Arsenic is a metalloid, having chemical and physical properties intermediate between a metal and a nonmetal. The importance of considering the form of arsenic to which people are exposed, arsenic metabolites, and exposure metrics used in studies has been well documented. How epidemiologic studies have assessed exposure to inorganic arsenic is an important element of study selection, interpretation, and use in dose–response analyses. Several studies and review articles concern the strengths and weaknesses of various exposure measures, including biomarkers, of arsenic (e.g., Vahter 2002; Hough et al. 2010; Basu et al. 2011; Maull et al. 2012; Calderon et al. 2013).
NATURAL AND ANTHROPOGENIC SOURCES
There are numerous potential sources of exposure to inorganic arsenic. Inorganic arsenic occurs in the environment as arsenate (iAs[V]) and arsenite (iAs[III]). Human exposure can result from naturally occurring sources, such as inorganic arsenic in drinking water; from occupational exposure; from the ingestion of contaminated food products, such as rice; and from industrial sources sometimes referred to as “arsenic and old waste” (Gorby 1994). The US Geological Survey, in a study of all major aquifers in the United States, estimated that 7% of household wells have arsenic concentrations that exceed the Environmental Protection Agency’s maximum contaminant level (MCL) of 10 ppb, leaving roughly 4.2 million people with the responsibility of taking corrective action themselves (Ayotte et al. 2011). In addition, an unknown number of public water supplies at times exceed the MCL. Moreover, of the 1,320 Superfund sites now on the national priority list (EPA 2013e), 901 name arsenic as a contaminant of concern (EPA 2013f).
There is increasing evidence that dietary inorganic arsenic makes an important contribution to total inorganic arsenic exposure, particularly when inorganic arsenic concentrations in water are low. In the United States, data from the National Health and Nutrition Examination Survey (NHANES) has proved to be extremely useful for the investigation of the food sources of inorganic arsenic and their relative contributions to overall exposure. Probabilistic exposure modeling that used NHANES dietary data from 2003–2004 coupled with duplicate diet and biomarker measurements estimated that the major contributors to dietary inorganic arsenic intake are vegetables (24%), fruit juices and fruits (18%), and rice (17%) (Xue et al. 2010). Indeed, the lay press has recently called attention to the issue of inorganic arsenic in fruit juices and rice (Consumer Reports 2012a,b), creating concern in the US public. Of all the food components of the US Market Basket Survey, rice—particularly rice grown in the south central United States—has the highest concentration of inorganic arsenic (Schoof et al. 1999; Williams et al. 2007). A recent evaluation of data on 2,323 children 6–17 years old who participated in 2003-2008 NHANES studies found that children who reported consuming rice had urinary arsenic concentrations that were nearly 40% higher than concentrations in those who did not consume rice (Davis et al. 2012). Rice consumption has also
been associated with urinary arsenic concentration, including inorganic arsenic and its metabolites monomethyl arsenic (MMA) and dimethyl arsenic (DMA), in pregnant women (Gilbert-Diamond et al. 2011). A recent report also indicates that the meat of broiler chickens fed a diet that included roxarsone, an organic arsenical feed additive that promotes growth and feed use, is a source of dietary arsenic, although it is not clear that the meat arsenic is the inorganic form (Gul Kazi et al. 2013).
Given that a great deal of epidemiologic research concerning the health effects of inorganic arsenic exposure from drinking water has been conducted in South Asian and Taiwanese populations, for whom rice is a dietary staple, the committee recommends that the contribution of inorganic arsenic intake from rice be considered in interpreting findings from those regions. For example, Kile et al. (2007) conducted a duplicate diet study to estimate daily arsenic intake in a region of Bangladesh with varied concentrations of inorganic arsenic in household drinking water. The authors concluded that when drinking-water arsenic exceeded 50 ppb, water was the dominant source of inorganic arsenic intake. However, at water arsenic concentrations less than 50 ppb, dietary arsenic is a more important contributor to overall intake. In the United States, an analysis of 252 participants in the National Human Exposure Assessment Survey-Arizona and Arizona Border Survey with diet, water, and urinary arsenic data concluded that 75% of inorganic arsenic exposure was attributed to diet in households that had arsenic in drinking water at 10 μg/L or lower (Kurzius-Spencer et al. 2013). Thus, whenever possible, exposure to inorganic arsenic from rice, rice flour, and other rice-based foods should be considered in estimating total daily exposure, a practice that has largely been ignored. It is critically important because biomarkers of inorganic arsenic exposure, such as urinary arsenic, capture exposures from all sources. However, the estimate of arsenic intake from rice is problematic because arsenic concentrations in rice vary widely by the type of rice and cooking method and because of variation in per capita rice consumption. The committee suggests that EPA consider a probabilistic approach to estimate daily arsenic intake from rice to account for such variability in both arsenic concentrations in rice and rice consumption.
