1
Introduction and Overview
At least 50 million U.S. women have used oral contraceptives since they were introduced in 1960, and currently at least 10.7 million women depend on them for contraception (Mosher and Pratt, 1990). Eighty percent of all 35-year-old women use or have used them (Table 1-1; Dawson, 1990). The pattern of oral contraceptive use has changed over the years, however: less than 0.5 percent of women now aged 45-50 years used the pill prior to age 20, compared with 25 percent of women who are now 23 years old. Such shifts complicate analytic epidemiological studies of the short- and long-term effects of oral contraceptives. Younger women who have not yet started or have not yet completed their planned childbearing increasingly rely on oral contraceptives and other reversible methods of contraception, and women who do not plan further pregnancies rely more and more on irreversible sterilization procedures for themselves or their partners.
Breast cancer has become more common among American women through the twentieth century and now accounts for more of their new cases of cancer than are ascribed to cancer of any other organ (Figure 1-1). The rate among all women increased by 28 percent between 1974 and 1986 (Table 1-2), but the rates increased more in older than in younger women. Among women over age 50, the incidence of breast cancer has risen by approximately 1.4 percent a year since 1973 (Devesa et al., 1987); women age 50 and older now have 10 times the annual rate of women between the ages of 18 and 50 (Table 1-3)
TABLE 1-1 Percentage of Women Who Have Ever Used Oral Contraceptives, by Birth Cohort and Marital Status, United States, 1987
|
Birth Cohort |
Age at Interview (years) |
All Women (percent) |
Ever-married Women (percent) |
|
1935-1939 |
48-52 |
46.9 |
47.9 |
|
1940-1944 |
43-47 |
67.9 |
70.7 |
|
1945-1949 |
38-42 |
80.6 |
81.9 |
|
1950-1954 |
33-37 |
80.6 |
82.7 |
|
1955-1959 |
28-32 |
79.4 |
82.9 |
|
1960-1964 |
23-27 |
78.2 |
85.4 |
|
1965-1969 |
18-22 |
49.5 |
80.4 |
|
SOURCE: Data from the 1987 National Health Interview Survey, adaptedfrom D. A. Dawson, "Trends in Use of Oral Contraceptives—Data fromthe 1987 National Health Interview Survey," Family Planning Perspectives 22(1990):169-172. |
|||
. Today, 1 in 9 women who live long enough develops the disease sometime in her life, and 1 in 18 can expect to die from it. Furthermore, approximately two-thirds of women over the age of 70 are reported to have abnormal cellular proliferation in their breast tissue (Kramer and Rush, 1973).
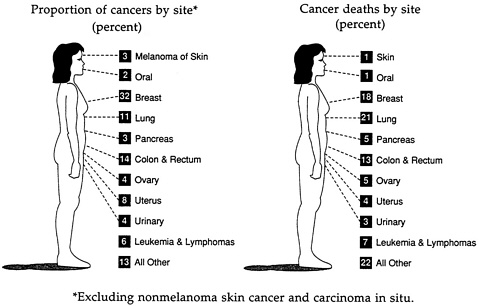
FIGURE 1-1 Estimates of cancer among American women, 1991. SOURCE: C. C. Boring, T. S. Squires, and T. Tong. “Cancer Statistics, 1991,” A Cancer Journal for Clinicians 41(1991):19-51. Reproduced with permission of the American Cancer Society.
TABLE 1-2 Increases in Breast Cancer Incidence Rates Between 1974 and 1986
|
Age at Diagnosis |
Percent Increase |
|
25-44 |
15 |
|
45-54 |
6 |
|
55-64 |
20 |
|
65-74 |
40 |
|
75-84 |
30 |
|
Total |
28 |
|
SOURCE: Emily White, Associate Member, Fred Hutchinson Cancer ResearchCenter, Seattle, Washington, personal communication, 1990. Nine standardSurveillance, Epidemiology, and End Results (SEER) registries wereused. Data were for females only, all races. |
|
Thus, the introduction and dissemination of the use of oral contraceptives in the United States were superimposed on steadily rising breast cancer incidence rates (Devesa et al., 1987). In the United States, the years since the introduction of oral contraceptives have also been years in which hysterectomy and oophorectomy rates were increasing, and the prescription of replacement hormones (estrogens and progestins) for these and other postmenopausal women became widespread (Hemminki et al., 1988; Kennedy et al., 1985).
