As envisioned by the Institute of Medicine (IOM) committee, the Clinical and Translational Science Awards (CTSA) Program has the potential to overcome many bottlenecks and pioneer new solutions that can be used to accelerate clinical and translational research. Accomplishing the tasks ahead, however, will require a revitalized approach to program leadership—one that builds on the academic homes that have been established and moves toward an integrated network of CTSAs that increasingly applies collaborative and systems-based approaches. Leadership opportunities and challenges facing the CTSA Program are outlined in this chapter with discussion and recommendations related to leadership strategies, organizational structure, collaborations and partnerships, leadership for individual CTSAs, evaluation, and communications. Leading the CTSA Program into its next phase, CTSA 2.0, will involve building on the strengths of individual CTSAs; leveraging the dedication of individuals working in clinical and translational science; and expanding successful collaborative endeavors, both within and outside of the National Institutes of Health (NIH).
Balancing the tensions and benefits of two seemingly contradictory approaches to leadership is one of the challenges inherent in an endeavor with the scope and structure of the CTSA Program. A variety of possible advantages and disadvantages exist for differing leadership approaches. For example, the grass-roots approach to leadership offers the potential for creativity and innovation. It harnesses the dedication and energy of multiple researchers and stakeholders, all with an interest in moving clin-
ical and translational research forward, but all of whom also have ties and obligations to their home institutions. The top-down leadership approach offers the potential for a systems-level perspective, greater focus and direction, and a commitment to progress for the overall research enterprise. However, this approach usually means fewer people will have direct decision-making responsibilities, and it requires careful oversight and coordination to ensure that multiple people and projects are on track and working to meet the same goals. Finding the correct balance between these two approaches will be an important element for future CTSA success.
As CTSA 2.0 moves forward, the IOM committee sees the need for a more centralized approach to leadership, one in which National Center for Advancing Translational Sciences (NCATS) plays a more active role. To date, the program has, for the most part, relied on the energy and efforts of individual CTSAs and their principal investigators (PIs). This has created a largely ad hoc structure and process for identifying next steps and overall management. Direction from the NIH (first through the National Center for Research Resources [NCRR] and more recently through NCATS) has been articulated primarily through the funding announcements. With each cycle of applications for new CTSAs or renewals, these announcements have emphasized specific key functions or priorities for the investigators to include in their applications.
The mechanism by which the CTSA Program is funded gives NCATS the opportunity to lead awardees toward fulfilling the NIH’s vision for the program both in the performance of individual institutions and in the program’s overall achievements. The NIH has three funding mechanisms for making research awards—grants, contracts, and cooperative agreements. The individual CTSAs and the coordinating center are funded through cooperative agreements. The salient feature of cooperative agreements is that NIH staff members provide assistance to awardees “above and beyond the levels usually required for program stewardship of grants. This level of stewardship is known as substantial involvement” (OIG, 2011, p. i). Substantial involvement can be achieved through various means, including technical assistance, advice, and coordination, and the most recent request for applications (RFA) for the CTSA Program noted that “substantial involvement means that, after award, NIH staff will assist, guide, coordinate, or participate in project activities” (NIH, 2012c).
Cooperative agreements provide the structure and mechanisms with which NCATS can exert a stronger leadership role while also promoting collaboration and innovation by individual CTSAs and researchers and
guiding the program forward. As described throughout this chapter, the IOM committee urges NCATS to take a more active role in the direction and oversight of the CTSA Program. A number of lessons can be learned from a report on the program’s early experience that was prepared by the Department of Health and Human Services’ (HHS’s) Office of the Inspector General (OIG) (discussed below), as well from insights gained from the management of other collectives of academic institutions working toward specific goals, such as the Human Genome Project (HGP). Although there are several differences between the HGP and the CTSA Program, they share many common elements, such as the following:
• an emphasis on innovation, supported by the development of new technologies and databases;
• the expectation of useful, actionable results and a need for ongoing evaluation;
• an emphasis on efficiency and timely outcomes;
• the development of a parallel research effort in bioethics and, in the CTSA case, community involvement; and
• a commitment to collaborative, team-based science and to widely sharing tools and results.
An analysis of the management of HGP identified five key factors in the project’s success. HGP had (1) a clear goal; (2) a flexible organizational structure (the “bottom-up” approach); (3) political support; (4) competition; and (5) strong leadership (the “top-down” approach) (Lambright, 2002). The Lambright analysis of the program says that, of these factors, “the fifth was the most important because it pulled the other factors together and made the most of them when it counted” (2002, p. 5). Leadership manifested in different ways over the life of the project and ultimately provided a balance between the top-down and bottom-up leadership approaches that not only promoted flexibility and innovation but also provided the direction and oversight needed to achieve outcomes.
Although the purpose and tasks of the two programs were widely different, a number of management and leadership lessons can be learned from the HGP that could be useful in the administration of the CTSA Program. For example, as with the HGP, a compelling vision, clearly articulated goals, and a mission-oriented approach could be used to organize and align the work of the individual CTSAs. In addition, the combination of flexibility and active leadership that was used in the HGP to promote innovation and excellence in a pool of talented, multidiscipli-
nary researchers collaborating across multiple research centers is also applicable to the CTSA Program.
Although collaboration was a key element of the HGP, the types of collaboration and partnerships necessary to define and achieve success for the CTSA Program are very different (e.g., community partnerships, collaboration with industry) and will require new leadership strategies.
In 2011, the HHS OIG conducted a review of the administration of the CTSA Program in which OIG reviewers assessed files for the 38 cooperative agreements awarded from 2006 through 2008 (when the program was administered by the NCRR). This assessment found numerous lapses in program oversight. In addition to the administrative critiques described in the report, the OIG found no evidence that NIH program staff provided the “substantial involvement” required by federal regulations and NIH policy with respect to cooperative agreements. In fact, OIG reviewers found no documentation of technical assistance by project scientists for any of the cooperative agreements. Further, they found no evidence that project scientists assisted awardees in performing project activities; stopped activities that were not meeting performance requirements; reviewed or approved the various stages of projects; approved the selection of key personnel, subawardees, or external contractors; conducted technical monitoring; or served on committees (OIG, 2011). The NCRR agreed that relevant information was not in the files in a meaningful way and presented its view that the NCRR worked with awardees “jointly in a partner role but did not assume direction, prime responsibility, or a dominant role” (OIG, 2011, p. 27). Although more project monitoring and aid may have taken place than the files reflect, there is no way to know. The OIG made several recommendations to remedy these shortcomings, including the following:
• “NIH must ensure that staff document awardee accomplishments toward meeting project goals; reasons for not meeting project goals, if applicable; and plans for activities during the coming year.”
• “NIH should ensure that staff document correspondence with awardees as they act to obtain delinquent progress reports and financial status reports.”
• “At a minimum, staff must clearly list the Project Scientists involved and include the annual summary of involvement within the award files” (OIG, 2011, pp. ii–iii).
In considering the next steps for the CTSA Program and the recommendations that are made in this report, the IOM committee believes that NCATS should learn from previous experiences and lessons and take a more active role in the direction and oversight of the CTSA Program. The goal would be to ensure the highest achievable performance of individual CTSAs and to provide stronger guidance toward the development of a national network of institutions engaged in accelerating clinical and translational science. As the program’s newly designated home, NCATS has the opportunity, mission, and purpose to provide leadership for CTSA 2.0 and subsequent program phases.
In addition to setting program direction through a revised mission and strategic goals for the CTSA Program (described in the following section), more active NCATS leadership will require that it take on significant responsibilities in promoting collaborations, conducting evaluations of progress, and ensuring that the program leverages the innovations provided by each of the individual CTSAs and their researchers, leaders, staff, and partners. Striking the right balance between top-down and grass-roots leadership will not be easy, and a number of challenges and possible unintended consequences need to be carefully considered as changes are made to the governance and leadership of the program. For example, imposing a top-down generated research agenda or priorities that do not meet the needs of local researchers, health care providers, and communities would conflict with the original intent and spirit of the program. In addition, an overly directive approach to leadership could dampen creativity, ingenuity, and collaboration among the on-the-ground researchers. More active, but appropriately balanced, leadership from NCATS, combined with the creative talents and leadership from the PIs, will be critical in moving the CTSA Program to a more systems- and network-based approach to clinical and translational research and ensuring that the program remains focused on the outcomes most relevant to its overarching mission.
Given the size of the CTSA Program, appropriate feedback loops and checks and balances should be incorporated into the program’s governance, particularly through the vice-chair and other PIs on the new steering committee that is recommended below. These bidirectional communication and governance strategies will help achieve balanced and informed leadership and will ensure that the active engagement of the onthe-ground perspectives and expertise is maintained and promoted.
Defining the Mission and Goals of the CTSA Program
When NCATS was created in December 2011, components of various NIH programs were moved into the new center. These components include the CTSA Program, the Office of Rare Diseases Research, a variety of programs and activities that are housed in the NCATS Office of the Director for re-engineering translational research,1 and the newly created Cures Acceleration Network (IOM, 2012; NCATS, 2013c). All of these diverse activities must work together in order to fulfill the NCATS mission. Maximizing the benefits of bringing these various elements together within NCATS requires rethinking the missions and goals of the individual pieces. The need for greater alignment within and across HHS agencies and activities was a major theme of a 2009 IOM report, HHS in the 21st Century: Charting a New Course for a Healthier America, which concluded that better alignment and focus on performance were essential to meeting departmental and agency goals (IOM, 2009).
Mission
As the CTSA Program matures, it is important to revisit the missions of NCATS and the CTSA Program to ensure alignment and that the CTSA Program supports the mission of NCATS. This IOM committee was specifically asked for its assessment of the appropriateness of the CTSA Program’s mission and goals. Box 3-1 contains the current mission statements of NIH, NCATS, and the CTSA Program. In considering the appropriateness of the CTSA mission, the committee heard both support for preserving coverage of the full spectrum of clinical and translational research from T0–T4 and concern about the feasibility of doing so, given the limited available resources (IOM, 2013a). Tension about the scope of mission and uncertainty about the adequacy of resources is also reflected in the input that NIH received, in response to a request for information (RFI), regarding ways to improve the program (Mulligan, 2012; NCATS, 2012c).
The IOM committee noted that the current mission of NCATS could be interpreted as being more narrowly focused on the development of diagnostics and therapeutics than on the more global mission of the
_____________________
1 For example, the tissue chip for drug screening initiative, activities related to rescuing and repurposing drugs, and activities related to identifying and validating drug targets.
BOX 3-1
Aligning the Missions
Current Mission Statements
NIH Mission: to seek fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce the burdens of illness and disability.
NCATS Mission: to catalyze the generation of innovative methods and technologies that will enhance the development, testing, and implementation of diagnostics and therapeutics across a wide range of human diseases and conditions.
CTSA Current Program Mission: seeks to strengthen the full spectrum of translational research. Institutional CTSA awards are the centerpiece of the program, providing academic homes for translational sciences and supporting research resources needed by local and national research communities to improve the quality and efficiency of all phases of translational research. Institutional CTSAs also support the training of clinical and translational scientists and the development of all disciplines needed for a robust workforce for translational research.
Proposed CTSA Program Mission Statement
A Suggested Streamlined Mission: to improve the quality and efficiency of the full spectrum of clinical and translational research and to speed the development and use of new diagnostics, therapeutics, and preventive interventions.
SOURCES: NCATS, 2013a,b; NIH, 2011.
CTSA Program, which focuses on the full spectrum of clinical and translational research including preventive interventions and translation into front-line clinical and community practice. The committee concurs with NCATS’s recent decision to allow increased flexibility for individual CTSAs in meeting program requirements, while ensuring that the CTSA Program as a whole continues to support the full spectrum of clinical and translational research.
The current CTSA Program mission statement conflates mission and goals. As indicated below, strategic goals should be separated from the mission, and both need to be clear and consistent. The most recent RFA refers to a slightly different mission than that shown in Box 3-1, stating that the program works toward “increased quality, efficiency, and de-
creased cost of all translational research within academic institutions and nationally” (NIH, 2012c).
A revamping of the mission should strive for simplicity and should reflect the program’s overarching purpose. In working to update the mission of the CTSA Program, the committee suggests that NCATS also consider whether revisions to its own mission statement would help achieve better alignment between the two missions, highlighting support for the full spectrum of clinical and translational research. The committee’s suggested streamlined mission for the CTSA Program also is provided in Box 3-1.