After ingestion, inorganic arsenic in drinking water is rapidly and almost completely absorbed from the gastrointestinal tract (Zheng et al. 2002). Inorganic arsenic in contaminated soils is more poorly absorbed and varies with the type of soil. In a swine model of soil arsenic bioavailability, the fraction of arsenic absorbed ranged from 7 to 75%, depending on soil type (Juhasz et al. 2007); this information could be of use in situations where the ingestion of soil via pica or ordinary hand-to-mouth activity in children is a concern. The absorption of inorganic arsenic in food is less well studied. However, using a similar in vivo swine model, a study of inorganic arsenic bioavailability in vegetables grown hydroponically in arsenic-contaminated water found that its bioavailability varied between 50 and 100%, depending on the type of vegetable (Juhasz et al. 2008). There is also evidence that the bioavailability of arsenic from ingested rice is high (Juhasz et al. 2006). Rice strains vary in arsenic content and in their relative proportions of inorganic arsenic and methylated arsenic species (Consumer Reports 2012b). A mass-balance study of the bioavailability of rice arsenic in humans involved two volunteers who ate a wheat-based diet and then switched for a week to a rice-based diet that had a known mass (and speciation) of arsenic (He and Zheng 2010). Of all of their dietary items, rice had the highest arsenic content, and 76% of the rice arsenic was inorganic arsenic. The mean urinary excretion fraction was about 58% in one subject and 63% in the other—indicating that a substantial fraction of the rice arsenic was absorbed. Several studies have identified increased concentrations of urinary arsenic metabolites after ingestion of rice, and studies that used more sensitive arsenic speciation methods have found elevated concentrations of urinary inorganic arsenic and DMA in people for whom rice is a dietary staple (e.g., Cascio et al. 2011). Thus, considerations of dose–response relationships derived from epidemiologic studies concerning the health effects of arsenic in drinking water should include the likelihood that the dose derived from drinking water alone does not represent the total arsenic dose.
ABSORPTION, DISTRIBUTION, METABOLISM, AND ELIMINATION
A summary of the evidence on the metabolism of arsenic and the implications for understanding arsenic’s toxicity at low doses was provided at the committee’s workshop by Thomas (2013). The metabolism of inorganic arsenic is complex and leads to the formation of various arsenic species that differmarkedly in toxicity, tissue distribution, and rate of elimination. Inorganic arsenic, both in the trivalent and pentavalent oxidation states, is easily absorbed in the gastrointestinal tract. Absorbed pentavalent arsenic is largely reduced to trivalent arsenic in the blood, particularly after low to moderate exposures (Vahter 2002). Trivalent arsenic is the main form taken up by the hepatocytes (Lerman et al. 1983) and subsequently metabolized to MMA and DMA, both of which may exist in the trivalent and pentavalent oxidation states. Arsenic methyl transferase (AS3MT) is the requisite enzyme, and S-adenosyl methionine serves as the methyl donor (Lin et al. 2002) (Figure 2). Arsenical thiols may also form (Fricke et al. 2005), but there is less evidence of their occurrence in human urine (Raml et al. 2007), perhaps in part because of their oxidation once eliminated (Currier et al. 2013). In short, human tissues, blood, and urine contain a mixture of arsenic metabolites that vary in acute and chronic toxicity.
There is strong evidence from cell and animal-model systems that the trivalent species of inorganic arsenic, MMA, and DMA are far more toxic than the less reactive pentavalent species. MMA(III) has been shown to be particularly cytotoxic in human cell cultures (Styblo et al. 2000). The extent to which DMA(V) can be reduced to the more toxic DMA(III) is unclear although there is some evidence that DMA(III) is also found in human biologic samples (Valenzuela et al. 2005). The retention of arsenic in tissues is influenced by a host of factors, particularly methylation capacity. In that regard, recent work with the AS3MT knockout mouse has been extremely revealing in that it clearly demonstrates the importance of arsenic methylation in facilitating elimination and decreasing tissue retention (Drobna et al. 2009).
