A Examples of Clinical Practice Guidelines and Related Materials1
This appendix, which is a collection of clinical practice guidelines and related materials,2 has three main purposes. First, for readers not familiar with guidelines, it presents samples that may make the text of this report more concrete. Second, it illustrates how guidelines can differ. Third, the appendix discusses the topic of formatting, which is in some ways a step between development and implementation.
Apart from the sponsoring agency or organization, guidelines can vary (as noted in Chapter 1) in at least five key ways:
-
Clinical orientation—whether the chief focus is a clinical condition, a technology (broadly defined), or a process.
-
Clinical purpose—whether they advise about screening and prevention, evaluation or diagnosis, aspects of treatment, or other dimensions, or more discrete aspects of health care.
-
Complexity—whether the guidelines are relatively straightforward in presentation and discussion or are marked by considerable detail, complicated logic, or lengthy narrative and documentation. For purposes of the descriptions in this appendix, complexity is indicated simply as high, medium, or low.
-
Format—whether the guidelines are formatted as free text, tables, if-then statements, critical pathways, decision paths, or algorithms.
-
Intended users—whether they are intended for practitioners, patients, or others.
The next section of this appendix discusses the ways guidelines may be formatted. The rest of the appendix presents, in whole or in part, 16 guidelines and related items (see Table A-1). Each example is preceded by an annotation indicating the principal information for the five variables just noted. In addition, a brief introduction highlights especially salient points about the item in terms of purpose, content, or presentation. These notes should in no way be considered a complete analysis or evaluation of the item in the example. At the end of each write-up is the complete reference or citation to the guideline.
Inclusion in this appendix does not imply endorsement of the content of these guidelines or of the process by which they were developed. Some of these materials are not, for example, the products of a systematic develop
TABLE A-1 List of Examples, by Main Purpose
|
Screening and Prevention 1. Screening for diminished visual acuity in children 2. Vaccination for pregnant women who are planning international travel |
|
Diagnosis and Pre-Diagnosis Management of Patients 3. Triage of the injured patient 4. Evaluating chest pain in the emergency room 5. Using erythrocyte sedimentation rate tests in diagnosis |
|
Indications for Use of Surgical Procedures 6. Indications for carotid endarterectomy 7. Indications for percutaneous transluminal coronary angiography 8. Managing labor and delivery after previous cesarean section |
|
Appropriate Use of Specific Technologies and Tests as Part of Clinical Care 9. Using autologous or donor blood for transfusions 10. Detecting or tracking deteriorating metabolic acidosis |
|
Guidelines for Care of Clinical Conditions 11. Using oral contraceptives to prevent pregnancy and manage fertility 12. Deciding on treatment for low back pain 13. Managing patients following coronary artery bypass graft 14. Guidelines for the management of patients with psoriasis 15. Acute dysuria in the adult female 16. Management of acute pain |
ment process. Others may result from such a procedure but do not include references to the scientific literature used in development.
FORMATTING GUIDELINES
Formatting is a step beyond the development of a guideline. It can be executed in many ways and at many stages in the process of moving guidelines from development to application. Congress, for instance, recognized the importance of formatting by requiring the Agency for Health Care Policy and Research (AHCPR) to present its guidelines in formats that are appropriate for use by practitioners, medical educators, and medical care reviewers.
Many formatting activities of most relevance to the persuasive and effective presentation of guidelines may occur after the initial formatting and dissemination of a set of guidelines by its sponsor or developer. For example, a professional society may initially present its guidelines in one format in a journal but then rework them into another format for use in continuing medical education. The initial guidelines may also be converted by target users--—hospitals, clinics, utilization review firms, health maintenance organizations, patient groups, and others—into formats ranging from a sophisticated computer-based algorithm to a simple chart that the patient can put on the refrigerator door or bathroom mirror. Voluntary associations such as the American Cancer Society and American Heart Association, as well as commercial firms, may reformat guidelines in various ways for dissemination to different groups.
Guidelines can vary quite dramatically, both logically and graphically, in their modes of presentation. The major approaches are free-text and formalized presentations, including if-then statements, algorithms, flowcharts, and decision trees. More recently, some clinical researchers and medical informatics experts are moving to more complex computer-based approaches. These approaches are discussed briefly below.
Free Text
The most common format for guidelines is free text; this report, for example, is presented in free text. Generally, free text is the starting point for most other formats and itself has many variants.
Shortened free-text versions of guidelines documents can be tailored for specific uses by specific types of practitioners. For instance, guidelines for the diagnosis and management of acute myocardial infarction might well be rendered into several different versions and formats depending on whether the target audience was to be emergency room physicians needing quick reference or cardiac specialists managing a patient over several weeks.
Narrative information may be collapsed into tables or graphs or summarized in highly technical terms with liberal use of acronyms, abbreviations, or symbols.3
To cite one example of a series of formatting steps, the U.S. Preventive Services Task Force took its guidelines to a commercial publisher, which created and copyrighted the graphic design and produced the guidelines for general publication. In addition to the basic text, that publication includes eight small, plasticized pull-out charts summarizing screening, counseling, and immunization schedules for different patient age groups. These tables include abbreviated references to nonroutine situations for which physicians may consider deviations from the schedules.
Guidelines can also be rendered into much simpler, less technical documents for use by patients, consumers, and their families. Although the free-text approach is likely to be preserved, some use may be made of lay terms (including colloquialisms), simple drawings, and such heuristic devices as introductory questions. One would expect such versions of guidelines to differ, depending on the target audience.
Formalized Presentations
Apart from translations into shorter, simpler, nontechnical versions, which may still be in a free-text format, guidelines documents may be formatted into stylized graphic representations such as flowcharts or decision trees. These, in turn, may become programs for computer-based clinical decision making tools.
One reason for translating free text into other formats is that some guidelines identify dozens, if not hundreds, of specific criteria for care; even creative free-text presentations may not allow practical, quick access to this volume of information. Instead, the free text may be reconfigured (or, less often, the initial guidelines may be drafted) using various related or overlapping formal approaches including flowcharts, decision tables, and if-then rules.
Algorithms
Typically, a preliminary step in the development of many such formats is the development of a clinical algorithm. Algorithms were invented in the ninth century by a Persian mathematician, Al-khaforizmi, to solve arithmetic problems. They were first applied to practical problem solving by the U.S. Army in job-training manuals, and they have been fundamental to the programming of electronic computers. More specifically in the health field, algorithms have been used since at least the 1960s to aid clinical problem solving (Gottlieb, 1990).
Strictly speaking, an algorithm is not a graphic representation but rather a presentation of information for decision making using step-by-step conditional logic rather than ordinary prose or lists of factors to be considered. This distinction is frequently ignored.
The clinical algorithm, even when not used as the format of choice for disseminating guidelines, may be used to compare guidelines and to identify missing or conflicting decision "branches." In developing methods for analyzing and comparing guidelines, Margolis et al. (1991) have identified categories of logical error in guideline construction and have described the complexity of different guidelines in quantitative terms. Applying such a process could lead to the significant reworking of a set of guidelines, not just a repackaging or interpretation of the same content.
Constructing algorithms and translating them into flowsheets and other tools can be a powerful learning process for practitioners. The process can (1) highlight differences in practice patterns and values that may need to be explored, (2) clarify key characteristics and weaknesses of processes of care, (3) identify gaps in clinical knowledge, and (4) contribute to redesign of systems of care.
Free-text versions of guidelines or review criteria usually precede the development of algorithms and similar formats, but one exception is worth noting. The Health Standards and Quality Bureau of the Health Care Financing Administration (HCFA) is developing quality-of-care and appropriateness algorithms for collecting and analyzing clinical data in its Uniform Clinical Data Set (UCDS; Krakauer, 1990; Krakauer and Bailey, 1991). The agency believes that the application of these computer-based algorithms will be a major tool for quality review for the Medicare peer review organizations (PROs), improving on the manual review of hospital charts by PRO nurse reviewers.
The UCDS development process began in the late 1980s with more than 3,000 complex software algorithms for direct collection of data and identification of hospital admissions that did not meet certain admission or quality-of-care criteria. However, little documentation of the programming or clinical logic was performed. Consequently, the procedures and rules against
which practitioners and hospitals may be judged to have provided poor or unnecessary care cannot be easily explained to clinicians or institutions. HCFA has sponsored some work to prepare free-text versions of the fraction of these algorithms that can generate "flags" about potential quality-of-care deficiencies.
Flowcharts and Similar Formats
Graphic representations of algorithms are of various kinds. They include flowcharts, decision tables (sometimes used to indicate appropriate health screening), protocol charts (e.g., for handling medical problems by telephone), and so-called influence diagrams (a decision clarification tool imported from the business world and now being adapted to medicine).
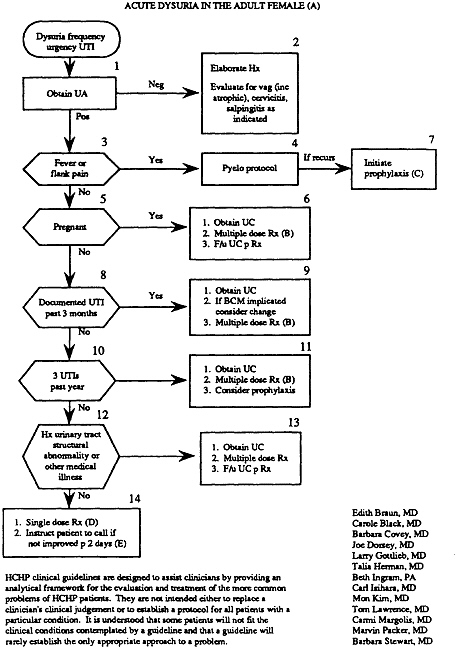
As used to assist clinical problem solving, flowcharts (which are commonly called algorithms) begin with a clinical condition or patient symptom and lead the reader through a series of branching, dichotomous choices based on the patient's risk status, medical history, or clinical findings. They also include action steps such as testing, treating, or scheduling further examinations. This appendix presents two flowcharts, one for patients (Example 12 on the treatment of low back pain) and one for practitioners (Example 15 on the treatment of dysuria). In clinical practice, flowcharts may help practitioners choose from among alternative actions the most efficient sequence (as in a diagnostic workup); they may also aid in reducing the likelihood of overlooking uncommon but important elements of care for specific patients.
The basic elements of flowcharts include boxes and arrows; the latter connect the boxes or direct the user to other parts of the algorithm. The boxes may be numbered and have internal text, and they may come in several shapes, depending on whether they describe a clinical state, ask a question (diagnostic assessment), or describe an action to be taken. Prose may be used to annotate the boxes. Experts recommend that flowcharts read from left to right and top to bottom and that only two arrows exit from a given box, corresponding to a yes or no response to the clinical question posed. (Further rules for creating flowcharts and algorithms are proposed below.)
Practitioners differ widely in their attitude toward flowcharts, decision trees, and other shorthand, visual formats for guidelines. Some see them as helpful reminders and a useful tool for assimilating or quickly locating information. Others are emphatic in their dislike of these formats, maintaining that the sequences do not represent the experienced clinician's mental processes or that the necessary complexity makes them impractical to use during care of a patient. Alternatively, they may argue that simplified formats do not adequately account for all the factors present in patient care.
Some practitioners are concerned that algorithms make patient care appear cut and dried and that they will become a series of rules to be applied, with mindless rigidity, by those without clinical expertise. As can happen with any format, some physicians may view any guidelines for familiar clinical conditions as insults that imply that without such a guideline they would not know what care to provide.
Proposed Standards
As noted, the clinical algorithm map, a type of practice guideline, has received increased attention in recent years and appears more frequently in various medical journals. However, format, style, graphics, and uses vary widely, posing an obstacle to widespread use and dissemination. To overcome this difficulty, Margolis, Gottlieb, and their associates (Margolis et al., 1991) advanced some suggestions for standardization of algorithms, which are briefly presented here. The proposals involve use of boxes, including clinical state boxes, decision boxes, action boxes, and link boxes; arrows; a numbering scheme; pagination; abbreviations; and various aspects of annotations.
TITLE
The title should define the clinical topic and intended users. Under it, the authors, their degrees, and institutional affiliations should be listed. The date of publication and revision (if applicable) should be specified. A footnote to the title should state the process by which the algorithm logic was decided; this might be, for instance, group consensus after literature review, individual recommendation based on clinical experience, or some other technique.
BOXES
Clinical state box—rounded rectangle or elliptical box. This box defines the clinical state or problem. It has only one exit path and may or may not have an entry path. This box always appears at the beginning of an algorithm. The initial clinical state box should describe the clinical problem to be addressed. Clinical state boxes in the body of the algorithm are used to clarify the status of the patient or diagnosis along the path of the algorithm (i.e., to describe a subset of patients with a particular clinical condition).
Decision box—hexagon. This box requires a branching decision, whose response will lead to one of two alternative paths. It always has an entry path and two exit paths. Statements in decision boxes should be phrased as questions punctuated with question marks. If two assessments are to be determined, then the developers should specify whether both (''and") or one
("or") must be positive for a "yes" response. Multiple questions can be asked in one box, and criteria are specified for a "yes" response to the entire box—for instance, whether two of three criteria must be present, whether all must be present, or whether any must be present.
Action box—rectangle. This box indicates an action, commonly either therapeutic or diagnostic. Several rules are suggested for action boxes, as follows: A single phrase within a box should not be punctuated with a period. Multiple actions that do not need to be sequenced in time may be listed in one box. When multiple actions are presented in one box, each action is to be listed on a separate line (preceded with an optional number, dash, or bullet). When two statements are to be joined by "and" or "or," the conjunction should be placed on a separate line for emphasis.
Link box—small oval. This box is used in place of an arrow, to link boxes for graphic clarity. This might be useful at page breaks or between separated nodes to maintain path continuity. The box itself should read "Go to Page ... Box ...."
ARROWS
Several rules for arrows have also been advanced. The flow should be from top to bottom. In general, the flow should be from left to right, except when a side branch rejoins the main stem. Arrows should never intersect. Link boxes (see above) can be used to avoid crossing paths. Arrows originating from decision boxes should be labelled "yes" or "no." No other text should be used over an arrow. Wherever possible (i.e., where clinical content will not be obscured), "yes" arrows should point to the right, and "no" arrows should point down.
NUMBERING SCHEME
Clinical state boxes, decision boxes, and action boxes should be numbered sequentially from left to right and from top to bottom. Link boxes are not numbered.
PAGING
Whenever possible, it is advisable to consolidate the algorithm so that it can be presented on one page. Page breaks are inserted where clinical logic indicates, and a single box should not be isolated on a page. For complex algorithms, the first page could best serve as a directory to clinical subsets of patients. In this case, each subset is identified as a clinical state box.
ANNOTATIONS
Citation of the annotation. Annotations are an intrinsic part of the algorithm. They are used to clarify the rationale of the decisions, cite the supporting literature, and expand on less essential details of the clinical
information contained in the box. Annotations would be cited following a single phrase using a capital letter at the end of the phrase. When multiple statements are contained in a single box, annotation(s) should appear at the end of the phrase(s) to which it is applicable. If an annotation is applicable to the entire box with multiple statements, then it should be cited using a capital letter centered on a separate line at the bottom of the box.
Annotation format. Annotations should be written in text format and should appear on pages that are separate from the algorithm. They should be referenced according to standard medical reference format, with references numbered using superscripts within the text.
ABBREVIATIONS
Except for units of measurement, abbreviations are discouraged.
Computer-based Formats
Some clinical researchers argue that clinical flowcharts are inefficient representations of algorithms because they are limited by yes/no branch points to arbitrary sequences and thus cannot accommodate the richer choices common to medicine. They have been looking for ways to avoid flowcharts and to move toward a "meta-language" by using a standard syntax to convert algorithms to different kinds of computer-based decision aids. The Arden Syntax, developed in medical centers with private-sector support, is an effort to create such a system (McDonald et al., 1991; Megargle, 1991).
Using this syntax, a well-defined algorithm can be transformed into various kinds of computer programming statements such as if-then statements or for-loop statements. Use of the Arden Syntax allows easy transfer of understandable contraindication alerts, management suggestions, data interpretations, treatment protocols, and similar aids from one computer system to another. Example 10, on detecting deteriorating metabolic acidosis, and Example 11, on the use of oral contraceptives, illustrate specific computer-based formats.
The rest of the appendix presents 16 examples of guidelines and related materials. A range of topics, formats, sponsors and users, clinical orientations and purposes, and levels of complexity are presented and discussed; cross-comparisons within the appendix are noted. As stated above, these discussions should not be taken as complete analyses of the items in question, nor should inclusion in this appendix be taken as endorsement of the content or development process of these guidelines.
Example 1
SCREENING FOR DIMINISHED VISUAL ACUITY
|
Clinical orientation: |
Clinical condition |
|
Clinical purpose: |
Screening and prevention |
|
Complexity: |
Medium |
|
Format: |
Free text and a stand-alone reference chart |
|
Intended users: |
Practitioners, perhaps patients |
In 1989, the U.S. Preventive Services Task Force (USPSTF) published a 419-page document intended mainly for primary care providers. The task force's objective was to develop comprehensive recommendations addressing preventive services for all age groups for 60 target conditions, using a systematic process and explicit criteria to review evidence and develop recommendations. The work of the task force was discussed extensively in Chapter 2 and elsewhere in the report.
This particular guideline is presented, in the USPSTF book, as part of a larger course of preventive care. It is one of 169 guidelines for specific preventive interventions, each of which may include recommendations for preventive care by age group (e.g., in favor of vision screening for children of younger ages and possibly for the elderly but not for adolescents and adults). Reproduced here are (1) the specific recommendations for vision screening and (2) the plasticized reference card for preventive care of children ages 2-6, which recommends vision screening.
In its concern with reliability of a particular test, this guideline is similar to the one on erythrocyte sedimentation rate tests (Example 5). As is true of several items in the appendix, this guideline cites the literature on which it is based. Educated patients might also make use of this guideline, as they could for the guidelines on, for instance, deciding what to do about low back pain or managing labor and delivery after a previous cesarean delivery (see Examples 12 and 8, respectively).
SOURCE: Reprinted (public domain) from: U.S. Preventive Services Task Force. Guide to Clinical Preventive Services: An Assessment of the Effectiveness of 169 Interventions. Baltimore, Md.: Williams & Wilkins, 1989.
Screening for Diminished Visual Acuity
Recommendation: Vision screening is recommended for all children once before entering school, preferably at age 3 or 4 (see Clinical Intervention). Routine vision testing is not recommended as a component of the periodic health examination of asymptomatic schoolchildren. Clinicians should be alert for signs of ocular misalignment when examining all infants and children. Vision screening of adolescents and adults is not recommended, but it may be appropriate in the elderly. Screening for glaucoma is discussed in Chapter 32.
