Page 163
6
VOCs and Nox: Relationship to Ozone and Associated Pollutants
Introduction
Ozone (O3) is produced in the troposphere as a result of a complex set of reactions that involve volatile organic compounds (VOCs) and oxides of nitrogen (NOx). These reactions are discussed in detail in Chapter 5. Because the initial atmospheric concentrations (and corresponding emissions) of VOCs and NOx are not directly proportional to the maximum ozone concentration ultimately formed, a principal question associated with the VOC-NOx-O3 system is ''What is the maximum amount of ozone that can form from a given initial mixture of VOCs and NOx?''
A.J. Haagen-Smit (see, for example, Haagen-Smit and Fox, 1954) first plotted maximum ozone concentrations that result from initial mixtures of VOCs and NOx on a graph, the axes of which are the initial VOC and NOx concentrations. Isopleths (lines of constant value) of the maximum ozone concentrations can be constructed by connecting points that correspond to various initial conditions. Each point on a particular isopleth represents the same ozone concentration. Because ozone isopleth diagrams are a concise way to depict the effect of reducing initial VOC and NOx concentrations on the peak ozone concentrations, they have been used quantitatively to develop control strategies for ozone reduction by the U.S. Environmental Protection Agency's EKMA (empirical kinetic modeling approach) (Dodge, 1977). Figure 6-1 shows a typical set of EKMA ozone isopleths.
In principle, isopleths can be generated directly from smog chamber experiments in which the initial VOC and NOx concentrations are systematically
Page 164
varied. However, for application to the atmosphere, such data need to be corrected, for example, for chamber-wall effects on the chemical reactions, the relatively high concentrations used, and the level of dilution. Although the ozone isopleth diagram was put forward by Haagen-Smit as an empirical representation of the VOC-NOx-O3 relationship, the chemistry that gives rise to the characteristic isopleth shape is now well understood. Isopleths are now generated by models that use photochemical reaction mechanisms, and they are tested against smog chamber data.
EKMA, which is largely being supplanted for use in ozone NAAQS attainment demonstration by grid-based models, simulates urban ozone formation in a hypothetical box of air that is transported from the region of most intense source emissions (a center city, for example) to the downwind point of maximum ozone accumulation. Emissions of VOCs and NOx are assumed to be well mixed in the box, which varies in height, to account for dilution caused by changes in the height of the mixed layer of air; ozone formation is simulated using a photochemical mechanism. By simulating an air mass as a box of air over its trajectory for a large number of predetermined combinations of initial VOC and NOx concentrations, EKMA generates ozone isopleths that are, to varying degrees, specific to particular cities. Once the maximum measured ozone concentration in a city has been identified, the VOC and NOx reductions needed to achieve the National Ambient Air Quality Standard (NAAQS) are determined in EKMA from the distances along the VOC and NOx axes to the isopleth that represents the 120 ppb (parts per billion) peak ozone concentration mandated by the NAAQS.
The location of a particular point on the ozone isopleth is defined by the ratio of the VOC and NOx coordinates of the point, referred to as the VOC/ NOx ratio. Figure 6-1 shows that the shape of the ozone isopleths depends on the VOC/NOx ratio. (The lines in Figure 6-1 correspond to VOC/NOx ratios of 15, 8, and 4 ppb carbon (C)/ppb.) The 0.32 parts per million (ppm) (320 ppb) ozone isopleth, for example, spans a wide range of VOC/NOx ratios. As a result, the degrees of VOC and NOx reductions required to move from the 320 ppb isopleth to the 120 ppb isopleth vary considerably depending on the VOC/NOx ratio at the starting point on the 320 ppb isopleth.
The VOC/NOx ratio is important in the behavior of the VOC-NOx-O3 system. Moreover, it has a major effect on how reductions in VOC and NOx affect ozone concentrations. This chapter examines how ozone isopleths depend on the VOC/NOx ratio. Chapter 8 discusses the data on ambient VOC/NOx ratios in urban, suburban, and rural areas of the United States, and Chapter 11 addresses the implications of these ratios for the effectiveness of VOC and NOx control in reducing ozone.
Page 165
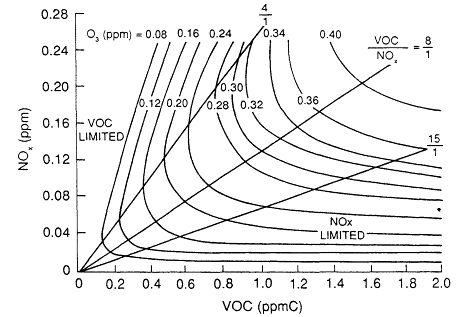
Figure 6-1
Typical ozone isopleths used in EPA's EKMA. The NOx-limited region is typical
of locations downwind of urban and suburban areas, whereas the VOC-limited
region is typical of highly polluted urban areas. Source: Adapted from Dodge, 1977.
Characteristics of Ozone Isopleths
An ozone isopleth diagram characteristically exhibits a diagonal ridge from the lower left to the upper right corner of the graph. The corresponding VOC/NOx ratio is typically about 8:1, although the shape of the isopleths, and hence the ratio, is sensitive to a number of factors.
