SESSION 2
WASTE TREATMENT
Alan D. Pasternak
Consultant
Lafayette, California
Rapporteur's Report
The session on waste treatment included five speakers. The topics covered were the fundamentals of waste treatment economics, high temperature photochemistry, the fundamentals of combustion kinetics, and research and development of techniques—not necessarily utilizing solar energy—for destroying hazardous and mixed radioactive and hazardous wastes. In summary, the session identified two kinds of applications for solar energy in waste treatment: as a source of solar photons for thermal photolytic destruction of organic compounds at high temperatures (but temperatures that are lower than would be required without solar photons) and as a source of thermal energy in thermal destruction processes. Research and development related to solar applications in waste treatment is needed in the mechanisms of photolysis, particularly at high temperatures, and the engineering of practical systems. Applications may be limited to certain waste streams; finding the right niche for solar energy in waste treatment is the key to commercialization of this application.
Peter Daley of Chemical Waste Management presented an overview of the hazardous waste management market. Mr. Daley described the waste business as the "world's biggest zoo." Chemical Waste Management manages over 130,000 different waste streams from over 10,000 customers. Successful waste technologies are low cost, simple, capable of accepting a range of feeds, adaptable, and entail no new problems. The cost of treatment, on a unit volume basis, is a very strong inverse function of the volume of the waste stream being treated. Therefore it is advisable to avoid "narrow processes"; processes should be adaptable to numerous waste streams.
As a background to the discussion of new waste processing techniques, Mr. Daley offered these conclusions:
-
Cost is the only real issue in destroying hazardous wastes.
-
Prices are dropping as technology improves.
-
Low-tech approaches work "acceptably well" for the great majority of applications:
-
Concentrated organics are destined to be fuels.
-
Incinerators will be increasingly cornered into a shrinking "toxics" niche.
-
Complex and dilute aqueous wastes are generally regarded as expensive to manage.
-
-
High-tech approaches must offer significant advantages in cost versatility to justify their development.
-
Inorganics will move increasingly toward recovery and thermal stabilization as those technologies become more cost-competitive with chemical stabilization.
Solar faces two major limitations in its applications to waste treatment: high capital costs and intermittent energy supply.
Barry Dellinger of the University of Dayton spoke on "High Temperature Photochemistry Induced by Concentrated Solar Radiation." Mr. Dellinger described his experimental studies of high temperature, photochemical, solar-induced reactions with potential applications in the destruction of toxic organic wastes. Mr. Dellinger finds that for a number of compounds, under conditions of thermal photolytic destruction, decomposition occurs at significantly lower temperatures than is the case for thermal decomposition alone. Also, under these conditions thermal decomposition by-products (products of incomplete combustion or PICs) are also destroyed at lower temperatures than is the case with purely thermal decomposition.
According to Mr. Dellinger, a potential advantage of using concentrated solar radiation is that rapid heating rates are possible, which suggests that high temperature photochemical processes may be induced. Knowledge of these reactions is in its infancy, and he recommends research to elucidate the fundamental mechanisms of the process and the influence of temperature.
In comparison to controlled thermal incineration, solar destruction offers several advantages, according to Mr. Dellinger. Lower temperatures provide better control of vaporization of toxic metals and formation of nitrogen oxides; increased destruction efficiency of the parent compound and its by-products; use of excess thermal energy for thermal desorption of solids and sludges; reduced production of carbon dioxide, carbon monoxide, and toxic organic emissions from conventional fuels; cost savings from lower fuel costs and lower capital costs due to increased materials lifetime and reduced need for air pollution control devices; and increased public acceptance through reduced pollution and use of nonincineration disposal technology. Apparent disadvantages include unreliability of solar radiation, cost of solar collection and concentration, lack of off-the-shelf technology to construct a working pilot-or full-scale system.
Dellinger suggests a hybrid two-stage system for detoxification of soils and other solids. Thermal desorption of organics from the solids would take place in the primary unit while a solar reactor would be used for thermal photolytic destruction of the desorbed organics.
Wing Tsang of the National Institute of Standards and Technology spoke on "Chemical Processes During Incineration and Implications of Detoxification of Hazardous Waste Using Solar Photons." Destruction of organic chemicals proceeds without thermodynamic hindrance, but kinetic effects can lead to failure in incineration. Like Dellinger, Tsang also called for more data on photochemical reactions particularly at high temperatures.
According to Tsang, photodecomposition may require an "optically thin" system, and this may be an important constraint on large systems. Similarly, large concentrations of hazardous wastes may lead to the formation of solids and increase the optical density of the media.
Tsang anticipates that the applications for solar powered destruction of hazardous wastes will include very dilute mixtures of photodecomposable materials in a variety of matrices.
John F. Cooper of the University of California's Lawrence Livermore National Laboratory (LLNL) presented a paper on "Molten Salt Processing of Mixed Wastes and Potential Solar-Thermal Applications., In molten salt processing, organic wastes are oxidized at temperatures of 700–900ºC in a bed of molten carbonate/halide salts. The alkaline properties of the salt prevent formation of the acid gases hydrogen chloride or sulfur dioxide which are tied up as chlorides and sulfates. Heavy metals are also chemically incorporated in the salt. For example, arsenic is converted to sodium arsenate.
According to Dr. Cooper, major benefits of molten salt processing when compared to incineration of radioactive wastes are that it has the potential to eliminate or greatly reduce the amount of radioactive material in fugitive form (free molecules or gas-levitated particulates) and the elimination of stack emissions of gases in favor of total retention. Lower temperatures (700ºC), instead of the 1200ºC typical of incineration, will inhibit formation and loss of volatile radionuclide molecules and aerosols.
