3
Addressing the Threats
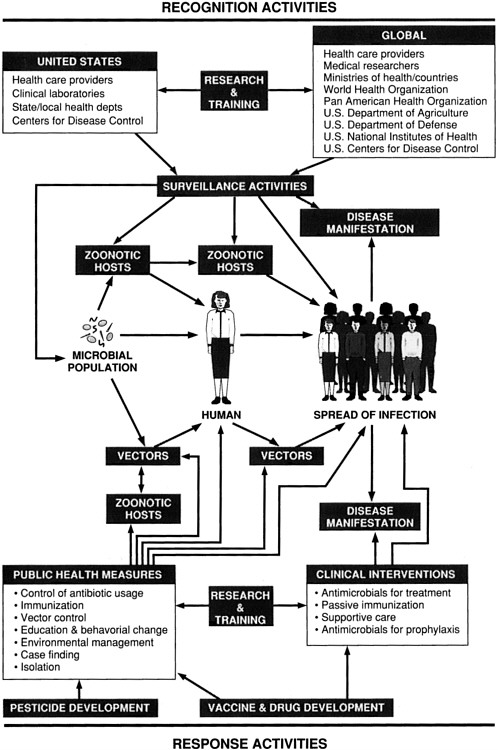
The process by which an infectious disease emerges and is recognized and responded to can be complex. Chapter 2 dealt with the many factors involved in emergence. This chapter addresses disease recognition and intervention and provides specific recommendations for improving the ability of the United States and the global community to respond to future microbial threats to health. The relationships between and among recognition activities and interventions are diagrammed in Figure 3-1.
Chapter 3 is divided into two sections. The first, on recognition, addresses domestic and international surveillance. The recommendations in this section, if implemented, would strengthen U.S. surveillance activities and encourage efforts to develop a global infectious disease surveillance network. The second section, on interventions, is divided into subsections that address the U.S. public health system, research and training, vaccine and drug development, vector control, and public education and behavioral change. Each subsection includes one or more recommendations directed at improving the current U.S. capability to respond to outbreaks of emerging infectious diseases.
RECOGNITION
The key to recognizing new or emerging infectious diseases, and to tracking the prevalence of more established infectious diseases, is surveillance. Surveillance and rapid response to identified disease threats are at the core of preventive medicine. A well-designed and well-implemented infectious disease surveillance program can provide a means to detect unusual clusters of disease, document the geographic and demographic spread of an outbreak,
estimate the magnitude of the problem, describe the natural history of the disease, identify factors responsible for emergence, facilitate laboratory and epidemiological research, and assess the success of specific intervention efforts.
Unfortunately, there is insufficient awareness of and appreciation for the value of comprehensive surveillance programs. Even among public health personnel, involvement in surveillance activities is often limited to collecting and transmitting disease-related data, a viewpoint that can mask the objectives and significance of the overall effort. Some health care and public health professionals are unfamiliar with surveillance methods, mainly because the topic is covered inadequately in medical schools and even in schools of public health (Thacker and Berkelman, 1988). The result is incomplete, underrepresentative, and untimely disease reporting. Poor surveillance leaves policymakers and practitioners without a basis for developing and implementing policies for controlling the spread of infectious diseases.
Surveillance can take many forms, from complex international networks involving sophisticated laboratory and epidemiological investigations, to small, community-based programs or a single astute clinician. Disease surveillance often is a passive process that is based on individual health care workers who report instances of unusual or particularly contagious human illnesses, usually to a government health agency. In other instances, more formal surveillance can take place, in which public health workers actively seek out cases of disease and report their findings regularly to a central data collection point.
The importance of surveillance to the detection and control of emerging microbial threats cannot be overemphasized. Active monitoring of such factors as population growth and migration, vector abundance, development projects that disturb the environment, and natural environmental factors (especially temperature and precipitation) is an essential component of surveillance and can influence the spread of emerging infectious diseases and the effectiveness of efforts to control them.
Surveillance is important to any disease control effort; it is absolutely essential if that effort's goal is eradication. Without the information obtained through disease surveillance, it is not possible to know how and where disease control efforts should be focused or to analyze the impact of ongoing efforts. The smallpox eradication program, discussed below, is an excellent example of the use of surveillance for case finding and program monitoring.
Surveillance in Action: The World Health Organization's Smallpox Eradication Program
An often overlooked but very significant contributor to the success of global smallpox eradication was disease surveillance. Of course, smallpox eradication would have been impossible had there not been an effective
vaccine and a simple, inexpensive means of delivering it—the bifurcated needle. The fact that humans were the only known reservoir for the smallpox virus also simplified the task of eradication, since no insect vector or nonhuman animal hosts were involved in disease transmission.
Smallpox is transmitted by the respiratory route, by contact with pox lesions, or by infective material, such as bed linens, recently contaminated with discharge from lesions. A distinctive rash and skin lesions develop within 10 to 14 days in virtually all who are infected by the smallpox virus. Because those infected are contagious only from the time the rash appears to the time the resulting scabs fall off, and because subclinical cases play no role in disease spread, tracing the chain of transmission is fairly straightforward. During the eradication period, when a case of disease was located, the affected individual was isolated and potential contacts were vaccinated. At the same time, an effort was made to find the person from whom the patient had presumably contracted the disease, and that individual's contacts were similarly vaccinated.
Perhaps the most difficult part of the eradication effort was the development of adequate national surveillance programs, since they were either nonexistent or nearly so in all participating countries when the program began. At the outset, it was evident that most smallpox cases were not being reported even though smallpox was, by international treaty, a reportable disease. It has been estimated that less than 1 percent of cases were being reported when the World Health Organization's (WHO) global smallpox eradication program got under way in 1967 (Henderson, 1976a,b).
Thus, one of the early steps in the eradication campaign was to establish disease reporting systems in countries that did not have them and to upgrade the quality of reporting systems in countries that did. It was a formidable task. In African countries and in Brazil, this was often done by assigning teams of two to four persons to an administrative area that encompassed a population of from 2 million to 5 million. The teams were charged with regularly visiting health centers and hospitals to encourage health personnel to report cases (or the absence of cases) each week, with investigating and containing outbreaks, and with distributing vaccine and vaccination supplies.
These teams played a vital role in the development and success of the reporting system. Not only did they discover unreported cases, but their prompt response to smallpox outbreaks also served to encourage case-reporting by health workers. As the incidence of the disease fell, periodic searches were conducted on a house-by-house basis. In some countries, such as India, Pakistan, and Bangladesh, rewards were offered for reporting cases.
Another key feature of the smallpox surveillance effort, and one that is common to all effective surveillance initiatives, was information dissemination.
Those taking part directly in the eradication effort, as well as others with a "need to know," were regularly supplied with surveillance reports. The reports contained weekly tallies of cases from each reporting unit, comments, and other items of interest, such as specimen collection procedures or information about other smallpox programs.
In 1971, four years after the global campaign had begun, the number of countries reporting smallpox cases had fallen from 44 to 16 (Henderson, 1976a,b). By 1975, only one country, Ethiopia, remained endemic for the disease; two years later, the last known case of naturally occurring smallpox was diagnosed. Finally, in 1979, after long and careful review, the WHO certified the world free of smallpox.
LESSONS FROM THE SMALLPOX EXPERIENCE
Because every disease is different, in terms of how it is diagnosed, whom it affects, and where it occurs, surveillance efforts must be individually tailored. The experience with smallpox eradication was unique in several respects. Most important, eradication is not the goal of most public health activities that use surveillance. The fact that vaccination was the primary tool used to combat the disease also sets smallpox apart from most other situations in which surveillance plays a role. Nevertheless, the eradication campaign illustrated a number of important principles about surveillance that might be applied to other efforts to monitor and control the spread of infectious diseases.
One of the most fundamental is that a reduction in disease incidence is the ultimate measure of success in disease control. In the case of smallpox, for example, tallying the number of vaccinations performed in order to gauge the campaign's success would have been of little value because the immune response to the vaccine was not the same for all who were vaccinated. Not everyone to whom vaccine was administered was effectively vaccinated in terms of protection from contracting smallpox.1 In addition, as the eradication campaign developed, it became clear that special efforts to vaccinate those at high risk, particularly contacts of infected individuals, were the most effective strategy. By focusing on disease incidence, it was possible to identify the epidemiological factors responsible for cases of disease that occurred despite ongoing efforts to prevent them. Once these
factors had been identified, disease control efforts could be modified accordingly.
The importance of flexibility in surveillance activities was underscored early in the eradication campaign. The initial strategy had been to conduct mass vaccinations in every endemic country and at the same time improve surveillance capabilities. It was felt that once 80 percent of a country's population was immunized, any remaining foci of infection could be rapidly identified, contained, and eliminated.
Once the campaign was under way, however, it became clear that achieving the 80 percent immunization goal might not be necessary. A more targeted approach, called surveillance-containment, was tried. Infected individuals were located and isolated, and known or suspected contacts were vaccinated, thus preventing the disease from spreading to others. The new strategy worked because smallpox infection is never silent, because it spreads slowly compared with many other infectious diseases, and because vaccination could produce immunity within the incubation period for the disease.
Current U.S.-Supported Surveillance Efforts
Current U.S. surveillance efforts include both domestic and international components. Although the domestic program, in which a number of federal government agencies participate independently, is fairly comprehensive, U.S. international surveillance activities at this time are fragmented and inadequate to detect emerging infectious disease threats on a timely basis.
DOMESTIC EFFORTS
Surveillance of infectious diseases in the United States is a passive process. It relies on physicians, hospitals, and other health care providers to report cases to state and local organizations that are responsible for disease surveillance. The Centers for Disease Control (CDC) works in cooperation with the states in monitoring the domestic incidence of specific infectious diseases (such as measles, mumps, rubella, pertussis, diphtheria, and hepatitis B). Each state has its own regulations regarding the reporting of specific diseases. These "notifiable" diseases may duplicate or expand on the list of 49 diseases that are reportable to the CDC (see Table 3-1).
Notifiable Diseases Surveillance
The bulk of the federal reporting requirements are implemented through the National Notifiable Diseases Surveillance System (NNDSS), established in 1961. The list of nationally notifiable diseases is maintained and revised as needed by the Council of State and Territorial Epidemiologists in collaboration
TABLE 3-1 Diseases Currently Reportable to the Center for Disease Control
|
Acquired immunodeficiency syndrome |
Amebiasis |
|
Anthrax |
Aseptic meningitis |
|
Botulism, food borne |
Botulism, infant |
|
Botulism, wound |
Botulism, unspecified |
|
Brucellosis |
Chancroid |
|
Cholera |
Congenital rubella syndrome |
|
Diphtheria |
Encephalitis, post chickenpox |
|
Encephalitis, post mumps |
Encephalitis, post other |
|
Encephalitis, primary |
Gonorrhea |
|
Granuloma inguinale |
Hansen disease |
|
Hepatitis A |
Hepatitis B |
|
Hepatitis, non-A, non-B |
Hepatitis, unspecified |
|
Legionellosis |
Leptospirosis |
|
Lyme disease |
Lymphogranuloma venereum |
|
Malaria |
Measles |
|
Meningococcal infections |
Mumps |
|
Pertussis |
Plague |
|
Poliomyelitis, paralytic |
Psittacosis |
|
Rabies, animal |
Rabies, human |
|
Rheumatic fever |
Rocky Mountain spotted fever |
|
Rubella |
Salmonellosis |
|
Shigellosis |
Syphilis, all stages |
|
Syphilis, primary and secondary |
Syphilis, congenital |
|
Tetanus |
Toxic shock syndrome |
|
Trichinosis |
Tuberculosis |
|
Tularemia |
Typhoid fever |
|
Yellow fever |
|
|
SOURCE: Wharton et al., 1990. |
|
with the CDC. Reporting of diseases on the list is voluntary, with the exception of the diseases that require quarantine: yellow fever, cholera, diphtheria, infectious tuberculosis, plague, suspected smallpox, and viral hemorrhagic fevers. Regulatory authority for disease surveillance in the United States is provided through state legislation.
Reportable disease data are provided to the CDC on a weekly basis by state health departments, New York City, the District of Columbia, Puerto Rico, the Virgin Islands, Guam, American Samoa, and the Commonwealth of the Northern Mariana Islands. Since 1984, disease reporting has been accomplished through a computer-based telecommunications system, the National Electronic Telecommunications System for Surveillance (NETSS). The CDC analyzes the data and disseminates it in its Morbidity and Mortality Weekly Report. As of June 1990, aggregate or case-specific data for a total of 49 infectious diseases were being reported to the CDC by all
U.S. states and territories. Individual states require reporting on more than 100 additional infectious diseases or infectious disease-related conditions (Centers for Disease Control, 1991k).
Data on disease incidence obtained through the NNDSS are important for public health decision making. Data supplied by private physicians and laboratories, the points of contact within the health care system for individuals who become ill, are critical elements in this process. In those instances in which a patient is diagnosed with a reportable disease, this information is supposed to be transmitted to the local or state health department. Unfortunately, this does not always happen. Laboratories may not have sufficient resources for reporting or may decide that reporting is unimportant. (Some states, however, require laboratories to report specific diseases.) Some physicians may be unaware of the requirement to report the occurrence of a specific disease or may not appreciate the importance of such a requirement.
Outbreaks of any disease that is not on CDC's current list of notifiable illnesses may go undetected altogether or may be detected only after an outbreak is well under way. In fact, except for food-borne and waterborne diseases, the United States has no comprehensive national system for detecting outbreaks of infectious disease. Emerging infectious diseases also are not usually detected and reported through established surveillance activities. Instead, private physicians who see small clusters of unusual cases may report them in the medical literature. What is needed is a way to bring these small clusters to the attention of the appropriate agencies in a timely manner.