TABLE 1-3 Age-adjusted Breast Cancer Incidence Rates (per 100,000 women) for White Females; Surveillance, Epidemiology, and End Results Program
|
Age |
||
|
Year of Diagnosis |
Under 50 |
50 and Above |
|
1973 |
29.1 |
252.1 |
|
1975 |
29.9 |
271.0 |
|
1977 |
28.8 |
258.4 |
|
1979 |
27.9 |
265.4 |
|
1981 |
28.8 |
280.3 |
|
1983 |
29.3 |
295.4 |
|
1985 |
32.0 |
326.7 |
|
Percent change |
2.8 |
16.9 |
|
Average yearly change |
0.2 |
1.4 |
|
SOURCE: Emily White, Associate Member, Fred Hutchinson Cancer ResearchCenter, Seattle, Washington, personal communication, 1990 (data fromNational Cancer Institute, 1988). |
||
The upward national trends in breast cancer incidence have continued and been consistently more marked in postmenopausal than in premenopausal women. The 1970s and 1980s were characterized by an acceleration of the increase in the U.S. postmenopausal breast cancer incidence rate, which has been more marked in women in their later 60s and 70s than in the earlier postmenopausal years. This is also the cohort of women who were too old ever to have taken oral contraceptives. The increase in annual numbers of cases, at least in postmenopausal women, is not completely explained by changes in age at first full-term pregnancy or wholly by increased rates of use of screening mammograms (The Cancer Letter, 1990; Marchant and Sutton, 1990; White et al., 1990). Nor, as yet, is any reason known for the apparently different experiences of younger and older postmenopausal women. Were older women exposed to an additional risk that bypassed younger cohorts? Has the effect of that additional risk yet to appear in younger cohorts? Were the younger cohorts exposed to that risk but protected against its effects by some new experience?
Against this background, the Institute of Medicine's Committee on the Relationship Between Oral Contraceptives and Breast Cancer assessed the role of oral contraceptives in breast cancer etiology. The committee met on three occasions through 1989 and 1990, convening an invitational conference in May 1990 to inform its deliberations. This report of the committee's efforts evaluates ways in which the etiology of breast cancer may relate to the use of oral contraceptives, identifies options for future research on several fronts, and lays out information for clinicians and women considering the use of the pill.
BIOLOGICAL PLAUSIBILITY OF A LINK BETWEEN ORAL CONTRACEPTIVES AND BREAST CANCER
The etiology of breast cancer is unknown. Growth and differentiation of breast tissue are regulated by a large number of factors, including steroid hormones such as estradiol and progesterone. Therefore, it is biologically plausible that exogenously administered steroidal hormones such as those in the pill could have an effect on breast carcinogenesis. On purely theoretical grounds it is impossible to say whether this effect might be adverse or beneficial. Estrogen causes proliferation of breast tissue and would be expected to increase the risk of breast cancer by stimulating growth of stem and intermediate cells (Thomas, 1984). Progestin causes not only alveolar cell growth in the estrogen-primed breast but also differentiation. It is thus unclear whether the net effect would be to increase or decrease breast cancer risk (see Appendix A).
The finding of an oral contraceptive effect on breast cancer, however small and whether harmful or beneficial, could throw some general light on the pathogenesis of this devastating disease. Although a comprehensive evaluation of the possible etiologic mechanisms of breast cancer is beyond the scope of this report, clearly the uncertainty that currently exists about a possible interaction between this too-common cancer and the method of contraception used at one time or another by 80 percent of women in the United States must be resolved.
