This chapter presents an overview of the exposure considerations for lead that are relevant to firing ranges, including an overview of the routes of exposure, factors that affect internal doses of lead, and exposure factors that influence health outcomes.
Several terms are used differently by toxicologists and epidemiologists, so definitions are provided for terms as they are used here. Dose is the amount of a substance to which a person is exposed over some period. An exposure dose is how much of a substance is encountered in the environment. An absorbed dose or internal dose is the amount of a substance that gets into the body through the eyes, skin, stomach, intestines, or lungs. One needs to differentiate exposure (lead outside the body) from the internal dose.
On a firing range, exposure to lead arises through two primary media: air and surfaces. Absorption in general can occur through four primary routes: inhalation, ingestion, transdermal, and percutaneous. The latter two routes are not relevant to firing ranges, because inorganic lead is not normally absorbed through the skin, nor is lead injected percutaneously in this setting. Concerning the inhalation route, particle size is an important determinant of internal dose. Smaller particles, like those associated with fumes (under 0.1 μm in aerodynamic diameter), have large relative surface areas and are generally better absorbed than larger particles by both the inhalation and ingestion routes. Fume and particles under 5 μm are also inhaled more deeply into the lungs and are better absorbed than larger particles at the alveolar-capillary interface. However, smaller fume particles may agglomerate into larger particles before inhalation or ingestion. About 50% of the lead deposited in the respiratory tract is absorbed and reaches the systemic circulation whereas net absorption of ingested lead from the adult digestive tract is appreciably lower (less than 8% to 10%) (O’Flaherty 1993).
Juhasz (1977) qualitatively demonstrated that lead-based ammunition was associated with the generation of particles ranging from under 0.3 to 100 μm; most of the particles were smaller than 1 μm. That observation is similar to results regarding the particle size of copper associated with the use of lead-based or lead-free frangible ammunition. For example, the Air Force Institute for Occupational Health (AFIOH 2008) reported that most airborne copper particles associated with an M4 rifle muzzle blast have an aerodynamic diameter under 5 μm. The committee was unable to obtain similar quantitative data on particle size distributions associated with lead-based ammunition used in different small-arms weapons.
The basis of the general industry lead standard of the Occupational Safety and Health Administration (OSHA) and a large scientific literature (IARC 2006; ATSDR 2007; EPA 2012; NTP 2012) document that several personal behaviors can increase lead dose, including tobacco-smoking, eating, and drinking in the workplace and inadequate personal hygiene before leaving the workplace. The OSHA standard therefore mandates no eating, drinking, or smoking in areas that have potential lead exposure and separate facilities for changing clothes and washing before returning home.
The toxicokinetics of lead—that is, its absorption, distribution, metabolism, and excretion—and its relevance to common biomarkers of exposure are schematically represented in Figure 3-1.
Understanding lead’s partitioning in the body provides a useful background for understanding the available biomarkers of lead. After gastrointestinal or pulmonary absorption, lead enters the bloodstream, in which the vast majority of circulating lead (over 95%) is bound to erythrocyte proteins and the remainder is associated with the plasma (Barltrop and Smith 1972; Cake et al. 1996; Bergdahl et al. 1997), before reaching target organs. Lead is distributed widely in the body and can gain access to sites in the central and peripheral nervous, cardiovascular, renal, reproductive, musculoskeletal, hematopoietic, and other organ systems (see reviews by Hu et al. 2007; EPA 2012; NTP 2012). Lead binds to sulfhydryl and carboxyl groups on a wide variety of structural and functional proteins (Rabinowitz et al. 1973), thereby altering their structure or function. Lead can also agonize or antagonize calcium and thereby alter its normal metabolic functions (Rabinowitz 1991). The ability to mimic calcium contributes to lead storage in the bone; at equilibrium, lead-exposed persons have a substantial body burden of lead: over 90% in the bone pool, 2-8% in various soft tissues, and 2-5% in blood (Rabinowitz et al. 1976). In addition, lead’s nonspecific binding to a variety of proteins and its involvement in calcium pathways explain in large part its myriad health effects. Lead is excreted primarily in urine; this pathway can be enhanced by intravenous chelating agents, such as calcium disodium ethylene diamine tetraacetic acid (commonly referred to as
CaNa2EDTA), and oral chelating agents, such as 2,3-dimercaptosuccinic acid (commonly referred to as DMSA) (Graziano et al. 1985). A much smaller proportion of absorbed lead is excreted in feces, sweat, breast milk, seminal fluid, and hair.
