4
Origin and Variable Composition of Present Day Riverborne Material
MICHEL MEYBECK
Universite de Paris VI
ABSTRACT
Rivers are a major pathway in the global geochemical cycles of elements. However, little consideration has been given so far to the geographic distribution of their loads and contents because most scientists use only global figures. Actually, river chemistry is highly variable. Ionic contents are highly dependent on the lithology of the basin area; total organic carbon (TOC) and nitrogen are closely related to climate and vegetation; and total suspended solids (TSS) level and composition are linked to relief and climate. Finally, oceanic aerosols may greatly contribute to the dissolved load of surface waters. Because of these different sources, the chemical composition of water from small watersheds is extremely variable: about 15 chemical types have been reported, and most elements vary by two to four orders of magnitude. However, these ranges are only from one to three orders of magnitude for major rivers (>100,000 km2). When global budgets are considered, crystalline rocks contribute to only a minor proportion of dissolved inputs to oceans. Carbonate rocks (16.3 percent of the continental surfaces) and evaporites (1.3 percent) are by far the major sources, together with soil and atmospheric CO2, and oceanic aerosols.
The geographic origin of river load results from the combined influences of lithology, relief, and climate. Dissolved silica originates mainly from the humid tropics, as does total organic carbon derived from soil erosion; ions are relatively more abundant in the temperate regions due to their higher proportions of limestone; TSS originates from mountainous and dry regions found in the temperate zone, and the Huang He river alone accounts for more than 6 percent of the total river load. If the solid transport rate per unit area (Ts) is globally four times the dissolved transport rate (Td), the ratio Ts/Td actually varies from 80 to 0.1 for major rivers, and for 40 percent of the world rivers Ts/Td is < 1. Global averages of river loads are therefore of little use in understanding the present-day pattern of element circulation and distribution, and should be considered carefully when used in geological models.
INTRODUCTION
When referring to riverine material, many sedimentologists, geochemists, and even hydrobiologists consider global or continental averages. This tendency is now reinforced by the increasing use of present-day global river inputs to oceans to model past geochemical cycles (Berner et al., 1983; Wilkinson and Walker, 1989). Actually, global averages mask the extended chemical diversity of river water and particulates as well as the wide range of river transport rates.
The purpose of this chapter is (1) to assess the variability of river chemistry, (2) to review the main environmental factors that control these variations, and (3) to estimate the contributions to the global river loads from various geographic, geologic, and climatic environments. Because of the amount of information available, the focus is put on major elements, nutrients, and organic carbon. The anthropogenic influence is not considered here, [i.e., river data have been screened so that most polluted rivers have been discarded and only predamming data on total suspended solids (TSS) have been retained]. Extended compilations of river chemistry started in the 1960s (Durum et al., 1960; Livingstone, 1963; Turekian, 1969). More recent basic data have already been published or previously referenced (Meybeck, 1979, 1982, 1983, 1986, 1987, 1988; Kempe, 1982; SCOPE CARBON, 1982, 1983, 1985, 1987). In addition to these, some regional studies on river water chemistry in unpolluted or less polluted environments have been used: the Mackenzie river watershed (Reeder et al., 1972), Japan rivers in 1943 to 1957 (Kobayashi, 1960), Thailand rivers in 1956/1957 (Kobayashi, 1959), and the entire Amazon River basin (Stallard, 1980).
ENVIRONMENTAL FACTORS CONTROLLING CHEMISTRY OF WATER AND SUSPENDED MATTER
River water chemistry is controlled by many environmental factors, most of them known for a long time (Erikson, 1960; Gorham, 1961; Drever, 1982; Stallard and Edmond, 1981, 1983, 1987; Meybeck, 1984, 1986; Berner and Berner, 1987). They can be presented in three major groups: sources (lithosphere, atmosphere, biosphere), sinks (vegetation uptake, settling), and rate-controlling factors (temperature, water circulation). In addition, the river basin size plays a major part through the integration of diverse environments.
Lithology is a key factor for most major dissolved elements (Si, Ca, Mg, Na, Cl, S, C). Carefully selected, pristine monolithologic watersheds are characterized by distinctly different water quality compositions (Table 4.1A),
TABLE 4.1 Variability of Dissolved Major Elements in Pristine Stream Waters Draining Various Rock Types
|
|
Elec. cond. |
pH |
TZ+ |
SiO2 |
Ca2+ |
Mg2+ |
Na+ |
K+ |
Cl- |
SO4- |
HCO3- |
|
A. Most common rock types |
|||||||||||
|
Granitea |
35 |
6.6 |
166 |
150 |
39 |
31 |
88 |
8 |
0 |
31 |
128 |
|
Gneissa |
35 |
6.6 |
207 |
130 |
60 |
57 |
80 |
10 |
0 |
56 |
135 |
|
Volcanic rocksa |
50 |
7.2 |
435 |
200 |
154 |
161 |
105 |
14 |
0 |
10 |
425 |
|
Sandstonea |
60 |
6.8 |
223 |
150 |
88 |
63 |
51 |
21 |
0 |
95 |
125 |
|
Shalea |
— |
— |
770 |
150 |
404 |
240 |
105 |
20 |
20 |
143 |
580 |
|
Carbonate rocka |
400 |
7.9 |
3,247 |
100 |
2,560 |
640 |
34 |
13 |
0 |
85 |
3,195 |
|
Evaporitic depositsb |
1,700 |
8.0 |
18,000 |
125 |
3,060 |
1,440 |
13,500 |
90 |
13,500 |
2,340 |
2,160 |
|
Evaporitic depositsc |
— |
— |
20,000 |
110 |
12,400 |
7,000 |
600 |
40 |
600 |
15,000 |
4,400 |
|
B. Influence of water-rock interaction on water chemistry |
|
|
|
|
|
|
|
||||
|
U.S. riversd |
|
|
2,800 |
232 |
1,600 |
700 |
430 |
70 |
300 |
650 |
1,800 |
|
U.S. groundwaterse |
|
|
4,914 |
282 |
2,500 |
950 |
1,400 |
64 |
450 |
700 |
3,700 |
|
Note: TZ+ is the sum of the cations µeq/l) SiO2 in µmole/l; ions in µeq/l. a Averages from survey of 250 pristine streams in France (Meybeck, 1986) and from 75 sites worldwide, both corrected for oceanic Cylic salts (Meybeck, 1987). b Mostly rock salt, 13 watersheds (Meybeck, 1987). c Mostly gypsum and anhydrite, 6 French streams (Meybeck, 1986). d Discharge-weighted average for the Mississippi, Columbia, and Colorado rivers; references in Meybeck (1979). e Median distributution from Davis (1964), potable groundwaters. |
|||||||||||
directly related to the mineralogy of surficial rocks exposed to weathering. Stallard (1988) has defined the sequence of mineral stability in tropical soils (from the more stable to the less stable): quartz >> K-feldspar, micas >> Na-feldspar > Ca-feldspar, amphiboles > pyroxene, chlorite > dolomite > calcite > gypsum, anhydrite >> halite. Water chemistry results from the combination of this weathering order and the relative abundance of the minerals. Meybeck (1987) has ordered the chemical weathering of common rock types found in France on the basis of total cations (TZ+) observed in pristine streams (in order of increasing weathering): granite, gneiss, micaschist < gabbro, sandstone < volcanic rocks (basalts mainly) < shales < serpentine, marble, amphibolite << carbonate rocks << gypsum < rock salt. The influence of lithology on TSS level and chemical composition is much less known. In regions where high mechanical erosion occurs, thus exceeding chemical erosion, river particulates are a mixture of rock debris and poorly weathered soil particles and closely reflect the chemical composition of parent rock (Table 4.2A). The following scale of rock sensitivity to mechanical erosion is tentative (in order of increasing erosion): pure limestones < granite and gneiss < micaschists < consolidated volcanic rocks < shales << volcanic ash, sands, glacial deposits < clays < loess. Based mainly on major rivers, it remains to be tested on small monolithologic watersheds.
