6
Epidemiologic Studies: Background on Multiply Referenced Populations
This chapter presents study-design information on populations of Vietnam veterans, occupational cohorts, and environmentally exposed groups that have been reported on repeatedly, often for many health outcomes, and on case-control studies that have generated multiple publications relevant to the Veterans and Agent Orange (VAO) series. One-time reports on given study populations that addressed only single health outcomes are not discussed in this chapter.
In drawing its conclusions, the committee synthesized the evidence from studies that have gathered data and published results over an extended period of time, taking into account the interdependence among related studies. In particular, if new results are based on updating or adding subjects to previously studied populations or concern a subset of original study populations, this synthesis considers redundancy among studies while recognizing that separately reported information can impart new relevance to other data on a study population. The design information provided in this chapter links repeated studies and clarifies their interdependence.
This chapter also provides design information on studies involving multiple health outcomes to avoid repetition in the health-outcome chapters (Chapters 7–13). Some of the populations have been studied previously and reviewed in previous VAO publications (thus, these populations are multiply referenced both over time and among health outcomes), and others have not been addressed in other VAO publications. The procedures used to identify relevant literature on health effects in human populations in conjunction with exposure to the chemicals of interest (COIs) are provided in Chapter 2. Details of exposure assessment in individual studies are presented in the present chapter, whereas generic issues of exposure assessment are discussed in Chapter 3 with the special challenges
involved in characterizing and reconstructing the herbicide exposures of Vietnam veterans.
The original VAO committee and the update committees up to that for Update 2006 have been satisfied with exposure characterization as nonspecific as “usual occupation” on a death certificate or “current occupation” from a census. With the passage of time, exposure assessments in epidemiology studies have been increasingly exact in both specificity and amount, and this has led the members of the more recent updates to establish stricter criteria for accepting exposure as sufficiently specific for results to be added to the evidentiary database. The current committee now seeks results expressed in terms of the five chemicals of interest for this project or their analogues and regards classification based only on job title as inadequate; restriction by the investigators to “herbicide” exposure is considered specific enough only to provide supporting evidence. According to the policy established by the Agent Orange Act of 1991, studies of Vietnam veterans are presumed to involve relevant exposure, as are studies of workers at a particular plant during a period when it is known to have been producing phenoxy herbicides or other chemicals recognized as having been contaminated with TCDD.
In Update 2010, the committee undertook a major change in the formatting of the tables of cumulative results on the health outcomes that was aimed at making relationships among publications more evident for its own deliberations and for the reader. The prior practice had been to insert findings from new publications in the results tables at the beginning of the sections on veteran, occupational, and environmental studies and so to create bands of studies reviewed in individual updates. Now, however, the reported findings on a given condition from a particular study population described in any of the VAO reports are gathered and presented in reverse chronologic order to provide the full history of the study of each endpoint in each group studied. The current update has attempted to shift the focus further to the total picture presented by a study population by clustering related findings and shifting the citations that were the source of particular results to the far right of the results tables. For instance, all incidence findings on the Seveso cohort over the successive followup periods are grouped first, and they are followed by all the analogous mortality findings, even when that means separating various sorts of results from the same publication.
Within the three general types of exposure that cohorts or cross-sectional study populations may have experienced, the order of the study populations (Vietnam veterans, occupationally exposed workers, and environmentally exposed people) roughly reflects the degree of importance attributed to the information generated. In the present update, the occupational-study populations have been partitioned into those involved in the production of herbicides and other industrial products contaminated with TCDD and those involved in occupational use of the herbicides of interest, because of substantial differences in the nature and intensity of their exposures. Doing so entailed splitting the findings on sprayers
cohorts from those on production workers in the large International Agency for Research on Cancer (IARC) cohort of phenoxy herbicide workers.
Studies of subgroups are presented after those on an overarching cohort. For example, when first reported (Saracci et al., 1991), the original IARC Cohort of Phenoxy Herbicide Workers was composed of 20 cohorts in 10 countries that had been studied separately. When mortality in those workers was followed up (Kogevinas et al., 1997), they were augmented with 16 additional cohorts—four German study populations and 12 groups of workers studied separately in US manufacturing facilities—which together make up the independently studied National Institute for Occupational Safety and Health (NIOSH) cohort. To simplify the location of underlying information on study populations, their discussion in this chapter follows the order in which their findings are presented in the results tables for each health outcome.
The section below on Vietnam veterans covers studies conducted in the United States by the Air Force, the Centers for Disease Control and Prevention (CDC), the Department of Veterans Affairs (VA), the American Legion, and individual states; it also covers studies of Australian and South Korean Vietnam veterans. The section “Occupational Studies” covers studies of workers other than Vietnam veterans exposed occupationally to the COIs, including production workers, agriculture and forestry workers (including herbicide and pesticide applicators), and paper and pulp workers. The section “Environmental Studies” covers studies of populations exposed to the COIs from nonoccupational sources, including the general population, such as the National Health and Nutrition Examination Survey cohort, and people who had usually high exposures because of industrial sources in their residential neighborhoods, such as residents of Seveso, Italy; southern Vietnam; suburban Taichung, Taiwan; Chapaevsk, Russia; and Times Beach, Missouri. This chapter ends with a section that addresses publications that are based on repeatedly mentioned case-control study populations; case-control studies that assessed Vietnam-veteran status, however, are included in the section on veteran studies, and nested case-control studies are presented in conjunction with the cohorts from which they were derived.
Studies of Vietnam veterans who might have been exposed to herbicides, including Agent Orange, have been conducted in the United States at the national and state levels and in Australia and Korea. Exposures have been estimated by various means, and health outcomes have been evaluated with reference to various comparison or control groups. This section is organized primarily by research sponsor because it is more conducive to a methodologic presentation of the studies. The specificity of exposure spans a wide range from individual exposures of Ranch Hand and Army Chemical Corps (ACC) personnel, as reflected in serum
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) measurements, to the use of service in Vietnam as a surrogate for TCDD exposure in some studies.
Several comparison groups have been used for veteran cohort studies: Vietnam veterans who were stationed in areas where herbicide-spraying missions were unlikely to have taken place; Vietnam-era veterans who were in the military at the time of the conflict but did not serve in Vietnam; veterans who served in other wars or conflicts, such as the Korean War and World War II; and various US populations (either state or national).
In all studies of Vietnam veterans (whether or not the study participants were American), the study participants are the target population of the committee’s charge, and they are assumed to have had a higher probability of exposure to the COIs than people who did not serve in Vietnam, whether or not their individual exposures are characterized beyond the mere fact that they were deployed to Vietnam.
The publication period considered in the present update saw a number of publications concerning psychologic outcomes in American and Australian Vietnam veterans, but these conditions do not fall in the spectrum of physical responses included in the VAO statement of task (Campbell and Renshaw, 2012; Franzen et al., 2012; Gellis and Gehrman, 2011; Renshaw and Caska, 2012; Yesavage et al., 2012). Conley and Heerwig (2012) investigated whether eligibility for military conscription (although not necessarily being conscripted or actually deployed to Vietnam) might be associated with mortality in later life by using the draft lottery for 1950–1952 birth cohorts as a natural experiment. Mortality data were obtained from the National Center for Health Statistics multiple-cause-of-death file, 1989–2002; the date of birth was used to determine draft status so that mortality in draft-eligible and draft-ineligible people could be compared. That study provides valid estimates of the effects of the Vietnam-era draft, but there is no specific information on actual deployment or exposure to the COIs. Wilmoth et al. (2010) examined the association between veteran status and trajectories of health conditions, limitations on activities of daily living, and self-rated health in 12,631 male participants from the 1992–2006 waves of the Health and Retirement Study; they compared nonveterans and veterans, veterans with and without wartime service, and war-service veterans who served during World War II, Korea, Vietnam, and multiple wars. Again, there is no specific information on exposure to the COIs.
US Air Force Health Study
Although no new reports from the Air Force Health Study (AFHS) were identified in the current literature review, reports and findings from the study have provided important information that was incorporated into the previous VAO reports and continues to play an important role in the committee’s assessment of the overall evidence for the current report. The data-gathering phase of this study is complete, but the committee remains
interested in seeing additional publications that provide longitudinal analysis of the vast amount of information assembled and make use of the collection of preserved biologic samples.
Major defoliation activities in Vietnam were conducted by Air Force personnel as part of Operation Ranch Hand. Veterans who took part in the defoliation activities became the first subpopulation of Vietnam veterans to receive special attention with regard to Agent Orange and have become known as the Ranch Hand cohort within the AFHS. To determine whether exposure to herbicides, including Agent Orange, had adverse health effects, the Air Force made a commitment to Congress and the White House in 1979 to conduct an epidemiologic study of Ranch Hand personnel (AFHS, 1982). Results of biologic-marker studies of Ranch Hand personnel have been consistent with their being exposed, as a group, to TCDD. When the Ranch Hand cohort was classified by military occupation, a general increase in serum TCDD was detected in people whose jobs involved more frequent handling of herbicides (AFHS, 1991a).
The exposure index initially proposed in the AFHS relied on military records of spraying of TCDD-containing herbicides (Agent Orange, Agent Purple, Agent Pink, and Agent Green) as reported in the Herbicide Reporting System (HERBS) tapes for the period starting in July 1965 and on military procurement records and dissemination information for the period before July 1965. In 1991, the exposure index was compared with the results of the Ranch Hand serum-TCDD analysis. The exposure index and the TCDD body burden correlated weakly.
Michalek et al. (1995) developed several indexes of herbicide exposure of members of the Ranch Hand cohort and tried to relate them to the measurements of serum TCDD from 1987 to 1992. Self-administered questionnaires completed by veterans of Operation Ranch Hand were used to develop three indexes of herbicide or TCDD exposure: number of days of skin exposure; percentage of skin area exposed; and the product of the number of days of skin exposure, the percentage of skin exposed, and a factor for the concentration of TCDD in the herbicide. A fourth index, which used no information gathered from individual study participants, was calculated by multiplying the volume of herbicide sprayed during a person’s tour of duty by the concentration of TCDD in herbicides sprayed in that period and then dividing the product by the number of crew members in each job specialty at the time.
Each of the four indexes tested was significantly related to serum TCDD, although the models explained only 19–27% of the variability in serum TCDD concentrations. Days of skin exposure had the highest correlation. Military job classification (non-Ranch Hand combat troops, Ranch Hand administrators, Ranch Hand flight engineers, and Ranch Hand ground crew), which is separate from the four indexes, explained 60% of the variability in serum TCDD. When the questionnaire-derived indexes were applied within each job classification, days of skin exposure added statistical significance, but not substantially, to the variability explained by job alone.
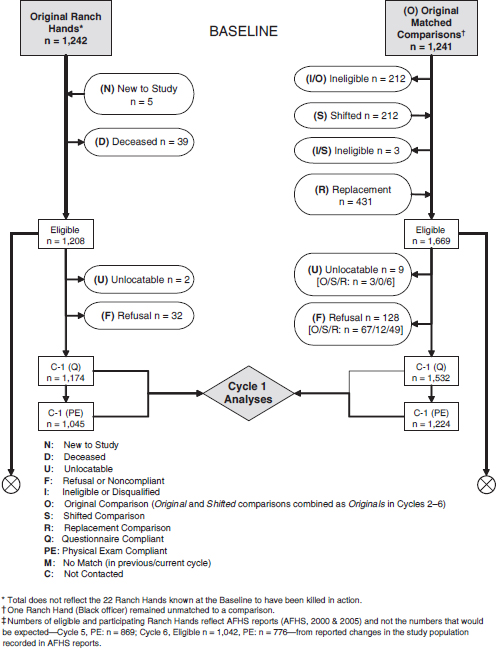
FIGURE 6-1 Flowchart of procedures followed and participant involvement in the Air Force Health Study.
NOTE: Flowchart numbers reflect what was known to AFHS investigators at any given cycle according to AFHS reports and do not reflect corrections made to earlier cycles due to the identification of misclassified subjects in later cycles. Identical study population counts vary on occasion within and across cycle reports. Thus, this reconstruction should
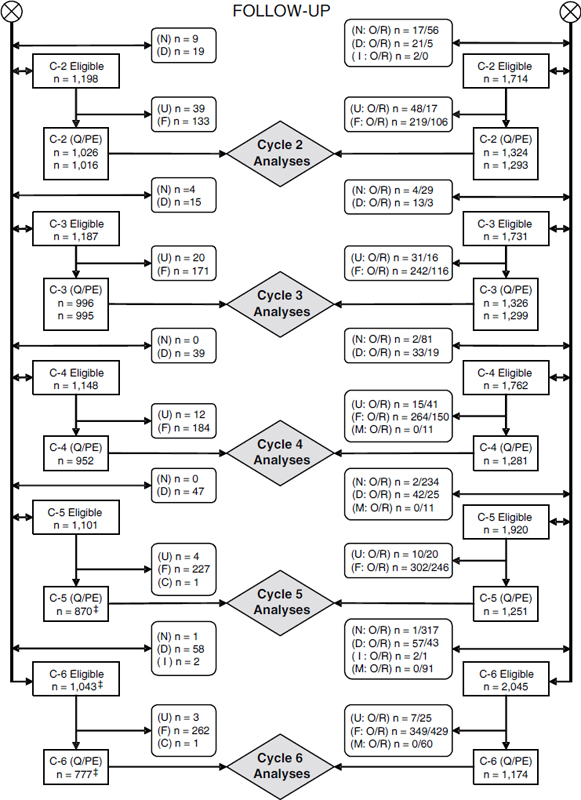
As depicted in Figure 6-1, a retrospective matched-cohort study design was used to examine morbidity and mortality; followup was scheduled to continue until 2002. Records from the National Personnel Records Center and the US Air Force Human Resources Laboratory were searched and cross-referenced to identify all Ranch Hand personnel (AFHS, 1982; Michalek et al., 1990). A total of 1,269 participants were originally identified (AFHS, 1983). A control population of 24,971 C-130 crew members and support personnel assigned to duty in Southeast Asia (SEA) but not occupationally exposed to herbicides (AFHS, 1983) was selected from the same data sources. Control participants were individually matched for age, type of job (based on Air Force specialty code), and race (white or not white) to control for age-related, educational, socioeconomic-status, and race-related differences in development of chronic disease. To control for many potential confounders related to the physical and psychophysiologic effects of combat stress and the SEA environment, Ranch Hands were matched to control participants who performed similar combat or combat-related jobs (AFHS, 1982). Rank also was used as a surrogate of exposure. Alcohol use and smoking were included in the analysis when they were known risk factors for the outcome of interest.
Ten matches formed a control set for each exposed participant. For the mortality study, the intent was to follow each exposed participant and a random sample of half of each participant’s control set for 20 years in a 1:5 matched design. The morbidity component of followup consisted of a 1:1 matched design; the first control was randomized to the mortality-ascertainment component of the study. If a control was noncompliant, another control from the matched “pool” was selected; controls who died were not replaced.
The baseline physical examination occurred in 1982, and examinations took place in 1985, 1987, 1992, 1997, and 2002. Morbidity was ascertained through questionnaires and physical examination, which emphasized dermatologic, neurobehavioral, hepatic, immunologic, reproductive, and neoplastic conditions. Some 1,208 Ranch Hands and 1,668 comparison participants were eligible for baseline examination. Initial questionnaire response rates were 97% for the exposed cohort and 93% for the nonexposed; baseline physical-examination responses were 87% and 76%, respectively (Wolfe et al., 1990). Deaths were identified and reviewed by using US Air Force Military Personnel Center records, the VA Beneficiary Identification Record Locator Subsystem (BIRLS), and the Internal Revenue Service database of active Social Security numbers. Death certificates were obtained from the appropriate health departments (Michalek et al., 1990).
Ranch Hands were divided into three categories on the basis of their potential exposure:
• Low potential. Pilots, copilots, and navigators. Exposure was primarily through preflight checks and spraying missions.
• Moderate potential. Crew chiefs, aircraft mechanics, and support personnel. Exposure could occur by contact during dedrumming and aircraft loading operations, onsite repair of aircraft, and repair of spray equipment.
• High potential. Spray-console operators and flight engineers. Exposure could occur during operation of spray equipment and through contact with herbicides in the aircraft.
Ostensibly, the AFHS was designed to answer exactly the question that the VAO project is asking, but the nature of the “exposed” (Ranch Hand veterans) and “comparison” (SEA veterans) groups and the evolving practices of VAO committees in endeavoring to fulfill the intention of their congressional mandate make interpretation less straightforward.
Results have been published for baseline morbidity (AFHS, 1984a), baseline mortality (AFHS, 1983), and for reproductive outcomes (AFHS, 1992; Michalek et al., 1998a; Wolfe et al., 1995). Mortality updates have been published for 1984–1986, 1989, and 1991 (AFHS, 1984b, 1985, 1986, 1989, 1991a). An interim technical report updated cause-specific mortality in Ranch Hands through 1993 (AFHS, 1996). Michalek et al. (1998b) and Ketchum and Michalek (2005) reported on 15-year and 20-year followup of postservice mortality, respectively, in veterans of Operation Ranch Hand, updating an earlier cause-specific mortality study by Michalek et al. (1990). Comparisons presented in the voluminous reports on the followup examinations of 1984, 1987, 1992, 1997, and 2002 (cited as AFHS, 1987, 1990, 1995, 2000, 2005) have been deemed not useful for the purposes of the VAO reviews because of the prevalence or cross-sectional nature of the data on only those in the cohort who were still alive and participated in a particular examination.
Blood samples for determination of serum TCDD concentrations were drawn at the periodic examinations conducted in 1982 from 36 Ranch Hands (Pirkle et al., 1989); in 1987 from 866 Ranch Hands (AFHS, 1991b); in 1992 from 455 Ranch Hands (AFHS, 1995); and in 1997 from 443 Ranch Hands (AFHS, 2000). For veterans whose TCDD was not measured in 1987 but was measured later, the later measurement was extrapolated to 1987 by using a first-order kinetics model with a constant half-life of 7.6 years. Analyses of the serum TCDD readings were included in the report on the 1987 followup examination (AFHS, 1991b), and other Ranch Hand publications have addressed the relationship between serum TCDD and reproductive hormones (Henriksen et al., 1996); diabetes mellitus, glucose, and insulin (Henriksen et al., 1997); skin disorders (Burton et al., 1998); infant death (Michalek et al., 1998a); sex ratios (Michalek et al., 1998c); skin cancer (Ketchum et al., 1999); insulin, fasting glucose, and sex-hormone–binding globulin (Michalek et al., 1999a); immunologic responses (Michalek et al., 1999b); diabetes mellitus (Longnecker and Michalek, 2000; Steenland et al., 2001); cognitive function (Barrett et al., 2001); hepatic abnormalities (Michalek et al., 2001a); peripheral neuropathy (Michalek et al., 2001b); hematologic results
(Michalek et al., 2001c); psychologic functioning (Barrett et al., 2003); correlations between diabetes and TCDD elimination (Michalek et al., 2003); thyroid function (Pavuk et al., 2003); cancer incidence (Akhtar et al., 2004; Pavuk et al., 2005); insulin sensitivity (Kern et al., 2004), prostate cancer (Pavuk et al., 2006); serum testosterone and risk of benign prostate hyperplasia (Gupta et al., 2006); and diabetes and cancer incidence (Michalek and Pavuk, 2008). All the VAO updates—Veterans and Agent Orange: Herbicide/Dioxin Exposure and Type 2 Diabetes (IOM, 2000), and Veterans and Agent Orange: Length of Presumptive Period for Association Between Exposure and Respiratory Cancer (IOM, 2004)—have discussed reports and papers that address the cohort in more detail.
The tendency of the AFHS researchers to use differing cutpoints and population definitions for analogous analyses suggests their a posteriori selection in a fashion that influences the results. For example, Michalek and Pavuk (2008) allude to the commonly held assumption that Agent Orange was more heavily contaminated earlier in the war as the motivation for making various temporal partitions in their analyses, but the choices were not consistent. For cancer, service in 1968 or before was considered to fall in the critical exposure period, whereas days of spraying were counted through 1967 and the variable for “days of spraying” was assigned the value “low” or “high” by partitioning the resulting distribution at 30 days. For diabetes, however, service in 1969 or before was regarded as being in the critical exposure period, and the variable “days of spraying” was split into “low” and “high” at 90 days or more, with no specification of the period over which the counting was done.
The AFHS is perceived by many to be the central piece of research for decision-making by the VAO committees, but it also has important limitations that all VAO committees have had to take into consideration. A recent Institute of Medicine (IOM) report, Disposition of the Air Force Health Study (IOM, 2006), which was undertaken by another IOM committee as the AFHS was approaching the end of its data-gathering phase, described the limitations of the AFHS effectively and was quoted in extensive detail in Updates 2006 and 2008. In summary, VAO committees have recognized the following features as the primary strengths and limitations of the AFHS:
• The AFHS is one of the most pertinent studies for the VAO reviews, with a study population that was directly exposed to the COIs in the Vietnam War theater.
• It can be argued that the AFHS population is not representative of the entire population of Vietnam veterans, so its findings might not be generalizable to all Vietnam veterans.
• The AFHS might be underpowered for detecting small effects, especially rare outcomes, because of its relatively small sample. Therefore, its findings are vulnerable to false negatives (failure to detect an important association). This also raises questions about the stability of positive findings;
this is somewhat less problematic if they are repeated over examination cycles, although the results of the examination cycles themselves are not fully independent repetitions.
• For AFHS analyses that used non-AFHS Vietnam veterans as the comparison group, the comparison group might also have been exposed to the COIs although the exposure was likely to be substantially higher in the AFHS group than in the comparison group. Therefore, the comparison is not an ideal “exposed vs unexposed” comparison but rather a “high exposure vs low exposure” comparison. The exposure in the comparison group might also make the study findings vulnerable to false negatives if the exposure differential between the AFHS group and the comparison group was not large enough to allow an association between exposure and outcome to be detected. However, that problem does not affect the validity of positive findings.
US Department of Veterans Affairs
VA Army Chemical Corps Cohort
The study of members of the US ACC was conducted by VA, whose other research efforts on Vietnam veterans are discussed together below. It is discussed immediately after the AFHS because of the importance that VAO committees have attributed to it. Like the Ranch Hand personnel, members of the ACC were involved directly in handling and distributing herbicides in Vietnam. Because the ACC personnel were expected to have been highly exposed to Agent Orange, VAO committees recommended study of this important group of Vietnam veterans (IOM, 1994) and later encouraged publication of its findings (IOM, 2004). The availability of serum TCDD concentrations in a subset of this cohort of Vietnam veterans has made its findings particularly useful in appraising possible associations with various health outcomes.
ACC troops performed chemical operations on the ground and by helicopter and were thereby involved in the direct handling and distribution of herbicides in Vietnam. The ACC population was belatedly identified for the study of health effects related to herbicide exposure (Thomas and Kang, 1990). In an extension, Dalager and Kang (1997) compared mortality in veterans of the ACC specialties, including Vietnam veterans and non-Vietnam veterans. Results of an initial feasibility study were reported by Kang et al. (2001). They recruited 565 veterans—284 Vietnam veterans and 281 non-Vietnam veterans—as controls. Blood samples were collected in 1996 from 50 Vietnam veterans and 50 control veterans, and 95 of the samples met CDC standards of quality assurance and quality. Comparison of the entire Vietnam cohort with the entire non-Vietnam cohort showed that the geometric mean TCDD concentrations did not differ significantly (p = 0.6). Of the 50 Vietnam veterans sampled, analysis of question-
naire responses indicated that those who reported spraying herbicides had higher TCDD concentrations than did those who reported no spraying activities. The authors concluded that Agent Orange exposure was a likely contributor to TCDD concentrations in Vietnam veterans who had a history of spraying herbicides.
Kang et al. (2006) reported findings of the main study. A health survey of 1,499 Vietnam veterans and 1,428 non-Vietnam veterans was administered by telephone. Exposure to herbicides was assessed by analyzing serum specimens from a sample of 897 veterans for dioxin. Veterans who reported spraying herbicides had significantly higher TCDD serum concentrations than did Vietnam veterans and other veterans who did not report herbicide spraying. The final analysis compared Vietnam-veteran sprayers with Vietnam-veteran nonsprayers in the entire study population.
Having determined the vital status of the ACC personnel through 2005, Cypel and Kang (2010) presented results on mortality from the following: cancers (oral and pharyngeal, digestive, respiratory, prostate, testicular, skin, brain, and lymphopoietic [leukemia]), diabetes, circulatory conditions (hypertension and cerebrovascular), respiratory conditions (pneumonia, influenza, and chronic obstructive pulmonary disease), and cirrhosis of the liver. The study compared 2,872 ACC personnel who served in Vietnam with 2,737 ACC personnel who did not serve in Vietnam, using survival analysis that controls for race, age at entry into followup, rank, and duration of military service. It also compared 662 ACC personnel who served in Vietnam and reported spraying herbicides with 811 who did not serve in Vietnam and did not report spraying, controlling for additional covariates obtained in the telephone survey—body-mass index (BMI) and smoking status. Mortality in both cohorts was also compared with the expected mortality in US males. Concerns were raised that the findings in Cypel and Kang (2010) regarding respiratory diseases were not adjusted for smoking status, probably an important confounding factor for respiratory diseases, in the analyses based on the entire ACC cohort that compared those who served in Vietnam with those who did not. (The subcohort analyses that compared sprayers with nonsprayers were adjusted for smoking status.)
The primary strengths and limitations of the ACC studies are similar to those of the AFHS. No new ACC studies were reported during the current review period.
VA Female US Vietnam-Veteran Cohort
Although estimates vary, 5,000–7,000 US women are believed to have served in Vietnam after volunteering for military service (Thomas et al., 1991). The vast majority of them served as combat nurses—mostly in the Army Nurse Corps—but some also served with the Women’s Army Corps and the Air Force, Navy, and Marine Corps (Spoonster-Schwartz, 1987; Thomas et al., 1991).
In 1986, Public Law (PL) 99-972 was enacted. It required that an epide-
miologic study be conducted to examine long-term adverse health effects on female Vietnam veterans as a result of their exposure to traumatic experiences, exposure to such herbicides as Agent Orange or other chemicals or medications, or any similar experience or exposure during such service. The first study that VA conducted to assess mortality in female Vietnam veterans was by Thomas et al. (1991). No comprehensive record of female personnel who served in Vietnam in 1964–1972 existed, so researchers gathered military service data from each branch of the armed forces to conduct the mortality study through December 31, 1987. Female Army and Navy personnel were identified from morning reports and muster rolls of hospitals and administrative support units where women were likely to have served. Military personnel were identified as female by their names, leaving open the possibility that some women may have been inadvertently excluded from the analysis. Women who served in the Air Force and Marine Corps were identified through military records. The combined roster of all female personnel from the military branches was considered by the researchers to be generally complete. A comparison group consisted of female veterans who were identified through the same process as the female Vietnam veterans but had not served in Vietnam during their military service. Demographic information and information on overseas tours of duty, unit assignments, jobs, and principal duties were abstracted from military records. Mortality information was obtained from VA’s BIRLS, the Social Security Administration, the Internal Revenue Service, the National Death Index, and military personnel records. When women whose service in the military fell outside the period of interest, whose records were lacking data, or who served in SEA but not in Vietnam were excluded, the analysis included 132 deaths in 4,582 female Vietnam veterans and 232 deaths in 5,324 comparison veterans who served in the military during July 4, 1965–March 28, 1973. Cause-specific mortality was derived for Vietnam veterans and comparison veterans and compared with mortality in US women with adjustment for race, age, and calendar period. Dalager et al. (1995b) updated mortality in the original cohort until December 31, 1991, using the same study protocol as Thomas et al. (1991). After updating of mortality figures and adjustment of the existing cohort on the basis of new information about the study groups based on the inclusion criteria, 4,586 Vietnam veterans and 5,325 comparison veterans were included in the final analyses (Dalager et al. 1995b).
VA also published studies of pregnancy outcomes and gynecologic cancers—namely, neoplasms of the cervix, uterus, and ovary—in US female Vietnam veterans (Kang et al., 2000a,b). Army veterans were identified from a list obtained by the US Army and Joint Services Environmental Support Group; computerized lists were also provided by the Air Force, Navy, and Marine Corps. Militaryservice data were abstracted from personnel records. Of 5,230 eligible veterans, 4,390 whose permanent tour of duty included service in Vietnam were alive on January 1, 1992. From a pool of 6,657 potential control participants whose military units did not serve in Vietnam, 4,390 veterans who were alive on January 1,
1992, were randomly selected as controls. After exclusion of 250 veterans and 250 nonveterans who participated in a pilot study, an attempt was made to locate the remaining 4,140 veterans in each group. Various location strategies were used, and fewer than 5% (370) were not located; another 339 were deceased. A full telephone interview was conducted on 6,430; 775 refused (13% of Vietnam veterans and 17% of non-Vietnam veterans), and another 366 completed only a short written questionnaire. A questionnaire was administered on demographic background, general health, lifestyle, menstrual history, pregnancy history, pregnancy outcomes, and military experience, including nursing occupation and combat exposure. Information on pregnancy risks and complications—including smoking, infections, medications, exposure to X-rays, occupational history, and exposure to anesthetic gases, ethylene oxide, herbicides, and pesticides—was collected for each pregnancy. In Kang et al. (2000a), the first pregnancy after the beginning of Vietnam service was designated as the index pregnancy of each woman. For the comparison group, the first pregnancy after July 4, 1965, was used as the index pregnancy of each woman. Odds ratios were calculated for reproductive history and pregnancy outcomes. The study analyzed data on 3,392 Vietnam and 3,038 non-Vietnam veterans and on 1,665 Vietnam and 1,912 non-Vietnam veteran index pregnancies. In Kang et al. (2000b), a self-reported history of gynecologic cancers (defined by the authors as cancers of the breast, ovary, uterus, and cervix) was collected. The authors attempted to “retrieve hospital records on all reported cancers as far back as 30 years.” Of records successfully found, 99% of the breast cancers and 90% of all cancers were confirmed. The authors did not provide data on validation of the three sites other than breast, but stated that Vietnam status was not associated with verification of the outcome.
After the publications by Kang et al. (2000a,b), Congress passed PL 106-419, which provides compensation for children of female Vietnam veterans who are born with birth defects unrelated to an existing familial disorder, to a birth-related injury, or to a fetal or neonatal infirmity with a well-established cause. Eighteen birth defects are covered by the legislation, including cleft lip or palate, congenital heart disease, hypospadias, neural-tube defects, and Williams syndrome. A complete list of covered birth defects can be found in Section 3.815 of the legislation.
Cypel and Kang (2008) conducted a mortality study of female Vietnam veterans and compared their mortality with that in a control group of women who were in military service but did not participate in the Vietnam War. Non-Vietnam veterans were selected randomly from among female veterans who never served in Vietnam and were matched to the Vietnam veterans according to rank and military occupation.
No reports on female Vietnam Veterans have been published since Update 2008.
VA Proportionate-Mortality Cohort
Among the earliest reports on Vietnam veterans was a proportionate-mortality study by Breslin et al. (1988). The participants were men who had served as ground troops in the US Army or Marine Corps at any time from July 4, 1965, through March 1, 1973. A list of 186,000 Vietnam-era veterans who served in the Army or Marine Corps and were reported deceased as of July 1, 1982, was assembled from VA’s BIRLS; 75,617 names were randomly selected from the list for inclusion in the study. Information extracted from the selected military records included the places, dates, and branch of military service; date of birth; sex; race; military occupation specialty codes; education level; type of discharge; and confirmation of service in Vietnam. Additional information was extracted on veterans who served in SEA, including the first and last dates of service in SEA, the military unit, and the country where the veteran served. For the final sample of 52,253 Army and Marine Corps veterans, cause of death was ascertained from death certificates or Department of Defense Report of Casualty forms for 51,421 men, including 24,235 who served in Vietnam and 26,685 men who did not serve in SEA; 501 deaths were excluded from the final analyses because service in SEA was in a country other than Vietnam or the location of military service was unknown. Each veteran’s cause of death was coded by a nosologist who used the 8th revision of the International Classification of Diseases.
On the basis of the proportionate-mortality study (Breslin et al., 1988), Burt et al. (1987) conducted a nested case-control study of non-Hodgkin lymphoma (NHL) with controls selected from among the cardiovascular-disease deaths. In a followup of the Breslin et al. study, Bullman et al. (1990) compared cause-specific proportionate mortality in 6,668 Army I Corps Vietnam veterans—veterans who served in the northernmost part of South Vietnam in a combat zone designated as Military Region I by the US military—with that in 27,917 Army Vietnam-era veterans who had not served in Vietnam. The study by Bullman et al. included the study population identified by Breslin et al. and an additional 9,555 Army Vietnam-era veterans whose deaths were identified after the BIRLS mortality data were extended through December 31, 1984. Similarly, Watanabe et al. (1991) updated the Vietnam-veteran mortality experience reported by Breslin et al. (1988) by extending the followup from January 1, 1982, to December 31, 1984. An additional 11,325 deceased Army and Marine Vietnam-era veterans were identified from the period and included in the study. The study population for Watanabe et al. consisted of 62,068 military veterans, of whom 29,646 served in Vietnam and 32,422 never served in SEA. Proportionate-mortality ratios were calculated for three referent groups: branch-specific (Army and Marine Corps) non-Vietnam veterans, all non-Vietnam veterans combined, and the US male population. A third followup proportionate-mortality study (Watanabe and Kang, 1996) used the veterans from Breslin et al. (1988) and Watanabe et al. (1991) and included an additional 9,040 randomly selected Vietnam-era veterans who died
from July 1, 1984, through June 30, 1988. The final study included 70,630 veterans—33,833 who served in Vietnam and 36,797 who never served in SEA—and the analyses were performed with the same referent groups described previously (Watanabe et al., 1991).
Other VA Studies
VA also conducted studies that focused on specific health outcomes, using data from VA’s Agent Orange Registry (AOR), a computer database containing health information on Vietnam veterans who voluntarily undergo examinations in a VA hospital. The AOR was set up in 1978 to monitor Vietnam veterans’ health complaints or problems that could be related to Agent Orange exposure during military service in Vietnam. The examinations consist of an exposure history, a medical history, laboratory tests, and an examination of body systems most commonly affected by toxic chemicals. As of June 1, 2008, the registry contained information from 506,184 examinations (Agent Orange Review, 2008).
Using early data from the registry, Bullman et al. (1991) examined the risk of posttraumatic stress disorder (PTSD) in a case-control study of veterans who received AOR medical examinations during January 1983–December 1987. The final analyses include 374 PTSD cases and 373 controls whose military records were used to verify Vietnam service, Military Occupational Specialty Codes (MOSCs), primary duties, military branch, dates of Vietnam service, medals, awards, and disciplinary actions for each veteran. Similarly, Bullman et al. (1994) studied the risk of testicular cancer by using the AOR health records of veterans who received Agent Orange medical examinations during March 1982–January 1991. The final analyses in that study included 97 testicular-cancer cases and 311 controls. A surrogate metric for Agent Orange exposure was developed by using branch of service, combat MOSCs, geographic area of service in Vietnam, location of military units in relation to herbicide-spraying missions, and the length of time between spray missions and military operations in sprayed areas.
Watanabe and Kang (1995) compared postservice mortality in Vietnam veterans in the Marine Corps with that in Vietnam-era marines who did not serve in Vietnam. All study participants were on active duty during 1967–1969 and were followed from their discharge date or from the date of the US military withdrawal from Vietnam until their date of death or December 31, 1991, whichever came first. The final study population included 10,716 Vietnam and 9,346 non-Vietnam veteran marines.
Kang et al. (1991) conducted a case-control study that compared dioxin and dibenzofuran concentrations in the adipose tissue of 36 Vietnam veterans with those in 79 non-Vietnam veterans and a sample of US men born in 1936–1954. All tissue samples were archived specimens from the US Environmental Protection Agency National Human Adipose Tissue Survey and had been collected by hospitals and medical examiners from men who died from external causes or
surgical procedures. Military service—branch of service, MOSC, and geographic service location in Vietnam, if applicable—was researched and verified with military records. Controls were matched by birth year and sample collection year (± 2 years), and the final analyses were adjusted by age and BMI.
Dalager et al. (1991) examined NHL in male Vietnam veterans in a hospital-based case-control study. Study participants were identified via inpatient discharge records from VA medical centers for fiscal years 1969–1985. Cases were identified as having a malignant lymphoma and a birth date during 1937–1954. Controls were identified from VA medical-center discharge records and were matched by hospital, discharge date, and birth date. The location and dates of each veteran’s military service were verified by using military records. A surrogate Agent Orange exposure opportunity was also developed for each Vietnam veteran according to branch of service, combat experience, and geographic location of the military unit assignment. The final analysis included 201 cases and 358 controls. Another study by Dalager et al. (1995a) examined the association between Hodgkin lymphoma (HL) and Vietnam service. It used the same method as the 1991 Dalager et al. study; the analysis included 283 HL cases and 404 controls.
VA has evaluated specific health outcomes, including case-control studies of soft-tissue sarcoma (STS) (Kang et al., 1986, 1987), testicular cancer (Bullman et al., 1994), and lung cancer (Mahan et al., 1997). It also has conducted a study of self-reported physical health (Eisen et al., 1991) and PTSD (Goldberg et al., 1990) in monozygotic twins who served during the Vietnam era.
VA has examined other outcomes in Vietnam veterans: PTSD (Bullman et al., 1991; True et al., 1988), suicide and motor-vehicle crashes (Bullman and Kang, 1996; Farberow et al., 1990), and tobacco use (McKinney et al., 1997). The studies have been included for completeness, but the outcomes that they address are outside the purview of this committee. VAO and Update 1998 discuss them in detail; most did not deal with exposure to Agent Orange, and exposure to “combat” was evaluated as the risk factor of interest.
US Centers for Disease Control and Prevention Studies
Surveys of US Vietnam veterans who were not part of the Operation Ranch Hand or ACC groups indicated that 25–55% believed that they were exposed to herbicides (CDC, 1989a; Erickson et al., 1984a,b; Stellman and Stellman, 1986). Several attempts have been made to estimate exposure of Vietnam veterans who were not part of the Ranch Hand or ACC groups. CDC has undertaken a series of studies to examine various health outcomes in Vietnam veterans as directed by Congress in the Veterans Health Programs Extension and Improvement Act of 1979 (PL 96-151) and the Veterans’ Health Care, Training, and Small Business Loan Act of 1981 (PL 97-72).
CDC Birth-Defects Study
The first was a case-control interview study of birth defects in offspring of men who served in Vietnam (Erickson et al., 1984a,b). In 1983, the US government asked CDC to conduct a study of possible long-term health effects in Vietnam veterans exposed to Agent Orange. The CDC Agent Orange study (CDC, 1985) attempted to classify veterans’ service-related exposures to herbicides. That involved determining the proximity of troops to Agent Orange spraying by using military records to track troop movement and the HERBS tapes to locate herbicide-spraying patterns. The CDC birth-defects study developed an exposure-opportunity index to score Agent Orange exposure (Erickson et al., 1984a,b).
CDC Agent Orange Validation Study
In 1987, CDC conducted the CDC Agent Orange Validation Study (AOVS) to test the validity of the various indirect methods used to estimate exposure of ground troops to Agent Orange in Vietnam. The study measured serum TCDD in a nonrandom sample of Vietnam veterans and in Vietnam-era veterans who did not serve in Vietnam (CDC, 1988a). Vietnam veterans were selected for the study on the basis of the number of Agent Orange hits that they were thought to have experienced given the number of days on which their company was within 2 km and 6 days of a recorded Agent Orange spraying event. Blood samples were obtained from 66% of 646 Vietnam veterans and from 49% of the eligible comparison group of 97 veterans. More than 94% of those whose serum was obtained had served in one of five battalions.
The median serum TCDD in Vietnam veterans in 1987 was 4 parts per trillion (ppt) (range, under 1 to 45 ppt). Only two veterans had concentrations above 20 ppt. The “low” exposure group consisted of 298 Vietnam veterans, the “medium” exposure group 157 veterans, and the “high” exposure group 191 veterans. The distribution of TCDD measurements was nearly identical with that in the control group of 97 non-Vietnam veterans. The CDC validation study concluded that study participants could not be distinguished from controls on the basis of serum TCDD. In addition, neither record-derived estimates of exposure nor self-reported exposure to herbicides could predict Vietnam veterans with currently high serum TCDD (CDC, 1988a, 1989a). The report concluded that it was unlikely that military records alone could be used to identify a large number of veterans who might have been heavily exposed to TCDD in Vietnam.
CDC Vietnam Experience Study
Using exposure estimates from the AOVS, CDC conducted the CDC Vietnam Experience Study (VES), a historical cohort study of the health experience of Vietnam veterans (CDC, 1989b). The study was divided into three parts: physi-
cal health, reproductive outcomes and child health, and psychosocial characteristics (CDC, 1987, 1988a,b,c, 1989b). Using VES data, CDC examined postservice mortality (through 1983) in a cohort of 9,324 US Army veterans who served in Vietnam and in 8,989 Vietnam-era Army veterans who served in Korea, Germany, or the United States (Boyle et al., 1987; CDC, 1987). Another study (O’Brien et al., 1991) combined the mortality and interview data to identify all veterans who had NHL. To evaluate whether self-reported assessment of exposure to herbicides influences the reporting of adverse health outcomes, CDC designed a study of VES participants (Decoufle et al., 1992). In a followup of CDC’s VES cohort, Boehmer et al. (2004) reported findings on mortality during 1965–2000.
The serum TCDD measurements in Vietnam veterans also suggested that exposure to TCDD in Vietnam was substantially lower, on the average, than that of persons exposed as a result of the industrial explosion in Seveso or that of the heavily exposed occupational workers who have been the focus of many of the studies evaluated by the present committee. The assessment of average exposure does not preclude heavy exposure of subgroups of Vietnam veterans.
CDC Selected Cancers Study
CDC undertook the CDC Selected Cancers Study (CDC, 1990a) to investigate the effects of military service in Vietnam and of exposure to herbicides on the health of American veterans, specifically NHL (CDC, 1990b), STS and other sarcomas (CDC, 1990c), HL (CDC, 1990d), and nasal, nasopharyngeal, and primary liver cancers (CDC, 1990d).
CDC National Vietnam Veterans Readjustment Study
The CDC National Vietnam Veterans Readjustment Study (NVVRS) investigated primarily psychological outcomes. It is now being updated to become the National Vietnam Veterans Longitudinal Study. To date the only resulting publication (Currier and Holland, 2012) on a sample from the NVVRS addressed psychologic outcomes in association with combat trauma and bereavement.
Other US Vietnam-Veteran Studies
American Legion Study
The American Legion, a voluntary service organization for veterans, conducted a cohort study of the health and well-being of Vietnam veterans who were members. Studies examined physical health and reproductive outcomes, social–behavioral consequences, and PTSD in veterans who had served in SEA and elsewhere (Snow et al., 1988; Stellman JM et al., 1988; Stellman SD et al., 1988). No additional studies have been published on the cohort.
State Studies
Several states have conducted studies of Vietnam veterans, most of them unpublished in the scientific literature. VAO and Update 1996 reviewed studies of veterans of Hawaii (Rellahan, 1985), Iowa (Wendt, 1985), Maine (Deprez et al., 1991), Massachusetts (Clapp, 1997; Clapp et al., 1991; Kogan and Clapp, 1985, 1988; Levy, 1988), Michigan (Visintainer et al., 1995), New Jersey (Fiedler and Gochfeld, 1992; Kahn et al., 1988, 1992a,b,c), New Mexico (Pollei et al., 1986), New York (Greenwald et al., 1984; Lawrence et al., 1985), Pennsylvania (Goun and Kuller, 1986), Texas (Newell, 1984), West Virginia (Holmes et al., 1986), and Wisconsin (Anderson et al., 1986a,b). Chamie et al. (2008) examined the association between Agent Orange and prostate cancer in all Vietnam-era veterans using the VA health system in northern California; the reliability of this study of about 13,000 men is limited by its reliance on self-reported exposure status and the exclusion of prostate cases diagnosed before 1998, when computerized records became available. No additional state studies have been published.
Additional studies have examined health outcomes that included spontaneous abortion (Aschengrau and Monson, 1989) and adverse outcomes late in pregnancy in spouses of Vietnam veterans (Aschengrau and Monson, 1990). After a published study indicated a potential association between testicular cancer in dogs and their service in Vietnam (Hayes et al., 1990), Tarone et al. (1991) conducted a case-control study of testicular cancer in male veterans. VAO summarized those studies, and no additional studies have been published on these study populations.
Australian Vietnam-Veteran Studies
Over many years the Australian government has commissioned studies to follow health outcomes in two sets of Australian veterans who served in Vietnam.
Australian Vietnam Veterans
The Australian Vietnam Veterans study population corresponds to the cohort defined by the “Nominal Roll of Vietnam Veterans,” which lists Australians who served on land or in Vietnamese waters from May 23, 1962, to July 1, 1973, including military and some nonmilitary personnel of both sexes. People who served in all branches of service in the “defence forces” and “Citizen Military Forces” (such as diplomatic, medical, and entertainment personnel) were considered. The comprehensive studies, however, are limited to male members of the military and most of the analyses focus on men in the “defence forces”—the Army (41,084), the Navy (13,538), and the Air Force (4,570). Association of Vietnam service with cancer incidence (ADVA, 2005b) was sought by comparing diagnoses from 1982–2000 among male Vietnam veterans with those in the general
population of Australia. The results in this report supersede those in the report of the Australian Department of Veterans’ Affairs (CDVA, 1998a). Morbidity in all female Vietnam veterans had been studied in an earlier report (CDVA, 1998b). Additional case-control studies of the incidence of adrenal gland cancers, leukemia, and NHL were conducted in this population (AIHW, 1999, 2000, 2001).
A related report (ADVA, 2005a) considered the causes of death of men in all branches of service through 2001. The numbers of deaths were 4,045 in the Army, 1,435 in the Navy, and 686 in the Air Force. The mortality experience of military personnel serving in Vietnam was compared with that of the general population of Australia and reported by branch of service. The findings of this study supersede those in the report on mortality from 1980 to 1994 (CDVA, 1998a). There had been several earlier studies of mortality among Austalian Vietnam veterans (CIH, 1984a,b,c; Crane et al., 1997a,b; Evatt, 1985; Fett et al., 1987a,b; Forcier et al., 1987).