The committee recommends the development and implementation of strategies that would strengthen state and federal efforts in U.S. surveillance. Strategy development could be a function of the Centers for Disease Control (CDC). Alternatively, the strategy development and coordination functions could be assigned to a federal coordinating body (e.g., a subcommittee of the Federal Coordinating Council for Science, Engineering, and Technology's [FCCSET] Committee on Life Sciences and Health,2 specifically constituted to address this issue. Implementation of the strategies would be assigned to the appropriate federal agencies
(e.g., CDC, National Institutes of Health, U.S. Department of Agriculture). Approaches for consideration could include simplifying current reporting forms and procedures, establishing a telephone hotline by which physicians could report unusual syndromes, and using electronic patient data collected by insurance companies to assist in infectious disease surveillance.
The committee believes that an excellent example of appropriate coordination of surveillance (and other) activities related to the emergence of a microbial threat to the U.S. population is the recent effort spearheaded by the CDC. Recognizing the seriousness of the emerging multidrug-resistant TB (MDRTB) epidemic, the CDC convened a federal task force in December 1991 at the request of James Mason, the Assistant Secretary for Health. This effort resulted in the National Action Plan to Combat Multidrug-Resistant Tuberculosis (National MDR-TB Task Force, 1992). The plan lays out a series of objectives, in the areas of epidemiology and surveillance, laboratory diagnosis, patient management, screening and preventive therapy, infection control, outbreak control, program evaluation, information dissemination/training and education, and research. These objectives are based on specific problems identified by the task force to meet these objectives. The plan specifies a series of activities, responsible organizations, and time frames for implementation. The committee feels that a similar task force could be convened to implement the above recommendation, as well as the one presented later in this chapter on U.S. international efforts in surveillance.
Nosocomial Infections Surveillance
A second major domestic disease surveillance effort is the National Nosocomial Infections Surveillance System (NNISS), which gathers data from approximately 120 sentinel hospitals. The NNISS is operated by the CDC's Hospital Infections Program (HIP); it is the nation's only database devoted to tracking nosocomial infections, which annually affect some 2 million hospitalized patients. The system allows estimates to be made about the incidence of nosocomial infections in the United States, and it provides data that help to detect changes in patterns of incidence, distribution, antibiotic drug resistance, sites of infection, outcomes of infection, and risk factors for nosocomial infections.
Each year, the HIP receives more than 5,000 inquiries about nosocomial infections, including a small number that involve the management of acute outbreaks. In the past 10 years, HIP staff have investigated approximately 120 hospital outbreaks of infectious disease (Centers for Disease Control, 1991b).
Hospitals must apply for membership in the NNISS, and their identity remains confidential. Membership is approved based on adequacy of personnel support for infection control, availability of a computer compatible with the NNISS software, and agreement of the hospital administration. The system has several limitations. For example, it cannot correct for differences among participating hospitals in diagnostic testing, intensity of surveillance, and provisions for post discharge surveillance. The requirement that NNISS member hospitals have at least 100 beds and the fact that a relatively small sample of hospitals is included in the system are potential sources of bias (Gaynes et al., 1991). Even so, the NNISS is the only national database for nosocomial infections, and it is a critical element in the CDC's program to monitor disease incidence.
The system is still evolving. Current plans call for improvements in the dissemination of NNISS data, the inclusion of a surveillance component for immunosuppressed patients, and the addition of more sentinel hospitals, among other efforts (Gaynes et al., 1991). These improvements should lead to better detection of outbreaks and widespread trends in the emergence of resistance among nosocomial pathogens. The limited participation of hospitals in the NNISS, however, remains a problem; as a result, little improvement will occur in nosocomial surveillance in the more than 6,000 hospitals that are not NNISS participants. Since hospital surveillance activities are not income generating, there is little financial motivation for hospitals to become involved. It is likely that accrediting agencies will have to mandate greater full-time-equivalents before the surveillance and control of these pathogens will improve in the majority of hospitals.
The committee recommends that additional resources be allocated to the Centers for Disease Control to enhance the National Nosocomial Infections Surveillance System (NNISS) in the following ways:
-
Include data on antiviral drug resistance.
-
Include information on morbidity and mortality from nosocomial infections.
-
Increase the number of NNISS member hospitals.
-
Strive to make NNISS member hospitals more representative of all U.S. hospitals.
-
Evaluate the sensitivity the specificity of nosocomial infection surveillance activities performed in NNISS member hospitals.
-
Determine the reliability of antimicrobial susceptibility testing performed in NNISS member hospitals.
Outbreak Surveillance
Since 1988, the CDC has participated with a number of states in a pilot project to develop a system for computerized surveillance of outbreaks of diseases that are not currently notifiable. For food-borne or waterborne outbreaks, reporting is required when two or more cases occur; for other outbreaks, the threshold for reporting is three cases. During a five-month period in 1990, nine participating states reported 233 outbreaks involving 6,241 individual cases of disease (Centers for Disease Control, 1991k). This initiative should also provide data to help identify factors that increase the risks of outbreaks and make it easier to assess the effectiveness of outbreak prevention and control measures.
Influenza Surveillance
To monitor influenza incidence and the prevalence of particular virus strains in this country, the CDC, in addition to participating in the WHO's global influenza surveillance network (see the later discussion), operates a domestic influenza surveillance program. Data for the program come from state and territorial health departments, U.S.-based WHO collaborating laboratories (see Figure 3-2), 121 key U.S. cities, and ''sentinel" U.S. physicians. The epidemiological information these sources gather is analyzed and released to public health officials, physicians, the media, and the public.
Access to Surveillance Information
Considerable effort and resources are being expended on the various surveillance activities in which U.S. government agencies and the private sector participate. Much of this information, however, is not readily accessible. There is currently no single database from which a physician, researcher, health care worker, public health official, or other interested party can obtain information on disease incidence, antibiotic drug resistance, drug and vaccine availability, or other topics that might be relevant to infectious disease surveillance, prevention, treatment, and control. The need for such a database is strong; given the current communications capabilities of personal computers and the relative ease with information on a multitude of topics can be accessed, a database is not only technologically feasible but could be a valuable addition to U.S. surveillance efforts.
The committee recommends that the U.S. Public Health Service develop a comprehensive, computerized infectious disease database. Such a database
might consolidate information from more specialized sources, such as the National Nosocomial Infections Surveillance System (NNISS), the National Electronic Telecommunications System for Surveillance (NETSS), and the influenze surveillance system; it could also include additional information, such as vaccine and drug availability. As an alternative, expansion of currently available databases and provisions for easy access to these sources should be aggressively pursued. Also included in the implementation of such a program should be expanded efforts to inform physicians, public health workers, clinical laboratories, and other relevant target groups of the availability of this information.
INTERNATIONAL EFFORTS
U.S.-supported overseas infectious disease laboratories have played a historic role in the discovery and monitoring of infectious diseases. The United States and other nations first created these disease surveillance posts, many of them in tropical and subtropical countries, in an effort to protect the health of their citizens who were sent to settle or administer recently acquired territory. During and after World War II, there was a second blossoming of U.S. government-supported international disease research and surveillance activities. Several overseas laboratories staffed by Department of Defense (DoD) personnel were established. The Middle America and Pacific Research Units of the National Institutes of Health (NIH) were founded, and later terminated. The Gorgas Memorial Laboratory, in Panama, was until 1991, supported by the United States. Privately funded activities, like those of the Rockefeller Foundation Virus Program, were important contributors to surveillance efforts. Other private foundations and universities also played a role in surveillance overseas.
Over the past two decades, the number of such facilities has declined, largely as a result of shifts in program priorities. This trend is of concern to the committee, particularly in view of the many important achievements of the laboratories that have been closed. The loss of these facilities has left a major gap in U.S. overseas infectious disease surveillance, research, and training capabilities. Brief histories of some U.S.-supported overseas laboratories, several of which no longer operate, appear below. (See also the section on research and training later in this chapter.) Table 3-2 is a list of current U.S. government-supported overseas infectious disease laboratories.
Past Successes
The Gorgas Memorial Laboratory The Gorgas Memorial Laboratory (GML) in Panama, founded by the Gorgas Memorial Institute in 1928, was
TABLE 3-2 Sponsors and Locations of Current U.S. Governmental-Supported Overseas Infectious Disease Laboratories
|
Department of Defense |
|
|
|
|
|
|
Brazil |
|
|
|
|
|
Egypt |
|
|
|
|
|
Indonesia |
|
|
|
|
|
Kenya |
|
|
|
|
|
Peru |
|
|
|
|
|
South Korea |
|
|
|
|
|
Thailand |
|
|
|
|
U.S. Public Health Service |
|
|
|
|
|
|
National Institute of Allergy and Infections Diseases (NIAID), National Institutes of Health |
|
||
|
|
|
International Centers for Tropical Disease Research |
||
|
|
|
|
International Collaboration in Infectious Diseases Research (ICIDR) |
|
|
|
|
|
|
Brazil—with Cornell University |
|
|
|
|
|
Brazil—with Harvard University |
|
|
|
|
|
Brazil—with University of Virginia |
|
|
|
|
|
Brazil—with Vanderbilt University |
|
|
|
|
|
Israel—with Columbia University |
|
|
|
|
|
Sudan—with Brigham Young University |
|
|
|
|
|
Venezuela—with Albert Einstein College of Medicine |
|
|
|
Tropical Medicine Research Centers (TMRC) |
||
|
|
|
|
|
Colombia—at Centro Internacional de Entranamiento e Investigaciones Medicas |
|
|
|
|
|
Brazil—at Federal University of Bahia |
|
|
|
|
|
Philippines—at Research Institute for Tropical Medicine |
|
|
|
International Collaborations in AIDS Research (ICAR) |
||
|
|
|
|
Brazil—with Cornell University |
|
|
|
|
|
Malawi—with Johns Hopkins University |
|
|
|
|
|
Mexico—with Harvard University |
|
|
|
|
|
Senegal—with Harvard University |
|
|
|
|
|
Uganda—with Case Western Reserve University |
|
|
|
|
|
Zaire—with Tufts University |
|
|
|
Centers for Disease Control |
|
|
|
|
|
|
Côte d'Ivoire |
|
|
|
|
|
Guatemala |
|
|
|
|
|
Kenya |
|
|
|
|
|
Sierra Leone |
|
|
|
|
|
Thailand |
|
|
|
|
|
Zaire |
|
|
|
U.S. Agency for International Development |
|
|
||
|
|
International Center for Diarrheal Disease Research—Bangladesh |
|||
|
|
Ain Shams University—Egypt (administered by NIAID) |
|||
|
|
Hebrew University—Israel (administered by NIAID) |
|||
throughout its existence funded directly by the U.S. Congress, although the Panamanian government donated the facilities in which the laboratory was housed. Named for General William C. Gorgas, a U.S. Army physician and engineer credited with controlling malaria and yellow fever during construction of the Panama Canal, the facility initially concentrated its research efforts on malaria, leishmaniasis, and yellow fever. Later, in fruitful collaboration with the Middle America Research Unit, an NIH field station (see below), the GML conducted studies of many arboviral infections indigenous to the American tropics. More recently, Gorgas scientists became known for their work on sexually transmitted diseases, human papillomavirus and cervical cancer, and hepatitis A.
In 1989, Congress decided that the money that historically had been given to the GML on a noncompetitive basis should be awarded through an open, national competition (U.S. Medicine, 1991). The laboratory was unable to enter the competition for funding, mainly because of its difficulty in retaining professional staff as a result of the political situation in Panama. The GML managed to survive through fiscal year 1990, while attempts were made to obtain funding through a cooperative U.S. Agency for International Development-Pan American Health Organization (USAID-PAHO) effort, or through the CDC. When these attempts failed, the Gorgas Memorial Institute relinquished the laboratory and its equipment to the Panamanian government and dismissed the staff. The Panamanian government has maintained the laboratory with a small cadre of scientists who survey dengue and leishmaniasis, and it is attempting to obtain funding from other sources to expand the laboratory's activities.
U.S. Army Medical Research Unit—Malaysia Investigations into the efficacy of chloramphenicol for the treatment and prophylaxis of scrub typhus were initiated by American military scientists in Malaya in 1948. From the time it was formally established five years later, the U.S. Army Medical Research Unit in Kuala Lumpur not only investigated diseases of importance to the U.S. military but also frequently assisted the Malaysian government in the investigation of disease outbreaks of known and unknown etiology. Over more than four decades of scientific studies, the laboratory was involved in research on scrub typhus, typhoid fever, leptospirosis, malaria, and other tropical diseases (Oaks et al., 1983). Much of what is known about the vector of scrub typhus (the Leptotrombidium mite) was the result of collaborative efforts between the U.S. Army Unit and the Institute for Medical Research, Kuala Lumpur, in which the unit was housed (Ramanathan et al., 1976). Despite these achievements, this DoD laboratory, which had a strong research record (particularly in the area of vector-borne diseases), was closed in 1989 because of lack of funding.
Rockefeller Foundation Virus Program The Rockefeller Foundation Virus Program was established in 1951 to investigate arthropod-borne viruses of vertebrates (Theiler and Downs, 1973). A number of foreign governments, including India, Brazil, Trinidad, South Africa, Colombia, and Nigeria, joined the effort. The program established a virus laboratory in each country in collaboration with a local university or government health agency. The costs of the research were split about equally between the foundation and the host country.
The program, through its surveillance of febrile and hemorrhagic diseases, was responsible for finding and characterizing scores of new infectious agents. In Belem, Brazil, for instance, more than 50 new tropical viruses were discovered, including eight in Groups C and Guama that were responsible for debilitating, nonfatal jungle fevers common in those living in the Amazon region. Kyasanur Forest disease was discovered at the Rockefeller laboratory in Puna, India. Crimean hemorrhagic fever in the former Union of Soviet Socialist Republics and Congo disease in East Africa were linked through studies by the Rockefeller arbovirus reference unit at Yale University (YARU). Several viruses related to rabies were discovered in Africa. Program scientists also searched for viruses in healthy wild animals and arthropods, an innovative approach to disease surveillance that identified a number of agents, such as the Oropouche virus in Trinidad and Brazil (Theiler and Downs, 1973). Oropouche virus in later years caused several major tropical epidemics (Pinheiro et al., 1981).
