6
Magnetic Resonance Imaging
New Breakthroughs in Medical Diagnosis
Biology in general and medicine even more so provide a natural—if anthropocentric—setting to address the question, How do the fruits of scientific progress concern the individual? Medicine itself consists of two broad pursuits; first, the clinical—concerned with applying the art and science of medicine to healing people. A second focus of modern medicine is the research conducted to address more general scientific dilemmas whose resolution might contribute to remedies for disease, illness, and incapacity per se, in a larger context than that of individual patients. As practiced, for example, at the National Institutes of Health (NIH)—headquartered in Bethesda, Maryland, and regarded as the most comprehensive U.S. medical research organization—medicine necessarily involves a myriad of patients and subjects who undergo tests, protocols, and experimental treatments as medical researchers address the larger questions of etiology and treatment. The Frontiers symposium session on magnetic resonance imaging (MRI) provided a vivid illustration of how these two sometimes distinct aspects of medicine often merge.
In the last 15 years, MRI has moved from the embryonic research stages into the thick of modern clinical practice. Most of the specialists in the Frontiers session nearly every day see—if not dozens of patients—scores of MRI pictures to aid diagnosis. Keynote presenter William Bradley, Jr., director of the MR Center at Long Beach Memorial Medical Center, specializes in MR imaging of the brain and together with David Stark—according to session organizer Peter
Dervan of the California Institute of Technology—"wrote the book [Stark and Bradley, 1988]" on clinical MRI. Stark specializes in imaging the liver and abdomen, where contrast agents have been particularly useful for enhancing MR images. He teaches at Harvard University and also works at the Radiology Department of Massachusetts General Hospital. Both authors are practicing physicians whose primary charge is to produce superior pictures of patients who are referred by other doctors for MR imaging.
Graeme Bydder from the Hammersmith Hospital in London was another of the session's presenters and is, said Dervan, "one of the true pioneers in this area." Bydder explained how the general problem of motion in the area under consideration has led to some refined MRI techniques. John Crues III provides another example of the range of MRI specialists. Teaching on the faculty at the UCLA Medical School and also serving as director of MRI at Santa Barbara Cottage Hospital, Crues told about the increasing value of MRI in imaging the musculoskeletal system, hitherto thought to be the province of the x ray. If there was an exception to the generalization that clinical MRI involves its practitioners in a continual process of analyzing and refining the pictures made of individual patients, Robert Balaban, chief of the Laboratory of Cardiac Energetics at NIH, is it. His laboratory is building new prototype machines in an effort to elucidate some of the subatomic phenomena even most MRI practitioners take for granted.
MRI provides a new way to look inside the body. When compared to the earlier x-ray systems—even sophisticated current versions such as computerized tomography (CT) and positron emission tomography (PET)—MRI is noninvasive, and often dramatically superior in discerning details and subtleties. The enhanced safety and results derive from a different approach altogether. The x ray invades the body in a series of electromagnetic pulses that are targeted to collide with the electrons in the tissue under examination. MRI, however, rests on different interactions: first, since most MR imaging targets the nuclei of hydrogen atoms (1H) found in water (H2O), the interaction is with protons; second, the "disruption" caused by the radiation is far more benign, since the radio frequency (RF) pulses generated travel in wavelengths many orders of magnitude longer—by photons that have energy many orders of magnitude less powerful—than those in the x-ray range of the electromagnetic spectrum. Instead of targeting electrons and thus generating ions in the body, MRI manipulates the inherent magnetic properties of spinning protons, detecting information from how the protons react to the RF pulses and magnetic fields directed toward the target tissue.
Even though Wilhelm Roentgen's discovery of the x ray astounded the world and revolutionized medical diagnosis, its benefits were not entirely benign. The radiation used was in the higher ranges of the electromagnetic spectrum and posed hazards. As it collided with atoms in the body, sometimes glancing off, sometimes being absorbed and creating ions, the radiation produced a significant energy transaction. Also, notwithstanding the use of contrast agents, the process revealed no depth and therefore showed sometimes confusing overlap of images in the frame. By the 1970s, the computer's power to generate, analyze, and combine images had overcome this deficiency, and CT scanning produced dramatically improved and enhanced x-ray images in three dimensions. These views of the body enhanced the power of medical diagnosis significantly, and won a Nobel Prize in 1979 for one of the pioneers, computer engineer Godfrey Hounsfield.
PET scanning techniques also rely on interactions with particles, but the emanating source comes from within the body by way of radioactive molecules introduced there by nuclear physicians. These chemicals are designed—insofar as is possible—to go to the area under scrutiny, and once there to begin to decay and emit positrons. The positrons then collide—in some instances combine—with electrons nearby and produce gamma rays. This radiation emerges from the body to be read by detectors that can pinpoint the source of the rays.
Another recently developed imaging technique is Doppler ultrasound, in which the potentially dangerous high-frequency (short-wavelength) x rays and gamma rays are supplanted by the much lower-frequency (longer-wavelength) sound waves. The waves are directed toward the target and then analyzed as they emerge for the pattern of interference they then carry.
MRI practitioners approach the formation of an image with a fundamentally different assumption than targeting electrons or tissues for collision. The imager uses magnetization to affect a range of tissue and imparts magnetic energy to all of the 1H protons in the target area. This contrasts with the x-ray process, in which increasing the (potentially harmful) amount of radiation is the only means for increasing the likelihood of impacts that lead to a successful image. Quantum physics also demonstrates that electrons vary far less in their ability to reveal density or spatialization than do water molecules in the body. Electrons are electrons, regardless of the atomic nuclei they are circling, and x-ray pictures often fail to discriminate very clearly between them. Water (and thus the presence of 1H) in the target tissues varies much more significantly. Concentration, density, and even more subtle measures having to do with H2O binding to
macromolecules all provide possible ways that MRI can exploit variations in water in the body—even in very nearby and only slightly different tissue targets—to produce better pictures.
As Bradley described the intricacies of MRI, he clarified which particular tissues and conditions in the body yield the best MRI pictures. From a strictly clinical perspective, many tissues and processes are ideal candidates for MRI, though some are not. He and his colleagues touched on the reasons for some of these limitations but also suggested how the expanding horizons of MRI research are encompassing more and more clinical diagnostic situations. As more sophisticated methods of scanning, more discerning contrast agents, and altogether new techniques are refined, the potential clinical scope of MRI will expand.
In the world of modern clinical medicine, however, practitioners face a number of practical considerations. The machinery and staff for a comprehensive MRI center cost millions of dollars a year and patently must be supported by larger institutions or hospitals, many of which have not installed MRI centers. Clinical and cost considerations aside, some patients are not suitable candidates for MRI because they cannot tolerate the conditions of the procedure, physically or psychologically. Usually the patient must climb into a large tunnel, hold still for 10 or 15 minutes, and perhaps remain for two or three times that long for several series of images. The noise and isolation of the machines have also presented problems for patients. Thus MRI is but one of a number of approaches to imaging the body. X-ray procedures continue to be refined, sonogram imaging has many applications, angiography and arthrography give doctors a direct approach to the problem area, and there may always be certain situations where the MRI procedure is categorically impossible. And yet, if cost and other practical impediments can be overcome, the future value of MRI to diagnosis is almost without limit. As Bradley described it, MRI "really has become the most powerful diagnostic tool in clinical medicine since the discovery of the x ray."