Atmospheric aerosols may greatly affect the chemistry of surface waters (Gorham, 1961; Sugawara et al., 1982), particularly where waters drain crystalline rocks and near the ocean coastline. Ocean aerosols are the major source of major elements (Na+, Cl-, Mg2+, SO42-) in atmospheric precipitation and can be traced as far as 2000 km inland, as in Central Amazonia where they still contribute to an important part of the so-called Black Waters, the less mineralized world waters (Stallard, 1980; Stallard and Edmond, 1981, 1983). Near the ocean, relatively high ionic contents may result from atmospheric inputs as in pristine streams draining sand deposits in the extended Landes pine forest in southwestern France (Figure 4.1). From the coastline to 20 km inland, stream waters are rich in NaCl, and all major ions (Ca2+, Mg2+, Na+, K+, Cl-, SO42-) show a steep exponential decrease. From 20 to 100 km, Na+ and Cl- still decrease at a lower rate, whereas Ca2+, Mg2+, SO42-, and K+ show a marked increase. This last feature is intriguing and could be due to the influence of continental aerosols partly produced by the pine forest itself. From 0 to 100 km, the atmospheric inputs far exceed the weathering products of these quartz and feldspar sands.
Evapotranspiration of water within river basins leads to the concentration of solute by a factor equal to the rain/ runoff ratio. In the previous example of Landes streams (Figure 4.1), the NaCl pattern mimics the Cl- double exponential decrease observed in rainfall with a stream/rain concentration factor of 3:1 similar to the rain/runoff ratio. In New Zealand, the stream/rain ratio for Cl- is (47.5 mg/l)/ (3.9 mg/l), close to the rain/runoff ratio of 10 (Claridge, 1973). Where waters are concentrated through evapotranspiration, the CaCO3 saturation limit may be reached; and only the Ca2+—SO42- or Na+—Cl- are left in these saline rivers (TZ+ > 10 meq/l), which are generally not perennial.
Rainfall pattern is a major factor in mechanical erosion and TSS transport. Where annual rainfall is distributed during a few rainstorm events (arid regions) or during a short intense rainy period (wet subtropics), the TSS levels found in rivers are the highest, as in the southwestern United States or in monsoonal southeastern Asia.
TABLE 4.2 Chemical Composition of River Suspended Matter (wt.%)[a]
|
|
SiO2 |
Al2O3 |
Fe2O3 |
MnO |
MgO |
CaO |
Na2O |
K2O |
TiO2 |
IL (1000°C) |
|
A. Influence of lithology |
||||||||||
|
Migmatite |
81.5 |
9.22 |
1.82 |
0.03 |
0.51 |
trace |
0.96 |
2.82 |
0.55 |
2.09 |
|
Basalt |
62.2 |
14.8 |
7.53 |
0.17 |
2.87 |
4.97 |
3.01 |
2.34 |
1.96 |
0.0 |
|
Limestone |
45.2 |
6.67 |
2.49 |
0.04 |
1.42 |
24.9 |
0.35 |
1.07 |
0.34 |
17.8 |
|
B. Influence of climate and geologyb |
||||||||||
|
Tropical and arid zone |
56.5 |
21.5 |
8.8 |
0.11 |
1.6 |
1.05 |
0.69 |
2.19 |
1.2 |
|
|
Cold and temperate zone |
60.6 |
14.2 |
6.65 |
0.14 |
2.1 |
4.4 |
1.1 |
2.75 |
0.8 |
|
|
Huang He |
57.8 |
15.1 |
4.57 |
0.10 |
2.17 |
8.4 |
1.2 |
(2.5) |
0.66 |
|
|
Note: The ignition loss (IL) accounts for CO2 from particulate organic carbon and carbonate minerals. a A Monolithologic French Streams, average altitude of watersheds is 1000 m. b Average values based on about 10 major rivers each (Meybeck, 1988). |
||||||||||
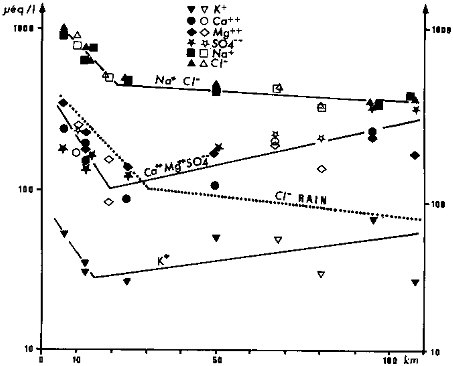
FIGURE 4.1 Evolution of stream water chemistry in the Landes forest (southwest France) as a function of the average distance of the watersheds from the ocean. The ocean and continental (forest origin?) aerosols that mask the chemical weathering of quartz and feldspars sands are evident from the exponential decrease of Na+ and Cl-.
Temperature influence on weathering is best evidenced for silica and for total cations (TZ+) originating from crystalline rocks (Figure 4.2). This positive relation is probably due to the combined influence of feldspars dissolution kinetics and of higher bacterial activity in soil of the tropical belt. For carbonate rocks, an inverse relationship between temperature, Ca2+ and HCO3- has been observed (Harman et al., 1975).
Contact between water and rock (water residence time, nature or contact) is an important factor. As a result, phreatic groundwaters are usually one to three times more mineralized than surface waters depending on the element considered (Table 4.1B).
