nuisance species, many of which are exotics, or have lost important species (with no replacement) because of toxic stresses from heavy metals, synthetic organic compounds, or acidity.
Funds for EPA's Clean Lakes Program have not been included in the president's budget since fiscal year 1981, but Congress has recognized the program's importance and annually restored some funds to it. The 1991 budget for the CLP is $8 million. Although this amount will help to maintain or initiate a few restoration programs, it is minuscule relative to the large task of restoration facing the United States. The annual uncertainty in CLP funding has led some states to postpone the development of full-scale lake programs. Moreover, EPA apparently will not recommend the CLP for continuation in the reauthorization of the Clean Water Act, which Congress began to consider in 1991.
The need for an expanded, well-funded Clean Lakes Program to provide the nation with adequate supplies of safe, protected surface waters in the future is apparent. The next century is very likely to witness increased agriculture, urbanization, and release of toxic substances, all of which will add to the current impaired state of the waters we depend upon for portable water supplies, irrigation, recreation, and industrial uses. Nonetheless, this valuable program is scheduled once again for termination, in part due to a (mistaken) philosophical viewpoint that lake restoration is a problem for state and local governments and not a federal responsibility. As noted repeatedly in this chapter, in many cases lakes do not cleanse or restore themselves. They are sinks for incoming contaminants, which recycle and maintain the impaired conditions.
The status of restoration programs within the variety of federal agencies that have responsibilities to protect and manage the nation's lakes and reservoirs needs to be enhanced, not diminished. Moreover, a better understanding must be developed within federal agencies of the importance of lakes for the wide range of uses and benefits described in this chapter: potable water supply, recreation, wildlife habitat, irrigation, water storage, and flood control. A failure to protect, manage, and restore these systems is likely to mean that their usefulness for such purposes will be even more diminished in coming decades. Their continued usefulness as economic resources requires
an active and continuous federal program. In addition, the knowledge and experience gained from a U.S. program will be vital to developing countries, where a shortage of clean surface water already hinders economic progress.
By far the most widespread problem affecting lakes and reservoirs is agricultural nonpoint runoff of silt and associated nutrients and pesticides. This problem and its manifestations are within the purview of numerous federal agencies, and coordination of nonpoint source control programs would profit from oversight by an interagency task force or committee.
State lake programs are a key to long-term monitoring and assessment of the nation's lakes, as well as to their restoration, protection, and management. Currently, the CLP provides a 50 percent match to state and local funds for lake restoration. Administrators in the relevant state sgencies are the best informed and equipped to determine state needs for lakes. In many cases, the existence of a state program is directly dependent on the continued existence of the federal program, in part because states, as well as various federal agencies, often emphasize stream and river quality and protection in their programs. The states need a continuing federal commitment to lake management and restoration to stimulate and support their efforts.
Lake and reservoir water quality standards are needed for nutrients and related parameters, based on ecoregional attainable lake quality. Criteria for toxic substances are also necessary, which must take into account the trapping and recycling capacities of lake systems. The development and enforcement of standards will help to prevent impairment of lake use. A more complete discussion of this issue was given by Duda et al. (1987).
The quality of drinking water withdrawn from surface impoundments is another interagency issue that would benefit from the development of lake standards and from cooperative activities among federal programs or between federal and state programs. The present emphasis on restoring and managing lakes and reservoirs for their recreational value ignores and may conflict with managing lakes to ensure their roles as water supplies. With our growing population and the increasing popularity of aquatic recreation, multiuse conflicts may require that additional interagency efforts be made to achieve resolution. Appropriate standards for raw potable water may be too stringent to allow multiple uses of lakes in some areas.
Project Selection and Design
Because of limited resources, it will be impossible to undertake all lake restoration projects. Criteria thus are needed to set priorities, select projects, and evaluate project design. A ''triage" framework is a minimum initial step. In this approach, systems would be divided into three categories: (1) those that will recover without intervention, (2) those that cannot be restored even with extensive intervention, and (3) those that can be restored with appropriate action. Systems in the third group bear further consideration. Selections from that group should be based on criteria such as the likelihood of success, benefits, costs, and technical review of the restoration plan. It is imperative that project selection be based on these criteria, and not on political ones.
Lake restoration is still a developing science. Every project is an opportunity to learn. It is essential that projects be regarded as large-scale experiments (Matson and Carpenter, 1990). We cannot learn from them unless proper baseline and follow-up data are collected, analyzed, and published in a form accessible to others. These experimental aspects of restoration projects are as important as the other technical components, and they should be designed with the same care. A peer-review system is crucial for maintaining the rigor and quality of restoration ecology.
Need for Integration of Management Programs
Effective lake restoration demands an ecosystem perspective. It often depends on land use in the surrounding watershed and interacts with the management of connecting streams and wetlands. Ironically, agency structures frequently dictate a piecemeal approach to management or restoration. Training is similarly fragmented among specialties such as limnology, water chemistry, fisheries science, and wildlife management. Ecosystem scientists are trained at few institutions. Moreover, there is no single governmental agency responsible for ensuring an integrated, ecosystem approach to lake restoration and management.
Better-coordinated efforts to manage water quality, fisheries, and wildlife are needed at both state and federal levels. Mechanisms for coordination will vary among restoration projects. At a minimum, coordinated planning of restoration projects and regular communication among the agencies involved are essential.
CONCLUSIONS AND RECOMMENDATIONS
Use the Ecoregion Concept to Restore Lakes
Morphometric features and hydrologic factors can vary widely from lake to lake even within a small region, but nonetheless the earth can be characterized as containing ecological regions (or "ecoregions") that have broad similarities of soil, relief, and dominant vegetation. Omernik (1987) divided the conterminous United States into 76 ecoregions, or areas of regional similarity in soils, land use, land surface forms, and potential natural vegetation.