The process under R&D at Livermore is similar to a process developed by Rockwell. Rockwell has demonstrated destruction of PCBs at temperatures as
low as 700ºC. The LLNL approach employs a two-stage process: pyrolysis and oxidation. This avoids polymerization of alumina and silica species which can otherwise occur at high oxygen pressures.
The molten salt process relies on the heat of combustion and electric resistance heating to maintain melt temperatures. A possible application of solar energy to this process would be to provide inexpensive heat that would allow molten salt treatment of trace organic contaminants in dilute aqueous or gas waste streams.
Another process for destruction of hazardous wastes was described by Terry Galloway of Synthetica Technologies, Inc. in his presentation, "Waste Destruction by Very High Temperature Steam Reforming." The Synthetica process relies almost completely on external sources of thermal energy (electric resistance heating) for the destruction of organics by steam reforming, an endothermic process. The primary destruction mechanism is neither incineration or oxidation. (Oxygen is introduced only in a final catalytic clean up step to convert carbon monoxide to carbon dioxide.) The system is designed for treatment of small quantities of organic wastes on the waste generator's site. According to Dr. Galloway, it is possible to achieve greater than 99.99% removal efficiency at temperatures of 2000 to 3000ºF.
Reliance on external sources of thermal energy may provide an application for heat from solar collectors. The trick will be to design a system which allows this heat to be delivered to a high temperature reaction chamber where steam reforming takes place. During discussion on this point, engineers from Solar Energy Research Institute expressed their judgement that this can be done.
HIGH-TEMPERATURE PHOTOCHEMISTRY INDUCED BY CONCENTRATED SOLAR RADIATION
Barry Dellinger, John Graham, Joel H. Berman, and Don Klosterman
Environmental Sciences Group
University of Dayton Research Institute
Background
Concentrated solar radiation is a unique source of energy in that it contains radiation of a broad enough spectrum to produce very high temperatures while simultaneously promoting molecules to electronically excited states. The intense infrared (IR) radiation in this spectrum can be converted to thermal energy, which has traditionally been used for electrical power generation. However, the even greater quantities of available ultraviolet and visible radiation (UV-VIS) have proven more difficult to use efficiently due to low conversion efficiencies to thermal or electrical energy. This shorter wavelength radiation has been primarily used for photovoltaic generation.
There has been a relatively recent flurry of activity to utilize the unique properties of solar radiation to produce useful chemical reactions. The rapid heating rates possible in a concentrated radiation field suggest that high-temperature, photochemical processes may be induced. Our knowledge of these reactions is only in its infancy, and there are many fundamental physical, chemical, and engineering aspects that deserve close attention and serious research. Destruction of toxic organic wastes is one emerging, potentially important, and viable application of concentrated solar radiation. A discussion of the fundamental aspects of this process can also provide a framework for investigation of other high-temperature, photochemical, solar-induced reactions.
Figure 1 displays some typical data obtained for the environmentally significant compound 3,3',4,4'-tetrachlorobiphenyl (TCB). This figure compares the purely thermal destruction of the TCB with the thermal-photolytic destruction. These data were obtained on a specially designed flow reactor system designated as the Thermal Photolytic Reactor System (TPRS). With this system the exposure temperature of the sample can be varied independently of the incident, simulated solar radiation which is provided using a filtered xenon arc lamp. There is clear evidence of the photochemically enhanced destruction of both the parent TCB and the toxic reaction by-products, e.g., tetrachlorodibenzofuran (TCDF). Similar solar-induced, photolytic destruction enhancement (vs. purely thermal destruction) has been demonstrated for over a dozen compounds.
Fundamental Research Aspects
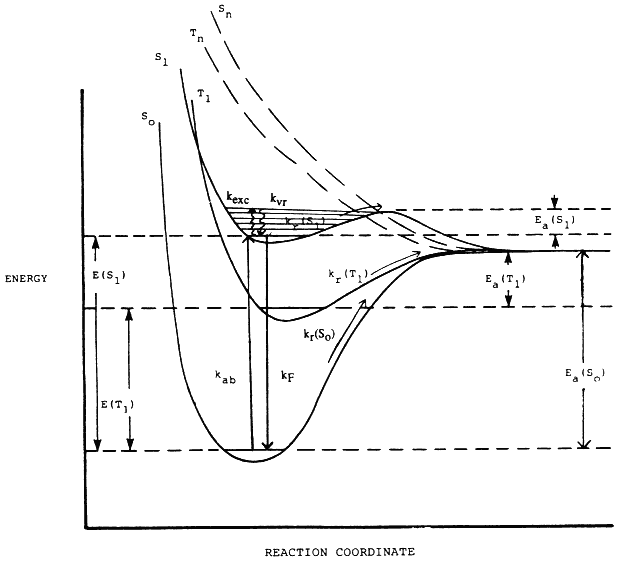
The elementary processes involved in the primary photochemical and thermal processes are displayed in the energy-reaction coordinate diagram in Figure 2. The rate of the ground state thermal, unimolecular reaction is related to the passage over a relatively high energy barrier of activation, Ea(So). The rate of the primary photochemical process from S1 is related to the rate of energy absorption, kab, and the rate of passage over the reduced energy barrier, Ea(S1). Of course, this simple picture is complicated by the presence of other possibly reactive states, T1, and even dissociative states, Sn and Tn, as well as secondary reactions.
Several issues of fundamental interest that can be readily identified are enumerated below:
-
Are excited vibronic states thermally equilibrated?
-
Will the absorption spectra shift to lower energy and the oscillator strength increase with increasing temperature?
-
Do excited state deactivation rates vary with temperature?
-
Does reactivity correlate with τ(S1) or τ(T1)?
-
Does reactivity correlate with ΔHrxn,-E (S1) or ΔHrxn-E (T1)?
