This chapter provides an overview of the technologies commonly used to detect substandard and falsified drugs, ranging from inexpensive field assays to highly sophisticated laboratory methods. It does not describe every technique used or the pharmaceutical application of each technology, but rather explains how technology can be used to identify illegitimate drugs.
Modern science has opened up immensely powerful and expensive forensic chemistry techniques that can give investigators information on the unique fingerprints manufacturers leave on their products. Such an analysis can give prosecutors the evidence necessary to tie falsified drugs to particular sources, but such sensitivity comes at a cost. Forensic chemistry assays cost $5,000 to $15,000 per test on average (personal communication, Ben Paulson, Chemir, January 25, 2013). They are not practical for routine product quality market surveillance in any country and may be out of reach entirely in many of the low- and middle-income countries most affected by the problem (Fernandez et al., 2008; Power, 2008). Keeping in mind the high costs of these laboratory analyses, this chapter discusses inexpensive and sustainable detection technologies that can be used for routine product quality assessments in all markets.
QUALITATIVE AND QUANTITATIVE METHODS
Detection technologies provide varying degrees of qualitative and quantitative data about medicines. Qualitative techniques provide information about a drug’s identity, such as its active ingredient, color, or labeling. Quantitative techniques provide information about a drug’s content and how that content will be absorbed in the body. Qualitative assays may be used to quickly detect the least sophisticated falsified drugs, such as those with the wrong or no active ingredient. Quantitative deficiencies, such as an unacceptably high level of impurities or an unacceptably low or high dosage of active ingredient, are more common among substandard drugs. Tests for drug quality use both qualitative data (e.g., the identity of ingredients, the presence and nature of any packaging and inserts, the presence or absence of impurities, and any data referring to the drug’s appearance) and quantitative data (e.g., the amount of an ingredient present, tablet hardness, the rate and extent of disintegration and dissolution, and measured levels of impurities).
Key Findings and Conclusions
• As criminals become more sophisticated, there will be an increased need for expensive technologies to detect falsified medicines.
• There are several categories of techniques to analyze pharmaceuticals. They include visual inspection of product and packaging; tests for physical properties such as disintegration, reflectance spectroscopy, and refractive index; chemical tests including colorimetry and dissolution; chromatography; spectroscopic techniques; and mass spectrometry.
• Novel technologies are constantly being developed to detect falsified and substandard medicines.
A full evaluation of drug quality requires a range of qualitative and quantitative testing to verify the identities and amounts of active ingredients, check for impurities, and ensure acceptable disintegration, dissolution, stability over time, and sterility (USP, 2007). Identifying falsified and substandard drugs does not always follow the same process as a rigorous quality evaluation. A few simple tests can identify a product with no active ingredient or one made under gross manufacturing negligence. More sophisticated fakes resist easy detection. Appearance, content, and therapeutic effect can all be considered in classifying falsified drugs. Box 6-1 outlines one method for making categories.
Criminals in the business of making falsified drugs can buy crude active ingredients, chemicals that have not undergone the appropriate purification steps required to meet pharmacopeial standards or manufacturer’s dossier requirements, for example. The drugs made from such chemicals would pass most tests. Only highly sophisticated and expensive assays could detect trace contaminates. Figure 6-1 gives an overview of the different levels of technology needed to catch progressively more complex falsified drugs.
BOX 6-1
Classifying Falsified Medicines
One way to classify falsified medicines is to assign categories based on the sophistication of the fake. This is an example of such categorization.
• Category 1: Completely fraudulent products with unknown contents and therapeutic effects significantly different from the genuine drug.
• Category 2: Look somewhat similar to the drug being imitated, but the drug composition is not known.
• Category 3: Look very similar or identical to the genuine product but contain an entirely different drug, if any.
• Category 4: Look very similar or identical to the actual product but contain an alternative drug or synthetic analogue providing similar therapeutic value to that of the authentic product; intended to create repeat business.
• Category 5: Visually identical, highly sophisticated copies or synthetic analogues with some therapeutic value that cannot be detected using most field and laboratory methods.
Overview of Detection, Screening, and Analytical Techniques
The main categories of techniques for pharmaceutical analysis can be broken down as follows: visual inspection of product and packaging; tests for physical properties such as disintegration, reflectance spectroscopy, and refractive index; chemical tests including colorimetry and dissolution; chromatography; spectroscopic techniques; and mass spectrometry. Within each of these categories, some technologies are appropriate for use in the field with minimal training, while others require sophisticated lab equipment and a high level of technical expertise.
Visual Inspection and Package Technologies
An expert can identify some drug quality problems by sight. Therefore, visual inspection of a product and its packaging by someone who knows the properties of the authentic drug or is able to compare the sample to the authentic product is the standard first step in any drug quality analysis (Martino et al., 2010). These visual inspections provide qualitative data about drugs’ identities. Differences from the authentic materials in color, size, shape, tablet quality, and packaging indicate a possible falsified or substandard drug. These differences range from subtle to obvious. An educated consumer could probably identify a very-poor-quality fake, such as a pill of entirely the wrong color or shape, if they knew some properties of the authentic product, but even experts struggle to recognize more subtle inconsistencies. The Global Pharma Health Fund’s Minilab toolkit promotes visual inspection as the first step to identifying falsified and substandard drugs but admits that this is challenging even for experts (Jähnke et al., 2008; Sherma, 2007). In recent years, criminals have produced very accurate reproductions of legitimate packaging. And, as Chapter 4 mentions, poor-quality drugs can sometimes be hidden in legitimate packaging (Sherma, 2007).

FIGURE 6-1 More sophisticated fakes require more sophisticated technologies to detect them.
NOTE: HPLC= high-performance liquid chromatography; TLC= thin layer chromatography; UV= ultraviolet.
Visual inspection of a product can yield useful information, however. Some substandard drugs are of visibly low quality. Tablets that are cracked or falling apart are products of poor manufacturing practices (Kaur et al., 2010). Falsified drugs’ packaging may have missing or misplaced expiry dates, lack instructions or manufacturing information, not have a batch number, or differ from the genuine packaging in many other ways. Sometimes poorly written instructions and spelling errors expose fake medicines; poor-quality inks may dissolve in water (Kaur et al., 2010). Similarly, the drugs may be the wrong color, size, or shape, have the wrong markings on them, have a different coating or texture, or be otherwise different from what is expected (Kaur et al., 2010). Sometimes the differences are obvious: fake Viagra seized in Hungary was pink instead of the well-known blue color of the genuine product. Further analysis revealed that the tablets contained 15 milligrams of amphetamine instead of the correct active ingredient (U.S. Drug Enforcement Administration Office of Forensic Sciences, 2004).
Visual inspections are often unreliable because substandard and falsified drugs and their packaging often appear identical or very similar to the genuine products. Criminals have copied holograms, barcodes, packaging styles, and tablet colors and markings with astonishing accuracy (Lim, 2012). Microscopic packaging analysis can identify some of these very careful copies. Under magnification, fine differences in printing, imprints, and alignment become clear. Figure 6-2 shows a high-magnification comparison of the lettering on a legitimate and fake blister pack. As this illustration suggests, visual inspection alone is not adequate to test for drug quality (Lim, 2012; Martino et al., 2010). Though a trained inspector can draw conclusions about drug quality by visual inspection, physical analysis is generally a more reliable way to identify fakes.
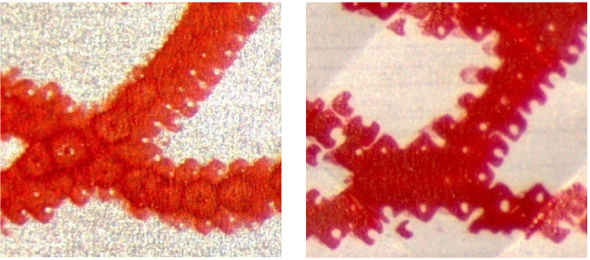
FIGURE 6-2 The printing on a fake Cialis blister pack is less crisp at 32× magnification.
SOURCE: Lim, 2012.
Physical and Bulk Property Testing
As Chapter 4 explains, active ingredients are the most expensive component of drugs; dilute or impure active ingredients can translate into vastly increased profits for an unscrupulous manufacturer. Some tests that rely on pH and other bulk properties can help identify active ingredients. Bulk properties, also called intensive properties, are properties that do not depend on the amount of the chemical sampled. Density, solubility, reflectance spectra, refractive indices, and optical rotation are examples of bulk properties (Brown et al., 2011). The malaria drug artesunate, for example, has some distinctive physical properties: It yields characteristic crystals when precipitated from water, and its extract acidifies water (Deisingh, 2005; Newton et al., 2006). These properties can be used to distinguish some authentic and fake antimalarials.
The refractive index, the measure of how light passes through a substance relative to the speed at which light passes through a vacuum, is a similarly useful bulk property. The refractive index can be used to measure the purity of pure liquids and can detect materials separated by liquid chromatography. Field inspectors can use handheld refractometers to measure the refractive index and use it as a quantitative test for some active ingredients (Kaur et al., 2010). Green and colleagues explored the practical use of refractive index to measure the amount of active ingredients selectively dissolved in certain solvents (Green et al., 2007). They found that while the refractive index can measure the amount of an unknown active ingredient, colorimetry can be used to help confirm its presence (Green et al., 2007).
