The Intersection of Genomics and Health Economics
Important Points Made by the Speaker
- Health economics can provide a variety of tools and frameworks to help guide the implementation of genome sequencing in clinical practice.
- The majority of new medical interventions improve outcomes and increase cost.
- A cost-effectiveness framework for whole genome sequencing could consider the prevalence and penetrance of a genetic variant, the effects of that condition, the cost and accuracy of a test, the cost of an intervention, the outcomes of an intervention, and the severity of the disease.
- Greater understanding of patient-centered outcomes is needed to determine the value of genome sequencing.
- Patient and provider responses to genome sequencing will require new investments in services, new pathways of care, genetic counseling, decisions about what will and will not be covered by insurance, and provisions for dealing with incidental findings.
- Quicker approaches that incorporate qualitative assessments need to be devised for the economic evaluation of genetic information.
David Veenstra, professor in the Pharmaceutical Outcomes Research and Policy Program at the University of Washington in Seattle, began his overview of health economics by reiterating the point Evans made about health economics not really being about costs; rather, he said, it is about value and understanding utility. Through a consideration of value, health economics can help people clarify the assumptions that are being made, consider uncertainties, and evaluate trade-offs. Thus, health economic evaluations are primarily used to inform decision making.
A basic tenet of economics is that people make decisions to improve their well-being. For most commodities, price is a measure of perceived improvements in well-being, or value, and people make decisions on the basis of value. These principles, however, often do not apply in health care, Veenstra observed. The individual receiving the health care is generally not the person who makes the decision about what health care will be received. The person receiving the health care typically does not have a good idea of the potential benefits and harms of a decision. And patients generally do not pay out of pocket for the services they receive.
Health care economics tries to gain a better understanding of the value of one health care intervention compared to an alternative approach, taking into consideration all the impacts across patients, providers, and the health care system. This value can be measured in terms of price, improvements in quality of life, a longer life expectancy, resources saved, health state, and so on. The key components of the evaluation, Veenstra said, are that all relevant factors are included in the analysis and that the same approach is applied to all decisions that are being assessed.
Economists use several different tools to carry out economic evaluations of health care interventions, including cost-minimization analysis, cost-benefit analysis, cost-effectiveness analysis (CEA), and cost-utility analysis (CUA). All these approaches consider the cost of the intervention as well as downstream costs, but they differ in how they measure the outcome or utility of an intervention (see Table 3-1).
Cost-minimization assumes that the outcome of two different interventions is the same. The goal in this case is to reduce costs, and thus the cheapest intervention with the same effect on outcome can be determined. Still, outcomes tend to be different to some degree, said Veenstra, so this approach cannot be commonly used.
Cost-benefit analyses consider everything in terms of costs. But this can be difficult to do in health care, Veenstra said, because people tend to resist putting a monetary value on health, and thus it is extremely hard to measure accurately.
TABLE 3-1 Types of Economic Evaluations in Health Care
| Study Design | Costs Measured? | Outcomes Measured? | Strengths | Weaknesses |
| Cost-minimization | Yes | Not necessary | Easy to perform | Useful only if outcomes are the same for both interventions |
| Cost-benefit | Yes | Yes, in monetary terms | Good theoretical foundation; can be used within health care and across sectors of the economy | Less commonly accepted by health care decision makers; evaluation of benefits methodologically challenging |
| Cost-effectiveness | Yes | Yes, in clinical terms (events, life years) | Relevant for clinicians; easily understandable | Cannot compare interventions across disease areas when using disease-specific end points |
| Cost-utility | Yes | Yes, in quality-adjusted life years | Incorporates quality of life; comparable across disease areas and interventions; standard | Requires evaluation of patient preferences; can be difficult to interpret |
SOURCE: David Veenstra, IOM workshop presentation, July 17-18, 2012.