One of the deadliest of the agents identified by the program was Lassa virus, which was isolated in 1969 at YARU from the blood of a sick mission nurse who had been air-evacuated from Africa (Buckley and Casals, 1970). The discovery of this virus was the direct result of a surveillance program to find new agents that were infecting African missionaries.
The Rockefeller Foundation withdrew funding for the virus program in 1971, based on a policy decision of its board of trustees. During its two decades of operation, the program was an outstanding catalyst for international surveillance and research. YARU continues with support from the NIH, DoD, and WHO. Host country governments and international agencies assumed financial support for each of the field laboratories when Rockefeller withdrew its support. In most countries, these laboratories are now central national resources for disease surveillance and infectious disease research (R. Shope, Director, YARU, personal communication, 1992).
National Institutes of Health In 1958, the NIH established the Middle America Research Unit (MARU) to study tropical infectious diseases, especially those of viral origin, in the U.S. Canal Zone. (A component of this effort was the U.S. Army Medical Research Unit—Panama, which was charged with research on histoplasmosis and other fungal diseases of military importance.)
Some of the MARU field studies were collaborations with the Gorgas Memorial Laboratory. MARU researchers conducted definitive studies on Bolivian hemorrhagic fever, Venezuelan equine encephalitis in Central America, and several viral infections that are transmitted by tropical sandflies. In the mid-1970s, as a harbinger of decreasing U.S. government commitment to international health research, MARU left the NIH to become part of the Gorgas Laboratory.
In 1960, the National Institute of Allergy and Infectious Diseases (NIAID) established the International Centers for Medical Research and Training (ICMRT) program to further support tropical disease research of benefit to U.S. citizens. In collaboration with foreign universities and government agencies, the program provided long-term overseas research training for U.S. scientists. ICMRT grants resulted in broadly productive research programs that studied a wide variety of infectious and noninfectious diseases. In 1979, as part of an overall plan to scale back its involvement in research training activities, the NIAID discontinued the ICMRT program.
Loss of Capacity
The establishment of a new laboratory (particularly on foreign soil), its staffing, and the development of a reputation for carefully conducted, rigorous scientific work are tasks that cannot be accomplished overnight. It is unfortunate that the U.S.-supported overseas laboratories discussed above were, for varying reasons, either discontinued or forced to scale back their efforts. Their achievements had a profound impact on the level of scientific knowledge of many previously known and newly recognized infectious diseases and their causative agents. A further loss is the many opportunities they provided for U.S. scientists to develop overseas field experience and to collaborate with foreign scientists and institutions, thereby acquiring infectious disease surveillance information of importance to U.S. monitoring activities.
Current Efforts
The purposes and entities discussed briefly below constitute current U.S. efforts in international infectious disease surveillance, most of which is conducted through passive monitoring.
-
The NIAID's International Centers for Tropical Disease Research (ICTDR) program. Established as a means to provide more cohesion to existing and newly initiated programs in tropical infectious disease research, the ICTDR program laboratories, because of their geographic distribution (see Table 3-2 above), are well situated to conduct surveillance for new and
-
emerging diseases. (This program and its subordinate elements are discussed in more detail in the research and training section later in this chapter.)
-
The CDC's participation in the WHO global influenza surveillance network. Information obtained through the network allows the CDC to predict the potential impact of influenza on the United States in any given year. This information is crucial for influenza vaccine manufacturers, who need a minimum of six months' lead time to prepare and distribute adequate quantities of new vaccine.
As part of its international efforts, the CDC produces and sends (free of charge) WHO influenza virus detection and identification kits to 117 foreign national WHO collaborating laboratories and to 68 U.S. collaborating laboratories. These laboratories collect and identify influenza virus isolates and forward information about their findings, as well as actual virus samples, to the CDC on a weekly basis. The CDC also receives influenza virus isolates and information from about 50 foreign laboratories, provides the WHO with information collected from U.S. collaborating laboratories, and receives weekly reports from the WHO on the level of influenza activity in the other reporting countries.
Laboratories and research groups in several key areas of the world, such as the People's Republic of China, Hong Kong, Singapore, and the Pacific Basin, the areas in which most new strains originate, are also in regular contact with the CDC. Recently, the global influenza surveillance system improved its coverage of the Far East. In cooperation with the Chinese National Influenza Center in Beijing, the CDC has supported a national surveillance network in the People's Republic of China. This network has greatly increased the number and timeliness of influenza isolates that are available for analysis at the CDC from that country.
-
The CDC's foreign field stations. Similar to the previously mentioned NIAID ICTDR program, the CDC overseas affiliates (see Table 3-2 above) provide passive surveillance information and expertise that is available to the host country for assistance in investigating outbreaks.
-
Rockefeller Foundation's International Clinical Epidemiology Network. The network trains physicians from other nations in medical epidemiology, including infectious disease epidemiology. Through these efforts, a continuing interaction with U.S. universities is fostered, and collaborative activities aimed at infectious disease surveillance and response to emerging diseases are possible. (This program is discussed in greater detail in the research and training section later in this chapter.)
-
USAID-supported International Center for Diarrheal Disease Research, Bangladesh (ICDDR, B). Of almost equal importance to its contributions in cholera epidemiology and treatment have been the pioneering efforts of this laboratory in the surveillance of diarrheal diseases throughout the Asian
-
region. (This program is discussed in greater detail in the research and training section later in this chapter.)
-
USAID's Program in Worldwide Control of Sexually Transmitted Diseases (STD)/HIV. This is a single-source contract to Family Health International, a non-profit organization committed to family planning; contraceptive safety, acceptability, effectiveness, and ease of use; maternal and child health; interventions to reduce the transmission of STDs; and other related issues.
Although the CDC appears to have a mandate for U.S. disease surveillance, other government agencies (e.g., the NIAID, U.S. Department of Agriculture [USDA], DoD, and USAID), private foundations, and universities may also independently play major or minor roles. Currently, there is little coordination among these agencies and organizations regarding infectious disease surveillance. The committee concludes that the effectiveness of their surveillance activities, particularly those pertaining to recognition of and response to emerging microbial threats, could be greatly improved by designating a central focus for such efforts.
The committee recommends that international infectious disease surveillance activities of U.S. government agencies be coordinated by the Centers for Disease Control (CDC). To provide the necessary link between U.S. domestic and international surveillance efforts, the body that is established for this purpose should be the same as that suggested earlier in the recommendation on domestic surveillance. Alternatively, a federal coordinating body (e.g., a subcommittee of the Federal Coordinating Council for Science, Engineering, and Technology's [FCCSET] Committee on Life Sciences and Health, specifically constituted to address this issue) could be assigned the coordinating function. Implementation of surveillance activities, however, should remain with the appropriate federal agencies (e.g., the CDC, Department of Defense, National Institutes of Health, U.S. Department of Agriculture).
Multilateral International Surveillance Efforts
The coordination efforts of multilateral international organizations, such as the WHO, are critical to infectious disease surveillance. Without these organizations, programs such as the successful worldwide eradication of smallpox and the interruption of polio transmission in the Americas would be little more than dreams. Any implementation of a global surveillance system for emerging infectious diseases must draw upon the capabilities of such organizations. Some of the ongoing and past programs of two of these bodies are discussed below.
WORLD HEALTH ORGANIZATION
The WHO is a focal point for surveillance data on global infectious diseases. Under the International Health Regulations, all countries (except Australia, Papua New Guinea, North Korea, and South Africa) must report to the WHO within 24 hours all cases of cholera, plague, and yellow fever (or any isolation of yellow fever virus from monkeys or mosquitoes). This information is published in the WHO's Weekly Epidemiological Record. Despite the requirement to do so, however, some countries are reluctant to release surveillance data. As a result, some outbreaks of these diseases are never discovered or are discovered only retrospectively after they have subsided.
The WHO also operates a number of networks, composed of selected laboratories worldwide (collaborating centers), that report and investigate outbreaks of specific diseases, such as influenza and HIV disease. The influenza surveillance network is designed to monitor newly emerging strains and subtypes of influenza virus. As noted earlier, the information it collects is used to determine the antigenic makeup of each year's influenza vaccines.
Among other activities, the HIV disease network is encouraging participating countries to do seroprevalence studies at sentinel sites (rather than just reporting numbers of cases) and to develop trend data on infection; it is also collecting geographically representative strains of HIV. More informally, the WHO gathers data about disease outbreaks through its contacts with tourist agencies and international companies, whose clients and employees often inadvertently act as sentinels for new or emerging diseases when they become infected while traveling in other countries.
As is true for many similar efforts, WHO disease surveillance activities are hindered by incomplete reporting and a frequent failure to obtain laboratory confirmation of reported cases of disease. Most cases of yellow fever, for example, are diagnosed on the basis of clinical symptoms alone and often occur in areas in which hepatitis or other tropical diseases with similar symptoms are prevalent. Although the WHO makes every attempt to obtain clinical specimens to allow a definitive diagnosis of reported cases, this is not always possible.
The WHO often is involved in early investigative efforts of newly emerging or reemerging infectious diseases, such as Ebola and Lassa fevers, yellow fever, and dengue fever. For example, when Ebola fever outbreaks occurred in Zaire and Sudan, the WHO provided rapid-response teams, composed of consultants from a number of countries, to help the governments of these nations determine the origin of the outbreaks and develop control strategies.
At one time, the WHO supported a series of serum banks, established in 1960 by John Paul, a physician-epidemiologist at Yale University who is
considered the father of clinical and serological epidemiology; the banks contained thousands of well-documented serum samples from many areas of the world. The collections were located in the Department of Epidemiology and Public Health at Yale University; the Institute of Epidemiology and Microbiology, Prague, Czechoslovakia; the National Institute of Health, Tokyo, Japan; and the South African Institute for Medical Research, Johannesburg. The sera in these collections were useful for retrospective studies of specific disease agents. For example, using serum collected in Barbados, investigators were able to estimate vaccine coverage for childhood diseases and, recently, to document HTLV-I antibody. The WHO withdrew its support for these efforts in 1989, however, and in 1990, most of the sera from the Yale collection were transferred to the National Cancer Institute. The overseas serum banks apparently are receiving minimal support from their governments. Without the funding and coordination provided by an international organization like the WHO, it is doubtful whether the serum banks will continue to be maintained. Expansion of these collections at this time is uncertain at best (A. Evans, Professor of Epidemiology and Past Director, WHO Serum Reference Bank, Department of Epidemiology and Public Health, Yale University, personal communication, 1992).
PAN AMERICAN HEALTH ORGANIZATION
In 1985, PAHO proposed a program to interrupt the transmission of poliomyelitis in the Americas by 1990. In 1986, the year prior to the actual start of the campaign, there were more than 900 confirmed cases of polio in the region. By the end of 1991, as a result of extensive immunization campaigns with oral polio vaccine, transmission appeared to be confined to only one country in the entire Western Hemisphere, Peru (De Quadros et al., 1991). In 1991, only eight isolates of wild poliovirus were detected in the Americas: six in Colombia (the last one in April 1991) and two in Peru (the last one in September 1991).
As with the smallpox eradication effort nearly 20 years earlier, surveillance has played a critical role in the PAHO polio eradication strategy. From the outset of the PAHO effort in September 1985, surveillance was a major component of the program. A number of important indicators were monitored by health facilities, including the proportion of sites reporting each week, the interval between diagnosis and the start of control measures, and the follow-up of cases (De Quadros et al., 1991). Reporting of cases of acute flaccid paralysis (including negative reports) was required in all countries, and by the end of 1991, there were nearly 20,000 health units involved in the reporting system, with approximately 80 percent of them reporting every week.
A cadre of epidemiologists was trained to do case investigation and follow-up to collect stool specimens and institute control measures. Eight
diagnostic laboratories were identified and their personnel trained to conduct DNA-probe and polymerase chain reaction (PCR) assays for poliovirus identification and characterization. Between 1989 and 1991, a yearly average of 4,000 stool specimens were tested in this laboratory network. Twenty-four, 18, and 8 wild poliovirus isolates were identified in 1989, 1990, and 1991, respectively. This surveillance and laboratory network is being expanded to include one or two other vaccine-preventable diseases. The network has already proved to be of great assistance in the detection and follow-up of the cholera epidemic that recently struck the Western Hemisphere.
The Concept of Global Surveillance
Current U.S. and worldwide surveillance efforts are useful for detecting known infectious and noninfectious diseases. They fall short, however, in their ability to detect the emergence of infectious diseases. Although there are isolated examples of how such a system could work, there has been no effort to develop and implement a global program of surveillance for emerging diseases or disease agents (including agents with newly acquired drug resistance). Current surveillance efforts (even when adequate in specific areas for specific diseases) are not effectively linked; consequently, knowledge of small clusters of emerging diseases, even if detected, is not widely disseminated. Added to these factors is declining interest in studying, treating, and preventing infectious diseases as increasing attention has focused on chronic degenerative diseases.
To be effective, any global infectious disease surveillance network must be interactive and reciprocal. It is especially important that U.S.-funded laboratories engaged in infectious disease surveillance in foreign countries operate in partnership with host-country facilities. Developing countries, for their part, contribute surveillance data, but they must also be provided with a base of training and expertise, as well as with upgraded local surveillance, data acquisition, and analysis capabilities. The partnerships of U.S. and local facilities can work toward eliminating deficiencies in these areas. Global surveillance thus involves providing not only case numbers but the knowledge, skills, and tools necessary to improve disease surveillance and response within and among countries and regions. Such an effort, of necessity, will be multinational and will require regional and global coordination, advice, and resources from participating nations. These activities would not only benefit each participating country but, in the opinion of the committee, constitute the most economical means by far for developing and supporting a global surveillance network.