Colorimetry and Other Chemical Testing
A variety of simple chemical reactions can test for the presence of active ingredients. Colorimetry is one such technique. It relies on chemicals that undergo color changes when reacted with certain compounds to provide qualitative data about a drug’s identity. Colorimetry protocols exist for the active ingredients in many essential drugs. Fast Red TR dye tests for the active ingredient in some antimalarials by turning yellow in the presence of artesunate (Green et al., 2001). In addition to verifying the presence of an active ingredient, colorimetry can serve as a semi-quantitative technique to provide information about tablet potency; a more drastic color change or deeper color generally indicates a larger amount of ingredient. More precise colorimetric testing is possible with a handheld photometer, a spectroscopic device that measures absorbance of light through a substance (Newton et al., 2006). Colorimetry gives limited information and destroys the sample under investigation, but it is invaluable to field inspectors because it is an inexpensive technique that requires very little training.
Disintegration and dissolution testing may identify common formulation problems. Disintegration tests measure how rapidly solid dosage forms disintegrate in a solution; dissolution tests analyze the rates at which drugs dissolve (USP, 2007). Dissolution tests require more training than colorimetry and disintegration testing but may help predict the bioavailability of drugs, an important aspect of their efficacy. If a drug has poor dissolution, then the target dose of active ingredient may not be available to the patient. Incorrect excipient formulation, poor-quality manufacturing, and improper storage conditions can all lead to poor dissolution (Kaur et al., 2010). Even if the drug contains the correct dose of active ingredient, disintegration and dissolution tests may be able to identify an illegitimate drug (Deisingh, 2005). Disintegration tests are fairly simple and can be done in the field, but dissolution tests require sophisticated equipment (Kaur et al., 2010).
Chromatography separates mixtures into their constituent parts based on a variety of chemical and physical properties. It can be used to separate drug ingredients for further testing and, when used with appropriate detectors, provides both qualitative and quantitative information about active ingredients and impurities (Kaale et al., 2011). Chromatography is therefore the most common analytical method used in drug evaluations (Martino et al., 2010). Chromatographic techniques range from basic techniques, such as thin layer chromatography (TLC) with visual inspection, to more specialized laboratory methods, such as high-performance liquid chromatography
(HPLC) coupled with mass spectrometry. Like colorimetric tests, chromatographic analysis destroys the drug sample.
TLC is a planar chromatographic technique that is ideal for field drug testing (Martino et al., 2010). In TLC comparisons, authentic samples travel the same distance on a TLC plate and yield main spots of highly similar shapes, colors, intensities, and sizes as reference standards. TLC is a qualitative and, when used with visual detection, semi-quantitative technique. The distance the sample travels is associated with its identity; the intensity of the spot correlates with the amount of the drug present. High concentrations of impurities may be visible on a TLC plate as well (Kaur et al., 2010). In a convenience sample of tuberculosis drugs in Botswana, TLC indicated 31 percent of the samples tested were substandard (Kenyon et al., 1999). In China, researchers used TLC to distinguish between authentic and falsified versions of several antibiotics (Hu et al., 2005).
TLC is an uncomplicated assay useful in developing countries because it yields “versatile and robust” results at a low cost (Kaale et al., 2011). Each TLC plate costs about $2, and most solvents used in TLC are common and inexpensive. The plates are only used once, preventing contamination and limiting maintenance requirements (Kaale et al., 2011). Compared to other chromatographic techniques such as HPLC, TLC requires significantly less equipment and expertise. Modern instrumental TLC applications give quantitative assessments similar to those obtained with other instrumental chromatography procedures. High-performance TLC is a more effective and efficient version of TLC. Disposable HPLC plates cost about $15 each but can run 18-36 samples at the same time (Kaale et al., 2011).
The main drawbacks to TLC are its limited semi-quantitative data (when used with visual detection) and the need for accurate technique (Kaale et al., 2011). TLC solvents are often toxic or flammable, so these chemicals may be difficult to transport for field use. Furthermore, TLC provides limited information about a drug’s identity; two samples that travel different distances are definitely not the same substance, but two different substances could appear identical using any chromatography technique if they are chemically similar enough. The inspector running the TLC assay must spot the plate correctly with the sample, which requires some training, and then compare the results to those obtained with reference standards. Accurately estimating the amount of drug on a TLC plate can be difficult without experience (Kaale et al., 2011). Despite its limitations, a trained operator can glean significant information from a TLC experiment with visual detection (Jähnke et al., 2001; Kaale et al., 2011).
Advanced chromatography techniques HPLC is a more selective technique and, when coupled with sensitive detectors, is generally regarded as the definitive technique for drug content analysis (Martino et al., 2010).
Depending on the associated detection technology, it can be expensive and require skilled operators and expensive, often scarce, solvents. The systems also require reliable electrical power, which can be an obstacle in developing countries.
Figure 6-3a shows an HPLC chromatogram that clearly distinguishes between the antimalarials chloroquine, mefloquine, and quinine. Although the drugs are chemically similar (see Figure 6-3b), mefloquine is significantly more expensive, and the cheaper drugs are sometimes sold labeled as mefloquine (Gaudiano et al., 2006). HPLC can identify and measure active ingredients and many impurities, but may not detect excipients that are not soluble in the mobile phase. It can be used with an array of detection technologies such as mass spectrometry and UV-visible spectroscopy (Martino et al., 2010).
Diode array detection is now standard with many HPLC assays and can be used to confirm the presence of active ingredients. It is a type of UV spectroscopy that is particularly useful because it can operate at varying wavelengths, allowing it to be fine-tuned for analyses, and can help detect the presence of several components hidden in a single HPLC peak (Kazakevich and McNair, 1996). Titier and colleagues developed an HPLC with diode array detection method to detect and quantify eight antidepressants for use in cases of suspected poisonings (Titier et al., 2003). The main advantages of the method were its speed, ease of use, and accuracy.
Gas chromatography, the most powerful chromatographic technology, provides similar information as the other chromatography systems. However, it may only be used for separation of volatile materials, such as residual solvents, undeclared ingredients, and any volatile impurities. This technique can only be used when the compounds of interest are gaseous in the analytical temperature range and do not degrade at or before the assay’s minimum temperature. For example, artemisinin derivatives for treating malaria are too unstable for gas chromatography (Martino et al., 2010).
Investigators can use gas chromatography to develop profiles of drugs’ volatile impurities and use those profiles to link batches of drugs from the same source. The great deal of natural variation in impurities allows this; even batches of genuine product from different sources are distinguishable, and the same is true among different falsified and substandard versions. In a review of the forensic applications of impurity profiles, Mulligan and colleagues concluded that drugs with very similar impurity profiles may be from the same place. Statistical analysis of impurity data can determine the probability that different samples have a common source (Mulligan et al., 1996).
Unlike TLC, advanced chromatography techniques require considerable investment; the equipment needed is expensive to buy and maintain (Kaale et al., 2011). These tests can only be done in central laboratories, and countries most affected by falsified and substandard drugs have limited access to such facilities (IOM, 2012). HPLC and gas chromatography are time-consuming, especially considering the time spent preparing the samples for analysis. The return on the time investment is mixed, as chromatography separates a minimum number of components present in a sample. A peak assumed to represent one compound may be hiding several other compounds.
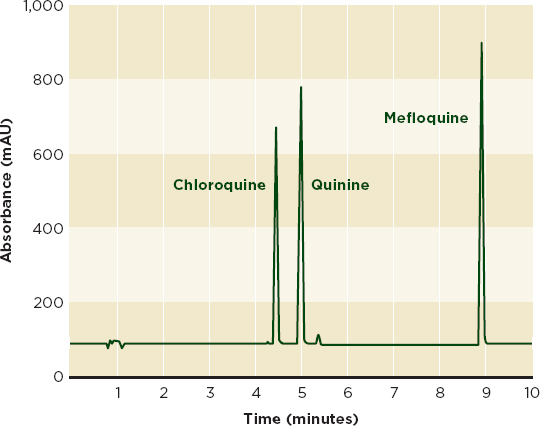
FIGURE 6-3a An HPLC chromatogram with distinct peaks for chloroquine, quinine, and mefloquine can be used to identify cheap chloroquine and quinine treatments labeled as the more expensive mefloquine.
NOTE: HPLC = high-performance liquid chromatography;
mAU = milli absorbance unit.
SOURCE: Adapted from Gaudiano et al., 2006. Reprinted with permission from Elsevier.
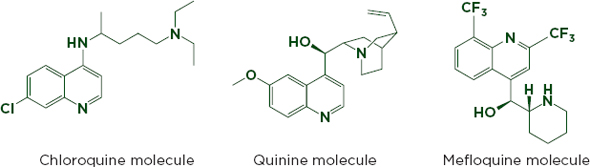
FIGURE 6-3b The similar chemical structures of chloroquine, quinine, and mefloquine.
Spectroscopy is a class of analytical techniques that measures the interaction of matter and radiation, thereby giving insight into chemical structure and contents. These techniques all provide qualitative data, and some provide significant quantitative data as well. Often referred to as the chemical fingerprints of drugs, the various spectra produced using these techniques elucidate different aspects of drug composition; characteristic absorption or emission peaks correspond to aspects of chemical composition and molecular structure. A chemist can extract detailed chemical and structural information from a spectrum, and an inspector with minimal training can also identify falsified and substandard medicines by comparing the drug spectra to reference materials in drug spectra databases (Kaur et al., 2010). The WHO maintains a digital version of the International Pharmacopoeia with drug quality determination protocols for many common medicines (WHO, 2011). This guide includes a reference infrared spectrum for each drug.
