Appendix E
Synopsis of Brain Injury Detection Methods
SCOPE
Methods for detection of brain injury range from observation of the victim’s behavior to advanced noninvasive imaging methods, including the following: report of symptoms and responses to questions that test awareness and memory; sophisticated computer-based neuropsychology computer tests; and advanced sensing methods of magnetic resonance, positron tomography, acoustic, electroencephalographic and impedance measurements that enable noninvasive sensing of blood flow, brain metabolism, brain inflammation, brain accumulation of markers of injury, and brain electrical properties.
The status of these methods relative to detection of traumatic brain injury (TBI) is reviewed here. Blood tests for biomarkers of nerve damage are not discussed because, despite extensive investigations in the search for definitive markers of TBI, none has emerged as specific, timely, and sufficiently sensitive for diagnosis within hours of the concussive incidents (Svetlov et al., 2009).
COGNITIVE TESTS
Detection of brain trauma in the battlefield is based on the signs and symptoms of mental status ranging from unconsciousness to symptoms such as confusion, memory loss, slurred speech, headaches, and dizziness. An assignment of concussion is based on these symptoms. The concept of concussion is imprecise and not related to a specific neurological mechanism, nor have methods of quantification of the severity of a concussive event been available until recently.
The most commonly used method for detection of concussion in combat zones and during sports events is neuropsychological testing. The assessment tool for concussion in the battlefield is the Neuropsychological Assessment Metrics (ANAM). The method is a 20-minute computer based evaluation that tests reflex times and some measures of memory and cognitive abilities. After development by the military more than 10 years ago, it has been used to assess sports injuries. Recently, ANAM was validated in the combat environment. Sixty-six cases and 146 controls were studied with the result that the simple reaction test, if applied within 72 hours of the injury, is a relatively sensitive method to differentiate concussed from non-concussed individuals in the combat environment (Kelly et al., 2012).
There are a multitutude of cognitive tests that neuropsychiatrists and psychologists use to assess and score mental capabilities. Before the computerization of cognitive tests, these were applied in controlled studies of cognitive abilities in old and young subjects years after experiencing TBI. For example, cognitive impairments 10 years following TBI were found to be associated with injury severity using tests of attention, mental processing speed, memory, and executive functions (Draper and Ponsford, 2008). An instrument that specifically assesses the quality of life in patients with TBI (Quality of Life after Brain Injury) has been developed (von Steinbüchel et al., 2010). The European Brain Injury Questionnaire (EBIQ) is a clinically reliable instrument to determine the subjective well-being of individuals with brain injury and to assess changes over time (Sopena et al., 2007).
MRI IMAGING
Of the major methods that have known efficacy in the examination of the brain in vivo (i.e., electroencephalogram [EEG], x-ray computer tomography [CT], emission tomography, magnetic resonance imaging [MRI]). MRI is the one that can provide noninvasive information specific to most of the pathologies (e.g., Gutierrez-Cadavid, 2005; Benson et al., 2012). MRI can provide a wealth of information regarding organ changes associated with ballistic trauma to the body, as has already been shown in studies of blast-injured veterans (Van Boven et al., 2009). Below is a synopsis of the specific capabilities for noninvasive measurements by MRI.
• Brain contusion. Edema is an expected early sign of contusion and will appear as a bright signal on T2-weighted or fluid-attenuated inversion recovery (FLAIR) MRI. The appearance of edema on MRI is variable (Gutiereex-Cadavid, 2005). T1-weighted protocols might give a sensitive diagnosis, as will other protocols.
• Brain edema. Edema resulting from vascular compromise (i.e., air emboli from lung damage), pressure impulse transmitted from the periphery to the brain, or ischemic damage from other causes can be detected by MRI diffusion-weighted imaging sequences, FLARE, and possibly by T1-weighted protocols.