Geomorphologic features control both TSS and dissolved organic carbon (DOC). In similar climatic types with identical dominant vegetation, those river basins with a greater proportion of wetlands (peat bogs, swamps, etc.) have much higher DOC levels. In the well-drained Mamai basin, New Zealand, the average DOC is 4.5 mg/l compared to 43.5 mg/l for the proximate Larry basin draining a peat bog (Moore, 1987). Although much less documented, the geomorphologic influence on nitrogen and phosphorous levels and transport rates by rivers is likely. Relief pattern, particularly the slope distribution, is a key factor in mechanical erosion: all mountain ranges are drained by rivers with high TSS levels (Milliman and Meade, 1983).
Vegetation influence on river chemistry is still poorly known, except for the well-known nutrient increase in waters after a forest fire. Concerning TSS, the vegetal cover is one of the four major controlling factors in mechanical erosion, along with bedrock, slopes, and rainfall pattern: unprotected bare soils are sensitive to rainsplash impact, and developed root system lower mass wasting and subsequent erosion.
Past geological history in a river basin is seldom considered although post glacial features may affect chemical and physical weathering in many ways. Glacial deposits in those mountain previously glaciated (Alps, Rockies, Himalayas) are still an important source, if not the major one, of river particulates. The Quaternary eolian loess deposits are a peculiar case: they form the major TSS source of the Huang He River, the most turbid river (TSS average > 20 g/l, about 6 percent of the present global TSS input to oceans). Past geological events may also limit chemical weathering: (1) when former glacial abrasion has left only bare rock, as in Canadian and Scandinavian Shields, the chemical weathering is limited; (2) in lowland regions exposed to erosion for millions of years, as is common in Africa, the resulting soil layer extends over meters or more and is highly depleted in soluble elements and the subsequent chemical erosion is limited. When these important soil layers have developed, the river TSS is generally low and enriched in the less soluble elements (Al, Fe, Ti, Mn), whereas Ca, Mg, and Na are strongly depleted; Si and K are generally similar to parent rock. The average TSS composition of active mountain ranges is close to the average shale (Table 4.2B). The Huang He TSS has a loess composition.
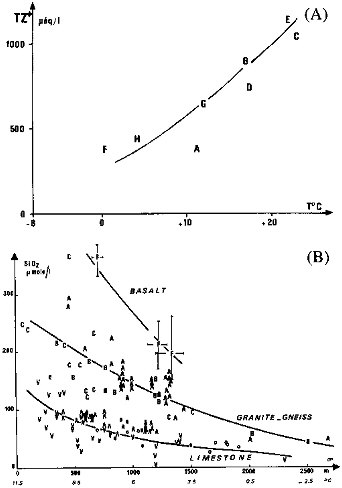
FIGURE 4.2 Influence of temperature on stream and river chemistry (monolithologic basins). (A) Total cations (TZ+) in volcanic basins. Columbia River tributaries. A (Santos, 1965); Kenyan rivers, B; Java rivers, C (Kobayashi et al., 1974); La Reunion rivers, D; Uganda rivers, E (Viner, 1975); Icelandic rivers, F; Japan rivers, G (Kobayashi, 1960); French Streams, H (Meybeck, 1986). (B) Dissolved silica in French monolithologic streams at various altitudes (Meybeck, 1986). Three sets of watersheds: basalts, gneiss and granite, limestone.
Lake retention of calcite, nutrients (organic C, N, P, Si), and of TSS may greatly affect river chemistry. In many hard-water lakes, calcite precipitates during the planktonic production period, when pH increases. In such systems, 25 to 40 percent of Ca2+ and HCO3-may be retained. The biological uptake of nutrients decrease NO3-, PO43-, and dissolved SiO2 at lake outlets. In alpine French lakes, 40 to 80 percent of dissolved silica inputs are stored in lake sediments. The settling rate of TSS in lakes commonly varies between 90 and 99 percent, and only the finest and most organic particles may escape the trap.
Tectonic and volcanic controls on river chemistry and transport rates are multiple. Active volcanism, an event barely studied, is probably important on a long-term scale as a primary source of ions (Ca2+, Na+, SO42-, F-, Cl-, NH4+, etc.) and of TSS, which do not originate from the recycling of sedimentary material, the major source of river material. Uplift rates are probably a key factor in long-term river transport, as Stallard (1988) described for the humid tropics: in the South American Shield (uplift rates of 10 to 20 mm/1000 yr) and in tectonically active environments such as Taiwan (rates of 103 to 104 mm/1000 yr), the erosion rates are of the same order of magnitude or slightly lower than the uplift rates. Other environmental factors rarely encountered may also influence river chemistry, for example, hydrothermal springs.
Globally, the control of water chemistry—mainly by thermodynamic equilibrium at the mineral scale (Garrels and Christ, 1965)—is much more complex than sometimes indicated (Gibbs, 1970) when streams and rivers are considered. Total suspended solids are also regulated by numerous factors that prevent any simple correlation between TSS levels or TSS transport rate (Ts, in t/km2/yr) with runoff, temperature, relief, etc. (Walling and Kleo, 1979; Walling and Webb, 1983). A multiple regression approach is needed, as proposed by Janssen and Painter (1974), under the condition that river basin size is taken into consideration, because the amount of particulate matter transported downstream by a river is only a small part, the sediment delivery ratio, of the material produced by upland erosion (Walling and Kleo, 1979).
DISTRIBUTION OF RIVER WATER QUALITY
The distribution of ions, silica, organic carbon, and TSS in world river systems depends on the relative importance of the environmental factors mentioned and on the basin size. Because of the great diversity of environments, particularly of surface rock types, found on the scale of streams and small rivers (area < 1000 km2), their water chemistry can vary considerably: from two (DOC and SiO2) to four (Na+ and H+) orders of magnitude, depending on the element considered. In major rivers (area > 105 or 106 km2), the effect of extreme environments, such as evaporite outcrops, loess deposits, or peat bogs, is highly diluted by the influence of more common environments such as crystalline rock outcrops and well-drained systems. In these major systems, the water quality is more unified and ranges of concentrations are one order of magnitude less than for small streams. This evolution is illustrated on Figure 4.3 for dispersion of major cation sum (TZ +) as a function of basin size.