Powerful, and also sophisticated. The science of radiology, following the discovery by Roentgen in 1895 of x rays, created a formal branch of medicine devoted exclusively to diagnosis. Medicine, of course, has for millennia involved its practitioners in the process of ascertaining what it was they were undertaking to treat. Said Hippocrates: "The capacity to diagnose is the most important part of the medical art." But not until the 20th century did that part become so distinct as to involve separate, specialized doctors and technicians whose relationship with the patient was conducted primarily through the imaging machinery. This development has continued as other
imaging modalities were discovered and developed, and MRI technology continues this trend. As CT and PET scanning techniques have become more elaborate and computer-enhanced strategies more complex (both for obtaining and then for processing the images), specialists must develop more than the discernment to simply read the results. With MRI in particular, the choice of imaging strategies is fundamental to producing pictures with the hoped-for information. That choice, and the reading of the pictures, require on the part of the MRI practitioner a mastery of the physics and biology that underlie the process, a mastery that has not been so crucial with other imaging modalities.
Bradley has written, "Understanding the physical principles of MRI is often compared to understanding the operation of an internal combustion engine. One need not understand the intricate details of an engine to be able to drive a car. Similarly, one need not be conversant with the basic principles of nuclear magnetic resonance to understand certain simple aspects of an MR image" (Stark and Bradley, 1988, p. 108). However, he made clear in his presentation to the symposium that radiologists and other MRI practitioners must consider the various factors—both limiting and enhancing—inherent to the image they are after. These factors presume a fundamental knowledge of the principles of magnetic resonance, which he summarized for the scientists in his 1990 Frontiers keynote presentation, "Clinical Magnetic Resonance Imaging and Spectroscopy."
THE PHYSICS AND COMPUTATION BEHIND MRI
Like its predecessor the x ray, MRI grew out of studies in basic physics. Physics itself, however, was undergoing a dramatic growth into the new territory of quantum electrodynamics, leading to exploration of particle behaviors undreamed of even in Roentgen's time. The era just before the turn of the century would soon come to seem almost antiquated, after the views of Max Planck, Niels Bohr, Albert Einstein, and Max Born began to open up vast new horizons, though on a subatomic scale. For decades, much of the important work was done in America, as the European conflict spurred many scientists seeking both academic and personal freedoms to depart for American universities. Magnetic resonance pioneer Felix Bloch credited Otto Stern and I. Estermann with first elucidating the deflection of a molecular beam of hydrogen by an inhomogeneous magnetic field in 1934. I.I. Rabi in 1937 confirmed the magnetic moment of nucleons and then demonstrated that resonance could be induced using particle beams and further magnetic effects. The definitive conception
came in 1946 when—in separate efforts by Bloch and Edward Purcell—magnetic resonance was demonstrated and explained in solids and liquids.
The field was then called nuclear magnetic resonance (NMR) spectroscopy and bore immediate, fruitful advances in chemistry, the study of magnetism, and eventually biology. Paul Lauterbur in 1972 solved the problem of transforming the spectra data to a three-dimensional image by manipulating the magnetic field itself; what was to become MRI he called zeugmatography. Medical applications have proliferated in the two decades since. Techniques and computer power have both become more sophisticated. State-of-the-art MRI machines now provide scientists with a wide arsenal of approaches to produce spectacular pictures of not only the details, but also the condition, of soft tissue, fluid flow, even bone. As Dervan mused, looking back on this history: ''Whoever thought that that simple basic thread of research would ultimately lead to one of the most powerful clinical diagnostic tools now on planet earth? I think there are 2000 hospitals in the United States that are using this and probably 3000 worldwide.''
How MRI Generates Information
"Magnetic resonance imaging is based on a harmless interaction between certain nuclei in the body (when placed in a strong magnetic field) and radio waves," began Bradley in his explanation of the physics involved. The process works only with nuclei having an odd number of protons or neutrons, and works better when the nuclei are in great abundance. The hydrogen atom provides the ideal MRI target for these two reasons: it has a single proton, and is the most abundant nucleus of any species in the body.
To summarize the process: the MRI operator designs and controls an input in the form of radio waves directed toward the 1H protons in the target area. A further input involves the magnetic field environment applied to the area, where the area is usually limited to a thin slice of only several millimeters, and the information in such a voxel (volume element) is subsequently translated into a two-dimensional pixel (picture element) by the computer. The spin of all protons produces a characteristic magnetic moment, in this case one that is inherent to hydrogen. These spinning particles respond only to inputs adjusted according to the basic formula, called the Larmor equation: ω = γ · B0. This equation defines the frequency of the RF pulse (ω) needed to produce resonance in the 1H protons (which have a characteristic gyromagnetic ratio, γ) in a given magnetic field (B0). When such a frequency is transmitted, those and only those
nuclei absorb that predesigned energy input. "The Larmor equation provides the key to spatial localization of the NMR signal and subsequent image formation," emphasized Bradley.
The MRI practitioner influences the subsequent release of that energy by manipulating the magnetic field environment across a gradient so that only those protons at a very specific site are affected. As that energy is emitted, the protons return to their original energy state. The act of absorbing and emitting energy is repeated over and over again, very rapidly: the protons are said to be resonating, and they do so at the Larmor frequency. The resonance signal is picked up by a radio receiver tuned to that particular frequency. The information comes in the form of amplitude versus time. In order to produce an image, that information must be transformed into the domain of amplitude versus frequency. The Fast Fourier Transform (FFT) accomplishes this necessary conversion, as well as the task of distinguishing each proton's characteristic frequency from the composite signal.
Bradley explained the basic steps of the process in greater detail in order to clarify the factors he and other MRI practitioners consider when designing the inputs to target a particular area or tissue for imaging. The key to locating the precise area under examination is the nonuniformity of the primary magnetic field (which is created by the large, primary superconducting magnet). The MRI operator intentionally creates and designs this nonuniform field by superimposing over the main field additional magnetic fields produced by resistive electromagnets coaxial to the axis of the main field, continued Bradley, adding, "These additional electromagnets can provide linear spatial variation in the net magnetic field along the x-, y-, or z-axis, creating magnetic field gradients, gx, gy, and gz."
From this spatial arrangement comes another dramatic improvement in diagnostic power over CT, because the CT image is limited to the plane of the gantry and produces clinical images in the axial and semicoronal planes only. With the complex magnetic fields that magnetic resonance imagers can establish, the practitioner can acquire images in any plane—axial, sagittal, coronal, or oblique. The equation w = gxx where gx = dB/dx, explains how this is possible. The magnetic field strength through a combination of these various resistive magnets can be controlled at a precise point in three-dimensional space. Since protons in hydrogen will react to RF pulses that are a function of the field strength present (according to the Larmor equation), said Bradley, MRI experiments create "a one-to-one correspondence between frequency and position." Extremely precise localization is therefore possible.
To summarize: Within a given tomographic slice, magnetic field strength is established such that a 1H proton at any minute point locatable on the x-and y-axes will resonate to RF pulses of a particular frequency, while 1H protons at nearby and adjacent points will not absorb the RF pulses because their magnetic environment—and hence their Larmor frequency—is different. A series of slices are then each submitted to a similar MRI pulse sequence, and the z-axis is captured, allowing the computer to produce a fully three-dimensional image of any point in the body where hydrogen protons are free to resonate. Thus is the first MRI measurement obtained, which reflects hydrogen proton density. The ubiquity of water in and between almost all cells of the body explains why MRI can be directed virtually anywhere. However, just as all electrons are electrons, so too are all protons identical. And if their densities were the only information discernible, the MRI procedure would not have begun to revolutionize diagnostic imaging as it has.