It is useful to consider two areas on the graph: those to the right of the ridge line and those to the left. The variation of peak ozone concentration with the VOC/NOx ratio can be explained on the basis of the atmospheric chemistry discussed in Chapter 5 and summarized as follows:
Page 166
Page 167
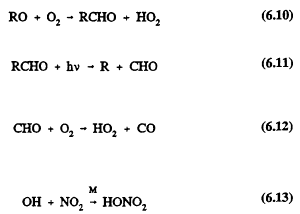
For VOC/NOx ratios to the right of the ridge line (characteristic of rural areas and of suburbs downwind of center cities), lowering NOx concentrations either at constant VOC concentration or in conjunction with lowering VOCs results in lower peak concentrations of ozone. (Chapter 8 investigates VOC/ NOx ratios of different geographic areas.) At these high VOC/NOx ratios, the system is said to be NOx-limited. In this region of an isopleth, there is an ample supply of organic peroxy radicals (RO2) and peroxy radicals (HO2) to convert nitric oxide (NO) to nitrogen dioxide (NO2). The only important tropospheric source of ozone is the photolysis of NO2 (Reactions 6.1 and 6.2), so that decreasing the available NOx leads directly to a decrease in ozone. When the system is NOx-limited, ozone concentrations are sensitive neither to reductions of VOC at constant NOx nor to the VOC composition.
At VOC/NOx ratios to the left of the ridge line (characteristic of some highly polluted urban areas) lowering VOC at constant NOx results in lower peak ozone concentrations; this is also true if VOCs and NOx are decreased proportionately and at the same time. However, the isopleths in Figure 6-1 indicate that lowering NOx at constant VOC will result in increased peak ozone concentrations until the ridge line is reached, at which point the ozone concentration begins to decrease. This seemingly contradictory prediction, that lowering NOx can, under some conditions, lead to increased ozone, results from the complex chemistry involved in ozone formation in VOC-NOx mixtures (see Chapter 5; Finlayson-Pitts and Pitts, 1986; Seinfeld, 1986). In this region of low VOC/NOx ratio, the radicals that propagate VOC oxidation and NO-to-NO2 conversion are scavenged (Reaction 6.13) by the relatively high concentrations of NOx. The NO2 effectively competes with the VOCs for
Page 168
the OH radical, slowing RO2 and HO2 radical production (Reactions 6.7 and 6.8) relative to that at lower NOx concentrations. As a result, as NOx is decreased, more of the OH radical pool is available to react with the VOCs, leading to greater formation of ozone. Ozone is also removed by its rapid reaction with NO, although during the day, the subsequent photolysis of NO2 (Reactions 6.1 and 6.2) regenerates ozone. The regeneration rate depends on the rate of NO2 photolysis and hence on solar zenith angle and other factors.
An additional source of free radicals for NO-to-NO2 conversion is aldehyde (RCHO) production from VOC oxidation (Reactions 6.7-6.10), followed by photolysis of the aldehyde (Reaction 6.11) and secondary Reactions 6.8, 6.9, and 6.12. Reducing the VOCs reduces aldehyde production, resulting in smaller RO2 and HO2 radical concentrations, which lowers the rate of NO-to-NO2 conversion by Reaction 6.9.
The increase in peak ozone concentration at relatively low VOC/NOx ratios that occurs when NOx is reduced has been a major issue in the development of ozone control strategies. It is one reason that historically the major emphasis has been on reductions of VOC. One issue that this report addresses is the effectiveness of the "VOC only" approach to ozone control. The isopleth graph shows that NOx reductions will have significantly different effects depending on the particular VOC/NOx ratio, which varies significantly within an air basin. Because NOx generally reacts more rapidly than VOCs in air masses, NOx is removed preferentially, and areas downwind of major VOC and NOx sources, such as rural areas, often have relatively high VOC/NOx ratios. These are the places to the right of the ridge line in Figure 6-1. VOC/NOx ratios smaller than those at the ridge line occur in some highly polluted urban areas.
Uncertainties and Sensitivities of Isopleths
Because ozone isopleths have been used to develop control strategies, it is important to understand the sensitivity of the shape and separation of the isopleths to uncertainties in the input variables and data used to generate them. These uncertainties include the details of the chemical mechanism used in the model and the initial VOC composition, the effects of which are discussed explicitly in the following sections. In addition, isopleths are sensitive to the ambient concentrations of VOCs, NOx, and ozone that are available for entrainment into the volume of air being studied, and the VOC and NOx composition of emissions into the air volume, which change the VOC/NOx ratio.
