In the United States, approximately 14 million people are cancer survivors and more than 1.6 million people are newly diagnosed with cancer each year (ACS, 2013). By 2022, it is projected that there will be 18 million cancer survivors and, by 2030, 2.3 million people are expected to be newly diagnosed with cancer each year (ACS, 2013; Smith et al., 2009). However, more than a decade after the Institute of Medicine (IOM) first addressed the quality of cancer care in the United States (IOM and NRC, 1999), the barriers to achieving excellent care for all cancer patients remain daunting. The growing demand for cancer care, combined with the complexity of the disease and its treatment, a shrinking workforce, and rising costs, constitute a crisis in cancer care delivery (see Box 1-1).
The complexity of cancer impedes the ability of clinicians, patients, and their families to formulate plans of care with the necessary speed, precision, and quality. As a result, decisions about cancer care are often not evidence-based (IOM, 2008b, 2012). Many patients also do not receive adequate explanation of their treatment goals, and when a treatment phase concludes, they frequently do not know what treatments they have received or the consequences of their treatments for their future health (IOM, 2011b). In addition, many patients do not receive palliative care to manage their cancer symptoms and the side effects from treatment. Most often this occurs because the clinician lacks knowledge of how to provide this care (or how to make referrals to palliative care consultants) or does not identify palliative care management as an important component of high-quality cancer care.
Complicating the situation further are the changing demographics in
BOX 1-1
The Crisis in Cancer Care Delivery
Studies indicate that cancer care is often not as patient-centered, accessible, coordinated, or evidence-based as it could be, detrimentally impacting patients. The following trends amplify the problem:
• The number of older adults is expected to double between 2010 and 2030, contributing to a 31 percent increase in the number of cancer survivors from 2012 to 2022 and a 45 percent increase in cancer incidence by 2030.
• Workforce shortages among many of the professionals involved in providing care to cancer patients are growing and training programs lack the ability to rapidly expand. The care that is provided is often fragmented and poorly coordinated. In addition, family caregivers and direct care workers are administering a substantial amount of care with limited training and support.
• The cost of cancer care is rising faster than are other sectors of medicine, having increased from $72 billion in 2004 to $125 billion in 2010; costs are expected to increase another 39 percent to $173 billion by 2020.
• Advances in understanding the biology of cancer have increased the amount of information a clinician must master to treat cancer appropriately.
• The few tools currently available for improving the quality of cancer care–– quality metrics, clinical practice guidelines, and information technology–– are not as widely used as they could be and all have serious limitations.
SOURCES: de Moor et al., 2013; He et al., 2005; IOM, 2008c, 2009b, 2011a; Mariotto et al., 2011; NCI, 2007; NRC, 2009; Reinhard and Levine, 2012; Smith et al., 2009; Spinks et al., 2012.
the United States that will place new demands on the cancer care delivery system, with the number of adults older than 65 rapidly increasing (He et al., 2005; Smith et al., 2009). The population of those 65 years and older comprises the majority of patients who are diagnosed with cancer and die from cancer, as well as the majority of cancer survivors (NCI, 2012, 2013; NVSS, 2012). In addition, there is a major structural crisis looming in cancer care delivery: the oncology workforce may soon be too small to care for the growing population of individuals diagnosed with cancer (IOM, 2009b). Meanwhile, the Centers for Medicare & Medicaid Services (CMS), the single largest insurer for this older population, is struggling with financial solvency (Goldberg, 2013; Medicare Trustees, 2013). In addition, the costs of cancer treatments are escalating unsustainably, making cancer care less affordable for patients and their families, and creating disparities in patients’ access to high-quality cancer care
(IOM, 2013; Kantarjian and experts in chronic myeloid leukemia, 2013; Stump et al., 2013; Sullivan et al., 2011).
To address the increasing challenges clinicians face in trying to deliver high-quality cancer care, this report charts a new course for cancer care. There is great need for high-quality, evidence-based strategies to guide cancer care and ensure efficient and effective use of scarce resources.
CHANGES IN CANCER CARE SINCE 1999
The IOM’s National Cancer Policy Board first examined the quality of cancer care in the United States in 1999. The resulting report, Ensuring Quality Cancer Care, concluded that “for many Americans with cancer, there is a wide gulf between what could be construed as the ideal and the reality of their experience with cancer care” (IOM and NRC, 1999, p. 2). The report recommended steps to improve cancer care and the evidence base for cancer care, and to overcome barriers of access to high-quality cancer care.
These recommendations led to a number of efforts targeted at improving the delivery of cancer care. The Secretary of the U.S. Department of Health and Human Services (HHS) established the Quality of Cancer Care Committee to work on issues identified in the report. A number of organizations used the report to develop core indicators of quality of cancer care and recommendations for improving the quality of cancer care, including the Agency for Healthcare Research and Quality (AHRQ), the National Quality Forum (NQF), and the National Dialogue on Cancer (a collaboration organized by former President George H.W. Bush and Senator Dianne Feinstein, now known as C-Change). In response to the report, the American Society of Clinical Oncology (ASCO) undertook a national study of the quality of care delivered by oncologists, called the National Initiative on Quality Cancer Care (ASCO, 2013). In addition, the Cancer Quality Alliance, a diverse group of stakeholders committed to advocating for improvements in the quality of cancer care, used the 1999 IOM report and several other reports to develop five cancer case studies depicting a vision for high-quality cancer care and a blueprint for action (Rose et al., 2008). The report also provided major input for the quality of cancer care legislation drafted by the Senate Health, Education, Labor, and Pension Committee.1
Box 1-2 provides examples of the progress to date in implementing the IOM’s 1999 recommendations and examples of the recommendations that are still relevant. However, cancer care has changed substantially since this report was released.
____________________
1 Quality of Care for Individuals with Cancer Act. S. 2965. 107th Cong. (2d Sess. 2002).
BOX 1-2
Examples of Progress to Date in Implementing the Institute of Medicine’s 1999 Recommendations
Recommendation 1: Ensure patients undergoing procedures that are technically difficult to perform and have been associated with higher mortality in lower volume settings receive care at facilities with extensive experience.
