Potential health effects of endocrine-disrupting chemicals have been an environmental health concern for decades because of their ability to interfere with normal hormone function of humans and wildlife populations. Endocrine-disrupting chemicals are exogenous substances or mixtures that alter function(s) of the endocrine system and so may cause adverse health effects in an intact organism or its progeny or subpopulations (EPA 2013). Endocrine disruptors are heterogeneous and include synthetic chemicals used as industrial solvents and their byproducts (such as polychlorinated biphenyls and dioxins), plastics (such as bisphenol A), plasticizers (such as phthalates), pesticides (such as chlorpyrifos and DDT), fungicides (such as vinclozolin), pharmaceutical agents (such as diethylstilbestrol), and natural chemicals found in human and animal food (such as phytoestrogens) (e.g., Diamanti-Kandarakis et al. 2009).
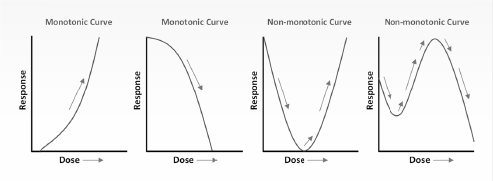
Because endocrine systems exhibit tissue-, cell-, and receptor-specific actions through the life course of organisms, the mechanisms by which endocrine disruptors may interfere with hormone function are complex and challenging to decipher (Zoeller et al. 2012). Evolving epidemiologic, toxicologic, mechanistic, and biomonitoring research on endocrine disruptors has invigorated the discussion with respect to their effects, the mechanisms by which the effects occur, and the interpretation of the data for science-policy implications. Concerns also have been raised about nonmonotonic dose–response (NMDR) relationships reported in in vitro, in vivo, and epidemiologic studies because conventional assumptions about chemical toxicity may not be applicable to chemicals that have such dose–response curves. NMDR curves are characterized by a change in the sign of their slope. They may be U-shaped, inverted U-shaped, or more complex (see Figure 1-1). NMDR curves could have implications for regulatory toxicity testing and risk assessment, which typically extrapolate from higher doses in animals to lower exposures in the environment for testing the safety of chemicals and thus assume that the slope of the dose–response curve does not change sign, that is, is monotonic. Controversy regarding this issue led the US Environmental Protection Agency (EPA) to review the evidence on NMDR curves and to request that the National Research Council conduct an independent scientific review of the draft of State of the Science Evaluation: Nonmonotonic Dose Responses as They Apply to Estrogen, Androgen, and Thyroid Path-
ways and EPA Testing and Assessment Procedures (referred to here as the SOTS evaluation). In response to EPA’s request, the National Research Council convened the Committee to Review EPA’s Draft State of the Science Paper on Nonmonotonic Dose Response, which prepared the present report.
BACKGROUND AND HISTORY OF ENDOCRINE-DISRUPTOR RESEARCH
EPA has a long history of involvement with endocrine-disruptor research. This section highlights a number of issues that have focused scrutiny on NMDR relationships and that have led to the development and review of EPA’s draft SOTS evaluation.
The Food Quality Protection Act of 1996 mandated that EPA “develop a screening program, using appropriate validated test systems and other scientifically relevant information, to determine whether certain substances may have an effect in humans that is similar to an effect produced by a naturally occurring estrogen, or other such endocrine effect” (EPA 2011). As a result, the Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC) was established to advise EPA on methods of screening and testing individual chemicals for endocrine-disruptor activity. EDSTAC (1998) proposed a two-tier system in which an initial battery of relatively short-term in vitro and in vivo assays to screen chemicals for potential endocrine-disruptor activity (activity that may interfere with estrogen, androgen, and thyroid hormones) would be used (Tier 1), followed by a second set of more refined tests (Tier 2). Since then, EPA’s Endocrine Disruptor Screening Program has further developed the Tier 1 assays and engaged in a validation process intended to ensure that the Tier 1 data would be reliable and reproducible among laboratories. More recently, EPA has embarked on the use of high-throughput screening in vitro assays that would be faster, ould be more efficient, and would make it possible to evaluate a greater number of chemicals (Rotroff et al. 2013).