Molecular vibration and rotation energies occur in the infrared regions of the electromagnetic spectrum, and these movements may be observed with infrared, near-infrared, or Raman spectrometers. These techniques are relatively straightforward to use and moderately expensive, and routine comparative applications do not require extensive training. Chemists analyze the absorption peaks in these spectra primarily to identify molecular functional groups; most active pharmaceutical ingredients and some organic excipients and impurities have characteristic spectral peaks or spectral fingerprints that can be used to help identify them.
Infrared spectroscopy The infrared range of the electromagnetic spectrum can be divided into three subregions: the near-infrared, mid-infrared, and far-infrared. The mid-infrared range is the more discerning and commonly used region (Deisingh, 2005). Figure 6-4 shows the different infrared spectra of the antimalarial artemisinin and its derivative, artemether. This comparison can identify the common substitution of artemisinin for more effective and expensive antimalarials (Kaur et al., 2010).
There are several ways to collect infrared spectra, each having advantages and disadvantages. Attenuated total reflectance and Fourier-transformation infrared (FT-IR) is particularly useful for drug quality analyses because it does not require sample preparation, does not destroy the sample, and provides information about the distribution of active ingredients and excipients on the surface of tablets (Martino et al., 2010). A creative application of FT-IR can distinguish between some types of real and falsified packaging. Some manufacturers label their packaging to take advantage of the fact that only inks that absorb in the infrared range will be visible under infrared radiation. In an example from Singapore (see Figure 6-5), an inspector could see only a small amount of writing on a genuine Levitra package under IR radiation but could see all of the text on a falsified package (Lim, 2012).
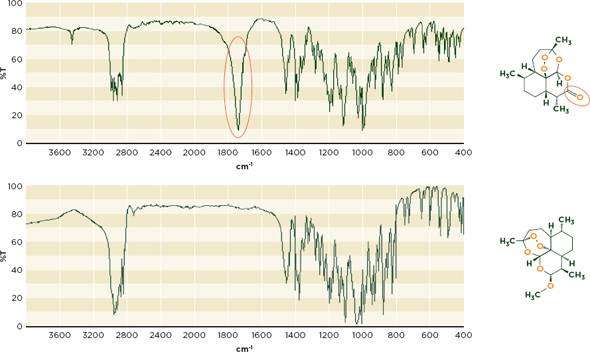
Figure 6-4 Infrared Spectra for artemisinin(top) and artemether, an artemisinin derivative(bottom) illustrate differences in their chemical composition. Artemisinin’s carbonyl(C=O) functional group, missing from the derivative molecule, produces a characteristic peak between 1700 and 1800 cm-1.
NOTE: cm-1= wave number;%T= percent transmittance.
SOURCE: Spectra from WHO, 2008.

FIGURE 6-5 Use of FT-IR spectroscopy to distinguish between real (left) and falsified (right) packaging in Singapore.
SOURCE: Lim, 2012.
Near-infrared and Raman spectroscopy Recent developments of portable near-infrared and Raman spectrometers have led to an increase in the use of these techniques for drug quality analysis (Fernandez et al., 2011). Both techniques are nondestructive, fast, and require no sample preparation; radiation can pass through samples in blister packs (Kaur et al., 2010; Martino et al., 2010).
Near-infrared is better suited than mid-infrared to quantitative analysis of drug contents. Computer modeling can produce limited quantitative characterization from all vibrational spectroscopy, but near-infrared and UV-visible spectroscopy yield more reliable quantitative data (Hsu, 1997). Near-infrared can identify active ingredients and is particularly useful for detecting incorrect concentrations of excipients, a common inconsistency in falsified and substandard drugs (Deisingh, 2005). When used with imaging techniques, near-infrared can yield information about a tablet’s composition. Koehler and colleagues demonstrated this by comparing images of a pain relief tablet, one captured using near-infrared imaging and the other not, and illustrating that the homogenous-looking tablet surface actually contained a heterogeneous mix of active and inactive ingredients (see Figure 6-6) (Koehler et al., 2002).
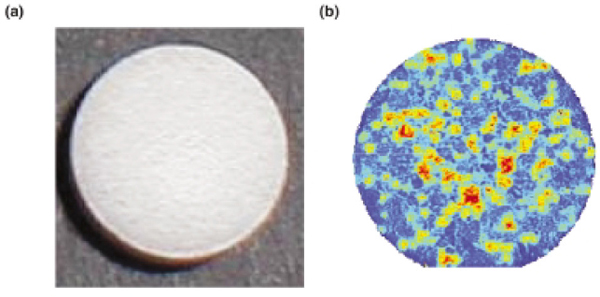
FIGURE 6-6 Image (a) is a pain relief tablet, image (b) its near-infrared spectra. The red spots indicate active ingredient and other colors indicate other ingredients.
SOURCE: Adapted from Koehler et al., 2002. Reprinted with permission from John Wiley & Sons, Ltd.
Near-infrared spectra of two different compounds are often only subtly different, and accurately interpreting results may require significant training (Martino et al., 2010). Portable, battery-powered near-infrared spectrometers are a more accessible alternative to traditional spectrometers (Dowell et al., 2008). Bate and colleagues compared the effectiveness of a handheld model to TLC and disintegration tests and found that the handheld spectrometer detected significantly more poor-quality antimalarial drugs and antibiotics than the other tests (Bate et al., 2009a). The model they used weighed 4 pounds and contained a battery that could operate for 10 hours after a full charge, making it a powerful field tool (Bate et al., 2009a).
Raman spectroscopy can readily identify many active ingredients and give further information about excipients, as well as the relative concentration of active ingredients to excipients (Deisingh, 2005). These ratios can be key to detecting falsified and substandard drugs, because criminal manufacturers often take care to use the correct amount of active ingredient but may not be as exacting about the excipients, which may vary even among genuine manufacturers (Deisingh, 2005; Nyadong et al., 2009). For example, artesunate tablets may contain either of the highly similar sugars lactose or sucrose, depending on the manufacturer (Nyadong et al., 2009). Raman can distinguish between these, and a Raman spectrum of Cialis identifies both the active ingredient, tadalafil, and the primary excipient, lactose (Lim, 2012). Raman spectroscopy is particularly useful for detecting
inorganic substances in drugs, such as titanium dioxide, a common component of tablet coatings (Witkowski, 2005).
On the other hand, some blister packs, capsule materials, and tablet coatings can interfere with Raman scattering and make readings difficult (Martino et al., 2010). If the materials used produce fluorescence, they interfere with Raman signals, especially those read with handheld Raman spectrometers. Though far more widely available and useful for field inspections, these portable devices have less tolerance for fluorescence than their full-sized counterparts. This is especially problematic in screening antimalarials, as artesunate is somewhat fluorescent (Martino et al., 2010). But some investigators maintain that the fluorescence of genuine artesunate can serve as a tool to distinguish between good- and poor-quality samples, as those without sufficient active ingredient will not produce as much fluorescence (Ricci et al., 2008). Ricci and colleagues found that fluorescence interfered more with their readings on the handheld scanner, but it ultimately produced as reliable results as the Fourier-transformed Raman scanner (Ricci et al., 2008).
Nuclear magnetic resonance Nuclear magnetic resonance (NMR) spectroscopy analyzes the interaction of nuclei with electromagnetic radiation while in magnetic fields. Like Raman and near-infrared spectrometry, it is a nondestructive, reliable technique, applicable to nuclei that have a non-zero spin, such as those in hydrogen and carbon-13, that yields quantitative data with little sample preparation. Figure 6-7 shows an NMR spectrum for o-acetoxybenzoic acid, the active ingredient in aspirin.

A handheld Raman spectrometer.
SOURCE: Zook, 2012.
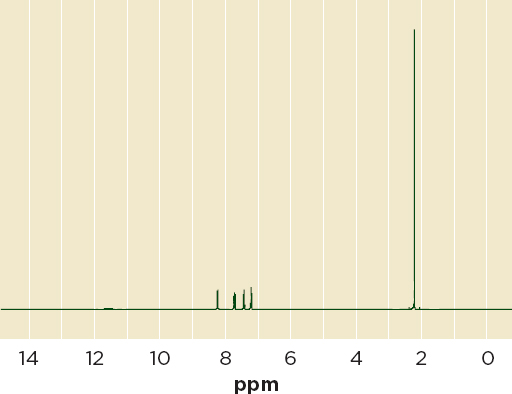
FIGURE 6-7 Proton NMR spectrum of aspirin (o-acetoxybenzoic acid).
NOTE: NMR = nuclear magnetic resonance; ppm = parts per million.
SOURCE: SBDSWeb.
However, NMR instruments are expensive and require stable electrical power supplies, controlled temperatures, and skilled analysts for their operation. Integrating the area under each absorption peak can provide detailed information about molecular composition and structure; the area under each peak corresponds to the number of nuclei (in protons or carbon-13 atoms) contributing to that particular signal. Many common chemical contaminants produce characteristic absorption peaks (Gottlieb et al., 1997).
In NMR analysis, all of the compounds in the mixture (including active ingredients, excipients, and impurities) that contain the nucleus under analysis will contribute to the spectrum. This can produce ambiguous spectra that may contain overlapping signals, so chemists typically isolate the components before analyzing them with NMR. However, newer, more sophisticated NMR technologies may be capable of separating drug components and producing clearer signals. Diffusion-ordered proton-NMR spectroscopy, for example, can identify the various types of ingredients in a mixture by taking advantage of differences in molecular mass (Martino et al., 2010). The downside to this type of technique is that it is not quantitative, like normal NMR is, but, by using the two techniques together, a
fuller, clearer molecular picture can be developed. Using these methods, scientists have successfully differentiated between many authentic and falsified versions of antimalarials, erectile dysfunction drugs, and antidepressants (Martino et al., 2010).
