The US Endangered Species Act (ESA) requires federal agencies to consult with the Fish and Wildlife Service (FWS) or the National Marine Fisheries Service (NMFS) when a federal action might affect a species that is listed as threatened or endangered (that is, a listed species) or its designated critical habitat. One such action that could potentially affect listed species or their critical habitats is the registration (or reregistration) of pesticides by the US Environmental Protection Agency (EPA) under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). Accordingly, EPA must first determine whether the registration (or reregistration) of a pesticide “may affect” a listed species. If so, EPA must initiate formal consultation or determine whether it is “likely to adversely affect” a listed species. If EPA determines that the pesticide registration is “not likely to adversely affect” a listed species—and FWS or NMFS, as appropriate, agrees—no further consultation is required. However, if EPA determines that the pesticide registration is “likely to adversely affect” a listed species, a formal consultation is required, and the product of that formal consultation is a biological opinion (BiOp) issued by FWS or NMFS. Over the last decade, several court cases have made it clear that formal or informal consultation is required when EPA registers or reregisters a pesticide that might affect a listed species. The consultations that have resulted from the court cases raise questions regarding the best approaches or methods for determining risks to listed species and their critical habitats. Because EPA, FWS, and NMFS have some fundamental differences in approaches, they and the US Department of Agriculture (USDA) asked the National Research Council (NRC) to examine scientific and technical issues related to determining risks to ESA-listed species from pesticides that are registered under FIFRA. As a result of the request, NRC convened the Committee on Ecological Risk Assessment under FIFRA and ESA, which prepared the present report.
THE FEDERAL INSECTICIDE, FUNGICIDE, AND RODENTICIDE ACT
FIFRA is the federal statute that governs the sale, distribution, and use of pesticides in the United States [7 U.S.C. §§ 136-136y]. EPA has the primary
responsibility for administering FIFRA, and the states play an important role in enforcing the act. Under FIFRA, the term pesticide is defined as “any substance or mixture of substances intended for preventing, destroying, repelling or mitigating any pest” [7 U.S.C. § 136 (u)(1)].
Pursuant to FIFRA Section 3(a), a pesticide may not be sold or distributed in the United States without a license, known as a registration, from EPA. To obtain a FIFRA registration, an applicant must demonstrate, among other things, that the pesticide will “perform its intended function without unreasonable adverse effects on the environment” [§ 136a (c)(5)(C)] and that when the pesticide is “used in accordance with widespread and commonly recognized practice it will not generally cause unreasonable adverse effects on the environment” [§ 136a (c)(5)(D)]. FIFRA defines environment as “water, air, land, and all plants and man and other animals living therein and the interrelationships which exist among these” [§ 136 (j)]. It defines the phrase unreasonable adverse effects on the environment as any “unreasonable risk to man or the environment taking into account the economic, social, and environmental costs and benefits of the use of any pesticide” [§ 136 (z)(bb)(1)]. In other words, when deciding whether a particular pesticide meets the standard for registration, EPA must consider the economic and social benefits of using the pesticide and the risks to humans and the environment posed by its use. EPA has interpreted the “unreasonable adverse effects on the environment” standard to require a balancing of costs and benefits in which EPA weighs the costs to human health and the environment resulting from pesticide use against social and economic benefits, such as the benefits of mitigating disease vectors and reducing crop damage.
To obtain a registration, an applicant must provide data demonstrating that its pesticide does not cause unreasonable adverse effects. FIFRA does not mandate that any particular tests be conducted or that any particular type of data be submitted to obtain a registration. However, FIFRA Section 3(c)(2)(A) directs EPA to publish guidelines “specifying the kinds of information which will be required to support the registration of a pesticide” and directs EPA to revisit and revise these guidelines “from time to time.” Pursuant to that section, EPA has promulgated rules in 40 C.F.R. Part 158 that establish data requirements for demonstrating that a particular pesticide product meets the standard for registration. Excerpts from Part 158 are provided in Appendix A of the present report. EPA has also developed a series of test guidelines that specify methods for conducting the studies that will generate the data to support registration.