Goals
Although the difference between mission and goals may seem largely semantic, the lack of separately articulated, achievable goals—versus a broad mission—weakens the ability to measure overall progress and establish accountability. As the CTSA Program has grown and evolved, variations on the goals have been cited, which indicates the goals of the program have not been communicated consistently and may not be well understood. For example, a CTSA fact sheet notes that “its goals are to accelerate the translation of laboratory discoveries into treatments for patients, to engage communities in clinical research efforts, and to train a new generation of clinical and translational researchers” (NCATS, 2012a), and the recent RFA said that “the goal of the CTSA Program remains focused on integrated academic homes for the clinical and translational sciences that increase the quality, safety, efficiency and speed of clinical and translational research, particularly for NIH supported research” (NIH, 2012c). Most recently, the program’s website describes goals for the “next phase” of the CTSA Program:
• “Building a better bridge between pre-clinical and clinical science;
• Providing a foundation of shared resources that could reduce costs, delays and difficulties experienced in clinical research, including trials;
• Developing partnerships for research to be better integrated across sites and into ongoing patient care; and
• Strengthening strategies for engaging patient communities into the research process” (NCATS, 2013b).
The issue of CTSA goals is further complicated by the CTSA Consortium’s separate set of five strategic goals:
1. National clinical and translational research capability;
2. Training and career development of clinical and translational scientists;
3. Consortiumwide collaborations;
4. The health of our communities and the nation; and
5. T1 translational research (CTSA Central, 2013a).
As noted in Chapter 1, these consortium-generated goals were developed through a strategic planning process conducted by the PIs in 2008, with the fifth goal added in 2009 (Disis, 2012; Reis et al., 2010). The IOM committee believes that these goals are overly broad and cannot be easily measured. In addition, because innumerable confounding factors in the clinical and translational ecosystem influence progress in these areas (see Chapter 2), the direct impact of the CTSA Program cannot be assessed. As the various lists of goals have grown apart, it is not clear which set, if any, accurately reflects the current and most pressing challenges facing the clinical and translational research ecosystem or the goals that NCATS has for CTSA 2.0.
Clearly defined, measurable goals directly tied to the mission and work of the CTSA Program will help better align the 61 awardees to achieve the vision for a more integrated network and will provide a basis for evaluation, reporting, and accountability. Clearer communication regarding the goals, as distinct from the mission, also would facilitate program management and increase understanding of the program among its stakeholders.
Reshaping and Reconciling Mission and Goals for the Future
NCATS needs to take a leadership role in shaping the CTSA Program’s future by engaging in a strategic planning process in collaboration with the CTSAs to revise the program’s mission and establish measurable goals. As noted, the work of the CTSA Program has numerous important audiences and touches people in many domains—researchers, educators, clinicians, health care providers, payers, policy makers, staff in other government departments and agencies (e.g., Department of Veterans Affairs, Agency for Healthcare Research and Quality [AHRQ], Centers for Medicare & Medicaid Services, Patient-
Centered Outcomes Research Institute, and the other NIH institutes and centers), private industry, nonprofit funding agencies, and, ultimately most important, communities, patients, and families. All these groups have a vital interest in achieving a more efficient and rapid clinical and translational research enterprise (see, for example, IOM, 2011), and they should be asked for input during the strategic planning process.
In the transition of the CTSA Program from NCRR to NCATS, the NIH sought internal and external advice through an 11-member NIH working group. This group was asked to “enumerate the roles and capabilities of the CTSAs that could support and enhance the mission of NCATS; identify CTSA needs and priorities; and propose processes for ensuring a smooth transition from the NCRR to NCATS” (Katz et al., 2011). The working group consulted widely with individuals involved in the program in developing its recommendations for a smooth integration. In addition, NIH issued an RFI for input from public stakeholders, NIH personnel, and CTSA PIs that generated 139 responses, mostly from CTSA institutions (Mulligan, 2012; NCATS, 2012c). Conducting such broad-based outreach (as well as using the input already collected as a departure point) might be a useful strategy in assuring that relevant viewpoints about the program’s mission and goals are considered.
A particular benefit of the collaborative approach to developing plans for integrating the CTSA program into NCATS was the positive response from the individual CTSAs, which demonstrated their “deep commitment to the NCATS mission,” willingness to move forward rapidly, and recognition of new opportunities that NCATS would create, including greater visibility and closer, more transparent working relationships with NIH institutes and centers (CTSA PIs, 2012).
Whatever process is adopted should result in a compelling mission statement and a single set of strategic goals that
• are clearly defined and measurable;
• reflect the full range of clinical and translational research;
• are targeted at overcoming specific, current research challenges and barriers;
• encourage clear decision points (go/no-go decisions) that promote a flexible and dynamically responsive program;
• build on program successes and reinforce areas of progress (e.g., training and education, community engagement, child health);
• are fully supported and consistently communicated by all those involved; and
• can serve as the basis for developing a set of common metrics for evaluating the individual CTSAs and the program as a whole.
All components of the CTSA Program—NCATS, CTSA consortium committees, the CTSA Coordinating Center, individual CTSAs, and the researchers whose work is supported through the program—should be focused on achieving these unified goals. This is essential in order to establish accountability and assess progress, as outlined in HHS in the 21st Century (IOM, 2009). As the CTSA Program moves forward, these goals should be reviewed and updated periodically as progress is made, as the research ecosystem continues to evolve, and as population health needs change.
The CTSA Program has grown rapidly, from 12 sites in 2006 to 61 in 2012. From the program’s outset, the NIH and the individual CTSAs have recognized the value of collaboration and the need for a crossinstitutional structure to advance the program’s efforts; this recognition was the beginning of the vision for a CTSA network. In the early stages of the program, the NIH charged CTSAs with developing a national consortium to promote and implement best practices, policies, and procedures (NIH, 2005). The first funding announcement stated that a National CTSA Consortium Steering Committee should be organized for PIs. In addition, as discussed in Chapter 1, it directed that subcommittees be formed to foster advances in the NIH-identified common themes (e.g., education, informatics, regulation) and that these committees meet annually and have representation from each CTSA (NIH, 2005).
In subsequent years, the CTSA Consortium developed largely as an unfunded grass-roots effort through the commitment and energy of PIs and researchers. It now has three leadership committees (Executive Committee, Steering Committee, and Child Health Oversight Committee); committees charged with making progress on each of the 5 CTSA Consortium strategic goals; 14 key function committees, 10 thematic special interest groups, and numerous working groups and task forces under each of those committees. In total, these committees involve more than 2,000 people (CTSA Central, 2013d; Reis et al., 2010) (see Chapter 1).
A 2011 addition is the CTSA Consortium Coordinating Center, which was awarded to Vanderbilt University through a competitive process (Vanderbilt University, 2011). A variety of collaborative informatics tools have been developed and are being disseminated through the Consortium Coordinating Center. One of the most widely adopted is REDCap (Research Electronic Data Capture), a Web-based tool for creating online surveys and databases used in clinical research. Currently, 602 institutional partners in 54 countries actively participate in REDCap (Vanderbilt University, 2013b). Other examples of research tools that are available through the CTSA Program are provided in Table 3-1. The full range of collaborative tools developed through the CTSA Program should be assessed, and those deemed successful can be deployed further in NIH-funded projects, with data being shared as openly and freely as possible.
TABLE 3-1 Examples of Collaborative Tools
| ROCKET (Research Organization, Collaboration, and Knowledge Exchange Toolkit) | A Web-based tool that provides a common platform for CTSA institutions to share documents and build web pages. ROCKET is designed to be easy for users to edit and maintain their private workspaces but allows specific pages to be made public in order to share information with a larger audience (CTSA Central, 2013g). |
| IRBshare | This shared IRB review model for multisite studies provides an established set of review documents and review processes as well as an IRBshare Master Agreement. Twenty-three sites currently use the model, and it is open to new NIH-funded studies (Vanderbilt University, 2013a). |
| ResearchMatch | An online registry aimed at bringing researchers and volunteers together for health-related research. Volunteers willing to participate in studies complete a questionnaire with their contact information and health history. Registered researchers search the database for individuals who qualify for a particular study. Individuals decide whether to allow ResearchMatch to release their contact information to the researcher. Currently, the service is limited to institutions affiliated with the CTSA Program, and in order to gain access to recruit volunteers, researchers must have IRB approval of their pro |
| posal. More than 35,000 research volunteers are currently registered, and more than 1,600 researchers conducting more than 350 studies at 78 institutions are using this resource (Vanderbilt University, 2012) | |
| eagle-i Network | An openly available online network that anyone can use to search for more than 50,000 biomedical resources at 25 member institutions. Resources available vary from biological specimens and reagents to software and physical laboratory space (Harvard College, 2012). |
| VIVO | An open-source Web application that allows researchers at seven participating institutions to describe their interests, activities, and accomplishments in order to create groups or networks of people with similar research goals within and across institutions (VIVO, 2013). |
| CTSA-IP | A website that compiles and publicly shares information on technology, intellectual property, and licensing opportunities available through more than 24 CTSA institutions. The goal is to promote research activity and collaboration opportunities among CTSAs (University of Rochester, 2012). |
Opportunities and Next Steps
Moving toward systems- and network-based approaches to resolving the challenges in clinical and translational research will require more hands-on leadership from NCATS than in the past; more focused, streamlined, and efficient centralized leadership of the program; and changes in its structure.
The IOM committee believes that CTSA Program governance should be markedly simplified from the current structure, which involves an executive committee and a steering committee, more than 200 committee members total, and separate monthly conference calls. The IOM committee envisions that the primary governance of the program would reside within a new NCATS-CTSA Steering Committee that would be responsible for
• program oversight and direction;
• trans-CTSA activities;
• collaborative efforts with external partners;
• promotion of collaborative opportunities within and outside the NIH;
• identification, dissemination, and implementation of best practices; and
• implementation of a proposed new innovations fund to promote collaboration with other NIH institutes and centers and external partners.
The steering committee should represent a cooperative program leadership effort between NCATS and the CTSAs that provides strategic direction and guides progress to ensure that individual CTSAs and the program as a whole are fulfilling their revised mission and strategic goals. The committee should be chaired by an NCATS lead staff member, with a CTSA PI as vice-chair, and should have a rotating membership representing diverse CTSA and stakeholder interests to ensure responsive and effective governance. The number of steering committee members should be small enough to enable the committee to be nimble and efficient. This steering committee should oversee the Coordinating Center and a streamlined structure of consortium committees (see Figure 3-1). Development of the specific details of committee membership, responsibilities, and operations should be a joint effort between NCATS and the CTSA PIs and should be part of the strategic planning process. This new governance model centralizes accountability for the program and provides a more active leadership role for NCATS, while enabling focused, stakeholder-based leadership that should fully leverage the experience, creativity, and commitment of the CTSA PIs and other stakeholders. As discussed previously, possible disadvantages to a more active leadership role for NCATS should be considered as the governance and structure of the program evolve.
Streamlining the current consortium committee structure is an urgent need. However, the structure and governance should evolve over the next year or two as a component of the recommended strategic planning process. Only those consortium committees that are most relevant to the program’s revised goals and priorities should be retained. The current unwieldy committee setup was perhaps a natural result of the rapid
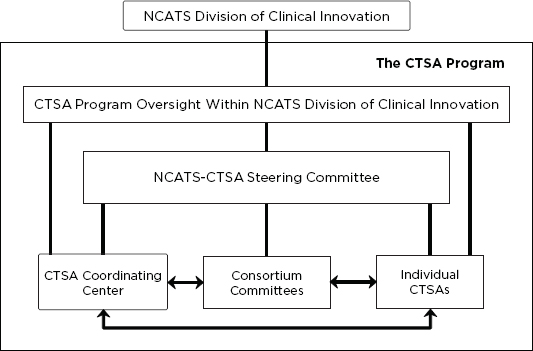
FIGURE 3-1 A revised structure for the CTSA Program in the Division of Clinical Innovation at NCATS. The components of a reorganized CTSA Program would include the staff and CTSA Program oversight activities at NCATS; individual CTSAs; a set of streamlined consortium committees; the CTSA Coordinating Center; and the new NCATS-CTSA Steering Committee, which would provide oversight and direction to the Coordinating Center, the consortium committees, and, to a lesser extent, the individual CTSAs. Leadership, collaboration, and communication among all these entities will be essential for the efficiency and effectiveness of the program overall.
increase in the number of CTSAs, with each wanting to ensure it had a voice in the program’s leadership. However, according to testimony received by the IOM committee and from interviews conducted as part of the Westat site visit evaluation, the current number and size of committees—some having more than 150 members—makes too many burdensome demands on researchers’ time (Westat, 2011).