WHAT IS—AND IS NOT—KNOWN
Even though, over the past 30 years, oral contraceptives have become one of the most intensively studied drugs in history, the observational data collected to date are inadequate to answer the basic questions of whether oral contraceptives have an effect on the course of breast cancer and whether they make it more common or less common. About a dozen factors are at the core of what is—and is not—known.
The United States has one of the world's highest annual rates of breast cancer incidence, a rate that was already increasing steadily when oral contraceptives were introduced. Whether susceptible women had already fulfilled their carcinogenesis potential (see Appendix B) and could not react further to an added carcinogen, or whether it will simply take many more observations to measure a relatively small increment against an escalating high background rate, is hypothetical.
The age at which women have chosen to start using oral contraceptives has decreased (Table 1-4), and their pattern of use before the first pregnancy (Table 1-5) and to space pregnancies has changed. Younger women will need effective contraception for more than the average 5 years' use by older birth cohorts (Table 1-6). Studies to date have largely focused on patterns of use in years past and, although reassuring overall, do not address more recent patterns of use. In fact, the best existing information about oral contraceptives is for women now in their late 40s and early 50s— the first generation of oral contraceptive users in the 1960s—who used the pill to space their second and later pregnancies. Exposure to oral contraceptives around the time of menarche or menopause, or preceding the first full-term pregnancy, may have quite different influences.
Neither a positive nor negative association between overall breast cancer risk and oral contraceptive use has been found among the first generation of oral contraceptive users from the 1960s. These women lived in developed countries and used the pill for relatively short
TABLE 1-4 Cumulative Percentage of Women Who Have Ever Used Oral Contraceptives Prior to Selected Ages, by Birth Cohort, United States, 1987
|
Percent of Women Who Have Ever Used Oral Contraceptives Prior to Age: |
||||||||||
|
Birth Cohort |
15 |
18 |
20 |
25 |
30 |
35 |
40 |
45 |
50 |
55 |
|
1935-1939 |
0.0 |
0.1 |
0.3 |
8.9 |
28.9 |
39.9 |
45.0 |
46.5 |
46.9 |
46.9 a |
|
1940-1944 |
0.1 |
0.4 |
4.8 |
43.5 |
62.0 |
66.9 |
67.9 |
67.9 |
67.9 a |
|
|
1945-1949 |
0.2 |
4.1 |
21.5 |
66.8 |
77.2 |
80.1 |
80.6 |
80.6 a |
||
|
1950-1954 |
0.5 |
9.6 |
34.2 |
72.3 |
78.4 |
80.4 |
80.6 a |
|||
|
1955-1959 |
1.1 |
18.6 |
44.9 |
72.1 |
78.6 |
79.4 a |
||||
|
1960-1964 |
2.2 |
22.9 |
47.1 |
76.0 |
78.2 a |
|||||
|
1965-1969 |
1.8 |
24.9 |
44.3 |
9.5 a |
||||||
|
a This figure is a conservative estimate because not all women in the cohort have reachedthe age in question. SOURCE: Data from the 1987 National Health Interview Survey, adaptedfrom D. A.Dawson,"Trends in Use of Oral Contraceptives—Data fromthe 1987 National Health Interview Survey," Family Planning Perspectives 22(1990):169-172. |
||||||||||
TABLE 1-5 Percent Distribution of Women Who Have Ever Used Oral Contraceptives, by Birth Cohort and by Timing of First Use, United States, 1987
|
Timing of Oral Conctaceptive Use |
||||
|
Birth Cohort |
Age at Interview (years) |
Before First Full-term Pregnancy (percent) |
Same Age as Full-term Pregnancy (percent) |
After First Full-term Pregnancy (percent) |
|
1935-1939 |
48-52 |
11.9 |
3.4 |
84.7 |
|
1940-1944 |
43-47 |
31.7 |
7.5 |
60.8 |
|
1945-1949 |
38-42 |
53.2 |
12.2 |
34.6 |
|
1950-1954 |
33-37 |
67.3 |
11.7 |
21.0 |
|
1955-1959 |
28-32 |
72.0 |
9.9 |
18.1 |
|
1960-1964 |
23-27 |
75.8 |
10.6 |
13.6 |
|
1965-1969 |
18-22 |
77.3 |
12.8 |
9.9 |
|
SOURCE: Data from the 1987 National Health Interview Survey, adaptedfrom D. A. Dawson, "Trends in Use of Oral Contraceptives—Data fromthe 1987 National Health Interview Survey," Family Planning Perspectives 22(1990):169-172. |
||||
TABLE 1-6 Women Who Have Ever Used Oral Contraceptives, by Birth Cohort, According to Average Months of Use, United States, 1987
|
Birth Cohort |
Age at Interview (years) |