• Vasospasm. Vasospasm is of major importance and perhaps the least understood. Vasospasm is a narrowing of the small arteries of the brain and frequently follows subdural hematoma, but also can occur as a consequence of blunt trauma without hematoma. The onset of vascular spasm can be a few days after trauma, and as vessel narrowing limits blood supply to parts of the brain, vasospasm is a major cause of morbidity. The importance of vasospasm has not been generally recognized (Ortell et al., 2005). It can be detected by magnetic resonance angiography (MRA) or Doppler ultrasound. The majority of cases of vasospasm reviewed at the National Naval Medical Center were blast trauma victims (Armonda et al., 2012).
• Hemorrhage. Early signs of hemorrhage usually occur due to tears in the tributary surface veins that bridge the brain surface to the dural venous sinus. T2-weighted MRI can show the accumulation of blood as a bright signal initially, with an evolution to a dark signal in 2 to 3 days and back again to a bright signal within the first 2 weeks (Taber et al., 2003, Tong et al., 2003). The choice of magnetic resonance (MR) protocol is important here as it has been shown that susceptibility-weighted MR imaging depicts significantly more small hemorrhagic lesions than does conventional gradient echo (GRE) MR imaging and, therefore, has the potential to improve the diagnosis of small hemorrhagic lesions as well as diffuse axonal injury (Tong et al., 2003).
Neuronal Architecture Imaging Methods
Neural axon injury might be the most subtle, yet the most important, pathology that requires early imaging for diagnosis (Mayorga, 1997). Experience has shown that this pathology occurs in the corpus callosum and brain stem. Diffusion-weighted imaging (Huisman et al., 2003) and T1-weighted protocols have been replaced by diffusion tensor imaging (DTI) because DTI has been found to be a sensitive indicator of white matter defects. DTI is able to detect damage to axonal tracts using a measure of directional water diffusion (fractional anisotropy). Fractional anisotropy metric varies from 0 to 1. Low values indicate less directional diffusion and relatively less fiber orientation suggestive of damage. This MRI method has been found to delineate white matter defects in TBI, and these defects were correlated with neuro-cognitive function (Lipton et al., 2008, Kumar et al., 2009; Jorge et al., 2012). Some caution should be exercised in making inferences from the MRI studies as being directly related to organic nerve injury. A recent study found DTI abnormalities in combat-exposed soldiers that normalized after 1.5 years, but the soldiers had neither posttraumatic stress disorder (PTSD) nor TBI (van Wingen et al., 2012).
As discussed in Chapter 10, a number of clinical imaging studies with MRI have shown associations between white matter neuronal track disruptions inferred from images and symptoms associated with blunt trauma and blast injuries in veterans months and years after return from the battlefield (Mac Donald et al., 2011; Yeh et al., 2013). However, in one study, white matter injuries were not revealed by magnetic resonance DTI on veterans with mild TBI, despite their symptoms of compromised verbal memory (Levin et al., 2010).
Functional MRI
Functional MRI (fMRI) involves evaluation of the changes in local blood flow and volume due to an external stimulus such as a visual challenge or memory test (Figure E-1). It is also known as blood oxygen level dependent (BOLD) MRI. This is an objective test of brain functioning and has been found to correlate with some post-concussion symptom metrics such as visual memory (Talavage et al., 2013).
Instrumentation availability and costs vary widely—from a permanent magnet system for small animals at less than $0.5 million to elaborate systems that combine magnetic resonance with PET at over $2 million. Most studies can be enabled through collaboration with medical clinics.
Magnetoencephalography
Mapping the origin of ionic current densities in the brain by detection of the induced magnetic fields at the surface of the human head has been employed in neurophysiological investigations and surgical applications to treat epilepsy as well as to identify functioning tissues in tumor surgery. The principal attribute of magnetoencephalography is its ability to provide high temporal fidelity information of the activity of parts of the brain with limited spatial resolution. The combination of magnetoencephalography with MRI methods, including MRI tractography (a method of displaying major nerve bundles in the brain through detection of proton diffusion principal tensor component), has promise for identification of late manifestions of neuronal dysfunction in TBI patients (Larson-Prior et al., 2013).
