The Clinical and Translational Science Awards (CTSA) Program has demonstrated progress in three crosscutting domains that the Institute of Medicine (IOM) committee believes are integral to effectively advancing clinical and translational science: training and education, community engagement, and child health research. These efforts, along with the program’s contributions in building infrastructure and providing a range of research resources, make the CTSA Program a unique national resource within the clinical and translational research landscape. As with all such activities, each of these functions can be strengthened in a variety of ways. The committee provides a brief overview of each of these areas, followed by its recommendations for next steps.
The health needs of the nation call for a generation of scientists trained in “interdisciplinary, transformative translational research” (Meyers et al., 2012, p. 132; Van Hartesveldt et al., 2008) and in the leadership and team skills to engage in effective collaborative partnerships. A major challenge in rapidly translating research findings into health care practice is the concomitant need for support of clinician scientists in order to overcome the growing divide between clinical (M.D.) and research (Ph.D.) careers (Roberts et al., 2012). Further, emerging and growing areas of research (including comparative-effectiveness and community-engaged research) are emphasizing skills and collaborations integral to both clinical and translational research.
Background and Context
Sustaining a vibrant clinical and translational research enterprise in the future depends on building and retaining a diverse research workforce. Education and training in clinical and translational research are priorities for the CTSA Program. All CTSA institutions are expected to provide robust postgraduate training (NIH, 2012c), and many have extensive training programs that often include undergraduate and predoctoral student training as well as training for research staff and community collaborators. In addition, the CTSA Consortium has identified “training and career development of clinical and translational scientists” as a consortium strategic goal and has devoted considerable resources to enhancing the effectiveness of training and education programs across institutions (CTSA Central, 2013a,e).
CTSA Training Awards and Programs
Since the inception of the CTSA Program, the training of new clinical and translational science investigators has been an integral part of the program. The KL2 Mentored Clinical Research Scholar Program is a required part of all individual CTSAs (NIH, 2012c). This career development program provides awardees who have a doctoral degree (M.D., Ph.D., or equivalent) with formal research training experience and funding support to help them become independent investigators (NCATS, 2013). The TL1 Clinical Research Training Program provides an introduction to clinical and translational research to pre- and postdoctoral candidates or others who want to learn more about these types of research. For example, the TL1 program can provide medical students with a structured year-long research opportunity. In fiscal year (FY) 2011, 501 scholars participated in the KL2 program, and 469 trainees participated in the TL1 program through the CTSA Program (Collier, 2013a). Many CTSAs offer a master’s level degree in clinical and translational research.
In 2011, Westat provided its findings from an online survey of CTSA-supported scholars, trainees, and mentors from CTSAs funded between 2006 and 2010 (Miyaoka et al., 2011). A total of 665 mentors (56 percent response rate) and 553 scholars and trainees (43 percent response rate) completed the surveys. Overall, the results were positive. Mentors reported providing a range of support in key areas for career development, and they reported benefits to their own professional development. Scholars and trainees reported developing more skills and hav-
ing enhanced opportunities for career development. Areas for improvement included the need for greater diversity among mentors, scholars, and trainees; increased emphasis on team science; and additional focus on the development of skills related to technology transfer, commercialization, and communicating with policy makers. Box 4-1 presents a few highlights from this evaluation.
CTSA Consortium Efforts on Training and Education
At the consortium level, the Strategic Goal Committee on Training and Career Development has developed and disseminated core competencies in clinical and translational science for master’s degree students (CTSA Central, 2011), as well as core competencies in specific areas, including child health translational research, T1 research, academia-industry drug development, and medical device innovation and technology transfer (CTSA Central, 2011).
BOX 4-1
Selected Highlights from the CTSA National Evaluation Education and Training Study
• CTSAs are engaging scholars and trainees across the spectrum of translational research (21 percent basic biomedical research; 52 percent clinical research; 26 percent postclinical research).
• Trainees report positive experiences in educational activities. For example, 92 percent responded that building relationships with mentors was useful; 96 percent were positive about working as a member of a research team.
• Evidence of success was found in obtaining R01 funding (47 percent of R01 applications were funded). The rate of submission was low, however (16 percent of scholars or trainees reported submitting an R01 application).
• Clear benefits were noted by mentors (97 percent of mentors rated their experience as positive) and trainees or scholars (83 percent assessed their levels of training and expertise in clinical research as moderate or high after participating in the CTSA Program, compared with a baseline 33 percent).
• Most scholars and trainees reported serving as the PI on their first (79 percent) and second (72 percent) grant/award applications.
SOURCE: Miyaoka et al., 2011.
The additional goals of the strategic goal committee are to provide CTSA-wide access to training resources, develop a core curriculum, implement a mentoring training program that shares best practices, and develop metrics and criteria for recognizing success and achieving career promotion in clinical and translational research (CTSA Central, 2013e).
Opportunities and Next Steps
CTSA 2.0 should build on the successes of the training and education components of individual CTSAs and the consortium-level work. This calls for new and innovative training and education approaches and methodologies. Training and education efforts are a prime area for collaboration with NIH institutes and centers to monitor, track, and adopt best practices and successful models in education, mentoring, and career development.
Presentations to the IOM committee and peer-reviewed publications highlighted a range of innovative training opportunities that could be brought to scale for greater impact. These include training and education across the educational spectrum (i.e., from undergraduate to postgraduate levels) and learning opportunities for community partners, faculty, and research administrators. Academic training options also exist across levels of intensity, from individual courses to certificate programs to advanced degrees.
Innovative Curricula and Team-Based Education and Training
The excitement and the challenge of clinical and translational science is that it requires approaches to training and education that are outside of traditional scientific fields. The focus on a truly team-based and interdisciplinary approach to science requires collaborations that go far beyond lip service and necessitates relationship building between and among professional schools (e.g., medicine, nursing, business, law, engineering, public health), as well as with a range of community partners (from patients and families to health care providers). Learning in this field is often through and by experience. Further, the topics to be covered stretch beyond traditional ones to include, for example, entrepreneurship, intellectual property, regulatory science, health equity, unconscious bias, and community engagement.
In a number of CTSAs, innovative efforts to develop new courses and curricula are already under way. For example, the CTSA partnership in which the University of Washington is involved implements a teambased translational educational program with a clinical and translational research boot camp workshop and several active seminar series, including a monthly clinical research education series focused on conveying practical information and tools (ITHS, 2013). The goal is for translational scientists to have competencies related to key questions at each phase of the translational research cycle, including
• Which problems will we tackle? (discovery phase);
• How will the handoff happen to move discoveries into human studies? (development phase);
• Can we scale up into clinics or communities? (delivery phase); and
• How will we know we are making a real impact? (outcomes phase) (Edwards, 2013; Kelley et al., 2012).
In another innovative training model, the University of Pennsylvania offers a variety of training options for undergraduates; predoctoral, graduate, and postdoctoral students; fellows; clinical residents; and faculty with several types of certificates or advanced degrees offered. In addition to general training in clinical and translational science, this program offers a concentration in translational therapeutics that includes publicprivate partnerships for industry internships and training in intellectual property and commercialization (Meagher, 2011; University of Pennsylvania ITMAT, 2013).
CTSA 2.0 should build on these and other innovative training and education programs that are bridging the gap between the basic and clinical sciences. Emphasis on experiential and team-based learning and incorporating topics outside the traditional realm will provide the solid foundation needed to spur clinical and translational research. CTSAs have the potential both to create learning cultures that embrace innovative teaching methods and content (e.g., gaming, flipped classrooms,1 mini-institutes) and to disseminate those innovations rapidly and effectively.
_____________________
1 Flipped classrooms typically offer instruction online and doing homework in the classroom.
Effective Mentoring and Coaching
An emphasis on effective mentoring has also been a strength and integral component of CTSA training programs. In 2008, the CTSA Education and Career Development Key Function Committee established a Mentor Working Group that has identified several key elements of successful mentoring programs: mentor selection and support, alignment of mentor and mentee expectations, mentor training, evaluation, and feedback (Fleming et al., 2012).
Recent surveys and interviews found that the active mentoring programs that are in place at CTSAs for the KL2 awards differ widely regarding policies on selecting mentors, criteria to qualify as a mentor, and processes to evaluate the mentoring relationships (Huskins et al., 2011; Silet et al., 2010; Tillman et al., 2013). Programs also varied on the formality of the mentoring program, with 30 percent reporting the use of mentoring contracts (Huskins et al., 2011). Two-thirds of the mentoring programs reported requiring or encouraging mentees to have multidisciplinary mentors, with mentors or the program taking the lead to coordinate this effort to varying degrees (Silet et al., 2010). The same survey found that CTSA institutions infrequently provide tangible support for mentors, such as salary support, institutional recognition, and research support.
Successful mentoring practices should be disseminated across the CTSA Program. While avoiding the pitfalls of just checking boxes, the CSTA Program should consider developing metrics for how mentoring is evaluated. Mentoring is not an inherent skill for many people, but it can be developed by training and alignment of incentives. Positive mentoring experiences appear to be linked to strong relationships with individual mentors. The time commitment made to mentoring should be recognized in decisions on the mentor’s career advancement.