-
Does reactivity correlate with localization of excitation energy?
-
Is reactivity wavelength dependent, and what is the role of forbidden, repulsive states in photoreactivity?
-
Will molecules undergo excited state oxidations?
-
What is the effectiveness of radical photoinitiators and energy or electron transfer sensitizers?
Some of these questions have been previously proposed by photochemists and spectroscopists; however, the temperature dependencies of the processes in question remains essentially unresearched. We have begun to address these issues using monochlorobenzene (MCBz) as a model chlorinated aromatic compound of environmental significance.
We are currently performing pressure and collisional energy transfer efficiency experiments on the TPRS. A simple three state thermal photolytic model has been developed to qualitatively explain the pressure and temperature dependence of the measured thermal-photolytic reaction rate (see Figure 3). Steady state analysis of this kinetic scheme for the case where the excited state reaction efficiency is high (i.e., k1kp/kFk-1 >> 1) indicates that the observed rate constant for unimolecular thermal-photolytic dissociation is given by
At low pressures ([M] ⇒ 0) the expression approaches the pressure dependent rate constant, kdiss = (ka/kf)k1[M]. At high pressures, the rate constant is independent of pressure and is limited by the rate of energy absorption, i.e., kdiss = ka. If kF increases faster with temperature than k1, then kdiss becomes more pressure dependent with increasing temperature. The effects of the key parameters, k ab, kF, and [M], are all subject to experimental determination. Initial results for monochlorobenzene indicate that the dissociation reaction is not thermally equilibrated and is thus pressure-and collision efficiency-limited. This observation suggests that the destruction efficiency of a solar destruction unit can be significantly increased under the proper operating conditions and choice of both gas or operating pressure. Temperature dependent lifetime experiments as well as QRRK and RRKM calculations are being conducted to further elucidate the key aspects of high temperature photochemical processes.
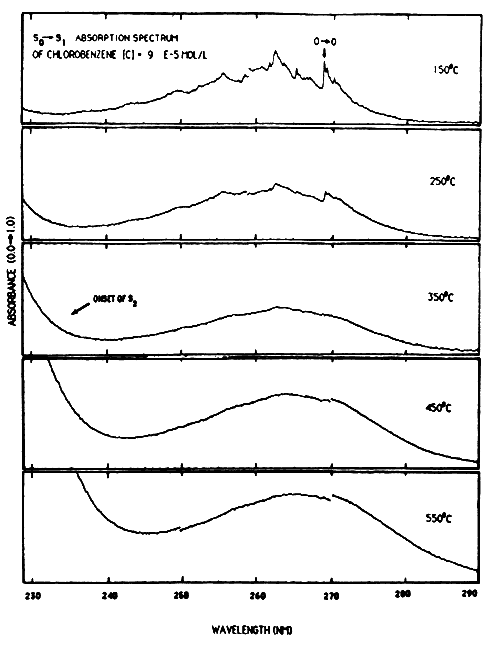
Temperature dependent absorption spectra for monochlorobenzene are depicted in Figure 4. These spectra clearly demonstrate a red-shift and increase in oscillator strength with increasing temperature. The red-shift is largely attributable to thermal population of totally symmetric ground state vibrational levels, reducing the energy required for excitation to S1. The increase in total oscillator strength may be attributable to thermal population of non-totally symmetric vibrations that can vibronically couple the ground state to other electronic states lending intensity to particular So ⇒ S1 vibronic optical transitions. The observed high temperature red-shift is sufficient to make the S1 accessible with solar radiation. This results in more molecules being amenable to solar destruction through direct excitation of S1 than might be expected based on room temperature absorption spectra.
These ongoing experiments are addressing issues 1–3. We also plan to study issues 4–6 by studying homologous series of polychlorinated benzenes and other halogenated benzenes. The wavelength dependence (issue 7) is directly addressable with the aid of scannable laser systems.
Figure 5 presents thermal decomposition data for monochlorobenzene in helium obtained on the Advanced Thermal Photolytic Reactor System (ATPRS) using a Nd:YAG pumped dye laser as the excitation source. Excitation at 280 nm with an average radiation intensity of 3.00 W/cm2 for 10.0 s residence time, resulted in 99% destruction of MCBz prior to any thermal destruction. As in the case of TCB, thermal decomposition by-products are destroyed at lower temperatures than for purely thermal destruction. Wavelength dependence studies are currently in progress.
Issues 8 and 9 are potentially complex to unravel since they include multi-step, non-primary, non-unimolecular reactions. We have begun to address these issues through development of detailed, elementary reaction kinetic models for data obtained on MCBz and dinitrotoluene (DNT). The well-known Chemkin kinetic code and Senkin kinetic sensitivity analysis code are being used in these analyses. The theoretical models being constructed include the full set of thermal initiation and secondary reactions necessary to model the parent decomposition and product formation. The roles of photochemically induced radical molecule and radical chain reactions are being addressed using this detailed modeling approach.
The impact of secondary radical molecules and chain reactions may be particularly evident in complex mixtures. This is particularly important from a practical viewpoint where molecules that do not directly absorb solar radiation may be efficiently destroyed by secondary radical molecule reactions. Thus, it may only be necessary to have one photoactive compound in a waste mixture which may act as a radical photoinitiator for the destruction of other nonabsorbing compounds in the waste. An example of one such mixture that we have investigated is shown in Figure 6. The principal absorbing species in this mixture is nitrobenzene; however, effective total destruction of all components of the mixture as well as products is observed.