Progress to date
• Mortality rates for select complex cancer operations declined after certain patients were redirected to high-volume cancer centers.
• Low-volume clinicians are participating in programs designed to improve the quality of their care.
Current gaps
• The capacity at high-volume centers is insufficient to provide care for all complex cancer cases.
Recommendation 2: Use systematically developed guidelines based on the best available evidence for prevention, diagnosis, treatment, and palliative care.
Progress to date
• The National Comprehensive Cancer Care Network, the American Society of Clinical Oncology, and the American Society of Radiation Oncology have worked with clinical experts to develop guidelines for more than 135 cancers or processes of care.
Current gaps
• Clinicians’ adoption and reporting of adherence to these guidelines is voluntary and not widespread.
• Existing guidelines are not comprehensive and were often developed using consensus processes, not always meeting current standards.
Recommendation 3: Measure and monitor the quality of care using a core set of quality measures.
Progress to date
• A select number of cancer care measures have been developed and endorsed for use in quality reporting.
• These measures are largely process oriented.
Current gaps
• There is no nationally mandated program to which clinicians report data for core measures related to cancer.
• There are pervasive gaps in existing cancer measures.
Recommendation 4: Ensure the following elements of quality care for each individual with cancer:
• Experienced professionals who make recommendations about initial cancer management, which are critical to determining long-term outcome
• An agreed-upon care plan that outlines goals of care
• Access to the full complement of resources necessary to implement the care plan
• Access to high-quality clinical trials
• Policies to ensure full disclosure of information about appropriate treatment options
• A mechanism to coordinate care
• Psychosocial support services and compassionate care
Progress to date
• Many clinicians use multidisciplinary care planning to provide coordinated care to cancer patients.
• Medicare, several states, and new insurance plans included in Health Insurance Marketplaces created by the Patient Protection and Affordable Care Act (ACA) cover standard or routine costs of clinical trials.
• Patient-focused educational materials are available to clinicians when discussing appropriate treatment options with patients.
Current gaps
• Continuing geographic, financial, and social barriers prevent patients from seeking and receiving multidisciplinary care planning and comprehensive cancer care.
• Many cancer patients are not informed about their treatment options and their preferences are not elicited.
• Palliative care is not integrated with cancer care across the continuum from diagnosis to end of life.
• Many cancer patients receive inadequate psychosocial support.
Recommendation 5: Ensure quality of care at the end of life, particularly the management of cancer-related pain and timely referral to palliative and hospice care.
Progress to date
• Screening tools are available to monitor the frequency and severity of patients’ symptoms and to guide patients to supportive and palliative care services.
• Most cancer centers in the United States have inpatient palliative care consult teams.
Current gaps
• Patients with advanced cancer frequently receive palliative care late in their disease course, which compromises quality of life and quality of care for them and their families.
• Patients with advanced cancer nearing the end of life are frequently referred to hospice only days to weeks before death, if at all, compromising quality of life and quality of care for them and their families.
Recommendation 6: Federal and private research sponsors, such as the National Cancer Institute, the Agency for Health Care Policy and Research (now called the Agency for Healthcare Research and Quality), and various health plans, should invest in clinical trials to address questions about cancer care management.
Progress to date
• This recommendation has not been implemented because of the current nature of clinical trials.
Current gaps
• Cancer care management is addressed in Recommendation 8.
Recommendation 7: A cancer data system that can provide quality benchmarks for use by systems of care (e.g., hospitals, provider groups, and managed care systems) is needed.
Progress to date
• Some large health care systems have implemented electronic health records (EHRs) that capture data fields relevant to cancer care.
Current gaps
• There is no standardized system for all cancer care providers to report on quality benchmarks.
• Current EHRs were not designed to collect and report quality metrics but rather as records of individual patient information.
Recommendation 8: Public and private sponsors of cancer care research should support national studies of recently diagnosed individuals with cancer, using information sources with sufficient detail to assess patterns of cancer care and factors associated with the receipt of good care; research sponsors should also support training for cancer care providers interested in health services research.
Progress to date
• The American Recovery and Reinvestment Act, which directed $1.1 billion to comparative effectiveness research (CER), has accelerated CER activity.
Cancer care has always been highly complex, due to diagnostic challenges (imaging, pathology); multimodal, multispecialty treatment strategies (surgery, radiation, chemotherapy); a narrow therapeutic/toxic ratio for many treatments; and long-term and late effects of disease and treatment that contribute to morbidity and mortality (Zapka et al., 2012). Recent results from The Cancer Genome Atlas project (NCI, 2013a), which
• The Patient-Centered Outcomes Research Institute (PCORI) was created by the ACA.
Current gaps
• CER for cancer is just beginning.
• There are shortages of funding for and investigators trained in health services research.
Recommendation 9: Services for the un- and underinsured should be enhanced to ensure entry to, and equitable treatment within, the cancer care system.
Progress to date
• State and federal programs are directing funds to screening for and early detection of cancer in underserved populations.
• The ACA introduced new programs to improve access for many uninsured individuals.
Current gaps
• The uninsured population continues to grow despite ongoing implementation of the ACA, and was exacerbated by the Great Recession.
• Uninsurance is associated with poorer outcomes and lower survival rates.
• Underinsurance is a growing problem with the increased cost of cancer treatments, including tiered copayments for expensive cancer therapies.
Recommendation 10: Studies are needed to examine why specific segments of the population (e.g., members of certain racial or ethnic groups, older patients) do not receive appropriate cancer care.
Progress to date
• Programs have been introduced to increase the involvement of cancer centers designated by the National Cancer Institute in developing research, education, and outreach programs to reduce cancer health disparities.
Current gaps
• There are ongoing disparities, including later stage diagnoses and poorer outcomes for racial and ethnic minorities with cancer.