While the IOM committee recognizes the dedication and commitment many people have given to the program’s work, now is the time to build on what has been accomplished and rechannel that commitment through a leaner structure. The result should be greater management efficiency as well as increased productivity. Larger numbers of stakeholders and CTSA leaders can be convened periodically to communicate progress, solicit input, and plan next steps. With this streamlined structure,
care should be taken to ensure that ample opportunities exist for communication; collaboration; and sharing best practices, available resources, and expertise within and across the CTSAs.
Opportunities abound for the CTSA Program to reengineer its structure and governance. A new NCATS-CTSA Steering Committee, along with the Coordinating Center and a simpler consortium committee structure, will position the program to better coordinate the advancement of clinical and translational research.
COLLABORATIONS AND PARTNERSHIPS
The CTSA Program is a facilitator of clinical and translational research. Its inherent function is to initiate and foster collaborations—including developing innovative tools, policies, and processes; removing barriers to research; training teams of investigators; engaging communities in the research process; and other efforts—that bring together researchers, research networks, NIH institutes and centers, community stakeholders, health care providers, industry partners, government research agencies, and others to advance clinical and translational science. NCATS, the CTSA Coordinating Center, and the individual CTSA sites need to ensure that the full range of potential collaborators understands the value that the CTSA Program brings to clinical and translational research and that the Program is responsive to their needs. Incentives for building these partnerships are also needed. The IOM committee urges the establishment of an innovations fund to promote further collaboration and emphasizes that the success of CTSA 2.0 will depend on the extent and strength of the partnerships and collaborations formed.
Collaborations with NIH Institutes and Centers
Some of the most natural partnerships are occurring and need to occur more frequently between the CTSA Program and NIH institutes and centers. A number of NIH institutes and centers have their own research centers, networks, and clinical trials (e.g., Comprehensive Cancer Centers, NeuroNext, Type 1 Diabetes TrialNet) already benefiting from the CTSA Program’s collaborative databases and tools. Nonetheless, individuals who provided testimony to the IOM committee reported that intra-NIH collaborations with the CTSA Program need to be strengthened.
Evidence of the potential for collaboration is shown in Figure 3-2, which reports the number of NIH grants using some form of CTSA support or resources, although the extent and depth of that interaction is not indicated. CTSA support or resources frequently come in the form of providing institute grantees with facilities, core resources, equipment, staff expertise, or administrative services. They may also take the form of providing specific tools, such as those described earlier. Box 3-2 illustrates some of the general CTSA resources that NIH institutes are finding helpful.
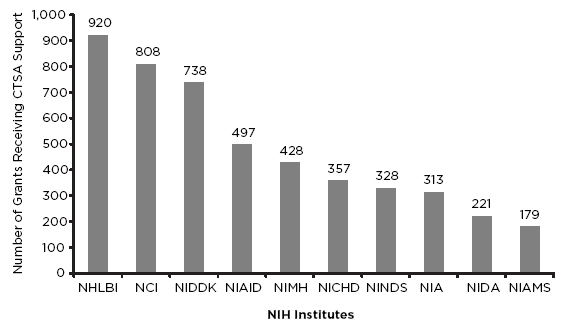
FIGURE 3-2 Top 10 NIH institutes and centers using CTSA resources. NOTE: NCI = National Cancer Institute; NHLBI = National Heart, Lung, and Blood Institute; NIA = National Institute on Aging; NIAID = National Institute of Allergy and Infectious Diseases; NIAMS = National Institute of Arthritis and Musculoskeletal and Skin Diseases; NICHD = National Institute of Child Health and Human Development; NIDA = National Institute on Drug Abuse; NIDDK = National Institute of Diabetes and Digestive and Kidney Diseases; NIMH = National Institute of Mental Health; NINDS = National Institute of Neurological Disorders and Stroke.
SOURCE: NIH, 2012b. Reprinted with permission from the National Institutes of Health/U.S. Department of Health and Human Services.
BOX 3-2
Selected Examples of NIH Institute Uses of CTSA Skills and Capacities
National Cancer Institute (NCI)
• Cancer centers have benefited from CTSAs’ experience in educating and mentoring investigators, community outreach, clinical facilities, and pilot project programs (Weiss, 2012).
• NCI projects and CTSAs invest in a range of shared resources, such as biostatistics and laboratory management tools and biorepositories and genomics resources. They also jointly support pilot projects, faculty and staff recruitment and training programs, clinical research infrastructure, and community-based research through practice-based research networks, mobile clinical research units, and e-health programs (Weiss, 2012).
National Institute of Allergy and Infectious Diseases (NIAID)
• NIAID Clinical Research Centers use CTSA support for research volunteer recruitment (e.g., 34 allergy and infectious disease–related studies used ResearchMatch, and 110 NIAID-related pilot projects were funded) (CTSA Central, 2011b).
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
• CTSAs provide support for many NIDDK-funded large clinical trials and networks. For example, most of NIDDK’s liver disease consortia and the Drug-Induced Liver Injury Network have used CTSA infrastructure for follow-up activities (Germino, 2012).
The way that skills and resources available through the CTSA Program can be translated into tangible support for institute and center grants and projects is further illustrated by these more detailed examples:
• NeuroNEXT, a National Institute of Neurological Disorders and Stroke network to conduct Phase 2 clinical trials in neurological disorders of children and adults, has 25 clinical sites, 23 of which also have CTSA awards. The network is in its early phases and is collaborating with the CTSA Program whenever possible in order to connect researchers with CTSA resources; the NeuroNEXT RFA specifically asked applicants to identify how they would interact with CTSAs. A number of overlapping goals between the network and the CTSAs include the use of central institutional review board (IRB) processes and standing trial agreements
across sites and early involvement of patient advocacy groups (Kaufmann, 2013).
• The University of Iowa’s CTSA provides support to meet the informatics needs of a multidisciplinary team that was awarded a 5-year, $3 million grant from the National Cancer Institute to develop image analysis tools that will be used in future clinical trials to better assess patient responses to cancer treatments (NIH, 2012b).
• Three California CTSAs are collaborating with the Stanford Synchrotron Radiation Lightsource2 to develop an automated and customizable drug discovery pipeline called “Auto-Drug,” which can screen samples that could be used to develop new pharmacological treatments for a variety of diseases (NIH, 2012b; Stanford University, 2012; Tsai et al., 2012).
• The National Heart, Lung, and Blood Institute’s ongoing multisite trial, the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA), used the CTSA resource IRBShare to help participating institutions share review documents and review processes on a secure web portal (Shurin, 2012).
• The National Institute on Drug Abuse used set-aside funds for three studies at CTSA sites: an examination of models to reduce disparities in clinical trials, including recruitment and retention of drug users and underrepresented populations; an experimental trial of a drug to reduce opioid overdose; and a study on how best to prevent drug abuse among Hispanic adolescents (Volkow, 2012).
The IOM committee believes that many of the challenges in clinical and translational research must be solved using systemwide approaches and that the CTSA Program is well positioned, perhaps uniquely so, to facilitate and implement those approaches. The combination of local, disease-specific resources and the more general collaborative approaches and tools developed under the CTSA Program strengthen the potential contribution CTSAs can make when partnered with projects funded by NIH institutes and centers. In addition, these types of partnerships and
_____________________
2 Stanford Synchrotron Radiation Lightsource is a research tool that uses extremely bright X-rays to study compounds and other samples at atomic and molecular levels (Stanford University, 2013).
collaborations provide opportunities to optimize available resources and to increase efficiency and cost-effectiveness. Collaborative efforts would go a long way toward “dissolving the artificial barriers that inevitably spring up in any large organization,” a benefit envisioned at the time of the program’s launch (Zerhouni, 2005, p. 1622). In a sense, this represents a scaling up of the program sites’ success at building academic homes for clinical and translational research, reducing intradepartmental boundaries to collaboration, and developing regional collaborations (Briggs and Austin, 2012).
Partnerships with Health Care Providers, Health Care Systems, and Practice-Based Research Networks
Beginning in 2007, the IOM, through its Roundtable on Evidence-Based Medicine (now the Roundtable on Value & Science-Driven Health Care), has examined a broad range of topics related to reengineering clinical research and health care delivery so as to support a continuously learning health care system (see Chapter 2) (IOM, 2007, 2013b). Built on the foundation of a strong digital infrastructure, innovative health care models, research on practice, and aligned incentives, the learning health care system offers the promise of more efficient and effective clinical care.
The work of CTSAs can bear directly on the nation’s ability to achieve a learning health care system through collaborations that strengthen ties between the realms of practice and research. Among the strongest candidates for these types of CTSA collaborations are practicebased research networks (PBRNs) and the HMO Research Network (HMORN).
PBRNs are “groups of primary care clinicians and practices working together to answer community-based health care questions and translate research findings into practice” (AHRQ, 2012). As of 2011, almost 13,000 primary care practices (providing care to approximately 47.5 million people) were involved in PBRNs, and just over half of them (52 percent) were affiliated with CTSAs (Peterson et al., 2012). Several surveys of the relationship between CTSAs and PBRNs have found disappointingly low levels of community engagement and collaboration. In a 2008 survey, PBRN directors noted that the value of CTSAs to PBRNs included funding, aid with IRB processes, biostatistics, training, and consultation. In return, PBRNs were in a position to help CTSAs move their research
into community settings and improve connections between CTSA home institutions and the community (Fagnan et al., 2010). Nevertheless, these relationships were not without challenges. For example, “CTSA leaders often sought PBRNs as study recruiting sites . . . but they seemed less aware of the need for cultivating ongoing relationships and the importance of engaging practitioners in the support and development of study protocols” (Fagnan et al., 2010, p. 482).
In 2011, PBRNs registering with the AHRQ’s National PBRN Resource Center noted that having a relationship with a CTSA made no significant difference in PBRN research opportunities or capacity (Peterson et al., 2012). The researchers reported that “although 63 registered PBRNs (52 percent) reported a formal affiliation with a CTSA, in 2011 these PBRNs conducted the same number of studies as PBRNs not affiliated with a CTSA” (Peterson et al., 2012, p. 568). Calmbach and colleagues (2012) characterized the relationship between CTSAs and PBRNs as “a process that still is developing” (p. 572). While partnerships with CTSAs may be helpful in increasing support for PBRNs and raising their visibility, these partnerships also “could be detrimental if the university tries to impose a top-down research agenda onto already busy primary care physicians” (Calmbach et al., 2012, p. 572). Further, the institutions may not fully appreciate the costs to the physicians’ practices of participation in CTSA projects.
As noted in these articles, support for the concept of collaboration between the academic researchers and clinicians working in the community remains strong despite the time and effort involved in forging strong relationships and the difficulties noted. The development of electronic health records and their use as part of a learning health care system, as well as the work of the Patient-Centered Outcomes Research Institute, may enable and motivate closer working relationships between researchers and clinicians in the future. The CTSA Program offers a venue and mechanisms for these types of partnerships, which are critical to the future of translational sciences.
Another potential source of clinician partnerships is offered by the HMORN, a network of 18 U.S. health care delivery organizations with recognized research departments or institutes that also have a defined patient population (HMO Research Network, 2013). Input to the IOM committee from the HMORN Governing Board indicated that 14 HMORN sites have established relationships with local CTSAs, and 1 HMORN site has two such relationships. One site has discontinued its CTSA relationship
(Steiner, 2013). Suggestions from HMORN board members for improved collaborations included the following:
• development of partnerships that are aligned by purpose and values;
• expanded pilot funding for community-based investigators;
• expanded bidirectional training opportunities; and
• ongoing education for members of academic leadership about the importance of research related to defined populations and then translating findings into community-based delivery systems (Steiner, 2013).
