4
Causes of Falsified and
Substandard Drugs
The committee recognizes that the factors that encourage the proliferation of substandard and falsified medicines are different but overlapping. In general, neglect of good manufacturing practices, both accidental and deliberate, drives the circulation of substandard drugs, while falsification of medicines has its roots in crime and corruption. Both types of products circulate because of the erratic supply and constant demand for medicines and weaknesses in the regulatory system. An inaccurate or inadequate understanding of the problem among health workers and the public contributes to the problem.
As Chapter 1 explains, substandard drugs are those products that fail to meet the specifications set by the regulatory authority and delineated in a pharmacopeia or the manufacturer’s dossier. Substandard medicines may, for example, be made in such a way that they do not dissolve properly; they may be of incorrect hardness or osmolarity; they may contain improper doses of the active ingredients; or be made from impure or unstable ingredients. Failure of good manufacturing practices is the root cause of substandard drugs.
Any company can make mistakes, but adherence to good manufacturing practices makes mistakes less likely and easier to correct. A factory run in accordance with best practices does not need to be the most technologically advanced or use state-of-the-art equipment, but there are costs to bring a factory up to standard, train staff on appropriate protocols, and observe them consistently. There are many exemplary manufacturers in developing countries that observe international best practices. There are also many that do not, but they operate anyway, either because the regulatory authority is unaware of the problem, or because regulators are under pressure to ignore it in the name of promoting industry.
Key Findings and Conclusions
• There are equipment, staffing, and process costs necessary to meet international good manufacturing practices in the pharmaceutical industry.
• Lack of investment capital and poor infrastructure hold back some small- and medium-sized drug companies in developing countries from meeting international standards.
• For want of investment in pharmaceutical manufacturing, the poor pay more for substandard medicines.
• Unscrupulous manufacturers will deliberately produce poor-quality drugs, if the odds of getting away with it are favorable.
• When regulatory checks on production are inconsistent, procurement practices can help ensure that honest manufacturers get the largest market share.
• The World Health Organization’s (WHO’s) Model Quality Assurance System for procurement is a useful independent standard for procurement agencies.
• National and international procurement agencies should follow the WHO’s guidelines for procurement. Small agencies should not procure directly from manufacturers.
Quality control is a part of good manufacturing practices sometimes neglected in developing countries. The WHO compendium on pharmaceutical manufacture describes the importance of having quality-control staff who are separate from production staff, working in an independent department (WHO, 2007b). A manager trained in quality control should supervise this department and run an equipped quality-control laboratory (WHO, 2007b). Quality-control staff should verify that everything that is a part of the factory’s product, including packaging, starting materials, intermediates, and finished products, meets requirements (WHO, 2007b). They may also do internal inspections and quality audits and evaluate the quality controls used by their suppliers (WHO, 2007b). The majority of the pharmaceutical industry in the poorest countries only formulates and
repackages finished medicines, also called secondary and tertiary production (see Box 4-1). Confirming the quality control measures used by suppliers, who are often in other countries, is particularly difficult for these firms.
Formulation companies have about a 6-month lag between placing an order for an active ingredient and selling a finished drug (Bumpas and Betsch, 2009). This delay can be even longer for firms in landlocked countries or places where customs clearance and transportation from the port of entry are slow or unpredictable (McCabe, 2009). It takes substantial working capital to cover costs during those lags (Bumpas and Betsch, 2009). Adding to the expense are the active ingredients themselves, which can cost thousands of dollars per kilogram; buying from WHO-prequalified or stringent-regulatory-authority–approved suppliers can add a 100 percent markup to the sale price (Bumpas and Betsch, 2009). The market for active ingredients has been especially volatile in recent years because of increasing costs of raw materials and growing environmental regulation in India and China (Bumpas and Betsch, 2009). Price volatility further complicates business for smaller firms, who tend to deal with less consistent (therefore cheaper) suppliers who are more vulnerable to market shocks. Although proper quality-control measures require purchasing only from suppliers who observe good manufacturing practices, supplier quality is often neglected because of logistical and financial obstacles. And, because the cost of active ingredients is by far the largest fraction of overall cost, a small reduction in active ingredient can vastly increase the profit margin.
Good quality comes at a price, either from equipment costs, better ingredients, or the higher process cost of quality assurance. The water filtration system is a high risk for microbial growth in any pharmaceutical plant and should be monitored vigilantly (WHO, 2007b). Microbial contamination is more of a threat in countries with poor water quality; much equipment cannot run on erratic power supplies (Anderson, 2010). Drug manufacturers also need an air handling system that will prevent dust and residue from one work area from contaminating other parts of the factory (WHO, 2007b). The adequacy of the air handling becomes more important in areas of the factory where different products are being processed at the same time and opportunities for cross-contamination abound (WHO, 2007b).
Some small-scale pharmaceutical companies make few finished formulations, but others make a wide range of products. Small firms are not generally able to dedicate equipment to specific products; equipment cleaning and cleaning validation become especially important. When equipment used for multiple products is not properly cleaned, and the cleaning not validated prior to changing the product line, the drugs produced can become contaminated. This type of contamination is difficult to detect. Quality-control assays generally test for the presence of the known ingredients, not the wide range of possible unknown contaminants. Good pharmaceutical manufacturing requires drug producers to follow a cleaning protocol laid out in their standard operating procedures and to follow cleaning with validation testing (APIC, 1999; WHO, 2007b).
BOX 4-1
The Medicines Manufacturing Process
Drugs are made with four or five main steps between the raw materials and the packaged final formulation (Figure 4-1). Medicines manufacture in the poorest countries is generally limited to the last steps in this process: formulation and packaging (Bumpas and Betsch, 2009; IFC, 2007). Of the 46 countries in sub-Saharan Africa, about 80 percent have local pharmaceutical industries, but only South Africa produces active ingredients (Bumpas and Betsch, 2009). South Africa alone accounts for 70 percent of the region’s medicines production (Bumpas and Betsch, 2009).
The firms that make final formulations in developing countries buy excipients and active ingredient from chemical suppliers abroad, mostly from China and India. China supplies about 43 percent of the world’s active ingredients for anti-infective medicines and exports 77 percent of the active ingredient made in the country, a $4.4 billion industry. India exports 75 percent of the $2 billion worth of active ingredients it produces (Bumpas and Betsch, 2009).
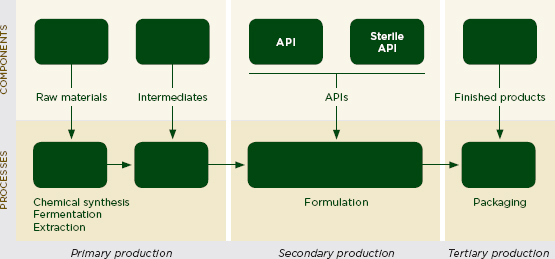
FIGURE 4-1 Schematic block diagram of a pharmaceutical manufacturing process.
NOTE: API = active pharmaceutical ingredient.
SOURCES: Adapted from Kaplan and Liang, 2005; Wilson et al., 2012.
There is significant expense necessary for pharmaceutical companies to follow good manufacturing practices. Multinational companies, both
innovator and generic, operate on a scale that allows them to recover the costs of running high-quality factories. This is not true for many smaller manufacturers in developing countries. In India, for example, large pharmaceutical companies supply medicines and vaccines of the highest quality to every country in the world, but thousands of small manufacturers struggle to implement quality-assurance and quality-control procedures (Kaplan and Laing, 2005). A World Bank study found that one-tenth of Indian registered pharmacies report substandard medicines, most of them coming from small- and medium-sized producers (Kaplan and Laing, 2005). Because the registered pharmacy is the most strictly regulated medicines outlet in India, the proportion of substandard medicines sold in the informal market is presumably much higher. The problem is not limited to India. In a survey of antibiotic quality in Indonesia, investigators found 89 percent of samples of one local company’s cotrimoxazole were substandard (Hadi et al., 2010).
Critics of local manufacture have cited these problems as reasons against pharmaceutical manufacturing in low- and middle-income countries (Ahmed, 2012; Bate, 2008). This may be a short-sighted argument. Domestic manufacture of medicines is an important part of health and industrial policy in many countries. Governments are understandably eager to ensure a safe drug supply for their population. In theory, locally made products could be cheaper because of lower shipping costs incorporated into the final price (Kaplan et al., 2012). Manufacturing medicines also gives people jobs and facilitates technology transfer (Wilson et al., 2012). Companies that start out packaging only finished drugs will slowly develop the trained workforce needed for more complicated secondary and primary manufacturing.
Initial capital investments and infrastructure problems stand between quality medicines and many small- and medium-sized medicine manufacturers. There are companies in developing countries that want to meet international quality standards and buy from reliable suppliers, but they fail to do so for reasons beyond their control. Governments alone cannot supply the technical depth or money to fix these problems (Wilson et al., 2012). The private sector must be involved. The International Finance Corporation (IFC) and the Overseas Private Investment Corporation (OPIC) can work to encourage private sector growth in developing countries. With the initial investments made, governments can take on the more manageable role of encouraging partnerships with foreign manufacturers.
Recommendation 4-1: The International Finance Corporation and the Overseas Private Investment Corporation should create separate investment vehicles for pharmaceutical manufacturers who want to upgrade to international standards. Governments can complement this effort by encouraging partnerships between local and foreign manufacturers.
Poor infrastructure, management problems, and insufficient training for staff can all hold back pharmaceutical manufacturers in low- and middle-income countries. While the extent to which each of these factors impedes progress varies among countries, there is a common problem of lack of capital (Cho et al., 2012; Patricof and Sunderland, 2005). Small- and medium-sized businesses have a particularly difficult time securing business improvement loans, as do firms in Africa (Patricof and Sunderland, 2005).
The only capital available to many small- and medium-sized drug manufacturers is the company’s already meager profits. Reinvesting profits in capital improvements is not a quick or reliable path to develop a modern manufacturing infrastructure (UNDP, 2004). In developed countries small-and medium-sized firms might mortgage their assets to raise money, but mortgage laws and bank policies often disallow this in low- and middle-income countries (UNDP, 2004). The equipment and supplies needed to observe good manufacturing practices must be bought on foreign markets with hard currency, which banks in poor countries may only have at certain times of year (McCabe, 2009).
Manufacturers in developing countries often have to absorb their customer’s debts, further reducing their working capital (McCabe, 2009). Therefore, small- and medium-sized companies are risky investments. Their national banks find the costs of the initial risk assessment both too costly and too complicated to make loans attractive (UNDP, 2004). Barriers to accessing capital hold back small- and medium-sized businesses, the “engines of job creation,” in the parts of the world most desperate for more and better jobs (Economist, 2012b; UNDP, 2004, p. 4). When these enterprises are drug companies, there is an added drawback. For want of investment capital, the poor pay higher prices for substandard drugs (UNDP, 2004).
The IFC and OPIC both promote private-sector development as a means to reduce poverty. The IFC’s goals include promoting open markets and jobs that deliver essential services in developing countries (IFC, 2012c). To this end, it provides investment services to help promote private-sector growth in developing countries. Through investments, advisory services, and asset management, the IFC aims to reduce poverty and encourage economic growth (IFC, 2012d). The IFC works with the World Bank Group, but with financial and legal autonomy. Its membership is made up of 184 developed and developing countries (IFC, 2012f).
The IFC accepts applications for ventures in member countries, often when a company cannot access the requisite capital in its home country (IFC, 2012a,d). The organization serves a wide range of industries, including health, education, infrastructure, agribusiness, and manufacturing (IFC, 2012b).
There is precedent for the IFC working with pharmaceutical companies in developing countries (IFC, 2012e). Alongside investment in upgrading pharmaceutical standards, its membership structure could be used to set up partnerships between pharmaceutical companies in developing countries and those in countries with strict regulatory authorities. The IFC does not lend directly to small- and medium-sized enterprises but can invest in organizations that will in turn lend to smaller companies (IFC, 2012a).
OPIC, the U.S. government’s development finance agency, does make loans to small businesses (OPIC, 2012a). Its loans and guaranties for small-and medium-sized business financing range from $350,000 to $250 million (OPIC, 2012a). OPIC often finances capital costs such as equipment and construction (OPIC, 2012a). It also funds national lenders to expand their lending capacity to small- and medium-sized enterprises (OPIC, 2012a). Although the agency does not grant requests that are solely for acquisitions or working capital, it will support the expenses if they are part of a larger project (OPIC, 2012a).
OPIC creates ways for investing in developing countries, to the benefit of both development abroad and private firms in the United States (OPIC, 2012a). OPIC’s investment policies promote sustainable development and human rights; investment in medicines manufacture is well aligned with these priorities (OPIC, 2012b).
Investment in upgrading pharmaceutical manufacturing standards advances the goals of both organizations; there is also precedent for such investments. In August 2012, the IFC invested $47 million in Fosun Pharma, a leading Chinese drug company that makes, among other products, anti-malarials for aid organizations (Yu and Hindenburg, 2012). OPIC supported the development of generic drug manufacturing in Afghanistan in 2005 (OPIC, 2006). The committee commends these projects and encourages OPIC and the IFC to make more similar investments in a wider range of companies.
Investment in pharmaceutical manufacturers in low- and middle-income countries has immediate benefits to the manufacturers trying to upgrade their production. There are also spillover benefits to a cohort of workers trained in good manufacturing practices and the use of modern equipment. These workers may eventually find new positions in other industries, sharing their knowledge about manufacturing, and contributing to a more competent workforce. IFC and OPIC investment will help buyers identify manufacturers who are serious about running a responsible business and willing to make expensive changes to their methods. Firms that make these investments are clearly trying to eliminate substandard production. Building responsible firms gives procurement agencies that are forced to buy locally produced medicines a high-quality alternative to the status quo.
Governments in low- and middle-income countries can complement investments in the private sector by encouraging partnerships between
foreign and local manufacturers who upgrade their production. Partnerships can continue the cross-fertilization of ideas that direct investment sparks. Manufacturing staff in developing countries who work with their counterparts abroad learn about regulatory science and business management, for example. This exposure benefits all parties and advances an international network of high-quality drug manufacturers.
In practice it is difficult to distinguish the quality failures that are to blame on a manufacturer’s inability to meet international best practices from those which come from a decision to cut corners and produce inferior products for poorly regulated markets. When a producer capable of meeting international standards fails to do so consistently and only in product lines sold to the poor, one may conclude that the noncompliance is part of a more insidious system.
Rich countries enforce high quality standards for medicines, and manufacturers recognize the need to use good-quality ingredients and good manufacturing practices to sell in these markets. United Nations (UN) agencies and the larger international aid organizations will also refuse to do business with companies that cannot meet stringent regulatory authority quality standards. Manufacturers are aware, however, that low- and middle-income countries are less likely to enforce these standards. Some companies exploit this and produce drugs of lower quality for the loosely regulated markets (Caudron et al., 2008). When a manufacturer produces medicines of inferior quality for less exacting markets, it is known as tiered or parallel production (Caudron et al., 2008; World Bank, 2007).
Tiered production is a complicated problem, in part because some kinds of tiered production are legal. International manufacturers may supply to multiple markets which use different legal product quality standards. For example, the British Pharmacopoeia monograph for amoxicillin gives no dissolution standard (British Pharmacopoeia, 2012); the U.S. Pharmacopeia does (USP-NF, 2010). Assay limits may also be different, making a product illegal by one pharmacopeia but legal by another. A manufacturer may supply to one country that stipulates a uniformity of dosage at 90-120 percent of declared dosage and to another country that stipulates 85-115 percent, for example. Both these standards aim to correct for the fact that drugs such as antibiotics degrade quickly, making a high initial dose acceptable. However, manufacturers could technically aim to fill only the lower bound of the dosage requirements and be within the letter of the law. A study of amoxicillin samples in Arab countries found that most samples’ active ingredient concentrations were bordering the U.S. Pharmacopeia lower limit (Kyriacos et al., 2008). The authors admitted, however, that many of
the problematic samples would have been judged acceptable by the wider British Pharmacopoeia standard (Kyriacos et al., 2008).