At the heart of MRI are several other more subtle phenomena that derive from the quantum physics of resonance. As a generalization, MRI studies are conducted to explore how individual 1H protons behave subsequent to their absorption of energy from the RF pulse. The protons are first distinguished by location, according to the field strength present. This feature explains only how a very precise location can be isolated in an image. The goal of MRI, however, is not to measure water or 1H protons, but rather to produce revealing images of tissue and internal processes. Fortunately, these targets are almost invariably associated with water and can very often be distinguished in fine detail by how individual water molecules that are within the tissue behave. One way of describing this behavior is to ascertain the net effect a given 1H proton's local chemical environment has on its otherwise predictable atomic activity—its return to equilibrium after absorbing the magnetization energy—compared to other protons nearby. Physicists classify as relaxation the primary behavior involved: over a measurable period of time, the 1H atoms lose the energy that was absorbed from the photons of the RF pulse(s). In losing this energy, all of the protons that were energized by the RF pulse are involved in two basic activities, and single MR images result from a series of pulse sequences that are designed by the operator to focus on one or the other.
T1and T2Relaxation Times as Data
Bradley referred to the fact that each atomic species has its own characteristic magnetic moment. Where does this come from? The
quantum quality of spin arises whenever a nucleus has an odd number of either nucleon. Even numbers of protons or neutrons cancel one another's magnetic potential, but when one of these particles is left unpaired, a potential magnetization arises (it possesses a quantum spin of 1/2) because it is spinning rapidly. In a large collection of such atoms, however, there is no net magnetization—species are only potentially magnetic—because the various small magnetic moments of millions of individual particles will statistically cancel one another out. When such a collection of atoms is put into the primary magnetic field of an MR imager, however, these particles are influenced and act something like atoms in a bar magnet, aligning with the magnetic vector of the field. In practice, however, not all of them align parallel to the field vector. "After a few seconds," Bradley explained, a slight majority of them align parallel to the field, the rest antiparallel. The difference is only about 1 proton for every 1 million magnetized, but "this excess gives the tissue [in which the 1H protons are embedded] a bulk property known as its magnetization."
When exposed to a single RF pulse at the Larmor frequency, this magnetization, referred to as longitudinal, disappears. On the quantum scale, the population of protons has responded to the energy by equally distributing themselves parallel and antiparallel to the main field. Equilibrium, however, requires that an excess of 1 proton in a million point with the field, rather than an equal number with and against it. The excess protons that were flipped into the antiparallel direction are therefore in a higher energy state and tend to return. As they do so, they lose this energy, in the form of heat, to their environment. Physicists refer to this local region as the lattice, a holdover from earlier studies of relaxation in solids that often had a crystal lattice structure. Thus the phenomenon is called, variously, thermal, longitudinal, or spin-lattice relaxation. It is designated T1 and is the time required for longitudinal relaxation to regain 63 percent of its equilibrium value. 1/T1 is the relaxation rate of a species.
A second phenomenon occurs as RF pulses are received by the 1H target protons, however. The energy of this pulse, as any beam of energy will, provides its own magnetic moment, which can be seen to arrive at the main field from a perpendicular direction. "When the system is exposed to the oscillating magnetic component of a radio wave at the Larmor frequency, the magnetization will begin to precess about the axis of that second, smaller magnetic field at the Larmor frequency," explained Bradley. It will move from pointing along the z-axis rotating toward 90°, and continue down to the-z-axis at 180°, at which point the magnetization is said to be reversed. The rotation about the z-axis will continue, and the precession thus in-
duces an oscillating magnetic signal. "An RF receiver coil placed such that its axis is perpendicular" to the main field, said Bradley, "will be sensitive to such fluctuating changes in magnetization, and a voltage will be induced, oscillating at the Larmor frequency. This signal is known as the free induction decay (FID)." Its exponential decay occurs with a time constant T2*.
In a perfectly homogeneous magnetic field, the magnetic effect of the RF pulse on transverse magnification would continue, once set into motion, for quite some time. This time, called T2 relaxation time, can be calculated. In practice, however, the effect is observed to end much sooner than T2. Why? "Differences in local magnetic susceptibility and imperfections in the magnet result in local hot and cold spots in the field, with resultant differences in magnetic frequency," explained Bradley. Each of the spinning protons producing its own little magnetic moment is exposed to a slightly different microscopic magnetic environment, which is itself changing rapidly. As an analogy, one wave will ripple smoothly outward on the surface of a lake, but a series of stones thrown in create multiple waves that eventually cancel out. So, too, do these protons having slightly different phase angles, and spinning at slightly different frequencies, cause the whole system to run down or dephase. The crucial factor in all of this atomic variation, however, is the tissue environment where these protons are being measured. In a given area, in the water associated with a group of similar cells—a tumor, for example—the relaxation rate of the protons will be similar enough to distinguish as a group from another nearby signal emitted by hydrogen protons in cells or tissue whose biological condition is different.
But, for a variety of reasons, T2 is the preferred measurement. What good is T2 if it only applies to homogeneous magnetic field conditions that cannot be attained? A major refinement in MRI was the development of the spin-echo technique, a way of purifying the signal so as to compensate for the irregularities of the field. It was established that when a given RF pulse is sent (calculated to rotate the magnetization by 90°), the decay of the signal is a function of T2*. If a second pulse of twice the duration is then sent (to rotate the magnetization an additional 180°), a so-called spin echo forms. This echo is seen to reach its maximum amplitude in exactly as much time as the period that elapsed between sending the first (90°) pulse and the second (180°) pulse. The time from the first pulse until this spin echo occurs is called the TE or echo delay time. The effect of this series of pulses is to bring the precession rates of the various randomly precessing protons back into phase, regardless of where they were at the time of the second RF pulse. Thus the spin-echo tech-
nique allows T2 to be measured. It is also referred to as the transverse, or spin-spin relaxation, time.
T1and T2Time as a Diagnostic Lens
Thus T1 is a measurement of how quickly a proton (in reality the average of millions of protons at a given site) that is resonating at the Larmor frequency recovers 63 percent of its equilibrium magnetization. T2 measures how quickly this collection of protons at a given site loses 63 percent of its phase coherence, once that coherence is induced by a pulse sequence designed to coordinate the spinning motion of precession of the various particles. T2 can never be longer than T1, since once a particle has lost its resonant energy, phase coherence becomes irrelevant. In pure water in vitro, both T1 and T2 are about 2.7 seconds. As water becomes more complex with solutes, these times shorten, since the presence of other materials provides additional magnetic forces that speed up the energy drain and the phase interference. Generally speaking, the 1H protons in water associated with tissues show T1 rates about 5 times greater, and T2 rates about 50 times greater, than the rates in pure water.
Exactly how these rates are affected by the environment of the protons is a question of manifold complexity, under continuing study, which dates back to the early days of NMR. The so-called BPP theory of relaxation unified work done by Nicholaas Bloembergen, Purcell, and R.V. Pound over four decades ago and provided a qualitative description of relaxation in solids and monomolecular solvents like water, ethanol, glycerol, and other oils (Fullerton, 1988, p. 42). But as NMR was being developed into MRI, the inadequacy of these concepts became manifest when applied to the extremely heterogeneous multicomponent solutions—consisting of very complex collections of proteins—that are found in human tissue. In particular, hydration-induced changes in the motion of water at or near the surface of macromolecules have a profound influence on relaxation times.
