5
Weaknesses in the Drug
Distribution Chain
The modern pharmaceutical supply chain is complex. Medicines are made from ingredients sourced from different countries. Final formulations are then exported. Packaging, repackaging, and sale can happen in many other countries. Drugs change hands many times between the manufacturer and patient; every transaction is an opportunity for falsified or substandard products to infiltrate the market. Changes to the drug distribution system could improve drug quality around the world.
This chapter gives an overview of the drug distribution chain, explaining differences between the systems in developed and developing countries. The drug wholesale system is a weak point where the licit and illicit supply chains mix. Better controls on the wholesale market could improve the security of the distribution chain. Drug tracking systems could also improve security by preventing products that leave the legitimate supply chain from returning to it. These solutions can improve drug safety as long as the supply chain does not disintegrate at the point closest to the patient. Disorganized drug markets, both real and on the internet, undermine regulatory checks on medicines distribution.
AN OVERVIEW OF DRUG DISTRIBUTION IN
DEVELOPED AND DEVELOPING COUNTRIES
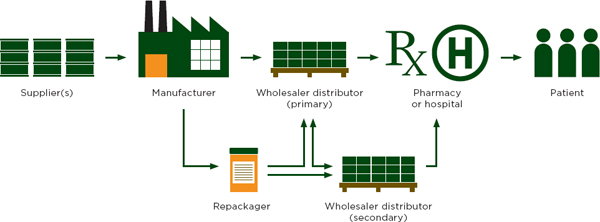
Figure 5-1 describes the drug distribution chain in developed countries, where most patients get medicine from a doctor’s office or a licensed pharmacy or dispensary (Yadav and Smith, 2012). For example, in the United States about three-quarters of all pharmaceuticals are bought in retail pharmacies, about half of which are national chains or food stores with an internal pharmacy (Yadav and Smith, 2012). These vendors handle a wide variety of products sold in an even wider variety of packaging. Retailers in developed countries would find it logistically impossible to buy their stock, in its many different packages, directly from manufacturers (Yadav et al., 2012). Therefore, most vendors buy their inventory from pre-wholesalers and wholesalers.
Key Findings and Conclusions
• A few national firms control most of the primary wholesale market in rich countries. In developing countries, hundreds, sometimes thousands, of firms control tiny shares of the same.
• Drug distribution chains in developing countries are often fragmented and complicated.
• The final leg of the drug distribution chain is exceptionally expensive and inefficient in developing countries.
The drug distribution system in low- and middle-income countries has the same basic steps as that described in Figure 5-1, but with more intermediaries between the manufacturer and patient (Yadav and Smith, 2012). Instead of having one coordinated distribution chain that reaches the whole country, there are many small chains and many small companies at every step (Yadav and Smith, 2012). Figure 5-2 describes the drug flow for public, private, and nongovernmental organizations, and their separate, but sometimes overlapping, intermediaries.
A comparison of Figures 5-1 and 5-2 and Table 5-1 illustrate some important differences in drug distribution in developing and developed countries. For example, a few large firms generally control the national wholesale market in developed countries. Cardinal Health, McKesson, and AmerisourceBergen distribute 90 percent of drugs sold in the United States; four or five major firms distribute to 90 percent of the market in Western Europe and Japan (Yadav and Smith, 2012). In developing countries, hundreds, even thousands, of companies control tiny shares of the drug wholesale market (Yadav and Smith, 2012).
Excessive fragmentation is an important difference between developed and developing countries’ drug distribution systems. In developed countries, comparatively few large firms control the market and regulatory authorities require some chain of custody documentation. In low- and middle-income countries, the system is vastly more complicated. Sometimes multiple parallel distribution systems of varying efficiency run in the same country. Box 5-1 describes the confusing drug distribution systems often found in humanitarian emergencies.
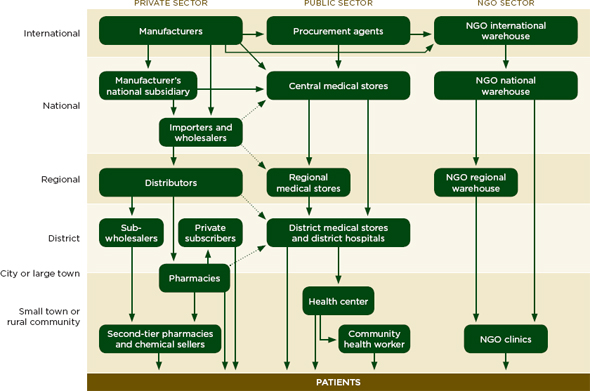
FIGURE 5-2 The private, public, and NGO drug distribution systems for essential medicines in developing countries.
NOTE: NGO= nongovernmental organization.
SOURCE: Yadav et al., 2011.
|
Factor |
Developed Countries |
Developing Countries |
|
Payer or reimbursement |
Strong presence of public or private insurance companies and limited out-of-pocket expenditure. |
Mostly payments are made out of pocket. Social health insurance systems are expanding in many emerging markets. Private insurance plans are also growing in some emerging market countries. |
|
Regulatory structure |
Strong, well-defined laws and overall good ability to enforce regulations. |
Weak fragmented regulatory structures, ill-defined laws in some instances, and poor ability to enforce regulations. |
|
Patented, generic vs. branded generic |
The market for prescription drugs consists of patented drugs and generics. |
Poor regulatory structure creates a strong market for branded generics (brand is used as a signal of quality by the patient). |
|
Prescription adherence |
Prescription drugs can only be dispensed with a formal prescription. |
Retail drug shops often dispense medicines and also act as the first point of health care contact for many patients. |
|
Balance of power in the system |
Buyer (insurance companies or national health system) monopoly creates good balance of power between the manufacturer and the patients. In the United States, pharmacy benefit managers and drug formularies are commonly used as a means to ensure further balance of power. |
Balance of power is tilted toward the manufacturer and the distribution channel. The large fraction of patients purchase with out-of-pocket funds and have little bargaining power. |
SOURCE: Adapted from Yadav and Smith, 2012.
In OECD countries, private companies ship and transport almost all pharmaceuticals, but in developing countries, despite their vastly smaller tax base, the government does (Yadav, 2010). In sub-Saharan Africa, a government-owned-and-operated central medical store manages the distribution of drugs, transporting goods around the country in a government-owned fleet. Donors and developing-country governments favor this system, wherein the central store manager can neither hire people with business experience nor fire incompetent workers (Yadav, 2010). Inefficient supply chain management directly drives up costs and causes drug stock-outs in low- and middle-income countries (Yadav, 2010). As Chapter 4 explained, this drug scarcity in turn creates a vacuum for poor-quality products to fill.
BOX 5-1
Drug Distribution in Humanitarian Emergencies
A donated batch of Ringer’s Lactate Infusions made its way to humanitarian aid workers in Darfur through a UN agency and other suppliers. Despite its many stops along the way, only at one of the final destinations did a volunteer doctor notice fungal spores contaminating the product (Caudron et al., 2008). The infusions had been distributed so widely and haphazardly that, despite a product recall, only 15 percent were ever collected (Caudron et al., 2008). Such problems are not uncommon during emergencies, when quality control throughout long supply chains becomes difficult.
Despite the good intentions of aid agencies, nongovernmental organizations (NGOs), and individual and corporate donors, the chaos inherent in humanitarian emergencies often leads to a proliferation of fake, substandard, and otherwise poorly regulated medical products. The dangers of poorly regulated drugs lead some bodies, such as the European Commission’s Directorate-General for Humanitarian Aid, to stipulate that quality-assurance guidelines not be relaxed during emergencies, even though quality-assurance steps can slow down response (Pomatto and Schuftan, 2006).
After the tsunami in Sri Lanka, only 50 percent of the drugs donated had expiration dates on them; of that half, 5 percent had already expired or would expire within days; 62 percent of the medication labels were not in English, the language of the Sri Lankan health system (Mahmood et al., 2011). Such inappropriate drug donations cause serious problems because disposing of such drugs, especially in large quantities, is a lengthy and expensive project (Pomatto and Schuftan, 2006). After the 2000 floods in Venezuela, 70 percent of the drugs donated for humanitarian assistance needed to be destroyed, requiring the government to pay $16,000 to cover the extra personnel needed to sort the donations (Hechmann and Dune-Birouste, 2007).
During emergencies, little about patients, their diagnoses, or medical history is collected at most health facilities. Drug quality signals can be difficult to spot when infrastructure is disrupted: Patients are seen quickly and only minimal information is recorded. NGOs often arrive with few or no pharmacists on staff, and although local health workers may be aware of substandard and falsified drugs, visiting doctors often are not (Villacorta-Linaza, 2009).
The World Health Organization published its “Guidelines for Drug Donations” in 1996 after particularly problematic donations during the Bosnian War (Berckmans et al., 1997; Hechmann and Dune-Birouste, 2007). At times the guidelines are followed closely; for example, humanitarian emergencies in East Timor and Gujarat State saw few inappropriate donations (van Dijk et al., 2011).
Of course, donor demands alone do not drive the costs of supply chain management in developing countries. It is expensive to transport products over rough terrain with poor roads. In India, for example, nearly 70 percent of the population lives in rural areas, where the health posts may be few and lacking in staff, electricity, and supplies (Langer and Kelkar, 2008). The costs of drug distribution in India are two to three times greater than in the United States or the European Union, despite vastly lower labor costs (Langer and Kelkar, 2008). Supply chain managers are always concerned with the last-mile problem: the disproportionately expensive and inefficient final leg on the distribution chain. In developing countries, the last mile is exceptionally long, extending to sparsely populated villages far from a paved road and farther from a supply center (USAID, 2011).
Managing the drug distribution system in developing countries means containing the costs of the last mile, moving medicines to patients quickly, and keeping records of all transactions between the manufacturer and the consumer. The first step on this chain is the drug wholesale market. Around the world, drug wholesale is a common point of vulnerability to falsified and substandard medicines.
There are two kinds of drugs wholesalers: primary wholesalers who have written distribution contracts with manufacturers and buy directly from them, and secondary wholesalers who buy from other intermediaries. In some countries, including the United States, there are also large regional wholesalers (Fein, 2012; White and Bothma, 2009). Regional wholesalers may be primary or secondary wholesalers (Fein, 2011). They often serve independent pharmacies or hospitals and may have strong distribution networks (Levy, 2006).
As Figure 5-1 suggests, the distinction between the primary and secondary wholesalers is not always clear. Primary wholesalers may, for example, buy products from secondary wholesalers as well as manufacturers (Ziance, 2008). The back-and-forth sales are common among drug wholesalers, who buy and sell medicines to accommodate market demand. That is, when they see a medicine is scarce in one region, they can buy the same medicine from other wholesalers that may be flush with it. The markets are constantly fluctuating; products change hands many times.
Sometimes secondary wholesalers fill a void; they supply to rural pharmacies or markets that national or regional wholesalers do not reach. But they choose stock based on demand forecasts, price, margin, and their customers’ willingness to pay (Yadav, 2009). The costs of the transactions required when dealing with many suppliers and their generally poorer bargaining power give the secondary wholesalers weak incentives to stock a wide variety of products or brands (Yadav, 2009).
Key Findings and Conclusions
• The U.S. drug wholesale market is made up of a combination of primary and secondary wholesalers. There are three major national wholesalers, a few regional wholesalers, and thousands of secondary wholesalers.
• Secondary wholesalers are the weakest point in the U.S. pharmaceutical distribution chain.
• Wholesalers buy and sell drugs in response to market demand, repeatedly repackaging products. In wholesale repackaging, illegitimate products can gain authentic packaging, and clean, authentic packaging is removed and not always destroyed.
• In the United States, state pharmacy boards or other state agencies license wholesalers. Their licensing requirements vary widely. Unscrupulous wholesalers seek out states with the most lenient requirements and move from state to state when caught in violations.
• There is no national database on drug wholesalers.
• Raising the minimum standards for drug wholesale in the United States could build momentum for increased control of the drug wholesale market in low- and middle-income countries.
Wholesalers may sell and resell medicines repeatedly among themselves before filling a pharmacy order. Wholesalers often repackage products with every sale, or at least repackage individual containers for final sale (Catizone, 2006; Laven, 2006). Through a process called salting, legitimate and fake drugs are mixed at wholesale, and in the wholesale repackaging, the fake products gain authentic labels (Donaldson, 2010; Liang, 2006). Salting can be done unknowingly, such as when primary wholesalers buy from other intermediaries, accidentally launder fake products, package them in authentic labels, and send them to pharmacies (Spies and VanDusen, 2003). In repackaging the manufacturer’s expensive fraud-protection packaging can be removed, and batch numbers reprinted (Satchwell, 2004). Not only does this interfere with tracking requirements, but it leaves the wholesaler repackagers with clean, unused packaging that is not always destroyed (Satchwell, 2004).
Manufacturers usually have no distribution agreements with secondary wholesalers (Ziance, 2008). The firms may trade in many kinds of products other than pharmaceuticals. Their staff are not required to show skills
in pharmaceutical warehousing and management, often with disastrous consequences (Ziance, 2008). In 2001, for example, a falsified version of Epogen, one of the most expensive drugs in the Medicare formulary, killed a 16-year-old boy in New York (Gressit, 2007; Ziance, 2008). Eleven secondary wholesalers had traded the Epogen that killed him (Engelberg et al., 2009; Gressit, 2007; Whoriskey, 2012). Though it is impossible to recreate the drug’s exact path, it was briefly stored in a drinks cooler above a Florida strip club (Brown, 2005).
Small secondary wholesalers act negligently in part because they do not have the reputational risks that major national or regional wholesalers do. There are thousands of secondary wholesalers in the United States, all legally supplying to pharmacies, the product of lax licensing requirements (Appleby, 2003). In a recommendation to the state legislature, a Florida grand jury described some of the states’ drug wholesalers as “uneducated, inexperienced, … rank amateurs, many with criminal records” (Appleby, 2003). As the grand jury description implies, many of these companies are looking to increase their profits at any cost.
These companies exploit problems in the regulated drug market, such as drug shortages. Hospital pharmacists are under pressure to fill prescriptions even during drug shortages, forcing them to buy from gray market vendors at up to 10 times the standard prices for some drugs, including anesthetics and cancer drugs (Aleccia, 2011; ISMP, 2011). More than half of surveyed hospitals in the United States buy cancer medicines from the gray market (Gatesman and Smith, 2011). A similar proportion of U.S. hospital pharmacists and drug buyers report daily inquiries from gray market pharmaceutical salesmen about their inventories (ISMP, 2011). One survey respondent told the Institute for Safe Medication Practices, an NGO, “You are hesitant to tell gray market vendors what you need because they will buy it all up if they find it, and then harass you [to buy it] for months afterwards” (ISMP, 2011). Box 5-2 describes one such gray market purchase.
