Workshop Summary
Rising health care costs are a central fiscal challenge confronting the United States (CBO, 2012b; IOM, 2012a; Sullivan et al., 2011). National spending on health care currently accounts for 18 percent of gross domestic product (GDP), but is anticipated to increase to 25 percent of GDP by 2037 (CBO, 2012a). The Bipartisan Policy Center argues that “this rapid growth in health expenditures creates an unsustainable burden on America’s economy, with far-reaching consequences” (BPC, 2012). These consequences include crowding out many national priorities, including investments in education, infrastructure, and research; stagnation of employee wages; and decreased international competitiveness (BPC, 2012; Emanuel et al., 2012; Milstein, 2012). In spite of health care costs that far exceed those of other countries, health outcomes in the United States are not considerably better (Fineberg, 2012; IOM, 2010b, NRC and IOM, 2013). In fact, the United States is in the lowest quartile for life expectancy among countries in the Organisation for Economic Co-operation and Development (OECD) (Fineberg, 2012).
The costs of cancer care are expected to increase as the aging of the population leads to a rapid influx of new cancer diagnoses and as new innovations in cancer treatment and care are deployed in practice. As more expensive targeted therapies and other new technologies in surgery and radiation become the standard of care, there are concerns that the costs of
cancer treatment could begin to outpace health care inflation as a whole (Sullivan et al., 2011).
Advances in early detection, prevention, and treatment have resulted in consistently falling cancer death rates (Eheman et al., 2012). Compared to other OECD countries, the United States has a lower cancer mortality rate for males and a similar cancer mortality rate for females (OECD, 2013). However, many indications suggest that cancer care is not optimal. Despite progress in reducing cancer death rates, disparities in cancer outcomes persist, problems of overuse and misuse contribute to a lack of evidence-based cancer screening and treatment, and many patients do not experience patient-centered cancer care, such as access to palliative care and use of treatment plans to help with patient–clinician communication and decision making (Goodwin et al., 2011; IOM, 2011; Schnipper et al., 2012; Siegel et al., 2011). In addition, there are missed opportunities to collect information that could help inform clinical practice decision making (IOM, 2012a), as electronic medical records (EMRs) are often not designed for this purpose.
With the goal of ensuring that patients have access to high-quality, affordable cancer care, the Institute of Medicine’s (IOM’s) National Cancer Policy Forum convened a public workshop, Delivering Affordable Cancer Care in the 21st Century, October 8–9, 2012, in Washington, DC.1 Workshop presentations and discussions examined the drivers of current and projected cancer care costs, including
- inappropriate financial incentives in the health care system;
- unrealistic expectations about the effectiveness of screening and treatments for cancer by both patients and clinicians;
- overuse and misuse of medical resources and inadequate adherence with treatment guidelines; and
- lack of evidence on what represents high-quality, affordable cancer care.
__________________
1This workshop was organized by an independent planning committee whose role was limited to the identification of topics and speakers. This workshop summary was prepared by the rapporteurs as a factual summary of the presentations and discussions that took place at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants, are not necessarily endorsed or verified by the Institute of Medicine or the National Cancer Policy Forum, and should not be construed as reflecting any group consensus.
Workshop participants also suggested ways to improve the affordability and quality of cancer care. Box 1 highlights possible solutions proposed by individual participants. Beginning on page 29, the workshop summary describes possible solutions in more detail, organized by:
- patient and clinician communication and education
- best practices in cancer care
- evidence base for clinical practice and reimbursement
- financial incentives aligned with affordable, high-quality cancer care
- delivery system and reimbursement changes
A recurring theme of the workshop was the need for all stakeholders—including patients, clinicians, private and government payers, and the pharmaceutical and device industries—to work together to address affordable cancer care. In addition, several workshop speakers suggested that strategies for controlling cancer care costs are likely to be applicable to reducing health care costs in general. “Because cancer is such a prevalent set of conditions and so costly, it magnifies what we know to be true about the totality of the health care system. It exposes all of its strengths and weaknesses,” explained Susan Dentzer, editor in chief at Health Affairs. Mark McClellan, director of the Engelberg Center for Health Care Reform at the Brookings Institution, concurred, adding, “Oncology is where the action is going to be. There will be modifications called for in the Affordable Care Act that will put more pressure on finding ways to lower cancer care costs while improving quality and innovation.” IOM President Harvey Fineberg agreed: “If we can find a way to solve this problem for cancer care, then we have the keys to solve it for health care more broadly.”
Workshop presentations and discussions will also inform an ongoing IOM consensus study, Improving the Quality of Cancer Care: Addressing the Challenges of an Aging Population. The study committee is examining issues in the quality of cancer care, including a specific focus on how the aging of the population will rapidly accelerate the number of new cancer diagnoses at a time when workforce shortages are predicted. The committee’s report is expected to be released in 2013.
A FINANCIAL CRISIS IN HEALTH CARE
Workshop speakers presented statistics that underscore the severity of the problem of health care spending and the need for immediate solutions.
• Improve the information patients have to make decisions about and manage their care
o Make available transparent quality metrics to help patients select their oncology providers
o Reimburse clinicians for communications with patients, including provision of accurate information on a patient’s prognosis; the costs, potential benefits, and side effects of various treatment options; and palliative care and hospice care considerations
• Improve training and information available to clinicians
o Ensure clinicians are well trained to communicate with patients, follow evidence-based guidelines, and convey the financial repercussions of different treatment options
o Promote adherence to the American Society of Clinical Oncology Top 5 list and encourage clinicians to stop using interventions with questionable value
o Incorporate cost information in clinical practice guidelines and treatment pathways
• Promote and facilitate best practices in cancer care
o Support team-based models of care that provide 24-hour support to cancer patients
o Ensure early integration of palliative care in cancer care delivery and better use of hospice care
The total cost of health care in the United States is currently about 18 percent of GDP. By 2037, total health care expenses are expected to account for 25 percent of the U.S. GDP (CBO, 2012a). “We really don’t have a choice in terms of reducing the amount of money we spend on health care. We have to face this problem,” stressed Scott Ramsey, full member of the Fred Hutchinson Cancer Research Center and professor of medicine at the University of Washington.
The United States spends far more than other nations on health care, in proportion to its earnings as a nation (see Figure 1). “We are on a different planet,” said Ezekiel Emanuel, Diane v.S. Levy and Robert M. Levy University Professor and vice provost for Global Initiatives at the University of Pennsylvania.
o Improve the functionality and interoperability of electronic medical records
o Provide feedback to patients, providers, and payers through population-based performance measurement of quality, outcomes, and costs
• Enhance research that informs clinical practice
o Develop learning health care systems to collect point-of-care data that can inform personalized medicine, comparative effectiveness, health care redesign, and quality cancer care
o Conduct pragmatic trials with real-world comparators and populations, as well as clinically relevant outcomes in pertinent patient subpopulations
• Reward the provision of affordable, high-quality cancer care through delivery system and reimbursement changes
o Evaluate delivery system changes, including capitation, episode-related payments, medical homes, accountable care organizations, and shared savings programs
o Support coverage with evidence development programs to assess new innovations in cancer care
o Reimburse clinicians for performance on quality measures and for patient–clinician communication
o Sever the relationship between treatment choice and physician income o Structure copayments based on the value of the service provided, to encourage patients to use higher-value treatments and discourage use of lower value interventions
Despite spending nearly twice as much on health care as many other developed countries, the United States is not reaping more benefits in terms of increasing life expectancy or lowering infant mortality, said Ramsey and Otis Brawley, the chief medical officer and executive vice president of the American Cancer Society (see also Fineberg, 2012; OECD, 2013). For example, the U.S. life expectancy is slightly lower and its infant mortality rate slightly higher than that of Canada or Switzerland (OECD, 2011, 2013), despite lower health care expenditures in both countries compared to the United States (OECD, 2011). “We don’t get what we pay for, even though we are the most expensive health care system in the world,” Brawley said.
Brawley noted that the $2.6 trillion spent on health care in the United States is more than twice what the nation spends on food. Emanuel added
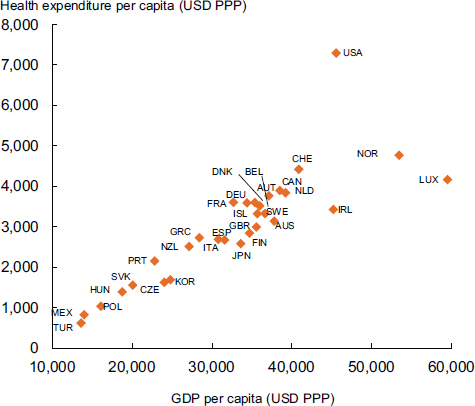
FIGURE 1 Total health expenditure per capita and gross domestic product per capita, 2007. The United States spends far more per capita on health care than the other Organisation for Economic Co-operation and Development countries.
NOTE: AUS = Australia; AUT = Austria; BEL = Belgium; CAN = Canada; CHE = Switzerland; CZE = Czech Republic; DEU = Germany; DNK = Denmark; ESP = Spain; FIN = Finland; FRA = France; GBR = United Kingdom; GDP = gross domestic product; GRC = Greece; HUN = Hungary; IRL = Ireland; ISL = Iceland; ITA = Italy; JPN = Japan; KOR = Korea; LUX = Luxembourg; MEX = Mexico; NLD = Netherlands; NZL = New Zealand; NOR = Norway; POL = Poland; PPP = purchasing power parities; PRT = Portugal; SVK = Slovak Republic; SWE = Sweden; TUR = Turkey; USA = United States; USD = U.S. dollar.
SOURCES: Emanuel presentation (October 8, 2012) and OECD, 2009, Health at a Glance 2009: OECD Indicators, OECD Publishing. http://dx.doi.org/10.1787/health_glance-2009-en (accessed March 6, 2013).
that about one-third of health care costs are hospitalization expenses—which is more than the United States spends on Social Security ($731 billion) or defense ($718 billion) (CBPP, 2012; KFF, 2009). Such high spending on health care affects other aspects of the nation’s economy and welfare. Emanuel argued that these rising health care costs jeopardize health coverage and access; state budgets and funding for education; middle-class wages; and the United States’ long-term fiscal stability and status as a world power. Emanuel noted that one state budget director predicted Medicaid
and other health care expenses would grow by as much as 40 percent of the state budget by 2015, forcing the state to cut higher education funding.
Wages are also linked to health care costs, with increasing costs causing employers to balance their budgets by lowering wages (Emanuel and Fuchs, 2008). Lee Newcomer, senior vice president of oncology for UnitedHealthcare, added that one recent projection suggested that in 2017, health insurance premiums and out-of-pocket health costs could account for half of all household income (see Figure 2).
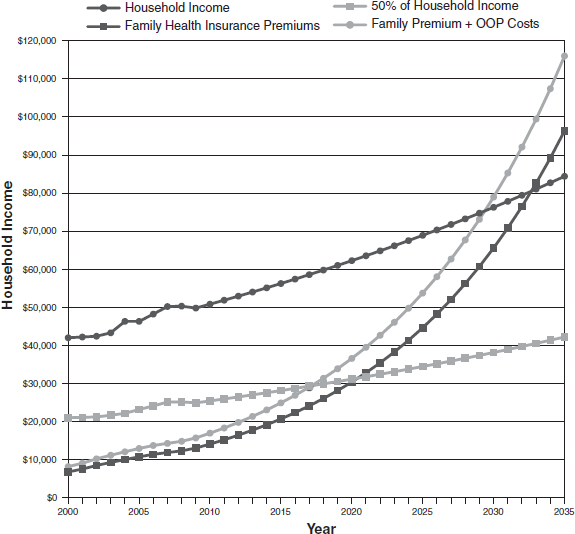
NOTE: OOP = out-of-pocket.
SOURCES: Newcomer presentation (October 9, 2012) and Young and DeVoe, 2012. Reprinted with permission from Who Will Have Health Insurance in the Future? An Updated Projection, March/April, 2012, Vol 10, No 2, Annals of Family Medicine. Copyright © 2012 American Academy of Family Physicians. All Rights Reserved.
The impact of health care costs on the overall economy is substantial, Ramsey stressed, quoting the Stanford health economist Victor Fuchs: “If we solve our health care spending, practically all of our fiscal problems go away. [If we don’t,] then almost anything else we do will not solve our fiscal problems” (Kolata, 2012).
The urgency of the health care financial crisis and the need to solve it was stressed by several speakers. “We have to do something in the very immediate future about this,” Newcomer said. “We do not have time to do a long controlled study about it any longer.” McClellan added, “We can’t keep doing things the way that we have been doing them because it is not financially sustainable. And if you look at the trends in health care costs, we don’t have 5 years to wait and see what works. We need something sooner.” Emanuel warned, “We don’t have a lot of time and we need to be proactive. Now is the time to take a risk.”
Fineberg stressed that total health care costs must be addressed, and that shifting costs to insurers, hospitals, providers, patients, or the government will not solve the problem of unsustainable health care costs. “It does not make sense to drive only toward making health care affordable for the person, affordable for the government, affordable for the employer,” said Fineberg. “If we are going to succeed in reaching affordable cancer care and an affordable health care system, we have to focus on the total costs.”
Emanuel attempted to dispel some common misperceptions about the drivers of escalating health care costs. He said physicians tend to blame much of the rising costs on the practice of defensive medicine,2 medical malpractice, insurance company profits, drug costs, and demanding patients. However, Emanuel pointed out that malpractice premiums, settlements, and administrative costs compose slightly more than 1 percent of health care spending, with defensive medicine estimated at 2.5 percent of total health care spending. Additionally, proposed tort reforms are estimated to reduce health care spending by only 0.5 percent (CBO, 2009). Similarly, the profits of the five biggest health insurers in 2010 was $11.7 billion, which he called “a drop in the bucket” of total health care spending (Emanuel, 2011). There
__________________
2Defensive medicine occurs when doctors order tests, procedures, or visits, or avoid high-risk patients or procedures, primarily (but not necessarily or solely) to reduce their exposure to malpractice liability (OTA, 1994).
also is no evidence that particularly demanding patients are driving up the costs of care substantially. Patients who use more than $1 million in health care services composed only 0.5 percent of total health care spending, and those who use more than $250,000 compose 6.5 percent, according to Emanuel.
However, Emanuel noted that 10 percent of the population spends more than 60 percent of our nation’s health care costs (KFF, 2009). “These are patients with multiple chronic illnesses and cancer, and that is who we have to focus on if we really want to control costs,” Emanuel said. Speakers discussed other drivers of rising health care costs, including financial incentives driven by fee-for-service reimbursement that reward volume of care rather than quality or efficiency of care, and a lack of focus on system and individual patient costs of treatment. Additionally, innovation and the diffusion of new technologies in care and a lack of coordination among providers, hampered by a lack of interoperability of EMRs, have also led to high health care costs.
Throughout the workshop, Emanuel and other speakers stressed that the complex reasons for these high costs necessitate a multifaceted solution. “The fault in the health care system lies with the doctors, hospitals, government, insurers, lawyers, patients, and their advocacy groups. There is no one who is not to blame for the problem in American health care,” Brawley said. Thomas Kean, chief executive officer and president of C-Change, added, “We all are the problem. The demonizing doesn’t really take us anywhere because we are all part of the solution.”
Total cancer care costs are estimated to comprise 5 percent of all health care expenditures and 10 percent of Medicare expenditures (ACS CAN, 2009; Sullivan et al., 2011). Robin Yabroff, an epidemiologist at the National Cancer Institute, reported that the estimated medical costs of cancer care were $125 billion in 2010, but costs are projected to increase to $173 billion in 2020, a 39 percent increase (Mariotto et al., 2011). However, she pointed out that there are numerous challenges for estimating and projecting cancer costs, including a lack of complete data, especially the costs for uninsured patients, as well as unanticipated changes in treatment practices that can significantly affect cost projections. Emanuel said that due to these challenges, both current and projected estimates in cancer care costs are probably underestimates.
Emanuel emphasized that although cancer patients represent 0.6 percent of the population, they accrue 5 to 6 percent of the total health care expenditures. Newcomer added that 11 percent of UnitedHealthcare’s budget accounts for cancer care—half is attributed to hospitalization costs, while the remaining half is split almost evenly between the costs of physicians and the cost of cancer drugs.
Leaders from the cancer community reviewed the evidence on international cancer costs to compose a public policy perspective on delivering affordable cancer care in high-income countries for Lancet Oncology, reported Jeffrey Peppercorn, associate professor of medicine at Duke University and faculty associate of the Trent Center for Bioethics. These authors concluded that both the burden of cancer and the costs of cancer care are continuing to increase for high-income countries (Sullivan et al., 2011). He added that in the past 30 years, the cost of cancer care in the United States has increased substantially.
Many speakers discussed the drivers of increasing cancer care costs, including innovation and technology diffusion; overuse and misuse of interventions; an insufficient evidence base to inform clinical decision making; regulatory and legal issues; increasing drug prices; and demographic and epidemiologic trends, including an aging population and an obesity epidemic, that are increasing the incidence of new cancer diagnoses. Ramsey noted from 2010 to 2030, total projected cancer incidence is estimated to increase by 45 percent (Smith et al., 2009). According to Peppercorn, the median age at diagnosis for all cancers is 66 years (SEER, 2012), and between 2010 and 2050, the number of Americans aged 65 and older is projected to double—from 40.2 million in 2010 to 88.5 million in 2050 (U.S. Census Bureau, 2010). Many cancers are also turning into chronic diseases that require use of more care over a patient’s lifetime, Ramsey said, further contributing to a rise in cancer care costs.
Given the link between obesity and the risk of developing a number of different cancers and other chronic diseases (IOM, 2012b), the growing obesity epidemic is expected to be a driver in rising cancer care costs, Peppercorn and Brawley emphasized. Brawley noted that in the past 30 years, the obesity rate in adults has doubled, and the childhood obesity rates have more than tripled (IOM, 2012d).
Another driver of cancer care costs are cancer drugs, due in part to the increasing unit prices of cancer drugs (Bach, 2009; see Figure 3). Medicare spending on such drugs has been rising steadily over the past few decades, noted Peter Bach, attending physician at Memorial Sloan-Kettering Cancer
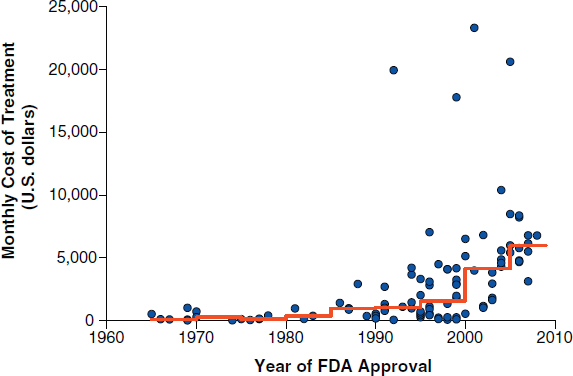
FIGURE 3 Monthly and median costs of cancer drugs at the time of Food and Drug Administration approval, 1965–2008. Dots represent the cost of 1 month of cancer treatment for an individual who weighs 70 kilograms or has a body-surface area of 1.7 m2. The line indicates median drug prices over 5-year time intervals.