In given river systems, the statistical distributions of ions and silica clearly reflect the prevailing regional conditions and the occurrence of some rare environments. Five regional distributions have been set up for medium-sized
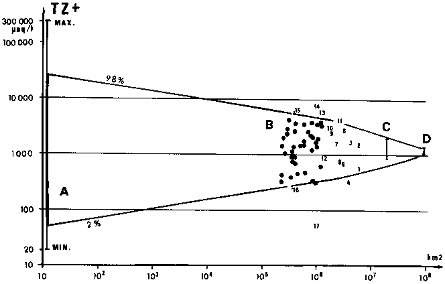
FIGURE 4.3 Range of total cations (TZ+) in streams and rivers as a function of basin size. (A) Distribution observed for small monolithologic watersheds from France and other regions, worldwide (Meybeck, 1986, 1987). (B) Distribution for major world rivers (Meybeck, 1979); 1 = Amazon, 2 = Ob + Yenissei, 3 = Lena + Kolyma + Indigirka, 4 = Zaire, 5 = Amur, 6 = Parana, 7 = White Nile, 8 = Mississippi, 9 = Chiang Jiang, 10 = Mackenzie, 11 = Volga, 12 = Zambezi, 13 = Murray, 14 = Texas, 15 = rivers in Spain and Portugal, 16 = North West Territories, 17 = Rio Negro (Brazil). (C) Range for the global watersheds of the four oceans. (D) World mean.
rivers, for the arctic and subarctic Mackenzie river watershed (data from Reeder et al., 1972), for the Lower and Central Amazon basin (Stallard, 1980), for the Andean tributaries of the Amazon (Stallard, 1980), and for the rivers of Japan and Thailand (Kobayashi, 1959, 1960). A global distribution is also considered based on 50 major world rivers (distribution weighted by water discharge, data from Meybeck, 1979). Three distributions for small streams are given: (1) a set of 250 monolithologic pristine French Streams located 20 to 600 km from the coastline (Meybeck, 1986); (2) a set of 75 monolithologic streams (Miscellaneous Streams) from various literature sources (chosen to be representative of global rock types) and grossly corrected for oceanic aerosol influence; (3) a set of pristine streams, the Temperate Stream Model, derived from set 1 after systematic correction of atmospheric inputs and selected as representative of the global distribution of rock types (Meybeck, 1987). The distribution of some ions and silica is given for these small, medium, and large rivers on Figure 4.4 and Table 4.3.
The K+ distribution (Figure 4.4A) can be considered as unimodal and log-normal. Potassium originates mainly from a few aluminosilicate minerals that weather at comparable rates. For the Temperate Stream Modal, K+ is much lower, due to an overcorrection of atmospheric inputs. Other distributions are similar, median values are within a factor of two and distributions are parallel, except for Thailand rivers for unknown reasons. The sample of major rivers is similar to the French Streams and Miscellaneous Streams, which indicates that the data used are still largely unaffected by pollution.
The dissolved silica pattern (Figure 4.4B) is similar to that of K+, which confirms a common origin. A major discrepancy is noted for the Mackenzie tributaries for which the SiO2 level is about half that in other regions and has a significant drop in the lower decile value. This is most probably due to the SiO2 uptake in numerous lakes of this basin, whereas K+ is not used by freshwater plants. As a result, the K-/SiO2 ratio of medians is 0.36 in the Mackenzie, compared to 0.04 to 0.07 for other pristine waters (Meybeck, 1987).
Bicarbonate distribution (Figure 4.4C) is usually bimodal. The first mode corresponds to the soil and atmospheric CO2 used in the weathering of noncarbonate rock (Garrels and Mackenzie, 1971), the second to the weathering of carbonate rocks, in which only 50 percent originate from calcite or dolomite. Concentrations related to the second mode are generally 5 to 10 times greater than those of the first one. In large basins where carbonate rocks are absent, as in Central and Lower Amazonia, the distribution is unimodal. In sedimentary regions, the HCO3- distribution shows an upper limit near 6 meq/l that corresponds to the CaCO3 saturation.
Sodium distribution (Figure 4.4D) is also complex, due to a triple source: weathering of aluminosilicate mineral, weathering of rock salt, and inputs of oceanic aerosols. The Central and Lower Amazon tributaries have an unimodal Na+ distribution reflecting the first source only. The influence of oceanic aerosols is well illustrated by Japanese rivers; the minimum Na+ values are much higher than those of the Amazon tributaries. Rock salt dissolution leads to the very high values, from 0.5 to 10 meq/l, noted for the Mackenzie tributaries, Andean rivers, French Streams, and Miscellaneous Streams.
The percentiles values of distributions are presented on Table 4.3A for small streams (French streams and literature
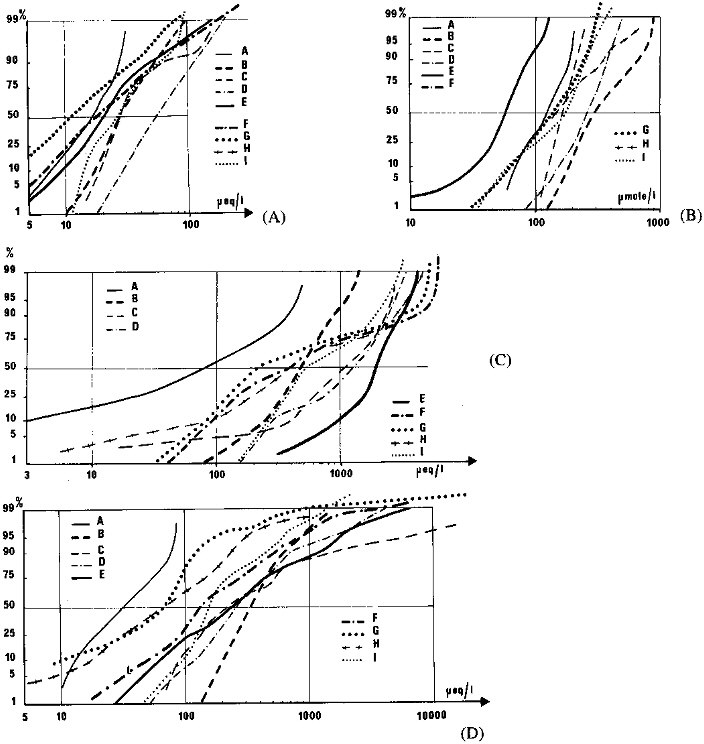
FIGURE 4.4 Cumulative distributions of major dissolved elements [(A) potassium, (B) silica, (C) bicarbonate, and (D) sodium] in rivers from selected regions: Central and Lower Amazon, A (n = 40. data from Stallard, 1980); Japan, B (n = 225, from Kobayashi, 1959); Andean tributaries of the Amazon, C (n = 42, from Stallard, 1980); Thailand, D (n = 31, from Kobayashi, 1960); Mackenzie, E (n = 100. from Reeder et al., 1972); monolithologic French Streams, F (n = 250, from Meybeck, 1986); Temperate Stream Model, G (Meybeck, 1987); monolithologic Miscellaneous Streams, H (n = 75, from various sources); major world rivers, I (n = 60, from Meybeck, 1979).
survey) and for the major rivers (discharge weighted distribution). If the 98 percent/2 percent ratio is taken as an index of the natural variability of water chemistry, the most variable elements in small streams are (in order of decreasing variability):
H+ = HCO3- > TSS > Cl- = Na+ = SO42- > Ca2+ > Mg2+ >> SiO2 = DOC > K+.