A new initiative being announced by the NIH Common Fund and the National Research Mentoring Network offers opportunities for focused efforts on mentoring and will aim to provide mentoring standards, training for mentors, and opportunities to increase the diversity of participants involved in being a mentor or mentee (NIH, 2013b). CTSA Program participation in this network could be a benefit to furthering CTSA mentoring opportunities. Consideration could be given to trans-CTSA mentorships where predoctoral students in one institution would have mentors in multiple CTSA institutions, thereby sharing specific expertise, creating venues for innovative partnerships, and opening up a poten-
tial pipeline for recruitment across institutions when their predoctoral training is completed.
Flexibility and Focus
Consistency in key components of training and education, such as core competencies and standards, can be balanced with flexibility in the elements and focus of the training experiences. Input received by the IOM committee from those who had participated in the CTSA training programs identified many positive aspects of the training experience, including protected time to develop a programmatic research agenda and grant proposals; exposure to multidisciplinary perspectives; committed mentoring relationships; high-quality courses, seminars, and workshops; support for participation in national conferences; and access to pilot grant funding and core resources (Ceglia, 2013; IOM, 2013; Shackelford, 2013). Areas of concern included the lack of awareness of the range of core resources available through the CTSA and the extensive time commitment for completing an advanced degree in the CTSA institutions that require this as part of the training program.2
In moving to CTSA 2.0, the IOM committee urges increased flexibility in training and education programs. The extensive list of competencies identified by the strategic goal committee (CTSA Central, 2011) offers many potential areas for program development. The objective should be to personalize training experiences to meet the needs and goals of individuals and focus on competency rather than on the absolute requirement of obtaining a master’s or other advanced degree. This flexibility will be valuable in attracting and retaining KL2 scholars and TL1 trainees and may be particularly pertinent to clinician-scientists, who can play a major role in the clinical and translational research enterprise.
Disseminating Education and Training Materials
The transformation of training and education is possible only through the dissemination of successful approaches and practices. Several efforts are under way to provide online repositories of CTSA training and education modules and materials. The Virtual University through the Uni-
_____________________
2 CTSA institutions have the flexibility to decide how to structure their career development programs. In some CTSAs the KL2 participants are required to obtain a master’s degree as part of the KL2 program, and the degree is optional at other CTSA sites (Collier, 2013b).
versity of Iowa offers access to online courses relevant to clinical and translational sciences (University of Iowa ICTS, 2013). The National CTSA Educational Resource Program, developed by the University of Rochester’s Clinical and Translational Science Institute, provides links to educational modules from a number of CTSAs (University of Rochester CTSI, 2013). Online access to course materials on mentoring is available through the Mentor Development Program at the University of California, San Francisco (University of California San Francisco CTSI, 2013).
Notable in the information obtained by the IOM committee regarding training and education is frequent sharing of courses, seminars, workshops, and other resources among the CTSA sites and other training and education programs within institutions. This sharing enhances crossdisciplinary training within individual institutions and across the CTSA institutions. The IOM committee notes that similarities in core curricula highlight opportunities for improving efficiencies in training as well as for exposing scholars and trainees to expertise in areas of particular strength from one CTSA institution to another.
Increasing Diversity and Growth of the Clinical and Translational Research Workforce
To date, scholars, trainees, and mentors in CTSA programs lack diversity. The Westat evaluation showed that most mentors are white males, and most scholars and trainees are white females (Miyaoka et al., 2011). Bringing the brightest minds to research, which is critical for new discoveries to improve health, depends on creating a training and education environment that attracts and retains a diverse pool of scientists. Innovative education programs such as Harvard University’s Summer Clinical and Translational Research Program for undergraduate scholars have the potential to create a pipeline of diverse clinical and translational scientists (Harvard Medical School DCP, 2013), particularly if partnerships are formed with colleges and universities that traditionally serve racial and ethnic minority students.
Opportunities may also be available for CTSAs to connect with STEM (science, technology, engineering, and mathematics) initiatives within and across institutions. CTSAs need to take full advantage of efforts sponsored by the NIH and others to build diversity, and, moreover, they should lead in the implementation of these initiatives. An example is the BUILD Program (Building Infrastructure Leading to Diversity) and the National Research Mentoring Network (NIH, 2013a). The National
Institute on Minority Health and Health Disparities sponsors Research Centers in Minority Institutions (RCMI), which provide additional opportunities for ongoing collaborations with the CTSA Program, particularly with the RCMI Translational Research Network (NIMHD, 2013).
Metrics and Incentives for Careers in Clinical and Translational Research
Traditional metrics have been used for the most part to measure the success of CTSA training and education programs. These metrics include
• number of scholars and trainees;
• conversion rate from K (training) to R (independent investigator) grants;
• number of publications; and
• number and types of degrees completed (Miyaoka et al., 2011).
These metrics do not measure or provide incentives for the teambased and interdisciplinary approaches needed to accomplish clinical and translational research. If the CTSAs are to be centers for innovations in clinical and translational research, they should also lead in innovations in mentoring and its evaluation, including assessment of the professional career trajectory of those who have participated in the training programs, creation of networking opportunities, active participation in national professional organizations, and commitment or intention of the scholars and trainees to engage in clinical and translational research.
Two groups within the CTSA Program have begun related efforts to examine the components of career success for clinical and translation scientists and the metrics needed to assess individual and organizational progress. The Research on Careers Workgroup at the University of Pittsburgh identified personal factors (e.g., demographic and psychosocial factors, research experience) and organizational factors (e.g., training opportunities, financial resources, balance of research and clinical responsibilities) contributing to career success for physician-scientists (Rubio et al., 2011). This information can provide training programs with insights on critical areas for working with scholars, trainees, and mentors. The CTSA Education Evaluation Working Group identified validated metrics and measures for assessing personal and organizational determinants of career success for clinical and translational scientists (Lee et al., 2012).
NCATS and individual CTSAs have the opportunity to lead changes in how training and education programs are assessed and in instituting incentives for the recognition and promotion of those involved. The traditional benchmarks for academic promotion and advancement are focused on individuals and products (e.g., publications, new grants). New benchmarks that value team-based efforts and collaborative approaches are needed to complement these traditional metrics. Changing those measures will be challenging because it is difficult to assess the depth or substance of collaborations. Identifying the right measures and incentives is a major challenge for CTSA 2.0. Examples of relevant measures might include the following:
• evidence of interdisciplinary collaborations and of teams that cross disciplines and include community partners;
• increases in the number of training and educational opportunities outside of KL2 and TL1;
• increases in the number and level of involvement of community-based health care providers and other community stakeholders in the CTSA’s activities;
• higher satisfaction with mentoring relationships and increases in trained mentors; and
• the extent of public communication and knowledge transfer.
Expanding Training Opportunities
To date, CTSAs have made substantial progress in developing graduate and postdoctoral training in clinical and translational research. In addition to sustaining and building on those efforts, further work is needed to expand those opportunities, including training and continuing education for faculty, professional staff, and community partners. For example, substantive involvement of community partners in clinical and translational science provides the opportunity for education in research methodologies and design, policy and regulatory aspects of clinical trials, and dissemination of clinical innovations.
Community partner training generally appears rather informal across CTSA institutions. One example of a formal program is the Community Engaged Scholars Program developed and implemented by the South Carolina Clinical and Translational Research Center for Community Health Partnerships. This 18-month program focuses on developing competencies in community-based participatory research among teams
that must include at least one academic and one community partner. Program components include monthly sessions focused on problem-based learning, mentorship, and funding for pilot projects. An early evaluation found the program successfully recruited and retained teams that identified and implemented community-based translational research pilot studies (Andrews et al., 2012).
As CTSAs continue to develop as strong networks of diverse stakeholders, there will be important opportunities to provide all participants with training and education on clinical and translational science. Meaningful involvement and collaboration among diverse groups require some common starting points, and CTSAs are the prime location for the training needed.
Conclusions and Recommendation
Training and education in clinical and translational research is a core element of all CTSAs. To date, significant progress has been made in identifying core competencies and in developing curricula in clinical and translational research. CTSA 2.0 will require further efforts to develop and implement innovative education and training approaches that emphasize the unique aspects of clinical and translational science. The full range of stakeholders needs to have expertise so they can contribute fully to the accelerated development and implementation of new therapies, preventive measures, and devices to improve health.
Recommendation 5: Advance Innovation in Education and Training Programs
The CTSA Program should provide training, mentoring, and education as essential core elements. To better prepare the next generation of a diverse clinical and translational science workforce, the CTSA Program should
• emphasize innovative education and training models and methodologies, which include a focus on team science, leadership, community engagement, and entrepreneurship;
• disseminate high-quality online offerings for essential core courses for use in CTSA and other institutions;
• champion the reshaping of career development pathways for researchers involved in the conduct of clinical and translational science; and
• ensure flexible and personalized training experiences that offer optional advanced degrees.