NOTE: Prices are adjusted to 2007 U.S. dollars.
SOURCES: Bach presentation (October 9, 2012) and Bach, 2009. From New England Journal of Medicine, P. B. Bach, Limits on Medicare’s ability to control rising spending on cancer drugs, Volume No. 360, Page Nos. 626–633. Copyright © (2009) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Center. After the implementation of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA),3 the spending on Medicare Part B drugs dropped in 2005 (see Figure 4), but not as much as most people expected, in part because manufacturers responded by raising their unit prices, he said.
Peppercorn suggested that the increasing drug prices may be in part due to the rise of innovative targeted treatments. Pharmaceutical companies may be pricing these therapies higher to recoup research and development costs,
__________________
3MMA changed the way physician-administered (Medicare Part B) drugs are reimbursed. Many Medicare Part B drugs are used in oncology care. Prior to implementation, oncologists were reimbursed at a percentage of a drug’s average wholesale price (AWP). Now oncologists are reimbursed based on the drug’s average sales price (ASP) plus 6 percent and an administrative fee (Jacobson et al., 2006).
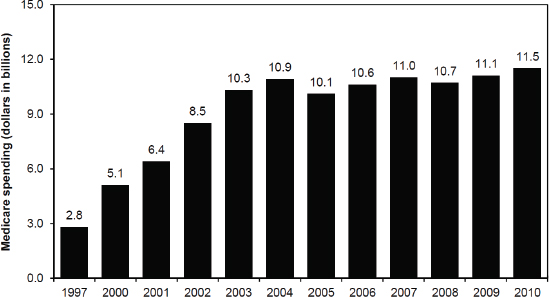
FIGURE 4 Medicare spending for Part B drugs administered in physicians’ offices or furnished by suppliers. From 1997 to 2003, Medicare spending on Part B drugs increased at an average rate of 25 percent. With the implementation of the MMA’s new reimbursement system for Part B drugs in 2005, spending declined by 8 percent. From 2006 to 2010, spending has increased at an average rate of 2.3 percent per year.
SOURCES: Bach presentation (October 9, 2012) and MedPAC, 2012.
since they are typically effective in smaller subgroups of cancer patients. He noted, for example, that adjuvant trastuzumab treatment for the 20 percent of patients with breast cancer whose tumors express human epidermal growth factor 2 (HER2) costs around $50,000 per year (Falconi, 2012).
However, Deborah Schrag, associate professor of medicine at Harvard Medical School and Dana-Farber Cancer Institute, and Newcomer noted that the price of a new drug is predominantly dependent on the price of the last drug released and not tied to therapeutic benefit. Bach agreed, and added that in the United States, “Nobody sets prices. The most powerful predictor of the entry price of a new drug is the entry price of the drug that came on before it. It has nothing to do with innovation, accelerated approval, [or] number of patients served.”
For example, Bach mentioned that Zaltrap, a new drug for colorectal cancer was priced at around $11,000, about double the cost compared to another medicine, even though patient outcomes were similar to those achieved with the less expensive medicine.4 A lack of competition,
__________________
4After the workshop, Memorial Sloan-Kettering Cancer Center announced that it was not going to be providing Zaltrap to its patients (Bach et al., 2012). Following this announcement, Sanofi decided to lower the price of Zaltrap by half (Goldberg, 2012).
combined with state and federal laws specifying that insurers must pay for the costs of cancer drugs, enables sellers to determine their price without constraints and drives up their cost, according to Schrag and Newcomer (see also the section on regulatory and legal challenges). Cancer drug costs are increasing 10 percent each year, Newcomer added.
“We have good medicines, but we are going to have to figure out how to get them delivered to the marketplace at a lower cost,” Newcomer said, noting that the profit margins for biological therapies can be significant. “There is plenty of room here to begin lowering prices and begin thinking about other strategies,” he said.
The cost of hospital care for cancer patients is also a factor, Newcomer said. He added that hospitals have an unfair negotiating advantage compared to physicians because hospitals can link bed access and other essential services to their oncology pricing, and health plans do not have alternative sources for these linked services. With this bundling, hospitals can charge more than physicians for chemotherapy treatments, despite hospitals’ ability to acquire these drugs at a significantly lower cost through a mechanism known as 340B,5 according to Newcomer. UnitedHealthcare figures suggest that hospital markups on drugs average about 250 percent, he said. Robert Green, a medical oncologist and the chief medical officer of Cancer of Excellence, added that the migration of community-based oncology practices to hospital-based health care systems is also increasing the costs of cancer care.
Financial Burden on Patients with Cancer
Veena Shankaran, assistant member of the Fred Hutchinson Cancer Research Center, stressed the growing financial burden of cancer on patients and their families. She said cancer patients face significantly greater health care costs compared to those with other chronic conditions (see Figure 5).
The rising out-of-pocket costs of cancer care are due to rising health insurance premiums and deductibles, as well as copayments for treatments, she said. In addition, oral chemotherapeutics are often an out-of-pocket expense unless they fall under outpatient prescription plans. There also can be high patient spending for off-label diagnostics and treatments. She
__________________
5The 340B Drug Pricing Program is a federal program that requires drug manufacturers participating in the Medicaid drug rebate program to provide outpatient drugs to enrolled “covered entities” at or below the statutorily-defined ceiling price (HRSA, 2013).
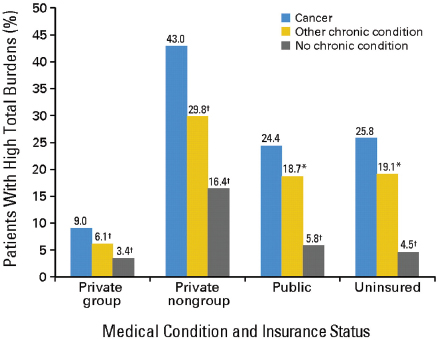
NOTE: High financial burdens were defined as health-related spending accounting for more than 20 percent of income.
*Difference from the reference category (cancer) is significant at the 5% level.
†Difference from the reference category (cancer) is significant at the 1% level.
SOURCES: Shankaran presentation (October 8, 2012) and Bernard et al., 2011. Reprinted with permission © (2011) American Society of Clinical Oncology. All rights reserved. Bernard, D. et al.: J Clin Oncol 29(20), 2011:2821–2826.
noted that between 1999 and 2012, workers’ contributions to health insurance premiums increased by 180 percent and health insurance premiums increased by 172 percent (KFF and HRET, 2012). Workers earnings rose only slightly during the same time period.
In addition to these costs, many cancer patients are financially vulnerable because their illness and/or treatment impedes their ability to work, with some patients losing employment altogether. They may also have increased transportation, childcare, or other expenses related to meeting their medical care needs (Kim, 2007).
Some cancer care costs may not be necessary or can be avoided by opting for lower cost, but equally effective, options. However, as Jessie Gruman, president of the Center for Advancing Health, pointed out,
because they are facing a life-threatening illness, cancer patients tend to be more inclined to seek treatments, no matter what the cost. “A cancer diagnosis sets off a fairly predictable first reaction, which is ‘I’ll pay anything,’” she said. “I don’t think that we, as cancer patients, are ever going to act like consumers and make rational price-quality risk trade-offs in the purchase of care to [try to] save our lives.”
Shankaran added, “We have seen reports of significant patient financial hardships, such as going into debt, depleting all assets to pay for cancer treatment, and personal bankruptcy.” A study of patients undergoing adjuvant treatment for regional colon cancer found 38 percent reported at least one treatment-related financial hardship (Shankaran et al., 2012). Another study found that cancer patients, particularly if they are younger than 65, had a higher rate of personal bankruptcy compared with controls (Ramsey et al., 2011). Shankaran pointed out that there is no evidence that patients who spend more on their cancer treatments necessarily do better clinically.
At a national level, increased cancer care expenditures are also not necessarily translating into improvements in cancer outcomes. Peppercorn noted that “we have poor outcomes in too many settings, despite lots of exciting new interventions that fill our conferences. The median survival for metastatic triple-negative breast cancer6 is still a little more than 1 year and is the same or worse in many other major cancers.” Ramsey added, “A lot of what we do in cancer is very expensive and actually offers very little for patients. In some cases, it may actually hurt patients.” Lowell Schnipper, the Theodore and Evelyn Berenson Professor of Medicine at Harvard Medical School and clinical director of the cancer center at Beth Israel Deaconess Medical Center, added that “for the common types of cancer, the expenses are rising inexorably along with new innovations, but the extent to which they really make meaningful impact is not always clear.”
Fineberg stressed, “The crisis we have reached in health care [necessitates] finding out how to provide value with better outcomes and reduced costs. There is no point in thinking about lowering costs if you don’t simultaneously have in mind maintaining and improving the quality of care. We have to think about both.”
__________________
6Describes breast cancer cells that do not have estrogen receptors, progesterone receptors, or large amounts of HER2/neu protein (NCI, 2013).
At the workshop, many speakers pointed out numerous obstacles in the health care system that must be overcome in order to provide affordable, high-quality cancer care, including
- unrealistic expectations of patients and clinicians with regard to the benefit of certain tests and treatments;
- inappropriate financial incentives in the health care system;
- overuse and misuse of medical resources and care that does not align with clinical practice guidelines;
- an insufficient evidence base to facilitate rational clinical and reimbursement decisions;
- legal and regulatory challenges; and
- lack of consensus on how to assess value in medical care.
McClellan noted that as insurers have decreased their physician payments in order to cut costs, many oncologists’ practice revenues have been driven by the profit margin made on the chemotherapies they administer to their patients. The current fee-for-service reimbursement structure provides an incentive to prescribe more chemotherapy and other expensive treatments even when the patient may not be likely to benefit from them, several speakers noted. “Giving the more expensive drug always gets us or our cancer center more money,” Schrag said.
Thomas Smith, the Harry J. Duffey Family Professor of Palliative Medicine at Johns Hopkins University, added that the Medical Group Management Association has calculated the median salary for medical oncologists at $400,000, almost double the salary of most cognitive-care7 internists. “Medical oncology salaries continue to go up rather than down, and we need to readjust that,” Smith said.
Peter Eisenberg, an oncologist at Marin Specialty Care, noted that in his community practice, six doctors purchase about $8 million worth of chemotherapy each year, and sell it for about $10 million. The difference pays their salaries and practice expenses. “Without selling chemotherapy, we
__________________
7Cognitive care refers to evaluation and management services in medicine (Smith and Hillner, 2011).
wouldn’t be in business,” he said. “Doctors, like everybody else, respond to incentives.” Green added, “We don’t get paid for multiple support systems that go on in our community practice, and we have no motivation to consider the cost to the system.”
Hospitals also depend on the earnings from radiation oncology procedures performed onsite, and encourage their physicians to prescribe such procedures, noted Justin Bekelman, assistant professor of radiation oncology, member of the Abramson Cancer Center, and senior fellow at the Leonard Davis Institute for Health Economics at the University of Pennsylvania Perelman School of Medicine. He added that reimbursement decisions also influence which radiation oncology procedures physicians order (see Figure 6). For example, brachytherapy following breast-conserving surgery was quickly incorporated into clinical practice, coinciding with Food and Drug Administration device approval and Medicare reimbursement (Smith et al., 2011). In addition, the rapid adoption of expensive intensity-modulated radiotherapy (IMRT) for prostate cancer replaced much less expensive 3-D conformal radiotherapy, despite limited evidence to support its clinical superiority, he said. From 2001 to 2005, the proportion of patients with nonmetastatic prostate cancer who received IMRT increased
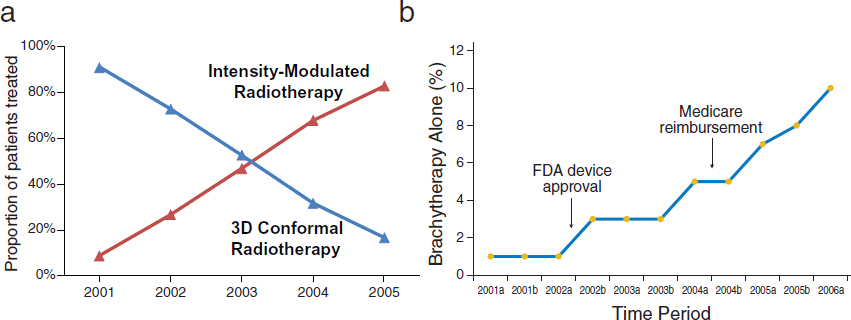
NOTE: In Figure 6(b), the “a” after year refers to January through June, and “b” refers to July through December.
SOURCES: Bekelman presentation (October 8, 2012), Yeboa et al., 2010, and Smith et al., 2011. Reprinted with permission © (2011) American Society of Clinical Oncology.
All rights reserved. Smith, G. et al.: J Clin Oncol 29(2), 2011:157–165.
from 9 percent to 83 percent (Yeboa et al., 2010). By 2008, 95.9 percent of patients received IMRT rather than 3-D conformal radiotherapy (Sheets et al., 2012). Proton beam therapy, which is even more costly, is now being rapidly implemented, several speakers noted.
Another example of an unintended and costly consequence of fee-for-service reimbursement is overtreatment of uncomplicated bone metastases using radiotherapy. Analyzing Surveillance, Epidemiology and End Results (SEER)-Medicare data, Bekelman found that a small minority of men with prostate cancer that had metastasized to the bone were treated with a single fraction of radiation, while the majority of patients received greater than 10 fractions of radiotherapy, despite a previous clinical trial that showed the two treatments were equally effective. Hospital facilities were more likely to overtreat than free-standing centers, he found.
Differences in Incentives and System Costs Between Canada and the United States
Health system characteristics can also influence reimbursement incentives and the total costs of care. Craig Earle, professor of medicine at the University of Toronto and the director of health services research at Cancer Care Ontario, compared aspects of the Canadian health care system with the U.S. system.
Canada’s health care system is a publicly administered, single-payer system that provides free point-of-care health care and is supported by general tax revenues. “You never have to reach into your pocket for anything, aside from your health card—there are no copays [or] user fees and it’s portable across the country,” he said. Earle noted that health care currently comprises about half of most provinces’ budgets, but is only around 10 percent of the nation’s GDP. Canada spends half as much per capita on health care as the United States, but Canadian physicians are paid about as much as U.S. physicians, and health outcomes in Canada are good, according to Earle.
Earle suggested that one reason health care costs are lower in Canada than in the United States for comparable quality is because Canada has substantially lower administrative costs (Woolhandler et al., 2003). In addition, hospitals have global funding, or budgets set by the government, which saves costs but also provides an incentive to treat patients more conservatively, Earle noted. But he added that the country is moving toward more activity-based funding for hospitals.
Canada also has price controls on drugs because provinces negotiate with drug makers to get a lower price, and some provinces are bound by law not to pay more than the median price for drugs that is offered in OECD countries. Compared to the United States, Earle said that there are no financial incentives to provide more chemotherapy to patients: “Drugs that are publicly paid for have to be given in public institutions. No one makes money or has an incentive to give a more expensive drug.” Expensive drugs that have come on the market in the past 20 years are reimbursed separately in a more controlled way, according to specific treatment guidelines. That is also true for tests, whose costs come out of the budget of the pathology department. “Pathologists can’t just run up tests, send in a bill, and have someone pay for it,” Earle said.
The Canadian health care system is not perfect, Earle acknowledged. There can be long wait times for medical interventions as well as overtreatment issues. There is a need to improve its preventive services, and there are some inappropriate financial incentives, as most physicians still receive fee-for-service reimbursements. Even with these challenges, Earle stressed the value of having a single-payer health care system. Without it, “the market becomes health insurance, instead of health or health care, and there is a middle man having to take money out of the system for that.” He added that it is assumed high drug prices and reimbursements in the United States are needed to support medical innovation, “but there are other ways to support research and innovation, without bankrupting the health care system,” he said.
Another factor that reduces the likelihood that affordable, high-quality cancer care will be provided is patients’ poorly informed or unrealistic expectations about the benefit of certain interventions or their likelihood of survival. Ninety percent of patients with advanced cancer report that they want to be told the truth about their illness (Yun et al., 2004), noted Jennifer Temel, associate professor of medicine at Harvard Medical School and clinical director of thoracic oncology at Massachusetts General Hospital Cancer Center. However, a study of terminally ill patients found that although their actual median survival was 26 days, clinicians estimated that their median survival would be 75 days, and communicated that their median survival would be 90 days (Lamont and Christakis, 2001). “Patients with serious illness desire prognostic information, [but] the reality is we do not disclose prognostic information to them,” said Temel.
In some instances, patients “expect to be cured when in fact cure is often not possible,” Ramsey said. However, it is not always clear whether patients are informed that their disease is incurable, whether they are informed effectively, or whether patients choose not to believe the message (Smith and Longo, 2012). One study found that 81 percent of patients with metastatic colorectal cancer and 69 percent of patients with metastatic lung cancer did not report understanding that chemotherapy was not at all likely to cure their cancer (Weeks et al., 2012). When patients thought their physicians were really good communicators, they were more likely to think their cancer was curable. “It is a trade-off. You want to be liked, but your patient gets it wrong,” said Schrag. Another study found that despite having just signed a consent form that stated seven times that their cancer is incurable, a third of patients checked affirmatively on a form that they thought their cancer was curable, and nearly 70 percent thought the goal of the therapy was to get rid of all their cancer (Temel et al., 2011).
Some speakers added that unrealistic expectations of patients are also fueled by direct-to-consumer marketing as well as problems with innumeracy. Brawley pointed out that survival statistics are often quoted in advertisements for health centers, but improvements in survival rates can be misleading because they can include individuals who were overdiagnosed, or whose cancers may not have required treatment. “There is the perception that all cancer is bad, must be found, and if found, aggressively treated. There is a belief among doctors that there is no such thing as overdiagnosis, and most think that an increase in 5-year survival statistics is evidence that screening is beneficial.” Barry Kramer, director of NCI’s Division of Cancer Prevention, agreed, noting “a large proportion of physicians incorrectly look to survival after the date of diagnosis as the strongest indicator of benefit of a screening test when it is well known that it is the weakest indicator.” Brawley also noted that people confuse relative risk with absolute risk.8
Physicians can also be reluctant to counter patients’ unrealistic beliefs about their prognosis because they do not want to make their patients emotionally distraught or take away their hope, others pointed out. However, some studies show that patients who engage in end-of-life care discussions are no more likely to feel worried, sad, or distressed, Temel said (Smith et al., 2010; Wright et al., 2008). She added that this is “a major problem
__________________
8Absolute risk is a measure of the risk of a certain event happening. Relative risk is a measure of the risk of a certain event happening in one group compared to the risk of the same event happening in another group (NCI, 2013).
because how patients perceive their illness and prognosis impacts decisions that they make about their care.” Patients who overestimated their chance of survival in one study were two and a half times more likely to receive intensive anti-cancer therapy, but they lived no longer than the patients who did not receive such treatment. Patients who are unrealistic about their prognosis are also less likely to receive hospice care, Temel said (Huskamp et al., 2009; Weeks et al., 1998).
