3
Physiology of Normal Pregnancy and the Effects of Undernutrition
The physiology of pregnancy presents well-defined challenges to the maternal organism that are unparalleled in the physiology of nonpregnant women. The fetus develops and grows at a rapid rate unequalled to that in postnatal life, and the mechanisms by which this growth occurs and the demands on the maternal organism imposed by this growth are only beginning to be understood. While a detailed review of maternal, placental, and fetal physiology and metabolism is beyond the scope of this report, the following presents a brief synopsis of the physiology of normal pregnancy, with emphasis on physical activity and diet, and in particular, undernutrition.
BODY WEIGHT AND COMPOSITION
The magnitude and pattern of weight gain during pregnancy is quite variable (Pitkin and Spellacy, 1978). Some of this variance is due to the manipulation of weight gain by restriction of food intake; the influence of age, parity, and pregravid body weight, and the presence or absence of excessive water retention. Total weight gain in healthy women who eat to fulfill their appetite probably averages between 10 and 12 kg (Pitkin and Spellacy, 1978). In developing countries, however, where less favorable conditions regarding food intake prevail, weight gains during pregnancy as low as 5 kg have been reported (Lawrence et al., 1985). Maternal weight gain during the first trimester may be minimal; at the end of the first trimester, weight begins to accumulate, and the rate of maternal weight gain is essentially linear throughout the second and third trimesters, at 350 to 400 g/week (Pitkin and Spellacy, 1978) (Figure 3-1).
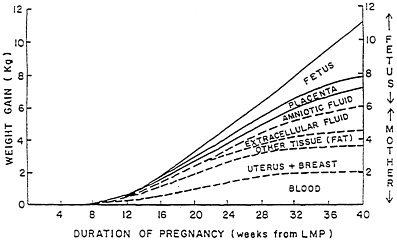
Figure 3-1 Pattern and components of maternal weight gain during pregnancy.
Source. Pitkin, 1976.
Accumulation of maternal tissue (i.e., blood, uterine, and breast tissues and tissue stores) occurs primarily during the second trimester as does growth of the placenta, while growth of the fetus and increases in the amount of amniotic fluid occur most rapidly in the third trimester. Of an average 11 kg of total weight gain, the maternal compartment represents about 6 kg and the conceptus (fetus, placenta, and amniotic fluid) represents about 5 kg.
The composition of the weight gain also varies, but an 11-kg gain includes, on the average, about 7 kg of water, 3 kg of fat, and nearly 1 kg of protein (Hytten, 1980b). Of the total 7 kg of water gain, about 2–3 kg consist of maternal extracellular fluid. Pregnant women with edema, particularly generalized edema, may gain substantially more water. Sodium and other minerals also accumulate during pregnancy, but their direct contribution to the total weight gain is minimal.
Fat metabolism is affected substantially by pregnancy. Starting early in human pregnancy, women begin to store large amounts of fat, with sustained fat deposition occurring through the first two trimesters. Fat accumulation averages 3 kg, or approximately 15–25 percent of the prepregnant fat content (Hytten, 1980a). The rapid rate of maternal fat deposition occurs in many species (Widdowson and Spray, 1950), including the rabbit (Elphick et al., 1978), guinea pig, and sheep (Vernon et al., 1981), and the maternal total
body fat content in these animals may increase by as much as 50 percent (Widdowson and Spray, 1950).
This rapid deposition of fat represents an efficient form of storage for large amounts of energy. Such fat deposition requires that energy intake go beyond basal needs, and this may result from spontaneous increases in energy intake, endocrine changes promoting fat deposition, and decreases in energy expenditure, including resting metabolic rate (RMR). In humans, the rate of fat deposition slows and may decline slightly during the third trimester. The human fetus normally accumulates up to 16 percent of its body weight as fat. This rapid accumulation of fat by the human fetus is unusual among mammals (Sparks, 1984; Widdowson and Spray, 1950) and is related to slow growth and long gestation.
In contrast to body fat, lean body mass appears to change relatively little in women during the course of pregnancy. While some individual organs, particularly the breast and uterus, increase in size substantially during pregnancy, the overall change in lean body mass appears to be of a smaller magnitude and more difficult to detect. Nitrogen balance studies in humans have shown only minimal changes, on average, in lean body mass (Johnstone et al., 1981). Carbohydrates are stored as glycogen in the liver and in muscle tissue.
Maternal Energy Expenditure and Energy Balance
Studies from The Gambia suggest that the nutritional state of the mother may influence the change in resting metabolism. At term, the cumulative change in RMR totaled 13,000 kcal in supplemented women, whereas RMR increased by only 1,000 kcal in unsupplemented women. In studies done in the Philippines (Tuazon et al., 1987) and Thailand (Thongprasert et al., 1987); where the maternal energy supply was not as limited as that in The Gambia, the cumulative increase in RMR totaled 23,900 and 18,900 kcal, respectively. It appears that prepregnancy undernutrition and/or underfeeding during pregnancy limit the increase in resting metabolism that occurs during pregnancy. This is consistent with the response to semistarvation in adult men. Grande et al. (1958) found that a 50 percent reduction in energy intake caused RMR to fall about 20 percent after 13 days. This rapid initial decline in RMR with undereating is associated with a fall in circulating levels of active thyroid hormones and the urinary excretion of catecholamine metabolites (Jung et al., 1980). A reduction in lean, or actively metabolizing, tissue among the underfed women also accounted for a reduction in their RMR. However, based on measurements of total body water, Gambian women have about the same amount of lean tissue as the
Cambridge, England, women, (43 and 41 kg, respectively) (Prentice, 1984). If there were no differences in the amount of active lean tissue, the lower RMR of the Gambian women must have been due to a reduced rate of metabolism per gram of lean tissue.
Women living in some developing countries may be exposed to an annual cycle of food shortages and the seasonal energy demands of subsistence farming (Roberts et al., 1982). Maternal weight gain is affected by this cycle. After adjustment for seasonal effects, Gambian women gained on average 6.3 kg by 35 weeks; those women receiving a food supplement that increased their total energy intake by about 430 kcal/day gained, on average, 7.9 kg (Lawrence et al., 1987). Approximately 10.7 kg are gained by well-nourished women at the same time of gestation. The mean total weight gain of Taiwanese pregnant women is 7.6 kg (Adair, 1983). Colombian and Guatemalan pregnant women gain about 7 kg (Lechtig et al., 1975; Mora et al., 1979), and Filipino and Thai women gain about 8.5 kg (Thongprasert et al., 1987; Tuazon et al., 1987). In general, it appears that women who live in developing countries gain 2.5 to 4.5 kg less than do well-fed women who live in developed countries. There is some evidence that a reduction in maternal weight gain is associated with a reduction in fat gain. For example, the Filipino and Thai pregnant women who gained about 8.5 kg overall gained only 1.3 kg of fat in comparison with the 3.3 kg of fat gained by well-fed women who live in developed countries. The measurements of change in fat mass during pregnancy are fraught with methodological limitations. Furthermore, there is a possibility that average gains in developed countries are higher than needed (Thongprasert et al., 1987; Tuazon et al., 1987).
