2
Photosynthesis
Artificial Photosynthesis: Chemical and Biological Systems for Converting Light to Electricity and Fuels
''Natural photosynthesis is a process by which light from the sun is converted to chemical energy," began Mark Wrighton in his presentation to the Frontiers symposium. Wrighton directs a laboratory at the Massachusetts Institute of Technology's Chemistry Department, where active research into the development of workable laboratory synthesis of the process is under way. As chemists have known for many decades, the chemical energy he referred to comes from the breakdown of carbon dioxide (CO2) and water (H2O), driven by photons of light, and leads to production of carbohydrates that nourish plants and of oxygen (O2), which is vital to aerobic organisms. What is not known in complete detail is how this remarkable energy-conversion system works on the molecular level. However, recent advances in spectroscopy, crystallography, and molecular genetics have clarified much of the picture, and scientists like Wrighton are actively trying to transform what is known about the process into functional, efficient, synthetic systems that will tap the endless supply of energy coming from the sun. "Photosynthesis works," said Wrighton, "and on a large scale." This vast natural phenomenon occurring throughout the biosphere and producing an enormous amount of one kind of fuel—food for plants and animals—Wrighton described as "an existence proof that a solar conversion system can produce [a different, though] useful fuel on a scale capable of meeting the needs'' of human civilization. Photovoltaic (PV) cells already in use around the world provide a functional (if more costly per kilowatt-hour)
source of electricity but will not begin to compete with the major fossil fuel source plants until costs come down further.
Wrighton's presentation, "Photosynthesis—Real and Artificial," was a closely reasoned, step-by-step discussion of the crucial stages in the chemical and molecular sequence of photosynthesis. His colleagues in the session were chosen for their expertise in one or another of these fundamental specialized areas of photosynthesis research. By the end of the session, they had not only provided a lucid explanation of the process, but had also described firsthand some of the intriguing experimental data produced. Douglas Rees of the California Institute of Technology (on the molecular details of biological photosynthesis), George McLendon of the University of Rochester (on electron transfer), Thomas Mallouk of the University of Texas (on the arrangement of materials to facilitate multielectron transfer chemistry), and Nathan Lewis of the California Institute of Technology (on synthetic systems using liquid junctions) all supplemented Wrighton's overview with reports about findings in their own area of photosynthesis research.
The science of chemistry is predicated on the atomic theory of matter. No matter how intricate the structure of an atom or molecule, its constituent parts will be conserved after the exchanges of a chemical reaction. In fact it was the development of the balance scale in the 18th century that led to the birth of modern chemistry. Once it was realized that the laws of thermodynamics and the principle of the conservation of energy provide an elegant set of constraints, chemistry became the ultimate puzzle-solving science. One could feel fairly confident—once most of the basic elements and compounds and their simple proportional relationships had been discovered—that the answer could be found in the laboratory, if only the pieces could be assembled into the proper, coherent picture. For chemists, this usually means recreating an interaction under conditions that are precisely repeatable.
The enabling paradigm was developed by British chemist John Dalton, who proposed the atomic theory of matter around the turn of the 19th century. Notwithstanding subsequent refinements due to quantum physics and to scientists' increasing ability to probe and examine these reactions directly, Dalton's basic description of the behavior and transfer of protons and electrons among and between elements and compounds—the opening salvo fired at every high school chemistry student—still sets the stage for the most advanced chemical research. Photosynthesis provides a vivid example of the type of drama that is played out effortlessly in nature but reenacted elaborately in chemical laboratories with painstaking concern for the intri-
cate details. It is hardly an oversimplification to say that if scientists could figure out exactly how the electrons liberated by light striking plants are so efficiently put to work in subsequent chemical transformations, analogous systems might well be developed that could help substantially to meet the world's energy needs. Thus the stakes for society are high, and the contrast is dramatic: a chemist works on precisely controlled and intricately choreographed reactions in a laboratory—usually on a very small scale—and yet the implications and applications of his or her work may lead to dramatic transformations, on a vast scale, of the material world.
In the case of research on artificial photosynthesis, such work could lead to the economical production of an alternative to the dwindling supply of fossil fuels. And a further benefit might be a reduction in the sulfurous products emitted by the combustion of carbon-based fuels. Wrighton explained that these fuels are themselves "the result of photosynthetic processes integrated over the ages." It must be kept in mind that long before plants developed the ability to produce molecular oxygen as a byproduct of photosynthesis, they were always about their real business of converting carbon dioxide from the atmosphere into carbohydrates for their own sustenance. In fact some of these anaerobic plants still exist today in certain specialized ecological niches. The system for photosynthesis evolved to its present state during the earth's natural history, and it exploits materials that are naturally abundant and inexpensive, Wrighton pointed out. As designed by nature, it is the ultimate recycling process—since it uses the planet's two most abundant resources, CO2 and H2O, providing fuel and breaking down a pollutant.
