5
Quality of Life and Mechanical Circulatory Support Systems
A human being would certainly not grow to be 70 or 80 years old if this longevity had no meaning for the species. The afternoon of human life must also have a significance of its own and cannot be merely a pitiful appendage to life's morning.
Carl Jung, The Stages of Life
HEALTH CARE IS BUT ONE of many determinants of the health and quality of life of an individual. Genes, environment, and personal behavior, among other personal, social, and economic factors, have an appreciable impact. Goals and expectations of health care, from the perspectives of the health care provider, patients, and their families, include preventing, managing, and curing disease as well as improving the levels of function and well-being (Stewart et al., 1989).
The use of health technologies, in particular mechanical circulatory support systems (MCSSs), in treating end-stage heart disease raises several important issues about assessing the quality and outcomes of care within the context of the goals and expectations of treatment. In this chapter we focus on one important aspect of treatment alternatives for end-stage heart disease—the quality of life of the patient. A Texas newspaper columnist (Greene, 1990) writing in first person about his experience of heart transplantation shares these thoughts on quality of life:
One of the meanest things we can say of a person is that he or she is heartless. Coldhearted equals ruthless. . . . Heart and soul are inextricably mixed in phrase and fable. Where the heart is, . . . there will the soul be also. To tell a male he has heart trouble attacks him in his manhood. . . . (pp. 28-29)
The discussion that follows attempts to answer such questions as: Why is quality of life important? What is meant by quality of life? What domains of quality of life are relevant to end-stage heart disease patients? What is known about the quality of life of these patients? The final sec
tions of this chapter explore the use of health-related quality-of-life measures in MCSS clinical trials and studies, examine the importance of disease-specific domains and measures, and set forth conclusions of the Institute of Medicine (IOM) committee.
THE IMPORTANCE OF QUALITY-OF-LIFE CONSIDERATIONS
From the Perspective of the Patient
Researchers and clinicians evince much interest in knowing about the quality of life of patients exposed to unconventional forms of therapy for life-threatening conditions. Many of these patients have prolonged hospitalizations, extensive diagnostic testing, and multiple failures of drug therapy. Patients with life-threatening conditions frequently report feelings of anxiety, fear, depression, and loss of control (Cooper et al., 1986); these feelings and the importance patients attach to them need to be considered in assessing treatment options and their outcomes.
Quality-of-life measures can be especially important in assessing the outcomes of care for patients receiving therapies that carry high risks of negative consequences (Falotico-Taylor et al., 1989). Families of these patients are also subject to a considerable range of emotions (e.g., anger, depression, denial, disengagement, guilt) that, in turn, may affect the quality of life of the patient (Christopherson, 1986).
The history of earlier efforts to treat end-stage heart disease patients with mechanical circulatory support devices reflects many emotional and ethical concerns about the clinical and technological environments (Galletti, 1984; Shaw, 1984; Swazey et al., 1989). That history has heightened concern for the “person” with an illness, such as end-stage heart disease, and has confirmed the importance of patients' preferences for balancing quantity of life with quality of life. These preferences are particularly significant when MCSS implantation limits or restricts future treatment options, should the device fail or the patient elect to stop maintaining the device.
“Proximate clinical indicators” or clinical endpoints combine measures of both outcomes and processes of care (IOM, 1991a). They assess care close to the time that treatment is provided and thus take on critical meaning for patients whose life expectancy may be no greater than six months. Patient preferences for health-related quality-of-life outcomes at these interim points of treatment can yield valuable information for care. Additionally, the interaction of the patient and physician has itself been documented as affecting the health status of patients (IOM, 1990; IOM, 1991a).
Methods for Assessing the Quality of Care
Issues relating to the adequacy and equity of the allocation of health care resources challenge the concepts and tools used to measure quality and outcomes of care at a time when medical care and high technology are frequently integrated for achieving a common goal of prolonging life (Furman et al., 1987; Stason et al., 1987; Wenger et al., 1987). For example, Patrick and Bergner (1990) state that health-related quality-of-life measures will be used in the coming decade for “ . . . improving the quality of health care and reducing inequities in health” (p. 175).
Therapeutic interventions may have similar biological effectiveness but still differ in terms of quality-of-life outcomes of patients (Falotico-Taylor et al., 1989). Using measures to assess patients ' health status provides an opportunity for taking individual preferences for outcomes into account; thus, measuring the net benefit of health care is tailored to the particular patient and extends beyond clinical or physiological measures. This approach allows for more comprehensive technology assessments as well. Worded another way, “more important than the presence or absence of signs, symptoms, or laboratory test values is the patient's response to how treatment affects his or her life” (IOM, 1991a, p. 281).
The conceptual and methodological challenge of assessing the quality and outcomes of care for end-stage heart disease patients is of particular importance. Heart disease not only is the most common cause of death in the United States but also is one of the major causes of sudden death. Patients frequently have high hopes for treatments such as MCSSs, including delay of imminent death or the improvement of quality of life prior to death. As occurred in the mid-1980s with the use of the Jarvik-7 total artificial heart (TAH) for Barney Clark and William Schroeder, this technology attracts much media attention, including major newspapers, tabloids, television, and radio. This attention tends to raise expectations for improved outcomes from the use of high-technology medicine. On the one hand, assessment methods need to be sensitive to the unrealistic expectations of patients, providers, and the public. On the other hand, methods for assessing quality and outcomes of care should include patient-oriented measures that provide more information than the single outcome of death or life.1
Additionally, MCSSs pose various challenges for assessing quality-of-life aspects of health outcomes. First, they are more mechanical than physi
|
1 |
The recently released IOM report on computer-based patient records (IOM, 1991b) discusses the opportunities such records provide for improving both the care patients receive and the ability to retrieve information about that care, for purposes such as research. |
ological, working in a fashion similar to a pump. Second, their use has the possible unintended consequence of prolonging life for patients who suffer an adverse event and end up in a coma or dependent on another form of long-term mechanical life-sustaining treatment such as a ventilator. Third, in the case of a TAH, the device replaces an organ that has much cultural symbolism. These characteristics are not totally peculiar to MCSSs, but their compounded impact on the conceptual and methodological issues related to assessing outcomes of care should not be overlooked.