SOURCE: Adapted from Spinks et al., 2012. Reprinted with permission from John Wiley and Sons.
has characterized hundreds of individual tumors originating from common cancer sites (e.g., breast, lung, prostate, ovary), using state-of-the-art genomic, molecular, and proteomic technologies, have provided startling information about the extreme heterogeneity of cancers that were once thought to have a more uniform biology (Hayano et al., 2013; Joung et al., 2013; Liang et al., 2012; Wang et al., 2013). Cancer treatments have
evolved to reflect this new information on the nature of the disease, with more treatments targeting specific molecular aberrations.
Large randomized clinical trials of muli-agent chemotherapy, the standard at the time of the 1999 report on quality cancer care, have given way to smaller trials of targeted agents, in which companion diagnostic tests are often needed to assess whether the patient’s tumor is likely to be susceptible to the planned treatment. Today, many patients need to be screened in order to identify patients whose tumors have the relevant mutations for trials that study new targeted treatments or combinations of treatments.
In addition, as noted above, there has been a major expansion in the number of individuals receiving treatment, and the population is older and more diverse than it was in 1999. Moreover, a number of recent federal laws, including the Patient Protection and Affordable Care Act of 2010 (ACA),2 have changed the context in which cancer care is practiced. Thus, the factors creating an imperative for change in the cancer care system today are not the same as during the drafting of the 1999 report (see Chapter 2 for a detailed discussion of these trends).
The charge to the committee was to revisit the quality of cancer care more than a decade after publication of the first IOM report, Ensuring Quality Cancer Care (1999). The committee examined what has changed, what challenges remain, whether new problems have arisen, and how health care reform might affect quality care, with a specific focus on the aging U.S. population (see Box 1-3). Although the committee was not asked to undertake an examination of the barriers to adoption of the previous 1999 recommendations, the committee invited Joe Simone, President, Simone Consulting, and chair of the 1999 study, to discuss the challenges associated with implementation of the earlier recommendations.
The IOM appointed an independent committee with a broad range of expertise, including patient care and cancer research, patient advocacy, health economics, ethics, and health law. Brief biographies of the 17 members of the Committee on Improving the Quality of Cancer Care: Addressing the Challenges of an Aging Population are presented in Appendix B. This report, which updates the 1999 report in response to the new and continuing challenges described above, presents the committee’s findings and recommendations.
____________________
2 Patient Protection and Affordable Care Act, Public Law 111-148, 111th Congress (March 23, 2010).
An Institute of Medicine (IOM) committee will examine issues related to the quality of cancer care with a specific focus on the demographic changes that will rapidly accelerate the number of new cancer diagnoses at a time when workforce shortages are predicted. The study will consider quality of care from the perspectives of key stakeholders, including patients, health care providers, and payers. Using other foundational IOM reports as a starting point, the committee will examine opportunities for and challenges to the delivery of high-quality cancer care to an aging population and formulate recommendations for improvement. The committee will
• Review various aspects of quality cancer care, including the coordination and organization of care, outcomes reporting, quality metrics, and disparities in care;
• Consider the growing need for survivorship care, palliative care, and informal caregiving;
• Consider the increasing complexity and cost of cancer care, for example through incorporation of biomarkers to predict response to therapy;
• Consider potential opportunities to improve the quality of care by aligning incentives to promote more effective models of care delivery or through specific payment reforms; and
• Consider how patients can identify, find, and access high-quality cancer care.
This report presents a conceptual framework for improving the quality of cancer care. Two concepts important for understanding the scope of the report include (1) the continuum of cancer care and (2) the importance of addressing the unique needs of older adults with cancer.
THE CONTINUUM OF CANCER CARE
The committee’s recommendations aim to ensure the delivery of high-quality cancer care across the care continuum from diagnosis and treatment to maintaining the health of survivors and providing end-of-life care consistent with patients’ needs, values, and preferences. The provision of patient-centered care planning, palliative care, and psychosocial care; the prevention and management of long-term and late effects of cancer treatment; and family caregiver support should span the cancer care continuum
from diagnosis through end-of-life care. The full cancer care continuum also includes the domains of prevention and risk reduction and screening; however, these domains are outside the scope of this report (see Figure 1-1). An opportunity to improve the quality of cancer care exists in each of the steps of care delivery, as well as in the transitions between the types of care (Zapka et al., 2003). Although the diagram is linear, a patient might enter the cancer care continuum at any of the stages and might not necessarily progress through each of the stages in sequence.
Another way to conceptualize the period of the cancer care continuum that is the focus of this report is through the three overlapping phases of cancer care: (1) the acute phase, (2) the chronic phase, and (3) the end-of-life phase. These phases correspond to the three phases commonly used in the NCI’s studies on the cost of cancer care (i.e., the initial, continuing, and last year of life phases) (Brown et al., 2002; Yabroff et al., 2011). The relationship of the three phases to the overall cancer care continuum is depicted by the green arrow in Figure 1-1.
The acute phase of cancer care occurs immediately after a person is diagnosed with cancer, and generally includes surgical interventions and initial chemotherapy and radiation therapies, as well as palliative and psychosocial care as needed by the patient. Although acute care is often associated with hospitalization for complex conditions, newly diagnosed cancer patients will generally have minimal contact with the inpatient hospital setting. Even many surgical treatments for cancer require only short hospital stays. A large proportion of cancer care is delivered by individual medical oncology practices, where chemotherapy is administered and other treatments are coordinated with surgeons and radiation oncologists.
Cancer treatment and management follow the acute period of care. This period can be conceptualized as the chronic phase, similar to what might be applied to the management of diabetes or congestive heart failure. The goal of care is to provide patients with long-term surveillance for cancer recurrence and, in some patients, prolonged adjuvant or maintenance therapies (e.g., adjuvant endocrine therapy for breast cancer, daily oral tyrosine kinase treatment for chronic myelogenous leukemia). Patients can also receive palliative and psychosocial care during this phase to manage residual effects of the cancer and its treatment. This period can continue for months to years after the initial diagnosis. It includes both patients who are disease-free, as well as the growing number of cancer patients whose disease is controlled but not cured (as in chronic myelogenous leukemia). This phase usually includes multiple clinicians who may or may not be working in the same system of care. Coordination of care with primary care clinicians during this time is variable.
