In comparison with most other chemicals evaluated by the Integrated Risk Information System (IRIS) program for hazard identification and dose–response assessment, there is a wealth of epidemiologic evidence on both cancer and noncancer end points after oral exposure to inorganic arsenic. The focus of this dose–response analysis chapter is on specific issues around the use of the epidemiologic data. As noted previously, the scope of the present National Research Council study did not include exposure by inhalation or dermal pathways, but issues addressed here could be applicable to epidemiologic studies involving those routes. On the basis of the extensive epidemiologic data, the committee expects that the results of animal and in vitro mechanistic studies will facilitate the understanding of the biologic plausibility or mechanisms of arsenic causation of effects observed in epidemiologic studies and interpretation of low-dose effects rather than be the focus of the dose–response analysis. Due consideration of the marked species differences in arsenic kinetics and toxicity would have to be factored into analyses using animal data.
The draft Environmental Protection Agency (EPA) development plan for the IRIS assessment of inorganic arsenic indicates that multiple cancer and noncancer end points will be evaluated and that dose–response analyses for the end points will be developed when feasible. The committee supports that approach, particularly in light of the substantial growth in epidemiologic information about a variety of noncancer end points. The implications of arsenic exposure for public health are much broader than the historical focus on cancer and skin lesions.
The following sections provide perspectives and recommendations on topics that the committee judged would play important roles in the dose–response analyses for health end points associated with inorganic arsenic. These considerations are specific to analyzing inorganic arsenic and may not be applicable or appropriate for other chemical assessments.
DOSE–RESPONSE ANALYSIS FOR NONCANCER AND CANCER END POINTS
The committee recognizes that quantitative dose–response analyses are one of the major functions of the IRIS program and that the extrapolations involved in this process are among the most controversial aspects of arsenic risk assessment. There has been a substantial expansion of epidemiologic studies of associations between arsenic exposure and a variety of health outcomes, including cardiovascular disease, diabetes, nonneoplastic respiratory changes, skin lesions, pregnancy, child development, skin cancers, bladder cancers, and lung cancers; the studies increasingly characterize risks of the health outcomes at low to moderate arsenic exposures.
Therefore, the committee recommends that EPA evaluate data on multiple outcomes to assess whether they are appropriate for direct estimation of risks of health outcomes in the range of epidemiologic observations. Such analyses would be undertaken as appropriate given the methods in the epidemiologic studies and as consistent with EPA technical guidance for evaluating dose–response relationships in the range of observations, such as with model-fitting approaches described in the EPA Benchmark Dose Technical Guidance (EPA 2012). Existing data may allow risk close to the range of background exposures for inorganic arsenic to be estimated directly from the epidemiologic findings, although limitations of the epidemiologic data will need to be considered; analyses by the European Food Safety Authority may constitute a useful example
(EFSA 2009). Given the importance of maximizing the understanding and credibility of the health risks associated with arsenic and moving protection of public health beyond debates over the shape of the dose–response curve below the range of observation of positive findings for selected cancer end points, the committee believes that a critical review of the existing data and a focusing of attention of the IRIS program and its many stakeholders on the observed evidence for arsenic health effects is essential.
Background concentrations of arsenic exposure vary, but the committee judged that urinary arsenic at 1–5 μg/L (summing inorganic, monomethyl, and dimethyl arsenic forms) was a reasonable estimate for the US population for purposes of the present report (Karagas et al. 2001b; Navas-Acien et al. 2009b; Zheng et al. 2013). That range was estimated on the basis of published epidemiologic studies, current work that committee members were aware of, and values that are obtained from the National Health and Nutrition Examination Survey (NHANES) that exclude populations that consume substantial amounts of fish (to eliminate a large contribution by arsenobetaine and other seafood arsenicals in the NHANES total urinary arsenic measurements) (Navas-Acien et al. 2011). Background concentrations would exclude substantial exposure to arsenic from drinking water and so would arise from a variety of sources that potentially include very low concentrations in water, diet, dust, and other sources. The committee does not assume that the background concentrations are with or without health effects; rather, it assumes that the needs of assessing health risks can be facilitated by characterizing dose–response relationships down to the background concentrations by using observed data.