Some changes to the drugs wholesale system could protect the American consumer. One option would be requiring all organizations that sell wholesale medicines to hold National Association of Boards of Pharmacy (NABP) accreditation. The NABP wholesale accreditation process reviews wholesalers’ record keeping, licensing, and drug verification procedures (NABP, 2012a). Accreditation also involves criminal background checks on the most senior operations, buying, and inventory staff, their supervisors, and anyone owning greater than 10 percent interest in the company if it is not publically held (NABP, 2012a). Indiana, North Dakota, and Wyoming require NABP accreditation for wholesalers; wholesalers in other states may voluntarily seek out certification as evidence of their standards (Cherici et al., 2011).
Direct-to-pharmacy distribution is another alternative to the current wholesale systems. In this system, manufacturers eliminate secondary wholesalers and use logistics companies to ship directly to the vendor. It has been used, with varying success, in Europe and Australia (Galve and Campos, 2011; Kanavos et al., 2011; Taylor, 2011). There is some concern, however, that direct distribution drives up medicines costs (Exel, 2003; OFT, 2007). It also puts impractical storage and warehousing demands on retailers (Exel, 2003). If direct-to-pharmacy distribution replaces wholesalers with an equally porous network of transport and logistics companies, then it is no improvement.
BOX 5-2
Falsified Avastin’s Circuitous Path to the United States
A fake drug originally manufactured in Turkey took a winding path to the United States in late 2011 and early 2012, where it found its way to several physicians’ offices. The drug, Avastin, is manufactured by Roche Holding AG of Switzerland and is often used alongside chemotherapy to treat certain lung, colon, and kidney cancers (Faucon and Whalen, 2012). The fake batches contained salt, starch, and various chemicals, but no active ingredients (Blair, 2012). In February and March 2012, the FDA warned approximately 20 practices that they may have received fake Avastin. Later that spring, they expanded the number to 76 potentially affected practices in 22 U.S. states (Weaver and Whalen, 2012).
The precise origins of the drugs are unknown, as the Turkish company listed on relevant paperwork was not registered with the Turkish authorities, and a trip to its stated address led investigators to a textiles warehouse (Faucon and Whalen, 2012). A Swiss drug distributor, apparently unaware of the problem, purchased the Avastin from Turkey from a Syrian middleman in Egypt, and subsequently sold it to another distributor in Copenhagen (Faucon and Whalen, 2012). From there, the drugs traveled through several companies in Britain and the United States under the parent company Canada Drugs, which operates an online pharmacy that often uses overseas companies to source discount drugs. Ultimately, two U.S. companies sold the drug directly to physicians (Weaver and Whalen, 2012). The high cost of such drugs, at times exacerbated by shortages, may tempt physicians to seek out alternative suppliers to lower their own and their patients’ costs and assure a steady supply. At a price several hundred dollars lower per vial than the standard, the falsified Avastin was a good deal for such practices (Weaver and Whalen, 2012). The same forces that lead patients to buy unreliable drugs can lead doctors to do the same, creating yet another vulnerable link in the medicines supply chain.
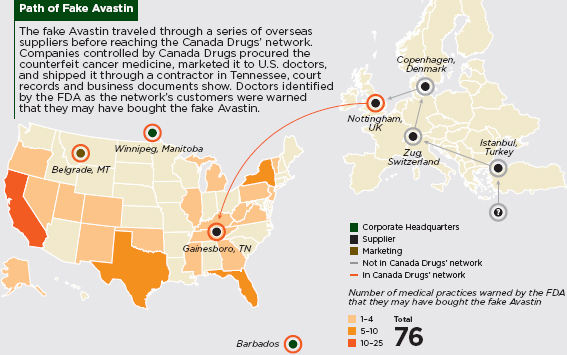
SOURCE: Weaver and Whalen, 2012.
More rigorous licensing and regulation of the wholesale market, especially the secondary wholesalers, is another solution. The committee believes the secondary wholesale market is the weakest link in the U.S. drug distribution system. Improvements to the secondary wholesale system could reduce the number of transactions in the drug distribution chain, thereby improving security.
Recommendation 5-1: State licensing boards should only license wholesalers and distributors that meet the National Association of Boards of Pharmacy accreditation standards. The U.S. Food and Drug Administration, in collaboration with state licensing boards, should establish a public database to share information on suspended and revoked wholesale licenses.
The committee finds that peculiarities of the American wholesale system account for much of the United States’ vulnerability to falsified and substandard drugs. Limiting the wholesale market to vetted firms would make the drug distribution chain less permeable to criminals (Donaldson, 2010). Similar weaknesses plague the wholesale system in developing countries, and action in the American market might give regulators around the world an example and encouragement to tighten controls on the chaotic wholesale and distribution systems.
This recommendation should be implemented in phases over the next 2 years. Collaboration between state licensing boards and the FDA should happen first. Next, the regulators should design the database and publish the processes for collecting accurate, reliable, and timely information about the suspension or revocation of wholesale licenses.
In the United States, state governments control professional practice, including the practice of pharmacy, which includes medicine distribution and wholesale. Some states have enacted tighter regulations on the market, with unintended spillover effects (Laven, 2006). After the state of Nevada increased oversight of drug wholesale, for example, “some wholesalers simply moved operations across the state line into California” (Flaherty and Gaul, 2003). When unscrupulous businesses can seek out the softest regulatory systems to work in, they do. As the previous section explains, the wholesale trade depends on buying and selling medicines in response to shortages and gluts in different parts of the country. Therefore, the weaknesses in one state licensing system can become vulnerabilities for the others. The committee recognizes the authority of states to license wholesalers but believes that public health will be best protected if all businesses adhere to the strict standards laid out by the National Association of Boards of Pharmacy accreditation process.
Every state has an interest in promoting high minimum standards for medicine sale and manufacture. The recent fungal meningitis outbreak from an steroid injection compounded under unhygienic conditions at New England Compounding Center in September 2012 is a reminder of the risks of competing state standards (Grady et al., 2012; Tavernise and Pollack, 2012). The outbreak and associated infections, which as of January 2013 had killed 45 patients and sickened 693 others in 19 states, was driven by the interstate sale of a compounded steroid (CDC, 2013). Compounding pharmacies are not held to the same standards as big pharmaceutical manufacturers; courts have questioned the FDA’s authority over them (Grady et al., 2012). As in the wholesale market, states regulate these businesses in isolation. Though the Massachusetts Department of Health registered three complaints against New England Compounding Center, there is no mandatory national system for sharing these complaints (Grady et al., 2012).
Similarly, there is no way for state authorities to share information on
criminal or negligent wholesalers. As part of a stronger wholesale system, states should report violations and revocations of wholesale licenses to a national, public database. This will impede unscrupulous wholesalers from moving from state to state and starting over when caught in violation of one state’s rules. The FDA should facilitate the sharing of this information among states and with the public. The recent tragic meningitis outbreak has brought to light the importance of sharing information on dangerous actors in the drug distribution chain. Although the states have the authority to license wholesalers, the nation’s interests are best served by enabling communication among the states.
The Wholesale Market in Low- and Middle-Income Countries
As the previous section explains, the wholesale market is a common vulnerability in medicines distribution around the world. One potential positive outcome of raising the standards for U.S. wholesalers is that it would build international momentum for a leaner, more organized wholesale drug market. Other countries are already working toward more controlled drug wholesale. For example, in 2004 the Chinese drug regulatory authority cut the number of drug wholesalers in the country from 16,000 to 7,445 (Yadav et al., 2011). This is still many more than in the United States, Europe, or Japan, but it is an admirable move in a more sustainable direction.
Proponents of the current drug wholesale system maintain that a small number of wholesalers cannot serve the drugs market of developing countries. They reason that a system of three or four large primary wholesalers may work in Europe or North America, but in developing countries a few companies could never guarantee fine-mesh distribution (Foundation Strategy Group, 2005; McCabe, 2009). Medicine shops in Kenya, for example, report buying from a range of pharmaceutical and general wholesalers both in and outside of the shop’s district, as well as mobile vendors and manufacturers (Amin and Snow, 2005).
Others argue that raising the quality standards for drug distribution carries an inherent trade-off of decreased access to medicine (OFT, 2007). Analysis of successful distribution chains, such as the Coca-Cola distribution chain, suggests this is a false dichotomy, however (Yadav et al., 2013). ColaLife, a nonprofit, has been using Coca-Cola’s fine-mesh distribution chain to bring oral rehydration and zinc supplements to remote areas since 2008 (ColaLife, 2012). Steps toward a more controlled and efficient wholesale market can protect patients in the markets most hurt by bad-quality drugs. A reduction in the number of licensed wholesalers and use of more efficient distribution chains can help the wholesale market around the world.
More stringent licensing requirements can improve the wholesale system, but drugs will still need to move from the factory to the vendor, passing through many hands before reaching the patient. With every transaction on the chain, there is a risk of the drug supply’s being compromised. Criminals take advantage of places where the distribution chain breaks down and medicines depart from documented chain of custody. Drugs that leave the proper distribution system are called diverted drugs; the markets that trade diverted drugs, or more generally, markets that trade with little authorized oversight, are called gray markets.
Drug diversion is the means through which medicines approved for sale in one country are sold in others, where they may not be registered. These schemes depend on false statements, forged customs declarations, or smuggling (PSI data shared with the committee, Thomas Kubic, PSI-Inc., July 11, 2012). On the surface, drug diversion is not the public health threat that falsified and substandard medicines are (Bate, 2012). Some countries have made legal provisions for importation of unregistered lifesaving drugs that are not available in local markets (Zaza, 2012). Others argue that thieves bring good-quality drugs to otherwise neglected markets, and that, issues of fraud aside, the end consumer is no worse off (Bate et al., 2010a). If thieves trafficked solely in quality-assured medicines, then this point might be valid. Once a medicine leaves the responsible chain of custody, there is no way to ensure that it has been properly stored. As Chapter 3 explains, drug quality research indicates that unregistered medicines are sometimes dangerous (Bate et al., 2010b; Lon et al., 2006; Stanton et al., 2012; Wondemagegnehu, 1999). By chance, drug diversion may bring good
products to some patients, but it hurts many more, not only by defrauding the official channels.
Key Findings and Conclusions
• When stolen drugs are reintroduced to the legitimate supply chain, there are no records of the products’ handling or storage conditions. Diverted drugs are often sold abroad and are of dubious quality.
• A drug pedigree is a record of the drugs’ chain of custody. Pedigree requirements prevent stolen drugs from entering the legitimate markets and facilitate efficient recalls.
• There are many methods to create a drug pedigree; all depend on unique serial numbers on the primary pack label.
• A reliable system for tracking and tracing drugs through the distribution chain would reduce the likelihood of illegitimate medicines reaching patients.
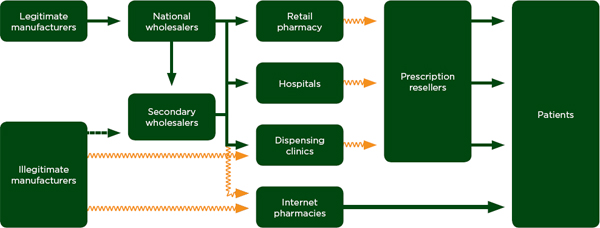
Drug diversion is roughly synonymous with theft, and trade in diverted drugs is an indicator of the relative ease with which criminals exploit weaknesses on the distribution chain. Figure 5-3 shows common diversion points in the distribution chain. In the United States, for example, the resale of prescription drugs is a common problem, but illicit vendors also circumvent the regulated distribution chain at other points. In developing countries, the sale of donated drugs for profit is a common type of diversion (World Bank, 2005). Small-scale theft, also called pilfering, happens mostly between the vendor and patients; larger cargo heists tend to happen to bulk drug packages, generally between the manufacturer and the vendor.
Many diverted drugs are donated ones, pilfered and resold by health workers (Ferrinho et al., 2004; Vian, 2008). The theft and resale of free drugs is engrained in the pharmacy and clinical culture in some countries, where it is seen as a professional perk for otherwise underpaid government health workers (Lim et al., 2012). This theft defrauds donors and contributes to drug shortages at legitimate dispensaries (Bate, 2012), thereby encouraging the distal causes of poor-quality drugs.
Medicines can also be stolen in large quantities earlier in the supply chain. In March 2010, $75 million worth of medicines were stolen from an Eli Lilly warehouse in Connecticut (Efrati and Loftus, 2010) and later partially recovered in Florida (Muskal, 2012). Freight Watch International, a supply chain security company, estimates that theft of pharmaceuticals in the United States increased 283 percent between 2006 and 2008 and have remained roughly constant since then (FreightWatch, 2011b). Warehouse heists such as the Lilly theft are relatively difficult to orchestrate; by far the more common route is theft of a loaded trailer (see Table 5-2) (FreightWatch, 2011b). The companies and the FDA issue warnings about batch and lot numbers of stolen products, but warnings are of limited value when some of the stolen goods are either laundered back into the legitimate supply chain or sold abroad (Burnham, 2012).
Cargo theft is not confined to the United States; Freight Watch International sees it as a serious problem in Brazil, Great Britain, India, Mexico, Russia, and South Africa as well (Fischer, 2012; FreightWatch, 2011a). Indeed, cargo security is generally more of a problem in low- and middle-income countries, where poor roads and slow transit times put shipments at risk for a long time, and in politically volatile places (SCMS, 2012). In any case, the goal of the theft is to sell the diverted shipment.
Sometimes diverted drugs in the market are easy to spot. The Global Fund finances a line of artemisinin combination therapies for the Affordable Medicine Facility in eight countries, including Nigeria and Ghana (Global Fund, 2012). These drugs are packaged differently from those meant for the public sector (Bate, 2012; Bate et al., 2010a). Roger Bate was therefore able to recognize Global Fund products meant for Nigeria and Ghana in Lomé, Togo (Bate, 2012). Thirty percent of the diverted samples he collected in Togo failed quality tests, a failure his team attributed to degradation (Bate, 2012).
TABLE 5-2 Number of Pharmaceutical Thefts in the United States, 2006-2011
|
Event Type |
Number of Thefts |
|
Theft of a trailer |
167 |
|
Theft from a trailer |
4 |
|
Hijacking |
12 |
|
Facility burglary |
9 |
|
Facility robbery |
1 |
SOURCE: FreightWatch, 2011b.
Outward evidence of diversion is not always so clear, but a drug sold in a country where it is not registered is often diverted and therefore suspect. A national sample of essential medicines in Cambodia found that unregistered drugs are six times more likely to be falsified than registered ones (Khan et al., 2011). Similarly, in Ghana, researchers found unregistered oxytocin samples to be uniformly substandard (Stanton et al., 2012). Diverted drugs are dangerous partly because there is no reliable record of what conditions they have been transported in. The uterotonic drugs analyzed in Ghana are unstable at room temperature, for example (Stanton et al., 2012). They might have failed quality testing because of exposure to tropical temperatures and humidity in travel.
Drug Resale and Late Diversion
Drugs can also be diverted late in the distribution chain, after the drug has reached the patient. This is a far less common point of diversion than diversion at the vendor level and earlier. Figure 5-3 refers to this problem as prescription resale. Drug diversion through resale is a growing concern in the United States, where a 2008 survey estimated that between 5 and 10 percent of American high school students take prescription pain killers,
sedatives, tranquilizers, and Ritalin for nonmedical uses (DuPont, 2010). A study of American college students found that more than one-third of those taking a prescription drug had diverted it at some time, but generally this diversion was infrequent sharing among friends, not predictable sales (Garnier et al., 2010).
