Polymer Membranes for Water Filtration

Today, about 783 million people—11 per cent of the world’s population—live without access to clean drinking water.21For these people, many of whom live in the developing world, taking a drink of water means risking exposure to bacteria, viruses, arsenic, and other chemicals. Every year, there are 3.4 million deaths from water-, sanitation-, and hygiene-related causes, many of them preventable.22
The problem is that for much of the world, sources of clean, fresh water are scarce. About 97.5 percent of the water on our planet is salty, and only 0.5 percent of the fresh water is accessible.23Even within the United States, freshwater supplies in places such as the West are rapidly dwindling,24and drought threatens agriculture. At the same time, increasing contamination of water supplies from pesticides, fertilizers, hormones, pharmaceuticals, and shale gas extraction is a serious problem that threatens health. A lack of access to water supplies has the potential to trigger conflict, both within the United States and around the world.

To help solve these problems, materials scientists and chemical engineers are working to develop inexpensive, scalable, and sustainable methods to harvest and purify water, for example with new polymer membranes that can filter contaminants from the water25 (see box). One research team is working on a fuel-efficient method for turning salty seawater into clean, drinkable freshwater. Currently, salt is removed from seawater either by thermal desalination, which involves boiling seawater above 212°F and then distilling the vapors, or by reverse osmosis, which uses hydraulic pressure to force water through a membrane that filters out salt. Both methods require large inputs of energy. In 2011, Oasys Water announced a desalination method that is at least 10 times more fuel-efficient.26 The new method is based on the principle of simple forward osmosis: Without any input of energy, water molecules will naturally move from fresh solutions to saltier ones. The researchers developed a specially formulated solution that is saltier than seawater, drawing water molecules out of seawater across a porous membrane and leaving the sea salt residue behind. The resulting solution contains a mix of salt compounds that vaporize at much lower temperatures than seawater salts. That means the salty liquid can be made into freshwater by thermal desalination at only 122°F, a significant energy saving over traditional thermal desalination methods.
Another team of researchers is developing new membranes that make the process of reverse osmosis more efficient by fabricating a matrix of polymers and nanoparticles that allows water molecules to flow through but repels contaminants.27 That means less energy is needed to pump water through the membrane.
Striving to develop technologies that could help increase access to clean drinking water was one of the goals of the late MIT polymer science professor Anne M. Mayes. Her research group designed and synthesized novel polymer materials that helped solve one of the more intractable problems of water filtration systems: the buildup of contaminants and salt at the surfaces of the filters and membranes. This fouling can clog membrane pores, decreasing the amount of water that flows through the system. As a result, the membranes must be cleaned and replaced frequently—at a high cost.
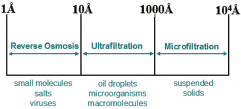
Schematic representation of the processes involved in the cleaning of water. Contaminated water contains many pollutants of various sizes, and an integrated approach is needed to remove them all. Initial states include treatment with sediment filters that remove larger particles and then ultrafiltration with hydrophobic/ hydrophilic membranes for the removal of oils and microorganisms. Typically, these steps are followed by subjecting the water to reverse osmosis through membranes that remove the smallest contaminants.
Every year, there are 3.4 million deaths from water-, sanitation-, and hygiene-related causes, many of them preventable.
The Mayes group developed copolymers that are studded with “comb” copolymers, which consist of a hydrophobic backbone lined with short hydrophilic extensions, like the teeth on a comb. The teeth attract water molecules and prevent the buildup of contaminants, creating membranes that are remarkably resistant to fouling (see box). Two of Mayes’s graduate students founded a new small business in Massachusetts, Clean Membranes (www.cleanmembranes.com) to bring these foul-resistant membranes to the market. With support from the Office of Naval Research, the company has developed membranes as a pretreatment step for shipboard desalination systems, attracting venture investment. Companies like these bring new technologies to help solve global problems, while providing jobs to bolster the U.S. economy.
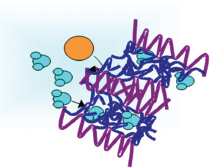
Efforts to improve water filtration systems include the development of membranes made up of combinations of materials that perform different functions in the filtration process. The example in this figure consists of two types of molecular chains. The first, poly(oxyethylene) side chains (dark blue), provides nanochannels for the selective transport of liquids. They are highly wettable by water and many organics and have low fouling propensity, allowing the passage of water (aqua) through the filter, while pushing away foulant molecules (orange). These are combined with poly(vinylidene fluoride) (PVDF) backbone chains that provide structural integrity and are insoluble in most organic liquids. SOURCE: Reprinted with permission from J.F. Hester, P. Banerjee, Y.-Y. Won, A. Akthakul, M.H. Acar, and A.M. Mayes, 2002. ATRP of amphiphilic graft copolymers based on PVDF and their use as membrane additives, Macromolecules 35: 7652. Copyright 2002, American Chemical Society.