A substantial number of cancer patients will eventually experience
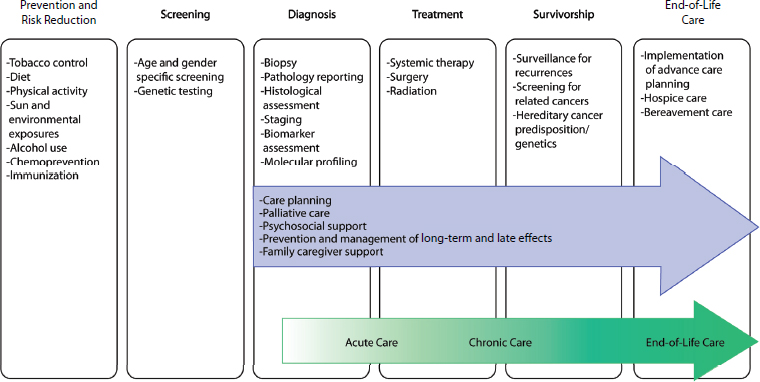
FIGURE 1-1 Domains of the cancer care continuum with examples of activities in each domain. The blue arrow identifies components of high-quality cancer care that should span the cancer care continuum from diagnosis through end-of-life care. The green arrow identifies three overlapping phases of cancer care, which are a way of conceptualizing the period of the cancer care continuum that is the focus of this report.
SOURCE: Adapted from National Cancer Institute figure on the “Cancer Control Continuum” (NCI, 2013b).
a cancer recurrence or progression of their disease. In addition, a minority of patients will have advanced, incurable disease from the time of diagnosis. When cancer-directed therapies are no longer beneficial for the patient, the primary focus of their care should be on palliative care, psychosocial support, and timely referral to hospice care. These patients are in the end-of-life phase of their care.
Cancer Care in Older Adults
Cancer care for older adults, as noted throughout this report, is especially complex. Age is one of the strongest risk factors for cancer. As mentioned above, the majority of cancer diagnoses and cancer deaths occur in individuals 65 years and older, and the majority of cancer survivors are in this age range (see Figures 1-2, 1-3, and 1-4) (NCI, 2012, 2013c; NVSS, 2012).
There are many important considerations to understanding the prognoses of older adults with cancer and formulating their care plans, such as altered physiology, functional and cognitive impairment, multiple coexisting morbidities, increased side effects of treatment, distinct goals of care, and the increased need for of social support. Their ability to participate in clinical trials has been limited, and thus the evidence base for informing treatment decisions in this population is lacking (Scher and Hurria, 2012). The current health care delivery system is poorly prepared to address
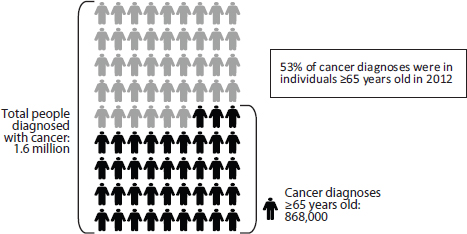
FIGURE 1-2 The majority of cancer diagnoses are in older adults.
SOURCE: NCI, 2012.
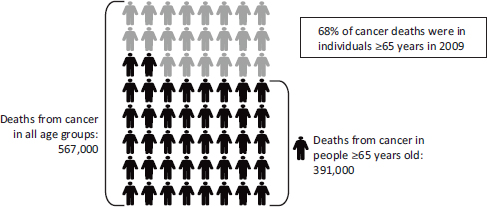
FIGURE 1-3 The majority of cancer deaths are in older adults.
SOURCE: NVSS, 2012.
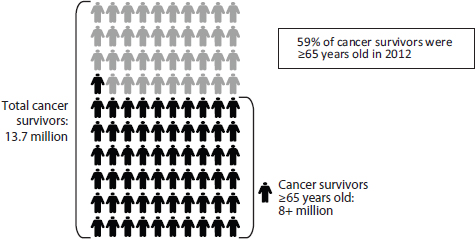
FIGURE 1-4 The majority of cancer survivors are older adults.
NOTE: The committee adopted the National Coalition for Cancer Survivorship’s definition of a cancer survivor, which states that a survivor is any person who has been diagnosed with cancer, from the time of diagnosis through the balance of life (IOM and NRC, 2005).
SOURCE: NCI, 2013c. Figure 1-4
these concerns comprehensively. Thus, meeting the needs of the aging population will be an integral part of improving the quality of cancer care.
The various stakeholders involved in cancer care bring different perspectives on quality. Patients, for example, tend to evaluate care based on whether they receive the most effective and timely treatment for their particular ailment so that they may return to normal life as soon as possible. Health care clinicians, on the other hand, may focus on technical competence and how well care is executed. A health plan might evaluate quality based on efficiency and appropriate use of resources (IOM and NRC, 1999).
The IOM has a long history of analyzing the quality of care and recommending improvements to the health care delivery system. Since the 1999 report was released, the IOM has produced a number of foundational consensus studies addressing particular aspects of high-quality cancer care (e.g., Interpreting the Volume-Outcome Relationship in the Context of Cancer Care [IOM, 2001]; From Cancer Patient to Cancer Survivor: Lost in Transition [IOM and NRC, 2005]; Cancer Care of the Whole Patient: Meeting Psychosocial Health Needs [IOM, 2008a]) and health care generally (e.g., Crossing the Quality Chasm: A New Health System for the 21st Century; Best Care at Lower Cost: The Path to Continuously Learning Health Care in America [IOM, 2001, 2012]) as well as the impact of changing demographics on the health care workforce (Retooling for an Aging America: Building the Health Care Workforce [IOM, 2008c]). In addition, past workshops hosted by the IOM’s National Cancer Policy Forum (NCPF) have addressed a number of issues relevant to improving the quality of cancer care, including the oncology workforce, survivorship care, informal caregiving, assessing value in cancer care, molecularly targeted therapies, treatment planning, a learning health care system for cancer, and the affordability of cancer care (IOM, 2007, 2009a,b, 2010a,b, 2011b, 2013). IOM forums convene workshops in which stakeholders examine policy issues, but they are not formulated to generate consensus recommendations.