Effective translational research requires effective community engagement across the full spectrum of research from basic science and first-in-human studies (T0–T1) to community and population health research (T4). For the purposes of this report, the IOM committee has adopted a widely cited CDC definition of community engagement: “the process of working collaboratively with and through groups of people affiliated by geographic proximity, special interest, or similar situations to address issues affecting the well-being of those people” (CDC and ATSDR, 1997). The committee considers that the term “community” can include all stakeholders connected to clinical and translational research. This broad definition encompasses the people who are served by the individual CTSAs, including patients and families, community organizations, and disease advocates, as well as clinicians and health professionals, including physicians, nurses, dentists, nutritionists, social workers, and many others. The committee also recognizes the “research community,” which includes the full range of researchers—basic, clinical, and locally based researchers who work both inside and outside of academic settings. In this section of the chapter, however, when the word “community” is used, it denotes the people who seek and provide health care in community, academic, and private settings, as well as individuals and organizations working in communities to improve the health and well-being of local populations.
Community engagement in clinical and translational research varies in terms of both level of engagement and the stage(s) of research in which public participants are involved. The type of research in which community members are most deeply involved is community-based participatory research (CBPR), which engages local participants as partners and involves them in shared leadership roles throughout the entire research process, from concept development to protocol design to dissemination of the research findings. The least involvement includes outreach mechanisms that are primarily unidirectional and may entail a researcher
providing information about research results or ongoing research in the region (Hood et al., 2010; Task Force on the Principles of Community Engagement, 2011).
Communities can contribute to the full range of clinical and translational research in important ways that are not always recognized (see Box 4-2). For example, partnerships with community representatives can identify community health needs and priorities, provide critical input and data on clinically relevant questions, develop culturally appropriate clinical research protocols, promote successful enrollment and retention of research participants, and, ultimately, disseminate and implement research results more effectively. In addition, community engagement at early stages of research helps to ensure that ethical considerations are taken into account and facilitates early establishment of trust (Horowitz et al., 2009; Martinez et al., 2012; Woolf, 2008).
The benefits of community engagement therefore are numerous and can lead to a more robust research enterprise, stronger community support for research and research funding, and attract more, and more diverse, young people to careers in research (Freeman and Seifer, 2013; Staley, 2009; Task Force on the Principles of Community Engagement, 2011; Yarborough et al., 2012).
BOX 4-2
Examples of Community Engagement in Clinical and Translational Research
For basic research (T0-T1), communities and patient advocacy organizations can play a vital role in identifying research areas, providing resources and specimens to support research, and putting a human face on the diseases and disorders being studied. For example, the Hermansky-Pudlak Syndrome (HPS) Network is a nonprofit advocacy organization that supports patients and families with HPS, a genetic disorder associated with albinism, bleeding, visual impairment, inflammatory bowel disease, and pulmonary fibrosis (HPS Network, 2013). The HPS Network has worked to identify research questions, recruit researchers, build partnerships, and fund research studies that have used animal models to investigate cell lines in the lungs of mice with HPS that are most susceptible to pulmonary fibrosis (Young et al., 2007). Likewise, PXE International, Inc., a nonprofit advocacy organization, provides support for research and for individuals and families affected by pseudoxanthoma elasticum (PXE), a genetic disorder that can lead to changes in vision, skin elasticity, and the cardiovascular and gastrointestinal systems. To expand research in this area, PXE International worked with patients and families to establish a biobank of blood and tissue samples made available to researchers conducting genetic research (PXE International,
2012). Both of these organizations work to bridge the gap between patients and basic science researchers studying these rare disorders.
In the clinical trial phases of clinical and translational research (T2-T3), community organizations can play a significant role in developing appropriate research protocols, helping researchers understand the needs and culture of the patient population, and recruiting prospective research participants. The HIV/AIDS community has played an active role in research since the beginning of the HIV/AIDS epidemic. To facilitate these interactions, the National Institute of Allergy and Infectious Diseases (NIAID) made Community Advisory Boards (CABs) a requirement for all HIV/AIDS clinical trial networks and sites it funds (Community Partners, 2009). The CABs have provided a venue for dialog between the community and clinical researchers and provided an opportunity for community representatives to participate in trial design and recruitment. NIAID continues to make community engagement a priority in HIV/AIDS research by supporting a number of other mechanisms to foster community participation in clinical trials. For example, the Legacy Project and Community Partners work to build trust, cultivate partnerships, and ensure effective community representation in clinical trials with an emphasis on engaging underrepresented communities (Dieffenbach, 2011; HANC, 2013a,b; Kagan et al., 2012).
Community engagement is an inherent part of community health and population health research (T4). The Healing Canoe Project is an example of a multiphase collaborative project that applies the community-based participatory research model. The project is a partnership between the Suquamish Tribe, the Port Gamble S’Klallam Tribe, and the Alcohol and Drug Abuse Institute (ADAI) at the University of Washington and is funded by the National Center on Minority Health and Health Disparities (Healing of the Canoe, 2013; Thomas et al., 2010). The first phase of the project (2005-2008) focused on the partnership between ADAI and the Suquamish Tribe. During that phase, researchers conducted a community assessment that involved interviews and focus groups to identify needs related to substance abuse prevention and cultural identity among youth. They used a team-based approach to develop a curriculum that blended elder community members’ experiences, the community’s traditions and culture, cognitive-behavioral skills, and information about alcohol and drugs. The second phase of the project (2008-2013) is continuing this work and using the same methods to adapt the project for the Port Gamble S’Klallam Tribe (Healing of the Canoe, 2013; Thomas et al., 2010).
An array of information has been developed on the principles, best practices, and need and potential for community engagement in all aspects of the research process, and studies have delineated the best methods to achieve authentic engagement, including defining community, identifying partners, learning the etiquette of community engagement, building sustainable networks of community engagement researchers, developing new engagements, and refining translation and dissemination plans (Hatcher and Nicola, 2008; IOM, 2012a; Michener et al., 2012).
Nevertheless, as noted by Hood and colleagues (2010), “to date, there is a paucity of research about the prevalence of community engagement in research, especially among clinical and translational research studies traditionally funded by NIH” (p. 19). This report will not review the literature on best practices and principles of community engagement. The committee recognizes the many valuable contributions to the field made by such reports as Principles of Community Engagement (Task Force on the Principles of Community Engagement, 2011), Communities as Partners in Cancer Clinical Trials: Changing Research, Practice and Policy (ENACCT and CCPH, 2008), and Recommendations for Community Involvement in National Institute of Allergy and Infectious Diseases HIV/AIDS Clinical Trials Research (Community Partners, 2009), along with the work conducted, for example, through the CTSA Program, the Community-Campus Partnerships for Health (CCPH), the Centers for Disease Control and Prevention, the Patient-Centered Outcomes Research Institute, and various NIH institutes and centers.
The CTSA Program and Community Engagement
Community engagement was identified as a priority area from the earliest stages of the CTSA Program and became a required key function for CTSA sites as administered by the National Center for Research Resources (NIH, 2009a,b, 2012b). Shifts in the requirements of the most recent RFA, which were made to provide increased flexibility for the individual CTSAs, have caused concern among some community organizations and advocacy groups that community engagement is being downplayed (CCPH, 2012b; Seifer, 2013; Thomas, 2013). Although the key functions that were previously required are no longer explicit requirements, the recent RFA does indicate that all CTSAs must have core resources across the full spectrum of translational research. It also encourages individual CTSAs to build a program to “meet the needs of their own investigative and public communities and to develop and build upon unique institutional and community strengths,” which implies some level of necessary community engagement (NIH, 2012c).
The CTSA Consortium has adopted a set of competencies for clinical and translational research that includes community engagement as a core competency (CTSA Central, 2011). The “Frequently Asked Questions” section of the recent RFA refers to future solicitations in 2014 and highlights community engagement as an area of interest for NCATS
(NCATS, 2012a). NCATS staff confirmed that “the NCATS Advisory Council approved concept clearance for [a new initiative titled] ‘Strengthening Community-Engaged Research in the CTSA Program’ at its September 2012 meeting” but indicated that “no decision has been made on what form this initiative will take” (Parsons, 2013). Initial information regarding future partnerships with NIH institutes and centers for demonstration projects also highlights community engagement as an area of focus (NCATS, 2012a).
On October 15, 2012, NCATS released an RFI focused on how community engagement research could be enhanced through the CTSA Program with the end goal being “the development of a research agenda that would leverage the community engagement capability of the CTSA institutions to solve critical roadblocks in the translational research process” and that would build “on the CTSA community engagement projects, collaborations, and infrastructure to facilitate the conduct of translational research” (NIH, 2012b). The RFI asked for stakeholder input on possible research questions and opportunities to advance research, tools, and techniques for community engagement and on the role of community engagement and community-based participatory research in clinical and translational research. The responses to the RFI were still being reviewed by NCATS just prior to the IOM committee’s final meeting in March 2013, but the authors of a few of the responses shared their comments with the committee and emphasized a commitment to community engagement and a continued need for it within the CTSA Program (CCPH, 2012a; Emmons, 2012; Parsons, 2013).
