The adverse effects of lead on human health are well documented. Effects seen after lead exposure depend on the exposure dose and the absorbed dose, the duration of exposure, the timing of exposure during critical life stages, and host factors. The committee used the recent compilations of the toxicologic and epidemiologic studies of lead performed by the National Toxicology Program (NTP) and the US Environmental Protection Agency (EPA). Those reviews were used as a basis for identifying the primary noncancer health end points that would be of concern for firing-range personnel, including adverse effects on the adult nervous, hematopoietic, renal, reproductive, immune, and cardiovascular systems. Adverse effects in the developing fetus were also of concern. This chapter is organized along those lines.
As noted in Chapters 1 and 2, the committee specifically sought health-effects data on blood lead levels (BLLs) under 40 μg/dL because the current standard of the Occupational Safety and Health Administration (OSHA) aims to maintain BLLs below that concentration. Evidence on health effects at a corresponding estimated cumulative blood lead index (CBLI) of 1,600 μg-years/dL (that is, 40 years at 40 μg/dL) and tibia lead levels of 40-80 μg/g were also specifically sought.
ENVIRONMENTAL PROTECTION AGENCY AND NATIONAL TOXICOLOGY PROGRAM ASSESSMENTS
Three previous assessments were used by the committee for identifying key literature: the 2012 NTP Monograph on Health Effects of Low-Level Lead, the 2006 EPA Air Quality Criteria Document [AQCD] for Lead, and the 2012 EPA Integrated Science Assessment for Lead (Second External Review Draft). Each of the assessments provides background on lead exposure and lead toxicokinetics and includes a review of the primary epidemiologic or experimental literature for evidence that lead exposure is associated with adverse health effects. NTP’s assessment focuses on epidemiologic evidence at BLLs of under 5 or under 10 μg/dL and presents specific conclusions regarding each category of
health effect. EPA’s AQCD (2006) also identified health effects associated with BLLs under 10 μg/dL. EPA’s Integrated Science Assessment for Lead (2012) affirmed many of the conclusions reached in the AQCD (2006). The reader is referred to specific conclusions reached by those organizations and the committee’s conclusions with respect to their relevance to Department of Defense personnel who work on firing ranges. The committee also performed its own search for recent relevant literature on the health effects of lead to supplement those evaluations.
The adult nervous system is a critical target for the toxic effects of lead. Effects on the central nervous system of lead workers include dose-related changes in cognitive and psychomotor performance and mood, neurodegenerative diseases, and neurophysiologic changes in the auditory, visual, and balance systems. Effects of occupational lead exposure on the peripheral nervous system at BLLs of 60-70 μg/dL are manifested as motor weakness with abnormalities in motor and sensory nerve conduction. No peripheral motor or sensory symptoms are known to occur at BLLs under 40 μg/dL, but sensory nerve function is associated with lead dose.
Potential modes of action for lead neurotoxicity include oxidative stress, inhibition of enzymes needed for energy production, decreased levels of neurotransmitters and altered neurotransmitter release, and increased permeability of the blood-brain barrier (EPA 2012). Ultimately, lead-induced neurotoxicity in adults consists of changes in brain structure and neurochemistry, including white-matter changes, reduction in gray matter, and alterations in brain metabolites.
Conclusions from the Environmental Protection Agency 2006 and 2012 and National Toxicology Program 2012 Lead Documents
Environmental Protection Agency 2006 Air Quality Criteria Document
EPA’s 2012 Integrated Science Assessment for Lead (Second External Review Draft) focused on updating the 2006 Air Quality Criteria Document for Lead (EPA 2006), so a summary of the key neurotoxic effects of lead in adults from the earlier document will be presented first.
Studies of the effects of aging and their relationship with environmental lead exposure included the Veterans’ Administration Normative Aging Study established in 1961 in Boston and consisting of 2,280 healthy men 21-80 years old who are examined every 3 years (Payton et al. 1998; Rhodes et al. 2003; Wright et al. 2003; Weisskopf et al. 2004), the Kungsholmen Project on aging and dementia in Sweden (Nordberg et al. 2000), and the third National Health and Nutrition Examination Survey (NHANES III) (Krieg et al. 2005). There was
mixed evidence of a relationship between environmental lead exposure, as judged by current BLL, and impaired cognitive performance in adults. However, when bone lead was used as the measure of lead dose, the Normative Aging Study found significant associations with impaired neurocognitive performance. Bone lead measurements capture both long-term cumulative exposure and past high lead exposure, which may be more important than current BLL.
In contrast, EPA noted that occupational lead exposure measured by BLL, CBLI, and bone lead was associated with decreased cognitive and psychomotor performance, diminished peripheral sensory nerve function, slowing in visual evoked potentials and brainstem auditory evoked potentials, and abnormalities in postural sway. Evidence in support of EPA’s conclusion included onset of diminished cognitive function and diminished psychomotor speed at a BLL of 18 μg/dL (Schwarz et al. 2001). However, in some studies, it was not the current BLL (under 30 μg/dL) but the measures of CBLI or bone lead concentration that were associated with poorer neurobehavioral performance (Lindgren et al. 1996; Bleecker et al. 1997; Hänninen et al. 1998; Bleecker et al. 2005a). The same relationship was found for peripheral sensory nerve studies most commonly associated with CBLI (Chia et 1996a,b; Kovala et al. 1997; Yokoyama et al. 1998). Changes in sensory nerve function occurred at BLLs of 28-30 μg/dL (Chuang et al. 2000; Bleecker et al. 2005b). Visual evoked potentials, measuring speed of conduction in the optic nerves, were prolonged beginning at BLLs of 17-20 μg/dL (Abbate et al. 1995). Slowed brainstem auditory evoked potentials were found to be associated with CBLI or weighted average BLL (Discalzi et al. 1992, 1993, Bleecker et al. 2003). A calculated benchmark dose for postural sway (measure of balance) was a current BLL of 14 μg/dL (Iwata et al. 2005).
EPA identified a few publications that reported an increased risk of amyotrophic lateral sclerosis (ALS) and motor neuron disease associated with past occupational lead exposure (Roelofs-Iverson et al. 1984; Armon et al. 1991; Gunnarsson et al. 1992; Chancellor et al. 1993; Kamel et al. 2002). The presence of the delta-aminolevulinic acid dehydratase (ALAD) 2 allele (ALAD2) increased that risk (odds ratio [OR] = 1.9; 95% confidence interval [CI]: 0.60, 6.3) (Kamel et al. 2003). Essential tremor, another neurodegenerative disorder, was associated with low concurrent BLL (3 μg/dL) caused by exposure to environmental lead (Louis et al. 2003), but there was no information about past exposures, which might have been higher. The presence of the ALAD2 allele increased the odds of essential tremor by a factor of 30 compared with subjects that had only the ALAD1 allele (Louis et al. 2005).
Environmental Protection Agency 2012 Integrated Science Assessment for Lead (Second External Review Draft)
Neurobehavioral and Mood Effects
EPA (2012) reviewed epidemiologic evidence of associations between environmental lead exposure and neurobehavioral outcomes primarily from two
studies—the Baltimore Memory Study and the Normative Aging Study. Results of those studies strengthened the association between cognitive performance and bone lead and probably reflect the effect of cumulative lead exposure on the brain (Shih et al. 2006; Weuve et al. 2006; Wang et al. 2007; Weisskopf et al. 2007; Rajan et al. 2008; Bandeen-Roche et al. 2009; Glass et al. 2009). Analysis of data from NHANES III revealed an association between concurrent BLL and lower neurobehavioral performance in particular age and genetic-variant subgroups (Krieg and Butler 2009; Krieg et al. 2009, 2010). Mood disorders in young adults in the survey increased with a BLL of 2.11 μg/dL or above (Bouchard et al. 2009). However another publication that used data from NHANES III but was not included in the EPA 2012 document examined all adults (20 years old or older) and found no consistent relationship between environmental lead exposure and depression (Golub and Winters 2010).
In adults who had past occupational lead exposure, BLL and bone lead were associated with decrements in cognitive performance years after the cessation of occupational exposure. The relationship between bone lead and cognitive performance was significant in workers older than 55 years old (Khalil et al. 2009a).
Neurodegenerative Disease
Two case-control studies published after 2006 found that BLL was associated with ALS, but EPA had concerns about the contribution of “reverse causality”. ALS decreases the ability to move the limbs, and this leads to increased demineralization of bone and release of lead from bone, which in turn increase BLLs. Thus, the disease could cause the increased BLL. In addition, there was bias in the study in that survival time increased with higher BLLs (Kamel et al. 2008; Fang et al. 2010). Parkinson disease was also reported to be associated with bone lead and whole-body lifetime exposure (Coon et al. 2006; Weisskopf et al. 2010), but EPA commented on the need to establish temporality between exposure and the onset of the disease and on the potential contribution of past exposure to manganese, a metal known to be associated with parkinsonism. Two additional studies reported the association of BLL and essential tremor, but the temporality between exposure and development of tremor was not established (Dogu et al. 2007; Louis et al. 2011)
Sensory Organ Function
New analyses have found an increase in hearing thresholds associated with bone lead in subjects in the Normative Aging Study (Park et al. 2010). In the occupational setting, people who had higher BLLs had significantly greater hearing loss (Chuang et al. 2007; Hwang et al. 2009).
National Toxicology Program 2012 Monograph on Effects of Low-Level Lead
A recent NTP report examined the literature of neurotoxic outcomes associated with a BLL under 10 μg/dL. NTP concluded that the evidence that BLLs under 10 μg/dL were associated with the diagnosis of essential tremor was sufficient but that the evidence that BLLs under 5 μg/dL were associated was limited. NTP also found limited evidence of an association between BLLs under 10 μg/dL and impaired cognitive function in older adults, psychologic effects, ALS, and reduced sensory function and auditory function. There were no studies of an association between BLLs of 10 μg/dL or lower and Alzheimer disease, Parkinson disease, or sensory function or visual function.
Neurobehavioral and Mood Effects
NTP noted that studies of BLL and cognitive performance in older adults who had environmental lead exposure had mixed results (Payton et al. 1998; Nordberg et al. 2000; Wright et al. 2003; Gao et al. 2008). According to data from NHANES III, neurobehavioral test performance in younger adults had no significant relationship with BLL (Krieg et al. 2005, 2009). However, studies that reported no association between neurologic outcome and BLL often found decreased neurobehavioral performance significantly associated with BLL (Weisskopf et al. 2004; Shih et al. 2006; Weuve et al. 2009). BLLs were associated with psychiatric symptoms and mood disorders in young and older adults (Rhodes et al. 2003; Rajan et al. 2007; Bouchard et al. 2009). NTP concluded that the evidence was limited because of the small number of studies and because there were multiple studies of a given cohort. However, as with all outcomes in adults, NTP noted that there were no data on whether BLLs were always under 10 μg/dL from birth until the time of study.
Neurodegenerative Effects
NTP had the same concern as EPA (2012) that the association of BLL with ALS was influenced by reverse causality and by bias due to the increase in survival time with higher BLL (Kamel et al. 2008; Fang et al. 2010). NTP’s conclusion that there was sufficient evidence of an association between essential tremor and BLLs under 10 μg/dL was based on case-control studies conducted in two countries (Louis et al. 2003, 2005, 2011; Dogu et al. 2007). The evidence that essential tremor is associated with a BLL of 3 μg/dL is based on a small sample (300 essential tremor patients) in the two studies. Thus, NTP concluded that evidence of an association with a concurrent BLL under 5 μg/dL was limited.
Sensory Organ (Auditory) Effects
In occupational studies, diminished hearing occurred primarily at frequencies over 3,000 Hz and began at a BLL of 7 μg/dL (Chuang et al. 2007; Hwang et al. 2009). The pattern of hearing loss was not the typical pattern seen in noiseinduced hearing loss. The authors concluded that BLLs under 10 μg/dL might enhance noise-induced hearing loss. In people who had environmental lead exposure, hearing loss was associated with bone lead (Park et al. 2010).
Other Studies Considered
Mood and Occupational Lead Exposure
Mood is evaluated with a neurologic-symptom questionnaire and a mood checklist or mood scale, such as the Center for Epidemiological Studies Depression Scale (CES-D) and the Profile of Mood States (POMS), which screen on moods such as anger, confusion, depression, fatigue, anxiety and tension, and vigor. Those mood-rating scales differ slightly in content depending on the country in which they were developed. Mood change might be a primary outcome associated with exposure, but its evaluation is also necessary in administering neuropsychologic testing, inasmuch as mood may influence performance. In some occupational studies, mean BLLs of 29-43 μg/dL were associated with POMS subscales or items on a mood checklist (Maizlish et al. 1995; Hänninen et al. 1998; Niu et al. 2000), whereas other studies found no relationship between BLLs of 27-38 μg/dL and measures of mood (Stollery et al. 1989; Chia et al. 1997; Osterberg et al. 1997; Lucchini et al. 2000). Results of administration of the CES-D screen for depression to 803 lead-exposed Korean workers were significantly associated with tibia lead (mean 37 μg/g) but not with BLL (mean 32 μg/dL) after adjustment for covariates (Schwartz et al. 2001).
In some studies, difficulty in concentrating, irritability, fatigue, and muscle and joint pain were reported in workers who had a mean BLL of 43 μg/dL (Maizlish et al. 1995) or 27 μg/dL (Lucchini et al. 2000), whereas other studies with mean BLLs in the high 30s found no association with symptoms (Chia et al. 1997; Osterberg et al. 1997). Lucchini et al. (2000) estimated a BLL threshold of 12 μg/dL for a statistically significant increase in neurologic symptoms.
Neurobehavioral Effects
Tests are often used in neurobehavioral batteries to measure effects of lead exposure in different domains, such as attention and concentration (Digit Span), conceptual and executive functioning (Stroop and Trails B), visuoperceptive and visuoconstructive (Block Design), visuomotor (Reaction Time, Pegboard Test, Digit Symbol Substitution, and Trails A), verbal memory (Rey Auditory Verbal
Learning Test, Logical Memory, and Paired Associated Learning), and nonverbal memory (Rey-Osterreith Complex Figure and Benton Visual Retention). In analyzing the association between lead exposure and test performance, adjustment for confounders is critical. Confounders include age, education (preferably a measure of verbal intelligence), depressive symptoms, alcohol use, and smoking.
A study by Lindgren et al. (1996) of 467 Canadian lead-smelter workers was one of the first to evaluate the effects of cumulative lead exposure on the nervous system. The mean number of years of employment was 18, the mean BLL was 28 μg/dL, the time-weighted average BLL over a working lifetime was 40 μg/dL, and the mean CBLI was 765 μg-years/dL. CBLI exposure groups differed significantly in digit symbol, logical memory, Purdue dominant hand, and Trails A and B. No dose-effect relationship between BLL and neuropsychologic performance was found. In the smelter population, 256 currently employed workers had a median score of 29 (range 19-30) in the screening test called the Mini-mental State Examination (MMSE). A dose-effect relationship between CBLI and MMSE was found only in the 78 workers who had a reading grade level less than 6 in the Wide Range Achievement Test (Revised). The absence of a dose-effect relationship in workers who had higher reading grade levels and the same CBLI was attributed to increased cognitive reserve (Bleecker et al. 2002). An in-depth examination of verbal learning and memory in the same population found no association with BLL, but with increasing CBLI or time-weighted average BLL over a working lifetime there was poorer storage and retrieval of previously learned verbal material. Alterations in the ability to organize materials in long-term memory interfered with retrieval efficiency. Those changes occurred in the group that had a mean time-weighted-average BLL of 41.2 ± 11.09 μg/dL and a CBLI of 813.1 ± 409.68 μg/g (Bleecker et al. 2005a). The one test sensitive to BLL in the population was Simple Reaction Time (SRT), which had a curvilinear relationship with increasing reaction time beginning at a BLL of about 30 μg/dL (Bleecker et al. 1997).
Hänninen et al. (1998) studied neuropsychologic effects in lead-battery workers who had current BLLs under 50 μg/dL compared with those who had BLLs over 50 μg/dL in the past. They found that overall high, past exposure had the greatest effect on tests that required the encoding of complex visually presented stimuli. The authors concluded that the effect of lead on brain function is better reflected by the history of the BLL, such as the CBLI, than by bone lead content.
Some studies, particularly cross-sectional ones, that included measures of cumulative lead and current lead exposures found the strongest association between BLL and neurobehavioral performance when the concurrent BLLs were high. Schwartz et al. (2001) reported that bone lead concentration was not associated with neurobehavioral performance in 803 Korean lead-exposed workers. In contrast, lead-exposed workers performed significantly worse than controls on SRT, Digit Span, Benton Visual Retention, Colored Progressive Matrices,
Digit Symbol, and Purdue Pegboard after controlling for age, sex, and education. BLL was the best predictor of significant decrements in neurobehavioral performance on Trails B, Purdue Pegboard (four measures), and Pursuit Aiming (two measures). For those effects, an increase in BLL of 5 μg/dL was equivalent in its effects to an increase of 1.05 years in age. Use of Lowess lines for Purdue Pegboard (assembly) and Trails B suggested a threshold BLL of 18 μg/dL.
Hwang et al. (2002) evaluated 212 consecutively enrolled workers from the above cohort of 803 Korean workers for protein kinase C (PKC) activity and the relationship between BLL and neurobehavioral performance. BLLs of 5-69 μg/dL were significantly associated with decrements in Trails B, SRT, and Purdue Pegboard (three measures). PKC activity was measured by back-phosphorylation of erythrocyte membrane proteins and found not to be associated with neurobehavioral test scores. However, dichotomization at the median revealed significant effect modification; the association of higher BLLs with poorer neurobehavioral performance occurred only in workers who had lower back-phosphorylation levels (which correspond to higher in vivo PKC activity). The authors suggested that PKC activity may identify a subpopulation at increased risk for neurobehavioral effects of lead.
The cohort of Korean lead workers was studied longitudinally. The relationship between occupational lead exposure and longitudinal decline in neurobehavioral performance was assessed in 576 current and former Korean lead workers who completed testing at three visits at about yearly intervals (Schwartz et al. 2005). Cross-sectional associations of BLL and short-term change occurred with Trails A and B, Digit Symbol, Purdue Pegboard (four measures), and Pursuit Aiming after adjustment for covariates. However, longitudinal BLL was associated only with poorer performance on Purdue Pegboard (four measures). Tibial bone lead was associated with Digit Symbol and Purdue Pegboard (dominant hand). For those effects, the effect of an increase in lead concentration from the 25th to the 75th percentile was equivalent to an increase of 3.8 years of age for cross-sectional BLL, 0.9 year of age for historical tibia lead, and 4.8 years for longitudinal BLL.
Long-term effects of occupational lead exposure have been evaluated in other studies. Khalil et al. (2009a) evaluated 83 lead-exposed workers and 51 controls 22 years after their initial neuropsychologic evaluation when the mean BLL was 40 μg/dL in workers and 7.2 μg/dL in controls. Twenty-two years later, their mean BLLs were 12 and 3 μg/dL, respectively. Mean bone lead obtained only at followup was 57 μg/g in workers and 12 μg/g in controls. BLL was not associated with any of the scores in five cognitive domains. Peak tibia lead was calculated to reflect bone lead level at the time that lead exposure ended. Peak bone lead predicted lower cognitive performance and cognitive decline over 22 years. A statistically significant association of peak bone lead with performance on spatial ability, learning and memory, and total cognitive score was found only in workers who were over 55 years old. The results support a decline in cognitive performance with aging in lead-exposed workers.
Brain Anatomic and Biochemical Effects
Eighty workers at the primary lead smelter previously described by Lindgren et al. (1996) underwent magnetic resonance imaging (MRI) of the brain. MRIs were graded by a neuroradiologist for white matter change (WMC) on a scale of none to lesions larger than 10 mm. Only the 61 workers under 50 years old were used in the analysis because of the large effect of age on WMC. Mean BLL in the group was 29 μg/dL, CBLI was 826 μg-years/dL, and bone lead was 39 μg/g. Logistic regression of WMC on lead exposure after controlling for age, hypertension, triglycerides, C-reactive protein, smoking, and drinking found CBLI and bone lead significantly associated with WMC. A measure of psychomotor speed and dexterity, grooved pegboard, was significantly related to WMC and measures of lead exposure. Path analysis supported that the effect of CBLI and bone lead on psychomotor speed and dexterity was mediated by WMC (Bleecker et. al. 2007).
Magnetic resonance spectroscopy (MRS) of the brain was used to examine the biochemical changes caused by lead (Hsieh et. al. 2009). Twenty-two lead workers (mean BLL 16.99 μg/dL, tibia lead 61.55 μg/g, and patella lead 66.29 μg/g) in a paint factory were compared with 18 healthy volunteers (mean BLL 3.4 μg/dL, tibia lead 18.51 μg/g, and patella lead 7.14 μg/g). Measures that reflected neuronal loss and myelin alterations were lower in the lead-exposed workers primarily in the frontal and occipital lobes. Multiple linear regression for each MRS measure and lead after adjustment for sex, age, and smoking found significant associations of increasing BLL and bone lead levels with decreases in gray and white matter in the occipital lobe. The strongest of the associations was of neuronal loss in the frontal lobe with BLL and patella lead level. It was suggested that those changes may contribute to poorer outcome in tests of memory and visual performance.
Peripheral Nerve Function
A meta-analysis of 32 publications of nerve-conduction studies and occupational lead exposure found BLL to be a weak predictor of peripheral nerve impairment (Davis and Svendsgaard 1990). Nerve-conduction testing includes analysis of latent period (time it takes for stimulatory impulse to initiate an evoked potential), conduction velocity, and amplitude. Reduced nerveconduction velocities in lead-exposed subjects revealed that the median motor nerve was most sensitive.
Nerve-conduction studies of workers in a lead-battery factory (Kovala et al. 1997) found that sensory amplitudes of the median and sural nerves correlated negatively with long-term exposure (CBLI and duration of exposure). Chia et al. (1996b) also found the strongest dose-effect relationship between median sensory conduction velocity and CBLI, whereas He et al. (1988) found sensory-conduction
abnormalities related to BLL. Yokoyama et al. (1998) measured the distribution of conduction velocities in large myelinated fibers of the sensory median nerve twice (at a 1-year interval) in 17 gun-metal workers. They reported that measurements of chelatable lead (readily mobilized lead from soft tissue) were more strongly predictive of peripheral nerve impairment than BLL.
Other studies examined peripheral sensory nerve function in the extremities with a quantitative sensory test, vibration threshold, that measures the integrity of large myelinated nerve fibers. Kovala et al. (1997) found vibration threshold at the ankle to be related to CBLI and duration of exposure, whereas finger vibration threshold was associated with BLL (mean BLL 26 μg/dL and average BLL over the preceding 3 years 29 μg/dL). Overall, historical BLLs were more closely associated with peripheral nerve function than was bone lead in this population. In contrast, Schwartz et al. (2001) examined vibration thresholds and bone lead in 803 Korean workers and 135 controls and found that after adjustment for covariates tibia lead concentration (mean 37 μg/g) but not BLL (mean 32 μg/dL) was significantly associated with poorer vibration threshold in the dominant great toe but not the finger. In a followup study of 576 lead workers who completed three visits at yearly intervals, vibration threshold in the toe was associated with current BLL (mean 31 μg/dL), longitudinal BLL, and tibia lead (38 μg/g) after adjustment for covariates (Schwartz et al. 2005). Chuang et al. (2000) reported on vibration perception in the foot in 206 lead-battery workers. There was a significant association of BLL in the past 5 years (mean 32 μg/dL) and time-weighted average BLL over a working lifetime (mean 32 μg/dL) with vibration perception in the foot after adjustment for covariates, including the use of vibrating hand tools. Data analyses used a hockey-stick regression that uses two different curves to fit two regions of a dataset (Hudson 1966). The curve of foot vibration threshold vs mean BLL for the preceding 5 years showed an inflection point around 30 μg/dL; a positive linear relation above this point suggested a potential threshold.
Bleecker et al. (2005b) examined peripheral nerve function in 80 smelter workers with Current Perception Threshold (CPT), a neuroselective test that measures integrity of the large and small myelinated nerve fibers and unmyelinated nerve fibers. CPT was not associated with BLL (mean 26 μg/dL) or bone lead (mean 40 μg/g). CPT for large myelinated nerve fibers had a curvilinear relationship with time-weighted average BLL over a working lifetime (mean 42 μg/dL), with an apparent threshold at 28 μg/dL. In regression analyses, CBLI and its associated exposure variables explained the increasing variance in CPT of large myelinated fibers and suggested that cumulative lead exposure intensity is more important than duration of exposure with regard to the peripheral nervous system. At the highest BLL criterion, both large and small myelinated nerve fibers were impaired. Ergonomic stressors (used as a surrogate for active motor units) enhanced the effect of lead on the peripheral nervous system.
