10
Linking Helmet Protection to Brain Injury
The relationships between helmet deformation and brain injury are not well known. Most of the studies in biomechanical engineering and medicine are related to sports and vehicle collisions, and these investigations are based on a different range of stresses and stress rates from those encountered in the battlefield. The aim of this chapter is to present information on what is known, and the gaps, about the linkage between brain injury and current battlefield threats. The major finding is that helmet protection from penetration and backface deformation (BFD) greater than a particular value does not protect the brain from occurrence of many categories of tissue injury. Recommendations that can help focus research range from determination of the prevalence of reversible declines in hormonal function years after brain trauma to acceleration of research in computational modeling and simulation that can show shear stress fields associated with the known spectrum of threats and the protective capabilities of helmets.
The transmission of stress to the brain from any substantial impact on the head can lead to traumatic brain injury (TBI). Acute brain injury, even mild injuries, may severely influence or restrict military operational capabilities, and long-term consequences will have an impact on individual quality of life.
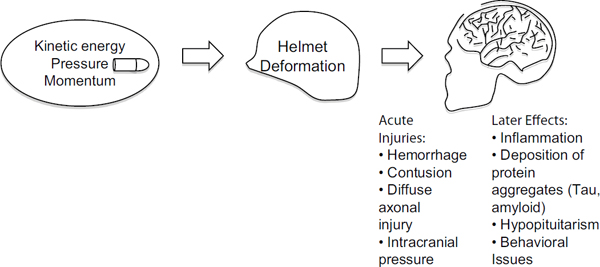
The effects on brain function depend on the magnitude and direction of the force impacting the head. Therefore, it is important to understand linkages between blunt trauma and brain injury and how the helmet attenuates the effect of the impact (see Figure 10-1). For example, it is known that for lower severity ballistic or blunt inputs, the transfer of momentum and rate of change of momentum (force) from an impact can be sufficiently attenuated by the helmet to prevent brain tissue injuries. Thus, an understanding of brain tissue and brain physiological tolerance must be linked to the magnitude of the transfer of force or other mechanical parameters—from the impact to the helmet onto the head and into the brain.
For helmeted service personnel, nonpenetrating injuries may be caused by local contact of the deforming undefeated helmet onto the head/underlying skull or from more regional helmet/head contact with forces transmitted through the helmet webbing or padding to the skull (Bass et al., 2003). These forces may result in direct, local deformation of the skull and translation and/or rotation of the head, leading to brain injuries. Some mechanisms of brain injury, such as abrupt acceleration changes of the body due to an improvised explosive device (IED) blast or a paratrooper hard landing, are not necessarily attenuated by helmets, but the injury mechanisms are likely similar to injuries from blunt head trauma. Blast pressure stress from IEDs and artillery can directly or indirectly transmit pressure fields to the head that result in shear stresses in the brain (Panzer et al., 2012; Shridharani et al., 2012a).
The subject of this chapter is the right side of Figure 10-1. The committee presents what is known (and the gaps) about brain injury tolerances relative to current standards of helmet protection. This is an essential component in determining how much the helmet must attenuate the impact force to prevent brain trauma. Box 10-1 provides a glossary of terms used in this chapter.
Types of Nonpenetrating Brain Injuries
Blunt trauma can lead to various types of brain injuries, ranging from concussion, hemorrhaging, hematoma (blood clots), skull fracture, anoxic injury (lack of oxygen), and diffuse axonal injury or DAI (damage to the brain neurons). Table 10-1 provides a listing of 13 major categories of brain injuries and potential causes.
Many of these injuries are caused by differential motions/strains within the soft tissues of the brain. The motion of the surface of the brain against the bony structures of the head leads to tissue contusions, vascular tears, and hemorrhages. These initiating injuries may degrade brain function through various mechanisms such as the restriction of blood supply or damage to cells. It is thought that compression (hydrostatic) alone is not an initiating cause of tissue injury unless it results in shear stress. (See Panzer et al., 2012, for results with high rate blast impacts.)
TABLE 10-1 Categories of Brain Injuries
|
Categories |
|
|
1 |
Direct contusion of the brain from skull deformation or fracture |
|
2 |
Brain contusion (including coup) from movement against interior surfaces of the skull |
|
3 |
Indirect (countercoup) contusion from mechanical response of the brain opposite the side of the impact |
|
4 |
Reduced blood flow due to infarction or pressure-based occlusion |
|
5 |
Disruptive and non-disruptive diffuse axonal injury from shear stresses |
|
6 |
Tissue stresses and strains produced by motion of the brain hemispheres relative to the skull |
|
7 |
Subdural and epidural hematomas produced by rupture of bridging vessels between the brain and the dura mater |
|
8 |
Pressure-based rupture of small blood vessels leading to petechial hemorrhages |
|
9 |
Strains beyond material tolerances of nerves and blood vessels |
|
10 |
Vasospasm resulting in diminished blood flow |
|
11 |
Trauma induced hypopituitarism |
|
12 |
Perturbations in brain biochemistry functioning with pathologic signs and symptoms long after the injury |
|
13 |
Temporary or permanent changes in visual, verbal, and motor functioning |
Quasistatic compression as high as 50 MPa (7300 psi) or more does not result in injury to mammalian cells (Grundfest, 1936). Nerves and blood vessels are susceptible to stresses with strain tolerances usually less than 10 to 20 percent for functional failure of neural tissues such as neurons/axons/glia and probably less for some arterial networks (Margulies and Thibault, 1992; Smith et al., 1999).
The susceptibility of the brain to shearing forces, and its very high incompressibility, may lead to contusions or hemorrhaging at the surface of the brain. Rotational acceleration and change in acceleration cause blood vessel ruptures leading to bleeding between the brain covering (dura mater) and the skull with the result of increased intracranial pressure. Bleeding may also arise in the space between the dura mater and the brain (subdural hemorrhage). Injuries associated with the rapid acceleration and deceleration of the head result in forces that produce stretching and tearing of axons (causing DAI). Such strains and potentially large pressure or stress waves in small blood vessels can lead to small hemorrhages (petechial hemorrhages) deep within the brain. Even when not life threatening, such injuries have the potential for delayed injury, including local brain swelling, as well as long-term consequences with symptoms persisting many years after the initial brain injury.
Important and frequently undiagnosed effects include alterations in microcirculation that can lead to hypoperfusion or regional vasospasm with the result of inadequate delivery of vital metabolites to neural tissue. These mechanisms are believed to contribute to the short-term as well as long-term effects from ballistic helmet hits, head collisions, and exposures to high-intensity blasts. Other long-term effects from brain trauma may include declines in hormonal function related to disruption of the pituitary gland (e.g., growth hor-
|
Blast |
Detonation of liquid or solid explosive material results in the generation of gaseous products in the pressure range of 150,000 atmospheres or 1.5 billion Pascals (1.5 GPa) and temperature of 3000 Kelvin. |
|
DTI |
Diffusion tensor imaging—a MRI method that maps the magnitude of water diffusion in different directions. The method gives a value of diffusion anisotropy (DA), which will decline if the normal orientation of fiber in white matter is disrupted by edema or tears, for example. |
|
Epidural hematoma |
Collection of blood from rupture of vessels between the brain dura mater and the skull. |
|
FEM |
Finite element modeling—a computational system that provides the means to simulate the effects of forces on structures such as the skull and brain tissues. |
|
fMRI |
functional magnetic resonance imaging—fMRI is similar to MRI, but the image gives information regarding blood flow changes in the brain after some stimulation. |
|
G or g |
Symbol for the acceleration of gravity magnitude of 9.8 m s-2. |
|
Hypopituitarism |
Dysfunction of the pituitary organ manifested by low secretion of hormones such as ACTH, growth hormone, thyroid stimulating hormone, oxytocin, vasopressin, etc. |
|
J |
Joule is energy or force times the distance over which force acts. It is the unit for kinetic energy defined as mass times velocity squared/2. |
|
kPa |
(kiloPascal) is a unit of pressure equal to a 1000 Pascals (10 kPa is 1 atmosphere of pressure). |
|
Momentum |
Defined as the product of mass and velocity. The rate of change of momentum is force. |
|
MRI |
Magnetic resonance imaging |
|
N |
Newton is the unit of force or the product of mass times acceleration. |
|
NHTSA |
National Highway Traffic Safety Administration. |
|
National Institute of Justice Standard |
This standard, designated “0101.04” stipulates the maximum deformation a soft armor vest can undergo without penetration is 44-mm as measured in a clay substrate after a live fire test of the armor. |
|
PET |
Positron emission tomography—an imaging method that uses radioactive tracers that specifically target proteins and other functions of the body. It differs from SPECT in the types of tracers used and the characteristics of the instrumentation. |
|
Pituitary organ |
A 7-mm diameter organ suspended on a stalk from the base of the brain into a well at the floor of the skull. It secretes 9 hormones into the bloodstream in response to stimuli from the hypothalamus also at the base of the brain. These hormones include growth hormone and thyroid stimulating hormone. |
|
Shear modulus |
The ratio of the tangential force per unit area to the angular deformation in radians. |
|
Strain |
The fractional change in a physical dimension of matter in response to stress. It is frequently given as a percentage (e.g., 5 percent) and can be over 100 percent. |
|
Stress |
The force per area or volume with dimensions of newtons per meter squared or Pascal. |
|
Stress waves |
Compression waves in a material due to an impulse or sudden load change. |
mone and thyroid function deterioration) and the occurrence of abnormal proteins in the brain years after trauma.
Some data on injury thresholds exist for low-rate skull fracture, concussion, and diffuse axonal injury. But these have been derived from animal and human studies using experiences from vehicle collisions and laboratory experiments with stresses and rate of change of stress (i.e., strain rate) much lower than those associated with projectile and blast threats in the battlefield. Thus, a translation of these low-stress-rate data from animals, physical models, and mathematical simulations to the ballistic blunt trauma case is not expected to be reliable. As a consequence, design of protection from typical military threats is compromised because we do not know the injury thresholds.
A study by the Institute of Medicine found evidence for association between TBI and various disorders that included adverse social-functions, endocrine dysfunction, depression, aggressive behavior, and dementias for moderate or severe TBI (Ishibe et al., 2009). Further, concussion is no longer accepted as a threshold for diagnosis of potential brain trauma. Modern diagnostic methods reviewed in Appendix F show signatures of mild TBI (mTBI) unrelated to presence of concussion.
Once the acute medical events are treated, current clinical practice is not capable of effectively enhancing natural recovery or diminishing long-term effects after the blunt trauma (Giza et al., 2013). Thus, the best approach is protection from blunt brain trauma. This chapter presents relevant physiological and biomechanical aspects of blunt trauma, the state of knowledge regarding injury tolerances, and perspectives on detection of mTBI through noninvasive imaging. Current noninvasive methods of brain injury detection are in Appendix F. Aspects of helmet design and the threat characteristics are given in Chapters 2 and 3.
Historical Data
TBI can result from a number of events: falls, motor vehicle accidents, bicycle accidents, collisions, blast exposure, and blunt head trauma in the battlefield. More than 5 million Americans alive today have had a TBI, and the associated medical care cost is around $56 billion per year in the United States. Cognitive, communicative disabilities and social behavior abnormalities as well as medical complications, such as hormonal deficiencies that affect functioning of the brain, thyroid, and gonads, are prevalent in survivors of TBI.