The initial commitment to community engagement within the CTSA Program should be commended. However, NCATS’s vision for how community engagement will be a part of the CTSA Program moving forward remains unclear. Although indications point to community engagement remaining an important feature of the program, there are serious concerns that if it is not an explicit requirement for all CTSAs, it may fade in importance. These concerns were expressed clearly by the CCPH in its response to the RFI on community engagement, highlighted at the IOM committee’s January 2013 meeting. The CCPH sees “troubling signs that CTSAs are already responding to a perceived lack of NCATS support for community engagement” by reducing resources supporting community engagement and putting a hold on community engagement activities (CCPH, 2012a). In developing its plans for implementing the CTSA Program and how community engagement will fit within it, NCATS must carefully consider unintended consequences of
its decisions in terms of developing and sustaining fragile partnerships that have been and continue to be built.
Progress in Community Engagement
Because community engagement has been a required part of the CTSA Program, individual CTSAs and the CTSA Consortium have dedicated time and resources to building partnerships with community organizations and representatives, developing and sharing tools and resources to facilitate community engagement, educating researchers and communities, building trust, and engaging communities in the research process. The CTSA Consortium has two main committees focused on community engagement—Strategic Goal Committee 4, which focuses on “enhancing the health of our communities and the nation,” and the Community Engagement Key Function Committee, which has eight working groups devoted to a range of related areas, including practicebased research network collaborations, health policy, resource development, and community partner integration (CTSA Central, 2013c). These two committees work “to identify and develop effective partnerships among researchers and community stakeholders” and “to implement a successful broad plan of community and practice engagement among the CTSA sites by sharing knowledge, expertise and resources” (CTSA Central, 2013c,f). These committees and working groups have facilitated the development of a range of tools and resources for CTSAs and researchers that promote effective community engagement (see Box 4-3) (Brady, 2012).
In addition to activities facilitated through the CTSA Consortium, individual CTSAs are making progress in community engagement efforts. A recent survey of involvement of community representatives in CTSA activities found that, of the 47 CTSAs responding (out of 60 surveyed), almost 90 percent have established a community advisory board (Spofford et al., 2012). However, these boards are used primarily to advise the community engagement cores at the CTSAs and are involved to a lesser extent in advising CTSA leadership. The survey also revealed few opportunities for community representatives to participate in leadership roles beyond those within the community engagement core or on CTSA leadership committees.
To fully engage community representatives throughout the CTSA Program, strategies should be developed and implemented that integrate
community representatives beyond community engagement projects. This effort should include having community representatives actively participate in leadership and governance committees of the CTSA Program and individual CTSAs and obtaining substantive input from them on how to improve community engagement.
BOX 4-3
Examples of Community Engagement Tools
• Sentinel Network for Community-Based Participatory Research: a collaborative project with five CTSAs and several partner organizations that works to identify strategies to increase community participation in clinical research through education and referrals. Since the start of the program, more than 5,000 individuals have been surveyed on topics related to barriers to participating in research, local health concerns and needs, and the types of research in which they would be willing to participate (NIH, 2012a).
• Community Engagement Consultative Service (CECS): a two-phase program that provides consultations and referrals to help individual CTSAs and researchers “develop the knowledge, skills, and attitudes to successfully engage with internal and external groups and communities” (Carter-Edwards et al., 2013, p. 34). Phase I of the program tested the feasibility and utilization of the service, and Phase II paired individual CTSAs with consultants to promote improvements in community engagement (Carter-Edwards et al., 2013; Duke Center for Community Research, 2013).
• Community Research Utilities and Support (CORUS): an online database designed for sharing resources and tools related to community-engaged research. Resources include evaluation tools and strategies, education modules, stakeholder registries, communications tools, and ethics resources (Indiana University CTSI, 2012).
• The Research Toolkit (formerly known as PRIMER or Partnership-driven Resources to IMprove and Enhance Research): an online library of available resources and tools meant to facilitate multisite research involving community organizations and PBRNs. The toolkit is organized by phase of research to provide investigators with a complete guide. A collaborative team of researchers that included members from three CTSAs, PBRNs, and the HMO Research Network developed the toolkit (Dolor et al., 2011; Research Toolkit, 2012).
• Principles of Community Engagement: an almost 200-page primer developed by a task force of the Community Engagement Key Function Committee. This resource compiles definitions, principles, examples, and best practices, along with challenges and mechanisms for evaluating community engagement (Task Force on the Principles of Community Engagement, 2011).
As noted by the NIH CTSA/NCATS Integration Working Group, to develop or strengthen community-based research, though this is “The CTSA requirement for community outreach has led many institutions one of the most highly variable aspects of the CTSAs” (Katz et al., 2011). Examples of community engagement efforts at individual CTSAs are provided in Box 4-4.
BOX 4-4
Examples of Community Engagement Projects at CTSAs
• Chicago Consortium for Community Engagement: a partnership between Northwestern University, the University of Chicago, and the University of Illinois at Chicago designed to facilitate coordination and synergy in order to enhance the capacity of each of the institutions for community engagement through a range of activities, including having regular meetings for the Chicago community-based participatory research network, providing education and training for researchers and community organizations, developing a map of research opportunities across the city, and hosting a citywide summit to discuss challenges and opportunities for improving the health of Chicagoans (C3, 2013; CTSA Central, 2013b).
• Clinical and Translational Science Collaborative of Cleveland at Case Western Reserve University: a collaboration of support of local PBRNs with stabilizing funding and micropilot grants that have supported more than 115 projects. One PBRN encompasses 50 practices and is supported by the Robert Wood Johnson Foundation. In 3 years the work of this PBRN has lowered hemoglobin A1c levels by 1 percentage point—enough to reduce risks of complications—in a population of 27,000 patients with type II diabetes (Case Western Reserve University CTSC, 2012; Pulley, 2013a).
• University of Cincinnati’s Community Leaders Institute: a 6-week training program for community leaders designed to “assist agencies that engage and empower communities to reduce health, social and educational disparities in leveraging funding and learning how to use data to improve services and programs.” Since the program started in 2010, 41 community leaders have completed it. The first cohort of the program, which had 9 people, has secured more than $1.3 million in grants for their community organizations. Previous participants included individuals from such diverse local organizations as the YMCA of Greater Cincinnati, the Cincinnati Health Department, Lincoln Heights Missionary Baptist Church, and Sickle Cell Affected Families of Greater Cincinnati (Pulley, 2013a; University of Cincinnati CCTST, 2013).
• Scripps Translational Science Institute Community Engagement Program: a partnership with the Scripps Whittier Diabetes Institute developed to improve prevention and treatment strategies for diabetes in a high-risk population—individuals in the San Diego area with Mexican
ancestry. To achieve these goals, the program leverages genomic sciences, wireless technologies, established community partnerships, and culturally appropriate approaches to community education and health care. The program also includes a diabetes gene bank and a study on gestational diabetes (NIH, 2012a; STSI, 2013).
Although many compelling examples of community engagement exist, assessing how widespread community engagement really is and at what level it is occurring is difficult. Hood and colleagues (2010) conducted a survey to establish a baseline of community engagement across research being funded by the NIH at one midwestern university with a CTSA. Of the 194 NIH-funded studies (out of 480) for which responses were received, fewer than half (43 percent) included community engagement activities at any level. Of the studies that included a community engagement component, only 17 percent reported meaningful community engagement that involved significant collaborative actions. These results prompted the investigators to recommend that CTSAs clarify the goals of community engagement and determine “whether community engagement programs should strive to increase the number of authentic CBPR studies, increase less intensive community engagement activities in all NIHfunded research, or both” (Hood et al., 2010, p. 22).
Metrics and Evaluation
Community engagement should be evaluated, just as any other aspect of the CTSA program, using clear and innovative metrics that can be applied uniformly and consistently at individual CTSAs and across the program. To date, a formal evaluation of the community engagement aspects of the CTSA Program as a whole has not been conducted. In recent stakeholder input provided by the Center for Community Health Education Research and Service and the CCPH, one respondent wrote that the lack of common metrics “hurts [the] CSTA [community engagement] programs’ ability to measure, document, [and] communicate their value in a way that’s understood by CTSA leadership locally and nationally” (Freeman and Seifer, 2013).
The CTSA Community Engagement Key Function Committee has a working group to develop a uniform set of outcomes and measurements. This working group identified examples of community-engaged research and developed a logic model to guide development of community engagement metrics (Eder et al., 2013) (see Figure 4-1). Additional possible
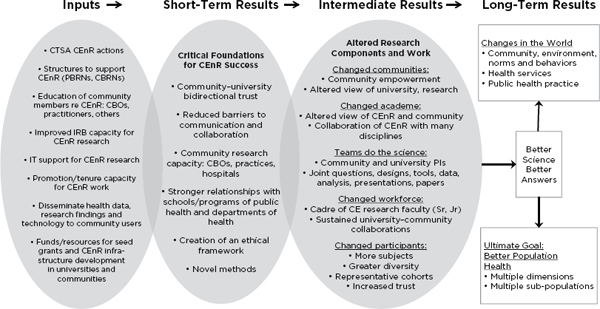
FIGURE 4-1 A logic model to guide community engagement metrics.