The committee recommends that EPA develop risk estimates for the variety of health effects on which there is adequate epidemiologic evidence and then derive risk-specific doses to address the needs of analyses that would typically use a reference dose (RfD). A risk-specific dose for a noncancer end point is an estimate of the dose associated with a degree of risk based on the dose–response function for that end point (NRC 2009). In the committee’s workshop, Sande (2013) noted that stakeholders, such as state agencies, have used risk-based estimates only for cancer end points and would need some guidance in implementing the use of risk values for noncancer end points. Thus, EPA should provide guidance on how an RfD might be selected from among the risk-specific doses on the basis of such factors as end-point severity, interindividual variability, and policy considerations (NRC 2009). Candidate values for multiple end points will provide the basis for conducting cumulative risk assessment of chemicals that have common adverse outcomes, even when the end points are not the most sensitive. By providing both the dose–response function and a series of risk-specific doses, the IRIS assessment for inorganic arsenic will go beyond typical IRIS assessments, which develop a single RfD, and will facilitate an array of analyses, including cumulative risk assessment, benefit–risk ratios, and other comparative and economic analyses.
For both cancer and noncancer end points, consideration of the methods used in the epidemiologic study analyses will be important in developing the dose–response analysis in the observed range. For example, it may be preferable to consider exposure–response relationships by using categorical associations rather than the continuous data because they allow flexible evaluation of the dose–response relationships and do not simply assume that they are linear. Selection of the model should consider appropriate statistical methods for alternative models and biologic considerations. The IRIS program will also need to consider the extent to which published information can be used and whether it would be beneficial to collaborate with study authors to obtain study data. Choices made throughout these processes need to be clearly explained to provide transparency.
In the range of lower drinking-water exposures, the contribution of dietary sources of inorganic arsenic can be important or even dominant, so the total oral dose of inorganic arsenic needs to be considered regardless of source, as discussed previously. The committee recognizes that that is a difficult challenge. To address it, the committee recommends that EPA consider study-selection options to facilitate the dose–response analyses. Preference would be given to studies that best characterize exposures in the low to moderate range by measuring arsenic-exposure biomarkers (such as urinary inorganic arsenic and its methylated metabolites), which reflect exposures regardless of pathway or source, while considering standards for such biomarkers (Altar et al. 2008). Lower preference would be given to studies that characterize only water exposures although effects observed at moderate to higher exposure, in which water dominates, could be used by carefully considering likely dietary exposures and their influence on the lower end of the dose–response
curve. For example, a National Research Council (NRC 2001) report carried out a sensitivity analysis of the effect of alternative assumptions concerning dietary exposures on risk estimates. A challenge that the IRIS program will need to address in using these studies is that they have characterized arsenic exposures by using different metrics (such as urinary arsenic or water concentrations), but a common exposure metric is needed to integrate the information across epidemiologic studies or with in vitro or animal data to inform dose–response assessment. Measures of internal doses would be desirable and could be obtained from pharmacokinetic models, but it may be necessary to use a simpler analysis based on oral dose. The IRIS assessment will need to provide a clear description of the approach that is used to convert reported exposures in the studies to whatever common metric is employed. Other factors will also affect which lower-dose studies are ultimately chosen for dose–response assessment and how they are used, including numbers of subjects, methods of end-point assessment (such as odds ratios and standardized mortality ratios), control for confounders, potential for exposure misclassification to lead to underestimation or overestimation of risks, and other factors that could affect study interpretation and sensitivity to detect a low-dose effect. Finally, the committee notes that EPA will consider epidemiologic studies that involve various exposures, some of which will be fairly high and others of which will span a broad range; some studies will focus on low to moderate exposures. The quality of the studies—assessed on the basis of their exposure assessments, their potential for bias or confounding, and many other methodologic details—will determine which ones are used in the dose–response analyses. Although the committee sees new opportunities in the growing database of studies that include low to moderate exposures, characterizing the dose–response relationship over the broad range of exposures available might be useful, particularly for assessing sensitive populations and interaction with other risks factors (as discussed in the committee’s workshop by Cantor [2013]).
If health-assessment needs cannot be fully met with modeling of the data in the range of observation, the committee recommends that EPA take one of the following approaches and provide the rationale for its choice:
• The preferred approach would be to extrapolate from the dose–response relationship in the range of epidemiologic observations by using mechanistic data and models to define the individual and population risks and their associated uncertainty on the basis of analyses of adverse outcome pathways or modes of action and human variability in susceptibility to arsenic-induced effects (see Chapter 6). This approach could describe human pharmacokinetics, biomarkers of exposure ( such as urinary or toenail measures), tissue doses of relevant arsenic forms (such as monomethyl arsenic), and the multiple toxicodynamic processes that lead to the adverse outcomes for which extrapolation below the range of observation is desired. It would appropriately account for any dose-dependent transitions in the mechanisms of toxicity that exist (Slikker et al. 2004a,b).
