Assessment of LED and OLED Technologies
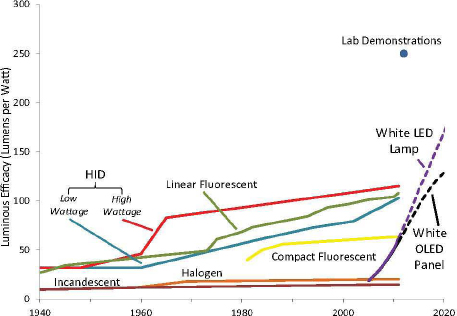
Both inorganic and organic light-emitting diodes (LEDs) offer dramatically new sources of illumination with the potential of greater efficiency, longer lifetimes, exceptional control over the colors generated, and differing form factors. In aggregate, these sources promise to redefine how lighting that is both economically and energy efficient can be integrated into our daily lives. Inorganic LEDs, fabricated from light-emitting semiconductors, leverage a great deal of fabrication and manufacturing equipment developed for electronic semiconductor devices. Although red, green, and yellow LEDs have been available since the 1970s, the advent of high-brightness blue LEDs in 1993 made high-efficiency white lighting sources possible. There has been continual progress in the wall plug efficiency of these lighting sources, resulting in current values of ~150 lumens per watt (lm/W) for commercial samples and the best in-lab values of 254 lm/W (Cree, 2012). Organic LEDs (OLEDs) for lighting can build on manufacturing experience gained from the recent and large-scale production of OLED displays— which in 2012 represent a $3 billion market. Today white OLEDs with color rendering indices greater than 80 have been reported (i.e., they can be made to emit with almost any color; therefore generation of high-quality white light is easily achieved) with greater than 100 lm/W efficacy (D’Andrade et al., 2008; Reineke et al., 2009). Figure 3.1 illustrates the progress in lighting efficiency and the role that LEDs and OLEDs have played in driving that progress. The succeeding sections of this chapter will provide a basic introduction to both LED and OLED technologies and will discuss the major challenges for each technology in achieving widespread, low-cost, higher-efficiency lighting sources. The technical details that underlie performance of LEDs and OLEDs have important impacts on the efficacy and reliability of the performance of the total lighting system and also determine the subtleties of color quality of the lighting. Ultimately, understanding these details will allow better strategies for developing lower-cost manufacturing technologies. Although specific findings and recommendations are integrated into the entirety of this chapter, some of the committee’s major findings and recommendations are stated at the outset of this chapter to provide some perspective for the reader and to set the tone for the rest of the chapter.
FINDING: LEDs and OLEDs are complementary lighting sources that can together offer a wide range of lighting solutions. OLEDs can provide large-area diffuse lighting, while, in the same venue, LEDs form intense point sources, useful for spot illumination and downlighting. The committee finds value in supporting rapid developments in both technologies, because they both represent large possible markets, new applications, and tremendous energy savings.
FINDING: LED and OLED efficiency and performance are still limited by fundamental materials issues. Improvements in efficiency at the device and materials level, as targeted by the Department of Energy (DOE) solid-state lighting (SSL) roadmap, will have a “lever effect”—influencing the design, performance, and cost of the luminaires. Therefore, improvements in efficiency and performance of the entire SSL system are linked to further fundamental investigations in core technology on emitter materials.
While inorganic LEDs have been manufactured and widely available commercially for some time, there is as yet no commensurate large-scale manufacture of OLEDs. Nevertheless, LED yield, cost, and performance would still benefit enormously from further fundamental exploration and improvements in the basic technology of materials growth.
FINDING: Current LED dies used in SSL lighting suffer from inhomogeneities in the light output, color, and operating voltage that necessitate “binning” (hence testing) of dies from a single wafer. This variability severely constrains the yield of the manufacturing process and raises the cost of the technology. These inhomogeneities are in turn related to fundamental materials and materials growth issues.
FIGURE 3.1 Progress in lighting efficacy. SOURCE: DOE (2012b, p. 38).
RECOMMENDATION 3-1: The Department of Energy should continue to make investments in LED core technology, aimed at increasing yields, and in fundamental emitter research to increase efficacy, including improvements in the controlled growth and performance of the emitter material. DOE should carefully consider the range and depth of funding in its portfolio of investments in these areas, given the existing technological challenges, in order to determine how the targeted goals of device performance can indeed be met.
The remainder of this chapter will provide an introduction to both inorganic and organic LEDs in a parallel approach. The LED and OLED primers will first focus on the basic device structure and metrics of device performance. This will be followed by discussions on the control of the color output of these devices and the important influence of materials on device performance. Because OLEDs for SSL have not yet been scaled up for large-scale manufacture, the discussion for OLEDs will also encompass issues of reliability and manufacturabililty. The chapter will conclude with a comparison and summary of promises and challenges for both technologies.
Semiconductor LEDs are a special kind of electronic device that emits light upon the application of a voltage across the device. Silicon (Si) is probably the best-known semiconductor material and the basis of the integrated circuits that underlie the fast and compact electronic devices, such as computers and cell phones, that are so critical to our daily lives. LEDs are based on a semiconductor material comprised of several different elements. This material is known as a compound semiconductor. The tremendous power of semiconductors lies in their ability to take on a wide range of conductivities, from metallic to insulator. This is brought about by “doping” the semiconductor with other elements that will donate either positively or negatively charged carriers to achieve a desired conductivity.
Semiconductors can also absorb and emit light, and the relevant wavelengths are related to the bandgap of the semiconductor (see Box 3.1). The general process for light emitted in this manner is referred to as electroluminescence. The first high-efficiency light-emitting devices were developed in the 1960s utilizing gallium arsenide (GaAs), aluminum gallium arsenide (AlxGa1xAs), gallium phosphide (GaP), and gallium arsenide phosphide (GaAsxP1-x) (Hall et al., 1962; Nathan et al., 1962; Pankove and Massoulie, 1962; Woodall et al., 1972; Herzog et al., 1969). GaAs and AlGaAs LEDs produced light with infrared wavelengths, ~850 nanometers (nm), while the gallium phosphide-based LEDs produced light in the red and green wavelengths. In the early 1990s, efficient blue LEDs based on III-nitride materials began to appear based on the work of Akasaki et al. (1992) and Nakamura et al. (1994). (The III refers to elements in the third column of the periodic table, indicating that these LEDs can be comprised of alloys of aluminum nitride (AlN), gallium nitride (GaN), and indium
BOX 3.1
Light Emission Mechanism
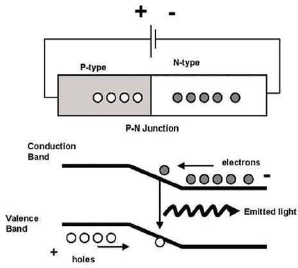
Figure 3.1.1 gives a simple description of the basic light-emission process. Electrons fill up energy states in a valence band, which is separated in energy from a conduction band by an energy gap, with energy Eg (where there are generally no allowed states in which electrons can reside). Providing energy to an electron in the valence band can promote that electron to the higher-energy conduction band, also creating a hole (lack of electron) in the valence band. The electron can subsequently return to its lower-energy state: in radiative recombination, the electron returns to the valence band and releases a photon with the energy of the photon approximately equal to the energy Eg. In an LED, radiative recombination is the desirable outcome for an “energized electron,” but there are also numerous non-radiative recombination processes where the electron or hole may be trapped at defects or imperfections in the material. Such imperfections limit the efficiency of the light generation and, therefore, of the LED.
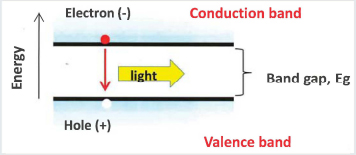
FIGURE 3.1.1 Light emission process.
nitride (InN)). The bandgaps of these III-nitrides produce light emission across a range of wavelengths spanning the infrared to ultraviolet (UV) parts of the spectrum. The IIInitride LEDs have had an unusually rapid development and huge impact on appearance of SSL. Although the first GaN LED was reported by Pankove et al. (1971), almost two decades transpired before substantial further progress was made by Akasaki and Nakamura. Akasaki demonstrated that high crystal quality GaN could be grown by metal organic chemical vapor deposition (MOCVD) using a novel low-temperature buffer (Amano et al., 1986). In 1992, Nakamura, working at Nichia, developed an industrially robust process for p-doping of GaN that led to the first high-brightness blue LEDs. This provided the understanding of the mechanisms that had limited the conductivity of P-type material and allowed for the first time the fabrication of low-voltage p-n junction LEDs and eventually led to the commercialization of high-brightness blue and white LEDs for SSL. The wider bandgaps of the III-nitrides enabled the development of efficient LEDs that emit light at blue wavelengths, which together with green and red LEDs provided the basis for white light as well as full-color displays. The nitride blue emitters can also be coupled with phosphors to generate white light, which is currently the dominant approach to an SSL technology. The later introduction of blue LEDs, compared to their green and red counterparts, is the result of materials issues that are still of importance today: the lack of a well-matched material (substrate) upon which to form the LED structures and some difficulties in controlling the electrical properties of the material. Nonetheless, the III-nitride materials have been pivotal in the success of inorganic SSL, and thus the committee will focus on LEDs formed from those materials. There are several good reviews of LED device technology (see, for example, Schubert [2006]) as well as III-nitride materials technology (Pankove and Moustakas, 1998)).
The basis of the LED device is a p-n junction diode, shown schematically in Figure 3.2. As the name implies, there is a junction between the N-type material (rich in electrons) and P-type material (rich in holes). Under forward bias (positive voltage applied to the P-region and negative voltage applied to the N region) large numbers of electrons are injected into the N region and large numbers of holes are injected into the P region.
Current flows in the device and the large number of injected electrons and holes can combine radiatively, producing significant light emission. The basic structure is modified in actual LEDs to (1) improve the efficiency of injection of electrons and holes and to (2) “localize” the electrons and
FIGURE 3.2 Schematic of p-n junction diode.
holes and improve the likelihood of radiative recombination. This localization is accomplished by introducing quantum wells in the region of the junction. These are thin slivers of lower bandgap-materials that, as their name implies, serve as wells that confine pools of electrons and holes to increase the probability that they will recombine radiatively.
The external view of the typical LED structure is given in Figure 3.3, showing the N-type GaN, the InGaN quantum wells, and the P-type GaN. Most GaN LED devices are formed on a sapphire substrate through the MOCVD process. Typically, one 4-inch-diameter sapphire wafer can produce 5,000 individual devices or “dies.” The 16 percent mismatch in natural lattice size between the sapphire substrate and the GaN overlayers has important consequences on device performance and on the uniformity of the dies grown from a single wafer. This is further discussed in the section “Materials Issues.” In order to connect the device to the outside world, metal contacts must be deposited by evaporation on the N and P regions. Figure 3.3 shows these metal contacts, as well as the transparent and conductive indium tin oxide (ITO) layer that extends the top-side electrical contact over the device surface. Both the sapphire substrate and the ITO spreader contact are transparent to the emitted light, as is necessary for the light to leave the device. High-quality electrical contacts are important to reduce loss due to resistance (R) to current flow (I) in the contact region. This is even more important when the device is operated at high currents or current densities, because loss of power due to resistive heating scales as I2R. In the III-nitride materials, it is a challenge to dope the materials to a sufficient level so that resistances are low, particularly for P-type materials. The formation of the device structure shown in Figure 3.3 is just a starting point for the fabrication of the final solid-state “light-bulb.” An individual device must be further “packaged” to better control its chemical, thermal, and electrical environment and to better integrate it into the final luminaire.