X-ray diffraction and X-ray fluorescence are other techniques that can give substantial information about drug contents. X-ray diffraction can be used to analyze active ingredients and excipients, while X-ray fluorescence is used for elemental analyses that can often distinguish real from falsified drugs (Kaur et al., 2010; Martino et al., 2010).
Mass spectrometry, generally called mass spec, is a sophisticated analytical technique that requires extensive training and expertise to use. It provides abundant structural information and the precise molecular weight of the compound under investigation. Mass spec can identify many active ingredients and excipients, as well as some impurities (Kaur et al., 2010; Martino et al., 2010). This technique successfully detected falsified halofantrine syrup, an antimalarial, in West Africa that instead contained a sulphonamide antibiotic (Wolff et al., 2003). When mass spectrometers were the size of a dishwasher (Stroh, 2007), their value in the poorest countries was hard to realize, but newer, portable machines can take this sophisticated technology into the field (Yang et al., 2008). However, mass spectrometers require a stable electrical power source, which may be difficult to obtain in some developing countries.
An isotope ratio mass spectrometer provides detailed information about the abundance of various elemental isotopes. Many elements have naturally occurring isotopes that are present in minute quantities in any sample. The exact ratio of isotopes varies over time and space and with different production techniques. Isotopic ratios have been able to distinguish different sources of drugs and therefore may be useful for combating highly sophisticated copies (Lim, 2012). Regulators and law enforcement can use isotopic ratios to connect seemingly disparate events and build evidence that separate drug seizures have a common source. Documenting the isotopic ratios of a selection of common elements, such as carbon, hydrogen, oxygen, or nitrogen, can help identify these patterns (Lim, 2012).
Other kinds of mass spectrometry (e.g., direct ionization, tandem, time-of-flight, secondary ion, and electrospray ionization [ESI]) can be used alone and in combination with other analyses to detect illegitimate drugs (Deisingh, 2005; Martino et al., 2010; Wolff et al., 2003). Direct ionization mass spec, for one, is a relatively new class of mass spectrometric analysis that does not require lengthy sample preparation. Other techniques, such as direct analysis in real time (DART) mass spec and desorption ESI mass spec,
can identify correct and incorrect active ingredients and some excipients. Desorption ESI mass spec in particular provides information about tablet surface homogeneity and the distribution of active ingredients and excipients in or on the surface of a tablet (Martino et al., 2010). For example, an artesunate sample with homogeneous surface distribution of lactose and paracetamol, a fever reducer, is illegitimate; an authentic, good-quality sample should have homogeneous distribution of artesunate and scattered distribution of lactose (Martino et al., 2010).
The most sophisticated drug copies may resist identification with any technology other than mass spectrometry. Among these are very close analogues of genuine active ingredients. These analogues can be so chemically and structurally similar that they behave the same under nearly any analysis. Mass spectrometry’s ability to precisely measure molecular weight and compare fragmentation patterns can help distinguish between compounds that differ by only one or two atoms. For example, the erectile dysfunction drug Cialis is often copied with varying degrees of sophistication (Putze et al., 2012; Trefi et al., 2008). U.S. Food and Drug Administration (FDA) forensic chemists have discovered several analogues of the active ingredient, tadalafil, in so-called herbal remedies (Gamble et al., 2008). Figure 6-8 compares the molecular structure of one such analogue, aminotadalafil, to tadalafil. The two differ only by the substitution of an amino (-NH2) group for a methyl (-CH3) group, making aminotadalafil slightly heavier. The health threats posed by such products have led researchers to investigate ways of reliably detecting and identifying these illicit drug compounds; other sophisticated techniques have been shown to detect some analogues, but the high specificity and sensitivity of mass spec makes it the most popular method (Singh et al., 2009; Venhuis and Kaste, 2012).
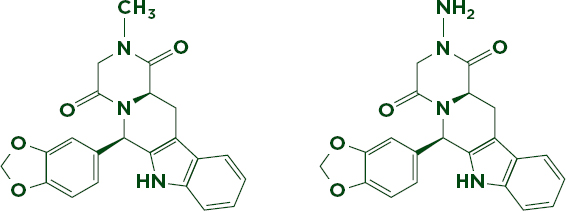
FIGURE 6-8 Nearly identical analogues of tadalafil (left, 389.40 g/mol), aminotadalafil (right, 390.39 g/mol). The only difference between the two compounds is the substitution of an amino group for a methyl group.
Innovative technologies to detect falsified and substandard drugs are constantly emerging. Many of the most promising examples draw from a range of scientific disciplines. Researchers in Pakistan found that the relative susceptibility of ofloxacin-sensitive bacteria to various samples of ofloxacin is a good indicator of drug quality (Iqbal et al., 2004). A team of researchers from U.S. Pharmacopeia and Boston University is developing another new technology called PharmaCheck (see Box 6-2). PharmaCheck uses microfluidics, the control of fluids at a sub-millimeter scale, for rapid field drug testing (EurekAlert, 2012). PharmaCheck, which will weigh less than 10 pounds and fit in a shoebox, promises to greatly reduce the need for confirmatory laboratory testing (Barlow, 2012; Gaffney, 2012).
Capillary electrophoresis, a separation technique, has recently been demonstrated to be a useful tool in the process of analyzing suspect pharmaceuticals (Marini et al., 2010). Staub and colleagues developed a capillary electrophoresis system paired with time-of-flight mass spectrometry for analyzing protein-based drugs, such as insulin, without sample preparation (Staub et al., 2010).
In a combined effort with the U.S. Pharmacopeial Convention, the United States Agency for International Development (USAID), and the Wallace H. Coulter Foundation, Muhammad Zaman of Boston University has been developing a PharmaCheck, a portable drug analysis device (Barlow, 2012). Called a “pharmaceutical lie detector” by the campus newspaper BU Today, Zaman’s machine uses fluorescence and imaging technologies to measure a sample’s potency (Barlow, 2012). The current prototype is the size of a shoebox, uses solar energy or battery power, and is designed as an “easy-to-use, robust system” for drug companies, nongovernmental organizations, and government agencies, among others (Barlow, 2012; Seiffert, 2012). Although originally developed for testing often copied malaria drugs, PharmaCheck will also be able to test other kinds of medications (Barlow, 2012). Zaman has said that the device should be undergoing testing in developing countries by early 2013 (Seiffert, 2012).
Saving Lives at Birth, a project developed by USAID, the Norwegian Ministry of Foreign Affairs, the Bill & Melinda Gates Foundation, Grand Challenges Canada, and the UK Department for International Development, runs a competition designed to find and support innovations in care for mothers and newborn children in developing countries (Saving Lives at Birth, 2012a,b). The organization recognized PharmaCheck’s potential with a $250,000 grant given to Zaman and his partners over the next 2 years to further develop the device, one of only 15 projects chosen out of the more than 500 applications (Saving Lives at Birth, 2012c; Seiffert, 2012).
Researchers at King’s College London and Lund University in Sweden have received a Translation Award from the Wellcome Trust to help bring their portable nuclear quadrupole resonance (NQR) device to market (Wellcome Trust, 2012). Based on technology similar to nuclear magnetic resonance spectroscopy, NQR uses radiofrequencies to provide qualitative and quantitative information about medicines and can scan them through packaging (Wellcome Trust, 2012; Wilkinson, 2012). Unlike most other techniques, NQR can analyze large quantities of medicine (an entire bottle or package) at one time (Barras et al., 2012). Radio wave technologies similar to those used in bomb detection are also being tailored for pharmaceutical analysis (Sprey, 2010).
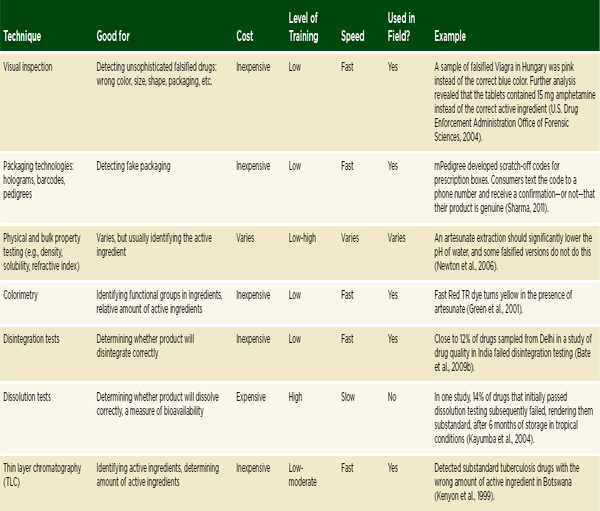
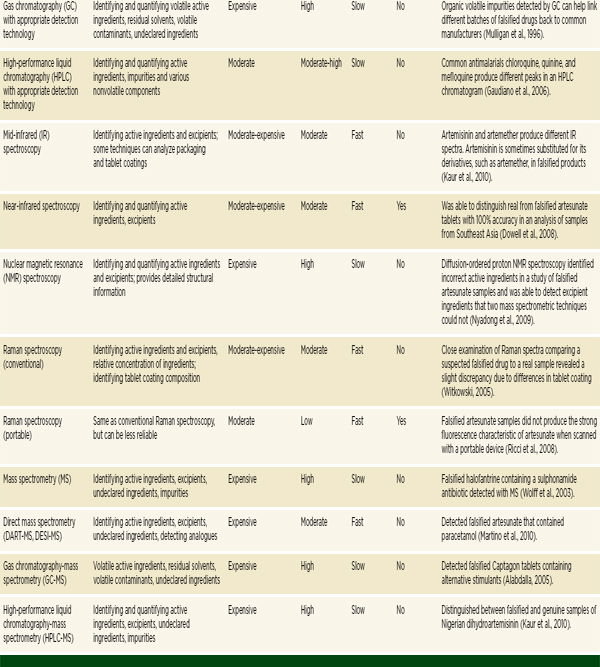
The previous section outlined the main categories of techniques for detecting falsified and substandard drugs. A summary of a selection of the techniques discussed is presented in Table 6-1. Understanding when, where, and why to use the various techniques can be difficult. The information a technique provides, as well as its reliability, cost, required expertise, speed, and portability make it more or less appropriate in any given situation.
