3
The Global Environment and Its Evolution
ESSAY
A familiar image shows the Earth hanging against the black marbled coldness of deep space. The blues sparkle, barely dulled by patches of brown. Swirling white whorls veil the bright sphere only slightly. Twenty-five years ago that image had not yet been seen. Furthermore, the idea of the Earth as an integral unit was not a prevalent one. Study of the planet proceeded at local or regional scales. Then plate tectonics began to weave regional studies into one planetwide dynamic model.
The past two decades have also seen the emergence of a new perspective in the earth sciences—or to use a more recent term, earth system science—emphasizing changes in the global environment that occur over spans of geological time. The changing environments leave geological evidence that permits investigation of a wide range of geographic, oceanographic, climatic, and biotic transitions. Such evidence includes information about environmental changes that cannot be directly observed today. The record of the rocks reveals that certain factors force changes in the global environment and that some ecosystems are more sensitive than others to those changes.
The surface has been changing for over 4.5-billion-years. Many of these changes are fluctuations within definable extremes. A familiar example is that of water. Sediments record changes in the hydrologic cycle during which rising seas engulfed extensive continental tracts and then drained away. Some of these changes resulted from ice ages that left wide swaths of continental shelf exposed to the air during glacial accumulation and sent torrents to the oceans during intervals of melting. Other cyclic fluctuations recorded in the geological record include geochemical exchanges through reservoirs: atmosphere, ocean, biomass, sediment, crust, and mantle. Ocean basins rise and sink and expand and contract in cycles, and mountain ranges thrust upward and then waste away.
While cyclic changes leave recognizable patterns in the geological record, they continually alter the components that are recycled. Inevita-
bly, new conditions are created by these natural movements. The original state can never be exactly regained. The new conditions are the result of what is referred to as secular change—change with time.
The most obvious secular change that has taken place during earth history is the early transformation of its surface from a landscape of naked rock, barren seas, and toxic atmosphere to a landscape seething with life, with organisms that exist on a variety of scales and in a medley of forms. As the cycles have churned away and new secular changes have occurred, sporadic catastrophic events have thrown the whole dynamic system into chaos.
Geoscientists use an assortment of techniques and instruments to investigate the complex interactive systems that have created the surface environment. A 200-year-old tradition of field mapping and detailed description offers a solid foundation—called ground truth—for new technologies like remote sensing and for new conceptual models such as ones that explain how biological evolution has altered the chemistry of the atmosphere.
Remote-sensing technology is not limited to satellite imagery and geodesic laser measurements; remotely sensed magnetic and gravitational anomalies help trace the vertical and horizontal movements of the crust.
Fine-tuned seismic reflection research has also provided a new way to "see" inside the Earth. Seismic reflection can now produce subsurface images that rival, in an areal extent, drilled cores for locating geological boundaries; the cores, however, are often needed to provide ground truth. Tomography combines sets of seismic reflectance data to create cross sections through various planes.
Mapping also reveals patterns of past environments, ranging from tropical seas to jungles and deserts. These environments are identified by a great variety of evidence, including fossil occurrences, key sedimentary rocks, and the isotopic composition of shells. Maps of fossil occurrences show how organisms were distributed in space and where they lived bears directly on how they evolved.
Once geologists have determined what the patterns are, they can study how those patterns changed; on a planetary scale, this dual effort is at the heart of studies of global change. Geochronology provides the framework for arranging temporal sequences in the geological record. It also provides estimates for rates of chemical, physical, and biological change. Paleontology and stratigraphy led to the first arrangement of geological time scales. Intervals of the Earth's past gained names like Precambrian, Devonian, Cretaceous, and Pleistocene. Today, new quantitative methods for analyzing fossil occurrences are improving our ability to compare ages of strata throughout the world.
Techniques that use naturally occurring radioactive isotopes can now provide dates within a 2-million-year range of accuracy for events that affected particular materials 2.5-billion-years ago. At the other extreme of recency and resolution, close documentation of tree-ring patterns yields chronologies accurate to less than 1 year.
Reconstruction of ancient seas, climates, and continental geography for key intervals of the past provides data for constructing and testing
numerical and conceptual models that portray global circulation patterns for ancient oceans and atmospheres. Such models are shedding light on the agents that triggered ice ages and other shifts to new environmental states on a global scale.
Ancient life has tracked changes in habitats—sometimes migrating, sometimes evolving, and sometimes disappearing from the Earth. In fact, fossils provide the only direct record of large-scale evolution and extinction, and this record can be understood only in the context of past global change. Evolutionary theorists depend on the paleontological record to test their hypotheses, and the application of innovative quantitative techniques is providing new insights into rates, patterns, and modes of evolution.
The unifying theory of a dynamic Earth and the vast scale of coverage provided by satellite imagery complement recent advances in international cooperation at all levels. Geoscientists have organized the International Geological Correlation Program (IGCP) and the Global Sedimentary Geology Program (GSGP) and have helped set up the International Geosphere-Biosphere Program (IGBP). Over the past 25 years, internationally based ocean drilling programs have concentrated on obtaining cores from the ocean floor—nearly three-quarters of the surface—that have improved patchy data and supported stratigraphic correlations with an unexpected degree of success. The drill ship has been to geologists what the telescope is to astronomers, allowing geologists to study the most remote parts of their domain. Such systematic examinations of the Earth are filling in gaps in knowledge as though they were pieces of a giant spherical jigsaw puzzle.
Investigators who correlate the geological information from remote regions like the deep ocean find signs of environmental change that affected the whole planet. Some of these sweeping changes periodically caused widespread extinction of species. The study of such extensive occurrences is called global event stratigraphy. The most familiar example of global event stratigraphy is the evaluation of the iridium anomaly found in widely distributed strata that date to 66-million-years ago. The iridium anomaly may signify a meteorite impact that resulted in the extinction of as much as 66 percent of the species, including all the dinosaurs. The most pervasive extinction occurred 225-million-years ago when perhaps as much as 95 percent of all species died off.
More recently, 75 percent of the giant mammal species that roamed the spacious North American plains south of the Laurentide ice sheet disappeared 11,000 years ago as the ice sheet melted away. This extinction may have had other causes besides the climatic fluctuations that occurred in the wake of the retreating ice front. Additional threats were posed by the hunting skills of human beings.
Anthropogenic factors—those caused by the activities of humans—affect the surface environment on a vast scale. As modern technology offers striking images of a bright blue globe against a black abyss, it also provides evidence of environmental crises, some caused by humankind.
While efforts continue to monitor global change and to attribute the causes to humans or to nature, no consequences can be predicted without
an understanding of the records of past global change. Through the record in the rocks, the past has become the key to the future.
THE GLOBAL ENVIRONMENTS: A GEOLOGICAL PERSPECTIVE
The Changing Land Surface
The processes of weathering, erosion, and soil formation that together degrade upland areas have operated throughout earth history. Variations in the way the processes operate have generally been dominated by climate. Glaciers form in polar or subpolar regions or at high altitudes; deserts develop around 20° from the equator; and rain forests, with their great rivers, grow in equatorial and temperate latitudes.
Against this image it has been surprising to learn from new methods of dating and from quantitative studies of material fluxes that the times in which we live are unusual—not for one but for two reasons. The first is that since Northern Hemisphere glaciation developed about 2.5-million-years ago, fluctuations in ice volume have been large enough to repeatedly change world sea level by as much as 100 m over time scales of tens to hundreds of thousands of years. Despite recent fluctuations, Antarctic ice caps have remained stable for as long as 40-million-years. For this reason the dominant major cause of sea level change during the past 2.5-million-years has been the accumulation and ablation of Northern Hemisphere ice sheets. As an example of fast change, consider that 18,000 years ago, when sea level was about 100 m lower, rivers reached the sea at the edge of the continents. Since then they have retreated so much in response to rising sea level that some river mouths (e.g., the Susquehanna) now lie far back from the continental shelves in estuaries like Chesapeake Bay. Such short-term changes prevent the establishment of an equilibrium in weathering, erosion, and deposition, and during the past 2.5-million-years they have rendered the surface an unusually dynamic place.
We ourselves provide the other reason for unusual conditions. The condition of the soil and processes of erosion have been substantially changed since agriculture began. These changes have increased in recent decades because of such processes as dam building, forest destruction, widespread irrigation, and flood control. For instance, sediments that formerly were carried to the Mississippi delta are now being impounded behind dams; this is contributing to the encroachment of the Gulf of Mexico upon the delta. The problems of marine transgression along the Gulf Coast are discussed throughout this report; here, attention is drawn to progress in understanding the major processes that dominate the change in land surface above sea level, with emphasis on the peculiar problems and opportunities that result from the apparently exceptional time in which we live.
Landforms
Landforms are continually changing, but most changes are subtle and generally escape notice. Although great attention is rightly given to catastrophic events such as earthquakes, volcanic eruptions, and landslides, the time scales of importance in geomorphology—the study of landforms and the processes that shape them—range from seconds to millions of years, and the space scales range from single hillsides to global dimensions. The challenge is to characterize the ways in which landforms respond to both common and uncommon events. The geomorphic record contains information about the ways in which present and past environmental changes have modified the processes operating at the surface, both in intensity and duration. A long-term view is essential because the time spans of contemporary monitoring are too short to represent the range of possible conditions. Long-range perspectives from the geomorphic record permit testing of models of environmental change, whether they apply at global, regional, or local scales.
A landscape can provide information about the magnitudes and return frequencies of natural processes. This information can lead to identification of geomorphological thresholds that precede disastrous events. Some responses are immediate—floods, landslides, and debris flows—but others can be spread over years or decades—upland soil erosion, glacial to interglacial cycles, river-channel change, and sea level change. The rates of some of these processes have been greatly accelerated by human activities.
Geomorphic events of the past, which are recorded in landforms and stratigraphy, can provide usable analogs of anticipated environmental change.
For example, the rate of sea level rise in the next century could become 1 cm per year, a rate not unlike that at which sea level rose at the end of the last ice age. Study of the coasts drowned at that time will provide information relevant both to biotic response to a future sea level rise and to the anticipated acceleration of sedimentation in the lower reaches of river systems.
Geomorphologists and geochemists have been using particular isotopes, produced in the atmosphere and in rocks by cosmic radiation, to determine ages of landforms and to date events such as floods, landslides, fault movements, lava and debris flows, and the onset of glaciation. The application of accelerator mass spectrometry to carbon-14 (14C) dating provides a means of dating samples both older and smaller—by a thousand times—than the type of sample conventionally used.
Various new methods are being developed to determine the age of a landform, measuring the time elapsed either since the rocks forming it were deposited or since they were exposed by erosion. These methods represent a breakthrough in quantifying landform dynamics, permitting more precise dating of key events in the evolutionary development of landforms and yielding erosional rates for the various features of a landscape.
The Himalaya, for example, have long been considered not only the highest but also the fastest rising mountain range in the world (Figure 3.1). Just how fast they are rising is something we are only beginning to understand. Uplift rates may be as great as 5 mm per year—5 km per million years—in places along the front of the mountain belt during the past 20-million-years. Crystals of microcline were eroded from the surface and deposited in the sediments of the Bengal deep-sea fan south of Sri Lanka only a few million years after they became cool enough to stop losing radiogenic argon by diffusion, which occurred at a depth of 5 km inside the mountain belt.
Weathering and Soil Formation
The interaction of the atmosphere and hydrosphere—in the form of groundwater—with the rocks at the surface is very complex, partly because organisms ranging in size from bacteria to trees are involved but largely because the relevant time scales vary so widely. Water has a residence time as short as a single storm and typically cycles on an annual scale; trees have lives of decades to centuries; and the minerals formed in the weathering processes can have residence times in the soil of as high as thousands to millions of years. Varieties of soil are strongly controlled by local climate, and with the growing interest in global change soils are being looked at anew to learn what they record about past climatic changes on time scales from decades to millions of years.
Soils lie at the interface between the geosphere, biosphere, and atmosphere. They have unique properties that derive from the intimate mixing of partly weathered geological substrata, dead organic matter, live roots, microorganisms, and an atmosphere high in carbon dioxide, nitrogenous gases, and moisture. Ions of potassium, sodium, calcium, magnesium, sulfur, and phosphorus are released from minerals by hydrolytic weathering. Many of these ions, as well as carbon and nitrogen, are also released from dead organic matter by microbial digestion. The geological composition of the soil passively influences soil biota through ionic deficiencies or release of toxic elements. The soil biota actively influence weathering rates through respiration and production of organic acids.
Soils are open systems that gain and lose energy and matter as they evolve through time (Figure 3.2). Gains, losses, translocations, and transformations occur continuously in the soil column; the relative magnitudes of these processes determine the types of horizons formed along the column. If the vectors of these processes remain relatively constant, the intensity of their expression in horizons is time dependent. Time-dependent soils are useful for correlating geographically separated geomorphic surfaces. This property of soils has been widely used in studies concerned with the rates and timing of tectonically and climatically driven geomorphological processes.
In the past, most research on rates and pathways of soil development centered on careful chemical, physical, and microscopic dissection of soil horizons developed during known time intervals. Future research will emphasize collection of in situ measurements to document fluxes of gases and solutes, rates of mineral weathering, and biological interactions. Already, these studies reveal the physiology of soil to be modulated by complex feedback mechanisms that are nonetheless subject to catastrophic breakdown under external influences such as fire, erosion, or dramatic climatic change. If we are to understand the contribution of soils to ecological stability, we must also study the effect of anthropogenic disruptions, such as toxic chemical discharges, on soil processes. Soils are great purifiers, but we do not know what level of disruption constitutes a lethal dose.
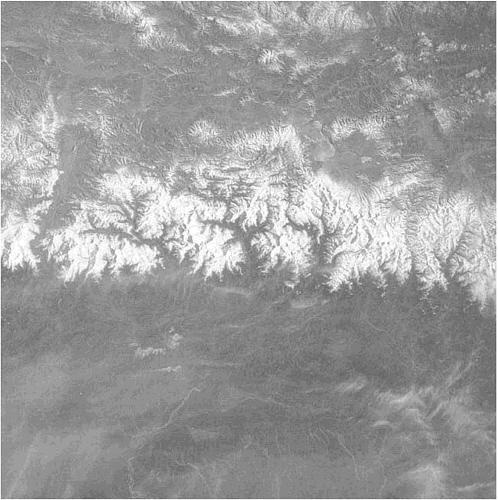
FIGURE 3.1 View of the Himalaya.
Rivers and Material in Transit from Mountains to Sedimentary Basins
The surface is shaped locally by weather. The temperature and moisture regimes, with the weather intensities and rates of change, represent climate. The effects of climate change are becoming more clearly understood through the use of general circulation models. Climatologists, who develop general circulation models to predict the consequences of global change, test their models by comparing results obtained with variables that define past conditions against the evidence found in the geological record. That evidence includes the landforms themselves, sedimentary sequences, and fossil biotas. Closer study of the response generated on the surface by climatic changes that last for short intervals, up to hundreds of years, or long intervals,
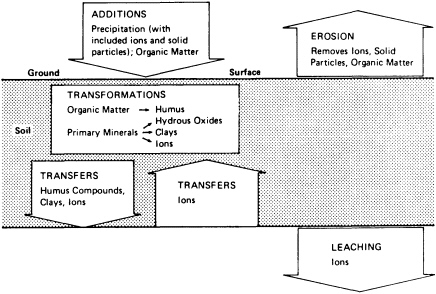
FIGURE 3.2 Flow of the major processes in soil development.
hundreds of thousands of years, allows prediction of how future climatic fluctuations can alter the landscape. For example, the sizes of river channels formed in the past 10,000 years and the character of their contained sediments have been used to reconstruct a history of long-term change in the magnitudes of high-frequency floods in the Upper Mississippi valley (Figure 3.3). That record helps to test general circulation models of climatic fluctuations over the past ten centuries.
Stratigraphic evidence also extends the existing record of river behavior beyond the limits of data collected through observation over the past 100 years. Advances have recently been made in estimating the size and frequency of ancient floods, effectively extending hydrologic records for up to thousands of years, by combining interpretations of river and sediment behavior with results from geological dating methods.
The intensity and sequence of climatic events are crucial factors in molding the landscape into a distinctive form and producing a recognizable geological pattern. The lone occurrence of a moderate flood might accomplish little erosion and deposition, but the clustered occurrence of two or three moderate floods can destabilize entire channel systems and cause them to become sensitive to the erosional effects of even small floods.
Studies of the erosion, transportation, and deposition of sediments in contemporary drainage sys-
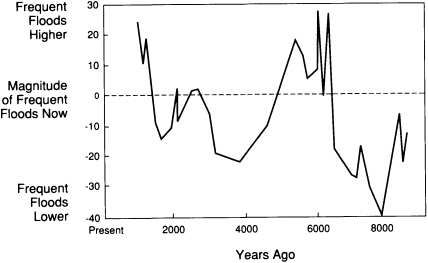
FIGURE 3.3 Variations in the size of annual and biennial floods in the upper Mississippi Valley over the past 9,000 years; the size is expressed as a percentage of the present flood size.
tems are showing departures from the steady state. Modern watersheds are accumulating more sediment than they pass on. In many rivers a large proportion of the sediment is transported by flood events that prevail only a few days of the year. How the climatic conditions that favor these events relate to the magnitudes and frequencies of erosional and transportation episodes is emerging as an area of great scientific interest.
Erosion by other agents, such as glaciation and wind, plays some part in the degradation of topography, but most eroded material is carried to the sea by rivers either in solution or as detrital sediment. River transport varies enormously with both climate and source area; the Huang He (Yellow River) alone, for example, accounts for 6 percent of the world's total suspended matter river load, because this river drains the readily eroded windblown material of the loess plateau in the Chinese interior. In addition, the beginnings of agriculture more than 4,000 years ago appears to have greatly increased the rate at which sediment is moving into the river.
Winds and Glaciers: More Material in Transit
Although most of the material carried into sedimentary basins is transported by rivers, appreciable amounts are carried by wind and glaciers. Deserts, where wind erosion and deposition in the form of sand dunes are most important, have become the subject of increased research activity. This activity has been sparked by such diverse influences as the rapid extension of the Sahara into the Sahel within the past 30 years, the discovery of wind-made landforms on Mars, and the availability of satellite and radar images of the Earth's remote deserts (Figure 3.4). Again, new techniques of age determination have yielded exciting results.
Recent measurements of the thermoluminesence of rocks from surfaces buried beneath dunes in the high plains of the American West have shown that the dunes were moving in response to desert winds much more recently than had previously been realized. Windblown dust mixed into the deep-sea sediments of the North Pacific helps to show how continental climatic fluctuations in China relate to the orbitally induced climatic changes that are well known from the deep-sea record. On a longer time scale, windblown dust in sediments from the deep Atlantic indicates that the Sahara first became a huge desert about 10-million-years ago, possibly as a result of changes in atmospheric circulation related to the uplift of the Tibetan plateau.