Figure 10-2 shows the annual incidence of TBIs in war-fighters during the period 2000-2011.1 It is likely that the increasing numbers of mild and moderate TBI relative to severe TBI may be partly attributable to greater awareness of TBI risk among military clinicians (Okie, 2005; Warden, 2006). During this period, 220,430 service members had sustained TBI, with 169,209 classified as concussion/mTBI (Kelly et al., 2012). In a study of 3,973 soldiers who served in Iraq, 23 percent percent had a clinician-confirmed history of TBI (Terrio et al., 2009). In a separate study, mTBI in soldiers deployed in Iraq was found to be strongly associated with posttraumatic stress disorder and depression (Hoge et al., 2008). The deployment of magnetic resonance imaging methods to the evaluation of brain injury related to blast exposure of warfighters (Mac Donald et al., 2011; Yeh et al., 2013) can potentially provide a refinement in diagnoses of brain injury in warfighters exposed to non-concussive blast and blunt trauma events. However, in one study white matter injuries were not revealed by magnetic resonance diffusion tensor imaging (DTI) on veterans with mTBI, despite their symptoms of compromised verbal memory (Levin et al., 2010).
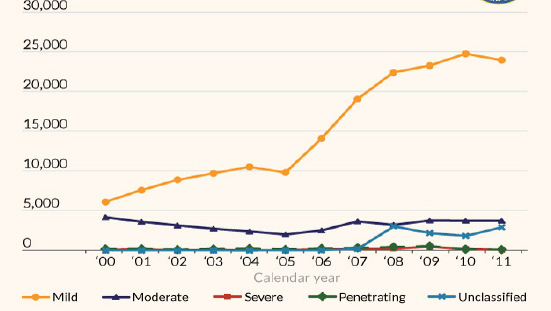
Figure 10-2 Incidence of traumatic brain injury classified by severity for warfighters. SOURCE: DoD Worldwide Numbers for Traumatic Brain Injury, http://semanticommunity.info/Binary_at_LandWarNet_2011/Defense_and_Veterans_Brain_Injury_Center_Site_Map/DoD_Worldwide_Numbers_for_Traumatic_Brain_Injury.
10.3 HEAD AND BRAIN INJURY TOLERANCES
Brain response and brain injury tolerances are not well established for high-rate impacts such as those from BFD or blasts (Bass et al., 2003, 2012; Rafaels et al., 2012).
Head Injury Tolerance Standards for Vehicle Collisions
Early work on low-rate blunt trauma brain injury tolerance (Gurdjian et al., 1966; Ommaya and Hirsch, 1971; Ono et al., 1980) emphasized that acceleration of the head and the time duration of the acceleration are important parameters for assessing injury severity (Prasad and Mertz, 1985). Such criteria are in wide use in the automobile impact community (FMVSS-208, EuroNCAP), but the injury risk functions using these parameters have not been universally accepted. The most widely used criterion is known as the Head Injury
_________________
1Armed Forces Surveillance Program information available at http://semanticommunity.info/Binary_at_LandWarNet_2011/Defense_and_Veterans_Brain_Injury_Center_Site_Map/DoD_Worldwide_Numbers_for_Traumatic_Brain_Injury. Last accessed on January 31, 2014.
Criterion (HIC) severity index. Although it is widely used, it is recognized as inadequate to fully explain brain injury outcome (Versace, 1971). For military helmets, HIC and similar concepts incorporating global skull rotational parameters (e.g., Newman et al., 2000) assume rigid body motion of the head/brain system and do not incorporate local deformations that may be crucial for assessing the injury potential from ballistic impacts (Bass et al., 2003).
Some measures based on internal stresses and/or strains have been proposed as the injury criteria for the brain (e.g., Stalnaker et al., 1971; Takhounts et al., 2003). However, there is still no universally acknowledged criterion, and the situation today is much the same as that articulated by Goldsmith (1981):
Thus, the state of knowledge concerning trauma of the human head is so scant that the community cannot agree on new and improved injury criteria even though it is generally admitted that present designations are not satisfactory. Minimally, there is an urgent need to differentiate skull fracture and mechanical and/or physiological damage to the central nervous system, with a replacement of a critical acceleration level for the former by a limiting stress value.
In the past 30 years, experimental data and models have been accumulating from animal, cadaver, physical models, and computational modeling and simulation studies (discussed later in this chapter). With further research, these data and models can lead to injury risk evaluations such as those done for the risk of a skull fracture for 9-mm bullet impacts to the helmet as detailed below. A goal is to determine the injury risk function for the major brain tissue injuries of Table 10-1 relevant to militarily relevant injuries such as those associated with BFD and blunt and blast neurotrauma.
Recommendation 10-1. There is an urgent need to establish stress and stress rate or other parameters as metrics for categories of brain tissue injuries from ballistic and blast-based head exposures.
Nonmilitary Helmet Protection Standards
There have been major advances in blunt head protection over the past 30 years. Some of these advances are be due to widespread use of helmets in athletics and the subsequent reduction in both frequency and severity of head and neck injuries. Many improvements in helmet technology have followed from the development of standardized test methodologies based on mechanical blunt impact injury criteria. The Advisory Group for Aerospace Research and Development (AGARD) Report AR-330 lists 29 blunt impact test standards (AGARD, 1996), and each of these standards has some form of translational impact acceleration limiting criterion. Of these standards, 19 are based on acceleration or force peaks alone, and 10 are based on acceleration/duration levels. The levels specified in these standards range from 150 to 400 g, with more recent standards tending to the 150 g peak limit.
Studies of football impacts suggest that an acceleration standard of approximately 80 g should be used to provide protection below the threshold for changes in mentation (Duma, et al., 2005). Other relevant results include: the Advanced Combat Helmet standard (CO/PD-05-04), which is based on the motorcycle helmet Federal Motor Vehicle Safety Standard-218 (49 CFR Sec. 571.218); and the National Operating Committee on Standards for Athletic Equipment and standards incorporating the International Standards Organization headforms. Virginia Polytechnic Institute’s star rating system for helmets2 involves extensive impact tests and risk analysis to establish a rating for commercial football helmets.
These criteria are based, in part, on underlying assumptions that are not realistic, especially for military use with ballistic protective helmets. The first is that the head acts as a rigid body so that acceleration or some derivative may be correlated with injury and that head injury of any type is associated with skull fracture (Hodgson and Thomas, 1973). Previous studies show a poor correlation between skull fracture and brain injury (Viano, 1988). For ballistic BFD injuries, local deformations invalidate the rigid body assumption, and injuries seen from BFD are not well correlated with acceleration-based measures.
10.4 BRAIN TISSUE INJURY: EXPERIMENTAL RESULTS
Over the past 70 years, researchers have attempted to understand the relationships between head, skull, and brain injury mechanisms and blunt trauma using cadavers, physical models, animals, and computer simulations. This has been stimulated largely by the automobile industry in an effort to improve vehicle occupant safety. More recently, sports injuries have triggered international efforts to improve helmet protection and to make measurements on human subjects involved in collision sports. Currently, there is no satisfactory experimental model that can produce the complete spectrum of brain injuries that are seen clinically while also being sufficiently well controlled and quantifiable for defining brain injury tolerances. Some data do exist for the stress associated with skull fracture, but this is only part of the spectrum of short- and long-term consequences of ballistic impacts to the helmeted soldier, and the low-rate tests generally available may not be applicable to ballistic impacts.
Early Investigations of Mechanisms
In the early 1940s, investigators proposed that brain injury from skull fractures was from intracranial pressure. However, physical studies using photoelastic models of the head dem-
_________________
2Additional information is available at http://www.sbes.vt.edu/nid.php.
onstrated that the likely cause of diffuse brain injury is from tissue strains induced by rotational acceleration of the head (Holbourn, 1943). This was confirmed by Gurdjian et al. (1955). The investigation of the relative roles of translational and rotational accelerations using more elaborate experimental models of the subhuman primate led to the conclusion that diffuse injuries to the brain occurred only in the presence of head rotational motion (Gennarelli et al., 1972; Gennarelli and Thibault, 1989; Ommaya and Gennarelli, 1974). Diffuse brain injuries occurred at lower angular deceleration levels as the pulse duration increased (Gennarelli and Thibault, 1989).
In coronal plane rotational acceleration, the critical shear strain associated with the onset of diffuse axonal injury was about 10 percent, and the rotational acceleration threshold for severe diffuse axonal injury was about 16,000 rad/sec2 (Margulies et al., 1990). Inertial loading alone to the head can cause DAI, which is an important cause of fatality or late onset of disabilities due to head injury (Gennarelli et al., 1972).
Modern Experimental Investigations of Injury Criteria
To simulate the impact response of the human, the automotive industry developed the Hybrid III 50th Percentile Male anthropometric test device (ATD) in the early 1970s. Originally developed by General Motors, the ATD is now regulated by the National Highway Traffic Safety Administration (NHTSA) in conjunction with the committees from the Society of Automotive Engineers. It has become a validated tool for the evaluation of automotive impacts and can accommodate a wide range of instrumentation and transducers. It is also robust enough for repeated ballistic experiments (Bass et al., 2003).
A collaborative effort between Natick laboratories, DRDC-Valcartier, and the University of Virginia (UVA) led to the development of a ballistic version of the Hybrid III head augmented with impact pressure sensors (Bass et al., 2003). The UVA headform is shown in Figure 10-3a. Instrumentation for the Hybrid III head and neck region consisted of three linear accelerometers and angular rate sensors at the center of the ATD headform and six-axis upper and lower neck load cells. Using the Hybrid III headform modified to accept surface pressure sensors, the pressure measurements at various locations were recorded, analyzed, and compared to human cadaver results (e.g., Bass et al., 2003). Injury metrics assessed using this headform include force/pressure, the HIC injury criterion, and the National Institute of Justice Neck Injury Criteria. The force/pressure results correlated well with injury in the paired cadaver model, while HIC was poorly correlated with injury. This concept has been recently modified in a rigid headform with regional loadcell sensing under the ballistic impact by Biokinetics (Figure 10-3b).
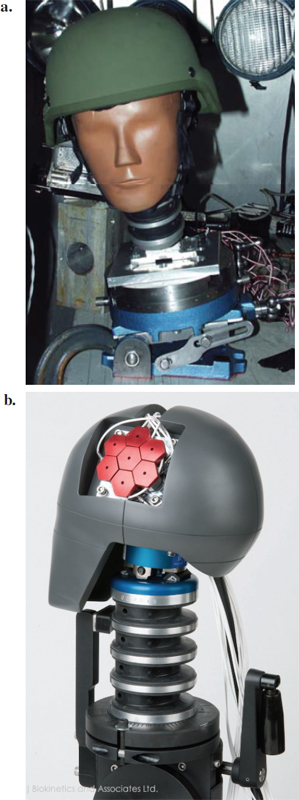
FIGURE 10-3 (a) The University of Virginia’s Hybrid III head model used for laboratory simulations and measurements. (b) Biokinetics headform variant of the Hybrid III headform for ballistic impact. SOURCE: Courtesy of Biokinetics and Associates, Ltd.
Brain Injury/Concussion Risk/Thresholds
Concussion is a symptom of the state of awareness or consciousness and is not a category of pathological brain tissue injury. The linkage between a diagnosis of concussion and a specific brain injury has been the subject of controversy among neurologists and neurosurgeons since the mid-1920s (Saucier, 1955). For example, one cannot say to what extent structural damage, such as vascular ruptures or neuronal strains, cause loss of consciousness. What have been defined experimentally are the relations between stress and animal consciousness over a limited range of stress rates that have not included the rates associated with a high-velocity, ballistic, nonpenetrating hit to a helmet. The threshold for concussion increases as the duration of impact decreases (Guardjian et al., 1955). See Table 10-2 for the median concussion levels trauma given in dimensions of energy, power, and pressure.
The criteria for concussion in the animal laboratory studies reflected in most of the studies of Table 10-2 are much different from concussions diagnosed in sports, vehicle collisions, falls, and battlefield events. The majority of concussions do not result in a loss of consciousness. In particular, for sports injuries, a concussion is diagnosed if the athlete is confused, complains of dizziness, headaches, blurred vision, or sensitivity to light, sound, or odors or by the physical signs of motor coordination dysfunction (cf. Appendix F). Ninety-five percent of high school football concussions did not involve loss of consciousness (Meehan et al., 2010). In the battlefield, a diagnosis of mTBI or equivalently “concussion” involves a protocol called Military Acute Concussion Evaluation (MACE). This examination is given as soon as possible after a warfighter has been exposed to blast, projectile blunt trauma, or vehicle collision. It measures orientation, recent memory, concentration, and memory recall.
