8
Atmospheric Science
Research and Regulation: Science's Contribution to the Public Debate
Regional and global air pollution problems have become highly sensitive and politicized subjects. Potential consequences to the biosphere, the health of its inhabitants, and the very future of the planet are the subjects of intense debate. Currently, roughly 100 cities in the United States—home to about 80 percent of the U.S. population—are out of compliance with air quality standards designed to protect public health and welfare. At the same time control costs now exceed $30 billion annually. Politicians are always debating next year's budget, Gregory J. McRae of Carnegie Mellon University has learned, and "in times of competing economic priority there is a terrible temptation to reduce the costs" of pollution study and control. An interesting problem for scientists, he believes, is to figure out how to lower both exposure to pollution and the costs of its control."
In his Frontiers symposium presentation, "Using Supercomputing and Visualization in Los Angeles Smog Simulation," McRae emphasized the growing need to bring knowledge of science to bear on the public policy domain. "People may try to pull the problems of pollution apart politically, but the underlying phenomena in fact are chemically coupled. We need to think about the chemical coupling at the time we are devising regulations," he maintained. Aware of pressing environmental problems, atmospheric scientists work with a sense of urgency to sort out and understand the intricacies of complex physical interactions. McRae, who has become a recognized authority on computer modeling of atmospheric phenomena, also "puts
on his public policy hat" and spends an average of 3 days a month in Washington, D.C., translating and transmitting the science into its political context.
Explained McRae: "The role of the science is to try to understand what is going on in the atmosphere, and that involves three things: the chemical interactions that take place in the atmosphere, the emissions from mobile and stationary sources, and the meteorology that moves the materials around," The traditional role for atmospheric science has been, "given these inputs, to unravel the science that governs what happens, and to predict what will happen." Very often, however, the political process involves other nonscientific goals that inevitably color the way an atmospheric scientist works, often influencing his or her charge and data-gathering focus. "Atmospheric science is not practiced in isolation. The whole air-quality planning process involves science as well as social issues," according to McRae, who emphasized that scientists not only need to think about doing good science and engineering, but also need to venture a little beyond just the science and think about what that work and the results really mean to society. "With a bit of luck," he added in his presentation to the symposium's scientists, ''we might perhaps convince you that that is an important thing to do.''
McRae is a chemical engineer with what he described as "a fairly simple-minded view of the world, [the need for] what I call getting the sign right." To illustrate, he discussed chlorofluorocarbons (CFCs), engineered to be "extraordinarily good materials" and a very useful industrial product. But because of their particular chemical nature, CFCs turned out to have an unanticipated negative impact on stratospheric ozone. Millions of dollars were spent to create CFCs, but millions more will be spent to eliminate them and to mediate their adverse effect on the atmosphere. Thus there is a need, McRae emphasized, to go beyond concentrating on the physics and chemistry of how to make new materials and to think also about "the impact and pathways of chemicals after they leave the lab." McRae is also sensitive to the possibility that studies to elucidate phenomena in the atmosphere may not be balanced with a search for control strategies to deal with them. The urgency that attends the problems of pollution, he thinks, confers a responsibility on atmospheric scientists to consider issues like cost-effectiveness and adverse impacts whenever their results might be central to a political debate, which—in atmospheric science—is very often.
McRae has developed this awareness of the political arena as his model—begun in graduate school at Caltech—has evolved using a historical database of Los Angeles weather and pollutant data. That
growing and coherent real-world test case permits him to evaluate what has now become a generic model, which he continues to develop, perfect, and apply from his present post at Carnegie Mellon University. The model's historical database provides useful comparative and baseline information for future simulations, of Los Angeles and of other urban applications. Because of the severity of pollution in Southern California, McRae and his model have been drawn deeply into the political decision-making process. Alan Lloyd, another session participant and chief scientist for the South Coast Air Quality Management District in California, emphasized that "it is critical to have available a wide group of scientists and institutions that regulators can turn to for unbiased and impartial information."
McRae credited the session's third participant, Arthur Winer, an atmospheric chemist at the University of California, Los Angeles, with "unraveling some of the very interesting things—the chemistry—going on at nighttime in the atmosphere." Winer brought to the symposium discussion his perspective as a longtime scientific advisor to California's regulators, and he also singled out as a challenge for atmospheric science the need "to arrest the declining numbers of really gifted young scientists" entering the field. He found it ironic that the surge in interest and academic programs in his field in the 1970s withered during the 1980s, just when dramatic environmental problems called for galvanizing society's resources.
HELPING REGULATORS DEFINE THE PROBLEM
Atmospheric scientists use the phrase photochemical oxidant air pollution to refer to a mixture of chemical compounds commonly known as smog. "The oxidants are not emitted directly, but rather are formed as products of chemical reactions in the atmosphere. It is this latter property that makes their control so difficult," explained McRae, because time of day and meteorological conditions directly affect the formation of pollution, as do the characteristics of the chemical compounds themselves. The precursors of smog are primarily nitrogen oxides, referred to by the symbol NOx to indicate a sum of nitric oxide (NO) and nitrogen dioxide (NO2). products of high-temperature fuel combustion, and reactive hydrocarbons resulting from solvent use, vegetative emissions, motor vehicles, and a broad spectrum of stationary sources.