In 2000, EPA and the National Institute of Environmental Health Sciences (NIEHS) held a workshop on low doses of endocrine-disrupting chemicals (EDCs) to provide an independent peer review of the available data, including a statistical reanalysis of studies that suggested the existence of NMDR relationships for EDCs. Among the findings from the workshop were that there was sufficient evidence of low-dose reproductive and developmental effects and that some estrogenic compounds exhibit NMDR curves (NTP 2001).
In 2011, EPA held a workshop on low-dose effects of EDCs—with participation by EPA scientists of the Office of Chemical Safety and Pollution Prevention (OCSPP), the Office of Water, and the Office of Research and Development (ORD)—to review the state of the science and discuss its potential implications. Workshop participants emphasized the need for a comprehensive scientific review given the rapid advances in this field (Schoeny 2013).
In 2012, a number of scientific publications on NMDR curves and EDCs received a great deal of attention from the scientific, nongovernment, and industry communities. Vandenberg et al. (2012) reviewed the extensive literature on NMDR relationships, and concluded that “fundamental changes in chemical testing and safety determination are needed to protect human health.” In an editorial in Environmental Health Perspectives, the director of NIEHS cited that review and indicated that future research should be directed at answering the question of “which dose-response shapes should be expected from specific environmental chemicals and under what specific circumstances” (Birnbaum 2012). A commentary by Rhomberg and Goodman (2012) on the Vandenberg et al. review was critical of the approach used to evaluate the literature and draw conclusions. The Endocrine Society published a position statement in 2012 that provided guidelines for applying fundamental principles of endocrinology to identify EDCs and to assess their potential risks (Zoeller et al. 2012). Those and other publications and the discourse surrounding them highlight the critical need for using a transparent, well-defined, and clearly articulated strategy in seeking to assess the effect of NMDR relationships on toxicity-testing strategies and how they affect human health and environmental risk assessments.
Several European and international organizations have evaluated whether the evidence on EDCs and NMDR curves requires changes in testing strategies. For example, the Centre on Endocrine Disrupters of the Danish National Food Institute evaluated the REACH (Registration, Evaluation, Authorisation, and Restriction of Chemicals) testing guidelines (Hass et al. 2013) and included critiques of the Vandenberg et al. (2012) paper. It concluded that “the current information requirements in REACH are not designed for the identification of endocrine disrupters, although certain endpoints and assays may give some indication of endocrine disrupting effects. It is, however, evident that important endpoints needed for the detection of [endocrine-disrupter] effects are not included.” The UN Environment Programme and World Health Organization (UNEP/WHO 2013) published State of the Science of Endocrine Disrupting Chemicals—2012. The report noted the need for revising current testing strategies because of questions, raised by the evidence on EDCs, about the adequacy
of typical testing exposure paradigms and the array of end points considered. A scientific committee of the European Food Safety Authority (EFSA) conducted a review of the scientific criteria for identifying EDCs and of methods for assessing effects mediated by such chemicals (EFSA 2013). The EFSA committee concluded that “a reasonably complete suite of standardized assays for testing the effects of [EDCs] is (or will soon be) available for the oestrogenic, androgenic, thyroid and steroidogenic modalities in mammals and fish, with fewer tests for birds and amphibians.” It also stated that it “cannot conclude whether the test methods are adequate to fully define dose response relationships. However, the available information is equally insufficient to conclude that current dose response analysis in regulatory (eco)toxicology should be modified on a routine basis.”
EPA’s ORD began work on the draft SOTS evaluation in 2012 in response to a request from OCSPP. The agency convened a cross-program, cross-agency working group that engaged other federal partners (for example, the Food and Drug Administration and the National Institute of Child Health and Human Development) to provide input for the evaluation. The working group adopted consensus definitions of four key terms for the SOTS evaluation: NMDRs, EDC, low-dose effect, and adverse effect. The definitions were:
• NMDRs: “measured biological effects with dose response curves that contain a point of inflection where the slope of the curve changes sign at one or more points within the tested range” (EPA 2013).
• EDC: “an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub)populations” (adopted from WHO 2002).
• Low-dose effect: “a biological change occurring in the range of typical human exposures or at doses lower than those typically used in standard testing protocols” (adopted from NTP 2001).
• Adverse effect: “a measured endpoint that displays a change in morphology, physiology, growth, development, reproduction, or life span of a cell or organism, system, or population that results in an impairment of functional capacity, an impairment of the capacity to compensate for additional stress, or an increase in susceptibility to other influences” (adopted from Keller et al. 2012).