Many of the data requirements in Part 158 address general information about a pesticide, such as its chemical composition and chemical and physical properties. Other data requirements focus on mammalian testing that can be used to evaluate the human health effects of pesticide exposure. Most important for purposes of this report, Part 158 includes a number of sections related to environmental risk, including risks to species that are not the targets of the pesticide (that is, nontarget species). For example, Subpart G requires avian oral toxicity testing, avian dietary toxicity testing, and avian reproduction testing and might require wild-mammal toxicity testing and simulated or actual field testing. Addi-
tional data on wildlife are required only case by case. Subpart G also requires acute toxicity tests on honeybees and various toxicity tests on freshwater fishes, freshwater invertebrates, and estuarine and marine organisms. Subpart L sets forth requirements for spray-drift data, and Subpart N sets forth requirements for environmental fate data, which are targeted at assessing “the presence of widely distributed and persistent pesticides in the environment which may result in loss of usable land, surface water, ground water, and wildlife resources, and…the potential environmental exposure of other nontarget organisms, such as fish and wildlife, to pesticides” [40 C.F.R § 158.130(h)(l)].
If, after evaluating the data submitted, EPA determines that the applicant has demonstrated that the standard for registration has been met, it will issue a registration. The registration will specify use restrictions that EPA has determined are necessary to meet the standard for registration. Most important, the registration will require that the pesticide be labeled with specific product information, directions for use, and hazard information. The product label dictates legal use of the pesticide. FIFRA provides that it is a violation of federal law “to use any registered pesticide in a manner inconsistent with its labeling” [§ 136 (j)(a)(2)(G)], and every registered pesticide product is required to bear a label containing this warning. Accordingly, the label is the vehicle not only for providing important information to end users but for mandating the purposes for which and the manner in which end users may use the pesticide product. The label instructions are necessary to ensure that the pesticide meets the standard for registration. A pesticide that might have an unreasonable adverse effect on the environment if used at a particular dosage, for a particular crop type, or in a particular manner might not have an unreasonable adverse effect if its use is restricted to other specified crops or specified application rates or restricted in other ways to minimize human health or environmental risks. Thus, the label language is EPA’s primary regulatory tool for reducing pesticide risk under FIFRA. Users who fail to comply with label directions can incur penalties, although in practice it is extremely difficult to monitor every pesticide application to determine whether it was carried out according to the label.
Once a pesticide is registered, EPA does not require a permit or any other approvals before it is used. That is, there is no evaluation of specific pesticide applications; thus, the geographic and temporal factors specific to an application site or timing are not evaluated before the pesticide is released into the environment. However, some states have their own pesticide-permitting programs that apply to specific types of pesticide use (for example, aerial application). Furthermore, EPA has the authority under FIFRA to classify specific pesticides as “restricted use pesticides.” Those pesticides can be used only under the supervision of a certified applicator who has received training in the proper handling and use of the pesticide in question. However, even when there are state permitting requirements and certified-applicator-training requirements, most pesticide use is regulated only by label restrictions without a requirement for a permit or other approval before use.
After a pesticide product is registered, FIFRA continues to impose responsibilities on the registrant, and EPA can require additional data submission. FIFRA Section 6(a)(2) requires that if at any time after the issuance of a registration a registrant obtains information that a pesticide has unreasonable adverse effects on the environment, the registrant is required to submit the information to EPA. And FIFRA Section 3(c)(2)(B) states that “if [EPA] determines that additional data are required to maintain in effect an existing registration of a pesticide, [EPA] shall notify all existing registrants of the pesticide to which the determination relates.” If EPA invokes Section 3(c)(2)(B), referred to as a “data call-in,” each registrant must provide evidence to EPA within 90 days that it is “taking steps to secure the additional data required.” If EPA determines that a registrant has failed to take appropriate steps to secure the required data, it may initiate proceedings to suspend the registration of the pesticide. EPA can cancel a registration if it determines that a pesticide or its labeling does not comply with FIFRA or if the pesticide “generally causes unreasonable adverse effects on the environment when used in accordance with widespread and commonly recognized practice” (75 Fed. Reg. 68297[2010]). FIFRA Section 6(c) authorizes the suspension of a registration if EPA determines that suspension is necessary to prevent an imminent hazard during the time required for cancellation. FIFRA Section 2(l) defines imminent hazard to include a “situation which exists when the continued use of a pesticide during the time required for cancellation proceeding… will involve unreasonable hazard to the survival of a species declared endangered or threatened by the Secretary pursuant to the Endangered Species Act of 1973.”