The LED package is the structure in which the LED chip is mounted and through which access to the LED terminals is provided. It is an important part of the finished device. The package serves the following functions: (1) it passivates or protects the active semiconductor material from degradation due to the environment (principally moisture); (2) it integrates an optical lens structure, which determines the optical emission pattern of the structure; (3) it removes heat from the device, protecting against degradation due to overheating; and (4) it protects the device from electrostatic discharge
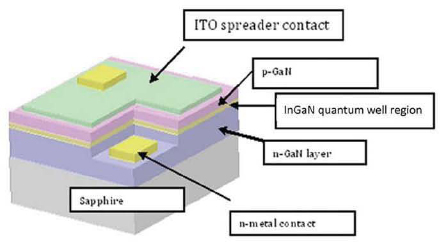
FIGURE 3.3 A typical GaN LED chip.
failure. The packaging processes include placement of the device in the chip carriers, attachment of the optical lens, as well as electrical and optical device testing and “binning.” Because of the variability in the color accuracy, color quality, and color stability (see section “Controlling the Color Output of the LED”), each device must be individually tested and placed in performance bins. In addition, if phosphor coatings are used in connection with the LED to control the output color, the phosphor must be added to the device or package.
A schematic of a typical LED package is shown in Figure 3.4. The LED semiconductor chip or “die” is bonded to a ceramic substrate, which provides mechanical support and thermally connects the LED to a thermal pad on the bottom of the substrate. An electrical interconnect layer connects the LED chip to the voltage leads on the bottom of the substrate (one of the voltage leads, the cathode, is shown). A silicone lens above the LED extracts the light that is generated within the chip. Also shown is a transient voltage suppressor (TVS) chip which protects the LED chip against electrostatic discharge events.
Efficiency is an important metric of LED device performance, and some insights into efficient operation can be gleaned by tracing the life cycle of the LED operation beginning with the injection of electrons and holes, shown in Figure 3.2, leading to the generation of photons within the device, and culminating with the emission (or extraction) of the photons from the device. A simple summary of the total external quantum efficiency (EQE or ƞEQE) of an LED can be expressed as:
ƞEQE = ƞIQE • ƞout
where ƞIQE is the internal quantum efficiency, and ƞout is the outcoupling (or light extraction) efficiency, which will be further discussed below.
Internal Quantum Efficiency
Not all electrons and holes that are injected into the LED (e.g., from a battery) will produce photons; for example, defects in the LED material can trap an electron or a hole, and prevent the formation of a photon. The percentage of photons generated, relative to current (of electrons or holes) that is injected into the device is reflected in the IQE. can ƞIQE be maximized by using quantum well structures as described above, by utilizing defect-free semiconductor material, and by ensuring high-quality, very-low-resistance metal contacts to the device. ƞIQE also sensitively depends on the quality of the LED material. Because the quantum well composition avnardiesstrwaiinth v waraievselwenitghthth. eAdltehsoiruegdh e çmission wavelength, ƞIQE IQE of today’s best LEDs
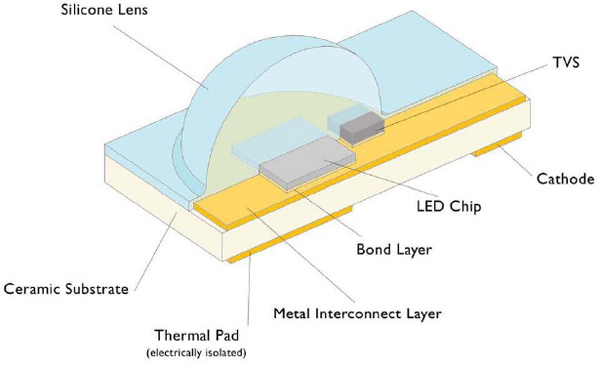
FIGURE 3.4 Schematic of an LED module. NOTE: TVS = transient voltage supression. SOURCE: Figure provided courtesy of Sudhir Subramanya, Philips Lumileds Lighting Company.
has reached as high as 80 percent for blue LEDs and 38 percent for green LEDs (DOE, 2011a, p. 71), equal efficiency of LEDs at all colors is important, and further improvements toward 100 percent ƞIQE will require far better control of the material defects.
Current and Thermal Droop
Two of the most important issues holding back efficiency at high illumination levels is the droop in efficiency as the LED is driven at higher currents (e.g., operation at 100 A/cm2 compared to operation at 35 A/cm2 (DOE, 2011a, Table A1.2, p. 71), and the effect of temperature. These issues are known in the industry as “current droop” and “thermal droop.” The causes and solutions to current droop are still not widely known. Thermal droop is influenced by the choice of IIInitride alloy bandgaps and the active layer design, which is limited to thin quantum wells. As was discussed above, ƞIQE of green LEDs is much lower than that of blue LEDs. Similarly, the “droop” at higher current operation is more pronounced for green LEDs. All major LED companies have active research in these areas.
Outcoupling (or Light Extraction) Efficiency
Once the photons have been formed in the LED structure, care must be taken to ensure that they will exit the device. The ratio of photons leaving the device to the number generated within the device is called the outcoupling (or light extraction) efficiency. Because the LED material has a higher index of refraction (n ~ 2.5) than air (n = 1), most photons incident on the GaN-air interface will be internally reflected and trapped within the LED structure or absorbed (lost) by other materials comprising the device (see Figure 3.5). A thin metal film can serve as a mirror to direct the light out through the “front surface” of the LED. The internal reflection and trapping of the light can be mitigated by forming a rough, rather than smooth top LED surface; one way of achieving this is through the immersion of the device structure in a simple wet chemical etchant (Fujii et al., 2004). Such techniques can improve the extraction efficiency from a few percent to values of 80 percent (Krames et al., 2007). Finally, the external power efficiency (ƞP) is defined as the ratio of the total optical power output of the LED to the electrical power input. Low resistive power loss, high ƞIQE, and good design to maximize ƞout produce high power efficiency in LEDs. Maximizing the power efficiency not only increases the efficacy of the LED but also reduces the heat removal problem.
FINDING: Efficient operation of LEDs depends on a number of critical factors related to materials defects, structure, and strain. Such factors not only limit device efficiencies, but also lead to thermal and current droop; all have a major impact on the cost and performance of LED lighting.
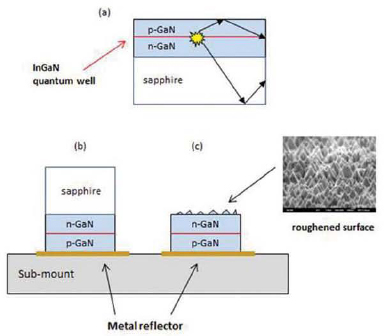
FIGURE 3.5 Improving light extraction efficiency. (a) Much of the light emitted from the quantum well is internally reflected (not extracted). (b) Flipping the LED and placing it above a reflective surface helps to direct the light outwards. (c) Removing the sapphire substrate and then roughening the top of the LED surface. NOTE: p-GaN is P-type (i.e., positive) gallium nitride material (rich in holes); n-GaN is N-type (i.e., negative) gallium nitride material (rich in electrons).
CONTROLLING THE COLOR OUTPUT OF THE LED
An important metric of LED device operation is the control over the accuracy, quality, and stability of its color or peak emission wavelength. Three well-established approaches to generating white light using LEDs are shown in Figure 3.6. These include a blue LED with yellow phosphors; an ultraviolet (UV) LED with blue and yellow phosphors (or red, green, and blue phosphors); and a device that combines red, green, blue LEDs.
The color of emitted light from an LED depends on the structure and composition of the LED. Achieving a desired photon frequency (hence color) from an LED requires sensitive control over the thicknesses and material composition of the LED.
Quantum Well Thickness and Composition
The active layers of the current blue LEDs used in SSL are extremely small, 3 nm thick, which classifies them as quantum wells. In other words, these nanostructures fall in the class of devices in which the light generation mechanism is controlled at the atomic level. Small changes in the indium composition and well thickness affect the emission wavelength and width of the emission. Currently, blue LEDs have a peak wavelength of 455 nm and a width of 15 nm. Any changes in the peak position or width can visibly affect the hue of white light obtained. The MOCVD deposition machines used in the manufacture of the LEDs have a huge influence on the uniformity of the wavelength and yield of white LEDs (see the section “Materials Growth”). One way to improve the color consistency and make wider, more reproducible quantum wells is to look at alternative substrates for the growth of the GaN LED structures.
FINDING: The color output of LEDs is extremely sensitive to the control of materials composition and thicknesses of the LED structure, which in turn are influenced by the control of the MOCVD growth process.
Another means of controlling the LED color output is through the use of phosphors. The phosphors absorb the (typically) blue light from the GaN LED and re-emit light at longer wavelengths. The phosphors are chosen so that the combination of the direct light from the LED and the light emitted from the phosphor will produce the desired white light. A selected few phosphors have garnered considerable attention, including for example rare-earth (RE) doped yttrium aluminum garnets (YAG:RE, Y3Al5O12(RE)). The cerium-doped YAG can absorb blue and UV light and emit it as yellow light with high efficiency. A critical aspect of this process is that the higher-energy light (e.g., UV or blue) is being converted into lower energy (e.g., yellow or red). As a consequence, LEDs emitting red light—the color having the lowest energy in the visible spectrum—cannot be used with phosphors to generate white light; instead, a short-wavelength UV, violet, or blue LED is required (Denbaars et al., 2013).
Phosphors are typically directly added on top of the LED in the encapsulation material, which is either silicone or epoxy-based. The uniformity of the phosphor coating and mixture selection can drastically affect the efficacy and quality
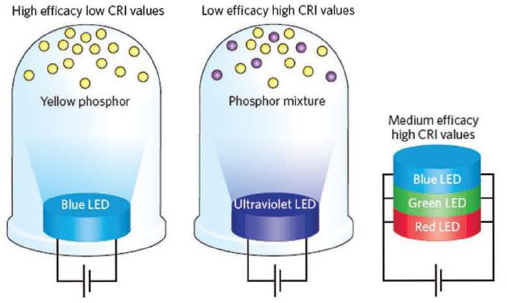
FIGURE 3.6 Three types of white LEDs for lighting: (a) blue LED plus yellow phosphors, (b) ultraviolet LED plus three phosphors, (c) three LEDs: red, green, blue connected in parallel. SOURCE: Pimputkar et al. (2009). Reprinted by permission from Macmillan Publishers Ltd.
of light. For example, the use of just a yellow-emitting YAG phosphor with a single blue GaN LED results in high luminous efficacies but relative poor quality (color rendering index, CRI < 75) light. This has led to the perception that all LED lighting is blueish-white and cold. UV LEDs with phosphor mixtures provide a better CRI value, but at the expense of poorer efficacy. A combination of three (or more) LEDs having different wavelengths (red, green, and blue) may be used if one wishes to dynamically control white light. This approach may lead to higher efficacies than the UV-phosphor LED. In addition, moving the phosphor layer away from the chip and tailoring the optics between the LED chip and the remote phosphor layer has resulted in significant improvement in light output and luminous efficacy ( Narendran et al., 2005).