Page 169
Sensitivity to Chemical Mechanism
To predict ozone concentrations that will result from specific concentrations of VOCs and NOx requires a chemical reaction mechanism. To represent accurately the complete chemistry of the VOC-NOx-O3 system, one would need, in principle, to know which VOCs were present and all of their atmospheric reaction mechanisms and kinetics. Even if such detailed data were available, it is not now practical to include such detailed chemistry for each VOC in air quality models. Consequently, the approach to developing photochemical reaction mechanisms has involved the lumping of VOCs into groups. All reactions of a certain class of VOCs may be represented by those of a single species, or VOCs may be segmented according to the kinds of carbon bonds in the molecules. Because different mechanisms use somewhat different approximations in lumping the VOC chemistry, the ozone concentrations predicted for a given set of initial conditions by different chemical mechanisms will not agree exactly, and the resulting isopleths can differ.
Of the many chemical mechanisms developed in recent years (see, for example, a comparison of 20 models by Hough [1988]), three have gained wide acceptance in the modeling community: the carbon bond mechanism (CBM) (Gery et al., 1989), the CAL mechanism (Carter et al, 1986; Lurmann et al., 1987; Carter, 1990a), and the regional acid deposition model (RADM) (Stockwell 1986, 1988; Stockwell et al., 1990). These mechanisms include periodically updated descriptions of gas-phase reactions that closely reflect current understanding of atmospheric chemistry. The mechanisms have been evaluated against a common, large set of experimental smog chamber data. Statistical analysis of experimental data and model calculations with the CAL mechanism, for example, indicate agreement to ±30% for ozone concentrations (Carter and Atkinson, 1988). Because all of the current generation of models generally rely on the same kinetic and mechanistic data base and evaluations, however, it has been pointed out that agreement among them could be coincidental and cannot guarantee that the mechanisms are correct (Dodge, 1989).
The sensitivity of ozone isopleths to the chemical mechanism used was especially significant as the mechanisms were being developed, when they diverged significantly in their treatment of VOC chemistry (Dunker et al., 1984; Shafer and Seinfeld, 1986; Hough, 1988; Hough and Reeves, 1988; Dodge, 1989). Agreement of the predictions for ozone formation among various chemical mechanisms is much better now, although there are significant uncertainties in the mechanisms, and the model predictions for secondary pollutants other than ozone (e.g., hydrogen peroxide, H2O2) disagree more than do the predictions for ozone (Hough, 1988; Hough and Reeves, 1988; Dodge, 1989, 1990).
Page 170
The most significant uncertainties are associated with aromatic oxidations and the treatment of carbonyl photolysis. (Until recently, the temperature dependence of peroxyacetyl nitrate (PAN) formation also was controversial, and this had an effect on ozone isopleths especially at low temperatures. However, recent measurements of the temperature dependence of the ratio of the rate constants for the reaction of the acylperoxy radical [CH3CO3] with NO vs. NO2 (see Chapter 5) have confirmed that there is no significant temperature dependence of this rate constant ratio.) The sensitivity to the aromatic oxidation mechanism of model predictions for peak ozone concentrations (as well as sensitivity to the earlier PAN temperature dependence discrepancy) has been examined in some detail by Dodge (1989, 1990), and the effect of VOC composition on the ozone isopleths predicted using each mechanism has been studied by F.W. Lurmann (pers. comm., Sonoma Technology, Santa Rosa, Calif., April 1990).
Sensitivity to VOC Composition
Because different VOCs show widely varying reactivities in terms of ozone formation (see Chapter 5), the peak ozone generated in a given VOC-NOx mixture and hence the shapes of the ozone isopleths, particularly when the VOC/NOx ratio is low, are sensitive to the initial VOC composition.
Figure 6-2, for example, shows the ozone isopleths predicted by F.W. Lurmann (pers. comm., Sonoma Technology, Santa Rosa, Calif., April 1990) using the LCC (Lurmann, Carter, and Coyner) mechanism for the base case, in which 5% of the initial VOCs in the boundary layer and 10.7% aloft are aldehydes and also for the case in which only 2% of the initial VOCs in the boundary layer and 4.3% of VOCs aloft are aldehydes. Lurmann generated these isopleths (and those in Figure 6-3) using baseline conditions for a trajectory leading to Glendora, California, on August 24, 1984, when a 390 ppb peak ozone concentration was observed. The VOC composition for the base case was the "all-city average" (see Table 6-1) reported by Jeffries et al. (1989). The mixing height was allowed to increase from 250 meters at 8:00 a.m. to 700 meters at 1:00 p.m., and the concentrations of ozone and VOCs aloft were taken to be 0.10 ppm (100 ppb) and 0.10 ppmC (100 ppbC), respectively. The ozone does not drop to 0 in the low-concentration portions of the graphs in Figures 6-2 and 6-3 because of the assumption of ozone and VOC entrainment into the air mass from aloft.
