Page 211
8
Atmospheric Observations of Voc, Nox, and Ozone
Introduction
Chapter 6 contains a discussion of the central role of the VOC/NOx ratio (the ratio of volatile organic compounds to oxides of nitrogen) in determining the chemical character of the VOC-NOx-ozone system. Two important points were made about the VOC/NOx ratio: First, the atmospheric boundary layer (defined here as a well-mixed layer extending from the surface to a height of about 1 or 2 km during the day) cannot be characterized by a single ratio because this ratio varies significantly with location and time of day. Second, ambient ratios often exceed by a substantial mount those calculated from emissions inventories. The goal of this chapter is to examine data gathered from atmospheric observations to determine if ambient VOC, NOx, and O3 concentrations follow a regular pattern as one moves from an urban or suburban area to a rural area and then to a remote area. By comparing these patterns with those observed in smog-chamber experiments, it may be possible to establish to what degree smog-chamber experiments, and the chemical models based on these experiments can be applied to the atmospheric VOC-NOx-ozone system. By comparing patterns found in urban and suburban areas with those found in rural and remote locations, it may be possible to infer the relative effectiveness of controlling VOC versus NOx in different parts of the country.
The second point above—that discrepancies between ambient and emission-inventory-derived VOC/NOx ratios have major implications concerning the accuracy of emissions inventories—is addressed in Chapter 9.
Page 212
It is useful to focus on four regions of the atmospheric boundary layer, each of which has a distinct mix of anthropogenic and natural VOC and NOx emissions:
• The urban-suburban atmosphere, which is the area most strongly affected by anthropogenic emissions
• The rural atmosphere, which is somewhat less affected by anthropogenic emissions and more affected by natural emissions than is the urban-suburban atmosphere
• The atmosphere over remote tropical forests, which is essentially free of anthropogenic VOC and NOx emissions and strongly affected by natural emissions
• The remote marine atmosphere, which is not only free of anthropogenic emissions, but also has relatively small biogenic sources of VOCs and NOx
Because we are most interested in the conditions that foster episodes of high concentrations of ozone, our discussion concentrates on observations made during the daylight hours of the summer months. In the sections below, we first briefly examine the typical concentrations of ozone in these four regions and then turn to the more complex issues of NOx and VOC concentrations and their variability.
Observations of Ozone
Compared with those for NOx and VOCs, the data base of ozone observations is fairly extensive, especially for urban and suburban areas. At most rural surface sites, ozone concentrations have been found to vary over a diurnal cycle with a minimum in the early morning hours before dawn and a maximum in the late afternoon (Figure 8-1). This pattern is believed to result from daytime photochemical production or downward transport of ozone-rich air from above, combined with ozone loss by dry deposition and reaction with nitric oxide (NO) at night, when photochemical production ceases and vertical transport is inhibited by an inversion of the normal temperature profile. In locations near large sources of NO, the nighttime minimum in ozone can be quite pronounced because of the rapid reaction between ozone and NO. In fact, in many urban areas the NO source is strong enough to cause the complete nighttime disappearance of ozone. A somewhat different pattern has been observed at high-altitude sites (i.e., sites located 1 km or more above the local terrain). At these sites, ozone often exhibits a shallow maximum rather than a minimum at night (Figure 8-1b). This diurnal pattern is thought to
Page 213
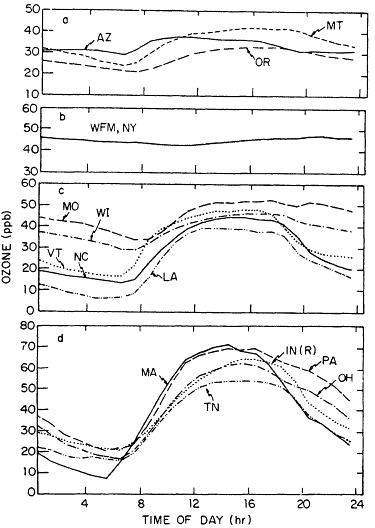
Figure 8-1
Diurnal behavior of ozone at rural sites in the United States in July.
Sites are identified by the state in which they are located. (a) Western
National Air Pollution Background Network (NAPBN); (b) Whiteface
Mountain (WFM) located at 1.5 km above sea level; (c) easter NAPBN
sites; and (d) sites selected from the Sulfate Regional Air Quality study.
IN(R)refers to Rockport. Source: Logan, 1989.
Page 214
reflect the contrasting dynamic conditions typically encountered at high altitude sites, with upslope flow bringing ozone-poor air from the boundary layer to the site during the day and downslope flow bringing ozone-rich air from the free troposphere to the site at night.
In addition to variations over a diurnal cycle, ozone concentrations at a given location also can vary significantly from one day to the next. It is not uncommon for the daily maximum ozone concentration at an urban site, for instance, to vary by a factor of two or three from day to day as local weather patterns change.
Despite the variable nature of ozone, the data base of ozone observations suggests a systematic pattern of decreasing daily maximum concentrations as one moves from urban-suburban locations to rural locations and then to remote locations. Table 8-1 shows that daily maximum ozone concentrations within the atmospheric boundary layer tend to be largest in the urban-suburban atmosphere, where 1-hour ozone concentrations most often exceed the National Ambient Air Quality Standard (NAAQS) concentration of 120 parts per billion (ppb), and maxima well above 200 ppb have been observed. Although the NAAQS can be exceeded in rural areas, ozone concentrations in these regions tend to be more moderate and rarely exceed 150 ppb. In remote locations, ozone concentrations tend to be quite low, typically ranging from 20 to 40 ppb.
| Table 8-1 | ||
| Region | Ozone, ppb | |
| I | Urban-suburban | 100-400 |
| II | Rural | 50-120 |
| III | Remote tropical forest | 2040 |
| IV | Remote marine | 20-40 |
| Sources: Cleveland et al. (1977); Hov (1984b); Gregory et al. (19881990); Kirchoff (1988); LeFohn and Pinkerton (1988); Janach (1989); Logan (1989). | ||
Page 215
Observations of Nox
There is a sizable body of data on the concentrations of NOx (the combined concentrations of NO and nitrogen dioxide (NO2)) in the atmosphere, but caution must be exercised in drawing conclusions from these measurements. As noted in Chapter 7, most measurements of NOx have been made by devices that convert NO2 to NO, which is then measured by chemiluminescence. Comparison of these measurements with more specific techniques suggests that all surface converters that can convert NO2 to NO also convert other reactive nitrogen oxide species, such as peroxyacetyl nitrate (PAN), to NO, thereby causing interference (Singh et al., 1985; Fehsenfeld et al., 1987, 1990; Gregory et al., 1990). Because PAN concentrations can vary considerably depending on other pollutant concentrations and the temperature, the potential error associated with PAN interference will depend strongly on the location, season, and altitude at which samples are taken. In urban locations, where the local NO sources are typically large, NO and NO2 are probably the dominant constituents of the total reactive nitrogen or NOy (NOx + HNO3 [nitric acid] + NO3 [nitrate radical] + N2O5 [dinitrogen pentoxide] + HONO [nitrous acid] + PAN + other organic nitrogen compounds). Thus, in urban areas, interference from PAN and other oxides of nitrogen is believed to be relatively small. In rural and remote locations, however, the interference can be substantial. For this reason, all nonurban NOx measurements made with surface converters must be considered upper limits (biased toward a high measurement); these measurements are so indicated in this discussion.
Urban Nox
Given the dominant role of anthropogenic emissions in the budget of atmospheric NOx and the fact that the sources of these emissions tend to be located in or near urban areas, elevated concentrations of NOx are to be expected in these locations. Observations of NOx support this expectation.