TABLE 10-2 Brain Injury Criteria and Median Values for Concussion for Low-Rate Blunt Impact
|
Brain Injury Criteria |
Median Values for Concussion |
Source |
|
Energy |
22-24 J |
Denny-Brown and Russell (1941) |
|
Power |
13 kW |
Newman et al. (2000) |
|
Strain |
0.2 |
Bain and Meaney (2000); Morrison et al. (2003)a |
|
Strain x strain rate |
30 s-1 |
Viano and Lövsund (1999) |
|
Stress (von Mises) |
6-11 kPa |
Shreiber et al. (1997) |
|
Cumulative strain damage measure |
0.55 |
Takhounts et al. (2003) |
|
Strain energy density |
0.8-1.9 kJ/m3 |
Shreiber et al. (1997) |
|
Pressure |
173 kPa |
Ward et al. (1980) |
a Strains less than 0.15 can cause diffuse axonal injury.
Modern Football Helmet Instrument Data versus Concussion Symptoms
In the early 1970s, head-bands of suspension-style football helmets were instrumented with an accelerometer and electroencephalogram system (Moon et al., 1971; Reid et al., 1974) that allowed records from a single player at a time. Around 2000, hockey and football helmets were instrumented with three-dimensional accelerometers, and these measurements gave an average of 29 g from 158 impacts from high school athletes with no observed symptoms of TBI. Addition of video analysis and dummy reenactments allowed laboratory simulations and measurements of head acceleration, although there are substantial limitations in inferring accelerations directly from video (Newman et al., 2005; Pellman et al., 2003). Velocities and changes in velocities were interpreted from video recordings, and threshold values for concussion were given based on analyses simulating the video impacts with the Hybrid III dummy headform. These studies did not clear up potential distinction between injuries from rotational and translational accelerations (Genarelli and Thibault, 1989; King et al., 2003). But it is important to note that: (1) purely translational or rotational accelerations of the head are not likely for a head tethered to the inertial mass of the body (King et al., 2003); and (2) even purely translational acceleration of the head produces rotational behavior in the brain tissue, and purely rotational excitation of the brain produces local translational behavior in the brain tissue. Thus, the debate regarding the severity of rotational acceleration versus translational acceleration brain trauma is largely artificial and is based on a rigid body view of the head.
Actual measurements of direction and magnitude of head accelerations football players receive became available when sensors and telemetry units were provided to multiple players using an in-helmet 6-accelerometer system that transmits data via radio frequency to a sideline receiver and laptop computer system (Duma et al., 2005). Using this commercial system, a risk of sustaining a concussion for a given impact was derived from data collected from 63,011 impacts including 244 concussions (Rowson and Duma, 2013). Both linear and rotational accelerations as well as the combination of linear and rotational accelerations were used in the derivation of a concussion risk function. The predictive capability of linear acceleration was about the same as that for the combined probability.
A study of the linkage of impact severity was done on high school football players using cognitive tests and magnetic resonance imaging (MRI) before and after two seasons of football while wearing accelerometer instrumented helmets (Breedlove et al., 2012). A relationship was found between the number of impacts and cognitive tests and the number of hits and functional MRI changes (see also Talavage et al., 2013). It is expected that an expansion of these types of study will improve the development of head injury criteria
and clinical evaluation techniques as well as enhance return-to-play decision making.
Military helmet sensor instrumentation programs in the United States were initiated in 2008 in order to collect battlefield data that could then be used by medical epidemiologists as well as design and manufacturing communities to improve design. These data should help significantly in the quest to understand the linkages between stresses on the helmet and brain injuries.
Skull Fracture
Modern ballistic protective helmet materials (McManus et al., 1976; Carey et al., 2000) can deform sufficiently so that the backface of the helmet contacts the head, potentially causing head injuries (Bass et al., 2002). Potential injuries include both depressed and long-linear skull fractures and closed-head brain injuries. Substantial work has been done on skull fracture injury, especially at low rates, but most of it is not directly applicable to military helmet injury criteria.
Skull fracture is a measure of head injury that can be related to the forces applied and thus can provide one of the needed links between level of protection and threats. But most of the existing measurements are restricted to low velocities and large impact areas (Yoganandan et al., 1995; Bass and Yoganandan, 2013) and have limited relevance to the goal of linking battlefield threats to required protection for head and brain injury protection.
Whatever is known is based on cadaver measurements of skull fracture and recordings from internally placed pressure sensors and accelerometers. The mechanical properties of stiffness, force deflection, and energies to fracture were measured on 12 unembalmed cadaver skulls (Yoganandan et al., 1995) at low rates typical of blunt trauma from conventional falls and vehicle crashes. Impact loading at 7 to 8 m/s revealed failure loads of 6.4 kN (±1.1) and energies averaging 33.5 J (±8.5). Quasistatic loading at 2.5mm/s showed failure at 12 mm (±1.6). Variability was great in all parameters with, for example, a range of stiffness of 467 to 5,867 N/mm. Delye et al. (2007) found skull fracture energy level in the range of 22 to 24 J for dynamic loading of the cadaver head having one degree of freedom. A human cadaver study of fracture thresholds for 37-mm diameter projectiles of 25 to 35 g gave force values of 6 kN for the forehead, 1.9 kN for the mandible, and 1.6 kN for the zygoma (Viano et al., 2004). Impact stress values for the adult skull are given as 43 MPa (Ommaya et al., 2002) and are age and size related.
Two series of ballistic impact tests used human cadaver heads with protective helmets (Bass et al., 2003). These tests used UHMWPE helmets with 9-mm full-metal-jacket test rounds under various impact velocities to 460 m/s (1,510 ft/s). Measurements taken from cadavers with and without skull fracture show no correlation with existing blunt trauma injury models based on the Wayne State Concussive Tolerance Curve or similar concepts, including HIC. For the skull fracture tests, the calculated injury assessment value was well below the usual low-rate blunt trauma injury reference value. Further, there was no obvious association of acceleration-based responses to the occurrence of BFD and fracture. This study developed injury criteria for both test round velocity and cadaver peak-impact pressure. For this injury risk function, there is a 50 percent risk of skull fracture for a peak impact pressure of 51 MPa as measured by the force/strain instrumentation (Figure 10-4). Using a simple velocity correlation between the dummy and cadaver, a dummy injury risk function is developed that has a 50 percent risk of skull fracture for dummy peak impact pressure of 15,220 kPa. This injury risk function may be used with a general helmet and the Hybrid III dummy discussed earlier in this section.
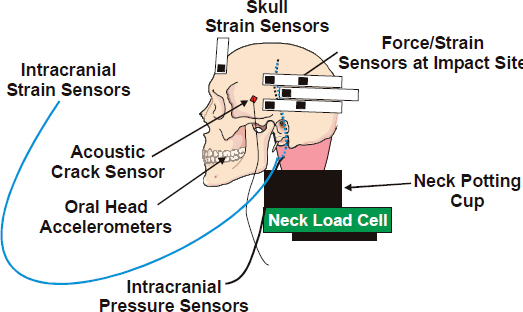
FIGURE 10-4 Instrumented cadaver head. SOURCE: NRC (2012).
Automobile injury criteria, including the HIC, were not found to be a good predictor of cadaveric injury. Skull fracture from ballistic BFD is an intrinsically high rate event. Energy is deposited locally, and local skull deformations are significant. Use of HIC requires essentially rigid body motion of the head at relatively low rate compared to ballistic events.
Finding 10-1. Most of the experimental work that links brain injury to blunt trauma is related to vehicle collisions and football collisions. The data from these studies are not directly relevant to BFD and blast TBI because the rate of momentum change is higher and contact times shorter for military TBI situations.
Brain Intracranial Pressure and Edema
Symptoms from intracranial pressure (ICP) increases can be acute and an immediate consequence of the stress wave from blunt trauma to the brain or transmitted pressures from trauma delivered to remote parts of the body. The experimental data that link ICP elevations to blunt trauma to the surface of a helmet or surrogate protective material come from a limited number of experiments that used live animal models, cadavers, and physical models (Engelborghs et al., 1998;
Shridharani et al., 2012b; Rafaels et al., 2012; Sarron et al., 2004). The models differ in characteristics, and the ballistic trauma mechanism varies from dropping masses from varying heights in order to vary the velocities of projectiles (e.g., 9-mm rounds from 300 to 800 m/s). These types of data can be used to extrapolate a threshold for ICP elevation versus armor characteristics and threat velocity. Although there are limitations in animal model biofidelity with human skulls, these types of experimental data are needed to better assess brain injury tolerances and risk levels for defined threats. Some models, although illustrative of the sequence of events after brain trauma (e.g., occurrence of edema, blood brain barrier changes, ion concentration variations), are difficult to interpret relative to the ballistic threats and even collision impacts as they use impactors systems of low velocity (3m/s) and poorly or undefined energy or force metrics (e.g., Cernak et al., 2004).
Brain Shear Stress and Diffuse Axonal Injury
Diffuse brain injury from low-rate traumatic impacts to the head results in both destructive and nondestructive axonal injury. Destructive axonal injury was first described for cases of collision-based injuries leading to limited periods of survival with autopsy findings of disrupted white matter tracks and normal grey matter (Strich, 1956, 1961). It is unknown whether such injuries can arise from ballistic BFD. Morphological studies of axonal injuries using non-human primates subjected to head acceleration have shown that shear forces create varying degrees of axonal damage, including fragmentation. Nondisruptive or reactive axonal injuries manifest over long time periods and are ascribed to axonal membrane damage. It is now recognized that animal models do not reflect the spatial and temporal patterns of axonal injury in human brains (Maxwell et al., 1997; Bain and Meaney, 2000).
Margulies and Thibault (1992) is one of the most detailed experimental and modeling studies relative to thresholds of brain injury, and it showed that a combination of a peak rotational acceleration of more than 10 krad/s2 and a peak change in rotational velocity of more than 100 rad/s causes diffuse axonal injury. These criteria are proposed to be valid only for pure rotational accelerations, but the experimental model incorporates translational accelerations about the brain center of gravity and the effect of these accelerations is uncertain. Lower injurious risk levels for rotational acceleration were proposed by others (Ueno and Melvin, 1995; Meaney et al., 1995). Thresholds for human brain injuries from Margulies and Thibault (1992) are shown in Figure 10-5.
Animal studies, physical model experiments, and analytical model simulations have been employed to determine the critical tolerances in terms of strain (relative elongation) and deterioration in function (Gennarelli et al., 1972; Lewis et al., 1996; Bain et al., 2001). Based on animal studies, the strain tolerance for frank axonal injury that may lead to DAI occurs when neuronal axons are stretched by more than about 20 percent. The results from simulations presented in the discussion of modeling and simulations later in this chapter show maximum strain levels of 14 percent and lower (from 9-mm rounds) at 360 m/s striking helmets (Aare and Kleiven, 2007). However, it is not clear if these thresholds are safe for injury effects that might manifest years after the injury.