Lawrence et al. (1987) also evaluated the effect of food supplementation and seasonal energy demands on total fat gain during gestation in Gambian women. In unsupplemented women, there was no gain in body fat, as estimated from changes in total body water, throughout pregnancy. In supplemented women, approximately 2.5 kg of fat were gained by the end of the second trimester, of which 0.5 kg was subsequently mobilized during the third trimester. There were marked seasonal influences, however, on tissue fat changes in both groups. In unsupplemented women whose second and third trimesters coincided with the worst time of the year (from the point of view of food availability and workload), total weight gain averaged 2.3 kg, and there was an overall fat loss of 4.7 kg. During the best time of the year, the unsupplemented women gained up to 11.2 kg and had a maximal net fat gain of 3 kg. In supplemented women, total weight and fat gain were greater than in their unsupplemented counterparts at the worst and best times of the year, but the seasonal differences in their weight gain were narrower than in the unsupplemented ones. Thus, during periods of limited energy
intake, pregnant women conserve energy for fetal growth by mobilizing maternal stores to the maximum extent possible. Because weight gains were the greatest among women with the most depleted reserves (Lawrence, 1997), these stores seem to be repleted when the energy supply improves.
Potential Energy-Sparing Mechanisms
Besides the already indicated changes in RMR among the less nourished groups, savings in energy expenditure seem to occur also through a reduction in physical activity. Two types of reductions are most likely: a reduction in the number and duration of activities or a change in the efficiency with which various physical activities are accomplished. There is some evidence that underfed pregnant women adjust both the amount of physical activity as well as the intensity of that activity. Studies of the activity patterns of women in developing countries show that they perform the same tasks whether or not they are pregnant. If any changes occur, they are in the duration and intensity of work and not in the type of work. Activity diaries of pregnant Gambian women (Roberts et al., 1982) showed that they reduced their energy expenditure to 75 percent of that of nonpregnant women in the last month of pregnancy by reducing the amount of housework and heavy farm work. The total time spent farming was unchanged, however.
The response to food supplementation is also of interest. A net increase of 723 kcal/day during lactation did not affect breast milk volume or milk energy content (Prentice, 1984). There was a slight increase in maternal body weight during the first 2 months after initiation of the supplement, but thereafter, weight change patterns were similar to those in unsupplemented women. Those changes in body weight accounted for only 7 percent of the supplemental energy. It is unclear what happened to the remaining energy. The women reported reduced fatigue and an increased feeling of well-being, but it is unlikely that changes in physical activity could account for an additional 700 kcal/day, as this would require that they double their physical activity (Prentice, 1984). Hormonal and energy substrate levels in the blood suggested that overall metabolic efficiency was reduced.
It is possible that adaptations in metabolism allow underfed women to achieve an energy balance when consuming energy-limited diets. With improved intakes, this efficiency is not maintained, and the additional energy is expended without a sufficient weight gain.
The energy costs of various activities have been measured in pregnant and nonpregnant women to determine whether the activity is performed in a more economical, energy-conserving manner. The energy costs of 40 domestic and agricultural activities were assessed recently in 142 nonpreg-
nant, pregnant, and lactating Gambian women (Lawrence et al., 1985). Since the energy expenditures of nonpregnant and nonlactating women, pregnant women in the first trimester, and lactating women did not differ, these women were grouped and compared with pregnant women in their second and third trimesters. With one exception, there was no significant change in the energy cost for activities with advancing gestation. The exception was for bending and digging, for which the total energy cost decreased during late pregnancy. When the data are expressed per unit of body mass, the energy expenditure of pregnant women was significantly less than that of nonpregnant women. This is because the pregnant women weighed 5 kg more than the nonpregnant women. Since RMR increased about 0.1 kcal/minute during weeks 20–33 of gestation, the net energy cost for all of these activities would have decreased in the second and third trimesters. This reduction in the net cost for activity in late pregnancy suggests that the intensity of work declined, particularly for bending and digging activities. The energy cost of physical activity did not increase with food supplementation (Lawrence et al., 1985). It is possible that all pregnant women, regardless of nutritional state, reduce their work intensity. However, studies of East Javanese peasant farmers showed that energy-deficient people perform work more efficiently (Edmundson, 1977).
Because women in developing countries weigh about 6 percent less than women from developed countries, the energy cost for weight-bearing activities should be reduced accordingly. This is evident from measurement of energy expenditure for walking during pregnancy. Reductions in nonshivering thermogenesis may contribute to energy balance in underfed people (Prentice, 1984). This hypothesis is supported by the fact that adult Kalahari (African) Bushmen and Australian Aborigines have a reduced response to the cold and that they allow their core body temperatures to fall during the night, thus reducing the temperature gradient and the heat loss (Prentice, 1984). Nonshivering thermogenesis may result from futile cycles in the muscle or from the uncoupling of adenosine triphosphate (ATP) synthesis in brown adipose tissue. The role of these cycles in energy balance in humans is uncertain. Recent work in mice shows that energy can be conserved very efficiently during pregnancy and lactation by the uncoupling of ATP synthesis in brown adipose tissue (Trayhurn et al., 1982). The additional heat resulting from fetal growth and milk production may obviate the need for thermoregulatory heat production, or there may be a physiologic adaptation in energy metabolism mediated by a hormone such as prolactin (Prentice, 1984). Prolactin levels in pregnant and lactating women in developing countries tend to be higher than those in women in developed countries. It is uncertain, however, whether this hormonal change mediates an adaptation to thermogenesis in humans.
Blood Volume and Composition
Blood volume and composition change profoundly in pregnant women. Plasma volume increases in healthy, pregnant women by at least 1,250 ml, or about 48 percent above the usual volume of 2,600 ml in nonpregnant women (Hytten and Lind, 1973). The increase in plasma volume follows a sigmoid pattern with little change during the first 10 weeks, but it increases linearly during the second and the early part of the third trimesters until a plateau is reached at about 30–34 weeks of gestation. In general, the larger the increase in plasma volume, the larger the fetus. Erythrocyte volume also increases, but to a smaller extent than the plasma volume. In a healthy, pregnant woman who is not receiving iron supplements, the erythrocyte volume increases about 18 percent (250 ml) above that of a nonpregnant woman (1,400–1,650 ml). With iron supplementation, hemopoiesis is more rapid, and the erythrocyte volume increases about 30 percent through the end of gestation (an increase of from 400 to 450 ml). Erythrocyte volume also begins to expand at about 10 weeks of gestation, but unlike the plasma volume, it continues to expand until the end of gestation. Without iron supplementation, the erythrocyte count, hemoglobin concentration, and hematocrit fall during pregnancy, reaching a nadir at 28–32 weeks of gestation.