During the summer of 1986, NOx measurements were made from 6:00 to 9:00 a.m. Daylight Saving Time (DST) at six locations in Philadelphia, Pennsylvania (Meyer, 1987). Four of the sites were downtown, where the average measured NOx concentration ranged from 40 to 99 ppb. At two suburban sites, the average concentrations were 33 ppb (upwind of the core city) and 65 ppb (downwind of the core city). The average for the six-station network was 60 ppb. However, the NOx concentrations at all locations exhibited a high degree of variability—standard deviations ranged from 37% to 50% of the average NOx mixing ratio measured at the site.
Page 216
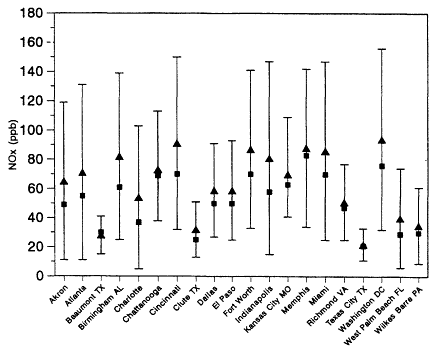
Figure 8-2a
NOx concentrations measured in urban locations in the United States during
the summer of 1984. All measurements were made between 6:00 a.m. and 9:00
a.m. daylight savings time. The triangles are the averages for each site, the
squares are the medians, and the bars show the standard deviations of the
averages. Adapted from Baugues, 1986.
The range and variability found in the Philadelphia study's measurements are reflected in measurements made in 29 other cities across the eastern and southern United States during the summers of 1984 and 1985 (Baugues, 1986). NOx measurements were made in 10 of the cities in both years. The measurements were made during the morning rush hour, 6:00 a.m. and 9:00 a.m. DST. Figure 8-2a and 8-2b show the average and median NOx mixing ratios and the standard deviations for each city. The average for all the cities studied varied between 18 ppb in Texas City, Texas (1985), and 114 ppb in Cleveland, Ohio (1985).
Page 217
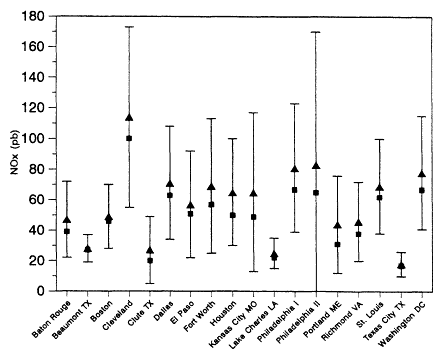
Figure 8-2b
NOx concentrations measured in urban locations in the United States during
the summer of 1984. All measurements were made between 6:00 a.m. and 9:00
a.m. daylight savings time. The triangles are the averages for each site, the
squares are the medians, and the bars show the standard deviations of the averages.
Adapted from Baugues, 1986.
Because urban areas have concentrated sources of NOx, urban measurements allow study of the rate of temporal and spatial decline of the NOx concentration with distance downwind of a source. Spicer et al. (1982) observed NOx concentrations in the Boston, Massachusetts, pollution plume a short distance from the city ranged from 27 to 131 ppb—concentrations similar to those typically found in surface measurements in urban areas. However, concentrations declined rapidly as the plume traveled away from the urban core. NOx concentrations in air masses 4-7 hours downwind of Boston were found to be 5-10 ppb. From this and similar plume studies (Spicer, 1977, 1982; Spicer et al., 1978; Spicer and Sverdrup, 1981) made over several cities
Page 218
in the United States, it has been estimated that the characteristic time for conversion of NOx to other NOy species is 4-20 hours.
Nonurban Nox
Only during the past 10 years have techniques been available with sufficient sensitivity and range of detectability to measure NOx in nonurban locales (NOx concentrations below 1.0 ppb), and as a result the size and reliability of the data base needed to define nonurban NOx concentrations are limited. Altshuller (1986) compiled and reviewed a series of NOx measurements made at a number of rural sites in industrial regions of the United States; the results of these measurements are summarized in Table 8-2. Because of the proximity of these sites to urban and industrial sources, the NOx concentrations usually exceeded 1 ppb and exhibited a high degree of short-term variability.
Measurements taken at more isolated rural sites in the United States are listed in Table 8-3. NOx concentrations at these sites also can be dominated by anthropogenic NOx emissions when meteorological conditions favor rapid transport of pollutants from urban and industrial centers to the site, but nevertheless tend to be significantly lower than concentrations measured at less-isolated rural sites (Table 8-2) and generally range from a few tenths to 1 ppb. Measurements of NOx in the atmospheric boundary layer and lower free troposphere in remote maritime locations have generally yielded concentrations of 0.02-0.04 ppb (Bottenheim et al., 1986, Gregory et al., 1988, 1990; Montzka et al., 1989). Although the data base is still quite sparse, concentrations in remote tropical forests (not under the direct influence of biomass burning) appear to range from 0.02 to 0.08 ppb; the somewhat higher NOx concentrations found in remote tropical forests, as compared with those observed in remote marine locations, could result from biogenic NOx emissions from soil (Kaplan et al., 1988; Torres and Buchan, 1988).
A summary of the NOx measurements made in the four areas considered here is presented in Table 8-4. It can be seen that, even more than is the case for ozone, NOx concentrations decrease sharply as one moves from urban and suburban to rural sites in the United States and then to remote sites over the ocean and tropical forests. The striking difference of three orders of magnitude or more between NOx concentrations in urban-suburban areas and remote locations is compelling evidence for the dominant role of anthropogenic emissions of NOx over North America and suggests that NOx concentrations in the United States would be significantly lower than their current concentrations in the absence of these emissions.
Page 219
| TABLE 8-2 | ||||
| Reference | Location | NO, ppb | NO2, ppb | NOx, ppb |
| Research | Fort McHenry, Maryland | NDa | 6b | ND |
| Triangle | Dubois, Pennsylvania | ND | 10b | ND |
| Institute, | McConnelsville, Ohio | ND | 6b | ND |
| 1975 | Wilmington, Ohio | ND | 6b | ND |
| Wooster, Ohio | ND | 6b | ND | |
| Decker et | Bradford, Pennsylvania | 2 | 3b | 5b |
| al., 1976 | Creston, Louisiana | 4 | 2b | 6b |
| Deridder, Louisiana | 1 | 3b | 4b | |
| Martinez | Montague, Massachusetts | 2 | 3b | 5b |
| and Singh, | Scranton, Pennsylvania | 3 | 11b | 14b |
| 1979 | Indian River, Delaware | 3 | 5b | 8b |
| Research Triangle Park, North Carolina | 10 | 13b | 23b | |
| Lewisburg, West Virginia | 1 | 5b | 6b | |
| Duncan Fails, Ohio | 1 | 8b | 9b | |
| Fort Wayne, Indiana | 3 | 7b | 10b | |
| Rockport, Indiana | 3 | 7b | 10b | |
| Giles County, Tennessee | 3 | 10b | 13b | |
| Jetmore, Kansas | 1 | 4b | 5b | |
| Pratt et al., | Lamoure County, North | 2.4 | 1.7b | 4.1b |
| 1983 | Dakota | 4.8 | 1.5b | 6.3b |
| 3.3 | 2.8b | 6.1b | ||
| 2.7 | 2.1b | 4.8b | ||
| Wright County, Minnesota | 3.2 | 5.4b | 8.6b | |
| 3.0 | 6.7b | 9.7b | ||
| 3.5 | 5.8b | 93b | ||
| 2.9 | 4.7b | 7.6b | ||
| Pratt et al., | Traverse County, Minnesota | 3.6 | 3.7b | 7.3b |
| 1983 | 4.8 | 3.6b | 8.4b | |
| 4.0 | 2.9b | 6.9b | ||
| 2.0 | 2.2b | 4.2b | ||
(Table continued on next page)
Page 220
(Table continued from previous page)
| Parrish et al., 1986 | Scotia, Pennsylvania | 3.0b | ||
| Parrish et al., 1988 | Scotia, Pennsylvania | 3.1b | ||
| aNo data. | ||||
| bUpper limit for NO2 and NOx | ||||
| TABLE 8-3 | ||||
| References | Location | NO, ppb | NO2, ppb | NOx, ppb |
| Kelly et al., 1980 | Niwot Ridge, Colorado | 0-2a | ||
| Kelly et al., 1982 | Pierre, South Dakotab | 1.2a | ||
| Kelly et al., 1984 | Schaeffer Observatory Whiteface Mountain, New York | < 0.2 | 1.1a | |
| Bollinger et al., 1984 | Niwot Ridge, Colorado | 0.80 | ||
| Fehsenfeld et al., 1987 | Niwot Ridge, Colorado | 0.56 | ||
| Parrish et al., 1985 | Point Arena, California | 0.37 | ||
| aUpper limit for NO2 and NOx | ||||
| bMeasurement site located 40 km WNW of Pierre. | ||||
Page 221
| TABLE 8-4 | |
| Region | NOx, ppb |
| Urban-suburban | 10-1000 |
| Rural | 0.2-10 |
| Remote tropical forest | 0.02-0.08 |
| Remote marine | 0.02-0.04 |
Observations of Noy
In addition to examining the measurements of atmospheric NOx, it is instructive to consider the observed concentrations of atmospheric NOy. The ratio of NOx to NOy reflects the chemical processing that occurs in an air mass after the initial introduction of NOx. Thus this quantity is indicative of the oxidant formation that has occurred in the air mass.