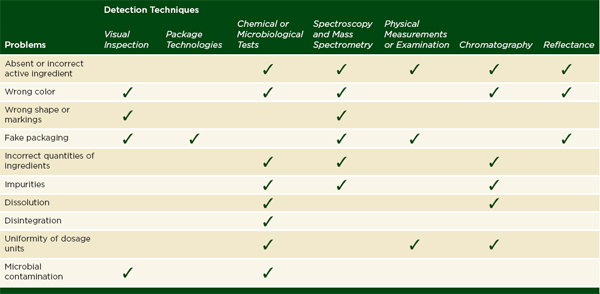
In order to conclude that a drug is of good quality, an inspector must test a sample for all of the main deficiencies of substandard and falsified drugs: fake packaging, incorrect color, shape, or markings, absent or incorrect active ingredients, incorrect quantities of ingredients, impurities, and reduced dissolution or disintegration. Table 6-2 outlines which classes of analytical techniques can test for these problems and how well they can be used in the field. In general, field use describes a relatively straightforward assay or technique that depends on portable or sturdy equipment. Most field methods can be used by professionals such as regulators, pharmacists, or health workers, but some, like mobile verification, are accessible to a layperson.
The Counterfeit Drug Forensic Investigation Network (CODFIN) uses a systematic analytical process to detect and classify substandard and falsified drugs (Fernandez et al., 2011). Figure 6-9 shows how investigators in the network test samples in national drug quality surveys (Fernandez et al., 2011). The steps shown in green can be done in the field, but samples are generally sent to a central laboratory for the steps show in brown (Fernandez et al., 2011). Fernandez and colleagues have used this system to investigate malarial drug quality in developing countries (Fernandez et al., 2011).
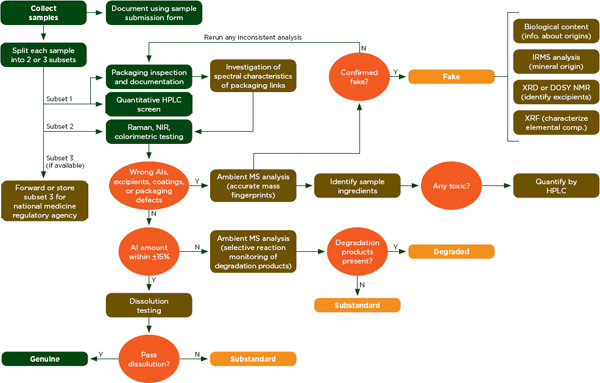
FIGURE 6-9 CODFIN analysis fl owchart.
NOTE: AI= active ingredient; CODFIN= Counterfeit Drug Forensic Investigation Network; DOSY NMR= diffusion ordered spectroscopy nuclear magnetic resonance; HPLC= high-performance liquid chromatography; IRMS= isotope-ratio mass spectrometry; MS= mass spectrometry; NIR= near infrared; XRD= x-ray diffraction; XRF= x-ray fluorescence.
SOURCE: Fernandez et al., 2011.
Key Findings and Conclusions
• There is no single analytical technique that provides enough information to confirm that a drug is genuine, but combining techniques gives more precision.
• It is often difficult to test for drug quality in low- and middle-income countries. Poorly trained chemists and dilapidated infrastructure are common obstacles in performing accurate drug quality testing.
• Making detection technologies easily accessible in low- and middle-income countries will help curtail the trade in falsified and substandard medicines.
• Field technologies and techniques are useful for detecting most falsified and substandard drugs. They should be easy to use and maintain, cheap, and durable.
• The costs associated with developing new detection technologies are a barrier to having robust, sustainable, easy-to-use, and inexpensive technologies available in the field.
Although any one test may suffice to label a drug substandard or falsified, no single analytical technique provides enough information to confirm that a drug is genuine. Similarly, while colorimetry and TLC are field techniques for testing for the presence of a particular ingredient, knowing a sample’s full content requires more testing. Spectroscopic techniques are useful for identifying active ingredients but cannot rule out the presence of countless possible impurities. Chromatographic techniques may suggest that the drug contains sufficient active ingredient, but they do not provide any information about how much of that active ingredient will reach the patient. Time and budget allowing, the best understanding of drug quality comes from the several complementary experiments.
Even combinations of techniques from within a class, such as spectroscopy, can be helpful. One study illustrated how, due to differences in the ranges of their spectral regions, infrared spectroscopy may at times be better at identifying organic substances in tablet coatings, whereas Raman spectroscopy may better identify the inorganic components (Witkowski, 2005). Experiments that looked at the coating on Cialis tablets found that Raman spectroscopy did not distinguish between the real coating and falsified coating, but infrared spectroscopy did (Lim, 2012).

Klaus Boehm of Merck presents minilabs to Hiti Sillo, head of the Tanzanian Food and Drugs Authority.
SOURCE: GPHF, 2012e.
Chemists typically pair mass spec with separation techniques, such as HPLC, to achieve a more definitive analysis. These hyphenated techniques have broad capabilities. For example, liquid chromatography-mass spectrometry is a highly reliable separation technique, but does not directly provide quantitative data about the amount of active ingredient present; analysts must compare results to standards to determine content (Kaur et al., 2010). A type of combined gas chromatography and mass spec (GC-MS) can provide information missing in HPLC-MS analysis. Mulligan and colleagues found that automated equilibrium headspace sampling with capillary gas chromatography provides information about volatile impurities, but adding mass spec analysis provides extra qualitative information about the identity of any impurities present (Mulligan et al., 1996).
When Captagon, a stimulant drug popular in the Middle East, was outlawed, illegal manufacturers began selling the drug (Alabdalla, 2005). The copies were generally falsified drugs containing amphetamines and caffeine meant to mimic Captagon’s therapeutic effects. Early investigations primarily relied on ultraviolet, infrared, and TLC analysis to determine the active ingredients in suspect tablets (Alabdalla, 2005). In 2005, Alabdalla and
colleagues used GC-MS analysis to further identify substitute ingredients, including chloroquine, ephedrine, caffeine, amphetamine, and methamphetamine (Alabdalla, 2005). The combined analysis also indicated, with reasonable certainty, which drugs were from the same batches (Alabdalla, 2005). Where applicable, GC-MS plots are an unequivocal way to identify substances (Rivier, 2003). Courts prefer them to other analytical techniques as forensic evidence (Rivier, 2003).
Combining analytical techniques is a challenge both in the field and in the laboratory. It is difficult to determine which tests can be combined to allow inspectors to use the minimum number of different techniques. It is usually best to work through tests beginning with the easiest or least expensive ones and to only move on to the more expensive or difficult tests if the sample passes the earlier ones. For example, a drug that fails an identity test does not need to be tested for the amount of incorrect active ingredient. This is the basis of the minimum testing scheme used by the Pharmaceutical Security Institute (USP, 2007).
Using Technology in Developing Countries
The question remains as to how to use analytical methods in parts of the world with limited laboratory capacity and trained chemists. Reliable reference materials to test samples against are often scarce in poor countries (Fernandez et al., 2011). Manufacturers are reluctant to release reference standards when they fear the information could be used to make an illegitimate drug. In any case, the most sophisticated analytic technologies were not designed for the field.
Technologies for field detection of falsified and substandard drugs in developing countries must be portable, relatively simple to use, sturdy, and inexpensive to buy, use, and maintain. They must also provide reliable, useful data. Field techniques (including visual inspection, colorimetry, disintegration tests, TLC, and handheld spectrometry) can detect many falsified and substandard drugs. As the previous section explains, these techniques are durable, fast, relatively inexpensive, and fairly easy to use, making them attractive to regulators interested in monitoring drug quality. Box 6-3 describes the Chinese regulatory authority’s mobile verification labs. Package verification technologies can also aid in field detection of falsified drugs, although these methods are useful more to the patient at the point of use than to the regulator.
The more reliable field analytic tools are also more expensive. Although fairly inexpensive TLC and disintegration testing are useful field techniques, according to Bate they are less reliable than handheld spectrometric devices (Bate et al., 2009a). Of 78 samples tested in one study, 17 passed both TLC and disintegration tests but did not pass either Raman or near-infrared spectroscopic analysis (Bate et al., 2009a). Field tests are no substitute for definitive laboratory techniques and cannot test all aspects of a product’s quality, including its drug content, impurity profile, and dissolution profile.
BOX 6-3
Chinese Mobile Laboratories
The Chinese drug regulatory authority uses mobile labs for drug surveillance (Jin, 2007). First used in Henan province in March 2006, mobile labs quickly spread to 29 provinces (Jin, 2007). The mobile lab program also trained 760 technicians to operate the labs, which bring drug screening technology to rural areas (Jin, 2007).