-
The committee believes that goals for restoration of lakes need to be realistic and should be based on the concept of expected conditions for individual ecoregions. Further development of project selection and evaluation techniques based on ecoregion concepts and refinement of ecoregion definitions and descriptions should be encouraged and supported by the U.S. Environmental Protection Agency.
Research Needed
Lake restoration is a relatively new and developing field. This is especially true for holistic approaches that consider lakes as components of a landscape and treat their restoration at the watershed scale. Although numerous techniques are available to restore lakes and manage the consequences of degradation from certain stresses, many of them require further development to improve their efficiency and effectiveness and to identify situations in which they are best applied. For certain kinds of lake problems (e.g., contaminants, macrophytes), suitable restoration techniques are lacking.
In addition, the current base of knowledge about the nation's lakes is grossly inadequate, depending largely on questionnaires characterized by incomplete and qualitative responses.
Therefore the committee recommends the following:
-
The federal government should support research and development for watershed-scale restorations that integrate lake, stream, and wetland components. State agencies and university researchers should participate in planning, implementing, and evaluating restoration projects. In addition, an interagency program under the Federal Coordinating Council for Science, Engineering, and Technology could be formed to coordinate the selection, planning, and evaluation of these demonstration projects. The research and implementation
-
of the projects would be managed by the participating agencies.
-
Research and development are needed in several areas of applied limnology, and these programs should take an experimental approach (one that emphasizes manipulation of whole-lake systems or large in-lake enclosures in controlled fashion).
-
Improved techniques for littoral zone and aquatic macrophyte management need to be developed. Research should go beyond the removal of nuisance macrophytes to address the restoration of native species that are essential for waterfowl and fish habitat. Basic research is necessary to improve understanding of fundamental limnological processes in littoral zones and the interactions between littoral and pelagic zones of lakes.
-
Biomanipulation (food web management) has great potential for low-cost and long-term management of lakes, and research in this emerging field must be stimulated.
-
Innovative and low-cost approaches to contaminant cleanup in lakes need to be developed, especially for such widespread problems as contamination by mercury and PCBs.
-
The relationships between loadings of stress-causing substances and responses of lakes need to be understood more precisely. This is true even for such well-studied phenomena as phosphorus and algal bloom problems. Research should be undertaken to improve predictions of trophic state from nutrient loading relationships. In particular, phosphorus loading should be evaluated in terms of both its biological availability, which can be estimated chemically, and its effects on plant communities in receiving waters.
-
Improved assessment programs are needed to determine the severity and extent of damage in lakes and their change in status over time. Innovative basic research is required to improve the science of assessment and monitoring. There is a great need for cost-effective, reliable indicators of ecosystem function, including those that will reflect long-term change and response to stress. Research on indicators should include traditional community and ecosystem measurements, paleoecological trend assessments, and remote sensing.
-
Procedures such as food web manipulation, introduction of phytophagous insects and fish, liming, and reintroduction of native species show promise for effective and long-lasting results when used alone or in combination with other restoration measures. Further research and development should be undertaken on these techniques.
-
Paleolimnological approaches should be used to infer the past trophic history of lakes and to decide whether lakes should
-
be restored. Paleolimnological approaches also should be used to infer whether a lake has been restored to its predisturbance condition.
Education and Training
The public needs to be better informed about the rationales, goals, and methods of aquatic ecosystem restoration. In addition, scientists with the broad training needed for aquatic ecosystem restoration are in short supply. The committee recommends the following:
-
Public education and outreach should be components of aquatic ecosystem restorations. Lake associations and citizen monitoring groups have proved helpful in educating the general public, and efforts should be made to ensure that such groups have accurate information about the causes of lake degradation and various lake restoration methods.
-
Funding is needed for both undergraduate and graduate programs in aquatic ecosystem restoration. Training programs must cross traditional disciplinary boundaries such as those between basic and applied ecology; between water quality management and fisheries or wildlife management; and among lake, stream, and wetlands ecology.
REFERENCES AND RECOMMENDED READING
Association of State and Interstate Water Pollution Control Administrators (ASIWPCA). 1984. America's Clean Water: The State's Evolution of Progress 1972–1982. ASIWPCA, Washington, D.C.
Association of State and Interstate Water Pollution Control Administrators (ASIWPCA). 1985. America's Clean Water: The States' Nonpoint Source Assesment. ASIWPCA, Washington, D.C.
Baker, L. A., and E. B. Swain. 1989. Review of lake management in Minnesota. Lake Reservoir Manage. 5:10–10.
Barrett, S.C.H. 1989. Waterweed invasions. Sci. Am. (October):90– 97.
Barten, J.M. 1987. Stormwater runoff treatment in a wetland filter: Effects on the water quality of Clear Lake. Lake Reservoir Manage. 3:297– 305.
Bauman, L. R., and R. A. Soltero. 1978. Limnological investigation of eutrophic Medical Lake, Wash. Northwest Sci. 52:127– 136.
Benndorf, J., and K. Putz. 1987. Control of eutrophication of lakes and reservoirs by means of pre-dams—I . Mode of operation and calculation of nutrient elimination capacity. Water Res. 21:829–838.
Benndorf, J., H. Schultz, A. Benndorf, R. Unger, E. Penz, H. Kneschke, K. Kossatz, R. Dumke, U. Hornig, R. Kruspe, and S. Reichel. 1988. Foodweb manipulation by enhancement of piscivorous fish stocks: Longterm
effects in the hypertrophic Bautzen Reservoir. Limnological 19:97–110.