Congress has on several occasions directed EPA to review the human health and environmental effects of pesticides registered before some specified date. In 1972, revisions of FIFRA mandated that EPA re-evaluate registered pesticides—a process known as reregistration—by using current scientific and regulatory standards to ensure that the data used to register the pesticides originally meet current standards. In 1988, Congress imposed specific reregistration requirements that were intended to improve the speed and the nature of reregistration. The 1988 provisions established a multistep process with various deadlines intended to ensure that registrants submit required data to EPA in a timely manner. Under the 1988 amendments, failure to meet the data-submission deadlines could result in suspension or cancellation of a registration.
In 1996, Congress passed the Food Quality Protection Act (FQPA), which also amended FIFRA. The FQPA was focused on providing additional protections for humans, not wildlife, and required EPA to re-evaluate many food-use pesticides under new human-health standards. As a result of the re-evaluation, EPA canceled some pesticide uses, changed allowable application rates, and imposed use restrictions on others that were not aimed at reducing risk to wildlife but had that result.
The ESA is the federal statute that creates the authority to designate species as threatened or endangered and governs the activities that might affect those species (Endangered Species Act 16 U.S.C. §§ 1531–1544). The ESA is administered and enforced by two federal agencies that have jurisdiction for species in different ecosystems. FWS, in the Department of the Interior, typically is responsible for freshwater and terrestrial species, and NMFS, in the Department of Commerce, typically is responsible for marine and anadromous species (species that migrate from marine to freshwater environments to spawn, such as Pacific salmonids). The two agencies—referred to collectively as the Services and individually as the Service—are responsible for listing species as endangered or threatened under the ESA.
An endangered species is defined as a “species which is in danger of extinction throughout all or a significant portion of its range” [16 U.S.C. § 1532 (6)]. A threatened species is defined as a species that is “likely to become… endangered…within the foreseeable future throughout all or a significant portion of its range” [§ 1532 (20)]. Subspecies of “fish or wildlife or plants and any distinct population segment of any species of vertebrate fish or wildlife which interbreeds when mature” [§ 1532 (16)] are also included in the ESA’s definition of species and thus can be listed. In this report, the terms endangered species, threatened species, and listed species can refer to subspecies or distinct population segments as defined by the ESA. Once a species is listed, the ESA requires that the Services designate critical habitat for each listed species. As of October 15, 2012, critical habitat had been designated for 653 of the 1,434 listed species that occur in the United States.
Endangered species are subject to several protections under the ESA, and threatened species are for the most part subject to the same protections. ESA Section 9 prohibits the “take” of listed species. The statute defines take as “harass, harm, pursue, hunt, shoot, wound, kill, trap, capture, or collect or attempt to engage in any such conduct” [16 U.S.C. § 1532 (19)]. The Services have further defined harm to include acts that involve substantial habitat modification or degradation that kills or injures listed species by substantially impairing essential behavior patterns, including breeding, feeding, and sheltering. That broad interpretation of harm has been upheld by the US Supreme Court [Babbitt v Sweet Home Chapter of Communities for a Greater Oregon, 515 U.S. 687, 698 (1995)]. The ESA authorizes the Services to assess penalties for unauthorized take of listed species and authorizes courts to impose injunctions to prevent a take from occurring or continuing. A federal agency (such as EPA) is liable for its actions, including, at least according to one court, the issuance of FIFRA registrations that result in a take of a listed species [Defenders of Wildlife v Administrator, EPA, 882 F. 2d 1294 (8th Cir. 1989)].