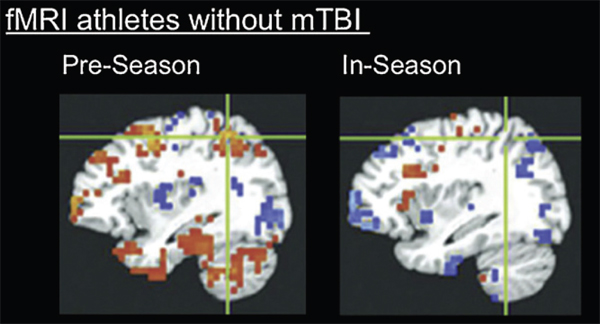
FIGURE E-1 Brain alterations shown on functional imaging without behavioral changes. fMRI image of highschool football players without clinically diagnosed concussion, performing neurocognitive testing before football season and during football season: Even in the absence of concussion (in 8 out of 21 athletes), fMRI shows changes in stimulated blood flow that are correlated with a poorer performance in neurocognitive testing. SOURCE: Talavage et al. (2013). The publisher of this copyrighted material is Mary nn Liebert, Inc., publishers.
PET AND SPECT IMAGING
Whereas magnetic resonance spectroscopy of specific volumes of the brain can define the chemical status of, for example, bioenergetic molecules (e.g., adenosine triphosphate [ATP], creatine phosphate, etc.) for most studies of brain metabolism and neuroreceoptor concentrations, emission tomography (single photon emission tomography [SPECT] and positron tomography [PET]) is the sensitive measurement method. Pathophysiological perturbations in the following parameters can be imaged by PET:
• Oxygen utilization,
• Regional glucose metabolism,
• Regional blood flow and vasospasm detection,
• Permeability,
• Neuroreceptor concentrations,
• Inflammation,
• Beta amyloid deposits associated with dementia, and
• Tau protein associated with brain trauma and dementia.
The methods are noninvasive and can be repeated over the course of hours or days. Whereas PET and SPECT are readily available in medical centers, not all experimentalists will have these instruments and the required radioisotopes available, particularly for small animal studies. The spatial resolution in instruments designed for animal studies can be 2 mm or less. Normally, the spatial resolution for large animals and human subjects is 4 to 6 mm. The tracers available allow studies of blood flow, glucose uptake (commonly interpreted as cerebral metabolism), dopamine transporters and receptors, muscarinic system activity, and blood brain permeability. PET and SPECT instrumentation for small animal studies is available from a number of vendors. Large animal studies can be accomplished through collaborators at medical institutions where the requisite approvals for use of radionuclides are already in place.
Metabolism Imaging
Since the early 1980s, cerebral glucose metabolism associated with dementia has been quantitatively imaged in patients using 18F-fluoro-deoxyglucose and positron tomography. Recent human studies in boxers showed patterns of hypometabolism using the accumulation of 18F-deoxy-glucose (Provenzano et al., 2010), but one must be careful not to interpret hypometabolism when the reason for less apparent tracer uptake is tissue atrophy or decreases in blood flow rather than a decrease in the metabolic uptake mechanism. An important application of PET evaluation of brain glucose uptake is to study the effects of low growth hormone associated with trauma-induced hypopituitarism because brain glucose metabolism increases after growth
hormone stimulation in patients with hypopituitarism. PET using 13N-labeled ammonia was shown to be an important method for detection of pituitary dysfunction in a limited study (Zang et al., 2005).