Total serum lipids increase progressively throughout pregnancy, reaching a level about 0.4 g/dl higher than that in nonpregnant women at the end of gestation (Hytten and Lind, 1973). The concentrations of triglycerides, cholesterol, and phospholipids increase, with the concentrations of phospholipids and triglycerides tending to level off in the third trimester and the cholesterol concentration continuing to rise (Knopp et al., 1978).
Undernutrition, Blood Volume Expansion, and Placental Blood Flow in Animal Models
Acute food restriction in pregnant rats limits the expansion of blood volume (Rosso and Streeter, 1979), and this, in turn, limits the increase in uterine blood flow (Rosso and Kava, 1980). An alternative explanation is that undernutrition reduces uteroplacental metabolism and then uterine blood flow, which leads to accommodating adjustments in central cardiovascular functions, including blood volume. In a comparison of chronically and acutely malnourished pregnant rats, the percent absolute increase in maternal plasma volume expansion in chronically malnourished animals was nearly twice that seen in the acutely malnourished ones (Fellows, 1985).
Adjustment to chronic undernutrition before conception may improve the rats' adaptation to pregnancy.
The timing of food restriction during pregnancy alters the distribution of the cardiac output to the uteroplacental unit in rats (Ahokas et al., 1983a). When food restriction was initiated late in gestation (day 14 of 22 days), cardiac output and uteroplacental blood flow were equally reduced, showing that the distribution of the cardiac output to the uterus was maintained. Restriction early in gestation (up to day 5), however, showed that uteroplacental blood flow was, at term, reduced more than the cardiac output was, suggesting that the cardiac output is redistributed away from the conceptus. Restriction during midpregnancy only (days 5–14) caused a redistribution of the cardiac output toward the conceptus at term. The timing of the onset of maternal food restriction can have different effects on uteroplacental blood flow, depending on the distribution of the cardiac output.
It is unclear whether a reduction in placental blood flow caused by food restriction significantly reduces nutrient transfer to the fetus. Measurements of placental nutrient clearance in sheep show that the clearance of substances such as ethanol is asymptotic; that is, there is little change in clearance with changes in blood flow at high rates of flow (Wilkening et al., 1985). Thus, a 50-percent reduction in placental blood flow caused only a 20-percent decrease in the clearance of ethanol. In rats, malnutrition reduced placental blood flow by 43 percent (Rosso and Kava, 1980); the associated reduction in nutrient clearance may be much less—possibly less than 20 percent. Assumptions about fetal nutrient supply cannot be made from measurements of uteroplacental blood flow without information about nutrient clearance since the two are not linearly related. For a discussion of the effects of maternal undernutrition on fetal and maternal outcomes in animal models, the reader is referred to Rosso and Lederman, 1983.
GESTATIONAL EFFECTS ON ORGAN SYSTEMS IN PREGNANT WOMEN
Renal plasma flow is continuously increased throughout pregnancy from about 200 to 250 ml/minute (Hytten and Lind, 1973). The glomerular filtration rate (GFR) similarly increases by about 50 percent during the last two trimesters, and a small fall in GFR may occur during the last 4 weeks of pregnancy. The rise in GFR may merely be the result of the increased renal plasma flow; it does not appear to be regulated hormonally during pregnancy.
The general tone and motility of the stomach and the small and large intestines are reduced during pregnancy because of smooth muscle relaxation by progesterone (Hytten and Lind, 1973). Consequently, gastric emptying time and intestinal transit time are prolonged. This relaxation of muscle tone may explain the nausea that occurs during pregnancy; constipation is also a common complaint. It is not known whether this prolonged intestinal transit time contributes to improved nutrient absorption.
Pregnancy-Related Changes in Maternal Metabolism
Maternal metabolism meets two important goals: maintaining concentrations of key substrates in blood within a certain range and dynamically producing and consuming sufficient substrate quantities to meet ongoing tissue needs. The dynamic disappearance of glucose to meet tissue energy and carbon requirements balances the dynamic appearance of glucose from endogenous and exogenous sources, maintaining blood glucose within a relatively narrow range.
Metabolic Adaptations
When women fast during pregnancy, placental steroid secretion is altered. The human placental lactogen (hPL), a lipolytic hormone that mobilizes maternal fatty acids and thus spares glucose for the fetus and placenta (Kitzmiller, 1980), is increased in fasting pregnant women (Kim and Felig, 1971). This is associated with a rapid rise in maternal fatty acid and ketone levels (Schreiner et al., 1980). Placental hPL levels also increase when the maternal blood glucose level is low (Prieto et al., 1976). Placental estrogen and progesterone secretion alter maternal insulin levels, fat deposition, and plasma and red blood cell volume expansion (Kitzmiller, 1980). Maternal malnutrition may also alter the synthesis of these placental hormones. Chronic undernutrition in rats decreased estradiol levels on day 18 of gestation, but there was no change in the progesterone level (Alexander et al., 1988). Indian women of low socioeconomic status have decreased levels of urinary estrogen (Iyengar, 1968) and progesterone (Rosso, 1980). Thus, undernutrition may alter the mother's ability to accumulate nutrient stores early in pregnancy and, subsequently, to deliver them to the fetus late in gestation (Jones and Crnic, 1986). This is supported by the fact that impaired fat deposition in women during the second trimester is predictive of poor fetal growth (Viegas et al., 1987). Also, interpregnancy supplementation from the special Supplemental Food Program for Women, Infants, and
Children of the U.S. Department of Agriculture and, therefore, an assumed increase in maternal stores at conception, improves birth weight significantly more than supplementation only during pregnancy (Caan et al., 1987).
Pregnancy imposes significant alterations on substrate metabolism, resulting in considerable changes in the dynamic rates of metabolism and lesser changes in substrate concentrations in the basal state. From the point of view of physical activity and diet, these changes in metabolism are particularly important for glucose, lactic acid, and oxygen and non-esterified fatty acids.
Oxygen
While oxygen is not typically considered to be a nutrient, energy production to perform metabolic and physical work requires oxygen consumption. The relationship of oxygen consumption to work is basic to nutritional studies and provides a foundation for indirect calorimetry. Like that of glucose, the concentration of oxygen does not appear to change greatly during pregnancy. Oxygen consumption, analogous to the disposal of carbon substrates, appears to increase gradually throughout pregnancy. In resting women during late gestation, oxygen consumption is 16–32 percent greater than that of nonpregnant controls, apparently because of the effects of the conceptus, rather than a large increase in the oxidation rate of nonuterine tissues (kidneys, heart, liver, and gut) (Lotgering et al., 1985). This increase in the level of absolute oxygen consumption while resting is consistent among species, and it appears reasonable in view of not only the continued growth of the metabolically active conceptus but also the high cost of the extra maternal cardiac and respiratory work and the considerable cost of reasborbing the extra sodium filtered by the raised glomerular filtration rate (Block et al., 1985; Gilbert et al., 1982). Resting oxygen consumption in early and mid-gestation appears to be highly variable, being lowered or increased depending on previous maternal nutritional status and energy intake.
