D
Animal Models of Sex Steroid Hormones and Mammary Cancer: Lessons for Understanding Studies in Humans
DIANA B. PETITTI
For many years, studies in animals have been used to evaluate the carcinogenicity of various chemicals, including drugs. The effect of chemicals on the occurrence of cancer in animals is the basis for regulating human exposure in the workplace, in the environment, in food, and in pharmaceuticals. Studies of cancer in animals are often the sole input to the growing field of risk assessment. Most important, animal models of cancer have provided useful insights into the mechanisms of chemical carcinogenesis that have enlarged our understanding of the biology of human cancer.
The effect of various sex steroid hormones on the occurrence of mammary cancer has been studied in a variety of species, but the most extensive studies have been conducted in mice, rats, and beagle dogs. There have also been some studies in monkeys, which were conducted on a scale considered large for such studies. The low spontaneous rate of mammary tumors in primates, however, seriously limits the usefulness of the monkey as a model for studying sex steroid hormones and mammary cancer.
In theory, sex steroid hormones might cause mammary cancer because they are intrinsically carcinogenic or because they have hormonal effects that lead to cancer (Roe, 1976). This paper considers studies in mice, rats, and beagle dogs that were designed as direct
Diana B. Petitti is an associate professor in the Department of Family and Community Medicine, in the School of Medicine, University of California, San Francisco.
tests of the carcinogenicity of sex steroid hormones. It also reviews studies in these three species designed to elucidate the hormonal mechanisms of mammary cancer in general and in relation to exogenous administration of sex steroid hormones.
DIRECT TESTS OF CARCINOGENICITY
When the first steroid contraceptives were developed, they were submitted to the same toxicological tests that had been routinely conducted with other drugs. These tests were performed to identify the acute and chronic toxic effects of the drugs, and they were generally carried out in one rodent and one nonrodent species.
Because contraceptive steroids were to be taken by a large number of healthy young women for a considerable length of time, and because many important cancers in women were considered to be hormonally mediated, the carcinogenic risk of contraceptive steroids was of particular concern. This concern led the U.S. Food and Drug Administration (FDA) to require long-term tests of tumorigenicity in at least two species as a precondition for marketing approval. Later, FDA and other drug regulatory agencies began to require additional evaluation of contraceptive steroids in long-term studies in beagle dogs and rhesus monkeys.
Information from the long-term tests conducted to obtain marketing approval constitutes a valuable data resource and is reviewed here in some depth. It is important to recognize, however, that in 1932, Lacassagne (1932) demonstrated that exogenous estrogens caused mammary cancer in mice. By the late 1950s, it was already well established that exogenous estrogens caused mammary cancer in at least some strains of mice and rats.
Mice and Rats
A 1972 report by the U.K. Committee on Safety of Medicines (CSM, 1972) constitutes the largest data base on tumorigenicity of contraceptive steroids in mice and rats. The studies reported by the CSM involved 7,000 mice and 6,500 rats who were given six different estrogen/progestogen combinations at three doses and were examined for the presence or absence of neoplasia at 18 different sites. The tests of different estrogens and estrogen/progestogen combinations were carried out according to a standard protocol. Almost all experiments in mice used the CF-LP mouse strain; all of the experiments used 120 mice per treatment group. Doses of contraceptive steroids were 2-5, 50, and 200-400 times the human contraceptive dose. The
rat experiments used the same three dose groups, but the strain of rat and the number of rats per dose group varied from experiment to experiment.
The CSM report itself presented no tests of statistical significance in the comparisons of tumors in the treated and control animals. The analysis presented in the CSM report also did not take into account mortality during the course of the experiment, nor was any attempt made to determine whether there were dose-response relationships. These problems hamper interpretations of the results of the study, but the information is useful depite its limitations.
Mice who were treated with estrogen alone showed no excess of malignant mammary tumors. Mice treated with any of the six progestogens alone or with any of the combinations of estrogen and progestogen also showed no excess of malignant mammary tumors. In some experiements, female rats treated with ethinyl estradiol or mestranol alone had an increase in malignant mammary tumors (Table D-1). An excess of malignant mammary tumors also appeared in male rats treated with norethynodrel alone (Table D-2). In both male and female rats, an increase in malignant mammary tumors occurred following treatment with a norethynodrel/mestranol combination and norethindrone/mestranol combination, but not following treatment with other combinations (Table D-3). Female rats showed an excess of malignant mammary tumors following treatment with a combination of ethynodiol diacetate and mestranol.