Inflammation Imaging
Detection of inflammation in the brain is facilitated by PET compounds that localize in the receptors on the surface of brain cells that are part of the inflammation response (Cagnin et al., 2001; Figure E-2). Amyloid depositions seen in nontrauma-based dementia (e.g., Alzheimer’s disease) can be quantified by a 11C- PET agent (Klunk et al., 2001) and recently a 18F agent. Because autopsy and spinal fluid assays have demonstrated that a biomarker for dementias and blunt brain trauma is phosphorylated tau protein, a quest for a suitable ligand that would specifically accumulate in regions of the brain having excesses of tau protein has led to some successes. Tau protein is the main component in neurofibrillary tangles seen in Alzheimer’s disease and the pathologic protein associated with dementias such as Pick’s disease, corticobasal degeneration, and progressive supranuclear palsy.
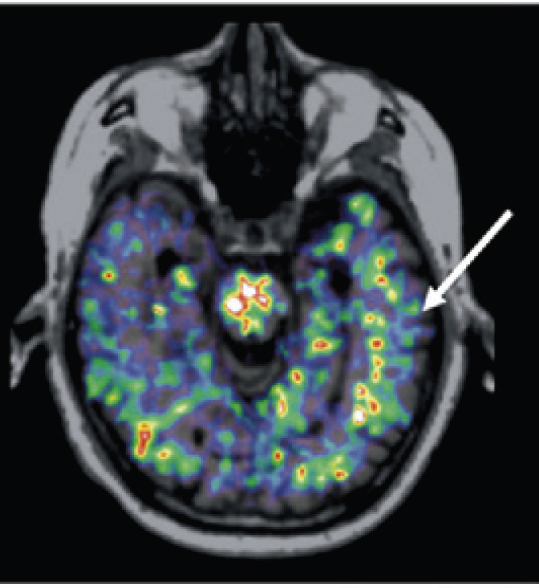
FIGURE E-2 Positron tomography image showing sites of inflammation using the tracer 11C-PK11195 with superposition of the positron emission tomography emission on a magnetic resonance imaging anatomical image. SOURCE: Cagnin et al. (2007), with kind permission from Springer Science & Business Media.
Tau Protein Imaging
Studies at autopsy have shown the occurrence of tissue responses to trauma, including tau (T-tau) hyperphosphorylated protein (Blennow et al., 1995; Zetterberg et al., 2001), c-Fos and c-Myc expression, deposition of β-APP (Säljö et al., 2002), glial fibrillar acidic protein (GFAP), and fibrillar light protein (FL-P). The most recent and promising noninvasive detection method for neurochemistry of the brain in mild cognitive impairments (MCIs) and behavioral disorders subsequent to multiple episodes of blunt trauma is 18F-ligands for aggregates of the protein tau known to accumulate in injured brain tissue. A few years ago, a successful study in vitro and in small animals revealed the potential of PET to visualize tau protein using a fluoroethoxyquinoline compound and the positron emitter 18F (Fodero-Tavoletti et al., 2011). The first human studies with another agent for amyloid and tau protein, called FDDPN, was associated with the pattern of glucose accumulation deficits in Alzheimer’s disease patients (Barrio et al., 2008) and shortly thereafter, the accumulation in the brains of symptomatic pro-football veterans was demonstrated (Small et al., 2013).
ULTRASOUND FOR BRAIN BLOOD FLOW
Measurements of blood flow in the brain basal arteries and the carotids by transcranial Doppler (Jaffres et al., 2005; Visocchi et al., 2007) are surrogates for estimating cerebral vascular resistance and are effective methods for detection of vasospasm associated with abnormally high velocities. These measurements rely on the skill of the operator. Vascular spasm can occur late after brain injury (Armonda et al., 2012) and will result in a change in the flow characteristics (Jaffres et al., 2005; Kochanowicz et al., 2006; Oertel et al., 2005) with eventual change in electrical impedance (Fritz et al., 2005). Ultrasound instrumentation is generally more available than the other radiological imaging systems for human studies. Specialized small animal systems are now available to the researcher.