At a VOC concentration of 1,000 ppbC and a NOx concentration of 100 ppb, the peak ozone concentration changes from 300 ppb in the base case to 280 ppb for the lower aldehydes case; at 500 ppbC VOCs and 100 ppb NOx,
Page 171
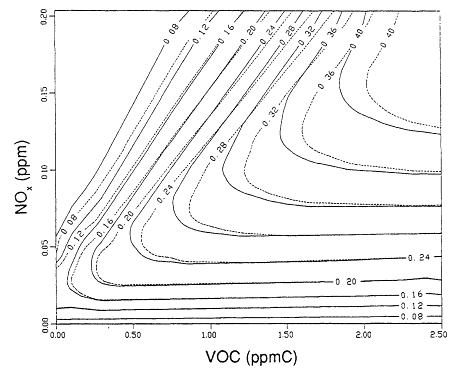
Figure 6-2
Ozone (ppm) isopleths generated using the Lurman, Carter, and Coyner (LCC)
mechanism and assuming that of the total VOCs (excluding methane), the following
percentages are aldeydes: for solid lines 5% in the atmospheric boundary layer (ABL),
10.7% aloft (base case) and for broken lines 2% in the ABL, 4.3% aloft. Source: F.W.
Lurmann, Sonoma Technology, Santa Rosa, Calif., pers. comm., April 1990.
the change is from 160 to 120 ppb ozone. These reductions in the peak ozone concentration are expected because of the photochemical reactivity of aldehydes and their efficiency at generating free radicals. A similar sensitivity of the predicted peak ozone concentration to the initial aldehyde concentration and speciation has been noted by Dodge (1990).
Differences in the speciation of VOCs have been shown, using mixtures with compositions very different from those found in the atmosphere, to give significantly different isopleths, although the reactivity of the mixture with
Page 172
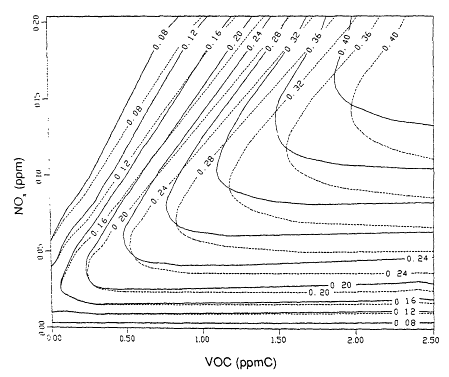
Figure 6-3
Ozone (PPM)isopleths generated using the Lurman, Carter, and Coyner (LCC)
mechanism and VOC compositions (including methane) typical (Jeffries et al., 1989)
of Washington D.C. (higher aromatic, lower alkane and alkene content), solid lines,
and Beaumont, Texas (higher alkane and ethene, lower aromatic content), broken
lines. Source: F.W. Lurmann, Sonoma Technology, Santa Rosa, Calif., pers. comm.,
April 1990.
Page 173
respect to OH is kept constant (Carter et al., 1982). For changes in VOCs more typical of those observed in urban areas, there also is some sensitivity of the isopleths to changes in VOC composition. For example, Figure 6-3 shows the isopleths predicted using the LCC mechanism for two VOC profiles that differ significantly from an all-city average VOC composition (Jeffries et al., 1989). (The base case is shown in Figure 6-2). These two profiles are typical of two extremes: Washington, D.C. (solid link in Figure 6-3a), which has a higher aromatic but lower alkane and alkene content compared with the all-city average, and Beaumont, Texas, which has a higher than average alkane and ethene but lower aromatic content (see composition data in Table 6-1). The isopleths are particularly sensitive to VOC composition at low VOC/NOx ratios. For example, at concentrations of 1.00 ppmC (1,000 ppbC) for VOCs and 0.15 ppm (150 ppb) NOx, peak ozone concentrations of ˜220 ppb and 160 ppb were predicted for Washington, D.C., and Beaumont, Texas, mixtures, respectively.
Other Limitations of Isopleths for Evaluation of Control Strategies
The ozone isopleth diagram, as generated in EPA's EKMA, has been used for determining the percentage reductions in VOCs and NOx needed to attain the NAAQS for urban areas. This approach has several well-recognized limitations. One is that such isopleth diagrams reflect a 1-day simulation in one location and do not apply to multiday episodes of high concentrations of ozone and downwind areas. Furthermore the predicted ozone concentrations from 1-day simulations can be quite sensitive to the initial conditions used. Another limitation in the application of these isopleth diagrams lies in the difficulty of selecting the appropriate VOC/NOx ratio in defining the base-year point on the peak ozone isopleth. VOC/NOx ratios are discussed in detail in Chapters 8 and 11. Ambient VOC/NOx ratios vary not only from one location to another in a particular area, but also with time of day. For example, VOC/NOx ratios from 2 to 40 have been measured in different locations in various urban areas (Baugues, 1986). In addition, the ratio can vary significantly from ground level to aloft as a result of emissions from tall stacks and pollutant carry-over from previous days (Altshuller, 1989).