Other research suggests that Medicaid recipients and other patients sell their medicines for profit in unregulated street markets (Inciardi et al., 2007). Pill brokers may buy medicines from patients, especially elderly ones, or work with unscrupulous doctors to arrange prescriptions for kickbacks (Inciardi et al., 2007). In some ways, drug resale is similar to pilfering as both methods of drug diversion happen in small amounts and attract little attention from the authorities.
Small thefts and large diversions compromise the integrity of the drug distribution chain and confidence in the quality of medicines. In rich and poor countries alike, drugs often circulate outside of the main distribution channels without a drug pedigree, a record of “each prior sale, purchase, or trade of a drug, including the date of those transactions and the names and addresses of all parties to them” (FDA, 2011a). Between the factory and the patient, drugs change hands many times. A drug pedigree controls diversion and gray market sales by preventing a stolen product from coming back into commerce and by recording every merchant who handles the product, thereby deterring prospective thieves.
Tracking and Tracing Products Through the Supply Chain
A strong chain of custody through the drug distribution system can reduce the risks introduced with product diversion and porous supply chains. Track-and-trace systems allow all interested parties to know where the product is at any time and see a record of where it has been previously (Altunkan et al., 2012). These systems allow manufacturers and others to track their products, meaning to follow drugs forward in the distribution chain. They also allow patients or pharmacists to trace the drug, or to verify its past locations.
Track-and-trace systems rely on serialization, the assigning of unique identifying numbers to products. Products that lack identification numbers, or products with identification numbers that cannot be accounted for throughout the distribution chain, must be treated as falsified and removed from the market, even if they come from licensed manufacturers (Altunkan et al., 2012). The unique identifier may be stored in a barcode, electronic product code, or radio frequency chip, or it may be a long-digit serial number.
Mass-produced items such as packaged foods and electronics use machine-readable barcodes to store product information. Some countries require the pharmaceutical industry to mark drugs with unique product codes that contain the product’s tracking and identification number. The FDA, for example, requires all human drugs to carry a 10-digit universal identifier called a national drug code (FDA, 2012b). The first digits of the number identify the firm that manufactures, repackages, or relabels the product; the second segment identifies the product, its dosage form, and formulation, and the last digits identify the packaging (FDA, 2012b). The use of national drug codes predated the widespread use of electronic readers (HIMSS, 2003; Simonaitis and McDonald, 2009). In 2004 the FDA issued a rule requiring some human drugs and biologics to carry the national drug code in a linear barcode (FDA, 2011b).1
Machine-readable barcodes have many advantages. When used in the hospital or at the point of dispensing medication, these codes can verify that the drug is of the correct dose and dosage form (Pedersen et al., 2003). There is a limit to how much data a simple linear barcode can hold, however. Two-dimensional barcodes can encode more information in a small space and are therefore gaining popularity for supply chain management (McCathie and Michael, 2005).
Two-dimensional barcodes Two-dimensional barcodes, also called matrix barcodes, carry a product serial number, expiration date, batch code, and other information, and they are compatible with older barcode technologies (Lefebvre et al., 2011). Any camera, or even a smartphone, can read a matrix barcode (Altunkan et al., 2012). The camera has to be within the line of sight of the barcode to read it, however, so technicians scan them slowly and one at a time.
Matrix barcodes are printed onto primary packages, and the manufacturer keeps track of the code in a corporate database (Barlas, 2011a). The unique serial numbers carried in the barcode can be downloaded into a regulatory agency database accessible to pharmacists and medicine vendors (Barlas, 2011a). When intermediaries scan the matrix, they record the product’s transfers in the database. Information in the barcode should link the bulk and primary packaging. When it fails to do so, much time is wasted in packing, scanning, and repacking shipments (Davison, 2011).
In 2011 the Turkish drug regulatory authority implemented a mandatory pharmaceutical track-and-trace system using two-dimensional barcodes
______________________
1 Bar Code Label Requirement for Human Drug Products and Biological Products, 69 Fed. Reg. 9120 (Feb. 26, 2004).
(Barlas, 2011b). Multinational pharmaceutical companies are obliged to provide two-dimensional barcodes for all products bound for Turkey, though some may print serial labels separately and attach them to packages in-country (Taylor, 2010). The logistics company DHL manages the Turkish labels for some companies (Taylor, 2010). Brazil has a similar requirement, rolled out over 3 years starting in 2009 and allowing a 1-year grace period to sell all warehoused products that predated the requirement (Taylor, 2010).
The use of matrix barcodes for tracking and tracing is not foolproof; barcodes can be forged. They are also not helpful when a patient does not receive the manufacturer’s packaging. The system also demands active participation from every intermediary on the distribution chain. If a pharmacist fails to scan the barcode, the information it carries is of no use. Nevertheless, electronic track-and-trace can do much to thwart criminals and protect the drug supply. The systems in Brazil and Turkey give vendors and motivated consumers a way to verify the safety of their products, and they allow regulators to better understand where and how frequently products leave the distribution chain.

A data matrix or two-dimensional barcode on a medicine package.
SOURCE: Altunkan et al., 2012, reprinted with permission. © 2012 IEEE.
Electronic Product Codes and Radio Frequency Identification
Electronic product codes are a form of product code stored in radio frequency identification tags about the size of a grain of rice. The radio frequency tag contains an antenna and a chip (EPCglobal, 2007; Wunder and Roach, 2008). The chip holds the product’s unique serial number, expiry date, batch code, and information about its previous transactions; the antenna, when activated by the tag reader, conducts radio energy to the chip to send and receive data (Lefebvre et al., 2011; RFID Journal, 2012). The technician reading the chip does not need to position the reader within sight of the tag to read it; the signal is sent by radio waves, not sight. The amount of information encoded in electronic product codes and the ease of accessing this information make the system attractive for drug pedigrees (Lefebvre et al., 2011).
Though some see radio frequency identification (also called RFID) as one of the greatest technological achievements of recent times (Bendavid et al., 2007; Srivastava, 2004), others call it disruptive and over-hyped (Bendavid et al., 2007). The technology clearly has innovative potential, but a critical mass of intermediaries on the drug distribution chain need to upgrade their systems for it to be useful (Lefebvre et al., 2011). Consumer electronics and other expensive products are commonly labeled with radio frequency tags, but using the technology for medicines presents obstacles.
Radio frequency tags are expensive. A 2008 estimate put the cost at $0.11 per tag when bought in lots of 1 million (Wunder and Roach, 2008). After marking each primary package (the smallest unit of packaging) with a radio frequency tag, access to the electronic product code database necessary to decipher the information in the chip costs about $50,000 in the first year (Wunder and Roach, 2008). RFID infrastructure can cost a medium-sized hospital between $200,000 and $600,000 (Yao et al., 2010). The high costs led the U.S. Generic Pharmaceutical Association to call unique serialization “prohibitively expensive” (GPhA, 2012).
Generics companies in many parts of the world share this sentiment, although the generics industry is not at consensus on the question (Barlas, 2005; Jagdale, 2010; Wolinsky, 2006). Even if the technology were cheaper, it is unclear that it would be practical in the markets most hurt by falsified drugs. Chapter 3 explains that the burden of falsified medicines is borne mostly by the poor, especially the poor in low- and middle-income countries, who buy drugs at unlicensed drug stores and unregulated street markets. As a packaging expert explained to Express Pharma, an Indian trade publication, “If you imagine a rural town or village in India—are we really talking sense when we expect an RFID scanner at the outlet?” (Jagdale, 2010).
For the time being, the poorest countries are not likely to use electronic tracking systems below the tertiary or bulk packaging at the warehouse level. Mobile phone verification, an ingenious form of mass serialization, can fill in for an electronic pedigree at a drug’s last step to the consumer. Mobile verification companies such as Sproxil take subscriptions from drug companies and wholesalers. Sproxil provides labels to their clients; each label is marked with a visible serial number and secret code hidden under the scratch-off surface. When the label is attached to the final package, the manufacturer enters the visible serial number in the Sproxil database through a secure web portal. The visible serial number links the product manufacturer, batch number, manufacture, and expiry dates to the secret scratch-off code.
At the point of purchase, the consumer sends a text message or, in some systems, an e-mail to the verification company, the company that makes the scratch-off labels and manages the linked database. The message is sent to a secure server, usually for no charge. An immediate text message response confirms if the secret code number is registered with the manufacturer, or if it is from a shipment reported to have left the legitimate supply chain. Mobile verification of pharmaceuticals is gaining users in 17 sub-Saharan African countries and India (Mukherjee, 2012; Sproxil, 2012; Versel, 2012). An elegant system for assigning unique product numbers, mobile verification empowers consumers to act for their own safety.
Mobile verification cannot prevent fraud, nor is it a substitute for pharmacovigilance and postmarket surveillance. A product could be substandard at the factory but still gain a valid mobile verification label. Mobile verification, however, appeals to good-quality manufacturers, who see the service as an investment in their brand or as a way for consumers to have
confidence in the quality their internal records already show. A more likely problem would be a wholesaler assigning a legitimate label to a falsified drug. Also, the verification service only confirms a product’s identity at the end of the distribution chain, at purchase. These systems cannot track the chain of custody or monitor if the product has been stored and transported properly.

Sproxil standard labels with visible serial number and scratch-off covering the secret code number.
SOURCE: Ashifi Gogo.
A reliable system for tracking and tracing drugs through the distribution chain would greatly reduce the likelihood of falsified and substandard medicines reaching patients. Recent technological advances, such as the use of radio frequency identification and the expansion of mobile phones in developing countries, hold promise for supply chain security. The committee believes that manufacturers and governments should use these technologies to integrate all records of a drug’s chain of custody.
Recommendation 5-2: Congress should authorize and fund the U.S. Food and Drug Administration (FDA) to establish a mandatory track-and-trace system. In the interim, the FDA should convene a working group of stakeholders, including the International Federation of Pharmaceutical Manufacturers and Associations and the Generic Pharmaceutical Association, to promote voluntary track-and-trace for all supply chain actors in accordance with existing guidance.
A mandatory track-and-trace system for drugs is the best way to monitor the chain of custody and protect patients from unsafe drugs. A full track-and-trace system would allow all parties in the drug distribution chain to see a complete record of the product’s path from the manufacturer to the patient (Rappeport and Jack, 2012). Track-and-trace systems place unique demands on drug manufacturers, retailers, and wholesalers. Some may see the imposition of a drug pedigree system as a matter of pharmacy practice, and therefore under the jurisdiction of state boards of pharmacy, the state health department, or another state authority. To avoid confusion on this question, Congress should clearly authorize the FDA to require manufacturers to trace back finished dosage forms to their constituent ingredients. This authority should accompany an increase in funding to allow the agency, which has received many unfunded mandates in recent years, the staffing and technical upgrades necessary to monitor compliance (McCain, 2011; Palmer, 2010).
A track-and-trace system would allow pharmacists to identify suspicious drugs before dispensing them and would facilitate more efficient product recalls (Buynak, 2011; DeCardenas, 2007). Some versions of track-and-trace exist in the system already. Companies tag drug pallets or other bulk packages with radio frequency tags, for example, but use barcodes or other identifiers on smaller units (Lefebvre et al., 2011). Full track-and-trace will require changes to drug primary pack labels and changes to the packaging and repackaging practices at wholesale. These changes have delayed acceptance of full track-and-trace (Yukhananov, 2012).
Nevertheless, consumers and governments have demanded a stronger chain of custody (DeCardenas, 2007). This problem has been lingering for years and should be addressed promptly (Palmer, 2012). Without a clear federal mandate on the problem, companies and state governments work in a state of uncertainty, not knowing where and how to make the necessary investments that track-and-trace will require. If Congress does not set a mandatory requirement, then the competing demands of state track-and-trace systems will create an unmanageable burden for manufacturers, wholesalers, and retailers. For example, in 2015 California will require unique serial numbers on pill bottles and drug vials (GPhA, 2011; Norman, 2012).
In 2011, the FDA held a workshop on tracking and tracing prescription drugs. Stakeholder comments on the workshop mentioned the importance of track-and-trace and “the need for one standard, without variations imposed, for example, by individual states” (Ducca, 2011). There is risk to allowing a piecemeal approach to pharmaceutical track-and-trace. Any track-and-trace system will be an expense to manufacturers and industry, but the expense can be contained by making one national requirement.
Other stakeholders commented on the expense of implementing a national track-and-trace system (GPhA, 2011). Generic manufacturers and drug wholesalers operate on lean margins (Berndt and Newhouse, 2010; CBO, 2007). An increased track-and-trace requirement will put a financial burden on these companies, even if the added cost is low. There are also costs to pharmacies, between $84,000 and $110,000, about 0.88 percent of annual sales (RFID Update, 2008). Therefore, the committee recommends that the FDA bring all industry stakeholders together to work toward voluntary use of track-and-trace technology. This can help control the burden an inevitable shift to drug tracking will put on these businesses.
Tracking primary packages through the drug distribution chain with unique serial numbers is a good defense against criminal infiltration (Ludwig, 2012; Pellek, 2009; Power, 2008). A method of tracking medicines from the factory to the consumer could greatly reduce the chances of a dangerous product being sold at a reputable pharmacy. These solutions are of limited value in the vast pharmaceutical gray markets, however. Ignorance, convenience, and desperation, or some combination thereof, drive patients to unlicensed pharmacies in street bazaars and on the internet. Medicines retail, the last leg of the drug distribution system, is often the most chaotic.
The drug distribution system becomes more disordered as the products leak out of regulated distribution chains. The risk increases as drugs move farther from the manufacturer en route to the vendor. Licensed pharmacies and dispensaries can control the quality of their stock, at least insomuch as they can trust their wholesalers. There are no such efforts at quality control in the unlicensed market. Unlicensed vendors are often minimally educated. They may approach medicines dispensing as any other sales job and not want a customer to leave without making a purchase. In general, these vendors exploit the chaos inherent to street markets and dry goods shops in low- and middle-income countries and to online drug stores in middle- and high-income ones. Their stock is poor because the stockists are either unable or unwilling to judge quality.
Their customers are similarly ill-equipped to evaluate the dangers of buying medicine outside of controlled chains. Unlicensed medicine vendors fill a need, especially in poor countries, when time, expense, and distance impede access to registered pharmacies. Internet pharmacies can fill a similar void, appealing to customers eager to save time and money or to purchase discretely. Both types of market are dangerous and more similar than they may appear at first glance. A Chinese military pharmacist described the appeal of unlicensed medicine shops: “There are people who choose to seek medical help from these places, possibly because of lower prices or privacy concerns, which may increase their chances of getting counterfeit products” (Quingyun, 2012). The observation is true of all unregulated pharmacies. Street markets and the internet are a main source of falsified and substandard medicines for patients around the world (WHPA, 2011). The committee believes some changes to medicines retail could improve the world’s vast and disorganized pharmaceutical bazaars.