Burden of Suffering
About 2-5% of American children suffer from amblyopia ("lazy eye") and strabismus (ocular misalignment), and nearly 20% have simple refractive errors by age 16.1-4 Amblyopia and strabismus usually develop between infancy and ages 5-7.3 Since normal vision from birth is necessary for proper eye development, failure to treat amblyopia and strabismus before school age may later result in irreversible visual deficits, permanent amblyopia, loss of depth perception and binocularity, cosmetic defects, and educational and occupational restrictions.1,4,5 In contrast, refractive errors such as myopia become common during school age but rarely carry serious prognostic implications.1,3,6,7 Experts disagree on whether uncorrected refractive errors cause diminished academic performance among schoolchildren. 1,3,5,7,8,
The majority of vision disorders occur in adults; over 8.5 million Americans suffer from visual impairment 9 Visual disorders such as presbyopia (decreased ability to focus on near objects) become more common with age10 and therefore the prevalence of visual impairment is highest in those over age 65. Preliminary statistics from recent surveys suggest that nearly 13% of Americans age 65 and older have some form of visual impairment, and almost 8% of this age group suffer from severe impairment: blindness in both eyes or inability to read newsprint even with glasses.11 Vision disorders in the elderly may be associated with injuries due to falls and motor vehicle accidents, diminished productivity, and loss of independence.12 Many older adults are unaware of changes in their visual acuity, and up to 25% of them may be using an incorrect lens prescription.12
Efficacy of Screening Tests
Although screening for strabismus and amblyopia is most critical at an early age, screening tests to detect occult vision disorders in children under age 3 have
generally been unsuccessful due to the child's inability to cooperate, the time required for testing, and the inaccuracy of the tests.13-15 Promising techniques such as alternate stimulation (cover testing), preferential-looking, grating acuity cards, and refractive screening are currently being developed for this age group.14,16,17 Although refractive errors detected during infancy can predict some cases of amblyopia and strabismus, the sensitivity of this form of screening is quite poor.2
Screening tests for detecting strabismus and amblyopia in preschool children over age 3 include simple inspection, visual acuity tests, and stereograms. Visual acuity tests include the Snellen eye chart, the Landolt C, the tumbling E, the Sheridan-Gardner STYCAR test, Allen picture cards, grating cards, and other techniques.15 The specificity of most acuity tests, however, is imperfect for detecting strabismus and amblyopia because diminished visual acuity can occur in other conditions, such as simple refractive error or visual immaturity. 2 In addition, many children with nonamblyopic strabismus often have normal visual acuity but are at risk for serious complications 2,18 Thus, although simple acuity tests are inexpensive and easy to administer, they may miss many cases. Snellen letters, for example, are estimated to have a sensitivity of only 25-37%.2,18,19 Refractive screening has also been criticized as not being a direct test for either amblyopia or strabismus.2
Stereograms such as the Random Dot E (RDE) have been proposed as more effective than visual acuity tests in detecting strabismus and amblyopia in preschool children.2,18,20,21 The test, in which the patient views test cards through Polaroid glasses, requires about one minute to perform.18,20 When compared with a battery of visual tests, the RDE has an estimated sensitivity of 64%, specificity of 90%, positive predictive value of 57%, and negative predictive value of 93%.18
A more effective but less efficient strategy is the combination of more than one visual test.2,19 The Modified Clinical Technique (MCT), for example, includes retinoscopy, cover and Hirschberg tests, the Snellen acuity test, a color vision test, and external observation of the eye. The MCT has gained acceptance among optometrists since its introduction in the Orinda Study of 1959.18,22-24 Sensitivity and specificity in excess of 90% were found in that study and have since been reproduced in screening programs involving as many as 50,000 children.18 The MCT cannot be used routinely by primary care physicians for screening purposes, however, because it requires about 12 minutes to perform and the examiner must be a skilled eye care specialist.18,23
Vision screening of older children and adults is a means of detecting unrecognized refractive errors. Tests of visual acuity are often used for this purpose, but few studies have examined the sensitivity, specificity, and predictive value of these tests in adult age groups.
Effectiveness of Early Detection
There is convincing evidence that early detection and treatment of vision disorders in infants and young children improve the prognosis for normal eye development.21 A prospective study has demonstrated that preschool children who receive visual acuity screening have significantly less visual impairment than controls when reexamined 6-12 months later.25 Detection and treatment of strabismus and amblyopia by age 1-2 can increase the likelihood of developing normal or near-normal binocular vision and may improve fine motor skills.2,4 Interventions for amblyopia and strabismus are significantly less effective if started after age 5, and such a delay increases the risk of irreversible amblyopia, ocular misalignment, and other visual deficits.1,3 It is widely held that clinical screening tests can detect
these disorders earlier than parents or teachers; only about 50% of children with ocular misalignment have a cosmetically noticeable defect.2,8
There is little evidence that bilaterally equal refractive errors among older children and adolescents are associated with significant morbidity, such as diminished academic performance.1,3,6,7 This is true in young adults as well, and, in addition, uncorrected vision disorders are quite uncommon among young adults.26 Vision screening for older adults is defended on the grounds that the prevalence of abnormal visual acuity is considerably greater among the elderly10 and these deficits are more commonly left uncorrected.26 Among persons aged 65-74, a visual acuity of 20/50 or less has been measured in 11% of those who wear glasses and in 26% of those who do not.26 Some forms of visual impairment in the elderly are associated with difficulties in ambulation,27 and early correction of refractive errors may serve a role in preventing injuries and facilitating the performance of daily living functions. However, there have been no prospective studies documenting these benefits in an elderly cohort receiving vision screening.
Recommendations of Others
The American Academy of Ophthalmology recommends an ophthalmological examination of newborns who are premature or at risk for eye disease; an examination of fixation preference and ocular alignment by age 6 months; an examination of visual acuity, ocular alignment, and ocular disease at age 3-4; annual screening of schoolchildren for visual acuity and ocular alignment; occasional examinations from puberty to age 40; and an examination for presbyopia at age 40 and every two to five years thereafter.1 The American Academy of Pediatrics recommends external examination and tests of following ability and the pupillary light reflex in the newborn period and once during the first six months.5 Testing of visual acuity, ocular alignment, and ocular disease is recommended by the Academy at ages 4, 5-6, and at less frequent intervals thereafter.5 The Canadian Task Force recommends an eye examination and cover test at ages 1 week, 2 months, and, along with a vision chart test, at age 2-3 years and 5-6 years. Testing at age 10-11 is considered discretionary, and no adult screening is recommended.28 The American Optometric Association recommends screening schoolchildren every three years and annual eye examinations in adults after age 35.29 Screening guidelines have also been issued by other organizations, such as the National Society to Prevent Blindness, the National Association of Vision Program Consultants, Volunteers for Vision, and the American Public Health Association.2,8 Vision screening of preschool and school children is also required by law in some states and in a number of Federal programs.2,22
Discussion
Although it is established that early detection of strabismus and amblyopia is most beneficial for children under age 3, a practical and effective screening test is not yet available for this age group. Clinicians should, of course, be alert to signs of ocular misalignment when examining infants and young children. Screening tests for preschool children are available but, with the exception of a comprehensive battery (e.g., the MCT), most tests for amblyopia and strabismus lack the sensitivity, specificity, and predictive value that are expected of good screening tests. Of these, the Random Dot E stereogram appears to have the best performance and is recommended by many experts. 2,21 Due to the high rate of false-negative results with this test, however, it would need to be repeated throughout the preschool period to achieve optimal effectiveness.
Screening of schoolchildren by primary care clinicians is not recommended because the procedure is usually performed by the public school system, and there is little scientific evidence that early detection of myopia is of greater benefit than detection when symptoms first become apparent. Similarly, there is no basis for screening asymptomatic adolescents or adults below age 40 who lack specific risk factors for vision disorders. With increasing age, there is a stronger argument for the early detection of uncorrected visual impairment to help prevent injury and improve independent living. The performance characteristics of acuity tests at this age are poorly described, and the claimed benefits of screening have not been proved. Repeated acuity testing can, however, improve sensitivity with presumably little cost or inconvenience to the patient. There are no available data for any age group on the optimal interval for vision screening; recommended frequencies are selected arbitrarily on the basis of expert opinion.
Clinical Intervention
Testing for amblyopia and strabismus is recommended for all children once before entering school, preferably at age 3 or 4. Stereotesting (e.g., Random Dot E stereogram) is more effective than visual acuity testing (e.g., Snellen optotype cards) in detecting these conditions. Routine screening for refractive errors is not recommended as a component of the periodic health examination of asymptomatic schoolchildren. Clinicians should be alert for signs of ocular misalignment when examining all infants and children. Vision screening of asymptomatic adolescents and adults is not recommended. It may be appropriate in the elderly, but there is insufficient evidence to recommend an optimal interval. All patients with abnormal test results should be referred to an eye specialist for further evaluation. Screening for glaucoma is discussed in Chapter 32.
REFERENCES
1. American Academy of Ophthalmology. Infants and children's eye care. Statement by the American Academy of Ophthalmology to the Select Panel for the Promotion of Child Health, Department of Health and Human Services. San Francisco, Calif.: American Academy of Ophthalmology, 1980.
2. Ehrlich MI, Reinecke RD, Simons K. Preschool vision screening for amblyopia and strabismus: programs, methods, guidelines, 1983. Surv Ophthalmol 1983; 28:145-63.
3. Cross AW. Health screening in schools. Part I. J Pediatr 1985; 107:487-94.
4. Sanke RF. Amblyopia. Am Fam Physician 1988; 37:275-8.
5. American Academy of Pediatrics. Vision screening and eye examination in children. Committee on Practice and Ambulatory Medicine. Pediatrics 1986; 77:918-9.
6. Rosner J, Rosner J. Comparison of visual characteristics in children with and without learning difficulties. Am J Optom Physiol Opt 1987; 64:531-3.
7. Helveston EM, Weber JC, Miller K, et al. Visual function and academic performance. Am J Ophthalmol 1985; 99:346-55.
8. APHA resolution number 8203: children's vision screening. Am J Public Health 1983; 73:329.
9. National Center for Health Statistics. Prevalence of selected chronic conditions, United States, 1979-81. Vital and Health Statistics, series 10, no. 155. Washington, D.C.: Government Printing Office, 1986. (Publication no. DHHS (PHS) 86-1583.)
10. Idem. Monocular visual acuity of persons 4-74 years, United States, 1971-1972. Vital and Health Statistics, series 11, no. 201G. Washington, D.C.: National Center for Health Statistics, 1977:60. (Publication no. DHEW (HRA) 77-1646.)
11. Nelson KA. Visual impairment among elderly Americans: statistics in transition. J Vis Impair Blind 1987; 81:331-4.
12. Stults BM. Preventive health care for the elderly. West J Med 1984; 141:832-45.
13. Hall SM, Pugh AG, Hall DMB. Vision screening in the under-5s. Br Med J 1982; 285: 1096-8.
14. Jenkins PL, Simon JW, Kandel GL, et al. A simple grating visual acuity test for impaired children. Am J Ophthalmol 1985; 99:652-8.
15. Fern KD, Manny RE. Visual acuity of the preschool child: a review. Am J Optom Physiol Opt 1986; 63:319-45.
16. Jacobson SG, Mohindra I, Held R. Visual acuity of infants with ocular diseases. Am J Ophthalmol 1982; 93:198-209.
17. Brown AM, Yamamoto M. Visual acuity in newborn and preterm infants measured with grating acuity cards. Am J Ophthalmol 1986; 102:245-53.
18. Hammond RS, Schmidt PP. A Random Dot E stereogram for the vision screening of children. Arch Ophthalmol 1986; 104:54-60.
19. Lieberman S, Cohen AH, Stolzberg M, et al. Validation study of the New York State Optometric Association (NYSOA) vision screening battery. Am J Optom Physiol Opt 1985; 62:165-8.
20. Simons K. A comparison of the Frisby, Random-Dot E, TNO, and Randot Circles stereotests in screening and office use. Arch Ophthalmol 1981; 99:446-52.
21. Reinecke RD. Screening 3-year-olds for visual problems: are we gaining or falling behind? Arch Ophthalmol 1986; 104:33.
22. Nussenblatt H. Symposium on optometry's obligation in vision screening. Opening remarks. Am J Optom Physiol Opt 1984; 61:357-8.
23. Peters HB. The Orinda Study. Am J Optom Physiol Opt 1984; 61:361-3.
24. Woodruff ME. Vision and refractive status among grade 1 children of the province of New Brunswick. Am J Optom Physiol Opt 1986; 63:545-52.
25. Feldman W, Milner R, Sackett B, et al. Effects of preschool screening for vision and hearing on prevalence of vision and hearing problems 6-12 months later. Lancet 1980; 2:1014-6.
26. National Center for Health Statistics. Refraction status and motility defects of persons 4-74 years, United States, 1971-1972. Vital and Health Statistics, series 11, no. 206. Washington, D.C.: National Center for Health Statistics, 1978: 89-93. (Publication no. DHEW (PHS) 78-1654.)
27. Idem. Aging in the eighties, impaired senses for sound and light in persons age 65 and over. Advance Data from Vital and Health Statistics, no. 125. Hyattsville, Md.: National Center for Health Statistics, 1986:4-5. (Publication no. DHHS (PHS) 86-1250.)
28. Canadian Task Force on the Periodic Health Examination. The periodic health examination. Can Med Assoc J 1979; 121:1194-254.
29. Miller SC, American Optometric Association. Personal communication, October 1988.
Example 2
VACCINATION FOR PREGNANT WOMEN
|
Clinical orientation: |
Clinical states (protection against certain disorders, in the context of pregnancy, an existing clinical condition); use of a technology (vaccination guidelines) |
|
Clinical purpose: |
Prevention, in the context of managing a clinical condition |
|
Complexity: |
Low |
|
Format: |
Free text; summary tables; maps (excerpts included) |
|
Intended users: |
Practitioners, perhaps patients |
This is an example of guideline development by a federal agency-here the Centers for Disease Control (CDC) of the U.S. Public Health Service (PHS)---as a direct product of its mandate. Example 1 on screening vision also came from a PHS agency and Example 9 on blood transfusions is the product of a state mandate. Chapter 2 discusses more fully the development of various guidelines at CDC and other federal agencies. Although aimed at clinicians, this guideline could be used by well-informed patients and consumers.
Of interest is the inclusion in the guideline of maps indicating areas in the world that are probably infected with specific diseases. An example concerning yellow fever in the Americas is included here. Since yellow fever vaccination is contraindicated except in cases of likely exposure, this map provides additional information for pregnant women to consider as they contemplate travel outside the United States. This guideline is the only one in the appendix to categorize levels of care according to ''sociogeographic" considerations (i.e., level of health, common health perils, or socioeconomic level of a particular locale)—a reflection of its concern with providing advice for international travel and with controlling the entry of infectious diseases into the United States.
Finally, this guideline was also included because it has two very different orientations: it can be seen as a guideline for a broadly defined technology (immunization) that includes information for the care of quite specific patients, or as a guideline combining two clinical states (pregnancy and potential exposure to disease) and providing recommendations on the intersecting territory.
SOURCE: Reprinted (public domain) from: Centers for Disease Control. Health Information for International Travel, 1990. Washington, D.C.: U.S. Government Printing Office, 1990.
VACCINATION DURING PREGNANCY
On the grounds of a theoretical risk to the developing fetus, live, attenuated-virus vaccines are not generally given to pregnant women or to those likely to become pregnant within the next 3 months after receiving vaccine(s). With some of these vaccines—particularly rubella, measles, and mumps—pregnancy is a contraindication. Both yellow fever vaccine and OPV can be given to pregnant women at substantial risk of exposure to natural infection. When a vaccine is to be given during pregnancy, waiting until the second or third trimester is a reasonable precaution to minimize any concern over teratogenicity. Although there are theoretical risks, there has been no evidence of congenital rubella syndrome in infants born to susceptible mothers who inadvertently received rubella vaccine during pregnancy.
Since persons given measles, mumps, or rubella vaccine viruses do not transmit them (although virus shedding does occur), these vaccines can be administered safely to children of pregnant women. Although live polio virus is shed by persons recently immunized with OPV (particularly following the first dose), this vaccine also can be administered to children of pregnant women. Polio immunization of children should not be delayed because of pregnancy in close adult contacts. Experience to date has not revealed any risks of polio vaccine virus to the fetus.
There is no convincing evidence of risk to the fetus from immunization of pregnant women using inactivated viral or bacterial vaccines, or toxoids. A previously unimmunized pregnant woman who may deliver her child under unhygienic circumstances or surroundings should receive two properly spaced doses of Td before delivery preferably during the last two trimesters. Incompletely immunized pregnant women should complete the three-dose series. Those immunized more than 10 years previously should have a booster dose.
There is no known risk to the fetus from passive immunization of pregnant women with IG (see above).
TABLE 5 Vaccination during pregnancy
|
|
Vaccine |
Indications for vaccination during pregnancy |
|
Live virus vaccines |
|
|
|
Measles |
Live-attenuated |
Contraindicated. |
|
Mumps |
|
|
|
Rubella |
|
|
|
Yellow fever |
Live-attenuated |
Contraindicated except if exposure is unavoidable. |
|
Poliomyelitis |
Trivalent live-attenuated (OPV) |
Persons at substantial risk of exposure may receive live-attenuated virus vaccine. |
|
Inactivated virus vaccines |
|
|
|
Hepatitis B |
Plasma derived or recombinant produced. purified hepatitis B surface antigen |
Pregnancy is not a contraindication. |
|
Influenza |
Inactivated type A and type B virus vaccines |
Usually recommended only for patients with serious underlying disease. It is prudent to avoid vaccination during the first trimester. Consult health authorities for current recommendations. |
|
Poliomyelitis |
Killed virus (IPV) |
OPV not IPV, is indicated when immediate protection of pregnant females is needed. |
|
Rabies |
Killed virus Rabies IG |
Substantial risk of exposure. |
|
Inactivated bacterial vaccines |
|
|
|
Cholera Typhoid |
Killed bacterial |
Should reflect actual risks of disease and probable benefits of vaccine. |
|
Plague |
Killed bacterial |
Selective vaccination of exposed persons. |
|
Meningococcal |
Polysaccharide |
Only in unusual outbreak situations. |
|
Pneumococcal |
Polysaccharide |
Only for high-risk persons. |
|
Toxoids |
|
|
|
Tetanusdiphtheria (Td) |
Combined tetanusdiphtheria toxoids, adult formulation |
Lack of primary series, or no booster within past 10 years. It is prudent to avoid vaccination during first trimester. |
|
Immune globulins, pooled or hyperimmune |
Immune globulin or specific globulin preparations |
Exposure or anticipated unavoidable exposure to measles, hepatitis A, hepatitis B, rabies, or tetanus. |
YELLOW FEVER ENDEMIC ZONES IN THE AMERICAS
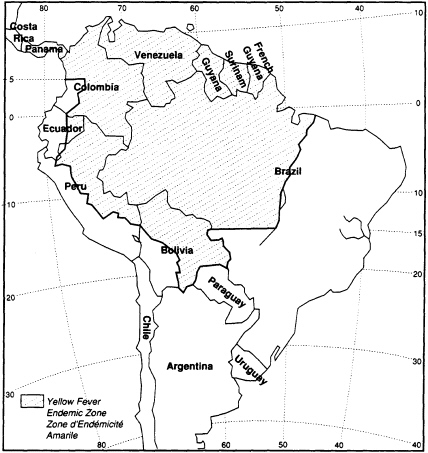
NOTE: Although the "yellow fever endemic zones" are no longer included in the International Health Regulations, a number of countries (most of them being not bound by the Regulations or bound with reservations) consider these zones as infected areas and require an International Certificate of Vaccination against Yellow Fever from travelers arriving from those areas. The above map based on information from WHO is therefore included in this publication for practical reasons.
In addition to areas shaded, CDC recommends vaccination for entire state of Mato Grasso in Brazil.
Example 3
TRIAGE OF THE INJURED PLANT
|
Clinical orientation: |
Clinical conditions |
|
Clinical purpose: |
Evaluation and management |
|
Complexity: |
Medium |
|
Format: |
Triage decision chart, free text, and trauma scoring table (Chapter 3 is provided as an excerpt) |
|
Intended users: |
Practitioners and prehospital care personnel |
This item is actually a chapter from a longer document that is concerned with optimal care of the injured patient. It was produced by the American College of Surgeons' Committee on Trauma. The excerpt deals with field triage (essentially the decision of whether to move an injured patient to a trauma center or to evaluate and manage the patient at a local hospital) and with calculation of a well-known, widely used trauma score (which can be the first step in the triage decision as well as a factor in deciding on interhospital transfers).