One of the biggest potential barriers to the implementation of a global surveillance network is the transfer of information from and to remote sites
in many developing countries that have inadequate telecommunications capabilities. A new satellite technology is currently being tested that may help resolve this dilemma. The system uses a low-level communications satellite that has two-way communications with remote ground stations (each costing approximately $5,000). The requisite satellite, which is now in orbit, passes over every point on the globe at least twice each day. On each pass, it accepts information passed to it from files stored in a remote station's computer. The satellite stores the received information and then transfers it to the appropriate station on its next pass.
The system offers researchers and physicians in the developing world a simpler and less costly alternative for communicating with their peers and accessing information (e.g., scientific and medical journals). Tests of the system are ongoing in several East African sites, and licenses for additional testing sites are pending. Eventually, additional satellites will be placed in orbit to augment the system and provide more opportunities for data transfer each day (Pool, 1991; Clements, 1992). This technology may allow the earlier inclusion of many remote areas in a global infectious disease surveillance network.
A surveillance network must do more than detect cases of disease. It must also collect data on those cases, analyze them in some useful fashion, and disseminate the findings of the analyses to people who can use the information. Surveillance alone, however, is insufficient to address emerging infectious diseases adequately. A response mechanism is necessary as well. Thus, the committee believes that a global surveillance network for detecting emerging microbial threats should have four basic components:
-
a mechanism, based on clinical presentation, for detecting clusters of new or unusual diseases or syndromes (see Box 3-1);
-
laboratories capable of identifying and characterizing infectious agents;
-
an information system to record and analyze reportable occurrences and to disseminate summary data; and
-
a response mechanism to provide feedback to reporting agencies and individuals and, if necessary, to mobilize investigative and control efforts of local and international agencies.
Specific elements of a global infectious disease surveillance system are as follows:
-
sustainability through continuity of funding;
-
locally staffed surveillance centers to promote regional self-reliance and train local personnel;
-
a research component with links to academic centers and other regional facilities involved in basic research;
|
BOX 3-1 Clinical Circumstances That Require High-Priority Surveillance Efforts
|
-
a network of laboratories/diagnostic facilities with people trained to examine specimens, identify isolates, search for clinical syndromes, prepare and distribute reagents, and develop physical and molecular markers for identification (these facilities should have discretionary capability to respond appropriately to emerging diseases by, for example, identifying causative agents and notifying appropriate national health authorities);
-
full clinical documentation of unsolved cases, with a system for archiving sera and pathological specimens;
-
a clinical arm for hospital-based surveillance and drug and vaccine trials;
-
a targeted disease approach with broad reporting criteria for maximum retrieval of data (e.g., ''disease targeted: polio; reporting criterion: acute flaccid paralysis");
-
an effective specimen collection and transport system; and
-
an active system of data analysis and dissemination, with feedback to those providing data.
The WHO's global influenza surveillance network and its collaborating centers for specific diseases, PAHO's polio eradication program, and previous efforts such as the WHO's smallpox eradication program and the Rockefeller Foundation's virus program, although all limited in scope, are nevertheless useful models to consider in the design of a global infectious disease surveillance system. The strengths and weaknesses of each component of these past and current programs should be carefully evaluated.
In the case of current programs, when withdrawal of support threatens to close down a surveillance network, consideration should be given to preserving those components that prove to be of value. The infrastructure of a successful program can in some cases be continued and put to use in the cause of monitoring other diseases. The smallpox eradication surveillance
network is a good example. With appropriate planning and support, that network might have been shifted to surveillance for other diseases and now be useful as a basis for a global infectious disease surveillance system.
The committee recommends that the United States take the lead in promoting the development and implementation of a comprehensive global infectious disease surveillance system. Such an effort could be undertaken through the U.S. representatives to the World Health Assembly. The system should capitalize on the lessons from past successes and on the infrastructure, momentum, and accomplishments of existing international networks, expanding and diversifying surveillance efforts to include known diseases as well as newly recognized ones. This effort, of necessity, will be multinational and will require regional and global coordination, advice, and resources from participating nations.
INTERVENTION
The response to an emerging infectious agent or disease necessitates coordinated efforts by various individuals, organizations, and industries. The committee believes that the current U.S. capability for responding to microbial threats to health lacks organization and resources. This section addresses these deficiencies. It begins by discussing elements of response that actually precede intervention (the U.S. public health system and the research and training infrastructure), and it concludes with a discussion of and recommendations for specific interventions (in vaccine and drug development, vector control, and public education and behavioral change).
The U.S. Public Health System
Disease assessment, which includes the early recognition of emerging microbial threats, is the foundation on which knowledgeable public health policy decisions are based. In the United States, principal responsibility for protecting the public's health rests with the 50 state health departments, or their counterparts, and more than 3,000 local health departments. At the federal level, the national focus for disease assessment is the CDC.
A 1988 Institute of Medicine (IOM) report, The Future of Public Health, described the U.S. public health system as being in a state of disarray, which resulted in "a hodgepodge of fractionated interests and programs, organizational turmoil among new agencies, and well-intended but unbalanced appropriations—without coherent direction by well-qualified professionals." The report also cited several other problems.
-
Many state and local facilities lack the capability for assessing health status.
-
Policy at all levels often develops as a result of immediate and pressing needs rather than from analysis of carefully collected data.
-
Unequal access to public health services means that certain populations, such as the poor, receive inadequate medical care.
-
Public Health leadership, particularly at the state and local levels, suffers from inadequate technical knowledge and rapid turnover, among other things.
It is the perception of this committee that there has been little positive change in the state of U.S. public health since the release of the 1988 IOM report. As partial evidence for this statement, the recent rapid increase in measles incidence (which is now beginning to subside) and the current upswing in cases of tuberculosis (TB) (including multidrug-resistant disease) can be offered. These emerging disease problems are largely the result of complacency—a misguided perception that the advanced U.S. health care system with its array of medical technologies is able to disarm almost any infectious disease.
In the case of measles, successful vaccination programs had diminished disease incidence to such a degree that the public, health care professionals, and public health organizations reduced their levels of vigilance and effort. The result was a resurgence in the disease that only last year reached a peak. Partly as a response, Congress appropriated an additional $40 million in 1992 (a 19 percent increase over 1991) to support the CDC immunization program. The money was targeted at children under the age of two living in communities in need, such as inner cities (National Foundation for Infectious Diseases, 1991).
As discussed earlier, the declines in incidence of TB since the early 1950s led to a belief held by many public health officials, beginning in the early 1980s, that the disease no longer posed a significant health problem. Research efforts waned, and in 1986, the CDC's surveillance program for tracking TB drug resistance trends was terminated. Increases in homelessness, poverty, substance abuse, HIV infection, and active TB among immigrants have now contributed to a resurgence in TB cases (Fox, 1992), which has been further complicated by outbreaks of multidrug-resistant TB (MDRTB) and poor availability or unavailability of some antituberculosis drugs. As recently as 1989, the Department of Health and Human Services developed a national plan to eliminate TB as a health problem in the United States, and at that time, the prospects appeared excellent for success. The plan was not implemented, however, because of both insufficient resources and a lack of conviction regarding the plan's effectiveness.
An aggressive response to the current TB/MDRTB crisis is now being pursued. A national coalition of more than 40 patient and provider organizations has been formed to address TB elimination issues (U.S. Department
of Health and Human Services, 1992). Senior NIH and CDC officials are devoting more attention to the disease, in the form of research and public education. In April 1992, the Food and Drug Administration (FDA) arranged for a limited supply of streptomycin and para-aminosalicylic acid manufactured outside the United States to be available through the CDC under an investigational new drug agreement (Centers for Disease Control, 1992a). The FDA has also recently identified U.S. pharmaceutical companies that have agreed to manufacture these drugs and make them commercially available by late 1992 (Centers for Disease Control, 1992a). In addition, the FDA has promised to expedite the review process for TB-related products (Fox, 1992). Most recently, the CDC published a National Action Plan to Combat Multidrug-Resistant Tuberculosis. The plan lays out a series of specific activities (with organizational responsibility and time frames for action) that address nine objectives identified by the federal task force (National MDR-TB Task Force, 1992).
These responses, like those related to the resurgence of measles, are potentially of value in resolving the current problems with TB and MDRTB but they are reactive, not proactive. It is the committee's view that prevention of infectious diseases must be continually stressed if the U.S. public health system is to be maintained or, preferably, improved. Efforts directed at the recognition of and responses to emerging public health problems, particularly emerging infectious diseases, would help to achieve this goal. The country's recent episodes of measles and TB resurgence should reinforce the importance of upgrading and maintaining the U.S. public health system at all levels. Experience has taught that, in the long run, preventive action is generally more cost-effective than reactive response. For example, the current cholera epidemic, as of mid-1991, had cost Peru's economy an estimated $43 million in medical costs alone. Had that amount been spent over the past few years to provide clean water and adequate sanitation to the people of Peru, it is likely that the epidemic would not have progressed to its current state (Misch, 1991). Other examples of cost effectiveness include measles vaccination and the global eradication of smallpox. The benefit-cost ratio for measles prevention ranges from 11.9:1 to 14.4:1, depending on whether the vaccine administered is measles antigen alone or a combined vaccine (measles, mumps, and rubella) (Hinman et al., 1985). It has been estimated that, in 1967, global expenditures on smallpox annually were $1.35 billion. The 13-year (1967-1979) global smallpox eradication campaign totaled $299 million ($23 million per year), almost a 60-fold annual savings (Fenner et al., 1988).
Microbial disease assessment is a shared function. State and local health departments; the CDC; health care providers; private laboratories; schools of medicine, public health, and veterinary medicine; the FDA; the U.S. Department of Agriculture (USDA); and the NIH all contribute. The existing
system for assessing microbial threats in the United States is based on a myriad of laws, practices, organizational structures, and shared responsibilities. Assessment capabilities, resources, and levels of commitment vary widely among the participants.
The nation's capacity for assessing microbial threats could be improved by strengthening the public health infrastructure to carry out assigned functions of disease assessment, policy development, and assurance of health with respect to microbial threats. Improving cooperation through the formation of consortia of schools of medicine, public health, and veterinary medicine, and departments of public health might also be an effective strategy, as would the availability of emergency funds to investigate, conduct research and surveillance on, and control major new or reemerging infectious diseases.
The quality of infectious disease surveillance varies according to the quality of disease reporting required by states from health care providers. Alert and capable clinical and, especially, laboratory staff are also crucial. In addition to surveillance, effective assessment of microbial threats requires epidemiological and laboratory research, and investigative capabilities at all levels of the health infrastructure. Without each of these, a public health system has little chance of succeeding.
The current U.S. economic climate has done little to help public health initiatives, which for years have lacked sufficient resources. Declining budgets have forced many local and state organizations to cut back on public health programs. Without strong local and state programs, the ability of federal agencies to promote the public health is greatly diminished. Diminishing resources have particularly threatened the state laboratories, which early in this century were major contributors to public health microbiology. The holes in the fabric of diagnostic, investigative, and research capabilities created by the dwindling activities of state laboratories are seldom repaired.
There is some indication that the United States' weakened public health infrastructure has become a concern to policymakers. Recently, the U.S. Public Health Service (PHS) published a plan designed to strengthen the U.S. public health infrastructure (Assistant Secretary for Health's Public Health Service Task Force to Strengthen Public Health in the United States, 1991). This document apparently comes as a response to The Future of Public Health (Institute of Medicine, 1988) and the national prevention objectives set out in Healthy People 2000 (U.S. Department of Health and Human Services, 1990) and Healthy Communities 2000 (American Public Health Association et al., 1991). The proposed PHS plan lays out strategies to improve surveillance, epidemiology, and communication, the three key areas identified in the 1988 IOM report. In reviewing these strategies, the committee found that a number of them were particularly applicable to emerging disease issues (see Box 3-2); moreover, if implemented, these
|
BOX 3-2 Extracts from Plan to Strengthen U.S. Public Health Assessment strategy 1: Developing health information and health information systems that are useful to legislative and executive governmental bodies at the Federal, State, and local levels, and to other groups and organizations.
Assessment strategy 3: Building the capacity of States and local health departments and other relevant organizations to use health information systems to prevent disease, promote health, and increase access to services in their community.
Policy development strategy 2: Developing strategies and programs to realize the goals.
Assurance strategy 1: Developing and maintaining the capacity of public health agencies at the State and local levels, and other organizations, to plan, implement, and assure the quality of the services that they provide or need to provide.
|
|
Assurance strategy 3: Helping to ensure an adequate supply of appropriately trained health personnel.
Source: U.S. Department of Health and Human Services, 1991. |
strategies will, in part, respond to recommendations made in this report. Consequently, the committee supports the implementation of these strategies (Assistant Secretary for Health's Public Health Service Task Force to Strengthen Public Health in the United States, 1991).
Research and Training
Many of the factors that are responsible for, or that contribute to, emergence of infectious diseases are now known. However, our understanding of these factors and of how they interact is incomplete. We are a considerable way from being able to develop strategies to anticipate the emergence of infectious diseases and prevent them from becoming significant threats to health. Nevertheless, the committee sees the development of such strategies as a desirable long-term goal and concludes that research to achieve it should be strongly encouraged. Research of this kind will often be interdisciplinary in nature and might include, for example, the development of strategies to determine the potential for certain microorganisms to emerge or of methods to assess the potential environmental and microbiological consequences of development projects. Basic research in support of this goal should also be encouraged.
Because emerging microbes are not limited by geographic boundaries, research focusing on emerging infectious diseases must involve scientists worldwide. Although this report focuses on U.S. public health, the importance of international research links and collaborations must not be forgotten. Furthermore, the success of global surveillance for these microbes depends in part on an infrastructure that includes viable research programs in nations on all continents. The United States could take a leading role, through the WHO, to develop a program of international infectious disease research
and to enlist the participation of other nations and of foundations. This program could be targeted to research on specific emerging microbes, in addition to those already addressed by two extrabudgetary programs of the WHO: Special Programme for Research and Training in Tropical Diseases (TDR) and the Vaccine Development Programme (VDP). The TDR encompasses research on selected parasitic diseases and leprosy. The VDP supports research on vaccine development using molecular approaches. The international aspect of these efforts is one of their most prominent features. The funding is multinational, the review steering committees are composed of scientists from many countries, and scientists from any United Nations member nation may compete for funding under either program. These efforts may be useful models for a global infectious disease research program.