Key Findings and Conclusions
• There are few high-quality, licensed drug shops in developing countries, especially outside of cities.
• Drug sellers in developing countries often do not have the training to oversee the purchasing and dispensing of medicines.
• Drug seller accreditation and franchising programs have improved drug retail in some developing countries. Task shifting and vocational training in medicines retail can alleviate the shortage of pharmacists. Government incentives can help keep trained staff in underserved areas.
• Internet pharmacies are often the disorganized drug markets of developed countries. Only 7 percent of countries have a system for verifying legitimate online drug stores.
• In the United States, the expense of drugs contributes to the draw of online drug stores.
Unregistered Pharmacies in Low- and Middle-Income Countries
The packaging of falsified drugs contains clues that are lost in unregulated pharmacies (Dondorp et al., 2004). Epidemiological research suggests that falsified medicines are often sold without packaging (Basco, 2004), by street vendors (Tipke et al., 2008) or by patent medicine dealers (Onwujekwe et al., 2009). The dangers of these vendors are clear: Some sell loose pills from large plastic bags or cut apart and subdivide blister packs; none has training in the proper storage, buying, or dispensing of medicines. Even when packaged medicines happen into these markets, their customers are not often sophisticated enough to analyze packages for irregularities. Illiteracy is a known predictor of buying falsified and substandard drugs (Erhun et al., 2001), and it is the poorest and least educated patients who buy medicines from unauthorized dealers (Nkamnebe, 2007). As David Peters and Gerald Bloom observed, “The wealthiest people in developing
nations tend to use highly regulated services. The poor, by contrast, usually seek care elsewhere” (Peters and Bloom, 2012, p. 164).

Medicine for sale in a Côte d’Ivoire street market.
SOURCE: Issouf Sanogo/Getty Images.
Shortage of Quality-Assured Drug Shops
A simple lack of alternatives pushes the poorest consumers to buy medicine at unregulated shops. High taxes and overhead costs make a difficult business environment for pharmacists; there are few incentives to work in underserved areas (McCabe, 2009). Research on drug shops in rural Tanzania found that despite gross regulatory violations, including stocking of controlled medicines, selling loose tablets, selling of unregistered drugs, and near universal lack of qualified staff in sales, the shops operated with the government’s tacit permission (Goodman et al., 2007).
The regulatory authority might not have enough inspectors to monitor all drug shops on the prescribed timetable (Goodman et al., 2007; MSH, 2012). The Ghanaian Pharmacy Council, for example, inspects only about 20 percent of all drug sellers annually (Segrè and Tran, 2008). Inspectors commonly find the shops selling restricted medicines, the products that bring in about half of the stores’ total revenues (Segrè and Tran, 2008). The low likelihood of being caught in a violation and the social and financial incentives to ignore regulations outweigh the threat of punishment for many shopkeepers (Segrè and Tran, 2008). When infrequent inspection does identify violations, regulators are loath to enforce the rules, as this would remove from many communities their only medicine store (Goodman et al., 2007).
These inspectors realize that even unlicensed drug shops serve a purpose in developing countries, especially outside of cities, where there are no licensed pharmacies (MSH, 2012). People in rural areas use these shops for more than just retail; the shopkeepers are a source, sometimes the sole source, of health advice in their communities (Anderson et al., 2009; Azhar et al., 2009; Bustreo et al., 2003; Goel et al., 1996; Peters and Bloom, 2012). The accuracy of the information they give is doubtful, however (McCabe, 2009). In some parts of the world, so-called pharmacy assistants may have less than a middle-school education (Goel et al., 1996). These shopkeepers are not properly trained for medicines retail, let alone patient counseling.
Shortage of Trained Pharmacy Staff
Poor supervision of medicines retail allows falsified and substandard products to circulate. Pharmacists oversee the responsible purchase of drugs from legitimate wholesalers. They watch for suspicious products in the licit supply chain, educate patients on warning signs of problem drugs, and are
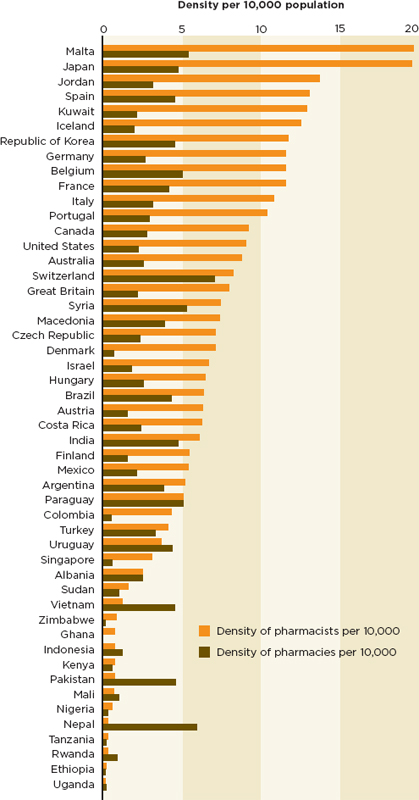
the first line of postmarketing surveillance (Ziance, 2008). Too few people are trained to do this job in the parts of the world where falsified and substandard medicines are a systemic problem. As Figure 5-4 shows, poorer countries often have more pharmacies than pharmacists, sometimes many times more; in some counties even these estimates may be inflated (FIP, 2009). The International Pharmaceutical Federation (known by the French acronym FIP) estimates that only slightly more than half of all pharmacists are active in the workforce (FIP, 2009).
The WHO commented on this problem in a 2010 report, observing that many types of medicines outlets in sub-Saharan Africa are not managed by pharmacists (WHO, 2010). In general, the region has a pharmacist for every 23,375 people; 75 percent of these pharmacists live in Nigeria or South Africa (Kome and Fieno, 2006). After excluding these countries, the ratio is closer to 1:64,640 (Kome and Fieno, 2006). Though the shortage is especially acute in sub-Saharan Africa, the ratio of pharmacists to population in most low- and middle-income countries falls far short of the WHO-recommended 1:2,000 (Azhar et al., 2009). National estimates in Malaysia (1:6,207) and Pakistan (≈ 1:19,748) also suggest serious problems (Azhar et al., 2009).
The world distribution of pharmacists shown in Figure 5-5 indicates a dearth of pharmacy professionals in sub-Saharan Africa and Southeast Asia. This map fails to capture the relative privation of rural areas, where far fewer pharmacists per person work (Hawthorne and Anderson, 2009). In India, for example, most pharmacists work in the country’s drug manufacturing sector (Mohanta et al., 2001). So, although the national average ratio of pharmacists to population is 1:1,785, this number masks regional disparities (Basak et al., 2009). In states with less manufacturing the ratio hovers around 1:4,000 (Basak et al., 2009). All states struggle with a pronounced rural-urban imbalance. Few pharmacists work outside of cities, and almost none works in remote areas (Basak et al., 2009). This problem is not unique to India. Survey data indicate that managers around the world find it difficult to fill pharmacy positions in the public sector and outside of urban areas (FIP, 2006).
Rural disadvantage starts with education. Pharmacy schools are in cities and therefore attract urban students who have little interest in working in the countryside or reason to move there after graduation (Anderson et al., 2009). Furthermore, pharmacy training in many low- and middle-income countries, especially in Asia, qualifies people to work in industry (Azhar et al., 2009; Mohanta et al., 2001). A critic of the Indian pharmacy education system observed, “Community pharmacy practice does not exist in its true sense, only drug selling” (Mohanta et al., 2001, p. 810).
Improvements to the practice of community pharmacy would curtail the sale of poor-quality drugs in low- and middle-income countries. However, having practicing community pharmacists oversee all pharmacies is an unrealistic solution in the parts of the world most hurt by falsified and substandard pharmaceuticals. Viable short-term solutions should aim to increase the reach of legal drug shops staffed by sellers with appropriate minimal training. The committee believes that governments and the private sector both have important roles in assuring a safe medicine supply in underserved areas.
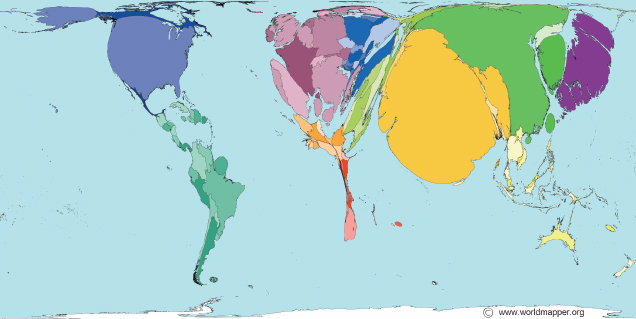
FIGURE 5-5 The world’s pharmacists, pharmacy technicians, and pharmacy assistants, 2006 data.
SOURCE: Worldmapper, 2006. © Copyright SASI Group (University of Sheffield) and Mark Newman (University of Michigan).
Recommendation 5-3: Governments in low- and middle-income countries should provide an environment conducive to the private sector establishing high-quality medicines retail in underserved areas. Government incentives could encourage this. To the same end, governments, the World Health Organization, and the International Pharmaceutical Federation should support national pharmacy councils and education departments to train tiers of pharmaceutical personnel.
The committee recognizes two main problems with medicines retail in low- and middle-income countries. First, there are not enough high-quality vendors, driving customers to street markets and unlicensed shops. Second, there are not enough trained staff to oversee the responsible purchasing and
dispensing of medicines. This is a problem in both rural areas and slums (Azhar et al., 2009; Riley et al., 2007).
The committee recognizes that supplying cheap, quality-assured drugs to the population is not a realistic goal for many governments, especially in poor countries. These countries can encourage private-sector investment in medicines and facilitate task shifting among pharmaceutical staff, however. Examples of successful programs that improved medicines access follow.
Providing safe, affordable medicine to the population is not within the budget of many countries. The private sector, however, will invest in medicines retail if there is a good business reason to do so. Governments can take steps that would encourage private-sector investment and create an environment where responsible private drug sellers will thrive.
One promising example of government and private-sector investment in medicine retail is the Accredited Drug Dispensing Outlet (ADDO) program in Tanzania (MSH, 2012). The Tanzanian regulatory authority was eager to discourage illegal stocking and improve dispensing practices at unregistered drug stores through an accreditation program (MSH, 2012; Rutta et al., 2011). The Bill & Melinda Gates Foundation funded the program, which used a combination of training, incentives, and creation of consumer demand to drive changes in the private sector (MSH, 2005; Rutta et al., 2011). Trainers from the Tanzanian Ministry of Health educated drug shopkeepers on proper dispensing techniques, medicines storage, national regulations, business skills, and ethics (MSH, 2005; Rutta et al., 2011). The government offered low-interest loans for improving drug shops, many of which had been stuffy, hot, humid (therefore unsuitable for medicine storage), and not properly secured against theft (MSH, 2012). Participants who met the program’s standards were rewarded with legal authority to sell some controlled drugs (MSH, 2005). The government has made efforts to increase the ADDO customer base, allowing ADDOs in some districts to dispense subsidized artemisinin combination therapies (Rutta et al., 2011). The subsidy also ensures that good-quality antimalarials are as affordable to poor customers as the ubiquitous falsified ones.
The ADDO certification program conferred a competitive advantage on participating shopkeepers. A widespread social marketing campaign on access to malaria drugs promoted the outlets as reliable vendors (Hetzel et al., 2007). This publicity helps build consumer confidence in the program and create demand for the outlet’s services. An emphasis on customer service and good management in the accreditation process gave the shops a professional quality that enhanced consumer satisfaction.
Franchising is another private-sector approach to improving drug retail. The Ghanaian Social Marketing Foundation, a national NGO, founded the CareShop franchising program to improve access to good-quality medicines in Ghana (Segrè and Tran, 2008). The foundation recruited franchisees from among licensed chemical sellers, attracting them with an improved supply chain. The drug sellers had been spending an average of 30 percent of their time purchasing from an unreliable wholesale market (Segrè and Tran, 2008). The franchiser guaranteed supply and direct delivery of the shop’s entire inventory, thereby saving the shopkeeper time and about $227 per year in travel expenses (Segrè and Tran, 2008). This system also puts wholesale buying in the hands of a purchaser qualified to judge product quality. The purchaser’s frequent large orders command a collective buying power that controls costs.
Customer loyalty to the CareShop franchise grew quickly in the program’s first 4 years (Segrè and Tran, 2008). With 270 outlets, CareShop is one of the largest drug store franchises in Africa (Segrè and Tran, 2008). Box 5-3 present profiles of two typical CareShop franchisees.
Drug seller accreditation improves medicine quality at the place most patients will, from convenience and habit, turn to first (MSH, 2012). In Kenya, the United States Agency for International Development funded a public–private drug seller accreditation program that increased the rational dispensing of antimalarials among participating shopkeepers (MSH, 2012; Tavrow et al., 2002). Drug seller accreditation requires making the best use of the shopkeepers already selling medicines. Part of the project’s success came from its training of motivated drug shopkeepers.
Training and credentialing of drug shop staff must accompany any successful accreditation program. Task shifting, delegating responsibilities from doctors, nurses, and pharmacists to less specialized lay health workers, is a way to improve the shortage of health professionals in developing countries (Fulton et al., 2011; WHO, 2008). There is international support for task shifting in pharmacy, especially in the training of pharmacy technicians, which is often a kind of post–high school vocational training in dispensing medicines (Bureau of Labor Statistics, 2012; Hawthorne and Anderson, 2009).
International organizations such as the FIP and the WHO cannot dictate the best training programs and levels of pharmacy staff needed in hundreds of different countries (Anderson et al., 2009). They can, however, help ministries of education and national pharmacy councils identify the competencies a vocational pharmacy worker would need in their country. Their efforts in-country should aim to identify the competencies and minimum training necessary to work in medicines retail. They might also consider developing chains of supervision wherein minimally educated staff manage stock and then report their needs up to someone who is qualified to identify good-quality wholesalers and buy from them. The committee believes that national pharmacy councils are best able to articulate what the proper reporting chain should be in their country and what minimum qualifications their countries’ patients will accept. The minimum training for a drug dispenser or pharmacy technician in rural Canada will be different from what is suitable to rural Nepal. In any case, there should be emphasis on vocational training to credential medicine shopkeepers and include them in the health system.
BOX 5-3
CareShop Franchisee Profiles
Rose Kaade Apreku
“Upon the completion of primary school, Ms. Apreku worked on her family farm prior to saving enough money to start her own licensed chemical shop. She estimates that it cost $300 to open her shop. Ms. Apreku explained that the CareShop franchise drastically improved her business in several ways. First, she is able to advise her customers more confidently on the nature and appropriate treatment of their afflictions. Second, she is able to offer her patients better customer service through complementary selling techniques. Lastly, she is able to track her sales and pricing using a ledger. Ms. Apreku’s sales are five times higher than they were prior to conversion, and she runs the store from 7 am to 10 pm every day with the help of Adams, her son (also pictured)” (Segrè and Tran, 2008, p. 31).