The two more commonly used approaches in the field, said Veenstra, are CEA and CUA. CEA is a quantitative framework for evaluating the complex and often conflicting factors involved in the evaluation of health care technologies. CEA seeks to determine whether an intervention used to prevent, diagnose, or treat an illness improves clinical outcomes enough to justify the additional dollars spent compared with alternative uses of the same money. CEA is not a method to show which interventions reduce cost. Rather, it aims to inform which interventions provide the greatest value for the amount of money that is spent. Also, CEA is not a method that removes individual or group responsibility for making clinical and financial decisions. Rather, it provides information that is incorporated into larger decisions involving additional considerations, such as issues of equity.
CEAs measure outcomes in terms of clinical events such as cost per
heart attack avoided or cost per life year saved. This approach works well, said Veenstra, and people are reasonably accepting of it. CEAs, however, do not easily allow for cross-intervention comparisons, for example, whether to spend $50,000 to prevent a heart attack or spend $50,000 to prevent breast cancer. Answering this question would require further considerations of how long a person would have lived, what that person’s quality of life would have been with a given intervention, as well as other downstream costs.
The gold standard in the field has become CUA because of this limitation of CEA analysis, said Veenstra. CUA typically measures outcomes through a metric called a quality-adjusted life year (QALY) and allows for comparisons across interventions. For example, if $50,000 spent to prevent a heart attack produces 10 QALYs, and $50,000 spent to prevent a breast cancer produces 20 QALYs, a decision can be informed by that information. “That is what we produce in health care,” said Veenstra. “We don’t make cars; we don’t make phones. We increase people’s length of life, and we improve their quality of life—at least that is our goal. And the QALY captures those.”
INCREMENTAL COST-EFFECTIVENESS RATIOS
Another standard measure in health economics is the incremental cost-effectiveness ratio, which is defined as the difference in cost between two interventions divided by the difference in their effectiveness. This metric can fall into four different quadrants on what is called a cost-effectiveness plane (see Figure 3-1). The best result is when outcomes improve and costs go down. The worst is when outcomes become worse and costs increase. Most interventions in health care result in higher costs with improved outcomes, Veenstra said, which makes CUAs useful for comparing these interventions. For example, the cost per life year saved may be $10,000 for one intervention and $200,000 for another intervention. In this case, money may be more effectively spent on the first intervention.
In the United States, however, there is not a clear threshold on how much money society is willing to spend to save a life for 1 year, said Veenstra. “You might hear people [say] $50,000 per QALY. In reality, it is probably closer to $100,000 or more in this country.” Nevertheless, this approach provides a way to determine whether an intervention is reasonable.
Whether genome sequencing is cost-effective depends on the outcome that is being measured, Veenstra said. These outcomes could be measured in
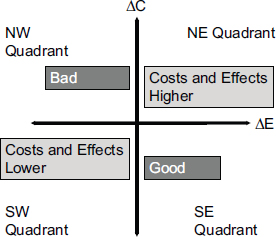
FIGURE 3-1 The change in costs and change in effectiveness compared with current practice divides the results of cost-effectiveness analyses into four quadrants.
NOTE: ΔC, change in cost; ΔE, change in effectiveness.
SOURCE: David Veenstra, IOM workshop presentation, July 17-18, 2012.
terms of base pairs sequenced per dollar, the number of clinically meaningful genetic variants identified, diagnoses received, clinical actions taken, or patient outcomes. The other important factor is the comparator. Is genome sequencing being compared to nothing, to observing the patient in the clinic, or to a targeted sequencing approach?
Flowers and Veenstra (2004) developed a framework for factors that could influence cost-effectiveness in pharmacogenomic testing (see Table 3-2). Important factors include the prevalence and penetrance of the genetic variant, the cost and accuracy of the test, the prevalence of the disease and the outcomes if left untreated, and the effectiveness and cost of treatments. A similar framework could be constructed for whole genome sequencing to examine benefits and harms, according to Veenstra. That framework would consider the prevalence of the variant of interest, the penetrance of the condition, the cost of a test, the cost and outcomes of an intervention, and the severity of the disease. A major complication is that a typical economic evaluation of a single test or single genetic variant can take a year. “We don’t have time for that,” Veenstra said. “We need to have quicker approaches that use more of a qualitative assessment.” Yet, if enough examples of this type of analysis can be completed, he added, “we can get a sense of where good value may be provided.”