Bernhardt, H. 1980. Reservoir protection by in-river nutrient reduction. Pp. 272–277 in Restoration of Lakes and Inland Waters. EPA 440/5-81-010. U.S. Environmental Protection Agency, Washington, D.C.
Bjork, S. 1988. Redevelopment of lake ecosystem — A case-study approach. Ambio 17:90–90.
Brezonik, P. L., S. King, and C. E. Mach. 1988. The influence of water chemistry on metal bioaccumulation and toxicity. Chapter 1 in M. C. Newman and A. W. McIntosh, eds., Metal Ecotoxicology: Concepts and Applications. Lewis Publishers, Chelsea, Mich.
Brezonik, P. L., K. E. Webster, and J. A. Perry. 1991a. Effects of acidification on benthic community processes in Little Rock Lake, Wisconsin. Verh. Int. Ver. Limnol. 24:445–448.
Brezonik, P.L., K. E. Webster, W. A. Swenson, B. Shelley, C.J. Sampson, W. A. Rose, J.A. Perry, J. H. McCormick, T. K. Kratz, P. J. Garrison, T. M. Frost, and J.G. Eaton. 1991b. Responses of Little Rock Lake to experimental acidification: Chemical and biological changes over the pH range 6.1 to 4.7. Can. J. Fish. Aquat. Sci. (in review).
Brocksen, R. W., H. W. Zoettl, D. B. Porcella, R.F. Huettl, K-H. Feger and J. Wisniewski. 1988. Experimental liming of watersheds: An international cooperative effort between the United States and West Germany. Water, Air, Soil Pollut. 41:455–471.
Brooker, M. P., and R. W. Edwards. 1975. Aquatic herbicides and the control of water weeds. Water Res. 9:1–15.
Brown, D.J. A., G. D. Howells, T. R. K. Dalziel, and B. R. Stewart. 1988. Loch Fleet. A research watershed liming project. Water, Air, Soil Pollut. 41:25–42.
Brown, L. R., and E. C. Wolf. 1984. Soil Erosion: Quiet Crisis in the World Economy. Worldwatch Paper 60. Worldwatch Institute, Washington, D.C.
Brown, R. M., N. I. McClelland, R. A. Deininger, and M. F. O'Connor. 1972. A water quality index — Crashing the psychological barrier. Paper No. 29. Proceedings of the Sixth International Conference on Water Pollution Research, Jerusalem. Pergamon, Oxford.
Bruton, M. N. 1990. The conservation of the fishes of Lake Victoria, Africa: An ecological perspective. Environ. Biol. Fishes 27:161–176.
Buckler, J. H., T. M. Skelly, M. J. Luepke, and G. A. Wilken. 1988. Case study: The Lake Springfield sediment removal project. Lake Reservoir Manage. 4:143–152.
Camanzo, J., C. P. Rice, D. J. Jude, and R. Rossmann. 1987. Organic priority pollutants in nearshore fish from 14 Lake Michigan tributaries and embayments, 1983. J. Great Lakes Res. 13:296–309.
Campbell, P. C. G., and P. Stokes. 1985. Acidification and toxicity of metals to aquactic biota. Can J. Fish. Aquat. Sci. 42:2034–3049.
Canfield, D. E., and R. W. Bachmann. 1981. Prediction of total phosphorus concentrations, chlorophyll a, and Secchi depths in natural and artificial lakes. Can. J. Fish. Aquat. Sci. 38:414–423.
Canfield, D. E., Jr., M. J. Maceina, and J. V. Shireman. 1983. Effect of hydrilla and grass carp on water quality in Florida lake. Water Resour. Bull. 19:773–778.
Carlson, R. E. 1977. A trophic state index for lakes. Limnol. Oceanogr. 22:-361-369.
Carpenter, S. R. 1980. The decline of Myriophyllum spicatum in a hardwater eutrophic lake. Can J. Bot. 58:527–535.
Carpenter, S. R. ed. 1988. Complex Interactions in Lake Communities. Springer Verlag, New York.
Carpenter, S. R., and J. F. Kitchell. 1988. Consumer control of lake productivity. BioScience 38:764–769.
Carpenter, S. R., J. F. Kitchell, J. R. Hodgson, P. A. Cochran, J. J. Elser, M. M. Elser, D. M. Lodge, D. Kretchmer, X. He, and C. N. von Ende. 1987. Regulation of lake primary productivity by food web structure. Ecology 68: 1863–1876.
Center, T. D., A. F. Confrancesco, and J. K. Balciunas. 1988. Biological control of aquatic and wetland weeds in the southeastern United States. Proceedings of the Seventh International Symposium on Biological Control Weeds, Rome, 239–262.
Charaduttan, R. 1986. Integrated control of waterhyacinth (Eichhornia crassipes) with a pathogen, insects, and herbicides. Weed Sci. 34 (Suppl. 1):26–30.
Clark, J. R., J. H. VanHassel, R. B. Nicholson, D. S. Cherry, and J. Cairns, Jr. 1981. Accumulation and depuration of metals by duckweed (Lemna perpusilla). Ecotoxicol. Environ. Saf. 5:87–96.
Clasen, J. 1989. Wahnbach Reservoir—Lont-term experience with phosphorus removal. Pp. 130–132 in W. Lampert and K.-O. Rothhaupt, eds., Limnology in the Federal Republic of Germany. International Association of Theoretical Applications in Limnology, Plon, Germany.
Clean Air Act Amendments. P.L. 101-549, Nov. 15, 1990.