NOTE: CBOs = community-based organizations; CBRNs = community-based research networks; CE = community engagement; CEnR = community engagement research; IRB = institutional review board; IT = information technology; PBRNs = practice-based research networks.
SOURCE: Adapted with permission from Eder et al., 2013.
measures that have been suggested by researchers and community leaders include the following: extent of community partner integration into the research teams, documented research outcomes (e.g., community health improvements and outcomes, policy changes, successful translation), allocation of funds to community partners, and number of new and sustained community partnerships (Freeman and Seifer, 2013).
Opportunities and Next Steps
Because involving the community in the continuum of research is a new experience for many researchers, the CTSA Program and NCATS must provide clear guidance and leadership that effectively defines and communicates their goals and expectations. A number of barriers need to be overcome in order to establish effective community partnerships, including issues related to trust and respect, understanding the benefits and value of community engagement, challenges within academic cultures, a lack of clear expectations and protocols for engagement, and a lack of funding to compensate and provide training and education for community partners (Freeman and Seifer, 2013; Spofford et al., 2012). Models from individual CTSAs that have effectively incorporated members of the community throughout the research and translation process should be actively disseminated.
Although there is still much work to be done to integrate communities fully into all aspects of the CTSA Program, opportunities to embrace community engagement fully are increasing and are showing great promise. Electronic health records, social networking, and an increasingly active and sophisticated disease and health advocacy community can and should accelerate progress toward improvements in health status and reductions in health disparities. Partnerships with community engagement efforts sponsored by other NIH institutes and centers (e.g., the Research Centers in Minority Institutions program) offer potential for increasing the reach of CTSA’s investments and strengthening the program’s community engagement initiatives.
Throughout the study, the IOM committee heard overwhelming support for community engagement as an integral part of the CTSA program. An NIH working group on CTSA/NCATS integration, leaders of the CTSA Consortium, CTSA PIs, Congress, and a range of diverse stakeholders have all voiced support for the broad focus of the program, which encompasses the full spectrum of translational research, including
community engagement (CTSA PIs, 2012; Katz et al., 2011; NCATS, 2012b; Pulley, 2013b; U.S. Congress, 2011). The IOM committee fully supports community engagement and involvement throughout the entire research process and believes that this program component is essential and needs to be preserved, nurtured, and expanded.
Community engagement should not be a stand-alone program requirement; it must be crosscutting and embedded in leadership, implementation, research, and communication strategies across all levels of the CTSA Program. Each CTSA site has unique strengths to build on, in addition to the opportunities afforded by the special interests, characteristics, and needs of the surrounding population or specific patient populations served. Inevitably, geographic and program diversity will lead to variations in the nature of community engagement from one site to another. But without clear incentives, metrics, and evaluation internally and from NCATS, the potential value in engaging community participants in the full range of activities—from basic discovery through patient outcomes—will not be realized.
Recommendation 6: Ensure Community Engagement in All Phases of Research
NCATS and the CTSA Program should ensure that patients, family members, health care providers, clinical researchers, and other community stakeholders are involved across the continuum of clinical and translational research. NCATS and the CTSA Program should
• define community engagement broadly and use this definition consistently in RFAs and communications about the CTSA Program;
• ensure active and substantive community stakeholder participation in priority setting and decision making across all phases of clinical and translational research and in the leadership and governance of the CTSA Program;
• define and clearly communicate goals and expectations for community engagement at the individual CTSA level and across the program and ensure the broad dissemination of best practices in community engagement; and
• explore opportunities and incentives to engage a more diverse community.
For too long, research examining the safety and efficacy of medications and other health interventions has focused on adults, and too little has been known about health- and development-related impacts of medications, devices, and preventive measures on children3 (IOM, 2012b). Because of the paucity of pediatric-specific research, health care providers caring for children often use their own personal experiences in clinical practice, rather than published evidence, as the basis for treatment decisions (Kon, 2008). Thus, clinical and translational research is urgently needed in the area of child health. The IOM committee was specifically asked to look at child health research efforts in the CTSA Program and concluded that the program has placed an appropriate emphasis on accelerating clinical and translational research to improve child health.
The lack of pediatric studies results from a number of factors. Safety and ethical considerations for children participating in clinical trials are of primary concern, including the potential risks of exposing healthy volunteers to medications or other treatments that may have health or developmental side effects. Moreover, although some rare childhood diseases can be catastrophic, most children are healthy, which makes case finding for trials difficult. Additional challenges are the small market for pediatric drugs and devices, the smaller number of potential research participants, and variability among children because of age and developmental factors (IOM, 2012b). Further, because drugs and devices are often already approved for adults, they can be legally prescribed for children and may be widely used off label, before pediatric studies can be completed or even started (Portman, 2012).
Context and Background
Child health has been a focus of the CTSA Program. The NIH Reform Act of 2006 (Public Law 109-482) stipulated that independent
_____________________
3 In 1998 the NIH released policy guidelines for the inclusion of children in research (NIH, 1998). In the past two decades legislation aimed at increasing clinical trials involving children has included the Best Pharmaceuticals for Children Act (Public Law 107-109), the Pediatric Research Equity Act of 2003 (Public Law 108-155), and the Food and Drug Administration Safety and Innovation Act (Public Law 112-144). These initiatives support progress in pediatric clinical studies; however, empirical studies still need to be done in many areas.
funding and infrastructure for pediatric clinical research centers (formerly in the GCRCs) can be maintained. Subsequent to that legislation, the funding announcements for CTSAs have noted that applications can include a second principal investigator with authority for child health research and proposals for designating a separate budget for child health research (Huskins et al., 2012; NIH, 2009a). As of March 2013, 9 of the 61 CTSAs have a pediatrician as the principal investigator, and many have a designated pediatric or child health lead (Collier, 2013a). In addition, 53 CTSAs have some degree of partnership with children’s hospitals (Collier, 2013a). Most CTSAs have pediatric researchers participating on the CTSA Consortium Child Health Oversight Committee (CC-CHOC).
The CC-CHOC was established in 2006 as one of the CTSA Consortium’s leadership committees. Its mission and goals are to provide a national forum to identify collaborative opportunities for facilitating clinical and translational research on child health; to set priorities for the development of collaborative efforts and standard approaches; and to coordinate CTSA-wide efforts on child health research (CTSA Central, 2012a).
Several recent projects suggest the breadth of efforts spearheaded by CC-CHOC with participation by individual CTSAs:
• The Point Person Project works to overcome barriers that have hindered past efforts in pediatric research by ensuring that connections are made to respond to collaborative opportunities among industry, research networks, and investigators with relevant expertise for protocol and trial development and implementation (see Box 4-5).
• CC-CHOC is working toward the harmonization of policy and regulatory aspects of child health research, including efforts to standardize relevant terminology, case definitions, diagnostic criteria, and core outcome measures (CTSA Central, 2012a; Davis, 2012).
• CC-CHOC and participating CTSAs have developed a federated IRB model to provide a thorough and flexible IRB process to facilitate multisite pediatric clinical trials (CTSA Central, 2012a). For example, CC-CHOC has collaborated with the Rare Disease Clinical Research Network in the use of the centralized IRB model to test a treatment for infantile Pompe disease (CTSA Central, 2012a).
BOX 4-5
The Point Person Project
In an effort to increase the efficiency and response rate in multisite pediatric clinical trials, the CTSA Consortium Child Health Oversight Committee initiated the Point Person Project in 2012 (Davis, 2012). This program allows research sponsors, industry representatives, or individual developers to propose and explore interest in a range of child health research studies (CTSA Central, 2012b). A synopsis of the protocol is submitted to the CTSA Coordinating Center and reviewed by the CC-CHOC Operations Group, and, if approved, it is sent to the point person at each of the 55 CTSA sites with a pediatric program (Davis, 2012). The point person gives the synopsis link to investigators in their CTSA with related interests, and these investigators indicate whether they are interested, not interested, or need more information. The responses are entered into a central database, and those who are interested are invited to join a weekly conference call where the project is discussed with the sponsor (Children’s National Medical Center CTSI, 2013). In the first 5 months of this program, 20 protocols were reviewed (Davis, 2012). In addition to facilitating collaborations and expediting the initiation of clinical trials, an additional benefit may be identifying and recruiting experts and collaborators for protocol development (Davis, 2012).
Interdisciplinary and multisite collaborations are particularly important for child health research. Multicenter studies are often necessary because of the small number of children who meet eligibility criteria for clinical trials. The developmental and physiological needs of children differ from those of adults and require the expertise of multiple disciplines in the design and conduct of clinical studies. One of CC-CHOC’s shortterm goals is to enhance interactions with child health research partners in the United States and globally. Efforts are under way to coordinate with NIH-related networks (e.g., Pediatric Trials Network), practicebased research and other research networks (e.g., the American Academy of Pediatrics’ Pediatric Research in Office Settings Network, the Global Alliance for Pediatrics Therapeutics); and international networks (e.g., European Network of Pediatric Research at the European Medicines Agency) (Davis, 2012; Portman, 2012).