For major rivers, this order is somewhat different:
TSS > Cl- > Ca2+ = SO42- > Na+ = HCO3-= Mg2+ > PO43- = NH4+ > SiO2 > NO3-.
The great variability of H+ and HCO3- in streams is due to the occurrence of the acidic Black Waters of the Amazon and Orinoco basins (Stallard, 1980, 1988). In major rivers,
TABLE 4.3 Statistical Distributions of Dissolved Concentrations, TSS, and of Rates of Total Dissolved Transport (Td) and Particulate Transport (Ts)
|
|
Ma |
1% |
10% |
25% |
50% |
75% |
90% |
99% |
|
A. Distribution of selected major ions (µeq/l) |
||||||||
|
Small streamsb |
||||||||
|
K+ |
|
3.3 |
7 |
11 |
19 |
30 |
60 |
170 |
|
Na+ |
|
<3 |
19 |
32 |
70 |
140 |
220 |
≥800 |
|
Ca2+ |
|
10 |
40 |
100 |
210 |
800 |
2,100 |
5,500 |
|
Major riversc |
||||||||
|
K+ |
33 |
12 |
15 |
19 |
27 |
40 |
65 |
100 |
|
Na+ |
225 |
50 |
95 |
125 |
160 |
250 |
520 |
1,600 |
|
Ca2+ |
670 |
90 |
160 |
250 |
400 |
900 |
1,500 |
2,700 |
|
B. Transport rates of major rivers (t/km2/yr)d |
||||||||
|
Td |
40 |
<2 |
6.0 |
14 |
27 |
45 |
64 |
160 |
|
Ts |
175 |
3.2 |
6.4 |
10 |
45 |
100 |
500 |
1,900 |
|
Td/Ts |
4.4 |
<0.1 |
0.3 |
0.5 |
1.3 |
4 |
8 |
50 |
|
C. Total suspended solids (TTS) (g/l) |
||||||||
|
Streamse |
|
|
40 |
|
700 |
|
6,000 |
|
|
Medium-sized riversf |
|
|
70 |
|
600 |
|
4,500 |
|
|
Major riversg |
||||||||
|
M = 415 |
|
|
40 |
|
180 |
|
1,000 |
|
|
a M is equal to discharge weighted natural content (DWNC, Meybeck and Helmer, 1989) b Based on a set of 75 Monolithologic basins, chosen to be representative of global lithology distribution, corrected from ocean atmospheric inputs. c Based on a set of 60 major rivers (Meybeck, 1979), distribution weighted by water discharges. d Same source as footnotes c (Meybeck, 1976, 1979), distribution weighted by drainage area. Ts values prior to river damming. e Area from 10 to 103 km2., f area from 104 to 106 km2., g area from 104 to 106 km2. From a set of 128 world rivers (Fournier, 1969). |
||||||||
the pH is commonly between 6.5 and 8.2. The TSS distribution presented in Table 4.3B is tentative because it is highly dependent on the sediment delivery ratio (SDR). Because SDR is a function of basin size, the TSS distribution is presented for three classes of basin area. Median TSS value and TSS range are both greatly reduced in major rivers.
The chemistry of streams and small rivers in very different environments is highly variable (Stallard, 1980; Meybeck, 1986) and more than 15 different water types and subtypes have been observed (Ca2+— HCO3-, Ca2+—SO42-, Mg2+—HCO3-, Mg2+—SO42-, Na+—HCO3-, Na+ —SO42-, Na+—Cl-, etc.).
The chemical types of major river waters are much less variable than for small basins. When the water discharge to oceans is taken into account, the Ca2+—HCO3- type of water is largely dominant, about 97.3 percent (46.7 percent for the Ca2+ > Mg2+ > Na+ > K+ and HCO3- > SO42- > Cl- subtypes; 33.1 percent for the Ca2+ > Na+ > Mg2+ > K+ and HCO3- > Cl- > SO42- subtypes; and 17.5 percent for other Ca2+—HCO3- subtypes). Other major types are Ca2+—SO42-, about 1 percent, and Na+—HCO3-, about 1.4 percent.
CONTROL AND DISTRIBUTION OF RIVERINE TRANSPORT RATES
Transport rates (t/km2/yr) of individual elements i (Tdi), of total dissolved solids (Td, sum of major ions and dissolved silica rates), and of total suspended solids (Ts) are the products of discharge-weighted by annual mean concentrations Cd (mg/l), by annual river runoff q (mm/yr or l/s/km2). Some major ions show an inverse correlation between Cdi and q: Cdi = aqb with -1 < b < 0. Most other water quality descriptors either are not directly correlated with q or present a positive correlation. Therefore, in a given homogeneous environment, where q is the only variable, all of the transport rates of any riverine material would be positively correlated with q. If the region is heterogeneous, the environmental factors (lithology, tem-
parature, morphology, etc.) will scatter the Tdi or Ts versus q relationships. Two examples of such scattering are presented in Figure 4.5 for the calcium transport rate (TdCa2+) in major rivers, still controlled by the lithological nature of the watersheds, and for the dissolved silica transport rate (TdSiO2), still influenced by annual air temperature.
The plot of Td versus Ts plot for major rivers is highly scattered, but when the biggest rivers are considered, the correlation is positive (Figure 4.6) and quite different from the one observed by Judson and Ritter (1964) for U.S. rivers. Maximum Td and Ts are both observed for high mountain watersheds in humid climate due to high runoff, steep slopes, and occurrence of sedimentary rocks. Minimum Td is noted in arid lowlands and in crystalline shields; minimum Ts is also noted in shields or in very flat sedimentary lowlands. When the Ts/Td ratio is plotted against average runoff q (Figure 4.6B), the figure is much more complex than the inverse trends described by Langbein and Dawdy (1964) and Leopold et al. (1964) due to a major control by basin morphology. For specific elements, relationships may also be drawn between dissolved and particulate transport. Although this data base is much less documented than total transport rates, the correlation Tdi versus Tsi is also positive, except for organic carbon.