Glaciers and glacial deposits reflect the tremendous fluctuations in the climate of the current ice age. Only 20,000 years ago glaciers extended as far south as New York City, and there may have been as many as a dozen comparable advances and retreats of Northern Hemisphere ice during the past 2.5-million-years. A new research effort to integrate continental and oceanic data from the past 2.5-million-years should produce a picture of how the surface environment adapts to rapid climate change. Glacier ice provides a unique record of short-term change, which is discussed in the part of this chapter dealing with cyclical change.
Lakes: Interruptions in Transit
Lakes represent a peculiar part of the earth system. If the solar-driven heat engine entails erosion of mountains, transit of eroded material from the mountains to the sea, and deposition of sediments at the edges of the continents and on the ocean floor, then lakes represent an interruption to the smooth flow of the system. As such, lakes are usually quite short lived because they fill with sediments. The familiar outlines of North America's Great Lakes are less than 20,000 years old and are unlikely to last more than another 20,000 years. Only a few of the world's existing lakes are more than a million years old, and the oldest, Lake Baikal in Siberia and the Great Rift Valley lakes of East Africa, are found in places where the continents are being ripped apart by tectonic forces.
Lakes and lake sediments provide crucial information about climatic change during the past 2.5-million-years. In recently glaciated areas, some lakes have produced annual cycles in sedimentary layers called varves. Some of these varve-layer exposures indicate thousands of years of continuous deposition. The most productive method of retrieving environmental information from lake deposits is the analysis of fossil pollen, which provides a record of changes in nearby vegetation. In tropical areas, lakes expand during intense monsoonal episodes, while in temperate latitudes high lake levels may indicate more rain or less evaporation. Fluctuations in shoreline elevations are used with the pollen record to reconstruct recent environmental changes.
The long-term rock record contains scattered evidence of ancient lakes; the oldest of these is more than 2-billion-years old, half as old as Earth itself. The study of old lake deposits has become a major research activity. Interest has been stimulated not only by studies of the geologically recent lakes but also by the discovery that lake beds are commonly
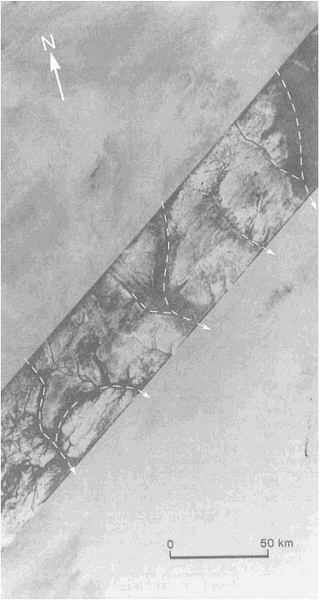
FIGURE 3.4 SIR-A radar scan (diagonal band) reveals aggraded valley segments that were barely perceptible on Landsat images of eastern Sahara in northwest Sudan (19.7°N, 25.2°E).
a source of petroleum—an idea promoted by Chinese geologists and now broadly accepted. Some of the finest remains of early humans have been found around the shores of old lake beds, and often the richest sites for dinosaur fossils are associated with the rocks along ancient shores.
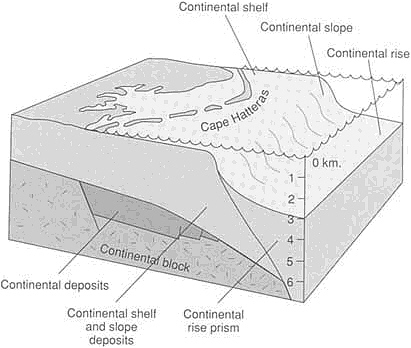
FIGURE 3.5 Atlantic-type (passive) continental margin along the eastern United States. Continental deposits accumulated in fault-bounded basins as the early stages of rifting separated North America from Africa. When the continents separated enough so that seawater could enter to form the Atlantic Ocean, marine deposition commenced.
Deltas and Estuaries
Rivers reach the sea in deltas and estuaries. Deltas form at river mouths when the prevailing current becomes too slow to carry detritus, so that enormous amounts of sediment are dumped close to the continental margin. Where deltaic deposition has continued for tens of millions of years, deltas have extended onto the deep ocean floor. Huge petroleum resources in the Mississippi, Niger, Orinoco, and other deltas are currently being developed, and related understanding has contributed to the way in which deltas are perceived from the complementary resource, environment, and hazard viewpoints.
Estuaries, in contrast to deltas, are broad embayed river mouths that have been flooded since the end of the most recent glaciation when sea level rose and the river's sediment load diminished. Water circulation in estuaries is much more restricted than in the open sea, and, as a result, sensitivity to environmental modification is very high. For this reason estuaries are important targets for interdisciplinary research in biogeochemical dynamics.
Beneath the Sea
The large-scale structure of the ocean basins has been established by the operation of the Earth's internal heat engine, which causes rupture and drift of continents and island arcs, formation of new ocean floor at spreading centers, and establishment of new arc systems where plates converge. The operation of these processes leads inexorably to the opening and closing of oceans, to island-arc and continental collisions, to the assembly of continents, to the addition of new arc material to existing continents, and to recycling of both ocean-floor rocks and continental material into the mantle. Solar heat modifies the ocean floor mainly by deposition of detrital sediment eroded from the land and by precipitation of calcium carbonate and silica from oceanic waters—partly by marine organisms—to form limestone and chert. Together, these processes degrade the thermally generated submarine relief, not so much by erosion, the process dominant above sea level, as by deposition that smooths the topography.
Deposition at Atlantic-Type Margins
Sedimentary deposition below sea level is controlled primarily by the tectonic framework of the ocean basins. The largest volumes of sediments accumulate at the rifted continental margins, called Atlantic-type margins because they are best developed around the Atlantic Ocean (Figure 3.5). The most rapid additions to these types of margins at present come from rivers draining the world's largest areas of high elevation—from the Mississippi and Mackenzie rivers that drain western North
America and from the Ganges and Indus rivers that drain the Himalaya and Tibet. Accumulations of rock as much as 15 km thick can develop along Atlantic-type margins. These, the greatest thicknesses of sediments in the world, are accommodated by subsidence as the lithosphere—thin and hot at continental rupture—cools and thickens. The load of accumulating sediments depresses the lithosphere farther and amplifies this subsidence.
Broad continental shelves extending for many tens of kilometers from the edge of the ocean at depths of only a few tens of meters below sea level are characteristic of Atlantic-type oceanic margins, because of deposition by powerful river systems and perhaps because of extensive lithospheric thinning at the time of continental rupture. Those off the coast of New England and the Alaskan coast of the Bering Sea are typical. The recent sea level changes that mark responses to glaciation and deglaciation have led to repeated erosive episodes of the unconsolidated sediments of continental shelves. Large masses of such sediments have been off-loaded through submarine canyons onto deep-sea fans and abyssal plains.
Limestone shelves develop where there is little sediment eroded from the land and in areas of abundant biological activity. Sediments originate mainly from the calcareous skeletons of shallow-water, bottom-dwelling marine organisms. These limestone shelves respond to sea level change in a way very different from sand and mud shelves. When sea level falls, exposure to fresh water as rain or runoff produces cementation that binds the loose skeletal sediment. As a result, carbonate-dominated banks and shelves reflect conditions of deposition during high stands of the sea and of subsequent cementation during low stands. Much of Florida and all of the Bahama banks were produced by this process.
Submarine canyons carry sediments from the continental shelves to the deep oceans and the submarine fans, where that sediment settles. When they were first recognized about 50 years ago, the prime question asked was how such enormous features could form. Computation of the huge volume of sediments in the fans showed that turbid sediment-laden flows pouring from the continental shelves in times of glacially controlled low stands of the sea could have readily carved even the greatest of submarine canyons, many of which are much larger than the Grand Canyon. Modern research on both submarine fans and canyons is accelerating because of the availability of new instrumental capabilities. Deep-sea drilling, multibeam echo sounders, side-look scanning sonars, and manned and remotely controlled submersibles are providing a much more detailed picture than was formerly obtainable.
Research on the sedimentary development of the Atlantic-type margins has expanded enormously during the past decade, largely in response to two stimuli: an appreciation, following the plate tectonic revolution, of how continental rupture happens and an understanding of how the sediment wedge at the continental margin evolves through time. The latter owes much to oil exploration, which led to the development of the technique of sequence stratigraphy, where coherent packages of distinctive strata in reflection seismic data—calibrated against the record of local oil wells—can be used to establish a detailed history of the transgression and regression of the sea. Lively controversy persists as to exactly how and whether the seismic stratigraphic records can be linked to global sea level fluctuations.
Deposition at Convergent Plate Boundaries
The greatest variations in topographic relief are produced at convergent plate boundaries. The giant peaks of the Himalaya, 8 km high, result from the collision of India with Asia, and the 11-km extreme of oceanic depth is found in the Marianas Trench, where subduction is carrying the Pacific plate into the mantle. Two dominant sedimentary depositional environments are associated with these immense topographic contrasts: trenches and foreland basins.
Subduction-zone trenches contain substantial sediment accumulations only where the supply of sediments is large enough to exceed the rate of removal by subduction (Figure 3.6). This type of accumulation is occurring, for example, at the eastern end of the Aleutian Trench close to sediment sources in the North American continent and at the southern end of the Lesser Antilles Trench close to the South American continent at the mouth of the Orinoco River. Sediments accumulate at the front of the related arc system to form a thick wedge, or accretionary prism, that extends along the trench. An exciting challenge—currently being addressed by deep-sea drilling, multibeam echo sounding, and other new techniques—is to establish exactly how unconsolidated sediment entering a deep-sea trench becomes solid rock in the accretionary prism, a process that involves both intense deformation and the expulsion of vast quantities of water.
The other sites of substantial sediment accumulation in convergent plate boundary zones are the
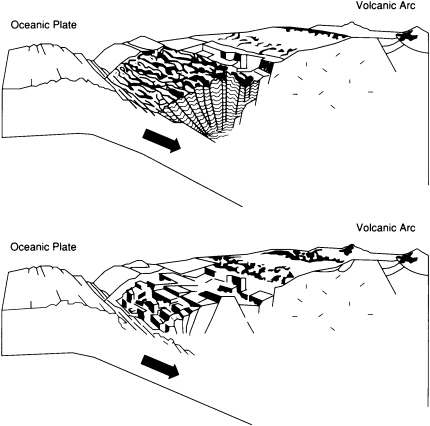
FIGURE 3.6 Accretionary (top) and erosional (bottom) end-members of convergent margins. Accretionary prisms form only where sufficient sediment is supplied to a trench.
foreland basins that develop next to continental margin mountain belts in areas where the load of the mountains depresses the lithosphere profoundly. Sedimentary thicknesses in the foreland basin depressions are huge and may rival those of the Atlantic-type margins, especially in places where the mountains are being actively thrust over the basin. At present, many of the best-developed foreland basins are accumulating sediments above sea level. This is the case in the finest examples of all, the foreland basins lying to the east of the 5,000-km-long Andean chain, which has been rising for the past 3-million-years.
During most of the geological past, when sea level was higher, foreland basins accumulated marine sediments. Recent research in foreland basins emphasizes an integrated approach that models how episodes of uplift in the mountain belts modify sediment supply and interact with sea level changes and with thrusting of the mountain load over the basin. The methods of sequence stratigraphy address these problems. Further pursuits in oil exploration have led to studies of how fluids migrate through the foreland basins for distances of up to hundreds of kilometers.
Deposition in the Deep Ocean
Very little of the material eroded from the continents reaches the central areas of the oceans, and much of what does is in the form of windblown dust. In these remote regions, far from the continents, accumulation of the skeletons of microorganisms that live in the oceanic waters dominates the depositional process. While calcareous skeletons are most important at shallower depths, they dissolve in the deepest and coldest waters faster than they can accumulate. Beneath the deepest waters siliceous skeletons form a significant part of the sediment pile.
The calcareous sediments located around the ocean's abyssal plains are proving to contain an astonishingly informative record of the history of the water masses of the world ocean. The oxygen-isotopic compositions of the skeletons of foraminifera, which make up most of the calcareous oozes, reflect the isotopic composition of the water in which they lived. The ratios between different isotopes reflect the size of the world's ice sheets and the temperature and salinity of the water in which the organisms grew. Cores from the deep seafloor show
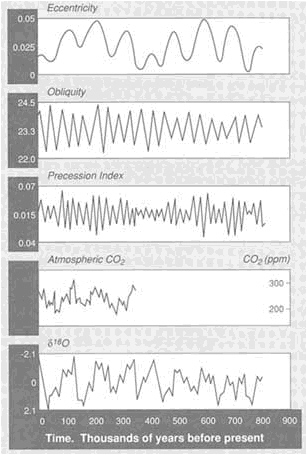
FIGURE 3.7 Variations in insolation caused by changes in the Earth's orbital parameters (eccentricity, obliquity, precession) can be correlated with variations in fossil atmospheric CO2 contents (from ice sheets) and global ice volume (as determined from δ18 O of ocean sediments).
isotopic variations that record the history of the ice ages and yield persuasive evidence of relations between ice ages and the Earth's orbital parameters (Figure 3.7).
The deep-sea sedimentary record reveals long-term alterations in oceanic circulation that reflect changes in the way the Sun's energy has been dissipated in the oceanic waters. Cycles of change, secular variations, and catastrophic perturbations of the system can all be discerned in this remarkable record.
CHANGE IN THE GLOBAL ENVIRONMENT
Interest in global changes focuses especially on time scales of decades to centuries and in attempts to characterize human-induced change and to discriminate between that change and natural variability.
The geological record provides a unique perspective on change because it extends back for more than 3.5-billion-years. This is the extent of the cumulative record built up from shorter individual sequences of rock, each of which rarely spans more than a few tens of millions of years. The overall record is integrated from numerous and disparate sources by correlation. In ancient rocks the precision of correlation is poor, with deviations exceeding a million years, but in younger rocks it is more reliable. Because of new dating techniques, all anchored to time scales based on the decay of natural radioactive isotopes, better temporal resolution in both young and old rocks can now be attained.
The most recent past preserves the best record, so study of the youngest parts of earth history is intense. But high-resolution evidence of annual and more frequent events has been preserved in very old rocks. There are annual layers in ancient lake beds dating to more than 2-billion-years, and 14-day tidal cycles have been discerned in rocks 700-million-years old. But even global correlation cannot patch together a complete record spanning the more than 4-billion-years of active geological processes. Many of the processes acting in the system are as likely to destroy the record as to preserve it. Comparisons of sediment accumulation rates in modern environments with the isotopically dated rates for ancient intervals in similar environments have always shown that the preserved sediments could have been deposited in a fraction of the measured interval. The apparent rates are much lower than modern ones because the duration represented by a preserved section includes times of nondeposition and erosion as well as deposition.
Herein lies a unique challenge of the earth sciences—the lack of conspicuous evidence can be a clue, as well as the less subtle direct testimony. When expected evidence is missing from the record, that absence poses new questions and suggests new mysteries about the geological processes at work.
Cyclical Change in the Global Environment
One way of looking at the geological record is to consider it as preserving evidence of cyclical processes. The rise and fall of sea level take place on time scales ranging from days to tens of millions of years; superimposed on these cyclical changes are noncyclic or secular changes, such as biological evolution, that have been occurring throughout earth history. The way in which the cyclical processes operate has been modified to some extent by secular changes, but it is one of the more exciting
recent developments in the study of earth history that so many of the cyclical processes have changed very little since the formation of the earliest preserved rocks.
A cyclical change can be chaotic but fluctuates between two or more distinct extremes. Time elapses during all cyclical processes, so when the cycle is complete conditions can never be identical to those at the start, but the repetition of similar phenomena is usually considered a cycle. The rock record contains evidence of cycles operating on daily scales, such as tidally controlled sedimentation; annual scales, such as varved lake deposits; and tens to hundreds of thousands of years, such as cycles controlled by variations in the Earth's orbit around the Sun. Sea levels change over millions of years due to variations in the average age of the ocean floor; ocean basins open and close over tens to hundreds of millions of years; and the isolation of major compositional reservoirs in the mantle may be the result of cycles that take billions of years.
These cycles involve transfers of energy and material among reservoirs of different sizes. Materials can be concentrated and even isolated in individual reservoirs for varied intervals. Usually the longer the interval the larger the reservoir. The largest parts of the Earth constitute the largest reservoirs: the core, mantle, crust, asthenosphere, lithosphere, hydrosphere, atmosphere, and biosphere.
The idea of cycles in earth activity is at least 200 years old. However, the strength of the cyclical concept has been fully realized only since we have come to appreciate the Earth as a complex system consisting of a multitude of interacting subsystems. Its recently achieved strength stems from new analytical and observational capabilities that allow characterization of the discrete reservoirs within the earth system in a way that was not formerly possible. Modern data-handling and especially computational capabilities are essential for the interpretations now being made.
The Rock Cycle
The processes operating at the surface constitute part of an enormous cycle. Mountains form and material is eroded from them, which is eventually deposited in basins. The sediments in the basins become caught up in new mountain building and are then eroded themselves to repeat the cycle. This is the rock cycle, and it involves interaction among all the outer parts of the Earth—atmosphere, ocean, biosphere, crust, and upper mantle.
Arbitrarily, we can start the rock cycle at the surface with weathering: liberation of a chemical element to solution and its inclusion within secondary minerals. During weathering some elements are also exchanged with the atmosphere, a process involving the active participation of plants and soil biota. The liberated element is carried in solution, together with eroded particles traveling in suspension, by streams that eventually empty into the ocean. Here it is deposited on the seafloor, either as eroded and transported particles or as part of a newly formed precipitate from seawater, which is often biological in origin, such as a calcium carbonate seashell.
Materials accumulate on the seafloor as sediments. Upon burial these become sedimentary rocks, which may themselves become involved in mountain-building processes. They may be transformed into metamorphic rocks by increased temperature and pressure or into igneous rocks by partial melting. Igneous material erupts onto the surface of the continents or onto the seafloor to form volcanic rocks, which can react with seawater to change the chemical composition of both. Other igneous material may crystallize slowly at depth to form plutonic rocks. During the formation of igneous and metamorphic rocks, volcanic gases migrate upward to the surface to enter the atmosphere. Sedimentary, metamorphic, and igneous rocks are uplifted by tectonism into the zone of weathering to begin the cycle anew. The process entails weathering, erosion, river transport, deposition, burial, metamorphism, melting, volcanism, degassing, uplift, and weathering again—a rock cycle that has been repeated many times during the history of the Earth.