One extremely important problem in evaluating control strategies with any air-quality model is that measured ambient VOC/NOx ratios are significantly larger than those calculated on the basis of current emissions inventories. This phenomenon, which could be a result of underestimation of anthropogenic VOC emissions, failure to sufficiently include biogenic VOCs, the trapping of NOx emissions aloft, differences in averaging times between measure-
Page 174
| TABLE 6-1 | |||
| Species in LLC Mechanisml | Washington | Beaumont | All-city averagea |
| ALK4 | 0.219 | 0.305 | 0.214 |
| ALK7 | 0.257 | 0.285 | 0.280 |
| ETH | 0.033 | 0.047 | 0.037 |
| PRPE | 0.032 | 0.040 | 0.048 |
| TBUT | 0.017 | 0.024 | 0.029 |
| TOL | 0.108 | 0.042 | 0.080 |
| XYL | 0.102 | 0.048 | 0.079 |
| TMB | 0.055 | 0.027 | 0.042 |
| FROM | 0.020 | 0.020 | 0.020 |
| ALD | 0.030 | 0.030 | 0.030 |
| NRHC | 0.127 | 0.132 | 0.141 |
| aRepresents 47 city sites. (Five cities had two sites.) | |||
| Source: Jeffries et al., 1989 | |||
merits and emissions profiles, and VOC and NOx measurement errors (Seinfeld, 1988; Altshuller, 1989), is addressed in Chapter 9.
It must be concluded that the concept of a single VOC/NOx ratio for an
Page 175
urban area has severely limited utility in quantitative assessment of control strategy options. The major limitation in quantitatively applying the conventionally generated isopleths, such as those in Figure 6-1, to the development of control strategies is the difficulty in adequately representing an entire air basin or region, where the VOC/NOx ratio can vary significantly from areas close to emissions sources to those downwind. This limitation has been overcome through the use of three-dimensional airshed models that are used to generate isopleths as a function of location in the region. This second- generation approach to isopleth development for an air basin has major advantages in being able to take into account transport, meteorology, and local emissions far more realistically than is possible with a model like EKMA, and hence to allow control strategies to be examined from the perspective of an entire air basin rather than separate locations. It allows the effect of precursor controls on peak ozone concentrations to be examined regardless of where the maximum occurs in an air basin.
For example, Milford et al. (1989) applied a grid-based airshed model to the Los Angeles basin for Aug. 30-31, 1982. The effects of reducing precursor VOC and NOx emissions were examined for five locations within the air basin, from downtown Los Angeles in the western part of the air basin to San Bernardino in the east (see detailed discussion in Chapter 11.) Figure 6-4 shows the effect of VOC and NOx controls on peak ozone formation in the Los Angeles air basin as a whole, irrespective of the location of the peak. The isopleths in Figure 6-4 show that when the air basin as a whole is considered, as much as 80% control of VOCs alone will not result in attainment of the NAAQS; if biogenic VOC emissions were included, VOC control would be even less effective. In addition, in contrast to what the EKMA isopleths show, the greater number of ''L'' shaped isopleths in Figure 6-4 show that basin-average peak concentrations of ozone will not necessarily increase as NOx is decreased.
The second-generation methods also have limitations. For example, because by their nature they are episode-specific, carrying out such calculations for all regions in the United States is not now feasible. In addition, many of the uncertainties associated with the more conventionally generated isopleths, such as those associated with the chemical mechanisms, also apply to this approach.
Effects of Voc and Nox Control on Other Species
Although this report focuses on ozone control, reducing concentrations of VOCs, NOx, or both can affect the concentrations of many secondary pollutants formed in VOC-NOx mixtures, some of which can also be hazardous to human health, plants, or materials. It is thus important to recognize that
Page 176
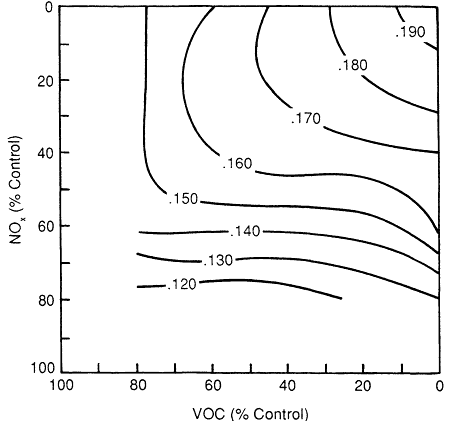
Figure 6-4
Ozone isopleths for peak ozone concentrations (ppm) regardless of lcoation in the
Los Angeles air basin. A decrease in percent control along an axis corresponds to a
higher concentration of a precursor in the atmosphere. Source: Milford et al., 1989.
concurrent with ozone control, changes in other secondary pollutants will occur as well. The next sections discuss some of the most important secondary pollutants. The intent is not to review all secondary pollutants in detail or to describe how each will respond to lower VOC and NOx concentrations, but rather to illustrate other significant changes that may accompany reductions in ozone.
Page 177
No2, Hno3 and Particulate (Inorganic) Nitrate
Decreasing NOx emissions will lower concentrations of nitrogen dioxide, NO2, which is also a criteria pollutant. Russell et al. (1988b) showed in simulations for Los Angeles that reducing NOx emissions should decrease both peak NO2 and the associated nitric acid and nitrate aerosols that form from NO2
Nitrates from nitric acid, HNO3, are a significant component of acid aerosols, which are under consideration for inclusion on the list of criteria air pollutants (Lipfert et al., 1989). These particles are typically in the respirable 0.1-1.0 micrometer (mm) size range, the size that also contributes the most to degradation of visibility. Nitric acid also is a significant component of acid rain and fog formed in ambient air from NO2 through a variety of reactions (see Chapter 5):
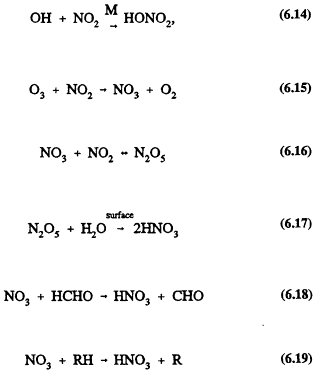
Page 178
Reaction of HNO3 with ammonia, NH3, or absorption into aerosol and fog and cloud droplets incorporates nitrate into the condensed phase.