Applied Research Aspects
Available results for the destruction of hazardous wastes using concentrated solar radiation clearly suggest that many compounds are amenable to destruction through direct absorption and unimolecular decomposition, while other weak or nonabsorbing species may also be amenable to destruction through secondary photoinduced, radical molecule reaction pathways. However, for a technology to compete successfully in the waste disposal market, it must demonstrate a technological, cost, or possible social advantage over the available techniques. The principal competition at this point is controlled thermal incineration.
There appear to be several advantages of solar destruction over thermal destruction which include
-
increased destruction efficiency of the parent and by-products;
-
control of vaporization of toxic metals through lower operating temperatures;
-
control of Nox formation through lower operation temperatures;
-
availability of excess thermal energy that can be used for thermal desorption of solids and sludges;
-
control of CO2, CO, and toxic organic emissions through substitution of solar energy for conventional fuels;
-
cost savings due to lower fuel costs, increased materials lifetime, and reduced size and complexity of air pollution control devices; and
-
increased public acceptance through use of a renewable, nonpolluting energy source for a nonincineration waste disposal technology.
Apparent major disadvantages include
-
the unreliable availability of solar radiation;
-
cost of collection and concentration of solar radiation; and
-
lack of off-the-shelf technology to construct a working pilot-or full-scale system.
Our task is to develop an approach that utilizes the advantages to offset or minimize these disadvantages.
One approach is to develop a hybrid two-stage system targeted for detoxification of contaminated soil and other solids. With this concept, a hybrid primary unit (possible an indirectly fired rotary drum design) may be used to thermally desorb toxic organics from solids, while a secondary solar reactor would be used to thermal-photolytically destroy the desorbed organics. The excess thermal energy generated in the photoreactor would be used to heat the solid waste in the primary unit. This solar generated thermal energy can be supplemented with auxiliary indirect heating from a gas-fired burner. The auxiliary heat source is necessary to operate the process continuously during intermittent cloud cover and maintain nighttime operation. The desorbed organic matter during dark operation may be stored by cryogenic trapping or sorption on carbon for destruction during light periods. Since the total volume of material desorbed is small, the photolytic reactor should readily handle the stored off-gases during light operation.
This approach maintains the previously listed advantages for solar-based waste destruction while minimizing two of the three disadvantages. The hybrid primary unit allows continuous operation, thus eliminating the concern over the unreliability of sunlight. It also uses available technology for construction of the hybrid rotary drum, as the indirect fired kiln and off-gas storage approach has already been developed by Chemical Waste Management, Inc.
Further research on development of applied system is, of course, necessary. Specifically, experiments combined with prudent calculations should be performed to prove the listed advantages for a solar-based technology. It is felt that a bench scale, rotary drum-photoreactor system should be constructed and tested using a small solar concentrator to further research the practical aspects of hazardous waste treatment using concentrated solar radiation.
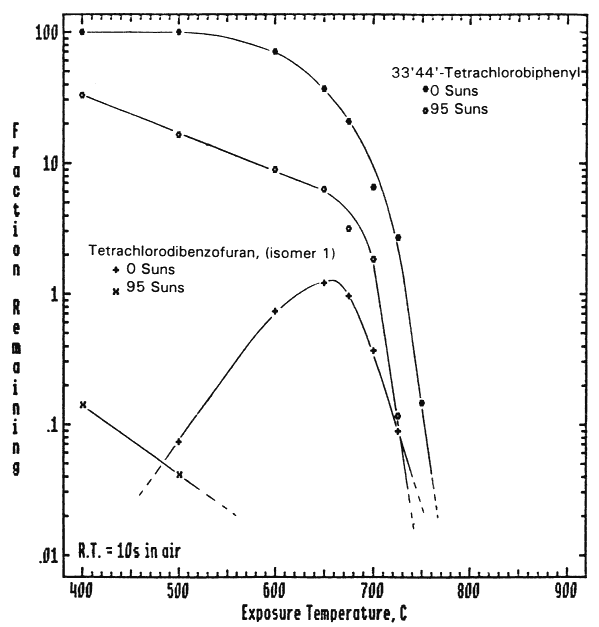
Figure 1 Thermal versus photolytic destruction of 3,3,4,4'-tetrachlorobiphenyl. Data were obtained on the Thermal Photolytic Reactor System (see text) in an atmosphere of flowing air at a gas phase residence time of 10.0 s and simulated solar flux of 95 suns(~9.5W/cm2). Also shown is the photochemically enhanced destruction of the toxic reaction by-product, tetrachlorodibenzofurzan.
Reprinted with Permission from: Environmental Sciences Group/University of Dayton Research Institute
PHYSICAL MODEL
Figure 2 Energy-reaction coordinate diagram for the thermal and thermal-photolytic destruction of an organic compound. The ground electronic state (purely thermal) reaction rate constant is given by kr(So) with an activation energy Ea(So). The excited state (thermal photolytic) reaction rate is related to the rate constant of energy absorption, kab; the rates of reaction from excited singlet and triplet states, e.g. kr(Sl) and kr(T1); the rates of competing deactivation process, e.g. kF; and the rate of achieving excited state thermal equilibrium, e.g., kexc and kvr. Some processes are omitted for clarity.
Reprinted with Permission from: Environmental Sciences Group/University of Dayton Research Institute
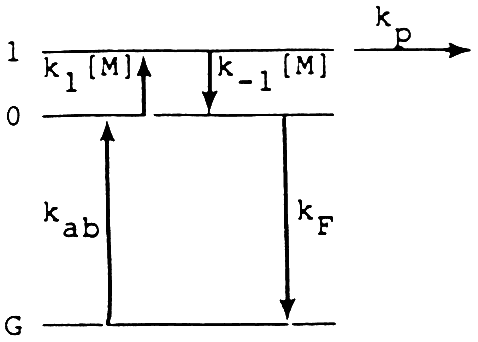
Figure 3 Three state model for thermal-photolytic dissociation in which kab is the rate of absorption; kF is the rate of excited state deactivation; and kl[M] and k-1[M] are the pressure dependent rates of thermal activation and deactivation, respectively. State 1 is the reactive state corresponding to the top of the energy barrier depicted in Figure 2, with the rate of passage over the barrier of kp.