Once the polluting sources have been identified, the next logical and traditional step taken by regulators has been to mediate the problem by reducing or redirecting the emitting source. But McRae pointed out one of the basic truths of this field that applies to most ecosys-
tems: remedial actions may have unforeseen consequences. In the 1950s and 1960s, for example, regulators addressed the problem of pollution from coal-fired electrical power plants by mandating the construction of tall stacks—chimneys that soared hundreds of feet into the air to disperse the pollutants from the plants over a wide area. The "tall stacks solution" was counted a success because pollution near the power plants was dramatically reduced. Unfortunately, the sulfur-containing smoke from these plants, many of which were built in the American Midwest near high-sulfur coal deposits, traveled with the prevailing winds to New England and southeastern Canada. The material in the plumes then underwent chemical reactions and resulted in acid rain falling over large areas.
Thus, requiring tall stacks did not solve the pollution problem. Regulators were more successful, however, in responding to the problems of lead, which "is known to cause neurological malfunctioning, learning disabilities, and increased blood pressure. Almost all regulatory efforts in the past have been based on the simple notion that controlling the release of pollutant emissions will result in improved air quality. Regulatory activity designed to phase out the use of lead compounds as an antiknock additive in gasoline has been quite successful and has resulted in drastic reduction of ambient concentration levels" of lead in our air, said McRae. And so far the sign seems to be right: no significant or problematic unintended consequences have surfaced after the removal of lead from gasoline. Smog, however, is a larger, more complex problem.
REGULATION-ARE THE CORRECT QUESTIONS BEING POSED?
McRae and his colleagues have drawn some conclusions from their latest model that might influence regulators to redirect a current, major control strategy: the reduction of reactive organic gases. As with the tall stacks strategy, McRae wants to be sure "we aren't getting the sign wrong" and actually mandating a control strategy with unforeseen consequences that could be avoided. The environmental awareness that blossomed in the 1960s helped to establish the U.S. Environmental Protection Agency (EPA) in 1970. Atmospheric pollution was evident even before the dawn of the environmental movement: many cities were cloaked in a brown shroud of smog. Studies were sanctioned and conducted, and a series of regulatory guidelines developed to—again, logically—attack pollution at its source. With urban pollution, one major source appears to be the hydrocarbons that come from automobile engine combustion and from the use
of solvents, together classified as reactive organic gases (ROGs). The EPA established a limit on these emission sources, and when McRae began modeling the atmosphere over Los Angeles in the early 1980s, one of the inevitable elements of his work was an evaluation of the effectiveness of lower emissions of ROGs. That evaluation, as it developed in the larger context of his model and in the work of other atmospheric scientists, surprised everyone.
"EPA's approach was basically right if you're looking very close to the source. But the role of hydrocarbons is essentially that of controlling the speed of the chemistry" that is leading to atmospheric pollution, said McRae. Other elements, primarily NOx, turn out to be more instrumental in whether the pollution is actually generated. "If you put only organic controls on a city, it slows the reactions so that ozone forms downwind, outside the central city," McRae remarked. This generally means that regions and counties away from the center of a city—such as the Riverside and San Bernadino areas east of Los Angeles—are thus inundated with the smog. Because the formation of the pollutant was slower, ''our national policy was leading to a situation where we may have reduced ozone in cities," but at the expense of suburban and rural areas downwind, so that "we were creating a problem on the regional scale." The sins of the generating region—as with the acid rain experience—were being visited upon nearby or faraway neighbors, depending upon the meteorology transporting them.
Running his model suggested to McRae an even more startling possibility. Regulating ROGs was not reducing ozone formation at all, because the role of NOx had been misperceived and as a consequence, underestimated. Although, as Lloyd pointed out, "ozone levels in Los Angeles have been cut by about half over the last 15 years," the model indicated that in the rest of the country where NOx was not being regulated, uncontrolled emissions of NOx could confound the intended results of ROG reduction. "Despite massive control efforts," concluded McRae, "the average ozone levels in the eastern United States and in western cities such as Houston, Phoenix, and Denver have not been lowered. Part of the reason is that the chemistry of oxidant production is highly nonlinear. There is no simple relationship between the emissions of the major precursors and the resulting ozone."
Lloyd noted that the EPA is now turning to control of NOx as well as ROGs, an approach taken early on by regulators in California, and he emphasized as well the amount of work still to be done by scientists in addressing problems of air pollution. Especially important, he maintained, is the need to obtain adequate amounts of high-
quality experimental data, the lack of which will compromise even the best efforts to model and understand complex physical and chemical phenomena basic to environmental change.