Collectively, inorganic arsenic and its metabolites have many targets of toxicity and carcinogenicity. For example, IARC (2012) lists the lung, urinary bladder, and skin as known targets and the prostate, liver, and kidney as three probable targets for carcinogenicity. Chapter 4 of the present report describes many other organ systems in which noncancer toxicity is manifested. Tissues vary extensively in their arsenic methylation efficiency (Kobayashi et al. 2007), which probably affects their susceptibility to toxic insult. However, the cellular uptake of the various arsenic metabolites and the intracellular distribution and extrusion of them might also vary extensively among tissues and contribute to the variation in toxicity (e.g., Dopp et al. 2010). Those variations imply that the mode of action of inorganic arsenic might depend on the type of tissue, as well as exposure factors. Predictions of tissue concentrations of inorganic arsenic and its metabolites may be obtained by using physiologically based pharmacokinetic models, although human data to parameterize such models is limited (El-Masri and Kenyon 2008).
The relative toxicity of the various arsenic metabolites is related to both their inherent reactivity and their circulating half-lives. The administration of a single oral dose of radioactive 74As as the trioxide to human volunteers revealed half-lives of 2, 10, and 38 days (Pomroy et al. 1980); because some arsenic distributes to bone (Lindgren et al. 1982), it is conceivable that with chronic long-term exposure there is an even longer half-life at a steady state. Indeed, a recent report that describes a case of osteoresorptive arsenic intoxication in a 47-year-old woman who had osteoporosis and had been exposed to arsenic as a child provides support for earlier animal studies that indicated that bone can be a reservoir of inorganic arsenic (arsenate) from which arsenic can be released later in life (Dani 2013). Buchet et al. (1981a) administered 500 μg of arsenic as arsenite, MMA, or DMA to human volunteers. Within 4 days, urinary excretion totaled 46, 78, and 75% of the administered dose, respectively. Another study by the same investigators administered sodium meta-arsenite (NaAsO2) to volunteers at various doses up to 1 mg/day for 4 days and reported that arsenic methylation was not saturable even at the dose of 1 mg/day (Buchet et al. 1981b).
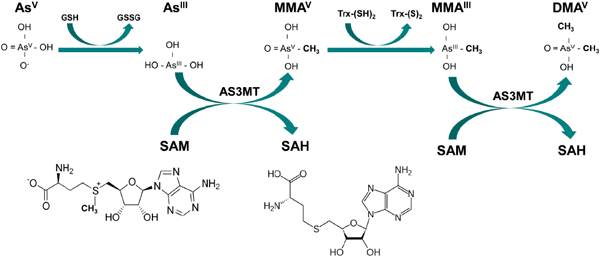
FIGURE 2 Arsenic metabolism: Inorganic arsenate (AsV) can be reduced by glutathione (GSH) or other reductants to yield inorganic arsenite (ASIII) and glutathione disulfide (GSSG). AsIII is converted to pentavalent monomethylarsonic acid (MMAV), a reaction catalyzed by arsenic-3-methyltransferase (AS3MT), with S-adenosyl methione (SAM) serving as the methyl donor; in the process, SAM is hydrolyzed to S-adenosyl homocysteine (SAH). After the reduction of MMAVto MMAIII by thioredoxin (Trx), a second methylation step result in the synthesis of dimethylarsinic acid (DMAV). Source: Figure courtesy of Megan Hall.
People vary widely in their ability to metabolize inorganic arsenic, as reflected by the widely varying proportions of inorganic arsenic, MMA, and DMA in urine and blood. Women are more efficient than men in converting inorganic arsenic to DMA, particularly during pregnancy, when arsenic metabolism is facilitated (Vahter et al. 2006), and children appear to methylate arsenic as well as adults (e.g., Wasserman et al. 2004). There is consistent evidence from epidemiologic studies in populations exposed to high arsenic concentrations in drinking water that people who can efficiently convert inorganic arsenic to DMA are at lower risk for arsenic-induced disease than those who are “poor methylators”, who have higher proportions of MMA and inorganic arsenic in urine. In particular, in comparison with those who have a low proportion of MMA (III + V) in urine, those who have a higher proportion have been observed to be at higher risks for heart disease, atherosclerosis, peripheral vascular disease, hypertension, and bladder, lung, and skin cancer (reviewed in Steinmaus et al. 2010). Those findings are derived from studies in Taiwan, Argentina, and Bangladesh. There is compelling evidence that arsenic metabolism and toxicity vary because of genetic factors (see discussion in Chapter 5).