Kofi Asiam
“Mr. Asiam inherited his chemical shop, a converted space attached to his home, from his father and was a licensed chemical seller for nearly 20 years prior to his conversion to CareShop. Commenting on the difference between his business before and after conversion, Mr. Asiam notes, ‘It is a tremendous difference. CareShop has enlightened us. Our customers now see our place as a beautiful place.’ During his renovation process, Mr. Asiam spent roughly $200 on improvements, which include ceiling fans, a refrigerator, and glass display cases. These are important differentiators because Mr. Asiam has four competing LCS [licensed chemical sellers] within a kilometer of his own shop” (Segrè and Tran, 2008, p. 31).

There is evidence that task shifting can alleviate the pharmacist shortage in developing countries. In Malawi, an emergency training and credentialing program for health workers increased the number of pharmacy technicians by 84 percent between 2004 and 2009 (O’Neil et al., 2010). Malawian pharmacy technicians supervise pharmacy attendants, the lower-level staff who stock and dispense drugs, allowing the technician more time for stock management and other more complicated tasks (Shulman et al., 2009). Because of task shifting, pharmacy technicians monitor adherence to antiretroviral treatment in Zambia and tuberculosis treatment in urban Uganda (Bolton-Moore et al., 2007; Mafigiri et al., 2011; Stringer et al., 2006).
Training and task shifting programs that recruit minimally educated shopkeepers are also promising. For example, Kilifi, Kenya, is a rural area of 70,000 people with 15 licensed dispensaries and pharmacies and 316 general stores that sell medicine (Marsh et al., 2004). A training program for Kilifi shopkeepers more than doubled the proportion of antimalarials sold in adequate dosage (Marsh et al., 2004). A similar Kenyan program trained mobile wholesalers or wholesaler counter attendants to teach drug retailers about correct malaria drug dosing (Tavrow and Shabahang, 2002). After 6 months, mystery shoppers were nine times more likely to receive the correct drugs in the correct dose from retailers who had participated in the program (Tavrow and Shabahang, 2002).
Giving Incentives to Pharmaceutical Personnel
Using workers more efficiently could do much to remedy chaotic drug retail in low- and middle-income countries, but there is also a problem of retaining trained staff in underserved posts. Even minimal technical training confers a competitive advantage in the labor market, especially in poor countries (Attanasio et al., 2009; GTZ, n.d.; Kasipar et al., 2009). Newly minted pharmacy technicians or drug dispensers can easily leave their rural
assignment for better paying jobs in places with a higher standard of living and better opportunities for their children. This pattern can undermine the best efforts to improve rural-urban equity and should be discouraged, while respecting the individual right to emigrate.
Governments should reward service in underserved areas and attempt to mitigate the hardships of these posts. The people who take advantage of training programs are bright and ambitious. They naturally want their children to be at least as educated as they are. Scholarships for the children of pharmacy staff in underserved areas could assuage fears that a rural posting puts their children at a disadvantage. Efforts to guarantee good schooling for children, possibly through boarding schools or scholarships, could remove a barrier to rural service (Rao et al., 2010). Better salaries can draw trained staff to cities, but tax breaks and hardship pay can alleviate this obstacle (CSG, 2008; Rao et al., 2010).
Health workers also have concerns about quality of life and physical hardships in rural posts (Rao et al., 2010). Subsidized housing or provision of modern living quarters could help in places where this is a common concern. It is also possible to recruit pharmacy technicians and pharmacy assistants from underserved communities. Training students from rural and remote areas is a known way to reduce attrition in these posts (Rabinowitz et al., 1999). The Australian Rural and Remote Pharmacy program has successfully increased service to rural and isolated communities, in part through giving scholarships to students from rural backgrounds (see Box 5-4).
Internet Pharmacies in Middle- and High-Income Countries
Disorganized medicines retail is not confined to developing countries. The previous section describes the large gray market for medicines in bazaars and unlicensed drug shops in low- and middle-income countries. The internet serves the same purpose, but mostly in middle- and high-income countries. Illegitimate internet pharmacies are similar to unlicensed drug shops both in the quality of the products they stock, which is poor, and in the lack of official oversight of their operations (Crawford, 2003). And, because the internet facilitates easy international sales, online drug stores have spread the problem of falsified and substandard drugs “from small, unprofitable, markets in developing nations to the [drug] industry’s most lucrative markets” (Lybecker, 2007, p. 512).
The Legality of Internet Drugs Retail
A 2011 survey of 114 WHO member states found that the majority of countries had no laws governing the operation of internet pharmacies (WHO, 2011). Of those countries that have legislation about internet pharmacies, more disallow (19 percent) than allow them (7 percent) (WHO, 2011). Figure 5-6 shows the geographic breakdown of the 30 countries that have legislation on the operation of internet pharmacies.
BOX 5-4
The Australian Rural Pharmacy Workforce Program
The Australian government began the Rural Pharmacy Workforce Program in 1999 as part of a broader effort to improve rural and indigenous people’s health care (Australian Government, 1999). The program aims to improve access to pharmacy services in rural or remote regions and includes a variety of initiatives to improve recruitment and retention of rural pharmacists.
One part of recruitment is raising awareness of rural pharmacy as an attractive career choice (KPMG, 2010). Recruitment materials emphasize the benefits of a rural career, including increased patient interaction, diverse career paths, a more laid-back lifestyle, close-knit community life, altruism, and excitement (PGA, 2011). A 2009 DVD campaign promotes the same messages to high school students (KPMG, 2010).
The program also increases pharmacy students’ exposure to rural work during their training. Australian pharmacy students work in community or hospital pharmacies as part of their studies. Most will do so in an urban area (PGA, 2012b). By paying housing and transportation costs, the program allows universities to place students in rural internships (PGA, 2012b). Positive internship experiences encourage students to practice in rural areas during their careers (FIP, 2009).
The program also supports students from rural backgrounds to pursue pharmacy degrees. Rural students are twice as likely to return to rural areas after graduation as students from cities (FIP, 2009). The Rural Pharmacy Scholarship Scheme awards rural students $10,000 per year of study and pairs them with a mentor who also works in rural pharmacy (PGA, 2012a).
Professional isolation can lower retention of rural pharmacists (KPMG, 2010). A continuing education program aims to avoid this pitfall by funding rural pharmacists’ professional development (KPMG, 2010). Another successful initiative to improve retention is the program’s emergency locum service. This offers rural pharmacists direct, 24-hour access to replacement pharmacists in emergency situations when they need to leave their practices (KPMG, 2010). The service ensures that rural pharmacies remain open and that communities have continued pharmacy access (FIP, 2009).
The FIP has praised the Australian program as the world’s most comprehensive rural pharmacy improvement (FIP, 2009). An independent evaluation found that both rural pharmacists and consumers valued its initiatives (FIP, 2009). The program is credited with expanding pharmacy service in rural Australia by 13 percent, eight times faster than the national average (FIP, 2009).
One of the most common tools for governing internet pharmacy is accreditation and certification, though only 7 percent of responding countries have a national process for certifying, accrediting, or regulating internet pharmacies (WHO, 2011). Most of these countries are in Europe, and all are either in the World Bank’s high-income or upper-middle-income groups (WHO, 2011). A few countries have an accreditation process for their own internet pharmacies, but internet commerce is transnational. Legislation on internet pharmacies outside of a country’s jurisdiction is even less common; only 25 percent of the 144 countries surveyed have legislation governing the purchase of medicine from foreign countries (WHO, 2011). The countries that allow purchasing from online pharmacies abroad are mostly in Europe (WHO, 2011), where the single market has hastened consideration of trans-national commerce. Perhaps concern about the practicality of enforcing laws against internet drug sales prevents countries from passing them. It may also seem futile to ask internet drug sellers to observe the same standards registered pharmacies do, such as requiring doctor’s prescription for controlled medicines, when “national rules banning the sale of drugs without a prescription can be easily overcome” (Levaggi et al., 2012, p. 245).
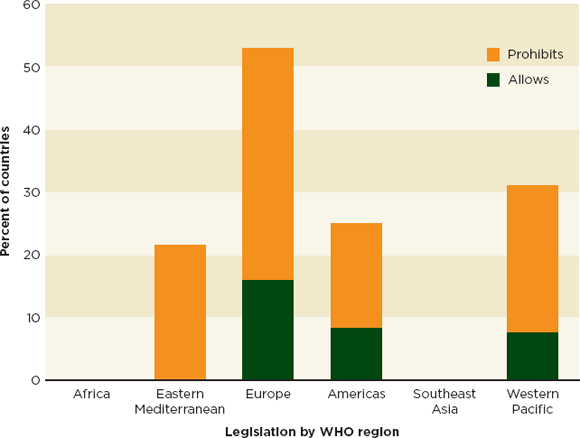
FIGURE 5-6 Legislation of internet pharmacy operations by WHO region.
NOTE: WHO = World Health Organization.
SOURCE: WHO, 2011.
Just as often, restrictions and quality controls for online pharmacies are not, in fact, violated because many internet pharmacies operate out of countries that have no such restrictions. Rogue internet pharmacies, those that sell dangerous products and avoid inspections, commonly operate from low- and middle-income countries (Baert and De Spiegeleer, 2010; FDA, 2005). The United Nations Office on Development and Crime (UNODC) reckons India supplies the most drugs for illegal online pharmacies (UNODC, 2010). Although regulatory agencies can ask foreign governments to close online drug stores, it is difficult to prevent them from reopening at a different address (Ivanitskaya et al., 2010). Because the products are sent through courier or postal services, customs and border officers may also stop the imported drugs at the port of entry (Ivanitskaya et al., 2010).
The Attraction of Internet Pharmacies
Some of the more reputable-looking internet drug sellers keep up the pretense of having patients complete a health questionnaire before buying drugs, but many do not (Ivanitskaya et al., 2010; Orizio et al., 2011). Far more variable is the requirement for a doctor’s prescription (Orizio et al., 2011). A European study found 62 percent of medicines bought online to be falsified or substandard or both (EAASM, 2008). The U.S. Government Accountability Office found that only about 20 percent of the 21 online pharmacy samples were falsified, though another three samples were possibly degraded (GAO, 2004).
Bostwick and Lineberry proposed four main categories of customers at internet pharmacies: bargain hunters, the poor or elderly, the “lifestyle libertines” who prefer to self-prescribe, and drug addicts (Baert and De Spiegeleer, 2010; Bostwick and Lineberry, 2007). Of these groups, addicts are the least likely to purchase prescription drugs online (Inciardi et al., 2010). Internet drug stores cater to people who like to buy drugs without, or even against, a physician’s advice (Levaggi et al., 2012), or to those who cannot otherwise afford their medication (Baert and De Spiegeleer, 2010). Table 5-3 shows other perceived advantages and disadvantages of online pharmacies.
Other online shoppers seem motivated by a belief, sometimes a mistaken one, that internet pharmacies sell cheaper drugs. In a sample of 19 websites, Levaggi and colleagues found that one-third of their drug orders never arrived, though no company issued refunds (Levaggi et al., 2012). On average, the investigators paid more for the drugs that never arrived than for those that arrived (€0.83 per pill for those that arrived, €1.27 for those that did not). Investigators cited other hidden costs, including shipping and customs fees, as well as the cost of time spent waiting for the slow transactions to process (Levaggi et al., 2012). More importantly, of the 13 pharmacies that filled orders, only two were not of substandard quality (Levaggi et al., 2012).
TABLE 5-3 Advantages and Disadvantages of Internet Pharmacies
|
Advantages of Online Pharmacies |
Disadvantages of Online Pharmacies |
|
Available 24 hours per day, 7 days per week |
Limited participation by third-party payers |
|
Lessened perceptions of intimidation when obtaining embarrassing or sensitive drugs |
Pharmacists not always immediately available online to answer important questions patients may have |
|
Some sites allow patients to check medication profiles online |
Concerns about issues of privacy of information |
|
Medications delivered directly to patient’s home via standard or special mail |
Concerns about security of financial information transmitted |
|
Convenience |
Questions about the integrity of drugs shipped |
|
Medication availability to patients with physical or other disabilities that hinder retail patronage |
Questions about the quality of drug information provided |
|
|
May bypass the pharmacist/patient and physician/patient relationship |
|
|
Difficult to ascertain whether licensed practitioners are dispensing drugs or providing consultations |
SOURCE: Crawford, 2003.
Levaggi and colleagues are an Italian research group. They criticized the false economy of online drug sellers in part because the products they bought sell for less in Italian regulated, storefront pharmacies (Levaggi et al., 2012). For consumers in the United States, the cost-to-benefit analysis is not as clear. A 2001 study of Parkinson’s disease medications found online drug stores offered substantial savings off U.S. list prices; brand-name drugs were 7 to 58 percent cheaper, generics 31 to 76 percent less expensive (Wagner et al., 2001). A study of American shoppers at internet pharmacies found that 37.6 percent perceive the costs as one of the main advantages of internet drug sellers (Crawford, 2003).
Some internet pharmacy shoppers choose to bypass the regulated medicine channels out of arrogance or ignorance. Others understand the risks but have no better alternative. A Forbes magazine contributor explained, “My wife needs the meds to stave off a recurrence of cancer, so avoiding [online pharmacies] is not an option” (Wasik, 2012). The risks of online purchases are, especially in the United States, inextricable from larger questions of affordable drug pricing (Financial Times, 2012). Every year more Americans, and others accustomed to using the internet for bargain shopping, import “incremental amounts” of medicines to their countries though gray market internet purchases (Laven, 2006; Shepherd, 2007b). Many of these patients presumably struggle with the dilemma the UNODC described: “In some cases, cheaper but lesser quality medication is better than nothing; in other cases, it clearly is not” (UNODC, 2010, p. 184).
Distinguishing Rogue Pharmacies from Legitimate Ones
In late September 2012, Interpol, an intergovernmental organization for police cooperation, organized an international raid of online pharmacies
(Interpol, 2012a). The operation, known as Pangea V, is part of Interpol’s enforcement against pharmaceutical crime. Regulatory, customs, and law enforcement agencies in 100 other countries took part in the operation, which shut down over 18,000 internet pharmacies and led to 79 arrests (FDA, 2012a; Interpol, 2012b; Shelton, 2012; TechNewsDaily, 2012). In the United States, the FDA estimates that Pangea V led to closing of more than 4,100 illegal online drug sellers (FDA, 2012a).
A GlaxoSmithKline ad campaign about the dangers of online pharmacies purporting to sell Canadian medicines.
SOURCE: Lybecker, 2007.
By the agency’s own admission however, such efforts are futile. Ilisa Bernstein, acting director of the FDA Center for Drug Evaluation and Research, explained, “We don’t know how many websites are out there, but there are a lot more … they can pop up days or weeks later using another URL and another way to deceive consumers” (Shelton, 2012). Although there is value to shutting down criminal online drug stores, in the longer term, it may be more helpful to recognize the e-commerce division of legitimate pharmacies.