Average Months of Use |
|
1935-1939 |
48-52 |
51.3 |
|
1940-1944 |
43-47 |
60.8 |
|
1945-1949 |
38-42 |
60.6 |
|
1950-1954 |
33-37 |
56.2 |
|
1955-1959 |
28-32 |
52.7 |
|
1960-1964 |
23-27 |
38.0 |
|
1965-1969 |
18-22 |
24.3 |
|
SOURCE: Data from the 1987 National Health Interview Survey, adaptedfrom D. A. Dawson, "Trends in Use of Oral Contraceptives—Data fromthe 1987 National Health Interview Survey," Family Planning Perspectives 22(1990):169-172. |
||
periods of time—10 years being the longest exposure. Although there are no firm biological estimates of the longest latent periods between exposure and evidence of carcinogenesis, the existing 10 to 20 years of follow-up are probably sufficient to provide confidence in these results. An additional 5 to 10 years of follow-up of these first users would provide complete assurance of the safety of their use of oral contraceptives.
There is uncertainty about the relationship between oral contraceptive use and the relatively small number of breast cancers that arise in women under the age of 35. To date, studies have yielded inconsistent findings; some suggest an increased relative risk of breast cancer of as high as 1.6. Interpreting the positive findings in several recent case-control studies (see Appendix A, Appendix B, and Appendix E) is difficult principally for two reasons. First, the positive subgroups keep changing. Second, the relative risk estimates are close to 1.0. Skegg (Appendix E, in this volume) warns that the relationship between oral contraceptives and breast cancer will not be clarified by chasing after “shifting goalposts” (i.e., positive subgroups). Rather, future studies should cover the full range of ages of women who have used the pill.
An important part of the information needed to resolve some of the uncertainties in the breast cancer/oral contraceptive question relates to duration and timing of exposure. Much more information is also needed about the effect on breast cancer incidence rates of short-term exposures at earlier ages (particularly before age 25 and/or before the first full-term pregnancy) on breast-cancer-incidence rates
both before and after age 35. A further gap in knowledge is whether an increased risk detected before age 35 persists throughout the pre-and postmenopausal years. In line with these observations, more information is needed to learn whether oral contraceptive use increases risk in young women with diagnoses of benign breast disease even though breast cancer risk is increased only for women with specific histologic types of benign breast disease. The same information is needed about other breast cancer risk factors.
Although some information has been collected about the influence on breast cancer rates of relatively short-term exposures to oral contraceptives, whether long-term use beginning at any age increases pre-, peri-, or postmenopausal breast cancer risk has yet to be determined. And, the possibility has been raised that oral contraceptive use may increase breast cancer risk in relatively underdeveloped countries with low background breast cancer rates. This possibility also requires further inquiry.
If oral contraceptive use is associated with an increased risk of breast cancer at young ages, is this a promotional effect with a subsequent decrease in risk at older ages, or is this a cohort effect with an increased risk throughout the remainder of life? This question raises an issue of significant public health concern, given the current state of knowledge about oral contraceptives and breast cancer. Data from the Cancer and Steroid Hormone study support a promotional effect, but many other studies have not included women over the age of 35 or 45 and therefore do not have data to address the question. Furthermore, if changing patterns of oral contraceptive use (i.e., starting in the teenage years to delay a first pregnancy) or changing oral contraceptive composition (i.e., changing estrogen and progestin potencies) are related to increased risk at young ages, then it is only now—when the birth cohort who first experienced those use patterns enters its middle-aged years—that a cohort effect on breast cancer risk could be identified.