Thus, the hazard assessment of oral exposure to inorganic arsenic should take into account the fact that some people are more susceptible than others because of their relative inability to metabolize arsenic. Susceptibility factors, discussed more fully in Chapter 5, include life stage, genetics, sex, nutritional characteristics (such as folate, selenium, and body-mass index), pre-existing diseases, smoking, alcohol consumption, and exposure to mixtures of chemicals.
With regard to animal studies, it is important to note that the metabolism of inorganic arsenic varies widely among species (Vahter et al. 1995a; Vahter 1999; Loffredo et al. 2003). Dogs and mice, for example, are extensive methylators of inorganic arsenic and excrete 80% or more of a given dose as DMA in urine and less than 5% as MMA. Humans excrete more MMA on the average than other species, and this suggests that humans may be poorer methylators of inorganic arsenic. There is extensive evidence from cellular and animal models that MMA (III) and DMA (III) are more toxic than inorganic arsenite (e.g., Styblo et al. 2002), although the in vivo toxicities reflect the overall balance both among forms of arsenic and the extent and rate of its elimination. Arsenic excreted by humans tends to be 10-20% MMA, whereas that of dogs, hamsters, mice, rabbits, and rats is 1-5% MMA (Vahter 1999). Guinea pigs, marmoset monkeys, and chimpanzees do not methylate inorganic arsenic (Vahter 1999). Several species metabolize inorganic arsenic to a trimethyl form as well, but this form has not been observed in humans. After ingestion of DMA, however, a few percent of the arsenic excreted in the urine was in the form of trimethylarsine oxide (Marafante et al. 1987).
Finally, considerable work in mice has described the tissue distribution of various arsenic metabolites. For example, the oral administration of radiolabeled arsenate (73As) led to accumulation in organs known to be targets for inorganic arsenic–induced cancers (bladder, kidney, skin, and lung). MMA was found in all organs except the bladder. In the kidney, inorganic arsenic was the predominant species (Hughes et al. 2003), possibly because arsenate undergoes renal tubular reaborption via the phosphate transporter (Ginsberg and Lotspeich 1963; Ginsberg 1965). Bladder and lung had the highest percentage of DMA; this is of interest in that mouse studies of DMA suggest that it might be a tumor promoter, albeit at concentrations of 10-100 ppm, concentrations that are not relevant to human exposures (reviewed by Hughes et al. 2003). It is notable that in AS3MT knockout mice, which have markedly impaired arsenic methylation, dramatic cytotoxic urothelial cell changes occur within hours after the administration of sodium arsenite (Arnold et al. 2013).
There are various ways to estimate exposure to environmental chemicals. Environmental sampling can be conducted in air, water, soil, house dust, and food to estimate exposure. To estimate exposure, statistical models can be used to relate environmental concentrations to internal dose, estimated over various time frames, but such processes rely on an assumption that environmental concentrations correlate with amounts of arsenic ingested and inhaled (e.g., Nasreddine and Parent-Massin 2002). A more direct way to
assess exposure to arsenic from all sources is to assess biomarkers of internal dose, although such measures also have limitations, as discussed below. The term biomarkers can refer to analytic measures that reflect mechanistic biologic effects of an environmental exposure or reflect an estimate of the internal dose of exposure to an exogenous agent. This section will concentrate on the latter type of biomarker with regard to arsenic. It should be noted that whether using environmental measures of exposure or biomarkers of exposure, similar issues arise regarding the constancy or variability of exposure, population mobility, the relevant period of exposure (e.g., long latency would make exposures long in the past the relevant information), and other factors, so repeated measures over time are valuable.