Physicians may also benefit financially from patients’ unrealistic expectations by providing more treatment, even if it is not likely to be effective, several speakers noted. “We in medicine have overpromised our magic,” said Brawley. “In the United States people have decided that because of all the promises, there is no reason for anybody ever to die.”
Overuse and Misuse of Interventions
Unrealistic expectations and misaligned financial incentives are contributing to the overuse and misuse of interventions in cancer care, a number of speakers noted. Overuse is particularly problematic in individuals with advanced cancer, several speakers suggested, noting a high rate of treatment with chemotherapy close to the end of life, more time spent in the emergency room and the hospital, and less time in hospice care. “Such aggressive care at the end of life is bad for patients and their family members,” Temel said. She noted that patients receiving intensive or aggressive interventions near the end of life have a worse quality of life, as reported by their professional and family caregivers, and are more likely to have psychological distress (Wright et al., 2008).
Not only is such aggressive care near the end of life not in the patient’s best interest, it is also usually costly. For example, patients who engaged in end-of-life discussions with their physicians were much less likely to receive aggressive cancer therapy at the end of life, and that corresponded with a cost savings of approximately $1,000 per patient, one study found (Zhang et al., 2009). Another study found palliative care consults reduced the cost of cancer care (Morrison et al., 2008). In addition to higher costs, Wright et al. (2008) found that more aggressive medical treatment near the end of life was associated with worse patient quality of life, whereas longer hospice stays were associated with better patient quality of life.
Various screening and treatment interventions are also often overused or prematurely used in cancer care, several speakers pointed out. Premature use of screening interventions often occurs before clinical benefits of the
screening are proven, Brawley noted. For example, ovarian cancer screening and prostate cancer screening are still advocated by some practitioners, despite a prospective, randomized trial that showed ovarian screening was not effective (Buys et al., 2011), and despite four organizations recommending against routine prostate cancer screening due to the lack of evidence that the benefits outweigh the risks. Overuse of imaging technology also occurs. Kramer highlighted the increased use of imaging among Medicare beneficiaries (see Figure 7), and Brawley noted that the United States has more per capita computed tomography (CT) and magnetic resonance imaging (MRI) machines than most other OECD countries. Only Austria and Belgium have more per capita CT scanners, and only Japan has more MRI units (Anderson and Squires, 2010).
Denise Aberle, professor of radiology and vice chair of research in the University of California, Los Angeles, Department of Radiological Sciences, discussed the results of the National Lung Screening Trial, which found that screening with low-dose CT reduced mortality from lung cancer for those at high risk,9 and discussed the challenges of implementing screening in practice (National Lung Screening Trial Research Team et al., 2011). According to Aberle, these challenges include (1) defining the scope of the screening programs, including the clinicians involved, incorporation of tobacco cessation therapy, standardization of screening and image analysis, and workflow issues; (2) implementing screening in primary care, given the limited time in a clinician’s office visit to explain the potential risks and benefits of screening, and the number of abnormal screens that will require follow-up; and (3) overcoming the stigma associated with lung cancer and educating patients and providers about those who are likely to benefit from lung cancer screening.
Aberle added that more research will be needed to better determine who should be screened, and acknowledged that preventing indiscriminate overutilization of CT screening “will require considerable communication within the medical disciplines and within the community at risk.” Brawley added that such overutilization is already occurring. One hospital in Atlanta advertised the availability of low-dose spiral CT scanning for those at risk for lung cancer, which the hospital claimed included 40-year-old nonsmoking women who have lived in an urban area for 10 years. The hospital
__________________
9High-risk individuals included those between 55 and 74 years of age who had a history of cigarette smoking of at least 30 pack years, and if former smokers, had quit within the previous 15 years.
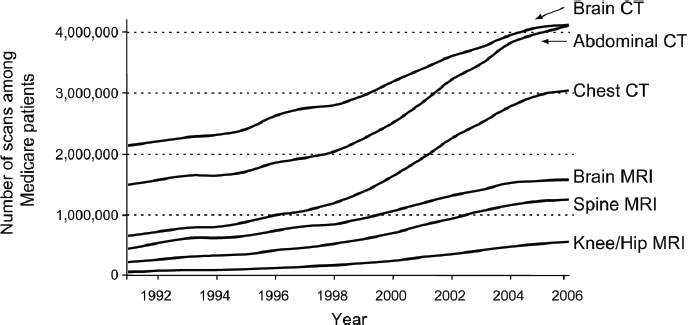
FIGURE 7 Trends in radiology scans in the United States, 1991–2006.
SOURCES: Kramer presentation (October 9, 2012) and Welch, H. G., and W. C. Black, Overdiagnosis in cancer, Journal of the National Cancer Institute, 2010, Volume 102, Issue 9, 605–613, by permission of Oxford University Press.
offered the CT at a low cash price, knowing insurance would not cover this questionable procedure, but assumed insurance would pay for the follow-up of those with abnormal screens, Brawley reported. “This is a subtle form of corruption in medicine,” he said.
Brawley also gave examples of several treatments that were used extensively before they were shown to be ineffective, and in some cases, even harmful. These treatments include postmenopausal hormonal therapy, high doses of vitamins, lidocaine after a heart attack, and erythropoietin to stimulate red blood cell production in cancer patients. “We used [erythropoietin] for 20 years before we figured out it stimulates tumor growth,” he said. “We could decrease the waste and improve overall health if we simply got scientific [about how we treat patients].”
Brawley also noted the excessive use of newer, more expensive drugs, such as esomeprazole (Nexium), which is 1 of the 10 most prescribed drugs in the United States, even though patient outcomes are similar to omeprazole (Prilosec), a drug that costs a fraction of what Nexium costs. “We are overconsuming health care because we ignore known science, ignore the scientific method,” Brawley emphasized. He called for more evidence-based medicine, which will foster rational use of health care as opposed to rationing of health care. “Rational use of health care is necessary for the future of our economy,” he said, as well as in the best interest of patients.
Variable Care, Lack of Best Practices, and an Inadequate Evidence Base
Several speakers stressed that the tremendous variability in the costs of cancer care for the same diagnosis suggests a lack of consensus on the most effective treatment, a lack of adherence to clinical practice guidelines, and is perhaps an indicator of low-quality care. Ramsey noted his study of reimbursement rates for local-stage breast cancer patients found a fivefold difference in expenditures between the lowest and the highest quartiles, without clear reasons for why there was such tremendous variation. Similarly, a high degree of variability was seen in the number of positron emission tomography (PET)/CT tests ordered during the diagnosis and staging period for these same patients, despite guidelines that specify such scans are not warranted in local stage breast cancer.
James Goodwin, the George and Cynthia Mitchell Distinguished Chair in Geriatric Medicine at University of Texas Medical Branch, said his analyses of Texas Medicare data showed that about 20 percent of primary care physicians have rates of colorectal cancer screening among their patients that are significantly higher than the mean, and an almost equal number have rates that are significantly lower than the mean. This high degree of variability in screening was consistently maintained over time. Similarly, he also found a high degree of variability in how frequently practitioners were conducting colonoscopies on their patients aged 70 and older (Goodwin et al., 2011). These examples of such variable care suggest high-quality care is often not provided, Ramsey stressed.
Some of that variability stems from an inadequate evidence base (see also the section on the evidence base for clinical practice and reimbursement). Several speakers noted the difficulty in assessing interventions due to a lack of comparative effectiveness studies for various cancer treatments. Determining the treatment with the most value for specific subgroups of patients can also be difficult if clinically meaningful endpoints were not determined in the clinical trials that led to drug approval, Schrag noted. “We need to distinguish, when we do our trials, those interventions that provide a little bit of incremental benefit for many people versus those that provide an enormous benefit for a very distinct subset,” she said. She also called for more publicly funded clinical trials to address questions that pharmaceutical companies are reluctant to address, such as whether it is better to treat patients with fewer interventions versus more interventions.
Eisenberg added, “We lack studies that show the best practices and
values for patients.” He also called for decision support tools, such as treatment pathways, that can help clinicians manage the complexity of treating cancers that have multiple possible interventions. He also suggested that physicians need a way to compile and analyze patient treatment information collected in clinical practice in order to estimate the benefits and risks of various interventions when choosing the best options for their patients.
Surgeons also need more guidance on what new surgical techniques and technologies they should adopt, noted Jim Hu, the Henry E. Singleton chair in urology and director of minimally invasive urology at UCLA. He said that the tremendous variability in the results associated with new surgical interventions, due to the learning curve practitioners have with new techniques and technologies, makes it difficult to assess their value, and to determine which surgical options are the best to employ. Hu added, “It is very difficult to use study designs like randomized controlled trials to evaluate new technologies in surgery.”
Kramer disagreed with Hu’s perspective on randomized controlled trials in surgery, and noted that these trials have uncovered “some very rude surprises through medical history,” including radical mastectomy versus lumpectomy and radical prostatectomy versus active surveillance. “A randomized trial may be the most efficient way and subject to the fewest confounders,” said Kramer. “I think trying to rely on epidemiological evidence opens up the study design to a far larger range of confounding factors.”
Hu said that a randomized controlled trial with a single surgeon and third-party collection of outcomes data might be better suited to assessing new surgical innovations, but Kramer argued that this would lead to problems of generalizability. Hu added that the main driver of surgical outcomes for robot-assisted prostatectomies is surgeon volume (Hu et al., 2003). “The challenge for patients isn’t so much to find the best robot, because there is only one manufacturer, but rather how to find the surgeon who can optimize the trifecta—outcomes of continence, potency, and cancer control,” Hu said.
When Hu reviewed patient outcomes at his center, the plateau for preservation of sexual function took more than 750 cases (Freire et al., 2010). Although Sanda et al. (2008) found a difference between a nerve-sparing approach for sexual function versus non-nerve-sparing, this study averaged the outcomes of all surgeons in the study, he added. The heterogeneity is more apparent in studies that compare the outcomes of individual surgeons. For example, one study of 11 surgeons found tremendous variation in the likelihood of recovery of continence, erectile function, and cancer control
(Vickers et al., 2011). “The heterogeneity in surgical technique and outcomes often is larger than the difference in the new technology that is being adopted,” Hu said.
The challenge of evaluating new surgical procedures for effectiveness has huge implications for system costs given that new surgical technologies are often more expensive, and because patients and providers rapidly adopt these new procedures. Prostate cancer patients are quickly migrating to hospitals that offer robotic prostatectomies, and because many as four out of five radical prostatectomies now use the da Vinci Surgical System robot (NCI, 2011). One study found that robotic surgery adds about 13 percent to the costs of prostatectomies, and estimated that the replacement of open surgery with robotic surgery in all procedures where it is currently used would add $2.5 billion annually to the health care expenditures (Barbash and Glied, 2010).
Several legal and regulatory restraints also impede the provision of affordable, high-quality cancer care. The Centers for Medicare & Medicaid Services (CMS) is unable to consider the cost of interventions when making reimbursement decisions, Schrag noted, nor can it negotiate with drug companies about pricing. Due to a lack of price controls and negotiations, drugs can cost twice as much or more in the United States than they do in the United Kingdom and European nations, which set price limits via cost-effectiveness cut-offs, Bach said. Federal law also makes it illegal to exclude certain drugs from formularies, said Emanuel and Joanne national clinical lead for cancer for the Kaiser Permanente Care Management Institute. In addition, almost 75 percent of the U.S. population resides in states that have laws mandating the coverage of cancer drugs by private payers (Bach, 2009).
The Patient Protection and Affordable Care Act calls for an Independent Payment Advisory Board that is currently moving forward. This board has a mandate to control costs, but is explicitly prohibited from rationing, from making cuts in service, from implementing cost sharing, and from making changes in hospital reimbursement, Peppercorn said. “What tools it has left and how effective these will be remains to be seen,” he said.
Schrag was critical of contrary crosstalk among federal agencies within HHS, such as CMS, the Food and Drug Administration (FDA), the Centers for Disease Control and Prevention (CDC), the Agency for Health
Research and Quality (AHRQ), and the National Cancer Institute (NCI). “Sometimes they are not rowing together and it would be great for the research community if we had more cooperation and coordination between them,” she said.
FDA is responsible for ensuring that interventions on the market are safe and effective, but does not consider clinical utility or cost. Schnipper noted FDA only judges the scientific evidence, but does not consider the impact of an intervention at the level of the patient or the health care system, and does not have to consider cost effectiveness. Often, developers of devices and diagnostics just have to show equivalence with what is already on the market. Schrag added, “The FDA is not charged with determining the value of a specific intervention.”
In addition, Schrag observed, “The evidence base is always changing. You draw the line and then you learn something new, some marker that predicts response and you have to move the line. It is a Sisyphean task. You are never done.” Peppercorn discussed bevacizumab (Avastin) for breast cancer as an example of the changing evidence base. Initial studies indicated it improved progression-free survival and overall response rate for breast cancer, which led FDA to grant accelerated approval for this indication. However, subsequent studies showed no overall survival benefit or improvement in quality of life in breast cancer patients. Based on the later study findings, the approval of the drug for breast cancer was ultimately withdrawn, but by then, this expensive drug had already been widely prescribed for breast cancer patients.
A great deal of discussion focused on how to assess the value of a specific medical intervention. Ramsey argued that value assessments for cancer interventions should consider the multiple perspectives of patients, providers, and payers. He added that an intervention is valuable “if patients, their families, physicians, and health insurers all agree that the benefits afforded by the intervention are sufficient to support the total sum of resources expended for its use” (Ramsey and Schickedanz, 2010).
When assessing value, Peppercorn noted that “while data from randomized controlled trials is essential, it is not sufficient because it may not always apply to the general population. We need better health care outcomes databases to evaluate utilization, effectiveness, and toxicity.”
Peppercorn pointed out that the United Kingdom is considered to
be one of the most successful nations in controlling health care costs by applying an explicit threshold of 30,000 pounds, or $50,000, for each quality-adjusted life year added by an intervention, above which medical interventions will not be reimbursed by the national health care plan. This threshold was developed by the National Institute of Health and Clinical Excellence (NICE), but he noted that even this system faces pushback when it comes to cancer care, and has allocated a special cancer fund and value-based pricing, which is leading to cancer care that varies by region. “The jury is out yet on how successful NICE will be over the coming years with controlling costs of new interventions,” Peppercorn said.
Emanuel noted that “getting rid of low-value treatment raises the question of low value to who; how low is low value? If it extends life by 2 weeks, is it worth it?” He suggested it is easier to limit the use of no-value treatments and treatments that have not demonstrated value compared to lower-cost alternatives, such as Avastin for metastatic breast cancer or proton beam therapy for prostate cancer, versus low-value treatments that offer some marginal benefits. He suggested that the threshold for assessing value of medical interventions should include whether they are proven to (1) increase survival, (2) reduce side effects, (3) improve quality of life, or (4) reduce costs. Interventions that have none of these benefits should not be used, Emanuel said.
Bach pointed out that treatments that the FDA has not approved for certain cancers may still be listed as off-label options in the National Comprehensive Cancer Network (NCCN) guidelines or other compendia. In regard to breast cancer screening, he added, there is still no consensus on the ages at which screening should begin and end. In addition, he noted that it is hard to claim a treatment has zero benefit if it has a tiny, yet discernible, benefit. “We may comfortably mistake 2 or 3 percent with 0 percent or comment about other things as not being important without going through some sort of formal analysis,” he cautioned. “We need to wrestle with the distinction between something and nothing. We need clearly defined parameters that we will stop doing these interventions collectively as a society because they are not beneficial enough to justify the costs, rather than having the doctor and individual patient make that societal decision and cause inequities in treatment and other problems,” Bach said.
Green also noted that drug treatments are often a mixed bag of benefits and risks, which ultimately determine value, and each patient may weigh the combination of the two differently, or not even consider the financial costs in comparison to the benefits. For example, for metastatic lung cancer,
the combination of paclitaxel and cisplatin is similar in efficacy compared to pemetrexed and cisplatin, but the latter is less likely to cause hair loss. “I give pemetrexed and cisplatin to most of my patients with advanced adenocarcinoma of the lung primarily because of the toxicity profile, and if you talk to patients about it, no one wants to lose their hair if they don’t have to. But no one would probably write a check for $6,000 a month to not lose their hair,” Green said. Similarly, different chemotherapies for gastric cancer are equally effective, but have different quality-of-life issues. An oral regimen is more convenient but is much more costly, for example. “How do you trade off the costs and values of that?” Green asked.
Bach agreed that assessing value in these examples does involve complex decision making that must consider costs, survival prolongation, quality of life, inconvenience, and other factors before equivalence between treatments can be drawn. “It is a little too fast and potentially too dangerous to say two things are the same that have never been compared along all of these dimensions,” Bach said. Kean added, “We need an agreement of the value of what we are doing.”
Even when interventions are determined to be of high value, there are still unanswered questions of how they can be most efficiently allocated, Peppercorn noted. “Is health care a commodity or something special where it is our obligation to provide it to everybody? There is a wide debate on this in the United States. Is efficiency really what we should aim for as a society, or should we be thinking about equitable allocation?” he said.
Peppercorn added that when interventions are considered to be of low value, it is not clear who—insurers, states, federal agencies, physicians, or hospitals—should curb the overuse of these interventions. “How are we going to handle exceptions, how burdensome will the appeal process be, and where is the room for physician discretion, recognizing the complexity of cancer care? It may come down to how do we balance cost control with clinical judgment,” Peppercorn said.
Fineberg stressed that multiple strategies working synergistically will be needed to deliver affordable, high-quality cancer care. “We have to be prepared to use every tool in our toolbox and be creative about inventing new tools,” said Fineberg. “We have to be willing to experiment and pilot test. We have to be prepared to extend and implement what works, and what saves money at the same time.”
There was also acknowledgment from many speakers that culture change may be necessary to make cancer care affordable. That culture change will require greater consideration of costs when making health care decisions, and balancing the needs of individual patients, physicians, insurers, and pharmaceutical companies with the need to improve health care and lower societal costs of such care. Renzo Canetta, vice president of oncology global clinical research at Bristol-Myers Squibb, added that there is a need to recognize that “we are all in the same type of boat, even though we may have different employers. Progress is going to come only from collaboration and not from creating little parishes where we fight against each other on petty issues.”