Wrighton and others in the hunt to develop a synthetic version of the photosynthetic process that would generate commercially viable energy accept these two fundamental provisos: it must be cheap, and its raw material inputs must be abundant. Present PV technology has developed solar efficiencies (that is, a certain percentage of the energy received from the sun deliverable as electricity) of 28.5 percent for point-contact crystalline silicon solar cells and 35 percent for a gallium arsenide-gallium antimonide stacked junction cell (Brodsky, 1990), but the manufacturing costs of these products do not allow them to compete where conventional alternative sources are available. If the two natural photosynthetic inputs, CO2 and H2O, could be harnessed, said Wrighton, "fuel mixtures that would be useful within the existing technological framework—where combustion processes dominate the use of our existing fuels"—are foreseeable. One ancillary benefit of such a process could be to "bring global CO2 concentrations to a steady-state value," manifestly a desirable goal,
said Wrighton. But as he pointed out, there are other candidates for an input source that are ubiquitous, including SiO2 (silicon dioxide in rocks), N2 and O2 (molecular nitrogen and oxygen from the air), and NaCl (common table salt).
If one of Earth's abundant natural resources could be energized by sunlight to produce (probably by the breakdown and release of one of its elements) a source that could be used for fuel, the entire fossil fuel cycle and the problems associated with it might be obviated. If that resource were water, for example, and the resultant fuel source were hydrogen, burning liquid hydrogen in the air would produce only water as a combustion product. Liquid hydrogen is already in use as a fuel source and has always been the primary fuel powering space vehicles, since it produces more heat per gram of weight than any other known fuel. If a photosynthetic system delivering usable hydrogen could be developed, the process would regenerate the original water source, and an entirely new recycling of natural resources could be established. This time, however, cultural rather than natural evolution would call the shots. With such a major new fuel production process, science would hopefully be able to provide a methodology to better anticipate and control the global impact of any byproducts or emissions.
The search for a new way to harness the sun's energy involves first the study of how photosynthesis works in nature, and then the attempt to devise a new system that to some extent will probably mimic or model the successful example. Wrighton and his colleagues provided a lucid description of both efforts.
PHOTOSYNTHESIS IN NATURE
Photons and the Physics of Light Energy
Photosynthesis is made possible by the flood of free energy raining down on the planet from the sun. Clearly this energy is put to good use by plants and certain bacteria that have developed analogous photosynthesizing abilities. Many studies of photosynthesis are conducted on these organisms, which are hardy and genetically manipulable under laboratory conditions.
But of what does this energy shower of light consist? How can certain structures convert it to chemical energy that is useful to them? The background to answering this question involves two of the giants of 20th-century physics—Planck and Einstein—whose work at the beginning of this century provided important fundamental insights into the energy of light. German physicist Max Planck in 1900
derived what is called Planck's constant (universally referred to by the symbol h) to demonstrate his theory of the quantization of energy. He realized that the light energy given off by burning bodies varies with the frequency of their vibrating atoms, and produced the formula E = nvh, n = 1, 2, 3, . . . .
Thus the energy (E) of a vibrating atom will vary according to its frequency of vibration (v), but can assume only specific quantity values, namely whole integers times h (approximately 6.63 × 10-34 Joule-second). These values, the various products of hv times whole integers, are known as quantum numbers. When one says the energies of atoms are quantized, one means that they can assume values from this set of numbers only. Thus a quantum of energy—whether it be light or electromagnetic energy outside the optical spectrum—provides scientists a measure of the smallest piece of energy that seems to be involved in the transfer events they are trying to understand. "These concepts have been extended to molecules, where absorption of quanta of energy—photons—occurs at particular energy levels and gives rise to molecules that then possess very different properties," and most importantly, Wrighton continued, "they can be useful in effecting oxidation and reduction reactions."
These "particles" of electromagnetic energy were observed to be proportional to the frequency of light in which they were traveling. Thus when a photon of a particular energy strikes a metal, for instance, that metal's outer electron(s) will be ejected by the photoelectric effect only when the incoming photon has sufficient energy to knock it loose. Light and the energy value of the photons it transmits vary according to its wavelength frequency; materials vary according to how easy it is to displace a valence electron. When this does occur, the photon is said to be absorbed by the substance, and actually ceases to exist as a particle. Aerobic plants absorb photons of light from the sun within a certain frequency range, and this drives the movement of electrons that yields the synthesis of carbohydrates and oxygen. This is the theoretical physics underlying photosynthesis. But it is the physical chemistry that interests Wrighton and his colleagues, who hope to develop analogous systems that would produce usable energy.