• If the preferred extrapolation approach is judged to be unfeasible without additional data and modeling, the basis for that judgment needs to be provided. As an alternative, the committee recommends that EPA evaluate the dose–response relationship in the range of epidemiologic observation for a given health end point by using, if it is feasible, multiple reasonably fitted models and then extrapolating at most over a short distance below that range by using the modeled curve shape approaching the low range of observations (see Box 7 for example). Fitting of alternative models could indicate how far below the range of observation the extrapolation is essentially independent of model choice and provide greater confidence in the extrapolation to that point. Model averaging approaches might also prove useful (Morales et al. 2006; Wheeler and Bailer 2009). Nonmechanistic extrapolations further from the range of observation may be increasingly likely to overlook dose-dependent transitions that would substantially alter risk estimates (Slikker et al. 2004a,b). It should be considered whether mechanistic and biologic information can inform this process, and all choices in the process should be clearly explained. It would be reasonable for the IRIS assessment to stipulate the range over which the dose–response relationship derived for cancer or noncancer end points is useful for risk assessment and ranges in which it is considered inapplicable. Because epidemiologic observations have extended to lower doses in recent years, their utility for some end points will extend into the range of background exposure, so extrapolation may not be necessary.
BOX 7 Illustration of Proposed Strategy for Estimating Risk at Low Doses
The various approaches presented and implications for risk assessment are for illustrative purposes only, and no risk estimates should be inferred from the examples. To illustrate a strategy for estimating risk at low doses, an example is provided on the basis of hypothetical data on cardiovascular-disease mortality in relation to arsenic exposure (Table A). In this example, the relative risks (RRs) based on the dataset were adjusted for appropriate covariates, but additional data are not available.
TABLE A Summary Data on Cardiovascular-Disease Mortality from a Hypothetical Cohort Study
|
|
||
| Urinary Arsenic, µg/g of creatinine | Midpoint | Relative Risk |
|
|
||
| <5 | 3.5a | 1.0 |
| 5-10 | 7.5 | 1.05 |
| 10-15 | 12.5 | 1.2 |
| >15 | 18 | 2.2 |
|
|
||
aWithout additional information about the urinary arsenic concentrations below 5 μg/g of creatinine, a midpoint was set to 5/√2, following guidelines for adjusting for limits of detection.
The committee’s proposed strategy follows four steps:
Step 1: Fit a general nonlinear model to observed data. The nonlinear model can accommodate a linear fit if supported by the data. Evaluate the goodness-of-fit of the model to the observed data graphically or more formally with a statistical test.
Step 2: Test the hypothesis for linearity.
Step 3: Extrapolate below the observable range to the range of interest (roughly an order of magnitude in this example).
Step 4: Obtain an estimate of RR at low doses within the range of extrapolation.
In the hypothetical data, the reference group (Table A) had urinary arsenic concentrations below 5 μg/g of creatinine. The model is first fitted with this group represented at a “midpoint” value similar to what is commonly used for values below the limit of detection (LOD/√2). A comparison is made with the risk estimate when the model did not include the reference group with a value of RR = 1; this results in a negligible change. The results of models without the reference group are presented below.
Step 1: Fit a nonlinear exponential model. The model is fitted to the mean RR as a function of arsenic (x) constrained to a mean of RR of 1.0 as x approaches 0:
![]()
On the basis of visual inspection, the predicted model adequately fitted the observed RR estimates (Figure A). Two vertical reference lines are included in the figure to denote the region of observed data and the extrapolation region. The slope parameter was positive and significant (p <0.001), indicating that RR increases as baseline urinary arsenic increases.
A nonlinear logistic model was fitted to the same data for comparison (results not shown). There was a negligible difference in the prediction of RR in the extrapolation region when an exponential or logistic nonlinear model was used. The estimated model and resulting confidence intervals presented herein are based only on the RR estimates and not the variability around the estimates. When the numbers of cases and controls are available for each group, biologic variability can be incorporated into the analysis.
Step 2: Test for linearity. The nonlinear model in equation (1) is linear with an intercept of 1.0 and a slope of exp(β0) when β1 = 1. Thus, a test of linearity is based on comparing the model in (1) to the (reduced) linear model by using a likelihood-ratio statistic compared with a chi-squared distribution with one degree of freedom. The hypothesis of linearity was rejected (p < 0.001).