CHEMISTRY IN THE ATMOSPHERE
Ozone as a Protective Shield
Ozone formation in the lower atmosphere may be one of the more popularly misunderstood of scientific phenomena, but its importance to the Earth's fate cannot be overestimated. Part of the popular confusion involves a crucial distinction that must be kept in mind: the environmental impact of ozone depends on the altitude at which one considers it. In One Earth, One Future (Silver and DeFries, 1990), authors Cheryl Simon Silver and Ruth S. DeFries explain (pp. 104–109) that ozone tends to accumulate in a layer in the Earth's stratosphere, with the maximum concentrations occurring at a height of roughly 25 to 35 kilometers above the Earth's surface. Although it is only a small component of the Earth's stratosphere, ozone nonetheless performs a vital function: the layer where it accumulates filters the Sun's rays and blocks out much of the radiation in the ultraviolet-B wavelength range, thereby contributing to an environment on the planet's surface where life could and did evolve. Even though its density averages only 3 parts per million (ppm) throughout the atmosphere and at its stratospheric peak only about 9 ppm, at that level it screens out more than 99 percent of the potentially damaging radiation.
Oxygen as a molecule is composed of two atoms (O2); ozone (O3) is a highly reactive molecule composed of three oxygen atoms. The atmosphere is rich with O2 molecules, which in the upper stratosphere are exposed to large amounts of the Sun's radiant energy that is relatively unaffected by its journey through space. Photolysis is the process whereby this energy is absorbed by a reactive chemical species that changes as a result. Once split into two atoms by photolysis, oxygen provides the raw materials, because these liberated atoms can then join with other O2 molecules to form ozone. The so-called ozone layer of the stratosphere with the heaviest concentrations develops at the altitudes it does because of a natural balance: higher up, above the stratosphere, the density of oxygen is such that liberated oxygen atoms encounter relatively few oxygen molecules to join to; lower down in the troposphere, insufficient light energy arrives due to the shielding effect of the ozone that has been created above.
When McRae referred to engineers failing to "get the sign right"
with CFCs, he was referring to damage done to the ozone layer by this man-made chemical creation (Box 8.1). CFCs are classified as synthetic halocarbons, and by photolysis can be broken down to release fluorine and chlorine atoms. When liberated, a single chlorine atom can trigger a catalytic reaction and destroy thousands of ozone molecules, thus contributing to a breakdown of the Earth's protective ozone shield.
Ozone as a Pollutant
Ozone in the upper stratosphere is created largely by natural processes, and its presence serves a vital function. Lower down in the troposphere, however, ozone is classified as a pollutant, with adverse health effects. "In the Earth's atmosphere, the primary tendency of the chemistry is to oxidize any molecules emitted into the air," said McRae, who pinpointed ozone as one of the two primary species that mediate the process. Published work by McRae and Armistead G. Russell discusses some of the chemistry basic to models of air pollution and explains some of the primary phenomena (Box 8.2).
|
BOX 8.1. CHLOROFLUOROCARBONS CFC molecules consist of either a single carbon atom or a pair of carbon atoms bonded to a fluorine and a chlorine atom, and have an atomic structure that confers on them a collection of beneficial properties. They are light in weight, neither flammable nor toxic, and are largely impervious to degradation by microorganisms or to reactions with other chemicals. Their applications are many: refrigerants in air conditioners and refrigerators, propellants for aerosol cans, foam blowing agents, and solvents for cleaning electronic components and other industrial products, to name only the most significant. But these same chemical qualities make them problematic, McRae explained. Since they do not react or degrade and are lighter in weight than nitrogen or oxygen, once they escape from their earthbound containers or have served their utilitarian function, they endure and migrate very slowly over a number of years or decades into the stratosphere. It is upon their eventual arrival in the stratosphere that CFCs turn deadly—in McRae's terminology, reverse their positive sign. Again, photolysis occurs when radiant light energy reacts with valence electrons to dissociate atoms and break down chemical bonds. |
|
BOX 8.2 OZONE PRODUCTION IN THE TROPOSPHERE Ozone production in the troposphere comes from the photolysis of nitrogen dioxide (NO2), NO2 + light ⇒ NO + O(3P), (1) which generates nitric oxide (NO) and a species of oxygen atom, O( 3P), that is highly reactive, referred to as the triplet state. In the presence of a spectator molecule like nitrogen (N2) or oxygen (O2)—designated M in this reaction—the triplet oxygen reacts with molecular oxygen to form ozone (O3): (2) In turn, the ozone can react with the nitric oxide produced in the first step O3 + NO ⇒ NO2 + O2 (3) to produce nitrogen dioxide and molecular oxygen, thus circling back to the original atmospheric components. In this cycle of reactions, no net ozone is produced. Under typical atmospheric conditions, McRae said, the cycle is very rapid, so that the three key species are approximately in equilibrium: (4) "Since most nitrogen oxide emissions are in the form of nitric oxide (NO), these relationships suggest that ozone levels should be quite low, on the order of 0.02 ppm," according to McRae and Russell. They noted that the levels that are actually observed can be an order of magnitude higher than this. Los Angeles has peak ozone levels above 0.30 ppm, and Mexico City's levels are often higher. Thus, "one of the key questions in atmospheric photochemistry is to understand how ozone can accumulate to these high levels in urban and rural regions. Part of the answer," the authors said, derives from the other primary atmospheric species involved in oxidation, the hydroxyl radical. The hydroxyl radical is chemically adept at oxidizing organic species present in the urban atmosphere and as a consequence is largely responsible for elevated ozone levels in and around cities. Hydroxyl radicals can be produced in several ways. When an ozone molecule absorbs a relatively high-energy photon, it dissociates to form molecular oxygen and a relatively long-lived, electronically excited atom known as the singlet D state (1D): |