General Considerations: Mixed LEDs or Phosphors?
Using narrowband (colored) components to create white light, like the examples in Figure 1.9 in Chapter 1, allow manufacturers to manipulate the luminous efficacy of radiation (LER) and color qualities of a light source depending on their goals and priorities. At these relatively early stages of the technology, there are a number of technical difficulties with the use of multi-color LEDs (red, green, blue; red, green, blue, yellow) to produce a white source in SSL products. If one of the colored components ages differently from the others or responds to heat differently, the color properties of the light source will change. Furthermore, inadequate mixing of the light will result in colored shadows. Nonetheless, multi-color LED lighting has some advantages. The spectrum can be tuned to optimize LER. A wide range of chromaticities can be achieved by adjusting the relative intensities of the component colors. In fact, some SSL products currently on the market allow users to adjust the chromaticity—a heavily marketed feature (Philips Solid-State Lighting Solutions, 2012)—and some in the industry believe that eventually all consumers will expect their lighting products to offer such functionality (Thompson et al., 2011).
Even though entire portions of the visible spectrum are missing from such light sources, narrowband multi-color lights can achieve good (three components) or excellent (four or more components) color rendering. These types of lights can even exhibit some desirable color-rendering properties that broadband light sources cannot, such as inducing increases in the colorfulness/vividness of object colors beyond what the CRI deems to be “perfect.” Research on this effect suggests that people find these slightly enhanced object colors to appear more attractive (Jost-Boissard, 2009).
Phosphor white LEDs have a clear ease-of-use advantage and dominate the current SSL general illumination market. Although the resultant products do not offer the same flexibility in chromaticity as those from multi-color white LEDs, the phosphors themselves are available in a wide range of chromaticities. Color rendering varies depending on the particular phosphor involved, but can be disappointing, particularly compared to incandescent-based light sources. Over the past few years, manufacturers have found that adding some additional energy in the long (red) wavelengths, either with a red LED or remote phosphor, vastly improves the color quality of typical white phosphor LEDs (Hum, 2011). This solution is now widely used.
Quantum Dot “Phosphors”
There has recently emerged another interesting approach for the color control of both LEDs and OLEDs involving semiconductor nanocrystals or “quantum dots.” These materials are governed by the light emission mechanism described earlier, but these semiconductors, like the phosphors, can also absorb higher-energy photons and emit photons of lower energy or longer wavelength. What is distinctive about these chemically synthesized materials, with diameters of a few nanometers, is that the size of the nanocrystals will influence the wavelength of emission. Initial work on CdSe nanocrystals began in the late 1980s (Brus, 1991): the color tunability of these structures, their small size, the relative ease of production, and their optical robustness encouraged researchers to utilize these quantum dots in a variety of applications, such as selective tagging and in vivo imaging of features in cells (Michalet et al., 2005). Although much further assessment will be required to understand the full potential of this technology, initial results look promising.
FINDING: A number of approaches have successfully been used to achieve and modulate color rendition for LED lighting. Phosphor-converted and color-mixed LEDs show promise but face different challenges. The ultimate choice of approach will depend on a multiplicity of issues regarding sensitivity of color control, efficiency, reliability, manufacturability, and cost.
RECOMMENDATION 3-2: The Department of Energy has provided excellent guidance in its roadmap targets for both phosphor-converted and color-mixed light-emitting diodes. Core investment in these technologies should be continued, with consideration for promising new technologies (e.g., quantum dot layers replacing phosphors).
MATERIALS ISSUES FOR WHITE LEOS
Materials issues in white LED technology affect the cost, yield, and reliability of the resulting luminaire at the most fundamental level. A particularly critical issue is that the current white LED materials substrate and growth technology do not produce LED devices that are uniform with respect to their color quality or efficiency. This in turn places an additional burden on the evaluation of individual devices: “Variability in lumen output, correlated color temperature (CCT), and forward voltage, is currently handled by testing
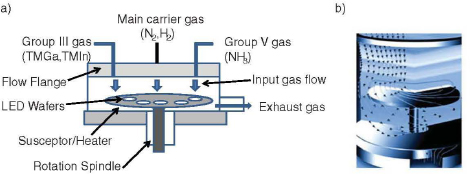
FIGURE 3.7 Schematic of an metal organic chemical vapor deposition system. Panel (b) is a three-dimensional diagram of the gas flow around and under the wafer stage. Panel (b) image courtesy of Veeco Instruments Inc. NOTE: TMGa = trimethyl gallium; TMIn = trimethyl indium.
each package and placing it into a specific performance bin” (DOE, 2009, p. 15). The technology of growth of LED devices and the choices of substrates for that growth form the early components of LED manufacturing and can have a profound “lever effect” with long-term implications for device yield and reliability (DOE, 2011b, p. 14).
There are two principal approaches to mitigation or elimination of the materials-related cost and performance issues. The first approach is to improve the uniformity of the epitaxial growth process. In the second approach, a fundamental breakthrough in native GaN substrate technology would allow the elimination of a vast number of crystal defects and would revolutionize the materials growth process. The two approaches are discussed below and build on an understanding of the MOCVD growth process, which is one of the steps in forming the LED devices.
Materials Growth: Mechanisms, Reactors, and Monitoring
As was made evident in the preceding section, the formation of the materials that comprise the LED plays a critical role in determining the color output and the efficiency of the device. Sensitive control over the composite layers of the crystalline LED device structure, some of which are only nanometers in thickness, is achieved through the use of epitaxial growth processes. In these processes, a single-crystal material (the overlayer) is grown on a crystalline substrate, and there is a registry, or relationship, between the structure of the overlayer and the substrate. The most commonly used process is MOCVD. These complex MOCVD machines are basically very sophisticated “ovens” used to produce the wafers that are later fabricated into individual LED chips. The typical MOCVD machine costs more than $2 million and can carry out growth on 60 2-inch wafers at a time.
Technology leadership in this field is still based in the United States (VEECO, Applied Materials) and Europe (AIXTRON). In MOCVD technology, ammonia gas and trimethylgallium (called a metal organic gas) are combined in a stainless steel growth chamber. In order for the reaction between ammonia and trimethylgallium to occur, forming the GaN material, the sapphire substrate must be heated to temperatures of about 1,000°C. This is done using a heated metal plate (called a susceptor). There are several possible configurations for this growth system; however, MOCVD growth based on a vertical rotating disk design has had broad acceptance. The generic rotating disk design is shown in Figure 3.7. The sample sits on the rotating disk, which is also a susceptor. Gases are injected vertically into and through a showerhead, and the high-speed rotating disk produces stable gas flow, aiding the uniformity of the material composition produced. The MOCVD technology can be used for all of the III-nitride materials (Ga, In, Al) utilizing a specific metal organic gas for each element (for example, trimethyindium for indium compounds and trimethyaluminum for aluminum compounds). Therefore, all of the elements of the LED structure can be grown in a single run. MOCVD technology has the following several advantages: (1) the ability to grow all of the III-nitride materials and alloys, (2) the ability to produce abrupt junctions between dissimilar regions of materials, and (3) the ability to produce thin (almost single atom layer) quantum well regions.
Prior research on MOCVD technology has established the fundamental understanding of reactor design and scale-up. Excellent numerical codes are available to simulate the gas flow and gas chemistry in the reactor. Therefore, scale-up in reactor size to accommodate larger substrates (and potentially lower-cost manufacturing) should be straightforward. The major challenge in MOCVD technology is control of this complicated growth process over the entire area of the substrate. Complicating the issues of MOCVD control and monitoring for the III-nitride materials is the substantial material differences between the overlayers (GaN LED structure) and the substrate (sapphire). The low thermal conductivity of the sapphire means that substrate and overlayer might not be at the same temperature during the growth process. It is therefore important to accurately measure the temperature of the surface of the growing material. The substrate and the overlayer have different thermal coefficients of expansion,
producing a strain at the interface between the substrate and overlayer that can result in a curvature or “bowing” of the wafer. The curvature is accentuated by the mismatch in lattice constant between the sapphire and the LED overlayers (see section below). This curvature in turn aggravates non-uniformity in temperature and the flux of materials seen by each wafer. The most important issue for the MOCVD growth of nitride LEDs is control of the growth temperature. Wafer temperature is important for determining the growth rate as well as the composition of the indium-containing layers in the structure. Even small changes in temperature are enough to change the optical emission (color) of the LED.
The technology that has been developed to accomplish monitoring of MOCVD is based largely on optical reflectivity. Early technology development was supported by DOE through Sandia National Laboratories (Sandia National Laboratories, 2004) and has resulted in a U.S.-based start-up company, k-space Associates, that manufactures MOCVD monitoring products. There is a similar company in Europe, LayTec, which manufactures similar technology. Both k-Space1 and LayTec2 have developed specific systems, which can in “real time” monitor either the wafer temperature or the growth rate or the curvature (hence the strain) of the growing layer. However, a complete picture of the state of the reactor, and therefore of the material being grown, requires information about all of these parameters. It is possible to incorporate several of these monitors into a single reactor, but this requires the careful design of the reactor to best accommodate and integrate the monitors. In addition, careful cross-calibration of the monitor outputs with the actual grown materials is required in order to extract meaningful data about the monitored growth conditions and the actual material characteristics. There are currently no systems, which can directly monitor the composition of the growing film. Initial efforts have been made to implement many of systems with feedback reactor control (Haberland, 2008). However, for commercial reactors only wafer temperature has been implemented as part of the control loop.
Current sapphire substrates are 4 inches in diameter. As the production reactors scale up to 8-inch-diameter sapphire substrates, the interrelated problems of substrate temperature, wafer bow, and change in materials composition can only get worse. In order to achieve the desired wafer yield, optical monitoring needs to continue to develop. Goals for future optical monitoring system include the following: (1) integration of growth rate and wafer bow measurements with reactor control in commercial systems, (2) development of tools to make real time composition measurements on the growing layers, and (3) development of tools that will provide full wafer maps of the important parameters (temperature, composition, strain, and growth rate).
FINDING: Production-scale MOCVD growth of LEDs is a complex process. The uniformity and yield of the structures grown (and hence of the optical performance of the LEDs) is strongly and negatively affected by small variations in the MOCVD growth process. The thermal and lattice mismatch between substrate and overlayer exacerbates the sensitivity of the growth process. Further difficulties of growth control are anticipated with use of substrates with increased diameter.
RECOMMENDATION 3-3: The Department of Energy should fund research to develop instrumentation for in situ monitoring and dynamic control of the metal organic chemical vapor deposition growth process.
The Search for an Improved Subslrale
Epitaxial growth processes work best when the substrate (e.g., sapphire) that serves as the template for the material growth has a structure (lattice constant) that matches that of the finally formed material. Without the one-to-one registry of the overgrown material to the template, there will be a strain in the overlayer that may eventually give rise to dislocations and defects in the material (107 to 108 cm-2 dislocations in the best case for GaN on sapphire). Such defects will compromise the performance and reliability of the devices formed from the material, and this in turn leads to “uncertainty in the long-term performance of the luminaire system” and “makes it difficult to estimate and warrant the lifetime of LED-based luminaires” (DOE, 2012a, p. 30).