Global statistics of Td, Ts, and Ts/Td for 60 major world rivers are presented in Table 4.3C. As ionic concentrations were weighted by river discharge (Table 4.3A), the transport rates presented here are weighted by the river drainage area. The total river sample corresponds to 45 to 65 percent of the nonglaciated continental exorheic area (99.9 x 106 km2) and of the river water discharged to the oceans (37,400 km3/yr; Baumgartner and Reichel, 1975). Because of the skewed distributions of Ts, the dissolved transport predominates in more than 40 percent of the exorheic area, whereas the global Ts/Td ratio is about 4.
GLOBAL ORIGINS OF RIVERBORNE MATERIAL
An important part of the continental area (148.9 x 106 km2) is not drained by rivers to the oceans: (1) 22.3 percent is drained toward the interior of continents, as the Caspian Sea of Lake Chad, which corresponds to a water discharge of 2000 km3/yr (which ultimately evaporates)
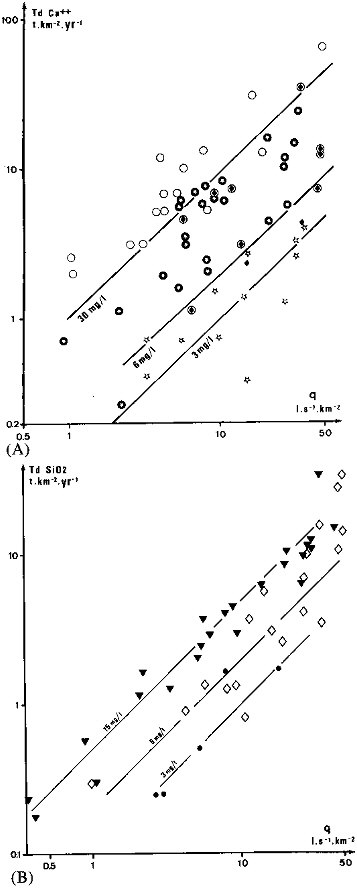
FIGURE 4.5 Evolution of annual dissolved transport rates of calcium and silica (Td in t/km2/yr) as a function of annual runoff (q in l/s/km2) for major rivers. (A) Calcium transport is influenced by the watershed lithology ( granite and gneiss basins; ♦; volcanic;
granite and gneiss basins; ♦; volcanic;  sedimentary). (B) Silica transport is influenced by average basin temperature (• cold regions; à temperate;
sedimentary). (B) Silica transport is influenced by average basin temperature (• cold regions; à temperate;  tropical).
tropical).
and (2) 10.5 percent is covered by ice caps, mostly Antarctica, which discharge about 2300 km3/yr of ice to oceans (Baumgartner and Reichel, 1975).
What is the influence of environmental factors on riverine transport on a global scale? This question was raised a long time ago for dissolved materials. Erikson (1960) and Garrels and Mackenzie (1971) tried to estimate the relative contributions of atmospheric inputs and of the weathering of major rock types. The question was readdressed by Meybeck (1987) through consideration of the chemical composition of waters from small monolithologic watersheds and of the relative abundance of a dozen rock types at the continental surface. Atmospheric inputs were also reassessed (Meybeck, 1983). The breakdown of major ions, Sr2+, and dissolved silica by rock weathering and atmospheric inputs is given in Table 4.4A. The influence of rare evaporitic outcrops (about 1.2 percent of the Earth's surface) on Ca2+, Na+, SO42-, Cl -, and Sr2+
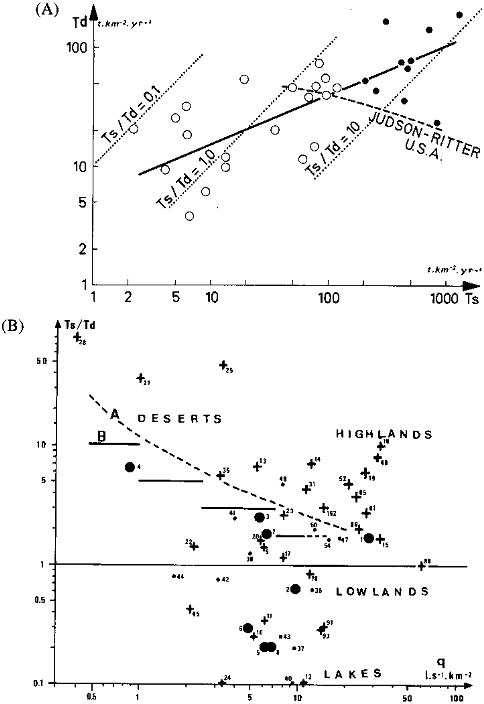
FIGURE 4.6 Global evolution of total dissolved (Td) and particulate (Ts) transport rates of worldwide rivers. (A) Influence of basin relief on Td versus Ts relationship (• mountainous watersheds,  others), compared to the Judson and Ritter (1964) variations for U.S. rivers. (B) Influence of basin morphology on the Ts/Td ration versus runoff q compared to relationships from (A) Langbein and Dawdy (1964) and (B) Leopold et al. (1964). (• river basin > 2.4 x 106 km2; + river basins from 0.5 x 106 km2 to 2.4 x 106 km2;
others), compared to the Judson and Ritter (1964) variations for U.S. rivers. (B) Influence of basin morphology on the Ts/Td ration versus runoff q compared to relationships from (A) Langbein and Dawdy (1964) and (B) Leopold et al. (1964). (• river basin > 2.4 x 106 km2; + river basins from 0.5 x 106 km2 to 2.4 x 106 km2;  other rivers).
other rivers).
TABLE 4.4 Origins of Global Riverborne Material (Exorheic Drainage)
|
A. Lithological Origins of Dissolved Matter (percent of total natural inputs)a |
|||||||||
|
Rock Type |
Area |
SiO2 |
Ca2+ |
Na+ |
Sr2+ |
Cl- |
SO42- |
HCO3- |
|
|
Atmospheric CO2 |
— |
— |
— |
— |
— |
— |
— |
67.2 |
|
|
Plutonic, metamorphic, and volcanic |
33.5 |
36.1 |
6.3 |
12.9 |
14.3 |
0.0 |
8.7 |
0.0 |
|
|
Sandstones and shales |
48.9 |
51.6 |
21.9 |
20.1 |
39.3 |
3.9 |
38.0 |
2.4 |
|
|
Carbonate rocks |
16.3 |
11.3 |
62.0 |
2.5 |
18.6 |
0.0 |
8.2 |
28.9 |
|
|
Evaporites |
1.3 |
0.6 |
9.4 |
35.5 |
27.8 |
49.2 |
40.2 |
1.5 |
|
|
Oceanic aerosols |
— |
0.0 |
0.4 |
29.0 |
29.0 |
46.9 |
4.9 |
— |
|
|
B. Geographic Origins of Riverborne Material (percent of total input to oceans)b |
|||||||||
|
|
|
|
Dissolved |
|
|
|
|
|
|
|
|
Area |
Runoff |
SiO2 |
Icons |
TOC |
SMc |
|
|
|
|
Cold regions |
23.4 |
14.7 |
5.4 |
15.5 |
17.5 |
2.7 |
|
|
|
|
Temperate |
22.4 |
27.5 |
19.9 |
39.9 |
28.5 |
56.5 |
|
|
|
|
Tropical |
37.0 |
57.2 |
73.6 |
41.8 |
52.0 |
34.2 |
|
|
|
|
Arid |
17.2 |
0.65 |
1.0 |
2.8 |
1.3 |
6.6 |
|
|
|
|
a Meybeck (1987, 1988). Marble is included in carbonate rocks; area refers to the percentage of surficial rocks. b Meybeck (1979, 1988). Total organic carbon (TOC) budget has been reviewed. The Huang He River is in the temperate Regions. c Smis suspended matter. |
|||||||||
budgets is evident. Although 62 percent of Ca2+ originate from carbonate rock weathering, 67 percent of HCO3- originates from soil and atmospheric CO2.