HMORN board members made several suggestions for ways in which the CTSA Program could become a more effective incubator for innovation. These suggestions included more coordinated outreach by CTSA-HMORN partners to underserved populations; broader use of supplemental funds to support HMORN dissemination of “translatable” research findings; strategic application of pilot funds to codevelop innovations; and increased incentives for community-based clinical trials (Steiner, 2013). HMORN board members suggested that the NIHinitiated Health Care Systems Research Collaboratory may be an example of how collaborative work between CTSAs and HMORN could work in the future.3
Partnerships with Industry
As with community-based clinician involvement, partnerships with industry have been viewed as essential components of the CTSA Program from the beginning. In a 2010 meeting organized by the NIH, senior industry executives formally met with CTSA awardee scientists for the first time to discuss ways to advance both groups’ goals. Although general consensus exists about the desirability of moving useful innovations quickly and safely into clinical care, how to achieve this goal is much less obvious (Fine, 2012; Wadman, 2010).
Among the obstacles to greater CTSA-industry partnerships are intellectual property constraints and concerns about conflicts of interest.
_____________________
3 The Health Care Systems Research Collaboratory, housed at Duke University, is a coordinating center for a set of pragmatic randomized trials involving HMORN sites. The center is currently developing and disseminating collaborative research tools to the investigator community, including the NIH Distributed Research Network (Steiner, 2013).
“We are not able to work effectively together because of the perception and reality of conflict of interest,” one industry participant said (Wadman, 2010, p. 256), although some instances were cited in which these concerns have been overcome. One initiative described at the conference is Eli Lilly and Company’s not-for-profit tuberculosis drug consortium,4 which links academic investigators to expertise and tools donated by Eli Lilly, Merck, private foundations, the National Institute of Allergy and Infectious Diseases, and the nonprofit Infectious Disease Research Institute (NIAID, 2012). Another is Merck’s new Calibr (California Institute for Biomedical Research) initiative, a nonprofit organization intended to speed the development of innovative new medicines (Fine, 2012). Similarly, the Indiana Clinical and Translational Science Institute formed a partnership with Veeda Clinical Research and Eli Lilly to develop a shared research facility for first-in-human studies and other early phase studies (NIH, 2012b).
At the IOM committee’s December 2012 meeting, Jacqueline Fine of Merck Research Laboratories spoke specifically about collaborative opportunities in early phases of translational research (Fine, 2012). She pointed out that, from industry’s point of view, successful translation is not achieved until an innovation becomes part of the standard of care. The activities that will facilitate this process, including regulatory approval and other favorable policy actions, should be thought about at the front end of a project. It is not clear to industry leaders that academic researchers, accustomed to nondirected discovery, are sufficiently focused on those end-of-the-process considerations (Fine, 2012). Successful partnerships in this arena will require identification of shared goals and an understanding of the roles and value that each of the partners brings to the table.
The CTSA Program provides a unique venue to build strategic partnerships and collaborations between academia and private-sector partners, including pharmaceutical and biotech companies and companies involved in the development of medical devices, technologies, and diagnostics. These partnerships and collaborations will be imperative to realizing the program’s streamlined mission. Characteristics that may facilitate effective industry-CTSA relationships include a simple and transparent interface for partnering including shared strategies and priorities, a clear mission for CTSAs and their multisite collaboratives, the willing-
_____________________
4 See http://www.tbdrugdiscovery.org (accessed March 26, 2013).
ness of individual CTSAs or CTSA groups to align their focus with that of an industry partner, and an ethical and transparent relationship.
The CTSA Program needs to explore and implement new approaches for collaboration with industry that foster the translation of research into products while remaining within legal and regulatory boundaries. In addition, the CTSA Program can serve as a leader in promoting innovation, entrepreneurship, and cultural changes; developing and testing new approaches for managing conflict of interest and intellectual property; and engaging the Food and Drug Administration and other regulators in collaborations. As these partnerships are established and grow, it will be important for NCATS and the CTSAs to share and implement best practices.
Opportunities and Next Steps
Collaborations across and among researchers and research networks are core to building an integrated network that supports the work of clinical and translational science. Because the CTSA Program is a facilitator and accelerator of this research, NCATS, the CTSA Program, and individual CTSA sites need to ensure that they are working to initiate, nurture, and strengthen collaborations across CTSA institutions, with NIH institutes and centers, with private- and public-sector research institutions and networks, and with community stakeholders. With their core focus on strengthening the infrastructure of clinical and translational research, CTSAs are ideal partners for all these entities.
Mutually beneficial collaborations take time, energy, and initiative to build and sustain. All sides need to find value in the partnership, and all need to work to maintain and further shared goals. Sufficient incentives for collaboration may be inherent in the partnering organizations’ ongoing priorities, but in many cases, external incentives may be needed in order to provide the impetus to collaborate.
The IOM committee urges NCATS to establish a CTSA innovations fund that would provide incentives for creating new collaborations and supporting collaborative pilot studies or resource-sharing initiatives. For example, such projects might require the collaboration of multiple CTSAs, might jointly involve CTSAs and PBRNs or other research networks, or might engage groups of CTSAs and NIH institutes or centers. These collaborative projects might also involve new industry or community partners, other government agencies (e.g., the AHRQ, Food and Drug Administration, Department of Veterans Affairs, or public and pri-
vate research entities. The fund could be used to support collaborative initiatives to develop novel strategies for implementation research that would be tested in multiple locations. This fund could be created through a set-aside mechanism within the CTSA Program that provides flexible funding to foster collaborative efforts that are pioneering and have great potential to accelerate clinical and translational research. Projects associated with this fund should include clear metrics and evaluation measures.
The NIH developed the CTSA Program in order to increase the speed and efficiency with which new ideas and technologies would move from the research laboratory into clinical and community practice. Accomplishing this objective has required strengthening many research activities in academic health centers (and other institutions), such as biomedical informatics and training for researchers, as well as a focused effort to find efficient and effective ways to share resources and develop common tools, databases, and research processes, all with the ultimate goal of improving human health. Building an active and productive CTSA at an academic health center or other institution often involves not only the funds from the CTSA cooperative agreement but also substantial financial and staff commitments from the institution; although institutional cost sharing is not required. The committee could not identify any data to quantify these institutional contributions but heard testimony from many individuals about the depth of efforts and the commitment to the CTSA Program from top leaders at health research institutions across the nation.
In its first 7 years, the CTSA Program has done much to develop and nurture the CTSA sites as academic homes for clinical and translational research with an emphasis on training researchers, providing shared resources, streamlining and improving clinical research management, and developing community and research partnerships. Zerhouni (2005) defined an academic home as a place that provides intellectual and physical resources for original clinical and translational science. He envisioned CTSAs as creating “environments that over time provide the theoretical underpinnings of the discipline, provide much needed education programs, contribute to the growth of well structured and well recognized career pathways, and provide a research environment more nimble, conducive to and responsive to the demands of modern translational and
clinical research” (Zerhouni, 2005, p. 1622). Progress has been made in creating these academic homes. The challenges ahead will be to capitalize on these efforts and refocus the program so that it becomes a network that is responsive, nimble, and innovative in accelerating clinical and translational research.
The early funding announcements for the CTSA Program identified a set of key functions that evolved over time, some of which became a required part of the program. Some of the key functions were as follows:
• Development of novel clinical and translational methodologies
• Research education, training, and career development
• Pilot and collaborative translational and clinical studies
• Biomedical informatics
• Design, biostatistics, and clinical research ethics
• Regulatory knowledge and support
• Clinical research resources and facilities
• Community engagement
• Evaluation
• Translational technologies and resources (NIH, 2005, 2009).
In its most recent CTSA funding announcement, the NIH changed the criteria so that applicants were given greater flexibility to build on the strengths of their home institutions in specific phases of clinical and translational research (T0–T4) or in a specific area of research (e.g., child health research) (NCATS, 2012b; NIH, 2012c).
The 61 CTSA sites provide a wide array of training and research resources to help researchers identify promising therapeutics and interventions and move them forward as rapidly as feasible, as discussed in Chapter 1. These resources often require large institutional investments in purchasing equipment and instruments, renovating space, and providing at least partial support for staff. Despite the availability of these resources, researchers in some institutions have reported that they were unaware of the core resources or found them too expensive or cumbersome to use (Curley, 2013; Raue et al., 2011). A central database cataloging CTSA resources has been developed (CTSA Central, 2013b), but questions regarding the cost of using the resources and visibility continue, according to testimony received by the IOM committee from a number of involved individuals.
CTSAs have worked to facilitate and simplify clinical research management through the use of project managers or navigators and other
mechanisms and tools. For example, CTSA sites are attempting to ensure efficient protocol development processes and limit required review steps to those that actually add value to the final research protocol. In a partial inventory of the process improvements that had been achieved, as of 2010, 15 sites reported they had developed process maps, and at least 20 reported improvements that, in some cases, reduced processing times by more than 30 percent. Some of these improvements resulted from streamlining IRB approvals (Rosenblum and Alving, 2011).
Variation in the activities of individual CTSAs has grown up across the program due in part to the range of CTSA site funding (from $4 million to $23 million in fiscal year [FY] 2012) (Briggs and Austin, 2012) and in part to differences in home institution governance structures, management styles, and the areas of focus and depth in research portfolios (Rosenblum and Alving, 2011). Although the CTSA Program is disease agnostic in its overall resources and approach, the individual CTSA sites may facilitate research on specific conditions, and their home institutions may be research leaders in those areas. Thus the individual CTSAs and the program as a whole provide great potential to augment and facilitate the disease-specific work of NIH institutes and centers, as well as that of their home institutions (see Box 3-3). The CTSA Program supports disease-specific research in a variety of ways, which may include funding, research tools and facilities, expert consultation, research participant recruitment, and other research resources and support mechanisms described in Chapter 1. In its assessment of the CTSA Program, the committee identified numerous examples of disease-specific research connected to the Program. The types of CTSA resources that were used to support the research were rarely identified or described, however.
BOX 3-3
Examples of Disease-Specific Research Aided by CTSA Resources
Cancer: An international team of researchers led by Weill Cornell Medical College investigators identified two inherited gene deletions that more than triple the risk of aggressive prostate cancer (Demichelisa et al., 2012; Woods, 2012).
Heart disease: Using financial support and an information network provided by Harvard’s CTSA, investigators at Beth Israel Deaconess Medical Center used a mouse model to develop molecular evidence that helps explain why preeclampsia and multiple gestation are risk factors for peripartum cardiomyo-
pathy, a sometimes fatal condition that develops in 1 in 3,000 pregnant women with no known heart disease history (Patten et al., 2012; Prescott, 2012).
Lung disease: Leading a 175-person research team in 13 sites in Japan, Canada, and the United States, with partial CTSA funding, University of Cincinnati researchers identified a treatment for a rare, severe lung disease (lymphangioleiomyomatosis) that affects women in their childbearing years (McCormack et al., 2011; Pence, 2012) Muscular dystrophy: Using a national database developed by the University of Rochester Medical Center, researchers explored the impact of symptoms on the lives of patients with muscular dystrophy and found that those symptoms affecting daily life (e.g., fatigue, limited mobility) are more important to patients than those most commonly associated with muscular dystrophy (e.g., myotonia). Study results are being used to improve patient outcome measures and could be used to target future treatment (Heatwole et al., 2012; Michaud, 2012).
Stroke: Physicians at Barnes-Jewish Hospital used partial funding through Washington University in St. Louis’s CTSA to reduce the average time between a stroke patient’s arrival and treatment from 58 to 37 minutes by eliminating inefficient steps in the care process. Rapid administration of anticlotting medication is key to preventing brain damage from stroke (Purdy, 2012).
Opportunities and Next Steps
Individual CTSAs have made progress in establishing academic homes for clinical and translational research. The challenge for CTSA 2.0 will be to create a national network of institutions that are engaged in accelerating clinical and translational science. In its assessment of the program, the IOM committee heard many questions from stakeholders about the optimal number of individual CTSAs. The committee did not choose to specify an ideal number, but rather believes that over time the number could change as strategic goals and priorities are set and as determinations are made about future program directions. The focus should not be on the absolute number of CTSAs but on whether the CTSAs are achieving progress as measured by defined goals and priorities.