Section 7 of the ESA includes another important provision that specifically applies to actions of federal agencies. It mandates that federal agencies use their existing authorities to conserve endangered and threatened species and
consult with the Services to ensure “that any action authorized, funded, or carried out by such agency is not likely to jeopardize the continued existence of any endangered species or threatened species or result in the destruction or adverse modification of [critical habitat] of such species” [16 U.S.C. § 1536 (a)(2)]. The phrase “jeopardize the continued existence of [a listed species]” means “to engage in an action that reasonably would be expected, directly or indirectly, to reduce appreciably the likelihood of both the survival and recovery of a listed species in the wild by reducing the reproduction, numbers, or distribution of that species” [50 CFR § 402.02].
Any proposed federal agency action that “may affect” listed species is subject to ESA Section 7 and could require a formal consultation (see Figure 1-1). The term “may affect” is defined broadly to include beneficial and adverse effects. For any action that “may affect” listed species, the action agency has two options: it may choose to initiate formal consultation or may determine whether the action is “likely to adversely affect” listed species. If the action agency determines, with written concurrence of FWS or NMFS, that the action is “not likely to adversely affect” a listed species or its critical habitat, no further consultation is required. However, if the action agency determines that the action is “likely to adversely affect” a listed species or its critical habitat, formal consultation is required. Through the formal consultation process, FWS or NMFS determines whether the proposed federal agency action is likely to jeopardize listed species; if so, FWS or NMFS will develop “reasonable and prudent alternatives” (RPAs) that, if implemented, are expected to avoid jeopardy. It is at the action agency’s discretion whether to adopt the RPAs. However, the agency will be liable under Section 9 if a take results from its action and the take was not provided for by an incidental take statement (ITS) in the BiOp, the final document issued by FWS or NMFS. An ITS describes actions that will not be considered prohibited takes and describes “reasonable and prudent measures” that must be complied with to be covered by the ITS.
Unlike FIFRA and its implementing regulations, the ESA does not prescribe specific studies that must be conducted or specific data that must be collected or submitted in the consultation process. Instead, in several provisions of the ESA, Congress has directed the Services to make determinations based on the “best scientific and commercial data available.” Similarly, the Services’ rules on consultation state that
the Federal agency requesting formal consultation shall provide the Service with the best scientific and commercial data available or which can be obtained during the consultation for an adequate review of the effects that an action may have upon listed species or critical habitat. This information may include the results of studies or surveys conducted by the Federal agency or the designated non-Federal representative. The Federal agency shall provide any applicant with the opportunity to submit information for consideration during the consultation [50 C.F.R. 402.14(d)].
In formulating its biological opinion, any reasonable and prudent alternatives, and any reasonable and prudent measures, the Service will use the best scientific and commercial data available [50 C.F.R. 402.14(g)(8)].
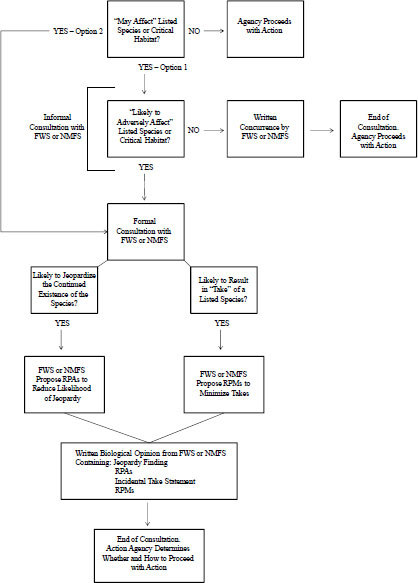
FIGURE 1-1 Consultation process under ESA Section 7 for a federal action that potentially could affect a listed species or critical habitat. If the agency determines that the action “may affect” the listed species or critical habitat, it has two options: (1) determine whether the action is “likely to adversely affect” or (2) go directly to formal consultation with the appropriate Service. Abbreviations: FWS, Fish and Wildlife Service; NMFS, National Marine Fisheries Service; RPA, Reasonable and Prudent Action; RPM, Reasonable and Prudent Measure.