Australian Conscripted Army National Service
The Australian Conscripted Army National Service study population is a subset of the veterans considered in the overall Australian Vietnam Veterans study group. The 19,240 conscripted male Army veterans deployed to Vietnam (“National Service” veterans) were compared with their 24,729 non-deployed counterparts (“National Service non-veterans”). This comparison between contemporaries who had been sufficiently healthy to enter the service provided a means of adjusting for a possible “healthy-warrior” effect. The results on death and cancer in the Australian conscripted Army National Service veterans (ADVA, 2005c) supersede those of earlier internal comparisons of deployed and non-deployed Vietnam War–era National Service veterans (CIH, 1984a; Crane et al., 1997b; Fett et al., 1987a,b). Those government-sponsored studies of Australian Vietnam veterans did not characterize the veterans’ exposure to the herbicides sprayed in Vietnam beyond the fact that they served on land or in Vietnamese waters during May 23, 1962–July 1, 1973. It is the convention of VAO committees to regard Vietnam veterans in general as being more likely to have received higher exposures to the COIs than the general public, but it would have been informative to validate that assumption by gathering biomarkers of exposure, such as serum measurements, in a sample of Australian Vietnam veterans.
Update 2000 had moved the occurrence of acute myeloid leukemia in offspring of Vietnam veterans to the limited or suggestive category of association primarily on the basis of findings reported by the Australian Institute of Health and Welfare (AIHW, 2000) but rescinded in a revised report (AIHW, 2001). The reversal of the conclusion on this matter by the committee for Update 2000 is discussed in Veterans and Agent Orange: Herbicide/Dioxin Esposure and Acute Myelogenous Leukemia in the Children of Vietnam Veterans (IOM, 2002).
Sample of 1,000 Australian Vietnam Veterans
O’Toole et al. (1996a,b,c) studied a broad spectrum of health issues in a random sample of 1,000 Australian Vietnam veterans (both regular enlisted and conscripted Army National Service members) selected from Australia’s comprehensive roster of 57,643 service members deployed to Vietnam. In wave 1, conducted in 1990–1993, 641 members of the sample were located and interviewed. In wave 2, conducted in 2005–2006, O’Toole et al. (2009) obtained responses from 450 (51.4% of those not known to have died); 391 responded to both waves. The Australian Bureau of Statistics National Health Survey was administered in both waves with collection of additional data on combat experience, PTSD, and general psychiatric status. The veterans’ self-reported health status was compared with that of the general male Australian population gathered during the government’s administration of the same survey in 1989–1990 and 2004–2005; it is not clear that this instrument was administered to the two groups under comparable conditions. The low response rates make the findings vulnerable to nonresponse bias, and the use of self-report measures of health conditions might be of low validity and subject to recall bias. The committee for Update 2010 was skeptical about the reliability of the nearly uniform findings of statistically increased prevalence of nearly 50 health conditions. O’Toole et al. (2010) reported on the mortality in the sample through 2004 as related to previously gathered information on psychosocial factors that are not within the scope of VAO reviews. It is of interest, however, that they found that 11.7% of the veterans in the sample had died by the end of 2004.
Case-Control Study of Birth Defects in Australian Infants
The Australian government sponsored a case-control study of 8,517 infants with congenital anomalies born in 1966–1979 at 34 hospitals in New South Wales, Victoria, and the Australian Capital Territory matched by period of birth, mother’s age, hospital, and means of hospital payment to live-born infants without diagnosed birth defects (Donovan et al., 1983, 1984; Evatt, 1985). The fathers of both groups were identified and their names compared to the roster of men who had served in the Australian Army in 1962–1972; additional means of verification were used to determine whether the child’s father had been in the Army during this interval (329 cases and 338 controls) and also whether he had been deployed to Vietnam (127 cases and 123 controls). Adjusting for maternal age, infant sex, multiple births, and father’s place of birth, conditional logistic regression was used to compare the Vietnam veterans (National Service or regular Army) to other era veterans and to all other fathers for all birth anomalies and for seven diagnostic groups.
Korean Vietnam-Veteran Studies
Study of TCDD Concentrations in Korean Vietnam Veterans
Military personnel of the Republic of Korea served in Vietnam during 1964–1973. Kim JS et al. (2001) attempted to use serum dioxin concentrations to validate an index for estimating group exposure. The study involved 720 veterans who served in Vietnam and 25 veterans who did not. The exposure index was based on Agent Orange spraying patterns in military regions in which Korean personnel served, time–location data on the military units stationed in Vietnam, and an exposure score derived from self-reported activities during service. A total of 13 pooled samples were submitted to CDC for serum dioxin analysis. One analytic sample was prepared from the pooled blood of the 25 veterans who did not serve in Vietnam. The remaining 12 samples were intended to correspond to 12 exposure categories; each was created by pooling blood samples from 60 veterans. The 12 exposure categories ultimately were reduced to four exposure groups, each representing a quartile of 180 Vietnam veterans but characterized by only three serum TCDD measurements.
The paper by Kim JS et al. (2001) reported highly significant Pearson correlation coefficients and results of multiple logistic-regression analysis. The statistical analyses apparently were based on the assignment of the pooled serum dioxin value to each person in the exposure group and thereby inflated the true sample size. The multiple regression analysis evaluated such variables as age, BMI, and consumption of tobacco or alcohol. In a later report on the same exposure groups and serum dioxin data, the authors corrected their analysis (Kim JS et al., 2003). A correlation was observed between serum dioxin concentrations and ordinal exposure categories, but the correlation was not statistically significant. The authors attributed the lack of statistical significance to the small sample, and they noted that the data exhibited a distinct monotonic upward trend; average serum dioxin concentrations, 0.3, 0.6, 0.62, 0.78, and 0.87 pg/g (lipid-adjusted) for exposure categories 0–4, respectively. The decision to pool blood samples from a large number of persons in each exposure set (Kim JS et al., 2001) greatly reduced the power of the validation study. Instead of 180 samples in each of the final exposure categories, the pooled analysis produced only three samples in each category. The lipid-adjusted serum TCDD concentrations in the 12 pooled samples from Vietnam veterans ranged from 0.25 to 1.2 pg/g, whereas the single sample from the non-Vietnam veterans contained 0.3 pg/g. The narrow range of results makes the biologic relevance of any differences questionable.
Thus, it appears that there was not a clear separation between Korean Vietnam veterans and non-Vietnam veterans. Furthermore, the range of mean values in the four Vietnam-veteran exposure categories was narrow, and all concentrations were relatively low (less than 1 pg/g). The relatively low serum dioxin concentrations observed in the 1990s in those people are the residual of substan-
tially higher initial concentrations, as has been seen in other Vietnam-veteran groups. However, the concentrations reported in the Korean-veterans study are significantly lower than are those reported in American Vietnam veterans in the 1988 CDC AOVS, which was nonetheless unable to distinguish Vietnam veterans from non-Vietnam veterans on the basis of serum dioxin (CDC, 1988a). The Korean authors were able to construct plausible exposure categories based on military records and self-reporting, but they were unable to validate the categories with serum dioxin measurements.
Study of Role of Vietnam Service in Recovery of Koreans with Acute Coronary Syndrome
Kim JB et al. (2012) reported on the association between exposure to TCDD and recovery outcomes (HT, hyperlipidemia, and the rate and severity of major adverse coronary events) in men who presented with acute coronary syndrome (obstruction of coronary ateries and chest pain) during 2004–2009 at Gwangju Veterans Hospital. The age range was limited to 50–70 years to reflect the current age of Korean veterans of the Vietnam War. There were 251 patients: 121 were Vietnam veterans (assumed to have been exposed to TCDD), and 130 were not. Medical records were reviewed to determine a variety of cardiovascular recovery outcomes. T tests, chi-square tests, and logistic regression were used to determine whether measures of recovery differed between the acute coronary patients who had served in Vietnam and those who had not. The study findings are not informative about associations between TCDD and acute coronary syndrome itself, as the researchers allege.
Other Studies of Korean Vietnam Veterans
Epidemiologic studies have also looked at immunotoxicologic outcomes (Kim HA et al., 2003) and skin and general disease patterns (Mo et al., 2002) in Korean Vietnam veterans who were exposed to Agent Orange during the Vietnam War.
Several occupational groups in the United States and elsewhere have been exposed to the COIs. Exposure characterization varies widely in the metric used, the extent of detail, confounding by other exposures, and whether individual, surrogate, or group (ecologic) measures are used. Some studies use job titles as broad surrogates of exposure; others rely on disease-registry data.
The committee reviewed many epidemiologic studies of occupationally exposed groups for evidence of an association between exposure to TCDD or to the herbicides used in Vietnam—primarily the phenoxy herbicides 2,4-dichloro-
phenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T)—and health risks. TCDD is an unwanted byproduct of 2,4,5-T production but not of 2,4-D production. Other contaminants, including other dioxins (such as 1,3,6,8-tetrachlorodibenzo-p-dioxin), have been reported at low concentrations in 2,4-D, but those identified do not have the toxicity of TCDD (ATSDR, 1998; Huston, 1972; Norström et al., 1979). In reviewing the studies, the committee considered two types of exposure separately: exposure to 2,4-D or 2,4,5-T and exposure to TCDD from 2,4,5-T or other sources. That separation is necessary because some health effects could be associated with exposure to 2,4-D or 2,4,5-T in the absence of substantial TCDD exposure. After recognition of the problem of dioxin contamination in phenoxy herbicides, production conditions were modified to minimize contamination, but use of the products most subject to containing specifically TCDD (2,4,5-T and Silvex) was banned. As a result, study participants exposed to phenoxy herbicides only after the late 1970s would not be assumed to have been at risk for exposure to TCDD.
The distinction is particularly important for workers in agriculture and forestry, including farmers and herbicide appliers, whose exposure is primarily the result of mixing, loading, and applying herbicides. In addition to those occupational groups, the committee considered studies of occupational exposure to dioxins, focusing on workers in chemical plants that produced phenoxy herbicides or chlorophenols, which tend to be contaminated with polychlorinated dibenzo-p-dioxins (PCDDs). Waste-incineration workers were also included in the occupation category because they can come into contact with dioxin-like compounds while handling byproducts of incineration. Other occupationally exposed groups included were pulp and paper workers exposed to dioxins through bleaching processes that use chlorinated compounds, and sawmill workers exposed to chlorinated dioxins that can be contaminants of chlorophenates used as wood preservatives.
Studies of Herbicide Production Workers
International Agency for Research on Cancer Phenoxy Herbicide Cohort
A multisite study by IARC involved 18,390 production workers and phenoxy herbicide sprayers working in 10 countries (Saracci et al., 1991). The full cohort was established by using the International Register of Workers Exposed to Phenoxy Herbicides and Their Contaminants. Twenty cohorts were combined for the analysis: one each in Australia, Austria, Canada, Finland, and Sweden; two each in Denmark, Italy, the Netherlands, and New Zealand; and seven in the United Kingdom. There were 12,492 production workers and 5,898 sprayers in the full cohort.
Questionnaires were constructed for workers who were manufacturing chlorophenoxy herbicides or chlorinated phenols and for herbicide sprayers; the
questionnaires were completed with the assistance of industrial hygienists. Information from production records and job histories was examined when available. Workers were classified as exposed, probably exposed, with unknown exposure, or nonexposed. The exposed-workers group (13,482) consisted of all those known to have sprayed chlorophenoxy herbicides and all who worked in particular aspects of chemical production. Two subcohorts (totaling 416) had no job titles available but worked in chemical-production facilities that were likely to produce TCDD exposure, so they were deemed probably exposed. Workers with no exposure information (541) were classified as of unknown exposure. Nonexposed workers (3,951) were those who had never been employed in parts of factories that produced chlorophenoxy herbicides or chlorinated phenols and had never sprayed chlorophenoxy herbicides. Two nested case-control studies were undertaken with the IARC cohort to evaluate the relationship between STSs and lymphomas (Kogevinas et al., 1992, 1995). Kogevinas et al. (1993) presented the information available on the subcohort of 701 women who were occupationally exposed to chlorophenoxy herbicides, chlorophenols, and dioxins included in 11 of the cohorts in seven of the countries; nine deaths and 29 incident cancer cases were reported (too few to tabulate results).
An expanded and updated analysis of the IARC cohort with an emphasis on cancer mortality was published in 1997 (Kogevinas et al., 1997). The researchers added herbicide production workers in 12 plants in the United States (the NIOSH cohort) and four plants in Germany. The 21,863 male and female workers exposed to phenoxy herbicides or chlorophenols were classified in three categories of exposure to TCDD or higher-chlorinated dioxins: those exposed (13,831), those not exposed (7,553), and those with unknown exposure (479). Several exposure metrics were constructed for the cohort—years since first exposure, duration of exposure (in years), year of first exposure, and job title—but detailed methods were not described. The overall results were for mortality in 1939–1992, but for some of the subcohorts, followup had begun as late as 1975, and at the time of publication, mortality in some had been tracked only through 1983. For nonneoplastic causes of death, Vena et al. (1998) repeated the grouped statistics for all phenoxy-herbicide workers in the updated IARC cohort (as previously presented in Kogevinas et al., 1997) and provided results partitioned according to whether the workers had the potential for exposure to TCDD and more highly chlorinated dioxin contaminants.
No new studies of the IARC cohort have been published since Update 1998.
International Agency for Research on Cancer Subcohorts
In addition to the NIOSH cohort and its component subcohorts (discussed below), several of the subcohorts that make up the IARC cohort have generated independent reports that have been evaluated separately by VAO committees to garner additional insights, such as results associated with TCDD concentrations
measured in some subjects: Austrian production workers (Jäger et al., 1998; Neuberger et al., 1998, 1999); British production workers (Coggon et al., 1986, 1991); Danish production workers (Lynge, 1985, 1993); Dutch production workers (Boers et al., 2010, 2012; Bueno de Mesquita et al., 1993; Hooiveld et al., 1998); German production workers (Becher et al., 1996; Flesch-Janys, 1997; Flesch-Janys et al., 1995; Manz et al., 1991); and New Zealand production workers (McBride et al. 2009a,b; Smith et al., 1981, 1982; ’t Mannetje et al., 2005). Several of the component cohorts have not been the subject of any separate publications: Australian herbicide sprayers, Canadian herbicide sprayers, Finnish production workers, two cohorts of Italian production workers, and Swedish production workers. The international production-worker cohorts are discussed below in alphabetical order, followed by the NIOSH cohort and its subcohorts. The section on studies of herbicide-using workers, which follows discussion of all production-worker studies, includes consideration of the separate reports on the New Zealand herbicide sprayers.
Dutch production workers The two Dutch subcohorts of the IARC cohort consist of 2,106 male workers employed in two manufacturing factories producing and formulating chlorophenoxy herbicides: 2,4,5-T in factory A during 1955–1985 and 2-methyl-4-chlorophenoxyacetic acid (MCPA), 2-(2-methyl-4-chlorophenoxy)propionic acid (Mecoprop, MCPP); and 2,4-D in factory B during 1965–1986. Accordingly, members of both subcohorts had potential exposure to phenoxy herbicides, but only those in factory A could have been exposed to TCDD. The study populations were defined as all workers who worked in factory A during 1955–1985 or factory B during 1965–1986.
Hooiveld et al. (1998) updated the mortality experience (1955–1991) of production workers in the two Dutch chemical factories in the Netherlands with known exposure to dioxins: workers in herbicide production, nonexposed production workers, and workers known to have been exposed as a result of an accident that occurred in 1963. On the basis of an assumption of first-order TCDD elimination with an estimated half-life of 7.1 years, measured TCDD concentrations were extrapolated to the time of maximum TCDD exposure of a group of 47 workers. A regression model was then used to estimate, for each cohort member, the effect on estimated maximum TCDD exposure attributable to exposure as a result of the accident, duration of employment in the main production department, and time of first exposure (before or after 1970).
Boers et al. (2010) conducted updated analyses based on the third followup of the Dutch subcohorts of the IARC cohort, examining cause-specific mortality (cancer and noncancer) in 2,106 male workers employed in factories A and B. Both cohorts were followed through 2006, accumulating 65,087 person-years, with 567 deaths observed. Sample loss was minimal (< 1% lost to followup, < 5% emigrated). Death certificates obtained by linkage to Statistics Netherlands were used to ascertain cause-specific mortality, including various cancers, endocrine or
blood diseases, nervous system, ischemic heart disease, other heart disease, cerebrovascular diseases, respiratory diseases, digestive diseases, and genitourinary diseases. Exposure to chlorophenoxy herbicides was determined on the basis of the type of work experience (such as production vs office) and involvement in the accident of 1963 in factory A (factory A: 539 exposed, 482 nonexposed; factory B: 411 exposed, 626 nonexposed). TCDD measures taken in 1993 support that exposure classification; the highest mean TCDD concentrations were found in workers involved in the 1963 accident (1,841.8 ppt) and those who worked in main production (608.2 ppt), whereas concentrations in nonexposed workers were much lower (7.6 ppt). Cox proportional-hazards models with attained age as the time scale were used to assess hazard ratios for exposed vs nonexposed workers. Exposure to phenoxy herbicides and dioxins was expected to be different between factory A and factory B, and the factories were therefore analyzed separately. Further nested case-control studies were conducted for the factory A cohort by using all cancer cases (112) and three controls per case matched on age and employment period; analysis used conditional logistic regression.
Since Update 2010, several new studies based on this cohort have been published. Boers et al. (2012) conducted more detailed dose–response analyses of the updated mortality data on the cohorts reported in Boers et al. (2010). From May 2007 to September 2008, blood was drawn for the determination of plasma TCDD concentrations in a systematically selected subsample of 187 workers (101 in factory A, 86 in factory B). Serum concentrations measured in the workers in factory B (geometric mean = 0.4 ppt) confirmed they had not experienced TCDD exposures above background. The combination of linear regression on the log-transformed serum results and work-history details was used to derive a model to predict current TCDD in the entire cohort, from which back-extrapolation predicted each person’s concentration when he left employment in factory A or B. There were considerable individual differences from the previously assigned exposure groups, but overall the exposures predicted by the empirical model had a high rank correlation (Spearman’s r = 0.79) with the exposure statuses used in previous analyses. A Cox proportional-hazards model was used to assess exposure–outcome relationships on the basis of the predicted exposures as a time-varying covariate. To allow for latency, a 1-year lag was used for noncancer endpoints and a 10-year lag for cancer outcomes. The log-linear TCDD model was applied to the workers in factory A only and to the entire cohort, including workers from factory B, who had been exposed only to phenoxy herbicides as confirmed by the serum samples from the 86 factory B subjects who had only background concentrations of TCDD.
Saberi Hosnijeh et al. (2011) examined the association between TCDD exposure and outcomes, including humoral immunity (serum immunoglobulin and complement factor concentrations) and atopic diseases (self-reported asthma, hay fever, eczema, and allergy) in a subsample of 153 workers, including 45 who had TCDD exposure in factory A, matched individually with a nonexposed compari-
son group consisting of 39 in factory A and 69 in factory B. TCDD exposure was characterized by using exposure status (exposed vs nonexposed), current serum concentration, and back-extrapolated serum concentration at the time of last exposure. Logarithmic transformation was used for TCDD and immune-marker concentrations. Statistical analyses were conducted with t tests, chi-square tests, and linear regression. Similarly, Saberi Hosnijeh et al. (2012) examined the association between TCDD exposure and serum cytokine concentrations in a subsample of workers in factory A (47 with high exposure, 38 with low exposure).
German production workers Becher et al. (1996) conducted an analysis of the four German cohorts added to the IARC cohort as of 1997: the Boehringer–Ingelheim cohort (also reported on in more detail by Manz et al., 1991 and later researchers); a cohort in the BASF Ludwigshafen plant that did not include those involved in a 1953 accident; and cohorts in a Bayer plant in Uerdingen and a Bayer plant in Dormagen. Preliminary information on the four cohorts had been published earlier (Becher et al., 1992). All the plants were involved in production of phenoxy herbicides or chlorophenols. Additional information is available only on the Boehringer–Ingelheim cohort, and the workers involved in the 1953 accident have been studied separately.
Boehringer–Ingelheim Cohort in Hamburg As first reported by Manz et al. (1991), workers in the Boehringer–Ingelheim plant in Hamburg had high potential for TCDD exposure because of production of trichlorophenol (TCP) and 2,4,5-T from 1951 to 1954 and from 1957 to 1984. The hiatus was motivated by a chloracne outbreak, and production recommenced when a process that resulted in less TCDD contamination became available. The cohort consisted of 1,184 men and 399 women, who had been employed for at least 3 months during 1952–1984. Vital status of all but 46 workers (2.9%) through 1989 was established; 313 deaths were observed in the men and 54 in the women. Detailed results were reported only for the men. Mortality from all causes did not differ from what would be predicted by rates for West Germany (standardized mortality ratio, [SMR] = 1.00, 95% confidence interval [CI] 0.89–1.12); compared with what was probably a more appropriate occupational cohort of Boehringer gas workers, however, mortality from all causes (only through 1985 because of limitations of available information) was significantly higher (SMR = 1.34, 95% CI 1.18–1.51). The risk of death from all cancers was marginally higher than the West German rates (SMR = 1.24, 95% CI 1.00–1.52) and more definitively so compared with the gas workers (SMR = 1.39, 95% CI 1.10–1.75).
Flesch-Janys et al. (1995) updated the cohort’s vital staus through 1992 and added a quantitative exposure assessment based on blood or adipose-tissue measurements of PCDDs and polychlorinated dibenzofurans (PCDFs). The authors estimated maximum PCDD and PCDF exposure of 190 workers with a first-order kinetics model, half-lives with an elimination study of 48 workers in the cohort,
and background concentrations in the German population. They then regressed the estimated maximum PCDD and PCDF exposures of the workers against the length of time that they worked in each production department in the plant. The working-time weights were then used with work histories of the remainder of the cohort to estimate PCDD and PCDF exposure of each person at the end of his or her employment. Those values were used to estimate TCDD doses in the population. (At this the stage of updating, the Hamburg cohort was discussed with three other German cohorts by Becher et al. [1996] and became a subcohort of the IARC phenoxy-herbicide cohort as updated by Kogevinas et al. [1997].)
Manuwald et al. (2012) updated the mortality experience of 1,191 men and 398 women in the Hamburg cohort. Subjects entered the cohort on the date of their first employment in the plant, and vital status was sought through 2007; loss to followup was only 3.2%. SMRs calculated relative to the population of Hamburg showed that death from all causes was slightly higher in men (698 deaths, SMR = 1.14, 95% CI 1.06–1.23); in the entire cohort, the increase in mortality was significant (SMR = 1.08, 95% CI 1.01–1.16), but not in women (180 deaths, SMR = 0.91, 95% CI 0.78–1.05). Similarly, mortality from all malignant neoplasms was slightly higher in men (226 cancer deaths, SMR = 1.14, 95% CI 1.06–1.23), and the increase in mortality was significant in the entire cohort (SMR = 1.33, 95% CI 1.18–1.49), but not in women (65 cancer deaths, SMR = 0.91, 95% CI 0.78–1.05). Individual cumulative exposure was estimated from work history on the basis of company records, and the intensity of TCDD exposure in workplaces was based on previous analyses of serum and fat-tissue dioxin concentrations. Cochran–Armitage trend tests on quartiles of cumulative exposure were conducted for deaths from all causes, all malignancies, breast cancer, cancers of digestive organs, respiratory cancers, and circulatory diseases.
BASF Ludwigshafen Plant Workers Involved in Accident Cleanup (not in IARC cohort) An accident on November 17, 1953, during the manufacture of TCP in a BASF plant in Germany resulted in extreme exposure of some workers to TCDD. VAO, Update 1996, Update 1998, and Update 2000 summarized studies of those workers, including a mortality study of persons initially exposed or later involved in cleanup (Thiess et al., 1982), an update and expansion of that study (Zober et al., 1990), and a morbidity followup (Zober et al., 1994). In addition, Ott and Zober (1996) and Zober et al. (1997) examined cancer incidence and mortality in workers exposed to TCDD after the accident or during reactor cleanup, maintenance, or demolition. No new studies have been published on these workers since Update 2000.
New Zealand Production Workers
The mortality status of the New Zealand cohort that was incorporated into the original IARC cohort was followed up through 2000 by ’t Mannetje et al. (2005).
The New Plymouth plant produced phenoxy herbicides from the “late” 1950s through the “mid-1980s.” It is of interest to note that this plant also produced picloram, one of the COIs about which very little information is available. Complete employment records for 1969–1984 were available, so the study included anyone who had worked at least 1 month in that period—a cohort of 713 men and 100 women (the 1984 cohort).
Burns et al. (2010), Collins et al. (2009c), and McBride et al. (2009a,b) examined the New Zealand production-worker subcohort of the IARC cohort, which comprised employees who worked at the Dow AgroSciences (formerly Ivon Watkins-Dow) plant in New Plymouth that manufactured diverse agrochemical products, including phenoxy herbicides. McBride et al. (2009a) conducted expanded analyses and updated previous analyses of cause-specific mortality (from both cancer and other conditions). The cohort was inflated to 1,599 participants (referred to hereafter as the 1988 cohort), including a substantial number of people who had minimal opportunity for exposure, by extending the employment period for eligibility to November 1, 1988, and removing the requirement that employment lasted at least 1 month. McBride et al. (2009b) further expanded the cohort to 1,754 participants (the 2003 cohort) by further extending eligibility to anyone who worked at the site at any time until October 1, 2003. Both enlarged cohorts were followed through 2004. The New Zealand Health Information Service Mortality Collection was used to identify deaths (247 in both cohorts; there seem to have been no deaths in the increment of 155 workers who were in the 2003 cohort but not in the 1988 cohort). Exposure status was classified according to work experience. A subsample of the 1988 cohort participated in a serum-dioxin analysis (346, 70% exposed).
Collins et al. (2009c) described the group’s serum TCDD concentrations overall, and Burns et al. (2010) performed analyses to determine what factors might predict serum TCDD: age, BMI, and employment history were found to be significant determinants. In particular, the exposed group had significantly (p = 0.03) higher concentrations (9.9 ppt) than did the nonexposed group (4.8 ppt); number of years since termination is associated significantly (p = 0.002) with lower TCDD; and serum TCDD is also associated significantly (p < 0.0001) with predicted cumulative TCDD exposure on the basis of area-under-the-curve in a pharmacokinetic model of the accumulation and elimination of dioxins. Both studies reported SMRs that were derived by using the Occupational Cohort Mortality Analysis Program with the New Zealand population as the reference population and adjusted for age, sex, and calendar age. For the 1988 cohort, SMRs were stratified by exposure status (ever exposed and never exposed) and by predicted cumulative exposure categories. For the 2003 cohort, SMRs were reported for the entire cohort and stratified by employment duration (less than 3 months and at least 3 months) and by latency (15 years and less than 15 years of latency). For the 1988 cohort, proportional-hazards survival analysis was also
used to test the association between mortality and predicted cumulative exposure categories.
The New Zealand studies have several important limitations. The sample loss was substantial: 13% were lost to followup in both cohorts, and 8% of the 1988 cohort and 9% of the 2003 cohort emigrated. If sample loss was nonrandom, the study findings might be vulnerable to sample selection bias. In addition, the inclusion in the 2003 cohort of the employees hired as recently as 2003 is questionable. It appears that no deaths were observed in the increment between the 1988 cohort and the 2003 cohort (those hired since 1988), presumably because these participants are relatively young. The inclusion of the incremental participants might dilute the power of the study to detect effects of TCDD exposure on health outcomes that require a long latent period; participants who have not yet “matured” through the latent period might be contributing noise rather than signal to the analyses. The committee, therefore, did not give substantial weight to the dose–response findings of McBride et al. (2009b).
The serum concentrations of dioxins and furans observed in a subset of the workers in the Dow phenoxy-herbicide plant in New Zealand have been used in estimating individual exposure (Aylward et al., 2010; Collins et al., 2009c).
National Institute for Occupational Safety and Health Studies
NIOSH PCP Cohort Ruder and Yiin (2011) reported findings on mortality in 2,122 pentachlorophenol (PCP) production workers in four plants—Midland, Michigan; Sauget, Illinois; Tacoma, Washington; and Wichita, Kansas—in the NIOSH dioxin registry. For analytic purposes, the cohort was partitioned into a subcohort of 1,402 workers (PCP-only group) who were employed only in production of PCP, which has dioxin and furan contaminants that do not include the most toxic 2,3,7,8-TCDD congener, and a subcohort of 720 (PCP-plus-TCDD group) who also worked in TCP production and so did have exposure to TCDD). The cohort was followed through December 31, 2005. Exposure was specified both as exposure status (exposed vs not exposed, for cohort members vs reference population) and as cumulative duration of exposure stratified into four quartiles. Statistical analyses were based on SMRs with the US population as the reference, and standardized rate ratios were used to compare workers in cumulative duration categories.
NIOSH Cross-Sectional Medical Study Before the first publication of mortality results in the main cohort, the NIOSH Cross-Sectional Medical Study gathered comprehensive medical histories, conducted medical examinations, and measured the pulmonary function of workers employed in chemical-manufacturing at plants in Newark, New Jersey (1951–1969), and Verona, Missouri (1968–1972). Control participants were recruited from surrounding neighborhoods (Sweeney et al., 1989, 1993). The New Jersey plant manufactured 2,4,5-TCP and 2,4,5-T; the
Missouri plant manufactured 2,4,5-TCP, 2,4,5-T, and hexachlorophene. Specific health outcomes were evaluated in the members of this subcohort, including porphyria cutanea tarda (Calvert et al., 1994); effects on pulmonary function (Calvert et al., 1991); effects on hepatic and gastrointestinal function (Calvert et al., 1992); mood (Alderfer et al., 1992); effects on the peripheral nervous system (Sweeney et al., 1993); and effects on reproductive hormones (Egeland et al., 1994). Sweeney et al. (1996, 1997/1998) reviewed and updated noncancer outcomes, including effects on hepatic function, gastrointestinal disorders, chloracne, diabetes, and serum glucose, hormone, and lipid concentrations. The data gathered from the two plants were also examined for cardiovascular effects (Calvert et al., 1998); diabetes mellitus, thyroid function, and endocrine function (Calvert et al., 1999); immune characteristics (Halperin et al., 1998); and cancer incidence (Kayajanian, 2002). Halperin et al. (1995) investigated the relationship between serum TCDD concentrations and cytochrome P450 induction in 400 of the original 586 subjects in the cohort. Lawson et al. (2004) studied three birth outcomes—birth weight, preterm delivery, and birth defects—in offspring of the cohort members by comparing serum TCDD concentrations with those in a reference population. TCDD exposures at conception were estimated by using physiologically based pharmacokinetic modeling (Dankovic et al., 1995; Thomaseth and Salvan, 1998).
NIOSH TCDD Mortality Cohort Since 1978, an extensive set of data on chemical production workers potentially contaminated with TCDD in 1942–1984 has been compiled by NIOSH. More than 5,000 workers who were involved in production or maintenance in any of 12 companies were identified from personnel and payroll records; 172 additional workers identified previously by their employers as being exposed to TCDD were also included in the study cohort (Suskind and Hertzberg, 1984). The employees’ possible exposure resulted from working with substances of which TCDD was a contaminant: 2,4,5-TCP, 2-(2,4,5-trichlorophenoxy) propionic acid (Silvex, 2,4,5-TP), 2-(2,4,5-trichlorophenoxy) ethyl 2,2-dichloropropionate (Erbon), O,O-dimethyl O-(2,4,5-trichlorophenyl) phosphorothioate (Ronnel®), and hexachlorophene. The 12 plants involved were large manufacturing sites of major chemical companies, so many of the participants were potentially exposed to many other compounds, some of which could be toxic and carcinogenic. The NIOSH cohort was added to the IARC cohort as of the 1997 publication by Kogevinas et al.
Exposure status was determined initially through a review of process operating conditions, employee duties, and analytic records of TCDD in industrial-hygiene samples, process streams, products, and waste (Fingerhut et al., 1991). Occupational exposure to TCDD-contaminated processes was confirmed by measuring serum TCDD in 253 cohort members. Duration of exposure, defined as the number of years worked in processes contaminated with TCDD, was used as the primary exposure metric in the study. The use of duration of exposure as a
surrogate for cumulative exposure was based on a correlation (Pearson correlation efficient, 0.72) between log-transformed serum TCDD and number of years worked in TCDD-contaminated processes. Duration of exposure of individual workers was calculated from work records, and exposure-duration categories were created: less than 1 year, 1 to less than 5 years, 5 to less than 15 years, and 15 years and longer. In some cases, information on duration of exposure was not available, so a separate metric—duration of employment—was defined as the total time that each worker was employed at the study plant. Fingerhut et al. (1991) used the exposure measures in assessing mortality through 1987.
A followup study (Steenland et al., 1999) examined the association between TCDD exposure and cause of death through 1993; it examined specific health outcomes, including cancer (all and site-specific), respiratory disease, cardiovascular disease, and diabetes. The researchers used a more refined exposure assessment than that used in previous analyses; it excluded workers whose records were inadequate to determine duration of exposure, and this reduced the number of study participants to a subcohort of 3,538 workers (69% of the overall cohort). The exposure assessment for the subcohort was based on a job–exposure matrix (JEM) that assigned each remaining worker a quantitative exposure score for each year of work (Piacitelli and Marlow, 1997).
No new studies on the entire NIOSH cohort were published during the current review period.
Subcohorts of the NIOSH TCDD Mortality Cohort
Monsanto The NIOSH study cohort (Fingerhut et al., 1991) included employees of the Monsanto facility in Nitro, West Virginia, that produced 2,4,5-T in 1948–1969. Zack and Suskind (1980) examined the mortality experience of the 121 men who had chloracne associated with an unintentional release that occurred on March 8, 1949. Other studies considered mortality and other health outcomes in additional workers involved in numerous aspects of 2,4,5-T production at the Monsanto plant (Collins et al., 1993; Moses et al., 1984; Suskind and Hertzberg, 1984; Zack and Gaffey, 1983). The Monsanto studies were discussed in more detail in VAO. No additional studies on those participants alone have been published; they have since been followed as part of the NIOSH and IARC cohorts.
Dow 2,4-D Production Workers Since Update 2010, Burns et al. (2011) have reported on cancer incidence in 2,4-D production workers in the Dow Midland plant. The exposed cohort consisted of 1,316 men who worked in 2,4-D operations during 1945–1994 and were alive on January 1, 1985, when the Michigan statewide cancer registry was initiated. Exposure was considered both as a category (exposed [cohort members] vs not exposed [reference population]) and as a cumulative variable estimated as (job-specific exposure estimate) × (duration
on the job) summed over all jobs held since 1945. Workers were stratified into three categories according to estimated cumulative exposure. The cohort was followed in 1985–2007. Cancer incidence was ascertained from the Michigan statewide cancer registry and data linked to Arizona and Ohio, states where cohort members might reside. Three nested cohorts were used for statistical analyses to address potential problems with data that were missing because of migration outside the three states with data linkage. Cohort 1 consisted of the entire exposed cohort (1,316 who had 25,267 person-years of followup). Cohort 2 required Michigan residency; followup was terminated when a person was known not to be a Michigan resident, either because company records showed a permanent non-Michigan address or a death certificate showed a state other than Michigan as the state of residency (1,256 who had 23,354 person-years). Cohort 3 had a more stringent residency requirement; followup was terminated when a person was no longer known to be a Michigan resident (1,108 who had 18,897 person-years). For Cohort 2, people of unknown residency status were assumed to remain Michigan residents and were included in the followup; for Cohort 3, such people were assumed to be nonresident and were excluded. Standardized incidence ratios (SIRs) were derived for all three cohorts with Michigan white males as the reference population; Fisher’s exact confidence interval was used to characterize uncertainty. For Cohort, 2 additional analyses were conducted by using the NCI Surveillance, Epidemiology, and End Results (SEER) registry population and a regional population as the reference populations and by stratifying the cohort according to cumulative duration and cumulative exposure categories.
There are concerns that the study findings might be biased, for several reasons. First, the study cohort might be healthier than the general population being used as the reference population. Second, the lack of a latent period in the study design might lead to an attenuation effect on the risk estimates; this is similar to the Villeneuve and Steenland (2010) criticism of the Dow-Midland mortality study reported in Collins et al. (2009a). Third, Cohort 2, used as the researchers’ focus of the study, might be vulnerable to an attenuation effect because of the uncertainty of residency status. For the present VAO review, the results on Cohort 3 are considered least subject to bias, and hence most reliable, although this smallest group is subject to the most variability; consistency in results among the three cohorts is considered confirmatory.
All Dow TCP-Exposed Workers TCP was produced in Dow’s facility in Midland, Michigan, from 1942 to 1979, and 2,4,5-T was produced there from 1948 to 1982. The cohort of TCP workers who were potentially exposed specifically to TCDD is one of the eight cohorts in the NIOSH cohort of dioxin-exposed US workers that were entered into the IARC phenoxy herbicides cohort.
Collins et al. (2009a) updated the vital status through 2003 of 1,615 people who had worked with TCP or 2,4,5-T during 1942–1982; 58,743 person-years were accumulated, and 662 deaths were observed. SMRs for cause-specific mor-
tality in the cohort—with and without the overlap of 196 people with the PCP cohort in Collins (2009b)—were calculated by using the US population as the reference population and using the Occupational Mortality Analysis Program.
Dow PCP Production Workers This set of people were engaged in the manufacture of PCP from 1937 to 1980 in the same plant where the TCP cohort worked. Unlike TCP, PCP did not contain TCDD, but it did contain other highly chlorinated dioxin congeners, and 20% of the PCP workers had suffered from chloracne. Those who had no TCDD exposure are not in IARC or NIOSH cohorts. This group is one of four cohorts included in NIOSH’s PCP cohort (Fingerhut et al., 1984; Ruder and Yiin, 2011).
Dow has tracked a cohort of its manufacturing workers who were exposed to PCP (Ramlow et al., 1996). The exposure assessment evaluated the available industrial-hygiene and process data, including recollections from employees about processes and jobs, information about changes in processes and engineering controls, measurements from surface wipes, and exposure-monitoring data from area sampling and personal breathing zones. Jobs in the “flaking/prilling/packaging area” were determined to have higher potential exposure because of dermal exposure to airborne PCP; the industrial-hygiene data suggested a difference of about a factor of 3 between the areas of highest and lowest potential exposure. An estimated exposure-intensity score of 1–3 (from lowest to highest potential exposure intensity) was assigned to each job. Information concerning the use of personal protective equipment was deemed to be unreliable. For each participant, cumulative PCP and TCDD exposure indexes were calculated by multiplying the duration of each exposed job by its estimated exposure intensity and then summing across all exposed jobs.
Collins et al. (2009b) conducted a mortality study of the Dow PCP production workers with the accrual of years at risk starting at the beginning of 1940. The cohort was followed for “up to 64 years.” Although the date of closure of the followup was not provided explicitly, it appears that the cohort was followed through 2003, as were the TCP workers (Collins et al., 2009a). The cohort consisted of 773 PCP workers; 27,035 person-years were accumulated, and 370 deaths were observed. SMRs for the PCP cohort (with and without the overlap of 196 people in the TCP cohort) were given for cause-specific mortality with the US population as the referent population. Proportional-hazards survival analysis was also used to assess the association between mortality and predicted cumulative exposure as total toxic equivalent (TEQ) to TCDD.
Dow TCDD-Exposed Production Workers Dow conducted a study of 204 workers engaged in the production of 2,4,5-T (Ott et al., 1980) and one of 61 TCP manufacturing workers who had chloracne (Cook et al., 1980). Industrial hygienists developed a JEM that ranked employee exposures as low, moderate, or high on the basis of available air-monitoring data and professional judgment. The
matrix was merged with employee work histories to assign an estimate of exposure to each job. A cumulative dose was then developed for each of the 878 employees by multiplying the representative 8-hour time-weighted average (TWA) exposure value for each job by the number of years in the job and then adding the products for all jobs. A 2,4-D TWA of 0.05 mg/m3 was used for low, 0.5 mg/m3 for moderate, and 5 mg/m3 for high exposure. The role of dermal exposure in the facilities does not appear to have been considered in the exposure estimates. It is not clear to what extent the use of air measurements alone can provide accurate classification of workers into low-, moderate-, and high-exposure groups. Biologic monitoring of 2,4-D apparently was not included in the study.
Bond et al. (1983) investigated potential exposure to TCDD and morbidity in the sets of workers reported on by Cook et al. (1980) and Ott et al. (1980). Potential TCDD exposure and reproductive outcomes were studied in the offspring of 930 men who worked with chlorophenol during 1939–1975 (Townsend et al., 1982). Dow employees who had a diagnosis of chloracne or who were classified as having chloracne on the basis of a clinical description were followed prospectively for mortality (Bond et al., 1987). There was a succession of mortality studies of workers involved in 2,4-D production in several of the plants (Bloemen et al., 1993; Bond et al., 1988; Burns et al., 2001), which also were conducted with the same exposure-assessment procedures.
Dow assembled a large cohort at the Midland, Michigan, plant (Bond et al., 1989a; Cook et al., 1986, 1987). Exposure to TCDD in the cohort was characterized on the basis of chloracne diagnosis (Bond et al., 1989b). Within the cohort, a subcohort study of women (Ott et al., 1987) and a case-control study of STS (Sobel et al., 1987) were conducted. The Dow cohorts have been followed as part of the NIOSH and IARC cohorts since 1991 and 1997, respectively.
Bodner et al. (2003) published a 10-year followup of the work of Cook et al. (1986), comparing the mortality experience of 2,187 male Dow workers who were potentially heavily exposed to dioxin before 1983 with that of the NIOSH and IARC cohorts. Dow researchers have published a study of serum dioxin concentrations measured in 2002 in former chlorophenol workers (Collins et al., 2006). Most of the workers in the study were included in the NIOSH and IARC cohorts. The authors used their data to estimate worker exposure at the time of exposure termination by using several pharmacokinetic models. They concluded that their findings were consistent with those of other studies that reported high serum dioxin concentrations in chlorophenol workers after occupational exposure.
Czech Worker Studies
Several studies of Czech workers have been reviewed by VAO committees. The original committee reviewed a 10-year followup study of 55 men in Czechoslovakia who were exposed to TCDD during the production of 2,4,5-T
(Pazderova-Vejlupková et al., 1981). The exposure occurred because of excessive temperature and pressure in the production process over an extended period (1965–1968) rather than as a consequence of a major release at a single time. More than 80 workers were affected, but the researchers provided little information about those who were not included in the study. Researchers observed several disorders in the workers, including chloracne, metabolic disturbances, abnormal results of glucose-tolerance tests, evidence of a mild hepatic lesion, nervous system focal damage, and psychologic disorders. In a 30-year followup, Pelclová et al. (2001, 2002) examined biochemical, neuropsychologic, neurologic, and lipid-metabolism abnormalities in the surviving Czech cohort. Previous VAO committees concluded that there were methodologic problems of selection bias; lack of control for confounding by educational achievement, tobacco use, or alcohol use; the use of self-reported symptoms; and the lack of an objective measure of exposure. In 2004, Pelclová and colleagues (2007) compared vascular function of 15 exposed workers with that of 14 healthy male health-care workers who had no history of occupational exposure to TCDD. Urban et al. (2007) evaluated the same set of workers, looking at over-all health effects. Further details on those studies were given in Update 2006 and Update 2008.
Pelclová et al. (2009) reported on an update on the exposed cohort that was based on examination and testing of 11 participants in a followup visit in 2008, including internal and neurologic examination, eye fundus examination, TCDD in plasma, thyroid-stimulating hormone, testosterone and serum lipids, ultrasonography of the carotid artery, nerve-conduction study, electroencephalography, visual-evoked potential, Lanthony test of acquired visual impairment, single-photon emission computed tomography of the brain, neuropsychologic examination (eight consented), and carbohydrate-deficient transferrin, an index of long-term alcohol consumption. Mean TCDD concentration remained high (274.0 pg/g of blood lipids), with a wide dispersion (53–756 pg/g) among the 11 participants. Prevalences of health conditions were compared with those in the male population of comparable age. Paired t tests and F tests were used to test for changes in assessments obtained repeatedly during followup visits; Spearman’s rank correlation coefficient was used to test the association between health outcomes (such as color-vision impairment) and risk factors (such as concentrations of TCDD and carbohydrate-deficient transferring). This study has important limitations. With a low retention rate (11 participants of the original cohort of 80), the study findings are vulnerable to nonresponse bias. No description of sample loss was given, even regarding the loss of four participants from the 2004 followup reported in Pelclová et al. (2007). The comparison with the prevalence in the male population of comparable age is important in the interpretation of the study findings, but no description of the comparison group is given beyond citations of its (presumed) sources.
Since Update 2010, Pelclová et al. (2011) have reported on further comparisons of markers of oxidative or nitrosative stress and inflammation in plasma,
urine, and exhaled breath condensate of the 11 exposed workers studied previously in Pelclová et al. (2009) compared with 16 health-care workers (seven men, nine women). This study has similar limitations as in Pelclová et al. (2009). In particular, the mixed-sex comparison group might not be appropriate for the all-male cohort of exposed people.
Studies of Other Industrial Cohorts
Other Chemical Plants
Studies have reviewed health outcomes in UK chemical workers exposed to TCDD as a result of an industrial accident in 1968 (Jennings et al., 1988; May, 1982, 1983), 2,4-D production workers in the former Soviet Union (Bashirov, 1969), 2,4-D and 2,4,5-T production workers in the United States (Poland et al., 1971), white men employed at a US chemical plant that manufactured flavors and fragrances (Thomas, 1987), and US chemical workers engaged in the production of PCP, lower-chlorinated phenols, and esters of chlorophenoxy acids (Hryhorczuk et al., 1998). The long-term immunologic effects of TCDD were examined in 11 industrial workers involved in production and maintenance operations in a German chemical factory that produced 2,4,5-T (Tonn et al., 1996), and immunologic effects were studied in a cohort of workers formerly employed at a German pesticide-producing plant (Jung et al., 1998). VAO, Update 1998, and Update 2000 detailed those studies. Garaj-Vrhovac and Zeljezić (2002) conducted a study of workers occupationally exposed to a complex mixture of pesticides (atrazine, alachlor, cyanazine, 2,4-D, and malathion) during their production.
Waste-Incineration Worker Studies
A study in Japan examined the association between serum-dioxin concentrations (TEQ values for PCDDs, PCDFs, and coplanar polychlorinated biphenyls) and oxidative DNA-damage markers in municipal-waste–incineration workers (Yoshida et al., 2006).
A Korean study evaluated immunologic and reproductive toxicity (DNA damage and sperm quality) in 31 waste-incineration workers and 84 control participants (Oh et al., 2005). Rather than measuring serum dioxin, both studies inferred dioxin exposure of individual workers on the basis of dioxin concentrations in air and estimated exposures to polycyclic aromatic hydrocarbons by analyzing two urinary metabolites: 1-hydroxypyrene and 2-naphthol.
No studies of waste-incineration workers relevant to the COIs have been published since Update 2006.
Paper and Pulp Cohorts
Workers in the paper and pulp industry can be exposed to TCDD and other dioxins that can be generated by the bleaching process during the production and treatment of paper and paper products. VAO described mortality studies of pulp and paper-mill workers potentially exposed to TCDD in five mills in Washington, Oregon, and California (Robinson et al., 1986) and in a New Hampshire mill (Henneberger et al., 1989); of vested members of the United Paperworkers International Union (Solet et al., 1989); and of cancer incidence in male papermill workers in Finland (Jappinen and Pukkala, 1991). Rix et al. (1998) studied cancer incidence through 1993 in 11,130 male and 3,232 female workers employed at three Danish paper mills anytime in 1943–1990.