What are the effects on breast cancer risk of long-term oral contraceptive use followed by hormone replacement therapy? This question assumes particular importance because of the resurgence in popularity of hormone (i.e., estrogen or estrogen plus progestin) replacement therapy (HRT), and its recognized beneficial effects in prevention of osteoporosis and, for estrogen replacement therapy alone, prevention of cardiovascular disease. HRT is widely used: at least 15 percent of eligible women use it, or have used it, at some time; the percentage varies by geographic region within the United States. Furthermore, oral contraceptive users may be more likely than nonusers to use HRT subsequently. HRT itself has been evaluated in numerous stud-
ies for an effect on breast cancer risk. The findings have varied; some studies show small increased relative risk estimates (around 1.5 for long-term use), and others show no increase. However, there is only minimal information about the effects of HRT on the breast following prolonged use of oral contraceptives. The cohort of women with prolonged oral contraceptive use is just now entering the menopausal years when HRT is prescribed. This affords both the opportunity for and the necessity of studying breast cancer risk in relation to the use of oral contraceptives followed sequentially by HRT.
The preceding two questions are amenable to traditional epidemiological research designs—that is, case-control or cohort studies. An obvious strategy is a multicenter, case-control study of women aged 45-65. With this age group both types of hormonal products could be studied, and various details of HRT and oral contraceptive types and use patterns could be evaluated. Characteristics of the tumors—histology, stage, and receptor status—could be evaluated, provided the hypothesis, design, and power calculations allow for subgroup analyses. Furthermore, potential biases, such as source of controls and method of diagnosis, could be addressed in developmental phases and thus avoided in the main study.
Changes in oral contraceptive formulations stimulated a few of the existing epidemiological studies. It is now apparent that ethinyl estradiol and 19-nortestosterone derivatives are the most commonly used synthetic estrogen and progestin (see Appendix C). To date, no differences in possible associations with breast cancer have been observed between oral contraceptive formulations containing ethinyl estradiol and mestranol. Moreover, no 19-nortestosterone-derived progestin studied thus far has been shown to be more strongly associated with breast cancer than any other. Nevertheless, comprehensive studies of their specific effects are needed.
As a practical matter, physicians would like to be able to identify those women who could use the pill with absolute safety. Much more information is needed to help physicians identify women who are least likely to have their risk of breast cancer increased. Data are also necessary to determine oral contraceptive patterns of use that are least likely to increase risk and the influence of prior oral contraceptive use on the course of subsequent breast cancers.
Some of the questions noted above can be answered relatively quickly; others could take several decades of study in developed nations. A few of the questions may be best answered, and some can only be answered, in countries with relatively low background rates of breast cancer.
The epidemiology of breast cancer clearly suggests that hormone-
related events during puberty, during fertile years around menopause, and after menopause can independently or synergistically influence breast cancer. Exposure information must therefore be collected throughout a majority of a woman's adult years, and the consequences of exposure must be monitored until the longest potential latent period has passed. Margins of 20 to 30 years must be used until there are better estimates of the shortest and longest intervals between exposures to carcinogens and promoters, and clinical diagnosis of breast cancer.
SEEKING ANSWERS
Premarketing drug testing is funded by the drug's developer or manufacturer. For safety's sake, all new drugs are tested on experimental animals prior to approval by the Food and Drug Administration (FDA) for first use in carefully controlled studies of human volunteers. Additional safety studies in animals are conducted, while human trials are ongoing, using many multiples of the human doses. Even so, safety is not necessarily ensured—for several reasons. The most important relates to the fact that different species of animals sometimes respond differently to the same drug, especially in the case of the reproductive system, for which variations among species are often widest. Human beings, for example, are the only primates whose breasts develop at puberty, instead of during the first pregnancy. Before manufacturers receive marketing approval from the FDA for a new drug or contraceptive, they must conduct trials of efficacy and safety on several hundred volunteers. Irrespective of cost, the scale and duration of such trials are insufficient to detect many rare, but potentially important, adverse or beneficial side effects. Although it is essential to gather as much relevant information as possible, every new drug widely used by the human species is an experiment that must be closely monitored. Nowhere is this fact more important than in the case of contraceptives, which are likely to be used at some time by a majority of adult women.