Reprinted with Permission from: Environmental Sciences Group/University of Dayton Research Institute
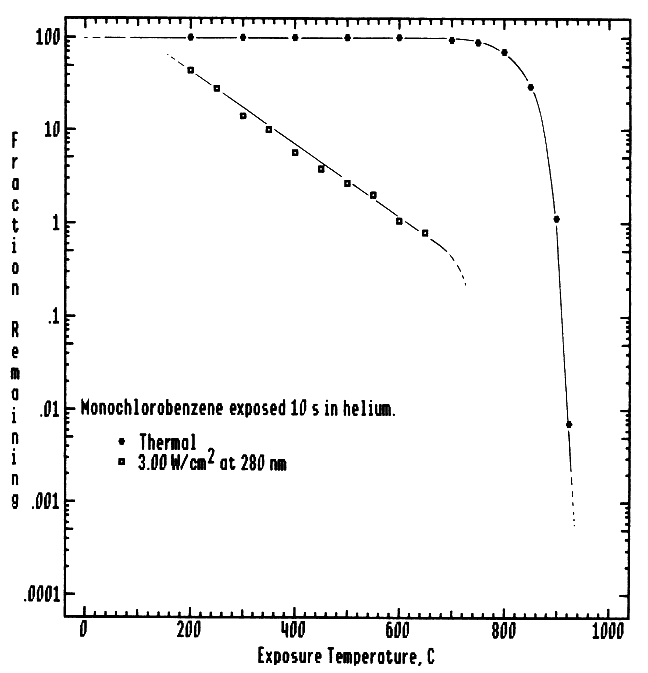
Figure 5 Thermal versus thermal photolytic destruction of monochlorobenzene. Data were obtained on the Advanced Photolytic Reactor System in an atmosphere of flowing helium, gas phase residence time of 10.0 s, and laser flux of 3.00 W/cm2 at 280 nm.
Reprinted with Permission from: Environmental Sciences Group/University of Dayton Research Institute
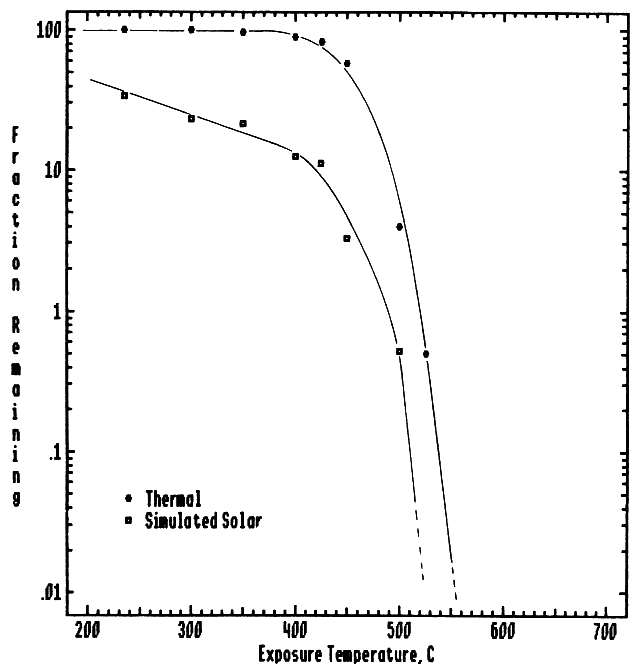
Figure 6 Thermal photolytic destruction of an eight component mixture. The plots are for the total of all undestroyed parent and all observed products. Data were obtained on the TPRS at a gas phase residence time of 10.0 s in air and a simulated solar flux of ~19.0 W/CM2. The mixture contains methylene chloride, trichloroethylene, pyridine, aniline, and nitrobenzene at individual, nominal, gas phase concentrations of 300 ppm and naphthalene, nitronaphthalene, and xanthane at 30 ppm.
Reprinted with Permission from: Environmental Sciences Group/University of Dayton Research Institute
CHEMICAL PROCESSES DURING INCINERATION AND IMPLICATIONS OF DETOXIFICATION OF HAZARDOUS WASTES USING SOLAR PROTONS
Wing Tsang
Chemical Kinetics Division
National Institute of Standards and Technology
Gaithersburg, Maryland
The use of incineration for hazardous waste destruction has been hindered by environmental concerns regarding the effluents from such systems[1]. Important issues are the capability of incinerators to effect the high levels of destruction that are desired and the possibility that other hazardous chemicals may be formed during the process itself. This discussion is a review of the chemistry of incineration. The aim of the work is to demonstrate that it is possible to explain in semiquantitative terms the nature of failure mechanisms. Comparison with the situation when solar photons are used can lead to conclusions regarding the extra dimensions that such an approach offers.
There are no thermodynamic barriers to the transformation of any organic molecules during incineration to their thermodynamic end states[2]. In other words, if one can confine an organic molecule in a hypothetical incineration environment for sufficient time the destruction is total. Failure must, therefore, be due to kinetic effects. These can be divided into physical or chemical. We will be interested in the latter. A consequence of this is the need to examine the reaction pathways.