Participants at the public meetings for this study mentioned concerns with parallel production, but evidence for it is largely anecdotal. There is reason to suspect tiered manufacturing when the dose of active ingredient is consistently lower, never higher, than the label claim (Bate et al., 2009b). Drugs, especially tablets, of less than half the labeled potency before the expiry date are particularly dubious. In a hospital dispensary in rural Nepal, a bottle of pediatric amoxicillin from a WHO-certified producer with many obvious labeling and packaging defects also suggests either parallel manufacturing or diversion, a problem discussed in Chapter 5 (Brhlikova et al., 2007).
Tiered manufacturing is a rising problem in emerging manufacturing nations. A 2006 Lancet report described a shift in Russia from most bad medicines being falsified drugs made “in basements and small backroom enterprises” to ones coming from legitimate companies running “a ‘night shift’ to produce extra quantities of a certified drug that does not pass quality control, or sophisticated copies of well-known drugs … often with reduced levels of expensive active ingredients” (Parfitt, 2006, p. 1481). The United Nations Office on Drugs and Crime (UNODC) described a similar case in India. The U.S. Food and Drug Administration (FDA) revoked market authorization from an Indian drug manufacturer found to be producing antibiotics with no active ingredients (UNODC, 2010). After losing its license, “the factory continued to operate at night, until an evening raid by police uncovered an underground cellar in the factory, where exact look-alikes of several popular, fast-moving, high-cost medicines were being manufactured, most of which contained no active ingredient” (UNODC, 2010, p. 187).
Jiben Roy reported on a similar case: A Bangladeshi company deliberately kept the active ingredients in paracetemol, ampicillin, and cotrimoxazole below the labeled concentrations after repeated warnings from the regulatory authority (Roy, 1994). In the same paper he attributed the manufacturer’s quality failures in their cheaper product lines to negligence alone. Their B-vitamins, for example, contained the proper ingredients, but in erratic doses (Roy, 1994). This paper was able to make distinctions between the deliberate quality failures and negligence because the author had close knowledge of the manufacturer and its history. Usually only the national regulatory authority could have the information needed to make this distinction. In many countries, even the regulatory authority would not have that information or the political will to act on it (Christian et al., 2012b).
Pinpointing cases of deliberate tiered manufacturing is difficult to do, though it is easier to see practices that allow it happen. Poor oversight of
contract manufacturers is one such practice. A combination of technological sophistication and low labor costs in some developing countries attract drug companies, both innovator and generic, to contract with manufacturers abroad (PWC, 2010). Setting up a drug factory in India, for example, costs companies about 40 percent of what they would pay in North America or Europe (PWC, 2010).
Companies provide contract manufacturers with the materials, including packaging, to make their products. As Dilip Shah, Secretary General of the Indian Pharmaceutical Alliance, explained to a committee delegation in India, “Very few companies, foreign or domestic, monitor the [contract manufacturer’s] process loss of raw materials, active ingredients, and packaging materials. I have known of cases of 15 to 20 percent packaging material losses and companies are not bothered.” These contract manufacturers have established distribution channels; it is not difficult for them to introduce falsified drugs into the market. Because the contract manufacturers have the processes and materials needed to produce a proper drug, they will sometimes sell perfectly made drugs outside of the licit distribution system. More often, they will use legitimate packaging to disguise a false product.
Pharmaceutical exporting countries can also unintentionally facilitate tiered manufacturing by not requiring the same evaluations for exported drugs as for those sold domestically (Caudron et al., 2008). In general, regulatory agencies are responsible for protecting their country’s domestic medicine supply; ensuring quality for exported products is often beyond their mandate and budget. Importing countries’ regulatory agencies have the right to inspect producers abroad, but the breadth of international supply chains makes this a difficult job even for the most mature agencies (IOM, 2012). It is more difficult for low- and middle-income countries to ensure checks on drug quality during manufacture, a problem discussed later in this chapter.
Procurement and Substandard Medicines
When regulatory checks on production are inconsistent, procurement practices can help ensure that quality medicines get the largest market share. The Global Fund explains the goal of good procurement as supplying medicine “meeting agreed quality standards at the lowest possible price and in accordance with national and international laws” (Global Fund, 2009, p. 6). Government agencies procuring medicines have to reconcile a tension between quality and price (Torstensson and Pugatch, 2012). The WHO Operational Principles for Good Pharmaceutical Procurement discusses the hidden costs of cheap drugs, including poor shelf life and health threats (WHO, 1999, 2002a). The firms that offer the cheapest prices may do so by buying impure ingredients or cutting corners in formulation.
Good procurement dictates that the cheapest tenders are not accepted
if they are of dubious quality, but it is difficult not to be swayed by price, especially for provincial health offices and other small procurement agencies (Bate, 2007; Harper et al., 2007). Chinese provincial procurement, for example, is known for “pressuring manufacturers to produce the lowest cost possible while preserving their profits” (Burkitt, 2012). These agencies face pressure to supply medicines for entire populations on tight budgets; sometimes there is added demand to support local manufacturers (Dickens, 2011; Torstensson and Pugatch, 2012). Openness in procurement can balance these pressures by exposing unnecessarily high costs or bad quality, but transparency, which also includes vetting procurement officers for conflicts of interest, auditing suppliers, documenting decisions, and scrutinizing procurement agents, adds costs to the process (Torstensson and Pugatch, 2012). For these reasons the Organisation for Economic Co-operation and Development (OECD) recommends “an adequate degree of transparency in the entire procurement cycle to promote fair and equitable treatment for potential suppliers” (OECD, 2009, p. 11).
Over the longer term, more openness is a good investment. In Argentina, for example, a health transparency program brought down the procurement costs of medicines (Lewis, 2006). Reducing costs of procurement would be especially helpful in the poorest countries, which tend to spend a higher proportion of their health budget on drugs and where medicines are often expensive (Torstensson and Pugatch, 2012). In a study of 36 low-and middle-income countries, Cameron and colleagues found that public procurement agencies in the western Pacific, Africa, and the former Soviet bloc pay an average of 34 to 44 percent above the international reference prices (Cameron et al., 2008).
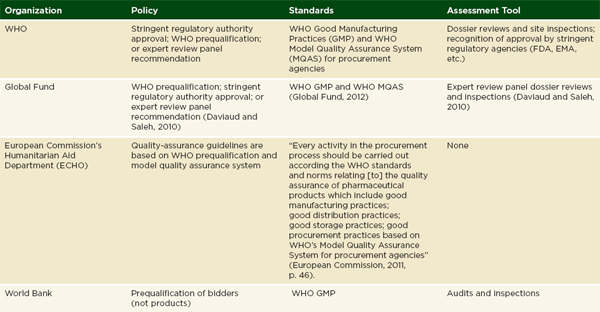
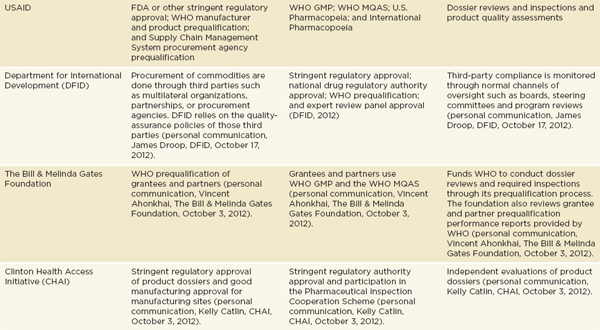
Donors may attempt to cover unmet demand for drugs, though donor procurement also has problems. Methods for assuring the quality of donated medicines vary by donor. The United States Agency for International Development (USAID) requires FDA, or other stringent regulatory agency, approval on donated medicines. It also has a prequalification process to vet the wholesalers it works with (GAO, 2012). USAID contractors are often responsible for implementing these rules in the field (Moore et al., 2012). The Global Fund will accept WHO prequalification, the approval of stringent regulatory authorities, or the review of an expert panel, especially for finished pharmaceuticals that are not prequalified by the WHO (GAO, 2012). Many European donors ask their recipients to assure quality of medicines procured with donated funds (Moore et al., 2012). Table 4-1 gives an overview of different agencies’ quality assurance policies.
Proper precaution in the medicines procurement process can prevent poor-quality products from infiltrating the market. Good procurement involves separating the various steps of procurement process identified in Table 4-2. Good procurement also puts a strong emphasis on controlling corruption and promoting transparency. The WHO’s Model Quality Assurance
System for procurement lays out the steps necessary for efficient and open procurement of the best-quality medicines possible (WHO, 2007a).
Recommendation 4-2: Procurement agencies should develop a plan, within the next 3 to 5 years, to comply with the World Health Organization’s Model Quality Assurance System for procurement agencies and work to remove any barriers to compliance.
The technical aspects of good pharmaceutical procurement and distribution practices have always been part of training courses on medicine supply management (MSH, 2012). The most complete and modern procurement guideline is the 2006 Model Quality Assurance System for Procurement Agencies, a United Nations interagency document endorsed by the WHO, Unicef (the United Nations Children’s Fund), the UN Development Program and Population Fund, and the World Bank (WHO, 2007a). The model draws on the accumulated experience of these agencies’ procurement experts and combines advice on the procurement of medicines, vaccines, diagnostic kits, and devices. The model focuses on four key activities: prequalification of pharmaceutical products and manufacturers and drug purchase, storage, and distribution. It presents the recommended practices in great detail (WHO, 2007a).
At its launch in 2006, the model had an aspirational element; it described standards that few if any of the international procurement agencies were able to maintain at that time. In the past 6 years, large procurement agencies have made great progress toward meeting the standards laid out in the model (van Zyl et al., 2012). The committee sees the model quality assurance system as a useful independent standard to assess procurement agencies. The system is a practical tool that can be used by national and international procurement agencies. Eventually, agencies can use the WHO tool to prequalify suppliers; prequalification is a recommended piece of a procurement system (MSH, 2011).
Modern pharmaceutical chains are international. No country is self-sufficient in its medicine supply. Pharmaceutical procurement almost always means working with foreign suppliers; a practice that exceeds capacity of national regulators, who cannot hope to inspect foreign manufacturers as they would domestic ones. Good procurement also means that only organizations that follow the model system should import medicines. Small-scale importation and procurement by small actors threaten the medicines supply chain. This risk is not only present in developing countries. In many OECD countries, pharmacies and private clinics import drugs directly from suppliers, greatly increasing the risks of introducing a poor-quality product to the market.
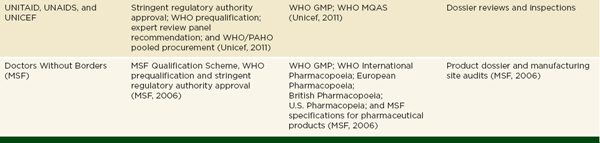
NOTE: DFID = Department for International Development; ECHO = European Commission’s Humanitarian Aid Department; EMA = European Medicines Agency; FDA= U.S. Food and Drug Administration; GMP = Good Manufacturing Practice; MQAS = Model Quality Assurance System for procurement agencies; PAHO = Pan American Health Organization; UNAIDS = Joint United Nations Programme on HIV/AIDS; Unicef= United Nations Children’s Fund; USAID = United States Agency for International Development; WHO= World Health Organization.
SOURCE: Adapted from Moore et al., 2012.
Applying the Model Quality Assurance System to Secondary Procurement
The requirements for infrastructure, policies and documentation, prequalification, purchasing, receipt, and distribution of medicines laid out in the model quality assurance system are written for large national or international agencies (WHO, 2006a). Much of the most problematic procurement happens at subordinate levels, however. District hospitals and health posts in poor countries will not likely meet the model standards for premises, equipment, or staffing any time in the near future (Dickens, 2011).
In the meantime, if full preselection of quality suppliers is not possible, interim solutions such as a two-envelope system can help reduce bias toward the cheapest firms. In this system, used by the Delhi hospital system, bidders submit their technical statement of work and their costs in separate, sealed envelopes (Chaudhury et al., 2005). The reviewers evaluate the quality controls in the statement of work. Only if the quality controls are sufficient do they open the second envelope, containing the project budget.
Ultimately, medicine procurement is complicated and requires considerable investment in staff and procedures. While the WHO model system should guide drug procurement at the national level, small agencies will never command the economies of scale necessary for good and open procurement (Dickens, 2011; Rao et al., 2006; WHO, 1998). Cutting corners in procurement creates opportunities for substandard products to infiltrate the supply chain. Therefore, smaller organizations such as district health offices should be free to choose the products and amounts they need from licensed, national wholesalers or importers, but they should not procure directly from manufacturers.
The committee recognizes that licensing wholesalers and importers requires political will. It might take time to build momentum for this step, as discussed further in Chapter 5. Therefore, the committee recommends that national and international procurement agencies take 3 to 5 years to develop and implement their compliance plans. These plans will identify those agencies with the technical depth and buying power necessary to comply with the WHO system. These agencies can develop their quality-assurance system within the next 5 years. The regulatory authority can then license national procurement agencies to buy medicines directly from manufacturers. Agencies that are not able to comply with the WHO’s minimum standards will not be licensed for procurement. Instead, these organizations will be able to order their medicines from licensed procurement agencies, thereby making more efficient use of their staff and budgets and avoiding the dangers of primary procurement.
TABLE 4-2 Pharmaceutical Procurement Best Practices
|
Pre-Procurement Stage |
|
Ensure an adequate procurement infrastructure is in place. Ensure health professional and technical capacity is high among officials. Use written quality manual and written standard operating procedures. Use of prescreening and prequalification is recommended for procurement agencies with limited capabilities. Prequalification must include quality assurance and quality testing through product and manufacture assessments, including testing of batches. Have management information systems in place to monitor actual supply and payment of drugs as well as post-supply quality. Estimates of medicines needed should be based on data like past use, morbidity records, and consumption predictions. Separate duties of pre-procurement process. |
|
Procurement Stage |
|
Procurement should be transparent, following formal written procedures and clear public selection criteria. International competitive bidding ensures economy efficiency and transparency and should be used. Separate duties of selection, product specification, and adjudication. Quality assessment of drugs upon receipt, including lab testing, inspection of shipments, and certificate of analysis of delivered products. Domestic preferencing should not compromise quality standards. Ensure technical specifications are right (e.g., dosage, storage, shelf life, delivery expectations, etc.) in bidding documents. |
|
Post-Procurement Stage |
|
Continue to monitor quality of received drugs through independent testing. Establish pharmacovigilance and adverse event reporting. Conduct independent and transparent audits of procurement and supplier performance. Regular new tenders should open to new bidders. |
SOURCE: Adapted from Torstensson and Pugatch, 2012.
As Chapter 1 explains, the drug regulator, having the authority to license manufacturers and register medicines, can act against products made by known manufacturers. When the manufacturer is falsely represented this is not possible. The regulator can only confirm that the producer is unknown and turn the case over to law enforcement. The police and detectives who inherit these cases have a difficult job gathering sufficient evidence for a prosecution; there is usually little if anything to tie the falsified drug in the market to the culprit (see Box 4-2). It is also hard to convince agents to investigate pharmaceutical crime when they are under immediate pressure to prosecute murders and other violent felonies. For all these reasons, falsifying medicines has been called the perfect crime (Dobert, 2012; Kontnik, 2004; Nelson et al., 2006).
Key Findings and Conclusions
• Making fake medicine is an opportunistic crime, more common in places where regulatory oversight is weak or inconsistent.
• Corruption allows for the manufacture, trade, and distribution of falsified medicines. Complicit government officials are often bribed with revenue from the illicit pharmaceutical business.
• Criminals may intentionally price falsified products slightly lower than legitimate drugs in order to guarantee their market share but avoid consumer suspicion.
• Major pharmaceutical companies have security departments that work with regulators and law enforcement agencies. These departments gather 80 percent of the evidence for criminal prosecution.
• Law enforcement agencies are cracking down on pharmaceutical crime. Seizures of falsified medicine have tripled in Brazil and led to 1,900 arrests in China.
Corruption and Organized Crime
Making fake medicine is not difficult. The least sophisticated operations manage with empty capsules bought in the open market or a hand-held pill press and any powder to load into it. Production costs on fake drugs are low (Clark, 2008; Perrone, 2012). And, because the licit and illicit supply chains mix in unregulated markets (described in Chapter 5), the odds of getting away with the crime are good. As Chapter 3 describes, the global burden of falsified and substandard medicines is borne disproportionately by low- and middle-income countries. There is wide evidence that criminals frequently target inexpensive anti-infective medicines, mostly because they are bought often and by the largest segment of the population. The UNODC therefore describes making falsified medicines as an “opportunistic crime, emerging where regulatory capacity is low, not where profits would be highest” (UNODC, 2010, p. vi).