|
O3 + light → O(1D) + O2. (5) This species can react with water vapor to form two hydroxyl radicals: O(1D) + H2O → 2OH. (6) "A second source of OH radicals," they said, "is from oxygenated organics. Formaldehyde (HCOH) provides the simplest example," using photolysis to produce hydroperoxyl radicals (HO2): HCOH + light → H + HCO, (7) (8) and (9) These HO2 radicals themselves can interact to produce hydrogen peroxide (H2O2), which in turn can also undergo photolysis to produce OH radicals": HO2 + HO2 → H2O2 + O2 (10) and H2O2 + light → 2OH. (11) Thus the generation of hydroxyl radicals is understood, but less apparent is why they should generate excess ozone, since the basic equilibrium equation (4) is a function of the ratio of nitrogen dioxide to nitric oxide. The answer is in the propensity of the hydroperoxyl radicals to interact with and oxidize nitric oxide: HO2 + NO → OH + NO2. (12) The products of this reaction are important. Not only are more hydroxyl radicals produced, but newly formed nitrogen dioxide also pushes the numerator of the ozone equilibrium equation up, and along with it the concentration of ozone. Without these excess concentrations, it normally requires an ozone molecule to oxidize nitric oxide into nitrogen dioxide, but equation (12) describes a reaction that, unlike equation (3), "provides a way to oxidize NO without consuming an ozone molecule, which in turn implies that the equilibrium in (4) is shifted to higher concentration levels," according to McRae and Russell. SOURCE: McRae and Russell (1990). |
To summarize the chemistry: certain photolytically driven reactions (such as the interaction of hydroxyl radicals with organics) tend to oxidize NO to NO2 and/or to produce more hydroxyl radicals. Such chemical reactions can contribute to a cycle or chain reaction that intensifies the concentration of ozone, and hence increases smog. Knowing how to identify and apply the reactions is the important framework, but obtaining accurate measurements of these substances—which can exist in a transient condition, swirling around the atmosphere, in concentrations as small as a few parts per trillion—presents a formidable challenge to atmospheric chemists.
GETTING THE NUMBERS RIGHT
Winer necessarily brings a historical perspective to any consideration of atmospheric science. For 20 years he has been a prominent participant in a number of important developments in the field, watching the science of measurement, the use of computers, and the role of the scientist in the public forum all evolve. In his early work, Winer was concerned "that too much attention was being given to six so-called criteria pollutants under the Clean Air Act" in the mid- to late-1970s and that as a consequence not much was known about a whole spectrum of other species such as formaldehyde (HCHO), nitrous acid (HONO), nitric acid (HNO3), the nitrate radical (NO3), dinitrogen pentoxide (N2O5), and formic acid (HCOOH). "These species either had never been measured in the atmosphere, or had, at best, been detected by wet chemical methods of notorious unreliability," said Winer, who added that, "not surprisingly, almost nothing was known about the possible health impacts of these compounds." Then at the Statewide Air Pollution Research Center (SAPRC), a research institute of the University of California based on the Riverside campus, Winer and a SAPRC team led by James N. Pitts, Jr., began a series of experiments to "try to understand what's really in a smog cloud," using Fourier transform infrared (FT-IR) spectroscopy for the first time over long optical paths of a kilometer or more. The parts-per-billion detection sensitivities afforded by such pathlengths led to the first spectroscopic detection of HCHO and HNO3 in polluted air.
This success spurted Winer and his colleagues to collaborate with German physicists Uli Platt and Dieter Perner, who had devised a novel spectroscopic instrument operating in the UV-visible region. By exploiting long optical paths—up to 17 kilometers—and a rapid scanning computer-based capability in which a signal-averaging algorithm was applied to thousands of superimposable spectral records, Winer, Platt, and their collaborators were able to achieve parts-per-
trillion detection limits and hence the first observation of the nitrate radical and nitrous acid in the polluted troposphere (Platt et al., 1980a, b). Thus the SAPRC-German teams established the methodological basis for later, more sophisticated models predicated on accurate measurements of atmospheric components. They worked on what Winer called "an historical antecedent" to McRae's powerful model, based on the premise that the more definitive the measurable data used as input, the more likely the model will be to mimic real-world processes.
NIGHTTIME CHEMISTRY
The long optical-path measurements by the SAPRC-German collaborators opened inquiry into an area that had been previously overlooked, nighttime chemistry. Sunlight drives most of the chemical reactions that atmospheric scientists model, and thus conventional wisdom held that nighttime—with its absence of hydroxyl radicals—might offer a respite from the creation of secondary pollutants. General pollution measurements taken after dark seemed to confirm that nighttime chemistry "was of minor importance," said McRae, who added that it "was even disregarded in some models." NO3, for example, is a trace nitrogen-containing radical with such small predicted concentrations (in the parts-per-trillion range) in the troposphere that scientists largely ignored its possible significance. But Platt, Winer, and Pitts and their co-workers focused their special spectrograph (Figure 8.1) on just such overlooked yet potentially significant species. They were the "first to discover the nitrate radical in the polluted troposphere," and then went on to detect it in the cleaner troposphere as well, said McRae. "We have now shown systematically in cleaner atmospheres that after sunset the nitrate radical concentration grows into more or less steady-state levels," said Winer, ''which persist throughout the night and disappear at sunrise due to photolysis'' (Platt et al., 1984).