Bradley explained how the practitioner develops a pulse sequence—a series of RF pulses—based on a feeling for how the chemical environment in a given area will influence the local magnetic effects on the target protons. This general awareness, based on clinical experience, guides the design of a series of sequences intended to elicit proton density, T1-, or T2-weighted images (Figure 6.1). One variable is the period of time between 90° pulses, the repetition time (TR). When the spin-echo technique is used, another time parameter is the echo delay time (TE), which "is the time between the 90° pulse and
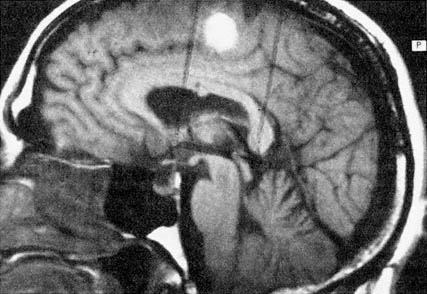
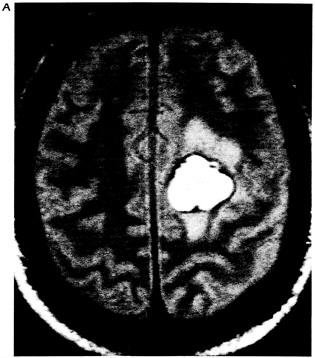
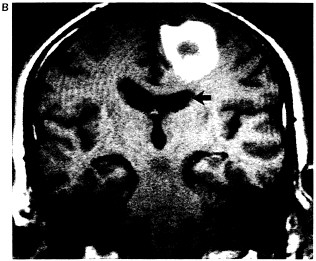
Figure 6.1 Multiplanar display capabilities for magnetic resonance imaging. (A) Proton-density-weighted (long-TR/short-TE) axial image through a chronic hematoma. This is the same plane visualized by x-ray computed tomography, which is parallel to the ground with the patient erect. (B) T1-weighted (short-TR/short-TE), coronal plane through the hematoma demonstrates local mass effects, displacing the left frontal horn (arrow) inferiorly. (Coronal images are obtained in the plane of the face with the patient facing us.) (C) T1-weighted image acquired in the sagittal plane, i.e., looking at the brain from the side in the midline with the nasal pharynx to the reader's left. By drawing a nearly horizontal line connecting the anterior and posterior commissures (the "bicommissural line"), and drawing perpendiculars to them, the central sulcus can be identified. Since the bright hematoma involves the precentral gyrus (the motor strip), we can predict on the basis of these images that motor deficits would be present as they were in the right leg. The combination of appearances on T1- and T2-weighted images allows us to determine that the central portion of the hematoma (which remains bright on both T1- and proton-density-weighted images) contains extracellular methemoglobin, while on the proton-density-weighted image there is a dark rim surrounding the hematoma due to hemosiderin macrophages. This places the hematoma in the "chronic" class, which is at least 2 weeks old. (Courtesy of W. Bradley.)
the middle of the spin echo. Typically," Bradley continued, "tissues containing more water have longer T1 relaxation times, while those containing lipid or paramagnetic species capable of a dipole-dipole interaction cause T1 shortening." When the diagnostician examines the resultant images, "tissues with short T1 relaxation times appear brighter, particularly on images acquired with short repetition times. Such short TR images tend to enhance the differences based on T1 and are, therefore, known as T1-weighted images," he explained. Conversely, because the longitudinal magnetization increases exponentially to a plateau, he added, "long TR images are thus said to be proton density weighted." When the spin-echo sequence is used, a similar variable is at work, and scans with a long TR and long TE are known as T2-weighted images. Such T2-weighted images appear brighter where T2 is longer, as opposed to the T1-weighted images, where shorter T1 times produce a higher signal intensity and therefore a brighter image.
Looking More Closely at the Complexities of Relaxation
Robert Balaban and his team at the NIH are doing research into the question of how water molecules actually interact on or near the surface of the macromolecules that constitute so much of human tissue. They are examining extremely small magnetic interactions to explore how this mechanism might really work and to understand "why we get such good contrast with the different types of imaging sequences," explained Balaban. It was known that the relaxation time of a given proton depended on where it was located, its molecular neighborhood, so to speak. In pure water in the laboratory, T1 and T2 are identical. In tissue, they vary. The simple explanation is that the "freer the water, the longer the relaxation time." So-called bound water refers to water molecules tightly packed near protein structures classified as macromolecules. The "general dogma was that cross-relaxation or through-space interaction," said Balaban, is roughly "analogous to just getting two magnets close to each other. They don't have to touch, but of course they are going to interact through space as these dipoles get close enough."
Balaban's team wanted to see if this was really the primary cause of T1 relaxation and also to come up with a description more chemically precise than just saying it was due to "cross-relaxation, or chemical exchange." They began with the basic methodology of saturation transfer but added a crucial variant. By setting the irradiation frequency only 5 to 10 kilohertz off the water frequency line, they were able to target the protons in the macromolecule without directly af-
fecting the protons in free water (Eng et al., 1991). Thus any change in the water proton signal would be due to magnetization transfer between the macromolecule and water protons. The results clearly confirmed that the magnetization transfer with macromolecules was indeed the dominant source of T1 relaxation for water in tissues. Moreover, since tissues vary as to their macromolecular makeup (and in turn also vary from the changes found in disease), this refinement yielded a predictable effect for each tissue type (Figure 6.2). What
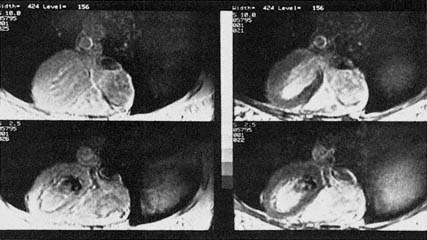
Figure 6.2 MRI image of the human heart collected at the National Insti tutes of Health on a normal volunteer. The images on the left are standard MRI images collected at different points in the cardiac cycle. The bottom of the image is the front of the chest in these axial images. The heart is in the center of the image with the large left ventricle on the left and the right ventricle below it to the right. The images on the right were collected under identical conditions, except that the macromolecules within the tissue were irradiated, revealing where water is in magnetic communication with these structures. A decrease in signal intensity occurs where the interaction is strongest; notice the interaction in the heart and skeletal muscle of the chest. The bright area in the center of the left and right ventricles is the blood within the heart chambers. This effect not only improves the quality of the MR image, but also promises to improve the diagnostic potential of MRI since this form of contrast is specifically linked to biochemical structures in the cell. (Reprinted with permission from Balaban et al., 1991. Copyright © 1991 by the Radiological Society of North America, Inc.)
was being measured was the rate constant for the interaction of a particular tissue's macromolecules with protons from adjacent water molecules. ''So these studies seem to confirm that indeed, there is a relaxation pathway through the macromolecules in biological tissues, and,'' he continued, citing a large effect in skeletal muscles and very little effect in the kidney, "that it is a tissue-specific phenomenon." Thus by irradiating the sample with RF energy slightly off the expected Larmor frequency, Balaban and his colleagues devised a new method that produced heightened contrast where the macromolecular interactions were significant.