Glucose
Glucose is a major fuel for the maternal organism and is required for the proper functioning of vital maternal organs, particularly the brain. Pregnancy alters glucose concentration in the fed state only minimally, resulting in small and gradual, but real, decreases in the glucose concentration in blood throughout pregnancy. In the starved state, changes are
rapid and dramatic, giving rise to the concept of accelerated starvation, which is discussed later in this chapter. Such changes have been described in several species, including human, rabbits, monkeys, sheep, and guinea pig (Lotgering et al., 1985).
In contrast to the relatively small changes in the glucose concentration in blood during pregnancy, relatively large changes in glucose kinetics do occur. The brain, which is relatively insensitive to insulin, accounts for a major portion of glucose disposal in nonpregnant animals. Glucose use by other tissues such as muscle and adipose tissue is quantitatively less important, but it is more variable in time and is subject to insulin regulation. Tracer studies in unstressed guinea pigs, rabbits, and sheep have shown that substantial changes in the disposal rate of glucose occur in the pregnant animals, and more limited stable isotope studies have demonstrated that considerable increases in glucose turnover occur in pregnant women as well. In guinea pigs, animals that typically have a larger fetal mass per body weight, the absolute turnover of glucose during pregnancy approximately doubles (Gilbert et al., 1982); there are lesser effects in other species. Recent studies in guinea pigs have related the increase in glucose turnover directly to fetal number and mass (Lotgering et al., 1985). Studies in sheep with one and two fetuses (Bergman, 1974; Hay et al., 1983) support the concept that most of the increase in glucose turnover during pregnancy is a direct result of the increasing mass of the metabolically active conceptus that is being supported by the mother and increased feed intake (Steel and Leng, 1973).
In view of the complex endocrine changes that occur during pregnancy, the question of possible alterations in glucose appearance and disposal of nonreproductive maternal tissues is of great interest but remains largely unresolved. Increases in cortisol, hPL, human chorionic gonadotropin (hCG), estrogens and progesterone during pregnancy alter maternal glucose metabolism. Cortisol increases gluconeogenesis and glucose release, but its effects may be offset by the effects of increased progesterone and its metabolites. Estrogen may also increase gluconeogenesis and glucose turnover. While under nonstressed conditions, pregnant women maintain glucose concentrations near the levels found in nonpregnant women. The altered endocrine milieu appears to predispose women toward having difficulties in handling metabolic stress during pregnancy.
Insulin and glucagon are major regulators of glucose metabolism in nonpregnant animals. In pregnant animals, glucagon appears to play a minor role in metabolic adaptation to pregnancy (Knopp, 1981). In contrast, considerable interest has been paid to the concept of insulin resistance in which, despite increased levels during pregnancy, insulin appears to be less effective in maintaining glucose homeostasis. Insulin resistance appears to
develop early in pregnancy, and a variety of hypotheses have been proposed to explain it. It has been suggested that there may be an increased gluconeogenic set point induced by other hormones during pregnancy and that there is an alteration in insulin receptor function and number. More recently, new methodologies such as the glucose clamp have helped investigators assess glucose homeostasis. (In the glucose clamp, the glucose concentration is maintained within very narrow boundaries by careful intravenous infusion of glucose with frequent monitoring; concentrations of insulin, glucagon, somatostatin, and other hormones are altered experimentally. The effect of the alteration can then be quantitated by determining the change in the glucose infusion rate required to maintain the glucose concentration within the desired range.) Newer glucose clamp techniques are beginning to elucidate further the phenomenon of insulin resistance during pregnancy (Leturque et al., 1984; Ryan, et al., 1985).
Lactic Acid
Lactic acid metabolism during pregnancy deserves particular attention in view of its importance during acute exercise and conditioning. Lactic acid is produced by the reduction of pyruvate and is an intermediate in many biological reactions. As such, it can be viewed as a central intermediary metabolite by a number of glycolytic tissues, including red cells, the placenta, and probably, muscle. Lactic acid production does not necessarily imply hypoxia or ischemia. Lactic acid is not normally ingested in significant amounts by adults, and therefore, its metabolism can be viewed primarily as the balanced endogenous production and endogenous consumption of lactic acid (Johnson et al., 1986; Kreisberg, 1980). Ordinarily, the rates of both consumption and production of lactic acid are rapid and balanced. Carbon cycling among organs in the form of lactic acid and other compounds may be important in the internal economy of the organism, but it need not have caloric implications for the economy of the whole organism, as both lactic acid production and consumption are endogenous within the organism.
When the body is at rest, lactic acid concentration remains relatively constant over time because rapid production of lactic acid is balanced by rapid consumption. During periods of short-term activity as well as strenuous activity, increased lactic acid production from muscular tissues may lead to significant, but transient, increases in the lactic acid concentration, which is then cleared to normal levels as activity is reduced. In pregnant women in labor, lactic acid concentration normally increases in relation to muscular work with each contraction.
Little is known about the specific factors regulating the production and consumption of lactic acid in nonpregnant or pregnant animals. However, net production of lactic acid in large amounts by the gravid uterus appears to be a general property of pregnancy in a number of species, including humans, sheep, rabbits, and guinea pigs (Block et al., 1985; Johnson et al., 1986; Sparks et al., 1982). The effect of this large load of lactic acid on either normal maternal metabolism or maternal exercise physiology remains to be determined.
Other Substrates
Basal concentrations of amino acids, free fatty acids, and keto acids do not appear to change substantially during pregnancy in well-fed humans or experimental animals (Gilbert et al., 1984; Lotgering et al., 1985; Sparks et al., 1982).
Undernutrition in Pregnancy: ''Accelerated Starvation''
While the basal circulating concentrations of major substrates appear to change over a relatively narrow range during pregnancy when food intake is adequate, the combination of increased absolute rates of fuel consumption and the endocrine changes associated with pregnancy appear to predispose pregnant animals toward intolerance of brief fasts that nonpregnant animals would easily accommodate. During pregnancy, the normal metabolic responses to a fast appear exaggerated in both time and quantity. This exaggerated response has been described as accelerated starvation. Studies in humans have demonstrated that even a brief fast is associated with rapid changes of large magnitude in gluconeogenic amino acids, keto acids, and free fatty acids, confirming a series of previous studies in experimental animals that document similar exaggerated responses to even brief periods of starvation (Metzger, 1982; Freinkel et al., 1972). Recently, these studies have been repeated in guinea pigs along with studies of glucose kinetics and substrate levels. Guinea pigs are notable because of their extremely large fetal mass, and starvation for as short as 2 hours results in significant decreases in glucose turnover, as well as a rapid elevation of keto acids levels. In particular, the magnitude of the changes in glucose concentration and turnover directly relate to the fetal mass (Gilbert et al., 1985).