A number of factors can influence the toxicokinetics of lead. Several aspects of nutrition—including iron, zinc, and calcium status and supplementation—can influence the gastrointestinal absorption and distribution of lead. Diets low in calcium or high in lactose or fat have been reported to enhance lead accumulation (Goyer 1995). Studies have shown that iron-deficient children have higher gastrointestinal absorption of lead (Barton et al. 1978). Sex differences in lead toxicokinetics have also been reported. Some studies have shown that blood lead levels (BLLs) varied by geographic areas, were higher in men than in women, and were higher in smokers than in nonsmokers (Friberg and Vahter 1983). Analysis of BLLs in monozygotic and dizygotic twins provided evidence of a genetic factor’s regulating BLLs in females but not in males (Bjorkman et al. 2000). Yang et al. (2007) reported a small but statistically significant increase in BLLs of about 7.6% from a baseline of 2.64 μg/dL in teenage girls during menstruation, but the underlying factors related to this observation remain unknown. Lactating and postmenopausal women can mobilize lead stores from bone (Hernandez-Avila et al. 2000; Ettinger et al. 2004).
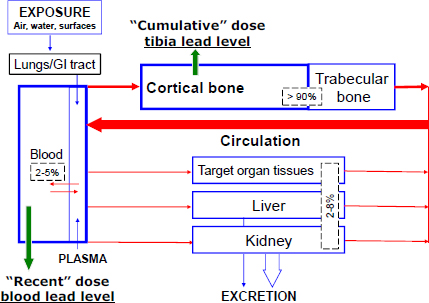
FIGURE 3-1 Compartmental model for lead (modified from O’Flaherty 1993). The percentages shown represent the fractions of lead found in different tissue compartments. Used with permission of Brian Schwartz, Johns Hopkins Bloomberg School of Public Health.
A number of genetic polymorphisms can influence the toxicokinetics of lead (Wetmur et al. 1991; Wetmur 1994; Lee et al. 2001a; Hu et al. 2007). A large number of genetic polymorphisms have been studied in relation to lead, including polymorphisms of the delta-aminolevulinic acid dehydratase (ALAD), the vitamin D receptor (VDR), apolipoprotein E (APOE), Na+,K+-ATPase, endothelial nitric oxide synthase (eNOS), and human hemochromatosis protein (HFE) genes. The two best-studied polymorphisms are those of the ALAD and VDR genes, which are relatively common; the less common allele is present in 5-25% of people, depending on race and ethnicity (Lee et al. 2001b). In a number of study populations and designs, the ALAD2 allele has been associated with higher BLLs (although recent studies with lower average BLLs are inconsistent on this point [Krieg et al. 2009]), lower DMSA-chelatable lead levels, lower zinc protoporphyrin levels for a given BLL, lower plasma aminolevulinic acid levels (Sithisarankul et al. 1997), and a variety of renal effects (Smith et al. 1995). In addition, there is evidence that the ALAD2 allele may contribute to selection factors: it has been associated with longer work duration in the lead industry and is more prevalent in factories that have higher exposure to lead (Schwartz et al. 1995). In studies of lead workers, the VDR B allele has been associated with higher blood pressure (Lee et al. 2001b), tibia lead, DMSAchelatable lead, and BLL (Schwartz et al. 2000a), and there are different associations of age and work duration with tibia lead levels (Schwartz et al. 2000b). Thus, there is compelling evidence that genetic polymorphisms modify the toxicokinetics of lead. However, the committee agreed with a prior review that concluded that “although recent studies suggest that polymorphisms in specific genes may modify the toxicokinetics [of lead] … research findings at present are insufficient to conclusively identify genotypes that confer increased [health] risk” (Kosnett et al. 2007, p. 464).
The committee believed it important to note that the studies of gene-lead interaction, although providing interesting and compelling mechanistic information, must be considered in relation to prevailing ethical and policy contexts. It is standard practice in the United States and around the world to develop occupational exposure and dose standards that protect all workers, including the most susceptible, rather than to perform genetic screening and exclude higher-risk people from work settings.
Because of its wide distribution in the body, biologic measures of lead dose in a number of tissues—including blood, plasma, umbilical cord blood, hair, fingernails and toenails, breast milk, urine, semen, soft tissue, and bone—are available (see review by Hu et al. 2007). The excretion of lead in urine can be enhanced by CaNa2EDTA or DMSA, and chelatable lead has been used to estimate lead dose (Schütz et al. 1987; Tell et al. 1992; Lee et al. 1995, 2000; Schwartz et al. 2001). Because CaNa2EDTA can partially chelate bone lead
stores, EDTA-chelatable lead has been used as a surrogate for cumulative dose in some studies, but this is probably inadequate. DMSA chelates lead primarily from soft tissues, so DMSA-chelatable lead has been offered as an estimate of soft-tissue lead stores (Lee et al. 1995, 2000; Schwartz et al. 2001).