The Services have also issued two policy statements on implementing the “best scientific and commercial data available” mandate. The first is the Notice of Interagency Cooperative Policy on Information Standards [59 Fed. Reg. 34271 (July 1, 1994)]. It applies to, among other things, decisions made in the Section 7 consultation process and states that biologists employed by the Services must evaluate all information to “ensure that any information used by the Services to implement the Act is reliable, credible, and represents the best scientific and commercial data available.” It also expresses a preference that the Services use primary and original sources of information as the basis of its recommendations.
The second policy statement is the Notice of Interagency Cooperative Policy for Peer Review in Endangered Species Act Activities [59 Fed. Reg. 3270 (July 1, 1994)]. It provides that in making listing decisions and developing recovery plans under the ESA, the Services will seek independent peer review. It does not explicitly apply to decisions made in the Section 7 consultation process.
Neither the ESA nor its implementing regulations or policies provide detailed guidance on what is meant by “best scientific and commercial data available.” Moreover, the legislative history of the ESA does not provide any clear direction on what Congress intended by using that language. However, experts who have studied the ESA, its legislative history, and circumstances surrounding the passage of the act have stated that the “best scientific and commercial data” mandate was generally intended to “ensure objective, value-neutral decision making by specially trained experts” (Doremus 2004). As one expert has opined, “taking the best available science mandate at face value, its most obvious purpose would seem to be to ensure that agency decisions are substantially as ‘good’ as can be” (Doremus 2004). Experts who have analyzed the case law involving the use of the best-available-science mandate have concluded that the cases suggest “no consistent thread or logic” (Brennan et al. 2003). Thus, there is little guidance in the ESA, its legislative history, the Services’ rules and policies, or court cases to elaborate the meaning of the “best scientific and commercial data available” mandate in the ESA.
THE RELATIONSHIP BETWEEN THE TWO ACTS
At least one court has held that EPA can be liable for a take under the ESA if its registration of a pesticide results in the take of a listed species [Defenders of Wildlife v Administrator, EPA, 882 F. 2d 1294 (8th Cir. 1989)]. More important for the purposes of the present report, courts have held that EPA is required to comply with the ESA Section 7 consultation process when registering or taking other regulatory actions on pesticides under FIFRA. The requirement that EPA comply with the ESA when registering pesticides under FIFRA presents a number of challenges. First, pesticides, by their very nature, are intended to harm or disrupt a living organism in some way. Pesticides intended for out-
door agriculture, forestry, weed control, and other uses are also intentionally released into the environment. Consequently, if any listed species nest, roost, migrate through, or otherwise exist in a particular geographic location where pesticides are released, they could be exposed to potentially harmful substances, and takes could occur.
As described above, the ESA prohibits any take of a listed species and requires formal consultation for any agency action that is likely to affect any listed species adversely. FIFRA, in contrast, requires a cost-benefit balancing of the risks associated with the use of a pesticide and the social and economic benefits to be gained by its use. The ESA prohibits takes of listed species and seeks to ensure that federal agency actions do not jeopardize the continued existence of a listed species. Economic considerations do not come into play in ESA listing, take, or jeopardy evaluations as they do under FIFRA. The FIFRA cost-benefit standard applies whether or not listed species are at issue, although presumably harm to a listed species would be considered a high cost. In fact, the only place where FIFRA mentions threatened or endangered species is in Section 6(c)(1) of FIFRA, which authorizes EPA to “suspend the registration of a pesticide [if that] is necessary to prevent an imminent hazard during the time required for a cancellation proceeding.” As noted above, FIFRA Section 2(l) defines imminent hazard to include a “situation which exists when the continued use of a pesticide during the time required for cancellation proceeding … will involve unreasonable hazard to the survival of a species declared endangered or threatened.” FIFRA does not provide EPA with any other direction concerning listed species.