This guideline was chosen for several reasons. First, the formatting facilitates quick evaluation of a patient and timely decision making—critical elements for the circumstances in which the guideline would be used. Second, the report also explicitly notes that it "replaces similar documents published in 1976, 1979, 1983, and 1986/87. It is generally recognized that this document is a set of guidelines representing current thinking for optimal care of the injured patient. Further revisions will be published at timely intervals as new information becomes available" (p. 1). In keeping with the emphasis on providing up-to-date information, the book arrives with a sheet of emendations on self-sticking label paper and directions for placing them in the report.
Third, the guideline addresses a broad category of clinical conditions—those that result from injury. The full report focuses on reducing preventable deaths, and it notes the need to balance surgical education and the provision of optimal care. The text also offers discussion of such issues as: systems development; treatment protocols; specific subspecialties of trauma care such as musculoskeletal, pediatric, or eye care; and issues more closely related to policy than to clinical care such as quality assurance concerns, geographically disparate resources, populations, and personnel, and cost-effectiveness considerations. As an example of the last (policy) category, see the discussion in the excerpted free text (p. 18) of acceptable levels of undertriage and overtriage and the relationship between the two-the stated assumption being that in minimizing undertriage (i.e., minimizing the provision of inadequate care to injured patients), some level of overtriage (and therefore overuse of resources) may be inevitable.
CHAPTER 3
FIELD CATEGORIZATION OF TRAUMA PATIENTS (FIELD TRIAGE) 
Triage is the classification of patients according to medical need. There are three applications of this process in the early management of the trauma patient: 1) field triage: 2) interhospital triage to specialized care facilities: and 3) mass casualty triage.
Trauma patients who, because of injury severity, require care at Level I or Level II trauma centers, constitute a fraction of all patients hospitalized each year for trauma. In 1983. approximately 3.75 million patients were hospitalized for injury. In the same year. a study revealed 450 patients per million had an Injury Severity Score (ISS) of 15 or more, accounting for only 5.7 percent of all patients who were discharged from the hospital. Only 8.9 percent of the patients had severities greater than ISS 10. which incorporates just one serious body injury personnel. Even with high over-triage rates, it is unlikely that the number of patients entering trauma centers will exceed 1.00 per million per year. 1.000 per million per year.
It is a substantial challenge for field personnel to identify that small proportion of patients who require prompt access to trauma centers. Furthermore, time is critical. Of the trauma victims who are going to die, 50 to 60 percent do so before reaching a hospital. Of the remaining who die in-hospital, about 60 percent do so within the first four hours.
The following factors must be considered in field triage: 1) the actual or potential level of severity of the injured patient: 2) medical control: and 3) the regional resources available to treat the patient. including time and distance.
ASSESSMENT OF PATIENT SEVERITY
For the purpose of field triage, assessment of patient severity is based on examination of the patient for 1) abnormal physiological signs; 2) obvious anatomic injury; 3) mechanism of injury; and 4) concurrent disease
A triage decision scheme based on current scientific knowledge is illustrated in Table 1.
MEDICAL CONTROL
The triage decision determines the level and intensity of initial management of the major or multiple trauma patient. The vast majority of trauma deaths occur within a few hours of injury. The triage decision is often germane to patient survival or death. It is for this reason that the highest available level of medical expertise should be brought into the triage decision-making process. Usually this process will involve advice and guidance from physicians who provide medical control to prehospital personnel. On-line physician medical control is vitally important in emergency medical systems for the trauma patient.
Surgeons, emergency physicians, and prehospital-care should work together to develop prehospital triage protocols for trauma patients. In most instances of triage based on potentially severe injuries. the patient "triage based on potentially severe injuries, the patient is unable to make an informed decision in selecting appropriate hospital care. The "system" is often responsible for this decision. The system must, therefore. make surrogate decisions. In no instance may these decisions prejudice patient outcome. Disposition decisions at the scene must hold the patient's interests and needs paramount.
RANGE OF RESOURCES; TIME AND DISTANCE FACTORS
Both the level of available hospital resource and time/ distance factors also are considered in making the triage decision. It must be recognized that Level I through III trauma facilities are stratifications in a continuum of capability of commitment to trauma patient care. The system for trauma triage in an urban environment is considerably different from that in a rural environment. In the latter case access to any level of trauma care may involve a significant distance and time.
Each region must, therefore, structure a trauma system in a manner that ensures the prompt access to appropriate care and minimizes the risk of delay in diagnosis, delay in surgical intervention, and inadequately focused care, which are responsible for most of the preventable deaths that occur.
URBAN TRIAGE
In most urban communities in the United States, prompt access to a Level I or Level II trauma center should be feasible within 30 minutes of activation of the EMS feasible within 30 minutes of activation of the EMS system. Many urban populations have more than reasonable access to sophisticated care because of the distribution of tertiary care hospitals that function as Level I trauma centers. Other hospitals that do not offer this level of care or commitment should be bypassed in favor of access to a trauma center.
RURAL TRIAGE
In the rural environment, an injured patient may be at substantial distances from a trauma center. Such patients should be initially treated at a Rural Trauma Hospital. In more remote rural areas, where Level III facilities are not available, staff should at least be trained in ATLS. Patients with major severe injuries should then be secondarily triaged to Level I or II trauma centers, should local resources prove inadequate for continued care (see chapter 15)
Just as the Level II trauma center provides the highest level of care available within most communities across the country, the importance of the Level I trauma the country, the importance of the Level III trauma facility cannot be overemphasized. Between rural and facility cannot be overemphasized. Between rural and urban environments there are geographic areas with urban environments, there are geographic areas with increasing distances between hospitals and decreasing population density. Some patients may require initial triage and resuscitation at a Level III Rural Trauma Hospital. This action may be preferred to primary patient transport from the scene to an urban tertiary care referral center. The EMS system should be structured to provide the patient timely access to the best available level of care indicated by the extent and nature of injuries received.
NOTES TO TABLE 1
Step I Physiologic status thresholds are values of the Glascow Coma Score, blood pressure. and respiratory rate from which further deviations from normal are associated with less than a 90 percent probability of survival. Used in this manner, prehospital values can be included in the admission trauma score and the quality assessment process.
A variety of physiologic severity scores have been used for prehospital triage and have been found to be accurate. The scores contained in the triage guidelines, however are believed to be the simplest to perform and provide an accurate basis for field triage based on physiologic abnormality.
Step II Even in the presence of normal physiology. it is important to evaluate the likely presence of injuries that should be treated in a trauma center. A patient who has normal vital signs at the scene of the accident may still have a serious or lethal injury. Accurate diagnosis of life. threatening injury at the accident scene is unlikely. Thus. it is essential to look for indications that significant forces were applied to the body.
Evidence of damage to the automobile can be a helpful guideline to the change in velocity 'V). A 'V of 20 mph will produce an ISS of greater than 15 in 90 percent of automobile crash occupants 'V can be estimated if one inch of vehicular deformity is equated toe approximate one mph of 'V
Step III Certain other factors that might lower the threshold at which patients should be treated in trauma centers must be considered in field triage.. These include the following:
A. Age Patients over 55 have an increased risk of death from even moderately severe injuries. Patients younger than 5 have certain characteristics that may merit treatment in a trauma center with special resources for children
B. Co-morbid Factors. The presence of significant cardiac, respiratory or metabolic diseases are additional factors that may merit the triage of with moderately severe head injury to trauma centers.
Step IV It is the general intention of these triage guidelines to select patients with an ISS of greater than 15 for trauma center care. Patients with this level of ISS have at least a 10 percent risk of dying from a single severe or multiple serious injuries. When there is doubt. the patient is often best evaluated in a trauma center.
CONTINUING EDUCATION AND EVALUATION
Because of acknowledged imperfections of current field triage and the importance of this process in the delivery of trauma patient care, it is essential to involve surgeons in the continuing education of prehospital care personnel, as well as in feedback to those personnel on the accuracy of their patient triage decisions. Undoubtedly, as decision rules are reviewed, and the results are reported back to the prehospital care personnel, the process of triage will improve.
OVER-TRIAGE AND UNDER-TRIAGE
A system has yet to be developed that reliably and correctly selects the patients for appropriate levels of care that might be available in a given region. As a result, there will always be a certain number of patients selected for trauma center care who could very adequately be handled at a community hospital (85 to 90 percent of all injured patients do not need trauma center care). These patients are referred to as over-triaged. Conversely, patients who are in need of trauma center care but fail to gain timely access to such care are referred to as under-triaged. Together, over-triaged and under-triaged patients combine to form a misclassification rate for any triage decision scheme or rule.
Over-triage and under-triage are interdependent. Considerable medical effort should be made to minimize the number of patients who are under-triaged in a trauma system, because these patients are at risk of dying. Lives may be saved or cost of care may be reduced by prompt access to the needed level of definitive care. There is also concern about the over-triage of patients; over-triage can produce overuse of trauma centers and may divert patients away from community hospitals.
Not all patients with apparent minor injuries can clearly be grouped as not needing trauma center evaluation. For example, a patient who suffers high-deceleration injuries is found to have a wide mediastinum on X-ray film in a rural emergency department. Because of the risk of a ruptured aorta, the standard of care would dictate that such a patient be promptly evaluated in a trauma center where an arteriogram and necessary surgical care were immediately available. A large number of patients who undergo X-ray studies for a wide superior mediastinum after trauma will not have a ruptured aorta. These patients might eventually exhibit only minimal injuries. They could represent an over-triage on trauma system statistics, yet the medical prudence of transferring such a patient group for trauma center evaluation could not be argued.
Studies have shown that a 35 to 50 percent over-triage may be required to maintain a minimum level of under-triage in a community. It also has been estimated that because of the small number of patients who really need to be in trauma centers, the impact of patient flow on an individual institution will be minimal, should this degree of over-triage exist. Clearly, the surgical community needs to be more concerned about under-triage and the medical consequences that result from inadequate use of a trauma system.
Example 4
EVALUATION OF CHEST PAIN IN THE EMERGENCY ROOM
|
Clinical orientation: |
Clinical condition (symptom state) |
|
Clinical purpose: |
Diagnosis |
|
Complexity: |
Medium |
|
Format: |
Free text, tables, and algorithm (excerpts provided) |
|
Intended users: |
Emergency room physicians and other physicians; patients and families |
In 1984, the Massachusetts chapter of the American College of Emergency Physicians (MACEP) developed a set of guidelines focused on continuous monitoring of patient care in high-risk clinical areas as a part of the Massachusetts Emergency Medicine Risk Management Program. The guideline reproduced here is part of this large-scale effort (which includes medical record auditing, data analysis, and feedback) to obtain a malpractice discount for emergency room physicians insured by the state's Joint Underwriters Association. The guidelines, which are developed by consensus, are published as one-page summaries with commentary and/or references from the literature and as individual algorithms. The algorithms are meant to indicate the critical actions that physicians in the emergency room should document for the high-risk diagnostic problem covered by the algorithm.
The excerpt given here is for the assessment of chest pain in the diagnosis of (possible) ischemic heart disease (see the guideline's ''Appendix A. MI/Unstable Angina/New Onset Angina"). It indicates what the physician should take account of and document; it is basically intended to "alert the emergency physician to think of ischemic chest pain in the adult patient in terms of ischemic equivalents in addition to pain itself (p. 289). It also includes an instruction sheet for those patients for whom ischemic disease has presumptively been ruled out and who are therefore being discharged (the "Chest Pain Instruction Sheet" of the guideline's Appendix A). Finally, a separate portion of the guideline gives the algorithm related to this particular guideline (the guideline's "Appendix B. Chest Pain Algorithm").
SOURCE: Karcz, A., and Holbrook, J. The Massachusetts Emergency Medicine Risk Management Program. QRB (Quality Review Bulletin) 17:267292. 1991. Used with permission.
|
MI/Unstable Angina/New Onset Angina History: Pain in chest, jaw, upper abdomen (indigestion), arms Quality: burning, crushing, tight, pleuritic, sharp Radiation: left/right arm, jaw, back OR neurologic, respiratory, gastrointestinal symptoms without pain. Associated symptoms: SOB, nausea, diaphoresis, syncope, vomiting Risk factors: smoking, ASVD, hypertension, family history, obesity, diabetes, cocaine use, cardiac history Physical examination: Chest wall abnormalities/tenderness Lungs: rubs, adventitial sounds Cardiac: rubs, clicks, murmurs EKG: Helpful if abnormal or changed from previous EKG. All bets are off if EKG normal. Defend your diagnosis: Support your diagnosis from history, physical examination, associated symptoms, and risk factors. Watch out for: pneumothorax, aortic dissection, pulmonary embolus. If sending home: Document history, physical exam, and EKG as appropriate for discharge diagnosis. Give specific followup instructions. Assessment of Chest Pain as the Presentation of Ischemic Heart Disease It needs to be stated from the outset that at the present state of the art, it is not possible to diagnose ischemic heart disease with 100% accuracy. The best of clinicians will miss a certain percentage of cases and will undoubtedly admit many cases in which acute myocardial infarction will be ruled out. Given this, perhaps the most important element relating to proper evaluation of chest pain in the Emergency Department is a thorough and thoroughly documented history, physical examination, and appropriately evaluated EKG. The decision to admit a patient should not be dependent on an abnormal electrocardiogram, since, in fact, a normal electrocardiogram does not rule out acute ischemic heart disease. History The history should specifically note the presence or absence of the following: Chest pain: (or its equivalent, e.g., heartburn, indigestion, discomfort, arm or jaw pain.) Associated symptoms: Diaphoresis, nausea, anxiety, palpitations, shortness of breath, "sense of doom," weakness. Other symptoms without chest pain or equivalent: Syncope, shortness of breath, weakness, dizziness. Past medical history: Known coronary artery disease (history of angina or myocardial infarction), nitroglycerin use. It may also be useful to elicit information regarding the duration, type, location, radiation and aggravating/relieving factors relating to the pain. Risk factors (sex, age, hypertension, family history, cigarette smoking, diabetes mellitus, cholesterol) may also be elicited and recorded. |
|
Chest Pain Instruction Sheet You have been evaluated for chest discomfort and even though you are being allowed to go home, please follow the instructions below. Rest at home today. Take medications prescribed as instructed. Return to the Emergency Department by ambulance: 1. If chest pains, heaviness or pressure should develop and lasts longer than several minutes. 2. If you have known Angina and your chest discomfort is worse, lasts longer, comes on with less exertion, or is not relieved by the usual amounts of Nitroglycerin. 3. If you develop any shortness of breath, sweats, vomiting or nausea with your chest discomfort. 4. If your chest discomfort seems to travel into either of your arms, neck, back, jaw or stomach or otherwise changes in nature. Even if you feel better and have no further discomfort, you should follow up with your own doctor tomorrow. |
Example 5
USING ERYTHROCYTE SEDIMENTATION RATE TESTS IN DIAGNOSIS
|
Clinical orientation: |
Technology (diagnostic test) |
|
Clinical purpose: |
Diagnosis |
|
Complexity: |
Medium |
|
Format: |
Free text, tables, and figures (excerpts provided) |
|
Intended users: |
Practitioners |
This item, which consists of excerpts from a longer piece, is taken from a landmark monograph published by the American College of Physicians, Common Diagnostic Tests, which was discussed in Chapter 2. As is true of the entire monograph in its original 1987 version and in the revised edition of 1990, the intent of this guideline is to clarify the appropriate use of a long-established (and perhaps overused) test.
The recommendations are organized according to different patient states or characteristics: asymptomatic persons; problems of interpretation in symptomatic patients; patients with vague, unsubstantiated illness; cancer; temporal arteritis and polymyalgia rheumatica; estimating iron stores; inflammatory arthritis; suspected infection; an extreme or unexplained increase; and monitoring disease activity. Like certain of the other items in the appendix, it specifically focuses on the questions of when the service (here a diagnostic test) is indicated and when it is not.
Apart from its clinical significance, this guideline is of interest for formatting, as it makes use of free text, graphics, and tables. As is true of several other items in the appendix, it cites directly the literature on which its conclusions and recommendations are based. Shown here are the discussion of problems of interpretation in symptomatic patients and in patients with vague, unsubstantiated illness; a figure; and a summary table.
SOURCE: Sox, H.C., Jr., and Liang, M.C. The Erythrocyte Sedimentation Rate. Guidelines for Rational Use. In H.C. Sox, Jr., ed. Common Diagnostic Tests. Philadelphia, Pa.: American College of Physicians, 1987; 2nd ed., 1990. (Excerpts are from pages 209-212, 214.) Used with permission.
PROBLEMS OF INTERPRETATION IN SYMPTOMATIC PATIENTS
The ESR is sometimes used to provide confirmation when the history and physical findings point toward a diagnosis. The test is also used when the patient's chief complaint is not supported by evidence for a specific disease. In this situation, the physician uses the ESR to screen for any serious disease that may be present. Clinical studies have not provided sufficient information to define the role of the test in these two applications.
To evaluate the ESR in symptomatic patients, one must ask how well it predicts disease. The probability of a disease corresponding to an ESR result may be calculated with a Bayes theorem (14). Bayes theorem requires that the pretest probability of the disease and the sensitivity and specificity of the ESR for the disease be known. Unless both sensitivity and specificity are known, a test cannot be interpreted in all situations.
The sensitivity of the ESR has been measured in many diseases, but its specificity has been measured accurately only a few times (15,16). To understand why past studies are so limited, consider the design of an ideal study. The ESR is measured in all patients suspected of having a disease. All patients, regardless of the ESR results, undergo a definitive diagnostic procedure. Some study patients have the disease and the sensitivity of the test is measured in them. The specificity of the ESR is measured in study patients who do not have the disease. In contrast to this ideal study design, the study populations in past studies have comprised only patients with a disease and have not included patients who were suspected of having the disease but did not. Because the specificity of the ESR for a disease has seldom been measured in the appropriate population, the frequency of a normal ESR in healthy persons is sometimes used as a proxy. This approach leads to error because the specificity of the ESR for a disease will be higher in healthy persons than in patients suspected of having the disease, who often have other diseases that increase the ESR. In one study, the frequency of an ESR greater than 20 mm per hour was zero in 32 normal reference subjects, 0.42 in 149 cancer-free reference subjects, and 0.62 in 68 patients with cancer (17). The frequency of an increased ESR in the cancer-free reference subjects shows the lack of specificity of an increased ESR in sick people.
The shortcomings of studies of the ESR affect only the interpretation of an abnormal ESR. As shown in Figure 1A, test specificity largely determines the probability of disease when the ESR is abnormal. Because the specificity of the ESR for most diseases is not known, the post-test probability when the ESR is abnormal cannot be calculated. When the ESR is normal, the sensitivity of the test determines the post-test probability of a disease (Figure 1B). Because the sensitivity of the ESR for many diseases is known, a normal ESR can be interpreted, even if its specificity is not known.
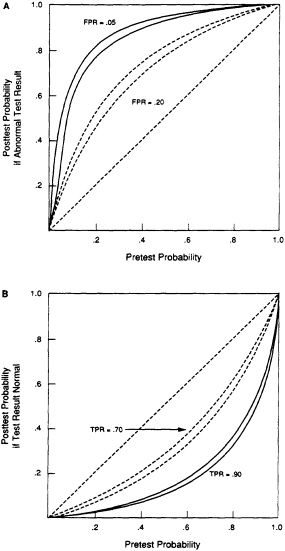
FIGURE 1. Relation between pretest probability of disease and post-test probability. The post-test probability was calculated with Bayes theorem. Figure 1A. The probability of disease in a patient with an abnormal test result. Two values for the false-positive rate (FPR) were assumed. For each value, the sensitivity of the test was assumed to be 0.9 (top curve) and 0.7 (bottom curve). Figure 1B. The probability of disease for a normal (or negative) test result. Two values for the sensitivity of the test (true-positive rate, TPR) were assumed. For each value, the false-positive rate of the test was assumed to be 0.2 (top curve) and 0.05 (bottom curve).