Because urban areas have large sources of NOx and because it takes several hours to convert NOx to other NOy compounds, NOy. concentrations in urban locations are generally dominated by NO For this reason, the NOy concentrations in urban and suburban locations should be approximately represented by the urban and suburban NOx concentrations described above.
Because the ability to measure NOy was developed only recently (see Chapter 7), the rural and remote NO data base is even more limited than that for NOx. However, there are enough data to establish a rough indication of the NOy distribution. During the summer of 1986, NOy was measured at several rural sites in North America: Brasstown Bald Mountain, Georgia; Whitetop Mountain, North Carolina; Bondville, Illinois; Scotia, Pennsylvania; Egbert, Ontario; and Whiteface Mountain, New York. These were all rural sites in the industrial regions of the eastern United States or southern Canada. The NOy concentrations recorded at these sites (c.f., Parrish et al., 1988), along with the period of the measurements at the various sites and their latitudinal locations, are shown in Figure 8-3. The concentrations covered a large range and were quite variable. In general, the low-elevation sites, where air from the atmospheric boundary layer was sampled, were closer to anthropogenic sources. These sites exhibited somewhat higher concentrations of NOy than did the mountain sites, which were more remote and where the samples usually were from the free troposphere. However, the average NOy concentrations observed at all the sites were quite similar; median values ranged from 3 to
Page 222
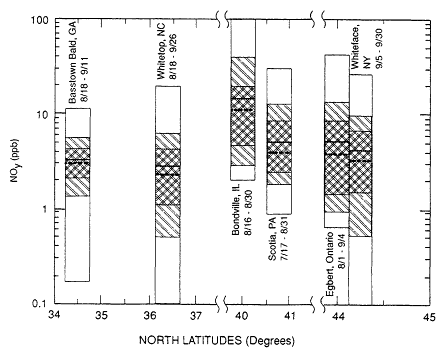
Figure 8-3
NOy concentrations measured during the summer of 1986 at several rural
sites in North America. The period of the measurements and the location
of each site are indicated. Each rectangle encompasses the range of NOy
concentrations recorded at a site. The 90% and 67% ranges of the data are
shown as lightly and heavily shaded areas, respectively. The heavy solid
line is the average, and the dashed line is the median concentration.
Source: Parrish et al., 1988.
10 ppb. These concentrations are somewhat lower than NOx concentrations typically observed in urban and suburban locations, which range from 10 to 1000 ppb.
The contrast in NOy concentrations found in rural areas of the continental United States with those observed in the remote troposphere is illustrated in Figure 8-4. The measurement sites are Scotia (Parrish et al., 1988), a rural site in the eastern United States; Niwot Ridge, Colorado (Fahey et al., 1986; Parrish et al., 1988), an isolated inland site in the western United States; Point Arena, California (Parrish et al., 1985), a site on the West Coast that often receives maritime air from the Pacific Ocean; and Mauna Loa, Hawaii (Car
Page 223
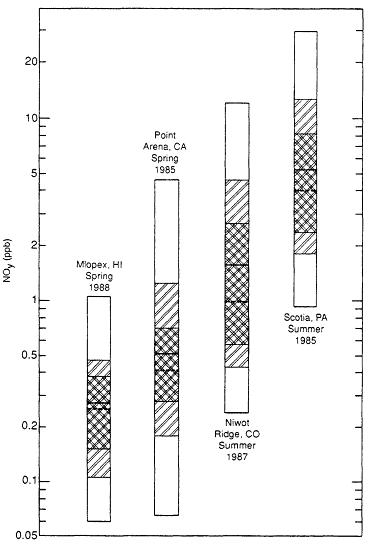
Figure 84
NOy measurements made at Mauna Loa (Carroll et al., in press), a remote site,
and Point Arena, CA (Parrish et al., 1985), Niwot Ridge, CO (Parrish et al., 1988;
Fahey et al., 1986b), and Scotia, PA (Parrish et al., 1988), three rural sites. Bars
show range of measurements made. Dashed line = median; solid line = average;
shaded area = central 68% of the data.
Page 224
roll et al., in press), a remote maritime site. Two of the sites, Mauna Loa and Niwot Ridge, are at high elevations (10,000 feet or approximately 3 kin), and thus the air sampled there is not necessarily representative of the boundary layer.
As was the case for NOx, the observations summarized in Figure 8-4 show the progressive decrease in NOy with increasing isolation from anthropogenic sources of NOx. For example, the median NOy mixing ratio decreases from 3.6 ppb at Scotia to 0.28 ppb at Mauna Loa. There is also a progressive decrease in the contribution of NOx to NOy as one moves toward more remote regions. On average, NOx at Scotia accounted for 59% of the observed NOy (Williams et al., 1987). At Niwot Ridge in 1987, NOx accounted for 32% of the NOy (Williams et al., 1987), and at Mauna Loa, NOx accounted for only 15% of the NOy (Carroll et al, in press) Because NOy enters the atmosphere as NOx, the decrease in the ratio of NOx to NO. as one moves to more remote sites can be understood in terms of the increasing chemical conversion of NOx to organic nitrates (principally PAN) and to inorganic nitrates (principally HNO3) with increasing distance of the site from major anthropogenic sources. This is why accurate measurements of NOx at rural and remote locations must be free of interference from other NOy species.
Observations of VOCs
Determining the variation in VOC concentrations from one area to another is a much more complex task than is tracking variations in NOx and ozone. There are scores of different VOCs in the atmosphere—some primary pollutants and others secondary—and the rate at which these compounds react in the atmosphere varies by orders of magnitude. We simplify the task somewhat by focusing on a subset of all the VOCs in the atmosphere, the nonmethane hydrocarbons, and by focusing primarily on midday. This subset of VOCs is composed of primary pollutants (i.e., those species that are emitted directly by sources and are not subsequently transformed in the atmosphere), and thus their concentrations probably most closely reflect local emissions. We exclude methane because its low reactivity precludes it from playing a significant role in urban and regional ozone formation. We focus on data collected at midday because this is the photochemically active period and also typically the time of the most intense vertical mixing; data from this period are most likely to reflect the balance between emissions and photochemical oxidation and least likely to be affected by inhomogeneities caused by local sources. On the other hand, because anthropogenic VOC concentrations are usually largest during the early morning and biogenic emis-
Page 225
sions tend to be most intense during the afternoon, VOC data from midday tend to overemphasize the contribution of biogenic compounds to the overall VOC mix. For this reason, some VOC data from other periods of the day are also included in our discussion.