TABLE 3-2 Factors That Influence the Cost-Effectiveness of Genomic Testing Strategies
| Factors to Assess | Features That Favor Cost-Effectiveness | |
| Gene | Prevalence |
|
| Penetrance |
|
|
| Test | Sensitivity, specificity, cost |
|
| Disease | Prevalence |
|
| Outcomes and economic impacts |
|
|
| Treatment | Outcomes and economic impacts |
|
David Veenstra, IOM workshop presentation, July 17-18, 2012.
COMPARATIVE-EFFECTIVENESS RESEARCH
Comparative-effectiveness research, which is an amalgamation of previous approaches in technology assessment and health economics, also relates to the issue of how people use information from genomic tests. According to Veenstra, comparative-effectiveness research includes all of the following components:
- Stakeholder-informed prioritization and design of studies
- Direct, head-to-head comparisons
- A broad range of beneficiaries, including patients, clinicians, purchasers, and policy makers
- Study populations representative of clinical practice
- A focus on patient-centered decision making
The emphasis on patient-centered outcomes in comparative-effectiveness research is especially relevant to whole genome sequencing, Veenstra said. The new Patient-Centered Outcomes Research Institute plans to fund studies that investigate the key determinants of outcomes patients experience following treatment decisions, with a special emphasis on studies considering that results may differ among patient groups on the basis of patient characteristics. The center also will be supporting studies that compare the use of prognostication and risk stratification tools with usual clinical approaches. “These are the types of things that we are going to need to get a better understanding of the value that whole genome sequencing brings,” Veenstra noted.
Veenstra cited three challenges at the intersection of genomics and health economics. The first is whether decreasing sequencing costs can reduce the incentives for test development. If whole genome tests cost less than $1,000, individual genetic tests may no longer make sense. At that point, does the provision of clinical interpretation become the value proposition, with each condition a patient presents with eliciting a new review of relevant whole genome sequence data? And if so, are reimbursement systems designed to reward this? As Ramsey et al. (2006) noted, value-based payment policies would provide greater incentives for continued innovation in test development, Veenstra said.
The second challenge involves the development of policies to incorporate personal utility into test assessments. Diagnoses do not necessarily increase life expectancy, but individuals may value this information. What is the value to an individual of knowing something, asked Veenstra. Standard approaches in health economics are limited in that they do not sufficiently capture patient-centered outcomes. New techniques and tools, such as conjoint analysis and discrete choice experiments, have been developed to get a better sense of the value of knowledge to patients (Basu and Meltzer, 2007; Grosse et al., 2008; Regier et al., 2009). These tools need to be further developed so that consideration can be given regarding whether to incorporate such measures into guidelines or into reimbursement policies.
A related policy issue involves evidence thresholds. Does a lower cost of obtaining information lead to a lower evidence threshold for using that information?, Veenstra asked. For example, he said, genetic testing for warfarin dosing is rarely done today even after 10 years of evidence development, but if a test result were already available, clinicians would likely
take the result into account in choosing an initial dose. He noted, “There is this issue that if it takes time and money to get the information, it is not worth it. But if you have it sitting there in front of you, you are going to go ahead and use it.” In an environment of limited evidence, an economic tool called value-of-information analysis may provide a framework for “evidence-based” decision making. This tool gives a sense of whether having information today is worthwhile as compared to developing the evidence base further.
The third and final challenge Veenstra described is the impact of whole genome sequencing on the health care system. We do not know how patients and providers will respond to the information available from genomic tests, specifically as it impacts their decisions about receiving medical care. Any response may necessitate further investment in services, the development of new pathways of care, genetic counseling, decisions about what will and will not be covered by insurance, and provisions for dealing with incidental findings.