Review of these experiments leads to several conclusions. First, estrogens alone, some progestogens, and some estrogen/progestogen combinations cause an increase in the occurrence of malignant mammary tumors in at least some strains of rats and mice. Second, there is strong evidence that progestogens modify the effect of estrogen on the occurrence of malignant mammary tumors in rats, as is also shown in experiments by other investigators (Schardein, 1980). Last, the effects of estrogens and progestogens on mammary cancer in mice and rats are strain specific (see also Rudali et al., 1971).
Beagle Dogs
The FDA began to require long-term studies of contraceptive steroids in beagles as a precondition for approval for marketing following the observation that one progestogen (MK-665, or ethynerone) caused high rates of mammary tumors in beagles even at doses that were a low multiple of the human contraceptive dose. Benign mammary tumors are considered an established precursor of malignant mammary tumors in beagles, and the beagle mammary response to
TABLE D-1 Malignant Mammary Tumors in Rats Treated with Mestranol or Ethinyl Estradiol Alone
|
Percent Increase |
||
|
Experiment |
Males |
Females |
|
8 |
N.a. |
16.7 |
|
8 |
N.a. |
19.2 |
|
10 |
N.a. |
0.9 |
|
11 |
0.0 |
2.6 |
|
12 |
1.4 |
3.7 |
|
N.a.: Not applicable. SOURCE: Committee on Safety of Medicines, 1972. |
||
TABLE D-2 Malignant Mammary Tumors in Rats Treated with Various Progestogens Alone
TABLE D-3 Malignant Mammary Tumors in Rats Treated with Estrogen/Progestogen Combinations
|
Percent Increase |
||
|
Combination a |
Males |
Females |
|
Norethynodrel + Mestranol |
19.2 |
14.3 |
|
Ethyno/idiol diacetate + Mestranol |
4.2 |
15.0 |
|
Norethindrone acetate + Ethinyl estradiol |
0.0 |
2.1 |
|
Norethindrone + Mestranol |
11.7 |
25.5 |
|
dl Norgestrel + Ethinyl estradiol |
0.0 |
0.7 |
|
Megestrol acetate + Ethinyl estradiol |
4.1 |
6.3 |
|
a High-dose levels. SOURCE: Committee on Safety of Medicines, 1972. |
||

FIGURE D-1 Excess percentage of dogs with tumors. SOURCE: Larsson and Machin (1989).
progestogen was considered a promising screening test for mammary carcinogenicity of contraceptive steroids.
Much has been written about the appropriateness, and inappropriateness, of the beagle as a model for studying the carcinogenicity of steroid hormones in humans (see El Etreby et al., 1979, 1989). These arguments are discussed later. First, however, let us consider the findings of studies in beagles that were summarized in a systematic form by Larsson and Machin (1989) for a conference sponsored by the World Health Organization (held in Geneva, Switzerland in January 1987). These studies are representative of the other published studies of sex steroid hormones and mammary tumors in beagles.
The experimental studies were all carried out according to a standard protocol. Three dose groups of 1-2, 10, and 25 times the human contraceptive dose were used in each experiment, together with an untreated control group. The mandated minimum number of dogs in each dose group and in the control group was 12; most experiments included 16 or 20 dogs in each group. All dogs were treated and followed for seven years, half the estimated life span of the beagle. Because even relatively low doses of some progestogens cause fatal pyometria in beagles, all experimental animals were hysterectomized.
Larsson and Machin (1989) compiled the results of 26 different
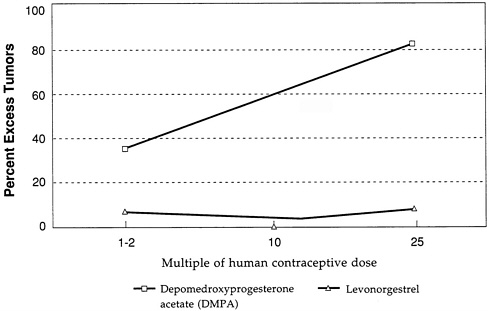
FIGURE D-2 Excess percentage of dogs with tumors. SOURCE: Larsson and Machin (1989).
experiments using estrogen, various progestogens, and estrogen/progestogen combinations. These experiments involved mestranol and ethinyl estradiol given alone and 16 different progestogens, both alone and in combination with mestranol or ethinyl estradiol. In the beagle, neither ethinyl estradiol nor mestranol alone caused an increase in mammary tumors, as shown in Figure D-1, which gives the excess percentage of dogs with tumors following treatment with ethinyl estradiol and mestranol for three multiples of the human contraceptive dose. Statistical analysis of these data taking into account survival time and dose-response yields the same conclusion about the mammary tumorigenicity of estrogen alone in beagle dogs (Larsson and Machin, 1989).