IARC Paper and Pulp Cohort Update 2006 reviewed a collaborative study of cancer mortality (McLean et al., 2006) led by IARC that was composed of cohorts in 11 countries and had followup through 1990 to 1996 (depending on the country). The pooled data included several cohorts that had been evaluated individually in VAO and earlier updates. For departments in each company, industrial-hygiene experts estimated exposure to 27 agents over time. The 60,468 pulp and paper industry workers employed during 1920–1996 were assigned to the “nonvolatile organochlorines” (potential contamination with TCDD assumed) group (58,162) and the “volatile organochlorines” group (60,468). It is unclear how the entire cohort was portioned into apparently overlapping groups for “volatile” and “nonvolatile” organochlorines and these populations were subdivided for analyses into sets that “ever” or “never” had exposure to the chemicals, the “never exposed.”
Sawmill Workers Sawmills use PCP (which has some contamination with dioxins but not the TCDD congener) as a fungicide, so the exposures experienced are more like “herbicide-use” than those encountered in herbicide production or in pulp and paper processes. Workers in sawmills might have been exposed to pentachlorophenates, which are contaminated with higher-chlorinated PCDDs (Cl6–Cl8), or to tetrachlorophenates, which are less contaminated with higher-chlorinated PCDDs. Wood is dipped into those chemical preservatives and then cut and planed in the mills. Most exposure is dermal, but some exposure can occur by inhalation (Hertzmann et al., 1997; Teschke et al., 1994).
McLean et al. (2009) studied serum dioxin concentrations in 94 former sawmill workers in New Zealand who were classified as exposed (71) and non-exposed (23) according to their work history. In addition, the serum-dioxin test results on 23 former sawmill workers in Sawmill Workers Against Poisons (SWAP) were provided for the study. A semiquantitative estimate of exposure intensity was also developed by using a PCP exposure algorithm that incorporated the participants’ job titles and specific work tasks: mixing of PCP solutions,
cleaning sludge, and spraying. Serum concentrations of PCDDs and PCDFs were analyzed; the total TEQ was calculated by using the World Health Organization (WHO) toxic equivalence factors (TEFs) (van den Berg et al., 2006). Mean concentrations in exposed workers were higher than those in the nonexposed: 1,2,3,6,7,8-hexachlorodibenzodioxin, 1,2,3,4,6,7,8-heptachlorodibenzodioxin, and octachlorodibenzodioxin concentrations were 2–3 times higher and the WHO TEQs about 40% higher (13.67 pg/g vs 9.56 pg/g). The congener profiles in serum were consistent with those in PCP solutions, and dioxin concentrations increased with both employment duration and estimated exposure intensity. The averages in the SWAP members were 2–3 times those in the exposed study participants (37.74 pg/g).
Studies of Herbicide-Using Workers
Various methods have been used to estimate occupational exposure of agricultural workers to herbicides or TCDD. The simplest method derives data from death certificates, cancer registries, or hospital records (Burmeister, 1981), in which information on “usual occupation” is used to construe likely exposure to the COIs. Although such information is relatively easy to obtain, it does not provide information on duration or intensity of exposure, and it cannot even be used to determine whether a worker was exposed to a specific agent. In some studies of agricultural workers, examination of differences between occupational practices has allowed identification of subsets of workers who were likely to have had higher exposures (Hansen et al., 1992; Musicco et al., 1988; Ronco et al., 1992; Vineis et al., 1986; Wiklund, 1983; Wiklund and Holm, 1986; Wiklund et al., 1988a). In other studies, county of residence was used as a surrogate for exposure, and agricultural censuses of farm production and chemical use were relied on for characterizing exposure in individual counties (Blair and White, 1985; Cantor, 1982; Gordon and Shy, 1981), exposure was estimated on the basis of the number of years of employment in a specific occupation as a surrogate for exposure duration, or information on herbicide use at each farm was used as a surrogate of its operator’s exposure (Morrison et al., 1992; Wigle et al., 1990). Still others used self-reported information on exposure that recounted direct handling of a herbicide, whether it was applied by tractor or hand-held sprayer, and what types of protective equipment or safety precautions were used (Hoar et al., 1986; Zahm et al., 1990). A set of studies validated self-reported information with written records, signed statements, or telephone interviews with co-workers or former employers (Carmelli et al., 1981; Woods and Polissar, 1989).
Forestry and other outdoor workers, such as highway-maintenance workers, are also likely to have been exposed to herbicides and other chemicals. Exposure of those groups has been classified by using approaches similar to those noted above for agricultural workers, for example, by using the number of years employed, job category, and occupational title.
American Herbicide-User Studies
Agricultural Health Study
The US Agricultural Health Study (AHS) is a prospective investigation of cohorts of private pesticide applicators (farmers), their spouses, and commercial pesticide applicators in Iowa and North Carolina, with a total of 89,658 participants, including 57,311 applicators (82% of those seeking licensing) and 32,347 spouses (75% of all spouses). The applicators are predominantly but not exclusively male, and the spouses are predominantly but not exclusively female. The AHS is sponsored by the National Cancer Institute (NCI), the Environmental Protection Agency, and the National Institute of Environmental Health Sciences. Enrollment in the study was offered to applicants for applicator certification in Iowa and North Carolina. The project’s website (www.aghealth.org) provides many details about the study, including specification of which pesticides were the subject of information gathered from the enrollment forms and mailed questionnaires (Alavanja et al., 1994).
In phase I (1993–1997), the enrollment form for both commercial (8.6%) and private (largely farmers) applicators asked for the details of use of 22 pesticides (10 herbicides, including 2,4-D; nine insecticides; two fungicides; and one fumigant) and yes–no responses as to whether 28 other pesticides (eight herbicides, including 2,4,5-T and Silvex, 2,4,5-TP; 13 insecticides; four fungicides; and three fumigants) had ever been used.
A subset of 24,034 applicators also completed and mailed back a take-home questionnaire. The questionnaire asked for details about use of the 28 pesticides with yes–no information on the enrollment form and for yes–no responses as to whether 108 other pesticides (34 herbicides, including organic arsenic, which would cover cacodylic acid; 36 insecticides; 29 fungicides; and nine fumigants) had ever been “frequently” used. Dosemeci et al. (2002) published an algorithm designed to characterize personal exposures of that population. Weighting factors for key exposure variables were developed from the literature on pesticide exposure. This quantitative approach has the potential to improve the accuracy of exposure classification for the cohort but has not yet been used in published epidemiologic studies.
The response rate for the take-home questionnaire, 42%, is rather low. Although no pronounced differences in demographics, medical histories, or farming practices were found between those who completed and did not complete the questionnaire (Tarone et al., 1997), selection bias might compromise the validity of studies based on the questionnaire because of differences that might not have been captured in the enrollment form.
Phase II was a 5-year followup conducted in 1999–2003. Computer-assisted telephone interviews (CATIs) were completed by 60,138 participants. The interviews specified “pesticides” in general to include herbicides. They asked about
specific pesticides on individual crops; for several crops, only if atrazine or 2,4-D was specified was a participant asked whether it had been used alone or as part of the manufacturer’s mixture. A full pesticide list was not posted on the website with the followup questionnaire. In addition, dietary histories were completed by 35,164 respondents, and buccal-cell samples were gathered from 34,810 participants. The rate of response to the phase II survey—67% overall and 63% of the original cohort of 55,748 male applicators—is modest and leaves some room for selection bias to compromise the validity of studies based on the survey. In phase III (2005–2010), responses to an updated CATI were provided by 43,426 participants.
Numerous reports on the AHS cohort have been considered in earlier updates. All have developed pesticide-exposure estimates or exposure categories from self-administered questionnaires. Using various subsets of the study population, they have addressed a variety of health outcomes: doctor visits resulting from pesticide exposure (Alavanja et al., 1998), chemical predictors of wheeze (Hoppin et al., 2002), prostate cancer incidence (Alavanja et al., 2003, 2005), lung cancer incidence (Alavanja et al., 2004), reproductive effects (Farr et al., 2004, 2006), cancer risk in the 21,375 children of pesticide appliers born in 1975 or later (Flower et al., 2004), mortality (Blair et al., 2005a), morbidity (Alavanja et al., 2005; Blair et al., 2005b), rheumatoid arthritis (De Roos et al., 2005a), breast-cancer incidence (Engel et al., 2005), neurotoxicity of chronic exposure to modest amounts of pesticides (Kamel et al., 2005), and prevalence of wheeze (Hoppin et al., 2006a). Three additional publications have discussed pesticide-use patterns in the population (Hoppin, 2005, Hoppin et al., 2006b; Kirrane et al., 2004; Samanic et al., 2005). The AHS questionnaire collected detailed information regarding herbicide use; 2,4-D was the most commonly reported herbicide. Kamel et al. (2007a) evaluated questionnaire responses from more than 18,000 AHS participants, who listed a variety of neurologic symptoms, including memory and concentration problems. Another study by Kamel et al. (2007b) evaluated Parkinson disease (PD) in participants in the AHS. Lee WJ et al. (2007) analyzed incident colorectal cancers diagnosed in AHS participants in 1993–2005. Associations with self-reported exposures to 50 pesticides (including 2,4-D, 2,4,5-T, and 2,4,5-TP) were studied. Samanic et al. (2006) reported on the incidence of all cancers combined and selected individual cancers in male pesticide applicators in the AHS particularly with respect to reported exposures to the benzoic acid herbicide dicamba (3,6-dichloro-2-methoxybenzoic acid). Dicamba was used in combination with other herbicides, such as 2,4-D and Agent Orange. Montgomery et al. (2008) discussed the relationship between self-reported incident diabetes and pesticide and herbicide exposure in 31,787 licensed pesticide applicators and their spouses. Saldana et al. (2007) reported on the cross-sectional relationship between pesticide and herbicide exposure and a history of gestational diabetes in the wives of licensed applicators. Of 11,273 women asked about their pregnancies closest to enrollment, 506 (4.5%) reported gestational diabetes. Hop-
pin et al. (2006c) evaluated participants who experienced wheeze, Hoppin et al. (2007a) evaluated farmer’s lung (hypersensitivity pneumonitis), Hoppin et al. (2007b) and Valcin et al. (2007) evaluated chronic bronchitis, and Hoppin et al. (2008) evaluated atopic and nonatopic asthma in women.
Andreotti et al. (2009) conducted a case-control analysis of pancreatic cancer in participants who completed the enrollment form (93 incident cases in 64 applicators and 29 spouses and 82,503 cancer-free controls). Ever use of 24 chemicals and intensity-weighted lifetime days—(lifetime exposure days) × (exposure intensity score)—of 13 chemicals were assessed. Risk estimates were calculated by using unconditional logistic regression for various exposures and controlling for age, smoking, and diabetes.
Hoppin et al. (2009) reported on pesticide use and 127 cases of allergic and 314 cases of nonallergic adult-onset asthma in 19,704 male private applicators at least 20 years old in the AHS who completed both the enrollment form and the take-home questionnaire, with full information on smoking, asthma history, age, BMI, and high pesticide-exposure events. The researchers excluded 487 female applicators with 19 cases of asthma because of the small sample. Logistic regression was used to evaluate the association between farming exposures and adult-onset asthma, allowing for separate associations with allergic and nonallergic asthma and adjusting for age, state (Iowa or North Carolina), smoking status (current, past, or never), and BMI. For each of 48 pesticides, exposure status was specified as ever use vs never use. Further exposure–response analyses were conducted with a three-level specification for exposure—never used, median use or less, and greater than median use—according to the distribution for intensity-adjusted days of use for the specific pesticide. As noted previously, the findings from this study might be vulnerable to selection bias because of the low response rate (42%) for the take-home survey.
Mills et al. (2009) reported on the association between lifetime use of 49 pesticides and the incidence of and mortality from myocardial infarction (MI) in the AHS cohort: 476 deaths in 54,069 male participants who completed the enrollment form, and 839 nonfatal events in 32,024 male participants who completed the phase II telephone interview. Deaths from MI, as either a primary or a contributing cause, were recorded from state and national death records starting at enrollment and going through December 31, 2006. The incidence of nonfatal MI was determined on the basis of a positive response on the 5-year followup questionnaire to the question “Has a doctor or other health professional ever told you that you had a heart attack (or myocardial infarction)?” First MIs that occurred after enrollment were counted as incident MIs. Separate analyses for mortality and incidence were conducted by using Cox regression and adjusting for state (Iowa or North Carolina), age, and smoking status (whether or not the participant had smoked 100 cigarettes in his or her lifetime). The incidence analysis also adjusted for BMI. The analyses were conducted for each pesticide specified as ever used and as lifetime days of exposure. As noted previously, the validity of the findings
for the incidence analysis might be compromised because of the modest rate of response to the phase II survey—63% according to the committee’s calculation (35,088 respondents of 55,748 in the original cohort), reported as 70% in Mills et al. (2009). In particular, for incidence analyses reported in Mills et al. (2009), this survey is vulnerable to selection bias because of left truncation, that is, missing participants who died before the survey.
Goldner et al. (2010) examined the association between organochlorine exposure and thyroid disease in 19,529 female spouses in the AHS. The analysis was limited to female spouses of private applicators who completed both the take-home survey in phase I (pesticide use) and the followup interview in phase II (thyroid disease) and had complete data on all covariates. Thyroid-disease status (none in 14,486, hyperthyroidism in 369, hypothyroidism in 1,114, and other in 560) was ascertained from self-reported history of physician diagnoses obtained during phase II interviews. Logistic regression was used to estimate the association between use of herbicides (including 2,4-D and 2,4,5-T) and insecticides and thyroid-disease status (with no disease as the reference group) with adjustment for education, age, smoking (never, past, or current), BMI, and hormone-replacement therapy (ever or never). As noted previously, the findings from this study might be vulnerable to selection bias because of the low overall rate of response to the combination of the take-home survey and the followup interview.
Dennis et al. (2010) reported on 150 cases of cutaneous melanoma diagnosed after enrollment in the AHS of pesticide applicators who completed both the enrollment form and the take-home questionnaire during phase I, excluding 24,704 who had a cancer diagnosis before enrollment. Cases were identified through linkage to cancer registries, state death registries, and the National Death Index with a cutoff date of December 31, 2005. Dichotomous measures (ever or never used) were used for arsenic pesticides (lead arsenate and inorganic and organic arsenic). Categorical measures (no, low, or high) based on intensity-weighted lifetime days of exposure were used for other chemicals, including 2,4-D, 2,4,5-T, and 2,4,5-TP. Unconditional logistic regression was used to estimate the association between melanoma and exposure with adjustment for age, sex, and other variables “as indicated” (apparently selection through an unspecified variable selection procedure), including sun exposure, tendency to burn, red hair, and BMI.
Thomas et al. (2010) reported on a monitoring study of 2,4-D and chlorpyrifos exposures in a sample of AHS participants. For 69 2,4-D applicators, geometric mean values were 7.8 and 25 mg/L in preapplication and postapplication urine, respectively (p < 0.05 for difference), and 0.37 mg/m3 in personal air. The estimated amounts of dermal absorption through the hands (hand loading) and through total skin surface (body loading) were 0.39 mg and 2.9 mg of 2,4-D, respectively; the readings for individual applicators were correlated across these media. Glove use and the mode of application were found to be associated with the degree of exposure.
Slager et al. (2009) reported on current rhinitis in commercial pesticide
applicators in the AHS (excluding private applicators, such as farmers). Of the 4,916 commercial pesticide applicators in the full AHS cohort, the 2,245 who had provided information on all the variates in the analysis model constituted the sample for this investigation. Current rhinitis was ascertained with the following question in the take-home questionnaire: “During the past 12 months have you had a stuffy, itchy, or runny nose?” Exposure to individual pesticides was specified both as a dichotomous measure (ever vs never in the preceding year) and as a categorical measure (days per year). Logistic regression was used to estimate the association between exposure and current rhinitis, with adjustment for age, education, and having grown up on a farm. As noted previously, the findings from this study might be vulnerable to selection bias because of the low rate of response to the take-home survey (46% by commercial applicators, slightly higher by the entire AHS cohort).
Crawford et al. (2008) reported on hearing loss in white male licensed pesticide applicators in the AHS, considering the hypothesis that some pesticides are neurotoxic and could potentially affect hearing. The study sample consisted of participants who completed the enrollment form and the take-home questionnaire during phase I and the followup telephone interview in phase II. Hearing loss was ascertained with the following question in the phase II interview: “Do you have trouble with your hearing in one or both ears (this is without a hearing aid)?” Potential cases of hearing loss attributable to a congenital condition or to infection or injury (determined by responses to survey questions) were excluded. The analysis also excluded participants who reported never using pesticides and excluded nonwhite and female respondents. Of 16,246 participants who completed all three surveys, 14,229 were retained in the final analysis sample. Logistic regression was used to estimate the associatons between exposure and hearing loss with adjustment for state, age, and exposures to noise, solvents, and metals. The overall low rate of response (less than 30%) to the combination of the three surveys raises concerns about the validity of the study findings. The authors argued that there were too few nonwhites and females (1.5% of eligible participants) for analysis. Although it might be reasonable to consider those participants to be too few to be analyzed as subgroups, it is unclear why they needed to be excluded from the main analysis. (Limited analysis for nonwhites is mentioned in the discussion.)
Although health outcome results in the whole cohort or entire subgroups are not fully relevant for the COIs and might be regarded as of marginal interest to more recent VAO committees, Blair et al. (2005b) have reported that 2,4-D is the pesticide most frequently used by the Iowa farmers and is often used by the rest of the applicators. Consequently, the results on relative rates of individual conditions seem comparable in exposure specificity with findings in production cohorts in which not all the workers included were necessarily exposed to the COIs and may have had additional toxic exposures. Therefore, the findings on mortality from enrollment through 2000 (Blair et al., 2005a) and on cancer incidence
through 2002 (Alavanja et al., 2005) have been retained in the results tables for health outcomes. Accordingly, the committee for this update has added mortality findings through 2007 on various causes of death (Waggoner et al., 2011) and comparisons with state populations for cancer incidence updated through 2006 (Koutros et al., 2010a) to the health-outcomes results tables. Conventional SMRs and SIRs were calculated adjusted for age, calendar year, race, sex, and state. In an effort to compensate for the pronounced healthy-worker effect evident in the AHS cohorts, both Waggoner et al. (2011) and Koutros et al. (2010a) also calculated, in addition to conventional SMRs and SIRs, “relative” counterparts of these statistics—rSMR and rSIR, respectively. To obtain the relative rates, the standardized ratio for incidence or mortality was divided by the standardized ratio for all causes excluding it. Because even the usual SMRs and SIRs from these non-pesticide specific findings on the entire cohort are minimally informative, the committee opted not to consider the relative versions.
Waggoner et al. (2011) reported 4,880 deaths in the applicators (private and commercial) and 1,539 in the spouses, significantly fewer than expected for both (SMR = 0.54, 95% CI 0.52–0.55; and SMR = 0.52, 95% CI 0.50–0.5). Similarly, deaths from all types of cancer were significantly lower than the state rates in both applicators (SIR = 0.61, 95% CI 0.58–0.864) and spouses (SIR = 0.65, 95% CI 0.60–0.70). Koutros et al. (2010a) found 4,316 cancer cases in the private applicators, 219 in the commercial applicators, and 1,896 in the spouses. Findings on the commercial applicators were set aside, and the cancer incidence rates in both the private applicators (SIR = 0.85, 95% CI 0.83–0.88) and the spouses (SIR = 8.2, 95% CI 0.79–0.86) were again significantly lower than expected.
Several recent AHS publications (Andreotti et al., 2012; Barry et al., 2011, 2012; Koutros et al., 2010b, 2011) reported on a nested case-control substudy that examined the relationship of pesticide exposure (including herbicides of interest, such as 2,4-D and 2,4,5-T) and of genetic markers with the risk of prostate cancer. All men eligible for inclusion in the study were white applicators who had not had any cancer other than non-melanoma skin cancer before enrollment in the AHS and had provided a buccal-cell sample. Two controls matched on age to each case had to have been alive at the time of the case’s diagnosis. The final study sample consisted of 776 prostate-cancer cases diagnosed in 1993–2004 and 1,444 controls. Although the primary focus of this substudy was on the interaction between pesticide exposure and genetic markers (how pesticide exposure modified the association between genetic markers and prostate cancer), some useful information about the association between exposure to particular pesticides and prostate cancer can still be gleaned as a by-product of the interaction analyses. Intensity-weighted lifetime exposure days are used in Andreotti et al. (2012) and Barry et al. (2011, 2012). Genotyping for an array of 26,512 single-nucleotide polymorphisms (SNPs) in 1,291 candidate genes was performed at the NCI’s Core Genotype Facility. Unconditional logistic regression was used to estimate odds ratios and 95% confidence intervals for the associations between prostate
cancer and the main effect for pesticide exposure, the main effect for genetic markers, and the interaction between pesticide exposure and genetic markers, adjusted for age and state. Only Koutros et al. (2011), who reported the findings for this substudy on 1,913 SNPs in 149 candidate genes known to play a role in the metabolism of xenobiotic substrates, also adjusted for family history of prostate cancer and provided main effects for exposure to individual pesticides and the incidence of prostate cancer. Koutros et al. (2010b) reported the findings on 211 SNPs in the 8q24 region known to be associated with prostate cancer. Andreotti et al. (2012) reported the findings on 220 SNPs in 59 genes involved in lipid metabolism. Barry et al. (2011) reported the findings on 394 SNPs in 31 base-excision repair genes involved in repairing oxidative DNA damage that are hypothesized to be possibly important for populations exposed to pesticides or other putative oxidative stress–inducing agents. Barry et al. (2012) reported findings on 324 SNPs in 27 necleotide excision repair (NER) genes thought to be important in repairing damages induced by putative prostate carcinogens. The false discovery rate (Benjamini and Hochberg, 1995) method is used to account for multiple comparisons involving a large number of pesticides and genetic markers.
Tanner et al. (2011) conducted a case-control study of PD in AHS participants. Suspect cases (170) were identified from self-reports and state mortality files and confirmed (115; 110 with pesticide data included in study) by a neurologist during home visits. Potential controls (644) were sampled randomly from the AHS cohort and frequency-matched about 3:1 to cases by age, sex, and state. Controls were confirmed (383; 358 with pesticide data included in study) by a neurologist or a neurologist-trained technician during home visits. CATIs were used to obtain detailed information on use, since the age of 14 years, of 31 selected pesticides expected to be possibly associated with PD (oxidative stressors and mitochondrial inhibitors), and key covariate information, including smoking and family history of PD. Participant characteristics were compared between cases and controls by using Fisher’s exact test or Pearson’s chi-square test for categorical variables and Wilcoxon’s rank-sum test for continuous variables. Logistic regression was used for pesticides reported by at least 10 participants, controlling for potential confounding factors, including age, sex, state, and cigarette-smoking (ever or never).
Several recent AHS studies examined a variety of exposure issues. Blair et al. (2011) examined the effect of exposure misclassification, which is likely to occur when self-reported exposure assessment is used, on the relative risks estimated in the AHS and showed substantial attenuation toward the null. Similar results are likely for other studies that use self-reported exposure status. Coble et al. (2011) reported on an updated version of an estimation algorithm for pesticide exposure intensity, developed previously in Dosemeci et al. (2002) for the AHS, to incorporate new data obtained in two exposure-monitoring studies to modify the weighting factors used in the algorithm. Payne et al. (2012) conducted a Cox
proportional-hazards regression to assess the risk posed by high pesticide exposure in the AHS cohort.
California United Farm Workers of America Study
Mills and Yang (2005) and Mills et al. (2005) analyzed lymphohematopoietic cancer and breast cancer, respectively, in nested case-control studies of Hispanic workers drawn from a cohort of 139,000 Californians who were members of the United Farm Workers of America (UFW). Estimates of exposure to specific pesticides, including 2,4-D, were developed through linkage of the union’s job histories with the California Pesticide Use Reporting Database of the state’s Department of Pesticide Regulation, which has records of all agricultural applications of pesticides in the state since 1970. Vital status and cancer incidence were ascertained through a probabilistic record linkage to the California Cancer Registry for the period 1988–2001. Mills and Yang (2007) conducted a nested case-control gastric cancer study embedded in the UFW cohort and identified cases of gastic cancer newly diagnosed in 1988–2003.
No reports relevant to the COIs have been published on the California UFW population since Update 2008.
Other US Studies of Agricultural Workers
Studies of proportionate mortality were conducted in Iowa farmers (Burmeister, 1981) and male and female farmers in 23 states (Blair et al., 1993). Mandel et al. (2005) reported results of urinary biomonitoring of farm families in Minnesota and South Carolina as a part of CropLife America’s Farm Family Exposure Study. Curwin et al. (2005) measured 2,4-D concentrations in urine and hand-wipe samples to characterize exposures of farmers and nonfarmers in Iowa.
Studies in Other Countries
Australian Herbicide-User Studies
Fritschi et al. (2005) used CATIs and occupational histories reviewed by an industrial hygienist to estimate exposures to phenoxy herbicides in an Australian study.
Canadian Herbicide-User Studies
Ontario Farm Family Health Study The Ontario Farm Family Health Study (OFFHS) has produced several reports on exposure to phenoxyacetic acid herbicides, including 2,4-D. A study of male pesticide exposure and pregnancy outcome (Savitz et al., 1997) developed an exposure metric based on self-reports
of mixing or application of crop herbicides, crop insecticides, and fungicides; livestock chemicals; yard herbicides; and building pesticides. Study participants were asked whether they participated in those activities during each month, and their exposure classifications were based on activities in 3-month periods. Exposure classification was refined with answers to questions about use of protective equipment and specificity of pesticide use.
A related study included analysis of 2,4-D residues in semen as a biologic marker of exposure (Arbuckle et al., 1999a). The study began with 773 potential participants, but only 215 eventually consented to participation. Of the 215, 97 provided semen and urine samples for 2,4-D analysis.
The OFFHS also examined pregnancy outcomes of stillbirth, gestational age, and birth weight (Savitz et al., 1997) and the effects of exposure to pesticides, including 2,4-D, on time to pregnancy (Curtis et al., 1999) and on the risk of spontaneous abortion (Arbuckle et al., 1999b, 2001). About 2,000 farm couples participated in the study. Exposure information was pooled from interviews with husbands and wives to construct a history of monthly agricultural and residential pesticide use. Exposure classification was based on a yes–no response for each month. Data on such variables as acreage sprayed and use of protective equipment were collected but were not available in all cases. Other studies have used herbicide biomonitoring in a subset of the population to evaluate the validity of self-reported predictors of exposure (Arbuckle et al., 2002). Assuming that the presence of 2,4-D in urine was an accurate measure of exposure and that the results of the questionnaire indicating 2,4-D use were more likely to be subject to exposure-classification error (that is, assuming that the questionnaire results were less accurate than the results of urinalysis), the questionnaire’s prediction of exposure, compared with the urinary 2,4-D concentrations, had a sensitivity of 57% and a specificity of 86%. In multivariate models, pesticide formulation, protective clothing and gear, application equipment, handling practice, and personal-hygiene practice were valuable as predictors of urinary herbicide concentrations in the first 24 hours after application was initiated.
Urinary concentrations of 2,4-D and MCPA were measured in samples from farm applicators (Arbuckle et al., 2005) and from women who lived on Ontario farms (Arbuckle and Ritter, 2005). Indirect sources of herbicide exposure of farm families were evaluated through wipe sampling of surfaces and drinking-water samples (Arbuckle et al., 2006). Weselak et al. (2008) examined occupational exposures and birth defects in the offspring of OFFHS participants. Spouses completed questionnaires that requested the history of pesticide use on the farm. Pregnancies resulting in birth defects were reported by the female study participants. All birth defects were combined for study analyses, and exposure was examined by pesticide class, family, and active ingredient for two 3-month periods—before and after conception.
No reports on the OFFHS relevant to the COIs have been published since Update 2008.
Canadian Farm Operator Study The Canadian Farm Operator Study assembled a cohort of 156,242 male farmers from 1971 Canadian census data on the provinces of Manitoba, Saskatchewan, and Alberta and linked to the national mortality database to identify deaths occurring during June 1971–December 1987. The cohort was also matched to the Central Farm Registers for 1966, 1976, 1981, and 1986 to gather information on reported exposures and farm practices. Information on the amount of acreage on each farm sprayed in 1970 with herbicides (without product specificity) was used as surrogate for its operator’s exposure in determining the risk of specific causes of death: NHL (Morrison et al., 1994; Wigle et al., 1990), prostate cancer (Morrison et al., 1993), brain cancer (Morrison et al., 1992), multiple myeloma (MM) (Semenciw et al., 1993), and leukemia (Semenciw et al., 1994). In the one-third sample that completed the census long form, people most likely to have been exposed (no employees or custom expenses reported) could be identified. The age at which years at risk began to accumulate for each person varied for the various causes of death assessed, and this resulted in different numbers of eligible subjects. No reports on relevant health outcomes have been published on participants in this study population since Update 1996.
Other Canadian Studies of Agricultural and Forestry Workers Faustini et al. (1996) evaluated the immune, neurobehavioral, and lung function of residents in an agricultural area of Saskatchewan, Canada, and focused on immunologic changes in 10 farmers who mixed and applied commercial formulations that contained chlorophenoxy herbicides. Studies have been conducted in forestry workers potentially exposed to the types of herbicides used in Vietnam. A cohort mortality study examined men employed by a Canadian public utility (Green, 1987, 1991). Senthilselvan et al. (1992) investigated asthma’s relationship to pesticide use with self-reported data gathered in a cross-sectional servey completed by 1,939 of the 2,375 male farmers approached in Saskatchewan. Mortality and reproductive effects have been studied in British Columbia sawmill workers potentially exposed to chlorophenate wood preservatives used as fungicides (Dimich-Ward et al., 1996; Heacock et al., 1998; Hertzman et al., 1997); PCP, which would be a frequently used fungicide, is expected to have dioxin and furan contamination, but the 2,3,7,8-TCDD congener is unlikely to have been present.
Danish Herbicide-User Studies
Records of the Danish Union of General Workers for 10 trade unions of gardeners were used to identify 3,156 male members on May 1, 1975; similarly, 859 women were identified. The workers were known to be highly exposed to pesticides. Most of the women worked in greenhouses, where herbicides are not routinely used; for the men, however, exposure was mainly to herbicides, which included the phenoxy herbicides 2,4-D, 2,4,5-T, and MCPA. Matching of union
records to the Danish Central Population Registry permitted establishment of the vital status of the entire cohort through 1984 for determination of person-years at risk; this provided for a latent period of 10–15 years by starting accumulation when people reached the age of 30 years. Using the Danish Cancer Registry, Hansen et al. (1992) determined cancer incidence in this cohort of Danish gardeners from 1975 to 1984 compared with the general Danish population and adjusted for age, sex, and calendar period.
Hansen et al. (2007) used analogous methods to extend the followup period for the men through 2001. The updated information was analyzed by using year of birth as a surrogate for intensity of exposure, with high exposure assumed for those born before 1915, low exposure for those born in 1934 or later, and intermediate exposure for those born in between.
Drawing from the same cohort of Danish gardeners and further stipulating that people were alive and living in Denmark at the beginning of 1977, Kenborg et al. (2012) established a cohort of 3,124 men who were monitored in the Danish Hospital Register for hospitalization for PD as a primary diagnosis during 1977–2008 and compared the results with the observed incidence of PD in all Danish men by calendar period and age group. Revisiting the Danish Cancer Registry, they also investigated the incidence of lung, larynx, and bladder cancers, which are recognized as smoking-related. The incidence of those cancers was compared by age and calendar period with the incidence in the general male Danish population, and the rates were used as a proxy for smoking frequency in the cohort. The birth cohorts defined by Hansen et al. (2007) were used again to stratify degree of exposure.
Ronco et al. (1992) studied mortality in Danish farmers. The utility of the findings was limited by their being largely unanalyzed products of linking the country’s cancer registry with census records to garner information on recent occupation.
Dutch Herbicide-User Studies
A Dutch study of forestry workers exposed to 2,4,5-T investigated the prevalence of acne and hepatic dysfunction (van Houdt et al., 1983). No reports on forestry workers have been published since 2000.
Mortality from cancer and other causes in Dutch male herbicide applicators has been studied by Swaen et al. (1992, 2004).
Finnish Herbicide-User Studies
Asp et al. (1994) conducted a followup through 1989 on mortality and cancer morbidity in Finnish men who had applied 2,4-D and 2,4,5-T for at least 2 weeks in 1955–1971. This group of 1,971 was assembled in 1972 from records of the four Finnish employers primarily responsible for brush removal and assessed for
mortality through 1980 (Riihimaki et al., 1982) and for cancer morbidity (Riihimaki et al., 1983) through 1978.
German Herbicide-User Studies
Barthel (1981) studied cancer incidence and overall mortality through 1970–1978 in 1,658 male agricultural plant-protection workers in the former German Democratic Republic who spent a portion of at least 5 years in 1948–1972 applying pesticides. Unlike most of the many pesticides thought to have contributed to the exposure of these workers, the phenoxy herbicides were available for use throughout this period. It was not known, however, which individuals used the the COIs, so exposure characterization was not as specific as current VAO committees require for results to be considered fully relevant. Among the cancers, only lung cancer had a large enough number of cases to permit analysis by the amount and time period of pesticide use.
Iceland Herbicide-User Studies
Using the national cancer and death registries, Zhong and Rafnsson (1996) determined cancer incidence from entry into a pesticide using occupation through 1993 for 2,449 mean and women in Iceland. A listing of the amount of specific pesticides sold for agricultural use in Iceland between 1976 and 1993 ranked was led by 2,4-D, but it was not characterized to which of these individual subjects had been exposed, so the results of this study do not constitute fully relevant evidence according to the criteria of recent VAO committees.
Italian Herbicide-User Studies
Ronco et al. (1992) also studied the incidence of specific types of cancer in Italian farmers. The utility of the findings was limited by their being the largely unanalyzed products of linking the country’s cancer registry with census records to garner information on recent occupation.
Cancer mortality in a cohort of rice growers in the Novara Province of northern Italy was investigated by Gambini et al. (1997).
A cohort of male farmers in Italy’s southern Piedmont region who were licensed to use agricultural pesticides in 1970–1974 was established. The use of phenoxy herbicides in the area was reported to be twice the national average. Corrao et al. (1989) evaluated cancer incidence in 25,945 on the basis of new diagnoses from hospital admissions in 1976–1983. In a continuation of that study, Torchio et al. (1994) reported on mortality through 1986 in the 23,401 who were residents of the Piedmont area at the time of registration; cause of death was abstracted from death certificates. The cohort was partitioned into people who lived near arable land, those who lived near woodlands, and those who lived near
mixed-use land; separate results were reported for the first two groups. No reports on this cohort have been published since 1994.
New Zealand Herbicide-User Studies
A study evaluated cancer incidence in a group of New Zealand forestry workers (Reif et al., 1989). No reports on forestry workers have been published since 2000. ’t Mannetje et al. (2005) evaluated a study population that included herbicide production workers and was a subcohort of the IARC cohort.
Norwegian Herbicide-User Studies
Kristensen et al. (1997) tested whether cancers or birth defects were increased in the offspring of Norwegian farmers who worked on farms with pesticide use documented by agricultural censuses.
South American Herbicide-User Studies
Lerda and Rizzi (1991) studied the incidence of sperm abnormalities in Argentinian farmers. The utility of the findings was limited by their being the largely unanalyzed products of linking each country’s cancer registry with census records to garner information on recent occupation.
Swedish Herbicide-User Studies
The Swedish Cancer-Environment Register (CER) linked the cancer cases entered in the Swedish Cancer Registry with the records of people who responded to the 1960 and 1970 national censuses, which had obtained data on current occupation. The resulting database has been used in studies that evaluated cancer mortality and farm work (Wiklund, 1983); STS and malignant lymphoma in agricultural and forestry workers (Wiklund and Holm, 1986; Wiklund et al., 1988a); and the risk of NHL, HL, and MM in relation to occupational activities (Eriksson et al., 1992). No new studies using the Swedish CER that are relevant to the COIs have been published since the original VAO report.
Cancer mortality in Swedish railroad workers has been studied (Axelson and Sundell, 1974; Axelson et al., 1980). Another study examined mortality and cancer incidence in a cohort of Swedish lumberjacks (Thörn et al., 2000). Cancer in Swedish pesticide and herbicide applicators has been studied repeatedly (Dich and Wiklund, 1998; Wiklund et al., 1987, 1988b, 1989a,b).
Other Studies of Workers Using Herbicides
Other studies of the agricultural use of pesticides have not provided specific information on exposure to 2,4-D, TCDD, or other compounds relevant to Vietnam veterans’ exposure (Bell et al., 2001a,b; Chiu et al., 2004; Duell et al., 2001; Garry et al., 2003; Gorell et al., 2004; Hanke et al., 2003; van Wijngaarden et al., 2003).
A series of papers from a workshop focused on methods of assessing pesticide exposure in farmworker populations (Arcury et al., 2006; Barr et al., 2006a,b; Hoppin et al., 2006b; Quandt et al., 2006). They provide a helpful review of current methodologic issues in exposure science for those populations but do not address the COIs directly.
Industrial accidents have led to the evaluation of long-term health effects in neighboring nonworker populations of exposure to fairly high concentrations of the COIs. Effects on residents around normally performing industrial operations, such as waste incinerators, and even on people exposed only to “background” concentrations have also been studied. Because the systematic followup studies that have been conducted on the Seveso population and the numerous analyses of the large database generated by the continuing US National Health and Nutrition Examination Survey (NHANES) have contributed so prominently to the evidence base considered by VAO committees, this section opens with discussions of these two study populations. Other environmental studies follow alphabetically by country.
People’s environmental exposures to dioxin-like chemicals and their non–dioxin-like counterparts are to mixtures of components that tend to correlate, so it is not surprising that specific chemicals measured in a person’s serum also tend to correlate; this collinearity means that it will be difficult for epidemiologic studies to attribute any observed association to a particular chemical configuration (Longnecker and Michalek, 2000). Analyses in terms of TEQs circumvent that problem to some extent.
Seveso, Italy
A large industrial accident that resulted in environmental exposure to TCDD was caused by an uncontrolled reaction during TCP production in Seveso, Italy, on July 10, 1976. The degree of TCDD contamination in the soil has been used extensively as a means of imputing exposures of members of the population. Three areas were defined on the basis of soil sampling: Zone A (556 people), the most heavily contaminated, from which all residents were permanently evacuated within 20 days; Zone B (3,920), an area of lower contamination that all children
and women in the first trimester of pregnancy were urged to avoid during daytime; and Zone R (26,227), a region with some contamination in which consumption of local crops was prohibited (Bertazzi et al., 1989a,b). The sample sizes differ among followup studies, presumably because of migration; the sample sizes given above were reported in Bertazzi et al. (1989b).
Cohort of Entire Exposed Population
Data on serum TCDD concentrations in Zone A residents have been presented by Mocarelli et al. (1990, 1991) and by CDC (1988d). In the 10 who had severe chloracne, TCDD concentrations were 828–56,000 ppt of lipid weight. In 10 without chloracne, TCDD concentrations were 1,770–10,400 ppt. TCDD was undetectable in all control participants but one. The highest of the concentrations exceeded any that had been estimated at the time for TCDD-exposed workers on the basis of backward extrapolation and a half-life of 7 years. Data on nearby soil concentrations, number of days that a person stayed in Zone A, and whether local food was consumed were considered in evaluating TCDD. That none of those data correlated with serum TCDD suggested strongly that the important exposure was from fallout on the day of the accident. The presence and degree of chloracne did correlate with TCDD. Adults seemed much less likely than children to develop chloracne after acute exposure, but surveillance bias could have affected that finding. Recent updates (Bertazzi et al., 1998, 2001) have not changed the exposure-assessment approach.
A number of studies of the Seveso population have used lipid-adjusted serum TCDD concentrations as the primary exposure metric (Baccarelli et al., 2002; Eskenazi et al., 2002a,b, 2003a, 2004; Landi et al., 2003). Fattore et al. (2003) measured current air concentrations of PCDDs in Zones A and B and compared them with measurements in a control area near Milan. The authors concluded that release from PCDD-contaminated soil did not add appreciably to air concentrations in the Seveso study area. Finally, Weiss et al. (2003) collected breast milk from 12 mothers in Seveso to compare TCDD concentrations with those in a control population near Milan. The investigators reported that the TCDD concentrations in human milk from mothers in Seveso were twice as high as those in controls. The authors concluded that breastfed children in the Seveso area were likely to have higher body burdens of TCDD than children in other areas.
Several cohort studies have been conducted on the basis of the exposure categories. Seveso residents have had long-term followup of their health outcomes, especially cancer. Bertazzi and colleagues conducted 10-year mortality followup studies of adults and children who were 1–19 years old at the time of the accident (Bertazzi et al., 1989a,b, 1992), 15-year followup studies (Bertazzi et al., 1997, 1998), and a 20-year followup study (Bertazzi et al., 2001). Pesatori et al. (1998) also conducted a 15-year followup study to update noncancer mortality. Consonni et al. (2008) reported on the 25-year followup (through 2001) vital
status of residents (“present”) in the Seveso area and reference territory at the time of the Seveso accident and of immigrants and newborns (“non-present”) in the 10 years thereafter. Cause-specific mortality was determined for each zone, compared with that in the comparison cohort and adjusted for presence at the accident, sex, period, age, and time since the Seveso accident.
In addition to a 2-year prospective controlled study of workers potentially exposed to TCDD during cleanup of the most highly contaminated areas after the accident (Assennato et al., 1989a), studies have examined specific health effects associated with TCDD exposure in Seveso residents—chloracne, birth defects, and spontaneous abortion—and crude birth and death rates (Bisanti et al., 1980); the distribution of chloracne in Seveso children (Caramaschi et al., 1981); chemicals in the blood and urine of children who had chloracne (Mocarelli et al. 1986); chloracne and peripheral nervous system conditions (Barbieri et al., 1988); dermatologic and laboratory tests in a group of the children who had chloracne and in a group of controls (Assennato et al., 1989b); health status and TCDD concentrations in chloracne cases and noncases recruited previously by Landi et al. (1997, 1998) and followed by Baccarelli et al. (2005a); hepatic-enzyme–associated conditions (Ideo et al., 1982, 1985); abnormal pregnancy outcomes (Mastroiacovo et al., 1988); cytogenetic abnormalities in maternal and fetal tissues (Tenchini et al., 1983); neurologic disorders (Boeri et al., 1978; Filippini et al., 1981); cancer (Bertazzi et al., 1993; Pesatori et al., 1992, 1993); sex ratio of offspring who were born in Zone A (Mocarelli et al., 1996); immunologic effects (Baccarelli et al., 2002); aryl hydrocarbon receptor–dependent (AHR-dependent) pathway and toxic effects of TCDD in humans (Baccarelli et al., 2004); effects of TCDD-mediated alterations in the AHR-dependent pathway in people who lived in Zones A and B (Landi et al., 2003); and NHL-related t(14;18) translocation prevalence and frequency in dioxin-exposed healthy people in Seveso (Baccarelli et al., 2006). Baccarelli et al. (2005b) reviewed statistical strategies for handling nondetectable readings or readings near the detection limit in dioxin-measurement datasets. They recommended that a distribution-based multiple-imputation method be used to analyze environmental data when substantial proportions of observations have nondetectable readings.
Baccarelli et al. (2008) reported on crude sex ratios, birth weight, and neonatal thyroid function for all births in 1994–2005 to women who were less than 18 years old at the time of the Seveso accident. Mocarelli et al. (2008) investigated TCDD’s effects on reproductive hormones and sperm quality in a comparison of 135 young men exposed to TCDD by the 1976 Seveso accident with 184 age-matched healthy men who lived outside the contamination zones. Both groups were divided into three categories that reflected their ages at the time of the Seveso accident: infancy to prepuberty (1–9 years), puberty (10–17 years), and adulthood (18–26 years).
Pesatori et al. (2008) investigated the incidence of pituitary tumors in the Seveso population (804 in Zone A, 5,941 in Zone B, and 38,624 in Zone R)
compared with the reference population in the surrounding, noncontaminated area (232,745). The hospital discharge-registration system of the Lombardy Region (where the study area is) was used to identify incident cases of pituitary adenoma during 1976–1996. All relevant medical records were reviewed to confirm the diagnosis for each case. Risk ratios and 95% confidence intervals were estimated by using Poisson regression and adjusting for age, sex, and calendar period and an assumed 10-year latent period for dioxin effects. Pesatori et al. (2009) reported on cancer incidence in a 20-year followup of the Seveso cohort covering the period 1977–1996. The study included all participants 0–74 years old who lived in the study area (723 in Zone A, 4,821 in Zone B, 31,643 in Zone R, and 181,574 in the reference zone) at the time of the accident. Participants who moved outside the study area were traced with a success rate of over 99% (Consonni et al., 2008). Emigration was homogeneous among zones and ranged from 4.7% to 6.7%. The difference in exposure among zones was corroborated by soil TCDD measurements, serum concentrations of TCDD, and TEQs. In the absence of a regionwide cancer registry, incident cancer cases were ascertained from the 120-hospital network of the Lombardy region, where the study area is. Original medical records were examined to identify true cases, to retrieve diagnoses as accurately as possible, and to determine the dates of occurrence. The study covered malignant tumors at any site and benign tumors of liver, bladder, and central nervous system first diagnosed after the date of the accident. For cohort members who were not hospitalized or who emigrated outside Lombardy, cancer cases were identified solely from death certificates, so nonfatal incident cases were missed. Risk ratios and 95% confidence intervals for Zones A, B, and R vs the reference zone were derived by using Poisson regression and adjusting for sex, age, and period.
Since Update 2010, Mocarelli et al. (2011) have reported on the sperm quality and hormone concentrations of sons born from March 1977 to January 1984 to women exposed to dioxin in Seveso (78 invited, 39 participated) compared with men of similar age and socioeconomic status whose mothers did not live in the dioxin-contaminated areas and were recruited from healthy volunteer permanent blood donors (123 invited, 58 participated). The exposed group was exposed both in utero (39) and parinatally through breastfeeding (21). Mothers’ serum TCDD concentrations were measured by using serum samples collected in 1976–1977 and kept frozen since and extrapolated to the time of conception. The outcome measures included sperm concentration, total count, progressive motility, and total motility count based on semen samples and follicle-stimulating hormone concentration based on fasting blood sample. A general linear model was used to analyze sperm and hormone data—including exposure group, lactation class, and group × lactation interaction—adjusted for age, days in abstinence, smoking, chemical exposures, BMI, alcohol use, education level, and employment status. Scale transformations were taken on the outcome measures to achieve approximate normal distribution and homoscedasticity. Although the study was
carefully designed and implemented, the low response rate raises concerns about possible selection bias.