Cooke, G. D., and R. E. Carlson, 1989. Reservoir Management for Water Quality and THM Precursor Control. American Water Works Association Research Foundation, Denver, Colo.
Cooke, G.D., and R. H. Kennedy. 1989. Water Quality Management for Reservoirs and Tailwaters. Rept. 1. In Reservoir Water Quality Management Techniques. Tech. Rept. E-89-1. U.S. Army Corps of Engineers, Vicksburg, Miss.
Cooke, G. D., and A. B. Martin. 1989. Long-term evaluation of the effectiveness of phosphorus inactivation (abstract). Annual Meeting of the North American Lake Management Society, Austin, Tex.
Cooke, G. D., R. T. Heath, R. H. Kennedy, and M. R. McComas. 1982. Change in lake trophic state and internal phosphorus release after aluminum sulfate application. Water Resour. Bull. 18:699–705.
Cooke, G. D., E. B. Welch, S. A. Peterson, and P. R. Newroth. 1986. Lake and Reservoir Restoration. Butterworth, Stoneham, Mass.
Council on Environmental Quality (CEQ). 1989. Environmental trends. Chapter 2 in Water. Washington, D.C.
Czuczwa, J. M., B. D. McVeety, and R. A. Hites. 1984. Polychlorinated dibenzo-p-dioxins and dibenzofurans in sediments from Siskiwit Lake, Isle Royale. Science 226:568–569.
Czuczwa, J. M., B. D. McVeety, and R. A. Hites. 1985. Polychlorinated dibenzodioxins and dibenzofurans in sediments from Siskiwit Lake, Isle Royale. Chemosphere 14:62–626.
Dalziel, T. R. K., M. V. Proctor, and A. Dickson. 1988. Hydrochemical budget calculations for parts of Loch Fleet Catchment, before and after watershed liming. Water, Air, Soil Pollut. 41:417–434.
Dierberg, F. E., V. P. Williams, and W. H. Schneider. 1988a. Water Quality Effects of Lake Enhancement Techniques Used in Florida. Water Resources Research Center, University of Florida, Gainesville. 64 pp. plus app.
Dierberg, F. E., V. P. Williams, and W. H. Schneider. 1988b. Evaluating the water quality effects of lake management in Florida. Lake Reservoir Manage. 2:101–111.
Duda, A. M., and F. J. Johnson. 1984. Lakes are losing the battle in clean water programs. J. Water Pollut. Control Fed. 56:815–822.
Duda, A. M., M. L. Iwanski, R. J. Johnson, and F. A. Joksch. 1987. Numerical standards for managing lake and reservoir water quality. Lake Reservoir Manag. 3:1–16.
Edmondson, W. T. 1979. Lake Washington and predictability of limnological events. Arch. Hydrobiol. 13:234–241.
Edmondson, W. T. 1991. The Uses of Ecology: Lake Washington and Beyond. University of Washington Press, Seattle, Wash. 312 pp.
Eisenreich, S. J. 1987. The chemical limnology of nonpolar organic contaminants: Polychlorinated biphenyls in Lake Superior. Pp. 393–470 in S. J.
Eisenreich and R. A. Hites, eds
., Sources and Fate and Aquatic Pollutants. Adv. Chem. Ser. 216. American Chemical Society, Washington, D.C.Elton, C. S. 1958. The Ecology of Invasions by Animals and Plants. Methuen, London.
Federal Water Pollution Control Act Amendments of 1972. P.L. 92-500, Section 314, Oct 18, 1972, 86 Stat. 816.
Fiala, I., and P. Vasata. 1982. Phosphorus reduction in a man-made lake by means of a small reservoir on the inflow. Arch. Hydrobiol. 94:24–37.
Fordham, G.F., and C. T. Driscoll. 1989. Short-term changes in the acid/ base chemistry of two acidic lakes following calcium carbonate treatment. Can. J. Fish. Aquat. Sci. 46:306–314.
Forsberg, C. 1987. Evaluation of lake restoration in Sweden. Schweiz. Z. Hydrol. 49:260–274.
Fowler, M. C. and R. O. Robson. 1978. The effects of the food preferences and stocking rates of grass carp (Ctenopharyngodon idella Val.) on mixed plant communities. Aquat. Bot. 5:261–276.
Fulmer, D. G. and G. D. Cooke. 1990. Evaluating the restoration potential of Ohio reservoirs. Lake Reservoir Manage. 6:197–206.
Gakstatter, J. H. A. F. Bartsch, and C. A. Callahan. 1978. The impact of broadly applied effluent phosphorus standards on eutrophication control. Water Resour. Res. 14:1155–1158.
Garrison, P. J., and D. R. Knauer. 1984. Long-term evaluation of three alum treated lakes. Pp. 513–517 in Lake and Reservoir Management. EPA 440/ 5-84-001. U.S. Environmental Protection Agency, Washington, D.C.
Garrison, P. J., W. J. Rose, C. J. Watras, and J. P. Hurley. 1992. Mitigation of acid rain by ground water addition: An alternative to liming. In V. D. Adams and E. Morgan, eds., Acid Rain Mitigation. Lewis Publishers, Chelsea, Mich. (in review).
Goldman, C. R. 1988. Primary productivity, nutrients and transparency during the early onset of eutrophication in ultra-oligotrophic Lake Tahoe, California-Nevada. Limnol. Oceanogr. 33:1321–1333.
Gulati, R. D., E. H. R. R. Lammens, M.-L. Meijer, and E. van Donk. 1990. Biomanipulation: Tool for Water Management. Kluwer, Boston.