Health State Utilities
The role of health state utilities in cost-effectiveness analysis (CEA) is another example of the importance of quality-of-life assessment (see Chapter 6 and a later section of this chapter). Utility is a concept from economics and decision analysis. It refers to the preference for, or desirability of, a particular outcome. The values or preferences assigned to descriptions of the health status of end-stage heart disease patients are utilities. These preferences range from most desirable to least desirable, including states of health that may be considered less desirable than death. Utility assessment provides a means of integrating the value attributed to the worth of life at a given point (state) in time with the quantity of time (months, years) spent in various states (Williams and Wood-Dauphinee, 1989).
Rothenberg and Koplan (1990) summarized the growing interest in utility assessment:
Finally, although currently our major measures of burden and progress in the chronic diseases remain mortality and longevity, an improved quality of life is likely to emerge as the primary goal of our efforts. The concept of “quality-adjusted life years,” now used as a measurement in cost-utility analyses, may well become a concept that has practical significance for the general public. The public may become increasingly sophisticated at health equivalents and valuation, e.g., five years at full activity is worth ten years in a nursing home. Such thinking by both health care providers and consumers may well influence the way medicine is practiced. (p. 292)
Descriptions of the health states of end-stage heart disease patients provide the basis for assigning utilities to these states. As discussed later in this chapter, these health states are operational definitions of the health and quality of life of patients under different treatment options, including conventional medical treatment, MCSS, and heart transplantation.
HEALTH, HEALTH STATUS, AND QUALITY OF LIFE
Health is more than the absence of disease or illness. Thus the accurate assessment of health is not limited to the traditional clinical factors based on anatomical and physiological variables. The variables chosen to evaluate health depend primarily on what aspects of life are considered relevant to health; in turn, the variables used to evaluate the effects of health care interventions depend on the goals of the intervention.
Outcome measures might include the assessment of health-related quality of life in key facets of life experience. Patrick and his colleagues have identified five such facets: (1) life expectancy (or duration of life); (2) impairments, including symptoms, signs, and clinical indicators; (3) physical, psychological, and social functioning; (4) general health perceptions, including self-perceived health status and satisfaction with health; and (5) opportunities, including the stigma or disadvantage arising from ill health and the resilience or future capacity for health (Patrick and Elinson, 1984; Patrick and Erickson, 1988; Patrick and Bergner, 1990).
Health-related quality of life (sometimes called health status) is a concept that incorporates these key facets of life experience in what are called “domains.” The most commonly assessed domains are clinical status, physical functioning, mental health or psychological and cognitive functioning, social functioning, role functioning, and general health perceptions. Within these health domains are varying states of health, illness, disease, and well-being. Individuals and groups may assign values or preferences, both positive and negative, to these domains. These values are what distinguish “health-related quality of life” from quantity of life (Patrick and Erickson, 1988; Patrick, 1990).
A broader composite of factors contributes to or influences health-related quality of life than are included directly in the more commonly adopted set of domains of health-related quality of life. The influences of this broader set of factors can be positive or negative, and the degree and direction of influence of a given factor will vary among patients and across time for the same patient. Several of these determinants are mentioned in the literature as having particular significance to the outcomes of patients undergoing heart or kidney transplantation, hemodialysis, or coronary artery bypass graft surgery. Such determinants include (1) intensity of health care, personal health habits and attitudes, and use of health services; (2) social resources and networks of personal relationships; and (3) various economic, educational, and psychological resources (Patrick and Erickson, 1988; Patrick et al., 1988; see also Bergner, 1985).
These determinants are the context in which patients seek health care (or, as may be the case, do not seek or receive health services), and thus they, too, affect the outcomes of the encounter (IOM, 1991a). The success of the
Stanford heart transplant program during the 1970s has been attributed, in part, to stringent patient selection criteria that included some of these broader determinants, such as the presence of strong family and social support systems (Lubeck and Bunker, 1982; Christopherson, 1986). A positive outlook on life by the patient, accompanied by intra-psychic strength and interpersonal support systems, creates synergic forces to help the patient, caregivers, and professionals cope with the burdens of end-stage heart disease.
The broader concept of quality of life (in contrast to health-related quality of life) extends the relevant domains to include areas such as the environment and living situation (e.g., housing, neighborhood), employment, religious beliefs, and attitudes toward life and death. Quality-of-life domains might also include the patient's perception of the impact of his or her illness on family members and close friends (Christopherson, 1986). Figure 5.1 depicts the interrelationships of the various factors, including those in the broader quality-of-life construct, that influence the health-related quality-of-life domains.
DOMAINS OF QUALITY OF LIFE RELEVANT TO END-STAGE HEART DISEASE PATIENTS
No consensus or body of empirical research literature exists on the essential set of domains of quality of life for end-stage heart disease patients. Research findings on several diseases and conditions indicate the importance of using both generic and disease-specific tools in assessing quality of life for some patients (Rector et al., 1987; Patrick, 1990; Spilker, 1990). A particular domain, such as role functioning, may be assessed by both or only one of these types of measurement tools. Testing proposed domains through research is one major way of confirming their relevance.
Domains for Utility Assessment
In order to reflect quality of life in CEAs, such as that described in Chapter 6, several steps are necessary; this discussion focuses only on the classification of health states for the assignment of utilities or preferences (the two terms are interchangeable in this context). One of the early activities in a CEA process is the identification or selection of relevant domains of health-related quality of life. Once identified, the domains guide the development of descriptive functional attributes and perceptions of patients in the various health states undergoing analysis. The domains selected for use in this study's CEA of three patient-treatment groups are listed in Table 5.1.