ELECTROENCEPHALOGRAPHY AND ELECTRICAL IMPEDANCE
Electroencephalography and electrical impedance tomography are two techniques that might be used to assess parenchymal integrity through measurement of electrical properties both during the acute phase of ballistic trauma and during posttrauma intervals up to years. Both approaches require sensitive instruments and are plagued with electrode coupling noise. However, in previously successful large and small animal experiments, EEG measurements (Drobin et al., 2007) and impedance measurements (Klein et al., 1993; Olsson et al., 2006; Harting et al., 2010) have shown the kinetics of brain physiologic response to blunt trauma. Methods for field measurements of brain electrical potentials
and impedance are available, and their development has promise using modern electrode systems and signal processing (Budinger, 1996).
REFERENCES
Armonda, R.A., T.A. Tignob, S.M. Hochheimera, F.L. Stephens, R.S. Bell, A.H. Vo, M.A. Severson, S.A. Marshall, S.M. Oppenheimer, R. Ecker, and A. Razumovsky. 2012. Posttraumatic vasospasm and intracranial hypertension after wartime traumatic brain injury. Perspectives in Medicine 1:261-264.
Barrio, J.R., V. Kepe, N. Satyamurthy, S.C. Huang, and G. Small. 2008. Amyloid and tau imaging, neuronal losses and function in mild cognitive impairment. Journal of Nutrition Health and Aging 12(1):61S-65S.
Benson, R.R., R. Gattu, B. Sewick, Z. Kou, N. Zakariah, J.M. Cavanaugh, and E.M. Haacke. 2012. Detection of hemorrhagic and axonal pathology in mild traumatic brain injury using advanced MRI: Implications for neurorehabilitation. NeuroRehabilitation 31:261-279.
Blennow, K., K. Walllin, H. Agren, C. Spenger, J. Siegfied, and E. Vanmechelen. 1995. Tau protein in cerebrospinal fluid: A biochemical marker for axonal degeneration in Alzheimer’s disease? Molecular and Chemical Neuropathology 26:231-245.
Budinger, T.F. 1996. Neuroimaging applications for the study of Alzheimer’s disease. Pp. 145-174 in Alzheimer’s Disease: Cause(s), Diagnosis, Treatment, and Care (Z.S. Khachaturian and T.S. Radebaugh, eds.). CRC Press, Boca Raton, Fla.
Cagnin, A., M. Kassiou, S.R. Meikle, and R.B. Banati. 2007. Positron emission tomography imaging of neuroinflammation. Neurotherapeutics 4(3):443-452.
Draper, K., and J. Ponsford. 2008. Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychology 22(5):618-625.
Drobin, D., D. Gryth, J. Persson, D. Rocksen, U. Arborelius, L. Olsson, J. Bursell, and B. Kjellstrom. 2007. Electroencephalogram, circulation, and lung function after high-velocity behind armor blunt trauma. Journal of Trauma 63(2):405-413.
Fodero-Tavoletti, M.T., N. Okamura, S. Furumoto, R.S. Mulligan, A.R. Connor, C.A. McLean, D. Cao, A. Rigopoulos, G.A. Cartwright, G. O’Keefe, S. Gong, P.A., et al. 2011. 18F-THK523: A novel in vivo tau imaging ligand for Alzheimer’s disease. Brain 134(Pt 4):1089-1100.
Fritz, H., B. Walter, M. Holzmayr, M. Brodhun, S. Patt, and R. Bauer. 2005. A pig model with secondary increase of intracranial pressure after severe traumatic brain injury and temporary blood loss. Journal of Neurotrauma 22(7):807-821.
Gutierrez-Cadavid, J.E. 2005. Head trauma. Pp. 869-901 in Imaging of the Nervous System. Diagnostic and Therapeutic Applications (Latchaw, Kucharczyk, Mosebey, eds.). Elsevier Mosby, Philadelphia, Pa.