This discovery, notwithstanding the small amount of the nitrate radical they found, could hardly have been more confounding and significant to the atmospheric sciences community. Suddenly, the formation of nitric acid in the troposphere at night could be explained. Previously, it was believed that nitric acid could be produced only by the reaction of the hydroxyl radical with NO2 during daylight hours. With the discovery of the nitrate radical concentration arising after dark, the leftover pollutants O3 and NO2 were provided a pathway leading to the nighttime production of nitric acid.
Winer, as a Californian, immediately recognized the implications of this discovery, since "nitric acid is, in fact, two-thirds of our acid
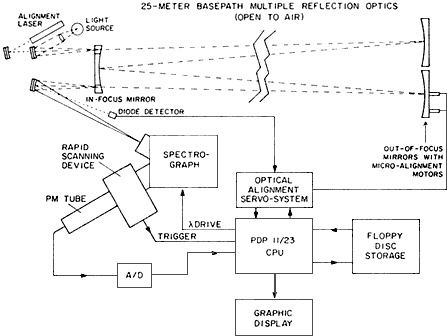
Figure 8.1 Schematic diagram of differential optical spectroscopy absorption systems used to measure atmospheric concentrations of the nitrate radical. (Courtesy of A.M. Winer.)
rain problem in the western United States. We don't burn many high-sulfur fuels, and consequently don't have much of the sulfur dioxide problem" that is so prominent in the East. The nitrate radical held even more significance, however, once its nighttime presence was established. Scientists had long puzzled over the absence of accumulated nighttime concentrations of compounds emitted from vegetation in large amounts. Isoprene and the monoterpenes were shown to react rapidly and efficiently with the nitrate radical, which effectively becomes a nighttime sink to mediate their buildup in the atmosphere (Winer et al., 1984).
The final discovery was even more significant, said Winer, solving "a longstanding problem in marine science." Dimethyl sulfide (DMS) is produced in large quantities from the world's oceans but does not accumulate during the day because of the presence and interaction of the hydroxyl radical. Again, since the OH radical disappears at night, atmospheric chemists were unable to explain why
DMS concentrations did not swing higher after dark. The nitrate radical turns out to be extremely efficient at titrating DMS, said Winer, and thus suppresses its buildup over the oceans at night (Winer et al., 1984).
The spectroscopic measurements made by Winer and his colleagues explained one final piece of the nighttime puzzle, which is suggestive of a larger pattern of diurnal pollution cycling that may be fairly independent of the meteorology. The ubiquitous hydroxyl radical, he said, "is the single most important reactive intermediate in the atmosphere. It catalyzes and drives smog formation." Winer and his colleagues confirmed the accumulation of substantial amounts of nitrous acid (HONO) at night in the Los Angeles basin. Since HONO is a photolytic species that readily breaks apart into the hydroxyl radical and nitric oxide during the day, Winer continued, "the accumulated nitrous acid that photolyzes at sunrise leads to a pulse of OH radicals that represents a kicking off of the next day's smog." In the fall and winter seasons in California when ozone and formaldehyde (which also photolyze to produce OH radicals) are reduced, this OH radical kickoff by nitrous acid can become a dominant source of OH radicals.
McRae clarified for the symposium three important implications from discoveries by Winer, Pitts, and others that have helped to redefine the urban pollution problem. First, nitric acid can be created in a way that requires neither sunlight nor OH radicals; second, he said, "previous model simulations that did not include this reaction would underpredict the importance of controlling NOx emissions; and finally, peroxyacetyl nitrate (PAN) can lead to long-distance transport of reactive nitrogen," a phenomenon that had been known about previously but was further clarified here.
And they also demonstrate the utility of computer models, which, in this case, revealed a crucial part of the picture that scientists had overlooked. Compared to the immediate troposphere where pollutants often accumulate in high and easily detectible concentrations, the upper atmosphere where these reactions occur was harder to observe. Thus the trace concentrations of the nitrogen-containing NO3 radical, to most scientists, had seemed less significant to their models than had the more apparent phenomena. Computer models can be constructed to exclude such judgmental criteria, however. Therefore serendipities can emerge such as this detection of a chemically rapid path by which NO3 can produce nitric acid and PAN without the sunlight's driving energy, especially in the upper portion of the polluted layer. There is something particularly compelling about such discoveries, which seem bred of the inherent complexities of a dy-
namical system like the atmosphere when explored with the comparably inexhaustible power of the computer.
COMPUTATIONAL EXPERIMENTATION
McRae appreciates that this use of the computer represents a new stage in the history of the scientific method: "This is where high-performance computing has played a very critical role, because we can now begin to think in terms of computational experiments. Traditionally, in the past, scientists had a theoretical formulation and then went into the lab to verify it." But meteorology has always been difficult to fit into that tradition because one cannot simply design and conduct experiments in the laboratory of a real city. With advanced models like the one McRae has spent the better part of a decade developing and refining, however, atmospheric science is beginning to utilize the experimental method more as some of the hard sciences do.