Health state utilities provide the means for assessing trade-offs in health-
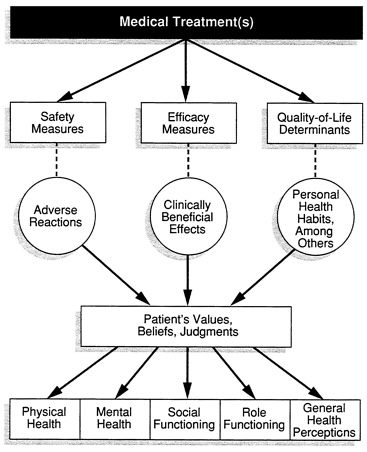
FIGURE 5.1 Model showing, from the perspective of the patient's values, how the interrelationships of clinical aspects of care and a broad composite of quality-of-life determinants influence key health-related quality-of-life domains. SOURCE: Adapted with permission from Spilker (1990).
related quality of life over time. These trade-offs occur among two or more health states defined with two or more domains. For example, social functioning might improve and self-care might deteriorate for a patient between two points in time. Additionally, changes in the patient's expectations can influence the patient's perception of the outcome. Irrespective of the degree and direction of change in any one or more domains, three general health state outcomes are possible. The determination of whether improvement, deterioration, or “no change” has occurred in health status is subjective, depending on the weights and values that are assigned by the individual making the trade-offs among the various states described.
The results or products of trade-offs can be converted to a quantitative
TABLE 5.1 Domains for Assessing Utility Measures for End-Stage Heart Disease Health States
|
Physical Vitality or energy Maximum physical activity or limitation Percent of time in bed during daytime Mental Anxiety or depression Affect or mood Cognitive Social Interpersonal contacts Role Role functioning Self-care Bathing, dressing, etc. General health perceptions Current health Discomfort or shortness of breath |
value, namely preference (utilities) weights to health states.2 In the committee's CEA (Chapter 6) these weights form the basis for calculating quality-adjusted life years. Appendix E provides more details on the CEA and how utility measures fit into the broader methodology.
Other Domains Relevant to These Quality-of-Life Issues
The study committee identified several health-related quality-of-life domains of potential significance to patients considering or having received MCSS treatment, in addition to those used in the health states for the CEA in Chapter 6. The domain that may be unique to the MCSS patient population, given the cultural symbolism of the heart, is that of machine dependence and societal reaction. Two other domains may be relevant not only to MCSS patients but also to other patients treated by exceptional therapies such as transplantation or dialysis: these domains are first, the meaning and purpose of life, and second, spiritual well-being. These three domains are
|
2 |
A more detailed explanation of health state preferences can be found in Froberg and Kane, 1989a, 1989b, 1989c, 1989d; Patrick and Erickson, in press. |
discussed here for the purpose of stimulating new research directions and conceptualization on health-related quality of life for patients considering or choosing unconventional forms of therapy in an effort to sustain life.
Machine Dependence and Societal Reaction
The TAH has two characteristics as a medical device that may be of particular significance to the patient's quality of life: It replacesrather than supportsor assistsa natural organ, and the organ replaced is highly symbolic. These characteristics may have a compounded effect on the MCSS patient. The patient's quality of life may change, positively or negatively, because of the patient's reaction to being machine dependent and to the partial or total excision of his or her heart. The responses of other people, such as family, friends, and even strangers, to the patient as well as the patient's perceptions of these responses may have both positive and negative influences on the patient's self-image.
In some instances, particularly when the TAH technology is new, the MCSS recipient may be perceived as exceptionally brave and appealing to know, and may receive frequent positive responses from friends and acquaintances; the recipient may also find satisfaction from attention provided by the media. In other instances, particularly if TAH use becomes common, recipients may be perceived as “different ” or “handicapped” when compared with a “well person.” Goffman's term, “spoiled identity” (1963), depicts the potentially negative influence that societal stigmatization can have on one's self-image, an influence that may be quite powerful for TAH patients. The costs that are borne by society or third-party payers to provide MCSS treatment may also raise questions for others as to whether the patient is “worth” the expense; such questions may create doubts in the patient of his or her worthiness.
Other, more philosophical aspects of health status, including some perhaps not yet identified, may be significant for patients treated with a TAH because of the unusualness of a machine heart. For example, will the patient feel like a “whole person”? Will the patient anticipate disengagement from emotions perceived as being centered in the natural heart, such as love? What, if any, relationship will exist between these subjective experiences and the patient's health-related quality of life? How will these experiences be manifest in the patient's behavior and affect other domains such as social functioning?
Meaning and Purpose of Life Versus Fear of Death
The value of living is a personal concept and not one easily measured in quantitative terms. Individuals confronted with life-threatening conditions
may choose treatments that sustain life because, on the one hand, they perceive their present and future life as having meaning and purpose, or, on the other hand, they have a fear of dying. Some patients may struggle with both feelings and, through the therapeutic process, find an increase in the meaning to life and a decrease in fear of dying.
This subjective sense of meaning to life is similar to a sense of personal dignity; one's life has meaning irrespective of external adversities. The domain of meaning and purpose of life differs conceptually from the global domain of life satisfaction. The former implies an actively initiating role of the self (George and Clipp, 1991); the latter implies an act of judgment of an individual on his or her interaction with external or objective life resources or conditions such as illness, socioeconomic status, and relationships to others (Patrick and Erickson, 1988).
Additional concepts in this area of potential relevance to MCSS patients are the finiteness of life, will to live, imminence of death, and readiness for death. These concepts overlap somewhat, but they also have unique elements; to what extent and in what time frame the differences across these concepts are significant and relevant to health-related quality of life for the MCSS patient remain to be confirmed.