The mobile labs, housed in vans, can carry out rapid on-site screening of suspicious drugs (NICPBP, 2012). Each van carries chemical analysis technologies, including TLC systems, a near-infrared spectrometer, and portable computers (NICPBP, 2012). The vans also house information on 200,000 manufacturers, including names, addresses, and licensed products, as well as a provincial “Drug Quality Bulletin” with annually updated information on known poor-quality drugs (Jin, 2007). The labs’ operations are designed to be simple, fast, and easily executed (NICPBP, 2012). A mobile lab can test the quality of more than 800 drugs, including antimalarials, antiretroviral, tuberculosis medication, other essential drugs, and traditional Chinese herbal medicines (Jin, 2007; NICPBP, 2012).
In the first 6 months of operation, mobile labs screened 110,426 batches of drugs and confirmed 3,122 of them to be substandard (Jin, 2007). The project’s success has inspired the regulatory authority to help other countries develop similar mobile labs and to use them in drug procurement (Jin, 2007). Members of the Thai FDA, for example, visited Chinese mobile labs in 2006 (Jin, 2007).

A mobile lab in China.
Noting the cost of laboratory pharmaceutical testing and the dearth of qualified laboratories in developing countries, the German Pharma Health Fund (now known as the Global Pharma Health Fund) developed the Minilab, a portable quality-analysis laboratory described in Box 6-4 (Jähnke et al., 2001; Kaale et al., 2011). During a November 2012 Minilab training session in Angola, trainees tested an illegal shipment of various pharmaceuticals seized by customs officials along the African coast (Minilab Saves Lives, 2012; World Customs Organization, 2012). Using the TLC and visual inspection techniques, they identified many drugs with no or little active ingredient (Minilab Saves Lives, 2012). Merck S.A. in Portugal provided 10 Minilabs to Angola, which has no drug testing labs (Minilab Saves Lives, 2012). Other similar field kits also exist, such as the Thermo Scientific FirstDefender and TruDefender field laboratory devices used by the Singaporean regulatory authority (Lim, 2012).
There is a wide range of technology available to detect falsified and substandard drugs; a good prevention strategy makes use of a wide variety of them. As Chapter 5 describes, some technologies, such as scratch-off codes, can be used by the consumer. There are also package technologies manufacturers may use to distinguish their products at the point of purchase. Holograms and reactive ink are examples of such package technologies. Holograms can be convincingly copied, as illustrated in Figure 6-10, but may give customers an extra level of assurance. Similarly, Brazil requires all drug companies to mark packages with a scratch-off label made from a reactive ink (Filho et al., 2010), though participants at the São Paulo site visit for this study expressed consistent doubt that consumers were adequately informed about how to use the label.
Informed patients can assist in identifying falsified and substandard drugs. Visual inspection of drug packages and color can identify gross differences between authentic and fake medicines. Similarly, patients might detect microbial contamination, seen as black specks on the surface of the product with the naked eye, or notice defects in a drug’s hardness when handling it. Table 6-2 describes the limits of visual inspection and other types of inspection.
Pharmacists are able to run a wider variety of tests to detect problems with medicine quality. If properly equipped, a pharmacist can run colorimetric tests and TLC on suspect samples in the pharmacy. The pharmacist, or a lower-level pharmacy worker, is also key in monitoring the chain of custody in track-and-trace systems. Field inspectors can take a similar role, especially in places where there are few trained pharmacists. As Boxes 6-3 and 6-4 explain, mobile testing is an important piece of drug quality monitoring in much of the world. Field inspectors can use handheld spectrometers and Minilabs to evaluate drug quality.
BOX 6-4
The Global Pharma Health Fund Minilab
The Global Pharma Health Fund Minilab is a portable drug quality analysis toolkit (Kaale et al., 2011). The Minilab was designed to help control the proliferation of substandard and falsified drugs in countries with weak or absent regulatory systems (Jähnke et al., 2001).
The Minilab relies on a combination of accessible techniques for simple, fast, and reliable detection of falsified and substandard drugs. With the exception of running water and a flat surface on which to work, the kit contains all the labware, reagents, standards for comparison, and instructions necessary to run quality tests on many common medicines. Each Minilab fits into two suitcases for durable portability. Price and simplicity guided the kit’s design; the solvents and reagents used in the assessments are safe for use with very little training and are widely available and inexpensive. Each Minilab quality test costs no more than $3 to run (GPHF, 2012c; Kaale et al., 2011).

SOURCE: GPHF, 2012d.
The kit includes equipment and instructions for thin layer chromatography (TLC), chemical colorimetry, and disintegration tests, as well as a visual inspection protocol. Testing and inspection protocols and materials are included for more than 50 World Health Organization essential medicines, including reference standards for 63 drug compounds (GPHF, 2012a; Kaale et al., 2011). By using colorimetry, which tests for the identity of active ingredients, and TLC, which provides information about potency, the kit is capable of testing for the top three kinds of substandard and falsified drugs: those that contain no active ingredient, those that contain too little active ingredient, and those that contain the wrong active ingredient (GPHF, 2012c; Jähnke et al., 2001). Since the reliability of TLC is based in large part on the tester’s level of training, the Minilab attempts to simplify the analysis by providing reference tablets that can be used to prepare 100 percent and 80 percent dosage strengths for comparison (Kaale et al., 2011).
Currently, there are more than 500 Minilabs in 80 countries, and many prominent national and international organizations recommend the kits for field testing (GPHF, 2012c). The U.S. Pharmacopeial Convention distributes and administers trainings for Minilabs in developing countries through its Drug Quality and Information program, in collaboration with USAID (Smine and Hajjou, 2009). The lab has also been used by the WHO’s Roll Back Malaria program and by several local nongovernmental organizations in countries such as Tanzania and Ghana (Jähnke et al., 2001).
Field inspectors feed useful information about drug quality into the regulatory system. Regulators have higher-level controls to detect poor manufacturing and product quality in the market. Ultimately, no detection
technology can replace stringent drug regulation in the fight against falsified and substandard drugs. The sentiment that no one can test quality into drugs is true to a certain extent. It is important to be able to test drug quality, but also important to impose good manufacturing practices on companies to prevent quality problems before they arise. However, effective use of technology can help improve drug quality. A study on drug quality in Nigerian pharmacies before and after handheld spectrometers were distributed indicated that drug quality improved when testing became more reliable and convenient (Bate and Mathur, 2011).
Making detection technology more accessible in low- and middle-income countries is invaluable to controlling the trade in falsified and substandard drugs. Technologies can protect consumers and also help generate accurate estimates of the magnitude of the problem. An understanding of the technological landscape, the range and gaps in available technologies, and the likely improvements in the near future is necessary for using technologies in developing countries.

FIGURE 6-10 Genuine (left) and falsified (right) holograms on artesunate blister packs found in Southeast Asia.
SOURCE: Newton et al., 2008.
Technology is a constantly evolving field. New techniques developed specifically for detection and analysis are always emerging. As some of the standard assessment techniques become smaller, lighter, cheaper, and more durable, the boundary between field and laboratory testing is blurring. Navigating the technological landscape is a formidable challenge, especially in low- and middle-income countries. The committee believes that interdisciplinary collaboration yields the best and most efficient advances in detection technologies, especially technologies that can be useful in developing countries.
Regulators in these countries have relatively infrequent opportunities to interact with academic and industry experts (IOM, 2012). Working in relative isolation translates into few opportunities to advocate for research on their behalf. This chapter gives some overview of the detection technologies that exist now, but a different expert working group could better articulate what technologies will be useful in the future. It is also unclear under what conditions the cost-to-benefit analysis favors the use of different detection technologies.
Recommendation 6-1: The National Institute of Standards and Technology should fund the development of a central repository for existing and newly innovative detection, sampling, and analytical technologies, ranging from field and rapid screening technology to sophisticated laboratory-based assessments, to identify substandard and falsified medicines.
The cost of development is the main barrier to having robust, sustainable, easy-to-use, and inexpensive detection technologies available in the field. The committee believes that public funding for development would direct academic interest and attention to this important problem. The National Institute of Standards and Technology (NIST), a division of the Department of Commerce, has the depth in physical and materials science necessary for developing and adapting drug testing technologies (NIST, 2008). The institute is committed to innovative interdisciplinary research for bioscience and health (NIST, 2010). Drug quality analysis draws from
material, basic, and computer science, and a range of engineering disciplines. The FDA and the pharmaceutical industry also have technical depth in these areas, and they should work with NIST on a technical working group about drug detection technologies. The NIST has worked closely with the FDA before, such as in their work on the measurement of drug delivery systems with secondary ion mass spectrometry (NIST, 2009a).

At a Minilab training session in Angola, field inspectors learn how to test drug quality.
SOURCE: Minilab Saves Lives, 2012.
Every year, the institute’s Small Business Innovation Research (SBIR) program awards contracts to small businesses for science and engineering research (NIST, 2009b). Proposals need to respond to the specific terms set out in the SBIR annual solicitation (NIST, 2009b). Although an emphasis on field technologies that are useful in developing countries would be a departure from the Department of Commerce’s charge of promoting American industry, there is enough of a shared stake in drug safety that they might consider a SIBR solicitation for innovative technologies to detect poor-quality drugs.