The VOC data sets analyzed here are listed in Table 8-5. The analysis includes data gathered from 11 different areas: 5 urban-suburban areas in the United States, 2 rural sites in the eastern United States, 3 sites in the tropical forests of Brazil, and a region of the North Atlantic Ocean. From these 11 areas, 43 separate data sets have been formed for analysis. Each consists of a lengthy list of VOC species in the C2 through C10 range and the concentrations of these species, as measured through analysis of whole-air samples using gas chromatography-flame ionization detection (Greenberg and Zimmerman, 1984; Westberg and Lamb, 1985; Christian and Riley, 1986; Rasmussen and Khalil, 1988). The concentration for each VOC species in each data set is an average of at least three measurements, and in many cases more than ten measurements were made at different times over the period shown in Table 8-5 for that particular data set.
Each data set has been assigned a data code: a Roman numeral, which shows the region; a capital letter, to indicate the area, and a number, to show the specific location and period of data collection. Thus, the data code for the Georgia Tech site in Atlanta is I.A1. The data code for the measurements made in Glendora (a suburb of Los Angeles) from 1200-1600 during the period of 8/12-8/17/86 (when moderately high temperatures, averaging 31 ºC, were encountered) is denoted by I.C1, while the equivalent data set for the period from 8/18 o 8/20/86 (when extremely high temperatures, averaging 37 ºC, were encountered) is denoted by I.C2. In some cases, a lower-case letter is also used to denote analyses for the same site during the same period of days but at different hours of the day. Thus data codes I.C2a, I.C2b, and I.C2c are for the Glendora site for 8/17 to 8/20/86 but for time intervals from 0800-1200, 1200-1600, and 1600-2000, respectively. In the case of the Baton Rouge data, a separate analysis was carried out for each hour of the day between 0600 and 1800 for each of the two sites considered here. A lower-case letter is used in the code to indicate each hourly measurement; the code I.E1a is used to denote the LSU data gathered at 0600, I.E1b is for data gathered at 0700, and so on.
The information listed in Table 8-5 is probably among the highest quality speciated VOC data available, but the data are not without problem areas and potential flaws. For instance, in all of the data sets, a significant fraction (generally about 10% by mass) of the VOCs in the air samples could not be identified. Furthermore, although many of the species identified in one data set appeared in others, there were some notable exceptions. In the case of
Page 226
| TABLE 8-5 | |||||||
| Region | Area | Site | Data | Time period | Comments | Data | |
| Days | Hours | ||||||
| Urban- | Atlanta | Georgia | I.A1 | 7/13-8/03/81 | 1100-1400 | ''Midtown'' site p-cymene identified | W. Lonneman |
| Fulton | I.A2 | 7/13-8/03/81 | 1100-1400 | "Downtown" site p-cymene identified | |||
| DeKalb Comm. | I.A3 | 7/13-8/03/81 | 1100-1400 | "Perimeter" site p-cymene identified | |||
| College | a-pinene identified | ||||||
| Detroit | Downtown | I.B1 | 7/20-8/03/81 | 1200-1500 | Avg. T = 31 VC | W. Lonneman | |
(Table continued on next page)
Page 227
(Table continued from previous page)
| Region | Area | Site | Data | Time period | Comments | Data | |
| Days | Hours | ||||||
| Los Angeles | Glendora | I.C1 | 8/12-8/17/86 | 1200-1600 | Avg. T = 37 ºC | D. Lawson., | |
| Glendora | I.C2a | 0800-1200 | R. Rasmussen, | ||||
| Glendora | I.C2b | 8/18-8/20/86 | 1200-1600 | and W. Lonneman | |||
| Glendora | I.C2c | 1600-2000 | |||||
| Claremont | I.C3 | 8/27-8/29/87 | 1400-1700 | High isoprene days | |||
| Columbus | Fort Hayes | I.C3 | 8/20-8/29/87 | 1200-1600 | "Downtown" site | W. Lineman | |
| WVCORadio Tower | I.D2 | 7/20-8/14/80 | 1200-1600 | "Suburban" site | |||
(Table continued on next page)
Page 228
(Table continued from previous page)
| Region | Area | Site | Data | Time period | Comments | Data | |
| Days | Hours | ||||||
| Baton Rouge | La. State University | I.EI a...m | 7/18-7/26/89 | 0600-1800 | "Downtown" site, analysis for each hour of period | M.O. Rodgers | |
| Pride | I.E2 a...m | 7/27-8/20/89 | 0600-1800 | "Suburban" site analysis for each hour of period | |||
| Rural | Pennsylvania | Scotia | II.A | Summer, 1988 | 0800-1700 | N=200 samples | D. Parrish |
| Georgia | Brasstown Bald Mtn. | II.B | Summer, 1988 | 0800-1700 | High altitude site N=30 samples | M.O. Rodgers | |
(Table continued on next page)
Page 229
(Table continued from previous page)
| Region | Area | Site | Data | Time period | Comments | Data | |
| Days | Hours | ||||||
| Remote Tropical Forest | Brazil | Ducke Forest | III.A | Dry season, 1985 | 24 hr | 10km North of Manaus, N = 81 samples | |
| Amazon | III.B | September 1980 | 24 hr | Samples collected after biomass burning N = 12 samples | |||
| Lago Calado | IIIC. | Wet season, 1984 | 24 hr | 50km north of Manaus, N = 9 samples | P. Zimmerman, et al., 1988b | ||
(Table continued on next page)
Page 230
(Table continued from previous page)
| Region | Area | Site location | Data codea | Time Period | Comments | Data | ||
| Day | Hours | |||||||
| Remote marine | N. Atlantic Ocean | 26-34ºN 72-80ºW | IV.A | 2/3, 2/8, 2/10/86 | 1300-1500 | P. Zimmerman and J. Greenberg | ||
| aEach data set has been assigned a data code: a Roman numeral (which shows the region); a capital letter (to indicate the area); and a number (to show the specific location and period of data collection). Source: Parrish et al., 1988 | ||||||||
| bPersonal communications in April 1990: W. Lineman (EPA), D. Lawson (California Air Resources Board), R. Rasmussen (Oregon Graduate Center), M.O. Rodgers (Georgia Institute of Technology), D. Parrish (National Oceanic and Atmospheric Administration), P. Zimmerman and J. Greenberg (National Center for Oceanic Research). | ||||||||
Page 231
the urban data sets, isoprene was generally the only biogenic VOC identified. In the Atlanta data, however, relatively large concentrations of p-Cayman appear, and in the Detroit data small concentrations of a-pinene were reported. Although these differences could reflect the actual chemical variability of the atmosphere, the possibility that they are caused by analytical problems with one or more of the measurements cannot be ruled out. All of the VOC data analyzed here were gathered from sites located at or near the earth's surface. Because virtually all VOC sources also are located near the surface and because concentrations tend to decrease as VOCs disperse in the atmosphere at a rate proportional to their reactivity, the data analyzed here could be somewhat biased in favor of reactive as opposed to less reactive VOCs. It is difficult to assess the magnitude of this bias, but the limited measurements of Zimmerman et al. (1988b) and Rasmussen and Khalil (1988) over tropical forests suggest that it is not especially large. (When averaged over the atmospheric boundary layer, it is less than a factor of two.) Furthermore, as noted earlier, we have focused on midday data in an attempt to minimize bias.
Analysis of VOC Data Sets
The analysis of the data sets was done with two contrasting methods. The first, which is the simplest and the one probably most often adopted, is a concentration-based method in which the various species are ranked in importance according to their concentrations (on a carbon-atom basis), and data sets from different locations and times are compared according to the total VOC concentration (the sum of the concentrations of all the individual VOCs).