Thus, a key issue in the growth of III-nitrides is the lack of a native or lattice-matched substrate. Currently, the substrates used for the III-nitrides are sapphire, SiC, or Si; at the moment, there are no GaN substrates of suitable size and quality available. Were they available, they might provide a better match to the overlayer III-nitride material, within the limitations imposed by different lattice constants for InGaN, GaN, and so forth. Besides improvement in device efficiency and reliability, a better thermal match between the substrate and overlayer would reduce wafer bowing, increasing the yield of fabricated devices.
From the discussion above it is easy to see that there is a great impetus for the development of GaN native substrates— among which is the growth of GaN bulk substrates. DOE is considering several competing technologies for substrates. There are a variety of approaches to the growth of GaN bulk substrates, which have been recently reviewed in a special issue of the Proceedings of the IEEE and in other journals (Avrutin et al., 2010; Ehrentraut and Fukuda, 2010; Paskova et al., 2010). Further progress in the formation of GaN bulk substrates has considerable technological challenges; furthermore, GaN substrates will have to compete with the lower-cost availability of (lattice-mismatched) substrates such as 8-inch sapphire.
________________
1 See, for example, Data Sheets KSA 400, KSA MOS, KSA Rate Rat Pro, and KSA BandiT (k-Space Associates, 2012).
2 See, for example, Application Notes 49, 53, 45, 50, and 34 at http://www.laytec.de/compounds-applicationnotes.html.
However, the committee believes that a breakthrough in native GaN substrate technology would allow the elimination of a vast number of crystal defects, revolutionize the materials growth process, and have profound benefits for LED efficiency, reliability, and yield. DOE workshop participants speculate that “in principle, the use of a GaN substrate, if it were available at reasonable cost, might simplify the buffer layer technology (thinner buffer layers with shorter growth times) and allow flat, uniform epiwafers to be manufactured” (DOE, 2012a, p. 35.).
FINDING: Significant improvements in LED efficiency, yield, and reliability are possible by using GaN substrates and latticed-matched epitaxial growth processes. Currently, there are no viable techniques for producing high-quality, low-cost GaN substrates. While realization of low-cost GaN substrates is not assured, the potential payoff of this research is immense.
RECOMMENDATION 3-4: The Department of Energy should make a long-term investment in the development and deployment of gallium nitride substrates.
CHALLENGES AND PROMISES FOR LEOs
The development of III-nitride LED technology has brought many surprises to the semiconductor community. Never before have production devices been formed in a materials system in which the light-emitting layers were produced on non-native substrates with thermal and lattice mismatch. Although GaN-based devices have worked well enough to initiate a lighting revolution, materials issues have re-emerged as defining elements in the technology. As has been discussed above, current devices suffer from a high concentration of defects and dislocations that limit the internal quantum efficiency achievable.
The DOE (2011a) roadmap goals relating to device efficiency, shown in Table 3.1, can only be achieved by substantial improvements in the control and quality of the materials growth and in the reduction of defects that arise through the growth and fabrication processes, which are aggravated by the strain between substrate and overlayers. Moreover, improvements in the basic technology that forms the starting materials of the LEDs will have a profound feed-forward effect that will influence yield, and thus cost, at every stage of the LED package formation and performance. For example, strain between the substrate and the overlayer results in the non-uniformity of LED characteristics across wafers, leading to the wasteful practice of “binning.” Fluctuations in the composition of the LED layers, and particularly in the quantum well region, compromise control over the LED emission wavelength. Defects have an impact on the electrical resistance of the LEDs, increasing power dissipation and limiting higher-temperature performance, as well as lifetime. Limitations at the device level necessitate compensating solutions (e.g., heat sinking) at the packaging level, which may increase the overall cost. For example, Krames et al. (2007) have calculated that an improvement in IQE from 2010 values (Table 3.1) to 2020 values could result in a fourfold reduction in the amount of wasteful heat generated in a 70 lm/W device. The ancillary issues of increased device lifetime and reliability will also have an impact on cost.
TABLE 3.1 Internal Quantum Efficiency Values of Light-Emitting Diodes
| Metric(s) | 2010 Status | 2020 Target(s) |
| IQE at 35 A/cm2 | 80% (blue) |
90% (blue, green, red) |
| EQE at 35 A/cm2 | 64% (blue) |
81% (blue, green, red) |
| Power Conversion Efficiency @ 35 A/cm2 | 44% (blue) |
73% (blue, green, red) |
| Relative EQE at 100 A/cm2 versus 35 A/cm2 (droop) | 77% | 100% |
SOURCE: DOE (2011a, Table 2.1, p. 71).
Thus, investments in improving the control and uniformity of the epitaxial growth process can have a profound effect on long-term device performance, reliability, and cost. Improvement in the cumulative manufacturing yield of the LED module, currently in the range of 50 to 70 percent to more than 95 percent, will further lower the cost and improve the quality of SSL. But although not directly shown in the projection of LED package costs (Figure 3.8), improvements in the cumulative yield will benefit enormously from improvements in the earlier part of the manufacturing process, such as improved uniformity in the epitaxial process. These improvements will exercise a “lever” effect on the cumulative yield and have a large impact on the final device cost and selling price through improved binning yield (DOE, 2011b).
FINDING: LED efficiency and performance is still limited by materials issues. Improvements in efficiency at the device level, as targeted by the DOE SSL roadmap, will have a “lever effect,” influencing design, performance, and cost of the luminaires. Improvements in efficiency and performance are linked to further fundamental investigations in core technology on emitter materials.
RECOMMENDATION 3-5: The Department of Energy should continue to make investments in light-emitting diode core technology and fundamental emitter research. Its portfolio of investments in these areas should be extensive enough to ensure that the targeted goals of device performance can indeed be met.
| Unit | 2010 | 2012 | 2015 | 2020 |
| Price ($/klm) | 18 | 7.5 | 2.2 | 1 |
| Efficacy (lm/W) | 96 | 141 | 202 | 253 |
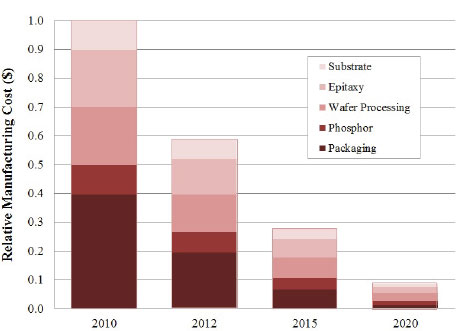
FIGURE 3.8 Light-emitting diode package cost trends. SOURCE: DOE (2011b).
OLEDs are a new source of illumination wherein light is emitted uniformly over a large planar surface. They are primarily deployed today in very large numbers for displays on handheld appliances such as smart phones. The excitement surrounding OLED technology stems from several unique aspects of its manufacture and performance. They are inherently ultrathin film devices that can be deposited on any smooth substrate such as glass, flexible metal foil, or even plastic, and the devices themselves have very high performance: 100 percent internal quantum efficiency, custom tunable color from the blue to the near infrared, and extremely low temperature rise, even when operated at very high brightness. In contrast to the inorganic semiconductor materials used for LEDs, organic materials are predominantly carbon-based, much the same as inks used in printing or dyes used to color fabrics. Hence, in principle they are abundant, inexpensive, and may have limited negative environmental impact. In addition, the materials used in fabricating OLEDs are used in very small quantities and are deposited over large areas using low energy consumption processes owing to their low sublimation temperatures. While there are currently no significant concerns regarding the toxicity of materials used in OLEDs and their packages, the committee can well appreciate that for any technology, what can begin as minor concerns can become more significant as volume of deployment increases. Hence, it may be necessary to monitor the potential negative toxic and environmental effects that OLED lighting may have as the technology becomes adopted to ensure that risks associated with their use is minimal.
The OLEO Device Structure and Operation
The first organic light-emitting device was demonstrated in the 1960s by Pope et al. (1963) and later by Helfrich and Schneider (1965). Sandwiching the organic material anthracene between contact electrodes, blue light was emitted at a relatively high efficiency (a few percent). Unfortunately, the voltage required was very high (~500 V). This situation changed dramatically in 1987 with the first low-voltage OLED. With an efficiency of approximately 1 percent, the voltage was dropped to <10 V, suggesting that a new and potentially efficient light source had been demonstrated (Tang and VanSlyke, 1987). While their first commercial applications of OLEDs have been in ultrathin, full color displays, their currently extremely high efficiency has led laboratories worldwide to explore their applicability as lighting sources.
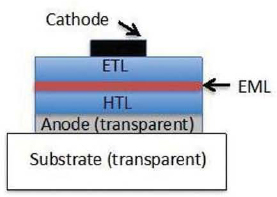
A simplified OLED structure is shown schematically in Figure 3.9, where the nomenclature used is typical of that used in OLEDs. Here, “ETL” is the organic electron
FIGURE 3.9 Archetype organic light-emitting diode structure. SOURCE: Willner et al. (2012). ©IEEE (2012). Reprinted, with permission from Proceedings of the IEEE.
transport layer that moves electrons from the cathode metal contact to the light emissive layer, or “EML.” This layer is typically composed of two different molecules, a charge conductive “host” molecule into which is doped a molecule at very small concentration (~1 to 8 percent by weight) that gives off light of the desired color (or wavelength) under excitation from electrons and holes in the device. This dopant is called the light emissive “guest.” The “HTL” is the hole transport layer whose purpose is to transport positively charged “holes” from the anode contact to the EML. The transparent conducting anode through which the light is viewed is invariably composed of ITO, and the cathode is a metal (such as aluminum doped with lithium) capable of forming an ohmic contact with the ETL for the efficient injection of electrons. Typical OLED structures used in high-efficiency and high-reliability applications are considerably more complex than the structure shown in Figure 3.9. However, in all cases, the total thickness of organic layers rarely exceeds 100 nanometers (1 nanometer = 10–7 centimeter) (Willner et al., 2012). The committee also notes that in contrast to LEDs, OLEDs can be made integral to the luminaire rather than being added to it, in contrast to all alternative lighting solutions. This structural adaptability provides new design possibilities for SSL.
The mechanism for light emission in organic, thinfilm OLEDs (Box 3.2) is fundamentally different than in inorganic semiconductor LEDs described earlier in this chapter. When an electron and its oppositely charged counterpart, the hole, are conducted to the same molecule within the EML, they put the molecule into an excited state. This excitation is maintained for a brief period of time (from nanoseconds to microseconds). While it exists, the excitation can hop from molecule to molecule, which are very densely packed within the EML. This mobile excitation (called an “exciton”) eventually decays by the recombination of the electron and the hole (i.e., the electron “falls into” the hole that is located on the same molecule as the electron). This decay process often emits light whose energy is equal to that of the difference in energies between the electron and hole. By changing the composition or structure of the molecule, the wavelength (color) of light emission can be varied. In fact, small chemical modifications can change the color emission from the ultraviolet, through the blue and green, to the red. In all cases, the light emission can be extremely efficient, with 100 percent conversion of electrons to photons having been reported across the visible light spectrum (Willner et al., 2012).