In July 1991, the NIAID convened a task force on microbiology and infectious diseases to identify promising research opportunities and to recommend research strategies for future NIAID programs. The report of the task force was released in January 1992 (U.S. Department of Health and Human Services, 1992). This committee has reviewed the report, believes that its and the work of the task force are complementary, and supports the conclusions and recommendations of the NIAID group. Following are 11 recommendations from the NIAID report that are particularly pertinent to dealing with emerging microbial threats to health:
-
Every effort should be made to continue and expand basic research on microbial pathogenesis. These studies, using state-of-the-art techniques, should provide a detailed knowledge of how microbes cause infection and disease.
-
More needs to be known about the insects that serve as vectors for infectious agents and about the interactions of microbes with their vectors.
-
Identify, through basic research on infectious agents, new molecular targets amenable for drug design, and improve methods for their cloning, expression, purification, and crystallization.
-
Establish a new mechanism to facilitate the production of experimental vaccines on a pilot-plant scale under conditions suitable for their subsequent use in clinical studies.
-
Fundamental studies should be aimed at providing ideal vaccines that would be entirely safe and would be as effective as current vaccines that are composed of infectious microbes.
-
Increase the research focus on prevention of infection.
-
Promote multifaceted approaches to disease control that cut across different disciplines.
-
Increase the research focus on insect and tick vectors of disease.
-
Research support for the surveillance of infectious diseases should be increased to enhance the detection of emerging infectious diseases in the United States.
-
New biomedical technology should be applied to the detection, identification, and control of emerging infectious pathogens.
-
There should be an increase in the support for international research units studying infectious disease outside the United States.
Much has been written about the present and projected future shortage of scientists, physicians, and others trained to conduct basic and applied research on infectious diseases. Previous reports from the National Research Council and the IOM, for example, have stressed that there are shortages of several kinds of crucial personnel: medical entomologists (National Research Council, 1983); clinical specialists trained in tropical disease diagnosis, prevention, and control; biomedical researchers (National Research Council and Institute of Medicine, 1987); and public health specialists (Institute of Medicine, 1988).
Although this committee was not charged with examining issues related to personnel, it considers it important to register its concern about these shortages. Particularly troubling is the personnel situation in very specific disciplines involving the study of uncommon organisms such as rickettsiae. In these instances, the committee is concerned that support for training and careers for interested students is insufficient to ensure that future research programs in these disciplines will be adequately staffed.
Recently, much infectious disease research has shifted toward an approach primarily based on molecular biology, a discipline that the committee believes is critical to the prevention and control of infectious diseases in general. As important, however, is that the nation maintain a core of generalists (who are well versed in molecular biology) to respond to emerging and other infectious disease problems. Therefore, the committee urges that future training in molecular biology be integrated with training in clinical infectious diseases, epidemiology, medical microbiology, entomology, tropical medicine, and public health.
There are a number of programs managed and supported by U.S. government agencies and U.S.-based foundations that conduct research related to, and train people in, the recognition, epidemiology, prevention, and control of emerging microbial threats. In addition to those programs that are discussed below, several others should be noted, all of which support international research and capacity building in epidemiology, health policy, and management. These include the National Epidemiology Boards (NEB), sponsored by the Rockfeller Foundation; the Community Epidemiology and Health Management Network (CEN), sponsored by the Ford Foundation; and the International Health Policy Program (IHPP), sponsored by the Pew Trusts and the Carnegie Foundation (Commission on Health Research for Development, 1990). Whether they involve U.S. or foreign scientists, have a broad or narrow focus, all of these programs contribute in some way to
the international capability to recognize and respond to emerging microbial threats to health.
THE ROCKEFELLER FOUNDATION
In 1980, the Rockefeller Foundation established the International Clinical Epidemiology Network (INCLEN) to train junior medical school faculty from developing countries in the discipline of epidemiology. After receiving their training, these individuals return to their home countries where they become part of a medical school-based Clinical Epidemiology Unit (CEU) that helps evaluate the availability, effectiveness, and efficiency of health care in that nation. Faculty who complete a program at one of the five Clinical Epidemiology Resource and Training Centers (CERTC) receive a master's degree in one of several disciplines related to clinical epidemiology. The five CERTCs are located at McMaster University, Ontario, Canada; University of Newcastle, New South Wales, Australia; University of North Carolina at Chapel Hill; University of Pennsylvania in Philadelphia; and University of Toronto, Ontario, Canada (International Clinical Epidemiology Network, 1990).
The INCLEN program has resulted in the establishment of more than 25 CEUs in medical schools in Africa, Asia, India, and Latin America. The goal of each unit is to provide training to at least six epidemiologists, a biostatistician, a health economist, and a social scientist. CEU staff are to conduct research in areas that have a measurable impact on health or health care policy. In addition to supporting training at the CERTCs, the INCLEN program organizes annual scientific meetings and conducts site visits to evaluate progress at CEUs (International Clinical Epidemiology Network, 1990).
The hope is that selected CEUs will eventually become CERTCs, thus expanding the network. Closer links with other international training programs (e.g., the CDC's Field Epidemiology Training and International Health and Policy Programs) are being pursued (International Clinical Epidemiology Network, 1990).
NATIONAL INSTITUTES OF HEALTH
As part of its mandate, the NIH conducts research and training that covers a broad range of infectious and tropical infectious diseases. Both inhouse and extramural programs contribute to this effort and are under the direction of the NIAID.
-
In 1978, the agency established the International Collaboration in Infectious Disease Research (ICIDR) program. With both an international and
-
a domestic component, ICIDR efforts focus on the study of tropical infectious diseases and are designed to promote collaboration and exchange of scientific knowledge between scientists from the United States and their overseas counterparts. The majority of the research under this program must take place in the country represented by the foreign collaborator, which allows U.S. scientists to develop overseas work experience, thereby increasing their understanding of endemic diseases in other countries and their value as a potential resource for investigating disease outbreaks.
-
The Tropical Disease Research Units (TDRU) program, initiated in 1980, encourages research in tropical infectious diseases. A wholly domestic program, the grants allow investigators to use state-of-the-art technology in the study of tropical infectious diseases including the six diseases—filariasis, leishmaniasis, malaria, leprosy, schistosomiasis, and trypanosomiasis—designated by the WHO as major health problems in tropical countries.
-
Recently, the NIAID initiated the International Centers for Tropical Disease Research (ICTDR) program, which is designed to coordinate the institute's efforts in tropical diseases and international health. It is anticipated that the program will create a forum to promote more efficient use of resources, provide a means to identify targets for further research, and streamline future planning. In addition to the ICIDR and TDRU programs, the ICTDR initiative will comprise the Tropical Medicine Research Centers (TMRC) and the Intramural NIAID Center for International Disease Research (INCIDR) programs established in 1991, as well as the AIDS Research Division and Office of Tropical Medicine of the NIAID. Table 3-2, in the earlier section on Recognition, showed overseas locations affiliated with the ICTDR program.
These NIH-supported programs do not specifically address the emergence of infectious diseases. Proposed research to investigate questions related to disease emergence currently is unlikely to receive a high priority for funding, an issue of concern to this committee. To deal with the problem, the committee suggests that the NIH and other funding organizations issue requests for proposals (RFP) that address specific issues related to infectious disease emergence, for example, those involving agent, host, vector, or environmental emergence factors.
The committee recommends the expansion and coordination of National Institutes of Health-supported research on the agent, host, vector, and environmental factors that lead to emergence of infectious diseases. Such research should include studies on the agents and their biology, pathogenesis, and evolution; vectors and their control; vaccines; and antimicrobial drugs. One approach might be to issue a request for proposals
(RFP) to address specific factors related to infectious disease emergence.
CENTERS FOR DISEASE CONTROL
The majority of research and training supported by the CDC in the area of infectious diseases is conducted by the National Center for Infectious Diseases (NCID). The CDC does not maintain formal overseas laboratories, but it does support a number of foreign field stations that carry out infectious disease research and training. These initiatives are collaborative efforts with the host country (see Table 3-2 in the earlier section on Recognition).
The CDC is home to more than 40 WHO collaborating centers, more than half of which are housed in the NCID. In addition, the CDC has nearly 50 employees stationed in foreign countries, many of whom are involved in activities related to infectious disease. In fiscal 1990, the agency mounted 25 international emergency responses, 10 of which were related to infectious disease outbreaks. Agency research personnel were sent to, among other locations, Brazil (Brazilian purpuric fever), Bolivia (yellow fever), Netherlands (filovirus in monkeys), and Uganda (meningitis). In fiscal 1991, CDC personnel were instrumental in investigations of the cholera epidemic in Latin America (ASM News, 1992).
From the mid-1960s to early-1970s, the CDC administered an extramural program that awarded grants to academia and other institutions for research in infectious disease prevention and control. This program, discontinued in 1973 by the CDC as a result of tight funding (the legislation for this program then lapsed), supported up to 102 separate research projects and varied from a high of approximately $3.9 million in fiscal year 1969 to a low of $1.7 million in its final year. Examples of areas in which support was provided included the evaluation of immunization techniques and the resulting effects on the immunity of populations, the development and evaluation of laboratory diagnostic tests, field studies on the epidemiology and control of specific diseases, and defining health hazards related to pesticide use. The committee concludes that the now defunct program filled a need for support in a critical area of research.
The committee recommends increased research on surveillance methods and applied control methods, on the costs and benefits of prevention, control, and treatment of infectious disease, and on the development and evaluation of diagnostic tests for infectious diseases. Reinstating and expanding (both in size and scope) the extramural grant program at the Centers for Disease Control, which ceased in 1973, would be one important step in this direction. Similarly, the FDA extramural grant
program should be expanded to put greater emphasis on the development of improved laboratory tests for detecting emerging pathogens in food.
The CDC's Epidemic Intelligence Service (EIS) provides health professionals with training and field experience in public health epidemiology. The two-year program graduated 70 EIS officers in 1991. EIS officers are assigned to CDC headquarters, one of CDC's seven domestic field stations, state and local health departments, or, on occasion, to other federal agencies such as the FDA or the NIH. Under the tutelage of an experienced epidemiologist, EIS officers carry out epidemiologic research and investigations. Over four decades, officers have participated in investigations of such problems as the Hong Kong influenza epidemics, Legionnaire's disease and toxic shock syndrome outbreaks, and the current HIV/AIDS pandemic (Thacker et al., 1990).
The EIS program is and will continue to be an important source of experienced public health epidemiologists. It is also the model for another evolving program, the joint CDC/WHO Field Epidemiology Training Program (FETP). Begun in 1980, FETP's first efforts were in Thailand. Other FETPs have followed in Indonesia, Mexico, Peru, the Philippines, Saudi Arabia, and Taiwan. The programs are funded by the host country and international organizations, such as the WHO. FETPs provide their host countries with field-oriented epidemiologists who can actively participate in the development and implementation of needed disease prevention and control programs (Music and Schultz, 1990).
The committee considers the EIS and FETP two of the nation's primary resources for the training of epidemiologists. Current and former EIS officers and FETP graduates are important sources of information on emerging diseases. Moreover, because these individuals form an informal global network, their participation in the implementation of a global surveillance system for infectious diseases could be particularly valuable. Currently, however, their distribution is geographically restricted because of the limited number of graduates each year.
The committee recommends the domestic and global expansion of the Center for Disease Control's (CDC) Epidemic Intelligence Service program and continued support for CDC's role in the Field Epidemiology Training Program.
DEPARTMENT OF DEFENSE
The seven overseas medical research laboratories maintained by the DoD are the most broadly based international infectious disease research
laboratories supported by the United States. DoD has maintained overseas research activities since 1900, when the Yellow Fever Commission was established in Cuba. The U.S. Army supported laboratories in the Philippines from 1900 to 1934, and in Panama from 1936 to 1945. During World War II, the Navy established a tropical disease research laboratory on Guam, which was later designated the Naval Medical Research Unit No. 2 (NAMRU-2). This was followed by the establishment of additional laboratories in Burma and Egypt, the latter being the forerunner of the current NAMRU-3 laboratory in Cairo (Armed Forces Epidemiological Board, 1991).
Since that time, the DoD has supported a total of 20 strategically located overseas laboratories, research teams, and research units. At present, the department operates laboratories in Thailand, Indonesia, Egypt, Brazil, Kenya, Peru, and Korea; all of them cooperate with scientists of the host country and serve as focal points for basic and applied disease research, especially on diseases of military significance. In addition to being well situated to recognize and study emerging disease threats, the laboratories are valuable sites for testing new drugs and vaccines, since they are located in areas in which the targeted diseases are endemic. The laboratories are also a vital resource for recruiting and training medical personnel for the U.S. military (Armed Forces Epidemiological Board, 1991). The committee is concerned that some of these laboratories have been closed in the past, for reasons related both to insufficient funding and changes in mission priorities, and that further closings could jeopardize the United States' ability to detect and respond to emerging infectious disease threats.
The committee recommends continued support—at a minimum, at their current level of funding—of Department of Defense overseas infectious disease laboratories.
OTHER PROGRAMS
An excellent model of U.S. involvement in tropical infectious disease research is the USAID-supported International Center for Diarrheal Disease Research, Bangladesh (ICDDR, B), earlier known as the Cholera Research Laboratory. The ICDDR, B was founded in 1959 in Dhaka with funds from the International Cooperation Agency, the USAID predecessor. Much of our current understanding of cholera epidemiology and treatment is the result of studies conducted in Dhaka. The center's involvement in the development of oral rehydration therapy for cholera was a major contribution to international health. Over time, with additional support from numerous countries, the facility has evolved into a high-caliber multinational research organization.