Safety and ethical considerations are of primary concern in child health research. CC-CHOC’s Pediatric Research Ethics Workgroup has reviewed protocols submitted by CTSA pediatric investigators to determine trends in IRB decision making and is encouraging shared IRB approaches for multisite trials. The group also facilitates a pediatric research ethics consultation service to link research ethics consultants
and pediatric investigators in order to strengthen requests for protocol approval (CTSA Central, 2013d).
CC-CHOC has ongoing efforts to develop evaluation metrics for child health research (Huskins et al., 2012). Further, it is surveying CTSAs regarding steps needed to enhance career pathways for child health investigators (Davis, 2012).
Opportunities and Next Steps
Leadership, Collaboration, and Evaluation in Child Health Research
The CTSA Program, through CC-CHOC, has made important steps in streamlining and accelerating clinical and translational research specific to the neglected area of child health. To strengthen these efforts, the IOM committee believes that the NCATS-CTSA Steering Committee should identify a relatively small number of CTSAs with established expertise and outstanding efforts in child health research as the leaders in this arena. Those designated CTSAs, in collaboration with CC-CHOC, would be charged with creating focused initiatives to develop key partnerships and collaborations across other CTSAs, and with the NIH and a variety of public and private sector research networks, including industry partners. The goal would be to strengthen the resources and leadership provided for child health research. These CTSAs would spearhead efforts to improve and accelerate clinical and translational research in child health, encourage research participation, and promote career pathways for child health investigators.
Identifying specific CTSAs to take the lead in child health research would not preclude other CTSAs from involvement in this area. Instead, the IOM committee hopes that such focused efforts would encourage and promote collaborations among CTSAs for multisite studies and other efforts. The committee also believes that the CTSAs should be engaged in a life-span approach that includes research on the transition from adolescence into adulthood.
As part of a learning health care system, child health researchers need to be sure that this area of investigation is well positioned to fully embrace the use of electronic health records for research purposes and to actively partner with PBRNs. Implementing these types of strategies will allow researchers to understand what is occurring in clinical practice and
will allow pediatric health care providers, patients, and families to learn about new medications, therapeutics, and preventive measures.
The Involvement of Children, Parents, Family Members, and Community Organizations
Efforts to advance child health research need the active and direct involvement of patients, parents, family members, pediatric and family health care providers, and other community stakeholders in all phases of research. As a part of the team that guides clinical and translational research and encourages participation in this vital area, informed family and community participants will bring practical insights and dedicated commitment to setting research priorities, reviewing protocols, modifying trial designs, and ensuring adherence to research subjects’ protection policies for clinical research involving children. Further, these groups can promote research participation. Recent study findings suggest that families know little about potential opportunities for participation in clinical research and often do not understand its potential benefits (Davis et al., 2013).
Conclusions and Recommendation
Research is needed on medications, devices, and preventive measures that specifically assesses their impact on children, whether they are targeted to specific diseases of children or are adult treatments used for pediatric patients. Primarily because of the much smaller population affected with pediatric diseases, many of these diseases are “orphan conditions,” yet they can be life limiting or result in lifelong mental, physical, or developmental disabilities.
Because of the burden of these conditions, clinical and translational research is of special importance, and the IOM committee believes that the CTSA Program has placed an appropriate emphasis on accelerating clinical and translational research to improve child health. As a strong and vital part of the CTSA Program, the individual CTSAs and CCCHOC have made important steps in this direction. The CTSA Program should continue its role in leading efforts to coordinate and advance child health research by building on the expertise of individual CTSAs and by ensuring that the CTSA Program continues to be a leader in developing and sustaining the collaborations necessary to move these efforts forward.
Recommendation 7: Strengthen Clinical and Translational Research Relevant to Child Health
NCATS should collaborate with CC-CHOC to strengthen clinical and translational research relevant to child health through efforts to
• identify and designate CTSAs with expertise in child health research as leaders in advancing clinical and translational research relevant to child health and as coordinators for CTSA programwide efforts and other collaborative efforts in this research; and
• promote and increase community engagement specific to child health by
![]() raising awareness of the opportunities for children and families to participate in research efforts with clear information conveyed on the risks and potential benefits; and
raising awareness of the opportunities for children and families to participate in research efforts with clear information conveyed on the risks and potential benefits; and
![]() involving parents, patients, and family members more fully at all stages of the research process, including identifying priorities and setting research agendas.
involving parents, patients, and family members more fully at all stages of the research process, including identifying priorities and setting research agendas.
Andrews, J. O., M. J. Cox, S. D. Newman, G. Gillenwater, G. Warner, J. A. Winkler, B. White, S. Wolf, R. Leite, M. E. Ford, and S. Slaughter. 2012. Training partnership dyads for community-based participatory research: Strategies and lessons learned from the community engaged scholars program. Health Promotion Practice., October 22. Published online before print, doi: 10.1177/1524839912461273.
Brady, K. 2012. CTSA strategic goal 4: Enhancing the health of our communities and the nation. PowerPoint presented at Meeting 1: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, October 29. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2012-OCT-29/CTSA%20presentations/5-Brady%20CTSA%20%20SGC4%20slides%20for%20%20IOM.pdf (accessed March 25, 2013).
C3 (Chicago Consortium for Community Engagement). 2013. Welcome to the C3 network. http://c3ctsa.org (accessed March 25, 2013).
Carter-Edwards, L., J. L. Cook, M. A. McDonald, S. M. Weaver, K. Chukwuka, and M. M. Eder. 2013. Report on CTSA consortium use of the community
engagement consulting service. Clinical and Translational Science 6(1):34– 39.
Case Western Reserve University CTSC (Clinical and Translational Science Collaborative). 2012. Clinical and Translational Science Collaborative: Key achievements, 2007–2012. http://casemed.case.edu/ctsc/calendar/news/achievements.cfm (accessed March 25, 2013).
CCPH (Community-Campus Partnerships for Health). 2012a. CCPH’s response to NOT-TR-13-001 Request for Information: Enhancing communityengaged research through the CTSA Program. http://depts.washington.edu/ccph/pdf_files/CCPH-RFI-Nov2012F3.pdf (accessed March 22, 2013).
———. 2012b. Letter to Christopher P. Austin, Director of the National Center for Advancing Translational Sciences. September 28. Submitted to the IOM Committee on October 12, 2012. Available by request through the National Academies’ Public Access Records Office.
CDC and ATSDR (Centers for Disease Control and Prevention and Agency for Toxic Substances and Disease Registry). 1997. Principles of community engagement. http://www.cdc.gov/phppo/pce (accessed March 22, 2013).
Ceglia, L. 2013. Presentation: KL2 award: One researcher’s experience. Remarks presented at Meeting 3: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, January 24.
Children’s National Medical Center CTSI (Clinical and Translational Science Institute). 2013. Introducing the Point Person Project. http://ctsicn.org/2013/01/introducing-the-point-person-project/ (accessed February 18, 2013).
Collier, E. 2013a. Responses to committee questions. Submitted to the IOM Committee on March 27. Available by request through the National Academies’ Public Access Records Office.
———. 2013b. Written comments regarding career development programs. Submitted to the IOM Committee on April 2. Available by request through the National Academies’ Public Access Records Office.
Community Partners (Community Recommendations Working Group of Community Partners). 2009. Recommendations for community involvement in National Institute of Allergy and Infectious Diseases HIV/AIDS clinical trials research. Washington, DC: National Institutes of Health. http://www.niaid.nih.gov/about/organization/daid/Networks/Documents/cabrecommendations.pdf (accessed March 22, 2013).
CTSA (Clinical and Translational Science Awards) Central. 2011. Core competencies for clinical and translational research. https://www.ctsacentral.org/education_and_career_development/core-competencies-clinical-and-translational-research (accessed March 22, 2013).
———. 2012a. 2012 Annual report: CTSA Consortium Child Health Oversight Committee. https://www.ctsacentral.org/sites/default/files/documents/2012%20CC%20CHOC%20Annual%20Report.pdf (accessed March 20, 2013).
———. 2012b. CC-CHOC Pediatric Point Person Project. www.ctsacentral.org/documents/point-person-project (accessed April 22, 2013).
———. 2013a. About the CTSA Consortium. https://www.ctsacentral.org/aboutus/ctsa (accessed February 13, 2013).
———. 2013b. Chicago Consortium for Community Engagement. http://www.ctsacentral.org/regional-consortia/chicago-consortium-community-engagement (accessed March 25, 2013).
———. 2013c. Community Engagement Key Function Committee.https://www.ctsacentral.org/committee/community-engagement (accessed March 25, 2013).
———. 2013d. Pediatric research ethics consultation service. https://www.ctsacentral.org/articles/pediatric-research-ethics-consultationservice (accessed March 20, 2013).
———. 2013e. Strategic Goal Committee 2—training and career development of clinical/translational scientists. https://www.ctsacentral.org/committee/sg2-training-and-career-development-clinicaltranslational-scientists (accessed March 11, 2013).