As described in Chapter 1, individual CTSAs, the CTSA Program, and NCATS need to continually push to identify effective therapeutics and interventions and move them to the individuals and populations that could benefit from them. The IOM committee realizes that this major undertaking will move forward in incremental steps as well as major leaps. For individual CTSAs, the challenge will be to keep their home
institutions engaged and active while reaching out to develop the collaborations with other CTSAs, research networks, industry, and community stakeholders. Effective clinical and translational research requires creativity and innovation that could be ignited by collaboration across disciplines and beyond the biomedical and health sciences. In order to tackle the most complex and pressing health challenges, CTSAs should attempt to create partnerships with schools of business, law, engineering, nursing, public health, and communications, as well as with relevant academic departments, such as anthropology and psychology.
The IOM committee supports the recent change to the CTSA RFA that allows greater flexibility for individual CTSA sites to focus on the strengths of their institutions. Recognizing that some CTSAs may excel in early discovery science, others in later development research, and others in implementation of findings in the community, it will still be critically important for NCATS to ensure that the CTSA Program, as a whole, covers the full spectrum of clinical and translational research. Further, sites should retain their emphasis on community engagement in order to ensure participation and breadth of input by community practitioners, patients, and other stakeholders (see Chapter 4).
The IOM committee urges NCATS and CTSAs to continue to foster integrated research communities of CTSAs with common interests and expertise, to share infrastructure further, to work on common projects, and to strengthen collaborations. Individual CTSAs are encouraged to identify and implement efficient and cost-effective ways to provide access to core facilities and resources. Further efforts are needed to promote awareness of their many resources, training, and services and to reduce their costs.
Stakeholders, including research funders, the public, and Congress, increasingly demand evidence of returns on investments in the health research enterprise, such as advances in clinical practice and increases in the availability of new therapeutics and interventions (Austin, 2013; Reed et al., 2012; Shuster, 2012). Evaluation can be an incentive or catalyst for positive change and improved outcomes and is necessary for accountability, transparency, informed decision making, and communication
about outcomes and the value of an investment.5 In a multifaceted and complex effort such as the CTSA Program, evaluation is a formidable undertaking, but one that is vital to ensuring accountability and planning for future directions. Over the life of the CTSA Program, the NIH has recognized the importance of evaluation by building it into the requirements for the first set of CTSA awards, maintaining the requirement for each of the subsequent CTSA awards, and initiating an external evaluation process that assessed the program as a whole, as described below.
Evaluating Individual CTSAs
As part of the application process for a CTSA award, applicants are required to have a plan in place to
• monitor the use, quality, and costs associated with the programs, resources, and services that are provided;
• assess data and modify programs, resources, and services as necessary in order to better meet the needs of researchers, increase quality and efficiency, and reduce costs; and
• track and assess innovative methods and practices related to the structure, aggregation, and provision of services, programs, and resources (NIH, 2012c).
The recent RFA also requests “a full description of tracking processes, metrics, and milestones proposed to ensure ongoing assessment and timely adjustment of activities of the CTSA” (NIH, 2012c). Individual CTSA evaluation plans correspond to the varying needs and capacities of an individual CTSA’s structure, available funding, and programs and activities supported. This diversity makes cross-CTSA evaluation a challenge that may become even more difficult as individual CTSAs are given more latitude to specialize. However, some salient summative measures are necessary in order to establish program accountability and to demonstrate its progress and value as a whole. Cross-CTSA evaluation
_____________________
5 The definition of “evaluation” used for this report is from Patton’s work on utilization-focused evaluation: “Program evaluation is the systemic collection of information about the activities, characteristics, and results of programs to make judgments about the program, improve or further develop program effectiveness, inform decisions about future programming, and/or increase understanding” (Patton, 2008).
will require high-level common metrics and measures that can be applied consistently at all sites.
Self-Evaluations of the Individual CTSAs
Each CTSA site conducts internal assessments of its own activities, processes, and performance. Individual CTSAs report a median of three members on their evaluation teams representing 1.3 full-time equivalents (FTEs) on average. Approximately three-quarters of evaluation teams, however, report significant assistance from other staff in evaluation efforts, which may not be captured in the FTEs reported (Alexander et al., 2013). Sites often use mixed-methods approaches that may include quantitative data collection, satisfaction surveys, social network analysis, focus groups, interviews, and case studies. Many use a systems approach that assesses programmatic components individually and collectively (Alexander et al., 2013; Rubio et al., 2012).
The 2012 National CTSA Evaluators Survey6 recognized robust evaluation efforts across the program cumulatively, but also identified significant heterogeneity in strategies and methods being employed at individual CTSAs. The authors concluded that the heterogeneity represents both a strength, in terms of diversity and flexibility, and a challenge with regard to obtaining standardized information (Alexander et al., 2013). The IOM committee noted variability in publicly available information on the individual CTSAs’ evaluation efforts and overall accomplishments—for example, some CTSAs post information about their broad evaluation plans on their websites,7 and some include impact information.8 However, descriptions of these evaluation processes, metrics, and outcomes are not consistently available for public information.
Although flexibility in the evaluation approaches and processes of the individual CTSAs is appropriate given their variation in size, structure, and focus, some level of standardization is also needed. The National CTSA Evaluators Survey highlighted challenges related to a lack
_____________________
6 For the last 3 years, the Shared Resources Working Group of the Evaluation Key Function Committee (described below) has used the National CTSA Evaluators Survey to assess the evaluation management and methods being applied across the individual CTSAs and to identify emerging challenges and new evaluation tools in use, such as dashboard technologies (Alexander et al., 2013; Rubio et al., 2012).
7 See, for example, http://casemed.case.edu/ctsc/cores/evaluation.cfm; http://dccweb2.bumc.bu.edu/wordpress/index.php/programs/program-tracking-and-evaluation (accessed April 10, 2013).
8 See, for example, http://ctsi.ucsf.edu/impact (accessed April 10, 2013).
of common metrics, clear definitions, and guidance from funders (Alexander et al., 2013). Overcoming these obstacles needs to be a priority in order to implement effective evaluation strategies that will ensure sufficient consistency and establish accountability.
Fostering Best Practices
In the early phases of the CTSA Program, the Evaluation Key Function Committee was established to provide “a forum for institutions to exchange information about their evaluation approaches, challenges, and progress” (CTSA Central, 2013f). This key function committee and its working groups are making strides to foster best practices and improve evaluations being conducted at the individual CTSA level. Every other month the committee produces a newsletter and hosts a cohort call during which two CTSAs present evaluation strategies and challenges. Members of the key function committee have found these presentations valuable for sharing best practices and identifying common challenges (and possible solutions) encountered in the sites’ self-evaluation processes (personal communication, D. Rubio, University of Pittsburgh, March 15, 2013).
The Evaluation Key Function Committee is attempting to develop common metrics in specific areas that could be used as benchmarks for individual CTSAs and the CTSA Program as a whole. It is currently working across the consortium committees to develop, test, and implement common metrics related to clinical research processes and outcomes. While in the early stages of development, this effort is focusing on 15 metrics in areas such as clinical research processes, careers, services, economic return, collaboration, and products (Rubio, 2013).
The committee has advised other key function committees on the development of common metrics also. For example, it worked with the Biostatistics, Epidemiology, and Research Design Key Function Committee to develop consensus on 56 performance items related to collaboration, use of existing methods, and discovery of new methods (Rubio et al., 2011a); it consulted with the Community Engagement Key Function Committee in developing the evaluation section of Principles of Community Engagement and with the Education and Career Development Key Function Committee to develop common metrics for measuring career success (Lee et al., 2012; Rubio et al., 2011b, 2012; Task Force on the Principles of Community Engagement, 2011).
Finally, the evaluation committee’s National Evaluation Liaison Working Group consulted with the American Evaluation Association to propose an evaluation framework for the CTSA Program (Rubio et al., 2012). The framework was developed to provide recommendations to NCATS and other program stakeholders on how the program could be evaluated effectively, given its complexity. The recommendations focus on a range of areas that include scope of evaluation, structure and organization, funding, methodology, utilization, policy, and capacity (CTSA Evaluation Key Function Committee, 2012). This framework has the potential to serve as a valuable resource for NCATS as it considers its next steps in implementing the CTSA Program and in identifying approaches to evaluating individual CTSAs and the program as a whole.
NCATS’s Role in Evaluating Individual CTSAs
As part of their award obligations, CTSA sites must submit annual progress reports to NCATS, describing their accomplishments, milestones, challenges, and the barriers affecting their work. The Office of Management and Budget is requiring that the progress report format be updated and standardized across agencies; the new format will capture information on accomplishments, products, participants, impact, changes, special reporting requirements, and budget (NIH, 2012a). The IOM committee understands, however, that the new format will reduce flexibility in the types of specific information that NCATS can request as part of the reporting process (personal communication, E. Collier, NCATS, March 20, 2013).
Despite a statement in the recent RFA that “the NIH is committed to transparency in the CTSA Program to ensure the program is delivering on its mission” (NIH, 2012c), no parts of the sites’ annual progress reports are publicly available (nor were they available to the committee). In addition, the progress reports are not currently used for evaluation purposes, according to NCATS staff (Parsons, 2013). The IOM committee cannot say whether these required progress reports are the most appropriate way to evaluate the individual CTSAs or the program or to communicate annual progress, not having inspected them. Nevertheless, the committee strongly believes that, given the size of this investment, there must be a mechanism in place that requires all of the individual CTSAs to regularly and publicly report on defined metrics, milestones, and accomplishments. From the IOM committee’s perspective, this lack of public information thwarts the need to ensure transparency and accountability.
During the December 2012 IOM committee workshop, NCATS staff reported that the NIH’s current primary evaluation mechanism for CTSA sites is the peer-review process associated with renewal applications for the cooperative agreements (Briggs, 2012). The recent RFA provided an extensive list of review criteria that will be used to assess these applications; however, it is unclear how NCATS evaluates the CTSAs during the 5-year award cycle and what mechanisms are in place for midterm assessments or any necessary corrective actions. The committee urges NCATS to assert leadership in improving transparency in reporting of metrics, milestones, and accomplishments at the individual CTSA level and to ensure that sufficient accountability monitoring is in place through a set of common metrics that reflect the program’s mission and strategic goals.
Evaluating the CTSA Program
Various aspects of the CTSA Program have been evaluated. External evaluations by Westat provided quantitative and qualitative baseline measures for the program. That 3-year evaluation used site visits to assess training and education, resource utilization, publications, and the overall progress of individual CTSAs (see Box 3-4). Westat’s final
BOX 3-4
Evaluations of the CTSA Program
Report on Field Visits to the CTSAs (Westat, 2011): Westat conducted site visits at 9 CTSA institutions with interviews of 369 individuals in various positions. Despite variation across the sites, Westat concluded that the CTSAs are making progress on meeting program goals related to building infrastructure, training and education, and translation of research findings into practice. Participating interviewees across the sites indicated that CTSA support had enabled progress in clinical and translational research that would not have been made otherwise. The report points out that at the time of these interviews, the program was still in its early stages and notes that many aspects of the program were still evolving, especially those related to building collaborations.
Findings from the CTSA National Evaluation Education and Training Study (Miyaoka et al., 2011): Westat surveyed 553 CTSA-supported scholars and trainees and 665 mentors and found that both groups were very positive in their review of the education and training components of the CTSA program. Areas identified for improvement included increasing the ethnic diversity of mentors, scholars, and trainees; expanding training in team science and tech-
nology transfer; and dedicating more resources to the online learning and career planning components of the program.
Findings from the CTSA National Evaluation Utilization Study (Raue et al., 2011): Westat conducted a survey of 302 users of CTSA resources and 537 nonusers, all of whom worked for one of the initial 46 CTSA sites that were established between 2006 and 2009. A large majority of nonusers (80 percent) conducted nonclinical research, and 48 percent indicated they did not need additional resources. The evaluators highlighted the wide range of research resources available and found that 79 percent of the users were satisfied with those resources. However, the report cited a lack of awareness and confusion about the program and its resources and noted a need for increased visibility and communication.
Final Report on CTSA-Supported Publications: 2006 to 2011 (Steketee et al., 2012): Westat analyzed the CTSA sites’ annual progress reports and online journal databases and found 17,038 publications identified as supported by CTSA resources. Evaluators indicated that publications per CTSA institution increased every year it had CTSA funding and found a growing number of publications that involved collaboration between more than one CTSA. The report identified a need for improvements in standardization in reporting CTSA-supported publications; more than 2,800 publications that cited CTSA support were not included in awardee annual progress reports, and 85 percent of publications listed in the annual reports did not cite CTSA funding.