Lead in whole blood is typically measured with atomic absorption spectrophotometry or other analytic techniques (Hu et al. 2007). In a one-compartment pharmacokinetic model, blood lead has an average elimination half-life of 30 days (Rabinowitz 1991) and is therefore thought to represent primarily variation in recent exposure but in equilibrium with bone lead stores (Hu et al. 2007). Lead can be measured in trabecular bone (such as patella, calcaneus, or finger phalanx) or cortical bone (such as tibia) with several techniques. In general, measurement of bone lead (in micrograms of lead per gram of bone mineral) with 109Cd K-shell x-ray fluorescence (XRF) has the greatest antemortem utility (Todd et al. 1992; Landrigan and Todd 1994; Todd and Chettle 1994), although XRF is a research tool and is not generally available for clinical or medical surveillance. Lead in patella has complex elimination kinetics with three phases of elimination; the longest clearance half-time is about 5-7 years (Kim et al. 1997). In contrast, lead in tibia has a clearance half-time of 25-30 years (Chettle et al. 1991; Todd and Chettle 1994), although recent longitudinal studies suggest that it may be considerably longer (Wilker et al. 2011). Thus, it is not surprising that patella lead and tibia lead have shown different associations with health outcomes in some studies—evidence that the former’s generally shorter elimination kinetics may make it inadequate for estimating lifetime dose (Hu et al. 2007).
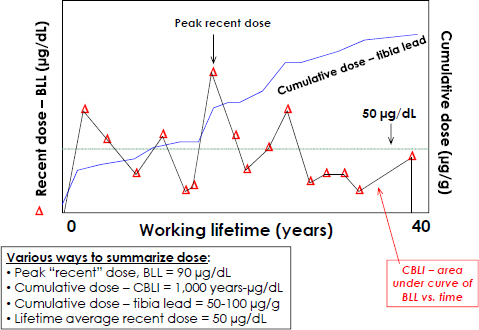
In 1978, when the OSHA lead standard was first promulgated, the average American had a BLL of about 15 μg/dL (CDC 1982; Annest et al. 1983). OSHA consequently set an occupational provision that a single BLL over 60 μg/dL or three BLLs averaging over 50 μg/dL would result in the medical removal of a worker until the BLL was under 40 μg/dL on two occasions. BLL is the preferred dose metric for studying health outcomes that are thought to be short-latency, acute health effects due to recent exposure. However, in persons who had high past exposures, BLL can also be moderately increased because of its equilibrium with bone lead stores. BLL may thus also reflect, to some degree, longer-term doses. In contrast, associations of health outcomes with cumulative blood lead index (CBLI, the area under the curve of blood lead vs time, Figure 3-2) or tibia lead concentrations are the preferred dose metrics for studying longer-latency, chronic health effects of cumulative dose. OSHA, however, did not consider lead bioaccumulation (in bone) and did not distinguish between short-latency, acute health effects and longer-latency, chronic health effects when setting the 1978 lead standard.
FIGURE 3-2 Schematic of a hypothetical worker’s dose over time. Used with permission of Brian Schwartz, Johns Hopkins Bloomberg School of Public Health.
Figure 3-2 illustrates additional complexities in relating lead dose to health outcomes. BLLs are highly variable throughout the work span, reflecting variation in exposure, the shorter elimination time from the blood compartment, and changes in work practices, hygiene, and related issues. Such data can be summarized in a variety of ways, but many health studies have relied on a single BLL in relation to health effects. Peak levels, average levels, and current levels could be expected to have different associations with health outcomes, depending on mechanism of action, latency, and other considerations. For some health outcomes, such as pregnancy, there are critical exposure periods during which a given lead dose could have a much greater deleterious effect than if it occurred at other stages of life. As the working lifetime increases and cumulative dose steadily rises, bone lead stores contribute more to current BLLs, so a single BLL later in the working career can reflect exposure earlier in employment, when BLLs may have been much higher. Thus, a single BLL measurement late in employment or at higher ages may reflect both recent external exposure and cumulative exposure. Finally, the CBLI can be estimated when rich longitudinal BLL data are available. Studies have validated the CBLI as an estimate of lifetime dose because it correlates strongly with tibia lead levels and its associations with health outcomes are more similar than BLL to the associations observed with tibia lead (Roels et al. 1995; Somervaille et al. 1988; Landrigan and Todd 1994).
Given the importance of cumulative lead dose to health (discussed in Chapters 4 and 5) and the lack of wide availability of XRF systems for measuring bone lead, this argues that more frequent longitudinal monitoring of BLLs, with attention to the CBLI over time, would have much greater utility than heretofore required under the OSHA lead standard.
PHARMACOKINETIC MODELS FOR LEAD
The relationship between air lead concentration and BLL is complex. Exposure to lead can occur through multiple pathways. BLL is the exposure metric most commonly described in association with health effects in humans, and lead exposure is typically assessed by using a pharmacokinetic model to relate air (or dietary) exposure concentrations to BLLs. The committee’s goal was not to review in depth the various dosimetry models available for lead but to explore how dosimetry models were used in the development of the OSHA general industry lead standard and to evaluate the models and their assumptions (see Table 3-1).