An atmospheric scientist may bring many theoretical considerations to bear in creating a model, but it receives its pedigree only by successfully matching nature. That is, given as inputs for its calculations the measurements and descriptive weather data that were actually observed at a given historical period, the model will predict a pattern of change. This simulation of an evolving weather and pollution scenario is then compared to the conditions that transpired and were measured, and the model's credibility can thereby be established. "Before a model can be used for control strategy calculations, it is critically important to demonstrate that it has the capability to accurately reproduce historical events," emphasized McRae. Once confirmed as a descriptive model, it has license as a prescriptive model. "You can with more confidence use the model in a predictive mode, the traditional role of science," said McRae. Lloyd cautioned, however, against overstating the reach of these newer methods: "Much more work is still needed to understand, for example, the meteorology and turbulence of the atmosphere. We can still only approximate."
MORE THAN A TOOL, THE COMPUTER PROVIDES A FRAMEWORK
In the political forum McRae makes use of some very illustrative video presentations of his work—which he showed to the symposium—that convey the dynamics of the atmosphere with a power and coherence undreamed of before the development of the sophisti-
cated modeling and visualization techniques that he and others now rely on. Atmospheric modeling on computers has proven to be a key to unlock possibilities that previous generations of scientists could not imagine. McRae's use of the computer provides a vivid illustration of how a working scientist can master this revolutionary tool to the point where his power to theorize and to predict virtually redefines the scope of problems he can tackle. Lloyd believes that McRae's greatest contributions to the field have been made as a modeler.
The session's organizer, Larry Smarr, who directs the National Center for Supercomputing Applications (NCSA) at the University of Illinois, reminded the symposium audience that McRae was the first winner, in 1989, of the Frontiers of Computational Science Award. Writing about McRae and other scientists breaking new ground with computers, Robert Pool (1984) anticipated a theme that was to be visited during the Computation session at the Frontiers symposium: "Not only can computer experiments substitute for many of the studies normally done in a lab, but they also are allowing scientists to gather data and test hypotheses in ways that were closed to them before. And the field is still in its infancy—as computers become more powerful and as more scientists become aware of their potential, computer experiments are likely to change the way research is done. The promise of computational science has led some researchers to suggest the field will eventually grow into a third domain of science, coequal with the traditional domains of theory and experimentation" (p. 1438).
McRae came to the California Institute of Technology from Melbourne, Australia, in the mid-1970s for graduate work on control theory, but experienced a sea change that altered his career path. "Once you fly into Los Angeles, you realize there's a very severe problem there," he recalled. He began working on a computational model of the chemistry and physics of air pollution, which in the dissertation stage brought him into contact with mechanical engineer Armistead G. Russell. (The two are now colleagues at Carnegie Mellon, where they make good use of the Pittsburgh Supercomputing Center's CRAY-YMP 8/832.) The model contains the formulas and equations necessary to capture the relevant variables and relationships based on what is understood about the atmospheric physics and chemistry. McRae conceived a set of hypotheses in the form of a strategy to reduce pollution. He set the emissions levels of the target pollutants at variable levels and then evolved their chemical interaction through time under the influence of the other major set of variables, the meteorology (wind speed and direction, cloud cover, temperature, and so on).
While he was learning more about modeling the atmosphere, he was also collecting more and more data from Los Angeles air monitoring studies. Thus, moving to Pittsburgh did not deflect the focus of his model, but did expand it into the supercomputing environment, where the Cray he uses harnesses eight processors that can perform up to 2.7 billion calculations per second at its peak capacity. With this engine to drive it, McRae's model makes far fewer concessions (than are made in some other models) to the inherent limitations and extrapolations necessary to deal with nonlinear mathematics. The Navier-Stokes equations are a complex set of such equations that have been developed to model the weather. McRae created a grid map of the air over the entire Los Angeles basin that marked out 12,000 so-called mesh points, where all of the relevant interactions and phenomena would be individually computed. (Smarr has noted that only a few historical data points exist to provide pollution input numbers to drive the predictive model.) Fifty chemical species and their interactions are modeled at each of these 12,000 points, and the equations are then coupled. The resulting model staggers the imagination, in fact would never have even been imagined before the advent of supercomputers: 500,000 coupled nonlinear equations, which McRae then manipulated into 200 different simulations, each for a different set of environmental conditions. The computer can run through one of these full 2-day simulations in about 40 minutes.