However, because the concentration-based method does not account for the different reactivities of the various VOC species, it can be misleading about the involvement of the various species in ozone formation. Recall from Chapter 5 that the rate of ozone production from a given VOC is essentially a function of three factors: the species' atmospheric concentration, its rate of reaction with OH (its OH-reactivity), and the number of ozone molecules produced each time the species is oxidized (its mechanistic reactivity). Although the concentrations and OH-reactivities of VOCs can vary by orders of magnitude from one species to another, the mechanistic reactivities of the VOC species generally found in the atmosphere are fairly uniform, varying only by factors of two or three from one species to another (see Table 5-5). The product of a VOC's concentration and its rate of reaction with OH will
Page 232
determine its relative role in an air mass as an ozone precursor. A species with a large concentration will not necessarily be an important precursor if it is unreactive; conversely, another with a small concentration can be important if it is extremely reactive. (An example is methane, typically the most abundant VOC in the atmosphere but of negligible importance in producing ozone on urban or regional scales over the contiguous United States because of its extremely low reactivity.) An air mass can have a large total VOC concentration but a low ozone-producing capacity if the VOCs present are relatively unreactive.
To account for the combined effect of OH-reactivity and concentration, we have adopted a second, OH-reactivity-based method. In this method, we define a propylene-equivalent concentration, Propy-Equiv(j), for each VOC species j. This equivalent concentration is given by
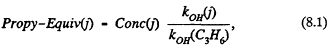
Conc(j) is the concentration of species j in ppb of carbon (C); kOH (j) is the rate constant for the reaction between species j and OH; and kOH(C3Hy) is the rate constant for the reaction between OH and propylene. Propy-Equiv(j) is a measure of the concentration of species j on an OH-reactivity-based scale normalized to the reactivity of propylene and is literally the concentration (in parts per billion carbon) required of propylene to yield a carbon oxidation rate equal to that of VOC species j. Thus if a VOC species has an atmospheric abundance of 10 ppbC and is twice as reactive as propylene, its Propy-Equiv is 20 ppbC; if the species is half as reactive as propylene, its Propy-Equiv is 5 ppbC.
Because the OH-reactivity-based method accounts for a species' rate of reaction as well as its atmospheric concentration, it provides a more accurate picture than does the concentration-based method of the relative contribution of each VOC species to the photochemical production of ozone at the specific time and location of the measurement. To the extent that the measurements from a given site are representative of the average concentrations throughout the air mass, the OH-reactivity-based method provides a more rational basis for assessing the relative importance of the various VOCs in the air mass to ozone formation as well as for comparing the VOC concentrations in different air masses. The reader should note that the use of propylene's reactivity as
Page 233
the normalization factor in the above formulation is completely arbitrary, and equivalent results would be obtained if the reactivity of any other species had been chosen. In addition, even though Equation 8.1 considers only VOC reactions with OH and does not account for ozone reactions, this has little effect on our conclusions; calculations using an alternate formulation to account for ozone as well as OH reactions with VOCs yield results essentially identical to those obtained from Equation 8.1. A ranking of the 35 most important species from data set I.A1 is presented in Table 8-6. In Part A of the table, the ranking was made using the concentration-based method, and in Part B the OH-reactivity-based method was used. (The OH rate constants used to calculate the Propy-Equiv concentrations in Table 8-6 and below were obtained from Middleton et al. [1990].) Although the 35 most important compounds obtained from both methods include a complex list of alkanes, alkenes, and aromatics ranging in concentration from 1 to 20 ppbC, the relative ranking of the species on the two lists is quite dissimilar. The two highest ranking species using the concentration-based method are i-pentane and n-butane, two relatively unreactive compounds; the third highest ranking species is toluene, a moderately reactive compound. Isoprene, a highly reactive species normally associated with biogenic emissions, is ranked fifteenth using this method, with an average concentration of 4.6 ppbC; it constitutes only about 2% of the total VOCs present during the sampling period. The total VOC concentration for the data set, obtained by adding the concentrations of each individual VOC, is about 200 ppbC.
In contrast to the above results, the highest ranking species obtained with the OH-reactivity-based method are isoprene and p-Cayman, two biogenic compounds, and m- and p-xylene, highly reactive aromatics associated with evaporative emissions. (An unusual feature of the data sets from Atlanta is the high concentration for reported p-Cayman, a reactive aromatic thought to be emitted biogenically.) Whereas i-pentane, n-butane, and toluene are the most abundant species in the data set, their rankings on an OH-reactivity-based scale are eleventh, eighteenth, and sixth, respectively. Furthermore, although the total VOC concentration for data set I.A1 is about 200 ppbC, the total Propy-Equiv concentration is only about 105 ppbC, indicating that the mix of VOCs in data set I.A1 is on average less reactive than propylene. (This conclusion is totally consistent with the data given in Chapter 5 concerning the incremental reactivities of an "all-city" urban mix and representative alkanes, alkenes, and aromatic VOCs.) These results illustrate the importance of accounting for a species' OH-reactivity in assessing its role in the photochemistry of an air mass. For instance, it might be concluded using a concentration-based approach that biogenics are unimportant in ozone formation in the Atlanta area because their ambient concentrations are only a few percent
Page 234
based scale indicates that a significant fraction of the total organic carbon being cycled through the atmosphere at the Atlanta site originated from biogenic VOCs and thus that these species could be quite important.
| TABLE 8-6 | |||
| Species sorted by concentration | Species sorted by OH reactivity | ||
| Species | Concentration, ppbC | Species | Propy-Equiv., ppbC |
| 1 i-pentane | 19.8 | 1 isoprene | 17.6 |
| 2 n-butane | 16.9 | 2 p-Cayman | 10.4 |
| 3 toluene | 14.7 | 3/m&p-xylene | 7.1 |
| 4 p-Cayman | 11.0 | 4 2-methyl 2-butene | 5.9 |
| 5 n-pentane | 9.4 | 5 1,3,5-trime-benzene | 4.3 |
| 6 benzene | 8.8 | 6 toluene | 3.5 |
| 7 m&p-xylene | 7.6 | 7 m&p-ethyl toluene | 3.4 |
| 8 2-me-pentane | 5.9 | 8 1,2,4-trime-benzene | 3.4 |
| 9 cyclohexane | 5.4 | 9 t-2-pentane | 3.1 |
| 10 2-me-hexane | 5.2 | 10 iso-butene | 3.1 |
| 11 ethane | 5.0 | 11 i-pentane | 3.1 |
| 12 undecane | 4.9 | 12 t-2-butene | 2.9 |
| 13 propane | 4.8 | 13 c-2-butene | 2.7 |
| 14 i-butane | 4.8 | 14 undecane | 2.3 |
(Table continued on next page)
Page 235
(Table continued from previous page)
| Species sorted by concentration | Species sorted by OH reactivity | ||
| Species | Concentration, ppbC | Species | Propy-Equiv., ppbC |
| 15 isoprene | 4.6 | 15 1,4-diethyl benzene | 2.3 |
| 16 acetylene | 4.3 | 16 c-2-pentene | 2.2 |
| 17 n-hexane | 3.8 | 17 cyclohexane | 1.8 |
| 18 m&p-eth-toluene | 3.6 | 18 n-butane | 1.7 |
| 19 3-me-pentane | 3.4 | 19 1,2,3-trime-benzene | 1.7 |
| 20 ethylene | 3.0 | 20 o-xylene | 1.6 |
| 21 me-cyclopentane | 2.9 | 21 2-methyl 1-butene | 1.6 |
| 22 ethylbenzene | 2.8 | 22 propene | 1.5 |
| 23 o-xylene | 2.8 | 23 2-methyl hexane | 1.4 |
| 24 3-me-hexane | 2.6 | 24 n-pentane | 1.4 |
| 25 2,3-dime-pentane | 2.5 | 25 2-methyl pentane | 1.2 |
| 26 1,4-dieth-benzene | 2.4 | 26 o-ethyl toluene | 1.0 |
| 27 iso-butene | 2.2 | 27 ethylene | 1.0 |
| 28 2,2,4-trimepentane | 2.2 | 28 1-pentene | 0.9 |
| 29 1,2,4-trime-pentane | 2.2 | 29 2,3-dimethyl pentane | 0.8 |
| 30 i-butyl-benzene | 2.2 | 30 ethylbenzene | 0.8 |
| 31 2-me-2-butene | 1.8 | 31 methyl cyclo-pentane | 0.8 |
| 32 1,3,5-trime-benzene | 1.8 | 32 n-hexane | 0.8 |
| 33 cyclopentane | 1.6 | 33 3-methyl hexane | 0.7 |
(Table continued on next page)
Page 236
(Table continued from previous page)
| Species sorted by concentration | Species sorted by OH reactivity | |||
| Species | Concentration, ppbC | Species | Propy-Equiv., bbpC | |
| 34 propene | 1.5 | 34 3-methyl pentane | 0.7 | |
| 35 i-propyl-benzene | 1.5 | 35 i-butyl benzene | 0.5 | |
| Totala | 197 | Totala | 105.0 | |
| aIncludes all measured VOCs and comprises more than the 35 species presented in the table. | ||||
The total VOC concentrations and total Propy-Equiv concentrations for each of the midday data sets included in our analysis are compared in Figure 8-5. (Note that data sets III.A, III.B, and III.C from the remote tropical forest actually represent diurnally averaged concentrations rather than midday concentrations. Because VOC concentrations in forests generally peak at midday, the values indicated in Figure 8-5 are underestimates of the midday concentrations to be expected in tropical forests.) The results obtained from the concentration-based method are fairly predictable. On average, the urban-suburban areas have the highest total VOC concentrations, ranging from somewhat less than 100 ppbC to slightly more than 500 ppbC. Lower total VOC concentrations in the range of a few tens of ppbC are found in the rural and remote areas, where anthropogenic influences are small to negligible. The highest total VOC concentration obtained for the remote tropical forest was from data set III.B. Note in Table 8-5 that this data set was gathered during a period following extensive biomass burning; the relatively large abundance of VOCs measured during this period perhaps indicated the sizable effect biomass burning can have on a region's air quality.