Four subgroups worked independently on the sections of the evaluation that dealt with estrogen, androgen, and thyroid (in human and nonhuman) hormone pathways. The groups used various approaches for searching and reviewing the literature (details of these approaches are in Chapter 2). They focused explicitly on EDCs that act through the estrogen, androgen, and thyroid hormone systems. Because of the limitations of the literature, the focus was limited to single chemical exposures. The draft SOTS evaluation states that the report is not intended to be a policy document or to reflect testing guidance but rather as a resource for informing decision-making. EPA intends to revise the document in response to recommendations made in the present report and after considering
public comments. To the committee’s knowledge, this is one of the few attempts to evaluate specifically the implications of the evidence on NMDR curves for testing strategies and risk-assessment practices, and the agency is commended for undertaking such a challenging task.
THE COMMITTEE’S TASK AND APPROACH
Given the complex nature of NMDR curves exhibited by EDCs and their possible importance in interpreting toxicity testing and risk assessment, EPA asked the National Research Council to review the draft SOTS evaluation. In response to the request, the Research Council convened the Committee to Review EPA’s Draft State of the Science Paper on Nonmonotonic Dose Response. The committee was charged with reviewing EPA’s draft SOTS evaluation, commenting on EPA’s analysis of the existence of NMDR curves, and considering the implications of NMDR curves for chemical-testing and risk-assessment practices. The complete statement of task is presented in Box 1-1.
An ad hoc committee will conduct a scientific review of EPA’s draft paper, State of the Science on Nonmonotonic Dose Response. Specifically the committee will review and provide a brief report on the following:
• EPA’s analysis of the potential existence of nonmonotonic dose-response (NMDR) curves for chemicals. Has EPA fairly and soundly evaluated the weight of evidence and has it reached conclusions supported by the available studies?
• EPA’s evaluation of the studies and expert opinion (including the completeness of the database) used to assess whether current chemical testing strategies capture adverse effects potentially represented by NMDR curves. To what extent do the available studies capture adverse effects?
• EPA’s scientific rationale used to evaluate whether the state of the science influences EPA’s weight of evidence conclusions and the implications for risk assessment.
For the issues addressed in the bullets above, the committee will consider:
– Is EPA’s State of the Science document scientifically sound and of high quality?
– Has EPA selected studies of suitable breadth relevance and quality?
– Has EPA fairly and soundly evaluated and integrated the weight of evidence from the diversity of studies (epidemiological, mode-of-action, animal testing)?
– Are the assumptions valid and reasonable?
– Are the conclusions valid and supported on the basis of EPA’s assessment and the literature?
– Are there potential limitations or data gaps that would substantially impact the conclusions?
To address its task, the committee held three meetings. At the first meeting, EPA officials provided an overview of the development process that led to the draft SOTS evaluation, and an open-microphone session was held to hear the views of interested stakeholders on the evaluation. The second and third meetings were used to draft this report. Throughout the course of its work, the committee considered input from interested stakeholders and additional input that it requested from EPA. As specified in its charge, the committee focused on determining whether the draft SOTS evaluation provides a credible overview of the evidence and adequately documents the methods used to analyze the evidence and on determining whether conclusions were appropriately drawn and justified. The committee did not conduct an independent literature review to draw its own conclusions about the evidence on NMDR curves.
The committee’s review of the SOTS evaluation is presented in the following two chapters. Chapter 2 reviews the approach and methods that EPA used for its literature search, study selection, study evaluation, and synthesis and integration of the evidence on NMDR curves. Chapter 3 reviews how EPA assessed the implications of NMDR curves for the agency’s testing strategies, its weight-of-evidence conclusions, and its risk-assessment determinations.
Birnbaum, L. 2012. Environmental chemicals: Evaluating low-dose effects. Environ. Health Perspect. 120(4):A143-A144.
Diamanti-Kandarakis, E., J.P. Bourguignon, L.C. Giudice, R. Hauser, G.S. Prins, A.M. Soto, R.T. Zoeller, and A.C. Gore. 2009. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr. Rev. 30(4):293-342.
EDSTAC (Endocrine Disruptor Screening and Testing Advisorty Committee). 1998. Final Report [online]. Available: http://www.epa.gov/endo/pubs/edspoverview/finalrpt.htm [accessed Sept. 2, 2013].