Spiritual Well-Being
Social scientists have developed the concepts of religion, religiosity, and spirituality within theoretical constructs useful in research (Ellison, 1983; Payne, 1990; Blazer, 1991). Health services researchers have been slow to adopt or adapt these concepts and theories, perhaps in part because the historical focus of social science in these areas has tended to be on operational measures such as membership and participation in organized religious functions.
Nevertheless, health services researchers recognize that the extended, more broadly defined domains of quality of life include spiritual or religious beliefs. For certain patients, such as those with life-threatening conditions, spiritual well-being may in fact be a significant health-related quality-of-life domain rather than a domain in the more extended general area of quality of life.
QUALITY OF LIFE AND ITS DETERMINANTS FOR PATIENTS WITH END-STAGE HEART DISEASE
Some studies have been published on patients' quality of life after receiving different therapies, procedures, and devices for treatment of heart disease. Little empirical research has been conducted on the quality of life of patients who have received MCSSs (see Ruzevich et al., 1990). The
literature does include anecdotal and case studies of MCSS-treated patients, and quality of life is an often-mentioned characteristic without a standardized definition. Limited inferences on quality of life can be drawn from studies of somewhat similar treatments (such as dialysis) and applied to MCSS patients (Lubeck and Bunker, 1982); broad inferences from studies of patients having illnesses or conditions other than end-stage heart disease should be made only with great caution.
Some of the more common domains of quality of life historically reported by patients include functional levels and symptoms, and recent research includes quantitative measures of these domains (Kaplan and Anderson, 1990). Decreases in functional levels and the presence of discomfort symptoms (e.g., fatigue, breathlessness, sleeplessness) are problems frequently noted by patients with congestive heart failure (Rector et al., 1987; Tandon et al., 1988).
The Medical Outcomes Study (Stewart et al., 1989) provided an opportunity to compare the functional status and well-being of patients with congestive heart failure with the same domains among eight other patient groups having different chronic diseases, including comorbidities. Interrelationships were found among emotional well-being, health perceptions, and physical functioning for both mental and physical disorders in all nine patient groups. Chronic conditions were found to be the factor, among all health measures, that had the greatest negative effect on functioning and well-being. Much of the variance in well-being was not accounted for by the presence or severity of the diseases themselves; other variables such as personal factors and medical care may contribute to the variances (Stewart et al., 1989; Greenfield, 1990).
One must also be careful when drawing conclusions about the impact of particular therapeutic interventions on the quality of life of patients (Walden et al., 1989). In elderly patients with cardiovascular disease, physical and psychosocial symptoms tend to overlap; depression is common and thought to have prognostic significance (Gentry et al., 1987). These researchers report that older people (65 years of age or older) have inappropriately low expectations for functional capacity and other outcome measures following treatment and rehabilitation from a myocardial infarction. These low expectations are correlated with passive rehabilitation behavior as well as behavioral and attitudinal tendencies that perpetuate “sick-role” dependency over long periods of time.
Heart Transplantation
Comparison of the quality-of-life outcomes of organ transplantation with those of other treatments provides some insight to the health status of different patient groups. However, the dearth of research involving control
groups, in particular control groups of patients not accepted for transplantation, leaves many questions unanswered about patient preferences and outcomes of alternate treatments to transplantation.
Similar to patients with congestive heart failure, heart transplant recipients also report impairment of physical functioning but tend to rate their overall quality of life as “good” on subjective evaluations (Evans et al., 1984; Lough et al., 1985). Walden and colleagues (1989) reported that the quality of life of transplant patients differed little from that of survivors of sustained medical therapy. Psychosocial functioning (feelings of anxiety, depression, and hostility) was among the domains studied; both groups scored poorly in this domain. Patients with stable heart failure reported more dysfunction in social and leisure activities than did transplant patients, and heart failure patients viewed their overall functional status to be lower than did heart transplant recipients.
These findings did not support the researchers' expectations that a higher level of emotional benefits would be reported among the heart transplantation study population. The authors are of the opinion that many patients are unwilling to undergo the rigors of transplant surgery to gain only an extension of life; patients want to be guaranteed that the transplant will also improve their quality of life (Walden et al., 1989).
Guarantees are rare in health care, however, and patients react differently to the “unknowns” of health care. For example, organ transplantation involves a high level of uncertainty for many patients: uncertainty of being accepted for a transplant, uncertainty of the availability of an organ within the time constraints, and uncertainty of outcome after transplantation (Christopherson, 1986). Uncertainty is frequently accompanied by fear; thus, the quality of life of implant and transplant patients may be related to the presence and degree of fear, e.g., “fear of sudden death or failure of the graft or machine leading inevitably to death” (Christopherson, 1986, p. 556).
Automatic Implantable Cardioverter Defibrillator
The psychosocial effects of implantation of an automatic implantable cardioverter defibrillator (AICD), a costly new treatment for intractable arrhythmias, have been studied by several investigators. Cooper et al. (1986) reported an association between AICD implantation and multiple physical, social, and psychological alterations; patients tended to report having fears of premature battery failure and shock resulting from the device discharging.
Other investigators of patients having AICD implantation found higher degrees of both anger and anxiety in pre- and postimplantation patients compared with normal controls or with other medically ill populations
(Vlay et al., 1989). These researchers speculate that personality traits of anxiety and anger have the potential to influence outcome adversely.
Pycha and colleagues (1986) also studied the relationship of postimplant adjustments to AICD patients' personality styles, attitudinal and philosophical beliefs about quality of life, severity of the illness, and ability to return to work. Many patients reported having unpleasant feelings during the postimplant stage: anxiety, fear, depression, and loss of security and control; moderate levels of self-doubt and helplessness; and high levels of emotional upset and distress. Most of these feelings decreased over time.