When a drug that had been on the market for 40 years killed a young, generally healthy woman in 2004 despite her six previous doses with no side effects, the technical director of the AstraZeneca subsidiary in Río Negra, Argentina, was alarmed and suspected impropriety. The drug was Yectafer, an injectable iron supplement given to the patient for her anemia. She died of liver failure within weeks of receiving the fatal injection, unable to undergo transplant surgery quickly enough to save her life (Loewy, 2007). A sample of the drug was sent for testing at the plant and was immediately identified as a fake: the package labels were applied incorrectly, the name of the drug written in a different font, and the color of the liquid significantly altered. Chemical analysis confirmed that the bottle did not contain iron sorbitol, the active ingredient in Yectafer, but a different form of iron at three times the stated dosage (Loewy, 2007). Despite an attempted recall, one more woman died in the ensuing months, and at least eight women undergoing the same treatment were hospitalized for liver damage, including a 22-year-old pregnant woman whose condition caused her to deliver her baby prematurely at 26 weeks (Loewy, 2007; WHO, 2006b).
Although some of the people involved in distributing the dangerous fake were charged for their crimes, lack of an effective paper trail prevented Argentine authorities from tracking down the manufacturer. The victims’ youth lent an emotional appeal to this incident, making it the public face of drug regulation agenda, but Argentina was no stranger to tragedy of this sort. Fake drugs for treating Parkinson’s disease circulated in 1997 and exacerbated the symptoms they were taken to prevent (Loewy, 2007). Weak regulation and the legal confusion made Argentina’s drug supply vulnerable and hampered efforts to prosecute those involved (WHO, 2006b).
This is not to say that profits generated from falsifying drugs are insignificant. In a study of fake malaria medications in Southeast Asia, Dondrop and colleagues found the falsified artesenuate to be cheaper, but only somewhat, than the authentic one (Dondorp et al., 2004). By pricing their product just slightly under the legitimate drug, criminals can guarantee market share, but they avoid pricing it so low as to arouse suspicion. Falsified medicines can be priced less cautiously in the wholesale market, however, because these markets are less regulated and customers are not the general public but buyers for retail who are sometimes complicit. Tempo, an Indonesian news magazine, reported on “astonishingly low” prices
in a medicines wholesale market in Jakarta (Silverman et al., 1992). The story described how pharmacists unwilling to buy from the illegal markets probably could not survive in business (Silverman et al., 1992). Box 4-3 describes the profit motive of one American pharmacist dealing in diluted cancer drugs.
Interpol uses the term pharmaceutical crime to describe “the manufacture, trade and distribution of fake, stolen or illicit medicines and medical devices” (Interpol, 2012b). Pharmaceutical crime includes theft, trade, and the money laundering criminals use to cover their tracks (Interpol, 2012b). Corruption allows the crime to continue. Complicit government officials are often bribed with revenue from the underground pharmaceutical business (UNODC, 2010); criminal executives may be embedded in the government hierarchy (Parfitt, 2006). Threats and bribery are the purview of members of organized crime, who are often responsible for trafficking falsified medicines, perhaps attracted by the mild punishments discussed below (Beken and Balcaen, 2006; Interpol, 2012a). Interpol has evidence linking the trade in falsified drugs to Al-Qaeda and transnational crime syndicates (Beken and Balcaen, 2006; Liberman, 2012).
BOX 4-3
Adulterated Cancer Drugs
Robert Courtney, a pharmacist in Kansas City, Missouri, made millions selling adulterated drugs to patients and physicians throughout the 1990s until 2001, when he was prosecuted for his crimes. Most famous for diluting chemotherapy drugs such as Taxol, Gemzar, Paraplatin, and Platinol, Courtney regularly sold tampered versions of 72 different prescription drugs. His first foray into pharmaceutical crime was illegally purchasing drugs at low cost and selling them at market value, as well as disguising generic drugs as their name-brand counterparts and charging the associated higher price. Seeking higher profits, he left the gray market and turned to dilution (Belluck, 2001; Draper, 2003).
Traditionally, oncologists purchase chemotherapy drugs and dissolve them in saline at their offices. Robert Courtney was one of the first pharmacists in the area to begin selling convenient, premixed cancer drugs. By adding extra saline he stretched out his drug supply and made enormous profit selling the expensive therapies. The practice was so lucrative that he began diluting more extensively, going so far that during the investigation it was found that all of the mixtures sampled contained 39 percent or less of the proper dose and one even contained less than 1 percent. The substantial profit margin on the diluted drugs was the motivating factor; in one case he allegedly made more than $700 from one prescription. Courtney has admitted that his actions were “out of greed” (Belluck, 2001).
Communication between Eli Lilly Corporation, which manufactures Gemzar, and a physician prescribing the drug brought the scandal to light. A sales representative noticed the discrepancy in the amount of Gemzar that Courtney was buying from them and the amount he was selling, which led to an investigation by the company (Belluck, 2001). Although Eli Lilly dropped the investigation when it found no evidence of Courtney’s buying drugs elsewhere, the representative mentioned the finding to an affected physician, who sent samples of some of the drugs for testing. When the samples contained approximately one-third of the stated amount, she alerted authorities. After more than a decade of selling poor-quality drugs to more than 4,000 patients, Robert Courtney was investigated by the Federal Bureau of Investigation and the FDA for his crimes and sent to federal prison (Draper, 2003).

A medicines seizure in Shagamu, Nigeria, 2007.
SOURCE: © 2007 Opara Adolphus, Courtesy of Photoshare.
When falsified medicines are also counterfeits that infringe on the trademarks of multinational pharmaceutical companies, the company targeted tries to respond. Major pharmaceutical firms have designated security departments that work with regulators and law enforcement to gather evidence for criminal prosecution (Cockburn et al., 2005). In general these companies collect evidence and build 80 percent of the case against the criminals, then hand the investigation over to law enforcement (Economist, 2012a).
Law enforcement agencies, for their part, are cracking down more on pharmaceutical crime. The Chinese government, perhaps driven to improve China’s reputation as the world leader in fake drugs, arrested more than 1,900 suspects from about 1,100 manufacturers in late July 2012 (Burkitt, 2012; Palmer, 2012; Quingyun, 2012). The 18,000 police officers working in simultaneous raids across the country seized a range of falsified products, including saline labeled as rabies vaccine and an obesity drug recalled from the Chinese market because of toxic side effects (Quingyun, 2012). It was not clear what products were destined for the domestic market and which were meant for export (Palmer, 2012).
In an analysis of the Brazilian federal police reports, Ames and Souza found that police seizure of falsified medicines roughly tripled between 2007 and 2010 (Ames and Souza, 2012). Most falsified products entered Brazil from Paraguay, and the arrests were made at the border (Ames and Souza, 2012). Some data suggest that arrests at the point of sale, manufacture, and distribution are more common, however (see Table 4-3). Box 4-4 presents the Pharmaceutical Security Institute’s (PSI’s) 2010 and 2011 data on arrests for pharmaceutical crime.
PSI data indicate that China and Brazil took the most police action against falsified medicines in 2011. Table 4-3 presents the number of arrests in the PSI incident database by country. The countries with the most serious problems might have no arrests in a year, as arrests depend on government motivation to marshal the police. The momentum for labor-intensive police raids is difficult to sustain. Only half of the countries on PSI’s 2006 arrests list appear on the same list in 2011 (see Table 4-4). In 2006 Russia led in arrests for pharmaceutical crime after a series of raids reported in the Lancet (Parfitt, 2006). At the time, Gennady Shirshov, director of a Russian pharmaceutical industry association, predicted that other criminal manufacturers would quickly replace the closed ones (Parfitt, 2006). Mr. Shirshov mentioned insufficient law enforcement interest in the problem but concluded, “The legislation is inadequate. It’s a civil liability, not a criminal one … and the fines are negligible” (Parfitt, 2006, p. 2).
TABLE 4-3 Top Countries for Arrests, 2011
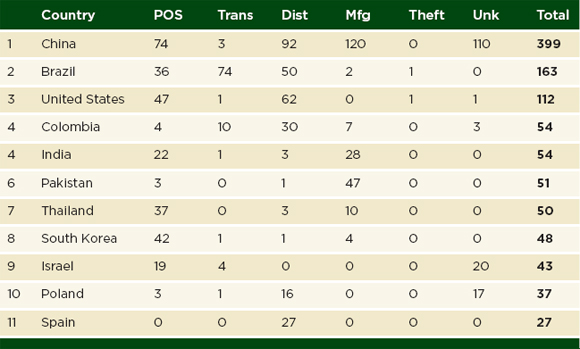
NOTES: Dist = distributing; Mfg = manufacturing; POS = point of sale; Trans = transporting; Unk = unknown.
SOURCE: PSI data shared with the committee, Thomas Kubic, PSI-Inc., July 11, 2012.
BOX 4-4
Pharmaceutical Security Institute Crime and Arrest Data
The Pharmaceutical Security Institute, a nonprofit network of 25 major pharmaceutical companies’ security departments, maintains a database on compromised medicines (PSI-Inc., 2012c). In PSI records, every report of a fake product, either from member companies or from public sources, is an incident. Incidents vary in their size and time frame (PSI-Inc., 2012a). PSI also keeps records on arrests, gathered from members, law enforcement officers, and open sources. These data indicate 1,311 arrests for pharmaceutical crime in 2011, a 14 percent increase from their 2010 records (PSI-Inc., 2012b). For 44 percent of their 2011 arrests data and 59 percent of 2010 arrests data, PSI has sufficient information to tie an arrest to an incident report in their database (PSI-Inc., 2011).
In both 2010 and 2011, about one-quarter of incidents ended in an arrest. In 2011 PSI identified an increase in arrests at the point of sale and during distribution (PSI-Inc., 2011). Figure 4-2 compares PSI data from 2011 and 2010, excluding 191 incidents for which PSI had insufficient information to confidently identify the point on the supply chain where the arrest was made.
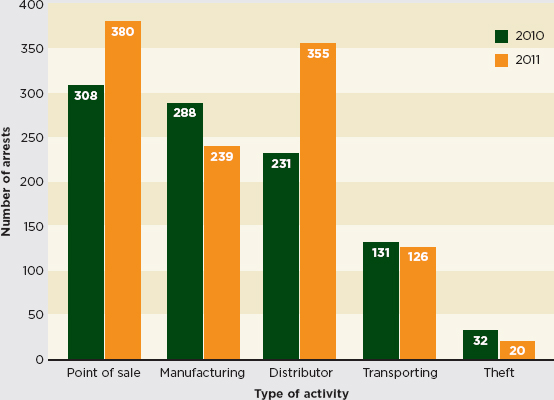
FIGURE 4-2 Arrests by activity, 2010–2011.
SOURCE: PSI-Inc., 2011.
TABLE 4-4 Top 10 Countries Ranked by Number of Counterfeit Drug Seizures and Discoveries in 2006
|
|
Country |
Number of Seizures |
|
1 |
Russia |
93 |
|
2 |
China |
87 |
|
3 |
South Korea |
66 |
|
4 |
Peru |
54 |
|
5 |
Colombia |
50 |
|
6 |
United States |
42 |
|
7 |
United Kingdom |
39 |
|
8 |
Ukraine |
28 |
|
9 |
Germany |
25 |
|
10 |
Israel |
25 |
NOTE: PSI uses the term counterfeit broadly, the way this report uses the word falsified. See page 23.
SOURCE: PSI-Inc., 2006.
As Box 4-5 mentions, perpetrators who are caught falsifying medicine are punished leniently in some countries (Kyriacos et al., 2008; WHO, 2012a). In the United States, the Food, Drug, and Cosmetic Act dictates a penalty of 1 year in prison, a fine of no more than $1,000, or both (Donaldson, 2010). Even repeat offenders are punished with no more than 3 years in prison or a fine of $10,000 (Donaldson, 2010). Considering that the profit margin for falsified drugs runs in the billions, the risk-to-profit analysis favors the crime. Table 4-5 shows the penalties for falsifying medicines in a selection of countries. The leniency in many countries may be a function of outdated laws. Tables 4-6 and 4-7 show penalties for patent and trademark infringement, which are dealt with more severely in some countries.
From 2005 to 2008, Manuel Calvelo operated internet pharmacies selling misbranded and falsified drugs for sale without prescription (DOJ, 2011). Calvelo sold $1.4 million worth of drugs on websites such as allcheapdrugs.com, cheapdrugspharmacy.com, and trustgeneric.com. He offered more than 40 products including Viagra, Zoloft, Lipitor, Cialis, and Xanax (Kake.com, 2011). Many were purported generic versions of patent-protected heart attack, stroke, and diabetes medications (PSM, 2011b).
Calvelo, a Belgian citizen, operated his business across borders. His customer service call center was in the Philippines; he paid his employees through wire transfers from Costa Rica, the Philippines, and the United States. Internet companies in Ohio and Kansas hosted his websites and he received payments through Dutch credit card processors from mostly American customers (DOJ, 2011).
In 2007, an undercover agent from the FDA’s Office of Criminal Investigation bought drugs from Calvelo’s websites (DOJ, 2011). These drugs appeared legitimate. Chemical testing, however, proved they were fake (PSM, 2011b). The agent later posed as a pharmaceutical wholesaler looking to establish an internet pharmacy (PSM, 2011a). Calvelo described the internet pharmacy scheme and the details of his operation to the agent (DOJ, 2011).
Calvelo was arrested in Costa Rica and extradited to Kansas. In January 2011, he plead guilty to one charge of conspiracy to commit drug trafficking and one charge of conspiracy to defraud the United States (DOJ, 2011). According to Patrick Holland, the special agent in charge of the FDA’s Office of Criminal Investigation’s Kansas City Field Office, “The investigation and [Manuel Calvelo’s] sentencing reflect the seriousness of importing counterfeit and misbranded pharmaceutical drugs into the United States” (DOJ, 2011). Calvelo was sentenced to 48 months in prison and, as part of his plea, agreed to pay $1.4 million in fines (DOJ, 2011; Kake.com, 2011).
Stricter and more consistent penalties could do much to fight the public health crime of producing and trading fake medicines. Chapter 7 discusses this solution in more detail, describing how a global code of practice could encourage consistent strict minimum punishments for these offenses.
As Chapter 1 explains, falsified and substandard medicines overlap a great deal. Much as poor-quality drugs are often both falsified and substandard, some potentiating factors encourage both kinds of problems. The high demand and erratic supply of drugs, weak regulatory systems, and lack of political will contribute to the trade on both falsified and substandard drugs.
Medicines are what economists describe as a comparatively inelastic good (Arnold, 2008); changes in the unit price of the medicine have proportionately little effect on the demand (Siminski, 2011). Price inelasticity, combined with a high relative price, make medicines a major expense for patients around the world. In the United States, health expenditures on medicine rise sharply in middle life and average between $1,000 and
$2,000 per person per year after age 45 (Paez et al., 2009). The cost of medicine is even more of a burden in low- and middle-income countries, where it accounts for 20-60 percent of health spending, and 90 percent of the population pays for medicine out-of-pocket (Cameron et al., 2008; WHO, 2004a,b).
Key Findings and Conclusions
• The demand for medicines is relatively consistent, though the supply is not. The private medicines market can be expensive and drug scarcity drives up prices.
• Reducing the costs and increasing the availability of medicines would remove some of the financial incentive to produce falsified and substandard drugs.
• A robust generics market can keep drug prices down, but there are cost barriers to market entry for many good-quality generics companies. A more straightforward registration and application process would reduce burdens on industry and regulators.
• Falsified and substandard medicines circulate because of weaknesses in the regulatory system. Regulators in low- and middle-income countries need training, equipment, and technology, as well as guidelines for strategic decisions about what to invest in first.
• In countries where state and federal governments share regulatory oversight, the division of responsibility is not always clear. Substandard drug production at the New England Compounding Center happened because of insufficient clarity between state and national responsibilities.
• Awareness of the problem of substandard and falsified medicines is uneven. Patients and providers need accurate information about the risks, communicated in way that empowers them to take reasonable precautions to protect their safety.