PATIENTS WITH VAGUE, UNSUBSTANTIATED ILLNESS
Physicians often obtain an ESR in patients whose history and physical findings do not suggest any cause for their illness. These patients' pretest probability of serious disease is presumably very low, perhaps nearly as low as that in asymptomatic persons. Although too little is known to be certain, several considerations suggest that the ESR is generally not useful in these patients.
In principle, either a normal or an increased ESR could be diagnostically useful. In practice, neither result is very useful A normal ESR can exclude temporal arteritis, but the test is too often normal in other diseases to be of much value in excluding serious disease. An increased ESR is a clue to unsuspected serious disease but it is seldom present in patients with vague, poorly characterized complaints. As discussed in the preceding section, too little is known to interpret an increased ESR with confidence. However, when the pretest probability of disease is low, the post-test probability will be low unless the ESR is markedly elevated. The probability of some form of serious disease is probably relatively high when the ESR exceeds 50 mm/h, because a markedly increased ESR seldom occurs in healthy people. For example, in one population survey the ESR exceeded this rate in only 4 of 1462 apparently healthy women (15). However, the probability of a markedly increased ESR is very low when the pretest probability of disease is very low (14). This reasoning is substantiated by the very low frequency of an increased ESR in persons with unsuspected disease (Table 3).
These considerations suggest that the ESR is not very useful when the patient's symptom is unsubstantiated by the other clinical data. However, clinical studies of the ESR have not been done in such patients, and a precise recommendation cannot be made at present. Many diagnosticians will choose to focus on possible psychophysiologic explanations for the symptom and allow the evolution of the symptom over time to determine the need for diagnostic testing.
Table 3 Effects of Doing the Erythrocyte Sedimentation Rate (ESR) as a Screening Procedure
|
Study Description (Reference) |
Definition of Abnormal ESR |
Patients with Increased ESR |
ESR as Only Clue to Diagnosis |
|
|
mm/h |
n/n (%) |
n(%) |
|
Random sample of Swedish women; 6-year follow-up (9) |
>30 |
78/1462 (5.3) |
0 |
|
Clinic patients: 10-year follow-up (10) |
>30 (men) |
|
|
|
|
>35 (women) |
790/9140 (8.6) |
|
|
Male clinic patients: no follow-up (11) |
>20 |
Not given |
1+ (0.05) |
|
Surgical admissions: 6 to 42-month follow-up (12) |
|
99/6148 (6.0) |
1§ (0.06) |
|
Israeli airmen age 18-33; yearly follow-up for 15 years (13) 44/1000 (4.4) |
|
44/1000 (4.4) |
|
|
§ Patient died of prostate cancer 28 months after the index visit, at which there was no evidence of cancer and the ESR was 28 mm/h.
|
|||
Example 6
INDICATIONS FOR APPROPRIATE USE OF CAROTID ENDARTERECTOMY
|
Clinical orientation: |
Clinical condition |
|
Clinical purpose: |
Evaluation |
|
Complexity: |
High |
|
Format: |
Free text, tables, and diagrams (excerpts provided) |
|
Intended users: |
Health sciences researchers, policy analysts, and practitioners |
In the mid-1980s, the RAND Corporation developed appropriateness criteria for the use of six specific clinical procedures; indications for carotid endarterectomy were one of the six topics. The procedures were chosen for evaluation according to the following criteria: they are frequently performed, use substantial medical resources, and exhibit significant variation in rates of use across large geographic areas of the United States.
The immense array of possible indications for carotid endarterectomy (excerpts of which are shown in the example) is not, strictly speaking, a guideline; rather it is a detailed analysis and categorization of indications for use of the procedure. Thus, it is closer to being a set of medical review criteria than a tool for shared decision making by physician and patient (the IOM definition of practice guidelines). Using these indicators requires translating the indications into computer algorithms, or learning to read tens of pages of charts such as those included here, or both.
Several of the examples in this appendix are products of a consensus or expert panel; in this case, the process also involved significant analytic and logistical support from the sponsoring organization. The development process included rigorous analysis of all the literature in the subject area, although individual recommendations (indications) are not tied directly to that literature. Like Example 3, on triage of injured patients, and Example 5, on the use of erythrocyte sedimentation rates, this guideline implicitly considers the cost-effectiveness of resource use. Finally, the initial definitions of clinical conditions, the literature analysis, and the process of getting data from practicing physicians are carefully and extensively documented.
SOURCE: Merrick, N.J., Fink, A., Brook, R.H., et al. Indications for Selecting Medical and Surgical Procedures—A Literature Review and Ratings of Appropriateness: Carotid Endarterectomy. R-3204/6-CWF/HF/PMT/RWJ. Santa Monica, Calif.: RAND, 1986. (Excerpts are from pages 48-55.) Used with permission.
RESULTS
The following is the final list of rated indications for carotid endarterectomy. Figure 3 provides a key to reading the results. Note that the first indication for carotid endarterectomy is for a patient with a single episode of carotid TIA or amaurosis fugax whose surgical risk is low and whose angiogram demonstrates an occlusion of the ipsilateral artery and less than 50 percent stenosis of the opposite artery.
This indication received a rating of 1 (extremely inappropriate) by all nine panelists; the median rating was 1.0. Because of the unanimity of the rating the dispersion was 0.0, and panelists agreed on the rating.
INDICATIONS AND RATINGS
DEFINITIONS USED BY THE PANELISTS AT THE TIME THEY RATED THE INDICATIONS FOR CAROTID ENDARTERECTOMY
-
Carotid Transient Ischemic Attack and/or Amaurosis Fugax—Single Episode: The patient's symptoms are consistent with hemispheric ischemia, the TIA episode occurred within the past three months, and the symptoms resolved within 24 hours of onset. The patient may or may not have been placed on medical therapy.
-
Carotid TIAs and/or Amaurosis Fugax—Multiple Episodes, Never Tried on Medical Therapy. The patient's symptoms are consistent with hemispheric ischemia, the most recent TIA episode occurred within the past three months, the symptoms resolved within 24 hours of onset, and the symptoms are different from those grouped separately as ''crescendo TIAs." The patient has never been placed on platelet inhibitors or anticoagulation for cerebrovascular symptoms in the past.
-
Carotid TIAs and/or Amaurosis Faugax—Multiple Episodes, At Least One Recurrence Since Initiation of Medical Therapy As above, the symptoms are consistent with hemispheric ischemia, the most recent TIA occurred within the past three months, the symptoms resolved within 24 hours and are different from "crescendo TIAs." The patient had at least one TIA subsequent to the initiation of treatment with platelet inhibitors or anticoagulation.
-
Carotid TIAs and/or Amaurosis Fugax—Multiple Episodes, No Recurrence Since Initiation of Medical Therapy: The symptoms are the same as those in #2. The patient has been without TIA recurrence while on platelet inhibitors or anticoagulation.
-
Vertebrobasilar TIAs: The patient has suffered symptoms that are not consistent with hemispheric ischemia, but that are consistent with a TIA episode; the most recent TIA episode occurred within the past one year; the symptoms resolved within 24 hours of onset; and the symptoms are different from those grouped separately as "crescendo TIAs." We group patients who have experienced only a single TIA episode with those who have experienced more than one. This category excludes isolated, nonspecific symptoms of dizziness or confusion.
-
Post-Atherothrombotic Stroke: The patient has suffered an atherothrombotic stroke at least three weeks previously. We assume that the patient has not suffered an incapacitating or profound neurologic deficit, but rather is a functional adult living within the community; the patient's symptoms are not consistent with the separately described category "stroke in evolution," and neurologic symptoms have fully stabilized.
-
Stroke in evolution: We used Goldstone and Moore's1 definition: Stroke in evolution is an acute neurological deficit of modest degree that may, within hours or days of the initial event, progress in a sequential series of acute exacerbations to a major stroke. Alternatively, after the initial episode, the neurological deficit may improve temporarily, only to reappear later, often with more widespread involvement, leading to a pattern of waxing and waning of signs and symptoms that occurs over hours to days with an incomplete recovery.
-
Crescendo TIAs: We used Goldstone and Moore's2 definition: Crescendo TIAs are those attacks abruptly increasing in frequency to at least more than one per day.
-
Ipsilateral artery (ipsi): The artery on the same side as the cerebral hemisphere with symptoms.
Contralateral (contra) artery: The artery on the side opposite the symptomatic cerebral hemisphere.
Example: For a patient whose TIA has resulted in a weakness in the right leg, a left-sided TIA, ipsi = left and contra = right.
-
Vessel diameter: The degree of stenosis of both carotid arteries is specified as none or 0%, 1-49%, 50-99%, or 70-99% reduction in luminal diameter.
-
Ulceration categories: Multicentric refers to a large ulceration having multiple cavities or more than one ulcer in a plaque or possessing a cavernous appearance on angiography.
-
Asymptomatic: We include all asymptomatic patients here (other than those undergoing other surgery) whether screening was on the basis of a carotid bruit, other peripheral vascular disease, or a contralateral carotid lesion. We also include here patients with vague symptoms (such as dizziness) not meeting the previous definitions of TIAs.
-
Asymptomatic, Patient to Undergo Other Surgery: Patients undergoing carotid endarterectomy prophylactically before other surgery. Separate ratings were made for two subgroups: intra-abdominal or intra-thoracic excluding coronary artery bypass surgery, and coronary artery bypass surgery.
-
“Dementia of Vascular Origin”: Any patient whose primary indication for endarterectomy is "dementia" that the physician feels is amenable to surgery. "Multi-infarct dementia" is included here.
-
Surgical Risk: Surgical risk is classified as low, elevated, or high Our classifications come from an index developed by Goldman, Caldera, Nussbaum et al.3 and the Dripps-American Surgical Association Classification.4 The first groups patients according to their scores on the following variables:
|
Patient Characteristics |
Score |
|
Age greater than 70 years |
5 |
|
Myocardial infarction in previous 6 months |
10 |
|
S3 gallop or jugular venous distention |
11 |
|
Significant valvular aortic stenosis |
3 |
|
Rhythm other than sinus, or premature atrial contractions on last EKG |
7 |
|
More than five premature ventricular contractions per minute 7 Poor general medical statusa |
3 |
|
Emergency operation |
4 |
|
Intraperitoneal, intrathoracic, or aortic operation |
3 |
|
a PO2 < 60, PCO2 > 50mm Hg, K < 3.0 or HCO3 < 20 mg/L, BUN > 50 or Creatinine > 3.0, abnormal SGOT, signs of chronic liver disease or patient bedridden from noncardiac causes. |
|
-
Class I patients are those with total scores of 0-5 points; Class II, 6-12 points; Class III, 13-25 points; and Class IV, 26 points or greater. Class I is described as "low surgical risk," Class II and III as "elevated surgical risk," and Class IV as "high surgical risk."
The Dripps-American Surgical Association Classification also correlates well with surgical outcome. Patients are categorized as follows by the Dripps system:
|
DRIPPS I DRIPPS II |
Normal healthy person. Person with mild systemic disease (hypertension, asthma, etc.). |
|
DRIPPS III |
Person with severe systemic disease that is not incapacitating (e.g., insulin-requiring diabetes, chronic obstructive pulmonary disease without CO2 retention). |
|
DRIPPS IV |
Person with an incapacitating systemic disease that is a constant threat to life. |
|
DRIPPS V |
Person who is moribund and not expected to survive for 24 hours with or without an operation. (No such patients were apparently found in the population studied by Goldman, Cal- dera, Nussbaum, et al. [3]). |
-
We considered patients in Dripps Classes I and II to represent low surgical risk; Classes III and IV, elevated; and Class V, high surgical risk.
-
Risk of stroke is either high or normal High stroke risk is defined as a probability of greater than 100 per 1000 patients of developing an atherothrombotic brain infarction in eight years based on data from the Framingham Study, 18 year follow-up.5 Calculations of probability take into account a patient's age, sex, presence of left ventricular hypertrophy, whether the patient is diabetic, a smoker, and his or her diastolic blood pressure and cholesterol level.
Example 7
INDICATIONS FOR PERCUTANEOUS TRANSLUMINAL CORONARY ANGIOPLASTY
|
Clinical orientation: |
Technology (surgical procedure) |
|
Clinical purpose: |
Treatment |
|
Complexity: |
Medium |
|
Format: |
Free text |
|
Intended users: |
Practitioners |
This guideline on percutaneous transluminal coronary angioplasty (PTCA) is the result of a collaboration between the American College of Cardiology and the American Heart Association (ACC/AHA); it was noted in the discussion in Chapter 2 on multiorganizational efforts at guidelines development. The guideline illustrated here builds on earlier ACC/AHA work on PTCA generally; this one focuses on indications for angioplasty in patients with acute myocardial infarction (AMI) and makes further clinical distinctions concerning, for example, evolving AMI.
Interestingly, this guideline classifies patients (as opposed to clinical symptoms or technologies) into subgroups, and recommendations are expressed in reference to those subgroups. (This can be contrasted with the subgroup approach to appropriateness indicators as illustrated in Example 6 on carotid endarterectomy from the RAND Corporation.) As is true for several items in this appendix, this guideline cites the relevant literature for its recommendations.
Like most journals the Journal of the American College of Cardiology uses a double column format. In order to include this example, however, it has been reproduced in a single column format. This clearly changes its appearance but probably does not affect its basic utility.
SOURCE: Gunnar, R.M., Passamani, E.R., Bourdillon, P.D., et al. Guidelines for the Early Management of Patients with Acute Myocardial Infarction. ACC/AHA Task Force Report. Journal of the American College of Cardiology 16:249-292, 1990. (Excerpt taken from pages 273-276.) Used with permission.
Percutaneous Transluminal Coronary Angioplasty
Introduction. The guidelines for the use of percutaneous transluminal coronary angioplasty have been previous published in an ACC/AHA Task Force Report (129). That report outlines the immediate and long-term effects of elective angioplasty. its risks and contraindications, the selection of patients and current indications for its use. The present report will elaborate on the indications for angioplasty in patients with acute infarction. The use of angioplasty alone in evolving acute myocardial infarction will be considered separately from the use of angioplasty as an adjunct to thrombolytic therapy.
Primary coronary angioplasty. Along with the increasing interest in thrombolysis for the treatment of acute myocardial infarction, there has been interest in mechanical reperfusion by coronary angioplasty. There have been a number of reports (130-135) describing the use of angioplasty alone in the treatment of acute myocardial infarction. These have all been relatively small series and only one (134) has been randomized in comparison with an alternative therapy (streptokinase). These studies have generally reported a beneficial effect on left ventricular function, but there has been no good large scale randomized study comparing this form of treatment with either conventional supportive therapy or the most effective forms of thrombolytic therapy given early during acute infarction.
Percutaneous transluminal coronary angioplasty as the primary treatment strategy suffers from the need to have facilities and personnel for cardiac catheterization and a physician qualified to perform angioplasty available at all times. Because of this, intravenous thrombolysis has become established as the first line of therapy in acute myocardial infarction in suitable patients.
With this background, angioplasty should be considered as primary therapy in acute myocardial infarction only when facilities are available for expeditious transfer to a cardiac catheterization laboratory and where the personnel have the technical expertise and experience in performing angioplasty in this acute situation. Primary coronary angioplasty may appropriately be considered when a hospitalized patient has
acute myocardial infarction, a patient presents within 4 h after onset of symptoms to an institution where adequate facilities and personnel are available or when thrombolytic therapy is contraindicated. Patients presenting in cardiogenic shock are a special group that may benefit from emergency angioplasty (vide infra).
Although intracoronary thrombolytic therapy is not usually as practical as primary therapy, the use of adjunctive intracoronary thrombolytic therapy during or after an angioplasty procedure may be appropriate when there is evidence of residual thrombus in the artery. In this situation, a smaller dose can be used than that used intravenously (such as 50,000 to 500,000 U of streptokinase or urokinase). Using a smaller dose, particularly < 100,000 U, has the advantage of avoiding a systemic lytic effect, therefore minimizing bleeding complications resulting from thrombolytic therapy.
Recommendations for Primary Angioplasty of Infarct-Related Artery Only
Class I
-
Patients presenting within 6 h of onset of pain and who meet the criteria for thrombolysis but in whom thrombolytic therapy is clearly contraindicated and only if facilities and personnel are immediately available. This recommendation is operative only when data indicate a large amount of myocardium is at risk.
Class IIa
-
Intermittent continuous pain indicating the possibility of "stuttering" infarction, especially if there are ECG changes, but without clear indication for thrombolytic therapy.
-
Within 18 h of acute infarction in patients developing cardiogenic shock or pump failure.
-
Patients who have had previous coronary artery bypass graft surgery in whom recent occlusion of a vein graft is suspected.
Class IIb
-
Patients with known coronary anatomy in whom thrombolytic therapy is not contraindicated, but who develop symptoms and ECG evidence of acute infarction in hos-
-
pital at a time when rapid access to a catheterization laboratory with personnel experienced in performing expeditious angioplasty for acute myocardial infarction is available (completion within I h).
-
Patients in whom thrombolytic therapy is not contraindicated who present within 4 h of onset of symptoms of acute infarction at a facility where rapid access to a catheterization laboratory with personnel experienced in performing expeditious angioplasty for acute myocardial infarction is available (completion within I h).
Class III
This category applies to patients with acute myocardial infarction who do not fulfill the Class I or II criteria. For example:
-
Patients with severe left main coronary artery disease when instrumentation of a more distal occluded artery may be hazardous.
-
Patients in whom only a small area of myocardium is involved, as evidenced by clinical data or previously known coronary anatomy.
-
Dilation of vessels other than the infarct-related artery within the early hours of infarction. (This may not apply to the patient in shock or pump failure.)
Angioplasty after thrombolytic therapy. Immediate angioplasty. Although intravenous thrombolysis offers the promise of early reperfusion in up to 75% of patients (136), more complete reperfusion may be possible by performing angioplasty in those with a high grade residual stenosis of the infarct-related artery and those who failed intravenous thrombolysis. Three well-controlled, relatively large prospective trials (79,136,137) have, however, cast doubt on the utility of this strategy when applied early after thrombolysis and in the absence of continued or recurrent ischemia. The TAMI trial (136), European Cooperative Study (137) and TIMI-IIA (79) trial of urgent angioplasty failed to demonstrate a significant improvement in global or regional ventricular function in patients undergoing emergency (immediate) angioplasty of infarct-related vessels with a residual stenosis after administration of tissue plasminogen activator compared with patients receiving intravenous tissue plasminogen activator alone and undergoing elective angioplasty (TAMI trial), de-
layed angioplasty (TIMI-IIA trial) or no angioplasty (European Cooperative Study). The incidence of complications and death associated with emergency angioplasty was significantly greater in those undergoing emergency angioplasty after intravenous rt-PA than in those undergoing intravenous rt-PA administration without emergency angioplasty in the summed results of the three trials. It therefore appears that urgent angioplasty of infarct-related vessels with a residual stenosis after rt-PA therapy has no significant benefit, but does have a significant increase in risk. The failure of angioplasty immediately after thrombolysis may be related to an increased risk of hemorrhagic infarction when angioplasty is performed after administration of tissue plasminogen activator or to an increased risk of rethrombosis. Thrombolytic agents such as streptokinase, urokinase or tissue plasminogen activator have been shown to cause platelet activation and release of thromboxane A2 (138,139).
Because thrombolysis is incomplete 1.5 to 3 h after the administration of an intravenous thrombolytic agent such as tissue plasminogen activator, it is not surprising that angioplasty performed under these circumstances may further predispose to platelet deposition on the residual thrombosis, with subsequent, distal platelet embolization, reocclusion and death. Whether a similar risk exists with other thrombolytic agents remains to be determined.