Seveso Women’s Health Study
The Seveso Women’s Health Study (SWHS) was undertaken to evaluate the association between individual serum TCDD concentrations and reproductive effects in women who resided in Seveso at the time of the 1976 accident. From a pool of 1,271 eligible women who were between infancy and 40 years old at the time of the accident, who had resided in Zone A or B, and for whom adequate serum remained from the samples collected shortly after the explosion, 981 were enrolled in the study group in 1996–1998. The fairly adequate 80% participation rate resulted from 17 women being lost to followup, 21 having died, 12 being seriously ill, and almost 250 refusing. All the women were interviewed by a nurse blinded as to their exposure status, and a subset received gynecologic examinations. Medical records of those who reported ever having received a diagnosis of cancer were obtained and subjected to blind review by a pathologist. The stored samples were used for new TCDD analyses with improved analytic techniques that became available in recent years.
As an initial step in the SWHS, Eskenazi et al. (2001) tested the validity of exposure classification by zone. Investigators measured serum TCDD in samples collected in 1976–1980 from 601 residents (97 in Zone A and 504 in Zone B). A questionnaire that the women completed in 1996–1998 included age, chloracne history, animal mortality in the vicinity, consumption of homegrown food, and location at the time of the explosion. Participants did not know their TCDD concentrations at the time of the interview, but most knew their zones of residence. Interviewers and TCDD analysts were blinded to participants’ zones of residence. Zone of residence explained 24% of the variability in serum TCDD. Addition of the questionnaire data improved the regression model, explaining 42% of the variability. Those findings demonstrate a significant association between zone of residence and serum TCDD, but much of the variability in TCDD concentration is still unexplained by the models. Warner et al. (2005) compared a chemical-activated luciferase-gene expression bioassay with an isotope-dilution high-resolution gas-chromatography-high-resolution mass-spectrometry assay to measure PCDDs, PCDFs, and polychlorinated biphenyls (PCBs) in serum of 78 women who resided near Seveso to determine average total dioxin-like chemical TEQs; similar results were obtained with the two methods.
The women enrolled in the SWHS were assessed for cancer incidence during the 20 years after the accident (Warner et al., 2002). A pathologist blinded as to exposure status reviewed medical records of the 21 women who reported in their initial interview (conducted between March 1996 and July1998) ever having received a cancer diagnosis; 15 of these diagnoses were for breast cancer, so analysis was limited to all cancers and to this cancer type. The remaining six can-
cers consisted of three cases of thyroid cancer, a melanoma, a kidney cancer, and an unspecified tumor. For each woman, the earliest post-accident blood samples with at least 0.5 mL remaining were analyzed for TCDD. The resulting readings were back-extrapolated to the time of the 1976 explosion, assuming a 9-year half-life, as derived from data obtained on Vietnam veterans in the AFHS (Pirkle et al., 1989). Cox estimation of hazard ratios (HRs) was conducted by using those values and the women’s ages at diagnosis or when they were interviewed for the controls, and a test for trend was conducted over four exposure categories with partitions at 10, 20, and 44 TCDD ppt; the results for both tests were marginally significant for breast cancer (p = 0.05 and p = 0.07, respectively) and slightly weaker for all cancers. A broad spectrum of possible confounders was assessed, but they had to be tested individually in the model. The small number of cases observed was a consequence of the cohort’s being relatively small (981) and young at the time of interview (72% less than 50 years old).
Warner et al. (2011) added more than 10 years of observation on cancer incidence in the women in the SWHS, updating the borderline significant results for breast cancer published earlier (Warner et al., 2002) to cover the period from the 1976 explosion through 2009. Of the 981 women participating in the earlier study, 833 were located, alive, and willing to participate. They all were reinterviewed, provided clinical measurements, and allowed access to medical records for confirmation; a subset was given bone-density tests. The average age was now 50.8 years. In the update, an additional 45 cancers had been diagnosed, making the total 66 cases, of which 33 were breast cancers. Thyroid cancer was the next most prevalent, with seven cases, and the 15 other types of cancer observed had at most three cases. After adjustment for age at the time of the accident and for marital status, the risk of any cancer in association with lipid-adjusted, log-transformed serum TCDD concentrations at the time of the accident was distinctly elevated (HR = 1.86; 95% CI 1.29–2.52). It was a small cohort, so the analyses that could be conducted were curtailed, but the availability of serum TCDD concentrations measured from blood samples gathered fairly soon after the single-substance accident (which minimizes uncertainty about what exposure had been experienced and reduces the need for back-extrapolation) contributes substantially to the value of the results.
A series of studies have examined associations between serum TCDD and a variety of endpoints related to female reproductive functioning: menstrual cycle (Eskenazi et al., 2002a); endometriosis (Eskenazi et al., 2002b); pregnancy outcome (Eskenazi et al., 2003a); age at exposure to the accident (Eskenazi et al., 2004); age at menarche and age at menopause (Eskenazi et al., 2005); and age at menarche in women who were premenarcheal at the time of the explosion (Warner et al., 2004). Eskenazi et al. (2007) and Warner et al. (2007) examined the incidence of fibroids and ovarian function, respectively, in SWHS participants. Eskanazi et al. (2007) excluded women who had received a diagnosis of fibroids before 1976, leaving a total of 956 women for analysis. Fibroids were
ascertained in 634 women by self-report, medical records, and ultrasonography. Analyses were adjusted for confounding by parity, family history of fibroids, age at menarche, current BMI, smoking, alcohol consumption, and education. Warner et al. (2007) studied menstrual function in SWHS participants who were 20–40 years old and not taking oral contraceptives; the evaluations included ultrasonography (96 women), serum hormone concentrations (87 women), and the occurrence of ovulation (203 women).
Eskenazi et al. (2010) examined the relationship between serum TCDD around the time of the accident and time to pregnancy (TTP) in 472 SWHS participants who had attempted pregnancy since the accident. In addition to other eligibility criteria for SWHS, participants were eligible for the study if they were no more than 40 years old at the time of the accident. Nine women were excluded because of fertility-related problems, leaving 463 eligible women in the analysis sample. The main analysis was restricted to the 278 women who delivered live births that were not the results of contraceptive failure. Alternative analyses included various subsamples excluded in the main analysis. TTP for the first postaccident pregnancy was determined from responses in interviews conducted in 1996–1998 to the question “How many months did it take to become pregnant? In other words, for how many months had you been having sexual intercourse without doing anything to prevent pregnancy?” Women whose TTP was 12 months or more were classified as infertile. Initial serum TCDD concentrations at the time of the accident were measured in stored samples from 444 participants (431 collected in 1976–1977 and 13 collected in 1978–1981). For 19 participants with insufficient stored samples, new samples were collected in 1996 or 1997. For the 27 women with detectable post–1977 TCDD measurements, TCDD was back-extrapolated to 1976 by using the Filser model (Kreuzer et al., 1997). Initial serum TCDD concentrations were extrapolated to the time when each woman initiated her attempt to become pregnant; Kreuzer et al. (1997) used a toxicokinetic model for women 16 years old or younger at the time of the accident, and Pirkle et al. (1989) used a first-order kinetic model that assumed a 9-year half-life. The association between serum TCDD and TTP was assessed by using a Cox proportional-hazards model to estimate the fecundability odds ratios (ORs) and 95% confidence intervals. The association between serum TCDD and infertility was assessed by using multiple logistic regression. Both models were adjusted for maternal age, maternal smoking in the year before conception, parity, menstrual-cycle irregularity, oral-contraceptive use in the year before attempt, paternal age near the time of conception, and history of reproductive and endocrine conditions, including pelvic infection and thyroid or urogenital problems. A variety of sensitivity analyses were conducted to investigate the consistency of study findings and to check for possible bias. Initial serum TCDD and extrapolated serum TCDD were specified as continuous variables on the logarithmic scale and as categorical variables.
US Environmental Studies
National Health and Nutrition Examination Survey
In the early 1960s, the CDC National Center for Health Statistics began the NHANES program as a means of monitoring and assessing the health and nutritional status of people of all ages living in the United States. In 1999, the survey became a continuous program that has a changing focus on a variety of health and nutrition measurements to meet emerging needs. A rich variety of data—demographic and socioeconomic data; dietary information; medical, dental, and physiologic assessments; and serum concentrations of persistent organic pollutants (POPs), including specific congeners of dioxins, furans, and PCBs—are collected through in-person interviews, health examinations, and blood samples obtained from a nationally representative sample of adults and children in the noninstitutionalized US population. Information obtained from NHANES data is used to determine prevalences of diseases, to assess nutritional status, and to establish national standards of height, weight, and blood pressure. Researchers also conduct analyses of the NHANES data for epidemiologic studies and health-science research on serum concentrations of various compounds in association with various health outcomes.
NHANES data from 1999–2002 were used to evaluate cardiovascular disease (Ha et al., 2007) and hypertension (Everett et al., 2008a,b). Lee DH et al. (2006, 2007a,b,c) used data from the same years to evaluate several health outcomes, including diabetes, the metabolic syndrome, insulin resistance, and arthritis. Turyk et al. (2007) analyzed NHANES data from 1999–2002 and 2001-2002 to evaluate associations with thyroid-hormone concentrations. Since Update 2008, several new publications have used NHANES data in reporting on associations between the COIs and various health outcomes.
Lee et al. (2008) examined the associations between serum concentrations of POPs and the prevalence of peripheral neuropathy and poor glycemic control (A1C ≥ 7.0%) in NHANES 1999–2002 participants who were at least 40 years old and had diabetes or impaired fasting glucose. Peripheral neuropathy is ascertained on the basis of one or more insensate sites on the foot. Diabetes is ascertained on the basis of high plasma glucose (≥ 126 mg/dL fasting or ≥ 200 mg/dL nonfasting) or on the basis of whether a person is taking insulin or an oral antidiabetes agent. Although 49 POPs were measured, analysis was restricted to 25, of which at least 60% of study participants had detectable concentrations: three PCDDs, four PCDFs, five dioxin-like PCBs, seven non-dioxin-like PCBs, and six organochlorine (OC) pesticides. Logistic regression was used to determine the OR between each outcome (peripheral neuropathy or poor glycemic control) and each exposure to POP subclass with adjustment for age, sex, race or ethnicity, poverty, duration of diabetes, hypertension (yes or no), BMI, cigarette-smoking (never, former, or current), cotinine concentration, alcohol consumption, leisure-
time physical activity (vigorous, moderate, or none), and A1C (neuropathy only). For each POP subclass, a cumulative measure was derived by summing the rank scores among individual chemicals that belonged to the subclass; the cumulative measure was then categorized into tertiles. Additional analyses were conducted for individual compounds by using the correlation coefficient between the rank score for each chemical and each outcome with adjustment for the same covariates listed above.
Ha et al. (2009) examined the association between serum concentrations of POPs and the prevalence of newly diagnosed hypertension in NHANES 1999–2002 adult participants 40 years old or older. After exclusion of 444 patients known to be hypertensive irrespective of antihypertensive medication, 165 diabetic patients, and 49 subjects whose blood-pressure values were missing, the final sample size was 524. Participants were considered to have hypertension if their systolic blood pressure was 140 mm Hg or higher or if their diastolic blood pressure was 90 mm Hg or higher. The analysis was restricted to 21 POPs of which at least 60% of study participants had detectable concentrations: three PCDDs, three PCDFs, five dioxin-like PCBs, six non-dioxin-like PCBs, and four OC pesticides. The discrepancy from Lee et al. (2008) in the number of POPs detected is probably due to the difference in the samples used. For each POP, participants whose serum concentrations were below the limit of detection were regarded as the reference group; participants who had detectable concentrations were categorized into quartiles. A cumulative measure for each POP subclass was derived by summing the category numbers (0 for nondetectable, 1 for detectable below the first quartile, and so on up to 4 for above the third quartile) of individual chemicals belonging to the subclass. The summary values were again categorized into quartiles. Logistic regression was used to derive adjusted ORs, which were stratified by sex and adjusted for age, race or ethnicity, poverty-income ratio, BMI, cigarette-smoking (never, former, or current), cotinine, alcohol consumption, and leisure-time physical activity (vigorous, moderate, or none).
Schreinemachers (2010) examined the association in healthy adults between exposure to 2,4-D, as indicated by its presence in urine, and biomarkers that are linked to the pathogenesis of acute MI and type 2 diabetes, namely, serum high-density lipoprotein (HDL), triglycerides, total cholesterol minus HDL, insulin, C-peptide, plasma glucose, and thyroid-stimulating hormone. Study participants, 20-59 years old, were selected from a subset of the NHANES III (1988–1994) sample. The study sample is regarded as a convenient sample rather than a representative sample of the US population because volunteers were recruited without a formal statistical sampling procedure. Among 1,338 candidate participants for the study, 375 were excluded because data on urinary 2,4-D were missing, and 236 were excluded on the basis of study exclusion criteria: history of congestive heart failure, heart attack, diabetes, thyroid disease, lupus, or cancer; a white blood cell count over 12 × 109 per liter, C-reactive protein over 10 mg/dL, or glycosylated hemoglobin (HbA1c) over 8%. Among the remaining 727 study
participants, urinary 2,4-D was detectable in 102 (14%), with concentrations of 1-28 mg/dL. The outcome variables were compared between participants with and without detectable urinary 2,4-D by using Wilcoxon’s rank-sum test. Further analysis was conducted with linear regression, and the outcome variables were transformed to a logarithmic scale. The linear-regression models included the following explanatory variables: 2,4-D (binary), HDL (continuous, log-transformed, and included in all models except when HDL itself was the dependent variable), urinary creatinine (continuous and log-transformed), sex, age, BMI, race or ethnicity, and smoking (none, past, and active). Alcohol consumption, education, household income, and hours of fasting before a blood sample was drawn were also checked for their effects on the regression coefficient for urinary 2,4-D. The analyses were conducted on the final study sample of 727 and on two subsamples that were expected to be more susceptible: participants who had HbA1c above the median (5.1%) of the total sample and participants who had thyroxine at or below the median (8.5 μg/dL) of the total sample.
Since Update 2010, several new NHANES studies have been published. Cho et al. (2011) reported on the associations between bone mineral density (BMD) and exposures to POPs, including OC pesticides, assessed from serum samples from NHANES participants in 1999–2004 (2,769 for OC pesticide analyses and 2,565 for POP analyses). The study also examined whether the POP levels modified the association between BMD and fat mass or lean mass. All analyses were stratified by sex and age group (cutoff 50 years). General linear models were used to derive adjusted means, adjusted for age, race or ethnicity, poverty-income ratio, fat mass, lean mass, height, smoking, physical activity, and postmenopausal hormone intake.
Elobeid et al. (2010) examined the association between POPs and obesity—BMI and waist circumference (WC)—in NHANES 1999–2002 participants (2,464 for BMI analysis and 2,448 for WC). Regression models were used to assess the association between the obesity measures and POPs, adjusted for sex, ethnicity, age, and age squared. An additional model for WC also adjusts for BMI.
Jones et al. (2011) examined the association between urinary arsenic and hypertension and blood pressure in NHANES 2003-2008 participants (4,167). Logistic-regression and linear-regression models were used for hypertension and blood pressure, respectively, adjusted for age, sex, race or ethnicity, urinary creatinine, education, BMI, and serum cotinine; models for blood pressure also adjusted for antihypertensive medication. All analyses accounted for the complex sample design for NHANES.
Anniston, Alabama, Community Health Survey In 2003, the Agency for Toxic Substances and Disease Registry (ATSDR) funded a study of the health effects of environmental exposures on the residents of Anniston, Alabama. Anniston housed a plant that produced PCBs from 1929 to 1971; it had been owned and operated by Monsanto since 1935. Residents of Anniston were known to have
high concentrations of PCBs although these have not been found to be associated with employment in the plant or consumption of local fish and produce. PCBs have spread in Anniston via air, soil, and water movement. Before the ATSDR study, there had been minimal study of the health effects of PCBs on Anniston residents. Residents were recruited into the study through a stratified random sampling of housing units across the city weighted by proximity to the plant and by race. Of the 1,110 who agreed to be interviewed, 772 had blood drawn for biochemical and PCB analyses and had blood pressure measured. Of the 758 subjects who provided sufficient data to be retained in the study, 364 were taking antihypertensive medications and 394 were not.
Two methods were considered for PCB analysis: wet-weight values and lipid-standardized values. The latter are thought to be more prone to bias, but both were considered. Having two approaches makes comparisons between studies difficult. Another issue was the problem of PCB values that were below the level of detection (LOD). They amount to left-censored covariates in a regression model. They were dealt with by crude imputation of the LOD divided by the square root of 2 (Goncharov et al., 2010; Silverstone et al., 2012) or by 2 (Goncharov et al., 2011). This is highly biased, and alternative methods (such as multiple imputation) would have been preferable. It is also potentially problematic that the studies took different approaches.
Goncharov et al. (2010) studied the association between all PCBs and blood pressure. After adjustment for age there was a significant association between PCB concentrations and risk of hypertension. When people on antihypertensive medications were included in the models, the associations between PCB concentrations and risk of hypertension were diluted; this could be due to an effect of the medications on PCB metabolism.
Goncharov et al. (2011) extended the 2010 report by considering the effect of PCBs on the entire range of continuous blood pressure and by investigating 5 PCB groups and 33 individual PCB congeners. Only the 394 people who were not on antihypertensive medications were included in this study. The study found that serum PCB concentration is associated with blood pressure even in the normotensive range. Furthermore, the relationship was found to be strongest for ortho-substituted PCB congeners that have two or more chlorines and with some that have some dioxin-like activity. The overall concentrations of PCBs are higher in the Anniston population than in the US population overall.
Silverstone et al. (2012) examined the association between PCB exposure and diabetes in the Anniston study. Diabetes was present in 27% of 774 participants; 75% of the 27 were taking glycemic control medications. People who had prediabetes were identified and were excluded from some regression analyses because they were intermediate between the diabetic and normoglycemic groups. There was a nonmonotonic increase in the prevalence of diabetes with high PCB congener concentrations, but some of the groups (for example, by age) were small. Women had a consistently higher likelihood of diabetes in each subset of
PCBs (except the estrogenic subset). The findings suggest a low-dose PCB effect inasmuch as the ORs increased in the second quintile of PCB exposure and remained high. Advantages of this study are its adjustment for family history of diabetes and exclusion of people who had prediabetes. Lipid metabolism in people who have diabetes may affect the metabolism of PCBs.
Center for the Health Assessment of Mothers and Children of Salinas Cohort Castorina et al. (2010) compared metabolites of current-use pesticides and other precursor compounds in 538 women in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) cohort with those in 342 pregnant women in NHANES 1999–2002. CHAMACOS (Eskenazi et al., 2003b) is a longitudinal birth-cohort study investigating the effect of in utero and postnatal environmental exposures on the health of children who live in the Salinas Valley of Monterey County, California. The study enrolled 601 pregnant women from September 1999 to November 2000 in six prenatal clinics in the largely agricultural Salinas area. Women were eligible if they were no more than 20 weeks into gestation, were at least 18 years old, were qualified to receive poverty-based government health insurance, and planned to continue receiving prenatal care in a participating clinic. Personal interviews were conducted during which information on demographics, household characteristics, health, and occupations of CHAMACOS participants was collected. Two interviews were conducted shortly after enrollment (mean, 13 weeks of gestation; SD, 5.2 weeks) and later in the second trimester (mean, 26 weeks of gestation; SD, 2.6 weeks) by bilingual (English and Spanish), bicultural study staff. At each prenatal interview, spot urine samples were collected from CHAMACOS participants and analyzed for metabolites, including organophosphorus, OC chemicals, pyrethroid pesticides, herbicides, and ethylene bisdithiocarbamate fungicides. Adequate urine samples with valid creatinine concentrations were collected from 538 (90%) of the 601 participants at the first sampling point and 481 (80%) at the second. In addition, pesticide-use data were extracted from the California-pesticide use reporting dataset and geocoded into square-mile units. NHANES reported concentrations of current-use pesticide metabolites measured in spot urine collected from representative samples of the US population stratified by age, sex, and racial or ethnic group (Barr et al., 2005; CDC, 2004). The NHANES comparison group consisted of 342 pregnant women 15-50 years old, a subset of the 3,048 US residents 6-59 years old who had metabolite concentrations measured in urine samples during NHANES testing in 1999 and 2002. The public-release versions of the NHANES data-sets, including demographic information and metabolite data, were used for the analyses. No sample weights were applied to the NHANES data. Descriptive analyses were conducted on the CHAMACOS and NHANES cohorts. Metabolite concentrations were compared between the two cohorts with a Wilcoxon rank-sum test and quantile regression at the 95th percentile adjusted for demographic variables, including age, current smoking (yes or no), ethnicity,
and socioeconomic status. Analysis of variance was used to compare differences in detection frequency.
Coronary Artery Risk Development in Young Adults Study Lee et al. (2010) reported on the association between low-dose POPs and type 2 diabetes in the Coronary Artery Risk Development in Young Adults (CARDIA) cohort. Serum samples were collected at year 2 (1987–1988) and were later used to measure POPs. This was a nested case-control study in which subjects were required to be free of diabetes at years 0 and 2. From 1988 to 2006, 116 received a diagnosis of diabetes; 90 were randomly selected as cases. Controls were randomly selected from those who had not received a diagnosis of diabetes. Cases and controls were frequency-matched on BMI strata. The authors analyzed summary measures of POPs. The second summary measure selected POPs on the basis of their effect in the current study; this invalidates standard inference. The authors asserted that summary measures are useful because appropriate controls are those with globally low concentrations of POPs. Other limitations are the single measure of POPs and the age of the samples at analysis (18 years). The authors stated that the study was underpowered and that extensive statistical testing was performed.
Lee et al. (2011a) further investigated the 90 controls selected for the nested case-control study reported in Lee et al. (2010). This investigation is concerned with associations between exposure to POPs and obesity, dyslipidemia, and insulin resistance in people who are free of diabetes. Multiple comparisons are of concern, as is the small sample size.
Great Lakes Fish Consumption Study The Great Lakes Fish Consumption Study was initiated in early 1992. Bloom et al. (2006) measured serum dioxin in New York sport fishermen as part of a study of thyroid function. A methodologic study by Petreas et al. (2004) found generally high correlations between concentrations of dioxins and related chemicals in breast and abdominal fat in the same woman; this suggested that they could be used interchangeably in epidemiologic studies. The same study, however, also found that adjusting concentrations according to lipid content rather than weight of the fat samples is important because of the presence of nonlipid components in the samples.
In 2001–2005, 1,788 of the 4,200 people who had participated in the original study were contacted and asked for updated information on health, reproductive history, and fish consumption. Blood samples gathered from 515 of them were analyzed for serum concentrations of various POPs, including a number of PCBs. Turyk et al. (2009) investigated whether the serum results were related to self-reported diabetes. Lambertino et al. (2011) studied the self-reported occurrences of uterine leiomyomas (benign fibroid tumors) in 580 women using the serum results from the 197 women who had provided blood samples. The exposure measure for the category “dioxin-like PCBs” consisted of the summed concentrations of only the mono-ortho PCBs 118 and 167. Because results were based
solely on mono-ortho PCBs, which typically contribute only a small percentage to total TEQs, the findings of these publications cannot be considered conclusive.
Pensacola, Florida Karouna-Renier et al. (2007) examined health effects related to dioxins and furans in soil at a Superfund site in Pensacola, Florida, that was contaminated by operations at a wood-treating company that operated from 1942 to 1982. In 2001, the study collected health and exposure histories and measured serum concentrations of 17 PCDD and PCDF congeners in 47 potentially exposed people who were selected nonsystematically from among former workers, their families, and residents. Logistic regression was used to predict the prevalence of health outcomes from TEQs with adjustment for age, race, sex, BMI, tobacco and alcohol use, and worker status.
Times Beach and Quail Run Cohorts
Several reports have provided information on environmental exposure to TCDD in the Times Beach area of Missouri (Andrews et al., 1989; Patterson et al., 1986), one of the incidents that heightened concerns about the health effects of dioxin. In 1971, TCDD-contaminated sludge from a hexachlorophene-production facility was mixed with waste oil and sprayed in various areas for dust control. Soil contamination in some samples exceeded 100 parts per billion. Among the Missouri sites with the highest soil TCDD concentrations was the Quail Run mobile-home park. Residents were considered exposed if they had lived in the park for at least 6 months during the time when contamination occurred (Hoffman et al., 1986).
Of 51 exposed participants, 87% had adipose-tissue TCDD concentrations below 200 ppt; however, TCDD concentrations in seven of the 51 were 250–750 ppt. In 128 nonexposed control participants, adipose-tissue TCDD ranged from undetectable to 20 ppt (median, 6 ppt). On the basis of a 7-year half-life, it is calculated that two study participants would have had adipose-tissue TCDD near 3,000 ppt at the time of their last exposure (Andrews et al., 1989).
Several studies evaluated health effects potentially attributable to exposure (Evans et al., 1988; Hoffman et al., 1986; Stehr et al., 1986; Stehr-Green et al., 1987; Stockbauer et al., 1988; Webb et al., 1987). Those studies were reviewed in VAO; no further work on the cohorts has been published.
Danish Environmental Studies
Halldorsson et al. (2009) studied the association between consumption of fatty fish, as a source of environmental exposure to dioxins and dioxin-like chemicals, and birth weight and development in 100 healthy pregnant women 25–35 years old selected from the Danish National Birth Cohort, which includes 101,046 women (Olsen et al., 2001). The 9,815 eligible women were stratified ac-
cording to the frequency of fatty-fish intake (low, zero meals per month; medium, one to three; and high, over three); 34, 33, and 33 were randomly sampled in three strata, respectively. Four standardized CATIs (at gestation weeks 12 and 30 and at 6 and 18 months postpartum) were used to collect information on parental lifestyle and health. Participants received a food-frequency questionnaire in week 25 of gestation, and two maternal blood samples were collected during routine visits to a general practitioner. The blood samples were analyzed for CALUX-TEQs in picograms per gram of lipid. Birth outcomes (weight, length, and head circumference) based on measurements taken by the midwives who attended the births were extracted from the Danish National Birth Registry. Developmental milestones (such as sitting without support and crawling) were obtained from the telephone interviews conducted when the children were 5.7–7 months old. A total-development scale was derived by summing the indicators of the 13 milestones. Linear mixed models (with the multiple plasma samples specified as an individual-level random effect) were used to estimate the association between CALUX-TEQ and birth weight with adjustment for gestational age, infant sex, and maternal smoking. Logistic regression was used for the association between CALUX-TEQ (dichotomized into high and low relative to the sample median) and infant development milestones with adjustment for gestational age, duration of breastfeeding, infant age at interview, and maternal fish intake. Spearman rank correlation was used for the association between CALUX-TEQ and the total-development scale.
Finnish Environmental Studies
Turunen et al. (2008) studied mortality in 6,410 fishermen and their 4,260 wives in Finland in comparison with national mortality figures (standardized by sex, age, and period), assuming that the difference in mortality reflects the high consumption of contaminated fish by fishermen and their wives. A small subsample (88 fishermen and 94 wives) participated in a substudy of fish consumption and life habit and provided blood samples that were analyzed for nutrients and environmental contaminants, including dioxins and PCBs. The substudy found higher fish consumption and higher serum dioxins and PCBs in fishermen and their wives than in the general population studied in the 2000 health survey. However, the validity of the findings of the mortality study is limited by various types of confounding, including possible health benefits of fish consumption by fishermen and their wives and a possible healthy-worker effect in the cases of fishermen.
French Environmental Studies
Viel et al. (2000) reported on an investigation of apparent clusters of cases of STS and NHL in the vicinity of a municipal solid-waste incinerator (MSWI) in
Doubs, France. The presumptive source of TCDD in the region is an MSWI in the Besançon electoral ward in western Doubs. Dioxin emissions from the incinerator were measured in international TEQ units at 16.3 ng/m3, far in excess of the European Union (EU) standard of 0.1 ng/m3. TCDD concentrations in cow’s milk measured on three farms near the incinerator were well below the EU guideline of 6 ng/kg of fat, but the concentrations were highest on the farm closest to the incinerator. Floret et al. (2003) examined the same population and investigated rates of NHL in Besançon, France. Cases were identified from a cancer registry of people who had a diagnosis of NHL in 1980–1995. Viel et al. (2008a) examined the same population and reported a case-control study conducted in 434 women who had breast cancer compared with 2,170 community controls selected according to the proximity of their residence to emissions from the waste incinerator.
Viel et al. (2008b) expanded the previous work and studied the association between NHL and dioxin exposure from MSWIs in four French administrative departments (Isère, Bas-Rhin, Haut-Rhin, and Tarn), which were covered by a population-based cancer registry. (The study did not include the area of previous studies, Doubs, which is a separate administrative department.) The study was conducted with geostatistical analysis at the level of block groups and compared exposures and outcomes in the 2,270 block groups in the area. The block groups had an average surface area of 9.45 km2. The cases considered for this study were in people 15 years old and older who had received a diagnosis of NHL during the period 1990–1999 and were living in the study area at the time of their diagnosis. Anonymous data were extracted from cancer registries on date of birth, sex, date of diagnosis, address at the time of diagnosis, and cancer category. The block group for each case was geocoded by using the residential address.
A second-generation Gaussian atmospheric-dispersion model (ADMS 3) was used to derive “immission” estimates (defined by the researchers as “the amount of pollutant reaching a particular location as a result of—and in contrast to—the emission coming out the chimney”) for dioxins, metals, and dusts in the area near each of 13 MSWIs operating in the study area. That involved a receptor grid of 200 m that was based on emission estimates for the MSWI, plant characteristics (chimney height and diameter, emission temperature, particle size, and density), topography indicators (roughness and relief), local meteorologic conditions, and so on. For each of the 2,270 block groups, the median of all immission estimates for receptors in the block group was used as the immission for the block group. For block groups under the plumes of multiple MSWIs, the sum of the immission estimates was used. A cumulative ground-level dioxin concentration estimate was derived for each block group by using the immission estimates transformed to account for the number of years that the plant had operated and the degradation rate in the soil. Poisson regression was applied at the block-group level to assess the association between observed number of NHL cases in each block group and the dioxin concentration (with a square-root transformation) estimated for the
block group and adjusted for population density, urbanization, socioeconomic level, airborne traffic pollution, and industrial pollution.
Since Update 2010, Viel et al. (2011) have reported a new case-control study of NHL in a study area consisting of three electoral wards (170,000 people) that contained the Besançon MSWI. Cases (53 eligible, 34 participated) were identified from the local university hospital. Controls (34) were matched 1:1, randomly selected from blood donors living in the area matched on sex, age (± 5 years), and date of blood draw (± 1 year); 5 refusals were replaced. A wide spectrum of OCs was measured in a fasting blood sample drawn from each participant. Exact logistic-regression models were used to assess the association between NHL and exposure measures.
Cordier et al. (2004, 2010) studied the risk of birth defects attributable to environmental dioxins released from MSWIs in the Rhône-Alpes region (Lyon and surrounding areas) in southern France. The studies partially overlapped the areas studied by Viel et al. (2008b): all three studies included the administrative department of Isère.
Cordier et al. (2004) conducted a geostatistical analysis at the level of communities (official municipalities), studying 2,872 communities, each with fewer than 50,000 residents. Birth defects during the study period, 1988–1997, were identified from a population-based birth-defects registry (the French Central-East Registry). There were 70 MSWIs that operated in the study region for at least a year during the study period. Immission scores were derived by using a Gaussian plume model (POLAIR) for dioxin concentrations in kilometer grids within 10 km of the plants and using plant emission estimates, chimney heights, and local meteorologic data. For each community, the immission score at the geographic point with the highest population density was used as the contemporaneous exposure index for the community. (That is a bit different from the usual practice of using the population centroid for the community.) In addition, a cumulative exposure index was derived by multiplying the contemporaneous exposure index by the number of years that the plant was in operation. A total of 194 communities were classified as exposed, and the remaining 2,678 communities as nonexposed. In the exposed communities, only births after the start of the MSWI were considered in the analysis. Poisson regression was used to derive the relative risk of congenital malformations with adjustment for year of birth, maternal age, department of birth, population density, average family income, and (when available) local road traffic.
Cordier et al. (2010) examined the same population with a case-control study in 2001–2003, comparing 304 infants who had urinary tract birth defects with a random sample of 226 population controls that were frequency-matched for infant sex and year and district of birth. Of 353 cases identified in the birth-defects registry, 304 were located, and 187 were interviewed. The modest response rate (53% of all cases, although the authors claimed a higher response rate of 62%, excluding 49 cases not located) may compromise the validity of the study find-
ings. The controls were recruited through CATIs that attempted to reach 3,000 telephone numbers in the region presumed to belong to families with children; this resulted in 226 control participants after 1,989 ineligible candidates were excluded. Exposure estimates for dioxins, furans, and metals in areas near each MSWI (in 100-m grids) were derived by using Gaussian modeling software (ADMS 3) that took into account emissions, plant characteristics (chimney height and diameter, emission temperature and speed, and distribution between gaseous and particulate phases), and local meteorologic conditions. Participants were classified as exposed or nonexposed; those exposed were further classified into above or below the median. Multiple logistic regression was used to estimate the association between dioxin exposure and urinary tract birth defects, with adjustment for stratification variables (child’s sex and year and district of birth). Potential confounders were selected by using backward selection, including community characteristics (population density, deprivation score, and industrial dioxin sources besides MSWIs), maternal age, parental geographic origin, educational level, employment status during pregnancy, treatment for chronic disease during the first trimester, folic acid supplementation, history of urinary tract birth defects in first-degree relatives, parity, obesity, tobacco and alcohol use during pregnancy, and environmental tobacco-smoke exposure.
Japanese Environmental Studies
From 2002 to 2006, Ueruma et al. (2008a,b) assembled a stratified sample of 1,374 Japanese 15–73 years old (627 men and 747 women) who represented urban, farming, and fishing areas of the entire country. The participants completed questionnaires on occupational, medical, smoking, and residential histories and height and weight. They also provided blood samples that were analyzed with isotope-dilution high-resolution gas chromatography-mass spectrometry for PCDDs, PCDFs, and dioxin-like PCBs. Ueruma et al. (2008a) investigated the relationship of those chemicals with the prevalence of diabetes, defined as self-reported physician-diagnosed diabetes or occurrence of plasma HbA1c greater than 6.1% as a predictor of fasting plasma glucose above 126 mg/dL. Ueruma et al. (2008b) presented summary statistics on the serum concentrations of the individual chemicals in the blood of the study participants and on their distributions with respect to various demographic characteristics; they also provided the results of log-transformed correlation analyses of all PCDDs and PCDFs combined, of all dioxin-like PCBs, and of total TEQ with total cholesterol, high-density lipoprotein, and triglycerides.
Uemura et al. (2009) conducted further studies of the same cohort and examined the association of body burdens of dioxins and related chemicals with the prevalence of metabolic syndrome, assessed by using a modification of the National Cholesterol Education Program Adult Treatment Panel III definition (NCEP, 2002) to accommodate differences between Asian and Caucasian populat-
ions (Ko et al., 2005; Tan et al., 2004). In particular, participants were classified as having metabolic syndrome if they satisfied three or more of the following five criteria: BMI of at least 25 kg/m2 (rather than abdominal waist circumference), serum triglycerides of at least 150 mg/dL, serum HDL under 40 mg/dL in men or under 50 mg/dL in women, systolic blood pressure of at least 130 mm Hg or diastolic blood pressure of at least 85 mm Hg or self-reported history of physician-diagnosed hypertension, and HbA1c of at least 5.6% (rather than fasting serum glucose) or self-reported history of physician-diagnosed diabetes. Logistic regression was used to assess the associations between exposures (TEQs for PCDDs, PCDFs, and dioxin-like PCBs and total TEQs) and the prevalence of metabolic syndrome, both adjusted and not adjusted for age, sex, smoking and drinking habits, regional block, residential area, and survey year. The analysis was conducted with and without prevalent diabetes cases. Further analyses were conducted for the adjusted associations of the TEQs with the five components of metabolic syndrome and the adjusted associations of the concentrations of the 16 selected congeners of which more than 75% of the subjects had detectable concentrations with the prevalence of metabolic syndrome.
Yusho Disease Group
Tsukimori et al. (2012) reported on the association between mother’s dioxin exposure and children’s birthweight in Japanese women affected by Yosho disease after an accidental exposure to rice oil contaminated with PCBs, PCDFs, and PCDDs in western Japan in 1968 that affected over 1,900 people. Of 737 affected women officially registered with the Study Group for Yusho, 206 reported having given birth after the Yusho incident. Of them, 101 (with 190 eligible births) had their dioxin concentrations measured and participated in the mother-child study. Maternal serum concentrations of contaminants were assessed, converted into TEQs, and extrapolated to the time of delivery. Multiple linear-regression models were used to examine the association between birth weight and maternal serum contaminant concentrations, log-transformed to account for their lognormal distributions. The models adjusted for potential confounders for birth weight, including maternal age, parity, maternal smoking during pregnancy, gestation age at delivery, infant sex, duration of breast feeding, number of births, and frequency of seafood consumption.
Norwegian Environmental Studies
Stolevik et al. (2011) reported on prenatal exposure to PCBs and increased risk of wheeze, eczema, and infections in newborns in the birth subcohort of the Norwegian Mother and Child Cohort Study. Maternal exposure to PCBs was determined from a validated food-frequency questionnaire that covered the first 4 months of pregnancy and that was adapted to include rarely eaten foods that are
known to have high concentrations of PCBs and dioxins. Pregnant women were invited to enter the study in 2007–2008. Outcomes were determined through a questionnaire sent to the mothers at 1 year. Confounders in the statistical analyses were taken from the questionnaires filled out by the mothers during pregnancy and 6 months after birth. They included previous breastfeeding, parity, history of atopy, age, smoking, education, BMI, child’s sex, and others. Logistic regression with backward selection was used to fit multivariate models. There is concern about selection bias because of the low participation rate (38.5%).
Russian Environmental Studies
Chapaevsk
Several studies in the Samara region of Russia have identified the Middle Volga Chemical Plant (also known as SZVH or Khimprom) in Chapaevsk, about 950 km southeast of Moscow, as a major source of TCDD pollution (Revazova et al., 2001; Revich et al., 2001). From 1967 to 1987, the plant produced γ-hexachlorocyclohexane (lindane) and its derivatives, and many of the workers experienced chloracne. Since then, it has produced various chlorinated products. Dioxins were detected in the small number of air, soil, drinking-water, and cow’s-milk samples gathered in the region, but no description of how these media were sampled was given. When Revich et al. (2001) compared them with measurements from four other Russian cities that had industrial facilities, the TCDD concentrations observed in Chapaevsk exceeded all reported maximums. Revich et al. (2001) presented rudimentary comparisons of cancer incidence and mortality and reproductive outcomes with regional and national rates; residence in the city of Chapaevsk was used as a surrogate for exposure, and no attempt was made to create exposure categories based on factors that might have influenced the degree of TCDD exposure. The analyses of chromosomal aberrations and other cytologic indicators of genetic damage did partition the women studied into three groups on the basis of worker status or distance of residence from the factory (Revazova et al., 2001).
Chapaevsk Children’s Study
Later research efforts on Chapaevsk residents have focused on quantifying serum concentrations of dioxins and TEQs associated with furans and PCBs. Akhmedkhanov et al. (2002) reported on a convenience sample of 24 volunteers. A cohort of 499 peripubertal boys (8–9 years old in 2003–2005) and their mothers has been established as the Russian Children’s Study for assessing the effect of in utero and childhood exposure on development. The information generated by this study will be relevant to VAO reports only in conjunction with effects in offspring after maternal exposure to the extent that the consequences of gesta-
tional and childhood exposure can be distinguished. To date, however, published findings have not involved health outcomes but have been limited to detailed characterizations of serum concentrations in the boys (Burns et al., 2009, 2011) and their mothers (Humblet et al., 2010).
Swedish Environmental Studies
Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS)
The PIVUS study recruited participants, within 2 months after their 70th birthdays, randomly from the registry of residents of the community of Uppsala, Sweden, from April 2001 to June 2004. The primary aim was to investigate cardiovascular disease in an elderly population with adjustment for sex. Of the 2,025 subjects who were invited to participate, 1,016 were included, for a participation rate of about 50%; 50% were female. All participants answered a questionnaire about medical history, medication, diet, and smoking habits. The burden of POPs, including several dioxin-like PCBs, was assessed from blood serum or plasma.
Salihovic et al. (2012a) reported on circulating concentrations of POPs in 992 participants with valid measurements. They found significant sex differences in the concentrations of 17 of 21 POPs; women had higher concentrations of five of them. Appropriate adjustment for multiple comparisons (via Holm’s method) was applied. Salihovic et al. (2012b) investigated a new method for extraction of POPs from human blood in the PIVUS study and found it to be robust.
Lee et al. (2011b) reported on the association between POPs and type 2 diabetes in the subjects of the PIVUS study. Of the 1,016 evaluated at baseline, 81% returned 5 years later at age 75. The cross-sectional study (baseline) included 989, and 725 were in the prospective analysis. The additional value of POPs on top of standard risk factors was evaluated with the C-statistic and the net reclassification index and the improved discrimination index. The cross-sectional analyses could not account for any measure of duration of exposure. The results are limited by the small number of incident diabetes cases (36), the multiple testing, and the correlation of POPs (and the ensuing difficulty of interpretation of association with diabetes).
Ronn et al. (2011) reported on associations of POPs with fat mass in the PI-VUS study. Multiple imputation was used to handle missing dietary assessments. A strength of the study is its use of DXA screening to measure fat. There was considerable multiple testing without adjustment. The findings are limited by the cross-sectional design and the limited age of participants inasmuch as age could affect the findings in several ways; the published paper could report on only one subset of findings for this age group.
Lee et al. (2012a) reported on associations of POPs with abdominal obesity in the PIVUS study. Abdominal obesity was treated as a binary variable. The authors note concerns with residual confounding due to diet and physical activ-
ity. Furthermore, greater food consumption may lead to obesity and increased concentrations of chemicals. There could be alterations in pharmacodynamics of POPs because of health disorders, which themselves may influence obesity.
Lee et al. (2012b) reported on associations of POPS with stroke in the PIVUS study. Only hospital-treated strokes were considered. Ischemic and hemorrhagic strokes were not distinguished. There were 35 incident strokes during the 5 years of followup. Sex might be an important effect modifier, but the sample was too small to assess this. The POPs in this study may not be causally related to stroke but rather their concentrations may be correlated with other, causal POPs. The study was not able to use time of stroke in a survival analysis, which would have been a more powerful analysis.
Lind et al. (2012) reported on associations of POPs with carotid atherosclerosis in the PIVUS study. Ordinal logistic regression was used to model the ordinal outcome of number of involved arteries. A Bonferroni adjustment for multiple testing was applied.
Taiwanese Environmental Studies
Taiwan Residents Around Closed PCP Factory
Chang et al. (2010) reported on exposure to PCDDs and PCDFs and hypertension in metabolic syndrome in 1,490 nondiabetic Taiwanese who lived near a highly dioxin-contaminated area. This was a cross-sectional study (2005–2007) that accrued subjects from a health center near a deserted PCP factory. The sample constituted about 80% of the invited residents of the community. Univariate analyses of association with several components of metabolic syndrome were conducted, as was principal component factor analysis to identify a set of uncorrelated factors from among components of metabolic syndrome. Multiple regression models were fitted for each component. In addition, an analysis of association between each congener and the prevalence of metabolic syndrome was conducted. The authors list the following limitations of the study: unknown age at first exposure to PCDDs and PCDFs and unknown duration of exposure, a cross-sectional design that evaluates current association, adjustment for obesity as one element of metabolic syndrome, rather than BMI, and some arbitrary choices inherent in factor analysis. In summary, the large size of this study is a strength, but the unknown age and duration of exposure are clear weaknesses.
Chang et al. (2011a) reported on the same cross-sectional study, restricted to 1,449 non-diabetic residents (the slight decrease in sample size from the 2010 study is due to slightly different starting population sizes and to a difference of 19 diabetics people between the studies). The study aimed to investigate the joint effects of exposure to dioxins and mercury on pancreatic endocrine function. People who lived near the deserted factory were exposed both to PCDDs and PCDFs and to mercury from eating contaminated seafood from the reservoir
near the factory. In multiple-regression models, the authors did not include PCDDs and PCDFs and mercury simultaneously but rather included each singly. They reported the correlation of PCDDs and PCDFs and mercury to be 0.14 (p < 0.001), which is low, so it is not apparent why they did not fit models that included both simultaneously and perhaps even with an interaction term to assess the magnitude of the contribution of each contaminant. They did, however, fit a model that included all combinations of tertiles of exposure to each. Again, a major limitation of this study is the absence of information on onset and duration of exposure. Furthermore, the authors note that the homeostasis model for insulin-resistance assessment is not the gold standard and that repeated testing may be needed for assessment in older people. The study did not address co-contamination with mercury.
Chang et al. (2011b) reported on the same cross-sectional study with enrollment extended to December 2009 and restricted to 914 residents who did not have cardiovascular disease (CVD) and who were 30–45 years old. The study aimed to investigate associations between PCDD and PCDF exposure and continuous measures of CVD within 10 years as measured by the Framingham risk score, a formula for combining established risk factors into a single number. Mercury concentrations were not adjusted for in the models, although seafood consumption was. One limitation is the use of the Framingham score; other factors are associated with risk but not included in the score (such as socioeconomic position, genetics, and imaging biomarkers). As in all the publications on this cohort, this one is limited by the lack of information about onset and duration of exposure.
Chang et al. (2012) reported on the same cross-sectional study during 20062009, with enrollment restricted to 1,167 residents who had fasted before blood sampling and were more than 50 years old. The study aimed to investigate the biochemical profiles of those exposed to PCDDs and PCDFs. Na-PCP, a widely used pesticide, had been used in the production process at the abandoned factory. After the factory shut down, a large quantity was improperly stored and released into the environment. Some of the retired workers moved away from the area and were not exposed by eating seafood, whereas others remained and were exposed, alongside other local residents. Thus, there were three exposure groups of retired Na-PCP workers: those who still lived locally (23), those who lived locally but did not knowingly eat polluted fish (37), and those who moved away (96). There were three control groups that did not include any Na-PCP workers: local residents who had eaten polluted fish (345), local residents who had not eaten polluted fish (666), and “background participants” in Taiwan’s general population (645). The first two of the control groups made up the 1,167 in the study population. Limitations of the study include unknown PCDD and PCDF concentrations in retired workers who moved away or when exposure ceased. There may be important unmeasured confounders related to which workers moved away and which ones did not.