Many workshop participants offered ways to meet the current challenges in delivering affordable, high-quality cancer care. Some of these solutions aim to improve patient and clinician education, with explicit recognition of costs and evidence-based use of interventions. Other solutions focus on improving care and making it more efficient and affordable by coordinating care, using more palliative care and better end-of-life care, and eliminating or reducing use of treatments with little or no value. In addition, several speakers suggested applying more appropriate financial incentives, developing and applying performance metrics, facilitating greater use of and adherence to standards and treatment guidelines, and focusing more on cancer prevention. To improve clinical practice guidelines, participants also suggested gathering more clinically relevant information about interventions as part of a learning health care system.
Patient and Clinician Communication and Education
A number of speakers suggested that facilitating better patient–clinician communication and improving education for both clinicians and patients could result in more affordable, high-quality cancer care.
Improving Patient–Provider Communication and Patient Education
Gruman stressed that patients need valid, reliable, readily available information that can help them find the best clinician, treatment, and hospital for their condition. Once a clinician has been found, patients need more time with that provider to discuss treatment options. “These are tough conversations. You can’t just have these conversations on the fly, and there is broad agreement among patients and doctors that we rarely have enough time. If
we understand our options, make our decisions with our doctors, have a sense of what to expect, we will make decisions about our care that not only will benefit us more, but simultaneously will be of high quality,” she said.
Several speakers noted the need for providers to realistically convey to patients their prognoses; the costs, potential benefits, and side effects of various treatment options; and palliative care and end-of-life care considerations. However, Schrag described the difficulty in acquiring information about the benefits of specific cancer treatments. Drug labels or consent forms provide information on risks and side effects, but rarely provide information on expected benefits that is quantified and tailored to a patient and his or her comorbidities. Schrag suggested revising the way chemotherapies are labeled and consent forms are written so that they provide clear, simple, and direct statements of the expected magnitude of benefit from the treatment. Instead of indicating response rates and confidence intervals, she suggested indicating median survival or the percentage of patients still alive 1 or 2 years after treatment.
The costs of various treatments also have to be part of the patient–clinician discussion, several speakers suggested, including Gruman. “We patients and families may not want to believe that our lives and our hope and our physician’s goodwill have a price,” Gruman said. “Increasingly, we have to come to terms with that uncomfortable fact. We need the tools and the leadership from our clinicians to consider price, both financial price and broad price, as part of our shared decision making about treatment.”
Shankaran pointed out that cancer patients are experiencing high unexpected out-of-pocket treatment expenses because physicians often neglect to discuss the financial risks involved with their therapies and end-of-life care. She noted that often there is no easily available information in the clinic about how much patients can anticipate paying for their treatments, such as chemotherapies. “We need a system that can provide both total and out-of-pocket costs up front to patients and oncologists,” Shankaran said.
She suggested incorporating cost information into clinical practice guidelines, which can indicate treatments as being equivalent, even though they have markedly different price tags. “Cost is one additional factor we should consider when choosing among multiple, equally supported evidence-based treatment options. Perhaps the most important thing we can do to help individual patients and their families is to not recommend treatments that are highly costly but have no evidence of benefit,” Shankaran said. Emanuel noted a recent American College of Physicians guideline that stated, “Physicians have an ethical obligation to be prudent with the use
of resources and not profligate.” He added, “There is an awful lot that the physician can and must do to be a responsible steward of the patient’s and society’s resources.”
Schnipper agreed that doctors should routinely discuss the costs of treatment with patients to help avoid what he termed “financial toxicity.” He added that Cancer.net makes available a pamphlet and website that explain the costs of cancer care and encourage patients to ask for the evidence supporting a physician’s treatment recommendation and its financial implications.
Although Peppercorn agreed that physicians should discuss the costs of care with their patients, he noted it is not clear when and how often those discussions should occur, especially given the competing interests in the typical 15- to 20-minute office visit. He also added that there is a lack of agreement on what types of costs should be discussed with patients—should only the patients’ out-of-pocket costs be discussed or should the discussion include the societal costs?
Although physicians may make concerted efforts to discuss the costs and benefits of treatments with their patients, some patients may still insist on a specific medical intervention, even if the risks or costs are likely to outweigh the benefits, Goodwin pointed out. “Patients demand those treatments or screenings and doctors prescribe them because they are afraid they will lose their business,” he said.
Patients may also receive more emergency room and other expensive care because providers do not adequately inform patients of the anticipated side effects and how to deal with them. Both Schrag and Gruman noted that cancer patients and their families are increasingly expected to provide complicated home care, but often there is not 24-hour support for home care, such as clinicians who can give patients advice after standard office hours. “When we have good support to care for ourselves at home, we are more satisfied, we do better, and we also rack up fewer costs to the system,” Gruman said.
Paula Rieger, chief executive officer of the Oncology Nursing Society agreed, stressing, “We have to teach patients and families how they can self-manage, because we have asked them to take on a bigger burden in their care. In order to try and prevent readmissions and unintended outcomes, they have to be knowledgeable about their treatments—to know what is normal and expected, what are the thresholds for things they should immediately report to the health care system, and how they can try to manage side effects.”
She added that nurses are uniquely positioned to help with such patient education and can facilitate patient-centered care. Nurses can also provide patient education about cancer prevention. Green added, “We need to invest the resources to figure out how we are going to answer phone calls and provide after-hours or weekend care for patients, other than the on-call physician, and we need a mechanism for getting those resources.”
Temel stressed that patients should also be educated about end-of-life care options and how various options will affect their quality of life and their own psychological well-being, as well as that of their families. She added that physicians frequently do not discuss advance directives with their cancer patients, nor do they often discuss hospice or other end-of-life care more than a few months to weeks before the patients die (Smith and Hillner, 2011). Temel conducted a study in which oncologists were sent email prompts to discuss advance directives and end-of-life care preferences early in the course of a patient’s disease. Email prompts to discuss preferences were also sent to the oncologists when their patients experienced disease progression. These reminders improved the documentation of cardiopulmonary resuscitation (CPR) preferences in the ambulatory care setting compared to historical controls (Temel et al., 2013). “Oncologists can also do some of the tasks that the palliative care clinicians are doing,” Temel said.
She noted that Angelo Volandes at Massachusetts General Hospital developed videos that help patients better understand end-of-life care options, such as receiving CPR. In his study of 150 advanced cancer patients who were randomized to see a video depiction of CPR or hear a verbal description of the same procedure, patients who watched the video were more likely to answer questions about CPR accurately, and less likely to choose it as an option (Volandes et al., 2012). “We need to educate patients so that they can make more informed decisions about their care,” she said.
When a workshop participant asked Temel if such discussions affect patient quality of life, she responded that an early palliative care study found that the majority of patients who became accurate in stating that their cancer was incurable experienced improved quality of life (Temel et al., 2011). However, Eisenberg noted that patients often want to be treated even if the treatment is likely to be futile. He frames advanced cancer care discussions with his patients by telling them, “I know I can make you sick, but I am not sure I can make you better. Maybe we ought to talk about changing our goals from making you live as long as you possibly can to
making you live as well as you possibly can.” Patients tend to be receptive to such statements, he said.
Dentzer stressed that at least for non-cancer-related elective procedures, such as knee and hip replacements, “We know from shared decision-making models that when you expose patients to careful, accurate evidence, that clearly lays out the risks and benefits of treatments, [a majority of] patients will pursue a less risky path than their clinicians would have recommended.” She also gave examples of well-informed patients choosing conservative treatment that gives them better quality of life, rather than pursuing only quantity of life. “We need to enable patients to make the best choices about their care and engage patients as true partners,” Rieger added.
Improving Clinician Education
Although physician fees comprise 15 percent of all Medicare spending, the decisions physicians make influence 80 percent or more of Medicare spending, McClellan said, making it imperative that physicians are better trained to practice high-quality, affordable cancer care. Emanuel suggested educating oncologists about the financial aspects of running a practice and making them more aware of the costs involved and how to mitigate them without compromising the care of their patients. “Most doctors in practice have no idea where they are spending the dough—how much is going to chemo, how much to hospitalization, to imaging, etc. There is a good reason why doctors don’t think about this—right now we are paid to not think about it because we make money by doing the most expensive thing. So we have to get the clinical practice aligned with the business practice. We need to educate doctors on where they are spending their money and where it is unnecessary,” he said.
Hu suggested better mentoring of surgeons using new techniques. He recommended that evaluation of new surgical technologies should initially be restricted to a few facilities whose physicians could then mentor surgeons at other sites about new surgical interventions and how to conduct them properly. For example, a few robots used for surgery could be purchased by acknowledged surgical centers of excellence, of which there are three or four in the country, he said. Surgeons at these centers will have good surgical technique and thus are best poised to evaluate whether these new robotic techniques should be disseminated, he said. Once these surgeons develop expertise with the robots, they could then mentor others. Such mentoring, termed “collaborative feedback,” can be effective at improving outcomes
among surgeons, Hu said. One study showed it led to a reduction in patient mortality (O’Connor et al., 1996). “Mentoring of others led to rapid and safe adoption of robotic surgery,” Hu said, adding that such mentoring is improved when there is online collection of data that can provide performance feedback. He added that health plans should be more involved with ensuring clinician competency; for example, they could require clinicians to pass tests showing they understand the benefits and limitations of the new technology prior to paying for new health care interventions.
Physicians also need to be better educated about new drugs, Eisenberg suggested. “We are faced with an onslaught of new drugs with new side effects that we really need to learn how to manage and use. I am not sure that we have the tools that will adequately enable us to do that,” he said. Schottinger said that Kaiser Permanente delineates care paths for their physicians to follow, including care paths for cancer survivors, and also encourages its physicians to use shared decision-making tools that have videos and links to websites.
Physicians also have to be better educated to interpret study results, suggested Kramer. To counter the excessive use of screening and overdiagnosis, he also suggested physicians be better educated about uncertainty. “We are the problem, but we are a product of our training. The major lesson in medical training is a failure to train our [physicians] for clinical uncertainty. That has led to the systematic overuse in practice of tests and procedures and treatments,” he said.
“We are diagnosing huge numbers of cancers without necessarily having a concomitant effect on the risk of actually dying, because many lesions are not life threatening. We created the world’s largest epidemic of prostate cancer by screening with a very simple screening test, the prostate-specific antigen [PSA] test, without having the evidence in hand about whether it would actually help people,” Kramer said. Although screening has increased the number of diagnoses made of various cancers, it has not led to a decrease of the same magnitude in the numbers of people dying from those diseases (Welch and Black, 2010).
“We need to start in medical school because that is where belief systems are formulated,” Kramer stressed. When expressing the evidence of a medical intervention to both physicians and patients, he suggested detailing the absolute magnitude of benefits and harms, and not conveying the information in relative terms. As an example of such effective communication, he cited an information sheet devised for the National Lung Screening Trial. Brawley noted that physicians’ knowledge may be improving in one regard,
citing the growth in the number of schools of public health and medical school curriculums that include epidemiology.
Robert Erwin, president of the Marti Nelson Cancer Foundation, pointed out that often knowledge and belief are confused when physicians communicate with their patients about the possible benefits of a treatment. “Unfortunately, it is belief about the compounds, interventions, and devices that lead to this willingness to overpay for things that just flat don’t work,” said Erwin. “We talk in terms of treatment benefit and cure. We don’t talk in terms of failure, but failure is a huge part of oncology. We need to embrace that reality and communicate it better.”
Given the rising importance of interdisciplinary team care, Rieger suggested education that gets health care professionals “out of their traditional silos. Oftentimes, we don’t have a full appreciation of what our colleagues can do, and that would help facilitate interdisciplinary coordinated care,” she said.
ASCO’s Physician Advisory Tool could also help inform clinician decision making. This pilot project provides information on regimen toxicities and efficacy—such as progression-free or overall survival statistics—as well as cost information on a particular regimen for a particular disease to participating physicians on their computer desktop. The aim of the pilot is to assess whether making such information readily available to physicians influences their decisions.
Workshop participants suggested a number of changes to clinical practice that could facilitate more affordable, high-quality cancer care. These changes include
- greater use of and adherence to treatment guidelines;
- reducing or eliminating use of procedures with little or no value;
- making care more coordinated and efficient;
- improving functionality of EMRs; and
- prioritizing cancer prevention.
Greater Use of and Adherence to Treatment Guidelines
Several participants suggested greater use of and adherence to standards and treatment guidelines and that these guidelines incorporate cost considerations.
Peppercorn has served on the ASCO Clinical Practice Guidelines Committee, and he said that within this committee, “there is an active effort to move toward including at least transparency with regard to costs in the guidelines, if not making decisions exclusively based on cost. That is clearly where things are going.” Ramsey noted that cost is not currently a factor considered when devising the NCCN guidelines.
Schrag noted that treatment pathways and guidelines can also help curb cancer drug costs when there is a menu of choices, including variably priced but equally effective options. Emanuel further suggested that treatment guidelines be required to rank chemotherapy by price, and recommend the least expensive treatments be used first when outcomes are similar. Schrag added that developing and curating these pathways and guidelines is resource intensive, and difficult to keep free from commercialism.
Emanuel also suggested supporting insurers who only pay for cancer treatments that adhere to clinical practice guidelines, standard of care, or evidence-based treatment pathways. “We don’t like to say no and be the bad guy, but we need to support insurers that say no,” he said. Newcomer added, “If we can shift to the less expensive medications that are equally effective, we are not harming patients, and we clearly could make a difference in the cost of treatment.”
Schrag suggested having tiered formularies for cancer drugs that clearly indicate those that are the most preferred, based on cost effectiveness. Another strategy is to have a step-up approach for drugs, in which more expensive drugs cannot be used without first trying equally effective, but less expensive, drugs. Requiring prior authorization can also help reduce cancer drug costs, some studies show, although Schrag recognized that most physicians dislike such required authorizations. She suggested streamlining prior authorization drug programs “so they are less painful.” Another option Schrag suggested is placing quantity limits on expensive oral chemotherapy drugs. These drugs, which can cost $10,000 per month, can be dispensed in 2-week supplies, she suggested. “Yes it is a pain to go back to the pharmacy to get more, but a lot of patients do not last 2 weeks on a particular drug. I cannot tell you how often a patient of mine dies, leaving behind a 3-month supply of sorafenib [Nexavar] that cannot be [given to] other patients.”
Smith pointed out that U.S. Oncology has strict treatment pathways that indicate which cancer drugs are appropriate for first-, second-, and third-line therapies. This medical group employs financial counselors who provide a recommended regimen’s total cost figure to patients, as well as the portion of that cost for which the patient will be responsible.
Newcomer noted that NCCN still recommends Avastin for breast cancer, despite the lack of evidence for this recommendation. However, by encouraging its medical groups to follow what the evidence supports, UnitedHealthcare has cut the use of this drug in half in just a few years (see Figure 8). “We need standard approaches,” he stressed, noting that John Sprandio, chief physician at Consultants in Medical Oncology and Hematology, for example, has cut hospitalization in his medical practice in half and emergency room visits by two-thirds by looking at standard ways to approach patient problems, such as making sure patients have after-hours access to clinicians.
Schottinger noted that due to adherence to treatment guidelines facilitated by Kaiser’s EMR system, the variation of its providers has been substantially reduced, with 90 percent adhering to protocols on the first round of therapy for cancer.
Schnipper reported that when the ASCO Quality Oncology Practice Initiative (QOPI; see Box 2) was first implemented at the University of Michigan Comprehensive Cancer Center, they found that half of patients received chemotherapy within 2 weeks of their death. After presenting these results at a faculty research conference, their oncologists better adhered to treatment guidelines and chemotherapy use at the end of life dropped by 30 percent (Blayney et al., 2009).
“If we can measure and [provide] the data back to those whose practice patterns are being measured, we have an opportunity to influence behavior
FIGURE 8 UnitedHealthcare use of Avastin (bevacizumab), 2009–2011.
NOTE: PMPM = per member per month.
SOURCE: Newcomer presentation (October 9, 2012).
BOX 2
ASCO’s Quality Oncology Practice Initiative (QOPI)
The American Society of Clinical Oncology’s Quality Oncology Practice Initiative (QOPI) is a practice-based quality improvement program developed by practicing oncologists and quality experts, using clinical guidelines and published standards. The goal of QOPI is to promote excellence in cancer care by helping practices create a culture of self-examination and improvement. QOPI assesses performance and then provides feedback and improvement tools for hematology and oncology practices to improve the quality of cancer care. Some of the QOPI performance metrics include having a discussion with a patient about the intent of chemotherapy, providing a patient with a chemotherapy treatment plan, and providing appropriate hospice enrollment.
SOURCES: IOM, 2011; QOPI, 2013a,b.
even independent of the financial incentives that we probably need as well,” Schnipper said. “We believe that doctors are inherently wanting to do the right thing and are mildly competitive in that regard. We can use these two characteristics to achieve our ends,” he added.
However, Schnipper noted that only a small percentage of oncology practices in the United States currently employ QOPI—probably less than 10 percent, he said. In addition, few QOPI-certified practices are at academic medical centers where future oncologists undergo training. “I strongly advise that academic programs become QOPI certified so as to give us a reasonable measure of what is being practiced in those institutions, as well as to influence those graduating from such institutions,” Schnipper said.
Gruman added, “We need good cancer treatment quality measures that are evidence-based. This information needs to be gathered and disseminated in places that we can find it in order to help us make good decisions about our doctors and hospitals.”
Reducing or Eliminating Use of Procedures with Little Value
Schnipper pointed out that many interventions have little or no benefit in cancer care. To address this, ASCO developed a “Top 5 List” of
questionable costly procedures used in oncology. ASCO estimated that if physicians limited or eliminated use of these procedures, they could result in high-quality care and substantial savings in cancer care costs (see Box 3). As Goodwin pointed out, “Something can’t be cost effective if it’s not effective. We can go far, in terms of stopping inappropriate treatments, without ever thinking about cost effectiveness, and that way encounter less resistance by
As a participant in the American Board of Internal Medicine Foundation’s Choosing Wisely® campaign, the American Society of Clinical Oncology (ASCO) issued a “Top 5” list of common, costly procedures in oncology that are not supported by evidence and that patients and their oncologist should question using. The development of this list was led by ASCO’s Cost of Cancer Care Task Force, a multidisciplinary group of oncologists, and selections were based on a comprehensive review of published studies and current guidelines from ASCO and other organizations. The final list also reflects input from more than 200 oncologists and patient advocates.
- For patients with advanced solid-tumor cancers who are unlikely to benefit, do not provide unnecessary anticancer therapy, such as chemotherapy, but instead focus on symptom relief and palliative care.