Providing Support in the Postoperative Phase
Assessing health-related quality-of-life outcomes implies that a relationship exists between health care interventions and the patient 's health status. Because health care is a continuum of events for the MCSS patient, the postimplantation processes of care, including the patient-provider relationship, have significant potential for influencing clinical indicators or clinical endpoints. Postoperative patient management that includes active interventions such as counseling and educational services can improve patients' quality of life. In many circumstances, family and other significant friends should participate in these sessions (Christopherson, 1986; Pycha et al., 1986; Ruzevich et al., 1990).
Learning in the postoperative phase is not limited to one direction, from health professionals to patients. Patients and family members are a vast resource of knowledge and support, a resource frequently overlooked by health care providers, researchers, and other patients and their families.
Perspectives from Prior Studies of the Artificial Heart
The National Heart and Lung Institute Artificial Heart Assessment Panel (NHLI, 1973) concluded that many patients who will eventually have TAHs will experience anxiety-related psychological burdens and perhaps psychotic reactions. The panel noted that patient concerns may focus on financial worries that in turn may create severe guilt and intrafamilial tensions, feelings of “dehumanization” because of the particular organ replaced with an artificial device, and negative health consequences because of the source of power for the device. (The last of these concerns was noted by the panel because nuclear power was one of three power modes then under consideration.)
An Office of Technology Assessment (OTA) report (Lubeck and Bunker, 1982) on the cost, risks, and benefits of the artificial heart draws some inferences from studies of heart transplant, hemodialysis, and kidney transplant patients for the quality of life of TAH-implanted patients. The study identified several burdens TAH patients may encounter:
-
feelings of depression, rapid mood changes, guilt, insecurities about self-image, and insecurities and stress about the potential for sudden death;
-
role and identity confusion such as reversal of dependency roles between spouses and difficulties of adjustment to the new role of not being a “sick” patient; and
-
inconvenience and anxiety about device maintenance.
The OTA report suggests that the TAH patient's ability to cope may relate to the degree to which society accepts the TAH, especially from the perspective of a general societal concern over a growing dependence on medical technology.
The National Heart, Lung, and Blood Institute (NHLBI) Working Group on MCSS (NHLBI, 1985) noted that even though MCSS recipients will be able to engage in many normal ambulatory activities and moderate exercise, they will not be able to forget about the device. The working group concludes:
There is no way of predicting with any certainty the quality of life. Considering the clinical circumstances of the recipient when the device is implanted, the anticipated benefits are substantial, but the possible complications and side effects are also significant. Only experience will establish how these balance out. However, it is quite plausible that with appropriate selection of recipients, they will look upon their lives as being of good quality. (p. 27)
IMPLICATIONS OF QUALITY-OF-LIFE CONSIDERATIONS IN CLINICAL TRIALS AND STUDIES OF MECHANICAL CIRCULATORY SUPPORT PATIENTS
Concepts and Methods
Assessing the quality of life in clinical trials and follow-up studies of patients receiving MCSSs is fraught with methodological, funding, and policy issues. For instance, although disease-specific measures for end-stage heart disease patients are important, no consensus exists on whether to incorporate such measures in existing generic instruments or develop unique instruments for this population (Evans et al., 1984; Lough et al., 1985; Wallwork and Caine, 1985; Patrick, 1990; Patrick, in press) On the one hand, some researchers and clinicians question the sensitivity of existing generic instruments to ascertain the preferences of condition-specific populations (Sechrest and Pitz, 1987; Cleary, 1990). On the other hand, most studies of cardiovascular patients use generic measures rather than disease-specific instruments to assess heart disease and treatment effects. Further work is needed in instrument development and validation to deter-
mine which domains are important to particular patient populations and, within domains, which components are sensitive to “burdens” perceived by patients (Miles et al., 1988).
The Nottingham Health Profile (NHP) is an example of a generic, validated instrument used to assess the quality of life in studies with cardiac patients (Wallwork and Caine, 1985; O'Brien et al., 1987; see also Falotico-Taylor et al., 1989). Part I of the NHP measures six dimensions of social functioning: physical mobility, pain, sleep, energy, social isolation, and emotional reaction; Part II consists of measures of the effects of health problems in areas such as occupation and sexual functioning.
In contrast, the Living with Heart Failure Questionnaire (LHFQ) was developed for a specific population, namely congestive heart failure patients (Rector et al., 1987). It is a self-administered questionnaire that covers many of the same domains as found in the NHP; the basic difference is in the wording of the individual measures. All 21 questions found in the LHFQ begin with a reference to the patient's condition: “Did your heart failure prevent you from living as you wanted during the last month by making your relating to or doing things with your friends or family difficult?” (Rector et al., 1987, p. 206)
Figure 5.2 depicts the theoretical relationships among different health-related quality-of-life concepts relevant to end-stage heart disease and shows the interactive influences of the disease and behavioral, perceptual, and social determinants (Patrick and Bergner, 1990). This figure reflects the complexity of the subject and, at the same time, points out the importance of a theoretical base for future research studies.
Four perspectives are relevant to the selection of instruments for clinical trials and evaluative studies, such as quality-of-life assessments for end-stage heart disease patients receiving MCSSs or conventional medical or surgical treatment: (1) adequacy of the conceptual content; (2) methodological issues of different measurement strategies; (3) practical considerations relating to data collection, editing, analysis, and interpretation; and (4) the interrelationship of the design and purpose of a study with conceptual, methodological, and practical considerations (Patrick, in press).
Another issue arises when a trial or study includes a cost-effectiveness analysis. Preference-based (i.e., utility) measures are desirable for cost-effectiveness analyses, such as the one described in Chapter 6. Nevertheless, they are frequently challenged for presumed lack of stability, validity, and reliability; further research is needed to determine the best way to undertake such measures.