In a manner similar to the calculation of the EQE of an inorganic LED, the EQE of an OLED depends on both an intrinsic efficiency, for the material and device, and an outcoupling or extraction efficiency, where
![]()
where ɸ is the absolute efficiency of a molecule to emit light once excited, Y is the probability that every injected electron and a hole can simultaneously exist on a light-emissive molecule, ƞout is the outcoupling efficiency to be discussed below, and X is the ratio of emissive molecular excited states that an electron and hole can reside on in a single molecule to the total number of possible excited states. X is also known as the excited state ratio. For the best emissive molecules, ɸ = 1, which is often the case with state-of-the-art materials. Furthermore, Y = 1 in properly engineered device structures.
The power efficiency (ƞP) of the light source is its most important operational parameter. Here the optical power out per the input electrical power is related to the quantum efficiency following the formula:
![]()
Here, ɵ is the overlap of the light source with the spectral sensitivity of the eye, and Vλ is related to the energy of the emitted photon. The operating voltage of the OLED is V— clearly the power efficiency decreases as V increases. For a given device geometry, the operating voltage is related to the device drive current and thus also has an important influence on the device lifetime.
In conventional OLEDs fabricated on glass substrates, through mechanisms similar to those in inorganic LEDs, much of the emitted light is trapped within the glass substrate or absorbed in the layers that comprise the device (see Figure 3.10), resulting in an extraction or outcoupling efficiency of only ~20 percent. However, low-cost schemes have been reported that can increase this efficiency to 40 to 60 percent (see below). Nevertheless, one of the grand challenges facing OLEDs is how to extract more of the emitted light in a cost-effective and highly efficient manner. This will
BOX 3.2
How Light Is Emitted in OLEDs
Shown in Figure 3.2.1 is a pictorial view of the light-emitting layer in an organic light-emitting diode (OLED). This layer is typically sandwiched between electron and hole transporting layers. The blue background represents the thin film that is comprised of a molecular species that transports the charges injected from contacts at the boundaries of the OLED itself. The red dots are the dopant molecules that are interspersed at low density within the charge transporting matrix These dopants can either be fluorescent moleaJles or phosphorescent molewles Ftlosphorescent molecules can produce devices with the highest internal quantum efficiency. The inset on the lower left shows a typical phosphor molecule. It can be very inexpensive and is only used in trace amounts. Ultimately, it consists of carbon, nitrogen, and hydrogen atoms (open circles) that are bonded together (lines) along with a heavy metal atom (typically iridium) in its center (red dot). Light emission occurs when an electron injected from the cathode travels to the same molew Ie as the ho Ie (positive charge) injected from the anode, forming a IllJ bile excitation or “exciton.” Light is then generated when the electron and hole (or exciton) recombine on the edges of the dopant molecule. This emission process is depicted by the yellow burst around the dopant molecule in the emitting region. By varying the structure of the molecule, the entire visible and near-infrared spectra can be accessed.
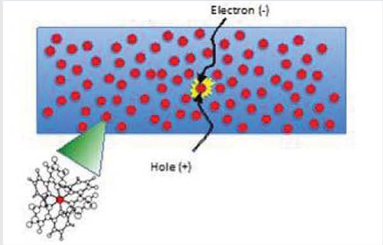
FIGURE 321 Pictorial view of the light--emitting layer of an OLEO
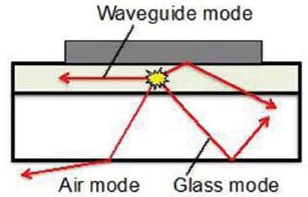
FIGURE 3.10 Illustration of the optical pathways taken by a photon following emission from a luminescent molecule (shown as yellow star).
be discussed further in the section on necessary technology developments.
Finally, the excited state ratio is X = 0.25 for fluorescent emitting molecules, and X = 1 for phosphors, as will be discussed in the following section (Baldo et al., 1999b). Putting all of the efficiencies together, it is demonstrated that ƞEQE is 20 to 60 percent in the very best cases. Even with these limitations, the power efficiency of phosphorescent white organic light-emitting devices can exceed 150 lm/W, making them especially attractive for use as efficient lighting sources.
CONTROLLING THE COLOR OUTPUT OF THE OLED
For OLEDs, changing the composition of the molecular components of the material influences the wavelength (color) of the light emitted. White light is generated by mixing red,
green, and blue emission from different regions of the OLED. That is, the emission spectrum of a particular molecule is insufficiently broad to efficiently generate the desired broadband white radiation to produce high-quality illumination, so the use of several different molecular species within the emission region of the OLED is required to achieve the desired white spectrum.
A given molecule within an EML will emit with a welldefined spectral shape. Hence, unlike the case for inorganic LEDs, binning is not needed to select those devices that emit at the appropriate wavelength. However, the white balance or chromaticity is ultimately determined not only by the welldefined spectra of the constituent molecules in the EML, but also by the details of the device structure, which may vary from run to run. Hence, several schemes have been developed for lighting applications that are both efficient and have a stable, predictable, and highly controllable white chromaticity. The highest performance is achieved using a variant of one of the three designs shown in Figure 3.11—the striped, white OLED (WOLED), the fluorescent/phosphorescent (F/P) WOLED, and the stacked WOLED (SOLED). The latter design is most effective in achieving long lifetime and high brightness and can be combined with the F/P design as well as others for illumination purposes.
The simple design of the striped WOLED places stripes of red (R), green (G), and blue (B) PHOLEDs (phosphorescent OLEDs) side by side. The R-G-B pattern is repeated on a very small scale so that the separate colors cannot be resolved by an observer. By injecting current into each stripe, the viewers will perceive the mixture of the three primary colors, which will appear white. An advantage of this design is that each of the three color elements can be separately optimized to emit with 100 percent internal efficiency, and variation of the current through each of the elements can be used to tune the color, from their constituent color to any desired white chromaticity. A disadvantage is the complexity of driving the WOLED with three different current sources.
The F/P WOLED is based on the recognition that approximately 25 percent of the color content of white light is blue. To achieve lower voltage operation and perhaps longer lifetime, which is currently limited by phosphorescent blue EMLs (see Box 3.3), and because 25 percent of the injected charge forms fluorescent states, this device uses a fluorescent blue segment and harvests the remaining green and red excited states using phosphorescent (i.e., heavy-metal-atom containing) molecular compounds. In principle, this particular device has the lowest drive voltage and hence highest efficacy of all alternative architectures. The F/P design can also be incorporated into stacked and striped architectures. Hence, the device still achieves 100 percent internal quantum efficiency because all excitons are harvested by a combination of blue fluorescent dopants and red and green phosphors.
The compact SOLED design stacks two or three white-emitting segments, with each segment separated by a very thin and transparent “charge generating layer.” In this case, a single injected electron can recombine with a positively charged hole in each segment, generating a photon. Thus, a 2 to 3 times higher quantum efficiency is achieved with this device compared to the other designs, but at 2 to 3 times higher voltage (where the multiplier is equal to the number of elements in the final stack). Hence, the efficacy of this device is no higher than that of the other designs shown, but there are significant benefits of increased device lifetime.
For example, the SOLED of Figure 3.11 comprises a B PHOLED as one stacked element and an R-G PHOLED as the second element. Other examples of SOLEDs include a complete white-emitting phosphorescent R-G-B EML in each element. This three-element SOLED is known as a W-W-W SOLED, as shown in Figure 3.11.
Finally, the committee notes that there are several other approaches to generating white light. Two alternatives that are often pursued are to use very broadly emitting white
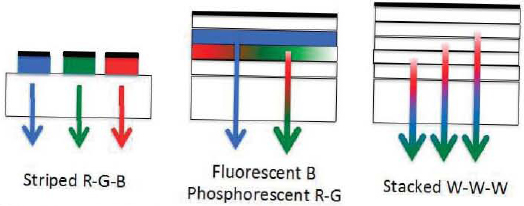
FIGURE 3.11 Three examples of white organic light-emitting diode designs.
According to Equation 3.2, the white organic light-emitting diode (WOLED) efficacy (i.e., power efficiency) is inversely proportional to the total voltage dropped across the device. Low-voltage operation is, therefore, desirable for high efficacy (and also results rn lower electrrcal power drssrpatron), because the conductivity of organic materials is low, this requires the use of thin device layers. Unfortunately, such thin layers can also decrease device yields, because any flaws in the film (e.g., pinholes, clusters, etc.) can lead to shorts between the closely spaced electrodes (typically, the organic layers are ~100-200 nm thick in total). A particular constraint for low voltage operation resides with OLEDs emitting in the blue, requiring at least ~3.5 V to excite the emitting (dopant) molecule. To transfer the excitation from the host material to the dopant molecule requires ~3.5 V for fluorescent emission in the blue, but >4 V to achieve phosphorescent emission in the blue. As discussed earlier, the excited state ratio, X is only 0.25 for fluorescent-emitting molecules, but fortuitously, only 25 percent of the color content of white light is blue. Thus using fluorescent molecules for blue OLED emission allows a lower-voltage operation.
Another effective means for decreasing voltage is to increase the conductivity of the charge transporting layers usrng conductrvrty-enhancrng dopants (Blochwrtz et al., 1998; D’Andrade etat, 2008, Pferffer et al., 2002). Thrs strategy has been successfully pursued by Novaled and the COMEDD Fraunhofer Institute in Dresden, Germany.
OLEDs (e.g., emission from a single molecular species that spans an unusually broad spectrum or uses a blue OLED to “pump” red and green organic phosphors located external to the OLED pump). Unfortunately, in both cases the efficiency is considerably less than direct PHOLED emission and, hence, are generally not viewed as adequate for meeting the stringent demands of advanced SSL sources.
All current significant manufacturing of OLEDs employs small-molecular-weight organic materials. These materials consist of a single molecular unit with a well-defined number of constituent atoms. This is in contrast to polymers that are chains of units of indeterminate length and, hence, number of atoms. Polymers are typically used in plastics, whereas small molecules are used as pigments in common dyes for clothing, ink-jet printing, and so on. Small-molecular-weight organic compounds are commonly deposited from the vapor phase (i.e., either vacuum thermal evaporation (VTE) or organic vapor phase deposition (OVPD); Shtein et al., 2002), although, like polymers, they can also be deposited from liquid solution.
Note that the demands placed on the deposition process (and tools) are quite high. As discussed above, a typical high-performance OLED display consists of at least five layers, with the thickness of the entire stack seldom exceeding 100 nm. White-emitting OLEDs used in lighting applications have at least double this number of layers. Hence, deposition must occur over very large substrate surfaces (exceeding 1 m2 in production environments), with minimum individual layer thicknesses of ~5-10 nm. To maintain device performance uniformity, layer thickness variations of only a few percent are tolerable across the entire substrate surface. Fortunately, in-line VTE and OVPD have been proven to achieve these demanding specifications in display production facilities, suggesting that such targets are realizable for lighting as well.
To produce high-quality, long-lived devices, it is imperative that the organic materials, most of which are easily synthesized using well-known methods, be highly purified prior to use. That is, small concentrations of molecular impurities can lead to rapid degradation in device performance, and hence considerable steps must be taken to ensure their purity. High purity is achieved by evaporation of the volatile (and lightweight) impurity molecules in a vacuum system that exhausts the volatile evaporants.
The layering of numerous materials with different functions needed to confine both charges and photons (see Figure 3.9) is a relatively simple task when deposition occurs from the vapor phase, and given that the process generally occurs in vacuum, this too aids in the prolonged operational lifetime of the OLEDs. As will be discussed below, extended lifetimes, particularly of the blue emitting molecules, remains a challenge.