Taiwanese Mother-and-Child Studies
A prospective study of healthy Tiawanese mothers and their children recruited during the mothers’ pregnancy is under way to study the associations between exposures to PCDDs, PCDFs, and PCBs and health outcomes (Chao et al., 2004, 2007; Su et al., 2010, 2012; Wang et al., 2004, 2005). The study enrolled pregnant women who had no clinical complications, were 25–35 years old, and delivered in the period between December 1, 2000-November 30, 2001, in a medical center in suburban Taichung in central Taiwan, where a solid-waste incinerator is. Participants completed a questionnaire concerning maternal age, occupation, disease history, cigarette-smoking, alcohol consumption, dietary habits, and baby’s stature. Biologic samples (including placenta, umbilical cord blood, mother’s venous blood, and breast milk) were collected for analysis of PCDDs, PCDFs, and PCBs. A total of 610 women were enrolled (80% of those invited). The placenta was collected from and the questionnaire completed by 430 participants. Of those, 250 provided sufficient venous blood for the chemical analyses. Of the 250, 175 provided adequate breast milk samples. Wang et al. (2004) reported on PCDDs, PCDFs, and PCBs in the biologic samples and correlations among specimens. Chao et al. (2004) reported on PCDDs, PCDFs, and PCBs in breast milk and the cumulative dose derived for infants exclusively breastfed vs those fed formula.
Wang et al. (2005) examined the association between in utero exposure to PCDDs, PCDFs, and PCBs and thyroid and growth hormones in the newborns. Hormone concentrations were compared between infants with high vs low dioxin/PCB TEQ (above vs below the median) and between females (62) and males (57) by using a two-sample t test or the Mann-Whitney U test (when the distribution deviated significantly from the normal distribution assumed for the t test). Spearman’s correlation was used to evaluate the association between hormone concentrations and PCDD, PCDF, and PCB concentrations. Further analyses were carried out with stepwise multivariate regression analysis to adjust for age and other covariates selected through the stepwise selection procedure. Wang et al. (2006) examined the association between PCDDs, PCDFs, and PCBs measured in the placenta samples and estrogens and metabolites measured in mothers’ blood samples by using Pearson correlations, linear and quadratic regressions, and multivariate regression analyses.
Su et al. (2010) reported on 2-year and 5-year followups of the mother-child pairs of Wang et al. (2005). Children’s anthropomorphic measures were obtained, including height, weight, BMI, head circumference, chest girth, bone age, and the ratio between bone age and chronologic age. Thyroid, sex-hormone, and growth-factor concentrations were measured in venous blood samples obtained from children whose mothers’ serum PCDD and PCDF TEQs were available. The anthropomorphic measures and thyroid, sex-hormone, and growth-factor concentrations were compared by sex (29 and 14 males at years 2 and 5, respectively,
and 41 and 27 females at years 2 and 5) and pooled across sexes; those who had high vs low in utero PCDD and PCDF concentrations (≥ 15 vs ≥ 15 pg-TEQ/g of lipid) were compared with a two-sample t test or (when not normally distributed) a Wilcoxon rank-sum test. Further analyses were conducted with multiple regression and stepwise selection for detecting factors that might affect growth or hormone concentrations.
Since Update 2010, Su et al. (2012) reported on the 8-year followup of the same cohort in a subset of 23 boys and 33 girls, substantially more than the numbers examined in the 5-year followup. In addition to anthropomorphic measures used in previous waves, reproductive development (breast, genital, and armpit stages) were assessed.
Vietnamese Environmental Studies
Various epidemiologic studies have been conducted in the Vietnamese population exposed to the spraying that occurred during the Vietnam War. In a review paper, Constable and Hatch (1985) summarized the unpublished results of studies conducted by researchers in Vietnam. They also examined nine reports that focused primarily on reproductive outcomes (Can et al., 1983a,b; Huong and Phuong, 1983; Khoa, 1983; Lang et al., 1983a,b; Nguyen, 1983; Phuong and Huong, 1983; Trung and Chien, 1983). Vietnamese researchers later published results of four additional studies: two on reproductive abnormalities (Phuong et al., 1989a,b), one on mortality (Dai et al., 1990), and one on hepatocellular carcinoma (Cordier et al., 1993). Ngo et al. (2006) published a meta-analysis that addressed an association between exposure to Agent Orange and birth defects and covered some reports reviewed previously by Constable and Hatch (1985), some new Vietnam studies, and studies on US and Australian veterans who served in Vietnam.
The committee has been interested in recent assessments of contaminant concentrations in Vietnam attributable to storage, distribution, and spraying of herbicides by the US military during the Vietnam War, but none has explored associations between the measured concentrations measured and health outcomes.
Dioxins and PCBs were among OC chemicals measured by Schecter et al. (2003) in food samples gathered in 2002 around Bien Hoa City, Vietnam, about 32 km north of Ho Chi Minh City (formerly Saigon). Bien Hoa City is known as a dioxin “hot spot,” with a substantial leak of more than 5,000 gal of Agent Orange at the nearby Bien Hoa air base about 30 years before the study. Marked increases in TCDD concentrations and TEQs were found in ducks, chickens, and fish, but not in pork or beef. The study concluded that food appeared to be responsible for the increase in TCDD in residents of Bien Hoa City even though the original Agent Orange contamination occurred 30–40 years before sampling.
Hansen et al. (2009) studied maternal serum concentrations of OC chemicals (including dioxin-like PCBs 118, 126, 156, and 169) at the time of delivery in
women from two communities in southern Vietnam: Nha Trang, a coastal city about 450 km northeast of Ho Chih Minh City, and Dien Khanh, a rural district about 10 km inland from Nha Trang. Of 246 women who delivered infants in May-July 2005, 94 in Nha Trang and 95 in Dien Khanh met the study’s residence requirements, agreed to participate, and provided blood specimens. Mean concentrations of the ordinarily prevalent non-dioxin-like PCB 153 were 0.15 μg/L in Nha Trang and 0.10 μg/L in Dien Khanh; other PCB congeners were low in both communities. Age and parity were the most important predictors of plasma concentrations of all chemicals, whereas community of residence was also predictive for PCB 153. Correlations with the health status of mothers or children were not reported.
Nhu et al. (2009) examined the correlations of dioxin concentrations in soil, sediment, and breast milk in an area in Vietnam that had been sprayed with herbicide during the war, Cam Chinh commune in Quang Tri province, and a control site that was not sprayed, Cam Phuc commune in Ha Tinh province. Soil and sediment samples were taken randomly throughout Cam Chinh commune and analyzed for PCDDs and PCDFs. Spatial distribution of PCDDs and PCDFs was estimated by using lognormal kriging (Saito and Goovaerts, 2000). Breast-milk samples were taken from lactating mothers 20–40 years old who lived in two communes (86 in Cam Chinh commune and 71 in Cam Phuc commune) in September 2002-July 2003. The participants were also interviewed to collect information on personal habits, such as smoking, alcohol drinking, contraceptive-drug use, history of pesticide contact, disease history, number of pregnancies, age at each pregnancy, and reason for pregnancy failure, if applicable. The mean dioxin concentrations in soil and breast milk in the sprayed area were significantly higher than were those in the nonsprayed area. There were no significant correlations between the estimated dioxin concentrations in soil obtained with the kriging method and those in breast milk. Again, no results were presented with respect to the health status of mothers or infants.
Other Environmental Studies
Additional outcomes of environmental exposure to the COIs were studied: NHL in Yorkshire, England (Cartwright et al., 1988); adverse health effects after an electric-transformer fire in Binghamton, New York (Fitzgerald et al., 1989); lymphomas and STSs in Italy (Vineis et al., 1991); cancer in Finland (Lampi et al., 1992); early-onset Parkinson disease in Oregon and Washington (Butterfield et al., 1993); neuropsychologic effects in Germany (Peper et al., 1993); mortality and cancer incidence in two cohorts of Swedish fishermen whose primary exposure route was assumed to be diet (Svensson et al., 1995); immunologic effects of prenatal and postnatal exposure to PCB or TCDD in Dutch infants from birth to the age of 18 months (Weisglas-Kuperus et al., 1995); effects of inhalation exposure to TCDD and related chemicals in wood preservatives on cell-mediated
immunity in German day-care center employees (Wolf and Karmaus, 1995); skin cancer in Alberta, Canada (Gallagher et al., 1996); immunologic effects in hobby fishermen in the Frierfjord in southeastern Norway (Lovik et al., 1996); HL, NHL, MM, and acute myeloid leukemia in various regions of Italy (Masala et al., 1996); NHL, HL, and chronic lymphocytic leukemia in a rural Michigan community (Waterhouse et al., 1996); cancer mortality in four northern wheat-producing US states (Schreinemachers, 2000); mortality and incinerator dioxin emissions in municipalities in Japan (Fukuda et al., 2003); prevalence of hypertension in Taiwanese who lived near municipal-waste incinerators (Chen HL et al., 2006); and adverse pregnancy outcomes in Japan on the basis of maternal residence at the time of birth (Tango et al., 2004).
Combustion records in the Zeeburg area of Amsterdam in the Netherlands were used as a surrogate for exposure to dioxins in a study of orofacial clefts (ten Tusscher et al., 2000). Location downwind or upwind of an incineration source was used to define exposed and reference groups for the study. A study of STS in the general population was conducted in and around the city of Mantua in northern Italy (Costani et al., 2000). Several industrial facilities are in Mantua, and residential proximity to them was presumed to result in increased TCDD exposure, but TCDD was not measured in the environment or in human tissues.
A study of dioxin exposure pathways in Belgium focused on longtime residents of the vicinity of two municipal-waste incinerators (Fierens et al., 2003a). Residents near a rural incinerator had significantly higher serum dioxin concentrations than did a control group (38 vs 24 TEQ pg/g of lipid). Concentrations in people who lived near the incinerators increased proportionally with intake of local-animal fat. A second study (Fierens et al., 2003b) measured dioxin body burden in 257 people who had been environmentally exposed to determine whether dioxin and PCB exposures were associated with type 2 diabetes and endometriosis. No difference in body burden was found between women who had endometriosis and women in a control group, but the risk of type 2 diabetes was significantly higher in those who had higher body burdens of dioxin-like chemicals and of PCBs. Another study of the correlation between dioxin-like chemicals in Italian and Belgian women and the risk of endometriosis used measurements of TCDD and other dioxins in blood (De Felip et al., 2004). There was no difference in body burden between women who had endometriosis and a control group, but serum-dioxin concentrations were substantially higher in the Belgian controls than in a similar group in Italy (45 vs 18 TEQ pg/g of lipid, respectively).
Cross-Canada Study of Pesticides and Health (Rare Tumors Study)
After a pilot study done for the Canadian government, McDuffie et al. (2001) initiated a full population-based case-control study of men in six Canadian prov-
inces that addressed several fairly uncommon malignancies—HL, NHL, MM, and STS—and the relationship of their occurrence with exposure to pesticides (both occupationally and domestically). A target number of cases of each cancer type was preset for each province; cases newly diagnosed starting on September 1, 1991, were gathered from the provincial cancer registries or hospital records in Quebec until the end of 1994 or until the target number was reached. Physician consent was obtained, diagnoses were confirmed with pathology reports and preserved tissues, and consent forms and questionnaires were sent to the cases. The controls were men at least 19 years old identified in the health-insurance records of Alberta, Saskatchewan, Manitoba, and Quebec; telephone listings for Ontario; and voter lists in British Columbia. The controls were selected randomly to obtain a stratified age distribution matching that of the cases. They, too, were sent consent forms and questionnaires. People who had died were dropped from the study, as were people who had Kaposi sarcoma or were HIV-positive. All 1,506 controls that responded were used in comparisons for each of four cancer groups: 316 HL cases, 517 NHL cases, 342 MM cases, and 357 STS cases.
The postal questionnaire gathered standard demographic information, personal and family medical histories, employment history, smoking behavior, and basic data on pesticide exposure. The pilot study had tested the reliability of self-reported pesticide use by comparison with purchase records. Any subject who reported at least 10 hours of pesticide exposure per year was asked to complete a telephone questionnaire on the details of pesticide exposure; in addition, 15% of the remaining subjects were randomly selected to answer the telephone survey. Conditional logistic regression stratified on age and province and adjusted for all covariates found to be associated with the outcome at the 0.05 level of significance was used to estimate ORs for specific active ingredients, including dicamba and the phenoxy herbicides 2,4-D, Mecoprop, MCPA, and diclofopmethyl. Dose–response relationships were investigated for cumulative categories of time spent in mixing or applying particular products.
A series of publications have addressed the relationship between each of the cancers and various risk factors. Those pertaining to herbicides overall or to the particular ones of interest are as follows:
• HL—Karunanayake et al., 2012; Pahwa et al., 2003
• NHL—Hohenadel et al., 2011; McDuffie et al., 2001
• MM—Pahwa et al., 2003, 2012
• STS—Pahwa et al., 2003, 2011
A number of other publications arising from that dataset have addressed topics somewhat more tangential to the interests of the VAO reports. For instance, McDuffie et al. (2005) and Pahwa et al. (2006) considered the possible interaction of exposure to insect repellents, particularly N,N-diethyl-m-toluamide (DEET) and phenoxy herbicides, in the genesis of the malignancies in question. McDuffie
et al. (2009) examined family histories of cancer in first-degree relatives of the study participants (1,528 cases and 1,506 controls) to assess the interacton between family history and pesticide exposure. Hohenadel et al. (2011) investigated how various combinations of pesticide exposures influenced the occurrence of NHL. Ghosh et al. (2011) investigated the association of occupational exposures other than to pesticides with the occurrence of MM.
Children’s Oncology Group Study (US)
In two related case-control studies, Chen Z et al. reported on exposure to pesticides (including herbicides) and the risk of childhood germ-cell tumors. One focused on parental occupational exposures (Chen Z et al., 2005) and the other on parental exposures to residential pesticides and chemicals (Chen Z et al., 2006), but they are based on the same overall case-control study.
No reports from the Children’s Oncology Group have been published since Update 2008.
National Birth Defects Prevention Study (10 US centers, 1997–2003 births)
Rocheleau et al. (2011) reports on the association between maternal occupational pesticide exposure and risk of hypospadias in the National Birth Defects Prevention Study. This was a case-control study with 647 cases of hypospadias and 1,496 controls with estimated delivery dates of October 1997-December 2002. Mothers were interviewed about job status, which was then formally coded. Typical pesticide ratings were assigned to the job codes. Duration and confidence in exposure were used to refine them. Complete case analysis was conducted, with some sensitivity analysis around missingness (creation of a missing category). Most exposure was to insecticides only or to all three types (insecticides, herbicides, and fungicides). The analysis did not include fetuses that died with hypospadias. Multiple comparisons are a concern. There was generally a low level of pesticide exposure in the study population. Other exposures of the population (for example, in agricultural workers) could cause the outcome in question.
Upper Midwest Health Study
The Upper Midwest Health Study (UMHS) was initiated by NIOSH as a population-based case-control study of cancer risk in a nonmetropolitan Midwestern US population. Several reports from the study were reviewed in previous updates. Chiu et al. (2004) and Lee et al. (2004a) conducted pooled (combined) analyses of two earlier case-control studies of NHL carried out by the UMHS in Iowa and Minnesota (Cantor et al., 1992) and Nebraska (Zahm et al., 1990). Chiu et al. (2004) examined the association of NHL with agricultural pesticide use and familial cancer, and Lee WJ et al. (2004a, 2006) looked at NHL in asthmatic people
who reported pesticide exposure. Data from Nebraska (Chiu et al., 2006, based on Zahm et al., 1990, 1993) were used to identify whether there was a higher risk of subtypes of NHL. Specifically, tissue samples were analyzed according to the presence of a specific chromosomal translocation (t[14;18][q32;q21]); only 172 of 385 cases were included.
Two studies focused on pesticide use and the risk of adenocarcinomas of the stomach and esophagus (Lee et al., 2004b) and the risk of gliomas (Lee et al., 2005). Cases were white Nebraska residents over 21 years old who were identified from the Nebraska Cancer Registry and matched to controls drawn from an earlier study by Zahm et al. (1990).
Researchers evaluated farm pesticide exposure in men (Ruder et al., 2004) and women (Carreon et al., 2005) in Iowa, Michigan, Minnesota, and Wisconsin in relation to gliomas as part of the UMHS. Ruder et al. (2006) reported a followup of Ruder et al. (2004) that evaluated gliomas in UMHS participants. The new analyses provided no evidence of greater use of pesticides in cases than in controls, and there was no breakdown by specific agents.
Ruder et al. (2009) reported another followup, which had similar findings and no breakdown by specific agents.
Since Update 2010, Yiin et al. (2012) has reported findings from new analyses of the UMHS sample that incorporated more detailed exposure information that was not used in previous analyses, including years of use and estimated cumulative exposures to categories of pesticides, including phenoxy herbicides, and use of specific agents, including 2,4-D and dicamba.
Other Case-Control Studies
Numerous case-control studies have been reviewed in previous updates. In 1977, case-series reports in Sweden (Hardell, 1977, 1979) of a potential connection between exposure to phenoxyacetic acids and STS prompted several case-control investigations (Eriksson et al., 1979, 1981, 1990; Hardell, 1981; Hardell and Eriksson, 1988; Hardell and Sandstrom, 1979; Wingren et al., 1990). After the initial STS reports (Hardell, 1977, 1979), case-control studies of other cancer outcomes were conducted in Sweden: of HL and NHL (Hardell and Bengtsson, 1983; Hardell et al., 1980, 1981; Persson et al., 1989, 1993), of NHL (Hardell and Eriksson, 1999; Olsson and Brandt, 1988), of nasal and nasopharyngeal carcinomas (Hardell et al., 1982), of gastric cancer (Ekström et al., 1999), and of primary or unspecified liver cancer (Hardell et al., 1984). To address criticism regarding potential observer bias in some of the case-control series, Hardell (1981) conducted another case-control study of colon cancer. Hardell et al. (1994) also examined the relationship between occupational exposure to phenoxyacetic acids and chlorophenols and various characteristics related to NHL—including histopathologic measures, stage, and anatomic location—on the basis of the NHL cases in a previous study (Hardell et al., 1981).
Prompted by the Swedish studies (Hardell, 1977, 1979), Smith and Pearce (1986) and Smith et al. (1983, 1984) conducted a set of case-control studies to evaluate the association between phenoxy herbicide and chlorophenol exposure and STS incidence and mortality in New Zealand. An expanded case series was collected, and additional case-control studies of exposure to phenoxy herbicides or chlorophenols and the risks of malignant lymphoma, NHL, and MM were conducted (Pearce et al., 1985, 1986a,b, 1987).
Geographic patterns of increased leukemia mortality in white men in the central part of the United States prompted a study of leukemia mortality in Nebraska farmers (Blair and Thomas, 1979). Additional case-control studies of leukemia were later conducted in Nebraska (Blair and White, 1985); in Iowa (Burmeister et al., 1982) on the basis of the cohort study of Burmeister (1981); and in Iowa and Minnesota (Brown et al., 1990). Another study investigated leukemia in association with NHL and 2,4-D in eastern Nebraska (Zahm et al., 1990).
Case-control studies have been conducted in various US populations for associations of herbicides with other cancers, including NHL (Cantor, 1982; Cantor et al., 1992; Hartge et al., 2005; Tatham et al., 1997; Zahm et al., 1993); MM (Boffetta et al., 1989; Brown et al., 1993; Morris et al., 1986); gastric cancer, prostate cancer, NHL, and MM (Burmeister et al., 1983); STS, HL, and NHL (Hoar et al., 1986); NHL and HL (Dubrow et al., 1988); and STS and NHL (Woods and Polissar, 1989; Woods et al., 1987). In a subset of participants in the Hartge et al. (2005) study, De Roos et al. (2005b) studied associations between overall TEQs of PCBs, furans, and dioxins but not TCDD alone.
Other case-control studies conducted outside the United States have addressed various cancers: STS and other cancers in the 15 regional cancer registries that constitute the National Cancer Register in England in connection with the chemicals of interest (COIs) (Balarajan and Acheson, 1984); ovarian cancer in the Piedmont region of Italy (Donna et al., 1984); STS in rice weeders in northern Italy (Vineis et al., 1986); mortality from esophageal cancer, pancreatic cancer, cutaneous melanoma, renal cancer, and brain cancer in three English counties (Magnani et al., 1987); brain gliomas in two hospitals in Milan, Italy (Musicco et al., 1988); lymphoid cancer in Milan, Italy (LaVecchia et al., 1989); primary lung cancer in pesticide users in Saskatchewan (McDuffie et al., 1990); STS and malignant lymphomas in the Victorian Cancer Registry of Australia (Smith and Christophers, 1992); oral-cancer risk in occupationally exposed workers in Sweden (Schildt et al., 1999); and renal-cell carcinoma in the Denmark Cancer Registry (Mellemgaard et al., 1994). Nanni et al. (1996) conducted a population-based case-control study—based on the work of Amadori et al. (1995)—of occupational and chemical risk factors for lymphocytic leukemia and NHL in northeastern Italy.
Noncancer health outcomes also have been investigated in case-control studies: spontaneous abortion (Carmelli et al., 1981); congenital malformations (García et al., 1998); immunosuppression and later decreased host resistance to
infection in AIDS patients who had Kaposi sarcoma (Hardell et al., 1987); mortality in US Department of Agriculture extension agents (Alavanja et al., 1988) and conservationists (Alavanja et al., 1989); PD associated with occupational risk factors (Semchuk et al., 1993); birth defects in offspring of agriculture workers (Nurminen et al., 1994); mortality from neurodegenerative diseases associated with occupational risk factors (Schulte et al., 1996); PD associated with various rural factors, including exposure to herbicides and wood preservatives (Seidler et al., 1996); spina bifida in offspring associated with paternal occupation (Blatter et al., 1997); PD associated with occupational and environmental risk factors (Liou et al., 1997); and mortality from neurodegenerative diseases, including Alzheimer disease and presenile dementia, PD, and motor neuron disease associated with occupational factors (Park et al., 2005). Those studies have been discussed in detail in previous updates.
Orsi et al. (2009) have studied the association between occupational exposures to pesticides and lymphoid neoplasms by using a hospital-based case-control study in the main hospitals of six French cities (Brest, Caen, Nantes, Lille, Toulouse, and Bordeaux) from September 2000 to December 2004. Cases were eligible if they were male, were 20–75 years old, were residing in the hospital’s catchment area (the administrative department where the hospital is or a neighboring department), lacked a history of immunosuppression or of taking immunosuppressant drugs, and had recently received a diagnosis of any lymphoid neoplasm except acute lymphoid leukemia. The diagnoses were classified by using the World Health Organization third edition of the International Classification of Diseases for Oncology codes and confirmed cytologically or histologically by a panel of pathologists and hematologists. Among 513 eligible incident cases, 491 (96%) participated: 87 with HL, 244 with NHL, 56 with MMs, and 104 with lymphoproliferative syndrome (LPS). The controls were male patients from the same hospitals who had no prior history of lymphoid neoplasm (LN), were residing in the hospital’s catchment area, and were not admitted to the hospital for conditions directly related to occupation, smoking, or alcohol abuse. The controls were individually matched with the cases by hospital and age (± 3 years). Among 501 eligible controls, 456 (91%) participated. Participants were given a self-administered questionnaire, had a face-to-face interview, and had a reinterview by an occupational hygienist and an agronomist when needed to collect socioeconomic and lifestyle information, personal and family medical history, residential and occupational histories, and detailed information on occupational and nonoccupational exposure to herbicides and pesticides. Dichotomous exposure measures (ever or never exposed) were constructed for each category (insecticides, fungicides, and herbicides) and for each chemical family (such as OC chemicals and phenoxy herbicides). Unconditional logistic regression was used to estimate the ORs and confidence intervals for each outcome (all LN, NHL, HL, LPS, and MM) and chemical exposure with adjustment for age, hospital, and socioeconomic category (white collar or blue collar). Logistic regression was
used for NHL subtypes (diffuse large B-cell lymphoma, follicular lymphoma, and other NHL) and LPS (chronic lymphocytic leukemia and hairy-cell leukemia).
Spinelli et al. (2007) conducted a population-based case-control study of histologically confirmed NHL in men and women 20–79 years old who lived in the greater metropolitan areas of Vancouver and Victoria, British Columbia, during 2000–2004. Population controls, frequency-matched to cases by 5-year age groups and area, were identified from the client registry of the provincial health care system. A random subset of controls was included in the analyses. The analyses were based on concentrations of OC and related chemicals in serum obtained from controls at the time of interview and from cases before chemotherapy. NHL patients who lost weight rapidly were excluded. Ng et al. (2010) examined the single-nucleotide polymorphisms (SNPs) in the aryl hydrocarbon receptor (AHR) gene that were genotyped for the same study cohorts (422 NHL cases and 459 controls) to measure the association between individual SNPs, haplotypes, and risk of NHL. Gene-environment interaction analyses were conducted for OC chemicals and AHR SNPs by using logistic regression.
Hartge et al. (2005) conducted a case-control study that used four NCI SEER registries (Detroit, Iowa, Los Angeles County, and Seattle) for associations of herbicides with NHL. In a subset of participants in the Hartge et al. study, De Roos et al. (2005b) studied associations between NHL and overall TEQs of PCBs, furans, and dioxins but not TCDD alone. Colt et al. (2009) studied whether the relationship between OC exposure and NHL is modified by immune-gene variation in the SEER study participants (1,172 cases and 513 controls). The study genotyped 61 polymorphisms in 36 immune genes and examined three exposures measured in plasma and dust: to PCB 180, to OC pesticides (TEQ), and to α-chlordane. Unconditional logistic regression was used to estimate the exposure-outcome association with stratification by genotype and adjustment for sex, age, race, education, and study region.
Firestone et al. (2005) reported on a population-based case-control study of incident PD cases in Washington state (250 cases and 388 controls). PD cases were identified in 1992–2002 at the Group Health Cooperative (GHC, a large managed-care organization) or the University of Washington. Control participants were sampled randomly from GHC enrollees who had no history of PD or other progressive neurologic disorder and were frequency-matched to cases by age, sex, GHC clinic location, and year of GHC enrollment. Participants were interviewed to obtain information on demographics, medical and occupational history, occupational and home-based pesticide use, drinking-water source, residential history, and smoking history. Both occupational exposures and residential exposures were reported. No specific COIs were reported beyond the broad category “herbicide.” Unconditional logistic regression was used to estimate the association between PD and exposure, with adjustment for age, sex, and smoking.
Firestone et al. (2010) provided an expanded update (404 cases and 526 controls) that extended the same recruitment protocol through 2006. The partici-
pation rates were good among eligible cases (70%) and modest among eligible controls (60%); this left some room for selection bias due to nonresponse. Only occupational exposures were reported. Exposures to specific chemicals were reported, including 2,4-D (nine exposed cases and 12 exposed controls).
ADVA (Australia, Department of Veterans’ Affairs). 2005a. The Third Australian Vietnam Veterans Mortality Study 2005. Canberra: Department of Veterans’ Affairs.
ADVA. 2005b. Cancer Incidence in Australian Vietnam Veteran Study 2005. Canberra: Department of Veterans’ Affairs.
ADVA. 2005c. Australian National Service Vietnam Veterans: Mortality and Cancer Incidence 2005. Canberra: Department of Veterans’ Affairs.
AFHS (Air Force Health Study). 1982. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides: Study Protocol, Initial Report. Brooks AFB, TX: USAF School of Aerospace Medicine. SAM-TR-82–44.
AFHS. 1983. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. Baseline Mortality Study Results. Brooks AFB, TX: USAF School of Aerospace Medicine. NTIS AD-A130–793.
AFHS. 1984a. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. Baseline Morbidity Study Results. Brooks AFB, TX: USAF School of Aerospace Medicine. NTIS AD-A138–340.
AFHS. 1984b. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. Mortality Update: 1984. Brooks AFB, TX: USAF School of Aerospace Medicine. NTIS AD-A162–687.
AFHS. 1985. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. Mortality Update: 1985. Brooks AFB, TX: USAF School of Aerospace Medicine. NTIS AD-A163–237.
AFHS. 1986. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. Mortality Update: 1986. Brooks AFB, TX: USAF School of Aerospace Medicine. USAFSAM-TR-86–43.
AFHS. 1987. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. First Follow-up Examination Results. Brooks AFB, TX: USAF School of Aerospace Medicine. USAFSAM-TR-87–27.
AFHS. 1989. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. Mortality Update: 1989. Brooks AFB, TX: USAF School of Aerospace Medicine. USAFSAM-TR-89–9.
AFHS. 1990. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. Brooks AFB, TX: USAF School of Aerospace Medicine. USAFSAM-TR-90–2.
AFHS. 1991a. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. Mortality Update: 1991. Brooks AFB, TX: Armstrong Laboratory. AL-TR-1991–0132.
___________________
1Throughout this report, the same alphabetic indicator after year of publication is used consistently for a given reference when there are multiple citations by the same first author in a given year. The convention of assigning the alphabetic indicators in order of citation in a given chapter is not followed.
AFHS. 1991b. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. Serum Dioxin Analysis of 1987 Examination Results. Brooks AFB, TX: USAF School of Aerospace Medicine. NTIS AD-A237–516.
AFHS. 1992. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. Reproductive Outcomes. Brooks AFB, TX: Armstrong Laboratory. AL-TR-1992–0090.
AFHS. 1995. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. 1992 Follow-up Examination Results. Brooks AFB, TX: Epidemiologic Research Division; Armstrong Laboratory. AL-TR-920107.
AFHS. 1996. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. Mortality Update 1996. Brooks AFB, TX: Epidemiologic Research Division; Armstrong Laboratory. AL/AO-TR-1996–0068.
AFHS. 2000. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. 1997 Follow-up Examination Results. Brooks AFB, TX: Epidemiologic Research Division. Armstrong Laboratory. AFRL-HE-BR-TR-2000–02.
AFHS. 2005. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. 2002 Follow-up Examination Results. Brooks AFB, TX: Epidemiologic Research Division. Armstrong Laboratory. AFRL-HE-BR-SR-2005–0003.
Agent Orange Review. 2008. IOM announces new Vietnam veterans and Agent Orange committee to prepare 2008 Update. Department of Veterans Affairs 20(2):1–8.
AIHW (Australian Institute of Health and Welfare). 1999. Morbidity of Vietnam Veterans: A Study of the Health of Australia’s Vietnam Veteran Community: Volume 3: Validation Study. Canberra: AIHW.
AIHW. 2000. Morbidity of Vietnam Veterans. Adrenal Gland Cancer, Leukaemia and non-Hodgkin’s Lymphoma: Supplementary Report No. 2. (AIHW cat. No. PHE 28). Canberra: AIHW.
AIHW. 2001. Morbidity of Vietnam Veterans. Adrenal Gland Cancer, Leukaemia and non-Hodgkin’s Lymphoma: Supplementary Report No. 2. Revised edition (AIHW cat. No. PHE 34). Canberra: AIHW.
Akhmedkhanov A, Revich B, Adibi JJ, Zeilert V, Masten SA, Patterson DG Jr, Needham LL, Toniolo P. 2002. Characterization of dioxin exposure in residents of Chapaevsk, Russia. Journal of Exposure Analysis and Environmental Epidemiology 12(6):409–417.
Akhtar FZ, Garabrant DH, Ketchum NS, Michalek JE. 2004. Cancer in US Air Force veterans of the Vietnam war. Journal of Occupational and Environmental Medicine 46(2):123–136.
Alavanja MC, Blair A, Merkle S, Teske J, Eaton B. 1988. Mortality among agricultural extension agents. American Journal of Industrial Medicine 14:167–176.
Alavanja MC, Merkle S, Teske J, Eaton B, Reed B. 1989. Mortality among forest and soil conservationists. Archives of Environmental Health 44:94–101.
Alavanja MC, Akland G, Baird D, Blair A, Bond A, Dosemeci M, Kamel F, Lewis R, Lubin J, Lynch C, et al. 1994. Cancer and non-cancer risk to women in agriculture and pest control: The Agricultural Health Study. Journal of Occupational Medicine 36(11):1247–1250.
Alavanja MC, Sandler DP, Mcdonnell CJ, Lynch CF, Pennybacker M, Zahm SH, Lubin J, Mage D, Steen WC, Wintersteen W, Blair A. 1998. Factors associated with self-reported, pesticide-related visits to health care providers in the Agricultural Health Study. Environmental Health Perspectives 106(7):415–420.
Alavanja MC, Samanic C, Dosemeci M, Lubin J, Tarone R, Lynch CF, Knott C, Thomas K, Hoppin JA, Barker J, Coble J, Sandler DP, Blair A. 2003. Use of agricultural pesticides and prostate cancer risk in the Agricultural Health Study cohort. American Journal of Epidemiology 157(9):800–814.
Alavanja MC, Hoppin JA, Kamel F. 2004. Health effects of chronic pesticide exposure: Cancer and neurotoxicity. Annual Review of Public Health 25:155–197.
Alavanja MC, Sandler DP, Lynch CF, Knott C, Lubin JH, Tarone R, Thomas K, Dosemeci M, Barker J, Hoppin JA, Blair A. 2005. Cancer incidence in the Agricultural Health Study. Scandinavian Journal of Work, Environment and Health 31(Suppl 1):39–45.
Alderfer R, Sweeney M, Fingerhut M, Hornung R, Wille K, Fidler A. 1992. Measures of depressed mood in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Chemosphere 25:247–250.
Amadori D, Nanni O, Falcini F, Saragoni A, Tison V, Callea A, Scarpi E, Ricci M, Riva N, Buiatti E. 1995. Chronic lymphocytic leukemias and non-Hodgkin’s lymphomas by histological type in farming-animal breeding workers: A population case-control study based on job titles. Occupational and Environmental Medicine 52(6):374–379.
Anderson HA, Hanrahan LP, Jensen M, Laurin D, Yick WY, Wiegman P. 1986a. Wisconsin Vietnam Veteran Mortality Study: Proportionate Mortality Ratio Study Results. Madison: Wisconsin Division of Health.
Anderson HA, Hanrahan LP, Jensen M, Laurin D, Yick WY, Wiegman P. 1986b. Wisconsin Vietnam Veteran Mortality Study: Final Report. Madison: Wisconsin Division of Health.
Andreotti G, Freeman LEB, Hou L, Coble J, Rusiecki J, Hoppin JA, Silverman DT, Alavanja MCR. 2009. Agricultural pesticide use and pancreatic cancer risk in the Agricultural Health Study cohort. International Journal of Cancer 124(10):2495–2500.
Andreotti G, Koutros S, Berndt SI, Barry KH, Hou L, Hoppin JA, Sandler DP, Lubin JH, Burdette LA, Yuenger J, Yeager M, Freeman LEB, Alavanja MCR. 2012. The interaction between pesticide use and genetic variants involved in lipid metabolism on prostate cancer risk. Journal of Cancer Epidemiology 2012:11 pps.
Andrews JS Jr, Garrett WA, Patterson DG Jr, Needham LL, Roberts DW, Bagby JR, Anderson JE, Hoffman RE, Schramm W. 1989. 2,3,7,8-Tetrachlorodibenzo-p-dioxin levels in adipose tissue of persons with no known exposure and in exposed persons. Chemosphere 18(1–6):499–506.
Arbuckle TE, Ritter L. 2005. Phenoxyacetic acid herbicide exposure for women on Ontario farms. Journal of Toxicology and Environmental Health Part A 68(15):1359–1370.
Arbuckle TE, Schrader SM, Cole D, Hall JC, Bancej CM, Turner LA, Claman P. 1999a. 2,4-Dichlorophenoxyacetic acid (2,4-D) residues in semen of Ontario farmers. Reproductive Toxicology 13(6):421–429.
Arbuckle TE, Savitz DA, Mery LS, Curtis KM. 1999b. Exposure to phenoxy herbicides and the risk of spontaneous abortion. Epidemiology 10:752–760.
Arbuckle TE, Lin Z, Mery LS. 2001. An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environmental Health Perspectives 109(8):851–857.
Arbuckle TE, Burnett R, Cole D, Teschke K, Dosemeci M, Bancej C, Zhang J. 2002. Predictors of herbicide exposure in farm applicators. International Archives of Occupational and Environmental Health 75:406–414.
Arbuckle TE, Cole DC, Ritter L, Ripley BD. 2005. Biomonitoring of herbicides in Ontario farm applicators. Scandinavian Journal of Work, Environment and Health 31(Suppl 1):90–97.
Arbuckle TE, Bruce D, Ritter L, Hall JC. 2006. Indirect sources of herbicide exposure for families on Ontario farms. Journal of Exposure Science and Environmental Epidemiology 16(1):98–104.
Arcury TA, Quandt SA, Barr DB, Hoppin JA, McCauley L, Grzywacz JG, Robson MG. 2006. Farmworker exposure to pesticides: Methodologic issues for the collection of comparable data. Environmental Health Perspectives 114(6):923–928.
Aschengrau A, Monson RR. 1989. Paternal military service in Vietnam and risk of spontaneous abortion. Journal of Occupational Medicine 31:618–623.
Aschengrau A, Monson RR. 1990. Paternal military service in Vietnam and the risk of late adverse pregnancy outcomes. American Journal of Public Health 80:1218–1224.
Asp S, Riihimaki V, Hernberg S, Pukkala E. 1994. Mortality and cancer morbidity of Finnish chlorophenoxy herbicide applicators: An 18-year prospective follow-up. American Journal of Industrial Medicine 26:243–253.
Assennato G, Cannatelli P, Emmett E, Ghezzi I, Merlo F. 1989a. Medical monitoring of dioxin clean-up workers. American Industrial Hygiene Association Journal 50:586–592.
Assennato G, Cervino D, Emmett E, Longo G, Merlo F. 1989b. Follow-up of subjects who developed chloracne following TCDD exposure at Seveso. American Journal of Industrial Medicine 16:119–125.
ATSDR (Agency for Toxic Substances and Disease Registry). 1998. Toxicological Profile for Chlorinated Dibenzo-p-dioxins (CDDs). Atlanta, GA: Centers for Disease Control.
Axelson O, Sundell L. 1974. Herbicide exposure, mortality and tumor incidence. An epidemiological investigation on Swedish railroad workers. Scandinavian Journal of Work, Environment and Health 11:21–28.
Axelson O, Sundell L, Andersson K, Edling C, Hogstedt C, Kling H. 1980. Herbicide exposure and tumor mortality: An updated epidemiologic investigation on Swedish railroad workers. Scandinavian Journal of Work, Environment and Health 6:73–79.
Aylward LL, Bodner KM, Collins JJ, Wilken M, McBride D, Burns CJ, Hays SM, Humphry N. 2010. TCDD exposure estimation for workers at a New Zealand 2,4,5-T manufacturing facility based on serum sampling data. Journal of Exposure Science and Environmental Epidemiology 20(5):417–426.
Baccarelli A, Mocarelli P, Patterson DG Jr, Bonzini M, Pesatori A, Caporaso N, Landi MT. 2002. Immunologic effects of dioxin: New results from Seveso and comparison with other studies. Environmental Health Perspectives 110(12):1169–1173.
Baccarelli A, Pesatori AC, Masten SA, Patterson DG Jr, Needham LL, Mocarelli P, Caporaso NE, Consonni D, Grassman JA, Bertazzi PA, Landi MT. 2004. Aryl-hydrocarbon receptor-dependent pathways and toxic effects of TCDD in humans: A population-based study in Seveso, Italy. Toxicology Letters 149(1–3):287–293.
Baccarelli A, Pesatori AC, Consonni D, Mocarelli P, Patterson DG Jr, Caporaso NE, Bertazzi PA, Landi MT. 2005a. Health status and plasma dioxin levels in chloracne cases 20 years after the Seveso, Italy accident. British Journal of Dermatology 152(3):459–465.
Baccarelli A, Pfeiffer R, Consonni D, Pesatori AC, Bonzini M, Patterson DG Jr, Bertazzi PA, Landi MT. 2005b. Handling of dioxin measurement data in the presence of non-detectable values: Overview of available methods and their application in the Seveso chloracne study. Chemosphere 60(7):898–906.
Baccarelli A, Hirt C, Pesatori AC, Consonni D, Patterson DG Jr, Bertazzi PA, Dölken G, Landi MT. 2006. t(14;18) translocations in lymphocytes of healthy dioxin-exposed individuals from Seveso, Italy. Carcinogenesis 27(10):2001–2007.
Baccarelli A, Giacomini SM, Corbetta C, Landi MT, Bonzini M, Consonni D, Grillo P, Patterson DG Jr, Pesatori AC, Bertazzi PA. 2008. Neonatal thyroid function in Seveso 25 years after maternal exposure to dioxin. PLoS Medicine 5(7):1133–1142.
Balarajan R, Acheson ED. 1984. Soft tissue sarcomas in agriculture and forestry workers. Journal of Epidemiology and Community Health 38:113–116.
Barbieri S, Pirovano C, Scarlato G, Tarchini P, Zappa A, Maranzana M. 1988. Long-term effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the peripheral nervous system. Clinical and neurophysiological controlled study on subjects with chloracne from the Seveso area. Neuroepidemiology 7:29–37.
Barr DB, Allen R, Olsson AO, Bravo R, Caltabiano LM, Montesano A, Nguyen J, Udunka S, Walden D, Walker RD, Weerasekera G, Whitehead RD Jr, Schober SE, Needham LL. 2005. Concentrations of selective metabolites of organophosphorus pesticides in the United States population. Environmental Research 99(3):314–326.
Barr DB, Landsittel D, Nishioka M, Thomas K, Curwin B, Raymer J, Donnelly KC, McCauley L, Ryan PB. 2006a. A survey of laboratory and statistical issues related to farmworker exposure studies. Environmental Health Perspectives 114(6):961–968.
Barr DB, Thomas K, Curwin B, Landsittel D, Raymer J, Lu C, Donnelly KC, Acquavella J. 2006b. Biomonitoring of exposure in farmworker studies. Environmental Health Perspectives 114(6):936–942.
Barrett DH, Morris RD, Akhtar FZ, Michalek JE. 2001. Serum dioxin and cognitive functioning among veterans of Operation Ranch Hand. Neurotoxicology 22:491–502.
Barrett DH, Morris RD, Jackson WG Jr, Stat M, Michalek JE. 2003. Serum dioxin and psychological functioning in US Air Force veterans of the Vietnam War. Military Medicine 168:153–159.
Barry KH, Koutros S, Berndt SI, Andreotti G, Hoppin JA, Sandler DP, Burdette LA, Yeager M, Freeman LEB, Lubin JH, Ma X, Zheng T, Alavanja MCR. 2011. Genetic variation in base excision repair pathway genes, pesticide exposure, and prostate cancer risk. Environmental Health Perspectives 119(12):1726–1732.
Barry KH, Koutros S, Andreotti G, Sandler DP, Burdette LA, Yeager M, Beane Freeman LE, Lubin JH, Ma X, Zheng T, Alavanja MCR, Berndt SI. 2012. Genetic variation in nucleotide excision repair pathway genes, pesticide exposure and prostate cancer risk. Carcinogenesis 33(2):331–337.
Barthel E. 1981. Increased risk of lung cancer in pesticide-exposed male agricultural workers. Journal of Toxicology and Environmental Health 8:1027–1040.
Bashirov AA. 1969. The health of workers involved in the production of amine and butyl 2,4-D herbicides. Vrachebnoye Delo 10:92–95.
Becher H, Wahrendorf J, Angerer R. 1992. A cohort study on persons exposed to phenoxy acid herbicides and their contaminants in Germany—Design and first results. Chemosphere 25(7–10):100–101.
Becher H, Flesch-Janys D, Kauppinen T, Kogevinas M, Steindorf K, Manz A, Wahrendorf J. 1996. Cancer mortality in German male workers exposed to phenoxy herbicides and dioxins. Cancer Causes and Control 7(3):312–321.
Bell EM, Hertz-Picciotto I, Beaumont JJ. 2001a. Case-cohort analysis of agricultural pesticide applications near maternal residence and selected causes of fetal death. American Journal of Epidemiology 154(8):702–710.
Bell EM, Hertz-Picciotto I, Beaumont JJ. 2001b. A case-control study of pesticides and fetal death due to congenital anomalies. Epidemiology 12(2):148–156.
Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B 57(1):289–300.
Bertazzi PA, Zocchetti C, Pesatori AC, Guercilena S, Sanarico M, Radice L. 1989a. Mortality in an area contaminated by TCDD following an industrial incident. Medicina Del Lavoro 80:316–329.
Bertazzi PA, Zocchetti C, Pesatori AC, Guercilena S, Sanarico M, Radice L. 1989b. Ten-year mortality study of the population involved in the Seveso incident in 1976. American Journal of Epidemiology 129:1187–1200.
Bertazzi PA, Zocchetti C, Pesatori AC, Guercilena S, Consonni D, Tironi A, Landi MT. 1992. Mortality of a young population after accidental exposure to 2,3,7,8-tetrachlorodibenzodioxin. International Journal of Epidemiology 21:118–123.
Bertazzi PA, Pesatori AC, Consonni D, Tironi A, Landi MT, Zocchetti C. 1993. Cancer incidence in a population accidentally exposed to 2,3,7,8-tetrachlorodibenzo-para-dioxin. Epidemiology 4:398–406.
Bertazzi PA, Zochetti C, Guercilena S, Consonni D, Tironi A, Landi MT, Pesatori AC. 1997. Dioxin exposure and cancer risk: A 15-year mortality study after the “Seveso accident.” Epidemiology 8(6):646–652.
Bertazzi PA, Bernucci I, Brambilla G, Consonni D, Pesatori AC. 1998. The Seveso studies on early and long-term effects of dioxin exposure: A review. Environmental Health Perspectives 106(Suppl 2):625–633.
Bertazzi PA, Consonni D, Bachetti S, Rubagotti M, Baccarelli A, Zocchetti C, Pesatori AC. 2001. Health effects of dioxin exposure: A 20-year mortality study. American Journal of Epidemiology 153(11):1031–1044.
Bisanti L, Bonetti F, Caramaschi F, Del Corno G, Favaretti C, Giambelluca SE, Marni E, Montesarchio E, Puccinelli V, Remotti G, Volpato C, Zambrelli E, Fara GM. 1980. Experiences from the accident of Seveso. Acta Morphologica Academiae Scientarum Hungaricae 28:139–157.
Blair A, Thomas TL. 1979. Leukemia among Nebraska farmers: A death certificate study. American Journal of Epidemiology 110:264–273.
Blair A, White DW. 1985. Leukemia cell types and agricultural practices in Nebraska. Archives of Environmental Health 40:211–214.
Blair A, Mustafa D, Heineman EF. 1993. Cancer and other causes of death among male and female farmers from twenty-three states. American Journal of Industrial Medicine 23:729–742.
Blair A, Sandler DP, Tarone R, Lubin J, Thomas K, Hoppin JA, Samanic C, Coble J, Kamel F, Knott C, Dosemeci M, Zahm SH, Lynch CF, Rothman N, Alavanja MC. 2005a. Mortality among participants in the Agricultural Health Study. Annals of Epidemiology 15(4):279–285.