The CTSA National Evaluation Phase 1 Final Report (Frechtling et al., 2012): This report concluded that the CTSA Program is enabling a new research infrastructure and encouraging the adoption of new practices that have the potential to streamline the clinical and translational research process. Westat recommended that, going forward, the program should support institutional pilot programs, increase awareness of the program and available resources, expand education and training opportunities, streamline the CTSA Consortium, increase incentives for collaboration and partnership, and conduct longterm evaluations.
summary report concluded that “the CTSA program is making important strides toward encouraging and enhancing a new kind of medical research infrastructure and re-engineering the scientific research process” (Frechtling et al., 2012, p. vii). In addition, the OIG evaluated the administration of the CTSA Program under the NCRR (discussed earlier in the chapter) (OIG, 2011).
Despite these previous efforts and the obvious commitment to evaluation at the CTSA site level, the committee is not aware of future plans to evaluate the overall program. The IOM committee believes that a formal, programwide evaluation process should be undertaken to measure progress toward NCATS’s vision for CTSA 2.0. Elements that could be
considered in developing metrics have been identified by NCATS staff (Briggs and Austin, 2012) and by the IOM committee and include the extent to which program awardees are facilitating clinical studies, with an emphasis on multisite studies; advancing the translation of research findings into therapeutics, diagnostics, and preventive interventions; developing innovative research methods; promoting community engagement; training and educating the next generation of clinical and translational researchers; protecting human subjects and reducing delays in clinical trials; developing informatics standards; and promoting data resources and research tools.
In addition to the evaluations described, additional reporting mechanisms document and communicate the accomplishments of CTSA sites and the CTSA Consortium. For example, the CTSA Consortium committees produce an annual report recounting the activities and achievements of each committee, working group, and task force. These reports detail a range of activities, from small-scale accomplishments, such as establishing committee-specific e-mail addresses, to larger efforts, such as developing a catalog of unique T1 resources available through the program (CTSA Central, 2011a). The CTSA Consortium compiled an extensive list of activities that demonstrates the breadth and capabilities of the program for the NIH CTSA/NCATS Integration Working Group (Katz et al., 2011; Pulley, 2013).
In addition, the NIH has produced two progress reports that cover accomplishments of individual CTSAs and the CTSA Consortium from 2006 to 2008 and 2009 to 2011 (NCRR, 2009; NIH, 2012b). The most recent report highlights specific examples of program accomplishments in six key areas: accelerating discoveries, improving clinical research efficiency, training the next generation of investigators, fostering collaboration and partnerships, enhancing the health of communities and the nation, and developing resources and networking for sharing data. Just as some individual CTSAs feature information on achievements on their websites, the NCATS website has a page that includes stories about successes attributed to the CTSA Program and other NCATS-supported programs (NCATS, 2013d).
These mechanisms, although not part of a formal evaluation process, are important for communicating the value of the program. They could be modified to include specific detail on how CTSAs contributed to particular accomplishments or which program resources were used to achieve them. They also could be used to report results of a more formalized evaluation process. The NIH and NCATS should report on any
programwide evaluation findings in pursuit of transparency, and as mentioned previously, there should be a mechanism in place for individual CTSAs to report regularly on defined metrics, milestones, and accomplishments.
Opportunities and Next Steps
The IOM committee believes that a set of clearly defined, measurable strategic goals is the starting point for shaping the CTSA Program’s future. These measurable goals should serve as a foundation for developing high-level common metrics and measures that could be applied and reported consistently to demonstrate progress. At this point, NCATS’s plans for evaluating individual CTSA sites and the CTSA Program as a whole are unclear. Progress is being made at the individual CTSA level in terms of self-evaluation, but the current lack of transparency in reporting and lack of high-level common metrics are barriers to overall program accountability.
Developing common metrics that can be implemented across the CTSAs will be a formidable challenge that will require input and cooperation from all the CTSAs. The complexity of the CTSA Program may also require the application of innovative metrics that go beyond standard academic benchmarks of publications and number of grants awarded.
The ultimate goal of clinical and translational research, and therefore the ultimate goal of the CTSA Program, is to improve human health. Although it would be ideal to evaluate the CTSA Program’s impact on clinical care and public health, currently this is neither feasible nor realistic given the numerous driving forces that shape the research enterprise (see Chapter 2) and the multitude of factors that affect health outcomes. In addition, there are multiple direct and indirect ways in which the CTSA Program contributes to research infrastructure and resources, collaborations, cultural changes, training, and community engagement that influence clinical and translational research but cannot be easily identified or measured. Despite these and other such challenges, the CTSA Program can be a leader in developing evaluation methodologies and metrics that could provide more real-time assessments of progress in advancing clinical and translational research, overcoming research barriers, fulfilling the program’s mission and strategic goals, and, whenever possible, changing clinical care and improving public health.
A common underlying theme to establishing leadership and accountability is effective communication. “The effective communication of scientific results and viewpoints to the public is an important responsibility of the scientific community. This is particularly so for science that has been publicly funded” (ICSU CFRS, 2010, p. 1). In this report, the IOM committee takes a broad perspective on the role of communications. It does not mean only an occasional news release or printed report, but rather the full array of activities and methods of communication undertaken to attract partners, achieve transparency and accountability, promote public support and understanding of findings, and ensure that findings are acted on, whether they relate to advances affecting clinical care or ways to improve the working of the scientific enterprise. Communication in this global sense is fundamental to clinical and translational research. It is likewise fundamental to conveying and achieving the value added from the CTSA Program.
In part, the need for a robust and diverse communications effort has been anticipated through the creation of two entities: the Communications Key Function Committee and the CTSA Consortium Coordinating Center. The key function committee provides the opportunity for CTSA and NIH information officers and staff “to share local and national CTSA communications best practices, activities and experiences; and to identify and generate ideas to address CTSA communications opportunities and challenges” (CTSA Central, 2013e). The committee’s Year in Review report highlights best practices from individual CTSAs in their use of a range of media, including social media, to highlight their work and attempt to educate the public generally about clinical and translational research (CTSA Central, 2013c).
When the CTSA Consortium Coordinating Center was established in November 2011, its role explicitly included enhancing communications through organizing networking resources and enabling outreach and dissemination of tools. Although some overlap may exist in mission between the Coordinating Center and the key function committee, the latter’s efforts appear more heavily focused on media dissemination, whereas the Coordinating Center’s role is more directed to providing technical assistance and facilitating communication within the program. To the extent that the Communications Key Function Committee and Coordinating Center, as they evolve, cannot take on all of the communications roles that might be desired, this structure might need reassessment by
NCATS. Further, a CTSA Program strategic communications plan is needed to fully implement this role.
Around the world, scientific communication efforts are slowly moving away from a strictly dissemination-based approach to a more participatory and collaborative one, as “the [Public Understanding of Science] ‘paradigm of science dissemination’ has been partially translated into what could be termed a ‘paradigm of dialogue and participation’ or Public Engagement with Science” (Felt et al., 2007, p. 55). This paradigm shift will require ongoing commitment and leadership from within the research enterprise. Signs of progress are evident at the NIH and, specifically, with the CTSA Program, wherein community engagement has been a strong part of the program from its inception. Community engagement provides a platform for collaborative dialogue and participation and encourages development of new strategies for scientific communication. Although scientists in general have resisted a more active role in communications, in part because they have not been trained for it and most likely see it as time-consuming with unclear benefit, “the lack of [professional] reward is a bigger issue” (Palmer and Schibeci, 2012, p. 12). NCATS is in a position to ameliorate this problem by, for example, including strength of communications and of community partnerships among its criteria to assess the quality of CTSA research efforts.
Both general oversight and various high-level communications activities—for example, with federal policy makers—are most appropriate for NCATS to carry out. Other, more program-specific communications activities and technical assistance might be appropriate at an intermediate level, such as by the Coordinating Center or through the communications committee. Finally, some communication activities are most effectively carried out by individual CTSA sites and projects. Box 3-5 provides examples of the opportunities at each of these levels.
BOX 3-5
Communications Opportunities Within the CTSA Program
At the broad NCATS level,
• Communicate the CTSA Program’s mission and goals clearly and consistently.
• Ensure that NIH institute and center leadership know about the CTSA Program, the opportunities it offers to add value to their research efforts, and how to connect with and use it.
• Ensure that CTSA PIs and researchers appreciate the importance that NCATS places on their ability to clearly articulate the goals of
their research and findings for various audiences, including community partners, and reward teams that do so effectively.
• Coordinate efforts where NCATS leverage may be needed to achieve institutional, governmental, or legislative policy changes, particularly with respect to the barriers to multi-institutional research projects (e.g., uncoordinated reviews, multiple IRB filings, and so on).
• Use what is learned from the individual CTSAs to distill best research practices that might be deployed across the nation’s biomedical research enterprise and disseminate the best practices in ways that encourage their adoption.
• Maintain essential transparency and accountability through a system of reporting on both program implementation and results (IOM, 2009).
• Provide funding for professional communications staff within the Coordinating Center.
At an intermediate level (e.g., the CTSA Coordinating Center or communications-focused committee),
• Provide consultation and support for individual CTSAs and projects in their website development, media outreach, and social media strategies.
• Coordinate with other key committees as well as CTSA leadership and NCATS on media-related topics and opportunities.
• Train site representatives on effective communication with the media and public.
• Identify and share communications best practices from the sites, as with the 2011–2012 Year in Review report (CTSA Central, 2013c) and develop tools to simplify site adoption of these best practices.
• Provide technical assistance to sites in identifying potential audiences and communications partners (e.g., community and industry).
• Continue promotion of the specific tools and databases that sites have developed and encourage their wider adoption (at present, the Coordinating Center’s website includes a listing of shareable informatics tools, and it conducts monthly webinars on how to use these tools).
• Work with sites to develop novel dissemination strategies aimed at achieving impact and sharing their findings (examples of impact would be a policy change, a clinical practice change, or an improvement in public understanding).
• Continue efforts to collect “success stories” from site efforts and developing strategies to deploy them effectively.
• Provide researchers, especially new researchers, training on appropriate communication strategies and preparing journal articles for publication.
At the individual CTSA level,
• Promote and disseminate intra-institutional communications about specific projects to maximize sharing of technical, information, and human resources within the CTSA institution and its collaborators.
• Ensure broad communication of available tools and resources across researchers within the home institution.
• Include in their teams a person with appropriate skills and defined responsibility for communications, including community outreach, collection of information on best practices, dissemination planning, and so on.
• Brief media, communications, and development offices at home institutions about projects, major milestones, and findings.
• Keep community and industry partners in the communications loop.
• Work with the Coordinating Center and NCATS on a plan for disseminating findings through the media and beyond.
• Consistently share success stories and research findings on the individual CTSA websites.
• Ensure that at least one member of the research team from each CTSA participant has had training on effective communication of scientific information.
CONCLUSIONS AND RECOMMENDATIONS
The CTSA Program has made progress in fulfilling its task of strengthening the nation’s infrastructure for clinical and translational science. In implementing the CTSA Program and moving it toward CTSA 2.0, NCATS has an obligation to ensure that the significant public investment that has been made thus far is effectively contributing to the research enterprise. The IOM’s 2009 report HHS in the 21st Century outlined a systematic approach to accountability that could be useful in leading the CTSA Program toward greater accountability and efficiency. This approach requires
• a small number of critical, measurable goals;
• clearly delineated lines of responsibility;
• quantifiable targets and time-specific milestones;
• identification of barriers and strategies to overcome them;
• a process of regular reporting and assessment;
• rewards and recognition for achieving goals;
• a clear understanding of whether progress is being made; and
• corrective action as needed (IOM, 2009).
The current clinical and translational science ecosystem presents many challenges in identifying and testing therapeutic and preventive interventions for safety and efficacy and moving them into clinical and community settings. The IOM committee believes that the CTSA Program has a major role to play in overcoming those challenges and facilitating and accelerating clinical and translational research. Meeting these tasks will necessitate a CTSA Program that meets the accountability standards outlined above, is nimble enough to be action oriented, has the ability to focus the disparate energies and talents of many institutions and individuals, and is able forge the partnerships and collaborations needed to move forward in a complex research ecosystem.
The committee urges NCATS to take a more active role in the direction of the CTSA Program and to build on its current strengths by setting clear and measurable goals, streamlining the program structure, establishing accountability and transparency, communicating its value, and instilling strong evaluation expectations.