Plate tectonics and hydrologic processes combine to drive the rock cycle at rates that vary over a wide range. This results in complex interconnections between very different earth processes. For example, at various times in the geological past—millions to billions of years ago—changes in the level of atmospheric carbon dioxide (CO2) may have resulted from increases in the rate of degassing of CO2 by volcanic activity and from decreases in the uptake of CO2 by rock weathering. An increase in atmospheric CO2 would have tended to elevate global temperatures through the greenhouse effect, which in turn might have influenced the evolution of life. There is some evidence that about 100-million-years ago just this situation developed. As interest grows in long-term changes in atmospheric CO2 content, a parallel research effort is focusing on short-term cycles on the order of tens of thousands
of years. These changes are well correlated with variations in the Earth's orbit around the Sun.
Orbital Cycles
It was recognized in the nineteenth century that variations in the Earth's orbit would cause changes in incoming solar radiation that could be important in controlling ice ages. Theoreticians first calculated how these variations would interact, and the study of deep-sea sediments has yielded persuasive evidence that the recurring ice ages of the past million years are indeed closely associated with orbital cycles. These cycles cause subtle changes, particularly in high latitudes, in the seasonal variation of the incoming solar radiation, called insolation, and may be reliably calculated from celestial mechanics. The ice ages themselves are recorded in the ratio of oxygen isotopes in deep-sea sediments. This is because the elevated fraction of light isotopes in fresh water evaporated from the ocean surface and stored in ice sheets is reflected by an increased fraction of heavy isotopes in the precipitated carbonate skeletons of microorganisms living in the remaining ocean water. Variations in the oxygen isotope (18 O/16O) ratio with depth in a sediment core are widely interpreted as indicating total land-ice volume as a function of time.
The glacial record, as revealed in ice cores, sedimentary sequences, landforms, and other related phenomena, is especially useful for understanding past changes and anticipating the characteristics of future changes. In some instances, fossil pollen and other specific environmental indicators are also present in stratigraphic records.
As better cores are examined and dating procedures are refined, it has become apparent that the changes in insolation correlate with subsequent changes in ice volume. However, a correspondence between the two records requires allowance for the slow buildup of ice sheets over several tens of thousands of years in contrast with their relatively rapid decay, which introduces a degree of nonlinearity into the system response. Presumably the periodic changes in insolation are the ultimate cause, but the precise mechanism remains obscure. The presence of ice, however, does not appear essential for a cyclical response. Recently compiled geological records of 200-million-year-old lake sediments in the eastern United States show a sequence of cycles of approximately the same intervals as the present orbital cycles, spanning a period of 40-million-years. These lakes were then in the tropics. No evidence of continental glaciers or sea ice exists for this period, but local climate and lake levels were apparently influenced by a strong stable external control.
The relationship of the orbital cycles to climatic variation is a fertile research field. The deep-sea sediment record indicates that about 900,000 years ago the governing periodicity of cycles switched from 40,000 years to 100,000 years. This sudden change has not yet been explained. High-resolution records of the glacial cycles come from fast-sedimentation-rate deep-sea cores, from cores in the Greenland and Antarctic ice sheets, and from cores of mountain glaciers at low and mid-latitudes. Cores from the Vostok station, high on the Antarctic ice cap, have extended the record back to about 160,000 years ago, so a remarkably complete record is now available of how temperature varied through the whole of the last glacial cycle. Analyses of air bubbles in ice cores show that temperature and atmospheric CO2 content generally varied sympathetically. Geologists are currently investigating whether orbital variations drove the system and whether changes in oceanic circulation and biology affected the atmosphere's trace gas content, amplifying the climatic oscillations.
Abrupt changes in environmental conditions are recorded in the Greenland record, where the Dye-3 core indicates a switch from glacial to interglacial conditions within one century. Some researchers suggest that such sudden changes during the last glacial period could have been triggered by major diversions of meltwater draining from the Laurentide ice sheet. In a more recent time frame, dust and oxygen isotopic records associated with an ice record from the Peruvian Andes indicate that local transition from the Little Ice Age to current conditions could have occurred about 100 years ago and in as short a time span as a few years.
These examples show that large climatic changes can occur on many time scales, including those of critical relevance to modern society. Climate change may be initiated by variations in atmospheric and oceanic circulation patterns driven by feedback connections to other terrestrial environmental factors, such as changes in vegetation cover or in physical composition of the atmosphere influenced by volcanic or human activity. Therefore, the geomorphic history, geographic distribution, and rates of glacial advances and retreats need to be documented to permit understanding of the interconnected global associations of environmental change and to seek causal connections. These data can provide very important independent tests of the atmospheric general circulation models (GCMs)
that are used to estimate consequences of future environmental change.
The most recent million years of earth history offer exceptional opportunities to reconstruct global environments. For the latter part of this interval, geological and human time scales overlap, and under certain circumstances radiocarbon dating can be pushed back to several tens of thousands of years before the present. Radiocarbon dating applies directly to fossil material, providing remarkably accurate dates for certain biotic events of the recent geological past. The vast majority of species that have lived during this interval are alive today, so that detailed knowledge of their ecological traits in the modern world can be used to reconstruct ancient environments. Such details help to improve the resolution of research on environmental change.
Deep-sea cores provide an extensive record for this interval, with radiocarbon dates scaling a chronology for the most recent few tens of thousands of years. Studies of tree rings, pollen sequences in lake and estuarine sediments, fossils of terrestrial insects, and cave deposits supply even greater detail, offering high-resolution for events that have occurred within the past 10,000 years. For the most recent past, ice cores from arctic and alpine glaciers exhibit yearly bands that allow events to be traced back from the present with a level of resolution that approximates 1 year. The record of this historic and barely prehistoric past reveals global events that produce very rapid change, not only when scaled against traditional geological chronologies but even when scaled against a human lifetime.
Remote sensing, the set of processes by which we observe large areas of the Earth from outer space, also provides valuable information for assessing environmental changes of the recent past. Combinations of different perspectives and frequent coverage inform us about rates of tectonism, volcanism, and other processes that have altered landforms. The images display the effects of past and ongoing climatic changes. For example, radar and laser altimeter data indicate the degree to which alluvial fans in arid regions have weathered and become mantled with windblown sediments, and visible, near-infrared, and thermal infrared imaging spectrometers reveal the degree to which exposed rocks and sediments have weathered to become clay minerals. Remote sensing also detects recent migration of sand dunes and changes in the levels of ancient lakes, both of which testify to climatic change. Satellite technology helps in distinguishing how much current deforestation, beach erosion, and desertification is a product of human activities and how much the result of other natural causes.
Cycles of Sea-Level Change
The level of the world's oceans has oscillated up and down over a range of more than 300 m in the course of earth history (for at least the past 100-million-years or so). Because continents generally slope gradually toward their margins, high stands of sea level sometimes have spread shallow marine waters rapidly over broad continental areas. In fact, for most of the time during the past 600-million-years, sea level stood higher than its present position, so that continents were more extensively inundated. For this reason, large volumes of ancient marine sediments and sedimentary rocks are currently exposed on modern continents.
Several factors control sea level. The growth or shrinkage of ice sheets cause seas to rise or fall as much as 100 to 150 m at rates that might have exceeded 10 m per 1,000 years. The desiccation of isolated ocean basins may generate sea level fluctuations at the same rate, but only within a range of about 15 m. Spreading rates of mid-ocean ridges produce much larger changes (in the range of 300 m) but more slowly—at rates of only 1 cm per 1,000 years or less.
Even changes in sea level of only a few meters leave their imprint in the rock record. In tropical areas of limestone deposition, for example, calcareous algae and other sediment producers tend to maintain the floors of shallow marine carbonate platforms within a few meters of the sea surface. A small lowering of sea level exposing the platform will terminate sediment deposition, subjecting these deposits to subaerial weathering that will be recognizable hundreds of millions of years later. Conversely, a rapid rise in sea level by several meters can virtually halt shallow-water carbonate production because such a sea level rise will exceed the rate at which carbonate-producing algae can produce sediment. Thus, minor fluctuations in sea level can leave imprints in the stratigraphic record. In ancient rocks deposited in lakes and on carbonate platforms, geologists are now recognizing cycles that seem to match distinctive orbital periodicity. It may be appropriate to invoke climatic forcing from orbital variations, even without evidence of glacial activity.
Deciphering sea level change in the rock record is currently one of the most active areas of geological research. Here a primary new tool is sequence stratigraphy, which uses seismic reflection data to study the spatial relationships and, indirectly, the
temporal relationships of sedimentary strata lying below the surface. Petroleum geologists are the pioneers in this field and use seismic data obtained from modern continental margins to interpret the history of global sea level change. The focus has been on the Atlantic-type margins, which form when continents rift apart. Once ocean spreading has carried a continental margin far from the uplifted center, tectonic subsidence becomes very slow. The structurally quiescent margin serves as a yardstick against which global sea level change can be measured.
Sequence stratigraphy makes use of the discovery that buried surfaces, appearing as lines in reflection profiles, have been formed simultaneously. Many appear to mark brief interruptions in deposition when compaction or lithification took place. The configuration of these surfaces, which can represent both subaerial and submarine topography, depicts the fluctuation of ancient shorelines through time. When adjustments are made for subsequent compaction and subsidence, positions of ancient shorelines reveal past positions of sea level. The chronology is established primarily by the study of microfossils recovered from well cores drilled in the area of the seismic data.
Because modern ocean basins and continental margins are no older than about 200-million-years, sea level analysis for older rocks using sequence stratigraphy focuses on rocks exposed on continents. Here other methods help. Deposits whose sedimentary characteristics and fossils represent very shallow marine conditions provide indications of past sea levels, as do unconformities that represent intervals of subaerial exposure.
Some indices of ancient sea levels in the rock record are ambiguous, so that the sea level curve for the past 600-million-years is under constant revision. The configuration of this curve has great significance, in part because the total area occupied by shallow seas has a controlling influence over certain climatic conditions. Shallow epicontinental seas supply moisture to surrounding terrestrial regions. They also absorb sunlight and conserve heat because they do not mix extensively with cool deep waters and because the heat capacity of water is so high that they do not lose heat rapidly to the atmosphere. Because of their thermal stability, shallow seas—like large lakes—moderate temperatures in nearby terrestrial regions.
Cycles of Opening and Closing of Oceans
Once earth scientists became aware of the plate structure of the lithosphere—the outer rigid layer of the Earth—it was a short step to recognizing that some of the world's oceans are opening and others are closing. Opening oceans, such as the Atlantic, have active spreading centers but lack major convergent plate boundaries. Closing oceans, such as the Pacific and the Mediterranean, are bounded largely by convergent plate boundaries. Researchers concluded that perhaps earth history operates within a framework of complex cycles of opening and closing ocean basins. The evidence for these ocean cycles is found in the sediments deposited in the oceans and along the ocean's margins. The depositional evidence of ocean development depends on the solar-atmosphere-ocean system and its associated latitudinal patterns of temperature and rainfall, which profoundly affect erosion and its consequences.
A continent drifting latitudinally records its transit in the character of its sediments. Use of these paleolatitudinal indicators—as well as magnetic evidence that can track paleolongitudinal drift—has allowed the construction of maps (Figure 3.8) that show the distribution of continents and oceans in the past. The record farther back than about 500-million-years ago is too patchy at present to justify the construction of world maps, but evidence from the better-known areas, such as North America, is consistent with the idea that similar ocean-opening and -closing processes were operating in the remote past.
Geochemical Cycles
Ingenious sampling methods often assist in estimating the distribution of individual elements, compounds, and nuclides among the various terrestrial reservoirs. Patterns are readily discernible: iron and nickel are concentrated in the core and volatile elements in the atmosphere and ocean. Many of the distribution patterns appear to be basically simple. But the study of how concentrations within discrete reservoirs change with time promises valuable information about how the Earth's chemical systems behave.
Isotopes of the same element, particularly radioactive isotopes, that travel by different paths through the reservoirs to a common end, can be especially informative about geochemical cycling. The change in the strontium isotopic ratio (87Sr/86Sr) of seawater as recorded in marine shells is an excellent example.
87Sr is a stable nuclide continuously formed by the decay of radioactive rubidium (87Rb). Like the other alkali metals, rubidium has become concentrated in
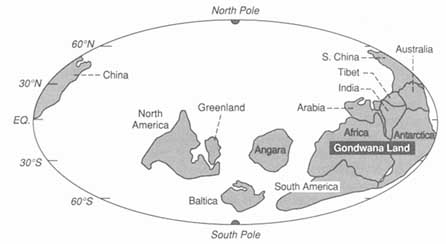
FIGURE 3.8 Map showing how the continental blocks were distributed on the Earth's surface about 530-million-years ago. The ability to construct high-resolution maps of this kind allows integration of geological information and the construction of models embodying a range of paleoenvironmental data. Numerous, now widely dispersed continental blocks were assembled in the great continent of Gondwanaland at this time.
the continents through the repeated partial melting processes that fractionate the mantle. 68Sr is a stable nuclide that remains more strongly linked to the mantle. The 87Sr/86Sr ratio of seawater has varied with time (Figure 3.9) over the past 500-million-years. The variation can be attributed to changes in the relative influence of erosion from the continents, which promotes concentration of the rubidium daughter 87Sr in seawater, and of volcanism beneath the sea, which samples the mantle and promotes the concentration of 86Sr. With the fall of sea level over the past 100-million-years, the continental contribution has generally been rising. A serendipitous application of the change in 87Sr/86Sr of marine shells is that stratigraphers are using the ratio to date sedimentary rocks.
A more familiar example involves the cycling of carbon and oxygen. The concentration of CO2 in the atmospheric reservoir has risen rapidly in recent decades. These concentrations are usually in chemical equilibrium with dissolved CO2 and bicarbonate ions in the ocean waters and with calcium carbonate in the oceanic sediment reservoir. The cyclical transfer of CO2 through these three reservoirs appears to be considerably perturbed by the rapid rise in the atmospheric component; model simulations indicate that it could take hundreds of years to restore equilibrium to this subsystem.
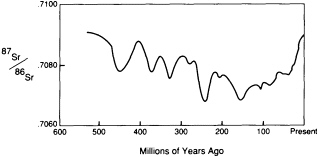
FIGURE 3.9 Variations in strontium isotopes of seawater.
The geological record offers information about a past scenario that involved disequilibrium among these same three reservoirs. About 100-million-years ago there was about twice as much underwater volcanism as there is at present because seafloor spreading was more rapid and the total volume of the oceanic ridges was about twice what it is now. This condition tended to perturb the CO2 cycle in two ways. The extra volcanism added CO2 to the ocean and the extra volume of young hot rock on the ocean floor displaced the oceanic waters so that they flooded the continents to an exceptional extent, thus reducing the area of rock available for the weathering that extracts CO2 from the atmosphere. There is a strong likelihood that the CO2 content of the atmospheric reservoir rose in response to these perturbations. The evidence indicates that climatic conditions were warmer than today, as the greenhouse principle would suggest.
A comforting general observation is that feedback mechanisms will come into play to ameliorate any extreme consequences of perturbations to cyclical processes. The shallow-waters of the flooded continents (some 100-million-years ago) were an ideal environment for the deposition of limestone, and the process of limestone deposition pulls CO2 out of the atmosphere and processes it through the oceanic waters into the rock reservoir. This feedback system would have brought the cycle back to a more normal state.
The geochemistry of carbon is uniquely exciting, primarily because of carbon's role in life. Carbon also forms economically important resources, in-
cluding oil, coal, and diamonds. And 14C, a radioactive isotope with a half-life of 5,000 years, is the most important means for timing environmental events over the past 40,000 years. The existence of two readily fractionated stable isotopes and a single short-lived radioactive isotope, along with the preservation of carbon from a wide range of environments throughout the geological record, means that interpretation of the geochemical cycles of carbon is particularly informative. The resulting understanding of the rates of past changes allows researchers to assess ongoing changes.
The carbon cycle has played a major role in the development of the global environment. In any body of water, dead organic matter settles to the bottom where animals and bacteria have a chance to oxidize the contained carbon. But mud and the minerals produced by organisms also settle to the bottom. Their accumulation may be rapid enough to trap and bury organic material before it can be consumed by oxidation, sometimes preserving it to become fossil fuel. The abundance of fossil fuels and other organic debris in the sedimentary shell is considerable, and every atom of that organic carbon, as it was buried, left behind a molecule of O2 that was released into the surface environment.
Balancing the accounts should be possible. From the inventory of elements in sedimentary rocks, we should be able to calculate the amount of oxidizing power that the buried accumulation of organic carbon left behind at the surface—and also the timing, or history, of the accumulation. There are two problems. We cannot collect samples of all buried rocks for analysis of carbon content, and even if we could take a perfect inventory of all existing sedimentary rocks we could not account for rocks destroyed by erosion, altered by metamorphism, or subducted into the mantle.
There is another way to approach the accounting. The problem can be restated usefully by asking: What fraction of the carbon passing through the system has been buried in the form of organic material? This turns out to be a question that we can answer with the help of the two stable carbon isotopes, 12C and 13C. The 12C isotope is more abundant, amounting to 98.895 percent of all terrestrial carbon; most of the remainder is the 13C isotope. Because both isotopes are stable, their abundances have not changed throughout earth history. At any time, the isotopic composition of the carbon entering the surface part of the system—the atmosphere, biosphere, and hydrosphere—is given by the terrestrial average, but the two processes of biomass synthesis and carbonate precipitation tend to slightly separate the carbon isotopes. At present, for example, carbonate forming in the ocean contains 1.113 percent 13C, and, on average, organic material being buried in sediments contains 1.086 percent 13C.
Measuring isotopic abundances at that level of precision is not simple, but it is incomparably easier than constructing a global inventory of carbonates and organic material, and it provides a way to monitor the behavior of the carbon cycle. By calculating the abundances of buried organic and inorganic carbon from the total carbon and 13C mass balances, indications are that at present about 30 percent of the carbon passing through the hydrosphere, atmosphere, and biosphere is being buried. Characteristics of ancient carbon cycles can be similarly determined. For every interval it is necessary only to obtain globally representative carbon isotopic abundances for carbonate sediments and organic carbon.
Paleoceanography: Cycles in the History of Oceanic Waters
Recognition of changes in variables such as the chemistry of the oceans, the global sea level, the configuration of ocean basins, the three-dimensional thermal structure of the ocean, and the history of marine organisms permits the description of ancient conditions, which even during the past 18,000 years have undergone remarkable transformations. On a broader scale of time, changes have been even more profound.