Harvesting Photons and Putting Them to Use
Two fundamental constraints govern the system: the plant or photosynthesizing organism must possess a mechanism to register or receive the incoming photon; and since the energy content of a single photon is small, a way must also be found to collect and aggregate
such energy. Plants have evolved mechanisms to overcome both of these problems. In plants, chlorophyll provides what chemists classify as a sensitizer, a species that absorbs light and effects subsequent chemical reactions. "The assembly of chlorophyll collects and aggregates light energy," Wrighton explained.
As Wrighton pointed out, "First, it is noteworthy that exposure of CO2 and H2O to sunlight alone does not lead to a reaction, since the photosynthetic apparatus involves light absorption by molecules other than CO2 and H2O, namely chlorophyll. Chlorophyll can therefore be regarded as a sensitizer, a light absorber which somehow channels the light to reaction pathways leading to the production of energy-rich products." It is the absorption by chlorophyll of light frequencies in the lower wavelengths that produces the green color of plants, he explained. But the crucial role played by chlorophyll and any sensitizer is to expand the bandwidth of the energy a system can accept. Wrighton added that since CO2 and H2O do not absorb in the visible frequency range, some sort of sensitization will be needed to exploit the full range of the sun's energy in the optical, or visible, range of the electromagnetic spectrum where photon energies tend to be sufficient to dislodge an electron, between 200 and 800 nanometers. This proviso is not limited to the carbon dioxide and water nature breaks down, however, but also applies, said Wrighton, to "all abundant, inexpensive fuel precursors" currently under consideration as candidates for a synthetic system. "Thus, efficient photoconversion systems will involve use of efficient visible light absorbers," he concluded.
"The second critical feature of the photosynthetic apparatus," Wrighton emphasized, is that "in order to achieve high solar conversion efficiency, the formation of a single fuel molecule will involve the energy from more than one photon." The ratio of photons to electrons released will probably be one to one in any system—as it is in nature—but there must be a way to harness the energy from several of these freed-up electrons to produce a chemical transformation. "If a one-photon mechanism were operative in nature, the process would be doomed to low efficiency," he explained, because a single photon that would break down H2O would have to be in the blue wavelength range, and "sunlight does not contain much energy of blue light, or of shorter wavelengths." Nature's way of aggregating and using more than one photon of the energy that is abundant, throughout the entire optical wavelength spectrum, is photosynthesis.
The photosynthesis process in nature involves two separate photosystems (called PS I and PS II), each of which contains the chorophyll whose electrons provide the vehicle for the energy that is creat-
ed. The sequence of molecular events occurs within a structure that biochemists classify as the Z-scheme (Figure 2.1). This molecular arrangement accomplishes an oxidation-reduction, or redox, reaction that involves the actual (or in some cases only the apparent) transfer of electrons between species. When these two phenomena occur together, the overall activity is described as a redox reaction, whereby in one half of the reaction a species loses electrons—is oxidized—and in the other half of the reaction a different species gains electrons—is reduced (Ebbing, 1990). Nature uses photons to free electrons from chlorophyll and—through a series of steps—to oxidize H2O, and in the process O2 is created as a product of the reduction of CO2.
The Z-scheme provides an architecture of molecules, located in what biochemists call the reaction center of the plant, that facilitates the redox reaction. Crucial to this arrangement is a mechanism that will serve not only to separate an electron from its atomic species, but will also move it, once it has been separated, in a timed and coordinated way along a known path. Summarizing the three essential elements of the Z-scheme, Wrighton said that the two natural photosystems found in all aerobic plants work in series to (1) absorb four photons of light to energize chlorophyll, (2) release electrons by charge separation and move them by unidirectional charge transport
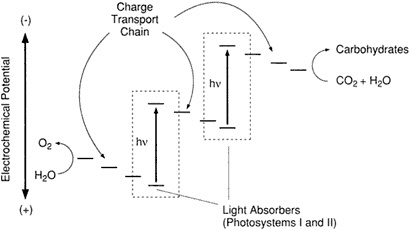
Figure 2.1 Z-scheme representation of the photosynthetic apparatus showing, on an electrochemical potential scale, components for light absorption, charge transport, and redox processes for oxidation of H2O to O2 and reduction of CO2 and H2O to carbohydrates. (Courtesy of M. Wrighton.)
away from the holes they left behind, and then (3) deliver them to sites distant enough from each other to prevent their mutual interference, where their respective oxidizing (of H2O) and reducing (of CO2) tasks can be fulfilled.