Hamelink, J. L., D. R. Buckler, F. L. Mayer, D.V. Palawski, and H. O. Sanders. 1986. Toxicity of fluridone to aquatic invertebrates and fish. Environ. Toxicol. Chem. 5:87–94.
Harkins, R. D. 1974. An objective water quality index. J. Water Pollut. Control. Fed. 46:588–591.
Hartig, J. H., and R. L. Thomas. 1988. Development of plans to restore degraded areas in the Great Lakes. Environ. Manage. 12:327–347.
Hasler, A. D. 1947. Eutrophication of lakes by domestic drainage. Ecology 28: 383–395.
Hawkes, C. L., D. L. Miller, and W. G. Layther. 1986. Fish ecoregions of Kansas: Stream fish assemblage patterns and associated environmental correlates. Environ. Biol. Fishes 17: 267–279.
Heiskary, S. A., C. B. Wilson, and D. P. Larsen. 1987. Analysis of regional patterns in lake water quality: Using ecoregions for lake management in Minnesota. Lake Reservoir Manage. 3:337–344.
Henning, T. 1989. Historical and areal deposition of mercury in NE Minnesota and Northern Wisconsin Lakes. M.S. thesis. University of Minnesota, Minneapolis, Minn.
Henrikson, L., H. G. Nyman, H.G. Oscarson, and J. A. E. Stenson. 1980. Trophic changes without changes in external nutrient loading. Hydrobiologia 68:257–263.
Hoar, S. K., A. Blair, F.F. Holmes, C. D. Boysen, R. J. Robel, R. Hoover, and J. F. Traument. 1986. Agricultural herbicide use and risk of lymphoma and soft-tissue sarcoma. J. Am. Med. Assoc. 256:1141–1147.
Hrbacek, J. M., M. Dvorakova, V. Korinek, and L. Prochazkova. 1961. Demonstration of the effect of the fish stock on the species composition of zooplankton and the intensity of metabolism of the whole plankton assemblage. Verh. Int. Limnol. 14:192–195.
Hsu, P. H. 1975. Precipitation of phosphate from solution using aluminum salts. Water Res. 9:1155–1161.
Hughes, R. M., and D. P. Larsen. 1988. Ecoregions: An approach to surface water protection. J. Water Pollut. Contr. Fed 60:486–493.
Hughes, R. M., D. P. Larsen, and J. M. Omernik. 1986. Regional reference sites: A method for assessing stream potentials. Environ. Manage. 10:629–635.
Hutchinson, G. E. 1975. A Treatise on Limnology. Vol. III. Aquatic Botany. John Wiley & Sons, New York.
Janus, L. L., and R. A. Vollenweider. 1981. The OECD Cooperative Programme on Eutrophication. Canadian Contribution Summary Report. Sci. Ser, No. 131. Canada Centre for Inland Waters, Burlington, Ont.
Johnson, B. M., R. S. Stewart, and S. J. Gilbert. 1992. Fisheries Biomanipulation in the Madison Lakes. Wisconsin Department on Natural Resources, Madison, Wis.
Kennedy, G. L., Jr. 1986. Biological effects of acetamide, formamide and the monomethyl and dimethyl derivatives. CRC Crit. Rev. Toxicol. 17:129–182.
Kennedy, R. A., and R. F. Gaugush. 1988. Assessment of water quality in Corps of Engineers reservoirs. Lake Reservoir Manage. 4:253–260.
Kennedy, R. H., J. W. Barko, W. F. James, W. D. Taylor, and G. L. Godshalk. 1987. Aluminum sulfate treatment of a reservoir: Rationale, application methods, and preliminary results. Lake Reservoir Manage. 3:85–90.
Ketelle, M. J., and P. D. Uttormark. 1971. Problem Lakes in the United States. U.S. Environmental Protection Agency. Project EHR 16010. Washington, D.C.
Kimbel, J. C., and S. C. Carpenter. 1981. Effects of mechanical harvesting on Myriophyllum spicatum L. regrowth and carbohydrate allocation to roots and shoots. Aquat. Bot. 11:121–127.
Kitchell, J. F., ed. 1991. Food Web Management: A Case Study of Lake Mendota, Wisconsin. Springer-Verlag, New York.
Knapp, S. M., and R. A. Soltero. 1983. Trout-zooplankton relationships in Medical Lake, Washington, following restoration by aluminum sulfate treatment. J. Freshwater Ecol. 2:1–11.
Kratzer, C. R., and P. L. Brezonik. 1981. A Carlson-type trophic state index for nitrogen in Florida lakes. Water Resour. Bull. 17:713–715.
Larsen, D. P., K. W. Malueg, D. W. Schults, and R. M. Brice. 1975. Response of eutrophic Shagawa Lake, Minnesota, U.S.A., to point source phosphorus reduction. Verh. Int. Ver. Limnol. 19:884–892.
Larsen, D. P., J. Van Sickel, K. W. Malueg, and D. P. Smith. 1979. The effect of wastewater phosphorus removal on Shagawa Lake, Minnesota: Phosphorus supplies, lake phosphorus and chlorophyll a. Water Res. 13:1259–1272.
Larsen, D. P., D. W. Schults, and K. W. Malueg. 1981. Summer internal phosphorus supplies in Shagawa Lake, Minnesota. Limnol. Oceanogr. 26:740–753.
Larsen, D. P., D. R. Dudley, and R. M. Hughes. 1988. A regional approach for assessing attainable surface water quality: An Ohio case study. J. Soil Water Conserv. 43:171–176.
Lathrop. R. C. 1979. Dane County water quality plan. Dane County Regional Planning Commission, Madison, Wis.