In addition to polymer and small-molecular-weight OLEDs, as mentioned in the section “How Light Is Emitted,” there are two systems of emissive dopants available: those that emit from fluorescent molecules and those from phosphorescent molecules. Note that while fluorescent devices (Tang and VanSlyke, 1987) can have a theoretical maximum emission efficiency of only 25 percent (because ÷ = 25 percent in Equation 3.1, in this case), phosphorescent devices can have 100 percent internal quantum efficiency. This implies that PHOLEDs are ideal for both displays and lighting. Following the demonstration of “electro phosphorescence” using metalorganic compounds (Baldo et al., 1998, 1999a) in 1998, the materials have found widespread adoption in all OLED devices currently introduced into the market.
As shown in Table 3.2, extremely high-efficiency emitting molecules (with 100 percent IQE) are available for emission across this entire spectral region.
Several other materials are used in high-efficiency PHOLED structures beyond the simplified design shown in Figure 3.9, including the conductive host, the electron and
TABLE 3.2 Representative Commercial Phosphorescent Molecules and Their Corresponding PHOLED Performances
| Operating Lifetime (hours) | ||||
| PHOLED Performance at 1000 cd/m2 | CIE 1931 Chromaticity Coordinates | Luminous Efficiency (cd/A) | L95 | L50 |
| Deep red | (0.69,0.31) | 17 | 14,000 | 250,000 |
| Red | (0.69,0.34) | 24 | 25,000 | 600,000 |
| Red | (0.64,0.36) | 30 | 50,000 | 900,000 |
| Green-yellow | (0.46,0.53) | 72 | 70,000 | 1,400,000 |
| Green | (0.34,0.62) | 78 | 18,000 | 400,000 |
| Light blue | (0.18,0.42) | 47 | 600 | 20,000 |
NOTE: Results are for bottom-emitting structures (with no cavities). Lifetime data are based on accelerated current drive conditions at room temperature without any initial burn-in. L95 and L50 are the time to which the luminance has decreased to 95 percent and 50 percent, respectively, of initial values.
SOURCE: Universal Display Corporation (undated).
hole transporting molecules, and the exciton blocking layer (EBL). This EBL layer, positioned at the cathode-side of the EML, is required in PHOLEDs because of their long excitedstate lifetimes and corresponding diffusion length. The EBL prevents diffusion of the excited states to the cathode contact where they may quench before they can radiatively emit light. Hence, the use of an EBL has greatly increased the efficiency of PHOLEDs such that 100 percent IQE is routinely obtained using optimized materials sets.
KEY ISSUES FOR IMPROVED DEVICE PERFORMANCE
Perhaps the largest efficiency gain that has yet to be achieved is through increased light outcoupling from the substrate. As noted in Equation 3.1 and the ensuing discussion, only 20 percent of the light emitted by the WOLED is coupled into the air and is, therefore, viewable if the device is deposited on a conventional glass substrate. The remainder is trapped in the glass, or is absorbed by the materials that comprise the device, as shown in Figure 3.10. Outcoupling of light in OLEDs is particularly difficult when compared with LEDs because of the large areal dimension and integrated form factor of the OLEDs. That is, they are “area” rather than “point” lighting sources, whereby a large surface area must be coated with emitting materials to generate the desired level of illumination. As noted above, this is a generally desirable feature because the lighting source (the OLED) and the luminaire form a single integrated unit. Yet, it also poses challenges because there is little access to the light trapped within the substrate and emissive layers. Numerous outcoupling efforts have therefore explored ways of costeffectively harvesting a greater proportion of the trapped light (Wang et al., 2011).
There are three principal optical pathways an emitted photon can take. The first is the air mode: i.e., the light that escapes from the substrate and can be viewed as useful light. As noted above, only 20 percent of the light is emitted into air modes because of total internal reflection when conventional, low-cost glass substrates are employed. A large remaining fraction (again about 20 percent) of the light is trapped in the glass substrate (glass modes). And finally, 33 percent of the light is emitted along the plane of the organic thin films, forming waveguide modes. Other losses due to absorption in the organic or transparent conducting oxide anode layers, as well as excitation of so-called plasmons at the metal cathode surface, can also lead to reductions in efficiency.
Because of the relative sizes of the effects and ease in modal access, most efforts have been directed at eliminating glass and waveguide modes. The effective elimination of these losses can result in a tripling of the external efficiency from 20 to 60 percent.
Any practical solution of the outcoupling problem must be extremely low cost to implement, and it must not affect the wavelength or angular intensity distribution of the emitted light. With these considerations in mind, glass modes are most effectively eliminated by the attachment of microlens arrays onto the substrate/air surface (Möller and Forrest, 2001; Sun and Forrest, 2008). Microlenses are typically 5-10 μm diameter transparent hemispheres that can be made by molding into large plastic sheets (see Figure 3.12, for example). This omnidirectional, wavelength-independent solution has been shown to increase the efficiency of the OLEDs by nearly a factor of 2 (with external efficiencies as high as 40 percent measured). Alternative solutions for extracting glass modes include roughening of the glass surface, placing OLEDs on the surfaces of plastic blocks with tapered edges, or using high index of refraction plastic substrates and a large hemispherical lens.
Extracting waveguide modes (i.e., that light emitted within the organic layers themselves and propagating in the plane of the substrate) that consume 33 percent of the emission is more difficult. It needs to be done very near to the point of emission (i.e., at the location of the excited molecular dopant) to avoid losses due to waveguiding within the absorptive organic or transparent conducting oxide anode layers. Such solutions, therefore, must be integrated into the
FIGURE 3.12 Polymer sheet of 5 ìm diameter microlenses attached to the glass surface of an OLED. Outcoupling enhancements of a factor of 2 are possible using this approach. SOURCE: Sun (2008). Reprinted by permission from Macmillan Publishers Ltd.
OLED structure itself without degrading device performance in other, unintended ways.
Generally, to couple waveguide modes into the glass or air, there must be a surface texture inserted at the transparent anode/organic interface. The length scale of the texture cannot be on the order of the emission wavelength; otherwise, an undesirable angular dependence of emission wavelength (i.e., color) and/or intensity may result. Low-index grids consisting of a dielectric such as silicon dioxide residing at this interface have been shown to outcouple almost all of the waveguide modes without significant losses (Sun and Forrest, 2008). The openings in the grids are typically 5 μm, with grid lines of only 1 μm. Combining the grid with the microlenses shown in Figure 3.12 has resulted in the demonstration of 34 percent external efficiency.
FINDING: A number of promising approaches have been developed to increase outcoupling efficiency.
RECOMMENDATION 3-6: The Department of Energy should focus on efforts that result in significant light outcoupling enhancements for OLED that are low-cost to implement and are independent of both wavelength and viewing angle.
As in the case of LEDs, OLEDs also suffer a loss of efficiency as the current (and corresponding brightness) is increased. This is readily apparent in Figure 3.13 where the external quantum efficiencies of archetype fluorescent and phosphorescent devices are shown as functions of drive current. This droop is fundamentally related to the molecular excited state (exciton) that, when de-excited, emits light. At very high intensity, a substantial fraction of the emitting molecules in the EML are excited. When the excitation migrates from molecule to molecule, it has a possibility of colliding with another excitation on the same molecule or on an electron or hole that is transiting the EML. This collision results in the loss (or de-excitation) of one of the two excited states, ultimately resulting in the loss of efficiency. This process is known as “exciton annihilation.” Importantly, this same process leads to the degradation of the molecules and hence a decrease in OLED operational lifetime, as discussed below. Hence, it is essential to find device architectures that minimize exciton annihilation processes. One method to effect this is, for example, extending the thickness or grading the dopant concentration within the EML. However, little work has been done to date to reduce or even eliminate droop.
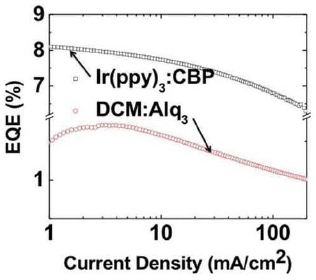
FIGURE 3.13 Efficiency droop in phosphorescent OLEDs (black rectangles) and fluorescent OLEDs (red circles).
One important difference between OLEDs and LEDs, however, is that in the former case, there is no thermally driven droop effect. That is, as the temperature is varied, the efficiency characteristics in Figure 3.13 are largely unaffected.
FINDING: OLEDs show a decrease in efficiency as the current is increased. This results in a reduction in efficiency at high brightness.
RECOMMENDATION 3-7: The Department of Energy should support research to understand the fundamental nature of efficiency droop at high currents in organic lightemitting diodes and to seek means to mitigate this effect through materials and device architectural designs.
ISSUES FOR OLEO DEVICE RELIABILITY AND MANUFACTURING
As in all electronic devices, there are numerous sources of OLED degradation that limit the operating lifetime of the lighting source. These mechanisms fall into two categories: extrinsic and intrinsic, or fundamental. Typically, the usable lifetime of a device is indicated by the time to which the light output has dropped to a given percentage of initial luminance (Lo) (see Table 3.2). Typically, lighting requires qualification to at least the so-called L70 limit set forth in the industry standard, LM-80, issued by the Illumination Engineering Society (2008). For displays, a differential luminance loss of only 10 percent on a highly used area of the display field is easily perceptible, rendering the appliance useless. Once again, experience being gained in the large-scale deployment of reliable displays has promoted the advances in lighting applications as well. However, in the case of white light sources, their exceptionally high surface brightness (typically 3,000 cd/m2 compared to 100 cd/m2 required for displays) places additional stress on the devices that results in a shortened lifetime. Furthermore, the differential aging of one color component versus another leads to perceptible and unacceptable shifts in the CRI or luminaire color temperature over time.
The primary extrinsic source of aging in OLEDs is degradation of materials and cathode interfaces due to exposure to moisture and oxygen (Burrows et al., 1994). For this reason, packaging of OLEDs is important in controlling the local environment. OLEDs are sealed against ambient ingress by packaging in an ultrahigh purity nitrogen environment. A conventional package consists of a glass substrate and a metal back cap that has a slight recess filled with a dessicant such as BaO to scavenge any residual oxygen or moisture in the package. The seal is typically made using a bead of UV-curable epoxy. Although it is by no means a perfect seal, the long-term degradation of most OLEDs is extended to well within acceptable industrial standards. Other routes to extrinsic failure include incorporation of impurities from source materials and chemical reactions in the guest-dopant conductive layer. All such extrinsic mechanisms can be reduced to acceptable levels through the proper handling and purification of source materials and by careful selection of materials sets for a particular OLED structure.
FINDING: The lifetime of OLEDs is very sensitive to extrinsic factors such as exposure to air and moisture. The low-cost fabrication of large area OLED lighting sources requires a high degree of fabrication competency that can ensure package hermiticity along the entire large package periphery and scavenge excess water and oxygen that might have been enclosed during the package manufacture.
RECOMMENDATION 3-8: To create a highly environmentally robust organic light-emitting diode (OLED) lighting technology, the Department of Energy should invest in materials and packaging technologies that make OLEDs resistant to degradation over their long operational lifetimes. In particular, important areas for investment include finding low-cost means to eliminate glass as a primary package constituent, devising molecules and device architectures that are resistant to degradation on exposure to atmosphere, and developing sealing technologies that are fast, precise, and robust to bending.