The beagle dog as a model for studying the mammary carcinogenicity of contraceptive steroids came under close scrutiny because of observations of the effects of depomedroxyprogesterone acetate (DMPA) in this system. The profound tumorigenic effect of DMPA in beagles is illustrated in Figure D-2, which again gives the excess percentage of dogs with tumors following treatment with DMPA at several multiples of the human contraceptive dose. The figure and analysis show that DMPA is a potent mammary tumorigen at doses that are close to the human contraceptive dose, whereas levonorgestrel shows almost
no effect on mammary tumorigenesis in the beagle in doses that are the same multiple of the human contraceptive dose. Again, statistical techniques that take into account survival of the dogs and dose-response yield the same conclusion (Larsson and Machin, 1989).
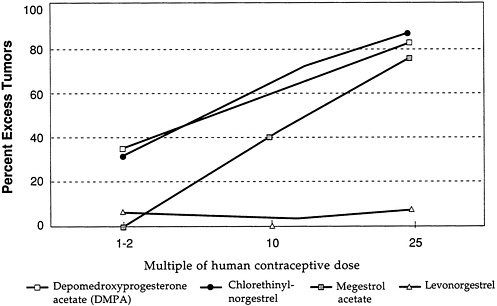
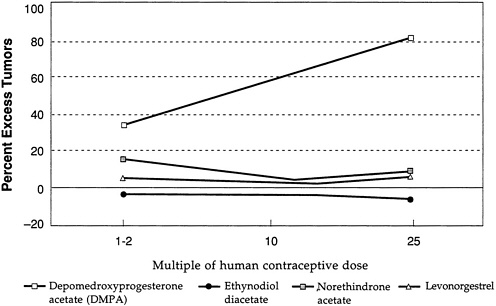
Figure D-3 and Figure D-4 illustrate further the variability in mammary tumorigenicity of different progestogens in beagles. Leaving DMPA and levonorgestrel as “reference” compounds, Figure D-3 shows the excess percentage of tumors after treatment with two C-21 hydroxy progestogens—chlorethinyl norgestrel and megestrol. Both are potent mammary tumorigens in beagles. In contrast, Figure D-4 shows that, like levonorgestrel, norethindrone acetate and ethynodiol diacetate do not cause an excess of mammary tumors in beagles even at high multiples of the human contraceptive dose. It is noteworthy that progesterone and medroxyprogesterone acetate are, like DMPA, potent mammary tumorigens in the beagle (Figure D-5).
Overall, the data support the conclusion that there are important differences in the mammary tumorigenicity of different progestogens in the beagle at comparable multiples of the human contraceptive dose.
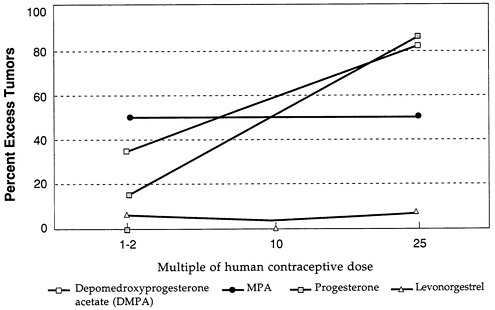
FIGURE D-5 Excess percentage of dogs with tumors. SOURCE: Larsson and Machin (1989).
PHARMACOKINETICS OF VARIOUS STEROIDS IN MICE, RATS, AND BEAGLES
Many of the arguments against use of the beagle as a model for studying the carcinogenicity of progestogens in humans center on a consideration of differences between dogs and humans in the pharmacokinetics of the steroids. A careful, direct comparative study of the pharmacokinetics of five steroids in rats, beagles, and humans was reported by Humpel (1989), who confirmed that there were both important similarities and important differences in various pharmacokinetic parameters between dogs and humans. This study also showed, however, that the pharmacokinetics of the five steroids were different in rats and humans. Thus, a consideration of differences between humans and other species in the pharmacokinetics of various steroids would lead one to reject the rat as well as the dog as a model for studying these compounds. For this reason, data from the experiments reported by the U. K.'s Committee on Safety of Medicines are no more reassuring about the effects of steroid hormones and mammary cancer than the beagle experiments are alarming.