Once a drug is approved for marketing, there is generally no obligation on the part of manufacturers to conduct additional large-scale studies. However, medical research institutions, with private or federal funding, often carry out ad hoc investigations if pathology is suspected of being associated with use of the drug. In the case of oral contraceptives, a prudent alternative to waiting for the occurrence of unexpected disease is a well-planned cohort approach to detect all forms of unexpected side effects—and possible benefits, as well. Some such studies have been conducted in the United States
and United Kingdom; investment in further cohort studies seems wise, particularly with new formulations of the pill becoming available and changes in use patterns.
Ecological data describing breast cancer incidence and mortality and oral contraceptive distribution and use are sparse, and they are likely to be of limited utility in countries with high rates of breast cancer. Whether they can be fully exploited worldwide will depend on the quality of national information systems. Chapter 2 explores this opportunity more fully. The chapter also discusses the need to plan a fresh cohort study as soon as a new oral contraceptive preparation is brought into general use, as well as the opportunity to capture essential new information that arises in countries that have relatively low background rates of breast cancer and that decide to introduce the pill as a generally available contraceptive.
The potential contributions of existing animal and in vitro human tissue models to questions of oral contraceptives and breast cancer have not been fully exploited, perhaps because they are mostly being used by investigators who are working within their own disciplines to answer other specific questions. Opportunities must be provided for interdisciplinary research that concentrates the expertise of investigators who use these models on efforts to get answers about oral contraceptives and breast cancer. These opportunities are discussed in Chapter 3. The existing evidence from animal models (see Appendix D) has been influential in continuing the effort to explore the possible influences of oral contraceptives on breast carcinogenesis.
Beside the rising rates of breast cancer incidence there is a virtual absence of definitive information about the pathological processes of breast carcinogenesis. Available experimental data from studies of different mammals are conflicting. However, the sum of the data suggests that some of the steroids used in oral contraceptives in certain dosage patterns are capable of inducing tumors in experimental animals. Both increased tumor incidence and decreased latency have been observed. Given the widespread use of these compounds, even a small increase in risk for lifetime cancer incidence would be expected to have a substantial effect on human populations using these hormones. This suggests that a careful, formalized risk assessment should be conducted and compared with the human epidemiological data. The assumptions for this risk assessment should be explicitly defined and delineated.
A formal, four-step cancer risk assessment methodology (comprising hazard identification, hazard characterization, exposure assessment, and risk characterization) has been used extensively over the past decade to protect the public health from known or suspected
carcinogens (National Research Council, 1983, 1984). A modification of this approach that reflects the biology of the hormones found in oral contraceptives may be especially useful.
As mentioned earlier, breast cancer incidence rates increase steadily with age so that breast cancer is mainly a disease of the postmenopausal years (Table 1-7). A minority of breast cancer cases occur among women under the age of 45; therefore, most of the women who have had breast cancer since 1960 passed through their fertile years before oral contraceptives were widely used. Women now in their 40s, however, have used the pill more extensively and need answers to their questions about any possible interaction between the pill and breast cancer risk. In addition, if they use postmenopausal replacement hormones, questions about interaction with prior oral contraceptive use could become even more important.
When oral contraceptives were first approved for marketing by the FDA in 1960, they were known to be highly efficacious and thought by many not to have common, short-term, serious side effects. Little else was known about the more general effects of their use. With longer duration of use, an increasingly detailed picture of oral contraceptive risks and benefits is emerging (see Chapter 4). Today, practically the only important area to be resolved relates to breast cancer.