Blair A, Sandler D, Thomas K, Hoppin JA, Kamel F, Cobel J, Lee WJ, Rusiecki J, Knott C, Dosemeci M, Lynch CF, Lubin J, Alavanja M. 2005b. Disease and injury among participants in the Agricultural Health Study. Journal of Agricultural Safety and Health 11(2):141–150.
Blair A, Thomas K, Coble J, Sandler DP, Hines CJ, Lynch CF, Knott C, Purdue MP, Zahm SH, Alavanja MC, Dosemeci M, Kamel F, Hoppin JA, Freeman LB, Lubin JH. 2011. Impact of pesticide exposure misclassification on estimates of relative risks in the Agricultural Health Study. Occupational and Environmental Medicine 68(7):537–541.
Blatter BM, Hermens R, Bakker M, Roeleveld N, Verbeek AL, Zielhuis GA. 1997. Paternal occupational exposure around conception and spina bifida in offspring. American Journal of Industrial Medicine 32(3):283–291.
Bloemen LJ, Mandel JS, Bond GG, Pollock AF, Vitek RP, Cook RR. 1993. An update of mortality among chemical workers potentially exposed to the herbicide 2,4-dichlorophenoxyacetic acid and its derivatives. Journal of Occupational Medicine 35:1208–1212.
Bloom M, Vena J, Olson J, Moysich K. 2006. Chronic exposure to dioxin-like compounds and thyroid function among New York anglers. Environmental Toxicology and Pharmacology 21(3):260–267.
Bodner KM, Collins JJ, Bloemen LJ, Carson ML. 2003. Cancer risk for chemical workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occupational and Environmental Medicine 60:672–675.
Boehmer TK, Flanders WD, McGeehin MA, Boyle C, Barrett DH. 2004. Postservice mortality in Vietnam veterans: 30-year follow-up. Archives of Internal Medicine 164(17):1908–1916.
Boeri R, Bordo B, Crenna P, Filippini G, Massetto M, Zecchini A. 1978. Preliminary results of a neurological investigation of the population exposed to TCDD in the Seveso region. Rivista di Patologia Nervosa e Mentale 99:111–128.
Boers D, Portengen L, Bueno de Mesquita HB, Heederik D, Vermeulen R. 2010. Cause-specific mortality of Dutch chlorophenoxy herbicide manufacturing workers. Occupational and Environmental Medicine 67(1):24–31.
Boers D, Portengen L, Turner WE, Bueno de Mesquita HB, Heederik D, Vermeulen R. 2012. Plasma dioxin levels and cause-specific mortality in an occupational cohort of workers exposed to chlorophenoxy herbicides, chlorophenols and contaminants. Occupational and Environmental Medicine 69(2):113–118.
Boffetta P, Stellman SD, Garfinkel L. 1989. A case-control study of multiple myeloma nested in the American Cancer Society prospective study. International Journal of Cancer 43:554–559.
Bond GG, Ott MG, Brenner FE, Cook RR. 1983. Medical and morbidity surveillance findings among employees potentially exposed to TCDD. British Journal of Industrial Medicine 40:318–324.
Bond GG, Cook RR, Brenner FE, McLaren EA. 1987. Evaluation of mortality patterns among chemical workers with chloracne. Chemosphere 16:2117–2121.
Bond GG, Wetterstroem NH, Roush GJ, McLaren EA, Lipps TE, Cook RR. 1988. Cause specific mortality among employees engaged in the manufacture, formulation, or packaging of 2,4-dichlorophenoxyacetic acid and related salts. British Journal of Industrial Medicine 45:98–105.
Bond GG, McLaren EA, Lipps TE, Cook RR. 1989a. Update of mortality among chemical workers with potential exposure to the higher chlorinated dioxins. Journal of Occupational Medicine 31:121–123.
Bond GG, McLaren EA, Brenner FE, Cook RR. 1989b. Incidence of chloracne among chemical workers potentially exposed to chlorinated dioxins. Journal of Occupational Medicine 31:771–774.
Boyle C, Decoufle P, Delaney RJ, DeStefano F, Flock ML, Hunter MI, Joesoef MR, Karon JM, Kirk ML, Layde PM, McGee DL, Moyer LA, Pollock DA, Rhodes P, Scally MJ, Worth RM. 1987. Postservice Mortality Among Vietnam Veterans. Atlanta, GA: Centers for Disease Control. CEH 86–0076.
Breslin P, Kang H, Lee Y, Burt V, Shepard BM. 1988. Proportionate mortality study of US Army and US Marine Corps veterans of the Vietnam War. Journal of Occupational Medicine 30:412–419.
Brown LM, Blair A, Gibson R, Everett GD, Cantor KP, Schuman LM, Burmeister LF, Van Lier SF, Dick F. 1990. Pesticide exposures and other agricultural risk factors for leukemia among men in Iowa and Minnesota. Cancer Research 50:6585–6591.
Brown LM, Burmeister LF, Everett GD, Blair A. 1993. Pesticide exposures and multiple myeloma in Iowa men. Cancer Causes and Control 4:153–156.
Bueno de Mesquita HB, Doornbos G, van der Kuip DA, Kogevinas M, Winkelmann R. 1993. Occupational exposure to phenoxy herbicides and chlorophenols and cancer mortality in the Netherlands. American Journal of Industrial Medicine 23:289–300.
Bullman TA, Kang HK. 1996. The risk of suicide among wounded Vietnam veterans. American Journal of Public Health 86(5):662–667.
Bullman TA, Kang HK, Watanabe KK. 1990. Proportionate mortality among US Army Vietnam veterans who served in Military Region I. American Journal of Epidemiology 132:670–674.
Bullman TA, Kang H, Thomas TL. 1991. Posttraumatic stress disorder among Vietnam veterans on the Agent Orange Registry: A case-control analysis. Annals of Epidemiology 1:505–512.
Bullman TA, Watanabe KK, Kang HK. 1994. Risk of testicular cancer associated with surrogate measures of Agent Orange exposure among Vietnam veterans on the Agent Orange Registry. Annals of Epidemiology 4:11–16.
Burmeister LF. 1981. Cancer mortality in Iowa farmers: 1971–1978. Journal of the National Cancer Institute 66:461–464.
Burmeister LF, Van Lier SF, Isacson P. 1982. Leukemia and farm practices in Iowa. American Journal of Epidemiology 115:720–728.
Burmeister LF, Everett GD, Van Lier SF, Isacson P. 1983. Selected cancer mortality and farm practices in Iowa. American Journal of Epidemiology 118:72–77.
Burns CJ, Beard KK, Cartmill JB. 2001. Mortality in chemical workers potentially exposed to 2,4-dichlorophenoxyacetic acid (2,4-D) 1945–1994: An update. Occupational and Environmental Medicine 58:24–30.
Burns CJ, Collins JJ, Humphry N, Bodner KM, Aylward LL, McBride D. 2010. Correlates of serum dioxin to self-reported exposure factors. Environmental Research 110(2):131–136.
Burns CJ, Bodner K, Swaen G, Collins J, Beard K, Lee M. 2011. Cancer incidence of 2,4-D production workers. International Journal of Environmental Research and Public Health 8(9):3579–3590.
Burns JS, Williams PL, Sergeyev O, Korrick S, Lee MM, Revich B, Altshul L, Patterson DG Jr, Turner WE, Needham LL, Saharov I, Hauser R. 2009. Predictors of serum dioxins and PCBS among peripubertal Russian boys. Environmental Health Perspectives 117(10):1593–1599.
Burns JS, Williams PL, Sergeyev O, Korrick S, Lee MM, Revich B, Altshul L, Del Prato JT, Humblet O, Patterson DG, Turner WE, Needham LL, Starovoytov M, Hauser R. 2011. Serum dioxins and polychlorinated biphenyls are associated with growth among Russian boys. Pediatrics 127(1): e59-e68.
Burt VL, Breslin PP, Kang HK, Lee Y. 1987. Non-Hodgkin’s lymphoma in Vietnam veterans. Washington, DC: Department of Medicine and Surgery, Veterans Administration.
Burton JE, Michalek JE, Rahe AJ. 1998. Serum dioxin, chloracne, and acne in veterans of Operation Ranch Hand. Archives of Environmental Health 53(3):199–204.
Butterfield PG, Valanis BG, Spencer PS, Lindeman CA, Nutt JG. 1993. Environmental antecedents of young-onset Parkinson’s disease. Neurology 43:1150–1158.
Calvert GM, Sweeney MH, Morris JA, Fingerhut MA, Hornung RW, Halperin WE. 1991. Evaluation of chronic bronchitis, chronic obstructive pulmonary disease, and ventilatory function among workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. American Review of Respiratory Disease 144:1302–1306.
Calvert GM, Hornung RW, Sweeney MH, Fingerhut MA, Halperin WE. 1992. Hepatic and gastrointestinal effects in an occupational cohort exposed to 2,3,7,8-tetrachlorodibenzo-para-dioxin. Journal of the American Medical Association 267:2209–2214.
Calvert GM, Sweeney MH, Fingerhut MA, Hornung RW, Halperin WE. 1994. Evaluation of porphyria cutanea tarda in US workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. American Journal of Industrial Medicine 25:559–571.
Calvert GM, Wall DK, Sweeney MH, Fingerhut MA. 1998. Evaluation of cardiovascular outcomes among US workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Environmental Health Perspectives 106(Suppl 2):635–643.
Calvert GM, Sweeney MH, Deddens J, Wall DK. 1999. Evaluation of diabetes mellitus, serum glucose, and thyroid function among United States workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occupational and Environmental Medicine 56(4):270–276.
Campbell SB, Renshaw KD. 2012. Distress in spouses of Vietnam veterans: Associations with communication about deployment experiences. Journal of Family Psychology 26(1):18–25.
Can N, Xiem NT, Tong NK, Duong DB. 1983a. A case-control survey of congenital defects in My Van District, Hai Hung Province. Summarized in: Constable JD, Hatch MC. Reproductive Effects of Herbicide Exposure in Vietnam: Recent Studies by the Vietnamese and Others. As cited in Constable and Hatch, 1985.
Can N, Xiem NT, Tong NK, Duong DB. 1983b. An epidemiologic survey of pregnancies in Viet Nam. Summarized in: Constable JD, Hatch MC. Reproductive Effects of Herbicide Exposure in Vietnam: Recent Studies by the Vietnamese and Others. As cited in Constable and Hatch, 1985.
Cantor KP. 1982. Farming and mortality from non-Hodgkin’s lymphoma: A case-control study. International Journal of Cancer 29:239–247.
Cantor KP, Blair A, Everett G, Gibson R, Burmeister LF, Brown LM, Schuman L, Dick FR. 1992. Pesticides and other agricultural risk factors for non-Hodgkin’s lymphoma among men in Iowa and Minnesota. Cancer Research 52:2447–2455.
Caramaschi F, Del Corno G, Favaretti C, Giambelluca SE, Montesarchio E, Fara GM. 1981. Chloracne following environmental contamination by TCDD in Seveso, Italy. International Journal of Epidemiology 10:135–143.
Carmelli D, Hofherr L, Tomsic J, Morgan RW. 1981. A Case-Control Study of the Relationship Between Exposure to 2,4-D and Spontaneous Abortions in Humans. SRI International. Prepared for the National Forest Products Association and the US Department of Agriculture, Forest Service.
Carreon T, Butler MA, Ruder AM, Waters MA, Davis-King KE, Calvert GM, Schulte PA, Connally B, Ward EM, Sanderson WT, Heineman EF, Mandel JS, Morton RF, Reding DJ, Rosenman KD, Talaska G, Cancer B. 2005. Gliomas and farm pesticide exposure in women: The Upper Midwest Health Study. Environmental Health Perspectives 113(5):546–551.
Cartwright RA, McKinney PA, O’Brien C, Richards IDG, Roberts B, Lauder I, Darwin CM, Bernard SM, Bird CC. 1988. Non-Hodgkin’s lymphoma: Case-control epidemiological study in Yorkshire. Leukemia Research 12:81–88.
Castorina R, Bradman A, Fenster L, Barr DB, Bravo R, Vedar MG, Harnly ME, McKone TE, Eisen EA, Eskenazi B. 2010. Comparison of current-use pesticide and other toxicant urinary metabolite levels among pregnant women in the CHAMACOS cohort and NHANES. Environmental Health Perspectives 118(6):856–863.
CDC (Centers for Disease Control and Prevention). 1985. Exposure Assessment for the Agent Orange Study. Interim Report Number 2. Atlanta, GA: Center for Environmental Health.
CDC. 1987. Postservice mortality among Vietnam veterans. Journal of the American Medical Association 257:790–795.
CDC. 1988a. Health status of Vietnam veterans. II. Physical health. Journal of the American Medical Association 259:2708–2714.
CDC. 1988b. Health status of Vietnam veterans. I. Psychosocial characteristics. Journal of the American Medical Association 259:2701–2707.
CDC. 1988c. Health status of Vietnam veterans. III. Reproductive outcomes and child health. Journal of the American Medical Association 259:2715–2717.
CDC. 1988d. Preliminary report: 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure in humans—Seveso, Italy. Morbidity and Mortality Weekly Report 37:733–736.
CDC. 1989a. Comparison of Serum Levels of 2,3,7,8-Tetrachlorodibenzo-p-dioxin with Indirect Estimates of Agent Orange Exposure Among Vietnam Veterans: Final Report. Atlanta, GA: US Department of Health and Human Services.
CDC. 1989b. Health Status of Vietnam Veterans: Vietnam Experience Study. Vols. I—V, Supplements A-C. Atlanta, GA: US Department of Health and Human Services.
CDC. 1990a. The Association of Selected Cancers with Service in the US Military in Vietnam: Final Report. Atlanta, GA: US Department of Health and Human Services.
CDC. 1990b. The association of selected cancers with service in the US military in Vietnam. I. Non-Hodgkin’s lymphoma. Archives of Internal Medicine 150:2473–2483.
CDC. 1990c. The association of selected cancers with service in the US military in Vietnam. II. Soft-tissue and other sarcomas. Archives of Internal Medicine 150:2485–2492.
CDC. 1990d. The association of selected cancers with service in the US military in Vietnam. III. Hodgkin’s disease, nasal cancer, nasopharyngeal cancer, and primary liver cancer. Archives of Internal Medicine 150:2495–2505.
CDC. 2004. 2001–2002 National Health and Nutrition Examination Survey (NHANES). http://www.cdc.gov/nchs/nhanes/nhanes2001–2002/nhanes01_02.htm (accessed April 1, 2011).
CDVA (Commonwealth Department of Veterans’ Affairs). 1998a. Morbidity of Vietnam Veterans: A Study of the Health of Australia’s Vietnam Veteran Community. Volume 1: Male Vietnam Veterans Survey and Community Comparison Outcomes. Canberra, Australia: Department of Veterans’ Affairs.
CDVA. 1998b. Morbidity of Vietnam Veterans: A Study of the Health of Australia’s Vietnam Veteran Community. Volume 2: Female Vietnam Veterans Survey and Community Comparison Outcomes. Canberra, Australia: Department of Veterans’ Affairs.
Chamie K, DeVere White R, Lee D, Ok J, Ellison L. 2008. Agent Orange exposure, Vietnam War veterans, and the risk of prostate cancer. Cancer 113(9):2464–2470.
Chang JW, Chen HL, Su HJ, Liao PC, Guo HR, Lee CC. 2010. Dioxin exposure and insulin resistance in Taiwanese living near a highly contaminated area. Epidemiology 21(1):56–61.
Chang JW, Chen HL, Su HJ, Liao PC, Guo HR, Lee CC. 2011a. Simultaneous exposure of non-diabetics to high levels of dioxins and mercury increases their risk of insulin resistance. Journal of Hazardous Materials 185(2–3):749–755.
Chang JW, Chen HL, Chang CC, Su HJ, Liao PC, Lee CC. 2011b. Predicting the risk of cardiovascular disease in people exposed to moderate to high levels of dioxin. Journal of Hazardous Materials 198:317–322.
Chang JW, Chen HL, Su HJ, Liao PC, Lee CC. 2012. Biochemical study of retired pentachlorophenol workers with and without following dietary exposure to PCDD/FS. Chemosphere 88(7):813–819.
Chao HR, Wang SL, Lee CC, Yu HY, Lu YK, Päpke O. 2004. Level of polychlorinated dibenzo-p-dioxins, dibenzofurans and biphenyls (PCDD/Fs, PCBs) in human milk and the input to infant body burden. Food and Chemical Toxicology 42:1299–1308.
Chao HR, Wang SL, Lin LY, Lee WJ, Päpke O. 2007. Placental transfer of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in Taiwanese mothers in relation to menstrual cycle characteristics. Food Chemical Toxicology 45:259–265.
Chen HL, Su HJ, Guo YL, Liao PC, Hung CF, Lee CC. 2006. Biochemistry examinations and health disorder evaluation of Tiawanese living near incinerators and with low serum PCDD/Fs levels. Science of the Total Environment 366:538–548.
Chen Z, Stewart PA, Davies S, Giller R, Krailo M, Davis M, Robison L, Shu XO. 2005. Parental occupational exposure to pesticides and childhood germ-cell tumors. American Journal of Epidemiology 162(9):858–867.
Chen Z, Robison L, Giller R, Krailo M, Davis M, Davies S, Shu XO. 2006. Environmental exposure to residential pesticides, chemicals, dusts, fumes, and metals, and risk of childhood germ cell tumors. International Journal of Hygiene and Environmental Health 209(1):31–40.
Chiu BC, Weisenburger DD, Zahm SH, Cantor KP, Gapstur SM, Holmes F, Burmeister LF, Blair A. 2004. Agricultural pesticide use, familial cancer, and risk of non-Hodgkin lymphoma. Cancer Epidemiology, Biomarkers and Prevention 13(4):525–531.
Chiu BC, Dave BJ, Blair A, Gapstur SM, Zahm SH, Weisenburger DD. 2006. Agricultural pesticide use and risk of t(14;18)-defined subtypes of non-Hodgkin lymphoma. Blood 108(4):1363–1369.
Cho MR, Shin JY, Hwang JH, Jacobs DR Jr, Kim SY, Lee DH. 2011. Associations of fat mass and lean mass with bone mineral density differ by levels of persistent organic pollutants: National Health and Nutrition Examination Survey 1999–2004. Chemosphere 82:1268–1276.
CIH (Commonwealth Institute of Health). 1984a. Australian Veterans Health Studies. Mortality Report. Part I. A Retrospective Cohort Study of Mortality Among Australian National Servicemen of the Vietnam Conflict Era, and An Executive Summary of the Mortality Report. Canberra, Australia: Australian Government Publishing Service.
CIH. 1984b. Australian Veterans Health Studies. The Mortality Report. Part II. Factors Influencing Mortality Rates of Australian National Servicemen of the Vietnam Conflict Era. Canberra, Australia: Australian Government Publishing Service.
CIH. 1984c. Australian Veterans Health Studies. The Mortality Report. Part III. The Relationship Between Aspects of Vietnam Service and Subsequent Mortality Among Australian National Servicemen of the Vietnam Conflict Era. Canberra, Australia: Australian Government Publishing Service.
Clapp RW. 1997. Update of cancer surveillance of veterans in Massachusetts, USA. International Journal of Epidemiology 26(3):679–681.
Clapp RW, Cupples LA, Colton T, Ozonoff DM. 1991. Cancer surveillance of veterans in Massachusetts, 1982–1988. International Journal of Epidemiology 20:7–12.
Coble J, Thomas KW, Hines CJ, Hoppin JA, Dosemeci M, Curwin B, Lubin JH, Beane Freeman LE, Blair A, Sandler DP, Alavanja MC. 2011. An updated algorithm for estimation of pesticide exposure intensity in the Agricultural Health Study. International Journal of Environmental Research and Public Health 8(12):4608–4622.
Coggon D, Pannett B, Winter PD, Acheson ED, Bonsall J. 1986. Mortality of workers exposed to 2-methyl-4-chlorophenoxyacetic acid. Scandinavian Journal of Work, Environment and Health 12:448–454.
Coggon D, Pannett B, Winter P. 1991. Mortality and incidence of cancer at four factories making phenoxy herbicides. British Journal of Industrial Medicine 48:173–178.
Collins JJ, Strauss ME, Levinskas GJ, Conner PR. 1993. The mortality experience of workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin in a trichlorophenol process accident. Epidemiology 4:7–13.
Collins JJ, Budinsky RA, Burns CJ, Lamparski LL, Carson ML, Martin GD, Wilken M. 2006. Serum dioxin levels in former chlorophenol workers. Journal of Exposure Science and Environmental Epidemiology 16(1):76–84.
Collins JJ, Bodner K, Aylward LL, Wilken M, Bodner CM. 2009a. Mortality rates among trichlorophenol workers with exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. American Journal of Epidemiology 170(4):501–506.
Collins JJ, Bodner K, Aylward LL, Wilken M, Swaen G, Budinsky R, Rowlands C, Bodnar CM. 2009b. Mortality rates among workers exposed to dioxins in the manufacture of pentachlorophenol. Journal of Occupational and Environmental Medicine 51(10):1212–1219.
Collins JJ, Wilken M, McBride D, Humphry NF, Herbison P, Burns CJ, Bodner K. 2009c. Serum concentrations of chlorinated dibenzo-p-dioxins and dibenzofurans among former New Zealand trichlorophenol workers. Chemosphere 76(11):1550–1556.
Colt JS, Rothman N, Severson RK, Hartge P, Cerhan JR, Chatterjee N, Cozen W, Morton LM, De Roos AJ, Davis S, Chanock S, Wang SS. 2009. Organochlorine exposure, immune gene variation, and risk of non-Hodgkin lymphoma. Blood 113(9):1899–1905.
Conley D, Heerwig J. 2012. The long-term effects of military conscription on mortality: Estimates from the Vietnam-era draft lottery. Demography 49:841–855.
Consonni D, Pesatori AC, Zocchetti C, Sindaco R, D’Oro LC, Rubagotti M, Bertazzi PA. 2008. Mortality in a population exposed to dioxin after the Seveso, Italy, accident in 1976: 25 years of follow-up. American Journal of Epidemiology 167(7):847–858.
Constable JD, Hatch MC. 1985. Reproductive effects of herbicide exposure in Vietnam: Recent studies by the Vietnamese and others. Teratogenesis, Carcinogenesis, and Mutagenesis 5:231–250.
Cook RR, Townsend JC, Ott MG, Silverstein LG. 1980. Mortality experience of employees exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Journal of Occupational Medicine 22:530–532.
Cook RR, Bond GG, Olson RA. 1986. Evaluation of the mortality experience of workers exposed to the chlorinated dioxins. Chemosphere 15:1769–1776.
Cook RR, Bond GG, Olson RA, Ott MG. 1987. Update of the mortality experience of workers exposed to chlorinated dioxins. Chemosphere 16:2111–2116.
Cordier S, Le TB, Verger P, Bard D, Le CD, Larouze B, Dazza MC, Hoang TQ, Abenhaim L. 1993. Viral infections and chemical exposures as risk factors for hepatocellular carcinoma in Vietnam. International Journal of Cancer 55:196–201.
Cordier S, Chevrier C, Robert-Gnansia E, Lorente C, Brula P, Hours M. 2004. Risk of congenital anomalies in the vicinity of municipal solid waste incinerators. Occupational and Environmental Medicine 61(1):8–15.
Cordier S, Lehebel A, Amar E, Anzivino-Viricel L, Hours M, Monfort C, Chevrier C, Chiron M, Robert-Gnansia E. 2010. Maternal residence near municipal waste incinerators and the risk of urinary tract birth defects. Occupational and Environmental Medicine 67(7):493–499.
Corrao G, Caller M, Carle F, Russo R, Bosia S, Piccioni P. 1989. Cancer risk in a cohort of licensed pesticide users. Scandinavian Journal of Work, Environment and Health 15:203–209.
Costani G, Rabitti P, Mambrini A, Bai E, Berrino F. 2000. Soft tissue sarcomas in the general population living near a chemical plant in northern Italy. Tumori 86:381–383.
Crane PJ, Barnard DL, Horsley KW, Adena MA. 1997a. Mortality of Vietnam Veterans: The Veteran Cohort Study. A Report of the 1996 Retrospective Cohort Study of Australian Vietnam Veterans. Canberra, Australia: Department of Veterans Affairs.
Crane PJ, Barnard DL, Horsley KW, Adena MA. 1997b. Mortality of National Service Vietnam Veterans: A Report of the 1996 Retrospective Cohort Study of Australian Vietnam Veterans. Canberra, Australia: Department of Veterans Affairs.
Crawford JM, Hoppin JA, Alavanja MCR, Blair A, Sandler DP, Kamel F. 2008. Hearing loss among licensed pesticide applicators in the Agricultural Health Study. Journal of Occupational and Environmental Medicine 50(7):817–826.
Currier JM, Holland JM. 2012. Examining the role of combat loss among Vietnam War veterans. Journal of Traumatic Loss 25:102–105.
Curtis K, Savitz D, Weinberg C, Arbuckle T. 1999. The effect of pesticide exposure on time to pregnancy. Epidemiology 10(2):112–117. [Comment in Epidemiology 1999. 10(3):470.]
Curwin BD, Hein MJ, Sanderson WT, Barr DB, Heederik D, Reynolds SJ, Ward EM, Alavanja MC. 2005. Urinary and hand wipe pesticide levels among farmers and nonfarmers in Iowa. Journal of Exposure Analysis and Environmental Epidemiology 15(6):500–508.
Cypel Y, Kang H. 2008. Mortality patterns among women Vietnam-era veterans: Results of a retrospective cohort study. Annals of Epidemiology 18(3):244–252.
Cypel Y, Kang H. 2010. Mortality patterns of Army Chemical Corps veterans who were occupationally exposed to herbicides in Vietnam. Annals of Epidemiology 20(5):339–346.
Dai LC, Phuong NTN, Thom LH, Thuy TT, Van NTT, Cam LH, Chi HTK, Thuy LB. 1990. A comparison of infant mortality rates between two Vietnamese villages sprayed by defoliants in wartime and one unsprayed village. Chemosphere 20:1005–1012.
Dalager NA, Kang HK. 1997. Mortality among Army Chemical Corps Vietnam veterans. American Journal of Industrial Medicine 31(6):719–726.
Dalager NA, Kang HK, Burt VL, Weatherbee L. 1991. Non-Hodgkin’s lymphoma among Vietnam veterans. Journal of Occupational Medicine 33:774–779.
Dalager NA, Kang HK, Burt VL, Weatherbee L. 1995a. Hodgkin’s disease and Vietnam service. Annals of Epidemiology 5(5):400–406.
Dalager NA, Kang HK, Thomas TL. 1995b. Cancer mortality patterns among women who served in the military: The Vietnam experience. Journal of Occupational and Environmental Medicine 37:298–305.
Dankovic DA, Andersen ME, Salvan A, Stayner LT. 1995. A simplified PBPK model describing the kinetics of TCDD in humans (abstract). Toxicologist 15:272.
De Felip E, Porpora MG, di Domenico A, Ingelido AM, Cardelli M, Cosmi EV, Donnez J. 2004. Dioxin-like compounds and endometriosis: A study on Italian and Belgian women of reproductive age. Toxicology Letters 150(2):203–209.
De Roos AJ, Hartge P, Lubin JH, Colt JS, Davis S, Cerhan JR, Severson RK, Cozen W, Patterson DG Jr, Needham LL, Rothman N. 2005a. Persistent organochlorine chemicals in plasma and risk of non-Hodgkin’s lymphoma. Cancer Research 65(23):11214–11226.
De Roos AJ, Cooper GS, Alavanja MC, Sandler DP. 2005b. Rheumatoid arthritis among women in the Agricultural Health Study: Risk associated with farming activities and exposures. Annals of Epidemiology 15(10):762–770.
Decoufle P, Holmgreen P, Boyle CA, Stroup NE. 1992. Self-reported health status of Vietnam veterans in relation to perceived exposure to herbicides and combat. American Journal of Epidemiology 135:312–323.
Dennis LK, Lynch CF, Sandler DP, Alavanja M. 2010. Pesticide use and cutaneous melanoma in pesticide applicators in the Agricultural Health Study. Environmental Health Perspectives 118(6):812–817.
Deprez RD, Carvette ME, Agger MS. 1991. The Health and Medical Status of Maine Veterans: A Report to the Bureau of Veterans Services, Commission of Vietnam and Atomic Veterans. Portland, ME: Public Health Resource Group.
Dich J, Wiklund K. 1998. Prostate cancer in pesticide applicators in Swedish agriculture. Prostate 34(2):100–112.
Dimich-Ward H, Hertzman C, Teschke K, Hershler R, Marion SA, Ostry A, Kelly S. 1996. Reproductive effects of paternal exposure to chlorophenate wood preservatives in the sawmill industry. Scandinavian Journal of Work, Environment and Health 22(4):267–273.
Donna A, Betta PG, Robutti F, Crosignani P, Berrino F, Bellingeri D. 1984. Ovarian mesothelial tumors and herbicides: A case-control study. Carcinogenesis 5:941–942.
Donovan JW, Adena MA, Rose G, Battistutta D. 1983. Case-Control Study of Congenital Anomalies and Vietnam Service: Birth Defects Study. Report to the Minister for Veterans’ Affairs. Canberra, Australia: Australian Government Publishing Service.
Donovan JW, MacLennan R, Adena M. 1984. Vietnam service and the risk of congenital anomalies: A case-control study. Medical Journal of Australia 140:394–397.
Dosemeci M, Alavanja MC, Rowland AS, Mage D, Zahm SH, Rothman N, Lubin JH, Hoppin JA, Sandler DP, Blair A. 2002. A quantitative approach for estimating exposure to pesticides in the Agricultural Health Study. Annals of Occupational Hygiene 46(2):245–260.
Dubrow R, Paulson JO, Indian RW. 1988. Farming and malignant lymphoma in Hancock County, Ohio. British Journal of Industrial Medicine 45:25–28.
Duell EJ, Millikan RC, Savitz DA, Schell MJ, Newman B, Tse CKJ, Sandler DP. 2001. Reproducibility of reported farming activities and pesticide use among breast cancer cases and controls: A comparison of two modes of data collection. Association of Emergency Physicians 11(3):178–185.
Egeland GM, Sweeney MH, Fingerhut MA, Wille KK, Schnorr TM, Halperin WE. 1994. Total serum testosterone and gonadotropins in workers exposed to dioxin. American Journal of Epidemiology 139:272–281.
Eisen S, Goldberg J, True WR, Henderson WG. 1991. A co-twin control study of the effects of the Vietnam War on the self-reported physical health of veterans. American Journal of Epidemiology 134:49–58.
Ekström AM, Eriksson M, Hansson LE, Lindgren A, Signorello LB, Nyren O, Hardell L. 1999. Occupational exposures and risk of gastric cancer in a population-based case-control study. Cancer Research 59(23):5932–5937.
Elobeid MA, Padilla MA, Brock DW, Ruden DM, Allison DB. 2010. Endocrine disruptors and obesity: An examination of selected persistent organic pollutants in the NHANES 1999–2002 data. International Journal of Environmental Research and Public Health 7:2988–3005.
Engel LS, Hill DA, Hoppin JA, Lubin JH, Lynch CF, Pierce J, Samanic C, Sandler DP, Blair A, Alavanja MC. 2005. Pesticide use and breast cancer risk among farmers’ wives in the Agricultural Health Study. American Journal of Epidemiology 161(2):121–135.
Erickson JD, Mulinare J, McClain PW, Fitch TG, James LM, McClearn AB, Adams MJ Jr. 1984a. Vietnam Veterans’ Risks for Fathering Babies with Birth Defects. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control.
Erickson JD, Mulinare J, McClain PW, Fitch TG, James LM, McClearn AB, Adams MJ Jr. 1984b. Vietnam veterans’ risks for fathering babies with birth defects. Journal of the American Medical Association 252:903–912.
Eriksson M, Hardell L, Berg NO, Moller T, Axelson O. 1979. Case-control study on malignant mesenchymal tumor of the soft tissue and exposure to chemical substances. Lakartidningen 76:3872–3875 [in Swedish].
Eriksson M, Hardell L, Berg NO, Moller T, Axelson O. 1981. Soft-tissue sarcomas and exposure to chemical substances: A case-referent study. British Journal of Industrial Medicine 38:27–33.
Eriksson M, Hardell L, Adami HO. 1990. Exposure to dioxins as a risk factor for soft tissue sarcoma: A population-based case-control study. Journal of the National Cancer Institute 82:486–490.
Eriksson M, Hardell L, Malker H, Weiner J. 1992. Malignant lymphoproliferative diseases in occupations with potential exposure to phenoxyacetic acids or dioxins: A register-based study. American Journal of Industrial Medicine 22:305–312.
Eskenazi B, Mocarelli P, Warner M, Samuels S, Needham L, Patterson D, Brambilla P, Gerthoux PM, Turner W, Casalini S, Cazzaniga M, Chee WY. 2001. Seveso Women’s Health Study: Does zone of residence predict individual TCDD exposure? Chemosphere 43(4–7):937–942.
Eskenazi B, Warner M, Mocarelli P, Samuels S, Needham LL, Patterson DG Jr, Lippman S, Vercellini P, Gerthoux PM, Brambilla P, Olive D. 2002a. Serum dioxin concentrations and menstrual cycle characteristics. American Journal of Epidemiology 156(4):383–392.
Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, Needham LL, Patterson DG Jr, Brambilla P, Gavoni N, Casalini S, Panazza S, Turner W, Gerthoux PM. 2002b. Serum dioxin concentrations and endometriosis: A cohort study. Environmental Health Perspectives 110(7):629–634.
Eskenazi B, Mocarelli P, Warner M, Chee WY, Gerthoux PM, Samuels S, Needham LL, Patterson DG Jr. 2003a. Maternal serum dioxin levels and birth outcomes in women of Seveso, Italy. Environmental Health Perspectives 111(7):947–953.
Eskenazi B, Bradman A, Gladstone EA, Jaramillo S, Birch K, Holland N. 2003b. CHAMACOS, a longitudinal birth cohort study: Lessons from the fields. Journal of Children’s Health 1(1):3–27.
Eskenazi B, Mocarelli P, Warner M, Needham LL, Patterson DG Jr, Samuels S, Turner W, Gerthoux PM, Brambilla P. 2004. Relationship of serum TCDD concentrations and age at exposure of female residents of Seveso, Italy. Environmental Health Perspectives 112(1):22–27.
Eskenazi B, Warner M, Marks AR, Samuels S, Gerthoux PM, Vercellini P, Olive DL, Needham L, Patterson D Jr, Mocarelli P. 2005. Serum dioxin concentrations and age at menopause. Environmental Health Perspectives 113(7):858–862.
Eskenazi B, Warner M, Samuels S, Young J, Gerthoux PM, Needham L, Patterson D, Olive D, Gavoni N, Vercellini P, Mocarelli P. 2007. Serum dioxin concentrations and risk of uterine leiomyoma in the Seveso Women’s Health Study. American Journal of Epidemiology 166(1):79–87.
Eskenazi B, Warner M, Marks AR, Samuels S, Needham L, Brambilla P, Mocarelli P. 2010. Serum dioxin concentrations and time to pregnancy. Epidemiology 21(2):224–231.
Evans RG, Webb KB, Knutsen AP, Roodman ST, Roberts DW, Bagby JR, Garrett WA Jr, Andrews JS Jr. 1988. A medical follow-up of the health effects of long-term exposure to 2,3,7,8-tetrachloro-dibenzo-p-dioxin. Archives of Environmental Health 43:273–278.
Evatt P. 1985. Royal Commission on the Use and Effect of Chemical Agents on Australian Personnel in Vietnam, Final Report. Canberra, Australia: Australian Government Publishing Service.
Everett CJ, Mainous AG 3rd, Frithsen IL, Player MS, Matheson EM. 2008a. Association of polychlorinated biphenyls with hypertension in the 1999–2002 National Health and Nutrition Examination Survey. Environmental Research 108(1):94–97.
Everett CJ, Mainous AG 3rd, Frithsen IL, Player MS, Matheson EM. 2008b. Commentary on association of polychlorinated biphenyls with hypertension. Environmental Research 108(3):428–429.
Farberow NL, Kang H, Bullman T. 1990. Combat experience and postservice psychosocial status as predictors of suicide in Vietnam veterans. Journal of Nervous and Mental Disease 178:32–37.
Farr SL, Cooper GS, Cai J, Savitz DA, Sandler DP. 2004. Pesticide use and menstrual cycle characteristics among premenopausal women in the Agricultural Health Study. American Journal of Epidemiology 160(12):1194–1204.
Farr SL, Cai J, Savitz DA, Sandler DP, Hoppin JA, Cooper GS. 2006. Pesticide exposure and timing of menopause: The Agricultural Health Study. American Journal of Epidemiology 163(8):731–742.
Fattore E, Di Guardo A, Mariani G, Guzzi A, Benfenati E, Fanelli R. 2003. Polychlorinated dibenzo-p-dioxins and dibenzofurans in the air of Seveso, Italy, 26 years after the explosion. Environmental Science and Technology 37(8):1503–1508.
Faustini A, Settimi L, Pacifici R, Fano V, Zuccaro P, Forastiere F. 1996. Immunological changes among farmers exposed to phenoxy herbicides: Preliminary observations. Occupational and Environmental Medicine 53(9):583–585.
Fett MJ, Adena MA, Cobbin DM, Dunn M. 1987a. Mortality among Australian conscripts of the Vietnam conflict era. I. Death from all causes. American Journal of Epidemiology 126:869–877.
Fett MJ, Nairn JR, Cobbin DM, Adena MA. 1987b. Mortality among Australian conscripts of the Vietnam conflict era. II. Causes of death. American Journal of Epidemiology 125:878–884.
Fiedler N, Gochfeld M. 1992. Neurobehavioral Correlates of Herbicide Exposure in Vietnam Veterans. New Jersey Agent Orange Commission.
Fierens S, Mairesse H, Heilier JF, De Burbure C, Focant JF, Eppe G, De Pauw E, Bernard A. 2003a. Dioxin/polychlorinated biphenyl body burden, diabetes and endometriosis: Findings in a population-based study in Belgium. Biomarkers 8(6):529–534.
Fierens S, Mairesse H, Hermans C, Bernard A, Eppe G, Focant JF, De Pauw E. 2003b. Dioxin accumulation in residents around incinerators. Journal of Toxicology and Environmental Health Part A 66(14):1287–1293.
Filippini G, Bordo B, Crenna P, Massetto N, Musicco M, Boeri R. 1981. Relationship between clinical and electrophysiological findings and indicators of heavy exposure to 2,3,7,8-tetrachlorodibenzodioxin. Scandinavian Journal of Work, Environment and Health 7:257–262.
Fingerhut MA, Halperin WE, Honchar PA, Smith AB, Groth DH, Russell WO. 1984. An evaluation of reports of dioxin exposure and soft tissue sarcoma pathology among chemical workers in the United States. Scandinavian Journal of Work, Environment and Health 10(5):299–303.
Fingerhut MA, Halperin WE, Marlow DA, Piacitelli LA, Honchar PA, Sweeney MH, Greife AL, Dill PA, Steenland K, Suruda AJ. 1991. Cancer mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. New England Journal of Medicine 324:212–218.
Firestone JA, Smith-Weller T, Franklin G, Swanson P, Longstreth WT Jr, Checkoway H. 2005. Pesticides and risk of Parkinson disease: A population-based case-control study. Archives of Neurology 62:91–95.
Firestone JA, Lundin JI, Powers KM, Smith-Weller T, Franklin GM, Swanson PD, Longstreth WT Jr, Checkoway H. 2010. Occupational factors and risk of Parkinson’s disease: A population-based case-control study. American Journal of Industrial Medicine 53(3):217–223.
Fitzgerald EF, Weinstein AL, Youngblood LG, Standfast SJ, Melius JM. 1989. Health effects three years after potential exposure to the toxic contaminants of an electrical transformer fire. Archives of Environmental Health 44:214–221.
Flesch-Janys D. 1997. Analyses of exposure to polychlorinated dibenzo-p-dioxins, furans, and hexachlorocyclohexane and different health outcomes in a cohort of former herbicide-producing workers in Hamburg, Germany. Teratogenesis, Carcinogenesis and Mutagenesis 17(4–5):257–264.
Flesch-Janys D, Berger J, Gurn P, Manz A, Nagel S, Waltsgott H, Dwyer JH. 1995. Exposure to polychlorinated dioxins and furans (PCDD/F) and mortality in a cohort of workers from a herbicide-producing plant in Hamburg, Federal Republic of Germany. American Journal of Epidemiology 142(11):1165–1175.
Floret N, Mauny F, Challier B, Arveux P, Cahn JY, Viel JF. 2003. Dioxin emissions from a solid waste incinerator and risk of non-Hodgkin lymphoma. Epidemiology 14(4):392–398.
Flower KB, Hoppin JA, Lynch CF, Blair A, Knott C, Shore DL, Sandler DP. 2004. Cancer risk and parental pesticide application in children of Agricultural Health Study participants. Environmental Health Perspectives 112(5):631–635.
Forcier L, Hudson HM, Cobbin DM, Jones MP, Adena MA, Fett MJ. 1987. Mortality of Australian veterans of the Vietnam conflict and the period and location of their Vietnam service. Military Medicine 152:9–15.
Franzen PL, Woodward SH, Bootzin RR, Germain A, Colrain IM. 2012. K-complexes are not preferentially evoked to combat sounds in combat-exposed Vietnam veterans with and without post-traumatic stress disorder. International Journal of Psychophysiology 83:393–398.
Fritschi L, Benke G, Hughes AM, Kricker A, Turner J, Vajdic CM, Grulich A, Milliken S, Kaldor J, Armstrong BK. 2005. Occupational exposure to pesticides and risk of non-Hodgkin’s lymphoma. American Journal of Epidemiology 162(9):849–857.
Fukuda Y, Nakamura K, Takano T. 2003. Dioxins released from incineration plants and mortality from major diseases: An analysis of statistical data by municipalities. Journal of Medical and Dental Sciences 50:249–255.
Gallagher RP, Bajdik CD, Fincham S, Hill GB, Keefe AR, Coldman A, McLean DI. 1996. Chemical exposures, medical history, and risk of squamous and basal cell carcinoma of the skin. Cancer Epidemiology, Biomarkers and Prevention 5(6):419–424.
Gambini GF, Mantovani C, Pira E, Piolatto PG, Negri E. 1997. Cancer mortality among rice growers in Novara Province, northern Italy. American Journal of Industrial Medicine 31(4):435–441.
Garaj-Vrhovac V, Zeljezić D. 2002. Assessment of genome damage in a population of Croatian workers employed in pesticide production by chromosomal aberration analyis, micronucleus assay and Comet assay. Journal of Applied Toxicology 22(4):249–255.
García AM, Benavides FG, Fletcher T, Orts E. 1998. Paternal exposure to pesticides and congenital malformations. Scandinavian Journal of Work, Environment and Health 24(6):473–480.
Garry VF, Holland SE, Erickson LL, Burroughs BL. 2003. Male reproductive hormones and thyroid function in pesticide applicators in the Red River Valley of Minnesota. Journal of Toxicology and Environmental Health 66:965–986.
Gellis LA, Gehrman PR. 2011. Cognitive behavioral treatment for insomnia in veterans with longstanding posttraumatic stress disorder: A pilot study. Journal of Aggression, Maltreatment and Trauma 20:904–916.
Ghosh S, McLaughlin JR, Spinelli JJ, Dosman JA, McDuffie HH, Pahwa P. 2011. Multiple myeloma and occupational exposures: A population-based case-control study. Journal of Occupational and Environmental Medicine 53(6):641–646.
Goldberg J, True WR, Eisen SA, Henderson WG. 1990. A twin study of the effects of the Vietnam War on posttraumatic stress disorder. Journal of the American Medical Association 263:1227–1232.
Goldner WS, Sandler DP, Yu F, Hoppin JA, Kamel F, Levan TD. 2010. Pesticide use and thyroid disease among women in the Agricultural Health Study. American Journal of Epidemiology 171(4):455–464.
Goncharov A, Bloom M, Pavuk M, Birman I, Carpenter DO. 2010. Blood pressure and hypertension in relation to levels of serum polychlorinated biphenyls in residents of Anniston, Alabama. Journal of Hypertension 28:2053–2060.
Goncharov A, Pavuk M, Foushee HR, Carpenter DO. 2011. Blood pressure in relation to concentrations of PCB congeners and chlorinated pesticides. Environmental Health Perspectives 119(3):319–325.
Gordon JE, Shy CM. 1981. Agricultural chemical use and congenital cleft lip and/or palate. Archives of Environmental Health 36:213–221.
Gorell JM, Peterson EL, Rybicki BA, Johnson CC. 2004. Multiple risk factors for Parkinson’s disease. Journal of the Neurological Sciences 217:169–174.
Goun BD, Kuller LH. 1986. Final Report: A Case-Control Mortality Study on the Association of Soft Tissue Sarcomas, Non-Hodgkin’s Lymphomas, and Other Selected Cancers and Vietnam Military Service in Pennsylvania Males. Pittsburgh, PA: University of Pittsburgh.
Green LM. 1987. Suicide and exposure to phenoxy acid herbicides. Scandinavian Journal of Work, Environment and Health 13(5):460.
Green LM. 1991. A cohort mortality study of forestry workers exposed to phenoxy acid herbicides. British Journal of Industrial Medicine 48:234–238.
Greenwald P, Kovasznay B, Collins DN, Therriault G. 1984. Sarcomas of soft tissues after Vietnam service. Journal of the National Cancer Institute 73:1107–1109.
Gupta A, Ketchum N, Roehrborn CG, Schecter A, Aragaki CC, Michalek JE. 2006. Serum dioxin, testosterone, and subsequent risk of benign prostatic hyperplasia: A prospective cohort study of Air Force veterans. Environmental Health Perspectives 114(11):1649–1654.
Ha MH, Lee DH, Jacobs DR Jr. 2007. Association between serum concentrations of persistent organic pollutants and self-reported cardiovascular disease prevalence: Results from the National Health and Nutrition Examination Survey, 1999–2002. Environmental Health Perspectives 115(8):1204–1209.
Ha MH, Lee DH, Son HK, Park SK, Jacobs DR. 2009. Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: Results from the National Health and Nutrition Examination Survey 1999–2002. Journal of Human Hypertension 23(4):274–286.
Halldorsson TI, Thorsdottir I, Meltzer HM, Strom M, Olsen SF. 2009. Dioxin-like activity in plasma among Danish pregnant women: Dietary predictors, birth weight and infant development. Environmental Research 109(1):22–28.
Halperin W, Kalow W, Sweeney MH, Tang BK, Fingerhut M, Timpkins B, Wille K. 1995. Induction of P-450 in workers exposed to dioxin. Occupational and Environmental Medicine 52(2):86–91.
Halperin W, Vogt R, Sweeney MH, Shopp G, Fingerhut M, Petersen M. 1998. Immunological markers among workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occupational and Environmental Medicine 55(11):742–749.