In the late 1970s, OSHA sought the development of a dosimetry model that could predict the distribution of BLLs in a given worker population in response to changing air lead concentrations. To that end, OSHA commissioned the development of a model by the Massachusetts Institute of Technology Center for Policy Alternatives (CPA) (Ashford et al. 1977; 43 Fed. Reg. 52962 [1978]). CPA used a physiologic model (developed by S. R. Bernard) to describe the response of BLLs to air lead exposure. Bernard constructed a kinetic model that included blood, bone, liver, kidney, and soft tissue compartments. Each compartment contained a variable-size “pool” of lead that was determined by metal transfer across compartment boundaries. Lead exchange across each compartment was represented as a first-order rate constant (in a transport-limited or diffusion-limited model). Transfer rate constants in the CPA model were obtained from experimental measurements of retention and excretion of lead in dogs, rats, baboons, and humans. A series of exponential equations were then generated to predict the time-dependent accumulation of lead in a compartment given a presumed air concentration. The relationship between BLL and air lead concentration (Figure 3-3) was given by the following equation (43 Fed. Reg. 52962 [1978]):
BLL = a(air lead concentration) + b,
where a is the BLL-air lead slope coefficient and b is the BLL at zero air lead. The coefficients a and b varied with job tenure.
TABLE 3-1 Assumptions Used by Occupational Safety and Health Administration for Center for Policy Alternatives Model and Committee’s Evaluation
| Parameter | Assumptions | Committee Evaluation |
| Particle size | First 12.5 μg/m3 of airborne lead consisted of lead particles with an aerodynamic diameter of ≤1 μm; remainder consisted of larger particles (≤1 μm) that would be deposited in upper respiratory tract. | Size of lead aerosol can influence deposition and absorption of lead from respiratory tract and delivery to systemic bloodstream (Froines et al. 1986; Park and Paik 2002). For example, lead fumes are more easily absorbed from lungs and result in higher BLLs than inhalation of larger lead particles. Smaller lead particles also appear to be more soluble regardless of chemical form of dust (Spear et al. 1998). |
| Deposition efficiency | 37% of all lead particles ≤1 μm are deposited in alveolar region. CPA model assumes that alveolar deposition of particles ≤1 μm does not occur. | It is now generally accepted that some alveolar deposition occurs with particles of 1-10 μm (Froines et al. 1986; ACGIH 2012). |
| Lung and gastrointestinal absorption | Complete (100%) absorption occurs in alveolar region. In contrast, larger particles (>1 μm) would be removed by mucociliary clearance and swallowed, and about 8% of lead would be absorbed in gastrointestinal tract. | Bioavailability of lead is influenced by chemical speciation, age of exposed person, level of lead exposure, matrix, and nutritional status of person. |
| Model linearity | Nonlinearity captured by using time-dependent mathematical terms. | Some models assume linearity in relationship between intake parameters and BLL. Available data suggest that relationship between BLL and lead intake is nonlinear (Leggett 1993). At low lead concentrations, kinetics are linear; nonlinear kinetics start when lead concentration in erythrocytes reaches 60 μg/dL, which corresponds to BLL of about 25 μg/dL (Leggett 1993). |
| Contributions from other routes of exposure | Inhalation exposure considered | It is critical to consider oral exposure to lead-based dusts during training and weapon cleaning. |
FIGURE 3-3 Time-dependent relationship between BLL and air lead concentration as estimated with the CPA model used by OSHA to develop the permissible exposure level. Estimated BLLs for lead workers exposed to airborne lead at 50 μg/m3.
The CPA model had several key assumptions (referred to as assumption C in Table 1 of the OSHA documentation). On the basis of those and other assumptions, the model predicts that 70% of lead-exposed workers would have a BLL under 40 μg/dL and 6% would have a BLL over 50 μg/dL (Snee 1982).
Several additional models have been developed to describe the relationship between air and BLLs. Their general form accounts for multiple sources of lead exposure and follows this relationship:
BLL = A[(air lead concentration) + (B1{food lead concentration}) + (B2{water lead concentration})]K,
where terms A, Bn, and K are derived from the data.
“Validation” of those models uses a variety of databases, two of which were from exposure studies by Azar et al. (1975) and Williams et al. (1969). In both studies, exposure durations were assumed to be sufficient to result in pseudo-steady-state BLLs. The Williams et al. study surveyed lead-acid battery factory workers and included the use of personal samplers. Personal samplers were worn for 2 weeks, and daily BLLs were determined for each worker during the second week. Personal lead exposures varied between job categories and ranged from 9 to 218 μg/m3. Mean BLLs varied between job categories and ranged from 27.2 to 74.2 μg/dL.
Snee (1982) provides equations based on the best fit to the Williams dataset. When it is applied to an air lead concentration of 50 μg/m3, the resulting
BLL is estimated to be 35 μg/dL, which shows good concordance with the CPA model used by OSHA. Fleming et al. (1999) reported the following relationship between BLL and air lead concentration for a population of Brunswick smelter workers:
BLL = 16.1[(0.24{Air Lead Concentration} + ({0.76}{0.5}) + 1.65]0.379,
where the background air lead concentration is assumed to be 0.5 μg/m3. Application of that model to an air lead concentration of 50 μg/m3 predicted a BLL of about 44 μg/dL. This result is also in good agreement with the model used by OSHA.