METABOLIC AND TRANSPORT FUNCTION OF THE PLACENTA
While the roles of the placenta in hormone synthesis and substrate transport are well known, the metabolic processes that fuel these roles are considered less often and are considerably less well understood. Generalizations about placental function and structure are somewhat difficult in view of the surprising diversity among species in placental types and perfusion patterns (Meschia, 1983). Placental tissues generally are rich in mitochondria, and recent in vivo studies of sheep placenta demonstrate surprisingly high weight-specific oxygen consumption, almost as high as that for brain tissue. In ruminants, glucose appears to be a major placental substrate, with the concomitant net placental production of lactate distributed into both the fetus and the mother. Placental tissue from many species consumes glucose and produces lactate in vitro.
This rapid placental metabolic rate may have significant consequence in considerations of placental nutrient flux. In sheep, the placenta normally consumes 50 percent of the oxygen and 75 percent of the glucose that leaves the uterine circulation (Meschia, 1983). Far from being a simple membrane diffusion system, the placenta appears to be actively involved in the metabolism of major substrates, and this has added considerable mathematical and practical complexity to metabolic studies that attempt to characterize and compare maternal and fetal rates of substrate metabolism in experimental animals (Hay et at., 1981; Sparks et al., 1982).
From the point of view of transport, the properties governing nutrient flux across the placenta also are receiving considerable attention. In general, nutrient transport can be considered to be limited by diffusion on the one hand and by blood flow on the other. More recent work has documented that the flux of actual nutrients appears to be more complicated and varies from simple diffusion as for water; facilitated diffusion, as for glucose; or active transport, as for some amino acids and calcium. Fluxes of some substrates, such as ethanol, appear to be directly related to placental blood flow, while fluxes of others, such as glucose and oxygen, are relatively independent of flow until it is markedly diminished (Wilkening and Meschia, 1985; Wilkening et al., 1985). The effect of alterations in uterine blood flow on actively transported substrates is unknown. Because substrates appear to be affected differently by changes in uterine blood flow and maternal substrate concentration, alterations in blood flow may affect the transport of some substrates more than others. Thus, there is a possibility that alterations in flow or concentration, such as those that occur with exercise or dietary interventions, may produce not only differences in nutrient flux but also some redistribution in the types of caloric sources available to the fetus.
The nonlinearity of nutrient flux to blood flow (e.g., glucose and oxygen) (Wilkening and Meschia, 1985; Wilkening et al., 1985) and to concentration (e.g., glucose) (Simons et al., 1979) significantly complicates the prediction of nutrient flux across the placenta from mother to fetus. The amounts of glucose and oxygen crossing the placenta do not change substantially with a change in blood flow until there is a marked reduction of flow, but they decrease rapidly with a further reduction in blood flow. Similarly, many substrates, including glucose, amino acids, and minerals, cross the placenta by carrier-mediated or active transport systems. Thus, changes in blood flow or nutrient concentration may have minimal or dramatic effects, depending on the precise nature of the transport system and the particular circumstances under study. Studies in chronically prepared sheep, discussed later in this report, suggest that exercise may result in decreases in uterine and umbilical blood flow unaccompanied by decreases in nutrient flux.
Placental Transport in Malnourished Women and Animals
The placentas of women from lower socioeconomic groups weigh less, have disproportionately large deficits in trophoblastic mass, and have reductions in the surface area of villi. The surface area of the villi of fetal capillaries is also reduced as compared with that of fetuses developing in women from higher socioeconomic groups (Laga et al., 1972). Reduced amounts of placental DNA also have been measured (Dayton et al., 1969). It is uncertain whether these placental defects alter fetal nutrition. It is thought that the fetal demand for nutrients is far less than the placental functional capacity for transport. For example, when rats were malnourished in early gestation and returned to ad libitum feeding on day 14, pup birth weight recovered to control levels, whereas placental weight did not (Ahokas et al., 1983a). In sheep, reduction in placental size due to chronic undernutrition is highly correlated with reduced fetal growth rate during late gestation (Mellor, 1983). In humans, degenerative placental changes are not associated with a reduction in birth weight (Shah et al., 1979). It seems that morphological changes in the placenta due to malnutrition may not necessarily impair transport capacity sufficiently to alter fetal nutrition (Jones and Crnic, 1986).
Effect on Nutrient Flux
In animals, chronic protein-energy malnutrition may significantly affect both the circulating nutrient concentration and maternal hemodynamics. As
noted earlier, numerous studies in sheep, rats, rabbits, and guinea pigs have demonstrated that there are decreases in blood glucose associated with elevations in keto acids and fatty acids.
The relationship of nutrient flux to either blood flow or maternal nutrient concentration appears to be nonlinear and is possibly saturable for nutrients whose transport is either carrier-mediated or actively transported (Wilkening and Meschia, 1985; Wilkening et al., 1985). However, the combination of diminished uterine blood flow and altered substrate concentration suggests that chronic fasting may diminish the flux of nutrients available to the fetus. Such data are limited, but direct measurements in sheep suggest that glucose flux may be substantially diminished during prolonged fasting: The decreases for lactic acid are less. Given the limited permeability of the cell to keto acids and fatty acids, the net result suggests that there is diminished total nutrient entry to the conceptus during fasting. Similarly, studies in small animals demonstrate the altered transport of nutrient analogs consistent with the hypothesis of diminished nutrient flux when the mother is undernourished.
Maternal protein-calorie malnutrition in rats can also limit the normal expansion in circulating blood volume and can result in diminished uterine blood flow (Rosso, 1981). Decreased uterine blood flow and decreased substrate concentration in combination result in diminished nutrient delivery consistent with diminished nutrient flux from mother to fetus.
FETAL METABOLISM AND NUTRITION
Studies of fetal body composition during pregnancy have established ranges for fetal nutrient requirements. Measurements of fetal body composition are available for several species, including rats (Lederman and Rosso, 1981), sheep (Rattray et al., 1974), guinea pigs (Sparks et al., 1985) and humans (Sparks, 1984; Widdowson and Spray, 1950). There are significant interspecies differences in nutritional requirements for fetal growth. For these reasons, one must be cautious in interpreting fetal growth data between human and nonhuman species.
Surprisingly, the more extensive data on fetal body composition are available for humans than for other species, and these cross-sectional data provide chemical analyses of body composition in a large number of fetuses over a range of gestational ages. These data, which have been reviewed extensively (Sparks, 1984; Widdowson and Spray, 1950; Ziegler et al., 1976), provide estimates of the net rate of accretion of calories and major macronutrients in the human fetus, although the data provide only minimal requirements for those nutrients. Growth of the human fetus in the third
trimester represents accretion rates of approximately 40 kcal/kg of fetal weight per day, of which approximately 80 percent is accounted for by fat accretion. Rates of nitrogen, nonfat (lean weight), and mineral accretion have also been calculated.