In clinical situations, it turned out that the presence of lipids in a given tissue was usually a dominant factor. Further studies suggested a very specific molecular reaction and isolated a specific OH group as the primary actor. What was being measured was the correlation time for the magnetic reaction in which the OH group was orienting the target water molecules. "It is not a chemical exchange," insisted Balaban, "but rather the action of an electric dipole" with a very long correlation time (10-9 seconds). "This model is much different from what we used to think about relaxation processes in biological tissues," Balaban summed up.
MAGNETIC RESONANCE IMAGING IN MEDICINE
Soft Tissues—Even Bone and Moving Joints—Are Prime MRI Targets
John Crues specializes in diagnosing musculoskeletal problems. When MRI began to reach clinical trials in the early 1980s, he reported, not many of his colleagues in orthopedics were optimistic that it would be of much use to their specialty because of an apparent major hurdle. Bones, explained Crues, "are made up of a matrix that is primarily calcium," an atom whose structure does not lend itself to imaging because it does not possess the mobile protons of hydrogen. Before long, however, surprisingly good pictures started to emerge (Figure 6.3) "because we had forgotten that the living elements of bone actually contain soft tissue that has a lot of hydrogen," said Crues. "When we started looking at the bones and the soft tissues of the musculoskeletal system with MR, we found that there really was a diagnostic revolution there," which he, too, compared to the vistas opened up by Roentgen's discovery of the x ray. Added Crues, "MRI has become the preferred imaging modality for the musculoskeletal system."
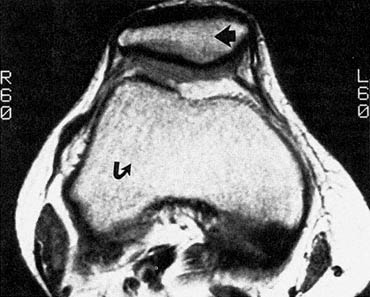
Figure 6.3 High-resolution MR image of the knee. This MR image shows the inside of a human knee painlessly and without the need for surgery. The patella (kneecap) can be seen anteriorly (fat arrow). The distal femur (thigh bone) is seen more posteriorly (curved arrow). The bright structures around the bones are fat. The dark structures are vessels, nerves, ligaments, ten dons, and muscles. (Courtesy of J. Crues.)
Biomechanical joints such as the human knee and shoulder represent systems with a very precise design, produced by evolution for purposes that people living in modern times now routinely exceed. Modern athletes may not be that much more physically adept than their human counterparts who—a million years ago—roamed the savannas in search of food and shelter and in flight from enemies, but the persistent stress of modern activity puts their bodies and joints to a greater test. Medicine responds to the challenge of these greater demands by studying the joint systems, for example, in search of warning precursors, indicators of disease and breakdown. Until recently, such studies were limited to anatomical observations made during surgery and after death, when the system could be laid bare and its parts examined. The biomechanics were inferred from how these parts seemed to be connected and constructed. MRI and other
modern diagnostics are now providing a much more scientific view of such systems. With contrast agents, the portable receivers Crues mentioned, and a version of MRI being refined that uses fluoroscopy, isotropic three-dimensional imaging can be done on a moving joint, functioning in real time under stresses designed by the diagnostician.
Twenty years ago, cartilage tears were often inferred from observing a characteristic presence of edema and interference with the range of motion. Mistakes were often made, however, given the complexity of the structure and the possible alternative kinds of damage that could be masked by the swelling. Although by the early 1980s the growing use of the arthroscope improved diagnosis significantly, a minor surgical procedure was still required just to confirm the diagnosis. But as MRI has been refined, it now reveals both anatomy and pathology, by "noninvasive diagnosis of joint and soft tissue injuries that was inconceivable 10 years ago. This has virtually eliminated diagnostic surgery in the musculoskeletal system," Crues continued, and now allows surgeons to devise a more definitive and sophisticated approach to specific pathologies. A compact and accessible area like the human knee allows physicians to use a variation on the large, body-size RF coils, which often produces very sharp pictures. Called a surface coil, this compact transmitter-receiver unit fits right over the knee itself, and by this close proximity to the target tissue improves the signal-to-noise ratio (SNR) and therefore image quality. Procedures have been developed so that diagnosis of meniscal and ligament tears is now performed easily in most cases.
Beyond enabling the fairly definitive evaluations of anatomy and pathology Bradley referred to, MRI gives Crues and other musculoskeletal MR specialists a tool with which to conduct proactive studies of the integrity of tissue in the knee. Torn or severed cartilage is a common injury, especially among athletes. Knee cartilage or menisci are small, curved tissue sacs that cushion the major ball-and-socket joint of the leg on either side of the knee joint. One of the underlying causes of meniscal tears is believed to be differences in the coefficient of friction between the top and bottom meniscal surfaces and the bones they slide against as the knee moves, said Crues. This pressure differential can cause shear stresses in the interior and tear collagen fibers, he continued, which the body responds to as it would to trauma or inflammation, leading to a "vicious cycle of . . . 'degenerative changes' and eventual mechanical breakdown." A physician who suspects the presence of such precursors to a rupture will design an MRI sequence to look for the longer T2 that would be expected as the
interior collagen fibrils uncoil and free up the water in and around their cells.
Crues believes that MRI goes beyond simple diagnosis, and he demonstrated how the views it provides have altered the fundamental medical approach to certain conditions. "We thought we understood these disease processes," he explained, until the subtleties and unprecedented detail of the MRI scans indicated complexities that were overlooked with previous diagnostic modalities. "One of the most common causes of knee symptoms in the population at large, especially in young women, is degenerative change in the articular cartilage of the patella [kneecap]," Crues continued. MR studies in the laboratory on this tissue revealed there are actually two layers of cartilage, usually with different water content. Studies over time of a given patient can reveal deterioration within these layers, and even a single MRI study may reveal more—since the cellular biochemistry revealed by MRI may indicate incipient damage—than would be discovered by a surgeon who cut in surgically for a look. ''Further studies need to be performed to see if the signal changes [revealed with MRI] accompany early biochemical changes" that are known to be associated with diseased cartilage; if so, predicted Crues, "early detection may lead to changes in patient behavior, or to the development of drugs to protect the cartilage."
Tears of the rotator cuff in the shoulder provide another vivid example of how MRI is revolutionizing the treatment of joints. Such tears have many different possible causes, and in the past, cutting in for repair or removal did not allow surgeons "to distinguish among the etiologies," said Crues. "A major breakthrough in understanding this disease stemmed from the surgical findings of Charles Neer, who postulated that 95 percent of rotator cuff tears were due to chronic trauma," continued Crues, caused by a congenitally abnormally shaped acromion, the bone at the top of the shoulder. Normally this bone surface is flat, but "some people are born with a congenital abnormal shape," said Crues, a kind of bony hook that often abrades and produces chronic trauma on the rotator cuff muscle laid over it. Before MRI, this process was almost unavoidable in people with the condition. Now, said Crues, we "can adequately differentiate between those patients whom surgery may help, and those" whose shoulder joint degeneration stems from other causes. Now the area can be imaged, using short TE times and T1-weighted strategies, and if the misshapen bone seems the likely cause, a quick arthroscopy to file it down will likely prevent altogether a tear that once—for lack of warning symptoms—was inevitable.