The IOM has defined quality of care as “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge” (IOM, 1990, p. 21). In its 1999 report on ensuring the quality of cancer care, the IOM elaborated on this definition and defined poor quality as “overuse (e.g., unnecessary tests, medication, and procedures, with associated risks and side effects); underuse (e.g., not receiving lifesaving surgical procedures); or misuse (e.g., medicines that should not be given together, poor surgical technique)” (IOM and NRC, 1999, p. 79). The IOM defined
good quality care as “providing patients with appropriate services in a technically competent manner, with good communication, shared decision making, and cultural sensitivity” (IOM and NRC, 1999, p. 79).
The 1999 report adopted Avedis Donabedian’s approach to evaluating quality based on structure, process, and outcomes (Donabedian, 1980). Structural quality refers to the ability of a health care system to meet the needs of patients or communities; process quality refers to the technical skills of health care clinicians and their interactions with patients; and outcomes quality refers to changes in patients’ health status (e.g., morbidity and mortality) (IOM and NRC, 1999).
The IOM’s report Crossing the Quality Chasm furthered the conceptualization of high-quality care by identifying six aims for the 21st-century health care system. It stated that health care should be (1) safe—avoiding injuries to patients from the care that is intended to help them; (2) effective—providing services based on scientific knowledge to all who could benefit and refraining from providing services to those not likely to benefit; (3) patient-centered—providing care that is respectful of and responsive to individual preferences, needs, and values, and ensuring that patient values guide all clinical decisions; (4) timely—reducing waits and sometimes harmful delays for both those who receive and those who give care; (5) efficient—avoiding waste, including waste of equipment, supplies, ideas, and human resources; and (6) equitable—providing care that does not vary in quality because of personal characteristics, such as gender, ethnicity, geography, and socioeconomic status (IOM, 2001a).
More recently, a number of other groups have identified additional components of high-quality health care. For example, in commissioning a new facility for Walter Reed National Military Center, Congress mandated that an independent committee oversee the development of the design plans. This committee initiated its task by developing a definition of a world-class medical facility. It determined that these facilities should (1) be designed using evidence-based design principles that facilitate care processes; (2) employ a well-trained, competent, and compassionate workforce; (3) provide coordinated, evidence-based care; (4) meet all relevant quality metric benchmarks and reporting requirements; and (5) appoint pragmatic and visionary leaders (Kizer, 2010; NCR BRAC HSAS, 2009).
AHRQ’s conceptualization of medical neighborhoods—which are oriented around patient-centered medical homes (PCMHs) and include all other clinicians involved in caring for patients, the community, and social services—also include key features of high-quality care. According to AHRQ, high-functioning medical neighborhoods (1) delineate the roles of the clinicians and institutions in the system; (2) share clinical information;
(3) develop individualized care plans for patients; (4) coordinate patients’ transition between care settings; (5) focus on patient preferences; and (6) link clinical and nonclinical services (e.g., personal care services, home-delivered meals, or school-based health care). For patients with cancer, a medical neighborhood could be centered on the cancer care team rather than a primary care PCMH (Taylor et al., 2011). Both of these efforts represent high-level examinations of structural and operational aspects of high-quality health care delivery.
In recent years there have also been several efforts to define high-quality of care for specific aspects of cancer care delivery. The IOM’s report Cancer Care for the Whole Patient concluded that “attending to psychosocial needs should be an integral part of quality cancer care” (IOM, 2008a, p. 8). Recently, Parry and colleagues (2013) developed a conceptual model for cancer survivorship care. Similar to the cancer care framework presented in this report, care planning and meeting the needs of patients and their families are at the center of their survivorship care framework. Their framework aims to use survivorship care plans to produce the short-term goals of improving patients’ adherence to follow-up care; clinicians’ management of long-term and late effects of treatment and comorbid conditions; and health care resources use, and the long-term goals of better health outcomes and lower costs.
Similarly, McCorkle and colleagues (2011) adapted the Chronic Care Model to cancer care because cancer patients increasingly need long-term surveillance and treatment. The primary features of this model are productive interactions between patients and their clinicians; enabled and empowered patients; proactive and prepared practice teams; a practice home for patients with cancer (i.e., a single clinical team that takes responsibility for meeting a patient’s care needs across the continuum of care); and collaborative care plans.
The committee’s conceptual framework for improving the quality of cancer care takes into account the heterogeneity of clinical settings where cancer care is delivered as well as the existing models of high-quality care summarized above. The central goal of its conceptual framework is to deliver patient-centered, evidence-based, high-quality cancer care that is accessible and affordable to the entire U.S. population regardless of the setting where cancer care is provided. The committee identified six components of a high-quality cancer care delivery system that will be integral to this transformation:
1. Engaged patients: A system that supports all patients in making informed medical decisions consistent with their needs, values, and preferences in consultation with clinicians who have expertise in patient-centered communication and shared decision making (see Chapter 3).
2. An adequately staffed, trained, and coordinated workforce: A system that provides competent, trusted, interprofessional cancer care teams that are aligned with patients’ needs, values, and preferences, as well as coordinated with the patients’ noncancer care teams and their caregivers (see Chapter 4).
3. Evidence-based cancer care: A system that uses scientific research, such as clinical trials and comparative effectiveness research (CER), to inform medical decisions (see Chapter 5).
4. A learning health care information technology (IT) system for cancer: A system that uses advances in IT to enhance the quality and delivery of cancer care, patient outcomes, innovative research, quality measurement, and performance improvement (see Chapter 6).
5. Translation of evidence into clinical practice, quality measurement, and performance improvement: A system that rapidly and efficiently incorporates new medical knowledge into clinical practice guidelines; measures and assesses progress in improving the delivery of cancer care and publicly reports performance information; and develops innovative strategies for further improvement (see Chapter 7).