An elegant experiment in beagles done by El Etreby and colleagues (1989) gives considerable insight into the mechanism for differences in mammary tumorigenicity of the various progestogens in the beagle. In this important experiment, the investigators explored the relationship between progestogen dose and mammary tumor response using doses of several progestogens that were larger or smaller than the “standard ” multiples of the human contraceptive dose. They were able to show that all of the progestogens tested were mammary tumorigens. The difference in mammary tumorigenicity of various progestogens was a matter of the dose at which the effect occurred and not a matter of some progestogens causing mammary tumors and some not causing mammary tumors. Levonorgestrel, which is very poorly absorbed by the dog, showed high rates of mammary tumorigenicity but only at doses that are many hundreds of times a multiple of the human contraceptive dose.
STUDIES OF MECHANISMS FOR MAMMARY CARCINOGENICITY OF SEX STEROID HORMONES IN MICE, RATS, AND DOGS
The most important lessons from animal studies of sex steroids come not from what must be considered fairly crude attempts to study their intrinsic carcinogenicity, but from studies that have gone further to define the mechanisms for steroid-induced mammary car-
TABLE D-4 Effect of Median Eminence Lesions on Prolactin Levels and Mammary Tumors in Rats
|
Effect |
||
|
Treatment |
Prolactin |
Percent Tumors |
|
Control |
50.9 |
19.0 |
|
Median eminence |
179.8 |
52.2 |
|
SOURCE: Welsch et al., 1970. |
||
cinogenicity in mice, rats, and beagles. It is now agreed that estrogen-induced mammary tumors in rats and mice are caused by increased levels of prolactin, which are a consequence of estrogen-induced benign pituitary adenomas. Thus, Welsch (1970) showed that lesions in the hypothalamus of the rat that destroyed inhibitors of pituitary prolactin release led both to increases in levels of prolactin and increases in malignant mammary tumors (Table D-4). Subsequently, Welsch (1977) showed that estrogen-induced mammary tumors in mice are prevented by simultaneous administration of a prolactin inhibitor, 2-bromo-α-ergocryptine (Table D-5). This prolactin inhibitor also prevented mammary tumors caused by simultaneous administration of estrogen and progestogen (Table D-6).
Studies in beagles have implicated progestogen-induced elevations in growth hormone in the pathogenesis of mammary tumors seen in experiments in dogs. Similar to the studies in rats and mice, this link rests first on the demonstration that progestogens that cause mammary tumors elevate growth hormone levels (Table D-7; Concannon et al., 1980). Later, El Etreby and coworkers (1989) showed that inhi
TABLE D-6 Mammary Tumors in Mice After Treatment with Enovid Mestranol (plus Norethynodrel) Alone and Enovid Plus a Prolactin Inhibitor
|
Treatment |
Percentage with Tumors |
|
Control |
14.0 |
|
Enovid |
30.0 |
|
Enovid + CB-154 a |
10.0 |
|
a CB-154 = 2-bromo-α-ergocryptine. SOURCE: Welsch et al., 1977. |
|
TABLE D-7 Serum Hormone Levels in Beagles After Treatment with Progesterone and Medroxyprogesterone Acetate (MPA)
|
Hormone |
Control |
Progesterone a |
MPA b |
|
Cortisol |
13.7 |
14.9 |
1.7 |
|
Prolactin |
12.6 |
13.6 |
13.7 |
|
Growth hormone |
0.4 |
0.6 |
9.5 |
|
a “Physiologic dose.” b Dose = milligrams per kilogram for 3 months. SOURCE: Concannon et al., 1980. |
|||
bition of progestogen-induced increases of growth hormone altered the beagle mammary response to the progestogen (data not shown).
CONCLUSIONS
Studies of the relationship of sex steriod hormones and mammary cancer in mice, rats, and beagles point to three main conclusions that apply to all three species. 1. Either estrogen alone, progestogen alone, or a combination of estrogen and progestogen have been shown to be mammary carcinogens. 2. The effect of estrogen on mammary cancer can be modified by the progestogen. 3. The mammary carcinogenic effects of sex steroid hormones are mediated by an effect of the sex steroid on a pituitary hormone, which is the mammary mitogen. This last observation, the most important, draws attention to a hypothesis put forth by El Etreby and colleagues (1989) that progestogen and
TABLE D-8 Comparison of Risk Factors for Mammary Cancer in Mice, Rats, Dogs, and Humans
estrogen in humans might affect mammary tumors through their effect on a novel pituitary mammary mitogen.