As can be observed in Table 3.2, both red- and greenemitting PHOLEDs used in analogous display applications have lifetimes of several hundreds of thousands to millions of hours. Devices emitting with these colors, therefore, significantly exceed the lifetimes required for practical lighting sources. However, blue PHOLEDs have a significantly shorter lifetime. Early blue failure is not due to environmental factors, but rather to properties intrinsic to organic molecules (Giebink et al., 2008). The principal intrinsic failure mode is excited state-charge and excited state-annihilation reactions that occur when, at the molecular scale, a charge ends up on an excited molecule (Giebink and Forrest, 2008)—a process similar to that responsible for a decrease in quantum efficiency as current is increased (see Figure 3.13). Today, the useful life of blue phosphorescent devices is only approximately 10,000 to 20,000 hours (Table 3.2), also setting the limit for the lifetime of white lighting sources. Fluorescent blue-emitting materials have somewhat longer lifetimes because of the very short existence of the blue fluorescent excited state (~nanoseconds) compared to that of blue phosphorescence (~tens of microseconds). Hence, the less efficient fluorophores support annihilating collisions with charges and other excited states for durations that are far shorter than for phosphors, increasing the operational lifetime of the device.
One significant challenge that must be overcome to ensure the widespread deployment of WOLEDs for lighting, therefore, is to increase the lifetime of the blue-emitting element to hundreds of thousands of hours. As noted, use of fluorescent blues is one avenue for improvement, as is designing phosphorescent blue molecules that have shorter excited state emission times. Further techniques might include extending the thickness of the blue EML, thereby reducing the density of excited molecules at high brightness. Indeed, the SOLED architecture does this effectively by distributing several of the blue-emitting regions within the several elements in the stack. Finally, white light does not require the use of deep blue (i.e., high-energy) emission. Rather, light blue (cyan) emission is desirable for this application. Because this is a lower energy of emission, the problem is partially resolved simply by the judicious choice of blue-emitting wavelength. Nevertheless, more rapid degradation of blue intensity versus red or green inevitably leads to color shifting during the
operational lifetime of the WOLED that must ultimately be minimized.
To summarize the operational lifetimes of both single element and stacked OLEDs, a comparison is shown in Figure 3.14. The advantage to using a SOLED that distributes the EML between elements, while reducing operating current to achieve a desired brightness, is readily apparent.
Finally, the committee notes that elevated temperature can significantly reduce the OLED operational lifetime. At surface luminances of 8,000 cd/m2, it has been found that a 10°C increase in temperature reduces the lifetime by as much as 30 percent (see panel a of Figure 3.15). Fortunately, because OLEDs are highly distributed lighting sources, their temperature rise during operation is minimal (Levermore et al., 2012). Indeed, with proper packaging, the rise in temperature even at high surface luminances of 3,000 cd/m2 can be less than 1°C using only natural convection present in the ambient surrounding the fixture, as shown in panel b of Figure 3.15.
FINDING: OLEDs are area light sources, and their rise in temperature, even at the highest drive currents (and hence brightness), is minimal. This is a major distinction from LEDs, which are intense point light sources and, hence, operate at high temperatures that require extensive heat sinking and care in their installation. Nevertheless, OLED operational lifetime is very sensitive to temperature increases. As the room temperature rises, the OLED lifetime can be expected to be noticeably decreased.
RECOMMENDATION 3-9: The Department of Energy should support the pursuit of material sets and device architectures that would increase the useful operational lifetimes of high-intensity white organic light-emitting diodes.
FIGURE 3.14 Comparison of lifetimes of three different white PHOLED emitters at two different surface luminance intensities. SOURCE: Courtesy of Universal Display Corporation.
There is as yet no large-scale manufacture of OLED lighting; however, major growth in OLED display technologies may provide both infrastructure and cost reduction and, thus, important incentives for the further development of manufacturing for OLED-based SSL. As of this writing, one company alone, Samsung Mobile Displays (SMD), is producing 30 million such displays per month, with plans to scale these devices to larger, three-dimensional displays. SMD’s major competitor in this space is LG Display, along with a handful of other display companies in Asia of varying
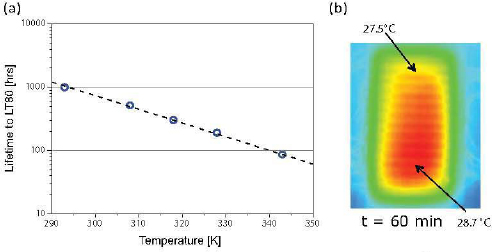
FIGURE 3.15 Time to LT80 for a white PHOLED with an initial surface luminance of Lo = 8,000 cd/m2. (b) Infrared image showing the surface temperature of a 7 cm × 15 cm panel as in (a) after 60 min operation in a 24°C ambient temperature. SOURCE: Levermore et al. (2012).
sizes. All of this manufacturing, today occurring on Gen 5.5 (1,300 × 1,500 × 0.6 mm) mother glass substrates, is resulting in a precipitous decrease in the cost of OLED technology, while increasing performance, as the industry grows, thereby positioning these early-entry companies to become competitive in producing low-cost, ultrahigh efficiency, easily dimmable OLED sources for the consumer lighting market. Indeed, several companies are concerned only with the lighting applications of OLEDs (i.e., not their uses in displays), such as General Electric, Osram, Moser-Baer, and Philips.
Equipment makers, providing key infrastructure that is required to provide a strong growth in manufacturing, are also starting to take notice of the possibilities for large, developing markets in OLED displays and lighting. Chief among the OLED manufacturing equipment suppliers is Aixtron, SE, the largest producer of MOCVD equipment for LED lighting, which also produces (on still a small scale) organic vapor phase deposition systems for OLEDs. Applied Materials is the world’s largest supplier of equipment for low-temperature polysilicon deposition on glass substrates used as active matrix display drivers (Nathan et al., 2005), and its division Applied Films supplies in-line deposition sources for front-plane OLED display materials deposition. The committee notes, however, that the current lack of a complete tool set for manufacture of OLEDs remains a limiting factor in their widespread and low-cost deployment as lighting sources.
Based on the foregoing discussion, phosphorescent WOLEDs provide an unusual opportunity to complement LEDs as an important solution for areal SSL. Yet there remain significant barriers to their adoption, and as a result their development still lags that of inorganic LEDs as the preferred white illumination source
The committee’s findings and recommendations on the major challenges that should be the focus of near-term investment are given below.
FINDING: This is potentially the single most important metric to meet in OLED lighting. It requires simplification of device structure, use of ultralow-cost substrates such as metal foils, development of replacements for costly transparent anodes (current technology is indium tin oxide), low-cost encapsulation technologies, and so on. Also, investment in equipment infrastructure is essential for the success of low-cost, manufacturable products. In-line vacuum deposition sources, roll-to-roll processes on flexible substrates, ultrahigh-speed organic vapor phase deposition, and in situ encapsulation techniques will all require substantial infrastructure development.
RECOMMENDATION 3-10: The Department of Energy should aggressively fund the development of all possible routes leading to significant (100×) cost reduction in organic light-emitting diode lighting sources.
FINDING: Extending the lifetime of blue phosphorescent OLEDs is a primary area where investment will have substantial payoff. It involves a combination of advances in the development of new materials, device architectures, encapsulation, and contact technologies, as well as a fundamental advance in the understanding of degradation processes. Interactions between the phosphor and the conductive host will have an influence on mitigating efficiency droop, or the de-excitation of the molecules in the OLED. The mechanisms for thermally induced degradation also require clarification. Encapsulation compatible with flexible, lightweight substrates is also an important area of development.
RECOMMENDATION 3-11: Given the interactions between the phosphor and the conductive host molecules, the Department of Energy should direct studies for determining what chemical structural combinations lead to the most robust materials sets. Fundamental studies of the degradation mechanisms should be carried out both at room and elevated temperatures. Research on understanding contact and ambient degradation routes and their minimization should also be supported.
FINDING: Increased outcoupling remains the single most beneficial route to increasing device efficiency from the current 100 lm/W to nearly three times that value. Methods to achieve this should be inherently very low cost and deployable over very large areas, even in the context of roll-to-roll manufacture. The outcoupling technology should have the additional attributes of being wavelength and intensity independent, and the light source should exhibit no color shifts as the viewing angle is varied from normal to highly oblique. Clearly, a viable outcoupling technology should not otherwise impact or degrade OLED performance.
Addressing the critical challenges for OLED lighting should enable the realization of increased power efficacy and the realization of the targets set by DOE, as shown in Table 3.3.
SUMMARY AND COMPARISON OF LEO AND OLEO SSL
The LED and OLED technologies explored in this chapter provide new, energy-efficient approaches to lighting with exceptional control over the chromaticity and the quality of the light produced. As shown in Table 3.3, both technologies
TABLE 3.3 Comparison of Lighting Sources by Various Metrics
| Incandescent | Fluoicscent | LEDs | OLEDs | |
| Efficacy (lab demo) | 231 InvW-white | 100lm/W | ||
| 150 lm/W - warni white | ||||
| Efficacy(commcrcial) | 17 lm/W | lOOlnVW | lOO-1201m/W-whitc | 50 lm/W panel |
| CMI | 100 | 8O-85 | 85 - white | Up to 95 |
| 95-warm white | ||||
| Form factor | Hcjt gencrating | Lorn; or compact gas tilled glass tube | Point source high-intensity lamp | Large area thin diffuse source Flexible, transparent |
| Safely concerns | Very hot | Contains nercury | Very hot operation | None to date |
| Lifetime (hours) | 1,000 | 20.000 | 50.000 | 30.000 |
| Dimmablc? | Yes, but much lower efficacy | Yes, efficiency decreases | Yes, efficiency increases | Yes. efficiency increases |
| Noise | No | Yes | No | No |
| Cost ($/klm) | 0.50 | 1.0 | 7.0 | 100-250 |
OLEDs can be designed and fabricated with exceptionally high internal efficiencies, rendering virtually any desired color, but there has not yet been a sufficient manufacturing infrastructure established to understand the practical issues and costs of scaling up this device technology into a practical and ubiquitous lighting source. Considerable additional efforts are needed to optimize light extraction, extend device lifetimes, reduce the roll off in efficiency at high brightness, and improve operational lifetime. The inorganic LEDs form “point-sources” of light while OLEDs lend themselves to unusual, conformable, and flexible form factors, offering diffuse lighting over large areas. Thus, the two technologies provide complementary lighting solutions, depending on particular lighting applications and venues. Investments in fundamental advances in OLED materials and device architectures, at this stage, may be the deciding factors in their ultimate success and the widespread adoption of this very promising lighting technology. On the other hand, the inorganic LEDs have already made the transition to widespread manufacturing and commercialization, yet still face substantial challenges to longer-term success. Issues of higher efficiency and more robust and reliable operation at lower cost similarly require continued investments into the technology, both at the fundamental level of materials and device improvement, as well as at the level of manufacturing and systems-level improvements.
Akasaki, I., H. Amano, K. Itoh, N. Koide, and K. Manabe. 1992. “GaN Based UV/blue Light-Emitting Devices.” Presentation to the Institute of Physics Conference Series: GaAs and Related Compounds. London: Institute of Physics Publishing.