Step 3: Extrapolation below the range of observation. The predicted nonlinear model in the observable range can be used for extension of the curve to the region of interest (Figure A). For comparison, the figure provides a benchmark-dose (BMD) analysis (green bar) of the data as represented by the point of departure and the use of a benchmark response of RR = 1.1.
Step 4: Estimate low-dose risk. To obtain an estimate of RR with low doses (as represented by the reference group), one could use this committee’s recommended approach of extending the dose–response curve below the range of observed data points and into the “reference” population exposure levels. If the predicted model of equation (1) is used, the estimated RR at a baseline urinary arsenic concentration of 0.75 μg/g of creatinine is 1.0000003. Thus, the extrapolation curve provides a probabilistic estimate of arsenicinduced RR of cardiovascular-disease mortality for exposures between 0.75 and 7.5 μg/g of creatinine that is consistent with the data in the observed range and allows the devel-opment of a candidate RfD on the basis of a risk-specific dose within the extrapolation range. If an RfD already exists, EPA would be able to estimate the RR associated with it (assuming that it falls within this range) and the benefit of mitigating exposure in the range of the RfD.
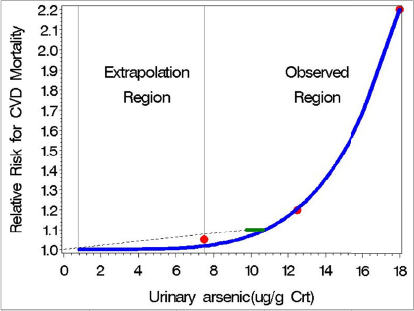
FIGURE A Hypothetical observed (red dots) and model-predicted mean relative risk (RR) for cardiovascular-disease mortality (blue curve) as a function of urinary arsenic (data presented in Table A) on the basis of a nonlinear exponential model. The model is parameterized to approach a mean RR of 1.0 as urinary arsenic approaches 0 but is extrapolated only 1 order of magnitude from the midpoint of the lowest group (excluding the reference group). The green bar represents the 95% one-sided confidence interval on the BMD associated with RR = 1.1. The dashed line connects the BMDL with the origin, in accordance with the traditional low-dose linear-extrapolation approach for cancer.
Those recommendations differ from some of the approaches outlined in EPA’s draft development plan for the toxicologic review of inorganic arsenic. EPA proposes to use linear low-dose extrapolation as the default for cancer and noncancer effects. The committee proposes limited extrapolation by using the modeled shape of the dose–response relationship to provide a data-informed estimate of the potential dose–response relationship below the range of observation. Risks posed by background concentrations of arsenic should be characterized to the extent feasible, but in the absence of a sound mechanistic understanding of dose–response relationships, further extrapolation to lower exposures does not appear useful or pragmatic. EPA could use different models and present the risk estimates that would result to illustrate the magnitude of the impact of selecting one model over another. Justification should be provided for the chosen model, including any policy considerations that were factored into the decision.
EPA’s plan discusses additivity to background disease processes, which the National Research Council report (NRC 2009) also discussed. Interpretation of this issue requires careful scientific justification and involves an understanding of adverse outcome pathways and their interactions with multifactorial disease processes at a level that would probably allow extrapolation based on a quantitative mode-of-action description (the preferred option noted above). Finally, EPA’s plan focuses on characterizing uncertainty in the selected dose–response model rather than acknowledging that much greater uncertainties are generally associated with the issues of selection of alternative models. As noted in the second extrapolation option above, comparisons of results of alternative models are useful for learning how model-dependent the extrapolation results are and the uncertainty in them.
The committee is aware that there will be continued publication of epidemiologic studies of low to moderate exposures over the next several years. By focusing on the dose–response relationship in the range of observation, it will be easier for EPA to update selected components of the dose–response assessment as additional information becomes available. Substantial time is required to draft an IRIS assessment and complete the review process, so inevitably an assessment will become out of date. The committee recommends that EPA update selected health outcomes as new information becomes available. It is likely that within the 2–3 years that it takes to develop the assessment, updating will be useful.
The committee’s recommendations are made specifically for the IRIS assessment of inorganic arsenic and should not be interpreted as applying to other IRIS assessments, given the robust epidemiologic data available related to exposures approaching background. Animal and in vitro studies have important roles to play in evaluating arsenic health risks by providing controlled experimental findings relevant to causality, mode of action, tissue dosimetry of specific metabolites, and other aspects of arsenic toxicology; they are not the focus of the dose–response analysis in the way that they are for many other chemicals. In addition, given the number of populations that are exposed to arsenic, epidemiologic data are increasingly available on concentrations directly relevant to the risk assessment. That is the case for a number of other chemicals, such as lead, ozone, and particulate matter. But in other cases, the available epidemiologic studies may involve exposures substantially higher than typical environmental exposures (for example, high occupational exposures), so analyses in the range of observations would not provide an adequate dose–response characterization for the needs of the IRIS program. Thus, some of the approaches recommended here for evaluating inorganic arsenic would not be feasible for many other chemicals.