The drug market is not stable; both price and supply fluctuate. Sometimes the supply falters because of shortages in the raw materials, as in 2004 when increased demand for artemisinin, combined with a poor Artemesia annua harvest, drove up the price and led to stock-outs (Kindermans et al., 2007; Newton et al., 2006b; Pilloy, 2009). More generally, drug supply problems are driven by the economy. In the United States, for example, manufacturers sometimes stop producing products with low profit margins, such as sterile injectables—inexpensive products that are complicated to make (Hoffman, 2012). Manufacturers also can lose interest in a drug after its patent expires, when revenues from the product drop (Hoffman, 2012). Although the United States has a more stable drug supply than most developing countries, there have been regular shortages for the past 15 years, especially among injectables, cancer drugs, and antibiotics (Hoffman, 2012).
Drug shortages are more common in developing countries (MDG Gap Task Force, 2008). Survey data from the WHO and Health Action International suggest that although medicines may be available free or cheaply in public health centers, these centers often do not have the medicines needed; availability is generally better in the private sector but for a much higher price (Cameron et al., 2008; MDG Gap Task Force, 2008). Figure 4-3 shows that although private-sector outlets have a higher percentage of drugs available than public-sector ones, there is still a great deal of unmet need. A month’s course of the lowest-priced generic ulcer medication, for example, is still more than 3 days’ wages for a low-paid government worker in much of Africa, Eastern Europe, and the Middle East (Cameron et al., 2008).
Reducing the costs and increasing the availability of medicines would remove some of the financial incentive to produce and procure falsified and substandard medicines. If patients had a plentiful supply of reliable, affordable medicines, there would be less need to shop at unregulated gray markets.
The WHO has recommended generic substitution as a way to keep medicines costs down (MDG Gap Task Force, 2008), but this depends on a supply of high-quality generic medicines on the market. For generic manufacturers, companies that generally run on low margins, the costs of proving bioequivalence and preparing a manufacturer’s dossier for regulatory review can be prohibitive to market entry (Lionberger, 2008). Different regulatory authorities have different, often widely divergent, requirements for establishing bioequivalence (Mastan et al., 2011). To complicate the problem, many small regulatory authorities lack the technical depth to evaluate the bioequivalence data that generics manufacturers submit (Hill and Johnson, 2004).
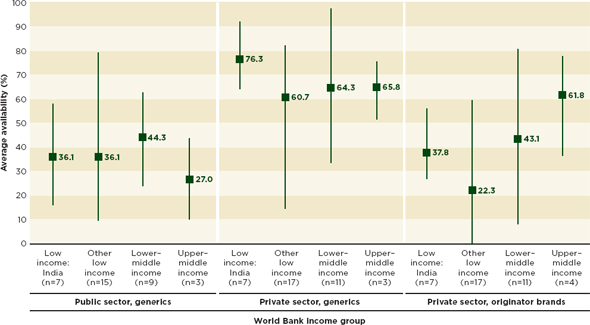
FIGURE 4-3 Average of country-level mean percentage availability of medicines by World Bank income group. Data are mean(maximum/minimum).
SOURCE: Cameron et al., 2008. Reprinted from the Lancet with permission from Elsevier.
Reducing the Costs of Market Authorization
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) brings together industry experts and regulators from Europe, Japan, and the United States to promote harmonized product registration requirements (ICH, 2010). To this end, ICH developed the Common Technical Document, a common application for medicines registration (ICH, 2012). The WHO has published guidance on preparation of generic product dossiers in keeping with the Common Technical Document format (Rägo, 2011; WHO, 2011). The committee believes this format could be useful to regulators and generics companies in low- and middle-income countries.
The use of a common form has made drug registration more efficient in Europe (Brousseau, 2012; ICH, 2010; Sahoo, 2008). It also controls the demands that registration puts on manufacturers. Harmonized applications also give regulators a common format to discuss their product registration process. Like sharing inspections and other harmonization efforts, the use of the common document increases efficiency and promotes a common language among regulators.
Recommendation 4-3: Regulatory authorities in low- and middle-income countries should use the International Conference on Harmonisation Common Technical Document format for product registration to better harmonize their procedures and reduce application costs for manufacturers. To the same end, they should also conduct joint inspections and use a common inspection report.
A more robust generic drug market in low- and middle-income countries could help prevent the drug shortages and price spikes that encourage the sale of poor-quality products. Regulatory authorities can work to better harmonize their procedures, thereby improving their own efficiency and reducing barriers to market entry for good-quality generics manufacturers. The use of the ICH Common Technical Document format for registration would ease the regulatory burden on generics companies. Regulators also reap a spillover benefit of more convergent regulatory systems without negotiating cumbersome mutual recognition agreements. The Singaporean drugs regulatory authority has promoted the common format, citing its ease of use and the way it facilitates sharing information among other regulators in the region (Poh, 2011). Similarly, Southeast Asian companies benefit
from the common format which allows them to prepare submissions for several countries at once (Poh, 2011).
The cost of bioequivalence testing runs from $50,000 to $200,000 (GIZ, 2012). Bioequivalence testing also requires sophisticated laboratories that are not available in many countries. This baseline cost to generic companies does not include several person-months of staff costs for revising registration application data into a new dossier. The costs of market authorization are prohibitively expensive, especially for entry into a small country’s market. When the overwhelmed regulatory authority will allow it, companies avoid the expense by submitting no proof of bioavailability; others falsify bioavailability data (Silverman, 2011).
Evidence suggests that these high costs keep generics companies out of the market and increase costs to the consumer (Mastan et al., 2011; Rawlins, 2004). Even multinational, innovator pharmaceutical companies struggle to convert applications between FDA and EMA formats. A 1996 industry study estimated that converting applications took between 2 and 10 months and significant staff time and expense (Molzon, 2009). Different standards for bioequivalence assessment also encourage the problem of widely divergent national drug quality standards (Mastan et al., 2011).
If the application and registration process were more straightforward then more good-faith companies could enter the market, increasing the supply of reliable drugs and controlling costs. The committee also believes that a consistent use of the common registration format could further the cause of regulatory harmonization, which would improve the drug regulatory systems in low- and middle-income countries. Harmonization also controls the burdens regulation puts on manufacturers; shared inspections are more efficient and less disruptive to industry. Generics companies, which generally have fewer staff than innovator companies, are disproportionately disturbed by frequent inspections.
A competitive generics market benefits consumers, as does a rigorous and unpredictable inspection regime (Mackintosh et al., 2011). In many developing countries, lack of confidence in the regulatory system breeds low enthusiasm for generic medicines (Hassali et al., 2009; Kaplan et al., 2012; Russo and McPake, 2010). Doctors and patients may perceive these products as lower quality (Chua et al., 2010; Gossell-Williams, 2007). An influx of generic medicines will only reduce the circulation in falsified and substandard drugs when there is a system to assure consumers of medicines quality. In their review of policy actions to promote generic medicines, Kaplan and colleagues conclude that a functioning medicines regulatory
authority is a necessary condition for a robust generic medicines market (Kaplan et al., 2012).
The drugs regulatory authority has the ultimate responsibility for the quality of medicines in the country. That includes registering medicines, issuing licenses and market authorization, postmarket surveillance, quality control testing, oversight of drug trials, and manufacturer and distributor inspections (IOM, 2012; WHO, 2010a). The regulatory authority also provides health workers and the public with accurate information on the rational and safe use of medicines and punishes illegal trade in drugs (WHO, 2012b). This range of responsibilities requires significant technical depth in staffing and political will to enforce regulations. Staffing shortages are often a problem in the public sector in low- and middle-income countries, where regulators are poorly paid and not well respected (IOM, 2012).
Staffing shortages at the regulatory authority are a particularly serious problem in India and China, two main pharmaceutical producing nations with massive industries to oversee. In 2003 the Mashelkar Commission estimated about 5,877 licensed manufacturers in India; other estimates cite as many as 20,000 Indian drug manufacturers, some very small (Government of India, 2003; KPMG International, 2006). In any case, only 250 to 300 of them are major producers (KPMG International, 2006). China has a comparatively more manageable 3,500 companies, down from roughly 5,000 in 2004; the reduction is partly the result of heightened enforcement in the wake of a series of drug contamination scandals (Reuters, 2008).
The pharmaceutical industry in both countries is exceptionally fragmented. The top 10 pharmaceutical companies in India cover about 30 percent of the domestic market (KPMG International, 2006); in China the top 10 companies account for only 10 percent (Sun et al., 2008). In contrast, the top 10 innovator pharmaceutical companies control about 42 percent of the international market (Sun et al., 2008). Inspecting and licensing so many factories would be an overwhelming task for a well-funded regulatory agency with sufficient staff. In both China and India, the understaffed provincial authorities oversee licensing and inspecting manufacturers, with uneven results. In 2007 a Chinese provincial regulator issued 67 forged manufacturing licenses for a bribe (Liu, 2010). Indian regulators sometimes approve medicines without trials or valid expert review and authorize irrational, even dangerous, fixed-dose formulations of multiple active compounds (Vaidyanathan, 2012). Drugs that neighboring countries ban are often available in India because the regulatory agencies cannot enforce bans or execute recalls (Shaji and Lodha, 2010).
There are similar problems in less industrialized countries. A WHO survey of 26 drug regulatory authorities in sub-Saharan Africa found that only one country’s regulator published guidelines on good distribution, while only 20 percent published internationally rigorous manufacturing practices (WHO, 2010a). The same study found that several regulatory authorities grant licenses and renewals with no inspections, that operating procedures for conducting inspections were woefully weak, and that 35 percent of the regulatory authorities have no legal authority for inspections (WHO, 2010a). Figure 4-4 shows the number of agencies out of the 26 surveyed that can perform drug regulatory functions. All of these weaknesses allow for falsified and substandard drugs to circulate. As one of the participants in the WHO study explained, “The illicit medicines market has become a real plague…. All therapeutic classes can be found, including psychotropic medicines, and there is no national strategy to combat this situation” (WHO, 2010a, p. 16).
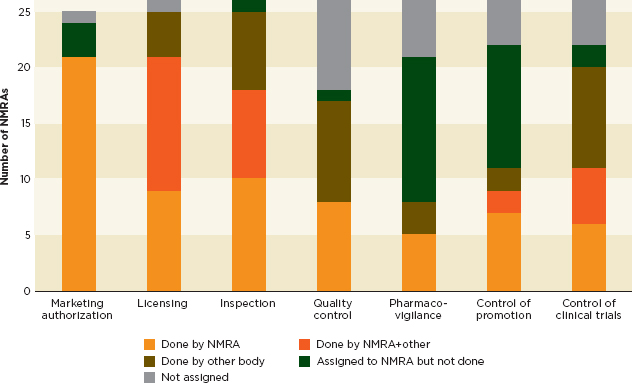
FIGURE 4-4 Number of sub-Saharan African countries out of 26 surveyed meeting the main functions of a regulatory authority.
NOTE: NMRA = National Medicines Regulatory Authority.
SOURCE: WHO, 2010a.
Governments in low- and middle-income countries need a strategy to act against falsified and substandard medicines. Any viable solution will include strengthening the drug regulatory system, including building the inspectorate, enforcing quality standards, and licensing in accordance with international standards. Without a competent regulatory authority to inspect wholesalers, distributors, and manufacturers, opportunities to corrupt the drug supply abound. Box 4-6 describes a patient safety disaster following the disbanding of the Pakistani national regulatory authority.
A 2012 Institute of Medicine report called for greater international investment in building food and drug regulatory systems in developing countries and for an international training and credentialing system for regulators (IOM, 2012). This committee supports these recommendations. It also recognizes that the magnitude of the task facing these agencies is overwhelming and that governments need to make drug quality a priority, and then empower their regulatory agencies to improve.
BOX 4-6
Dissolution of the Pakistani Drug Regulatory Authority
Over the course of several weeks in January 2012, more than 120 patients in Lahore, Pakistan, died of drug overdoses and hundreds more suffered adverse reactions after being treated with contaminated heart medicine at the Punjab Institute of Cardiology (Arie, 2012). The drug responsible was Isotab (isosorbide mononitrate, 20 mg), manufactured by Efroze Chemical in Karachi, Pakistan (Arie, 2012). Each Isotab tablet contained isosorbide mononitrate, as well as 14 times the normal dose of the antimalarial drug pyrimethamine. The overdose caused rapid bone marrow, white blood cell, and platelet depletion (BBC, 2012). The drug’s packaging did not contain dates of manufacture or expiration, and the drugs were given to patients for free (Arie, 2012). Drug pricing was a concern at Punjab Institute of Cardiology. Anonymous sources at the hospital reported significant pressure to buy the lowest cost drugs available. Under Pakistani law, when the lowest bidder does not win a sale, rejected firms can bring lawsuits against the hospital (BBC, 2012).
Pharmaceutical regulation in Pakistan is particularly weak. Though the government approved an independent drug regulatory authority in 2005, political tensions prevented action (Arie, 2012). In 2010, a constitutional amendment further debilitated regulation by abolishing the ministry of health. Provincial governments, many with weak infrastructures, were given sole responsibility for drug regulation. Manufacturers exploited the confused system by rapidly registering thousands of drugs (Khan, 2012).
Following the Isotab scandal, the Pakistan Supreme Court ordered action on the independent agency. Doctors have expressed doubts, fearing that insufficient regulatory expertise and ineffective execution will impede the new agency’s success (Khan, 2012). Their concerns appear to be well founded. The new agency’s board includes only one position for an expert in medicine or pharmacy (Khan, 2012).

Protesters in Lahore, January 2012.
SOURCE: Owasis Asam Ali, Demotix News.
Recommendation 4-4: Governments in low- and middle-income countries should support their regulatory agencies to develop strategic plans for compliance with international manufacturing and quality-control standards. In the least developed countries, international organizations should support their efforts.
International quality standards for drug manufacture depend on the competence of the national regulatory authority. Regulators in low- and middle-income countries need training, equipment, technology, and reference standards (IOM, 2012). The agencies’ budgets do not allow for improvements in all these areas, and the scope of the needs can overwhelm the agencies, leading to inaction. It is important for regulators to make strategic decisions about what to invest in first. A strategic plan can help identify an organization’s priorities and guide activities that advance these priorities (Tominaga, 2012).
The committee believes that making a strategic plan is feasible for almost all poor countries. The process of making the plan helps regulators advocate for better support from their ministers and identify places for donors to contribute. At a strategic planning workshop in 2010, for example, the Namibian health minister asked the regulatory authority to propose ways to build capacity in the agency and to advance harmonized regulatory systems in southern Africa (TIPC, 2010).
Agencies in the poorest countries should first enforce standards in manufacturing, wholesale, and retail. The WHO and more developed regulatory agencies should support these improvements. There is good precedent for such collaboration. The WHO prequalification program has a capacity-building function. As part of the program, regulators from low- and middle-income countries serve 3-month rotations at WHO headquarters (WHO, 2010b). Their rotations require close work with prequalification assessors and allow for sharing ideas about how to monitor manufactures (WHO, 2010b). A similar partnership among regulators could also be useful. Some regulatory agencies in emerging economies have made great progress in a relatively short time. These agencies are well positioned to help their counterparts in other developing countries set out their goals. For example, experts from the Brazilian drug regulatory agency, Anvisa, could work with their counterparts in Mozambique or Angola to help develop realistic plans.
A strategic plan for compliance with international standards can help reduce redundant work and fragmentation. Both industry and regulators
would agree to work toward the priorities identified on the strategic plan, and all work would be directly related to the plan, an openly shared document (Tominaga, 2012). For many smaller countries the plan should include a strategy for sharing work and pooling resources. At the regional level, the New Partnership for Africa’s Development recently published a 5-year strategic plan for regulatory harmonization (NEPAD, 2011). This document identified the technical barriers facing African regulators, clarified the mission of the African Medicines Regulatory Harmonization (AMRH) project, and identified objectives for 2011-2015 (NEPAD, 2011).
Multilateral agencies, such as development banks, should support the development and implementation of strategic plans for compliance with international standards. The pharmaceutical market is international, and everyone has an interest in promoting global standards. There is precedent for such investment. The Bill & Melinda Gates Foundation, the British Department for International Development, the World Bank, and the WHO all support the AMRH program (AMRH, 2012). Donor agencies can do similar work, as USAID has in support of postmarket surveillance in Latin America, Southeast Asia, and Africa (Miarlles, 2011).