About 70-million-years ago, shortly before mammals inherited the Earth from dinosaurs, the oceans supported a huge population of calcareous nannoplankton. They were so abundant that their minute skeletal remains rained down on the seafloor to produce thick deposits of chalk that stand now as the White Cliffs of Dover in England and the cliffs of the Selma Chalk in Alabama. Today, photosynthesizing calcareous nannoplankton survive as very important producers in the marine food chain but have never again generated such widespread deposits of chalk; they suffered severe losses at the same time the dinosaurs met their end. Probably part of the explanation is that they never rediversified fully because other taxa took their place. Certainly another important factor is that relatively cool climatic regimes, which do not favor calcareous nannoplankton, have prevailed during the past 60-million-years. On the other hand, diatoms—silica-precipitating organisms that thrive in cold water—expanded greatly during that time. The deep-sea,
which today is close to freezing, was warm between 100 million and 70-million-years ago. This is inferred from the isotopic composition of foraminifera that then lived on the deep-seafloor. Paleoceanographic research suggests mechanisms that may have caused the refrigeration of the deep-sea since that time. Geologists are investigating the effects of those thermal changes as well as related aspects of ocean evolution on time scales that range from thousands to billions of years.
Although the history of seawater is an important subject in its own right, it also serves as an indicator of processes that have shaped the outer parts of the Earth through time. Limits for the composition of ancient oceans are determined from the mineralogy and chemistry of marine evaporites, the sediments formed by the evaporation of seawater, but even these indicators leave a wide range of uncertainty. The most useful technique currently available to define the major compositional variation of seawater over the past 600-million-years requires extraction and analysis of brines trapped in the rock salt found within marine evaporite deposits. In many instances these brines appear to have suffered little, if any, alteration. Their composition is not that of seawater, but the mass compositional parameters of the parent seawater can be reconstructed from the brine chemistry by correcting for the effects of evaporation and for the precipitation of limestone, gypsum, and rock salt. The results of the analysis of more than 100 inclusion fluids from marine evaporites covering the past 550-million-years of earth history suggest that the chemical composition of seawater has not changed greatly. This observation has come as something of a surprise, because the isotopic compositions of sulfur, strontium, and carbon in seawater have varied significantly. During the next few years the chemical evolution of seawater should be defined much more precisely, and we anticipate gaining a clearer understanding of the mechanisms that have controlled the composition of seawater.
We know less about patterns of circulation for modern oceans than about those of the modern atmosphere because of the logistical difficulty of gathering oceanographic data. This deficiency limits the accuracy of paleoceanographic modeling. However, conditions within ancient oceans can be reconstructed by using patterns of modern oceanic circulation to reassemble the thermal structure and dominant currents in ancient oceans and by selecting especially important physical, chemical, and biological indicators in the geological record to plot distributions. Nowhere has this approach been undertaken more effectively than in the Climate: Long-Range Investigation, Mapping, and Prediction (CLIMAP) project and its successors, broad international initiatives inaugurated in 1971 to recreate the ice age world of the past million years.
Although CLIMAP's broad goal was to investigate global climates for the past million years, its crowning achievement was the production of a climatic map of the world as it existed 18,000 years ago. This was the time of the most recent glacial maximum. In the overall strategy of CLIMAP, the most important element was reconstruction of sea-surface temperatures for the time frame of 18,000 years ago. Fossil occurrences of living marine species were used to chart the geographic distribution of ancient temperatures.
The most general conclusion drawn from the CLIMAP model was that 18,000 years ago the average sea-surface temperature was 2.3°C cooler than it is today. The high spatial resolution of the analysis permitted many more specific results. The equatorial Atlantic and Pacific oceans did not cool as much. Waters near the sea surface were generally cooler and more mixed than they are today, with a less pronounced thermal contrast between surface and deep waters. Ice floes extended to much lower latitudes in the North Atlantic—the Gulf Stream flowed eastward toward Spain, not Great Britain. And in the North Pacific, radiolarian species that today are restricted to cool waters from northern California to Washington ranged at least 1,000 km farther south. From other evidence we know that glacial expansion took place primarily in the north, with ice caps centered in Hudson Bay, Greenland, and Scandinavia, but marked climatic changes occurred in the Southern Hemisphere as well.
Reconstructing ocean temperatures and current patterns for earlier times is more difficult. Nevertheless, certain striking oceanographic changes that occurred tens and even hundreds of millions of years ago are clear. Deep-sea conditions changed drastically over geological time in response to profound global changes in shallow marine thermal regimes and in terrestrial climates. Today, throughout the globe the deep sea remains only slightly above freezing because its waters are derived from polar regions. At those high latitudes, surface currents cool so severely that they become much more dense than the underlying water. The chilled water sinks to the bottom and spreads along the deep seafloor to equatorial latitudes, forming a cold basal layer in all the oceans.
Fifty-five-million years ago, many regions of the Earth were much warmer than they are today. At
that time, unlikely as it may seem, southeastern England was cloaked by tropical jungles like those of modern Malaysia. Fossils of deep-sea ostracodes, minute crustaceans that are distant cousins of crabs and lobsters, reveal that a major change took place in the deep-sea about 40-million-years ago. The types of ostracodes that occupy the oceanic abyssal plain today, having adapted to frigid conditions, made their first appearance at that time. Oxygen isotopes in foraminifera confirm the observation of this trend toward frigidity.
Before that cooling began, the deep-sea may have reached temperatures as warm as 15°C. It did not cool to its modern temperature immediately, of course, but gradually lost heat until 30-million-years ago when the cold basal currents of the modern oceans became firmly entrenched.
Plate tectonics offers a possible explanation for cooling in the Southern Hemisphere 40-million-years ago. In the vicinity of Antarctica, microfossils preserved in deep-sea sediments testify to a drastic change in thermal conditions. Millions of years earlier the supercontinent of Pangea had rifted apart to form many of the fragments that constitute the continents of the modern world. South America and Australia remained attached to what is now Antarctica, which was positioned on the South Pole close to its present location. While these connections remained, cool water was deflected equatorward and warm water poleward along the coasts. Microfossil and other data indicate that about 40-million-years ago South America began to drift away from Antarctica, allowing a continuous current to flow around Antarctica—the Circumantarctic Current. This current isolated the continent thermally, and the change in circulation marked the origin of the refrigeration system for the deep-sea that operates in this region today, trapping water and allowing it to cool and sink. About the same time this refrigeration system was supplemented by another. The Arctic Ocean became connected to the Atlantic over the Iceland sill, allowing cold Arctic surface waters to descend into the deep-sea.
Throughout earth history a cold basal layer of ocean water must have formed each time at least one of the poles became frigid. Fossil data verify the occurrence of such an event 450-million-years ago, when the supercontinent of Gondwana encroached on the South Pole and accumulated massive ice sheets that left extensive glacial deposits in what is now the Sahara Desert in Africa. Careful stratigraphic research into the period has shown that brachiopods and other creatures that had colonized the seafloor at cooler high latitudes progressively shifted into deep-water habitats at all latitudes, apparently tracking the movement of cool waters into the deep-sea.
Even after the origin of the modern cold basal layer and before the start of the modern ice age, the oceans experienced major thermal changes. Substantial alterations occurred between about 22 million and 5-million-years ago. Important clues have come from carbon isotopes in fossil foraminifera. Gradients of 13C/12C ratio, detected by the study of fossil foraminifera, reveal that prior to about 14-million-years ago water flowed from the Mediterranean Sea into the Indian Ocean and southward toward Antarctica. It traveled at intermediate depths, apparently having sunk below the surface because it was more saline than normal seawater. The intense salinity resulted from a high evaporation rate in the Mediterranean region. This water and others that it entrained apparently joined the Circumantarctic Current at depth. The outflow of this saline plume ended about 14-million-years ago, probably when collision of Arabia with Asia closed the eastern end of the Mediterranean. The cutoff of warm-water flow toward Antarctica may have resulted in the buildup of the West Antarctic ice cap, which has been documented to have occurred at this time period on the basis of other geological evidence. Apparently the tectonic movements that pinched off the flow from the Mediterranean had profound climatic repercussions in regions thousands of kilometers away.
Problems in distinguishing among the effects of glacial expansion, temperature change, and variation in salinity frustrate detailed investigations of paleoclimate that use the oxygen isotopes preserved within fossil skeletons. A partial remedy is now in sight, and it comes from an unexpected source. A particular family of calcareous phytoplankton includes several living species that produce lipids called alkenones. The degree of hydrogen saturation in these fatty compounds varies markedly with the temperature at the time of production. They retain their original chemical composition over millions of years, even after bacterial decay releases them and they end up in deep-sea sediment. Their changing patterns of chemical composition, as displayed in ice age cores, correlate closely with those of oxygen isotope ratios, but the alkenone composition can be scaled to approximate absolute temperature.
The analysis of fossil alkenones promises a large volume of ocean temperature data extending back tens of millions of years. One important controversy inviting resolution relates to the warm interval that preceded the origin of the modern cold basal
ocean waters. Climatic modeling and analysis of oxygen isotopes cannot yet produce a consensus as to whether the tropics were also warmer at that time or whether they were cooler than today, generating gentler latitudinal temperature gradients. In the near future, fossil alkenones may yield a general temperature map for the 50-million-year-old global ocean.
At any time in earth history, deep ocean water is generated from the densest water masses that develop at shallower levels and have access to the major ocean basins. The high density of these waters may result from either low-temperature or high salinity. So when deep waters are relatively warm, they probably are saline, after descending from marginal basins with high evaporation rates. When relatively warm waters occupy the deep ocean basins, thermal gradients are weak, deep-sea circulation is sluggish, and bottom waters become depleted of oxygen. In contrast, at times such as the present the dynamic descent of cool polar waters constantly replenishes the oxygen, which maintains biological respiration and the oxidation of organic and inorganic compounds. In addition, the spread of cold water masses into low latitudes scours out areas of the deep seafloor. Cores of deep-sea sediment display features that reflect these conditions. At some levels, cores contain a substantial amount of red oxidized sediment or evidence of depositional hiatuses. These particular anomalies represent intervals when cold currents from polar regions plowed through the deep seafloor, supplying oxygen or eroding sediments respectively.
A particularly interesting interval extended from about 110 million to 90-million-years ago, when huge concentrations of hydrocarbons accumulated. The organic matter that finds its way into marine sediments, and is the source of most petroleum, derives ultimately from phytoplankton. As primary producers, phytoplankton utilize solar energy to synthesize inorganic carbon sources into organic material. Their biomass fuels the marine food web, in which energy required by other organisms is produced by the metabolic oxidation of organic material. If the food web operated with perfect efficiency, all organic material would be oxidized and the underlying sediments would contain no organic carbon. Where high amounts of organic carbon are found in sediments, the food web must have operated at low efficiency, allowing organic material to escape oxidation. There are two possible explanations for the vast reservoirs of organic carbon remaining in the 100-million-year-old deposits. Either the conditions of the water column or sediments were not favorable for the growth of efficient recyclers, or the production rates were so high that the food web was unable to utilize all of the supply. Close study of the sediments from that interval favors the first alternative, although the second might have been important in places. Even though large amounts of organic carbon were preserved, production rates were low in comparison to present values.
Because the organisms most efficient at reoxidizing organic carbon require free oxygen, this combination of high preservation despite low production could characterize a deep ocean containing little or no dissolved oxygen. Sediments from this period show fine laminations that are completely undisturbed by trails or burrows, indicating that oxygen-dependent animals were unable to colonize the deep environments despite an abundance of available food. On the basis of this interpretation, episodes of global deposition of organic-carbon-rich sediments have been termed oceanic anoxic events.
The climate prevailing 100 million and 90-million-years ago was warmer than today, and seawater would thus have held less oxygen. Moreover, the positions of the continents were different at that time. North and South America were in the early stages of separation from Europe and Africa; the Atlantic Ocean already had some deep narrow basins but widened as the Mid-Atlantic Ridge created new seafloor and moved the continents apart. At the Mid-Atlantic Ridge and similar active spreading centers, free oxygen reacted with sulfides and other oxidizable materials that were being introduced at unusually high rates.
That warm world may have been completely free of ice caps; the north polar region is known to have been covered with lush forests. The inventory of water was almost entirely in liquid form, so sea level was elevated. Two additional factors made the high stand even more pronounced. First, water expands when it is warmed, and the average temperature of seawater was much higher than at present. Second, the volume of the ocean basins decreased because the rapid extrusion of new hot crust at spreading centers meant that large areas of ocean floor rose upward. The resulting elevation of the sea level produced maximal flooding of the continents. In some localities even the oxygen-poor deeper waters extended onto continents, depositing marine sediments rich in organic carbon. When such sediments became deeply buried and were heated to temperatures of 90° to 120°C, they became important sources of petroleum. In summary, a powerful combination of global conditions discouraged the availability of oxygen in deep ocean wa
ters, which in turn prevented the efficient consumption of organic carbon and eventually resulted in the accumulation and preservation of material now used as a nonrenewable resource.
Such episodes of enhanced burial of organic carbon represented globally significant perturbations of the carbon cycle. This change is reflected in the carbon isotope record, which indicates that the fraction of carbon buried in organic form was 50 percent higher during anoxic events than during the intervals immediately preceding and following them. As a result, atmospheric levels of oxygen must have risen even as the deep seas became oxygen depleted. Atmospheric levels of CO 2 declined, contributing to global cooling and the consequent termination of the conditions that had prevailed. Though many factors related to anoxic events can be identified and discussed, the details are not yet fully understood.
Paleoceanographers are investigating the changes over the past 100-million-years in the vertical thermal structure of the oceans at increasingly finer scales of resolution. The heart of this research, which addresses biogeographic patterns as well, concentrates on diagnostic elemental isotopes. The isotopes distinguish plankton living at particular water depths characterized by unique thermal conditions. Areas of upwelling leave their own kinds of evidence, including phosphate deposits along ancient continental margins. Many of these deposits have considerable economic value, and paleoceanographic models contribute to their discovery.
Paleoclimatology: Cycles in Past Climates
A number of important climatic indicators help to establish how climate has changed. Most of them are applicable to the record of the past few hundred-million-years, and some portray conditions that dominated billions of years ago. These indicators are coals, soils, evaporites and sand dunes, glacial deposits, marine reefs and bedded carbonate deposits, and land plants.
-
Coals: Present in the modern world as peat, coals form from organic accumulations in areas combining high rain and poor drainage. The optimal conditions are located in equatorial rain forests or in moist areas at higher latitudes best represented today in zones about 55° north and south of the equator.
-
Soils: Soils rich in the clay kaolinite develop in warm humid climates. Associated with these soils are laterites, in which iron and aluminum are concentrated. Bauxite, the main ore of aluminum, also characterizes very hot moist conditions. All of these climatic indicators are end products of protracted weathering processes and resist subsequent alteration. Despite later cooling or aridity, such evidence of tropical climate can be preserved in the rock record for vast stretches of geological time.
-
Evaporites and Sand Dunes: These sedimentary features reflect arid conditions. Thick evaporite deposits require continuing replenishment from seas or lakes and extremely dry air. Dunes imply strong winds, a source of sand, and a lack of stable vegetation. They are best developed in the subtropical dry climatic zones but can form at mid-latitudes in landlocked regions such as the Gobi Desert or in rainshadows of mountains such as the Sierra Nevada.
-
Glacial Deposits: Glaciers leave boulders in erratic deposits and produce icebergs that release dropstones to lake bottoms and seafloors. Their most characteristic traces, however, are striations—the scratch marks found on pebbles or boulders that glaciers transported or on bedrock that glaciers scoured. Only continental glaciers have broad climatic significance because mountain glaciers commonly form at high altitudes, even near the equator.
-
Marine Reefs and Bedded Carbonate Deposits: Today, limestones accumulate mainly within about 40° of the equator. Those formed primarily by calcareous algae are probably restricted to this zone in part by sunlight requirements. Others, including massive organic reefs built by organisms specific to different time periods, are limited by thermal requirements. Modern coral reefs are confined to within about 30° of the equator.
-
Land Plants: Terrestrial floras are excellent indices of paleoclimates. Flowering plants are especially useful because of their conspicuous fossil record, which extends for about 100-million-years. Climatic conditions are reflected in the basic leaf morphology (Figure 3.10) of flowering plants. Perhaps most valuable is leaf outline—a strong, positive, linear relationship exists between the percentage of species in fossil floras with smooth leaf margins and the mean annual temperature of the habitat. While the slope of the curve may have varied with time, the kind of gradient that we observe today has almost certainly characterized flowering plants since early in their history. The visible characteristics and the composition of fossil vegetation, as determined from pollen, spores, and seeds as well as leaves, give a general picture of climatic changes in North
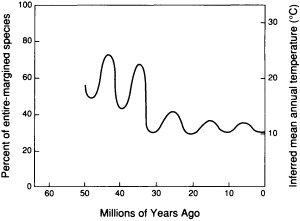
FIGURE 3.10 Entire-margined leaves became less common in rocks (30-million-years and younger)of the Pacific Northwest. This has been used as an indication of mean annual temperature and shows a cooling. Entire-margined leaves are common in hot, humid climates and those with incised margins in cool climates.
-
America for the past million centuries. Efforts are under way to extend this technique to vegetation that existed before the rise of flowering plants.
Paleoclimatic models for intervals of time tens or hundreds of millions of years before the present have been determined by these criteria. For more recent intervals, more detailed information is available. For example, in the 1980s the Cooperative Holocene Mapping Project (COHMAP) produced climatic simulations for time frames that ranged from 18,000 years ago, the most recent glacial maximum, to the present. Empirical input came from estimates of numerous variables. These included insolation controlled by orbital variations, aridity produced by mountains and glaciers, trace gas concentrations in the atmosphere, distribution of sea ice and snow cover, albedo fluctuations, and effective soil moisture. The simulations were based on a model constructed from data concerning those variables in the modern world. The simulations were then tested against other geological indices of terrestrial climates, including the distribution of fossil pollen, levels of ancient lakes, and the distributions of fossil plankton in nearby oceans that had been analyzed by the earlier CLIMAP project. For the most part, the empirical data supported the simulations. There were discrepancies that suggest imperfections in the models. Simulated July temperatures for the southeastern United States for the interval from 18,000 to 12,000 years ago were substantially lower than temperatures indicated by data from the stratigraphic record, and ancient dunes indicate that wind directions may not have been properly modeled. Discrepancies such as these call for further research.
Testing the models for earlier periods of earth history becomes even more difficult because climatic indicators are less precisely documented. But general climatic models developed for periods approaching half a billion years offer provocative results that must be taken seriously. One example is a model for 250-million-years ago (Figure 3.11). A linear version of a two-dimensional seasonal energy-balance model shows a temperature response that depends on differences in the heat capacities of
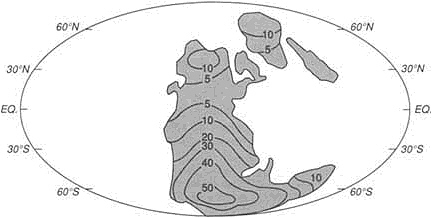
FIGURE 3.11 An energy-balance model based on this distribution of the continents about 250-million-years ago generates the temperature ranges indicated. The interior of Gondwana had an extreme range.
land and water. Other components of the model include the effective heat capacity of the earth-atmosphere column, the isotropic heat diffusion coefficient, the solar constant, the regional distribution of solar radiation, and the albedo of the earth-atmosphere system.