Unparalleled Views Inside the Skull
Perhaps in no subspecialty has MRI had such a profound impact as in neurology, where physicians understandably want as much information as possible short of cutting in for a direct examination. Until recently, the study of interior brain states was largely limited to the graphs of electrical activity discernible in electroencephalogram (EEG) studies. With the development of CT scanning techniques over the last two decades, clinical neurology took a big step forward in the diagnosis and localization of problems. Even more recently, PET studies (such as those that send a solution of glucose molecules labeled with fluorine-18 atoms to the brain) have provided real-time pictures of local activity as various regions of the brain metabolize other molecules for the energy to transmit their electrical signals. But, in many specifics, MRI has gone beyond even that level of detail. "With few exceptions, the sensitivity of MRI is greater than that of CT," said Bradley.
The brain is a very densely packed and convoluted system with some 10 billion constituent parts, its neurons. The vast terra incognita at the frontiers of brain research involves the intricate network of interconnections between these nerve cells, and how this network actually functions. But for clinical neurology, MRI can give surgeons and diagnosticians like Bradley extremely sophisticated information about the brain's anatomy, fluid flow, and the presence of hemorrhage, edema, and neoplasm. "Because of its sensitivity to small amounts of water, MRI is more sensitive to edematous lesions" than is CT, said Bradley. It can locate a lesion more precisely, and it "better demonstrates subtle mass effect, atrophy, and the presence of hemorrhage . . . and is currently the most sensitive technique available for detecting early brain tumors, strokes, or the demyelinated plaques in multiple sclerosis."
Although the brain is undoubtedly the most complex structure known to man, its component parts are not mysterious. MR images of the brain can distinguish clearly between the white matter, gray matter, the lipid-rich fat surrounding the scalp, and the cerebrospinal fluid (CSF). When tumors develop, the water they contain (and thus the protons emitting an MRI signal) is distinguishable from that in adjacent tissues. The CSF is largely water, and therefore an excellent target. One brain disease, hydrocephalus, can result from overproduction of CSF, but much more commonly from blockage (usually by a tumor) of the ventricles, the pathways allowing "old" CSF to flow out of the brain. "Any edematous process, any water-forming process in the brain or anywhere else in the body," explained Bradley,
"increases the T2 relaxation time, slows the rate of decay, and causes these processes to be highlighted on T2-weighted images." This feature of MRI provides a nearly custom-designed camera for studies, not merely of the brain's gross anatomical structure, but of the condition of brain tissue as well.
Because MRI allows physicians to look directly at biochemical effects and cell processes, many strategies have been developed, including new approaches to internal bleeding. Explained Bradley: "Hemorrhage has many different forms. We can be fairly specific about the age of hemorrhage based on its MR appearance. Acute hemorrhage is primarily deoxyhemoglobin, which has a short T2 and thus causes loss of signal. Typically, some days later, methemoglobin will be formed" and emit a different but characteristic signal that has "high intensity on any sequence and is therefore easily detectable."
The short T1 time associated with methemoglobin can be attributed to the fact that it contains iron, which reacts directly with the surrounding water protons in a dipole-dipole interaction. Deoxyhemoglobin can be clearly distinguished from methemoglobin, said Bradley, because its associated "water molecules are unable to approach the heme iron within 3 angstroms for a dipole-dipole interaction," the mechanism whereby T1 is shortened.
Paramagnetic substances act as contrast agents to enhance MR images. One such solid is ferritin, a "first cousin," explained Bradley, to "the hemosiderin that is a breakdown product of hemorrhage. Seeing this, we know that the only way it could have gotten there was from a prior bleed." Both of these show up as a conspicuous blackness due to short T2 relaxation times. "Early in the subacute phase of hemorrhage,'' continued Bradley, methemoglobin within the red cells is magnetically very susceptible when compared to the plasma just outside these cells, leading to a short T2. Later in the subacute stage, lysis of the red cells leads to a long T2 and high signal. Thus variation in the T1 and T2 relaxation times indicates the sequential stages that may develop—and between which it is important to distinguish—in intracranial hemorrhage.
Capturing Fluid Motion with MRI
"Few aspects of MR are as potentially confusing as the effect of motion on the MR image," wrote Bradley (Stark and Bradley, 1988, p. 108). But the detection of motion "has no correlate in CT" scanning, and so pictures of flow represent a fundamental advance in diagnostic imaging. An invasive diagnostic x-ray technique called angiogra-
phy has been developed to study blood vessels, but early indicators suggest MRI could largely supplant it, if techniques and cost considerations can be harnessed. "The routine angiogram requires sticking a needle about the size of a Cross pen into your groin, your femoral artery, where you feel the pulse, and then sending a catheter about a meter long up into the neck and injecting contrast into your carotid artery. It is not a pleasant procedure," commented Bradley, who has contributed to the development of "a painless procedure, MR angiography, that gives much the same information" in a matter of a few minutes, compared to the hour patients normally endure with the invasive and painful angiogram.
MRI studies can be directed not only toward the flow of blood, but also to the CSF in the central nervous system. Because of the abundance of protons in hydrogen and of water in the human body, MRI can detect and discern the movement of water anywhere in the body and is sensitive even at the smaller scales of molecules. MRI techniques are especially sensitive to the rate of flow. Blood traveling upwards from the heart through the aorta moves at nearly a meter a second. As it continues into the vascular network and moves into continually branching arteries, velocity is decreased proportional to the cumulative cross-sectional area. In major arteries in the brain, for example, blood is moving at about 10 centimeters per second. It continues to diffuse through the ever finer network until, reaching the capillaries, it is moving at about a millimeter per second. Generally, the more rapid the flow, the darker the MRI image: thus blood moving more slowly through the veins is lighter, and that pulsing through the arteries is darker on the image. Another convention used when imaging flow is to code for direction, which allows easy discrimination between inflowing and returning blood and CSF.
Before MRI practitioners could produce images of flow, techniques had to be developed to overcome a number of obstacles. Bradley pointed out that several "independent factors can result in decreased signal intensity of flowing blood: high velocity, turbulence, and odd-echo dephasing." Sometimes known as flow void, this decrease in signal can per se be revelatory, indicating under certain conditions the presence of aneurysms and arteriovenous malformations. To explain the mechanism behind the flow void, Bradley again described the spin-echo technique. Because the MRI equipment has been refined such that a thin slice on the order of 5 millimeters (or thinner) can be isolated for imaging, the speed of protons moving through the slice in a normal direction is problematic. "To give off a spin-echo signal," wrote Bradley, "a group of protons must be exposed to both a 90-and 180-degree RF pulse" (Stark and Bradley, 1988, p. 108). If
the slice at the appropriate resonant magnetic field is thin enough, and/or the movement of the fluid fast enough, the protons may not be within the slice long enough to receive the two pulses and to produce the spin-echo signal.
The situation, however, still contains much inherent information for the MRI practitioner, explained Bradley. Flow that runs through the slice in a normal direction, rather than along it, will experience the flow void for the following reason. Certain protons will be entering the slice from the top and others leaving the slice from the bottom during the time of the MRI exposure. These will all receive and react to portions of the spin-echo sequence depending on their position, thus revealing information about direction and speed of flow when multislice analysis on the computer is accomplished. Another potential image problem concerns blood flowing essentially parallel to, rather than through, the slice. Since these protons will experience a changing magnetic gradient between pulses, they will not experience the coordinated effect of the pulses. One of the techniques developed to clarify these pictures involves elimination of the cyclical pumping effects of the heart. This is accomplished by gating, a technique that compensates for the cycle by timing its image exposures such that the same moment in the pump cycle is captured for each successive heartbeat. So, by correlations of phase, and subtracting images one from another, real-time pictures can be filmed, and fluid flow can be coded by the computer to reveal both its rate and direction. Flow patterns within the blood vessel can also lead to the void. Laminar blood flow results when blood near the center of a vessel moves faster than blood near the walls, causing a so-called slippage of layers. Turbulence, a form of chaotic and hence unpredictable motion, may also develop when blood flows rapidly through expanding vessels and changes in direction.
Just as some situations produce a tendency to decrease the MRI signal, "three independent factors also result in increased signal intensity," wrote Bradley: "flow-related enhancement, even-echo rephasing, and diastolic pseudogating" (Stark and Bradley, 1988, p. 108). Flow-related enhancement occurs when the spinning protons first enter a slice and have full magnetization. If an MRI sequence employs a short TR (i.e., is T1-weighted), the protons in the flowing fluid have higher signal than the partially saturated (demagnetized) surrounding tissues. If a gradient echo technique is employed and only single slices are examined, "every slice is an entry slice," explained Bradley, referring back to the description of fluid flowing through, or normal to, the area under examination. This technique and the effect of "flow-related enhancement have recently been combined with a max-
imum-intensity, ray-tracing algorithm to provide the three-dimensional images of blood vessels" used in the MRI angiogram referred to above.
Contrast Agents
As David Stark observed, "Roentgen himself introduced the first contrast agents," and thus the principle of using contrast to enhance diagnostic images is as old as the x ray itself. "The general term" for the class of materials used, said Stark in his presentation to the symposium's scientists, is "diagnostic contrast media." They are pharmaceuticals that alter tissue characteristics [so as] to increase the information on diagnostic images. Each of the diagnostic imaging modalities—nuclear medicine, ultrasound, x-ray-based techniques—has its own family of dedicated contrast media."
The contrast agents used in MRI in certain situations can improve accuracy, reduce the number of possible diagnostic procedures, or shorten the course (and therefore the cost) of an MRI sequence. One significant distinction between MRI and CT contrast media is that with MRI the chemical agent is not itself being imaged. Rather, it produces an enhancing effect on one of the other MRI parameters already discussed, that is, relaxation. Stark pointed out that the key is to give a drug that goes to normal, but not abnormal, tissue and increases the signal difference between the two, thus giving a primary diagnostic test, which is to identify the abnormality as a result of the distribution of the drug. The fundamental point, he emphasized, is that these drugs can help us only if they target—or physically localize in—either the normal or the abnormal tissue but not both.
The only paramagnetic agent fully approved for clinical use by the Food and Drug Administration at this writing is gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA), although oral iron compounds and molecular oxygen also are being used in clinical studies. "In fact, some 30 percent of the patients who undergo NMR exams worldwide receive the drug," Stark said. Calling it, therefore, "a big ticket item," Stark reported that a number of corporations would like to break into this market, which "leads to some interesting issues in academia with sponsored research."
Stark explained that paramagnetic agents like Gd-DTPA serve to increase the parallel magnification of materials placed in the main field. "Paramagnetism is characterized by independent action of individual atomic or molecular magnetic moments due to unpaired electron spins" and "can occur in individual atoms or ions, as well as in collections of atoms or ions in solids such as ferritin and hemosid-
erin" and other substances in the body. Gadolinium-DTPA's potency is a measure of how much the proton relaxation rate is speeded up by a certain concentration of the drug. Gadolinium, which has a magnetic effect orders of magnitude larger than the effect of hydrogen nuclei on one another, provides a "built-in amplification step," said Stark, and can significantly shorten T1 and T2 relaxation times, T1 more than T2.
Since "tissue T1 relaxation is inherently slow compared with T2 relaxation, the predominant effect of paramagnetic contrast agents is on T1," explained Stark. Gadolinium-DTPA, indeed all paramagnetic contrast agents, as they shorten T1 serve to increase the signal intensity in the tissues that fall within the magnetic field gradient. When TE is short, T1-weighted spin-echo and inversion recovery techniques tend to "show the greatest enhancement," he said. And with an increased signal-to-noise ratio in the image, scan times can be reduced.
An important characteristic of these agents is their biodistribution, how they tend to accumulate in the target tissue. Gadolinium-DTPA is administered intravenously and thus moves through the vascular system. When it reaches a particular tissue, differentiation may be observed because of a characteristic perfusion, relative to adjacent tissues, and likewise by diffusion through the capillaries into the extracellular spaces in the local region. Thanks to the blood-brain barrier, Gd-DTPA will only arrive at central nervous system tissue that is abnormal. Conversely, a properly functioning renal system tends to collect low-weight molecular compounds such as Gd-DTPA (590 daltons).
Stark also described another specific subset of drugs that has been designed for MRI enhancement. "These drugs work wonders in the brain and kidney," explained Stark, because—as small molecules—they are generally excluded by the blood-brain barrier, "and pathology selectively accumulates them, and the kidney handily excretes them." Thus any problem or pathology in the blood-brain barrier will be tagged by the use of Gd-DTPA, and any pathology in the kidney likewise will accumulate it. This picture of how the agent arrives at the target area also describes enhanced CT imaging, but the image contrast produced is usually superior with MRI.
Most uses of Gd-DTPA involve comparing the same MRI imaging sequence with and without the contrast agent. Contrast imaging is also indicated when a first MRI result indicates the presence of something not clearly definable, for which greater contrast is desired in a subsequent sequence.
With all contrast media, clinicians are concerned about toxicity. One problem inherent to some of these contrast media, said Stark, is
that once introduced, they diffuse. ''They pickle the entire body, just like most drugs that you eat or are injected with—they go everywhere. The whole body gets exposed to them even though the therapeutic or diagnostic effect is [confined to] one organ and represents a small fraction of the drug" that was administered. In all organs, the goal is to design an agent that will react differentially between normal tissue and the tumor. Thus researchers looking at possible new MRI contrast agents are searching for a "silver bullet," a substance that will go directly and exclusively to the problem organ by utilizing monoclonal antibodies.
Often referred to as the body's housekeeper, the liver, he pointed out, performs a similar kind of chemical discernment on many of the body's products. The liver hepatocytes take up some materials that a liver tumor cannot, and thus some liver cancers are easily detectable. Manganese-DPTP has been developed in a form that will go selectively to the liver and heighten the signal intensity in a T1-weighted image, since it will not be present in the tumor. Researchers hope that a very precise and affordable test for liver imaging can be developed that will permit mass screening of the population for cancer. Also, such a process could provide insights that might lead to the development of additional contrast agents that would target selected areas of the body.
Spectroscopy and Diffusion Weighting
For decades, chemists have been using NMR principles to study atomic phenomena. With magnetic resonance spectroscopy (MRS), practitioners are beginning to explore the body through analysis of its chemistry in vivo. The process is similar to MRI, since the characteristic data come in the form of a unique resonant frequency, a response to input energy in the form of RF pulses rather than the laser light used in standard chemical spectroscopy. As with standard spectroscopy, however, each element in the periodic table as well as any variant with an alteration in the atomic composition of a molecule will produce a unique fingerprint. Therefore, even a single displaced electron will alter a target atom sufficiently to produce a slightly different resonant frequency, referred to as the chemical shift. Such a change in the atomic configuration will also alter the local magnetic neighborhood, so to speak, though the change in magnetism is very small compared to the power of the gradient field used in standard MRI. Since MRS is designed to detect these very small magnetic effects, the process requires an external magnetic field with much greater homogeneity (to within better than 0.1 ppm over the region
of interest). Chemical shift information is often used as an additional refinement to further clarify pictures produced through standard MRI.
In the body, ascertaining the presence of certain substances at a particular site can often provide important indicators of disease or abnormality. Inositol aspartate seems to be present in all neurons and can serve as a marker for mapping the nervous system using MRS. Choline and creatine are associated with much new growth and with tumor production, and can therefore provide useful targets in spectroscopic studies. N-acetyl aspartate acid (NAA) is another useful marker for neurons and demonstrates an intriguing use for MRS in tracking the course of disease. "It is possible to create maps of [the brain using] NAA," said Graeme Bydder, by first generating "a normal one as a baseline" and then a subsequent MRS map "to observe neuronal fallout, for example, in Pick's disease. This allows us to detect the absence of neurons even where the MR imaging is normal." Since NAA serves as a marker for neurons, MRS studies of newborns over time can map the growth of the brain, and possibly detect abnormalities that would show up in no other tests.
Bydder's latest research interest involves imaging the movement in vivo of anisotropically weighted water, what he described as "diffusion-weighted imaging," a very novel approach that Bydder nonetheless relates to the field: "Ideas in magnetic resonance have come from a lot of different sources, and this is another one that has actually been borrowed from spectroscopy." Bydder credited Michael Mosley from the University of California, San Francisco, with the important work necessary to turn the idea into a clinical application. The motion of the water molecules due to diffusion causes signal loss that allows MRI to differentiate particular tissues. The anisotropic quality means that water protons diffuse at different rates from different directions. When this diffusion factor is added to the other MRI parameters, certain biochemical phenomena in the body may be revealed that would not show up in a straightforward search for differences in proton density or in T1 or T2 times. Tissues can in this way be distinguished by their "diffusibility," a further marker to discriminate fine detail.
Bydder called the use of this new technique in brain studies "an interesting area. It brings together the molecular motion of water, the properties of myelinated nerve fibers, the gross anatomy of the brain in terms of being able to distinguish the directions of white-matter fibers, and the diffusion of water" as influenced by the presence of disease. It is MRI that unites all of these factors, he concluded, which could prove to be a revolutionary diagnostic advance. Bydder cited improved diagnosis of multiple sclerosis as another application.
Previously designed MRI studies of the disease revealed a certain level of detail that he and his colleagues had agreed was "the gold standard. When we apply this [new form of] imaging, we can see a lot of extra abnormality" and detail that was not discernible before. "We have got to conclude either that we have a whole lot of false positives here," said Bydder, or that the new technique is more sensitive than the standard one. The realization that the anisotropic diffusion of water could yield this deeper look is so recent that it is now under intensive investigation, and new revelations may be expected. What is certain, however, is that MRI researchers will continue the search for techniques that will yield ever finer detail, which in these experiments has already reached the level of microns (one millionth of a meter).
PROSPECTS
Over 2000 systems are up and running in America, another 1000 elsewhere. Their average price was closer to $2 million than $1 million, and since much of that cost is associated with the magnet system, an MRI machine is going to be costly to procure and maintain until economies in the manufacture of superconducting systems are achieved. In evaluating this investment, hospitals will inevitably think in terms of "patient throughput," or the number of procedures per unit of time, at what billable return of costs. Another cost factor is the system's complexity. Radiologists and technicians, simply put, need to be better trained and qualified than with other imaging systems. But while medicine is a business, it is also much more. The growth and role of MRI will undoubtedly be determined more by physicians than by accountants and insurance companies. To the extent that MRI becomes essential to the provision of life-enhancing quality of care and to saving human life, to this extent will it assume its proper place in the medical armamentarium.
There are, however, some very interesting and promising possibilities, many of which were mentioned by the session's presenters.
-
Fast scanning techniques. Because the utility of MRI in part depends on its ability to pay its way, faster scan times could lead to more images at a lower cost. These shorter imaging times will, in turn, increase the number of patients who can remain still and hold their breath for the duration of the process, and will simplify many of the longer gating procedures now required. Some in vivo work contemplates subsecond scan times. This is an area where funding for basic science, R&D, and theoretical studies could have a significant payback.
-
MR angiography. As described, current traditional angiography techniques are extremely invasive. As MRI flow-study techniques are perfected, the former system may become antiquated.
-
NMR spectroscopy. A number of biomedically interesting nuclei are being investigated to delve even more deeply into specific biochemical and intracellular processes.
-
MRI guided/assisted interventional diagnosis and therapy. At present, CT and ultrasound are occasionally used in operating theaters to better control biopsy, drainage, and retrieval procedures. Significant physical obstacles would have to be overcome, but a system based on MRI could provide enhanced guidance and analysis.
-
MRI fluoroscopy. Patients move certain joint systems—the jaw, the knee, the hip, the spine—through a graded series of positions, allowing MR imaging to produce studies of movement hitherto impossible. An analogous procedure is under investigation for cardiac studies.
-
New contrast agents. A host of oral and intravenous contrast agents are being developed and tested.
-
Clinical efficacy. MRI in many diagnostic situations has dramatically improved medicine's reach, penetrating even to the interior of the body's cells. Clinical horizons are expanding dramatically, and enhanced diagnosis of many areas and processes seems inevitable. Cost and other practical considerations are clearly an important element in this future. But the gold standard is as clearly in the process of being redefined.
BIBLIOGRAPHY
Balaban, R.S., S. Chesnick, K. Hedges, F. Samaha, and F.W. Heineman. 1991. Magnetization transfer contrast in and MR imaging of the heart. Radiology 180:367–380.
Eng, J., T.L. Cecker, and R.S. Balaban. 1991. Quantitative 1-H magnetization transfer imaging in vivo. Magnetic Resonance in Medicine 17:304–314.
Fullerton, Gary D. 1988. Physiologic basis of magnetic relaxation. Pp. 36–55 in Magnetic Resonance Imaging. David D. Stark and William G. Bradley, Jr. (eds.). Mosby, St. Louis.
Stark, David D., and William G. Bradley, Jr. (eds.). 1988. Magnetic Resonance Imaging. Mosby, St. Louis.
RECOMMENDED READING
Bradley, W.G., and G.M. Bydder. 1990. MRI Atlas of the Brain. Martin Dunity Publishers, London.
Crues, J.V. (ed.). 1991. MRI of the Musculoskeletal System. The Raven MRI
Teaching File. R.B. Lufkin., W.G. Bradley, Jr., and M. Brant-Zawadski, (eds.). Raven Press, New York.
Lehner, K.B., H.P. Rechl, J.K. Gmeinwieser, A.F. Heuck, H.P. Lukas, and H.P. Kohl. 1989. Structure, function, and degeneration of bovine hyalin cartilage: assessment with MR imaging in vitro . Radiology 170(2):495–499.
Mink, J.H., M.A. Reicher, and J.V. Crues. 1987. Magnetic Resonance Imaging of the Knee. Raven Press, New York.
Stark, D.D., and W.G. Bradley (eds.). 1992. Magnetic Resonance Imaging. Second edition. Volumes One and Two. Mosby, St. Louis.
Stoller, D.W. (ed.). 1989. Magnetic Resonance Imaging in Orthopedics and Rheumatology. Lippincott, Philadelphia.