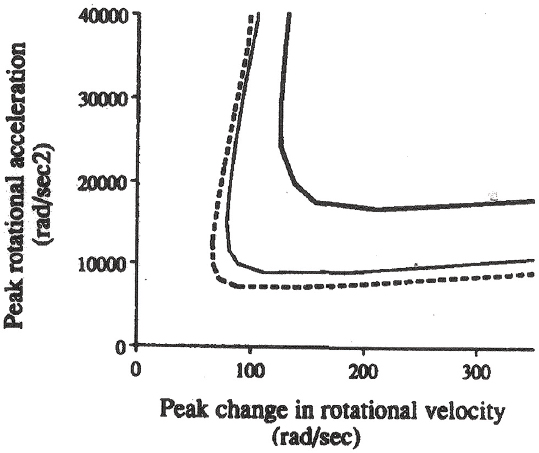
FIGURE 10-5 Thresholds for diffuse axonal injury based on nonhuman primate rotational acceleration experiments and scaling through computational modeling to human brain masses of 500 g (thick solid curve), 1,067 g (solid curve), and 1,400 g (dotted curve). Regions to the upper and right of each curve are regions of diffuse axonal injury. SOURCE: Reprinted from Margulies and Thibault (1992) with permission from Elsevier.
The threshold for nondisruptive axonal damage of 15 percent has been suggested by Maxwell and associates (Maxwell et al., 1997). But it is not clear that the 15 percent strain criterion should be an important benchmark, because tissue tolerance of the hippocampus and brainstem might be much lower, and the strain criteria are expected to be stress-rate dependent. Computational models and simulations can explore the structural strains of simulated brain tissues related to physical variables of a ballistic or blast impact (e.g., acceleration, stress rate, stress duration, etc.). But an important point is the understanding that nondestructive axonal damage can be the major cause of the high prevalence of posttraumatic stress syndrome months and years after brain trauma.
A summary of the current status of mechanisms, symptoms, and possible treatments of DAI is now available from the May 2011 workshop hosted by the National Institute of Neurological Disorders and Stroke (Smith et al., 2013).
Biological Response of Cells Exposed to Mechanical Forces
A key aspect of defining tissue tolerances is to describe the pathophysiological activation of cellular biochemical cascades that produce delayed cell damage and death. This can be accomplished by measurements of the consequences of mechanical injuries on living brain tissue through observations of cell viability and tissue biochemical changes using a tissue culture model of rapid stretch induced injury (Ahmed et al., 2000) or pulse pressure pulse exposure TBI (Morrison et al., 2003). Stretch-induced injuries associated with about 30 percent strains alter mitochondrial membrane potential and cellular bioenergetic molecules, as shown by chemical assay methods applied at various times after injury (Ahmed et al., 2000). Strains and strain rates can be precisely applied and responses measured by fluorescent imaging and immunostaining, including cell death quantification (Morrison et al., 2003). Cellular energy metabolism perturbations have been shown through standard molecular biology studies using in vitro and in vivo shock tube models of blast-induced TBI (Peethambaran et al., 2013). Blast exposures resulted in significant decreases in neuronal adenosine triphosphate levels at 6 h post-blast that returned towards normal levels by 24 h.
Finding 10-2. There are no data on axonal injuries from backface deformation. Also, currently there is no method to detect if diffuse axonal injury has occurred from head trauma in the battlefield.
Recommendation 10-2. Methods including blood sampling and brain imaging should be explored for feasibility of early detection of diffuse axonal injuries.
Evidence for Differential Motion of the Brain and Skull
A mechanism for many consequences of rapid accelerations and decelerations is the shearing caused by differential motion between the skull and local brain tissue. Typical injuries include contusions and meningeal hematomas seen in automobile accidents. The first definitive study of brain motion after a traumatic skull impact was done on live subhuman primates using a Lucite cover over the skull vertex. Blunt trauma was applied by a pneumatic impactor, and observations were made with cinephotography (Pudenz et al., 1946). These authors also provided a detailed review of theories and observations from the late 1800s regarding brain motion as well as contusion and hemorrhage mechanisms.
Although experimental studies demonstrate motion between brain and skull, little data exist regarding the base of the skull. Experiments on human subjects used MRI tagging techniques to show that the brain rotates relative to the skull (Kleiven and Hardy, 2002). Relative brain-skull displacements of 2 to 3 mm in some areas of the brain for induced linear and angular accelerations of 1.5 g and 120-140 rad s–2, respectively. These accelerations are orders of magnitude less than those associated with concussions. Small displacements were found in regions having brain-skull connections. Strain fields seen in this study exhibited significant areas with maximal principal strains of 5 percent or greater at these low experimental accelerations. Simple head flexion causes cerebellum rotation of a few degrees and a downward motion of up to 1.6 mm of the brain stem (Ji et al., 2004).
Hemorrhage: Petechial Disruption, Subdural Hematoma, and Epidural Hemorrhage
There are three principal types of internal vascular disruptions from shear stresses and rotational accelerations that cause shear strain on small and large blood vessels and lymphatics: petechial, subdural, and epidural hemorrhages.
Petechial hemorrhages can occur throughout the brain and give evidence of shear strain as well as a pressure-based disruption of capillaries and arterioles. The pressure can be from a remote stress such as a blunt trauma to any part of the body and possibly from blast stresses of high intensities (NRC, 2012). These hemorrhages appear as blood extrusions of a millimeter or less in diameter in the midbrain, but they can be extensive throughout the brain. They are not recognized as a clinical entity unless they disrupt sensory or motor functions of the brain. But they can cause some compromise of brain function and perhaps play a role in progressive brain deterioration. They can be detected by high-field MRI if the proper MRI pulse sequence is used. Subdural hemorrhages leading to subdural hematomas occur in the space between the dura (the outer cover over the brain) and the arachnoid space.
Epidural hemorrhages are bleedings from ruptured vessels between the skull and the outer layer of dura. The build-up of blood causes an increase in pressure within the intracranial space, with subsequent compression of brain tissue and obstruction of the flow of blood and cerebral spinal fluid. This is associated with particularly serious brain injury because 15 to 31 percent of patients die of the injury (Leitgeb et al., 2013).
Pituitary/Hypothalamus Damage
The pituitary gland is a pea-sized gland suspended from a pedicle at the base of the brain. It is surrounded by a skull base bone structure whose saddle-shaped structure is known as the sella turcica (Figure 10-6). This gland secretes nine hormones, some of which control the secretion of other hormones that are vital to growth and metabolism and whose dysfunctions have been related to disorders beyond metabolism, including behavioral and affective disorders. Pituitary gland dysfunction has been inferred from the occurrence of hypopituitarism in victims of head injury from low-rate impact.
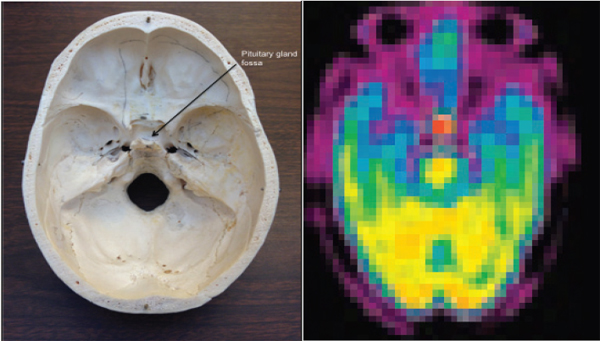
FIGURE 10-6 Left: The base of the human skull supports the bottom of the brain and the brain stem that descends through the large orifice in the center known as the foramen magnum. Right: Positron tomography of the uptake of ammonia-13N in the normal pituitary. SOURCE: (Left) Image provided courtesy of member Tom Budinger. (Right) This research was originally published in JNM. Xiangsong, Z., Y. Dianchao, and T. Anwu. Dynamic 13N-Ammonia PET: A new imaging method to diagnose Hypopituitarism. Journal of Nuclear Medicine. 2005;46:44-47. Copyright by the Society of Nuclear Medicine and Molecular Imaging, Inc.
Chronic hypopituitarism, defined as deficient production of one or more pituitary hormones at least 1year after injury, occurs in 40 percent of subjects who have sustained blunt brain trauma (Bondanelli et al., 2005). In contrast, the prevalence of hypopituitarism in the general population is estimated at 0.03 percent. As the hormones released from the pituitary are triggered by events in the hypothalamus, one cannot be certain of which tissue has been damaged. Growth hormone decreases develop in 15 to 20 percent of patients with complicated mild, moderate, or severe TBI and are associated with symptoms of posttraumatic stress disorder (Kelly et al., 2006; Powner et al., 2006). About 15 percent of TBI patients develop gonadal hormone deficiencies, and 10 to 30 percent of them develop hypothyroidism. After brain trauma, the short-term decline in hormones can recover in some cases, but there is a high prevalence of long-term deficiencies after severe TBI (Leal-Cerro et al., 2005; Agha et al., 2004). Chronic adrenal failure can occur because of low adrenocorticotropic hormone secretion from the pituitary in TBI patients.
Most studies found the occurrence of posttraumatic hypopituitarism to be unrelated to injury severity. In the past 2 years, researchers have found that about 42 percent of veterans with blast injuries showed abnormally low levels of at least one of the pituitary hormones (Wilkinson et al., 2012). Some veterans had abnormal levels of vasopressin and oxytocin, and these hormones are linked to psychological or behavioral abnormalities. It is not clear if this applies to ballistic BFD impacts.
Blood tests, some of which are complicated, can assess pituitary function. Positron emission tomography (PET) (Figure 10-6) and MRI, discussed in Appendix F, can noninvasively image metabolic function and structural abnormalities of the pituitary. MRI and PET can visualize anatomical and metabolic changes, respectively, as presented in Appendix F.
Recommendation 10-3. Modeling and simulation studies should incorporate the biomechanics of blunt brain trauma that affects the pituitary organ in the base of the brain in order to determine injury thresholds and tolerances for blunt trauma and for ballistic backface injuries.
Recommendation 10-4. The medical community should institute a data collection program to determine the prevalence of hypopituitarism in warfighters relevant to ballistic and blast blunt trauma with appropriate warfighter controls.
There is high prevalence of pituitary hypofunction in brain trauma from all causes. The recent discovery of low levels of pituitary hormones in TBI soldiers, coupled with the known replacement treatments for this disorder, mean that the medical community should launch a broad program of long-term periodic tests for veterans of head and blast injuries.
Neurobehavioral Effects from Traumatic Brain Injury
The linkages between the severity and frequency of blunt brain trauma to various physical injury classifications listed in Table 10-1 are the topics emphasized in this chapter. But there is another classification associated with brain trauma that has an association with TBI from all causes. Neurobehavioral changes include the specific neuropsychiatric syndromes of depression, mania, psychoses (e.g., paranoia and obsessive compulsive disease), aggressive behavior, and personality changes as well as cognitive decline. The causal associations have been debated for 100 years since the early papers on shell shock and also more recently because of the prevalence of psychiatric symptoms in veterans from wars of the past 70 years. Clear evidence of a causative relationship between negative neurobehavior and brain trauma has arisen in the past few years from pathological studies on athletes who have sustained TBI. Yet, despite some continuing skepti-
cism about the lack of objective studies, there is compelling evidence for associations between both behavioral and cognitive disorders and TBI. From the vast literature of reports of psychiatric and cognitive evaluations of TBI subjects, two cited below have measures of the prevalence.
Depression, anxiety, and low self-esteem were the principal disabilities in half of 360 head-injured individuals evaluated from the group who had survived for 7 years after an initial head injury (Whitnall et al., 2006). Another study showed the prevalence of depression is 6 to 39 percent with mTBIs (Schoenhuber and Gentilini, 1988).
Cognitive impairments 10 years following TBI were found to be associated with injury severity using tests of attention, processing speed, memory, and executive function (Draper and Ponsford, 2008). Even mTBI patients may perform worse than controls on some tests of reasoning (Borgaro et al., 2003). Long-term effects of mild head injury approximately 8 years post injury included complex attention and working memory defects (Vanderploeg et al., 2005). Early-onset dementia in particular is frequently associated with head injury history (McMurtray et al., 2006). Repeated concussions have been linked to dementia (Guskiewicz et al., 2005) and chronic traumatic encephalopathy (McKee et al., 2009).