Delayed angioplasty. In view of the increased risk of urgent angioplasty after thrombolysis, attention has focused on the role of delayed and elective angioplasty. The need for further revascularization after intravenous thrombolysis relates to the often incomplete thrombolysis and the high incidence of residual stenosis in the infarct-related artery after intravenous thrombolysis. This is in part due to the presence of residual thrombosis and in part to the underlying atherosclerotic lesion. Patients undergoing thrombolysis alone, such as in the GISSI trial (9) or the Western Washington trial (96), had a higher incidence of reocclusion and reinfarction than those not given a thrombolytic agent. The significant advantages of early reperfusion in patients with anterior myocardial infarction in the Western Washington trial of intracoronary streptokinase were lost over a year follow-up as a result of reocclusion of the infarct-related
artery and reinfarction. In a recent study, Mathey et al. (140) reported that patients undergoing coronary artery bypass graft surgery after reperfusion with streptokinase had a better survival rate than patients undergoing thrombolysis alone. The ISIS-2 study, in which aspirin was given in conjunction with intravenous streptokinase, suggested a reduced incidence of reinfarction compared with that from intravenous streptokinase alone (10). The beneficial result of the use of aspirin in conjunction with intravenous streptokinase in regard to survival, reocclusion and reinfarction may modify the need for delayed angioplasty. Nevertheless, a high grade residual stenosis with the potential for recurrent ischemia and infarction persists in many patients after intravenous thrombolysis, suggesting a potential role for delayed or elective angioplasty.
In the Johns Hopkins University trial (121) of delayed angioplasty, patients were first randomized to receive tissue plasminogen activator or placebo and then after 48 to 72 h were rerandomized to undergo or not undergo angioplasty. At follow-up study before hospital discharge, patients undergoing angioplasty had a significant improvement in exercise ejection fraction but not rest left ventricular ejection fraction compared with those not undergoing angioplasty. The risk of angioplasty under these circumstances 48 to 72 h after infarction does not appear to be appreciably greater than that for elective angioplasty. The advantages of this strategy include avoiding the risk of early angiography, avoiding the risk of emergency angioplasty and achieving a high incidence of final reperfusion, a decrease in the incidence of recurrent ischemic events and an improvement in exercise-stressed ventricular function. A disadvantage of this strategy is the possible overuse of angioplasty in low risk individuals.
The TIMI-IIB investigators (79) examined the strategy of delayed angioplasty in a relatively large number of patients and demonstrated that there was no advantage of this strategy on rest left ventricular ejection fraction or survival compared with a noninvasive strategy in which angioplasty was performed only for postinfarction angina or the development of ischemia on stress testing before hospital discharge (79). The noninvasive strategy avoids the risk of early angiography and urgent angioplasty. It restricts the use of coronary angioplasty to those at increased risk of ischemic events. The disadvantage
of this strategy relates to the failure to identify coronary anatomy and the argument that a submaximal prehospital discharge stress test may not reliably predict recurrent ischemic events, reinfarction and death.
In view of the failure of available data to demonstrate an advantage of salvage or rescue angioplasty and the failure to show a benefit of routine urgent or delayed angioplasty after successful thrombolysis, it appears that an elective or noninvasive strategy is preferred. Until further data are available from prospective controlled trials, a conservative approach after intravenous thrombolytic therapy seems indicated. This would reserve angiography and angioplasty for patients with postinfarction angina, severe left ventricular dysfunction or stress-induced myocardial ischemia detected before hospital discharge.
Recommendations for Angioplasty After Intravenous Thrombolysis
Class I
Dilation of a significant lesion suitable for coronary angioplasty in the infarct-related artery in patients who are in the low risk group for angiographic-related morbidity and mortality who have a type A lesion (see ACC/AHA Task Force Report on coronary angioplasty [129]) and:
-
Have recurrent episodes of ischemic chest pain particularly if accompanied by ECG changes (postinfarction angina).
-
Show evidence of myocardial ischemia while on optimal medical therapy during submaximal stress testing performed before hospital discharge or on maximal stress testing in the early posthospital period.
-
Have recurrent ventricular tachycardia or ventricular fibrillation, or both, convincingly related to ischemia while on antiarrhythmic therapy.
Class IIa
Dilation of significant lesions in patients who:
-
Are similar to those in class I but who have type B lesions (anticipated success rate 60% to 85%) (see ACC/AHA Task Force Report on coronary angioplasty [129]).
-
Are within 18 h of onset of acute infarction and have
-
cardiogenic shock or pump failure. These patients should be studied and undergo reperfusion as soon as possible.
-
Before hospital discharge in those who have survived cardiogenic shock or pump failure.
Class IIb
Dilation of a lesion in patients who:
-
Have an occluded coronary artery after attempted thrombolytic therapy.
-
Require multivessel angioplasty.
-
Have >90% diameter proximal narrowing of an infarctrelated artery with a large area of viable myocardium still at risk.
Class III
All patients in the immediate postinfarct period (during initial hospitalization) who do not fulfill Class I or II criteria. For example:
-
Dilation in patients who are within the early hours of an evolving myocardial infarction and have <50% residual stenosis of the infarct-related artery after receiving a thrombolytic agent.
-
Dilation of lesions in vessels other than the infarct-related artery within the early hours of infarction.
-
Dilation of residual lesions that are borderline in severity (50% to 70% diameter narrowing) of the infarct-related artery without demonstration of ischemia on functional testing.
-
Dilation of type C lesions (see ACC/AHA Task Force Report on coronary angioplasty for definition [129]).
-
Undertaking angioplasty in patients in the high risk group for morbidity and mortality (see ACC/AHA Task Force Report on coronary angioplasty for definition [129]).
Example 8
MANAGEMENT OF LABOR AND DELIVERY AFTER A PREVIOUS CESAREAN SECTION
|
Clinical orientation: |
Clinical condition |
|
Clinical purpose: |
Management of birth after previous cesarean birth |
|
Complexity: |
Low |
|
Format: |
Free text condensed from an extensive computer data base on CD-ROM disks (excerpt provided) |
|
Intended users: |
Practitioners (and perhaps educated patients) |
This guideline is one of many in the 400-page A Guide to Effective Care in Pregnancy and Childbirth. The book is a synopsis of the main conclusions of a systematic, 10-year analysis of clinical data conducted by physicians and researchers at Oxford University, England. The analysis was based on a large, continuously updated data base of information (managed and stored using computer systems), which led to a 1,500-page, two-volume reference book called Effective Care in Pregnancy and Childbirth. This example, therefore, is drawn from a summarizing publication that is independent of the data base and reference document.
The guideline was chosen chiefly for two reasons: (1) its relation to the CD-ROM data base and (2) the unusual amount of time and the rigor of analysis that went into its development. In addition, it addresses an area of care about which, in the United States at least, malpractice concerns are great (see the discussions of malpractice and the anesthesia guidelines developed by the American Society for Anesthesiology in Chapters 2 and 5 and case study 4 of Chapter 3). Malpractice is explicitly considered in Example 4 on evaluation of chest pain in the emergency room.
The excerpts shown here might be usefully contrasted with several others in the appendix that also concern the management of a clinical condition but that are presented in quite varied formats (e.g., Example 12 on low back pain and Example 13 on post-bypass surgery care).
SOURCE: Enkin, M., Keirse, M.J.N.C., and Chalmers, I. A Guide to Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1990. Used with permission.
39 Labour and delivery after previous caesarean section
This chapter is derived from the chapter by Murray Enkin (70) in EFFECTIVE CARE IN PREGNANCY AND CHILDBIRTH.
1 Introduction
2 Results of a trial of labour
3 Risks of caesarean section to the mother
3.1 Risks to the mother
3.2 Risks to the baby
4 Factors to consider in the decision about a trial of labour
4.1 More than one previous caesarean section
4.2 Reason for the primary caesarean section
4.3 Previous vaginal delivery
4.4 Type of previous incision in the uterus
4.5 Gestational age at previous caesarean section
4.6 Integrity of the scar
5 Care during a trial of labour
5.1 Use of oxytocics
5.2 Regional analgesia and anaesthesia
5.3 Manual exploration of the uterus
6 Rupture of the scarred uterus in pregnancy and labour
7 Gap between evidence and practice
8 Conclusions
1 Introduction
Although in recent years the dogma of 'once a caesarean always a caesarean has come under both professional and public scrutiny, in many countries the practice is still carried out, and remains a stated policy in many institutions.
Two general propositions underlie the widespread practice of repeat caesarean section: that trial of labour, with its inherent risk of uterine rupture, represents a significant hazard to the well-being of mother and baby; and that planned repeat caesarean operations are virtually free of risk. It is important to examine the validity of these propositions.
2 Results of a trial of labour
No controlled trials have compared the results of elective caesarean section versus trial of labour for women who have had a previous caesarean section. In the absence of such trials, the best available data on the relative safety of trial of labour comes from the prospective comparative studies that have been reported. In these studies, including a total of almost 9000 pregnant women with a history of one caesarean section, over two-thirds were allowed a trial of labour. Of these women almost 80 per cent gave birth vaginally. Thus, for the series for which total data are available, well over half of all women with a previous caesarean section gave birth vaginally.
A large number of retrospective studies have also compared the effects of elective caesarean section versus trial of labour in women who have had one previous caesarean section. There is far greater potential for bias in these retrospective studies than in the prospective studies, and one should be cautious in drawing conclusions from them; nevertheless, it is interesting to note that their results are similar to, and support the conclusions from the prospective studies.
Uterine dehiscence (wound breakdown) or rupture (the data available do not allow these two conditions to be quantified separately) occurred in 0.5 to 2.0 per cent of the women who had elective caesarean sections, and in 0.5 to 3.3 per cent of the women in the trial of labour groups in the prospective cohort studies. Most of these dehiscences were minor in nature, and had no sequelae.
Data from the prospective studies show that febrile morbidity rates were consistently and substantially higher in the groups of women who underwent elective caesarean section (range 11 to 38 per cent) than in the groups of women who had a trial of labour, including both those who had an emergency caesarean section and those who had a vaginal delivery (range 2 to 23 per cent). Although the febrile morbidity rates were highest among women who underwent caesarean section after a trial of labour, these were more than counterbalanced by the lower rate in the two-thirds of women who give birth vaginally after a trial of labour.
Blood transfusions, endometritis, abdominal wound infections, thrombo-embolic phenomena, anaesthetic complications, pyelonephritis, pneumonia, and septicemia were also less common in women who had a vaginal delivery following low transverse caesarean section than in women who underwent a repeat caesarean section.
Perinatal mortality and morbidity rates were similar with trial of labour and elective caesarean section in the studies that report these data. Such comparisons, however, are of little value, because the groups compared are not equivalent. The decision to perform a repeat caesarean section or to permit a trial of labour may be made on the
basis of whether or not the fetus is living or dead, anomalous, or immature.
3 Risks of caesarean section
3.1 Risks to the mother
Large series of caesarean sections have been reported with no associated maternal mortality. One should not be lulled into a false sense of security by this. The risk of a mother dying with caesarean section is small, but is still considerably higher than with vaginal delivery.
The rate of maternal death associated with caesarean section (approximately 40 per 100 000 births) is four times that associated with vaginal delivery (10 per 100 000 births). The maternal death rate associated with elective repeat caesarean section (18 per 100 000 births), although lower than that associated with caesarean sections overall, is still almost twice the rate associated with all vaginal deliveries, and nearly four times the mortality rate associated with normal vaginal delivery (5 per 100 000 births).
The rate of maternal mortality attributable to caesarean section per se is difficult to estimate, as some of the deaths observed are caused by the condition which necessitated the caesarean section in the first place. While it is not possible to quantitate exactly the extent of increased risk of death to the mother from elective caesarean section, the data available suggest that it is between two and four times that associated with vaginal delivery.
Most forms of maternal morbidity are higher with caesarean section than with vaginal delivery. In addition to the risks of anaesthesia attendant on all surgery, there are risks of operative injury, febrile morbidity, and effects on subsequent fertility, and of psychological morbidity as well.
3.2 Risks to the baby
The major hazards of caesarean section for the baby relate to the risks of respiratory distress contingent on either the caesarean delivery itself, or on preterm birth as a result of miscalculation of dates. Babies born by caesarean section have a higher risk of respiratory distress syndrome than babies born vaginally at the same gestational age.
The availability of more accurate and readily available dating with ultrasound may decrease the risk of unexpected preterm delivery. Nevertheless, it is unlikely that this risk can ever be completely eliminated.
4 Factors to consider in the decision about a trial of labour
A mathematical, utilitarian approach comparing the balance of risks
and benefits of trial of labour with those of planned caesarean section will not always be the best way to choose a course of action. Such an approach can, however, provide important data that may be helpful in arriving at the best decision.
The technique of decision analysis has been used to determine the optimal delivery policy after previous caesarean section. The probabilities and utilities of a number of possible outcomes, including the need for hysterectomy, uterine rupture, iatrogenic 'prematurity', need for future repeat caesarean sections, prolonged hospitalization and recovery, additional cost, failed trial of labour, discomfort of labour, and inconvenience of awaiting labour can be put into a mathematical model comparing different policies. Over a wide range of probabilities and utilities, which included all reasonable values, trial of labour proved to be the logical choice.
4.1 More than one previous caesarean section
Data on the results of trials of labour in women who have had more than one previous caesarean section tend to be buried in studies of trial of labour after previous caesarean section as a whole. The available data on delivery outcome for trial of labour in women who have had more than one previous caesarean section show that the overall vaginal delivery rate is little different from that seen in women who have had only one previous caesarean section. Successful trials of labour have been carried out on women who have had three or more previous caesarean sections.
The rate of uterine dehiscence (wound breakdown) in women who have had more than one previous caesarean section is slightly higher than the dehiscence rate for women with only one previous caesarean, but all dehiscences in the reported series were without symptoms and without serious sequelae. There was no maternal or perinatal mortality associated with any of the trials of labour after more than one previous caesarean section reported in these series. No data have been reported on other maternal or infant morbidity specifically associated with multiple previous caesarean sections.
While the number of cases reported is still small, the available evidence does not suggest that a woman who has had more than one previous caesarean section should be treated any differently from the woman who has had only one caesarean section.
4.2 Reason for the primary caesarean section
The greatest likelihood of vaginal delivery is seen when the first caesarean section was done because of breech presentation; vaginal delivery rates are lowest when the initial indication was failure to progress in labour, dystocia, or cephalopelvic disproportion. Even when the indication for the first caesarean section was disproportion,
dystocia, or failure to progress, successful vaginal delivery occurred over 50 per cent of the time in most published series, and the rate was over 75 per cent in the largest series reported. It is clear that a history of caesarean section for dystocia is not a contraindication to a trial of labour, and has only a small effect on the likelihood of vaginal birth when a trial of labour is permitted.
4.3 Previous vaginal delivery
Mothers who have had a previous vaginal delivery in addition to their previous caesarean sections are more likely to deliver vaginally after trial of labour than mothers with no previous vaginal deliveries. This advantage is increased even further in those mothers whose previous vaginal delivery occurred after rather than before the primary caesarean section.
4.4 Type of previous incision in the uterus
Modern experience with operative approaches other than the lower segment operation for caesarean section is limited. There is, however, a growing trend towards the use of vertical incisions in preterm caesarean sections. This, and the inverted T incision sometimes necessary to allow delivery, show that consideration of the type of uterine scar is still relevant.
The potential dangers of uterine rupture are related to the rapid 'explosive' rupture which is most likely to be seen in women who have a classical midline scar. The majority of dehiscences found following lower segment transverse incisions are 'silent', 'incomplete', or incidentally discovered at the time of repeat caesarean section. While scars found at repeat caesarean section can be described as 'dangerous' (meaning thin or 'windowed'), only a small proportion of them actually demonstrated a rupture. What the fate of these 'dangerous' scars would actually have been, had labour been permitted, can only be surmised.
Following a classical caesarean section, rupture of the scar is not only more serious than rupture of a lower segment scar, it is also more likely to occur. Rupture may occur suddenly during the course of pregnancy, prior to labour, and before a repeat caesarean section can be scheduled. A review of the literature at a time when classical caesarean section was still common showed a 2.2 per cent rate of uterine rupture with previous classical caesarean, and a rate of 0.5 per cent with previous lower segment caesarean sections. That is, the scar of the classical operation was more than four times more likely to rupture in a subsequent pregnancy than that of the lower segment incision.
Unfortunately, even in the older literature, there are very few data on the risk of uterine rupture of a vertical scar in the lower segment.
One 1966 study reported an incidence of rupture of 2.2 per cent in classical incision scars, 1.3 per cent in vertical incision lower segment scars and 0.7 per cent in transverse incision lower segment scars. The distinction between the risk of rupture of vertical and transverse lower segment scars may be related to extension of the vertical incision from the lower segment into the upper segment of the uterus.
The uncertain denominators in the reported series make it difficult to quantify the risk of rupture with a previous classical or vertical incision lower segment scar. It is clear, however, that the risk that such a rupture may occur, that it may occur prior to the onset of labour, and that it may have serious sequelae, are considerably greater with such scars than with transverse incision lower segment scars. It would seem reasonable that women who have had a hysterotomy, a vertical uterine incision, or an 'inverted T' incision should be treated in subsequent pregnancies in the same manner as women who have had a classical caesarean section, and that trial of labour, if permitted at all, should be carried out with great caution, and with acute awareness of the increased risks likely to exist.
4.5 Gestational age at previous caesarean section
During the past decade improved neonatal care has increased the survival rate of preterm babies, and this in turn has led to a reduction in the stage of gestation at which obstetricians are prepared to perform caesarean sections for fetal indications. This has resulted in caesarean sections being used to deliver babies at or even before 26 weeks. At these early gestations the lower segment is poorly formed, and so-called 'lower segment' operations at this period of gestation are, in reality, transverse incisions in the body of the uterus. Whether or not such an incision confers any advantage over a classical incision remains in doubt. Indeed, some obstetricians now recommend performing a classical incision under these circumstances.
Whichever of these incisions is used at these early gestational ages, their consequences for subsequent pregnancies are currently unknown. It is quite possible, in theory at least, that they may result in a greater morbidity in future pregnancies than that associated with the lower segment operation at term.
4.6 Integrity of the scar
The decision to advise for or against a trial of labour may be influenced by an assessment of the integrity of the scar. This assessment may be helped by knowledge of the operative technique used at the previous caesarean section, the operative findings at the time of surgery, whether an extension of the operative incision had occurred, and the nature of the postoperative course.
5 Care during a trial of labour
5.1 Use of oxytocics
The use of oxytocin or prostaglandins for induction or augmentation of labour in women who have had a previous caesarean section has remained controversial, because of speculation that there might be an increased risk of uterine rupture or dehiscence. This view is not universally held, nor is it strongly supported by the available data. A number of series have been reported in which oxytocin or prostaglandins were used for the usual indications with no suggestion of increased hazard. Review of the reported case series shows that any increased risk of uterine rupture with the use of oxytocin is likely to be extremely small.
Such comparisons, of course, are rendered invalid by the fact that the cohorts of women who received, or did not receive, oxytocin may have differed in many other respects in addition to the use of oxytocin. Nevertheless, the high vaginal delivery rates and low dehiscence rates noted in these women suggest that oxytocin can be used for induction or augmentation of labour in women who have had a previous caesarean section, with the same precautions that should always attend its use.
5.2 Regional analgesia and anaesthesia
The use of regional (caudal or epidural) analgesia in labour for the woman with a previous caesarean section has been questioned because of fears that it might mask pain or tenderness, which are considered to be early signs of rupture of the scar. The extent of the risk of masking a catastrophic uterine rupture is difficult to quantify. It must be minuscule; only one case report of this having occurred was located. In a number of reported series regional block is used whenever requested by the woman for pain relief, and no difficulties were encountered with this policy.
There does not appear to be any increased hazard from uterine rupture associated with the use of regional anaesthesia for women who have had a previous caesarean section. It is sensible, safe, and justified to use analgesia for the woman with a lower segment scar in the same manner as for the woman whose uterus is intact.