These models yield results for the annual climatic cycle that generally agree with observations. For 250-million-years ago, the model indicates remarkably pronounced seasonality, owing to the accumulation of continental crust into a single giant continent. Simulated subtropical mean temperatures for the Southern Hemisphere range from about 10°C in the winter to about 40°C in the summer, with summer temperatures occasionally reaching the value of 45°C (113°F). These results have profound implications for terrestrial life. A large group of mammal-like reptiles called therapsids—whose fossil record ranges from South Africa and Virginia to Russia—occupied the subtropics of the Southern Hemisphere, where it appears that temperatures underwent large great seasonal oscillations. This occurrence is inconsistent with the idea that therapsids, which have no living representatives, were cold-blooded like their reptilian relatives. The implication is that, like their mammalian descendants, they employed thermoregulation. In fact, fossil trackways of these creatures suggest that they were warm-blooded. Their footsteps were usually far apart, like those of fast-moving mammals and unlike those of cold-blooded reptiles.
A more general conclusion of this energy-balance model applied to the world 250-million-years ago is that continental glaciation in polar regions was favored by positioning of small continental areas near poles. Summer temperatures remained low because of the high heat capacity of neighboring seas, which caused abundant winter snow accumulation to persist throughout the year. If a very large dry continent were situated more centrally over a pole, pronounced seasonality would produce warm summers that could prevent the snow from persisting and glaciers from expanding.
Secular Change in the Global System
The numerous cyclical processes that interact to make up the entire earth system are superimposed on unidirectional, mostly gradual, processes of which radioactive decay and biological evolution are the most fundamental. Catastrophic events that affect large parts of the earth system are capable of interrupting the operation of familiar cycles and can greatly modify the established direction of gradual secular change. During the past decade, in one of the most exciting developments in the study of the Earth, there occurred a deviation from traditional assumptions of general gradualism to consideration of a possible role for catastrophes.
The history of the Earth can be conveniently divided into three major intervals distinguished by important secular changes in the geological record. Many surface processes have changed somewhat throughout the three phases, but the biggest changes are seen in the sedimentary and fossil evidence. The record from the oldest rocks used to be extremely sporadic in quantity and quality, but gradually it has been improved by the accumulation of superior data.
The Precambrian Record
The interval of time that preceded the development of a rich fossil record of invertebrates with skeletons, nearly 600-million-years ago, is called the Precambrian. Representing nearly 4-billion-years of earth history, its unique features have led geologists to use techniques that are given less emphasis in the evaluation of later intervals. One limitation of this most ancient record is that rocks older than about 1.4-billion-years lack any fossils that can be used to correlate strata and establish synchroneity from place to place. Limited correlation using fossils is possible among rocks between 1.4 billion and 600-million-years old. Nonetheless, global developments of environments and life forms during the Precambrian were so significant that many general features have been deciphered and assigned at least approximate dates on the basis of isotopic dating. Among these was a major radiation in phenotypic diversity in eukaryotes, organisms with a cell with a true nucleus, at about 1.6-billion-years ago or possibly even earlier—an event that may have been linked to changes in the proportion of atmospheric carbon dioxide and oxygen.
Both prokaryotic, or prenucleus, and eukaryotic single-celled organisms are remarkably well preserved in rocks of this period. Beginning around 550-million-years ago, preskeletal multicellular animals left an abundant fossil record consisting of tracks, trails, and body imprints.
Study of early environments, and the organisms that evolved in them, is intertwined with the study of secular trends. These earliest secular trends were more profound and influential than any of the changes characterizing the most recent 600-million-years. Not only was the Sun weaker and the greenhouse effect stronger, but calculations suggest that
the Moon was circling the Earth in a smaller orbit, which would produce stronger tides in the ocean. Fossils provide clues about less ancient Earth-Moon relationships. Corals preserved from 360-million-years ago have about 400 growth bands in each annual interval, revealing that the Earth rotated more rapidly and that days were shorter. Rotation must have been more rapid still during Precambrian time.
The oldest preserved sedimentary rocks exposed at the surface are about 3.8-billion-years old, although there has been a discovery in Australia of zircon grains, preserved in somewhat younger sediments, that were formed 4.2-billion-years ago. The oldest sediments do not look very different from later ones, which suggests that surface environments have not changed all that much. A notable exception is a recent discovery of a very high iridium content in rocks about 3.6-billion-years old from South Africa and Australia. This evidence has been interpreted as indicating that the flux of meteorites to the surface of the early Earth was much higher than had previously been considered likely. Meteorite impacts had a substantial role in the evolution of the earliest surface. But, by analogy with evidence from the Moon, it has generally been considered that the flux rapidly decreased at about the time the oldest preserved rocks formed.
Speculation about conditions at the surface before the oldest rocks formed has changed as ideas about the Earth's origin and earliest development have evolved. The possibility of an impact of a Marssized body on the Earth suggests a mechanism for Moon formation. Such a scenario would have occurred within a few tens of millions of years after the solar system's origin 4.56-billion-years ago. After the impact the Earth was probably wholly molten, although mantle and core would have remained separate with no great amount of chemical interaction. Volatiles, those elements and compounds that tend to vaporize, would have been lost to space or dissolved in the mantle silicates. In the former case the materials making up the subsequent atmosphere and ocean may have reached the surface in further meteoritic flux, originating farther out in the solar system, within a few more tens of millions of years. This material was probably dominated by water, nitrogen, and carbon dioxide, like the early atmospheres of Venus and Mars.
By about 4.46-billion-years ago, when the solar system was 100-million-years old, the Earth was approaching its present state. It had a core, a mantle, some kind of basaltic crust, and an atmosphere and ocean. Conspicuous differences from later times might have included the absence of a discrete inner core, much more heat dissipation from a hotter mantle and more vigorous convection, a much higher meteorite flux involving the generation and dissipation of more heat, and the repeated rupturing of the lithosphere. Unless some form of organism had been delivered by a meteorite or cometary dust, the Earth would have been still barren of life at this early stage.
The earliest erosion and deposition would have operated in much the way they do today, with the former dominant above sea level and the latter below. Rapid convection in the mantle would have led to rapid operation of cycles that open and close ocean basins, although there is no way of knowing when rigid extensive plates of lithosphere first characterized the surface. An intermediate arrangement could have involved a less organized convective system dominated by numerous Hawaii-like hot-spots.
These speculations suggest that the surficial processes operating when the oldest rocks formed were similar to processes operating today. The geologist studying the oldest rocks is less like a playgoer seeing the curtain rise on the first act of a drama than one walking in on a performance that has been in progress for some time.
The oldest preserved sediments, at Isua in Greenland, still portray the drama in progress at the time of their formation, despite having been heated to 800°C and deformed intensely during subsequent mountain building. Detrital sediments indicate that both weathering and erosion proceeded nearby and that deposition was under water. The presence of small amounts of limestone may indicate that organisms were active in the depositional basin. The oldest structures acknowledged as exhibiting bacterially controlled limestone deposition are about 3.5-billion-years old, 300-million-years younger than the rocks at Isua. But the presence of limestones among the oldest preserved sediments strongly suggests that life had already originated by 3.8-billion-years ago. Evidence involving the carbon cycle further implies that even then, as now, organisms played a substantial role in modifying the environment.
Solar energy reaching the Earth has probably increased over time because solar luminosity has risen as a consequence of the conversion of the Sun's hydrogen to helium. That conversion increases mean solar atomic weight and hence the temperature necessary to maintain thermal pressure against gravitational collapse. When the oldest rocks formed, the Sun's energy output was about 30 percent less than it is today. The surface would have
been warmed so gently that much of the ocean would have frozen. But the early atmosphere was rich in carbon dioxide, a strong absorbent of the infrared wavelengths that conduct heat reflected from the surface. The abundant carbon dioxide would have created the effect of an enhanced greenhouse, sheltering the fragile surface of the planet from a complete freeze that would have been difficult to reverse.
Development of the modern atmosphere from those primeval conditions accompanied biological evolution. The oldest carbon compound deposits, stored in limestones of the rock reservoir, were produced by organic precipitation. Those early organisms consumed carbon dioxide in photosynthesis, released free oxygen to the atmosphere, and left their carbonaceous skeletons to accumulate as limestone on the ocean floor.
The record of change in the oxidation state of the atmosphere-ocean system is fragmentary, and interpretation is necessarily speculative. Even so, a case can be built on a collection of evidence that suggests a gradual buildup toward saturation. There are remnants of detrital grains that could not have resisted oxidation in an oxygen-rich atmosphere. These grains are preserved in sediments that are at least 2.0-billion-years old, and the grains themselves may be 2.5-billion-years old. Also, abundant iron ore deposits chemically precipitated occur in sediments deposited between 2.8 billion and 1.8-billion-years ago. These ores are richly oxidized compounds that when formed would have acted as an oxygen sink; that means newly freed oxygen would have had to react with such exposed minerals before any atmospheric buildup could have become available for biological functions. Furthermore, the oldest uncontestable evidence indicating biological use of oxygen dates to 2.8-billion-years ago. Finally, the first evidence of persistent oxygenation of surface environments occurs in rocks that formed 2.2-billion-years ago.
The interpretation of much of this evidence is disputable, and active research, including a search for stable carbon isotope variations, is attempting to clarify the picture. Recent isotopic investigations emphasize the role of the carbon cycle in modifying the environment. These investigations focus on another episode that witnessed the burial of large quantities of organic carbon in sediments between 900 million and 550-million-years ago—a period that preceded an explosion of multicellular organisms throughout the world's oceans. Complementary release of oxygen from carbon dioxide would have driven atmospheric oxygen concentrations up to present levels or higher. This interpretation supports those evolutionary biologists who speculate that multicellular life forms could not develop until the atmospheric partial pressure of oxygen became high enough to diffuse the element across multiple cell layers. Paleontological evidence of prolific multicellular life fills the stratigraphic record immediately after the isotopic evidence of increasing oxygen.
Projecting the carbon isotope record forward to 550-million-years ago produces evidence of numerous smaller cyclical changes. For example, significant variations in 13C abundances have been linked to complementary shifts in the abundances of sulfates and sulfides, the oxidized and reduced forms of sulfur. It seems likely that, when larger-than-average quantities of organic material have been buried and the carbon cycle frees excess oxygen, the excess is consumed by the oxidation of sulfur minerals exposed at the surface. The oxygen release during the carbon burial event 900 million to 550-million-years ago was apparently so large that oxidation of sulfur could, at best, attenuate it.
Record of Change Between 600 Million and 150 Million Years Ago
During this period organisms colonized vastly differing environments through a variety of physical adaptations. Among the plants, ferns and conifers evolved, and animal life developed from marine invertebrates to fish, insects, amphibians, reptiles, and mammals. Interpretation of change between 600 million and 150-million-years ago profits from the richness of the fossil record and the relatively accurate reconstructions of former continental positions based in part on paleomagnetic data (see Figure 3.8). Sedimentary particles containing iron tend to settle in magnetic alignment with the Earth's magnetic field, and, similarly, iron-bearing lavas become magnetized as they crystalize while cooling. A rock magnetized in one of these ways is, in effect, a paleocompass that reveals its own orientation to the magnetic pole at the time when it formed. Reliably preserved data come from the magnetic declination, which shows directional orientation, and from the magnetic inclination, which increases at higher latitudes. These relative magnetic deviations are set into a rock body when it forms, allowing geologists to determine the orientations of ancient continents as well as their latitudinal positions.
Rock and fossil distributions can also indicate possible paleogeographies, and maps of ancient
continental positions are under constant revision. As might be expected, the paleogeography of older time intervals is the least certain. It has even been suggested that as long as 600-million-years ago nearly all continental lithosphere formed a single supercontinent—an earlier Pangea—which then separated into Gondwana and smaller fragments. Subsequent reassembly in a different configuration formed Pangea about 250-million-years ago.
Many forms of life that existed between 600 million and 150-million-years ago belonged to genera, or even families, that are now extinct, so only broad environmental inferences can be drawn from their geographic distributions. As a result, analysis of tectonic patterns remains the focal point of global environmental reconstructions for the entire interval. Since 150-million-years ago the dominant process has been the breakup of Pangea to form the modern continents and the oceans that separate them. The record is much better preserved, and paleoenviromental analysis is correspondingly more refined.
Record of Change from 150 Million Years Ago to the Current Ice Age
For the past 150-million-years, the breakup of Pangea into the modern continents and their subsequent distribution have dominated the processes recorded in the rocks. The changing plate positions can be projected for that interval from information preserved in the ocean-floor record. The high-quality of the youngest segment of the geological record allows earth scientists to use additional techniques in evaluating global change over the past 150-million-years. Many sediments of this age remain soft, so they readily yield their fossils for study. Furthermore, the fossils are commonly quite well preserved; some marine skeletons faithfully retain even the original ratio of stable oxygen isotopes, shedding light on temperature change during the recent past.
The oldest dated floor of modern ocean basins covers only a small area and is about 180-million-years old. Progressively larger areas of younger ocean floor are preserved within the ocean basins. The Ocean Drilling Program, like its predecessors, utilizes the relatively complete sedimentary record beneath the deep ocean where erosion is much less pervasive than on the land. Close examination of the microplankton fossils found in deep-sea cores, partly through the study of oxygen and carbon isotopes in microfossils and partly through analysis of microfossil paleoecology, has proved rewarding. Thanks to this work, paleoceanographic research that is focused on the past 150-million-years reveals dramatic changes in the thermal structure of the oceans, which have culminated in present patterns.
The method of correlation known as magnetic stratigraphy is used for sediments deposited during the growth of an ocean basin. The magnetic field, which is generated by fluid motions within the outer core, reverses itself episodically. These reversals of polarity are recorded in magnetization of sediments and volcanic rocks, as described previously, and they serve as valuable events for global correlation. Magnetic reversal sequences in sediments can match marine with nonmarine strata, which have few fossils in common. The magnetic reversal pattern recorded in igneous rocks formed at spreading centers is laid out as stripes on the floor of the world's oceans. Matching the characteristics of these patterns as they appear on either side of an ocean rift can be used to reconstruct the size and shape of the oceans and the distribution of the continents. For much of the past 150-million-years, the magnetic time scale has been calibrated by isotopic dating, so the scale is not simply a means of correlating strata but also a source of information about ages of sediments and fossils.
Fossils are not only important in correlating strata separated in space, but also they are invaluable for characterization of ancient environments. For example, flowering plants, which include not only plants with conspicuous flowers but also hardwood trees and grasses, have been called the thermometers of the past because of their diagnostic value in assessing ancient thermal regimes on the land since they became abundant forms of life 100-million-years ago.
Recognizing Environments from the Geological Record
Our picture of global environmental conditions is a composite of local studies. Many of these studies are rooted in detailed analyses of depositional environments—environments in which sediments, and often fossils, have accumulated.
During the past 25 years, sedimentologists have developed a set of tools for distinguishing particular environments of deposition and for unraveling the histories of these environments from clues left in sequences of sedimentary strata. Some of the diagnostic features are small in scale. Examples are accumulations of lens-like inclined sand beds draped with mud, produced by ripples migrating across a tidal flat that left a record of ancient oscillating currents. Other examples are beds in which scours
are filled by coarse sands at the base, which are covered by finer sands, then by silt, and finally by clay. This sequence, or package, indicates that the entire bed formed when a turbid flow charged with particles of many sizes—a turbidity current—swept down onto the deep seafloor at the margin of a continent. Upon deceleration it dropped its coarse debris first and its slower-settling fine debris later.
Key diagnostic features involve the associations between individual sediment units in the vertical sequence. For example, meandering rivers, as they migrate back and forth across a valley floor, produce repetitions of deposits that suggest cycles of sediment accumulation. Coarse sandy or even gravelly deposits with inclined bedding indicate an active stream channel. These pass upward into finer deposits that culminate in muds recording the lateral migration of the channel with deposition limited to flood events. The cycle is complete when evidence shows an abrupt cut through the sequence that is filled with another coarse deposit and subsequent fining upward.
By analyzing sedimentary sequences, geologists can recognize a wide range of ancient environments, ranging from alluvial fans that form along the bottom slopes of mountains to mountain belts that have incorporated sediments squeezed up when deep floors of ancient oceans converged with continents along subduction zones. Depositional environments bear witness to most ongoing earth processes—they only need accurate interpretation. And there are immediate applications. Reconstruction of sedimentary environments plays a major role in the search for petroleum and gas, indicating locations of natural traps for these fluids.
Accurate dating of rocks is critical to paleoenvironmental reconstruction on all spatial scales. When isotopic dates are not known, tight temporal correlations between areas must be dependable. Improvement of existing techniques continues, as does the invention of new ones. Only recently, for example, geologists have applied and improved quantitative techniques for correlating strata on the basis of the earliest and latest appearances of fossil species. Quantitative analysis of populations of fossil conodont—minute tooth-like structures of an extinct group of marine vertebrates—can correlate rocks more than 400-million-years old. Calculations indicate that these correlations are accurate to within just a few hundred thousand years.
Breakthroughs have come in other areas, too. Stratigraphers have lately discovered that many limestones exhibit sufficient paleomagnetism to display reversals in the magnetic field. This characteristic permits geologists to assign strata to positions in the global paleomagnetic time scale. Surprisingly, the magnetism results from forms of bacteria that colonize tropical seafloors and produce minerals containing iron. Using this approach, stratigraphers have dated limestone cores taken during drilling operations in the Bahama Banks. From these dates they can calculate rates of subsidence for this huge limestone platform and determine the times when global lowering of sea level left it standing far above marine waters.
New means of isotopic dating are also continually being developed and refined. Single grains of zircon from Precambrian rocks more than a billion years old can now be dated with a precision of just a few million years, as discussed previously. Other exciting techniques, still in the early stages of development, should soon permit dating of terrestrial sediments that are just a few tens or hundreds of thousands of years old—too old for radiocarbon dating, or lacking any carbon that might be dated, and too young for other dating methods.
Paleogeography
Researchers have proposed that not only the positions of the continents but also the uplift of mountains exert control over global climate patterns. Mechanisms that physically alter environments on a regional scale have traditionally been accepted, but theories that suggest that tectonic forces may cause climatic changes on a global scale still inspire controversy. For example, the uplift of the Sierra Nevada in eastern California exemplifies regional, and direct, effects. Today, the Sierra Nevada is an imposing structure, a block of granitic crust heaved upward to form a towering eastward-facing scarp that was a formidable barrier to pioneers attempting to reach California. Fossil plants dating from 10 million to 15-million-years ago that could not have lived as much as 1 km above sea level are found today on the crest of the range, nearly 3 km high. Only in the past 5-million-years has the Sierra Nevada approached its present height; the consequences of this elevation are enormous for the Basin and Range Province to the east, in Nevada and southern California. This area, which had been covered by broadleaf evergreen forests, came to lie in the rain shadow of the Sierra Nevada in the past 5-million-years and developed a savannah vegetation. During the past 1.8-million-years climates became drier on a global scale. The Basin and Range Province became the desert that we know today, although during glacial maxima it received substantial rainfall.