Other concerns about clinical trials relate to (1) statistical bias, in particular when either study populations do not include variables such as multi-geographic and treatment site representation or numbers in such cells are
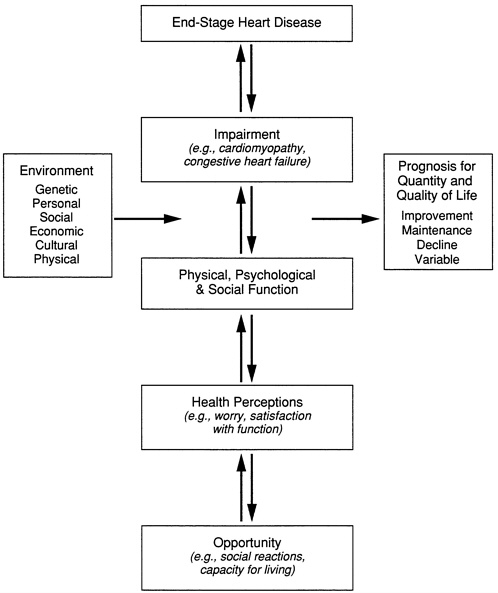
FIGURE 5.2 Theoretical relationships among health-related quality-of-life concepts. SOURCE: Adapted from Patrick and Bergner, 1990. Reproduced, with permission, from the Annual Review of Public Health, Vol. 11, © 1990 by Annual Reviews Inc.
small; (2) problems with establishing control groups, in particular when study designs are attempting to assess net gains or losses using several outcome measures such as quality of life; and (3) limitations on the follow-up period because of the short life expectancy of many in both the control and experimental groups (O'Brien et al., 1987; see also Tuteur and Tuteur,
1990). These concerns frequently can be addressed through the study design but resource limitations for conducting clinical trials create pressures to take shortcuts.
To the extent that policymakers judge it to be in the public interest to use public resources to develop the ventricular assist device and TAH, their commitment should include adequate support for comprehensive assessments of health-related quality of life in patients receiving MCSSs during clinical trials and even thereafter. If they do not follow through on such a commitment, major policy decisions addressing access to and equality of health care and patient-physician decision making on the appropriateness of care for end-stage heart disease patients will be made using “convenience” criteria and data that may perhaps be inappropriate or inadequate to the task.
Costworthiness: Patients' and Societal Perspectives
Patients need information that will assist them in making decisions that are costworthy, and individuals should be able to participate in determining which health care interventions are deemed to be costworthy. Just as patients have different goals and expectations of health care, they assign different values to the outcomes of care.3 Health providers also need information that will help in defining populations for whom clinically effective technologies are appropriate and in planning for the support systems that may enhance quality of life in the postintervention stages (Sechrest and Pitz, 1987; Kaplan and Anderson, 1990).
When differences occur between patients' and society's determinations of the costworthiness of specific health technologies, the greater is the need for information that delineates the domains or outcome measures in which the variations in values and preferences arise. The scarcity of information on outcomes that include health-related quality-of-life measures relative to specific technologies may hinder efforts to make comprehensive coverage decisions that include a criterion on costworthiness.
SUMMARY AND CONCLUSIONS
One important aspect of treatment alternatives for end-stage heart disease is the quality of life of the patient. This chapter discusses the meaning and importance of health-related quality of life within a conceptual frame-
|
3 |
The importance of considering patients' preferences in health care decisions is the subject of much work by Wennberg, Mulley, and their colleagues. For more details, see Barry et al., 1988; Wennberg et al., 1988; Mulley, 1989, 1990. |
work depicting the relevant domains to be measured. Additional potential domains are proposed that may have special significance for patients considering or choosing unconventional forms of therapy, such as the total artificial heart, for life-threatening conditions.
This review of the concepts and theoretical base of health-related quality of life in the context of end-stage heart disease patients and MCSS interventions supports the following conclusions:
-
Quality of life is an important criterion of successful treatment and should be assessed in clinical trials with heart disease technologies.
-
A core set of domains for quality-of-life assessment, similar to those used in the utility measures in this report, should be included in all MCSS clinical trials; clinical trials should receive adequate funding to assess health-related quality of life in the core domains. The committee is aware that the Office of the Director of the National Institutes of Health (NIH) has expressed interest in all institutes ' incorporating a standard core set of domains in all appropriate NIH-sponsored clinical trials; the committee endorses continued efforts of this nature.
-
The need exists to identify and verify a core set of disease-specific domains and respective measures for patients with end-stage heart disease. Such efforts should involve all parties having an interest in the measures, including clinicians treating end-stage heart disease patients.
-
The need exists for more research to identify and understand support systems and selected other determinants of health-related quality of life that might be helpful in identifying those patients, among the groups meeting certain clinical conditions, who are more likely to benefit from MCSS treatments.
-
The success of the post-MCSS treatment phase is influenced by several variables, including the psychosocial support provided by the team of health care professionals. Attention should be given to the importance of support services for enhancing the patient's quality of life and minimizing the damage or negative aspects of MCSS treatment; services such as primary nursing care in the home, educational and counseling programs to family and informal caregivers, and patient support groups are thought to have particular supportive value. Researchers should be encouraged to increase knowledge in these areas.
-
All NHLBI-sponsored research and clinical trials that include cost-benefit or cost-effectiveness analyses should include preference-weighted or utility measures in the analysis design. Utility assessments are important if CEA is to be done correctly.
REFERENCES
Barry, M. J., A. G. Mulley, Jr., F. J. Fowler, Jr., and J. W. Wennberg. 1988. Watchful waiting vs. immediate transurethral resection for symptomatic prostatism: The importance of patients' preferences. Journal of the American Medical Association 259:3010-3017.
Bergner, M. 1985. Measurement of health status. Medical Care 23:696-704.
Blazer, D. 1991. Spirituality and aging well. Generations (Winter) pp. 61-65.
Christopherson, L. K. 1986. Organ transplantation and artificial organs. International Journal of Technology Assessment in Health Care 2:553-562.