There is considerable scope for innovative research in drug detection
and analysis. All of the methods described in this chapter, for example, are relevant to small molecules, but hormones, oral contraceptives, low-dose vaccines, and biologics are also vulnerable to quality failures, failures that are much harder to detect. Even the existing technologies to detect falsified and substandard small molecules could be improved. For example, the Minilab, a useful and elegant kit, can test only 63 drugs (GPHF, 2012b). The Global Pharma Health Fund should expand this inventory; the WHO should help identify which products are the first priority for inclusion.
TABLE 6-1 Techniques for Detecting Poor-Quality Drugs
Similarly, expansion of the Raman active ingredient database would make handheld Raman spectrometers more useful in detecting falsified drugs. All drug detection technologies would be more powerful if there were a full authentication database with information about drug color, shape, size, weight, Raman and near-infrared reflectance, and a TLC procedure for assay. Drug companies may balk at releasing this information, but the committee believes that stringent regulatory agencies should require it. Sharing all drug authentication information in a drug quality library would vastly improve the power of existing drug detection technologies.
Alabdalla, M. A. 2005. Chemical characterization of counterfeit Captagon tablets seized in Jordan. Forensic Science International 152:185-188.
Barlow, R. 2012. A new counterfeit problem: Anti-malarial drugs. BU Today, July 26.
Barras, J., K. Althoefer, M. D. Rowe, I. J. Poplett, and J. A. S. Smith. 2012. The emerging field of medicines authentication by nuclear quadrupole resonance spectroscopy. Applied Magnetic Resonance 43(4):511-529.
Bate, R., and A. Mathur. 2011. Working paper: The impact of improved detection technology on drug quality: A case study of Lagos, Nigeria. Washington, DC: American Enterprise Institute.
Bate, R., R. Tren, K. Hess, L. Mooney, and K. Porter. 2009a. Pilot study comparing technologies to test for substandard drugs in field settings. African Journal of Pharmacy and Pharmacology 3(4):165-170.
Bate, R., R. Tren, L. Mooney, K. Hess, B. Mitra, B. Debroy, and A. Attaran. 2009b. Pilot study of essential drug quality in two major cities in India. PLoS ONE 4(6):e6003.
Brown, T., H. E. LeMay, B. Bursten, C. Murphy, and P. Woodward. 2011. Chemistry: The central science. 12th ed. Boston, MA: Prentice Hall.
Deisingh, A. K. 2005. Pharmaceutical counterfeiting. Analyst 130(3):271-279.
Dowell, F. E., E. B. Maghirang, F. M. Fernandez, P. N. Newton, and M. D. Green. 2008. Detecting counterfeit antimalarial tablets by near-infrared spectroscopy. Journal of Pharmaceutical and Biomedical Analysis 48:1011-1014.
EurekAlert. 2012. New technology represents next-generation tool for detecting substandard and counterfeit medicines. http://www.eurekalert.org/pub_releases/2012-07/up-ntr072612.php (accessed January 31, 2013).
Fernandez, F. M., M. D. Green, and P. N. Newton. 2008. Prevalence and detection of counterfeit pharmaceuticals: A mini review. Industrial and Engineering Chemistry Research 47:585-590.
Fernandez, F. M., D. Hostetler, K. Powell, H. Kaur, M. D. Green, D. C. Mildenhall, and P. N. Newton. 2011. Poor quality drugs: Grand challenges in high throughput detection, countrywide sampling, and forensics in developing countries. Analyst 136(15):3073-3082.
Filho, A. F. M., P. Blanco, and L. E. Ferreira. 2010. Pharmaceutical products traceability system pilot project in Brazil. 2010/2011 GS1 Healthcare Reference Book. http://www.gs1.org/docs/healthcare/case_studies/Case_study_Brazil_pharma_traceability.pdf (accessed March 7, 2013).
Gaffney, A. 2012. New portable anti-counterfeiting technology promises “paradigm shift. ” http://www.raps.org/focus-online/news/news-article-view/article/2020/new-portable-anti-counterfeiting-technology-promises-paradigm-shift.aspx (accessed March 7, 2013).
Gamble, B. M., S. R. Gratz, J. J. Litzau, K. J. Mulligan, and R. A. Flurer. 2008. FDA forensic investigations using mass spectrometry. Paper presented at Conference on Small Molecule Science, San Jose, CA.
Gaudiano, M. C., E. Antoniella, P. Bertocchi, and L. Valvo. 2006. Development and validation of a reversed-phase lc method for analysing potentially counterfeit antimalarial medicines. Journal of Pharmaceutical and Biomedical Analysis 42:132-135.
Gottlieb, H. E., V. Kotlyar, and A. Nudelman. 1997. NMR chemical shifts of common laboratory solvents as trace impurities. Journal of Organic Chemistry 62:7512-7515.
GPHF (Global Pharma Health Fund). 2012a. GPHF-Minilab®—fact sheet. http://www.gphf.org/web/en/minilab/factsheet.htm (accessed July 30, 2012).
———. 2012b. The GPHF-Minilab®—focusing on prevalent medicines against infectious diseases. http://www.gphf.org/web/en/minilab/wirkstoffe.htm (accessed November 5, 2012).
———. 2012c. The GPHF-Minilab®—protection against counterfeit medicines. http://www.gphf.org/web/en/minilab/index.htm (accessed July 30, 2012).
———. 2012d. The Global Pharma Health Fund (GPHF). http://www.gphf.org/web/en/start/index.htm (accessed October 24, 2012).
———. 2012e. Tanzanian food and drugs authority receives four unique mobile compact laboratories. http://www.gphf.org/web/en/news/pressemitteilungen.htm?showid=164 (accessed October 24, 2012).
Green, M. D., D. L. Mount, and R. A. Wirtz. 2001. Short communication: Authentication of artemether, artesunate and dihydroartemisinin antimalarial tablets using a simple colorimetric method. Tropical Medicine & International Health 6(12):980-982.
Green, M. D., H. Nettey, O. V. Rojas, C. Pamanivong, L. Khounsaknalath, M. G. Ortiz, P. N. Newton, F. M. Fernández, L. Vongsack, and O. Manolin. 2007. Use of refractometry and colorimetry as field methods to rapidly assess antimalarial drug quality. Journal of Pharmaceutical and Biomedical Analysis 43(1):105-110.
Hsu, C.-P. S. 1997. Infrared spectroscopy. In Handbook of instrumental techniques for analytical chemistry, edited by F. Settle. Arlington, VA: National Science Foundation. Pp. 247-283.
Hu, C.-Q., W.-B. Zou, W.-S. Hu, K.-K. Ma, M.-Z. Yang, S.-L. Zhou, J.-F. Sheng, Y. Li, S.-H. Cheng, and J. Xue. 2005. Establishment of a fast chemical identification system for screen of counterfeit drugs of macrolide antibiotics. Journal of Pharmaceutical and Biomedical Analysis 40:68-74.
IOM (Institute of Medicine). 2012. Ensuring safe foods and medical products through stronger regulatory systems abroad. Washington, DC: The National Academies Press.
Iqbal, M., S. T. Hakim, A. Hussain, Z. Mirza, F. Qureshi, and E. M. Abdulla. 2004. Ofloxacin: Laboratory evaluation of the antibacterial activity of 34 brands representing 31 manufacturers available in Pakistan. Pakistani Journal of Medical Science 20(4):349-356.
Jähnke, R. W. O., H. J. Kallmayer, C. Breyer, M. D. Green, V. Rubeau, and A. Paulke. 2008. A concise quality control guide on essential drugs and other medicines: Colour reaction tests. Germany: Global Pharma Health Fund.
Jähnke, R. W. O., G. Küsters, and K. Fleischer. 2001. Low-cost quality assurance of medicines using the GPHF-Minilab®. Drug Information Journal 35:941-945.
Jin, S. 2007. Mobile labs developed in China for detection of counterfeit drugs. Paper read at 3rd Global Forum on Pharmaceutical Anticounterfeiting, Prague, Czech Republic.
Kaale, E., P. Risha, and T. Layloff. 2011. TLC for pharmaceutical analysis in resource limited countries. Journal of Chromatography A 1218(19):2732-2736.
Kaur, H., M. D. Green, D. M. Hostetler, F. M. Fernández, and P. N. Newton. 2010. Antimalarial drug quality: Methods to detect suspect drugs. Therapy 7(1):49-57.
Kayumba, P. C., P. G. Risha, D. Shewiyo, A. Msami, G. Masuki, D. Ameye, G. Vergote, J. D. Ntawukuliryayo, J. P. Remon, and C. Vervae. 2004. The quality of essential antimicrobial and antimalarial drugs marketed in Rwanda and Tanzania: Influence of tropical storage conditions on in vitro dissolution. Journal of Clinical Pharmacy and Therapeutics 29:331-338.
Kazakevich, Y., and H. McNair. 1996. Diode-array detectors. In Basic liquid chromatography: Text book on high performance liquid chromatography (HPLC). http://hplc.chem.shu.edu/HPLC/index.html (accessed March 7, 2013).
Kenyon, T. A., A. S. Kenyon, B. V. Kgarebe, D. Mothibedi, N. J. Binkin, and T. P. Layloff. 1999. Detection of substandard fixed-dose combination tuberculosis drugs using thin-layer chromatography. International Journal of Tuberculosis and Lung Disease 3(11):5347-5350.
Koehler, F., E. Lee, L. Kidder, and E. N. Lewis. 2002. Near infrared spectroscopy: The practical chemical imaging solution. Spectroscopy Europe 14(3):12-19.