Overall, the pill has performed remarkably well as a relatively safe, effective, widely acceptable contraceptive (see Appendix E). Long-term follow-up has shown that oral contraceptive use can alter disease risk in pre- and perimenopausal women and that specific formulations modify these risks in different ways. The changes of greatest interest have been that certain types of cardiovascular disease have become more common and two reproductive cancers (i.e., ovarian and endometrial) have become less common. By developing different artificial hormones and reducing the amount of hormones in each tablet, some of the increased risk of cardiovascular disease observed in earlier, high-dose pills has been reduced without losing any of the cancer prevention benefits. Substantial gaps in data still exist, however. For example, although new progestins that are still making their way through the approval process in the United States have been widely used in Europe for a decade, there is no published body of epidemiological evidence on their influence on overall cardiovascular and cancer disease profiles. In addition, no postmenopausal women used oral contraceptives earlier in their reproductive lives for a period long enough to yield any information about systematic oral contraceptive influence on disease profiles. The lack of information on such influence applies not only to early but also to
TABLE 1-7 Breast Cancer Incidence (per 100,000 women), United States, 1986
|
Age |
Incidence Rate |
|
20-24 |
1 |
|
25-29 |
7 |
|
30-34 |
30 |
|
35-39 |
65 |
|
40-44 |
131 |
|
45-49 |
186 |
|
50-54 |
217 |
|
55-59 |
263 |
|
60-64 |
331 |
|
65-69 |
402 |
|
70-74 |
424 |
|
75-79 |
450 |
|
80-84 |
443 |
|
85+ |
391 |
|
SOURCE: Nine standard Surveillance, Epidemiology, and End Results(SEER) registries. Females only, all races, by five-year age groups. |
|
late postmenopausal years when coronary heart disease and breast and ovarian cancers become more and more common.
Studies in the United States and around the world show that many women—and some health care providers—are both misinformed and unnecessarily pessimistic about the effects of the pill. For example, fewer than a fifth of American women in 1987 (El Shafei et al., 1987) knew that the pill protects against ovarian and uterine cancer; some women considered oral contraceptive use to be more dangerous than childbirth. (In fact, when the full range of proven side effects is considered, childbirth is always more risky than using the pill [Fortney et al., 1986].) In Chapter 4, the committee summarizes the best available information for pill users and health care providers.
Most of the relatively few unplanned pregnancies that do occur during pill use are due to inconsistent or incorrect use, including contemporaneous use of other drugs that compromise the effectiveness of oral contraceptives (Mattison, 1984). It is important to remember that users have not only fewer deliveries but fewer abortions and ectopic pregnancies, each of which carries its own risk of mortality and morbidity. In the short term, most women on the pill have less menstrual loss, risk of anemia, and chance of acne, as well as a reduction in premenstrual symptoms. Fewer pill users are hos-
pitalized for treatment of benign breast cysts. Users commonly complain that they are gaining weight on the pill, but this complaint has not been confirmed by the results of controlled trials. Some women do, however, suffer an increase in blood pressure, which can become a reason to stop pill use.
Initial oral contraceptive use can be associated with nausea and breast tenderness. Serious side effects include an increased risk of heart attack and stroke, with their associated mortality and morbidity, and of clotting in the deep veins of the leg which, in the rare event that a large clot dislodges, can also lead to death. The risk of adverse cardiovascular effects begins more or less as soon as the user takes her first pill and is thought to disappear shortly after she takes her last tablet. The cardiovascular risk of using the pill rises steeply in the 40s and interacts in an especially powerful way with cigarette smoking. Even for women over 40, the benefits of the pill often continue to outweigh the risks, as long as they are nonsmokers. Smokers of this age—if they cannot stop smoking—should use an alternative method of contraception. Pill users have more gall bladder disease than nonusers of the same age, most probably because use advances a disease that would otherwise have appeared somewhat later in the life of the same woman. Oral contraceptives are associated with a marked increase in the relative risk of benign and malignant liver tumors, but these tumors are so exceedingly rare among U.S. women that a pill user's absolute risk of getting one remains low.
