APPENDIX E
Pressure Dependence of Photochemical Reactions
Augmentation of thermal reactions by excitation with photons is pressure dependent because of the activation and deactivation of excited states by collisions. This was illustrated by the simple example provided by Dellinger et al. [1] at the workshop associated with this study. For the simple model (Figure E-1) of a photolytic followed by thermal excitation to an energy level
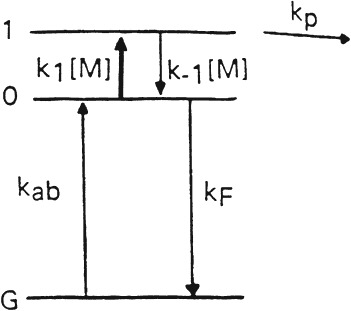
FIGURE E-1 Three State of thermal-photolytic model.
leading to dissociation, the rate constant kdiss for the overall reaction can be shown to be
where the rate coefficients are identified in Figure E-1, and [M] is the total molar concentration.
For the conditions favorable to dissociation, (k1/kF)(kp/k-1)>1, the rate constant can be approximated by
At high pressures the thermal reaction approaches equilibrium and the rate constant becomes equal to the absorption rate kab, independent of pressure. At low pressures the rate becomes proportional to pressure and can be approximated by
Experimental programs should be designed to provide the coefficients needed to model their temperature and pressure dependencies using the quantum Rice Rammsberg and Kassel or Rice Rammsberg Kassel and Marcus representations (1).
REFERENCE
1. Dellinger, B., Graham, J., Berman, J.M., and Klosterman, D. "High-Temperature Photochemistry Induced by Concentrated Solar Radiation." Workshop on Potential Applications of Concentrated Solar Photons, NRC, Nov. 7–8, 1990, Golden, Colo.