The committee recognizes that dosimetry models may need to be developed for firing-range personnel. Multiple models, including physiologically based pharmacokinetic (PBPK) models for lead, could be used for this purpose (O’Flaherty 1991a,b,c; 1993; 1995; Beck et al 2001). PBPK models allow experimental or environmental levels of exposure (or “applied dose”) to be re-expressed more usefully as corresponding levels of biologically effective concentrations in target tissuess that would potentially be affected by toxicity. PBPK models can be used to estimate corresponding levels of exposure associated with different BLLs of concern. Statistical and Monte-Carlo methods can also be used to estimate values of PBPK model parameters and to characterize uncertainty in PBPK model predictions.
For its evaluation of health effects associated with lead exposure in Chapters 4 and 5, the committee sought to find evidence that health effects could occur at exposures and doses lower than those specified in the OSHA lead standard. OSHA relied most heavily on BLLs, so the committee evaluated studies first for their associations of health effects with BLLs. Because the evidence base on health effects related to cumulative dose was insufficient in the 1970s, OSHA’s consideration of exposure duration and cumulative dose was inadequate. The committee therefore had to distinguish between BLL associations that were probably acute effects of recent dose from those in which BLLs were acting as surrogates for longer dose periods because of long exposure durations or higher exposure intensities in the past. The committee’s primary focus was on health effects that occur at BLLs under 40 μg/dL for short-duration exposures and on acute health effects. For longer-duration exposures and chronic health effects, BLLs had to be extrapolated in the context of what is known about tibia lead, CBLIs, and exposure duration. In evaluating relationships of the dose measures with health outcomes, dose-response relationships were evaluated, when available, for important characteristics, such as thresholds, U-shaped relations, log-linear relations, and linearity, especially in the dose range of interest.
The presence of log-linear relationships was deemed particularly important; they were emphasized in this report because they imply much greater incremental health risks with increasing lead dose at low levels than at higher levels.
An important assumption of the OSHA lead standard was that a population of workers exposed at OSHA’s permissible exposure limit (PEL) would have an average BLL of 40 μg/dL; that is, the PEL was selected to keep BLLs around 40 μg/dL on the average. Thus, if the committee found evidence to suggest that health effects occur below that BLL (or below the CBLIs or tibia lead levels that would result over time from long-duration BLLs at lower levels), it would have to conclude that the OSHA exposure standard is inadequate. Although OSHA did not explicitly address the issue of cumulative dose, another important implication of the OSHA standard, in allowing a BLL of 40 μg/dL for a working lifetime of 40 years, is that OSHA believed that this was an acceptable cumulative dose. As previously discussed, cumulative lead dose has most commonly been measured with the CBLI (in micrograms per deciliter times years of exposure [μg-years/dL]) or tibia lead. Although it was not explicitly stated, the OSHA lead standard presumes that a CBLI of 1,600 μg-years/dL (average BLL of 40 μg/dL × 40 years) provides adequate protection for lead workers. Although there is some uncertainty on this point, even if the most conservative estimates are used (Healey et al. 2008), that CBLI is roughly equivalent to tibia lead of 40-80 μg/g (on the basis that tibia lead can be estimated as 2.5-5% of the CBLI) (Hu et al. 2007; Healey et al. 2008). Thus, an important question is whether there is evidence that a CBLI under 1,600 μg-years/dL or a tibia lead concentration of 40-80 μg/g may be associated with adverse effects in lead workers.
A large and growing scientific literature documents a number of factors that modify the health effects of lead and make some people more susceptible to the effects of lead than others. For example, the decrement in cognitive function with higher tibia lead concentration is more severe in those who have the APOE ε-4 allele (Stewart et al. 2002). Other such factors include a number of genetic polymorphisms, coexposures (including noise for hearing loss), and comorbidities (Hu et al. 2007). Of those, only coexposure to noise was explicitly considered by the committee. Noise is an important coexposure on firing ranges and was identified by the Department of Defense as an appropriate factor to consider in addressing the committee’s charge. The other factors were not considered relevant, because the military population is typically healthy, having undergone medical screening that would minimize the likelihood that firing-range workers would have medical conditions that would make them more susceptible to lead (such as high blood pressure and diabetes mellitus).
ACGIH (American Conference of Governmental Industrial Hygienists). 2012. 2012 TLVs® and BEIs®. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
AFIOH (Air Force Institute for Occupational Health). 2008. Lead Free Frangible Ammunition Exposure at United States Air Force Small Arms Firing Ranges, 2005-2007. IOH-RS-BR-TR-2008-0002. Air Force Institute for Occupational Health, Brooks City-Base, TX [online]. Available: http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA487506 [accessed Dec. 19, 2011].
Annest, J.L., J.L. Pirkle, D. Makuc, J.W. Neese, D.D. Bayse, and M.G. Kovar. 1983. Chronological trend in blood lead levels between 1976 and 1980. N. Engl. J. Med. 308(23):1373-1377.
Ashford, N.A., R.D. Gecht, D.B. Hattis, and J.I. Katz. 1977. The Effects of OSHA Medical Removal Protection on Labor Costs of Selected Lead Industries. CPA Report No. CPA-77/11. NTIS PB-278653. Center for Policy Alternatives, Massachusetts Institute of Technology, Cambridge, MA.