The challenge of recognizing legitimate online pharmacies As Table 5-3 mentions, online pharmacies can be a boon to people who live in remote areas or who cannot manage in-person shopping. These consumers need reliable advice on how to navigate the confusing internet marketplace. There are many reputable pharmacies with licensed e-commerce divisions, but identifying them can be difficult.
Both the United States and Europe have accreditation programs for legitimate online pharmacies. In the United States, the National Association of Boards of Pharmacy runs the Verified Internet Pharmacy Practice Sites (VIPPS) accreditation program (NABP, 2012b). To earn accreditation, online pharmacies must comply with state licensing requirements for both the state the pharmacy is in and for all the states in which it dispenses medicines (NABP, 2012e). Chief among these requirements are the authentication of prescriptions, observance of quality-assurance standards, and submission to regular state inspections (NABP, 2011, 2012e). Accredited pharmacies are rewarded with the VIPPS seal, but because the seal would be easily copied, the project website lists both certified pharmacies (the good list) and known fraudulent ones (the bad list) (Ivanitskaya et al., 2010; NABP, 2012c,d). Most VIPPS-certified pharmacies are online divisions of national chain drug stores (deKieffer, 2006). The program also recognizes some independent internet businesses such as Medco’s Express Scripts (Medco Health, 2012). The certification process is not perfect, however. An online drug store later exposed for dispensing narcotics inappropriately briefly enjoyed VIPPS accreditation (deKieffer, 2006). As of 2012, 30 pharmacies have earned VIPPS accreditation (Bate et al., 2012).
Similarly, the European Medicines Agency (EMA) has made controlling internet sales one of the four pillars of their falsified medicines legislation (Cockburn, 2011). They are planning a consumer education campaign starting in the summer of 2013 that will empower consumers to give their business to a reputable online pharmacies
(Cockburn, 2011; EMA, 2011; personal communication, David Cockburn, January 18, 2013). In addition to patient education materials, the agency’s website will link patients to national regulatory authorities’ websites that will maintain a list of authorized online pharmacies. The authorized pharmacies will in turn post an accreditation logo that links back to the regulatory agencies’ website (Cockburn, 2011). The Royal Pharmaceutical Society of Great Britain also has a registration logo that legitimate online pharmacies can display (EAASM, 2008).

Customs and border police inspect packages during Pangea V.
SOURCE: © Carabinieri NAS (Italia).
The VIPPS system and its EMA counterpart rely on accreditation from trusted national health organizations. The committee agrees that independent accreditation is a useful tool for consumers trying to make sense of the chaotic world of online medicine retail. Even the accreditation can add to confusion, however. A 2008 study found that about 20 percent of online drug stores displayed an approval of a regulatory or accrediting agency, but about 80 percent of these approvals were phony (Mayor, 2008).
Although there are many good-quality online drug stores, the illicit
ones outnumber them. The American National Association of Boards of Pharmacy found the vast majority (97 percent) of a sample of more than 10,000 online drug sellers they examined to be in violation of pharmacy laws and standards (DrugTopics, 2012). Furthermore, the internet confuses the cues customers might usually use to judge quality. There is no pharmacist to counsel patients on online pharmacy, and a website claiming affiliation with a respected local chain might be lying. A 2005 study found that of the more than 11,000 online drug stores claiming to be Canadian, only 214 were registered with the Canadian authorities (Clabaugh, 2005).
Proponents of online drug stores maintain that consumer demand can keep online sellers to standards by driving the development of “private verification services” (Bate et al., 2012). Research indicates, however, that even patients with a sophisticated understanding of both health and technology are poorly equipped to judge the quality of online drug sellers. Between 2005 and 2008, 1,914 undergraduates completed the e-Health literacy assessment, rating highly suspect online drug sellers (Ivanitskaya et al., 2010). The investigators reasoned that if even American college students, who are savvy users of technology, and undergraduate students of health science, who are well educated about health, could be deceived by internet pharmacies, then how much greater the risk to the average consumer (Ivanitskaya et al., 2010)? They found that participants were quickly deceived by professional-looking websites and unsuspicious of very low list prices. Sixty percent of respondents attributed the low prices to fewer regulatory restrictions; 16 percent thought people should be advised to buy drugs on the internet to save money (Ivanitskaya et al., 2010).
This committee commends the NABP on the VIPPS accreditation system for online pharmacies. This system should be more widely promoted as a valuable consumer tool. Beyond promoting the verified pharmacies, it is unclear what novel actions could better control internet drug sales. One possible solution is asking Congress to make all online pharmacies illegal except for the VIPPS-accredited ones (Shelton, 2012). There is no value,
however, to an unenforceable law, as legislation about internet pharmacies would be.

The Verified Internet Pharmacy Practice Sites logo.
SOURCE: NABP, 2012.
To complicate the problem, even unlicensed internet pharmacies have advocates who believe the stores empower them to avoid artificially inflated medicine prices (Wasik, 2012). They may maintain that individual importation from foreign pharmacies improves the competiveness of the drug market (Shepherd, 2007b). Taking advantage of these countries’ price controls could, they reason, drive down prices in the United States (Shepherd, 2007b). However, internet importation is, at best, an exploitation of other countries’ price controls (Shepherd, 2007a). At worst, it exposes patients to an unregulated medicine supply. Encouraging internet importation is also a shortsighted solution to American problems with drug pricing. As the director of the University of Texas Center for Pharmacoeconomic Studies explained, “Our high drug prices are our problem … not the problem of Canada or any other country” (Shepherd, 2007a, p. 1290).
Trustworthy, accredited online drug stores do not sell medicine more cheaply than any other registered pharmacy would. Steep online discounts attract customers but come from illegitimate vendors. In the United States, reducing the draw of unlicensed drug stores requires either regulating the internet, a fool’s errand, or completely renegotiating national drug price controls, which is outside the scope of this report (deKieffer, 2006).
Controlling the sale of medicine is a complicated problem the world over. Some unlicensed vendors work in street bazaars; others sell on the internet. In either case, regulatory accreditation can help consumers by identifying the good-faith sellers. The NABP’s VIPPS accreditation does this for trustworthy online drugstores in the United States; the EMA is working on a similar program for Europe. In developing countries, the most useful drug-seller accreditation programs are those that work with the private sector to improve retail, especially in rural areas and slums. Training and task shifting could also improve the quality of patient counseling and drug dispensing in low- and middle-income countries.
Consumer confidence in drug safety could be improved by strengthening the ability of every intermediary on the supply chain to track drugs’ movement from the manufacturer to the patient. Understanding a drug’s history and path is important, especially as it moves through the unpredictable wholesale market. The United States needs stricter drug wholesale and tracking requirements. Implementing changes to the American system would build momentum for stronger medicines regulation around the world.
Aleccia, J. 2011. Half of hospitals buy back-door drugs, new survey shows. NBC News, August 26. http://www.nbcnews.com/id/44280296/ns/health-health_care/#.uvmyshzvtdr (accessed March 27, 2013).
Altunkan, S. M., A. Yasemin, I. T. Aykac, and E. Akpinar. 2012. Turkish pharmaceuticals track & trace system. Paper read at Health Informatics and Bioinformatics (HIBIT), 2012 7th International Symposium, April 19-22, 2012.
Amin, A., and R. Snow. 2005. Brands, costs and registration status of antimalarial drugs in the Kenyan retail sector. Malaria Journal 4(36). DOI: 10.1186/1475-2875-4-36.
Anderson, C., I. Bates, D. Beck, T. P. Brock, B. Futter, H. Mercer, M. Rouse, S. Whitmarsh, T. Wuliji, and A. Yonemura. 2009. The WHO UNESCO FIP pharmacy education taskforce. Human Resources for Health 7(45). DOI: 10.1186/1478-4491-7-45.
Appleby, J. 2003. Fake drugs show up in U.S. pharmacies. USA Today, May 14.
Attanasio, O. P., A. D. Kugler, and C. Meghir. 2009. Subsidizing vocational training for disadvantaged youth in developing countries: Evidence from a randomized trial. Bonn, Germany: Institute for the Study of Labor.
Australian Government. 1999. Looking after pharmacy in rural and remote Australia. http://www.health.gov.au/internet/main/publishing.nsf/Content/health-mediarel-yr1999-mw-mw99010.htm (accessed October 5, 2012).
Azhar, S., M. Hassali, M. Ibrahim, M. Ahmad, I. Masood, and A. Shafie. 2009. The role of pharmacists in developing countries: The current scenario in Pakistan. Human Resources for Health 7(1):54.
Baert, B., and B. De Spiegeleer. 2010. Quality analytics of internet pharmaceuticals. Analytical and Bioanalytical Chemistry 398(1):125-136.
Barlas, S. 2005. Drug companies on different RFID frequencies. http://www.packworld.com/package-type/thermoformed-packaging/drug-companies-different-rfid-frequencies (accessed September 28, 2012).
———. 2011a. FDA’s track-and-trace workshop talks Turkey about item-level pharmaceutical packaging. Healthcare Packaging, March 15.
———. 2011b. Track-and-trace drug verification: FDA plans new national standards, pharmacies tread with trepidation. Pharmacy and Therapeutics 36(4):203-204, 208, 231.
Basak, S., J. van Mil, and D. Sathyanarayana. 2009. The changing roles of pharmacists in community pharmacies: Perception of reality in India. Pharmacy World & Science 31(6):612-618.
Basco, L. K. 2004. Molecular epidemiology of malaria in Cameroon. XIX. Quality of antimalarial drugs used for self-medication. American Journal of Tropical Medicine and Hygiene 70(3):245-250.
Bate, R. 2012. Phake: The deadly world of falsified and substandard medicines. Washington, DC: AEI Press.
Bate, R., K. Hess, and L. Mooney. 2010a. Antimalarial medicine diversion: Stock-outs and other public health problems. Research and Reports in Tropical Medicine 1:19-24.
Bate, R., L. Mooney, and K. Hess. 2010b. Medicine registration and medicine quality: A preliminary analysis of key cities in emerging markets. Research and Reports in Tropical Medicine 1:89-93.
Bate, R., G. Z. Jin, and A. Mathur. 2012. Unveiling the mystery of online pharmacies: An audit study. Cambridge, MA: National Bureau of Economic Reasearch.
Bendavid, Y., E. Lefebvre, L. Lefebvre, and S. F. Wamba. 2007. B-to-B e-commerce: Assessing the impacts of RFID technology in a five layer supply chain. Proceedings of the 40th Hawaii International Conference on System Sciences. http://www.computer.org/csdl/proceedings/hicss/2007/2755/00/27550143c.pdf (accessed December 12, 2013).
Berckmans, P., V. Dawans, G. Schmets, D. Vandenbergh, and P. Autier. 1997. Inappropriate drug-donation practices in Bosnia and Herzegovina, 1992 to 1996. New England Journal of Medicine 337(25):1842-1845.
Berndt, E. R., and J. P. Newhouse. 2010. Pricing and reimbursement in U.S. pharmaceutical markets. Cambridge, MA: National Bureau of Economic Research.
Blair, E. 2012. Egyptian middleman bought fake Avastin from Turkey. Reuters, February 28. http://www.reuters.com/article/2012/02/28/us-egyptian-avastin-idUSTRE81R24120120228 (accessed March 7, 2013).
Bolton-Moore, C., M. Mubiana-Mbewe, R. A. Cantrell, N. Chintu, E. M. Stringer, B. H. Chi, M. Sinkala, C. Kankasa, C. M. Wilson, C. M. Wilfert, A. Mwango, J. Levy, E. J. Abrams, M. Bulterys, and J. S. A. Stringer. 2007. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA 298(16):1888-1899.
Bostwick, J. M., and T. W. Lineberry. 2007. Do cheap Internet drugs threaten the safety of the doctor-patient relationship? Expert Opinion on Drug Safety 6(1):9-13.
Brown, E. C. 2005. Pharmaceutical fakery: Counterfeit drugs threaten patients’ health. Long Island Press, June 9-15.
Bureau of Labor Statistics. 2012. Occupational outlook handbook, 2012-13 edition, pharmacy technicians. http://www.bls.gov/ooh/healthcare/pharmacy-technicians.htm (accessed October 11, 2012).
Burnham, T. 2012. Arrests made in $75 million prescription drug heist. NPR, May 4.
Bustreo, F., A. Harding, and H. Axelsson. 2003. Can developing countries achieve adequate improvements in child health outcomes without engaging the private sector? Bulletin of the World Health Organization 81(12):886-895.
Buynak, D. 2011. Docket no. FDA-2010-n-0633: Determination of system attributes for tracking and tracing of prescription drugs. AstraZeneca.
Catizone, C. A. 2006. Counterfeit drugs and states’ efforts to combat the problem. Journal of Pharmacy Practice 19(3):165-170.
Caudron, J. M., N. Ford, M. Henkens, C. Macé, R. Kiddle-Monroe, and J. Pinel. 2008. Substandard medicines in resource-poor settings: A problem that can no longer be ignored. Tropical Medicine & International Health 13(8):1062-1072.
CBO (U.S. Congressional Budget Office). 2007. Prescription drug pricing in the private sector. Washington, DC: CBO.
CDC (Centers for Disease Control and Prevention). 2013. Multistate fungal meningitis outbreak—current case count. http://www.cdc.gov/hai/outbreaks/meningitis-map-large.html (accessed January 28, 2013).
Cherici, C., P. McGinnis, and W. Russell. 2011. Buyer beware: Drug shortages and the gray market. Washington, DC: Premier Healthcare Alliance.
Clabaugh, J. 2005. Survey finds few online pharmacies sell drugs. Washington Business Journal, June 13.
Cockburn, D. 2011. Implementation of falsified medcines directive: Meeting with patients and conusmer organizations, 30 November 2011. London: European Medicines Agency.
ColaLife. 2012. About. http://www.colalife.org/about (accessed October 31, 2012).
Crawford, S. Y. 2003. Internet pharmacy: Issues of access, quality, costs, and regulation. Journal of Medical Systems 27(1):57-65.
CSG (Council of State Governments). 2008. Physician shortages and the medically underserved. Lexington, KY: CSG.
Davison, M. 2011. Indian pharmaceutical exporters must add 2D codes to all packs. http://www.bluespherehealth.com/wordpress/?p=236 (accessed October 4, 2012).
DeCardenas, R. 2007. Drug pedigree systems. Oncology Issues (March/April):38-41.
deKieffer, D. E. 2006. The internet and the globalization of counterfeit drugs. Journal of Pharmacy Practice 19(3):171-177.
Donaldson, A. 2010. Current development: “And the ones that mother gives you don’t do anything at all” combating counterfeit pharmaceuticals: The American and British perspectives. New England Journal of International and Comparative Law 16(1):145-168.
Dondorp, A. M., P. N. Newton, M. Mayxay, W. Van Damme, F. M. Smithuis, S. Yeung, A. Petit, A. J. Lynam, A. Johnson, T. T. Hien, R. McGready, J. J. Farrar, S. Looareesuwan, N. P. J. Day, M. D. Green, and N. J. White. 2004. Fake antimalarials in Southeast Asia are a major impediment to malaria control: Multinational cross-sectional survey on the prevalence of fake antimalarials. Tropical Medicine & International Health 9(12):1241-1246.