Another challenge for EPA in complying with the ESA for pesticide registrations is that FIFRA creates a national registration process whereas the ESA requires an evaluation of effects on the habitat of a listed species and individual members of a species. Under FIFRA, pesticide registration or cancellation decisions are made on a nationwide basis. The ESA, in contrast, is geographically and temporally focused. Although EPA typically considers geographic fate and exposure scenarios relevant to where and when a pesticide is expected to be used, it is challenging to design label restrictions and warnings to ensure that there is never an effect on a listed species.
Another difference between FIFRA and the ESA concerns data available for assessments. As indicated above, FIFRA requires the submission of data before registration, whereas under the ESA the Services are mandated to rely on the best data available (as opposed to requesting new data). Furthermore, under the ESA, decisions are not to be delayed because of a lack of data.
The differences between the statutes have led EPA and the Services to develop different approaches to ecological risk assessment that have often made it difficult for them to reach a scientific agreement. As a result, EPA and the Services decided to seek advice from the NRC on several scientific issues related to conducting an ecological risk assessment.
The committee that was convened in response to the request from EPA, FWS, NMFS, and USDA included experts on salmonid biology, ecology, hydrology, geospatial analysis, exposure analysis, toxicology, population dynamics, statistics, uncertainty analysis, environmental law, and ecological, pesticide, and mixture risk assessment (see Appendix B for biographical information). The committee was asked to evaluate EPA’s and the Services’ methods for determining risks to listed species posed by pesticides and to answer questions concerning the identification of the best scientific data, the toxicological effects of pesticides and chemical mixtures, the approaches and assumptions used in various models, the analysis of uncertainty, and the use of geospatial data. See Box 1-1 for a verbatim statement of the committee’s task.
THE COMMITTEE’S APPROACH TO ITS TASK
The committee held five meetings to assist it in accomplishing its task. The first three included open sessions during which the committee heard from the sponsors and invited speakers from academe, professional organizations, nonprofit organizations, and consulting agencies. The committee submitted written questions to the sponsors to clarify the charge questions, discussed their responses in an open session, and reviewed extensive literature on various aspects of ecological risk assessment and materials provided by the sponsors and stakeholders. As directed in its statement of task, the committee used the recent consultations between the NMFS and EPA as a reference for its evaluation of assessment methods used by EPA and the Services. It emphasizes that it did not specifically evaluate the biological opinions or EPA’s effect determinations on Pacific salmonids; that would have been outside its charge. For ease of discussion, the committee has designated the steps in the ESA process—“may affect,” “likely to adversely affect,” and “likely to jeopardize”—as Steps 1, 2, and 3 in this report.
The committee does not take a position on any legal or regulatory policy issue, provide any legal or policy advice, or comment on the merit of any particular court ruling or other legal or policy decision. Furthermore, it recognizes that the agencies must make regulatory policy choices, and it has consciously avoided commenting on regulatory policy. In fact, the committee concludes that science and regulatory policy need to be kept separate to the extent possible and that there should be transparency where policy is involved. The present report evaluates the science of ecological risk assessment. Once an assessment is conducted, the involved agencies are responsible for making policy decisions pursuant to their legal mandates. The committee uses the generic term decision-maker to indicate a person who will use the results of a risk assessment to inform a decision. The committee makes no statements on who such a person should be; that is a policy issue.
A committee of the National Research Council (NRC) will examine scientific and technical issues related to the methods and assumptions used by the U.S. Environmental Protection Agency (EPA), the U.S. Fish and Wildlife Service (FWS), and the National Oceanic and Atmospheric Administration (NOAA) to conduct scientific assessments of ecological risks from pesticides registered by EPA under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) to species listed under the Endangered Species Act (ESA). The range of scientific studies needed to make such assessments will be considered, including ecological, hydrological, toxicological, and exposure studies. The committee will develop conclusions reflecting the use of scientific principles and to facilitate a more holistic approach to assessing risks across the agencies, considering the intent of the ESA and of FIFRA. Policy issues related to decision making will not be addressed. Specific topics that the committee will consider to the extent practicable include the following:
• Best available scientific data and information. The Services and EPA approach the identification of “best available scientific information” using a variety of differing protocols pertaining to the type and character of scientific information that may be appropriate for these evaluations. Some of these approaches pertain to the character of the information as consensus information, peer-reviewed information, regulatory studies supporting pesticide registrations, or other published and unpublished information. The NRC will evaluate those protocols with respect to validity, availability, consistency, clarity, and utility.