The fairly astounding concept at the heart of this series of events is that, in order for photosynthesis to occur, evolution had to develop a structure that would facilitate a chemical series of steps that are, even today, reproducible in the laboratory only with great difficulty. Wrote McLendon (1988), "Just a few years ago, the idea of 'long-distance' chemical reactions, in which the reactants are held fixed at distances (10–30 Å) that preclude collisions, was viewed with suspicion or disbelief by many chemists. Fortunately, nature is not so skeptical, and biological energy is channelled in the photosynthetic and respiratory transport chains by just such long-distance reactions."
The Reaction Center
Wrighton reported that "remarkable progress in establishing the molecular structure" of the reaction center has been made in recent years, citing Douglas Rees of the California Institute of Technology as a contributor in this area. There remain some important unanswered questions, however, said Wrighton: Why is the electron transfer from the photoexcited chlorophyll so fast? Why does the electron transfer take only one of two quite similar paths? Rees has focused on "one of the simplest systems for studying biological photosynthetic electron transfer," a system with "only a single photosystem," a type of bacteria that—while it does not produce oxygen or reduce carbon dioxide—nonetheless does photosynthesize, and employs in certain of its cells an electron transfer chain that is comparatively clear to study, and that will most likely yield insights about artificial systems that might be designed and built in the laboratory.
The reaction center molecules are mostly proteins, specialized polypeptides, and strings of amino acid residues. The overall process begins, explained Rees, when "light is absorbed by a specialized pair of the bacterium's chlorophyll molecules." These molecules are called P680 (pigments that absorb light most strongly at the frequency of 680 nanometers) and react to the photon by giving up an electron. Such a structure is then said to be in an excited state and represents the positive component of the charge separation, referred to as a hole. As the electron moves through the transport chain, its negative charge and the positively charged hole are separated by ever greater distance, and potential energy is created. In essence, the reaction center acts as a tiny capacitor by separating and storing these
positive and negative charges on opposite sides of its architecture, figuratively like the poles of a battery. Four more or less distinct steps constitute the process, after the chlorophyll pigment has absorbed the photon and donated its electron. The moving electron is accepted by the pigment pheophytin very quickly, "in roughly 4 picoseconds," explained Rees, which passes it to a primary quinone, QA, and then on to a secondary acceptor quinone, QB. Finally, a secondary electron donor gives up an electron to replace the one lost by the original donor, which is thereby reduced (that is, gains an electron). The light energy captured by the reaction center is ultimately utilized to drive the metabolic processes necessary for life.
Much of the detail has been observed directly, said Rees, who pointed out that "crystallographic studies provide a nice framework for rationalizing and understanding the kinetic sequence of events. But also, they raise a number of questions." The most significant of these involves the rates of these various steps. The atomic electrical attraction of positively and negatively charged actors in the process always threatens to draw the liberated electron back into its hole, a process called back electron transfer. If a step proceeds forward at too slow a rate, back transfer will occur and will short-circuit the entire process. In addition to increasing their speed, emphasized McLendon, experimenters also have to steer these freed-up electrons. "It doesn't do any good to move an electron around in a picosecond if it goes to the wrong place, because you will just short-circuit your electron transport chain. Then every cellular component gets to a common free energy, and you have a death by entropy."
Genetic engineering has also been employed to create mutant reaction centers, where certain proteins and cofactors have been deleted or altered. Rees reported that, "rather surprisingly, in many of these mutant forms the reaction center still works," though with a reduced quantum efficiency. In sum, "this marriage of molecular biology and chemical physics has provided a good structural understanding of the reaction center," said Rees, who was also referring to major strides made in spectroscopy, theory, and x-ray crystallography. The dynamics and the energetics of the process still remain imperfectly understood, but, he predicted, "the prognosis looks quite good for unravelling these details in the next 5 years or so."
LABORATORY PHOTOSYNTHESIS: IMPORTANT FIRST STEPS
Wrighton and other chemists around the world are trying to use what has been learned about photosynthesis in nature to create a
synthetic system that might produce a renewable source of energy. The energetics Rees referred to have become an important area of inquiry called excited-state electron transfer, advances in which will aid chemists and molecular biologists who are already building actual molecular structures to achieve conversion of light to energy. Thus far, the most promising synthetic systems have exploited the chemistry and physics of liquid-semiconductor junctions.
Excited-state Electron Transfer in Synthetic Systems
Quantum physics explains how the light energy of a photon is absorbed by the right sort of receptor, kicking an electron loose and commencing the process of photosynthesis by creating a source of potential energy between the separated electron and the hole it formerly occupied. Gaining and losing electrons "is the name of the game in redox reactions," said Wrighton, who added, "It has long been known that the photoexcited molecules are both more potent oxidants and more potent reductants than the ground electronic state" (Figure 2.2). When a photon is absorbed to create an electron and a hole, something thermodynamically unstable is produced, and there's always the tendency for the electron and the hole to recombine. Back electron transfer is, metaphorically, a short circuit that bleeds the potential energy of the charge separation before it can aggregate at a distant site within the system and be put to use.
