Important Points Highlighted by Individual Speakers
• The creation of more certainty and predictability within the translational research pathway would make investment in this sector more desirable.
• Adjustment of the health care ecosystem to account for advances in technological innovations could improve the translational pathway through reductions in bottlenecks to product development in the later stages of the process.
• Obstacles to translation could be reduced by leveraging of new technologies, opportunities afforded by globalization, regulatory reform, and alternative research models.
• A successful business model for the translation of the findings of basic research to personalized medicine requires that patients be put first through the creation of well-designed clinical questions and the generation of sound clinical data for clinicians and payers.
• A shift in investments from pharmaceutical development to preventive diagnostics would result in a more efficient drug development process because it would provide cost-saving measures for care and new knowledge about disease.
• Patients who own and control their genomic data will benefit from data sharing, lessening the need for sharing of enforcement strategies.
The successful translation of biological discoveries into commercial applications depends on a partnership with industry. Geoffrey Duyk, partner and managing director of TPG Biotech, explained why venture capitalists have been reducing their investments in biomedical companies that are developing products for which the risk is uncertain. He also outlined opportunities for overcoming barriers to the commercialization of research discoveries. Randy Scott, chair and chief executive officer of InVitae, described innovative business models that provide new ways to translate discoveries in basic science to personalized medicine.
THE HIGH COST OF UNCERTAINTY FOR VENTURE CAPITAL
Over the past decade, venture capital has been the “worst returning subsection within private equity,” said Duyk. One of the reasons for its poor performance may be that the development of a new product costs more and takes longer than it did in the past. As a result, even if the product is successful, the return on investment is less than it was a decade ago. “It’s no surprise that capital is leaving the market,” Duyk said.
Duyk and other investors have been rethinking how they invest money in health care. Spending on health care as a percentage of the gross domestic product (GDP) is much higher for the United States than for any other industrialized country, yet health outcomes in the United States are in many ways worse (see Figure 4-1). Improvement of the cost-effectiveness of the system therefore provides a potential opportunity. Duyk mentioned the inefficiencies in the health care system, including the problem of the nonalignment of incentives in medicine, in which treatment of a patient for diabetes prevention today will not produce its full return to the health care system for decades. “This is an area where there needs to be scholarship.… We have to figure out an economic model to align incentives so you can deal with preventive care,” Duyk said.
Many obstacles to bringing a product to market exist, and most of those obstacles are not technological, Duyk said. “I worry less about technological problems because I have more confidence in the ability of the system to solve them. I worry more about the [uncertainty from] the regulators, the public and financial markets, and the reimbursement,” Duyk said. Duyk also recounted the central problem that others mentioned earlier in the workshop, that the translational pathway has challenges because “the early stages of research have become faster, better, and cheaper, but everything else downstream has not really caught up … and unless we make concomitant investments in these areas, what we’re doing is accelerating ourselves to a bottleneck,” said Duyk. These uncertainties have “a huge impact on the cost of doing business,” he said. If preclinical processes were more predictable, drugs could fail faster and with fewer costs. This
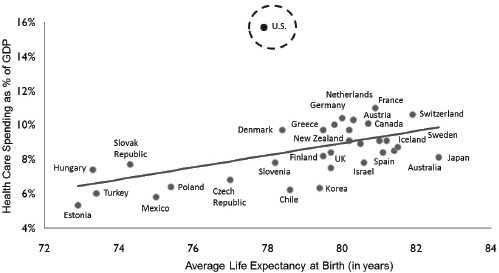
FIGURE 4-1 Health care spending as a percent of GDP by country.
SOURCE: Data from the Organisation for Economic Co-operation and Development, 2007 (http://www.oecd.org/els/health-systems/oecdhealthdata2013-frequentlyrequesteddata.htm [accessed October 3, 2013]).
predictability would then propagate through the rest of the system, bringing greater clarity to regulation, reimbursement, and health care delivery.
Duyk demonstrated the impact that uncertainty can have on investment by explaining a scenario from the work of Gneezy and colleagues (Gneezy et al., 2006). The average price that someone would pay to purchase a $50 gift certificate from a friend is $26. Similarly, someone would pay $45 for a $100 gift certificate, or about 50 percent of the original purchase price, Duyk noted. However, when uncertainty is introduced into the circumstance and the buyer knows only that the gift certificate is worth either $50 or $100 but not which amount specifically, he or she is willing to wager only $16. In this case, the risk is overvalued, resulting in an undervalued product. “Anything we can do to create certainty and predictability in translational research has enormous economic impact, [so] I wouldn’t underestimate [value] in the system,” Duyk said.
To improve the translational pathway, the entire translational research ecosystem needs to be considered, he said, explaining that all the parts need to be taken into account, just like a car needs to be cared for: it needs fuel, tires, and insurance so that it functions properly. The challenge is that the entire ecosystem needs to change around new technologies, such as genomics. “Sometimes, the technologies induce the change. Other times, you, as an innovator, have to figure out how … to allow change to occur.”