Data describing the diet available to the fetus for oxidative metabolism and tissue accretion are considerably more limited. Unstressed measurements of substrate uptake are available only in the ruminant. In summary, during normal pregnancy, the fetal lamb grows a lean fetus at approximately 6 percent per day on a diet consisting of glucose (35 percent), lactic acid (15 percent), and amino acids (50 percent). The placenta of the fetal lamb is somewhat impermeable to fatty acids, keto acids, and acetate, and these substrates appear to contribute minimally to oxidative metabolism and tissue growth in the fetal lamb.
For the human, the fetal diet is likely to differ somewhat from that of the lamb. Glucose appears to provide a larger share of the fetal diet, as high as 50 percent. In addition, the human placenta appears to be more permeable to fat, and placentally transferred fat may provide a meaningful portion of total fetal intake of calories and carbon.
The role of lactic acid as an exogenous fetal macronutrient in humans is less clear (Soothill et al., 1986), although for unstressed human fetuses, the data support the fact that lactic acid is a fetal nutrient (Stembera and Hodr, 1966). From the point of view of normal fetal nutrition, lactic acid appears to be produced by the placenta (derived from the glycolysis of fetal glucose) and consumed by the fetus, providing a major source of energy and carbon. As it was mentioned earlier in contrast, lactic acid accumulation in the fetus is regarded clinically as an indicator of the degree of fetal distress, as caused by asphyxia or hypoxia. In humans, lactic acid is produced by intensive exercise and is widely used as an indicator of exercise conditioning and fitness. These very different roles of lactic acid have not been reconciled, and the consequences of maternal lactic acidemia from vigorous maternal physical work on a fetus already consuming large amounts of lactic acid has, thus far, received only minimal scientific attention. However, Chandler et al. (1985) showed that in sheep, despite increased maternal and fetal blood concentration, fetal lactate metabolism is not grossly disturbed by moderate maternal exercise. This study also examined fetal responses to combined maternal under-nutrition and exercise.
Iron Status and Work Performance
Iron is an essential component of hemoglobin, which is responsible for transporting oxygen to the tissues. Iron deficiency can lead to reductions in
hemoglobin synthesis and impairment of oxygen transport to the tissues. Iron deficiency can also cause a reduction in the synthesis of cytochrome C, which is involved in transporting electrons and forming ATP during oxidative metabolism in the mitochondria. Iron deficiency may also reduce myoglobin synthesis, which is essential for storing oxygen in the muscles for use during work. If cytochrome C and myoglobin levels are low, the working muscle must rely primarily on anaerobic metabolism, which results in lactic acid production. As earlier discussed in this chapter, lactate accumulation during physical activity contributes to fatigue and impairs work performance (O'Neil et al., 1986). These reductions in hemoglobin, cytochrome C, and myoglobin caused by iron deficiency were hypothesized to have a negative effect on work performance.
The impairment of physical performance under conditions of iron deficiency is evident from a reduction in total exercise time, increased heart rate, decreased oxygen uptake (decreased maximal oxygen consumption), and increased lactate levels in blood (Gardner et al., 1977; O'Neil et al., 1986).
Severe iron deficiency markedly alters the metabolism of biogenic amines and the production of triiodothyronine (T3). Increases in the circulating levels and urinary excretion of norepinephrine and reductions in T3 production rates have been documented, together with diminished receptor sites in the brain for dopamine and serotonin. These changes are considered to be responsible for behavioral modifications, and for increased heartbeat loss and impaired temperature regulation in people with iron deficiency and anemia (Beard et al., 1984; Dallman, 1986).
The end result of iron deficiency on energy metabolism is a marked diminution of the efficiency of energy use and conservation. These consequences of iron deficiency and anemia may exaggerate the undesirable effects of energy deficiency during pregnancy and lactation on work performance and the outcome of pregnancy. These are areas in which research is needed.
Workers' productivity may also be negatively affected by iron deficiency. Iron supplementation for 2 months significantly improved the amount of tea female workers picked on a tea plantation in Sri Lanka (Edgerton et al., 1979). The degree of improvement was greater in more anemic women (those with hemoglobin concentrations were between 6.0 and 9.0 g/dl). Work tolerance, as estimated by changes in heart rate or actual work performance, was 20 percent lower in women with hemoglobin concentrations between 11.0 and 11.9 g/dl than it was in women with hemoglobin concentrations greater than 13.0 g/dl (Gardner et al., 1977). No data are available on the effect of moderate or severe iron deficiency and anemia in exercise performance or in physical work productivity during pregnancy and lactation.
References
Adair, L.S., E. Pollitt, and W.H. Mueller. 1983. Maternal anthropometric changes during pregnancy and lactation in a rural Taiwanese population. Hum. Biol. 83:771–787.
Ahokas, R.A., C.D. Anderson, and J. Lipshitz. 1983a. Cardiac output and uteroplacental blood flow in diet-restricted and diet-repleted pregnant rats. Am. J. Obst. Gynec. 146:6–13.
Alexander, M.H., and K.M. Rasmussen. 1988. Effect of chronic protein-energy malnutrition on fecundability, fecundity, and fertility in rats. J. Nutr. 118(7):883–887.
Beard, J., W. Green, L. Miller, and C. Finch. 1984. Effect of iron deficiency anemia on hormone levels and thermoregulation during cold exposure. Am. J. Physiol. 246:R380.
Bergman, E.N., R.P. Brockman, and C.F. Kaufman. 1974. Glucose metabolism in ruminants: Comparison of whole-body turnover with production by gut, liver, and kidneys. Fed. Proc. 33:1849–1854.
Block, S.M., J.W. Sparks, R.L Johnson, and F.C. Battaglia. 1985. Metabolic quotients of the gravid uterus of the chronically catheterized guinea pig. Pediatr. Res. 19:840–845.
Caan, B., D.M. Horgen, S. Margen, J.C. King, and N.P. Jewell. 1987. Benefits associated with WIC supplemental feeding during the interpregnancy interval. Am. J. Clin. Nutr. 45:29–41.
Chandler, K.D., B.J. Leury, A.R. Bird, and A.W. Bell. 1985. Effects of undernutrition and exercise during late pregnancy on uterine, fetal, and uteroplacental metabolism in the ewe. Br. J. Nutr. 53:625–635.
Dallman, P.R. 1986. Biochemical basis for the manifestations of iron deficiency. Ann. Rev. Nutr. 6:13.
Dayton, D.H., and L.J. Filer. 1969. Cellular changes in placentas of undernourished mothers in Guatemala. Fed. Proc. 28:488–492.