- Do not use positron emission tomography (PET), computed tomography (CT), and radionuclide bone scans in the staging of early prostate cancer at low risk for metastasis.
- Do not use PET, CT, and radionuclide bone scans in the staging of early breast cancer at low risk for metastasis.
- For individuals who have completed curative breast cancer treatment and have no physical symptoms of cancer recurrence, routine blood tests for biomarkers and advanced imaging tests should not be used to screen for cancer recurrences.
- Avoid administering colony stimulating factors to patients undergoing chemotherapy who have less than a 20 percent risk for febrile neutropenia.
SOURCE: ASCO, 2012b.
physicians and the general public” concerned about employing cost effectiveness thresholds as a way to curb costs.
Schnipper elaborated on the Top 5 List. The first item tries to counter aggressive, yet nearly always ineffective, therapies given to cancer patients with poor performance status. These patients spend at least half their time in bed or in a chair, and cannot walk into a clinic unassisted. Such patients should receive palliative treatment or hospice care, Schnipper noted. Although there are exceptions to this rule, such as patients with advanced cancer for whom an interventional target has recently been discovered due to genetic testing, Schnipper pointed out that these exceptions are rare. Other exceptions include patients whose poor functional status is caused by conditions other than cancer.
The next two items aim to reduce the excessive use of imaging tests for staging patients with early-stage prostate or breast cancer. Such imaging is not likely to help the patients, will expose them to unnecessary radiation, and may yield false positive results that will require follow-up procedures, Schnipper stressed.
Evidence supporting the fourth item on the list comes from two randomized trials conducted in the 1990s that compared different surveillance strategies for breast cancer patients who had completed curative breast cancer treatment and had no symptoms of recurrence. Women who had routine clinical office visits and mammograms had no difference in survival outcomes compared to women who had more intensive monitoring with bloodwork, chest films, and ultrasounds. In addition, chest and abdominal CT scans or whole-body PET scans have not been evaluated as surveillance strategies for follow-up of early-stage breast cancer. With the low prevalence of distant recurrence in early-stage breast cancer and the high risk of false-positive and incidental findings, there is no evidence to support the use of these other tests for surveillance, Schnipper stressed.
The last item on the list is to limit the inappropriate use of colony-stimulating factors (CSFs). This is based on an ASCO 2006 guideline (Smith et al., 2006), which recommends the use of CSFs only when the risk of febrile neutropenia10 is 20 percent or greater and there are no other equally effective regimens. “In some situations, primary prophylaxis with CSFs is essential and recommended to alleviate the toxicity of certain ‘dose-dense’ chemotherapy regimens,” said Schnipper. Despite this guideline, a
__________________
10A condition marked by fever and a lower-than-normal number of neutrophils in the blood (NCI, 2013).
CMS study found that nearly 20 percent of the time there is overuse of these CSFs in practice, Schnipper said, which is problematic because CSFs can also promote cancer growth.
Schnipper noted that ASCO is updating QOPI so that it incorporates ASCO’s Top 5 List. The variables on this list will be measured in QOPI audits of charts to assess the impact of the list on oncology practice.
A number of workshop participants spoke favorably about ASCO’s Top 5 List. Emanuel thought more should be added to the list. “It’s a good start, but we can’t stop at just the five that we have. We could probably easily add 20 to 30 based on those interventions not proven to do one of four things: increase survival, reduce side effects, improve quality of life, or reduce cost,” he said.
Bach added that interventions that are considered to have limited or no benefit are a moving target. He also questioned labeling these “zero-benefit” interventions, noting that more extensive scanning in prostate cancer patients considered at low risk for metastatic disease will detect metastases in 5 percent of those patients, who are likely to benefit by having more systemic interventions. “These are low numbers, but they are not zero,” he said.
Schnipper pointed out that the American Board of Internal Medicine has been collaborating with Consumer Reports to expand its Choosing Wisely campaign, of which the ASCO Top 5 List is a part. This collaboration has enlisted more than two dozen medical specialties to identify commonly used procedures or technologies in those specialties that add little to no value.11
Making Cancer Care More Coordinated and Efficient
A number of workshop participants offered suggestions for making cancer care more efficient and coordinated. These suggestions included eliminating duplicated tests and services; integrating palliative care throughout the continuum of cancer care, and improving care at the end of life; providing more efficient survivorship care; better aligning screening with the evidence base; and making greater use of nurses and other clinical specialists who are less expensive than physicians.
__________________
11See http://www.abimfoundation.org/Initiatives/Choosing-Wisely.aspx (accessed December 17, 2012). ASCO is in the process of creating an additional list of practices that should be avoided in oncology.
Schottinger pointed out that one advantage of being in an integrated health care system like Kaiser Permanente is that care is more efficient. She noted that if patients see any clinician in the system, and the clinician discovers that their patient needs an eye exam or has not had blood sugar levels checked recently, a nurse will make sure they have those needed tests at that time, rather than have them come in for another visit. “It is wasteful to say, ‘go see your primary care doctor and deal with that.’ When they are coming in to our system, we want to address every gap they have at every visit,” she said.
There was some debate at the workshop over who should be most responsible for the overall care a patient receives in a non-integrated system, with some suggesting that the clinician treating the disease of most importance oversee the coordination of a patient’s care, including overseeing the management or coordination of their patients’ comorbid conditions. Others were skeptical of whether an oncologist would have the time or expertise for such care coordination. Newcomer countered, “[Oncologists] may not be able to handle the angina, but we can at least make sure that the cardiologist is aware and adjusting medications as necessary, and that we are aware of the interactions. The oncologist can figure out if the diabetes is under control and then get help if needed, but that patient shouldn’t have to go see the endocrinologist every week, as well as the medical oncologist.”
Rieger advocated for more interdisciplinary team care that maximizes “how we use the education, knowledge, and skills of all professionals involved in the care of patients with cancer.” Coordinated care is especially important as cancer survivors transition from oncology care to primary care, and nurses are especially suited to aid in that coordination, she pointed out. Schottinger added that Kaiser is experimenting with offering survivorship clinics that provide follow-up care for their cancer patients, instead of having them continue to be seen by their oncologists. They are also trying to integrate primary care nurse practitioners into some of their oncology units to provide more affordable survivorship care.
Schottinger also pointed out that Kaiser clearly demonstrated that integrating palliative care physicians into the oncology clinic not only benefits patients, but also reduces costs. She added that Kaiser employs social workers because “they have more time than doctors and [may] do a much better job than the physicians in terms of addressing these difficult end-of-life issues.”
Several participants described the tremendous cost savings and benefits for patients that could be gained by enhanced use of palliative care
throughout the cancer continuum and better end-of-life care. Peppercorn pointed out that about a quarter of Medicare’s total spending accounts for health care people receive in the last year of their life (Hoover et al., 2002). Smith noted there are good data from randomized clinical trials showing that better, coordinated end-of-life care that is integrated into regular cancer care and regular medical care reduces the cost of end-of-life care. Brumley et al. (2007) showed that when Kaiser patients received interdisciplinary palliative team care, their end-of-life costs were reduced by 33 percent compared to those receiving usual care, according to Smith. “I haven’t found a single study in which palliative care added to usual care actually increased total end-of-life costs,” he said.
Temel added that a study evaluating the integration of palliative care into an oncology practice found that patients who received the palliative care intervention were more likely to accurately state that their cancer was incurable over time, as opposed to patients receiving standard care (Temel et al., 2011). “This new model of care was able to alter patients’ understanding of their illness and prognosis,” she said. In addition, early integration of palliative care has also been shown to prolong median survival among patients with metastatic non-small-cell lung cancer (Temel et al., 2010).
In another study, although palliative care did not alter the use of chemotherapy over the course of illness, those patients receiving the early palliative care were significantly less likely to receive chemotherapy at the end of life compared to those receiving standard care (Greer et al., 2012). Length of stay in hospice was also longer for those who received the palliative care, even though referrals to hospice were the same for both groups of patients, as was location of death. For patients who received early palliative care, the median length of stay was 24 days versus 9.5 for the others. There also were fewer hospitalizations and emergency room visits for patients receiving palliative care. These patients lived as long or longer than those receiving standard care.
When the researchers examined costs expended in the last 30 days of life, they found that the average cost difference was about $2,000 in favor of palliative care. The cost savings were primarily due to fewer hospitalizations and emergency room visits and less futile chemotherapy at the end of life.
Smith added that U.S. Oncology has also shown that earlier use of palliative care, provided by social workers, psychologists, or nurses, led to patients living at least as long if not longer, while reducing the cost of care by one-third. We can save costs while improving care, “but it means saying no to consumers, pharmaceutical companies, and other people within the system,” he stressed.
Improving Functionality and Interoperability of EMRs
Substantial discussion at the workshop centered on possible benefits and challenges of using EMRs to improve the quality and affordability of cancer care. Peppercorn noted that EMRs can help prevent duplicated tests and services, but how much that duplication contributes to the high costs of cancer care is not well documented.
Schrag suggested that EMR chemotherapy order entry systems be hard wired to indicate the most cost-effective regimens. “These make it easy for people to do the right thing and save money at the same time,” she said. However, she noted these systems are often missing molecular profiling information, which can indicate whether the appropriate regimen is ordered. This is important as researchers are increasingly finding that many targeted treatments are only effective and/or safe for a subset of patients with the right molecular markers on their tumors. “When you order trastuzumab (Herceptin), is it for somebody who is HER2 positive? This is an IT issue that we have to piece together,” Schrag said.
Aberle suggested EMRs could make lung cancer screening more cost-effective by tracking and helping to manage patients so they receive the appropriate follow-up care. Rieger stressed that “systems that capture data in real time for practitioners should also look across the spectrum of care and collect data on the care of all members of the team.”
Community oncologists at the workshop were highly critical of EMRs as they are currently deployed in their practices. Eisenberg said, “There has been a dismal failure of electronic medical records to do the things they are supposed to do. In my practice, it is very hard for me to find out what is happening with my patients because of the limitations of technology. The EMRs today are designed for coding and billing, and not designed for me to care for my patients.” He added that EMRs fail to impart important safety information to prevent medical errors and do not include reminder prompts that could help provide guideline-based care. “[EMRs do not] give me the kind of data that I want,” Eisenberg said.
Green added, “The EMR doesn’t force you to put in relevant data points, which is why we have so much difficulty actually knowing what is going on in our practices. Every time I see a patient and move onto the next room, I feel that I have thrown away information from that encounter that could have been useful to someone somewhere else. There is not really a good mechanism for either collecting or analyzing data.”
Green noted that a recent study found most EMR vendors failed to
innovate and do not even allow innovative uses of data and interoperation with other software (Mandl and Kohane, 2012). He quoted another article, which stated: “Swapping out the medical record cabinet and prescription pad for a computer is proving insufficient to realize the benefits of health IT” (Jones et al., 2012). He emphasized Mandl and Kohane’s (2012) conclusion: “Doctors become increasingly bound to documentation and communication products that are functionally decades behind those they use in their ‘civilian life.’”
Eisenberg recommended having “more intelligent EMRs that can talk to others.” Rieger agreed that EMRs need to be better integrated. She noted her hospital employs an EMR that is oncology specific, but is not compatible with the EMR system used by the primary care physicians employed by the same physician health organization. “They have read-only access to the information I enter, and I have read-only access to the information they enter. This makes no sense,” she said.
Newcomer acknowledged these criticisms as valid, but pointed out that with the right resources, EMRs can be invaluable tools for promoting high-quality and affordable care. “With the EMR, we can identify every diabetic in our system and hit all of our quality measures. With the right resources, you can use EMRs in a very positive way.”
Schottinger agreed, noting that the EMR system Kaiser employs is superior to most because of the large number of professionals devoted to building and updating it. “The EMR system we use is not the same one that came out of the box. A lot of what it does is what we have built and continue to build. We had a substantial investment, not only from IT, but a collaborative build team that consisted of dozens of doctors, nurses, and pharmacists from around the country,” she said.
Kaiser Permanente’s EMR captures every encounter with every patient and ensures that the care patients receive at Kaiser Permanente conforms to evidence-based practice guidelines that are built into their EMR software. “That means that if you are a member who is 55 years old and you tweaked your knee and go to see your orthopedic [doctor], if you have not been screened for colon cancer, the nurse in that office will give you a stool test that is already entered into the record by your primary care physician. The EMR will also help make sure you go down to the lab to get it done because it detects if the kit is not returned to the lab within 30 days, in which case the regional outreach program will call you and request you to come in.”
Schottinger noted that the combination of provider education with EMR reminders fostered a decline in the overuse of Pap tests from 42 percent
in the first half of 2006 to about 8 percent in 2012. Overall cervical cancer screening rates rose from 79 percent to about 90 percent during the same time frame.
In addition to overseeing preventive care, Kaiser Permanente’s EMR system monitors ordering of medical interventions and dispensing of medications with barcoding for safety assurance. The system also includes an oncology knowledge database built by pharmacists that is integrated with lab and pathology information. “It has reduced safety events, simplified our referrals to clinical trials, and helped increase enrollments in these trials. Information from our EMR is used to assess practice patterns and provides alerts when those patterns do not match with treatment guidelines,” Schottinger said. She added that the EMR also helps ensure adherence to medications by informing practitioners when patients last refilled their medications. Patients not properly refilling their medications are followed up with phone calls.
Kaiser Permanente has used the data collected in its EMR system to modify its practice protocols. For example, it reviewed the outcomes of patients with breast cancer given docetaxel/cyclophosphamide who were older and had more comorbid conditions, such as diabetes. In this population, the neutropenia rate with this regimen was around 28 percent, so Kaiser Permanente modified its CSF protocol for that patient population.
“Looking at our regimens with our EMR will really help us improve the safety of the care that we deliver,” Schottinger stressed.
Prioritizing Cancer Prevention
Another measure that could reduce costs is more effective cancer prevention. Brawley noted that “we don’t have a culture in American medicine that actually favors prevention. Instead we treat—even when we prevent heart disease, we treat hypertension.”
McClellan added that even when preventive measures are offered free of charge, as they are in Medicare, 30 to 40 percent of the population does not use screening tests for cancer, which the evidence base suggests would be helpful. Brawley noted that uninsured individuals and those with lower educational status tend not to receive potentially life-saving cancer screening measures, such as Pap tests. He added that about half of the women who die from cervical cancer never had a Pap test.
Schottinger said Kaiser Permanente has prioritized preventive care among its members, leading to greater tobacco cessation, for example.
Kaiser also provides resources for healthy eating and has included exercise as a vital sign in its EMRs. Schottinger reported that the percentage of patients over 18 who smoke has declined significantly, to about 9 percent, and that has been accompanied by a decline in lung cancer incidence rates, which are well below national averages. In addition, their breast and cervical screening rates have consistently been above 90 percent, and colorectal cancer screening rates are around 80 percent, Schottinger reported. Presumably due to these screening efforts, fewer Kaiser Permanente members are being diagnosed with colorectal cancer at any stage (see Figure 9). From 2008 to 2011, the total incidence rate for colorectal cancer among Kaiser Permanente members declined by 36 percent.
Schottinger attributed Kaiser Permanente’s successful cancer prevention efforts in part to their extensive EMRs that notify practitioners if their patients are not up to date on proper screening tests, are smoking, or are not getting sufficient exercise, for example.
Evidence Base for Clinical Practice and Reimbursement
Several speakers noted there is a lack information on the comparative effectiveness of cancer care interventions that can guide clinical practice. Erwin said that clinical trials are generally designed for publication or for
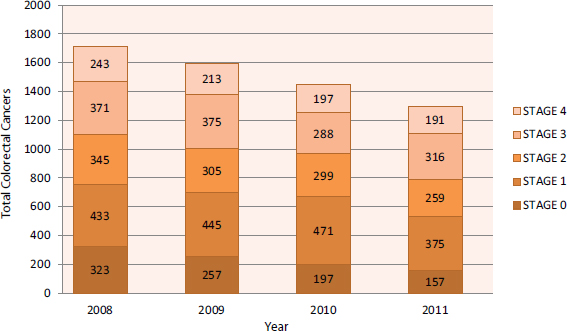
FIGURE 9 Reduction in diagnoses of colorectal cancers at Kaiser Permanente, 2008–2011.
SOURCE: Schottinger presentation (October 9, 2012).
product registration, and the patient eligibility criteria are so narrow for these trials that they do not really provide guidance to the physician about the population he or she is treating. “We need to realize that clinical trials and the kinds of evidence that we tend to consider to be the gold standard are not particularly good at guiding clinical practice,” he said.
Amy Abernethy, associate professor of medicine at Duke University School of Medicine, noted that once a drug enters the market for a FDA-approved indication, it is typically used more broadly for other indications. Off-label use12 is exceptionally common in oncology, with the NCCN estimating in 2005 that half to three-quarters of all cancer prescriptions were written for off-label indications, Abernethy reported. Such off-label prescribing is common in oncology because of the life-threatening nature of the illness, and the biologic plausibility that what works for one type of cancer might work for a similar cancer. Furthermore, once drugs enter the market based on efficacy for a narrow group of cancer patients, pharmaceutical companies are not likely to invest in more Phase III clinical trials to broaden that use, Abernethy said.
In addition, reimbursement by insurers fosters off-label prescribing. “Off-label prescribing in oncology is a real part of care and a substantial contributor to cost that is reinforced by the compendia-based reimbursement mechanism that tries to review rapidly evolving evidence and information without a mechanism to make sense of it all,” said Abernethy. “We need a strategy to define appropriate off-label use. It’s the Wild Wild West out there and we have not demanded that we figure out a way to make sense of it” (see Figure 10).
Abernethy said that drug compendia, such as the NCCN, often guide the use of off-label prescribing of cancer therapeutics and the reimbursement for such regimens (Abernethy et al., 2009). However, compendia are not designed for this purpose, nor are they designed to guide the choice of one drug compared to another drug (Abernethy et al., 2010a). “We are relying on the compendia system to help us essentially do comparative effectiveness research, but really their role still in their minds has been that of helping to understand toxicity around drugs, and what does it mean once a drug has been prescribed,” Abernethy said.
Abernethy found a number of subjective processes influenced the validity of the assessment, choice of citations, and the policy for equivocal evidence
__________________
12Off-label use is the prescribing of drugs already on the market for an indication, age group, dose, or form of administration that has not been approved by the FDA.