The detailed chemistry of the breakdown of organic chemicals during incineration is complex[3]. Nevertheless, by building upon what is known about hydrocarbon combustion processes and concentrating on the principal issues defined above, the problem can be rendered more tractable[4]. Incineration is essentially combustion with nonconventional fuels. In many, if not most, cases, destruction is carried out in the presence of standard fuels. It is not surprising that the concepts from the combustion kinetics of standard fuels should be a good starting point[5]. Hazardous waste compounds have different reactivity and reaction intermediates. When this is superimposed onto the basic fuel chemistry, one can derive information on mechanisms for hazardous waste destruction. This discussion will be confined to chlorinated organics. They are important components in hazardous waste mixtures. The general procedure is, however, applicable to all organics.
In a system containing carbon, hydrogen, and oxygen there are three principal pathways that affect the destruction of organics during combustion[6]. These involve radical attack by OH and H and unimolecular decomposition. There are many other radicals present. Many of the larger organics are not particularly reactive, while other radicals decompose. Small radicals such as O, methyl, and HO2 radicals can make contributions under specialized conditions. The OH radicals are particularly important in fuel-lean situations. They are less important as the fuel content becomes richer. The H atoms are important in fuel-lean and fuel-rich mixtures[7]. The importance of unimolecular mechanisms is solely dependent on the reaction temperature.
When chlorinated compounds are added into the fuel mixture at sufficiently low concentrations, the concentration of radicals will not be affected[8]. Destruction mechanisms will only be affected by the reactivities of the chlorinated compounds. The existing data show that chlorine substitution at combustion conditions does not have drastic effects on reactivity, except that thermochemistry prevents OH from abstracting Cl[9]. As the waste concentrations are increased, larger amounts of Cl will be formed. A new channel is now opened for waste destruction. Chlorine atoms
can arise directly from the decomposition of the waste. Another and more important source is the reaction of OH and H radicals with hydrogen chloride. This has the additional consequence of removing these radicals from the reacting system. Chlorine atoms are quite reactive as long as there are no thermodynamic constraints. This is particularly serious for chlorine attack on chlorinated compounds, since direct abstraction of chlorine is highly endothermic. Since H and OH radical concentrations are reduced, an extra degree of stability is offered to highly chlorinated compounds. The decrease in H atoms means that the chain branching reaction H + O2 ⇒ O + OH will be retarded. This is the basis for flame inhibition. However, if one tries to remedy the situation through addition of more oxygen, increasing amounts of chlorine will be released into the system. If organic destruction is total, this will have no consequences. However, in the presence of any organics, chlorination[10], being a very facile process, can occur at temperatures far below that in the combustor.
The residence times and temperatures quoted for hazardous waste incinerators are average values. Instead, one has a broad distribution of numbers. The physical picture is that of packets of gas of varying stoichiometries being heated and quenched. For packets with stoichiometric amounts of oxygen or above, the chain nature of the decomposition process means that reaction is either complete or does not proceed. Kinetic effects will therefore not be apparent. However, with insufficient oxygen, product distributions of effluents will be a reflection of the relative rates of reactions under pyrolytic situations. This means H-atoms and unimolecular decomposition are important. In the presence of large quantities of chlorine, the reactivity of Cl atoms must also be factored in. The sharp differences in reactivity may not be reflected in the effluents since some substances may not have reacted at all.
The use of solar photons can lead to direct photodecomposition or thermal decomposition through heating. For the former, photons are simply another reactant. The most important requirement is for the system to be optically thin. This may be an important constraint in large-scale systems. An important parameter is the quantum yield for photodissociation. For many of the possible hazardous wastes such data do not exist. Particularly interesting will be those at higher temperatures where one expects that the photo processes may be more efficient. Thermal decomposition with solar energy is equivalent to pyrolysis with photons as the extra reactant. As noted earlier, pyrolysis is not a truly efficient destruction mechanism. In any large concentrations of hazardous wastes, the formation of solids will increase the optical density of the media. However, the presence of the particulates may provide a more efficient means of heating the reaction mixture or providing reactive sites for destruction. Solar-assisted combustion will probably not offer unique advantages since, as noted above, the oxidation mechanism is already highly efficient and the true need is to obtain mixtures with the desired stoichiometry.
Proper assessment of the potentialities of solar powered destruction of hazardous waste is hindered by the lack of fundamental information. It probably cannot be as versatile a tool as incineration. However, there are many specific applications where it can play a major role. These will involve very dilute mixtures of photodecomposable materials in a variety of matrices. The fact that solar power can heat as well as photolyze represents an extra dimension that should be exploited. Particularly interesting candidates are nitro compounds (military wastes) or other compounds with chromophores. It may also be useful in hybrid systems where it can be used for pre-or posttreatment. In all cases, however, there is the need for additional photochemical data, particularly at the higher temperatures.
References
1. Oppelt, E.T. 1986. Hazardous Waste Destruction. Environmental Science and Technology 20:312–318.
2. Tsang, W., and W. Shaub. 1982. Chemical Processes in the Incineration of Hazardous Materials. in Detoxification of Hazardous Wastes, J. Exner ed. Ann Arbor Press, Ann Arbor, Michigan, p. 41.
3. Hucknall, D.J. 1985. Chemistry of Hydrocarbon Combustion. Chapman and Hall, London.
4. Tsang, W., and R.F. Hampson. 1986. Chemical Kinetics Data Base for Combustion Modeling: I: Methane and Related Compounds. J. Phys Chem. and Chem. Ref. Data 1087: 15.
5. Tsang, W. 1986. Fundamental Aspects of Key Issues in Hazardous Waste Incineration. ASME Publication 86-WA/HT-27.
6. Tsang, W. 1990. Mechanisms for the Formation and Destruction of Chlorinated Organic Products of Incomplete Combustion. Combustion Science and Technology (in press).
7. Cui, J.P., Y.Z. He, and W. Tsang. 1989. Rate Constants for Hydrogen Atom Attack on Some Chlorinated Benzenes at High Temperatures. J. Phys. Chem. 93:724.