Regulators will welcome the strategic investments this planning would bring. Governments need to support these investments as well. Compliance with international standards will demand a wide range of activities, including research, education, supply chain management, and incentives for the private sector. The regulatory agency alone cannot effect change and will need government support to marshal the involvement of all stakeholders.
Developed country governments also need to improve support for their regulatory agencies. At the time this report was prepared, substandard injectable drugs caused a fungal meningitis outbreak in the United States, bringing the topic of drug regulatory oversight to the forefront of the U.S. political discourse.
On September 21, 2012, the Tennessee Department of Health notified the Centers for Disease Control and Prevention (CDC) about an outbreak of meningitis caused by fungal infection through a contaminated epidural steroid injection from New England Compounding Pharmacy Center in Framingham, Massachusetts (CDC, 2012). By early 2013, the CDC had counted 693 illnesses and 45 deaths in 19 states from the contaminated drug (CDC, 2013). The FDA’s October 2012 inspection report indicated gross violations of good manufacturing practices, including visible contamination of equipment and drug ingredients at the New England Compounding Pharmacy (FDA, 2012b).
The outbreak brought to light a gap in the U.S. regulatory system. The
FDA’s MedWatch system had identified drug quality problems with methylprednisolone acetate, the steroid that caused the 2012 outbreak, at New England Compounding Center in 2002 and 2004 (Energy and Commerce Committee, 2012). The FDA and Massachusetts state inspectors uncovered sanitary violations in a joint inspection and issued the manufacturer a warning in 2006 (Energy and Commerce Committee, 2012). The problem is not confined to New England Compounding Center. In 2002, nonsterile practices at a South Carolina compounding pharmacy caused a similar, though smaller, outbreak (CDC, 2002). Since 2001, the FDA has issued 67 warning letters to various compounding pharmacies (Markey, 2012), but the FDA’s authority over these organizations is unclear and has been for some time. In 1996, David Kessler, then FDA commissioner, testified that compounding pharmacies threatened to create “a shadow industry” of unregulated drug manufacture (Kessler, 1996).
In the United States, professional practice, including the practice of medicine and pharmacy, is regulated by the states. Compounding pharmacies, which were traditionally small operations that prepared custom drugs for individual patients, fall under state jurisdiction (Burton et al., 2012). Pharmacy councils have long resisted federal interference in their practice, including oversight of compounding pharmacies (Calvan, 2012; Markey, 2012). At the same time, enforcement of the Food, Drug, and Cosmetic Act, which controls the marketing and manufacture of medicines, is the FDA’s responsibility. Large compounding pharmacies are in practice much closer to small manufacturers than pharmacies (Burton et al., 2012), though compounding pharmacies do not register with the FDA as manufacturers (Outterson, 2012). A 2007 bill aimed to increase FDA oversight of compounding pharmacies, but met the vociferous opposition of the International Association of Compounding Pharmacists and died in committee (Burton et al., 2012). Confusion over the regulation of compounding pharmacies was evident at congressional hearings on November 14, 2012 (Grady, 2012). New York Times reporter Denise Grady observed, “The hearing was titled ‘The Fungal Meningitis Outbreak: Could It Have Been Prevented?,’ but the question was never really answered” (Grady, 2012).
Disagreement over what authority the FDA has promotes a degree of paralysis. Neither the state of Massachusetts nor the FDA had clear control over the New England Compounding Center. Confusion about their responsibilities created a regulatory gap that the company exploited. Similar confusion causes regulatory gaps in other countries where national and local governments share responsibilities for drug regulation. In 2003, the Mashelkar Report raised concerns with Indian states’ uneven implementation of drug regulations (Government of India, 2003). More recent testing and sampling confirms that drug quality is still more reliable in states with stricter regulations (Bate et al., 2009a). Brazil, China, Russia, and many
other large countries face similar problems (Mooney, 2010; Vashisth et al., 2012).
As Chapter 3 explains, there is a dearth of reliable estimates of the scope of the problem of falsified and substandard medicines. Without a clear picture of the extent of the problem, which products are compromised, and where the products surface, it is difficult to develop an appropriate prevention strategy and monitor progress. An insufficient understanding of the scope of the problem also contributes to a lack of awareness about substandard and falsified drugs among health workers and the general population. Increasing public awareness will not in and of itself decrease falsified and substandard medicines, because consumers cannot distinguish safe and unsafe medicine in the marketplace. However, public awareness is a useful way to drive political will for correcting the problem and to educate people on warning signs of compromised medicines.
Starting in the early 2000s, medicines counterfeiting (as it was then called) has been the topic of some media attention. General awareness of the problem was still poor, however (Cockburn et al., 2005; Newton et al., 2006a). Reporting was “alarming[ly] low”: between 2002 and 2004 the WHO received no reports of fake drugs from any member states (Newton et al., 2006a). This began to change in 2006 when the International Medical Products Anti-Counterfeiting Task Force (IMPACT) made raising awareness one of its main goals (Liberman, 2012).
IMPACT, and the larger debate about pharmaceutical fraud that it was a part of, appears to have had success in raising awareness of the problem in some parts of the world. A 2010 Gallup poll in sub-Saharan African countries found that the majority of the public in 15 of the 17 countries surveyed were aware that fake medicines were a problem (see Table 4-8) (Ogisi, 2011). The leadership of drugs regulators in Nigeria, one of the largest and most influential African countries, might have contributed to the public consciousness in Africa (see Box 4-7). More recently, Interpol launched an awareness campaign featuring South Africa’s Yvonne Chaka Chaka and Senegal’s Youssou N’Dour, two of the continent’s biggest celebrities (Interpol, 2011). Awareness of the problem is also growing in Southeast Asia (Christian et al., 2012a; Gleeson, 2012).
Other research suggests gaps in awareness, especially among the poorest people in society. A qualitative study of Sudanese policy makers and pharmacists suggested that awareness of counterfeit products is lowest among the poor and people living in remote areas (Alfadl et al., 2012). Participants at overseas site visits for this study mentioned similar patterns in many developing countries. Often, well-educated urban consumers understand the threat of fake drugs and take precautions to avoid them. The poorest patients, and those living in areas with few to no reliable pharmacies, are often the least aware. Moreover, as Chapter 5 will discuss, they often have no choice but to buy medicines in the open market or have no money to buy from a registered pharmacy.
TABLE 4-8 Response to the Question “Are You Aware of the Presence of Fake Medicine in This Country?”
|
Country |
% Yes |
|
|
Cameroon |
91 |
|
|
Sierra Leone |
83 |
|
|
Nigeria |
83 |
|
|
Liberia |
79 |
|
|
Ghana |
74 |
|
|
Mali |
74 |
|
|
Central African Republic |
72 |
|
|
Burkina Faso |
71 |
|
|
Uganda |
70 |
|
|
Zimbabwe |
69 |
|
|
Tanzania |
66 |
|
|
Senegal |
65 |
|
|
Kenya |
63 |
|
|
Niger |
62 |
|
|
Chad |
58 |
|
|
Botswana |
32 |
|
|
South Africa |
25 |
|
NOTE: Data collected by Gallup in 2010. By fake medicine, we mean a product that looks like the real one but doesn’t provide the same effect and could even have bad side effects.
SOURCE: Ogisi, 2011.
It is not clear how well informed populations in other parts of the world are about falsified and substandard drugs. People in developed countries, who have long taken medicines regulation for granted, are among the least knowledgeable. An Inter-Press Service story reported that 20 percent of Western Europeans did not consider it dangerous to circumvent traditional pharmacies to buy medicine (Stracansky, 2010). The same behavior has long been normal in the United States, where pharmacy tourism to Canada and Mexico has been common since the 1970s (Rabinovitch, 2005). Chapter 5 will discuss the internet pharmacies that have largely replaced in-person cross-border shopping.
BOX 4-7
A National Awareness Campaign in Nigeria
In February 2005, the Nigerian drugs regulatory agency launched a national awareness campaign about fake medicines in Nigeria (Akunyili, 2005). The success of this program may account for Nigerians’ high (83 percent) awareness of the problem (Ogisi, 2011).
The awareness campaign had several pieces. The agency broadcast short public service announcements on television and radio in English and local languages. “There is a development,” a young businessman tells an obvious kingpin in one television piece; “you can no longer use my warehouse or any of my outlets for the distribution of your fake drugs!” (NAFDAC, 2011). The piece ends with the villain arrested at gunpoint to voiceover assurance of the agency’s commitment to protect the Nigerian public.
Other pieces of the public awareness campaign intended to change consumer behavior (Akunyili, 2005). The regulators reasoned that if consumers were informed about falsified medicines and empowered to make safe choices, they would. To this end, they published lists of known fake products and photos illustrating warning signs in daily newspapers (Akunyili, 2006; Raufu, 2006). High school consumer safety clubs helped enlist youth in the cause. Since 2002, the agency has sponsored an essay contest on medicine safety for students, awarding cash prizes to the winners and computers to their schools (Akunyili, 2006).

A public health campaign poster from Nigeria.
SOURCE: Jack, 2007. Reprinted with permission from BMJ Publishing Group LTD.
Educating the public on the problems of falsified and substandard medicines is important, but only insomuch as education empowers people to act. In an international site visit for this report, a procurement agency informed the IOM delegation that when they uncover manufacturers making substandard drugs they do not report the offense to the authorities. The reasons they gave included doubt that the regulator would act on their information and fear of litigation.
Similar attitudes may underlie a lack of reporting of adverse drug reactions among health workers in developing countries. Health workers are the first line for monitoring the safety of medicines. Their role in surveillance is important in low- and middle-income countries, where falsified and substandard drugs are common, and less than 27 percent have functional pharmacovigilance systems (Pirmohamed et al., 2007). Reporting of adverse drug events is generally low in these countries (Chedi and Musa, 2011; Fernandopulle and Weerasuriya, 2003; WHO, 2002b). Few staff are trained in pharmacovigilance, a practice sometimes seen as adding to the responsibilities of already overworked health professionals (Olsson et al., 2010; Sharma and Ahuja, 2010).
The increasing awareness of falsified and substandard medicines could drive improved pharmacovigilance in developing countries. Awareness campaigns and investigative reporting reach health workers as well as they reach the rest of the public. There is also a need for targeted health worker education on falsified and substandard medicines, emphasizing the correct reporting channels health workers can use to confirm suspected cases of falsified and substandard drugs. Much useful work has been done on the first steps of this process; clinicians struggling to broach the topic with their patients can consult the World Health Professionals Alliance guidelines on how to inquire about suspicious medicines (see Box 4-8).
Chapter 3 describes governments’ and drug companies’ reluctance to share information on substandard and falsified drugs (Cockburn et al., 2005). Pharmaceutical companies fear damage to their branding from rumors of poor quality, whereas governments can see such information as undermining confidence in the health system (Cockburn et al., 2005). These concerns are well grounded, and an appropriate communication strategy will convey accurate information is a way that is sensitive to all stakeholders. Falsified and substandard medicine is a sensitive and dynamic problem, and the public has a right to accurate information about it. This information can be presented in such a way as to empower the consumer to make safe choices and to build confidence in the regulatory system. A professional communication strategy provides the best guarantee that sensitive information is conveyed clearly and well.
BOX 4-8
Health Worker Guidelines
It is important for health care workers to query gently, by asking
1. Where patients will or did buy the medicine. Emphasis can be placed on the importance of buying medicine from a pharmacy or other known and reliable sources.
For example: “Did you purchase the medicine from a known and reliable source?”
2. What patients should look out for when they buy medicines. It can be suggested that patients check the packaging, the product, and the patient leaflet when they purchase medicine.
For example: “Was the packaging of the product intact, properly sealed, clearly labeled with dosing, manufacturer, batch number, and expiry date?”
3. How the medicine is expected to take effect. By explaining what should happen when patients take medicine, health professionals can help patients identify anything unusual.
For example: “Did the medicine cause any unexpected side effects?”
4. When the first improvements in condition should be experienced. If a medicine is supposed to start relieving symptoms within 24 hours, for example, then patients should know, so that if the medicine does not take effect, then can notify their health professional.
For example: “Has the medicine taken longer than anticipated to have an effect?”
SOURCE: WHPA, n.d.
Recommendation 4-5: Governments and donor agencies should fund development of effective communication and training programs for consumers and health workers on understanding the quality and safety of medicines.
Falsified and substandard drugs are a potential threat around the world, though risk varies widely from country to country. Awareness of the problem also varies and may be most limited in countries with strong regulatory systems but where, because of the global drug supply chain, substandard and falsified drugs still reach consumers. An effective communication campaign should present accurate information in a way that empowers patients to protect their own health. For example, the FDA website discourages buying drugs from foreign websites (see Figure 4-5) (FDA, 2012c). The CDC website gives similar guidance, discussing poor-quality antimalarials and alerting prospective travelers to avoid buying drugs abroad (CDC, 2010).
Education and communication are feasible in rich and poor countries alike. Representatives of 200 WHO member states stressed the importance of educational initiatives for consumers and health workers at the first meeting of the WHO global mechanism against falsified and substandard drugs (WHO, 2012c). Many developing countries have already made headway in consumer education. Figure 4-6, for example, shows a Cambodian health education poster promoting licensed pharmacies. Similarly, as Box 4-7 explains, the Nigerian drugs regulatory authority improved public understanding of the problem with relatively simple steps: public service announcements, newspaper ads, and school essay contests. This kind of campaign is realistic in many low- and middle-income countries.
While information about the problem is important, it is also important to link this information to action. The messages communicated and the action promoted will vary by country or region. In many countries, the most useful messages will be about specific drugs and vendors. Buying antimalarials from street markets, for example, is a dangerous behavior in most of Africa and Southeast Asia. Chapter 5 discusses some of the safe medicine outlets that the communication campaigns could promote.
The most wide-reaching communication strategies make use of many channels, including print media, television, radio, the internet, mobile devices, and social media. Governments and NGOs have made good progress using these channels to promote understanding of the problem (Besançon, 2008, 2012; Elliot, 2012; FIP, 2011). Educated consumers may now be more receptive to messages about the correct appearance or taste of medicines, the normal responses to it, and possible side effects. Patients who understand the correct attributes of their medication will be better able to identify suspicious products.

FIGURE 4-5 An FDA public service announcement that promotes the Verified Internet Pharmacy Practice certification discussed in Chapter 5. This is an example of an empowering consumer education message.
NOTE: The poster uses the term counterfeit broadly, the way this report uses falsified. See page 23.
SOURCE: FDA, 2012.

FIGURE 4-6 The English translation of a Cambodian poster encouraging consumers to buy medicines only from licensed pharmacies and to examine the drug’s color, shape, and taste for abnormalities.
NOTE: The poster uses the term counterfeit broadly, the way this report uses falsified. See page 23.
SOURCE: U.S. Embassy, Phnom Penh, Cambodia.
Therefore, governments and donors should consider developing medicine checklists that remind patients of dangers and help them identify problem drugs. A checklist or authentication database might include the reasonable price range for the drug (thereby reminding people that low costs are suspicious); a check for sealed, complete packaging; a check for the correct shape and markings on the pills; and a check for other physical properties such as stickiness or hardness. Mobile phones might be the most efficient way to disseminate this information. Consumers could also use their phones to photograph suspicious drugs and relay the image to a central site for review. Mobile phones and the internet have a wide reach and will be useful tools for promoting such a checklist. Patients and providers could use mobile phones to access a database with information about poor-quality drugs.
Health workers are the first line of pharmacovigilance and will be point persons in any consumer education campaign. Their training should include information on falsified and substandard drugs. Providers should be made
more aware of their role in the postmarket surveillance of medicines, a new responsibility in many developing countries (Sharma and Ahuja, 2010). A health worker checklist might remind providers to ask patients for information about lack of response to treatment, slow response, and appearance of unusual symptoms. The list would also remind health workers about the proper channels for reporting an adverse event.
The next 10 years will see the introduction of many new drugs and vaccines in low- and middle-income countries (Kaufmann et al., 2011; Lienhardt et al., 2012). Messages of caution about dangerous medicines should not be presented in such a way as to scare people or to discourage appropriate use of medicines (Larson et al., 2011). To this end, awareness and communication campaigns could take some inspiration from successful vaccine safety campaigns (Leitmeyer et al., 2006; Mansour-Ghanaei et al., 2008). Awareness campaigns should also be tailored for their audience. Programs for policy makers would include a broader summary of the conditions encouraging the trade in falsified and substandard medicines, as presented in this chapter.