Recommendation 1: Strengthen NCATS Leadership of the CTSA Program
NCATS should strengthen its leadership of the CTSA Program to advance innovative and transformative efforts in clinical and translational research. As it implements CTSA 2.0, NCATS should
• increase active involvement in the CTSA cooperative agreements and the CTSA Consortium;
• conduct a strategic planning process to set measurable goals and objectives for the program that address the full spectrum of clinical and translational research;
• ensure that the CTSA Program as a whole actively supports the full spectrum of clinical and translational research while encouraging flexibility for each institution to build on its unique strengths;
• form strategic partnerships with NIH institutes and centers and with other research networks and industry;
• establish an innovations fund through a set-aside mechanism that would be used for collaborative pilot studies and other initiatives involving CTSA institutions, other NIH institutes, and/or other public and private entities (e.g., industry, other government agencies, private foundations, community advocates and organizations);
• evaluate the program as a whole to identify gaps, weaknesses, and opportunities and create mechanisms to address them; and
• distill and widely disseminate best practices and lessons learned by the CTSA Program and work to communicate its value and accomplishments and seek opportunities for further efforts and collaborations.
Recommendation 2: Reconfigure and Streamline the CTSA Consortium
NCATS should reconfigure and streamline the structure of the CTSA Program by establishing a new multistakeholder NCATS-CTSA Steering Committee that would
• be chaired by a member of NCATS leadership team and have a CTSA principal investigator as vice-chair, and
• provide direction to the CTSA Coordinating Center in developing and promoting the use of available shared resources.
Recommendation 3: Build on the Strengths of Individual CTSAs Across the Spectrum of Clinical and Translational Research
Individual CTSAs, with the leadership of NCATS, should emphasize their particular strengths in advancing the program’s broad mission and goals. In doing so, CTSAs should
• drive innovation and collaboration in methodologies, processes, tools, and resources across the spectrum of clinical and translational research;
• emphasize interdisciplinary team-based approaches in training, education, and research;
• involve patients, family members, health care providers, and other community partners in all phases of the work of the CTSA;
• strengthen collaborations across the schools and disciplines in their home institutions;
• build partnerships with industry, other research networks, community groups, and other stakeholders; and
• communicate the resources available through the CTSA Program.
Recommendation 4: Formalize and Standardize Evaluation Processes for Individual CTSAs and the CTSA Program
NCATS should formalize and standardize its evaluation processes for individual CTSAs and the CTSA Program. The evaluations should use clear, consistent, and innovative metrics that align with the program’s mission and goals and that go beyond standard academic benchmarks of publications and number of grant awards to assess the CTSA Program and the individual CTSAs.
AHRQ (Agency for Healthcare Research and Quality). 2012. Practice-Based Research Networks. http://pbrn.ahrq.gov (accessed March 11, 2013).
Alexander, A., J. A. Hogle, C. Kane, H. M. Parsons, and L. Phelps. 2013. The Clinical and Translational Science Award National Evaluators Survey: Where are we now? Submitted to the IOM Committee by D. Rubio on March 28. Available by request through the National Academies’ Public Access Records Office.
Austin, C. P. 2013. National Center for Advancing Translational Sciences: Catalyzing translational innovation. PowerPoint presented at Meeting 3: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, January 24. http://www.iom.edu/~/media/Files/Activity%20Files/Research/ http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2013-JAN-24/Chris%20Austin.pdf (accessed February 13, 2013).
Briggs, J. 2012. Evaluation of the CTSA Program. Remarks presented at Meeting 2: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, December 12.
Briggs, J., and C. P. Austin. 2012. NCATS and the evolution of the Clinical and Translational Science Award (CTSA) Program. PowerPoint presented at
Meeting 1: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, October 29. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2012-OCT-29/IOM%20Briggs-Austin%20102912.pdf (accessed February 13, 2013).
Calmbach, W. L., J. G. Ryan, L.-M. Baldwin, and L. Knox. 2012. Practice-Based Research Networks (PBRNs): Meeting the challenges of the future. Journal of the American Board of Family Medicine 25(5):572–576.
CTSA (Clinical and Translational Science Awards) Central. 2011a. 2011 CTSA Consortium Committee annual reports. https://www.signup4.net/Upload/BOOZ12A/CTSA37E/ȀFile%202_2011%20CTSA%20Consortium%20Committee%20Annual%20Reports.pdf (accessed March 26, 2013).
———. 2011b. CTSA NIAID annual summary 2011. https://www.ctsacentral.org/sites/default/files/documents/NIAID_2011.pdf (accessed February 21, 2013).
———. 2013a. About the CTSA Consortium. https://www.ctsacentral.org/aboutus/ctsa (accessed February 13, 2013).
———. 2013b. Assets/Catalog. https://www.ctsacentral.org/reports/cataloging (accessed April 2, 2013).
———. 2013c. CKFC year in review: Communications best practices. https://ctsacentral.org/sites/default/files/documents/%2310_Communications_year_in_review.pdf (accessed March 26, 2013).
———. 2013d. Clinical and Translational Science Awards. https//www.ctsacentral.org/ (accessed March 26, 2013).
———. 2013e. Communications Key Functions Committee. https://www.ctsacentral.org/committee/communications (accessed March 26, 2013).
———. 2013f. Evaluation Key Function Committee. https://www.ctsacentral.org/committee/evaluation (accessed March 26, 2013).
———. 2013g. ROCKET (Research Organization, Collaboration, and Knowledge Exchange Toolkit). https://www.ctsacentral.org/rocket (accessedMarch 1, 2013).
CTSA Evaluation Key Function Committee. 2012. Evaluation guidelines for the Clinical and Translational Science Awards (CTSAs). Submitted to the IOM Committee by D. Rubio on March 28. Available by request through the National Academies’ Public Access Records Office.
CTSA PIs (Principal Investigators). 2012. Preparedness of the CTSA’s structural and scientific assets to support the mission of the National Center for Advancing Translational Sciences (NCATS). Clinical and Translational Science 5(2):121–129.
Curley, M. A. Q. 2013. Future directions for using CTSA programs and resources. PowerPoint presented at Meeting 3: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, January 24. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2013-JAN-24/Martha%20Curley.pdf (accessed March 26, 2013).
Demichelisa, F., S. R. Setlurd, S. Banerjeee, D. Chakravartya, J. Y. H. Chend, C. X. Chena, J. Huanga, H. Beltranf, D. A. Oldridgea, N. Kitabayashia, B.
Stenzelg, G. Schaeferg, W. Horningerg, J. Bekticg, A. M. Chinnaiyanh, S. Goldenbergi, J. Siddiquih, M. M. Regank, M. Kearneyl, T. D. Soongb, D. S. Rickmana, O. Elementob, J. T. Weij, D. S. Scherri, M. A. Sandal, G. Bartschg, C. Leed, H. Klockerg, and M. A. Rubin. 2012. Identification of functionally active, low frequency copy number variants at 15q21.3 and 12q21.31 associated with prostate cancer risk. Proceedings of the National Academy of Sciences of the United States of America 109(17):6686–6691.
Disis, N. 2012. CTSA strategic goal 5: Advancing T1 translational research. PowerPoint presented at Meeting 1: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, October 29. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2012-OCT-29/CTSA%20presentations/6-Disis%20IOM_CTSA_StrategicGoal5_Disis_10%2029%2012.pdf (accessed March 26, 2013).
Fagnan, L. J., M. Davis, R. A. Deyo, J. J. Werner, and K. C. Stange. 2010. Linking Practice-Based Research Networks and Clinical and Translational Science Awards: New opportunities for community engagement by academic health centers. Academic Medicine 85(3):476–483.
Felt, U., B. Wynne, M. Callon, M. E. Goncalves, S. Jasanoff, M. Jepsen, P.-B. Joly, Z. Konopasek, S. May, C. Neubauer, A. Rip, K. Siune, A. Stirling, and M. Tallacchini. 2007. Taking European knowledge society seriously: Report of the expert group on science and governance to the science, economy and society directorate, Directorate-General for Research, European Commission. Luxembourg: Office for Official Publications of the European Communities. http://ec.europa.eu/research/science-society/document_library/pdf_06/european-knowledge-society_en.pdf (accessed March 26, 2013).
Fine, J. 2012. Translation of basic science to human studies: Advancing T1 and T2 research. PowerPoint presented at Meeting 2: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, December 12. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2012-DEC-12/1-4%20Jacqueline%20Fine.pdf (accessed March 26, 2013).
Frechtling, J., K. Raue, J. Michie, A. Miyaoka, and M. Spiegelman. 2012. The CTSA national evaluation phase 1 final report. Rockville, MD: Westat. https://www.ctsacentral.org/sites/default/files/files/CTSANationalEval_FinalReport_20120416.pdf (accessed April 1, 2013).
Germino, G. G. 2012. Opportunities for NIDDK-CTSA cooperation. PowerPoint presented at Meeting 1: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, October 29. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2012-OCT-20/NIH%20presentations/4-%20Germino%20CTSA%20IOM%20NIDDK.pdf (accessed February 21, 2013).
Harvard College. 2012. About eagle-i. https://www.eagle-i.net/about (accessed March 1, 2013).
Heatwole, C., R. Bode, N. Johnson, C. Quinn, W. Martens, M. P. McDermott, N. Rothrock, C. Thornton, B. Vickrey, D. Victorson, and R. Moxley III.
2012. Patient-reported impact of symptoms in myotonic dystrophy type 1 (PRISM-1). Neurology 79(4):348–357.
HMO (Health Maintaince Organization) Research Network. 2013. HMO Research Network: About our organization. http://www.hmoresearchnetwork.org/about.htm (accessed February 28, 2013).
ICSU CFRS (International Council for Science Committee on Freedom and Responsibility in the Conduct of Science). 2010. Advisory note: “Science
communication.” http://www.icsu.org/publications/cfrs-statements/sciencecommunication/ICSU_Sci_Commn_Adv_Note_Dec2010.pdf (accessed March 26, 2013).
IOM (Institute of Medicine). 2007. The learning healthcare system: Workshop summary. Washington, DC: The National Academies Press.
———. 2009. HHS in the 21st century: Charting a new course for a healthier America. Washington, DC: The National Academies Press.
———. 2011. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academies Press.
———. 2012. Maximizing the impact of the Cures Acceleration Network: Workshop summary. Washington, DC: The National Academies Press.
———. 2013a. Responses to public input questions regarding the CTSA Program at NCATS. Submitted to the IOM Committee between December 17, 2012–March 1, 2013. Available by request through the National Academies’ Public Access Records Office.
———. 2013b. Roundtable on Value Science-Driven Health Care. http://www.iom.edu/Activities/Quality/VSRT.aspx (accessed April 10, 2013).
Kane, C., A. Alexander, J. A. Hogle, H. M. Parsons, and L. Phelps. 2013. Heterogeneity at Work: Implications of the 2012 Clinical Translational Science Award Evaluators Survey: Where are we now? Submitted to the IOM Committee by D. Rubio on March 28. Available by request through the National Academies’ Public Access Records Office.
Katz, S., J. Anderson, H. Auchincloss, J. Briggs, A. Guttmacher, K. Hudson, R. Hodes, W. Koroshetz, R. Ranganathan, G. Rodgers, and S. Shurin. 2011. NIH CTSA/NCATS Integration Working Group recommendations. http://www.ncats.nih.gov/files/recommendations.pdf (accessed April 8, 2013).
Kaufmann, P. 2013. NeuroNEXT. PowerPoint presented during Conference Call Meeting 3: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, January 30. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2013-JAN-30/Petra%20Kaufmann.pdf (accessed February 25, 2013).
Lambright, W. H. 2002. Managing “big science”: A case study of the Human Genome Project. Arlington, VA: PriceWaterhouseCoopers Endowment for the Business of Government. http://www.businessofgovernment.org/sites/default/files/HumanGenomeProject.pdf (accessed April 18, 2013).
Lee, L. S., S. N. Pusek, W. T. McCormack, D. L. Helitzer, C. A. Martina, A. M. Dozier, J. S. Ahluwalia, L. S. Schwartz, L. M. McManus, B. D. Reynolds, E. N. Haynes, and D. M. Rubio. 2012. Clinical and translational scientist career success: Metrics for evaluation. Clinical and Translational Science 5(5):400–407.