EFSA (European Food Safety Authority). 2013. Scientific opinion on the hazard assessment of endocrine disruptors: Scientific criteria for identification of endocrine disruptors and appropriateness of existing test methods for assessing effects mediated by these substances on human health and the environment. EFSA J. 11(3):3132.
EPA (U.S. Environmental Protection Agency). 2011. Endocrine Disruptor Screening Program. Endocrine Primer [online]. Available: http://www.epa.gov/endo/pubs/edspoverview/primer.htm [accessed Aug. 30, 2013].
EPA (U.S. Environmental Protection Agency). 2013. State of the Science Evaluation: Nonmonotonic Dose Responses as They Apply to Estrogen, Androgen, and Thyroid Pathways and EPA Testing and Assessment Procedures. U.S. Environmental Protection Agency. June 2013 [online]. Available: http://epa.gov/ncct/download_files/edr/NMDR.pdf [accessed Aug. 12, 2013].
Fagin, D. 2012. Toxicology: The learning curve. Nature 490(7421):462-465.
Hass, U., S. Christiansen, M. Axelstad, K. Dreisig Sorensen, and J. Boberg. 2013. Input for the REACH-review in 2013 on Endocrine Disrupters. Final report. Center on Endocrine Disrupters, Division of Toxicology and Risk Assessment, National Food Institute, Technical University of Denmark. March 21, 2013 [online]. Available: http://www.mst.dk/NR/rdonlyres/B865F94A-54C0-43DF-AB0F-F07A593E1FD0/0/ReachreviewrapportFINAL21March.pdf [accessed July 15, 2013].
Keller, D.A., D.R. Juberg, N. Catlin, W.H. Farland, F.G. Hess, D.C. Wolf, and N.G. Doerrer. 2012. Identification and characterization of adverse effects in the 21st century toxicology. Toxicol. Sci. 126(2):291-297.
NTP (National Toxicology Program). 2001. National Toxicology Program’s Report of the Endocrine Disruptors Low Dose Peer Review. National Toxicology Program, U.S. Department of Health and Human Services, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC [online]. Available: http://www.epa.gov/endo/pubs/edmvs/lowdosepeerfinalrpt.pdf [accessed Aug. 30, 2013].
Rhomberg, L.R., and J.E. Goodman. 2012. Low-dose effects and nonmonotonic dose-responses of endocrine disrupting chemicals: Has the case been made? Regul. Toxicol. Pharmacol. 64(1):130-133.
Rotroff, D.M., D.J. Dix, K.A. Houck, T.B. Knudsen, M.T. Martin, K.W. McLaurin, D.M. Reif, K.M. Crofton, A.V. Singh, M. Xia, R. Huang, and R.S. Judson. 2013. Using in vitro high throughput screening assays to identify potential endocrine-disrupting chemicals. Environ. Health Perspect. 121(1):7-14.
Schoeny, R. 2013. State of the Science Evaluation: Nonmonotonic Dose Responses as They Apply to Estrogen, Androgen, and Thyroid Pathways and EPA Testing and Assessment Procedures. Presentation at the First Meeting on Review of EPA’s Draft Paper State of the Science on Nonomonotonic Dose Response, July 23, 2013, Washington, DC.
UNEP/WHO (United Nations Environment Program/World Health Organization). 2013. State of the Science of Endocrine Disrupting Chemicals-2012, A. Bergman, J.J. Heindel, S. Jobling, K.A. Kidd, and R.T. Zoeller, eds. World Health Organization [online]. Available: http://www.who.int/ceh/publications/endocrine/en/ [accessed Aug. 30, 2013].
Vandenberg, L.N., T. Colborn, T.B. Hayes, J.J. Heindel, D.R. Jacobs, D.H. Lee, T. Shioda, A.M. Soto, F.S. vom Saal, W.V. Welshons, R.T. Zoeller, and J.P. Myers. 2012. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 33(3):378-455.
WHO (World Health Organization). 2002. Global Assessment of the State-of-the-Science of Endocrine Disruptors. International Programme on Chemical Safety. World Health Organization [online]. Available: http://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en/ [accessed Aug. 30, 2013].
Zoeller, R.T., T.R. Brown, L.L. Doan, A.C. Gore, N.E. Skakkebaek, A.M. Soto, T.J. Woodruff, and F.S. Vom Saal. 2012. Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinology 153(9):4097-4110.