6. Accessible, affordable cancer care: A system that is accessible to all patients and uses new payment models to align reimbursement to reward care teams for providing patient-centered, high-quality care and eliminating wasteful interventions (see Chapter 8).
Figure 1-5 illustrates the interconnectivity of the committee’s six components for a high-quality cancer care delivery system. Patients are at the center of the committee’s conceptual framework, recognizing that the system’s most important goal is to meet the care needs of patients with cancer and their families, through patient-centered communication and shared decision making. The workforce encircles the patients, depicting the idea that high-quality cancer care depends on the workforce to provide competent, trusted, interprofessional care aligned with patients’ needs, values, and preferences. The evidence base and a rapid learning IT system support patient-clinician interactions and provide patients and clinicians with the information and decision support necessary to make well-informed medical decisions. The arrows in the figure depict the cy-
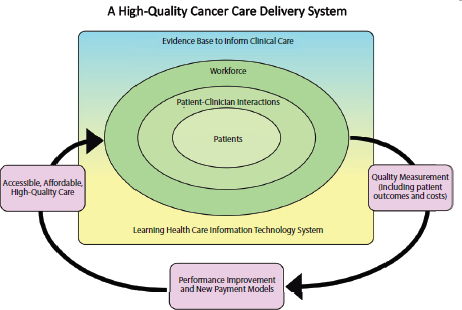
clical process of measuring the outcomes of patient-clinician interactions and implementing innovative strategies and new payment models to improve the accessibility, affordability, and quality of care.
Prioritizing the Components of the Framework
The committee recognizes that improving the quality of cancer care will take substantial time and effort to achieve and implementation will require efforts by all stakeholders in the cancer care community. The committee numbered its six components for high-quality cancer care in order of priority for implementation, taking into account both the need and the feasibility of achieving each component of the framework. Thus, achieving a system that supports patient decision making is the top priority, followed by an adequately staffed, trained, and coordinated workforce, evidence-based cancer care, a learning health care IT system, the translation of evidence into practice, measurement of outcomes, and performance improvement, and, finally, accessible and affordable cancer care. The top priorities for implementation are depicted within the rectangle in Figure 1-5, with the most important component in the center (i.e., patients). The committee recognizes the importance of access and affordability in a high-quality cancer care delivery system but expects the
ACA to make substantial changes in these areas of health care. Because much of the law has not yet been implemented, these issues will need to be revisited once the law’s full impact is known.
Approach to Implementing the Framework
The committee utilizes a variety of approaches in its recommendations to improve the quality of cancer care. In many circumstances, the recommendations provide specific direction to individual stakeholders. It directs recommendations to patients; members of the cancer care team (including both academic and community oncology clinicians, primary care clinicians, and other specialists); and health care delivery organizations that are directly involved in the provision of cancer care. It also targets the federal government, where appropriate, because the government is in a position to develop national strategies and to influence the policies that affect the behavior of those involved in the provision of cancer care. In addition, as the dominant health insurance provider for cancer patients and survivors, the federal government has a responsibility to assure that its payments for services meet quality standards and are not harmful to patients.
In many cases, change may start with individual organizations that undertake localized efforts or pilot projects to implement improvements in the cancer care delivery system. There are already many ongoing activities related to the committee’s recommendations that would fall in this category. In some cases, fully achieving the goals of the committee’s framework may also necessitate collaboration among relevant stakeholders to define the best path to implementation. Although there are numerous challenges to such collaboration, examples of ongoing collaborations among diverse stakeholders in the cancer community already exist, and there may be greater incentives for such coordinated efforts in the current environment. For example, the ACA is focusing national attention and resources on improving the coordination and quality of the U.S. health care system, such as promoting accountable care organizations and other innovative payment models that reward clinicians for working as a team and providing high-quality care. Many stakeholders are already making changes in response to health care reform, and the committee’s framework provides guidance on this process. In addition, the current financial situation in the United States is placing pressure on the health care delivery system to develop actionable solutions for eliminating waste in care while also maintaining or improving quality. Again, the committee’s conceptual framework charts a new course for achieving this task.
The committee deliberated during four in-person meetings and numerous conference calls between May 2012 and April 2013. During its second meeting, the committee met in conjunction with the NCPF’s workshop on Delivering Affordable Cancer Care in the 21st Century. The goals of the workshop included (1) summarizing current evidence on the overuse, underuse, and misuse of medical technology throughout the continuum of cancer care; (2) identifying modifiable problems in the cancer care delivery system and suggesting changes to address them; and (3) discussing policy issues related to the value, cost containment, and reimbursement of cancer care, as well as the economic incentives for innovation and technology diffusion in cancer care. As part of this study, the committee reviewed published literature, including the prior NCPF workshops and IOM consensus studies, and sought input from stakeholders in cancer care. The committee used the IOM’s Ensuring Quality Cancer Care report (1999) as a foundation for examining challenges to and opportunities for the delivery of high-quality cancer care and formulating recommendations for improvement.
The committee structured its report around the six components of its conceptual framework. This introductory chapter has described the background, charge to the committee, conceptual framework, and methods for the report. Chapter 2 provides additional background information on the current landscape and trends in cancer care. Chapters 3 through 8 elaborate on the committee’s six components for a high-quality cancer care system and present the committee’s recommendations for action.
Chapter 2: The Current Cancer Care Landscape: An Imperative for Change, focuses on demographic changes in the United States; trends in cancer diagnoses, cancer survivorship, cancer treatment, and cancer care costs; the unique needs of older adults with cancer; and policy initiatives that may impact cancer care. It also provides a summary of the key stakeholders involved in the cancer care delivery system.
Chapter 3: Patient-Centered Communication and Shared Decision Making, focuses on strategies and tools for improving patient-centered communication and shared decision making, as well as the unique communication and decision-making needs of patients with advanced cancers.
Chapter 4: The Workforce Caring for Patients with Cancer, focuses on ensuring that there is an adequate supply of clinicians to meet the rising demand for cancer care and that the workforce has the training and skills necessary to provide high-quality cancer care.