Finding 10-3. An increased prevalence of neurobehavioral abnormalities has been confirmed from many scientific evaluations of individuals involved in TBI incidents.
10.5 COMPUTATIONAL MODELING AND SIMULATION
Computational modeling and simulation (M&S) has long been considered an invaluable tool for analyzing engineering systems in a wide range of technology areas. Recently, M&S has also been used effectively in the broad field of injury biomechanics and to a limited extent in the evaluation and design of force protection systems.
M&S can provide a quantitative description of the relevant physical system response that can be used to assess system performance and inform potential improvements. Significant effort has been devoted in the past several decades to developing the basic science, algorithms, simulation software, and hardware infrastructure to meet this goal. However, owing to the unique complexities associated with the interplay between the physics and biology of injury, the full potential of M&S in understanding of injury biomechanics and the design of protection systems is yet to be realized.
Analytical and computational modeling of ballistic perforation of materials has been exhaustively reviewed up to 1978 (Backman and Goldsmith, 1978) with an update 10 years later (Anderson and Bodner, 1988). More recent reviews are provided by King et al. (1995). But the biomechanics of blunt trauma to tissues is a major added complexity to M&S because of the need to incorporate biophysical and biomedical parameters.
This section of the chapter, on linkages between a ballistic or blast threat and brain injury, is directed toward the important role of computational models, as it is through this tool that one can equate needed protection from brain injury to helmet design. One principal value of M&S in human injury biomechanics is its ability to obtain information in situations in which it is fundamentally impossible to conduct in vivo tests on the actual system (the human), although postmortem testing is possible using human cadaver tests. This approach may be supplemented by in vivo testing in animal surrogates to understand force effects on the human body and possible ways to mitigate them. There are cases in which this approach has provided useful insights into injury biomechanics such as blast lung injury criteria (Bass et al., 2008) and to develop test equipment for vehicle collision tests against tissue injury criteria. However, as discussed in this report, in the particular case of military helmets, evaluation and acceptance protocols are based exclusively on tests that use head surrogates with poor biofidelity.
It is therefore clear that M&S can play a significant role both in improving understanding of injury biomechanics and in guiding the design of protective systems with enhanced injury mitigation performance. Analytical approaches include mathematical modeling and computer simulations using advanced constitutive models and coupled fluid-solid mechanics. In the past, these approaches have been challenged as inadequate because of limitations in the fidelity of the computer simulations, realism of the tissue material properties, and the lack of validation.
Computational Simulations of Brain Injuries from Blunt Trauma
Ten years ago NHTSA developed a Simulated Injury Monitor (SIMon), based on a finite-element head model. This tool uses vehicle-dummy-head kinematics as an input and estimates the probability of three types of injuries: diffuse axonal injury, contusions, and subdural hematomas (Takhounts et al., 2003). This system is intended for vehicle crashes, and it is unclear how the results might apply to ballistic BFD injuries.
SIMon has been upgraded and recently did evaluations using input from instrumented helmets on professional football players (Takhounts et al., 2008) and vehicle collisions (von Holst and Li, 2013). A finite element model of the human head described the dynamic response of the brain during the first milliseconds after the impact with velocities of 10, 6, and 2 m/s, respectively. Their simulations show what is called a dynamic triple maxima sequence: (1) strain energy density, (2) intracranial pressure, (3) the first principal strain. Limitations of the NHTSA simulation system include limited spatial fidelity, uncertainty in brain material properties, and limited incorporation of potentially important brain structures such as the hippocampus and the amygdala. For example, the relative motion of the brain and
skull is not modeled well with current computational model mesh sizes that do not provide the opportunity for insertion of the anatomy and material properties of vessels and tether points between the brain and the inner table of the skull. For example, the tensile strength of the dura material is much larger than brain tissues.
Simulations of Brain Strains from Ballistic Impacts on Helmeted Head
Finite element simulations to determine expected skull and brain tissue injuries from ballistic BFD trauma were performed in Sweden (Aare and Kleiven, 2007). These were performed using a validated human head and brain model as well as a model of the coupling between helmets of various stiffnesses and the head, so that tissue trauma parameters could be assessed based on the ballistic kinetic energy (ca. 518 J) of an 8 g, 9-mm bullet impact and angle of impact. The trauma parameters measured were stress in the cranial bone, strain in brain tissue, pressure in the brain, change in rotational velocity, and translational and rotational acceleration, as shown in Figure 10-7.
Computational Simulations of Brain Injury from Blast
Recent efforts in computational modeling of traumatic physical effects on the central nervous system have focused on blast-induced TBI. A reason for this effort is the need to resolve the controversy regarding the mechanism for brain injury dating from World War I when soldiers with neurological and neuropsychological symptoms were labeled “shell shocked” (cf. Bass et al., 2012). The linkage between symptoms and blast exposures is not the subject for this chapter, but the role of the helmet and face shield in mitigating the strain field is of great importance.
Several papers (including Moore et al., 2009; Chafi et al., 2010; Panzer et al., 2011; Przekwas et al., 2011; Nyein et al., 2010; and Sharma and Zhang, 2011) developed human head models from medical imaging data to study the interaction of blast waves with the head, including various anatomical structures resolved to various scales. Work still remains to be done on material properties, especially at blast-different stress rates (Panzer et al., 2012), but the body of this work suggests that blasts are a plausible cause of TBI, including the potential for axonal injury at various locations within the brain.
It has been clearly demonstrated that blasts can lead to the development of significant levels of pressure, volumetric tension, and shear stress in focal areas on a short time scale and that stress patterns are dependent on the orientation of the blast wave and the complex geometry of the skull, brain, and tissue interfaces (Taylor and Ford, 2009; Moore et al., 2009; Panzer et al., 2012).
A numerical and experimental investigation into the effects of low-level blast exposure on pigs used a two-dimensional pig head model that consisted of a skull model (Teland et al., 2010). They found that the blast wave propagates directly through the skull and that the orientation of
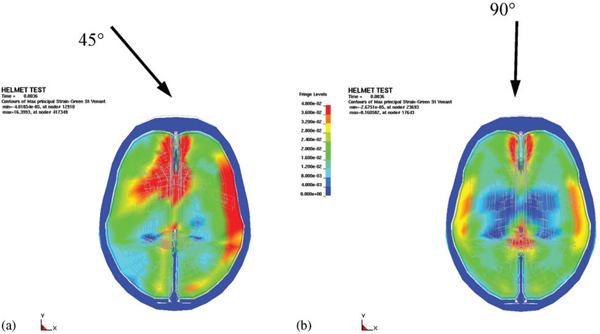
FIGURE 10-7 Principal strains in simulated brain material from projectile-induced kinetic energy striking a helmet at two angles. Blue is 0 percent, green is 2 percent, and red is >4 percent. SOURCE: Reprinted from Aare and Kleiven (2008), with permission from Elsevier.
the head is important. Another study constructed a better computational pig model consisting of skull, brain, cerebrospinal fluid, dura, and pia using computed tomography and MRI data (Zhu et al., 2013). The researchers found high pressures in the frontal and occipital regions, possibly due to wave reflection at the skull/brain interface. Examining strain, they found that the highest strains of 1.7 percent were in the brainstem, and the lowest strains of 0.2 percent were in the center of the brain. They also found that strains within the skull were two orders of magnitude lower than the strains within the brain and that the maximum deflection of the skull was less than 0.5 mm.
Very-high-resolution anisotropic models have been developed MRI T1 relaxation weighting and DTI with a three-dimensional, biofidelic finite-element volume mesh to conduct simulations of the stress and strain distributions after a frontal force of 7 kN impulse of 2.75 ms (Kraft et al., 2012). They then used a damage model based on data from rat experiments to predict cellular death based on axonal strain and strain rate. The temporal and occipital regions had the largest values of axonal strain and thus the highest amount of cellular death. Four days after injury, 19.7 percent of the network edges were fully degraded, but the network of axons remained intact. This type of analysis is new to blast-induced injury research and offers a promising route to connect biomechanical response to neurophysiological insight. It is unclear, however, what the brain material properties and detailed network behavior are in this basis, because the underlying experimental work has not been done.
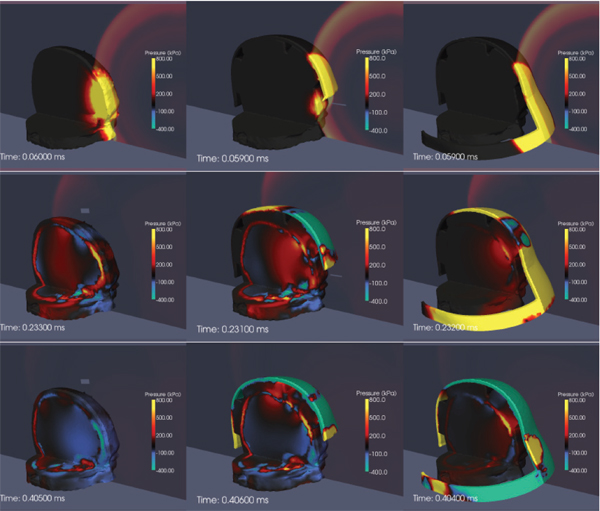
FIGURE 10-8 Computational simulations of the protective effect of the Advanced Combat Helmet (center column) and face shield (right column) show a significant attenuation of the transmitted pressure field when compared to the unprotected head (left column). SOURCE: Nyein et al. (2010).
Figure 10-8 shows results from large-scale computational simulations to compare the stress fields for blast exposures involving the head alone, head with helmet, and head with helmet and face shield (Nyein et al., 2010). Computer-aided design models of the actual ACH, including foam pads as well as a conceptual model of a mask protecting the face, were added to the detailed MRI-based model of the human head. For front blast conditions, the propagation of stress waves into the brain tissue is somewhat attenuated by the existing ACH and significantly attenuated by the addition of a face cover. This suggests a possible strategy to improve protection against blast-induced mTBI.
Other recent studies have considered the blast-mitigating effect of helmets (Panzer et al., 2010; Zhang et al., 2011; Shridharani et al., 2012a; Przekwas et al., 2011). These models and measurements have consistently shown strong mitigation of blast pressure behind the ACH.
Finding 10-4. Computational simulations of the protective effect of helmet and face shield show a significant attenuation of the transmitted pressure field.
In conclusion, M&S can prove a valuable tool in the analysis of the effects of mechanical threats (blast, impact) on brain tissue. Its main usefulness is in explaining mechanisms of momentum transfer from the external threat to the internal tissues, including the identification of areas of the brain that can be most vulnerable for particular threats. Simulations can also guide the design of protective gear and the assessment of the comparative effectiveness in mitigating the effect of the external threat on brain tissue. There is a clear opportunity to extend the existing use of M&S in the area of brain injury biomechanics and protective gear design, as in many other areas of science and engineering.
10.6 MECHANICAL AND CONSTITUTIVE PROPERTIES OF TISSUES
Characterization of the dynamic mechanical properties of brain tissue is important for developing a comprehensive knowledge of the mechanisms underlying brain injury and for developing computational models of potential ballistic and blunt neurotrauma. There are regional, directional, and age-dependent changes in the properties of the brain when it undergoes large deformations (Prange and Margulies, 2002). The frequency dependence of elastic properties must be included in comprehensive models, along with the frequency characteristics of the changing pressure field (Figure 10-9). Previous brain material characterizations at various stress rates suffer from wide experimental dispersion (Figure 10-9), nearly three orders of magnitude in the complex modulus. This has made comparison of computational results using these disparate data difficult (Panzer et al., 2012). Strain is dependent on the shear stress and stress rate, as shown in Figure 10-10. For a given shear stress, the strain on brain tissues is inversely related to the stress rate.