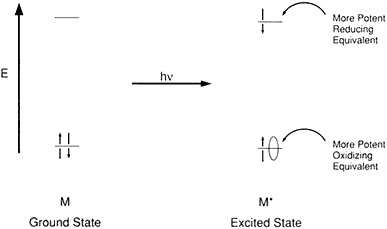
Figure 2.2 Orbital diagram rationalizing potent redox power of one-electron excited molecules. (Courtesy of M. Wrighton.)
Wrighton and others have been able to find and to build multi-component molecules with ''a donor and an acceptor system covalently linked to a light absorber, an assembly,'' he pointed out, "that does indeed resemble the heart of the Z-scheme" found in plants. But their system does not produce energy as efficiently as they would like, because of the timing of the reactions: "In solution the energy-rich molecules lose the stored energy by either intermolecular or intramolecular back electron transfer. In nature, the movement of the carriers away from one another is crucial to high efficiency. The unidirectional movement of electrons in the Z-scheme is a consequence of the components of the charge transport chain, and how they are arranged, both geometrically and energetically," Wrighton explained. Work in his group, he continued, did lead to construction of a complex molecule with all of the functional components, but the photoexcitation test showed that a 10-nanosecond time was required for the donor to deliver the molecule to the acceptor. "This is very sluggish compared to the 4 picoseconds demonstrated [in the natural systems]," Wrighton summarized, "and so one of the challenges is to prepare a molecule that will have more zip than our 10-nanosecond time." Thus chemists explore the quantum world, said Wrighton, narrowing in on several factors that might elucidate the transfer rates of the electrons: "the energetics for electrons, the distance dependence of electron transfer, and the structures of the donor and acceptor and their relationship" in space. George McLendon of the University of Rochester, said Wrighton, "has made important progress in understanding such factors."
McLendon is a chemist specializing in the quantum processes of moving electrons from one molecule to another. Not focusing exclusively on photosynthesis, he usually works with proteins and biological systems, but his laboratory has demonstrated phenomena crucial to all electron transfer systems. The basic physics involves the concept of conservation of energy, which, explained McLendon, shows that an electron's rate of transfer varies with the energy force driving it. Essential to the first step in photosynthesis, this relationship between rate and energy was analyzed theoretically some years ago by Rudy Marcus (1956) at Caltech, who predicted an anomaly that was first confirmed by John Miller at Argonne National Laboratory, and verified subsequently by McLendon and others. Up to a certain level of energy, the rate of electron transfer increases with the force driving it, but the initially proportional relationship changes. After the peak level is reached, additional driving force actually slows the electron down. "A funny thing," said McLendon, "is that you can have too much of a good thing."
Quantum physics provides a suggestive, if incomplete, explanation of why the electron does not transfer ever more quickly as more energy is applied. McLendon explained that when an electron is lost from one molecule (a donor) and gained by another (an acceptor), the lengths of the bonds in each molecule change, like a stretching spring. The energy for this stretching, called the "reorganization energy," said McLendon, "is supplied by the excess [free] energy of reaction. The fastest rate occurs when the reaction free energy exactly equals the reorganization energy. If less energy is available, the bonds can't be stretched enough for reaction to occur." Conversely, continued McLendon, ''if too much energy is available, the system must wait for this extra energy to 'dribble' away, since at the instant of transfer, the energy of the starting materials and products must be equal, by the law of conservation of energy."
McLendon provided an example: "Consider a system containing only ferrous ions (dipositive charged ion molecules) and ferric (tri-positive) ions. When an electron moves, one ferrous becomes ferric, and vice versa. Obviously the system, as a whole, remains unchanged. However, in each case the molecules' bonds have changed length—ferric bonds are shorter. If this length change occurred after the electron moved, energy would be released. With each subsequent electron transfer, the solution would become warmer, and there would be no [so-called] energy crisis. Obviously," continued McLendon, "nature doesn't work this way. Instead, heat is first taken from the system to stretch the bonds. Then, the electron jumps. Finally, the bonds relax, and heat is given back up. Overall, there is no change in heat, and energy is conserved. However, the slow step is getting the initial energy to stretch the bonds. If this can be provided by the energy of reaction, the reaction rate increases." The dependence of the separation rate on energy is approximately exponential, falling away from the peak value at lower, or higher, energies. Marcus' theoretical prediction of this phenomenon his colleagues believe was prescient, and he has continued to influence developments in the study of reorganizational energy (Marcus and Sutin, 1985).