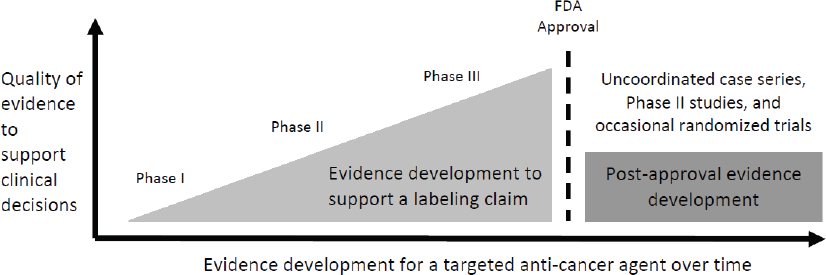
SOURCE: Abernethy presentation (October 8, 2012).
used in the compendia. “Each compendium had a different approach and said ‘we are trying to figure out how to do this better, but we have not figured this out,’” Abernethy said. In addition, the compendia noted that the rapidly developing evidence base in oncology made it difficult to update the monographs in a timely fashion.
For example, when Abernethy and her colleagues compared the compendia recommendations for the use of gemcitabine for bladder cancer, there was no consistency regarding what stage it should be prescribed for and whether it should be a first-, second-, or third-line treatment, and whether it should be combined with another drug. In addition, this comparison also demonstrated variation in whether a specific off-label disease indication was given for a specific drug.
The researchers also found compendia were attempting to do the equivalent of systematic reviews, but without fully reviewing all the relevant studies with a rigorous review process. Many of the studies available for review were of poor quality (i.e., were not randomized or were case series Phase II studies), and many of the studies were reported simultaneously in June due to the timing of ASCO’s annual meeting held during that month. “We saw a rapid accumulation of poor-quality evidence that the compendia were trying to continuously do systematic reviews on,” Abernethy said.
Gathering Clinically Relevant Information About Interventions
Limitations in the evidence base for making treatment decisions in clinical practice can inhibit the delivery of affordable, high-quality cancer care, several workshop participants stressed. “Clinicians need a method to make
sense of this rapidly accumulating evidence because we must move from a population-based question back to what works for individual patients and comparative effectiveness of the choices available,” Abernethy said.
One solution to this dilemma that Abernethy proposed is to have more pragmatic trials with real-world comparators and populations, as well as clinically relevant outcomes broken down according to patient subpopulations with the aid of biomarkers (Mullins et al., 2012). She also suggested developing learning health care systems that collect point-of-care data that can inform personalized medicine, comparative effectiveness, health care redesign, and high-quality cancer care (Abernethy et al., 2010b; IOM, 2010a).
Another strategy Abernethy suggested is to have temporary recommendations for off-label use, akin to what are applied in France (Emmerich et al., 2012). “These [temporary recommendations] balance the need for rapid access to oncology drugs against this reality that it takes a long time to get regulatory approval for off-label indications. Putting in place a system of continuous evidence collection for such temporary recommendations enables us to monitor what happens in real-world practice and to essentially start making rapid learning a reality,” she said. “We can’t cut off off-label use, but instead we can generate evidence to define appropriate off-label use in the future.” She stressed the need to conduct thoughtful, pragmatic with relevant comparators whenever possible and collect and learn from data recorded in clinical practices.
Such evidence collection should guide reimbursement decisions, Emanuel stressed. “I am a big fan of coverage with evidence development—we have to have a conditional approval whenever we agree to cover something and be more rigorous on the evidence development side of it—stop paying for treatments if the data supporting them are not provided within 3 years, for example. That would be a way to provide a very hard and fixed incentive for people to generate data,” said Emanuel. He noted that “there have been more than 1 million surgeries using the da Vinci robot and not a single randomized trial of this intervention. That is an embarrassment. We need to establish that we have to prove it before we are going to cover it in the long term.”
Bekelman said that CMS is generally reluctant to use coverage with evidence development because “it is a very blunt instrument and probably not appropriate for most technologies,” he said. He noted that a global market-based payment system would compel technology vendors to demonstrate the evidence of their therapies.
Bekelman suggested that FDA have a more rigorous approval process for new technologies, and that the radiation device industry be incentivized to invest in evidence generation alongside payers, including federal and non-federal insurers. Many devices and interventions, such as proton therapy for prostate cancer, are supplanting less expensive conventional alternatives in the clinic without evidence of their clinical superiority, Bekelman noted. The technology companies that devise these interventions tend not to do comparative-effectiveness studies and, compared to the pharmaceutical industry, invest much less in research and development.
“Equipment manufacturers need to have their skin into the game and be part of it and demonstrate the value of their machines with clinical trials,” Bekelman said. Once that value is demonstrated, insurers have to be willing to pay for the innovation demonstrated in the clinic, he added.
McClellan also stressed the importance of collecting data that are clinically relevant. He suggested being more rigorous about measuring clinical outcomes, such as complications, and suggested that medical practices collect data that can be used for performance metrics and evidence development. “If you can’t measure it, you can’t do very much to support it. Often in practice, as new drugs and combinations of treatments get used, there are opportunities to learn more about which types of patients are really responding the most.”
He noted that such collection of data might reinforce a payment system that has part of the payment tied to collection of information on results and complications for patients. “With better measures it is possible to change the way that we pay and change the way that benefits are designed, with better alignment between care that oncologists want to provide and how our financing systems are actually supporting our health care system,” he said. “That way we can move away from traditional measures like volume and intensity and instead pay for what really matters to patients.”
Newcomer quipped, “In God we trust, everyone else bring data. There is a transition period during which changes are made and [we need to] make sure you are measuring from day one during that transition so you can document improvements,” he stressed.
Therese Mulvey, physician in chief for the Southcoast Centers for Cancer Care, noted that the health care system is dynamic and changing rapidly. “We must collect data in real time because no amount of clinical trial work will solve this problem. So often we roll out our standard regimens into populations of patients who were not eligible for the trial. The ability for us to collate, analyze, aggregate, and feed back that data is critical.”
Dentzer reported on the benefits of data sharing by members of the High Value Health Care Collaborative, which is composed of 15 large health care systems, including the Cleveland Clinic and Mayo Clinic. “By pooling our data and looking at our results, we have identified a number of innovations that were then shared across the collaborative and made an immediate difference to the patients. The opportunity for this type of model to be tried across some of the major cancer treatment centers in this country would be extraordinary,” she said.
ASCO chief executive officer Allen Lichter added that physicians are acculturated to operate on an individual level, and are not used to collecting data on their patients that can be used to revise treatment guidelines and make care more effective and efficient. “We must begin to change our acculturation and collect data,” he said. He discussed ASCO’s new rapid learning project, CancerLinQ, as a means to accomplish this (see Box 4). The Commission on Cancer is also working to provide near-real-time feedback on adherence to National Quality Forum–endorsed breast and colorectal cancer quality metrics through its Rapid Quality Reporting System (American College of Surgeons, 2013).
Schrag emphasized that clinical trials should still remain the linchpin in evidence development. She suggested, however, that the accrual process needs to be improved so more patients enter the trials, noting that now roughly 5 percent of cancer patients go on a clinical trial. She also suggested designing studies with more meaningful endpoints and effect sizes and aiming for bigger benefits, rather than extending life by only 6 weeks, for example.
Rieger added the importance of conducting clinical trials in community-based clinics that better reflect patient populations compared to academic settings, because, as Eisenberg noted, about 80 percent of patients with cancer are treated in community settings.
Lichter noted that public funding of research will probably be required to get the comparative effectiveness, personalized medicine, and other evidence needed to make cancer care more cost-effective and provide clinical guidance to physicians and patients. “No one company is able or willing to do the studies that are needed for that,” he said.
Schrag also suggested that comparative effectiveness research and learning health care systems are strategies, but not necessarily solutions, to the high costs of cancer care. “We need to leverage our day-to-day experience, and the better health IT systems will facilitate that and give us a chance to examine the many patients who are left out of trials. But to do it, we need
BOX 4
ASCO’s CancerLinQ Rapid Learning Initiative
CancerLinQ is a multiphase initiative that aims to change the way cancer is understood and treated. The goals of this “rapid learning system” are to apply technological advances to connect oncology practices, measure quality and performance, and provide oncologists with decision support in real time. ASCO’s vision for CancerLinQ is to curate and analyze this information in a central knowledge base, which will learn over time. Specifically, the system will
- upload clinical data stored in electronic medical records (EMRs) from patients in multiple practices;
- aggregate information from EMRs, new clinical trials, and published guidelines;
- identify trends and associations among myriad variables in order to generate new hypotheses;
- enable physicians and researchers to evaluate those hypotheses and determine which ones may lead to improved care in real-world settings; and
- enable clinicians and researchers to quickly apply those conclusions, forming a continuous cycle of learning.
In practice, once the full technology platform is completed, CancerLinQ aims to
- improve personalized treatment decisions by cancer care teams by capturing patient information in real time at the point of care; providing real-time decision support tailored to each patient and his or her cancer; and automatically reporting on the quality of care compared with clinical guidelines and the outcomes of other patients;
- educate and empower patients by linking them to their cancer care teams and providing personalized treatment information at their fingertips; and
- create a powerful new data source for use in real-world quality and comparative effectiveness studies, and to generate new ideas for clinical research.
SOURCE: ASCO, 2012a.
better datasets that are more quickly and cheaply available, as well as better methods. We also need to better engage our patients and manage conflicts of interest because this research is subject to the same kinds of conflict issues that clinical trials are,” she said.
Schottinger noted that Kaiser Permanente has a comparative effectiveness and safety research institute that uses the extensive information collected in EMRs. This health care system has also conducted several randomized controlled trials, demonstrating the importance of integrating palliative care services.
Using Biomarkers
Several speakers emphasized the need for more biomarkers of tumor aggressiveness or responsiveness to make more effective use of cancer drugs. For example, sipuleucel-T (Provenge) is approved for prostate cancer, but it only provides a substantial benefit to a small proportion of prostate cancer patients, and there are currently no biomarkers for response to this drug, which costs $100,000 per patient, Peppercorn said.
McClellan noted that the variable responses cancer patients can have to the same drug suggests that instead of uniformly squeezing down prices of targeted therapies, they be priced differently for various patient subpopulations depending on how much value each of these subpopulations is likely to gain from the treatment. “We need to do a better job of encouraging high-value treatments that are individualized to particular patients,” he said. “Often diagnostic tests that could be highly valuable in influencing a treatment course for a patient are poorly reimbursed or not reimbursed at all. It is a misalignment between what matters most for patients with cancer or patients at risk for developing cancer, and the way that we pay for cancer care that is increasingly an issue as we have an increasingly broad array of treatment options available.”
Schrag emphasized the importance of embedding molecular correlates in cancer clinical trials. “We have to distinguish, when we do our trials, those treatments that provide a little bit of incremental benefit for many people versus those that provide an enormous benefit for a very distinct subset,” she said. She noted that crizotinib only benefits 8 percent of all lung cancer patients. However, Brawley cautioned against having subset analyses being the final word on a treatment’s effectiveness. “We need to be careful in using subset analyses to make clinical decisions. They usually should only be used to justify the next prospective randomized clinical trial,” he said.
Lichter stressed that only by gathering and combining data can progress be made on personalizing cancer care. “Individual institutions cannot sort this out and figure out what works for distinct patient subgroups,” he said. Canetta suggested more sharing of drug data among different pharmaceutical companies. Such sharing would enable greater understanding of which subgroups of patients are most likely to benefit from specific treatments. Data sharing can also be a cost-cutting measure in the drug development arena, for example, if it reveals early radiology endpoints that can be relied on to assess treatment effectiveness, he noted. In addition, precompetitive collaborations in early drug development with companies targeting the same cancer pathways can help to reduce the risks and costs of drug development that are factored into drug prices, Canetta noted.
Financial Incentives Aligned with Affordable, High-Quality Cancer Care
A number of speakers noted the need for better alignment of financial incentives to reward affordable, high-quality cancer care. Financial incentives can encourage both clinicians and patients to change their behavior and consider costs in decision making. In addition, tying reimbursement to performance on quality metrics could also incentivize affordable, high-quality care.
Provider Financial Incentives
Changes that would alter the financial incentives for providers include more reimbursement for cognitive care, elimination or reduced use of fee-for-service reimbursement, and better reimbursement for coordinated, cost-effective care. Reimbursement levels could also be based on performance metrics.
Peppercorn and others suggested increased reimbursement for clinicians spending more time with their patients discussing their medical intervention options (cognitive care). “If 80 percent of your income was based not on the profit margins from administering chemotherapy, and instead on having a great discussion with your patients, people would be really well informed and I bet we would see large declines in chemotherapy utilization and cost,” he said.
Earle deplored the U.S. fee-for-service system. “The idea of making half of your practice salary off of selling chemotherapy is crazy. There should be rational decisions, not rationing decisions,” he said.
Eisenberg added, “What we value is a doctor giving an attentive, listening ear to a patient and family. That certainly isn’t how the payment system is arranged. It does not reward that behavior.” In addition, Green noted, “We ought not to be getting paid for drug margins, but getting paid for what we do, such as cognitive services or after-hours support services.”
Peppercorn and others also said that more reimbursement of cognitive care is likely to reduce costs because it would lead to better thought-out intervention plans. “Providers often don’t have time to think and it is easier to just order a test or scan,” he said. Gruman added, “Our doctors need higher reimbursement for cognitive services. This will give them an incentive to spend more time talking to us and hopefully provide a disincentive to recommend useless or extra services and tests.”
Bekelman also called for separating cancer specialists’ income from treatment choices. “We need to move beyond fee-for-service payment,” he said, but also noted the difficulties in doing this in radiology. The fees for radiologic procedures help pay for physicians’ salaries, and also help reimburse the costs of the hospital or other facility that owns the radiology equipment. “We have to appreciate that whether you are a large hospital or a small hospital, a good portion of your bottom line comes from the technical fees from radiation oncology,” Bekelman said.
“To stop this spiral, we have to sever the relationship between treatment choice and reimbursement for treatment,” Bekelman stressed, recommending that instead provider incentives be aligned toward patient-centered, coordinated care among cancer specialists, such as radiologists and medical oncologists, and primary care physicians.
One way to do this is to link guideline-concordant care to shared savings from global payments. Instead of making payments modality based (i.e., a care pathway or bundled payment for uncomplicated bone metastasis), Bekelman suggested making payments diagnosis-based, such as a global payment for localized cancer (see also the section on episode-based payments). Such a payment scheme would require integrated diagnosis-based panels of surgical, radiation, and medical oncologists, and thus would have a greater impact on care coordination and linkage to primary care. “We have to think about radiation not as the blunt hammer, but as part of the types of therapies that can be applied to different subgroups of patients based on the value of that treatment,” Bekelman said.
He suggested differential reimbursement based on the complexity of treatment and care coordination, but added that having such a payment scheme for physicians who are not within the same facility would be challenging.
Schrag also suggested better alignment of financial incentives to promote teamwork and coordination.
Enabling nurses to practice to the full scope of their licenses would help reduce the cost of cancer care, Earle noted. However, nursing care is often viewed as an expense for a cancer clinic, whereas physician services are viewed as revenue, based on fee-for-service reimbursement. Thus, there is more incentive for a cancer center to avoid the expense of hiring a nurse by having the care delivered by a physician. “It is a prime example of how we don’t have the incentives aligned,” Earle said.
Reimbursement Aligned with Performance Metrics
Bekelman suggested providing feedback to patients, providers, and payers through population-based performance measurement of quality, outcomes, and costs. This could be accomplished by upgrading federally supported state cancer registries to provide near-real-time ascertainment of quality metrics, risk-adjusted outcomes, and costs by linking with claims databases.
Newcomer also suggested using performance metrics, such as complication rates and patient survival statistics, so that physicians can be financially rewarded when they provide high-quality care. “With a minimal amount of clinical information and a claims dataset, we can produce very valuable information about how oncologists are performing,” he said.
For example, UnitedHealthcare’s episode-based pilot program (see the episode-based payment section) calculates a physician’s expected profit margin for each drug regimen based on what it has been in the past. This margin is called a patient care fee and is paid to providers the first day they see the patient. The only way to raise that patient care fee is to reduce their costs by improving results. If the total cost of care is reduced, shares the savings gained with the employer and physician. Similarly, if any of the medical groups reduce their hospitalization rates, then UnitedHealthcare rewards them with higher fee schedules the following year. “They have to meet the targets that we set to receive the higher fee schedules,” Newcomer noted.
Bekelman also called for more transparency of hospital and cancer specialists’ profit margins. Such transparency will “tell hospitals to start thinking about how they are going to budget for the future. In addition, it signals to the radiation device industry that they have to begin to market value-based technology that deals with quality and safety in addition to the
bells and whistles that they are used to marketing now,” Bekelman said. Emanuel agreed and added that such pricing transparency should include quality metrics.
Patient Financial Incentives
A number of workshop participants suggested two types of changes that would alter the financial incentives for patients—that patients (1) pay more of the costs of their health care, or (2) share some of the savings gained when they choose less expensive options that are equally effective. Schrag discussed the moral hazard of health insurance. “Most of our patients are able to get very expensive drugs without feeling any effects directly in their own pockets. There is so much insurance that we are inured to it. We have very generous policies—Medigap, Medicaid, caps on insurance payments—all of which result in high costs that ultimately get passed on to our employers and to all of us U.S. taxpayers,” she said. She added that eliminating patient accountability for cost sharing incentivizes patients to use more care. However, Shankaran noted that patients are responsible for increasing proportions of treatment costs, through rising health insurance premiums, deductibles, and copayments, as well as stagnant wages attributed to increases in medical expenditures.
Ramsey suggested setting patient copayments based on the value of the service provided, to encourage patients to use higher value treatments and discourage use of lower-value interventions. Insurers could also provide financial incentives for eliminating futile treatments and increasing the use of palliative and hospice care.
McClellan noted that after the Medicare Part D drug program was implemented, people tended to choose a plan that had lower premiums with a more cost-effective tiered structure. Although participants pay more of the costs of their drugs with such a plan, they also receive a larger share of the savings gained by switching to less expensive generic drugs. The costs are currently running about 40 percent lower than projections with this drug plan, according to McClellan. “There are still a lot of health care costs, but they are a lot lower than they would have been according to the projections, based on traditional ways of using prescription drugs. A big contributor was that the use of generics among seniors went from about 50 percent at the beginning of 2006 to close to 80 percent today,” he said.
Similar cost-sharing plans have been set up by employers and private insurers, especially for some elective procedures, such as colonoscopies and
coronary bypass operations, in which good information about quality of care coupled with stronger financial incentives have led to significant cost savings. Some of these insurance designs provide a set amount for procedures and require patients to pay the difference for more expensive providers of that surgery. Depending on which provider you choose, “You could end up paying $10,000 or more for a bypass surgery—something that would never have happened under a traditional insurance design,” McClellan said.
“These approaches put consumers in a position where they can benefit financially from making more efficient decisions,” he added, noting that patients given these cost-sharing benefit plans tend to have shorter hospital stays, fewer readmissions, and earlier return-to-work times, all of which results in overall costs that are substantially lower than what is seen with standard benefits plans.