In summary, careless manufacturing, whether deliberate or accidental, causes substandard medicine. Making falsified medicines is driven by the interests of criminals, who weigh the millions of dollars in potential profits against low odds of getting caught. To complicate the problem, medicines are expensive and often scarce. There is a financial incentive to produce a poor-quality or imitation drug. These products circulate because national regulatory authorities are often poorly equipped to detect problems and act against them.
TABLE 4-5 Penalties for Falsifying Medicine*
|
Maximum Prison |
Country |
Maximum Civil Monetary Penalty (Quantified Penalties in U.S. Dollars) |
|
Up to 6 months |
Indonesiaa |
Up to $30 |
|
Up to 2 years |
Tanzaniab |
Up to $57,000 |
|
Up to 3 years |
Up to $40,000 |
|
|
|
Canadae |
Up to $5,000 |
|
|
Lebanonf |
Up to $30,000 |
|
|
Singaporeg |
Up to $100,000 |
|
Up to 5 years |
Jordanh |
Up to $15,000 |
|
|
$100,000 or more |
|
|
Up to 10 years |
Monetary penalty not disclosed |
|
|
|
Ugandap |
Up to $2,000 |
|
|
Pakistanq |
Up to $5,000 |
|
|
Up to $15,000 |
|
|
|
$100,000 or more |
|
|
Maximum Prison |
Country |
Maximum Civil Monetary Penalty (Quantified Penalties in U.S. Dollars) |
|
Up to 15 years |
Nigeriav |
Up to $5,000 |
|
|
Up to $98,000 |
|
|
|
Kenyay |
Up to 5x the value of the medicine |
|
Up to 20 years |
$100,000 or more |
|
|
Up to life imprisonment or death |
Up to 5x the value of the medicine |
|
|
|
Indiadd |
Up to $20,000 or 3x the value of the medicine |
|
|
$100,000 or more |
|
|
|
Thailandgg |
Up to $1,700 |
* Additional penalties and fines may be associated with specific infractions.
a WHPA. 2011. Background document on counterfeit medicines in Asia. Paper read at WHPA Regional Workshop on Counterfeit Medical Products, Taipei, Taiwan.
b The Tanzania Food, Drugs and Cosmetics Act, 2003. (Tanzania). Part IV, Sec. 76 (2).
c WHPA. 2011. Background document on counterfeit medicines in Asia. Paper read at WHPA Regional Workshop on Counterfeit Medical Products, Taipei, Taiwan.
d Business Monitor International. 2010. Malaysia pharmaceuticals and healthcare report 2010. London: Business Monitor International.
e Food and Drugs Act (R.S.C., 1985, c.F-27). (Canada). Sec. 31 (a);(b).
f Ghosn, Z. 2008. Lebanon launches campaign to counter fake drugs. http://www.scidev.net/en/news/lebanon-launches-campaign-to-counter-fake-drugs.html (accessed October 4, 2012).
g Health Products Act (Chapter 122D). (Singapore). 2007. Part IV, Art. 16, Sec. 1 (b); 2 (b).
h Saba & Co. IP. 2009. Jordan: Relentless efforts to curb counterfeit drugs. http://www.sabaip.com/NewsArtDetails.aspx?ID=514 (accessed October 4, 2012).
i Institute of Research Against Counterfeit Medicines. 2012. Tracking and condemning fake drug traffickers. Institute of Research Against Counterfeit Medicines.
j Counterfeit Goods Act 37 of 1997. (South Africa). Art. 19, Sec. 1 (a);(b).
k Betts, A. B. 2010. Fight against counterfeit medical products: The Medicrime Convention and the Swiss experience. Presentation given at International Conference of Drug Regulatory Authorities, Singapore.
l Therapeutic Medicines Act. (Switzerland). (December 15, 2000). Chap. 8, Art. 86 (1) a-g.
m Bate, R. 2012. Phake: The deadly world of falsified and substandard medicines. Washington, DC: AEI Press.
n Medicinal Products Act. (Germany). (2010). Chap. 17, Sec. 95 (3) 3.
o AEI. 2012. The deadly world of fake drugs. AEI.
p The National Drug Policy and Authority Act of 2003. (Uganda). Chap. 206, Part IV, Sec. 30.
q The Drugs Act, 1976. (Pakistan). Chap. IV, Sec. 27 (1);(2).
r AEI. 2012. The deadly world of fake drugs. AEI.
s Phana, C. 2007. Country presentation: Cambodia. Presented at First ASEAN–China Conference on combating counterfeit medicinal products. Jakarta, Indonesia.
t WHPA. 2011. Background document on counterfeit medicines in Asia. Paper read at WHPA Regional Workshop on Counterfeit Medical Products, Taipei, Taiwan.
u WHPA. 2011. Background document on counterfeit medicines in Asia. Paper read at WHPA Regional Workshop on Counterfeit Medical Products, Taipei, Taiwan.
v Counterfeit and Fake Drugs and Unwholesome Processed Food (miscellaneous provisions) Act of 1999. (Nigeria). Sec. 3 (1).
w Capell, K., S. Timmons, J. Wheatley, and H. Dawley. 2001. What’s in that pill? Bloomberg Businessweek Magazine.
x Lei No 6.437 De 20 De Agosto De 1977. (Brazil). Tit. 1, Art. 2, §1o.
y The Anti-Counterfeit Bill, 2008. (Kenya). Part VI, Sec. 35 (a); (b).
z AEI. 2012. The deadly world of fake drugs. AEI.
aa Ley General De Salud, 2012. (Mexico). Titulo Decimo Octavo, Capitulo VI, Artículo 464 Ter. (I); (II).
bb Drug Administration Law of the People’s Republic of China. (China). 2001, No. 45. 20th meeting, 9th Cong., Chap. IX, Art. 74.
cc Jailing, D. 2011. China broadens scope of counterfeit drugs criminal prosecution, but definition still murky. Elsevier Business Intelligence.
dd Sinha, K. 2009. From Monday, spurious drug sellers can be jailed. Times of India.
ee Special Law on Counterfeit Drugs. (Philippines). 1996. Republic Act No. 8203, Cong. of the Philippines Metro Manila, 2nd sess., Sec. 8 (b); (e); (f).
ff Counterfeit Drug Penalty Enhancement Act of 2011, HR 3468. 112th Cong., 1st Sess., Sec. 2 (a); (b).
gg Thailand Drug Act, B.E. 2510 (1967). (Thailand). Chap. X, Sec. 117.
TABLE 4-6 Penalties for Patent Infringement*
|
Maximum Prison Sentences |
Country |
Maximum Civil Monetary Penalty (Quantified Penalties in U.S. Dollars) |
|
No imprisonment for infraction |
Grenada,a India,b |
Damages are recovered |
|
|
Taiwani |
Infringer must may patentee profits earned |
|
|
Patentee may file a civil or criminal lawsuit |
|
|
|
$100,000 or more |
|
|
|
Mexicon |
$80,000 or more |
|
Up to 1 year |
Brazilo |
Monetary penalty not disclosed |
|
|
Canadap |
Up to $500 |
|
|
Singaporeq |
Up to $10,000 |
|
|
Switzerlandr |
$100,000 or more |
|
Maximum Prison Sentences |
Country |
Maximum Civil Monetary Penalty (Quantified Penalties in U.S. Dollars) |
|
Up to 2 years |
Thailands |
Up to $13,150 |
|
Up to 3 years |
Germanyt |
Monetary penalty not disclosed |
|
|
Lebanonu |
Up to $33,000 |
|
Up to 4 years |
Indonesiav |
Up to $50,000 |
|
Up to 5 years |
Cambodiaw |
Up to $5,000 |
|
|
Francex |
Up to $650,000 |
|
|
Japany |
Up to $100,000 with labor |
|
|
Kenyaz |
Up to $6,000 |
|
|
Tanzaniaaa |
Up to $300 |
|
5 or more years |
Monetary penalty not disclosed |
|
|
|
South Koreadd |
Up to $100,000 with labor |
* Additional penalties and fines may be associated with specific infractions.
a Patents Act (Cap. 227). (Grenada). (May 16, 1898). Art. 20.
b The Patents Act, 1970. (India). Chap. XVIII, Sec. 108.
c Malaysia Patents Act. Amended by Act 1264 of 2006. (Malaysia). (August 16, 2006). Par. XII, Sec. 60 (1).
d Patents Ordinance, 2000 as amended by Patents (Amendment) Ordinance, 2002. (Pakistan). Chap. XVII, Sec. 61.
e Intellectual Property Code of the Philippines. (Philippines). (June 6, 1997). Part II, Chap. VIII, Sec. 76 (1); (2).
f Patents Act No. 57 of 1978. (South Africa). (April 26, 1978). Chap. XI, Art. 65 (3); (6).
g The Patents Act. (Uganda). (October 15, 1993). Part V, Sec. 26 (2).
h U.S. Patent Law, 35 U.S.C. § 284. (2007).
i Patent Act. (2011). (Taiwan). Sec. 7, Art. 97 (2); (3).
j Patent Law, No. 32. (Jordan). 1999. Art. 33.
k Patents and Designs Act (Chapter 344). (Nigeria). Sec. 25 (1); (2).
l Patent Law of the People’s Republic of China. (China). No. 8. 11th Cong. (December 27, 2008). Chap. VII, Art. 63; 65.
m Peru Industrial Property Law. (Peru). (May 24, 1996). Tit. XVI, Art. 242.
n Industrial Property Law. (Mexico). (Last amended January 26, 2006). Chap. II, Art. 214 (I); (V).
o Law No. 9,279 of May 14, 1996. (Brazil). Tit. V, Chap. 1, Art. 183 (I).
p Canada Consolidation Patent Act, R.C.S., 1985, c. P-4. (Canada). (Last amended September 21, 2006.) Sec. 75 (a); (b); (c).
q Singapore Patents Act as amended by Act No. 2 of 2007. (Singapore). (April 1, 2007). Part XVIII, Sec. 99 (1).
r Loi fédérale sur les brevets d’invention. (Switzerland). (June 25, 1954). Tit. 3, Chap. 3, Art. 81 (1).
s Patents Act Consolidation. (Thailand). No. 3. (1999). Part VI, Chap. VI, Art. 85.
t Germany Patent Act. (Germany). (July 30, 2009). Part 9, Sec. 142 (1).
u Patents Law of Lebanon, Law No. 240. (Lebanon). (August 7, 2000). Chap. 2, Sec. 1, Art. 42.
v Law of the Republic of Indonesia Regarding Patents. (Indonesia). No. 14. 2001. Chap. XV, Art. 130.
w Law on the Patents, Utility Model Certificates and Industrial Designs. (Cambodia). 8th sess., 1st legis. (December 31, 2002). Chap. 7, Art. 133.
x Intellectual Property Code. (France). (July 1, 1992). Chap. V, Sec. II, Art. L615-14 (1).
y Patent Act (Act No. 121 of 1959). (Japan). Chap. XI, Art. 196-2.
z The Industrial Property Act, 2001. (Kenya). Part XVI, Sec. 109 (1); (2).
aa The Patents (Registration) Act. (Tanzania). Part XV, Sec. 70 (1).
bb Penal Code of Argentina. (Argentina). Law 11,179 (1984). Chap. IV, Art. 172.
cc Legal Intellectual Property Regime (Argentina). Law No. 11.723, Art. 71.
dd Patent Act (Act No. 950 of December 31, 1961, as last amended by Act No. 9985 of January 30, 2009). (Republic of Korea). Chap. XII, Art. 225 (1).
TABLE 4-7 Penalties for Trademark Infringement*
|
Maximum Prison Sentences |
Country |
Maximum Civil Monetary Penalty (Quantified Penalties in U.S. Dollars) |
|
No imprisonment for infraction |
Cambodia,a Germany,b |
Damages are recovered |
|
|
Koreaj |
Up to $47,000 |
|
|
Infringer must pay the trademark owner profits earned from the infringement or the amount of losses that the trademark owner has suffered |
|
|
|
Jordanm |
Up to $8,500 |
|
Up to 3 days |
Mexicon |
Up to $70,000 |
|
Up to 1 year |
Switzerlando |
Up to $110,000 |
|
|
Brazilp |
Monetary penalty not disclosed |
|
Up to 2 years |
Argentinaq |
Up to $30,000,000 |
|
Up to 3 years |
Lebanonr |
Up to $0.40 |
|
Up to 5 years |
Japans |
Up to $60,000 with labor |
|
|
Indonesiat |
Up to $105,000 |
*Additional penalties and fines may be associated with specific infractions.
a The Law concerning Marks, Trade Names and Acts of Unfair Competition. (Cambodia). Chap. 8, Art. 27.
b Germany Trademark Law (as amended on July 16, 1998). (Germany). Chap. 3, Sec. 14 (6).
c The Trade Marks Act, 1999. (India). No. 47 of 1999. Chap. XIII, Sec. 135 (1).
d Trade Marks Ordinance, 2001. (Pakistan). Chap. V, Sec. 46 (2).
e Intellectual Property Code of the Philippines. (Philippines). (June 6, 1997). Part III, Sec. 156.
f Trade Marks Act (Chapter 332). (Singapore). Part III, Sec. 31.
g Trade Marks Act No. 194 of 1993. (South Africa). Part VIII, Sec. 34, (3) c; d.
h The Trademarks Act, 2010. (Uganda). Part VIII, Sec. 79 (4).
i U.S. Trademark Law of 1946. § 1114 (2012).
j Trademark Act. (Korea). Chap. VI, Art. 67; 67-2.
k Trademark Law of the People’s Republic of China. (China). October 27, 2001. Chap. VII, Art. 56.
l Kuo, Y., and J. Wong (2012). Taiwan overhauling the trademark law, Formosa Transnational.
m Abu Ghazaleh Intellectual Property (2008). New amendments to Jordan’s trademark law. Retrieved December 28, 2012, from http://www.ag-ip-news.com/news.aspx?id=24580&lang=en.
n Arenas, A. (2012). Country correspondent: Mexico, Olivares & Cía.
o Federal Law of August 28, 1992 on the Protection of Trademarks and Indications Source (as last amended on March 24, 1995). (Switzerland). Tit. 3, Chap. 2, Art. 61.1 (a); (b).
p Industrial Property Law No. 9.279, of May 14, 1996 (as amended by Law 10.196 of February 14, 2001). (Brazil). Chap. 3, Art. 189.
q Law on Trademarks and Designations (No. 22,362 of December 26, 1980). (Argentina). Chap. III, Tit. 1, Sec. 31 (b).
r Resolution No.2385/1924 issued on January 17, 1924, (amended by the law of 31/1/1946). (Lebanon). Part 6, Chap. 2, Art. 105.
s Trademark Act (Act No.127 of April 13, 1959). (Japan). Chap. IX, Art. 78-2.
t Law of the Republic of Indonesia, No. 15/2001 Regarding Marks. (Indonesia). Chap. XIV, Art. 90.
Ahmed, R. 2012. India’s plan to distribute free medicines raises questions. Wall Street Journal India, July 6.
Akunyili, D. 2005. Counterfeit drugs and pharmacovigilance. Paper presented at 10th Pharmacovigilance Training Course held at Uppsala Monitoring Centre, Uppsala, Sweden, May 25.
———. 2006. Women leadership in emerging democracy—my NAFDAC experience. Address by the director general of the National Agency for Food and Drug Administration and Control, Dora Nkem Akunyili. Wilson Center, April 29.
Alfadl, A. A., M. A. Hassali, and M. I. M. Ibrahim. 2012. Counterfeit drug demand: Perceptions of policy makers and community pharmacists in Sudan. Research in Social and Administrative Pharmacy. [Epub ahead of print].
Ames, J., and D. Z. Souza. 2012. Counterfeiting of drugs in Brazil. Revista de Saúde Pública 46(1). DOI: 10.1590/S0034-89102012005000005.
AMRH (African Medicines Regulatory Harmonization). 2012. Partners. http://www.amrh.org/partners (accessed January 9, 2013).
Anderson, T. 2010. Tide turns for drug manufacturing in Africa. Lancet 375(9726):1597-1598. APIC (Association for Professionals in Infection Control and Epidemiology). 1999. Cleaning validation in active pharmaceutical ingredient manufacturing plants. Washington, DC: APIC.