———. 2013f. Strategic Goal Committee 4—enhancing the health of our communities and the nation. https://www.ctsacentral.org/committee/sg4-enhancing-health-our-communities-and-nation (accessed March 25, 2013).
CTSA PIs (Principal Investigators). 2012. Preparedness of the CTSA’s structural and scientific assets to support the mission of the National Center for Advancing Translational Sciences (NCATS). Clinical and Translational Science 5(2):121–129.
Davis, J. 2012. The role of CC-CHOC in maternal child research. PowerPoint presented at Meeting 2: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, December 12. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2012-DEC-12/2-1%20%20Jonathan%20Davis.pdf (accessed February 18, 2013).
Davis, M. M., S. J. Clark, A. T. Butchart, D. C. Singer, T. P. Shanley, and D. S. Gipson. 2013. Public participation in, and awareness about, medical research opportunities in the era of clinical and translational research. Clinical and Translational Science 6(2):88–93.
Dieffenbach, C. 2011. Community engagement in NIAID’s HIV/AIDS clinical trials networks. http://blog.aids.gov/2011/09/community-engagement-inniaid%E2%80%99s-hivaids-clinical-trials-networks.html (accessed March 22, 2013).
Dolor, R. J., S. M. Greene, E. Thompson, L.-M. Baldwin, and A. V. Neale. 2011. Partnership-Driven Resources to Improve and Enhance Research (PRIMER): A survey of community-engaged researchers and creation of an online toolkit. Clinical and Translational Science 4(4):259–265.
Duke Center for Community Research. 2013. Community Engagement Consultative Service (CECS). https://www.dtmi.duke.edu/about-us/organization/duke-center-for-community-research/community-engagement-consultative-servicececs (accessed March 25, 2013).
Eder, M., L. Carter-Edwards, T. C. Hurd, B. B. Rumala, N. Wallerstein. 2013. A logic model for community engagement within the CTSA Consortium: Can we measure what we model? Academic Medicine 88(10).
Edwards, K. 2013. Using CTSAs to leverage change: New investigators, new science. PowerPoint presented at Meeting 3: Committee to Review the CTSA Program at NCATS, Washington, DC, January 24. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2013-JAN-24/Kelly%20Edwards.pdf (accessed April 10, 2013).
Emmons, K. M. 2012. Harvard Catalyst response to RFI NOT-TR-13-001. Submitted to the IOM Committee on February 1, 2013. Available by request through the National Academies’ Public Access Records Office.
ENACCT and CCPH (Education Network to Advance Cancer Clinical Trials and Community-Campus Partnerships for Health). 2008. Communities as partners in cancer clinical trials: Changing research, practice and policy. Silver Spring, MD: ENACCT. http://www.enacct.org/sites/default/files/CommunitiesAsPartners_Report_12_18_08_0.pdf (accessed March 22, 2013).
Fleming, M., E. L. Burnham, and W. C. Huskins. 2012. Mentoring translational science investigators. JAMA 308(19):1981–1982.
Freeman, E. R., and S. Seifer. 2013. A Delphi process to solicit stakeholder feedback for the IOM Committee Review of the CTSA Program. Submitted to the IOM Committee on March 3. Available by request through the National Academies’ Public Access Records Office.
HANC (HIV/AIDS Network Coordination). 2013a. Community Partners. https://www.hanc.info/cp/Pages/default.aspx (accessed March 22, 2013).
———. 2013b. The Legacy Project. https://www.hanc.info/legacy/Pages/default.aspx (accessed March 22, 2013).
Harvard Medical School DCP (Office for Diversity Inclusion and Community Partnership). 2013. The Harvard Catalyst Program for Faculty Development and Diversity. Program for college students: Summer Clinical and Translational Research Program. http://www.mfdp.medp.med.harvard.edu/Catalyst/CollegeStudents.html (accessed April 1, 2013).
Hatcher, M. T., and R. M. Nicola. 2008. Building constituencies for public health. In Public health administration: Principles for population-based management. Vol. 2, edited by L. F. Novick, C. B. Morrow and G. P. Mays. Sudbury, MA: Jones and Bartlett. Pp. 443–458.
Healing of the Canoe. 2013. The Healing of the Canoe Project. http://healingofthecanoe.org (accessed March 19, 2013).
Hood, N. E., T. Brewer, R. Jackson, and M. E. Wewers. 2010. Survey of community engagement in NIH-funded research. Clinical Translational Science 3(1):19–22.
Horowitz, C. R., M. Robinson, and S. Seifer. 2009. Community-based participatory research from the margin to the mainstream: Are researchers prepared? Circulation 119(19):2633–2642.
HPS (Hermansky-Pudlak Syndrome) Network. 2013. Hermansky-Pudlak Syndrome Network, Inc. http://www.hpsnetwork.org (accessed March 18, 2013).
Huskins, W. C., K. Silet, A. M. Weber-Main, M. D. Begg, V. G. Fowler, J. Hamilton, and M. Fleming. 2011. Identifying and aligning expectations in a mentoring relationship. Clinical and Translational Science 4(6):439–447.
Huskins, W. C., C. D. Sullivan, J. Wang, M. Aitken, S. R. Alexander, L. G. Epstein, A. Hoberman, E. Neufeld, A. Philipps, T. P. Shanley, P. Szilagyi, M. Purucker, and S. L. Barkin. 2012. Tracking the impact of the National Institutes of Health Clinical and Translational Science Awards on child health research: Developing and evaluating a measurement strategy. Pediatric Research 71(5):619–624.
Indiana University CTSI (Clinical and Translational Sciences Institute). 2012. CORUS (Community Research Utilities and Support): Working together to advance community engaged research. https://ctsacorus.org/home (accessed March 25, 2013).
IOM (Institute of Medicine). 2012a. Primary care and public health: Exploring integration to improve population health. Washington, DC: The National Academies Press.
———. 2012b. Safe and effective medicines for children: Pediatric studies conducted under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. Washington, DC: The National Academies Press.
———. 2013. Responses to public input questions regarding the CTSA Program at NCATS. Submitted to the IOM Committee between December 17, 2012–March 1, 2013. Available by request through the National Academies’ Public Access Records Office.
ITHS (Institute of Translational Health Sciences). 2013. Education core. www.iths.org/ED (accessed April 10, 2013).
Kagan, J. M., S. R. Rosas, R. L. Siskind, R. D. Campbell, D. Gondwe, D. Munroe, W. M. K. Trochim, and J. T. Schouten. 2012. Communityresearcher partnerships at NIAID HIV/AIDS clinical trial sites: Insights for evaluation and enhancement. Progress in Community Health Partnerships: Research, Education, and Action 6(3):311–320.
Katz, S., J. Anderson, H. Auchincloss, J. Briggs, A. Guttmacher, K. Hudson, R. Hodes, W. Koroshetz, R. Ranganathan, G. Rodgers, and S. Shurin. 2011. NIH CTSA/NCATS Integration Working Group recommendations. http://www.ncats.nih.gov/files/recommendations.pdf (accessed April 8, 2013).
Kelley, M., K. Edwards, H. Starks, S. M. Fullerton, R. James, S. Goering, S. Holland, M. L. Disis, and W. Burke. 2012. Values in translation: How asking the right questions can move translational science toward greater health impact. Clinical and Translational Science 5(6):445–451.
Kon, A. A. 2008. Real pragmatism, kids, and the Clinical and Translational Science Award (CTSA). American Journal of Bioethics 8(4):45–47.
Lee, L. S., S. N. Pusek, W. T. McCormack, D. L. Helitzer, C. A. Martina, A. M. Dozier, J. S. Ahluwalia, L. S. Schwartz, L. M. McManus, B. D. Reynolds, E. N. Haynes, and D. M. Rubio. 2012. Clinical and translational scientist career success: Metrics for evaluation. Clinical and Translational Science 5(5):400–407.
Martinez, L. S., B. Russell, C. L. Rubin, L. K. Leslie, and D. Brugge. 2012. Clinical and translational research and community engagement: Implications for researcher capacity building. Clinical and Translational Science 5(4):329–332.
Meagher, E. A. 2011. Training translators in the PENN CTSA. Clinical and Translational Science 4(5):314–316.
Meyers, F. J., M. D. Begg, M. Fleming, and C. Merchant. 2012. Strengthening the career development of clinical translational scientist trainees: A consensus statement of the Clinical Translational Science Award (CTSA) research education and career development committees. Clinical and Translational Science 5(2):132–137.
Michener, L., J. Cook, S. M. Ahmed, M. A. Yonas, T. Coyne-Beasley, and S. Aguilar-Gaxiola. 2012. Aligning the goals of community-engaged research: Why and how academic health centers can successfully engage with communities to improve health. Academic Medicine 87(3):285–291.
Miyaoka, A., M. Spiegelman, K. Raue, and J. Frechtling. 2011. Findings from the CTSA National Evaluation Education and Training Study. Rockville, MD: Westat. https://ctsacentral.org/sites/default/files/documents/educationTrainingReport_20111228.pdf (accessed April 1, 2013).