The environmental changes produced on a global scale by uplift involve more complicated processes. Important simulations have been produced by researchers who used the National Center for Atmospheric Research's Community Climate Model with variables adjusted for three different orographic conditions. The three variations are plotted for no elevation, half elevation, and full mountain elevation in two separate areas: the extensive high ground that spreads from the Rockies to the Sierra Nevada and the Cascades in the western United States, and the Tibetan Plateau bordered to the south by the lofty Himalaya. The three variations of uplift correspond respectively to conditions that dominated 40-million-years ago, 8-million-years ago, and during modern times. The results suggest that the uplift of those two areas during the past 50-million-years modified the Northern Hemisphere's atmospheric circulation, in a domino-like series of events that could have promoted the glaciations of the current ice age. The uplifted plateaus would have rerouted dominant air circulation, establishing violent monsoonal weather systems. Those weather systems, in turn, would have accelerated chemical weathering from the mountains—weathering that draws carbon dioxide from the atmosphere. An atmosphere depleted in carbon dioxide would have a global cooling effect and would encourage ice buildup in the higher latitudes.
A differing hypothesis points out that increased weathering, induced by frost, could remove material from an already high plateau. Traditionally, geologists have considered coarse massive deposits as evidence of uplift. They have tended to assume that uplift exposes more rock to weathering and produces piles of boulders and cobbles downstream. The new hypothesis attributes the piles of rubble to climate change—unloading the upstream regions generates uplift because of isostatic compensation.
Another tectonic mechanism that may have contributed to the ice age in the Northern Hemisphere is the closing of the gap between North and South America by the uplift of the Isthmus of Panama. Changes in plankton preserved in deep-sea sediments date the uplift of the Isthmus to 3-million-years ago. Blockage of the westward-flowing equatorial currents from the Atlantic to the Pacific would deflect warm equatorial waters out of the Gulf of Mexico. The Gulf Stream delivers large masses of warm water to the northern Atlantic, where it evaporates and increases humidity. The humidity precipitates as snow at high latitudes. The hypothesis that a strong Gulf Stream engendered the ice age attributes the accumulation of continental ice sheets to that increase in precipitation (Figure 3.12).
The cause of the current ice age probably incorporated facets of all these changes and more. The implication is quite astounding. Small tectonic events plunged the entire Northern Hemisphere into a persistent ice age. This current ice age has persisted for the past 2.5-million-years. All human civilizations have occurred within one geologically brief interglacial interval, and there is no indication when this ice age will come to an end.
Throughout earth history, lateral plate movements have caused profound environmental changes. Paleolatitudinal data indicate that between about 450 million and 400-million-years ago, the great supercontinent of Gondwana encroached on the south pole and underwent major climatic changes that appear to have influenced all latitudes. The polar region of Gondwana, which now constitutes northern Africa, was the center of a vast glaciation. Today, in the Sahara Desert we find massive deposits 450-million-years old that bear striations produced by grinding along the base of glaciers and that contain boulders. Many species failed to adapt to the new thermal regimes or to migrate successfully to hospitable ones. This was the time of one of the most severe mass extinctions to have taken place in the marine realm during the past 600-million-years.
Anomalous distributions of fossils have been related to plate movement in quite a different way. The distribution of some fossil species simply does not make sense. During the 1970s such abnormal distributions provided the first clues in both eastern and western North America to the existence of exotic terranes—large blocks and fragments of lithosphere that have been sutured to the edge of the continent (Figure 3.13). The indication that these terranes had been rafted into place from far away came from the observation that their fossil marine faunas represented paleobiogeographic provinces quite different from those of neighboring continental regions. In this way a major mechanism of continental growth was demonstrated.
History of Life
Life on Earth develops in an ever-changing environment, and paleobiologists are reaching out to other disciplines for help in assessing the effects of environmental changes on ancient life. Prominent questions are those about the kinds of environmental change that influence the evolution and extinction of life and about the time scales on which the
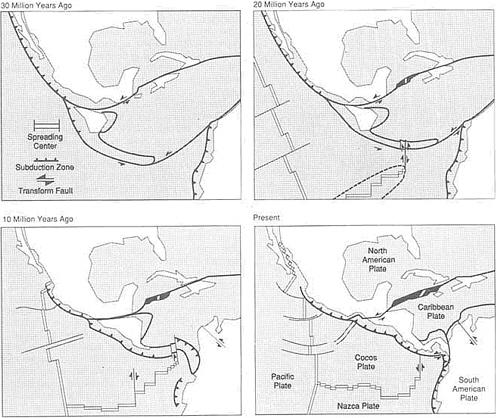
FIGURE 3.12 Evolution of Caribbean land bridge through plate tectonic processes over the past 30-million-years. This changed ocean circulation and may have led to the accumulation of continental ice sheets.
changes operate. These questions not only bear on past rates and patterns of evolution and extinction, as revealed in the fossil record, but also illuminate impending extinctions that humans may be able to prevent. Some predictions suggest that half of all living species may disappear within the next 50 to 100 years. This rapid disappearance is supposed to be biased toward loss of terrestrial species, especially those of the tropics. However, there has been little work on present rain forest diversity or on natural rates of extinction in rain forest areas during the past 50,000 years. The little research that has been done produced conflicting results. Conservation programs can only be improved by learning more about the environments and biology of these areas during the recent past.
Specifically, we need to find out the climatic and vegetational histories of the Amazon Basin and of similar rain forests in western Africa and Asia. The Amazon rain forest shrank and broke up into pockets of verdant growth separated by stretches of savannah as the building northern glaciers repeatedly removed much of the oceanic and atmospheric water from free circulation. Some researchers claim that there was a 90 percent reduction in rain forest area during this period of Northern Hemisphere glaciation, with the inevitable accompanying extinctions.
The modern world is impoverished in terrestrial species of mammals, the class to which humans belong. Only 12,000 years ago the American landmasses were populated by beavers the size of black
FIGURE 3.13 The shaded areas are among those terranes in western North America that have been added to the continent during the past 250-million-years. This process has been important in building continents throughout earth history.
bears, ground sloths the size of elephants, and lions much larger than their living relatives. Close to 11,000 years ago these giant mammals and many others disappeared. Large animals such as these tend to be especially vulnerable to extinction because they have small populations. Perhaps the appetites of humans accounted for the demise of the behemoths; one scenario for the extinctions implicates human hunters armed with advanced new weaponry. The other possible culprit is climatic change, which caused heavy extinction in the oceans earlier in the current ice age. In the North Atlantic and Caribbean, where climates became cooler and more seasonal beginning about 3-million-years ago, thousands of shallow-water marine species died out.
Whatever the causes of the various extinctions during the current ice age, the fossil record gives some indication that the modern world is biotically deprived. This paleontological perspective makes the faunas and floras that survive today especially precious. The magnificent mammals of the Serengeti Plain provide an unusual glimpse of a rich savannah fauna, but even this environment is depleted in diversity compared to the fauna that inhabited the same area 2 million or 3-million-years ago.
Rates of evolution and extinction show how the modern ecosystem came into being. The number of species in any higher biological category is a result of the positive process of the multiplication of species, called speciation, and the negative process of extinction. But speciation represents only one mode of evolution—another is phyletic evolution, the transformation of already existing species. Both multiplication and transformation of species result primarily from natural selection. Evolutionary processes became more fully elucidated in the twentieth century through progress in modern genetics. Still, the relative importance of phyletic evolution and speciation within segments of the tree of life for particular intervals of time remains controversial. Some scientists question whether speciation has contributed more to the total amount of evolutionary change—the punctuational model—or whether phyletic evolution has played the larger role-the gradualistic model. If phyletic evolution is sluggish, accounting for a relatively small fraction of all change, the traditional idea that many species respond to gradual environmental change by undergoing adaptive evolution should be reexamined. Instead, the punctuational model should be considered. This model, which emerged during the 1970s from interpretation of the fossil record, has engendered much research in both paleontology and biology. While insight has and will come from the field of biology, the fossil record will be the ultimate testing ground. On a scale of millions of years, evidence comes uniquely from the fossil record.
Traditionally, studies of rates, trends, and patterns of multiplication and extinction use the more general biological categories, genera and families, as units of analysis. During the past few years, however, numerous studies have been undertaken at the level of species, improving the fidelity of macroevolutionary research. Analogy with demography, which normally monitors individual organisms rather than species, contributes to assessments of increase and decline. Multivariate statistics and computers have made it relatively easy to handle morphological data and to test alternative models of evolutionary processes. Paleontologists have learned to use more than sophisticated mathematical-
tools to analyze the fossil record. Interpretation of trends and patterns involves familiarity with functional morphology, which brings extinct plants and animals to life; with local paleoecology, which places them in their environmental context; and with paleobiogeography, which reconstructs their broader distribution.
Speciation and Extinction
A variety of demographic techniques is available to assist in analyzing the waxing and waning of biological groups through analogy with the birth and death processes of individual organisms. One example is logistic growth. A population of cells in a petri dish that expands from a single original cell will grow at a rate of cell division that initially exceeds the rate of death. In a similar way, new groups of animals and plants have diversified because their rate of speciation exceeded their rate of extinction. The number of species expands almost exponentially at first and then suffers a decline in its rate of increase, so the diversity curve levels off. Growth rate in the petri dish declines because of crowding and perhaps because of waste accumulation. For a group in nature, ecological crowding may eventually reduce the rate of speciation to a level that approximates the rate of extinction. Alternatively, highly efficient predators or competitors may evolve or the habitat may deteriorate, increasing the rate of extinction. The demographic analogy extends to groups that arise during brief geological intervals. They are comparable to cohorts of individuals born more or less simultaneously within a population and are amenable to various methods of survivorship analysis.
The factors that govern rates of speciation and extinction for any group of organisms may change greatly from place to place or time to time. More fundamentally, there are also intrinsic differences in the rates characteristic of different taxa. Mammals, for example, have experienced higher rates of both speciation and extinction than many groups of fossil marine invertebrates. Sets of rates such as these reveal patterns, and patterns in turn suggest the nature of the factors that regulate the rates.
We now recognize that at times of biotic crisis some normal patterns of extinction have been altered. Statistically significant differences between the biological traits of victims and survivors serve to test extinction hypotheses. For example, in the mass extinction that swept away the dinosaurs, marine phytoplankton that could form biologically inactive resting spores were relatively unaffected. Though not definitive, this pattern is consistent with the hypothesis that during the crisis oceanic photosynthesis was drastically diminished, a condition that might have initiated a cascading trend of food chain collapse.
The basic nature of speciation and extinction must be understood in order to interpret the rates at which they occur. Species are separated from one another by reproductive barriers. Although biologists debate the importance of population divergence to form new species where there is no spatial separation from parent species, it seems clear that most speciation entails at least partial isolation of the diverging population. This isolation may occur by fragmentation of the parent species' population or by migration or transport of a subpopulation to a separate location. Simple arithmetic reveals that speciation is quite infrequent. If the average time span for a species has been reasonably estimated at 5-million-years, and if global diversity has not changed markedly during a 5-million-year period, then during this long interval an average species will have spawned only about one descendant species.
Extinction has been the fate of the vast majority of species that have inhabited the Earth. Usually it is caused by an accentuation of the limiting factors that naturally restrict the distribution and abundance of the species. Extinction amounts to an ultimate decline in both areal extent and population size. These limiting factors include competition with other species, efficiency of predators, availability of resources, conditions of the physico-chemical environment, and chance factors. Often two or more limiting factors conspire to cause extinction, with chance playing its most important role when species are rare and spatially confined.
To explore more fully how patterns suggest controls over probable speciation and extinction, controlling factors can be assigned to three categories: intrinsic traits of the species being considered, limiting factors of the biotic environment, and limiting factors of the physical environment.
Intrinsic Biological Traits
The fossil record reveals that rates of speciation and extinction correlate in predictable ways with geographic dispersal, characteristic size and stability of populations, and behavioral complexity. For example, weak dispersal can favor speciation by fostering the frequent isolation of small populations and can favor extinction by restricting geographic distribution. These relationships cannot be examined easily in the modern world, which represents
but a brief slice of geological time. However, some segments of the fossil record represent useful reality checks for the mathematical models that examine such relationships.
Biotic Interactions
Ecological relationships between groups can be positive or negative. On the positive side, diversification in one group can promote speciation in another. Furthermore, ecologic interactions between two groups can promote the diversification of both through a kind of evolutionary synergism—combined actions that produce enhanced results. For example, during the past 150-million-years the diversification of flowering plants went hand in hand with an expansion of insect pollinators. New varieties of plants offered new food resources for insects, while at the same time new varieties of pollinators promoted reproductive isolation, and hence speciation, in flowering plants. Unfortunately, this relationship has rendered both groups more vulnerable today. The two groups are so interdependent that extinction of species within either will often lead to extinction of species within the other.
Negative interactions between groups have included both competition and predation. As one example, during the period dominated by the dinosaurs, the progressive decline of several major groups of seafloor life is attributed to the expansion of predatory groups that remain prominent in modern seas: crabs, bony fishes, and carnivorous snails. The declining groups include certain kinds of bivalves, snails, and calcareous algae that were especially vulnerable to attack according to studies of functional morphology. The long-term result was a wholesale transformation of seafloor life.
Changes in the Physico-Chemical Environment
Nonbiological aspects of the environment also change in ways that can have positive or negative effects on particular forms of life. Some of these changes influence evolution and extinction by removing barriers to migration, thus allowing species to move to new regions. Tectonic events and changes in global sea level have had this effect by connecting landmasses or oceans that had previously been separated. The tectonic origin of the Isthmus of Panama, for example, served as a natural experiment for testing faunal equilibrium. Migration of mammals at first gave the appearance of maintaining equilibrium, but this has broken down in the past few hundred thousand years. Numerous northern forms infiltrated the South American system without having drastic effects on existing elements, until South American carnivores experienced heavy extinction. Apparently, the northern immigrants had some ability to migrate not possessed by their southern counterparts.
Environmental changes can also result in new habitats that tend to produce diversification of the groups that first gain access to them. The origins of islands and lakes epitomize this phenomenon. Just as modern mouth-breeding fishes proliferate rampantly in the lakes that have formed recently in rift valleys of Africa, primitive fish groups underwent spectacular diversifications in the rift valley lakes that developed as North America separated from Europe and Africa. Today, their 200-million-year-old fossils are preserved in lake sediments of eastern North America.
Other changes in the physical environment have had global effects. During the past 35-million-years or so, a decrease in mean annual temperature at the surface and an increase in seasonality and aridity promoted evolutionary changes in plants that propagated up the food chain. A proliferation of new species of seed-producing herbs and grasses contributed to a rampant diversification of seed-eating rodents and song birds, which in turn fostered a great increase in the diversity of predatory snakes (Figure 3.14).
Ironically, deterioration of a species' habitat can also promote evolutionary diversification if its effect is to fragment the habitat, producing isolation and eventual speciation. This occurred when the fragmentation of tropical rain forests in Africa and South America during dry intervals of the current ice age led to the origin of many new species in the small remnants of forest that survived until the return of better times.
Adaptive Radiation
Rapid proliferation of species within a group constitutes adaptive radiation. This process accounts for most evolutionary change. Typically, numerous distinctive new taxa arise during relatively brief intervals in the early stages of adaptive radiation. Fossil discoveries that date from 600-million-years ago, when organisms first developed hard skeletons, record the initial explosive radiation of animal life. But more recent, and more modest, radiations present special research opportunities when they can be studied with high-quality analytical tools that test diversification as well as spatial and temporal distri
FIGURE 3.14 A proliferation of new species of seed-eating rodents and song birds fostered a great increase in the diversity of predatory snakes.
bution. Most adaptive radiations have resulted from unusually high rates of speciation, but low rates of extinction, compared to those of closely related groups, have been important in some cases.
The adaptive shifts associated with radiations must be understood before the evolution of the biosphere can be adequately explained. Radiations often followed the evolution of adaptive innovations that greatly expanded the inhabitable domain of life. A few examples among many are the evolution of oxygen-mediating enzymes in Precambrian eubacteria, the protective roll-up reaction in trilobites, and advanced limb mechanics in mammal-like reptiles that were ancestral to dinosaurs.
When judiciously focused, functional morphological analysis—the study of anatomical forms that suit particular functions—underpins most identifications of adaptive breakthroughs. Adaptive radiations of some groups have been triggered by the extinction of competing groups or, in some cases, predatory groups. The adaptive radiation of mammals following the mass extinction of the dinosaurs may have resulted from decreases in both competition and predation. Lately, detailed studies of this adaptive radiation have revealed that a single mammal genus (Protangulum), after surviving that mass extinction, gave rise to some three dozen genera during the first 2 million or 3-million-years of the ensuing Age of Mammals.
Pulses of Extinction
Pulses of extinction are, in a sense, the negative equivalents of adaptive radiations. Normally, extinctions occur on local scales at a regular rate, but mass extinctions affect many groups throughout broad regions of the globe. The record of mass extinctions shows more sudden and drastic change than has ever been achieved by global radiations. No simple formula can be established for the optimal analysis of these events. During the 1980s, research into this exciting branch of paleobiology has found that the most fruitful approaches fall into two categories.
One approach investigates nonbiological evidence for causation at certain levels. This approach calls attention to iridium, an element generally very rare in the crust, and to shocked minerals, particularly quartz grains with distinctive sets of intersecting planar structures. Both are found worldwide in anomalously high concentrations at the level of strata that dates to about 66-million-years ago—the period of the dinosaur's extinction. These anomalies have been construed to be evidence of an extraterrestrial impact.
The second approach studies patterns of extinction—selective survival—in a search for clues to causation. This research investigates the characteristics of those species that remained after a mass extinction, like the marine phytoplankton with resting spore stages that survived the crisis of 66-million-years ago. This research indicates a tendency for mass extinction to strike most heavily in the tropics, which suggests an important causal role for climatic cooling. During climatic cooling, tropical organisms have no thermal refuge.
The dominant theme pervading both approaches to mass extinctions is the need for interdisciplinary research. Geochemical, sedimentological, tectonic, and even astronomical data have come into play. The endeavor to obtain the best possible stratigraphic resolution has helped to establish a reliable sequence of events. Paleoclimatic and paleoceanographic data provide frameworks that can support dependable interpretations.