Lessons for and from the Construction of Photodiodes
Thomas Mallouk, a chemistry professor at the University of Texas, Austin, wants to make certain that such theoretical insights are put to use. He sees an object lesson in the history of research on superconductors in the 1950s, when increasing knowledge led only very slowly to any "perturbation in the technology." So at his laboratory chemists are actually trying to build a photodiode from which
to obtain usable electric current. Using insights from "the two systems that really work—photosynthesis and photovoltaics that use semi-conductors—the first question in creating a multistep electron transfer mechanism," according to Mallouk, "is, What is the best way to organize the molecules?"
Because the cost of materials and the complexity of assembly are crucial determinants of the viability of a commercial system, Mallouk said, "we look for ways where we can get these things to self-assemble, hopefully into a matrix that will teach them how to line up usefully, even if we just chuck them all in there randomly." Several strategies are combined, including using electric field gradients to propel and direct freed electrons, creating a gate whose size will selectively admit only the desired species of molecule, and employing molecules whose electrochemical potentials are easily controlled. (Figures 2.3 and 2.4).

Figure 2.3 Analogy between semiconductor- and molecule-based electronic devices. (Courtesy of T.E. Mallouk.)
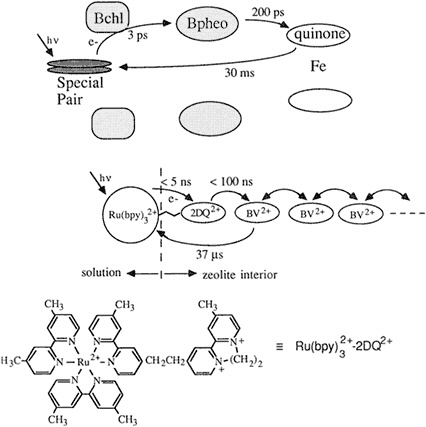
Figure 2.4 Analogy between natural and zeolite-based photosynthetic reaction centers. (Courtesy of T.E. Mallouk.)
Zeolites are a form of aluminosilicate. "There are many different polymorphs of this general formula," said Mallouk, "and they have the endearing property that the holes in them are about the size of a molecule, about 6 or 7 angstroms across. You have an array of linear tunnels that run through the structure, with the tunnels packed in next to one another." By exploiting the diameter of the tunnel to exclude molecules of a certain size, adding some useful catalysts, and charging the system with a calculated amount of energy, Mallouk's team was able to trap the target molecule inside the tunnel, where it could not escape while the scientists beamed their light and photolyzed it. The first transfer step occurs when the electron escapes
from a molecule in solution to interact with molecules that have been chemically attracted to the surface of the assembly; the second step involves the product of that step reacting with molecules inside the tunnel altogether. "So we have a self-assembling, three-molecule chain, where the viologen is inside, porphyrin is on the outside, and EDTA is ion-paired with the porphyrin out there," Mallouk explained. The assembly worked, in that it propelled the electron into the tunnel in 30 picoseconds, only 10 times slower than with natural photosynthesis. The efficiency of the electron-hole separation was very poor, however, said Mallouk, illustrating Wrighton's emphasis on the importance of back electron transfer pathways.
"We had to think more intelligently for our Mach II version," said Mallouk, who then added two more components to the system in order to better mimic nature. "Remember, the natural system is a sort of staircase arrangement of redox potentials," he said, and so they tried to create a multistep mechanism within their molecular assembly. Rather than speed up the process, they chemically blocked the back transfer pathway by manipulating the cationic charges and using the 7.1-angstrom-wide zeolite tunnel as a kind of trap. Their molecule was electrically propelled through, small-end first, but then became trapped like a pig in a fence as its other larger end reached the tunnel. Mallouk's team actually "slowed down the forward rate," which, he conceded, is "kind of a macho thing in electron transfer chemistry, where the basic rule is to get your forward rates as fast as possible." But, he said, citing McLendon's thesis, ''it really doesn't matter as long as your forward rate is fast enough compared to the competing back transfer pathway."
"There are many steps in a row, but the point is to get the electron safely away from where it came. With so much empty space in the zeolite, we can load up the rest of the channel with a molecule that is easier to reduce. Now we have two steps forward, which chemically puts the electron much further away from its hole, so the rate back down [the hill] decreases by a factor of 100 or so," Mallouk explained. How did it work? He continued, "The effect of putting in these more intelligent features is to increase the quantum efficiency by about four orders of magnitude. When you put this whole thing together, you really only have to stir it up, and you come out with a quantum yield of about 30 percent for photochemical separation of electrons and holes, and subsequent hydrogen evolution." Thus, Mallouk concluded, they have created another system—this one man made—that works, and that delivers electrons productively. However, even though his system has the advantage of self-assembly, Mallouk noted that it still employs components that are expensive to create.