The most commonly used biomarker of arsenic exposure is total arsenic in urine. Arsenic measured in urine is derived from recent exposures, largely the previous day, so consideration of whether exposures are constant or changing over longer durations involved in the development of arsenic-induced toxicities is needed. Total arsenic includes some forms of organic arsenic commonly occurring in various seafoods, such as arsenobetaine, that have short half-lives and little or no toxicity. The speciation of arsenic metabolites in urine is thus necessary to separate the contribution of arsenobetaine from fish and seafood from the metabolites of inorganic arsenic. After exposure to inorganic arsenic, the proportions of arsenic metabolites excreted in human urine are typically 10-30% inorganic arsenic, 10-20% MMA, and 60-70% DMA (Vahter 2002). A limitation of the use of urinary arsenic is that altered renal function might influence the extent to which the various arsenic metabolites (and creatinine) are excreted. If the urine collection is not a timed collection over 24 hours, it is essential to adjust the measured urinary arsenic concentrations for variation in urine dilution, which may vary widely. In that respect, creatinine excretion has been associated with arsenic methylation efficiency in several studies (Gamble et al. 2005; Nermell et al. 2008; Basu et al. 2011). Barr et al. (2005) has pointed out that using urinary creatinine to create a ratio with the urinary chemical concentration can introduce bias because of the undue influence that high or low ratios can produce. In recent years, the most common practice is to use urinary creatinine concentration as a covariate in a regression analysis to reduce this effect. Creatinine excretion also varies with age, sex, meat intake, and muscle mass. Adjusting by specific gravity is a useful alternative to creatinine adjustment, in that it is less influenced by those factors (Nermell et al. 2008). Several studies have examined interindividual and intraindividual variability in urinary arsenic measurements and have concluded that total urinary arsenic is relatively stable but that the concentrations of urinary arsenic metabolites are not (e.g., Calderon et al. 1999; Hopenhayn et al. 2003a; Steinmaus et al. 2005; Kile et al. 2009). Such studies have shown that pregnancy, life stage, and disease can influence metabolism.
Several other types of exposure biomarkers often used in human population studies involve primarily inorganic arsenic (Button et al. 2009). Among them are hair arsenic and toenail arsenic. Because arsenic accumulates in tissue that are rich in the protein keratin, such as hair and nails (Mandal et al. 2003; Raab and Feldmann 2005), these tissues can be used as matrices for exposure biomarkers. Measurements taken from the tissues are generally indicators of past exposure to arsenic, in contrast with urinary measurements, which reflect more recent exposure, although the period of exposure captured by these tissues can still be short compared with the latency periods between exposure and some arsenic-induced effects. Internal deposition of inorganic arsenic into hair and nails occurs through blood flow, but these structures are themselves external, so environmental contamination must be considered. Various cleaning techniques are used to remove external contaminants. They are adequate for toenails, but removal may not be complete, particularly for hair samples, which are more porous, or in populations that wear open footwear or go barefoot. Unlike blood and urine, these biomarkers can provide information on the timing and dose of past exposure. Hair grows at an average rate of 1 cm per month, so the 1 cm of hair nearest the scalp should correlate with the integrated level of exposure over the last month. Segmentation of hair in 1-cm blocks can provide repeated measures of exposure at monthly intervals. Because of the growth rate of toenails, the lag time between the formation of living nail (found in the cuticle area) and the clipped nail at the end of the toe takes about 6-12 months; thus, measures of toenail arsenic represent past exposure (Karagas et al. 2000; Slotnick and Nriagu 2006). There is evidence that toenail concentrations were fairly consistent over a period of several years (Garland et al. 1993; Karagas et al. 2001a). Fingernails grow
faster and can yield information on exposure about 6 months in the past. Thus, consideration should be given to the exposure metric’s relevance to health outcomes with respect to their latency.
Finally, since the advent of sensitive inductively coupled plasma mass spectrometry techniques, blood arsenic has been used in recent years but remains the least used biomarker. In particular, speciation of arsenic in blood is more complicated than that in urine. In at least two studies in Bangladesh, total arsenic in blood was found to be significantly associated with urine and water arsenic and with risk of skin lesions (Hall et al. 2006; Y. Chen et al. 2007a). Whole-blood arsenic would be most reflective of acute exposure; however, as with any biomarker of chemical exposure, chronic low-dose exposure would eventually lead to a steady state. Such a situation could exist in the case of drinking-water contamination or dietary exposure. Limitations of the methods of measuring blood arsenic include the relatively narrow range of values (in comparison with urine), its high cost, and its failure to exclude the relatively nontoxic species of arsenic derived from seafood. Its strength is related to its biologic proximity to organs of concern and a reduced risk of external contamination compared with hair and nails. Arsenic metabolites in blood differs from those in urine in that there is a substantially lower proportion of DMA (because of its shorter circulating half-life) and relatively more inorganic arsenic and MMA (Hall et al. 2006).
In summary, measurements of arsenic in urine, blood, toenails, and hair have been used as biomarkers of exposure to inorganic arsenic in various epidemiologic studies. The extent to which these biomarkers have been “calibrated” to external exposure to inorganic arsenic varies, and this sometimes poses a challenge in the interpretation of findings. Nevertheless, the Integrated Risk Information System assessment can benefit from careful examination of studies in which estimates of both external exposure (such as water concentrations) and biomarker data are provided.