Lehman, J. T. 1986. Control of eutrophication in Lake Washington. Pp. 301–316 in Ecological Knowledge and Environmental Problem Solving. National Academy Press, Washington, D.C.
Leslie, A. J., Jr., J. M. Van Dyke, R. S. Hestand III, and B. Z. Thompson. 1987. Management of aquatic plants in multi-use lakes with grass carp (Ctenopharyngodon idella). Lake Reservoir Manage. 3:266–276.
Lessmark, O. and E. Thornelof. 1986. Liming in Sweden. Water, Air, Soil Pollut. 31:809–815.
Lodge, D. M., J. J. Magnuson, and A. M. Beckel. 1985. Lake-bottom tyrant. Nat. Hist. 94:32–37.
Mackie, G. L., W. N. Gibbons, B. W. Muncaster, and I. M. Gray. 1989. The Zebra Mussel, Dreissena polymorpha: A Synthesis of European Experiences and a Preview for North America . Environment Ontario, Toronton, Canada.
Magnuson, J. J. 1976. Managing with exotics: A game of chance. Trans. Am. Fish. Soc. 105:1–9.
Maki, A. W., D. B. Porcella, and R. H. Wendt. 1984. The impact of detergent phosphorus bans of receiving water quality. Water Res. 18:893–903.
Marais, G. V. R., R. E. Loewenthal, and I. P. Siebritz, 1983. Observations supporting phosphate removal by biological excess uptake—A review. Water Sci. Technol. 15:15–41.
Martin, E. H. 1988. Effectiveness of an urban runoff detention pond-wetlands system. Am. Soc. Civ. Eng, J. Environ. Eng. Div. 114:810–827.
Matson, P. A., and S. R. Carpenter, eds. 1990. Special feauture on analysis of response to large-scale perturbations. Ecology 71:2037–2068.
McIntyre, S. C., and J. W. Naney. 1990. Reelfoot lake sedimentation rates and sources. Water Resour. Bull 26:227–232.
McKnight, D. M., S. W. Chisholm, and D. F. Harlemann. 1983. CuSO4 treatment of nuisance algal blooms in drinking water reservoirs. Environ. Manage. 7:311–320.
McQueen, D. J., and D. R. S. Lean. 1986. Hypolimnetic aeration: An overview. Water Pollut. Res. Can. 21:205–217.
Mires, J. W., R. A. Soltero, and G. R. Keizur. 1981. Changes in the zooplankton community of Medical Lake, Washington, subsequent to its restoration by a whole-lake alum treatment and the establishment of a trout fishery. J. Freshwater Ecol. 1:167–178.
Molot, L. A., J. G. Hamilton, and G. M. Booth. 1986. Neutralization of acidic lakes: Short-term dissolution of dried and slurried calcite. Water Res. 20:757–766.
Mooney, H. A., and J. A. Drake, eds. 1986. Ecology of Biological Invasions of North America and Hawaii. Springer-Verlag, New York.
National Acid Precipitation Assessment Program (NAPAP). 1990a. Current Status of Surface Water Acid-Base Chemistry. State of Science and Technology Rept 9. NAPAP Interagency Program, Washington, D.C.
National Acid Precipitation Assessment Program (NAPAP). 1990b. Intergrated Assessment: Questions 1 and 2. NAPAP Interagency Program, Washington, D.C.
National Research Council (NRC). 1982. Water resources, Pp. 255–286 in Outlook for Science and Technology: The Next Five Years. W. H. Freeman, San Francisco.
National Research Council (NRC). 1986. Ecological Knowledge and Environmental Problem Solving. National Academy Press, Washington, D.C.
National Research Council (NRC). 1987. The Mono Basin Ecosystem: Effects of Changing Lake Level. National Academy Press, Washington, D.C.
Newbold, C. 1975. Herbicides in aquatic ecosystems. Biol. Conserv. 7:97–118.
Nichols, S. A., R. C. Lathrop, and S. R. Carpenter. 1991. Macrophyte community dynamics —A vegetation history. In J. F. Kitchell, ed., Food Web Management: A Case History of Lake Mendota, Wisconsin. Springer-Verlag, New York.
Nicholson, S.A. 1981. Changes in submersed macrophytes in Chautauqua Lake, 1937–1975. Freshwater Biol. 11:523–530.
Noonan, T. 1986. Water quality in Long Lake, Minnesota, following Riplox sediment treatment. Lake and Reservoir Manage. 2:131–137, Proceedings Fifth Annual Conference, Symposium North American Lake Management Society, Nov. 13–16, 1985, Lake Geneva, Wis.
Nurnberg, G.K. 1987. Hypolimnetic withdrawal as a lake restoration technique. Am. Soc. Civ. Eng., J. Environ. Eng. Div. 113:1006–1017.
Ogutu-Ohwayo, R. 1990. The decline of the native fishes of lakes Victoria and Kyoga (East Africa) and the impact of introduced species, especially the Nile perch, Lates niloticus, and the Nile tilapia, Oreochromis niloticus. Environ. Biol. Fishes 27:81–96.
Omernik, J.M. 1987. Aquatic ecoregions of the conterminous United States. Ann. Assoc. Am. Geogr. 77:118–125.
Omernik, J.M., D.P. Larsen, C.M. Rohm, and S.E. Clarke. 1988. Summer total phosphorus in lakes: A map of Minnesota, Wisconsin, and Michigan, USA. Environ. Manage. 12:815–825.
Osborne, J.A. 1982. The potential of the hybrid grass carp as a weed control agent. J. Freshwater. Ecol. 1:353–360.