5.3 Manual exploration of the uterus
In many reports of series of vaginal births after previous caesarean section, mention is made of the fact that the uterus was explored postpartum in all cases, in a search for uterine rupture or dehiscence without symptoms. The wisdom of this approach should be seriously challenged.
Manual exploration of a scarred uterus immediately following a vaginal delivery is often inconclusive. It is difficult to be sure whether or not the thin, soft lower segment is intact. In any case, in the absence of bleeding or systemic signs, a rupture without symptoms discovered postpartum does not require any treatment, so the question of diagnosis would be academic.
No studies have shown any benefit from routine manual exploration of the uterus in women who have had a previous caesarean section. There is always a risk of introducing infection by the manual exploration, or of converting a dehiscence into a larger rupture. A reasonable compromise consists of increased vigilance in the hour after delivery of the placenta, reserving internal palpation of the lower segment for women with signs of abnormal bleeding.
6 Rupture of the scarred uterus in pregnancy and labour
Complete rupture of the uterus can be a life-threatening emergency. Fortunately the condition is rare in modern obstetrics despite the increase in caesarean section rates, and serious sequelae are even more rare. Although often considered to be the most common cause of uterine rupture, previous caesarean section is involved in less than half the cases.
Excluding symptomless wound breakdown, the rate of reported uterine rupture has ranged from 0.09 per cent to 0.22 per cent for women with a singleton vertex presentation who underwent a trial of labour after a previous transverse lower segment caesarean section. To put these rates into perspective, the probability of requiring an emergency caesarean section for other acute other conditions (fetal distress, cord prolapse, or antepartum haemorrhage) in any woman giving birth, is approximately 2.7 per cent, or 30 times as high as the risk of uterine rupture with a trial of labour.
Treatment of rupture of a lower segment scar does not require extraordinary facilities. Hospitals whose capabilities are so limited that they cannot deal promptly with problems associated with a trial of labour are also incapable of dealing appropriately with other obstetrical emergencies. Any obstetrical department that is prepared to look after women with much more frequently encountered conditions such as placenta praevia, abruptio placentae, prolapsed cord, and acute fetal distress should be able to manage a trial of labour safely after a previous lower segment caesarean section.
7 Gap between evidence and practice
Obstetric practice has been slow to reflect the scientific evidence confirming the safety of trial of labour after previous caesarean section. The degree of opposition to vaginal birth after caesarean section, in North America in particular, is difficult to explain, considering the
strength of the available evidence that trials of labour are, under proper circumstances, both safe and effective. Two national consensus statements and two national professional bodies, in Canada and the United States, have recommended policies of trial of labour after previous caesarean section.
Increasing numbers of pregnant women, as well as professionals, are vehemently protesting the status quo. For a variety of reasons many women prefer to attempt a vaginal birth after a caesarean section. Their earlier caesarean experience may have been emotionally or physically difficult. They may be unhappy because they were separated from their partners or from their babies. They may wonder if it was all necessary in the first place. They may be aware of the accumulated evidence on the relative safety and advantages of trial of labour, and simply be looking for a better experience this time.
In recent years a number of consumer 'shared predicament' groups have appeared, with the expressed purposes of demythologizing caesarean section, of combatting misinformation, and of disseminating both accurate information and their own point of view. Special prenatal classes are available for many parents who elect to attempt a vaginal birth after a caesarean section.
8 Conclusions
A trial of labour after a previous caesarean section should be recommended for women who have had a previous lower segment transverse incision caesarean section, and have no other indication for caesarean section in the present pregnancy. The likelihood of vaginal birth is not significantly altered by the indication for the first caesarean section (including 'cephalopelvic disproportion' and 'failure to progress'), nor by a history of more than one previous caesarean section.
A history of classical, low vertical, or unknown uterine incision or hysterotomy carries with it an increased risk of uterine rupture, and in most cases is a contraindication to trial of labour.
The care of a woman in labour after a previous lower segment caesarean section should be little different from that for any woman in labour. Oxytocin induction or stimulation, and epidural analgesia, may be used for the usual indications. Careful monitoring of the condition of the mother and fetus is required, as for all pregnancies. The hospital facilities required do not differ from those that should be available for all women giving birth, irrespective of their previous history.
Example 9
USE OF AUTOLOGOUS OR DONOR BLOOD FOR TRANSFUSIONS
|
Clinical orientation: |
Technology |
|
Clinical purpose: |
Treatment |
|
Complexity: |
Low |
|
Format: |
Free-text table |
|
Intended users: |
Patient or family, health care practitioners |
In 1990, the California legislature enacted a bill requiring that any patient undergoing treatment that might involve a blood transfusion be presented with written information about benefits, risks, and options. The written document used must be the standardized document approved by the state's Department of Health Services; furthermore, physicians are required to buy supplies of the guideline to make it available to patients.
This guideline is of interest for several reasons. First, its use was mandated by a state, a relatively unusual occurrence with guidelines (although see Example 4 on diagnosis of chest pain and its relationship to events in Massachusetts). Second, it is intended for use by both practitioners and patients and thus implicitly assumes that patient preferences are a critical factor in providing appropriate care. Like the guideline on deciding about low back pain (Example 12), this guideline is designed primarily for patient use, but its purpose is to convey a fairly sophisticated set of advantages and trade-offs so that any decisions patients make about the course of treatment are based on adequate information and their preferences. Finally, the formatting as a table is clear and concise. This guideline was referred to in Chapter 5 in the discussion on ethics and informed consent.
SOURCE: Reproduction of a public domain brochure from the State of California Department of Health Services, Sacramento, California.
The Safest Blood is Your Own.
Use It Whenever Possible.
Many surgeries do not require blood transfusions. However, if you need blood, you have several options. Although you have the right to refuse a blood transfusion, this decision may hold life-threatening consequences. Please carefully review this brochure and decide with your doctor which option(s) you prefer.
PLEASE NOTE: Your options may be limited by time and health factors, so it is important to begin carrying out your decision as soon as possible.
|
A Patient's Guide to Blood Transfusions |
-
ASK YOUR PHYSICIAN ABOUT NEW DEVELOPMENTS IN TRANSFUSION MEDICINE.
-
CHECK WITH YOUR INSURANCE COMPANY FOR THEIR REIMBURSEMENT POLICY.
This brochure was developed by
California Department of Health Services
714/744 P Street
Sacramento, CA 95814
Kenneth W. Kizer, M.D., MP.H., Director
For information about the contents, plea call:
(916) 445-1248
This brochure distributed by
Medical Board of California
1426 Howe Avenue
Sacramento, CA 9582-3236
Kenneth J. Wagstaif, Executive Director
|
TO ORDER ADDITIONAL COPIES, PLEASE WRITE TO THE FOLLOWING ADDRESS. Office of Procurement Publications Section P.O. Box 1015 North Highlands, CA 95660 Ask for the publication: ''IF YOU NEED BLOOD". Sold in bundles of 50 copies at $4.00 per bundle. [Note: This publication is not copyrighted. You may duplicate for distribution to your patients.] |
IF YOU NEED BLOOD...
The methods of using your own blood can be independently or together to eliminate or minimize the need for donor blood, as well as virtually eliminate transfusion risks of infection and allergic reaction
▇AUTOLOGOUS BLOOD - Using Your Own Blood
|
Option |
Explanation |
Advantages |
Disadvantages |
|
PRE-OPERATIVE DONATION Donating Your Own Blood Before Surgery |
The blood bank draws your blood and stores it until you need it, during or after surgery. For elective surgery only. |
|
• Requires advance planning. • May delay surgery. • Medical conditions may prevent pre-operative donation. |
|
INTRA-OPERATIVE AUTOLOGOUS TRANSFUSION Recycling Your Blood During Surgery |
Instead of being discarded, blood lost during surgery is filtered, and put back into your body during surgery. For elective and emergency surgery. |
|
• Not for use if cancer or infection is present. |
|
POST-OPERATIVE AUTOLOGOUS TRANSFUSION Recycling Your Blood After Surgery |
Blood lost after surgery is collected, filtered and returned. For elective and emergency surgery. |
|
• Not for use if cancer or infection is present. |
|
HEMODILUTION Donating Your Own Blood During Surgery |
Immediately before surgery, some of your blood is taken and replaced with I.V. fluids. After surgery, your blood is filtered and returned to you. For elective surgery. |
|
• Limited number of units can be drawn. • Medical conditions may prevent hemodilution. |
|
APHERESIS Donating Your Own Platelets and Plasma |
Before surgery, your platelets and plasma, which help stop bleeding, are withdrawn, filtered, and returned to you when you need it. For elective surgery. |
|
• Medical conditions may prevent apheresis. • Procedure has limited application. |
In some cases, you may require more blood than anticipated. If this happens and you receive blood other than your own, there is a possibility of complications, such as hepatitis or aids
▆DONOR BLOOD - Using Someone Else's Blood
Donor blood and blood products can never be absolutely 100% safe, even though testing makes the risk very small.
Example 10
DETECTION AND TRACKING OF METABOLIC ACIDOSIS
|
Clinical orientation: |
Clinical conditions (physiologic states) |
|
Clinical purpose: |
Detection of worsening clinical status |
|
Complexity: |
High |
|
Format: |
Decision path with text |
|
Intended users: |
Practitioners |
This guideline was developed by clinicians at a large academic medical center to clarify the appropriate use of serum bicarbonate levels as a means of alerting physicians to new or worsening metabolic acidosis. An alert is triggered when laboratory values for one or a series of these tests meet (or fall below or above) certain criteria; the guideline also alerts the physician to common causes of metabolic acidosis. This specific guideline does not go on to suggest any further diagnostic or therapeutic steps.
The guideline is a shortened version of a Medical Logic Module (MLM) being run at Columbia-Presbyterian Medical Center in New York; MLMs are essentially aggregations of the information necessary to make a single medical decision. Clinical alerts, management critiques, diagnostic scoring algorithms, protocols, and screening rules for research studies have been encoded in this fashion. An MLM is composed of a set of slots grouped into three larger categories: maintenance, library, and knowledge, of which only the knowledge slot is here presented. For clarity, comments on the slots and the three overall categories are imbedded within the guideline in italics-these comments would not appear on the practitioner's computer monitor.
The guideline was selected in part because it is written in Arden Syntax. This is a computer language specially designed to accommodate and promote the use of various health knowledge databases in the service of medical decision making. Hence, it is an interesting sample of a computer- driven algorithm.
SOURCE: Hripcsak, G. Screen for worsening metabolic acidosis based on serum bicarbonate. In the annual ASTM Book of Standards, copyright ASTM, 1916 Race Street, Philadelphia, PA 19103, forthcoming 1992. Used with permission.
SCREEN FOR WORSENING METABOLIC ACIDOSIS
BASED ON SERUM BICARBONATE
KNOWLEDGE: The knowledge category specifies the actual medical decision. The MLM is evoked whenever a serum bicarbonate is stored in the patient database, and the MLM alerts the health care provider to the development of worsening metabolic acidosis. If worsening acidosis is detected, then an alert is stored in the patient database where it can be seen by the provider. The first slot is the type slot, which will be used for future expansion of the syntax; it indicates which slots follow
type: data-driven;
data: The data slot maps the terms used in the rest of the MLM to entities in the patient database. The first statement is a query in which "currentbicarb," "sodium," "chloride,'' and "creatinine" are defined as laboratory values that are a part of the data that evoked this MLM (thus these are the data that have just been stored in the patient database). The second statement is a query that maps "raw_bicarbs" to the patient's last 10 bicarbonate values within the past year. The aggregation operator ("last 10 from") and the time constraint ("where they occurred within the past 1 year") are defined in the Arden Syntax. The part in curly brackets ("{serumbicarbonate}") is specific to the institution in which the MLM is used. When an MLM is shared, this part must be altered to match the institution's patient database. The last statement defines "bicarb_storage" as an event in which a serum bicarbonate is stored in the patient database.
/*----------------------------------------------------------------------------------------------------------------------------------------------------*/
/*get the data that evoked this MLM */
/*----------------------------------------------------------------------------------------------------------------------------------------------------*/
(current_bicarb, sodium, chloride, creatinine) := READ last
{serum_bicarbonate, serum_sodium, serum_chloride,
{serum_creatinine where they are evoking};
/*----------------------------------------------------------------------------------------------------------------------------------------------------*/
/* get the last 10 bicarbs (may or may not be valid) */
/*----------------------------------------------------------------------------------------------------------------------------------------------------*/
raw_bicarbs := READ last 10 from
( { serum_bicarbonate }
where they occurred within the past 1 year;
/*----------------------------------------------------------------------------------------------------------------------------------------------------*/
/* define the storage of serum bicarbonate */
/*----------------------------------------------------------------------------------------------------------------------------------------------------*/
bicarb_storage := EVENT
{insertion of serum_bicarbonate);
;;
evoke: The evoke slot defines the context in which the MLM is executed. In this case the term "bicarb_storage" is used to specify that this MLM is evoked whenever a serumbicarbonate is stored. The serum bicarbonate is usually stored as part of a panel of tests that includes the sodium, chloride, and creatinine. If the bicarbonate is stored by itself, then the first query in the data slot would assign a value of "null" to ''sodium," "chloride," and "creatinine," indicating that there are no valid values for these terms
/*----------------------------------------------------------------------------------------------------------------------------------------------------*/
/* this MLM is evoked by the storage of serum bicarbonate */
/*----------------------------------------------------------------------------------------------------------------------------------------------------*/
bicarb_storage;;
logic: The logic slot decides whether or not an action needs to be taken. Processing occurs in the logic slot until a "conclude" statement is reached; "conclude true" indicates that the action defined in the action slot should be performed, and "conclude false" indicates that it should not. In this logic slot, there is first a check of whether there is a valid value for "current_bicarb;" this ensures that the sample was not hemolyzed. Then there is a check of whether the bicarbonate is below a threshold. The threshold varies with the patient's renal function, as indicated by the creatinine.
Once it is determined that the bicarbonate is below a threshold, the rest of the logic slot checks whether the bicarbonate is worsening. The term "valid_bicarbs" is defined as only those "rawbicarbs" that are valid numbers. There is a check to make sure that the data being stored is not significantly older (in terms of the time that the sample was drawn from the patient) than others in the database. Then "comparisonbicarbs" is defined as those bicarbonates that occurred before the current one and after the last time the bicarbonate was as low as the current one. Finally, there is a check to see whether the bicarbonate has
fallen by at least 20% within the search interval. Note that rather than look for a drop of 20%, the MLM looks for a drop of 10% plus 2 units; this accounts for the absolute variability of the reported bicarbonate value. If all these conditions have been satisfied, then the logic slot concludes true, and the action slot is executed. The original version of this MLM contained additional logic to avoid duplicate alerts and to tailor the alert message to the patient's condition.
/*---------------------------------------------------------------------------------------------------*/
/* Decide whether to send an alert.*/
/*---------------------------------------------------------------------------------------------------*/
/*---------------------------------------------------------------------------------------------------*/
/* Is there a valid bicarbonate value to check (vs. hemolyzed)? */
/*-----------------------------------------------------------------------------------------------------------------------------------------------------*/
if current_bicarb is not number then
conclude false; /* no alert */
endif:
/*----------------------------------------------------------------------------------------------------------------------------------------------------*/
/* Check for evidence of significant metabolic acidosis. */
/*----------------------------------------------------------------------------------------------------------------------------------------------------*/
/*if creatinine >= 3 then /* check for renal insufficiency */
/*----------------------------------------------------------------------------------------------------------------------------------------------------*/
/* If there is renal insufficiency, expect some acidosis. */
/*----------------------------------------------------------------------------------------------------------------------------------------------------*/
if current_bicarb ñ= 15 then /* lower threshold */
conclude false; /* no significant acidosis so no alert */
endif;
else /* BUN normal or unknown */
if current_bicarb ñ= 18 then /* higher threshold */
conclude false; /* no significant acidosis so no alert */
endif;
endif;
/*-------------------------------------------------------------------------------------------------*/
/* Is the acidosis worsening? */
/*-------------------------------------------------------------------------------------------------*/
/*-------------------------------------------------------------------------------------------------*/
/* Define valid bicarbonates (vs. hemolyzed, .. .). */
valid_bicarbs := rawbicarbs where they are number;
/*--------------------------------------------------------------------------------------------------*/
/* Find out whether there are more recent bicarbonates */
/* than the current one being stored. If so, then do */
/* not alert on old data. Define "more recent" as more */
/* than the half time of the bicarbonate changing or of */
/* seeing an alert (about 1 hour). */
/*----------------------------------------------------------------------------------------------------*/
if currentbicarb occurred before
(1 hour before the time of last of valid_bicarbs) then
conclude false; /* do not alert */
endif;
/*----------------------------------------------------------------------------------------------------*/
/* Generate a list of bicarbonates for comparison to */
/* decide whether the value has fallen enough to merit */
/* an alert. Pick a search window that ends at the */
/* current bicarb and that begins at the last bicarb */
/* that was less than or equal to the current bicarb. */
/*----------------------------------------------------------------------------------------------------*/
previous_bicarbs := valid_bicarbs /* previous = before current */
where they occurred before the time of the current_bicarb;
start_time := time of last( /* last time it was <= current */
previous_bicarbs where they <= current_bicarb);
if start_time is present then
comparison_bicarbs := /* comparison = after start */
previous_bicarbs
where they occurred not before start_time;
else /* none of previous bicarbs is this low */
comparison_bicarbs :=
previous_bicarbs; /* so use them all */
endif;
/*--------------------------------------------------------------------------------------------------------*/
/* Make sure the value is dropping and that it has */
/* dropped by at least 20% since the last alert or since */
/* it was last this low. Note that 10%-2 mEq/l is used */
/* in place of 20% to better account for variability */
/* at very low bicarbs. If there are no comparison */
/* bicarbs, then an alert is sent. */
/*--------------------------------------------------------------------------------------------------------*/
if (max(comparison_bicarbs)*0.90)-2 < current_bicarb then
conclude false; /* has not dropped by 20% so no alert */
else
conclude true; /* drop 20% or no comparison, so alert */
endif;
;;
action: The action slot defines what the MLM should do if the logic slot's criteria are satisfied. Possible actions include storing a message in the patient database, sending an electronic mail message, printing a message, and evoking other MLMs. In this MLM the bicarbonate value and time are inserted into an alert message, and then they are stored in the patient database.
/*-------------------------------------------------------------------------------------------------------*/
/* send an alert warning new or worsening metabolic acidosis */
/*-------------------------------------------------------------------------------------------------------*/
write: The patient's serum bicarbonate level " II current_bicarb II " mEq/l at " II time of current_bicarb II ") shows evidence of new or worsening metabolic acidosis. Common causes of metabolic acidosis include: ketoacidosis (diabetic, alcoholic, starvation), lactic acidosis (sepsis, shock, toxins), poisoning (salicylates, ethylene glycol, methanol), renal failure, renal tubular dysfunction (RTA), loss of alkali (diarrhea, ureterosigmoidoscopy), medication, compensation for respiratory alkalosis.";
;;
end:
Example 11
USE OF ORAL CONTRACEPTIVES
|
Clinical orientation: |
Technology (pharmaceutical agent) |
|
Clinical purpose: |
Prevention of pregnancy, management of fertility |
|
Complexity: |
Medium |
|
Format: |
If-then decision statements |
|
Intended users: |
Practitioners |
This guideline was included mainly for reasons relating to formatting. Specifically, it clearly demonstrates the use of both a computer-based guideline and an if-then series of statements. As a guideline displayed on a screen and incorporated in computer software, its advantages lie in ease of use, likely frequency of use, feasibility of frequent or continuous updating, ability to print out specific information for patient reference or patient records, and the ability to bring important information to the physician's attention for needed action in a timely way. In addition, because the guideline is recorded in the computer, it can easily be updated or used to print out patient references or patient records. Note that the citations in parentheses (such as R:2255, R:2097) are to references listed in the computer system (not reproduced here). This approach might easily be employed to generate medical review criteria, such as those used for quality assurance purposes.