A very different pattern emerges in Figure 8-5 from the results obtained with the OH-reactivity-based method. In the urban-suburban areas, the total Propy-Equiv concentration is always less than the total VOC concentration, indicative of the large amounts of relatively unreactive VOCs typically present in the urban atmosphere. In rural areas and in the remote tropical forests, on the other hand, where emissions of highly reactive biogenic VOCs are the largest, the total Propy-Equiv concentration is always larger than the total VOC concentration. As a result, we find using the OH-reactivity-based meth-
Page 237
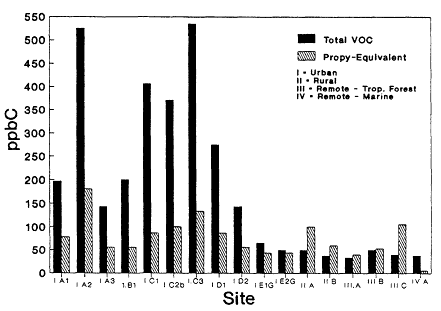
Figure 8-5
Total nonmethane VOC concentrations and propylene equivalents (Propy-Equiv)
concentrations measured at urban-suburban, rural, and remote sites from Table 8-5.
The urban-suburban data were gathered during the midday, the rural data are averages
from 0800 to 1700 local standard time (LST). The remote, tropical forest data are diurnally
averaged, and the remote marine data were gathered from 1300-1500 (LST).
od that the concentrations of VOCs in rural regions of the United States and in the remote tropical forests of Brazil are similar to those found in urban centers of the United States; the total Propy-Equiv concentrations from these three regions all range from 50 to 250 ppbC. In fact, the total Propy-Equiv concentration calculated for the tropical forests during the wet season (data set III.C) is actually larger than the total Propy-Equiv concentrations obtained from the majority of the midday data sets from the urban-suburban regime. The only data set from a remote regime that yielded a significantly smaller total Propy-Equiv concentration than that of the urban-suburban data sets was IV.A. This data set, however, was gathered in the remote marine atmosphere, where biogenic VOC emissions are negligible and anthropogenic influences are minimal.
Page 238
Suorce Apportionment
To gain insight about the relative importance of various kinds of sources in generating the VOCs observed in the atmosphere, the VOC data sets from the urban, suburban, and rural United States were further analyzed by assigning each species' Propy-Equiv concentration to an emission or source type. As described below, two broad categories of sources were considered for this analysis: biogenic and anthropogenic, with the anthropogenic sources further divided into mobile and stationary sources.
Biogenic VOCs were assumed to be characterized exclusively by isoprene and all of the terpenoid compounds (eg., a-pinene, b-pinene) normally associated with vegetative emissions (Chapter 9). Evidence in support of this assumption in the case of isoprene, the dominant biogenic VOC in all the data sets analyzed here (and for most of the urban-suburban data sets the only biogenic VOC identified), is presented in Figures 8-6, 8-7, and 8-8. Figure 8-6, which is based on data gathered from the Los Angeles, California, and Baton Rouge, Louisiana, areas, shows that although reactive VOCs normally associated with mobile sources, such as 2-methyl-2-pentene, cyclohexene, trans-2-pentene, and cis-2-butene (Middleton et al., 1990), are strongly correlated with one another, isoprene shows a weak negative correlation with these compounds. Figure 8-7 illustrates that the ratio between isoprene and a mobile-source VOC exhibits a minimum during the night and early morning hours, when biogenic emissions are suppressed, and a maximum in the late afternoon, when isoprene emissions are at their peak. Figure 8-8 illustrates that although the variability in urban isoprene concentrations is large, it exhibits a temperature dependence consistent with the laboratory-measured temperature dependence of biogenic isoprene emissions.
All VOCs not assigned to the biogenic category were assumed to originate exclusively from anthropogenic sources. These anthropogenic VOCs were further divided into mobile and stationary sources for all but the Los Angeles sites using the 1985 NAPAP (National Acid Precipitation Assessment Program) speciated VOC inventory for the United States (J. Wagner, EPA, and M. Saeger, Alliance Technologies, pets. comm., April 1990). For the Los Angeles sites, we used a speciated VOC inventory prepared by the California Air Resources Board (CARB) to simulate VOC emissions during an August day in the Los Angeles area (T. McGuire and P. Allan, pets. comm., California Air Resources Board, June 1990). If an anthropogenic VOC had a Propy-Equiv concentration of 10 ppbC and if, in the inventory, the source of this VOC was 50% from mobile sources and 50% from stationary sources, 5 ppbC of its Propy-Equiv concentration would be assigned to mobile sources and the other 5 ppbC to stationary sources. Any species that appeared in the ambient measurements but did not appear in the inventory was assumed to come exclusively from stationary sources. By adding up the contributions from all
Page 239
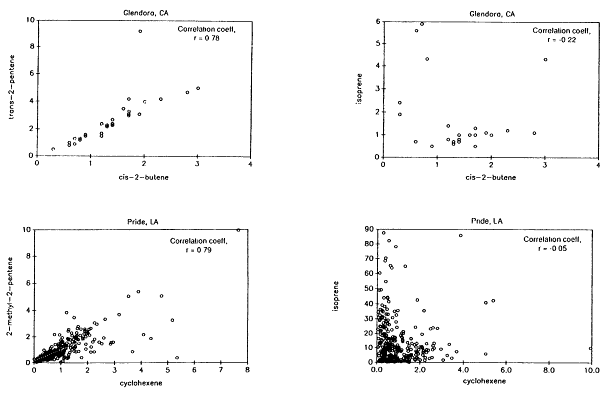
Figure 8-6
Observed atmospheric concentrations of trans-2-pentene, cis-2-butene, cyclohexene, 2-methyl-2-pentene,
and isoprene. The data from Pride, a suburb of Baton Rouge, were obtained from M.O. Rodgers (personal
communication). The data from Glendora, a suburb of Los Angeles were obtained from R. Rasmussen and