Painter, D.S., and K.J. McCabe. 1988. Investigation into the disappearance of Eurasian watermilfoil from the Kawartha lakes. J. Aquat. Plant Manage. 26:3–12.
Pastorok, R.A., M.W. Lorenzen, and T.C. Ginn. 1982. Environmental Aspects of Artificial Aeration and Oxygenation of Reservoirs: A Review of Theory, Techniques, and Experiences . Tech. Rept. E-82-3. U.S. Army Corps of Engineers, Vicksburg, Miss.
Perry, J.A., N.H. Troelstrup, Jr., M. Newsom, and B. Shelley. 1987. Results of a recent whole ecosystem manipulation: The search for generality. Water Sci. Technol. 19:55–72.
Porcella, D.B. 1989. Lake acidification mitigation project (LAMP): An overview of an ecosystem perturbation experiment. Can. J. Fish. Aquat. Sci. 46:246–248.
Postel, S. 1985. Managing freshwater supplies. Pp. 42–72 in State of the World 1985. Worldwatch Institute Report, Washington, D.C.
Prepas, E.E., D.J. Webb, C.L.K. Robinson, and T.P. Murphy. 1990. Impact of liquid oxygen injection on a deep, naturally eutrophic lake: Amisk Lake, Alberta, year one. Ver. Int. Ver. Limnol. 24:320.
Reddy, K.R., and T.A. DeBush. 1987. State-of-the-Art Utilization of Aquatic Plants. In Water Pollut. Control, Water Sci. and Tech. 19:61–79.
Reinertsen, H., A. Jensen, J.I. Koksvik, A. Langeland, and Y. Olsen. 1990. Effects of fish removal on the limnetic ecosystem of a eutrophic lake. Can. J. Fish. Aquat. Sci. 47:166–173.
Richardson, C.J. 1988. Freshwater wetlands: Transformers, filters, or sinks? Forem (Duke University, Durham, N.C.) 11:3–9.
Ripl, W., and G. Lindmark. 1978. Ecosystem control by nitrogen metabolism in sediment. Vatten 2:135–144.
Robertson, A., and D. Scavia. 1984. North American Great Lakes. Pp. 135–176 in F.B. Tait, ed., Lakes and Reservoirs. Elsevier, The Netherlands.
Rudd, J.W.M., C.A. Kelly, D.W. Schindler, and M.A. Turner. 1988. Disruption of the nitrogen cycle in acidified lakes. Science 240:1515–1517.
Sawyer, C.N. 1947. Fertilization of lakes by agricultural and urban drainage. J.N. Engl. Water Works. Assoc. 61:109–127.
Schindler, D.W. 1988. Effects of acid rain on freshwater ecosystems. Science 239:149–157.
Scholz, A.T., R.A. Soltero, K.O. McKee, E. Anderson, and J.K. Vehara. 1985. Biomanipulation of a trout fishery and its effect on zooplankton composition, phytoplankton biovolume, and water quality of Medical Lake, Spokane County, Washington, following restoration by treatment with alum. Lake Reservoir Manage. 1:48–56.
Semmens, M.J., T. Ahmed, and M. Voss. 1990. An evaluation of bubbleless membrane aeration for lake aeration. Presented at Second International Conference on Gas Transfer at Water Surfaces, Minneapolis, Minn., Sept. 9-14, 1990. American Society of Civil Engineers and U.S. Corps of Engineers.
Shannon, E.E., and P.L. Brezonik. 1972. Eutrophication analysis: A multivariate approach. Am. Soc. Civ. Eng., J. Sanit. Eng. Div. 98:37–57.
Shapiro, J. 1973. Blue-green algae: Why they become dominant. Science 179:382–384.
Shapiro, J. 1984. Blue-green dominance in lakes: The role and management significance of pH and CO2. Int. Rev. [Ges.] Hydrobiol. 69:765–780.
Shapiro, J. 1990a. Biomanipulation: The next phase—Making it stable. Hydrobiologia 200/201:13–27.
Shapiro, J. 1990b. Current beliefs regarding dominance by blue-greens: The case for the importance of CO2 and pH . Verh. Int. Ver. Limnol. 24.
Shapiro, J., and D.I. Wright. 1984. Lake restoration by biomanipulation: Round Lake, Minnesota, the first two years. Freshwater Biol. 14:371–383.
Shapiro, J., G.V. Levin, and H. Zea. 1967. Anoxically induced release of phosphate in wastewater treatment. J. Water Pollut. Control Fed. 39:1811–1818.
Shapiro, J., V. Lamarra, and M. Lynch. 1975. Biomanipulation: An ecosystem approach to lake restoration. Pp. 85–96 in P.L. Brezonik and J.L. Fox, eds., Proceedings of a Symposium on Water Quality Management Through Biological Control, University of Florida, Gainesville.
Shireman, J.V., and M.J. Maceina. 1981. The utilization of grass carp, Ctenophyaryngodon idella Val., for hydrilla control in Lake Baldwin, Florida. J. Fish. Biol. 19:629–636.
Shireman, J.V., R.W. Rottmann, and F.J. Aldridge. 1983. Consumption and growth of hybrid grass carp fed four vegetation diets and trout chow in circular tanks. J. Fish. Biol. 22:685–693.
Smith, C.S., and J.W. Barko. 1990. Ecology of Eurasian watermilfoil. J. Aquat. Plant Manage. 28:55–64.
Soltero, R.A., D.G. Nichols, A.F. Gasperino, and M.A. Beckwith. 1981. Lake restoration: Medical Lake, Washington. J. Freshwater Ecol. 2:155–165.