ATSDR (Agency for Toxic Substances and Disease Registry). 2007. Toxicological Profile for Lead. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA. August 2007 [online]. Available: http://www.atsdr.cdc.gov/toxprofiles/tp13.pdf [accessed Sept. 13, 2012].
Azar, A., R.D. Snee, and K. Habibi. 1975. An epidemiologic approach to community air lead exposure using personal air samplers. Environ. Qual. Saf. Suppl. 2:254-290.
Barltrop, D., and A. Smith. 1972. Lead binding to human haemoglobin, Experientia 28(1):76-77.
Barton, J.C., M.E. Conrad, S. Nuby, and L. Harrison. 1978. Effects of iron on the absorption and retention of lead. J. Lab. Clin. Med. 92(4):536-547.
Beck, B.D., R.L. Mattuck, T.S. Bowers, J.T. Cohen, and E. O’Flaherty. 2001. The development of a stochastic physiologically-based pharmacokinetic model for lead. Sci. Total Environ. 274(1-3):15-19.
Bergdahl, I.A., A. Grubb, A. Schutz, R.J. Desnick, J.G. Wetmur, S. Sassa, and S. Skerfving. 1997. Lead binding to delta-aminolevulinic acid dehydratase (ALAD) in human erythrocytes. Pharmacol. Toxicol. 81(4):153-158.
Bjorkman, L., M. Vahter, and N.L. Pedersen. 2000. Both the environment and genes are important for concentrations of cadmium and lead in blood. Environ. Health Perspect. 108(8):719-722.
Cake, K.M., R.J. Bowins, C. Vaillancourt, C.L. Gordon, R.H. McNutt, R. Laporte, C.E. Webber, and D.R. Chettle. 1996. Partition of circulating lead between serum and red cells is different for inernal and external sources of lead. Am. J. Ind. Med. 29(5):440-445.
CDC (Centers for Disease Control). 1982. Current Trends Blood Lead Levels in U.S. Population. MMWR 31(10):132-134.
Chettle, D.R., M.C. Scott, and L.J. Somervaille. 1991. Lead in bone: Sampling and quantitation using K X-rays excited by 109Cd. Environ. Health Perspect. 91:49-55.
EPA (U.S. Environmental Protection Agency). 2012. Integrated Science Assessment for Lead. EPA/600/R-10/075B. National Center for Environmental Assessment-RTP Division, Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, NC. February 2012 [online]. Available: http://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=235331 [accessed Apr. 17, 2012].
Ettinger, A.S., M.M. Téllz-Rojo, C. Amarasiriwardena, T. Gonzáles-Cossío, K.E. Peterson, A. Aro, H. Hu, and M. Hernαndez-Avila. 2004. Levels of lead in breast milk and their relation to maternal blood and bone lead levels at one month postpartum. Environ. Health Perspect. 112(8):926-931.
Fleming, D.E., D.R. Chettle, C.E. Webber, and E.J. O’Flaherty. 1999. The O’Flaherty model of lead kinetics: An evaluation using data from a lead smelter population. Toxicol. Appl. Pharmacol. 161(1):100-109.
Friberg, L., and M. Vahter. 1983. Assessment of exposure to lead and cadmium through biological monitoring: Results of a UNEP/WHO global study. Environ. Res. 30(1):95-128.
Froines, J.R., W.C. Liu, W.C. Hinds, and D.H. Wegman. 1986. Effect of aerosol size on the blood lead distribution of industrial workers. Am. J. Ind. Med. 9(3):227-237.
Goyer, R.A. 1995. Nutrition and metal toxicity. Am. J. Clin. Nutr. 61(3 Suppl.):646S-650S.
Graziano, J.H., E. Siris, N. LoIacono, S.J. Silverberg, and L. Turgeon. 1985. 2,3-Dimercaptosuccinic acid as an antidote for lead intoxication. Clin. Pharmacol. Ther. 37(4):431-438.
Healey, N., D.R. Chettle, F.E. McNeill, and D.E. Fleming. 2008. Uncertainties in the relationship between tibia lead and cumulative blood lead index. Environ. Health Perspect. 116(3): A109-A110.
Hernandez-Avila, M., C.G. Villalpando, E. Palazuelos, H. Hu, M.E. Villalpando, and D.R. Martinez. 2000. Determinants of blood lead levels across the menopausal transition. Arch. Environ. Health 55(5):355-360.
Hu, H., R. Shih, S. Rothenberg, and B.S. Schwartz. 2007. The epidemiology of lead toxicity in adults: Measuring dose and consideration of other methodologic issues. Environ. Health Perspect. 115(3):455-462.
IARC (International Agency for Research on Cancer). 2006. Inorganic and Organic Lead Compounds. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 87. Lyon, France: World Health Organization [online]. Available: http://monographs.iarc.fr/ENG/Monographs/vol87/mono87.pdf [accessed Sept. 20, 2012].
Juhasz, A.A. 1977. The Reduction of Airborne Lead in Indoor Firing Ranges by Using Modified Ammunition. NBS Special Publication 408-26. Washington, DC: Law Enforcement Standards Laboratory, National Bureau of Standards.
Kim, R., C. Landrigan, P. Mossmann, D. Sparrow, and H. Hu. 1997. Age and secular trends in bone lead levels in middle-aged elderly men: Three-year longitudinal follow-up in the Normative Aging Study. Am. J. Epidemiol. 146(7):586-591.
Kosnett, M.J., R.P. Wedeen, S.J. Rothenberg, K.L. Hipkins, B.L. Materna, B.S. Schwartz, H. Hu, and A. Woolf. 2007. Recommendations for medical management of adult lead exposure. Environ. Health Perspect. 115(3):463-471.
Krieg, E.F., Jr., M.A. Butler, M.H. Chang, T. Liu, A. Yesupriya, M.L. Lindegren, and N. Dowling. 2009. Lead and cognitive function in ALAD genotypes in the Third National Health and Nutrition Examination Survey. Neurotoxicol. Teratol. 31(6): 364-371.
Landrigan, P.J., and A.C. Todd. 1994. Direct measurement of lead in bone. A promising biomarker. J. Am. Med. Assoc. 271(3):239-240.
Lee, B.K., B.S. Schwartz, W. Stewart, and K.D. Ahn. 1995. Provocative chelation with DMSA and EDTA: Evidence for differential access to lead storage sites. Occup. Environ. Med. 52(1):13-19.
Lee, B.K., K.D. Ahn, S.S. Lee, G.S. Lee, Y.B. Kim, and B.S. Schwartz. 2000. A comparison of different lead biomarkers in their associations with lead-related symptoms. Int. Arch. Occup. Environ. Health 73(5):298-304.
Lee, B.K., G.S. Lee, W.F. Stewart, K.D. Ahn, D. Simon, K.T. Kelsey, A.C. Todd, and B.S. Schwartz. 2001a. Associations of blood pressure and hypertension with lead dose measures and polymorphisms in the vitamin D receptor and delta-aminolevulinic acid dehydratase genes. Environ. Health Perspect. 109(4):383-389.
Lee, S.S., B.K. Lee, G.S. Lee, W.F. Stewart, D. Simon, K. Kelsey, A.C. Todd, and B.S. Schwartz. 2001b. Associations of lead biomarkers and delta-aminolevulinic acid dehydratase and vitamin D receptor genotypes with hematopoietic outcomes in Korean lead workers. Scand. J. Work Environ. Health 27(6):402-411.
Leggett, R.W. 1993. An age-specific kinetic model of lead metabolism in humans. Environ. Health Perspect. 101(7):598-616.
NTP (National Toxicology Program). 2012. NTP Monograph on Health Effects of Low-Level Lead. Prepublication Copy. U.S. Department of Health and Human Services, National Institute of Environmental Health Sciences, National Institutes of Health. June 13, 2012 [online]. Available: http://ntp.niehs.nih.gov/?objectid=4F04B8EA-B187-9EF2-9F9413C68E76458E [accessed June 14, 2012].
O’Flaherty, E.J. 1991a. Physiologically based models for bone-seeking elements. I. Rat skeletal and bone growth. Toxicol. Appl. Pharmacol. 111(2):299-312.
O’Flaherty, E.J. 1991b. Physiologically based models for bone-seeking elements. II. Kinetics of lead disposition in rats. Toxicol. Appl. Pharmacol. 111(2):313-331.
O’Flaherty, E.J. 1991c. Physiologically based models for bone-seeking elements. III. Human skeletal and bone growths. Toxicol. Appl. Pharmacol. 111(2):332-341.
O’Flaherty, E.J. 1993. Physiologically based models for bone-seeking elements. IV. Kinetics of lead disposition in humans. Toxicol. Appl. Pharmacol. 118(1):16-29.
O’Flaherty, E.J. 1995. Physiologically based models for bone-seeking elements. V. Lead absorption and disposition in childhood. Toxicol. Appl. Pharmacol. 131(2):297-308.
Park, D.U., and N.W. Paik. 2002. Effect on blood lead of airborne lead particles characterized by size. Ann. Occup. Hyg. 46(2):237-243.
Rabinowitz, M.B. 1991. Toxicokinetics of bone lead. Environ. Health Perspect. 91:33-37.
Rabinowitz, M.B., G.W. Wetherill, and J.D. Kopple. 1973. Lead metabolism in the normal human: Stable isotope studies. Science 182(4113):725-727.
Rabinowitz, M.B., G.W. Wetherill, and J.D. Kopple. 1976. Kinetic analysis of lead metabolism in healthy humans. J. Clin. Invest. 58(2):260-270.
Roels, H., J. Konings, S. Green, D. Bradley, D. Chettle, and R. Lauwreys. 1995. Time-integrated blood lead concentration is a valid surrogate for estimating the cumulative lead dose assessed by tibial lead measurement. Environ. Res. 69(2):75-82.
Schütz, A., S. Skerfving, J.O. Christoffersonand, and I. Tell. 1987. Chelatable lead versus lead in human trabecular and compact bone. Sci. Total Environ. 61:201-209.
Schwartz, B.S., B.K. Lee, W. Stewart, K.D. Ahn, K. Springer, and K. Kelsey. 1995. Associations of delta-aminolevulinic acid dehydratase genotype with plant, exposure duration, and blood lead and zinc protoporphyrin levels in Korean lead workers. Am. J. Epidemiol. 142(7):738-745.
Schwartz, B.S., B.K. Lee, G.S. Lee, W.F. Stewart, D. Simon, K.T. Kelsey, and A.C. Todd. 2000a. Associations of blood lead, dimercaptosuccinic acid-chelatable lead, and tibia lead with polymorphisms in the vitamin D receptor and [delta]-aminolevulinic acid dehydratase genes. Environ. Health Perspect. 108(10):949-954.
Schwartz, B.S., W.F. Stewart, K.T. Kelsey, D. Simon, S. Park, J.M. Links, and A.C. Todd. 2000b. Associations of tibial lead levels with BsmI polymorphisms in the vitamin D receptor in former organolead manufacturing workers. Environ. Health Perspect. 108(3):199-203.
Schwartz, B.S., B.K. Lee, G.S. Lee., W.F. Stewart, S.S. Lee, K.Y. Hwang, K.D. Ahn, Y.B. Kim, K.I. Bolla, D. Simon, P.J. Parsons, and A.C. Todd. 2001. Associations of blood lead, dimercaptosuccinic acid-chelatable lead, and tibia lead with neurobehavioral test scores in South Korean lead workers. Am. J. Epidemiol. 153(5):453-464.
Sithisarankul, P., B.S. Schwartz, B.K. Lee, K.T. Kelsey, and P.T. Strickland. 1997. Aminolevulinic acid dehydratase genotype mediates plasma levels of the neurotoxin, 5-aminolevulinic acid, in lead-exposed workers. Am. J. Ind. Med. 32(1):15-20.
Smith, C.M., X. Wang, H. Hu, and K.T. Kelsey. 1995. A polymorphism in the deltaaminolevulinic acid dehydratase gene may modify the pharmacokinetics and toxicity of lead. Environ. Health Perpect. 103(3):248-253.
Snee, R.D. 1982. Models for the relationship between blood lead and air lead. Int. Arch. Occup. Environ. Health 50(4):303-319.
Somervaille, L.J., D.R. Chettle, M.C. Scott, D.R. Tennant, M.J. McKieman, A. Skilbeck, and W.N. Trethowen. 1988. In vivo tibia lead measurements as an index of cumulative exposure in occupationally exposed subjects. Br. J. Ind. Med. 45(3):174-181.
Spear, T.M., W. Svee, J.H. Vincent, and N. Stanisich. 1998. Chemical speciation of lead dust associated with primary lead smelting. Environ. Health Perspect. 106(9):565-571.
Stewart, W.F., B.S. Schwartz, D. Simon, K. Kelsey, and A.C. Todd. 2002. ApoE genotype, past adult lead exposure, and neurobehavioral function. Environ. Health Perspect. 110(5):501-505.
Tell, I., L.J. Somervaille, U. Nilsson, I. Bensryd, A. Schütz, D.R. Chettle, M.C. Scott, and S. Skerfving. 1992. Chelated lead and bone lead. Scand. J. Work Environ. Health 18(2):113-119.
Todd, A.C., and D.R. Chettle. 1994. In vivo X-ray fluorescence of lead in bone: Review and current issues. Environ. Health Perspect. 102(2):172-177.
Todd, A.C., F.E. McNeill, and B.A. Fowler. 1992. In vivo X-ray fluorescence of lead in bone. Environ. Res. 59(2):326-335.
Wetmur, J.G. 1994. Influence of the common human delta-aminolevulinate dehydratase polymorphism on lead body burden. Environ. Health Perspect. 102(Suppl. 3):215-219.
Wetmur, J.G., G. Lehnert, and R.J. Desnick. 1991. The delta-aminolevulinate dehydratase polymorphism: Higher blood lead levels in lead workers and environmentally exposed children with the 1-2 and 2-2 isozymes. Environ. Res. 56(2):109-119.
Wilker, E., S. Korrick, L.H. Nie, D. Sparrow, P. Vokonas, B. Coull, R.O. Wright, J. Schwartz, and H. Hu. 2011. Longitudinal changes in bone lead levels: The VA Normative Aging Study. J. Occup. Environ. Med. 53(8):850-855.
Williams, M.K., E. King, and J. Walford. 1969. An investigation of lead absorption in an electric accumulator factor with the use of personal samplers. Br. J. Ind. Med. 26(3):202-216.
Yang, Y.H., S.H. Liou, C.Y. Yang, F.C. Sung, C.C. Wu, and T.N. Wu. 2007. Increased blood lead concentration during menstruation in teen female students. Sci. Total Environ. 382(2-3):224-227.