D. Lawson (personal communication).
Page 240
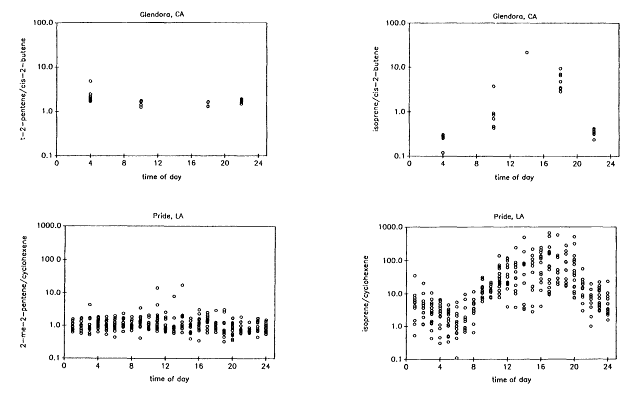
Figure 8-7
Observed atmospheric concentrations ratios of trans-2-pentene to cis-2-butene, 2-methyl-2-pentene to
cyclohexene, isoprene to cis-2-butene, and isoprene to cyclohexene as a function of time of day. The data
from Pride, a suburb of Baton Rouge, were obtained from M.O. Rodgers (personal communication). The
data from Glendora, a suburb of Los Angeles, were obtained from R. Rasmussen and D. Lawson (personal
communication).
Page 241
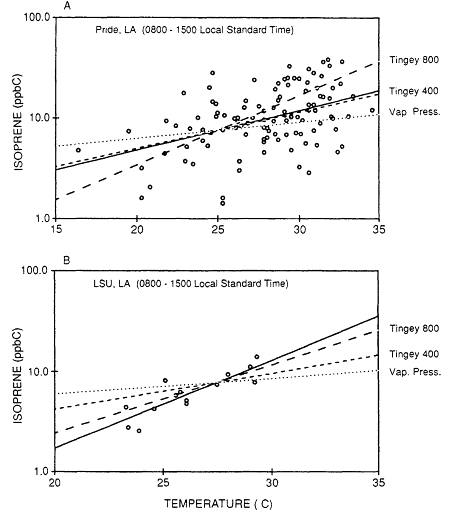
Figure 8-8
Isoprene concentrations as function of temperature at Pride, a suburb of
Baton Rouge, and at the Louisiana State University campus, in downtown
Baton Rouge. Data from M.O. Rodgers (personal communication). The solid
lines are the least-square linear-regression fits to the data. The dashed lines
are the normalized temperature variations predicted by Tingey's (1980) algorithm
for isoprene emissions from trees for solar insolations of 800 and 400 mEinsteins/m2-s.
The dashed line is the normalized temperature dependence of the isoprene vapor pressure.
Page 242
VOCs, a total Propy-Equiv concentration for mobile sources and for stationary sources was obtained.
The resulting Propy-Equiv concentrations from mobile, stationary, and biogenic sources for the urban-suburban midday data sets as well as the rural data sets are shown in Figure 8-9. In Figures 8-10 and 8-11, we illustrate how the contributions of the various sources to the total reactivity (Propy-Equiv concentrations) at single locations (downtown Baton Rouge and Glendora) vary as a function of time of day. Because the distinction between biogenic and anthropogenic VOCs in the urban-suburban and rural data sets is straightforward, the sum of the reactivity of VOCs apportioned to mobile and stationary sources in the figures is probably a fairly reliable estimate of the total anthropogenic contribution to the reactivity of VOCs concentrations at the various sites. However, the apportionment of these VOCs between mobile and stationary sources should be viewed only as a rough estimate because of the sizable uncertainties in the inventories used to make the apportionment (see Chapter 9). Furthermore, because isoprene was the only biogenic species measured in the urban samples and because it generally constitutes 30-50% of the total biogenic emissions in an area (Chapter 9), the total reactivity of VOCs assigned to biogenic sources should be viewed as a lower limit in these cases.
With the exception of the Baton Rouge data sets, mobile and stationary sources make the largest contributions to the total reactivity of VOCs at the urban sites. In Baton Rouge, biogenic sources contribute most to the total reactivity, and in the eastern U.S. rural data sets, biogenic sources dominate over anthropogenic sources. The anthropogenic and biogenic contributions to the total reactivity vary significantly over the course of the day. In Baton Rouge and Glendora, anthropogenic VOCs peak in the early morning hours; biogenic VOCs tend to peak during the mid- and late afternoon. In Los Angeles, the contribution from biogenic VOCs never equals the contributions from anthropogenic sources. In Baton Rouge, however, a very different pattern emerges; biogenic VOCs surpass the contributions from mobile and stationary sources by 1,000 hours and remain dominant for the rest of the daylight period.
Although mobile and stationary sources make the largest contributions to the reactivity of VOCs at most of the urban-suburban sites, the contribution from biogenic sources to these areas should not be discounted as negligible. In most of the urban-suburban data sets, a significant fraction of the total reactivity was found to arise from VOCs of biogenic origin. Except for downtown Detroit, Michigan, and Columbus, Ohio, where extremely low isoprene concentrations were reported, midday biogenic VOC concentrations in the urban-suburban data sets accounted for a Propylene-Equiv (reactivity) of at
Page 243
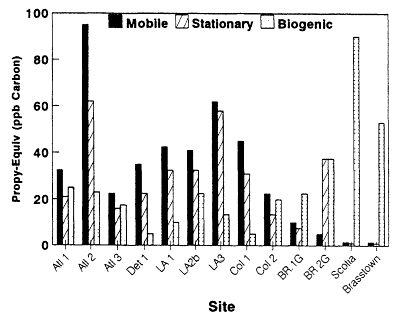
Figure 8-9
Total nonmethane VOC in propylene-equivalent concentrations in units
of ppb carbon observed at urban-suburban sites (midday) and rural sites
(daylight hours) and apportioned by source category. Atl is Atlanta, Det
is Detroit, LA is Los Angeles, Col is Columbus, BR is Baton Rouge, Scotia
is in Pennsylvania, Brasstown is Brasstown Bald in Georgia. Because of
uncertainties, the apportionment between source categories should be viewed
as an estimate. For instance, except for the Los Angeles sites, the splitting of
anthropogenic VOCs between mobile and stationary source VOCs was based on
a national rather than a local inventory. The assignment of biogenic VOCs is a
lower limit because isoprene was generally the only biogenic VOC identified in the
speciated data.
least 10 ppbC—and in many cases, more than 20 ppbC. Even in Glendora, in an area not generally noted for large biogenic emissions, midday isoprene concentrations were about 10 ppbC Propy-Equiv during a moderately hot period (see Table 8-5) and more than 25 ppbC during a particularly hot 3-day period. Furthermore, given that isoprene, which typically amounts to 30-50% of the total VOC emissions from vegetation (Chapter 9), was generally the only VOC of biogenic origin identified in the urban-suburban data sets, it is possible that the actual contribution of biogenic emissions to the reactivity of
Page 244
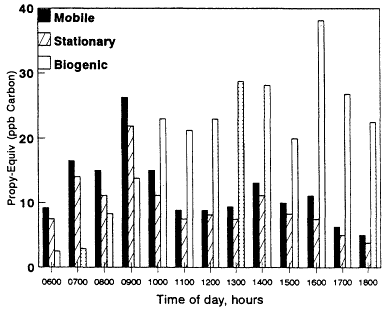
Figure 8-10
Total nonmethane VOC Propy-Equiv concentrations in units of ppb carbon
observed at the Louisiana State University campus as a function of time of
day and apportioned by source category. Sampling period was July 18-26, 1989.