Harting, M., C. Smith, R. Radhakrishnan, K. Aroom, P. Dash, B. Gill, and C. Cox, Jr. 2010. Regional differences in cerebral edema after traumatic brain injury identified by impedance analysis. Journal of Surgical Research 159(1):557-564.
Huisman, T.A., A.G. Sorensen, K. Hergan, R.G. Gonzalez, and P.W. Schaefer. 2003. Diffusion-weighted imaging for the evaluation of diffuse axonal injury in closed head injury. Journal of Computer Assisted Tomography 27:5-11.
Jaffres, P., J. Brun, P. Declety, J. Bosson, B. Fauvage, A. Schleiermacher, A. Kaddour, D. Anglade, C. Jacquot, and J. Payen. 2005. Transcranial Doppler to detect on admission patients at risk for neurological deterioration following mild and moderate brain trauma. Intensive Care Medicine 31(6):785-790.
Jorge, R.E., L. Acion, W. White, D. Tordesillas-Gutierrez, R. Pierson, B. Crespo-Facorro, and V.A. Magnotta. 2012. White matter abnormalities in veterans with mild traumatic brain injury. American Journal of Psychiatry 169:1284-1291.
Kelly, M.P., R.L. Coldren, R.V. Parish, M.N. Dretsch, M.L. Russell. 2012. Assessment of acute concussion in the combat environment. Archives of Clinical Neuropsychology 27:375-388.
Klein, H.C., W. Krop-Van Gastel, K.G. Go, and J. Korf. 1993. Prediction of specific damage or infarction from the measurement of tissue impedance following repetitive brain ischaemia in the rat. Neuropathology and Applied Neurobiology 19(1):57-65.
Kochanowicz, J., J. Krejza, Z. Mariak, M. Bilello, T. Lyson, and J. Lewko. 2006. Detection and monitoring of cerebral hemodynamic disturbances with transcranial color-coded duplex sonography in patients after head injury. Neuroradiology 48(1):31-36.
Kreipke, C.W., and J.A. Rafols, eds. 2013. Cerebral Blood Flow, Metabolism, and Head Trauma: The Pathotrajectory of Traumatic Brain Injury. Springer, New York.
Kumar, R., M. Husain, R.K. Gupta, K.M. Hasan, M. Haris, A.K. Agarwal, C.M. Pandey, and P.A. Narayana. 2009. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. Journal of Neurotrauma 26:481-495.
Larson-Prior, L.J., R. Oostenveld, S. Della Penna, G. Michalareas, F. Prior, A. Babajani-Feremi, J.M. Schoffelen, L. Marzetti, F. de Pasquale, F. Di Pompeo, J. Stout, M. Woolrich, et al. 2013. Adding dynamics to the Human Connectome Project with MEG. NeuroImage 80:190-201.
Lipton, M.L., E. Gellella, C. Lo, T. Gold, B.A. Ardekani, K. Shifteh, J.A. Bello, and C.A. Branch. 2008. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: A voxel-wise analysis of diffusion tensor imaging. Journal of Neurotrauma 25:1335-1342.
Magnotta, V.A. 2012. White matter abnormalities in veterans with mild traumatic brain injury. American Journal of Psychiatry 169:1284-1291.
Mayorga, M. 1997. The pathology of primary blast overpressure injury. Toxicology 121(1):17-28.
Oertel, M., W. Boscardin, W. Obrist, T. Glenn, D. McArthur, T. Gravori, J. Lee, and N. Martin. 2005. Posttraumatic vasospasm: The epidemiology, severity, and time course of an underestimated phenomenon: A prospective study performed in 299 patients. Journal of Neurosurgery 103(5):812-824.
Olsson, T., M. Broberg, K. Pope, A. Wallace, L. Mackenzie, F. Blomstrand, M. Nilsson, and J. Willoughby. 2006. Cell swelling, seizures and spreading depression: An impedance study. Neuroscience 140(2):505-515.