Most light extinction in urban atmospheres is the result of light scattering and absorption by particles, especially those in the 0.1-1.0 mm range. Several studies (see Finlayson-Pitts and Pitts, 1986) treat the relationship between the light-scattering coefficient (bsp) associated with these fine particles measured in a number of urban areas and their chemical composition in the following form:
![]()
where Mi is the mass concentration of the ith chemical species (g/m3), ai is the mass scattering coefficient (m2/g) associated with this particular species, and ao is a constant for the data set. Light scattering depends strongly on the concentrations of sulfate, nitrate, and carbon in the air.
Table 6-2 lists some mass-scattering coefficients found for particles collected in a variety of locations. The increase in the ai for nitrate with year could result from the collection of gaseous nitric acid as a filter artifact in earlier studies (Appel et al., 1985). The most recent studies, in which this artifact should be minimized, show that, on a mass basis, nitrate is of comparable importance to sulfate with respect to light scattering. Thus reductions in NOx associated with ozone control are expected to affect the nitrate component of light scattering.
Nitrous Acid
Photolysis of nitrous acid, HONO, is believed to be an important early-morning source of OH, and it acts as a major initiator of VOC oxidation at dawn. Figure 6-5 shows the contribution to OH generation from three major sources under typical conditions in a polluted urban atmosphere as a function of time of day (Winer, 1986). HONO is predicted to be the greatest source of OH at dawn. Similar conclusions were reached by Rodgers (1986) for Atlanta, based on HONO measurements in that area (Rodgers and Davis, 1989).
The effects of HONO on ozone formation have not been studied extensively because there is no reliable large data base on ambient HONO concentrations or their sources. One study (Lurmann et al., 1986b) suggests that chang-
Page 179
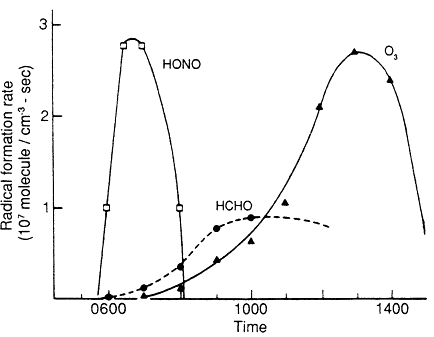
Figure 6-5
Predicted sources of HO radicals as a function of time of day for
a typical polluted urban atmosphere. Source: Winer, 1986.
es in HONO will be reflected primarily in the timing of the ozone peak, rather than in its absolute value. Such a shift in the location of the ozone peak could change the population exposed to this peak.
The sources of HONO in ambient air are not clear, although laboratory studies (see Finlayson-Pitts and Pitts (1986) for a review to 1985; Akimoto et al., 1987; Svensson et al., 1987; Jenkin et al., 1988; Lammel and Perner, 1988) suggest that heterogeneous reactions of NO2 are major contributors:
![]()
Page 180
| Table 6-2 | |||||
| Location | Sulfate as (NH4)2SO4º | Nitrate as NH4NO3º | Carbonaceous particlesa | DMass1 | Reference |
| Los Angeles | 5.4 | 2.0 + 4.6/m2 | N.S.b | 2.0 | White and Roberts, 1977 |
| Denver | 5.9 | 2.5 | 3.2 | 1.5 | Groblicki et al., 1981 |
| Detroit | 6.2 | N.S. | 3.1 | 1.7 | Wolff et al., 1982 |
| China Lake, California | 4.3 | d | 1.5 | 1.0c | Ouimette and Flagan, 1982 |
| Portland, Oregon | 4.9 | 4.4 | 5.0 | 2.1 | Shah et al., 1984 |
| Average of San Jose, Louisiana, and Riverside | 3.6 + 5.9 m | 4.9 + 4.8 m | 4.5a | 0.4f | Appel et al., 1985g |
(Table continued on next page)
Page 181
(Table continued from previous page)
| Location | Sulfate as (NH4)2SO4º | Nitrate as NH4NO3º | Carbonaceous particlesa | DMass1 | Reference |
| Western Netherlands | 4.8 | 8.6 | N.D.h | 4.7 | Diederen et al., 1985 |
| aCombination of organic and elemental carbon except in studies of Appel et al. (1985) where the coefficient is for elemental carbon only. | |||||
| bNot significant. | |||||
| cm = relative humidity/100. | |||||
| dPresent only at very low concentrations in these samples. | |||||
| eIn addition to sulfate carbonaceous aerosol and the remainder bap was found to be correlated to the crustal species Fe, Ca, and Si with ai = 2.4 m2/g. | |||||
| fNot significantly different from zero at p = 0.90. | |||||
| gData adjusted to reflect (NH4)2SO4 and NH4NO3 stoichiometry. Correlation of bsp with coarse sulfate also was found with ai = 13.4 m2/g; this could be due to a correlation of coarse sulfate with some other efficient light scatterer such as sea salt particles. | |||||
| hNot determined. | |||||
| iDMass = (Total mass sulfate nitrate carbonaceous particles) | |||||
| Source: Finlayson Pitts and Pitts, 1986. Adapted from Shah et al., 1984. | |||||
Page 182
(The reaction of OH with NO produces only a small steady-state concentration of HONO during the day because it is photolyzed rapidly). In addition, direct emissions of HONO from combustion sources have been observed (Pitts et al., 1984c, 1989).