Amano, H., N. Sawaki, I. Akasaki, and Y. Toyoda. 1986. Metalorganic vapor-phase epitaxial-growth of a high quality GaN Film. Applied Physics Letters 48(5):353-355.
Avrutin, V., D.J. Silversmith, Y. Mori, F. Kawamura, Y. Kitaoka, and H. Morkoc. 2010. Growth of bulk GaN and AlN: Progress and challenges. Proceedings of the IEEE 98(7).
Baldo, M.A., D.F. O’Brien, Y. You, A. Shoustikov, M.E. Thompson, and S.R. Forrest. 1998. High efficiency phosphorescent emission from organic electroluminescent devices. Nature 395:151.
Baldo, M.A., S. Lemansky, P.E. Burrows, M.E. Thompson, and S.R. Forrest. 1999a. Very high efficiency green organic light emitting devices based on electrophosphorescence. Applied Physics Letters 76:4.
Baldo, M.A., D.F. O’Brien, M.E. Thompson, and S.R. Forrest. 1999b. The excitonic singlet-triplet ratio in a semiconducting organic thin film. Physical Review B 60:14422.
Blochwitz, J., M. Pfeiffer, T. Fritz, and K. Leo. 1998. Low voltage organic light emitting diodes featuring doped phthalocyanine as hole transport material. Applied Physics Letters 73:729.
Brus, L. 1991. Quantum crystallites and nonlinear optics. Applied Physics A 53:465-474.
Burrows, P.E., V. Bulovic, S.R. Forrest, L.S. Sapochak, D.M. McCarty, and M.E. Thompson. 1994. Reliability and degradation of organic light emitting devices. Applied Physics Letters 65:2922.
Bush, S. 2008. Lumileds talks LED packaging—Interview. Electronics Weekly.com, March 26.
Cree, I. 2012. Cree Sets New R&D Performance Record with 254 Lumen-Per-Watt Power LED. Available at http://www.cree.com/news-andevents/cree-news/press-releases/2012/april/120412-254-lumen-perwatt. Accessed July 1, 2012.
D’Andrade, B.W., J. Esler, C. Lin, V. Adamovich, S. Xia, M.S. Weaver, R. Kwong, and J.J. Brown. 2008. Realizing white phosphorescent 100 lm/W OLED efficacy. Proceedings of SPIE 7051:70510Q.
DenBaars, S.P., D. Feezell, K. Kelchner, S. Pimputkar, C.-C. Pan, C.-C. Yen, S. Tanaka, Y. Zhao, N. Pfaff, R. Farrell, M. Iza, S. Keller, U. Mishra, J.S. Speck, and S. Nakamura. 2013. Development of gallium-nitride-based light-emitting diodes (LEDs) and laser diodes for energy-efficient lighting and displays. Acta Materialia 61(3):945-951.
DOE (U.S. Department of Energy). 2009. Solid State Lighting Research and Development: Manufacturing Roadmap 2009. Washington, D.C.: U.S. DOE.
DOE. 2011a. Solid-State Lighting Research and Development: Multi-Year Program Plan. Washington, D.C.: U.S. DOE.
DOE. 2011b. Solid State Lighting Research and Development: Manufacturing Roadmap 2012. Washington, D.C.: U.S. DOE.
DOE. 2012a. Solid-State Lighting GATEWAY Demonstration Results. Available at http://www1.eere.energy.gov/buildings/ssl/gatewaydemos_results.html. Accessed May 23, 2012.
DOE. 2012b. Solid-State Lighting Research and Development: Multi-Year Program Plan. Washington, D.C.: U.S. DOE.
Ehrentraut, D., and T. Fukuda. 2010. The ammonothermal crystal growth of gallium nitride—A technique on the up rise. Proceedings of the IEEE 98(7):1316-1323.
Fujii, T., Y. Gao, R. Sharma, E.L. Hu, S.P. DenBaars, and S. Nakamura.
2004. Increase in the extraction efficiency of GaN-based light-emitting diodes via surface roughening. Applied Physics Letters 84(6):855-857.
Giebink, N.C., B.W. D’Andrade, M.S. Weaver, P.B. Mackenzie, J.J. Brown, M.E. Thompson, and S.R. Forrest. 2008. Intrinsic luminance loss in phosphorescent small-molecule organic light emitting devices due to bimolecular annihilation reactions. Journal of Applied Physics 103:044509.
Giebink, N.C., and S.R. Forrest. 2008. Quantum efficiency roll-off at high brightness in fluorescent and phosphorescent organic light emitting diodes. Physical Review B 77:235215.
Haberland, K. 2008. Optical in situ monitoring in LED production. Solid State Technology, Volume 51, November 1.
Hall, R.N., G.E. Fenner, J.D. Kingley, T.J. Soltys, and R.O. Carlson. 1962. Coherent light emission from GaAs junctions. Physical Review Letters 9:366-368.
Helfrich, W., and W.G. Schneider. 1965. Recombination radiation in anthracene crystals. Physical Review Letters 14:229.
Herzog, A.H., W.O. Groves, and M.G. Craford. 1969. Electroluminescence of diffused GAs1-xPx diodes with low donor concentrations. Journal of Applied Physics 40.
Hum, D. 2011. Phosphor Converted Light Source Having an Additional LED to Provide Long Wavelength Light. U.S. Patent No. WO/2011/133312. October 27.
Illuminating Engineering Society. 2008. Approved Method: Measuring Lumen Maintenance of LED Light Sources. New York: Illuminating Engineering Society.
Jost-Boissard, S., M. Fontoynonta, and J. Blanc-Gonneta. 2009. Perceived lighting quality of LED sources for the presentation of fruit and vegetables. Journal of Modern Optics 56(13):1420-1432.
k-Space Associates, I. 2012. k-Space Products: Advanced tools for in-situ thin film characterization. Available at http://www.k-space.com/products/raterat-pro/. Accessed September 15, 2012.
Krames, M.R., O.B. Shchekin, R. Mueller-Mach, G.O. Mueller, L. Zhou, G. Harbers, and M.G. Craford. 2007. Status and future of high-power light-emitting diodes for solid-state lighting. Journal of Display Technology 3(2):160-175.
Levermore, P.A., A.B. Dyatkin, Z.M. Elshenawy, H. Pang, J. Silvernail, E. Krall, R. Kwong, M.S. Weaver, R. Ma, J.J. Brown, X. Qi, and S.R. Forrest. 2012. Phosphorescent OLEDs for high efficacy long lifetime solid state lighting. Journal of Photonics for Energy 2(1):021205.
Michalet, X., F.F. Pinaud, L.A. Bentolila, J.M. Tsay, S. Doose, J.J. Li, G. Sundaresan, A.M. Wu, S.S. Gambhir, and S. Weiss. 2005. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307(5709):538-544.
Möller, S., and S.R. Forrest. 2001. Improved light outcoupling in organic light emitting devices employing ordered microlens arrays. Journal of Applied Physics 91:3324.
Nakamura, S., T. Mukai, and M. Senoh. 1994. Candela-class high-brightness InGaN/AlGaN double-heterostructure blue-light-emitting diodes. Applied Physics Letters 64:1687-1689.
Narendran, N., Y. Gu, J. Freyssinier-Nova, and Y. Zhu. 2005. Extracting phosphor-scattered photons to improve white LED efficiency. Physica Status Solidi (A), Applied Research 202(6):R60-R62.
Nathan, A., G.R. Chaji, and S.J. Ashtiani. 2005. Driving schemes for a-Si and LTPS AMOLED displays. Journal of Display Technology 1:267-277.
Nathan, M.I., W.P. Dumke, G. Burns, F.H. Dill, Jr., and G.J. Lasher. 1962. Stimulated emission of radiation from GaAs p-n junctions. Applied Physics Letters 1:62-64.
Pankove, J.I., and M.J. Massoulie. 1962. Injection luminescence from GaAs. Bulletin of the American Physical Society 7:88.
Pankove, J.I., E.A. Miller, and H.E. Berkeyhe. 1971. GaN electroluminescent diodes. RCA Review 32(3):78.
Pankove, J.I., and T.D. Moustakas. 1998. Gallium Nitride (GaN) I (R.K. Willardson and E.R. Weber, eds.). Semiconductors and Semimetal, Volume 50. Academic Press.
Paskova, T., D.A. Hanser, and K.R. Evans. 2010. GaN substrates for III-N device. Proceedings of the IEEE 98(7):1324-1338.
Pfeiffer, M., S.R. Forrest, K. Leo, and M.E. Thompson. 2002. Electrophosphoresent p-i-n organic light emitting devices for very high efficiency flat panel displays. Advanced Materials 14:1633.
Philips Solid-State Lighting Solutions, I. 2012. Color Blast Lighting. Available at http://www.colorkinetics.com/ls/rgb/colorblast12/. Accessed July 31, 2012.
Pimputkar, S., J.S. Speck, S.P. DenBaars, and S. Nakamura. 2009. Prospects for LED lighting. Nature Photonics 3(4):179-181.
Pope, M., H. Kallmann, and P. Magnante. 1963. Electroluminescence in organic crystals. Journal of Chemical Physics 38:2042.
Reineke, S., F. Lindner, G. Schwartz, N. Seidler, K. Walzer, B. Lüssem, and K. Leo. 2009. White organic light-emitting diodes with fluorescent tube efficiency. Nature 459:234.
Sandia National Laboratories. 2004. Final Report on Grand Challenge LDRD Project: A Revolution in Lighting—Building the Science and Technology Base for Ultra-Efficient Solid-State Lighting. Albuquerque, N.M.
Schubert, E.F. 2006. Light-Emitting Diodes. Second edition. Cambridge, U.K.: Cambridge University Press.
Shtein, M., J.K. Mahon, T. Zhou, S.R. Forrest, M. Schwambera, and N. Meyer. 2002. Organic VPD shows promise for OLED volume production. Solid State Technology 45:131.
Sun, Y., and S.R. Forrest. 2008. Enhanced light outcoupling of organic light emitting devices using embedded low-index grids. Nature Photonics 2:483.
Tang, C.W., and S.A. VanSlyke. 1987. Organic electroluminescent devices. Applied Physics Letters 51:913.
Thompson, M.J.S., K. Larson, and H. Hall. 2011. Investigation of tunable LED lighting for general illumination employing preliminary activity recognition sensor network. Proceedings of SPIE 8123:812305.
Universal Display Corporation. Undated. Universal PHOLED Technology and Materials. Available at http://www.oled-info.com/files/UDC-Product-Sheets-sid-2011.pdf. Accessed September 15, 2012.
Wang, Z.B., M.G. Helander, J. Qiu, D.P. Puzzo, M.T. Greiner, Z.M. Hudson, S. Wang, Z.W. Liu, and Z.H. Lu. 2011. Unlocking the full potential of organic light-emitting diodes on flexible plastic. Nature Photonics 5:753.
Willner, A.E., R.L. Byer, C.J. Chang-Hasnain, S.R. Forrest, H. Kressel, H. Kogelnik, G.J. Tearney, C.H. Townes, and M.N. Zervas. 2012. Optics and photonics: Key enabling technologies. Proceedings of the IEEE 100:1604.
Woodall, J.M., R.M. Potemski, S.E. Blum, and R. Lynch. 1972. AlGaAs LED structures grown on GaP substrates. Applied Physics Letters 20.