Lim, C. C. 2012. Detection of counterfeit drugs—Singapore’s approach. Presentation at Understanding the Global Public Health Implications of Counterfeit, Falsified, and Substandard Drugs: Meeting One, Washington, DC, March 12-13.
Marini, R. D., E. Rozet, M. L. A. Montes, C. Rohrbasser, S. Roht, D.Rhème, P. Bonnabry, J. Schappler, J.-L. Veuthey, P. Hubert, and S. Rudaz. 2010. Reliable low-cost capillary electrophoresis for drug quality control and counterfeit medicines. Journal of Pharmaceutical and Biomedical Analysis 53(5):1278-1287.
Martino, R., M. Malet-Martino, V. Gilard, and S. Balayssac. 2010. Counterfeit drugs: Analytical techniques for their identification. Analytical and Bioanalytical Chemistry 398(1): 77-92.
Minilab Saves Lives. 2012. Caught in the act, first by customs, then by minilabs. https://www.facebook.com/media/set/?set=a.10151243315994666.481256.182507359665&type=1 (accessed November 14, 2012.
Mulligan, K. J., T. W. Bruggemeyer, D. F. Crockett, and J. B. Schepman. 1996. Analysis of organic volatile impurities as a forensic tool for the examination of bulk pharmaceuticals. Journal of Chromatography B 686:85-95.
Newton, P. N., F. M. Fernández, A. Plançon, D. C. Mildenhall, M. D. Green, L. Ziyong, E. M. Christophel, S. Phanouvong, S. Howells, E. McIntosh, P. Laurin, N. Blum, C. Y. Hampton, K. Faure, L. Nyadong, C. W. R. Soong, B. Santoso, W. Zhiguang, J. Newton, and K. Palmer. 2008. A collaborative epidemiological investigation into the criminal fake artesunate trade in South East Asia. PLoS Medicine 5(2):e32.
Newton, P. N., M. D. Green, F. M. Fernández, N. P. J. Day, and N. J. White. 2006. Counterfeit anti-infective drugs. Lancet Infectious Diseases 6(9):602-613.
NICPBP (National Institute for the Control of Pharmaceutical and Biological Products). 2012. Introduction to mobile labs. http://www.nicpbp.org.cn/en/CL0324 (accessed November 5, 2012).
NIST (National Institute of Standards and Technology). 2008. NIST general information. http://www.nist.gov/public_affairs/general_information.cfm (accessed November 5, 2012).
———. 2009a. Next generation metrology for drug delivery systems. http://www.nist.gov/mml/mmsd/83705-next-generation-metrology-for-drug-delivery-systems.cfm (accessed November 26, 2012).
———. 2009b. Small business innovation research program. http://www.nist.gov/tpo/sbir/index.cfm (accessed November 5, 2012).
———. 2010. Bioscience & health portal—overview. http://www.nist.gov/bioscience-and-health-portal.cfm (accessed November 26, 2012).
Nyadong, L., G. A. Harris, S. Balayssac, A. S. Galhena, M. Malet-Martino, R. Martino, R. M. Parry, M. D. Wang, F. M. Fernández, and V. Gilard. 2009. Combining two-dimensional diffusion-ordered nuclear magnetic resonance spectroscopy, imaging desorption electrospray ionization mass spectrometry, and direct analysis in real-time mass spectrometry for the integral investigation of counterfeit pharmaceuticals. Analytical Chemistry 81(12):4803-4812.
Power, G. 2008. Anti-counterfeit technologies for the protection of medicines. Geneva: IMPACT and WHO.
Putze, E., E. Conway, M. Reilly, and O. Madrid. 2012. The deadly world of fake drugs. Washington, DC: American Enterprise Institute.
Ricci, C., L. Nyadong, F. Yang, F. M. Fernandez, C. D. Brown, P. N. Newton, and S. G. Kazarian. 2008. Assessment of hand-held Raman instrumentation for in situ screening for potentially counterfeit artesunate antimalarial tablets by ft-Raman spectroscopy and direct ionizatoin mass spectrometry. Analytica Chimica Acta 623:178-186.
Rivier, L. 2003. Criteria for the identification of compounds by liquid chromatography-mass spectrometry and liquid chromatography-multiple mass spectrometry in forensic toxicology and doping analysis. Analytica Chimica Acta 492:69-82.
Saving Lives at Birth. 2012a. The challenge. http://savinglivesatbirth.net/challenge (accessed March 7, 2013).
———. 2012b. Partners. http://savinglivesatbirth.net/partners (accessed March 7, 2013).
———. 2012c. Round 2 innovators. http://savinglivesatbirth.net/innovation/2012/innovators (accessed November 14, 2012).
Seiffert, D. 2012. BU professor gets grant for counterfeit drug detector. Mass High Tech, August 1.
Sharma, Y. 2011. Fighting fake drugs with high-tech solutions. http://www.scidev.net/en/health/detecting-counterfeit-drugs/features/fighting-fake-drugs-with-high-tech-solutions-1.html (accessed March 7, 2013).
Sherma, J. 2007. Analysis of counterfeit drugs by thin layer chromatography. Acta Chromatographica 19:5-20.
Singh, S., B. Prasad, A. A. Savaliya, and R. P. Shah. 2009. Strategies for characterizing sildenafil, vardenafil, tadalafil, and their analogues in herbal dietary supplements, and detecting counterfeit products containing these drugs. TRAC 28(1):13-28.
Smine, A., and M. Hajjou. 2009. USP DQI workshop on basic tests of antimalarials using Minilabs®: Establishing drug quality monitoring in five sentinel sites in Ghana. Rockville, MD: U.S. Pharmacopeia Drug Quality and Information Program.
Sprey, K. 2010. Using radio waves to identify counterfeit drugs. http://www.gizmag.com/using-radio-waves-to-identify-counterfeit-drugs/16233 (accessed March 7, 2013).
Staub, A., S. Rudaz, J.-L. Veuthey, and J. Schappler. 2010. Multiple injection technique for the determination and quantitation of insulin formulations by capillary electrophoresis and time-of-flight mass spectrometry. Journal of Chromatography A 1217(51):8041-8047.
Stroh, M. 2007. Nose for trouble: A portable germ scanner exposes tainted food. Popular Science, September.
Titier, K., N. Castaing, E. Scotto-Gomez, F. Pehourchq, N. Moore, and M. Molimard. 2003. High-performance liquid chromatographic method with diode array detection for identification and quantification of the eight new antidepressants and five of their active metabolites in plasma after overdose. Therapeutic Drug Monitor 25(5):581-587.
Trefi, S., C. Routaboul, S. Hamieh, V. Gillard, M. Malet-Martino, and R. Martino. 2008. Analysis of illegally manufactured formulations of tadalafil (Cialis®) by 1h NMR, 2D DOSY 1h NMR and Raman spectroscopy. Journal of Pharmaceutical and Biomedical Analysis 47(1):103-113.
U.S. Drug Enforcement Administration Office of Forensic Sciences. 2004. Intelligence alert: Viagra® mimic tablet containing amphetamine in Fejer County, Hungary. Microgram Bulletin 37(6):106-107.
USP (U.S. Pharmacopeia). 2007. Ensuring the quality of medicines in resource-limited countries: An operational guide. Rockville, MD: U.S. Pharmacopeia Drug Quality and Information Program.
Venhuis, B. J., and D. d. Kaste. 2012. Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: A history, analytical aspects and health risks. Journal of Pharmaceutical and Biomedical Analysis 69:196-208.
Wellcome Trust. 2012. Enabling technlogy. http://www.wellcome.ac.uk/Funding/Technology-transfer/Funded-projects/Enabling-technology/index.htm (accessed March 7, 2013).
WHO (World Health Organization). 2008. The international pharmacopoeia. Second Supplement, Fourth Ed. Geneva: WHO.
———. 2011. The international pharmacopoeia: Fourth edition 2011 (incl. first and second supplements). http://apps.who.int/phint/en/p/about (accessed March 7, 2013).
Wilkinson, N. 2012. Briefcase encounter: An invention to detect fake drugs. Wellcome Trust. http://wellcometrust.wordpress.com/2012/11/12/briefcase-encounter-an-invention-to-detect-fake-drugs (accessed March 7, 2013).
Witkowski, M. R. 2005. The use of Raman spectroscopy in the detection of counterfeit and adulterated pharmaceutical products. American Pharmaceutical Review 8:56-62.
Wolff, J.-C., L. A. Thomson, and C. Eckers. 2003. Identification of the “wrong” active pharmaceutical ingredient in a counterfeit Halfan™ drug product using accurate mass electrospray ionisation mass spectrometry, accurate mass tandem mass spectrometry and liquid chromatography/mass spectrometry. Rapid Communications in Mass Spectrometry 17:215-221.
World Customs Organization. 2012. High-impact customs operation tackles illicit medicines in Africa. http://www.wcoomd.org/en/media/newsroom/2012/october/high-impact-customs-operation-tackles-illicit-medicines-in-africa.aspx (accessed November 14, 2012).
Yang, M., T.-Y. Kim, H.-C. Hwang, S.-K. Yi, and D.-H. Kim. 2008. Development of a palm portable mass spectrometer. Journal of the American Society for Mass Spectrometry 19:1442-1448.
Zook, A. 2012. Detection of counterfeit pharmaceuticals: Merck case study. Presentation at Committee on Understanding the Global Public Health Implications of Substandard, Falsified, and Counterfeit Medical Products: Meeting One, Institute of Medicine, Washington, DC.