NCATS (National Center for Advancing Translational Sciences). 2012a. FAQ about CTSA RFA-TR-12-006. http://www.ncats.nigh.gov/research/cts/ctsa/funding/faq/faq.html (accessed March 22, 2013).
———. 2012b. Request for information: Enhancing the Clinical and Translational Science Awards Program. http://www.ncats.nih.gov/files/report-ctsa-rfi.pdf (accessed April 8, 2013).
———. 2013. Scholar and research programs. http://www.ncats.nih.gov/research/cts/ctsa/training/programs/scholar-trainee.html (accessed March 11, 2013).
NIH (National Institutes of Health). 1998. NIH policy and guidelines on the inclusion of children as participants in research involving human subjects. http://grants.nih.gov/grants/guide/notice-files/not98-024.html (accessed March 20, 2013).
———. 2009a. RFA-RM-09-004: Institutional Clinical and Translational Science Award (U54). http://grants.nih.gov/grants/guide/rfa-files/RFA-RM-09-004.html (accessed March 22, 2013).
———. 2009b. RFA-RM-09-019: Institutional Clinical and Translational Science Award (U54). http://grants.nih.gov/grants/guide/rfa-files/RFA-RM-09-019.html (accessed March 22, 2013).
———. 2012a. Enhancing the health of our communities and the nation. Chapter 5. In Progress Report 2009–2011 Clinical and Translational Science Awards: Foundations for accelerated discovery and efficient translation. http://www.ncats.nih.gov/ctsa_2011/ch5.html (accessed April 1, 2013).
———. 2012b. Request for information: Enhancing community-engaged research through the Clinical and Translational Science Awards (CTSA) Program. http://grants.nih.gov/grants/guide/notice-files/NOT-TR-13-001.html (accessed March 22, 2013).
———. 2012c. RFA-TR-12-006: Institutional Clinical and Translational
Science Award (U54). http://grants.nih.gov/grants/guide/rfa-files/rfa-tr-12-006.html (accessed February 13, 2013).
———. 2013a. Increasing the diversity of the NIH-funded workforce: Program initiatives. http://commonfund.nih.gov/diversity/initiatives.aspx (accessed March 11, 2013).
———. 2013b. Notice of intent to publish a funding opportunity announcement for planning grants for the NIH National Research Mentoring Network. http://grants.nih.gov/grants/guide/notice-files/NOT-RM-13-009.html (accessed April 30, 2013).
NIMHD (National Institute on Minority Health and Health Disparities). 2013. NIMHD Research Centers in Minority Institutions Program. http://www.nimhd.nih.gov/our_programs/research_centers.asp (accessed April 30, 2013).
Parsons, S. 2013. Responses to committee questions. Submitted to the IOM Committee on February 26. Available by request through the National Academies’ Public Access Records Office.
Portman, R. 2012. Children’s health research: Role of the CTSA Program in pediatric drug development. PowerPoint presented at Conference Call Meeting 2: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, November 30. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2012-NOV-30/Ronald%20Portman.pdf (accesssed March 20, 2013).
Pulley, J. 2013a. CTSA essays and worksheets. Submitted to the NIH CTSA/NCATS Integration Working Group, July 2011. Submitted to the IOM Committee on January 7. Available by request through the National Academies’ Public Access Records Office.
———. 2013b. CTSA PI response to RFI NOT-TR-12-003. Submitted to the IOM Committee on January 6. Available by request through the National Academies’ Public Access Records Office.
PXE International. 2012. PXE International Blood and Tissue Bank. http://www.pxe.org/blood-tissue-bank (accessed March 22, 2013).
Research Toolkit. 2012. Research toolkit: An active, growing library of resources for conducting health research. http://www.researchtoolkit.org (accessed March 25, 2013).
Roberts, S. F., M. A. Fischhoff, S. A. Sakowski, and E. L. Feldman. 2012. Perspective: Transforming science into medicine: How clinician-scientists can build bridges across research’s “valley of death.” Academic Medicine 87(3):266–270.
Rubio, D. M., B. A. Primack, G. E. Switzer, C. L. Bryce, D. L. Seltzer, and W. N. Kapoor. 2011. A comprehensive career-success model for physicianscientists. Academic Medicine 86(12):1571–1576.
Seifer, S. D. 2013. It’s time to fully realize the potential of community engagement in the CTSA Program. PowerPoint presented at Meeting 3: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, January 24. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2013-JAN-24/Sarena%20Seifer.pdf (accessed March 22, 2013.
Shackelford, D. 2013. Panel 1 presentation. PowerPoint presented at Meeting 3: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, January 24. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2013-JAN-24/David%20Shackelford.pdf (accessed April 2, 2013).
Silet, K. A., P. Asquith, and M. F. Fleming. 2010. A national survey of mentoring programs for KL2 scholars. Clinical and Translational Science 3(6):299–304.
Spofford, M., C. Wilkins, C. McKeever, and N. Williams. 2012. Community representatives’ involvement in CTSA activities: Summary. Submitted to the IOM Committee on November 14. Available by request through the National Academies’ Public Access Records Office.
Staley, K. 2009. Exploring impact: Public involvement in NHS, public health and social care research. Eastleigh, UK: Involve. http://www.invo.org.uk/wpcontent/uploads/2011/11/Involve_Exploring_Impactfinal28.10.09.pdf (accessed March 22, 2013).
STSI (Scripps Translational Science Institute). 2013. Community engagement. http://www.stsiweb.org/index.php/community (accessed March 25, 2013).
Task Force on the Principles of Community Engagement (Clinical and Translational Science Awards Consortium Community Engagement Key Function Committee Task Force on the Principles of Community Engagement). 2011. Principles of community engagement: Second edition. NIH Publication No. 11-7782. http://www.atsdr.cdc.gov/communityengagement/pdf/PCE_Report_508_FINAL.pdf (accessed April 2, 2013).
Thomas, L. R., D. M. Donovan, and R. L. W. Sigo. 2010. Identifying community needs and resources in a native community: A research partnership in the Pacific Northwest. International Journal of Mental Health and Addiction 8(2):362–373.
Thomas, S. 2013. Presentation: Roundtable discussion: Future directions for the mission and goals of the CTSA Program. PowerPoint presented at Meeting 3: Committee to Review the CTSA Program at NCATS, Washington, DC, January 24.
Tillman, R. E., S. Jang, Z. Abedin, B. F. Richards, B. Spaeth-Rublee, and H. A. Pincus. 2013. Policies, activities, and structures supporting research monitoring: A national survey of academic health centers with clinical and translational science awards. Academic Medicine 88(1):90–96.
University of California, San Francisco CTSI (Clinical and Translational Science Institute). 2013. Mentor development program: Course materials. http://accelerate.ucsf.edu/training/mdp-materials (accessed April 10, 2013).
University of Cincinnati CCTST (Center for Clinical and Translational Science and Training). 2013. University of Cincinnati: Center for Clinical and Translational Science and Training. http://cctst.uc.edu/programs/community/cli (accessed March 25, 2013).
University of Iowa ICTS (Institute for Clinical and Translational Science). 2013. Virtual University. https://virtualu2.icts.uiowa.edu (accessed March 11, 2013).
University of Pennsylvania ITMAT (Institute for Translational Medicine and Therapeutics). 2013. CTSA ITMAT education. http://www.itmat.upenn.edu/ctsa/ctsa_education.shtml (accessed March 11, 2013).
University of Rochester CTSI (Clinical and Translational Science Institute). 2013. All courses. https://research.urmc.rochester.edu/ncerp/search (accessed March 11, 2013).
U.S. Congress, House of Representatives. 2011. Military Constructions and Veterans Affairs and Related Agencies Appropriations Act: Conference report to accompany HR 2055. 112th Cong., 1st sess. http://www.gpo.gov/fdsys/pkg/CRPT-112hrpt331/pdf/CRPT-112hrpt331.pdf (accessed May 6, 2013).
Van Hartesveldt, C., J. Giordan, and IGERT(Integrative Graduate Educate and Research Traineeship) Program Directors. 2008. Impact of transformative interdisciplinary research and graduate education on academic institutions. http://www.nsf.gov/pubs/2009/nsf0933/igert_workshop08.pdf (accessed April l0, 2013).
Woolf, S. H. 2008. The meaning of translational research and why it matters. Journal of the American Medical Association 299(2):211–213.
Yarborough, M., K. Edwards, P. Espinoza, G. Geller, A. Sarwal, R. R. Sharp, and P. Spicer. 2012. Relationships hold the key to trustworthy and productive translational science: Recommendations for expanding community engagement in biomedical research. Clinical and Translational Science, January 14. Published online before print, doi: 10.1111/cts.12022.
Young, L. R., R. Pasula, P. M. Gulleman, G. H. Deutsch, and F. X. McCormack. 2007. Susceptibility of Hermansky-Pudlak mice to bleomycin-induced type II cell apoptosis and fibrosis. American Journal of Respiratory Cell and Molecular Biology 37(3):67–74.






