Because NO2 is the most likely precursor to HONO, reductions in NO2 also should reduce ambient concentrations of this photochemically labile species. However, given the uncertainty in the kinetics and mechanisms of HONO production, quantitatively predicting the relationship between control of NO2 and HONO is not now possible.
Peroxyacetyl Nitrate
PAN is formed from the reaction of acetylperoxy radicals with NO2:
![]()
PAN thermally decomposes over the range of temperatures found in the atmosphere, reforming acetylperoxy radicals and NO2. If NO is present, the acetylperoxy radical reacts with NO, so that PAN is permanently removed.
PAN is important as a plant toxicant and, through Reaction 6.22, transports NOx over relatively large distances through the atmosphere. Its rate of decomposition significantly increases with temperature, so that it can be formed in colder regions, transported, and then decomposed to deliver NOx to warmer regions (Singh and Hanst, 1981; Hov, 1984a).
NO3 and N2O5
The nitrate rascal (NO3) and dinitrogen pentoxide (N2O5) are both formed from the reactions of NO2 with O3:
![]()
Page 183
![]()
Both NO3 and N2O5 are believed to be significant in nighttime atmospheric chemistry (NO3 photolyzes rapidly at dawn, depleting both NO3 and N2O5 by shifting the equilibrium between NO2, NO3, and N2O5 toward NO2). As discussed above, hydrogen abstraction reactions of NO3 form nitric acid, as does the hydrolysis of N2O5, which is a major source of nitric acid in the atmosphere (Russell et al., 1985).
NO3 works in the nighttime oxidation of naturally produced organic compounds, such as isoprene and the pinenes, as well as dimethylsulfide and methyl mercaptan (Finlayson-Pitts and Pitts, 1986).
From Reactions 6.23 and 6.24 forming NO3 and N2O5, if either ozone alone or ozone and NO2 are reduced, lower concentrations of NO3 and N2O5 would be expected, thus decreasing their contribution to acid deposition and the oxidation of organic compounds at night.
Other Nitrated Species
The formation of mutagenic nitrated polycyclic aromatic hydrocarbons (PAHs) has been observed in the atmospheric reactions of PAHs (see Finlayson-Pitts and Pitts, 1986; Pitts, 1987; Arey et al., 1989, for reviews). Some nitrated species are not found in significant mounts in particles directly emitted from combustion sources but are characteristic of atmospheric reactions of PAHs in air. For example, the OH-initiated oxidation of fluoranthene leads to formation of 2-nitrofluoranthene, which has been identified in organic extract of ambient particulate matter:
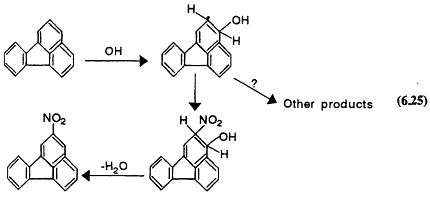
Page 184
This nitrated product is a direct mutagen, which in at least some samples of ambient PAHs, contributes significantly to the total observed mutagenicity (Pitts, 1987). Similarly, NO3 reacts, at least in part, to form nitrogen derivatives of some PAHs (Atkinson et al., 1990):
![]()
There also is evidence for the formation of one or more unidentified NOy species (the sum of the oxides of nitrogen) in the atmosphere. For example, Singh et al. (1985), Fahey et al. (1986), Buhr et al. (1990), and Ridley (1989) have observed in ambient air measurements that the sum of individual measurements of the species (NO + NO2 + HNO3 + particulate NO3 + PAN) was less than the total NOy measured by detecting NO (via its chemiluminescence with ozone) after reduction using a gold/CO converter. The discrepancy between the two measurements increases with the extent of photooxidation, suggesting that the unidentified species is a stable product of VOC-NOx reactions, possibly an organic nitrate or nitrates. Finally, several inorganic halogen-containing species, including ClNO, BrNO, ClNO2, and BrNO2, can be formed in coastal marine areas by the reactions of NO2, N2O5, and possibly NO3 with the components of sea salt (Finlayson-Pitts, 1983; Finlayson-Pitts et al., 1989a,b, 1990; Livingston and Finlayson-Pitts, 1991). These species are highly labile photochemically, and could play a role in ozone formation, in the case of the chlorine compounds, or ozone destruction, in the case of the bromine derivatives. For example, chlorine atoms will help initiate photochemical oxidation in much the same way that OH does, contributing to NO-to-NO2 conversion and hence to ozone formation:
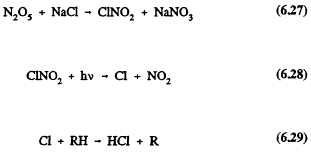
Page 185
Such oxidation of VOCs by chlorine atoms could account for 10% or more of the initiation of tropospheric VOC oxidation in marine urban areas such as Los Angeles (Livingston and Finlayson-Pitts, 1991). While the reaction of chlorine with ozone is also fast, the total concentration of VOCs in urban and rural areas is usually large enough compared with that of ozone that Reaction 6.29 is expected to dominate the tropospheric chlorine atom loss in those regions.