DrugTopics. 2012. Strategies to combat online counterfeit drug supply. http://drugtopics.modernmedicine.com/drugtopics/article/articleDetail.jsp?id=782394 (accessed October 22, 2012).
Ducca, A. 2011. Re: Determination of system attributes for the tracking and tracing of presription drugs; [docket no. FDA-2010-n-0633]. 76 Fed. Reg. 1182 (January 7, 2011). http://www.regulations.gov/#!documentDetail;D=FDA-2010-N-0633-0012 (accessed October 12, 2012).
DuPont, R. 2010. Prescription drug abuse: An epidemic dilemma. Journal of Psychoactive Drugs 42(2):127-132.
EAASM (European Alliance for Access to Safe Medicines). 2008. The counterfeiting superhighway. Surrey, UK: EAASM.
Efrati, A., and P. Loftus. 2010. Lilly drugs stolen in warehouse heist. Wall Street Journal, March 17.
EMA (European Medicines Agency). 2011. Falsified medicines. http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/general/general_content_000186.jsp&mid=WC0b01ac058002d4e8 (accessed November 7, 2012).
Engelberg, A. B., A. S. Kesselheim, and J. Avorn. 2009. Balancing innovation, access, and profits—market exclusivity for biologics. New England Journal of Medicine 361(20): 1917-1919.
EPCglobal. 2007. Electronic Product Code (EPC): An overview. http://www.gs1.org/docs/epcglobal/an_overview_of_EPC.pdf (accessed March 7, 2013).
Erhun, W. O., O. O. Babalola, and M. O. Erhun. 2001. Drug regulation and control in Nigeria: The challenge of counterfeit drugs. Journal of Health and Population in Developing Countries 4(2):23-34.
Exel. 2003. Role of pre-wholesalers in generic pharmaceutical manufacturers’ demand chain management strategy. http://www.touchbriefings.com/pdf/15/pg031_t_exel.pdf (accessed March 7, 2013).
Faucon, B., and J. Whalen. 2012. Fake Avastin took murky path to U.S. Wall Street Journal, April 5.
FDA (U.S. Food and Drug Administration). 2005. The possible dangers of buying medicines over the Internet. Washington, DC: FDA.
———. 2011a. CPG sec. 160.900 Prescription Drug Marketing Act—pedigree requirements under 21 CFR Part 203. http://www.fda.gov/ICECI/ComplianceManuals/CompliancePolicyGuidanceManual/ucm073857.htm (accessed October 2, 2012).
———. 2011b. Guidance for industry: Bar code label requirements—questions and answers. Washington, DC: FDA.
———. 2012a. FDA news release: FDA takes action against thousands of illegal Internet pharmacies. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm322492.htm#.UG2Q840J6nY.email (accessed October 22, 2012).
———. 2012b. National drug code directory. http://www.fda.gov/Drugs/InformationOnDrugs/ucm142438.htm (accessed October 24, 2012).
Fein, A. 2011. 2011 MDM market leaders: Top pharmaceutical wholesalers. Modern Distribution Management. http://www.mdm.com/2011_pharmaceutical_mdm-market-leaders (accessed January 15, 2013).
———. 2012. Trends and top distributors in the pharmaceuticals sector. Modern Distribution Management. http://www.mdm.com/2012_pharmaceuticals_mdm-market-leaders (accessed January 15, 2013).
Ferrinho, P., M. Omar, M. Fernandes, P. Blaise, A. Bugalho, and W. Lerberghe. 2004. Pilfering for survival: How health workers use access to drugs as a coping strategy. Human Resources for Health 2(1):4.
Financial Times. 2012. Chasing fakes. October 7.
FIP (International Pharmaceutical Federation). 2006. Part I: Workforce description. In Global pharmacy workforce and migration report. The Hague, The Netherlands: FIP. Pp. 11-16.
———. 2009. FIP global pharmacy workforce report. The Hague, The Netherlands: FIP.
Fischer, E. 2012. Medical cargo theft: Securing supply chain. http://www.drugdevelopment-technology.com/features/featuremedical-cargo-theft-securing-the-supply-chain (accessed October 30, 2012).
Flaherty, M. P., and G. Gaul. 2003. Nevada gets tough with mixed results. Washington Post, October 22.
Foundation Strategy Group. 2005. Beyond R&D: Leveraging the pharmaceutical industry on issues of drug access. Washington, DC: Foundation Strategy Group.
FreightWatch. 2011a. Global threat assessment. http://www.transportschaden.biz/html/documents/FreightWatch_Global_Threat_Assessment_2011.pdf (accessed March 7, 2013).
———. 2011b. U.S. Cargo theft: A five-year review. http://static.rmasecurity.com/wp-content/uploads/2012/05/US-Cargo-Theft-A-Five-Year-Review.pdf (accessed March 7, 2013).
Fulton, B., R. Scheffler, S. Sparkes, E. Auh, M. Vujicic, and A. Soucat. 2011. Health workforce skill mix and task shifting in low income countries: A review of recent evidence. Human Resources for Health 9(1):1.
Galve, Á., and P. R. R. Campos. 2011. Direct to pharmacy distribution in Spain: An operational and politico-economic analysis. MIT-Zaragoza International Logistics Program, University of Zaragoza, Spain.
GAO (U.S. Government Accountability Office). 2004. Internet pharmacies: Some pose safety risks for consumers. Washington, DC: GAO.
Garnier, L., A. Arria, K. Caldeira, K. Vincent, K. O’Grady, and E. Walsh. 2010. Sharing and selling of prescription medications in a college student sample. Journal of Clinical Psychiatry 71(3):262-269.
Gatesman, M. L., and T. J. Smith. 2011. The shortage of essential chemotherapy drugs in the United States. New England Journal of Medicine 365(18):1653-1655.
Global Fund. 2012. Affordable medicines facility—malaria. http://www.theglobalfund.org/en/amfm (accessed October 24, 2012).
Goel, P., D. Ross-Degnan, P. Berman, and S. Soumerai. 1996. Retail pharmacies in developing countries: A behavior and intervention framework. Social Science & Medicine 42(8):1155-1161.
Goodman, C., S. P. Kachur, S. Abdulla, P. Bloland, and A. Mills. 2007. Drug shop regulation and malaria treatment in Tanzania—why do shops break the rules, and does it matter? Health Policy and Planning 22(6):393-403.
GPhA (Generic Pharmaceutical Association). 2011. Comments of the Generic Pharmaceutical Association on the Food and Drug Administration’s public workshop: Determination of system attributes for the tracking and tracing of prescription drugs [docket no. FDA-2010-n-0633]. http://www.regulations.gov/#!documentDetail;D=FDA-2010-N-0633-0039 (accessed October 12, 2012).
———. 2012. Pedigree. http://gpha.hfwebdev.com/issues/pedigree (accessed September 28, 2012).
Grady, D., S. Tavernise, and A. Pollack. 2012. In a drug linked to a deadly meningitis outbreak, a question of oversight. New York Times, October 4.
Gressit, K.-B. 2007. How can we secure the U.S. pharmaceutical supply channel? IG Living 28-31.
GTZ (Deutsche Gesellschaft für Internationale Zusammenarbeit). n.d. GTZ’s cooperation in technical and vocational education and training. Bonn, Germany: GTZ.
Hawthorne, N., and C. Anderson. 2009. The global pharmacy workforce: A systematic review of the literature. Human Resources for Health 7(1):48.
Hechmann, R., and A. Dune-Birouste. 2007. Drug donations in emergencies, the Sri Lankan post-tsunami experience. Journal of Humanitarian Assistance, September 26. http://sites.tufts.edu/jha/archives/54 (accessed March 7, 2013).
Hetzel, M., N. Iteba, A. Makemba, C. Mshana, C. Lengeler, B. Obrist, A. Schulze, R. Nathan, A. Dillip, S. Alba, I. Mayumana, R. Khatib, J. Njau, and H. Mshinda. 2007. Understanding and improving access to prompt and effective malaria treatment and care in rural Tanzania: The ACCESS programme. Malaria Journal 6(1):83.
HIMSS (Healthcare Information and Management Systems Society). 2003. Implementation guide for the use of bar code technology in healthcare. Chicago, IL: HIMSS.
Inciardi, J. A., H. L. Surratt, T. J. Cicero, A. Rosenblum, C. Ahwah, J. E. Bailey, R. C. Dart, and J. J. Burke. 2010. Prescription drugs purchased through the internet: Who are the end users? Drug and Alcohol Dependence 110(1-2):21-29.
Inciardi, J. A., H. L. Surratt, S. P. Kurtz, and T. J. Cicero. 2007. Mechanisms of prescription drug diversion among drug-involved club- and street-based populations. Pain Medicine 8(2):171-183.
Interpol. 2012a. Media release: Global crackdown on illicit online pharmacies. London, October 4.
———. 2012b. Operations: Operation Pangea. http://www.interpol.int/Crime-areas/Pharmaceutical-crime/Operations/Operation-Pangea (accessed October 22, 2012).
ISMP (Institute for Safe Medication Practices). 2011. Gray market, black heart: Pharmaceutical gray market finds a disturbing niche during the drug shortage crisis. http://www.ismp.org/newsletters/acutecare/showarticle.asp?id=3 (accessed October 2, 2012).
Ivanitskaya, L., J. Brookins-Fisher, I. O’Boyle, D. V. D. Erofeev, and L. Fulton. 2010. Dirt cheap and without prescription: How susceptible are young U.S. consumers to purchasing drugs from rogue internet pharmacies? Journal of Medical Internet Research 12(2). http://www.jmir.org/2010/2/e11 (accessed May 1, 2013).
Jagdale, S. 2010. Catching the counterfeiters. http://www.expresspharmaonline.com/20100430/packagingspecial01.shtml (accessed September 28, 2012).
Kanavos, P., W. Schurer, and S. Vogler. 2011. The pharmaceutical distribution chain in the European Union and impact on pharmaceutical prices. European Commission. http://ec.europa.eu/enterprise/sectors/healthcare/files/docs/structimpact_pharmaprices_032011_en.pdf (accessed March 7, 2013).
Kasipar, C., M. V. Tien, S.-Y. Lim, P. L. Phuong, P. Q. Huy, A. Schnarr, W. Quanquan, X. Ying, and F. Bünning. 2009. Linking vocation training with the enterprises—Asian perspectives. Bonn, Germany: InWEnt and UNESCO-UNEVOC.
Khan, M. H., J. Okumura, T. Sovannarith, N. Nivanna, H. Nagai, M. Taga, N. Yoshida, M. Akazawa, T. Tanimoto, and K. Kimura. 2011. Counterfeit medicines in Cambodia—possible causes. Pharmaceutical Research 3:484-489.
Kome, G., and J. Fieno. 2006. New thinking in addressing the rising chllenges of human resources for health in sub-Saharan Africa. African Renaissance 3(4):83-97.
KPMG. 2010. Evaluation of the rural pharmacy programs: Final report. http://www.health.gov.au/internet/main/publishing.nsf/Content/5B1B138DA00BB9C7CA2578150083984E/$File/RPWP%20Report.pdf (accessed March 7, 2013).
Langer, E., and A. Kelkar. 2008. Pharmaceutical distribution in India. BioPharm International 21(10). http://www.biopharminternational.com/biopharm/India+Today/Pharmaceutical-Distribution-in-India/ArticleStandard/Article/detail/557245 (accessed May 1, 2013).
Laven, D. 2006. Indroduction: Drug diversion and counterfeiting, part 1. Journal of Pharmacy Practice 19(3):135-139.
Lefebvre, E., A. Romero, L.-A. Lefebvre, and C. Krissi. 2011. Technological strategies to deal with counterfeit medicines: The European and North-American perspectives. International Journal of Education and Information Technologies 5(3):275-284.
Levaggi, R., C. Marcantoni, L. Filippucci, and U. Gelatti. 2012. Not a good buy: Value for money of prescription drugs sold on the Internet. Health Policy (Amsterdam, Netherlands) 106(3):241-245.
Levy, S. 2006. Regional wholesalers are holding their own. DrugTopics, http://drugtopics.modernmedicine.com/news/regional-wholesalers-are-holding-their-own (accessed January 15, 2013).
Liang, B. A. 2006. Fade to black: Importation and counterfeit drugs. American Journal of Law and Medicine 32:279-323.
Lim, Z., C. Anderson, and S. McGrath. 2012. Professional skills development in a resource-poor setting: The case of pharmacy in Malawi. International Journal of Educational Development 32(5):654-664.
Lon, C. T., R. Tsuyuoka, S. Phanouvong, N. Nivanna, D. Socheat, C. Sokhan, N. Blum, E. M. Christophel, and A. Smine. 2006. Counterfeit and substandard antimalarial drugs in Cambodia. Transactions of the Royal Society of Tropical Medicine and Hygiene 100(11):1019-1024.
Ludwig, F. 2012. Fighting counterfeit pharmaceuticals: A primer. http://www.healthcarepackaging.com/archives/2012/01/fighting_counterfeit_pharmaceu.php (accessed October 11, 2012).
Lybecker, K. M. 2007. Rx roulette: Combatting counterfeit pharmaceuticals in developing nations. Managerial and Decision Economics 28(4-5):509-520.
Mafigiri, D., J. McGrath, and C. Whalen. 2011. Task shifting for tuberculosis control: A qualitative study of community-based directly observed therapy in urban Uganda. Global Public Health 7(3):270-284.
Mahmood, M., K. Riley, D. Bennett, and W. Anderson. 2011. The supply of pharmaceuticals in humanitarian assistance missions: Implications for military operations. Military Medicine 176(8):852-857.
Marsh, V. M., W. M. Mutemi, A. Willetts, K. Bayah, S. Were, A. Ross, and K. Marsh. 2004. Improving malaria home treatment by training drug retailers in rural Kenya. Tropical Medicine & International Health 9(4):451-460.
Mayor, S. 2008. More than half of drugs sold online are fake or substandard. British Medical Journal 337”a618.
McCabe, A. 2009. Private sector pharmaceutical supply and distribution chains: Ghana, Mali and Malawi. http://siteresources.worldbank.org/HEALTHNUTRITIONANDPOPULATION/Resources/281627-1095698140167/PvtSectorPharma0811.pdf (accessed March 7, 2013).
McCain, J. 2011. Doing more with less? Past and present challenges for the FDA. Pharmacy and Therapeutics 36(3):145-155.
McCathie, L., and K. Michael. 2005. Is it the end of barcodes in supply chain management? Paper read at Collaborative Electronic Commerce Technology and Research Conference LatAm, October 3-5, 2005, University of Talca, Chile.
Medco Health. 2012. Find out about us. http://www.medcohealth.com/medco/consumer/helpcenter/help_article.jsp?accessLink=SHP_ANON_01&faq=HelpCenterUR_FindAboutMedcoHealth-VIPPS (accessed January 9, 2013).
Mohanta, G., P. Manna, K. Valliappan, and R. Manavalan. 2001. Achieving good pharmacy practice in community pharmacies in India. American Journal of Health-System Pharmacy 58(9):809-810.