Arie, S. 2012. Contaminated drugs are held responsible for 120 deaths in Pakistan. British Medical Journal 344:e951.
Arnold, R. 2008. Economics. 9th ed. Mason, OH: Cenage Learning.
Bate, R. 2007. On the trail of a cure: Reality and rhetoric on treating malaria. Washington, DC: American Enterprise Institute for Public Policy Research.
———. 2008. Local pharmaceutical production in developing countries: How economic protectionism undermines access to quality medicines. London: Campaign for Fighting Diseases.
Bate, R., R. Tren, K. Hess, L. Mooney, and K. Porter. 2009a. Pilot study comparing technologies to test for substandard drugs in field settings. African Journal of Pharmacy and Pharmacology 3(4):165-170.
Bate, R., R. Tren, L. Mooney, K. Hess, B. Mitra, B. Debroy, and A. Attaran. 2009b. Pilot study of essential drug quality in two major cities in India. PLoS ONE 4(6):e6003.
BBC (British Broadcasting Company). 2012. Police investigate Pakistan heart drug deaths. BBC News, January 24.
Beken, T. V., and A. Balcaen. 2006. Crime oppotunities provided by legislation in market sectors: Mobile phones, waste disposal, banking, pharmaceuticals. European Journal on Criminal Policy Research 12:299-323.
Belluck, P. 2001. Prosecuters say greed drove pharmacist to dilute drugs. http://www.nytimes.com/2001/08/18/us/prosecutors-say-greed-drove-pharmacist-to-dilute-drugs.html?pagewanted=all&src=pm (accessed July 12, 2012).
Besançon, L. 2008. Country specific case studies—best practices to combat counterfeit medicines and to protect public health. The Hague, The Netherlands: International Pharmaceutical Federation.
———. 2012. Health professionals in the risk communication process on counterfeit medicines. Generics and Biosimilars Initiative Journal 1(3-4): 135-137.
Brhlikova, P., I. Harper, and A. Pollock. 2007. Good manufacturing practice in the pharmaceutical industry. Workshop on Tracing Pharmaceuticals in South Asia, University of Endinburgh, Centre for International Public Health Policy.
British Pharmacopoeia. 2012. Amoxicillin capsules. In British Pharmacopoeia Vol. III: Formulated preparations—specific monographs. http://bp2012.infostar.com.cn/Bp2012.aspx?tab=browse&a=display&n=53&id=6349 (accessed March 5, 2013).
Brousseau, Z. 2012. Mastering the ECTD format critical for regulatory submissions pros. http://www.raps.org/focus-online/news/news-article-view/article/1191/mastering-the-ectd-format-critical-for-regulatory-submissions-pros.aspx (accessed November 5, 2012).
Bumpas, J., and E. Betsch. 2009. Exploratory study on active pharmaceutical ingredient manufacturing for essential medicines. Washington, DC: The International Bank for Reconstruction and Development/The World Bank.
Burkitt, L. 2012. Beijing says counterfeit drugs seized. Wall Street Journal, August 5.
Burton, T., J. Grimaldi, and T. Martin. 2012. Pharmacies fought controls. Wall Street Journal, October 14.
Calvan, B. 2012. Compounding pharmacies have long evaded the tight oversight governing established drug makers. Boston Globe, October 29.
Cameron, A., M. Ewen, D. Ross-Degnan, D. Ball, and R. Laing. 2008. Medicines prices, availability, and affordability in 36 developing and middle-income countries: A secondary analysis. Lancet 373(9659):240-249.
Caudron, J. M., N. Ford, M. Henkens, C. Macé, R. Kiddle-Monroe, and J. Pinel. 2008. Substandard medicines in resource-poor settings: A problem that can no longer be ignored. Tropical Medicine & International Health 13(8):1062-1072.
CDC (Centers for Disease Control and Prevention). 2002. Exophiala infection from contaminated injectable steroids prepared by a compounding pharmacy. Morbidity and Mortality Weekly Report 51(49):1109-1112.
———. 2010. Counterfeit and substandard antimalarial drugs. http://www.cdc.gov/malaria/travelers/counterfeit_drugs.html (accessed November 6, 2012).
———. 2012. CDC and FDA joint telebriefing on investigation of meningitis outbreak. http://www.cdc.gov/media/releases/2012/t1004_meningitis_outbreak.html (accessed November 26, 2012).
———. 2013. Multistate fungal meningitis outbreak—current case count. http://www.cdc.gov/hai/outbreaks/meningitis-map-large.html (accessed January 28, 2013).
Chaudhury, R. R., R. Parameswar, U. Gupta, S. Sharma, U. Tekur, and J. Bapna. 2005. Quality medicines for the poor: Experience of the Delhi programme on rational use of drugs. Health Policy and Planning 20(2):124-136.
Chedi, J. F. O. E. A. A. B., and A. Musa. 2011. Knowledge, attitude and practice of adverse drug reaction reporting among healthcare workers in a tertiary centre in northern Nigeria. Tropical Journal of Pharmaceutical Research 10(3):235-242.
Cho, Y., D. Margolis, D. Newhouse, and D. Robalino. 2012. Labor markets in low- and middle-income countries. Washington, DC: World Bank.
Christian, L., L. Collins, M. Kiatgrajai, A. Merle, N. Mukherji, and A. Quade. 2012a. The problem of substandard medicines in developing countries. Madison, WI: La Follette School of Public Affairs, University of Wisconsin–Madison.
———. 2012b. The problem of substandard medicines in developing countries. Paper presented at Workshop in International Public Affairs, La Follette School of Public Affaris, University of Wisconsin–Madison, May 21.
Chua, G. N., M. A. Hassali, A. A. Shafie, and A. Awaisu. 2010. A survey exploring knowledge and perceptions of general practitioners towards the use of generic medicines in the northern state of Malaysia. Health Policy 95(2-3):229-235.
Clark, E. 2008. Counterfeit medicines: The pills that kill. Telegraph, April 5.
Cockburn, R., P. N. Newton, E. K. Agyarko, D. Akunyili, and N. J. White. 2005. The global threat of counterfeit drugs: Why industry and governments must communicate the dangers. PLoS Medicine 2(4):e100.
Daviaud, J., and A. Saleh. 2010. Quality assurance policy for pharmaceutical products. Joint WHO and UNAIDS consultation meeting with pharmaceutical companies, December 9-10, Geneva, Switzerland.
DFID (Department for International Development). 2012. DFID quality assurance policy for reproductive health commodities. London: DFID.
Dickens, T. 2011. The world medicines situation 2011: Procurement of medicines. Geneva: WHO.
Dobert, D. n.d. Pharmaceutical counterfeiting: A clear and present danger. http://www.atlco.com/pharma_counter.html (accessed August 15, 2012).
DOJ (U.S. Department of Justice). 2011. Press release: Belgian citizen sentenced for selling counterfeit, misbranded drugs. Washington, DC: DOJ and U.S. Food and Drug Administration Office of Criminal Investigations.
Donaldson, A. 2010. “And the ones that mother gives you don’t do anything at all,” combating counterfeit pharmaceuticals: The American and British perspectives. New England Journal of International and Comparative Law 16(1):145-168.
Dondorp, A. M., P. N. Newton, M. Mayxay, W. Van Damme, F. M. Smithuis, S. Yeung, A. Petit, A. J. Lynam, A. Johnson, T. T. Hien, R. McGready, J. J. Farrar, S. Looareesuwan, N. P. J. Day, M. D. Green, and N. J. White. 2004. Fake antimalarials in Southeast Asia are a major impediment to malaria control: Multinational cross-sectional survey on the prevalence of fake antimalarials. Tropical Medicine & International Health 9(12):1241-1246.
Draper, R. 2003. The toxic pharmacist. http://www.nytimes.com/2003/06/08/magazine/the-toxic-pharmacist.html?pagewanted=print&src=pm (accessed July 12, 2012).
Droop, J. 2012. DFID quality assurance/ procurement policies for medicines, October 17.
Economist. 2012a. Bad medicine, October 13.
———. 2012b. Bulging in the middle, October 20.
Elliot, J. 2012. Global interagency efforts stem counterfeit drugs in Greater Mekong Asia—update. http://casestudiesforglobalhealth.org/post.cfm/global-interagency-efforts-stem-counterfeit-drugs-in-greater-mekong-asia-update (accessed November 6, 2012).
Energy and Commerce Committee. 2012. Committee leaders request documents related to deadly meningitis outbreak. http://energycommerce.house.gov/press-release/committee-leaders-request-documents-related-deadly-meningitis-outbreak (accessed November 26, 2012).
European Commission. 2011. Guidelines for the award of procurement contracts within the framework of humanitarian aid actions financed by the European Union. European Commission.
FDA (U.S. Food and Drug Administration). 2012a. Counterfeit medicines: Filled with empty promises (print public service announcement). http://www.fda.gov/Drugs/ResourcesForYou/ucm079306.htm (accessed November 6, 2012).
———. 2012b. Department of Health and Human Services, Food and Drug Administration, inspection form for New England Compounding Pharmacy, Inc. http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofGlobalRegulatoryOperationsandPolicy/ORA/ORAElectronicReadingRoom/UCM325980.pdf (accessed November 26, 2012).
———. 2012c. Information for consumers (drugs): Buying medicines over the Internet. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/BuyingMedicinesOvertheInternet/default.htm (accessed November 6, 2012).
Fernandopulle, R. B. M., and K. Weerasuriya. 2003. What can consumer adverse drug reaction reporting add to existing health professional-based systems?: Focus on the developing world. Drug Safety 26(4):219-225.
FIP (International Pharmaceutical Federation). 2011. Pharmacists working at national levels. http://www.fip.org/menu_counterfeitmedicines_publications (accessed November 5, 2012).
GAO (U.S. Government Accountability Office). 2012. Ensuring drug quality in global health programs: Briefing for congressional requesters. Washington, DC: GAO.
GIZ (Deutsche Gesellschaft für Internationale Zusammenarbeit). 2012. Pharmaceuticals—East Africa: Establishment of a bioequivalence centre for East Africa in Addis Ababa. Bonn, Germany: GIZ.
Gleeson, S. 2012. Battle against counterfeit drugs inspires artists. Phnom Penh Post, June 6.
Global Fund. 2009. Guide to the Global Fund policies on procurement and supply management of health products. Geneva: Global Fund.
———. 2012. Guide to the Global Fund policies on procurement and supply management of health products. Geneva: Global Fund.
Gossell-Williams, M. 2007. Generic substitutions: A 2005 survey of the acceptance and perceptions of physicians in Jamaica. West Indian Medical Journal 56(5):458-463.
Government of India. 2003. Report of the Expert Committee on a Comprehensive Examination of Drug Regulatory Issues, Including the Problem of Spurious Drugs. New Delhi: Government of India.
Grady, D. 2012. Deaths stir a dispute on powers of FDA. New York Times, November 19.
Hadi, U., P. van den Broek, E. Kolopaking, N. Zairina, W. Gardjito, I. Gyssens, and the Study Group “Antimicrobial Resistance in Indonesia” (AMRIN): I. Prevalence, and Prevention. 2010. Cross-sectional study of availability and pharmaceutical quality of antibiotics requested with or without prescription (over the counter) in Surabaya, Indonesia. BMC Infectious Diseases 10(1):203.
Harper, I., P. Brhlikova, M. S. Subedi, and S. Bhattarai. 2007. Drug procurement in Nepal. Centre for International Public Health Policy, University of Edinburgh.
Hassali, M. A. A., A. A. Shafie, S. Jamshed, M. I. M. Ibrahim, and A. Awaisu. 2009. Consumers’ views on generic medicines: A review of the literature. International Journal of Pharmacy Practice 17(2):79-88.
Hill, S., and K. Johnson. 2004. Emerging challenges and opportunities in drug registration and regulation in developing countries. London: Health Systems Resource Centre, Department for International Development.
Hoffman, S. 2012. The drugs stop here: A public health framework to address the drug shortage crisis. Case Western Reserve School of Law: The Food and Drug Law Journal 67(1):1-21.
ICH (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use). 2010. The value and benefits of ICH to drug regulatory authorities—advancing harmonization for better health. Geneva: ICH.
———. 2012. M4: The Common Technical Document. http://www.ich.org/products/ctd.html (accessed November 2, 2012).
IFC (International Finance Corporation). 2007. The business of health in Africa: Partnering with the private sector to improve people’s lives. Washington, DC: IFC.
———. 2012a. How to apply for financing. http://www1.ifc.org/wps/wcm/connect/corp_ext_content/ifc_external_corporate_site/what+we+do/about_ifc_financing (accessed December 27, 2012).
———. 2012b. IFC’s industries. http://www1.ifc.org/wps/wcm/connect/industry_ext_content/ifc_external_corporate_site/industries (accessed December 27, 2012).
———. 2012c. IFC’s vision, values, & purpose. http://www1.ifc.org/wps/wcm/connect/corp_ext_content/ifc_external_corporate_site/about+ifc/vision (accessed November 2, 2012).
———. 2012d. IFC investment services. http://www1.ifc.org/wps/wcm/connect/CORP_EXT_Content/IFC_External_Corporate_Site/What+We+Do/Investment+Services (accessed December 12, 2012).
———. 2012e. Investing in life sciences in emerging markets. http://www1.ifc.org/wps/wcm/connect/Industry_EXT_Content/IFC_External_Corporate_Site/Industries/Health+and+Education/Health+Sector/Life_Sciences (accessed December 27, 2012).
———. 2012f. Organization. http://www1.ifc.org/wps/wcm/connect/CORP_EXT_Content/IFC_External_Corporate_Site/About+IFC/Organization (accessed December 12, 2012).
Interpol. 2011. Yvonne Chaka Chaka performs “Proud to Be” at Interpol General Assembly in Vietnam. http://www.interpol.int/News-and-media/News-media-releases/2011/N20111101 (accessed December 13, 2012).
———. 2012a. Fact sheet: Pharmeceutical crime. London: Interpol.
———. 2012b. Pharmaceutical crime. http://www.interpol.int/Crime-areas/Pharmaceutical-crime/Pharmaceutical-crime (accessed October 12, 2012).
IOM (Institute of Medicine). 2012. Ensuring safe foods and medical products through stronger regulatory systems abroad. Washington, DC: The National Academies Press.
Jack, A. 2007. Bitter pills. British Medical Journal 335(7630):1120-1121.
Kake.com. 2011. Belgian citizen pleads guilty to federal charges in Kansas. KAKEland, January 21.
Kaplan, W., and R. Laing. 2005. Local production of pharmaceuticals: Industrial policy and access to medicines—an overview of key concepts, issues and opportunities for future research. Washington, DC: The International Bank for Reconstruction and Development/ World Bank.
Kaplan, W. A., L. S. Ritz, M. Vitello, and V. J. Wirtz. 2012. Policies to promote use of generic medicines in low and middle income countries: A review of published literature, 2000-2010. Health Policy 106(3):211-224.
Kaufmann, J. R., R. Miller, and J. Cheyne. 2011. Vaccine supply chains need to be better funded and strengthened, or lives will be at risk. Health Affairs 30(6):1113-1121.
Kessler, D. 1996. Testimony on protecting the nation’s health and safety before the House Committee on Commerce, Subcommittee on Health and Environment.
Khan, A. A. 2012. Pakistani drug regulator “destined to be a failure.” Nature News, April 4.
Kindermans, J.-M., J. Pilloy, P. Olliaro, and M. Gomes. 2007. Ensuring sustained ACT production and reliable artemisinin supply. Malaria Journal 6(125). DOI: 10.1186/1475-2875-6-125.
Kontnik, L. 2004. Pharmaceutical counterfeiting: Preventing the perfect crime. http://www.fffenterprises.com/assets/downloads/fff_wht_ppr_111804.pdf (accessed December 10, 2012).
KPMG International. 2006. The Indian pharmaceutical industry: Collaboration for growth. The Netherlands: KPMG International.
Kyriacos, S., M. Mroueh, R. P. Chahine, and O. Khouzam. 2008. Quality of amoxicillin formulations in some Arab countries. Journal of Clinical Pharmacy and Therapeutics 33(4):375-379.
Larson, H. J., L. Z. Cooper, J. Eskola, S. L. Katz, and S. Ratzan. 2011. Addressing the vaccine confidence gap. Lancet 378(9790):526-535.