A critical issue in pooling epidemiologic studies for arsenic risk assessment for US populations is the relevance of arsenic concentrations in drinking water of the study populations and the evaluation of dose–response relationships. Meta-analytic approaches can be used to summarize reported results related to different magnitudes of exposure and to investigate the shape of the dose–response relationship (Rota et al. 2010). Available meta-analyses for arsenic-related disease have generally categorized the estimation of pooled estimates by dividing the studies of populations exposed to high arsenic concentrations (mean in drinking water above 50 μg/L, above 100 μg/L, or even above 150 μg/L) from studies of populations exposed to low to moderate concentrations (mean in drinking water less than 100 μg/L). That is a relatively simple technique that allows estimation of the association between arsenic and disease at relevant magnitudes of exposure, but it does not allow investigation into the shape of the dose–response curve across the range of exposures found in human populations. No dose–response meta-analysis has been conducted for arsenic-related disease. Multiple dose–response meta-analyses are, however, available in the environmental and public-health literature. For instance, dose–response meta-analyses have been conducted to evaluate occupational exposure to benzene and the risk of leukemia (Vlaanderen et al. 2010), the association of alcohol with esophageal squamous-cell carcinoma (Rota et al. 2010), and the association of selenium intake with cardiovascular disease (Flores-Mateo et al. 2006). Those meta-analyses could serve as examples for estimating the dose–response relationship of arsenic with relevant health end points, such as cancer and cardiovascular disease.
A number of methodologic issues need to be considered in conducting a dose–response metaanalysis. For studies reporting at least three category-specific relative risks and confidence intervals, the standard method of analysis is to fit a linear regression through the origin (reference category) weighted by using the estimated inverse variance of the log-relative risk. Alternative methods are used when the assumption that all relative risks in each study are independent is not met (Greenland and Longnecker 1992; Berlin et al. 1993; Orsini et al. 2012). In addition to linear dose–response meta-analyses, methods have been developed to evaluate nonlinear dose–response relationships by incorporating flexible splines, such as natural splines or restricted-cubic splines (Vlaanderen et al. 2010; Orsini et al. 2012). Those flexible methods provide excellent opportunities to evaluate the shape of the dose–response curve in epidemiologic studies across a wide range of arsenic exposure concentration although uncertainties may become greater at lower exposures, so careful consideration should be given when applying the methods. Although the direct incorporation of mode-of-action data into the analysis of dose–response data from human studies is challenging, biologic-response data could potentially inform the conduct and interpretation of the dose–response analysis on the basis of data from epidemiologic studies.
SENSITIVE POPULATIONS AND LIFE STAGES
IRIS assessments have long considered sensitive populations and life stages for noncancer end points but less frequently for cancer, although the Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens provided guidance on chemicals acting through a mutagenic mode of action (EPA 2005a). Epidemiologic studies of arsenic provide some evidence concerning sensitive life stages and populations in part owing to the unusual “natural experiments” that have resulted in relatively short well-defined periods of high exposure, as in northern Chile, or relatively well-defined times of using arsenic-contaminated water, such as the wells in Bangladesh. Life stages refers to different ages and development stages throughout life (such as infancy, pregnancy, and old age), whereas populations refers to groups of people who may have diverse or shared characteristics (such as genetic variations and phenotypic variations). The objective of IRIS assessments is to protect the general population, including those who may be more sensitive to toxicity induced by a chemical, particularly in comparison with those who were evaluated in the available epidemiologic studies. That implies assessing the potential health risks posed by arsenic in both men and women and considering the evidence of sex differences in both kinetics and susceptibility. As described throughout the health-effects sections presented earlier in this report, a number of studies and end points suggest that exposures during specific life stages need to be considered in the IRIS assessment and in the use of IRIS dose–response values in risk assessments.