Emanuel went so far as to suggest directly paying patients set amounts if they opt for less expensive but equally effective medical interventions. “It might appear unethical to pay people to do something cheaper, but it will be equivalent and give you the same results. We are not asking them to sacrifice any survival benefit,” he noted. Such payments could also compensate if the less expensive treatment has added inconvenience.
Earle agreed that it makes sense to incentivize patients to make market-based decisions, and noted there have been discussions about limiting Medigap insurance so people have such incentives. However, he cautioned that there needs to be a safety mechanism in such incentivizing plans to ensure payment for essential health care of the poor.
Delivery System and Reimbursement Changes
Participants discussed several strategies that aim to better align financial incentives and overcome current challenges to delivering affordable, quality cancer care. These strategies include capitation, bundled/episode-related payments, accountable care organizations and shared savings, medical homes, and the application of cost-effectiveness thresholds and value-based or performance-based care.
Capitation
Ramsey noted that one way of curbing cancer care costs is to have a capitation reimbursement system in which an insurer pays an oncologist a set amount per cancer patient based on expected care according to the
guidelines. Any expenses rendered above that amount would have to be paid for by the physician and/or patient. “We have some evidence that capitated systems do a much better job of not spending money on wasteful care. There are capitated systems such as Group Health and Geisinger, which look at the data and translate them to the doctors, who are usually salaried. For example, they were not using Avastin to treat breast cancer before FDA took it off the market for that indication because they didn’t believe the evidence,” Ramsey said.
Episode-Related Payments
Somewhat similar to capitation are bundled or episode-related payments, Bach reported. With this payment mechanism, providers are given a single payment for the care of the patient during a well-defined episode of care, usually based on the average cost of such care. For example, an episode could be defined as one month of care for metastatic lung cancer (Bach et al., 2011). This puts the provider at risk for proper performance rather than the insurer. Efficient use of the resources will enable the physician to make a profit, whereas non-efficient use will lead to financial loss. This differs from capitation, which includes an insurance risk (or the risk that a certain number of patients will be diagnosed with disease) when it calculates the amount insurers will reimburse per patient, according to Bach.
Episode-related payment systems are effective when there is competition, such as comparable treatment regimens that are available for the same indication at varying prices. This provides a financial incentive for physicians to choose the lower-cost options for their patients, Bach noted. “This incentive changes the market basket of drugs that are used, as well as lowers the average cost of care over time,” he said. With Bach’s model of episode-based payments, the bundle is recalibrated over time, as physicians are incentivized to choose lower-cost, equally effective therapies.
If it is not clear which treatments are comparable in effectiveness, it can be challenging to institute an episode-related payment system, Bach added. “We don’t have an adequate comparison between radical external radiation therapy, or watchful waiting for early-stage prostate cancer, for example. To say they are all the same is basically mistaking the absence of information about differences with proof that there are no differences. That is a common conceptual flaw that you see cropping up,” he said.
Episode-related payment systems can also be problematic due to patient variation in response to treatment. “We don’t say everyone with
pneumonia has to be out the door within 28 hours—that is just the average. You will have some patients who are gone in 25 and you will have some patients who will be in the hospital a week. That all goes into the same bucket,” Bach said.
Gruman advocated for an episode-related payment system for cancer care “that has the potential to give our doctors the flexibility to plan and spend time with us and make decisions with us about this shared project of our health through treatment and recovery,” she said.
UnitedHealthcare also is participating in a bundling pilot, which is arranged differently from Bach’s model. In this system, has removed financial profits from chemotherapy, and instead pays physicians a patient care fee. The only way to raise that patient care fee is to reduce their costs by improving results. If the total cost of care is reduced, UnitedHealthcare shares the savings gained with the employer and physician.
Newcomer noted that UnitedHealthcare has five medical groups that participate in their episode-related payment pilot. These groups worked with UnitedHealthcare to establish 68 measures for their oncology practices. These measures include total cost, survival, relapse rate, progression-free survival, hospitalizations, emergency visits, cost of drugs, etc. The group also established the best treatment pathway for each of 19 different cancer conditions.
Despite these constraints and incentives, Newcomer noted that there was still a great deal of practice variability, including what diagnostics and treatments were prescribed, and the total costs of treatments.
Medical Homes
CMS is evaluating patient outcomes for medical homes13 through pilot projects conducted through the CMS Innovation Center. In these pilots, there are financial incentives for coordinated care and providing higher-quality care, including care that adheres to guidelines, and avoiding some complications, such as emergency room visits. “There are quality and other modifiers to the payment system that try to get away from this ‘more treatment
__________________
13AHRQ defines the concept of a medical home as the organization of primary care that delivers the core functions of primary health care. The five functions and attributes of a medical home include patient-centeredness, comprehensive care, coordinated care, superb access to care, and a systems-based approach to quality and safety (AHRQ, 2011).
equals more money’ approach that is the backbone of fee-for-service,” Bach noted.
Several speakers stressed the need for oncology medical homes. “We need an oncology, patient-centered medical home that really supports patients throughout their cancer trajectory,” Gruman said. Sprandio discussed his practice’s move to become an oncology patient-centered medical home (Sprandio, 2010, 2012). He noted that after his medical practice transitioned into a medical home, his patients’ use of emergency rooms dropped by 68 percent over a 4-year period and hospital admissions were reduced by 51 percent for patients receiving chemotherapy over a 3-year period.
Schnipper also advocated for oncology homes as an outgrowth of medical homes in recognition that “the oncologist oversees an enormous complexity of care and care decisions.” He added, “This represents a way to work towards synchronizing care in a way that provides the best outcomes for our patients.”
Shared Savings Plans/Accountable Care Organizations
In shared savings plans, care providers are paid their traditional Medicare payments, but if they show improvement on agreed-upon measures of quality and reduce costs, they keep a portion of the savings above a certain threshold. Medicare has run shared-savings pilot projects, called Physician Group Practice Demonstrations. These pilots had about 30 measures, including some measures related to cancer screening, chronic disease management, and common cancer patient experience measures. These pilot projects led to the establishment of more than 250 of what are now termed accountable care organizations (ACOs) in the United States, including 116 public-sector ACOs that provide care for nearly 2.5 million Medicare beneficiaries, McClellan reported (see Figure 11).
In ACOs, physicians are reimbursed not just on volume and intensity, but also on their performance, using established and measured performance metrics. The private sector has ACOs in more than 40 states, according to McClellan. About half of ACOs are system-based, and the other half are led by physicians or more independent coordinated care efforts short of integrated ownership, he added.
Green reported that Cancer Clinics of Excellence is collaborating with others to develop a physician-led, high-quality shared savings model of care in oncology. “This model is founded on the belief that we need to invest heavily in resources to enable this to happen, both from the standpoint of
FIGURE 11 Dissemination of accountable care organizations (ACOs) in the United States. The public-sector ACOs include a few variations: Beacon Communities, the Medicare Physician Group Practice (PGP) Transition Demonstration, the Medicare Health Care Quality (MHCQ) Demonstration Programs, the Pioneer ACO Model, and the Medicare Shared Savings Program (MSSP). Beacon Communities aim to demonstrate how investments in health information technology and meaningful use of electronic medical records can promote patient-centered care. The Medicare PGP Transition Demonstration is evaluating how payment arrangements can help groups of physicians provide high-quality, coordinated care. The MHCQ Demonstration Programs are designed to examine how changes to health care delivery and financing can improve the quality of care without increasing total Medicare program expenditures. The Pioneer ACO model is a CMS Innovation Center initiative designed to assess the impact of different payment initiatives for experienced ACOs. The Medicare Shared Savings Program is designed to reward ACOs that lower the growth in health care costs and meet performance metrics.
SOURCE: McClellan presentation (October 8, 2012).
IT development to help us collect, interpret, and act on data, and to focus on quality outcomes and identify high-risk patients,” he said, adding that they are currently considering hiring care coordinators and psychological counselors in this model practice. Incorporating these individuals will require an investment in resources, but he stressed that most physician practices cannot afford this investment and suggested that there needs to be an external mechanism to support these providers.
McClellan noted that many medical homes found they were not able to pay their primary care providers more for delivering more coordinated care and better management of chronic disease using the traditional primary
care reimbursement system. Consequently, some have adopted an ACO method of payment, in which primary care physicians take on some accountability, not just for whether they meet all the criteria of a medical home, but whether they are actually lowering complication rates and cost trends for their patient population. McClellan added, “This is a new kind of risk to take on, one that takes some time and effort to build into, but it is happening.” These hybrid medical home-ACOs recognize that “you can’t get overall costs down, especially for conditions like cancer where the care predominantly involves specialists, without active involvement by specialists in the process,” McClellan said. So these medical home-ACO hybrids tend to rely on episode-based payments, he noted.
Cost-Effectiveness Thresholds
Other speakers suggested having cost-effective thresholds for medical interventions in oncology, akin to what is done in the national health care system offered by the United Kingdom (NHS, 2012). If interventions exceed cost-effectiveness thresholds (as measured by quality-adjusted life years), the National Health Service will generally not provide coverage for the interventions. “We have a whole literature on cost-effectiveness evaluation, much of it done in cancer, that puts a value on the various treatments that we do. We could set a national threshold and say we will only cover things that fall below that,” said Ramsey.
Accountable Drugs and Devices
Manufacturers of drugs, tests, and devices are increasingly being asked by insurers to be more accountable, with reimbursement rates being tied to the impact various tests and devices have on patient care. For example, instead of paying a set amount for a diagnostic test such as Oncotype DX,14 insurers could base the price on the value of avoiding unnecessary and costly chemotherapies, McClellan noted. Similarly, Betaseron is an expensive biologic drug for rheumatoid arthritis patients who are not responding to other treatments. In a new payment scheme devised by Health Alliance Medical
__________________
14A multigene expression test developed to predict the risk of recurrence for node-negative, estrogen-receptor-positive breast cancer. Oncotype DX may help identify women who are at such low risk of breast cancer recurrence that the risks of chemotherapy treatment would outweight the benefits of the treatment (IOM, 2012).
Plans and Bayer, the maker of Betaseron, the rebate for using Betaseron is tied to how often patients who are on it have complications requiring hospitalization for their rheumatoid arthritis, McClellan reported. “This is a way of shifting toward more accountability for results by manufacturers of the products,” he said.
Competitive Bidding
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 enabled CMS to conduct a competitive acquisition program (CAP) to acquire drugs at lower costs for their beneficiaries. This program failed due to administrative difficulties, according to Bach, but could be modified so it is more effective. Such a program could be applied to the acquisition of the top 10 Medicare Part B drugs administered by physicians, most of which are used in the cancer setting. He also suggested putting any drugs beyond a certain price into such a program.
Emanuel suggested expanding competitive bidding to drugs, imaging, and laboratory tests. He noted that the Affordable Care Act includes competitive bidding for durable medical equipment, such as prosthetics and orthotics. In the first year of its operation, prices for such commodities decreased by 42 percent, he said, and the program is to be expanded throughout the country by 2016.
Many workshop participants stressed that all stakeholders are responsible for the unsustainable rising costs of cancer care, which places affordable, high-quality cancer care in jeopardy. Tina Shih, director of the Program in the Economics of Cancer at the University of Chicago, emphasized, “Don’t think about the enemy as being out there. The take-home message from today’s meeting is that we are the ones creating the problem and we need to come together to solve it.” Peppercorn added, “The era of high-cost interventions for marginal benefit clearly must end, particularly if we are going to have any resources left to provide high-value interventions to the broader population and to spend on further scientific discovery, not to mention the other important social goals.”
Possible strategies for curbing the costs of cancer care include
- providing financial incentives aligned with patient-centered, high-quality, affordable, and coordinated care;
- better means of assessing and paying for medical interventions;
- greater use of and adherence to treatment guidelines; and
- better informed and educated patients and providers, so that their expectations are more realistic and cost conscious.
Summing up the various alternative delivery and reimbursement schemes, McClellan said, “All these different kinds of reforms—medical homes, bundled payments, ACOs, and new payments for drugs—hopefully will align the different pieces of our provider payment models in a way that reinforces a common set of goals for patients. You should not view these as competing alternatives, but rather as pieces that are incrementally but fundamentally changing the way that we pay for care so payment is much better aligned with paying for value.”
Abernethy, A. P., G. Raman, E. M. Balk, J. M. Hammond, L. A. Orlando, J. L. Wheeler, J. Lau, and D. C. McCrory. 2009. Systematic review: Reliability of compendia methods for off-label oncology indications. Annals of Internal Medicine 150(5):336-343.
Abernethy, A. P., R. R. Coeytaux, K. Carson, D. McCrory, S. Y. Barbour, M. Gradison, R. J. Irvine, and J. L. Wheeler. 2010a. Report on the evidence regarding off-label indications for targeted therapies used in cancer treatment: Technology assessment report. Rockville, MD: Agency for Healthcare Research and Quality.
Abernethy, A. P., L. M. Etheredge, P. A. Ganz, P. Wallace, R. R. German, C. Neti, P. B. Bach, and S. B. Murphy. 2010b. Rapid-learning system for cancer care. Journal of Clinical Oncology 28(27):4268-4274.
ACS CAN (American Cancer Society Cancer Action Network). 2009. Cancer and Medicare: A chartbook. http://www.allhealth.org/briefingmaterials/CancerandMedicareChartbookFinalfull documentMarch11-1412.pdf (accessed January 8, 2013).
AHRQ (Agency for Healthcare Research and Quality). 2011. What is the PCMH? http://pcmh.ahrq.gov/portal/server.pt/community/pcmh__home/1483/what_is_pcmh_ (accessed January 23, 2013).
American College of Surgeons. 2013. Rapid Quality Reporting System (RQRS). http://www.facs.org/cancer/ncdb/rqrs.html (accessed January 23, 2013).
Anderson, G. F., and D. A. Squires. 2010. Measuring the U.S. health care system: A cross-national comparison. http://www.commonwealthfund.org/~/media/Files/Publications/Issue%20Brief/2010 /Jun/1412_Anderson_measuring_US_hlt_care_sys_intl_ib.pdf (accessed January 8, 2013).
ASCO (American Society of Clinical Oncology). 2012a. CancerLinQ—building a transformation in cancer care. http://www.asco.org/ASCOv2/Practice+%26+Guidelines/Quality+Care/CancerLinQ+-+Building+a+Transformation+in+Cancer+Care (accessed December 18, 2012).
ASCO. 2012b. Choosing Wisely®: ASCO identifies five key opportunities in oncology to improve value of patient care. http://www.asco.org/ASCOv2/Practice+%26+Guidelines/Quality+Care/Access+to+ Cancer+Care/Cost+of+Cancer+Care (accessed December 17, 2012).
Bach, P. B. 2009. Limits on Medicare’s ability to control rising spending on cancer drugs. New England Journal of Medicine 360(6):626-633.
Bach, P. B., J. N. Mirkin, and J. J. Luke. 2011. Episode-based payment for cancer care: A proposed pilot for Medicare. Health Affairs (Millwood) 30(3):500-509.
Bach, P. B., L. B. Saltz, and R. E. Wittes. 2012. In cancer care, cost matters. New York Times, October 15, A25.
Barbash, G. I., and S. A. Glied. 2010. New technology and health care costs—the case of robot-assisted surgery. New England Journal of Medicine 363(8):701-704.
Bernard, D. S., S. L. Farr, and Z. Fang. 2011. National estimates of out-of-pocket health care expenditure burdens among nonelderly adults with cancer: 2001 to 2008. Journal of Clinical Oncology 29(20):2821-2826.
Blayney, D. W., K. McNiff, D. Hanauer, G. Miela, D. Markstrom, and M. Neuss. 2009. Implementation of the Quality Oncology Practice Initiative at a university comprehensive cancer center. Journal of Clinical Oncology 27(23):3802-3807.
BPC (Bipartisan Policy Center). 2012. What is driving U.S. health care spending? http://bipartisanpolicy.org/sites/default/files/BPC%20Health%20Care %20Cost%20Drivers%20Brief%20Sept%202012.pdf (accessed December 16, 2012).
Brumley, R., S. Enguidanos, P. Jamison, R. Seitz, N. Morgenstern, S. Saito, J. McIlwane, K. Hillary, and J. Gonzalez. Increased satisfaction with care and lower costs: Results of a randomized trial of in-home palliative care. Journal of the American Geriatrics Society 55(7):993-1000.
Buys, S. S., E. Partridge, A. Black, C. C. Johnson, L. Lamerato, C. Isaacs, D. J. Reding, R. T. Greenlee, L. A. Yokochi, B. Kessel, E. D. Crawford, T. R. Church, G. L. Andriole, J. L. Weissfeld, M. N. Fouad, D. Chia, B. O’Brien, L. R. Ragard, J. D. Clapp, J. M. Rathmell, T. L. Riley, P. Hartge, P. F. Pinsky, C. S. Zhu, G. Izmirlian, B. S. Kramer, A. B. Miller, J. L. Xu, P. C. Prorok, J. K. Gohagan, and C. D. Berg. 2011. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. Journal of the American Medical Association 305(22):2295-2303.
CBO (Congressional Budget Office). 2009. CBO’s analysis of the effects of proposals to limit costs related to medical malpractice (“tort reform”). http://www.cbo.gov/publication/24975 (accessed December 16, 2012).
CBO. 2012a. The 2012 long-term budget outlook. http://www.cbo.gov/sites/default/files/cbofiles/attachments/06-05-Long-Term_Budget_Outlook_2.pdf (accessed December 14, 2012).
CBO. 2012b. Health care. https://cbo.gov/topics/health-care (accessed December 16, 2012).
CBPP (Center on Budget and Policy Priorities). 2012. Policy basics: Where do our federal tax dollars go? http://www.cbpp.org/cms/index.cfm?fa=view&id=1258 (accessed January 25, 2013).
The Commonwealth Fund. 2012. Explaining high health care spending in the United States: An international comparison of supply, utilization, prices, and quality. http://www.commonwealthfund.org/~/media/Files/Publications/ Issue%20Brief/2012/May/1595_Squires_explaining_high_hlt_care_spending_intl_brief.pdf (accessed December 16, 2012).
Eheman, C., S. J. Henley, R. Ballard-Barbash, E. J. Jacobs, M. J. Schymura, A. M. Noone, L. Pan, R. N. Anderson, J. E. Fulton, B. A. Kohler, A. Jemal, E. Ward, M. Plescia, L. A. Ries, and B. K. Edwards. 2012. Annual report to the nation on the status of cancer, 1975-2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer 118(9):2338-2366.
Emanuel, E. J. 2011. Less than $26 billion? Don’t bother. New York Times, November 3. http://opinionator.blogs.nytimes.com/2011/11/03/less-than-26-billion-dont-bother (accessed March 6, 2013).