Leitmeyer, K., U. Buchholz, M. Kramer, K. Schenkel, H. Stahlhut, M. Köllstadt, W. Haas, and C. Meyer. 2006. Influenza vaccination in German health care workers: Effects and findings after two rounds of a nationwide awareness campaign. Vaccine 24(47-48):7003-7008.
Lewis, M. 2006. Tackling healthcare corruption and governance woes in developing countries. Washington, DC: Center for Global Development.
Liberman, J. 2012. Combating counterfeit medicines and illicit trade in tobacco products: Minefields in global health governance. Journal of Law, Medicine & Ethics 40(2):326-347.
Lienhardt, C., M. Raviglione, M. Spigelman, R. Hafner, E. Jaramillo, M. Hoelscher, A. Zumla, and J. Gheuens. 2012. New drugs for the treatment of tuberculosis: Needs, challenges, promise, and prospects for the future. Journal of Infectious Diseases 205(Suppl 2):S241-S249.
Lionberger, R. A. 2008. FDA critical path initiatives: Opportunities for generic drug development. AAPS Journal 10(1):103-109.
Liu, P. 2010. From decentralised developmental state towards authoritarian regulatory state: A case study on drug safety regulation in China. China: An International Journal 8(1):110-137.
Loewy, M. 2007. Deadly imitation. Perspectives in Health, June 5, Pp. 16-21.
Mackintosh, M., S. Chaudhuri, and P. Mujinja. 2011. Can NGOs regulate medicines markets? Social enterprise in wholesaling, and access to essential medicines. Globalization and Health 7(1):4.
Mansour-Ghanaei, F., H. Rahimi, F. Joukar, A. Bagherzadeh, A. Heidarzadeh, A. Rahbar, H. Rokhshad, S. R. H. Balou, and A. Sarshad. 2008. Mass vaccination of measles and rubella (MR) in Guilan, Northern Iran: Evaluation of coverage and complications. Iranian Red Crescent Society 10(3):173-179.
Markey, E. 2012. Compounding pharmacies compounding risk. Office of Congressman Edward J. Markey (D-Mass).
Mastan, S., T. B. Latha, and S. Ajay. 2011. The basic regulatory considerations and prospects for conducting bioavailability/bioequivalence (BA/BE) studies: An overview. Comparative Effectiveness Research 1:1-25.
McCabe, A. 2009. Private sector pharmaceutical supply and distribution chains: Ghana, Mali and Malawi. Health Systems for Outcomes.
MDG (Millenium Development Goal) Gap Task Force. 2008. Access to affordable essential medicines. In Millenium development goal 8: Delivering on the global partnership for achieving the millenium development goals.New York: United Nations.
Miarlles, M. 2011. USAID impact blog: Pharmaceutical management—assuring the quality and safety of medicines. http://blog.usaid.gov/2011/01/pharmaceutical-management-%E2%80%93-assuring-the-quality-and-safety-of-medicines (accessed January 8, 2013).
Molzon, J. A. 2009. Promoting efficient reviews: The influence of the ECTD. Paper presented at 22nd Annual DIA Conference for Electronic Document Management, February 10-13.
Mooney, P. 2010. China cracks down on counterfeiters. Nature Medicine 16(360). DOI: 10.1038/nm0410-360a.
Moore, T., D. Lee, N. Konduri, and L. Kassonde. 2012. Assuring the quality of essential medicines procured with donor funds. Washington, DC: The World Bank.
MSF (Médecins Sans Frontieres). 2006. MSF qualification scheme. http://www.msf.org/msf/articles/2006/07/en/msf-qualification-scheme.cfm (accessed October 1, 2012).
MSH (Management Sciences for Health). 2011. Pharmaceutical management: Managing the tender process. In MDS-3: Managing access to medicines and health technologies. Cambridge, MA: MSH.
———. 2012. Managing access to medicines and health technologies. Arlington, VA: MSH.
NAFDAC (National Agency for Food and Drug Administration and Control). 2011. NAFDAC war of fake drug peddlers.Mp4. http://www.youtube.com/watch?v=IYiOBaHv_Sw (accessed March 5, 2013).
Nelson, M., M. Vizurraga, and D. Chang. 2006. Counterfeit pharmaceuticals: A worldwide problem. The Trademark Reporter 26(5):1068-1100.
NEPAD (New Partnership for Africa’s Development). 2011. African medicines regulatory harmonization strategic plan: 2011-2015. Johannesburg, South Africa: NEPAD.
Newton, P. N., M. D. Green, F. M. Fernández, N. P. J. Day, and N. J. White. 2006a. Counterfeit anti-infective drugs. Lancet Infectious Diseases 6(9):602-613.
Newton, P. N., R. McGready, F. Fernandez, M. D. Green, M. Sunjio, C. Bruneton, S. Phanouvong, P. Millet, C. J. M. Whitty, A. O. Talisuna, S. Proux, E. M. Christophel, G. Malenga, P. Singhasivanon, K. Bojang, H. Kaur, K. Palmer, N. P. J. Day, B. M. Greenwood, F. Nosten, and N. J. White. 2006b. Manslaughter by fake artesunate in Asia—will Africa be next? PLoS Medicine 3(6):e197.
OECD (Organisation for Economic Co-operation and Development). 2009. OECD principles for integrity in public procurement. Paris: OECD.
Ogisi, M. 2011. Fake medicines common in many sub-Saharan African countries. Gallup, October 5. http://www.gallup.com/poll/149942/fake-medicines-common-sub-saharan-african-countries.aspx (accessed September 24, 2012).
Olsson, S., S. N. Pal, A. Stergachis, and M. Couper. 2010. Pharmacovigilance activities in 55 low-and middle-income countries: A questionnaire-based analysis. Drug Safety 33(8):689-703.
OPIC (Overseas Private Investment Corporation). 2006. Supporting private sector investment in Afghanistan. OPIC Highlights (October). Washington, DC: OPIC.
———. 2012a. What we offer: Financial products. http://www.opic.gov/what-we-offer/financial-products (accessed January 3, 2013).
———. 2012b. Who we are: Our investment policies. http://www.opic.gov/who-we-are/our-investment-policies (accessed November 2, 2012).
Outterson, K. 2012. Regulating compounding pharmacies after necc. New England Journal of Medicine 367(21):1969-1972.
Paez, K. A., L. Zhao, and W. Hwang. 2009. Rising out-of-pocket spending for chronic conditions: A ten-year trend. Health Affairs 28(1):15-25.
Palmer, E. 2012. China sweeps in on drug counterfeiters. FiercePharma, August 6.
Parfitt, T. 2006. Russia cracks down on counterfeit drugs. Lancet 368(9546):1481-1482.
Patricof, A., and J. Sunderland. 2005. Venture capital for development. Brookings Blum Roundtable: The Private Sector in the Fight Against Global Poverty. Washington, DC: Brookings Institution.
Perrone, M. 2012. Counterfeit drugs becoming big business worldwide. USA Today, February 16.
Pilloy, J. 2009. Artemisinin production and pricing. Paper presented at WHO/MMV Artemisinin Conference, Mumbai.
Pirmohamed, M., K. N. Atuah, A. N. O. Dodoo, and P. Winstanley. 2007. Pharmacovigilance in developing countries. British Medical Journal 335. DOI: 10.1136/bmj.39323.586123.
Poh, J. 2011 (April 26-28). Experience and value of CTD: Singapore’s experience. Paper presented at APEC Asia Regulatory Conference: Asia’s Role in Global Drug Development, Seoul, Korea.
PSI-Inc. (Pharmaceutical Security Institute). 2006. PSI 2006 situation report. Vienna, VA: PSI-Inc.
———. 2011. PSI 2011 situation report. Vienna, VA: PSI-Inc.
———. 2012a. Counterfeit situation. http://www.psi-inc.org/counterfeitSituation.cfm (accessed April 25, 2012).
———. 2012b. Counterfeit situation: Arrest data. http://www.psi-inc.org/arrestData.cfm (accessed October 25, 2012).
———. 2012c. Pharmaceutical Security Institute: Home. http://www.psi-inc.org/index.cfm (accessed October 16, 2012).
PSM (Partnership for Safe Medicines). 2011a. Feds shut down rogue online pharmacy operating out of Kansas City. http://www.safemedicines.org/2011/02/belgian-citizen-pleads-guilty-to-selling-counterfeit-drugs-via-online-pharmacy.html (accessed September 24, 2012).
———. 2011b. Peddling poison: The counterfeit drug problem in America. http://www.safemedicines.org/2012/01/peddling-poison-the-counterfeit-drug-problem-in-america-397.html (accessed September 24, 2012).
PWC (PriceWaterhouseCooper). 2010. Global pharma looks to India: Prospects for growth. http://www.pwc.com/gx/en/pharma-life-sciences/publications/india-growth.jhtml (accessed March 5, 2013).
Quingyun, W. 2012. Major crackdown in fake medicine scam. China Daily, August 6.
Rabinovitch, S. 2005. On the legitimacy of cross-border pharmacy. Alberta Law Review 43(2):327-368.
Rägo, L. 2011. Practical use of ICH CTD in facilitating approval of products by prequalification programme and beyond. Paper presented at APEC Asia Regulatory Conference: Asia’s Role in Global Drug Development, Seoul, Korea, April 26-28.
Rao, R., P. Mellon, and D. Sareley. 2006. Procurement strategies for health commodities: An examination of options and mechanisms within the commodity security context. Arlington, VA: DELIVER, for the U.S. Agency for International Development.
Raufu, A. 2006. Nigeria leads fight against “killer” counterfeit drugs. Bulletin of the World Health Organization 84(9). http://www.who.int/bulletin/volumes/84/9/06-020906/en (accessed May 1, 2013).
Rawlins, M. 2004. Cutting the cost of drug development? Nature Reviews Drug Discovery 3:360-364.
Reuters. 2008. Currently China has about 3,500 drug companies falling from more than 5,000 in 2004, March 18.
Roy, J. 1994. The menace of substandard drugs. World Health Forum 14:406-407.
Russo, G., and B. McPake. 2010. Medicine prices in urban mozambique: A public health and economic study of pharmaceutical markets and price determinants in low-income settings. Health Policy and Planning 25(1):70-84.
Sahoo, A. 2008. Drug approval trends at the FDA and EMEA. Business Insights. http://www.pharmatree.in/pdf/reports/Drug%20Approval%20Trends%20at%20the%20FDA%20and%20EMEA_Process%20improvements,%20heightened%20scrutiny%20and%20industry%20response.pdf (accessed May 1, 2013).
Shaji, J., and S. Lodha. 2010. Regulatory status of banned drugs in India. Indian Journal of Pharmaceutical Education and Research 44(1):86-94.
Sharma, V., and V. Ahuja. 2010. Training in post-authorization pharmacovigilance. Perspectives in Clinical Research 1(2):70-75.
Silverman, E. 2011. Will Teva sell a generic Lipitor in the US? Forbes, November 3.
Silverman, M., M. Lydecker, and P. Lee. 1992. Bad medicine: The prescription drug industry in the third world. Stanford, California: Stanford University Press.
Siminski, P. 2011. The price elasticity of demand for pharmaceuticals amongst high-income older Australians: A natural experiment. Applied Economics 43(30):4835-4846.
Stracansky, P. 2010. Fake medicines may kill a million a year. Global Issues, October 28.
Sun, Q., M. A. Santoro, Q. Meng, C. Liu, and K. Eggleston. 2008. Pharmaceutical policy in China. Health Affairs 27(4):1042-1050.
TIPC (Therapeutic Information and Pharmacovigilance Center). 2010. The Namibia Medicines Watch 2(2):5-8. Nambian Medicines Regulatory Council.
Tominaga, T. 2012. Pharmaceuticals and Medical Devices Agency. APEC MRCT roadmap: Regulatory authorities’ efforts to promote multi-regional clinical trials (MRCTs). Paper presented at 24th Annul EuroMeeting, Copenhagen, Denmark.
Torstensson, D., and M. Pugatch. 2012. What lies within? Procurement processes and the risk of substandard medicines. London: The Stockholm Network.
UNDP (United Nations Development Programme). 2004. Constraints on the private sector in developing countries. In Unleashing entrepreneurship: Making business work for the poor. New York: UNDP.
Unicef (United Nations Children’s Fund). 2011. Supplies and logistics. http://www.unicef.org/supply/index_39994.html (accessed October 3, 2012).
UNODC (United Nations Office on Drugs and Crime). 2010. The globalization of crime. New York: UNODC.
USP-NF (U.S. Pharmacopeia and The National Formulary). 2010. Amoxicillin capsules. USP Monographs. Rockville, MD: USP-NF.
Vaidyanathan, G. 2012. Failings exposed at India’s drug regulator. Nature News, May 18.
van Zyl, A., J. Daviaud, and S. Logez. 2012. Model quality assurance system for procurement agencies: Harmonized assessment tool. WHO Drug Information 26(3):5.
Vashisth, S., G. Singh, and A. Nanda. 2012. A comparative study of regulatory trends of pharmaceuticals in Brazil, Russia, India and China (BRIC) countries. Journal of Generic Medicines: The Business Journal for the Generic Medicines Sector 9(3):128-143.
WHO (World Health Organization). 1998. Drug supply choices: What works best? WHO Essential Drugs Monitor (25-26). http://apps.who.int/medicinedocs/en/d/Jwhozip10e/1.4.html#Jwhozip10e.1.4 (accessed May 1, 2013).
———. 1999. Operational principles for good pharmaceutical procurement. Geneva: WHO.
———. 2002a. Practical guidelines on pharmaceutical procurement for countries with small procurement agencies. WHO Regional Office for the Western Pacific, Manila, Philippines.
———. 2002b. Safety of medicines: A guide to detecting and reporting adverse drug reactions: Why health professionals need to take action. Geneva: WHO.
———. 2004a. Equitable access to essential medicines: A framework for collective action. Geneva: WHO.
———. 2004b. The world medicines situation. Geneva: WHO.
———. 2006a. Annex 6: A model quality assurance system for procurement agencies (recommendations for quality assurance systems focusing on prequalification of products and manufacturers, purchasing, storage and distribution of pharmaceutical products). Geneva: WHO.
———. 2006b. Counterfeit medicines. http://www.who.int/medicines/services/counterfeit/impact/ImpactF_S/en/index.html (accessed July 12, 2012).
———. 2007a. A model quality assurance system for procurement agencies. Geneva: WHO.
———. 2007b. Quality assurance of pharmaceuticals: A compendium of guideines and related materials (volume 2, 2nd updated edition). Geneva: WHO.
———. 2010a. Assessment of medicines regulatory systems in sub-Saharan African countries: An overview of findings from 26 assessment reports. Geneva: WHO.
———. 2010b. Fact sheet n°278: Prequalification of medicines by WHO. http://www.who.int/mediacentre/factsheets/fs278/en/index.html (accessed November 5, 2012).
———. 2011. Annex 15: Guidelines on submission of documentation for a multisource (generic) finshed product. General format: Preparation of product dossiers in common technical document format. Geneva: WHO.
———. 2012a. General information on counterfeit medicines. http://www.who.int/medicines/services/counterfeit/overview/en (accessed April 25, 2012).
———. 2012b. Medicines regulatory support. http://www.who.int/medicines/areas/quality_safety/regulation_legislation/en/index.html (accessed August 16, 2012).
———. 2012c. New global mechanism to combat substandard/spurious/falsely-labelled/ falsified/counterfeit medical products. Geneva: WHO.
WHPA (World Health Professions Alliance). n.d. Be aware: Helping to fight counterfeit medicines, keeping patients safer. France: WHPA.
Wilson, K. R., J. C. Kohler, and N. Ovtcharenko. 2012. The make or buy debate: Considering the limitations of domestic production in Tanzania. Globalization and Health 8(1):20.
World Bank. 2007. Policy note: Improving the competitiveness of the pharmaceutical sector in Bangladesh—draft. http://www.scribd.com/doc/97075636/Pharmaceutical-Bangladesh-Report (accessed March 5, 2013).
Yu, J., and H. v. Hindenburg. 2012. IFC invests in China’s Fosun Pharma, helping increase global supply of affordable drugs. IFC: News and Multimedia. http://www.ifc.org/IFCExt/pressroom/IFCPressRoom.nsf/0/A4EE3BD6C275AB0385257A69001C66A4?OpenDocument (accessed January 3, 2013).