Another reason for including this guideline was that it involves a technology (here, a pharmaceutical agent) that raises questions of whether and how to manage a clinical condition with long-term therapy that may have significant harmful side effects.
SOURCE: Reprinted, with permission, from Regenstrief Institute and Wishard Memorial Hospital, Indianapolis, Indiana.
ESTROGENS AND ORAL CONTRACEPTIVES
BEGIN BLOCK ESTROGEN CONTROL
If begun within a few years after the onset of menopause, treatment with exogenous estrogens greatly retards the development of osteoporosis (R:2255, R:2097). Estrogen therapy poses a high risk of endometrial cancer to menopausal women with intact uteri, but there is no such risk if the uterus has been removed. The following rule suggests estrogen replacement for "young" women who have had hysterectomy and oophorectomy. The risk of disabling osteoporosis is all the greater because of their young age, and estrogens have the additional advantage of improving their sense of well-being and preventing vaginal atrophy.
IF NO "ESTROGEN USE"
AND NO "ORAL CONTRACEPTIVE USE"
AND NO "ESTROGENS VAG USE"
THEN IF NO ''PREVIOUS VISIT"
AND (("HYSTERECTOMY SURG"
AND "CERVICAL PAP LAST" WAS =
"ATROPHIC PATTERN")
OR "SURGICAL HX" WAS = "OOPHORECTOMY")
AND "AGE" IS LT 45
AND NO "CAD RISK FACTORS"
AND NO "DX" IS = "BREAST CA"
THEN If patient had both ovaries removed, "estrogens" (with cycling) should be considered to retard osteoporosis. R:2255
AND EXIT
ELSE EXIT
Only patients who are currently using estrogens or oral contraceptives are admitted to the protocols below.
IF "ESTROGEN USE" EXISTS
OR "ORAL CONTRACEPTIVE USE" EXISTS
THEN CONTINUE
ELSE EXIT
The use of oral contraceptives is associated with an increased risk of stroke, myocardial infarction, and thromboembolic phenomena. The risk is proportional to the patient's age and is amplified by cigarette smoking. The medical community has long assumed that the estrogen half of the birth control pill caused this increased risk (R:1168). Estrogens do tend to raise the blood pressure, and to increase low-density and very-low-density lipoproteins (R:1159). In young women they increase cholesterol levels and
decrease the activity of antithrombin III. However, in postmenopausal women, pure estrogens decrease cholesterol levels and have no effect on the cardiovascular risk (R:2840, R:2815). Could it be that the progesterone half is the villain? A study performed at the Kaiser Permanente Clinics (R:3163) suggests this possibility.
Whatever the underlying mechanism, birth control pills have a substantial influence on the cardiovascular risk in women in their middle and upper reproductive years, particularly if they are smokers. The following warning protocol has 3 branches. The first deals with known smokers above the age of 40 in whom the cardiovascular risk of oral contraceptives is severe. The second deals with women over 35 in whom the smoking history is unknown. It generates a reminder once a year per patient. The third branch calls attention to the fact that the birth control pills could be causing the patient's hypertension.
IF "ORAL CONTRACEPTIVE USE" WAS ON AFTER "MOST
RECENT VISIT"
THEN IF ''AGE" IS GT 40
AND "CIGARETTE SMOKER"
THEN Smokers on "B/C" pills" over age 40 have a CV mortality risk 10X that of patients on traditional birth control & 2.5X the mortality risk of expected pregnancies off all birth control. Nonsmokers on B/C pills have a risk 3X that of nonsmokers on IUD's. Alternative contraception should be sought [494]. R:2229
AND EXIT
ELSE IF "MOST RECENT VISIT" WAS BEFORE 1/1
AND LAST "SMOKER 0-1" WAS NOT = 0
AND "AGE" IS GT 35
THEN If patient is a smoker, her CV mortality risk from "B/C pills" is 6X that of the mortality risk of patients on IUD's & 2X that of patients using traditional birth control and therefore alternative birth control method should be considered. R:2229
AND EXIT
ELSE IF
"DIAS BP SITTING LAST" WAS GT 100
THEN "oral contraceptives" may cause or aggravate hypertension (R:1168). Alternative contraceptive method should be sought.
AND EXIT
Estrogens are contraindicated in some forms of porphyria. This protocol simply reminds the clinician of that fact.
IF LAST "COPROPORPH T-URINE" WAS GT HIGH NORMAL
& ON AFTER "MOST RECENT VISIT"
OR LAST "UROPORPHYRIN" WAS GT HIGH NORMAL &
ON_AFTER ''MOST RECENT VISIT" OR "PORPHYRINS QUAL"
WAS NE "NEG" & ON AFTER "MOST RECENT VISIT"
OR "PORPHYRIA DX" EXISTS
THEN if lab results suggesting porphyria are verified, "estrogens" are contraindicated. R:12
The basis of the following rule is the clinical maxim that the least treatment is the best. In younger women, the most important adverse effect of birth control pills is venous thrombosis and pulmonary embolism. Some empirical data suggest that the risks of these complications are less with 50µg than with 80 µg doses of mestrinol. Extrapolating from these observations it seems likely (but is not certain) that the risk could be reduced even further by use of the 30 µg pills that are currently available.
IF "ESTRGN B/C'S LRG USE" WAS ON AFTER "MOST
RECENT VISIT"
THEN Note of interest: B/C pills with 30 mcg estrogen (e.g. "Lo/OVRAL 28") provide as effective birth control as patient's current B/C pills and may have less CV risk. R:803.
Replacement estrogens increase the risk of endometrial cancer (R:3101).
IF "ESTROGEN USE" WAS ON AFTER "MOST RECENT
VISIT" AND "AGE" IS GT 50
AND NO "HYSTERECTOMY SURG" EXISTS
THEN "Estrogens" increase the risk of endometrial ca 4-8 fold, yielding a net cancer risk greater than that of smoking. (If hysterectomy has been done, please disregard message and note date of hysterectomy = ________here.) R:1349
AND EXIT
The following is just a simple reminder about the potential causal effect of
estrogens on trigylceride elevations (R: 1159).
IF "HYPERTRIGLYCERIDE DX" OCCURRED ON_AFTER
"MOST RECENT VISIT"
THEN Reconsider need for "estrogens" in presence of hyperlipidemia since estrogens may be a contributing factor.
END BLOCK ESTROGEN CONTROL
END BLOCK GYNECOLOGY
Example 12
DECIDING ON TREATMENT FOR LOW BACK PAIN
|
Clinical orientation: |
Clinical condition (symptom state) |
|
Clinical purpose: |
Management |
|
Complexity: |
Low |
|
Format: |
Decision path graphic |
|
Intended users: |
Patients |
This simple guideline was designed specifically for patient use and is taken from a well-known book designed to help individuals decide whether to consult a physician, apply home treatment, or do nothing.
Like other items in the appendix aimed at patients, it uses formatting and graphics effectively, and the decision steps are depicted serially. It is oriented toward a clinical situation (actually, the symptom state of pain in the lower back as the patient would experience it), not toward the technologies (drugs, procedures, etc.) that clinicians might eventually use to care for the patient if the patient decided to seek care.
SOURCE: Vickery, D.M. and Fries, J.F. Take Care of Yourself. Copyright 1990, Reading, Pa.: Addison-Wesley Publishing Company. Used with permission.
Example 13
MANAGEMENT OF PATIENTS FOLLOWING CORONARY ARTERY BYPASS GRAFT
|
Clinical orientation: |
Clinical condition (care needed after a specific surgical procedure) |
|
Clinical purpose: |
Management |
|
Complexity: |
Medium |
|
Format: |
Critical pathway |
|
Intended users: |
Practitioners, particularly nurses |
Critical pathways for care are intended to indicate appropriate and efficient steps and timing of care. Typically, they reflect more the established practice norms—that is, the clinical experience and consensus—at the institution developing the pathway than conclusions based on rigorous review and analysis of the scientific literature. They usually cover (explicitly or otherwise) multiple caregivers and services in the institution or practice; in the case of pathways developed by inpatient medical centers, they may (as in this case) provide some guidance on the posthospitalization services to be arranged before discharge. Specific steps in the pathway such as drug therapy may then be covered by more detailed protocols.
Like several of the items in the appendix dealing with the management of a patient with a specific clinical state, this guideline is explicitly sequential in its approach to care; intermediate evaluations of the patient's state are necessary before a patient "moves" to the next step in the process. Presumably because of its intended audience (physicians and nurses within a single institution), it makes liberal use of specialized abbreviations.
This guideline provides a concrete example of the importance of formatting. What is here represented on four pages was originally a single 8.5" x 11" page printed sideways. Not only is the original easier to consult in an actual cardiac care unit, it provides a sense of progress as the plan of care moves across the page from many activities (DAY 1) to fewer (DAY 9). The formatting requirements of this book have in effect compromised the clarity of this guideline example.
Finally, this guideline illustrates how guidelines or protocols may be diffused; the staff of Holston Valley Hospital adapted (but only slightly) a guideline developed by the New England Medical Center in Boston.
SOURCE: Holston Valley Hospital and Medical Center, Kingsport, Tennessee. Used with permission.
Example 14
GUIDELINES FOR THE MANAGEMENT OF PATIENTS WITH PSORIASIS
|
Clinical orientation: |
Clinical condition |
|
Clinical purpose: |
Management (referral to consultant, treatment) and quality assurance audit |
|
Complexity: |
Medium |
|
Format: |
Free text, tables, photographs, lists (excerpts provided) |
|
Intended users: |
Practitioners ("everyone. . concerned in the manage- ment of patients with psoriasis" p. 829) |
This rather comprehensive guideline uses varied formats: free text, quick reference boxes, color photographs, tables, and a list of yes/no questions that parallels the guidelines in structure and is used for quality assurance. The photographs in particular present useful examples of chronic plaque, guttate, localized and generalized pustular, and erythrodermic psoriases. The specific pharmaceutical agents, the necessary pretreatment assessment, contraindications, response time, and areas for monitoring are summarized in a table (reproduced here).
Like Example 11 on the use of oral contraceptives, this guideline addresses the treatment of an ongoing condition; for both the treatment is "suppressive." Like Examples 1, 7, and 8 it summarizes the current information about a clinical condition in an easily read, free-text version. Like the graphic representations of the guidelines on low back pain and dysuria (Examples 12 and 15) this guideline takes an explicitly cumulative approach to treatment—topical treatments are discussed, as a first level of care, then phototherapy and photochemotherapy. The guideline explicitly takes patient concerns and preferences into consideration; it also addresses the question of when to refer a patient from a general practitioner to a consultant dermatologist. Finally, an interesting facet is the quality assurance checklist, which appears to be designed for use by the individual physician rather than by a hospital or other review organization.
SOURCE: Workshop of the Research Unit of the Royal College of Physicians of London; Department of Dermatology, University of Glasgow; British Association of Dermatologists. Guidelines for management of patients with psoriasis. British Medical Journal 303:829-835, 1991. Used with permission.
Types of psoriasis
CHRONIC PLAQUE PSORIASIS
Figure 1 illustrates chronic plaque psoriasis. Depending on the patient's wishes, appropriate management includes the option of no active treatment or the use of a simple emollient. If active treatment is required then the vast majority of patients can be adequately managed with topical agents of proved efficacy such as tar' and dithranol.' Under carefully monitored conditions, which are fully recorded below, the use of topical corticosteroid preparations is also appropriate.

Although each patient must be individually assessed, in general the larger the individual psoriatic plaques and the fewer their number the more appropriate is dithranol. The more numerous the lesions and the smaller they are, the more difficult the use of dithranol becomes, and tar and topical corticosteroids become more suitable. The effect of all topical treatments can be enhanced by suitably supervised treatment with ultraviolet B radiation.
Care must be exercised when a patient's psoriasis is in an inflammatory, eruptive, or unstable phase. In these circumstances the skin may display a general, non-specific irritancy to topical treatments, and therefore only emollients or low concentrations of tar or dithranol should be used.
Topical coal tar-Coal tar is extremely safe and can be used either as a refined product, of which there are many commercially available examples, or as cruder extracts such as crude coal tar in petroleum jelly. The cruder tar extracts are messier to use but are generally considered to be much more effective than more refined products such as coal tar solution. Although there is little published evidence to support the use of any particular concentration, a common treatment regimen is to start with concentrations of 0·5-10% of crude coal tar in petroleum jelly and increase the concentration every few days to a maximum of 10%.
Topical dithranol (anthralin)—Use of dithranol must be accompanied by adequate explanation of side effects, such as irritancy and staining of the skin and clothes. To minimise side effects treatment should normally be started at a concentration between 0·1% and 0·25% and increased in doubling concentrations as the response of psoriasis and development of drug induced irritancy allows. Great care should be taken with dithranol on sensitive body sites such as the face, flexures, and genitalia. It is reasonable to start treatment with a commercially available preparation, but for resistant lesions there may be an advantage in prescribing a similar concentration of dithranol in a different preparation such as modified Lassar's paste. The use of dithranol in the so called ''short contact mode," in which the preparation is left on the skin for only 15 to 45 minutes every 24 hours, can be of great social advantage to the patient without a significant reduction in efficacy.1
Topical corticosteroid—Although effective, cosmetically acceptable, and safe under proper supervision, the use of topical corticosteroids in psoriasis is accompanied by a risk of side effects such as dermal atrophy, tachyphylaxis, fast relapse times, precipitation of unstable and postular psoriasis6 and, in extreme cases, adrenal suppression due to systemic absorption. These risks are related to the potency and cumulative amount of steroid used and the concomitant use of occlusion. If appropriate guidelines are followed (box), however, the use of British National Formulary grade IV (mild) preparation on the face and a grade IV or grade III (moderately potent) preparation elsewhere remains a useful and acceptable therapeutic option.
|
Guidelines for the use of topical corticosteroids
|
Systemic agents used in treatment of psoriasis
|
Systemic agent |
Pretreatment assessment |
Contraindications |
Approximate response time |
Precautions and monitoring |
|
PUVA (psoralens + ultraviolet A) |
History and examination, liver function tests + eye examination |
Pregnancy or wish to conceive, clinically significant cataracts, age < 18 years, previous cutaneous malignancy, previously received ionising radiation or arsenicals, concomitant methotrexate or cyclosporin. Hypersensitivity to psoralens, psoriasis on shielded sites—for example, scalp- previously received PUVA with cumulative lifetime dose of > 1000 J/cm' ultraviolet A |
Four weeks |
Contraception, ultraviolet A eye protection, shielding of genitalia unless specific need to treat regular skin examination for premalignant and malignant changes |
|
Methotrexate |
History and examination, full blood count, liver function tests, serum urea and electrolytes, serum creatinine, creatinine clearance |
Pregnancy, breast feeding, wish to conceive, wish to father children, significant hepatic damage, anaemia, leucopenia, thrombocytopenia, excessive alcohol consumption, acute infectious diseases, diabetes or extreme obesity, immunodeficiency, interactive drugs, renal impairment (reduce dose) |
Two weeks |
Contraception (men and women), avoid drugs which interact (for example, non-streroidal anti-inflammatory drugs and co-trimoxazolc), full blood count, liver function tests, serum urea ant electrolytes, serum creatinine, consider liver biopsy |
|
Etretinate |
History and examination, full blood count, liver function tests, fasting serum lipids, spine radiography, pregnancy test |
Pregnancy or wish to conceive within two years of stopping treatment, Six weeks severe hypercholesterolaemia of hypertriglyceridaemia, severe hepatic or renal impairment, concomitant methotrexate |
Six weeks |
Contraception, liver function tests and fasting scrum lipids (one month after starting treatment and then every three to six months), annual lateral radiography of thoracic spine |
|
Cyclosporin |
History and examination, blood pressure, serum creatinine, measurement of glomerular filtration rate, serum urea and electrolytes, magnesium and uric acid |
Abnormal renal function, uncontrolled hypertension (diastolic blood pressure >95 mm Hg), previous or concomitant malignancy, concomitant radiation therapy, pregnancy and breast feeding, immunodeficiency and immunosuppression, interactive drugs, drug or alcohol misuse |
Three weeks |
Contraception, blood pressure (reduce dose if diastolic >95 mm Hg) , serum creatinine (reduce dose if value increases >30, of patient's ,own baseline value) |
|
Hydroxyurca |
History and examination, full blood count, serum urea and electrolytes, serum creatinine |
Pregnancy and breast feeding, severe anaemia or leucopenia or thrombocytopenia, hypersensitivity to hydroxyurea |
Four weeks |
Contraception, full blood count, liver function tests (weekly during first month and then monthly) |
|
Azathioprine |
History and examination, full blood count, serum urea and electrolytes, serum creatinine, liver function tests |
Pregnancy and breast feeding, severe anaemia, significant hepatic damage, interactive drugs |
Four weeks |
Contraception, full blood count, liver function test (weekly during first two months and then with decreasing frequency), avoid drugs which interact |
|
Systemic steroids |
Used only in extreme and very rare circumstances |
|
||
|
* Estimate of how long any particular treatment should be tried before deciding it is ineffective.
|
||||
Use of guidelines to derive audit measures
Diagnosis, assessment, and initial management
|
(1) Is the diagnosis in clinical doubt? |
Yes/No |
|
(2) Is the patient receiving any treatment likely to precipitate or aggravate psoriasis? |
Yes(what)/No |
|
(3) What in the patient's view is the most distressing or disabling aspect of the psoriasis? |
|
|
(4) Has the nature of psoriasis been explained to the patient? |
Yes/No |
|
(5) Have the treatment options been discussed in the light of (4)? |
Yes/No |
|
(6) Has the patient had an adequately documented 8-12 week trial of topical treatment? |
Yes/No |
|
(7) If topical steroids are included in the regimen is the potency and quantity used appropriate? |
Yes/No |
|
(8) Is referral to a consultant dermatologist appropriate? |
Yes/No |
|
Phototherapy |
|
|
(9) Has the minimal erythema dose been estimated? |
Yes/No |
|
(10) Is the patient receiving an appropriate treatment regimen? |
Yes/No |
|
Systemic treatment |
|
|
(11) Are the indications to move to systemic treatment appropriate? |
Yes/No |
|
(12) Have the options and side effects of possible regimens been fully discussed with the patient? |
Yes/No |
|
(13) If appropriate, has the need for contraception been fully discussed and appropriate provision arranged if required? |
Yes/No |
|
(14) Has the patient been given a psoriasis systemic treatment card? |
Yes/No |
|
(15) If yes, does the patient show this card to the general practitioner when receiving prescriptions for unrelated problems? |
Yes/No |
|
Photochemotherapy (PUVA) |
|
|
(16) Has the patient been given advice about appropriate eye protection? |
Yes/No |
|
(17) Have men been given advice about screening genitalia during PUVA? |
Yes/No |
|
(18) Has the minimal phototoxic dose been estimated? |
Yes/No |
|
(19) Is there a clear record of individual treatments and cumulative ultraviolet A dosage? |
Yes/No |
|
(20) Is the patient receiving an appropriate review and follow up programme? |
Yes/No |
|
Methotexate |
|
|
(21) Have pretreatment investigations excluded haematological, biochemical, and hepatic contraindications? |
Yes/No |
|
(22) Is the patient taking any drug known to have an adverse interaction with methotrexate? |
Yes/No |
|
(23) Are the arrangements for regular review and haematological and biochemical monitoring appropriate? |
Yes/No |
|
Etretinate |
|
|
(24) For women has the need for prolonged contraception (two years) after withdrawal of drug been fully discussed? |
Yes/No |
|
(25) Is the dosage regimen appropriate? |
Yes/No |
|
(26) Are review arrangements adequate? |
Yes/No |
|
Cyclosporin |
|
|
(27) Has the serum creatinine concentration been measured? |
Yes/No |
|
(28) Have potential drug interactions been considered? |
Yes/No |
|
(29) Is the current dose of cyclosporin appropriate? |
Yes/No |
Example 15
ACUTE DYSURIA IN THE ADULT FEMALE
|
Clinical orientation: |
Clinical condition |
|
Clinical purpose: |
Treatment and management |
|
Complexity: |
Medium |
|
Format: |
Algorithm |
|
Intended users: |
Practitioners |
The Harvard Community Health Plan (HCHP) is a multisite, group-model health maintenance organization. Over the past several years, HCHP has developed an extensive series of computer-accessible algorithms for ambulatory care management. Each algorithm is developed by a task force of clinicians based on a thorough review of the scientific literature. The task forces operate under the guidance of a research faculty and staff who are experienced in algorithm development. Each algorithm is followed by explanatory notes regarding options, patients at special risk, and recommended medications and dosages. Some are several pages long.