However, changing the ecosystem in health care is a significant hurdle because health care is a big business and many external forces affect the system by creating costs and obstacles to progress, Duyk said. The health care system is highly regulated, structured, and paternalistic, which has an enormous impact on making investment decisions. Health care is enabled by technology, but the technology is associated with high cost and risk. The status quo is not sustainable, and fundamental change may require structural change, he said.
Suggestions for Changing the Current Dynamics
Duyk described a range of opportunities that would begin to change the health care ecosystem to adjust for new technologies. Today, the areas in medicine that are some of the most innovative are those in which consumers provide most of the capital, such as laser vision correction surgery, cosmetic surgery, and in vitro fertilization. In the past, most economic decisions were made by physicians, whereas today most decisions may be influenced by reimbursement policies. Duyk suggested that in the future, more decisions and more capital will be derived from the consumer, and as more opportunities arise to empower patients as consumers, patients will expect more data and more transparency when they are responsible for more of the costs, said Duyk. This consumer demand can “force change and give you a place of market entry,” he said.
Mobile communications and cloud computing are other areas in which a market trend presents an opportunity for innovations in health care. Interconnected devices are creating vast stores of information about individuals, and new devices could monitor a wide range of health-related indicators and relay information to consumers, said Duyk.
The way in which people are thinking about privacy concerns and patient data is also changing. Privacy is a major issue, Duyk acknowledged; however, younger and older generations seem to have different perspectives about privacy concerns. Once a consumer can acquire his or her DNA sequence for a few hundred dollars, the dynamic between the holder of that information and the potential uses of that information will fundamentally change. More research is needed to address privacy issues, but “if we don’t adjust the system to reflect new norms of privacy, then the system will adjust,” Duyk said.
Globalization represents another opportunity to explore different types of health care systems, said Duyk, because the demand from emerging markets drives the growth of industry. Although the U.S. health care system is well established and the costs of making changes to this system are high, new types of health care solutions will be appealing in countries where the
GDP is rising rapidly. This trend is already occurring as demands from growing global markets have driven the development of the pharmaceutical industry. Duyk indicated that knowledge-based health care systems, such as those in the United States, are less scalable, but rule-based or evidence-based systems are more conducive to scaling, as exemplified by the cost-effectiveness of AIDS treatment in Africa.
Already, drug development increasingly occurs outside of the United States where regulatory agencies are more cooperative and collaborative, such as the biosimilars market, said Duyk. Other countries are improving their regulatory systems, and some drugs are now being approved elsewhere as a result. Investments need to be made in FDA, which is “underfunded and understaffed,” Duyk said. Rethinking a regulatory agency from the ground up is very difficult, he admitted, but time and effort need to be devoted to the task. One approach may be to restructure one of the divisions of FDA and use that as an example to spread reform through the rest of the agency. A workshop participant pointed out that FDA is preparing to handle genomics data and noted that similar regulatory systems around the world are not structured in the same way. Because genomic data are imperfect, for example, an error rate of even only 0.001 percent yields 3 million errors per haploid genome sequence, so the validation and qualification of the information are important.
Alternative research models and centers are also needed, said Duyk. “I honestly think we’re not going to fix translational research in the halls of a traditional university,” he said. Models such as the Massachusetts Institute of Technology Media Lab of the Center for Bits and Atoms, and the Santa Fe Institute demonstrate how to create different environments in which translational research can thrive.
Crowd sourcing also makes it possible to tap into the power of communities. “There are a lot of very smart people out there. If you give them access to data, they are going to figure things out.” Duyk went on to highlight a crowd-sourcing example from a game called Foldit, which originated at the University of Washington in Seattle. Essentially, a protein-folding problem was fashioned into a game whereby, while a person was playing it, competitive solutions for protein folding were simultaneously developed. “So what I would advocate is thinking about these out-of-the-box … ways of approaching education,” Duyk said. Students want to work on bioinformatics problems, Duyk insisted, but they are not aware of the problems that exist or of the rewards to be gained by solving those problems. “I don’t think that there is a deficit [of] people who want to work on these problems. I think there is a mismatch in our ability to teach them [that] those problems exist, and the tools aren’t readily available,” Duyk said.
A MODEL FOR TRANSLATIONAL MEDICINE IN GENOMICS
“I am an unashamed, unabashed optimist for the future of translational medicine,” Scott said. The next decade will bring tremendous technological and commercial progress, he suggested. He described how Genomic Health has built a model for translating science into personalized medicine on the basis of the success that it achieved when it brought the multigene, multianalyte Oncotype DX® test to the market to help breast cancer patients and their physicians make treatment decisions.