Hanke W, Romitti P, Fuortes L, Sobala W, Mikulski M. 2003. The use of pesticides in a Polish rural population and its effect on birth weight. International Archives of Occupational and Environmental Health 76:614–620.
Hansen ES, Hasle H, Lander F. 1992. A cohort study on cancer incidence among Danish gardeners. American Journal of Industrial Medicine 21:651–660.
Hansen ES, Lander F, Lauritsen JM. 2007. Time trends in cancer risk and pesticide exposure, a longterm follow-up of Danish gardeners. Scandinavian Journal of Work, Environment and Health 33(6):465–469.
Hansen S, Odland JO, Phi DT, Nieboer E, Sandanger TM. 2009. Maternal levels of organochlorines in two communities in southern Vietnam. Science of the Total Environment 408(2):225–232.
Hardell L. 1977. Malignant mesenchymal tumors and exposure to phenoxy acids: A clinical observation. Lakartidningen 74:2753–2754 [in Swedish].
Hardell L. 1979. Malignant lymphoma of histiocytic type and exposure to phenoxyacetic acids or chlorophenols. Lancet 1(8106):55–56.
Hardell L. 1981. Relation of soft-tissue sarcoma, malignant lymphoma and colon cancer to phenoxy acids, chlorophenols and other agents. Scandinavian Journal of Work, Environment and Health 7:119–130.
Hardell L, Sandstrom A. 1979. Case-control study: Soft-tissue sarcomas and exposure to phenoxyacetic acids or chlorophenols. British Journal of Cancer 39:711–717.
Hardell L, Bengtsson NO. 1983. Epidemiological study of socioeconomic factors and clinical findings in Hodgkin’s disease, and reanalysis of previous data regarding chemical exposure. British Journal of Cancer 48:217–225.
Hardell L, Eriksson M. 1988. The association between soft tissue sarcomas and exposure to phenoxyacetic acids: A new case-referent study. Cancer 62:652–656.
Hardell L, Eriksson M. 1999. A case-control study of non-Hodgkin lymphoma and exposure to pesticides. Cancer 85(6):1353–1360.
Hardell L, Eriksson M, Lenner P. 1980. Malignant lymphoma and exposure to chemical substances, especially organic solvents, chlorophenols and phenoxy acids. Lakartidningen 77:208–210.
Hardell L, Eriksson M, Lenner P, Lundgren E. 1981. Malignant lymphoma and exposure to chemicals, especially organic solvents, chlorophenols and phenoxy acids: A case-control study. British Journal of Cancer 43:169–176.
Hardell L, Johansson B, Axelson O. 1982. Epidemiological study of nasal and nasopharyngeal cancer and their relation to phenoxy acid or chlorophenol exposure. American Journal of Industrial Medicine 3:247–257.
Hardell L, Bengtsson NO, Jonsson U, Eriksson S, Larsson LG. 1984. Aetiological aspects on primary liver cancer with special regard to alcohol, organic solvents and acute intermittent porphyria: An epidemiological investigation. British Journal of Cancer 50:389–397.
Hardell L, Moss A, Osmond D, Volberding P. 1987. Exposure to hair dyes and polychlorinated dibenzo-p-dioxins in AIDS patients with Kaposi sarcoma: An epidemiological investigation. Cancer Detection and Prevention (Suppl 1):567–570.
Hardell L, Eriksson M, Degerman A. 1994. Exposure to phenoxyacetic acids, chlorophenols, or organic solvents in relation to histopathology, stage, and anatomical localization of non-Hodgkin’s lymphoma. Cancer Research 54:2386–2389.
Hartge P, Colt JS, Severson RK, Cerhan JR, Cozen W, Camann D, Zahm SH, Davis S. 2005. Residential herbicide use and risk of non-Hodgkin lymphoma. Cancer Epidemiology, Biomarkers and Prevention 14(4):934–937.
Hayes HM, Tarone RE, Casey HW, Huxsoll DL. 1990. Excess of seminomas observed in Vietnam service US military working dogs. Journal of the National Cancer Institute 82:1042–1046.
Heacock H, Hogg R, Marion SA, Hershler R, Teschke K, Dimich-Ward H, Demers P, Kelly S, Ostry A, Hertzman C. 1998. Fertility among a cohort of male sawmill workers exposed to chlorophenate fungicides. Epidemiology 9(1):56–60.
Henneberger PK, Ferris BG Jr, Monson RR. 1989. Mortality among pulp and paper workers in Berlin, New Hampshire. British Journal of Industrial Medicine 46:658–664.
Henriksen GL, Michalek JE, Swaby JA, Rahe AJ. 1996. Serum dioxin, testosterone, and gonadotropins in veterans of Operation Ranch Hand. Epidemiology 7(4):352–357.
Henriksen GL, Ketchum NS, Michalek JE, Swaby JA. 1997. Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology 8(3):252–258.
Hertzman C, Teschke K, Ostry A, Hershler R, Dimich-Ward H, Kelly S, Spinelli JJ, Gallagher RP, McBride M, Marion SA. 1997. Mortality and cancer incidence among sawmill workers exposed to chlorophenate wood preservatives. American Journal of Public Health 87(1):71–79.
Hoar SK, Blair A, Holmes FF, Boysen CD, Robel RJ, Hoover R, Fraumeni JF. 1986. Agricultural herbicide use and risk of lymphoma and soft-tissue sarcoma. Journal of the American Medical Association 256:1141–1147.
Hoffman RE, Stehr-Green PA, Webb KB, Evans RG, Knutsen AP, Schramm WF, Staake JL, Gibson BB, Steinberg KK. 1986. Health effects of long-term exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Journal of the American Medical Association 255:2031–2038.
Hohenadel K, Harris SA, McLaughlin JR, Spinelli JJ, Pahwa P, Dosman JA, Demers PA, Blair A. 2011. Exposure to multiple pesticides and risk of non-Hodgkin lymphoma in men from six Canadian provinces. International Journal of Environmental Research and Public Health 8(6):2320–2330.
Holmes AP, Bailey C, Baron RC, Bosanac E, Brough J, Conroy C, Haddy L. 1986. West Virginia Department of Health Vietnam-Era Veterans Mortality Study, Preliminary Report. Charleston: West Virginia Health Department.
Hooiveld M, Heederik DJ, Kogevinas M, Boffetta P, Needham LL, Patterson DG Jr, Bueno de Mesquita HB. 1998. Second follow-up of a Dutch cohort occupationally exposed to phenoxy herbicides, chlorophenols, and contaminants. American Journal of Epidemiology 147(9):891–901.
Hoppin JA. 2005. Integrating exposure measurements into epidemiologic studies in agriculture. Scandinavian Journal of Work, Environment and Health 31(Suppl 1):115—117.
Hoppin JA, Umbach DM, London SJ, Alavanja CR, Sandler DP. 2002. Chemical predictors of wheeze among farmer pesticide applicators in the Agricultural Health Study. American Journal of Critical Care Medicine 165:683–689.
Hoppin JA, Umbach DM, London SJ, Lynch CF, Alavanja MC, Sandler DP. 2006a. Pesticides associated with wheeze among commercial pesticide applicators in the Agricultural Health Study. American Journal of Epidemiology 163(12):1129–1137.
Hoppin JA, Adgate JL, Eberhart M, Nishioka M, Ryan PB. 2006b. Environmental exposure assessment of pesticides in farmworker homes. Environmental Health Perspectives 114(6):929–935.
Hoppin JA, Umbach DM, London SJ, Lynch CF, Alavanja MC, Sandler DP. 2006c. Pesticides and adult respiratory outcomes in the Agricultural Health Study. Annals of the New York Academy of Sciences 1076:343–354.
Hoppin JA, Umbach DM, Kullman GJ, Henneberger PK, London SJ, Alavanja MC, Sandler DP. 2007a. Pesticides and other agricultural factors associated with self-reported farmer’s lung among farm residents in the Agricultural Health Study. Occupational and Environmental Medicine 64(5):334–341.
Hoppin JA, Valcin M, Henneberger PK, Kullman GJ, Limbach DM, London SJ, Alavanja MCR, Sandler DP. 2007b. Pesticide use and chronic bronchitis among farmers in the Agricultural Health Study. American Journal of Industrial Medicine 50(12):969–979.
Hoppin JA, Umbach DM, London SJ, Henneberger PK, Kullman GJ, Alavanja MC, Sandler DP. 2008. Pesticides and atopic and nonatopic asthma among farm women in the Agricultural Health Study. American Journal of Respiratory and Critical Care Medicine 177(1):11–18.
Hoppin JA, Umbach DM, London SJ, Henneberger PK, Kullman GJ, Coble J, Alavanja MCR, Beane Freeman LE, Sandler DP. 2009. Pesticide use and adult-onset asthma among male farmers in the Agricultural Health Study. European Respiratory Journal 34(6):1296–1303.
Hryhorczuk DO, Wallace WH, Persky V, Furner S, Webster JR Jr, Oleske D, Haselhorst B, Ellefson R, Zugerman C. 1998. A morbidity study of former pentachlorophenol-production workers. Environmental Health Perspectives 106(7):401–408.
Humblet O, Williams PL, Korrick SA, Sergeyev O, Emond C, Birnbaum LS, Burns JS, Altshul L, Patterson DG Jr, Turner WE, Lee MM, Revich B, Hauser R. 2010. Predictors of serum dioxin, furan, and PCB concentrations among women from Chapaevsk, Russia. Environmental Science and Technology 44(14):5633–5640.
Huong LD, Phuong NTN. 1983. The state of abnormal pregnancies and congenital malformations at the Gyneco-Obstetrical Hospital of Ho Chi Minh City (formerly Tu Du Hospital). Summarized in: Constable JD, Hatch MC. Reproductive Effects of Herbicide Exposure in Vietnam: Recent Studies by the Vietnamese and Others. As cited in Constable and Hatch, 1985.
Huston BL. 1972. Identification of three neutral contaminants in production grade 2,4-D. Journal of Agricultural and Food Chemistry 20(3):724–727.
Ideo G, Bellati G, Bellobuono A, Mocarelli P, Marocchi A, Brambilla P. 1982. Increased urinary d-glucaric acid excretion by children living in an area polluted with tetrachlorodibenzo-para-dioxin (TCDD). Clinica Chimica Acta 120:273–283.
Ideo G, Bellati G, Bellobuono A, Bissanti L. 1985. Urinary d-glucaric acid excretion in the Seveso area, polluted by tetrachlorodibenzo-p-dioxin (TCDD): Five years of experience. Environmental Health Perspectives 60:151–157.
IOM (Institute of Medicine). 1994. Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. Washington, DC: National Academy Press.
IOM. 2000. Veterans and Agent Orange: Herbicide/Dioxin Exposure and Type 2 Diabetes. Washington, DC: National Academy Press.
IOM. 2002. Veterans and Agent Orange: Herbicide/Dioxin Exposure and Acute Myelogenous Leukemia in the Children of Vietnam Veterans. Washington, DC: The National Academies Press.
IOM. 2004. Veterans and Agent Orange: Length of Presumptive Period for Association Between Exposure and Respiratory Cancer. Washington, DC: The National Academies Press.
IOM. 2006. Disposition of the Air Force Health Study. Washington, DC: The National Academies Press.
Jäger R, Neuberger M, Rappe C, Kundi M, Pigler B, Smith AG. 1998. Chloracne and other symptoms 23 years after dioxin-exposure. Atemwegs-Und Lungenkrankheiten 24(Suppl 1):S101-S104.
Jappinen P, Pukkala E. 1991. Cancer incidence among pulp and paper workers exposed to organic chlorinated compounds formed during chlorine pulp bleaching. Scandinavian Journal of Work, Environment and Health 17:356–359.
Jennings AM, Wild G, Ward JD, Ward AM. 1988. Immunological abnormalities 17 years after accidental exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. British Journal of Industrial Medicine 45:701–704.
Jones MR, Tellez-Plaza M, Sharrett AR, Guallar E, Navas-Acien A. 2011. Urine arsenic and hypertension in US adults. The 2003–2008 National Health and Nutrition Examination Survey. Epidemiology 22:153–161.
Jung D, Berg PA, Edler L, Ehrenthal W, Fenner D, Flesch-Janys D, Huber C, Klein R, Koitka C, Lucier G, Manz A, Muttray A, Needham L, Päpke O, Pietsch M, Portier C, Patterson D, Prellwitz W, Rose DM, Thews A, Konietzko J. 1998. Immunological findings in formerly exposed workers to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds in pesticide production. Arbeitsmedizin Sozialmedizin Umweltmedizin (Suppl 24):38–43.
Kahn PC, Gochfeld M, Nygren M, Hansson M, Rappe C, Velez H, Ghent-Guenther T, Wilson WP. 1988. Dioxins and dibenzofurans in blood and adipose tissue of Agent Orange-exposed Vietnam veterans and matched controls. Journal of the American Medical Association 259:1661–1667.
Kahn PC, Gochfeld M, Lewis WW. 1992a. Dibenzodioxin and Dibenzofuran Congener Levels in Four Groups of Vietnam Veterans Who Did Not Handle Agent Orange. New Jersey Agent Orange Commission.
Kahn PC, Gochfeld M, Lewis WW. 1992b. Immune Status and Herbicide Exposure in the New Jersey Pointman I Project. New Jersey Agent Orange Commission.
Kahn PC, Gochfeld M, Lewis WW. 1992c. Semen Analysis in Vietnam Veterans with Respect to Presumed Herbicide Exposure. New Jersey Agent Orange Commission.
Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC, Sandler DP. 2005. Neurologic symptoms in licensed private pesticide applicators in the Agricultural Health Study. Environmental Health Perspectives 113(7):877–882.
Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC, Sandler DP. 2007a. Neurologic symptoms in licensed pesticide applicators in the Agricultural Health Study. Human and Experimental Toxicology 26(3):243–250.
Kamel F, Tanner C, Umbach D, Hoppin J, Alavanja M, Blair A, Comyns K, Goldman S, Korell M, Langston J, Ross G, Sandler D. 2007b. Pesticide exposure and self-reported Parkinson’s disease in the Agricultural Health Study. American Journal of Epidemiology 165(4):364–374.
Kang HK, Weatherbee L, Breslin PP, Lee Y, Shepard BM. 1986. Soft tissue sarcomas and military service in Vietnam: A case comparison group analysis of hospital patients. Journal of Occupational Medicine 28:1215–1218.
Kang HK, Enzinger FM, Breslin P, Feil M, Lee Y, Shepard B. 1987. Soft tissue sarcoma and military service in Vietnam: A case-control study. Journal of the National Cancer Institute 79:693–699 [published erratum appears in Journal of the National Cancer Institute 79:1173].
Kang HK, Watanabe KK, Breen J, Remmers J, Conomos MG, Stanley J, Flicker M. 1991. Dioxins and dibenzofurans in adipose tissue of US Vietnam veterans and controls. American Journal of Public Health 81(3):344–348.
Kang HK, Mahan CM, Lee KY, Magee CA, Mather SH, Matanoski G. 2000a. Pregnancy outcomes among US women Vietnam veterans. American Journal Industrial Medicine 38(4):447–454.
Kang HK, Mahan CM, Lee KY, Magee CA, Selvin S. 2000b. Prevalence of gynecologic cancers among female Vietnam veterans. Journal of Occupational and Environmental Medicine 42:1121–1127.
Kang HK, Dalager NA, Needham LL, Patterson DG, Matanoski GM, Kanchanaraksa S, Lees PSJ. 2001. US Army Chemical Corps Vietnam veterans health study: Preliminary results. Chemosphere 43:943–949.
Kang HK, Dalager NA, Needham LL, Patterson DG, Lees PSJ, Yates K, Matanoski GM. 2006. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. American Journal of Industrial Medicine 49(11):875–884.
Karouna-Renier NK, Rao KR, Lanza JJ, Davis DA, Wilson PA. 2007. Serum profiles of PCDDs and PCDFs, in individuals near the Escambia Wood Treatment Company Superfund Site in Pensacola, FL. Chemosphere 69:1312–1319.
Karunanayake CP, Spinelli JJ, McLaughlin JR, Dosman JA, Pahwa P, McDuffie HH. 2012. Hodgkin lymphoma and pesticides exposure in men: A Canadian case-control study. Journal of Agromedicine 17(1):30–39.
Kayajanian GM. 2002. The J-shaped dioxin dose response curve. Ecotoxicology and Environmental Safety 51:1–4.
Kenborg L, Lassen CF, Lander F, Olsen JH. 2012. Parkinson’s disease among gardeners exposed to pesticides—A Danish cohort study. Scandinavian Journal of Work, Environment and Health 38(1):65–69.
Kern PA, Said S, Jackson WG Jr, Michalek JE. 2004. Insulin sensitivity following agent orange exposure in Vietnam veterans with high blood levels of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Journal of Clinical Endocrinology and Metabolism 89(9):4665–4672.
Ketchum NS, Michalek JE. 2005. Postservice mortality of Air Force veterans occupationally exposed to herbicides during the Vietnam War: 20-year follow-up results. Military Medicine 170(5):406–413.
Ketchum NS, Michalek JE, Burton JE. 1999. Serum dioxin and cancer in veterans of Operation Ranch Hand. American Journal of Epidemiology 149(7):630–639.
Khoa ND. 1983. Some biologic parameters collected on the groups of people in an area affected by chemicals. Summarized in: Constable JD, Hatch MC. Reproductive effects of herbicide exposure in Vietnam: Recent studies by the Vietnamese and others. As cited in Constable and Hatch, 1985.
Kim HA, Kim EM, Park YC, Yu JY, Hong SK, Jeon SH, Park KL, Hur SJ, Heo Y. 2003. Immunotoxicological effects of Agent Orange exposure to the Vietnam War Korean veterans. Industrial Health 41:158–166.
Kim JB, Kang WY, Moon SG, Kim HJ, Kim KH, Kim YH, Hwang SH, Hwang SH, Kim W. 2012. Clinical outcome of veterans with acute coronary syndrome who had been exposed to Agent Orange. Chonnam Medical Journal 48(1):47–51.
Kim JS, Kang HK, Lim HS, Cheong HK, Lim MK. 2001. A study on the correlation between categorizations of the individual exposure levels to Agent Orange and serum dioxin levels among the Korean Vietnam veterans. Korean Journal of Preventative Medicine 34(1):80–88.
Kim JS, Lim HS, Cho SI, Cheong HK, Lim MK. 2003. Impact of Agent Orange exposure among Korean Vietnam veterans. Industrial Health 41:149–157.
Kirrane EF, Hoppin JA, Umbach DM, Samanic C, Sandler DP. 2004. Patterns of pesticide use and their determinants among wives of farmer pesticide applicators in the Agricultural Health Study. Journal of Occupational and Environmental Medicine 46(8):856–865.
Ko GT, Cockram CS, Chow CC, Yeung V, Chan WB, So WY, et al. 2005. High prevalence of metabolic syndrome in Hong Kong Chinese—Comparison of three diagnostic criteria. Diabetes Research and Clinical Practice 69:160–168.
Kogan MD, Clapp RW. 1985. Mortality Among Vietnam Veterans in Massachusetts, 1972–1983. Boston, MA: Massachusetts Office of the Commissioner of Veterans Services, Agent Orange Program.
Kogan MD, Clapp RW. 1988. Soft tissue sarcoma mortality among Vietnam veterans in Massachusetts, 1972–1983. International Journal of Epidemiology 17:39–43.
Kogevinas M, Saracci R, Bertazzi PA, Bueno de Mesquita BH, Coggon D, Green LM, Kauppinen T, Littorin M, Lynge E, Mathews JD, Neuberger M, Osman J, Pearce N, Winkelmann R. 1992. Cancer mortality from soft-tissue sarcoma and malignant lymphomas in an international cohort of workers exposed to chlorophenoxy herbicides and chlorophenols. Chemosphere 25:1071–1076.
Kogevinas M, Saracci R, Winkelmann R, Johnson ES, Bertazzi PA, Bueno de Mesquita BH, Kauppinen T, Littorin M, Lynge E, Neuberger M. 1993. Cancer incidence and mortality in women occupationally exposed to chlorophenoxy herbicides, chlorophenols, and dioxins. Cancer Causes and Control 4:547–553.
Kogevinas M, Kauppinen T, Winkelmann R, Becher H, Bertazzi PA, Bas B, Coggon D, Green L, Johnson E, Littorin M, Lynge E, Marlow DA, Mathews JD, Neuberger M, Benn T, Pannett B, Pearce N, Saracci R. 1995. Soft tissue sarcoma and non-Hodgkin’s lymphoma in workers exposed to phenoxy herbicides, chlorophenols and dioxins: Two nested case-control studies. Epidemiology 6:396–402.
Kogevinas M, Becher H, Benn T, Bertazzi PA, Boffetta P, Bueno de Mesquita HB, Coggon D, Colin D, Flesch-Janys D, Fingerhut M, Green L, Kauppinen T, Littorin M, Lynge E, Mathews JD, Neuberger M, Pearce N, Saracci R. 1997. Cancer mortality in workers exposed to phenoxy herbicides, chlorophenols, and dioxins. An expanded and updated international cohort study. American Journal of Epidemiology 145(12):1061–1075.
Koutros S, Alavanja MCR, Lubin JH, Sandler DP, Hoppin JA, Lynch CF, Knott C, Blair A, Freeman LEB. 2010a. An update of cancer incidence in the Agricultural Health Study. Journal of Occupational and Environmental Medicine 52(11):1098–1105.
Koutros S, Beane Freeman LE, Berndt SI, Andreotti G, Lubin JH, Sandler DP, Hoppin JA, Yu K, Li Q, Burdette LA, Yuenger J, Yeager M, Alavanja MCR. 2010b. Pesticide use modifies the association between genetic variants on chromosome 8q24 and prostate cancer. Cancer Research 70(22):9224–9233.
Koutros S, Andreotti G, Berndt SI, Hughes Barry K, Lubin JH, Hoppin JA, Kamel F, Sandler DP, Burdette LA, Yuenger J, Yeager M, Alavanja MCR, Freeman LEB. 2011. Xenobiotic-metabolizing gene variants, pesticide use, and the risk of prostate cancer. Pharmacogenetics and Genomics 21(10):615–623.
Kristensen P, Irgens LM, Andersen A, Bye AS, Sundheim L. 1997. Birth defects among offspring of Norwegian farmers, 1967–1991. Epidemiology 8(5):537–544.
Kreuzer PE, Csanady GA, Baur C, Kessler W, Päpke O, Greim H, Filser JG. 1997. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and congeners in infants. A toxicokinetic model of human lifetime body burden by TCDD with special emphasis on its uptake by nutrition. Archives of Toxicology 71(6):383–400.
Lambertino A, Turyk M, Anderson H, Freels S, Persky V. 2011. Uterine leiomyomata in a cohort of Great Lakes sport fish consumers. Environmental Research 111(4):565–572.
Lampi P, Hakulinen T, Luostarinen T, Pukkala E, Teppo L. 1992. Cancer incidence following chlorophenol exposure in a community in southern Finland. Archives of Environmental Health 47:167–175.
Landi MT, Needham LL, Lucier G, Mocarelli P, Bertazzi PA, Caporaso N. 1997. Concentrations of dioxin 20 years after Seveso. Lancet 349(9068):1811.
Landi MT, Consonni D, Patterson DG Jr, Needham LL, Lucier G, Brambilla P, Cazzaniga MA, Mocarelli P, Pesatori AC, Bertazzi PA, Caporaso NE. 1998. 2,3,7,8-Tetrachlorodibenzo-p-dioxin plasma levels in Seveso 20 years after the accident. Environmental Health Perspectives 106(5):273–277.
Landi MT, Bertazzi PA, Baccarelli A, Consonni D, Masten S, Lucier G, Mocarelli P, Needham L, Caporaso N, Grassman J. 2003. TCDD-mediated alterations in the AhR-dependent pathway in Seveso, Italy, 20 years after the accident. Carcinogenesis 24(4):673–680.
Lang TD, Tung TT, Van DD. 1983a. Mutagenic effects on the first generation after exposure to “Orange Agent.” Summarized in: Constable JD, Hatch MC. Reproductive effects of herbicide exposure in Vietnam: Recent studies by the Vietnamese and others. As cited in Constable and Hatch, 1985.
Lang TD, Van DD, Dwyer JH, Flamenbuam C, Dwyer KM, Fantini D. 1983b. Self-reports of exposure to herbicides and health problems: A preliminary analysis of survey data from the families of 432 veterans in northern Vietnam. Summarized in: Constable JD, Hatch MC. Reproductive effects of herbicide exposure in Vietnam: Recent studies by the Vietnamese and others. As cited in Constable and Hatch, 1985.
LaVecchia C, Negri E, D’Avanzo B, Franceschi S. 1989. Occupation and lymphoid neoplasms. British Journal of Cancer 60:385–388.
Lawrence CE, Reilly AA, Quickenton P, Greenwald P, Page WF, Kuntz AJ. 1985. Mortality patterns of New York State Vietnam veterans. American Journal of Public Health 75:277–279.
Lawson CC, Schnorr TM, Whelan EA, Deddens JA, Dankovic DA, Piacitelli LA, Sweeney MH, Connally LB. 2004. Paternal occupational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin and birth outcomes of offspring: Birth weight, preterm delivery, and birth defects. Environmental Health Perspectives 112(14):1403–1408.
Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, Jacobs DR Jr. 2006. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: Results from the National Health and Examination Survey 1999–2002. Diabetes Care 29(7):1638–1644.
Lee DH, Steffes M, Jacobs DR Jr. 2007a. Positive associations of serum concentration of polychlorinated biphenyls or organochlorine pesticides with self-reported arthritis, especially rheumatoid type, in women. Environmental Health Perspectives 115(6):883–888.
Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR Jr. 2007b. Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: Results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care 30(3):622–628.
Lee DH, Lee IK, Porta M, Steffes M, Jacobs DR Jr. 2007c. Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among non-diabetic adults: Results from the National Health and Nutrition Examination Survey 1999–2002. Diabetologia 50(9):1841–1851.
Lee DH, Jacobs DR Jr, Steffes M. 2008. Association of organochlorine pesticides with peripheral neuropathy in patients with diabetes or impaired fasting glucose. Diabetes 57(11):3108–3111.
Lee DH, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR Jr. 2010. Low dose of some persistent organic pollutants predicts type 2 diabetes: A nested case-control study. Environmental Health Perspectives 118(9):1235–1242.
Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR Jr. 2011a. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS ONE 6 (1):e15977.
Lee DH, Lind PM, Jacobs DR Jr, Salihovic S, van Bavel B, Lind L. 2011b. Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: The prospective investigation of the vasculature in Uppsala seniors (PIVUS) study. Diabetes Care 34(8):1778–1784.
Lee DH, Lind L, Jacobs DR Jr, Salihovic S, van Bavel B, Lind PM. 2012a. Associations of persistent organic pollutants with abdominal obesity in the elderly: The prospective investigation of the vasculature in Uppsala seniors (PIVUS) study. Environment International 40:170–178.
Lee DH, Lind PM, Jacobs DR, Salihovic S, van Bavel B, Lind L. 2012b. Background exposure to persistent organic pollutants predicts stroke in the elderly. Environment International 47:115–120.
Lee WJ, Cantor KP, Berzofsky JA, Zahm SH, Blair A. 2004a. Non-Hodgkin’s lymphoma among asthmatics exposed to pesticides. International Journal of Cancer 111(2):298–302.
Lee WJ, Lijinsky W, Heineman EF, Markin RS, Weisenburger DD, Ward MH. 2004b. Agricultural pesticide use and adenocarcinomas of the stomach and oesophagus. Occupational and Environmental Medicine 61(9):743–749.
Lee WJ, Colt JS, Heineman EF, McComb R, Weisenburger DD, Lijinsky W, Ward MH. 2005. Agricultural pesticide use and risk of glioma in Nebraska, United States. Occupational and Environmental Medicine 62(11):786–792.
Lee WJ, Purdue MP, Stewart P, Schenk M, De Roos AJ, Cerhan JR, Severson RK, Cozen W, Hartge P, Blair A. 2006. Asthma history, occupational exposure to pesticides and the risk of non-Hodgkin’s lymphoma. International Journal of Cancer 118(12):3174–3176.
Lee WJ, Sandler DP, Blair A, Samanic C, Cross AJ, Alavanja MC. 2007. Pesticide use and colorectal cancer risk in the Agricultural Health Study. International Journal of Cancer 121(2):339–346.
Lerda D, Rizzi R. 1991. Study of reproductive function in persons occupationally exposed to 2,4-dichlorophenoxyacetic acid (2,4-D). Mutation Research 262:47–50.
Levy CJ. 1988. Agent Orange exposure and posttraumatic stress disorder. Journal of Nervous and Mental Disorders 176:242–245.
Lind PM, van Bavel B, Salihovic S, Lind L. 2012. Circulating levels of persistent organic pollutants (POPs) and carotid atherosclerosis in the elderly. Environmental Health Perspectives 120(1):38–43.
Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC. 1997. Environmental risk factors and Parkinson’s disease: A case-control study in Taiwan. Neurology 48(6):1583–1588.
Longnecker MP, Michalek JE. 2000. Serum dioxin level in relation to diabetes mellitus among Air Force veterans with background levels of exposure. Epidemiology 11(1):44–48.
Lovik M, Johansen HR, Gaarder PI, Becher G, Aaberge IS, Gdynia W, Alexander J. 1996. Halogenated organic compounds and the human immune system: Preliminary report on a study in hobby fishermen. Archives of Toxicology Supplement 18:15–20.
Lynge E. 1985. A follow-up study of cancer incidence among workers in manufacture of phenoxy herbicides in Denmark. British Journal of Cancer 52:259–270.
Lynge E. 1993. Cancer in phenoxy herbicide manufacturing workers in Denmark, 1947–87—An update. Cancer Causes and Control 4:261–272.
Magnani C, Coggon D, Osmond C, Acheson ED. 1987. Occupation and five cancers: A case-control study using death certificates. British Journal of Industrial Medicine 44(11):769–776.
Mahan CM, Bullman TA, Kang HK, Selvin S. 1997. A case-control study of lung cancer among Vietnam veterans. Journal of Occupational and Environmental Medicine 39(8):740–747.
Mandel JS, Alexander BH, Baker BA, Acquavella JF, Chapman P, Honeycutt R. 2005. Biomonitoring for farm families in the Farm Family Exposure Study. Scandinavian Journal of Work, Environment and Health 31(Suppl 1):98–104.
Manuwald U, Garrido MV, Berger J, Manz A, Baur X. 2012. Mortality study of chemical workers exposed to dioxins: Follow-up 23 years after chemical plant closure. Occupational and Environmental Medicine 69(9):636–642.
Manz A, Berger J, Dwyer JH, Flesch-Janys D, Nagel S, Waltsgott H. 1991. Cancer mortality among workers in chemical plant contaminated with dioxin. Lancet 338:959–964.
Masala G, Di Lollo S, Picoco C, Crosignani P, Demicheli V, Fontana A, Funto I, Miligi L, Nanni O, Papucci A, Ramazzotti V, Rodella S, Stagnaro E, Tumino R, Vigano C, Vindigni C, Seniori Costantini A, Vineis P. 1996. Incidence rates of leukemias, lymphomas and myelomas in Italy: Geographic distribution and NHL histotypes. International Journal of Cancer 68(2):156–159.
Mastroiacovo P, Spagnolo A, Marni E, Meazza L, Bertollini R, Segni G, Borgna-Pignatti C. 1988. Birth defects in the Seveso area after TCDD contamination. Journal of the American Medical Association 259:1668–1672 [published erratum appears in the Journal of the American Medical Association 260:792].
May G. 1982. Tetrachlorodibenzodioxin: A survey of subjects ten years after exposure. British Journal of Industrial Medicine 39:128–135.
May G. 1983. TCDD: A study of subjects 10 and 14 years after exposure. Chemosphere 12:771–778.
McBride DI, Collins JJ, Humphry NF, Herbison P, Bodner KM, Aylward LL, Burns CJ, Wilken M. 2009a. Mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin at a trichlorophenol plant in New Zealand. Journal of Occupational and Environmental Medicine 51(9):1049–1056.
McBride DI, Burns CJ, Herbison GP, Humphry NF, Bodner K, Collins JJ. 2009b. Mortality in employees at a New Zealand agrochemical manufacturing site. Occupational Medicine 59(4):255–263.
McDuffie HH, Klaassen DJ, Dosman JA. 1990. Is pesticide use related to the risk of primary lung cancer in Saskatchewan? Journal of Occupational Medicine 32(10):996–1002.
McDuffie HH, Pahwa P, McLaughlin JR, Spinelli JJ, Fincham S, Dosman JA, Robson D, Skinnider LF, Choi NW. 2001. Non-Hodgkin’s lymphoma and specific pesticide exposures in men: Cross-Canada study of pesticides and health. Cancer Epidemiology, Biomarkers and Prevention 10(11):1155–1163.
McDuffie HH, Pahwa P, Robson D, Dosman JA, Fincham S, Spinelli JJ, McLaughlin JR. 2005. Insect repellents, phenoxyherbicide exposure, and non-Hodgkin’s lymphoma. Journal of Occupational and Environmental Medicine 47(8):806–816.
McDuffie HH, Pahwa P, Karunanayake CP, Spinelli JJ, Dosman JA. 2009. Clustering of cancer among families of cases with Hodgkin lymphoma (HL), multiple myeloma (MM), non-Hodgkin’s lymphoma (NHL), soft tissue sarcoma (STS) and control subjects. BMC Cancer 9(70).
McKinney WP, McIntire DD, Carmody TJ, Joseph A. 1997. Comparing the smoking behavior of veterans and nonveterans. Public Health Reports 112(3):212–217.
McLean D, Pearce N, Langseth H, Jappinen P, Szadkowska-Stanczyk I, Person B, Wild P, Kishi R, Lynge E, Henneberger P, Sala M, Teschke K, Kauppinen T, Colin D, Kogevinas M, Boffetta P. 2006. Cancer mortality in workers exposed to organochlorine compounds in the pulp and paper industry: An international collaborative study. Environmental Health Perspectives 114(7):1007–1012.
McLean D, Eng A, Walls C, Dryson E, Harawira J, Cheng S, Wong KC, ’t Mannetje A, Gray M, Shoe-mack P, Smith A, Pearce N. 2009. Serum dioxin levels in former New Zealand sawmill workers twenty years after exposure to pentachlorophenol (PCP) ceased. Chemosphere 74(7):962–967.
Mellemgaard A, Engholm G, McLaughlin JK, Olsen JH. 1994. Occupational risk factors for renal-cell carcinoma in Denmark. Scandinavian Journal of Work, Environment and Health 20:160–165.
Michalek JE, Pavuk M. 2008. Diabetes and cancer in veterans of Operation Ranch Hand after adjustment for calendar period, days of sprayings, and time spent in Southeast Asia. Journal of Occupational and Environmental Medicine 50(3):330–340.
Michalek JE, Wolfe WH, Miner JC. 1990. Health status of Air Force veterans occupationally exposed to herbicides in Vietnam. II. Mortality. Journal of the American Medical Association 264:1832–1836.
Michalek JE, Wolfe WH, Miner JC, Papa TM, Pirkle JL. 1995. Indices of TCDD exposure and TCDD body burden in veterans of Operation Ranch Hand. Journal of Exposure Analysis and Environmental Epidemiology 5(2):209–223.
Michalek JE, Rahe AJ, Boyle CA. 1998a. Paternal dioxin, preterm birth, intrauterine growth retardation, and infant death. Epidemiology 9(2):161–167.
Michalek JE, Ketchum NS, Akhtar FZ. 1998b. Postservice mortality of US Air Force veterans occupationally exposed to herbicides in Vietnam: 15-year follow-up. American Journal of Epidemiology 148(8):786–792.
Michalek JE, Rahe AJ, Boyle CA. 1998c. Paternal dioxin and the sex of children fathered by veterans of Operation Ranch Hand. Epidemiology 9(4):474–475.
Michalek JE, Akhtar FZ, Kiel JL. 1999a. Serum dioxin, insulin, fasting glucose, and sex hormonebinding globulin in veterans of Operation Ranch Hand. Journal of Clinical Endocrinology and Metabolism 84(5):1540–1543.
Michalek JE, Ketchum NS, Check IJ. 1999b. Serum dioxin and immunologic response in veterans of Operation Ranch Hand. American Journal of Epidemiology 149(11):1038–1046.
Michalek JE, Ketchum N, Longnecker MP. 2001a. Serum dioxin and hepatic abnormalities in veterans of Operation Ranch Hand. Annals of Epidemiology 11(5):304–311.
Michalek JE, Akhtar FZ, Arezzo JC, Garabrant DH, Albers JW. 2001b. Serum dioxin and peripheral neuropathy in veterans of Operation Ranch Hand. Neurotoxicology 22:479–490.
Michalek JE, Akhtar FZ, Longnecker MP, Burton JE. 2001c. Relation of serum 2,3,7,8-tetrachloro-dibenzo-p-dioxin (TCDD) level to hematological examination results in veterans of Operation Ranch Hand. Archives of Environmental Health 56(5):396–405.
Michalek JE, Ketchum NS, Tripathi RC. 2003. Diabetes mellitus and 2,3,7,8-tetrachlorodibenzo-p-dioxin elimination in veterans of Operation Ranch Hand. Journal of Toxicology and Environmental Health, Part A, 66:211–221.
Mills PK, Yang R. 2005. Breast cancer risk in Hispanic agricultural workers in California. International Journal of Occupational and Environmental Health 11(2):123–131.
Mills PK, Yang RC. 2007. Agricultural exposures and gastric cancer risk in Hispanic farm workers in California. Environmental Research 104(2):282–289.
Mills PK, Yang R, Riordan D. 2005. Lymphohematopoietic cancers in the United Farm Workers of America (UFW), 1988–2001. Cancer Causes and Control 16(7):823–830.
Mills KT, Blair A, Freeman LEB, Sandler DP, Hoppin JA. 2009. Pesticides and myocardial infarction incidence and mortality among male pesticide applicators in the Agricultural Health Study. American Journal of Epidemiology 170(7):892–900.
Mo HJ, Park HJ, Kim JH, Lee JY, Cho BK. 2002. A study about the skin and general disease patterns of the Vietnam veterans exposed to dioxin. Korean Journal of Dermatology 40(6):634–638.
Mocarelli P, Marocchi A, Brambilla P, Gerthoux P, Young DS, Mantel N. 1986. Clinical laboratory manifestations of exposure to dioxin in children. A six-year study of the effects of an environmental disaster near Seveso, Italy. Journal of the American Medical Association 256:2687–2695.
Mocarelli P, Patterson DG Jr, Marocchi A, Needham LL. 1990. Pilot study (phase II) for determining polychlorinated dibenzo-p-dioxin (PCDD) and polychlorinated dibenzofuran (PCDF) levels in serum of Seveso, Italy residents collected at the time of exposure: Future plans. Chemosphere 20:967–974.
Mocarelli P, Needham LL, Marocchi A, Patterson DG Jr, Brambilla P, Gerthoux PM, Meazza L, Carreri V. 1991. Serum concentrations of 2,3,7,8-tetrachlorodibenzo-p-dioxin and test results from selected residents of Seveso, Italy. Journal of Toxicology and Environmental Health 32:357–366.
Mocarelli P, Brambilla P, Gerthoux PM, Patterson DG Jr, Needham LL. 1996. Change in sex ratio with exposure to dioxin. Lancet 348(9024):409.
Mocarelli P, Gerthoux PM, Patterson DG Jr, Milani S, Limonta G, Bertona M, Signorini S, Tramacere P, Colombo L, Crespi C, Brambilla P, Sarto C, Carreri V, Sampson EJ, Turner WE, Needham LL. 2008. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environmental Health Perspectives 116(1):70–77.
Mocarelli P, Gerthoux PM, Needham LL, Patterson DG Jr, Limonta G, Falbo R, Signorini S, Bertona M, Crespi C, Sarto C, Scott PK, Turner WE, Brambilla P. 2011. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environmental Health Perspectives 119:713–718.
Montgomery MP, Kamel F, Saldana TM, Alavanja MC, Sandler DP. 2008. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study, 1993–2003. American Journal of Epidemiology 167(10):1235–1246.
Morris PD, Koepsell TD, Daling JR, Taylor JW, Lyon JL, Swanson GM, Child M, Weiss NS. 1986. Toxic substance exposure and multiple myeloma: A case-control study. Journal of the National Cancer Institute 76:987–994.
Morrison H, Semenciw RM, Morison D, Magwood S, Mao Y. 1992. Brain cancer and farming in western Canada. Neuroepidemiology 11:267–276.
Morrison H, Savitz D, Semenciw RM, Hulka B, Mao Y, Morison D, Wigle D. 1993. Farming and prostate cancer mortality. American Journal of Epidemiology 137:270–280.
Morrison HI, Semenciw RM, Wilkins K, Mao Y, Wigle DT. 1994. Non-Hodgkin’s lymphoma and agricultural practices in the prairie provinces of Canada. Scandinavian Journal of Work, Environment and Health 20:42–47.
Moses M, Lilis R, Crow KD, Thornton J, Fischbein A, Anderson HA, Selikoff IJ. 1984. Health status of workers with past exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in the manufacture of 2,4,5-trichlorophenoxyacetic acid: Comparison of findings with and without chloracne. American Journal of Industrial Medicine 5:161–182.
Musicco M, Sant M, Molinari S, Filippini G, Gatta G, Berrino F. 1988. A case-control study of brain gliomas and occupational exposure to chemical carcinogens: The risks to farmers. American Journal of Epidemiology 128:778–785.
Nanni O, Amadori D, Lugaresi C, Falcini F, Scarpi E, Saragoni A, Buiatti E. 1996. Chronic lymphocytic leukæmias and non-Hodgkin’s lymphomas by histological type in farming-animal breeding workers: A population case-control study based on a priori exposure matrices. Occupational and Environmental Medicine 53(10):652–657.
NCEP (National Cholesterol Education Program). 2002. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106:3143–3421.
Neuberger M, Kundi M, Jager R. 1998. Chloracne and morbidity after dioxin exposure (preliminary results). Toxicology Letters 96/97:347–350.
Neuberger M, Rappe C, Bergek S, Cai H, Hansson M, Jager R, Kundi M, Lim CK, Wingfors H, Smith AG. 1999. Persistent health effects of dioxin contamination in herbicide production. Environmental Research 81(3):206–214.
Newell GR. 1984. Development and Preliminary Results of Pilot Clinical Studies. Report of the Agent Orange Advisory Committee to the Texas Department of Health. University of Texas System Cancer Center.
Ng C, Janoo-Gilani R, Sipahimalani P, Gallagher R, Gascoyne R, Connors J, Weber J, Lai A, Leach S, Le N, Brooks-Wilson A, Spinelli J. 2010. Interaction between organochlorines and the AHR gene, and risk of non-Hodgkin lymphoma. Cancer Causes and Control 21:11–22.
Ngo AD, Taylor R, Roberts CL, Nguyen TV. 2006. Association between Agent Orange and birth defects: Systematic review and meta-analysis. International Journal of Epidemiology 35:1220–1230.
Nguyen HD. 1983. Pregnancies at the Polyclinic of Tay Ninh Province. Summarized in: Constable JD, Hatch MC. Reproductive effects of herbicide exposure in Vietnam: Recent studies by the Vietnamese and others. As cited in Constable and Hatch, 1985.
Nhu DD, Kido T, Naganuma R, Sawano N, Tawara K, Nishijo M, Nakagawa H, Hung NN, Thom LTH. 2009. A GIS study of dioxin contamination in a Vietnamese region sprayed with herbicide. Environmental Health and Preventive Medicine 14(6):353–360.
Norström A, Rappe C, Lindahl R, Buser HR. 1979. Analysis of some older Scandinavian formulations of 2,4-dichlorophenoxy acetic acid for contents of chlorinated dibenzo-p-dioxins and dibenzofurans. Scandanavian Journal of Work, Environment and Health 5:375–378.
Nurminen T, Rantala K, Kurppa K, Holmberg PC. 1994. Agricultural work during pregnancy and selected structural malformations in Finland. Epidemiology 1:23–30.
O’Brien TR, Decoufle P, Boyle CA. 1991. Non-Hodgkin’s lymphoma in a cohort of Vietnam veterans. American Journal of Public Health 81:758–760.
Oh E, Lee E, Im H, Kang HS, Jung WW, Won NH, Kim EM, Sul D. 2005. Evaluation of immuno- and reproductive toxicities and association between immunotoxicological and genotoxicological parameters in waste incineration workers. Toxicology 2(1):65–80.
Olsen J, Melbye M, Olsen SF, Sorenson TI, Aaby P, Anderson AM, Taxbol D, Hansen KD, Juhl M, Schow TB, Sorensen HT, Andresen J, Mortensen EL, Olesen AW, Sondergaard C. 2001. The Danish National Birth Cohort—Its background, structure and aim. Scandinavian Journal of Work, Environment and Health 29:300–307.
Olsson H, Brandt L. 1988. Risk of non-Hodgkin’s lymphoma among men occupationally exposed to organic solvents. Scandinavian Journal of Work, Environment and Health 14:246–251.
Orsi L, Delabre L, Monnerau A, Delva P, Berthou C, Fenaux P, Marti G, Soubeyran P, Huguet F, Mipied N, Leporrier M, Hemon D, Troussard X, Clavel J. 2009. Occupational exposure to pesticides and lymphoid neoplasms among men: Results of a French case-control study. Occupational and Environmental Medicine 66(5):291–298.
O’Toole BI, Marshall RP, Grayson DA, Schureck RJ, Dobson M, Ffrench M, Pulvertaft B, Meldrum L, Bolton J, Vennard J. 1996a. The Australian Vietnam Veterans Health Study: I. Study design and response bias. International Journal of Epidemiology 25(2):307–318.
O’Toole BI, Marshall RP, Grayson DA, Schureck RJ, Dobson M, Ffrench M, Pulvertaft B, Meldrum L, Bolton J, Vennard J. 1996b. The Australian Vietnam Veterans Health Study: II. Self-reported health of veterans compared with the Australian population. International Journal of Epidemiology 25(2):319–330.
O’Toole BI, Marshall RP, Grayson DA, Schureck RJ, Dobson M, Ffrench M, Pulvertaft B, Meldrum L, Bolton J, Vennard J. 1996c. The Australian Vietnam Veterans Health Study: III. Psychological health of Australian Vietnam veterans and its relationship to combat. International Journal of Epidemiology 25(2):331–340.
O’Toole BI, Catts SV, Outram S, Pierse KR, Cockburn J. 2009. The physical and mental health of Australian Vietnam veterans 3 decades after the war and its relation to military service, combat, and post-traumatic stress disorder. American Journal of Epidemiology 170(3):318–330.
O’Toole BI, Catts SV, Outram S, Pierse KR, Cockburn J. 2010. Factors associated with civilian mortality in Australian Vietnam Veterans three decades after the war. Military Medicine 175(2):88–95.