Chapter 5: The Evidence Base for High-Quality Cancer Care, focuses on improving the evidence base that supports cancer care decisions by improving the breadth and depth of data that are collected in clinical research and improving the use of IT to collect, organize, and assess data from various sources.
Chapter 6: A Learning Health Care Information Technology System for Cancer, focuses on using technological advancements to improve cancer care delivery, patient health, cancer research, quality measurement, performance improvement, and reimbursement for high-quality cancer care.
Chapter 7: Translating Evidence into Practice, Measuring Quality, and Improving Performance, focuses on translating evidence into practice through quality metrics, clinical practice guidelines, and performance improvement initiatives.
Chapter 8: Accessible and Affordable Cancer Care, focuses on access to cancer care and on the role of payers, clinicians, and patients in improving affordability and quality of cancer care.
ACS (American Cancer Society). 2013. Cancer facts and figures 2013. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf (accessed April 19, 2013).
ASCO (American Society of Clinical Oncology). 2013. National Initiative on Cancer Care Quality (NICCQ). http://www.asco.org/institute-quality/national-initiative-cancer-care-quality-niccq (accessed July 30, 2013).
Brown, M. L., G. F. Riley, N. Schussler, and R. Etzioni. 2002. Estimating health care costs related to cancer treatment from SEER-Medicare data. Medical Care 40(8 Suppl): IV-104-117.
de Moor, J. S., A. B. Mariotto, C. Parry, C. M. Alfano, L. Padgett, E. E. Kent, L. Forsythe, S. Scoppa, M. Hachey, and J. H. Rowland. 2013. Cancer survivors in the United States: Prevalence across the survivorship trajectory and implications for care. Cancer Epidemioliogy, Biomarkers, & Prevention 22(4):561-570.
Donabedian, A. 1980. Explorations in Quality Assessment and Monitoring. In The definition of quality and approaches to its assessment. Vol. 1. Ann Arbor, MI: Health Administration Press.
Goldberg, L. 2013. The Medicare trustees report in perspective. http://healthaffairs.org/blog/2013/06/07/the-medicare-trustees-report-in-perspective (accessed June 13, 2013).
Hayano, T., M. Garg, D. Yin, M. Sudo, N. Kawamata, S. Shi, W. Chien, L. W. Ding, G. Leong, S. Mori, D. Xie, P. Tan, and H. P. Koeffler. 2013. Sox7 is down-regulated in lung cancer. Journal of Experimental Clinical Cancer Research 32:17.
He, W., M. Sengupta, V. A. Velkoff, and K. A. DeBarros. 2005. 65+ in the United States: 2005. http://www.census.gov/prod/2006pubs/p23-209.pdf (accessed May 3, 2012).
IOM (Institute of Medicine). 1990. Medicare: A strategy for quality assurance. 2 vols. Washington, DC: National Academy Press.
———. 2001a. Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press.
———. 2001b. Interpreting the volume-outcome relationship in the context of cancer care. Washington, DC: National Academy Press.
———. 2007. Implementing cancer survivorship care planning. Washington, DC: The National Academies Press.
———. 2008a. Cancer care for the whole patient: Meeting psychosocial health needs. Washington, DC: The National Academies Press.
———. 2008b. Knowing what works in health care: A roadmap for the nation. Washington, DC: The National Academies Press.
———. 2008c. Retooling for an aging America: Building the health care workforce. Washington, DC: The National Academies Press.
———. 2009a. Assessing and improving value in cancer care: Workshop summary. Washington, DC: The National Academies Press.
———. 2009b. Ensuring quality cancer care through the oncology workforce: Sustaining care in the 21st century: Workshop summary. Washington, DC: The National Academies Press.
———. 2010a. A foundation for evidence-driven practice: A rapid learning system for cancer care: Workshop summary. Washington, DC: The National Academies Press.
———. 2010b. Policy issues in the development of personalized medicine in oncology: Workshop summary. Washington, DC: The National Academies Press.
———. 2011a. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
———. 2011b. Patient-centered cancer treatment planning: Improving the quality of oncology care: Workshop summary. Washington, DC: The National Academies Press.
———. 2012. Best care at lower cost: The path to continuously learning health care in America. Washington, DC: The National Academies Press.
———. 2013. Delivering affordable cancer care in the 21st century: Workshop summary. Washington, DC: The National Academies Press. IOM and NRC (National Research Council). 1999. Ensuring quality cancer care. Washington, DC: National Academy Press.
———. 2005. From cancer patient to cancer survivor: Lost in transition. Washington, DC: The National Academies Press.
Joung, J. G., D. Kim, K. H. Kim, and J. H. Kim. 2013. Extracting coordinated patterns of DNA methylation and gene expression in ovarian cancer. Journal of the American Medical Informatics Association 20(4):637-642.
Kantarjian, H., and experts in chronic myeloid leukemia. 2013. The price of drugs for chronic myeloid leukemia (CML); A reflection of the unsustainable prices of cancer drugs: From the perspective of a large group of CML experts. Blood epub ahead of print:doi:10.1182/blood-2013-1103-490003.
Kizer, K. W. 2010. What is a world-class medical facility? American Journal of Medical Quality 25(2):154-156.
Liang, H., L. W. Cheung, J. Li, Z. Ju, S. Yu, K. Stemke-Hale, T. Dogruluk, Y. Lu, X. Liu, C. Gu, W. Guo, S. E. Scherer, H. Carter, S. N. Westin, M. D. Dyer, R. G. Verhaak, F. Zhang, R. Karchin, C. G. Liu, K. H. Lu, R. R. Broaddus, K. L. Scott, B. T. Hennessy, and G. B. Mills. 2012. Whole-exome sequencing combined with functional genomics reveals novel candidate driver cancer genes in endometrial cancer. Genome Research 22(11):2120-2129.
Mariotto, A. B., K. R. Yabroff, Y. Shao, E. J. Feuer, and M. L. Brown. 2011. Projections of the cost of cancer care in the United States: 2010-2020. Journal of the National Cancer Society 103(2):117-128.