Like many of the items in this appendix, this guideline is designed for use by practitioners or other health care personnel in the patient's presence; the liberal use of abbreviations makes this easier. It also involves step-by-step decision making, whether by a practitioner or a patient. The relative complexity of this graphic—which is intended for physicians—might usefully be contrasted with the simplicity of the graphic for low back pain (Example 12)—which is intended for lay persons. It also illustrates some of the points made in the text concerning rules for formatting flowcharts and algorithms.
SOURCE: Harvard Community Health Plan, Cambridge, Massachusetts. Used with permission.
ACUTE DYSURIA IN THE ADULT FEMALE
-
A primary goal of this algorithm is to separate women with acute uncomplicated UTI that can be treated with single dose antibiotic therapy from women with complicated UTI that will require further evaluation or longer duration of therapy. Therefore, women who have symptoms longer than 2 or 3 days, women who have fever or flank pain, pregnant women and women with frequent recurrences or other underlying medical problems need to be eliminated from this algorithm. Initial steps in their management are suggested at branch points of this algorithm, but other algorithms will be necessary to more fully address the management of these groups of patients.
Stamm, W., Causes of the Acute Urethral Syndrome in Women, NEJM 1980; 303; 409-415.
-
Choices for multiple dose Rx include 7-10 day course of:
-
Trimethoprim sulfa DS BID (contraindicated in pregnancy, known G6PD deficiency or allergic Hx).
-
Amoxicillin 250 mg po tid (1st choice in pregnancy).
-
Nitrofurantoin 50 mg QID (alternative for patient with multiple allergies or pregnant patient with Hx Pen allergy).
-
Prophylaxis is usually continued for 6 months.
Options for prophylaxis include:
-
Trimethoprim sulfa 12 regular strength tab, QHS.
-
Nitrofurantoin 50 mg QHS (in pregnant patient or patient with Hx T/X allergy or known G6PD deficiency).
Ronald, A. and Harding, G., Urinary Infection Prophylaxis in Women, Annals Int. Med. 1981; 94(2) 268-269.
-
Options for single dose Rx include:
-
Trimethoprim sulfa DS 2 tabs x 1.
-
Amoxicillin 3 gm po x 1.
Kamaroff, A., Acute Dysuria in Women, NEJM 1984; 310; 368-375.
-
Patients who have failed single dose Rx should be considered to have upper tract infection and treated per pyelo protocol.
Example 16
MANAGEMENT OF ACUTE PAIN
|
Clinical orientation: |
Clinical condition |
|
Clinical purpose: |
Management of acute pain due to operative procedures, medical procedures, or trauma |
|
Complexity: |
Medium |
|
Format: |
Free text, flow charts, tables, graphic pain scales |
|
Intended users: |
Practitioners and patients |
The following pages are excerpts from the 1992 guideline, Acute Pain Management: Operative or Medical Procedures and Trauma, commissioned by the Agency for Health Care Policy and Research (AHCPR). The guideline addresses the "widespread inadequacy of pain management" (AHCPR, February 1992, p. 1) by providing clinicians and patients with approaches and tools for assessing and managing pain.
An interdisciplinary panel developed the guideline. They focused on the need for pain management, clinical practice patterns, and clinical and technological options for pain management. The development process included an extensive literature search, evaluation of the quality of clinical data, peer review of drafts of the guideline, tests of the guidelines in clinical situations, an open meeting for testimony, and use of external consultants.
The guideline is available in four forms. In addition to the full guideline cited above, there are two "quick reference guides for clinicians" Acute Pain Management in Infants, Children, and Adolescents: Operative and Medical Procedures and Acute Pain Management in Adults: Operative Procedures-and a patient's guide, Pain Control After Surgery, available in both English and Spanish. The guideline will also be incorporated into data bases at the National Library of Medicine and the National Technical Information Service and into the computer-based information systems.
The complete guide discusses: the need for aggressive postoperative control of pain; pain assessment and reassessment; options for preventing and controlling postoperative pain; control of site-specific pain; management of pain in infants, children, adolescents, and other patients with special needs (e.g., the elderly, known or suspected substance abusers); and institutional responsibility for effective pain relief. It also contains a significant list of references and appendices.
SOURCES: Reprinted (public domain) from: Acute Pain Management Guideline Panel. Acute Pain Management: Operative or Medical Procedures and Trauma. Clinical Practice Guideline. AHCPR Pub. No. 92-0032. Rockville, Md.: Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services. February 1992. Acute Pain Management Guideline Panel. Acute Pain Management in Adults: Operative Procedures. Quick Reference Guide for Clinicians. AHCPR Pub. No. 92-0019. Rockville, Md.: Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services. 1992.
Options to Prevent and Control Postoperative Pain
Patient education and reduction of any preexisting pain should occur before the operation. Because the goal of the treatment plan is to prevent significant postoperative pain from the outset, treatment alternatives, potential risks, dosage adjustments, and adjunctive therapies should be described to the patient and family. Teaching emphasizes what the patient is likely to experience postoperatively, including the specific method(s) of pain assessment, intervention(s) the staff will employ, and the level of patient participation required. Staff also should inform patients that it is easier to prevent pain than to "chase" or treat it once it has become established, and that communication of unrelieved pain is essential to its relief.
Pain control options include:
-
Cognitive-behavioral interventions such as relaxation, distraction, and imagery; these can be taught preoperatively and can reduce pain, anxiety, and the amount of drugs needed for pain control;
-
Systemic administration of nonsteroidal anti-inflammatory drugs (NSAIDs) or opioids using the traditional "as needed" schedule or around-the-clock administration (American Pain Society, 1989);
-
Patient controlled analgesia (PCA), usually meaning self-medication with intravenous doses of an opioid; this can include other classes of drugs administered orally or by other routes;
-
Spinal analgesia, usually by means of an epidural opioid and/or local anesthetic injected intermittently or infused continuously;
-
Intermittent or continuous local neural blockade (examples of the former include intercostal nerve blockade with local anesthetic or cryoprobe; the latter includes infusion of local anesthetic through an interpleural catheter);
-
Physical agents such as massage or application of heat or cold; and
-
Electroanalgesia such as transcutaneous electrical nerve stimulation (TENS).
|
Institutional Responsibility for Pain Management |
|
|
The institutional process of acute pain management begins with the affirmation that patients should have access to the best level of pain relief that may safely be provided. (See Table 3 for a summary of the scientific evidence for interventions to manage pain in adults.) Each institution should develop the resources necessary to provide the best and most modern pain relief appropriate to its patients and should designate who and/or which departments are responsible for the required activities. Optimal application of pain control methods depends on cooperation among different members of the health care team throughout the patient's course of treatment. To ensure that this process occurs effectively, formal means must be developed and used within each institution to assess pain management practices and to obtain patient feedback to gauge the adequacy of pain control. The institution's quality assurance procedures should be used periodically to assure that the following pain management practices are being carried out: |
• Patients are informed that effective pain relief is an important part of their treatment, that communication of unrelieved pain is essential, and that health professionals will respond quickly to their reports of pain. They are also told that a total absence of pain is often not a realistic or even a desirable goal. • Clear documentation of pain assessment and management is provided. • There are institution-defined levels for pain intensity and relief that elicit review of current pain therapy, documentation of the proposed modifications in treatment, and subsequent review of their efficacy. • Each clinical unit periodically assesses a randomly selected sample of patients who have had surgery within 72 hours to determine their current pain intensity, the worst pain intensity in the first 24 hours, the degree of relief obtained from pain management interventions, satisfaction with relief, and satisfaction with the staff's responsiveness. |
Table 3 Scientific Evidence for Interventions to Manage Pain in Adults
|
Pharmacologic Interventions |
|||
|
Intervention1 |
Type of Evidence |
Comments |
|
|
NSAIDs |
Oral (alone) |
LB, IV |
Effective for mild to moderate pain. Begin preoperatively. Relatively contraindicated in patients with renal disease and risk of or actual coagulopathy. May mask fever. |
|
|
Oral (adjunct to opioid) |
Ia, IV |
Potentiating effect resulting in opioid sparing. Begin preop. Cautions as above. |
|
|
Parenteral (ketorolac) |
Ib, IV |
Effective for moderate to severe pain. Expensive. Useful where opioids contraindicated, especially to avoid respiratory depression and sedation. Advance to opioid. |
|
Opioids |
Oral |
IV |
As effective as parenteral in appropriate doses. Use as soon as oral medication tolerated. Route of choice. |
|
|
Intramuscular |
Ib, IV |
Has been the standard parenteral route, but injections painful and absorption unreliable. Hence, avoid this route when possible. |
|
|
Subcutaneous |
Ib, IV |
Preferable to intramuscular for low-volume continuous infusion. Injections painful and absorption unreliable. Avoid this route for long-term repetitive dosing. |
|
|
Intravenous |
Ib, IV |
Parenteral route of choice after major surgery. Suitable for titrated bolus or continuous administration (including PCA), but requires monitoring. Significant risk of respiratory depression with inappropriate dosing. |
|
|
PCA (systemic) |
Ia, IV |
Intravenous or subcutaneous routes recommended. Good, steady level of analgesia. Popular with patients but requires special infusion pumps and staff education. See cautions about opioids above. |
|
|
Epidural and intrathecal |
Ia, IV |
When suitable, provides good analgesia. Significant risk of respiratory depression, sometimes delayed in onset. Requires careful monitoring. Use of infusion pumps requires additional equipment and staff education. |
|
Local Anesthetics |
Epidural and intrathecal |
Ia, IV |
Limited indications. Expensive if infusion pumps employed. Effective regional analgesia. Opioid sparing. Addition of opioid to local anesthetic may improve analgesia. Risks of hypotension, weakness, numbness. Use of infusion pump requires additional equipment and staff. |
|
|
Peripheral nerve block |
Ia, IV |
Limited indications and duration of action. Effective regional analgesia. Opioid sparing |
|
Nonpharmacologic Interventions |
|||
|
Intervention1 |
Type of Evidence |
Comments |
|
|
Simple Relaxation (begin preoperatively) |
Jaw relaxation Progressive muscle relaxation Simple imagery |
Ia, IIa, IIb, IV |
Effective in reducing mild to moderate pain and as an adjunct to analgesic drugs for severe pain. Use when patients express an interest in relaxation. Requires 3-5 minutes of staff time for instructions. |
|
|
Music |
Ib, IIa, IV |
Both patient-preferred and ''easy listening" music are effective in reducing mild to moderate pain. |
|
Complex Relaxation (begin preoperatively) |
Biofeedback |
Ib, IIa, IV |
Effective in reducing mild to moderate pain and operative site muscle tension. Requires skilled personnel and special equipment. |
|
|
Imagery |
Ib, IIa, IIb, IV |
Effective for reduction of mild to moderate pain. Requires skilled personnel. |
|
Education/ Instruction (begin preoperatively) |
|
Ia, IIa, IIb, IV |
Effective for reduction of pain. Should include sensory and procedural information and instruction aimed at reducing activity related pain. Requires 5-15 minutes of staff time. |
|
TENS |
|
Ia, IIa, III, IV |
Effective in reducing pain and improving physical function. Requires skilled personnel and special equipment. May be useful as an adjunct to drug therapy. |
|
1 Insufficient scientific evidence is available to provide specific recommendations regarding the use of hypnosis, acupuncture, and other physical modalities for relief of postoperative pain. |
|||
Summary
Summary recommendations 1-5 and 7, below, should be implemented in every hospital where operations are performed on inpatients. The Acute Pain Management Guideline Panel recommends that any hospital in which abdominal or thoracic operations are routinely performed offer patients postoperative regional anesthetic, epidural or intrathecal opioids, PCA infusions, and other interventions requiring a similar level of expertise, under the supervision of an acute pain service as described in summary recommendation 6, below. For pain management to be effective, each hospital must designate who or which department will be responsible for all of the required activities.
There are a number of alternative approaches to preventing or relieving postoperative pain, many of which can give good results if attentively applied. The following elements, however, apply to most cases and might serve as a focus for assessing the results of these guidelines:
-
Promise patients attentive analgesic care. Patients should be informed before surgery, verbally and in printed format, that effective pain relief is an important part of their treatment, that talking about unrelieved pain is essential, and that health professionals will respond quickly to their reports of pain. It should be made clear to patients and families, however, that the total absence of any postoperative discomfort is normally not a realistic or even a desirable goal.
-
Chart and display assessment of pain and relief. A simple assessment of pain intensity and pain relief should be recorded on the bedside vital sign chart or a similar record that encourages easy, regular review by members of the health care team and is incorporated in the patient's permanent record. The intensity of pain should be assessed and documented at regular intervals (depending on the severity of pain) and with each new report of pain. The degree of pain relief should be determined after each pain management intervention, once a sufficient time has elapsed for the treatment to reach peak effect. A simple, valid measure of intensity and relief should be selected by each clinical unit. For children, ageappropriate measures should be used.
-
Define pain and relief levels to trigger a review. Each institution should identify pain intensity and pain relief levels that will elicit a review of the current pain therapy, documentation of the proposed modifications in treatment, and subsequent review of its efficacy. This process of treatment review and followup should include participation by physicians and nurses involved in the patient's care.
-
Survey patient satisfaction. At regular intervals defined by the clinical unit and quality assurance committee, each clinical unit should assess a randomly selected sample of patients who have had surgery within 72 hours. Patients should
-
be asked their current pain intensity, the worst pain intensity in the past 24 hours, the degree of relief obtained from pain management interventions, satisfaction with relief, and their satisfaction with the staff's responsiveness.
-
Analgesic drug treatment should comply with several basic principles:
-
Non-opioid "peripherally acting" analgesics. Unless contraindicated, every patient should receive an around-the-clock postoperative regimen of an NSAID. For patients unable to take medications by mouth, it may be necessary to use the parenteral or rectal route.
-
Opioid analgesics. Analgesic orders should allow for the great variation in individual opioid requirements, including a regularly scheduled dose and "rescue" doses for instances in which the usual regimen is insufficient.
-
-
Specialized analgesic technologies, including systemic or intraspinal, continuous or intermittent opioid administration or patient controlled dosing, local anesthetic infusion, and inhalational analgesia (e.g., nitrous oxide) should be governed by policies and standard procedures that define the acceptable level of patient monitoring and appropriate roles and limits of practice for all groups of health care providers involved. The policy should include definitions of physician and nurse accountability, physician and nurse responsibility to the patient, and the role of pharmacy.
-
Nonpharmacological interventions: Cognitive and behaviorally based interventions include a number of methods to help patients understand more about their pain and to take an active part in its assessment and control. These interventions are intended to supplement, not replace, pharmacological interventions. Staff should give patients information about these interventions and support patients in using them.
-
Monitor the efficacy of pain treatment: Periodically review pain treatment procedures as defined in summary recommendations 1-4 above, using the institution's quality assurance procedures.

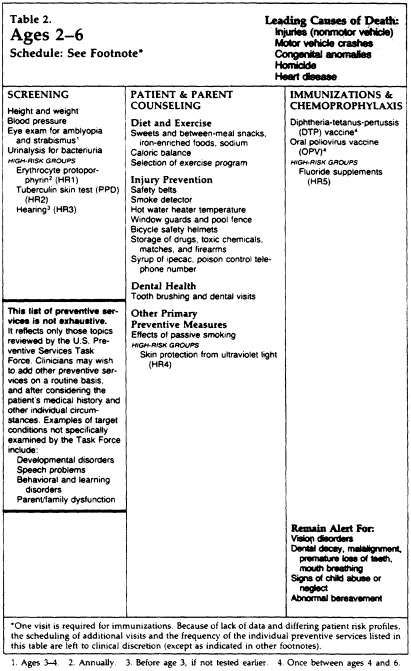
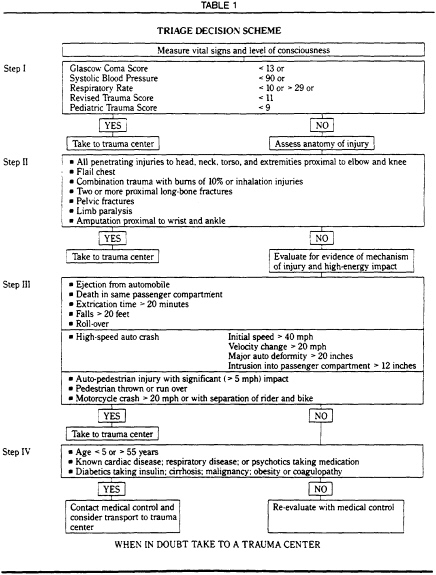
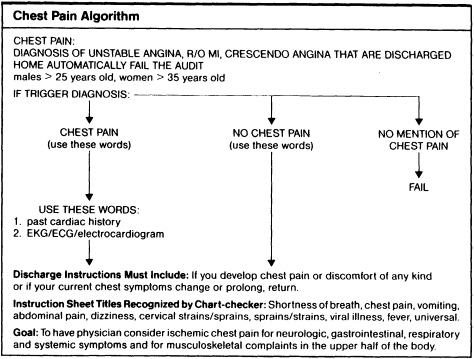
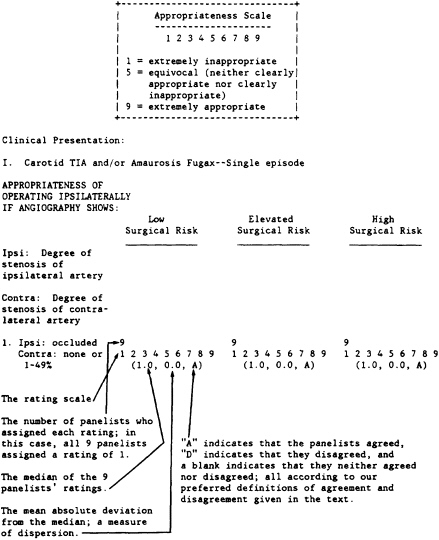



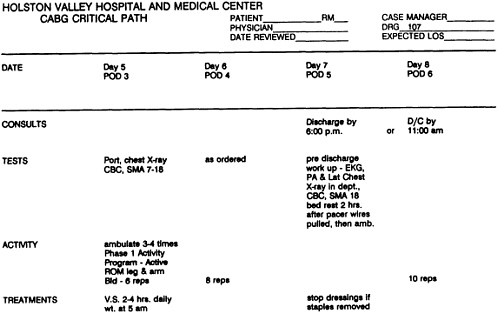
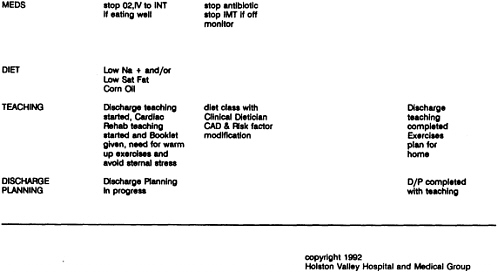
 Measurement of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase,
Measurement of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase,