4
Value Propositions for Drug Repurposing
Important Points Highlighted by Individual Speakers
- High-throughput genomics-based data are helping to elucidate disease mechanisms and targets, which provides optimism for the identification of new indications for a shelved compound.
- Opportunities for new indications for drugs need to be reevaluated throughout the discovery, development, and life-cycle management of a compound.
- Government incentives for drug development can be important for industry, but it is challenging to make an unattractive business opportunity more attractive.
- Understanding disease mechanisms and collecting clinical data are important components of identifying new indications for a drug.
Patients receive effective treatments when private companies are engaged and using their expertise to facilitate the regulatory approval, manufacturing, and distribution of drugs. Repurposing and repositioning therefore needs to make economic sense for companies if drugs are to become available for new indications. In a market where drug development is costly, pharmaceutical companies carefully consider their strategies for which drugs and targets to pursue, individual speakers said. The repurposing of thalidomide is provided as an example of the importance of understanding drug and disease mechanisms coupled with clinical data.
Drug repurposing offers grounds for pessimism and optimism, said Michael Ringel, partner and managing director for the Boston Consulting Group. For the past six decades, the number of new drugs developed per billion dollars invested in R&D has undergone an exponential decline—a trend known as Eroom’s law (or Moore’s law written in reverse) (see Figure 4-1) (Moore, 1965). As a result, the average cost to bring a drug to market has increased from a few hundred million dollars in the 1990s to more than $2 billion today, Ringel said. Today, for every dollar spent by the pharmaceutical industry on R&D, less than a dollar of value is returned, suggesting that future R&D expenditures by companies will decline.
One cause of the declining return on investment is the high failure rate of drug candidates in all phases of development. An obvious response would be for drugs to “fail earlier” in the development process so as to minimize the time and resources spent to determine that a drug will
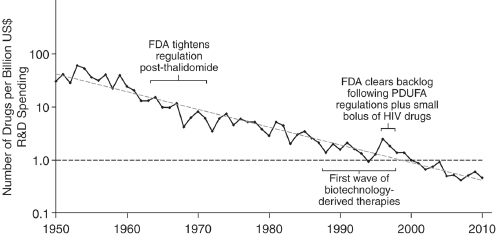
FIGURE 4-1 Eroom’s Law in pharmaceutical R&D (spending adjusted for inflation).
NOTE: FDA, U.S. Food and Drug Administration; HIV, human immunodeficiency virus; PDUFA, Prescription Drug User Fee Act; R&D, research and development; US, United States.
SOURCE: Reprinted by permission from Macmillan Publishers Ltd.: Nature Reviews Drug Discovery (Scannell et al., 2012), copyright 2012.
not be successful; however, behavioral disincentives (e.g., optimism about drug development data, market forces, job security) often interfere. The application of behavioral economics theory suggests that humans also are temperamentally biased toward continuing to invest in projects that already have considerable costs that may not be recouped. As Daniel Kahneman has written, there is a tendency to gamble on a gain even when taking that chance is more likely to lead to a greater loss (Kahneman, 2013).
These observations should point toward a cautious approach for drug repurposing, Ringel said. A drug that has already failed and is sitting on the shelf does not seem to be a good bet for alternative uses, he said, if, for example, the drug failed because of toxicity issues.
However, that challenge is balanced with reasons for optimism in genomics-enabled repurposing. GWAS data offer new ways to identify more promising targets. High-throughput biological data are uncovering the mechanistic pathways involved in disease. Precision medicine is becoming more successful, Ringel said—for example, in subdividing non-small-cell lung cancer into molecular subcategories responsive to targeted treatments (Ou et al., 2012).
Finding the Right Drug for a Disease Target
Thinking about drug development in a different way could establish a reasonable path forward for repurposing, Ringel suggested. Gaining a better understanding of disease targets and mechanisms can overcome market imperfections that impede repurposing. Some companies are developing platform tools to build matrices of diseases and molecules that might treat those diseases. Other companies are developing drugs specifically for out-licensing.
Drug developers could use imperfect drugs as probes to validate research hypotheses about the mechanisms of a disease, Ringel said. With this information, developers could then refine their research to design a molecule that works more effectively on the target to potentially treat the disease. As in the case of thalidomide (discussed later in this chapter), investigative molecular entities can act as probes for more effective or targeted molecules. Consortia, whether public or supported by government, can work on a larger scale than independent private efforts and can overcome loss-of-exclusivity issues.
Though returns on R&D investments have been declining in the pharmaceutical industry, Ringel said that he is an optimist about technol-
ogy curves. Insights into new therapeutic targets and pathophysiologic mechanisms portend new drug indications.
REPURPOSING DRUGS CURRENTLY IN DEVELOPMENT
Translating scientific insights into novel therapies requires identifying the area where scientific innovation, unmet needs, and commercial attractiveness overlap, observed Simeon Taylor, vice president for research and scientific affairs at Bristol-Myers Squibb (BMS).
At least two factors contribute to the commercial attractiveness of a therapeutic, Taylor said. One is the existence of a sufficiently large number of patients to make a drug profitable. In that context, rare diseases can present a challenge, though the challenge can be offset to a certain degree by the price of the drug per patient.
The second factor contributing to commercial attractiveness is the synergy of expertise that is created through collaborations between academia and industry, Taylor said. Academia can provide innovative insights linking pharmacologic and disease mechanisms to drug indications. Industry can facilitate execution across stage development, regulatory approval, manufacturing, and commercialization.
Evaluating Disease Indications
The terms purposing or positioning are preferable to repurposing and repositioning, Taylor said, because the process of selecting a disease indication should be evaluated at every point in the discovery, development, and life-cycle management of a product. Even when an initial disease indication is selected, knowledge about the mechanism or the target may be relatively limited. Over the course of drug development, there may be numerous opportunities to re-evaluate indications. In addition, a safety concern that may not be manageable in one context may be manageable or acceptable in another, such as a more serious disease that leads to negative outcomes in patients when a suitable treatment is not available.
Dasatinib, an antineoplastic agent, was originally developed from a BMS program targeting the tryosine kinase Lck. During the study of dasatinib, researchers noticed that the drug had off-target effects against other tyrosine kinases, including Abl, which is involved in Philadelphia chromosome positive leukemia. In collaboration with Charles Sawyer at
the University of California, Los Angeles, BMS researchers optimized dasatinib for Abl, which led to FDA approval for its use in treating imatinib-resistant Philadelphia chromosome-positive leukemia as well as consideration for first-line treatment (Das et al., 2006; Shah et al., 2004; Talpaz et al., 2006). In this case, academic–industry collaboration was essential for producing a dramatic shift from one target and indication early in the drug development process to another, Taylor said.
Another example involves lomitapide, which was recently approved as a treatment for familial hypercholesterolemia. The basic scientific research conducted largely at BMS relied on animal models and found that the compound effectively improved lipid profiles, Taylor said. However, the drug’s significant adverse reactions, especially the accumulation of fat in the liver, were deemed unacceptable because patients with common dyslipidemia had access to numerous therapeutic alternatives. At that point, an academic researcher at the University of Pennsylvania suggested that the drug could be used to treat patients with rare homozygous familial hypercholesterolemia in cases where effective treatments are not available. Researchers moved forward successfully (using the drug donated by BMS) and founded a separate biotechnology company, Aegerion Pharmaceuticals, for handling the regulatory approval, mass manufacturing, and efficient distribution of the drug. During a Phase III study, lomitapide was found to be effective in lowering low density lipoproteins in patients (Cuchel et al., 2013).
Understanding the reasons the development of a particular drug failed can help in the repositioning of the drug in a context where it is less likely to fail, such as using it for defined patient subgroups, Taylor said. Metreleptin, a modified version of the hormone leptin, was originally developed by Amgen to promote weight loss for people with a genetic deficiency in leptin (Farooqi and O’Rahilly, 2006; Farooqi et al., 1999; Zhang et al., 1994). However, most people who are obese have sufficient endogenous leptin and are resistant to its effects, which led Amgen to stop drug development. At the time, Taylor said, he was working at the National Institute of Diabetes and Digestive and Kidney Diseases, and Taylor and his team reached out to Amgen to consider repurposing metreleptin for patients with lipoatrophy who have no fat (except for significant deposits in the liver) and no leptin. It was subsequently demonstrated that metreleptin significantly reduced circulating triglyceride levels and deposition of fat in the liver, which reduced the prevalence of serious clinical sequelae, including steatohepatitis (Oral et al., 2002). The leptin program has been acquired by other companies over time and the
hope is that patients could be resensitized to leptin, thus learning from past failures in a way that repositions the drug for success.1
Considerations for Development
Taylor underscored the importance of financial incentives to companies by positing that the number of patients multiplied by the price for a drug must attain a certain minimum level in order for it to make financial sense for a company to invest in the drug (although companies may provide drugs for philanthropic reasons). The company must also take into account how long the drug will be under patent protection, Ringel said. If the patent on a drug has expired or is close to expiring, the cumulative revenues available from that drug will be much less than for a drug with significant patent life, he noted.