It is always easier to record a case of disease than the fact that a disease did not occur. Twenty years' work was required to demonstrate that oral contraceptives protect against developing ovarian and endometrial cancer years after oral contraceptive use, whereas it took less than 10 years to demonstrate some of the pill's adverse effects on the cardiovascular system. Fortunately, although cardiovascular risks arise when the pill is first used, they also fall soon after its use is stopped; on the other hand, the cancer protection benefits take some years of use to build up and, in contrast to the cardiovascular effects, persist for many years after taking the last pill. Seven years' use of oral contraceptives reduces the risk of ovarian cancer by 60 to 80 percent during the next 10 years. Only 4 percent of cancers among American women are ovarian, but the disease has already spread to other organs in 60 percent of women at the time of diagnosis. In addition to cancer protection, pill users also have less pelvic inflammatory disease, although this effect is probably a consequence of changes in the cervical mucus, which begin and end with oral contraceptive use.
In addition to the unresolved questions concerning the pill and
breast cancer, there is also a lack of scientific agreement about the possible influence of oral contraceptives on cervical cancer. The pill could have an effect on cervical changes, although a growing understanding of the etiology of cervical cancer points to involvement of the human papilloma virus (HPV). Of course, the involvements of HPV and oral contraceptives are not mutually exclusive. The overall incidence of death owing to invasive cervical cancer is decreasing, and it is less threatening because early detection can lead to its complete cure. Scientists disagree about the influence of the pill because the higher prevalence of cervical cancer reported among pill users in several studies may be due to confounding factors, in particular, the possibility that oral contraceptive users may have more sexual partners than nonusers and that they may have cervical smears taken more frequently.
Research into the causes and mechanisms of breast cancer, particularly in relation to the large increase in the incidence of the disease, has been conducted in an ad hoc fashion without any long-term investment in a coherent, national multidisciplinary research program. Further, the development of research resources and technologies needs to support such a program and carry it to the cutting edge of 1990s' state of the art. Chapter 5 addresses the critical question of resources for future research.
It is interesting to note that the relationship of oral contraceptives and breast cancer is not the only area of epidemiological investigation that has proved difficult and occasionally yielded contradictory results. In 1970, a large multicenter trial (MacMahon et al., 1970) was unable to demonstrate any relationship between breastfeeding and breast cancer. Further work was not conducted until the 1980s, when two studies from different parts of the world demonstrated a strongly protective, dose-dependent effect of lactation on the subsequent risk of breast cancer (McTiernan and Thomas, 1986; Yuan et al., 1988). Not long afterward, another group (London et al., 1990) was unable to confirm these results. Clearly, breastfeeding is a variable that needs to receive greater priority in all studies, including those that involve the pill and breast cancer.
Oral contraceptives have proved popular not only in America but in diverse cultures ranging from China to Indonesia to Tunisia to Colombia, and their use is likely to continue to grow rapidly in the coming decade. Globally, one woman a minute dies from pregnancy, childbirth, or abortion, and there is no doubt that oral contraceptives have saved and will continue to save a great many lives. Research continues, albeit at a low level, into new methods of contraception, and additional contraceptive choices would be welcome (NRC/IOM,
1990). At the same time, even though there remain key unanswered questions about oral contraceptives and breast cancer, it must be noted that any new methods, such as progestin-only subdermal implants, will require a whole new generation of investigations—and investigators —to illuminate the same set of unknowns that surrounded the introduction of the first oral contraceptives 30 years ago. It is imperative to fill in what is possibly the last substantial gap in knowledge of oral contraceptive side effects—namely, the effect, if any, on breast cancer. More than 80 percent of American women now in their 20s and 30s who have ever had intercourse have used the pill at some time or other in their fertile lives. Scientific study of the pill and of breast cancer, separately and combined, are therefore topics of immense and urgent importance to rapidly increasing numbers of American women.
