Cleary, P. D. 1990. Using patient reports of outcomes to assess effectiveness of medical care. In: Institute of Medicine. Effectiveness and Outcomes in Health Care. K. A. Heithoff and K. N. Lohr, eds. Washington, D.C.: National Academy Press, pp. 152-159.
Cooper, D. K., R. M. Luceri, R. J. Thurer, and R. J. Myerburg. 1986. The impact of the automatic implantable cardioverter defibrillator on quality of life. Clinical Progress in Electrophysiology and Pacing 4:306-309.
Ellison, G. W. 1983. Spiritual well-being: Conceptualization and measurement. Journal of Psychology and Theology 11:330-340.
Evans, R. W., D. L. Manninen, T. D. Overcast, L. P. Garrison, Jr., J. Yagi, K. Merrikin, et al. 1984. The National Heart Transplantation Study. Vol. 3. Survival, Quality of Life, Cost. Seattle, Wash.: Battelle Human Affairs Research Centers.
Falotico-Taylor, J., M. McClellan, and F. Mosteller. 1989. The use of quality-of-life measures in technology assessment. In: Institute of Medicine. Quality of Life and Technology Assessment. F. Mosteller and J. Falotico-Taylor, eds. Washington, D.C.: National Academy Press, pp. 7-44.
Froberg, D. G., and R. L. Kane. 1989a. Methodology for measuring health-state preferences—I: Measurement strategies. Journal of Clinical Epidemiology 42:345-354.
Froberg, D. G., and R. L. Kane. 1989b. Methodology for measuring health-state preferences—II: Scaling methods. Journal of Clinical Epidemiology 42:459-471.
Froberg, D. G., and R. L. Kane. 1989c. Methodology for measuring health-state preferences—III: Population and context effects. Journal of Clinical Epidemiology 42:585-592.
Froberg, D. G., and R. L. Kane. 1989d. Methodology for measuring health-state preferences—IV: Progress and a research agenda. Journal of Clinical Epidemiology 42:675-685.
Furman, S., E. A. Stead, Jr., H. J. C. Swan, and B. L. Zaret. 1987. Application of high technology in the diagnosis and treatment of the elderly. 18th Bethesda Conference: Cardiovascular Disease in the Elderly. Journal of the American College of Cardiology 10(Suppl. A):22A-24A.
Galletti, P. M. 1984. Replacement of the heart with a mechanical device: The case of Dr. Barney Clark. New England Journal of Medicine 310:312-314.
Gentry, W. D., M. K. Aronson, J. Blumenthal, P. T. Costa, Jr., and J. N. DiGiacomo. 1987. Behavioral, cognitive and emotional considerations. 18th Bethesda Conference: Cardiovascular Disease in the Elderly. Journal of the American College of Cardiology 10(Suppl. A):14A-17A.
George, L. K., and E. C. Clipp. 1991. Subjective components of aging well. Generations (Winter) pp. 57-60.
Goffman, E. 1963. Stigma: Notes on the Management of Spoiled Identity. Englewood Cliffs, N.J.: Prentice Hall, Inc.
Greene, A. C. 1990. Taking Heart. New York: Simon and Schuster.
Greenfield, S. 1990. Outcome assessment. Society of General Internal Medicine News 13(3):17.
IOM (Institute of Medicine). 1990. Medicare: A Strategy for Quality Assurance. Vol. 1. K. N. Lohr, ed. Washington, D.C.: National Academy Press.
IOM. 1991a. Kidney Failure and the Federal Government. R. A. Rettig and N. G. Levinsky, eds. Washington, D.C.: National Academy Press.
IOM. 1991b. The Computer-Based Patient Record: An Essential Technology for Health Care. R. S. Dick and E. B. Steen, eds. Washington, D.C.: National Academy Press.
Jung, C. 1975. The stages of life. In: J. Campbell, ed. The Portable Jung(translated by R. F. C. Hull). New York: Viking Press.
Kaplan, R. M., and J. P. Anderson. 1990. The general health policy model: An integrated approach. In: B. Spilker, ed. Quality of Life Assessments in Clinical Trials. New York: Raven Press, pp. 131-149.
Lough, M., A. Lindsey, J. Shinn, and H. Stotts. 1985. Life satisfaction following heart transplantation. Journal of Heart Transplantation 4:446-449.
Lubeck, D. P., and J. P. Bunker. 1982. Case Study #9: The Artificial Heart: Cost, Risk, and Benefits. In: Office of Technology Assessment. The Implications of Cost-Effectiveness Analysis of Medical Technology: Background Paper #2: CaseStudies of Medical Technologies. Washington, D.C.: U.S. Government Printing Office.
Miles, S. H., M. Siegler, D. L. Schiedermayer, J. D. Lantos, and J. La Puma. 1988. The total artificial heart—An ethics perspective on current clinical research and deployment . Chest 94:230-231, 409-413.
Mulley, A. G., Jr. 1989. Assessing patients' utilities. Can the ends justify the means? Medical Care 27(Suppl.):S269-S281.
Mulley, A. G., Jr. 1990. Applying effectiveness and outcomes research to clinical practice . In: Institute of Medicine. Effectiveness and Outcomes in Health Care. K. A. Heithoff and K. N. Lohr, eds. Washington, D.C.: National Academy Press, pp. 179-189.
NHLI (National Heart and Lung Institute). 1973. The Totally Implantable Artificial Heart: Legal, Social, Ethical, Medical, Economic, Psychological Implications. A Report of the Artificial Heart Assessment Panel of the National Heart and Lung Institute. Rockville, Md.: National Heart and Lung Institute.
NHLBI (National Heart, Lung, and Blood Institute). 1985. Artificial Heart and Assist Devices: Directions, Needs, Cost, Societal and Ethical Issues. The Working Group on Mechanical Circulatory Support of the National Heart, Lung, and Blood Institute. Rockville, Md.: National Heart, Lung, and Blood Institute.