The breakdown of river transport by geographic origins (Meybeck, 1979, 1982, 1988) is based on a typology of transport rates for a dozen morphoclimatic environments from tundra to mountainous wet tropics, defined by their average temperature (cold, temperate, desertic, tropical) and by their average runoff (five classes of runoff). It has been postulated that in these budgets, lithology is of secondary influence compared to runoff at scales on which these environments have been defined (106 to 107 km2). The relative importance of the four main climatic environments is presented in Table 4.4B. Because of its major contribution to the water budget, the tropical zone is the major source of silica and organic carbon. Given their relative area and water discharge, temperate regions contribute 60 percent more than tropical regions to the ionic budget: the hypothesis of equal distribution of rock types in morphoclimatic environments is effectively limited; most limestone outcrops are in temperate regions (Balazs, 1977).
In considering the global TSS budget to the oceans, Milliman and Meade (1983) found that Southeast Asia alone (from the Huang He to the Indus, 12 x 106 km2, islands included) is responsible for 35 percent of the global sediment discharge. Here, all major factors of high erosion rates (sedimentary rocks, volcanic ash in Indonesia, loess deposits in China, steep relief in Himalaya, monsoon climate) are combined with high sediment delivery ratio (steep slopes until river mouths in many cases). This rate (450 t/km2/yr) is twice that observed for the combined temperate and tropical zones (230 t/km2/yr).
When comparing the global riverine transport of major elements in dissolved and particulate states, the ratio of dissolved materials to total transport is highly variable, from 99 to 0.1 percent and the typical ranges are shown below:
|
|
90% |
|
50% |
|
10% |
|
1% |
|
|
Cl |
|
S, Na, C, Ca |
|
Ng, N, K |
|
P, Si, Mn |
|
Ti, Fe, Al |
Actually, these numbers may vary by nearly one order of magnitude, depending mainly on the amount of TSS. For example, the ratio of dissolved silica to total silica transport varies from 0.3 percent for regions of high mechanical erosion to 40 percent for lowlands.
CONCLUSIONS
The use of present-day river transport data for past geological times, particularly in ocean and climate modeling, should be undertaken cautiously. The following considerations need to be addressed:
-
the area contributing effectively to the ocean budget (i.e., the nonglaciated nondesertic exorheic area) should be known accurately;
-
the postglacial Quaternary period is peculiar and characterized by high sediment sources (fluvioglacial deposits, loess) and sinks (millions of lakes, floodplains) and moderate weathering rates;
-
water runoff and detailed lithology distribution (limestone and evaporites) are two essential factors in river transport that can never be well known for the past; and
-
the great variability of transport rates in today's rivers suggests that past global inputs to oceans of dissolved and particulate matter may well have varied by one order of magnitude within geologic evolution.
-
the great variability of transport rates in today's rivers suggests that past global inputs to oceans of dissolved and particulate matter may well have varied by one order of magnitude within geologic evolution.
Human influence is now an important one (Meybeck and Helmer, 1989). In this chapter, it has been avoided as much as possible in order to understand natural processes. The river particulate loads measured these past 30 years may not reflect natural processes, even in regions less affected by agriculture: (1) the proportions of accelerated erosion and accelerated storage are unknown, and (2) the storage-transport processes now observed may not reflect those occurring at short, medium, and long geological scales (Meade, 1988).
For the dissolved elements, man is already a very effective geologic agent who increases sources (e.g., through mining, deforestation, atmospheric fixation of N) and sometimes sinks (e.g., reservoir building, eutrophication), thus already modifying major global cycles (Na, Cl, S, N, P, F, etc). Deciphering natural processes in the actual cycles will be increasingly more difficult.
ACKNOWLEDGMENTS
This work was done while the author was a visiting professor at Northwestern University, Department of Geological Sciences, through a CNRS grant agreed to by the NSF, at the invitation of A. Lerman, who is warmly acknowledged for his support.
REFERENCES
Balazs, D. (1977). The geographical distribution of karst areas, Proceedings of the 7th International Congress on Speleology, Sheffield, 13-15.
Baumgartner, A., and E. Reichel (1975). The World Water Balance, Elsevier, New York, 179 pp.
Berner, E.A., and R.A. Berner (1987). The Global Water Cycle, Geochemistry, and Environment, Prentice-Hall, Englewood Cliffs, N.J., 397 pp.
Berner, R.A., A.C. Lasaga, and R.M. Garrels (1983). The carbonate, silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past million years, American Journal of Science283, 641-683.
Claridge, G.G.C. (1973). Studies on element balances in a small catchment at Taita, N.Z., in Studies and Reports in Hydrology12, Unesco Press, Paris, pp. 3245-3262.
Davis, S.N. (1964). Silica in streams and groundwaters, American Journal of Science262, 870-891.
Drever, J.I. (1982). The Geochemistry of Natural Waters, Prentice-Hall, Englewood Cliffs, N.J., 388 pp.
Durum, W.H., G. Heidel, and L.J. Tison (1960). Worldwide runoff of dissolved solids, International Association of Hydrological Science 51, 618-628.
Erikson, E. (1960). The yearly circulation of chloride and sulfur in nature: Meteorological, geochemical and pedological implications, Tellus12, 63-109.
Fournier, F. (1969). Transports solides effectues par les coursd'eau, International Association of Hydrological Science Bulletin14(3), 7-49.
Garrels, R.M., and C.L. Christ (1965). Solutions, Minerals, and Equilibria, Freeman Cooper, San Francisco, 450 pp.