8. Tsang, W., and D. Burgess. The Incinerability of Perchloroethylene and Chlorobenzene. Combustion Science and Technology (submitted).
9. Atkinson, R.A. 1990. Kinetics and Mechanisms of the Gas Phase Reactions of the Hydroxyl Radical with Organic Compounds. J. Phys. Chem. Ref. Data Monograph No. 1.
10. Poutsma, M. 1969. Free Radical Chlorination of organic Molecules. In Methods in Free Radical Chemistry, Vol. 1, E.S. Huyser, ed. Marcel Dekker, New York, p. 79.
SOLAR TECHNOLOGY APPLICATIONS IN CHEMICAL WASTE MANAGEMENT
Peter S. Daley
Chemical Waste Management, Inc.
Geneva, Illinois
Introduction
Using solar energy to destroy waste chemicals and toxic materials has great appeal to environmentalists, industrialists and the public. Using free sunlight to resolve one of the industrial age's most troublesome problems is almost magically attractive. For the magic to become reality, solar destruction must demonstrate that it competes favorably with current approaches in many ways:
|
Economic |
Environmental |
|
Capital cost |
Residuals' production |
|
Operating cost |
Air emissions |
|
Labor |
Public acceptance |
|
Chemicals |
Permitting difficulty |
|
Energy |
|
|
Versatility |
|
|
Reliability |
|
Among economic factors, versatility and reliability are truly components of both capital and operating Costs; they are separated here because of their great importance in chemical waste management applications. Regarding versatility, it is essential that waste management technologies tolerate extremely wide changes in waste-feed composition. Small changes in industrial processes may easily result in enormous (orders of magnitude) changes in the quantity and quality of waste produced. It is not unusual for operating changes to cause whole new classes of waste to be generated.
Demand for versatility in waste treatment is the key reason for success of rotary kiln incinerators for chemical waste destruction; they can accept virtually any solid, liquid, or gaseous feed and can destroy an extremely broad range of chemicals.
The versatility need is dramatized by the fact that our company alone manages over 100,000 substantially different waste streams from over 10,000 different customers. It is economically impossible to sort these streams into more than a few categories.
Reliability is crucial to waste technology success for reasons beyond simple economics. From an operating standpoint, waste generators demand reliability because they may be shut down if they can't dispose of their wastes. Because one disposal facility may service several plants, the impact of a shut-down can be especially serious. Perhaps more important, performance standards demanded of waste treatment systems by the public are uncompromising. The products produced by chemical waste treatment systems (clean air, water, and solids) must generally achieve zero defects' over long operating periods. Failure to do so will, at best, give the operator a public black eye and, at worst, may yield serious health or environmental risks or loss of operating permits.
Table I lists several technologies that have failed to live up to expectations and offers some reasons why. Factors related to both versatility and reliability are frequent.
The Vanishing-Niche Problem
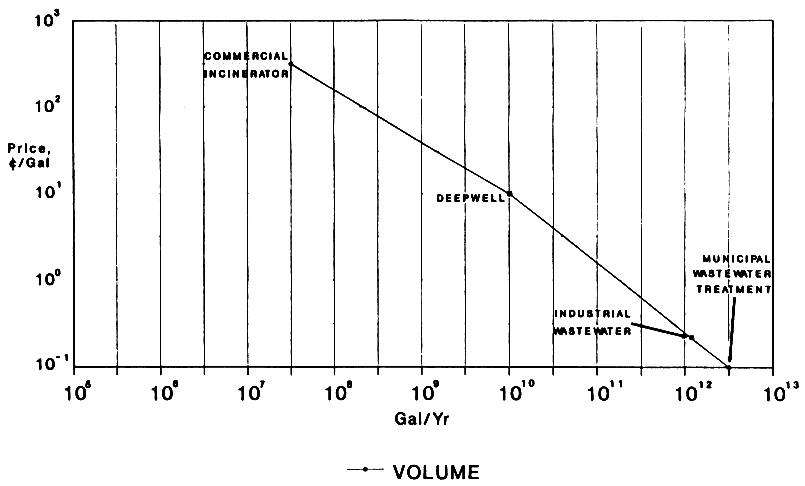
Figure 1 shows that there is strong and expected correlation between the price of a specific wastewater disposal service and the volume of wastewater treated by a specific technology. Similar figures can be constructed for other waste disposal markets. Technologies with costs below the line should be commercial successes, but they will not achieve volumes greater than those garnered by existing technologies unless they represent major technical breakthroughs.
The most important conclusion from the graph is that market size for high-cost technologies is small, on the order of 100 million gallons per year, and highly fragmented. The current price in this market is about $1.00 per gallon. Recalling that our company alone manages over 100,000 different waste streams, one can see that this market includes widely varying materials and each stream or group of streams only comprises a relatively small volume.
If one were to develop a technology that could address 10% of the market, several thousand streams, the market size would be $10 million. In our company it requires about $1.25 in capital to generate $1.00 in sales; therefore, one can afford to spend only about $12.5 million to develop and commercialize a technology to serve a 10 million gallon, many-thousand-waste-stream niche. For technologies capable of addressing fewer waste streams, the limit on development and commercialization cost is proportionally more restrictive, if one hopes to achieve a reasonable return on investment.
These observations hold true for all waste market segments. This generalization is born out by John Skinner's (U.S. EPA Deputy Assistant Administrator for Remedial Technology Development) observation that one of the biggest problems in the hazardous waste site clean up program is that the Agency cannot identify test sites compatible with the specific attributes of many innovative technologies; i.e., the size of the niche has diminished to zero, even when the entire inventory of waste sites is examined.