Although such systems may eventually evolve into competitive models, Wrighton and his team are currently at work on a prototype that will be compared to, he said, "the system to beat, the ones that work very well, semiconductor-based photoelectrochemical cells."
PHOTOVOLTAIC CELLS
As Wrighton said, not only does photosynthesis work—in nature and in the laboratory—but it can become commercially viable. Solar technology has been a growing phenomenon for two decades. The road to this growth, however, is really a two-lane highway, a fact that is often overlooked. Any alternative system that can produce electricity will be measured by how it compares to the current massive combustion of fossil fuels. On the one hand, scientists, ecologists, and even policymakers taking the long view recognize the finite and dwindling supply of these carbon-based sources and the harmful impacts of their sulfurous emissions. On the other hand, such considerations tend to be overshadowed by more immediate concerns, such as the cost of energy produced by alternative systems and how quickly that energy can be delivered. Market considerations, pragmatic politics, and the interests of the massive infrastructure now producing and selling electricity all deflect attention and, inevitably, resources away from the support of scientific efforts to develop a viable alternative source of energy.
Solar energy itself is multifaceted. Without the sun to evaporate the water that eventually returns to the earth's rivers and oceans, hydroelectric power would not have achieved the importance it has, providing approximately 17 percent of the energy used in industrialized countries and 31 percent in the developing countries. California's Altamont Pass is the site of the world's largest wind farm, indicating the feasibility and possibility of wind turbines powered by the earth's atmosphere, which in turn, of course, is powered by the sun. In addition, solar cells have been designed to collect heat and transfer it directly into heating and cooling uses at the site, as well as to heat a fluid that can be used to generate power in a system known as thermal electric generation. But the "system to beat" referred to by Wrighton, photovoltaic cells, creates energy with no noise and no pollution, and does so in a self-contained unit whose only drawback at the moment is cost of fabrication. If the design of photoelectrochemical cells could be improved to make the cost per kilowatt-hour of their generated electricity competitive, or to yield a fuel product from ubiquitous raw materials, the world's energy future could be reconsidered altogether.
Construction of Typical Solar Cells
A typical solar cell is made from two different types of semiconductor, each having a characteristic—and opposite—electrical tendency when perturbed by light. So-called p-type semiconductors, said Wrighton, "exhibit conductivity by virtue of having positively charged carriers, n-type semiconductors by virtue of having negatively charged carriers." The typical solar cell looks like a sandwich, engineered to exploit these preferences or atomic tendencies inherent in the silicon or other material of which the usually metallic semiconductor is fabricated. The base of the cell is a substrate of glass or plastic that insulates the cell. Above this cushion is the first of the two (outside, top and bottom) metal contacts that will eventually deliver the current in one direction each. Next comes the first of two silicon layers—say the p-type, designed to create and attract holes—and then an interface where an electric field is built into the cell as a type of particle accelerator. Above this is the second semiconductor, the n-type, through most of the upper layers of which photons from the sun will penetrate from above. This layer, including an antireflective coating at its upper edge, is designed so that photons moving through the material will be absorbed as close as possible to the magnetic interface with the other semiconductor. Thus, when an electron is released and creates a hole, each of these particles will be maximally positioned to begin their respective journeys through the respective semiconductors for which they have an inherent electrical affinity. Propelled by the electric field and their own charge, they migrate through the semiconductor layers to the metal contacts at top and bottom, from which a current can be tapped.
Use of Liquid Semiconductor Junctions
Nathan Lewis, now at Caltech, did his early work with Wrighton at MIT. Each of them is now working on a version of the photovoltaic (PV) cell described above, but with an important difference. Instead of a semiconductor-to-metal or solid-to-solid interface between the two particle-generating media, they are exploiting a liquid-semi-conductor junction. "There are many important practical advantages," said Wrighton, who conceded that one way of looking at the system is "as a solid-state PV device [arranged electrically] in series with an electrolysis cell. . . . The junction is easily prepared by simply immersing the semiconductor into the liquid electrolyte." Reminding the symposium's scientists that the payoff involves redox chemistry, Wrighton said that "further, the semiconductor surface
may be unique for certain redox processes that would be chemically inhibited on conventional metallic electrodes. . . . Most significant is the prospect that the liquid junction device could yield higher efficiencies than devices based on p-or n-semiconductor junctions." Added Lewis: "Really it is a cost-performance analysis. The physics of recombination can rigorously be demonstrated to be superior to that at the best semiconductor-semiconductor interfaces. We can control the interfacial chemistry."