The federal government incentivizes drug development for orphan diseases; however, Taylor said, some diseases are still more likely to attract interest than others. A disease affecting 199,000 patients is naturally a more attractive target than a disease with only 100 patients, he said. While government incentives certainly matter to companies, they may not make a clearly unattractive business opportunity attractive enough to pursue.
There is also some reluctance to explore repurposing collaborations, Taylor said. Transferring technology is also often time-consuming and costly, and biotechnology company partners may lack sufficient capital resources to successfully invest in the compound to see through its development. There is a perception that the odds are slim that a viable product line will result for any given drug. For these reasons, BMS infrequently engages in these types of repurposing efforts, Taylor said.
THALIDOMIDE: REPURPOSING A DRUG THAT WAS NOT SUCCESSFUL FOR ITS FIRST INDICATION
Thalidomide was initially marketed as a sedative to address symptoms of morning sickness in pregnant women and was first offered as an over-the-counter product in 1957 by the German pharmaceutical company
______________________
1On February 24, 2014, FDA approved Myalept (metreleptin) for patients with congenital generalized or acquired generalized lipodystrophy. See FDA approves Myalept to treat rare metabolic disease. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm387060.htm (accessed February 27, 2014).
Grünenthal, said Thomas Daniel, executive vice president for global research and early development at Celgene. However, sporadic reports had surfaced of phocomelia, a congenital deformity resulting in short limbs so that the hands and feet are in close proximity to the trunk, in children born of women who had taken the drug. The first affected patient was recognized in December 1956, but not until 1961 in a letter from William McBride in Lancet was there a published account of the correlation between thalidomide and birth defects (McBride, 1961). In the United States, FDA reviewer Frances Oldham Kelsey had delayed approval of the drug until a more complete data package was made available, which saved many U.S. women and families from devastating outcomes. For her role she was honored with the President’s Award for the Distinguished Federal Civilian Service by President Kennedy in 1962 (FDA, 2013c).
After its withdrawal as a sedative, thalidomide was discovered in the 1960s to be an effective anti-inflammatory treatment for patients with leprosy, Daniel said. In the 1990s, Gilla Kaplan at Rockefeller University had an interest in thalidomide as an active inhibitor of tumor necrosis factor (TNF)-alpha production. At the same time, there was interest from the international AIDS community for using thalidomide because HIV/AIDS patients have elevated levels of TNF-alpha, although FDA voiced concern about development in unauthorized markets. It was not until 1998 that thalidomide was approved by FDA for use in patients with erythema nodosum leprosum (ENL), a type of leprosy reaction. This fueled aggressive efforts to identify additional applications and alternative drugs with reduced safety liabilities. Celgene therefore set out to reposition thalidomide for new indications and to develop analogues that lacked teratogenicity and other side effects.
New Indications
The first two new indications for thalidomide were for hematologic applications far removed from its anti-inflammatory effects in ENL, Daniel said. Thalidomide proved to be effective in treating refractory multiple myeloma (Singhal et al., 1999) and refractory anemia with excessive blasts, a type of myelodysplastic syndrome. These successes enhanced development efforts for the second-generation compounds lenalidomide and pamolidomide, Daniel said.
A prominent part of that research endeavor was the identification of targets and mechanisms for thalidomide and related compounds. Because
thalidomide has pleiotropic effects on plasma cells, on the stromal cells that support them within bone marrow, and on the immune system, early evidence pointed to differential co-stimulation of T-cells by thalidomide and related compounds, Daniel said. In addition, in 2010, the primary target of teratogenicity was identified (Ito et al., 2010), which elucidated the immunomodulatory effects related to some of the common adverse reactions. This expanded understanding of the pharmacologic mechanisms facilitated the development of new thalidomide analogues, each with different pharmacodynamic properties, for further study. For example, a molecule called CC-122 is in late Phase I studies showing dramatic activity in B cell malignancies, in hepatocellular carcinomas, and in anaplastic astrocytoma, Daniel said.
Data Coupling Is Key
An understanding of disease mechanisms and therapeutic targets can dramatically narrow the hunt for clinical indications and provide paths for regulatory approval, Daniel said; however, clinical observations remain important for drug repurposing, as demonstrated by the use of thalidomide for leprosy and other conditions. Though the development of thalidomide and related compounds was not enabled by genomics, it is an example of how coupling systems biology to phenotypic assays can reveal differential activities, produce new intellectual property, and create new indications and value propositions.