Visual evoked potentials (VEPs) and brainstem auditory evoked potentials (BAEPs) measure speed of conduction in the nerves that run from the eyes and ears, respectively, to the relevant locations in the brain. On stimulation, nerves send signals in the form of “waves” that can be detected, and the time it takes for an impulse to initiate an evoked potential is latency. The VEP is the first positive wave and usually occurs at 100 ms (P100 latency) after the visual stimulus. That measure is very sensitive to demyelination of the optic nerve. BAEPs also have discrete waveforms. Wave I arises from the auditory nerve, and its latency reflects peripheral transmission time; wave III is generated predominantly from the auditory pathway in the lower brainstem; and wave V is generated from the upper brainstem. The use of interpeak latencies helps distinguish changes in peripheral auditory nerve latency from changes in brainstem transmission in the auditory pathway.
Abbate et al. (1995) studied VEPs in 300 lead-exposed men (30-40 years old) in good health who had no other neurotoxic exposure. Their BLLs ranged from 17 to 60 μg/dL and were stratified into four groups for data analyses. P100 latency of VEPs was significantly prolonged in all the BLL groups. Prolonged VEP began at BLLs of 17-20 μg/dL. The contribution of age was not a concern, and careful screening ruled out other medical and eye conditions and other potential exposures.
BAEPs in 49 lead-exposed workers (mean BLL 55 μg/dL; time-weighted average BLL over a working lifetime 54 μg/dL) and in age- and sex-matched controls were recorded (Discalzi et al. 1992). In workers who had a time-weighted average BLL over 50 μg/dL, conduction in the entire brainstem was slower. In a later publication, Discalzi et al. (1993) reported identical results in 22 battery storage workers who had a mean BLL of 47 μg/dL and a time-weighted average BLL of 48 μg/dL.
BAEPs were measured in 359 currently employed smelter workers who had mean indexes of exposure of 17 years, BLL of 28 μg/dL, and CBLI of 719 μg-years/dL (Bleecker et al. 2003). Linear regression, adjusted for age, found that BLL was significantly associated with peripheral auditory nerve conduction speed and CBLI was significantly associated with lower brainstem conduction speed. Groups were created on the basis of BAEP scores greater than clinical cut-off scores for peripheral auditory nerve conduction speed and brainstem conduction speed. For groups that had abnormal clinical BAEP values, the mean range of BLLs was 28.3 (± 7.8) to 34.8 (± 6.44) μg/dL and of CBLI was 723.0 (± 438.47) to 934.0 (± 352.80) μg-years/dL. Those results were all significantly higher than the ones in the group that had normal BAEPs.
A case-control study in Taiwan (Chuang et al. 2007) in which workers received periodic health examinations found 121 people who had hearing thresholds above 25 dB and 173 controls who had normal hearing. Geometric mean
BLL was 10.7 μg/dL for cases and 3.9 μg/dL for controls. In the final regression model with all-six-frequency thresholds for both ears, significant predictors of hearing loss were age and lead concentration (logarithmically transformed). Years of noise exposure at work had a nonsignificant, weak effect. The net effect of lead is 7.11 dB above the pooled all-six-frequency thresholds for both ears when logarithmically transformed lead level is increased by 0.1 μg/dL. Exposure to manganese or arsenic did not contribute to the model, but selenium was found to be protective against lead ototoxicity.
Another Taiwanese study (Hwang et al. 2009) examined 259 workers in a steel plant with audiograms and blood studies of lead, manganese, copper, zinc, arsenic, and cadmium. Noise levels were established in all work areas. Mean BLL was 5.43 μg/dL. Logistic regression adjusting for age and noise exposure found that BLLs of 7 μg/dL or higher were associated with hearing loss at sound frequencies of 3,000-8,000 Hz (p < 0.005 to p < 0.05). The OR was largest for 4,000 Hz (6.26) and 8,000 Hz (6.16). The pattern of hearing loss beginning with the greatest loss was 6,000 Hz, 4,000 Hz, 8,000 Hz, and 3,000 Hz—not the typical pattern for noise-induced hearing loss with a “notching” of the audiogram at 4,000 Hz. The authors conclude that BLL under 10 μg/dL may enhance noiseinduced hearing loss.
Postural Stability
Postural sway measures balance or steadiness on a force platform that requires integration of visual, vestibular, and peripheral sensory inputs and motor output. No standard protocol is used among studies.
One approach to determining the critical dose of lead that affects postural balance in the occupational setting is the benchmark-dose method in which a concentration of lead results in an increased probability of an abnormal end point—a benchmark response—and thereby places exposed people at increased risk (Iwata et al. 2005). Iwata et al. (2005) defined their benchmark dose level as the 95% lower confidence limit of the benchmark dose. In 121 lead-exposed workers who had a mean BLL of 40 μg/dL, almost all sway measures were significantly larger than those in controls. The mean benchmark dose level of the current BLL for postural sway was 14.3 μg/dL.
Postural sway evaluated in 49 chemical workers exposed to lead stearate (mean BLL 18 μg/dL, average working lifetime BLL 24 μg/dL, and mean CBLI 391 μg-years/dL) and 23 controls found significant increases in the exposed group in sway in all directions at high and low frequencies with eyes open and closed (Yokoyama et al. 1997). After adjustment for covariates, dose-dependent associations were observed between BLL and sway in the anterior-posterior direction and between time-weighted average BLL and right to left sway. The authors concluded that changes in the vestibulocerebellar pathways are affected by BLL whereas the anterior cerebellar lobe pathways are affected by time-weighted average BLL.
Postural sway characteristics were measured in 60 lead storage battery workers (mean BLL 36 μg/dL) and 60 controls (mean BLL 6 μg/dL). Computerized postural sway measurements showed that lead workers had poorer postural stability and that it decreased when their eyes were closed, but this deterioration in performance was not associated with BLL (Chia et al. 1994). A second publication examined cumulative BLL over 10 years and found that CBLI for the 2 years before testing was associated with all postural sway measures with eyes closed (Chia et al. 1996b).
When postural control was measured in 63 lead battery workers (mean past BLL 38 μg/dL), there were statistically significant increases in mean body oscillations with eyes closed and head tilted forward (Ratzon et al. 2000). Partial correlation after adjustment for education, coffee consumption, hours of sleep, and estimate of health was significant only for total lead exposure and increased body oscillations with head tilted forward. To maintain balance, lead-exposed workers required increased oscillations when visual and vestibular inputs were altered.
Autonomic Function and Electroencephalography
Effects on cardiac parasympathetic functioning were found in autonomic nervous system testing of 172 lead-exposed workers who had a mean BLL of 36 μg/dL (Teruya et al. 1991). A significant dose-related decrease in R-R interval (interval between the peak of one heart beat to the next) during deep breathing was reported in 132 workers who had a stable BLL over the preceding year. The decrease was most notable at BLLs of 30 μg/dL or higher, with a possible mild decrease first occurring at BLLs of 20 μg/dL or higher. Niu et al. (2000) reported similar findings in 44 lead-exposed workers who had a mean BLL of 29 μg/dL.
Sympathetic nerve function as seen in variations in R-R interval on electrocardiography and changes in finger blood flow with postural changes according to Doppler flowmetry were measured in 128 workers in the ceramic painting industry (mean BLL 13 μg/dL). The 46 workers in the lowest-exposure group, with BLLs under 10 μg/dL, served as the control group. The heat-recovery rate of erythrocyte ALAD in this group was over 80%, which was similar to rates seen in people who did not have obvious lead exposure. BLL, smoking, and body-mass index were statistically significant predictors of change in finger blood flow with postural change (Ishida et al. 1996).
Examination of 60 workers in a lead-battery factory (Kovala et al. 1997) with quantitative electroencephalography (EEG) found that alpha (8-13/sec) and beta (14-40/sec) frequencies were more abundant in workers who had higher long-term lead exposure as measured by tibia lead (mean 26 μg/g), calcaneus lead (mean 88 μg/g), CBLI (mean 546 μg-years/dL), and time-weighted average BLL (mean 32 μg/dL). The finding of slow alpha activity correlated positively with lead exposure may reflect increased episodes of “microdrowsiness” in
workers who had higher lead exposure. In Niu’s study (2000), quantitative EEG in 44 lead-exposed workers (mean BLL 29 μg/dL) found statistically significant increased beta activity and diminished amplitudes abnormalities in 81% of exposed workers compared with referents.
Essential Tremor
Essential tremor is a common neurologic disease with a prevalence in the general population of 1-6%. Prevalence is 4% in those over 40 years old and increases to 20.5% in those over 60 years old. The abnormal movement is related to involvement of the cerebellum and basal ganglia (Louis et al. 2003).
Louis et al. (2003) examined the relationship between BLL and essential tremor in 100 cases from a medical center in New York City (mean BLL 3.3 μg/dL) and 143 controls (mean BLL 2.6 μg/dL). Logistic regression adjusting for age and current cigarette-smoking found an association between BLL and essential tremor (OR per unit increase = 1.19; 95% CI: 1.03, 1.37; p = 0.02). BLL was higher in the 39 essential-tremor cases that had no family history. Both current prevalence and lifetime prevalence of occupational lead exposure were the same in essential-tremor cases and controls.
A second publication (Louis et al. 2005) examined whether an interaction between BLL and ALAD gene polymorphisms increases the odds of essential tremor. The study involved 63 essential-tremor cases that had a mean BLL of 3.5 μg/dL and 101 controls (similar in age, education, sex, and ethnicity) that had a mean BLL of 2.6 μg/dL. Of the 63 essential-tremor cases, 18 (29%) vs 17 (17%) of the controls had an ALAD2 allele (OR = 1.98; 95% CI: 0.93, 4.21; p = 0.077). When log BLL was examined according to the presence of ALAD2 allele in subjects who had essential tremor, log BLL was highest in cases that had an ALAD2 allele, intermediate in cases that did not, and lowest in controls (test for trend, β = 0.10; p = 0.001). When the ALAD2 allele was present, BLL was significantly associated with the odds of essential tremor (OR = 80.29; 95% CI: 3.08, 2.096; p = 0.008). The odds of essential tremor in people who had the ALAD2 allele were 30 times greater than in those who had only the ALAD1 allele. In the highest log BLL tertile, ALAD2 allele was present in 22% of essential-tremor cases and 5% of controls. It was proposed that increased BLL with the ALAD2 allele could affect the cerebellum and thereby increase the risk of tremor.
A similar study design was used in Mersin, Turkey, where 105 cases of essential tremor (mean age 52.9 ± 18.6 y) were compared with 69 spouse controls (mean age 50.9 ± 12.5 y) and 36 nonspouse controls (mean age 50.3 ± 15.9 y) (Dogu et al. 2007). Median BLL was 2.7 μg/dL in essential-tremor cases and 1.5 μg/dL in controls (p < 0.001). Logistic regression for BLL associated with essential tremor had an OR of 4.01 (95% CI: 2.53, 6.37; p < 0.001). Therefore, for each 1-μg/dL increase in BLL, there was a four-fold increase in the odds of essential tremor. The OR increased to 8.13 (95% CI: 3.05, 21.65; p < 0.001) when
the comparison was limited to nonspouse controls. This study replicated studies performed in New York City.
Another study by Louis et al. (2011) examined the interaction of harmane, a tremor-producing β-carboline alkaloid, and BLL in 106 cases of essential tremor (mean age 68.2 ± 15.2 y; median BLL 2.7 μg/dL, range 0.3-11.6 μg/dL) and 151 controls (mean age 64.1 ± 12.5 y; median BLL 2.4 μg/dL, range 0.3-11.9 μg/dL). Severity of tremor ranged from a score of 0 to 36. Tremor score correlated significantly with blood harmane concentrations and with BLL. The tremor score was low (8.4 ± 8.2) when both BLL and blood harmane were low, intermediate (10.5 ± 9.8) when one or the other was high, and highest (13.7 ± 10.4; p = 0.01) when both were high; this suggested an additive effect of exposure to the two toxicants.
Three of the four studies above were performed in New York City, and their case and control subjects overlapped. Therefore, the overall sample size in four studies at two locations may be only about 250. If low BLL is causally associated with the development of essential tremor, a much higher prevalence than 1-6% in the general population would be expected. Prospective studies of incident cases of essential tremor with measures of cumulative lead exposure are needed.
Summary Findings on Neurologic Effects
The committee concludes that the evidence is sufficient to infer causal relationships between BLLs under 40 μg/dL and adverse effects on nervous system function (see Table 4-1). Effects on both the central and peripheral nervous systems have been observed, including effects on cognitive function, peripheral nerve function, visual and auditory function, posture and balance, and autonomic nervous system function.
Neurobehavioral performance showed decrements in various domains in neurobehavioral testing, including verbal and visual memory, visuospatial ability, motor and psychomotor speed, manual dexterity, attention, and executive functioning associated with BLLs and measures of cumulative exposure (CBLI and bone lead levels). The committee focused on occupational studies, which it judged to be most relevant to the firing range. It found that decrements in neurobehavioral performance begin to occur at BLLs as low as 18 μg/dL. It also found that changes in mood were equivocal at BLLs of around 27-30 μg/dL, but lead-related symptoms could be detected at BLLs as low as 12 μg/dL despite the finding of some studies that there was no association with lead-related symptoms at BLLs over 30 μg/dL. Occupational lead exposure is associated with decrements in peripheral sensory nerve function beginning at BLLs around 28-30 μg/dL. BLLs over 10 μg/dL are associated with lead-induced hearing loss that might
TABLE 4-1 Key Studies of the Effects of Lead on Neurologic Outcomes
| Health Effect | Population Characteristics | Measures | Effect Estimate | Why Study Is Relevant to DOD | Reference |
| Cognitive Performance | |||||
| Neuropsychologic test battery in English or French; 14 neuropsychologic variables examined by MANCOVA | 467 Canadian former, current lead-smelter workers, French- and English-speaking; mean (SD) age = 43 (11.0) y; mean (SD) education = 10 (3.2) y | Mean (SD) BLL = 28 (8.4) μg/dL; mean (SD) employment = 18 (7.4) y; mean (range) timeweighted average BLL = 40 (4-66) μg/dL; mean (range) CBLI = 765 (1-1,626) μg-y/dL | MANCOVA (high, medium, low exposure); no significance with covariates (age, education, CES-D, alcohol use) until years of employment added suppressor variable; CBLI exposure groups differed significantly on some tests: digit symbol (p = 0.05), logical memory (p = 0.04), Purdue dominant hand (p = 0.01), Trails A (p = 0.02), Trails B (p = 0.04). | Study showed doseeffect relationship between CBLI and neuropsychologic performance when there was no association with current BLL. | Lindgren et al. 1996 |
| Simple reaction time | 80 currently employed smelter workers (from Lindgren cohort above); mean age = 44 y; mean (range) employment duration = 20 (1-26) y | Mean (SD) BLL = 26 (7.23) μg/dL; mean (SD) employment = 20 (5.6) y; mean (SD) tibia lead = 40 (25.17) μg/g bone mineral | Linear regression found BLL ± BLL2 accounted for 13.7% of variance after adjustment for age, education (p < 0.01). Bone lead was nonsignificant. | Curvilinear relationship found between BLL and SRT with threshold for increasing SRT at BLL of 30 μg/dL. Curvilinear relationship may explain why previous studies reported faster SRTs in groups with lead exposure. | Bleecker et al. 1997 |
| Mini-Mental State Examination (MMSE), reading section of Wide Range Achievement Test-revised (WRAT-R) | 256 lead-smelter workers: mean (SD) age = 41 (7.9) y; mean (SD) education = 10 (2.8) y; mean (SD) employment duration = 17 (8.1) y | Current mean (SD) BLL = 28 (8.8) μg/dL; mean (SD) CBLI = 725 (434) μg-y/dL | Multiple linear regression adjusting for age, WRAT-R, education, alcohol, smoking found significant CBLI ~ WRAT-R interaction (p = 0.01) and dose-effect relationship between CBLI and MMSE (p = 0.04), but only in 78 workers with WRAT-R reading grade level below 6 y. Overall, most workers had reading grade equivalent to or below their years of formal education. | Greater cognitive reserve, as measured by educational achievement, allowed some compensation for effects of lead on neurobehavioral measures. Because most military personnel have demonstrated greater educational achievement and probably have greater cognitive reserve, they might have less effect of lead exposure. | Bleecker et al. 2002 |
Abbreviations: BAEP, brainstem auditory evoked potential; BLL, blood lead level; BMD, benchmark dose; BMDL, benchmark dose level; CBLI, cumulative blood lead index; CES-D, Center for Epidemiological Studies-Depression Scale; CPT, current perception threshold; CV, coefficient of variation; ECG, electrocardiogram; EEG, electroencephalogram; GP, grooved pegboard; MANCOVA, multivariate analysis of covariance; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; OR, odds ratio; SD, standard deviation; SE, standard error; SRT, simple reaction time; WMC, white matter change; WRAT-R, wide range achievement test-revised.
enhance noise-induced hearing loss. BLLs of 17-20 μg/dL are associated with a decrease in conduction velocity in the visual pathway. The benchmark dose level of the BLL for postural sway is 14 μg/dL. Parasympathetic and sympathetic integrity is compromised in lead-exposed workers who have mean BLLs over 20 μg/dL. Quantitative EEG found increased beta activity in 81% of lead-exposed workers whose mean BLL was 29 μg/dL.
The committee also notes that cumulative lead dose that reflects past high lead exposure may be a strong predictor of decrements in neurobehavioral performance even in the absence of an association with current BLL. Cognitive effects of lead exposure may be present years after cessation of occupational lead exposure in older adults. Those finding are in general agreement with NTP and EPA reports.
Environmental and occupational lead poisonings have long been associated with anemia, whose mechanisms are complex and multifactorial. A review by Aub et al. (1925) concluded that severe lead poisoning was associated with anemia that was initially due to enhanced destruction of circulating erythrocytes followed by “bone marrow failure”. Those and other aspects of the anemia of lead poisoning have been well documented, and their mechanisms are now better understood. Exposure to lead has been associated with changes in erythrocyte structure and decreases in hemoglobin, hematocrit, mean corpuscular volume, and mean corpuscular hemoglobin concentration. This section will briefly review three aspects of the anemia of lead poisoning: shortened erythrocyte survival, impaired heme synthesis, and impaired renal production of erythropoietin. It will then provide estimates of the BLLs at which those phenomena occur.
Conclusions from the 2012 Environmental Protection Agency and 2012 National Toxicology Program Lead Documents
Environmental Protection Agency 2012 Integrated Science Assessment for Lead (Second External Review Draft)
EPA’s review of recent epidemiologic studies concerning environmental lead exposure and hematologic function concludes that there is strong evidence that exposure is associated with a variety of deleterious effects on hemoglobin concentration, mean corpuscular volume, mean corpuscular hemoglobin, and erythrocyte count and adverse effects on heme synthesis through the inhibition of several enzymes of the heme pathway. EPA’s draft report concludes that deleterious associations are observed in populations that have mean BLLs as low as about 5 μg/dL.
National Toxicology Program 2012 Monograph on Effects of Low-Level Lead
The NTP monograph did not consider effects of lead exposure on hematologic functioning.
Other Studies Considered
Erythrocyte Survival and Anemia
The use of radiolabeling techniques to measure erythrocyte survival times in men who worked in battery and lead-smelting plants and were heavily exposed to lead revealed that erythrocyte survival was shortened from a mean of 120 days in nonexposed men to 101 days in 17 workers, three of whom were symptomatic (Hernberg et al. 1967). BLLs were not measured in that landmark study, and the diagnosis of lead poisoning was made by measuring increased coproporphyrin in urine (over 500 μg/L). Heightened osmotic fragility and changes in erythrocyte shapes have long been thought to be responsible for the enhanced erythrocyte destruction; indeed, in experimental animals, removal of the spleen, the organ responsible for erythrocyte sequestration, temporarily reverses the anemia of lead poisoning (Aub et al. 1925).
Several reports of occupational lead poisoning from the 1970s suggested that a decrease in hemoglobin concentrations occurred only when the BLL reached about 50 μg/dL (Lilis et al. 1978; Baker et al. 1979; Grandjean 1979). However, more recent research indicates that effects may occur at substantially lower exposure. For example, in an attempt to establish the benchmark dose of lead that is associated with anemia in the workplace in Japan, 388 male leadexposed workers in a variety of industries were examined for BLL, erythrocyte counts, hemoglobin, and hematocrit. BLLs ranged from 1.0 to 113.3 μg/dL (mean 26.8 μg/dL). After controlling for age and working status, BLL was statistically significantly associated with small decrements in hemoglobin concentration, erythrocyte counts, and hematocrit. The benchmark BLLs “at an abnormal probability of 5% in unexposed workers and an excess risk of 5% in exposed workers” were estimated with the method of Budtz-Jorgensen et al. (2001) to be 19.5 μg/dL for hemoglobin, 19.4 μg/dL for erythrocytes, and 29.6 μg/dL for hematocrit (Karita et al. 2005, p. 957).
Measurements of bone lead and hemoglobin in 119 union members involved in the building trades have also been revealing. Patella lead concentrations were found to correlate significantly with a small decrease in hemoglobin and hematocrit; in the same men, BLLs were relatively low (mean 8.3 μg/dL) and were not associated with these outcomes (Hu et al. 1994). Those seemingly disparate findings suggest a subclinical effect of bone lead burden on erythro-poiesis
despite relatively low concurrent BLLs. A recent study of 15 exposed workers (mean BLL 74 μg/dL) and 15 nonexposed workers (mean BLL 9.9 μg/dL) found that the exposed workers had more than twice the erythrocyte intracellular calcium levels of nonexposed workers and that high intracellular calcium concentration was associated with increased osmotic fragility. In the same workers, lead exposure was associated with increased erythrocyte membrane lipid peroxidation as estimated with measurements of erythrocyte malondialdehyde (Quintanar-Escorza et al. 2007). Another recent study of 23 battery workers (mean BLL 50 μg/dL; range 5-90 μg/dL) and 36 controls (mean BLL 1.5 μg/dL) found an increase in several erythrocyte markers of oxidative damage but no change in hematologic measures (Conterato et al. in press). Several more recent studies have also suggested effects of lead on hematopoiesis at relatively low BLLs. A Nigerian study of 81 men moderately exposed to lead in manufacturing occupations reported a decrease in hemoglobin and increased circulating reticulocytes in men who had a mean BLL of only 7 μg/dL compared with controls (mean BLL 3 μg/dL) (Ukaejiofo et al. 2009), but nutritional and other risk factors for reduced hemoglobin were not discussed. Finally, a study in Sarajevo examined hematologic outcomes in a population of workers in the petrol industry whose mean BLL was 4.3 μg/dL. Associations were found between BLL and erythrocyte counts, hemoglobin concentrations, and mean corpuscular volume (Cabaravdic et al. 2010); however, exposures to other toxic chemicals cannot be ruled out.
Collectively, the large body of research on lead and anemia, only briefly explored here, consistently indicates that occupational exposure to lead is associated with biochemical and morphologic damage of erythrocytes. Moreover, the notion that BLLs over 50 μg/dL are required appears to have been put to rest by more recent research.
Impaired Heme Synthesis
The consequences of lead exposure for the biosynthesis of heme have been studied for decades; at times, various intermediates in the heme synthetic pathway have been used as biomarkers of exposure and effect. Indeed, in 1993, a National Research Council committee issued a report Measuring Lead Exposure in Infants, Children, and Other Sensitive Populations that reviewed the literature on lead and the heme biosynthetic pathway in a chapter titled “Biologic Markers of Lead Toxicity” (NRC 1993). The reader is referred to that chapter for a full description of the issue. A brief summary and interpretation of this large body of research are presented below.
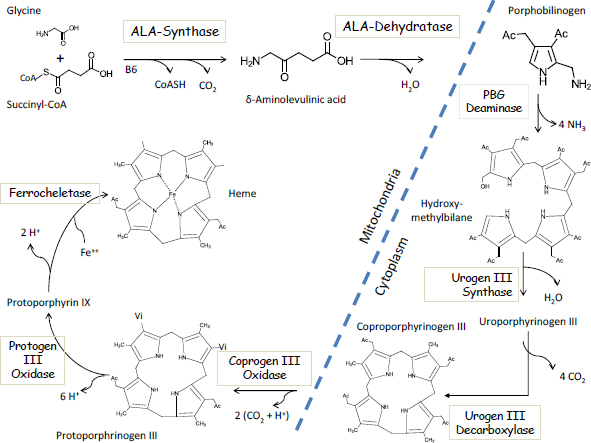
The first step in the heme pathway takes place inside mitochondria and involves the enzymatic condensation of succinyl-CoA with glycine to form deltaaminolevulinic acid (ALA) via the enzyme ALA-synthase (Figure 4-1). That is
FIGURE 4-1 The Biosynthetic pathway of heme.
also the rate-limiting step in heme synthesis. The second step involves the lead-sensitive enzyme ALAD, which combines two molecules of ALA to form porphobilinogen. Four molecules of porphobilinogen are enzymatically joined in the cytosol to form the first of a series of porphyrin molecules, including uroporphyrinogen, which is excreted in urine; coproporphyrinogen, which is excreted in urine and feces; protoporphyrinogen; and finally protoporphyrin IX, the precursor of heme. The final step in the synthesis of heme involves the insertion of an iron atom into protoporphyrin IX via the enzyme ferrochelatase, another lead-sensitive enzyme.
As early as 1947, the urinary excretion of porphyrins in urine was described as “the first symptom of lead poisoning” due to the inhibition of heme synthesis by lead (de Langen and ten Berg 1948). It is now known that other health effects precede the excretion of porphyrins in urine, which typically does not occur until BLLs exceed 40 μg/dL. Historically, other intermediates of heme synthesis have been used as diagnostic markers of occupational and environmental lead poisoning. Hernberg and Nikkanen (1970) published landmark findings in an urban population that the zinc metalloenzyme ALAD is exquisitely sensitive to lead, with 50% enzyme inhibition occurring at BLLs over 15 μg/dL. Measured erythrocyte ALAD or urinary ALA was later widely used as a lead biomarker. Several years later, Piomelli et al. (1973) developed the “FEP test” (free erythrocyte porphyrin test) as a screening test for childhood lead poisoning. Renamed as the ZPP test (zinc protoporphyrin test), this test took advantage of the fact that the partial inhibition of the final step in heme synthesis led to the accumulation of protoporphyin IX, a fluorescent biomarker that is easily detectable in a fingerstick blood sample. Widely used for many years, the test fell by the wayside in 1991 when the Centers for Disease Control (now the Centers for Disease Control and Prevention) lowered the BLL of concern to 10 μg/dL; a BLL of at least 17 μg/dL is required before porphyrins are increased in blood (Piomelli et al. 1982).
Thus, it is clear that the impairment of heme synthesis by lead exposure occurs at relatively low BLLs and that increased concentrations of ALA and protoporphyrin are associated with lead exposure. However, it is not widely recognized that the inhibition of heme synthesis cannot fully explain the anemia of lead poisoning. The elegant work of Piomelli et al. (1975) has demonstrated that even in patients with BBLs over 90 μg/dL, erythrocytes contain roughly 300 molecules of heme for every molecule of free protoporphyrin. Thus, other molecular mechanisms must play more important roles in the etiology of the anemia.
Impaired Production of Erythropoietin
Grandjean et al. (1989) conducted a simple experiment that demonstrated that after donating a unit (450 mL) of blood, workers occupationally exposed to lead (mean BLL 44 μg/dL) took longer to restore their predonation hemoglobin
concentrations than a group of age-matched controls. Over the 4 weeks after blood donation, the workers’ hemoglobin concentrations and reticulocyte counts dramatically lagged behind those of controls. The authors attributed the “delayed blood rejuvenation capacity” to impaired heme synthesis. But the work of Piomelli et al. (1975), described above, argues against that. Others have postulated that the failure to mount an adequate reticulocytosis to compensate for blood loss might be due to inadequate production of erythropoietin in the kidney, inasmuch as erythropoietin is produced by cells in the proximal tubule of the kidney, where lead is known to accumulate. Thus, Graziano et al. (1991) studied the relationships between BLL, hemoglobin concentration, and erythropoietin in a population of pregnant women in two towns in Kosovo (in the former Yugoslavia), one of which was the site of a lead smelter. It was demonstrated that lead-exposed women had inappropriately low circulating erythropoietin levels at any given level of hemoglobin. The relationship between BLL and erythropoietin was later described in lead workers in Austria (Osterode et al. 1999) and in a population of tricycle taxi drivers in Nepal (Sakata et al. 2007). Thus, the anemia associated with lead exposure may be partially due to lead nephrotoxicity and the failure to synthesize erythropoietin adequately to regulate erythropoiesis.
Summary Findings on Hematopoietic Effects
Occupational exposure to lead has consistently been associated with biochemical, morphologic, and physiologic effects that can impair erythrocyte formation and survival and ultimately lead to anemia. However, the literature varies in its estimates of the BLLs required to have clinically significant effects on those outcomes. Table 4-2 summarizes the studies and presents the BLL associated with each particular hematologic outcome.
The committee concludes that the evidence is sufficient to infer causal relationships between BLLs under 40 μg/dL and effects on heme synthesis. The evidence is suggestive with regard to possible effects of BLLs on circulating hemoglobin concentrations at a benchmark dose of 20 μg/dL. There is also convincing evidence that higher BLLs are associated with delayed blood rejuvenation after blood loss—an issue of possible concern in a population of military personnel. Those conclusions are generally in agreement with the conclusions of EPA (2012); the NTP review did not address hematologic effects of lead.
Adverse effects of lead exposure on renal function were first described in the 19th century (Lanceraux 1881). There is now a voluminous literature on the relationship between environmental and occupational lead exposure and renal function. It includes many epidemiologic studies and a broad array of mechanistic toxicology studies in animal models.
TABLE 4-2 Key Studies of the Hematopoietic Effects of Lead
| Health Effect | Population Characteristics | Measures | Effect Estimate | Why Study Is Relevant to DOD | Reference |
| Shortened erythrocyte survival | Battery and smelter workers (n = 17) and controls (n = 4) | Urinary coproporphyrin >500 μg/L | Erythrocyte survival was 101 days in workers vs 120 days in controls. | Clinically important outcome. | Hernberg et al. 1967 |
| Anemia | Smelter workers and a chemicals plant (n = 160) | BLLs about ≤50 μg/dL | Anemia (Hgb <14 g/dL) seen in 21% of workers. | Clinically important outcome. | Baker et al. 1979 |
| Decreased erythrocytes, HCT, and Hgb | Various industries in Japan | Estimated benchmark doses of about 20 μg/dL for hemoglobin, erythrocytes, about 30 μg/dL for HCT | Onsets of declines in these measures begin at these BLLs. | Facilitates risk assessment, management decisions. | Karita et al. 2005 |
| Decreased Hgb and HCT with patella lead but not BLL | Men in building trades | Patella bone lead in men with mean BLL of 8.4 μg/dL | Compared with those in lowest quintile of bone lead, those in highest had decrease in Hgb, HCT of 11 g/L, 0.03, respectively. | Suggests effects at relatively low exposures in range of interest. | Hu et al. 1994 |
| Increased erythrocyte calcium, fragility and lipid damage | Lead workers | Mean BLL >70 μg/dL | Erythrocyte calcium >2~, lipid peroxidation 1.7 ~ higher in lead workers than controls. | Suggests mechanism for shortened erythrocyte survival. | Quintanar-Escorza et al. 2007 |
| Inhibition of erythrocyte ALAD activity | Urban population | No apparent BLL threshold | Negative correlation between BLL and ALAD activity; y = 2.3-0.18x; r = -0.83. | Early biochemical evidence of toxicity. | Hernberg and Nikkanen 1970 |
| Inhibition of heme synthetase | Various | BLL threshold 17 μg/dL | Onset of rise in erythrocyte protoporphyrin begins at this BLL. | Basis of ZPP test; evidence of toxicity at BLLs that may occur on firing ranges. | Piomelli et al. 1973, 1982 |
Abbreviations: ALAD, delta-aminolevulinic acid dehydratase; ANOVA, analysis of variance; BLL, blood lead level; Hgb, hemoglobin; HCT, hematocrit; ZPP, zinc protoporphyrin.
Conclusions from the 2012 Environmental Protection Agency and 2012 National Toxicology Program Lead Documents
Environmental Protection Agency 2012 Integrated Science Assessment for Lead (Second External Review Draft)
EPA’s draft assessment concluded that recent and past basic toxicologic research and epidemiologic studies provided a strong body of evidence supporting the conclusion that nonoccupational lead exposure is causally associated with an increased risk of renal disease, as evidenced by increased serum creatinine, reduced creatinine clearance, and reduced glomerular filtration rate (GFR). EPA concluded that the evidence was sufficiently strong to support a causal relationship between lead exposure and renal disease, but the present committee judged that BLLs and duration of lead exposure at which the effects occur are uncertain because BLLs in adults probably reflect higher BLLs earlier in life.
National Toxicology Program 2012 Monograph on Effects of Low-Level Lead
Largely on the basis of a review of 13 large epidemiologic studies of the general population that examined associations between renal function and BLLs under 10 μg/dL, the NTP concluded that there was sufficient evidence that BLLs under 5 μg/dL are associated with adverse effects on renal function in adults. The 13 studies support relationships between concurrent BLL and renal function. The associations are typically stronger in susceptible populations (such as people who have diabetes or hypertension). However, the NTP report concluded that concurrent BLLs in adults may reflect higher BLLs in childhood or earlier adulthood. In the absence of a study of a population in which BLLs remained under 10 μg/dL for life, the effects of early vs late lead exposure on renal function cannot be discerned.
Other Studies Considered
Although the committee’s review focused on epidemiologic evidence, it is important to note that decades of studies in animal models have provided compelling evidence of lead-induced histopathologic changes in renal structure, particularly proximal tubular damage and sclerosis. They have also provided a suite of plausible molecular mechanisms of renal damage, including lead-induced mitochondrial dysfunction, inflammation, oxidative stress, and apoptosis (EPA 2012). Thus, the epidemiologic findings of lead-induced renal impairment, discussed below, are supported by biologic plausibility.
Renal function is characterized by the glomerular filtration or active tubular pumping of wastes and the simultaneous retention of essential molecules, such as water, glucose, amino acids, and electrolytes. Various techniques are
used to assess GFR clinically, including the measurement of creatinine clearance or serum cystatin C, a protein produced by all nucleated cells that undergoes glomerular filtration and tubular reabsorption in the kidney (Fried 2009). However, the measurement of GFR is not helpful in predicting early stages of clinical dysfunction. Recent research has also used the measurement of early-effect biomarkers, such as urinary B2-microglobulin, which normally is reabsorbed in the proximal tubules, and N-acetyl-β-D-glucosaminidase (NAG), a tubular enzyme that appears in urine as a result of cell death. Those and other so-called early-effect markers have not yet been sufficiently validated as predictors of clinical renal disease in populations exposed to nephrotoxic chemicals, but they serve as early indicators of toxicity.
Epidemiologic studies of the relationship between lead exposure and renal function can be divided into three categories: studies of the general population, which experiences environmental exposure; studies of the contribution of lead to disease progression in those who have chronic kidney disease (CKD); and studies of occupationally exposed workers. The evidence from studies in the first two categories is overwhelmingly convincing that lead exposure plays a role in the onset and progression of renal dysfunction. Epidemiologic studies of occupationally exposed workers are somewhat less consistent, in large part because they involve small samples and consequently have very poor statistical power and are unable to statistically adjust adequately for important confounding factors. Occupational studies also suffer from the healthy-worker effect (workers tend to be healthier than the general population and have lower mortality and morbidity rates, which could mask adverse effects of harmful exposures), other kinds of selection bias, and other methodologic issues.
Studies of the General Population
Numerous studies of the general US population derived from several NHANES evaluations have described associations between BLLs and renal function. They and the Normative Aging Study (Kim et al. 1996; Tsaih et al. 2004) and the Swedish Women’s Health Study (SWHS) (Åkesson et al. 2005) led NTP to conclude that “there is sufficient evidence available for an association between current [BLLs] <5 μg/dL in adults, measured at the time of study, and reduced kidney function in general populations” (NTP 2012, p. 102). For example, in an NHANES study of BLL and renal function in nearly 10,000 adults recruited in 1999-2002, Muntner et al. (2005) described an increased risk of CKD, defined as an estimated GFR under 60 mL/min/1.73 m2. Compared with those in the lowest quartile of BLL (under 1.06 μg/dL), people in the highest quartile (over 2.47 μg/dL) were 2.72 (95% CI: 1.47, 5.04) times more likely to have CKD. In support, Navas-Acien et al. (2009), in a comparable study of nearly 15,000 adults evaluated during 1999-2006, observed reduced GFR in those who had BLLs over 2.4 μg/dL vs those who had BLLs of 1.1 μg/dL or lower (adjusted OR = 1.56; 95% CI: 1.17, 2.08). The latter study also observed a
small but statistically significant trend for albuminuria. It controlled for more covariates, including blood cadmium concentration and blood pressure. Comparable findings of an association of remarkably low BLL with reduced GFR had been described in the SWHS (Åkesson et al. 2005). To put the SWHS study findings into perspective with regard to other factors that influence renal function, it should be noted that EPA calculated that the magnitude of the impact of a change in BLL from 1.1 μg/dL (the SWHS 5th percentile) to 4.5 μg/dL (the 95th percentile) on GFR “would be comparable to the loss of renal function associated with an increase in [body mass index] of 7 kg/m2 or an increase in age of 4.7 years” (EPA 2006, 2012).
For years, the idea of reverse causality (that impaired renal function results in reduced elimination of lead from blood and therefore a high BLL) could not be ruled out. Given the cross-sectional nature of the studies described above, it is not possible to rule it out on the basis of the studies alone. However, many earlier studies clearly suggested that renal dysfunction is caused by lead exposure (Batuman et al. 1981, 1983; Emmerson 1991). New lines of investigation also appear to rule out reverse causality. In Taiwan, in a 4-year longitudinal study of patients who had CKD, baseline BLL was associated with a decline in renal function (Yu et al. 2004). And in the Normative Aging Study, BLL and serum creatinine were associated even when serum creatinine was in the normal range (Kim et al. 1996; Tsaih et al. 2004). Thus, it does not appear that impaired renal function is required to drive the association between the two biologic measures.
At first glance, the very low BLLs associated with CKD in the NHANES and other large cross-sectional studies may appear to defy credibility. However, it is important to appreciate, as noted in Chapter 3, that BLL in adulthood probably captures cumulative dose to some extent.
Studies of Patients Who Have Chronic Kidney Disease
Several clinical studies of patients who have CKD have provided additional evidence of a causal relationship between lead exposure and a decline in renal function. In the above-mentioned longitudinal study in Taiwan, 121 patients who had well-controlled CKD were enrolled. One way to estimate the soft-tissue burden of lead is to administer a dose of calcium disodium ethylenediamine tetraacetic acid (CaNa2EDTA), a lead-chelating agent, and measure the amount of chelatable lead excreted in urine during the ensuing 72 h. Both CaNa2EDTA-chelatable lead and BLL at baseline were associated with statistically significant declines in serially measured GFR during the ensuing 4 years (Yu et al. 2004). Additional evidence was derived from a randomized clinical trial in 202 patients who had chronic renal insufficiency (serum creatinine 1.5-3.9 mg/dL). After a 24-month observation period, 64 patients who had increased CaNa2EDTA-chelatable lead and a mean BLL of 5.3 μg/dL were randomized to receive either chelation therapy with intravenous CaNa2EDTA or intravenous
placebo and were followed for more than 2 years. During the first 3 months of the trial, there was a statistically significant improvement in those who received CaNa2EDTA chelation therapy but not in those who received placebo. In addition, the later rate of decline in GFR was lower in the chelated group than in the placebo group (Lin et al. 2003). It is possible, however, that the improvement in renal function was due to effects of CaNa2EDTA treatment other than lead removal, inasmuch as antioxidant effects and improved blood flow have also been described in connection with this drug (Jacobsen et al. 2001; Saxena and Flora 2004; EPA 2012). Nevertheless, collectively, those and other studies of patients who had renal impairment indicate a role of low-level lead exposure and progression of disease in patients who have diabetes (Lin et al. 2006a) and who do not have diabetes (Lin et al. 2006b; EPA 2012).
Studies of Occupational Exposures
Chronic lead nephropathy in the occupational setting has been noted for many years, but the lead dosimetry in early studies was generally poorly characterized (Emmerson 1973; Cramér et al. 1974; Wedeen et al. 1975). Most of the occupational studies have been small and have failed to consider other important confounders of the association between lead exposure and renal function adequately. In addition, the healthy-worker effect, which is probably pronounced in industries in which health surveillance is required, may bias possible associations toward the null (Ekong et al. 2006).
A longitudinal study of a large population of current and former workers in 26 lead-using facilities in South Korea has to some extent been able to overcome those limitations. Workers were evaluated three times, roughly a year apart, for BLL, tibia bone lead, and markers of renal function (Weaver et al. 2009). In the initial cross-sectional analysis of 803 lead workers (mean BLL 32 μg/dL; standard deviation 15) and 135 controls, it appeared that lead exposure in the “moderate dose range” was adversely associated with renal function (as measured by serum creatinine, creatinine clearance, and blood urea nitrogen), especially in older workers (Weaver et al. 2003).
At the third evaluation, 537 current and former workers, 25% of whom were women, were available for analysis (Weaver et al. 2009). Using various statistical methods, the investigators attempted to separate the effects of recent dose (BLL) from cumulative dose (tibia lead) by controlling for baseline BLL and tibia lead. That effort was complicated by the fact that mean BLL did not differ among evaluations 1-3 in either sex. Nevertheless, both current and cumulative lead dose were associated with changes in renal function. In reviewing that work, EPA (2012) pointed out that the problem with setting a threshold for BLL regarding kidney outcomes is related to differential responses according to age. In young workers, there is a hyperfiltration pattern in which GFR increases as BLL (or tibia lead) increases. The opposite pattern, indicative of “traditional nephrotoxicity”, is observed in older workers.
An additional study of the Korean cohort has explored possible effect modification of the relationship between occupational lead exposure and renal function. A polymorphism of the vitamin D receptor (the variant B allele) was found to worsen the association between lead exposure and renal function. And in those who had the ALAD2 allele, higher BLLs were associated with higher calculated creatinine clearance (Weaver et al. 2006).
Several other studies published in the last 5 years have reported adverse associations between occupational lead exposure and impaired renal function. A study in Nigeria described impaired creatinine clearance in 190 lead workers (mean BLL 50 μg/dL) compared with 80 controls but did not adjust for any covariates (Alasia et al. 2010). A study of 87 industrial workers (mean BLL 29 μg/dL) and 61 controls in Pakistan reported statistically significant correlations between BLL and serum creatinine, uric acid, and several early biologic markers of renal dysfunction (Khan et al. 2008). Early biologic markers of tubular and glomerular function were explored in 155 battery workers (mean BLL 20 μg/dL) and 36 controls in China (Sun et al. 2008). The study reported a dose-response relationship between BLL and renal function, biomarkers of bone metabolism, and the prevalence of osteoporosis. Those and many other studies summarized by EPA (2012) have been rather consistent in making the link between occupational lead exposure and impaired renal function.
Studies of Renal Endocrine Function
In addition to its primary role as an excretory organ, the kidney has some endocrine functions, including the synthesis of the hormone erythropoietin (EPO) in the specialized epithelial-like cells in the peritubular capillary lining of the renal cortex and the synthesis, in the juxtaglomerular apparatus of the kidney, of renin, an enzyme that is intimately involved in the regulation of blood pressure via the renin-angiotensin-aldosterone system. EPO is responsible for the stimulation of erythropoiesis in the bone marrow and plays a role in preventing neuronal death after cerebral injury. Patients who have CKD typically require treatment with EPO to correct the anemia associated with the disease. There is clinical and epidemiologic evidence that environmental or occupational lead exposure adversely affects EPO production and results in delayed erythrocyte regeneration after blood loss or blood donation. The study by Grandjean et al. (1989) described earlier in the discussion of hematopoietic effects ultimately led to that discovery. Although the authors attributed the delay in erythrocyte regeneration to the impairment of heme synthesis by lead, it was later demonstrated in an environmentally exposed population of pregnant women that the effect was due to lead-induced impairment of EPO production (Graziano et al. 1991), in this case in response to the anemia of pregnancy. The relationship between BLL and EPO was later described in lead workers in Austria (Osterode et al. 1999). Thus, occupational lead exposure can have an adverse effect on renal EPO production and the regulation of hematopoiesis.
There is also evidence that lead exposure has adverse effects on the renin-angiotensin-aldosterone system and that these effects may contribute to leadinduced hypertension. A considerable body of literature concerning animal models indicates that lead exposure is associated with an increase in renal renin secretion (Vander 1988). Findings from human studies are more variable, although studies with larger samples have generally found increased plasma renin and increased serum aldosterone, which would be a logical consequence of increased renin secretion. For example, a study of 33 normotensive men, 25 of whom were occupationally exposed to lead for weeks to months (mean BLL 35.6 μg/dL), found positive exponential relationships between BLL and plasma renin activity, angiotensin, angiotensin-converting enzyme, and aldosterone levels (Campbell et al. 1985). A more recent study of 50 occupationally leadexposed and nonexposed adults in Egypt (BLLs not specified) reported higher serum aldosterone in the exposed than in the nonexposed group and higher plasma renin activity in male workers than in female workers (Shouman and El-Safty 2000).
Summary Findings on Renal Effects
The committee concludes that the evidence is sufficient to infer causal relationships between BLLs under 40 μg/dL (upper acceptable limit in the OSHA standard) and impaired renal function (see Table 4-3) (Staessen et al. 1992; Muntner et al. 2005; Navas-Acient et al. 2009). The adverse effects are manifested by increases in serum creatinine, impaired creatinine clearance, and GFR and by alterations in renal endocrine functioning that may contribute to delayed blood regeneration capacity and hypertension. The committee’s conclusions agree with those of NTP (2012) and those proposed by EPA (2012).
REPRODUCTIVE AND DEVELOPMENTAL EFFECTS
The adult reproductive system is a critical target for the toxic effects of lead. Men and women at the peak of their reproductive years serve as firing-range personnel. Occupationally relevant reproductive outcomes in lead-exposed men and women may include adverse effects on hormone concentrations, fertility, semen, menstruation, and gonadal histology and architecture. Potential leadinduced developmental effects (such as birth defects, spontaneous abortion, preterm birth, intrauterine growth retardation, low birth weight, and reduced spermatogenesis in exposed offspring) are also relevant.
Potential modes of action of lead reproductive toxicity include disruption of the hypothalamic-pituitary-gonadal axis through reduced luteinizing hormone secretion and reduction in the expression of the steroidogenic acute regulatory protein (Crain et al. 2008; EPA 2012). Lead may also interfere with cation-dependent secondary messenger systems that mediate pituitary hormone release
TABLE 4-3 Key Studies of the Renal Effects of Lead
| Health Effect | Population Characteristics | Measures | Effect Estimate | Why Study Is Relevant to DOD | Reference |
| Increase in serum creatinine | 459 men selected at random from Normative Aging Study of healthy veterans in greater Boston area | Mean BLL = 8.7 μg/dL | 10-fold increase in BLL was associated with increase of 0.08 mg/dL in serum creatinine. | Study of veterans with BLLs in range of interest. | Kim et al. 1996 |
| Impaired creatinine clearance | Random sample of 965 men, 1,016 women in areas with low or high environmental cadmium | Mean BLL = 11.4 μg/dL in men, 7.5 μg/dL in women | 10-fold increase in BLL was associated with reduction in creatinine clearance of 10-13 mL/min. | Strong evidence of impaired renal function at relatively low BLLs; well-done study. | Staessen et al. 1992 |
| CKD based on GFR clinical cutpoint | 10,000 adults from NHANES study (1999-2002) | Highest quartile of BLL was >2.47 μg/dL;a lowest quartile was <1.06 μg/dL | CKD was 2.72 times more likely in highest than in lowest quartile of BLL. | Strong evidence of effects in large US population. | Muntner et al. 2005 |
| CKD, albuminuria | 15,000 adults from 1999-2006 NHANES studies | Highest quartile of BLL was >2.4 μg/dL;a lowest quartile was <1.1 μg/dL | CKD was 1.56 times more likely in highest than in lowest quartile of BLL. | Strong evidence from NHANES data. | Navas-Acien et al. 2009 |
| Creatinine clearance, GFR | 820 women in Lund, Sweden | Mean BLL = 22 μg/dL (range 11-46 μg/dL) | For each 1 μg/dL in BLL, GFR declined by 0.20 mL/min, creatinine clearance by 0.18 mL/min. | Well-done study that adjusted for many covariates. | Akesson et al. 2005 |
| Decline in GFR over 4 y | 121 CKD patients in Taiwan | 3.4, 4.9 μg/dL in those with low, high chelatable lead burden, respectively, as assessed with CaNa2EDTA | Each increase of 1 μg/dL in BLL led to reduction in GRF of 4.0 mL/min. | Four-year longitudinal study. | Yu et al. 2004 |
Abbreviations: BLL, blood lead level; CaNa2EDTA, calcium disodium ethylenediamine tetraacetic acid; CKD, chronic kidney disease; DMSA, 2,3-dimercaptosuccinic acid; GFR, glomerular filtration rate; NHANES, National Health and Nutrition Examination Survey.
aIt is important to appreciate that BLL in adulthood probably captures cumulative dose to some degree.
and storage. Lead-induced production of reactive oxygen species (ROS), calcium mimicry, and binding to protein sulfhydryl groups are potential biochemical modes of action (EPA 2012). And lead can alter zinc, iron, and potassium function in cells (Sun and Suszkiw 1994; Lal et al. 1996; Lasley and Gilbert 1996).
Pharmacokinetic modes of action warrant consideration. Lead is consistently detected in human testes, epididymides, prostate, and seminal vesicles (Oldereid et al. 1993). Of particular concern is the accumulation of lead in the epididymides because this results in long-term exposure of semen to lead. Lead enters human sperm (and probably related cells at earlier stages of differentiation) through voltage-gated potassium and calcium channels (Benoff et al. 2007). Different isoforms of those channels differ in their ability to transport lead, and expression of channel isoforms likewise differ among men. Exposure to a given BLL may therefore result in different intracellular lead concentrations in male germ cells and have different effects on fertility. Pregnant women can transfer lead to their fetuses, as demonstrated by the strong correlation between maternal and umbilical cord BLLs (Gardella 2001). During pregnancy and after birth, skeletal lead stores are an important contributor to maternal BLLs (Gulson et al. 1999). Lead levels in breast milk increase with increasing maternal BLL, and this poses an additional risk to the neonate (Li et al. 2000).
Conclusions from the 2012 Environmental Protection Agency and 2012 National Toxicology Program Lead Documents
Environmental Protection Agency 2012 Integrated Science Assessment for Lead (Second External Review Draft)
EPA’s review of recent epidemiologic studies of environmental lead exposure and reproductive function concludes that there is strong evidence that increasing lead exposure is associated with reduced male fecundity or fertility, decreases in sperm count, and reduced sperm velocity and motility. EPA’s draft report further concludes that deleterious associations with sperm count and quality are observed in occupationally exposed men who have mean BLLs as low as 20-45 μg/dL. EPA concluded that there was some association between maternal lead exposure and low birth weight; toxicologic studies in animals have shown that lead exposure during early fetal development can result in abnormal retinal development and alterations in the developing hematopoietic and hepatic systems.
National Toxicology Program 2012 Monograph on Effects of Low-Level Lead
The NTP concluded that there was inadequate evidence to conclude that BLLs under 10 μg/dL are associated with adverse effects on reproduction in men. There was, however, sufficient evidence to conclude that BLLs of 15
μg/dL and over are associated with adverse effects on sperm or semen and that BLLs of 20 μg/dL and over are associated with delayed conception time. Decreases in sperm count, density, and concentration were seen in men who had mean BLLs of 15-68 μg/dL. The NTP also concluded that there was sufficient evidence that maternal BLLs under 5 μg/dL are associated with reduced fetal growth or lower birth weight. There is limited evidence that maternal BLLs under 10 μg/dL are associated with preterm birth and spontaneous abortion. Prospective studies reviewed by the NTP provided limited evidence that prenatal exposure to BLLs under 10 μg/dL is associated with reduced postnatal growth in children. The NTP recognized that its conclusions about prenatal lead exposure were confounded by possible continuing postnatal exposure to lead (associated with BLLs under 10 μg/dL) that is also associated with reduced postnatal growth in children.
Other Studies Considered
Animal Toxicology Studies
The committee considered several key pieces of evidence derived from animal studies in its deliberations. Those studies identified effects on hormone function, gonad structure, and developmental (teratogenic) responses that have been incompletely examined in human epidemiologic investigations and provided additional weight of evidence that supports the committee’s conclusions.
In male rodents and monkeys, long-term lead exposure resulting in BLLs over 20 μg/dL reduced serum concentrations of luteinizing hormone, follicle-stimulating hormone, testosterone, estradiol, and other reproductive hormones (Foster et al. 1993; Allouche et al. 2009; EPA 2012). Decreased reproductive-organ weight, histologic changes in the testes and germ cells, reduced fecundity, and decreased numbers of uterine and ovarian estrogen, luteinizing hormone, and follicle-stimulating hormone receptors occur in animals after lead exposure (Wiebe and Barr 1988; Wiebe et al. 1988; Singh et al. 1993; Batra et al. 2001; Wang et al. 2008; Anjum et al. 2011). Dumitrescu et al. (2008) demonstrated that exposure of female rats to lead at 150 ppb before mating and during pregnancy caused a concentration-dependent shift in the male-to-female sex ratio of the offspring, but this effect has not been replicated in other studies (Ronis et al. 1998).
Lead induces reproductive and developmental effects in laboratory rats after gestational or lactational exposure. Many of the effects occur in a concentration-dependent manner and have been observed at maternal BLLs that do not result in overt maternal toxicity (under 40 μg/dL). Animal studies have further demonstrated that effects of lead exposure during early development include impairment of retinal development and alterations in the developing hematopoietic and hepatic systems. Toxicology studies in male animals have reported delayed
sexual maturity, as seen in reduced prostate weight and other reproductive outcomes, reaching significance at BLLs of 34 μg/dL (Sokol et al. 1985).
Animal models are important for predicting human reproductive and developmental toxicity (Tyl 2005; Cooper and Doerrer 2010; Daston and Knudsen 201), but, because of differences in such things as pharmacokinetics and hormonal regulaton of parturition, their validity in modeling human pathologic conditions (in particular, conditions that are of a complex and multifactorial nature, such as preterm birth) must be considered in interpreting the data. The use of animal studies for lead risk assessment has important limitations. For example, human spermatogenesis is far less efficient than that of other mammals, with efficiency being defined as the estimated number of spermatozoa generated per day per gram of testicular parenchyma (Amann 1970; Johnson 1995; Johnson et al. 2000). Teratozoospermia (abnormal sperm morphology) is more common in humans than in animal models (Hafez 1987; WHO 2010).Although it remains unclear what animal species or strain is best for modeling lead effects on humans, the only consistent findings on the effects of lead exposure in the male animal and the human male are related to decreased sperm motility and increased spontaneous acrosome loss. Thus, one could infer that lead exposure may have a more deleterious effect on sperm function than on sperm production, and thus affect male fertility status, than previously thought.
Male Reproductive Effects
The committee considered two broad types of human epidemiologic studies during its deliberations: occupational studies and studies of male patients in infertility clinics. In general, the committee focused its review on studies in which BLLs were reported. It also considered studies in which lead concentrations in seminal fluid (total ejaculate, including fluids produced by the accessory sex glands—seminal vesicles and prostate—and sperm) or seminal plasma (fluid remaining after sperm are removed from the ejaculate) were measured.
Several studies report an association between occupational lead exposure and decreased sperm count, velocity, and motility; greater haploidy of sperm DNA; and morphologically apparent sperm abnormalities. The studies were of men who worked in battery- or paint-manufacturing plants for 10-15 years. Workers in the highest-exposure groups had mean BLLs of 68.26 μg/dL (Naha and Manna 2007) and 77.22 μg/dL (Naha and Chowdhury 2006). Nonoccupationally exposed controls had mean BLLs of 10-15 μg/dL. A Taiwanese study reported that male lead-battery workers who had BLLs of 45 μg/dL or over had more sperm head abnormalities, greater sperm DNA denaturation, and greater sensitivity to denaturation than workers who had BLLs under 25 μg/dL (Hsu et al. 2009). In contrast, no association was observed between lead exposure and changes in semen volume; sperm count, motility, or velocity; or ROS production in the same study. Kasperczyk et al. (2008) reported that Polish metalworkers who had a mean BLL of 53.1 μg/dL had lower sperm motility and higher seminal
lipid peroxidation than nonexposed office workers who had a mean BLL of 8.47 μg/dL. The authors also reported no difference in semen volume, sperm count, or sperm morphology among or between the groups. One problem associated with these occupational studies is that many of the control groups were reported to have BLLs over 8 μg/dL, so it was difficult to assess the effects of very low lead exposures and to determine whether there actually is a “safe” level of lead exposure. That may help to explain why epidemiologic studies have produced conflicting findings.
Several studies have examined men who were exposed to lead nonoccupationally. In general, BLLs of nonexposed, nonsmoking subjects recruited from the general population or from infertility clinics were generally lower than those seen in the occupational studies. BLLs (up to about 15 μg/dL) in Croatian men without occupational exposure to lead or other metals were associated with increased percentages of pathologic sperm, including wide sperm and round sperm (Telisman et al. 2007). Chia et al. (1992) reported that men who were attending an andrology clinic in Singapore and had reduced sperm motility had higher BLLs (mean 7.2 μg/dL) than men who had normal sperm motility (mean 5.1 μg/dL). However, blood cadmium concentration was also increased in this study population (Chia et al. 1992). Other studies of men who were attending infertility clinics reported no association between BLL (mean 8-15 μg/dL) and alterations in sperm characteristics (Xu et al. 1993; Meeker et al. 2008; Mendiola et al. 2011). The largely negative studies may reflect in part the use of sperm counts as the primary indicator of lead-associated infertility effects. There is strong evidence that human sperm count displays geographic, regional, and time-dependent decreases (Carlsen et al. 1992; Swan 2006). Spermatogenesis is testosterone-dependent. Consistent with the reports of declining sperm counts, Travison et al. (2007) reported that serum testosterone is declining over calendar time (for example, in birth cohorts) in American men and that the decline is age-independent. Therefore, serum hormone concentrations are also inappropriate as biomarkers of the potential antireproductive effects of lead in the human male. Another weakness of the studies is the failure to control for untreated fertility issues in the female partners of the study subjects and for the effects of other metal contaminants.
Several relatively recent investigations have focused on semen lead concentration as a biomarker, as has long been the case in the reproduction community (see review by Benoff et al. 2000). Mendiola et al. (2011) reported an association between semen lead concentration and increased percentage of immotile sperm; however, the analysis failed to adjust for exposure to other metals. Slivkova et al. (2009) reported a negative correlation between semen lead concentration and pathologic changes in sperm (specifically, flagellum ball), but no correlations with other sperm alterations were observed. There is additional evidence that increasing seminal plasma lead concentrations are associated with alterations in two sperm functions required for fertilization: decreased motility, which is the major determinant of pregnancy outcome (Shulman et al. 1998; Stone et al. 1999), and increased spontaneous acrosome loss (Benoff et al. 2000, 2003a,b). The latter process can be mimicked by incubating fertile donor sperm in medium containing lead at levels seen in seminal plasma (Benoff et al. 2000,
2003a,b). One study has shown that BLLs do not correlate with the concentration of lead in male reproductive tissues or any somatic tissues examined (Oldereid et al. 1993), but it remains unclear whether BLL or seminal plasma lead concentrations are equally predictive of lead-induced reproductive toxicity in men. Therefore, the committee focused its attention on the use of BLL, commonly assumed to have general applicability as a biomarker of lead exposure.
Female Reproductive Effects
Lead exposure was found to affect female reproductive function in both epidemiologic and toxicologic studies. BLL was associated with changes in hormone concentrations in women; however, study results varied in the magnitude and direction of the changes. The EPA report (2012) states that the selected studies are inconsistent with respect to associations between lead and fertility but suggest that there is evidence of a direct relationship between female lead concentrations and decreased fertility rates. Although the toxicologic and epidemiologic studies examined responsiveness to lead exposure during different exposure periods, both lines of evidence support the conclusion that exposure to lead affects some aspects of reproductive function in women.
The previous EPA (2006) report stated that lead exposure does not result in female sterility but can disrupt female fertility. That finding is supported by animal studies and epidemiologic investigations. Studies by Bloom et al. (2010, 2011) provided evidence that higher BLL was not associated with oocyte fertilization but that embryo cell number was lower in association with higher BLL. Chang et al. (2006) found that women who were seeking care at a fertility clinic had higher BLLs than women who delivered normally at a nearby medical center. In that study, the adjusted effect estimates threshold BLL was 2.5 μg/dL. Al-Saleh et al. (2008a) reported that lower rates of fertilization were associated with BLL but not with follicular lead concentrations. In contrast, Silberstein et al. (2006) found that lead concentrations in the follicular fluid exceeded those in blood and that even at low concentrations the presence of lead in follicular fluid was inversely associated with pregnancy. Those studies are limited in that they each used women who were seeking fertility assistance in an in vitro fertilization clinic where only a woman’s lead levels were considered and thus ignored the potential contribution of lead to sperm dysfunction. The study by Silberstein et al. suffers from a small sample, the fact that follicular and plasma lead concentrations did not correlate well with each other, and potentially confounding factors (such as smoking and occupation) that were not considered in the analysis.
Yin et al. (2008) examined whether there is an association between plasma lead concentrations and anembryonic pregnancies (spontaneous abortion in the first trimester associated with a normal amniotic sac but loss of the embryo). Women who delivered at term had mean plasma lead lower than that of women
who had anembryonic pregnancies. Lamadrid-Figueroa et al. (2007) found that women in the highest one-third for plasma:blood lead ratio had a greater risk of spontaneous abortion than women in the lowest one-third, but no associations were found if whole blood or plasma alone was used. Gundacker et al. (2010) reported that women who had miscarried during a previous pregnancy had higher placental lead concentrations (39 μg/kg) than women who had not miscarried in the past (27 μg/kg).
Most of the epidemiologic studies in the EPA report were cross-sectional, so it is difficult to ascertain the most sensitive period in a woman’s lifetime, when lead exposure would be most detrimental to reproductive health.
The NTP (2012) report points out that although current studies provide some level of evidence of an association between lead and adverse effects on female reproductive health, it cannot now be determined whether there is a critical window for exposure. Furthermore, it is difficult even to attempt to determine when a sensitive period might be inasmuch as older adults were likely to have had BLLs over 10 μg/dL as children. The NTP report cites a large cohort study of mother-infant pairs that did not find an association between BLLs (mean 2.1 μg/dL) and preterm birth (Zhu et al. 2010). The NTP’s conclusion of a limited association between low-level lead exposure and spontaneous abortion was based predominantly on a single case-control study of women who had occupational exposure to lead (Borja-Aburto et al. 1999). Another possible weakness in both studies was the small number of available datasets with which to study responses in humans. In addition, the use of in vitro fertilization clinic data takes a woman’s BLL into account only when there are problems with fertility without considering possible problems with male fertility that may be associated with BLL. Moreover, in vitro fertility clinic data represent only women who are actively seeking help for fertility and may not be representative of all women of child-bearing age.
The NTP concluded that there was limited evidence that BLL under 5 μg/dL is associated with delayed puberty. That conclusion was based in part on a South African longitudinal birth-to-20 cohort study that found that a BLL of 5 μg/dL or over was significantly associated with delayed breast and pubic-hair development (according to Tanner staging) and with age at menarche (Naicker et al. 2010). The association was significant even after adjustment for socioeconomic factors and anthropometric measurements. Evidence of an association between low lead exposure and hormone markers of delayed onset of puberty in girls is emerging (Gollenberg et al. 2010).
The NTP (2012) concluded that there was sufficient evidence of an association between maternal BLL under 5 μg/dL and reduced fetal growth and low birth weight (under 2,500 g after at least 37 weeks of gestation). That association is supported by several prospective studies that measured maternal BLL during pregnancy (Gundacker et al. 2010), a large retrospective cohort study (over 43,000 mother-infant pairs) that reported a mean maternal BLL of 2.1 μg/dL (Zhu et al. 2010), and a number of cross-sectional studies of maternal or umbilical cord blood lead at delivery (Bellinger et al. 1991).
Although the results are not entirely consistent among studies, the evidence on maternal or umbilical cord blood lead (under 10 μg/dL) and the large number of studies led the NTP to conclude that there was sufficient evidence of an association between maternal BLL under 10 μg/dL and reduced fetal growth and low birth weight. In contrast, the NTP concluded that there was only limited evidence that maternal BLL under 10 μg/dL is associated with spontaneous abortion and preterm birth. Although a number of prospective and cross-sectional studies have reported an association between prenatal BLL under 10 μg/dL and preterm birth, the conclusion of limited evidence was based primarily on inconsistency of the data and a large study of mother-infant pairs that failed to find the same relationship. EPA (2012) also concluded that there was little evidence to support an association between maternal or paternal lead exposure and the incidence of spontaneous abortion.
Prenatal and Postnatal Developmental Effects
Studies of the adverse developmental effects of lead exposure in utero and during the immediate postpartum neonatal period were considered relevant to the committee’s charge. The literature base is extensive, and a complete review was deemed outside the committee’s charge. Rather, it selected studies that examined different developmental end points. Many of the longitudinal studies measured umbilical BLL at birth and grouped infants as having had “low” exposure (for example, under 3 μg/dL), “medium” exposure (for example, 6-7 μg/dL), or “high” exposure (10 μg/dL or above). Postnatal development was then assessed at various intervals.
Detrimental effects of lead exposure on IQ and cognitive development have been observed in many studies (Baghurst et al. 1992; Bellinger 2000; Wasserman et al. 2000; Al-Saleh et al. 2009; Jedrychowski et al. 2009) and suggest the presence of a causal association between lead exposure and adverse developmental neurologic effects. Individual studies diverge in their ability to identify windows of susceptibility. In some, a lack of association between postnatal BLL and concurrent cognitive or other development scores was observed (e.g., Al-Saleh et al. 2009), but the finding suggests that in utero lead exposure may account for the effects seen. Other studies (e.g., Wasserman et al. 2000) have suggested that prenatal and postnatal exposures that occur at any time during the first 7 years of life are likely to be independently associated with small decrements in later IQ scores.
Studies have shown an association between umbilical blood lead concentration under 10 μg/dL and reduced head circumference (Al-Saleh et al. 2008b), effects on infant attention (Plusquellec et al. 2007), abnormal reflexes and abnormal results on neurologic soft signs scales (Ernhart et al. 1986), reduced body-weight gain (Sanin et al. 2001), and decreased body-mass index (NTP 2012). Deficits in visual function in children were also seen at umbilical blood lead levels as low as 10.5 μg/dL (Rothenberg et al. 2002). Increased maternal
tibia lead concentration 1 month after birth has been associated with decreased infant body weight at birth (Gonzαlez-Cossío et al. 1997). Results in a Mexico City cohort showed associations between maternal BLL at midpregnancy (mean 7.7 μg/dL) and brainstem auditory evoked responses in newborns, 3-month-old infants, and 67-month-old children (Rothenberg et al. 2000). Studies of human male sexual development are few, but the available data (for example, on timing of puberty onset based on testicular volume, delays in axillary and pubic-hair development, penile staging, and mean testosterone level) provide suggestive evidence that even low lead exposure (based on BLLs of over 5 μg/dL to under 10 μg/dL) is associated with delayed puberty in offspring (Hauser et al. 2008).
Summary Findings on Reproductive and Developmental Effects
The committee concludes that the evidence is sufficient to infer causal relationships between BLLs over 40 μg/dL and adverse effects on sperm and semen, including decreased sperm count, reduced sperm motility, and increased morphologic abnormalities (see Table 4-4). The committee concludes that the evidence to infer causal relationships between BLLs under 40 μg/dL and adverse effects on sperm and semen is limited. The committee also found strong evidence of a causal relationship between prenatal maternal BLLs under 10 μg/dL and adverse developmental effects in infants and children and sufficient evidence of an association between maternal BLLs under 5 μg/dL and reduced fetal growth and low birth weight (see Table 4-5). The committee’s conclusions are consistent with those of EPA and the NTP.
Animal studies that illustrate the capacity of lead at levels below those recognized as overtly toxic to modify immune function and compromise host resistance against infectious disease date back at least 50 years (Mishra 2009). Age-based exposure studies also suggest that BLLs previously thought safe (under 10 μg/dL) may be associated with later-life immune alterations (Dietert and Piepenbrink 2006). The potential for adverse human health effects of leadinduced alterations in the immune system is rapidly emerging as a matter of increasing scientific and public concern. Although studies of the effects of lead exposure on children are plentiful, few have examined effects on the adult immune system, and their results have often been contradictory. There are, however, compelling indications from epidemiologic and worker studies that lead can induce immune alterations in exposed humans (Fischbein et al. 1993; Undeger et al. 1996; Kuo et al. 2001; Qiao et al. 2001; Mishra et al. 2003; Mishra 2009; Garcia-Leston et al. 2011), particularly changes in B lymphocytes; in the nature, extent, and spectrum of circulating antibodies; and in T-lymphocyte profiles.
TABLE 4-4 Key Studies of the Male Reproductive Effects of Lead
| Health Effect | Population Characteristics | Measures | Effect Estimate | Why Study Is Relevant to DOD | Reference |
| Sperm abnormalities | Men (n = 80; mean age = 29.2 y) working at a Taiwanese battery plant for mean work duration of 1.7 y | Mean BLLs in groups were 21.3 (10.5-24.9), 37.3 (26.9-45.0), 53.3 (45.7-70.9) μg/dL; overall mean BLL = 40.2 μg/dL | ANOVA (p values <0.05) for: increased sperm head or neck abnormalities in highest-exposure group, lead-induced increases in sperm chromatin structure assay (áT, COMPáT) in both exposure groups. | Data suggesting negative outcome in group of relevant age. | Hsu et al. (2009) |
| Reduced sperm motility | Healthy, nonsmoking, fertile men (n = 63, mean age in three groups were 34.4 to 38.3 y) that worked at a Polish zinc and lead metalworks for an average of about 13-14 y | High-exposure workers: mean BLL = 53.1 (40-81) μg/dL, PbS = 2.02 μg/dL; low-exposure workers: mean BLL = 34.7 (25-40) μg/dL, PbS = 2.06 μg/dL; officeworker controls: mean BLL = 8.47 μg/dL, PbS = 1.73 μg/dL | 34% reduction in percentage of motile sperm after 1 h in highexposure group vs controls (p = 0.034). | Clinically important outcome in group of relevant age. | Kasperczyk et al. (2008) |
| Abnormal sperm | 240 Croatian men 19-52 years of age with no occupational exposure to lead or other metals | Median BLL = 4.92 (1.13-14.9) μg/dL | Increase in immature sperm concentration; in percentages of pathologic, wide, round, short sperm; in serum testosterone, estradiol. Decrease in seminal plasma zinc, in serum prolactin. | Data suggesting negative outcome in group of relevant age. | Telisman et al. (2007) |
Abbreviations: αT,extent of DNA denaturation per cell; BLL, blood lead level; COMP αT, percentage of sperm with increased sensitivity to DNA denaturation; PbS, seminal plasma lead concentration.
TABLE 4-5 Key Studies of the Female Reproductive Effects of Lead
| Health Effect | Population Characteristics | Measures | Effect Estimate | Why Study Is Relevant to DOD | Reference |
| Infertility | Women receiving care in fertility clinic in 2000-2001 or delivering normal infant in nearby medical center in 1999 | BLL >2.5 μg/dL | OR (95% CI): ≤2.5 μg/dL, 1.00 (reference); >2.5 μg/dL, 2.94 (1.18, 7.34). | Relevant BLL. | Chang et al. 2006 |
| Achieving fertilization or pregnancy | Women (19-50 y old) undergoing IVF | BLL = 3.34 ± 2.24 μg/dL | OR (95% CI) (unit not given, assume results are per 1 μg/dL): pregnancy, 0.55 (0.23, 1.31); fertilization, 0.30 (0.08, 1.03); in reduced adjusted model for fertilization, OR for BLL was 0.38 (0.14, 0.99). | Relevant BLL. | Al-Saleh et al. 2008a |
| Oocyte maturity, oocyte fertilization | Women in Study of Metals and Assisted Reproductive Technologies; women referred to Center for Reproductive Health at UCSF for infertility treatment and first IVF procedure | BLL = 0.34-1.5 μg/dL | RR (95% CI) per 1 μg/dL (controlled for cadmium): oocyte maturity (determined by metaphase II arrest), 0.54 (0.31, 0.93); oocyte fertilization, 0.97 (0.66, 1.43). | Relevant BLL. | Bloom et al. 2010 |
| Embryo cell number, embryo fragmentation score | Women in Study of Metals and Assisted Reproductive Technologies; women referred to Center for Reproductive Health of UCSF for infertility treatment and first IVF procedure and who generated embryos | BLL = 0.86 μg/dL | OR (95% CI) per 1 μg/dL (adjusted for mercury and cadmium): embryo cell number, 0.25 (0.07, 0.86); embryo fragmentation score, 1.71 (0.45, 6.56) | Relevant BLL. | Bloom et al. 2011 |
Abbreviations: BLL, blood lead level; CI, confidence interval; IRR, incidence rate ratio; IVF, in vitro fertilization; OR, odds ratio; RR, relative risk; SD, standard deviation; UCSF, University of California, San Francisco.
The exact mechanisms whereby lead interacts with the immune system remains unclear. However, several effects of lead on the immune system can be explained in the context of activation of the nuclear factor-κB (NF-κB) signal transduction pathway that represents a group of structurally related proteins. NF-κB plays a critical role in triggering and coordinating both innate and adaptive immune responses (Dietert and Piepenbrink 2006). In addition, Fischbein et al. (1993) suggested that lead may exert suppressive effects on lymphocyte functions because of its high affinity for the sulfhydryl groups on T-lymphocyte surface receptors and thus its interference with antigen processing from monocytes to T lymphocytes and consequently with cell-to-cell cooperation. Lead also appears in animal models to affect the immune system indirectly by altering regulation of the endocrine and nervous systems (Mishra 2009).
To provide a better understanding of the studies discussed in this section and to provide perspective on the relevance of such effects on human health, an overview of the immune system is provided below.
Immune System Overview and Early Biologic Effects
The immune system consists of a complex network of cells and soluble mediators that interact in a highly regulated manner to generate an immune response of appropriate magnitude and duration. In animals and humans, immunotoxicity can be manifested in several distinct immunopathologic conditions, including allergic disease, immunodeficiency (immunosuppression), and autoimmunity. On contact with the immune system, chemicals can exert direct toxicity to specific components of the immune system, which can lead either to malfunctioning of the system as a whole or to disruption of regulatory systems that in turn can give rise to immunosuppression or exaggerated responses (manifested possibly as atopy or autoimmunity). In the former case, the immune system responds to the agent as an allergen (of low or high molecular weight), and this results in such disorders as allergic contact dermatitis (Descotes et al. 1995; Luster et al. 2001). In immunodeficiency, the immune system acts as a passive target for the chemical, and the result may be increased incidence or severity of infectious disease or cancer. Autoimmunity, a breakdown in immune tolerance, occurs when the agent directly or indirectly induces an immune response to “self” constituents, such as specific proteins or DNA, that leads to pathologic conditions.
The immune system has three basic components: humoral, cell-mediated, and innate (nonspecific) immune responses. Humoral immunity is primarily associated with B lymphocytes and the production of antibodies, also known as immunoglobulins (Ig). Differentiated B cells produce five Ig isotypes, each with unique structure and function: IgG, IgM, IgD, IgE, and IgA. For example, IgE is associated with allergic type 1 immediate hypersensitivity reactions, and IgA (a secretory antibody) is found in bodily secretions.
In contrast, cell-mediated immunity is associated with T lymphocytes (such as CD3+, CD4+, and CD8+) that develop from pluripotent stem cells in bone marrow and migrate to the thymus to mature and differentiate. As these precursor cells proliferate and differentiate into mature T lymphocytes, they increase their expression of surface CD (cluster of differentiation) markers that confer cell-type specificity. Immature T cells termed double CD negative cells (CD4-CD8-) lack expression of CD4 and CD8. On activation, one class of mature T cells differentiates into cytotoxic T lymphocytes (CTLs), distinguished by a cell-surface CD8+ marker, that can release cytokines (small molecules that act on cells and stimulate or inhibit their function) and kill tumor- and virus-bearing cells. T cells bearing CD4+ markers recognize antigen on their surface and release signature cytokines. CD4+ cells, critical for immune protection, can be subdivided into T-helper (TH) cells (TH1, TH2, TH3, and TH17) that help other immune cells by activating or directing their activities. For example, TH cells are essential in B-cell antibody class switching, activation and growth of CTLs, and maximizing bactericidal activity of phagocytes (such as macrophages). CD4+ cell populations, classified as either naïve (not exposed to antigen) T lymphocytes (CD45RA+) or activated memory T cells (CD45RO+), also include regulatory cell types, such as natural killer (NK) T cells and T-regulatory cells. TH cells are subdivided (on the basis of the types of cytokines that they release) into TH1 and TH2 phenotypes. Under normal circumstances, the TH1: TH2 ratio is balanced. Skewing toward TH1 or TH2 responses can result in a hyperinflammatory state or autoimmunity, respectively.
The innate (also known as nonspecific) immune system comprises the cells and mechanisms that defend a host from infection in a nonspecific manner. The cells recognize and respond to pathogens and tumor cells, but, unlike the adaptive immune system, they do not confer long-lasting or protective immunity. A number of critical cell types and soluble mediators are essential for nonspecific immunity. Macrophages and neutrophils play an essential role in the uptake (phagocytosis) and killing of ingested bacterial pathogens, whereas NK cells, also essential for innate immunity, kill virus-infected cells and neoplasms without prior sensitization. Each of those cell types is also an important source of immunoregulatory cytokines that mediate a variety of immune responses and inflammation.
Many immune end points, such as serum cytokine and Ig concentrations, are often used as indicators to predict early biologic effects of chemical-induced immunotoxicity. However, such immune measurements can lack sensitivity, are poorly standardized, or are poorly linked quantitatively to a disease process, so their relevance in assessing immunotoxic effects in humans is poorly established. In addition, translation of such early effects to a pathologic response or a disease phenotype on the basis of the toxicologic paradigm exposure ![]() internal dose
internal dose ![]() biologically effective dose
biologically effective dose ![]() early biologic effects
early biologic effects ![]() altered structure
altered structure
and function ![]() clinical disease is often not possible, because of the lack of specificity and persistence of the response.
clinical disease is often not possible, because of the lack of specificity and persistence of the response.
Conclusions from the 2012 Environmental Protection Agency and 2012 National Toxicology Program Lead Documents
Environmental Protection Agency 2012 Integrated Science Assessment for Lead (Second External Review Draft)
Because of few data on the immunologic effects of lead in exposed adults, EPA’s draft assessment relied heavily on animal data and epidemiologic studies in children to support its overall conclusion of a causal relationship between lead exposures and immune system effects in adults. EPA’s draft assessment concluded that lead exposure is associated with a broad spectrum of changes in both cell-mediated and humoral immunity that cumulatively promote a TH2 phenotype and a hyperinflammatory state. The principal conclusions were that lead, at BLLs of 30 μg/dL or under, induced an increased production of TH2 cytokines, suppressed production of TH1 cytokines, increased neutrophil infiltration (a marker of inflammation), and increased circulating IgE. In the studies of adults (mostly males) who had occupational lead exposures, the most consistent finding was decreased neutrophil function in workers who had BLLs of 21-71 μg/dL (Valentino et al. 1991). Studies reviewed by EPA also reported a shift toward TH2 cytokines in workers who had a BLL of 5 μg/dL. Uncertainty exists regarding the contributions of current lead exposures and cumulative lead stores in bone, but recent evidence on adults who had no occupational exposure has demonstrated altered concentrations of allergic IgE and specific cytokines in populations of adults who had BLLs of 1.9-7.0 μg/dL. On the basis of the consistency and coherence of findings across the continuum of related immune measures that demonstrated a stimulation of TH2 responses, combined with the supporting epidemiologic evidence on children, EPA concluded that there is a causal relationship between lead exposure and immune system effects. No BLLs were noted as part of that conclusion although decreased neutrophil function, increased concentrations of autoantibodies, increased inflammation, and a shift to TH2 cytokines were observed at BLLs of 30 μg/dL or lower and for some end points as low as 5 μg/dL. Some conclusions drawn by EPA differ from those of the NTP. That probably reflects the fact that data from studies of children and rodent models were used to provide the weight of evidence for EPA’s conclusions.
National Toxicology Program 2012 Monograph on Health Effects of Low-Level Lead
The NTP concluded that there was inadequate evidence on adults to support a causal relationship between a BLL under 10 μg/dL and any immune end points, including IgE, allergy, and other hypersensitivity reactions. That conclusion was
reached because of the lack of studies of the relationship between immune function and lead in human adults and because most of the studies reported changes only in observational characteristics, such as immune-cell profiles or Ig concentrations, rather than effects on immune function (such as lymphocyte proliferation, phagocytosis of infectious agents, and NK tumoricidal activity), which is linked more closely with pathologic response or human disease. Even in those cases, however, there was inadequate evidence of an association owing to a general lack of investigations at lower BLLs and inconsistency in available data. The metric of bone lead could have been relevant in terms of a cumulative dose, but very few studies that examined changes in immune end points used non-BLL metrics.
Other Studies Considered
Alterations in the immune response that can be easily studied in humans are limited and are primarily reflective of changes in circulating leukocyte profiles and Ig concentrations. Some studies of lead-exposed workers have reported lead-associated differences in serum Ig concentrations, percentages of CD4+ TH cells, increased circulating B-lymphocytes, decreased NK cells, and impaired lymphoblastogenesis (Ewers et al. 1982; Horiguchi et al. 1992; Fischbein et al. 1993; Queiroz et al. 1993; Undeger et al. 1996; Pinkerton et al. 1998; Basaran and Undeger 2000; El-Safty and Metwally 2000; Kuo et al. 2001; Qiao et al. 2001; Ayatollahi 2002; Mishra et al. 2003, 2006; Heo et al. 2004; Valentino et al. 2007; Mishra et al. 2010; Garcia-Leston et al. 2011). In contrast, other human investigations at similar BLLs demonstrated opposite or no effects on the same characteristics (Horiguchi et al. 1992; Queiroz et al. 1994; Pinkerton et al. 1998; Heo et al. 2004; Mishra et al. 2006; Freije and Dairi 2009). Some results of relevant studies that demonstrated early immunotoxic effects in lead-exposed workers who had BLLs of 40 μg/dL or under are described in brief below.
El-Safty and Metwally (2000) found reduced serum IgA, IgM, and IgG concentrations in lead-exposed plumbers who had an average BLL of 39 μg/dL. Studies by Mishra et al. (2006) supported the finding on circulating Ig and further demonstrated that lead-exposed workers who had an average BLL over 10 μg/dL had increased serum IgA. Fischbein et al. (1993) reported alterations in circulating blood mononuclear-cell profiles and lymphocyte function in firearms instructors. Results of that highly relevant study demonstrated that people who had an average BLL of 25 μg/dL or under, and to a greater extent those who had a BLL over 25 μg/dL, had lower concentrations and lower functional integrity of CD4+ TH lymphocytes than healthy nonexposed controls. Specifically, the absolute percentage and number of CD3+ and CD4+ cells were statistically significantly reduced, whereas those of CD8+ cells were unchanged. Functional integrity of T cells, as determined by proliferative responses to mitogens, was impaired, whereas that of T-cell-dependent B-lymphocyte function appeared to be within the normal range at all stages of maturation. Another study (Sata et al. 1997) demonstrated that circulating immune cell profiles were increased in leadexposed male workers, concentrations of B lymphocytes were positively associated
with BLL (average 39 μg/dL), serum IgG was negatively associated with cumulative lead exposure, and naïve memory T cells (CD3+/CD45RA+) were positively associated with cumulative lead exposure. In a study of Portuguese workers that evaluated immune cell profiles, Garcia-Leston et al. (2011) demonstrated a significant decrease in percentages of CD8+ cells with a concurrent increase in the CD4+: CD8+ ratio in exposed people who had BLLs of 40 μg/dL or higher. In a clinical context, this finding could be significant (although the extent of CD8+ suppression needed for a clinical outcome is unknown) inasmuch as a decrease in CD8+ cells could increase susceptibility to viral infections or decrease antitumor immune mechanisms.
Studies of Turkish storage-battery workers who had a median BLL of 75 μg/dL indicated that it was associated with statistically significant decreases in TH cells, serum IgG, IgM, and some serum complement components (Basaran and Undeger 2000). In vitro functional aberrations, including changes in neutrophil chemotaxis and random movement, were also noted in the workers. The latter findings were in keeping with the study by Queiroz et al. (1993) that demonstrated defective neutrophil function (chemotaxis and production of ROS) in workers who had a lower average BLL of 41 μg/dL (range 14.8-91.4 μg/dL). Impaired neutrophil movement was thought to be due to lead-induced changes in cell membrane fluidity.
Although most worker studies have examined the immunotoxic effects of lead exposure on men, a study by Qiao et al. (2001) examined women who worked an average of 12 years in printing houses in China. The mean air lead concentration was about 25 (± 19) μg/m3, and average BLLs of the workers were about 29 (± 15) μg/dL; the study referent group had an average BLL of 12.4 μg/dL. Assessments of circulating lymphocyte subset concentrations in both referent and lead-exposed women indicated that “memory” TH cell levels were increased in the exposed workers, whereas NK-cell and B-cell concentrations were statistically significantly reduced. The reduction in blood NK cells in lead-exposed women agrees with the results of Sata et al. (1997), who examined circulating NK cells in male workers who had an average BLL of 18 μg/dL (range 7-35 μg/dL). Also in that study, BLL correlated significantly with serum IgA and IgE concentrations; IgE levels were greatest in workers who had BLLs over 60 μg/dL. In a study that examined battery workers in Korea (Heo et al. 2004), serum IgE concentration correlated significantly with BLL; values were statistically significantly higher in workers who had BLLs of 30 μg/dL or higher than in those who had BLLs under 30 μg/dL. In a study of lead-exposed workers who had BLLs of 9-46 μg/dL, Valentino et al. (2007) examined changes in cytokine concentrations. The results demonstrated that workers had statistically significantly higher concentrations of the anti-inflammatory cytokine inter-leukin-10 (IL-10) and a tendency toward higher concentrations of the proinflammatory cytokine tumor necrosis factor-α (TNF-α) in occupationally exposed male workers than in sex-matched and age-matched controls.
Summary Findings on Immunologic Effects
The committee concludes that the evidence is inadequate to infer a causal relationship between BLLs under 40 μg/dL and immunotoxicity related to clinical disease (see Table 4-6); this conclusion agrees with the NTP’s findings (2012). Although there is compelling evidence of early biologic effects and some effects linked with alterations in immune structure or function, there is insufficient evidence to link these early immune effects with clinical disease. Studies that can relate increased serum IgE concentrations to clinical indicators of allergy in lead-exposed adults would be needed to link immune measures with a specific pathology better. Because early biomarkers of immunotoxicity are observed in lead-exposed humans who have BLLs under 40 μg/dL, effects of lead on the human immune system should be re-evaluated as new research emerges.
The cardiovascular system is a primary target of lead toxicity. Experimental studies and epidemiologic studies have consistently suggested that lead exposure can increase the risk of cardiovascular disease, in particular increased blood-pressure readings and hypertension, even at low levels (BLLs under 10 μg/dL). Potential modes of action include oxidative stress through increased ROS production and inactivation of nitric oxide; hormonal and blood-pressure regulatory system dysfunction through alteration of the adrenergic and renin-angiotensin systems; vasomodulation through increases in vasoconstrictor prostaglandins and decreases in vasodilator prostaglandins; and cellular signaling disruption (Vaziri 2008; EPA 2012). Plasma total homocysteine, a documented risk factor for cardiovascular disease, has also been associated with lead exposure (Schafer et al. 2005; Chia et al. 2007; Yakub and Igbal 2010). Key cardiovascular outcomes and effects considered by the committee included changes in blood pressure, hypertension, pulse pressure, heart-rate variability, electrocardiogram (ECG) conduction abnormalities, peripheral arterial disease, coronary heart disease, myocardial infarction, stroke, and cardiovascular mortality.
Conclusions from the 2012 Environmental Protection Agency and 2012 National Toxicology Program Lead Documents
Environmental Protection Agency 2012 Integrated Science Assessment for Lead (Second External Review Draft)
EPA concluded that there is sufficient evidence of an association between lead exposure and adverse cardiovascular outcomes, especially increased blood pressure and hypertension. EPA’s draft report also suggested that bone lead concentration, an indicator of cumulative exposure, is associated with hypertension
TABLE 4-6 Key Studies of the Immunologic Effects of Lead
| Health Effect | Population Characteristics | Measures | Effect Estimate | Why Study Is Relevant to DOD | Reference |
| Significantly increased memory T cells; decreased percentage of NKC, B lymphocytes | Women working in printing houses in Shanxi Province, China (mean age 34 y) | Mean BLL = 28.6 μg/dL (mean air lead = 25 μg/m3) | p < 0.01 for NKC, B lymphocytes compared with controls. | Relevant to BLL of instructors on firing ranges. | Qiao et al. 2001 |
| Significantly increased plasma IL-10 TNF-α Male workers with occupational exposure to lead (30-61 y old) | Low-exposure group: BLL = 3.9 (± 1.8) μg/dL, ALAD = 265.2 U/mL, ZPP = 5.5 μg/dL; high-exposure group: BLL = 9.7 (± 4.2) μg/dL, ALAD = 224.6 U/mL, ZPP = 26.2 μg/dL | p < 0.05 for IL-10, TNF-á for both low and high BLL groups compared with nonexposed workers. | Relevant to BLL of instructors on firing ranges. | Valentino et al. 2007 | |
| Significantly increased serum IgA | Lead-exposed males in battery-recycling industries (median age 27 y) | BLL >10 μg/dL | p < 0.05 for serum IgA concentrations compared with controls. | Relevant to BLL of instructors on firing ranges. | Mishra et al. 2006 |
| Significantly increased percentage of CD4+ cells, decreased immune naive memory T cells (CD45+RA+) | Male workers driving 3-wheeler vehicles or working in battery-reconditioning plant | BLL = 6.7 μg/dL in drivers; BLL = 132 μg/dL in battery workers; BLL = 4.5 μg/dL in controls | p < 0.001 for percentage of CD4+, CD4+:CD8+ ratio; p < 0.05 for naive memory T-cell differences. | BLL in drivers relevant to that of instructors on firing ranges. | Mishra et al. 2010 |
| Significantly reduced salivary IgA; negative correlation between BLL and serum complement, IgG | Male workers in battery plant, lead smelter in West Germany | Mean BLL = 51.4 μg/dL in exposed workers; BLL range = 6.6-20.8 μg/dL in reference subjects | p = 0.008 for salivary IgA compared with referent group. | Relatively similar BLL to those of firingrange instructors. | Ewers et al. 1982 |
Abbreviations: ALAD, delta-aminolevulinic acid dehydratase; BLL, blood lead level; CD3+, CD4+, CD8+, cluster of differentiation (markers 3, 4, 8); CD45+RA+, naive memory T cells; CTL, cytotoxic T lymphocytes; HLA, human leukocyte antigen; IgA, IgG, IgE, immunoglobulin A, G, E; IL, interleukin; NKC, natural killer cells; TNF, tumor-necrosis factor; ZPP, zinc protoporphyrin.
in adults who have mean bone lead concentrations over 20 μg/g. Overall, the 2012 report suggested that the available data are sufficient to conclude that there is a causal association between lead exposure and several specific cardiovascular health effects. The report also pointed out that uncertainty exists as to the lead exposure level, timing, frequency, and duration that contribute to the observed associations because the adult populations examined in the epidemiologic research probably had high past lead exposures.
National Toxicology Program 2012 Monograph on Health Effects of Low-Level Lead
The NTP concluded that there is sufficient evidence that BLLs under 10 μg/dL are associated with increased blood pressure and hypertension in adults but that the evidence is limited with respect to associations with cardiovascular-related mortality and other cardiovascular end points, such as ECG conduction abnormalities, heart-rate variability, and clinical cardiovascular diseases. The NTP also indicated that bone lead has been more consistently associated with chronic cardiovascular outcomes, such as hypertension and cardiovascular mortality, than BLL; this suggests that long-term cumulative exposure as measured by bone lead is more critical than concurrent lead exposure reflected by BLLs with regard to chronic cardiovascular outcomes.
Other Studies Considered
Blood Pressure and Hypertension
Two meta-analyses integrated most published studies of the associations of blood pressure and hypertension with BLL and bone lead. Nawrot et al. (2002) examined 31 US and European studies published during 1980-2001 and used the following inclusion criteria: 50 or more subjects, ages 10 years and over, both blood pressure and BLL measurements presented with sufficient detail to estimate the magnitude of the association, and preference given to studies that adjusted for age, body-mass index, and additional factors of proven importance. The meta-analysis, whenever possible, analyzed sex-specific and race-specific associations separately. The combined analysis included 58,518 subjects for systolic blood pressure (SBP) and 58,491 subjects for diastolic blood pressure (DBP). The mean BLL ranged from 2.28 to 63.82 μg/dL (median 12.64 μg/dL; interquartile range 7.46-23.93 μg/dL). The combined overall association sizes for SBP and DBP for each two-fold increase in BLL were 1.0 mm Hg (95% CI: 0.5, 1.4) and 0.6 mm Hg (95% CI: 0.4, 0.8), respectively. Sex differences in the associations of BLL with SBP and DBP were not statistically significant.
Navas-Acien et al. (2008) performed a meta-analysis of the associations of bone lead concentration with blood-pressure outcomes based on three prospective
studies and seven cross-sectional studies published through 2007. The mean tibia lead concentrations ranged from 4.2 μg/g (a study of childhood lead exposure in a lead-smelter cohort in Silver Valley, Idaho, and a nonexposed cohort in Spokane, Washington) (Gerr et al. 2002) to 38.4 μg/g (a study of Korean lead workers) (Glenn et al. 2006), and mean patella lead concentrations ranged from 17.3 μg/g (Boston Nurses Health Study) (Korrick et al. 1999) to 32.1 μg/g (Normative Aging Study) (Hu et al. 1996). The combined summary estimates of SBP and DBP for a 10-μg/g increase in tibia lead were 0.26 mm Hg (95% CI: 0.02, 0.50) and 0.02 mm Hg (95% CI: -0.15, 0.19), respectively. The overall ORs for hypertension were 1.04 (95% CI: 1.01, 1.07) for tibia lead and 1.04 (95% CI: 0.96, 1.12) for patella lead.
Additional individual studies support a possible association of BLL over 10 μg/dL and 40 μg/dL and lower with higher blood pressure. A study in China compared 120 female crystal-toy workers (BLLs of 22.5-99.4 μg/dL) with 70 nonexposed controls (sewing workers, BLLs under 11.4 μg/dL) (Nomiyama et al. 2002). They found that workers who had BLLs of 60 μg/dL or higher had SBP, DBP, and pulse pressure 7.5 mm Hg (95% CI: 3.0, 12.0), 6.3 mm Hg (95% CI: 3.4, 9.1), and 3.4 mm Hg (95% CI: 0.5, 6.2), respectively, higher than those in the control group (BLLs under 11.4 μg/dL).
Glenn et al. (2006) explored whether the association between lead and blood pressure could be an acute response to lead or a long-term cumulative effect of lead. Their cohort consisted of 575 lead-exposed workers in South Korea with a baseline mean (standard deviation [SD]) BLL and tibia bone lead concentration of 31.4 (14.2) μg/dL and 38.4 (42.9) μg/g, respectively. They found that every increase of 10 μg/dL per year in concurrent BLL, as assessed with a longitudinal difference in BLL between visits, was associated with an average annual increase of 0.9 mm Hg (95% CI: 0.1, 1.6) in SBP during the 3-year followup. Tibia lead, however, was nonsignificantly inversely associated with a change in SBP. The authors suggested that SBP might be more responsive to circulating lead (as reflected by BLL), whereas lifetime cumulative dose may influence the risk of hypertension through other biologic pathways. Weaver et al. (2008) also examined the same cohort and observed a statistically significant cross-sectional association of SBP with concurrent BLL but not with patella lead. The significant association remained even after controlling for patella lead. There was no association of DBP or hypertension prevalence with either lead measure. A community-based cohort study conducted in the Baltimore, Maryland, area (Baltimore Memory Study) found that BLL was associated with blood pressure, whereas tibia lead was associated with hypertension; this suggested that “lead has an acute effect on blood pressure via recent dose and a chronic effect on hypertension risk via cumulative dose” (Martin et al. 2006).
Two studies examined BLL and blood pressure by using data from NHANES II. Sorel et al. (1991) reported age-adjusted BLLs of 13.2 μg/dL in black females, 12.1 μg/dL in white females, 20.1 μg/dL in black males, and 16.8 μg/dL in white males. They fitted linear BLL, but Schwartz (1991) constructed a log-linear model (natural log-transformation). Linear BLL was significantly associated with DBP only in males (β = 0.13 mm Hg; 95% CI: 0.04, 0.21, for every 1 μg/dL) (Sorel et al. 1991), whereas log-linear BLL was significantly
associated with DBP in both males (β = 2.93 mm Hg; 95% CI: 0.93, 4.98, for every 1 natural log unit of BLL) and females (β = 1.64 mm Hg; 95% CI: 0.27, 3.01) (Schwartz 1991). Those results suggest that a dose-response relationship between BLL and blood pressure is most likely log-linear.
Clinical Cardiovascular Outcomes
Navas-Acien et al. (2007) conducted a qualitative systematic review of lead exposure and cardiovascular end points except blood pressure and hypertension. They identified 12 studies of clinical cardiovascular end points in general populations and 18 studies of cardiovascular mortality in occupational cohorts. They concluded that the evidence was suggestive but not sufficient to infer causal relationships between lead exposure and clinical cardiovascular outcomes because of the small number of prospective studies, the lack of standardized assessment and information on outcomes, and methodologic limitations, such as exposure and outcome misclassification.
The committee identified one study of an important and specific clinical outcome—incident ischemic heart disease—and four studies of cardiovascular mortality. Jain et al. (2007) examined the association between bone lead and incidence of ischemic heart disease (myocardial infarction or angina pectoris) in a prospective cohort of veterans in the Boston, Massachusetts, area (Normative Aging Study: 83 cases and 754 noncases) with 10 years of followup. The mean (SD) concentrations of baseline BLL, patella lead, and tibia lead were 7.0 (3.8) μg/dL, 36.8 (20.8) μg/g, and 24.2 (15.9) μg/g in cases and 6.2 (4.3) μg/dL, 30.6 (19.7) μg/g, and 21.4 (13.6) μg/g in noncases, respectively. SD increases in BLL and patella lead were significantly associated with a 27% (95% CI of hazard ratio [HR]: 1.01, 1.59) and a 29% (95% CI of HR 1.02, 1.62) increased risk of ischemic heart disease. Compared with subjects who had BLLs under 5 μg/dL, those who had BLLs of 5 μg/dL or higher had an HR of 1.73 (95% CI: 1.05, 2.87). Weisskopf et al. (2009) conducted a survival analysis of mortality in the same cohort (an average of 8.9 years of followup) and found that men in the highest tertile of patella lead had HRs of 2.52 (95% CI: 1.17, 5.41) for all causes, 5.63 (95% CI: 1.73, 18.3) for cardiovascular disease, and 8.37 (95% CI: 1.29, 54.4) for ischemic heart disease. Baseline BLLs were not associated with cardiovascular mortality.
The committee identified other important studies. A study that used data from NHANES II reported a rate ratio of 1.39 (95% CI: 1.01, 1.91) for circulatory mortality associated with BLLs of 20-29 μg/dL compared with BLLs under 10 μg/dL (Lustberg and Silbergeld 2002). Lin et al. (2011) followed 927 dialysis patients in Taiwan for 18 months and found that after adjustment for confounders the upper two tertiles of BLLs (8.51-12.64 μg/dL and over 12.64 μg/dL) were associated with HRs of 3.70 (95% CI: 2.06, 6.48) and 9.71 (95% CI: 2.11,
23.26) compared with the first tertile (BLLs under 8.51 μg/dL). Khalil et al. (2009b) investigated a prospective cohort of 533 women in the Study of Osteoporotic Fractures at two US research centers (Baltimore, Maryland, and Monongahela Valley, Pennsylvania). The baseline mean (SD) BLL was 5.3 (2.3) μg/dL (range 1-21 μg/dL). Over 12 years of followup, women who had BLLs of 8 μg/dL or higher (n = 79) had an HR of 3.08 (95% CI: 1.23, 7.70) for coronary heart disease mortality and an HR of 1.78 (95% CI: 0.92, 3.45) for cardiovascular disease mortality compared with women who had BLLs under 8 μg/dL (n = 453). There was no association with stroke (HR = 1.13; 95% CI: 0.34, 3.81). Finally, many occupational-cohort studies published before 2000 examined mortality from stroke by comparing lead-exposed workers with nonexposed controls. The committee did not find the evidence from those studies compelling, because the associations were inconsistent and there was no lead dose assessment (reviewed by Navas-Acien et al. 2007).
Other Cardiovascular End Points, Including Subclinical Measures
As stated above, the NTP (2012) report suggested that the evidence regarding an association of low-level lead exposure with other cardiovascular outcomes, including subclinical measures, was limited. The committee identified 10 studies that examined subclinical measures—such as ventricular function, heart rate variability, and pulse pressure—and homocysteine, a biomarker of cardiovascular and neurodegenerative diseases.
Poręba et al. (2010, 2011a,b,c) published a series of papers that examined ventricular function, pulse pressure, and heart-rate variability by comparing lead-exposed workers and healthy controls in Poland. The average BLLs were 24.0-26.7 μg/dL in lead-exposed workers and 5.4-8.3 μg/dL in the healthy controls. A unit increase in BLL was associated with an increase of 0.02 (standard error [SE] = 0.01) to 0.03 (SE = 0.01) mm Hg in pulse pressure, an indicator of arterial stiffness, and a 13% increase in the odds of left ventricular diastolic dysfunction (95% CI of OR: 1.10, 1.15). They also found significant positive associations between ZPP and left ventricular hypertrophy (OR = 1.32; 95% CI: 1.26, 1.43, for every 1-μg/dL increase in ZPP) and augmentation index (β = 0.215; SE = 0.01). Lead-exposed workers had significantly lower measures of heart-rate variability, especially depressed vagal activity, than healthy controls (for example, root mean square of successive normal sinus RR interval difference [rMSSD] during the day activity hours: 52.94 ± 21.58 ms in copper-smelter workers vs 79.42 ± 31.14 ms; p < 0.01).
The committee believed that studies of lead and homocysteine were relevant to its work because of the large literature relating homocysteine to adverse cardiovascular outcomes (Stampfer et al. 1992; Perry et al. 1995; Verhoef et al. 1997; Humphrey et al. 2008). Chia et al. (2007) investigated an association between BLL and plasma homocysteine, a sulfur-containing amino acid and a risk factor for cardiovascular and neurodegenerative diseases (Shea et al. 2002; Martignoni et al. 2007; Blom and Smulders 2011), in 422 lead-exposed workers in
Singapore (159) and Vietnam (263). BLLs ranged from 2 to 66.9 μg/dL with a mean of 22.7 μg/dL. There was a borderline significant association in an analysis that included all subjects: a 1-μg/dL increase in BLLs was associated with a 0.04-μmol/L increase in homocysteine on the log scale (95% CI: -0.001, 0.082). A polynomial plot of BLL and log-transformed homocysteine showed that homocysteine did not increase until 20 μg/dL, and a clear positive dose-response relationship began to be apparent at BLLs above 20 μg/dL. Yakub and Igbal (2010) also examined 872 healthy adults (18-60 years old) in a low-income urban population of Karachi, Pakistan, whose mean (SD) BLL was 11.65 (5.5) μg/dL. Every 1-μg/dL increase in BLL was associated with a 0.09-μmol/L increase in plasma homocysteine. Compared with the first quartile, the upper three quartiles had significantly higher risks of hyperhomocysteinemia (defined as plasma homocysteine concentrations over 15 μmol/L), with ORs of 1.89, 2.21, and 1.69 for quartiles 2, 3, and 4, respectively. A community-based study also reported a significant association between lead exposure and homocysteine despite a mean BLL under 5 μg/dL (Schafer et al. 2005). In a cross-sectional analysis with 1,037 subjects in the Baltimore Memory Study, a 1-μg/dL increase in BLL was associated with a 0.35-μmol/L increase in plasma homocysteine after controlling for important potential confounding variables. However, as might be expected given mechanistic considerations, tibia lead was not associated with homocysteine concentrations.
The lead studies conducted as part of the Normative Aging Study consistently showed associations between bone lead and subclinical measures of cardiovascular disease. The participants in the cohort were veterans who had had military service and thus might have had lead exposure through the use of firearms. In different studies, the average tibia and patella bone lead concentrations in the Normative Aging Study were about 20 and 30 μg/g, respectively. The mean BLLs were about 6 μg/dL. The average age was about 70 years in 2000. The results of the studies suggest that low-level cumulative exposure to lead is associated with cardiac conduction abnormalities cross-sectionally (Cheng et al. 1998) and longitudinally (Eum et al. 2011) and that subjects who have metabolic syndrome may be more susceptible to cumulative lead exposure-related autonomic dysfunctions (Park et al. 2006).
Summary Findings on Cardiovascular Effects
The committee concludes that the evidence is sufficient to infer causal relationships between BLLs under 40 μg/dL, as well as cumulative dose measures, and increased blood pressure, hypertension, cardiovascular mortality, and subclinical cardiovascular outcomes (see Table 4-7). The committee also concludes that the evidence supporting a causal relationship between BLLs under 40 μg/dL and stroke is inconsistent. Those conclusions reinforce the conclusions of the NTP and EPA but suggest that further studies are needed to conclude whether the relationship between lead exposure and stroke is causal.
TABLE 4-7 Key Studies of the Effects of Lead on Cardiovascular Disease
| Health Effect | Population Characteristics | Measures | Effect Estimate | Why Study Is Relevant to DOD | Reference |
| Blood pressure or hypertension | Meta-analysis (31 US, European studies published in 1980-2001) (58,518) | Mean BLLs: minimum = 2.28 μg/dL, Q1 (25%) = 7.46 μg/dL, median = 12.64 μg/dL, Q3 (75%) = 23.93 μg/dL, maximum = 63.82 μg/dL | Pooled estimate per doubling of BLL (95% CI): SBP, 1 mm Hg (0.5, 1.4); DBP, 0.6 mm Hg (0.4, 0.8); male SBP, 1.2 mm Hg (0.6, 1.7); DBP, 0.6 mm Hg (0.4, 0.8); female SBP, 0.8 mm Hg (0.2, 1.4); DBP, 0.6 mm Hg (0.3, 0.9). | Most studies included have BLL >10 μg/dL, in both general populations and occupational cohorts. | Nawrot et al. 2002 |
| Meta-analysis (3 prospective studies, 5 cross-sectional studies, 1996-2007) | Mean (SD) bone lead in Normative Aging Study: tibia, 22 (13) μg/g; patella, 32 (19) μg/g Korean lead workers: tibia, 38 (40) μg/g Baltimore Memory Study: tibia, 18.8 (12.4) μg/g Boston Nurses Health Study: tibia, 13.3 (9) μg/g; patella, 17.3 (10.6) μg/g Postpartum women: tibia, 8.8 (11.4) μg/g; calcaneus, 11 (12) μg/g | For 10-μg/g increase in tibia, cross-sectional increase: SBP, 0.26 mm Hg (95 % CI: 0.02, 0.5); DBP, 0.02 mm Hg (95% CI: -0.15, 0.19). Hypertension: tibia, OR = 1.04 (95% CI: 1.01, 1.07); patella, OR = 1.04 (95% CI: 0.96, 1.12). | Reflecting cumulative exposure; summary estimates between bone lead markers and blood pressure and hypertension. | Navas-Acien et al. 2008 | |
| Cardiovascular mortality | 868 male veterans in Normative Aging Study, 8.9 (SD = 3.9) y of followup | Baseline tertile of patella lead: low <22, medium 22-35, high >35 μg/g | HR of all CVD mortality = 1.63 (95% CI: 0.51, 5.18) for medium group, 5.63 (95% CI: 1.73, 18.3) for high group compared with low group; HR of ischemic heart disease = 2.99 (95% CI: 0.40, 22.6) for medium group, 8.37 (95% CI: 1.29, 54.4) for high group. | Long-term exposure studies of veterans probably exposed to lead during military service. | Weisskopf et al. 2009 |
Abbreviations: BLL, blood lead level; CI, confidence interval; CHD, coronary heart disease; CV, cardiovascular; CVD: cardiovascular disease; DBP, diastolic blood pressure; HR, hazard ratio; OR, odds ratio; SBP, systolic blood pressure; SD, standard deviation.
Abbate, C., R. Buceti, F. Munaò, C. Giorgianni, and G. Ferreri. 1995. Neurotoxicity induced by lead levels: An electrophysiological study. Int. Arch. Occup. Environ. Health 66(6):389-392.
Åkesson, A, T. Lundh, M. Vahter, P. Bjellerup, J. Lidfeldt, C. Nerbrand, G. Samsioe, U. Strömberg, and S. Skerfving. 2005. Tubular and glomerular effects in Swedish women with low environmental cadmium exposure. Environ. Health Perspect. 113(11):1627-1631.
Alasia, D.D., P.C. Emem-Chioma, and F.S. Wokoma. 2010. Occupational and environmental lead exposure in Port Hartcourt, Nigeria: Analysis of its association with renal function indices. Niger J. Med. 19(4):407-414.
Allouche, L., M. Hamadouche, and A. Touabti. 2009. Chronic effects of low lead levels on sperm quality, gonadotropins and testosterone in albino rats. Exp. Toxicol. Pathol. 61(5):503-510.
Al-Saleh, I., S. Coskun, A. Mashhour, N. Shinwari, I. El-Doush, G. Billedo, K. Jaroudi, A. Al-Shahrani, M. Al-Kabra, and G. El Din Mohamed. 2008a. Exposure to heavy metals (lead, cadmium and mercury) and its effect on the outcome of in-vitro fertilization treatment. Int. J. Hyg. Environ. Health 211(5-6):560-579.
Al-Saleh, I., N. Shinwari, M. Nester, A. Mashhour, L. Moncari, G. El Din Mohamed, and A. Rabah. 2008b. Longitudinal study of prenatal and postnatal lead exposure and early cognitive development in Al-Kharj, Saudi Arabia: Preliminary results of cord blood lead levels. J. Trop. Pediatr. 54(5):300-307.
Al-Saleh, I., M. Nester, A. Mashhour, L. Moncari, N. Shinwari, G.D. Mohamed, and A. Rabah. 2009. Prenatal and postnatal lead exposure and early cognitive development: Longitudinal study in Saudi Arabia. J. Environ. Pathol. Toxicol. Oncol. 28(4):283-302.
Amann, R.P. 1970. Sperm production rates. Pp. 433-482 in The Testis, A.D. Johnson, W.R. Gomes, and N.L. Vandemark, eds. New York: Academic Press.
Anjum, M.R., S.B. Sainath, Y. Suneetha, and P.S. Reddy. 2011. Lead acetate induced reproductive and paternal mediated developmental toxicity in rats. Ecotoxicol. Environ. Saf. 74(4):793-799.
Armon, C., L.T. Kurland, J.R. Daube, and P.C. O’Brien. 1991. Epidemiologic correlates of sporadic amyotrophic lateral sclerosis. Neurology 41(7):1077-1084.
Aub, J.C., L.T. Fairhall, A.S. Minot, and P. Reznikoff. 1925. Effects of lead on blood cells. Medicine 4(1-2):126-151.
Ayatollahi, M. 2002. Study of the impact of blood lead level on humoral immunity in humans. Toxicol. Indust. Health 18(1):39-44.
Baghurst, P.A., A.J. McMichael, N.R. Wigg, G.V. Vimpani, E.F. Robertson, R.J. Roberts, and S.L. Tong. 1992. Environmental exposure to lead and children’s intelligence at the age of seven years. The Port Pirie Cohort Study. N. Engl. J. Med. 327(18):1279-1284.
Baker, E.L., P.J. Landrigan, A.G. Barbour, D.H. Cox, D.S. Folland, R.N. Ligo, and J. Throckmorton. 1979. Occupational lead poisoning in the United States: Clinical and biochemical findings related to blood lead levels. Br. J. Ind. Med. 36(4):314-322.
Bandeen-Roche, K., T.A. Glass, K.I. Bolla, A.C. Todd, and B.S. Schwartz. 2009. Cumulative lead dose and cognitive function in older adults. Epidemiology 20(6):831-839.
Basaran, N., and U. Undeger. 2000. Effects of lead on immune parameters in occupationally exposed workers. Am. J. Ind. Med. 38(3):349-354.
Batra, N., B. Nehru, and M.P. Bansal. 2001. Influence of lead and zinc on rat male reproduction at ‘biochemical and histopathological levels’. J. Appl. Toxicol. 21(6):507-512.
Batuman, V., J.K. Maesaka, B. Haddad, E. Tepper, E. Landy, and R.P. Wedeen. 1981. The role of lead in gout nephropathy. N. Engl. J. Med. 304(9):520-523.
Batuman, V., E. Landy, J.K. Maesaka, and R.P. Wedeen. 1983. Contribution of lead to hypertension with renal impairment. N. Engl. J. Med. 309(1):17-21.
Bellinger, D.C. 2000. Effect modification in epidemiological studies of low-level neurotoxicant exposures and health outcomes. Neurotoxicol. Teratol. 22(1):133-140.
Bellinger, D., A. Levion, M. Rabinowitz, E. Allred, H. Needlemann, and S. Schoenbaum. 1991. Weight gain and maturity in fetuses exposed to low levels of lead. Environ. Res. 54(2):151-158.
Benoff, S., A. Jacob, and I.R. Hurley. 2000. Male infertility and environmental exposure to lead and cadmium. Hum. Reprod. Update 6(2):107-121.
Benoff, S., G.M. Centola, C. Millan, B. Napolitano, J.L. Marmar, and I.R. Hurley. 2003a. Increased seminal plasma lead levels adversely affect the fertility potential of sperm in IVF. Hum. Reprod. 18(2):374-383.
Benoff, S., I.R. Hurley, C. Millan, B. Napolitano, and G.M. Centola. 2003b. Seminal lead concentrations negatively affect outcomes of artificial insemination. Fertil. Steril. 80(3):517-525.
Benoff, S., C.C. Chu, J.L. Marmar, R.Z. Sokol, L.O. Goodwin, and I.R. Hurley. 2007. Voltage-dependent calcium channels in mammalian spermatozoa revisited. Front. Biosci.12:1420-1449.
Bleecker, M.L., K.N. Lindgren, M.J. Tiburzi, and D.P. Ford. 1997. Curvilinear relationship between blood lead level and reaction time: Differential association with blood lead fractions derived from exogenous and endogenous sources. J. Occup. Environ. Med. 39(5):426-431.
Bleecker, M.L., K.N. Lindgren, D.P. Ford, and M.J. Tiburzi. 2002. The interaction of education and cumulative lead exposure on Mini-Mental State Examination. J. Occup. Environ. Med. 44(6):574-578.
Bleecker, M.L., D.P. Ford, K.N. Lindgren, K. Scheetz, and M.J. Tiburzi. 2003. Association of chronic and current measures of lead exposure with different components of brainstem auditory evoked potentials. Neurtoxicology 24(4-5):625-631.
Bleecker, M.L., D.P. Ford, K.N. Lindgren, V.M. Hoese, K.S. Walsh, and C.G. Vaughan. 2005a. Differential effects of lead exposure on components of verbal memory. Occup. Environ. Med. 62(3):181-187.
Bleecker, M.L., D.P. Ford, C.G. Vaughan, K.N. Lindgren, M.J. Tiburzi, and K.S. Walsh. 2005b. Effect of lead exposure and ergonomic stressor on peripheral nerve function. Environ. Health Perspect. 113(12):1730-1734.
Bleecker, M.L., D.P. Ford, C.G. Vaughan, K.S. Walsh, and K.N. Lindgren. 2007. The association of lead exposure and motor performance mediated by cerebral white matter change. Neurotoxicology 28(2):318-323.
Blom, H.J., and Y. Smulders. 2011. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J. Inherit. Metab. Dis. 34(1):75-81.
Bloom, M.S., P.J. Parsons, A.J. Steuerwald, E.F. Schisterman, R.W. Browne, K. Kim, G.A. Coccaro, G.C. Conti, N. Narayan, and V.Y. Fujimoto. 2010. Toxic trace metals and human oocytes during in vitro fertilization (IVF). Reprod. Toxicol. 29(3):298-305.
Bloom, M.S., P.J. Parsons, D. Kim, A.J. Steuerwald, S. Vaccari, G. Cheng, and V.Y. Fujimoto. 2011. Toxic trace metals and embryo quality indicators during in vitro fertilization (IVF). Reprod. Toxicol. 31(2):164-170.
Borja-Aburto, V.H., I. Hertz-Picciotto, M. Rojas Lopez, P. Farias, C. Rios, and J. Blanco. 1999. Blood lead levels measured prospectively and risk of spontaneous abortion. Am. J. Epidemiol. 150(6): 590-597.
Bouchard, M.F., D.C. Bellinger, J. Weuve, J. Matthews-Bellinger, S.E. Gilman, R.O. Wright, J. Schwartz, and M.G. Weisskopf. 2009. Blood lead levels and major depressive disorder, panic disorder, and generalized anxiety disorder in U.S. young adults. Arch. Gen. Psychiatry 66(12):1313-1319.
Budtz-Jorgensen, E., N. Keiding, and P. Grandjean. 2001. Benchmark dose calculation from epidemiologic data. Biometrics 57(3):698-706.
Cabaravdic, M., M. Mijanovic, J. Kusturica, and A. Cabaravdic. 2010. Occupational exposure of workers at gas stations to inorganic lead. Med. Arh. 64(2):107-109.
Campbell, B.C., P.A. Meredith, and J.J. Scott. 1985. Lead exposure and changes in the renin-angiotensin-aldosterone system in man. Toxicol. Lett. 25(1):25-32.
Carlsen, E., A. Giwercman, N. Keiding, and N.E. Skakkebaek. 1992. Evidence for decreasing quality of semen during past 50 years. BMJ 305(6854):609-613.
Chancellor, A.M., J.M. Slattery, H. Fraser, and C.P. Warlow. 1993. Risk factors for motor neuron disease: A case-control study based on patients from the Scottish Motor Neuron Disease Register. J. Neurol. Neurosurg. Psychiatry 56(11):1200-1206.
Chang, S.H., B.H. Cheng, S.L. Lee, H.Y. Chuang, C.Y. Yang, F.C. Sung, and T.N. Wu. 2006. Low blood lead concentration in association with infertility in women. Environ. Res. 101(3):380-386.
Cheng, Y., J. Schwartz, P.S. Vokonas, S.T. Weiss, A. Aro, and H. Hu. 1998. Electrocardiographic conduction disturbances in association with low-level lead exposure (the Normative Aging Study). Am. J. Cardiol. 82(5):594-599.
Chia, S.E., C.N. Ong, S.T. Lee, and F.H. Tsakok. 1992. Blood concentrations of lead, cadmium, mercury, zinc, and copper and human semen parameters. Arch. Androl. 29(2):177-183.
Chia, S.E., L.H. Chua, T.P. Ng, S.C. Foo, and J. Jeyaratnam. 1994. Postural stability of workers exposed to lead. Occup. Environ. Med. 51(11):768-771.
Chia, S.E., H.P. Chia, C.N. Ong, and J. Jeyaratnam. 1996a. Cumulative blood lead levels and nerve conduction parameters. Occup. Med. 46(1):59-64.
Chia, S.E., H.P. Chia, C.N. Ong, and J. Jeyaratnam. 1996b. Cumulative concentrations of blood lead and postural stability. Occup. Environ. Med. 53(4):264-268.
Chia, S.E., H.P. Chia, C.N. Ong, and J. Jeyaratnam. 1997. Cumulative blood lead levels and neurobehavioral test performance. Neurotoxicology 18(3):793-804.
Chia, S.E., S.M. Ali, B.L. Lee, G.H. Lim, S. Jin, N.V. Dong, N.T. Tu, C.N. Ong, and K.S. Chia. 2007. Association of blood lead and homocysteine levels among lead exposed subjects in Vietnam and Singapore. Occup. Environ. Med. 64(10):688-693.
Chuang, H.Y., J. Schwartz, S.Y. Tsai, M.L. Lee, J.D. Wang, and H. Hu. 2000. Vibration perception thresholds in workers with long term exposure to lead. Occup. Environ. Med. 57(9):588-594.
Chuang, H.Y., C.H. Kuo, Y.W. Chiu, C.K. Ho, C.J. Chen, and T.N. Wu. 2007. A casecontrol study on the relationship of hearing function and blood concentrations of lead, manganese, arsenic, and selenium. Sci. Total Environ. 387(1-3):79-85.
Conterato, G.M.M., R.P. Bulcao, R. Sobieski, A.M. Moro, M.F. Charao, F.A. de Feitas, F.L. de AAlmeida, A.P.L. Moreira, M. Roehrs, R. Tonello, B.L. Batista, D. Grotto, F. Barbosa, S.C. Garcia, and T. Emanuelli. In press. Blood thioredoxin reductase activity, oxidative stress and hematological parameters in painters and battery workers: Relationship with lead and cadmium levels in blood. J. Appl. Toxicol. Sep 9, 2011. doi: 10.1002/jat.1731
Coon, S., A. Stark, E. Peterson, A. Gloi, G. Kortsha, J. Pounds, D. Chettle, and J. Gorrell. 2006. Whole-body lifetime occupational lead exposure and risk of Parkinson’s disease. Environ. Health Perspect. 114(12):1872-1876.
Cooper, R.L., and N. Doerrer. 2010. Reproductive and developmental toxicity studies. Pp. 159-172 in Comprehensive Toxicology, C. McQueen, ed., Second Edition, Volume 3. Oxford, UK: Elsevier Ltd.
Crain, D.A., S.J. Janssen, T.M. Edwards, J. Heindel, S.M. Ho, P. Hunt, T. Iguchi, A. Juul, J.A. McLachlan, J. Schwartz, N. Skakkebaek, A.M. Soto, S. Swan, C. Walker, T.K. Woodruff, T.J. Woodruff, L.C. Giudice, and L.J. Guillette. 2008. Female reproductive disorders: The role of endocrine-disrupting compounds and developmental timing. Fertil. Steril. 90(4):911-940.
Cramér, K., R.A. Goyer, R. Jagenburg, and M.H. Wilson. 1974. Renal ultrastructure, renal function, and parameters of lead toxicity in workers with different periods of lead exposure. Br. J. Ind. Med. 31(2):113-127.
Daston, G.P., and T.B. Knudsen. 2010. Fundamental concepts, current regulatory design and interpretation. Pp. 3-9 in Comprehensive Toxicology, C. McQueen, ed., Second Edition, Volume 12. Oxford, UK: Elsevier Ltd.
Davis, J.M., and D.J. Svendsgaard. 1990. Nerve conduction velocity and lead: A critical review and meta-analysis. Pp. 353-376 in Advances in Neurobehavioral Toxicology, B.L. Johnson, ed. Chelsea, MI: Lewis Publishers Inc. de Langen, C.D., and J.A. ten Berg. 1948. Porphyrin in the urine as a first symptom of lead poisoning. Acta Med. Scand. 130(1):37-44.
Descotes, J., B. Nicolas, and T. Vial. 1995. Assessment of immunotoxic effects in humans. Clin. Chem. 41(12 Pt. 2):1870-1873.
Dietert, R.R., and M.S. Piepenbrink. 2006. Lead and immune function. Crit. Rev. Toxicol. 36(4):359-385.
Discalzi, G.L., F. Capellaro, L. Bottalo, D. Fabbro, and A. Mocellini. 1992. Auditory brainstem evoked potentials (BAEPs) in lead-exposed workers. Neurotoxicology 13(1):207-209.
Discalzi, G., D. Fabbro, F. Meliga, A. Mocellini, and F. Capellaro. 1993. Effects of occupational exposure to mercury and lead on brainstem auditory evoked potentials. Int. J. Psychophysiol. 14(1):21-25.
Dogu, O., E.D. Louis, L. Tamer, O. Unal, A. Yilmaz, and H. Kaleagasi. 2007. Elevated blood lead concentrations in essential tremor: A case-control study in Mersin, Turkey. Environ. Health Perspect. 115(11):1564-1568.
Dumitrescu, E., A. Trif, and S. Petrovici. 2008. Lead acetate impact on some markersof female reproductive system performances (litter size, sex ratio) and physical development (vaginal opening) in rats. Bull. USAMV Vet. Med. 65(2):283-287.
Ekong, E.B., B.G. Jaar, and V.M. Weaver. 2006. Lead-related nephrotoxicity: A review of the epidemiologic evidence. Kidney Int. 70(12):2074-2084.
El-Safty, A.M.K., and F.M. Metwally. 2000. Lead-associated health hazards and immunotoxic effects among plumbers. Egypt J. Occup. Med. 24(1):129-137
Emmerson, B.T. 1973. Chronic lead nephropathy. Kidney Int. 4(1):1-5.
Emmerson, B.T. 1991. Lead stores in patients with renal insufficiency. Nephron 58(2):233-234.
EPA (U.S. Environmental Protection Agency). 2006. Air Quality Criteria for Lead, Volume I. EPA/600/R-05/144aF. National Center for Environmental Assessment-RTP Division, Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, NC [online]. Available: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=158823 [accessed May 9, 2012].
EPA (U.S. Environmental Protection Agency). 2012. Integrated Science Assessment for Lead. EPA/600/R-10/075B. National Center for Environmental Assessment-RTP Division, Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, NC. February 2012 [online]. Available: http://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=235331 [accessed Apr. 17, 2012].
Ernhart, C.B., A.W. Wolf, M.J. Kennard, P. Erhard, H.F. Filipovich, and R.J. Sokol. 1986. Intrauterine exposure to low levels of lead: The status of the neonate. Arch. Environ. Health 41(5):287-291.
Eum, K.D., L.H. Nie, J. Schwartz, P.S. Vokonas, D. Sparrow, H. Hu, and M.G. Weisskopf. 2011. Prospective cohort study of lead exposure and electrocardiographic conduction disturbances in the Department of Veterans Affairs Normative Aging Study. Environ. Health Perspect. 119(7): 940-944.
Ewers, U., R. Stiller-Winkler, and H. Idel. 1982. Serum immunoglobulin, complement C3, and salivary IgA levels in lead workers. Environ. Res. 29(2):351-357.
Fang, F., L.C. Kwee, K.D. Allen, D.M. Umbach, W. Ye, M. Watson, J. Keller, E.Z. Oddone, D.P. Sandler, S. Schmidt, and F. Kamel. 2010. Association between blood lead and the risk of amyotrophic lateral sclerosis. Am. J. Epidemiol. 171(10):1126-1133.
Fischbein, A., P. Tsang, J.C. Luo, J.P. Roboz, J.D. Jiang, and J.G. Bekesi. 1993. Phenotypic aberrations of CD3+ and CD4+ cells and functional impairments of lymphocytes at low-level occupational exposure to lead. Clin. Immunol. Immunopathol. 66(2):163-168.
Foster, W.G., A. McMahon, E.V. YoungLai, E.G. Hughes, and D.C. Rice. 1993. Reproductive endocrine effects of chronic lead exposure in the male cynomolgus monkey. Reprod. Toxicol. 7(3):203-209.
Freije, A.M., and M.G. Dairi. 2009. Determination of blood lead levels in adult Bahraini citizens prior to the introduction of unleaded gasoline and the possible effect of elevated blood lead levels on the serum immunoglobulin IgG. Bahrain Med. Bull. 31(1):1-8.
Fried, L.F. 2009. Creatinine and cystatin C: What are the values? Kidney Int. 75(6):578-580.
Gao, S.J., Y.L. Jin, F.W. Unverzagt, F. Ma, K.S. Hall, J.R. Murrell, Y.B. Cheng, J.Z. Shen, B. Ying, R.D. Ji, J. Matesan, C. Liang, and H.C. Hendrie. 2008. Trace element levels and cognitive function in rural elderly Chinese. J. Gerontol. A Biol. Sci. Med. Sci. 63(6):635-641.
Garcia-Leston, J., J. Roma-Torres, M. Vilares, R. Pinto, M. Cunha, J. Prista, J.P. Teixeria, O. Mayan, E. Pasaro, J. Mendez, and B. Laffon. 2011. Biomonitoring of a population of Portuguese workers exposed to lead. Mutat. Res. 721(1):81-88.
Gardella, C. 2001. Lead exposure in pregnancy: A review of the literature and argument for routine prenatal screening. Obstet. Gynecol. Surv. 56(4):231-238.
Gerr, F., R. Letz, L. Stokes, D. Chettle, F. McNeill, and W. Kaye. 2002. Association between bone lead concentration and blood pressure among young adults. Am. J. Ind. Med. 42(2):98-106.
Glass, T.A., K. Bandeen-Roche. M. McAtee, K. Bolla, A.C. Todd, and B.S. Schwartz. 2009. Neighborhood psychosocial hazards and the association of cumulative lead dose with cognitive function in older adults. Am. J. Epidemiol. 169(6):683-692.
Glenn, B.S., K. Bandeen-Roche, B.K. Lee, V.M. Weaver, A.C. Todd, and B.S. Schwartz. 2006. Changes in systolic blood pressure associated with lead in blood and bone. Epidemiology 17(5):538-544.
Gollenberg, A.L., M.L. Hediger, P.A. Lee, J.H. Himes, and G.M. Louis. 2010. Association between lead and cadmium and reproductive hormones in peripubertal U.S. girls. Environ. Health Perspect. 118(12):1782-1787.
Golub, N.A., and P.C. Winters. 2010. A population-based study of blood lead in relation to depression in the United States. Int. Arch. Occup. Environ. Health 83(7):771-777.
González-Cossío, T., K.E. Peterson, L.H. Sanín, E. Fishbein, E. Palazuelos, A. Aro, M. Hernández-Avila, and H. Hu. 1997. Decrease in birth weight in relation to maternal bone-lead burden. Pediatrics 100(5):856-862.
Grandjean, P. 1979. Occupational lead exposure in Denmark: Screening with the haematofluorometer. Br. J. Ind. Med. 36(1):52-58.
Grandjean, P., B.M. Jensen, S.H. Sando, P.H. Jorgensen, and S. Antonsen. 1989. Delayed blood regeneration in lead exposure: An effect on reserve capacity. Am. J. Public Health 79(10):1385-1388.
Graziano, J.H., V. Slavkovic, P. Factor-Litvak, D. Popovac, X. Ahmedi, and A. Mehmeti. 1991. Depressed serum erythropoietin in pregnant women with elevated blood lead. Arch. Environ. Health 46(6):347-350.
Gulson, B.L., J.G. Pounds, P. Mushak, B.J. Thomas, B. Gray, and M.J. Korsch. 1999. Estimation of cumulative lead releases (lead flux) from the maternal skeleton during pregnancy and lactation. J. Lab. Clin. Med. 134(6):631-640.
Gundacker, C., S. Fröhlich, K. Graf-Rohrmeister, B. Eibenberger, V. Jessenig, D. Gicic, S. Prinz, K.J. Wittmann, H. Zeisler, B. Vallant, A. Pollak, and P. Husslein. 2010. Perinatal lead and mercury exposure in Austria. Sci. Total Environ. 408(23):5744-5749.
Gunnarsson, L.G., L. Bodin, B. Söderfeldt, and O. Axelson. 1992. A case-control study of motor neurone disease: Its relation to heritability, and occupational exposures, particularly to solvents. Br. J. Ind. Med. 49(11):791-798.
Hafez, E.S.E. 1987. Reproduction in Farm Animals. 5th Ed. Philadelphia, PA: Lea &Febiger.
Hänninen, H., A. Aitio, T. Kovala, R. Luukkonen, E. Matikainen, T. Mannelin, J. Erkkilä, and V. Riihimäki. 1998. Occupationl exposure to lead and neuropsychological dysfunction. Occup. Environ. Med. 55(3):202-209.
Hauser, R., O. Sergeyev, S. Korrick, M.M. Lee, B. Revich, E. Gitin, J.S. Burns, and P.L. Williams. 2008. Association of blood lead levels with onset of puberty in Russian boys. Environ. Health Perspect. 116(7):976-980.
He, F.S., S.L. Zhang, G. Li, S.C. Zhang, J.X. Huang, and Y.Q. We. 1988. An electroneurographic assessment of subclinical lead neurotoxicity. Int. Arch. Occup. Environ. Health 61(1-2):141-146.
Heo, Y., B.K. Lee, K.D. Ahn, and D.A. Lawrence. 2004. Serum IgE elevation correlates with blood lead levels in battery manufacturing workers. Hum. Exp. Toxicol. 23(5):209-213.
Hernberg, S., and J. Nikkanen. 1970. Enzyme inhibition by lead under normal urban conditions. Lancet 1(7637):63-64.
Hernberg, S., M. Nurminen, and J. Hasan. 1967. Nonrandom shortening of red cell survival times in men exposed to lead. Environ. Res. 1(3):247-261.
Horiguchi, S., I. Kiyota, G. Endo, K. Teramoto, K. Shinagawa, F. Wakitani, Y. Konishi, A. Kiyota, A. Ota, and H. Tanaka. 1992. Serum immunoglobulin and complement C3 levels in workers exposed to lead. Osaka City Med. J. 38(2):149-153.
Hsieh, T.J., Y.C. Chen, C.W. Li, G.C. Liu, Y.W. Chiu, and H.Y. Chuang. 2009. A proton magnetic resonance spectroscopy study of the chronic lead effect on the basal ganglion and frontal and occipital lobes in middle-age adults. Environ. Health Perspect. 117(6):941-945.
Hsu, P.C., H.Y. Chang, Y.L. Guo, Y.C. Liu, and T.S. Shih. 2009. Effect of smoking on blood lead levels in workers and role of reactive oxygen species in lead-induced sperm chromatin DNA damage. Fertil. Steril. 91(4):1096-1103.
Hu, H., H. Watanabe, M. Payton, S. Korrick, and A. Rotnitzky. 1994. The relationship between bone lead and hemoglobin. JAMA 272(19):1512-1517.
Hu, H., A. Aro, M. Payton, S. Korrick, D. Sparrow, S.T. Weiss, and A. Rotnitzky. 1996. The relationship of bone and blood lead to hypertension. The Normative Aging Study. JAMA 275(15):1171-1176.
Hudson, D.J. 1966. Fitting segmented curves whose joint points have to be estimated. J. Am. Stat. Assoc.. 61(316):1097-1129.
Humphrey, L.L., R. Fu, K. Rogers, M. Freeman, and M. Helfand. 2008. Homocysteine level and coronary heart disease incidence: A systematic review and meta-analysis. Mayo Clin. Proc. 83(11):1203-1212.
Hwang, K.Y., B.K. Lee, J.P. Bressler, K. Bolla, W.F. Stewart, and B.S. Schwartz. 2002. Protein kinase C activity and the relations between blood lead and neurobehavioral function in lead workers. Environ. Health Perspect. 110(2):133-138.
Hwang, Y.H., H.Y. Chiang, M.C. Yen-Jean, and J.D. Wang. 2009. The association between low levels of lead in blood and occupational noise-induced hearing loss in steel workers. Sci. Total Environ. 408(1):43-49.
Ishida, M., M. Ishizaki, and Y. Yamada. 1996. Decreases in postural change in finger blood flow in ceramic painters chronically exposed to low level lead. Am. J. Ind. Med. 29(5):547-553.
Iwata, T., E. Yano, K. Karita, M. Dakeishi, and K. Murata. 2005. Critical dose of lead affecting postural balance in workers. Am. J. Ind. Med. 48(5):319-325.
Jacobsen, C., K. Hartvigsen, M.K. Thomsen, L.F. Hansen, P. Lund, L.H. Skibsted, G. Hφlmer, J. Adler-Nissen, and A.S. Meyer. 2001. Lipid oxidation in fish oil enriched mayonnaise: Calcium disodium ethylenediaminetetraacetate, but not gallic acid, strongly inhibited oxidative deterioration. J. Agric. Food Chem. 49(2):1009-1019.
Jain, N.B., V. Potula, J. Schwartz, P.S. Vokonas, D. Sparrow, R.O. Wright, H. Nie, and H. Hu. 2007. Lead levels and ischemic heart disease in a prospective study of middle-aged and elderly men: The VA Normative Aging Study. Environ. Health Perspect. 115(6): 871-875.
Jedrychowski, W., F.P. Perera, J. Jankowski, D. Mrozek-Budzyn, E. Mroz, E. Flak, S. Edwards, A. Skarupa, and I. Lisowska-Miszczyk. 2009. Very low prenatal exposure to lead and mental development of children in infancy and early childhood: Krakow prospective cohort study. Neuroepidemiology 32(4):270-278.
Johnson, L. 1995. Efficiency of spermatogenesis. Microsc. Res. Tech. 32(5):385-422.
Johnson, L., D.D. Varner, M.E. Roberts, T.L. Smith, G.E. Keillor, and W.L. Scrutchfield. 2000. Efficiency of spermatogenesis: A comparative approach. Anim. Reprod. Sci. 60-61:471-480.
Kamel, F., D.M. Umbach, T.L. Munsat, J.M. Shefner, H. Hu, and D.P. Sandler. 2002. Lead exposure and amyotrophic lateral sclerosis. Epidemiology 13(3):311-319.
Kamel, F., D.M. Umbach, T.A. Lehman, L.P. Park, T.L. Munsat, J.M. Shefner, D.P. Sandler, H. Hu, and J.A. Taylor. 2003. Amyotrophic lateral sclerosis, lead and genetic susceptibility: Polymorphisms in the delta-aminolevulinic acid dehydratase and vitamin D receptor genes. Environ. Health Perspect. 111(10):1335-1339.
Kamel, F., D.M. Umbach, L. Stallone, M. Richards, H. Hu, and D.P. Sandler. 2008. Association of lead exposure with survival in amyotrophic lateral sclerosis. Environ. Health Perspect. 116(7):943-947.
Karita, K., E. Yano, M. Dakeishi, T. Iwata, and K. Murata. 2005. Benchmark dose of lead inducing anemia at the workplace. Risk Anal. 25(4):957-962.
Kasperczyk, A., S. Kasperczyk, S. Horak, A. Ostalowska, E. Grucka-Mamczar, E. Romuk, A. Olejek, and E. Birkner. 2008. Assessment of semen function and lipid peroxidation among lead exposed men. Toxicol. Appl. Pharmacol. 228(3):378-384.
Khalil, N., L.A. Morrow, H. Needleman, E.O. Talbott, J.W.Wilson, and J.A. Cauley. 2009a. Association of cumulative lead and neurocognitive function in an occupational cohort. Neuropsychology 23(1):10-19.
Khalil, N., J.W. Wilson, E.O. Talbott, L.A. Morrow, M.C. Hochberg, T.A. Hillier, S.B. Muldoon, S.R. Cummings, and J.A. Cauley. 2009b. Association of blood lead concentrations with mortality in older women: A prospective cohort study. Environ. Health 8:15.
Khan, D.A., S. Qayyum, S. Saleem, and F.A. Khan. 2008. Lead-induced oxidative stress adversely affects health of the occupational workers. Toxicol. Ind. Health 24(9): 611-618.
Kim, R., A. Rotnitsky, D. Sparrow, S. Weiss, C. Wager, and H. Hu. 1996. A longitudinal study of low-level lead exposure and impairment of renal function. The Normative Aging Study. JAMA 275(15):1177-1181.
Korrick, S.A., D.J. Hunter, A. Rotnitzky, H. Hu, and F.E. Speizer. 1999. Lead and hypertension in a sample of middle-aged women. Am. J. Public Health 89(3):330-335.
Kovala, T., E. Matikainen, T. Mannelin, J. Erkkilä, V. Riihimäki, H. Hänninen, and A. Aitio. 1997. Effects of low level exposure to lead on neurophysiological functions among lead battery workers. Occup. Environ. Med. 54(7):487-493.
Krieg, E.F., Jr., and M.A. Butler. 2009. Blood lead, serum homocysteine, and neurobehavioral test performance in the Third National Health and Nutrition Examination Survey. Neurotoxicology 30(2):281-289.
Krieg, E.F., Jr., D.W. Chrislip, C.J. Crespo, W.S. Brightwell, R.L. Ehrenberg, and D.A. Otto. 2005. The relationship between blood lead levels and neurobehavioral test performance in NHANES III and related occupational studies. Public Health Rep. 120(3):240-251.
Krieg, E.F., Jr., M.A. Butler, M.H. Chang, T. Liu, A. Yesupriya. M.L. Lindegren, and N. Dowling. 2009. Lead and cognitive function in ALAD genotypes in the Third National Health and Nutrition Examination Survey. Neurotoxicol. Teratol. 31(6):364-371.
Krieg, E.F., Jr., M.A. Butler, M.H. Chang., T. Liu, A. Yesupriya, N. Dowling, and M.L. Lindegren. 2010. Lead and cognitive function in VDR genotypes in the Third National Health and Nutrition Examination Survey. Neurotoxicol. Teratol. 32(2):262-272.
Kuo, H.W., T.Y. Hsiao, and J.S. Lai. 2001. Immunological effects of long-term lead exposure among Taiwanese workers. Arch. Toxicol. 75(10):569-573.
Lal, B., G. Goldstein, and J.P. Bressler. 1996. Role of anion exchange and thiol groups in the regulation of potassium efflux by lead in human erythrocytes. J. Cell. Physiol. 167(2):222-228.
Lamadrid-Figueroa, H., M.M. Téllez-Rojo, M. Hernández-Avila, B. Trejo-Valdivia, M. Solano-González, A. Mercado-Garcia, D. Smith, H. Hu, and R.O. Wright. 2007. Association between the plasma/whole blood lead ratio and history of spontaneous abortion: A nested cross-sectional study. BMC Pregnancy Childbirth 7:22.
Lanceraux, E. 1881. Nephrite et arthrite saturnines: Coincidence de ces affections: Parallele avec la nephrite et l’arthrite gouteusses. Arch. Gen. Med. 6:641-647.
Lasley, S.M., and M.E. Gilbert. 1996. Presynaptic glutamatergic function in dentate gyrus in vivo is diminished by chronic exposure to inorganic lead. Brain Res. 736(1-2):125-134.
Li, P.J., Y.Z. Sheng, Q.Y. Wang, L.Y. Gu, and Y.L. Wang. 2000. Transfer of lead via placenta and breast milk in human. Biomed. Environ. Sci. 13(2):85-89.
Lilis, R., J. Eisinger, W. Blumberg, A. Fischbein, and I.J. Selikoff. 1978. Hemoglobin, serum iron and zinc protoporphyrin in lead-exposed workers. Environ. Health Perspect. 25:97-102.
Lin, J.L., D.T. Lin-Tan, K.H. Hsu, and C.C. Yu. 2003. Environmental lead exposure and progression of chronic renal diseases in patients without diabetes. N. Engl. J. Med. 348(4):277-286.
Lin, J.L., D.T. Lin-Tan, C.C. Yu, Y.J. Li, Y.Y. Huang, and K.L. Li. 2006a. Environmental exposure to lead and progressive diabetic nephropathy in patients with type II diabetes. Kidney Int. 69(11):2049-2056.
Lin, J.L., D.T. Lin-Tan, Y.J. Li, K.H. Chen, and Y.L. Huang. 2006b. Low-level environmental exposure to lead and progressive chronic kidney diseases. Am. J. Med. 119(8):707e1-707e9.
Lin, J.L., D.T. Lin-Tan, C.W. Hsu, T.H. Yen, K.H. Chen, H.H. Hsu, T.C. Ho, and K.H. Hsu. 2011. Association of blood lead levels with mortality in patients on maintenance hemodialysis. Am. J. Med. 124(4):350-358.
Lindgren, K.N., V.L. Masten, D.P. Ford, and M.L. Bleecker. 1996. Relation of cumulative exposure to inorganic lead and neuropsychological test performance. Occup. Environ. Med. 53(7):472-477.
Louis, E.D., E.C. Jurewicz, L. Applegate, P. Factor-Litvak, M. Parides, I. Andrews, V. Slavkovich, J.H. Graziano, S. Carroll, and A. Todd. 2003. Association between essential tremor and blood lead concentration. Environ. Health Perspect. 111(14):1707-1711.
Louis, E.D., L. Applegate, J.H. Graziano, M. Parides, V. Slavkovich, and H.K. Bhat. 2005. Interaction between blood lead concentration and delta-amino-levulinic acid dehydratase gene polymorphisms increases the odds of essential tremor. Mov. Disord. 20(9):1170-1177.
Louis, E.D., P. Factor-Litvak, M. Gerbin, V. Slavkovich, J.H. Graziano, W. Jiang, and W. Zheng. 2011. Blood harmane, blood lead, and severity of hand tremor: Evidence of additive effects. Neurotoxicology 32(2):227-232.
Lucchini, R., E. Albini, I. Cortesi, D. Placidi, E. Bergamaschi, F. Traversa, and L. Alessio. 2000. Assessment of neurobehavioral performance as a function of current and cumulative occupational lead exposure. Neurotoxicology 21(5):805-811.
Lustberg, M., and E. Silbergeld. 2002. Blood lead levels and mortality. Arch. Intern. Med. 162(21):2443-2449.
Luster, M.I., P.P. Simeonova, and D.R. Germolec. 2001. Immunotoxicology. Encyclopedia of Life Sciences, Nature Publishing Group [online]. Available: http://immuneweb.xxmu.edu.cn/reading/innate/13.pdf [accessed Sept. 26, 2012].
Maizlish, N.A., G. Parra, and O. Feo. 1995. Neurobehavioral evaluation of Venezuelan workers exposed to inorganic lead. Occup. Environ. Med. 52(6):408-414.
Martignoni, E., C. Tassorelli, G. Nappi, R. Zangaglia, C. Pacchetti, and F. Blandini. 2007. Homocysteine and Parkinson’s disease: A dangerous liaison? J. Neurol. Sci. 257(1-2):31-37.
Martin, D., T.A. Glass, K. Bandeen-Roche, A.C. Todd, W. Shi, and B.S. Schwartz. 2006. Association of blood lead and tibia lead with blood pressure and hypertension in a community sample of older adults. Am. J. Epidemiol. 163(5):467-478.
Meeker, J.D., M.G. Rossano, B. Protas, M.P. Diamond, E. Puscheck, D. Daly, N. Paneth, and J.J. Wirth. 2008. Cadmium, lead, and other metals in relation to semen quality: Human evidence for molybdenum as a male reproductive toxicant. Environ. Health Perspect. 116(11):1473-1479.
Mendiola, J., J.M. Moreno, M. Roca, N. Vergara-Juarez, M.J. Martinez-Garcia, A. Garcia-Sanchez, B. Elvira-Rendueles, S. Moreno-Grau, J.J. Lopez-Espin, J. Ten, R. Bernabeu, and A.M. Torres-Cantero. 2011. Relationships between heavy metal concentrations in three different body fluids and male reproductive parameters: A pilot study. Environ. Health 10(1):6.
Mishra, K.P. 2009. Lead exposure and its impact on immune system: A review. Toxicol. In Vitro 23(6):969-972.
Mishra, K.P., V.K. Singh, R. Rani, V.S. Yadav, V. Chandran, S.P. Srivastava, and P.K. Seth. 2003. Effect of lead exposure on the immune response of some occupationally exposed individuals. Toxicology 188(2-3):251-259.
Mishra, K.P., U.K. Chauhan, and S. Naik. 2006. Effect of lead exposure on serum immunoglobulins and reactive nitrogen and oxygen intermediate. Hum. Exp. Toxicol. 25(11):661-665.
Mishra, K.P., R. Rani, V.S. Yadav, and S. Naik, S. 2010. Effect of lead exposure on lymphocyte subsets and activation markers. Immunopharmacol. Immunotoxicol. 32(3):446-449.
Muntner, P., A. Menke, K.B. DeSalvo, F.A. Rabito, and V. Batuman. 2005. Continued decline in blood lead levels among adults in the United States: The National Health and Nutrition Examination Surveys. Arch. Intern. Med. 165(18):2155-2161.
Naha, N., and A.R. Chowdhury. 2006. Inorganic lead exposure in battery and paint factory: Effect on human sperm structure and functional activity. J. UOEH 28(2):151-171.
Naha, N., and B. Manna. 2007. Mechanism of lead induced effects on human spermatozoa after occupational exposure. Kathmandu University Medical Journal 5(17):85-94 [online]. Available: http://www.kumj.com.np/issue/17/85-94.pdf
Naicker, N., S.A. Norris, A. Mathee, P. Becker, and L. Richter. 2010. Lead exposure is associated with a delay in the onset of puberty in South African adolescent females: Findings from the Birth to Twenty cohort. Sci. Total Environ. 408(2):4949-4954.
Navas-Acien, A., E. Guallar, E.K. Silbergeld, and S.J. Rothenberg. 2007. Lead exposure and cardiovascular disease–a systematic review. Environ. Health Perspect. 115(3): 472-482.
Navas-Acien, A., B.S. Schwartz, S.J. Rothenberg, H. Hu, E.K. Silbergeld, and E. Guallar. 2008. Bone lead levels and blood pressure endpoints: A meta-analysis. Epidemiology 19(3):496-504.
Navas-Acien, A., M. Tellez-Plaza, E. Guallar, P. Muntner, E. Silbergeld, B. Jaar, and V. Weaver. 2009. Blood cadmium and lead and chronic kidney disease in US adults: A joint analysis. Am. J. Epidemiol. 170(9):1156-1164.
Nawrot, T.S., L. Thijs, E.M. Den Hond, H.A. Roels, and J.A. Staessen. 2002. An epidemiological re-appraisal of the association between blood pressure and blood lead: A meta-analysis. J. Hum. Hypertens. 16(2):123-131.
Niu, Q., S.C. He, H.Y. Li, J.Y. Wang, F.Y. Dai, and Y.L. Chen. 2000. A comprehensive neurobehavioral and neurophysiological study for low level lead-exposed workers. G. Ital. Med. Lav. Ergon. 22(4):299-304.
Nomiyama, K., H. Nomiyama, S.J. Liu, Y.X. Tao, T. Nomiyama, and K. Omae. 2002. Lead induced increase of blood pressure in female lead workers. Occup. Environ. Med. 59(11):734-738.
Nordberg, M., B. Windblad, L. Fratiglioni, and H. Basun. 2000. Lead concentrations in elderly urban people related to blood pressure and mental performance: Results from a population-based study. Am. J. Ind. Med. 38(3):290-294.
NRC (National Research Council). 1993. Biologic markers of lead toxicity. Pp. 143-190 in Measuring Lead Exposure in Infants, Children and Other Sensitive Populations. Washington, DC: National Academy Press.
NTP (National Toxicology Program). 2012. NTP Monograph on Health Effects of Low-Level Lead. Prepublication Copy. U.S. Department of Health and Human Services, National Institute of Environmental Health Sciences, National Institutes of Health. June 13, 2012 [online]. Available: http://ntp.niehs.nih.gov/?objectid=4F04B8EA-B187-9EF2-9F9413C68E76458E [accessed June 14, 2012].
Oldereid, N.B., Y. Thomassen, A. Attramadal, B. Olaisen, and K. Purvis. 1993. Concentrations of lead, cadmium and zinc in the tissues of reproductive organs of men. J. Reprod. Fertil. 99(2):421-425.
Osterberg, K., J. Börjesson, L. Gerhardsson, A. Schütz, and S. Skerfving. 1997. A neurobehavioral study of long-term occupational inorganic lead exposure. Sci. Total Environ. 201(1):39-51.
Osterode, W., U. Barnas, and K. Geissler. 1999. Dose dependent reduction of erythroid progenitor cells and inappropriate erythropoietin response in exposure to lead: New aspects of anaemia induced by lead. Occup. Environ. Med. 56(2):106-109.
Park, S.K., J. Schwartz, M. Weisskopf, D. Sparrow, P.S. Vokonas, R.O. Wright, B. Coull, H. Nie, and H. Hu. 2006. Low-level lead exposure, metabolic syndrome, and heart rate variability: The VA Normative Aging Study. Environ. Health Perspect. 114(11):1718-1724.
Park, S.K., S. Elmarsafawy, B. Mukherjee, A. Spiro, III, P.S. Vokonas, H. Nie, M.G. Weisskopf, J. Schwartz, and H. Hu. 2010. Cumulative lead exposure and age-related hearing loss: The VA Normative Aging Study. Hear Res. 269(1-2):48-55.
Payton, M., K.M. Riggs, A. Spiro, III, S.T. Weiss, and H. Hu. 1998. Relations of bone and blood lead to cognitive function: The VA Normative Aging Study. Neurotoxicol. Teratol. 20(1):19-27.
Perry, H.M., Jr, J.P. Miller, J.R. Fornoff, J.D. Baty, M.P. Sambhi, G. Rutan, D.W. Moskowitz, and S.E. Carmody. 1995. Early predictors of 15-year end-stage renal disease in hypertensive patients. Hypertension 25(4 Pt. 1):587-594.
Pinkerton, L., R.E. Biagini, E.M. Ward, R.D. Hull, J.A. Deddens, M.F. Boeniger, T.M. Schnorr, B.A. MacKenzie, and M.I. Luster. 1998. Immunologic findings among lead exposed workers. Am. J. Ind. Med. 33(4):400-408.
Piomelli, S., B. Davidow, V. Guinee, P. Young, and G. Gay. 1973. The FEP [free erythrocyte porphyrin] test: A screening micromethod for lead poisoning. Pediatrics 51(2):254-259.
Piomelli, S., A.A. Lamola, M.B. Poh-Fitzpatrick, C. Seaman, and L.C. Harber. 1975. Erythropoietic protoporphyria and lead intoxication: The molecular basis for difference in cutaneous photosensitivity. I. Different rates of disappearance of protoporphyrin from the erythrocytes, both in vivo and in vitro. J. Clin. Invest. 56(6): 1519-1527.
Piomelli, S., C. Seaman, D. Zullow, A. Curran, and B. Davidow. 1982. Threshold for lead damage to heme synthesis in urban children. Proc. Natl. Acad. Sci. USA 79(10):3335-3339.
Plusquellec, P., G. Muckle, E. Dewailly, P. Ayotte, S.W. Jacobson, and J.L. Jacobson. 2007. The relation of low-level prenatal lead exposure to behavioral indicators of attention in Inuit infants in Arctic Quebec. Neurotoxicol. Teratol. 29(5):527-537.
Poreba, R., P. Gac, M. Poreba, and R. Andrzejak. 2010. The relationship between occupational exposure to lead and manifestation of cardiovascular complications in persons with arterial hypertension. Toxicol. Appl. Pharmacol. 249(1):41-46.
Poreba, R., P. Gac, M. Poreba, J. Antonowicz-Juchniewicz, and R. Andrzejak. 2011a. Relationship between occupational exposure to lead and local arterial stiffness and left ventricular diastolic function in individuals with arterial hypertension. Toxicol. Appl. Pharmacol. 254(3):342-348.
Poreba, R., M. Poreba, P. Gac, and R. Andrzejak. 2011b. Ambulatory blood pressure monitoring and structural changes in carotid arteries in normotensive workers occupationally exposed to lead. Hum. Exp. Toxicol. 30(9):1174-1180.
Poreba, R., M. Poreba, P. Gac, A. Steinmetz-Beck, B. Beck, W. Pilecki, R. Andrzejak, and M. Sobieszczanska. 2011c. Electrocardiographic changes in workers occupationally exposed to lead. Ann. Noninvasive Electrocardiol. 16(1):33-40.
Qiao, N., M. Di Gioacchino, H. Shuchang, L. Youxin, R. Paganelli, and P. Boscolo. 2001. Effects of lead exposure in printing houses on immune and neurobehavioral functions of women. J. Occup. Health 43(5):271-277.
Queiroz, M.L., M. Almeida, M.I. Gallao, and N.F. Hoehr. 1993. Defective neutrophil function in workers occupationally exposed to lead. Pharmacol. Toxicol. 72(2):73-77.
Queiroz, M.L., R.C. Perlingeiro, C. Bincoletto, M. Almeida, M.P. Cardoso, and D.C. Dantas. 1994. Immunoglobulin levels and cellular immune function in leadexposed workers. Immunopharmacol. Immunotoxicol. 16(1):115-128.
Quitanar-Escorza, M.A., M.T. Gonzalez-Martinez, L. Navarro, M. Maldonado, B. Arevalo, and J.V. Calderon-Salinas. 2007. Intracellular free calcium concentration and calcium transport in human erythrocytes of lead-exposed workers. Toxicol. Appl. Pharmacol. 220(1):1-8.
Rajan, P., K.T. Kelsey, J.C. Schwartz, D.C. Bellinger, J. Weuve, D. Sparrow, A. Spiro, III, T.J. Smith, H. Nie, H. Hu, and R.O. Wright. 2007. Lead burden and psychiatric symptoms and the modifying influence of the delta-aminolevulinic acid dehydratase (ALAD) polymorphism: The VA Normative Aging Study. Am. J. Epidemiol. 166(12):1400-1408.
Rajan, P., K.T. Kelsey, J.D. Schwartz, D.C. Bellinger, J. Weuve, A. Spiro, III, D. Sparrow, T.J. Smith, H. Nie, M.G. Weisskopf, H. Hu, and R.O. Wright. 2008. Interaction of the delta-aminolevulinic acid dehydratase polymorphism and lead burden on cognitive function: The VA Normative Aging Study. J. Occup. Environ. Med. 50(9):1053-1061.
Ratzon, N., P. Froom, E. Leikin, E. Kristal-Boneh, and J. Ribak. 2000. Effect of exposure to lead on postural control in workers. Occup. Environ. Med. 57(3):201-203.
Rhodes, D., A. Spiro, III, A. Aro, and H. Hu. 2003. Relationship of bone and blood lead levels to psychiatric symptoms: The Normative Aging Study. J. Occup. Enivon. Med. 45(11):1144-1151.
Roelofs-Iverson, R.A., D.W. Mulder, L.R. Elveback, L.T. Kurland, and C.A. Molgaard. 1984. ALS and heavy metals: A pilot case-control study. Neurology 34(3):393-395.
Ronis, M.J., J. Gandy, and T. Badger. 1998. Endocrine mechanisms underlying reproductive toxicity in the developing rat chronically exposed to dietary lead. J. Toxicol. Environ. Health A. 54(2):77-99.
Rothenberg, S.J., A. Poblano, and L. Schnaas. 2000. Brainstem auditory evoked response at five years and prenatal and postnatal blood lead. Neurotoxicol. Teratol. 22(4):503-510.
Rothenberg, S.J., L. Schnaas, M. Salgado-Valladares, E. Casanueva, A.M. Geller, H.K. Hudnell, and D.A. Fox. 2002. Increased ERG a- and b-wave amplitudes in 7- to 10-year-old children resulting from prenatal lead exposure. Invest. Ophthalmol. Vis. Sci. 43(6):2036-2044.
Sakata, S., S. Shimizu, K. Ogoshi, K. Hirai, Y. Ohno, T. Kishi, J.B. Sherchand, M. Utsumi, M. Shibata, M. Takaki, M. Ueda, and I. Mori. 2007. Inverse relationship between serum erythropoietin and blood lead concentrations in Kathmandu tricycle taxi drivers. Int. Arch. Occup. Environ. Health 80(4):342-345.
Sanín, L.H., T. González-Cossío, I. Romieu, K.E. Peterson, S. Ruíz, E. Palazuelos, M. Hernández-Avila, and H. Hu. 2001. Effect of maternal lead burden on infant weight and weight gain at one month of age among breastfed infants. Pediatrics 107(5):1016-1023.
Sata, F., S. Araki, T. Tanigava, Y. Morita, S. Sakurai, and N. Katsuno. 1997. Changes in natural killer cell subpopulations in lead workers. Int. Arch. Occup. Environ. Health 69(5):306-310.
Sata, F., S. Araki, T. Tanigawa, Y. Morita, S. Sakurai, A. Nakata, and N. Katsuno. 1998. Changes in T cell subpopulations in lead workers. Environ. Res. 76(1):61-64.
Saxena, G., and S.J. Flora. 2004. Lead-induced oxidative stress and hematological alterations and their response to combined administration of calcium disodium EDTA with a thiol chelator in rats. J. Biochem. Mol. Toxicol. 18(4):221-233.
Schafer, J.H., T.A. Glass, J. Bressler, A.C. Todd, and B.S. Schwartz. 2005. Blood lead is a predictor of homocysteine levels in a population-based study of older adults. Environ. Health Perspect. 113(1):31-35.
Schwartz, B.S., B.K. Lee, G.S. Lee, W.F. Stewart, S.S. Lee, K.Y. Hwang, K.D. Ahn, Y.B. Kim, K.I. Bolla, D. Simon, P.J. Parsons, and A.C. Todd. 2001. Associations of blood lead, dimercaptosuccinic acid-chelatable lead, and tibia lead with neurobehavioral test scores in South Korean lead workers. Am. J. Epidemiol. 153(5): 453-464.
Schwartz, B.S., B.K. Lee, K. Bandeen-Roche, W. Stewart, K.I. Bolla, J. Links, V. Weaver, and A. Todd. 2005. Occupational lead exposure and longitudinal decline in neurobehavioral test scores. Epidemiology 16(1):106-113.
Schwartz, J. 1991. Lead, blood pressure, and cardiovascular disease in men and women. Environ. Health Perspect. 91:71-75.
Shea, T.B., J. Lyons-Weiler, and E. Rogers. 2002. Homocysteine, folate deprivation and Alzheimer neuropathology. J. Alzheimers Dis. 4(4):261-267.
Shih, R.A., T.A. Glass, K. Bandeen-Roche, M.C. Carlson, K.I. Bolla, A.C. Todd, and B.S. Schwartz. 2006. Environmental lead exposure and cognitive function in community-dwelling older adults. Neurology 67(9):1556-1562.
Shouman, A.E., and I.A. El-Safty. 2000. Effect of occupational lead-exposure on blood pressure, serum aldosterone level and plasma renin activity. J. Egypt Public Health Assoc. 75(1-2):73-91.
Shulman, A., R. Hauser, S. Lipitz, Y. Frenkel, J. Dor, D. Bider, S. Mashiach, L. Yogev, and H. Yavetz. 1998. Sperm motility is a major determinant of pregnancy outcome following intrauterine insemination. J. Assist. Reprod. Genet. 15(6):381-385.
Silberstein, T., O. Saphier, O. Paz-Tal, J.R. Trimarchi, L. Gonzalez, and D.L. Keefe. 2006. Lead concentrates in ovarian follicle compromises pregnancy. J. Trace Elem. Med. Biol. 20(3):205-207.
Singh, A., C. Cullen, A. Dykeman, D. Rice, and W. Foster. 1993. Chronic lead exposure induces ultrastructural alterations in the monkey testis. J. Submicrosc. Cytol. Pathol. 25(4):479-486.
Slivkova, J., M. Popelkova, P. Massanyi, S. Toporcerova, R. Stawarz, G. Formicki, N. Lukac, A. Puta3a, and M. Guzik. 2009. Concentration of trace elements in human semen and relation to spermatozoa quality. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 44(4):370-375.
Sokol, R.Z., C.E. Madding, and R.S. Swerdloff. 1985. Lead toxicity and the hypothalamic-pituitary-testicular axis. Biol. Reprod. 33(3):722-728.
Sorel, J.E., G. Heiss, H.A. Tyroler, W.B. Davis, S.B. Wing, and D.R. Ragland. 1991. Black-white differences in blood pressure among participants in NHANES II: The contribution of blood lead. Epidemiology 2(5):348-352.
Staessen, J.A., R.R. Lauwerys, J.P. Buchet, C.J. Bulpitt, D. Rondia, Y. Van Renterghem, and A. Amery. 1992. Impairment of renal function with increasing blood lead concentrations in the general population. N. Engl. J. Med. 327(3):151-156.
Stampfer, M.J., M.R. Malinow, W.C. Willett, L.M. Newcomer, B. Upson, D. Ullmann, P.V. Tishler, and C.H. Hennekens. 1992. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. J. Am. Med. Assoc. 268(7):877-881.
Stollery, B.T., H.A. Banks, D.E. Broadbent, and W.R. Lee. 1989. Cognitive functioning in lead workers. Br. J. Ind. Med. 46(10):698-707.
Stone, B.A., J.M. Vargyas, G.E. Ringler, A.L. Stein, and R.P. Marrs. 1999. Determinants of the outcome of intrauterine insemination: Analysis of outcomes of 9963 consecutive cycles. Am. J. Obstet. Gynecol. 180(6 Pt 1):1522-1534.
Sun, L.R., and J.B. Suszkiw. 1994. Pb2+ activates potassium currents in bovine adrenal chromaffin cells. Neurosci. Lett. 182(1):41-43.
Sun, Y., D. Sun, Z. Zhou, G. Zhu, H. Zhang, X. Chang, and T. Jin. 2008. Estimation of benchmark dose for bone damage and renal function in a Chinese male population occupationally exposed to lead. Ann. Occup. Hyg. 52(6):527-533.
Swan, S.H. 2006. Does our environment affect our fertility? Some examples to help reframe the question. Semin. Reprod. Med. 24(3):142-146.
Telisman, S., B. Colak, A. Pizent, J. Jurasović, and P. Cvitković. 2007. Reproductive toxicity of low-level lead exposure in man. Environ. Res. 105(2):256-266.
Teruya, K., H. Sakurai, K. Omae, T. Higashi, T. Muto, and Y. Kaneko. 1991. Effect of lead on cardiac parasympathetic function. Int. Arch. Occup. Environ. Health 62(8):549-553.
Travison, T.G., A.B. Araujo, A.B. O’Donnell, V. Kupelian, and J.B. McKinlay. 2007. A population-level decline in serum testosterone levels in American men. J. Clin. Endocrinol. Metab. 92(1):196-202.
Tsaih, S.W., S. Korrick, J. Schwartz, C. Amarasiriwardena, A. Aro, D. Sparrow, and H. Hu. 2004. Lead, diabetes, hypertension, and renal function: The normative aging study. Environ. Health Perspect. 112(11):1178-1182.
Tyl, R.W. 2005. Toxicity Testing, Developmental. Encyclopedia of Toxicology. W. Philip. New York, Elsevier: 262-276.
Ukaejiofo, E.O., N. Thomas, and S.O. Ike. 2009. Haematological assessment of occupational exposure to lead handlers in Enugu urban, Enugu State, Nigeria. Niger. J. Clin. Pract. 12(1):58-64.
Undeger, U., N. Basaran, H. Canpinar, and E. Kansu. 1996. Immune alterations in leadexposed workers. Toxicology 109(2-3):167-172.
Valentino, M., M. Governa, I. Marchiseppe, and I. Visonă. 1991. Effects of lead on polymorphonuclear leukocyte (PMN) functions in occupationally exposed workers. Arch. Toxicol. 65(8):685-688.
Valentino, M., V. Rapisarda, L. Santarelli, M. Bracci, M. Scorcelletti, L. Di Lorenzo, F. Cassano, and L. Soleo. 2007. Effect of lead on the levels of some immunoregulatory cytokines in occupationally exposed workers. Hum. Exp. Toxicol. 26(7):551-556.
Vander, A.J. 1988. Chronic effects of lead on the renin-angiotensin system. Environ. Health Perspect. 78: 77-83.
Vaziri, N.D. 2008. Mechanisms of lead-induced hypertension and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 295(2): H454-H465.
Verhoef, P., F.J. Kok, D.A. Kruyssen, E.G. Schouten, J.C. Witteman, D.E. Grobbee, P.M. Ueland, and H. Refsum. 1997. Plasma total homocysteine, B vitamins, and risk of coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 17(5):989-995.
Wang, F.T., H. Hu, J. Schwartz, J. Weuve, A.S. Spiro, III, D. Sparrow, H.L. Nie, E.K. Silverman, S.T. Weiss, and R.O. Wright. 2007. Modifying effects of the HFE polymorphisms on the association between lead burden and cognitive decline. Environ. Health Perspect. 115(8):1210-1215.
Wang, L., P. Xun, Y. Zhao, X. Wang, L. Qian, and F. Chen. 2008. Effects of lead exposure on sperm concentrations and testes weight in male rats: A meta-regression analysis. J. Toxicol. Environ. Health A 71(7):454-463.
Wasserman, G.A., X. Liu, D. Popovac, P. Factor-Litvak, J. Kline, C. Waternaux, N. LoIacono, and J.H. Graziano. 2000. The Yugoslavia Prospective Lead Study: Contributions of prenatal and postnatal lead exposure to early intelligence. Neurotoxicol. Teratol. 22(6):811-818.
Weaver, V.M., B.K. Lee, K.D. Ahn, A.C. Todd, W.F. Stewart, J. Wen, D.J. Simon, P.J. Parsons, and B.S. Schwartz. 2003. Associations of lead biomarkers with renal function in Korean lead workers. Occup. Environ. Med. 60(8):551-562.
Weaver, V.M., B.K. Lee, A.C. Todd, K.D. Ahn, W. Shi, B.G. Jaar, K.T. Kelsey, M.E. Lustberg, E.K. Silbergeld, P.J. Parsons, J. Wen, and B.S. Schwartz. 2006. Effect modification by delta-aminolevulinic acid dehydratase, vitamin D receptor, and nitric oxide synthase gene polymorphisms on associations between patella lead and renal function in lead workers. Environ. Res. 102(1):61-69.
Weaver, V.M., L.R. Ellis, B.K. Lee, A.C. Todd, W. Shi, K.D. Ahn, and B.S. Schwartz. 2008. Associations between patella lead and blood pressure in lead workers. Am. J. Ind. Med. 51(5):336-343.
Weaver, V.M., M. Griswold, A.C.Todd, B.G.Jaar, K.D. Ahn, C.B. Thompson, and B.K. Lee. 2009. Longitudinal associations between lead dose and renal function in lead workers. Environ. Res. 109(1):101-107.
Wedeen, R.P., J.K. Maesaka, B. Weiner, and G. A. Lipat. Occupational lead nephropathy. Am. J. Med. 59(5):630-641.
Weisskopf, M.G., R.O. Wright, J. Schwartz, A. Spiro, III, D. Sparrow, A. Aro, and H. Hu. 2004. Cumulative lead exposure and prospective change in cognition among elderly men: The VA Normative Aging Study. Am. J. Epidemiol. 160(12):1184-1193.
Weisskopf, M.G., S.P. Proctor, R.O. Wright, J. Schwartz, A. Spiro, III, D. Sparrow, H. Nie, and H. Hu. 2007. Cumulative lead exposure and cognitive performance among elderly men. Epidemiology 18(1):59-66.
Weisskopf, M.G., N. Jain, H. Nie, D. Sparrow, P. Vokonas, J. Schwartz, and H. Hu. 2009. A prospective study of bone lead concentration and death from all causes, cardiovascular diseases, and cancer in the Department of Veterans Affairs Normative Aging Study. Circulation 120(12):1056-1064.
Weisskopf, M.G., J. Weuve, H. Nie, M.H. Saint-Hilaire, L. Sudarsky, D.K. Simon, B. Hersh, J. Schwartz, R.O. Wright, and H. Hu. 2010. Association of cumulative lead exposure with Parkinson’s Disease. Environ. Health Perspect. 118(11):1609-1613.
Weuve, J., K.T. Kelsey, J. Schwartz, D. Bellinger, R.O. Wright, P. Rajan, A. Spiro, III, D. Sparrow, A. Aro, and H. Hu. 2006. Delta-aminolevulinic acid dehydratase polymorphism and the relation between low level lead exposure and the Mini-Mental Status Examination in older men: The Normative Aging Study. Occup. Environ. Med. 63(11):746-753.
Weuve, J., S.A. Korrick, M.A. Weisskopf, L.M. Ryan, J. Schwartz, H.L. Nie, F. Grodstein, and H. Hu. 2009. Cumulative exposure to lead in relation to cognitive function in older women. Eniviron. Health Perspect. 117(4):574-580.
WHO (Word Health Organization). 2010. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th Ed. World Health Organization, Geneva [online]. Available: http://whqlibdoc.who.int/publications/2010/9789241547789_eng.pdf [accessed Sept. 27, 2012].
Wiebe, J.P., and K.J. Barr. 1988. Effect of prenatal and neonatal exposure to lead on the affinity and number of estradiol receptors in the uterus. J. Toxicol. Environ. Health 24(4):451-460.
Wiebe, J.P., K.J. Barr, and K.D. Buckingham. 1988. Effect of prenatal and neonatal exposure to lead on gonadotropin receptors and steroidogenesis in rat ovaries. J. Toxicol. Environ. Health 24(4):461-476.
Wright, R.O., S.W. Tsaih, J. Schwartz, A. Spiro, III, K. McDonald, S.T. Weiss, and H. Hu. 2003. Lead exposure biomarkers and mini-mental status exam scores in older men. Epidemiology 14(6):713-718.
Xu, B., S.E. Chia, M. Tsakok, and C.N. Ong. 1993. Trace elements in blood and seminal plasma and their relationship to sperm quality. Reprod. Toxicol. 7(6):613-618.
Yakub, M., and M.P. Iqbal. 2010. Association of blood lead (Pb) and plasma homocysteine: A cross sectional survey in Karachi, Pakistan. PLoS One 5(7):e11706.
Yin, Y., T. Zhang, Y. Dai, Y. Bao, X.Chen, and X. Lu. 2008. The effect of plasma lead on anembryonic pregnancy. Ann. N.Y. Acad. Sci. 1140:184-189.
Yokoyama, K., S. Araki, K. Murata, Y. Morita, N. Katsuno, T. Tanigawa, N. Mori, J. Yokota, A. Ito, and E. Sakata. 1997. Subclinical vestibulo-cerebellar, anterior cerebellar lobe and spinocerebellar effects in lead workers in relation to concurrent and past exposure. Neurotoxicology 18(2):371-380.