The reason has to do with quantum physics at the surfaces. If the semiconductor is in contact with a metal, said Lewis, "you find that the resultant voltages are less than thermodynamics analysis predicts," because of interfacial reactions. A similar problem plagues the interface between two semiconductors of different electrical characters. "If you try to match their surfaces with atomic precision, you will pay a price" to do so, said Lewis, and thus drive up the economic cost of the system. "When you miss at certain spots, those spots become recombination sites," and some of the free charge meant to be collected as electricity is drained into these surface reactions. Using a liquid at the interface obviates both of these problems. First, one can add into the liquid something else that will have a high affinity for the defective sites that could have led to problematic recombination, and can thereby passivate these sites. Second, one can reduce the danger of back electron transfer by choosing a solvent that draws electrons more strongly and increases their forward rate.
There is a trade-off, however, because the solvent used in the basic solution determines the wavelength of light necessary to kick loose an electron. Water, it turns out, oxidizes silicon, essentially "poisons it," said Lewis. "You can suppress that reaction by going to a nonaqueous solvent," he continued, "but you then surrender a key property, the ability to extract simple fuels of H2 and O2." For water, Lewis and his co-workers substituted an iron-containing molecule (ferrocene), an ideal donor to accept the holes generated as light kicks loose the silicon electrons. By carefully mixing their solution, Lewis and his team were able to produce a solvent that maximized the forward rate of electron transfer. The advantage of their cell, Lewis pointed out, is that it has an efficiency in the sun of over 14 percent and has been shown to be stable for months. However, it is not capable of simultaneously producing chemical fuel. Lewis sees the challenge as three-fold: achieving stability, efficiency, and simultaneous fuel formation, two-thirds of which has been demonstrated in the silicon cell.
Wrighton described the strontium-titanate cell as "extraordinarily efficient for the wavelengths of light that excite strontium titan-
ate," the downside from the practical perspective being that those wavelengths are shorter and "fall in the ultraviolet range, which contains only 5 percent of the available solar energy." Thus, like other experimental systems and cells, Wrighton's assembly works impressively under laboratory conditions, but it uses an energy source (or in other cases, molecular components) that nature does not have an abundance of. "Short of changing the sun itself," said Wrighton, the problem remains to find a stable solution that will provide a complete cycle of oxidation and reduction, and a donor to complement it that will react to visible light. A real frontier in research on PV cells is finding or constructing materials and solvents that will balance all of these considerations: bandgap, passivation to counter deficits at the surface junctions, ionic acceleration of particles to avoid back transfer, and a material that will provide both sides of the redox reaction so as to produce a usable fuel.
Wrighton summarized the challenge: "Although the semiconductor-based photoelectrochemical cells represent the best chemical systems for conversion of light to electricity or chemical fuel, there are many shortcomings. Long-term durability for any chemical system remains a serious question. Nonetheless, the SrTiO3-semiconductor-based cell for photoelectrolysis of H2O remains as a good demonstration that sustained conversion of light to useful energy is possible. In trying to duplicate the function of the natural photosynthetic apparatus," he continued, "semiconductor-based approaches are far ahead of molecular-based approaches. . . . But before we see a mature solar conversion technology based on the excitation of electrons in materials, advances in chemistry and materials science must overcome present obstacles to efficient, large-area, inexpensive systems."
BIBLIOGRAPHY
Brodsky, Marc H. 1990. Progress in gallium arsenide semiconductors. Scientific American 262(February):68–75.
Ebbing, Darrell D. 1990. General Chemistry. Third edition. Houghton Mifflin, Boston.
Grätzel, Michael. 1990. Artificial photosynthesis. Pp. 83–96 in Frontiers of Science. Andrew Scott (ed.). Basil Blackwell, Oxford.
McLendon, George. 1988. Long-distance electron transfer in proteins in model systems. Accounts of Chemical Research 21 (April):160–167.
Marcus, R.A. 1956. On the theory of oxidation-reduction reactions involving electron transfer. Journal of Chemical Physics 24:966–978.
Marcus, R.A., and Norman Sutin. 1985. Electron transfers in chemistry and biology. Biochimica et Biophysica Acta 811:265–322.
RECOMMENDED READING
Gust, D., and T.A. Moore. 1989. Mimicking photosynthesis. Science 244:35–41.
Krueger, J.S., C. Lai, Z. Li, J.E. Mayer, and T.E. Mallouk. 1990. Artificial photosynthesis in zeolite-based molecular assemblies. Pp. 365–378 in Inclusion Phenomena and Molecular Recognition. J.L. Atwood (ed.). Plenum Press, New York.




