McCormack, F. X., Y. Inoue, J. Moss, L. G. Singer, C. Strange, K. Nakata, A. F. Barker, J. T. Chapman, M. L. Brantly, J. M. Stocks, K. K. Brown, J. P. Lynch, III, H. J. Goldberg, L. R. Young, B. W. Kinder, G. P. Downey, E. J. Sullivan, T. V. Colby, R. T. McKay, M. M. Cohen, L. Korbee, A. M. Taveira-DaSilva, H.-S. Lee, J. P. Krischer, and B. C. Trapnell. 2011. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. New England Journal of Medicine 364(17):1595–1606.
Michaud, M. 2012. In muscular dystrophy, what matters to patients and doctors can differ. http://www.urmc.rochester.edu/news/story/index.cfm?id=3567 (accessed March 1, 2013).
Miyaoka, A., M. Spiegelman, K. Raue, and J. Frechtling. 2011. Findings from the CTSA National Evaluation Education and Training Study. Rockville, MD: Westat. https://ctsacentral.org/sites/default/files/documents/EducationTrainingReport_20111228.pdf (accessed April 1, 2013).
Mulligan, L. 2012. Compliation of Request for Information responses from May 5, 2012. Submitted to the IOM Committee on September 17. Available by request through the National Academies’ Public Access Records Office.
NCATS (National Center for Advancing Translational Sciences). 2012a. Clinical and Translational Science Awards factsheet. http://www.ncats.nih.gov/files/ctsa-factsheet.pdf (accessed March 26, 2013).
———. 2012b. FAQ about CTSA RFA-TR-12-006. http://www.ncats.nih.gov/research/cts/ctsa/funding/faq/faq.html (accessed March 22, 2013).
———. 2012c. Request for information: Enhancing the Clinical and Translational Science Awards Program. http://www.ncats.nih.gov/files/report-ctsa-rfi.pdf (accessed April 8, 2013).
———. 2013a. About NCATS. http://www.ncats.nih.gov/about/about.html (accessed March 26, 2013).
———. 2013b. About the CTSA Program. http://www.ncats.nih.gov/research/cts/ctsa/about/about.html (accessed April 8, 2013).
———. 2013c. NCATS: Program index. http://www.ncats.nih.gov/about/program-index/program-index.html (accessed March 26, 2013).
———. 2013d. News and events: Feature stories. http://www.ncats.nih.gov/news-and-events/features/features.html (accessed March 26, 2013).
NCRR (National Center for Research Resources). 2009. Progress report 2006–2008 Clinical and Translational Science Awards: Advancing scientific discoveries nationwide to improve health. http://www.ncats.nih.gov/files/2008_ctsa_progress_report.pdf (accessed March 26, 2013).
NIAID (National Institute of Allergy and Infectious Diseases). 2012. Not-forprofit partnership with Eli Lilly and Company for TB early phase drug
discovery. http://www.niaid.nih.gov/tipics/tuberculosis/research/pages/lilly.aspx (accessed April 10, 2013).
NIH (National Institutes of Health). 2005. RFA-RM-06-002: Institutional Clinical and Translational Science Award (U54). http://grants.nih.gov/grants/rfa-files/RFA-RM-06-002.html (accessed February 13, 2013).
———. 2009. RFA-RM-09-004: Institutional Clinical and Translational Science Award (U54). http://grants.nih.gov/grants/guide/rfa-files/RFA-RM-09-004.html (accessed March 22, 2013).
———. 2011. About NIH: Mission. http://www.nih.gov/about/mission.htm (accessed March 26, 2013).
———. 2012a. NIH research performance progress report (RPPR) instruction guide. http://grants.nih.gov/grants/rppr/rppr_instruction_guide.pdf (accessed March 26, 2013).
———. 2012b. Progress report 2009–2011 Clinical and Translational Science Awards: Foundations for accelerated discovery and efficient translation. http://www.ncats.nih.gov/ctsa_2011 (accessed March 26, 2013).
———. 2012c. RFA-TR-12-006: Institutional Clinical and Translational Science Award (U54). http://grants.nih.gov/grants/guide/rfa-files/rfa-tr-12-006.html (accessed February 13, 2013).
OIG (Office of the Inspector General). 2011. NIH administration of the Clinical and Translational Science Awards Program. https://oig.hhs.gov/oei/reports/oei-07-09-00300.pdf (accessed April 8, 2013).
Palmer, S. E., and R. A. Schibeci. 2012. What conceptions of science communication are espoused by science research funding bodies? Public Understanding of Science, August 24. Published online before print, doi: 10.1177/0963662512455295.
Parsons, S. 2013. Written comments regarding CTSA evaluations. Submitted to the IOM Committee on February 14. Available by request through the National Academies’ Public Access Records Office.
Patten, I. S., S. Rana, S. Shahul, G. C. Rowe, C. Jang, L. Liu, M. R. Hacker, J. S. Rhee, J. Mitchell, F. Mahmood, P. Hess, C. Farrell, N. Koulisis, E. V. Khankin, S. D. Burke, I. Tudorache, J. Bauersachs, F. del Monte, D. Hilfiker-Kleiner, S. A. Karumanchi, and Z. Arany. 2012. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 485(7398):333–338.
Patton, M. Q. 2008. Utilization-focused evaluation. 4th ed. Thousand Oaks, CA: SAGE Publications, Inc.
Pence, K. 2012. Discovery of treatment for rare lung disease earns U.C. researcher a national award. http://www.uc.edu/news/NR.aspx?id=15589 (accessed March 1, 2013).
Peterson, K. A., P. D. Lipman, C. J. Lange, R. A. Cohen, and S. Durako. 2012. Supporting better science in primary care: A description of Practice-Based Research Networks (PBRNs) in 2011. Journal of the American Board of Family Medicine 25(5):565–571.
Prescott, B. 2012. Researchers uncover important clues to a dangerous complication of pregnancy. http://www.bidmc.org/News/InResearch/2012/May/Arany_PPCM.aspx (accessed March 1, 2013).
Pulley, J. 2013. CTSA essays and worksheets. Submitted to the NIH CTSA/NCATS Integration Working Group, July 2011. Submitted to the IOM Committee on January 7. Available by request through the National Academies’ Public Access Records Office.
Purdy, M. C. 2012. Stroke patients benefit from carmaker’s efficiency. http://news.wustl.edu/news/Pages/24442.aspx (accessed March 1, 2013).
Raue, K., A. Miyaoka, M. Spiegelman, and J. Frechtling. 2011. Findings from the CTSA national evaluation utilization study. Rockville, MD: Westat. https://ctsacentral.org/sites/default/files/documents/EducationTrainingReport_20111228.pdf (accessed April 1, 2013).
Reed, J. C., E. L. White, J. Aube, C. Lindsley, M. Li, L. Sklar, and S. Schreiber. 2012. The NIH’s role in accelerating translational sciences. Nature Biotechnology 30(1):16–19.
Reis, S. E., L. Berglund, G. R. Bernard, R. M. Califf, G. A. FitzGerald, and P. C. Johnson. 2010. Reengineering the national clinical and translational research enterprise: The strategic plan of the National Clinical and Translational Science Awards Consortium. Academic Medicine 85(3):463–469.
Rosenblum, D., and B. Alving. 2011. The role of the Clinical and Translational Science Awards program in improving the quality and efficiency of clinical research. Chest 140(3):764–767.
Rubio, D. 2013. Evaluation Key Function Committee’s outline of next steps for common metrics. Submitted to the IOM Committee on March 28. Available by request through the National Academies’ Public Access Records Office.
Rubio, D. M., D. J. del Junco, R. Bhore, C. J. Lindsell, R. A. Oster, K. M. Wittkowski, L. J.Welty, Y.-J. Lih, and D. DeMetsi. 2011a. Evaluation metrics for biostatistical and epidemiological collaborations. Statistics in Medicine 30(23):2767–2777.
Rubio, D. M., B. A. Primack, G. E. Switzer, C. L. Bryce, D. L. Seltzer, and W. N. Kapoor. 2011b. A comprehensive career-success model for physician-scientists. Academic Medicine 86(12):1571–1576.
Rubio, D. M., M. Sufian, and W. M. Trochim. 2012. Strategies for a national evaluation of the Clinical and Translational Science Awards. Clinical and Translational Science 5(2):138–139.
Shurin, S. B. 2012. CTSA V. 2.0 perspective from the NLHBI. PowerPoint presented at Meeting 1: IOM Committee to Review the CTSA Program at NCATS, Washington, DC, October 29. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2012-OCT-29/NIH%20presentations/2-Shurin-IOM%20CTSA%202012%2010%2018%20rev%20JW.pdf (accessed February 21, 2013).
Shuster, J. J. 2012. U.S. government mandates for clinical and translational research. Clinical and Translational Science 5(1):83–84.
Stanford University. 2012. Macromolecular crystallography at SSRL: AutoDrug. http://smb.slac.stanford.edu/research/developments/autodrug (accessed April 10, 2013).
———. 2013. About the Stanford Synchrotron Radiation Lightsource. http://www-ssrl.slac.stanford.edu/content/about-ssrl/about-stanford-synchrotron-radiation-lightsource (accessed April 10, 2013).
Steiner, J. F. 2013. Written comments to IOM Committee to Review the CTSA Program at NCATS for panel presentation on January 24. Submitted to the IOM Committee on February 21. Available by request through the National Academies’ Public Access Records Office.
Steketee, M., J. Frechtling, D. Cross, and J. Schnell. 2012. Final report on CTSA-supported publications: 2006 to 2011. Rockville, MD: Westat.
Task Force on the Principles of Community Engagement (Clinical and Translational Science Awards Consortium Community Engagement Key Function Committee Task Force on the Principles of Community Engagement). 2011. Principles of community engagement: Second edition. NIH Publication No. 11-7782. http://www.atsdr.cdc.gov/communityengagement/pdf/PCE_Report_508_FINAL.pdf (accessed April 2, 2013).
Tsai, Y., S. E. McPhillips, T. M. McPhillips, A. González, and S. M. Soltis. 2012. AutoDrug: An automated pipeline for drug discovery at the Stanford Synchrotron Radiation Lightsource (SSRL). http://smb.slac.stanford.edu/research/developments/autodrug/handout.html (accessed April 10, 2013).
University of Rochester. 2012. CTSA IP: Intellectual Property information exchange. http://www.ctsaip.org (accessed March 1, 2013).
Vanderbilt University. 2011. Vanderbilt University Medical Center awarded $20 million to coordinate science consortium. http://www.mc.vanderbilt.edu/news/releases.php?release=2152 (accessed April 10, 2013).
———. 2012. ResearchMatch. https://www.researchmatch.org (accessed March 1, 2013).
———. 2013a. IRBshare. https://www.irbshare.org (accessed February 13, 2013).
———. 2013b. REDCap (Research Electronic Data Capture). http://projectredcap.org (accessed March 1, 2013).
VIVO. 2013. About VIVO. http://vivoweb.org/about (accessed March 1, 2013).
Volkow, N. 2012. Building drug abuse research at NIDA in cooperation with the CTSA consortium. PowerPoint presented during Conference Call Meeting 1: IOM Committee to review the CTSA program at NCATS,
Washington, DC, November 19. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2012-NOV-19/Nora%20Volkow.pdf (accessed February 22, 2013).
Wadman, M. 2010. NIH encourages translational collaboration with industry. Nature Reviews Drug Discovery 9(4):255–256.
Weiss, L. 2012. Written comments to IOM Committee to Review the CTSA Program at NCATS for panel presentation. Submitted to the IOM Committee on October 29. Available by request through the National Academies’ Public Access Records Office.
Westat. 2011. Report on field visits to CTSAs. Rockville, MD: Westat. https://www.ctsacentral.org/sites/default/files/documents/FieldVisit_FinalReport_2011June.pdf (accessed April 1, 2013).
Woods, L. 2012. Two genetic deletions in human genome linked to the development of aggressive prostate cancer. http://weill.cornell.edu/news/releases/wcmc/wcmc_2012/04_09_12.shtml (accessed March 1, 2013).
Zerhouni, E. A. 2005. Translational and clinical science—time for a new vision. New England Journal of Medicine 353(15):1621–1623.




















