O'Brien, B. J., M. J. Buxton, and B. A. Ferguson. 1987. Measuring the effectiveness of heart transplant programmes: Quality of life data and their relationship to survival analysis. Journal of Chronic Disease 40(Suppl. 1):137S-153S.
Patrick, D. L. 1990. Assessing health-related quality of life outcomes. In: Insti
tute of Medicine. Effectiveness and Outcomes in Health Care. K. A. Heithoff and K. N. Lohr, eds. Washington, D.C.: National Academy Press, pp. 137-151.
Patrick, D. L. In press. Quality of life concepts and measures in cardiovascular research. In: S. A. Shumaker, C. Furberg, and N. Wenger, eds. Research on Quality of Life and Cardiovascular Disease. Special Issue . The American Journal of Preventive Medicine, New York: Oxford University Press.
Patrick, D. L., and M. Bergner. 1990. Measurement of health status in the 1990s. Annual Review of Public Health 11:165-183.
Patrick, D. L., and J. Elinson. 1984. Sociomedical approaches to disease and treatment outcomes in cardiovascular care. Quality of Life in Cardiovascular Care 1:53-62.
Patrick, D. L., and P. Erickson. 1988. What constitutes quality of life? Concepts and dimensions. Clinical Nutrition 7(2):53-63.
Patrick, D. L., and P. Erickson. In press. Health Status and Quality of Life: A Guide to Measurement. New York: Oxford University Press.
Patrick, D. L., J. Stein, M. Porta, C. W. Porter, and T. C. Ricketts. 1988. Poverty, health services, and health status: Lessons from rural America . Milbank Quarterly 66:105-136.
Payne, B. P. 1990. Research and theoretical approaches to spirituality and aging. Generations (Fall) pp. 11-14.
Pycha, C., A. D. Gulledge, J. Hutzler, N. Kadri, and J. Maloney. 1986. Psychological responses to the implantable defibrillator: Preliminary observations. Psychosomatics 27:841-845.
Rector, T. S., S. H. Kubo, and J. N. Cohn. 1987. Patients' self-assessment of their congestive heart failure. Heart Failure (October/November) pp. 198-209.
Rothenberg, R. B., and J. Koplan. 1990. Chronic disease in the 1990s. Annual Review of Public Health 11:267-296.
Ruzevich, S. A., M. T. Swartz, J. E. Reedy, D. F. Termuhlen, L. R. McBride, S. M. Frese, et al. 1990. Retrospective analysis of the psychologic effects of mechanical circulatory support. Journal of Heart Transplantation 9:209-212.
Sechrest, L., and D. Pitz. 1987. Commentary: Measuring the effectiveness of heart transplant programmes . Journal of Chronic Disease 40(Suppl. 1):155S-158S.
Shaw, M. W., ed. 1984. After Barney Clark: Reflections on the Utah Artificial Heart Program. Austin, Tex.: University of Texas Press.
Spilker, B. 1990. Introduction. In: B. Spilker, ed. Quality of Life Assessments in Clinical Trials. New York: Raven Press, pp. 3-9.
Stason, W. B., C. A. Sanders, and H. C. Smith. 1987. Cardiovascular care of the elderly: Economic considerations. 18th Bethesda Conference: Cardiovascular Disease in the Elderly. Journal of the American College of Cardiology 10(Suppl. A):18A-21A.
Stewart, A. L., S. Greenfield, R. D. Hays, K. Wells, W. H. Rogers, S. D. Berry, et al. 1989. Functional status and well-being of patients with chronic conditions . Journal of the American Medical Association 26:907-913.
Swazey, J. P., R. C. Fox, and J. C. Watkins. 1989. The artificial heart: A case study of social and ethical issues posed by advanced medical technology. Final report to the National Science Foundation and the National Heart, Lung and Blood Institute. Grant No. BBS 8418994 .
Tandon, P. K., H. Stander, S. H. Dyke, T. J. Massey, R. DiBianco, and R. P. Schwarz. 1988. Assessment of the quality of life of patients with heart failure: A randomized, controlled drug trial. Heart Failure 4:39-48, 53-54.
Tuteur, P. G., and S. D. Tuteur. 1990. Life-sustaining therapies in elderly persons. Journal of the American Medical Association 264:2118.
Vlay, S. C, L. C. Olson, G. L. Fricchione, and R. Friedman. 1989. Anxiety and anger in patients with ventricular tachyarrhythmias. Responses after automatic internal cardioverter defibrillator implantation . PACE 12:366-373.
Walden, J. A., L. W. Stevenson, K. Dracup, J. Wilmarth, J. Kobashigawa, and J. Moriguchi. 1989. Heart transplantation may not improve quality of life for patients with stable heart failure. Heart & Lung 18:497-506.
Wallwork, J., and N. Caine. 1985. A comparison of the quality of life of cardiac transplant patients and coronary artery bypass graft patients before and after surgery . Quality of Life in Cardiovascular Care 1:317-324, 331.
Wenger, N. K., F. I. Marcus, and R. A. O'Rourke. 1987. Cardiovascular disease in the elderly. 18th Bethesda Conference: Cardiovascular Disease in the Elderly. Journal of the American College of Cardiology 10(Suppl. A):80A-87A.
Wennberg, J. E., A. G. Mulley, Jr., D. Hanley, R. P. Timothy, F. J. Fowler, Jr., N. P. Roos, et al. 1988. An assessment of prostatectomy for benign urinary tract obstruction: Geographic variations and the valuation of medical care outcomes . Journal of the American Medical Association 259:3027-3030.
Williams, J. I., and S. Wood-Dauphinee. 1989. Assessing quality of life: Measures and utility. In: Institute of Medicine. Quality of Life and Technology Assessment. F. Mosteller and J. Falotico-Taylor, eds. Washington, D.C.: National Academy Press, pp. 65-115.






