Another potentially useful program model is the National Health Service Corps (NHSC). The NHSC was created in 1970 by Public Law 91-623 to
improve the delivery of medical services in medically underserved areas of the United States. In 1972, the NHSC Scholarship Program was initiated. This program underwrites the costs of medical education and in return requires physicians trained with NHSC money to repay their debt by serving in areas in which health services are inadequate (Brown and Stone, 1990). The committee is unaware of any similar program directed toward those who wish to train for careers in public health and related disciplines, such as epidemiology, infectious disease, and medical entomology. Because more individuals with training in these disciplines are likely to be needed to fulfill the United States' commitments to the implementation of a global infectious disease surveillance network, the establishment of such a program, modeled after the NHSC, might help to attract individuals who otherwise would not consider public health careers.
The committee recommends that Congress consider legislation to fund a program, modeled on the National Health Service Corps, for training in public health and related disciplines, such as epidemiology, infectious diseases, and medical entomology.
Vaccine and Drug Development
Vaccines and antimicrobial drugs have led to dramatic improvements in public health in the United States and in much of the rest of the world during the latter half of this century. Despite this encouraging history, the committee is concerned that many of the vaccines and drugs available today have been used for decades. It believes that there is a need to review the present vaccine and drug armamentaria with a view toward improving availability and surge capacity (potential for emergency response), as well as safety and efficacy.
VACCINES
Vaccines are one of the most cost-effective means now available for preventing disease. The Haemophilus influenza type B (Hib) vaccine is a good example. With its newly approved use (the vaccine is given at 2 months of age instead of at 18 months) and assuming an effectiveness rate of 72 percent, the total cost savings for vaccinating a one-year cohort of infants is estimated to be more than $359 million. (Including the cost of providing and administering the vaccine, this works out to $106 million in vaccine costs versus $465.3 million in disease/morbidity/mortality costs [M. Rowe, Policy Analysis and Legislation Branch, NIAID, personal communication, 1992].)
In addition to protecting the individual who has been vaccinated, the
effects of immunization can extend to unvaccinated persons through so-called herd immunity. Herd immunity protects nonimmune individuals by reducing the number of infected individuals in the community (either because of previous exposure/natural immunity or vaccination) below the critical level needed to sustain transmission. Within such a community, the likelihood of a susceptible individual coming into contact with someone who has the specific disease is thus reduced. Vaccines can also have significant secondary public health and economic benefits. For example, hepatitis B vaccination may prevent the development of hepatocellular carcinoma, and influenza vaccine may prevent secondary bacterial pneumonia.
In many countries, including the United States, the use of vaccines has reduced or eliminated death and illness from infectious diseases. There are now effective vaccines against a number of once common childhood illnesses, including diphtheria, pertussis, measles, mumps, rubella, and polio. The global eradication of smallpox was possible because of the availability of a vaccine; polio is on the verge of being eradicated from the Western Hemisphere for the same reason. Influenza vaccine reduces morbidity in the young and prevents fatal disease in the elderly. Newer vaccines, against Hemophilus influenza B, hepatitis A and B, and Streptococcus pneumoniae, when used to full advantage, will significantly reduce morbidity and mortality as well. Altogether, there are more than 20 infectious diseases that can be prevented through the use of vaccines; nevertheless, there are many diseases for which no vaccine is available. These facts constitute a strong argument for making vaccine development an important first consideration for controlling microbial threats to health.
The route by which vaccines move from the research laboratory into the doctor's office is quite complex; it involves many government agencies and private organizations, and is only very loosely coordinated. There are multiple steps in the process, each with different decision makers who respond to diverse political, social, and economic forces (see Table 3-3). Federal vaccine development efforts are the responsibility of the PHS's National Vaccine Program Office, but there is considerable autonomy for program direction within the principal agencies—the NIH, CDC, FDA, and DoD.
The foundation for developing new or improved vaccines is basic research in microbiology, immunology, and disease pathogenesis. This research is largely carried out at the NIH, DoD (understandably, DoD's efforts are oriented toward military needs), universities, and biotechnology firms; it is funded by federal grants, private foundations, and the biotechnology industry. The level of effort reflects the priority decisions of the funding organizations—principally, the NIAID and DoD.
Applied research, which leads directly to vaccine development, is also funded by the NIAID, often through contracts, and by DoD. There is also significant investment at this level by industry. The rate of progress in any
TABLE 3-3 The U.S. Domestic Vaccine Research, Development, and Utilization Process
|
RDU Activity |
Major Support |
Major Performers |
|
Basic research |
NIH, NSF |
Academia |
|
|
Private foundations |
NIH |
|
Applied research |
NIH, CDC, FDA Private foundations Industry |
Academia NIH, CDC, FDA Industry |
|
(Transition to development) |
— |
FDA regulates |
|
Pilot manufacturing |
Industry |
Industry |
|
Preclinical testing |
Industry, NIH, FDA |
Industry, NIH, FDA, CDC, academia |
|
IND application |
Industry |
Industry |
|
Phase 1-3 clinical and field testing |
Industry, NIH |
Academia, CDC, NIH |
|
Large-scale manufacturing |
Industry |
Industry |
|
(Licensing) |
— |
FDA |
|
Postlicensure operational testing |
CDC, industry, FDA |
CDC, academia, industry |
|
Postlicensure safety evaluation |
CDC, industry, FDA |
CDC, FDA, industry |
|
Purchasing and utilization |
CDC, states, private medicine |
CDC, states, private medicine |
|
RDU, research, development, and utilization; NIH, National Institutes of Health; NSF, National Science Foundation; CDC, Centers for Disease Control; FDA, Food and Drug Administration; IND, application for investigational new drug. |
||
given field can be strongly influenced by the level of NIAID and DoD funding, as is the case with HIV vaccine research.
The decision to move a vaccine candidate from laboratory research to industrial development is in the hands of the private sector vaccine manufacturers. It is based on an assessment of technical feasibility, estimated development costs, and market analysis (including the potential for liability problems). In most cases, public policy only indirectly influences the decision to pursue vaccine development and thus has little effect on the character of the vaccines that eventually enter the marketplace.
During the course of vaccine development but before licensure, there is a requirement for a series of studies to prove clinical safety and efficacy. Government support for this phase of the development process is becoming increasingly common and varies in extent, depending on the priorities of NIH and the willingness of the vaccine's developers to cooperate with
government agencies. Government support of these studies is an important subsidization of the vaccine development process.
On the demand side, the purchase, distribution, and administration of vaccines are carried out through a mixture of federal, state, and private sector activities. The FDA subserves the regulatory role in vaccine licensing; the CDC is a major buyer of vaccines for federal and state programs, purchasing more than half of the vaccines used for childhood immunizations in the United States. The cost to consumers of vaccines purchased by the CDC is much lower than the cost of vaccines sold by the private sector market. Policies for the use of vaccines are developed by the Immunization Practices Advisory Committee, a CDC advisory committee, within the licensed-use guidelines set by the FDA.
Many decisions influence the life cycle of a new vaccine. In the public sector, such decisions are made independently by a number of agencies or committees (e.g., the FDA, CDC, Immunization Practices Advisory Committee [ACIP]) and are loosely coordinated by the PHS through its National Vaccine Program Office. (This agency is a coordinating office for the PHS but has no directive authority.) Corporate decision making responds primarily to market forces. The relationship between the public and private sectors is defined mainly by FDA guidelines and federal purchasing regulations and, as a result, is as often confrontational as cooperative.
Advances in immunology, molecular biology, biochemistry, and drug delivery systems have stimulated major new initiatives in vaccine development. The generation of vaccines that will come into use in the next decade is likely to be different from previous generations of vaccines. Some will contain more than one highly purified antigen and will rely on new delivery methods. Programmed-release biodegradable microspheres offer the possibility of single-dose regimens for parenteral vaccines. New oral vaccination methods will improve our ability to protect against enteric and respiratory agents.
Extensive investigations are also centering on vaccines that use attenuated viruses and bacteria as vectors to introduce specific antigenic components of disease-causing microbes. For example, a fowlpox virus recombinant, which has had parts of the genome of rabies virus inserted into its DNA, has been tested in animals to determine its ability to induce immunity to rabies. In two of five vertebrate species examined in one study, inoculation of the fowlpox recombinant vaccine candidate resulted in the induction of an immune response that protected against subsequent challenge with live rabies virus (Taylor et al., 1988). Other attenuated organisms being considered for use as vaccine vectors are vaccinia virus, baculovirus, poliovirus, Salmonella typhimurium, and bacille Calmette-Guerin (BCG). A major advantage of the vectored vaccine concept is that the vector genome can accommodate genetic material from more than one agent (perhaps as many
as six or more); thus, it might be possible to develop a single vaccine that would immunize a person against multiple agents. This area offers much promise for the future of vaccine development.
For all of their potential, however, vaccines should not be viewed as so-called magic bullets for defeating emerging microbial threats. The potential value of vaccination and the speed with which vaccines can be developed depend on many factors. Especially important are the existing scientific knowledge of the agent (or similar organisms), its molecular biology, rate of transmission, pathogenesis, how the human immune system responds to natural infection, and the nature of the protective immunity the vaccine induces.
Successful vaccines were first developed against organisms (such as smallpox and yellow fever viruses) that produce acute infections and generate a natural immune response that protects against reinfection. For such diseases, it was only necessary to induce an immune response through vaccination that was similar to that induced by the natural infection. Bacterial diseases like diphtheria and tetanus, whose clinical effects are the result of exotoxins, were good first targets for vaccine development because of the strong immune reaction stimulated by the toxins. For a number of viral diseases (such as polio), attenuated vaccines, which mimic the wild-type virus's ability to produce protective immunity, have been quite effective.
Vaccine development for other infectious diseases, particularly those caused by protozoans, helminths, and fungi, has proved to be quite difficult, often because the responsible pathogens are able to evade the body's normal immune defenses. In such cases, even natural infection does not always induce protective immunity. In malaria, for example, the protozoa that cause the disease go through a multistage life cycle. At each stage, the antigens exposed to the immune system are different; these changes effectively create a ''moving target" that is difficult for the body, and for vaccine developers, to combat effectively. An additional problem in malaria is that the body is "tricked" into mounting an immune response against noncritical parts of the organism rather than against those parts that are capable of inducing effective antimalarial immunity (Institute of Medicine, 1991a).
Vaccine development may be impeded by economic factors as well as by inherent mechanisms in the pathogens under study. The development of vaccines requires an extensive, up-front investment in research that most vaccine manufacturers (and policymakers) are reluctant to make, since few vaccines are highly profitable and the very strict FDA requirements for proof of a vaccine's safety and efficacy make the risk of failure an important consideration. This reluctance of vaccine manufacturers to invest in research contrasts with the attitude of drug manufacturers, who invest considerable funds in research and development. One reason for the difference may be that, as a group, drugs have a much better record of profitability.
Vaccine developers must also take into account the extra costs that may arise from liability claims for injuries or deaths owing to vaccine administration. This concern has forced a number of U.S. vaccine manufacturers out of the market over the past decade. Whereas in 1985, there were 10 licensed manufacturers of human vaccines (seven commercial, two state laboratories, and a single university) (Institute of Medicine, 1985), today there are only five.
Industry currently lacks economic incentives to stimulate efforts at preventing infectious diseases with vaccines for which there is little or no foreseeable market. Nor does the public health sector (with specific exceptions) have a mechanism for setting development programs in motion. There are ways in which industry might be encouraged to assume a greater role in vaccine development. A comprehensive strategy is urgently needed.
One approach would be to establish public/private sector collaborations in vaccine research and development, a strategy exemplified by the National Cooperative Vaccine Development Groups (NCVDG), whose goal is to address the problem of HIV. The NCVDGs represent the core of the investigator-initiated HIV vaccine development effort sponsored by the Vaccine Research and Development Branch (VRDB) of NIAID's Division of AIDS. These collaborative research teams are composed of scientists from industry, academia, and government working to develop and test experimental HIV vaccines. Current vaccine strategies being evaluated in animal models include inactivated virus, recombinant proteins, live recombinant viruses, synthetic peptides, anti-idiotypic antibodies, and passive immunization (Marta Glass, Division of AIDS, NIAID, personal communication, 1992). An alternative approach would be to offer industry economic incentives to develop vaccines. These incentives could range from partial or complete "socialization" of responsibility (government cost sharing and involvement in development decisions) to long-term guaranteed purchases of minimum amounts of a vaccine at a price that would allow the manufacturer to recover the costs of development and production.
Another current study at the Institute of Medicine is exploring issues that are likely to influence the participation and cooperation of American private and public sector organizations in the international initiative to accelerate development of new, improved childhood vaccines. The IOM Committee on the Children's Vaccine Initiative is examining legal, regulatory, economic, and practical impediments to optimal application of available national resources to the International Children's Vaccine Initiative. The results of this study, which is due to be completed in 1993, may have implications for the development of vaccines for microbial diseases in both children and adults.
Emerging microbes offer a different challenge for vaccine development than that presented by a well-established pathogen, and there are potentially catastrophic consequences if the development process is left entirely to free
enterprise (see Box 3-3). It is understandably difficult to promote private investment in vaccine development for diseases that may not materialize for 5, 10, or 20 years, if at all. If a company did stockpile vaccines for potentially emergent diseases, it would either lose its investment if the disease threat never materialized or be forced to charge extraordinary prices, when the need arose, to compensate for research (if applicable—vaccine research often is done by other than commercial manufacturers) and development costs and wasted inventory—a requirement that probably would not be tolerated by society.