Edgerton, V.R., G.W. Gardner, Y. Ohira, K.A. Gunawardena, and B. Senewiratne. 1979. Iron-deficiency anaemia and its effect on worker productivity and activity patterns. Br. Med. J. 2:1546–1549.
Edmundson, W. 1977. Individual variations in work output per unit energy intake in East Java. Ecol. Food Nutr. 6:147–151.
Elphick, M.C., J.L. Edson, J.P. Lawlor, and D. Hull. 1978. Source of fetal-stored lipids during maternal starvation in rabbits. Biol. Neonate 34:146–9.
Fellows, W.D., and K.M. Rasmussen. 1985. Does nutrient partition between rat dam and fetuses differ during acute and chronic underfeeding. Fed. Proc. 44:1957.
Freinkel, N., B.E. Metzger, M. Nitzan, J.W. Hare, G.E. Shambaugh, III, R.T. Marshall, B.Z. Surmaczynska, and T.C. Nagel. 1972. 'Accelerated starvation' and mechanisms for the conservation of maternal nitrogen during pregnancy. Isr. J. Med. Sci. 8:426–439.
Gardner, G.W., V.R. Edgerton, B. Senewiratne, R.J. Barnard, and Y. Ohira. 1977. Physical work capacity and metabolic stress in subjects with iron deficiency anemia. Am. J. Clin. Nutr. 30:910–917.
Gilbert, M., J.W. Sparks, J. Girard, and F.C. Battaglia. 1982. Glucose turnover rate during pregnancy in the conscious guinea pig. Pediatr. Res. 16:310–313.
Gilbert M., W.W. Hay, Jr., R.L. Johnson, and F.C. Battaglia. 1984. Some aspects of maternal metabolism throughout pregnancy in the conscious rabbit. Pediatr. Res. 18(9)854–859.
Gilbert, M., J.W. Sparks, and F.C. Battaglia. 1985. Effects of fasting on glucose turnover and metabolite levels in conscious pregnant guinea pigs. Biol. Neonate 48:85–89.
Grande, F., J.T. Anderson, and A. Keys. 1958. Changes of basal metabolic rate in man in semistarvation and refeeding. J. Appl. Physiol. 12(2):230–238.
Hay, Jr., W.W., J.W. Sparks, B.J. Quissell, F.C. Battaglia, and G. Meschia. 1981. Simultaneous measurements of umbilical uptake, fetal utilization rate, and fetal turnover rate of glucose. Am. J. Physiol. 240:E662–E668.
Hytten, F.E. and T. Lind. 1973. Diagnostic indices in pregnancy. Ciba-Geigy, Basel.
Hytten, F.E. 1980a. Nutrition. Pp. 163–192 in F.E. Hytten and G. Chamberlain, eds. Clinical Physiology in Obstetrics. Blackwell Scientific Publ. , Oxford.
Hytten, F.E. 1980b. Weight gain in pregnancy. Pp. 193–233 in F.E. Hytten and G. Chamberlain, eds. Clinical Physiology in Obstetrics. Blackwell Scientific Publ., Oxford.
Iyengar, L. 1968. Urinary estrogen excretion in undernourished pregnant Indian women. Am. J. Obstet. Gynecol. 102:834.
Johnson, R.L., M. Gilbert, S.M. Block, and F.C. Battaglia. 1986. Uterine metabolism of the pregnant rabbit under chronic steady state conditions. Am. J. Obstet. Gynecol. 154:1146–1151.
Johnstone, F.D., D.M. Campbell, and I. MacCillivray. 1981. Nitrogen balance studies in human pregnancy. J. Nutr. 111:1884–1893
Jones, A.P., and L.S. Crnic. 1986. Maternal mediation of the effects of malnutrition. Pp. 409–426 in E.P. Riley and C.V. Vorhees, eds. Handbook of Behavioral Teratology. Plenum Press, New York.
Jung, R.T., P.S. Shetty, and W.P.T. James. 1980. Nutritional effects on thyroid and catecholamine metabolism. Clin. Sci. 58:183–191.
Kim, Y.J., and P. Felig. 1971. Plasma chorionic somatomammotropin levels during starvation in mid-pregnancy. J. Clin. Endoc. Metab. 32:864–866.
Kitzmiller, J.L. 1980. The endocrine pancrease and maternal metabolism. Pp. 58–83 in D. Tulchinsky and K.J. Ryan, eds. Maternal-fetal Endocrinology. Saunders, Philadelphia.
Knopp, R.H., A. Montes, and M.R. Warth. 1978. Carbohydrate and lipid metabolism. Pp. 35–38 in Laboratory Indices of Nutritional Status in Pregnancy. Report of the Committee on Nutrition of the Mother and Preschool Child, Food and Nutrition Board, National Research Council. National Academy of Sciences, Washington, D.C.
Knopp, R.H., A. Montes, M. Childs, and H. Mabuchi. 1981. Metabolic adjustments in normal and diabetic pregnancies. Clin. Obstet. Gynecol. 24:21–49.
Kreisberg, R.A. 1980. Lactate homeostasis and lactic acidosis. Ann. Int. Med. 92:227–237.
Laga, E.M., S.G. Driscoll, and H.N. Munro. 1972. Comparison of placentas from two socioeconomic groups: I—Morphometry. Pediatr. 50:24–33.
Lawrence, M., J. Singh, F. Lawrence, and R.G. Whitehead. 1985. The energy cost of common daily activities in African women: Increased expenditure in pregnancy? Am. J. Clin. Nutr. 42:753–763.
Lawrence, M., W.A. Coward, F. Lawrence T.J. Cole, and R.G. Whitehead. 1987. Fat gain during pregnancy in rural African women: The effect of season and dietary status. Am. J. Clin. Nutr. 45:1442–1450.
Lechtig, A., J.P. Habicht, H. Delgado, R.E. Klein, C. Yarbrough, and R. Martorell. 1975. Effect of food supplementation during pregnancy on birthweight. Pediatr. 56:508–520.
Lederman, S.A., and P. Rosso. 1980. Effects of food restriction on fetal and placental growth and maternal body composition. Growth. 44:77–48.
Lederman, S.A., and P. Rosso. 1981. Effects of obesity, food restriction and pregnancy on fetal and maternal weight and on body composition in rats. J. Nutr. 111:2162–2171.
Leturque, A., A.F. Burnol, P. Ferre, and J. Girard. 1984. Pregnancy induced insulin resistance in the rat: Assessment by glucose clamp technique. Am. J. Physiol. 246:E25–E31.
Lotgering, F.K., R.D. Gilbert, and L.D. Longo. 1985. Maternal and fetal responses to exercise during pregnancy. Physiol. Rev. 65:1–36.
Mellor, D.J. 1983. Nutritional and placental determinants of fetal growth rate in sheep and consequences for the newborn lamb. Br. Vet. J. 139:307–324.