Efforts directed at explaining the extinctions that
mark the geological record beginning 34-million-years ago exemplify these points. This crisis is especially amenable to study because it happened relatively recently. The stratigraphic record is of high-quality, and the well-established pattern of magnetic reversals provides an excellent temporal framework. Fossil records of planktonic foraminifera, calcareous nannoplankton, and terrestrial mammals show that the crisis was protracted, suggesting multiple pulses of extinction. As we have seen, changes in terrestrial paleofloras indicate that climates cooled in many areas; shifts in oxygen isotopes of foraminiferan shells reveal that water masses underwent major changes; and plate reconstructions suggest that the Antarctic cooling system for the deep-sea originated at this time. Nonetheless, the ultimate cause of this crisis remains controversial.
Pulses of extinction, when they have removed large numbers of species by imposing unusual and lethal conditions, can reset biological systems in ways that may have had nothing to do with the victim's ability to adapt successfully to ordinary conditions. This circumstance is epitomized by the extinction of the dinosaurs, which emptied ecospace to the benefit of the mammals. In recent years, dinosaurs have been recast as active, ecologically adept creatures—not backward lumbering forms that were inherently inferior to mammals.
A continuing question about global biotic crises in general is whether, through geological time, they form the tip of one tail in a unimodal distribution of extinction rates or whether they represent a statistical outlier; a sparse data base currently frustrates valid statistical testing. The second condition would imply unique causation rather than simply an accentuation of normal agents of extinction. On the other hand, periodic spacing of mass extinctions may suggest a highly abnormal cause. The possible regularity of these crises stimulates current debates.
At the other end of the distribution of extinction rates lies another question: When the record shows extinctions occurring at minimal rates, do species still disappear in groups or do they die off independently in piecemeal fashion? The former condition would require an expansion of catastrophic ideology to embrace even relatively calm intervals of biotic history. Finer resolution of the stratigraphic record may solve this basic problem of normal, or background, extinction levels.
Macroevolutionary Trends
Macroevolution transcends species boundaries, involving changes in the more generalized taxonomic levels, such as genus or family. Macroevolutionary changes include those trends in the marine realm that have been driven by the expansion of sophisticated predators on the seafloor over the past 100-million-years. Phyletic evolution can produce macroevolutionary trends, as can differences in rates of extinction and speciation among groups of species. An important question here relates to the relative importance of phyletic evolution in producing trends in the history of life. If it has been of secondary importance, as asserted in the punctuational model, differential rates of extinction and speciation gain importance. A further question concerning phyletic evolution addresses the degree to which it has been gradual and the degree to which it has followed a stepwise course, in which established species have undergone most of their changes during very brief intervals of geological time.
Trends can be documented only on the basis of careful taxonomic and biostratigraphic studies, and they can be interpreted only by considering the functional morphology and ecology of component species.
Origins of Major Biological Categories
Fossil data provide estimates of the times when higher biological groups evolved. For groups with excellent fossil records, early estimates remain unchanged even though large volumes of new data have accumulated. For those with poor fossil records, the estimates are vulnerable to new discoveries. Thus, although Archaeopteryx is remarkable for its intermediate morphological position between dinosaurs and birds, the early fossil record of birds is so poor that a recent claim for a much earlier bird cannot be rejected out of hand. The fossil record offers a unique opportunity to assess the origins of taxonomic groups at general levels—the origins of kingdoms in Precambrian time and then of phyla.
Times of origin for the more general groups, which are estimated from molecular data, do not always agree with those from fossil data. Molecular data clearly have great potential for the assessment of relative times of origin, but only well-dated fossils will estimate actual rates of divergence between forms. The environmental sites of major evolutionary breakthroughs are difficult to predict. These events may be concentrated in relatively unstable habitats, such as those of high latitudes or nearshore marine habitats, or in more stable habitats, such as those of the tropics or offshore marine habitats at middle or low latitudes. Hostile habitats generally offer more vacant ecospace, but more
hospitable habitats sustain more varieties of life and harbor more biological groups, which means that they can support greater total rates of speciation.
Origins of higher groups frequently reflect the evolution of adaptive innovations. Studies of ancestors and descendants in the fossil record can reveal the morphogenetic mechanisms that gave rise to innovations. Among these mechanisms are changes in the relative timing for development of different morphological features. This area of research offers great potential for fruitful interaction between paleobiologists, developmental biologists, and geneticists.
Phylogenetic Reconstruction
During the past two decades, cladistic analysis—the study of similarities resulting from common origins—has emerged as a powerful quantitative method for reconstructing the genealogical relationships called phylogenies. It is based on assessment of how traits that have evolved only once are distributed among taxonomic groups. Some proponents of cladistic analysis disregard the stratigraphic distribution of groups, grounding reconstruction of phylogenies on judgment as to which characteristics are primitive and which are derived. An alternative approach reconstructs phylogenies by evaluating stratigraphic and morphological distances between groups. Comparisons have shown that the two approaches sometimes yield identical results. Such comparisons are of great value, and methods that combine the two approaches warrant further examination. Molecular data also can reconstruct phylogenies; however, some of the techniques, including DNA hybridization, remain controversial, and their results must be compared to those achieved with the other approaches.
Catastrophes in Earth History
Geologists call sudden violent changes catastrophes, and they contrast catastrophes with the changes in the rock record attributed to constant gradual processes. There is obviously a continuum between frequent events, moderate events, and the occasional violent happening, and this simple relationship is readily expressed in empirical laws. Such relationships can, for example, link earthquake frequency with earthquake magnitude.
Nearly two centuries ago polarized positions were assigned to geologists—they were either catastrophists or uniformitarians. Since then geological interpretation has accommodated the occurrence of occasional violent events, of the kinds experienced within our own lifetimes—hurricanes, earthquakes, and volcanic eruptions. A uniformitarian approach has dominated because it is very effective in analyzing the rock record and in making correlations.
The possibility that the four or five great biological extinctions of the past few hundred-million-years marked catastrophic events never received serious attention because no evidence of a promising catastrophic mechanism had been recognized. That changed a decade ago when researchers, studying a few sites scattered over the globe, reported that strata that marked the mass extinction of about 66-million-years ago contained anomalously high concentrations of iridium and related platinum-group metals. Similarily, high concentrations have now been reported from dozens of localities worldwide of the same age. They are considered strong evidence of a catastrophic event close to the time of extinction of many groups of animals, of which dinosaurs are the most notable. This latter extinction event is one of the five largest extinction events to have occurred since fossils became abundant—the largest, 250-million-years ago, is represented by a less detailed record. But many things were happening 66-million-years ago; evidence indicates that the atmosphere and ocean were cooling and that the sea level was rising again after having fallen. Because the last vestiges of some life forms ceased to appear in strata that date to before the time of the iridium anomaly, it is argued that a catastrophic event may have been the final blow at a time of general environmental deterioration.
Two kinds of nonbiological catastrophic perturbations that have been suggested are a global volcanic episode and the impact of a meteorite. A high platinum-metal concentration could indicate origins from within the mantle, though such a volcanic episode would have to be extremely intense, and one of the largest lava eruption events of the past few hundred-million-years formed the Deccan basalts in India at just the right time. Catastrophic perturbation by extraterrestrial impact seems more probable, not only because meteorites exhibit high concentrations of platinum-group metals in the right proportion but also because of an association of the iridium-rich horizon with quartz grains showing the effects of intense shock. Shock features are commonly found with meteorite impacts but are unknown in volcanos. And perhaps even more significant is the existence of small beads of glassy material of the type produced by the heat released by meteorite impacts—microtectites. Several mechanisms have been proposed that show how meteorite impact could
result in sufficient environmental modification to cause catastrophic mortality. One computer simulation portrays the ejection of a dust layer into the high atmosphere that would cut out sunlight for months at a time, ending photosynthesis and sinking all latitudes into a deep cold. After the dust settled, high levels of atmospheric carbon dioxide would warm the Earth by producing a greenhouse effect. Another model suggests that the fast-traveling ejecta from the impact site could have been at temperatures high enough to cause atmospheric oxygen and nitrogen to combine, forming clouds that would precipitate into nitric acid rain. A third hypothesis, which is gaining increased acceptance, is that an impact of the proper age, formed the 180-km-diameter Chicxulub crater in the Yucatan of Mexico. Chicxulub strata is composed of thick sulfate-rich evaporite and carbonate deposits; an impact into such deposits could eject huge amounts of sulfate aerosols into the stratosphere, resulting in large-scale global cooling and several years of acid-rich rains as the aerosols settled out of the atmosphere—both the cooling and acid rains could have devastated the food chain. Several issues complicate the impact scenario—one strong argument cites evidence of animal groups that suffered heavy extinction before the primary iridium anomaly settled into place.
Regardless of what the eventual consensus on its cause may be, the iridium anomaly has stimulated intense geological research. Not only have the contemporary rocks been studied closely—the anomaly has been documented at about 100 sites throughout the world—but the deep ocean record has been scrutinized, revealing a perturbation of the earth system that lasted half a million years. The most significant result of this intense geological research may be that serious consideration of sudden global catastrophes is now acceptable.
All meteorite impacts are now receiving great attention. The roughly 100 known impact craters on Earth are being looked at anew, their ages are being reassessed, and attempts are being made to see whether their incidence could be periodic. An impact site in Sweden, 50 km in diameter, has been drilled to a depth of more than 6 km, allowing observations about the effects of large-body impact at depth. The discovery of iridium anomalies in some of the oldest rocks strongly suggests a flurry of meteorite impacts as late as 3.6-million-years ago. And the most drastic of catastrophes, collision between the Earth and a Mars-sized body before 4-billion-years ago, is now considered a possible explanation for the Moon's formation.
Observations of asteroids show that the current meteorite flux is likely to be higher than had been considered, and in 1989 one carefully observed asteroid passed closer to us than any other in the past 50 years. There is a calculable, if remote, chance that the Earth will be struck by an object more than a few kilometers in diameter in the foreseeable future. Such an event would cause massive destruction, and a case can be made for assessing the possibility of diverting an incoming object to avert such a potential collision.
No other global iridium anomalies have been recognized, although a locally defined anomaly dated at 34-million-years ago corresponds to a substantial extinction. No iridium or shocked quartz horizons have been found for the greatest extinction ever, which was 240-million-years ago; however, the absence of evidence, especially in older rocks, should not lead to a positive conclusion. There is certainly evidence that other ancient mass extinctions were complex events, extending over intervals of several million years.
The mainstream of the earth sciences has shifted away from the extreme uniformitarian position, which attempts to explain all phenomena in terms of directly observed processes. Now researchers, confident in the soundness of their inductive methodology, can consider the possibility of exceptional events. There is, of course, an appropriate reluctance to invoke exceptional events as causal mechanisms, and intense skepticism deservedly awaits all such suggestions. The study of catastrophic effects on earth systems requires further work. Promising directions for general research include their continuing consequences, such as how perturbations propagate through the earth system and how secular change is altered permanently by catastrophe.
MODELING THE EARTH SYSTEM
An Incomplete Record
Solid-earth scientists, like all other scientists, have long used conceptual models as an aid in understanding. For example, it was recognized in ancient times that the occurrence of seashells in rocks on the continents required a model in which the disposition of sea and land changed with time. By the nineteenth century this idea had developed further with the recognition that there had been widespread episodes of continental flooding that were correlatable over great distances. In the mid-twentieth century the repeated flooding and emergence of North America over the past 550-million-years were found to provide a strong framework for under-
standing historical geology, and, during the past 20 years, flooding and emergence have been found to be approximately simultaneous for at least three continents. This coincidence has led to the suggestion that the main driver of sea level change is within the world ocean. The amount of seafloor spreading going on at a particular time seems a strong candidate for the dominant control. Young seafloor is hot and shallow, and aged seafloor is cold and lies deep. The average rate of spreading and the total length of spreading center at any one time can be quantitatively associated with the extent of flooding of the continents.
Conceptual models that are progressively refined may be considered successful for parts of the earth system but are not really comprehensive. The question of continental flooding helps to show why this is so. The amount of water that is locked up in ice has decreased since about 20,000 years ago with the melting of North American and Eurasian ice sheets. This decrease has accounted for about 100 m of sea level rise over the same interval. This is a very large change in sea level compared with the range of continental elevation. About 80 percent of the continental area lies within 1 km of the present sea level. Although we can estimate the change in the average age of the ocean floor for the past 100-million-years we cannot do so well for the amount of water locked up in ice sheets. Evidence that there were no major ice sheets 100-million-years ago is strong, but it is not clear how long ago the great Antarctic ice sheet formed, nor can we tell whether it has fluctuated much or even disappeared and been renewed. This example illustrates a general situation. Comprehensive modeling of earth systems is very difficult, because although some parts of the system are understood, fluctuations in critical variables for other parts of the system are too poorly known to justify the construction of elaborate models. The challenge represented by the fact that some parts of the earth system can be readily modeled whereas others cannot has been met by concentrating on modeling those subsystems that can be handled well and by seeking innovative ways of quantifying information about subsystems that cannot. A further problem is linking the models of system parts that operate at different rates, such as the atmosphere, which changes on a very short time scale, and the ocean, which changes more slowly. The most successful and productive models reconstruct cyclic changes involving limited components of the earth system. Only a few components, or variables, can be tested at one time, but every successful simulation provides further understanding.
Some aspects of paleoceanography and paleoclimatology lend themselves to quantitative modeling in isolation from the whole. However, most are not, and, because of the interdependencies, the models should be global in scale, link adjacent water masses, and couple the atmosphere with the oceans, so great is the interdependence of these two great fluid envelopes. Atmosphere and ocean are linked especially by exchanges of heat, momentum, carbon dioxide, and, of course, water. The surface temperatures of the ocean influence these fluxes. The salinity of waters that reach the ocean bottom, a variable related to climate, also affects oceanic circulation. And changes in global sea level affect the levels of atmospheric carbon dioxide, which in turn influence global climate through the greenhouse effect. Outstanding successes include modeling of the glacial cycles of the past 0.8-million-years, as controlled by variations in orbital parameters, and modeling of sedimentary basin fill, as controlled by loads applied to the lithosphere.
Modeling of atmospheric systems, which mainly use energy balance models and general circulation models (GCMs), has grown to be extremely sophisticated in recent years, and solid-earth scientists have found some community of interest with atmospheric modelers. For example, at the time of the most recent glacial maximum, the solar energy input at the surface was less than it is today. A GCM of that time also indicates wind and temperature distributions that are very different from those of today. These indications have been compared with the geological record of the most recent glacial maximum with some success. Simplified energy balance models for the atmosphere for 100 million and 375-million-years ago, when continental distributions were very different and much of the surficial climate was warmer than it is now, have also met with some qualified success. There are some problems, too—for example, existing GCMs for the glacial maximum will not predict conditions that would grow the large ice sheets that existed then.
Intellectual Frontiers
Understanding the processes that are active today in establishing the surface environment and understanding how those processes have operated throughout geological time are the basic challenges addressed in this chapter. Intellectual frontiers relate to questions about the surface environment that have not been asked in the past, either because it was not possible to ask them or because it was not
recognized that they had meaningful answers. A distinctive new development is the perspective that views the environment as a complex of interactive systems. Now specific problems are posed, and solved, as part of a broader framework of global understanding. Remotely sensed imagery from space and organized international cooperation have done much to stimulate the global approach. In the next decade the operation of higher-resolution instruments on advanced space platforms, such as those envisaged for the Earth Observing System, will enhance the global perspective. But perhaps the most important efforts toward global understanding are made through programs that depend on international cooperation among scientists. The Ocean Drilling Program exemplifies this trend, as does the innovative International Geological Correlation Program and the International Geosphere-Biosphere Program.
Dating Past Events
Because geochronology scales physical, chemical, and biological events against time, it plays a fundamental role in the earth sciences. To appreciate what has happened, we need to know the sequence of events and the rates of change.
Quantitative biostratigraphic techniques yield correlations with accuracies approaching a few hundred thousand years for bodies of rock that are hundreds of millions of years old and lie thousands of kilometers apart. These methods of correlation are integrated with others, including paleomagnetic methods and radiometric dating of marker beds such as volcanic ashes. Together they encourage the search for high-frequency events and for regular patterns in such events. Correlation techniques and isotopic dating serve as checks on each other. Isotopic dating methods can focus on scales from billions to mere thousands of years, but when possible they should be integrated with other dating methods.
Global event stratigraphy correlates the worldwide expression of certain events. It provides a framework of additional instantaneous markers against which intervening events can be calibrated. The stratigraphic evidence of rapid global sea level change falls within this category, as does the identification of global chemical signals. The chemical signals include both narrow stratigraphic markers that formed during brief moments of geological time and long-term secular trends that trace continuing developments. The iridium anomaly, which marks the mass extinction of 66-million-years ago and may signal the impact of a huge meteorite, is the most famous geochemical marker in the stratigraphic record. But others have been, and will continue to be, discovered.
All of the challenging areas of research described in the following paragraphs—historical studies of oceans and atmospheres, terrestrial environments, and life on Earth—depend on advances in geochronology.
Atmospheric and Oceanic Chemistry
On the longest time scale, the geological record indicates a gross change from a reducing to an oxidizing state of the linked atmosphere-ocean system. The details of timing and the reason for this secular change still provide topics for lively debate. On shorter time scales, the record of the rocks preserves evidence of cyclical changes. Geologists can trace variable concentrations of carbon dioxide in the atmosphere and ocean on time scales ranging up to hundreds of millions of years. They have also distinguished episodes during which much of the deeper ocean was anoxic. Intervals when widespread anoxia in deep waters expanded to flood broad areas of the continents are especially interesting, because they resulted in the massive accumulation of valuable hydrocarbons from sources in black anoxic sediments. Some researchers think that the storage of so much organic carbon implies the possibility of an increased oxygen content in the atmosphere at such times in the past. Others consider that an inflammatory concept.
Past oceanic composition, recorded within ancient sediments, reflects many aspects of the global environment. These include the mantle contributions through volcanism and continental input through erosion, the global climate, the presence or absence of ice caps, and the level and kinds of biological activity. The history of ocean chemistry can be established from the rock record-an endeavor that is rewarding because it has been so successful. For the past 150 million years, the interval when the sediment now carpeting the deep-seafloor has been accumulating, ocean chemists can study changes among the individual water masses that together make up the world ocean.
The most fundamental variable controlling atmospheric and oceanic chemistry has been the temperature of the deep sea. But patterns of upwelling and shallower currents and high biological productivity have undergone dramatic shifts at frequent intervals. Changes in oceanic conditions, especially sea level, have exerted a strong control over evolution
and extinction and also over the formation of valuable resources.
Dynamics of the Global Environment
The evolution of the environment is an important area of research in modern earth sciences. The study of ancient conditions, when climates were warmer than today, offers insight into potentials of modern greenhouse warming. In addition, studies of different global ecosystems of the past may reveal peculiarities of the present world that render it especially vulnerable to certain forcing factors. The most recent segment of the geological record provides the temporal resolution and geographic control needed to identify very sudden environmental changes.