The approach to assessing less than lifetime exposures for cancer risk from nonmutagenic carcinogens is typically to prorate the risk equally regardless of age; if exposure were for 10 years of a lifetime of 70 years, the lifetime cancer risk would be reduced by a factor of 1/7. There is an alternative approach for chemicals that have a mutagenic mode of action, which may not be the case for arsenic (Nesnow et al. 2002). Some findings with arsenic in humans and animals suggest that the appropriateness of the prorating approach needs to be considered particularly for early-life exposures. Although the Chilean studies may not be the best for characterizing the dose–response relationship, because they involve essentially a single high-concentration exposure for a specific duration, the committee recommends that these studies and others that yield evidence of life-stage sensitivity, including mechanistic studies, be considered in determining an appropriate approach for evaluating less than lifetime exposures. Although extremely high water concentrations were used in the early-life cancer studies of rodents, the consistency of the finding with the findings of epidemiologic studies provides at least supportive information (Tokar et al. 2010c, 2011b). The IRIS program needs to consider carefully the studies used to evaluate the cancer dose–response relationship, as well as the evidence of differential life-stage sensitivity, to ensure that both are appropriately addressed without inadvertently “double-counting”. For example, if the epidemiologic dose-response relationships are characterized for populations exposed throughout life, including pregnancy and early life, the higher risks encountered in that period would be built into the overall dose–response relationship.
Some reports of early-life sensitivity to some noncancer effects—such as those found in studies of cardiovascular disease, infections, lung function and disease, and body size—have been discussed previously (Smith et al. 2006, 2011; Dauphiné et al. 2011; Rahman et al. 2011). Depending on the dose–response analysis for the different noncancer end points, the approach for addressing sensitive populations in the risk estimates may vary. The IRIS program could provide approaches for addressing those subpopulations given the derivations used for the IRIS toxicity values. For example, if risks arise from a specific window of susceptibility, they could indicate the appropriate period for the exposure assessment to be consistent with using a particular toxicity value.
The success of the IRIS assessment of inorganic arsenic will depend in part on its usefulness to IRIS partners in EPA and to stakeholders, including state, local, and tribal agencies throughout the country and internationally. The committee recommends a number of approaches that are not typical of IRIS assessments, such as providing risk estimates for noncancer outcomes for which a risk-specific dose could be derived. To ensure that a wide array of users can understand and apply the values derived in the inorganic arsenic assessment, it will be essential to implement recommendations offered by previous National Research Council committees, notably the report on the draft IRIS assessment of formaldehyde (NRC 2011).
The IRIS program has to address two major needs beyond the general need to create a well-organized document with clear summaries and supporting materials placed in technical appendixes to provide detail. The first is to advance the ability of users of the IRIS assessment to understand the scientific basis of its decisions so that they can evaluate them from the perspectives of the programs that partners and stakeholders are addressing. The second is to provide examples of how values might be used if they are different from those typically derived by the IRIS program; these would be particularly valuable if nonlinear analyses for cancer or dose–response characterization of risk for noncancer end points are used in the IRIS assessment. As stated above, the committee judges that a continuous dose–response function for noncancer end points based on the epidemiologic database is feasible and scientifically defensible for inorganic arsenic. For users unfamiliar with measures used in epidemiologic studies, ib contrast with toxicologic studies, it will be valuable to provide careful explanations and reference materials to ensure understanding of the dose–response analysis. An acceptable daily exposure level, such as an RfD, may be needed in some settings. Thus, the committee recommends that EPA derive a series of risk-specific doses based on the dose–response relationship for a given end point and provide guidance for choosing an RfD. Furthermore, EPA should stipulate the range over which the dose–response slope is reasonable for use in risk assessment and the exposure range over which the slope might be different or stipulate that the uncertainty is too great to allow a slope estimate. These features should, on the one hand, improve the flexibility and utility of IRIS values (for example, use for multiple end points, use for cumulative risk assessment, use in risk–benefit analysis, and use in traditional RfD calculations) and, on the other, show the limitations of the assessment (that is, the range in which the slope is highly uncertain and not recommended).
The hyperlinking capabilities of the Web present tremendous opportunities for the IRIS program to enrich the usability of its assessments. Summary presentations of cancer and noncancer analyses for oral or other routes of exposure could readily be linked to technical appendixes or examples as suggested above.
In closing, the committee recognizes the challenges that lie ahead for EPA in responding to the issues raised in this report and looks forward to reviewing a new draft IRIS assessment of inorganic arsenic. The new IRIS assessment, conducted with EPA’s proposed approaches to improve analysis and transparency, will provide a critical data-based assessment of the health risks posed by exposure to inorganic arsenic, facilitate better understanding of the basis of decisions, and provide stakeholders with a valuable and user-friendly new resource.