Meschia, G. 1983. Circulation to female reproductive organs. Pp. 241–269 in J.T. Shepard and F.M. Abboud, eds. Handbook of Physiology: Section II, The cardiovascular system. Volume III, part I. Bethesda, Md.: Am. Physiological Society.
Meschia, G., F.C. Battaglia, W.W. Hay, Jr., and J.W. Sparks. 1980. Utilization of substrates by the ovine placenta in vivo. Fed. Proc. 39:245–249.
Metzger, B.E., V. Ravnikar, R.A. Vileisis, and N. Freinkel. 1982 "Accelerated starvation" and the skipped breakfast in late normal pregnancy. Lancet 1:588–592.
Mora, J.O., B. de Paredes, M. Wagner, L. de Navarro, J. Suescun, N. Christiansen, and M.G. Herrera. 1979. Nutritional supplementation and the outcome of pregnancy. I. Birth weight. Am. J. Clin. Nutr. 32:455–462.
O'Neil, F.T., M.T. Hynak-Hankinson, and J. Gorman. 1986. Research and application of current topics in sports nutrition. J. Am. Diet. Assoc. 86:1007–1015.
Pitkin, R.M., and W.N. Spellacy. 1978. Physiologic adjustments in general. Pp. 1–8 in Laboratory Indices of Nutritional Status in Pregnancy. Report of the Committee on Nutrition of the Mother and Preschool Child, Food and Nutrition Board, National Research Council. National Academy of Sciences, Washington, D.C.
Prentice, A.W. 1984. Adaptations to long-term low energy intake. Pp. 3–31 in E. Pollitt and P. Amante, eds. Energy Intake and Activity. Current Topics in Nutrition and Disease, Vol 11. Alan R. Liss, New York.
Prieto, J.C., and M. Serrano-Rios. 1976. hCS regulation during pregnancy. Obstet. Gynecol. 48:297–301.
Rattray, P.V., W.N. Garrett, N.E. East, and N. Hinman. 1974. Growth, development and composition of the ovine conceptus and mammary gland during pregnancy. J. Ann. Sci. 38:613–626.
Roberts, S.B., A.A. Paul, T.J. Cole, and R.G. Whitehead. 1982. Seasonal changes in activity, birth weight, and lactational performance in rural Gambian women. Trans. R. Soc. Trop. Mod. Hyg. 76:668–678.
Rosso, P. 1980. Placental growth, development, and function in relation to maternal nutrition. Fed. Proc. 39:250–254.
Rosso, P. 1981. Prenatal nutrition and fetal growth development. Pediatr. Ann. 10:21–26,28.
Rosso, P., and R. Kava. 1980. Effects of food restriction on cardiac output and blood flow to the uterus and placenta in the pregnant rat . J. Nutr. 110:2350–2354.
Rosso, P., and S.A. Lederman. 1983. Nutrition and pregnancy in other mammals: A valuable source of data to understand the human situation. Pp. 115–130 in D.M. Campbell and M.D.G. Gillmer, eds. Nutrition in Pregnancy. London: Royal College of Obstetrics and Gynaecologists.
Rosso, P., and M.R. Streeter. 1979. Effects of food or protein restriction on plasma volume expansion in pregnant rats. J. Nutr. 109:1887–1892.
Ryan, E.A., M.J. O'Sullivan, and J.S. Skyler. 1985. Insulin action during pregnancy. Studies with the euglycemic clamp technique. Diabetes 34:380–389.
Schreiner, R.L., P.A. Nolen, P.W. Bonderman, H.C. Moorehead. E.L. Gresham, J.A. Lemons, and M.B. Escobedo. 1980. Fetal and maternal hormonal response to starvation in the ewe. Pediatr. Res. 14(2):103–108.
Shah, A.K., V.J. Rajput, K.K. Bansil, and K.K. Kaul. 1979. Observation on some neonatal anthropometric and gestational characteristics in relation to placental morphology and histopathology . Indian Pediatr. 16:387–394.
Simons, M.A., F.C. Battaglia and G. Meschia. 1979. Placental transfer of glucose. J. Dev. Physiol. 1:227–243.
Soothill, P.W., K.H. Nicolaides, C.H. Rodeck, and H. Gamsu. 1986. Blood gases and acid-base status of the human second-trimester fetus. Obstet. Gynecol. 68:173–176.
Sparks, J.W. 1994. Human Intrauterine growth and nutrient accretion. Sem. Perinatol. 8:74–93.
Sparks, J.W., W.W. Hay, Jr., D. Bonds, G. Meschia, and F.C. Battaglia. 1982. Simultaneous measurements of lactate turnover rate and umbilical lactate uptake in the fetal lamb. J. Clin. Invest. 70:179–192.
Sparks, J.W., J.R. Girard, S. Callikan, and F.C. Battaglia, 1985. Growth of the fetal guinea pig: Physical and chemical characteristics. Am. J. Physiol. 248:E132–E139.
Stembera, Z.K., and J. Hodr. 1966. I: The relationship between the blood levels of glucose, lactic acid and pyruvic acid in the mother and in both umbilical vessels of the healthy fetus. Biol. Neonate 10:227–238.
Steel, J.W., and R.A. Leng. 1973. Effects of plane of nutrition and pregnancy on gluconeogenesis in sheep: The kinetics of glucose metabolism. Br. J. Nutr. 30:451–473.
Trayhurn, P., J.B. Douglas, and M.M. McCuckin. 1982. Brown adipose tissue thermogenesis is 'suppressed' during lactation in mice. Nature 298:59–60.
Tuazon, M.A.G., J.M.A. van Raaij, J.G.A.J. Hautvast, and C.V.C. Barba. 1987. Energy requirements of pregnancy in the Philippines. Lancet 2(8568):1129–1133.
Vernon, R.G., R.A. Clegg, and D.J. Flint. 1981. Metabolism of sheep adipose tissue during pregnancy and lactation. Adaptation and regulation. Biochem. J. 200:307–314.
Viegas, O.A.C., T.J. Cole, and B.A. Wharton. 1987. Impaired fat deposition in pregnancy: An indicator for nutritional intervention. Am. J. Clin. Nutr. 45:23–28.
Widdowson, E.M., and C.M. Spray. 1950. Chemical development in utero. Arch. Dis. Child. 26:205–214.
Wilkening, R.B., and G. Meschia. 1985. Fetal oxygen uptake, oxygenation and acid-base balance as a function of uterine blood flow. Am. J. Physiol. 244:H749–H755.
Wilkening, R.B., F.C. Battaglia, and O. Meschia. 1985. The relationship of umbilical glucose uptake to uterine blood flow. J. Devel. Physiol. 7:313–319.
Ziegler, E.E., A.M. O'Donnell, S.E. Nelson, and S.J. Fomon. 1976. Body composition of the reference fetus. Growth 40:329–341.






