This problem is compounded by the high cost of waste technology development. Development-related permitting and regulatory compliance can easily cost hundreds of thousands of dollars, and these costs for the commercialization phase are likely to reach millions. In addition, regulatory issues should be expected to double or triple development time, adding more costs as people and equipment go underutilized and interest accumulates.
High Associated Costs:
Chemical waste treatment operations are laden with high Costs not directly related to the treatment technology used. Failure to recognize this can lead to many misdirected development efforts. The simple conclusion from the ''vanishing niche'' discussion above is that even if the niche is only $10 million, a dollar a gallon is a lot; a small business should be able to do well. Unfortunately the $1.00 is price not cost. The price must obviously cover the basic treatment cost, regulatory compliance, feed and product analysis, feed preparation, residuals' disposal, profit, and a host of other factors.
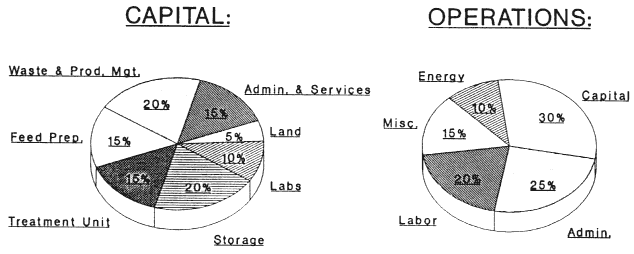
In our experience, the central disposal unit process in a chemical waste disposal facility accounts for only about 15% of capital and a third of operating costs (Figure 2 shows some generalized cost distributions as well as actual cost distributions for X*TRAX, a CWM thermal separation technology to clean soils). Thus, the amount one may spend to develop a new technology is substantially less than the $12.5 million discussed earlier; a few million is a much more realistic estimate.
Application to Solar Treatment Technologies
Solar technologies necessarily target markets that are energy intensive and compatible with the intermittent nature of sunlight. The chemical waste
market, with very few exceptions, is adequately served by the wide range of technologies now available. Prices, exclusive of taxes, are generally stable or slowly dropping as engineers adjust to the rapidly changing treatment rules. Each new regulation tends to engender near-term price increases, but prices drift downward as waste reduction efforts and evolutionary process improvements take hold.
Certain versatile and low-cost technologies are now underutilized because of social and regulatory barriers. The two most important are chemical incineration in cement and aggegate kilns, and use of wastes in building materials. Cement and aggregate kiln destruction and/or stabilization of organic and inorganic wastes is growing rapidly with dozens of kilns now permitted versus only a few five years ago.
For solar applications to be commercially successful, they must offer waste generators and treaters sufficient incentive to switch disposal technologies. As Figure 2 shows, energy is not a large part of the typical cost of chemical waste disposal. Since 'typical' encompasses a wide range of actual technologies, there are some niches in which energy is critical. For example, the treatment of groundwater at remote sites generally involves high pumping costs. Also, the destruction of dilute contaminants in high-volume exhaust air systems is energy intensive, especially if afterburners are used.
The intermittent nature of sunlight presents a major problem for solar applications given the importance of reliability in chemical waste treatment. To be compatible with this importance, solar-based systems must shut down during low-sun periods or be equipped with alternative energy sources. Since most chemical waste treatment technologies are capital intensive, additional cost for solar equipment is not attractive.
The present incineration business structure is instructive in gauging the importance of energy in solar-based chemical waste treatment. Few U.S. chemical incinerators operate with energy recovery in spite of the fact that they may release a large amount of energy. The reasons are several. First, to get a significant value for recovered energy, the supply must be reliable. This is inconsistent with chemical waste incinerators which typically operate about 85% of the time. Incinerators are very complex and are typically subject to about 50 automatic shutdown conditions, and high performance standards demand frequent maintenance. Complexity is the second reason operators reject energy recovery; adding more complex systems threatens to reduce operating rates and adversely affect profitability.
Given the current situation as described above, it will be difficult to find the right combination of energy needs, reliability requirements, and versatility to justify investment in solar-based technologies for the chemical waste business. The best opportunity may well be the groundwater treatment market cited earlier. This market is energy intensive and compatible with intermittent energy supply. In fact, there is some move toward intermittent pumping of aquifers for cleanup as the optimum approach.
Conclusion
Solar-based technologies offer limited opportunities for chemical waste applications because they add additional capital costs and system complexity without providing proportional energy savings. This is largely because energy is not a major factor in most chemical waste disposal activity. Overhead associated with regulatory compliance, chemical analysis, feed preparation, residuals' disposal, environmental permit, etc., typically dominates the cost equation. In addition, the great importance given to treatment reliability in the eyes of operators, regulators, and the public means that solar energy sources would require conventional energy backup in most applications. This would add significant costs to an already capital-intensive business and may compromise reliability by adding more complexity to the system.
Further reducing the likelihood of success for solar-based applications is that the niches open to the approach are likely to be small and not big enough to justify the development costs.
Table 1 Lagging Chemical Waste Treatment Technologies
|
Technology |
Prime Reason Not Implemented |
|
Plasma destruction |
High fundamental cost and complexity |
|
In situ vitrification |
Cost, by-products, uncertain destruction performance |
|
Electric furnaces |
Energy cost, capital cost |
|
Moving hearth furnaces |
Narrow applicability. |
|
Supercritical separators |
Narrow applicability, cost |
|
Freeze purification |
Narrow applicability |
|
In situ soil cleaning |
Narrow applicability, uncertain performance |
|
Alkali-metal polyethylene glycol dechlorination |
Narrow, cost, residuals management |
|
Sodium metal dechlorination |
Safety and cost |
|
White-rat fungus for halocarbon destruction |
Narrow applicability |
|
Biotreatment, general |
Narrow, failure to achieve low residual standards |