Sonzogni, W.C., G.P. Fitzgerald, and G.F. Lee. 1975. Effects of wastewater diversion on the lower Madison lakes. J. Water Pollut. Control Fed. 47:535–542.
Stanley, J.G., W.W. Miley, and D.L. Sutton. 1978. Reproduction requirements and likelihood for naturalization of escaped grass carp in the United States. Trans. Am. Fish. Soc. 107:119–128.
Stefan, H.G., S. Dhamothoran, F.R. Schiebe, A.Y. Fu, and J.J. Cardoni. 1990. Dynamic simulation of turbidity and its correction in Lake Chicot, Arkansas. Pp. 193–250 in B. Henderson-Sellers, ed., Water Quality Modeling. Vol. 4. Decision Support Techniques for Lakes and Reservoirs. CRC Press, Boca Raton, Fla.
Sverdrup, H., and I. Bjerle. 1983. The calcite utilization efficiency and the long term effect in several Swedish lakes liming projects. Vatten 39:41–54.
Swain, E.B., and D.D. Helwig. 1989. Mercury in fish from northeastern Minnesota lakes: Historical trends, environmental correlates, and potential sources. J. Minn. Acad. Sci. 55:103–109.
Swain, W.R. 1978. Chlorinated organic residues in fish, water, and precipitation from the vicinity of Isle Royale, Lake Superior. J. Great Lakes Res. 4:398–407.
Thomann, R.V. 1989. Bioaccumulation model of organic chemical distribution in aquatic food chains. Environ. Sci. Technol. 23:699–707.
Tiedje, J.M., R.K. Colwell, Y.L. Grossman, R.E. Hodson, R.E. Lenski, R.N. Mack, and P.J. Regal. 1989. The planned introduction of genetically engineered organisms: Ecological considerations and recommendations. Ecology 70:298–315.
U.S. Environmental Protection Agency (EPA). 1980. Economic Benefits of the Clean Lakes Program. EPA-440-5-80-081. U.S. EPA, Washington, D.C.
U.S. Environmental Protection Agency (EPA). 1985. Clean Lakes Program: A Review of the First Decade. EPA 440/5-85-033. U.S. EPA, Washington, D.C.
U.S. Environmental Protection Agency (EPA). 1989. Report to Congress. Water Quality of the Nation's Lakes. EPA 440/5-89-003. U.S. EPA, Washington, D.C.
U.S. Environmental Protection Agency (EPA). 1990a. The Lake and Reservoir Restoration Guidance Manual. 2nd ed. EPA 440/4-90-006. U.S. EPA, Washington, D.C.
U.S. Environmental Protection Agency (EPA). 1990b. National Water Quality Inventory. 1988 Report to Congress. EPA 440/4-90-003. U.S. EPA, Washington, D.C.
Uttormark, P.D., and J.P. Wall. 1975. Lake classification—A trophic Characterization
of Wisconsin lakes. EPA-660/3-75-033. U.S. Environmental Protection Agency, Corvallis, Ore, 165 pp.
Vollenweider, R.A., and J. Kerekes. 1981. OECD eutrophication programme. Synthesis Report. Organization for Economic Cooperation and Development, Paris.
Walker, W.W., Jr. 1987. Phosphorus removal by urban runoff detention basins. Lake Reservoir Manage. 3:314–326.
Walski, T.M., and F.L. Parker. 1974. Consumers water quality index. Am. Soc. Civ. Eng., J. Environ. Eng. Div. 100:593–611.
Walters, C.W. 1986. Adaptive Management of Renewable Resources. MacMillan, New York.
Water Quality Act of 1987. P.L. 100-4.
Welch, E.B. 1981. The dilution/flushing technique in lake restoration. Water Resour. Bull. 17:558–564.
Welch, E.B., and E.R. Weiher. 1987. Improvement in Moses Lake quality from dilution and sewage diversion. Lake Reservoir Manage. 3:58–65.
Welch, E.B., D.E. Spyridakis, J.I. Shuster, and R.R. Horner. 1986. Declining lake sediment phosphorus release and oxygen deficit following waste water diversion. J. Water Pollut. Control Fed. 58:92–96.
Welch, E.B., C.L. DeGasperi, D.E. Spyridakis, and T.J. Belnick. 1988. Internal phosphorus loading and alum effectiveness in shallow lakes. Lake Reservoir Manage. 4:27–33.
Wiley, M.J., and L.D. Wike. 1986. Energy balances of diploid, triploid, and hybrid grass carp. Trans. Am. Fish. Soc. 115:853–863.
Wilson, C.B., and W.W. Walker, Jr. 1989. Development of lake assessment methods based upon the aquatic ecoregion concept. Lake Reservoir Manage. 5:11–22.
Wirth, T. 1988. Lake Aeration in Wisconsin Lakes. Lake Management Program PUBL-WR-196. Wisconsin Department of Natural Resources, Madison. 76 pp.
Wright, R.F. 1985. Liming and reacidification of Hovvatan, a chronically acidified lake in southernmost Norway. Can. J. Fish. Aquat. Sci. 42:1103–1113.
Young, S.N., W.T. Clough, A.J. Thomas, and R. Siddall. 1988. Changes in plant community at Foxcote Reservoir following use of ferric sulphate to control nutrient levels. J. Inst. Water Environ. Manage. 2:5–12.
Young, T.C., J.V. DePinto, J.R. Rhea, and R.D. Scheffe. 1989. Calcite dose selection, treatment efficiency, and residual calcite fate after whole-lake neutralization. Can. J. Fish. Aquat. Sci. 46:315–322.