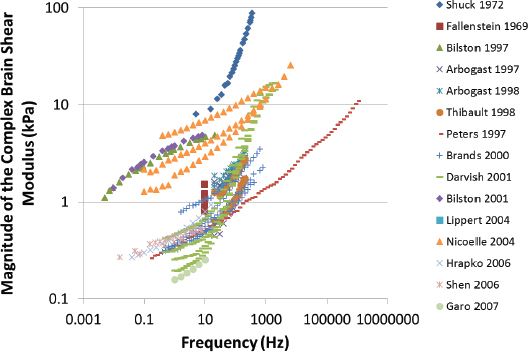
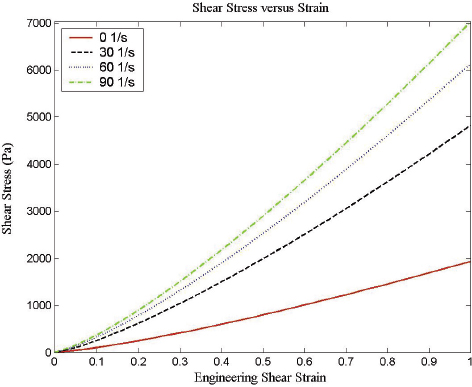
FIGURE 10-10 Dependence of shear strain on stress rate shows the importance of correct simulation of the shear stress rate in simulations. SOURCE: Adapted from Donelly and Medige (1997).
There has recently been significant progress in the experimental characterization and constitutive modeling of the mechanical response of brain tissue (Pervin and Chen, 2009; Prevost et al., 2011a, b). Tissue response exhibits moderate compressibility, substantial nonlinearity, hysteresis, conditioning, and rate dependence. A large-strain nonlinear viscoelastic model has been described that successfully captures the observed complexities of the material response in loading, unloading, and relaxation (Prevost et al., 2011a). This model covers strain rates—from quasistatic to
dynamic rates—comparable or exceeding those in blast and ballistic events with stress rates from 0.01 to 3000 s-1. But the low-strain-level behavior of brain tissue at high stress rates is not well known, and currently available results are not reliable because of the experimental methods employed to date. The results gathered to date on bovine and porcine tissue properties have been obtained mostly in vitro (Pervin and Chen, 2009; Prevost et al., 2011a). Previous studies on brain properties of note were on the juvenile pig (Gefen and Margulies, 2004). These results might differ quantitatively from those encountered in vivo, and this knowledge is critical for the development of biofidelic brain models. Further, different regions of the brain respond differently to identical mechanical stimuli, as shown in culture studies of the rat cortex and rat hippocampus. The cortex was less vulnerable to stretch-induced injury than the hippocampus (Elkin and Morrison, 2007).
Recently, Prevost et al. (2011b) measured the nonlinear dynamic response of the cerebral cortex to indentation of the exposed frontal and parietal lobes of anesthetized porcine subjects. Measurements included nonlinear, rate-dependent, hysteretic, and conditioning white and gray matter response in vivo, in situ, and in vitro. Results showed similar responses between in vivo and in vitro studies with respect to load versus indent and a “stiffening” with increase rate of stress. The data raise concerns regarding doing measurements in situ, wherein central circulation and cerebral spinal fluid pressures are much less than in vivo. Without the intact dura mater, whose tensile strength is much greater than other brain membranes, in vivo or in vitro measurements can be questioned, thus characterization of brain material properties might best be done by elastography using magnetic resonance techniques in vivo. But elastography does not have the spatial resolution to give region specific elastic properties and published values might be too low for studies of brain-surface-to-cortex relative motion or strains (cf. Coats et al., 2012).
Magnetic resonance elastography enables the visalization and measurement of mechanical waves propagating in three dimensions throughout a sample (Muthupillai et al., 1995; Manduca et al., 2001). From this information, the shear stiffness of the sample can be inferred. In MRE, oscillating shear displacements are generated by harmonic vibrations induced mechanically or acoustically on the skull or brain surface. The displacements are measured from phase images obtained by modulating the gradient field of the magnetic resonance scanner at the vibration frequency. These measurements have already shown the skull acts as a low-pass filter for frequencies of 45, 60, and 80 Hz. Skull transmission decreases, and shear-wave attenuation in the brain increases with increasing frequency (Clayton et al., 2012).
Further work is required to continue to improve and validate constitutive models—not just for brain but also for bone and other tissues. These models are essential for simulations of dynamic transients (impact from ballistic BFD/blunt trauma, blast/shock wave propagation) leading to TBI.
Finding 10-5. For models and simulations of brain trauma to be meaningful for injury assessments, they should include constitutive models of brain tissue response that account for nonlinear and rate-dependent viscoelastic effects. Viscoelastic brain properties for high rate, low strain levels necessary for ballistic BFD calculations are not established.
The protection of the warfighter afforded by helmets from threats ranging from bullets, shrapnel, blasts, vehicle collisions, and parachute landings has improved with improved helmet design and materials. However, the level of protection from nonfatal brain tissue injuries, which may have health consequences beyond the acute phase, is not known. This chapter and Chapter 3 give information regarding what is known about brain injury from blunt trauma and what is known about injury tolerances. In addition, this chapter defines the types of injuries that occur and most of the methods for diagnosis of both near- and long-term-onset medical conditions.
The principal finding is that there is not a known relationship between brain injury to the ballistic parameters of momentum, rate of change of momentum, acceleration, and time duration of the impact force. Findings in Chapter 3 emphasize that there is no known relationship between the measure of BFD by helmet evaluation protocols and skull fracture and brain trauma. This finding is known to the U.S. Army Medical Research and Materiel Command. Research is already underway on skull fracture injury criteria.3 Linkage of the ballistic threats whose physical parameters are known to brain injury must include knowledge of the protective attenuation of the helmet. The degree to which the listed types of brain-injury parameters are moderated by the helmet is not known.
Vehicle and sports collisions have been studied and modeled with attendant animal experiments. But parameters for the rate of change of momentum (i.e., force) and duration of contact are orders of magnitude different from those for ballistic injuries. Therefore, considerations in the design of sports and vehicle head protective devices as well as the parameters of injury tolerance are not the same as those encountered by the warfighter. The committee notes a broad effort to define mechanisms, develop diagnostic methods for evaluating organic damage to the brain, and methods for treatment. But the current principal approach is protection from transfer of injurious forces afforded by the helmet.
_________________
3Karin Rafaels, Army Research Laboratory, Survivability/Lethality Analysis Directorate, “Joint Live Fire Test Program Behind Helmet Blunt Trauma Skull Injury,” presentation to the committee on January 24, 2013.
Aare, M., and S. Kleiven. 2007. Evaluation of head response to ballistic helmet impacts using the finite element method. International Journal of Impact Engineering 34(3):596-608.
AGARD (Advisory Group for Aerospace Research and Development). 1996. Anthropomorphic Dummies for Crash and Escape System Testing. AGARD AR-330. Neuilly-Sur-Seine, France.
Agha, A., B. Rogers, M. Sherlock, P. O’Kelly, W. Tormey, J. Phillips, and C.J. Thompson. 2004. Anterior pituitary dysfunction in survivors of traumatic brain injury. Journal of Clinical Endocrinology and Metabolism 89(10):4929-4936.
Ahmed, S.M., B.A. Rzigalinski, K.A. Willoughby, H.A. Sitterding, and E.A. Ellis. 2000. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. Journal of Neurochemistry 74(5):1951-1960.
Anderson, Jr., C.E., and S.R. Bodner. 1988. Ballistic impact: The status of analytical and numerical modeling. International Journal of Impact Engineering 7(1):9-35.
Backman, M.E., and W. Goldsmith. 1978. The mechanics of penetration of projectiles into targets. International Journal of Engineering Science 16(1):1-99.
Bain, A.C., and D.F. Meaney. 2000. Tissue-level thresholds of axonal damage in an experimental model of central nervous system white matter injury. Journal of Biomechanical Engineering 122(6):615-622.
Bain, A.C., R. Raghupathi, and D.F. Meaney. 2001. Dynamic stretch correlates to both morphological abnormalities and electrophysiological impairment in a model of traumatic axonal Injury. Journal of Neurotrauma 18(5):499-511.
Bass, C.R., and N. Yoganandan. 2013. Skull and facial bone injury biomechanics. In Accidental Injury (N. Yoganandan, ed.). Springer Verlag, London, U.K.
Bass, C.R., M. Bolduc, and S. Waclawik. 2002. Development of a nonpenetrating, 9-mm, ballistic trauma test method. Pp. 18-22 in Proceedings of the Personal Armor Systems Symposium (PASS 2002), The Hague, Netherlands, November 18-22, 2002. Prins Maurits Laboratorium, Rose International Exhibition Management and Congress Consultancy, The Hague, Netherlands.
Bass, C.R., B. Boggess, B. Bush, M. Davis, R. Harris, M.R. Rountree, S. Campman, J. Ecklund, W. Monacci, G. Ling, G. Holborow, E. Sanderson, and S. Waclawik. 2003. “Helmet Behind Armor Blunt Trauma.” Paper presented at the RTO Applied Vehicle Technology Panel/Human Factors and Medicine Panel Joint Specialists’ Meeting held in Koblenz, Germany, May 19-23, 2003. NATO Science and Technology Organization, Neuilly-Sur-Seine, France.
Bass, C.R., K. Rafaels, and R. Salzar. 2008. Pulmonary injury risk assessment for short duration blasts. Journal of Trauma 65(3):604-615.
Bass, C.R., M.B. Panzer, K.A. Rafaels, G. Wood, and B. Capehart. 2012. Brain injuries from blast. Annals of Biomedical Engineering 40(1):185-202.
Bondanelli, M., M.R. Ambrosio, M.C. Zatelli, and L. De Marinis. 2005. Hypopituitarism after traumatic brain injury. European Journal of Endocrinology 152(5):679-691.
Borgaro, S.R., G.P. Prigatano, C. Kwasnica, and J.L. Rexer. 2003. Cognitive and affective sequelae in complicated and uncomplicated mild traumatic brain injury. Brain Injury 17(3):189-198.
Breedlove, E.L., Robinson, M., Talavage , T.M., Morigaki.K.E., Yoruk, K., O’Keefe, U., King, J. Leverenz, L J., Gilger, J.W., and Nauman, E.A. 2012. Biomechanical correlates of symptomatic and asymptomatic neurophysiological impairment in high school football. Journal of Biomechanics 45(7):1265-1272.
Carey, M.E., M. Herz, B. Corner, J. McEntire, D. Malabarba, S. Paquette, and J.B. Sampson. 2000. Ballistic helmets and aspects of their design. Neurosurgery 47(3):678-689.
Cernak, I., R. Vink, D.N. Zapple, M.I. Cruz, F. Ahmed, T. Chang, S.T. Fricke, and A.I. Faden. 2004. The pathobiology of moderate diffuse traumatic brain injury as identified using a new experimental model of injury in rats. Neurobiology of Disease 17(1):29-43.
Chafi, M.S., G. Karami, and M. Ziejewski. 2010. Biomechanical assessment of brain dynamic responses due to blast pressure waves. Annals of Biomedical Engineering 38(2):490-504.
Clayton, E.H., G.M. Genin, and P.V. Bayly. 2012. Transmission, attenuation and reflection of shear waves in the human brain. Journal of the Royal Society Interface 9(76):2899-2910.
Coats, B., S.A. Eucker, S. Sullivan, and S.S. Margulies. 2012. Finite element model predictions of intracranial hemorrhage from non-impact rapid head rotations in the piglet. International Journal of Developmental Neuroscience 30(3):191-200.
Delye, H., P. Verschureen, I. Verpoest, D. Berckmans, J. Vander-Sloten, G. Van Der Perre, and J. Goggin. 2007. Biomechanics of frontal skull fracture. Journal of Neurotrauma 24(10):1576-1586.
Denny-Brown, D., and W.R. Russell. 1941. Experimental cerebral concussion. Brain 64(2-3):93-164.