As stated by other speakers, Scott said, success begins with asking not just interesting scientific questions but also the right clinical question, and that requires listening to physicians and their patients. Bringing translational medicine to clinical practice is feasible, but “it’s really hard, it’s really painful, it costs a lot of money, and it’s not nearly as easy as we all hoped it would be a decade ago.” Cancer patients have always been at the center of Genomic Health’s business model, and “we fundamentally believe that if we do great things for patients that are really transformational in that patient-physician conversation and drive the way those patients are going to be treated, we’ll find a way to get that paid for,” Scott said, “Focus on your customers.”
Scott discussed how Genomic Health designed its model for product development. “You really need to go sit down with a physician and [his or her] patient and listen to that conversation because that’s the conversation that you have to affect,” he said. Once the right clinical questions are identified, Genomic Health focuses on high-quality, well-controlled clinical trial data, combined with sound statistical analysis and algorithm development, along with attention to other methodological details. Working closely with physicians is critical, because “you can’t go anywhere in health care unless you get the physicians on board.” Physicians will make the right decisions for their patients, if they are provided with strong data, Scott said. How physicians view the importance of what is accomplished for their patients matters more than the exact economics, he said.
Genomic Health is seeing successes in translational medicine because it is focused on providing sound clinical data. In theory, Oncotype DX™ should have been very attractive to payers because it lowers the cost of cancer care by providing cost-saving information about whether chemotherapy will be useful, but the reason payers supported the test in the end was because it was backed by good clinical data and had the support of physicians, said Scott. By using this model of asking the right clinical questions, Genomic Health now has a similar test for colon cancer, and a test for prostate cancer is under development, said Scott.
The reason for the lack of success in diagnostics development is that the United States is currently underinvesting in this area, Scott said. Devel-
opment of a new drug typically takes more than a decade, costs more than $1 billion, and has an 80 to 90 percent failure rate, he said. In contrast, development of a new diagnostic typically takes 3 to 4 years, costs $100 million to $200 million (when retrospective samples from completed clinical trials are used), and has a success rate close to 90 percent. The diagnostics model is “a much better business model. What if we stopped spending 50 billion or 100 billion dollars a year on research and development of the drugs for diseases we don’t understand and spent 10 billion to 20 billion dollars on understanding the disease so we can make that drug development more efficient?” asked Scott.
Change is occurring in the global payment market for diagnostic tests, Scott noted. Regions where health care is not covered—in South America and in parts of Europe and Asia—patients have incentives to spend $3,000 on a test that would provide more information about the chance that they should take on chemotherapy that could cost $20,000, Scott said. “This really will become a global economy as the costs come down very fast.”
Technology can be a solution when financing for the development of diagnostic tests is difficult to obtain because of increases in capital costs and hesitance on the part of investors to take risks that they may have been willing to take a few years ago, Scott said. “The cost of DNA sequencing is coming down rapidly and so rapidly that it’s having a dramatic shift in how we think of the cost of genetic information,” Scott said. The continued development of technologies will make it easier to finance diagnostics companies in the future, he said.
The exponentially declining cost of genome sequencing will have the same transformative effect on translational medicine that the exponentially increasing power of computing has had on digital technologies. Within 10 years, the cost of a complete genome sequence will be less than the price of a single genetic test today. Furthermore, the value of a single genome sequence is much less than the value of many genome sequences, just as the value of a single computer is dwarfed by the power of computers interconnected through the Internet. “Disruption is going to come very, very fast in this field. In fact, it’s going to be almost unstoppable, and we should start preparing for when that disruption comes,” Scott said.
A New Business Model
Scott said that he had recently joined a new company named InVitae that has the goal of “aggregating all the world’s genetic tests into a single assay with better quality and lower cost than [those of] most single gene assays today.” Within a few years, the comprehensive test will be able to look at all known Mendelian inherited genetic traits at a low cost. The company believes that genetic testing should be broadly available to everyone.
The price of genetic testing therefore needs to be consumer friendly, and the test needs to be globally available. “There’s no country in the world that’s going to be able to simply close the walls and say, ‘no, our population can’t have access to genetic information.’ … This will become a global economy, especially as the costs come down.”
The company also embraces the idea that data should be freely available; patients should have ownership and control over their data, and data should be shared so that others can learn from the data. “As we like to say at InVitae, we don’t patent the genes; we set them free. We believe very strongly that patients should own and control their own genetic information, that they should have access to the information, and they should have the rights to determine how it is used. And if that means not at all, that’s fine.” Data sharing will eventually become part of the medical system, he said. Scott continued, saying that he is not in agreement with the enforcement model of data sharing. It is not needed once people begin to see the incentives and value in sharing their data, as occurred with social media. “I’m fine if [developers] don’t want to share their data.… I just think it is going to be bad business 10 years from now,” said Scott.