Emanuel, E. J., and V. R. Fuchs. 2008. Who really pays for health care? The myth of “shared responsibility.” Journal of the American Medical Association 299(9):1057-1059.
Emanuel, E., N. Tanden, S. Altman, S. Armstrong, D. Berwick, F. de Brantes, M. Calsyn, M. Chernew, J. Colmers, D. Cutler, T. Daschle, P. Egerman, B. Kocher, A. Milstein, E. Oshima Lee, J. D. Podesta, U. Reinhardt, M. Rosenthal, J. Sharfstein, S. Shortell, A. Stern, P. R. Orszag, and T. Spiro. 2012. A systemic approach to containing health care spending. New England Journal of Medicine 367(10):949-954.
Emmerich, J., N. Dumarcet, and A. Lorence. 2012. France’s new framework for regulating off-label drug use. New England Journal of Medicine 367(14):1279-1281.
Falconi, M. 2012. Studies: Cancer drug best taken for a year. Wall Street Journal, October 2, B5.
Fineberg, H. V. 2012. Shattuck Lecture. A successful and sustainable health system—how to get there from here. New England Journal of Medicine 366(11):1020-1027.
Freire, M. P., W. W. Choi, Y. Lei, F. Carvas, and J. C. Hu. 2010. Overcoming the learning curve for robotic-assisted laparoscopic radical prostatectomy. Urologic Clinics of North America 37(1):37-47, Table of Contents.
Goldberg, P. 2012. Zaltrap price cut in half effective immediately as Sanofi responds to criticism from oncologists. Cancer Letter 38(42):1-4.
Goodwin, J. S., A. Singh, N. Reddy, T. S. Riall, and Y. F. Kuo. 2011. Overuse of screening colonoscopy in the Medicare population. Archives of Internal Medicine 171(15):1335-1343.
Greer, J. A., W. F. Pirl, V. A. Jackson, A. Muzikansky, I. T. Lennes, R. S. Heist, E. R. Gallagher, and J. S. Temel. 2012. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. Journal of Clinical Oncology 30(4):394-400.
Hoover, D. R., S. Crystal, R. Kumar, U. Sambamoorthi, and J. C. Cantor. 2002. Medical expenditures during the last year of life: Findings from the 1992-1996 Medicare Current Beneficiary Survey. Health Services Research 37(6):1625-1642.
HRSA (Health Resources and Services Administration). 2013. 340B Drug Pricing Program & Pharmacy Affairs FAQs. http://www.hrsa.gov/opa/faqs/index.html#1 (accessed February 13, 2013).
Hu, J. C., K. F. Gold, C. L. Pashos, S. S. Mehta, and M. S. Litwin. 2003. Role of surgeon volume in radical prostatectomy outcomes. Journal of Clinical Oncology 21(3):401-405.
Huskamp, H. A., N. L. Keating, J. L. Malin, A. M. Zaslavsky, J. C. Weeks, C. C. Earle, J. M. Teno, B. A. Virnig, K. L. Kahn, Y. He, and J. Z. Ayanian. 2009. Discussions with physicians about hospice among patients with metastatic lung cancer. Archives of Internal Medicine 169(10):954-962.
IOM (Institute of Medicine). 2010a. A foundation for evidence-driven practice: A rapid learning system for cancer care: Workshop summary. Washington, DC: The National Academies Press.
IOM. 2010b. The healthcare imperative: Lowering costs and improving outcomes: Workshop series summary. Washington, DC: The National Academies Press.
IOM. 2011. Patient-centered cancer treatment planning: Improving the quality of oncology care: Workshop summary. Washington, DC: The National Academies Press.
IOM. 2012a. Best care at lower cost: The path to continuously learning health care in America. Washington, DC: The National Academies Press.
IOM. 2012b. The role of obesity in cancer survival and recurrence: Workshop summary. Washington, DC: The National Academies Press.
IOM. 2012c. Evolution of translational omics: Lessons learned and the path forward. Washington, DC: The National Academies Press.
IOM. 2012d. Accelerating progress in obesity prevention: Solving the weight of the nation. Washington, DC: The National Academies Press.
Jacobson, M., A. J. O’Malley, C. C. Earle, J. Pakes, P. Gaccione, and J. P. Newhouse. 2006. Does reimbursement influence chemotherapy treatment for cancer patients? Health Affairs (Millwood) 25(2):437-443.
Jones, S. S., P. S. Heaton, R. S. Rudin, and E. C. Schneider. 2012. Unraveling the IT productivity paradox—lessons for health care. New England Journal of Medicine 366(24):2243-2245.
KFF (Kaiser Family Foundation). 2009. Health care costs: A primer. Key information on health care costs and their impact. http://www.kff.org/insurance/upload/7670_02.pdf (accessed December 16, 2012).
KFF and HRET (Health Research & Educational Trust). 2012. Employer health benefits. http://ehbs.kff.org/pdf/2012/8345.pdf (accessed January 8, 2013).
Kim, P. 2007. Cost of cancer care: The patient perspective. Journal of Clinical Oncology 25(2):228-232.
Kolata, G. 2012. Knotty challenges in health care costs. New York Times, March 6. http://www.nytimes.com/2012/03/06/health/policy/an-interview-with-victor-fuchs-on-health-care-costs.html (accessed December 16, 2012).
Lamont, E. B., and N. A. Christakis. 2001. Prognostic disclosure to patients with cancer near the end of life. Annals of Internal Medicine 134(12):1096-1105.
Mandl, K. D., and I. S. Kohane. 2012. Escaping the EHR trap—the future of health IT. New England Journal of Medicine 366(24):2240-2242.
Mariotto, A. B., K. R. Yabroff, Y. Shao, E. J. Feuer, and M. L. Brown. 2011. Projections of the cost of cancer care in the United States: 2010-2020. Journal of the National Cancer Institute 103(2):117-128.
MedPAC (Medicare Payment Advisory Commission). 2012. A data book: Health care spending and the Medicare program. http://www.medpac.gov/documents/Jun11DataBookEntireReport.pdf (accessed February 1, 2013).
Milstein, A. 2012. Code Red and Blue—safely limiting health care’s GDP footprint. New England Journal of Medicine 368(1):1-3.
Morrison, R. S., J. D. Penrod, J. B. Cassel, M. Caust-Ellenbogen, A. Litke, L. Spragens, and D. E. Meier. 2008. Cost savings associated with US hospital palliative care consultation programs. Archives of Internal Medicine 168(16):1783-1790.
Mullins, C. D., R. Montgomery, A. P. Abernethy, A. Hussain, S. D. Pearson, and S. Tunis. 2012. Recommendations for clinical trials of off-label drugs used to treat advanced-stage cancer. Journal of Clinical Oncology 30(6):661-666.
National Lung Screening Trial Research Team, D. R. Aberle, A. M. Adams, C. D. Berg, W. C. Black, J. D. Clapp, R. M. Fagerstrom, I. F. Gareen, C. Gatsonis, P. M. Marcus, and J. D. Sicks. 2011. Reduced lung-cancer mortality with low-dose computed tomographic screening. New England Journal of Medicine 365(5):395-409.
NCI (National Cancer Institute). 2011. Tracking the rise of robotic surgery for prostate cancer. NCI Cancer Bulletin 8(16). http://www.cancer.gov/ncicancerbulletin/080911/page4 (accessed April 26, 2013).
NCI. 2013. NCI dictionary of cancer terms. http://www.cancer.gov/dictionary (accessed January 8, 2013).
NHS (National Health Service). 2012. Measuring effectiveness and cost effectiveness: The QALY. http://www.nice.org.uk/newsroom/features/ measuringeffectivenessandcosteffectivenesstheqaly.jsp (accessed December 17, 2012).
NRC (National Research Council) and IOM. 2013. U.S. health in international perspective: Shorter lives, poorer health. Washington, DC: The National Academies Press.
O’Connor, G. T., S. K. Plume, E. M. Olmstead, J. R. Morton, C. T. Maloney, W. C. Nugent, F. Hernandez, Jr., R. Clough, B. J. Leavitt, L. H. Coffin, C. A. Marrin, D. Wennberg, J. D. Birkmeyer, D. C. Charlesworth, D. J. Malenka, H. B. Quinton, and J. F. Kasper. 1996. A regional intervention to improve the hospital mortality associated with coronary artery bypass graft surgery. The Northern New England Cardiovascular Disease Study Group. Journal of the American Medical Association 275(11):841-846.
OECD (Organisation for Economic Co-operation and Development). 2009. Health at a glance 2009: OECD indicators. http://www.oecd.org/health/healthpoliciesanddata/44117530.pdf (accessed January 17, 2013).
OECD. 2011. OECD factbook 2011-2012. http://www.oecd-ilibrary.org/economics/oecd-factbook-2011-2012_factbook-2011-en (accessed January 8, 2013).
OECD. 2013. Health at a glance 2011: OECD indicators. http://www.oecd-ilibrary.org/sites/health_glance-2011-en/01/07/g1-07-01.html?contentType=&itemId=/content/chapter/health_glance-2011-10-en&containerItemId=/content/serial/19991312&accessItemIds=/content/book/health_glance-2011-e (accessed January 8, 2013).
OTA (Office of Technology Assessment). 1994. Defensive medicine and medical malpractice, OTA-H-602. Washington, DC: U.S. Government Printing Office.
QOPI (The Quality Oncology Practice Initiative). 2013a. ASCO quality programs. http://qopi.asco.org/index (accessed January 8, 2013).
QOPI. 2013b. Program details. http://qopi.asco.org/program (accessed January 8, 2013).
Ramsey, S. D., and A. Schickedanz. 2010. How should we define value in cancer care? The Oncologist 15:s1.
Ramsey, S. D., C. R. Fedorenko, K. S. Snell, A. C. Kirchhoff, W. Hollingworth, and D. K. Blough. Cancer diagnosis as a risk factor for personal bankruptcy. Journal of Clinical Oncology 29(Suppl):abstr 6007.
Sanda, M. G., R. L. Dunn, J. Michalski, H. M. Sandler, L. Northouse, L. Hembroff, X. Lin, T. K. Greenfield, M. S. Litwin, C. S. Saigal, A. Mahadevan, E. Klein, A. Kibel, L. L. Pisters, D. Kuban, I. Kaplan, D. Wood, J. Ciezki, N. Shah, and J. T. Wei. 2008. Quality of life and satisfaction with outcome among prostate-cancer survivors. New England Journal of Medicine 358(12):1250-1261.
Schnipper, L. E., T. J. Smith, D. Raghavan, D. W. Blayney, P. A. Ganz, T. M. Mulvey, and D. S. Wollins. 2012. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. Journal of Clinical Oncology 30(14):1715-1724.
SEER (Surveillance, Epidemiology and End Results). 2012. SEER stat fact sheets: All sites. http://seer.cancer.gov/statfacts/html/all.html (accessed December 16, 2012).
Shankaran, V., S. Jolly, D. Blough, and S. D. Ramsey. 2012. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: A population-based exploratory analysis. Journal of Clinical Oncology 30(14):1608-1614.
Sheets, N. C., G. H. Goldin, A. Meyer, Y. Wu, Y. Chang, T. Stürmer, J. A. Holmes, B. B. Reeve, P. A. Godley, W. R. Carpenter, and R. C. Chen. 2012. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. Journal of the American Medical Association 307(15):1611-1620.
Siegel, R., E. Ward, O. Brawley, and A. Jemal. 2011. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: A Cancer Journal for Clinicians 61(4):212-236.
Smith, B. D., G. L. Smith, A. Hurria, G. N. Hortobagyi, and T. A. Buchholz. 2009. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. Journal of Clinical Oncology 27(17):2758-2765.
Smith, G. L., Y. Xu, T. A. Buchholz, B. D. Smith, S. H. Giordano, B. G. Haffty, F. A. Vicini, J. R. White, D. W. Arthur, J. R. Harris, and Y. T. Shih. 2011. Brachytherapy for accelerated partial-breast irradiation: A rapidly emerging technology in breast cancer care. Journal of Clinical Oncology 29(2):157-165.
Smith, T. J., and B. E. Hillner. 2011. Bending the cost curve in cancer care. New England Journal of Medicine 364(21):2060-2065.
Smith, T. J., and D. L. Longo. 2012. Talking with patients about dying. New England Journal of Medicine 367(17):1651-1652.
Smith, T. J., J. Khatcheressian, G. H. Lyman, H. Ozer, J. O. Armitage, L. Balducci, C. L. Bennett, S. B. Cantor, J. Crawford, S. J. Cross, G. Demetri, C. E. Desch, P. A. Pizzo, C. A. Schiffer, L. Schwartzberg, M. R. Somerfield, G. Somlo, J. C. Wade, J. L. Wade, R. J. Winn, A. J. Wozniak, and A. C. Wolff. 2006. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. Journal of Clinical Oncology 24(19):187-205.
Smith, T. J., L.A. Dow, E. Virago, J. Khatcheressian, L. J. Lyckholm, and R. Matsuyama. 2010. Giving honest information to patients with advanced cancer maintains hope. Oncology 24(6):521-525.
Sprandio, J. D. 2010. Oncology patient-centered medical home and accountable cancer care. Community Oncology 7(12):565-572.
Sprandio, J. D. 2012. Oncology patient-centered medical home. American Journal of Managed Care 18(4 Spec. No.):SP191-SP192.
Sullivan, R., J. Peppercorn, K. Sikora, J. Zalcberg, N. J. Meropol, E. Amir, D. Khayat, P. Boyle, P. Autier, I. F. Tannock, T. Fojo, J. Siderov, S. Williamson, S. Camporesi, J. G. McVie, A. D. Purushotham, P. Naredi, A. Eggermont, M. F. Brennan, M. L. Steinberg, M. De Ridder, S. A. McCloskey, D. Verellen, T. Roberts, G. Storme, R. J. Hicks, P. J. Ell, B. R. Hirsch, D. P. Carbone, K. A. Schulman, P. Catchpole, D. Taylor, J. Geissler, N. G. Brinker, D. Meltzer, D. Kerr, and M. Aapro. 2011. Delivering affordable cancer care in high-income countries. Lancet Oncology 12(10):933-980.
Temel, J. S., J. A. Greer, A. Muzikansky, E. R. Gallagher, S. Admane, V. A. Jackson, C. M. Dahlin, C. D. Blinderman, J. Jacobsen, W. F. Pirl, J. A. Billings, and T. J. Lynch. 2010. Early palliative care for patients with metastatic non-small-cell lung cancer. New England Journal of Medicine 363(8):733-742.
Temel, J. S., J. A. Greer, S. Admane, E. R. Gallagher, V. A. Jackson, T. J. Lynch, I. T. Lennes, C. M. Dahlin, and W. F. Pirl. 2011. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: Results of a randomized study of early palliative care. Journal of Clinical Oncology 29(17):2319-2326.
Temel, J. S., J. A. Greer, E. R. Gallagher, V. A. Jackson, I. T. Lennes, A. Muzikansky, E. R. Park, and W. F. Pirl. 2013. Electronic prompt to improve outpatient code status documentation for patients with advanced lung cancer. Journal of Clinical Oncology [epub ahead of print].
U.S. Census Bureau. 2010. The next four decades: The older population in the United States: 2010 to 2050. Population estimates and projections. http://www.census.gov/prod/2010pubs/p25-1138.pdf (accessed December 16, 2012).
Vickers, A., C. Savage, F. Bianco, J. Mulhall, J. Sandhu, B. Guillonneau, A. Cronin, and P. Scardino. 2011. Cancer control and functional outcomes after radical prostatectomy as markers of surgical quality: Analysis of heterogeneity between surgeons at a single cancer center. European Urology 59(3):317-322.
Volandes, A. E., M. K. Paasche-Orlow, S. L. Mitchell, A. El-Jawahri, A. D. Davis, M. J. Barry, K. L. Hartshorn, V. A. Jackson, M. R. Gillick, E. S. Walker-Corkery, Y. Chang, L. Lopez, M. Kemeny, L. Bulone, E. Mann, S. Misra, M. Peachey, E. D. Abbo, A. F. Eichler, A. S. Epstein, A. Noy, T. T. Levin, and J. S. Temel. 2012. Randomized controlled trial of a video decision support tool for cardiopulmonary resuscitation decision making in advanced cancer. Journal of Clinical Oncology [epub ahead of print].
Weeks, J. C., E. F. Cook, S. J. O’Day, L. M. Peterson, N. Wenger, D. Reding, F. E. Harrell, P. Kussin, N. V. Dawson, A. F. Connors, Jr., J. Lynn, and R. S. Phillips. 1998. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. Journal of the American Medical Association 279(21):1709-1714.
Weeks, J. C., P. J. Catalano, A. Cronin, M. D. Finkelman, J. W. Mack, N. L. Keating, and D. Schrag. 2012. Patients’ expectations about effects of chemotherapy for advanced cancer. New England Journal of Medicine 367(17):1616-1625.
Welch, H. G., and W. C. Black. 2010. Overdiagnosis in cancer. Journal of the National Cancer Institute 102(9):605-613.
Woolhandler, S., T. Campbell, and D. U. Himmelstein. 2003. Costs of health care administration in the United States and Canada. New England Journal of Medicine 349(8):768-775.
Wright, A. A., B. Zhang, A. Ray, J. W. Mack, E. Trice, T. Balboni, S. L. Mitchell, V. A. Jackson, S. D. Block, P. K. Maciejewski, and H. G. Prigerson. 2008. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. Journal of the American Medical Association 300(14):1665-1673.
Yeboa, D. R., K. Sunderland, K. Liao, K. Armstrong, and J. Bekelman. 2010. Trends in treatment with intensity modulated (IMRT) vs. 3D conformal (CRT) radiotherapy for non-metastatic prostate cancer. International Journal of Radiation Oncology, Biology, and Physics 78(3):s342-s343
Young, R. A., and J. E. DeVoe. 2012. Who will have health insurance in the future? An updated projection. Annals of Family Medicine 10(2):156-162.
Yun, Y. H., C. G. Lee, S. Y. Kim, S. W. Lee, D. S. Heo, J. S. Kim, K. S. Lee, Y. S. Hong, J. S. Lee, and C. H. You. 2004. The attitudes of cancer patients and their families toward the disclosure of terminal illness. Journal of Clinical Oncology 22(2):307-314.
Zhang, B., A. A. Wright, H. A. Huskamp, M. E. Nilsson, M. L. Maciejewski, C. C. Earle, S. D. Block, P. K. Maciejewski, and H. G. Prigerson. 2009. Health care costs in the last week of life: Associations with end-of-life conversations. Archives of Internal Medicine 169(5):480-488.










































