The United States, with only five vaccine manufacturers, is in a precarious position should an infectious disease emergency occur. Although there are vaccine manufacturing facilities outside the United States, obtaining vaccines from these facilities in an emergency would be complicated and time-consuming. In addition, overseas regulations for licensure may differ from those of the United States, another factor that must be considered when attempting to import vaccines. New technologies and production facilities need to be developed in this country for rapid response capability.
|
BOX 3-3 Are We Prepared? A Hypothetical Case Consider the city of New Orleans, with a population of about 500,000 people. Early in this century, in cities along the lower Mississippi River, deaths from yellow fever were as high as 50 percent of those infected. We know that the insect vector for yellow fever, the mosquito Aedes aegypti, is still in the area in abundance, as is a newly introduced potential vector, Ae. albopictus. An effective vaccine exists but is not manufactured in the United States; only small stocks are available in North America, from a Canadian manufacturer. Larger stocks are stored in Brazil but would take time to mobilize. Were yellow fever to break out in New Orleans and a determination be made to vaccinate the city's population, the existing North American vaccine supply would be exhausted within several days. "Acceptable" pesticidal approaches effective for control of the vector are not available, and it would probably be necessary to undertake massive spraying and source reduction to stem the epidemic. If that approach proved to be unacceptable, because there are no effective drugs and because no U.S. manufacturer could produce sufficient vaccine in a timely fashion, we could project with some confidence that 100,000 people would become ill with yellow fever and that 10,000 would likely die within a 90-day period. In addition to the loss of life, monetary costs to the health care system and to the New Orleans economy can be predicted to be in the tens of millions of dollars. |
|
BOX 3-4 Responding to the 1976 Influenza Outbreak
|
||||||||||||||||||||||||
There is no infrastructure in place today that allows for anticipatory vaccine development in response to future pandemics. The current system barely suffices for vaccines that have a predictable, established demand. The FDA does not have primary responsibility for ensuring that needed innovations are promptly developed and marketed; instead, the innovations are expected to emerge as firms pursue their organizational goals. Vaccines against future (some would say speculative) threats are looked upon by manufacturers as offering little promise for recovery of the investment needed to drive the system.
The overall process of vaccine development, manufacturing, and use is fragmented. There is no direct connection between research and development on the one hand and the purchasing and use of vaccines on the other. The various decision makers do not work together; in fact, they respond to different pressures. This imperfect system for the development of new vaccines could easily fail to produce new products rapidly enough in the face of an emerging disease threat.
The example of influenza vaccines is instructive. The sequence of events that constituted the response to the 1976 influenza epidemic began with the initial recognition of the new virus and culminated in the production and use of a vaccine (see Box 3-4 above). It should be noted that the time intervals can vary, as a result of both technical and political factors, and
that this is a class of viruses with which researchers and manufacturers have had previous experience. In cases in which the agent is unknown, the timetable would be extended. Also of note is that these actions would have blunted a winter epidemic but not an earlier one.
The basic technology for the production of influenza vaccine is 50 years old, and global surveillance for influenza viruses was initiated by the WHO in 1947. Since then, several new subtypes of influenza A virus have emerged or reemerged, each posing the threat of pandemic disease. The system in place for responding to these threats requires the combined and integrated efforts of the CDC, academic laboratories, private industry, and the FDA to recognize a new antigenic variant of influenza virus, fabricate (through genetic reassortment) an acceptable vaccine strain, distribute the strain to manufacturers, and monitor production lots. The system works reasonably well. But as with so many other vaccine programs, influenza vaccines are underused—only a fraction of those at increased risk of fatal outcome are vaccinated. Influenza thus remains essentially an uncontrolled disease.
To bring a new vaccine rapidly from the research laboratory into general use—a necessary criterion if one hopes to prevent or control an emerging infectious disease—will require an integrated national process that
-
defines the need for a vaccine and its technical requirements;
-
defines the target populations and delivery systems;
-
ensures the purchase and use of the developed product, through purchase guarantees and targeted immunization programs;
-
relies as much as possible on the capability of private industry to manage the vaccine development process, through the use of contracted production if necessary;
-
utilizes the capacity of the NIAID to manage and support basic and applied research and to conduct clinical studies and field evaluations;
-
utilizes the capacity of the CDC and academia to conduct field evaluations and develop implementation programs;
-
is centrally coordinated to take maximal advantage of the capabilities of the public and private sectors and ensures the continued existence of a competitive, efficient, reliable vaccine manufacturing industry within the United States; and
-
is prepared for the possible rapid emergence of novel disease threats, such as occurred in the 1918–1919 influenza pandemic.
The committee recommends that the United States develop a means for generating stockpiles of selected vaccines and a "surge" capacity for vaccine development and production that could be mobilized to respond quickly to future infectious disease emergencies. Securing this capability
would require development of an integrated national process, as described above. The committee offers two options for implementation of this recommendation:
-
Develop an integrated management structure within the federal government and provide purchase guarantees, analogous to farm commodity loans, to vaccine manufacturers that are willing to develop the needed capacity.
-
Build government-supported research and development and production facilities, analogous to the National Cancer Institute's program for cancer therapeutics and the federal space, energy, and defense laboratories. The assigned mission of these new facilities would be vaccine development for future infectious disease contingencies.
ANTIMICROBIAL DRUGS
Since the 1940s, antimicrobial agents have served to control many previously life-threatening infections. Antimicrobials have the unique ability to cure certain diseases, to provide prophylaxis for others, and to reduce sources of infection. The usefulness of these drugs must be protected by careful and responsible use, and by continuing to encourage the development of new antimicrobial drugs. The development of resistance by microorganisms (see Chapter 2), as well as the emergence of new organisms, will require replacement drugs to be in the pipeline even while existing drugs are still effective. Success depends on the alertness of the clinical community in identifying resistant organisms through surveillance and in reaching consensus on the need for new drugs. Data from the CDC's NNISS will be crucial to surveillance efforts and for developing guidelines for the rational use of antimicrobial drugs, as a means to delay the development of resistance. Should a global infectious disease surveillance system be put in place, such as the one suggested in this report, tracking antimicrobial resistance worldwide may be possible.
The development of public/private sector alliances, along the lines of the National Cooperative Drug Development Groups at the NIH (similar to the vaccine groups discussed above), may be desirable. There may also be circumstances similar to the current shortage of antituberculosis drugs in which the active involvement of the FDA may be necessary to encourage manufacturers to produce specific drugs or to pursue the development of drugs for a specific purpose.
The committee recommends that clinicians, the research and development community, and the U.S. government (Centers for Disease Control, Food and Drug Administration, U.S. Department of Agriculture,
and Department of Defense) introduce measures to ensure the availability and usefulness of antimicrobials and to prevent the emergence of resistance. These measures should include the education of health care personnel, veterinarians, and users in the agricultural sector regarding the importance of rational use of antimicrobials (to preclude their unwarranted use), a peer review process to monitor the use of antimicrobials, and surveillance of newly resistant organisms. Where required, there should be a commitment to publicly financed rapid development and expedited approval of new antimicrobials.
Vector Control
The United States and other developed countries have been able to free themselves to a remarkable degree from the burden of vector-borne diseases using a variety of methods of vector control. If that level of vigilance is maintained, there is a chance of minimizing new outbreaks of vector-borne disease. The potential for vector-borne disease to emerge in the United States still exists, however, because of the abundance of certain vectors, such as Aedes albopictus mosquitoes. And even in Lyme disease, a vector-borne illness with a known vector—the Ixodes tick—there is currently no agreement on intervention strategies.
Vector control generally includes the use of one or more measures to reduce vector abundance, vector longevity, and human-vector contact. Depending on the type of vector, common control measures include, but are not limited to, indoor and outdoor spraying of chemical pesticides, application of biological control agents, destruction or treatment of larval development sites, and personal protective measures, such as covering exposed areas of the body, application of repellents, sleeping under bednets, or reducing human contact with infective insects by remaining away from areas inhabited by the vectors. Innovative methods of vector control, such as genetic modification of vectors, the development of antivector vaccines, and the use of biological control techniques are currently being examined, particularly for use in the control of mosquito vectors of malaria (Institute of Medicine, 1991a).
The transovarial transmission (from infected female vectors through their eggs to succeeding generations) of pathogens, such as arboviruses, poses some unique problems for the development of control programs. A transovarially infected adult mosquito vector can transmit infection immediately after it emerges. In the case of the LaCrosse virus, for example, it is important to preclude adult emergence and/or reduce the abundance of adult vectors that emerge in the spring or early summer. Any reduction in vector-control efforts is likely to be followed by a resurgence of the vector population.
For a disease agent that is known or suspected to be transmitted by an
arthropod vector, efforts to control the vector can be crucial in containing or halting an outbreak. This is true even for those vector-borne diseases, such as yellow fever or malaria, for which there is or may eventually be an effective vaccine. To be effective, a vaccine must have time—often several weeks—to elicit an immune response in recipients. Vector control may provide this opportunity (see Box 3-5).
|
BOX 3-5 Vector Control in Action Venezuelan equine encephalomyelitis was introduced into Texas in 1971. This was not a new virus but a highly pathogenic (in both equines and people) strain that had emerged in Central America in 1969. The disease advanced from Guatemala through Mexico and into Texas, a distance of more than 4,000 kilometers, in two years. The virus produced high-titer viremias in equines and was isolated from many species of mosquitoes that fed on equines and people. Most of these mosquito species previously had been considered to be pests rather than vectors of disease (Sudia et al., 1975). The initial approach to containing the epidemic was to immunize equine populations (horses, mules, donkeys, and burros) across extensive areas of Central America and Mexico. The objective was to create an immunological barrier to prevent further spread. Fortunately, a vaccine developed by U.S. Army researchers (Berge et al., 1961) had been stockpiled, and additional doses were rapidly prepared. Although more than 4 million equines were vaccinated in Mexico in a two-year period, the virus continued to spread. There were tens of thousands of equine cases and 8,000 to 10,000 equine deaths in Mexico alone. Almost 17,000 cases (but no deaths) were reported in humans (Sudia et al., 1975). Once it was recognized that the disease had invaded Texas, a massive campaign to eliminate the virus was initiated (Pan American Health Organization, 1972). A total of 2.25 million equines were vaccinated over an 11-state area, and a quarantine was established to prevent movement of the equines out of infected areas. Malathion and dibrom pesticides were applied over 8 million acres in Texas and Louisiana to control mosquito populations. With completion of these activities in 1972 and the onset of winter, the pathogenic strain of the virus disappeared from Texas, Mexico, and Central America. The program's cost exceeded $30 million (Sudia et al., 1975). The virus has not reappeared, and it must be assumed that the vaccinated equine population has, after 20 years, been replaced by susceptible animals. Thus, this region is now receptive to the reintroduction of a pathogenic virus from South America or to the reemergence of a virulent strain from the Venezuelan equine encephalomyelitis viruses endemic in Central America and Florida. |
In temperate zones, epidemic onset of a newly emergent vector-borne disease occurs most often in the spring or summer, since both vector and pathogen depend on higher temperatures to maintain a rapid rate of reproduction. The spread of infection during the summer months may be rapid, particularly if humans are an effective source of vector infection or if the agent has become widespread in a nonhuman reservoir population. Thus, to be effective, vector control efforts must be launched shortly after the disease is first recognized or, ideally, before the disease is apparent.
For most vector-borne infectious diseases, the onset of winter dampens transmission or can even eliminate the vector or infectious agent. The exception is pathogens that can survive in humans for long periods and produce chronic infection (e.g., malaria and typhus). Vectors native to temperate areas, if introduced into new regions, may be able to survive at low temperatures, while those native to the tropics may not. In much of North America, cold weather is a second line of defense against most newly emerged or introduced pathogens that depend on vectors to be transmitted to humans. A sudden decrease in incidence of an unidentified disease at the start of winter may be the first epidemiological evidence that the disease is vectorborne.
VECTOR-CONTROL RESOURCES
North America has extensive vector-control resources. In fact, vector control is an essential part of environmental health programs in many communities. California's mosquito control, for example, covers most of the state and involves some 72 agencies with a 1991 budget of more than $48.9 million for an area with a population of more than 20 million (California Mosquito and Vector Control Association, Inc., 1991). Statewide surveillance for mosquito-borne encephalitis, plague, malaria, and Lyme disease is coordinated by the California Department of Health Services.
There are approximately 1,000 additional regional and community vector-control and vector-surveillance programs in the United States and Canada (American Mosquito Control Association, 1991). Most of these programs are geared to protecting local populations from indigenous vector-borne diseases and arthropod pests. They may also provide an early line of defense against newly introduced or resurgent vector-borne diseases. In the United States, responsibility for organizing surveillance data and investigating epidemics of emerging vector-borne diseases, such as encephalitis, plague, and Lyme disease, rests with the CDC's Division of Vector-Borne Infectious Diseases in Fort Collins, Colorado.
The control methods used in a particular region depend on the vectors that are present and on what is known about their biology and behavior. Chemical and biological agents and environmental modification can be
used individually or together in an integrated control effort. Although many local and regional vector-control programs can effectively combat local and even regional outbreaks of vector-borne disease, they are not equipped to deal with outbreaks that are national in scope. For example, regional vector-control programs cannot declare a health emergency or bypass the many legal restrictions that now limit the use of certain pesticides that are potentially useful for vector-control efforts. That authority rests with health and environmental agencies at the state and federal levels.
PESTICIDES FOR VECTOR CONTROL
A growing problem in controlling vector-borne diseases is the diminishing supply of effective pesticides. Federal and state regulations increasingly restrict the use and supply of such chemicals, largely as a result of concerns over human health or environmental safety. All pesticides must be registered with the U.S. Environmental Protection Agency (EPA) before they can be offered for sale in the United States. A 1972 amendment to the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), called for all pesticides to be re-registered by 1975 in order to meet new health and safety standards (Public Law No. 92-516). By 1986, only one of approximately 1,200 previously registered pesticides had met all of the re-registration requirements. A 1988 amendment to FIFRA moved the re-registration deadline to 1997, giving manufacturers additional time to locate or develop scientific data necessary for re-registration that were not in the original registration materials for their products. If adequate data are not submitted by the cut-off date, pesticide makers face the loss of registration (Moses, 1992).