Provenzano, F.A., B. Jordan, R.S. Tikofsky, C. Saxena, R.L. Van Heertum, and M. Ichise. 2010. F-18 FDG PET imaging of chronic traumatic brain injury in boxers: A statistical parametric analysis. Nuclear Medicine Communications 31(11):952-957.
Säljö, A., F. Bao, J. Shi, A. Hamberger, H. Hansson, and K. Haglid. 2002. Expression of c-Fos and c-Myc and deposition of b-APP in neurons in the adult rat brain as a result of exposure to short-lasting impulse noise. Journal of Neurotrauma 19:379-385.
Shenton, M.E., H.M. Hamoda, J.S. Schneiderman, S. Bouix, O. Pasternak, Y. Rathi, M.A. Vu, M.P. Purohit, K. Helmer, I. Koerte, and A.P. Lin. 2012. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging and Behavior 6:137-192.
Small, G.W., V. Kepe, P. Siddarth, L.M. Ercoli, D.A. Merrill, N. Donoghue, S.Y. Bookheimer, J. Martinez, B. Omalu, J. Bailes, and J.R. Barrio. 2013. PET scanning of brain tau in retired National Football League players: Preliminary findings. American Journal for Geriatric Psychiatry 21(2):138-144.
Sopena, S., B.K. Dewar, R. Nannery, T.W. Teasdale, and B.A. Wilson. 2007. The European Brain Injury Questionnaire (EBIQ) as a reliable outcome measure for use with people with brain injury. Brain Injury 21(10):1063-1068.
Taber, K.,. S. Rauch, R. Lanius, and R. Hurley. 2003. Functional magnetic resonance imaging: Application to posttraumatic stress disorder. Journal of Neuropsychiatry and Clinical Neurosciences 15(2):125-129.
Talavage, T.M., E.A. Nauman, E.L. Breedlove, U. Yoruk, A.E. Dye, K. Morigaki, H. Feuer, and L.J. Leverenz. 2013. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. Journal of Neurotrauma. April 11. E-pub ahead of print, doi:10.1089/neu.2010.1512.
Tong, K., S. Ashwal. B. Holshouser, L. Shutter, G. Herigault, E. Haacke, and D. Kido. 2003. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: Improved detection and initial results. Radiology 227(2):332-339.
Van Boven, R., G. Harrington, D. Hackney, A. Ebel, G. Gauger, J. Bremner, M. D’Esposito, J. Detre, E. Haacke, C. Jack Jr., W. Jagust, et al. 2009. Advances in neuroimaging of traumatic brain injury and posttraumatic stress disorder. Journal of Rehabilitation Research and Development 46(6):717-757.
van Wingen, G.A., E. Geuze, M.W.A. Caan, T. Kozicz, S.D. Olabarriaga, D. Denys, E. Vermetten, and G. Fernández. 2012. Persistent and reversible consequences of combat stress on the mesofrontal circuit and cognition. Proceedings of the National Academy of Sciences U.S.A. 109:15508-15513.
Visocchi, M., A. Chiaretti, D. Cabezza, and M. Meglio. 2002. Hypoflow and hyperflow in diffuse axonal injury. Prognostic and therapeutic implications of transcranial Doppler sonography evaluation. Journal of Neurosurgical Sciences 46(1):10-17.
von Steinbüchel, N., L. Wilson, H. Gibbons, G. Hawthorne, S. Höfer, S. Schmidt, M. Bullinger, A. Maas, E. Neugebauer, J. Powell, K. von Wild, et al. 2010. Quality of Life after Brain Injury (QOLIBRI): Scale validity and correlates of quality of life. Journal of Neurotrauma 27(7):1157-1165.
Zhang, X., D. Yue, and A. Tang. 2005. Dynamic 13N-ammonia PET: A new imaging method to diagnose hypopituitarism. Journal of Nuclear Medicine 46:44-47.