McCorkle, R., E. Ercolano, M. Lazenby, D. Schulman-Green, L. S. Schilling, K. Lorig, and E. H. Wagner. 2011. Self-management: Enabling and empowering patients living with cancer as a chronic illness. CA: A Cancer Journal for Clinicians 61(1):50-62.
Medicare Trustees (The Boards of Trustees, Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds). 2013. Annual Report. Washington, DC: The Centers for Medicare & Medicaid Services.
NCI (National Cancer Institute). 2007. Cancer trends progress report— 2007 update: Costs of cancer care. http://progressreport.cancer.gov/2007/doc_detail.asp?pid=1&did=2007&chid=75&coid=726&mid= (accessed May 13, 2013).
———. 2012. SEER Stat Fact Sheets: All Sites. http://seer.cancer.gov/statfacts/html/all.html (accessed April 19, 2013).
———. 2013a. The Cancer Genome Atlas. http://cancergenome.nih.gov/ (accessed July 30, 2013).
———. 2013b. Cancer control continuum. http://cancercontrol.cancer.gov/OD/continuum.html (accessed June 13, 2013).
———. 2013c. Estimated U.S. Cancer Prevalence Counts: Method. http://dccps.nci.nih.gov/ocs/prevalence/prevalence.html (accessed April 19, 2013).
NCR BRAC HSAS (National Capital Region Base Realignment and Closure Commission Health Systems Advisory Submcommittee of the Defense Health Board). 2009. Achieving world class: An independent review of the design plans for the Walter Reed National Military Medical Center and the Fort Belvoir Community Hospital. Washington, DC: Department of Defense.
NRC (National Research Council). 2009. Computational technology for effective health care: Immediate steps and strategic direction. Washington, DC: The National Academies Press.
NVSS (National Vital Statistics System). 2012. Deaths: Leading Causes for 2009. http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_07.pdf (accessed April 19, 2013).
Parry, C., E. E. Kent, L. P. Forsythe, C. M. Alfano, and J. H. Rowland. 2013. Can’t see the forest for the care plan: A call to revisit the context of care planning. Journal of Clinical Oncology 31:1-3.
Reinhard, S. C., and C. Levine. 2012. Home alone: Family caregivers providing complex chronic care. http://www.aarp.org/home-family/caregiving/info-10-2012/home-alone-family-caregivers-providing-complex-chronic-care.html (accessed March 29, 2013).
Rose, C., E. Stovall, P. A. Ganz, C. Desch, and M. Hewitt. 2008. Cancer Quality Alliance: Blueprint for a better cancer care system. CA: A Cancer Journal for Clinicians 58(5):266-292.
Scher, K. S., and A. Hurria. 2012. Under-representation of older adults in cancer registration trials: Known problem, little progress. Journal of Clinical Oncology 30(17):2036-2038.
Smith, B. D., G. L. Smith, A. Hurria, G. N. Hortobagyi, and T. A. Buchholz. 2009. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. Journal of Clinical Oncology 27(17):2758-2765.
Spinks, T., H. W. Albright, T. W. Feeley, R. Walters, T. W. Burke, T. Aloia, E. Bruera, A. Buzdar, L. Foxhall, D. Hui, B. Summers, A. Rodriguez, R. Dubois, and K. I. Shine. 2012. Ensuring quality cancer care: A follow-up review of the Institute of Medicine’s 10 recommendations for improving the quality of cancer care in America. Cancer 118(10):2571-2582.
Stump, T. K., N. Eghan, B. L. Egleston, O. Hamilton, M. Pirollo, J. S. Schwartz, K. Armstrong J. R. Beck, N. J. Meropol, and Y. Wong. 2013. Cost concerns of patients with cancer. Journal of Oncology Practice doi:10.1200/JOP.2013.00092.
Sullivan, R., J. Peppercorn, K. Sikora, J. Zalcberg, N. J. Meropol, E. Amir, D. Khayat, P. Boyle, P. Autier, I. F. Tannock, T. Fojo, J. Siderov, S. Williamson, S. Camporesi, J. G. McVie, A. D. Purushotham, P. Naredi, A. Eggermont, M. F. Brennan, M. L. Steinberg, M. De Ridder, S. A. McCloskey, D. Verellen, T. Roberts, G. Storme, R. J. Hicks, P. J. Ell, B. R. Hirsch, D. P. Carbone, K. A. Schulman, P. Catchpole, D. Taylor, J. Geissler, N. G. Brinker, D. Meltzer, D. Kerr, and M. Aapro. 2011. Delivering affordable cancer care in high-income countries. Lancet Oncology 12(10):933-980.
Taylor, E. F., T. Lake, J. Nysenbaum, G. Peterson, and D. Meyers. 2011. Coordinating care in the medical neighborhood: Critical components and available Mechanisms. White paper. Rockville, MD: Agency for Healthcare Research and Quality.
Wang, C., T. Pecot, D. L. Zynger, R. Machiraju, C. L. Shapiro, and K. Huang. 2013. Identifying survival associated morphological features of triple negative breast cancer using multiple datasets. Journal of the American Medical Informatics Association 20(4):680-687.
Yabroff, K. R., J. Lund, D. Kepka, and A. Mariotto. 2011. Economic burden of cancer in the United States: Estimates, projections, and future research. Cancer Epidemiology, Biomarkers & Prevention 20(10):2006-2014.
Zapka, J. G., S. H. Taplin, L. I. Solberg, and M. M. Manos. 2003. A framework for improving the quality of cancer care: The case of breast and cervical cancer screening. Cancer Epidemiology, Biomarkers & Prevention 12(1):4-13.
Zapka, J., S. H. Taplin, P. Ganz, E. Grunfeld, and K. Sterba. 2012. Multilevel factors affecting quality: Examples from the cancer care continuum. Journal of the National Cancer Institute Monographs 44:11-19.
