Ott MG, Zober A. 1996. Cause specific mortality and cancer incidence among employees exposed to 2,3,7,8-TCDD after a 1953 reactor accident. Occupational and Environmental Medicine 53(9):606–612.
Ott MG, Holder BB, Olson RD. 1980. A mortality analysis of employees engaged in the manufacture of 2,4,5-trichlorophenoxyacetic acid. Journal of Occupational Medicine 22:47–50.
Ott MG, Olson RA, Cook RR, Bond GG. 1987. Cohort mortality study of chemical workers with potential exposure to the higher chlorinated dioxins. Journal of Occupational Medicine 29:422–429.
Pahwa P, McDuffie HH, Dosman JA, Robson D, McLaughlin JR, Spinelli JJ, Fincham S. 2003. Exposure to animals and selected risk factors among Canadian farm residents with Hodgkin’s disease, multiple myeloma, or soft tissue sarcoma. Journal of Occupational and Environmental Medicine 45(8):857–868.
Pahwa P, McDuffie HH, Dosman JA, McLaughlin JR, Spinelli JJ, Robson D, Fincham S. 2006. Hodgkin lymphoma, multiple myeloma, soft tissue sarcomas, insect repellents, and phenoxyherbicides. Journal of Occupational and Environmental Medicine 48(3):264–274.
Pahwa P, Karunanayake CP, Dosman JA, Spinelli JJ, McLaughlin JR. 2011. Soft-tissue sarcoma and pesticides exposure in men: Results of a Canadian case-control study. Journal of Occupational and Environmental Medicine 53(11):1279–1286.
Pahwa P, Karunanayake CP, Dosman JA, Spinelli JJ, McDuffie HH, McLaughlin JR. 2012. Multiple myeloma and exposure to pesticides: A Canadian case-control study. Journal of Agromedicine 17:40–50.
Park RM, Schulte PA, Bowman JD, Walker JT, Bondy SC, Yost MG, Touchstone JA, Dosemeci M. 2005. Potential occupational risks for neurodegenerative diseases. American Journal of Industrial Medicine 48(1):63–77.
Patterson DG Jr, Hoffman RE, Needham LL, Roberts DW, Bagby JR, Pinkle JL, Falk H, Sampson EJ, Houk VN. 1986. 2,3,7,8-Tetrachlorodibenzo-p-dioxin levels in adipose tissue of exposed and control persons in Missouri. Journal of the American Medical Association 256(19):2683–2686.
Pavuk M, Schecter AJ, Akhtar FZ, Michalek JE. 2003. Serum 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) levels and thyroid function in Air Force veterans of the Vietnam War. Annals of Epidemiology 13(5):335–343.
Pavuk M, Michalek JE, Schecter A, Ketchum NS, Akhtar FZ, Fox KA. 2005. Did TCDD exposure or service in Southeast Asia increase the risk of cancer in Air Force Vietnam veterans who did not spray Agent Orange? Journal of Occupational and Environmental Medicine 47(4):335–342.
Pavuk M, Michalek JE, Ketchum NS. 2006. Prostate cancer in US Air Force veterans of the Vietnam War. Journal of Exposure Science and Environmental Epidemiology 16(2):184–190.
Payne K, Andreotti G, Bell E, Blair A, Coble J, Alavanja M. 2012. Determinants of high pesticide exposure events in the Agricultural Health Cohort Study from enrollment (1993–1997) through phase II (1999–2003). Journal of Agricultural Safety and Health 18(3):167–179.
Pazderova-Vejlupková J, Lukás E, Nĕmcova M, Pícková J, Jirásek L. 1981. The development and prognosis of chronic intoxication by tetrachlorodibenzo-p-dioxin in men. Archives of Environmental Health 36:5–11.
Pearce NE, Smith AH, Fisher DO. 1985. Malignant lymphoma and multiple myeloma linked with agricultural occupations in a New Zealand cancer registry-based study. American Journal of Epidemiology 121:225–237.
Pearce NE, Smith AH, Howard JK, Sheppard RA, Giles HJ, Teague CA. 1986a. Case-control study of multiple myeloma and farming. British Journal of Cancer 54:493–500.
Pearce NE, Smith AH, Howard JK, Sheppard RA, Giles HJ, Teague CA. 1986b. Non-Hodgkin’s lymphoma and exposure to phenoxyherbicides, chlorophenols, fencing work, and meat works employment: A case-control study. British Journal of Industrial Medicine 43:75–83.
Pearce NE, Sheppard RA, Smith AH, Teague CA. 1987. Non-Hodgkin’s lymphoma and farming: An expanded case-control study. International Journal of Cancer 39:155–161.
Pelclová D, Fenclová Z, Dlasková Z, Urban P, Lukás E, Procházka B, Rappe C. 2001. Biochemical, neuropsychological, and neurological abnormalities following 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure. Archives of Environmental Health 56:493–500.
Pelclová D, Fenclová Z, Preiss J, Procházka B, Spácil J, Dubská Z, Okrouhlík B, Lukás E, Urban P. 2002. Lipid metabolism and neuropsychological follow-up study of workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. International Archives of Occupational and Environmental Health 75(Suppl l):S60-S66.
Pelclová D, Prazny M, Skrha J, Fenclová Z, Kalousova M, Urban P, Navratil T, Senholdova Z, Smerhovsky Z. 2007. 2,3,7,8-TCDD exposure, endothelial dysfunction and impaired microvascular reactivity. Human and Experimental Toxicology 26(9):705–713.
Pelclová D, Fenclová Z, Urban P, Ridzon P, Preiss J, Kupka K, Malik J, Dubska Z, Navratil T. 2009. Chronic health impairment due to 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. Neuroendocrinology Letters 30(Suppl 1):219–224.
Pelclová D, Navratil T, Fenclová Z, Vlckova S, Kupka K, Urban P, Ridzon P, Zikan V, Landova L, Syslova K, Kuzma M, Kacer P. 2011. Increased oxidative/nitrosative stress markers measured non-invasively in patients with high 2,3,7,8-tetrachloro-dibenzo-p-dioxin plasma level. Neuro Endocrinology Letters 32(Suppl 1):71–76.
Peper M, Klett M, Frentzel-Beyme R, Heller WD. 1993. Neuropsychological effects of chronic exposure to environmental dioxins and furans. Environmental Research 60:124–135.
Persson B, Dahlander AM, Fredriksson M, Brage HN, Ohlson CG, Axelson O. 1989. Malignant lymphomas and occupational exposures. British Journal of Industrial Medicine 46:516–520.
Persson B, Fredriksson M, Olsen K, Boeryd B, Axelson O. 1993. Some occupational exposures as risk factors for malignant lymphomas. Cancer 72:1773–1778.
Pesatori AC, Consonni D, Tironi A, Landi MT, Zocchetti C, Bertazzi PA. 1992. Cancer morbidity in the Seveso area, 1976–1986. Chemosphere 25:209–212.
Pesatori AC, Consonni D, Tironi A, Zocchetti C, Fini A, Bertazzi PA. 1993. Cancer in a young population in a dioxin-contaminated area. International Journal of Epidemiology 22:1010–1013.
Pesatori AC, Zocchetti C, Guercilena S, Consonni D, Turrini D, Bertazzi PA. 1998. Dioxin exposure and nonmalignant health effects: A mortality study. Occupational and Environmental Medicine 55(2):126–131.
Pesatori AC, Baccarelli A, Consonni D, Lania A, Beck-Peccoz P, Bertazzi PA, Spada A. 2008. Aryl hydrocarbon receptor-interacting protein and pituitary adenomas: A population-based study on subjects exposed to dioxin after the Seveso, Italy, accident. European Journal of Endocrinology 159(6):699–703.
Pesatori AC, Consonni D, Rubagotti M, Grillo P, Bertazzi PA. 2009. Cancer incidence in the population exposed to dioxin after the “Seveso accident”: Twenty years of follow-up. Environmental Health 8:1 of 11.
Petreas M, Smith D, Hurley S, Jeffrey SS, Gilliss D, Reynolds P. 2004. Distribution of persistent, lipid-soluble chemicals in breast and abdominal adipose tissues: Lessons learned from a breast cancer study. Cancer Epidemiology, Biomarkers and Prevention 13(3):416–424.
Phuong NTN, Huong LTD. 1983. The effects of toxic chemicals on the pregnancy of the women living at two localities in the South of Vietnam. Summarized in: Constable JD, Hatch MC. Reproductive Effects of Herbicide Exposure in Vietnam: Recent Studies by the Vietnamese and Others. As cited in Constable and Hatch, 1985.
Phuong NTN, Thuy TT, Phuong PK. 1989a. An estimate of differences among women giving birth to deformed babies and among those with hydatidiform mole seen at the Ob-Gyn hospital of Ho Chi Minh City in the south of Vietnam. Chemosphere 18:801–803.
Phuong NTN, Thuy TT, Phuong PK. 1989b. An estimate of reproductive abnormalities in women inhabiting herbicide sprayed and non-herbicide sprayed areas in the south of Vietnam, 1952–1981. Chemosphere 18:843–846.
Piacitelli LA, Marlow DA. 1997. NIOSH 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure matrix. Organohalogen Compounds 33:510–514.
Pirkle JL, Wolfe WH, Patterson DG, Needham LL, Michalek JE, Miner JC, Peterson MR, Phillips DL. 1989. Estimates of the half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Vietnam veterans of Operation Ranch Hand. Journal of Toxicology and Environmental Health 27:165–171.
Poland AP, Smith D, Metter G, Possick P. 1971. A health survey of workers in a 2,4-D and 2,4,5-T plant with special attention to chloracne, porphyria cutanea tarda, and psychologic parameters. Archives of Environmental Health 22:316–327.
Pollei S, Mettler FA Jr, Kelsey CA, Walters MR, White RE. 1986. Follow-up chest radiographs in Vietnam veterans: Are they useful? Radiology 161:101–102.
Quandt SA, Hernandez-Valero MA, Grzywacz JG, Hovey JD, Gonzales M, Arcury TA. 2006. Workplace, household, and personal predictors of pesticide exposure for farmworkers. Environmental Health Perspectives 114(6):943–952.
Ramlow JM, Spadacene NW, Hoag SR, Stafford BA, Cartmill JB, Lerner PJ. 1996. Mortality in a cohort of pentachlorophenol manufacturing workers, 1940–1989. American Journal of Industrial Medicine 30(2):180–194.
Reif JS, Pearce N, Fraser J. 1989. Occupational risks of brain cancer: A New Zealand cancer registry-based study. Journal of Occupational Medicine 31(10):863–867.
Rellahan WL. 1985. Aspects of the Health of Hawaii’s Vietnam-Era Veterans. Honolulu: Hawaii State Department of Health, Research, and Statistics Office.
Renshaw KD, Caska CM. 2012. Relationship distress in partners of combat veterans: The role of partners’ perceptions of posttraumatic stress symptoms. Behavior Therapy 43:416–426.
Revazova J, Yurchenko V, Katosova L, Platonova V, Sycheva L, Khripach L, Ingel F, Tsutsman T, Zhurkov V. 2001. Cytogenetic investigation of women exposed to different levels of dioxins in Chapaevsk town. Chemosphere 43:999–1004.
Revich B, Aksel E, Ushakova T, Ivanova I, Zhuchenko N, Klyuev N, Brodsky B, Sotskov Y. 2001. Dioxin exposure and public health in Chapaevsk, Russia. Chemosphere 43:951–966.
Riihimaki V, Asp S, Hernberg S. 1982. Mortality of 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid herbicide applicators in Finland: First report of an ongoing prospective cohort study. Scandinavian Journal of Work, Environment and Health 8:37–42.
Riihimaki V, Asp S, Pukkala E, Hernberg S. 1983. Mortality and cancer morbidity among chlorinated phenoxyacid applicators in Finland. Chemosphere 12:779–784.
Rix BA, Villadsen E, Engholm G, Lynge E. 1998. Hodgkin’s disease, pharyngeal cancer, and soft tissue sarcomas in Danish paper mill workers. Journal of Occupational and Environmental Medicine 40(1):55–62.
Robinson CF, Waxweiler RJ, Fowler DP. 1986. Mortality among production workers in pulp and paper mills. Scandinavian Journal of Work, Environment and Health 12:552–560.
Rocheleau CM, Bertke SJ, Deddens JA, Ruder AM, Lawson CC, Waters MA, Hopf NB, Riggs MA, Whelan EA. 2011. Maternal exposure to polychlorinated biphenyls and the secondary sex ratio: An occupational cohort study. Environmental Health 10:20.
Ronco G, Costa G, Lynge E. 1992. Cancer risk among Danish and Italian farmers. British Journal of Industrial Medicine 49:220–225.
Ronn M, Lind L, Bavel BV, Salihovic S, Michaelsson K, Lind PM. 2011. Circulating levels of persistent organic pollutants associate in divergent ways to fat mass measured by DXA in humans. Chemosphere 85(3):335–343.
Ruder AM, Yiin JH. 2011. Mortality of US pentachlorophenol production workers through 2005. Chemosphere 83(6):851–861.
Ruder AM, Waters MA, Butler MA, Carreon T, Calvert GM, Davis-King KE, Schulte PA, Sanderson WT, Ward EM, Connally LB, Heineman EF, Mandel JS, Morton RF, Reding DJ, Rosenman KD, Talaska G. 2004. Gliomas and farm pesticide exposure in men: The Upper Midwest Health Study. Archives of Environmental Health 59(12):650–657.
Ruder AM, Waters MA, Carreon T, Butler MA, Davis-King KE, Calvert GM, Schulte PA, Ward EM, Connally LB, Lu J, Wall D, Zivkovich Z, Heineman EF, Mandel JS, Morton RF, Reding DJ, Rosenman KD, The Brain Cancer Collaborative Study Group. 2006. The Upper Midwest Health Study: A case-control study of primary intracranial gliomas in farm and rural residents. Journal of Agricultural Safety and Health 12(4):255–274.
Ruder AM, Carreon T, Butler MA, Calvert GM, Davis-King KE, Waters MA, Schulte PA, Mandel JS, Morton RF, Reding DJ, Rosenman KD. 2009. Exposure to farm crops, livestock, and farm tasks and risk of glioma. American Journal of Epidemiology 169(12):1479–1491.
Saberi Hosnijeh F, Boers D, Portengen L, Bueno de Mesquita HB, Heederik D, Vermeulen R. 2011. Long-term effects on humoral immunity among workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Occupational and Environmental Medicine 68(6):419–424.
Saberi Hosnijeh F, Boers D, Portengen L, Bueno de Mesquita HB, Heederik D, Vermeulen R. 2012. Plasma cytokine concentrations in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Frontiers in Oncology 2:37.
Saito H, Goovaerts P. 2000. Geostatistical interpolation of positively skewed and censored data in a dioxin contaminated site. Environmental Science and Technology 34:4228–4235.
Saldana TM, Basso O, Hoppin JA, Baird DD, Knott C, Blair A, Alavanja MC, Sandler DP. 2007. Pesticide exposure and self-reported gestational diabetes mellitus in the Agricultural Health Study. Diabetes Care 30(3):529–534.
Salihovic S, Lampa E, Lindström G, Lind L, Lind PM, van Bavel B. 2012a. Circulating levels of persistent organic pollutants (POPs) among elderly men and women from Sweden: Results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Environment International 44:59–67.
Salihovic S, Mattioli L, Lindström G, Lind L, Lind PM, van Bavel B. 2012b. A rapid method for screening of the Stockholm Convention POPs in small amounts of human plasma using SPE and HRGC/HRMS. Chemosphere 86:747–753.
Samanic C, Hoppin JA, Lubin JH, Blair A, Alavanja MC. 2005. Factor analysis of pesticide use patterns among pesticide applicators in the Agricultural Health Study. Journal of Exposure Analysis and Environmental Epidemiology 15(3):225–233.
Samanic C, Rusiecki J, Dosemeci M, Hou L, Hoppin JA, Sandler DP, Lubin J, Blair A, Alavanja MC. 2006. Cancer incidence among pesticide applicators exposed to dicamba in the Agricultural Health Study. Environmental Health Perspectives 114(10):1521–1526.
Saracci R, Kogevinas M, Bertazzi PA, Bueno de Mesquita BH, Coggon D, Green LM, Kauppinen T, L’Abbe KA, Littorin M, Lynge E, Mathews JD, Neuberger M, Osman J, Pearce N, Winkelmann R. 1991. Cancer mortality in workers exposed to chlorophenoxy herbicides and chlorophenols. Lancet 338:1027–1032.
Savitz DA, Arbuckle T, Kaczor D, Curtis K. 1997. Male pesticide exposure and pregnancy outcome. American Journal of Epidemiology 146(12):1025–1036.
Schecter A, Quynh HT, Pavuk M, Päpke O, Malisch R, Constable JD. 2003. Food as a source of dioxin exposure in the residents of Bien Hoa City, Vietnam. Journal of Occupational and Environmental Medicine 45:781–788.
Schildt EB, Eriksson M, Hardell L, Magnuson A. 1999. Occupational exposures as risk factors for oral cancer evaluated in a Swedish case-control study. Oncology Reports 6(2):317–320.
Schreinemachers DM. 2000. Cancer mortality in four northern wheat-producing states. Environmental Health Perspectives 108(9):873–881.
Schreinemachers DM. 2010. Perturbation of lipids and glucose metabolism associated with previous 2,4-D exposure: A cross-sectional study of NHANES III data, 1988–1994. Environmental Health 9:11.
Schulte PA, Burnett CA, Boeniger MF, Johnson J. 1996. Neurodegenerative diseases: Occupational occurrence and potential risk factors, 1982 through 1991. American Journal of Public Health 86(9):1281–1288.
Seidler A, Hellenbrand W, Robra BP, Vieregge P, Nischan P, Joerg J, Oertel WH, Ulm G, Schneider E. 1996. Possible environmental, occupational, and other etiologic factors for Parkinson’s disease: A case-control study in Germany. Neurology 46(5):1275–1284.
Semchuk KM, Love EJ, Lee RG. 1993. Parkinson’s disease: A test of the multifactorial etiologic hypothesis. Neurology 43:1173–1180.
Semenciw RM, Morrison HI, Riedel D, Wilkins K, Ritter L, Mao Y. 1993. Multiple myeloma mortality and agricultural practices in the prairie provinces of Canada. Journal of Occupational Medicine 35:557–561.
Semenciw RM, Morrison HI, Morison D, Mao Y. 1994. Leukemia mortality and farming in the prairie provinces of Canada. Canadian Journal of Public Health 85:208–211.
Senthilselvan A, McDuffie HH, Dosman JA. 1992. Association of asthma with use of pesticides: Results of a cross-sectional survey of farmers. American Review of Respiratory Disease 146:884–887.
Silverstone AE, Rosenbaum PF, Weinstock RS, Bartell SM, Foushee HR, Shelton C, Pavuk M. 2012. Polychlorinated biphenyl (PCB) exposure and diabetes: Results from the Anniston community health survey. Environmental Health Perspectives 120(5):727–732.
Slager RE, Poole JA, LeVan TD, Sandler DP, Alavanja MCR, Hoppin JA. 2009. Rhinitis associated with pesticide exposure among commercial pesticide applicators in the Agricultural Health Study. Occupational and Environmental Medicine 66(11):718–724.
Smith AH, Pearce NE. 1986. Update on soft tissue sarcoma and phenoxyherbicides in New Zealand. Chemosphere 15:1795–1798.
Smith AH, Matheson DP, Fisher DO, Chapman CJ. 1981. Preliminary report of reproductive outcomes among pesticide applicators using 2,4,5-T. New Zealand Medical Journal 93:177–179.
Smith AH, Fisher DO, Pearce N, Chapman CJ. 1982. Congenital defects and miscarriages among New Zealand 2,4,5-T sprayers. Archives of Environmental Health 37:197–200.
Smith AH, Fisher DO, Giles HJ, Pearce N. 1983. The New Zealand soft tissue sarcoma case-control study: Interview findings concerning phenoxyacetic acid exposure. Chemosphere 12:565–571.
Smith AH, Pearce NE, Fisher DO, Giles HJ, Teague CA, Howard JK. 1984. Soft tissue sarcoma and exposure to phenoxyherbicides and chlorophenols in New Zealand. Journal of the National Cancer Institute 73:1111–1117.
Smith JG, Christophers AJ. 1992. Phenoxy herbicides and chlorophenols: A case-control study on soft tissue sarcoma and malignant lymphoma. British Journal of Cancer 65:442–448.
Snow BR, Stellman JM, Stellman SD, Sommer JF. 1988. Post-traumatic stress disorder among American Legionnaires in relation to combat experience in Vietnam: Associated and contributing factors. Environmental Research 47:175–192.
Sobel W, Bond GG, Skowronski BJ, Brownson PJ, Cook RR. 1987. A soft tissue sarcoma case-control study in a large multi-chemical manufacturing facility. Chemosphere 16:2095–2099.
Solet D, Zoloth SR, Sullivan C, Jewett J, Michaels DM. 1989. Patterns of mortality in pulp and paper workers. Journal of Occupational Medicine 31:627–630.
Spinelli JJ, Ng CH, Weber JP, Connors JM, Gascoyne RD, Lai AS, Brooks-Wilson AR, Le ND, Berry BR, Gallagher RP. 2007. Organochlorines and risk of non-Hodgkin lymphoma. International Journal of Cancer 121(12):2767–2775.
Spoonster-Schwartz L. 1987. Women and the Vietnam experience. Journal of Nursing Scholarship 19(4):168–173.
Steenland K, Piacitelli L, Deddens J, Fingerhut M, Chang LI. 1999. Cancer, heart disease, and diabetes in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Journal of the National Cancer Institute 91(9):779–786.
Steenland K, Calvert G, Ketchum N, Michalek J. 2001. Dioxin and diabetes mellitus: An analysis of the combined NIOSH and Ranch Hand data. Occupational and Environmental Medicine 58:641–648.
Stehr PA, Stein G, Webb K, Schramm W, Gedney WB, Donnell HD, Ayres S, Falk H, Sampson E, Smith SJ. 1986. A pilot epidemiologic study of possible health effects associated with 2,3,7,8-tetrachlorodibenzo-p-dioxin contaminations in Missouri. Archives of Environmental Health 41:16–22.
Stehr-Green P, Hoffman R, Webb K, Evans RG, Knusten A, Schramm W, Staake J, Gibson B, Steinberg K. 1987. Health effects of long-term exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chemosphere 16:2089–2094.
Stellman JM, Stellman SD, Sommer JF. 1988. Social and behavioral consequences of the Vietnam experience among American Legionnaires. Environmental Research 47:129–149.
Stellman SD, Stellman JM. 1986. Estimation of exposure to Agent Orange and other defoliants among American troops in Vietnam: A methodological approach. American Journal of Industrial Medicine 9:305–321.
Stellman SD, Stellman JM, Sommer JF Jr. 1988. Health and reproductive outcomes among American Legionnaires in relation to combat and herbicide exposure in Vietnam. Environmental Research 47:150–174.
Stockbauer JW, Hoffman RE, Schramm WF, Edmonds LD. 1988. Reproductive outcomes of mothers with potential exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. American Journal of Epidemiology 128:410–419.
Stolevik SB, Nygaard UC, Namork E, Haugen M, Kvalem HE, Meltzer HM, Alexander J, van Delft JHM, Loveren HV, Lovik M, Granum B. 2011. Prenatal exposure to polychlorinated biphenyls and dioxins is associated with increased risk of wheeze and infections in infants. Food and Chemical Toxicology 49(8):1843–1848.
Su PH, Chen JY, Chen JW, Wang SL. 2010. Growth and thyroid function in children with in utero exposure to dioxin: A 5-year follow-up study. Pediatric Research 67(2):205–210.
Su PH, Huang PC, Lin CY, Ying TH, Chen JY, Wang SL. 2012. The effect of in utero exposure to dioxins and polychlorinated biphenyls on reproductive development in eight year-old children. Environment International 39(1):181–187.
Suskind RR, Hertzberg VS. 1984. Human health effects of 2,4,5-T and its toxic contaminants. Journal of the American Medical Association 251:2372–2380.
Svensson BG, Mikoczy Z, Stromberg U, Hagmar L. 1995. Mortality and cancer incidence among Swedish fishermen with a high dietary intake of persistent organochlorine compounds. Scandinavian Journal of Work, Environment and Health 21(2):106–115.
Swaen GMH, van Vliet C, Slangen JJM, Sturmans F. 1992. Cancer mortality among licensed herbicide applicators. Scandinavian Journal of Work, Environment and Health 18:201–204.
Swaen GMH, van Amelsvoort LGPM, Slangen JJM, Mohren DCL. 2004. Cancer mortality in a cohort of licensed herbicide applicators. International Archives of Occupational and Environmental Health 77:293–295.
Sweeney MH, Fingerhut MA, Connally LB, Halperin WE, Moody PL, Marlow DA. 1989. Progress of the NIOSH cross-sectional study of workers occupationally exposed to chemicals contaminated with 2,3,7,8-TCDD. Chemosphere 19:973–977.
Sweeney MH, Fingerhut MA, Arezzo JC, Hornung RW, Connally LB. 1993. Peripheral neuropathy after occupational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). American Journal of Industrial Medicine 23:845–858.
Sweeney MH, Calvert G, Egeland GA, Fingerhut MA, Halperin WE, Piacitelli LA. 1996. Review and update of the results of the NIOSH medical study of workers exposed to chemicals contaminated with 2,3,7,8-tetrachlorodibenzodioxin. Presented at the symposium Dioxin Exposure and Human Health—An Update, Berlin, June 17.
Sweeney MH, Calvert GM, Egeland GA, Fingerhut MA, Halperin WE, Piacitelli LA. 1997/98. Review and update of the results of the NIOSH medical study of workers exposed to chemicals contaminated with 2,3,7,8-tetrachlorodibenzodioxin. Teratogenesis, Carcinogenesis, and Mutagenesis 17(4–5):241–247.
’t Mannetje A, McLean D, Cheng S, Boffetta P, Colin D, Pearce N. 2005. Mortality in New Zealand workers exposed to phenoxy herbicides and dioxins. Occupational and Environmental Medicine 62(1):34–40.
Tan CE, Ma S, Wai D, Chew SK, Tai ES. 2004. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care 27:1182–1186.
Tango T, Fujita T, Tanihata T, Minowa M, Doi Y, Kato N, Kunikane S, Uchiyama I, Tanaka M, Uehata T. 2004. Risk of adverse reproductive outcomes associated with proximity to municipal solid waste incinerators with high dioxin emission levels in Japan. Journal of Epidemiology 14(3):83–93.
Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW. 2011. Rotenone, paraquat, and Parkinson’s disease. Environmental Health Perspectives 119(6):866–872.
Tarone RE, Hayes HM, Hoover RN, Rosenthal JF, Brown LM, Pottern LM, Javadpour N, O’Connell KJ, Stutzman RE. 1991. Service in Vietnam and risk of testicular cancer. Journal of the National Cancer Institute 83:1497–1499.
Tarone RE, Alavanja MC, Zahm SH, Lubin JH, Sandler DP, McMaster SB, Rothman N, Blair A. 1997. The Agricultural Health Study: Factors affecting completion and return of self-administered questionnaires in a large prospective cohort study of pesticide applicators. American Journal of Industrial Medicine 31:233–242.
Tatham L, Tolbert P, Kjeldsberg C. 1997. Occupational risk factors for subgroups of non-Hodgkin’s lymphoma. Epidemiology 8(5):551–558.
ten Tusscher GW, Stam GA, Koppe JG. 2000. Open chemical combustions resulting in a local increased incidence of orofacial clefts. Chemosphere 40:1263–1270.
Tenchini ML, Crimaudo C, Pacchetti G, Mottura A, Agosti S, De Carli L. 1983. A comparative cytogenetic study on cases of induced abortions in TCDD-exposed and nonexposed women. Environmental Mutagenesis 5:73–85.
Teschke K, Hertzman C, Fenske RA, Jin A, Ostry A, van Netten C, Leiss W. 1994. A history of process and chemical changes for fungicide application in the western Canadian lumber industry: What can we learn? Applied Occupational and Environmental Hygiene 9:984–993.
Thiess AM, Frentzel-Beyme R, Link R. 1982. Mortality study of persons exposed to dioxin in a trichlorophenol-process accident that occurred in the BASF AG on November 17, 1953. American Journal of Industrial Medicine 3:179–189.
Thomas KW, Dosemeci M, Hoppin JA, Sheldon LS, Croghan CW, Gordon SM, Jones ML, Reynolds SJ, Raymer JH, Akland GG, Lynch CF, Knott CE, Sandler DP, Blair AE, Alavanja MC. 2010. Urinary biomarker, dermal, and air measurement results for 2,4-D and chlorpyrifos farm applicators in the Agricultural Health Study. Journal of Exposure Science and Environmental Epidemiology 20(2):119–134.
Thomas TL. 1987. Mortality among flavour and fragrance chemical plant workers in the United States. British Journal of Industrial Medicine 44:733–737.
Thomas TL, Kang HK. 1990. Mortality and morbidity among Army Chemical Corps Vietnam veterans: A preliminary report. American Journal of Industrial Medicine 18:665–673.
Thomas TL, Kang H, Dalager N. 1991. Mortality among women Vietnam veterans, 1973–1987. American Journal of Epidemiology 134:973–980.
Thomaseth K, Salvan A. 1998. Estimation of occupational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin using a minimal physiologic toxicokinetic model. Environmental Health Perspectives 106(Suppl 2):743–753.
Thörn A, Gustavsson P, Sadigh J, Westerlund-Hannestrand B, Hogstedt C. 2000. Mortality and cancer incidence among Swedish lumberjacks exposed to phenoxy herbicides. Occupational and Environmental Medicine 57(10):718–720.
Tonn T, Esser C, Schneider EM, Steinmann-Steiner-Haldenstatt W, Gleichmann E. 1996. Persistence of decreased T-helper cell function in industrial workers 20 years after exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Environmental Health Perspectives 104(4):422–426.
Torchio P, Lepore AR, Corrao G, Comba P, Settimi L, Belli S, Magnani C, di Orio F. 1994. Mortality study on a cohort of Italian licensed pesticide users. The Science of the Total Environment 149(3):183–191.
Townsend JC, Bodner KM, Van Peenen PFD, Olson RD, Cook RR. 1982. Survey of reproductive events of wives of employees exposed to chlorinated dioxins. American Journal of Epidemiology 115:695–713.
True WR, Goldberg J, Eisen SA. 1988. Stress symptomatology among Vietnam veterans. Analysis of the Veterans Administration Survey of Veterans II. American Journal of Epidemiology 128:85–92.
Trung CB, Chien NT. 1983. Spontaneous abortions and birth defects in area exposed to toxic chemical sprays in Giong Trom District. Summarized in: Constable JD, Hatch MC. Reproductive Effects of Herbicide Exposure in Vietnam: Recent Studies by the Vietnamese and Others. As cited in Constable and Hatch, 1985.
Tsukimori K, Uchi H, Mitoma C, Yasukawa F, Chiba T, Todaka T, Kajiwara J, Yoshimura T, Hirata T, Fukushima K, Wake N, Furue M. 2012. Maternal exposure to high levels of dioxins in relation to birth weight in women affected by Yusho disease. Environment International 38(1):79–86.
Turunen AW, Verkasalo PK, Kiviranta H, Pukkala E, Jula A, Mannisto S, Rasanen R, Marniemi J, Vartiainen T. 2008. Mortality in a cohort with high fish consumption. International Journal of Epidemiology 37(5):1008–1017.
Turyk M, Anderson HA, Persky VW. 2007. Relationships of thyroid hormones with polychlorinated biphenyls, dioxins, furans, and DDE in adults. Environmental Health Perspectives 115(8):1197–1203.
Turyk M, Anderson HA, Knobeloch L, Imm P, Persky VW. 2009. Prevalence of diabetes and body burdens of polychlorinated biphenyls, polybrominated diphenyl ethers, and p,p’-diphenyldichloroethene in Great Lakes sport fish consumers. Chemosphere 75(5):674–749.
Uemura H, Arisawa K, Hiyoshi M, Satoh H, Sumiyoshi Y, Morinaga K, Kodama K, Suzuki T, Nagai M, Suzuki T. 2008a. Associations of environmental exposure to dioxins with prevalent diabetes among general inhabitants in Japan. Environmental Research 108(1):63–68.
Uemura H, Arisawa K, Hiyoshi M, Satoh H, Sumiyoshi Y, Morinaga K, Kodama K, Suzuki T, Nagai M, Suzuki T. 2008b. PCDDs/PCDFs and dioxin-like PCBs: Recent body burden levels and their determinants among general inhabitants in Japan. Chemosphere 73(1):30–37.
Uemura H, Arisawa K, Hiyoshi M, Kitayama A, Takami H, Sewachika F, Dakeshita S, Nii K, Satoh H, Sumiyoshi Y, Morinaga K, Kodama K, Suzuki TI, Nagai M, Suzuki T. 2009. Prevalence of metabolic syndrome associated with body burden levels of dioxin and related compounds among Japan’s general population. Environmental Health Perspectives 117(4):568–573.
Urban P, Pelclová D, Lukas E, Kupka K, Preiss J, Fenclová Z, Smerhovsky Z. 2007. Neurological and neurophysiological examinations on workers with chronic poisoning by 2,3,7,8-TCDD: Follow-up 35 years after exposure. European Journal of Neurology 14(2):213–218.
Valcin M, Henneberger PK, Kullman GJ, Umbach DM, London SJ, Alavanja MC, Sandler DP, Hoppin JA. 2007. Chronic bronchitis among nonsmoking farm women in the Agricultural Health Study. Journal of Occupational and Environmental Medicine 49(5):574–583.
van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. 2006. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicological Sciences 93(2):223–241.
van Houdt JJ, Fransman LG, Strik JJ. 1983. Epidemiological case-control study in personnel exposed to 2,4,5-T. Chemosphere 12(4):575.
van Wijngaarden E, Stewart PA, Olshan AF, Savitz DA, Bunin GR. 2003. Parental occupational exposure to pesticides and childhood brain cancer. American Journal of Epidemiology 157(11):989–997.
Vena J, Boffetta P, Becher H, Benn T, Bueno de Mesquita HB, Coggon D, Colin D, Flesch-Janys D, Green L, Kauppinen T, Littorin M, Lynge E, Mathews JD, Neuberger M, Pearce N, Pesatori AC, Saracci R, Steenland K, Kogevinas M. 1998. Exposure to dioxin and nonneoplastic mortality in the expanded IARC international cohort study of phenoxy herbicide and chlorophenol production workers and sprayers. Environmental Health Perspectives 106(Suppl 2):645–653.
Viel JF, Arveux P, Baverel J, Cahn JY. 2000. Soft-tissue sarcoma and non-Hodgkin’s lymphoma clusters around a municipal solid waste incinerator with high dioxin emission levels. American Journal of Epidemiology 152(1):13–19.
Viel JF, Clement MC, Hagi M, Grandjean S, Challier B, Danzon A. 2008a. Dioxin emissions from a municipal solid waste incinerator and risk of invasive breast cancer: A population-based case-control study with GIS-derived exposure. International Journal of Health Geographics [Electronic Resource] 7:4.
Viel JF, Daniau C, Goria S, Fabre P, De Crouy-Chanel P, Sauleau EA, Empereur-Bissonnet P. 2008b. Risk for non Hodgkin’s lymphoma in the vicinity of French municipal solid waste incinerators. Environmental Health 7(51): 9 pps.
Viel JF, Floret N, Deconinck E, Focant JF, De Pauw E, Cahn JY. 2011. Increased risk of non-Hodgkin lymphoma and serum organochlorine concentrations among neighbors of a municipal solid waste incinerator. Environment International 37(2):449–453.
Villeneuve PJ, Steenland K. 2010. Re: “Mortality rates among trichlorophenol workers with exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin.” American Journal of Epidemiology 171(1):129–130; author reply 130–121.
Vineis P, Terracini B, Ciccone G, Cignetti A, Colombo E, Donna A, Maffi L, Pisa R, Ricci P, Zanini E, Comba P. 1986. Phenoxy herbicides and soft-tissue sarcomas in female rice weeders. A population-based case-referent study. Scandinavian Journal of Work, Environment and Health 13:9–17.
Vineis P, Faggiano F, Tedeschi M, Ciccone G. 1991. Incidence rates of lymphomas and soft-tissue sarcomas and environmental measurements of phenoxy herbicides. Journal of the National Cancer Institute 83:362–363.
Visintainer PF, Barone M, McGee H, Peterson EL. 1995. Proportionate mortality study of Vietnam-era veterans of Michigan. Journal of Occupational and Environmental Medicine 37(4):423–428.
Waggoner JK, Kullman GJ, Henneberger PK, Umbach DM, Blair A, Alavanja MCR, Kamel F, Lynch CF, Knott C, London SJ, Hines CJ, Thomas KW, Sandler DP, Lubin JH, Beane Freeman LE, Hoppin JA. 2011. Mortality in the Agricultural Health Study, 1993–2007. American Journal of Epidemiology 173(1):71–83.
Wang SL, Lin CY, Guo YL, Lin LY, Chou WL, Chang LW. 2004. Infant exposure to polychlorinated dibenzo-p-dioxins, dibenzofurans and biphenyls (PCDD/Fs, PCBs)—correlation between prenatal and postnatal exposure. Chemosphere 54:1459–1473.
Wang SL, Su PH, Jong SB, Guo YL, Chou WL, Päpke O. 2005. In utero exposure to dioxins and polychlorinated biphenyls and its relations to thyroid function and growth hormone in newborns. Environmental Health Perspectives 113:1645–1650.
Wang SL, Chang YC, Chao HR, Li CM, Li LA, Lin LY, Päpke O. 2006. Body burdens of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls and their relations to estrogen metabolism in pregnant women. Environmental Health Perspectives 114(5):740–745.
Warner M, Eskenazi B, Mocarelli P, Gerthoux PM, Samuels S, Needham L, Patterson D, Brambilla P. 2002. Serum dioxin concentrations and breast cancer risk in the Seveso Women’s Health Study. Environmental Health Perspectives 110(7):625–628.
Warner M, Samuels S, Mocarelli P, Gerthoux PM, Needham L, Patterson DG Jr, Eskenazi B. 2004. Serum dioxin concentrations and age at menarche. Environmental Health Perspectives 112(13):1289–1292.
Warner M, Eskenazi B, Patterson DG, Clark G, Turner WE, Bonsignore L, Mocarelli P, Gerthoux PM. 2005. Dioxin-like TEQ of women from the Seveso, Italy area by ID-HRGC/HRMS and CALUX. Journal of Exposure Analysis and Environmental Epidemiology 15(4):310–318.
Warner M, Eskenazi B, Olive DL, Samuels S, Quick-Miles S, Vercellini P, Gerthoux PM, Needham L, Patterson DG, Mocarelli P. 2007. Serum dioxin concentrations and quality of ovarian function in women of Seveso. Environmental Health Perspectives 115(3):336–340.
Warner M, Mocarelli P, Samuels S, Needham L, Brambilla P, Eskenazi B. 2011. Dioxin exposure and cancer risk in the Seveso Women’s Health Study. Environmental Health Perspectives 119(12):1700–1705.
Watanabe KK, Kang HK. 1995. Military service in Vietnam and the risk of death from trauma and selected cancers. Annals of Epidemiology 5(5):407–412.
Watanabe KK, Kang HK. 1996. Mortality patterns among Vietnam veterans: A 24-year retrospective analysis. Journal of Occupational and Environmental Medicine 38(3):272–278.
Watanabe KK, Kang HK, Thomas TL. 1991. Mortality among Vietnam veterans: With methodological considerations. Journal of Occupational Medicine 33:780–785.
Waterhouse D, Carman WJ, Schottenfeld D, Gridley G, McLean S. 1996. Cancer incidence in the rural community of Tecumseh, Michigan: A pattern of increased lymphopoietic neoplasms. Cancer 77(4):763–770.
Webb K, Evans RG, Stehr P, Ayres SM. 1987. Pilot study on health effects of environmental 2,3,7,8-TCDD in Missouri. American Journal of Industrial Medicine 11:685–691.
Weisglas-Kuperus N, Sas TC, Koopman-Esseboom C, van der Zwan CW, De Ridder MA, Beishuizen A, Hooijkaas H, Sauer PJ. 1995. Immunologic effects of background prenatal and postnatal exposure to dioxins and polychlorinated biphenyls in Dutch infants. Pediatric Research 38(3):404–410.
Weiss J, Päpke O, Bignert A, Jensen S, Greyerz E, Agostoni C, Besana R, Riva E, Giovannini M, Zetterstrom R. 2003. Concentrations of dioxins and other organochlorines (PCBs, DDTs, HCHs) in human milk from Seveso, Milan and a Lombardian rural area in Italy: A study performed 25 years after the heavy dioxin exposure in Seveso. Acta Paediatrica 92(4):467–472.
Wendt AS. 1985. Iowa Agent Orange Survey of Vietnam Veterans. Iowa State Department of Health.
Weselak M, Arbuckle TE, Wigle DT, Walker MC, Krewski D. 2008. Pre- and post-conception pesticide exposure and the risk of birth defects in an Ontario farm population. Reproductive Toxicology 25(4):472–480.
Wigle DT, Semenciw RB, Wilkins K, Riedel D, Ritter L, Morrison HI, Mao Y. 1990. Mortality study of Canadian male farm operators: Non-Hodgkin’s lymphoma mortality and agricultural practices in Saskatchewan. Journal of the National Cancer Institute 82:575–582.
Wiklund K. 1983. Swedish agricultural workers: A group with a decreased risk of cancer. Cancer 51:566–568.
Wiklund K, Holm LE. 1986. Soft tissue sarcoma risk in Swedish agricultural and forestry workers. Journal of the National Cancer Institute 76:229–234.
Wiklund K, Dich J, Holm LE. 1987. Risk of malignant lymphoma in Swedish pesticide appliers. British Journal of Cancer 56:505–508.
Wiklund K, Lindefors BM, Holm LE. 1988a. Risk of malignant lymphoma in Swedish agricultural and forestry workers. British Journal of Industrial Medicine 45:19–24.
Wiklund K, Dich J, Holm LE. 1988b. Soft tissue sarcoma risk in Swedish licensed pesticide applicators. Journal of Occupational Medicine 30:801–804.
Wiklund K, Dich J, Holm LE, Eklund G. 1989a. Risk of cancer in pesticide applicators in Swedish agriculture. British Journal of Industrial Medicine 46:809–814.
Wiklund K, Dich J, Holm LE. 1989b. Risk of soft tissue sarcoma, Hodgkin’s disease and non-Hodgkin lymphoma among Swedish licensed pesticide applicators. Chemosphere 18:395–400.
Wilmoth JM, London AS, Parker WM. 2010. Military service and men’s health trajectories in later life. Journal of Gerontology: Social Sciences 65B(6):744–755.
Wingren G, Fredrikson M, Brage HN, Nordenskjold B, Axelson O. 1990. Soft tissue sarcoma and occupational exposures. Cancer 66:806–811.
Wolf N, Karmaus W. 1995. Effects of inhalative exposure to dioxins in wood preservatives on cell-mediated immunity in day-care center teachers. Environmental Research 68(2):96–105.
Wolfe WH, Michalek JE, Miner JC, Rahe A, Silva J, Thomas WF, Grubbs WD, Lustik MB, Karrison TG, Roegner RH, Williams DE. 1990. Health status of Air Force veterans occupationally exposed to herbicides in Vietnam. I. Physical health. Journal of the American Medical Association 264:1824–1831.
Wolfe WH, Michalek JE, Miner JC, Rahe AJ, Moore CA, Needham LL, Patterson DG. 1995. Paternal serum dioxin and reproductive outcomes among veterans of Operation Ranch Hand. Epidemiology 6(1):17–22.
Woods JS, Polissar L. 1989. Non-Hodgkin’s lymphoma among phenoxy herbicide-exposed farm workers in western Washington State. Chemosphere 18:401–406.
Woods JS, Polissar L, Severson RK, Heuser LS, Kulander BG. 1987. Soft tissue sarcoma and non-Hodgkin’s lymphoma in relation to phenoxy herbicide and chlorinated phenol exposure in western Washington. Journal of the National Cancer Institute 78:899–910.
Yesavage JA, Kinoshita LM, Kimball T, Zeitzer J, Friedman L, Noda A, David R, Hernandez B, Lee T, Cheng J, O’Hara R. 2012. Sleep-disordered breathing in Vietnam veterans with posttraumatic stress disorder. American Journal of Geriatric Psychiatry 20(3):199–204.
Yiin JH, Ruder AM, Stewart PA, Waters MA, Carrean T, Butler MA, Calvert GM, Davis-King KE, Schulte PA, Mandel JS, Morton RF, Reding DJ, Rosenman KD. 2012. The Upper Midwest Health Study: A case-control study of pesticide applicators and risk of glioma. Environmental Health: A Global Access Science Source 11(1):13 pps.
Yoshida J, Kumagai S, Tabuchi T, Kosaka H, Akasaka S, Kasai H, Oda H. 2006. Negative association between serum dioxin level and oxidative DNA damage markers in municipal waste incinerator workers. International Archives of Occupational and Environmental Health 79(2):115–122.
Zack JA, Suskind RR. 1980. The mortality experience of workers exposed to tetrachlorodibenzodioxin in a trichlorophenol process accident. Journal of Occupational Medicine 22:11–14.
Zack JA, Gaffey WR. 1983. A mortality study of workers employed at the Monsanto company plant in Nitro, West Virginia. Environmental Science Research 26:575–591.
Zahm SH, Weisenburger DD, Babbitt PA, Saal RC, Vaught JB, Cantor KP, Blair A. 1990. A case-control study of non-Hodgkin’s lymphoma and the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) in eastern Nebraska. Epidemiology 1:349–356.
Zahm SH, Weisenburger DD, Saal RC, Vaught JB, Babbitt PA, Blair A. 1993. The role of agricultural pesticide use in the development of non-Hodgkin’s lymphoma in women. Archives of Environmental Health 48:353–358.
Zhong Y, Rafnsson V. 1996. Cancer incidence among Icelandic pesticide users. International Journal of Epidemiology 25(6):1117–1124.
Zober A, Messerer P, Huber P. 1990. Thirty-four-year mortality follow-up of BASF employees exposed to 2,3,7,8-TCDD after the 1953 accident. International Archives of Occupational and Environmental Health 62:139–157.
Zober A, Ott MG, Messerer P. 1994. Morbidity follow-up study of BASF employees exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) after a 1953 chemical reactor incident. Occupational and Environmental Medicine 51:479–486.
Zober A, Messerer P, Ott MG. 1997. BASF studies: Epidemiological and clinical investigations on dioxin-exposed chemical workers. Teratogenesis, Carcinogenesis, and Mutagenesis 17(4–5):249–256.