During the first 4-billion-years of earth history, life arose and evolved through many intermediate stages to a point at which a variety of multicellular plants and animals existed. Evolution and extinction during this interval were tightly interwoven with profound changes in the physical nature of our planet, especially its atmospheric chemistry. This intimate relationship between life and environment serves to underscore an important point about future directions of research. Different intervals of earth history require distinct scientific strategies, because the intervals were characterized by different kinds and degrees of environmental change and are represented by different kinds of geological records.
The prospects are exciting. But they require a prodigious amount of research to chronicle global environmental change for a variety of past intervals with the resolution required. Once models can accurately simulate environmental conditions of key intervals during earth history, they can be used for predicting future conditions. Collaborative modeling projects that unite workers in the geological sciences with meteorologists and oceanographers are beginning to yield results.
Life Through Time
Numerous advances have breathed new life into paleontology. The contributions of the fossil record to the study of evolution and extinction uniquely document myriad forms unknown in the modern world. A cumulative chronology indicates the rates of evolution and extinction, and the timing of major events in the context of environmental change. Such a chronology establishes fluctuations and patterns that can point to new questions about the history of life. The development and application of quantitative techniques will continue to play a prominent role in future studies of life through time. Key methods will assess morphological change; patterns of evolution and extinction; and theoretical modeling of taxonomic, stratigraphic, and environmental data obtained from the fossil record.
The fossil record also provides evidence of the timing of evolutionary branching. The molecular clocks used to estimate the times when certain extant groups branched from others must be calibrated against fossil data, and conclusions must be tested against macroscopic evidence. Of special importance are fossil data that reveal the occurrence of adaptive breakthrough—evolutionary innovations that ushered in new modes of life that transformed the ecosystem. These can be recognized in the fossil record only by inferring function from morphology, an activity that merits increased support. Many adaptive breakthroughs have triggered adaptive radiations—diversification from a single life form that results in the invasion of a variety of ecological niches. Such radiations have special significance because they account for most of the major evolutionary changes in the history of life. Understanding the general diversification of life on Earth requires an understanding of adaptive radiations.
Sudden extinctions—the negative equivalent of adaptive radiations—have repeatedly reordered the global ecosystem and opened the way for new evolutionary directions. Mass extinctions can be understood only in the context of global environmental change. The taxonomic, temporal, and geographic patterns that have characterized these events are especially significant; compilation of new data demands the continued generation of high-quality biostratigraphic and taxonomic information.
Whether global biotic catastrophes have occurred in combination with background extinction or instead have been quantitatively or qualitatively distinct is a question that can be answered only by understanding the patterns and causes of noncrisis extinctions.
The Most Recent Past
Geologists know that the record of the most recent past is exceptional because its complex history can be better established than that of any earlier period. Powerful new techniques are determining the ages, isotopic compositions, and temperatures of materials deposited during the past 2.5-million-years. But the most significant recent development is the worldwide awareness of changes in the global environment caused by humanity. This new awareness has stimulated a need to better understand the
TABLE 3.1 Research Opportunity Framework
|
|
Research Opportunities |
|
|
|
|
Research Areas |
A. Understand Processes |
B |
C |
D |
|
I. Paleoenvironment and Biological Evolution |
■: Soil development, history, and contamination ■: Glacier ice and its inclusions ■: Quaternary record ■: Recent global changes ■: Paleogeography and paleoclimatology ■: Paleoceanography ■: Forcing factors in environmental change ■: History of life ■: Discovery and curation of fossils ■: Abrupt and catastrophic changes ■: Organic geochemistry |
|
|
|
|
II. Global Geochemical and Biogeochemical Cycles |
■: Geochemical cycles: atmospheres and oceans |
|
|
|
|
III. Fluids in and on the Earth |
■: Analysis of drainage basins ■: Mineral-water interface geochemistry |
|
|
|
|
IV. Crustal Dynamics: Ocean and Continent |
■: Landform response to change ■: Quantification of thresholds, response rates and feedback mechanisms for landforms ■: Mathematical and computer modeling of landform changes ■: Sedimentary basins ■: Sequence stratigraphy |
|
|
|
|
V. |
|
|
|
|
|
|
Facilities, Equipment, Data Bases |
|
|
|
|
|
■: Exploit new tools and techniques (e.g., isotopes, trace compounds, DNA sequencing and hybridization, digitizing techniques) ■: Exploit new dating techniques (e.g., radiometric methods, trends in isotope ratios, biostratigraphic correlation, chemical markers in stratigraphy) ■: Acquire high-quality data bases and establish information systems |
|||
immediate past that is forging closer links between geologists and other earth scientists. The history of human evolution is beginning to unfold in sufficient detail to reveal the kind of environmental influences that affected human ancestors. All the challenges that have been identified here as intellectual frontiers have special possibilities for resolution when addressed in light of our understanding of the ongoing global changes during this most recent geological period.
RESEARCH OPPORTUNITIES
The Research Framework (Table 3.1) summarizes the research opportunities identified in this chapter, with reference also to other disciplinary reports and recommendations. These topics, representing significant selection and thus prioritization from a large array of research projects, are described briefly in the following section. The relevant processes operate near the surface for the most part, although there is no sharp boundary between surficial geology and the deep-seated processes covered in Chapter 2. There are excellent prospects for generating better models of the earth system, both present and past, including global tectonic models, coupled ocean-atmosphere biogeochemical models of the fluid envelope, and paleoecological models of the biosphere. The fossil record of the biosphere provides information about evolution as well as evidence of environmental changes and migration of continents.
The relevant Research Areas for this chapter are interrelated, and many of the research topics relate to more than one area. The carbon cycle (Area II),
for example, links to life and biological evolution (Area I) and has influenced paleoatmospheres and paleoceans theme (Area I), as well as playing a major role in continental weathering theme (Area III). It also has connections to the mantle through the igneous and metamorphic processes in the lithosphere and crust (Area IV), as discussed in Chapter 2. Some of the many applications of these Research Areas are outlined in Chapters 4 and 5.
Research Area I. Paleoenvironment and Biological Evolution
Soil Development, History, and Contamination
If extended to soils, the new age-determination capabilities that are being applied to landforms and drainage basins could quantify temporal aspects of soil development that have been the subject of much speculation. More complete understanding of soil processes as aspects of environmental, especially climatic, evolution will improve both paleosoil and soil-contamination studies.
Glacier Ice and Its Inclusions
Given the important role of glaciers in controlling sea level and climate, and considering the evident instability of glacial volume, it is essential that we gain a clearer picture of the history of glaciation since the onset of the recent ice age about 2.5-million-years ago. Especially important too is further study of the annual layers in ice cores, including analysis of oxygen isotopes, carbon dioxide, and dust to obtain a record of the past few thousand years. We need a much better understanding of glacial expansion and contraction and the role of environmental thresholds in governing these processes.
Quaternary Record
The record of change in the Quaternary (the past 1.6-million-years) is important because it is the most complete available record for any part of the past and thus provides the best picture of environmental change. Synthetic and snyoptic studies of Quaternary history (e.g., CLIMAP and COHMAP) have led the way. Extending studies of this kind with better spatially distributed data and higher temporal resolution will help to show how phenomena occurring simultaneously in different areas were related to each other, and will indicate sequences of events. This kind of information is needed to test atmospheric and oceanic models that are being used to assess what might happen in future global environments.
Recent Global Changes
The evolution of the ocean, atmosphere, and life during the first 4-billion-years of the planet's history is of great intellectual appeal and deserves continued emphasis. Nonetheless, of greatest practical value are studies of global change during the most recent interval of geological time. The geological record of the past 10,000 years has great potential for shedding light on the ways in which climatic changes affect life. In light of the global warming anticipated for the coming decade, warm intervals of the past should be scrutinized for possible lessons for the future. In addition, intervals exhibiting large-scale environmental change or mass extinction of life warrant detailed scrutiny for patterns and causes.
Paleogeography and Paleoclimatology
The past few years have seen major advances in paleoclimatology and terrestrial paleogeography. Sedimentary indicators and fossil biotas, especially floras, continue to shed light on climates of the past, but there is also a need to refine the existing kaleidoscopic picture of changing continental configurations, especially for the long pre-Mesozoic (older than 66-million-years) interval of earth history. Global paleogeographic reconstructions must continue to be developed and refined in all possible ways, including paleogeographic interpretations, global paleobathymetric reconstructions, identification of accreted terranes and microcontinents both through anomalous fossil distributions and paleomagnetic determinations. Determination of the rates of geochemical cycling requires knowledge of the sizes, elevations, and positions of continents in the past.
Paleoceanography
Changing climatic regimes, plate positions, and levels of land and sea have had powerful effects on the thermal structure and current patterns of the world ocean. At the forefront of paleoceanography today are studies of the three-dimensional oceanic structure, including thermohaline circulation, conditions in the deep-sea, upwelling, and vertical zonation of plankton. The Ocean Drilling Program should continue to play a key role in this kind of research for the most recent 150-million-years of
earth history, as should the use of isotopic data. The global influence of events in polar regions is in special need of further study.
Forcing Factors in Environmental Change
We are only beginning to understand the interrelationships between continental configurations, the dynamics of the ocean and atmosphere, and the distributions of life on Earth. In modeling global environmental change, sensitivity experiments that suggest what forcing factors have pushed environmental conditions across thresholds to new states are often more successful than detailed global simulations. At present, models are outstripping the data needed to constrain and test them, and research that will provide additional data is badly needed.
History of Life
Inasmuch as the fossil record represents a unique store of information on rates, trends, and patterns of evolution and extinction, we must continue to exploit it to understand these aspects of the history of life. There is still no consensus on such issues as the incidence or cause of evolutionary stasis, the degree to which extinction occurs in pulses, or what environmental changes trigger rapid evolution, including bursts of speciation. New quantitative techniques, including morphometric methodologies, must play an important role in research here. There is also a need to develop methods of phylogenetic analysis that integrate morphological and molecular approaches with stratigraphic data. Interactions between life and the environment—for example, how much the changes in atmospheric composition have been responses to evolutionary change and how atmospheric change has influenced evolution—must be established.
Discovery and Curation of Fossils
Our understanding of the processes of biological evolution continues to be refined by the discovery and description of fossils that fill gaps in the record. Examples of great steps forward made within the past decade are the discovery of new localities and material indicative of the diversity of marine life 550-million-years ago, identification of the conodont animal and appreciation that it was a vertebrate, critical new specimens of the first terrestrial vertebrates, and (stepping back into the ocean?) a whale with vestigial limbs. The kind of relatively unglamorous fieldwork that leads to these successes requires ongoing commitment.
Abrupt and Catastrophic Changes
Sudden events have a lot to teach earth scientists, particularly where their record extends over the whole world or at least very large areas. What are the causes of these events? Are they of impact, volcanic, or other origins? Does the rock record indicate precursory phenomena? Was there subsequent environmental change? If there is evidence of change, how long did it last? The behavior of the environment under stressed or extreme conditions is likely to be informative and rapid climatic changes are of special interest in the field of global change research. It is worth noting that had this report been written a dozen years ago little emphasis would have been given to catastrophes.
Organic Geochemistry
There are diverse ways in which organic geochemistry is yielding new information. New techniques for isotopic analysis of specific organic compounds, ''chemical fossils," provide opportunities for reconstructing the temperatures, compositions, and oxidation states of the ancient ocean. Working out the role of ancient microbial communities in sediments and illuminating the thermal histories of sedimentary basins are some of the challenges.
Research Area II.
Global Geochemical and Biogeochemical Cycles
Geochemical Cycles: Atmospheres and Oceans
It is crucial that we improve our understanding of geochemical cycles to learn how the atmosphere and oceans have changed in the past and how they may change in the future. Controls of atmospheric carbon dioxide, and the resulting greenhouse effect, are of especially great significance. Geochemical cycles are complex dynamic systems that entail geological, biological, and extraterrestrial processes. Changes in fluxes and in sizes of chemical reservoirs must be more accurately quantified for the geological past. Among the controls needing further study are the compositions and abundances of sedimentary rocks, magmatic and metamorphic degassing, biological uptakes and emissions, sea level change, rates of weathering, and isotopic shifts for key elements. Even fluxes to and from the modern ocean are poorly known, as are the contributions of relevant
organic biogeochemical processes. Mathematical techniques for modeling biogeochemical cycles can be greatly improved.
Research Area III Fluids in and on the Earth
Analysis of Drainage Basins
To understand past changes in our terrestrial habitat and to anticipate future changes and their consequences, it is imperative that geomorphologists undertake quantitative analysis of drainage basins. This analysis should quantify linkages and pathways of weathering sediment erosion, storage, transportation, and contamination. Links to sequence stratigraphy (see Chapter 4) will come from identifying the impact of base-level changes on fluvial systems. One important question is how the history of river flooding for the past few thousand years has related to climatic change. On long time scales there is a need to relate the rates of surficial processes to tectonic activity.
Mineral-Water Interface Geochemistry
The rapid growth in this field will lead to a more fundamental understanding of weathering, the chemistry and physics of mineral-water interfacial phenomena, how chemical species partition between minerals and aqueous fluids in crust, and how the hydrosphere interacts with crustal rocks.
Research Area IV Crustal Dynamics: Ocean and Continent
Landform Response to Change
Landforms represent the products of tectonic, climatic, and hydrologic processes. The ability to date surfaces and shallow sediments using cosmogenically generated nuclides and other direct and indirect methods provides the opportunity for improving understanding of how these processes generate landforms. The current strong interest in how tectonic, climatic, and hydrologic processes change with time makes this opportunity particularly timely.
Quantification of Thresholds, Response Rates, and Feedback Mechanisms for Landforms
Further definition is needed for stability/instability thresholds, response and recovery times for landforms following destabilizing events, and the significance of return frequencies of key climatic events for landform stability.
Mathematical and Computer Modeling of Landform Changes
Modeling will elucidate landform changes over longer periods of time than it is possible to observe them. Process-based models are essential for understanding mechanisms and for predicting future changes. Exciting progress has been made with, for example, hillslopes and river channel patterns.
Sedimentary Basins
Depositional basins are likely to reward detailed study because they record so many diverse kinds of information. The integrated approach to basin studies—which embraces structural, erosional, and depositional evolution as well as thermal, chemical, and fluid-flow history and uses field, seismic, well-log, geochemical, and isotopic data—is proving extremely powerful. Foreland basins and rifted continental margin basins, including great deltas, harbor the world's largest volumes of hydrocarbons. We are learning about how the sediment accumulation in these environments relates to tectonic and sea level changes and apparently to orbital parameters.
Sequence Stratigraphy
The identification of sediment packages that are separated by what appear to be abrupt temporal boundaries is an exciting new tool because it permits characterization of environmental changes that can be linked to episodes outside the area of deposition. Some researchers correlate boundaries in sequence stratigraphy globally, but this practice is questioned by others. Improved access to well-calibrated sequence data may help to resolve this issue.
Facilities, Equipment, and Data Bases
Data Bases in Well-Managed Computer-Based Information Systems
The major problem facing paleogeographers, paleoclimatologists, and paleoceanographers is that the ability to predict and understand the earth system at high-resolution is outstripping the availability of the data required to test the methods and models. Vigorous collection of and rigorous quality control on data from the ancient record is urgently
needed. Data and samples taken on continental margins and from wells drilled by industry represent an invaluable resource, as do many kinds of fossil data. The data base available to scientists could be expanded by several orders of magnitude.
New Tools and Techniques
New equipment offers much potential that has yet to be exploited in paleontology in the areas of isotopic analysis; identification of trace organic and inorganic compounds, DNA sequencing and hybridization, new multivariate techniques, digitizing techniques for three-dimensional quantification of morphology, application of geographic information systems (GIS) to quantitative historical biogeography, and computer-interfaced digitizers (some three-dimensional and some linked to light microscopes or scanning electron microscope systems) for morphometric studies.
New Dating Techniques
During the past few years, new dating techniques have greatly improved resolution in the temporal correlation of geological and biological events and in the measurement of rates for a wide variety of processes. These techniques include identification of key chemical markers in stratigraphic sequences, quantitative biostratigraphic correlation, application of new radiometric methods, and utilization of secular trends in stable isotope ratios. Not only the development of new dating techniques, but also the refinement and ingenious application of existing methodologies, will benefit numerous areas of research in the decades to come.
BIBLIOGRAPHY
National Research Council Reports
NRC (1982). Studies in Geophysics: Climate in Earth History, Geophysics Study Committee, Geophysics Research Board, National Research Council, National Academy Press, Washington, D.C., 198 pp.
NRC (1983). Opportunities for Research in the Geological Sciences, Committee on Opportunities for Research in the Geological Sciences, Board on Earth Sciences, National Research Council, National Academy Press, Washington, D.C., 95 PP.
NRC (1986). Global Change in the Geosphere-Biosphere: Initial Priorities for an IGBP, U.S. Committee for an International Geosphere-Biosphere Program, Commission on Physical Sciences, Mathematics, and Resources, National Research Council, National Academy Press, Washington, D.C., 91 pp.
NRC (1989). Technology and Environment, Advisory Committee on Technology and Society, National Academy of Engineering, National Academy Press, Washington, D.C., 221 pp.
NRC (1989). Margins: A Research Initiative for Interdisciplinary Studies of Processes Attending Lithospheric Extension and Convergence , Ocean Studies Board, National Research Council, National Academy Press, Washington, D.C., 285 pp.
NRC (1990). Research Strategies for the U.S. Global Change Research Program, Committee on Global Change, U.S. National Committee for the IGBP, National Research Council, National Academy Press, Washington, D.C., 291 PP.
NRC (1990). Studies in Geophysics: Sea Level Change, Geophysics Study Committee, Board on Earth Sciences and Resources, National Research Council, National Academy Press, Washington, D.C., 234 pp.
NRC (1991). Toward Sustainability: Soil and Water Research Priorities for Developing Countries, Committee on International Soil and Water Research and Development, Water Science and Technology Board, National Research Council, National Academy Press, Washington, D.C., 65 pp.
Other Reports
The International Geosphere-Biosphere Programme: A Study of Global Change (IGBP) of the International Council of Scientific Unions (1992). The Pages Project: Proposed Implementation Plans for Research Activities, Pages Scientific Steering Committee, 110 pp.
NASA (1988). Earth System Science: A Closer View, Earth System Sciences Committee, NASA Advisory Council, Washington, D.C., 208 pp.
Office of Science and Technology Policy (1992). Our Changing Planet: The FY 1992 U.S. Global Change Research Program, Committee on Earth and Environmental Sciences, 90 pp.














