VOCs in urban areas is considerably larger than we estimate here.
It is also important to note that the biogenic contribution is a background VOC blanket that cannot be removed from the atmosphere through emissions controls. If VOC emissions from anthropogenic sources are reduced in the future, this background will be a larger and more significant fraction of the total reactivity of VOCs. Our analysis suggests that in many cities, even if anthropogenic VOC emissions are totally eliminated, a background of reactive biogenic VOCs will remain; on hot summer days, this background can be equivalent to 10 or 20 ppbC, or perhaps more, of propylene. Without control of NOx emissions, this VOC background should be able to generate ozone concentrations that exceed the NAAQS concentration of 120 ppb (Chameides et al., 1988).
Another interesting aspect of our results relates to the relative contributions of mobile- and stationary-source VOCs. In the NAPAP and CARB inventories, stationary-source VOC emissions are estimated to be significantly larger than are mobile-source emissions. For instance, in the CARB invento-
Page 245
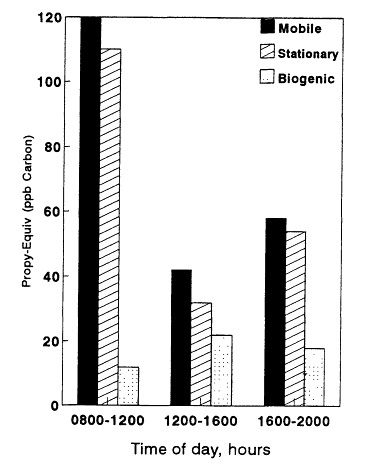
Figure 8-11
Total nonmethane VOC Propy-Equiv concentrations in units of ppb Carbon
observed at Glendora, a site near Los Angeles, as a function of time of day
and apportioned by source category. Sampling period was August 18-20,
1986, a period of extremely high temperatures.
ry, daily stationary-source emissions in the Los Angeles area are estimated to total 1,881,000 kilograms (kg); mobile source emissions total only 732,000 kg/day. By contrast, our analysis based on the ambient concentrations of mobile-source and stationary-source VOCs indicates that mobile sources contribute as much as or perhaps somewhat more than stationary sources (Figures 8-9, 8-10, and 8-11). Moreover, as illustrated in Figure 8-12, this finding appears to be essentially independent of whether the NAPAP invento-
Page 246
ry or the CARB inventory is used in the analysis. The fact that the apportionment of the observed anthropogenic VOCs yields a relatively larger role for mobile sources than implied by the emissions inventories suggests that mobile emissions could have been underestimated in these inventories.
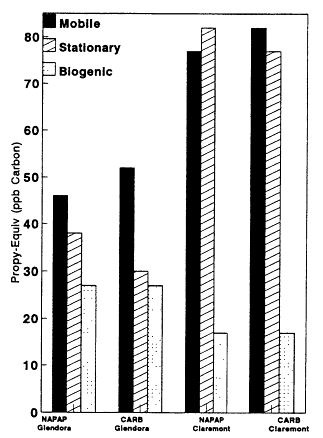
Figure 8-12
Nonmethane VOC Propy-Equiv concentrations in units of ppb Carbon
apportioned by source category using the 1985 National Acid Precipitation
Assessment Program (NAPAP) speciated VOC inventory for the nation and
the California Air Resources Board (CARB) speciated VOC inventory for the
Los Angeles area during an August day. Results are shown for Glendora
(data set I.C2b) and Claremont (data set I.C3). Data sets are described in
Table 8-5.
Page 247
Summary of Voc, Nox, and Ozone Observations
The ranges of VOC. NOx, and ozone concentrations measured in the four atmospheric boundary layer regions are summarized in Figure 8-13. The format for this figure was chosen to resemble the traditional ozone isopleth diagram (Chapter 6). However, although the x-axis in the ozone isopleth typically adopts a concentration-based scale (total VOC concentration), an OH-reactivity-based scale (Propy-Equiv) is adopted in Figure 8-13.
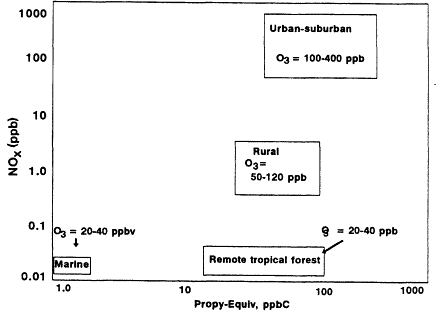
Figure 8-13
VOC, NOx and ozone concentrations in the atmospheric boundary layer at four
locations. VOC is shown as Propy-Equiv concentrations in units of ppb carbon.
The position of the four regions in the diagram shows a strong relationship between observed ozone and NOx concentrations but little or no consistent relationship between ozone and VOC reactivity. Although ozone and NOx concentrations increase substantially as one moves from the tropical forest to rural areas and then to urban and suburban regions, VOC reactivity as measured in Propyl-Equiv remains essentially the same in all three. Similarly,
Page 248
although VOC reactivity increases by more than an order of magnitude from the remote marine region to the tropical forest, ozone concentrations remain the same; the NOx concentrations in these two areas also are quite similar.
Summary
An analysis of observed concentrations of ozone, oxides of nitrogen (NOx), and volatile organic compounds (VOCs) in remote, rural, and urban-suburban areas implies the following:
• A large gradient in NOx concentrations exists between remote, rural, and urban-suburban areas. This gradient suggests that anthropogenic sources dominate the NOx atmospheric budget in the continental United States and that the greatest domination usually occurs in urban centers.
• On an OH-reactivity-based scale, the VOC concentrations observed at surface sites in the remote tropical forests of Brazil, in rural areas of the eastern United States, and in urban-suburban areas of the United States are comparable. All three regions tend to exhibit total VOC concentrations equivalent to 50-150 ppbC of propylene.
• In urban-suburban and rural areas of the United States, VOCs from mobile and stationary sources contribute about equally to the total ambient VOC reactivity. Comparison of this observation with VOC inventories suggests that the inventories have underestimated the contribution of mobile sources.
• In urban-suburban areas of the United States at midday, biogenic VOCs can account for a significant fraction of the total ambient VOC reactivity. Under some conditions in Atlanta and Los Angeles, isoprene alone was found to be present in near-surface air at concentrations equivalent to 25 ppbC of propylene on an OH-reactivity scale. In Baton Rouge, isoprene concentrations equivalent to 40 ppbC of propylene were observed.
• In rural areas of the eastern United States, biogenic VOCs contribute more than 90% of the total ambient VOC reactivity in near-surface air and dominate over anthropogenic VOCs.
• As one moves from remote forests to rural areas in the eastern United States and then to urban and suburban areas in the United States, ozone concentrations are found to correlate with NOx but not with VOC reactivity. This suggests that, in the gross average, NOx and not VOCs is the limiting factor in ozone photochemical production.
Page 249
These conclusions have been drawn from an analysis of a limited data base. It is hard to establish that the data are representative, particularly in the case of the VOC measurements, and it is likely that there are specific urban, suburban, and rural areas in the United States that do not follow the trends implied by the data analyzed here. A more representative analysis would be possible with a more complete data base, including a more comprehensive set of VOC measurements that more accurately establishes the horizontal and vertical variability of these species in the troposphere. In addition, one cannot exclude the possibility that the VOC analysis has been significantly biased by the inability of current techniques to identify and quantify the concentrations of all VOCs in the atmosphere. For these reasons, an important research priority for the coming decade should be the development and application of accurate and reliable techniques for the measurement of VOCs that react to form ozone.








