Atomic bromine, on the other hand, reacts only very slowly with organics but rapidly with ozone:
![]()
The fate of the BrO radical depends in part on the concentration of NO2; for example, in typical urban areas, NO2 sequesters the bromine in the form of BrONO2, so that significant chain destruction of O3 does not occur. However, under NOx-depleted conditions in remote areas, BrO could photolyze or react with itself or with HO2, thus regenerating reactive bromine atoms and leading to a chain destruction of ozone. Reductions in NOx and ozone would be expected to decrease the formation of these photochemically labile species.
Secondary Organic Particles and Acids
Recent atmospheric measurements suggest that organic acids constitute a significant fraction of total acidity, even in areas such as Los Angeles, which has large NOx emissions (Keene et al., 1983; Grosjean, 1989, 1990; Grosjean et al., 1990a; Grosjean and Parmar, 1990). The sources are not well established, but both direct emissions from mobile sources and atmospheric oxidation of VOCs (reactions of Criegee biradicals from ozone-alkene reactions, for example) have been suggested. Reduction of VOCs and ozone will thus reduce the concentrations of these organic acids as well.
Organic particles are believed to be largely the result of atmospheric photooxidation of VOCs, particularly aromatic hydrocarbons and long-chain and cyclic alkenes (Grosjean and Seinfeld, 1989). Their ambient concentrations are expected to depend on the concentrations not only of precursor VOCs, but also of the oxidants ozone, OH, and perhaps NO3. However, the relationship between organic particle concentrations and reductions in VOCs and NOx cannot be predicted quantitatively, given the current lack of understanding of the mechanisms of organic particle generation in the atmosphere.
Page 186
Summary
Isopleth diagrams are a convenient means of representing the complex relationship between initial concentrations of volatile organic compounds (VOCs) and oxides of nitrogen (NOx) and peak concentrations of ozone subsequently formed via chemical reactions in the troposphere. Ozone isopleth diagrams generated for urban areas using laboratory experiments or models (either EKMA [empirical kinetic modeling approach] or three-dimensional, grid-based models) show that the extent of reduction of peak ozone concentrations resulting from reductions of precursor emissions depends on the initial VOC/NOx ratio. At higher VOC/NOx ratios (greater than ˜8-10), ozone concentrations are relatively insensitive to VOC concentrations, and NOx control is more effective in lowering ozone. Measurements of 6:00 a.m. to 9:00 a.m. average VOC and NOx show that most urban areas appear to fall into this category, with measured ![]() (see chapter 8). At VOC/NOx ratios less than ˜8-10, found in some highly polluted urban areas, such as Los Angeles, lowering VOC reduces ozone, whereas NOx control might actually increase ozone at some locations.
(see chapter 8). At VOC/NOx ratios less than ˜8-10, found in some highly polluted urban areas, such as Los Angeles, lowering VOC reduces ozone, whereas NOx control might actually increase ozone at some locations.
Isopleth diagrams generated in the traditional manner, using EKMA, have several shortcomings, especially their failure to treat the effects of VOC and NOx controls throughout an airshed. Because the VOC/NOx ratio generally increases as an air mass moves downwind from major NOx sources, control strategies derived from the isopleths for upwind locations often are inappropriate for downwind areas within the same air basin.
This problem has recently been overcome by applying three-dimensional urban airshed models to generate ozone isopleth diagrams for some air basins where the requisite detailed model input is available. However, because of the limitations associated with developing information for urban airshed models (see Chapter 10), generating such isopleth diagrams for all regions in the United States is not now feasible. The relationship between VOC and NOx control and ozone concentrations, as determined by three-dimensional models, is discussed further in Chapter 11.
Changes in VOCs and NOx will, because of their complex chemical interactions, also lead to changes in a variety of other pollutants associated with ozone, such as nitric acid, peroxyacetyl nitrate, nitrogen dioxide, and aerosol particles. Some of these pollutants have known harmful effects on human health and welfare. Hence, it is important to recognize that control strategies implemented for ozone will simultaneously affect other species.
