With this vast sea of data under control, McRae worked with graphics specialists at NCSA to develop a computer-generated video presentation that uses scientific visualization techniques to illuminate the results of the model. Simulations of Los Angeles, over several days under specific environmental conditions, can be compressed into a few moments with modern visualization tools (Figure 8.2). But now that the model has earned its stripes as a predictor, it is the scientists who are defining the levels of various hypothetical pollutant emissions and the weather influencing them. The model then produces a vision of what such pollution and weather in Los Angeles would look like that has been captured in a video, produced by the Pittsburgh Supercomputing Center, called "Visualizing Los Angeles Air Quality." A second video called "Smog" was developed and produced by the National Center for Supercomputing Applications "to try to understand the physics and the chemistry," where the heart of McRae's work lies. ''We can work backwards from a condition, unraveling the meteorological transport. If this is the worst air quality experienced, what are the sources that might be responsible for it?'' he asks. Armed with these tools, then, McRae, his colleagues,

Figure 8.2 Computer-generated simulation of the air over Los Angeles. (Courtesy of L.L. Smarr.)
and other atmospheric scientists venture forth from their laboratories into the pragmatic, political world of pollution control.
REFRAMING THE LAWS OF POLLUTION CONTROL
McRae's long history of involvement with colleagues in the field and regulators in Southern California, along with the demonstrable cogency of his methods and his model, have given him a role in recent regulatory reform. Said Lloyd, "His work gave technical credence to strong regulations that were needed to reduce emissions." The South Coast Air Quality Management District, said Lloyd, is well served by the views and resources provided by individuals like Winer and McRae, although Lloyd's premise is that "the scientists and the scientific community have to be more proactive." Thus, when the model McRae has spent nearly a decade developing and refining was finally put to the test, and its results suggested many fairly dramatic implications, the battle was just being joined. Industry and other interested parties suggested, supported, and then promoted alternative studies that turned out to contradict McRae's work, and a fairly heated political and media debate ensued, fueled by major lobbying interests that had a lot at stake.
Ultimately, the decisions to regulate ROGs and NOx that California's legislators and regulators had previously made were ratified by
the superiority and demonstrable conclusions of McRae's and Russell's work, which further buttressed legislative and policy plans for the state's environmental strategy. McRae pointed out one of the essential ingredients in this vindication of their model: their determination to certify the work as solid science. "[The industry modelers] simply claimed their conclusions without exposing the details of the calculations to scientific peer review," McRae was quoted as saying (Schneider, 1990, p. 44). McRae and his colleagues, however, invited the test of scientific scrutiny by publishing their results in an established journal (Milford et al., 1989). Although purely scientific, that paper indicates the nature of pollution politics and reads like a manifesto for change, based on the strong demonstrative evidence developed by the authors from the model of Los Angeles. The following paraphrase of that paper matches the tone and summarizes the contents of the published version:
The Problem. More than half of the people living in the United States reside in areas where ozone concentrations exceed the National Ambient Air Quality Standard of 0.12 ppm mandated by the U.S. government, and many cities, like Los Angeles, also fail to comply with the standards governing particulate matter (PM10) pollution. In the face of this shortcoming, science suggests that the standards should be even tighter, and many agencies already feel that they have exhausted the technical, political, and economically feasible control measures currently available to address the problem. Moreover, there is a growing awareness that the scope of the problem is not fully captured by the historical convention of concentrating on cities, since recent evidence and modeling suggest downwind problems are sometimes more severe than in the areas whence the pollution was generated.
The Traditional Response. Given a mandated, numerical air quality goal, strategies to reduce ROG emissions to a level that leads to air that meets that standard have been evaluated for cost-effectiveness. The Empirical Kinetics Modeling Approach employs a simplified, single-cell trajectory formulation to model the impact of a number of possible ROG and NOx emission level combinations on the resultant ozone level, and then to derive a new ROG reduction goal. Lowering NOx has not been mandated (except in some jurisdictions such as California), since observations suggest that sometimes lower NOx can paradoxically lead to higher ozone. The model fails to incorporate the significance of other pollutants, such as gas-phase pollutants other than ozone, and particulate matter components less than 10 micrometers in diameter. Further, it is not clear whether peak ozone concentra-
tions observed within the urban area are the best measures of air quality for use in designing control strategies.
A New Modeling Approach. The use of new Cray Research supercomputers permits simulations predicated on a different approach to modeling that were formerly computationally infeasible. Current photochemical airshed models accurately describe the physical and chemical processes responsible for chemical transformation, transport, and fate. Advances in computational technology now permit the exploration of more control scenarios at a greater number of sites at smaller time intervals. Considering the entire Los Angeles basin as a rectangular grid 200 by 100 kilometers, 45 different combinations of control levels (most of them limited to 80 percent or less of an improvement over current standards, those more likely to be implemented over the next decade) were simulated under expected atmospheric conditions for thousands of adjacent but discrete locations within the shed over a 2-day period, each simulation requiring 40 minutes of computer time. The choice of issues and methods was designed to widen the applicability of the model to other urban locations.
The model must first demonstrate its reliability by reproducing air quality outcomes and readings for O3, NO2, HNO3, NH4NO3, and PAN that were historically experienced. August 30 and 31, 1982, were chosen for this purpose for a number of meteorological reasons. When compared to the data collected for those days, the model's performance was in excellent agreement for ozone and PAN, and for nitrogen dioxide was better than with previous models. Also, the gas-phase and particulate predictions were in excellent agreement with observations. This base-case performance established the model's reliability, and simulations of differing control strategies were run.