Garrels, R.M., and F.T. Mackenzie (1971). Evolution of Sedimentary Rocks, W.W. Norton, New York, 397 pp.
Gibbs, R.J. (1970). Mechanism controlling world water chemistry, Science170, 1088-1090.
Gorham, E. (1961). Factors influencing the supply of major ions to inland waters with special reference to the atmosphere, Geological Society of America Bulletin72, 795-840.
Harman, R.S., W.B. White, J.J. Drake, and J.W. Hess (1975). Regional hydrochemistry of North American carbonate terrains, Water Resources Research11(6), 963-967.
Jansen, J.M.L., and R.B. Painter (1974). Predicting sediment yield from climate and topography, Journal of Hydrology21, 371-380.
Judson, S., and D.F. Ritter (1964). Rates of regional denudation in the United States, Journal of Geophysical Research69(16), 3395-3401.
Kempe, S. (1982). Long-term record of CO2 pressure fluctuations in fresh water, Mitt. Geol. Paläont. Institut Hamburg52, 91-332.
Kobayashi, J. (1959). Chemical investigation on river waters of Southeastern Asiatic countries, Bericht Ohara Institut für Lanwirtschaft Biologie II(2), 167-223.
Kobayashi, J. (1960). A chemical study of the average quality and characteristics of river water of Japan, Bericht Ohara Institut für Lanwirtschaft BiologieIII(3), 313-357.
Kobayashi, J., et al. (1974). Chemical Properties of River Waters in the Southeastern Asian Countries, Report to the Ministry of Education, 30 pp.
Langbein, W.B., and D.R. Dawdy (1964). Occurrence of dissolved solids in the surface waters in the United States, U.S. Geological Survey Professional Paper501D, D115-D117.
Leopold, L.B., M.G. Wolman, and J.P. Miller (1964). Fluvial Processes in Geomorphology, Freeman, San Francisco, 522 pp.
Livingstone, D.A. (1963). Chemical composition of rivers and lakes, U.S. Geological Survey Professional Paper440G, G1-G64.
Meade, R.H. (1988). Movement and storage of sediments in river systems, in Physical and Chemical Weathering in Geochemical Cycles, A. Lerman and M. Meybeck, eds., Kluwer, Dordrecht, pp. 165-179.
Meybeck, M. (1979). Concentration des eaux fluviales en elements majeurs et apports en solution aux oceans, Rev. Geo. Dyn. Géogr. Phys.21(3), 215-246.
Meybeck, M. (1982). Carbon, nitrogen and phosphorus transport by world rivers, American Journal of Science282, 401-450.
Meybeck, M. (1983). Atmospheric inputs and river transport of dissolved substances, in Symposium on ''Dissolved Loads of Rivers,"International Association of Hydrological Science Publication141, 173-192.
Meybeck, M. (1984). Variabilité géographique de la composition chimique naturelle des eaux courantes, Verh. Int. Verein. Limnol.22, 1766-1774.
Meybeck, M. (1986). Composition chimique naturelle des ruisseaux non pollués en France, Sci. Geol. Bull.Strasbourg39, 3-77.
Meybeck, M. (1987). Global chemical weathering of surficial rocks estimated from river dissolved loads, American Journal of Science 287, 401-428.
Meybeck, M. (1988). How to establish and use world budgets of river material, in Physical and Chemical Weathering in Geochemical Cycles, A. Lerman and M. Meybeck, eds., Kluwer, Dordrecht, pp. 247-272.
Meybeck, M., and R. Helmer (1989). The quality of rivers: From pristine stage to global pollution, Palaeogeography, Palaeoclimatology, Palaeoecology 75, 283-309.
Milliman, J.D., and R.H. Meade (1983). World-wide delivery of river sediment to the oceans, Journal of Geology91, 1-21.
Moore, T.M. (1987). Dissolved organic carbon in forested and cutover drainage basins, Westland, N.Z., International Association of Hydrological Science167, 481-487.
Reeder, S.W., B. Hitchon, and A.A. Levinson (1972). Hydrogeochemistry of the surface waters of the Mackenzie river drainage basin, Canada, I. Factors controlling inorganic composition, Geochimica et Cosmochimica Acta36, 825-865.
Santos, J.F. (1965). Quality of surface waters in the Lower Columbia river basin, U.S. Geological Survey Water Supply Paper1784, 78 pp.
SCOPE Carbon Unit (1982, 1983, 1985, 1987). Transport of carbon and minerals in major world rivers, Mitt. Geol. Paläont.Institut Hamburg 52, 55, 58, 64.
Stallard, R.F. (1980). Major Element Geochemistry in the Amazon River System , Ph.D. thesis, Woods Hole Oceanographic Institution, WHOI-80-29, 362 pp.
Stallard, R.F. (1988). Weathering and erosion in the humid tropics, in Physical and Chemical Weathering in Geochemical Cycles, A. Lerman and M. Meybeck, eds., Kluwer, Dordrecht, pp. 225-246.
Stallard, R.F., and J.M. Edmond (1981). Geochemistry of the Amazon I. Precipitation chemistry and the marine contribution to the dissolved load at the time of peak discharge, Journal of Geophysical Research 86, 9844-9858.
Stallard, R.F., and J.M. Edmond (1983). Geochemistry of the Amazon II, The influence of the geology and weathering environment on the dissolved load, Journal of Geophysical Research88, 9671-9688.
Stallard, R.F., and J.M. Edmond (1987). Geochemistry of the Amazon III, Weathering chemistry and limits to dissolved inputs, Journal of Geophysical Research92, 8293-8302.
Sugawara, K.T. Yoshihara, K. Yanagi, and M. Ambe (1982). A new chemical approach to the study of waters of Mt. Fuji environs, Archiv. Hydrobiol. 94, 269-285.
Turekian, K.K. (1969). Oceans, streams, and atmosphere, in Handbook of Geochemistry, K.H. Wedepohl, ed., Springer-Verlag, Berlin, pp. 297-323.
Viner, A.B. (1975). The supply of minerals to tropical rivers and lakes (Uganda), in Coupling of Land and Water Systems, A. D. Hasler, ed., Springer, New York, pp. 227-261.
Walling, D.E., and A.H.A. Kleo (1979). Sediment yields of rivers in areas of low precipitation, International Association of Hydrological Science Publication128, 479-493.
Walling, D.E., and B.W. Webb (1983). The dissolved load of rivers: A global overview, International Association of Hydrological Science Publication141, 3-20.
Wilkinson, B.H., and J.C.G. Walker (1989). Phanerozoic cycling of sedimentary carbonates, American Journal of Science289, 525-548.