MSH (Management Sciences for Health). 2005. Tanzania: Accredited drug dispensing outlets—Duka la Dawa Muhimu. Cambridge, MA: MSH.
———. 2012. Drug seller initiatives. Cambridge, MA: MSH.
Mukherjee, R. 2012. Now send SMS to find if pill is genuine. Times of India, September 23.
Muskal, M. 2012. $80-million mystery solved? Brothers held in pharmaceutical heist. Los Angeles Times, May 3.
NABP (National Association of Boards of Pharmacy). 2011. VIPPS information and verification site of the National Association of Boards of Pharmacy. http://vipps.nabp.net (accessed October 22, 2012).
———. 2012a. Accreditation process. www.nabp.net/programs/accreditation/vawd/accreditation-process (accessed October 24, 2012).
———. 2012b. Buying medicine online: Online pharmacies and you. http://www.nabp.net/programs/consumer-protection/buying-medicine-online (accessed October 22, 2012).
———. 2012c. Find a VIPPS online pharmacy. http://www.nabp.net/programs/accreditation/vipps/find-a-vipps-online-pharmacy (accessed October 22, 2012).
———. 2012d. Not recommended sites. http://www.nabp.net/programs/consumer-protection/buying-medicine-online/not-recommended-sites (accessed October 22, 2012).
———. 2012e. VIPPS: Set yourself apart from rogue sites! http://www.nabp.net/programs/accreditation/vipps (accessed October 22, 2012).
Nkamnebe, A. 2007. Attitude of Nigerian consumers toward made-in-India pharmaceutical products. 2nd IIMA Conference on Research in Marketing. Ahmedabad, India, January 3-5.
Norman, B. 2012. User fee bill may hinge on drug tracking system. Politico, May 17.
O’Neil, M., Z. Jarrah, L. Nkosi, D. Collins, C. Perry, J. Jackson, H. Kuchande, and A. Mlambala. 2010. Evaluation of Malawi’s emergency human resources program. Department for International Development, Management Sciences for Health, and Management Solutions Consulting, Ltd.
OFT (Office of Fair Trading). 2007. Medicines distribution: An OFT market study. London: OFT.
Onwujekwe, O., H. Kaur, N. Dike, E. Shu, B. Uzochukwu, K. Hanson, V. Okoye, and P. Okonkwo. 2009. Quality of anti-malarial drugs provided by public and private healthcare providers in south-east Nigeria. Malaria Journal 8(22). DOI: 10.1186/1475-2875-8-22.
Orizio, G., A. Merla, P. Schulz, and U. Gelatti. 2011. Quality of online pharmacies and websites selling prescription drugs: A systematic review. Journal of Medical Internet Research 13(3). http://www.jmir.org/2011/3/e74 (accessed May 1, 2013).
Palmer, E. 2012. FiercePharma: It is RIP for track and trace. http://www.fiercepharma.com/story/it-rip-track-and-trace/2012-06-19 (accessed January 9, 2013).
Palmer, R. 2010. FDA strengthens its stance against unethical researchers. Nature Medicine 16(7):728.
Pedersen, C. A., P. J. Schneider, and D. J. Scheckelhoff. 2003. ASHP national survey of pharmacy practice in hospital settings: Dispensing and administration—2002. American Journal of Health-System Pharmacy 60(1):52.
Pellek, A. 2009. Drug serialization and supply chain security. http://www.pharmtech.com/pharmtech/IT/Drug-Serialization-and-Supply-Chain-Security/ArticleStandard/Article/detail/601169 (accessed October 11, 2012).
Peters, D. H., and G. Bloom. 2012. Developing world: Bring order to unregulated health markets. Nature 487(7406):163-165.
PGA (Pharmacy Guild of Australia). 2011. Meet a rural pharmacist. http://www.ruralpharmacy.com.au/ACareerinRuralPharmacy/MeetaRuralPharmacist/tabid/472/language/en-US/Default.aspx (accessed October 4, 2012).
———. 2012a. Pharmacy student scholarships. http://www.ruralpharmacy.com.au/AllowancesScholarships/PharmacyStudentScholarships/tabid/644/language/en-US/Default.aspx#Rural (accessed October 5, 2012).
———. 2012b. Rural pharmacy student placement allowance and administrative support to pharmacy schools. http://www.ruralpharmacy.com.au/AllowancesScholarships/Allowances/RuralPharmacyStudentPlacementAllowance/tabid/707/language/en-US/Default.aspx (accessed October 6, 2012).
Pomatto, V., and C. Schuftan. 2006. Review of quality assurance (QA) mechanisms for medicines and medical supplies in humanitarian aid. European Commission for Humanitarian Aid. http://ec.europa.eu/echo/files/evaluation/drugs_quality_guidelines.pdf (accessed March 7, 2013).
Power, G. 2008. Anti-counterfeit technologies for the protection of medicines. Geneva: IMPACT and World Health Organization.
Quingyun, W. 2012. Major crackdown in fake medicine scam. China Daily, August 6.
Rabinowitz, H. K., J. J. Diamond, M. Hojat, and C. E. Hazelwood. 1999. Rural health research: Demographic, educational and economic factors related to recruitment and retention of physicians in rural Pennsylvania. Journal of Rural Health 15(2):212-218.
Rao, K. D., S. Ramani, S. Murthy, I. Hazarika, N. Khandpur, M. Chokshi, S. Khanna, M. Vujicic, P. Berman, and M. Ryan. 2010. Health worker attitudes toward rural service in India: Results from qualitative research. Washington, DC: World Bank.
Rappeport, A., and A. Jack. 2012. FDA calls for drug tracking system. Financial Times, March 8.
RFID Journal. 2012. RFID Journal glossary of terms: Radio frequency identification. http://www.rfidjournal.com/site/glossary-of-terms (accessed October 4, 2012).
RFID Update. 2008. NACDS study puts price tag on pharmacy RFID systems. http://www.rfidjournal.com/articles/view?7003 (accessed March 7, 2013).
Riley, L. W., A. I. Ko, A. Unger, and M. G. Reis. 2007. Slum health: Diseases of neglected populations. BMC International Health and Human Rights 7. DOI: 10.1186/1472-698X-7-2.
Rutta, E., B. Kibassa, B. McKinnon, J. Liana, R. Mbwasi, W. Mlaki, M. Embrey, M. Gabra, E. Shekalaghe, S. Kimatta, and H. Sillo. 2011. Increasing access to subsidized artemisinin-based combination therapy through accredited drug dispensing outlets in Tanzania. Health Research Policy and Systems 9(1):22.
Satchwell, G. 2004. A sick business: Counterfeit medicines and organized crime. London: The Stockholm Network.
SCMS (Supply Chain Management Society). 2012. SCMS in brief: SCMS freight and logistics update. http://scms.pfscm.org/portal/pls/portal/!PORTAL.wwpob_page.show?_docname=2416074.PDF (accessed March 7, 2013).
Segrè, J., and J. Tran. 2008. What worls: CareShop Ghana. Washington, DC: World Resources Institute.
Shelton, D. 2012. Fake medicine poses growing threat to consumers. Chicago Tribune, October 16.
Shepherd, M. 2007a. Drug importation and safety of drugs obtained from Canada. Annals of Pharmacotherapy 41(7):1288-1291.
———. 2007b. Impact of drug importation on community pharmacy and patient care. Journal of the American Pharmacists Association 47:319-327.
Shulman, D., K. Jobarteh, E. Makani, M. Mtwea, A. Mapwelemwe, A. Manjomo, J. Crocker, and K. Joseph. 2009. Task-shifting in the pharmacy: A framework for expanding and strengthening services in rural Malawi. 5th IAS Conference on HIV Pathogenesis and Treatment.
Simonaitis, L., and C. J. McDonald. 2009. Using national drug codes and drug knowledge bases to organize prescription records from multiple sources. American Journal of Health-System Pharmacy 66(19):1743-1753.
Spies, A., and V. VanDusen. 2003. Counterfeit drugs: A menace keeps growing. U.S. Pharmacist 28(1). http://legacy.uspharmacist.com/index.asp?show=article&page=8_1014.htm (accessed May 1, 2013).
Sproxil. 2012. Countries. http://sproxil.com/countries.html (accessed October 1, 2012).
Srivastava, B. 2004. Radio frequency ID technology: The next revolution in SCM. Business Horizons 47(6):60-68.
Stanton, C., A. Koski, P. Cofie, E. Mirzabagi, B. L. Grady, and S. Brooke. 2012. Uterotonic drug quality: An assessment of the potency of injectable uterotonic drugs purchased by simulated clients in three districts in Ghana. BMJ Open 2(3):1-7.
Stringer, J., I. Zulu, J. Levy, E. Stringer, A. Mwango, B. Chi, V. Mtonga, S. Reid, R. Cantrell, M. Bulterys, M. Saag, R. Marlink, A. Mwinga, T. Ellerbrock, and M. Sinkala. 2006. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: Feasibility and early outcomes. JAMA 296(7):782-793.
Tavernise, S., and A. Pollack. 2012. FDA details contamination at pharmacy. New York Times, October 26.
Tavrow, P., Y.-M. Kim, and L. Malianga. 2002. Measuring the quality of supervisor–provider interactions in health care facilities in Zimbabwe. International Journal for Quality in Health Care 14(Suppl 1):57-66.
Tavrow, P., and J. Shabahang. 2002. Vendor-to-vendor education to improve malaria treatment by the private sector: A “how-to” manual for district managers. Bethesda, MD: Quality Assurance Project.
Taylor, L. 2011. Pfizer’s DTP system “threatens Australia’s drugs supply.” Pharma Times Online, July 11.
Taylor, P. 2010. Safety in numbers: Brazil’s medicine serialisation initiative. http://www.securingindustry.com/pharmaceuticals/safety-in-numbers-brazil-s-medicine-serialisation-initiative-/s40/a440 (accessed October 4, 2012).
TechNewsDaily. 2012. Internet pharmacy crackdown shutters 18,000 sites. October 5.
Tipke, M., S. Diallo, B. Coulibaly, D. Störzinger, T. Hoppe-Tichy, A. Sie, and O. Müller. 2008. Substandard anti-malarial drugs in Burkina Faso. Malaria Journal 7(95). DOI: 10.1186/1475-2875-7-95.
UNODC (United Nations Office on Drugs and Crime). 2010. The globalization of crime. New York: UNODC.
USAID (United States Agency for International Development). 2011. Using last mile distribution to increase access to health commodities. Arlington, VA: USAID DELIVER Project.
van Dijk, D. P. J., G.-J. Dinant, and J. A. Jacobs. 2011. Inappropriate drug donations: What has happened since the 1999 WHO guidelines? Education for Health 24(2):462.
Versel, N. 2012. Sproxil deal offers free mobile drug authentication in 17 African countries. MobiHealthNews, August 29.
Vian, T. 2008. Review of corruption in the health sector: Theory, methods and interventions. Health Policy and Planning 23:83-94.
Villacorta-Linaza, R. 2009. Bridging the gap: The role of pharmacists in managing the drug supply cycle within non-governmental organizations. International Journal of Health Planning and Management 24:73-86.
Wagner, M., J. Alonso, and A. Mehlhorn. 2001. Comparison of Internet and community pharmacies. Annals of Pharmacotherapy 35(1):116-119.
Wasik, J. 2012. Fake medicine bought online: How to avoid counterfeits. Forbes, October 18.
Weaver, C., and J. Whalen. 2012. How fake cancer drugs entered U.S. Wall Street Journal, July 20.
White, S., and J. Bothma. 2009. Pharmintercom: 7-11 September 2008 (part 3). South African Pharmaceutical Journal 76(2). http://www.sapj.co.za/index.php/SAPJ/article/view/521/478 (accessed May 1, 2013).
WHO (World Health Organization). 2008. First global conference on task shifting. http://www.who.int/mediacentre/events/meetings/task_shifting/en/index.html (accessed October 11, 2012).
———. 2010. Assessment of medicines regulatory systems in sub-Saharan African countries: An overview of findings from 26 assessment reports. Geneva: WHO.
———. 2011. Safety and security on the Internet: Challenges and advances in member states (executive summary). Geneva: WHO.
Whoriskey, P. 2012. Medicare overspending on anemia drug. Washington Post, August 9.
WHPA (World Health Professions Alliance). 2011. World Health Professions Alliance (WHPA) brief to WHO member states on spurious/falsely-labelled/falsified/counterfeit medical products (SFFC). France: WHPA.
Wolinsky, H. 2006. Tagging products and people. EMBO Reports 7(10):965-968.
Wondemagegnehu, E. 1999. Counterfeit and substandard drugs in Myanmar and Viet Nam: Report of a study carried out in cooperation with the governments of Myanmar and Viet Nam. Geneva: WHO.
World Bank. 2005. HNP brief #2: Pharmaceuticals: Counterfeits, substandard drugs, and drug diversion. Washington, DC: World Bank.
Worldmapper. 2006. Pharmacists working (map no. 17). http://www.worldmapper.org/display.php?selected=217 (accessed November 28, 2012).
Wunder, G., and B. Roach. 2008. Electronic pedigrees and counterfeit pharmaceuticals: The U.S. Experience with RFID. Washburn University School of Business Working Paper Series 104.
Yadav, P. 2009. Countering drug resistance in the developing world: An assessment of incentives across the value chain and recommendations for policy intervention. Working Paper No. 183. Washington, DC: Center for Global Development.
———. 2010. In-country supply chains: The weakest link in the health system. Global Health Magazine 5:18-19.
Yadav, P., and L. Smith. 2012. Pharmaceutical company strategies and distribution systems in emerging markets. Elsevier Encyclopedia on Health Economics.
Yadav, P., R. Smith, and K. Hanson. 2012. Pharmaceuticals and the health sector. In Health systems in low- and middle-income countries: An economic and policy perspective, edited by R. Smtih and K. Hanson. Oxford, NY: Oxford University Press.
Yadav, P., O. Stapleton, and L. v. Wassenhove. 2013. Learning from Coca-Cola. Stanford Social Innovation Review. http://www.ssireview.org/articles/entry/learning_from_coca_cola (accessed March 7, 2013).
Yadav, P., H. L. Tata, and M. Babaley. 2011. The world’s medicines situation 2011: Storage and supply chain management. Geneva: WHO.
Yao, W., C.-H. Chu, and Z. Li. 2010 June 17-19. The use of RFID in healthcare: Benefits and barriers. Paper presented at IEEE International Conference on RFID-Technology and Applications, Guangzhou, China.
Yukhananov, A. 2012. Congress fails to agree on national drug trace plan. Reuters, June 18.
Zaza, A. 2012. Importation of unregistered drugs in the UAE. http://www.tamimi.com/en/publication/publications/section-3/may/importation-of-unregistered-drugs-in-the-uae.html (accessed October 17, 2012).
Ziance, R. 2008. Roles for pharmacy in combatting counterfeit drugs. Journal of the American Pharmacists Association 48(4):e71-e91.