Some manufacturers have chosen not to re-register their products because of the expense of gathering the required safety data. Partly as a result, many effective pesticides developed over the past 40 years to control agricultural pests and vectors of human disease are no longer available because their registrations have been canceled or suspended in the United States.
For example, malathion, a pesticide used worldwide for both agricultural and public health purposes, is currently registered in the United States but must be re-registered in accordance with the provisions of FIFRA. The manufacturer (American Cyanamid Corporation) has sold the rights to malathion to a Danish company, which may or may not apply for re-registration in the United States. Because malathion is an effective, relatively inexpensive broad-spectrum pesticide, a failure to re-register would be considerable cause for concern.
Pyrethrum, a plant product that has been used successfully to control adult vectors for many years, is currently being reviewed for its potential environmental and health hazards. This product is not produced in the
United States, and supply is often a problem. Nevertheless, its failure to be re-registered would be a serious loss to the vector-control armamentarium in this country.
In addition, the new registration frequently limits the circumstances under which products may be applied. In many instances, compounds that were once approved for pest-control applications are now restricted to certain narrow agricultural uses, such as for pest control in a single crop. The result is that many pesticides that might have been used to control emerging vector-borne diseases are either no longer registered or are not available in sufficient quantity.
In accordance with federal endangered species legislation, the EPA further restricts pesticide use through its Endangered Species Protection Plan. The plan prohibits the use of a wide range of pesticides within the habitat of any endangered species. Prohibitions extend in some cases to urban and suburban environments, in which outbreaks of vector-borne disease pose a particular threat. Efforts have been made to develop a workable, legal strategy for vector control in the event of a public health emergency. Specifically, EPA has developed an emergency exemption procedure in collaboration with the California Mosquito and Vector Control Association and the American Mosquito Control Association. The plan calls for specific steps to be followed when surveillance data suggest that the possibility of an outbreak of a vector-borne disease is great. After the local vector-control agency has determined a need to invoke the exemption, it must follow a 12-step procedure that includes review of the area for endangered species, consultation with the U.S. Fish and Wildlife Service (FWS), submission of a request for a public health exemption to the state public health agency or the CDC, a review and determination by the state agency or the CDC (which must be performed within 10 days if an emergency is anticipated or within 24 hours if the emergency is in progress), review and revision (if necessary) of the original plan and submission of a final plan to the state or the CDC, submission (within 15 days) by the CDC of a request to the EPA for an exemption, EPA consultation with the FWS, EPA approval or denial of the request (within 15 days), and, finally, implementation of the plan (B. Eldridge, Director, Mosquito Research Program, Department of Entomology, University of California at Davis, personal communication, 1992).
The committee recommends that the Environmental Protection Agency develop and implement alternative, expedited procedures for the licensing of pesticides for use in vector-borne infectious disease emergencies. These procedures would include a means for stockpiling designated pesticides for such use.
As with vaccines, there is little economic incentive for firms to develop new pesticides for public health use because such use makes up a very
small part of the pesticide market. The committee feels strongly, however, that pesticide development in this area needs to be given some priority. Pesticide development is now driven mainly by the demands of agriculture. Moreover, as pesticide development has become ever more specialized, there are fewer compounds available that have both agricultural and public health uses.
Agricultural applications account for about 75 percent of pesticide use in the United States. Approximately 407,000 tons of pesticide were used in 1987, of which about 89,500 tons were insecticides. Public health use accounts for about 10 percent of all pesticides globally; the major public health uses are for control of malaria, filariasis, schistosomiasis, onchocerciasis, and trypanosomiasis (Moses, 1992).
Dichlorodiphenyl trichloroethane (DDT), one of the most effective and economical pesticides ever developed, was first marketed in 1942, three years after Swiss chemist Paul Mueller discovered that the compound had insecticidal properties. In 1972, all agricultural use of DDT in the United States was banned because of its adverse environmental effects. Its use is now restricted by the EPA to public health emergencies, as defined under FIFRA. DDT is still used in many developing countries for public health purposes, particularly malaria control. Currently, aldrin, benzene hexachloride, chlordane, chlordimeform, DBCP, diazinon, dieldrin, dinogeb, ethylene dibromide, andrin, EPN, heptachlor, lindane, mirox, nitrofen (TOK), 2,4,5-T/silvex, and toxaphene also are banned, suspended, or severely restricted in their use as pesticides within the United States (Moses, 1992).
The use of insect growth regulators (so-called biorational or third-generation pesticides) to control vector populations is being investigated. These compounds affect certain biological processes of insects, such as metamorphosis, that are not present in mammals and other vertebrates. Biological control agents (the use of one organism to control another) are also considered biorational pesticides. Once licensed, many such materials will be used to control the immature stages of a number of insect vectors. They are likely to be of limited value as adulticides, however, since compounds used to control adult insects usually must produce mortality quickly. So far, only conventional broad-spectrum pesticides possess this characteristic. Resistance to biorational pesticides has recently been demonstrated in laboratory settings, even in the case of microbial pesticides.
The lack of a sufficient stockpile of effective pesticides, which might be required in the event of a major epidemic, continues to be a serious problem. The public health community has played a minor role in the formulation of pesticide use policy, which is mainly influenced by agricultural and environmental lobbying efforts. Until there are adequate alternative means for controlling disease-carrying vectors, it is critical that public health requirements for pesticides be considered when pesticide policy is being
debated. There may well be instances in which the limited application of pesticides, such as DDT, to deal with a public health emergency may be acceptable—as long as the overall burden on the environment is not excessive. The committee believes that the current EPA contingency plan that addresses this issue is ineffective: the approval process for emergency use of pesticides is so cumbersome that approval would likely come after the critical period in which application of the pesticide could avert the outbreak. Under emergency circumstances, a tradeoff must be made, so that the process can be more expedient.
Several arboviruses (St. Louis, western, and eastern equine encephalitis) are examples of diseases that could erupt suddenly into emergency proportions that might require pesticide use. These arboviruses are enzootic in North America and are maintained in a cycle of infection between wild birds and vector mosquitoes, with little or no transmission to humans. Periodically, however, excessive rain or snow, followed by high summer temperatures, favors the emergence of increased vector populations, which may lead to the rapid spread of infection to humans.
These events can occur in both urban and rural communities, and when they do, there is an immediate need to implement a control program. The primary goal at the onset of mosquito-borne disease epidemics is to eliminate the infective mosquitoes as quickly as possible. Transmission can only be stopped by the effective application of a pesticide that kills adult mosquitoes. A control program directed against the preadult aquatic and adult stages of the vector would not have an immediate effect on virus transmission but might be valuable for preventing a prolonged epidemic.
St. Louis encephalitis (SLE) exemplifies the above scenario. It has frequently reemerged as an epidemic infection in the United States (Monath, 1980), most recently in Florida and Texas in 1990 (Centers for Disease Control, 1990d). In 1966, an effort was made, in the middle of an epidemic in Dallas, Texas, to evaluate the effectiveness of controlling populations of adult mosquitoes that transmit this disease. There were 545 suspected and 145 confirmed cases of SLE in a period of a few weeks (Hopkins et al., 1975). In an eight-day period, 475,000 acres of the area were aerially sprayed with 12,000 gallons of malathion in an ultra-low-volume, high-concentration mist. Observations made before and after the application indicated that there was a significant reduction in the vector population and its infection rate. Few new cases were detected during the two to three weeks after the spraying. This is one of the few epidemics of a reemerging infection for which a study was conducted on its economic impact. It was estimated that the SLE outbreak cost the community $796,500, of which almost $200,000 was spent on vector control (Schwab, 1968). The economic and public health consequences would certainly have been greater had pesticides not been available.
Alternative strategies for the control of epidemics of SLE and western equine encephalitis are considered in detail elsewhere (Reeves and Milby, 1990). In the event of an epidemic caused by one of these enzootic viruses, the control of adult vectors is probably the best approach for stopping the spread of disease. To be successful, it has been estimated that pesticide application should achieve a 90 percent or greater reduction in the infected vector population (W. Reeves, Professor of Epidemiology Emeritus, School of Public Health, University of California at Berkeley, personal communication, 1992).
As in the drug arena, resistance to pesticides can present serious problems to disease control. Mosquitoes, flies, and other disease-carrying insects have relatively short life cycles and produce many generations per year. This is a major factor in the development of pesticide resistance, and it is usually in these groups that resistance to a given chemical is seen. There are many strategies that can be used to delay or prevent pesticide resistance. So-called pesticide resistance management can include the rotation of chemicals, avoidance of sublethal doses, and the use of biodegradable materials. More research is needed, however, to hone the usefulness of these approaches.
The committee recommends that additional priority and funding be afforded efforts to develop pesticides (and effective modes of application) and other measures for public health use in suppressing vector-borne infectious diseases.
Public Education and Behavioral Change
The areas of public education and behavioral change in relation to emerging infectious diseases currently show visible activity; the media, for example, have been presenting information to the public about the control of Lyme disease and HIV transmission. The committee was not constituted to address these two issues; however, because the topics represent potentially important aspects of emerging infectious disease prevention and control, it was considered appropriate to address them briefly here.
Public policy discussions and scientific efforts sometimes focus on vaccine and drug development and fail to give appropriate consideration to education and behavioral change as means for preventing and controlling infectious diseases. This is unfortunate, since it is often only by changing patterns of human activity—from travel and personal hygiene to sexual behavior and drug abuse—that the spread of disease can be halted.
For many infectious disease problems, however, particularly those that result from emerging microbes, the use of vaccines and drugs is not practical. Often, for newly recognized diseases, the causative agent is unknown,
making vaccine and drug development essentially impossible. Because of the long development process, vaccines and drugs can contribute little to disease control at the onset of an outbreak of a newly emergent disease. Only in a case in which an effective drug has already been developed for use against another organism and is found be efficacious against the newly discovered agent will drugs be of use in such circumstances.
HIV disease illustrates these problems quite clearly. It has been almost a decade since HIV was isolated, yet there is no vaccine and few drugs that have been shown to slow the disease process. Since the major modes of transmission of HIV are behaviorally based, the pandemic offered a unique opportunity to put public education and behavior modification to use. Initially, officials were highly reluctant to provide candid information to the public on how to prevent the spread of HIV. Recently, however, efforts at education on HIV and AIDS, much of it from nongovernmental organizations, have been more straightforward. Among the more visible of the federal government efforts were the mailing of an AIDS information pamphlet to every household in the country in 1988 and the current television spots that provide a toll-free number to call to learn more about HIV disease. The concern of the committee is that these efforts are targeted to a general audience rather than to specific risk groups, and do not use the terminology that is most understandable to these populations.
Nevertheless, despite a disappointing beginning, the experience with HIV demonstrates that human behavior can be modified in part through education. Condom use has increased and numbers of sexual partners have decreased in most male homosexual populations that have been studied (National Commission on Acquired Immune Deficiency Syndrome, 1991). Evidence for similar behavioral change among those using intravenous drugs or crack cocaine is less encouraging.
Even when scientists and public health officials rely on education and encourage behavioral change to prevent or limit the spread of infectious disease, the public may not be convinced. Although scientists may see emerging microbes as a very real threat to public health, the average citizen may be unaware of the potential danger or may consider those dangers to be less important than other health risks, for example heart disease or cancer. In such instances, carefully conceived media campaigns may have a beneficial effect on behavior in relation to disease transmission.
The committee recommends that the National Institutes of Health give increased priority to research on personal and community health practices relevant to disease transmission. Attention should also be focused on developing more effective ways to use education to enhance the health-promoting behavior of diverse target groups.
* * * *
It is the committee's hope that this report will be an important first step in highlighting the growing problem of emerging microbial threats to health and focus attention on ways in which the United States and the global community will attempt to address such threats, now and in the future. The major emphasis in the American health care system has always been on curing rather than prevention. The committee strongly believes that the best way to prepare for the future is by developing and implementing preventive strategies that can meet the challenges offered by emerging and reemerging microbes. It is infinitely less costly, in every dimension, to attack an emerging disease at an early stage and prevent its spread than to rely on treatment to control the disease.
In some instances, what this report proposes will require additional funds. The committee recognizes and has wrestled with the discomforts that such recommendations can bring—for example, the awareness that there are other compelling needs that also justify—and require—increased expenditures. But everyone must realize and understand the potential magnitude of future epidemics in terms of human lives and monetary costs.
The 1957 and 1968 influenza pandemics killed 90,000 people in the United States alone. The direct cost of medical care was $3.4 billion (more than three times the NIAID budget for fiscal year 1992), and the total economic burden was $26.8 billion 3—almost three times the total NIH budget for fiscal year 1992 (Kavet, 1972). A more current example offers a similar lesson. The recent resurgence of TB (from 22,201 cases in 1985 to 26,283 cases in 1991, or 10.4 per 100,000 population) (Centers for Disease Control, 1992g), after a steady decline over the past several decades, will be costly. Every dollar spent on TB prevention and control in the United States produces an estimated $3 to $4 in savings; these savings increase dramatically when the cost of treating multidrug-resistant tuberculosis is factored in. We also have a recent example of what results when early prevention and control efforts are lacking. The costs of AIDS/HIV disease—in human lives as well as dollars—have been staggering, and the end is not yet in sight. The objective in the future should be earlier detection of such emerging diseases, coupled with a timely effort to inform the population about how to lower their risk of becoming infected.
Obviously, even with unlimited funds, no guarantees can be offered that an emerging microbe will not spread disease and cause devastation. Instead, this committee cautiously advocates increased funding and proposes some more effective ways for organizations—domestic and international, public and private—as well as individuals—both health professionals and the lay public—to work together and, in some cases, combine their resources. These efforts will help to ensure that we will be better prepared to respond to emerging infectious disease threats of the future.