INTERESTING QUESTIONS RAISED BY ANIMAL STUDIES
A comparison of information about mammary cancer in mice, rats, beagles, and humans (Table D-8) shows that our knowledge of risk factors for mammary cancer in mice and rats is very poor. It would be easier to judge how much one could extrapolate between these two species and humans if there was as much information about spontaneous mammary cancer in these animals as there was for humans.
Consideration of both the epidemiology of mammary cancer in animals, including humans, and the material presented here raises some interesting questions. Does pregnancy alter the risk of mammary cancer in rats and mice? If so, does it do so by altering prolactin levels, suggesting that prolactin is a unifying mechanism for mammary cancer in mice and rats? Similarly, are increasing rates of mammary cancer with age in free-living beagle dogs accompanied by increasing levels of growth hormone? Are endogenous levels of prolactin predictive of mammary cancer in rats and mice, and are endogenous levels of growth hormone predictive of mammary cancer in beagles? Do estrogens, progestogens, and estrogen/progestogen combinations affect prolactin levels and growth hormone levels in humans? Last, if progestogens importantly modify the effect of estrogen on mammary carcinogenesis of sex steroid in mice, rats, and dogs, is it possible that the secular changes in the balance of estrogen and progestogen in commonly used oral contraceptives in different places explain differences in some of the risk estimates that have been derived from these studies?
Better integration of information from animal studies of mammary cancer and steroid contraceptives with thinking about human epidemiological data would undoubtedly advance our understanding of
the epidemiological data. Similarly, epidemiological approaches to the study of mammary cancer in animals would help establish the suitability and unsuitability of the animal models for humans.
REFERENCES
Committee on Safety of Medicines (CSM). 1972. Carcinogenicity Tests of Oral Contraceptives. London: Her Majesty's Stationery Office.
Concannon, P., N. Altszuler, J. Hampshire, W. R. Butler, and W. Hansel. 1980. Growth hormone, prolactin, and cortisol in dogs developing mammary nodules and an acromegaly-like appearance during treatment with medroxyprogesterone acetate Endocrinology 106: 1173-1177
El Etreby, M. F., K.-J. Graf, S. Beier, W. Elger, P. Gunzel, and F. Neumann. 1979. Suitability of the beagle dog as a test model for the tumorigenic potential of contraceptive steroids: A short review. Contraception 20: 237-256
El Etreby, M. F., S. Beier, and P. Gunzel. 1989. Comparative endocrine pharmacodynamics of contraceptive steroids. Pp. 179-192 in Safety Requirements for Contraceptive Steroids, F. Michal, ed. Cambridge: Cambridge University Press.
Humpel, M. 1989. Comparative pharmacokinetics of selected steroids in animal species and man. Pp. 193-210 in Safety Requirements for Contraceptive Steroids, F. Michal, ed. Cambridge: Cambridge University Press.
Lacassagne, A. L. 1932. Apparition de cancers de la mamelle chez la souris malé soumise à des injections de folliculine Comptes Rendus des Séances de la Société de Biologie et des Filiales 195: 632-638
Larsson, K. S., and D. Machin. 1989. Predictability of the safety of hormonal contraceptives from canine toxicological studies. Pp. 230-269 in Safety Requirements for Contraceptive Steroids, F. Michal, ed. Cambridge: Cambridge University Press.
Roe, F. 1976. Possible carcinogenic hazards of oral contraception. Proceedings of the Royal Society of Medicine 69: 349-350
Rudali, G., E. Coezy, F. Frederic, and F. Apiou. 1971. Susceptibility of mice of different strains to the mammary carcinogenic action of natural and synthetic oestrogens. Revue Europeene d'Estudes Cliniques et Biologiques 16: 425-439
Schardein, J. L. 1980. Studies of the components of an oral contraceptive in albino rats. 2. Progestogenic component and comparison of effects of the components and the combined agent Journal of Toxicology and Environmental Health 6: 895-906
Welsch, C. W., H. Hagasawa, and J. Meites. 1970. Increased incidence of spontaneous mammary tumors in female rats with induced hypothalamic lesions. Cancer Research 30: 2310-2313
Welsch, C. W., C. Adams, L. K. Lambrecht, C. C. Hassett, and C. L. Brooks. 1977. 17-β -oestradiol and enovid mammary tumorigenesis in c3H/HeJ female mice: Counter-action by concurrent 2-bromo-α-ergocryptine British Journal of Cancer 35: 322-328