Donnelly, B.R., and J. Medige. 1997. Shear properties of human brain tissue. Journal of Biomechanical Engineering 119(4):423-432.
Draper, K., and J. Ponsford. 2008. Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychology 22(5):618-625.
Duma, S.M., and S.J. Manoogian, W.R. Bussone, P.G. Brolinson, M.W. Goforth, J.J. Donnenwerth, R.M. Greenwald, J.J. Chu, and J.J. Crisco. 2005. Analysis of real-time head accelerations in collegiate football players. Clinical Journal of Sport Medicine 15(1):3-8.
Elkin, B.S., and B. Morrison. 2007. Region-specific tolerance criteria for the living brain. Stapp Car Crash Journal 51:127-138.
Engelborghs, K., J. Verlooy, J. Van Reempts, B. Van Deuren, M. Mies Van de Ven, and M. Borgers. 1998. Temporal changes in intracranial pressure in a modified experimental model of closed head injury. Journal of Neurosurgery 89(5):796-806.
Gefen, A., and S.S. Margulies. 2004. Are in vivo and in situ brain tissues mechanically similar? Journal of Biomechanics 37(9):1339-1352.
Gennarelli, T., and L. Thibault. 1989. Clinical rationale for a head injury angular acceleration criterion. In Proceedings of Head Injury Mechanisms: The Need for an Angular Acceleration Criterion. Washington D.C. Available at http://www-nrd.nhtsa.dot.gov/pdf/esv/esv16/98S8O07.PDF.
Gennarelli, T.A., L.E. Thibault, and A.K. Ommaya. 1972. Pathophysiological responses to rotational and translational accelerations of the head. Stapp Car Crash Journal 16:296-308.
Giza, C.C., J.S. Kutcher, J. Barth, T.S.D. Getchius, G.A. Gioia, G.S. Grpnsethj, K. Guskiewicz, S. Mandel, G. Manley, D.B. McKeag, D.J. Thurman, and R. Zaafonte. 2013. Summary of evidence-based guideline update: Evaluation and management of concussion in sports. Neurology 80(24):2250-2257.
Goldsmith, W. 1981. Current controversies in the stipulation of head injury criteria. Journal of Biomechanics 14(12):883-884.
Grundfest, H. 1936. Effects of hydrostatic pressures upon the excitability, the recovery, and the potential sequence of frog nerve. Cold Spring Harbor Symposia on Quantitative Biology 4:179-187.
Gurdjian, E.S., J.E. Webster, and H.R. Lissner. 1955. Observations on the mechanism of brain concussion, contusion, and laceration. Surgery, Gynecology and Obstetrics 101(6):680-690.
Gurdjian, E.S., V.L. Roberts, and L.M. Thomas. 1966. Tolerance curves of acceleration and intracranial pressure and protective index in experimental head injury. Journal of Trauma and Acute Care Surgery 6(5):600-604.
Guskiewicz, K.M., S.W. Marshall, J. Bailes, M. McCrea, R.C. Cantu, C. Randolph, and B.D. Jordan. 2005. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 57(4):719-724.
Hodgson, V.R., L.M. Thomas, and J. Brinn. 1973. Concussion levels determined by HPR windshield impacts. SAE Technical Papers.
Hoge, C., D. McGurk, J. Thomas, A. Cox, C. Engel, and C. Castro. 2008. Mild traumatic brain injury in US soldiers returning from Iraq. New England Journal of Medicine 358(5):453-463.
Holbourn, A.H.S. 1943. Mechanics of head injuries. Lancet 2:438-441.
Ishibe, N., R.C. Wlordarczyk, and C. Fulco. 2009. Overview of the Institute of Medicine’s Committee search strategy and review process for Gulf War and Health: Long-term consequences of traumatic brain injury. Journal of Head Trauma Rehabilitation 24(6):424-429.
Ji, S., Q. Zhu, L. Dougherty, and S.S. Margulies. 2004. In vivo measurements of human brain displacement. Stapp Car Crash Journal 48:227-237.
Kelly, D.F., D.L. McArthur, H. Levin, S. Swimmer, J.R. Dusick, P. Cohan, C. Wang, and R. Swerdloff. 2006. Neurobehavioral and quality of life changes associated with growth hormone insufficiency after complicated, mild, moderate or severe traumatic brain injury. Journal of Neurotrauma 23(6):928-942.
Kelly, M.P., R.L. Coldren, R.V. Parish, M.N. Dretsch, and M.L. Russell. 2012. Assessment of acute concussion in the combat environment. Archives of Clinical Neuropsychology 27(4):375-388.
King, A.I., J.S. Ruan, C. Zhou, W.N. Hardy, and T.B. Khalil. 1995. Recent advances in biomechanics of brain injury research: A review. Journal of Neurotrauma 12(4):651-658.
King, A.I., K.H. Yang, L. Zhang, W. Hardy, D.C. Viano. 2003. Is head injury caused by linear or angular acceleration. Pp. 1-12 in Proceedings of the 2003 International IRCOBI Conference on the Biomechanics of Impact. IRCOBI, Lisbon, Portugal.
Kleiven, S., and W.N. Hardy. 2002. Correlation of a FE model of the human head with experiments on localized motion of the brain- consequences for injury prediction. Stapp Car Crash Journal 46:123-144.
Kraft, R.H., P.J. McKee, A.M. Dagro, and S.T. Grafton. 2012. Combining the finite element method with structural connectome-based analysis for modeling neurotrauma: Connectome neurotrauma mechanics. PLoS Computational Biology 8(8):pcbi.1002619.
Leal-Cerro, A., J.M. Flores, M. Rincon, F. Murillo, M. Pujol, F. Garcia-Pesquera, C. Dieguez, and F.F. Casanueva. 2005. Prevalence of hypopituitarism and growth hormone deficiency in adults long-term after severe traumatic brain injury. Clinical Endocrinology 62(5):525-532.
Leitgeb, J., W. Mauritz, A. Brazinov, M. Majdan, and I. Wilbacher. 2013. Outcome after severe brain trauma associated with epidural hematoma. Archives of Orthopaedic and Trauma Surgery 133(2):199-207.
Levin, H.S., E. Wilde, M. Troyanskaya, N.J. Petersen, R. Scheibel, M. Newsome, M. Radaideh, T. Wu, R. Yallampalli, Z. Chu, and X. Li. 2010. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. Journal of Neurotrauma 27(4):683-694.
Lewis, S.B., J.W. Finnic, P.C. Blumbergs, G. Scott, J. Manavis, C. Brown, P.L. Reilly, N.R. Jones, and A.J. McLean. 1996. A head impact model of early axonal injury in the sheep. Journal of Neurotrauma 13(9):505-514.
Mac Donald, C.L., A.M. Johnson, D. Cooper, E.C. Nelson, N.J. Werner, J.S. Shimony, A.Z. Snyder, M.E. Raichle, J.R. Witherow, R. Fang, S.F. Flaherty, and D.L. Brody. 2011. Detection of blast-related traumatic brain injury in U.S. military personnel. New England Journal of Medicine 64(22):2091-2100.
Manduca, A., T.E. Oliphant, M.A. Dresner, J.L Mahowald, S.A. Kruse, E. Amromin, J.P. Felmlee, J.F. Greenleaf, and R.L. Ehman. 2001. Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Medical Image Analysis 5(4):237-254.
Margulies, S.S., and L. Thibault. 1992. A proposed tolerance criteria for diffuse axonal injury in man. Journal of Biomechanics 25(8):917-923.
Margulies, S.S., L. Thibault, and T. Gennarelli. 1990. Physical model simulation of brain injury in the primate. Journal of Biomechanics 23(8):823-836.
Maxwell, W.L., J.T. Povlishock, and D.L. Graham. 1997. A mechanistic analysis of nondisruptive axonal injury: A review. Journal of Neurotrauma 14(7):419-440.
McKee, A.C., R.C. Cantu, C.J. Nowinski, E.T. Hedley-Whyte, B.E. Gavett, A.E. Budson, V.E. Santini, H.-S. Lee, C.A. Kubilus, R.A. Stern. 2009. Chronic traumatic encephalopathy in athletes: Progressive tauopathy following repetitive head injury. Journal of Neuropatholology and Experimental Neurology 68(7):709-735.
McManus, L.R., P.E. Durand, and W.D. Claus. 1976. Development of a One Piece Infantry Helmet. Report 76-30-CEMEL. U.S. Army Natick Research and Development Command, Natick, Mass.
McMurtray, A., D.G. Clark, D. Christine, and M.F. 2006. Early-onset dementia: Frequency and causes compared to late-onset dementia. Dementia and Geriatric Cognitive Disorders 21(2):59-64.
Meaney, D.F., D.H. Smith, D.L. Schreiber, A.C. Bain, R.T. Miller, D.T. Ross, and T.A. Gennarelli. 1995. Biomechanical analysis of experimental diffuse axonal injury. Journal of Neurotrauma 12(4):689-694.
Meehan, W., P. d’Hemecourt, D. Comstock. 2010. High school concussions in the 2008-2009 academic year: Mechanism, symptoms, and management. American Journal of Sports Medicine 38(12):2405-2409.
Moon, D.W., C.W. Beedle, and C.R. Kovacic. 1971. Peak head acceleration of athletes during competition. Medicine and Science in Sports 3(1):44-50.
Moore, D.F., A. Jerusalem, M. Nyein, L. Noels, M.S. Jaffee, and R. Radovitzky. 2009. Computational biology, modeling of primary blast effect on the central nervous system. NeuroImage 47(Suppl. 2):T10-T20.
Morrison III, B., H.L. Cater, C.C.-B. Wang, F.C. Thomas, C.T. Hung, G.A. Ateshian, and L.E. Sundstrom. 2003. A tissue level tolerance criterion for living brain developed with an in vitro model of traumatic mechanical loading. Stapp Car Crash Journal 47:93-105.
Muthupillai, R., D.J. Lomas, P.J. Rossman, J.F. Greenleaf, A. Manduca, and R.L. Ehman. 1995. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science 269(5232):1854-1857.
Newman, J.A., N. Shewchenko, and E. Welbourne. 2000. A proposed new biomechanical head injury assessment function—The maximum power index. Stapp Car Crash Journal 44:215-246.
Newman, J.A., M.C. Beusenberg, N. Shewchenko, C. Withnall, and E. Fournier. 2005. Verification of biomechanical methods employed in a comprehensive study of mild traumatic brain injury and the effectiveness of American football helmets. Journal of Biomechanics 38(7):1469-1481.
NRC (National Research Council). 2012. Testing of Body Armor Materials: Phase III. The National Academies Press, Washington, D.C.
Nyein, M., A.M. Jason, L. Yua, C.M. Pita, J.D. Joannopoulos, D.F. Moore, R.A. Radovitzky. 2010. In silico investigation of intracranial blast mitigation with relevance to military traumatic brain injury. Proceedings of the National Academy of Sciences U.S.A. 107:20703-20708.
Okie, S. 2005. Traumatic brain injury in the war zone. New England Journal of Medicine 352:2043-2047.
Ommaya, A.K., and R.A. Gennarelli. 1974. Cerebral concussion and traumatic unconsciousness: Correlation of experimental and clinical observations on blunt head injuries. Brain 97(4):633-654.
Ommaya, A.K., and A.E. Hirsch. 1971. Tolerances for cerebral concussion from head impact and whiplash in primates. Journal of Biomechanics 4(1):13-21.
Ommaya, A.K., W. Goldsmith, L.E. Thibault. 2002. Biomechanics and neuropathology of adult and paediatric head injury. British Journal of Neurosurgery 16(3):220-242.
Ono, K., Kikuchi, A., Nakamura, M., Kobayashi, H., and N. Nakamura. 1980. Human head tolerance to sagittal impact reliable estimation deduced from experimental head injury using subhuman primates and human cadaver skulls. SAE Technical Paper 801303, doi:10.4271/801303. SAE International, Warrendale, Pa.