The Results and Implications for Control Strategies. A number of surprising spatial patterns were observed for the first time, as this modeling technique revealed interactions and time-sensitive patterns for the targeted substances that had not been captured by previous models. In particular, the model demonstrated that changing relative emissions standards very often had the impact not of reducing peak ozone concentrations, but of merely moving them to a different region within the airshed. Another strongly supported observation involves the effect of reductions of emissions on concentrations of other pollutants, such as PAN and inorganic nitrate. The modeling seems to suggest several important premises for regulators trying to balance the cost and effectiveness of control strategies:
-
Trade-offs will be inevitable, because various choices among control strategies will have differential impacts on different receptor sites throughout the region. Also, the ratio of ROG to NOx reductions will affect pollutants other than ozone, in differing degrees, and for different receptor sites.
-
NOx reductions may actually increase ozone in regions of the airshed with high emissions. But as receptor sites further downwind are considered, the impact of reducing NOx throughout the basin tends to reduce ozone. The crossover point seems to be 3 to 4 hours of downwind travel time. Associated ROG controls are most effective in those areas where high NOx levels are maintained and radical concentrations suppressed through midday.
-
PAN concentrations seem to follow the same general pattern as ozone. Thus a combination of ROG and NOx controls—such as have been in effect for about a decade in California—is advisable.
-
Inorganic nitrate levels follow base-line levels of NOx and OH. Thus reducing NOx reduces inorganic nitrate pollution.
-
The foregoing points suggest that a strategy solely reliant on ROG emission reductions may be inadequate, as the EPA now recognizes, and under certain conditions could never succeed in attaining the required ozone standard, without correlative substantial reductions in NOx Moreover, only by a strategy that combines both ROG and NOx controls can simultaneous reductions be expected in ozone, PAN, and inorganic nitrates.
-
Any conclusions drawn about the effects of pollution based only on peak receptor sites will distort the picture that emerges from more revealing models. These suggest that only by considering numerous receptor sites throughout the region and over time, and evaluating at least PAN and inorganic nitrates in addition to ozone, can a strategy be developed that embraces the known major trade-offs.
SCIENCE IN THE PUBLIC INTEREST
Thus it can be seen from the work authored by Milford, Russell, and McRae that atmospheric scientists very often assume a strongly proactive stance about their conclusions. As McRae affirmed at the outset of his presentation at the Frontiers symposium, convincing people about the right thing to do in response to the problems he is analyzing is part of his charge. He and Winer and Lloyd each in his own way revealed a consciousness informed by science, but fueled with a passion for implemented solutions. And as McRae's career and his model attest, modern supercomputing provides a very useful
and powerful tool that has helped to revolutionize the study of meteorology and to create a more comprehensive vision of atmospheric science.
BIBLIOGRAPHY
McRae, Gregory J., and Armistead G. Russell. 1990. Smog, supercomputers, and society. Computers in Physics (May/June):227–232.
McRae, G.J., J.B. Milford, and J.B. Slompak. 1988. Changing roles for super-computing in chemical engineering. International Journal of Supercomputing Applications 2:16–40.
Milford, Jana B., Armistead G. Russell, and Gregory J. McRae. 1989. A new approach to photochemical pollution control: Implications of spatial patterns in pollutant responses to reductions in nitrogen oxides and reactive organic gas emissions. Environmental Science and Technology 23(10):1290–1301.
Platt, U., D. Perner, A.M. Winer, G.W. Harris, and J.N. Pitts, Jr. 1980a. Detection of NO3 in the polluted troposphere by differential optical absorption. Geophysical Research Letters 7:89–90.
Platt, U., D. Perner, G.W. Harris, A.M. Winer, and J.N. Pitts, Jr. 1980b. Observation of nitrous acid in an urban atmosphere by differential optical absorption. Nature 285:312–314.
Platt, U., A.M. Winer, H.W. Biermann, R. Atkinson, and J.N. Pitts, Jr. 1984. Measurements of nitrate radical concentrations in continental air. Environmental Science and Technology 18:365–369.
Pool, Robert. 1989. Is it real, or is it Cray? Science 244:1438–1440.
Schneider, Mike. 1990. Visualizing clean air in Los Angeles. Supercomputing Review 3(March):44–48.
Silver, Cheryl Simon, and Ruth S. Defries. 1990. One Earth, One Future. National Academy Press, Washington, D.C.
U.S. Environmental Protection Agency. 1988. Review of the National Ambient Air Quality Standards for Ozone. Staff Paper. Office of Air Quality Planning and Standards. EPA, Research Triangle Park, N.C.
Warneck, P. 1988. Chemistry of the Natural Atmosphere. Academic Press, San Diego, Calif.
Winer, A.M., R. Atkinson, and J.N. Pitts, Jr. 1984. Gaseous nitrate radical: Possible nighttime atmospheric sink for biogenic organic compounds. Science 224:156–159.
RECOMMENDED READING
Finlayson-Pitts, Barbara, and James Pitts. 1986. Atmospheric Chemistry—Fundamentals and Experimental Techniques. John Wiley and Sons, New York.
Seinfeld, John. 1986. Atmospheric Chemistry and Physics of Air Pollution. John Wiley and Sons, New York.





