• Sublethal, indirect, and cumulative effects. The ESA requires the consideration of direct, indirect, and cumulative effects on listed species and habitats in the consultation process. The Services and EPA have used differing approaches on how to characterize indirect, sub-lethal, and cumulative effects. The NRC will review the best available scientific methods for projecting these types of effects and consider options for the development of any additional methods that are likely to be helpful.
• Mixtures and inerts. Assessing the effects of the use of chemical mixtures, either in formulated products or as used at the field level, remains a complex and difficult challenge, as is assessing the effects of mixtures of pesticides and other environmental contaminants. Projecting the effects of inert ingredients such as adjuvants, surfactants, and other pesticide product additives is also an area of continuing challenge. The NRC will consider the scientific information available to assess the potential effects of mixtures and inert ingredients.
• Models. There is a range of approaches to the development and use of modeling to assist in analyzing the effects of actions such as using pesticides or alternatives to that use, and active issues remain about the use of unpublished models or the assumptions used in the choice of the available models for any particular analysis of effects. The NRC will assess the protocols governing the development of assumptions associated with model inputs and the use of sensitivity analyses to evaluate the impact of multiple assumptions on the interpretation of model results.
• Interpretation of uncertainty. There are a variety of methods for documenting and interpreting uncertainties and evaluating the extent to which uncertainties impact confidence in the scientific conclusions associated with a jeopardy decision. In particular, the NRC will consider the selection and use of uncertainty factors to account for lack of data on formulation toxicity, synergy, additivity, etc., and how the choice of those factors affects the estimates of uncertainty.
• Geospatial information and datasets. Location of the habitat is an important component of successfully protecting the impacted species. Much variability in datasets, geospatial layers, and scale contributes to uncertainty. The NRC will consider what constitutes authoritative geospatial information, including spatial and temporal scale that most appropriately delineates habitat of the species and the duration of potential effects.
In its deliberations, the NRC will focus on the scientific and technical methods and approaches the agencies use in determining risks to endangered and threatened species associated with the use of pesticides. The NRC will provide conclusions as appropriate about techniques the agencies might apply or use to improve those methods and approaches using scientific principles to support their decision-making.
As examples, the NRC will consider three recent consultations between NOAA and EPA on the effects of EPA’s proposed FIFRA actions on Pacific salmonids as reference points for its work. The NRC will use the consultations as examples of the various agencies’ scientific approaches and methods but will not evaluate the consultations themselves or the decisions resulting from them, and it will not limit its considerations strictly to aquatic species.
The committee’s report is organized into five chapters. Chapter 2 presents a common approach to the assessment process and discusses some overarching issues regarding uncertainty and best data available. Chapters 3 and 4 focus on exposure and effects analysis, respectively; each describes models and issues associated with uncertainty. Chapter 5 addresses the risk characterization process, which combines the results of the exposure and effects analyses. Excerpts of CFR Part 158 are provided in Appendix A, and Appendix B presents biographical information on the committee.
Brennan, M.J., D.E. Roth, M.D. Feldman, and A.R. Greene. 2003. The Endangered Species Act: Thirty years of politics, money, and science. 387 square pegs and round holes: Application of the “best scientific data available” standard in the Endangered Species Act. Tulane Environ. Law J. 16(Sumer):387-444.
Doremus, H. 2004. The purposes, effects, and future of the Endangered Species Act’s best available science mandate. Environ. Law 34:397-450.