Panzer, M.B., C.R. Bass, and B.S. Myers. 2010. Numerical study on the role of helmet protection in blast brain injury. Presented at Personal Armor Systems Symposium (PASS 2010), Quebec City, Calif.
Panzer, M.B., B.S. Myers, and C.R. Bass. 2011. Mesh considerations for finite element blast modeling in biomechanics. Computer Methods in Biomechanics and Biomedical Engineering 16(6):612-621.
Panzer, M.P., B.S. Myers, B.P. Capehart, and C.R. Bass. 2012. Development of a finite element model for blast brain injury and the effects of CSF cavitation. Annals of Biomedical Engineering 40(7):1530-1544
Peethambaran, A., R. Abu-Taleb, S. Oguntayo, Y. Wang, M. Valiyaveettil, J.B. Long, and M.P. Nambiar. 2013. Acute mitochondrial dysfunction after blast exposure: Potential role of mitochondrial glutamate oxaloacetate transaminase. Journal of Neurotrauma 30(19):1645-1651.
Pellman, E.J., D.C. Viano, A.M. Tucker, I.R. Casson, and J.F. Waeckerle. 2003. Concussion in professional football: Reconstruction of game impacts and injuries. Neurosurgery 53:799-814.
Pervin, F., and W. Chen. 2009. Dynamic mechanical response of bovine gray matter and white matter brain tissues under compression. Journal of Biomechanics 42(6):731-735.
Powner, D.J., C. Boccalandro, M.S. Alp, and D.G. Vollmer. 2006. Endocrine failure after traumatic brain injury in adults. Neurocritical Care 5(1):61-70.
Prange, M.T., and S.S. Margulies. 2002. Regional, directional, and age-dependent properties of the brain undergoing large deformation. Journal of Biomechanical Engineering 124(2):244-252.
Prasad, P., and H.J. Mertz. 1985. The position of the United States delegation to the ISO working group on the use of HIC in the automotive environment. SAE Transactions 94(5):106-116.
Prevost, T.P., A. Balakrishnan, S. Suresh, and S. Socrate. 2011a. Biomechanics of brain tissue. Acta Biomaterialia 7(1):83-95.
Prevost, T.P., G. Jin, M.A. de Moya, H.B. Alam, S. Suresh, and S. Socrate. 2011b. Dynamic mechanical response of brain tissue in indentation in vivo, in situ, and in vitro. Acta Biomaterialia 7(12):4090-4101.
Przekwas, A., X.G. Tan, V. Harrand, D. Reeves, Z.J. Chen, and K. Sedberry. 2011. Integrated experimental and computational framework for the development and validation of blast wave brain biomechanics and helmet protection. Pp. 34-1–34-20 in HFM-207—A Survey of Blast Injury Across the Full Landscape of Military Science. RTO Human Factors and Medicine Panel (HFM) Symposium, Halifax, Canada.
Pudenz, R.H., and C.H. Sheldon. 1946. The Lucite calvarium—A method for direct observation of the brain. II. Cranial trauma and brain movement. Journal of Neurosurgery 3(6):487-505.
Rafaels, K.A., C.R. Bass, M.B. Panzer, R.S. Salzar, W.W. Woods, S. Feldman, T. Walilko, R. Kent, B. Capehart, J. Foster, B. Derkunt, and A. Toman. 2012. Brain injury risk from primary blast. Journal of Trauma and Acute Care Surgery 73(4):895-901.
Reid, S.E., H.M. Epstein, T.J. O’Dea, and M.W. Louis. 1974. Head protection in football. Journal of Sports Medicine 2(2):86-92.
Rowson, S., and S.M. Duma. 2011. Development of the STAR Evaluation System for football helmets: Integrating player head impact exposure and risk of concussion. Annals of Biomedical Engineering 39(8):2130-2140.
Sarron, J.C., M. Dannawi, A. Faure, J.-P. Caillou, J. Da Cunha, and R. Robert. 2004. Dynamic effects of a 9 mm missile on cadaveric skull protected by aramid, polyethylene or aluminum plate: An experimental study. Journal of Trauma—Injury, Infection and Critical Care 57(2):236-242.
Saucier, J. 1955. Concussion: A misnomer. Canadian Medical Association Journal 72(11):816-820.
Schoenhuber, R., and M. Gentilini.1988. Anxiety and depression after mild head injury: A case controlled study. Journal of Neurology Neurosurgery and Psychiatry 51(5):722-724.
Schreiber, D.I., A.C. Bain, and D.F. Meaney. 1997. In vivo thresholds for mechanical injury to the blood brain barrier. SAE Technical Paper 973335. SAE International, Warrendale, Pa.
Sharma, S., and L. Zhang. 2011. “Prediction of Intracranial Responses from Blast Induced Neurotrauma Using a Validated Finite Element Model of Human Head.” Bioengineering Centre, Wayne State University, Detroit, Mich.
Shridharani, J.K., G.W. Wood, M.B. Panzer, K.A. Matthews, C. Perritt, K. Masters, and C.R. Bass. 2012a. Blast effects behind ballistic protective helmets. Presented at the Personal Armor Systems Symposium (PASS 2012), Nuremburg, Germany.
Shridharani, J.K., G.W.Wood, M.B. Panzer, B.P. Capehart, M.K. Nyein, R.A. Radovitzky, and C.R. Bass. 2012b. Porcine head response to blast. Frontiers in Neurology, Article 70, May.
Smith, D.H., J.A. Wolf, T.A. Lusardi, V.M.Y. Lee, and D.F. Meaney. 1999. High tolerance and delayed elastic response of cultured axons to dynamic stretch injury. Journal of Neuroscience 19(11):4263-4269.
Smith, D.H., R. Hicks, and J.T. Povlishock. 2013. Therapy development for diffuse axonal injury. Journal of Neurotrauma 30(5):307-323.
Stalnaker, R.L., J.H. McElhaney, and V.L. Roberts. 1971. MSC tolerance curve for human heads to impact. ASME Paper No. 71WA/BHF-10. American Society of Mechanical Engineers, New York.
Strich, S.J. 1956. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. Journal of Neurology Neurosurgery and Psychiatry 19(3):163-185.
Strich, S.J. 1961. Shearing of nerve fibres as a cause of brain damage due to head injury. The Lancet 278(7200):443-448.
Takhounts, E.G., R.H. Eppinger, J.Q. Campbell, R.E. Tannous, E.D. Power, and L.S. Shook. 2003. On the development of the SIMon Finite Element Head Model. Stapp Car Crash Journal 47:107-133.
Takhounts, E.G., S.A. Ridella, V. Hasija, E. Rabih, R.E. Tannous, J.Q. Campbell, D. Malone, K. Danelson, J. Stitzel, S. Rowson, S. Duma. 2008. Investigation of traumatic brain injuries using the next generation of simulated injury monitor (SIMon) Finite Element Head Model. Stapp Car Crash Journal 52:1-31.
Talavage, T.M., E.A. Nauman, E.L. Breedlove, U. Yoruk, A.E. Dye, K. Morigaki, H. Feuer, and L.J. Leverenz. 2013. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. Journal of Neurotrauma. April 11. E-pub ahead of print, doi:10.1089/neu.2010.1512.
Taylor, P.A., and C.C. Ford. 2009. Simulation of blast-induced early-time intracranial wave physics leading to traumatic brain injury. Journal of Biomechanical Engineering 131(6):061007.
Teland, T., A. Hamberger, M. Huseby, A. Säljö, and E. Svinsås. 2010. Numerical simulation of mechanisms of blast-induced traumatic brain injury. Journal of the Acoustical Society of America 127(3):1790.
Terrio, H., L. Brenner, B. Ivins, J. Cho, K. Helmick, K. Schwab, K. Scally, R. Bretthauer, D. Warden, and L. French. 2009. Traumatic brain injury screening: Preliminary findings in a U.S. army brigade combat team. Journal of Head Trauma Rehabilitation 24(1):14-23.
Ueno, K., and J. Melvin. 1995. Finite element model study of head impact based on hybrid III Head acceleration: The effects of rotational and translational acceleration. Journal of Biomechanical Engineering 117(3):319-328.
Vanderploeg, R.D., G. Curtiss, and H.G. Belanger. 2005. Long-term neuropsychological outcomes following mild traumatic brain injury. Journal of the International Neuropsychological Society 11(3):228-236.
Versace, J. 1971. A Review of the Severity index. Pp. 771-796 in Proceedings of the Fifteenth Stapp Car Crash Conference. Stapp Car Crash Conference, Atlanta, Ga.
Viano, D.C. 1988. Cause and control of automotive trauma. Bulletin of the New York Academy of Medicine: Journal of Urban Health 64(5):367.
Viano, D.C., and P. Lövsund. 1999. Biomechanics of brain and spinal-cord injury: Analysis of neuropathologic and neurophysiologic experiments. Journal of Crash Prevention and Injury Control 1(1):35-43.
Viano, D.C., C. Bir, T. Walilko, and D. Sherman. 2004. Ballistic impact to the forehead, zygoma, and mandible: Comparison of human and frangible dummy face biomechanics. Journal of Trauma—Injury, Infection and Critical Care 56(6):1305-1311.
von Holst, H., and X. Li. 2013. Consequences of the dynamic triple peak impact factor in traumatic brain injury as measured with numerical simulation. Frontiers in Neurology 4:1-8.
Ward, C.C., M. Chan, and A.M. Nahum. 1980. Intracranial pressure—A brain injury criterion. SAE Technical Paper 801304. SAE International, Warrendale, Pa.
Warden, D. 2006. Military TBI during the Iraq and Afghanistan wars. Journal of Head Trauma Rehabilitation 21(5):398-402.
Whitnall, L., T.M. McMillan, G.D. Murray, and G.M. Teasdale. 2006. Disability in young people and adults after head injury: 5-7 year follow up of a prospective cohort study. Journal of Neurology, Neurosurgery and Psychiatry 77(5):640-645.
Wilkinson, C.W., Pagulayan, K.F., petrie, E.C., Mayer, C.L., Colasurdo, E.A., Shofer, J.B., Hart, K. L., Hoff, D., Tarabochia, M.A., and Peskind, E.R. 2012. High prevalence of chronic pituitary and target-organ hormone abnormalities after blast-related mild traumatic brain injury. Frontiers in Neurology, February, Article 11.
Xiangsong, Z., Y. Dianchao, and T. Anwu. 2005. Dynamic 13N-Ammonia PET: A new imaging method to diagnose Hypopituitarism. Journal of Nuclear Medicine 46(1):44-47.
Yeh, P.-H., B. Wang, T.R. Oakes, L.M. French, P. Hai, J. Graner, W. Liu, and G. Riedy. 2013. Postconcussional disorder and PTSD symptoms of military-related traumatic brain injury associated with compromised neurocircuitry. Human Brain Mapping, doi:10.1002/hbm.22358.
Yoganandan, N., F.A. Pintar, A. Sances, Jr, P.R. Walsh, C.L. Ewing, D.J. Thomas, and R.G. Snyder. 1995. Biometrics of skull fracture. Journal of Neurotrauma 12(4):659-668.
Zhang, L., R. Makwana, and S. Sharma. 2011. Comparison of the head response in blast insult with and without combat helmet. Pp. 33-1–33-18 in HFM-207—A Survey of Blast Injury Across the Full Landscape of Military Science. RTO Human Factors and Medicine Panel (HFM) Symposium, Halifax, Canada.
Zhu, F., P. Skelton, C.C. Chou, H. Mao, K.H. Yang, and A.I. King. 2013. Biomechanical responses of a pig head under blast loading: A computational simulation. International Journal for Numerical Methods in Biomedical Engineering 29(3):392-407.





















